Note: Descriptions are shown in the official language in which they were submitted.
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
AMNTOTIC CELLS AND METHODS FOR USE THEREOF
FIELD OF THE INVENTION
The present invention relates to the fields of molecular biology, gene
therapy,
immunology and virology. More particularly, the invention relates to human
amniotic cells
comprising lentiviral vectors with an exogenous gene element, methods for
malcing such cells
and methods for treating diseases.
RACKGROUND OF THE INVENTION
Humans can suffer from such central nervous system diseases as cerebral
ischemia,
cerebral vascular disease, central nervous system injuries, hereditary
diseases of the nervous
system, degenerative diseases of the cerebral nervous system (for example,
Parlc.inson's
disease, Huntington's disease and Alzheimer's disease) and tumors of the
cerebral nervous
system. To date, there are few effective therapeutic treatinents or drugs for
central nervous
system diseases. Typically, the death and cripple rates for central nervous
system diseases are
very high. For example, every year 16,400,000 people suffer from brain
strokes, brain
injuries and spinal cord injuries throughout the world. Moreover, 4,100,000 of
these people
die each year due to such maladies.
Recently, gene therapy has developed as a potentially potent method for
treating many
neurological diseases previously considered refractory to conventional
approaches. In general,
there are three strategies in gene therapy for treating central nervous system
diseases. One
strategy is to decrease the activity of mutation recessive gene proteins with
antisense technic
and RNAi technic. For example, mutation recessive genes of central nervous
system diseases
express proteins and gene therapy can be used to down-regulate expression of
theses proteiuls
via antisense or RNAi to the mutated mRNA. Another strategy is to compensate
for loss-
funetion enzymes or proteins of the brain by transferring genes. Lastly,
neurons can be
protected by delivery of proteins such as growth factors, antioxidants, HSP or
anti-apoptotic
molecules. Natsume et al., Exp. Neurol., 2001, 169: 231-33; Berry et al.,
Curr. Opin. Mol.
Ther., 2001, 3: 338-49.
1
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
Five types of virus vectors are currently used in gene therapy: (1) retrovirus
vectors,
which only infect cells that can proliferate; (2) adenoviral (Ad) vectors that
do not insert into
the chromosomes of host cells and cannot express stably (Ad vectors can also
produce
immunogenicity); (3) adeno-associated viral (AAV) vectors, which cannot
transport large
exogenous fragments (less than 5 kb) or have high titers; (4) herpes simplex
viruses (HSV)
that only infect neuronal cells; and (5) lentiviral vectors. At present, there
are many issues
relating to efficacy, stability, regulatability and safety of directly using
genetically-engineered
virus vectors for in vivo gene therapy. These issues include, for example, the
stability and
specificity of virus vectors in vivo. Alternatively, using embryonic or
somatic stem cells for
ex vivo gene tlierapy poses concerns relating to safety, inunune rejection,
cell sources and
ethical implications. Georgievska et al., Eur. J. Neurosci., 2004, 20(11):
3121-30; Englund
et al., Exp. Neurol., 2002, 173(1): 1-2; Kahn et al., Blood, 2004, 103(8):
2942-9; De Pa1ma
et al., Nat. Med., 2003, 9(6): 789-95; Imren et al., J. Clin. Invest., 2004:
953-62.
SUMMARY OF THE INVENTION
The present invention, in one aspect, relates to a population of human
amniotic cells
(HAC) comprising lentiviral vectors with at least one exogenous gene element.
Preferably,
the exogenous gene elements of the vector are capable of being expressed by
the cells.
Another aspect the invention features a composition comprising these cells and
at least one
pharmaceutically acceptable carrier. Another object of the present invention
is to provide a
method for transducing human amniotic cells. Another object of the invention
is to provide a
method for treating central nervous system diseases using the human amniotic
cells
comprising lentiviral vectors with at least one exogenous gene element. For
example, a
method of the invention comprises administering human amniotic cells
comprising lentiviral
vectors with at least one exogenous gene element to a patient.
The invention also provides transfected human amn.iotic cells, which can be
useful for
gene therapy. Preferably, the exogenous gene element can be highly expressed
in the humasi
amniotic cells comprising lentiviral vectors. These ceLls can effectively
treat central nervous
system diseases such as, for example, cerebral ischemia, cerebral vascular
disease, central
nervous systenl injuries, hereditary diseases of the nervous system,
degenerative diseases of
the cerebral nervous system (for example, Parkinson's disease, Huntington's
disease and
2
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
Alzheimer's disease), tumors of the cerebral nervous system and combinations
thereof. The
cells can also be used to treat any of the diseases disclosed herein. In one
embodiment,
human amniotic cells according to the invention can minimize or eliminate
rejection reactions
or topical 'uiflammations. Prior to transduction, the human amniotic cells can
be derived from,
without limitation, a donor, tissue-cultures or subject (for example,
patient).
In one embodiment, lentiviral vector transduced HAC may also be used in the
treatment or prophylaxis of diseases that include, without limitation,
cerebral ischemia,
cerebral vascular disease, central nervous system injuries, hereditary
diseases of the
nervous system, degenerative diseases of the cerebral nervous system
(Parkinson's disease,
Huntington's disease and Alzheimer's disease), tumors of the cerebral nervous
system and
combinations thereof. The lentiviral vector transduced HAC of the invention
can also be
used in the treatment or prophylaxis of any disease state or malady disclosed
herein.
Generally, "prophylactic" or "prophylaxis" relates to a reduction in the
likelihood of the
patient developing a disorder such as AD or proceeding to a diagnosis state
for the disorder.
For example, the lentiviral vector transduced HAC of the invention can be used
prophylacticly as a measure designed to preserve health and prevent the spread
or
maturation of disease in a patient. The invention also provides for methods of
administering lentiviral vector transduced HAC to a patient in an effective
amount for the
treatment or prophylaxis of a disease such as, for example, CNS diseases.
The lentiviral vector transduced HAC of the invention can also be administered
to a
patient along with other conventional therapeutic measures or agents that may
be useful in the
treatment or prophylaxis of, for example, CNS diseases. In one embodiment, a
method is
provided for administering an effective amount of lentiviral vector transduced
HAC of the
invention to a patient suffering from or believed to be at risk of suffering
from a disease. The
method also comprises administering, either sequentially or in combination
with lentiviral
vector transduced HAC of the invention, a conventional therapeutic agent in an
amount that
can potentially be effective for the treatment or prophylaxis of a CNS
disease.
Preferably, lentiviral vector transduced HAC of the invention can be
adininistered to a
patient in an amount or dosage suitable for treating a CNS disease. Generally,
a dosage or
composition comprising lentiviral vector transduced HAC of the invention will
vary
depending on subject considerations. Such considerations include, for example,
age,
condition, sex, extent of disease, contraindications and concomitant
therapies. An exemplary
3
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
dosage or composition based on these considerations can also be adjusted or
modified by a
person of ordinary skill in the art. Administration of lentiviral vector
transduced HAC of the
invention to a subject may be local or systemic and accomplished
intravenously,
intraarterially, intrathecally (via the spinal fluid) or the like.
Administration may also be
intradennal or intracavitary, depending upon the body site wider therapy.
A"subject" is a
mammal such as, for example, a human, and, preferably, a human suspected of
having one or
more CNS diseases. The terms "subject" and "patient" are used interchangeably
herein.
The lentiviral vector transduced HAC of the invention can also be administered
in
the form of a composition such as an injectable composition, but may also be
formulated
into well known drug delivery systems such as, for example, topical, oral,
rectal, parenteral
(intravenous, intramuscular or subcutaneous), intracisternal, intravaginal,
intraperitoneal,
local (powders, ointments or drops) or as a graft, buccal or nasal spray. As
described,
administration of lentiviral vector transduced HAC or compositions thereof may
be local or
systemic and accomplished intravenously, intraarterially, intrathecally (via
the spinal fluid)
or via a graft. The administration of lentiviral vector transduced HAC to a
subject can be
by a general or local administration route. For example, the lentiviral vector
transduced
HAC or compositions thereof may be administered to the patient such that it is
delivered
throughout the body. Alternatively, the lentiviral vector transduced HAC or
compositions
thereof can be administered to a specific organ or tissue of interest.
