Note: Descriptions are shown in the official language in which they were submitted.
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
INHIBITION OF INTERMEDIATE-CONDUCTANCE CALCIUM ACTIVATED
POTASSIUM CHANNELS IN THE TREATMENT ANDIOR
PREVENTION OF ATHEROSCLEROSIS
RELATED APPLICATION
This patent application claims priority to United States Provisional
Patent Application Serial No. 60/716,859 filed September 13, 2005, the
entirety of which is expressly incorporated herein by reference.
FIELD OF THE INVENTION
The present invention relates generally to the fields of biology and
medicine and more particularly to compositions and methods for treating or
preventing atherosclerosis.
STATEMEMT REGARDING FEDERALLY SPONSORED RESEARCH
This invention was made with Government support under Grants
HL65203 and HL62852 awarded by the National Institutes of Health as well
as Veterans Administration Merit Award Grant Program 36 by the Department
of Veterans Affairs. The Govemment may have certain rights in this
invention.
BACKGROUND
A group of drugs knows as "statins" have become widely used as
cholesterol-lowering agents. Statins act by competitively inhibiting HMG-CoA
reductase, an 'enzyme of the metabolic pathway by which the body
synthesizes cholesterol. Commercially available statin drugs include
atorvastatin (Lipitor ), fluvastatin (Lesco{ ), lovastatin (Mevacor@,
Altocort ), pravastatin (Pravacol , Selektine , Lipostat ), rosuvastatin
(Crestor ) and simvastatin (Zocor , Lipex@).
It has been suggested that statins are the most promising drugs to
prevent the development or progressiori 'of atherosclerosis due to their
cholesterol lowering effect in combination with other beneficial effects
including stabilization of plaques, vascular protective effects, anti-
proliferative
and migratory effects, anti-inflammatory effects, and anti-oxidative effects.
However, multiple clinical studies revealed that the reduction in cardiac
events
I
SUBSTITUTE SHEET (RULE 26)
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
in subjects with coronary risk factors by statins is only 30%. In addition,
statins have been associated with side effects such as muscle symptoms or
myopathies (e. g., Myalgia-muscle ache or weakness without elevation of
creatine kinase (CK) and/or Myositis-muscle ache or weakness with
increased CK levels and Rhabdomyolysis-muscle symptoms with marked
elevation of CK as well as creatinine elevation and hepatotoxicity). There are
also certain contraindications to the use of at least some statin drugs, such
as
cholestasis, active liver disease or the concomitant administration of certain
drugs that increase the potential for serious myopathy.
Thus, there remains a need for the development of new potent drugs
for the treatment or prevention of athersclerosis without the potential for
the
side effects associated with statin therapy (e.g., rhabdomyolysis or injury to
cardiac muscles) and/or for use in subjects for whom statin drug therapy is
contraindicated.
A change of expression in calcium-activated potassium channels (KCa)
from large conductance KCa (BKCa = KCa1.1) to intermediate conductance
KCa (IKCa1 = KCa3.1) occurs concomitantly with the phenotypic change of
VSMCs from contractile to proliferative; a key process of vascular remodeling
during atherosclerosis. Therefore, Applicants have hypothesized that up-
regulation of IKCa1 activity plays a critical role in the progression of
atherosclerosis. Compounds that may effectively inhibit IKCa1 activity have
previously been described in United States Patent No. 6,903,375 (Chandy et
al.) entitled Non-Peptide Inhibition Of T-Lymphocyte Activation And Therapies
Related Thereto, which is expressly incorporated herein by reference.
Included among the compounds known to effectively inhibit activity of
IKCa1 is 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34). TRAM-
34 inhibits KCa3.1 channels which are predominantly expressed in
proliferative VSMCs, activated T cells and macrophages but not in contractile
VSMCs and non-activated inflammatory ceils, leading to the selective anti-
proliferatory and anti-inflammatory effects, and consequent vascular
protective effect. In addition, appropriate levels of plasma cholesterol are
still
controversial, although short-term treatment with statins has been reported to
2
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
reduce the incidence of ischemic cardiac events in subjects with normal
cholesterol levels by about 30%, KCa3.1 inhibiting compounds such as
TRAM-34 may offer advantages over statin drugs or other therapies in
preventing or treating atherosclerosis in non-hyperlipidemic patients.
