Note: Descriptions are shown in the official language in which they were submitted.
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
1
SMOKING DEVICE INCORPORATING A BREAKABLE CAPSULE, BREAKABLE
CAPSULE AND PROCESS FOR MANUFACTURING SAID CAPSULE
This invention relates to a smoking device
incorporating a breakable capsule, more particularly to a
filtered smoking device incorporating such a capsule in its
filter element.
In this invention, the term "capsule" means a
delivery system of a substance, said substance being
hereinafter referred to as "the core", which is enclosed into
a shell. The term "breakable capsule" refers to a capsule as
hereabove defined, wherein the shell can be broken by means
of a pressure to release the core, more specifically the
shell can be ruptured under the pressure imposed by the
smoker's fingers when the smoker wants to release the core of
the capsule.
In the prior art, some smoking devices
incorporating a breakable capsules are known. For example,
US2004/0261807 describes a cigarette comprising a tobacco rod
and a filter element connected to the tobacco rod, said
filter having a cavity wherein a capsule is disposed. Said
capsule comprises an outer gelatin shell, and an inner liquid
composition including flavoring agents. The goal of
US2004/0261807 is to make it possible for the smoker to break
the capsule, during the combustion of the cigarette, to allow
the release of the core of the capsule.
The gelatin capsule is a good storage capsule, but
is sensitive to moisture, and may soften during the smoking
time. This softening results in that the gelatin shell loses
its breakability, i.e. its ability of being ruptured under
the pressure imposed by the smoker's fingers when the smoker
wants to release the core of the capsule. Typically, the
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
2
gelatin capsule of the prior art has a very important
deformation when placed in smoking moisture conditions, which
prevents the smoker from breaking it through a pressure of
his/her fingers.
The ability to rupture is measured through the
crush strength to be exerted to rupture the capsule and
through the deformation of the capsule when pressure is
applied.
For the present invention, the crush strength at a
dry state shall not exceed 2.5 kp: a crush strength of more
than 2.5 kp appears too high to achieve the expected results.
There is therefore a need to build up new capsules
having a crush strength of at most 2.5kp and able to keep
their breakability even when exposed to the moisture brought
into the filter during the smoking.
The Applicant has now found that the incorporation
of selected hydrocolloids in the outer shell of the capsule,
and/or the coating of the outer shell by a moisture barrier
layer, results in capsules keeping their ability to rupture
within a smoking device even after exposition to the moisture
brought in said smoking device by the smoker.
Thus, the invention relates to a smoking device
comprising:
-a recipient including or able to receive burning
products, preferably tobacco,
- a filter element which is connected to the
recipient,
wherein said filter comprises at least one
breakable capsule, said capsule
- having a crush strength Ci of 0.5 to 2.5 kp,
- keeping a crush strength Cf of 0.5 to 2.5 kp and
presenting a deformation of less than two third of its
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
3
diameter prior to rupture after having been submitted to a
smoking test A. (It is known from one skilled in the art that
1 kp is 9.8 N).
The recipient may be the rod of a cigarette,
wherein the burning product is tobacco.
The initial crush strength Ci of the capsule is
measured before smoking, by continuously applying a load
vertically onto one particle until rupture using a LLOYD -
CHATILLON Digital Force Gauge, Model DFIS 50, having a
capacity of 25Kg, a resolution of 0.02 Kg, and an accuracy of
+/- 0,15 The force gauge is attached to a stand; the
capsule is positioned in the middle of a plate that is moved
up with a manual thread screw device. Pressure is then
applied manually and the gauge records the maximum force
applied at the very moment of the rupture of the capsule,
(measured in Kg or in Lb). Rupture of the capsule results in
the release of the core.
The smoking test A is performed on the smoking
Machine HEINR BORGWALDT RM 4/cs. The cigarette containing
breakable capsule is positioned on the smoking machine in the
standard starting position. The adjustable parameters are set
up as follows:
Puff volume: 35 ml (as defined in internationally
standard method for smoking machine)
Puff period: 60 seconds
Puff duration: 2 seconds
Puff sweep time: 1.8 second
Exhaust sweep time: 1 second
The test is finished when the cigarette is
completely smoked or after 7 or 8 puffs. The final crush
strength Cf is measure after having completing the smoking
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
4
test A, following the same measurement procedure as used for
the measure of Ci.