A typical composition for administration can comprise a pharmaceutically
acceptable carrier for one or more lentiviral vector transduced HAC of the
invention. A
pharmaceutically acceptable carrier includes such carriers as, for example,
aqueous
solutions and non-toxic excipients including salts, preservatives and buffers.
Remington's
Pharmaceutical Sciences, 15th Edition, Mack Publishing Co., 1975: 1405-1487;
The
National Formulary XIV., 14th Edition, American Pharmaceutical Association,
1975.
Exemplary pharmaceutically acceptable carriers for lentiviral vector
transduced HAC of
the invention can also include non-aqueous solvents such as propylene glycol,
polyethylene glycol, methoxypolyethylene glycol and vegetable oil or
injectable organic
esters such as ethyl oleate. In one embodiment, lentiviral vector transduced
HAC can be
conjugated to at least one pharmaceutically acceptable carrier. An aqueous
carrier can
include, without limitation, water, alcoholic/aqueous solutions, saline
solutions and
parenteral vehicles such as sodium chloride or Ringer's dextrose. Intravenous
carriers for
.4
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
administration of lentiviral vector transduced HAC of the invention can
include, for
example, fluid and nutrient replenishers. The pH and exact concentration of
the various
components for a composition comprising lentiviral vector transduced HAC can
also be
adjusted according to routine techniques known to those of ordinary skill in
the art.
Goodman and Gilman's The Pharmacological Basis for Therapeutics, 7th Edition.
In one embodiment, the invention provides a kit comprising lentiviral vector
transduced HAC. The invention also provides a method for the treatment or
prophylaxis of a
CNS disease comprising administering to a patient in need thereof an effective
amount of
lentiviral vector transduced HAC. For example, the method can include
providing a patient
suffering from or believed to be at risk of suffering from a CNS disease. The
method may
also comprise administering to the patient an effective amount of lentiviral
vector transduced
HAC of the invention. The lentiviral vector transduced HAC of the invention
can also be
administered as part of a composition comprising a pharmaceutically acceptable
carrier.
"Effective ainount" refers to the amount required to produce a desired effect.
One
example of an effective amount includes amounts or dosages that can be used to
alleviate or
minimize the effects of a CNS disease. Another example of an effective amount
includes
amounts or dosages that yield acceptable toxicity and bioavailability levels
for therapeutic use
including, without limitation, the treatment or prophylaxis of a CNS disease.
Another
exatn.ple of an effective anzount includes amounts or dosages that are capable
of minimizing
or preventing neuronal degeneration. When desired, lentiviral vector
transduced HAC of the
invention or compositions thereof may contain an additive such as pH
controlling agents (for
example, acids, bases, buffers), stabilizers (for example, ascorbic acid) or
isotonizing agents
(for example, sodium chloride).
A person of ordinary skill in the art can readily deterlnine an effective
amount of
lentiviral vector transduced HAC or compositions thereof by simply
administering the cells
or composition to a subject in increasing amounts over a period of time until
effects of a
disease are lessened. The determination of an effective amount for a
particular subject is
well known to those of ordinary skill in the art. The invention also
contemplates that
lentiviral vector transduced HAC can be used for ex vivo or in vivo gene
therapy.
Preferably, for in vivo gene therapy of a CNS disease, retrovirus, AAV,
lentiviral,
pseudotyped lentiviral or Ad vectors for the transduction of HAC can be
administered to a
subject in an effective amount. These vectors can be administered in a
composition
S ~
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
comprising a pharmaceutically acceptable carrier. Moreover, these vectors can
be
administered topically, orally, rectally, parenterally (intravenously,
intramuscularly or
subcutaneously), intracisternally, intravaginally, intraperitoneally, locally
(powders,
ointments or drops) or as a graft, buccal or nasal spray. As described,
administration can
also be local or systemic and accomplished intravenously, intraarterially,
intrathecally (via
the spinal fluid) or via a graft.
In addition to using promoters to drive expression in HAC, an enhancer
sequence may
be used to increase the level of expression. Armelor et al, Proc. Natl. Acad.
Sci., 1973, 70:
2702. Exemplary growth factors encoded by exogenous gene elements include
nerve growth
factor, neurotrophic factors, brain derived growth factor (BDNF), neurotrophin
(NT)-3, NT-4
and ciliary neuronal trophic factor (CNTF).
The invention also contemplates analogs, homologs, derivatives and variants of
nerve
growth factor, brain-d.erived neurotrophic factor, hypoxanthine guanine
phosplloribosyltransferase, glia-derived neurotrophic factor, ciliary
neurotrophic factor,
choline acetylase, tyrosine hydroxylase, aromatic L-amino acid decarboxylase,
bcl-2 or
tetrahydrobiopterin synthase. Similarly, the invention contemplates analogs,
hoinologs,
derivatives and variants of exogenous gene elements encoding, for example,
nerve growth
factor, brain-derived neurotrophic factor, hypoxanthine guanine
phosphoribosyltransferase,
glia-derived neurotrophic factor, ciliary neurotrophic factor, choline
acetylase, tyrosine
hydroxylase, aromatic L-amino acid decarboxylase, bcl-2 or tetrahydrobiopterin
synthase.
Exogenous elements can also be used to encode tyrosine hydroxylase, GTP-
cyclohydrolase I,
aromatic amino acid dopa decarboxylase, vesicular monoamine transporter 2
(VMAT2) or
any suitable protein or antibody.
Additionally, the invention contemplates transduction of HAC via, without
limitation,
one or more retrovirus, AAV, lentiviral, pseudotyped lentiviral, Ad vectors
and combinations
thereof. For example, these vectors can be used either sequentially or in
combination to
transduce HAC. A vector can also comprise one or more exogenous elements.
In one embodiment, a population of human aniniotic cells comprising one or
more
lentiviral vectors is provided. For example, the lentiviral vectors can
comprise at least one
exogenous gene element, which is expressed by the cells. Exemplary exogenous
gene
elements can encode one of nerve growth factor, brain-derived neurotrophic
factor,
hypoxanthine-guanine phosphoribosyltransferase, glia-derived neurotrophic
factor, ciliary
-6
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
neurotrophic factor, choline acetylase, tyrosine hydroxylase, aromatic L-amino
acid
decarboxylase, bcl-2 or tetrahydrobiopterin synthase. Preferably, the
lentiviral vectors can
comprise at least one controlling transcription fragment of an RNAi inducible,
Cre-loxP or
doxycycline-inducible system. The invention also provides a composition
comprising one or
more lentiviral vectors that include at least one exogenous gene.
Moreover, a method of transducing a population of human amniotic cells with
one or
more lentiviral vectors is provided. Preferably, the method comprises
culturing the human
amniotic cells. For example, an amnion can be separated from a placenta
obtained from a
donor. The placenta can be extensively scraped out to remove the underlying
tissues. The
amnion can optionally be treated with at least one enzyme. In one embodiment,
the isolated
cells can be cultured in a cell culture medium. The method also comprises
incubating at least
one vector and the population of human amniotic cells.
In one embodiment, a method for treating central nervous systein diseases in a
subject
is provided. Preferably, the method involves the use of a population of hunian
amniotic cells
comprising a lentiviral vector with an exogenous gene element. Exemplary CNS
diseases
include cerebral ischemia, cerebral hemorrhage, central nervous system trauma,
hereditary
diseases of the nervous system, degenerative diseases of the central nervous
system
(Parkinson's disease, Huntington's disease and Alzheimer's disease),
neurodegenerative
diseases, spinal cord trauma and neoplasms of the central nervous system.
Transduction or transfection is generally used to refer to the introduction of
genetic
material into a cell such as a human amniotic cell by using a vector, for
example, a retrovirus,
AAV, lentiviral, pseudotyped lentiviral or Ad vector. Different types of
vectors can be used
for the transduction or transfection of HAC. These vectors include plasmid or
viral vectors.
Retroviral vectors such as those based on Moloney murine leukemia virus
(MoMLV) can be
used. Moreover, other murine retroviral vectors that can be used include those
based on
murine embryonic stenz cell virus (MESV) and murine steni cell virus (MSCV).
Lentiviral vectors, a subclass of the retroviral vectors, can also be used for
high-
efficiency transduction and are able to transduce non-dividing cells,
increasing the
likelihood that the cells can be pluripotent. Other groups of retroviruses
such as
spumaviruses are also capable of efficiently transducing non-dividing cells.