SUMMARY OF THE INVENTION
The present invention provides methods for treating or preventing
atherosclerosis in human or animal subjects. These methods generally
comprise the step of inhibiting or blocking intermediate-conductance calcium
activated potassium channels (e.g., KCa3.1, KCNN4, IKCa1, IK1, SK4)
located in vascular smooth muscle cells or other tissues associated with the
pathogenesis of atherosclerotic lesions. Such inhibition or blocking of
intermediate-conductance calcium activated potassium channels may be
accomplished by administering to the subject an effective amount of a
substance that comprises a compound that inhibits- or blocks intermediate-
conductance calcium activated potassium channels. Compounds that may be
effective for this purpose include those having the structural formula:
x
~ (R)n
Q
(R)n
(R)n
z
m
wherein,
X,Y and Z are same or different and are independently selected
from CH2, 0, S, NRI, N=CH, CH=N and R2-C=C-R3, where R2
and R3 are H or may combine to form a saturated or unsaturated
carbocyclic or heterocyclic ring, optionally substituted with one
or more R groups;
R, is selected from H, alkyl, alkenyl, alkynyl, cycloalkyl, aryl, acyl
and aroyl, optionally substituted with hydroxy, amino, substituted
amino, cyano, alkoxy, halogen, trihaloalkyl, nitro, thio, alkylthio,
carboxy and alkoxycarbonyl groups;
3
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
R is selected from H, halogen, trihaloalkyl, hydroxy, acyloxy,
alkoxy, alkenyloxy, thio, alkylthio, nitro, cyano, ureido, acyl,
carboxy, alkoxycarbonyl, N-(R4)(R5) and saturated or
unsaturated, chiral or achiral, cyclic or acyclic, straight or
branched hydrocarbyl group with from I to 20 carbon atoms,
optionally substituted with hydroxy, halogen, trihaloalkyl,
alkylthio, alkoxy, carboxy, alkoxycarbonyl, oxoalkyl, cyano and
N-(R4)(R5) group,
R4 and R5 are selected from H, alkyl, alkenyl, alkynyl, cycloalkyl
and acyl or R4 and R5 may combine to form a ring, wherein a
carbon may be optionally substituted by a heteroatom selected
from 0, S or N-R6,
R6 is H, alkyl, alkenyl, alkynyl, cycloalkyl, hydroxyalkyl or
carboxyalkyl,
n is 1-5; m is 1 or 2; with the proviso that
when m is 1, Q is selected from OH, CN, carboxyalkyl', N-
(R7)(R8), where R7 and R8 are selected from H, lower alkyl (1-
4C), cycloalkyl, aryl, acyl, amido, or R7 and R8 may combine to
form a saturated or unsaturated heterocylic ring and optionally
substituted with up to 3 additional heteroatoms selected from N,
0, and S; or -NH-heterocycle, where the heterocycle is
represented by thiazole, oxazole, isoxazole, pyridine, pyrimidine,
and purine and
where U and V are selected from H and 0; and
~
-N ~ ~
V
when m is 2, Q is a spacer of from 2-10 carbons as a straight or
branched, chiral or achiral, cyclic or acyclic, saturated or
unsaturated, hydrocarbon group, such as phenyl.
Further information regarding these compounds, and method for synthesis are
described in United States Patent No. 6, 803,375 entitled Non-Peptide
Inhibition Of T-Lymphocyte Activation And Therapies Related Thereto and
copending United States Patent Application Serial No. 10/533,060 entitled
Compounds, Methods and Devices for Inhibiting Neoproliferative Changes in
Blood Vessel Walls, both of which are expressly incorporated herein by
reference.
In accordance with the present invention, non-limiting examples of
4
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
compounds having the above-set-forth structural formula include but are not
necessarily limited to: 1-[(2-chlorophenyl)diphenylmethyl]-1 H-pyrazole (TRAM
34); 1-[(2-fluorphenyl)dipheny[methyl]-1 H-pyrazole; 1-[(4-
chlorophenyl)diphenylmethyl]-1 H-pyrazole; 1-[(2-fluorphenyl)diphenylmethyl]-
1 H-pyrazole and 1-[(2-chlorophenyl)dipheny[inethyl]-H-1,2,3,4-tetrazole.
Further in accordance with the invention, there are provided methods
of the foregoing character wherein the substance administered to the subject
substantially blocks or inhibits KCa3.1 channels that are predominantly
expressed in proliferating vascular smooth muscle cells (VSMCs), endothelial
cells, activated T cells and macrophages but not in contractile VSMCs. This
selective KCa3.1 channel inhibition or blockade has a selective anti-
proliferative and anti-inflammatory effect, and a consequent vascular
protective effect.
Still further in accordance with the invention, substances that inhibit or
block intermediate-conductance calcium activated potassium channels may
be administered to the subject by any suitable route of administration
including but not limited to injection or infusion (e.g., intravenous,
intramuscular, subcutaneous), transdermal, transmucosal, via an implantable
drug delivery device, etc.
BRIEF DESCRIPTION OF THE DRAWINGS
The following detailed description and examples, and the
accompanying figures, are intended to describe certain embodiments or
examples of the invention and are not intended to limit the scope of the
invention in any way.
Figures 1A-1 C show differential expression of calcium-activated
potassium channels in the human coronary microcirculation. Figure 1A shows
that IKCa1 protein expression is remarkably increased in subjects with
coronary artery disease (CAD), compared to those without CAD. In contrast,
BKCa expression is decreased in CAD subjects. Three subjects were
examined in each group. The membrane protein samples (BKCa; 20 pg and
IKCa; 40 pg) were analyzed by Western blot method (dilutions of primary
5
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
antibodies; BKCa 1:500 and IKCa 1:1,000). Figure 1B shows localization of
IKCa1 protein using immunohistochemistry. a) In the tissue from non-CAD
subjects (representative image from 47 year-old female with valvular disease),
endothelial cells (ECs) were strongly stained, while the staining in VSMCs
was faint. b) In the tissue from CAD subjects (52 year-old female with CAD),
VSMCs showed strong immunostaining for IKCa1. c) There was no staining in
the negative control. d) In an isolated small coronary artery (internal
diameter
300 m) from CAD subjects (71 year-old male with CAD), it is notable that
VSMCs were heterogeneously stained. Positive staining appears in brown.