The deformation of the capsule is also measured
after smoking test A, just before the rupture of the capsule.
The deformation corresponds to the ratio between the capsule
diameter and the width of the capsule when pressed to the
limit of rupture.
According to one embodiment of the invention, the
smoking device includes a capsule having a crush strength Ci
of 0.6 to 2 kp, preferably of 0.8 to 1.2 kp and keeping a
crush strength Cf in the range of 0.6 to 2 kp, preferably in
the range of 0.8 to 1.2 kp when submitted to the smoking test
A.
According to another embodiment of the invention,
the deformation of the breakable capsule within the smoking
device at the limit of rupture, before and after its
submission to the smoking test A, is less than 2 mm,
preferably less than 1 mm.
According to a preferred embodiment of the
invention, the capsule is such that it claps or makes an
audible "pop", when ruptured.
Advantageously, the shell thickness of the capsule
is 10-500 microns, preferably 30-150 microns, more preferably
50-80 microns; the outer diameter of the capsule is in the
range of 2 to 8 mm, preferably 3 to 5 mm, more preferably 3.4
to 4.8, and even more preferably 3.5 to 4.5 mm; the ratio
diameter of the capsule / thickness of the shell is in the
range of 10 to 100, preferably 50 to 70.
The shell comprises at least one hydrocolloid
selected from gellan gum, agar, alginates, carrageenans,
pectins, arabic gum, ghatti gum, pullulan gum, mannan gum or
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
modified starch, alone or as a mixture thereof or in
combination with gelatin. The amount of said hydrocolloid(s)
present in the shell is 1.5 to 95% w/w, preferably 4% to 75%
w/w, and even more preferably 20 % to 50 % w/w of the total
5 dry weight of the shell. In a preferred embodiment, the
selected hydrocolloid is gellan.
In a preferred embodiment of the invention, when
used in combination with at least another gelling agent, the
weight ratio between gellan gum and the other gelling
agent(s) is from 80/20 to 20/80, preferably 75/25 to 25/75,
and even more preferably from 60/40 to 50/50.
According to another embodiment of the invention,
the shell contains less than 80 % gelatin, preferably less
than 75 % gelatin, and even more preferably less than 70%
gelatin by weight of the total dry weight of the shell.
In an alternative embodiment of the invention, the
capsule includes a moisture barrier coating. In this
embodiment, the shell of the capsule is coated with at least
one moisture barrier layer comprising at least one moisture
barrier agent dispersed in an organic solvent or in an
aqueous solution or suspension. In this embodiment, the shell
can be made of any hydrocolloid, including gelatin which can
even constitutes in that case the only gelling agent of the
shell. But preferably, even with the presence of the
hydrophobic coating, the shell comprises also an amount of
gellan, or agar, or carragheenan or alginates, or arabic gum,
or pectins, or pullulan gum or mannan gum sufficient to bring
a certain resistance to moisture; in this case the shell may
include 1.5 to 95 % preferably 4% to 75% w/w, and even more
preferably 20 % to 50 % w/w of the total dry weight of the
shellof at least one hydrocolloid selected from the group
CA 02612665 2012-07-10
WO 2007/010407 PCT/IB2006/002818
6
consisting of gellan agar carragheenan and pullulan gum.
According to another embodiment of the invention, the shell
of the coated capsule includes gellan, or arabic gum, or
pectines, or agar, or alginates, or carragheenan or ghatti
gum, or pullulan gum or mannan gum or a mixture thereof, but
does not include gelatin.
According to an embodiment, the weight of the shell
is of between 8-50 %, preferably 8-20%, more preferably 8-15
% by weight/total weight of the capsule.
Advantageously, the at least one moisture barrier
agent is at least one hydrophobic agent selected from those
suitable for confectionery or pharmaceutical products,
preferably selected from the group consisting of waxes,
especially carnauba wax, candelilla wax or beeswax, carbowax,
shellac (in alcoholic or aqueous solution), ethyl cellulose ,
hydroxypropyl methyl cellulose, hydroxypropylcellulose, latex
composition, polyvinyl alcohol, or a combination thereof.
More preferably, the at least one moisture barrier agent is
ethyl cellulose or a mixture of ethyl cellulose and shellac.