Additional
types of viral vectors that can be used in the invention include adenoviral
vectors, adeno-
associated viral (AAV) vectors, SV40 based vectors or forms of hybrid vectors.
A wide
7
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
variety of expression vectors are available for transferring gene elements,
for example,
endogenous or exogenous gene elements, encoding bioactive materials into HAC.
These
expression vectors can be viral vectors such as modified or recombinant
retroviruses,
adenoviruses, lentiviruses, pseudotyped lentiviruses and adeno-associated
viruses.
Alternatively, the expression vectors can be transfected to the cells via non-
viral routes
such as, without limitation, physical methods including electroporation,
ultrasound,
chemical, liposome-mediated, activated-dendrimer-mediated and calcium-
phosphate
techniques.
Examples of promoters useful in the invention include constitutive and
inducible
promoters. Without limitation, such promoters can comprise CMV, SV40,
retroviral LTR,
EF, tetracycline inducible, inflammation induced, TNF-a and IL-1 promoters. In
addition to
HAC, the invention may comprise various other components and means to
facilitate the
delivery of cells to a subject. For example, HAC can be provided within a
medium in which
the cells are preserved or maintained. Examples of such a medium include PBS,
DMEM and
any suitable type of cell culture medium. In one embodiment, the invention
contemplates
buffers such as, without limitation, HEPES, PBS or citrate-based buffers.
Furthermore, the
invention can include dyes, packagings, kits, instructions for using
transduced HAC, DMSO
and glycerols for cryopreservation. The transduced HAC of the invention can be
used for,
without limitation, therapy, diagnosis, cosmetics or any other applications.
In one embodiment, exogenous elements can include, without limitation, growth
factors, anti-microbial proteins, anti in.flamniatory protein or protease
inhibitors. Examples
of growth factors can include PDGF, FGF 2, EGF, KGF-2, GM-C SF, TGF b, IGF-I
and
HGH. Moreover, examples of anti-microbial proteins can include
bactericidallpermeability-
increasing proteins, defensin, collectin, granulysin, protegrin-1, SMAP-29,
lactoferrin and
calgranulin C. Preferably, examples of anti-inflammatory proteins can include
interleukin-1
receptor antagonists, interleukin-10, soluble TNF receptors and soluble CTLA4.
Protease
inhibitors can also include, for example, TIlVIP-1, -2, -3, -4, PAI-1, PAI-2
and ecotin.
The invention also contemplates that each of the vectors for transfection of
HAC
can comprise at least one promoter. The exogenous gene elements of a vector
can also
comprise a marker sequence. In one embodiment, cells that can be transduced
with a
vector include those derived from or comprising, without limitation, mammalian
tissues,
epithelial cells, alveolar cells, bone marrow, cardiac muscles, connective
tissues,
8
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
ependymal cells, epithelial tissues, epithelial cells, epidermis, esopliagus,
fibroblasts, glial
cells, hepatic cells, keratinocytes, leukocytes, lymphocytes, macrophages,
mammary
glands, melanocytes, monocytes, myoblasts, neurons, osteoblasts, osteogenic
cells,
pituicytes, plasma cells, skeletal muscles, smooth muscles, synoviocytes,
umbilical tissues,
HAC or combinations thereof. The exogenous gene elements of a vector can also
comprise a sequence encoding for a detectable marlcer sucll as, for example, a
green
fluorescent protein (GFP).
In one embodiment, lentiviral vector transduced HAC can be used to prepare a
graft
composition. A graft composition can include cells transfected by a vector
comprising
endogenous or exogenous gene elements. For example, a graft composition can
also
comprise a biocompatible matrix. The HAC of a graft composition can be talcen
from a
subject and transfected in vitro prior to reintroduction to the subject.
Exemplary
biocompatible matrices for a graft composition include those that are natural
or synthetic.
A graft composition can also comprise functional elements that can be either
constitutive
or inducible via physical or chemical stimuli to effect up or down regulation
during therapy.
Preferably, a graft composition can be administered, without limitation, via
the
gastrointestinal tract (orally or as a suppository), parentexally
(intramuscular, intravenous
or subcutaneous) or topically.
The invention also contemplates composition comprising vector transduced HAC
and
additional components. For examples, such components can include cells,
genetically
inodified cells, proteins, peptides, non-protein bioorganic substances,
therapeutic agents
(antiinflammatories, antibiotics, antivirals, antineoplastics or antimycotics)
and combinations
thereof. In one embodiment, the vector transduced HAC of the invention can
comprise at
least one exogenous gene element that encodes for glia or brain-derived
neurotrophic factors,
which can be used in the prophylaxis or treatment of CNS diseases.
A population of transduced HAC or compositions thereof can be administered to
a
patient, for example, locally, at exemplary dosage levels in the range of
about 103 cells to
about 1010 cells per day. The specific dosage used, however, can vary or may
be adjusted
as considered appropriate by a person of ordinary skill in the art. Without
limitation, the
dosage can depend on a number of factors including method of administration,
requirements of the patient and severity of the disease being treated. The
determination of
9-
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
optimum dosages for a particular patient is also well known to those of
ordinary slcill in the
art.
DESCRIPTION OF THE DRAWINGS
Other features and advantages of the invention may also be apparent from the
following detailed description thereof, taken in conjunction with the
accompanying drawings.
Fig. 1, a map of lentiviral vectors used herein. Vectors contain the post-
transcriptional regulatory element of woodchuck hepatitis virus (WPRE or WHV)
and central
polypurine tract (cPPT), which enhance transgene expression as well as cis-
acting elements,
improving the efficiency of gene transfer. All vectors contain promoters:
elongation factor
1-alfa promoter (EF1-a) or human cytomegalovirus (CMV), which are robust
transcriptional
elements in most cell types.
Fig. 2, efficient transfer, integration and sustained long-term expression of
EGFP in
HAC. EGFP expression in HAC at 7 days after transduction with lentiviral
vectors or
DOTAP, (Fig. 2A). EGFP was evaluated on photographs that were talcen with a
fluorescence
microscope (x 10) and transduction efficiency was measuxed by FACS. The EGFP
expression was sustained for 5 weeks without obvious change, (Fig. 2B). The
EGFP was
integrated into genomic DNA and transcripted into mRNA that was detected by
PCR analysis,
(Fig. 2C). The sanze percent of GO-Gl cells before and after infection with
vectors were
shown, (Fig. 2D).
Fig. 3, lentiviral vector-mediated siGFP suppression in HAC. Photographs were
taken with a fluorescence microscope (x 10) at 7 days after transfection of
HAC with EGFP-
expressing lentiviral vectors, (Fig. 3A), and efficiency was measured by FACS,
(Fig. 3B). At
7 days after transfection of HAC with EGFP-expressing lentiviral vectors
together with
lentiviral vectors expressing siGFP, (Fig. 3C), absence of EGFP expression can
be seen only
in the group of siGFP-expressing vectors. The suppression of EGFP
transcription was
detected by RT-PCR analysis, (Fig. 3D).
Fig. 4, the EGFP was efficiently regulated by Cre-loxP system base on
lentiviral
vectors in HAC. EGFP was observed by fluorescence microscopy (x 10) after
being
transduced with lenti EGFP, (Fig. 4A). Fluorescence was silenced by
cotransfection of lenti-
EGFP and lenti-Cre after 7 days, (Fig. 4B). EGFP efficiency treated with lenti-
Cre was
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
scored by FACS, (Fig. 4C). PCR of Genomic DNA demonstrated that EGFP was
deleted by
lenti Cre, (Fig. 4D), and positive control was the result of RT-PCR of EGFP.
Fig. 5, lentiviral vector-mediated DOX-induced gene expression. The EGFP was
expressed in HAC based on PLVTHM, (Fig. 5A). The HAC were cotransduced with
LVtTR ICRAB and PLVTHM, (Fig. 5B), as well as LVtTR-KRAB and PLVTHM in the
presence of DOX, (Fig. 5C). HAC were observed with a fluorescence microscope
(x 10).
Fig. 6, the expression of viral protein GAG and EGFP in the HAC and Hela cell
cultured with HAC conditional medium. RT-PCR of EGFP showed HAC transduced
with
lenti-EGFP sustain EGFP expression, but the Hela cell cultured with HAC
conditional
medium has no EGFP expression, (Fig. 6A). Structure gene GAG cannot be
detected either
in the lenti-EGFP transduced HAC or in the Hela cell cultured with HAC
conditional
medium, (Fig. 6B).
