Note: Descriptions are shown in the official language in which they were submitted.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
METERING AND PACKAGING OF CONTROLLED
RELEASE MEDICATION
Field of the Invention
The present invention relates to the metering and packaging of precise
quantities of pharmaceuticals and drugs for medical uses. The invention has
particular utility in the metering and packaging of combinations of two or
more pharmaceuticals and drugs for the same or c-morbid therapy, and will
be described in connection with such utility, although other utilities are
contemplated.
Related Art
The conveni.ence of administering a single dose of a medication which
releases multiple active ingredients in a controlled fashion and in a chose-n
location over an extended period of time, as opposed to the admixiistration of
a number of single doses at regular intervals, has long been recognized in the
pharmaceutical arts. The advantage to the patient and clinician in having
consistent and uniform blood levels of medication over an extended period of
time are likewise recognized. The advantages of a variety of controlled-
release dosage forms are well known. Among the most important advantages
are: (1) increased contact tinie for the drug to allow for local activity in
the
stomach, small intestine, colon, or other locus of activity; (2) increased and
more efficient absorption for drugs which have specific absorption sites; (3)
the ability to reduce the number of dosages per period of time; (4)
employment of less total drug; (5) m;,,im;7ation or elimination of local and f
or
systemic side effects; (6) minimization of drug accumulation associated with
chronic dosing; (7) improved efficiency and safety of treatment; (8) reduced
fluctuation of drug level; and (9) better patient compliance with overall
disease management.
In accordance with the present invention there is provided a
pharmaceutical delivery package comprising fixed unit dose quantities of
fixed quantities of two or more different active pharmaceutical ingredients
(a)
combined in a single delivery package,-and (b) segregated from one another
within the package.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
2
Additionally, many experts believe controlled release drug delivery
has many important non-therapeutic ramifications as well, including a
financial saving to the patient in terms of fewer lost work days, reduced
hospitalization and fewer visits to the physician.
It is known that certain design parameters are critical to proper drug
delivery. Typically, they are: (1) delivering the drug to the target tissue;
(2)
supplying the drug for a predetermined period of tirne; and (3) fabricating a
delivery system that provides drug in the desired spatial and temporal
pattern. Controlled release drug delivery systems are interided to utilize
these parameters to achieve the aforementioned advantages as compared to
conventional pharmaceutical dosing.
Previously direct placement of medication onto a substrate generally
was linv.ted to medical placement of large doses or required technology
where the active pharmaceutical was mixed with the substrate or matrix to
provide differential delivery, or coated with a material with desired release
characteristics.
As used herein "controlled-release" is used to describe a system, i.e.
method and materials for making an active ingredient available to the patient
in accordance with a preselected condition, i.e. time, site, etc.. Controlled-
release includes the use of instantaneou.s release, delayed release and
sustained release. "Instantaneous release" refers to immediate release to the
patient. "Delayed release" means the active ingredient is not made available
until some time delay after administration. Typically; dosages are
administered by oral ingestion, although other forms of administration are
contemplated in accordance with the present invention. "Sustained release"
refers to release of active ingredient whereby the level of active ingredient
available to the patient is maintained at some level over a period of time.
The
method of effecting each type of release can be varied. For example, the
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
3
active-ingredient can be placed on a semi-permeable membrane having
predetermined diffusion, dissoltition, erosion or breakdown characteristics.
Alternatively, the active ingredient can be masked by a coating, a
laminate, etc. Regardless of the method of providing the desired release
pattern, the present invention contemplates delivery of a controlled-release
system which'utilizes one or more of the "release" methods and materials.
Moreover, the present invention advantageously can be employed in the
development of multiple different release system(s).
The patent and scientific literature is replete with various sustained
release (SR) methods and materials. For common methods of obtaining SR
systems, see "Sustained and Controlled Release Drug Delivery Systems,"
Robinson, Joseph R., Ed., PP 138-171,1978, Marcel Dekker, Inc. New York,
NY. For example it is known to filI polymeric cappules with a solid, liquid,
suspension or gel containing a therapeutic agent which is slowly released by
diffusion through the capsule walls. Heterogeneous matrices, for example,
compressed tablets, control the release of their therapeutic agents either by
diffusion, erosion of the matrix or a combination of both. Other SR systems
focus on the fabrication of laminates of polymeric material and therapeutic
agent which are then formed into a sandwich, relying on different diffusion or
erosion rates to control xelease of the therapeutic agent. Liquid liquid
encapsulation in a viscous syrup-like solution of polymer also has been
known to be useful in controlling release of the therapeutic agent.
Additionally, it is generally known that heterogeneous dispersions or
solutions of therapeutic agents in water-swellable hydrogen matrices are
useful in controlling the release of the agent by slow surface-to-center
swelling of the matrix and subsequent diffusion of the agent from the water-
swollen part of the matrix.
During dissolution of a controlled-release matrix tablet, the dosage
form generally remains as a non-disintegrating, slowly eroding entity from
CA 02614899 2007-12-20
WO 02/096347 PCTIUS02/16185
4
which the therapeutic agent leaches out, through a diffusion controlled
process. Conventional SR formulations are generally designed to release their
active ingredients over an extended period of time, usually 8-24 hours.
Conventional SR formulations use waxes or hydrophilic gums as the primary
drug carriers to prolong the release of the active ingredients.
Starch USP (potato or corn) is commonly used as a component in
conventional tablet or hard shell capsule formulations.
