Note: Descriptions are shown in the official language in which they were submitted.
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
1,2,3,5-TETRAHYDRO-CYCLOPENTA[C]QUINOLIN-4-ONE DERIVATIVES AS
RXR AGONISTS FOR THE TREATMENT OF DYSLIPIDEMIA,
HYPERCHOLESTEROLEMIA AND DIABETES
FIELD OF THE INVENTION
The present invention relates to certain novel compounds, methods for
preparing compounds, compositions, intermediates and derivatives thereof and
for treating cancer and metabolic disorders. More particularly, the compounds
of
the present invention are Retinoid X Receptor (RXR) agonists useful for
treating,
ameliorating or inhibiting the onset of cancer and metabolic disorders such as
diabetes, dyslipidemia, and hypercholesterolemia.
BACKGROUND OF THE INVENTION
Diabetes is a chronic disorder affecting carbohydrate, fat and protein
metabolism in animals.
Type I diabetes mellitus, which comprises approximately 10% of all
diabetes cases, was previously referred to as insulin-dependent diabetes
mellitus
("IDDM") or juvenile-onset diabetes. This disease is characterized by a
progressive loss of insulin secretory function by beta cells of the pancreas.
This
characteristic is also shared by non-idiopathic, or "secondary," diabetes
having
its origins in pancreatic disease. Type I diabetes mellitus is associated with
the
following cliriical signs or symptoms: persistently elevated plasma glucose
concentration or hyperglycemia; polyuria; polydipsia and/or hyperphagia;
chronic
microvascular complications such as retinopathy, nephropathy and neuropathy;
and macrovascular complications such as hyperlipidemia and hypertension which
can lead to blindness, end-stage renal disease, limb amputation and myocardial
infarction.
Type II diabetes mellitus (non-insulin-dependent diabetes mellitus or
NIDDM) is a metabolic disorder involving the dysregulation of glucose
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
metabolism and impaired insulin sensitivity. Type II diabetes mellitus usually
develops in adulthood and is associated with the body's inability to utilize
or
make sufficient insulin. In addition to the insulin resistance observed in the
target
tissues, patients suffering from type II diabetes mellitus have a relative
insulin
deficiency -- that is, patients have lower than predicted insulin levels for a
given
plasma glucose concentration. Type II diabetes mellitus is characterized by
the
following clinical signs or symptoms: persistently elevated plasma glucose
concentration or hyperglycemia; polyuria; polydipsia and/or hyperphagia;
chronic
microvascular complications such as retinopathy, nephropathy and neuropathy;
and macrovascular complications such as hyperlipidemia and hypertension which
can lead to blindness, end-stage renal disease, limb amputation and myocardial
infarction.
Dyslipidemia, or dislipidemia, includes lipoprotein overproduction or
deficiency; sometimes associated with diabetes, it is a common cause of
lipidemia. For example, it is recommended for adults with diabetes to have
their
levels of LDL, HDL, total cholesterol, and triglyceride measured regularly.
The
desirable levels for such adults can be: LDL - less than 1,00 mg/dL (2.60
mmol/L), HDL - no less than 40 mg/dL (1.02 mmol/L), and triglyceride - less
than 150 mg/dL (1.7 mmol/L). When blood cholesterol is too high, the condition
is referred to as hypercholesterolemia. In one instance, dyslipidemia can
include
hypertriglyceridemia, and mixed hyperlipidemia. In terms of the above indices,
dyslipidemia (including hyperlipidemia) may be one or more-of the following
conditions: low HDL (< 35 or 40 mg/dl), high triglycerides (> 200 mg/dl), and
high LDL (> 150 mg/dl).
Compounds having retinoid-like activity are useful for preventing, treating
or at least alleviating the symptoms and conditions of numerous diseases and
conditions. There are two main types of retinoid receptors: the Retinoid X
Receptors (RXRs) including their subtypes RXRa, P, y, and the Retinoic Acid
Receptors (RARs), also including their subtypes RARa, P, y. Retinoid receptor
modulators are useful in a variety of conditions including, but not limited
to,
metabolic disorders, such as type II diabetes, dyslipidemia,
2
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
hypercholesterolemia, and atherosclerosis, and various cancerous and
precancerous conditions in the breast, skin, prostate, cervix, uterus, colon,
bladder, esophagus, stomach, lung, larynx, oral cavity, blood and/or lymphatic
systems. For example, RXRs belong to the nuclear receptor superfamily and
consist of a large number of ligand-regulated transcription factors that
mediate
the diverse physiological functions of steroid hormones, retinoids, thyroid
hormone, and vitamin D in embryonic development, growth, differentiation,
apoptosis, and homeostasis (Mangelsdorf, D. J., et al., Cell 83, 841-850
(1995);
Kastner, P., et al., Cell 83, 859-869 (1995)).
RXR modulators have been identified as insulin sensitizing drugs. All
diabetics, regardless of their genetic and environmental backgrounds, have in
common an apparent lack of insulin or inadequate insulin function. Because
transfer of glucose from the blood into muscle and fatty tissue is insulin
dependent, diabetics lack the ability to utilize glucose adequately, which
leads to
undesired accumulation of glucose in the blood, or hyperglycemia. Chronic
hyperglycemia leads to decrease in insulin secretion and contributes to
increased
insulin resistance, and as a result, the blood glucose concentration is
increased
so that diabetes is self-exacerbated (Diabetologia, 1985, "Hyperglycaemia as
an
inducer as well as a consequence of impaired isle cell function and insulin
resistance: implications for the management of diabetes", Vol. 28, p. 119);
Diabetes Cares, 1990, Vol. 13, No. 6, "Glucose Toxicity", pp. 610-630).
Therefore, by treating hyperglycemia, the aforementioned self-exacerbating
cycle
can be interrupted so that prophylaxis or treatment of diabetes is made
possible.
US Patent No. 6048873 to Vasudevan et al. is directed to novel
compounds having retinoid-like biological activity. More specifically, it is
directed
to compounds that include a substituted tetrahydroquinoline moiety and a 2,4-
pentadienoic acid moiety and have selective activity for retinoid X receptors.
US Patent No. 5739338 to Beard et al. is directed to novel compounds
having retinoid-like, retinoid antagonist and/or retinoid inverse-agonist-like
biological activity. More specifically, it is directed to aryl substituted
3
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
tetrahydroquinoline derivatives which bind to retinoid receptors and have
retinoid-
like, retinoid antagonist or retinoid inverse agonist-like biological
activity.
There is a continuing need for new RXR agonists. There is also a need
for RXR agonists useful for the treatment of conditions including but not
limited to
cancer and metabolic disorders such as diabetes, dyslipidemia, and
hypercholesterolemia.
SUMMARY OF THE INVENTION
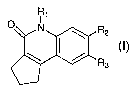
One aspect of the present invention features a compound of Formula (I)
R1
O N R2
R
3
15, (I)
wherein
R1 is H or C1_3alkyl;
(~a)n
R2 is -Z-X-C(O)OH ' wherein
- 0 s
0\/ 0/;
Z is selected from , and X is selected from a bond, optionally substituted -O-
C1_5alkylene, and
optionally substituted Ci_6alkylene;
Ra is H or optionally substituted C1_3alkoxy; and
nisl,2,or3;
4
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
or alternatively R2 is H or optionally substituted C1_3alkyl; with the proviso
(Ra)n
that when R2 is -Z X C(O)OH then R3 cannot be
(Ra)n
Z-X-C(O)OH, provided further that when R2 is H or optionally
substituted C1_3alkyl, then R3 cannot be H or optionally substituted Ci_
3alkyl; and
(Ra)n
R3 is -~ X-C(O)OH wherein
- o
0\/ o
Z is selected from and
X is selected from a bond, optionally substituted -O-C1_5alkylene, and
optionally substituted C1_6alkylene;
Ra is H or optionally substituted C1_3alkoxy; and
nis1,2,or3;
or alternatively R3 is H or optionally substituted C1_3alkyl;
(Ra)n
with the proviso that when R3 is X-C(O)OH s then R2 cannot be
(Ra)n
ZX-C(O)OH provided further that when R3 is H or optionally
substituted C1_3alkyl, then R2 cannot be H or optionally substituted Cl_
3alkyl;
or an optical isomer, enantiomer, diastereomer, racemate, or pharmaceutically
acceptable salt thereof.
Another aspect of the present invention features a pharmaceutical
composition comprising at least one compound of Formula (I) and at least one
pharmaceutically acceptable carrier.
5
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
One embodiment of the invention is a method for treating, preventing or
ameliorating a RXR mediated condition in a subject in need thereof comprising
administering to the subject a therapeutically effective amount of at least
one
compound of Formula (I). Particularly, it is an embodiment of the invention to
provide a method for treating, preventing or ameliorating a condition selected
from cancer, diabetes, dyslipidemia, hypercholesterolemia, and associated
symptoms or complications thereof in a subject in need thereof, comprising
administering to said subject a therapeutically effective amount of (a) at
least one
compound of Formula (I); and (b) at least one additional agent selected from a
retinoid receptor agonist, an anti-diabetic agent, a lipid lowering agent, an
anti-
thrombotic agent, and a blood pressure lowering agent, said~co-administration
being in any order. In one embodiment the additional agent is a RXR agonist.
Another embodiment of the invention is a method for inhibiting the onset
of a RXR condition in a subject in need thereof, comprising administering to
said
subject a therapeutically effective amount of (a) at least one compound of
Formula (I); and (b) at least one additional agent selected from a retinoid
receptor agonist, an anti-diabetic agent, a lipid lowering agent, an anti-
thrombotic
agent, and a blood pressure lowering agent, said co-administration being in
any
order and the combined amounts providing the desired prophylactic effect. In
one embodiment the additional agent is a RXR agonist.
It is a further embodiment of the invention to provide a process for making
a pharmaceutical composition comprising admixing any of the compounds
according to Formula (I) and a pharmaceutically acceptable carrier.
In the disclosed methods, the diabetes, dyslipidemia,
hypercholesterolemia, and associated symptoms or complications thereof can be
selected, for example, from IDDM, NIDDM, IGT (Impaired Glucose Tolerance),
IFG (Impaired Fasting Glucose), Syndrome X (or Metabolic Syndrome), insulin
resistance, obesity, hyperlipidemia (including, phase I hyperlipidemia, pre-
clinical
hyperlipidemia, and phase II hyperlipidemia), hypercholesteremia,
hypertriglyceridemia, insulin resistance, dyslipidemia, nephropathy,
neuropathy,
6
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
retinopathy, atherosclerosis, low HDL, non-alcoholic steatohepatitis,
polycystic
ovary syndrome or polycystic ovarian syndrome, hypertension, ischemia, stroke,
high blood pressure, heart disease (e.g., acute coronary syndromes or ACS,
including but not limited to, non-ST segment myocardial infarction and ST-
segment elevation myocardial infarctions), irritable bowel disorder,
inflammation,
cardiovascular disorders and cataracts.
Another aspect of the invention relates to treating hypertriglyceridemia,
raising levels of HDL, lowering levels of LDL, and/or lowering total
cholesterol.
Additional embodiments and advantages of the invention will become
apparent from the detailed discussion, examples, and claims below.
DETAILED DESCRIPTION OF THE INVENTION
This invention relates to novel RXR agonists and compositions thereof for
treatment or prophylaxis of conditions such as diabetes, dyslipidemia, and
hype rcholesterolemia, and associated symptoms or complications thereof.
One aspect of the present invention features a compound of Formula (I)
Ry
N
R2
\ I / R
3
(I)
wherein
R1 is H or C1_3alkyl;
(Ra)n
R2 is independently-Z-X-C(O)OHwherein
7
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
C), o S
\ / \ / Z is selected from , and
X is selected from a bond, optionally substituted -0-C1_5alkylene, and
optionally substituted C1_6alkylene;
Ra is H or optionally substituted C1_3alkoxy; and
nisl,2,or3;
or alternatively R2 is H or optionally substituted C1_3alkyl; with the proviso
(Ra)n
that when R2 is Z X C(O)OH, then R3 cannot be
(Ra)n
Z X.C(O)OHprovided further that when R2 is H or optionally
substituted C1_3alkyl, then R3 cannot be H or optionally substituted Ci_
3alkyl; and
(Ra)n
R3 is independently-Z-X-C(O)OH , wherein
- 0 S
0\/ ~ ~
Z is selected from , , and ,
X is selected from a bond, optionally substituted -O-C1_5alkylene, and
optionally substituted C1_6alkylene;
Ra is H or optionally substituted C1_3alkoxy; and
n is 1, 2, or 3;
or alternatively R3 is H or optionally substituted C1_3alkyl;
(Ra)n
with the proviso that when R3 is -Z-X-C(O)OH then R2 cannot be
(Ra)n
-Z-X-C(O)OH, provided further that when R3 is H or optionally
substituted C1_3alkyl, then R2 cannot be H.or optionally substituted C1_
3alkyl;
or an optical isomer, enantiomer, diastereomer, racemate, or pharmaceutically
acceptable salt thereof.
8
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Particularly, the present invention features a compound of Formula (I)
wherein R1 is H or C1_3alkyl. More particularly, Ri is C1_3alkyl.
