Note: Descriptions are shown in the official language in which they were submitted.
CA 02629410 2008-05-12
1
Method for the production of insulating materials made of mineral fibers
and filling for a melting aggregate for the production of a mineral melt
The invention relates to a method for the production of insulating materials
made of mineral fibers, particularly from glass and/or rock wool, in which a
melting aggregate, especially a cupola furnace, is filled with a meltable
starting
material for the production of a silicate melt and a combustible. The
combustible
comprises a primary energy carrier, especially foundry coke, and a substituent
and the melt produced from the starting material is supplied to a defibrator
wherein the melt is defibrated into preferably micro-fine fibers and the
fibers are
placed on a conveyor belt as a non-woven. The invention further relates to a
filling for a melting aggregate, especially a cupola furnace for the
production of
a mineral melt which is supplied to a defibrator for the production of mineral
fibers, particularly rock wool or glass wool fibers, wherein the mineral
fibers are
placed on a collection belt for the formation of a fibrous web consisting of a
meltable starting material and a combustible which is formed by a primary
energy carrier, especially foundry coke, and a substituent.
Insulating materials made of mineral fibers are produced from silicate melts.
For
this purpose, a silicate starting material, for instance glasses, natural
stone or
artificial stone are supplied to melting aggregate, for instance a cupola
furnace
or a shaft furnace. The silicate melt which is obtained there from is then fed
to a
defibration unit, in which micro-fine mineral fibers are produced from this
silicate
melt. The mineral fibers which are immediately fed to a collecting chamber are
normally wetted with binding and/or impregnating agents and are deposited on
a conveying device, usually a conveyor belt, arranged below the collecting
chamber. On said conveyor belt the mineral fibers which have been wetted with
binding and/or impregnating agents form a fibrous web which is processed in a
manner known per se in downstream thermal and/or mechanical installations, in
order to produce insulating materials in the form of webs, boards, molded
bodies or the like. Accordingly, mineral fiber insulating materials consist of
CA 02629410 2008-05-12
2
glassily solidified fibers which are connected to each other pointwise by
small
amounts of binding agents.
In the case of insulating materials made of mineral fibers a difference is
made
between those from glass wool and those from rock wool. Mineral fiber
insulating materials from rock wool are produced from silicate melts having
relatively high fractions of network transformers, especially alkalis and
borons.
The raw materials are melted in oil or gas-fired tank furnaces. The fiber-
making
takes places for instance with the aid of the so-called TEL process in which
the
melt is passed through openings in the walls of a rotating body under the
influence of centrifugal forces. By this method relatively long and smooth
fibers
are produced.
Rock wool insulating materials have usually been melted from rock like
diabase,
basalt and limestone, dolomite. In the meantime, these natural stones are
increasingly replaced by artificial stones or are subject to the melting
process
together with artificial stones. In these artificial stones particularly
production
wastes are processed, which may consist of solidified melts that occur at the
regular emptying of the melting aggregate. In addition to these production
wastes also defective production batches are processed. The wastes are
comminuted in a first step, thereafter mixed with Portland cement as a binding
agent and with crushed stones and finally pressed into artificial blocks, so-
called
shaped blocks.
The coarse-grained stones and/or the correspondingly formed shaped blocks
are filled into the melting aggregate, usually the cupola furnace, together
with
coarse pieces of coke as a primary energy source. Further, additives are
supplied which have a grain size spectrum of approx 80 to 200 mm. By
charging the raw material-coke column from the bottom, i.e. according to the
counter flow principle, with the air that is required for combustion, the coke
is
burnt above the bottom of the furnace. In the region where the air is
introduced,
CA 02629410 2008-05-12
3
the furnace temperature reaches a level at which the stones and the additives
are melted. During this process, the fraction of already glassily solidified
wastes
increases the melting speed. The temperature inside the furnace decreases
towards the upside, since the heat energy is delivered to the stones and to
the
primary energy source. At the same time, the oxygen content in the furnace
decreases.
By a subsequent heating of the exhaust air harmful carbon compounds are
transformed into less harmful compounds. The energy contained in the exhaust
air is thereafter delivered to the combustion air with the aid of heat
exchangers.
The molten constituents of the raw materials which are introduced into the
melting aggregate sink to the bottom of the melting aggregate. During this
process a segregation takes place in which the iron which is predominantly
reduced from the stones accumulates at the bottom and the melt which has a
lower specific weight and which is required for fiber making is discharged
through a discharge which is arranged above the bottom. The melt which is
discharged here is thereafter fed to the defibration unit and is defibrated.
