Note: Descriptions are shown in the official language in which they were submitted.
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
S-OMEPRAZOLE STRONTIUM OR HYDRATE THEREOF,
METHOD FOR PREPARING SAME, AND PHARMACEUTICAL
COMPOSITION COMPRISING SAME
FIELD OF THE INVENTION
The present invention relates to S-omeprazole strontium or a hydrate
thereof having improved optical purity, thermal stability, solubility and
non-hygroscopicity, a method for preparing same, and a pharmaceutical
lo composition comprising same.
DESCRIPTION OF THE PRIOR ART
Omeprazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)
methyl]sulfinyl]-IH-benzimidazole having the structure of formula (II) is
known as a H+/W-ATPase or proton pump inhibitor which is effective in
inhibiting gastric acid secretion to protect gastrointestinals cells (see EP
Patent No. 0 005 129), and its commercial formulations, Losec and
Prilosec (AstraZeneca AB), are marketed as medicaments for prevention
and treatment of gastric acid-related disorders. This omeprazole should be
formulated as enteric coated form because it has a structurally neutral
molecule and thus it is thermally and chemically unstable under a condition
below neutral pH value.
MOO
0
cc N_
Me OMe (II)
US Patent No. 4,738,974 discloses omeprazole salts and hydrates
thereof, e.g., lithium, sodium, potassium, magnesium, calcium, titanium,
ammonium, and guanidine salts. Such omeprazole salts are much more
stable than omeprazole in the neutral form.
Omeprazole is a racemic mixture composed of equal amounts of R-
and S-enantiomers. S-omeprazole of formula (III) is much more preferred
1
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
over the R-isomer in the treatment of gastric or duodenal ulcer,
gastroesophageal reflux disease, etc., because the R-isomer tends to be
metabolized as inactive metabolites in the irregular variations. Accordingly,
there have been many attempts to develop a method for preparing pure
S-omeprazole which is substantially free of R-omeprazole.
MoO
N (S) C Mo
Me OMo (III)
For example, racemic omerprazole has been resolved to isolate the
S-isomer by a high performance liquid chromatography (see Erlandsson et
al., Journal of Chromatography, 535, 305-319 (1990)), and a process for
lo preparing each of the omeprazole enantiomers is disclosed in PCT
Publication No. WO 1992/08716. However, the separated S-omeprazole
products have not been regarded as a stable solid of the pharmaceutically
required purity. US patent No. 6,162,816 discloses a crystalline
S-omeprazole, but even this crystalline form of S-omeprazole is not
sufficiently stable.
US Patent Nos. 5,714,504 and 5,693,818 disclose S-omeprazole salts
and hydrates thereof, e.g., lithium, sodium, potassium, magnesium, calcium,
and ammonium salts. US Patent Nos. 6,369,085 and 6,511,996 disclose the
crystalline potassium salt as well as magnesium salt dihydrate and trihydrate
of S-omeprazole, together with their polymorphs. These S-omeprazole
salts have stability superior to S-omeprazole itself.
Now, S-omeprazole salts with sodium, potassium and magnesium, or
hydrates thereof are commercially marketed with the trade name Nexium
(AstraZeneca AB) as a medicament for prevention and treatment of ulcer.
The sodium and potassium salts are preferred for injectable administration
because of their good solubility, but they are unsuitable for oral
administration due to their hygroscopicity. On the other hand,
non-hygroscopic S-omeprazole magnesium trihydate is preferred in terms of
oral administration of a solid form of omeprazole, but it is not easy to
3o achieve the optical purity required pharmaceutically. Accordingly,
S-omeprazole magnesium trihydate has been subjected to the salt exchange
2
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
with the optically pure sodium or potassium salt prepared in advance to
achieve satisfactory therapeutic effects (see Cotton et al., Tetrahedron
Asymmetry, 11, 3819-3825 (2000)).
PCT Publication Nos. WO 2004/099182, WO 2005/011692, WO
2003/074514, WO 2005/023796 and WO 2005/023797 disclose
S-omeprazole salts of barium, zinc, t-butylamine, adamantaneamine and
a-methylcyclohexanemethane amine, but, these salts are no better than
S-omeprazole magnesium trihydrate in terms of solubility, crystallinity,
hygroscopicity, stability and optical purity.
In view of the previous art, therefore, there has been a need to develop
an improved salt of S-omeprazole suitable for both oral and injectable
administration.
Strontium is an alkaline earth metal of IIA group and it exists in
nature in the form of 4 isotopes, "Sr (82.58%), "Sr (7.00%), 86Sr (9.86%)
and 84Sr (0.56%). It is also known that strontium exerts no safety problems
even at a dose of 633 mg/kg/day in rats (see P. J. Marie et al., Mineral &
Electrolyte Metabolism, 11, 5-13 (1985)). Strontium is reported to be
ingested by people in an average amount of about 3.3mg/day per 70 kg body
weight during the course of everyday life (see Report of Toxicological
Profile for Strontium, U.S. Department of Health and Human Services, 2004).
