Note: Descriptions are shown in the official language in which they were submitted.
CA 02612958 2007-11-30
TS 1904
- 1 -
PROCESS TO PREPARE A SWEET CRUDE
The present invention is directed to a process to
prepare a sweet crude from an ash containing and heavy
fraction of a tar sand oil.
US-A-3537977 and US-A-5958365 describe a process to
upgrade a crude mineral oil by means of hydroconversion.
The required hydrogen may be prepared by gasification of
the bottoms of a solvent deasphalting unit, which is fed
by the vacuum residue of the crude mineral oil. It is
know that crude oil contains only a very low amount of
ash.
US-A-6702936 describes a process wherein various
distillate fractions of a tar sands feed are subjected to
a hydroprocessing step to obtain a sweet synthetic crude.
From a vacuum residue fraction of the tar sands feed an
asphalt fraction is isolated and fed to a gasification
unit to obtain a mixture of carbon monoxide and hydrogen.
From this mixture substantially pure hydrogen is
recovered and used in the hydroprocessing unit.
A disadvantage of this process is that it is
sensitive for process failure. For example the
hydroprocessing unit requires a very high availability of
hydrogen. On the other hand it is known that gasification
units and de-asphalting units do not have the high
reliability to ensure a high hydrogen availability.
The present invention provides a solution to the
above problem.
A process to prepare a sweet crude from an ash
containing and heavy fraction of a tar sand oil by:
CA 02612958 2007-11-30
- 2 -
(a) supplying an atmospheric distillation bottoms of a
tar sands originated feed to a vacuum distillation to
obtain a vacuum gas oil and a vacuum bottoms,
(b) contacting the vacuum gas oil with hydrogen in the
presence of a hydrocracking catalyst to obtain a sweet
synthetic crude
(c) separating the vacuum bottoms obtained in step (a)
into an asphalt fraction comprising between 0.1 and 4 wt%
ash and a de-asphalted oil,
(d) feeding said asphalt fraction to a burner of a
gasification reactor where the asphalt fraction is
partial oxidised in the presence of an oxidiser gas in a
burner to obtain a mixture of hydrogen and carbon
monoxide,
(e) performing a water gas shift reaction on the mixture
of hydrogen and carbon monoxide,
(f) separating hydrogen sulphide and carbon dioxide from
the shifted gas in an acid removal unit thereby obtaining
crude hydrogen,
(g) purifying the crude hydrogen to obtain pure hydrogen
and
(h) using part of the pure hydrogen in step (b), wherein
in step (d) the asphalt fraction is provided to the
burner in a liquid state and wherein in case separation
step (c) fails to provide a feed for step (d), step (d)
is performed by feeding the vacuum bottoms of step (a) to
the burner in a liquid state.
Applicants found that by performing step (d) on a
liquid asphalt feed it is possible to quickly change to a
liquid vacuum bottoms feed in case the de-asphalting
operation fails. If separation step (c) fails to provide
sufficient feed for step (d) the hydrogen manufacture is
CA 02612958 2007-11-30
- 3 -
not disturbed because step (d) can be then performed on
the vacuum bottoms of step (a).
In order to further improve the reliability of the
hydrogen production it is preferred to perform step (d)
in n parallel-operated gasification reactors, wherein n
is at least 2, preferably at least 3 more preferably at
least 4. In case one gasification reactor would fail the
hydrogen availability would then only be reduced by at
least 33% or 25% in the latter cases. By designing some
extra capacity for these gasification reactors one can
also avoid any loss in hydrogen production by increasing
the production in the remaining reactors in case one of
the reactors fails.
The reliability may be further improved by
positioning in parallel a spare gasification reactor in
addition to the parallel-operated asphalt fed
gasification reactors. In the event of a failure of one
of the asphalt fed gasification reactors additional
mixture of hydrogen and carbon monoxide can be provided
by partial oxidation of a methane comprising gas in the
spare gasification reactor. The use of a methane fed
gasification reactor is advantageous because these
reactors are not very complicated and because they can
advantageously make use of the oxidiser which is at that
moment not used in one or more of the asphalt fed
reactors. The methane feed is preferably natural gas,
coal bed methane or the off-gas as separated from the
effluent of hydroprocessing step (b). The gasification
process for such methane comprising feeds are for example
the "Shell Gasification Process" (SGP) as described in
the Oil and Gas Journal, September 6, 1971, pp 5-90.
Other publications describing examples of such processes
CA 02612958 2015-02-05
- 4 -
are EP-A-291111, WO-A-9722547, WO-A-9639354 and
WO-A-9603345.