The existing sustained release technologies generally involve relatively
complicated formulations and manufacturing processes which often are
difEicult and expensive to precisely control. For example, one well known SR
delivery system, OROS, marketed by the Alza Corporation, involves laser
drilling through a tablet to create passages for the release of the drug from
the
tablet core. In controlled release technologies, it is desirable to be able to
incorporate the active ingredient in its controlled-release pattern in a
single
dosage unit without deteriorating the active ingredient. Moreover, the dosage
unit should be able to deliver the system without interfering with -its
release
.pattern.
Various methods have been devised to enable controIled-release
systems to be delivered to a patient without destruction of the delivery
system during manufacturing, handling and distribution. For example,
controlled-release systems have been provided in the form of beads or
particles which are packaged in a gelatin capsule for oral dosage. This
method of delivery of the controlled-release system prevents damage to the
coating on the beads.
Furthermore, when controlled-release active ingredients are
incorporated in compression tablets, it may be difficult for many people to
swallow such tablets. -Moreover, dissolution of high compression tablets often
initially is slow and erratic and may result in localized hot =spots of
alimentary
tract irritation where disintegration and release of the active ingredient
finally
CA 02513392 2008-01-22
WO 02/096347 PCT/US02/16185
occurs. And, present systems do not allow for the accurate deposition of
doses of powdered medication onto different substrates either in single
packets, layered packet, or multipackets on the same plane of the base
substrate. The present invention overcomes the disadvantages of the prior art
by offering, a simple and inexpensive means of incorporating active ingredient
(the drug) with a multitude of controlled-release systems.
In our earlier U.S. Patent 5,699,649, granted December 23, 1997, we
describe a method and apparatus for packaging microgram quantities of fine
powders such as pharmaceuticals using electrostatic phototechnology
techniques. More particularly, as described in our aforesaid U.S. Patent
5,699,649, the ability of powders to acquire an electrical charge
advantageously is utilized for precisely measuring exact microgram
quantities of the powder, whereupon these exact microgram quantities are
then placed in individual containers, and the containexs sealed.
Electrostatic chaige has been employed to attract a given quantity of
powder to a surface. An example of this is the laser printer or the
electrostatic
copy device where a drum is charged and toner particles are attracted and
held in position by the charge. The charge on the drum is neutralized by the
attracted toner powder, thus limiting the amount of toner in accordance with
the charge image on the drum. The charged powder on the printer drum is
then transferred to a sheet of paper or other carrier to give a final image.
In
our U.S. Patent 5,699,649, electrostatic charge technology is employed for
transferring a predetermined amount of a finely powdered pharmaceutical or
drug to a'carrier or an intermediate such as a drum, carrying a charge of
predetermined intensity and area, rotating the charged druin surface,
carrying the predetermined amount of powdered pharmaceutical or drug on
its surface, to a transfer station where the charge is overcome and the dry
powder is transferred to a package which is then sealed. In lieu of a drum, a
belt, or other movable surface is charged to a given potential in a localized
CA 02614899 2007-12-20
6
area. Alternatively, a predetermined amount of powdered pharmaceutical or
drug may be deposited directly in a package using electrostatic charge
technology.
When a given amount of a powdered pharmaceutical or drug is to be
packaged ; the charge and area of charge can be determined experimentally for
each dose of pharmaceutical or drug and each particle size distribution. This
can be done by controlling either the charged area for a given charge density
or the total electrostatic charge on any individual charged area. These
conditions can be adjusted to provide essentially the exact desired amount of
the particular pharmaceutical or drug to be transferred at the transfer
station.
In our U. S. Patent No. 5,960,609, we describe another
electrostatic charge technology which may be adopted to be used for
measuring and packaging unit doses of a pharmaceutical or drug in a readily
ingestible form, i. e. as a tablet or capsule. The technology thus described
also
permits reproducible precise measurement and packaging of a pharmaceutical
or drug, and which may be scaled from laboratory to pilot plant to full scale
production without the need for recertification.
Summary of the Invention
In accordance with one aspect of the invention, a pharmaceutical
delivery package comprising two or more active pharmaceuticals (a)
combined in a single delivery package, and (b) segregated from one another,
wherein the single delivery package comprises an unitary structure for
repeatable administration of fixed quantities of the two or more active
ingredients to the user, wherein the pharmaceuticals are encapsulated within
inert coatings, and wherein the two or more active ingredients comprise
Ketoconazole and testosterone; Valacylovir and one or both of Cimetidine
and Probenecid; Enalapril and a beta adrenergic-blocking agent, methyldopa,
nitrate, a calcium blocking agent, hydrazine, Prazosin or Digoxin;
Omeprazole and B 12; of Omeprazole and Clarithoromycin; Tamoxifen and a
diuretic; Isotretinoin and an oral contraceptive; Metformin HCI and
Solfonylurea; a diuretic and an Angiotensin converting enzyme inhibitor
(ACE inhibitor); a diuretic and an Angiotensin II Receptor Antagonist; a Beta
Adrenergic Blocking Agent; a diuretic and a Calcium channel block; a
CA 02614899 2007-12-20
6A
diuretic and a Periferal Adrenergic Blocking Agent; a diuretic and an
Adrenergic central stimulant; a diuretic and Endothelin A; an ACE inhibitor
and
a beta blocker; a biguanide and a sulfonylurea; a biguanide and a
thiazolidinedione; Metaformin and an alpha glucosidase inhibitor; a short
acting
oral insulin with a sustained release oral insulin; an HMG-CoA reductase
inhibitor with a bile acid sequestrant; an HMG-CoA reductase inhibitor with a
niacin compound; an HMG-CoA reductase inhibitor with a hypolipidemia agent;
an HMG-CoA reductase inhibitor, a niacin compound and a hypolipidemia
agent; digitalis plus an ACE inhibitor; digitalis plus an ACE inhibitor and a
diuretic; digitalis plus an ACE inhibitor, a diuretic and a beta blocker; a
rapid
onset anti-histamine plus a sustained release anti-histamine; an antihistamine
plus a Leukotriene modifier; a rapid acting 5-HT1 receptor agonist plus a long
acting 5-HT1 receptor agonist; an anti-nausea plus a steroid; a quick onset H
blocker plus a proton pump inhibitor; a selective serotonin reuptake inhibitor
(SSRI) fluoxetine and an Aminoketon; a protease inhibitor plus a nuclear
reverse
transcriptase inhibitor plus 2 d NRTI-Ziduvudine or Azidothymidine;
cyclosporine plus a steroid; cyclosporine plus a steroid, plus a PPI/H2;
Isoniazid,
Pyrazidamide and Rifampin; a Calcium channel block plus a vasodilator; a
Gamma Aminobutyric analog or a Gamma Aminobutyric stimulator plus a
Benzodiazepine; an opioid and a non-opioid analgesic; an opioid and an
antiemetic; an opioid and a bowel softener or evacuant; a cyclooxygenase-2
inhibitor plus Omeprazole; an anti-inflammatory plus Omeprazole; prednisone
plus testosterone; prednisone plus estrogen; a selective serotonin reuptake
inhibitor plus a benzodiazepinel; and an aminoketone plus Lorazepam.
