Note: Descriptions are shown in the official language in which they were submitted.
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 1 -
DEVICES, SYSTEMS, AND METHODS FOR STABILIZATION
OR FIXATION OF MAGNETIC FORCE DEVICES USED
IN OR ON A BODY
Related Applications
This application claims the benefits of United
States Provisional 'Patent Application Serial No.
60/739,519, filed November 23, 2005.
Field of the Invention
The invention is directed to devices, systems, and
methods for improved stabilization of magnetic force
devices used in and/or on a body. The improved
stabilization may be realized both during placement and
at an implanted position.
Background of the Invention
I. Characteristics of Sleep Apnea
First described in 1965, sleep apnea is a breathing
disorder characterized by brief interruptions (10 seconds
or more) of breathing during sleep. Sleep apnea is a
common but serious, potentially life-threatening
condition, affecting as many as 18 million Americans.
There are two types of sleep apnea: central and
obstructive. Central sleep apnea, which is relatively
rare, occurs when the brain fails to send the appropriate
signal to the breathing muscles to initiate respirations,
e.g., as a result of brain stem injury or damage.
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 2 -
Mechanical ventilation is the only treatment available to
ensure continued breathing.
Obstructive sleep apnea (OSA) is far more common.
Normally, the muscles of the upper part of the throat
keep the airway open to permit air flow into the lungs.
When the muscles of the soft palate at the base of the
tongue and the uvula (the small fleshy tissue hanging
from the center of the back of the throat) relax and sag,
the relaxed tissues may vibrate as air flows past the
tissues during breathing, resulting in snoring. Snoring
affects about half of men and 25 percent of women - most
of whom are age 50 or older.
In more serious cases, the airway becomes blocked,
making breathing labored and noisy, or even stopping it
altogether. In a given night, the number of involuntary
breathing pauses or "apneic events" may be as high as 20
to 30 or more per hour. These breathing pauses are almost
always accompanied b,y, snoring between apnea episodes,
although not everyone who snores has the condition. Sleep
apnea can also be characterized by choking sensations.
Lack of air intake into the lungs results in lower
levels of oxygen and increased levels of carbon dioxide
in the blood. The altered levels of oxygen and carbon
dioxide alert the brain to resume breathing and cause
arousal. The frequent interruptions of deep, restorative
sleep often lead to early morning headaches, excessive
daytime sleepiness, depression, irritability, and
learning and memory difficulties.
The medical community has become, aware of the
increased incidence of heart attacks, hypertension and
strokes in people with moderate or severe obstructive
sleep apnea. It is estimated that up to 50 percent of
sleep apnea patients have high blood pressure.
Upon an apneic event, the sleeping person is unable
to continue normal respiratory function and the level of
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 3 -
oxygen saturation in the blood is reduced. The brain will
sense the condition and cause the sleeper to struggle and
gasp for air. Breathing will then resume, often followed
by continued apneic events. There are potentially
damaging effects to the heart and blood vessels due to
abrupt compensatory swings in blood pressure. Upon each
event, the sleeping person will be partially aroused from
sleep, resulting in a greatly reduced quality of sleep
and associated daytime fatigue.
Although some apneic events are normal in all
persons and mammals, the frequency of blockages will
determine the seriousness of the disease and opportunity
for health damage. When the incidence of blockage is
frequent, corrective action should be taken.
II. Sleep and the Anatomy of the Upper Airway
The upper airway consists of a conduit that begins
at the nasal valve, situated in the tip of the nose, and
extends to the larynx. Although all tissue along this
conduit is dynamic and responsive to the respiratory
cycle, only the pharynx (the portion that starts behind
the nasal cavity and ends in its connections to the
supraglottic larynx is totally collapsible.
The cross sectional area of the upper airway varies
with the phases of the respiratory cycle. At the
initiation of inspiration (phase I), the airway begins to
dilate and then to remain relatively constant through the
remainder of inspiration (Phase II). At the onset of
expiration (Phase III) the airway begins to enlarge,
reaching maximum diameter and then diminishing is size so
that at the end of expiration (Phase IV), it is at its
narrowest, corresponding to the time when the upper
airway dilator muscles are least active, and positive
intraluminal pressure is lowest. The upper airway,
therefore, has the greatest potential for collapse and
closure at end- expiration. [ref: Schwab RJ, Goldberg AN.
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 4 -
Upper airway assessment: radiographic and other imaging
techniques. Otolaryngol Clin North Am 1998;31:931-968]
Sleep is characterized by a reduction in upper
airway dilator muscle activity. For the individual with
obstructive sleep apnea (OSA) and perhaps the other
disorders which comprise much of the group of entities
called obstructive sleep-disordered breathing (SDB), it
is believed that this change in muscle function causes
pharyngeal narrowing and collapse. Two possible
etiologies for this phenomenon in OSA patients have been
theorized. One is that these individuals reduce the
airway dilator muscle tone more than non-apneics during
sleep (the neural theory). The other is that all
individuals experience the same reduction in dilator
activity in sleep, but that the apneic has a pharynx that
is structurally less stable (the anatomic theory). Both
theories may in fact be contributors to OSA, but current
studies seem to support that OSA patients have an
intrinsically structurally narrowed and more collapsible
pharynx [ref: Isono S. Remmers J, Tanaka A Sho Y, Sato J,
Nishino T. Anatomy of pharynx in patients with
obstructive sleep apnea and in normal subjects. J Appl
Physiol 1997:82:1319-1326.] Although this phenomenon is
often accentuated at specific sites, such as the
velopharyngeal level [Isono], studies of closing
pressures [Isono] supports dynamic fast MRI imaging that
shows narrowing and collapse usually occurs along the
entire length of the pharynx. [ref: Shellock FG, Schatz
CJ, Julien P, Silverman JM, Steinberg F, Foo TKF, Hopp
ML, Westbrook PR. Occlusion and narrowing of the
pharyngeal airway in obstructive sleep apnea: evaluation
by ultrafast spoiled GRASS MR imaging. Am J of
Roentgenology 1992:158:1019-1024.].
III. Treatment Options
To date, the only modality that addresses collapse
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 5 -
along the entire upper airway is mechanical positive
pressure breathing devices, such as continuous positive
airway pressure (CPAP) machines. All other modalities,
such as various surgical procedures and oral appliances,
by their nature, address specific sectors of the airway
(such as palate, tongue base and hyoid levels), but leave
portions of pharyngeal wall untreated. This may account
for the considerably higher success rate of CPAP over
surgery and appliances in controlling OSA. Although CPAP,
which in essence acts as an airway splint for the
respiratory cycle, is highly successful, it has some very
significant shortcomings. It can be cumbersome to wear
and travel with, difficult to accept on a social level,
and not tolerated by many (for reasons such as
claustrophobia, facial and nasal mask pressure sores,
airway irritation). These factors have lead to a
relatively poor long-term compliance rate. One study has
shown that 65% of patients abandon their CPAP treatment
in 6 months.
