Note: Descriptions are shown in the official language in which they were submitted.
CA 02612320 2010-07-15
PLASMA-CATALYTIC CONVERSION OF CARBONACEOUS MATTERS
Technical Field
[0003] The present invention relates to a process and a plasma-catalytic
device of
converting various carbon containing liquid or gas matter in the presence of
added
oxygen (02).
Summary of the Invention
[0004] The process and the device of assistance by electric discharge plasma
for a
partial oxidization of various liquids or gas, has for object the gas rich
production of CO
and H2 (syngas) can contain the CH4 and C2H4 also, this without soot
formation.
Carbonaceous matters considered here are fossil origin (as the diesel oil,
gas, the
kerosene, the naphtha, the heavy oil, the natural gas, etc.) or renewable (as
the rape oil,
the ethanol, the glycerol, the biooil, the molasses, the biogas, etc.).
[0005] Products conversion is obtained in a device by electric discharge
plasma
GlidArc-I, installed in a superior compartment of the device and communicating
directly
with its lower compartment filled by a refractory porous containing oxides of
nickel. The
GlidArc-I first of all serves to light the electro-reinforced total combustion
of a flux
reduce a carbonaceous (fuel) mixed with a gas combustive base of oxygen (for
example
air). This combustion warms the then catalytic refractory of post -plasma
until a
favourable temperature is obtained to the partial catalytic oxidization of
fuel toward the
syngas or the mixed syngas with methane and the ethylene (reformates).
Finally, these
discharges maintain and consolidate this partial oxidization without
production of soot
and with a total conversion of fuel. The power dissipated in the GlidArc-I is
negligible
(2% to the maximum) in relation to the power thermal lower reach by the flux
of the
syngas or of reformats it thus products.
Brief Description of the Drawings
[0006] FIG. 1 is a graph depicting the composition of commercial Diesel fuel;
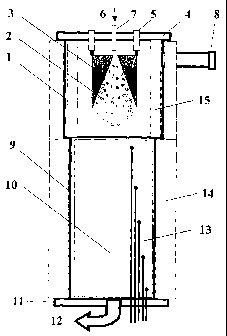
[00071 FIG. 2 is a simplified schematic of a device in accordance with the
present
invention;
[0008] FIG. 3 is a graph depicting temperature rise over time;
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
[0009] FIG. 4 is a graph depicting the heating value of a reformulate, and its
products
according to the fuel flow entering;
[0010] FIG. 5 is a graph depicting the composition of dry reformate according
to the
ra; and,
[0011] FIG. 6 is a graph depicting component concentration in the outgoing
gas, to the
proportion of the air/oil, of a device in accordance with the present
invention;
[0012] FIG. 7 is a graph depicting the exit flux of the syngas as well as the
thermal
power corresponding to this flux; and,
[0013] FIG. 8 is a graph depicting the flux and the thermal power of exiting
syngas.
Detailed Description of the Preferred Embodiments
[0014] The present invention relates to a process and a plasma-catalytic
device of
converting various carbon containing liquid or gas matter in the presence of
added
oxygen (02). According to this process, matter is converted mainly to a
gaseous mixture
of hydrogen H2, carbon monoxide CO, carbon dioxide CO2 and steam H2O,
accompanied
by light gaseous hydrocarbons. These gases may be diluted with nitrogen N2 if
air is
used as the source of oxygen. Such mixtures are referred to as reformate in
general and
synthesis gas or "syngas" when the content of hydrocarbons is limited.
[0015] The process is illustrated first by the conversion of a hydrocarbon
mixture such
as commercial Diesel fuel (diesel oil) which is derived from fossil matter.
The
composition of this liquid is indicated in Fig. 1; it contains hydrocarbons C7
to C27 (a
weighted average of C15.6 with a simplified chemical formula of CH1883) of the
paraffin,
olefin and aromatic types. Its relative density is 0.826 and contains 310 ppm
sulfur.
Another example is given for converting heavy naphtha. These two liquids
represent a
class of liquid fuels from fossil source such as gasoline, kerosene, heavy oil
and even
extra heavy oil.
[0016] The rape oil conversion illustrates that the process is applicable to
renewable
carbon compounds containing the oxygen in its molecular structure. This
example
represents a class of liquids derived from the biomass (generally known as "
renewable
source") such as ethyl alcohol either concentrated or diluted with water,
pyrolysis flash oil
from biomass (which often is called the "bio oil", containing up to 40% of
water),
molasses (which also contains water), concentrated glycerol or glycerol
diluted with
water, etc.