In accordance with another aspect of the present invention, controlled
quantities of powdered medication are formed in controlled release packages
using electrostatic metering technology. The present invention also provides,
in
another aspect, combination medication delivery systems in which the active
ingredients are segregated from one another.
Brief Description of the Drawings
Further features and objects of the present invention will become clear
from the following detailed description taken in conjunction with the
accompanying drawings, wherein like numerals depict like parts, and wherein:
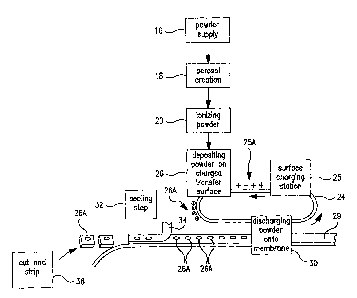
Fig. 1 is a schematic flow diagram showing the various steps involved in
practicing the present invention;
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
7
Fig. 2 is an enlarged cross-sectional view of one embodiment of a
controlled release tablet made in accordance 'with the present invention;
Fig. 3 is a view, sinmilar to Fig.1; and showing alternative steps
involved in practicing the present invention;
Fig. 4 is a view, similar to Fig. 2, and showing an altemative form of a
controlled release tablet made in accordance with the present invention;
Fig. 5 is a view similar to Fig. 2, and showing yet another alternative
embodiment of the present invention;
Fig. 6 is a view, sunilar to Fig. 2, and showing yet another embodiment
of the invention; and
Figs.7-9 are views similar to Fig. 2, and showing yet other embodiments
of the present invention.
Detailed Description of the Preferred Embodiments
Referring now to Fig. 1, there is a schematic flow diagram of the
various pieces of equipment needed- to perform in the total process from
powder supply to packaged pharmaceutical or drug, i.e. in controlled release
tablet form, containing a specified amount of ph.armaceutical or drug powder
..in the tablet or package. At 16 is indicated the pharmaceutical or drug
powder supply which is fed into a device 18 for creating an aerosol of the
powder. Next the powder particles are ionized at 20. As wiIl be indicated
later, a number of these steps and pieces of equipment can be conibined_ At
24 is indicated a carrier surface capable of maintaining a space charge on its
surface. This can be a plastic belt, for example, or a selenium drt.ln-i of
the
type used in Xerox TM photocopiers. This carrier surface 24 is passed through
a charging station 25 where a predetermined electrostatic charge 25A (an
electrostatic "image") is created on a predetermined area of the transfer
surface. This charged surface 25A then passes through a step 26 wherein
powder is deposited on the carrier surface in a sufficient amount 26A to
neutralize the charge carried by the carrier surface. Thereafter, the carrier
surface, carrying the predetermined amount 26A of powder on its surface, is
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
S
passed to a powder discharging device 30 which discharges the powder 26A
from the surface 24 onto a membrane 29. Alternatively, the powder may be
placed directly onto the membrane 29. The membrane 29 containing its
charge of powder 26A, then passes through a sealing step 32 wherein a
second membrane 34 which may be porous, permeable or semi-permeable
covers and seals the discharged powder 26A on the membrane 29. There is
thus produced an aliquot of powdered medicine 26A sandwiched between
semi-permeable or permeable membranes 29 and 34.
This sandwiched material is then passed to a cutting station 38 wherein
the sandwich is cut into individual tablets or wafers 36.
As mentioned previously in discussing Fig. 1, the carrier surface with
the electrostatic charge carries a known amount of charge on its surface and
the polarity of this charge is opposite to that of the powder particles
suspended in the chamber. The charged particles migrate to the charged
surface because of the attraction by the opposite nature of the charges. This
migration of the particles continues until the charge on the carrier surface
is
neutralized.
