Note: Descriptions are shown in the official language in which they were submitted.
CA 02613826 2007-12-07
Radiant Heating System Utilizing Circulated Heat Transfer Medium from a Pulsed
Electrolysis
System and Method of Using Same
FIELD OF THE INVENTION
The present invention relates generally to radiant heating systems.
BACKGROUND OF THE INVENTION
Radiant heating systems are known in the art. This type of heating system,
which
delivers heat by radiation from the heated surface to the people and objects
in proximity to the heated
surface, is typically installed within the floor of a structure although it
can also be used to heat the
structure's walls and/or ceilings. In general, radiant heating systems utilize
either electric radiators or
hydronic (i.e., liquid-based) radiators. Due to the high cost of electricity,
electric radiators that consist of
electric cables, grids or conductive plastic mats are typically only used for
small areas, e.g., a bathroom
floor, or in additions to existing structures where it may be impractical to
install a hydronic system.
Hydronic systems pump a heated fluid, usually water, through a series of tubes
that are located under, or
integrated within the floor and, in a few circumstances, within a wall or
ceiling. The water or other fluid
used in the hydronic system is typically heated using an electric burner, gas-
fired burner, oil-fired burner
or a wood-fired burner. The water can also be heated using a solar water
heater.
Unless solar water heating is used or the system happens to be located in one
of the few
regions of the world relying on alternative energy, a conventional radiant
heating system relies on a
fossil fuel source. As such, the use of a radiant heating system contributes
to the world's dependence on
fossil fuels, an energy source of finite size and limited regional
availability. Dependence on fossil fuels
not only leads to increased vulnerability to potential supply disruption, but
also continued global
warming due to carbon dioxide emissions.
Within recent years there has been considerable research in the area of
alternative fuels
that provide a`green' approach to the development of electricity. Clearly the
benefit of such an
approach, besides combating global warming and lessening the world's
dependence on fossil fuels, is
that the energy provided by the alternative source can then be used to power a
host of conventional
electrically powered devices without requiring any device modification.
Unfortunately, until such an
alternative source is accepted and tied in to the existing power grid, there
is little for the end consumer to
do to lessen their contribution to the world's dependence on fossil fuels
other than to simply lessen their
overall power consumption. To date, such an approach has had limited success
with most people
refusing to limit their power consumption.
1
CA 02613826 2007-12-07
Accordingly, what is needed is a means of helping end users to lower their
power
consumption without requiring actual sacrifice. The present invention, by
providing a high efficiency
radiant heating system utilizing an alternative heat source, provides such a
system.
SUMMARY OF THE INVENTION
The present invention provides a radiant heating system and a method of
operating the
same, the system utilizing an electrolytic heating subsystem. The electrolytic
heating subsystem is a
pulsed electrolysis system that heats a heat transfer medium contained within
a conduit, the conduit
coupled to the radiant heat tubing. As the heat transfer medium is circulated
through the conduit and the
radiant heat tubing, the radiant heat tubing becomes hot and radiates heat. In
at least one embodiment,
the system includes multiple and distinct sections of radiant heat tubing
which are either serially or
independently coupled to the electrolytic heating subsystem.
In one embodiment of the invention, the radiant heating system includes an
electrolytic
heating subsystem comprised of an electrolysis tank, a membrane separating the
electrolysis tank into
two regions, at least one pair of low voltage electrodes, at least one pair of
high voltage electrodes, a low
voltage source, a high voltage source, and means for simultaneously pulsing
both the low voltage source
and the high voltage source. The system is further comprised of a conduit
containing a heat transfer
medium, at least a portion of the conduit being in thermal communication with
the electrolytic heating
subsystem, radiant heat tubing coupled to the conduit, and a circulation pump
for circulating the heat
transfer medium through the conduit and the radiant heat tubing. The conduit
can surround a portion of
the electrolysis tank or be integrated within the electrolysis tank or be
integrated within the walls of the
electrolysis tank. The system can also include a control valve, for example a
variable flow valve, for
controlling flow of the heat transfer medium. The system can also include one
or more of a variety of
sensors (e.g., electrolysis medium temperature monitor(s), heat transfer
medium temperature monitor(s),
temperature monitors for monitoring the temperature in the region affected by
the radiant heating system,
electrolysis medium level sensors, electrolysis medium pH sensors,
electrolysis medium resistivity
sensors, etc.). The system can also include a system controller that can be
coupled to one or more of the
low and high voltage sources, the simultaneous pulsing means, the circulation
pump, and/or the system
sensors. The radiant heat tubing can be comprised of a single section of
radiant heat tubing or multiple
and distinct sections of radiant heat tubing. The system can further be
comprised of at least one
electromagnetic coil capable of generating a magnetic field within a portion
of the electrolysis tank. The
system can further be comprised of at least one permanent magnet capable of
generating a magnetic field
within a portion of the electrolysis tank.
2
CA 02613826 2007-12-07
In one embodiment of the invention, the radiant heating system includes an
electrolytic
heating subsystem comprised of an electrolysis tank, a membrane separating the
electrolysis tank into
two regions, at least one pair of high voltage electrodes, a plurality of
metal members contained within
the electrolysis tank and interposed between the high voltage electrodes and
the membrane, a high
voltage source, and means for pulsing the high voltage source. The system is
further comprised of a
conduit containing a heat transfer medium, at least a portion of the conduit
being in thermal
communication with the electrolytic heating subsystem, radiant heat tubing
coupled to the conduit, and a
circulation pump for circulating the heat transfer medium through the conduit
and the radiant heat tubing.
The conduit can surround a portion of the electrolysis tank or be integrated
within the electrolysis tank or
be integrated within the walls of the electrolysis tank. The system can also
include a control valve, for
example a variable flow valve, for controlling flow of the heat transfer
medium. The system can also
include one or more of a variety of sensors (e.g., electrolysis medium
temperature monitor(s), heat
transfer medium temperature monitor(s), temperature monitors for monitoring
the temperature in the
region affected by the radiant heating system, electrolysis medium level
sensors, electrolysis medium pH
sensors, electrolysis medium resistivity sensors, etc.). The system can also
include a system controller
that can be coupled to one or more of the high voltage source, the pulsing
means, the circulation pump,
and/or the system sensors. The radiant heat tubing can be comprised of a
single section of radiant heat
tubing or multiple and distinct sections of radiant heat tubing. The system
can further be comprised of at
least one electromagnetic coil capable of generating a magnetic field within a
portion of the electrolysis
tank. The system can further be comprised of at least one permanent magnet
capable of generating a
magnetic field within a portion of the electrolysis tank.