It is further known that strontium supports calcium metabolism in bone
tissues to promote the bone formation and inhibit the resorption of bone
tissues (see S. P. Nielsen, Bone, 35, 583-588 (2004)). As a typical example
of strontium salts which have been pharmaceutically used, strontium ranelate,
2-5 the salt of strontium with ranelic acid is known. However, there is so far
no
salt of strontium with weak acidic benzimidazole derivatives including
omeprazole.
The present inventors have endeavored to develop a novel salt of
S-omeprazole and found that S-omeprazole strontium or a hydrate thereof
3o has much improved optical purity, thermo-stability, non-hygroscopicity and
solubility over conventional salts.
SUMMARY OF THE INVENTION
35 It is a primary object of the present invention to provide S-omeprazole
3
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
strontium or a hydrate thereof and a method for preparing same.
In accordance with one aspect of the present invention, there is provided
S-omeprazole strontium of formula (I) or a hydrate thereof:
MeO N O
"-CC />-S N-
N (S) / Me = Sr2+
Me OMe 2 (I)
In accordance with another aspect of the present invention, there is
provided a method for preparing S-omeprazole strontium of formula (I) or a
hydrate thereof, which comprises the step of adding strontium hydroxide or
lo another strontium salt to a neutral or basic solution containing S-
omeprazole
and stirring the resulting mixture.
In accordance with still another aspect of the present invention, there is
provided a pharmaceutical composition comprising S-omeprazole strontium of
formula (I) or a hydrate thereof as an active ingredient and a pharmaceutical
acceptable carrier for preventing or treating a gastric acid-related disorder.
BRIEF DESCRIPTION OF THE DRAWINGS
The above and other objects and features of the present invention will
2o become apparent from the following description of the invention taken in
conjunction with the accompanying drawings, which respectively show:
FIG. 1: an X-ray powder diffraction (XPRD) spectrum of the crystalline
S-omeprazole strontium tetrahydrate (Crystalline Form A) obtained
according to a preferred embodiment of the present invention in Example 1;
FIG. 2: a differential scanning calorimeter (DSC) curve of the
crystalline S-omeprazole strontium tetrahydrate (Crystalline Form A)
obtained according to a preferred embodiment of the present invention in
Example 1;
FIG. 3: an XPRD spectrum of the crystalline S-omeprazole strontium
anhydrate (Crystalline Form B) obtained according to another preferred
4
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
embodiment of the present invention in Example 9;
FIG. 4: a DSC curve of the crystalline S-omeprazole strontium
anhydrate (Crystalline Form B) obtained according to another preferred
embodiment of the present invention in Example 9;
FIG. 5: an XPRD spectrum of the crystalline S-omeprazole strontium
hydrate (Crystalline Form C) obtained according to still another preferred
embodiment of the present invention in Example 10;
FIG. 6: a DSC curve of the crystalline S-omeprazole strontium
hydrate (Crystalline Form C) obtained according to still another preferred
lo embodiment of the present invention in Example 10;
FIG. 7: an XPRD spectrum of the amorphous S-omeprazole strontium
obtained according to yet another preferred embodiment of the present
invention in Example 11; and
FIG. 8: a DSC curve of the amorphous S-omeprazole strontium
obtained according to yet another preferred embodiment of the present
invention in Example 11.
DETAILED DESCRIPTION OF THE INVENTION
The inventive S-omeprazole strontium of formula (I) or a hydrate
thereof is a novel salt of S-omeprazole, which is optically purer, thermally
more stable, less hygroscopic and more soluble than any of the
corresponding magnesium salts.
The S-omeprazole strontium according to the present invention has
two S-omeprazole molecules coordinated to strontium ion (II), to which at
least one H2O molecule may be coordinated. Such S-omeprazole strontium
or a hydrate thereof can be produced in an amorphous form or crystalline
form, preferably a crystalline form, which may be confirmed by X-ray
powder diffraction (XRD) or differential scanning calorimeter (DSC)
3o analysis.
