Note: Descriptions are shown in the official language in which they were submitted.
CA 02623817 2008-03-25
WO 2007/038235
PCT/US2006/036914
THREE SECTION PIG FOR RADIO-PHARMACEUTICALS
BACKGROUND OF THE INVENTION
[0001] Field of the Invention
[0002] The present invention relates to medical devices and, more
specifically, to a
device for transporting radio-pharmaceuticals used in positron emission
tomography.
[00031 Description of the Prior Art
[0004] Positron emission tomography (PET) imaging is a diagnostic
examination that
involves acquiring physiologic images based on the detection of positron
radiation.
Positrons are particles emitted from radioactive substances. The radioactive
substances
used are injected into a patient and positrons from the radioactive substance
are detected
and imaged by a PET scanner. The resulting images are used to evaluate a
variety of
diseases.
[0005] Pharmaceutical compositions used in PET scans are administered in
liquid form
by injection into the patient. Radio-pharmacists typically calculate a unit
dose based on the
amount of decay that a dose will undergo during transport to the hospital at
which the dose
will be administered. In preparing the dose, the radio-pharmacist places the
dose into a
syringe and then places the syringe into a "pig" that shields those handling
the dose from
the radioactive contents of the syringe. The pig is then transported to the
hospital for
administration to a patient.
[0006] With many types of radio-pharmaceuticals, a lead pig is sufficient
to shield
those handling the dose. However, for radio-pharmaceuticals designed for PET
scans, a
typical lead-shielded pig does not provide sufficient protection by itself. To
compensate,
existing PET radio-pharmaceutical transport systems require an extra level of
shielding.
This is accomplished by providing a secondary shielded case for the pig. Such
a shielded
case includes lead shielding about a cylindrical opening into which the pig
fits.
CA 02623817 2008-03-25
WO 2007/038235
PCT/US2006/036914
[0007] Existing PET radio-pharmaceutical pigs tend to need to be replaced
on a regular
basis. This is because the shielding quality of lead breaks down in the
presence of PET
pharmaceutical radiation. Thus, the cost is increased. Also, there is a danger
of
insufficient protection if a radio-pharmacist continues to use a pig past its
designed life
span.
[0008] Because most pigs are roughly cylindrical in shape, rolling of such
pigs is a
problem. If a pig is allowed to roll, it could roll off of the surface on
which it is placed and
fall, causing injury, destroying the dose of the radio-pharmaceutical
composition contained
therein, or both. Some existing pigs include a flat surface milled into the
outer surface of
the pig. The milling is done by removing several exterior chords of the
cylinder forming
the pig, thereby forming flat surfaces. Also, several small evenly-spaced
bumps may be
added to the exterior surface of the pig. Both of these methods of preventing
rolling may
be satisfactory for ordinary conditions, but they do not provide a sufficient
anti-roll
capability in situations in which a pig is accidentally bumped with
considerable force.
Furthermore, most pigs include two sections that must be separated when
accessing the
syringe inside. However, many existing pigs usually include anti-roll
texturing on only one
section. Thus, if the section without the anti-roll texturing is placed on a
table, it may roll
off and cause injury.
[0009] Typical radio-pharmaceutical pigs have an inner chamber into which a
filled
syringe is placed. A plastic liner is frequently employed to prevent spillage
from the
syringe from accumulating inside the pig. Such a liner is typically made from
rigid plastic
and used only once. Because the liner is rigid, it takes up a considerable
amount of space
to store and to dispose of.
[0010] Typically, when administering a radio-pharmaceutical to a patient,
the syringe is
removed from the pig and placed in a shielded holder that protects the
physician's hands
from radiation while injecting the radio-pharmaceutical into an IV bag. The
transfer of the
syringe causes a brief exposure to the user and increases the risk that the
syringe could fall
and be harmed.
2
CA 02623817 2008-03-25
WO 2007/038235
PCT/US2006/036914
[0011] Therefore, it would be desirable for a radio-pharmaceutical pig to
be capable of
providing a mechanism for administering a radio-pharmaceutical without having
to transfer
it to a shielded holder.