In another aspect of the invention, a method of operating a radiant heating
system is
provided, the method comprising the steps of receiving an instruction to
initiate radiant heating,
performing electrolysis within an electrolysis tank of an electrolytic heating
subsystem, heating a heat
transfer medium contained within a conduit, wherein a portion of the conduit
is in thermal
communication with the electrolysis tank, circulating the heated heat transfer
medium through the
conduit and through radiant heat tubing coupled to the conduit, and suspending
the circulating step in
response to the receipt of an instruction to suspend radiant heating.
Operation of the electrolytic heating
subsystem can be in response to the instruction to initiate radiant heating or
the electrolytic heating
subsystem can operate continuously. If the electrolytic heating subsystem
operates continuously, it can
either operate at a single output level, or it can operated at multiple
levels, for example a low level prior
to receipt of the instruction to initiate radiant heating and at a higher
level after receipt of the instruction
to initiate radiant heating. The step of receiving an instruction to initiate
radiant heating can be further
3
CA 02613826 2007-12-07
comprised of the steps of measuring a temperature in a region impacted by the
radiant heating system,
comparing the measured temperature to a preset temperature, and transmitting
the instruction to initiate
radiant heating when the measured temperature is less than the preset
temperature. The step of receiving
an instruction to suspend radiant heating can be further comprised of the
steps of measuring a
temperature in a region impacted by the radiant heating system, comparing the
measured temperature to
a preset temperature, and transmitting the instruction to suspend radiant
heating when the measured
temperature is greater than the preset temperature. In at least one
embodiment, the method further
comprises the steps of periodically measuring the temperature of the
electrolysis liquid, comparing the
measured temperature with a preset temperature or temperature range, and
modifying at least one process
parameter of the electrolytic heating subsystem if the measured temperature is
outside (lower or higher)
of the preset temperature or temperature range. In at least one embodiment,
the step of performing
electrolysis further comprises the steps of applying a low voltage to at least
one pair of low voltage
electrodes contained within the electrolysis tank of the electrolytic heating
subsystem and applying a
high voltage to at least one pair of high voltage electrodes contained within
the electrolysis tank, wherein
the low voltage and the high voltage are simultaneously pulsed. In at least
one embodiment, the step of
performing electrolysis further comprises the steps of applying a high voltage
to at least one pair of high
voltage electrodes contained within the electrolysis tank, the high voltage
applying step further
comprising the step of pulsing said high voltage, wherein at least one metal
member is positioned
between the high voltage anode(s) and the tank membrane and at least one other
metal member is
positioned between the high voltage cathode(s) and the tank membrane. In at
least one embodiment, the
method further comprises the step of generating a magnetic field within a
portion of the electrolysis tank,
wherein the magnetic field affects a heating rate corresponding to the
electrolytic heating subsystem.
A further understanding of the nature and advantages of the present invention
may be
realized by reference to the remaining portions of the specification and the
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. I is an illustration of an exemplary embodiment of the invention;
Fig. 2 is an illustration of an alternate exemplary embodiment with multiple
radiant heat
tubing sections;
Fig. 3 is an illustration of an alternate exemplary embodiment with multiple
radiant heat
tubing sections, wherein each section is independently coupled to the
electrolytic heating subsystem;
Fig. 4 is a detailed view of an embodiment of the electrolytic heating
subsystem;
Fig. 5 is a detailed view of an alternate embodiment of the electrolytic
heating subsystem
shown in Fig. 4;
4
CA 02613826 2007-12-07
Fig. 6 is a detailed view of an alternate embodiment of the electrolytic
heating subsystem
shown in Fig. 4 utilizing an electromagnetic rate controller;
Fig. 7 is a detailed view of an alternate embodiment of the electrolytic
heating subsystem
shown in Fig. 5 utilizing an electromagnetic rate controller as shown in Fig.
6;
Fig. 8 is a detailed view of an alternate embodiment of the electrolytic
heating subsystem
shown in Fig. 6 utilizing a permanent magnet rate controller;
Fig. 9 is a detailed view of an alternate embodiment of the electrolytic
heating subsystem
shown in Fig. 7 utilizing a permanent magnet rate controller;
Fig. 10 illustrates one method of operating the radiant heating system of the
invention;
Fig. 11 illustrates an alternate method of system operation;
Fig. 12 illustrates another alternate method of system operation;
Fig. 13 illustrates another alternate method of system operation;
Fig. 14 illustrates another alternate method of system operation;
Fig. 15 illustrates another alternate method of system operation; and
Fig. 16 illustrates another alternate method of system operation.
DESCRIPTION OF THE SPECIFIC EMBODIMENTS
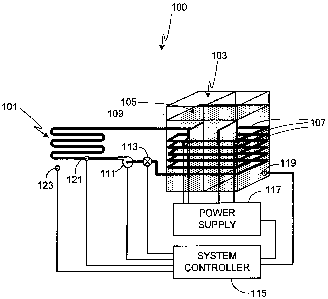
Fig. 1 is an illustration of an exemplary system 100 in accordance with the
invention.
System 100 is comprised of two primary subsystems; radiant heat tubing 101
which radiates the heat and
a pulsed electrolytic heating subsystem 103 which heats the fluid that is
pumped through tubing 101. As
will be described in detail, there are numerous configurations of electrolytic
heating subsystem 103
applicable to the invention.
During operation, electrolytic heating subsystem 103 becomes very hot, the
temperature
dependent on the operating conditions of subsystem 103 (e.g., on/off cycling
time, electrode size, input
power, input frequency and pulse duration). Typically subsystem 103, and more
specifically fluid 105
within subsystem 103, is maintained during operation at a relatively high
temperature, typically on the
order of at least 40 - 50 C, more preferably on the order of 60 - 75 C,
and still more preferably on the
order of 75 - 95 C. It some embodiments, even higher temperatures are used,
for example on the order
of 100 - 150 C, or on the order of 150 - 250 C, or on the order of 250 -
350 C.
Coupled to radiant heat tubing 101 is a conduit 107. Conduit 107 is contained
within
electrolysis tank 109, or mounted around electrolysis tank 109, or integrated
within the walls of
electrolysis tank 109. Although typically conduit 107 is a separate conduit
that is coupled to tubing 101,
it will be appreciated that in some embodiments conduit 107 is comprised of
the same material as tubing
101 and, additionally, in some embodiments conduit 107 is simply comprised of
a portion of tubing 101.
