Note: Descriptions are shown in the official language in which they were submitted.
CA 02613806 2007-12-05
1
PROCESS OF HYDROCRACKING HEAVY OIL
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0001]
The present invention belongs to the technical field
relating to a process of hydrocracking heavy oil,
specifically relates to a process of hydrocracking heavy
oil containing heavy metal components produced in a
refining process of crude oil (hereinafter also referred to
as petroleum heavy oil containing heavy metal components).
In particular, the present invention belongs to the
technical field relating to a process in which petroleum
heavy oil containing heavy metal components such as vacuum
residue is hydrogenerated in the presence of catalyst to
obtain highly decomposed products.
2. Description of the Related Art
[0002]
In the background of rapid changes in demand structure
in which supply of heavier crude oil and demand for lighter
products proceed simultaneously, an attention has been
drawn to decomposition techniques of heavy oil for
producing lighter products in short supply from excess
heavy oil, as the limited reserves of petroleum inevitably
CA 02613806 2007-12-05
2
diminish, its importance is increasing more than ever.
[0003]
A lot of processes for thermal cracking and
hydrocracking of heavy oil have been proposed. However,
these processes have problems of some kind for
hydrocracking heavy oil containing heavy metal components
such as vacuum residues.
[0004]
In more detail, such heavy oil contain a large amount
of nitrogen compounds and sulfur compounds therein. In the
case where decomposition of heavy oil are conducted in the
presence of catalyst, a large amount of organometallic
impurities hazardous to the catalyst is contained. As
organometallic impurities (hereinafter also referred to as
metal impurities), although the ones containing nickel (Ni)
or vanadium (V) are most popular, there are some that
contain other metals. These metal impurities are
chemically bounded to organic compounds with relatively
high molecular weight such as asphaltene in heavy oil. If
they are present, catalytic activities for decomposition
and removal of compounds containing nitrogen, sulfur and
oxygen are markedly hindered.
[0005]
As a method for treating the'heavy oil without using
catalyst, there is a thermal cracking process. So called
CA 02613806 2007-12-05
3
coker process is known. The process poses a problem on
treatment of a large amount of coke produced as by-product.
Also, the yield of distillate oil obtained is inevitably
lowered due to increase in gas generation resulting from
overcracking. In addition thereto, it has drawbacks that
there are a lot of aromatic and olefinic components,
resulting in poor quality.
[0006]
In a hydrocracking process in a fixed bed method by
filling granular catalysts in a reactor, in the case of
being highly decomposed, cokes as by-product and heavy
metal components are gradually deposited on catalyst layers
while being influenced by asphaltene and heavy metal
components such as V and Ni in feedstock as mentioned above.
As a result, there is a limit for a long-period continuous
operation, causing the lowering of catalytic activity and
clogging of catalyst layer.
[0007]
In a hydrocracking process in a reactor of ebullated
bed method using extrusion-molded granular catalysts of Co-
Mo type and the like, there is no pressure-drop increase
problem due to accumulation of coke etc. because of an
intensive mixing state inside the ebullated bed reactor.
Also, it has an advantage over a fixed bed method because a
continuous operation can be done for a long period while
CA 02613806 2007-12-05
4
keeping the catalytic activity constant by being capable of
charging and discharging catalysts in operation. The
operation, however, is more difficult than a fixed bed
method because the catalyst is circulated in operation.
The catalyst is expensive, the reaction pressure is
generally as high as 150-200 kg/cmZ (15-20 MPaG), and
desulfurization and denitrogenation of reaction products
are insufficient.
[0008]
As an improving technique to solve the conventional
drawbacks described above, there has been proposed a
process in which petroleum heavy oil containing heavy metal
components are supplied together with an inexpensive iron-
based catalyst on a throw-away basis into a suspended bed
(slurry bed) reactor for hydrocracking. Such a process
(hereinafter also referred to as a hydrocracking process in
a suspended bed method using an iron-based catalyst) is,
for example, described in Japanese Unexamined Patent
Publication No. 2001-89772.