According to another embodiment of the invention,
the hydrophobic moisture barrier agent may be a combination
of hydroxypropylmethylcellulose, plasticizer,
microcrystalline cellulose and color or any other
TM
commercialized combination known under the name of Seppifilm
TM TM TM
from SEPPIC, or Opadry from COLORCON.
According to another embodiment of the invention,
the hydrophobic moisture barrier agent is a filmogen gelling
agent, preferably gellan gum itself.
The shell may further comprise at least one
plasticizer, which may be glycerol, sorbitol, maltitol,
triacetine or polyethylene glycol, or another polyalcohol
with plasticizing properties, and optionally one acid of the
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
7
monoacid, diacid or triacid type, especially citric acid,
fumaric acid, malic acid, and the like. The amount of
plasticizer ranges from 1% to 30% by weight, preferably from
2% to 15% by weight, and even more preferably from 3 to 10%
by weight of the total dry weight of the shell.
The shell can advantageously comprise a coloring
agentwhich renders easier the location of the capsule within
the filter during the manufacturing process of filters. The
coloring agent is preferably chosen among colorants and
pigments.
Fillers can also be included in the composition of
the shell; by filler is meant any suitable material that can
increase the percentage of dry material in the external
liquid phase and thus after co-extrusion in the obtained
shell. Increasing the dry material amount in a shell results
in solidifying the shell, and in making it physically more
resistant. Preferably, the filler is selected from the group
comprising starch derivatives such as dextrin, maltodextrin,
cyclodextrin (alpha, beta or gamma), or cellulose derivatives
such as hydroxypropylmethylcellulose (HPMC),
hydroxypropylcellulose (HPC), methylcellulose (MC),
carboxymethylcellulose (CMC), polyvinyl alcohol, polyols or
mixture thereof. Dextrin is a preferred filler. The amount of
filler in the shell is at most 98.5%, preferably from 25 to
95% more preferably from 40 to 80% and even more preferably
from 50 to 60 % by weight on the total dry weight of the
shell.
The core of the capsule included within the smoking
device of the invention may include a mixture of materials or
products which are lipophilic or partially soluble in
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
8
ethanol, or of molecules formulated as oil/water/oil
emulsions.
According to a preferred embodiment of the
invention, the core of a breakable capsule according to the
invention represents by weight 50 to 92% of said capsule,
preferably 80 to 92%, more preferably 85 to 92%.
The core of the capsule may include one or more
lipophilic solvents conventionally used in the food,
pharmaceutical or cosmetic industries. In a preferred
embodiment, these lipophilic solvents may be triglycerides,
especially medium chain triglycerides, and in particular
triglycerides of caprylic and capric acid, or mixtures of
triglycerides such as vegetable oil, olive oil, sunflower
oil, corn oil, groundnut oil, grape seed oil, wheat germ oil,
mineral oils and silicone oils. The amount of lipophilic
solvent in the core of a capsule according to the invention
is of the order of 0.01 to 90%, preferentially 25 to 75% by
weight of the total weight of the capsule.
The core may also comprise one or more aromatic or
fragrance molecules as conventionally used in the formulation
of flavoring or fragrance compositions, preferably aromatic,
terpenic and/or sesquiterpenic hydrocarbons, and more
particularly essential oils, alcohols, aldehydes, phenols,
carboxylic acids in their various forms, aromatic acetals and
ethers, nitrogenous heterocycles, ketones, sulfides,
disulfides and mercaptans which may be aromatic or non
aromatic. The core may also comprise one or more molecules or
extracts for cosmetic use.
The core may also comprise one or more fillers as
used in aromatic emulsions. Mention will be made of dammar
gum, wood resins of the ester gum type, sucrose acetate
isobutyrate (SAID) or brominated vegetable oils. The function
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
9
of these weighting agents is to adjust the density of the
fluid core.
The core or the shell may also comprise one or more
sweeteners, which may be provided in the form of a solution
or suspension in ethanol. Examples of suitable sweeteners may
be, but is not limited to, aspartame, saccharine, NHDC,
sucralose, acesulfame, neotame, etc.
The core may also comprise one or more "sensate"
aromatic agents, which provide either a freshening effect or
a hot effect in the mouth. Suitable freshening agents may be,
but are not limited to, menthyl succinate and derivatives
thereof, in particular Physcool marketed by the Applicant. A
suitable hot effect agent may be, but is not limited to,
vanillyl ethyl ether.