5
CA 02613826 2007-12-07
The primary considerations for the location of conduit 107 are (i) the
efficiency of the thermal
communication between the electrolytic heating subsystem and the conduit (and
heat transfer medium
contained therein) and (ii) minimization of conduit erosion. As most materials
used for the electrolysis
tank are poor thermal conductors, typically conduit 107 is either contained
within the tank or integrated
within the tank walls.
During electrolysis, the heat generated by the process heats electrolysis
fluid 105 which,
in turn, heats conduit 107 and the heat transfer medium contained within
conduit 107. The heat transfer
medium, typically water, is pumped through tubing 101 using pump 111. A
control valve 113 controls
the flow of heat transfer medium. In at least one embodiment, control valve
113 is a simple on/off valve
and pump 111 is a single speed pump. In at least one other embodiment, control
valve 113 is a variable
flow valve that allows a range of flow, thus allowing the amount of heat
radiated by tubing 101 to be
regulated. Alternately, in at least one other embodiment, pump 111 is a
variable speed pump, thus
providing another means of regulating the amount of heat radiated by tubing
101.
In a preferred embodiment of the invention, a system controller 115 controls
the
performance of the system, including heat output from radiant heat tubing 101,
preferably by varying one
or more operating parameters (i.e., process parameters) of electrolytic
heating subsystem 103 to which it
is attached via power supply 117. Varying operating parameters of power supply
117 and thus
subsystem 103, for example cycling the subsystem on and off or varying other
operational parameters as
described further below, allows the subsystem to be operated at the desired
temperature. Preferably a
temperature monitor 119, coupled to subsystem 103, allows controller 115 to
obtain feedback from the
system as the operational parameters are varied. Preferably a second
temperature monitor 121, coupled
to radiant heat tubing 101, monitors the temperature of tubing 101 and/or the
heat transfer medium
contained therein, thus allowing system operation to be monitored. Preferably
a third temperature
monitor 123, mounted in proximity to radiant heat tubing 101, provides
additional insight into system
performance. Additionally, in at least one preferred embodiment, circulation
pump 111 and flow valve
113 are also coupled to, and controlled by, controller 115.
It will be appreciated that there are many potential applications for radiant
heating
system 100 and that the exact configuration of system 100 (e.g., system size,
operating temperature,
tubing configuration, etc.) depends upon the selected application. For
example, if radiant heat tubing
101 is associated with a process heater (e.g., drying system), the tubing may
be configured such that it
surrounds the part/component to be heated. Alternately, if the system is
configured to provide room
heating, tubing 101 will preferably be configured to be located within, or
under, the floor, or integrated
within a wall or ceiling.
6
CA 02613826 2007-12-07
In addition to heating the heat transfer medium for a single radiant heat
tubing
application, it should be understood that the system can be configured to
allow multiple processes or
multiple rooms to be heated. For example, system 200 shown in Fig. 2 includes
multiple and distinct
radiant heat tubing sections 201-203. Sections 201-203 can be associated with
different portions (e.g.,
rooms) of a structure, or different process heaters. As shown, system 200 does
not allow control over
individual radiant heat tubing sections as they are serially coupled. System
300, shown in Fig. 3, also
allows for multiple radiant heat tubing sections 201-203, but couples each of
them individually to the
electrolytic heating subsystem 103. By using individual flow valves 301-303
and/or individual pumps
305-307 as shown, the operation and preferably the temperature of each section
can be independently
controlled. Preferably in such a configuration individual temperature sensors
(i.e., tubing/transfer
medium temperature sensors 309-311 and proximity temperature sensors 313-315)
are used to provide
performance feedback, and thus individual control, of each section 201-203.
Particulars of the electrolytic heating subsystem will now be provided. Fig. 4
is an
illustration of a preferred embodiment of an electrolytic heating subsystem
400. Note that in Figs. 4-9
only a portion of conduit 107 is shown (conduit 617 in Figs. 6-9), thus
allowing a better view of the
underlying electrolytic subsystem. Additionally, for illustration clarity, the
portions of conduit 107 (or
conduit 617) that are included are shown mounted to the exterior surface of
the electrolysis tank even
though as previously noted, conduit 107 is typically integrated within the
tank walls or mounted within
the tank, thereby improving on the transfer of heat from the electrolytic
subsystem to the heat transfer
medium contained within the conduit.
Tank 401 is comprised of a non-conductive material. The size of tank 401 is
primarily
selected on the basis of desired system output, i.e., the level of desired
heat, which is based on the
desired heat output of the radiant heat tubing as well as the volume and flow
rate of the heat transfer
medium flowing through the radiant heat tubing. Although tank 401 is shown as
having a rectangular
shape, it will be appreciated that the invention is not so limited and that
tank 401 can utilize other shapes,
for example cylindrical, square, irregularly-shaped, etc. Tank 401 is
substantially filled with medium
105. In at least one preferred embodiment, liquid 105 is comprised of water,
or more preferably water
with an electrolyte, the electrolyte being an acid electrolyte, a base
electrolyte, or a combination of an
acid electrolyte and a base electrolyte. Exemplary electrolytes include
potassium hydroxide and sodium
hydroxide. The term "water" as used herein refers to water (HZO), deuterated
water (deuterium oxide or
D20), tritiated water (tritium oxide or T20), semiheavy water (HDO), heavy
oxygen water (H2180 or
H2 170) or any other water containing an isotope of either hydrogen or oxygen,
either singly or in any
combination thereof (for example, a combination of H20 and D20).
7
CA 02613826 2007-12-07
A typical electrolysis system used to decompose water into hydrogen and oxygen
gases
utilizes relatively high concentrations of electrolyte. Subsystem 103,
however, has been found to work
best with relatively low electrolyte concentrations, thereby maintaining a
relatively high initial water
resistivity. Preferably the water resistivity prior to the addition of an
electrolyte is on the order of I to 28
megohms. Preferably the concentration of electrolyte is in the range of 0.05
percent to 10 percent by
weight, more preferably the concentration of electrolyte is in the range of
0.05 percent to 2.0 percent by
weight, and still more preferably the concentration of electrolyte is in the
range of 0.1 percent to 0.5
percent by weight.