Steps (a), (b), (c), (f), (g) and (h) may be
performed as described in for example US-A-6702936.
The burner in step (d) is preferably a multi-orifice
burner provided with an arrangement of separate co-
annular passages, wherein the hydrocarbon feed flows
through a passage of the burner, an oxidiser gas flows
through a separate passage of the burner and wherein the
passage for hydrocarbon feed and the passage for oxidiser
gas are separated by a passage through which a moderator
gas flows and wherein the exit velocity of the moderator
gas is greater than the exit velocity of the oxidiser
gas.
Applicant found it advantageous to perform step (d)
with said burner in said manner to avoid burner damage. A
problem with the gasification of an asphalt fraction
originating from a tar sands is that the feed will
contain ash and that the feed will be very viscous. The
highly viscous feed will require high feed temperatures
in order to improve the ability to flow of the feed. In
addition the feed may contain next to the ash also solid
hydrocarbon agglomerates and lower boiling fractions. The
high feed temperatures and/or the presence of lower
boiling fractions or solids in the feed could give cause
to a short burner life-time because of burner tip damage.
By operating step (d) as above it has been found that
burner damage can be avoided.
Without wishing to be bound to the following theory
but applicants believe that the more stable and less
damaging operation of the burner results by using a
moderator gas having a high velocity as a separate medium
_
CA 02612958 2007-11-30
- 5 -
between oxidiser gas and hydrocarbon feed. The moderator
gas will break up the hydrocarbon feed and act as a
moderator such that reactions in the recirculation zone
at the burner tips are avoided. The result will be that
the hydrocarbon droplets will only come in contact with
the oxidiser gas at some distance from the burner
surface. It is believed that this will result in less
burner damage, e.g. burner tip retraction. The invention
and its preferred embodiments will be further described
below.
As explained above the relative velocity of the
hydrocarbon feed and the moderator gas is relevant for
performing the present invention. Preferably the exit
velocity of the moderator gas is at least 5 times the
velocity of the hydrocarbon feed in order to achieve a
sufficient break up of the liquid feed. Preferably the
exit velocity of the hydrocarbon feed is between 2 and
40 m/s and more preferably between 2 and 20 m/s. The exit
velocity of the moderator gas is preferably between 40
and 200 m/s, more preferably between 40 and 150 m/s. The
exit velocity of the oxidiser gas is preferably between
and 120 m/s, more preferably between 30 and 70 m/s.
The respective velocities are measured or calculated at
the outlet of the said respective channels into the
25 gasification zone.
Oxidiser gas comprises air or (pure) oxygen or a
mixture thereof. With pure oxygen is meant oxygen having
a purity of between 95 and 100 vol%. The oxidiser gas
preferably comprises of a mixture of said pure oxygen and
30 moderator gas. The content of oxygen in such a
moderator/oxygen mixture the oxidiser gas is preferably
between 10 and 30 wt% at standard conditions. As
moderator gas preferably steam, water or carbon dioxide
CA 02612958 2007-11-30
- 6 -
or a combination thereof is used. More preferably steam
is used as moderator gas.
The asphalt feed is liquid when fed to the burner and
preferably has a kinematic viscosity at 232 C of between
300 and 6000 cSt more preferably between 3500 and
5000 cSt, having a bulk density of between 650 and
1200 Kg/m3. The ash content is between 0.1 and 4 wt%,
especially between 1 and 4 wt%. The ash may comprise
silicium, aluminium, iron, nickel, vanadium, titanium,
potassium, magnesium and calcium. The feed may comprise
halogen compounds, such as chloride. The sulphur content
is between 1 and 10 wt%.
An example of a typical asphalt as obtained in
step (c) is provided in Table 1.
ak 02612958 2007-11-30
- 7 -
Table 1
Specific Density
Bulk Density Kg/m3 1181
Chloride
Kg/m3 670
PPmw 10
Carbon %w 85.7
Hydrogen %w 6.7
Sulphur %w 4.4
Nitrogen %w 1.6
Ash %w 1.3
Oxygen %w 0.2
Ash %w 1.3
Viscosity
@ 330 F cP 26700
@ 410 F cP 1340
@ 232 C cSt 4660
The multi-orifice burner is provided with an
arrangement of separate, preferably co-annular passages.