SUMMARY OF THE INVENTION
[0012] The disadvantages of the prior art are overcome by the present
invention which,
in one aspect, is a radio-pharmaceutical pig for transporting a syringe
containing a radio-
pharmaceutical. A first cylindrical member includes a first tungsten body
defining a first
cavity therein opening to a first end. A second cylindrical member includes a
second
tungsten body defining a second cavity therethrough and includes a proximal
end and an
opposite distal end. The proximal end is capable of engagement with the first
end of the
first cylindrical member so that the first cavity is in substantial alignment
with the second
cavity. An anti-roll structure extends outwardly from a portion of the second
cylindrical
member. The second cylindrical member also includes an external stainless
steel sleeve
covering a portion of the second cylindrical member. The stainless steel
sleeve includes a
gripping surface cut into the second external stainless steel sleeve. A third
cylindrical
member includes a third tungsten body defining a third cavity opening to a
primary end.
The primary end is capable of engagement with the distal end of the second
cylindrical
member so that the third cavity is in substantial alignment with the second
cavity, wherein
the first cavity, the second cavity and the third cavity are shaped so as to
be complimentary
in shape of the syringe.
[0013] In another aspect, the invention is a method for using a three
section pig. The
pig includes a first member, a second member and a third member. A radio-
pharmaceutical
filled syringe, having a needle extending therefrom, is placed in the pig. The
second
member is disengaged from the first member and the third member. The second
member is
gripped with the syringe disposed in the second member so that the needle
extends from
one end of the second member. At least a portion of the radio-pharmaceutical
is injected
into a receptacle.
[0014] These and other aspects of the invention will become apparent from
the
following description of the preferred embodiments taken in conjunction with
the following
3
CA 02623817 2008-03-25
WO 2007/038235 PCT/US2006/036914
drawings. As would be obvious to one skilled in the art, many variations and
modifications
of the invention may be effected without departing from the spirit and scope
of the novel
concepts of the disclosure.
BRIEF DESCRIPTION OF THE FIGURES OF THE DRAWINGS
[0015] FIG. 1 is a top perspective view of one illustrative embodiment of
the
invention.
[0016] FIG. 2A is an elevational view of one embodiment.
[0017] FIG. 2B is a cross-sectional view of the embodiment shown in FIG.
2A, taken
along line 2B-2B.
[0018] FIG. 2C is an expanded cross-sectional view of the embodiment shown
in FIG.
28.
[0019] FIG. 2D is a bottom plan view of the embodiment shown in FIG. 2A.
[0020] FIG. 2E is a bottom plan view of an alternate embodiment of the
invention.
[0021] FIG. 2F is a cross sectional view of a detail of the embodiment
shown in FIG.
2B.
[0022] FIG. 3 is a plan view of a plastic insert.
[0023] FIG. 4A is a cross-sectional view of the insert shown in FIG. 3,
taken along line
4-4.
[0024] FIG. 4B is a second cross-sectional view of the insert shown in FIG.
3, taken
along line 4-4.
[0025] FIG. 5 is a cross-sectional view of an insert disposed within a pig.
4
CA 02623817 2008-03-25
WO 2007/038235
PCT/US2006/036914
[0026] FIG. 6 is a plan view of a plastic insert including stiffening ribs.
[0027] FIG. 7 is a side view of a roll of plastic inserts.
[0028] FIG. 8 is a plan view of a strip of plastic inserts.
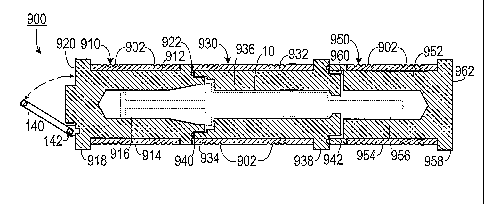
[0029] FIG. 9 is a cross sectional view of a three section pig.
[0030] FIG. 10 is a cross sectional view of the center section of a three
section pig as it
is ready for administration of a radio-pharmaceutical.
[0031] FIG. 11 is a plan view of the center section shown in FIG. 10 being
employed.
DETAILED DESCRIPTION OF THE INVENTION
[0032] A preferred embodiment of the invention is now described in detail.
Referring
to the drawings, like numbers indicate like parts throughout the views. As
used in the
description herein and throughout the claims, the following terms take the
meanings
explicitly associated herein, unless the context clearly dictates otherwise:
the meaning of
"a," "an," and "the" includes plural reference, the meaning of "in" includes
"in" and "on."
[0033] As used herein, "plastic" means capable of being deformed without
rupture and
a "plastic material" includes materials that are deformable. Plastic
materials, as used
herein, include, but are not limited to, synthetic polymer materials, natural
latex materials,
thin metal sheets and combinations thereof. It will be readily understood that
many other
materials, not specifically listed herein, will meet the criteria for being a
plastic within the
scope of the present invention.