From
the melt which is fed to the defibration unit only a fraction of 50% is
transformed
into fibers. The coarser non-fibrous constituents are separated from the
fibrous
constituents by wind sieving.
The iron which accumulates in the bottom area must be regularly discharged.
During this discharging of the iron the fiber production is interrupted. The
melt
which is contained in the melting aggregate at this point of time is not
suitable
for direct insulating material production after re-starting the melting
aggregate
and is therefore processed as a waste material during the recycling and is
supplied to the production.
During the melting process which is mostly carried out in cupola furnaces a
strong dependency exists between the viscosity and the temperature. Further,
CA 02629410 2008-05-12
4
the germination number and hence the crystallization tendency are relatively
high. At the fiber making on so-called cascade spinning machines these
properties result in mineral fibers which are relatively short and which are
swirled in themselves. The individual mineral fibers itself are glassily
solidified.
Due to their composition the temperature resistance of the mineral fibers made
of a stone melt is higher than in insulating materials which are made of glass
wool.
An important factor at the production and rating of mineral fibers is the bio-
solubility, i.e. the residence time of the mineral fibers in the human
organism.
The bio-solubility of insulating materials made of rock wool is decisively
influenced by the content of A1203. With increasing fractions of A1203 the
temperature resistance of the fibers increases on one side and, surprisingly,
also the bio-solubility on the other side.
A typical composition of bio-soluble mineral fibers made of rock wool includes
a
fraction of Si02 of between 35 and 43 percent by weight, a fraction of A1203
of
between 17.5 to 23.5 percent by weight, a fraction of TiO2 of 0.1 to 3 percent
by
weight, a fraction of FeO of 1.7 to 9.3 percent by weight, a fraction of CaO +
MgO of 23.5 to 32 percent by weight and a fraction of K20 + Na20 of 1.3 to 7
percent by weight.
For the economy of these rock wool insulating materials which are used as a
mass product the use of raw materials which include a high fraction of A1203
is
important. Although natural stones contain in many cases alumo-silicates, the
same are frequently not present in the required concentration or only together
with undesired minerals. On the other hand, calcinated bauxites are
comparatively expensive. For this reason residues are often exploited which up
to present could only be dumped and which include a considerable risk for the
environment because of the content of soluble matter. At the same time, these
residues, which occur for instance at the production of rock wool, in the form
of
CA 02629410 2008-05-12
melt remainders, separated non-fibrous particles, filtering dusts, defective
productions or the like are almost completely recycled in a primary waste
material cycle. These residues are prepared prior to their recycling so as to
correspond to the requirements of the machine equipment, particularly the
melting aggregates. For their recycling the residues are for instance
comminuted and mixed together in different grain sizes or are mixed with other
splintery raw materials and are spiked with binders like cement for instance
and
pressed into sufficiently large shaped bodies before the same are supplied to
a
shaft furnace or a cupola furnace as coarse-piece raw materials. From the
document EP 0 765 295 C1 it is known for instance to bind suitable shaped
bodies from fine-grained raw materials also with the aid of lignin. In the
document WO 94/12007 corresponding shaped bodies with molasses-
containing binders are described.
As it has already been mentioned above, coke is used as a primary energy
carrier. The energy which is required for melting the raw materials amounts to
approximately 2 megawatts per ton of melt. Depending on the origin of the coal
which is used for coking, the content of inorganic constituents of the coke
(ash
content) is between 6 and 10 percent by weight.
Foundry coke as a primary energy carrier turned out as a particularly suited
combustible. Foundry coke has a high calorific value which approximately
amounts to 30,000 kJ/kg at a water content lower than 5 percent by weight. In
addition to that, foundry coke is characterized by a low ash content of less
than
per cent by weight, a low sulfur content of less than 1 percent by weight as
well as a low content of volatile constituents of less than 1 percent by
weight
and at the same time a high drum strength M80 of more than 75%. It must be
taken into consideration here that the non-combustible constituents of the
coke
are embedded in the silicate melt. For this reason, combustibles are required,
of
which the non-combustible constituents are low and do not have any impact on
the final product.
CA 02629410 2008-05-12
6
From the document US 4 822 388 it is known to replace the coke predominantly
by a furnace cladding of furnaces for the production of aluminum. In this case
it
is described as desirable to completely replace the coke, but at least 60% of
the
primary energy carrier shall consist of the residues of the furnace cladding.