The actual amount of powder mass transferred to the carrier surface is
a function of the mass-to-charge ratio of the charged particles. Although it
is
difficult to achieve a linear relationship between the mass and the actual
charge, it is possible to establish a fixed relationship between the surface
area
of the powder particles and the charge the powder pa.rticle is carrying at
charge saturation. However, the surface area of a mixed group of powder
particles of different sizes and shapes can be extremely difficult to
calculate
mathematically, particularly when the shapes are irregular, (e.g. non-
spherical, microcrystalline, etc.) As mentioned earlier, the simplest method
of determining the amount and area of charge to attract a given weight of
particles is to estimate the correct area and charge and then apply the
estimated charge to the estimated area on the carrier surface 24 and expose
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
9
this selectively charged area to a mass of powder which has been ionized in
the ionizing step. The amount of powder deposited can then be readily
measured at the discharge step. Thereafter, either the size of the charged
area
or the amount of charge applied to the area at the charging station 25 can be
adjusted upwardly or downwardly to provide the correct amount of charge,
both in area and charge intensity, for picking up a desired weight of
oppositely charged powder. Likewise, using the technology of our co-
pending application Serial No. 09/097,104, larger quantities of medication
may be deposited.
A feature and advantage of the present invention is to produce
carefully controlled doses of controlled release medication. Electrostatic
metering and packaging as above described permits exact dosing. And, by
employing selected porous, permeable or semi-permeable membranes for
encapsulatirig the powdered medicine aliquots, drug release rate and also site
of drug release can be determined by adjusting membrane material and/ or
membrane thickness.
The membranes should be formed of ingestible materials having a
selected permeability porosity to fluids at a selected site or sites within
the
alimentary canal, so as to permit controlleci release of the medication. By
way
of example, one or both membranes 29, 34 may comprise.acid-dissolvable
materials when it is desired to release the medication into the stomach or the
membranes 29, 34 may be alkaline-dissolvable materials at differing pH's to
release into chosen locations within the intestine. Porosity, membrane
thickness, etc., may be selected to provide desired rate of dissolution at the
site of interest.
The invention is susceptible to modification. For example, referring to
Figs. 3 and 4 by adding a second powdered medicine supply and discharge
station (shown generally at 40), a two-component controIled release tablet 48
may be formed (see Fig. 4) incorporating two different powdered medicines
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
50,52, encapsulated between membranes 29 and 34 for simultaneous
controlled release.
Alternatively, as shown in Fig. 5, two different drugs 60, 62 may be
layered on one another, separated by a membrane 64 so the two medications
may be delivered sequentially either in the same location, or in different
locations within the alimentary canal. Another feature and advantage of the
multi-drug tablet of Fig. 4 and Fig. 5, as wiIl be discussed in detail herein
below, is that two normally incompatible drugs may be to be safely packaged
in a single tablet.
The. invention is susceptible to modification. For example, individual
doses may be formed by electrostatic deposition in accordance with U.S.
Patent No. 5,714,007.
Other possibilities are possible. For example, referring to Fig. 6, the
tablet 70 may incorporate an adhesive layer 72 such as a mucosal adhesive,
which in turn is covered by an acid or.alkaline dissolvable protective
membrane 74, which dissolves at a selected site allowing the adhesive to
adhere, for example, to the intestinal wall, thereby increasing residence time
of the medication in a chosen location. Alternatively, an acid or alkaline
activatable adhesive may be applied to the outer surface of the tablet. In yet
another possibility, the membrane may be a material which expands on
contact with the acid or alkaline in the alimentary canal and becomes more
porous whereby to slowly release medication in a chosen location within the
alimentary canal.
As mentioned above, a particular feature and advantage of the present
invention is that it permits packaging, within a single tablet of two or more
different drugs normally considered to be incompatible. Certain drugs are
known to cause undesirable side effects which need to be countered by a
second drug. Fdr example, Omeprazolel which finds substantial utility as an
oral antiulcer agent, also is known to block the release of B12 from its
protein
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
11
binding site in food. This can lead to perniczous anemia. The present
invention permits packaging of time-release Omeprazole with Vitamin B12 in
an appropriate dosage of, e.g. 25 gm -1 mg. After taking the medication, one
membrane will dissolve allowing absorption of the B12, while the remaining
membrane package carrying the Omeprazole will pass into the small intestine
where the drug is released and absorbed.
The invention is susceptible to modification. For example, while the
membranes have been described as being preformed, permeable,
semipermeable or porous material, one or both membranes could be formed
in place from a gel or liquid.
The ability to accurately place the dose of medication onto a plurality
of substrates and seal the dose with other membranes in accordance with the
present invention, allows for the fabrication of many different dosage forms;
by altering the substrates and encapsulating material a single unit dose form
Can be fabricated with a plurality of different drugs in different coverings,
membranes and barriers. This will provide a single dosage form with
-multiple active ingredients each being delivered to the appropriate site for
absorption. Altexnatively; two or more active medicaments may be combined
in a single delivery container, i.e. pill, capsule or caplet without actually
mixing the two or more ingredients. For example, referring to Fig. 7, the
active ingredients are segregated from one another in a compartmentalized
capsule 100. Alternatively, two or more tablets 102,104 each containing only
one active ingredient, could be placed in a larger absorbable capsule or
encased in a larger tablet 106. Or, as shown in Fig. 9, two or more active
ingredients could each be formulated as encapsulated particles 10SA,108B,
and the encapsulated mixed particles placed iri a capsule 110 where the only
contact is between the particle inert coatings, etc.
There are many drugs which could benefit from combinations to
improve patient benefit. However, with many active ingredients, there is a
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
12
question of chemical interaction. Thus, several drugs are normally prescribed
as separate tablets or capsules which presents a problem in terms of patient
compliance, e.g. TB triple therapy, AIDS multi-drug therapy, anti-infectives,
etc. Also, delivery of two or more active medicaments could reduce side
effects, and/or improve therapeutic response which may in turn permit a
decrease in the required dosage.