Particularly, the present invention features a compound of Formula (I)
(Ra)n
wherein R2 or R3 is -Z-X-C(O)OH wherein Z is More particularly, Z
isz
Particularly, the present invention features a compound of Formula (I)
(Ra)n o S
X C(O)OH 0\/ r ~ ~ M . r..
wherein R2 or R3 is wherein Z is o o e
O s
particularly, Z is CD/ or
Particularly, the present invention features a compound of Formula (I)
(Ra)n
wherein R2 or R3 is-Z-X-C(O)OH wherein X is a bond.
Particularly, the present invention features a compound of Formula (I)
(Ra)n
wherein R2 or R3 is-Z-X-C(O)OH wherein X is optionally substituted -O-C1_
5alkylene. More particularly, the C1_5alkylene is saturated. More
particularly, the
C1_5alkylene is or More
particularly, 1, 2, 3, 4, or 5 of the hydrogen atoms in the C,_5alkylene is
further
9
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
substituted with halo. Preferably, 1, 2 or 3 of the hydrogen atoms in the C1_
5alkylene is further substituted with halo. Particularly, the halo is F.
Particularly, the present invention features a compound of Formula (I)
(Ra)n
wherein R2 or R3 is-Z X C(O)OH wherein X is optionally substituted C1_
6alkylene. More particularly, the C1_6alkylene is saturated. More
particularly, the
C1_6alkylene is.
a a a
a a a_ a a
or More particularly, 1, 2, or 3 of the
hydrogen atoms in the C1_6alkylene is further substituted with halo.
Particularly,
the halo is F.
Particularly, the present invention features a compound of Formula (I)
(Ra)n
wherein R2 or R3 is -Z X C(O)OH , wherein X is optionally substituted -0-
C1_5alkylene or optionally substituted C1_6alkylene, wherein the alkylene is
unsaturated. More particularly, the alkylene contains a double or triple bond.
Particularly, the present invention features a compound of Formula (I)
wherein Ra is -OCF3, -OCH3, -OCH2CF3, or. -CH=CH-C(O)OH.
Particularly, the present invention features a compound of Formula (I)
wherein
R1 is -CH2CH3;
(Ra)n
R~ is -Z X C(fl)OH ~ wherein
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Z is
X is -CH2CH2- or -CH=CH-; and
Ra is -OCF3; and
nis1;
or alternatively R2 is H;
(Ra)n
with the proviso that when R2 is -Z-X-C(O)OHthen R3 cannot be
(Ra)n
-Z X C(O)OH , provided further that when R2 is H, then R3 cannot be
H; and
(Ra)n
R3 is -Z-X-C(O)OH wherein
0-
Zis/
X is -CH2CH2- or -CH=CH-; and
Ra is -OCF3; and
n is 1;
or alternatively R3 is H,
(Ra)n
with the proviso that when R3 is -Z ~ C(O)OH then R2 cannot be
(Ra)n
-Z-X-C(O)OH, provided further that when R3 is H, then R2 cannot be
H.
Another aspect of the present invention features a pharmaceutical
composition comprising at least one compound of Formula (I) and at least one
pharmaceutically acceptable carrier. In another aspect of the invention, the
pharmaceutical composition further comprises at least one additional agent,
11
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
drug, medicament, antibody and/or inhibitor for treating, ameliorating and/or
preventing a RXR mediated disease. More particularly, at least one compound
/I F
0 N F*F 0
O
0
.. / H 0 N \ \ i / O
F~ \ I / H~0
of Formula (I) is selected from F_
0 o F ~IJ/F F .. ..
0
0
H 0 N \\ 0
r \ I / H~0
F F , and
In another embodiment, at least one compound of Formula (I) is either
0
0 N \ 0 O
\ I / \ O \ I / \ \ 0
H I / H
F~-F F-4,F
F F
or
In another embodiment of the invention a method is disclosed for treating,
preventing or ameliorating a RXR mediated condition in a subject in need
thereof
comprising administering to the subject a therapeutically effective amount of
at
least one compound of Formula (I). An embodiment of the invention includes a
method for treating, preventing or ameliorating a RXR mediated condition
selected from cancer, diabetes, dyslipidemia, hypercholesterolemia, and
associated symptoms or complications thereof in a subject in need thereof,
comprising administering to said subject a therapeutically effective amount of
at
least one compound of Formula (I).
A further embodiment of the invention is a method for treating, preventing
or ameliorating a RXR mediated condition selected from IDDM, NIDDM, IGT,
IFG, Syndrome X (or Metabolic Syndrome), insulin resistance, obesity,
hyperlipidemia (including, phase I hyperlipidemia, pre-clinical
hyperlipidemia, and
phase II hyperlipidemia), hypercholesteremia, hypertriglyceridemia, insulin
12
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
resistance, dyslipidemia, nephropathy, neuropathy, retinopathy,
atherosclerosis,
low HDL, non-alcoholic steatohepatitis, polycystic ovary syndrome or
polycystic
ovarian syndrome, hypertension, ischemia, stroke, high blood pressure, heart
disease (e.g., acute coronary syndromes or ACS, including but not limited to,
non-ST segment myocardial infarction and ST-segment elevation myocardial,
infarctions), irritable bowel disorder, inflammation, cardiovascular disorders
and
cataracts in a subject in need thereof, comprising administering to said
subject a
therapeutically effective amount of at least one compound of Formula (I).
One embodiment of the invention is a method of treating
hypertriglyceridemia, raising levels of HDL, lowering levels of LDL, and/or
lowering total cholesterol.
Furthermore, RXR agonists can be co-administered with a second agent
other than a retinoid receptor agonist; such second agent can be, for example,
an anti-diabetic agent, a lipid .lowering agent, a blood pressure lowering
agent,
and an anti-thrombotic agent (e.g., aspirin, heparins, glycoprotein IIb-Illa
inhibitors, or Factor Xa inhibitors).
Particularly, it is an embodiment of the invention to provide a method for
treating, preventing or ameliorating a condition selected from cancer,
diabetes,
dyslipidemia, hypercholesterolemia, and associated symptoms or complications
thereof in a subject in need thereof, comprising administering to said subject
a
thereapeuctically effective amount of (a) at least one compound of Formula
(I);
and (b) at least one adittional agent selected from a retinoid receptor
agonist, an
anti-diabetic agent, a lipid lowering agent, an anti-thrombotic agent, and a
blood
pressure lowering agent, said administration being in any order. In one
embodiment, the additional agent is a second RXR agonist. In a further
embodiment, the additional agent is an anti-diabetic agent. In another
embodiment, the additional agent is a lipid lowering agent. In still another
embodiment, the additional agent is an anti-thrombotic agent. In yet another
embodiment, the additional agent is a blood pressure lowering agent. In
another
emobodiment, the additional agent is a RAR agonist.
13
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Another, embodiment of the invention is a method for inhibiting the onset
of a RXR condition in a subject in need thereof, comprising administering to
said
subject a therapeutically effective amount of at least one compound of Formula
(I). Another embodiment of the invention is a method for inhibiting the onset
of a
condition selected from cancer, diabetes, dyslipidemia, hypercholesterolemia,
and associated symptoms or complications thereof in a subject in need thereof,
comprising administering to said subject a therapeutically effective amount of
(a)
at least one compound of Formula (I); and (b) at least one compound selected
from the group consisting of a retinoid receptor agonist, an anti-diabetic
agent, a
lipid lowering agent, an anti-thrombotic agent, and a blood pressure lowering
agent, said co-administration being in any order and the combined amounts
providing the desired prophylactic effect.
A further embodiment of the invention is a method for inhibiting the onset
of a RXR condition selected from IDDM, NIDDM, IGT, IFG, Syndrome X (or
Metabolic Syndrome), insulin resistance, obesity, hyperlipidemia (including,
phase I hyperlipidemia, pre-clinical hyperlipidemia, and phase II
hyperlipidemia),
hypercholesteremia, hypertriglyceridemia, insulin resistance, dyslipidemia,
nephropathy, neuropathy, retinopathy, atherosclerosis, low HDL, non-alcoholic
steatohepatitis, polycystic ovary syndrome or polycystic ovarian syndrome,
hypertension, ischemia, stroke, high blood pressure, heart disease (e.g.,
acute
coronary syndromes or ACS, including but not limited to, non-ST segment
myocardial infarction and ST-segment elevation myocardial infarctions),
irritable
bowel disorder, inflammation, cardiovascular disorders and cataracts in a
subject
in need thereof, comprising administering to said subject a therapeutically
effective amount of at least one compound of Formula (I). In one embodiment,
the additional agent is a second RXR agonist. In a further embodiment, the
additional agent is an anti-diabetic agent. In another embodiment, the
additional
agent is a lipid lowering agent. In still another embodiment, the additional
agent
is an anti-thrombotic agent. In yet another embodiment, the additional agent
is a
blood pressure lowering agent. In another emobodiment, the additional agent is
a RAR agonist.
14
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
It is a further embodiment of the invention to provide a process for making
a pharmaceutical composition comprising admixing any of the compounds
according to Formula (I) and a pharmaceutically acceptable carrier.
In a further embodiment of the invention, a method for treating, preventing
or ameliorating a RXR mediated condition in a subject in need thereof
comprising
administering to the subject a therapeutically effective amount of at least
one
compound of Formula (I), wherein the therapeutically effective amount of the
compound of Formula (I) is from about 0.001 mg/kg/day to about 5 mg/kg/day.
In a further embodiment of'the invention, a method for inhibiting the onset
of a RXR mediated condition in a subject in need thereof comprising
administering to the subject a therapeutically effective amount of at least
one
compound of Formula (I), wherein the therapeutically effective amount of the
compound of Formula (I) is from about 0.001 mg/kg/day to about 5 mg/kg/day.
The invention is further described below.
A) Terms
Some terms are defined below and by their usage throughout this
disclosure.
Unless otherwise noted, "alkyl" as used herein, whether used alone or as
part of a substituent group, includes straight, cyclic, and branched-chain
alkyl
having 1 to 6 carbon atoms, or any number within this range. For example,
alkyl
radicals include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, t-
butyl, 2-butenyl, 2-butynyl, n-pentyl, 3-(2-methyl)butyl, 2-pentyl, 2-
methylbutyl,
neopentyl, n-hexyl, 2-hexyl and 2-methylpentyl. "Alkoxy" radicals are oxygen
ethers formed from the previously described straight, branched, or cyclic
chain
alkyl groups.
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
The term "alkylene" denotes straight, branched, or cyclic alkyl, or straight
or branched alkenyl, or straight or branched alkynyl, optionally substituted
with
one to five, preferably one to three groups including, but not limited to,
optionally
substituted C1_3alkyl and F.
The term "oxo" whether used alone or as part of a substituent group refers
to an 0= to either a carbon or a sulfur atom. For example, phthalimide and
saccharin are examples of compounds with oxo substituents.
The term "substituted" refers to a radical in which one or more hydrogen
atoms are each independently replaced with the same or different
substituent(s).
Preferred substituents include hydroxy, halogen, oxo, amino, carboxyl, and
alkoxy.
With reference to substituents, the term "independently" means that when
more than one of such substituent is possible, such substituents may be the
same or different from each other.
The term "composition" is intended to encompass a product comprising
the specified ingredients in the specified amounts, as well as any product
which
results, directly or indirectly, from combinations of the specified
ingredients in the
specified amounts.
The term "subject" as used herein, refers to an animal, preferably a
mammal, most preferably a human, who is the object of treatment, observation
or experiment.
The term "RXR" as used herein refers to Retinoid-X Receptors.
It is intended that the definition of any substituent or variable at a
particular
location in a molecule be independent of its definitions elsewhere in that
molecule. It is understood that substituents and substitution patterns on the
compounds of this invention can be selected by one of flrdinary skill in the
art to
provide compounds that are chemically stable and that can be readily
16
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
synthesized by techniques known in the art as well as those methods set forth
herein.
Diabetes and associated symptoms or complications include such
conditions as IDDM, NIDDM, Syndrome X, IGT (impaired Glucose Tolerance),
IFG (Impaired Fasting Glucose), obesity, nephropathy, neuropathy, retinopathy,
atherosclerosis, polycystic ovary syndrome, polycystic ovarian syndrome,
hypertension, ischemia, stroke, heart disease, irritable bowel disorder,
inflammation, and cataracts. IGT and IFG are also known as "prediabetic
state."
Methods are known in the art for determining effective doses for
therapeutic and prophylactic purposes for the disclosed pharmaceutical
compositions or the disclosed drug combinations, whether or not formulated in
the same composition. For therapeutic purposes, the term "therapeutically
effective amount" as used herein, means that amount of each active compound
or pharmaceutical agent, alone or in combination, that elicits the biological
or
medicinal response in a tissue system, animal or human that is being sought by
a
researcher, veterinarian, medical doctor or other clinician, which includes
alleviation of the symptoms of the disease or disorder being treated. For
prophylactic purposes (i.e., inhibiting the onset or progression of a
disorder), the
term "therapeutically effective amount" refers to that amount of each active
compound or pharmaceutical agent, alone or in combination, that treats or
inhibits in a subject the onset or progression of a disorder as being sought
by a
researcher, veterinarian, medical doctor or other clinician. Thus, the present
invention provides combinations of two or more drugs wherein, for example, (a)
each drug is administered in an independently therapeutically or
prophylactically
effective amount; (b) at least one drug in the combination is administered in
an
amount that is sub-therapeutic or sub-prophylactic if administered alone, but
is
therapeutic or prophylactic when administered in combination with the second
or
additional drugs according to the invention; or (c) both (or more) drugs are
administered in an amount that is sub-therapeutic or sub-prophylactic if
administered alone, but are therapeutic or prophylactic when administered
together.