Separating tank 401 into two regions is a membrane 403. Membrane 403 permits
ion/electron exchange between the two regions of tank 401. Assuming medium 105
is water, as
preferred, small amounts of hydrogen and oxygen are produced during operation.
Accordingly
membrane 403 also keeps the oxygen and hydrogen bubbles produced during
electrolysis separate, thus
minimizing the risk of inadvertent recombination of the two gases. Exemplary
materials for membrane
403 include, but are not limited to, polypropylene, tetrafluoroethylene,
asbestos, etc. Preferably tank 401
also includes a pair of gas outlets 405 and 407, corresponding to the two
regions of tank 401. The
volume of gases produced by the process can either be released, through
outlets 405 and 407, into the
atmosphere in a controlled manner or they can be collected and used for other
purposes.
As the electrolytic heating subsystem is designed to reach relatively high
temperatures,
the materials comprising tank 401, membrane 403 and other subsystem components
are selected on the
basis of their ability to withstand the expected temperatures and pressures.
As previously noted, the
subsystem can be designed to operate at temperatures ranging from 40 C to 350
C or higher.
Additionally, at elevated temperatures higher pressures are typically required
to prevent boiling of liquid
105. Accordingly, it will be understood that the choice of materials for the
subsystem components and
the design of the subsystem (e.g., tank wall thicknesses, fittings, etc.) will
vary, depending upon the
intended subsystem operational parameters, primarily temperature and pressure.
Replenishment of medium 105 can be through one or more dedicated lines. Fig. 4
shows
a portion of two such conduits, conduit 409 and 411, one coupled to each of
the regions of tank 401.
Alternately, a replenishment conduit can be coupled to only one region of tank
401. Although medium
replenishment can be performed manually, preferably replenishment is performed
automatically, for
example using system controller 115 and flow valve 413 within line 409 and
valve 415 within line 411.
Replenishment can be performed periodically or continually at a very low flow
rate. If periodic
replenishment is used, it can either be based on the period of system
operation, for example replenishing
the system with a predetermined volume of medium after a preset number of
hours of operation, or based
8
CA 02613826 2007-12-07
on the volume of medium within tank 401, the volume being provided to
controller 115 using a level
monitor 417 within the tank or other means. In at least one preferred
embodiment system controller 115
is also coupled to a monitor 419, monitor 419 providing either the pH or the
resistivity of liquid 105
within the electrolysis tank, thereby providing means for determining when
additional electrolyte needs
to be added. In at least one embodiment and as previously noted, preferably
system controller 115 is also
coupled to a temperature monitor 119, monitor 119 providing the temperature of
the electrolysis
medium.
In at least one embodiment of the electrolytic heating subsystem, two types of
electrodes
are used, each type of electrode being comprised of one or more electrode
pairs with each electrode pair
including at least one cathode (i.e., a cathode coupled electrode) and at
least one anode (i.e., an anode
coupled electrode). All cathodes, regardless of the type, are kept in one
region of tank 401 while all
anodes, regardless of the type, are kept in the other tank region, the two
tank regions separated by
membrane 403. In the embodiment illustrated in Fig. 4, each type of electrode
includes a single pair of
electrodes.
The first type of electrodes, electrodes 421/423, are coupled to a low voltage
source 425.
The second type of electrodes, electrodes 427/429, are coupled to a high
voltage source 431. In the
illustrations and as used herein, voltage source 425 is labeled as a`Iow'
voltage source not because of the
absolute voltage produced by the source, but because the output of voltage
source 425 is maintained at a
lower output voltage than the output of voltage source 431. Preferably and as
shown, the individual
electrodes of each pair of electrodes are parallel to one another; i.e., the
face of electrode 421 is parallel
to the face of electrode 423 and the face of electrode 427 is parallel to the
face of electrode 429. It
should be appreciated, however, that such an electrode orientation is not
required.
In one preferred embodiment, electrodes 421/423 and electrodes 427/429 are
comprised
of titanium. In another preferred embodiment, electrodes 421/423 and
electrodes 427/429 are comprised
of stainless steel. It should be appreciated, however, that other materials
can be used and that the same
material does not have to be used for both the low and high voltage
electrodes. Additionally, the same
material does not have to be used for both the anode(s) and the cathode(s) of
the low voltage electrodes,
nor does the same material have to be used for both the anode(s) and the
cathode(s) of the high voltage
electrodes. In addition to titanium and stainless steel, other exemplary
materials that can be used for the
low voltage and high voltage electrodes include, but are not limited to,
copper, iron, steel, cobalt,
manganese, zinc, nickel, platinum, palladium, aluminum, lithium, magnesium,
boron, carbon, graphite,
carbon-graphite, metal hydrides and alloys of these materials. Preferably the
surface area of the faces of
the low voltage electrodes (e.g., electrode 421 and electrode 423) cover a
large percentage of the cross-
9
CA 02613826 2007-12-07
sectional area of tank 401, typically on the order of at least 40 percent of
the cross-sectional area of tank
401, and more typically between approximately 70 percent and 90 percent of the
cross-sectional area of
tank 401. Preferably the separation between the low voltage electrodes (e.g.,
electrodes 421 and 423) is
between 0.1 millimeters and 15 centimeters. In at least one embodiment the
separation between the low
voltage electrodes is between 0.1 millimeters and 1 millimeter. In at least
one other embodiment the
separation between the low voltage electrodes is between 1 millimeter and 5
millimeters. In at least one
other embodiment the separation between the low voltage electrodes is between
5 millimeters and 2
centimeters. In at least one other embodiment the separation between the low
voltage electrodes is
between 5 centimeters and 8 centimeters. In at least one other embodiment the
separation between the
low voltage electrodes is between 10 centimeters and 12 centimeters.
In the illustrated embodiment, electrodes 427/429 are positioned outside of
the planes
containing electrodes 421/423. In other words, the separation distance between
electrodes 427 and 429
is greater than the separation distance between electrodes 421 and 423 and
both low voltage electrodes
are positioned between the planes containing the high voltage electrodes. The
high voltage electrodes
may be larger, smaller or the same size as the low voltage electrodes.