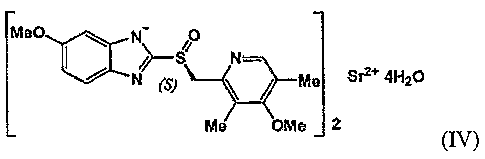
A preferred embodiment of the present invention is the crystalline
tetrahydrate of S-omeprazole strontium, represented by formula (IV):
5
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
MeO ,~ hey 0
Nom:
a S2 4H2+t
Me +Me 2
(IV)
The XRD spectrum of the crystalline S-omeprazole strontium
tetrahydrate shows major peaks having I/Is values of at least 3% (I is the
intensity of each peak; I0 is the intensity of the highest peak) at 20 0.2 of
5.6,
11.1, 13.5, 14.8, 16.2, ' 17.5, 18.0, 20.1, 20.4, 21.2, 22.2, 24.5, 25.2,
26.3,
27.5, 29.8, 31.1, 32.8 and 36.5 (FIG. 1). Also, a DSC scan of the crystalline
tetrahydrate obtained at 5C/min shows an endothermic peak of about 179
J/g which starts at about 100 C and reaches its maximum at about 118'C, as
lo well as an exothermic peak of about 451 J/g which starts at about 2031C and
reaches its maximum at about 211 C (FIG. 2). The actually observed
melting point of the crystalline tetrahydrate is around 202'C, and the
moisture content thereof determined by loss-on-drying test is 8.0 to 9.5%
which is within the experimental error range of 8.49% of the theoretical
value.
The present invention also covers a partial or heterogenic crystalline
form of S-omeprazole strontium or a hydrate thereof.
Accordingly, another preferred embodiment of the present invention
provides a partial or heterogenic crystalline anhydrate of S-omeprazole
strontium whose XRD spectrum shows a major peak having an I/lo value of
100% at 20 0.2 of 5.8 (FIG. 3). A DSC scan of the crystalline anhydrate
obtained at 5C /min shows an exothermic peak which starts at about 186 C
and reaches its maximum at about 197 C (FIG. 4) while no substantial
endothermic peaks are observable. The crystalline anhydrate is observed to
decompose at a temperature of about 196 C or higher.
Still another preferred embodiment of the present invention provides a
partial or heterogenic crystalline hydrate of S-omeprazole strontium whose
XRD spectrum shows a major peak having an I/Io value of 100% at 20 0.2 of
25.2 (FIG. 5). A DSC scan of the crystalline hydrate obtained at 5C/min
shows an endothermic peak which starts at about 37 C and reached its
6
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
maximum at about 68 C, as well as an exothermic peak which starts at about
161 C and reaches its maximum at about 189 C (FIG. 6). The crystalline
hydrate is observed to decompose at a temperature of about 160 C or higher.
In accordance with yet another preferred embodiment of the present
invention, S-omeprazole strontium of formula (I) is also provided in an
amorphous form, and its XRD spectrum shows no distinctively characteristic
peak (FIG. 7). A DSC scan of the amorphous form obtained at 5C/min
shows an endothermic peak which starts at about 29 C and reaches its
maximum at about 56 C, as well as an exothermic peak which starts at about
183 C and reaches its maximum at about 208 C (FIG. 8). This suggests
that a phase transition occurs at about 1961C. The amorphous form is
observed to decompose at a temperature of about 180 C or higher.
The inventive S-omeprazole strontium of formula (I) or a hydrate
thereof satisfies the pharmaceutically required stability since it can
maintain
the initial moisture content, purity and crystallinity under a long-term
storage
condition (25 C under 60% relative humidity), an accelerated aging
condition (40 C under 75% relative humidity) or an stressed condition (60 C
and 75% relative humidity) conducted for 4 weeks or more in a closed state.
Particularly, the crystalline S-omeprazole strontium tetrahydrate (crystalline
form A) is non-hygroscopic and it is capable of maintaining its initial
moisture content under an exposed condition at 25 to 40 C and 40 to 90%
relative humidity for 2 weeks or more.
Further, the inventive S-omeprazole strontium of formula (I) or a
hydrate thereof may be pharmaceutically preferred in terms of water
solubility over other salts of S-omeprazole. For example, it has a water
solubility of about 17.6 mg/ml, which is at least 10 times higher than that of
S-omeprazole magnesium trihydrate.
In accordance with the present invention, S-omeprazole strontium of
formula (I) or a hydrate thereof may be prepared by adding strontium
3o hydroxide or another strontium salt to a neutral or basic solution
containing
S-omeprazole, followed by, if necessary, converting the crystal structure
thereof.
Specifically, the crystalline S-omeprazole strontium tetrahydrate of
formula (IV) may be prepared by adding strontium hydroxide to a neutral
solution containing S-omeprazole of formula (III), stirring the resulting
7
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
mixture until precipitates form, and filtering and drying the resulting
precipitates by a conventional method:
IeU
Me
Me OMe (III)
Mao N- /10
~CCN (s) /r me Sr2* 4H20
Oe 2
(IV)
The neutral solution used herein means a solution prepared by
dissolving or suspending S-omeprazole in an organic solvent selected from
methanol, ethanol, 1-propanol, 2-propanol, acetonitrile, tetrahydrofuran and
acetone, preferably, methanol and acetone, or in a mixture of one of said
organic solvents and water, preferably, having a mix ratio having a mix ratio
of 99:1 to 50:50 (v/v). In the present invention, strontium hydroxide is
preferably used in an amount of 0.5 to 0.75 molar equivalent based on
S-omeprazole of formula (III). Also, the stirring procedure may be carried
out at a temperature ranging from O 'C to the boiling point of the solvent
used
for 30 minutes to 24 hours.
