Note: Descriptions are shown in the official language in which they were submitted.
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
ESTRIOL THERAPY FOR AUTOINIlVIUNE AND NEURODEGENERATIVE
DISEASES AND DISORDERS
[0001] This invention was made with Government support under Grant No. NS
36680 awarded by the National Institute of Health and the National Multiple
Sclerosis
Society Grant Nos. JF 2094, RG 3016, RD3407 and CA1028. This invention was
also made under Grant No. NS 45443 from the National Institutes of Health-
National
Institute of Neurological Disorders and Stroke Grant. The government has
certain
rights in this invention.
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0002] This invention relates generally to steroidal therapies for treating
autoimmune diseases, and, more particularly, to administering primary agents
being
estrogens or estrogen receptor active agents for the treatment of cell
mediated
diseases. Optionally, secondary agents which effect the immune and/or nervous
system may also be co-administered or tapered onto. This therapy may be used
in
patients, including post-partum patients. This invention also relates to
steroidal
therapies for the treatment of neurodegenerative diseases and disorders,
including cell
mediated diseases. Finally, treatment kits are provided containing at least
one
primary agent and at least one secondary agent for treating a patient
presenting with
syinptomology of an autoimmune disease or a neurodegenerative disease or
disorder.
2. General Background and State of the Art
[0003] There is a distinct female preponderance of autoimmune diseases during
the reproductive ages including multiple sclerosis (MS), rheumatoid arthritis
(RA),
uveitis, myasthenia gravis (MG), Sjogren's syndrome, and Hashimoto's
thyroiditis.
[0004] For example, MS is a chronic, and often debilitating disease affecting
the
central nervous system (brain and spinal cord). MS affects more than I million
people
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
worldwide and is the most common neurological disease among young adults,
particularly woman. The exact cause of MS is still unknown. MS attacks the
nervous
system resulting in myelin sheaths surrounding neuronal axons to be destroyed.
This
demyelinization can cause weakness, impaired vision, loss of balance, and poor
muscle coordination. MS can have different patterns, sometimes leaving
patients
relatively well after episodes of acute worsening, sometimes leading to
progressive
disability that persists after episodes of worsening. In the worst cases the
disease can
lead to paralysis or blindness.
[0005] Steroid hormones or sex-linked gene inheritance may be responsible for
the
enhanced susceptibility of women to these autoimmune diseases. A role for
steroid
hormones in susceptibility to autoimmune disease is supported by observations
of
alternations in disease symptomatology, with alterations in sex hormone levels
such
as during pregnancy, menopause or exogenous hormone administration (in the
form
of hormone replacement (HRT) or oral contraceptives (ORC)). For example, women
with MS and RA have been reported to experience remission of symptoms during
late
gestation. Particularly, MS patients have been reported to show a decrease in
relapse
rate in pregnancy.
[0006] Normally, cell-mediated immunity is mediated by T helper cell (Thl)
secretion of interferon gamma (IFN-.gamma.) and tumor necrosis factor beta
(TNF-
b). In contrast, humoral immunity is mediated by another group of T helper
cells
(Th2) secreting interleukin (IL)-10, IL-4, IL-5 and IL-6. A systemic shift
toward
humoral immunity (or Th2-mediated immunity) has been noted during pregnancy.
During pregnancy, cell-mediated immunity is decreased and humoral-mediated
immunity is increased thereby promoting fetal survival. Thus, this systemic
shift in
the immune system may explain why cell-mediated diseases, including MS and RA
have been reported to improve during pregnancy.
2
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0007] Although a shift toward humoral-mediated immunity has been
demonstrated during human pregnancy, mechanisms which induce this shift remain
unclear. One possibility is local production of Th2 (or humoral mediated)
cytokines
by the placenta. Another possibility is the production of Th2 cytokines by
immune
cells, consequent to changed levels of steroid horinones during pregnancy.
Consistent
with the latter possibility, in vitro studies have demonstrated the ability of
the steroid
progesterone to increase IL-4 production and the ability of the steroid
17.beta.-
estradiol to increase IL-10 production during T-lymphocyte responses. However,
it
remains unclear what cellular mechanisms are involved in regulating in vivo
amelioration of autoimmune symptomology.
[0008] Examples of potential candidates which effect may effect MS during
pregnancy include: Sex hormones (estrogens, progesterone), cortisol, vitamin
D,
alpha-fetoprotein, human chorionic gonadotropin and pregnancy specific
glycoproteins.
[0009] Further, some studies have suggested that a unique pregnancy factor
termed
"early pregnancy factor" is responsible for improved progression of cell-
mediated
autoimmune diseases during pregnancy. Other studies have suggested a role for
microchimerism. Still others suggest a role for local factors such as
TGF.beta. or
estriol (E3) which is known to be produced by the placenta during pregnancy.
Of
note, E3 is at its highest serum levels in the third trimester of pregnancy.
However,
E3's role in ameliorating symptoms of autoimmune diseases in humans is
unclear.
[0010] Studies in laboratory animals have established that experimental
autoimmune encephalomyelitis (EAE) and other Thl (cell-mediated) autoimmune
diseases in mice improve during pregnancy.
[0011] Specifically, treatment with late pregnancy levels of estriol or
supraphysiological doses of estradiol (5 times pregnancy levels) were shown to
delay
3
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
the onset of clinical EAE after disease was experimentally induced by
immunization
of mice (Jansson et al. 1994). However, there was no investigation as to how
estrogens delayed the day of onset of disease, nor as to whether disease
severity was
effected in these animals once symptomology occurred.
[0012] In another study, it was shown that EAE disease severity could be
reduced
by treatmeilt with estriol, either before or after disease onset. Treatment of
EAE mice
with 90 day release pellets of 5 milligrams or 15 milligrams of estriol (E3)
was shown
not only to decrease disease severity but also to enhance autoantigen specific
humoral-immunity, increase production of the Th2 cytokine IL-10 and reduced
inflammation and demyelination in EAE mice. Importantly, these changes in the
disease were induced by a dose (5 mg) which was shown to yield estriol levels
in
serum that were similar to those which occur during late pregnancy (Kim et
al.,
Neurology, 50(4 Supp. 4):A242-245, April 1998, FASEB Journal 12(4):A616, March
1998 and Neurology 52(6):1230-1238, April 1999; herein incorporated by
reference).
Thus, these results suggested that steroid hormones, and estriol in
particular, may be
involved in the amelioration of autoimmune reactions in the EAE animal model.
[0013] Other groups later demonstrated that estrogen potentiated the effects
of
treatment with TCR proteins to reduce autoimmune reactions in EAE mice.
Offner, et
al. FASEB Journal 14(6):A1246, April 2000; Int. Journal of Mol. Medicine 6
(Supp.
1): S8, October 2000 and Journal of Clin. Invest. 105(10):1465-1472, May
2000).
Further, it was shown in animal studies that estrogen suppressed the onset EAE
in
mice (Ito, et al. Journal of Immunology, 167(1): 452-52, 2001) and that
presumed
diestrus levels of estrogens reduced some manifestations of active EAE in
mice. Bebo
et al. Journal of Immunology 166(3): 2080-9, 2001.
[0014] However, the etiology and disease progression oftEAE and MS are not
identical, thus it is unclear that estrogens alone would be effective in
ameliorating
4
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
..... ...... ...
autoimmune responses in human patients. Indeed, not only is it unknown whether
pregnancy doses of estrogens might be protective in humans with autoimmune
disease, it is unclear even in mice whether low doses of estrogens are
protective. For
example, it has been reported by some that ovariectomy of female mice makes
EAE
disease worse (Matejuk et al., 2001), while others have found that ovariectomy
had no
effect on disease severity (Kim et al., 2001; Voskuhl and Palaszynski, 2001 a;
Voskuhl
and Palaszynski, 2001b). Thus, it is controversial whether low levels of
estrogens, as
they exist during the menstrual cycle, are protective even in mice.
[0015] Data from human studies to date have shown no clear benefit of steroids
in
treating any autoimmune disease. In humans, administration of available
hormone
therapies (including HRTs and OCPs) containing a mixture of sex hormones cause
some autoimmune diseases to improve while others worsen.
[0016] For example, there has been no conclusive evidence that women are
protected from or have a decrease in symptomology or relapse rates due to sex
steroids. One study noted that past use of oral contraceptives in healthy
women had
no effect on subsequent risk to develop MS (Hernan et al. 2000). Further,
another
study found that the incidence rates for MS in current users were not
decreased as
compared to never-users (Thorogood and Hannaford, 1998). Thus, low dose of the
estrogens in oral contraceptives are not of sufficient type or dose to
ameliorate the
immunopathogenesis of MS even temporarily during intercurrent use. At best, in
one
study, patients had the subjective impression that pre-existing MS symptoms
(as
opposed to relapse rates) worsen during the premenstrual period and that the
use of
oral contraceptives may have decreased this worsening (Zorgdrager and De
Keyser,
1997). Importantly, the lack of reports of an effect of oral contraceptive
therapy on
MS relapses is in marked contrast to what has been observed during pregnancy.
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0017] In contrast, it has been shown that women had a lower the risk of
developing MS during pregnancy compared to non-pregnant states (Runmarker and
Andersen, 1995). Due to the numerous changes that occur during pregnancy,
hormonal and nonhormonal (as listed above), the etiology of the beneficial
effect of
pregnancy may or may be related to sex steroid fluctuations. It has also been
reported
for decades that pregnancy decreases MS relapses (Abramsky, 1994; Birk et al.
1990;
Birk et al, 1998; Damek and Shuster, 1997; Runmarker and Andersen, 1995;
Confavreux et al., 1998). These studies have shown that the latter part of
pregnancy is
associated with a significant reduction in relapses, while there is a rebound
increase in
relapses post partum. In contrast, the absence of such an effect on relapses
during
OCP or HRT indicate that low level sex steroids are not adequate to treat
these
symptoms.
[0018] Further, women having rheumatoid arthritis that were treated with HRT
did
not show significant improvement in their symptomology. DaSilva and Hall,
Baillieres Clinical Rheumatology 1992, 6:196-219; Bijlsma at al. Journal of
Repro.
Imm. 28(3-4):231-4, 1992; Hall et al. Annals of the Rheumatic Diseases, 53(2):
112-
6, 1994.
[0019] Thus, the low doses of hormones found naturally during the menstrual
cycle or in ORT and HRT have not been shown to be effective at ameliorating
the
symptomology of autoimmune diseases. This is in spite of the observation that
women having MS have a decreased relapse rate during late pregnancy. Thus, a
challenge has been to identify a hormone and a treatment dose that is
therapeutic in
treating particular autoimmune diseases, while minimizing undesirable side
effects.
Obviously, the dose and method of administration of steroids in humans differs
from
steroid treatment in laboratory animals due to toxic effects of prolonged
exposure by
patients to steroid hormones. In particular, there are clinical concerns of
inducing
6
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
breast or endometrial cancers in women requiring long term exposure to steroid
hormones.
[0020] The actions of estrogen are mediated primarily by nuclear estrogen
receptors (ER) ER alpha and ER beta, although non-genomic membrane effects
have
also been described previously. Originally it was thought that ER alpha and ER
beta
would each have distinct tissue distributions, thereby providing a means
through
which use of selective estrogen receptor modifiers. However, the relationship
between ER alpha and ER beat became complex, with most tissues expressing some
detectable level of each of these receptors. The two receptors at times did,
and at
other times did not, co-localize to the same cells within a given tissue.
Furthermore,
in some issues the two receptors were shown to act synergistically, whereas in
the
other tissues they act antagonistically. However, any selective effects by ER
alpha
and ER beta on MS and other auto-immune and Neurodegerative diseases have yet
to
fWM4min&rther, the direct and indirect neuroprotective mechanisms by estrogens
in
EAE are not necessarily mutually exclusive, and have yet to be fully explored.
The
finding that estrogens are neuroprotective in EAE, regardless of mechanism,
has
relevance to estrogen treatment in MS, as well as pregnancy, a time when
circulating
estrogens are very high. Indeed, multiple pregnancies have been associated
with a
decrease in long-term disability accumulation in MS (Runmarker and Andersen,
1995; Damek and Shuster, 1997). Because it is known that up to 5 years of
continuous treatment with immunomodulatory treatments are needed to impact
disability in MS, a temporary anti-inflammatory effect of the third trimester
of
pregnancy would not necessarily be expected to improve long-term disability.
While
the efficacy of estrogen treatment appears to depend critically on its
administration
early, as a preventative therapy, before neurodegeneration has occurred
(Mulnard et
al., 2000), this therapeutic measure has yet to be explored.
7
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0022] Further, neurodegenerative diseases and disorders in addition to MS
comprise a substantial clinical problem for which existing treatments have
been
ineffective at ameliorating the clinical symptomology or preventing the
progression of
the disease or disorder.
[0023] Estrogen treatment has been shown previously to be neuroprotective in a
variety of neurodegenerative disease models including Parkinson's disease,
cerebellar
ataxia, stroke, and spinal cord injury (Leranth et al., 2000; Dubal et al.,
2001; Wise et
al., 2001; Jover et al., 2002; Rau et al., 2003; Sierra et al., 2003; Sribnick
et al., 2003,
2005). Estrogens are lipophilic, readily traversing the blood-brain barrier,
with the
potential to be directly neuroprotective (Brinton, 2001; Garcia-Segura et al.,
2001;
Wise et al., 2001). Estrogen-mediated protection of neurons has been
demonstrated in
a variety of in vitro models of neurodegeneration including those induced by
excitotoxicity and oxidative stress (Behl et al., 1995; Goodman et al., 1996;
Behl et
al., 1997; Harms et al., 2001). Estrogens have also been shown to decrease
glutamate-induced apoptosis and preserve electrophysiologic function in
primary
cortical neurons (Sribnick et al., 2003, 2004). In addition, in vitro studies
have
demonstrated the ability of estrogen to modulate the astrocytic response to
injury
(Azcoitia et al., 1999; Garcia-Segura et al., 1999) and protect
oligodendrocytes from
cytotoxicity (Sur et al., 2003; Cantarella et al., 2004; Takao et al., 2004).
However,
the role of estrogen and estrogen receptor subtypes involved neuroprotection
has yet
to be fully explored.
INVENTION SUMMARY
[0024] A general object of the present invention is to provide a method of
administering steroid hormones to mammals to treat autoimmune related
diseases,
more particularly, Thl-mediated (cell-mediated) autoimmune diseases including:
multiple sclerosis (MS), rheumatoid arthritis (RA), autoimmune thyroiditis,
uveitis
8
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
and other autoimmune diseases in which clinical symptomology has shown
improvement during the third term of pregnancy. The method may also include
the
treatment of post-partum patients having been diagnosed with, or at risk for
developing autoimmune diseases, including MS. The method may also include the
treatment of patients having been diagnosed with, or at risk for developing
neurodegenerative diseases, including MS.
[0025] In accordance with one aspect of the present invention, these
objectives are
accomplished by providing a treatment for autoimmune related diseases with a
selected dose and course of a primary agent being an estrogen or estrogen
receptor-
effective composition. The primary agent may include estrogen receptor
selective
ligands, such as agonists which mimic the effect of estrogens.
[0026] In accordance with one aspect of the present invention, these
objectives are
accomplished by providing a patient with a therapeutically effective amount of
estriol,
comprising from about 4 to 16 milligrams per day, or more specifically, about
8
milligrams once daily via oral administration. .
[0027] In accbrdance with another aspect of the present invention, these
objectives
are accomplished by providing a therapeutically effective amount of a primary
agent
in combination with a therapeutically effective amount of a secondary active
agent,
such as progesterone, glucocorticoids and/or known or experimental drugs used
to
treat autoimmune diseases.