Such burner arrangements are known and for example
described in EP-A-545281 or DE-OS-2935754. Usually such
burners comprise a number of slits at the burner outlet
and hollow wall members with internal cooling fluid (e.g.
water) passages. The passages may or may not be
converging at the burner outlet. Instead of comprising
internal cooling fluid passages, the burner may be
provided with a suitable ceramic or refractory lining
applied onto or suspended by a means closely adjacent to
the outer surface of the burner (front) wall for
resisting the heat load during operation or heat-up/shut
down situations of the burner. Advantageously, the
ak 02612958 2007-11-30
- 8 -
exit(s) of one or more passages may be retracted or
protruded.
The burner preferably has 4, 5, 6 or 7 passages. In a
preferred embodiment the burner has 6 or 7 passages. In
an even more preferred embodiment the burner has 7
passages wherein a shielding gas flows through the outer
most passage at a velocity of between 5 and 40 m/s. The
shielding gas is preferably the same gas as used for the
moderator gas. In the embodiment wherein the number of
passages are 7, preferably the following streams flow
through the below listed passages:
an oxidiser flow through the inner most passage 1 and
passage 2,
a moderator gas flow through passage 3,
a hydrocarbon feed flow through passage 4,
a moderator gas flow through passage 5,
an oxidiser flow through passage 6, and
a shielding gas flow through outer most passage 7,
preferably at a velocity of between 5 and 40 m/s.
Alternatively the number of passages is 6 wherein the
passage 1 and 2 of the above burner is combined or
wherein the passage 7 is omitted.
The process according to the present invention is
preferably performed at a syngas product outlet
temperature of between 1000 and 1800 C and more
preferably at a temperature between 1300 and 1800 C. The
pressure of the mixture of carbon monoxide and hydrogen
as prepared is preferably between 0.3 and 12 MPa and
preferably between 3 and 8 MPa. The ash components as
present in the feed will form a so-called liquid slag at
these temperatures. The slag will preferably form a layer
on the inner side of the reactor wall, thereby creating
an isolation layer. The temperature conditions are so
ak 02612958 2007-11-30
- 9 -
chosen that the slag will create a layer and flow to a
lower positioned slag outlet device in the reactor. The
slag outlet device is preferably a water bath at the
bottom of the gasification reactor to which the slag will
flow due to the forces of gravity.
The temperature of the syngas is preferably reduced
by directly contacting the hot gas with liquid water in a
so-called quenching step. Preferably the slag water bath
and the water quench are combined. A water quench is
advantageous because a water-saturated synthesis gas is
obtained which can be readily used in the water shift
step (e). Furthermore a water quench avoids complicated
waste heat boilers, which would complicate the
gasification reactor.
The direct contacting with liquid water is preferably
preceded by injecting water into the flow of syngas
steam. This water may be fresh water. In a preferred
embodiment a solids containing water may partly or wholly
replace the fresh water. Preferably the solids containing
water is obtained in the water quenching zone as will be
described below and/or from the scrubber unit as will be
described below. For example the bleed stream of the
scrubber unit is used. Use of a solids containing water
as here described has the advantage that water treatment
steps may be avoided or at least be limited.
In a preferred embodiment of the present invention
the liquid water of the quenching step and the water bath
for receiving the slag for is combined. Such combined
slag removing means and water quench process steps are
known from for example in US-A-4880438, US-A-4778483,
US-A-4466808, EP-A-129737, EP-A-127878, US-A-4218423,
US-A-4444726, US-A-4828578, EP-A-160424, US-A-4705542,
EP-A-168128.
CA 02612958 2007-11-30
- 10 -
The temperature of the synthesis gas after the water
quench step is preferably between 130 and 330 C.
The process is preferably performed in a reactor
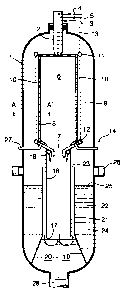
vessel as illustrated in Figure 1. The Figure shows a
gasification reactor vessel (1), provided at its upper
end with a downwardly directed multi-orifice burner (2).
Burner (2) is provided with supply conduits for oxidiser
gas (3), hydrocarbon feed (4) and moderator gas (5). The
burner (2) is preferably arranged at the top end of the
reactor vessel (1) pointing with its outlet in a
downwardly direction. The vessel (1) preferably comprises
a combustion chamber (6) in the upper half of the vessel
provided with a product gas outlet (7) at its bottom end
and an opening for the outlet of the burner (2) at its
top end. Between the combustion chamber (6) and the wall
of vessel (1) an annular space (9) is provided. The wall
of the combustion chamber protects the outer wall of
vessel (1) against the high temperatures of the
combustion chamber (6). The combustion chamber (6) is
preferably provided with a refractory lined wall (8) in
order to reduce the heat transfer to the combustion
chamber wall. The refractory wall (8) is preferably
provided with means to cool said refractory wall.