2
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
[0017] Finally, the conversion of natural gas, composed mainly of methane CH4
and
lesser quantities of ethane C2H6, propane C3H8 and butanes C4H10, illustrates
the process
to the gases of natural or industrial origin, of fossil or renewable, such as
the propane or
butane (called LPG), of oil well associated gases, of flare gases, of
pyrolysis gases from
fossil or renewable matter (such a gas sometimes containing steam), coal seam
methane
(firedamp of mines), or dry or humid biogases, etc.
[0018] The production of syngas from saturated light hydrocarbons (paraffins)
was the
object of an earlier French patent, see A. CZERNICHOWSKI, P. CZERNICHOWSKI,"
Assistance dlectrique d'oxydation partielle d'hydrocarbure legers par
l'oxygene ", No.
97.10989, (2 768 424). The present invention widens the types of carbonaceous
matters
that can be converted, bringing some substantial improvements: notably a
reduction in the
specific electric power to assist the conversion, the use of catalytic
refractory substances
in a post-plasma zone, and most effective starting and stopping modes of the
device.
[0019] The production of syngas is a very important process in the use of the
natural
gas, cf. the patent previously quoted. Besides, in recent years there is a
growing interest
in the production of syngas or reformate from liquid fuels of fossil or
renewable origins,
as a feed to a fuel cell. For fuel cells that operate at low temperatures, one
considers the
syngas as a source from which pure hydrogen may be extracted, while fuel cells
operating
at high temperatures, such as the "Solid Oxide Fuel Cell" (SOFC) or the
"Molten
Carbonate Fuel Cell" (MCFC), accept syngas or reformate directly as fuel.
These types
of fuel cells are of immediate interest for this invention.
Process and Plasma-Catalytic Device
[0020] The plasma-catalytic process is achieved in our device (called
"reactor" or
"reformer"), the process and the device being object of the present invention.
The device
is presented schematically in Fig. 2. This diagram illustrates an example of
the invention
but is not restricted in its use with modifications as an industrial reactor.
The device is
composed of a compartment (zone) 1 to generate plasma by gliding electric
discharges 2,
produced between two metallic divergent electrodes 3 having the shape of a
knife or a
dagger. This type of discharge is already known under name of "GlidArc-I ".
Electrodes
are connected to a high voltage source (typically 10 kV, 50 - 60 Hz or
suitable line
frequency), producing a limited current (typically <0.2 A). The electric power
in plasma
may be measured by a wattmeter. Electrodes 3 are attached to a top lid 4 by
means of
insulators 5. A mixture of reagents 6 to convert is introduced by the tube 7
disposed
3
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
symmetrically in relation to electrodes 3, the latter being placed in the
plasma
compartment 1. Reagents are composed of carbonaceous matter that needs to be
converted (called " fuel ") and air, oxygen enriched air or technical quality
oxygen (these
oxidizers called "oxidants"). Reagents can contain steam added to the oxidant
or already
present in an oxidizing gas, or some water added or initially present in a
fuel. Gas flows
are controlled by a mass flow meter, while the liquids are fed using a
metering pump.
Carbon matter is possibly preheated and can be pre-mixed with oxidizing gas
for this
operation. Reagents are injected in zone 1 of the reactor by the tube 7. The
reagents jet
pushes the electric discharge 2 starting initially between the nearest points
of electrodes 3,
provoking a fast displacement of this discharge along the jet. This discharge
glides
quickly along the electrodes and detaches when the electrode separation (that
increases
progressively) becomes too great in relation to the voltage delivered by the
electric
source. A new discharge then starts and follows the path of the previous
discharge.
[00211 The description of these gliding discharges has been given in details
in the
patent cited above. The successive discharges form an "electric flame" 2 in
presence of
an oxidant alone. One can observe this by incorporating a view-port in the
reactor 8.
This flame becomes a real thermal flame 2 electro-reinforced in presence of a
mixture of
a fuel and an oxidant, and the flame being ignited and maintained by the
GlidArc-I. The
second zone 9 called "post-plasma" is located downstream the plasma zone 1,
which is
filled with a refractory solid 10 granular shape or any other packing shape,
allowing the
outgoing gas flow of the plasma zone 1 to readily flow through this post-
plasma zone.