[0028] In accordance with one aspect of the present invention, the invention
comprises the use of a primary agent comprising an estrogen receptor alpha
ligand
having anti-inflammatory and/or neuroprotective effects to prevent or
ameliorate
clinical symptoms of autoimmune and/or neurodegenerative diseases or
disorders,
including multiple sclerosis.
9
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0029] In accordance with one aspect of the present invention, the invention
comprises the use of a primary agent comprising an estrogen receptor beta
ligand
having neuroprotective effects to prevent or ameliorate clinical symptoms of
neurodegenerative diseases or disorders, including multiple sclerosis.
[0030] The above described and many other features and attendant advantages of
the present invention will become apparent from a consideration of the
following
detailed description when considered in conjunction with the accompanying
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] FIG. la is a schematic depicting the trial design described in Example
1;
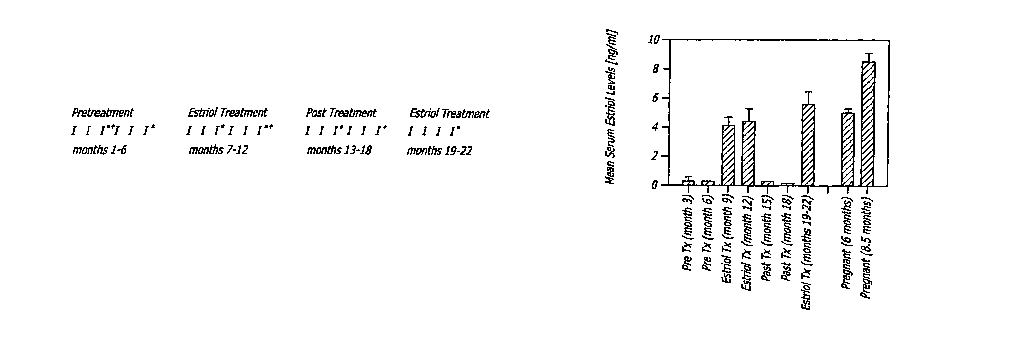
FIG. lb is a bar graph depicting human serum levels during pregnancy, estriol
treatment (Tx), and pretreatment (Pre Tx levels).
[0032] FIG. 2a is a bar graph describing the Delayed Type Hypersensitivity
(DTH)
responses to tetanus and to candida; FIG. 2b is a bar graph depicting levels
of
IFN.gamma. between treatment groups.
[0033] FIG. 3a-f are bar graphs depicting each patient's gadolinium enhancing
lesion volumes on serial cerebral MRIs which were assessed at each month
during the
pretreatment, estriol treatment and post treatment periods.
[0034] FIG. 4 is a bar graph depicting mean percent change in PASAT scores
during treatment with estriol as compared to pretreatment.
[0035] FIGs. 5A-B are bar graphs showing the uterine weights of wild type
(WT),
ER beta knock-out (KO), or ER alpha KO in mice treated with a control
(vehicle),
estrogen receptor alpha ligand (PPT) or estradiol treated animals (y-axis =
uterine
weight in grams).
[0036] FIGs. 6A-C are graphs showing the effect of ER alpha selective ligand
on
clinical scores in wild type (WT), ER beta knock-out (KO), or ER alpha KO in
mice
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
treated with a control (vehicle), estrogen receptor alpha ligand (PPT) or
estradiol
treated animals.
[0037] FIGs. 7A-D are bar graphs showing proinflammatory cytokine production
by peripheral immune cells in ovariectomized, wild type (WT) C57BL/6 female
mice
with EAE.
[0038] FIGs. 8A-E depict various measures of estrogen receptor alpha ligand
reduced inflammation and deinyelination in spinal cords of mice with EAE. FIG.
8A
are thoracic spinal cord sections from normal, or treated mice (vehicle,
estradiol (E2)
or estrogen receptor alpha ligand (PPT)); FIG. 8B depicts luxol fast-blue
stained
magnified regions of the dorsal spinal column for the same sections as shown
in 8A
(40x magnification); FIG. 8C depicts anti-BMP immunostained magnified regions
of
the dorsal spinal column for the same sections as shown in 8A; FIG. 8D is a
bar graph
showing white matter cell density by treatment group; and FIG. 8E is a bar
graph
showing myelin density by treatment group.
[0039] FIGs. 9A-E depict various measures of estrogen receptor alpha ligand
reduced inflammation and demyelination in spinal cords of mice with EAE. FIGs.
9A-D are split images of thoracic spinal cord sections stained with NeuN+
(red) in I
and Nissl in ii at 4x magnification, derived from mice from each treatment
group
(normal, vehicle, estradiol (E2) or estrogen receptor alpha ligand (PPT)).
FIG. 9E is
a bar graph showing the number of NeuN+ immunolabeled neurons in the
delineated
10O40*atteEIGs. 10A-D depict various measures of estrogen receptor alpha
ligand
reduced inflammation and demylination in spinal cords of mice with EAE. FIGs.
l0A
and B are images of thoracic spinal cord sections shown in FIG. 5 co-
immunostained
with NF200 (green) and CD45 (red) at l Ox magnification, derived from mice
from
each treatment group (normal, vehicle, estradiol (E2) or estrogen receptor
alpha
11
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
ligand (PPT)). FIG. 10C is a bar graph showing the axon number and FIG. 10D is
a
bar graph showing Mac-3 cell density measurements.
[0041] FIGs. 1 lA-B are bar graphs showing the uterine weights of wild type
(WT), estrogen receptor alpha ligand (PPT) and estrogen receptor beta ligand
(DPN)
treated animals (y-axis = uterine weight in grams).
[0042] FIGs. 12A-G are graphs showing the effect on clinical scores of wild
type
(WT), estrogen receptor alpha ligand (PPT) and estrogen receptor beta ligand
(DPN)
treated animals.
[0043] FIGs. 13A-C are bar graphs showing the effect of treatment with a
estrogen
receptor selective ligand (DPN), vehicle or estradiol on proliferation or
cytokine
production.
[0044] FIGs. 14A-F depict various measures of estrogen receptor alpha ligand
reduced inflammation and demyelination in spinal cords of mice with EAE. FIG.
14A and C are early and late thoracic spinal cord sections from normal, or
treated
mice (vehicle, estrogen receptor alpha (PPT) or estrogen receptor beta ligand
(DPN));
FIG. 14B depicts early white matter cell density for each treatment group;
FIG. 14D
depicts late white matter cell density for each treatment group; 14 E and F
depict early
and late sections co-immunostained with NF200 (green) and CD45 (red) at l Ox
magnification, derived from mice from each treatment group.
[0045] FIGs. 15A-H depict various measures of estrogen receptor alpha and beta
ligand preservation of MBP and spare axonal pathology in spinal cords of EAE
mice.
FIGs. 15A and C are images of thoracic spinal cord sections stained with NeuN
(red)
lOx magnification, derived from mice at early and late time points from each
treatment group (normal, vehicle, estrogen alpha ligand (PPT) or estrogen
receptor
beat ligand (DPN)). FIGs. 15E and G are images of thoracic spinal cord
sections co-
immunostained with anti-NF200 (green, i) and anti-BMP (red, ii), shown merged
in
12
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
iii, derived from mice at early and late time points from each treatment group
(normal,
vehicle, estrogen alpha ligand (PPT) or estrogen receptor beat ligand (DPN));
FIG. 15
B and D are a bar graphs showing myelin density, early and late, respectively,
while
FIG. 15 F and H show axon number, early and late, respectively.
[0046] FIGs. 16A-D depict various measures of estrogen receptor alpha and beta
ligand preservation of neuronal staining of gray matter in spinal cords of
mice with
EAE. FIGs. 9A-D are split images of thoracic spinal cord sections stained with
NeuN
(red) in I and Nissl in ii at 4x magnification, derived from mice from each
treatment
group (normal, vehicle, estradiol (E2) or estrogen receptor alpha ligand
(PPT)). FIG.
9E is a bar graph showing the number of NeuN+ immunolabeled neurons in the
delineated gray matter.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0047] This description is not to be taken in a limiting sense, but is made
merely
for the purpose of illustrating the general principles of the invention. The
section titles
and overall organization of the present detailed description are for the
purpose of
convenience only and are not intended to limit the present invention.
[0048] Generally, the invention involves a method of treating mammal
exhibiting
clinical symptoms of an autoimmune disease comprising administering a primary
agent at a therapeutically effective dosage in an effective dosage form at a
selected
interval. The treatment is aimed at reducing the symptomology and/or
progression of
the disease. In the preferred embodiment of the invention, human patients
clinically
diagnosed with MS (including both relapsing remitting or secondary progressive
type
patients) are treated with an oral preparation of 8 milligrams estriol daily
and have
ameliorated symptomology.
[0049] Amelioration of the autoimmune disease refers to any observable
beneficial
effect of the treatment. The beneficial effect can be evidenced by a delayed
onset or
13
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
progression of disease symptomology, a reduction in the severity of some or
all of the
clinical symptoms, or an improvement in the overall health.
[0050] For example, patients who have clinical symptoms of an autoimmune
disease often suffer from some or all of the following synlptoms: worsening of
pre-
existing syinptoms (such as joint pain in rheumatoid arthritis), the
appearance of new
symptoms (new joints affected in rheumatoid arthritis) or increased
generalized
weakness and fatigue. MS patients in particular suffer from the following
symptoms:
weakness, numbness, tingling, loss of vision, memory difficulty and extreme
fatigue.
Thus an amelioration of disease in MS would include a reduction in the
frequency or
severity of onset of weakness, numbness, tingling, loss of vision, memory
difficulty
and extreme fatigue. On imaging of the brain (MRI) amelioration of disease
would be
evidenced by a decrease in the number or volume of gadolinium enhancing
lesions, a
stabilization or slowing of the accumulation of T2 lesions and/or a slowing in
the rate
of atrophy formation. Immunologically, an increase in Th2 cytokines (such as
IL- 10)
a decrease in Thl cytokines (such as interferon gamma) would be associated
with
disease amelioration.
[0051] Patients may also express criteria indicating they are at risk for
developing
autoimmune diseases. These patients may be preventatively treated to delay the
onset
of clinical symptomology. More specifically, patients who present initially
with
clinically isolated syndromes (CIS) may be treated using the treatment
paradigm
outlined in this invention. These patients have had at least one clinical
event
consistent with MS, but have not met full criteria for MS diagnosis since the
definite
diagnosis requires more than one clinical event at another time (McDonald et
al.,
2001). Treatment of the present invention would be advantageous at least in
preventing or delaying the development of clinically definite MS.
14
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0052] PRIMARY AGENT. The primary agent useful in this invention is a steroid
hormone, more particularly a estrogen or a steroidal or non-steroidal estrogen
receptor
active agent. Most preferably the primary agent is estriol (estra-1,3,5(10)-
triene-
3,16,17-triol), E3, such as estriol succinate, estriol dihexanate or estriol
sulfinate.
However, the primary agent may be precursors or analogs of estriol (such as
nyestriol), estrone (E1) or precursors or analogs of estrone, 17.beta.-
estradiol (E2) or
precursors (including aromatizable testosterone) or analogs of 17.beta.-
estradiol, or
estranges.
[0053] The primary agent may also be a metabolite or derivatives of E1, E2 or
E3
which are active at the estrogen receptor alpha. or beta. Metabolites and
derivatives
may have a similar core structure to E1, E2 or E3 but may have one or more
different
groups (ex. hydroxyl, ketone, halide, etc.) at one or more ring positions.
Synthetic
steroids which are effective at estrogen receptor are also useful in this
invention, such
as those described in WO 97/08188 or U.S. Pat. No. 6,043,236 to Brattsand,
which is
hereby incorporated by reference herein.
[0054] The primary agent may also be an estrogen receptor alpha. or .beta.,
agonists and/or antagonist. These agonists or antagonists may be steroidal or
non-
steroidal agents which bind to and/or cause a change in activity or binding of
at least
one of the estrogen receptor alpha. or beta. subtypes. For example, specific
agonists
of ER alpha and ER beta may be useful in this invention (Fritzmeier, et al.).
Doses of
these agonists may be titrated to achieve an effect on disease similar to that
which is
observed during pregnancy and during treatment with pregnancy doses of estriol
by
methodologies lcnown to those skilled in the art of steroid pharmacology.
[0055] Any one or combination of these estrogens or estrogen receptor active
agents may be used to treat the selected autoimmune disease. The selection of
the
estrogens or estrogen receptor active agents can be made considering secondary
side
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
effects of the treatment to the patient. For example, estriol may be selected
over
17.beta.-estradiol, because estriol causes minimal endometrial proliferation
and is not
associated with increased risk of breast cancer. Minimal endometrial
proliferation is
observed when the long-acting estriol derivative, nyestriol is used. Indeed,
because
estriol has partial antagonist action on the binding of 17.beta.-estradiol to
the estrogen
receptor in vivo, estriol was at one point in the past considered as a
therapeutic agent
for treatment and prevention of breast cancer.
[0056] THERAPEUTICALLY EFFECTIVE DOSAGE OF THE PRIMARY
AGENT. A therapeutically effective dose of the primary agent is one sufficient
to
raise the serum concentration above basal levels, and preferably to pregnancy
levels
or above pregnancy levels. Most preferably, the therapeutically effective
dosage of
the primary agent is selected to result in serum levels in a patient
equivalent to the
steroid hormone level of that agent in women in the second or third trimester
of
pregnancy.
[0057] For example, during the normal female menstrual cycle estradiol levels
are
in the range of about 350 pg/mi serum. During pregnancy, there is about a 100
fold
increase in the level of estradiol to about 10,000 to about 35,000 pg/mi
serum.
(Correale, et al. Journal of Immunology 161:3365 (1998) and Gilmore, et al.
Journal
of Immunology 158:446). In contrast, estriol levels are undetectable during
the
menstrual cycle in the non-pregnant state. Estradiol levels rise progressively
during
pregnancy to levels from 3,000 to 30,000 pg/mi (3 to 30 ng/ml) (www.il-st-acad-
sci.org/steroidl.html#se3t).
[0058] In one embodiment, where the primary agent is estriol, the preferable
dose
is from about 4 to 16 milligrams daily, and more specifically, about 8
milligrams
daily. In this embodiment, blood serum levels preferably reach at least about
2 ng/ml,
may reach about 10 to about 35 ng/ml, or most preferably about 20-30 ng/ml.
(Sicotte
16
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
et al. Neurology 56:A75). In some embodiments, estradiol (E2) levels would
preferably reach at least about 2 ng/ml and most preferably about to 10-35
ng/ml. In
some embodiments, estrone (E1) levels would preferably reach at least about 2
ng/ml
and most preferably about 5-18 ng/ml (DeGroot and Jameson, 1994).
[0059] The dosage of the primary agent may be selected for an individual
patient
depending upon the route of administration, severity of disease, age and
weight of the
patient, other medications the patient is taking and other factors normally
considered
by the attending physician, when determining the individual regimen and dosage
level
as the most appropriate for a particular patient.
[0060] The use of this group of primary agents is advantageous in at least
that
other known or experimental treatments for cellular mediated autoimmune
diseases
are chemotherapeutic immunosuppresants which have significant risks and side
effects to patients, including decreasing the ability of the patient to fight
infections,
inducing liver or heart toxicity which are not caused by estrogen treatment.
Other
agents used in MS do not cause these side effect, but are associated with flu-
like
symptoms or chest tightness. Further, these previously used agents are
associated with
local skin reactions since they entail injections at frequencies ranging from
daily to
once per week.