As previously noted, the voltage applied to the high voltage electrodes is
greater than
that applied to the low voltage electrodes. Preferably the ratio of the high
voltage to the low voltage
applied to the high voltage and low voltage electrodes, respectively, is at
least 5:1, more preferably the
ratio is between 5:1 and 100:1, still more preferably the ratio is between 5:1
and 33:1, and even still more
preferably the ratio is between 5:1 and 20:1. Preferably the high voltage
generated by source 431 is
within the range of 50 volts to 50 kilovolts, more preferably within the range
of 100 volts to 5 kilovolts,
and still more preferably within the range of 500 volts to 2.5 kilovolts.
Preferably the low voltage
generated by source 425 is within the range of 3 volts to 1500 volts, more
preferably within the range of
12 volts to 750 volts, still more preferably within the range of 24 volts to
500 volts, and yet still more
preferably within the range of 48 volts to 250 volts.
Rather than continually apply voltage to the electrodes, sources 425 and 431
are pulsed,
preferably at a frequency of between 50 Hz and 1 MHz, more preferably at a
frequency of between 100
Hz and 10 kHz, and still more preferably at a frequency of between 150 Hz and
7 kHz. The pulse width
(i.e., pulse duration) is preferably between 0.01 and 75 percent of the time
period defined by the
frequency, and more preferably between 0.1 and 50 percent of the time period
defined by the frequency,
and still more preferably between 0.1 and 25 percent of the time period
defined by the frequency. Thus,
for example, for a frequency of 150 Hz, the pulse duration is preferably in
the range of 0.67
microseconds to 5 milliseconds, more preferably in the range of 6.67
microseconds to 3.3 milliseconds,
CA 02613826 2007-12-07
and still more preferably in the range of 6.67 microseconds to 1.7
milliseconds. Alternately, for
example, for a frequency of 1 kHz, the pulse duration is preferably in the
range of 0.1 microseconds to
0.75 milliseconds, more preferably in the range of 1 microsecond to 0.5
milliseconds, and still more
preferably in the range of 1 microsecond to 0.25 milliseconds. Additionally,
the voltage pulses are
applied simultaneously to the high voltage and low voltage electrodes via
sources 425 and 431,
respectively. In other words, the voltage pulses applied to high voltage
electrodes 427/429 coincide with
the pulses applied to low voltage electrodes 421/423. Although voltage sources
425 and 431 can include
internal means for pulsing the respective outputs from each source, preferably
an external pulse generator
433 controls a pair of switches, i.e., low voltage switch 435 and high voltage
switch 437 which, in turn,
control the output of voltage sources 425 and 431 as shown, and as described
above.
In at least one preferred embodiment, the frequency and/or pulse duration
and/or low
voltage and/or high voltage can be changed by system controller 115 during
system operation, thus
allowing the operation of the electrolytic heating subsystem to be controlled.
For example, in the
configuration shown in Fig. 4, low voltage power supply 425, high voltage
power supply 431 and pulse
generator 433 are all connected to system controller 115, thus allowing
controller 115 to control the
amount of heat generated by the electrolytic heating subsystem. It will be
appreciated that both power
supplies and the pulse generator do not have to be connected to system
controller 115 to provide heat
generation control. For example, only one of the power supplies and/or the
pulse generator can be
connected to controller 115.
As will be appreciated by those of skill in the art, there are numerous minor
variations of
the electrolytic heating subsystem described above and shown in Fig. 4 that
can be used with the
invention. For example, and as previously noted, alternate configurations can
utilize tanks of different
size and/or shape, different electrolytic solutions, and a variety of
different electrode configurations and
materials. Exemplary alternate electrode configurations include, but are not
limited to, multiple low
voltage cathodes, multiple low voltage anodes, multiple high voltage cathodes,
multiple high voltage
anodes, multiple low voltage electrode pairs combined with multiple high
voltage electrode pairs,
electrodes of varying size or shape (e.g., cylindrical, curved, etc.), and
electrode pairs of varying
orientation (e.g., non-parallel faces, pairs in which individual electrodes
are not positioned directly across
from one another, etc.). Additionally, alternate configurations can utilize a
variety of input powers, pulse
frequencies and pulse durations as previously noted.
In an exemplary embodiment of the electrolytic heating subsystem, a
cylindrical
chamber measuring 125 centimeters long with an inside diameter of 44
centimeters and an outside
diameter of 50 centimeters was used. The tank contained 175 liters of water,
the water including a
11
CA 02613826 2007-12-07
potassium hydroxide (KOH) electrolyte at a concentration of 0.1 % by weight.
The low voltage
electrodes were 75 centimeters by 30 centimeters by 0.5 centimeters and had a
separation distance of
approximately 10 centimeters. The high voltage electrodes were 3 centimeters
by 2.5 centimeters by 0.5
centimeters and had a separation distance of approximately 32 centimeters.
Both sets of electrodes were
comprised of titanium. The pulse frequency was maintained at 150 Hz and the
pulse duration was
initially set to 260 microseconds and gradually lowered to 180 microseconds
during the course of a 4
hour run. The low voltage supply was set to 50 volts, drawing a current of
between 5.5 and 7.65 amps,
and the high voltage supply was set to 910 volts, drawing a current of between
2.15 and 2.48 amps. The
initial temperature was 28 C and monitored continuously with a pair of
thermocouples, one in each side
of the tank. After conclusion of the 4 hour run, the temperature of the tank
fluid had increased to 67 C.
Illustrating the correlation between electrode size and heat production
efficiency, the
high voltage electrodes of the previous test were replaced with larger
electrodes, the larger electrodes
measuring 9.5 centimeters by 5 centimeters by 0.5 centimeters, thus providing
approximately 6.3 times
the surface area of the previous high voltage electrodes. The larger
electrodes, still operating at a voltage
of 910 volts, drew a current of between 1.73 and 1.9 amps. The low voltage
supply was again set at 50
volts, in this run the low voltage electrodes drawing between 0.6 and 1.25
amps. Although the pulse
frequency was still maintained at 150 Hz, the pulse duration was lowered from
an initial setting of 60
microseconds to 15 microseconds. All other operating parameters were the same
as in the previous test.