[00221 The refractory chosen is characterized by its porous structure and by
having a
point of fusion (melting or sintering) above 1250 C. The two zones communicate
directly (no separation so that the extremity of the GlidArc-I's discharge is
near (or in
contact) with the top layer of refractory granules (or of other catalytic
refractory filling).
The outside metallic body of zones 1 and 9 is connected to an electrical earth
ground
potential for safety reasons. The post-plasma zone is covered by a lower lid
11 which is
located near the exit 12 of products of the conversion. Several thermocouples
13 are
inserted in the filling of post-plasma zone. The two zones of the reformer are
thermally
isolated 14 from environment. Besides, the thermal isolation of the zone of
G1idArc-1 is
reinforced f om the inside 15. Finally, no part of the reactor is forced
cooled.
[00231 Chemical analyses of the outgoing gas (syngas or reformate) may be done
by
gas chromatography (GC). We used a p-GC with two channels, one channel
dedicated to
4
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
the H2, 02, N2, CH4 and CO and the other to C02, C2H4, C2H6, C2H2, C3H6+C3Hg
and
H2O (humidity). The steam flow in products is calculated from mass balance. A
complete analysis of gas only takes 255 seconds.
[0024] The two compartments 1 and 9 are therefore connected in series in
relation to
the flow of reactants and products. The first phase of the partial oxidation
conversion of
carbon matter having a chemical average formula CpHqOr (r = 0 for
hydrocarbons), starts
in the active plasma zone and ends on the first layer of the filling in the
post-plasma zone,
this filling being with the shape of a granular matter allowing passage of the
flow of
reactive products through it. Besides, whatever is the shape or the filling
technique - it
must always allow passage of the flow of reactants and products in contact
with all the
mass.
[0025] The advancement of this conversion phase can be described in a
simplified way
as a fraction f of total combustion of a carbon load
fCpHgO, + [f(p+q/4)-r/2] 02 = fp CO2 + fq/2 H2O. (1)
[0026] This reaction is greatly exothermic and the heat dissipated in the zone
I which
is thermally isolated generates the rapid warming up of this zone as well as
the warming
up of the top layer of the filling (granules); this temperature then can reach
1200 C. The
part of carbon matter that is not consumed in these conditions pyrolyzes,
producing
mainly H2, CO, methane CH4, ethylene C2H4, acetylene C2H2 and elementary solid
carbon C.
[00271 The gas flux containing products of the pyrolysis mixed with products
of
reaction (1) then cross the successive layers of the filling where the
endothermic reactions
take place that transform reagents successively to syngas mainly, or some
reformates:
C02 + CH4 = 2 CO + 2 H2 (2)
2 C02 + C2H4 = 4 CO + 2 H2 (3)
2 CO2 + C2H2 = 2 CO + H2 (4)
C02 + C = 2 CO (5)
H2O + CH4 = CO + 3 H2 (6)
2 H2O + C2H4 = 2 CO + 4 H2 (7)
2 H2O + C2H2 = 2 CO + 3 H2 (8)
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
H20 +C=H2+CO (9)
[0028] The reactions that consume heat (endothermic) provoke a progressive
lowering
of the temperature of the gas stream (and of the successive layers of the
filling crossed by
the fluid), slowing down the progression of reactions (2) to (9). The
heterogeneous
reactions (5) and (9) are especially slow. In the case of natural gas
reformation in the
referenced patent, formation of solid carbon is not a problem, even if a loose
packing of
metallic sticks are used. The quantity of soot was minimal by virtue the
refractory nature
of methane against pyrolysis. However, in the case of liquid carbonaceous
matter
presented here, their pyrolysis toward the soot is especially strong and
intolerable for the
targeted applications, such as the feeding of fuel cells like SOFC and MCFC.
[0029] We tried to replace these sticks by nickel wire to increase the surface
of contact
between the solid matter and the gaseous fluid initially. However, at the time
of the
starting of the reactor the top layer was then exposed to too high a
temperature of the
plasma jet. Indeed, the thermal conduction of these wires was not sufficient
to drive the
heat toward the deeper layers of such an arrangement. The wire deformed
dramatically.