[Patent Document 1]Japanese Unexamined Patent Publication
No. 2001-89772
[Disclosure of Invention]
[Technical Problems to be Solved]
[0009] In this way, there has been proposed a hydrocracking
CA 02613806 2007-12-05
process in a suspended bed method using an iron-based
catalyst, even in this process, however, further economical
improvement is being desired.
[0010]
BRIEF SUMMARY OF THE INVENTION
The present invention has been accomplished in such a
situation above mentioned, and its object is to provide a
process of hydrocracking heavy oil, which is capable of
obtaining decomposed oil with higher yields and/or milder
reaction conditions than the conventionally proposed
hydrocracking process in a suspended bed method using an
iron-based catalyst when hydrocracking petroleum heavy oil
containing heavy metal components into lighter oil.
The above objects can be achieved by a process of
hydrocracking heavy oil containing heavy metal components
produced in a refining process of crude oil, comprising:
a vacuum distillation step to obtain the heavy oil as
distillation residue by vacuum distillation; and
a reaction step to hydrocrack the heavy oil in the
presence of an iron-based catalyst in a suspended bed
reactor,
wherein the distillation is conducted at 350 C or less
in the vacuum distillation step.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 02613806 2007-12-05
6
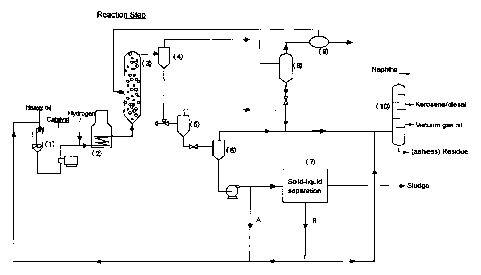
Fig. 1 is a schematic drawing showing an example of
embodiment of process of hydrocracking heavy oil containing
heavy metal components according to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0011]
The present inventors have earnestly studied to
achieve the foregoing object and accomplished the present
invention. According to the present invention, the above
object can be achieved.
[0012]
The present invention thus accomplished pertains to a
process of hydrocracking heavy oil, and is specified to the
process of hydrocracking heavy oil according to Claims 1 to
8 in CLAIMS (the process of hydrocracking heavy oil in the
first through the eighth inventions), which is based on the
following constituent.
[0013]
A process of hydrocracking heavy oil of Claim 1 is a
process of hydrocracking heavy oil containing heavy metal
components produced in a refining process of crude oil,
comprising:
a vacuum distillation step to obtain the heavy oil as
distillation residue by vacuum distillation; and
a reaction step to hydrocrack the heavy oil in the
CA 02613806 2007-12-05
7
presence of an iron-based catalyst in a suspended bed
reactor,
wherein the distillation is conducted at 350 C or less
in the vacuum distillation step (the first invention).
[0014]
A process of hydrocracking heavy oil of Claim 2 is the
process of hydrocracking heavy oil according to Claim 1,
comprising: a recycling step in which a liquid-phase
fluid obtained by gas-liquid separation of the reaction
products from the reaction step is recycled to the
suspended bed reactor; and
a recycling step in which after separation of a solid
component from the liquid-phase fluid, the resultant fluid
is recycled to the suspended bed reactor (the second
invention).
[0015]
A process of hydrocracking heavy oil of Claim 3 is the
process of hydrocracking heavy oil according to Claim 2,
wherein the content of heavy oil components with a boiling
point of 525 C or more in the liquid-phase fluid recycling
in the recycling step is 10-100 mass % of the amount of
heavy oil supplied into the suspended bed reactor in the
reaction step (the third invention).
[0016]
A process of hydrocracking heavy oil of Claim 4 is the
CA 02613806 2007-12-05
8
process of hydrocracking heavy oil according to any one of
Claims 1 to 3, wherein the reaction conditions in the
reaction step are a reaction pressure of 60-160 kg/cm2; a
reaction temperature of 430-455 C; and a reaction time of
30-180 minutes (the fourth invention).
