Note: Descriptions are shown in the official language in which they were submitted.
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
CHEMOSENSITIVITY TESTER
BACKGROUND OF THE DISCLOSURE
The invention relates to a chemosensitivity tester used with biological
artificial tissue slices, including those derived from any major organ, organ
system,
cloned tissue using somatic cell transfer, or any other stem cell based
regimen,
including cord blood, or any manner of neoplasms. The chemosensitivity tester
may be used to evaluate, detect, and test drug candidates, drugs, and drug
metabolites as a method of providing personalized medical treatments. Finally,
the
chemosensitivity tester can be used study diseases, such as carcinogenesis, in
1-0 tissue that has been selected based upon phenotypic analysis, or any other
proteomics, genomics, or metabonomic analysis methods, including nano-system
biological approaches.
It has been established that current regimens have two major failings with
respect to pre-market and post-market testing: By way of example, the VIOXXO
debacle made it clear that efforts to screen candidates for specific disease
state
treatments is needed to find out if segments of the populace have the
potential for
adverse reactions. Using currently available genomic and proteomic analysis
methods, high risk patients and=categories of patients could have tissue
screened
in advance of being subject to such potentially harmful and morbidly toxic
compounds.
Likewise, escalating costs impact this calculus and underscore and
highlight the needs for the teachings of the present disclosure. This becomes
crystal clear upon review of the historical numbers which have been
established in
this space.
In 2001, the average cost to develop a new drug exceeded $800 million,
according to a study by the Tufts Center for the Study of Drug Development. Of
this, approximately $16 million on average per company was used for pre-
clinical
1
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
research. Reduction of testing time and cost in drug development is therefore
a
critical factor to the survival of most pharmaceutical companies. In addition;
since
there is usually more than one company competing in the same drug arena, any
competitive advantage is welcome. A major portion of drug development costs is
borne during the FDA approval process. However, much of this cost cannot be
managed in the same way that pre-clinical costs can. To address soaring pre-
clinical costs, more efficient, affordable, and timely methods of in vivo and
in vitro
testing and selection of potential new drug candidates are of significant
interest in
the industry.
In developing a new drug, toxicity is always an important consideration.
Since the liver metabolizes most drugs, liver damage is of great concern.
Likewise,
other organs and systems, and how they react to foreign substances, is
extremely
important. Conventional in vivo and in vitro tests utilizing small animals and
cell
culture techniques are therefore widely used to assess liver function in drug
development. However, these conventional tests have particular disadvantages,
such as individual variation, high costs to use large animals, and loss of
naturally
existing characteristics of liver in situ. The same is true for other organs.
As our
knowledge base increase on these other organs and how they bring insight into
how mammals respond to various pro-drugs, drugs, compounds and systems the
relative significance of the instant disclosure becomes more prominent and
significant.
To overcome these disadvantages, cell culture systems have been used.
However, with these models cell-to-cell connective interactions cannot be
maintained for a desired length of time. Once cell-to-cell connectivity is
lost, failure
of the testing scheme soon follows because it is no longer directed to organ,
system, or organism level response.
Bioartificial organ devices are currently in development. It is believed that
organ function can only be replaced with the biological substrate, that is,
for
2
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
example, liver slices or a whole liver specimen, which requires the
availability of
liver tissue from xenogenic or human sources. Recent efforts have combined
mechanical and biologic support systems in hybrid liver support devices. The
mechanical component of these hybrid devices serves both to remove toxins and
to create a barrier between the patient's serum and the biologic component of
the
liver support device. The biologic component of these hybrid liver support
devices
may consist of liver slices, granulated liver, or hepatocytes from low-grade
tumor
cells or porcine hepatocytes. -These biologic components are housed within
chambers often referred to as bioreactors. However problems remain with
respect
to maintaining the functionality of the individual cell lines used in these
devices.
Most devices use immortalized cell lines. It has been found that over time
these
cells lose specific functions.
There are several groups developing bioartificial liver devices, for
example, Circe Biomedical (Lexington, MA), VitagenO (La Jolla, CA), Excorp
Medical (Oakdale, MN), and Algenix (Shoreview, MN). The Circe Biomedical
device integrates viable liver cells with biocompatible membranes into an
extracorporeal, bioartificial liver assist system. Vitagen's ELAD
(Extracorporeal
Liver Assist Device) Artificial Liver is a two-chambered hollow-fiber
cartridge
containing a cultured human liver cell line (C3A). The cartridge contains a
semipermeable membrane with a characterized molecular weight cutoff. This
membrane allows for physical compartmentalization of the cultured human cell
line
and the patient's ultrafiltrate. Algenix provides a system in which an
external liver
support system uses porcine liver cells. Individual porcine hepatocytes pass
through a membrane to process the human blood cells. Excorp Medical's device
contains a hollow fiber membrane (with 100kDa cutoff) bioreactor that
separates
the patient's blood from approximately 100 grams of primary porcine
hepatocytes
that have been harvested from purpose-raised, pathogen-free pigs. Blood passes
though a cylinder filled with hollow polymer fibers and a suspension
containing
billions of pig liver cells. The fibers act as a barrier to prevent proteins
and cell
3
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
byproducts of the pig cells from directly contacting the patient's blood but
allow the
necessary contact between the cells so that the toxins in the blood can be
removed.