Magnification 60x. (Antibody dilution; a and b 1:250 and d 1:160). L indicates
lumen. Morphological changes in the human coronary microcirculation were
examined by electron microscopy (Figure IC). Left panel) In vessels from
non-CAD subjects, VSMCs are spindle shaped (arrowhead). Right panel) In
vessels from CAD subjects, in the luminal overpopulations of VSMCs that
appear in the tunica media, the cells are irregular in size and cubic in shape
like cobblestones (blue arrow), whereas the main VSMCs are spindle shaped
(red arrowhead). Magnification; 2,500x. Scale bars; 1 m. L; lumen, E;
endothelial cell, I; intimal layer, and M; medial layer.
Figures 2A and 2B show the induction of IKCa1 message by platelet-
derived growth factor-BB (PDGF) in cultured human coronary artery smooth
muscle cells (HCSMCs). Figure 2A shows that IKCa1 mRNA expression is
increased in response to PDGF treatment. Figure 2B shows that Western blot
analysis also revealed increased IKCa1 protein expression in HCSMCs after
48-hour stimulation with PDGF (40 pg membrane proteins, IKCa1 antibody
1:1,000 dilution).
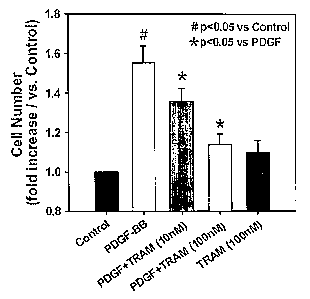
Figures 3A-D show the inhibitory effects of TRAM-34 on proliferation
and migration of cultured HCSMCs. Figure IA shows that TRAM-34 reduces
the increase in cell number of HCSMCs in the presence of PDGF. Figure 1 B
shows that the BrdU incorporation method revealed that PDGF-induced
increase in DNA synthesis is also decreased by TRAM-34. Figure 1 C shows
that treatment with TRAM-34 significantly inhibits c-fos up-regulation induced
by PDGF (20. g whole cell lysates and IKCa antibody 1:1,000 dilution).
6
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
PDGF-induced VSMC migration is also inhibited by TRAM-34 (Figure 1 D).
Figures 4A-4C show IKCa1 up-regulation and VSMC migration in
atherosclerotic lesions of apolipoprotein E (ApoE) knockout mice. Figure 4A
shows Western blot analysis indicating that IKCa1 channels are strongly
expressed in aortas from ApoE knockout mice, whereas BKCa channels are
down-regulated (IKCa; 40 g membrane protein and 1:1,000 antibody
dilution, and BKCa; 30 g and 1:500). Figure 4B shows that IKCa1 protein
expression is restricted to the endothelial layer of aortas of wild type (WT)
mice (panels a and c of Figure 4B). In contrast, IKCa1 expression is
extensively observed in aortic atherosclerotic lesions including ECs and
migrated cells into the thickened intimal lesions (panel b of Figure 4B). Note
that VSMCs in luminal area of medial layer are also strongly stained (panel d
of Figure 4B). (antibody 1:100 dilution). Figure 4C shows that the expression
of SM a-actin is seen only in medial layer of aortas from wild type mice
(panels a and c of Figure 4C). In aortas of ApoE knockout mice, not only
medial layer but also thickened intimal lesions are positively stained for SM
a-
actin (panel b of Figure 4C). The stained areas in the intima overlap with
those for IKCa1, indicating migrated VSMCs into the intima (panel d of Figure
4C). (antibody 1:100 dilution).
Figures 5A and 5B show altered vasodilator response to KCa
stimulation in ApoE KO mice. Figure 5A shows an enhanced vasodilation to
IKCa1 stimulation with EBIO in carotid artery segments of ApoE knockout
mice. Figure 54B shows that, in contrast, vasodilator response to BKCa
stimulation with pimaric acid is reduced. # p<0.05 compared to wild type mice.
Figures 6A and 6B show the effects of long-term inhibition of IKCa1
activity on the progression of atherosclerosis in ApoE KO mice. Figure 6A
shows representative images of aortic atherosclerotic formation. In wild type
mice, no formation of atherosclerotic lesions was observed. On the other
hand, ApoE KO mice treated with vehicle displayed extensive atherosclerotic
lesions throughout aortic trees from the aortic root to the iliac arteries,
while a
much smaller area was stained in the aorta from ApoE mice treated with
TRAM-34. Figure 6B shows that, in summary, treatment with TRAM-34
7
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
markedly reduced the lesion area (atherosclerotic lesion area / whole aortic
area) by approximately 60%.
Figure 7 is a table (also referred to below as Table 1) showing the
effects of long-term IKCa1 blockade by TRAM-34 on body weight, heart
weight, systemic blood pressure, heart rate, and plasma cholesterol levels in
mice. -
DETAILED DESCRIPTION AND EXAMPLES
The following detailed description and the accompanying drawings are
intended to describe some, but not necessarily all, examples or embodiments
of the invention. The contents of this detailed description do not limit the
scope of the invention in any way.
Unlike drugs that act by inhibiting cholesterol biosynthesis (e.g.,
statins) the treatments of the present invention act to prevent the
development
of atherosclerosis irrespective of the subject's plasma cholesterol levels.