17
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
The term "protecting groups" refer to those moieties known in the art that
are used to mask functional groups; protecting groups may be removed during
subsequent synthetic transformations or by metabolic or other in vivo
administration conditions. During any of the processes for preparation of the
compounds of the present invention, it may be necessary and/or desirable to
protect sensitive or reactive groups on any of the molecules concerned. This
may be achieved by means of conventional protecting groups, such as those
described in Protective Groups in Organic Chemistry, ed. J.F.W. McOmie,
Plenum Press, 1973; and T.W. Greene & P.G.M. Wuts, Protective Groups in
Organic Synthesis, Third Edition, John Wiley & Sons, 1999. The protecting
groups may be removed at a convenient subsequent stage using methods known
in the art. Examples of hydroxyl and diol protecting groups are provided
below.
Protection for the hydroxyl group includes methyl ethers, substituted methyl
ethers, substituted ethyl ethers, substitute benzyl ethers, and silyl ethers.
Substituted Methyl Ethers
Examples of substituted methyl ethers include methyoxymethyl,
methylthiomethyl, t-butylthiomethyl, (phenyldimethylsilyl)methoxymethyl,
benzyloxymethyl, p-methoxybe nzyloxym ethyl, (4-methoxyphenoxy)methyl,
guaiacolmethyl, t-butoxymethyl, 4-pentenyloxymethyl, siloxymethyl, 2=
methoxyethoxymethyl, 2,2,2-trichloroethoxymethyl, bis(2-chloroethoxy)methyl, 2-
(trimethylsilyl)ethoxymethyl, tetrahydropyranyl, 3-bromotetrahydropyranyl,
tetrahydrothiopyranyl, 1-methoxycyclohexyl, 4-methoxytetrahydropyranyl, 4-
methoxytetrahydrothiopyranyl, 4-methoxytetrahydrothiopyranyl S,S-dioxido, 1 +2-
chloro-4-m ethyl)phe nyl]-4-methoxypipe rid in-4-yl, 1,4-dioxan-2-yl,
tetrahydrofuranyl, tetrahydrothiofuranyl and 2,3,3a,4,5,6,7,7a-octahydro-7,8,8-
trimethyl-4,7-methanobenzofuran-2-yl.
Substituted Ethyl Ethers
Examples of substituted ethyl ethers include 1-ethoxyethyl, 1-(2-
chloroethoxy)ethyl, 1-methyl-1 -methoxyethyl, 1-methyl-1 -benzyloxyethyl, 1-
78
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
methyl-l-benzyloxy-2-fluoroethyl, 2,2,2-trichloroethyl, 2-trimethylsilylethyl,
2-
(phenylselenyl)ethyl, t-butyl, allyl, p-chlorophenyl, p-methoxyphenyl, 2,4-
dinitrophenyl, benzyl, and polyethyleneglycol ethers.
Substituted Benzyl Ethers
Examples of substituted benzyl ethers include p-methoxybenzyl, 3,4-
dimethoxybenzyl, o-nitrobenzyl, p-nitrobenzyl, p-halobenzyl, 2,6-
dichlorobenzyl,
p-cyanobenzyl, p-phenylbenzyl, 2- and 4-picolyl, 3-methyl-2-picolyl N-oxido,
diphenylmethyl, p, p'-dinitrobenzhydryl, 5-dibenzosuberyl, triphenylmethyl, a-
naphthyidiphenylmethyl, p-methoxyphenyldiphenylmethyl, di(p-
methoxyphenyl)phenylmethyl, tri(p-methoxyphenyl)methyl, 4-(4'-
bromophenacyloxy)phenyldiphenylmethyl, 4,4',4"-tris(4,5-
dichlorophthalimidophenyl)methyl, 4,4',4"-tris(Ievulinoyloxyphenyl)methyl,
4,4',4"-tris(benzoyloxyphenyl)methyl, 3-(/midazol-1-ylmethyl)bis(4',4"-
dimethoxyphenyl)methyl, 1,1-bis{4-methoxyphenyl)-1'-pyrenylmethyl, 9-anthryl,
9-(9-phenyl)xanthenyl, 9-(9-phenyl-10-oxo)anthryl, 1,3-benzodithiolan-2-yl,
and
benzisothiazolyl S,S-dioxido.
Silyl Ethers
Examples of silyl ethers include trimethylsilyl, triethylsilyl,
triisopropylsilyl,
dimethylisopropylsilyl, diethylisopropylsilyl, dimethylthexylsilyl, t-
butyldimethylsilyl,
t-butyidiphenylsilyl, tribenzylsilyi, tri-p-xylyisilyl, triphenylsilyl,
diphenylmethylsilyl,
and t-butylmethoxyphenylsilyl.
Esters
In addition to ethers, a hydroxyl group may be protected as an ester.
Examples of esters include formate, benzoylformate, acetate, chloroacetate,
dichloroacetate, trichloroacetate, trifluoroacetate, methoxyacetate,
triphenylmethoxyacetate, phenoxyacetate, p-chlorophenoxyacetate, p-P-
phenylacetate, 3-phenylpropionate, 4-oxopentanoate(levulinate), 4,4-
(ethylenedithio)pentanoate, pivaloate, adamantoate, crotonate, 4-
methoxycrotonate, benzoate, p-phenylbenzoate, 2,4,6-
trimethylbenzoate(mesitoate), and polyethyleneglycol esters.
19
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Carbonates
Examples of carbonates include methyl, 9-fluorenylmethyl, ethyl, 2,2,2-
trichloroethyl, 2-(trimethylsilyl)ethyl, 2-(phenylsulfonyl)ethyl, 2-
(triphenylphosphonio)ethyl, isobutyl, vinyl, allyl, p-nitrophenyl, benzyl, p-
methoxybenzyl, 3,4-dimethoxybenzyl, o-nitrobenzyl, p-nitrobenzyl, S-benzyl
thiocarbonate, 4-ethoxy-1 -naphthyl, methyl dithiocarbonate, and
polyethyleneglycol carbonates.
Assisted Cleavage
Examples of assisted cleavage include 2-iodobenzoate, 4-azidobutyrate,
4-nitro-4-methylpentanoate, o-(dibromomethyl)benzoate, 2-
formylbenzenesulfonate, 2-(methylthiomethoxy)ethyl carbonate, 4-
(methylthiomethoxy)butyrate, and 2-(methylthiomethoxymethyl)benzoate.
Miscellaneous Esters
Examples of miscellaneous esters include 2,6-dichloro-4-
methylphenoxyacetate, 2,6-dichloro-4-(1,1,3,3-tetramethylbutyl)phenoxyacetate,
2,4-bis(1,1-dimethylpropyl)phenoxyacetate, chiorodiphenylacetate, isobutyrate,
monosuccinoate, (E)-2-methyl-2-butenoate(tigloate), o-
(methoxycarbonyl)benzoate, p-P-benzoate, a-naphthoate, nitrate, alkyl
N,N,N',N'-tetramethylphosphorodiamidate, N-phenylcarbamate, borate,
dimethylphosphinothioyl, and 2,4-dinitrophenylsulfenate
Sulfonates
Examples of sulfonates include sulfate, methanesulfonate(mesylate),
benzylsulfonate, and tosylate.
PROTECTION FOR 1,2- AND 1,3-DIOLS
Cyclic Acetals and Ketals
Examples of cyclic acetals and ketals include methylene, ethylidene, 1 -t-
butylethylidene, 1-phenylethylidene, (4-methoxyphenyl)ethylidene, 2,2,2-
trichloroethylidene, acetonide (isopropylidene), cyclopentylidene,
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
cyclohexylidene, cycloheptylidene, benzylidene, p-methoxybenzylidene, 2,4-
dimethoxybenzylidene, 3,4-dimethoxybenzylidene, and 2-nitrobenzylidene.
Cyclic Ortho Esters
Examples of cyclic ortho esters include methoxymethylene, ethoxymethylene,
dimethoxymethylene, 1 -methoxyethylidene, 1 -ethoxyethylidine, 1,2-
dimethoxyethylidene, a-methoxybenzylidene, 1-(N,N-dimethylamino)ethylidene
derivative, a-(N,N-dimethylamino)benzylidene derivative, and 2-
oxacyclopentylidene.
Silyl Derivatives
Examples of silyl derivatives include di- t-butylsilylene group, and 1,3-
(1,1,3,3-tetraisopropyldisiloxanylidene) derivative.
B) Compounds
STRUCTURE COMPOUND# NAME
0
1 3-[3-(5-Ethyl-4-oxo-2,3,4,5-
~ a tetrahydro-1 H-.
cyclopenta[c]quinolin-8-yl)-4-
F~'F trifluoromethoxy-phenyl]-acrylic
F acid
F'i/F
'II0' 2 3-[3-(5-Ethyl-4-oxo-2,3,4,5-
o 'r tetrahydro-1 H-
~ I e , cyclopenta[c]quinolin-7-yl)-4-
" trifluoromethoxy-phenyl]-acrylic
acid
3 3-[3-(5-Ethyl-4-oxo-2,3,4,5-
~ o tetrahydro-1 H-
H cyclopenta[c]quinolin-8-yl)-4-
F~-F trifluoromethoxy-phenyl]-propionic
F acid
21
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
F~~~F
'~0 4 3-[3-(5-Ethyl-4-oxo-2,3,4,5-
0 1 o tetrahydro-1 H-
I ,o cyclopenta[c]quinolin-7-yl)-4-
" trifluoromethoxy-phenyl]-propionic
acid
C) Synthesis
The invention provides methods of making the disclosed compounds
according to traditional organic synthetic methods as well as matrix or
combinatorial synthetic methods. Schemes 1 through 5 describe suggested
synthetic routes. Using these Schemes, the guidelines below, and the examples,
a person of skill in the art may develop analogous or similar methods for a
given
compound that is within the invention. These methods are representative of the
synthetic schemes, but are not to be construed as limiting the scope of the
jnvention.
The present invention includes within its scope prodrugs of the
compounds of this invention. In general, such prodrugs will be functional
derivatives of the compounds which are readily convertible in vivo into the
required compound. Thus, in the methods of treatment of the present invention,
the term "administering" shall encompass the treatment of the various
disorders
described with the compound specifically disclosed or with a compound which
may not be specifically disclosed, but which converts to the specified
compound
in vivo after administration to the subject. Conventional procedures for the
selection and preparation of suitable prodrug derivatives are described, for
example, in "Design of Prodrugs", ed. H. Bundgaard, Elsevier, 1985.
Where the compounds according to this invention have at least one chiral
center, they may accordingly exist as enantiomers. Where the compounds
possess two or more chiral centers, they may additionally exist as
diastereomers.
22
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Where the processes for the preparation of the compounds according to the
invention give rise to mixtures of stereoisomers, these isomers may be
separated
by conventional techniques such as preparative chromatography. The
compounds may be prepared in racemic form or as individual enantiomers or
diasteromers by either stereospecific synthesis or by resolution. The
compounds
may, for example, be resolved into their component enantiomers or
diastereomers by standard techniques, such as the formation of stereoisomeric
pairs by salt formation with an optically active base, followed by fractional
crystallization and regeneration of the free acid. The compounds" may also be
resolved by formation of stereoisomeric esters or amides, followed by
chromatographic separation and removal of the chiral auxiliary. Alternatively,
the
compounds may be resolved using a chiral HPLC column. It is to be understood
that all stereoisomers, racemic mixtures, diastereomers and enantiomers
thereof
are encompassed within the scope of the present invention.
During any of the processes for preparation of the compounds of the
present invention, it may be necessary and/or desirable to protect sensitive
or
reactive groups on any of the molecules concerned. This may be achieved by
means of conventional protecting groups, such as those described in Protective
Groups in Organic Chemistry, ed. J.F.W. McOmie, Plenum Press, 1973; and
T.W. Greene & P.G.M. Wuts, Protective Groups in Organic Synthesis, John
Wiley & Sons, 1991. The protecting groups may be removed at a convenient
subsequent stage using methods known in the art.
Furthermore, some of the crystalline forms for the compounds may exist
as polymorphs and as such are intended to be included in the present
invention.
In addition, some of the compounds may form solvates with water {i.e.,
hydrates)
or common organic solvents, and such solvates are also intended to be
encompassed within the scope of this invention.
Examples of the described synthetic routes include Examples 1 through 4
and Schemes 1-5. Compounds analogous to the target compounds of these
examples can be made according to similar routes. The disclosed compounds
are useful as pharmaceutical agents as described in the next section.