[0017]
A process of hydrocracking heavy oil of Claim 5 is the
process of hydrocracking heavy oil according to any one of
Claims 1 to 4, wherein the iron based catalyst is a natural
limonite iron ore catalyst (the fifth invention).
[0018]
A process of hydrocracking heavy oil of Claim 6 is the
process of hydrocracking heavy oil according to any one of
Claims 1 to 5, wherein the iron based catalyst is a natural
limonite iron ore catalyst of 2pm or less in an average
particle size being mechanically pulverized in a petroleum
solvent (the sixth invention).
[0019]
A process of hydrocracking heavy oil of Claim 7 is the
process of hydrocracking heavy oil according to Claim 5 or
Claim 6, wherein the natural limonite iron ore catalyst is
a natural limonite iron ore catalyst containing essentially
no iron oxide (the seventh invention).
[0020]
A process of hydrocracking heavy oil of Claim 8 is the
CA 02613806 2007-12-05
9
process of hydrocracking heavy oil according to any one of
Claims 5 to 7, wherein on supplying the natural limonite
iron ore catalyst into the suspended bed reactor, the
amount supplied is 0.3-2 mass % as an ion component of the
amount of heavy oil supplied (the eighth invention).
[Effect of the Invention]
[0021]
The process of hydrocracking heavy oil of the present
invention makes it possible to give decomposed oil with
higher yields and/or milder reaction conditions than the
conventionally proposed hydrocracking process in a
suspended bed method using an iron-based catalyst when
hydrocracking petroleum heavy oil containing heavy metal
components produced in a refining process of crude oil into
lighter oil.
[Best Mode for Carrying out the Invention]
[0022]
In an oil refinery, generally, crude oil is fed to an
atmospheric distillation tower to separate it into oil
fractions such as naphtha, kerosene and diesel, as well as
atmospheric residue, which is discharged from the under-
part of the atmospheric distillation tower, and the
atmospheric residue is fed to a vacuum distillation tower.
In the vacuum distillation tower, vacuum gas oil fraction
is mainly collected. Simultaneously, vacuum residue is
CA 02613806 2007-12-05
discharged from the under-part of the vacuum distillation
tower, which is further decomposed in a thermal cracking
process (coker process) or a hydrocracking process to
produce naphtha, kerosene, diesel and vacuum gas oil
fractions.
[0023]
The present inventors have found that upon
hydrocracking vacuum residue by a hydrocracking process in
a suspended bed method using an iron-based catalyst, the
use of vacuum residue cut by higher vacuum distillation
temperature yields a low decomposition ratio, and the
decomposition ratio tends to decrease with increase in
vacuum distillation temperature. In the case of using
vacuum residue obtained in a high vacuum distillation
temperature, it is necessary to adopt severe conditions
such as higher pressure and longer reaction time in the
reaction step of hydrocracking, which leads to high plant
costs.
[0024]
In contrast, in the case of using vacuum residue
obtained in a low vacuum distillation temperature, the
decomposition ratio becomes high, and the decomposition
ratio increases with decrease in vacuum distillation
temperature. Specifically, it has been found that it is
preferable to set a maximum temperature at 350 C or less in
CA 02613806 2007-12-05
11
a vacuum distillation tower, thereby the decomposition
ratio becomes sufficiently high upon hydrocracking when
this vacuum residue drawn off from the under-part of vacuum
distillation tower is used. When the decomposition ratio
becomes sufficiently high in this way, it becomes possible
to obtain decomposed oil with high yield, and also
decomposed oil with a satisfying yield in mild reaction
conditions in a reaction step of hydrocracking. Namely, it
is possible to obtain decomposed oil by hydrocracking in a
higher yield and/or milder reaction conditions than the
conventionally proposed hydrocracking process in a
suspended bed method using an iron-based catalyst.
[0025]
Therefore, the process of hydrocracking heavy oil of
the present invention is a process of hydrocracking heavy
oil containing heavy metal components produced in a
refining process of crude oil, comprising:
a vacuum distillation step to obtain the heavy oil as
distillation residue by vacuum distillation; and
a reaction step to hydrocrack the heavy oil in the
presence of an iron-based catalyst in a suspended bed
reactor,
wherein the distillation is conducted at 350 C or less
in the vacuum distillation step.