[0034] As shown in FIG. 1, one illustrative embodiment of a radio-
pharmaceutical pig
100 includes a tungsten cylinder 110 having a first elongated member 112 and a
second
elongated member 114. The outer surface of the first elongated member 112 and
the
second elongated member 114 includes a rough textured portion 120 that
facilitates
CA 02623817 2008-03-25
WO 2007/038235
PCT/US2006/036914
gripping of the pig 100 by a user. Typically, the pig 100 is formed from
titanium stock and
formed on a metal lathe using conventional methods.
[0035] As shown in FIGS. 2A-2C, the first elongated member 112 terminates
in a first
engagement surface 150 and an opposite first distal end 118, the second
elongated member
114 terminating in a second engagement surface 152 that is complimentary to
the first
engagement surface 150 and an opposite second distal end 116. The pig 100
defines an
elongated cavity 113 therein that is substantially coaxial with the tungsten
cylinder 110.
The cavity 113 is of sufficient size to receive a syringe 10 therein. The
tungsten cylinder
110 is thick enough to shield users from a PET radio-pharmaceutical without
requiring
additional shielding. The actual thickness may be calculated easily by
referring to standard
radiological shielding tables and depends on the type and amount of radio-
pharmaceutical
being used.
[0036] A recess 160 may be milled into either the first engagement surface
150 or the
second engagement surface 152 (or both) to receive the finger grip tabs of the
syringe 10,
thereby preventing the syringe 10 from rocking during transport. An 0-ring 154
may be
embedded in one of the engagement surfaces 150 or 152 to prevent leakage from
the pig
100.
[0037] Returning to FIG. 1, a first anti-roll member 132 extends outwardly
from the
first distal end 118 and a second anti-roll member 130 extends outwardly from
the second
distal end 116. The first anti-roll member 132 and the second anti-roll member
130 each
include at least three (and in the embodiment shown, four) flat surfaces 134
that inhibit
rolling. Including an anti-roll member 132 and 130 on each of the first
elongated member
112 and the second elongated member 114, ensures that neither member will roll
if left
unattended on a flat surface. This may be a substantial advantage, given that
each
elongated member 112 and 114 will likely be quite heavy due to the thickness
of the
tungsten employed.
[0038] The second distal end 116 is supplied with a lifting ring 140 that
facilitates
lifting of the pig 100 out of any carrying container (not shown) used to
transport the pig
100. The lifting ring may be affixed to the top surface 136 of the pig 100
with an
6
CA 02623817 2008-03-25
WO 2007/038235 PCT/US2006/036914
attachment 146 and a spacing plug 142 may be affixed to the top surface 136
inside the ring
140 when the ring 140 is in the down position.
[0039] As shown in FIG. 2D, the anti-roll members 132 are blocks of
titanium that
could include four flat sides 134, or, as shown in FIG. 2E, only three flat
sides 134, or even
more than four flat sides 134, so long as the flat side 134 has dimensions
sufficient to
prevent rolling. The anti-roll members 132 may be formed from the same
titanium stock as
the rest of the pig 100.
[0040] As shown in FIG. 2F, a stainless steel sleeve 220 may disposed about
a portion
of the tungsten cylinder 110. The textured portion 222 may be cut into the
stainless steel
sleeve 220 and may include diamond patterned scoring.
[0041] As shown in FIGS. 3-5, a disposable plastic insert 300 is disposed
within the
cavity 113 to prevent leakage of radio-pharmaceutical materials into the
cavity 113. Each
insert 300 includes an elongated plastic envelope 310 made from a first
plastic sheet 309
and an oppositely-disposed second plastic sheet 311 that are sealed together
along a sealing
surface 312 (through thermal sealing, for example) and that open to a top side
316, thereby
defining passage therein 318. The passage is of sufficient size to allow a
syringe 10 to fit
therein. The disposable plastic insert 300 could be made of materials
including
polyethylene and polyvinyl chloride, but should be thick enough to resist
punctures from
any exposed needles placed into the insert 300.
[0042] A first adhesive tab 320 is placed on the first sheet 309 adjacent
the top side 316
and a second adhesive tab 320 is placed on the, second sheet 311 adjacent the
top side 316.