The combination of drugs of the present invention can be grouped into
polypharmacy for a therapeutic area, and into polypharmacy for treatment of
co-morbid diseases. The invention will now be described with reference to
the following non-limiting examples.
(1) Omeprazolel and analogs and isomers - As noted above
Omeprazole is an inhibitor of gastric secretion and also inhibits the
absorption
of certain drugs/compounds that require stomach acid such as Vitamin B12,
the deficit of which results in pernicious anemia. A combination of B12 with
Omeprazole would elirninate the potential problem.
(2) Valacyclovir2 and analogs and is used to treat Herpes Zoster. It
is well known that two drugs Cimetidines and Probenecid4 both increase the
AUC (area under curve) and Cmax. A combination drug can be constructed
with a combination of either one or more of these components to provide
more efficacy.
(3) Enalapril5 and analogs and isomers is an ACE inhibitor used for
the treatment of hypertension. This drug has been used with the following
and analogs and isomers beta adrenegic-blocking agents, methyldopa, nitrate,
calcium blocking agents, Hydralazine6, Prazosin7 and Digoxin$ without
clinically significant side effects. One or more of these agents may be
combined with Enalapril to improve the compliance of patient with
hypertension and hypertension and other cardiac diseases.
(4) Ketoconazole9 and analogs and isomers is used to treat fungal
infections. One of the side effects is the reduction of Testosterone. This
side
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
13
effect could be mitigated by the combination of Testosterone or one of its
isomers or analogs to overcome the side effect.
(5) Omeprazolel and analogs and isomers is also used in
combination with ClarithoromycinlO for ulcer treatment. These two drugs
may be combined as a single dose for patient compliance.
(6) Tamoxifenll and analogs and isomers used in treatment of
breast cancer has a +/- 30% incident of water retention with weight gain >
5%. This can be a disturbing consequence for patients with an even more
disturbing disease. The addition of a diuretic or combination diuretic
provides a single dosage form for reduction in side effect and compliance.
(7) Isotretinoin12 and analogs and isomers used for the treatment of
postular acne has a severe danger if taken by a woman who is pregnant. The
incorporation of oral contraceptive medication eliminates the potential for
pregnancy while medicated.
(8) Metformin HCI13 and analogs and isomers are hypoglycemic
agents which have been used in combination with Sulfonylurea14 and analogs
and isomers to treat Type 2 Diabetes. These two agents act in different ways
on reducing glucose levels. A combination is helpful for those patients
requiring more aggressive oral therapy for their diabetes.
(9) This example provides various drug combinations for treating
hypertension.
Combinations for treating hypertension include:
Combination ## 113iuretic + Angiotensin converting enzyme inhibitor
(ACE inhibitor)
An example includes the following classes of diuretics:
1. Carbonic anhydrase ffiliibitors - e.g.
Dichlorophenamide15.
2. Loop diuretics, e.g. Purosemide16.
3. Potassium sparing diuretics, e.g. AldactoneW.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
14
4. Thiazides and related drugs, e.g. Hydrochlorthiazide18
and Chlorthalidone'9.
5. A diuretic which is already formulated as a combination
diuretic, e.g. Aldactazide, a combination of Spironolactone20 (potassium
sparing diuretic + hydrochlorothiazide). This combination makes use of the
different methods of action of two different diuretics separated by a barrier
from an ACE inhibitor such as Enalapril maleate21, Fosinopril sodium22, or
Lisinopril23.
Combination diuretics such as Zestoretic AstraZeneca a combination of
Lisinopri12710 or 20 mg and Hydrochlorthiazide1712.5 or 25 mg, exist in tablet
form comprising mixed active ingredients in the pill or tablet form. The
present invention segregates the Lisinopril and Hydrochlorthiazide.
In accordance with the present invention, we can form e.g. 10 mg and
20 mg Lisinopri122 pills, and 12.5 and 25 mg Hydrochlorthiazidel7 pills and
then put them together with a barrier between two active ingredients. Pills
can be in the form of tablets, pills, capsules or other solid oral dosage
forms.
Combination # 2 Diuretic + Angiotensin It Receptor Antagonist
Diuretics as described in combination drug # 1 plus an angiotensin II
receptor antagonist such as Losartan potassium24 and/or Valsartan25.
These combinations also permit administration of two or more drugs
which, if in direct contact, have an unacceptable reaction.
Combination # 3 Diuretic + Beta Adrenergic Blocking Agent
Diuretic as described in combination #1, plus a beta adrenergic
blocking agent such as Bioprolol fumarate26 or Metoprolol succinate27.
Combination # 4 Diuretic + Calcium chanel block
Diuretic as described in combination #1, plus a Calcium chanel block
such as Amlodipine28 or Nifedipine29.
Combination # 5 Diuretic + Periferal Adrenergic Blocking A ent
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
Diuretic as described in #11, plus a periferal adrenergic blocking agent
such as: Prazosin hydrochloride7.
Combination # 6 Diuretic + Adrenergic central stimulant
Diuretic as described in #1, plus an adrenergic central stimulant such
as: Methyldopa30 or Clonidine31.
Combination # 7 Diuretic + Endothelin A
This is a new class of drugs.
The drug barrier system of the present invention allows further drug.
combinations such as a Calcium chanel block combined with: beta blockers,
ACE inhibitors, long acting nitrates, Digoxin8, oral hypoglycemic drugs as
well as multiple combinations, and combinations with a diuretic and
combination drugs # 2, 3, 4 or more of the above-mentioned compounds.