An inert refractory granular matter such as alumina, silica or zirconia was
unsatisfactory
because soot was always present in products, and it relatively quickly blocked
all the
post-plasma zone and therefore obstructing flow through the reactor.
[0030] We finally found the solution to this problem: it consists in
dispersing nickel on
inner and outer surfaces of a porous granular matter capable of handling high
temperatures. We used the "chamotte" (a natural aluminosilicate cooked to high
temperature, of beige color with stains indicating the presence of other
oxides) but other
granulates (or other shapes) of refractory can be also used. The utilization
of a solid
matter filling the post-plasma zone, to facilitate contact between the ionized
species and
excited radicals exiting the active zone of plasma has been evoked in the
previous patent
but the nature of this filling was not catalytic and very insufficient for
light hydrocarbon
conversions.
[0031] The preparation of the catalytic filling of zone post-plasma is
described here, as
an example, for the case of granules.
[0032] These are activated the following way: granules of a chamotte, of size
between
and 10 mm, are soaked for 10 to 30 minutes in a boiling concentrated solution
of nickel
nitrate (no precious metal is added). The filled granules are then dried and
are calcined in
6
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
an oxidant atmosphere (air) in the oven until they become black (the color of
certain
oxides of nickel), after the decomposition of the nickel nitrate and its
transformation to
finely dispersed oxides of nickel. Other soluble compounds of nickel
(chloride, sulfate,
acetate, etc.) were also successfully used to infiltrate the granules and to
char them
[0033] The prepared granules (pre-activated) are then filled into the post-
plasma zone
of the reactor. During the first use, these granules will become active by the
following
mechanism: at the time of the starting of the cold reactor filled with
granules is heated
inside (especially the first layer of the zone 9) until the temperature is
between 600 and
1000 C (according to the composition of the carbon matter that needs to be
converted)
while using the gliding discharges of the GlidArc-I to ignite and maintain the
complete
combustion flame (1) of carbon matter. The post-plasma compartment 9 filled
with the
pre-activated granules is then in a flowing stream of combustion products
containing an
excess of oxygen. In these conditions, all nickel (whatever its initial shape
or chemical
state) on the surface of and inside the granules become oxidized completely.
One knows
oxides of nickel mainly of NiO, Ni203 and Ni304 (otherwise NiONi2O3) that
change one
toward the other according to the temperature and the oxygen activity. It is
exactly this
property that we use judiciously for our plasma-catalytic process of partial
oxidization of
carbon matter, the object of the present innovative application.
[0034] When the temperature of the top layer of granules (measured for example
with
the help of one of thermocouples used 13) reaches 600 to 1000 C, we increase
the flow
rate of carbon matter to convert (fuel) and/or we decrease the flow of oxidant
gas, for
example air, while observing a small diffusion flame (in air) of a small side
stream taken
from the product gas 12. During the starting phase, the outgoing gas is not
flammable.
[0035] Following the adjustment of flow rates to achieve a fuel rich mixture,
such that
the atomic ratio of oxygen to carbon ra=O/C becomes near to 1, the flame will
have the
characteristics of one of the following cases:
[0036] A difficult flame to maintain when ra > 1.7 or when the flammable
products are
diluted strongly in a neutral gas matrix as nitrogen, steam and/or carbon
dioxide.
[0037] A flame hardly visible by eyes but steady when ra - 1.3 to 1.7. This is
the
ideal case for the process presented here, indicating that the outgoing gas is
composed
mainly of H2 and CO with only traces of other flammable compounds (one calls
such a
mixture "syngas").
7
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
[0038] A blue steady flame presenting a faint yellow border when ra - 1.0 -
1.3. This
is an acceptable case for our process when the outgoing gas composed mainly of
H2 and
CO also contains significant quantities of other flammable components as CH4
or C2H4;
one calls such a mixture a reformate.
[0039] A steady flame of yellow color when ra << 1Ø This is a dangerous case
for
our process because it is generally accompanied by soot deposition in the post-
plasma
zone. The product gas then contains easily detectable quantities of acetylene
that confirms
this danger. The value of ra must be increased until the flame of control
becomes
acceptable.