[0026]
CA 02613806 2007-12-05
12
According to the process of hydrocracking heavy oil of
the present invention, as is clear from the foregoing
founding, the decomposition ratio becomes sufficiently high
upon hydrocracking vacuum residue, so that it is possible
to obtain decomposed oil in a higher yield and/or milder
reaction conditions than the conventionally proposed
hydrocracking process in a suspended bed method using an
iron-based catalyst.
[0027]
It is preferable in the process of hydrocracking heavy
oil of the present invention to have a recycling step in
which a liquid-phase fluid (hereinafter also referred to as
liquid-phase fluid a) is obtained by gas-liquid separation
of the reaction products obtained from a hydrocracking
reaction step in a suspended bed reactor (hereinafter also
hydrocracking reaction step), and after separation of a
solid component from the liquid-phase fluid a, the
resultant liquid-phase fluid (hereinafter also referred to
as liquid-phase fluid b) is recycled into the suspended bed
reactor (the second invention). It also preferable to have
a recycling step in which the liquid-phase fluid a is
recycled into the suspended reactor. The reason is as
follows. Both the liquid-phase fluid a and the liquid-
phase fiuid b contain a heavy oil component (heavy residue)
with a boiling point of 525 C or more (+525 C). By
CA 02613806 2007-12-05
13
recycling them to the suspended bed reactor, re-
decomposition of the heavy oil components takes place, a
yield of oil fraction (C5-525 C) is improved. Main
difference between the liquid-phase fluid a and the liquid-
phase fluid b is that liquid-phase fluid a contains a
catalyst component, whereas liquid-phase fluid b hardly
contains a catalyst component.
[0028]
It is preferable that the content of heavy oil
components with a boiling point of 525 C or more in the
liquid-phase fluid recycling in the above recycling step is
10-100 mass % of the amount of petroleum heavy oil supplied
into the suspended bed reactor in the reaction step (the
third invention). The reason is as follows. When the
content of heavy oil components with a boiling point of
525 C or more is less than 10 mass %, a yield of oil
fraction (C5-525 C) is hardly improved, and a bottom
recycling effect cannot be displayed. On the other hand,
when the content of heavy oil components with a boiling
point of 525'C or more is more than 100 mass %, although a
yield of oil fraction (C5-525 C) is remarkably enhanced,
the rate of increase in the yield of oil fraction (C5-
525 C) is lower than that in the above-mentioned content of
heavy oil components with a boiling point of 525 C or more
of 10-100 mass %, and the recycling efficiency is lowered.
CA 02613806 2007-12-05
14
[0029]
Reaction conditions in the above reaction step, namely,
the reaction conditions in a suspended bed reactor are not
particularly limited, for example, they are set to be a
reaction pressure of 60-160 kg/cm2; a reaction temperature
of 430-455 C; and a reaction time of 30-180 minutes (the
fourth invention). The severer the conditions (higher
reaction pressure, longer the reaction time), the higher
the yield (conversion rate of residue, yield of oil
fraction (C5-525 C) is. In the present invention, however,
milder conditions such as pressure of 60-120 kg/cm2,
temperature of 430-455 C and time of 30-120 minutes can be
adopted to give sufficient yield. The condition for
providing hydrogen is generally 1 to 8 mass % although
depending on flow of freedstocks.
[0030]
In the process of hydrocracking heavy oil of the
present invention, a natural limonite iron ore catalyst is
preferably used as an iron-based catalyst (the fifth
invention). The reason is as follows. Such a natural
limonite iron ore catalyst has higher activities than iron-
based catalysts such as Fe203 (hematite), FeS2 (pyrite),
FeSO4 (iron sulfate), and is an inexpensive, natural
catalyst being mined.
[0031]
CA 02613806 2007-12-05
As a natural limonite iron ore catalyst, it is
preferable to use a natural limonite iron ore catalyst of
2pm or less in an average particle size being mechanically
pulverized in a petroleum solvent (the sixth invention).