While some antihyperlipidemic agents (e.g., certain statins) have been
reported to reduce the incidence of ischemic cardiac events even by
approximately 30% in subjects with normal cholesterol levels, the treatments
of the present invention (e.g., inhibiting or blocking intermediate-
conductance
calcium activated potassium channels (e.g., KCa3.1, KCNN4, IKCa1, IK1,
SK4) may provide better means for treating subjects who exhibit symptoms of
atherosclerosis, or are at risk for developing atherosclerosis, even though
they may have normal or low plasma cholesterol levels.
Applicants have found that expression of the intermediate-conductance
calcium activated potassium channel KCa3.1 (KCNN4, IKCa1, IK1, SK4) is
significantly increased in T lymphocytes, macrophages and vascular smooth
muscle cells from atherosclerotic lesions in both humans and mice with
atherosclerosis. In cultured human coronary artery smooth muscle cells
(HCSMCs) the platelet-derived growth-factor-BB (PDGF) increased
proliferation and migration concomitant with an up-regulation of KCa3.1
(IKCa1). In view of this finding, Applicants tested whether KCa3.1 blockers,
such as TRAM-34, could suppress the proliferation and migration of these
8
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
cells thereby deterring the formation of atherosclerotic lesions.
Through the in-vitro studies described here below, Applicants have
determined that TRAM-34, a KCa3.1 blocker, inhibited PDGF induced
proliferation and migration of cultured HCSMCs. Additionally, Applicants
tested whether TRAM-34 would prevent atherosclerosis development in the
ApoE-knockout mouse, a widely used animal model of atherosclerosis. Long-
term treatment with TRAM-34 reduced the development of atherosclerotic
lesions (consisting of proliferating and migrating VSMCs, macrophages and T
lymphocytes) in these mice by 60% compared to ApoE KO mice treated with
vehicle (peanut oil) when the animals were fed a high-cholesterol diet. An
nitric oxide-mediated component of endothelium-dependent vasodilation was
restored in these animals due to the reduced superoxide production from
VSMCs. Plasma levels of macrophage chemoattractants (MCP-1 and TNF-
alpha) were also reduced, concomitant with the decreased accumulation of
macrophages in the plaques. These results demonstrate that KCa3.1
blockade constitutes a novel therapeutic approach to the prevention and
treatment of atherosclerosis.
Materials and Methods
Tissue acquisition: Human coronary arteries. Human small coronary
arteries (n=26) were isolated as reported previously. Procedures for
harvesting tissue samples were in accordance with guidelines established by
the local Institutional Review Boards. Mouse carotid vessels. Mice
anesthetized with sodium pentobarbital (50 mg/kg, i.p. Abbott Laboratories,
North Chicago, IL) were sacrificed by collecting blood from the hearts. Under
a microscope, 1 st - 2nd branches of external carotid arteries (150-250 pm in
internal diameter, 1-2 mm in length) were carefully removed and placed
immediately into cold (4 C) HEPES buffer.
Western blot analysis: Total cell lysates or membrane fractions were
harvested and protein samples separated on an electrophoresis gel by SDS-
PAGE and then transferred to a PVDF membrane. The gels were stained in
Coomassie blue to confirm equal protein loading. Membranes were blocked
with 10% nonfat dried milk, blotted with primary antibodies (BKCa a-subunit
9
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
[Affinity BioReagents], c-fos [Santa Cruz, Inc.] and IKCa) and subsequently
probed with a horseradish peroxidase-labeled donkey anti-rabbit antibody
(1:5,000 - 10,000 [Santa Cruz, Inc.]). The bound antibody was detected by
chemiluminescence (ECL Plus, Amersham). The polyclonal primary antibody
against human and mouse IKCa was obtained from sera of rabbits immunized
using oligopeptides with following amino acids sequences; H-
LNASYRSIGALNQVRC-NH2 (S4-5 of human and mouse IKCa).
Immunohistochemistry: Immunohistochemistry was performed to
localize IKCa and SM a-actin in the blood vessels as previously described.
Briefly, tissues were fixed, and frozen in OCT compound. Sections (8 m
thick) were immunolabelled with primary antibodies (IKCa and SM a-actin
[AnaSpec, Inc.]). Immunostains were visualized by Vectastain Universal Quick
kit, Vector Laboratories. As a control for non-specific binding, the primary
antibody was omitted.
Electron microscopy: Electron microscopy was performed as
previously reported.
Cell culture: Human coronary artery smooth muscle cells (HCSMCs,
Camblex, inc.) were maintained according to manufacturer's instructions. To
achieve a quiescent state, cells were incubated in serum-free SmBM for 48
hours. All experiments were performed between passages 5 and 7.