In this test, during the course of a 5 hour run, the temperature of the tank
fluid increased from 28 C to
69 C. Given the shorter pulses and the lower current, this test with the
larger high voltage electrodes
exhibited a heat production efficiency approximately 8 times that exhibited in
the previous test.
Fig. 5 is an illustration of a second exemplary embodiment of the electrolytic
heating
subsystem, this embodiment using a single type of electrodes. Subsystem 500 is
basically the same as
subsystem 400 shown in Fig. 4 with the exception that low voltage electrodes
421/423 have been
replaced with a pair of metal members 501/503; metal member 501 interposed
between high voltage
electrode 427 and membrane 403 and metal member 503 interposed between high
voltage electrode 429
and membrane 403. The materials comprising metal members 501/503 are the same
as those of the low
voltage electrodes. Preferably the surface area of the faces of members 501
and 503 is a large percentage
of the cross-sectional area of tank 401, typically on the order of at least 40
percent, and often between
approximately 70 percent and 90 percent of the cross-sectional area of tank
401. Preferably the
separation between members 501 and 503 is between 0.1 millimeters and 15
centimeters. In at least one
embodiment the separation between the metal members is between 0.1 millimeters
and 1 millimeter. In
at least one other embodiment the separation between the metal members is
between 1 millimeter and 5
12
CA 02613826 2007-12-07
millimeters. In at least one other embodiment the separation between the metal
members is between 5
millimeters and 2 centimeters. In at least one other embodiment the separation
between the metal
members is between 5 centimeters and 8 centimeters. In at least one other
embodiment the separation
between the metal members is between 10 centimeters and 12 centimeters. The
preferred ranges for the
size of the high voltage electrodes as well as the high voltage power, pulse
frequency and pulse duration
are the same as in the exemplary subsystem shown in Fig. 4 and described
above.
In a test of the exemplary embodiment of the electrolytic heating subsystem
using metal
members in place of low voltage electrodes, the same cylindrical chamber and
electrolyte-containing
water was used as in the previous test. The metal members were 75 centimeters
by 30 centimeters by 0.5
centimeters and had a separation distance of approximately 10 centimeters. The
high voltage electrodes
were 3 centimeters by 2.5 centimeters by 0.5 centimeters and had a separation
distance of approximately
32 centimeters. The high voltage electrodes and the metal members were
fabricated from stainless steel.
The pulse frequency was maintained at 150 Hz and the pulse duration was
initially set to 250
microseconds and gradually lowered to 200 microseconds during the course of a
2 hour run. The high
voltage supply was set to 910 volts, drawing a current of between 2.21 and
2.45 amps. The initial
temperature was 30 C and monitored continuously with a pair of thermocouples,
one in each side of the
tank. After conclusion of the 2 hour run, the temperature of the tank fluid
had increased to 60 C.
As with the previously described set of tests, the correlation between
electrode size and
heat production efficiency was demonstrated by replacing the high voltage
electrodes with larger
electrodes measuring 9.5 centimeters by 5 centimeters by 0.5 centimeters. The
larger electrodes, still
operating at a voltage of 910 volts, drew a current of between 1.6 and 1.94
amps. The pulse frequency
was still maintained at 150 Hz, however, the pulse duration was lowered from
an initial setting of 90
microseconds to 25 microseconds. All other operating parameters were the same
as in the previous test.
In this test during the course of a 6 hour run, the temperature of the tank
fluid increased from 23 C to
68 C, providing an increase in heat production efficiency of approximately 3
times over that exhibited in
the previous test.
As with the previous exemplary embodiment, it will be appreciated that there
are
numerous minor variations of the electrolytic heating subsystem described
above and shown in Fig. 5
that can be used with the invention. For example, and as previously noted,
alternate configurations can
utilize tanks of different size and/or shape, different electrolytic
solutions, and a variety of different
electrode/metal member configurations and materials. Exemplary alternate
electrode/metal member
configurations include, but are not limited to, multiple sets of metal
members, multiple high voltage
cathodes, multiple high voltage anodes, multiple sets of metal members
combined with multiple high
13
CA 02613826 2007-12-07
voltage cathodes and anodes, electrodes/metal members of varying size or shape
(e.g., cylindrical,
curved, etc.), and electrodes/metal members of varying orientation (e.g., non-
parallel faces, pairs in
which individual electrodes are not positioned directly across from one
another, etc.). Additionally,
alternate configurations can utilize a variety of input powers, pulse
frequencies and pulse durations.
In at least one preferred embodiment of the invention, the electrolytic
heating subsystem
uses a reaction rate controller to help achieve optimal performance of the
heating subsystem relative to
the radiant heater. The rate controller operates by generating a magnetic
field within the electrolysis
tank, either within the region between the high voltage cathode(s) and the low
voltage cathode(s) or
metal member(s), or within the region between the high voltage anode(s) and
the low voltage anode(s) or
metal member(s), or both regions. The magnetic field can either be generated
with an electromagnetic
coil or coils, or with one or more permanent magnets. The benefit of using
electromagnetic coils is that
the intensity of the magnetic field generated by the coil or coils can be
varied by controlling the current
supplied to the coil(s), thus providing a convenient method of controlling the
reaction rate.
Fig. 6 provides an exemplary embodiment of an electrolytic heating subsystem
600 that
includes an electromagnetic rate controller. It should be understood that the
electromagnetic rate
controller shown in Figs. 6 and 7, or the rate controller using permanent
magnets shown in Figs. 8 and 9,
is not limited to a specific tank/electrode configuration. For example,
electrolysis tank 601 of system
600 is cylindrically-shaped although the tank could utilize other shapes such
as the rectangular shape of
tank 401. As in the previous embodiments, the electrolytic heating subsystem
includes a membrane
(e.g., membrane 603) separating the tank into two regions, a pair of gas
outlets (e.g., outlets 605/607),
medium replenishment conduits 609 and 611 (one per region in the exemplary
embodiment illustrated in
Fig. 6), flow control valves (e.g., valves 613 and 615) coupled to the system
controller, and heat removal
conduits 617 which are functional equivalents to conduits 107). As in the
embodiments shown in Figs. 4
and 5, only a portion of the conduits are shown, thus providing a better view
of the underlying system.
Preferably the system also includes a water level monitor (e.g., monitor 619),
a pH or resistivity monitor
(e.g., monitor 621), and a temperature monitor 623. This embodiment, similar
to the one shown in Fig.