The reason is that such catalyst has an excellent catalytic
activity. The average particle size of limonite iron ore
catalyst, for example, can be determined in the following
method. Limonite iron ore catalyst is pulverized in oil by
a ball mill and the like. The particle size of the
catalyst thus pulverized is measured by a laser diffraction
type particle size distribution analyzer. As a dispersion
solvent for the analyzer, ethanol, isopropyl alcohol and
the like are used. A sample (a mixture of oil and the
above pulverized catalyst) is put into the solvent. Then,
a 50% particle size (DpSO) is read out from a particle size
distribution curve (particle size vs. mass % integrated
value) outputted by the analyzer. The 50% particle size is
defined as an average particle size.
[0032]
It is preferable to use a natural limonite iron ore
catalyst containing essentially no iron oxide (the seventh
invention). The reason is that such catalyst has an
excellent catalytic activity. Essentially no iron oxide
means the content 'of Fe203 of 10% or less.
When the heavy oil is hydrocracked in the presence of
CA 02613806 2007-12-05
16
an iron-based catalyst in a suspended bed reactor, a co-
catalyst is used together with the catalyst in order to
change the catalyst to pyrrhotite (Fel_XS) which exhibits
catalytic activities. As a co-catalyst, sulfur is
generally used and added at an amount of approximately 1 to
3 times as much as iron content by atomic ratio. If the
co-catalyst is not used, since the function as a
haydrocracking catalyst is not demonstrated, a
polycondensation reaction occurs and it becomes hard to
achieve high yield.
[0033]
On supplying the above natural limonite iron ore
catalyst into a suspended bed reactor, the amount supplied
is preferably 0.3-2 mass % as an iron component of the
amount of heavy oil supplied (the eighth invention). The
reason is as follows. When the amount supplied is less
than 0.3 mass %, the amount of coke generation tends to
increase rapidly, whereas when the amount supplied is more
than 2 mass %, the oil yield is saturated, i.e. no increase
thereof in more than 2 mass %, which would tend to become
higher costs.
[0034]
In the present invention, the heavy metal components
of heavy oil containing heavy metal components produced in
a refining process of crude oil are Ni, V (one or more
CA 02613806 2007-12-05
17
kinds of Ni or V) and the like. The content of the heavy
metal components is not particularly limited. The vacuum
residue obtained in a vacuum distillation tower means the
distillation residue that atmospheric residue of crude oil
is fed to a vacuum distillation tower, distilled under
vacuum pressure, and drawn off from the under-part of
vacuum distillation tower, namely a vacuum residue of
atmospheric residue of crude oil. The atmospheric residue
of crude oil means the distillation residue that crude oil
is fed to an atmospheric distillation tower, distilled
under atmospheric pressure, and drawn off from the under-
part of atmospheric distillation tower.
[0035]
The pressure condition in the vacuum distillation
process is generally 10 mmHg to 100mmHg. In the present
invention, the vacuum distillation is carried out at 350 C
or less. For example, the maximum temperature of vacuum
distillation tower is controlled in a temperature at 350 C
or less. Namely, the upper limit of the maximum
temperature of vacuum distillation tower is regulated at
350 C. The lower limit of the maximum temperature of
vacuum distillation tower is neither regulated nor limited,
because it depends on the composition of vacuum gas oil
fraction to be obtained by vacuum distillation. The
maximum temperature of vacuum distillation tower is
CA 02613806 2007-12-05
18
suitably set by selecting a temperature of 350 C or less
according to the yield of oil content obtained in the
hydrocracking reaction step. Ordinarily it is 300 to 350 C.
[0036]
In a vacuum distillation tower, the highest
temperature is the temperature of a liquid-phase part in
the under-part of vacuum distillation tower. The highest
temperature in a vacuum distillation tower means the
temperature of the above liquid-phase part.
[0037]
Regarding a process of hydrocracking heavy oil of the
present invention, one example of more specific embodiment
will be described below using a flow chart of FIG. 1. The
present invention is not limited to the description shown
in FIG. 1.