Real-time PCR: HCSMCs were seeded onto 6-well plates at a density
of 12x104 / well in SmGM-2 and cultured up to 70% confluence (3 days). After
achieving a quiescent state, cells were stimulated for 48 hours with or
without
20 ng/ml platelet-derived growth factor-BB (PDGF, R&D Systems,
Minneapolis, MN). RNA was isolated with TRIZOL Reagent (Invitrogen),
reverse-transcribed to cDNA with iScript cDNA synthesis kit (Bio-Rad). Real-
time PCR (iCycler, Bio-Rad) was used for quantification of transcripts for
hIKCa (Gen bank Accession No. NM 002250) and GAPDH (AF 106860) using
iQ SYBR Green Supermix (Bio-Rad). Primers were designed (Beacon
Designer software 3.0, PREMIER Biosoft International, Palo Alto, CA) and
synthesized (Integrated DNA Technologies, Inc., Coralville, IA) as follows:
for
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
hlKCa, 5'- GGC CAA GCT TTA CAT GAA CAC G -3' (sense) and 5'- GTC
TGA AAG GTG CCC AGT GG 4(antisense); for GAPDH, 5'- CCT GCC AAG
TAT GAT GAC -3' (sense) and 5'- GGA GTT GCT GTT GAA GTC -3'
(antisense). Each 25 ~l PCR reaction consisted of 10"7 M forward and reverse
primers. The reaction conditions were as follows: 3 minutes at 95 followed by
40 cycles at 95 for 60 seconds, 60 for 60 seconds. All reactions were
carried
out in duplicate and included no template controls. Threshold cycles (Ct) were
calculated by iCycler iQ (Bio-Rad). Real-time RT-PCR signals for hlKCa were
standardized to GAPDH by use of the equation CtX - CtrGAPoH =ACt. Relative
quantification and the fold change were calculated according to the formula
ACtW/ - CfiX = ACt and 2 ct respectively (w/o = without stimulus).
Cell proliferation assays: Cell proliferation assays were performed as
previously reported. Briefly, quiescent HCSMCs seeded at a density of
4x104 /weii in 6-well plates were stimulated by 20 ng/mL PDGF in the
presence or absence of 10-' M TRAM-34, a selective IKCa blocker. Forty
eight hours after stimulation, the number of cells was counted with a
hemocytometer (MARIENFELD, Lauda-Konigshofen Germany). In another set
of experiments, a BrdU cell proliferation assay was also performed with
quiescent cells in 96-well plates at a density of 1x104/well according to the
manufacturer's instructions (Colorimetric Cell Proliferation ELISA, Roche,
Penzberg Germany). In this study, BrdU (10-5 M in medium) was applied 24
hours prior to the measurements.
Cell migration assay: A Cell migration assay was carried out with the
Transweli system (Corning, Acton, MA) as previously reported. Briefly, cells
(3x105 cefls/mL) were seeded onto the upper chamber of Transweils, and the
lower chamber was filled with serum-free medium containing 20 ng/mi PDGF.
TRAM-34 (10"8 - 10"7 M) was added to both chambers. After 8-hour
stimulation, migrated cells were fixed and stained with the Diff-Quick Stain
(IMEB Inc. Chicago, IL) and counted under a microscope.
Mouse treatment: C57BL/6J male mice (wild type [WT] n=1 1 and
ApoE deficient type [EKO] n=38, The Jackson Laboratory) were used. EKO
mice were weaned at 4 weeks of age onto a high-cholesterol diet (1.3%
tl
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
cholesterol; TD 96121, Harlan/Teklad) and treated with daily subcutaneous
injection of TRAM-34 (120mg/kg/day) or vehicle (peanut oil) for 12 weeks.
Littermate WT mice were used as the control group in the experiments. Mice
were provided diet and water ad libitum and maintained on a 12-hour
light/dark cycle. All animal experiments were conducted according to the
Guidelines for Animal Experiments at Medical College of Wisconsin.
Hemodynamic analysis of mice: At 16 weeks of age, mice were
anesthetized, and right femoral arteries were cannulated for continuous
measurement of arterial pressure and heart rate (pressure transducer;
Bioresearch Center, Nagoya, Japan) and recorded continuously by computer
for 30 min.
Plasma lipid analysis: Plasma was obtained by centrifugation of
blood and stored at -80 C until each assay was performed. Plasma
cholesterol levels were analyzed by General Medical Laboratories (Madison,
WI).
Histological analysis of atherosclerosis in mouse aortas: Isolation
of aortas and quantification of atherosclerosis were performed as previously
described. Briefly, aortas (from aortic arch to iliac bifurcation) were opened
longitudinally, pinned onto a silicon-coated dish, fixed with 4%
paraformaldehyde, and stained in 1.0% (v/w) Sudan III solution (The Science
Company, Denver, CO). Images were acquired using a digital camera (C-755,
Minolta), and the surface area of atherosclerotic lesions was measured as the
percentage of total area of the opened aorta using imaging software,
MetaMorph (Universal Imaging Corp).
Videomicroscopy: The preparation for videomicroscopy has been
previously described. Vasomotor and endothelial function was confirmed by
measuring constriction to 50 mM KCl and dilation to acetyicholine (ACh, 10-4
M, mouse vessels pressurized at 40 mmHg) or to bradykinin (10-' mol/L,
human vessels at 60 mmHg). Vessels were preconstricted with U46619 (10-9
- 10-$ M for mouse vessels) or ACh (10"8 - 5x10"' M for human vessels) to
adjust tone to a level between 30% to 50% of passive diameter. Dose-
dependent vasodilation to 1-ethyl-2-benzimidazolinone (EBIO, an IKCa
12
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
opener, 10"5 _ 10"4 M) and to pimaric acid, a BKCa opener (10"6 - 10"5 M)
were measured in isolated and pressurized vessels from human or mouse. In
some experiments, endothelial cells (ECs) were denuded.