[0061] DOSAGE FORM. The therapeutically effective dose of the primary agent
included in the dosage form is selected at least by considering the type of
primary
agent selected and the mode of administration. The dosage form may include the
active primary agent in combination with other inert ingredients, including
adjutants
and pharmaceutically acceptable carriers for the facilitation of dosage to the
patient as
known to those skilled in the pharmaceutical arts. The dosage form may be any
form
suitable to cause the primary agent to enter into the tissues of the patient.
17
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0062] In one embodiment, the dosage form of the primary agent is an oral
preparation (liquid, tablet, capsule, caplet or the like) which when consumed
results in
elevated serum estrogen levels. The oral preparation may comprise conventional
carriers including dilutents, binders, time release agents, lubricants and
disinigrants.
[0063] In otlier embodiments of the invention, the dosage form may be provided
in
a topical preparation (lotion, creme ointment or the like) for transdermal
application.
Alternatively, the dosage form may be provided in a suppository or the like
for
transvaginal or transrectal application.
[0064] That estrogens or estrogen receptor active agents can be delivered via
these
dosage fortns is advantageous in that currently available therapies, for MS
for
example, are all injectables which are inconvenient for the user and lead to
decreased
patient compliance with the treatment. Non-injectable dosage forms are further
advantageous over current injectable treatments which often cause side effects
in
patients including flu-like symptoms (particularly, beta. interferon) and
injection site
reactions which may lead to lipotrophy (particularly, glatiramer acetate
copolymer-1).
[0065] However, in additional embodiments, the dosage form may also allow for
preparations to be applied subcutaneously, intravenously, intramuscularly or
via the
respiratory system.
[0066] SECONDARY ACTIVE AGENTS. Any one or a combination of
secondary active agents may be included in the dosage form with the primary
agent.
Alternatively, any one or a combination of secondary active agents may be
administered independently of the primary agent, but concurrent in time such
that the
patient is exposed to at least two agents for the treatment of their
immunological
disease.
[0067] The secondary agents are preferably immunotherapeutic agents, which act
synergistically with the primary agent to diminish the sytnptomology of the
18
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
patient is exposed to at least two agents for the treatment of their
immunological
disease.
[0067] The secondary agents are preferably immunotherapeutic agents, which act
synergistically with the primary agent to diminish the symptomology of the
autoimmune disease. Secondary active agents may be selected to enhance the
effect of
the estrogen or estrogen receptor active agent, reduce the effect of the
estrogen or
estrogen receptor active agent or effect a different system than that effected
by the
estrogen or estrogen receptor active agent.
[0068] Secondary active agents include immunotherapeutic agents which cause a
change in the activity or function of the immune system.
[0069] In one embodiment, a secondary agent may be a therapeutically effective
amount of progesterone, precursor, analog or progesterone receptor agonist or
antagonist. Most preferably, the secondary agent is 100-200 milligrams of
progesterone administered daily. Progesterone in combination with estrogen or
estrogen receptor active agent treatment is advantageous in at least
protecting patients
against risks associated with long term estrogen exposure, including, but not
limited
to endometrial proliferation and breast cancers.
[0070] In another embodiment, a secondary agent may be a therapeutically
effective amount of glucocorticoid, precursor, analog or glucocorticoid
receptor
agonist or antagonist. For example, prednisone may be administered, most
preferably
in the dosage range of about 5-60 milligrams per day. Also, methyl prednisone
(Solumedrol) may be administered, most preferably in the dosage range of about
1-2
milligrams per day. Glucocorticoids are currently used to treat relapse
episodes in MS
patients, and symptomatic RA within this dosage range.
[0071] In other embodiments, a secondary agent may be selected from the group
immunotherapeutic compounds. For example, as .beta.-interferon (Avonex®
19
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
(interferon-beta la), Rebiff® (by Serono); Biogen, Betaseron®
(interferon-
beta ib) Berlex, Schering), glatiramer acetate copolymer-1 (Copaxone®;
Teva),
antineoplastics (such as mitoxantrone; Novatrone® Lederle Labs), human
monoclonal antibodies (such as natalizumab; Antegren® Elan Corp. and
Biogen
Inc.), immonusuppressants (such as mycophenolate mofetil; CellCept®
Hoffman-LaRoche Inc.), paclitaxel (Taxol®; Bristol-Meyers Oncology),
cyclosporine (such as cyclosporin A), corticosteroids (glucocorticoids, such
as
prednisone and methyl prednisone), azathioprine, cyclophosphamide,
methotrexate,
cladribine, 4-aminopyridine and tizanidine and natalizumab (Tysabri)
[0072] By way of example, which is consistent with the current therapeutic
uses
for these treatments, Avonex® in a dosage of about 0 to about 30 mcg may
be
injected intramuscularly once a week. Betaseron® in a dosage of about 0 to
about
0.25 mg may be injected subcutaneously every other day. Copaxone® in a
dosage of about 0 to about 20 mg may be injected subcutaneously every day.
Finally,
Rebiff® may be injected at a therapeutic dose and at an interval to be
determined
based on clinical trial data. Further, any of these secondary agents may be
used in
increasing, constant or decreasing dose in combination with a primary agent,
such as
estriol or an ER alpha or beta receptor ligand. However, dosages and method of
administration may be altered to maximize the effect of these therapies in
conjunction
with estrogen treatment. Dosages may be altered using criteria that are known
to those
skilled in the art of diagnosing and treating autoimmune diseases.
[0073] Preferably, secondary agents would be administered in the dosage ranges
currently used to treat patients having autoimmune diseases, including MS
patients.
Alternatively, the secondary agents may be administered at a reduced dose or
with
reduced frequency due to synergistic or duplicative physiological effects with
the
primary agent.
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0074] Preferably, patients exhibiting symptomology of autoimmune diseases are
treated with the above agents (estrogen or estrogen receptor active agents
with or
without secondary agents). Most preferably, patients exhibit autoimmune
diseases
marked by improvement in symptomology at least during a treatment regimen,
including but not limited to that reflecting patterns observed during the
second or
third trimester of pregnancy.
[0075] Treatment of Post-Partum Patients. In a recent clinical study, a
dramatic
decrease in the relapse rate during pregnancy, especially in the third
trimester was
noted, with a rebound increase in the three months post partum (such as a
patient who
has given birth, including until the following year from the date of birth).
These data,
in addition to confirmatory animal testing using the EAE model suggest that
sex
steroids have profound effects in autoimmune disease progression and
symptomology,
and could also have an effect on myelinating and re-myelinating the peripheral
and
possibly the central nervous system.
[0076] In another embodiment of the invention, the invention may include
methods of steroidal therapies for preventing or treating female post-partum
patients,
expressing symptoms of or at risk for autoimmune diseases. The invention may
include the method of preventing or treating a subject having been diagnosed
with at
least one symptom of an autoimmune disease to reduce the symptomology of/and
or
slow the progression of the disease. The method according to the invention may
comprise administering primary agents being estrogens or estrogen receptor
active
agents for the treatment of cell mediated diseases. The invention may further
include
the treatment with secondary agents which effect the immune system, which may
be
co-administered or tapered onto. In other embodiments, the use of the primary
agents,
combinations of primary agents with secondary agents, at the doses and in the
dosage
forms may be administered as described above for auto immune diseases.
21
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0077] In one embodiment of the invention, human post-partum patients who are
clinically diagnosed with an autoimmune disease, such as MS (including both
relapsing remitting or secondary progressive type patients) may be treated
with an
oral preparation of 8 milligrains estriol daily, resulting in ameliorated
symptomology.
Additionally, patients could be administered an estriol or an estrogen
following birth,
then tapered onto a conventional FDA approved therapy, such as Copaxone.
[0078] Amelioration of the post-partum autoimmune disease refers to any
observable beneficial effect of the treatment. The beneficial effect can be
evidenced
by a delayed onset or progression of disease symptomology, a reduction in the
severity of some or all of the clinical symptoms, or an improvement in the
overall
health.
[0079] For example, patients who have clinical symptoms of an autoimmune
disease often suffer from some or all of the following symptoms: worsening of
pre-
existing symptoms (such as joint pain in rheumatoid arthritis), the appearance
of new
symptoms (new joints affected in rheumatoid arthritis) or increased
generalized
wealcness and fatigue. Multiple sclerosis patients in particular suffer from
the
following symptoms: weakness, numbness, tingling, loss of vision, memory
difficulty
and extreme fatigue. Thus an amelioration of disease in multiple sclerosis
would
include a reduction in the frequency or severity of onset of weakness,
nunlbness,
tingling, loss of vision, memory difficulty and extreme fatigue. On imaging of
the
brain (MRI) amelioration of disease would be evidenced by a decrease in the
number
or volume of gadolinium enhancing lesions, a stabilization or slowing of the
accumulation of T2 lesions and/or a slowing in the rate of atrophy formation.
Immunologically, an increase in Th2 cytokines (such as IL-10) a decrease in
Thl
cytokines (such as interferon gamma) would be associated with disease
amelioration.
22
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0080] Patients may also express criteria indicating they are at risk for
developing
autoimmune diseases. These patients may be preventatively treated to delay the
onset
of clinical symptoinology. More specifically, patients who present initially
with
clinically isolated syndromes (CIS) may be treated using the treatment
paradigm
outlined in this invention. These patients have had at least one clinical
event
consistent with MS, but have not met full criteria for MS diagnosis since the
definite
diagnosis requires more than one clinical event at another time (McDonald et
al.,
2001). Treatment of the present invention would be advantageous at least in
preventing or delaying the development of clinically definite MS.
[0081] Treatment with primary agents being ER alpha receptor agonists. In
one embodiment, the invention comprises the use of a primary agent comprising
an
estrogen receptor alpha ligand, such as an agonist, having an anti-
inflammatory and
neuroprotective effect to prevent or ameliorate clinical symptoms of auto
immune
diseases including multiple sclerosis.
[0082] As above, multiple sclerosis is an inflammatory, neurodegenerative
disease
for which experimental autoimmune encephalomyelitis (EAE) is a model.
Treatments
with estrogens have been shown to decrease the severity of EAE through anti-
inflammatory and neuropreservation mechanisms. More recently, it has been
determined that estrogen receptor alpha (ER alpha) ligand could recapitulate
the
estrogen-mediated protection in clinical EAE. As described in the examples
below,
EAE treatment with a highly selective ER alpha agonist (propyl pyrazole triol)
ameliorated clinical disease in botli wild-type and ER beta knock-out mice,
but not in
ER alpha knock-out mice, suggesting that the ER alpha ligand maintained ER
alpha
selectivity in vivo during disease. Anti-inflammatory and neuroprotective
effects
included, reduced auto-antigen-specific pro-inflammatory cytokine production,
increased anti-inflainmatory cytokines, reduced nervous system inflammation,
23
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
reduced demyelination, reduction in neuronal cell loss, reduction in axonal
transaction, decreased white matter lesions, decreased loss in axonal number,
reduced
nervous system monocyte activation and reduced nervous system microglial
activation. See Examples 5 and 6 and FIGs. 5-10.
[0083] Treatment of Patients with Neurodegenerative Diseases/Disorders. In
another embodiment of the invention, the invention comprises the treatment of
neurodegenerative diseases and disorders, including MS. The invention may
include
the method of preventing or treating a subject having been diagnosed or
exhibiting at
least one clinical symptom of a neurodegenerative disease or disorder.
[0084] The method according to the invention may comprise administering a
primary agent at a therapeutically effective dosage in an effective dosage
form at a
selected interval to prevent, reduce the frequency or reduce the severity of
the
symptoms and/or progression of the disease or disorder.
[0085] In one specific embodiment, the method may comprise administration of 8
milligrams estriol daily, such as in an oral preparation and result in
ameliorated
symptomology. In one other embodiment, the method may comprise treating the
patent with a combination of estrogen and progestin or progesterone, as a
secondary
agent. In other embodiments, the use of the primary agents, combinations of
primary
agents with secondary agents, at the doses and in the dosage forms may be
administered as described above for auto immune diseases.
[0086] In other embodiments, the primary agent may comprise an estrogen
receptor beta ligand, such as a estrogen receptor beta agonist. In the EAE
animal
model, an estrogen receptor beta agonist was found to have significant
neuroprotective effects, including reduced demyelination, reduces axon loss,
reduces
neuronal abnormalities and reduced motor impairment, and reduced relapses. See
Example 6 and FIGs. 11-18, below.
24
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0087] Neurodegenerative diseases and disorders for which the invention may be
effective include, but are not limited to: Alzheimer's disease, Parkinson's
disease,
multiple sclerosis, stroke, amyotrophic lateral sclerosis (Lou Gehrig's
Disease),
frontotemporal dementia (Pick's Disease), prion disease and Huntington's
disease.
Additional disorders that may be treated on the basis of the pharmacological
results
with estrogens or estrogen receptor active agents, include, but are not
limited to
cerebral ischemia, idiopathic Morbus Parkinson, topically- or drug-induced
Parkinson
syndrome, Morbus Alzheimer and cerebral dementia syndromes of different
origin,
Huntington's chorea, infectious-induced neurodegeneration disorders such as
AIDS-
encephalopathy, Creutzfeld-Jakob disease, encephalopathies induced by rubiola
and
herpes viruses and borrelioses, metabolic-toxic neurodegenerative disorders
such as
hepatic-, alcoholic-, hypoxic-, hypo- or hyperglycemically-induced
encephalopathies
as well as encephalopathies induced by solvents or pharmaceuticals,
degenerative
retina disorders of various origin, traumatically-induced brain and bone
marrow
damage, cerebral hyperexcitability symptoms of varying origin such as after
the
addition of and/or withdrawal of medicaments, toxins, noxae and drugs,
mentally and
traumatically-induced cerebral hyperexcitability states, neurodegenerative
syndromes
of the peripheral nervous system, such as metabolism, medicament, toxically-
and
infectiously-induced polyneuropathies and polyneuritis, and the
bronchospasmolytic
effect.
[0088] KITS. In another aspect of this invention kits are provided for use by
the
treating physician in the clinic or prescribed patient for self-administration
of
treatment. The kits of this invention include at least one primary agent and
one
secondary agent in the appropriate dosages and dosage form for the treatment
of the
patient's clinical symptoms.
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[0089] In a first embodiment of the kit, the primary agent is estriol in doses
of
about 4-16 milligrams and the secondary agent is progesterone in doses of
about 100
to about 200 milligrams. In a second embodiment of this kit, the primary agent
is
estriol in doses of about 4-16 milligrams and the secondary agent is a
glucocorticoid,
such as prednisone (about 5-60 milligrams per day) or methyl prednisone (1-2
milligrams per day).
[0090] In a third embodiment of this invention, the primary agent is estriol
in
doses of about 4-16 milligrams and the secondary agent is .beta.-interferon in
doses of
about 0.25 milligrams of Betaseron® or 30 mcg of Avonex® In a fourth
alternate embodiment of the kit, the primary agent is estriol in doses of
about 4 to
about 16 milligrams and the secondary agent is glatiramer acetate copolymer in
doses
of about 20 milligrams of Copaxone®
[0091] The kit also preferably contains instructions for use of the kit by the
use by
the treating physician or patients to treat their autoimmune disease. Such
information
would include at least the schedule for the administration of the primary
agent dose
and the secondary agent dose.
[0092] Although the present invention has been described in terms of the
preferred
embodiment above, numerous modifications and/or additions to the above-
described
preferred embodiments would be readily apparent to one skilled in the art.