From the document EP 1 241 395 A2 it is further known to form the primary
energy carrier at the production of insulation materials from mineral fibers
of at
least 50 percent by weight of coke and a mixture of a carbon carrier and
refractory bricks, wherein said mixture is obtained from furnace clearing,
especially the cathodic cladding of furnaces for the production of aluminum.
This pre-known method turned out worthwhile in the practice, since it taps new
energy sources for the production of insulation materials from mineral fibers
which are much cheaper than coke.
In view of this prior art, the invention is based on the pro blem of further
developing a method in accordance with the invention in such a way that a
method in accordance with the invention can be carried out in a more
economical way by tapping new raw material sources, while taking into account
the usual procedural parameters for the production of a suitable melt, so that
good melt results without impurities of the melt are achieved. The invention
is
further based on the problem of providing an inexpensive filling for a
melting aggregate which allows preparing an inexpensive melt for fiber making
of mineral fibers for insulation materials, so that the production of
insulation
materials is possible with an increased economy and constant product quality.
In a method of the above-mentioned kind, the so lution of this problem
provides that the combustible has admixed to it as a substituent anodes which
are used in a melting electrolysis in a proportion of at least 15% in relation
to
the total amount of the combustible. For the so I ution of the problem it is
further provided that in a filling for a melting aggregate said substituent
consists
CA 02629410 2011-07-26
7
of used-up anodes that are employed in the melting electrolysis and of which a
fraction of at least 15% in relation to the total amount of the combustible is
contained in the combustible.
In accordance with the invention it is hence provided that in a method for
producing insulating materials from mineral fibers, particularly from glass
and/or
rock wool, in which a melting aggregate, especially a cupola furnace, is
filled with
a meltable starting material for the preparation of a silicate melt and with a
combustible, said combustible includes in addition to a primary energy
carrier,
e.g. foundry coke, a substituent which has admixed to it as used-up electrodes
which are employed in a melting electrolysis especially the remainders thereof
in
a proportion of at least 15% in relation to the total amount of the
combustible.
According to a further feature it is provided that a fraction of the
substituent of up
to 70% in relation to the total amount of the combustible is added to the
combustible. The anodes are especially anode remainders from the melting
electrolysis. The anodes re mostly produced in a so-called prebake method and
they consist of coal blocks which are pressed from highly viscous c calcinated
petrol coke, coal tar pitch and anode remainders and are prebaked. Here,
petrol
coke is a product from petro-chemistry which is produced during crude oil
refining. Coal tar pitch is a side product of black coal coking in coking
plants of
the steel industry. Accordingly, such anodes can be formed essentially from
recyclable residues from other branches of industry.
More particularly, this disclosure relates to a method for the production of
insulating materials made of mineral fibers from glass or rock wool or both,
wherein a melting aggregate comprising a fill of meltable starting material is
supplied for the production of a silicate melt with a combustible comprising a
primary energy carrier and a substituent, and the melt produced is supplied to
a
defibrator and the melt is defibrated into micro-fine fibers and the fibers
are
placed on a conveyor device as a non-woven, wherein a substituent comprising
CA 02629410 2011-07-26
7a
used-up anodes is admixed with the combustible, in a proportion of at least
15%
in relation to the total amount of the combustible and, before being admixed
with
the melting aggregate, the used-up anodes are comminuted to a grain size of at
least 50 mm, in order to achieve optimum mixing and combustion behavior of the
filling, to form a loose bulk with sufficient open pores, so that gases
produced in
the lower part of the melting aggregate during combustion can be diffused at a
high temperature through the fill and can heat the fill.
The anodes are formed in a block-shape and are used in the melting
electrolysis
for instance for the production of aluminum. During this process, aluminum is
produced at the cathode, e.g. the cathodic cladding of a melting furnace.
Oxygen
is produced at the anode of the melting furnace. The anode is therefore
subject
to a process of wear in which it becomes oxidized into CO At the end of the
process of wear a remainder of the used-up anode is left which can be recycled
up to present exclusively at the production of corresponding anodes.
Corresponding anodes constitute a very hard carbon product having a
* CA 02629410 2008-05-12
8
calorific value of approximately 33,000 kJ/kg at an ash content of max 4% by
weight, a carbon content of max 99% by weight, a sodium content of max 0.8%
by weight, a sulfur content of max 1.5% by weight, an aluminum content of max
1% by weight, and a fluorine content of max 1.8% by weight.
Surprisingly it has shown that due to their chemical composition and
especially
due to the high carbon content the anode remainders can be used as primary
energy carriers in connection with the usually employed constituents of the
combustible, namely foundry coke for instance for the production of insulating
materials from mineral fibers, especially glass and/or rock wool in a melting
aggregate.