The first adhesive tab 320 and the second adhesive tab 320 each include a peel-
off cover
that may be peeled off to allow exposure of the adhesive tabs 320 so as to
facilitate sealing
the top side (as shown in FIG. 4B). This facilitates easy sealing of the
syringe 10 for
disposal after use.
[0043] As shown in FIG. 6, the disposable plastic insert 300 may also
include a
plurality of elongated rib structures 610 embedded in one of the plastic
sheets 310 and 311
to provide structural support to the plastic insert 300. The elongated ridge
structures 610
7
CA 02623817 2008-03-25
WO 2007/038235 PCT/US2006/036914
could include wires embedded in one of the plastic sheets 310 and 311, or
could be
thickened plastic that is molded into the plastic sheets 310 and 311 using
commonly-known
plastic sheet forming methods.
[0044] As shown in FIG. 7, because the inserts 300 are flexible, a
plurality of inserts
300 may be formed continuously and stored in the form of a roll 700. This
facilitates easy
manufacturing and storage of the inserts. As shown in FIG. 8, when the inserts
are formed
as a continuous strip 800, a serration 810 may be cut in the sealed portion
between each
successive insert 300 in the strip 800 to facilitate separation of the inserts
300.
[0045] A three section embodiment is shown in FIGS. 9-11. As shown in FIG.
9, the
radio-pharmaceutical pig 900 includes a first cylindrical member 910, a second
cylindrical
member 930 and a third cylindrical member 950. The first cylindrical member
910
includes a first tungsten body 912 defining a first cavity 916 that opens to a
first end 922.
A first anti-roll structure 918 extends outwardly from a second end 920. The
first
cylindrical member 910 also includes a first external stainless steel sleeve
914 that covers a
portion of the first cylindrical member 910. Cut into the first external
stainless steel sleeve
914 is a gripping surface 902, which could be a diamond pattern scored
surface.
[0046] The second cylindrical member 930 includes a second tungsten body
932 that
defines a second cavity 936 therethrough. T he second cylindrical member 930
includes a
proximal end 940 and an opposite distal end 942. The proximal end 940 is
capable of
engaging (such as with complimentary threading, etc.) the first end 922 of the
first
cylindrical member 910 so that the first cavity 916 is in substantial
alignment with the
second cavity 936. A second anti-roll structure 938 extends outwardly from a
portion of
the second cylindrical member 930. As demonstrated in FIG. 11, the second anti-
roll
structure 938 also prevents slippage of the second cylindrical member 930 when
it is being
used to shield the user during delivery of the radio-pharmaceutical. Returning
to FIG. 9,
the second cylindrical member 930 also includes a second external stainless
steel sleeve
934 that covers a portion of the second cylindrical member 930, with a
gripping surface
902 cut into the second external stainless steel sleeve 934.
8
CA 02623817 2013-05-10
CA. 2,623,817
Agent Ref. 73673/00002
1 100471 The third cylindrical member 950 includes a third tungsten
body 952 that defines a
2 third cavity 956 that opens to a primary end 960. The primary end 960 is
capable of engaging
3 the distal end 942 of the second cylindrical member 930 so that the third
cavity 956 is in
4 substantial alignment with the second cavity 936. A third anti-roll
structure 958 extends
outwardly from a secondary end 962 of the third cylindrical member 950. The
third cylindrical
6 member 950 also includes a third external stainless steel sleeve 954 that
covers a portion of the
7 third cylindrical member 950 with a gripping surface 902 cut into the
third external stainless
8 steel sleeve 954. The first cavity 916, the second cavity 936 and the
third cavity 956are shaped
9 so as to be complimentary in shape of the syringe 10.
11 100481 As shown in FIG. 10, the second cylinder member 930 may be
disengaged from the
12 first cylinder member 910 and the third cylinder member 950. The second
cylinder member 930
13 has dimensions that allow it to be used to shield the syringe 10 while
the syringe 10 is being used
14 to deliver the radio-pharmaceutical to a receptacle. This is shown in
FIG. 11, wherein the
physician 14 grips the second cylinder member 930 with the syringe 10 disposed
therein. The
16 needle 12 extends from the second cylinder member 930 so that the
physician 14 can inject the
17 contents of the syringe 10 into a receptacle 16 (such as an injection
port of an IV bag).
18
19 100491 The scope of the claims should not be limited by the
preferred embodiments set forth
in the examples, but should be given the broadest interpretation consistent
with the description as
21 a whole.
21750739.2 9