[0038]
As shown in FIG. 1, to a slurry preparation tank (1)
are added a petroleum heavy oil containing heavy metal
components, an iron-based catalyst (for example, natural
limonite iron ore catalyst) and sulfur as a co-catalyst to
prepare a slurry mixture, then hydrogen gas is added
thereto, the slurry is heated by a preheater (2), and
supplied into a suspended bed reactor (3), which is a
hydrocracking reactor in a reaction step. In this case, as
a petroleum heavy oil containing heavy metal components,
CA 02613806 2007-12-05
19
there are used an atmospheric residue of crude oil (the
distillation residue in which crude oil is fed to an
atmospheric distillation tower, distilled under atmospheric
pressure and drawn off from the under-part of atmospheric
distillation tower) and a vacuum residue (the distillation
residue in which atmospheric residue is fed to a vacuum
distillation tower, distilled under vacuum pressure and
drawn off from the under-part of vacuum distillation
tower ). The maximum temperature in this vacuum
distillation tower is controlled at a temperature of 350 C
or less. A bubble tower type reactor is used as a typical
suspended bed reactor. Reaction conditions in the
suspended bed reactor (3) are preferably a reaction
temperature of 440-450 C; a reaction pressure of 80-120
kg/cm2; and a reaction time of 1-2 hours. Hydrocracking
reaction is conducted in the suspended bed reactor (3) to
give a hydrocracking reaction product therein.
[0039]
The hydrocracking reaction product obtained in the
suspended bed reactor (3) is introduced to a first gas-
liquid separator (4), and a gas-phase component is
separated under conditions of high temperature and high
pressure. This gas-phase component is passed to a high
pressure/low temperature gas-liquid separator (8) and a gas
purification step (9). A part of the gas is used as fuel
CA 02613806 2007-12-05
gas. The rest is used as a recycle gas and a cooling gas
for a reactor in the reaction step.
[0040]
In the above first gas-liquid separator (4), a liquid-
phase fluid containing catalyst is separated as well as a
gas-phase component. Light fractions in the liquid-phase
fluid containing catalyst are separated by a low pressure
gas-liquid separator (5) and a reduced pressure gas-liquid
separator (6), then a part of the liquid-phase fluid is
recycled either to the slurry preparation tank (1) or
directly to the suspended bed reactor (3) in the reaction
step (the fluid is called recycling fluid A). The rest is
transported to a solid-liquid separation step (7), and
separated into a liquid fraction, and a solid component
mainly containing a catalyst and coke. The liquid fraction
is the one mainly having a boiling point of 343 C or more,
and is recycled either to the slurry preparation tank (1)
or directly to the suspended bed reactor (3) in the
reaction step (the liquid fraction is called recycling
fluid B).
[0041]
Both the recycling fluid A and the recycling fluid B
contain heavy residues whose boiling point is 525 C or more
(+525 C). By recycling them to the reaction step, the re-
decomposition of the heavy residues takes place to improve
CA 02613806 2007-12-05
21
a yield of oil fraction (C5-525 C). Main difference
between the recycling fluid A and the recycling fluid B is
that the recycling fluid A contains a catalyst component in
the fluid, whereas the recycling fluid B has hardly
contains a catalyst component.
[0042]
in FIG. 1, a pre-heater and a distillation tower are
denoted as the numeral (2) and the numeral (10)
respectively.
[Examples]
[0043]
Examples of the present invention and Comparative
examples will be explained below. The present invention is
not limited to the examples, can suitably be modified and
carried out to the extent consistent with the spirit of the
present invention, and these are all included in the
technical scope of the present invention.
[0044]
Example 1
A process of hydrocracking petroleum heavy oil
containing heavy metal components was conducted in the same
process as in the above-mentioned FIG. 1.