[0093] EXAMPLE 1
[0094] Methods: Trial Design. A crossover design was used with monthly brain
MRIs during the six month pretreatment period, the six month treatment period
with
oral estriol (8 milligrams/day) and the six month post treatment period, with
clinical
and laboratory evaluations as demonstrated (FIG. 1A).
[0095] Inclusion Criteria. Women with clinically definite MS, ages 18-50, with
an
EDSS 0-6.5 who had been off interferon beta and copolymer-1 for at least six
months,
26
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
and had no steroid treatment for at least three months were eligible. At least
5 cm3 of
lesion burden on a screening T2 weighted brain MRI was required. Subjects who
were
pregnant or nursing, on oral contraceptives or hormone replacement therapy, or
who
had a history of thrombosis, neoplasm or gynecologic disease, or who had been
treated in the past witli total lymphoid irradiation, monoclonal antibody, T
cell
vaccination, cladribine or bone marrow transplantation were excluded.
[0096] Patients. Twelve female patients with clinically definite MS were
enrolled.
Six had RR disease and six had SP disease. All six RR and four of six SP
patients
completed the entire 18 month study period. One SP patient was discontinued
from
the study because of prolonged treatment with steroids for tonic spasms by an
outside
neurologist and the other did not wish to go untreated in the post treatment
period. Of
the ten patients who completed the entire study, the mean age was 44 years
(range 28
to 50 years) and the mean EDSS was 3.3 (range 1.0 to 6.5). The mean EDSS score
for
the SP patients was 5.0 while the mean EDSS for the RR patients was 2.2. The
18
month trial was extended in RR patients whereby treatment was re-instituted.
Medication. For the initial treatment phase, micronized, U.S.P. graded estriol
powder
(Medisca, Inc., Plattsburg, NY) was put into capsules by UCLA Pharmaceutical
Services. During the extension re-treatment phase in the RR patients, all but
one
received a capsule of estriol (8 milligrams/day) plus progesterone (100
milligrams/day),while the single RR patient who had a hysterectomy received
only
estriol (8 milligrams/day) (Women's International Pharmacy,lVladison, Wis.).
[0097] Clinical and Safety Measures. Subjects were evaluated using the
Kurtzke's
Expanded Disability Status Scale (EDSS) by the same neurologist (RV)
throughout
the study. At each visit the study nurse (RK) administered the paced auditory
serial
addition test (PASAT) and the 9-hole peg test. Blood was drawn for SMA12,
cholesterol panel, blood counts and hormone levels (estriol, estradiol,
estrone, LH,
27
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
FSH, cortisol, progesterone). Estriol levels in serum were determined by ELISA
according to manufacturer's instructions (Oxford Biomedical, Oxford, Mich.).
[0098] Delayed Type Hypersensitivity Responses (DTH). DTH to tetanus (Tetanus
Toxoid, Wyeth Laboratories, Marietta, PA) and candida (Candin, Allermed
Laboratories, San Diego, CA) were tested at two timepoints, once in the
pretreatment
period at study month 3 and once at the end of the treatment period at study
month 12
(FIG. 1 a). A group of six untreated healthy control women were also tested
twice,
spanning the same time interval (9 months). 0.1 ml of each solution was
injected
intradermally on the anterior surface of the forearm. Induration at each
injection site
was read after 48 hours. Each site was measured twice, once vertically and
once
horizontally with the average recorded. The same nurse (RK) administered all
injections and read all responses on all subjects at both time points.
[0099] Reverse Transcription and Polymerase Chain Reaction. Peripheral blood
mononuclear cells (PBMCs) were isolated from heparinized venous blood and
cryopreserved. PBMCs were thawed in parallel from a given patient during the
two
pre-treatment timepoints and the two treatment timepoints. Total RNA was
isolated,
DNA was removed and mRNA was reverse transcribed. Both IFN-.gamma. and actin
were amplified from the same cDNA, however, the cDNA was diluted 1:9 prior to
amplification for actin. Amplification was done in 1 mM MilligramsCl2 using
IFN.y
and actin primer sequences (Life Technologies, Rockville, Md.). Complementary
DNA was amplified for 35 cycles: 45" @ 95 C, 60" @ 54 C and 45" @ 72 C. PCR
products were separated on a 1.5% agarose gel containing ethidium bromide and
densitometry performed.
[00100] MRIs. Scans were performed on a 1.5T G.E. scanner. The pulse sequences
obtained were a Tl-weighted scan with and without gadolinium (Omniscan 0.1
mmol/kg) and a PD/T2 weighted scan. Digitized image data was transferred to a
SGI
28
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
workstation (Silicon Graphics, Inc) for further processing. The number and
volume of
new and total gadolinium enhancing lesions was determined using a
semiautomated
threshold based technique (Display, Montreal Neurological Institute) by a
single
experienced operator (NS). The operator was blinded as to whether patients had
RR or
SP disease. To calculate T2 volumes, a custom semiautomated, threshold based,
seed-
growing algorithm was used to deterinine lesion volume after skull stripping,
rf
correction and spatial normalization. All scans were counted by the same
technician
who was blinded as to whether patients had RR or SP disease.
[00101] Statistical Analysis. One sample, paired, t tests were used to
ascertain
significance of percent changes in DTH responses, IFN.gamma. levels and PASAT
cognitive testing scores during treatment as compared to pretreatment. The
nonparametric, Wilcoxon's signed rank test was used for statistical
comparisons in
enhancing lesion numbers and volumes on MRI between the six month baseline
period and each treatment period, post treatment period and re-treatment
period.
[00102] Results. Estriol levels and tolerability. Serum estriol levels during
treatment
and re-treatment approximated those observed in women who were six months
pregnant, but were lower than those who were 8.5 months pregnant (FIG. 1B).
Consistent with previous reports, estriol was well tolerated with only
menstrual cycle
abnormalities. There were no significant alterations in any laboratory
measures
including LH, FSH, cortisol, progesterone, estradiol and estrone.
[00103] Immune Responses. Skin testing to tetanus and-candida were performed
once in the pretreatment period and once at the end of the treatment period to
determine whether they might be decreased with treatment. DTH responses to
tetanus
were significantly, P=0.006, decreased at study month 12, when patients had
been on
estriol for six months, as compared to DTH responses at study month 3, the
pretreatment baseline (FIG. 2A). DTH responses to candida were decreased less
29
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00104] IFN.gamma. is a signature cytokine for Thl responses. Therefore, we
assessed IFN.gamma. levels by RT-PCR of unstimulated peripheral blood
mononuclear cells (PBMCs) derived ex vivo from patients during the
pretreatment
and the treatment periods. In the six RR patients, levels of IFN.gamma. were
variably
decreased at study month 9 (after three months of estriol treatment) and then
significantly decreased, P=0.003, at study month 12 (after six months of
estriol
treatment) as compared to baseline pretreatment levels (months 3 and 6) (FIG.
2B). In
contrast, there was no decrease in IFN.gamma. in the four SP patients. These
data are
consistent with the concept that the immune system of RR patients, as compared
to SP
patients, may be more amenable to treatments that aim to decrease Thl
responses.
Also, the observation that estriol treatment can alter cytokine production by
PMBCs is
consistent with reports demonstrating estrogen receptors alpha. and beta. in
immune
tissues and cells.
[00105] MRIs. Based on the protective effect of pregnancy on relapse rates in
MS
patients and the association of gadolinium enhancing lesions with relapses, we
hypothesized that estriol treatment would have an anti-inflammatory effect as
manifested by decreases in enhancing lesions on serial brain MRIs. Compared to
the
six month pretreatment baseline period, the total volume and number of
enhancing
lesions for all ten MS patients (6RR, 4SP) decreased during the treatment
period. This
improvement in the group as a whole was driven by the beneficial effect of
estriol
treatment in the RR, not the SP, group (FIGS. 3A and 3B). Therapeutic effects
of
estriol treatment in the RR group were therefore examined in further detail.
Within the
first three months of treatment of RR patients, median total enhancing lesion
volumes
were decreased by 79%, P=0.02, and numbers were decreased by 82%, P=0.09
(FIGS.
3C and 3D). They remained decreased during the next three months of treatment,
with
lesion volumes decreased by 82%, P=0.01, and nunlbers decreased by 82%,
P=0.02.
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
In the post treatment period, median total enhancing lesion volumes and
numbers
became variable in the first three months off treatment, before returning to
near
baseline levels in the last three months of the post treatinent period. During
the four
month re-treatment extension phase, enhancing lesion volumes decreased again
by
88%, P=0.008, and numbers decreased again, this time by 48%, P=0.04, as
compared
to original baseline (FIGS. 3C and 3D). Changes in median new enhancing lesion
volumes and numbers followed similar patterns as median total lesion numbers
and
volumes (FIGS. 3E and 3F).
[00106] Median T2 lesion volumes for the whole group were 15.3 cm<sup>3</sup> (range
6.1-33.8), with no significant differences in median T2 volumes between RR and
SP
groups. Consistent with enhancing lesion data, serial T2 lesion volumes
revealed that
estriol treatment tended to be most beneficial in RR patients. In the RR
group, median
T2 lesion volumes remained stable during the six month treatment period (0%
change), increased during the six month post treatment period (7.4% higher),
and then
declined in the four month re-treatment extension period (2.0% lower).
[00107] Clinical Measures. Relapses were few and showed no significant changes
during the study. In the six RR patients, one relapse occurred during the
pretreatment
period, one in the treatment period, two in the post treatment period and none
in the
re-treatment period. No relapses occurred in SP patients. EDSS and 9 Hole Peg
Test
scores showed no significant changes during the study (Table 1).
31
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
TABLE I
Clinical Measures
EDSS scores
Pretreatment Estriol 'Iteatment Post Treatment
3 mo. 6 mo. 9 mo. 12 mo. 15 mo. 18 mo.
6 RR 2.2 2.0 1.5 1.7 1.8 1.8
(0.6) (0.5) (0.7) (0.6) (0.6) (0.5)
4SP 5.0 5.0 4.9 5.0 5.1 5.0
(0.9) (0.9) (1.0) ((J.9) (1.1) (0.8)
9 Hole Peg Test scores
Pretreatment Estriol Treatment Post Treatment
3 mo. 6 mo. 9 mo. 12 mo. 15 mo. 18 mo.
RR
R 22.2 21.8 22.5 21.5 21.0 21.4
(2.4) (1.6) (2.3) (1.9) (1.7) (2.4)
L 24.8 22.9 24.3 23.3 23.0 22.7
(3.2) (1.6) (2.5) (2.1) (2.1) (2.3)
4 SP
R 26.8 29.9 30.2 31.7 29.4 34.0
(0.4) (2.4) (1.4) (4.8) (5.2) (8.7)
L 23.5 25.6 22.7 24.8 26.7 25.0
(1.4) (2.5) (1.7) (2.6) (0.7) (1.8)
[00108] Interestingly, PASAT cognitive testing scores were significantly
improved
in the RR-group, but not in the SP group (FIG. 4). This improvement in PASAT
scores in RR patients by 14.0% during treatment as compared to baseline,
reached
statistical significance, P=0.04. It is unlikely that this improvement was
entirely due
to a practice effect of repeated testing because of the long time interval
between
testing (9 months) and because alternate versions of the test were used in
each patient.
This beneficial effect of estriol treatment on PASAT scores of RR MS patients
is
consistent with previous reports describing a beneficial effect of estrogen
replacement
therapy in surgically menopausal women and high dose estrogen treatment in
Alzheimer's disease. Sicottte, et al. Treatment of Women with Multiple
Sclerosis
Using Pregnancy Hormone Estradiol: A Pilot Study. Neurology, 56 (8 Supp.
3):A75,
April 2001, and Sicottte, et al. Treatment of Multiple Sclerosis with the
Pregnancy
32
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
Hormone Estradiol, Submitted to Neurology 2002, are herein incorporated by
reference in their entirety.
EXAMPLE 2. Progesterone in combination with estrogen treatments has been
shown to protect against endometrial proliferation and cancer. Indeed,
estrogen cannot
be given for a lengthy period of time in an "unopposed" fashion in any woman
with a
uterus. Thus, seven of the 12 patients wanted to remain on estriol after
completion of
the 18 month study. These patients were then put back on 8 milligrams of
estriol and
100 milligrams of progesterone per day. In an extension phase of the study
which
began after completion of the post treatment phase. This extension phase was 4
months in duration. Each of the seven patients had an MRI every month during
the 4
montli extension phase. Additionally, each of the seven patients was examined
neurologically and had serologic studies done at the end of this phase. No
known
negative effects 100 milligrams of progesterone in combination therapy with 8
milligrams of estriol treatment were noted.
[00109] EXAMPLE 3.
In a pilot clinical trial, non-pregnant female MS patients were treated with
estriol to
induce a pregnancy level in serum. This treatment reduced the prototypic in
vivo Thl
response, the delayed type hypersensitivity response, as well as reduced Th1
(TNFa,
IFNy) and increased TH2 (IL5, IL10) cytokine production by peripheral blood
monuclear cells (Siotte et al., 2002; Soldan et al., 2003). Also, gadolinium-
enhancing
lesions on serial brain magnetic resonance images (MRIs) were reduced by >80%
(Sicotte et al., 2002). Because enhancing lesion activity on brain MRI is a
putative
biomarker for relapses in MS, these reports together suggested that estriol
treatment
may recapitulate the anti-inflammatory effect of pregnancy in relapsing
remitting MS
(RRMS).
[00110] EXAMPLE 4.
33
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
A 33 year old white female patient was diagnosed as having relapsing remitting
multiple sclerosis. Following the delivery of her first child (now age 7), the
patient
was treated only with Copaxone and relapsed at 6 weeks. Following the delivery
of
her second child (now age 3), the patient was again treated with Copaxone
alone and
again relapsed, this time at 4.5 months. Following a subsequent pregnancy, the
patient
was treated witli 8 mg estriol/day in an attempt to prevent her post partum
relapses.
[00111] Following the birth of the patient's third child (now 6 months), the
patient
resumed treatment with Copaxone as before. However, on day 10 post-partum she
began taking estriol 8 mg/daily in an oral dosage form The patient had no
relapses
for 6 months post-partum, and her neurologic exam is unchanged with minimal
disability (EDSS = 1). Since monthly brain MRIs with gadolinium to detect
enhancing MS lesions are more sensitive for inflammatory disease activity than
relapses, the patient underwent serial monthly MRIs at post partum months 4,
5, and
6. There was no enhancement at month 4, only one small enhancing lesion at
month 5,
and at 6 months only a small residual, less robust enhancement of the single
lesion
from the previous month. No new enhancement was observed at month 6. The T2
lesion load has been stable throughout.
[00112] The patient has had increased irregular menstrual bleeding despite
using the
progesterone minipill (norethindrone, 0.35 mg daily), one pill per day since
day 10, to
stabilize the uterine endometrium and for birth control. Uterine ultrasounds
at month
3 and 6 showed a thin, not thick, endometrium, consistent with an unstable
lining, not
suggestive of hyperplasia. The patient doubled the progesterone minipill for 2
weeks
to stabilize the endometrium. Otherwise no adverse events have been reported.
[00113] EXAMPLE 5.
34
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
...... .. ....... ....._
[00114] Arzirraals. Female C57BL/6 mice, 8 weeks of age, were purchased from
Taconic (Gertnantown, NY). ERa KO mice backcrossed onto the C57BL/6
background for 16 generations were a generous gift from Dr. Dennis Lubahn
(University of Missouri, Columbia, MO) (Lubahn et al., 1993). Wild-type
littermates
from F 16 crosses served as ERa KO matched controls. ER(3 KO mice, a generous
gift from Dr. Jan Ake Gustafsson (Karolinska Institute, Stockholm, Sweden)
(Krege
et al., 1998), were backcrossed onto the C576BL/6 background for eight
generations.