[0040] The processes of total conversion (100%) of carbon load (no trace of
solid
carbon at the exit of the reformer) toward syngas or reformate are explained
by the
following mechanism: oxides of nickel (NiOx global formula) initially present
on and in
the pores of the granules filling the post-plasma zone, react with certain
products of
oxidation and pyrolysis created in the plasma zone. We can distinguish two
cases:
these oxides increase their state of oxidization, for example:
2 NiO + H2O = Ni203 + H2, (10)
2 NiO + CO2 = Ni203 + CO, (11)
or decrease it, for example:
2 Ni2O3 + C2H4 = 4 NiO + 2 CO + 2 H2, (12)
2NiO+C2H2=2Ni+2 CO+H2. (13)
[0041] depending on the location, temperature and oxygen activity where these
oxides
of nickel are present in the post-plasma zone. Indeed, thermocouples 13
inserted in the
zone 9 indicate a strong negative gradient of temperatures through the post-
plasma zone
bed. This fall of temperature is not the result of insufficient thermal
insulation of the
zone but is rather due to the endothermic reactions (2) to (9) and those of
the type (10) to
(13). The distribution of temperatures stabilizes after an initial startup
transient to an
operating steady state. The GlidArc-I must remain in operation to maintain the
steady
state, otherwise one observes a progressive displacement of the normal
temperature
toward the exit 12 of the reactor, which initiates a cooling of the first
layers of the bed
which in a short time causes thermo-catalytic deactivation. If the GlidArc is
turned off, a
8
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
deposit of soot inevitably is deposited in the filling, which in a short time
stops the
process.
[0042] We must maintain the filling therefore to a sufficiently high
temperature to
maintain the process needed for consumption of the carbon formed at by
pyrolysis of a
portion of the carbon load within the plasma zone:
C + NiOõ -+ CO (or C02) + NiO,,_ (14)
[0043] where s takes a value> 0 and <_ x. This very efficient process happens
to the
surface of contact between the soot and oxides of nickel, thanks to migration
property of
02_ in the structure of oxides when taken to a high temperature. As the NiOx
sub-oxide is
formed then an inverse process of oxidization of the following types occur:
NiOx_+cH2O=sH2+NiOXor (15)
NiOX_ + c CO2 = E CO + NiO,. (16)
[0044] They are thus indeed the new catalytic processes of a heterogeneous
conversion
of the soot, like the one of reactions (5) and (9). The oxide of nickel, put
down on rings of
alumina filled with 10 to 16% (mass percentage) of Ni is well known as a
catalyst of
methane steam reforming (6) into synthesis gas at 800 - 900 C and to 3.3 MPa.
Nevertheless we don't have information concerning its use for a process of
partial
oxidization of a carbon matter heavier and more complex, at atmospheric
pressure.
Experimental Results
[0045] Example A Reforming of diesel oil is a very difficult process. The
characteristic of fuel used was given previously in the present description.
The reactor
used is schematically drawn in Fig. 2. The body of the reactor is made of
stainless steel.
The external diameter of the zone 1 GlidArc-I is I10 mm while the one of the
post-
plasma zone 9 is 90 mm. Lengths of the two zones respectively are 15.5 cm and
75 cm.
Six electrodes 3 disposed symmetrically of 60 of angular distance around the
injection
tube 7 (instead of two electrodes of the Fig. 2) are powered by a six phase
high voltage
system. A cylindrical insert of steel of interior diameter of 53 mm post-
plasma zone
(length of 75 cm) is placed; the volume of this zone is therefore 1.6 liters.
The space
between the insert and the outside cylinder is filled with sand, creating a
thermal barrier.
9
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
Additionally, a ceramic fiber insulation blanket isolates this zone from the
outside. The
plasma zone is also isolated thermally inside and outside.
[0046] Atmospheric air is used as oxidizer to a constant flow of 50 L(n)/ nin,
controlled by a mass flow meter. A gear pump sends the Diesel fuel toward a
mixing tee
connector after passing through a rotameter to control the flow rate. The
absolute flow
(in g/min) is measured frequently from the difference in mass of the liquid
aspirated by
the pump. The range of flows explored is 0.65-1.0 Liter per hour. Air and fuel
are mixed
in this tee fitting and the mixture is then sent toward a stainless steel coil
heated by an
electric current of adjustable intensity. We maintain the temperature of exit
of the
mixture then to about 200 C. The thus preheated mixture is injected then by
the tube 7 of
interior diameter of 4 mms directly in the reformer.