The flavoring agents that can be solubilized in the
solvent of the core of the capsule include, but are not
limited to, natural or synthetic aromas and/or fragrances.
Examples of suitable fragrances are fruity., confectionery,
floral, sweet, woody fragrances. Examples of suitable aromas
are vanilla, coffee, chocolate, cinnamon, mint.
According to an embodiment of the invention, the
total weight of the capsule is in the range of between 5-60
mg, preferably 10-50 mg, more preferably 20-40 mg.
The invention also relates to a breakable capsule
suitable for being incorporated in the filter of a smoking
device, which is substantially moisture stable.
"Substantially moisture stable" means that the outer shell or
outer coating of the capsule has the ability to retard
degradation of the capsule due to the water brought into the
smoking device by a smoker.
The breakable capsule according to the invention
comprises a core as described hereinbefore, and a shell, said
CA 02612665 2012-07-10
WO 2007/010407 PCT/IB2006/002818
capsule presents a crush strength from 0.5 to 2.5 kp, and
keeps a crush strength from 0.5 to 2.5 kp and a deformation
of less than two third of its diameter after having been
submitted to the smoking test A.
5 According to the invention, the hereabove described
technical features of the breakable capsule within the
smoking device are those of the breakable capsule of the
invention as such.
According to a preferred embodiment, the capsule of
10 the invention is a seamless capsule, obtained trough a co-
extrusion process. The co-extrusion process is a synchronous
extrusion of two liquids: the external and hydrophilic liquid
phase, and the internal and lipophilic liquid phase. The co-
extrusion process comprises three main stages: compound drop
formation, shell solidification and capsule collection. The
compound drop is a sphere of the liquid fill phase inside the
shell phase. The liquid fill phase is hereinafter referred to
as "the core". The shell phase is hereinafter referred to as
"the shell". The capsules of the invention may be produced by
any suitable co-extrusion process. Preferably, the capsules
are produced by an apparatus and a process as described in EP
513603.
According to an embodiment of the invention, after
the co-extrusion step, the solidification step is performed
by keeping cold the capsules in order to ensure correct
gelling of the shell for example by contacting them with a
cold bath. The cold bath is preferably cold oil. By cold in
the meaning of this invention, is meant a temperature of
between 1 and 15 C, preferably 2 and 10 C, more preferably
of between 4 and 6 C.
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
11
The capsules may then be centrifuged in order to
remove the surplus oil, optionally washed with organic
solvent (such as acetone, ethyl acetate, ethanol, petroleum
ether, etc.) also to remove the surplus oil, and dried.
According to one embodiment of the invention, after
the co-extrusion step, and eventually the solidification
step, the capsules are centrifuged.
According to another embodiment of the invention,
the capsules are co-extruded, centrifuged and optionally
immersed into a solution or an emulsion containing a curing
agent.
The curing agent may also be ethanol or any other
anhydrous organic solvent, such as ethyl acetate or
isopropanol, maintained at a temperature of between 0 and
25 C, more particularly between 10 and 20 C.
The curing agent can alternatively or also be a
bath of calcium ions, for example of calcium chloride,
dicalcium phosphate or calcium sulfate or a bath of acid
containing calcium ions of pH less than 5, preferably of 3 to
4. Examples of acids may be adipic acid, fumaric acid,
gluconic acid or glucono-delta-lactone. The calcium ion or
acid bath is preferably at a temperature of 0 to 25 C,
preferably 10 to 20 C.
The effect of the immersion step is to wash out the
oil remaining at the periphery of the capsule, and to
gradually strengthen the shell, notably through dehydration
and osmotic equilibrium.
According to an embodiment of the invention, after
immersion, the capsules are dried in a current or air at
controlled temperature and humidity. The relative humidity of
the drying air is 20o to 60%, preferably 30 to 50%; the
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
12
temperature of the drying air is of 15 to 60 C, preferably
35 to 45 C.
According to an embodiment of the invention, the
capsules can be obtained using the following method: co-
extrusion of the external and hydrophilic liquid phase on the
one hand, and of the internal and lipophilic liquid phase and
components of the core on the other hand, optionally
centrifugation, optionally immersion of the capsules so
obtained in a bath of calcium ions or acid, optionally
followed by drying.