Wild-type littermates from these crosses served as ER(3 KO matched controls.
Animals were housed under guidelines set by the National Institutes of Health,
and
experiments were conducted in accordance with the University of California,
Los
Angeles Chancellor's Animal Research Committee and the Public Health Service
Policy on Humane Care and Use of Laboratory Animals.
[00115] Reagents. PPT was purchased from Tocris Bioscience (Ellisville, MO),
and E2 was purchased from Sigma-Aldrich (St. Louis, MO). Miglyol 812 N, a thin
liquid oil, was obtained from Sasol North America (Houston, TX). Myelin
oligodendrocyte glycoprotein (MOG) peptide, amino acids 35-55, was synthesized
to
> 98% purity by Chiron Mimotopes (San Diego, CA). .
[00116] EAE. Active EAE induction ensued with subcutaneous injection of an
emulsion containing the autoantigen MOG peptide, amino acids 33-55 (300
jig/mouse) and Myobacterium tuberculosis (500 jig/mouse) in complete Freund's
adjuvant, as described previously (Suen et al., 1997; Liu et al., 2003). Mice
underwent
hormonal treatments as described below and were monitored daily for EAE
disease
severity using the standard EAE grading scale, as described previously
(Pettinelli and
McFarlin, 1981). Briefly, to determine the clinical score for each mouse on
each day,
each mouse was graded using the standard 0-5 scale: 0, unaffected; 1, tail
limpness; 2,
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
failure to right on attempt to roll over; 3, partial paralysis; 4, complete
paralysis; and
5, moribund. On each day, the mean of the clinical scores of all mice within a
given
treatment group were determined, thereby yielding the mean clinical score for
that
treatment group. Some mice were followed clinically for up to 40 d after
disease
induction, and others were killed earlier for mechanistic studies, 1-2 d after
the onset
of clinical signs in the vehicle-treated group (day 16-19 after disease
induction).
[00117] Treatnaents. Isoflurane-anesthetized female mice were ovariectomized
and
allowed to recuperate for 10 days. Daily treatments of oil vehicle alone,
estradiol, or
PPT began 7 days before EAE immunization. Estradiol and PPT were dissolved in
10% ethanol and 90% oil to give the final proper concentration of 0.04
mg/kg/day of
estradiol (Jansson et al., 1994) and 10 mg/kg/d of PPT per mouse (Harris et
al., 2002).
Estradiol, PPT or vehicle alone were given by daily subcutaneous injections
along the
midbackline and continued for the entire disease duration (up to 40 days after
disease
induction).
[00118] Pezfusion. Mice were deeply anesthetized with isoflurane and perfused
transcardially with ice-cold 0.9% saline, followed by 10% formalin. Spinal
cord
columns were removed and postfixed overnight in 10% formalin and cryoprotected
with 20% sucrose solution, in PBS. Spinal cords were removed from the column,
cut
in three parts (cervical, thoracic, and lumbar), and embedded in
gelatin/sucrose mix.
Spinal cord regions in gelatin were further postfixed and stored in 20%
sucrose. Free-
floating sections (25 m thick) were cut coronally with a sliding microtome
and
collected serially in PBS.
[00119] Uterine weights. After the mice were killed, each uterus was
extracted, and
the fat, connective tissue, and excess fluid were removed to obtain each
uterine
weight, as described previously (Frasor et al., 2003).
36
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00120] Inamune responses. Spleens were harvested during deep anesthesia
before
perfusion. Splenocytes were stimulated with the autoantigen, MOG peptide 35-
55, at
25 g/ml. Supernatants were collected after 48 and 72 h, and levels of TNFa,
interferon-y (IFNy) interleukin-6 (IL6), and IL5 were determined by cytometic
bead
array (BD Biosciences Pharmingen, San Diego, CA) as described previously (Liu
et
al., 2003).
[00121] Histopathology and irnnaunohistochemistry. Serial sections ere mounted
on
slides and stained with hematoxylin and eosin (H&E), Nissl, or Luxol fast blue
(LFB)
-cresyl violet. Consecutive sections were also examined by
immunohistochemistry.
Briefly, 25 m free-floating sections were permeabillized in 0.3% Triton X-
100 in
PBS and blocked with 10% normal goat serum. White matter immunostaining was
enhanced by treating sections with 95% ethanol/5% acetic acid for 15 min
before
permeabilization and blocking. To detect specific cell types and structures,
sections -
were preincubated with primary antibodies in PBS solution containing 2% NGS
for 2
h at room temperature, and then overnight at 40 C. The following primary
antibodies
were used: anti-(33 tubulin and anti-neurofilament-NF200 [monoclonal
(Chemicon,
Temecula, CA); polyclonal (Sigma Biochemical)], anti-neuronal-specific nuclear
protein (NeuN), anti-CD45 (Chemicon), anti-myelin basic proteins (MBP;
Chemicon)
and anti-Mac 3 (BD Biosciences Pharmingen). The second antibody step was
performed by labeling with antibodies conjugated to TRITC, FITC, and Cy5
(Vector
Laboratories and Chemicon). IgG control experiments were performed for all
primary
antibodies, and no staining was observed under these conditions. To assess the
number of cells, a nuclear stairs 4', 6'-diamidino-2-phenylindole
dihydrochloride
(DAPI; 2 ng/ml; Invitrogen, Eugene, OR) was added for 15 min before final
washes
37
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
after secondary antibody addition. The sections were mounted on slides, dried,
and
coverslipped in fluoromount G (Fisher Scientific, Hampton, NH).
[00122] Microscopy. Stained sections were examined and photographed using a
confocal microscope (TCS-SP; Leica, Mannheim, Germany) or a fluorescence
microscope (BX51WI; Olympus, Tokyo, Japan) equipped with Plan Fluor objectives
connected to a cainera (DP7O; Olympus). Digital images were collected and
analyzed
using Leica confocal and DP7O camera software. Images were assembled using
Adobe Photoshop (Adobe Systems, San lose, CA).
[00123] Quantification. To quantify immunostaining results, sections from
spinal
cord levels Tl-T5 were examined, six from each mouse, with n = 3 mice per
treatment
group, for a total of 18 sections per treatment group. Images were captured
under
microscope (4X, l OX, or 40X) using the DP7O Image software and'a DP70 camera
(both from Olympus). Identical light intensity and exposure times were applied
to all
photographs from each experimental set. Images from the same areas of spinal
cord
were compared (T1-T5) and were acquired separately from delineated whole gray
and
white matter regions. The middle region of the ventral horn was the focus for
gray
matter analysis, whereas the area lateral to the ventral horn was the focus
for white
matter analysis. Six gray matter and six white matter pictures were collected
from the
two sides of Tl-T5 sections (100 m apart) from three animals in each
treatment
group. All images were converted to grayscale and then analyzed by density
measurement with ImageJ version 1.29 (the Windows version of NIH Image),
downloaded from http://rsb.info.nih.gov/ij. A fixed threshold range of 0-160
was
chosen to highlight the staining signals in normal spinal cord sections, and
the total
area within this range was measured, averaged, and compared.
[00124] Increase in total number of infiltrating cells after induction of EAE
was
measured by density measurements of DAPI + nuclei in the whole white matter.
38
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
Neuronal cells were quantified by counting the NeuN +/(33-tubulin+ /DAPI +
cells per
square millimeter in the whole gray matter. Both white and gray matter
assessments
occurred its the Tl-T5 spinal cord sections. Laser-scanning confocal
microscopic
scans at 40X were performed on Mac 3+/(33-tubulin+ immunostained spinal cord
sections corresponding to levels T1-T5 ventral horn. The results for each
experimental condition were averaged from four unilateral levels per mouse
(100 m
apart, three mice in each treatment group, total of 12 sections per treatment
group)
and were expressed as mean fold change compared with healthy match controls.
[00125] Statistical analysis. EAE disease severity was compared between groups
using the Friedman test, histopathological changes were assessed using 1 x 4
ANOVAs, and uterine weights and cytokine levels were compared between
treatment
groups using Student's t test, as described previously (Dalal et al., 1997).
[00126] Results.
[00127] Treatment with an ERa ligand remains highly selective for ERa in vivo
during EAE.
[00128] The dose of the ERa-selective ligand for use in our RAE experiments
which could induce a known biological response on a control tissue (the
uterus).
Estrogen-induced increases in uterine weight had been shown previously to be
mediated by ERa, and doses of the ERa ligand PPT needed for this in vivo
treatment
effect had been described (Frasor et al., 2003). Daily subcutaneous injections
of PPT,
at a dose previously shown to increase uterine weight (10 mg/kg/d), resulted
in a
significant increase in uterine weight in female C57BL/6 mice with EAE at day
40
after disease induction, FIG. 1A. Sensitivity of this technique was shown by
the
decrease in uterine weight in ovariectomized compared with sham-operated,
vehicle-
treated mice. Treatment with injections of high doses of estradiol (to induce
39
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
pregnancy levels in serum) served as a positive control, whereas treatment
with
injections of vehicle alone served as a negative control. To further
demonstrate the in
vivo selectivity of this dose of PPT, uterotrophic responses were also
examined during
PPT treatment of ERa or ER(3 knock-out mice. Significant increases in uterine
weight
were observed in PPT-treated ER(3 knock-out mice (FIG. 1B) but not in ERa
knock-
out mice (FIG. 1C). Together, these data demonstrated that the method of
administration of the ERa ligand PPT induced an expected biological response
in vivo
on a positive control tissue.
[00129] FIG. 5 depicts results showing results showing treatment with an ERa-
selective ligand is highly selective in vivo during EAE. As shown in FIG. 5A,
treatment with the ERa ligand PPT induced expected biological responses on
uterine
weight (y-axis = uterine weight in grams). Uterine weight was increased with
PPT
given as daily subcutaneous injections at 10 mg/kg/day. The decrease in
uterine
weight with ovariectomy compared with sham surgery demonstrated the
sensitivity of
the technique in detecting differences in uterine weights associated with
differences in
estrogen levels. Treatment with a dose of estradiol known to induce a late
pregnancy
level of estradiol was used ad a positive control for an increase in uterine
weight,
whereas treatment with vehicle alone served as the negative control. The uteri
were
removed at day 35-40 during EAE treatment with the indicated hormone (sham
vehicle, n = 6;OVX vehicle, n = 12;OVX estradiol, n = 18;OVX PPT, n = 18). OVX
PPT and OVX Estradiol, each as compared with OVX Vehicle, ***p<0.0001. WT<
Wild type. As shown in FIG. 5B, Uterine weights were examined in
ovariectomized
ER(3 knock-out mice as in FIG. 5A. Uterine weights were increased with PPT
treatment in ER(3 knock-out mice (OVX vehicle, n= 9; OVX estradiol, n = 12;
OVX
PPT, n = 12). OVX PPT and OVX Estradiol, each as compared with OVX Vehicle,
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
*** p<0.0001. As shown in FIG. 5C, Uterine weights were examined in
ovariectomized ERa knock-out mice as in FIG. 5A. Uterine weights were not
increased with PPT treatment in ERa knock-out mice (OVX vehicle, n = 6;OVX
estradiol, n= 4; OVX PPT, n=6).
[00130] Treatment with an ERa ligand reduces the clinical severity of EAE.
Using the above dose and method of administration, PPT treatment was assessed
for
its effect on the clinical course of EAE. Ovariectomized, C57BL/6 wild-type
female
mice with MOG 35-55 peptide-induced active EAE were treated with the ERa-
selective ligand PPT. PPT treatment significantly reduced the clinical
severity of EAE
(FIG. 6A). Treatment with injections of estradiol served as a positive
control, whereas
treatment with injections of vehicle alone served as the negative control.
[00131] When ovariectomized ER(3 knock-out C57BL/6 female mice were treated
with PPT-during active EAE, clinical disease severity was also significantly
decreased (FIG. 6), These data demonstrated that the presence of ER(3 was not
required for disease protection mediated by treatment with PPT. In contrast,
when
PPT was administered to ovariectomized ERa knock-out mice induced with active
EAE, the disease-ameliorating effect of PPT treatment was abolished, as
evidenced by
the lack of a difference in mean clinical scores when comparing PPT-treated
and
vehicle-treated ERa knock-out mice (FIG. 6C). Similar results were obtained
when
castrated male mice were used instead of ovariectomized females (data not
shown),
consistent with a previous publication demonstrating that estrogen-medicated
improvements in clinical EAE in castrated male mice were abrogated in the ERa
knock-out (Liu et al., 2003). ERa knock-out female mice have high circulating
estradiol levels; hence, estrogen unresponsiveness in this mouse could be
attributable
to the ERa genetic modification or the estrogen history of the mouse before
41
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
ovariectomy at 4 weeks. Because male ERa knock-out mice do not have high
circulating levels of estradiol, similar results in both the female and male
ERa knock-
outs make the ERa genetic modifications, not the estrogen history of the
mouse, most
lilcely responsible for effects observed.
[00132] These data demonstrated that the estrogen-medicated protection from
EAE
could be recapitulated by treatment with a highly selective ERa ligand, and
that this
protection was not dependent on an interaction with ERP.
[00133] FIG. 6. Treatment with an ERa-selective ligand is sufficient to reduce
the
clinical severity of EAE. As shown in FIG. 6A, EAE clinical severity was
decreased
in ovariectomized, wild-type (WT) C57BL/6 female mice treated with PPT. Daily
treatments of ovariectomized mice with injections of vehicle (negative
control),
estradiol (positive control), or PPT (10 mg/kg/day) began, and then 7 d later,
active
EAE was induced with MOG 35-55 peptide. Mean clinical scores were
significantly
reduced in both estradiol-and PPT-treated mice compared with vehicle treated
(p<
0.0001, Friedman test). Data are representative from experiments repeated a
total of
five times. As shown in FIG. 6B, the decrease in the mean clinioal scores of
EAE by
PPT treatment was not dependent on the presence of ER(3. Ovariectomized, ER(3
knock-out C57BL/6 female mice were treated with either PPT, estradiol, or
vehicle as
in A. Mean clinical scores were significantly reduced in both estradiol-and
PPT-
treated mice compared with vehicle treated (p< 0.0001, Friedman test). Data
are
representative from experiments repeated a total of three times. As shown in
FIG. 6C,
PPT treatment in vivo during EAE remains highly selective for ERa.
Ovariectomized
female ERa knock-out C57BL/6 mice were treated as in FIG. 6A. InERa knock-out
mice, mean clinical scores were not significantly different in PPT-treated
compared
with vehicle-treated. PPT-treated wild-type mice served as a positive control
for a
42
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
PPT treatment effect within the experiment. Data are representative from
experiments
repeated a total of three times. Error bars indicate variability of clinical
scores
between mice within a given treatment group. n = 5 mice per each treatment
group.
[00134] Treatment with an ERa ligand reduces autoantigen-specific
proinflammatory cytokine production. Because it had been shown previously
using ERa knock-out mice that both disease protection and a reduction in
proinflammatory cytokines (TNFa and IFNy) were dependent on ERa, we next
determined whether treatment with an ERa ligand could reduce proinflammatory
cytokine production. As demonstrated in FIG. 7, PPT treatment significantly
reduce
TNFa, IFNy, and IL6 production. Interestingly, we had shown previously that
production of the Th2 cytokine IL5 was increased with estrogen treatment and
that
this was only partially, but not completely, abolished in the ERa knock-out
(Liu et al.,
2003). In the present study, when wild-type mice were treated with the ERa
agonist
PPT, treatment significantly increased IL5 production., Together, these data
demonstrated that treatment with an ERa agonist induced changes in cytokine
production during autoantigen-specific immune responses in the peripheral
immune
system that would be anti-inflammatory with respect to EAE immunopathogenesis.