Alternatively, the crystalline S-omeprazole strontium tetrahydrate may
be prepared by adding a reactive strontium salt to a basic solution of
S-omeprazole of formula (III) containing a base, stirring the resulting
mixture until precipitates form, and filtering and drying the resulting
precipitates by a conventional method. The reactive strontium salt may be
selected from strontium chloride, strontium bromide, strontium sulfate,
strontium nitrate, strontium perchlorate, strontium acetate, strontium
carbonate and strontium oxalate, preferably strontium chloride and strontium
acetate. The basic solution means a solution prepared by dissolving or
suspending S-omeprazole and a base in an organic solvent selected from
methanol, ethanol, 1-propanol, 2-propanol, acetonitrile, tetrahydrofuran and
8
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
acetone, preferably, methanol and acetone or in a mixture of one of said
organic solvents and water, preferably, having a mix ratio of 99:1 to 50:50
(v/v). The base may be selected from lithium hydroxide, sodium hydroxide,
potassium hydroxide, ammonia, methylamine, ethylamine, propylamine,
dimethylamine, diethylamine, trimethylamine and triethylamine, preferably
sodium hydroxide and potassium hydroxide. In this embodiment, the base
is preferably used in an amount ranging from 1 to 3 molar equivalents based
on 1 mole of S-omeprazole of formula (III) and the amount of the reactive
strontium salt is preferably in the range of 0.5 to 0.75 molar equivalent
based
on the base. The stirring may be conducted at a temperature ranging from
O 'C to the boiling point of the solvent used for 30 minutes to 24 hours.
It is preferred that the neutral or basic solution contains I g of
S-omeprazole of formula (III) in a volume of 1 to 20M, preferably 3 to 1W.
In accordance with the above method of the present invention,
S-omeprazole salts having high optical purity can be obtained even when an
optically impure S-omeprazole is used as a starting material.
Meanwhile, the partial or heterogenic crystalline anhydrate of
S-omeprazole strontium may be prepared by drying the crystalline
S-omeprazole strontium tetrahydrate at 80 to 130 C for 30 minutes to 24
2o hours to dehydrate, if necessary, under a reduced pressure.
The partial or heterogenic crystalline hydrate of S-omeprazole
strontium may be prepared by suspending such crystalline anhydrate in water,
stirring the suspension at room temperature for 1 to 24 hours, and filtering
and drying the resultant by a conventional method.
Also, the amorphous form of S-omeprazole strontium may be
prepared by dissolving the crystalline S-omeprazole strontium tetrahydrate in
an organic solvent such as acetone and removing the organic solvent from
the resulting solution by evaporation under a reduced pressure or spray
drying.
The inventive S-omeprazole strontium of formula (I) or a hydrate
thereof as mentioned above has a high optical purity of at least 99.0%
enantiomeric excess (ee), non-hygroscopicity and good stability against
moisture and heat, so that it can be pharmaceutically used for the prevention
or treatment of gastric acid-related disorders such as gastroesophageal reflux
disease, gastroenteritis and gastric ulcer due to hyperacidity.
9
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Accordingly, the present invention provides a pharmaceutical
composition comprising the inventive S-omeprazole strontium of formula (I)
or a hydrate thereof as an active ingredient.
The pharmaceutical composition according to the present invention
may be administered via various routes including oral, rectal and injectable
application, preferably the oral route.
For oral administration, the pharmaceutical composition of the present
invention may be in the form of tablets, capsules, pills, and the like, and
may
be formulated with pharmaceutically acceptable carriers, diluents or
excipients.
lo Examples of suitable carriers, diluents and excipients are excipients such
as
starches, sugar and mannitol; filling agents or increasing agents such as
calcium phosphate and silica derivatives; binding agents such as cellulose
derivatives of carboxymethylcellulose or hydroxypropylcellulose, gelatin,
arginic acid salt, and polyvinylpyrrolidone; lubricating agents such as talc,
magnesium or calcium stearate, hydrogenated castor oil and solid
polyethylene glycol; disintegrants such as povidone, croscarmellose sodium,
and crospovidone; and surfactants such as polysorbate, cetyl alcohol and
glycerol monostearate. Further, various pharmaceutical composition
comprising a specific amount of active ingredient, together with or without
2o additives such as said excipients, diluents or additives, may be prepared
in
accordance with any of the conventional procedures (see Remington's
Pharmaceutical Science, Mack Publishing Company, Easton, Pa., 19th
Edition, 1995).