An alternative method would "splint" the airway
during sleep that would give the benefits afforded by
CPAP without some of its shortcomings would therefore be
advantageous. In this method magnetic energy is used
either attractively (opposite poles of two or more
magnets facing one another, resulting in attractive
forces) or repulsively (like poles of two or more magnets
facing one another, resulting in forces which repel one
another). Magnets implanted in the tongue interact either
by attractive or repulsive forces with other magnets
implanted in various organs of the upper airway system or
external to the body within a neck collar.
Since the "splint" method using magnetic forces did
not eliminate all magnetic interaction, implants within
the tongue and pharyngeal wall often were often difficult
to stabilize in their specified locations. The magnetic
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 6 - -
implants could interact with one another causing the
implants to fold or lose their shape, as well as with
magnetic instruments. The implants could also rotate or
migrate from their original implant position.
The need remains for simple, cost-effective devices,
systems, and methods for improved stabilization of
magnetic force devices used in and/or on a body,
including improved stabilization during placement and at
an implanted position.
Summary of the Invention
The invention provides devices, and methods to
improve implant tolerance generally, prevent implant
migration, and stabilize a magnetic implant in tissue,
e.g., the tongue, oropharynx, and pharyngeal wall. The
invention is particularly useful to prevent sleep
disordered diseases such as Obstructive Sleep Apnea (OSA)
and hypopnea (a partial obstruction of the.airway during
sleep).
One aspect of the invention provides an implant
device comprising at least two ferromagnetic components
carried by a support structure in a spaced apart
relationship. The implant device includes at least one
opening formed in the support structure between the
ferromagnetic components. The openings can provide
stabilization after implantation, e.g., by providing
flexibility, and/or tissue in-growth, or placement of
external fixation elements, such as a suture, or a
staple, or glue.
In one embodiment, the support structure comprises a
net-like array of openings.
In one embodiment, the opening occupies a geometric
center of the support structure.
In one embodiment, the support structure is either
generally U-shaped or 0-shaped.
Another aspect of the invention provides an implant
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 7 -
device comprising a ferromagnetic component carried on a
a support structure. According to this aspect of the
invention, at least one protrusion extends from the
support structure. The protrusion is sized and configured
for engaging tissue to stabilize the support structure.
The protrusion can comprise, e.g., a barb, or a hook. In
one embodiment, the implant device includes means for
selectively withdrawing and extending the protrusion
relative to the support structure.
Another aspect of the invention provides an implant
device comprising a ferromagnetic component carried by a
support structure. According to this aspect of the
invention, the support structure includes a first side
having a textured surface sized and configured for
contact with tissue and a second side having a generally
smooth surface. Contact between the textured first side
and tissue within an airway stabilizes the implant, while
the generally smooth surface, which faces the airway,
minimizes interference with normal functions such as
swallowing or speech.
Another aspect of the invention provides an implant
device comprising a ferromagnetic component carried on a
support structure. According to this aspect of the
invention, the implant is shaped to prevent motion,
migration and extrusion while implanted in tissue. The
support structure can be sized and configured, e.g., with
rounded corners, and/or irregular outer edges forming
alternating wide and narrow areas, and/or regions of
different thickness.
According to another aspect of the invention, an
implant device includes multiple magnetic arrays, and
means for preventing attraction between the arrays to
facilitate placement of the device in or on a tissue
region.
According to another aspect of the invention, a
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 8 -
system is provided that comprises a magnetic implant
device, and a pocket surgically created in tissue. The
pocket is sized and configured with an irregularly shape
such that, when the magnetic implant is placed in the
pocket, intact tissue around the implant prevents motion
of the magnetic implant.
Another aspect of the invention provides a system
comprising first, second, and third magnetic structures,
each having a north magnetic pole. The first and second
magnetic structures are sized and configured for
placement in or on a first tissue region in a spaced
apart relationship. The magnetic north poles or the first
and second magnetic structures are mutually oriented
toward a second tissue region. According to this aspect
of the invention, the third magnetic structure is sized
and configured for placement in or on the second tissue
region. The magnetic north pole of the third magnetic
structure is oriented toward the first tissue region
between the first and second magnetic structures. The
offset between the third magnetic structure and the first
and second magnetic structures lends stability to the
repelling interaction among the magnets in the system.
Another aspect of the invention provides a system
for implanting a magnetic implant comprising side-by-side
arrays of magnets that can flip or fold upon itself to
form a folded-up structure. The system comprises first
means for separating the folded-up structure and
positioning the magnetic implant in tissue, and second
means for holding the magnetic implant in place while the
first means separates the folded-up structure.
Another aspect of the invention provides a method
for stabilizing a magnetic implant comprising side-by-
side first and second magnetic sections. The method
threads a placement suture through two adjacent inner
holes in the first magnetic section and ties the
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 9 -
placement suture to form a loop. The method folds the
implant so that the first section overlaps the second
section and places the implant while folded through the
incision into a pocket formed in wall tissue. The method
positions a first instrument to hold the second section
against fascia while placing a second instrument through
the suture loop. The method pulls the ends of the
placement suture to apply force to separate the first and
second sections, while using the second instrument to
guide the first section into a side-by-side relationship
with the second section. The method places anchoring
sutures at the four corners of the separated magnetic
implant and then cuts the loop to remove the placement
suture.
Another aspect of the invention provides methods for
inserting a various shaped implants in soft tissue.
One method implants a U-shaped implant. The method
cuts two incisions in the soft tissue, and cuts a U-
shaped pocket in the soft tissue. The method uses a tool
to push suture through one incision into the U-shaped
pocket, until one end of the suture comes out through the
other incision. The method ties one end of the suture to
the U-shaped implant. The method uses a tool to push from
one end of the implant, while pulling the suture at the
other end of the implant, to fit the U-shaped implant
into the specified pocket. The method closes the two
incisions.
Another method implants an L-shaped implant. The
method cuts an incision in the soft tissue and cuts an L-
3 0 shaped pocket in the soft tissue. The method uses a tool
to push the L-shaped implant into the L-shaped pocket and
closes the incision.
Another method implants an 0-shaped implant. The
method cuts an incision into the soft tissue and cuts an
0-shaped pocket into the tissue. The method inserts an 0-
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 10 -
shaped implant with an open link into the pocket. The
method closes the open link of the 0-shaped implant in
the pocket and closes the incision.
The implant devices, systems, and methodologies that
embody technical features of the invention are well
suited for placement in structures of the airway, such as
the tongue, soft palate/uvula, and pharyngeal wall.
Other inventions and technical features shall be
apparent based upon the accompanying description,
drawings, and claims.
Brief Description of the Drawings
Fig. 1 is an anatomic view of a magnetic force
system that includes a first magnetic component implanted
in the back of the tongue and a second magnetic component
implanted in a posterior region of the pharyngeal wall,
the first and second magnetic components having the same
polarity to magnetically interact by the generation of a
repelling force between them, which prevents the tongue
from moving in a posterior direction and closing or
restricting the pharyngeal conduit or airway.