4, utilizes both low voltage and high voltage electrodes. Specifically,
subsystem 600 includes a pair of
low voltage electrodes 625/627 and a pair of high voltage electrodes 629/631.
In the electrolytic heating subsystem illustrated in Fig. 6, a magnetic field
of controllable
intensity is generated between the low voltage and high voltage electrodes
within each region of tank
601. Although a single electromagnetic coil can generate fields within both
tank regions, in the
illustrated embodiment the desired magnetic fields are generated by a pair of
electromagnetic coils
633/635. As shown, electromagnetic coil 633 generates a magnetic field between
the planes containing
14
CA 02613826 2007-12-07
low voltage electrode 625 and high voltage electrode 629 and electromagnetic
coil 635 generates a
magnetic field between the planes containing low voltage electrode 627 and
high voltage electrode 631.
Electromagnetic coils 633/635 are coupled to a controller 637 which is used to
vary the current through
coils 633/635, thus allowing the strength of the magnetic field generated by
the electromagnetic coils to
be varied as desired. As a result, the rate of the reaction driven by the
electrolysis system, and thus the
amount of heat generated by the subsystem, can be controlled. In particular,
increasing the magnetic
field generated by coils 633/635 decreases the reaction rate. Accordingly, a
maximum reaction rate is
achieved with no magnetic field while the minimum reaction rate is achieved by
imposing the maximum
magnetic field. It will be appreciated that the exact relationship between the
magnetic field and the
reaction rate depends on a variety of factors including reaction strength,
electrode composition and
configuration, voltage/pulse frequency/pulse duration applied to the
electrodes, electrolyte concentration,
and achievable magnetic field, the last parameter dependent primarily upon the
composition of the coils,
the number of coil turns, and the current available from controller 637.
Although the subsystem embodiment shown in Fig. 6 utilizes coils that are
interposed
between the low voltage electrode and the high voltage electrode planes, it
will be appreciated that the
critical parameter is to configure the system such that there is a magnetic
field, preferably of controllable
intensity, between the low voltage and high voltage electrode planes. Thus,
for example, if the coils
extend beyond either, or both, the plane containing the low voltage
electrode(s) and the plane controlling
the high voltage etectrode(s), the system will still work as the field
generated by the coils includes the
regions between the low voltage and high voltage electrodes. Additionally it
will be appreciated that
although the embodiment shown in Fig. 6 utilizes a single controller 637
coupled to both coils, the
system can also utilize separate controllers for each coil (not shown).
Similarly, while the illustrated
subsystem utilizes dual coils, the invention can also use a single coil to
generate a single field which
affects both tank regions, or primarily affects a single tank region.
Additionally it will be appreciated
that the electromagnetic coils do not have to be mounted to the exterior
surface of the tank as shown in
Fig. 6. For example, the electromagnetic coils can be integrated within the
walls of the tank, or mounted
within the tank. By mounting the electromagnetic coils within, or outside, of
the tank walls, coil
deterioration from electrolytic erosion is minimized.
The magnetic field rate controller is not limited to use with electrolytic
heating
subsystems employing both low and high voltage electrodes. For example, the
electromagnetic rate
controller subsystem can be used with embodiments using high voltage
electrodes and metal members as
described above and shown in the exemplary embodiment of Fig. 5. Fig. 7 is an
illustration of an
exemplary embodiment based on the embodiment shown in Fig. 6, replacing low
voltage electrodes
CA 02613826 2007-12-07
625/627 with metal members 701/703, respectively. As with the electromagnetic
rate controller used
with the dual voltage system, it will be appreciated that configurations using
high voltage electrodes and
metal members can utilize internal electromagnetic coils, electromagnetic
coils mounted within the tank
walls, and electromagnetic coils mounted outside of the tank walls.
Additionally, and as previously
noted, the electromagnetic rate controller is not limited to a specific tank
and/or electrode configuration.
As previously noted, although electromagnetic coils provide a convenient means
for
controlling the intensity of the magnetic field applied to the reactor,
permanent magnets can also be used
with the electrolytic heating subsystem of the invention, for example when the
magnetic field does not
need to be variable. Figs. 8 and 9 illustrate embodiments based on the
configurations shown in Figs. 6
and 7, but replacing coils 633 and 635 with permanent magnets 801 and 803,
respectively. Note that in
the view of Fig. 8, only a portion of electrode 625 is visible while none of
electrode 631 is visible.
Similarly in the view of Fig. 9, only a portion of metal member 701 is visible
while none of electrode
631 is visible.
As previously described, the radiant heating system of the invention can be
operated in a
variety of ways, depending primarily upon the desired level of system control.
Further detail regarding
the primary and preferred methodologies will now be provided.
In one preferred method of operation, electrolytic heating subsystem 103 is
operated on
a continuous basis (step 1001 of Fig. 10). The primary advantage of this
approach is that it allows the
radiant heat tubing to be quickly heated to the desired operating temperature.
The initial operational step
is when a demand for heat is placed on the system (step 1003), the demand
being either automatic, for
example initiated by a timer, or manual, for example initiated by a user.
After receiving the demand, the
system begins to pump heat transfer medium through the conduit and the radiant
heat tubing coupled to
the conduit (step 1005), resulting in heat production (step 1007). Eventually
the demand for heat is
terminated (step 1009), termination being either automatic, for example using
a timer, or manual, for
example through action of the user. At this point heat transfer medium pumping
is terminated (step
1011).
Fig. 11 illustrates a slight modification of the methodology shown in Fig. 10.
In this
method the temperature in proximity to the radiant heater is monitored (step
1101), for example using
monitor 123, and compared to a preset temperature (step 1103), for example a
temperature set by the
end-user with a thermostat. As long as the monitored temperature is higher
than the preset temperature
(step 1105), the system simply continues monitoring the temperature. Once the
system determines that
the temperature is lower than the preset temperature (step 1107), the system
begins to pump heat transfer
medium through the conduit and the radiant heat tubing coupled to the conduit
(step 1005), resulting in
16
CA 02613826 2007-12-07
heating (step 1007). The temperature continues to be monitored (step 1109) and
compared to a second
preset temperature (step 1111). The second preset temperature can either be
the same as the first preset
temperature or it can be different from the first preset temperature. As long
as the monitored temperature
is lower than the second preset temperature (step 1113), the system continues
to pump heat transfer
medium through the radiant heat tubing and continues to monitor the
temperature. When the system
determines that the monitored temperature is greater than the second preset
temperature (step 1115), heat
transfer medium pumping is terminated (step 1011) and the system goes back to
monitoring temperature
(step 1101).