Various aspects of these devices represent improvements over pre-
existing technology, but they still have particular disadvantages. The
effectiveness
of these devices, all of which use individual hepatocytes, is limited due to
the lack
of cell-to-cell interactions, which characterize the liver in its in vivo
state.
Accordingly, a bioartificial organ, for example a liver with improved
efficiency,
viability, and functionality for use in drug development would be beneficial.
This
longstanding need is addressed by the instant teachings, which provide for
drug
testing with bio-artificial tissue slices.
As the technology of bioartificial organ systems continues to advance,
improved methods screening compounds also develop. Disclosed in this
application are methods that utilize the recent improvements to bioartificial
organ
systems.
SUMMARY OF THE DISCLOSURE
Disclosed is a novel method for testing the chemosensitivity of a
substance in a bioartificial organ system or cell culture by treating the
bioartificial
organ system or cell culture with at least one compound and observing the
effect
on the bioartificial organ system or cell culture. Likewise, those skilled in
the art
readily understand that further disclosed is a business method for using the
apparatus and methods of the present disclosure to provide for tissue and
organ
specific screening for patients in complement with cutting edge genomic,
proteomic, and metabonomic analysis.
Disclosed herein is a method for providing simulated in vivo conditions
comprising providing a bioreactor for substantially duplicating in vivo tissue
4
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
function in vitro, providing for the bioreactor to hold at least one aliquot
of a tissue
sample, and allowing at least one aliquot to generate useful data.
Likewise, a method for substantially simulating in vivo conditions is
disclosed comprising, obtaining a bioreactor for substantially duplicating in
vivo
tissue function in vitro, obtaining a tissue sample, and using at least one
aliquot of
the tissue sample to generate useful data.
Still further disclosed is a method for substantially simulating in vivo
conditions comprising providing a tissue sample, dividing the tissue sample
into
tissue slices, including the tissue slices as a part of a bioartificial tissue
system,
and allowing the bioartificial tissue system to be used by treating the tissue
slices
with at least one drug regimen to generate useful data.
A similar method is disclosed comprising obtaining a bioartificial tissue
system containing tissue slices, using the bioartificial tissue system by
treating the
tissue slices with at least one drug regimen to generate useful data, and
comparing the data to determine mitotic activity, toxicity of a compound, or
histopathology, choosing a drug regimen based on the comparisons of data.
Finally, a business method for chemosensitivity testing which comprises
providing a bioreactor-based system for housing tissue slices, populating the
bio-
reaction based system with tissue, testing a regimen on the tissue, and
collecting
results.
BRIEF DESCRIPTION OF THE FIGURES
The above-mentioned features and objects of the present disclosure will
become more apparent with reference to the following description taken in
conjunction with the accompanying drawings wherein like reference numerals
denote like elements and in which:
Fig. 1 is a schematic diagram of a system of an embodiment of a
bioartificial organ system;
5
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
Fig. 2 is a perspective view of an embodiment of a complete bioartificial
organ system;
Fig. 3 is a perspective view of an embodiment of a bioreactor installed in
a bioartificial organ system;
Fig. 4 is a perspective view of an embodiment of a bioreactor unit;
Fig. 5 is an perspective view of an embodiment of a bioreactor unit
showing the placement of a tissue slice apparatus;
Fig. 6 is a exploded view of an embodiment of an tissue slice apparatus
with containing an tissue slice;
Fig. 7A is a side sectional view of an tissue slice arrangement of an
embodiment of a bioartificial organ system;
Fig. 7B is a perspective view of the tissue slice arrangement of Fig. 7A;
Fig. 8 is a graphical representation of in vitro lidocaine clearance with
continuous and intermittent perfusion using a bioartificial organ system;
Fig. 9 is a graphical representation of in vitro lidocaine clearance with a
6-hour and a 24-hour run using the bioartificial organ system;
Fig. 10 is a graphical representation of in vitro DMX concentration with a
6-hour and a 24-hour run using the bioartificial organ system; and
Fig. 11 is a graphical representation of in vitro ammonia clearance with a
6-hour and a 24-hour run using the bioartificial organ system;
Fig. 12 is a schematic showing the process undertaken by the tissue slice
apparatus of the present disclosure;
Fig. 13 is a flowchart of an embodiment demonstrating a method of using
a bioartificial organ system to obtain useful results, according to
embodiments of
the present disclosure.