Fig. 2 is an anatomic view of a magnetic force
system that includes a magnetic (or ferrous) array
implanted near the posterior surface of the tongue and an
external magnet that is mounted in a form fitting collar
below the mandible and located forward, near the anterior
surface of the chin, the magnetic or ferrous array and
the external magnet being of opposite polarities to
magnetically attract the implanted magnets forward,
pulling the tongue in an anterior direction and opening
the airway.
Figs. 3 to 6 are alternative views of a magnetic
force system of the type shown in Fig. 2.
Fig. 7A diagrammatically shows an array of three
repelling magnets oriented in a relatively stable
repelling position, due to the creation of a magnetic
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 11 -
force field saddle shown in Fig. 7B.
Figs. 8A, 8B, and 8C show various implantable
magnetic arrays desirably shaped to provide both
stability after implantation, as well as the healing rate
post-operatively.
Figs. 9A, 9B, and 9C show representative embodiments
of magnetic implants having at least one side with
variegations to provide a tissue gripping surface,
thereby providing stability after implantation.
Figs. 10A, 10B, lOC, and 10D show various types of
magnetic implants with hooks, barbs, or a combination of
the two, or equivalent components, to prevent migration
and folding of the magnetic implant upon itself.
Figs. 11A(1) to 11A(4) ; 11B(1) to 11B(5) ; and 11C(1)
to 11C(4) show various representative alternative
embodiments of stabilized magnetic implant structures
especially adapted for implantation in a posterior
pharyngeal wall.
Figs. 12 and 13 show various types of magnetic
implants that include apertures through which external
fixation means, e.g., suture or staples, can be passed to
attach the implant to surrounding tissue.
Figs. 14A and 14B show an implant of the type shown
in Fig. 8A, which includes a network of holes that can be
filled with a growth-stimulating medium to encourage the
in-growth of tissue to stabilize the implant.
Fig. 15 shows an implant of the type shown in Fig.
8A, which includes a network of holes filled a tissue
adhesive or glue to give immediate post-op tissue
stability. ,
Figs. 16 to 18 show various types of magnetic net
array implants, including magnets or ferrous discs linked
together by a net-like webbing with flanges which provide
large areas in which the opposing surfaces of the
surgically produced pocket may be closed (sutured or
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- - 12 -
otherwise) for fast rejoining and healing of the tissue.
Figs. 19 to 22 show surgically formed pockets into
which magnetic net array implants of the type show in
Figs. 16 to 18 can be implanted for'use.
Fig. 23 shows a surgically formed pocket that, with
respect to the lateral and longitudinal dimensions of a
given implant is laterally-tight but longitudinally-loose
to accommodate anterior-posterior movement of the
implant, but restrict lateral movement of the implant.
Fig. 24 shows a U-shaped implant placed in a tissue
pocket of the same shape (e.g., in a tongue), the implant
shape being keyed to prevent migration and limit relative
tissue-to-implant motion.
Figs. 25A to D illustrate a way of inserting a U-
shaped implant as shown in Fig. 24 in the tongue.
Fig. 26A shows an 0-shaped implant placed in a
tissue pocket of the same shape (e.g., in a tongue), the
implant shape being keyed to prevent migration and limit
relative tissue-to-implant motion.
Figs. 26B and 26C show an 0-shaped implant of the
type shown in Fig. 26A where magnets are positioned on
only one side, the side without magnets acts as a rudder
to distribute the force of the tongue and to stabilize
the implant.
Figs. 27A to 27D illustrate a way of inserting an 0-
shaped implant, as shown in Figs. 26A or 26B, in a
tongue.
Figs. 28 and 29 show magnetic implants having a
structure that prevents folding during implantation.
Figs. 30A to 30C show a magnetic implant which
includes flexible hinges between arrays of magnetic
discs, which allow the arrays to pivot into a serpentine
shape (see Fig. 30C), but prevent the arrays from folding
upon themselves.
Fig. 31 shows a magnetic implant (shown prior to
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 13 -
implantation) having magnetic arrays that are prone to
folding or flipping upon itself in response to magnetic
interaction.
Figs. 32A to 32D show tools and related methodology
for controlling the separation of the magnetic arrays of
the magnetic implant shown in Fig. 31, to prevent folding
or flipping during implantation.
Fig. 33 shows a magnetic implant having preferential
flexibility allowing the implant to remain in position
because it closely mimics the movements of the
surrounding anatomy.
Figs. 34A and 34B a magnetic implant having a
support brace to help stabilize the implant.
Fig. 35 shows an alternative, circular design for
the magnetic posterior pharyngeal wall implant.
Fig. 36 shows a magnetic implant having preferential
flexibility that takes into account the shape and
movement of the tongue.
Description of the Preferred Embodiments
This Specification discloses various magnetic-based
devices, systems, and methods for improved stabilization
of magnetic forces both during implantation and at an
implanted position. For example, the various aspects of
the invention have application in procedures requiring
the restriction of tissue collapse in and/or around the
body, such as a passageway within the body. The devices,
systems, and methods that embody features of the
invention are also adaptable for use with devices,
systems, and methods that are not restricted to tissue
based applications.
The devices, systems, and methods are particularly
well suited for treating sleep disordered breathing,
including sleep apnea. For this reason, the devices,
systems, and methods will be described in this context.
Still, it should be appreciated that the disclosed
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 14 -
devices, systems, and methods are applicable for use in
treating other dysfunctions elsewhere in the body, which
are not necessarily sleep disorder related.
1. Magnetic Force Systems
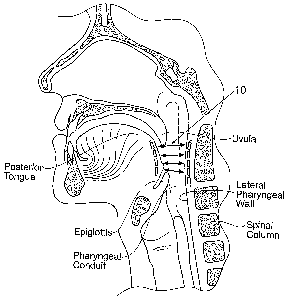
Fig. 1 shows, in an anatomic view, an illustrative
magnetic force system 10. The magnetic force system 10
resists the collapse of tissue in a targeted passageway,
such as a pharyngeal structure and the individual
anatomic components within the pharyngeal conduit during
sleep. As generally shown in Fig. 1, the magnetic force
system includes a first magnetic component 12 implanted
in the back of the tongue and a second magnetic component
14 implanted in a posterior region of the pharyngeal
wall. The first and second magnetic components 12 and 14
have the same polarity. They magnetically interact by the
generation of a repelling force between them. The
magnetic repelling force prevents the tongue from moving
in a posterior direction and closing or restricting the
pharyngeal conduit or airway.
It should be appreciated that the magnetic force
system 10 can be differently configured and arranged,
both anatomically and with respect to the position and
polarity of the magnets.