Although the electrolytic heating subsystem can be operated continuously, as
in the
processes illustrated in Figs. 10 and 11, in at least one embodiment the
electrolytic heating subsystem is
only run after the system receives a demand for radiant heating. In the
simplest configuration, once a
demand for radiant heat is received by the system (step 1201 in Fig. 12),
electrolysis in the electrolytic
heating subsystem is initiated (step 1203). The step of pumping the heat
transfer medium through the
conduit and the radiant heat tubing coupled to the conduit (step 1205) can
either begin immediately, or it
can be delayed while allowing the electrolytic heating subsystem to heat-up.
Radiant heat is supplied
(step 1207) until the demand for heating is terminated (step 1209) at which
time heat transfer medium
pumping is terminated (step 1211) as is electrolysis (step 1213). The process
illustrated in Fig. 11 can be
similarly modified, as shown in Fig. 13.
In a minor modification of the previously described processes, the
electrolytic heating
subsystem is continuously operated at a low output level until a demand for
heat is placed on the system,
at which time heat output is increased. This approach provides rapid heat
output from the radiant heat
tubing while limiting the inefficiency of operating the electrolytic heating
subsystem continuously.
As shown in Fig. 14, the electrolytic heating subsystem operates at a low
output level
continuously (step 1401). Once a demand for radiant heat is received by the
system (step 1403), heat
transfer medium pumping through the conduit and the radiant heat tubing
coupled to the conduit is
initiated (step 1405). Additionally, the level of heat output by the
electrolytic heating subsystem is
increased (step 1407). Radiant heat continues (step 1409) until the demand for
heating is terminated
(step 1411), at which time heat transfer medium pumping is terminated (step
1413) and the heat output
from the electrolytic heating subsystem is decreased to the pre-demand level
(step 1415).
The method of continuously operating the electrolytic heating subsystem at a
low level
until a demand for heat is received can also be applied to the processes
described relative to Figs. 11 and
13. Specifically and as illustrated in Fig. 15, the electrolytic heating
subsystem operates at a low output
level continuously (step 1501). Additionally, the temperature in proximity to
the radiant heater is
17
CA 02613826 2007-12-07
monitored (step 1503) and compared to a preset temperature (step 1505). The
preset temperature is
preferably set by the end-user, for example using a thermostat. As long as the
monitored temperature is
higher than the preset temperature (step 1507), the system simply continues
monitoring the temperature.
Once the system determines that the temperature is lower than the preset
temperature (step 1509), heat
transfer medium pumping through the conduit and the radiant heat tubing
coupled to the conduit is
initiated (step 1511) and the heat output level of the electrolytic heating
subsystem is increased (step
1513). While radiant heat is supplied (step 1515) the temperature in proximity
to the radiant heat tubing
is continually monitored (step 1517) and compared to a second preset
temperature (step 1519). The
second preset temperature can either be the same as the first preset
temperature or it can be different
from the first preset temperature. As long as the monitored temperature is
lower than the second preset
temperature (step 1521), the system continues to pump heat transfer medium
through the radiant heat
tubing and continues to monitor the temperature. When the system determines
that the monitored
temperature is greater than the second preset temperature (step 1523), heat
transfer medium pumping is
terminated (step 1525), the heat output from the electrolytic heating
subsystem is decreased to the pre-
demand level (step 1527), and the system goes back to monitoring temperature
(step 1503).
As previously described, if desired the system can be configured to adjust the
operating
parameters of the electrolytic heating subsystem during operation, for example
based on the temperature
of the fluid within the electrolysis tank or the temperature within the
radiant heat tubing. This type of
control can be used, for example, to insure that the temperature of the
electrolytic heating subsystem
remains within a preset range, even if the system output varies with age.
Typically this type of process
modification occurs periodically; for example the system can be configured to
execute a system
performance self-check every 30 minutes or at some other time interval. As
process modification is used
to optimize the system, it will be appreciated that it is done in addition to,
not as a replacement for, the
processes described relative to Figs. 10-15.
Fig. 16 illustrates a preferred method of modifying the output of the
electrolytic heating
subsystem. In this aspect of operation, when the system is in use and
operating at full, not reduced, heat
output (step 1601), it periodically undergoes a self-checking process (step
1603). The first step of the
self-check process is to determine the temperature of the electrolysis
subsystem or another representative
region (step 1605). The measured temperature is then compared to a preset
temperature or temperature
range (step 1607), the preset temperature/temperature range set by the end-
user, the installer, or the
manufacturer. If the temperature is acceptable (step 1609), for example within
the preset temperature
range, the system simply goes back to standard operation until the system
determines that it is time for
another system check. If the measured temperature is unacceptable (step 1611),
for example it falls
18
CA 02613826 2007-12-07
outside of the preset range, the electrolysis process is modified (step 1613).
During the electrolysis
process modification step, i.e., step 1613, one or more process parameters are
varied. Exemplary process
parameters include pulse duration, pulse frequency, system power cycling,
electrode voltage, and, if the
system includes an electromagnetic rate control system, the intensity of the
magnetic field. Preferably
during the electrolysis modification step, the system controller modifies the
process in accordance with a
series of pre-programmed changes, for example altering the pulse duration in
10 microsecond steps until
the desired temperature is reached. Since varying the electrolysis process
does not have an immediate
affect on the monitored temperature, preferably after making a system change a
period of time is allowed
to pass (step 1615), thus allowing the system to reach equilibrium, or close
to equilibrium, before
determining if further process modification is required. During this process,
the system controller
continues to monitor the temperature of the electrolytic heating subsystem or
another representative
temperature (step 1617) while determining if further system modification is
required (step 1619) by
continuing to compare the monitored temperature with the preset
temperature/temperature range. Once
the temperature reaches an acceptable level (step 1621), the system goes back
to standard operation.
As will be understood by those familiar with the art, the present invention
may be
embodied in other specific forms without departing from the spirit or
essential characteristics thereof.
Accordingly, the disclosures and descriptions herein are intended to be
illustrative, but not limiting, of
the scope of the invention which is set forth in the following claims.
19