Preferably such cooling means are conduits (10) through
which water flows. Such conduits may be arranged as a
spirally wound design in said tubular formed refractory
wall (8). Preferably the cooling conduits (10) are
arranged as a configuration of parallel-arranged vertical
conduits, which may optionally have a common header at
their top (11) and a common distributor at their bottom
(12) for discharging and supplying water respectively
from the cooling means. The common header (11) is fluidly
connected to a steam discharge conduit (13) and the
ak 02612958 2007-11-30
- 11 -
common header (12) is fluidly connected to a water supply
conduit (14). More preferably the cooling conduits (10)
are interconnected such that they form a gas-tight
combustion chamber (6) within the refractory wall as
shown in Figure 2. Such interconnected conduits type
walls are also referred to as a membrane wall.
The cooling by said conduits (10) may be achieved by
just the cooling capacity of the liquid water, wherein
heated liquid water is obtained at the water discharge
point. Preferably cooling is achieved by also evaporation
of the water in the conduits (10). In such an embodiment
the cooling conduits are vertically arranged as shown in
Figure 1 such that the steam as formed can easily flow to
the common header (11) and to a steam outlet conduit (13)
of the reactor vessel (1). Evaporation is preferred as a
cooling method because the steam may find use in other
applications in the process, such as process steam for
shift reactions, heating medium for liquid feed or, after
external superheating, as moderator gas in the burner
according to the process according to the present
invention. A more energy efficient process is so
obtained.
The gasification vessel (1) preferably comprises a
vertically aligned and tubular formed outlet part (16)
fluidly connected to the lower end of the combustion
chamber (6), which tubular formed outlet part (16) is
open at its lower end, further referred to as the gas
outlet (17) of the tubular outlet part (16). The outlet
part (16) is provided at its upper end with means (18) to
add a quenching medium to the, in use, downwardly flowing
mixture of hydrogen and carbon monoxide. Preferably the
vessel (1) is further provided at its lower end with a
combined water quenching zone (19) and slag discharge
ak 02612958 2014-11-18
- 12 -
water bath (20) as described above. The water quenching
zone (19) is present in the pathway of the synthesis gas
as it is deflected at outlet (17) in an upwardly
direction (see arrows) to flow upward through, preferably
an annular space (21) formed between an optional tubular
shield (22) and outlet part (16). In annular space (21)
the synthesis gas will intimately contact the water in a
quenching operation mode. The upper end (23) of the
annular space is in open communication with the
space (24) between outlet part (16) and the wall of
vessel (1). In space (24) a water level (25) will be
present. Above said water level (25) one or more
synthesis product outlet(s) (26) are located in the wall
of vessel (1) to discharge the quenched synthesis gas.
Between space (24) and annular space (9) a separation
wall (27) may optionally be present.
At the lower end of vessel (1) a slag discharge
opening (28) is suitably present. Through this discharge
opening (28) slag together with part of the water is
charged from the vessel by well known slag discharge
means, such as sluice systems as for example described in
US-A-4852997 and US-A-6755980.
Figure 3 illustrates how the process according to the
present invention and the reactor of Figure 1 can be
applied in the production of pure hydrogen. In this
scheme to a gasification reactor 105 an asphalt feed 101,
oxygen 102 and super heated steam 119 from a gas
turbine/steam turbine utilities block 114 are fed to a
burner according to the process of the present invention
as present in combustion chamber 106. Oxygen 102 is
prepared in air separation unit 104. Nitrogen 103 as
prepared in the same unit is used as purge gas in the
gasification reactor 105. In gasification reactor 105
ak 02612958 2014-11-18
- 13 -
slag 108 flows to a water quench 107 to be disposed as
slag via 110. The flash gas 112 separated from the
slag 110 is send to Claus unit 109. A water bleed 111 is
part of the process as illustrated.