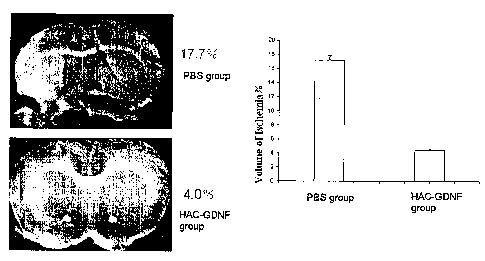
Fig. 7, the average iuifarct volumes in the experimental groups. A reduction
in the
volume of ischemic damage was detected in the cell transplanted groups
compared to the PBS
group when measured at 16 days after ischeniia.
Fig. 8, immunostaining of HAC-GDNF in vivo. 14 days after transplantation
immunohistochemical staining of GDNF from a xenografted rat brain showed more
GDNF
positive HAC in the brain of the HAC-GDNF group, (Fig. 8A). Fig.BB shows high-
power
photomicrographs depicting the boxed areas in Fig. 8A. DAB and hematoxylin
counterstaining are shown in Fig. 8C and 8D. As shown, there was no nestin
expression in
the contralateral area.
Fig. 9, 3 weeks after transplantation, strong MAP2 expression was detected in
the
injection tract with immunohistochemistry, (Fig. 9A), and DAB and hematoxylin
counterstaining, (Fig. 9C). Fig. 9B is the same site of contralateral tissue.
DAB and
hematoxylin counterstaining are shown in Fig. 9D.
Fig. 10, neurological test.
Fig. 11, beam walking test to detect coordination function.
DETAI_LED DESCRIPTION OF THE IWENTION
The present invention may be understood more readily by reference to this
detailed
description as well as the drawings and examples herein. The invention
provides a population
11
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
of human amniotic cells comprising lentiviral vectors in which each lentiviral
vector includes
at least one exogenous gene element. As used herein, lentiviral vectors are
agents that can
transport a gene of interest into a cell without degradation in all cells.
Lentiviral vectors can
also include a promoter yielding expression of a gene in cells such as, for
example, amniotic
cells. Lentiviral vectors are based on the nucleic acid backbone of a virus
from the lentiviral
fainily of viruses. A lentiviral vector can also comprise first, second and
third generation
lentiviruses. In one embodiment, lentiviral vectors can provide for gene
transfer more
efficiently than Ad and AAV. Moreover, lentiviral vectors can stably express a
foreign
transgene without detectable pathogenesis or irnrnunogenicity from viral
proteins. Human
amniotic cells transduced by lentiviral vectors according to the invention can
be particularly
effective for gene therapy.
In one embodiment, lentiviral vectors are third generation lentiviruses. For
example,
third generation lentiviruses have lentiviral packaging genes split into at
least 3 independent
plasnmids. Moreover, lentiviral vectors such as third generation lentiviruses
can lack the HN-
1 tat gene (a strong transcriptional activator of the HI.V-1 LTR promoter
essential for viral
replication) and accessory genes (vpr, vpu, vif and nef). Preferably, the EMV
gene of H1V-1
can be replaced with the VSVG gene and the residua] HW genome may be divided
into two
expression constructs. Exemplary lentiviruses can comprise a PWPT, PLVTHM or
PLV
vector.
HAC develop from early inner cell masses of blastula about 8 days after
fertilization.
Human amniotic cells can be obtained from human amnion. Exemplary HAC comprise
amniotic epithelial and mesenchymal stem cells. Human anuiiotic cells can
express some
marlcers nomially present on stenz cells such as, for example, GFAP, MAP2,
nestin and AFP.
Knezevic et al., Anat., 1996, 189: 1-7; Yuge et al., Transplantation, 2004,
77(9): 1452-4;
Takashima et al., Cell Struct. Funct., 2004, 29(3): 73-84; Sakuragawa et al.,
Neurosci. Lett.,
1996, 209(l): 9-12; Wei et al., Cell Transplant., 2003, 12(5): 545-52.
Moreover, evidence
may suggest absence or poor expression of HLA-A, HLA-B, HLA-C and HLA-DR
antigens
and 0 2 microglobulin on the surface of amniotic cells. Akle et al., Lancet,
1981, 2(8254):
1003-5; Adinolfi et al., Nature, 1982, 295: 28. The HAC according to the
invention can
express BLA class lb (HLA-G), which may provide for low ixnmune rejection and
a long
period of survival in a host. Similarly, HAC can undergo differentiation after
xeno- or allo-
transplantation. Ueta et al., Clin. Exp. Irnmunol., 2002, 129(3): 464-70.
12
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
Promoters controlling transcription from vectors in mammalian host cells may
be
obtained from various sources such as, for example, RNAi inducible, Cre-loxP
and
doxycycline-inducible systems. A vector for transduction of HAC can be
designed to carry
exogenous gene elements that may be expressed by the cells. An exogenous gene
element
can, for example, comprise a marker gene. Such a marker gene can produce a
product and be
used to determine whether a gene has been delivered to a cell and expressed
thereby.
Exenlplary marker genes can include the E. Coli lacZ gene, which encodes P-
galactosidase,
green fluorescent protein (GFP) or the enhanced green fluorescent protein
(EGFP).
In one embodiment, exogenous gene elements can also include genes that are
replacing or supplementing a native gene, which may be capable of treating a
central nervous
system disease. For example, the exogenous gene element can encode nerve
growth factor,
brain-derived neurotrophic factor, hypoxanthine guanine
phosphoribosyltransferase, glia-
derived neurotrophic factor, ciliary neurotrophic factor, choline acetylase,
tyrosine
hydroxylase, aromatic L-amino acid decarboxylase, bcl-2, tetrahydrobiopterin
synthase or
combinations thereof. Bemelmans et al., Hum. Gene Ther., 1999, 10: 2987-97;
Ghodsi et al.,
Hum. Gene Ther., 1998,9: 2331-40; Snyder et al., Nature, 1995, 374: 367-70;
Palella et al.,
Gene, 1989, 80: 137-44; Bjorklund et al., Brain Res., 2000, 886: 82-98; Kang
et al., Hmn.
Cell, 2001, 14: 39-48; Yamada et al., Proc. Natl. Acad. Sci., 1999, 96: 4078;
Tuszynski et al.,
Exp. Neuro1.,1998,154: 573-82; Saille et al., Neuroscience, 1999, 92: 1455-63;
Haase et al.,
Ann. Neuro1.,1999, 45: 296-04; Mohajeri et al., Hum. Gene Ther., 1999,10: 1853-
66;
Azzouz et al., Hum. Mol. Genet., 2000, 9: 803-811; Adachi et al., Hum. Gene
Ther., 2000,
11: 77-89. Preferred exogenous gene elements can comprises glia-derived
neurotrophic
factor (BDNF) or brain-derived neurotrophic factor (GDNF) genes.
Neurotrophic factors are responsible for the growth and survival of nerve
cells during
development as well as the maintenance of adult nerve cells. For example,
animal studies and
in vitro models have shown that certain neurotrophic factors are capable of
making damaged
nerve cells regrow. In one embodiment, amniotic cells comprising lentiviral
vectors in which
the lentiviral vectors include at least one exogenous gene element that
encodes for a
neurotrophic factor. Exemplary neurotrophic factors can be used to treat or
reverse the effects
of central nervous system diseases. Brain-derived neurotrophic factor (BDNF)
belongs to the
neurotrophin family of trophic factors. BDNF is widely and abundantly
expressed in the CNS
and is available to some peripheral nervous system neurons that uptake the
neurotrophin
13
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
produced by peripheral tissues. BDNF promotes survival and differentiation of
certain
neuronal populations during development.
During adulthood, BDNF can modulate neuronal synaptic strength and has been
implicated in hippocampal mechanisms of learning and memory as well as the
spinal
mechanisms for pain. Several CNS disorders are also associated with a decrease
in trophic
support. Given that BDNF and its high affinity receptors are abundant
throughout the whole
CNS, BDNF can be a potent neuroprotective agent that is effective for the
treatment of,
without limitation, Parldnson's disease, Alzheimer's disease, depression,
epilepsy and chronic
pain. Glial cell derived neurotrophic factor, GDNF, also belongs to the family
of
neurotrophic factor proteins. GDNF enhances the survival and morphological
differentiation
of dopaminergic neurons and increases their uptalce of dopamine. GDNF can
rescue motor
neurons from programmed cell death and death caused by axotomy. GDNF is a
particularly
potent factor for survival and axonal growth of inesencephalic dopaminergic
neuron and has
been shownto ameliorate motor deficits and reduce brain damage in several
aivmal models.