6
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
DETAILED DESCRIPTION
As used in the present disclosure, the term "regimen" shall be understood
to mean one or more drugs, compounds, therapeutic agents, nucleic acids,
peptides, metabolites, viruses, bacteria, or other agents that may be applied
to a
cell or tissue.
The present inventor has discovered an improved modular system for
circulating plasma about slices of organs from animals to create a system for
chemosensitivity testing, inter alia.
Another object of the present disclosure is to provide an effective method
using a platform for testing of the efficacy of compounds prior to actual
administration of the compounds to live patients. The _.compound's toxic and
pharmacologic effects are realized through in vivo and in vitro animal
testing.
However, the present disclosure allows for the use of tissues, both human and
animal, to be cultured and for the testing of the compound on the tissue. For
new
drug compounds, the FDA will ask, at a minimum, the new drug applicant to: (1)
develop a pharmacologic profile of the drug; (2) determine the acute toxicity
of the
drug in at least two species of animals; and (3) conduct short-term toxicity
studies
ranging from 2 weeks to 3 months, depending on the proposed duration and use
of the substance in the proposed clinical studies. The process is complicated
and
costly, with hundreds and sometimes thousands of compounds being tested.
A further object of the present disclosure is to provide a method upon
which a drug regimen, for example, a chemotherapeutic regimen, can be
personalized for individual users. Generally speaking, with chemotherapeutic
regimens, not all regimens work the same way in all patients. A drug cocktail
that
is effective in one patient will be ineffective in a different patient. The
present
disclosure provides a method to test the efficacy of a drug regimen prior to
administration to patients, thereby administering only the most effective
regimen to
each patient.
7
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
A further object of the present disclosure is to provide a research plafform
and method for the study of disease and the ways in which compounds affect a
tissue.
For the purposes of this disclosure, the term tissue sample, tissue slice, or
tissue aliquot refers to a bioartificial organ system or cell cultures of
tissue cells
that are not part of a bioartificial organ system.
In accordance with an -embodiment of the present disclosure, there is
provided a chemosensitivity testing method that uses a bioartificial organ
system
for evaluation, detection, and testing of drug candidates, drugs and drug
metabolites as incorporated by reference. The system has an tissue slice
culture
apparatus. Other similar systems that allow for testing of a bioartificial
organ are
expressly contemplated. Moreover, in other embodiments, the methods of the
present disclosure may be used with conventional tissue samples.
The present disclosure provides a method for chemosensitivity testing
that provides a way to personalize chemotherapeutic regimens in individual
patients, to predict toxicity of a compound in normal tissues, and to study
disease.
According to an embodiment, during surgery, for example a biopsy, a sample of
tissue is extracted. The sample is sliced into a plurality of tissue slices.
Various
chemotherapeutic regimens are applied to each tissue slice. After the regimen
is
complete, the results are compared to determine the efficacy of each regimen.
By
comparing various regimens, personalized chemotherapeutic regimens may be
designed based on the results from the tests. Other applications for a
chemosensitivity tester are to study and evaluate toxicity, and study and
evaluate
histopathology.
Fig. 1 is a schematic representation of drug testing system 10 in
accordance with the present disclosure. From reservoir 12 culture medium 13 is
introduced into the bioreactor 15. Within bioreactor 15 is at least one tissue
slice
apparatus 20, which comprises at least one tissue slice 23 arranged between
two
8
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
wire meshes 21 (see Figs. 7A and 7B) and placed vertically parallel within
bioreactor 15. As culture medium is introduced into the bioreactor, the
culture
medium level begins to rise until it comes into contact with the tissue
slices, which
allows tissue slices 23 to contact a myriad of compounds that are introduced
via
the culture medium.
Oxygenated gas is introduced by gas valve 151 in the top of the chamber.
Although the gas valve is shown in the top of the chamber, it is also
contemplated
herein that the gas valve could be on the side or bottom of the chamber,
provided
with an appropriate seal to prevent leakage of liquid medium. The gas is
preferably
a mixture of 95% 02 by volume and 5% CO2 by volume, and is supplied at a
pressure ranging from 1 to 10 ATM to the chamber through the gas valve and
discharged therefrom, while controlling the pressure by a pressure controller
(not
shown). A solenoid valve (also not shown) may be coupled with the pressure
controller to maintain a pre-set gas pressure. Gas sterilizing device 18, for
example, a syringe filter having a pore size of about 0.22pm, is preferably
installed
in gas valve 151 to filter out microbes, thereby sterilizing the supply gas to
the
chamber. Gas check valve. 11 with gas sterilizing device 18 is positioned on
the
medium reservoir and serves to equalize the pressure between the reservoir and
atmosphere.
Stabilization of the tissue slices is an important feature of the invention.