[00135] As shown in FIG. 7, treatment Treatment with an ERa ligand reduced
proinflammatory cytokine production by peripheral immune cells in
ovariectomized,
wild-type C57BL/6 female mice with EAE. EAE was induced as in FIG. 6, and then
at day 40 after disease induction, mice were killed, and cytokine production
by MOG
35-55 stimulated splenocytes was determined. TNFa, IFNy, and IL6 levels were
each
significantly reduced with PPT treatment, whereas IL5 levels were increased
with
PPT treatment. Error bars indicate variability of cytokine values for
splenocytes
between individual mice within a given treatment group, with n= 5 mice for
each
43
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
treatment group. Data are representative of experiments repeated three times.
*p<0.05.
[00136] Treatment with an ERa ligand reduces inflammation and
demyelination in EAE. Because we had observed that treatment with the ERa
ligand PPT recapitulated the protective effect of estrogen treatment on the
clinical
course of EAE and was anti-inflammatory with respect to the autoantigen-
specific
immune response in the periphery, we next ascertained the effect of treatment
with
PPT on inflammation and demyelination in the CNS of EAE mice. Spinal cord
sections of ovariectomized, C57BL/6 mice at the acute phase of EAE (1-2 days
after
onset of clinical signs in vehicle-treated mice) were assessed for
inflammation and
demyelination. Mice from all treatment groups were killed at the same time
point, to
permit their examination in parallel. Compared with vehicle-treated EAE, both
inflammation and demyelination were markedly reduced by treatment with the ERa
ligand PPT or E2 (FIG. 4). H&E-stained vehicle-treated EAE mice, compared with
normal healthy controls, had numerous multifocal to coalescing inflammatory
cell
infiltrates in the spinal cord. Infiltrates were present in the leptomeninges,
around
blood vessels in the leptomeninges, and in the parenchyma of the white matter
(FIG.
4A). Inflammatory cell infiltrates were associated with pallor and
vacuolation,
consistent with demyelination. Quantification of white matter cell density by
counting DAPI+ cells revealed a 60% increase in infiltrates of vehicle-treated
EAE
group. In contrast, both estradiol and PPT treated mice had no detectable
inflanimation, with white matter cell densities similar to those in the normal
control
(FIG. 4D).
[00137] The degree of myelin loss was assed by Luxol fast blue and confirmed
by
MBP immunostaining. Luxol fast blue staining revealed demyelination at the
sites of
44
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
inflammatory cell infiltrates (FIG. 4B). Also, myelin staining of dorsal
column
regions of vehicle-treated spinal cord section had significantly less MBP
immunostaining compared with normal control, E2-, and PPT-treated sections,
FIG.
4C. Quantification of demyelination by density analysis of Luxol fast blue-
stained
spinal cord sections revealed a 25% decrease in myelin density in vehicle-
treated EAE
mice. In contrast, both estradiol- and PPT-treated mice had much less
demyelination,
with myelin densities not significantly different from those in the normal
control
(FIG. 8E).
[00138] As shown in FIG. 8, treatment with an ERa ligand reduced inflammation
and demyelination in spinal cords of mice with EAE. In FIG. 8A, representative
H&E-stained thoracic spinal cord sections (4X magnification) from normal
(healthy
control), as well as vehicle-, E2-, and PPT-treated EAE mice are shown.
Vehicle-
treated EAE mouse spinal cord shows multifocal to coalescing areas of
inflammation
in the leptomeninges and white matter, around blood vessels, and in the
parenchyma
of the white matter (areas of inflammation shown byarrows). No inflammation
was
observed in either E2-or PPT-treated EAE spinal cords. As shown in FIG. 8B,
luxol
fast blue-stained region of dorsal column (square in A) of spinal cords (40X
magnification). Intense deinyelination in the white matter is seen in vehicle-
treated
EAE sections only. As shown in FIG. 8C, anti-MBP immunostained dorsal column
demonstrated demyelination in the white matter of vehicle-treated EAE sections
only.
As shown in FIG. 8D, increase in total number of infiltrating cells after
induction of
EAE was semiquantified by counting DAPI+ cells in the entire delineated white
matter (including dorsal, lateral, and ventral funiculi) and presented as
percentage of
normal. Vehicle-treated EAE mice had a significant increase in white matter
cell
density compared with healthy normal control, whereas E2-treated and the ERa
ligand (PPT)-treated groups did not. As shown in FIG. 8E, the extent of
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
demyelination was compared by staining thoracic spinal cord sections with
Luxol fast
blue. Myelin density is presented as percentage of normal. Vehicle-treated
mice EAE
mice had a significant decrease in myelin density in the entire delineated
white matter
as compared with normal control, whereas E2-treated and PPT-treated groups did
not.
Number of mice, three per treatment group; number ofT1-T5 sections per mouse,
six;
total number of sections per treatment group, 18. **Statistically significant
compared
with normals(p< 0.001), 1 x 4 ANOVAs. Data are representative of experiments
repeated in their entirety on another set of EAE mice with each of the
treatments.
[00139] Treatment with an ERa ligand is neuroprotective in EAE. In light of
the significant anti-inflammatory effect induced by PPT treatment of mice with
EAE,
the preservation of neuronal and axonal integrity was examined. A combination
of
Nissl stain histology and anti-NeuN/[33-tubulin immunolabeling was used to
identify
and semiquantify neurons, and neurofilament antibody (anti-NF200) was used to
identify axons. At the acute phase of EAE, 1-2 d after the onset of clinical
signs in
vehicle-treated mice, thoracic spinal cord sections of all treatment groups of
EAE
mice were assessed for NeuN+/R3 tubulin+ neurons in the gray matter and NF200
axons in the white matter. A surprising decrease in neuronal staining(NeuN
+/Nissl
+) in gray matter occurred at this early time point in vehicle-treated EAE
mice (FIG.
9B) compared with normal, healthy, age- and gender-matched control mice (FIG.
9B).
This significant decrease in neuronal staining in gray matter of vehicle-
treated EAE
mice was not observed in EAE mice treated with either estradiol (FIG. 9C) or
the
ERa ligand (FIG. 9D). Quantification of NeuN+ cells in gray matter confirmed
the
significant loss in vehicle-treated EAE mice compared with normal controls,
whereas
estradiol- and PPT-treated mice had NeuN + cell numbers that were no different
from
the normal control (FIG. 9E).
46
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00140] As shown in FIG. 9, treatment with an ERa ligand preserved neuronal
staining in gray matter of spinal cords of mice with EAE. As shown in FIG. 9A-
D,
split images of thoracic spinal cord sections stained with NeuN (red) in i and
Nissl in
ii at 4X magnification, derived from normal healthy control mice(A),vehicle-
treated
EAE (B), E2-treated EAE mice (C),and ERa ligand (PPT)-treated EAE mice(D),each
killed very early during EAE, 1-2 days after the onset of clinical signs. iii,
Merged
confocal scan at 40X of NeuN+ (red) and [33-tubulin+ (green) colabeled neurons
from
an area represented by dotted white square area in i. iv, A40X magnification
of Nissl-
stained area in solid black square in ii. A decrease in NeuN+ immunostaining
and
Nissl staining was observed in the dorsal horn, intermediate zone, and ventral
horn of
vehicle-treated EAE mice (FIG. 9B) compared with normal controls (FIG. 9A).
White arrows in Biii denote loss of NeuN+ staining. In contrast, EAE mice
treated
with either estradiol (FIG. 9C) or PPT (FIG. 9D) had preserved NeuN and Nissl
staining. After quantification of neurons in the entire delineated gray matter
of T1-T5
sections, NeuN+ immunolabeled neurons were significantly decreased, by nearly
25%,
in vehicle-treated EAE mice compared with normal controls, but E2-and PPT-
treated
EAE mice were not statistically different from normal controls (FIG. 9E).
Number of
mice, three per treatment group; number of T1-T5 sections per mouse, six;
total
number of sections per treatment group, 18. * *Statistically significant
compared with
normals (p<0.001); 1 x 4 ANOVAs. Data are representative of experiments
repeated
in their entirety on another set of EAE mice with each of the treatments.
[00141] Immunostaining for neurofilament (NF200) resulted in clear
identification
of axons within the spinal cord of normal mice (FIG. I OA). A significant
decrease in
axonal NF200 staining (NF200+) in white matter occurred in vehicle-treated EAE
mice compared with normal controls in areas positive for CD45 staining,
consistent
47
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
with previous observations of axonal transection within inflammatory white
matter
lesions in EAE (Wujek et al., 2002). EAE mice treated with either estradiol or
the
ERa ligand demonstrated not decrease in axonal NF200+ staining and only an
occasional single cell positive for CD 45 (FIG. 10A). Quantification of axon
numbers
in white matter confirmed the significant loss in vehicle-treated EAE mice,
but no
significant axonal loss occurred in EAE mice treated with either estradiol or
the ERa
ligand (FIG. lOC). These immunohistoligical data are consistent with our
observation
of markedly reduced inflammatory lesions by H&E in white matter with these
treatments (FIG. 8A). Notably, at this early time point in EAE, there was no
loss in
axon numbers in white matter areas devoid of inflammatory lesions, even in the
vehicle-treated EAE group, thereby providing no evidence for Wallerian
degeneration
of white matter tracts in these regions of the cord at this very early time
point in EAE.
[00142] Treatment with an ERa ligand reduces microglial/monocyte activation
in white and gray matter of mice with EAE. Gray matter axonal pathology has
been described in cortex of MS patients, which was characterized by activated
microglia closely opposed to and ensheathing apical dendrites, neuritis, and
neuronal
perikarya (Peterson et al., 2001). In light of our observation of a decrease
in NeuN+
/(33-tubulin+/Nissl+ neuronal staining in the gray matter of spinal cords in
EAE, we
next addressed the microglial reaction in this gray matter.
Microglia/monocytes were
stained for Mac 3, a lysosomal antigen equivalent to LAMP-2 (lysosomal-
associated
membrane protein 2)/CD 107b, present on the surface of microglia and mature
mononuclear phagocytes, and sections were coimmunolabeled with anti-B3-tubulin
(FIG. 1013). Striking Mac 3+ reactivity was observed in gray matter of mice at
this
very early time point in EAE, only 1-2 days after the onset of clinical signs
in the
vehicle-treated group. Most of the MAC 3+ cells demonstrated a morphology
similar
48
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
to that of activated microglia (FIG. 10B, inset). They were in close vicinity
to, and in
direct contact with, gray matter neurons that had reduced and punctuate (33-
tubulin
staining (FIG. l OB). In contrast, EAE mice treated with either the ERa ligand
PPT,
or estradiol, which were killed and examined in parallel, had some, but
significantly
less, immunoreactivity (FIG. l OB). Quantification of MAC 3+ cells revealed an
-65%
decrease when E2- and PPT-treated spinal cords were compared with those from
vehicle-treated EAE mice (FIG. 10D).
[00143] As shown in FIG. 10, treatment with an ERa ligand reduced CD45+ and
Mac 3+ cells in white and gray matter of mice with EAE. As shown in FIG. 10A,
thoracic spinal cord sections from mice used in FIG. 9 were coimmunostained
with
NF200 (green) and CD45 (red) at l OX magnification. Shown are partial images
with
white and gray matter from normal control, vehicle-treated EAE, E2-treated
EAE, or
ERa ligand (PPT)-treated EAE mice. LF, Lateral funiculus of white matter; GM,
gray matter. The vehicle-treated EAE cords had large areas of CD45+ cells
associated with reduced NF200 axonal staining in white matter compared with
the
normal control, whereas estradiol and ERa ligand-treated EAE mice had only
occasional CD45 positivity, with intact NF200 axonal staining. As shown in
FIG.
I OB, consecutive sections from the same mice were also coimmunostained with
(33-
tubulin (green) and Mac 3(red), with the section of the ventral horn
designated by the
dotted line square area in FIG. 10A scanned at 40X magnification by confocal
microscopy. Vehicle-treated EAE mice demonstrated markedly increased Mac 3
staining in ventral horn gray matter compared with normal control mice, with
most of
these Mac 3+ cells having the morphology of microglia (inset, 100X
magnification).
They were surrounding neuronal structures (white arrows). In contrast, E2-and
ERa
ligand (PPT)-treated EAE cord sections demonstrated less Mac 3 immunostaining
49
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
compared with vehicle-treated EAE mice. As shown in FIG. l OC, after
quantification, neurofilament-stained axon numbers in white matter were
significantly
lower in vehicle-treated EAE mice compared with normal mice, whereas E2-and
PPT-
treated EAE mice demonstrated no significant reduction in axon numbers. Axon
number is presented as percentage of norinal. **Statistically significant
compared
with normal (p< 0.001); 1 x 4 ANOVAs. FIG. lOD, Mac 3X cells were analyzed by
density measurements and represented as percentage of vehicle-treated groups.
Compared with vehicle-treated EAE mice, both the E2-treated and PPT-treated
had
significantly lower Mac 3+ immunoreactivity in gray matter. Number of mice,
three
per treatment group; number of T1-T5 sections per mouse, four; total number of
sections per treatment group, 12. **Statistically significant compared with
normal (p<
0.001); 1 x 4 ANOVAs. Data are representative of experiments repeated in their
entirety on another set of EAE mice with each of the treatments.
[001441 EXAMPLE 6. The neuroprotective effects of estrogen receptor (ER)
Beta. Methods. Animals. Female wild type C57BL/6 mice, as well as female ERJ3
1(0 mice on the C57BL 16 background, age 8 weeks, were obtained from 'laconic
(Germantown, NY). Wild type SI1L female mice, age S weeks, were obtained from
Harlan laboratories (Indianapolis, IN). Animals were maintained in accordance
with
guidelines set by the National Institutes of Health and as mandated by the
University
of California Los Angeles Office for the Protection of Research Subjects and
the
Chancellor's Animal Research Committee.
(00145] Reagents. Propyl pyrazole triol (PPt and Diarylpropionitrile (DPN), an
ERa
and an ERI3 agonist, respectively, were purchased from Tocris Bioscience
(Ellisville,
MO). Estradiol was purchased from Sigma-Aldrich (St. Louis, MO). Miglyol 812
N, a
thin liquid oil, was obtained from Sasol North America (Houston, TX). Myelin
oligodendrocytes glycoprotein (MOO) peptide, amino acids 35-55, proteolipid
protein
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
(PLP) peptides 139-151 and 179-191. and myelin basic protein (MBP) peptide 83-
102
were synthesized to >98% purity by Mimotopes (Clayton, Victoria, Australia).
[00146] Uterine weights to assess dosing. Uterine weight was used as a
positive
control to assess dosing of estrogen agonists. Daily subcutaneous injections
of
vehicle, estradiol, PPT, or DPN, as well as a combination of ITT with DPN,
were
administered for ten days at indicated doses to ovariectomized mice. Following
euthanasia, the uterus was extracted, then fat, connective tissue, and excess
fluid
removed in order to obtain the uterine weight, as described.
[00147] Hormone manipulations during EAE. Isotlurane-anesthetized female mice
were ovaricctornized and allowed to recuperate for 7-10 days. Daily
subcutaneous
injections of vehicle, estradiol, PPT, or DPN began seven days prior to EAE
immunization, and continued throughout the entire disease duration. Estradiol
was
delivered at a concentration of 0.04 mg/kg/day, DPN at 8 mg/kg/day and ITT at
10mg/kg/day. Vehicle alone treatments consisted of 10% Ethanol and 90%
Migylol.