23
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Abbreviations or acronyms useful herein include:
Boc (tert butyl carbamate)
BuLi (butyllithium)
.5 DMAP (4-(dimethylamino)pyridine)
DMF (dimethylformamide)
DMPU (1,3-Dimethyl-3,4,5,6-tetrahydro-2(1 H)-pyrimidinone)
DMSO (methyl sulfoxide)
EDCI (1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride)
EtOAc (ethyl acetate)
LCMS (high pressure liquid chroatography with mass spectrometer)
LHMDS (lithium hexamethyl disilazide)
NaHMDS (sodium hexamethyl disilazide)
NaOtBu (sodium tert-butoxide)
15. NBS (N-Bromosuccinimide)
NMP (N-Methyl Pyrroidinone)
TEMPO (2,2,6,6-tetramethyl-1-piperdinyloxy, free radical)
TFA (trifluoroacetic acid);
SPE (solid phase extraction)
THF (tetrahydrofuran)
TLC (thin layer chromatography)
General Guidance
24
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Scheme 1 a
RO ::0Y0
H KOH DMSO Ri (HO)2B 0 N Br R1-I O N I~ Br A R3 R3 Pd(PPh3)4,K2CO3
Tol./EtOH
(I) (II)
RO
Ri / I R1 RO O N O ::1s
O N
C02H
q
R
s R3
a)
(iv) (l
As demonstrated in Scheme 1 a above, wherein A represents hydrogen
(H), C1_6alkyl, or C1_6alkoxy, and R represents C1_6alkyl, and Ri and R3 are
as
described hereinabove, the aniline nitrogen of (i) can be treated with an
alkyl
iodide resultant aryl bromide (ii) can undergo a Suzuki reaction with an aryl
boronic acid (iii) containing a carbonyl functionality to yield intermediate
(iv). The
intermediate (iv), where A is Ci_6alkoxy, can be directly hydrolyzed to give
benzoic acid derivatives (Ia).
Scheme 1 b below, wherein A represents H, C1-6alkyl, or aryl, and Rx and
Ry represent straight or branched C1_4alkyl, A1, A2, and A3 represent
independently H, straight or branched C1-4alkyl optionally substituted by
halo, or
halo, and Rx and Ry represent independently straight or branched C1_4alkyl,
and
R1 and R3 are as described hereinabove, shows the intermediate (iv) can be
treated with a Wadsworth-Emmon's reagent (modified Witting reagent) to yield
substituted phenylacrylic esters or phenyldienoic esters, either of which can
be
hydrolyzed to the corresponding substituted acids (lb and Ic) under either
acidic
or basic conditions.
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Scheme 1 b
O a
1) RR OORy Ri RO / I Ai
X A1 O N I" / CO2H
BuLi, THF/DMPU
A
2Hydrolysis R3
R1 RO / I (Ib)
0 N 0
A
R3
(iv)
(A= H, C1_6alky or aryl)
RO
O A2 R1 I Ai A3
1) RxO-p~/C02Ry O N C02H
R~O lAi A13 R3 A A2
BuLi, THF/DMPU
2)Hydrolysis (Ic)
Scheme 2
H
O=C=N I H
~
~ N~ p N I~ H2SO41100 C O N I~
~ p
~ ~ ~
Y Y Y
(viii) (ix) (x)
As demonstrated in Scheme 2 wherein Y is Cl, Br, or I, desired quinolone
intermediates (x) can be made using a published procedure, Bioorg. Med. Chem.
Lett. 2002, 12, 2561. Eneamine additiori to substituted aryl isocyanates
(viii)
followed by acid mediated cyclization gives quinolones (x). The quinolone
intermediates (x) can be used as the aryl bromide component (i) in the
synthetic
sequence demonstrated in Scheme 1 a.
26
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Scheme 3
Ri RO / A1 Ri RO Ai
O N CO2H C02H
H2, Pd/C O N ~ ~. I
A I / A
.R3 R3
(vi) (id)
In accordance with Scheme 3 wherein A, Ai, R, Ri, and R3 are as
described hereinabove, the double bonds of the phenylacrylic acids and phenyi
dienoic acids, such as (vi) and (vii) synthesized by a sequencesimilar to the
one
shown in Scheme la and 1 b, can be reduced by hydrogenation with metal
catalysts such as palladium on carbon to give corresponding carboxylic acids
(Id). The same acids can also be synthesized from the corresponding esters by
hydrogenation followed by hydrolysis of the ester functionality.
Scheme 4
O
Ri RO / I A1 ~~ R RO
g~ 1
O N C02t Bu O N ,\C02t Bu
R3 A NaH, D O A~
R3
(XII)
(Xili)
RO
Hydrolysis O Ni
\COOH
Al
R3
(le)
In accordance with Scheme 4 wherein A, A1, R, R1, and R3 are as
described hereinabove, the cyclopropyl carboxylic acid (xiv) can be
synthesized
by a 1,4-addition of the ylide generated from trimethylsulfoxonium iodide and
sodium hydride into the phenylacrylate ester (xii). The cyclopropyl ester
(xiii) is
then hydrolyzed to give the corresponding carboxylic acid (le). Several other
methods have been reported for the conversion of an acrylate ester into a
cyclopropyl carboxylate ester such as the reaction of (xii) with diazomethane
in
the presence of a Palladium or Copper catalyst.
27
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Scheme 5
RO RO
R 0 N1 \ \ I O 1)A-MgBr- O Ni \ \~ O
\ I/ R3 H 2)oxidation R A
g
(iv) (xv)
In accordance with Scheme 5 wherein R, R1, and R3 are as described
hereinabove, the benzaldehyde (iv) can be converted into a corresponding
ketone (xv) by -additionof an alkyl or aryl Grignard reagent or an alkyl or
aryllithium reagent into the aidehyde followed by oxidation of the
corresponding
secondary alcohol.
When R2 is H or optionally substituted C1_3alkyl and R3
(Ra)n
is -Z-X-C(O)OH wherein Z, Ra, X are as described hereinabove,
compounds of Formula I can be made in a similar fashion.
Examples
Example 1
O N O
\ I / \ ~ OH
O
CF3
3-[3-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cyclopentafclguinolin-8-yl)-4-
trifluoromethoxy-phenyll-acrylic acid (Compound 1)
28
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
A. 8-Bromo-1,2,3,5-tetrahydro-cyclopentafclguinolin-4-one (Compound 1A
This was made made from 4-bromophenyl isocyanate according to Jaroch
et al, Bioorg. Med. Chem. Lett. 2002, 12, 2561. MS (electrospray): mass
calculated for C12H10BrNO, 262.99; m/z found 264, [M+H]+.
B. 8-Bromo-5-ethyl-1,2,3,5-tetrahydro-cyclopentafclguinolin-4-one (Compound
1B
A solution of Compound 1A (2.3 g, 8.7 mmol) was dissolved in 35 mL of
DMSO and added to a prestirred 2.0 M solution of potassium hydroxide (730 mg,
13.1 mmol) in DMSO. Next, ethyl iodide (01.45 mL, 17.4 mmol) was added and
the reaction stirred at room temperature overnight. The reaction was quenched
with 100 mL of water and extracted with dichloromethane. The combined
organic extracts were dried over magnesium sulfate, filtered and
excess.solvent
removed to yield 2.13 g (84%) of desired product. MS. (electrospray): mass
calculated for C14H14BrNO, 291.03; m/z found 292, [M+H]+.
C. 3-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cyclopentafclguinolin-8-yl)-4-
trifluoromethoxy-benzaldehyde (Compound 1 C)
A round bottom flask was charged with Compound 1 B (200 mg, 0.69
mmol), 2-trifluoromethoxy-5-formylphenylboronic acid (200 mg, 0.86 mmol) and
tetrakis(triphenylphosphine)palladium(0), (40 mg, 0.035 mmol). The flask was
sealed and 3mL of toluene and 1 mL of ethanol was added. The resulting
solution was stirred to dissolve the reactants and then 1 mL of a 2 M K2CO3
solution was added via syringe. The reaction mixture was heated to 80 C for 4
hours. After cooling, the reaction mixture was partitioned between ethyl
acetate
(50 mL) and water (25 mL). The water layer was further extracted (2 x 25 mL)
and the combined organic layers were washed with water followed by brine,
dried
over magnesium sulfate, filtered and excess solvent removed on the rotary
evaporator. The crude product was purified by flash chromatography
(EtOAc/Hexanes; gradient 10% to 25%) to give 185 mg (67%) of Compound 1 C,
an off white solid. MS (electrospray): mass calculated for C22H18F3NO3i 401.1;
m/z found 402.1, [M+H]+.
D. 3-f3-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cvclopenta[clguinolin-8-yl)-4-
29
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
trifluoromethoxy-phenyll-acrylic acid tert-butyl ester (Compound 1 D
A flask charged with dimethyl t-butoxycarbonyl-methylphosphonate (0.21
mL, 1.06 mmol) in 2 mL of dry THF/DMPU (10:1) was cooled to -78 C where
0.52 mL of n-butyl lithium (2.5 M hexane solution, 32mmol) was added slowly
via
syringe. The reaction mixture was stirred at -78 C for 10 minutes and then a
solution of Compound 1 C (185 mg, 0.48 mmol) in 2 mL of dry THF was added.
After warming to room temperature and an additional 20 minutes of stirring,
the
reaction was quenched with 5 mL of water and passed through a solid phase
extraction column (SPE). The column was washed with EtOAc_ (50 mL) and the
collected eluant was evaporated to give crude product which was purified by
flash chromatography (EtOAc/Hexanes; gradient 15% to 30%) to give 205 mg
(86%) of Compound 1 D as a foamy white solid. MS (electrospray): mass
calculated for C28H28F3NO4a 499.2; m/z found 500.2, [M+H]+.
E. 343-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cyclopentafclauinolin-8-yl)-4-
trifluoromethoxy-phenyll-acrylic acid (Compound 1)
A solution of Compound 1 D (50 mg, 0.1 mmol) in 2mL of dichloromethane
and 1 mL of trifluoroacetic acid was stirred at room temperature overnight.
The
reaction mixture was then concentrated on the rotary evaporator and
redissolved
in 5 mL of diethyl ether. To this solution, hexanes were added to give a
cloudy
solution from which 43 mg (100%) of Compound 1 precipitated out as a white
solid. MS (electrospray): mass calculated for C24H2OF3N04, 443.1; m/z found
444.1, [M+H]+.
Example 2
CF3
O
01
C 2H
3-[3-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cyclopentafclguinolin-7-yl)-4-
trifluoromethoxy-phenyll-acrylic acid (Compound 2)
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
This was prepared using a procedure similar to that described to make
Compound 1, except using 3-bromophenyl isocyanate in Step 1 A. MS
(electrospray): mass calculated for C24H2oF3N04, 443.1; m/z found 444.1,
[M+H]+.
Example 3,
O N O
OH
0
I
CF3
3-f3-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cyclopenta[clguinolin-8-yl)-4-
trifluoromethoxy-phenyll-propionic acid (Compound 3)
Compound 1 (25 mg, 0.056 mmol) was dissolved in 5 mL of EtOAc and
hydrogenated with a catalytic amount (5 mg) of 10'% Pd/C-and H2 balloon for 4
hours. Pd/C was then filtered off and solvent was evaporated to afford product
as colorless oil. Evaporation from hexane or ether afforded 23 mg of Compound
3 (100%) as white sticky foam. MS (electrospray): mass calculated for
C24H22F3NO4, 445.2; m/z found 446.2, [M+H]+.
Example 4
CF3
O
O N C02H
3-[3-(5-Ethyl-4-oxo-2,3,4,5-tetrahydro-1 H-cyclopentafclguinolin-7-yl)-4-
trifluoromethoxy-phenyll-propionic acid (Compound 4)
This was prepared from Compound 2 using the procedure described to
make Compound 3. MS (electrospray): mass calculated for C24H22F3N04a 445.2;
m/z found 446.2, [M+H]+.
31
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
D) General Administration, Formulation, and Dosages
The present compounds are RXR agonists and are therefore useful in
treating, preventing, or inhibiting the progression of RXR mediated
conditions,
such as metabolic disorders including diabetes, dyslipidemia,
hypercholesterolemia, and associated symptoms or complications thereof, as
well as various cancerous and precancerous conditions in the breast, skin,
prostate, cervix, uterus, colon, bladder, esophagus, stomach, lung, larynx,
oral
cavity, 'blood and/or lymphatic systems.
The invention features a method for treating a subject with a RXR
mediated disease, said method comprising administering to the subject a
therapeutically'effective amount of a pharmaceutical'composition comprising a
compound of the invention. The invention also provides a method for treating
or
inhibiting the progression of diabetes, dyslipidemia, hypercholesterolemia,
and
associated symptoms or complications thereof in a subject, wherein the method
comprises administering to the subject a therapeutically effective amount of a
pharmaceutical composition comprising a compound of the invention.
Pharmaceutically acceptable salts include the therapeutically active non-
toxic salts of disclosed compounds. The latter can conveniently be obtained by
treating the base form with an appropriate acid. Appropriate acids comprise,
for
example, inorganic acids such as hydrohalic acids, e.g. hydrochloric or
hydrobromic acid; sulfuric; nitric; phosphoric and the like acids; or organic
acids
such as, for example, acetic, propanoic, hydroxyacetic, lactic, pyruvic,
oxalic,
malonic, succinic, maleic, fumaric, malic, tartaric, citric, methanesulfonic,
ethanesulfonic, benzenesulfonic, p-toluenesulfonic, cyclamic, salicylic,
p-aminosalicylic, palmoic and the like acids. The term salt also comprises the
solvates which the disclosed compounds, as well as the salts thereof, are able
to
form. Such solvates are for example hydrates, alcoholates and the like.