For example, Fig. 2 shows a cross section of a human
head showing the nasal and oral cavities, tongue,
oropharynx, chin and neck. A magnetic (or ferrous or
ferromagnetic) array 16 is implanted near the posterior
surface of the tongue. An external magnet 18 is mounted
in a form fitting collar 20 such that the magnet is
positioned below the mandible and located forward, near
the anterior surface of the chin. A soft pad 22 provides
comfort for the wearer, preventing the magnet 18 from
pressing directly against the flesh of the chin. An outer
covering 24 encases the magnet and wraps around for the
collar 20 to stabilize and anchor the magnet 18 in the
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 15 -
desired location. The collar 20 can include a closure
means such as a buckle or Velcro strap for ease of use.
The strap may further be elastic to provide a degree of
stretch in the collar 20 for head movement, etc. The
collar 20 may be comprised of a foam interior with a
stretchable fabric covering for softness and
breathability.
In use, the magnet 18 has a polarity that is
opposite the polarity of the magnetic or ferrous or
ferromagnetic array 16. As a result, the magnet 18 will
attract the implanted magnets or ferrous or ferromagnetic
array 16, pulling the tongue in an anterior direction and
opening the airway. This will prevent closure and
occlusion of the airway during sleep.
Fig. 3 shows an alternative embodiment of a neck
collar 20 with the magnet 18 placed just under the chin.
Fig. 4 shows another alternative embodiment. The
magnetic or ferrous or ferromagnetic array 16 is
implanted near the posterior surface of the tongue. The
external magnet 18 of opposite polarity is mounted in a
form fitting collar 20 such that the magnet is positioned
against the anterior surface of the chin. This
arrangement will cause the direction of the attractive
force on the implanted array 16 to be directly forward,
as opposed to a more-downward direction as in Fig. 2.
Fig. 5 shows an alternative embodiment, in which the
external magnet 18 is held in place by a form fitting
appliance 26 and collar 20. Closure and adjustability can
be provided by a buckle and strap arrangement or by a
Velcro strap 32.
Fig. 6 shows yet another embodiment, in which a
headgear 30 is provided consisting of flexible webbing
straps. Side straps 32 extend downwardly to cup the
magnet and chin cup 34 with the magnet 18 fixed within
the chin cup 34. This arrangement has the further
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 16 -
advantage of preventing the mouth from falling open
during sleep. Open mouth breathing is blamed by some in
loud snoring, drying of the mouth and exacerbation of the
tendency of the tongue to fall backward into the airway.
Magnetic'forces field systems (repelling and/or
attracting) can create a magnetic field to resist the
collapse of tissue in targeted pharyngeal structures and
individual anatomic components within the pharyngeal
conduit during sleep. The targeted pharyngeal structures
and individual anatomic components within this region can
include the pharyngeal walls; the base of the tongue; the
vallecula; the hyoid bone and its attachments; the soft
palate with uvula; the palatine tonsils with associated
pillar tissue; and the epiglottis.
The implanted ferromagnetic material and/or the
source of magnetic force can each comprise a single or
discrete source of magnetism having a given desired
orientation. For example, a single permanent magnet,
comprising a body of a ferromagnetic material, can
comprise a single source of magnetism having a given
orientation.
As another example, a flexible or compliant array of
magnets can also comprise individual sources of magnetism
carried as a unit on a support carrier, or otherwise
directly linked together, as will be described.
II. Magnetic Stabilization.
As previously described, when two or more magnets
are placed near each other, a repelling or attracting
force will be present and will act upon the two or more
magnets.
An attracting force can also be generated between a
ferrous alloy/ferromagnetic material and a magnet. The
force, when properly directed, provides the benefit of
the system 10 in its various embodiments, as described.
The magnetic force can also create difficulty in
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 17 -
implanting or positioning the magnets at the targeted
tissue region, and can also contribute to the unwanted
movement (i.e., migration or extrusion) of the magnets in
the tissue region after implantation or positioning. It
is desirable to provide magnetic field systems that are
stabilized, both during implantation or positioning and
after implantation during use.
A. Prevention of Migration and Extrusion After
Implantation
1. Offset Repelling Pole Orientation
A repelling magnetic force system is inherently less
stable than a counterpart attracting magnetic system. The
inherent instability can be mitigated, e.g., by the
relative orientation of repelling magnets to provide a
preferred repelling position.
For example, Fig. 7A shows an array 300 of three
repelling magnets 302a, 302b, and 302c in a relatively
stable repelling position. The array 300 orients two
magnets 302a and 302b in a laterally spaced-apart
relationship, with the magnetic north poles (N) in
parallel side-by-side axial alignment. A lateral space
304 separates the magnets 302a and 302b.
The array 300 places the third magnet 302c in an
indirect facing relationship with the two magnets 302a
and 302b. As shown in Fig. 7A, the magnetic north pole
(N) of the magnet 302c is oriented parallel to the
magnetic north poles (N) of the magnets 302a and 302b,
but does not directly face the north poles (N) of the
magnets 302a and 302b. Instead, the north pole (N) of the
magnet 302c is offset and faces the lateral space 304
separating the magnets 302a and 302b. The offset array
300 creates a repelling force saddle 306 (see Fig. 7B) in
the magnetic force field, which serves to stabilize or
give a preferred repelling position.
2. Shapes that Promote Stabilization
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 18 -
The shape of a magnetic implant's outer edge
influences both the stability of an implant in its chosen
location, as well as the healing rate post-operatively.
Fig. 8A shows an implant 36 comprising flexible or
compliant array of magnets 38 arranged in a polymer
matrix having an outer profile or shape that is
representative of a shape that provides stability in
tissue after implantation. As Fig. 8A shows, the magnetic
implant 36 has an irregular outer edge 40, with
alternating wide and narrow areas. The wide areas prevent
motion of the implant as healing occurs around the
margins. The capsule that forms around the implant 36
after implantation will contract, grabbing the narrow
areas. Holes 43 may be provided to allow tissue in-
growth. The rounded corners of the implant 36 allow for
faster healing of the surrounding tissues.
Fig. 8B shows an alternate embodiment of a magnetic
implant 56 having a profile that is also designed to
discourage migration. The implant's flowing curves permit
a large area of the surrounding tissues to grow around
and grip the implant thus providing a natural anchor.
This implant 56 is particularly well suited for
implantation in the tongue, which has a naturally curved
morphology that matches the profile of the implant 56.
The rounded corners 60 and beveled edges 62 further allow
for faster healing of the surrounding tissues.
3. Integrated Protrusions for Soft Tissue
Fixation
Fig. 8C shows a magnetic implant 36 of a type shown
in Fig. 8A having a textured underside 42, or "bottom
treads," to grip tissue. Stabilization of the implant 36
(or any implant in general) can also be achieved through
attachment of implant parts to the underlying tissue
using, e.g., sutures or staples or glue, as will be
described in greater detail later. The treads 42 will
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 19 -
limit motion relative to the tissue to encourage rapid
healing.