[00148] EAE Induction. Active EkE was induced by immunizing with 300 gg of
myelin oligodenrocyte glycoprotein (MOO) peptide, amino acids 35-55, and 500
pg
of Mycobacterium tuberculosis in complete Freund's adjuvant as described.
Active
EAE was induced in SiT, mice with 100 jig of proteolipid protein (PLP)
peptide,
amino acids 139-15 1, and 100 jig of Mycobacterium tuberculosis in complete
Freund's adjuvant as described. Mice were monitored and scored daily for
clinical
disease severity according to the standard 0-5 EAE grading scale: 0,
unaffected; 1, tail
limpness; 2, failure to right upon attempt to roll over; 3, partial paralysis;
4, complete
paralysis; and 5, moribund. On each day, the mean of the clinical scores of
all mice
within a given treatment group were determined, thereby yielding the mean
clinical
score for that treatment group. Some mice were followed clinically for up to
50 days
after disease induction, while others were sacrificed earlier for mechanistic
studies at
51
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
day 19 after disease induction, corresponding to day 4-6 after the onset of
clinical
signs in the vehicle treated group.
[00149] Rotarod Testing. Motor behavior was tested up to two times per week
for
each mouse using a rotarod apparatus (Med Associates mc, St. Albans, VT).
Briefly,
animals were placed on a rotating horizontal cylinder for a maximum of 200
seconds.
The amount of time the mouse remained walking on the cylinder, without
falling, was
recorded. Each mouse was tested on a speed of 3-30 rpm and given three trials
for any
given day. The three trials were averaged to report a single value for an
individual
mouse, and then averages were calculated for all animals within a given
treatment
group. The first two trial days, prior to immunization (day 0), served as
practice trials.
[00150] Immune responses. Spleens were harvested either after deep anesthesia
prior to perfusion or after euthanasia. Splenocytes were stimulated with the
indicated
autoantigens at 25 pg/mI, and proliferation assessed using standard H3
incorporation
assays, as described. Supernatants were collected after 48 and 72 hours, and
levels of
TNF-i, IFN-y, 11,6, and 1L5 were determined by cytometric bead array (BD
Biosciences), as described.
[00151] Perfusion. Mice were deeply anesthetized with isoflurane and perfused
transcardially with ice-cold 0.9% saline, followed by 10% formalin. Spinal
cord
columns were removed and post-fixed overnight in 10% formalin and
cryoprotected
with 20% sucrose solution in PBS. Spinal cords were removed from the column
and
cut in 3 parts (cervical, thoracic and lumbar) and embedded in gelatin/sucrose
mix.
Spinal cord regions in gelatin were further postfixed and stored in 20%
sucrose. Free-
floating sections (25 pm thick) were cut coronally with a sliding microtome
and
collected serially in PBS.
52
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00152] Histopathology and Immunohistochemistry. Serial sections were mounted
on slides and stained with Hematoxylin & eosin (H&E) or Nissl. Consecutive
sections
were also examined by immunohistochemistry. Briefly, 25 m free-floating
sections
were permeabilized in 0.3% Triton X-100 in PBS and blocked with 10% normal
goat
serum. White matter immunostaining was enhanced by treating sections with 95%
ethanol/5% acetic acid for 15 minutes prior to permeabilization and blocking.
To
detect specific cell types and structures, sections were pre-incubated with
primary
antibodies in PBS solution containing 2% NGS for 2 hours at room temperature,
then
overnight at 40 C. The following primary antibodies were used: anti-03 tubulin
and
anti-neurofilament-NF200 (monoclonal, Chemicon; polyclonal Sigma Biochemical),
anti-neuronal specific nuclear protein (NeuN), anti-CD4S (Chemicon), and anti-
MW
(Chemicon). The second antibody step was performed by labeling with antibodies
conjugated to TRITC, FITC and Cy5 (Vector Labs and Chemicon). IgG-control
experiments were performed for all primary antibodies, and no staining was
observed
under these conditions. To assess the number of cells, a nuclear stain 4',6-
Diamidino-
2-phenylindole, DAPI (2ng/ml; Molecular Probes) was added for 15 minutes prior
to
final washes after secondary antibody addition. The sections were mounted on
slides,
dried and coverslipped in fluoromount G (Fisher Scientific).
[00153] Microscopy. Stained sections were examined and photographed using a
confocal microscope (Leica TCS-SP, Mannheim, Germany) or a fluorescence
microscope (BX51 WI; Olympus, Tokyo, Japan) equipped with Plan Fluor
objectives
connected to a camera (DP70, Olympus). Digital images were collected and
analyzed
using Leica confocal and DP70 camera software. Images were assembled using
Adobe Photoshop (Adobe Systems, San Jose, CA).
[00154] Quantification. To quantify immunostaining results, sections from
spinal
cord levels Tl-T5 were examined, six from each mouse, with n=3 mice per
treatment
53
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
group, for a total of 18 sections per treatment group. Iniages were captured
under
microscope (4X, IOX or 40X) using the DP70 Image software and a DP70 camera
(both from Olyinpus). Identical tight intensity and exposure times were
applied to all
photographs froin each experimental set. Images from the same areas of spinal
cord
were compared (TI-IS) and were acquired separately from delineated whole gray
and
white mailer regions. The middle region of the ventral horn was the focus for
gray
matter analysis, while the area lateral to the ventral horn was the focus for
white
matter analysis. Six gray matter and six white matter pictures were collected
from the
two sides of TI-IS sections (100 jim apart) from three animals in each
treatment
group. All images were converted to grayscale and then analyzed by density
measurement with ImageJ vi .29 (the Windows version of NIH Image), downloaded
from http://rsb.info.nth.gov/iy. A fixed threshold range of 0 to 160 was
chosen to
highlight the staining signals in normal spinal cord sections, and the total
area within
this range was measured, averaged, and compared.
[001551 Increase in total number of infiltrating cells after induction of EAE
was
measured by density measurements of DAPIk nuclei in the whole white matter.
Neuronal cells were quantified by counting the NeuN}/(33-tubulin+/DAPI'- cells
per
mm2 in the whole gray matter. Both white and gray matter assessments occurred
in
the TI-IS spinal cord sections. Laser scanning confocal microscopic scans at
40X
were performed on Mac 3"'/(33-tubulin+ immunostained spinal cord sections
corresponding to levels 11-15 ventral horn. The results for each experimental
condition were averaged from four unilateral levels per mouse (100 pm apart,
three
mice in each treatment group, total of 12 sections per treatment group) and
were
expressed as mean fold change as compared to healthy matched controls, as
described.
54
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00156] Statistical Analysis. EAE clinical disease severity was compared
between
treatment groups using the Friedman test; histopathological changes were
assessed
using 1 x 4 ANOVAs; uterine weights, proliferative responses and cytokine
levels
were compared between treatment groups using Student t-test, and time on
rotorod
was compared between treatment groups using ANOVA.
[00157] RESULTS.
[00158] Selected doses of ERa and ER(3 ligands induced known biological
responses on a positive control tissue, the uterus. Before beginning EAE
experiments, the uterine response was used to assess whether a known in vivo
response would occur during treatment with each of our dosing. regimens. It
was
known that estrogen treatment increased uterine weight primarily though ERa,
and it
had also been shown that treatment with the ER(3ligand Diarylpropionitrile
(DPN)
could antagonize the ERa mediated increase in uterine weight. The ERa ligand
propyl pyrazole triol (PPT) was given to ovariectomized C7BL/6 females for 10
days
at either an optimal (10 mg/kg/day) or suboptimal (3.3 mg/kg/day) dose, and a
significant increase in uterine weight as compared to vehicle treated was
observed
(FIG. 11). For the ER(3ligand DPN, a dose was selected which was shown to be
neuroprotective in an animal model of global ischemia. When this DPN dose (8
mg/kg/day) was given in combination with PPT'treatment, the increase in
uterine
weight mediated by PPT treatment was significantly reduced. Doses of the ERa
and
ER[3ligands induce known biological responses on a positive control tissue.
C57BLI6 mice were ovariectomized, then treated for 10 days with indicated
doses of
ERa or ER(3ligands as daily subcutaneous injections to determine the effect of
this
dosing regimen on uterine weight. As shown in FIG. 11, uterine weight was
increased with PPT treatments at both 10 mg/kg/day and 3.3 mg/kg/day, as
compared
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
to vehicle treated controls. Treatment with DPN alone at 8mg/kg/day had no
effect on
uterine weight, while this DPN dose antagonized the PPT 3.3 mg/kg/day mediated
increase in uterine weight. Each treatment group, n=4. * indicates p < 0.05,
student t-
test.
[00159] These data demonstrated that the method and dose of delivery of the
ERa
and ER(3 ligands induced a known biological response in vivo on a positive
control
tissue, the uterus.
[00160] Differential effects of treatment with ERa and ERP ligands on clinical
EAE. We compared and contrasted effects between ERa and ER(3 treatment during
EAE. When the ERa ligand was administered one week prior to active EAE
induction
with MOG 35-55 peptide in ovariectomized C57BL/6 female mice, clinical disease
as
measured by the standard EAE grading scale was completely abrogated, p<0.0001
(FIG. 12A). This was consistent with our previously findings in this EAE model
(described above), as well as findings in adoptive EAE in SJL mice by others.
In
contrast, ER(3 ligand treatment had no significant effect early in disease (up
to day 20
after disease induction), but then demonstrated a significant protective
effect later in
disease (after day 20), p<0.001 (FIG. 12B).
[00161] The protective effect using the ERP ligand DPN in active EAE in
C57BL/6
mice were surprising given that another ER(3 ligand (WAY-202041) was shown to
have no effect in adoptive EAE in SJL mice. Since WAY-202041 was shown to have
a 200 fold selectivity for ER(3 as compared to ERa, while DPN has a 70 fold
selectivity, it was possible that DPN was not sufficiently selective for ER(3
in vivo in
our studies. To assess the in vivo selectivity of DPN during EAE, DPN was
administered to ER(3 KO mice. When OPN was administered to ovariectomized ER(3
KO C57BL/6 mice with active EAE, the treatment was no longer protective (FIG.
56
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
12C). These data demonstrated the in vivo selectivity of DPN for ERP during
EAE at
the dose used.
[00162] Together these results indicate that treatment with an ERa ligand is
protective throughout the course of EAE, while treatment with an ERP ligand is
protective during the later phase of the disease, alter the acute initial
phase.
[00163] Differential effects of treatment with ERa and ERP ligands on
autoantigen specific cytokine production in C57BL/6 mice with EAE. To further
investigate differences between treatments with the ERa versus the ERP ligand,
the
autoantigen specific cytokine production during both early and later stages of
EAE in
C57BL/6 mice was assessed. ERa ligand treatment significantly reduced levels
of
proinflammatory cytokines (TNFa, IFNy, and IL6), while increasing the anti-
inflammatory cytokine IL5, during both early (FIG. 12D) and later (FIG. 12F)
stages
of EAE. In contrast, ERP ligand treatment was not statistically different from
vehicle
treatYnent in all measured cytokines (TNFa, IFNy, and IL6, and IL5) at either
the
early (FIG. 12E) or later (FIG. 12G) time points. Treatment with ERa versus
ERP
selective ligands has differential effects on chronic EAE and autoantigen
specific
immune responses in C57BL/6 mice. Ovariectomized C57BL/6 female mice were
given daily subcutaneous injections of an ER ligand during active EAE and
graded
using the standard EAE grading scale. FIG. 12A, Mean clinical scores of PPT
treated
mice as compared to vehicle treated mice were significantly reduced during the
entire
disease course, p<0.0001, Friedman test. Each treatment group had an n = 4,
and data
are representative of a total of five repeated experiments. FIG. 12B, DPN
treated
mice, as compared to vehicle treated mice, were not significantly different
early in
disease (up to day 20 after disease induction), but then became significantly
improved
later during EAE, (following day 30 after disease induction) p<0.001, Friedman
test.
57
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
Number of mice in each group were vehicle, n= 4; estradiol, n= 4; DPN, n = 8.
Data
are representative of experiments repeated twice. c, DPN treatment in vivo
during
EAE remains highly selective for ERf3. Clinical scores in ovariectomized ERP
1(0
C57BL/6 mice with active EAE were no different when comparing DPN treated with
vehicle treated. Each treatment group had an n 4, and data are representative
of
experiments repeated twice. Estradiol treated mice served as a positive
control for a
treatment effect in each experiment (FIG. A-C).
[00164] At day 19 (FIG. 12 D, E) or day 40 (FIG 12 F, G) after disease
induction,
mice were sacrificed and cytokine production by MOO 35-55 stintulated
splenocytes
was determined. ITT treatment significantly reduced TNFa, LFNy, and LL6, and
increased LU during early
[00165] EAE (FIG. 12D) and late EAE FIG 12. In contrast, no significant
differences with DPN treatment were seen in measured cytokine levels at either
the
early stage (FIG. 12E) or late stage (1) of EAE disease. Error bars indicate
variability
of cytokine values for individual mice within a given treatment group, with n
= 4 mice
for each treatment group. Data are representative of two to five experiments
for each
time point. (FIG. D-G) No differences were observed with either ERa or ER(3
ligand
treatment, as compared to vehicle, for IL1O production, while 11,4 and 1L12
levels
were too low to detect (not shown).
[00166] These results indicated that while ERa ligand treatment induced
favorable
changes in cytokine production during the autoantigen specific immune
response,
ER(3 ligand treatment did not.
[00167] Treatment with an ER(3 ligand reduces clinical relapses, but does not
alter autoantigen specific immune responses in SJL mice with EAE.
58
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00168] Next, proteolipid protein (PLP) 139-151 induced active EAE in SJL mice
were treated with either DPN or vehicle control. While there was no difference
in the
incidence, the day of onset, or the peak clinical scores, there was a
significant
decrease in relapses in DPN treated mice (5/13, 33%) as compared to vehicle
treated
(10/13, 77%), p<0.01. These relapses occurred between days 36 and 52 after
disease
induction. Notably, the previous report stating that the ER(3 ligand WAY-
202041 was
not protective in EAE in SJL mice followed mice for only the first 27 days
after
disease induction, a duration including only the first episode of acute EAE,
and a time
when no effect of DPN treatment was observed.
[00169] The immune responses in this EAE model were then assessed. Since
epitope spreading had been previously described in SJL mice with PLP 139-151
induced EAE, the immune response to the disease initiating autoantigen (PLP
139-
151) was assessed, as well as the response to possible epitope spreading
autoantigens
(PLP 179-191 and MBP 83-102). There was no significant effect of ER(3legand
treatment, as compared to vehicle treatment, on immune responses to the
disease
initiating autoantigen (FIG. 13), and no epitope spreading occurred, even m
vehicle
treated EAE mice, consistent with some reports not detecting epitope
spreading.