Combination #8 ACE Inhibitors + Beta Blockers
The drug barrier system of the present invention also allows drug
combinations such as ACE Inhibitors combined with Beta blockers,
methyldopa nitrates, calcium channel blockers, Hydralazine6, Prazosin7,
Digoxin8 as well as multiple combinations, and combinations with a diuretic
and combination drugs # 2, 3, 4 or more of the above-mentioned compounds.
(10) This example provides various'drug combinations for treating
diabetes.
Combination # 9
Biguanide such as Metaformin13 with a sulfonylurea such as
Glipizide32.
Combination # 10
Biguanide such as Metaformin13 with a thiazolidinedione such as
Rosiglitazone maleate33.
Combination # 11
Metaforrninl3 with an alpha glucosidase inhibitor such as
Cerivastatin34.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
16
Combination # 12
Short acting oral insulin with sustained release oral insulin.
(11) This example provides various drug combinations for treating
hyperlipidernia.
Combination # 13
HMG-CoA reductase inhibitor such as Simvastatin35, Atorvastatin36, or
Pravastatin37 with a bile acid sequestrant such as Colestipol hydrochloride38.
Combination # 14
A HMGCoA reductase inhibitor with a niacin compound.
Combination # 15
A HMG-CoA reductase inhibitor or combination #14 with a
hypo]ipidemia agent such as Gemfibrozi139.
(12) This example provides various drug combinations for treating
congestive heart failure.
Combination # 16
Digitalis plus an ACE inhibitor with or without a diuretic, and
optionally including a beta blocker.
Combination # 17
Digitalis plus any of the combination drugs #1-#7.
(13) This example provides various drug combinations for treating
asthma/allergy.
Combination # 18
Rapid onset anti-histamine plus sustained release anti-histamine.
Combination # 19
Antihistamine plus Leukotriene modifier, such as Loratadine40 plus
Montelukast41.
(14) This example provides a drug combination for treating
migraine.
Combination # 20
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
17
Rapid acting 5-HT1 receptor agonist such as Naratriptin HC142 plus a
long acting 5-HT1 receptor agonist such as Sumatriptan43.
(15) This example provides a drug combination for treating post-
operative/ post-chemotherapy nausea.
Combination # 21
Anti-nausea such as Doperidol44 plus steroid such as Dexamethasone45.
(16) This example provides various drug combinations for treating
gastric/ duodenial ulcer.
Combination # 22
Quick onset H blocker such as Famotidine46 plus a proton pump
inhibitor such as Omeprazolel.
Combination # 3
Selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac47) and
Aminoketon - Buptopion48.
(17) This example provides a drug combination for treating HIV.
Combination # 24
Protease inhibitor - Indinavir (Crixivan49) plus nuclear reverse
transcriptase inhibitor - Efavirenz (Sustiva50) plus third drug, i.e. 2nd NRTI-
Ziduvudine51 or Azidothymidine52.
(18) This example provides various drug combinations for treating
anti-rejection cocktail after organ transplant.
Combination # 25
Cyclosporine-'~3 plus steroid - Prednisone54.
Combination # 26
Combination drug # 25 plus PPI/H2 for ulcer pxevention -
Omeprazole1.
(19) This example pirovides a drug combination for treating
infections with combination therapy such as tuberculosis.
Combination #27
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
18
Triple combination Isoniazid55 and Pyrazidamide56 and Rifampin57.
(20) This example provides a polypharmacy for treatment of co-
morbid diseases.
Combination # 28
80% + of diabetics are also hypertensive. Therefore a combination of
any of combination drugs # 7-12 which are the combinations for control of the
diabetes with any of combination drugs #1-7 or the single component
medicaments used in the anti-hypertensive combinations.
Combination # 29
Hyperlipidemia is frequently concurrent with cardiac disease therefore
any of combination drugs # 13-17 plus any of combination drugs # 1-7.
(21) This example provides a polypharmacy for treatment of Angina.
Combination #30
A Calcium channel block such as Nifedipine29 plus a vasodilator such
as nitroglycerin.
(22) This example provides a polypharmacy for treatment of seizure
disorders.
Combination #31
A Gamma Aminobutyric analog such as Gabapentin58 or a Gamma
Aminobutyric stimulator such as Divalproex Sodium59 plus a Benzodiazepine
such as Alprazolam6o.
(23) This example provides various drug combinations for treating
pain and the side effects of opioids:
Combination #32
An opioid and a non-opioid analgesic such as codeine and
acetominophine.
Combination #33
An opioid and an antiemetic.
Combination #34
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
19
An opioid and a bowel softener or evacuant.
(24) This example provides polypharmacy for e]imimating or
minimizing gastric irritation caused by a primary drug.
Combination #35
A cyclooxygenase-2 inhibitor such as Celecoxib61 plus Omeprazolel.
Combination #36
An anti-inflainmatory such as Naproxen62 plus Omeprazolel.
(25) This example provides polypharmacy for countering the effect
of long term use of Prednisone54.
Combination #37
Prednisone54 plus testosterone to prevent muscle mass loss.
Combination #38
Prednisone-' 4 plus estrogen or progesterone to prevent bone mass loss.
It is also possible to package two or more doses of the same active
ingredient in slow and fast release forms.
(26) This example provides polypharmacy for treating anxiety or
panic disorder.
Combination #39
A selective serotonin reuptake inhibitor such as Paroxetine63 plus a
Benzodiazepine such as Lorazepam64.