[0047] Eleven type K thermocouples 13 measure temperatures in the different
points
of the device. We especially observe points T6, T7, T8, T9 and T10 of post-
plasma zone,
situated respectively at 7.5, 15, 30, 45 and 60 cm underneath the top layer of
the filling 10
(all the length of this zone is filled by chamotte's granules) while counting
from the top
extremity of the filling.
[0048} Granules of non-infiltrated chamotte 5 to 10 mms are first used to see
the
difference between the activated and not activated filling. We took a
proportion of 9.6
g/min of fuel for 50 L(n)/min of air corresponding to ra = O/C = 1.35.
Operation of the
reactor was stopped at the end of 4 hours following a strong rise in pressure
inside the
reactor. After cooling (under nitrogen) and the dismantling of the reactor, we
found soot
settled down on these raw granules. The deposit of soot was especially massive
between
25 and 70 centimeters from the top of filling 10. This test showed that the
inert filling
granules didn't catalyze (or insufficiently catalyzed) the conversion of soot
into useful
products such as H2 or the CO.
[0049] Example B The following test was conducted using nearly identical
conditions to those of the example A. The only difference concerns the nature
of
granules; this time they were activated by infiltration of nickel as described
previously.
For the same 9.5 g/min of fuel and 50 L(n)/min of air (ra = 1,36) we operated
for 12
hours, without difficulty and without progressive rise in pressure inside the
reactor. The
test was ended voluntarily. After the cooling (under nitrogen) and dismantling
of the
reactor, no soot was found on the active granules or on other parts of the
reactor. The
Fig. 3 presents the temperature rise during this test. A strong gradient of
temperature
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
through the post-plasma zone indicates an endothermic reaction taking place
quickly in
the top layer 10 of the activated granules. The exit gas analysis from the
reactor confirms
this visual observation (no soot or tar put down on a ceramic wool exposed to
the flux of
the exit gas) and chemical analysis. The syngas had the following composition
(% on dry
basis): H2 = 13.8, CO = 18.6, C02 = 4.5, CHI = 2.1, C2H4 = 0.3, C2H6 = 0.004,
C2H2, C3
and 02-absent, the remainder being N2 and Ar. Methane is present in reformate
from
liquids carbon fuel because it is the hardest molecule to crack or to reform.
It appears at
the time of pyrolysis, methane is synthesized in the cooler lower portion of
the bed by the
methanation reaction
C0+3H2=CH4+H20. (17)
[0050] The product gas from the device possesses a lower heating value of 5.1
kW for
an electric discharges power equal to 0.9 kW. This last value is high because
we used six
electrodes 3 of the GlidArc-I, all powered.
[0051] In examples that follow, we will show that two electrodes are
sufficient, which
means a reduction in the value of electric power assistance, then equivalent
to 1 or 2% of
the heating value of the syngas (or of reformates).
[0052] Example C The same Diesel fuel is reformed in another B reactor of the
interior diameter the post-plasma zone 9 of 82 mm and length of 22 cm only
(volume of
this zone then equal to 1.2 L). The zone 1 of GlidArc-I between two electrodes
3 has the
similar interior diameter and the length of 12.5 cm (the volume of this zone
is then 0.6 L).
The interior diameter of the injection tube 7 vaporizing mixture+ air is 8 mm;
we don't
use any nozzle or spraying to disperse fuel in air. On the contrary, we
sometimes see by
the port-hole 8 fuel droplets that don't affect our electric discharge 2.
[0053] We did several tests data in/out are the following:
Flow of the compressed air ................................... 48 - 146
L(n)/min
Diesel oil flow .................................................. 11 30 g/min
Preheating Temperature of mixture diesel oil + air ........ 140 - 200 C
GlidArc-I electric power ....................................... 310 W
Absolute pressure .............................................. 1.1 bar
11
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
[0054] Analysis of outgoing reformate (dry basis):
H2 .......... 16-20%vol.
CO ......... 19 - 22
H2+CO ..... 3 8 - 41
CO2 ......... 2.4-4.8
CH4 .......... 0.8-3.3
C2H4 ........ 0.0-2.1
N2+Ar ...... 52 - 58
C2H2, C3, 02 absent
H2S detected with the help of a specific reagent
Power (PCI) of outgoing reformats ......................... 7 - 22 kW.