Conversely the salt ferm can be converted by treatment with alkali into the
free
base form.
32
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Stereoisomeric forms define all the possible isomeric forms which the
compounds of the invention may possess. Unless otherwise mentioned or
indicated, the chemical designation of compounds denotes the mixture of all
possible stereochemically isomeric forms, said mixtures containing all
diastereomers and enantiomers of the basic molecular structure. More in
particular, stereogenic centers may have the (R)- or (S)-configuration;
substituents on bivalentcyclic saturated radicals may have either the cis- or
trans-configuration. The invention encompasses stereochemically isomeric
forms including diastereoisomers, as well as mixtures thereof in any
proportion of
the disclosed compounds. The disclosed compounds may also exist in their
tautomeric forms. Such forms although not explicitly indicated in the above
and
following formulae are intended to be included within the scope of the present
invention.
The next section includes detailed information relating to the use of the
disclosed compounds and compositions.
E) Use
The compounds of the present invention are pharmaceutically active, for
example, as RXR agonists. According to one aspect of the invention, the
compounds are preferably selective RXR agonists.
Examples of RXR-mediated diseases include IDDM, NIDDM, IGT, IFG,
Syndrome X (or Metabolic Syndrome), insulin resistance, obesity,
hyperlipidemia
(including, phase I hyperlipidemia, pre-clin.ical hyperlipidemia, and phase II
hyperlipidemia), hypercholesteremia, hypertriglyceridemia, insulin resistance,
dyslipidemia, nephropathy, neuropathy, retinopathy, atherosclerosis, low HDL,
non-alcoholic steatohepatitis, polycystic ovary syndrome or polycystic ovarian
syndrome, hypertension, ischemia, stroke, high blood pressure, heart disease
(e.g., acute coronary syndromes or ACS, including but not limited to, non-ST
33
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
segment myocardial infarction and ST-segment elevation myocardial
infarctions),
irritable bowel disorder, inflammation, cardiovascular disorders and
cataracts.
According to one aspect of the invention, the disclosed compounds and
compositions are useful for the amelioration of symptoms associated with, the
treatment of, and the prevention of, the following conditions and diseases:
phase
I hyperlipidemia, pre-clinical hyperlipidemia, phase II hyperlipidemia,
hypercholesteremia, hypertriglyceridemia, diabetes, insulin resistance,
impaired
glucose tolerance, dyslipidemia, and cardiovascular disorders. Preferred
compounds of the invention are useful in lowering serum levels of low-density
lipoproteins (LDL), intermediate density lipoprotein (IDL), and/or small LDL
and
other atherogenic molecules, or molecules that cause atherosclerotic
- complications, thereby reducing cardiovascular disorders and/or
complications
-thereof. Preferred compounds are also useful in elevating serum levels of
high-
density lipoproteins (HDL), as well as in lowering serum levels of
triglycerides
and/or free fatty acids.
According to one aspect of the invention, the disclosed compounds may
be used in a method for treating or inhibiting the progression of an RXR
mediated
condition and, optionally, an additional Retinoid A Receptor mediated
condition,
said method comprising administering to a patient in need of treatment a
pharmaceutically effective amount of a composition of the invention.
Another aspect of the invention is a method of use wherein the RXR
mediated condition is acute coronary syndromes such as non-ST segment
myocardial infarction and ST-segment elevation myocardial infarctions.
A further aspect of the invention is a method for treating at least one RXR
mediated condition and at least one Retinoid A Receptor mediated condition in
a
patient, said method comprising administering to a patient in need of
treatment a
pharmaceutically effective amount of a composition of the invention.
34
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
The invention also features pharmaceutical compositions which include,
without limitation, one or more of the disclosed compounds, and
pharmaceutically acceptable carriers or excipients.
1. Dosages
Those of skill in the treatment of disorders or conditions mediated by RXR
could easily determine the effective daily amount from the test results
presented
hereinafter and other information. The exact dosage and frequency of
administration depends on the particular compound of invention used, the
particular condition being treated, the severity of the condition being
treated, the
age, weight and general physical condition of the particular patient as well
as
other medication the patient may be taking, as is well known to those skilled
in.
the art. Furthermore, it is evident that said effective, daily amount' may be
lowered or increased depending on the response of the treated patient and/or
depending on the evaluation of the physician prescribing the compounds of the
instant invention. The effective daily amount ranges mentioned herein are
therefore only guidelines in practicing the present invention.
The pharmaceutical compositions herein will contain, per dosage unit,
e.g., tablet, capsule, powder, injection, teaspoonful and the like, an amount
of the
active ingredient necessary to deliver an effective dose as described above.
The
pharmaceutical compositions herein will contain, per unit dosage unit, e.g.,
tablet,
capsule, powder, injection, suppository, teaspoonful and the like, of frorri
about
0.01 mg/kg to about 300 mg/kg (preferably from about 0.01 mg/kg to about 100
mg/kg; and, more preferably, from about 0.01 mg/kg to about 30 mg/kg) and may
be given at a dosage of from about 0.01 mg/kg/day to about 300 mg/kg/day
(preferably from about 0.01 mg/kg/day to about 100 mg/kg/day and more
preferably from about 0.01 mg/kg/day to about 30 mg/kg/day). Preferably, the
method for the treatment of metabolic disorders described in the present
invention using any of the compounds as defined herein, the dosage form will
contain a pharmaceutically acceptable carrier containing between from about
0.01 mg to about 100 mg; and, more preferably, from about 5 mg to about 50 mg
of the compound, and may be constituted into any form suitable for the mode of
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
administration selected. The dosages, however, may be varied depending upon
the requirement of the subjects, the severity of the condition being treated
and
the compound being employed. The use of either daily administration or post-
periodic dosing may be employed.
Preferably these compositions are in unit dosage forms from such as
tablets, pills, capsules, dry powders for reconstitution or inhalation,
granules,
lozenges, sterile parenteral solutions or suspensions, metered aerosol or
liquid
sprays, drops, ampoules, autoinjector devices or suppositories for
administration
by oral, intranasal, sublingual, intraocular, transdermal, parenteral, rectal,
vaginal, dry powder inhaler or other inhalation or insufflation means.
Alternatively, the composition may be presented in a form suitable for once-
weekly or once-monthly administration; for example, an insoluble salt of the
active compound, such as the decanoate salt, may be adapted to provide a
depot preparation for intramuscular injection.
For preparing solid pharmaceutical compositions such as tablets, the
principal active ingredient is mixed with a pharmaceutical carrier, e.g.
conventional tableting ingredients such as diluents, binders, adhesives,
disintegrants, lubricants, antiadherents and gildants. Suitable diluents
include,
but are not limited to, starch (i.e. corn, wheat, or potato starch, which may
be
hydrolized), lactose (granulated, spray dried or anhydrous), sucrose, sucrose-
based diluents (confectioner's sugar; sucrose plus about 7 to 10 weight
percent
invert sugar; sucrose plus about 3 weight percent modified dextrins; sucrose
plus
invert sugar, about 4 weight percent invert sugar, about 0.1 to 0.2 weight
percent
cornstarch and magnesium stearate), dextrose, inositol, mannitol, sorbitol,
microcrystalline cellulose (i.e. AVICEL T~~ microcrystalline cellulose
available from
FMC Corp.), dicalcium phosphate, calcium sulfate dihydrate, calcium lactate
trihydrate and the like. Suitable binders and adhesives include, but are not
limited to acacia gum, guar gum, tragacanth gum, sucrose, gelatin, glucose,
starch, and cellulosics (i.e. methylcellulose, sodium carboxymethylcellulose,
ethylcellulose, hydroxypropylmethylcellulose, hydroxypropylcellulose, and the
like), water soluble or dispersible binders (i.e. alginic acid and salts
thereof,
36
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
magnesium aluminum silicate, hydroxyethylcellulose [i.e. TYLOSE T~~available
from Hoechst Celanese], polyethylene glycol, polysaccharide acids; bentonites,
polyvinylpyrrolidone, polymethacrylates and pregelatinized starch) and the
Iike.
Suitable disintegrants include, but are not limited to, starches (corn,
potato, etc.),
sodium starch glycolates, pregelatinized starches, clays (magnesium aluminum
silicate), celluloses (such as crosslinked sodium carboxymethylcellulose and
microcrystalline cellulose), alginates, pregelatinized starches (i.e. corn
starch,
etc.), gums (i.e. agar, guar, locust bean, karaya, pectin, and tragacanth
gum);
cross-linked polyvinylpyrrolidone and the like. Suitable lubricants and
antiadherents include, but are not limited to, stearates (magnesium, calcium
and
sodium), stearic acid, talc waxes, stearowet, boric acid, sodium chloride, DL-
leucine, carbowax 4000, carbowax 6000, sodium oleate, sodium benzoate,
sodium acetate, sodium lauryl sulfate, magnesium lauryl sulfate and the Iilee.
Suitable gildants include, but are not limited to, talc, cornstarch, silica
(i.e. CAB-
O-SIL T~~silica available from Cabot, SYLOID T~~ silica available from W.R.
Grace/Davison, and AEROSIL T~~ silica available from Degussa) and the like.
Sweeteners and flavorants may be added_to chewable solid dosage forms to
improve the palatability of the oral dosage form. Additionally, colorants and
coatings may be added or applied to the solid dosage form for ease of
identification of the drug or for aesthetic purposes. These carriers are
formulated
with the pharmaceutical active to provide an accurate, appropriate dose of the
pharmaceutical active with a therapeutic release profile.
Generally these carriers are mixed with the pharmaceutical active to form
a solid preformulation composition containing a homogeneous mixture of the
pharmaceutical active of the present invention, or a pharmaceutically
acceptable
salt thereof. Generally the preformulation will be formed by one of three
common
methods: (a) wet granulation, (b) dry granulation and ~c) dry blending. When
referring to these preformulation compositions as homogeneous, it is meant
that
the active ingredient is dispersed evenly throughout the composition so that
the
composition may be readily subdivided into equally effective dosage forms such
as tablets, pills and capsules. This solid preformulation composition is then
subdivided into unit dosage forms of the type described above containing from
37
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
about 0.1 mg to about 500 mg of the active ingredient of the present
invention.
The tablets or pills containing the novel compositions may also be formulated
in
multilayer tablets or pills to provide a sustained or provide dual-release
products.
For example, a dual release tablet or pill can comprise an inner dosage and an
outer dosage component, the latter being in the form of an envelope over the
former. The two components can be separated by an enteric layer, which serves
to resist disintegration in the stomach and permits the inner component to
pass
intact into the duodenum or to be delayed in release. A variety of materials
can
be used for such enteric layers or coatings, such materials including a number
of
polymeric materials such as shellac, cellulose acetate (i.e. cellulose acetate
phthalate, cellulose acetate trimetllitate), polyvinyl acetate phthalate,
hydroxypropyl methylcellulose phthalate, hydroxypropyl methylcellulose acetate
succinate, methacrylate and ethylacrylate copolymers, methacrylate and methyl
methacrylate copolymers and the like. " Sustained release tablets may also be
made by film coating or wet granulation using slightly soluble or insoluble
substances in solution (which for a wet granulation acts as the binding
agents) or
low melting solids a molten form (which in a wet granulation may incorporate
the
active ingredient). These materials include natural and synthetic polymers
waxes, hydrogenated oils, fatty acids and alcohols (i.e. beeswax, carnauba
wax,
cetyl alcohol, cetylstearyl alcohol, and the like), esters of fatty acids
metallic
soaps, and other acceptable materials that can be used to granulate, coat,
entrap or otherwise limit the solubility of an active ingredient to achieve a
prolonged or sustained release product.
The liquid forms in which the novel compositions of the present invention
may be incorporated for administration orally or by injection include, but are
not
limited to aqueous solutions, suitably flavored syrups, aqueous or oil
suspensions, and flavored emulsions with edible oils such as cottonseed oil,
sesame oil, coconut oil or peanut oil, as well as elixirs and similar
pharmaceutical
vehicles. Suitable suspending agents for aqueous suspensions, include
synthetic and natural gums such as, acacia, agar, alginate (i.e. propylene
alginate, sodium alginate and the like), guar, karaya, locust bean, pectin,
tragacanth, and xanthan gum, cellulosics such as sodium
38
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
carboxymethylcellulose, methylcellulose, hydroxymethylcellulose,
hydroxyethylcellulose, hydroxypropyl cellulose and hydroxypropyl
methylcellulose, and combinations thereof, synthetic poiymers such as
polyvinyl
pyrrolidone, carbomer (i.e. carboxypolymethylene), and polyethylene glycol;
clays
such as bentonite, hectorite, attapulgite or sepiolite; and other
pharmaceutically
acceptable suspending agents such as lecithin, gelatin or the like. Suitable
surfactants include but are not limited to sodium docusate, sodium lauryl
sulfate,
polysorbate, octoxynol-9, nonoxynol-1 0, polysorbate 20, polysorbate 40,
polysorbate 60, polysorbate 80, polyoxamer 188, polyoxamer 235 and
combinations thereof. Suitable deflocculating or dispersing agent include
pharmaceutical grade lecithins. Suitable flocculating agent include but are
not
limited to simple neutral electrolytes (i.e. sodium chloride, potassium,
chloride,,
and the like), highly charged insoluble polymers and polyelectrolyte species,
water soluble divalent or trivalent ions (i.e. calcium salts, alums or
sulfates,
citrates and phosphates (which can be used jointly in formulations as pH
buffers
and flocculating agents). Suitable preservatives include but are not limited
to
parabens (i.e. methyl, ethyl, n-propyl and n-butyl), sorbic acid, thimerosal,
quaternary ammonium salts, benzyl alcohol, benzoic acid, chlorhexidine
gluconate, phenylethanol and the like. There are many liquid vehicles that may
be used in liquid pharmaceutical dosage forms, however, the liquid vehicle
that is
used in a particular dosage form must be compatible with the suspending
agent(s). For example, nonpolar liquid vehicles such as fatty esters and oils
liquid vehicles are best used with suspending agents such as low HLB
(Hydrophile-Lipophile Balance) surfactants, stearalkonium hectorite, water
insoluble resins, water insoluble film forming polymers and the like.