For sterile injectable administration, the pharmaceutical composition
of the present invention may be prepared by directly filling the inventive
S-omeprazole strontium or a hydrate thereof and a pharmaceutically
acceptable carrier in vials under a sterile condition, or by filling the
amorphous powder obtained by dissolving the inventive S-omeprazole
strontium or a hydrate thereof and a pharmaceutically acceptable carrier in
sterile water and then freeze-drying in vials, which is dissolved in sterile
water to be administered.
In a preferred embodiment, the pharmaceutical composition for oral
administration of the present invention may contain S-omeprazole strontium
of formula (I) or a hydrate thereof in an amount ranging from 0.1 to 95% by
weight, preferably 1 to 70% by weight based on the total weight of the
CA 02626085 2010-09-20
composition.
A typical daily dose of S-omeprazole strontium of formula (I) or a
hydrate thereof for a mammalian including human may range from about 0.5
to 500 mg/kg body weight, preferably 5 to 100 mg/kg body weight, and can
be administered in a single dose or in divided doses.
The present invention will be described in further detail with reference
to Examples. However, it should be understood that the present invention
is not restricted by the specific Examples.
1 o Example
The analysis conditions of HPLC employed in Examples are listed
below, and the unit "% ee" as used herein means enantiomeric excess.
Condition A: For the measurement of the amount of omeprazole
- Column: ZorbaxMC8 XDB, 5,um (150mmx4.6mm)
- Detector: 281 rim
- Flow rate: 1.0 me/minute
- Elution condition: Na2HPO4-NaH2PO4 buffer solution/CH3CN=75/25
(v/v)
Condition B: For the measurement of the optical purity of S-omerprazole
strontium
- Column: Chiral-AGP, 5mn (150mmx4mm)
- Detector:280run
- Flow rate: 0.8 mi/minute
- Elution condition: NaH2PO4 buffer solution (pH 6.5)/CH3CN=10/90
(v/v)
<Preparation of S-omerprazole strontium or hydrate thereof>
Examples 1 to 8: Preparation of crystalline tetrahydrate of S-omerprazole
strontium (Crystalline Form A)
Example 1
11
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
S-Omeprazole (30.0g, 86.9mmol) having an optical purity of 95% ee
was dissolved in 200mn of methanol, and strontium hydroxide octahydrate
(13.8g, 51.9mmol) dissolved in 1000 of methanol was slowly added thereto,
followed by stirring the mixture at room temperature for 3 hours. The
precipitate formed was filtered, washed with 100in of methanol and dried at
45 C for 12 hours, to obtain 33.8 g of the title compound (yield: 92%) as an
white crystalline powder.
M.P.: 201-203'C
lo Moisture content (Loss-on-Drying Test): 9.0% (calculated for tetrahydrate,
8.49%)
Strontium content (EDTA titration): 11.1% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.5% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.9% ee
Specific rotation, [a]D20:-31.1' (c=1.0, acetone)
1H-NMR (DMSO-d6): S 8.26(s, 1H), 7.38(d, 1H), 7.02(bs, 1H), 6.54(dd, 1H),
4.58(d, 2H, J=13.3), 4.46(d, 2H, J=13.4), 3.68(s, 3H), 3.66(s, 3H), 2.22(s,
3H), 2.10(s, 3H)
IR (KBr, cm-'): 3422, 2991, 2831, 2364, 1638, 1611, 1569, 1561, 1476,
1444.4, 1390, 1365, 1271, 1204, 1156, 1077, 1027, 1000, 855, 844, 798, 637,
487
The result of X-ray powder diffraction analysis for the S-omeprazole
strontium tetrahydrate showed that the S-omeprazole strontium tetrahydrate
was crystal having the distinctively characteristic diffraction pattern as
shown in FIG. 1. The main peaks having I/Is values of at least 3% are listed
in Table 1.
12
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Table 1
(%)
20 ( 2) D I/Io (%) 20 ( 2) d 1/10
5.6 15.9 100 22.2 4.0 4.3
11.1 8.0 8.6 24.5 3.6 15.4
13.5 6.5 23.5 25.2 3.5 11.6
14.8 6.0 4.5 26.3 3.4 5.4
16.2 5.5 66.5 27.5 3.2 6.3
17.5 5.1 3.4 29.8 3.0 9.8
18.0 4.9 3.2 31.1 2.9 4.2
20.1 4.4 4.4 32.8 2.7 4.3
20.4 4.3 5.3 36.5 2.5 3.2
21.2 4.2 7.8
20: angle of diffraction, d: distance within each crystal face,
I11o (%): relative intensity of peak
Also, as can be seen from FIG. 2, the differential scanning calorimeter
(DSC) curve obtained at 5C /min of the crystalline S-omeprazole strontium
tetrahydrate showed an endothermic peak of 178.9 J/g which starts at
100.04 C and reaches its maximum at 118.33 C and an exothermic peak of
451.3 J/g which starts at 203.06 C and reaches its maximum at 210.68C.
lo Example 2
S-Omeprazole (10.4g, 30.lmmol) having an optical purity of 90% ee
was dissolved in 100rn of methanol, and strontium hydroxide octahydrate
(4.6g, 17.3mmol) dissolved in 100ni of methanol was slowly added thereto,
followed by stirring the resulting mixture at room temperature for 3 hours.