[0055] The Fig. 4 represent the heating value of reformate, and its products
according
to the fuel flow entering. We note that is very easy to modify the quantity
(and the power)
of exit reformate by changing the flow of the fuel entering and associating to
this flow a
suitable flux of air to maintain the O/C atom ratio (ra) around the value 1Ø
This change
of the thermal power of the device can be made in less than one minute. The
composition
of dry reformate according to the ra is presented on the Fig. 5 for all our
reforming tests
done in the same day. A point's dispersion is due by the lack of the thermal
balance of
the device that requires more time to equilibrate. However it also
demonstrates easiness
and flexibility of the device.
[0056] A remark relates to the fate of sulfur initially present in a
relatively elevated
level of 310 ppm (weight) in this commercial fuel. The presence of H2S in the
reformate
12 indicates that the sulfur is not kept in post-plasma zone, that could
deactivate the
nickel oxide catalyst filling. It is an important fact which facilitates the
use of high sulfur
fuels, avoiding the need for prior desulfurization. It is indeed very
difficult to desulfurize
carbon matter containing many sulfur atoms, while it is simpler to extract
only H2S from
reformate or from syngas.
[0057] The other important remark concerns how to intentionally stop the
device at the
end of its run in order to prepare for future use. We recommend a quick stop
of the
device while stopping the flow of fuel followed by cutting of the electric
power for the
GlidArc-I, followed immediately by the drastic reduction of the air flow (or
of other
12
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
oxidant) until a level of about 2 L(n)/min for every 1 L of volume of the zone
post-plasma
of the reformer.
[0058] So we create a sequential oxidization through all the nickel sub-oxides
to NiO
and then Ni203, in the filling when its temperature decreases below 600 C
Ni0 + 02 -> NiO - Ni203. (18)
[0059] This process is exothermic. The heat release is controlled by limiting
the flow
rate of oxidant in order to prevent a sudden rise of temperatures of the
successive layers
of the filling at the time of such " stratified combustion ". Temperatures
observed during
such an operation can reach 1200 C without causing any problem to the filling.
The peak
bed temperature moves slowly in the direction of the combustive flux while
leaving
behind it the successive layers of nickel oxidized. Once the nickel is fully
oxidized the
device begins to cool to the ambient temperature.
[0060] The restarting of the cooled down device takes between 10 and 30 min to
the
point of which we are getting a syngas or a reformate suitable to feed for
example, an
SOFC or a MCFC. Then, about 20 to 60 min of operating the device will be
required to
reach the optimal reforming and thermal balance of the device, characterized
by a
stability of temperatures through the filling, cf. the Fig. 3.
[0061] The reforming can be stopped also of another way if one wants then to
start
again it quickly in order to pursue this reforming. In this case, one
decreases the fuel
flow, then immediately cuts the electric power of the GlidArc-I and decreases
very
sharply the oxidant flow to match the reaction (1) of total combustion of
carbon load.
The heat brought by this reaction compensates for the thermal losses of the
device and
maintains sufficiently high temperature of the post-plasma zone filling while
waiting for a
restart of reforming mode of operation.
In the case of shutdown or standby mode we also observe reactions (10) and
(11) that
regenerate the filling by complete removal of carbon before the restart of
normal
operation. It takes then only between 1 and 3 minutes. That is, in the case of
a complete
stop of the process or "hold" we oxidize traces of carbon or soot (if present,
deposited in
the device) to CO or CO2 and/or we transform sulfides of nickel (if there are
some in the
filling) into NiO (or Ni203) and SO2. This short operation of reactivation of
the catalytic
13
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
refractory can be done if necessary periodically, in order to recover the
catalytic initial
properties of the refractory filling.
[00621 Example D Another C reformer of an interior total volume of 0.6 L is
used for
the conversion of heavy naphtha. This reactor is, as the previous devices,
constructed
according to the diagram of the Fig. 2. The reactor contains two electrodes 3
and the
dissipated power in the GlidArc-I discharge is equal to 50 W. Only one
thermocouple is
inserted close to the lateral exit 12 of products where the temperature can
reach 780 C.
To simplify, we eliminated the porthole and "observed" the discharge 2 by
simple electric
means. The post-plasma zone 9 has been filled by 0.34 L of activated granules.