According to a further feature of the invention it is provided that the anodes
or
the remainders of the anodes are cleaned before they are admixed to the
combustible. In particular, a mechanical cleaning process is carried out in
which
adhering residues of the melt on the surfaces of the anodes or remainders of
the anodes are removed, so that an anode remainder having a carbon content
which is a high as possible is admixed to the combustible as a substituent of
a
fraction of the primary energy carrier.
For employing the anodes in a cupola furnace that is provided as a preferred
melting aggregate in the present invention, the anodes must exhibit a
particular
grain size, depending on the dimensions of the cupola furnace, in order to
achieve an optimum mixing and combustion behavior of the filling of the cupola
furnace. In this connection, the components of the filling shall form a loose
bulk
with sufficient open pores, so that the gases which are produced in the lower
part of the cupola furnace during the combustion can be diffused at a high
temperature through the fill and can heat the fill. Therefore, it turned out
advantageous in usual cupola furnaces to comminute the anodes to a medium
grain size of between 50 and 200 mm prior to filling the melting aggregate.
) CA 02629410 2008-05-12
9
According to a further feature of the invention it is provided that the
starting
material and the combustible including the substituent form a filling for the
melting aggregate which is composed up to 30% of combustible including the
substituent and of the meltable starting material for the rest of it. Hence,
the
meltable starting material comprises up to 70% of the filling.
According to a further feature of the invention the meltable starting material
consists up to 50% of natural stones, especially diabase and/or basalt and of
a
rest of artificial shaped blocks, wherein the shaped blocks particularly
consist up
to 70% of recyclable mineral fiber material from the production and a rest of
recovered mineral fiber insulation materials. The recycled mineral material
from
the production consists for instance of segments or minor-quality products
which are removed from the production process. The shaped blocks are
pressed into lumpy bodies from fine-grained material and the solids which are
required for the shaped blocks together with stones with latent-hydraulic
substances used as a supporting grain. As latent-hydraulic substances there
may be used for instance Portland cement or other similarly effective binders,
while crushed stones may be used as a supporting grain.
In the melting aggregate, especially in the cupola furnace, a filling which is
prepared in this way is arranged in a column-like fashion, wherein the filling
contains the raw material, i.e. the meltable starting material and the
combustible
in the form of the primary energy carrier and the substituent. The required
combustion air is supplied to the filling according to the counter flow
principle,
so that the primary energy carrier burns down above a shaft floor of the
melting
aggregate. In the zone of the introduction of the combustion air the
atmosphere
in the melting aggregate reaches a temperature which is sufficient for melting
the meltable starting material. The meltable starting material is discharged
as a
melt through a drain and is forwarded to a fiber making device which usually
comprises several discs which are driven for rotation, wherein the melt
impinges
on the outer shell of said discs and is defibrated by the rotary movement into
, CA 02629410 2008-05-12
1.0
single mineral fibers which thereafter are charged with a binding and/or
impregnating agent and are placed on a collection belt.
In the production of aluminum, aluminum oxide is melted in electrolysis
furnaces
under the use of a flux like for instance cryolite (Na3AIF6) and is reduced to
metallic aluminum. For this purpose, electrolytic cells are employed which
consist of a steel tub which is lined with refractory bricks, for instance
firebrick
and/or aluminum oxide bricks. On this ceramic layer a layer of electrically
conducting carbon or graphite stones and/or masses is arranged which forms
the cathodic part.
In the same way as the anodes which have been described above and which
are used according to the invention, also this cathodic cladding of the
electrolytic cells is subject to wear which necessitates a revision every five
years on average. During this revision the furnaces must be shut off and the
cathodic cladding must be renewed. The worn-out cladding is comprised of a
mixture which consists for one half of graphite and for the other half of
refractory
bricks. It is not possible to completely separate these components from each
other during removal, but the components can be screened in a technically
expedient way.
In an advantageous embodiment of the invention it is provided that in addition
to
the anodes high-carbon residues, especially recovered cathodic claddings of
melting aggregates for the production of aluminum, are admixed as further
substituents to the combustible. It is provided in particular that the
fractions of
the different substituents are equal in relation to the amount or the carbon
content of the individual fractions of the different substituents.