[0045]
In this case, a vacuum residue (hereinafter referred
to as VR) was used as a petroleum heavy oil containing
CA 02613806 2007-12-05
22
heavy metal components. Namely, VR was used being obtained
in that the atmospheric residue of the fraction composition
shown in Table 1 (hereinafter referred to as AR) was fed to
a vacuum distillation tower and distilled under the
pressure of 10 mmHg at 325 C of liquid temperature. The
maximum temperature in the vacuum distillation tower is
325 C. In Table 1, "wt% on feed AR" means a weight ratio
in mass % relative to the amount of AR fed into the vacuum
distillation tower.
The kinds and amount of metals contained in AR are as
follows:
Ni:l5ppm, V:20ppm, Ca:3ppm, Fe:5ppm
[0046]
As an iron-based catalyst, a limonite iron ore
catalyst containing essentially no iron oxide was used.
The limonite iron ore catalyst used was a natural limonite
iron ore (Fe content: 53 mass %) mechanically pulverized in
a petroleum solvent to have 1 pm in average particle size.
The amount of limonite iron ore catalyst added, i.e., the
amount supplied was set to be 1 mass % as an iron component
of the amount of petroleum heavy oil supplied. The amount
of co-catalyst (sulfur) added was set to be 1.2 times the
amount of the iron component in atomic ratio.
[0047]
Reaction conditions in the reaction step, namely, the
CA 02613806 2007-12-05
23
reaction conditions in the suspended bed reactor (3)
employed a reaction pressure of 10 MPa (100 kg/cmz), a
reaction temperature of 450 C, and a reaction time of 60
minutes.
[0048]
A liquid-phase fluid obtained by gas-liquid separation
of the reaction product obtained in the reaction step and,
a liquid-phase fluid obtained by separating a solid
component from the liquid-phase fluid were recycled to the
suspended bed reactor (3). Namely, in the first gas-liquid
separator (4), a liquid-phase fluid was separated as well
as a gas-phase component, light fractions in the liquid-
phase fluid were separated by the low pressure gas-liquid
separator (5) and reduced pressure gas-liquid separator (6),
then a part of the liquid-phase fluid (recycling fluid A)
was recycled to the suspended bed reactor (3). The rest
was transported to the solid-liquid separation step (7),
separated into a solid component and a liquid-phase fluid,
and the liquid-phase fluid (recycling fluid B) was recycled
to the suspended bed reactor (3). In this time, the amount
of liquid-phase fluids (the recycling fluid A and the
recycling fluid B) recycled into the suspended bed reactor
(3) was set so that the sum of the amount of heavy oil
component with a boiling point of 525 C or more in the
recycling fluid A and the amount of heavy oil component
CA 02613806 2007-12-05
24
with a boiling point of 525 C or more in the recycling
fluid B would be 50 mass % of the amount of VR (vacuum
residue) supplied into the suspended bed reactor (3).
[0049]
As a result, conversion rate of residue was 92%; yield
of heavy residue (+525 C) was 5.8 mass % VR amount
(hereinafter, mass % of VR amount is also called %VR); and
yield of oil fraction (C5-525'C) was 84.9%VR (84.9 mass %
of VR amount). The conversion rate is obtained by the
following formula (1).
[0050]
Conversion rate of residue (%) = 100x[(mass % of
+525'C components in feedstock VR) minus (yield of heavy
residue)]/(mass % of +525 C components in feedstock VR) ----
---- Formula (1)
[0051]
Example 2
The maximum temperature in the vacuum distillation
tower was set to be 350 C. Namely, VR was used being
obtained in that AR of the fraction composition shown in
Table 1 was fed to the vacuum distillation tower and
distilled under the pressure of 10 mmHg at 350 C of liquid
temperature. Except for this point, the process of
hydrocracking petroleum heavy oil (VR) containing heavy
metal components was carried out in the same conditions and
CA 02613806 2007-12-05
the same method as in Example 1.
[0052]
As a result, conversion rate of residue was 86%; yield
of heavy residue (+525 C) was 11.5%VR (11.5 mass % of VR
amount); and yield of oil fraction (C5-525 C) was 78.5%VR.