Statistical Analysis: All data are expressed as mean SE. Data
acquired by either real-time PCR, cell proliferation and migration assays, or
histological analysis of atherosclerotic lesion were compared by using paired
Student's t test. Percent dilation was calculated as the percent change from
the preconstricted diameter to the passive diameter in Ca2+-free Krebs
containing 10"4 M papaverine. Percent constriction or basal tone was
determined by calculating the percent reduction in the passive diameter. To
compare dose-response relationships between treatment groups, a two-way
ANOVA supported by a Bonferroni post hoc test was used. Statistical
comparisons of maximal percent vasodilation and basal tone under different
treatments were performed by paired Student's t test. All procedures were
done using 'proc mixed' or 'proc gim' programs of SAS for Windows version
8.2. Statistical significance was defined as a value of P < 0.05.
Results
Differential expression of KCa and morphological changes in
diseased human coronary microvessels
IKCa1 protein expression was markedly increased in small coronary
arteries from subjects with coronary artery disease (CAD) compared to those
from subjects without CAD. In contrast, BKCa expression was comparatively
decreased in CAD subjects (Fig. 1A).
lmmunohistochemistry demonstrated that endothelial cells (ECs) were
positively stained for lKCa protein in vessels (=100 pm in diameter) from
subjects without CAD, while VSMCs showed little staining (Fig. 1 B-a). In
subjects with CAD, VSMCs showed marked staining (Fig. 1 B-b). In a larger
artery (internal diameter = 300 m) from a subject with CAD, heterogeneous
staining was observed among VSMCs of the medial layer (Fig. 1 B-d).
Morphological changes in vessels were examined by electron
microscopy. Microvessels from subjects without CAD displayed a single
13
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
endothelial layer and two layers of spindle-shaped VSMCs (arrowhead) with
extracellular spaces narrow and regular in width, representing normal
architecture (Fig. 1 C left panel). In vessels from subjects with CAD (Fig. 1
C
right panel), the medial layer was thickened and included spindle-shaped
VSMCs and irregularly-shaped and disarranged VSMCs surrounded by
excess extracellular matrix. Elastic components between ECs and VSMCs
became thicker and continued on to the inner elastic lamina. These findings
provide morphological evidence of VSMC phenotypes present in the human
coronary microcirculation in atherosclerosis. Taken together, these results
support the hypothesis that IKCal up-regulation is involved in the
morphological or phenotypic changes of VSMCs in atherosclerosis in humans.
Role of IKCal in VSMC proliferation and migration in vitro
IKCal expression was determined during VSMC proliferation in
response to PDGF in cultured HCSMCs. Real-time RT-PCR showed that
PDGF increased IKCa mRNA expression in a time-dependent manner (Max
response at 6h, 4.2 1.0-fold, p<0.05 vs Control, n=5) (Fig. 2A). Western blot
analysis also revealed that membranous expression of IKCa proteins was
increased after 48-hour exposure to PDGF (Fig. 2B). BKCa expression was
not detectable before or after treatment with PDGF. These findings suggest
that IKCal up-regulation is concomitant with the progression of VSMC
proliferation.
The role of IKCal in cultured HCSMC proliferation was examined by
blocking the channel activity with TRAM-34, a selective IKCal blocker. Figure
3A shows the effect of blocking IKCa activity with TRAM-34 on PDGF-
stimulated HCSMC proliferation. Treatment of HCSMC for 48 hours in the
presence of PDGF induced a significant increase in cell number (PDGF alone;
1.6 0.1-fold of control, n=7). The proliferation was significantly reduced by
TRAM-34 in a dose-dependent manner (PDGF+TRAM-34; 1.1 0.1-fold of
control at 10-7 M, p<0.05 vs PDGF alone, n=7). TRAM-34 in the absence of
PDGF had no effect on HCSMC proliferation. Glibenclamide, an ATP-
sensitive potassium channel blocker had no effect on PDGF-induced HCSMC
proliferation (data not shown, n=4). Treatment with either PDGF alone,
14
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
PDGF+TRAM-34, or TRAM-34 alone, did not affect cell viability. The role of
IKCa activity in DNA synthesis was determined by BrdU incorporation assay
(Fig. 3B). PDGF significantly increased DNA synthesis in HCSMCs (PDGF
alone; 2.8 0.3-fold of control, n=26). TRAM-34 suppressed PDGF-BB-
induced DNA synthesis of HCSMCs (PDGF+TRAM-34; 2.2 0.2-fold of
control, p<0.05 vs PDGF alone, n=26). TRAM alone had no effect on DNA
synthesis (n=6).
To provide additional support for the inhibitory effect of IKCa1 blockade
on cell proliferation and DNA synthesis, the expression of c-fos, a proto-
oncogene intimately involved in cell proliferation, was examined in HCSMCs.
PDGF induced up-regulation of c-fos protein in HCSMCs (Fig. 3C) that was
markedly reduced by TRAM-34.
A transwell migration assay was employed to test the role of IKCa in
VSMC migration. As shown in Fig. 3D, PDGF stimulated HCSMC migration
(32 4-fold of control n=10). TRAM-34 inhibited PDGF-induced migration
(PDGF+TRAM-34; 23 2-fold of control n=4, p<0.05 vs PDGF alone). These
findings indicate that increases in IKCa1 expression and activity are
associated with VSMC proliferation and migration, a key step in the early
stage of the development of atherosclerosis.
Up-regulation of IKCa1 in atherosclerotic mouse aortas
The expression of IKCa1 and BKCa were examined in ApoE KO mice.