The tissue slices are cultured under the supplies of the culture medium and an
oxygenated gas. The liquid culture medium, or the plasma, is supplied through
the
reservoir into the chamber and the oxygenated gas is supplied through the top
of
the chamber. Each is supplied at regular intervals so that each of the tissue
slices
is exposed alternately to the medium and to the gas at an exposure-time ratio
ranging from about 1:1 to about 1:4. A ratio of about 1:2.5 to about 1:3.5 has
been
found to be effective, and a ratio of about 1:1 or 1:3 has also been found to
be
effective, although changing these parameters are certainly within the normal
skill
9
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
level of an artisan. Pump 19 controls the flow of the culture medium. The rate
in
which a tissue slice is alternately exposed to gas and culture medium
corresponds
roughly to the rate of metabolism.
In the present disclosure Waymouth MB 752/1 culture medium is
preferred over plasma. The particular choice of the type of culture medium or
plasma will be known to a person of ordinary skill and may vary from cell type
to
cell type. To prevent central necrosis, the.gas mixture described above is 95%
02
and 5% COZ. Since this mixture may produce free oxygen radicals, which are
often toxic to tissue culture cells. For example, with liver samples a high
concentration of glutathione and vitamin E, as oxygen free radical scavengers
and
anti-oxidants, are added and supplemented with 10% inactivated fetal bovine
serum and L-glutamine.
Referring now to Fig. 2, an embodiment of bioartificial organ system 10 is
shown. Bioartificial organ system 10 comprises one or more bioreactors 15
disposed in incubator 32 that regulates temperature and humidity within the
chamber. Incubator 32 allows users to regulate the conditions of a
bioartificial
organ, ensuring that the bioartificial organ is exposed to optimal conditions
for
viability over time. The choice of a suitable incubator system for tissue
culture is
well known in the art and requires no further recitation.
Disposed in an incubation chamber are one or more bioreactors 15. Each
bioreactor 15 holds one or more tissue slices or samples. Rotator 30 turns
bioreactor 15 for wet and dry phases respectively. The wet phase corresponds
to
the time that the tissue slice is substantially exposed to culture medium.
Likewise,
the dry phase corresponds to the time the tissue slice is substantially
exposed to
gas. Control module 34 provides an interface for controlling the parameters of
bioartificial organ system 10 operation. For example, using control modules,
the
time period that tissue slices are exposed to gas and culture medium may be
regulated, which roughly corresponds to metabolism rates. Similarly the gas to
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
culture medium ratios may be regulated using control module 34, as well as
other
necessary operating parameters. Within incubator 32, gas and culture medium
supplies are provided as would be understood by a person of ordinary skill in
the
art.
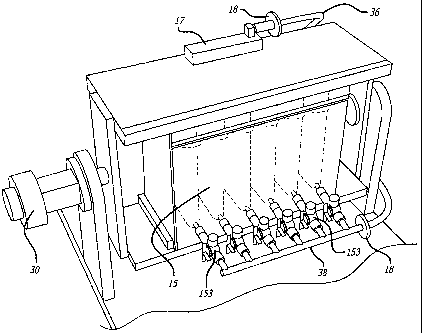
Turning now to Fig. 3, there is shown a close-up view of a bioreactor 15
installed in incubator 32. For ease of ingress and egress, bioreactor 15 and
rotator
30 may be affixed to a platform that slides into and out of incubator 32. When
installed in incubator 32, each bioreactor 15 is connected to gas and culture
medium supplies, 36 and 38 respectively. As shown in the embodiment of Fig. 3,
gas valves 151 (one for each tissue slice apparatus chamber 155) connects to
gas
supply 36. As shown in Fig. 3, manifold system 171s disposed between gas
valves
151 and gas supply 36. Gas filter 18, as previously described, is installed
between
gas supply 36 and gas valves 151. Similarly, culture medium valves 153 are
connected to culture medium supply 38. Culture medium filter 18, as previously
described, is disposed between culture medium supply 38 and culture medium
valves 153.
Turning attention now to Fig. 4, there is shown an embodiment of
bioreactor 15. Although many configurations are available and will be
understood
by a person of ordinary skill in the art, the embodiment shown in Fig. 4
comprises
a plurality of tissue slice apparatus chambers 155 (see Fig. 5). Each tissue
slice
apparatus chamber 155 is formed by a sealed cavity when bioreactor base 157
and bioreactor cover 158 are interconnected. Gas valves 151 may are typically
connected to gas supply 36 and are in gas communication with tissue slice
apparatus chamber 155 for the purpose of providing tissue slices contained in
tissue slice apparatus chamber 155 with a supply of a desired gas mixture.
Culture
medium valves 153 are in fluid communication with tissue slice apparatus
chamber 155 and serve the same function for culture medium as gas valve 151
does for gas. According to embodiments, bioreactor cover sealers 159 seal
11
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
bioreactor cover 158 and bioreactor base 157. Bioreactor cover sealer 159 may
be
an 0-ring or other similar device that prevent fluid leakage when bioreactor
base
157 and bioreactor cover 158 are in an interconnected configuration, and when
inverted the actual choice of device serving as bioreactor cover sealer 159
will be
understood and appreciated by a person of ordinary skill in the art.