[00170] FIG 13. Treatment with an ER[3 selective ligand did not affect
peripheral
immune cells in SJL mice with EAE. Active EAE was induced with PLP 139-151
peptide in ovariectomized SJL female mice treated with either vehicle, DPN or
estradiol. At day 52 after disease induction, mice were sacrificed and splenic
immune
responses to the disease initiating antigen (PLP 139-151), as well as to
possible
epitope speading antigens (PLP 178-191 and MBP 83-102) were assessed. The only
detectable response in all three treatment groups was to the disease
initiating antigen
(PLP 139-151), while responses to possible epitope speading antigens were
undetectable. No significant differences were observed in proliferation or
cytokine
59
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
(TNFa or LENy) production during the PLP 139-151 specific response in the DPN
treated group as compared to the vehicle treated group. Estradiol treatment
served as
the positive control for a treatment effect on immune responses, demonstrating
decreases in the proliferative response, as well as in TNFa and IFNy cytokine
production, when compared to vehicle treated, consistent with previous
reports. Error
bars indicate variability of values for individual mice within a given
treatment group,
with n= 4 mice for each treatment group, and data are representative of
experiments
repeated twice.
[00171] Together these data indicated that while ER(3ligand treatment mediated
a
reduction in relapses in SW mice with EAE, the mechanism for this effect on
relapses
did not include a significant effect on cytokine production or epitope
spreading.
[00172] Treatment with an ERa ligand, but not an ER(3ligand, reduces CNS
inflammation in EAE.
[00173] The comparison of the effect of ERa versus ERP ligands in
neuropathology
was assessed. At both early (day 19) and later (day 40) stages of EKE, spinal
cord
sections from mice treated with either vehicle, ERa or ERP ligand were
assessed for
inflammation and demyelination. On hemotoxylin and eosin (H&E) staining,
vehicle
treated C578L16 EAE mice had extensive white matter inflammation at both the
early
(FIG. 14A) and later (FIG. 14C) time points as compared to the healthy
controls. As
compared to vehicle treated EAE, this inflammation was significantly reduced
by
treatment with the ERa ligand PPT. In contrast, extensive white matter
inflammation
was present in the ERj3ligand treated group at both the early and late
timepoints.
Quantification of white matter cell density by counting DAPI+ cells revealed
that
ERa ligand treated mice at the early stage of EAE had a significant, p<0.001,
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
reduction in inflammation in white matter of the thoracic cord as compared
with
vehicle treated EAE, while white matter cell densities in DPN treated EAE mice
were
not significantly different from those in vehicle treated, FIG. 14B. At the
later time
point, quantification revealed a lesser, but still significant, p<0.05,
reduction in
inflammation with ERa ligand treatment as compared to vehicle, while
inflammation
in ER(3 ligaiid treated was no different from that in vehicle treated, FIG.
14D.
[00174] Double immunohistochemistry using anti-CD4S and anti-NIF200
antibodies was then used to stain inflammatory cells and axons, respectively.
ERa
ligand treated EAE mice, as compared to vehicle treated EAE, had less C045
staining
in white matter. This reduction in C045 staining was most marked at the early
time
point in EAE (FIG. 14E), while at the later time point, some C045 staining was
detectable in the ERa ligand treated, albeit still less than in vehicle
treated (FIG. 14F).
In contrast, ERP ligand treated EAE mice did not have reduced CD45 staining in
white matter, at either the early or the later time points.
[00175] Additionally, CD45 staining of cells in gray matter of vehicle treated
EAE
mice was observed at both the early and later time points, and these cells had
a
morphology suggestive of activated microglia (FIG. 13E and F insets), ERa
ligand
treatment, but not ER(3 ligand treatment, reduced this CD4S staining in gray
matter.
[00176] Together these data indicated that ERa ligand treatment, but not ER(3
ligand
treatment, reduced inflammation in the CNS of mice with EAE. Notably, the lack
of
a reduction in CNS inflammation with ER(3 ligand treatment was consistent with
the
lack of an immunomodulatory effect of ER(3ligand treatment on the autoantigen
specific immune response in the periphery (FIG. 12).
[00177] Treatment with both an ERa ligand and an ER(3 ligand reduces
demyelination and axonal transaction in white matter in EAE. The degree of
myelin loss was then assessed by myelin basic protein (MBP) immunostaining in
the
61
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
dorsal columns of thoracic cords. Extensive demyelination occurred at the
sites of
inflammatory cell infiltrates in vehicle treated EAE mice while less
demyelination
occurred in ERa and ERP ligand treated (FIG. 15A, C). Quantification of
demyelination by density analysis of MBP immunostained spinal cord sections
revealed a 32% (p<0.01) and 34% (p<0.005) decrease in myelin density in
vehicle
treated EAE mice, at the early and later time points, respectively, as
compared to
normal controls (FIG. 17B, D). Myelin staining was relatively preserved in
both ERa
and ERP ligand treated mice, at both the early and later time points in
disease, with
reductions ranging from 7-19%, not significantly different than healthy
controls.
[00178] Staining with anti-NF200 antibody revealed axonal loss in white matter
of
vehicle treated mice at both early and later time points of disease as
compared to
normal controls, while both ERa ligand and ERP ligand treatment resulted in
less
axonal loss, as compared to that in vehicle treated EAE mice (FIG. 15 E, G).
Quantification of NF200 staining in anterior fununculus revealed a 49 12% (p<
0.01)
and 40 8% (p <0.005) reduction in vehicle treated EAE, at the early and later
time
points, respectively, as compared to healthy controls (FIG. 15F, H) Axon
numbers in
ERa ligand and ERP ligand treated EAE mice were not significantly reduced as
compared to those in healthy controls.
[00179] FIG. 15. Treatment with an ERa ligand and an ERP ligand each preserved
myelin basic protein immunoreactivity and spared axonal pathology in white
matter
of spinal cords of mice with EAE. Dorsal columns of thoracic spinal cord
sections
were imaged at lox magnification from mice in FIG. 14 that were immunostained
with
antiMBP (red). At day 19 (FIG. 15A) and day 40 (FIG. 15C) after disease
induction,
vehicle treated mice had reduced MBP immunoreactivity as compared to normal
controls, while PPT treated EAE and DPN treated EAE mice showed relatively
preserved MBP staining. Upon quantification (FIG. 15B, D), MBP
immunoreactivity
62
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
in dorsal column was significantly lower in vehicle treated EAE mice as
compared to
normal mice, while PPT and DPN treated EAE mice demonstrated no significant
decreases. Myelin density is presented as percent of norinal. Statistically
significant
compared witli norinal (*p<0.01; p<0.005), 1 x 4 ANOVAs.
[00180] Part of the anterior funniculus of thoracic spinal cord sections was
imaged
at 40X magnification from mice in FIG. 15 that were co-immunostained with anti-
NF200 (green, i) and anti-MBP (red, ii). Merged images of smaller (i) and (ii)
panels
are shown in (iii). Distinct green axonal centers surrounded by red myelin
sheaths
can be seen in normal controls, PPT and DPN treated EAE mice from 19 day (FIG.
15E) and 40 day (FIG. 15G) after disease induction. Vehicle treated mice show
reduced axonal numbers and myelin, along with focal demyelination (white
stars) and
loss of axons. Upon quantification (FIG. 15 F, H), neurofilament stained axon
numbers in white matter were significantly lower in vehicle treated EAE mice
as
compared to normal mice, while PPT and DPN treated EAE mice demonstrated no
significant reduction in axon numbers. Axon number is presented as percent of
normal. Statistically significant compared with normal (*p<0.01; **p<0.005), 1
x 4
ANOVAs.
[00181] Together these data demonstrated that ERa ligand treatment reduced
inflammation, demyelination and axonal transection in white matter during EAE,
while ER(3ligand treatment did not reduce inflammation, but nevertheless still
was
capable of reducing demyelination and axonal transection.
[00182] Treatment with both an ERa ligand and an ER(3 ligand reduces neuronal
pathology in gray mailer of mice with EAE.
[00183] In Example 5 above, we demonstrated neuronal abnormalities
surprisingly
early during EAE (day 15), which were prevented by treatment with either
estradiol or
PPT. Whether ER(3 ligand treatment might preserve neuronal integrity at both
the
63
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
early (day 19) and later (day 40) time points of EAE was examined. Using a
combination of Nissl stain histology and anti NeuN/(33-tubulin immunolabeling
of
neurons in gray matter were identified and quantified, at both the early and
later time
points in EAE. A decrease in neuronal staining in gray matter occurred at both
time
points in vehicle treated EAE mice as compared to normal controls, while
neuronal
staining in gray matter was well preserved in EAE mice treated with either the
ERa or
the ER(3ligand at the early and the later time points (FIG. 16A,C).
Quantification of
NeuN+ cells in gray matter demonstrated a 41+13% (p<0.05) and 31 8% (p<0.05)
reduction, at the early and later time points respectively, in vehicle treated
EAE mice
as compared to normal controls, while PPT and DPN treated mice had NeuN+ cell
numbers that were fewer, but not significantly different from those in healthy
controls
(FIG. 16B,D).
[00184] FIG 16. Treatment with an ERa ligand and an ER(3 ligand each preserved
neuronal staining in gray matter of spinal cords of mice with EAE. Split
images of
thoracic spinal cord sections stained with NeuN+ (red) in (i) and Nissl in
(ii) at 4X
magnification, derived from normal healthy control mice, vehicle treated EAE,
ERa
ligand (PPT) treated EAE and ER[i ligand (DPN) treated EAE mice, each
sacrificed at
either day 19 (early; FIG. 16A) or at day 40 (late; FIG. 16C) after disease
induction.
Panel (iii) is a merged confocal scan at 40X of NeuN+ (red) and (33-tubulin+
(green)
co-labeled neurons from an area represented by dotted white square area in
(i). Panel
(iv) is a 40X magnification of Nissl stained area in solid black square in
(ii). A
decrease in NeuN+ immunostaining and Nissl staining was observed in the dorsal
horn, intermediate zone and ventral horn of vehicle treated EAE mice as
compared to
normal control. White arrows in panel (iii) denote loss of NeuN+ staining. In
contrast, EAE mice treated with either PPT or DPN had preserved NeuN and Nissl
staining. Upon quantification of neurons in the entire delineated gray matter
of T1-T5
64
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
sections, NeuN+ immunolabeled neurons were significantly decreased, by nearly
41%,
in vehicle treated EAE mice at day 19 (FIG. 16B) and nearly 31 % at day 40
(FIG.
16D) as compared to normal controls, while PPT and DPN treated EAE mice were
not statistically different from normal controls. Number of mice 3 per
treatment
group, number of T1-T5 sections per mouse = 6, total number of sections per
treatment group = 18. Statistically significant compared with normals
(*p<0.05), 1 x
4 ANOVAs. Data are representative of experiments repeated in their entirety on
another set of EAE mice with each of the treatments.
[00185] Protection from neuropathology is mediated by ER(3.
[00186] To confirm whether the effect of DPN treatment in vivo on CNS
neuropathology was indeed mediated through ER(3, we next assessed white and
gray
matter neuropathology in DPN treated EAE mice deficient in ER(3. At day 38
after
disease induction, inflammation, demyelination and reductions in axon numbers
were
present in white matter, while neuronal staining was decreased in gray matter
of
vehicle treated EAE mice (FIG. 17). In contrast to the preservation of myelin,
axon
numbers and neuronal staining observed during DPN treatment of wild type mice
(FIG. 15, 16), DPN treatment of ERP knock out mice failed to prevent this
white and
gray matter pathology (FIG. 17).
[00187] FIG. 17. DPN treatment mediated protection from neuropathology during
EAE is dependent upon ER(3. As shown in FIG. 17A, part of the anterior
funniculus
of thoracic spinal cord sections from ERP knock out control mice, vehicle
treated EE(3
knock out with EAE and DPN treated ERP knock out with EAE at day 40 after
disease induction were imaged at 40X magnification upon co-immunostaining with
anti-NF200 (green, i) and anti-MBP (red, ii). Merged images are shown in panel
iii.
ERP knock out control sections showed robust NF200 and MBP immunostaining
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
similar to wild type normal controls in FIG. 18, whereas vehicle and DPN
treated
EAE sections had decreased myelin and axonal staining. FIG. 17B shows split
images of thoracic spinal cord sections, derived from mice in FIG. 17A,
stained with
NeuN (red) in (i) and Nissl in (ii) at 4X magnification, showed neuronal
losses in gray
matter of both the vehicle treated and DPN treated ERP knock out mice with
EAE.
(FIG. 17C-F) Quantification of white matter cell density, myelin density,
axonal
numbers and NeuN+ cells revealed that DPN treatment does not prevent white and
gray matter pathology during EAE in ERP knock out mice. Number of mice = 3 per
treatment group, number of Tl-T5 sections per mouse = 6, total number of
sections
per treatment group = 18. Statistically significant compared with normals (**p
<
0.001), 1 x 4 ANOVAs. These data demonstrate that direct neuroprotective
effects
mediated by DPN treatment in vivo during EAE are mediated through ER(3.
[00188] Treatment with an ER(3ligand induces recovery of motor
performance.
[00189] Since treatment with an ERP ligand was found to be neuroprotective in
EAE, the clinical significance of this neuroprotective effect was assessed.
The
clinical outcome frequently used in spinal cord injury, rotarod performance
was used.
Vehicle treated C57BL/6 EAE mice demonstrated an abrupt and consistent
decrease
in the number of seconds they were able to remain on the rotarod, beginning at
day 12
after disease induction (FIG. 18A). This disability remained throughout the
remainder of the observation period in vehicle treated EAE mice. In contrast,
ERP
ligand treated mice had an abrupt decrease in the number of seconds they could
remain on the rotarod apparatus, beginning at day 12, but later during EAE, at
days
30-40, they had significant recovery of their ability to remain on the
rotarod. These
66
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
data demonstrated that ER(3ligand treatment induces functional clinical
recovery in
motor performance at later time points of disease during EAE.
[00190] Finally, to assess whether the improvement in rotarod performance with
DPN treatment was mediated through ER(3, rotarod performance studies were
conducted in ER(3 KO female mice. The improvement in rotarod performance late
during EKE with DPN treatment was no longer observed in the ER(3 KO (FIG.
18B).
[00191] FIG. 18. Treatment with an ER(3 selective ligand results in recovery
of
motor function late during EAE. Ovariectoniized C57BL/6 female mice with EAE
were treated with DPN and assessed for motor performance on a rotarod
apparatus.
As shown in FIG. 18A, while mean time on rotarod decreased abruptly at day 12
after
disease induction in both the vehicle and DPN treated EAE mice, after day 30
the
DPN treated group demonstrated significant recovery of motor function, while
the
vehicle treated did not improve. *p <0.01 and ** p <0.005, ANOVA. Estradiol
treatment served as a positive control for a treatment effect. Nuinber of mice
in each
treatment group, vehicle n=4; DPN n = 8; estradiol n = 4. Data are
representative of
experiments repeated twice. As shown in FIG. 18B, in contrast to the
improvement
observed with DPN treatment of wild type mice, no improvement was observed at
the
later phase of disease in DPN treated ER(3 KO mice. Again, vehicle served as a
negative control, and estradiol served as a positive control, for a treatment
effect.
Number of mice in each treatment group, vehicle n = 4; DPN n = 4; estradiol n
= 4.
[00192] These data demonstrated that the DPN induced recovery in motor
performance later in disease was mediated through ER(3.
[00193] In closing, it is noted that specific illustrative embodiments of the
invention
have been disclosed hereinabove. However, it is to be understood that the
invention is
not limited to these specific embodiments.
67
CA 02623905 2008-03-26
WO 2007/038435 PCT/US2006/037259
[00194] Accordingly, the invention is not limited to the precise embodiments
described in detail hereinabove. With respect to the claims, it is applicant's
intention
that the claims not be interpreted in accordance with the sixth paragraph of
35 U.S.C.
Section 112 unless the term "means" is used followed by a functional
statement.
[00195] While the specification describes particular embodiments of the
present
invention, those of ordinary skill can devise variations of the present
invention
without departing from the inventive concept.
68