Fig. 9A shows a magnetic implant 48 having a
posterior, tissue-facing, side that includes variegations
44 to provide a tissue gripping surface. In Fig. 9A, the
opposite anterior side 46 of the implant 48 (which
typically faces an airway) can also be variegated, but in
Fig. 9A the anterior side 46 is shown to be smooth, to
aid the epithelial tissue in gliding over the implant
during dynamic movement of the surrounding tissue, e.g.,
during swallowing or speech. In the two-sided arrangement
shown in Fig. 9A, the implant 48 provides both
stabilizing for the magnetic implant 48 (due to the
presence of the variegations 44 on the posterior tissue-
facing side), as well as increasing tolerance in patients
by avoiding interference with the process of swallowing
(due to the relatively un-variegated anterior airway-
facing side 46).
Figs. 9B and 9C show further embodiments, which are
particularly useful for soft tissue fixation in the
posterior pharyngeal wall. The posterior pharyngeal wall
implants 36 each includes a caudal (inferior) -facing
protrusion 47 and 49, shown in Figs. 9B and 9C,
respectively. The caudal-facing protrusions 47 and 49
allow the magnetic implants 36 to become stabilized in a
therapeutically-effective caudal-to-cranial orientation
(i.e., inferior-to-superior) within the posterior
pharyngeal wall, while also avoiding misalignment with
respect to the associated magnetic implant or implants in
the tongue and/or soft palate/uvula placed to
magnetically interact with the pharyngeal wall implants
36.
Treatment of sleep apnea may necessitate insertion
of a wide, flat implant in order to generate an effective
magnetic field and, at the same time, limit bulking the
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 20 -
tissue and making the obstruction worse. In such a case,
protrusions such as hooks and barbs are desirably
provided to grab the top tissue and limit motion.
Figs'. 10A and 10B show a representative embodiment
of a generally flat implant 50 with hooks 52 that dig
into tissue, e.g., in the tongue or oropharynx. Figs. 10C
and 10B show a representative embodiment of a generally
flat implant 50 with barbs 52 that provide the same
function. The hooks, barbs, or a combination of the two,
or equivalent components, prevent migration and folding
of the magnetic implant upon itself.
Figs. 11A(l) to 11A(4) show a representative
embodiment of an implant 54 having at least one tissue
piercing barb or hook 61, which is especially adapted for
implantation in a posterior pharyngeal wall. Fig. 11A(1)
shows the implant 54 that includes at least one anchoring
assembly 55. Either or both cranial (superior) and/or
caudal (inferior) ends of the implant 54 may be straight,
gently rounded or curved. The anchoring assembly 55
comprises, at one end, a loop 57 sized and configured for
accommodating passage of a tissue suture or staple. In
use, the loop 57 is intended to project beyond the
cranial edge of the implant 54 for this purpose. The
anchoring assembly 55 includes, at the opposite end, a
sharp, tissue-piercing or anchoring barb or hook 61. In
use, the barb 61 is intended to project beyond the caudal
edge of the implant 54. The barb or hook 61 can be
manufactured. e.g., from resilient shape memory NiTi
wire, resilient formed stainless steel 316L, or any other
medical grade metal. The barb or hook 61 can be
resiliently straightened by the application of external
pressure (as Fig. 11A(2) shows), and will resiliently
return toward its curved hook shape in the absence of
applied pressure (as Fig. 11A(1) shows).
In the illustrated embodiment (see Figs. 11A(1) and
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 21 -
11A(2)), the anchoring assembly 55 is sized and
configured to be passed, hook end 61 first, through a
constricted cranial-caudal channel 63 formed in the
implant 54. The channel 63 can be formed, e.g., from NiTi
tubing. In use, the channel 63 extends in a cranial-
caudal direction, parallel to the longitudinal anatomic
axis of the pharyngeal conduit. The channel 63 may extend
through holes formed through the individual magnets 65
carried by the implant 54. Alternatively, as shown in
Fig. 11A(1), the channel 63 passes through the flexible
polymer matrix material of the implant 54 itself.
When introduced into the cranial end of the channel
63 (see Fig. 11A(3)), the hooked end 61 will resiliently
straighten within the confines of the channel 63. The
hooked end 61 will resiliently return to its hook shape
(see Fig. 11A(4)) when freed of the caudal end of the
channel 63. It should be appreciated that a given implant
54 can include more than one channel 63 to accommodate a
plurality of anchoring assemblies 55, each with a suture
loop 57 and a barbed end 61 for fixation of the implant
54 in tissue.
During implantation (see Fig. 11A(3)), the implant
device 54 can be placed within a pocket P, e.g.,
surgically created in tissue in the pharyngeal conduit
wall. An X-ray or any other suitable image is desirably
taken to ensure that the position of the implant 54
within the tissue pocket P is correct. Once the correct
position of the implant 54 in the tissue pocket P is
confirmed, the desired number of anchoring assemblies 55
is passed, hook end 61 first, through a channel 63, from
cranial end toward the caudal end (see Fig. 11A(3)). Free
of the channel 63, the end(s) 61 resiliently return(s) to
the hook shape (see Fig. 11A(4)), piercing pharyngeal
wall tissue within the pocket P, to anchor the caudal
portion of the implant 54 within the pocket P. The
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 22 -
cranial end of the implant 54 can then be anchored by
suture material S passed through the loop 57 (as Fig.
11A(4) also shows).
Should the implant 54 need to be re-positioned or
removed, the suture material S can be removed from the
loop 57. By then pulling on the freed loop 57, the hook
61 can be withdrawn from tissue and back into the caudal
end of the channel 63 (as Fig. 11A(2) shows) . Once the
hook 61 is withdrawn and straightened within the channel
63, the implant 54 can be completely removed from the
pocket P, or it can be re-positioned and then re-affixed,
according to the patient's needs.
Fig. 11B(1) shows another representative embodiment
of an implant 54 having at least one tissue piercing barb
or hook 67, which is especially adapted for implantation
in a posterior pharyngeal wall. In Fig. 11B(l), two hooks
67 are shown. Each barb or hook 67 can be manufactured.
e.g., from NiTi wire, stainless steel 316L, or any other
medical grade metal. In the embodiment shown in Fig.
11B(1), each barb or hook 67 is permanently affixed to
the implant 54, e.g., by coupling to one or two of the
magnetic components. The barb or hook 67 extends from the
caudal end of the implant 54. A removable protective
cover 69 (e.g., made from u-shaped nitinol or any other
biocompatible material) is desirably fitted over the barb
or hook 67 prior to use (as Fig. 11B(1) shows) and/or
during implantation (as shown in Fig. 11B(2)).