Bjorklund et al., Brain Res., 2000, 886: 82; Gash et al., Nature, 1996, 380:
252; Kordower et
al., Science, 2000,290: 767; Zurnet al., Brain Res. Rev., 2001, 36: 222.
One example of an exogenous gene element comprises the GDNF gene. Figure 1
shows lentiviral constructs that comprise a GDNF exogenous gene element.
Exemplary
exogenous gene elements such as those described herein can comprise sequences
obtained
from, without limitation, the references provided herein and GenBank. In one
embodiment,
the exogenous gene elements can be constTUcted or modified through
conventional
recombinant techniques.
Another aspect of the invention includes a composition comprising the human
amniotic cells and at least one pharmaceutically acceptable carrier. For
example, HAC
comprising lentiviral vectors with exogenous gene elements and compositions
thereof can be
transplanted into the body of a subject for gene therapy. Preferably, the HAC
comprising
lentiviral vectors with exogenous gene elements can be used in the therapy of
cerebral
nervous system diseases. Diseases of the central nervous system include
disorders of the
brain, spinal cord, cranial nerves, nerve roots and autonomic nervous system.
Central nervous
system (CNS) diseases can also comprise, for example, cerebral ischemia,
cerebral
hemorrhage, central nervous system trauma, hereditary diseases of the nervous
system,
14
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
neurodegenerative diseases, spinal cord trauma, Parkinson's disease and
neoplasms of the
central nervous system.
Cerebral ischemia is an ischemic condition in whicli the brain or parts
thereof do not
receive enough blood flow to maintain normal neurological function. The
condition can be
the result of various diseases or arterial obstructions such as strangulation.
Cerebral ischemia
can bring about neuron death and induce brain disorder. Moreover, cerebral
hemorrhage is a
sudden loss of consciousness resulting from the rupture or occlusion of a
blood vessel, leading
to oxygen laclc in the brain. CNS trauma is damage to the brain and spinal
cord that results
from direct injury to them or from indirect injury due to damage of the bones,
soft tissues or
blood vessels surrounding the brain and spinal cord.
Hereditary diseases of the nervous system are a group of inherited, slowly
progressive
disorders that can result from progressive damage to nerves such as, for
example,
Huntington's chorea (HD), amaurotic family idiocy (Tay-Sachs),
dentatorubropallidoluysian
atrophy (DRPLA) and Machado-Joseph disease (MJD). A neurodegenerative disease
is a
disorder caused by the deterioration of certain nerve cells (neurons). Changes
in these cells
can cause them to function abnormally and eventually bring about their death.
Alzheimer's
disease, Parkinson's disease and amyotrophic lateral sclerosis (ALS) as well
as multiple
sclerosis are due to neuronal degeneration in the central nervous system.
Neoplasms of the central nervous system include, without limitation, the
cerebral
hemispheres, basal ganglia, hypothalamus, thalamus, brain stem and cerebellum.
Brain
neoplasms can be subdivided into primary (originating from brain tissues) and
secondary
(metastatic) forms. Primary neoplasms can also be subdivided into benign and
malignant
forms. In general, brain tuinors may be classified by age of onset, histologic
type or
presenting locations.
Spinal cord trauma is damage to the spinal cord that results from direct
injury to the
cord itself or from indirect injury due to damage to the bones, soft tissues
or blood vessels
surrounding the spinal cord. Parkinson's disease is a chronic progressive
nervous disease that
is linked to decreased dopamine production in the substantia nigra and can be
marked by.
tremors and weakness in resting muscles as well as by a shuffling gait. The
symptoms of
Parkinson's disease are caused by a loss of nerve cells in a part of the brain
called the
substantia nigra, resulting in a decrease in dopaniine (a neurochemical)
throughout the brain.
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
This destruction occurs due to genetic or environmental factors as well as
combinations
thereof.
In one embodiment, intracerebral grafting of GDNF-transduced HAC (for example,
epithelial) into ischemic rats prepared by middle cerebral artery occlusion
(MCAo) can
significantly ameliorate behavioral dysfunctions and reduce infarct volumes.
Furthermore,
neuronal markers and markers of neuronal stem cells can be detected in these
transplanted
HAC. Similarly, a number of these transplanted HAC survive and migrate to the
infarct area.
For example, the BDNF gene, which is an important member of the growth factors
for
the nervous system, plays a physiological role in the development of the
central nervous
system and regulation of adult neurons. The BDNF gene also provides for potent
neuroprotective effects on a variety of damaged neurons (irrespective of the
reasons for
damage). In the brain, the BDNF protein of AD or PD subjects is less than that
of those that
are normal. Intracerebral grafting of BDNF-transduced HAC (for example,
epithelial) into a
PD subject (rat) can significantly ameliorate behavioral dysfunction and
increase the BDNF
protein. According to the invention, grafting of BDNF-transduced HAC into a
Rhesus
monkey with incomplete dorsal spinal cord injury can also significantly
ameliorate behavioral
dysfunction.
As used herein, "optional" or "optionally" can mean that the subsequently
described
event or circumstance may or may not occur and that the description includes
instances in
which the event or circumstance occurs and instances in which it does not.
As described, pharmaceutically acceptable carriers may include sterile aqueous
solutions, suspensions, and emulsions. Aqueous solution carriers can also
include, without
limitation, PBS or Hankas' solutions. Exemplary emulsions or suspensions can
include
collagen, hydroxyproxyl cellulose, microcrystalline cellulose, amylum, PVP,
agar, pectin,
magnesium aluniinate silicate or magnesium aluminate.
The number of cells to be transplanted can be appropriately determined
depending on
the conditions of the patient and on the ability to produce the desired gene
product of the cells
according to the invention. In one embodiment, the number of cells
transplanted can be about
105 to 1010. Therapy methods can also refer to a method of transplantation of
cells and
compositions thereof. Moreover, therapy methods may also include sterile
methods such as,
for example, direct injection or encephalic transplantation.
1G
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
The examples herein are provided to illustrate advantages of the present
invention that
have not been previously described and to further assist a person of ordinary
skill in the art
with preparing and using the HAC thereof. The examples can include or
incorporate any of
the variations or embodiments of the invention described above. The
embodiments described
above may also further each include or incorporate the variations of any or
all other
embodiunents of the invention. Moreover, biological methods involving
conventional
techniques are described herein. Such conventional techniques are generally
known in the art
and are described generally in, for example, Molecular Cloning: A Laboratory
Manual, 3rd
Edition, vol. 1-3, ed. Saiiibrook et al., Cold Spring Harbor Laboratory Press,
Cold Spring
Harbor, N.Y., 2001 and Current Protocols in Molecular Biology, ed. Ausubel et
al., Greene
Publishing and Wiley-Interscience, N.Y., 1992. Similarly, exeniplary methods
for the
chemical synthesis of nucleic acids are described, without limitation, in
Beaucage et al., Tetra.
Letts., 1981, 22: 1859-12 and Matteucci et al., J. Am. Chem. Soc., 1981, 103:
3185. For
exan-iple, chemical synthesis of nucleic acids can be performed using
coinmercial automated
oligonucleotide synthesizers. Immunological methods are also described in,
without
limitation, Methods of hnmunological Analysis, ed. Masseyeff et al., John
Wiley & Sons,
N.Y., 1992. Conventional methods of gene transfer and gene therapy can also be
adapted for
use in accordance with the present invention. Gene Therapy: Principles and
Applications, ed.
Blackenstein et al., Springer Verlag, 1999; Gene Therapy Protocols (Methods in
Molecular
Medicine), ed. Robbins et al., Humana Press, 1997; Retro-vectors for Hiunan
Gene Therapy,
ed. Hodgson et al., Springer Verlag, 1996.
EXAMPLE I
Culturing of HAC
An amnion membrane was mechanically peeled from the chorion of a placenta and
was extensively scraped out to remove the underlying tissues (the spongy and
fibroblast
layers) to obtain a pure epithelial layer with basement membrane. The membrane
was cut
with a razor to yield a segment. Enough enzyme solution to obtain a signal
cell was added.