IKCa protein was increased and BKCa reduced in aortas of ApoE KO mice
(Fig. 4A). Endothelial denudation did not alter the differential expression of
KCa in mouse aortas (data not shown).
The localization of IKCa1 was examined by immunohistochemistry. As
shown in Fig. 4B, IKCa protein was localized in the endothelial layer in
aortas
of WT mice, whereas IKCa were detected in the endothelial layer, intimally-
migrated cells, and some VSMCs in the luminal area of medial layer in aortas
of ApoE KO mice.
SM a-actin localization was determined in mouse aortas (Fig. 4C).
While only VSMCs in the medical layer were positively stained in aortas of WT
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
mice (Fig. 4C-a and c), SM a-actin expression was observed both in the
medial layer and in the intimal atherosclerotic lesions in those of ApoE-KO
mice (Fig. 4C-b and d). The intimal staining overlapped with that for IKCa1
(Fig. 4B-d and 4C-d), indicating the presence of intimally-migrated VSMCs,
which express IKCa1. Thus, IKCa1 up-regulation in atherosclerotic vessels
results from VSMCs that proliferate and migrate into the intima.
Differential activity of KCa in vessels from atherosclerotic
subjects
In endothelium-denuded mouse carotid artery segments, little dilation
to EBIO, an IKCa1 opener was observed in WT mice (%max. dilation;
13 12% at 10-4 M), while the vasodilation was significantly enhanced in ApoE
KO mice (66 4% p<0.05 vs WT) (Fig. 5A). In contrast, pimaric acid, a BKCa
opener elicited potent vasodilation in WT mice in a dose-dependent manner
(%max. dilation; 55 10% at 10-5 M), but the dilation was markedly reduced in
ApoE KO mice (9 3% p<0.05 vs WT) (Fig. 5B).
When patients were stratified according to the presence or absence of
CAD (no CAD [57 13y.o.] n=8 and CAD [65 11y.o.] n=12), vasodilation of
human coronary arterioles to EBIO was identical between the groups (%max.
dilation; no CAD 59 12 and CAD 61 8% at 10-4 M). However, endothelial
denudation significantly reduced the dilation only in vessels from non-CAD
subjects (no CAD 22 14 vs CAD 58 9%, p<0.05). Vasodilation of
endothelium-denuded vessels to 3x10-6 M pimaric acid in CAD subjects
(31 3%, p<0.05 vs non CAD, n=3) was significantly lower than that in non-
CAD subjects (59 6%, n=3). These results suggest greater IKCa1 activity and
relatively less BKCa activity in VSMCs of vessels in humans and mouse with
atherosclerosis, consistent with the differential expression of KCa.
Role of IKCa1 in the development of atherosclerosis in ApoE
knockout mice in vivo
The effect of long-term IKCa1 blockade on the development of
atherosclerosis was determined in mice. Representative images of aortic
atherosclerotic lesions (stained in yellow - orange) are shown in Fig. 6A. In
16
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
ApoE KO mice treated with vehicle, atherosclerotic lesions were observed
extensively from the aortic root to the iliac arteries. In ApoE KO mice
treated
with TRAM-34, much less staining was observed but in a similar distribution
along the aorta. Quantitative measurements of atherosclerotic lesions are
summarized in Fig. 6B. Aortas of ApoE KO mice displayed extensive lesions
of atherosclerosis with 34 4% (18 to 53% n=6, p<0.05 vs WT) of lesion area
(atherosclerotic lesion area / whole aortic area), while no lesions were seen
in
WT mice (0%, n=3). Treatment with TRAM-34 significantly reduced % lesion
area approximately by 60% (14 1%, 11 to 17% n=7, p=0.001 vs ApoE KO
mice treated with vehicle). Thus, IKCa1 activity plays an important role in
the
development of atherosclerosis.
The effects of long-term IKCa1 blockade with TRAM-34 on body
weight, heart weight, systemic blood pressure, heart rate, and plasma
cholesterol levels are shown in Figure 7 (Table 1). One mouse in each group
(vehicle or TRAM-34) died due to unknown reasons during the 14-week
treatment. Plasma cholesterol levels were higher in ApoE KO mice treated
with vehicle or TRAM-34 than in WT mice, while there was no significant
difference of cholesterol levels between ApoE KO mice treated with vehicle
and those with TRAM-34. There were no significant differences of body and
heart weight among the groups. Blood pressure and heart rate were also
unaltered by the treatment.
Summary and Discussion
This study examines the role of IKCa1 in the development of
atherosclerosis. The findings are four-fold. First, IKCa1 expression and
activity are increased in the coronary circulation of patients with CAD and in
aortas from mice with atherosclerosis. BKCa are down-regulated under the
same conditions. Second, the increased expression of IKCa1 is associated
with the proliferation and migration of VSMCs, macrophages and T
lymphocytes in vivo and in vitro. Third, blockade of IKCa1 activity inhibits
proliferation and migration of HCSMCs by suppressing c-fos expression and
DNA synthesis. Finally, long-term IKCa1 blockade inhibits the development of
atherosclerosis in mice. Taken together, these findings demonstrate that up-
17
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
regulation of IKCa1 activity plays a crucial role in the proliferation and
migration of VSMCs and inflammatory cells, an early step in the development
of atherosclerosis and suggests that IKCa1 channels are a potential
therapeutic target for preventing vascular morphological remodeling during
atherosclerosis.