Conversely,
polar liquids such as water, alcohols, polyols and glycols are best used with
suspending agents such as higher HLB surfactants, clays silicates, gums, water
soluble cellulosics, water soluble polymers and the like. For parenteral
administration, sterile suspensions and solutions are desired. Liquid forms
useful
for parenteral administration include sterile solutions, emulsions and
suspensions. Isotonic preparations which generally contain suitable
preservatives are employed when intravenous administration is desired.
39
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Furthermore, compounds of the present invention can be administered in
an intranasal dosage form via topical use of suitable intranasal vehicles or
via
transdermal skin patches, the composition of which are well known to those of
ordinary skill in that art. To be administered in the form of a transdermal
delivery
system, the administration of a therapeutic dose will, of course, be
continuous
rather than intermittent throughout the dosage regimen.
Compounds of the present invention can also be administered in the form
of liposome delivery systems, such as small unilamellar vesicles, large
unilamellar vesicles, multilamellar vesicles and the like. Liposomes can be
formed from a variety of phospholipids; such as cholesterol, stearylamine,
phosphatidylcholines and the like.
Compounds of this invention may be administered in any of the foregoing
compositions and dosage regimens or by means of those compositions and
dosage regimens established in the art whenever treatment of RXR mediated
disorders is required for a subject in need thereof.
The daily dose of a pharmaceutical composition of the present invention
- may be varied over a wide range from about 0.7 mg to about 500 mg per adult
human per day; preferably, the dose will be in the range of from about 0.7 mg
to
about 100 mg per adult human per day; most preferably the dose will be in the
range of from about 0.7 mg to about 50 mg per adult human per day. For oral
administration, the compositions are preferably provided in the form of
tablets
containing, 0.01, 0.05, 0.1, 0.5, 1.0, 2.5, 5.0, 10.0, 15.0, 25.0, 50.0, 100,
150,
200, 250 and 500 milligrams of the active ingredient for the symptomatic
adjustment of the dosage to the subject to be treated. An effective amount of
the
drug is ordinarily supplied at a dosage level of from about-0.01 mg/kg to
about
300 mg/kg of body weight per day. Advantageously, a compound of the present
invention may be administered in a single daily dose or the total daily dosage
may be administered in divided doses of two, three or four times daily.
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Optimal dosages to be administered may be readily determined by those
skilled in the art, and will vary with the particular compOund used, the mode
of
administration, the strength of the preparation, and the advancement of the
disease condition. In addition, factors associated with the particular subject
being treated, including subject age, weight, diet and time of administration,
will
result in the need to adjust the dose to an appropriate therapeutic level.
2. Formulations
To prepare the pharmaceutical compositions of this invention, one or more
compounds of Formula (I) or salt thereof as the active ingredient, is
intimately
admixed with a pharmaceutical carrier according to conventional pharmaceutical
compounding techniques, which carrier may take a wide variety of forms
depending of the form of preparation desired for administration (e.g. oral or
parenteral). Suitable pharmaceutically acceptable carriers are well known in
the
art. Descriptions of some of these pharmaceutically acceptable carriers may be
found in The Handbook of Pharmaceutical Excipients, published by the American
Pharmaceutical Association and the Pharmaceutical Society of Great Britain.
The compounds of the present invention may be formulated into various
pharmaceutical forms for administration purposes. Methods of formulating
pharmaceutical compositions have been described in numerous publications
such as Pharmaceutical Dosage Forms: Tablets, Second Edition, Revised and
Expanded, Volumes 1-3, edited by Lieberman et al; Pharmaceutical Dosage
Forms: Parenteral Medications, Volumes 1-2, edited by.Avis et al; and
Pharmaceutical Dosage Forms: Disperse Systems, Volumes 1-2, edited by
Lieberman et al; published by Marcel Dekker, Inc.
3. Combination Therapy
The compounds of the present invention may be used in combination with
one or more pharmaceutically active agents. These agents include other RXR
modulators, other RAR modulators, other anti-diabetic agents, other lipid
41
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
lowering agents, as well as blood pressure lowering agents such as statin
drugs
and the fibrates.
Other RXR modulators include, but are not limited to:
(1) bexarotene (4 - (1 - (3,5,5,8,8 - pentamethyl - 5,6,7,8 - tetrahydro - 2 -
naphthalenyl) ethenyl) benzoic acid, known as TARGRETIN,
TARGRETYN, TARGREXIN; also known as LGD 1069, LG 100069,
LG 1069, LDG 1069, LG 69, RO 264455);
(2) 9-cis-retinoic acid;
(3) AGN-4326 (also known as ALRT -4204,,AGN -4204, ALRT -326,
ALRT-324, or LGD 1324);
(4).LGD 1324 (ALRT 324);
(5) LG 100754;
(6) LY-510929;
(7) LGD 1268 (6 - (1,1,4,4,6 - pentamethyl -*1,2,3,4 - tetrahydro - naphth -
7 - ylcycloprop - 1 - yl) nicotinic acid, known as ALRT 268 or LG
100268);
(8) LG 100264; and
(9) substituted heterocycles as disclosed in PCT publications WO
01/16122 and WO 01/16123 by Maxia.
One preferred example of substituted heterocycles is MX-6054, which is
2,4-thiazolidinedione, 5-[[3-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-
naphthalenyl)-4-(trifluoromethoxy)phenyl]methylene]-, (5z')-, also named 3-
(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-4-
trifluoromethoxybenzylidene-2,4-thiazolidinedione, reperesented by the
following
formula:
N
O ~
O
F F
F
42
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Another preferred example of substituted heterocycles is 2,4-
thiazolidinedione, 5-[[3-(1-ethyl-1,2,3,4-tetrahydro-4,4,6-trimethyl-2-oxo-7-
quinolinyl)-4-(trifluoromethoxy)phenyl]methylene]-, (5Z)-, reperesented by the
following formula:
F
F*F
0
O //
O N \ /S-'~
N
-
I \ O
Prefered substituted heterocycles are selected from:
3-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahyd ro-2-naphthyl)-4-
trifluoromethoxybenzylidene-2,4-thiazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-ne~phthyl)-1,3-
dioxolane]benzylidene-2,4-thiazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahyd ro-2-naphthyl)-2-
propyl]benzylidene-2,4-thiazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahyd ro-2-naphthyl)-1,3-
dioxolane]benzylidene-2-thioxo-2,4-thiazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)-2-
propyl]benzylidene-2-thioxo-2,4-thiazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-1,3-
dioxolane]benzylidene-2-thioxo-2,4-imidazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)-2-propyl]
benzylidene-2-thioxo-2,4-imidazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-1,3-
dioxolane]benzylidene-2,4-imidazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)-2-propyl]
benzylidene-2,4-imidazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-1,3-
dioxolane]benzyl-2,4-thiazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)-2-propyl]be.nzyl-
2,4-thiazolidinedione;
43
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-1,3-
dioxolane]benzyl-2-thioxo-2,4-thiazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahyd ro-2-naphthyl)-2-propyl]benzyl-2-
thioxo-2,4-thiazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-1,3-
dioxolane]benzyl-2-thioxo-2,4-imidazolidinedione;
4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl)-2-propyl]benzyl-2-
thioxo-2,4-imidazolidinedione;
4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)-1,3-
dioxolane]benzyl-2,4-imidazolidinedione; and
4-[2-(5,5,8,8-tetramethyl-5,6,7,8=tetrahydro-2-naphthyl)-2-propyl]benzyl-
2,4-imidazolidinedione.
Other anti-diabetic agents include thiazolidinedione and non-
thiazolidinedione insulin sensitizers, which decrease peripheral insulin
resistance
by enhancing the effects of insulin at target organs and tissues.
The following agents are known to bind and activate the nuclear receptor
peroxisome proliferator-activated receptor-gamma (PPARy) which increases
transcription of specific insulin-responsive genes. Examples of PPAR-gamma
agonists are thiazolidinediones such as:
(1) rosiglitazone (2,4 - thiazolidinedione,5 - ((4 - (2 - (methyl - 2 -
pyridinylamino) ethoxy) phenyl) methyl) -, (Z) - 2 - butenedioate (1:1) or
5 - ((4 - (2 - (methyl - 2 - pyridinylamino) ethoxy) phenyl) methyl) - 2,4 -
thiazolidinedione, known as AVANDIA; also known as BRL 49653,
BRL 49653C, BRL 49653c, SB 210232, or rosiglitazone maleate);
(2) pioglitazone (2,4 - thiazolidinedione, 5 - ((4 - (2 - (5 - ethyl - 2 -
pyridinyl) ethoxy) phenyl) methyl) -, monohydrochloride, (+ - ) - or 5 -
((4 - (2 - (5 - ethyl - 2 - pyridyl) ethoxy) phenyl) methy) - 2,4 -
thiazolidinedione, known as ACTOS, ZACTOS, or GLUSTIN; also
known as AD 4833, U 72107, U 72107A, U 72107E, pioglitazone
hydrochloride (USAN));
44
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(3) troglitazone (5 - ((4 - ((3,4 - dihydro - 6 - hydroxy - 2,5,7,8 -
tetramethyl
- 2H - 1 - benzopyran - 2 - yl) methoxy) phenyl) methyl) - 2,4 -
thiazolidinedione, known as NOSCAL, REZULIN, ROMOZIN, or
PRELAY; also known as Cl 991, CS 045, GR 92132, GR 92132X);
(4) isaglitazone ((+)-5-[[6-[(2-fluorophenyl)methoxy]-2-
naphthalenyl]metfiyl]-2,4-thiazolidinedione or 5 - ((6 - ((2 -
fluorophenyl) methoxy) - 2 - naphthalenyl) methyl - 2,4 -
thiazolidinedione or 5 - (6 - (2 - fluorobenzyloxy) naphthalen - 2 -
ylmethyl) thiazolidine - 2,4 - dione, also known as MCC-555 or
neoglitazone); and
(5) 5-BTZD.
Additionally, the non-thiazolidinediones that act as insulin sensitizing
agents include, but are not limited to:
(1) JT-501 (JTT 501, PNU-1827, PNU-716-MET-0096, or PNU 182716::
isoxazolidine - 3, 5 - dione, 4 - ((4 - (2 - phenyl - 5 - methyl) - 1,3-
oxazolyl) -
ethylphenyl - 4) methyl -);
(2) KRP-297 (5 - (2, 4 - dioxothiazolidin - 5 - ylmethyl) - 2 - methoxy - N -
(4 - (trifluoromethyl) benzyl) benzamide or 5 - ((2,4 - dioxo - 5 -
thiazolidinyl) methyl) - 2 - methoxy - N - ((4 - (trifluoromethyl) phenyl) m
ethyl) benzamide); and
(3) Farglitazar (L - tyrosine, N - (2 - benzoylphenyl) - o - (2 - (5 - methyl -
2
- phenyl - 4 - oxazolyl) ethyl) - or N - (2 - benzoylphenyl) - O - (2 - (5 -
methyl - 2 - phenyl - 4 - oxazolyl) ethyl) - L - tyrosine, or GW2570 or
GI-262570).