HAC were then cultured in a cell culture medium.
Vectors
17
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
Metllods of malcing paclcaged retroviral and leiitiviral packaging systems are
well
laiown to those of ordinaiy slcill in the art. Briefly, lentiviral vectors
were produced by
transient transfection of 293T cells. 20 g of lentiviral vectors (PWPT, LVTHM
or PWPT-
GDNF, Fig. 1), l0,ug of pMDlg/pRRE (or pCMVdR 8.2), 51u.g of pMD2 G and 51tg
of pRSV-
REV were mixed and adjusted to 250 l with water. 250 l of CaC12 0.5 M were
mixed
therewith and to the resultirig mixture was added 500 l of HeBS2X (0.28 M
NaCI, 0.05 M
HEPES, 1.5 M Na2HPO4), wllich was still for 30 minutes on a bencll.
The dishes were placed in a 37 C humidified incubator with a 5 % CO2
atmosphere.
The medium was aspirated. 14 hours later, 10 ml of fiesh DMEM-10 % FBS (PAA
Austria)
prewarmed to 37 C was gen.tly added, followed by incubation for 28 llours. The
virus was
collected and cleared via centrifiigation at 1500 ipm for 15 minutes and
filtered through a
0.45 m filter. Ultracentrifi.lgation occurred for 90 ininutes at 80,000 g and
4 C. An aliquot
of the supeniatant was resuspended as pellets with 1 ml PBS and stored at -80
C. Titration of
the concentrated supenlatants was perfonned by serial dilutions of vector
stocks on 1x 105
Hela cells, followed by fluorescence-activated cytometric, Beckton Dickinson
hrununocytometry Systems, analysis. According to the fonnula, 1 x 105 Hela
cell x (%)
EGFP positive cells x 1000/ l virus, titers of lentiviral vectors were
calculated among 0.1-1 x
10' TU/ml.
HAC infected with lentiviral vectors
The viral vector was added to cultured amniotic cells on the following day for
2 days
in the presence of 1-10 g/rnl polybrene (Sigma-Aldricli). After washing with
PBS, HAC
continued to be cultured in a cell culture medium such that exogenous gene
elements could be
introduced into HAC.
EXAlVIl'LE II
Lentiviral vector infection
Studies and use of lluinan amnion were approved by both patient and etlucs
conunittee review. For example, an ainnion meinbrane was mechanically peeled
from the
chorion of a placenta obtained from a woman with an uncomplicated cesarean
section and
was extensively scraped out to remove the underlying tissues (the spongy and
fibroblast
layers) to obtain a pure epithelial layer with basement membrane. The membrane
was placed
18
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
in a 250 ml wide-mouthed flask containing a RPMI 1640 mediunl and cut witli a
razor to
yield a 0.5-1.0 segment. Enough 37 C trypsin/EDTA solution was added to the
culture to
cover the membrane twice, the first and second times for about 30 and 15
minutes,
respectively. The obtained cells were seeded within six well plates in a RPMI-
1640 medium
supplemented with 10 % fetal calf serum (PAA Austria), streptomycin 100 gg/ml,
penicillin
100 U/ml and glutamine 0.3 mg/ml, followed by incubation under a humidified
atiuosphere of
5 % CO2 in air at 37 C. HAC were plated on a 6-well plate (2 x 105 cells/well,
Costar) and
polybrene (Sigma-Aldrich) was added to the wells for a final concentration of
8 g/ml.
Lentiviral vectors (PWPT) were added (MOI = 100), after 48 hours of
incubation. DOTAP
(Boehringer Mannheim) was also used to transfect HAC.
Transduced HAC was cultured for 1 weelc. EGFP expression was visualized by
fluorescent micropliotograph and analyzed by fluorescence-activated cell
sorting (FACS).
Genoinic DNA was isolated as described by, for example, Promega and total RNA
isolation
and reverse transcription were performed (Promega). To measure the relative
expression of
EGFP, a semi-quantitative PCR for EGFP and (3-actin (internal reference)
analysis was
performed with PCR amplification for 28 cycles. Primer sequences were EGFP
(upstream
5'-cgagctggacggcgacgtaaac-3' and downstream 5'-gcgcttctcgttggggtctttg-3') and
(3-actin
(upstream 5'-aacgagcggttccgatgccctgag -3' and downstream 5'-
tgtcgccttcaccgttccagtt 3').
Amplification conditions were 94 C for 5 minutes, 28 cycles of 94 C for 1
minute, 58 C for
1 minute, 72 C for 1 minute and 72 C for 10 minutes. The expected length of
the EGFP RT-
PCR products was 597 bp. The expression difference was normalized by the
respective (3-
actins (590 bp). Ten microliters from each RT-PCR product were loaded on a 1.5
% agarose
gel containing 0.5 g/ml of ethidium bromide and separated by electrophoresis.
Results
EGFP can be observed with fluorescence microscopy in the 4th day after
transduction.
As a control, HAC was transduced with DOTAP and these cells were scored by
fluorescence-
activated cell sorting (FACS) in the 7th day. Transduction of lentiviral
vectors was detected
to be more efficient than DOTAP with about 90 % of the cells successfully
transduced with
lentiviral vectors, (Fig. 2A), and less than 5 1o with DOTAP (not shown). EGFP
expression
was measured for 5 weeks in succession and EGFP-positive HAC were maintained
during
the culture period, (Fig. 2B). To evaluate integration of EGFP into HAC,
genomic DNA and
19,
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
mRNA was extracted in the 7tli day after transduction and subjected to PCR and
RT-PCR
amplification. The results showed that EGFP was inserted into the genome of
HAC with
stable expression at the mRNA level, (Fig. 2C). Lentiviral vectors did not
affect the percent
of GO-Gl stage cells after transfection, (Fig. 2D), demonstrating that
lentiviral vector
modified HAC were steady gene transniitters. As such, the lentiviral vector
transduced HAC
of the invention can provide for steady gene transmission, which may be useful
in the
prophylaxis or treatment of CNS diseases.
EXAMPLE III
Lentiviral. vectors-mediated siGFP suppression in HAC
HAC were plated on a 6-well plate (2 x 105 cells/well, Costar) aild polybrene
(Sigma -
Aldrich) was added to the wells. With a final concentration of 8 g/ml,
lentiviral vectors
(pLVTHM and pLVTHMsiGFP) were added (MOI =100). EGFP expression was visualized
by fluorescent microphotograph. and analyzed by fluorescence-activated cell
sorting (FACS).
To measure the relative expression of EGFP, a semi-quantitative PCR for EGFP
and J3-actin
(internal reference) analysis was performed with PCR amplification for 28
cycles. Primer
sequences were EGFP (upstream 5'-cgagctggacggcgacgtaaac-3' and downstream 5'-
gcgcttctcgttggggtctttg-3') and 0-actin (upstream 5'-aacgagcggttccgatgccctgag-
3' and
downstream 5 ' tgtcgccttcaccgttcca.gtt 3'). Amplification conditions were 94 C
for 5 minutes,
28 cycles of 94 C for 1 minute, 58 C for 1 minute, 72 C for 1 minute aud 72 C
for 10
minutes. The expected length of the EGFP PCR products was 597 bp. The
expression
difference was normalized by the respective (3-actins (590 bp). Ten
microliters from each
PCR product were loaded on a 1.5 % agarose gel containing 0.5 g/ml of
ethidium bromide
and separated by electrophoresis.
Results
RNA interference was the best gene-silencing pathway of RNIA mediated gene
regulation in post-transcription. Expression of siRNAs delivered by lentiviral
vectors can be
used to functionally silence EGFP expression in HAC. A sequence of siGFP in
the lentiviral
vector (PLVTHMsiGFP) was transcripted into dsRNA, which mediates, sequence
specific
cleavage with the formation of a ribonucleoprotein complex. In the first
experiment, the
ability of lentiviral vector PLVTHM to express EGFP in HAC, (Fig. 3A), was
probed. MOI
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
= 100 ensured good rates of transduction. The HAC-EGFP cells transduced with
PLVTHM
had strong EGFP expression irrespective of culture conditions. In contrast,
HAC-siGFP cells
cotransduced with the constitutively active PLVTHM and PLVTHMsiGFP exhibited a
strong
down regulation of EGFP, (Fig. 3C). FACS results demonstrated that PLVTHMsiGFP
silenced EGFP expression to less than 15 % in HAC-siGFP cells, (Fig. 3B).