Combination #40
An aminoketone such as Bupropion65 plus Lorazepam64.
Various analogs and isomers of the foregoing drugs also
advantageiously may be employed.
It should be noted that certain combination drugs, including some of
the above-hsted combination drugs, also may be blended and packaged in a
single tablet or capsule, when chemical interaction is not a problem.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
The present invention also allows for the rapid production of different
dosage medications using the same active ingredient, and aIlows for the
development of medications with longer resident time.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
21
APPENDIX
1. Omeprazole: 5-methoxy-2[[(4-methoxy-3,5-dimethyl-2-pyrindinly)
methyl] sulfinyl]-1H-benziinidazole.
2. Valacyclovir: L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)
methoxy] ethyl ester, monohydrochloride.
3. Cimetidine: N"-cyano-N-methyl-N'-[2-[[(5-methyl-l-H-imidazol-4-yl)
methyl]thio]-ethyl]-quanidine.
4. Probenecid: 4-[(dipropylamino) sulfonyl] benzoic acid (molecular
weigh 285.36).
5. ' Enalapxil: (S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-
proline, (Z)-2-butenedioate salt.
6. Hydralazine: 1-Hydrazinophthalazine monohydrochloride.
7. Prazosin HCI: hydrochoride salt of 1-(4-amino-6,7-dimethoxy-2-
quinazolinyl)-4-(2-furoyl) piperazine.
8. Digoxin:, 3B-[(o-2,6-dideoxy-B-D-ribo-hexopyranosyl-(lrj4)-0-2, 6-
dideoxy-B-D-ribo-hexopyranosyl - (1jI4)-2,6-dideoxy -B-D-ribo-
hexopyranosyl) oxy] -12B, 14-dihydroxy-5B-card-20(22) enolide.
9. Ketocanozole: CIS -1- acetyl - 4[4 -[[2,4-dicheorophenyl -2-(1H-
imidazol-1-ylmethyle) -1,3-dioxolan-4-yl] methocy] phenyl] piperazive.
10. Clarithoromycin:6-0-methylerythromycin.
11. Tamoxifen: (Z)2-[4=(1,2-diphenyl-l-butenyl)phenoxy]-N, N-
dimethylethanaxnine 2 hydroxy-1,2,3-propanetricarboxylate.
12. Isotretinoin: 13-cis-retinoic acid.
13. Metformin: N,N-dimethylimidodcarbonimidic diamide hydrochloride.
14. Sulfonylurea: 1-[[P-[2-(5-chloro-o-anisamido) ethyl] phenyl] sulfonyl-]-
3-cyclohexylure.
15. Dichlorophenamide: 4,5-dichloro-1,3- benzenedisulfonamide.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
22
16. Furosemide: 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid.
17. Aldactone:17-hydroxy-7aliha-mercapto-3-oxo-l7a4L)k-pregn-4-ene-21-
carboxylic acid ag mma-lactone acetate.
18. Hydrochlorthiazide: 6-chloro-3, 4-dihydro-2H-1, 2, 4-benzothiadiazine-
7-sulfonamide 1,1-dioxide.
19. Chlorthalidone:2-Chloro-5-(2,3-dihydro-l-hydroxy-3-oxo-lH-isoindol-
1-yl)benzenesulfonanmide.
,
20. Spirolactone:17-hydroxy-7alpha-mercapto-3-oxo-l7atpha-pregn-4-ene-
21-carboxylic acid ag mma -lactone acetate.
21. Enalapril maleate: (S)-1-[N-[1-(ethoxycarbonyl) -3-phenylpropyl]-L-
alanyl] -L-proline, (Z)-2-butenedioate salt (1:1).
22. Fosinopril sodium: L-proline, 4-cyclohexyl-l-[[[2-methyl-l-(1-
oxopropoxy) propoxyl](4-phenylbutyl) phosphinyl]acetyl]-,sodium
salt, trans-.
23: Lisinopril: (S)-1=[N2-(1-Carboxy-3- phenylpropyl)-L-lysyl]-L-proline
dihydrate.
24. Losartan=potassium.:2-butyl-4-chloro-1[p-(o-1H-tetrazol-5-
ylphenyl)benzyl]imidazole-5-methanol monopotassium salt.
25. Valsartan: as N (1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-
yl]methyl]-L valine.
26. Bioprolol fumarate: ( )-1-(4-((2-(1-
Methylethoxy)ethoxy)methyl)phenoxy)-3-((1 methylethyl)amino)-2-
propanol (E)-2-butenedioate (2:1) (salt).
27. Metoprolol succinate: (t)1-(isopropylamino)-3-[p-(2 methoxyethyl)
phenoxy]-2-propanol succinate (2:1) (salt).
28. Amlodipine: (R.S.) 3-ethyl-5-methyl-2-(2-am.inoethoxymethyl)-4-(2
chlorophenyl)-1,4-dihyxo-6-methyl-3,5-pyridinedicarboxylate
benzenessulphonate.
29. Nifedipine: 3,5-pyridinedicarboxylic acid,1,4-dihydro-2,6-dimethyl-4- =
(2-nitrophenyl)-,dimethyl ester.
' Trademark
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
23
30. Methyldopa: levo-3-(3,4- dihydroxyphenyl)-2-methylalanine
sesquihydrate.
31. Clonidine HCL: (2,6-dichlorophenylamino)-2- imidazoline
hydrochloride.
32. Glucotrol:1-cycloli.exyl-3-[[p-(2-(5-
methylpyrazinecarboxamido) ethyl]phenyl]sulfonyl]urea.