Other anti-diabetic agents have also been shown to have PPAR modulator
activity such as PPAR gamma, SPPAR gamma, and/or PPAR delfa/gamma
agonist activity. Examples are listed below:
(1) AD 5075;
(2) R 119702 ((+ - ) - 5 - (4 - (5 - Methoxy - 1 H - benzimidazol - 2 -
ylmethoxy) benzyl) thiazolin - 2, 4 - dione hydrochloride, or Cl 1037 or
CS 011);
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(3) CLX-0940 (peroxisome proliferator-activated receptor alpha agonist /
peroxisome proliferator-activated receptor gamma agonist);
(4) LR-90 (2,5,5 - tris (4 - chlorophenyl) - 1,3 - dioxane - 2 - carboxylic
acid, PPARdelta/y agonist);
(5) Tularik (PPARy agonist);
(6) CLX-0921 (PPARy agonist);
(7) CGP-52608 (PPAR agonist);
(8) GW-409890 (PPAR agonist);
(9) GW-7845 (PPAR agonist);
(10) L-764406 (PPAR agonist);
(11) LG-1 01280 (PPAR agonist);
(12) LM-4156 (PPAR agonist);
(13) Risarestat (CT-112);
(14) YM 440 (PPAR agonist);
(15) AR-H049020 (PPAR agonist);
(16) GW 0072 (4 - (4 - ((2S,5S) - 5 - (2 - (bis (phenylmethyl) amino) - 2 -
oxoethyl) - 2 - heptyl - 4 - oxo - 3 - thiazo lidinyl) butyl) benzoic acid);
(17) GW 409544 (GW-544 or GW-409544);
(18) NN 2344 (DRF 2593);
(19) NN 622 (DRF 2725);
(20) AR-H039242 (AZ-242);
(21) GW 9820 (fibrate);
(22) GW 1929 (N - (2 - benzoylphenyl) - 0 - (2 - (methyl - 2 -
pyridinylamino) ethyl) - L - tyrosine, known as GW 2331, PPAR alpha/y
agonist);
(23) SB 219994 ((S) - 4 - (2 - (2 - benzoxazolylmethylamino) ethoxy) -
alpha - (2,2,2 - trifluoroethoxy) benzen epropanoic acid or 3 - (4 - - .(2 -
(N - (2 - benzoxazolyl) - N - methylamino) ethoxy) phenyl) - 2 (S) - (2,
2, 2 - trifluoroethoxy) propionic acid or benzenepropanoic acid,4 - (2 -
(2 - benzoxazolylmethylamino) ethoxy) - alpha - (2,2,2 - trifluoroethoxy)
-, (alphaS) -, PPARalpha/y agonist);
(24) L-796449 (PPAR alpha/,y agonist);
46
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(25) Fenofibrate (Propanoic acid, 2-[4-(4-chlorobenzoyl)phenoxy]-2-
methyl-, 1-methylethyl ester, known as TRICOR, LIPCOR, LIPANTIL,
LIPIDIL MICRO PPAR alpha agonist);
(26) GW-9578 (PPAR alpha agonist);
(27) GW-2433 (PPAR alpha/y agonist);
(28) GW-0207 (PPARy agonist);
(29) LG-1 00641 (PPARy agonist);
(30) LY-300512 (PPARy agonist);
(31) NID525-209 (NID-525);
(32) VDO-52 (VDO-52);
(33) LG 100754 (peroxisome proliferator-activated receptor agonist);
(34) LY-510929 (peroxisome proliferator-activated receptor agonist);
(35) bexarotene (4 - (1 - (3,5,5,8,8 - pentamethyl - 5,6,7,8 - tetrahydro - 2
naphthalenyl) ethenyl) benzoic acid, known as TARGRETIN,
TARGRETYN, TARGREXIN; also known as LGD 1069, LG 100069,
LG 1069, LDG 1069, LG 69, RO 264455); and
(36) GW-1536 (PPAR alpha/y agonist).
Other insulin sensitizing agents include, but are not limited to:
(1)1NS-1 (D-chiro inositol or D- 1, 2, 3, 4, 5, 6 -
hexahydroxycyclohexane);
(2) protein tyrosine phosphatase 1 B(PTP-1 B) inhibitors;
{3) glycogen synthase kinase-3 (GSK3) inhibitors;
(4) beta 3 adrenoceptor agonists such as ZD 2079 ((R) - N-(2 (2,-
(carboxymethyl) phenoxy) ethyl) - N - (2 - hydroxy - 2 - phenethyl)
ammonium chloride, also known as ICI D 2079) or AZ 40140;
(5) glycogen phosphorylase inhibitors;
(6) fructose-1,6-bisphosphatase inhibitors;
(7) chromic picolinate, vanadyl sulfate (vanadium oxysulfate);
(8) KP 102 (organo-vanadium compound);
(9) chromic polynicotinate;
(10) potassium channel agonist NN 414;
47
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(11) YM 268 (5, 5' - methylene - bis (1, 4 - phenylene) bismethylenebis
(thiazolidine - 2, 4 - dione);
(12)TS971;
(13) T 174 ((+ - ) - 5 - (2, 4 - dioxothiazolidin - 5 - ylmethyl) - 2 - (2 -
naphthylmethyl) benzoxazole);
(14) SDZ PGU 693 ((+) - trans - 2 (S - ((4 - chlorophenoxy) methyl) -
7alpha - (3, 4 - dichlorophenyl) tetrahydropyrrolo (2,1 - b) oxazol - 5
(6H) - one);
(15) S 15261 (( - ) - 4 - (2 - ((9H - fluoren - 9 - ylacetyl) amino) ethyl)
10. benzoic acid 2 - ((2 - methoxy - 2 - (3-(trifluoromethyl) phenyl) ethyl)
amino) ethyl ester);
(16) AZM 134 (Alizyme);
(17) ARIAD;
(18) R 102380;
(19) PNU 140975 (1 -(hydrazinoiminomethyl) hydrazino) acetic acid;
(20) PNU 106817 (2 -(hydrazinoiminomethyl) hydrazino) acetic acid;
(21) NC 2100 (5 - ((7 - (phenylmethoxy) - 3 - quinolinyl) methyl) - 2,4 -
thiazolidinedione;
(22) MXC 3255;
(23) MBX 102;
(24) ALT 4037;
(25) AM 454;
(26) JTP 20993 (2 - (4 - (2 - (5 - methyl - 2 - phenyl - 4 - oxazolyl) ethoxy)
benzyl) - malonic acid dimethyl diester);
(27) Dexlipotam (5 (R) - (1, 2 - dithiolan - 3 - yl) pentanoic acid, also
known as (R)-alpha lipoic acid or (R)-thioctic acid);
(28) BM 170744 (2, 2 - Dichloro - 12 - (p - chlorophenyl) dodecanoic acid);
(29) BM 152054 (5 - (4 - (2 - (5 - methyl - 2 - (2 - thienyl) oxazol - 4 - yi)
ethoxy) benzothien - 7 - ylmethyl) thiazolidine - 2, 4- dione);
(30) BM 131258 (5 - (4 - (2 - (5 - methyl - 2 - phenyloxazol - 4 - yl) ethoxy)
benzothien - 7 - ylmethyl) thiazolidine - 2, 4 - dione);
(31) CRE 16336 (EML 16336);
48
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(32) HQL 975 (3 - (4 - (2 - (-5 - methyl - 2 - phenyloxazol - 4 - yl) ethoxy)
phenyl) - 2 (S) - (propylamino) propionic acid);
(33) DRF 2189 (5 - ((4 - (2 - (1 - Indolyl) ethoxy) phenyl) methyl)
thiazolidine - 2, 4 - dione);
(34) DRF 554158;
(35) DRF-NPCC;
(36) CLX 0100, CLX 0101, CLX 0900, or CLX 0901;
(37) IkappaB Kinase (IKK B) Inhibitors
(38) mitogen-activated protein kinase (MAPK) inhibitors
p38 MAPK Stimulators
(39) phosphatidyl-inositide triphosphate
(40) insulin recycling receptor inhibitors
(41) glucose transporter 4 modulators
(42) TNF-a antagonists
(43) plasma cell differentiation antigen-1 (PC-1) Antagonists
(44) adipocyte lipid-binding protein (ALBP / aP2) inhibitors
(45) phosphoglycans
(46) Galparan;
(47) Receptron;
(48) islet cell maturation factor;
(49) insulin potentiating factor (IPF or insulin potentiating factor-1);
(50) somatomedin C coupled with binding protein (also known as IGF-
BP3, IGF-BP3, SomatoKine);
(51) Diab II (known as V-411) or Glucanin, produced by Biotech Holdings
Ltd. or Volque Pharmaceutical;
(52) glucose-6 phosphatase inhibitors;
(53) fatty acid glucose transport protein;
(54) glucocorticoid receptor antagonists; and
(55) glutamine:fructose-6-phosphate amidotransferase (GFAT)
modulators.
49
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Anti-diabetic agents also include biguanides, which decreases liver
glucose production and increases the uptake of glucose. Examples of
biguanides include metformin such as:
(1) 1, 1 - dimethylbiguanide (e.g., Metformin - DepoMed, Metformin -
.5 Biovail Corporation, or METFORMIN GR (metformin gastric retention
polymer)); and
(2) metformin hydrochloride (N,N -dimethylimidodicarbonimidic diamide
monohydrochloride, also known as LA 6023, BMS 207150,
GLUCOPHAGE, or GLUCOPHAGE XR.
Anti-diabetic agents also include alpha-glucosidase inhibitors, vvhich
inhibit alpha-glucosidase. Alpha-glucosidase converts fructose to glucose,
thereby delaying the digestion of carbohydrates. The undigested carbohydrates
are subsequently broken down in the gut, reducing the post-prandial glucose
peak. Examples of alpha-glucosidase inhibitors include, but are not limited
to:
(1) acarbose (D - glucose, O - 4,6 - dideoxy - 4 - (((1 S -
(1 alpha,4alpha,5beta,6alpha)) - 4,5,6 - trihydroxy - 3 - (hydroxymethyl)
- 2 - cyclohexen - 1 - yl) amino) - alpha - D - glucopyranosyl - (1 - 4) - 0
- alpha - D - glucopyranosyl - (1 - 4) -, also known as AG - 5421, Bay -
g-542, BAY-g-542, GLUCOBAY, PRECOSE, GLUCOR, PRANDASE,
GLUMIDA, or ASCAROSE);
(2) Miglitol (3,4,5 - piperidinetriol, 1 - (2 - hydroxyethyl) - 2 -
(hydroxymethyl) -, (2R (2alpha, 3beta, 4alpha, 5beta)) - or
(2R,3R,4R,5S). - 1 - (2 - hydroxyethyl) - 2 - (hydroxymethyl - 3,4,5 -
piperidinetriol, also known as BAY 1099, BAY M 1099, BAY-m-1099,
BAYGLITOL, DIASTABOL, GLYSET, MIGLIBAY, MITOLBAY,
PLUMAROL);
(3) CKD-711 (0 - 4 - deoxy - 4 - ((2,3 - epoxy - 3 - hydroxymethyl - 4,5,6 -
trihydroxycyclohexane - 1 - yl) amino) - alpha - b - glucopyranosyl - (1 -
4) - alpha - D - glucopyranosyl -(1 - 4) - D - glucopyranose);
(4) emiglitate (4 - (2 - ((2R,3R,4R,5S) - 3,4,5 - trihydroxy - 2 -
(hydroxymethyl) - 1 - piperidinyl) ethoxy) benzoic acid ethyl ester, also
known as BAY o 1248 or MKC 542);
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(5) MOR-14 (3,4,5 - piperidinetriol, 2 - (hydroxymethyl) - 1 - methyl -, (2R -
(2alpha,3beta,4alpha,5beta)) -, also known as N-
methyldeoxynojirimycin or N-methylmoranoline); and
(6) Voglibose (3,4 - dideoxy - 4 - ((2 - hydroxy - 1 - (hydroxymethyl) ethyl)
amino) - 2 - C - (hydroxymethyl) - D - epi - inositol or D - epi -
Inositol,3,4 - dideoxy - 4 - ((2 - hydroxy - 1 - (hydroxymethyl) ethyl)
amino) - 2 - C - (hydroxymethyl) -, also known as A 71100, AO 128,
BASEN, GLUSTAT, VOGLISTAT.
Anti-diabetic agents also include insulins such as regular or short-acting,
intermediate-acting, and long-acting insulins, non-injectable or inhaled
insulin,
tissue selective insulin, glucophosphokinin (D-chiroinositol),
insulin.analogues
such as insulin molecules with minor differences in the natural amino acid
sequence and small molecule mimics of insulin (insulin mimetics), and
e'ndosome
modulators. Examples include, but are not limited to:
(1) Biota;
(2) LP 100;
(3) (SP - 5 - 21) - oxobis (1 - pyrrolidinecarbodithioato - S, S') vanadium,
(4) insulin aspart (human insulin (28B - L - aspartic acid) or B28-Asp-
insulin, also known as insulin X14, INA-X14, NOVORAPID, NOVOMIX,
or NOVOLOG);
(5) insulin detemir (Human 29B - (N6 - (1 - oxotetradecyl) - L - lysine) - (1A
- 21A), (1 B - 29B) - Insulin or NN 304);
(6) insulin lispro ("28B - L - lysine - 29B - L - proline human insulin, or
Lys(B28), Pro(B29) human insulin analog, also known as lys-pro
insulin, LY 275585, HUMALOG, HUMALOG MIX 75/25, or HUMALOG
MIX 50/50);
(7) insulin glargine (human (A21 - glycine, B31 - arginine, B32 - arginine)
insulin HOE 901, also known as LANTUS, OPTISULIN);
(8) Insulin Zinc Suspension, extended (Ultralente), also known as
HUMULIN U or ULTRALENTE;
51
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(9) Insulin Zinc suspension (Lente), a 70% crystalline and 30% amorphous
insulin suspension, also known as LENTE ILETIN II, HUMULIN L, or
NOVOLIN L;
(10) HUMULIN 50/50 (50% isophane insulin and 50% insulin injection);
(11) HUMULIN 70/30 (70% isophane insulin NPH and 30% insulin
injection), also known as NOVOLIN 70/30, NOVOLIN 70/30 PenFill,
NOVOLIN 70/30 Prefilled;
(12) insulin isophane suspension such as NPH ILETIN II, NOVOLIN N,
NOVOLIN N PenFill, NOVOLIN N Prefilled, HUMULIN N;
(13) regular insulin injection such as ILETIN II Regular, NOVOLIN R,
VELOSULIN BR, NOVOLIN R PenFill, NOVOLIN R Prefilled,
HUMULIN R, or Regular U-500 (Concentrated);
(14) ARIAQ;
(15) LY 197535;
(16) L-783281; and
(17) TE-1 7411.