Based on RT-
PCR results, it was found that inhibition was posed in the mRNA level, (Fig.
3D).
EXAMPLE IV
Lentiviral vectors based Cre-loxP systein delete EGFP transduced into HAC
HAC were plated on a 6-well plate (2 x 145 cells/well, Costar) and polybrene
(Sigma-
Aldrich) was added to the wells. With a final concentration of 8 g/ml,
lentiviral vectors (PWPT
and PWPT-Cre) were added (MOI = 100). Transduced HAC were cultured for 1
weelc. EGFP
expression was visualized by fluorescent microphotograph and analyzed by
fluorescence-activated
cell sorting (FACS). Genomic DNA was isolated as described by, for example,
Promega. To
measure the relative expression of EGFP, a semi-quantitative PCR for EGFP and
0-actin (internal
reference) analysis was performed with PCR amplification for 28 cycles. Primer
sequences were
EGFP (upstream 5'-cgagctggacggcgacgtaaaa-3' and downstream 5'-
gcgcttctcgttggggtctttg-3') and
P-actin (upstream 5'-aacgagcggttccgatgccctgag-3' and downstream 5'-
tgtcgccttcaccgttccagtt 3').
Amplification conditions were 94 C for 5 minutes, 28 cycles of 94 C for 1
minute, 58 C for 1
minute, 72 C for 1 minute and 72 C for 10 minutes. The expected length of the
EGFP PCR
products was 597 bp. The expression difference was normalized by the
respective (3-actins (590 bp).
Ten microliters from each PCR product were loaded on a 1.5 % agarose gel
containing 0.5 g/ml of
ethidium bromide and separated by electrophoresis.
Cre is a member of the integrase family of site-specific recombinases that
catalyzes
recombinationbetween loxP DNA elements. Sternberg et al., J. Mol. Biol., 1981,
150: 467-86. An
attempt was made to create a line of HAC in which Cre catalyzed loxP-flanked
specific gene
deletions based on lentiviral vectors. For example, there is a loxP in the
3'LTR of lentiviral vectors
and, after reverse transcription, loxP integrated into both ends of LTR. Cre
catalyzed the deletion of
loxP-flanked lentiviral fragments in the genome of HAC.
Results
21
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
FACS results demonstrated that EGFP was silenced to less than 10 %, (Fig. 4A,
4B
and 4C). Moreover, it was also found that this deletion was posed in the
genomic DNA,
(Fig.4D). As such, the specific gene with PWPT-Cre in HAC can be lrnoclced
out.
EXAMPLE V
Lentiviral vector based doxycycline-inducible systems regulate EGFP expression
HAC were plated on a 6-well plate (2 x 105 cells/well, Costar) and polybrene
(Sigma-Aldricli) was added to the wells. With a final concentration of 8
g/ml, lentiviral
vectors (pLVTH1VI and lenti-tTRKRAB) were added (MOI = 100). After 48 hours of
incubation, doxycycline (final concentration of 5gg /ml, Sigma) was added to
PLVTHM and
the lenti-tTRKRAB cotransduced cells.
Results
During the absence of doxycycline, the tTR-KRAB protein binds specifically to
tetO,
providing a means for suppression of the activity of the nearby promoter (up
to 31cb from its
DNA-binding site). Conversely, in the presence of doxycycline, tTR-KRAB was
sequestered away from tetO, thereby permitting gene expression. Lentiviral
vector mediated
doxycycline-induced system was tested for the doxycycline induced regulation
of EGFP in
HAC, after EGFP was observed in PLVTHM transduced HAC, (Fig. 5A). The tTRKRAB
based on lentiviral vectors suppressed the expression of EGFP in the absence
of doxycycline,
(Fig. 5B). In contrast, addition of doxycycline to the dually transduced cells
resulted in
EGFP (re)expression, (Fig. 5C).
EXAMPLE VI
Biosafety detection
To detect the intergrated vector in HAC, the cells were transduced with PWPT
for 3
days and washed intensively. Thereafter, the medium was collected and filtered
through a
0.45 gm filter. Hela cells with the medium were cultured for 3 days and
genomic DNA of the
Hela cells and HAC were isolated as described by, for example, Promega.
Genomic DNA
(100 ng) was then subjected to PCR using primers EGFP (upstream 5'-
cgagctggacggcgacgtaaac,--3' and downstream 5'-gcgcttctcgttggggtctttg-3') and 0-
actin
(upstream 5'-aacgagcggttccgatgccctgag-3' and downstream 5'-
tgtcgccttcaccgttccagtt 3') as
.22-
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
well as primers homologous to the HIV-1 gag gene (upstream 5'-
gagtatctgatcatactgtcctac-3'
and downstream 5'-ggaactactagtacccttcaggaa-3'). Amplification conditions were
94 C for 5
minutes, 28 cycles of 94 C for 1 minute, 58 C for 1 minute, 72 C for 1 minute
and 72 C for
minutes. The expected length of EGFP and gag products was 597 bp and 912 bp,
5 respectively. The expression difference was normalized by the respective (3-
actins (590 bp).
Ten microliters from each RT-PCR product were loaded on a 1.5 % agarose gel
containing
0.5 ggJml of ethidium bromide and separated by electrophoresis.
Results
10 It was fou.nd that EGFP integrated well into the HAC and EGFP did not exist
in the
genome of the Hela cells, (Fig. 6A). GAG, a structure protein in lentiviruses,
which can be
necessary for virus formation, was not detected in the EGFP expressed HAC or
Hela cells,
(Fig. 6B). These results demonstrated that lentiviral infected HAC can have a
checlc-up for
biosafety before transplantation.
EXAMPLE VII
HAC iuifected and transplanted into cerebral ischemic rats
Lenti-GDNF (MOI = 50) was added to DMEM F 12 cultured HAC on the following
day for 2 days in the presence of 8 g/ml polybrene (Sigma-Aldrich). After
growing for 5
days in a DMEM-F12 medium without fetal calf serum, HAC were washed with PBS
and
then incubated in 0.25 % trypsin (Sigma)/0.05 % DNase I (Sigma)/PBS at 37 C
for 20
minutes. The cells were rinsed 2-3 times with 0.05 % DNase I/PBS and
mechanically
dissociated into a single cell suspension. An aliquot of the cell suspension
was assessed with
regard to cell viability (trypan blue) and concentration. The viability of the
cell suspension
prior to grafting was more than 95 %. Using a stereotaxic frame (Narishige)
and a 26- gauge
Hamilton syringe, 8 x 105 GDNF modified HAC (HAC-GDNF) in 5 l of PBS were
injected
into the right dorsolateral striatum of MCAo rats. Markgraf et al., Brain
Res., 1992, 575(2):
238-46. Proximally, the injection was 4 mm beneath the skull surface, 1 mm
posterior and 3
mm lateral to the bregma over a 10-minute period, which approximated the
ischemic
boundary zone. Anterior-posterior (AP) = -1 mm, medial-lateral (ML) = 3 mm and
dorsal-
ventral (DV) = 4 mm from the bregma.
23
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
Three rats of each group were anesthetized and sacrificed with excess
phenobarbital
16 days after the surgery. Their brains were carefully removed and sliced to 2
inm slices
using a mold. The slices were stained with 2,3,5-triphenyltetrazolium chloride
(TTC, 2 %
solution in PBS) for 30 minutes. The slices were also photographed with an
image
acquirement (Leica) system. Iruage analysis software AutoCAD (AutoDesk) was
used for au
estimation of the infracted volume. The results were expressed as a percentage
of the
hemisphere.
Results
In all the groups, the infarct volume decreased from day 2 to day 16. Two days
after
MCAo, there were no significant differences among the two groups. 16 days
after MCAo,
there was a significant reduction in the percentage of infarct volume in the
HAC-GDNF
group compared to rats in the control PBS group, (Fig. 7).
Immunohistochemistry detection
For GDNF and MAP2 staining 16 days after transplantation, 3 rats of each group
were
killed and examined by immunohistochemistry. The brains of the rats were fixed
with 4 %
paraformaldehyde fixative for 2 days. After which, 30 gm of frozen sections
(near the
injection tract) were cut with a cryostat at -20 C and subjected to
immunohistochemistry.