The precipitate formed was filtered, washed with 50M of methanol and
dried at 45 C for 12 hours, to obtain 10.7 g of the title compound (yield:
84%) as an white crystalline powder.
M.P.: 201203 C
Moisture content (Loss-on-Drying Test): 8.9% (calculated for tetrahydrate,
8.49%)
13
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Strontium content (EDTA titration): 11.2% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.4% (calculated for anhydrite,
88.7%)
Optical purity (HPLC, condition B): 99.9% ee
Example 3
S-Omeprazole (10.4g, 30.1mmol) having an optical purity of 80% ee
was dissolved in 100mg of methanol, and strontium hydroxide octahydrate
(4.6g, 17.3mmol) dissolved in 500 of methanol was slowly added thereto,
1o followed by stirring the resulting mixture at room temperature for 3 hours.
The precipitate formed was filtered, washed with 50in of methanol and
dried at 45 C for 12 hours, to obtain 9.3 g of the title compound (yield:
73%) as an white crystalline powder.
M.P.: 201-203 C
Moisture content (Loss-on-Drying Test): 8.7% (calculated for tetrahydrate,
8.49%)
Strontium content (EDTA titration): 11.2% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.5% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.7% ee
Example 4
Sodium hydroxide (3.8g, 95.Ommol) was dissolved in 150mi of water,
and S-omeprazole (27.5g, 79.6mmol) having an optical purity of 90% ee was
dissolved therein. Thereto, strontium chloride hexahydrate (12.7g,
47.8mmol) dissolved in 150ni of methanol was slowly added, and the
resulting mixture was stirred at room temperature for 3 hours. The
precipitate formed was filtered, washed with a mixture of water (2W) and
methanol (80ui) and dried at 45 C for 12 hours, to obtain 29.7 g of the title
compound (yield: 88%) as an white crystalline powder.
M.P.: 201-203'C
Moisture content (Loss-on-Drying Test): 8.9% (calculated for tetrahydrate,
8.49%)
14
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Strontium content (EDTA titration): 11.35% (calculated for anhydrate,
11.3%)
Omerprazole content (HPLC, condition A): 88.6% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.8% ee
Example 5
Potassium hydroxide (5.3g, 94.5mmol) was dissolved in 150mg of
water, and S-omeprazole (27.5g, 79.6mmol) having an optical purity of 95%
lo ee was dissolved therein. Thereto, strontium chloride hexahydrate (12.7g,
47.8mmol) dissolved in 150mi of methanol was slowly added, and the
resulting mixture was stirred at room temperature for 3 hours. The
precipitate formed was filtered, washed with a mixture of water (20M) and
methanol (80M) and dried at 45 C for 12 hours, to obtain 28.7 g of the title
compound (yield: 85%) as an white crystalline powder.
M.P.: 201-203'C
Moisture content (Loss-on-Drying Test): 9.0% (calculated for tetrahydrate,
8.49%)
Strontium content (EDTA titration): 11.3% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.5% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.8% ee
Example 6
Potassium hydroxide (5.3g, 94.5mmol) was dissolved in 150ni of
water, and S-omeprazole (27.5g, 79.6mmol) having an optical purity of 95%
ee was dissolved therein. 150n of methanol and strontium acetate (12.7g,
47.8mmol) dissolved in 50M of water were slowly added thereto, and the
3o resulting mixture was stirred at room temperature for 3 hours. The
precipitate formed was filtered, washed with a mixture of water (20n) and
methanol (80m~) and dried at 45 C for 12 hours, to obtain 28.0 g of the title
compound (yield: 83%) as an white crystalline powder.
M.P.: 201203 C
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Moisture content (Loss-on-Drying Test): 8.9% (calculated for tetrahydrate,
8.49%)
Strontium content (EDTA titration): 11.2% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.5% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.9% ee
Example 7
(S)-(-)-binol (25.0g, 87.3mmol) was dissolved in a mixture of 400mi
of ethanol and 10W of water at 60 C and cooled to 50 to 55 C, to which
5.W of triethylamine (35.9mmol) and omeprazole (50.0g, 144.8mmol)
were successively added. The resulting solution was slowly cooled to room
temperature and stirred for 12 hours. The precipitate formed was filtered,
washed with a mixture of 85m. of ethanol and 15m., of water and then
100ni of hexane and dried at 40 C, to obtain an inclusion complex of
(S)-(-)-binol and S-omeprazole (optical purity: 97.0% ee).