Anti-diabetic agents also include insulin secretion modulators such as:
(1) glucagon-like peptide-1 (GLP-1) and its mimetics;
(2) glucose-insulinotropic peptide (GIP) and its mimetics;
(3) exendin and its mimetics;
(4) dipeptyl protease (DPP or DPPIV) inhibitors such as
(4a) DPP-728 or LAF 237 (2 - pyrrolidinecarbonitrile,l - (((2 - ((5 -
cyano - 2 - pyridinyl) amino) ethyl) amino) acetyl), known as NVP -
DPP - 728, DPP - 728A, LAF - 237);
(4b) P 3298 or P32/98 (di - (3N - ((2S, 3S) - 2 - amino - 3 - methyl -
pentanoyl) - 1, 3 - thiazolidine) fumarate);
(4c) TSL 225 (tryptophyl - 1,2,3,4 - tetrahydroisoquinoline - 3 -
carboxylic acid);
(4d) Valine pyrrolidide (valpyr);
(4e) 1-aminoalkylisoquinolinone-4-carboxylates and analogues thereof;
(4f) SDZ 272-070 (1 - (L - Valyl) pyrrolidine);
(4g) TMC-2A, TMC-2B, or TMC-2C;
52
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
(4h) Dipeptide nitriles (2-cyanopyrrolodides);
(4i) CD26 inhibitors; and
(4j) SDZ 274-444;
(5) glucagon antagonists such as AY-279955; and
(6) amylin agonists which include, but are not limited to, pramlintide (AC-
137, Symlin, tripro-amylin or pramlintide acetate).
Known anti-diabetic agents include insulin, sulfonylureas, biguanides,
meglitinides, AGI's (Alpha-Glucosidase Inhibitors; e.g., Glyset), PPAR alpha
agonists, and PPAR gamma agonists, and dual PPAR alpha/gamma agonists.
Examples of lipid lowering agents include, bile acid sequestrants, fibric acid
derivatives, nicotinic acid, and HMGCoA reductase inhibitors. Specific
examples
include statins such as LIPITOR , ZOCOR , PRAVACHOL , LESCOL , and
MEVACOR , and pitavastatin (nisvastatin) (Nissan, Kowa Kogyo, Sankyo,
Novartis) and extended release forms thereof, such as ADX-159 (extended
release lovastatin), as well as Colestid, Locholest, Questran, Atromid, Lopid,
and
Tricor.
Examples of blood pressure lowering agents include anti-hypertensive
agents, such as angiotensin-converting enzyme (ACE) inhibitors (Accupril,
Altace, Captopril, Lotensin Mavik, Monopril, Prinivil, Univasc, Vasotec, and
Zestril), adrenergic blockers (such as Cardura, Dibenzyline, Hylorel, Hytrin,'
Minipress, and Minizide) alpha/beta adrenergic blockers (such as Coreg,
Normodyne, and Trandate), calcium channel blockers (such as Adalat, Calan,
Cardene, Cardizem, Covera-HS, Dilacor, DynaCirc, Isoptin, Nimotop, Norvace,
Plendil, Procardia, Procardia XL, Sula, Tiazac, Vascor, and Verelan),
diuretics,
angiotensin II receptor antagonists (such as Atacand, Avapro, Cozaar, and
Diovan), beta adrenergic blockers (such as Betapace, Blocadren, Brevibloc,
Cartrol, Inderal, Kerlone, Lavatol, Lopressor, Sectral, Tenormin, Toprol-XL,
and
Zebeta), vasodilators (such as Deponit, Dilatrate, SR, lmdur, Ismo, lsordil,
Isordil
Titradose, Monoket, Nitro-Bid, Nitro-Dur, Nitrolingual Spray, Nitrostat, and
53
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Sorbitrate), and combinations thereof (such as Lexxel, Lotrel, Tarka, Teczem,
Lotensin HCT, Prinzide, Uniretic, Vaseretic, Zestoretic).
In addition, a second RXR or RAR modulator, as described above in
Section B), may also be utilized as a third antidiabetic agent, provided that
it is
different from the first RXR or RAR modulator.
F) Biological Examples
ABCA1 bDNA Assay
THP-1 cells, a human monocytic cell line, were obtained from ATCC and
maintained in RPMI (Gibco) supplemented with 10% fetal bovine serum (Gibco),
2mM L-glutamine and 1% antibiotic-antimycotic in 5% CO2 at 37 C. For ABCA1
mRNA induction assays, the cells were pelleted and resuspended in RPMI
- supplemented with 0.5% charcoal treated serum (Hyclone), 2mM glutamine and
1% antibiotic-antimycotic. The cells were plated at a density of 40,000
ceNs/90 1
and incubated as above for at least 4 hours before the initiation of
treatments.
Compounds were prepared as 10mM stocks in DMSO. For treatments, the
compounds were diluted in medium and 10 I of 10X stocks were added to the
cells at a final concentration 0.1% DMSO. The cells were incubated for the
desired amount of time (usually 18-24 hrs) and then lyzed with 50 l of
Quantigene HV bDNA lysis buffer which also contained the ABCA1 bDNA probes
(probe sequences shown below):
ABCA1 bDNA probe sequences
Primer Name Sequence
hABC1001 CGGGTAACGGAAACAGGGGTTGTTTTTCTCTTGGAAAGAAAGT
hABC1002 TCCGGGAGCCTCCCCAGGAGTTTTTTCTCTTGGAAAGAAAGT
hABC1003 GCCAGTTTCTCCCTTGGTAGTTTTTCTCTTGGAAAGAAAGT
hABC1004 CTCCTTGCTCGGGAAGGGTTTTTCTCTTGGAAAGAAAGT
hABC1005 AACAGCTCCTGGGCCAGAGTTTTTCTCTTGGAAAGAAAGT
hABC1006 TTCAGCCCCCCTCCCTCGGGATTTTTTCTCTTGGAAAGAAAGT
54
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
hABC1007 ATGCGGGAAAGAGGACTAGTTTTTCTCTTGGAAAGAAAGT
hABC1008 GTTCACCTCAGCCATGACCTTTTTTCTCTTGGAAAGAAAGT
hABC1009 CATGCCTTCCAGATCATGGAACTTTTTCTCTTGGAAAGAAP:GT
hABC1010 GCGAGCCACAATGGATTTTTTTTAGGCATAGGACCCGTGTCT
hABC1011 CTCCGAGCATCTGAGAACAGTTTTTAGGCATAGGACCCGTGTCT
hABC1012 TCAGAACTTTGCGCATGTCCTTTTTTAGGCATAGGACCCGTGTCT
hABC1013 GGATTTCTTGATCTGCTGTAATGTTCTTTTTAGGCATAGGACCCGTGTCT
hABC1014 AAGGTTTCATTGTCCACCAGGAATTTTTAGGCATAGGACCCGTGTCT
hABC1015 GGTTGTGATACAGGAACCCAGAGTTTTTAGGCATAGGACCCGTGTCT
hABC1016 GTCCACAGTAGACTTTGGGAGAGAGATTTTTAGGCATAGGACCCGTGTCT
hABC1017 TGACATCAGCCCTCAGCATCTTTTTTTAGGCATAGGACCCGTGTCT
hABC1018 CTTGTCAAATGTAACTGGTAGCCTTTTTTTAGGCATAGGACCCGTGTCT
hABC1019 CTGATTTTGATCCATTGCACAGATTTTTAGGCATAGGACCCGTGTCT
hABC1020 GGCTTCAGGATGTCCATGTTGTTTTTAGGCATAGGACCCGTGTCT
15' hABC1021 AGATGTAGAGTTTAGTGTTCTCAGGATTTTTTTAGGCATAGGACCCGTGTCT
hABC1022 TTTTGTGGCTTCGGCCAGTTTTTAGGCATAGGACCCGTGTCT
hABC1023 TCCCAAGACTATGCAGCAATGTTTTTTAGGCATAGGACCCGTGTCT
hABC1024 GTCACTCCAGCTTCTCATGCTGTTTTTAGGCATAGGACCCGTGTCT
hABC1025 GAAACATCACCTCCTGTCGCATTTTTTAGGCATAGGACCCGTGTCT
hABC1026 GCCTGGTAGATTTGGGTGGTTTTTAGGCATAGGACCCGTGTCT
hABC1027 GCCCGCAGACAATACGAGACACATTTTTAGGCATAGGACCCGTGTCT
hABC1028 -GGCTTTGTAGTTGTTGTCCTCTTTTTAGGCATAGGACCCGTGTCT
hABC1029 TGCCATTGCCTCCAAAGAGTTTTTAGGCATAGGACCCGTGTCT
hABC1030 AAGGTTTCAGCATCTTCCTCAGTTTTTAGGCATAGGACCCGTGTCT
hABC1031 TGCAGTAAGGAGTTGTAGAGTTGTCATAGTTTTTAGGCATAGGACCCGTGTCT
hABC1032 ACTCCAAATTCTTCATCAAATCATTTTTTAGGCATAGGACCCGTGTCT
hABC1033 CGGCTTCAGAGCTTTCCAGATATTTTTAGGCATAGGACCCGTGTCT
hABC1034 GTTAAAGTTTCCAACAAC
hABC1035 TGTATAAAAGAAGC
hABC1036 TCATGCTGGTGTCTTTCTGGC
hABC1037 ATCTTGAAGCTTCAAGTTTGAGCT
hABC1038 GCAAAAATACCTTGTGGAGAA
hABC1039 GGTCACCAAGTTGAATCATCTCTT
hABC1040 GCCACAAAGCTCAGAAACTTCTT
hABC1041 GAACGAAGTACTCGCTCTGCTGCA
hABC1042 AGGAGCTGGAGCTGTTCACATTGGTCA
hABC1043 ATACCAGTTGAGAGACTTGATC
hABC1044 ATACAGGATCTTCCCAACGAGCAG
hABC1045 GCCTTGTGGCTGGAGTGTCAGGTGT
hABC1046 ACAGCCAGTTCCTGGAAGGTCTT
RXR Co-transfection Assay
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
The nucleic acid sequence from 676-1464bp (accession number X52773)
encoding the ligand binding domains was subcloned into the pM vector (BD
Biosciences Clontech, Palo Alto, CA) and were fused with the DNA binding
domain of yeast GAL4. HEK293 cells were cultured in DMEM/F12 medium
supplemented with 10% FBS and 1% 1-glutamine (growth medium). Cells were
seeded at a density of 5-10x106 cells in 50 ml of growth medium and left
overnight. The medium was removed and the cells were washed with 15 ml of
OptiMEM serum free medium (Invitrogen Corp). The cells were transfected
using OptiMEM serum free medium and DMRIE transfection reagent (Invitrogen
Corp). Approximately 10-30 ng of DNA for the different receptors and 5-10 ng
of
the luciferase reporter (1:1 ratio for RAR and 4:1 ratio for RXR of receptor
DNA:reporter DNA) were gently mixed with 51 l of DMRIE reagent in a total
volume of 17 ml of OptiMEM medium. Eighteen hours after transfection, the
15. cells were washed once with growth medium and then incubated for 6 hrs in
30
ml of growth medium. The cells were then trypsinized, and reseeded at a
density
of 50,000 per well in 96 well plates and left overnight. The medium was
replaced
with 90 l of medium containing DMEM/F12 supplemented with 0.5% charcoal-
treated FBS (HyClone; Logan, UT) and 1% glutamine. Compounds or vehicle
were added in 10 l of 10X concentration of compound or vehicle (0.1 %
dimethyl
sulfoxide). The cells were treated for 16-18 hours, lysed and assayed for
luciferase activity using the Steady Glo luciferase assay kit (Promega,
Madison,
WI).
Compounds listed in Table II below were tested in the above assay(s):
56
CA 02624345 2008-03-28
WO 2007/041076 PCT/US2006/037321
Table II
RXR co-transfection EC50 in nM (% of
2,4-thiazolidinedione, 5-[[3-(1-ethyl-
ABCA1 EC50 in nM 1,2,3,4-tetrahydro-4,4,6-trimethyl-2-oxo-
Compound # (%max MX-6054) 7-quinolinyl)-4-(trifluoro-
methox hen I meth lene -, 5
Y)p Y ~ Y l ( Z)-)
>2000 96.6
~
(125%)
>2000 -1600
2
>2000 400.4
3
(10.76% @3 M) (121%)
>2000 -
4
While the foregoing specification teaches the principles of the present
invention, with examples provided for the purpose of illustration, it will be
understood that the practice of the invention encompasses all of the usual
variations, adaptations and/or modifications as come within the scope of the
following claims and their equivalents.
57