IKCa1 up-regulation in proliferatory and migratory VSMCs
Recent in-vivo studies demonstrated IKCa up-regulation during the
process of vascular remodeling (VSMC proliferation) following myocardial
infarction or chronic inhibition of NO synthesis in rats and rabbits. Other
investigators also reported IKCa1 up-regulation in VSMCs migrated to
neointima in carotid arteries following balloon catheter injury (Kohler et
al). In
the present study, we found that IKCa expression is increased in proliferating
VSMCs in atherosclerotic vessels and in cultured HCSMCs stimulated with
PDGF-BB. This is consistent with results reported by Neylon et al who
demonstrated in cultured rat aortic SMCs that enhanced IKCa activity is
closely related to cellular proliferative rate. In addition, IKCa are up-
regulated
and critically participate in the process of proliferation and migration in a
variety of activated cells including activated T cells, macrophages and cancer
cells. Thus, IKCa may serve a fundamental role in cellular activation common
among several cell types.
Role of IKCa1 in cellular proliferation
In the present study, PDGF-induced HCSMC proliferation was inhibited
with TRAM-34 in vitro. Similarly the proliferation of rat aortic VSMC cell
lines
induced by epidermal growth factor is blocked by IKCa1 blockers. IKCa1
blockers also inhibit the proliferation of cancer cells, T and B cells. The
intracellular calcium concentration ([Ca2+]i) plays a critical role in
initiating and
maintaining the cellular activation process through the regulation of
intracellular signaling cascades. Ca2+ influx through voltage-gated calcium
channels and Ca2+ release from ryanodine receptors in response to mitogens
initiates the activation of the mitogen-activated protein kinase (MAPK)/
extracellular signal regulated kinase (ERK1/2) cascade followed by the
activation of transcription factors, induction of early response genes and DNA
18
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
synthesis concomitant with phenotypic changes in VSMCs. An increase in
[Ca2+]i following membrane depolarization by high extracellular concentration
of KCI induces VSMC differentiation marker genes via activation of Rho
kinases. However, it is unlikely that membrane depolarization by blockade of
IKCal with TRAM-34 inhibits the early process of VSMC proliferation, since
very few IKCal channels are expressed in contractile or quiescent VSMCs.
Neylon et al reported evidence for differential membrane potentials
from contractile and proliferative VSMC phenotypes. Contractile VSMCs,
which express BKCa, have less negative resting membrane potential than
proliferative VSMCs, which express IKCa1. In contractile VSMCs, exposure to
endothelin-1 induces an elevation in [Ca2+]i and membrane depolarization,
and pharmacological blockade of potassium channels does not modulate the
depolarization. In contrast, when [Ca2+]i is elevated by the same agonists in
proliferative VSMCs, there is a pronounced hyperpolarization due to the
subsequent IKCal activation. IKCal plays a more important role than BKCa
in shaping Ca2+ signals of proliferating cells, because of its higher Ca2+
affinity
(EC50 of IKCa1; _300 nM, BKCa; =6 iaM). Indeed, IKCal up-regulation
enhances the electrochemical driving force for Ca2+ influx through membrane
hyperpolarization and thus sustains high [Ca2+]i levels required for gene
transcription to promote mitogenesis in lymphocytes, erythrocytes, and
fibroblasts. These data suggest that IKCal channels actively participate in
the
regulation of cell proliferation by controlling [Ca2+]i and subsequently
regulating the activities of Ca2+/calmodulin-dependent protein kinases and
transcription factors responsible for mitogenesis. Thus, blockade of IKCal
may reduce [Ca2+]i, leading to the inhibition of mitogenesis and VSMC
proliferation, thereby producing an anti-atherosclerotic effect.
Alternative mechanisms for the anti-atherosclerotic effect of
IKCal blockade
It has been reported that proliferative VSMCs generate more reactive
oxygen species (ROS) such as superoxide than contractile VSMCs, which
might scavenge nitric oxide released from ECs. Similar observations were
observed in vivo, where reduced endothelium-dependent vasorelaxation is
19
CA 02620923 2008-02-29
WO 2007/033307 PCT/US2006/035789
due to excess oxidative stress generated in the media of atherosclerotic
rabbit
aortas. ApoE KO mice also exhibit reduced nitric oxide bioavailability. Thus,
IKCa1 blockade might act by reducing oxidative stress and preserving nitric
oxide bioavailability. However, IKCa1 channels also play an important role in
the function of macrophages and T cells, and it is thus likely that inhibition
of
atherogenic inflammatory processes contributes to the anti-atherosclerotic
effect of IKCa1 blockade.
It is to be appreciated that the invention has been described hereabove
with reference to certain examples or embodiments of the invention but that
various additions, deletions, alterations and modifications may be made to
these examples and embodiments without departing from the intended spirit
and scope of the invention. For example, any element or attribute of one
embodiment or example may be incorporated into or used with another
embodiment or example, unless otherwise indicated and/or unless doing so
would render the embodiment or example unsuitable for its intended use.
Also, where steps of a method or process have been described or recited in a
certain order, the order of such steps may be changed unless otherwise
indicated and/or unless doing so would render the method or process
unsuitable for its intended use. All reasonable additions, deletions,
20' modifications and alterations are to be considered equivalents of the
described examples and embodiments and are to be included within the
scope of the following claims.