According to another embodiment, the process
according to the invention further comprises a coating step
during which the moisture barrier outer layer is applied on
the capsules. Preferably, said coating step is performed by
dipping the capsules in a coating solution, or by spraying a
coating solution onto the capsules. Said coating step is
preferably performed after the drying step.
The capsules manufactured by means of the process
of the invention are essentially or perfectly spherical and
very homogeneous in size.
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
13
The invention is hereunder illustrated by the
following examples, which should not be considered as
limiting the scope of the invention, and shall be read with
reference to the figures.
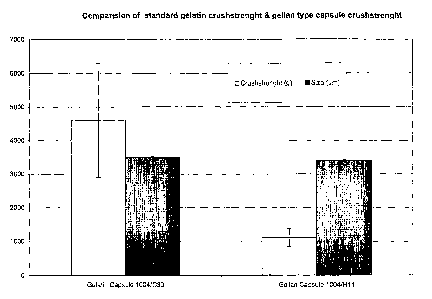
Figure 1 is a diagram comparing the crushstrength
of a capsule having for sole hydrocolloid gelatin (herein
referred to as "the gelatin capsule", and a capsule of the
invention.
Figure 2 is a diagram comparing the crushstrength
of a capsule having for sole hydrocolloid gelatin (herein
referred to as "the gelatin capsule", and a capsule of the
invention after smoking.
EXAMPLES
Example 1
Two types of capsules presenting the same size are
prepared by co-extrusion as disclosed in patent EP 513603.
The composition of capsules according to the present
invention, referenced as 1004/Hil (having less than 80 %
gelatin and including gellan gum) is given in Table 1 below,
and the composition of prior art capsules containing 80%
gelatin, referenced 1004/C30, is given in Table 2 below.
Weight of each capsule: 20.56 mg, in which :
weight of the shell: 3.68 mg (17,89%)
weight of the core: 16.88 mg (82.11%)
Table 1 - Capsules 1004/H11
External liquid phase %/total %/dry matter
Dry matter : 15.5% weight
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
14
Gellan gum Kelco F 1.800% 11.62%
Gelatin 260A 4.000% 25.82%
Sorbitol 1.000% 6.46%
Glycerol 0.500% 3.23%
Dextrin Cristal Tex T648 8.000% 51.65%
Sodium bicarbonate 0.180% 1.16%
brilliant blue FD&C#l 0.010% 0.06%
processing water 84.510%
100.000% 100%
Internal liquid phase
Ethanol 6.0000%
Mygliol 65.5000%
Menthol 28.5000%
Total 100.0000%
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
Table 2 - Capsules 1004/C30
External liquid phase %/total %/dry matter
Dry matter: 24.5% weight
Gelatin 260A 19.800 80.82
Sorbitol 2.7000 11.02
Caramel color, 2.000 8.16
processing water 75.500
100.000% 100%
Internal liquid phase
Ethanol 5.0000
Mygliol 8125 87.0000
Spearmint flavor #831661 8.0000
supplied by MANE
Total 100,0000%
5 Capsules 1004/C30 and capsules 1004/H11 present a diameter of
respectively 3489 +/- 40 and 3394 +/- 35 m.
The crush strength of the capsules is measured as follows
using a LLOYD-CHATILLON Digital Force Gauge - Model DFIS 50
10 Capacity = 25 Kgf (501b)
Resolution = 0.02Kgf (0.051b)
Accuracy = +/- 0.15%
Sampling Rate = 1000 times per second
15 Methodology:
Force Gauge is attached to a stand.
The capsule is positioned in the middle of a plate that is
moved up with a manual thread screw device. Pressure is then
applied manually and once the capsule wall fails, the gauge
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
16
records the maximum force applied prior to rupture (measure
in Kg or in lb). Sample of 10 to 20 capsules is measured.
The results are as follows:
capsules JCrush strength (Kp) Standard deviation
1004/C30 14.60 1.11
1004/H11 11.70 0.26
The results are also represented on Figure 1.
Example 2
Capsules are prepared according to example 1.