The wet raw synthesis gas 113 as prepared is
optionally treated in a scrubber unit to remove any
solids and ash particles which have not been removed in
the water quench before being further processed in a sour
water gas shift step 122 yielding a shifted gas 123 and
sour water, which is recycled via 124 to water quench
107. Between sour water gas shift step 122 and the gas
turbine/steam turbine utilities block 114 heat
integration 121 takes place. The shifted gas 123 is sent
to an acid gas removal step 126 yielding a carbon dioxide
rich gas 131, crude hydrogen 130, H2S 129 and steam
condensate 128. The carbon dioxide rich gas 131 is
compressed in compressor 136 to yield compressed carbon
dioxide gas 137. The carbon dioxide may be advantageously
disposed of by CO2 sequestration in for example sub-
surface reservoirs. The crude hydrogen 130 is further
processed in a pressure swing absorber (PSA) unit 138 to
yield pure hydrogen 140. Part 134 of the crude hydrogen
130 may be used as feed in the gas turbine/steam turbine
utilities block 114. The hydrogen rich PSA off-gas 139 is
compressed in compressor 133 and used, optionally blended
with nitrogen 132, as feed in the gas turbine/steam
turbine utilities block 114. Gas turbine/steam turbine
utilities block 114 is further provided with a fuel gas,
natural gas, feed 115, a water feed 116 and a flue gas
outlet 117 and an optional high pressure outlet 150.
In Table 2 an example is provided of the composition
of the streams of Figure 3 when a feed according to
ak 02612958 2007-11-30
- 14 -
Table 1 is used. The numerals in Table 2 refer to
Figure 1.
The process according to the invention is further
illustrated by means Figure 4. Tar sand derived oil 31 is
diluted with naphtha 32 to obtain diluted tar sand
derived oil 33, which is supplied to atmospheric
distillation unit 34. In atmospheric distillation unit
34, diluted tar sand derived oil 33 is distilled and two
atmospheric distillate streams, i.e. naphtha stream 32
and atmospheric gasoil stream 35, and atmospheric residue
36 are obtained. Atmospheric residue 36 is vacuum
distilled in vacuum distillation unit 37. Vacuum gasoil
stream 38 is obtained as distillate stream and vacuum
residue 39 as bottoms stream. Vacuum residue 39 is
supplied to solvent deasphalting unit 40 to obtain
deasphalted oil 41 and liquid asphaltic fraction 42. The
liquid asphaltic fraction 42 is fed to a gasification
unit 43. In case of a failure of solvent deasphalting
unit 40 vacuum residue 45 is fed directly to gasification
unit 43. In Gasification unit 43 hydrogen 44 is prepared
as illustrated in Figures 1 and 2. Distillate stream 35
and 38 are combined with deasphalted oil 41 to form
combined hydrocracker feedstock 48. Combined feedstock 48
is hydrodemetallised in hydrodemetallisation unit 49 in
the presence of hydrogen 50. The hydrodemetallised
combined feedstock 51 and additional hydrogen 52 are
supplied to hydrocracking unit 53 comprising a first
catalytic zone 54 comprising preferably a non-noble metal
hydrotreating catalyst for hydrodesulphurisation of the
feedstock and a second catalytic zone 55 comprising
preferably a non-noble metal hydrocracking catalyst. The
effluent 56 of the second catalytic zone 55 is separated
in gas/liquid separator 57 into upgraded sweet crude oil
CA 02612958 2007-11-30
- 15 -
product 58 and a hydrogen-rich gas stream 59 that is
combined with make-up hydrogen 60 to form hydrogen stream
52 that is supplied to the first catalytic zone 54. Make-
up hydrogen 60 and/or hydrogen 50 are hydrogen 44 as
produced in gasification unit 43. Upgraded sweet crude
oil product 58 may be fractionated into several upgraded
distillate fractions (not shown).
- 16 -
Table 2
Component Wet raw Gas ex Sour CO2 137 Raw
Pure PSA
syngas shift gas 112
Hydrogen Hydrogen offgas
113 section 130
140 139
123
Methane %mol 0.05 0.07 <0.01 0.05 0.11
- 0.74
Argon %mol 0.02 0.03 - - 0.04
0.04 0.06
0
COS %mol 0.04 - - -
- - 0
1.,
m
H2S %mol 0.48 0.74 61 5 PPm -
_
1.,
ko
01
H20 %mol 56.18 5 0.05 -
- _
m
1.,
0
H2 %mol 15.36 59.23 <0.01 0.7 93.81
99.82 60.87 0
...3
1
1-,
17'
N2 %mol 0.53 0.76 - - 1.20
0.14 7.04 w
0
CO2 %mol 0.54 38.01 34 99.1 3.01
- 19.53
CO
%mol 26.79 1.15 <0.01 0.1 1.82
11.78
HCN
%mol 0.01 - - -
-
NH3
%mol 0.01 0.02 0.01 - -
-