According to an embodiment shown in Fig. 5, each tissue slice apparatus
chamber 155 accommodates at least one tissue slice apparatus 20. When
uninterconnected, tissue slice apparatus 20 may be inserted into a cavity
forming
a part of tissue slice apparatus chamber 155 in bioreactor base 157. According
to
the exemplary embodiment, a single tissue slice apparatus 20 is inserted into
each
cavity; a plurality of tissue slice apparatuses 20, however, may be employed
in a
single bioreactor 15 by providing a plurality of tissue slice apparatus
chambers 155
in a bioreactor 15. Nonetheless, the present disclosure contemplates
configurations of bioreactor 15 that comprise various numbers of tissue slice
apparatus chambers 155, and various numbers of tissue slice apparatuses 20 per
tissue slice apparatus chamber 155. After each tissue slice apparatus 20 is
inserted into tissue slice apparatus chambers 155, bioreactor cover 158 is
interconnected with bioreactor base 157. Once interconnected, bioreactor 15 is
sealed with bioreactor cover sealer 159. Bioreactor 15 may then be placed into
incubator 52 and connected with gas supply 36 and culture medium supply 38 and
experiments accordingly conducted.
As previously described and as shown by an embodiment in Fig. 6, tissue
slice apparatus 20 may comprise a plurality of meshes 21. Meshes 21 may be
made of stainless steel or other materials that would be known by a person of
ordinary skill in the art, as described previously. One or more tissue slices
23 are
placed between adjacent meshes 21 and meshes 21 are clasped together with
tissue slice apparatus clips 24. It will be understood by artisans that size
and
12
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
thickness of tissues slices may need be optimized for each protocol and may
vary
from experiment to experiment and tissue to tissue.
According to the exemplary embodiment shown in Fig. 6, two meshes 21
form tissue slice apparatus 23. The tissue slices 23 are disposed in the lower
40%
of tissue slice apparatus 20 according to the exemplary embodiment. This
configuration of tissue slices 23 in tissue slice apparatus 20 ensures that
the tissue
slice is fully exposed to both the dry and wet cycles.
Figs. 7A and 7B show similar embodiments of tissue slice apparatus 20.
Two stainless steel meshes 21, the size of which can be chosen based on the
dimensions of the chamber. These two meshes are preferably arranged in
parallel.
In an embodiment, the meshes have about a 0.26 mm pore size. Also, in an
embodiment, the meshes are pressed to ensure consistent flatness. Between
meshes 21 is a plurality of tissue slices 23, such as liver slices arranged in
an
orderly fashion. The two meshes are positioned on each side of the tissue
slices
with enough room so as to not crush the tissue slices, but also to hold them
sufficiently so that they do not get washed away by the culture medium.
Although
Figs. 7A and 7B show a relatively small number of tissue slices positioned
between the meshes, it is to be understood that the efficiency of the
apparatus is
dependent upon the number of tissue slices and sizes of the tissue slices
employed. Additionally, although two meshes 21 are shown, it is contemplated
that
any number of meshes 21 may be used. If a single mesh 21 is used, it is formed
to
surround, at least partially, tissue slices 23 thereby forming a space and to
retain
them in that space. For example, the mesh could be formed in a suitably
dimensioned U-shape.
Tissue slices 23 used in the present disclosure may be obtained from a
suitable animal, for example, a rabbit, pig, dog, rodent, or human, depending
on
the intended use of the apparatus. Human tissues are expressly contemplated
and
may be used interchangeably with animal tissues. Tissue slices 23 may be of
any
13
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
size or shape suitable for maintaining the viability and essential functions
thereof.
In the present disclosure thickness of tissue slices 23 shown to be effective
have a
thickness ranging from about 10 m to about 2,000 m. A thickness is from
about
100 m to about 500 m has been determined to effective in particular
experiements.
The present disclosure is ideally suited to methods of testing the toxicity
and efficiency of a drug. The testing is accomplished by exposing tissue
slices to a
drug or drug candidate and observing the ability of the tissues, such as
liver, to
metabolize a compound, which compound or its metabolites can be detected. For
example, ammonia and lidocaine are common compounds that can be
metabolized by healthy liver. The following examples show this testing, as
applied
to liver-slices. Those skilled in the art will recognize the utility of the
present
disclosure as applied to other organs.
In order to test chemotherapeutic regimens on the tissue slices or
aliquots, at least one compound is applied to at least one tissue aliquot in
the
bioartificial organ system. After a predetermined time elapses, data is
gathered. In
each experiment, the conditions may be duplicated.