Core content is about 89 % and shell content is
about 11 %, corresponding to a thickness of dried shell of
about 50 microns. Capsules are then dried to obtain 3.5 mm
spherical capsules with a crush-strength of 1 kp. Average
weight of capsule is about 20 mg.
A prior art capsule containing gelatin (ref
1004/Cl) which 72 % gelatin as sole hydrocolloid, and a
capsule as described in example 1 above are incorporated into
a cavity filter of a cigarette. Crush strength value Ci is
measured for both capsules according to the herein described
method.
The obtained cigarettes are then smoked on a
smoking machine (smoking test A) according to international
standard procedure. Filters containing the capsules are
collected and capsules are extracted from the filter to
measure crush strength value Cf after smoking process.
In the case of gelatin capsules, capsules have
melted into the filter during the smoking process and crush
strength value is not measurable. Capsules are deformed and
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
17
cannot burst when press on them. Gellan-including capsule, on
the contrary, still burst when pressure on the filter.
Weight of each capsule: 20.57 mg,
in which weight of the shell: 2.37 mg (11,51%)
weight of the core: 18.20 mg (88.49%)
Instrumentation:
= Smoking Machine HEINR BORGWALDT RM 4/cs
The cigarettes containing breakable capsules are
positioned on the smoking machine as standard position used
for other type of analysis.The adjustable parameters are set
up as follows :
Puff volume: 35m1 (as defined in
internationally standard method for smoking
machine)
Puff period: 60 seconds
Puff duration: 2 seconds
Puff sweep time: 1.8 second
Exhaust sweep time: 1 second
Crush strength are measured according to the
procedure described in example 1 above.
Capsules 1004/C1
Internal liquid phase
Dry matter: 25,00
Components % %/dry
matter
Gelling agent Gelatin 18.000% 72.00%
Plasticizer Sorbitol 4.000% 16.00%
filler Calcium 1.000% 4.00%
Carbonate
CA 02612665 2012-07-10
WO 2007/010407 PCT/1B2006/002818
18
Color Caramel 2.000% 8.00%
Solvent water 75.000%
Total 100.000% 100%
Internal liquid phase
Name
Solubilising agent Ethanol 5.0000%
carrier Miglyol 87.0000%
Aroma Spearmint flavor 8.0000%
#831661 supplied
by MANE
I Total 100.0000%
The percentages in the third column of this table are calculated
as outlined in the following example :
In the case of gelatine, the internal liquid phase comprises 25%
of dry matter, including 18% of gelatine, with respect to the
total weight of the internal liquid phase.
Therefore, the percentage of gelatine with respect to the total
weight of the dry matter is calculated as (18%/25%)*100=72%.
The gelatine thus represents 72% by weight of the dry matter.
CA 02612665 2012-07-10
WO 2007/010407 PCT/IB2006/002818
18 a
The results are as follows:
capsules Crush strength Ci Crush strength Cf
1004/Cl 1.70kp Not measurable
(capsule has
melted)
1004/H11 1.2kp 1.2kp
After smoking, prior art capsules are no longer breakable.
Example 3
Capsules are prepared according to example 1, using the
following composition:
Capsules 1004/H6
CA 02612665 2007-12-18
WO 2007/010407 PCT/IB2006/002818
19
External liquid phase
Dry Matter:16,5%
Components % %/dry matter
gelling agent 1 Gellan gum 2.200% 13.33%
gelling agent 2 Gelatin 4.000% 24.24%
filler Dextrin CT648 10.000% 60.61%
acid or salt Sodium bicarbonate 0.200% 1.21%
Color brilliant blue FD&C #1 0.10% 0.61%
Solvent Osmosed water 83.500%
Total 100.000%
Internal liquid phase
components %
Solubilizing Ethanol 5.0000%
agent
carrier Miglyol 812S 90.0000%
aroma Spearmint flavor 5.0000%
Total 100.0000%
Weight of each capsule: 20.96mg in which
weight of the shell: 3.72 mg (17,74%)
weight of the core: 17.24 mg (82.26%)
One capsule was introduced in a cavity of a
cigarette filter. Crush strength Ci was measured according to
the procedure described in example 1 above.
Ci = 0.80 +/- 0.20 Kp
Then the cigarette was submitted to Test A as
described in example 2 above, for 8 puffs. After 8 puffs, the
capsule breaks with an audible `pop'.