The sections were rinsed 3 tiunes in PBS (pH 7.4). Endogenous peroxidase
activity was
quenched with H20Z (0.3 %) for 30 minutes. After blocking with 10 % normal
goat serum or
horse serum for one night in 4 C, the slides were incubated for 48 hours at 4
C with a first
antibody specific against MAP2 (1:200, Sigma M4403) and GDNF (1:100, Santa
Cruz SC-
9010). The sections were then rinsed 3 times in PBS (pH 7.4), followed
bybiotin conjugated
antimouse or antirabbit IgG (Vector Laboratories). Thereafter, sections were
washed in PBS
and incubated with an avidin-biotin-horseradish peroxidase complex. The
preparations were
also stained using a Vectastain ABC kit (Vector Laboratories). Lastly, the
slide was colorized
with diaminobenzidine (DAB). The immunohistochemical studies were repeated at
least
three times.
Results
24
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
GDNF engineered HAC (HAC-GDNF) were injected into the lateral striatum and
cortex of
MCAo rats and the grafts were detected by immunohistochemistry in the brains
of the
subjects. There were no positive signals for'GDNF in the cortical area of the
normal
nontransplanted rat (not shown). GDNF-positive cells were detected in the
injection tract of
the ischemic rats brains including in the cortex and striatum. A number of
GDNF positive
cells were also found in HAC transplanted MCAo rats, (Fig. 8). 16 days after
transplantation,
MAP2 positive cells were found in the injection tracts, (Fig. 9),
demonstrating that the HAC
have the potential to differentiate into neurons.
Neurological examination
The neurological findings were scored on a modified scoring system that was
developed by Longa et al. For example, a score of 0 indicates no neurological
deficits, 1
indicates that the rat had difficulty in fully extending the contralateral
forelimb, 2 indicates
that the rat could not extend the contralateral forelimb, 3 indicates a mild
circling to the
contralateral side, 4 indicates a severe circling to the contralateral side
and 5 indicates falling
to the contralateral side. The severity of neurological deficits was observed
in the three stroke
groups. Treatment was examined and analyzed statistically by mean :LSE with a
significance
level of P < 0.05.
No neurological deficits were observed before MCAo. Rats in the strolce groups
(stroke with PBS and stroke with GDNF transduced HAC) were examined at 4 time
points
after cell transplantation, up to 16 days.
Results
The neurological findings scored on a six-point scale were demonstrated and
data
indicated significant differences in the two groups, (Fig. 10). Follow-up
comparison analyses
revealed that there was significant difference between HAC-GDNF groups and the
PBS
group (p < 0.05) in the fourth day. These results indicate that HAC-GDNF
significantly
reduced the severity of neurological deficits for rats especially in the early
stage.
Moving test (beam-walking test)
The beam walking test was described by Ohlson et al. The beam was 1750 mm long
and 19 mm wide. The beam was placed 700 mm above the floor. A wall was
alternately
- 25
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
placed 2 cm near the beam (rats are more willing to wallc when a wall is
placed next to the
beam). Scoring was from 0 to 6. In particular, for 0, the rat falls down, for
1, the rat is unable
to traverse the beam, but remains sitting across the beam, for 2, the rat
falls down while
wallcing, for 3, the rat can traverse the beam, but the affected hindlimb does
not aid in forward
locomotion, for 4, the rat traverses the beam with more than 50 % footslips,
for 5, the rat
crosses the beam with a few footslips and for 6, the rat crosses the beam with
no footslips.
When the rat walked on the beam, such scoring was conducted. Treatinent was
examined and
analyzed statistically by mean +SE with a significance level ofP < 0.05.
Results
Performance of the beam-walking test sllowed differences among the tliree
animal
groups at four time points from day 4 to day 16, after traiisplantation.
Statistically significant
improvement effects were detected in HAC-GDNF transplantation over time, (Fig.
11).
Furthermore, the performances of the HAC-GDNF groups were significantly better
than the
control group in the early stage. Moreover, impaired coordination function in
the non-treated
ischemic rats (control group) did not recover.
These results suggest that HAC produced GDNF can rapidly rescue the deficits
of a
subject after MCAo and HAC also have a significant role in the following
recovery period.
These results may be caused by the neurotrophic factors or anti-inflammation
factors secreted
by HAC as well as differentiation to neuronal cells.
EY_AMPLE VIII
Preparation of cell injection
As described herein, HAC-GDNF were prepared and suspended in PBS.
EXAMPLE IX
Grafting HAC infected with PLVTHM-BDNF in rats with Parkinson's disease
PLVTHM BDNF (MOI = 100) was added to DMEM-F12 cultured cells on the
following day for 2 days in the presence of 8 g/ml polybrene (Sigma-Aldrich).
After
growing for 5 days in a DMEM-F12 medium without fetal calf serum, HAC were
washed
with PBS and then incubated in 0.25 % trypsin (Sigma)/0.05 % DNase
I(Sigma)/PBS at 37 C
for 20 minutes. The cells were rinsed 2-3 times with 0.05 % DNase I/PBS and
mechanically
26
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
dissociated into a single cell suspension. The cell concentration was adjusted
to 1-2 x 108.
Experimental PD was produced in adult rats with the intracerebral injection of
a neurotoxin,
6-hydroxydopamine. The toxin was injected in the medial forebrain bundle of
one side of a
rat brain under stereotaxic guidance. Three weelcs later, the subjects were
tested with
apomorphine, which causes aberrant rotation behavior in recipients with a
successful
biochemical lesion of the nigral-striatal tract. In the cell transplantation
group, 8 x 105 BDNF
modified HAC in 5 l of PBS were injected into the striatum of the 6-OHDA
model of the
PD rat (AP = -5.0 mm, ML = 2.5 mm, DV = -6.5 mm). In the control group, 5 l
of PBS
was injected into the striatum of the 6-OHDA model of the PD rat (AP = -5.0
mm, ML =
2.5 mm, DV =--6.5 mm). Thereafter, at 2, 4 and 8 weelcs later, apomorphine-
induced
rotations were observed.
Results
In the PD rats, HAC infected with PLVTHM-BDNF led to significant reductions in
apomorphine-induced rotations, (Table 1).
Table 1
Comparison of the total apomorphine-rotations in the two groups
Time (week) Cell transplantation group Control group
0 260.2 :0.6 233.3 12.2
2 297.6 5.4 96.5 8.8
4 243.7 2.8 99.4 5.9
8 233.5 8.6 52.9 10.2
EXAMPLE X
Grafting HAC infected with PLVTHM BDNF in a Rhesus monkey with incomplete
dorsal
spinal cord injury
PLVTHM-BDNF (MOI = 80) was added to DMEIVI-F12 cultured cells on the
following day for 2 days in the presence of 8 g/ml polybrene (Sigma-Aldrich).
After
growing for 5 days in a DMEM-F12 medium without fetal calf serum, HAC were
washed
with PBS and then incubated in 0.25 % trypsin (Sigma)/0.05 % DNase
I(Sigma)/PBS at 37 C
for 20 minutes. The cells were rinsed 2-3 times with 0.05 % DNase IUPBS and
mechanically
- 27
CA 02620946 2007-12-21
WO 2006/136114 PCT/CN2006/001437
dissociated into a single cell suspension. The cell concentration was adjusted
to 1-2 X 108. A
rhesus monkey with incomplete dorsal spinal cord injury was studied according
to the Tator
method. Basso et al., J. Neurotrauma, 1995,12(1): 1-21. In the cell
transplantation group, 1
x 10' BDNF modified HAC were injected into the injured position of 2 monkeys.
In the
control group, PBS was injected into the injured position of one monlcey. Two
months later, a
BBB locomotor rating scale was evaluated. Basso et al., J. Neurotrauma,
1995,12(1): 1-21.
Results
The BBB score of the cell transplantation group was 9.7. The BBB score of the
control group was 5.2. As such, the HAC infected with PLVTHM BDNF were shown
to
improve hindlimb motor function of the subject.
While the present invention has been described herein in conjunction with a
preferred
embodiment, a person with ordinary skill in the art, after reading the
foregoing, can effect
changes, substitutions of equivalents and other types of alterations to that
set forth herein.
Each embodiment described above can also have included or incorporated
therewith such
variations as disclosed in regard to any or all of the other embodiments.
Thus, it is intended
that protection granted by Letter Patent hereon be limited in breadth and
scope only by
definitions contained in the appended claims and any equivalents thereof.
28