Once the testing on each tissue slice or aliquot is completed, the data are
compared. Comparison of the data provides for various utilities of the present
disclosure, including, for example, detection of mitotic activity, cyto-
toxicity
parameters, and histopathology. Once derived from the data, these results are
useful in formulation of the most effective chemotherapeutic regimen for a
patient,
for general prediction of the toxicity of a given compound or compounds, or
for
study of carcinogenesis, for example. Naturally, other inferences may be
derived
from the data, and variations in the design of the experiments using the
tissue
slices or aliquots allow for variations in the information derived.
Figs. 8-11 demonstrate the utility of the principles and apparatuses
disclosed herein using liver slices as the tissue sample. The data presented
in
14
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
Figs. 8 and 9 demonstrates the ability of the bioartificial organ system to
clear
lidocaine over time. Similarly, Fig. 10 shows the increase in concentration
being
managed over time, which substantially simulates in vivo physiology of
samples.
Finally, Fig. 11 demonstrates the ability of the teachings of the present
disclosure
to detoxify ammonia. The data presented in Figs. 8-11 are not intended to be
limiting or to demonstrate the actual results the teachings of the present
disclosure
will achieve, but merely to demonstrate the achieved utility of the teachings
of the
present disclosure. It is intended that various configurations will accomplish
similar
results from configuration to configuration that are not exactly duplicative
of the
data presented herein.
Turning now to Fig. 12, there is shown an embodiment of a method for
use of the apparatuses disclosed herein. According to an embodiment,
bioreactor
is loaded with tissue slices 23 and sealed. Bioreactor 15 may be identical to
embodiments disclosed herein or other apparatuses with similar functionality.
15 Bioreactor is connected to at least one culture medium reservoir 12 which
contains
a supply of culture medium. According to embodiments in which bioreactor
comprises a plurality of tissue slice apparatus chambers 155, different
culture
medium reservoirs may be used to supply culture medium depending on the
specific goals of the experiment sought. For example, according to an
embodiment, bioreactor 15 comprises 6 tissue slice apparatus chambers 155
(see,
e.g., Fig. 5). The first chamber may be used as a control wherein culture
medium
is supplied with no additives. The other 5 chambers, may be supplied with
culture
medium containing a compound to be tested on the tissue slices 23 such as
lidocaine or ammonia in various concentrations. Similarly, all or some of
tissue
slice apparatus chambers 155 may be supplied with the exact same compounds in
order to have multiple sets of results or to prevent fouling of a particular
tissue
slice apparatus chamber 155 from preventing retrieval of results. The exact
experimental design and protocol, however, are configurable in many variations
as
would be known and understood by a person of ordinary skill in the art.
According
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
to embodiments, filter 18 may be disposed between culture medium reservoir 12
and bioreactor 15.
Bioreactor 15 is also connected to a gas supply 40. Gas supply may
supply gasses in various combinations and concentrations according to
experimental designs and protocols. Typically, a single gas supply 40 may be
connected to all tissue slice apparatus chambers 155. Nevertheless, a
plurality of
gas supplies 40 may be used if desired and called for by experimental design
or
protocol. According to embodiments, filter 18 may be disposed between gas
supply 40 and bioreactor 15.
After culture medium and gas is supplied to each tissue slice apparatus
chamber 155 bioreactor 15 is incubated for a given period of time. During
incubation, tissue slices are alternately exposed to culture medium and gas.
This
may be accomplished in multiple ways. For example, culture medium and gas may,
be injected and recovered alternately so that either gas or culture medium is
in
tissue slice apparatus chamber 155 at any one given time. Alternately,
bioreactor
15 may be rotated so that tissue samples are alternately exposed to culture
medium and gas, which are both held in tissue slice apparatus chamber 155.
This
may be accomplished by ensuring that tissue samples 23 occupy only a certain
volume of tissue slice apparatus chamber 155 so that it is fully submerged in
culture medium in one configuration, but upon rotation is fully exposed to
gas.
Fig. 6 demonstrates an embodiment reflecting this idea, wherein tissue sample
23
occupies 40% of tissue slice apparatus 20. Other variations on this idea will
be
understood by a person of ordinary skill in the art.
At various points during an experiment samples of culture medium may
be removed for testing. According to the embodiment shown in Fig. 12, drain
pump 60 may remove an aliquot of culture medium. Once removed, culture
medium may have any gas extracted at the same time captured in bubble trap 70.
Thereafter, the culture medium aliquot is held and tested in a processed
culture
16
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
medium reservoir 50. Once tested, it may be returned to bioreactor 15 or
discarded. Filters 18 disposed within the system maintain sterility.