[00631 The studied liquid is similar to aviation fuels JP8 or Jet A; it is a
mixture of
aliphatic saturated hydrocarbons and of other alicyclic (naphthalene) C7 to
C12, with a
content of about 25% of alkyl-aromatic hydrocarbons. The relative molecular
mass of
this naphtha is roughly 150 (middle value) and its average formula can be
written as
CH1,94. The boiling point of the liquid is between 130 C and 220 C and mass
density (at
ambient temperature) is equal to 780 kg/m3. The range of flow of the naphtha
explored
was 4.8 - 11 g/min; no preheating was applied this time to the liquid by
reason of its
better volatility in relation to Diesel fuel. The studied air flow was between
23 and 42
L(n)/min.
[00641 Several tests have been done giving the following results,
concentration (%
vol., dry basis) of gas components of outgoing reformats:
H2 .............................. 15 - 21
CO .............................. 17- 22
H2+CO .......................... 32 - 42
N2+Ar ........................... 53 - 62
CO2 ............................ 2.9-5.5
CH4 ............................ 1.1-2.7
C2H4 ........................... 0.1-0.9
C2H6, C2H2, 02, C3+ .......... absent.
[0065] Once again the conversion of the naphtha is total, without any
formation of
soot or tars. From the measured inlet and exit flow rates and compositions
mass and
energy balances were obtained for these tests. The thermal power (heat rate)
carried by
the reformate is then in the range 5-10kW according quasi-linear to the flow
of entering
naphtha. Then the power of the Glidarc-1 represents only 1% max.
14
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
[0066] Example E Commercial rape oil, with an average chemical formula
C18.1H34.1O2,0 and density of 913 kg/m3, is converted into syngas (reformate)
in our
device; this conversion demonstrates the feasibility of using an alternative
and renewable
fuel. These liquids contain contaminants, which make conventional reforming
technologies unsuitable without previous deep purification of such
carbonaceous loads in
order to avoid the classic catalyst poisoning.
[0067] We use here the small C reactor described in example D. External
preheating
of the oil was used during accumulated 6 hours of testing, without changing
any part of
the reformer. The air flow is between 35 and 102 L(n)/ruin and between 10 and
27 g/min
for the oil.
[0068] The Fig 6 represents the main component concentrations (dry basis) in
the
outgoing gas of the device according to the proportion of the air/oil for all
tests. The other
gases are in negligible quantity: CH4 0.5-1.0, C2H4 0.1-0.5 and C2H6 0.01-
0.03% vol.
[0069] A precise mass balance based on results of a representative test can be
explained by the following reaction:
C18.11434.102.0 + 10.53 02 =
= 15.09 CO + 12.54 H2 + 2.31 CO2 + 0.47 CH4 + 0.11 C2H4 + 3.35 H2O (19)
[0070] This reaction indicates that the atomic ratio O/C ra = 1.16 is in the
optimal
range (only using the added oxygen).
[0071] Fig 7 represent the exit flux of the syngas (H2+CO pure only) in
L(n)/min for
all tests as well as the thermal power (heat rate) corresponding to this flux.
[0072] Example F This example presents our new process applied to the
reforming of
the natural gas (NG) taken from the city network. The composition of the NG
(in % vol.)
done by micro-CG analysis before our tests is the following (percent): I N2,
0.5 C02, 0.1
C2H6, and 0.05 C3H8, the remainder being methane. Our tests are done in an A'
reactor
that is nearly identical to the A. reactor. This A' reactor is shorter and the
steel insert
previously placed in post-plasma zone is replaced by a larger ceramic tube. It
gives the
total volume of the reactor equal A' to about 1 L.
[0073] The starting of the reformer is very easy and we get the first out-flow
of syngas
after only 8 min. The Fig 8 represent the flux and the thermal power (heat
rate) of the
exiting syngas (H2+CO only) in m3(n)/h and in kW, respectively. This gas
carries
between I and 3% of methane that is either not converted and/or regenerated
following a
CA 02612320 2007-12-13
WO 2007/002719 PCT/US2006/025092
methanization reaction (17). Anyway, it is not bothersome for the SOFCs or
MCFCS that
"burn" this molecule CH4 easily. This low content of methane is also
acceptable for the
Fischer-Tropsches syntheses of synthetic fuels indicating, another potential
application of
our plasma-catalytic device and our process using this device to transform the
natural
gases or associated gases in these fuels.
16