During implantation (see Fig. 11B(2)), an implant
pocket P is surgically created in the pharyngeal conduit
tissue. In this arrangement, the pocket P that is formed
is desirably longer than the implant 54 itself, by a
distance designated D in Fig. 11(B)(2), e.g., by at least
3mm. The implant 54 is placed into the pocket P, caudal
end first, as Fig. 11B(2) shows. An. X-ray or any other
suitable image is taken to ensure that the position of
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 23 -
the implant 54 is correct. Once the correct position of
the implant 54 in the tissue pocket is confirmed, the
implant 54 is lowered by a distance less than D (e.g., by
approximately 2mm) into the pocket P, and the protective
cover 69 is removed (see Fig. 11B(3)). Each barb or hook
67 pierces pharyngeal wall tissue within the pocket P, to
anchor the caudal portion of the implant 54 within the
pocket P. The cranial end of the implant 54 can then
anchored by suture material S passed through the
apertures in the cranial end of the implant 54 (as Fig.
11B(3) shows ) .
Should the implant 54 need to be removed or re-
positioned, the implant pocket P is re-opened, again re-
creating a pocket P at least 3mm longer than the implant
54. The sutures S at the cranial end of the implant 54
are cut and the implant 54 is lowered within the pocket P
to release each barb or hook 67 from the surrounding
tissue, so that the protective cover 69 can be fitted
back over the barbs or hooks 67.
As Fig. 11B(4) shows, a special spatula tool 300 can
be used to facilitate of the release of the barbs or
hooks 67. The spatula tool 300 has a distal end 302 that
is generally the same width as the implant 54. The distal
end 302 includes a soft polymer material, sized and
configured to engage the sharp ends of the barbs or hooks
67. In use, as Fig. 11B(4) shows, the spatula tool 300 is
inserted behind the implant 54 to the implant device 54
to help free the barbs or hooks 67 from the tissue. Once
the barbs or hooks 67 of the implant 54 are free of
tissue, they will grab the soft polymer material of the
distal end 302. The spatula tool 300 and the attached
implant 54 can now be readily removed from the pocket P
as Fig. 11B(5) shows. Once removed from the pocket P, the
barbs or hooks 67 can be disengaged from the distal end
302, and the protective cover 69 can be fitted back over
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 24 -
the barbs or hooks 67. The implant 54 is again ready to
be re-positioned into the pocket, if desired, in the
manner previously described.
The anchoring systems described, with one or more
barbs or hooks, allow posterior pharyngeal wall implants
to stabilize in desired positions so as to maximize the
therapeutic effects of the implant systems.
Figs. 11C(l) and 11C(2) show another representative
embodiment of an implant 54 having at least one tissue
piercing barb or hook 71, which is especially adapted for
implantation in a posterior pharyngeal wall.
As shown in Fig. 11C(1), the implant 54 includes an
anchoring assembly 73 comprising a U-shaped carrier 75,
which carries at least one tissue piercing barb or hook
71. In the illustrated embodiment, the carrier 75 carries
a plurality of barbs or hooks 71, As before described,
each barb or hook 71 can be manufactured, e.g., from
resilient shape memory NiTi wire, resilient formed
stainless steel 316L, or any other medical grade metal.
The U-shaped carrier 75 slides within tracks 79 formed
within the implant 54 between a first position (shown in
Fig. 11C(l)) and second position (shown in Fig. 11C(2)).
In the first position (Fig. 11C(l)), the barbs or hooks
71 are retracted within the implant 54. In the second
position (Fig. 11C(2)), the barbs or hooks 71 extend
through holes in the track 79 outward from the implant
54.
During implantation, the implant 54 is placed within
a surgically formed tissue pocket P (see Fig. 11C(3))
(e.g., formed in a posterior pharyngeal wall), with the
carrier 75 in the first position, retracting the barbs or
hooks 71. Once the desired position for the implant 54 is
achieved, the carrier 75 is moved to the second position
(see Fig. 11C(4)), advancing the barbs or hooks 71 into
piercing contact wit tissue within the pocket P. One or
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 25 -
more sutures S can be applied to the carrier 75 at the
cranial end of the implant 54. Should repositioning or
removal of the magnetic posterior pharyngeal wall implant
54 be necessary, the carrier 75 can be pulled up to the
first position, retracting the barbs or hooks 71, so that
the implant 54 re-positioned within or removed from the
pocket P.
In an alternative arrangement, the U-shaped carrier
75 need not include side barbs or hooks 71, but comprise
an elongated staple that slides within the tracks 79 and
exits the caudal end of the implant 54 to engage tissue.
In this arrangement, should repositioning or removal of
the implant 54 be necessary, the carrier 75 can be pulled
up to retract the staple at the caudal end, so that the
implant 54 re-positioned within or removed from the
pocket P. As before described, one or more sutures can be
applied to the carrier 75 at the cranial end of the
implant 54.
4. External Fixation Means
Implants need to have features to reduce the stress
on the implant, but still allow them to maintain the
device shape. Another way to limit stress on a given
implant 62 is to include apertures 64 through which
external fixation means, e.g., suture or staples, can be
passed to attach the implant to surrounding tissue, as
illustrated in Fig. 12. This attachment may be to tissue
either on the cranial end or the caudal end of the
implant. Additionally, if the thickness of the underlying
tissue permits, barbs such as silicone extensions (as
previously described) may be also incorporated in the
implant 62. This design will limit the amount of force
applied at the implant edges and prevent motions that can
lead to extrusion. Rounded corners 60 are also provided
(as previously described) to allow for faster healing of
the surrounding tissues.
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 26 -
Fig. 13 shows an implant 66 whose inner edge 68
contains holes 70 to allow the use of surgical thread or
suture 72 to anchor the implant to tissue, e.g., into the
pharyngeal wall.
5. Tissue In-Growth
The implant 36 shown in Fig. 8A includes a network
of holes or cutouts 43 that allow tissue in-growth. The
in-growth of surrounding tissue that the holes or cutouts
43 allow further stabilizes the implant. The implant 36
shown in Fig. 8A can be used, e.g., as a tongue implant,
with the predetermined cut-outs 43 strategically
positioned to promote tissue in-growth. Promoting tissue
in-growth is beneficial in providing a lock-in position
that further discourages implant migration.
The curved implant 56 shown in Fig. 8B also
incorporates an opening 58 in the center of the implant
56 allow for tissue in-growth, further stabilizing the
implant. As before stated, this embodiment is
particularly well suited for implantation in the tongue.
The implant's flowing curves permit a large area of the
surrounding tissues to grow around and grip the implant
thus providing a natural anchor.
6. Stimulating Tissue In-Growth
Figs. 14A and 14B show an implant 36 of the type
shown in Fig. 8A, in which the network of holes 43 is
filled with a growth-stimulating medium 74, which bridges
the gap between the upper and lower tissue layers,
encouraging rapid healing. Fig. 14B shows a close-up of
the growth media 74 used in the implant. The growth-
stimulating substance 74 could be bio-absorbable, or act
as a scaffold for cell growth. The tissue in-growth will
help stabilize the implant.