An embodiment shown in - FIG. 13 illustrates a method of testing
chemosensitivity of various compounds and substances. In the exemplary method,
a tissue sample is extracted during surgery. Tissue may be human or animal
depending on the desired use of the tissue. For example, to create a
personalized
chemotherapeutic regimen, tissue is removed from the patient for whom the
regimen is to be created. For example, cancerous tissue may be removed and
exposed to a variety of cancer fighting drug cocktails to determine the best
cocktail
for the particular cancer tested. Similarly, in toxicity testing applications,
tissue
from any suitable human or animal host may be used. According to embodiments,
animal tissue may be used initially. After a compound is deemed safe in animal
studies, human tissue sample may then used to further test toxicity of the
compound or substance.
Tissue slices may be obtained_ incidental to other surgeries or in
procedures designed specifically to obtain the tissue sample, for example a
biopsy. For personalized chemotherapeutic regimens, tissue must be obtained
from the patient, necessitating taking a tissue sample directly from the
patient
either during a non-related surgery or during an operation specifically
designed to
obtain a tissue sample. Other chemosensitivity applications may use other
sources
of tissue samples depending on the particular protocol.
According to embodiments, adequately sized tissues samples are used to
obtain results when various compounds are tested on them. Such results,
including personalized medicine related matters, are within the normal skill
level of
artisans and likewise may be found in at least one of U.S. Letters Patents
Nos. 6,678,669; 6,905,816; 6,983,227 and 6,999,607 each of which are expressly
incorporated herein by reference as if fully set forth herein.
17
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
Once removed from the patient, the tissue sample is sliced or otherwise
divided into at least one aliquot of tissue. In an embodiment, each slice or
aliquot
is then individually cultured in tissue slice apparatus chamber 155 and
bioreactor
15 as previously described, such as using the method shown in Fig. 12. The use
of multiple, duplicative tissue slices allows researchers and doctors to
expose the
same tissue slices to compounds at the organ level where the only variable at
play
is the regimen administered.
Thereafter, the results are analyzed. Because the only variable is the
regimen administered, the present disclosure provides a powerful tool for
evaluation the efficacy of each given regimen compared to other viable
regimens.
Moreover, because the present system and methods are designed to allow testing
at the organ, system, and in some cases, organism level, as described in the
examples below. Thus, using the methods and apparatus of -the present
disclosure, researchers and doctors have a powerful tool to evaluate mitotic
activity, cyto-toxicity parameters, histopathology, and many other related
applications at the organ, system, and organism level.
According to an embodiment, a system or organism can be recreated in
vitro using the instant techniques, but provide results that are indicative of
in vivo
processes by using tissue slices from a variety of tissues in a system and
connecting them in parallel. Connection occurs via transfer of culture medium
from
one tissue type to the next, which allows researchers to observe the stepwise
effects of a regimen on various organ samples.
According to similar embodiments, tissue slices or different tissue types
from tissue slices may be combined in single tissue slice apparatus 20 in
various
permutations. Experiments of this type would allow researchers to control for
tissue type in an in vivo system in an in vitro environment for study and
therapeutic
applications. The many variations in the use of the apparatuses and methods of
the present disclosure in the observation of the effects and efficacy of
regimens
18
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
will be understood by a person of ordinary skill in the art. Similarly, the
various
methods of controlling variables in a in vivo system will appeal to artisans
as a
powerful means of obtaining results that would otherwise be impossible short
of
human or animal experiments.
For example, according to an embodiment, a plurality of animal liver
slices positioned securely within bioreactor 15 so as to maximize the surface
area
of the liver slices exposed to a culture medium. There is a means for
selectively
supplying and removing culture medium to the tissue slice apparatus chamber so
that the culture medium in the chamber rises to come into contact with the
tissue
slices. The culture medium rises in the chamber so that the liver slices are
completely immersed. The same process reversed may also be used to remove
the culture medium from contact with the tissue slices. There is also a means
for-
supplying a gas to the top of the chamber so that the tissue slices are
exposed
alternately to the gas and to the culture medium. This is done as described
previously. Additionally, a reservoir is provided for containing the culture
medium
as it enters and exits the chamber. The chamber is preferably thermoregulated.
For human tissue slices, the temperature is preferably kept at about 36.5
degrees
C. For rodent tissue slices, it is kept between about 36 to 38 degrees C.
However,
pig tissue slices are very sensitive to temperature fluctuation and it must be
maintained at 38 degrees C, the normal body temperature of pigs.
EXAMPLE 1
In an embodiment, a doctor may use the teachings of the present
disclosure to determine the optimum concentrations of a chemotherapy drug
cocktail for a cancer patient. The doctor takes a biopsy of a cancerous tissue
from
the patient and divides the tissue sample into aliquots. The doctor divides
aliquots
into two groups. The doctor uses the first group to determine the most
effective
19
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
drug cocktail for the patient in question. The doctor then uses the second
group of
aliquots to determine the optimal concentration of the drug cocktail.
With the first group of aliquots, the doctor uses the group to determine the
most effective drug cocktail. Tissue aliquots are cultured as described
previously.