[0053]
Comparative example 1
The maximum temperature in the vacuum distillation
tower was set to be 360 C. Namely, VR was used being
obtained in that AR of the fraction composition shown in
Table 1 was fed to the vacuum distillation tower and
distilled under a pressure of 10 mmHg at 360 C of liquid
temperature. Except for this point, the process of
hydrocracking petroleum heavy oil (VR) containing heavy
metal components was carried out in the same conditions and
the same method as in Example 1.
[0054]
As a result, conversion rate of residue was 81%; yield
of heavy residue (+525 C) was 15.8%VR; and yield of oil
fraction (C5-525 C) was 73.7%VR.
[0055]
Comparative example 2
The maximum temperature in the vacuum distillation
tower was set to be 385 C. Namely, VR was used being'
obtained in that AR of the fraction composition shown in
CA 02613806 2007-12-05
26
Table 1 was fed to the vacuum distillation tower and
distilled under the pressure of 10 mmHg at 385 C of liquid
temperature. Except for this point, the process of
hydrocracking petroleum heavy oil (VR) containing heavy
metal components was carried out in the same conditions and
the same method as in Example 1.
[0056]
As a result, conversion rate of residue was 81%; yield
of heavy residue (+525 C) was 16.6%VR; and yield of oil
fraction (C5-525 C) was 73.5%VR.
[0057]
Comparative example 3
The maximum temperature in the vacuum distillation
tower was set to be 360 C. Namely, VR was used being
obtained in that AR of the fraction composition shown in
Table 1 was fed to the vacuum distillation tower and
distilled under the pressure of 10 mmHg at 360 C of liquid
temperature. Also, reaction conditions in the reaction
step, that is, the reaction conditions in the suspended bed
reactor (3) employed reaction pressure of 15 MPa (150
kg/cm2), reaction temperature of 450 C, and reaction time
of 90 minutes. Except for this point, a process of
hydrocracking petroleum heavy oil (VR) containing heavy
metal components was carried out in the same conditions and
the same method as in Example 1.
CA 02613806 2007-12-05
27
[0058]
As a result, conversion rate of residue was 95%, yield
of heavy residue (+525 C) was 4.3%VR; and yield of oil
fraction (C5-525 C) was 80.7%VR.
[0059]
As is clear from the above Examples 1 to 2, and
Comparative examples 1 to 2, as the vacuum distillation
temperature (the maximum temperature in the vacuum
distillation tower) is raised from 325 C to 360 C, the
reaction efficiency becomes worse, namely, the yield of
heavy residue increases, the conversion rate of residue and
the yield of oil fraction decrease. The reaction
efficiency remains constant in a poor level in the case
where the vacuum distillation temperature is 360 C or more.
[0060]
The reason is thought as follows. Polycondensation
reaction (polymerization reaction) takes place as the
temperature in the vacuum distillation operation step rises
to produce a lot of heavy materials being hardly decomposed.
In order to prevent such deterioration, it is required that
the vacuum distillation temperature is controlled at 350 C
or less, and VR obtained from such the condition is used as
VR fed to a suspended bed reactor.
[0061]
As shown in Comparative example 3, when VR obtained in
CA 02613806 2007-12-05
28
a vacuum distillation temperature of more than 350 C is
used, i.t is necessary to set a higher reaction pressure and
a longer reaction time in order to obtain a relatively high
yield of oil fraction of about 80%VR,. In this case, plant
costs for the hydrocracking process become high.
[0062]
Table 1
Fraction composition, wt% on feed AR
171-232 C 232-343 C 343-450"C 450-525 C +525 C
Feedstock 0.0 3.0 18.2 24.8 53.9
[Industrial Applicability]
[0063]
The process of hydrocracking heavy oil of the present
invention makes it possible to give decomposed oil with
higher yields and/or milder reaction conditions than the
conventionally proposed hydrocracking process in a
suspended bed method using an iron-based catalyst when
hydrocracking petroleum heavy oil containing heavy metal
components produced in a refining process of crude oil into
lighter oil. Therefore the present invention is useful
since it can be preferably adopted as a process of
hydrocracking petroleum heavy oil containing heavy metal
components.