80g of the inclusion complex of (S)-(-)-binol and S-omeprazole
(optical purity: 97.0% ee) obtained above was dissolved in 400mm of
methanol, and strontium hydroxide octahydrate (20g, 75.3mmol) was added
thereto, followed by stirring the resulting mixture at room temperature for 3
hours. The precipitate formed was filtered, washed with 15W of
methanol and dried at 45 C for 12 hours, to obtain 49.0 g of the title
compound (yield: 91 %) as an white crystalline powder.
M.P.: 201-203'C
Moisture content (Loss-on-Drying Test): 9.0% (calculated for tetrahydrate,
8.49%)
Strontium content (EDTA titration): 11.2% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.5% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.9% ee
Example 8
50g of the inclusion complex of (S)-(-)-binol and S-omeprazole
(optical purity: 97.0% ee) obtained in Example 7 was dissolved in 500mZ of
16
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
isopropyl acetate, and sodium hydroxide (3.8g, 95.Ommol) dissolved in 150
im of water was thereto, followed by stirring the resulting mixture at room
temperature for 3 hours. After separating isopropyl acetate, the aqueous
layer was washed with 200iu of isopropyl acetate. To the basic aqueous
layer including S-omeprazole, a strontium chloride hexahydrate (12.6g, 47.5
mmol) dissolved in 150M of methanol was slowly added. The solution
suspended was stirred for 3 hours. The precipitate formed was filtered,
washed with a mixture of water (20ni) and methanol (80n) and dried at
45 C for 12 hours, to obtain 28.5 g of the title compound (yield: 85%) as an
lo white crystalline powder.
M.P.: 201203 C
Moisture content (Loss-on-Drying Test): 8.8% (calculated for tetrahydrate,
8.49%)
Strontium content (EDTA titration): 11.3% (calculated for anhydrate, 11.3%)
Omerprazole content (HPLC, condition A): 88.6% (calculated for anhydrate,
88.7%)
Optical purity (HPLC, condition B): 99.7% ee
2o Example 9: Preparation of partial or heterogenic crystalline anhydrate of
S-omerprazole strontium (Crystalline Form B)
30.Og of crystalline S-omeprazole strontium tetrahydrate (Crystalline
Form A) obtained in Example 1 was dried at 100 C for 5 hours, to obtain
27.0 g of the title compound.
M.P.: decomposition at 196 C or higher
Moisture content (Loss-on-Drying Test): 0.9%
Optical purity (HPLC, condition B): 99.9% ee
The result of XRD analysis for the S-omerprazole strontium obtained
showed a major peak having an I/Io value of 100% at 20 0.2 of 5.8 (FIG. 3),
and DSC curve obtained at 5C /min showed an exothermic peak which starts
at 186.09 C and reaches its maximum at 197.23 C without a substantial
endothermic peak, which was a partial or heterogenic crystalline anhydrate
form.
17
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Example 10: Preparation of partial or heterogenic crystalline hydrate of
S-omeprazole strontium (Crystalline Form C)
20.Og of crystalline anhydrate of S-omerprazole strontium (Crystalline
Form B) obtained in Example 9 was suspended in 150 ni of water and
stirred at room temperature for 12 hours. The precipitate formed was
filtered and dried at 45 C for 12 hours, to obtain 16.5 g of the title
compound.
lo M.P.: phase transition at 105-1071C and decomposition at 160 C or higher
Moisture content (Loss-on-Drying Test): 8.5%
Optical purity (HPLC, condition B): 99.9% ee
The result of XRD analysis for the S-omerprazole strontium obtained
showed a major peak having an I/Io value of 100% at 20 0.2 of 25.2 (FIG. 5),
and DSC curve obtained at 5C/min showed an endothermic peak which
starts at 37.11 C and reaches its maximum at 68.09 C, as well as an
exothermic peak which starts at 161.3 C and reaches its maximum at
188.81 C (FIG. 6), which was a partial and heterogenic crystalline hydrate
form.
Example 11: Preparation of amorphous form of S-omerprazole strontium
25.Og of crystalline S-omeprazole strontium tetrahydrate (Crystalline
Form A) obtained in Example 1 was dissolved in 250 ui of acetone, and the
resulting solution was subjected to solvent-evaporation under a reduced
pressure, to obtain 21.0 g of the title compound.
M.P.: decomposition at 180 C or higher
Moisture content (Loss-on-Drying Test): 6.0%
Optical purity (HPLC, condition B): 99.8% ee
The result of XRD analysis for the S-omerprazole strontium obtained
showed an amorphous form having no distinctively characteristic peak as
shown in FIG. 7.
Also, as can be seen from FIG. 8, DSC curve of the amorphous form
18
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
obtained at 5 C /min showed an endothermic peak which starts at 29.16 C
reaching maximum heat absorption point at 55.88 C, as well as an
exothermic peak which starts at 182.85 C reaching its peak at 207.78 C in
which phase transition occurred at 195.13'C.