Various drug cocktails are administered to the tissue aliquot. The percent of
apoptosis of the cancerous tissue in the aliquots is measured by methods that
would be common to a person of ordinary skill in the art. The doctor then
selects
the drug cocktail inducing the greatest degree of apoptosis in cancerous
tissue
compared to the healthy tissue.
The doctor then uses the second group of tissue aliquots to determine the
optimal concentration of the drug cocktail to use on the cancerous tissues.
Tissue
aliquots are cultured in the same manner as the first group of tissue
aliquots. The
doctor applies various concentrations of the drug cocktail to each tissue
aliquot
and selects the concentration of the drug cocktail that imparts the greatest
degree
of apoptosis in cancerous tissue as compared to healthy tissue.
As a result of optimizing the chemotherapeutic regimen that the patient
will receive to treat the cancer, the patient will receive the treatment that
imparts
the greatest benefit while minimizing undesirable side effects. If the doctor
wishes,
the same result may be obtained in a single experiment where various
concentrations of a plurality of drug cocktails is applied to a plurality of
tissue
aliquots.
EXAMPLE 2
In another embodiment, the teachings of the present disclosure may be
used to predict the toxicity of compounds to healthy tissue. Such results
would be
useful in data generated and submitted to the FDA pursuant to approval of a
new
drug application or abbreviated new drug application. An animal or human
tissue
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
sample is obtained by taking a biopsy or as part of surgery. Usually two
species of
animals, one rodent and one non-rodent are used because a drug may affect one
species differently than another. Other organs likewise provide key data and
are
useful within the scope of the present disclosure. In another embodiment, the
tissue is taken as part from a deceased organ donor, cloned, regenerated or
otherwise supplied by techniques known to those skilled in the art ranging
from
cord blood to stem cells by somatic cell transfer, among other things.
Tissue aliquots are derived from the tissue sample and cultured as
previously described. Once the tissue is cultured, a compound is applied to
each
tissue aliquot to determine the efficacy of the compound to achieve a desired
result as described previously. Data is gathered and interpreted as prescribed
by
the FDA or as according to parameters set by a person of ordinary skill in the
art in
the determination of a compound's efficacy in a tissue.
EXAMPLE 3
Similarly, disease studies may be performed by using various compounds
in the study of a disease. For example, inhibitors and stimulators of
compounds in
the tissues may be used to study their effects on chemical pathways in the
tissue.
Moreover, compounds may be applied to the tissue in an effort to observe their
effects on the tissue level as opposed to the cellular level or organism
level. The
present disclosure provides the methods to use a bioartificial organ system;
experimental parameters would be readily apparent to a person of ordinary
skill in
the art without the need for undue experimentation.
The testing may be performed by an independent third party in order to
rule out any appearance of bias. Every effort is made to ensure that as few
animals as possible are used as a source of tissue samples, and that they are
treated humanely. Alternately, the present disclosure also contemplates the
use of
21
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
human tissues, samples of which may be obtained from organ donors. Since most
drugs are metabolized in the liver, toxicity studies naturally focus on the
effects on
the liver.
EXAMPLE 4
The present disclosure also provides a novel way to observe the
interaction between organ systems. Tissue slices may be obtained from a
variety
of organs. These then are placed in parallel into multiple bioreactor tissue
slice
apparatus chambers. Culture medium is applied to a first chamber and permitted
to come into contact with the tissue slice for a time period. Following the
initial time
period, the culture medium is removed from the first tissue slice apparatus
chamber and moved into a second tissue slice apparatus chamber containing a
tissue sample from a different organ or the same organ under different
experimental conditions, such as increased metabolism, a different primary
cell
type, or a different concentration of cell type of interest. Prior to or
concurrently
with moving the culture medium to the second tissue slice apparatus chamber,
samples of the culture medium may be obtained to interim testing, in
embodiments. The culture medium moved into the second tissue slice apparatus
chamber is then reacted for a time period. The procedure is repeated for each
tissue slice_apparatus chamber until the experiment is concluded.
For example, a researcher may be interested in protein digestion.
Samples of tissue may therefore be taken from inside the mouth, esophagus,
stomach, small intestine sections corresponding to the duodenum, jejunum, and
ileum, and the large intestine. A sample of culture medium with a protein
sample
may be therefore reacted with each disparate tissue type to determine the
effect of
a particular organ on the proteins to be digested on a system level.
22
CA 02620837 2008-02-22
WO 2007/025154 PCT/US2006/033263
While the apparatus and method have been described in terms of what
are presently considered to be the most practical and preferred embodiments,
it is
to be understood that the disclosure need not be limited to the disclosed
embodiments. It is intended to cover various modifications and similar
arrangements included within the spirit and scope of the claims, the scope of
which should be accorded the broadest interpretation so as to encompass all
such
modifications and similar structures. The present disclosure includes any and
all
embodiments of the following claims.
23