7. Bio-Compatible Glue
Fig. 15 shows an implant 36 of the type shown in
Fig. 8A, in which the network of holes 43 are filled a
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 27 -
tissue adhesive 76 or glue (e.g. fibrin glue,
cyanoacrylate) . Such glue may be used by itself or in
conjunction with growth stimulating media 74 (shown in
Figs. 14A and 14B) to give immediate post-op tissue
stability.
8. Net Array Implants
Fig. 16 is a plan view of a net array implant 98.
Magnets or ferrous discs 100 are linked together by a
net-like webbing with flanges 102 surrounding each of the
magnets or ferrous shapes. Each disk 100 is linked to the
adjacent disc by a cross web 104, providing protection
and isolation from body fluids and tissue.
Openings 106 provide large areas in which the
opposing surfaces of the surgically produced pocket may
be closed (sutured or otherwise) for fast rejoining and
healing of the tissue. Further, the narrow flanges
surrounding the discs provide clearance for further
approximation of the tissue faces. The periphery of the
discs (see Fig. 17) is sloped 108 to allow the tissue to
form closely around the discs and provide maximum surface
tissue contact between the opposing faces of the tissue
pocket in which the implant 98 will reside.
The material of which the net array web is produced
will preferably be a polymer or compound providing a
predictable flexural modulus to allow normal speech and
swallowing without discomfort or otherwise affecting
these functions. Certain medical grades of silicone
rubber, PTFE (polytetrafluoroethylene) Teflon and
certain laminates using Gore Tex are suitable candidates
for this application. An additional and desirable
characteristic of the material of which the array web is
made will be providing a surface that supports attachment
by the surrounding tissue (in-growth). Expanded PTFE and
Gore Tex are known to exhibit this characteristic.
Fig. 18 shows an alternative embodiment of a net
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 28 -
array implant 98. In this embodiment, the flanges 110 are
linked together around the outside of the array. Also,
cross ties 112 diagonally join the discs 100, to provide
further stabilization.
Many different configurations of the webbing may be
employed to provide varying flexibility or stiffness. For
instance, all cross webbing and peripheral links may
follow a serpentine path instead of a straight line. This
will allow the disks to move toward or away from one
another when the muscular tongue tissue lengthens or
shortens during speech, swallowing, etc.
Furthermore, the magnetic or ferrous shapes may be
other than circular, such as (but not limited to) square,
rectangular, oval, elliptical, etc.
The magnetic net array 98 provides a highly stable
implanted magnetic or ferrous device, overcoming
difficulties related to migration magnet flipping and
inadequate forces needed to prevent occlusion of the
airway during a sleep related obstructive breathing
event. Furthermore, the magnetic net array will allow the
healing of the surgical implantation site prior to the
application of any attractive or repelling forces and
promote speedy healing through close approximation of the
wound surfaces.
The net array 98 can be implanted in a stable manner
in various ways.
Fig. 19 shows a surgically produced pocket 114 with
an opening 116 from the left posterior surface of the
tongue. A magnet or ferrous load net array 98 is
positioned for placement into the open pocket. Fig. 20
shows the net array 98 inserted and the opening 116
closed using sutures, staples, tissue adhesive or other
accepted wound closure means 118. Further suturing 120 or
other tissue securing means can be applied in the open
areas of the net array 98 to provide tight approximation
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 29 -
of the opposing internal surfaces of the pocket. A
template may be provided to the surgeon to aid in
accurate placement of the sutures in the openings in the
array 98.
Fig. 21 shows a surgically produced pocket 114 that
is oriented vertically instead of the horizontal
orientation described above. This approach may be
preferred by the surgeon, may be less difficult to
perform or may result in improved surgical result. Either
approach or other orientation such as angular will be
within the intent of the present invention. The opening
116 of the pocket 114 is upward and the net array 98 is
positioned into the pocket 114. Fig. 22 shows a net array
98 implanted and the opening closed with suitable closure
means 118 as described above. Further, additional sutures
or other anchoring means 120 are placed in areas of
openings in the net array 98. A template may be provided
to the surgeon to aid in accurate placement of the
sutures in the openings in the array 98.
9. Specially Dimensioned Surgical Pockets
As Fig. 23 shows, a surgically formed pocket 96 may
be formed that, with respect to the lateral and
longitudinal dimensions of a given implant (for example,
implant 36 shown in Fig. 8A), is laterally-tight but
longitudinally-loose. Fig. 23 shows, for the purposes of
illustration, the implant 36 to be of the type shown in
Fig. 8A, but the pocket 96 can be sized to accommodate
other types of implants. The pocket dimensions
accommodate anterior-posterior movement of the implant
36, but restrict lateral movement of the implant 36. The
dimensions of the pocket prevent implant migration, while
allowing the implant 36 to move with the tissue, e.g. the
tongue, during normal activities.
In addition to the laterally-tight but
longitudinally-loose surgical pocket, many different
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 30 -
embodiments of surgical pockets are contemplated for
"keyed" shapes implants. Such embodiments include, but
are not limited to, U-, 0-, and L-shaped surgical
pockets.
10. Open Implants
Preceding embodiments stabilize various styles of
implants by allowing and/or encouraging surrounding
tissue to grow through a net-like structure of the
magnetic implant's polymer matrix. Another way to
stabilize implants is by leaving the tissue in the center
of the implant substantially intact.
Fig. 24 shows a U-shaped implant 78 placed in a
tissue pocket 82 of the same shape (e.g., in a tongue).
The tissue 81 in the center of the implant 78 is left
substantially intact. Implant shape is keyed to prevent
migration and limit relative tissue-to-implant motion.
Figs. 25A to 25D illustrate a way of inserting a U-
shaped implant 78 in the tongue. In Fig. 25A two
incisions 80 are made in the tongue. The two incisions 80
are used to cut out a U-shaped implant pocket 82 in the
tissue. In Fig. 25B, using curved forceps, suture 84 is
pushed through the U-shaped pocket 82. One end of the
suture is then tied to the implant 78. In Fig. 25C, using
curved forceps at' one end for pushing the implant and
gently pulling the implant from the other end, the U-
shaped implant 78 is fitted into the pocket 82. During
this process, one leg of the U-shaped pocket becomes
enlarged as the implant turns in the pocket. In Fig. 25D,
the two incisions 80 are closed up with stitches 84. This
method allows the implant to effectively stabilize in its
specified location.
Fig. 26A shows an 0-shaped implant 86 placed in a
tissue pocket 88 of the same shape (e.g., in a tongue).
As with the U-shaped implant 78, the 0-shaped implant 86
leaves tissue 90 in the center of the implant 86
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 31 -
substantially intact. This implant shape is also keyed to
prevent migration and limit relative tissue-to-implant
motion.
Fig. 26B shows an 0-shaped implant 86 where magnets
87 are positioned on only one side. The side 89 without
magnets acts as a rudder to distribute the force of the
tongue and to stabilize the implant. The opening in
center incorporates intact tongue raphe tissue to resist
de-centering. Fig. 26C shows a side view of the
interaction between the one-sided 0-shaped implant 86 of
the type shown in Fig, 26B and a corresponding repelling
pharyngeal wall implant 101.