Consequently, at
least two substituents are admixed to the combustible in equal amounts or,
according to their carbon content, in different amounts. This technique
therefore
requires to first determine the carbon content of the substituent and
thereafter to
compute a mixing ratio, so that the substituents to be admixed have a
, CA 02629410 2008-05-12
11
predetermined carbon content. In this connection, a larger amount of a
substituent having a low carbon content is mixed with a smaller amount of a
substituent having a higher carbon content. In this connection, it turned out
=
advantageous that the used-up cathodic cladding of an electrolysis furnace in
conjunction with the anodes can be used in a highly economical way as a
primary energy carrier in mineral wool production.
Normally the high-carbon fraction of the cathodic cladding which is preferably
used in accordance with the invention contains 40 to 60% by weight of carbon
as well as 4 to 12% by weight of Si02, 26 to 56% by weight of A1203, 6 to 14%
by weight of Fe0/Fe203, 21 to 41% by weight of Na20 and 1.8 to 3.6% by
weight of S03.
When using the cathodic cladding in connection with the anodes as a part of
the
primary energy carrier the cyanogen compounds contained in the cladding are
destroyed during the melting process and are converted into less harmful, at
least non-toxic nitrogen compounds. Therefore, residues which are produced
here can be disposed and dumped much easier and hence cheaper and
environmentally friendlier.
Further features of the method according to the invention or the melt
according
to the invention will become apparent from the subclaims and the subsequent
description of the attached drawing showing a schematic structure of a filling
in
a cupola furnace. Further features will also become apparent from the
following
description of one embodiment.
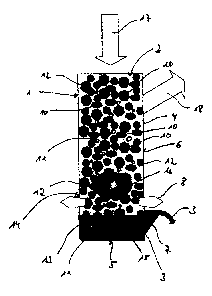
In the drawing there is schematically represented a cupola furnace 1 in which
a
filling 2 for the production of a mineral melt 3 is arranged which can be
supplied
to a fiber making device (not further illustrated) for the production of
mineral
fibers which are deposited on a collection belt for forming a fibrous web.
CA 02629410 2008-05-12
12
The cupola furnace 1 consists of a cylindrical housing having a bottom 5 and a
side wall 6 including in the lower part thereof, namely in the part facing the
bottom 5, a discharge opening 7 through which the melt 3 leaves the cupola
furnace 1. Above the discharge opening 7 a closed circular pipeline 8 is
arranged which includes inlet nozzles which are equally spaced over the
circumference of the side wall 6 and through which primary wind is blown into
the cupola furnace 1.
The filling 2 consists of a combustible which includes a primary energy
carrier in
the form of foundry coke 10 and a substituent 11 which consists of anodes from
the melting electrolysis.
The combustible contains 70% by weight of foundry coke 10 and 30% by weight
of substituent 11. In addition to the combustible a meltable starting material
12
is a constituent of the filling. The meltable starting material 12 consists
for 40%
by weight of natural stones, namely diabase and basalt, and for 60% by weight
of artificial shaped blocks which in turn consist for 60% by weight of
recycling
material originating from the production process and for 40% by weight of
recovered mineral fiber insulation materials. Additionally, these shaped
blocks
include latent-hydraulic substances, namely Portland cement and crushed
stones as a supporting grain, which components permit or facilitate the
pressing
of the artificial shaped blocks.
In the cupola furnace 1, in the lower part thereof above the bottom 5, the
sump
13 is distinguished from the coke bed 14, which coke bed 14 comprises the
sump 13 and the bottom area with a layer 15 of reduced iron. The sump 13 is
the area in the cupola furnace in which the melt 3 is contained in addition to
the
combustible. Above the sump 13 in the area of the closed circular pipeline 8
there is a section of the coke bed 14 in which exclusively combustible is
present
which is burnt in this area and which provides the melting temperature which
required for melting the starting material 12. Above the coke bed a melting
zone
= & CA 02629410 2008-05-12
13
16 is arranged in which the meltable starting material 12 is melted which is
then
immediately discharged to the sump and is drained through the discharge
opening 7 upon reaching a certain level which is predetermined by the
discharge opening 7.
The filling 2 is charged into the housing 4 of the cupola furnace 1 in
accordance
with arrow 17, wherein the constituents of the filling are usually heaped up
alternating according to a predetermined pattern. But it may also be provided
that the constituents of the filling 2, namely the starting material 12 and
the
combustible consisting of the foundry coke 10 and the substituent 11 are mixed
before being charged into the cupola furnace 1.
Smoke gases which are produced during the combustion of the combustible are
discharged in the upper area of the cupola furnace 1 in accordance with arrow
18 and are used for heating the cupola furnace 1, where appropriate.