33. Rosiglitazone maleate: ( )-5-[[4-[2-(methyl-2-
pyridinylamino)ethoxy]phenyl]methyl]- 2,4-thiazolidinedione,(Z)-2-
butenedioate.
34. Cerivastatin: [S-[ R*, S'-( E)] -7-[ 4-(4-b fluorophenyl)-5-
methoxymethyl)- 2,6bis(1-met ylethyl) 3-pyridinyll-3,5-dihydroxy+
heptenoate.
35. Siunvastatin:2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-
(tetrahydro=4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl
ester, [1S'E [1a,3a,7b,8b(2S';4S); 8ab]].
36. Atorvastatin: [R-(R*,R*)]-2-(4-fluorophenyl)-b,s-dihydroxy-5-(1-
methylethyl)-3-phenyl-4 [(phenylamino)carbonyl]-1H-pyrrole-l-
heptanoic acid, calcium salt (2:1) trihydrate.
37. Pravastatin:l-Naphthalene-heptanoic acid, 1,2,6,7,8,8a-hexahydro-b,
d,6-trihydroxy-2-methyl -8-(2-methyl -1- oxobutoxy)-, monosodium
salt,[1S-[1a(bS*, d S*),2a,6a,8b(R*),8aa]]-.
38. Colestipol hydrochloride: diethylenefriamine and 1 chloro-2,3-
epoxypropane.
39. Gemfibrozil: 5- (2,5-dimethylphenoxy)-2,2-dinnethylpentanoic acid.
40. Loratadine: ethyl4-(8-chloro-5,6-dihydro-11H benzo(5,6]cydohepta[1,2
b]pyridin-ll-ylidene)-1-piperidinecarboxylate.
41. Montelukast: [R-(E)]-I-[[[1-[3-[2-(7-chloro-2-
quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-l-
methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid,
monosodium salt,
42. Naratriptin HCL: N-methyl-3-(1-methyl-4-piperidinyl)-1H-indole-5-
ethanesulfonamide monohydrochloride.
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
24
43. Sumatriptan: 3-[2-(dimethylamino)ethyl]-N-methyl indole -5-
methanesulfonamide succinate (1:1).
44. Doperidol:1-(1-[3-(p-fluorobenzoyl)propyl]-1,2,3,6-tetrahydro-4-
pyridyl)-2-benzimidazolinone.
45. Dexamethasone: 9-fluoro-1l P,17,21-trihydroxy-l6a-methylpregna-1,4-
diene-3,20-dione.
46. Famotidine: N'-(arninosulfonyl)-3-[[[2= [(diaminomethylene)amino]-4-
thiazolyl]methyl]thio]propanimidamide.
47. Prozac": ( )-N-methyl-3-phenyl-3-[(a,a,a-trifluoro p-tolyl)-
oxy]propylamine hydrochloride.
48. Buptopion: (t)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-
propanone hydrochloride.
49. Cxixivari is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2- hydroxy-
1H-inden-1-yl)-5-[2-[ [ (1,1-dimethylethyl) amino] carbonyl]-4-(3-
pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-
pentonamide sulfate (1:1) salt.
50. Sustiva (S)-6-chloro-4=(cyclopropylethynyl)-1,4-clihydro-4-
(trifluoromethyl)-2H-3,1-benzoxazin 2-one.
51. Ziduvudine: 3'-azido-3'-deoxythymidine.
52. Azidothymidine:3'-azido-3'-deoxythymidine.
53. Cyclosporine: [R-[R.R*(E)}] cyclic(L-alanyl-D-alanyl-N-methyl-L-leucyl-
N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4-dirnethyl-L-2-
?imino-6-octenoyl-Is-a-amino-butyryl-N-methylglycyl-N-rnethyl-L-
leucyl-L-valyl-N-methyl Irleucyl).
54. Prednisone: pregna-1,4-diene-3,11,20-trione, 17,21-dihydroxy.
55. Isoniazid: isonicotinic acid hydrazide.
56. .Pyrazinarnide: pyrazinecarboxamide.
57. Rifampin:3-(4-methyl-l-piperazinyl-iminomethyl)-rifamycin.
~ Trademark
CA 02614899 2007-12-20
WO 02/096347 PCT/US02/16185
.58. Gabapentin 1-(aminomethyl)cyclohexanacetic add.
59. Div-alproex Sodium: sodiuin hydrogen bis (2-propylpentanoate).
60. Alprazolam 8-Chloro-].-mefihyl-6-phenyl-4H-s-triazolo [4,3-a] [1,4]
benzodiazepine.
61. Celecoxib: 4-[5-(4-methylphenyl)-3- (trifluoromethyl)-1H pyrazol=1-yl]
benzenesulfonamide.
62. Naproxen: 2-naphthaleneacetic acid, 5 methoxy- a-methyl-,(+).
63. Paroxetine available as Immediate-Release Tablets and Oral Suspension as:
(-)-trans-4R-(4' fluorophenyl)-3S-[(3',4'-methylenedioxyphenoxy)
methyl] piperidine hydrochloride heniihydrate and as Controlled-
Release Tablets as: (-) - (3S,4R)-4-[(p-fluorophenyl)-3-[(3,4-
methylenedioxy) phenoxy]methyl]piperidine hydrochloride
hemihydrate.
64. Lorazepa.m:7-chloro-5-(0-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-
1,4-benzo-d.iazepin-2-one.
65. Bupropion: (f)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-
propanone hydrochloride.