<Preparation of S-omeprazole magnesium trihydrate>
Comparative Example 1
Magnesium trihydrate of S-omeprazole was prepared according to
lo Example 7 of US Patent No. 6,369,085.
Specifically, potassium hydroxide (1.26g, 22.5mmol) was dissolved in
30ni of water, and S-omeprazole (5.18g, 15.0 mmol) having an optical
purity of 95% ee was added thereto. Then, magnesium sulfate (1.81g,
15.Ommol) dissolved in 10M of water was slowly added to the solution,
followed by stirring the resulting mixture at room temperature for 3 hours.
The precipitate formed was filtered, washed with 15M of water, and was
dried by blowing warm air at 45 C for 12 hours, to obtain S-omeprazole
magnesium trihydrate as a white crystalline powder in a yield of 95%.
Comparative Examples 2 and 3
The procedure of Comparative Example 1 was repeated except for
using S-omeprazole having an optical purity of 90% ee and 80% ee,
respectively.
Experimental Example 1: Optical purr test
The S-omeprazole strontium tetrahydrates obtained in Examples 1 to 3
and the S-omeprazole magnesium trihydrates obtained in Comparative
Examples 1 to 3, respectively, were subjected HPLC analyses under the
previously described condition B, to measure the optical purities thereof.
The results are shown in Table 2.
19
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Table 2
Effect of increasing optical purity
Starting material Obtained salt of S-omeprazole
(S-omeprazole) Magnesium trihydrate Strontium tetrahydrate
80% ee 81.4% ee (Com. Ex. 1) 99.9% ee (Ex. 1)
90% ee 91.1% ee (Com. Ex. 2) 99.9% ee (Ex. 2)
95% ee 95.0% ee (Com. Ex. 3) 99.7% ee (Ex. 3)
As shown in Table 2, the optical purities of the inventive
S-omeprazole strontium tetrahydrates were markedly higher than those of the
starting materials and the S-omeprazole magnesium trihydrates.
Experimental Example 2: Water-solubility test
The S-omeprazole strontium or a hydrate thereof prepared according
to the present invention and the S-omeprazole magnesium trihydrate were
each dissolved in deionized water to saturation. The water-solubility of
each of the saturated solutions was analyzed by HPLC under the previously
described condition A and the amount of each salt hydrate dissolved was
measured. The results are shown in Table 3.
Table 3
Solubility
Salt (mg/M, 25 C) Saturation pH
(mg/
Magnesium trihydrate 1.5 9.9
Strontium tetrahydrate (Crystalline Form A) 17.6 10.2
Strontium anhydrate (Crystalline Form B) 12.9 -
Strontium hydrate (Crystalline Form C) 8.1 -
Amorphous form of Strontium salt 11.6
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
As shown in Table 3, the solubility of the inventive S-omeprazole
strontium and hydrates thereof are at least 10 times higher than that of the
known S-omeprazole magnesium trihydrate, which suggests that the
inventive strontium salt and hydrates thereof are more suitable for injectable
application.
Experimental Example 3: Hygroscopicity test
The S-omeprazole strontium tetrahydrate prepared according to the
1 o present invention was exposed in the naked state at 25 to 40 C and 40 to
90%
relative humidity for a period of over 15 days. The moisture contents of the
inventive salt measured by loss-on-drying test at storage time 0, 3, 7 and 15
days are shown in Table 4.
Table 4
Moisture content (wt%)
40% (25 C) 60% (25 C) 75% (40 C) 90% (3 5 -C)
O day 9.0 9.0 9.0 9.0
3 days 8.9 9.0 8.9 9.2
7 days 8.7 9.2 9.0 9.3
15 days 8.8 9.1 8.9 9.2
As shown in Table 4, the inventive S-omeprazole strontium
tetrahydrate was less hygroscopic under a highly humid condition, and its
initial moisture content was maintained under a low humidity condition.
Experimental Example 4: Heat stabili test
The S-omeprazole strontium tetrahydrate prepared according to the
present invention was stored in the sealed state under a stressed condition of
60 C and 75% relative humidity, and the remaining amounts of active
S-omeprazole after 7, 14, 21 and 28 days were measured by HPLC condition A.
The results are shown in Table 5.
21
CA 02626085 2008-04-15
WO 2007/049914 PCT/KR2006/004369
Table 5
Amount of titrated S-omeprazole
(ag/mg)
Initial 997
7 days 997
14 days 998
21 days 997
28 days 997
As shown in Table 5, the inventive S-omeprazole strontium
tetrahydrate is highly stable as witnessed by the result obtained under the
accelerated aging condition.
While the invention has been described with respect to the specific
embodiments, it should be recognized that various modifications and changes
lo may be made by those skilled in the art to the invention which also fall
within
the scope of the invention as defined as the appended claims.
22