Figs. 27A to 27D illustrate a way of inserting an 0-
shaped implant 86, as described. The 0-shaped implant 86
may have magnets 87 on both sides, as for example the
embodiment shown in Fig. 26A, or only on one side, as for
example the embodiment shown in Fig. 26B. Both types of
0-shaped implants would use the same insertion method. In
Fig. 27A, an incision 92 is cut in the tongue and the 0-
shaped pocket 88 is created in the tissue. In Fig. 27B,
the 0-shaped implant 86 with open links L1 and L2, i.e.,
in an open configuration, is inserted into the 0-shaped
pocket 88. In Fig. 27C, the open link L2 of the 0-shaped
implant 86 is inserted around a posterior corner of the
0-shaped pocket 88, drawing the other open link L1 to the
opposite posterior corner. The links Li and L2 adjoin (as
Fig. 27C shows), thus changing the implant 86 to a closed
configuration. In Fig. 27D, the incision 92 is closed up
with stitches 94. With this method as well, the implant
is firmly stabilized in its specified location.
B. Prevention of Implant Folding or "Flipping"
During and After Implantation
Arrays of side-by-side magnets can attract each
other during implantation and (if not suitably
stabilized) after implantation, causing the implant to
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 32 -
fold or flip inward upon itself.
Such implant assemblies can be stabilized by
providing more rigid cross-support structures between the
arrays to prevent the motion of attracting the two arrays
together. Fig. 13, previously described, shows an implant
66 with stiff sections 122 between the two magnetic
arrays 124 and 126. The stiff section 122 prevents
migration of the two sections 124 and 126 of the implant
toward one another via attraction during implantation.
Fig. 28 shows an alternative embodiment of a
magnetic implant 128 having a structure that prevents
folding during implantation. The magnetic implant 128
consists of two main magnetic sections 130 and 132,
flexibly joined together by two smaller rigid structures
134 and 136. The flexible juxtaposition of the two
smaller rigid structures 134 and 136 provides four
potential twisting points through which the implant 128
may flexibly twist, but the implant 128 will avoid
folding.
Fig. 29 shows an alternative embodiment of a
magnetic implant 138 which includes middle webbing 140
integrated between two magnetic sections 142 and 144 into
the magnetic implant device to keep the implant 138 from
folding upon itself during implantation. The middle
webbing 140 contains a rigid structure for increased
rigidity during the insertion process. Once the implant
138 is in a desired (and stabilized) position (e.g., by
suturing through the holes 146 provided), the middle
webbing 140 may be cut and removed. The implant 138 is
thereby rendered flexible after implantation, while
resisting folding during implantation.
Figs. 30A to 30C show an alternative embodiment of a
magnetic implant 148 which includes flexible hinges 150
and 152 between two arrays 154 and 156 of magnetic discs
158. Figs. 30B and 30C show the north (N)-south (S)
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 33 -
polarity of the magnetic discs 158. The flexible hinges
150 and 152 allow the arrays 154 and 156 to pivot into a
serpentine shape (see Fig. 30C), but prevent the arrays
154 and 156 from folding upon themselves.
Some of the implant assemblies described above are
stiffened by the presence of rigid cross-support
structures between the magnetic arrays to prevent the
attracting forces between the arrays from flipping or
folding the arrays upon themselves. However, it may be
desirable for certain implants to have a desired degree
of flexibility, even if they are thereby made prone to
flipping. For these implants, it is desirable, during
implantation, to control the separation of the magnetic
arrays until fixation and stabilization of the implant at
the implant site can be accomplished, e.g., by suturing
or other forms of fixation.
Figs. 31 and 32A to 32D show tools and related
methodology for controlling the separation of the
magnetic arrays 200 and 202 of a magnetic implant 190
(shown prior to implantation in Fig. 31) that is prone to
folding or flipping upon itself in response to magnetic
interaction. As Fig. 32A shows, suture 192 is threaded
through one of the magnetic arrays 200 of the implant 190
and tied to form a loop 194. As Fig. 32A also shows,
after the suture loop 194 is formed, the implant 190 is
folded so that the magnetic arrays 200 and 202 overlap.
Folded over, the implant 190 is placed through an
incision into a tissue pocket (e.g., like the pockets
shown in Figs. 19, 21, or 23).
As Fig. 32B shows, within the pocket, a first non-
magnetic surgical instrument 196 holds the magnetic array
202 of the implant 190 against the tissue fascia. A
second non-magnetic surgical instrument 198 is placed
through the suture loop 194. As Figs. 32B and 32C show,
the suture loop 194 is pulled as the first instrument 196
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 34 -
holds the magnetic array 202 against tissue, and as the
second instrument 198 pulls the ends of the suture 192 up
and slightly to the side to separate the magnetic array
200 from the magnetic array 202. As the loop 194 is
pulled, the second instrument 198 continues to guide the
magnetic array 200 to separate the magnetic arrays 200
and 202 within the pocket. As Fig. 32D shows, with the
magnetic arrays 200 and 202 separated, suitable anchoring
sutures 206 are threaded through suture holes 204
provided in the arrays 200 and 202 to secure each
magnetic array 200 and 202 to tissue within the pocket.
The placement suture 192 is then cut and removed. The
instruments 196 and 198 are withdrawn and the pocket
closed.
The instruments 196 and 198 that can be used for
separating magnetic arrays include: forceps, compass-like
spreaders, forceps, tongue-blades and needle-holders.
They are manufactured out of non-magnetic materials,
e.g., titanium.
C. Other Technical Features
1. Implants for the Pharyngeal Wall
The pharyngeal wall is a dynamic structure that
undergoes considerable movement on a daily basis. For a
pharyngeal wall implant to be well tolerated, such an
implant must be able to be stabilized effectively, while
remaining flexible in a posterior-anterior direction.
Fig. 33 shows an implant 158 having spanning members
160 between the magnetic array sections 162, extending
along the vertical (elongated) axis on both sides of the
centerline. The spanning members 160 each have a reduced
thickness, compared to the thickness of the magnetic
array sections 162. The thinner cross section of the
spanning members 160 facilitates flexibility in the
anterior-posterior direction, while the thicker magnetic
array sections 162 discourage flexibility in the medial-
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 35 -
lateral direction. This preferential flexibility allows
the implant to remain in position because it closely
mimics the movements of the surrounding anatomy.
The implant 158 has other features described above
to impart stability and comfort while implanted, e.g.,
holes for accommodating passage of sutures or fasteners
for fixation, and rounded corner edges and beveled side
edges 166 to promote faster healing.
Posterior pharyngeal wall implants present
special challenges due to the difficulty associated
with the attachment/suturing of the caudal end of the
implants to the tissue in the posterior wall.
Rectangular posterior pharyngeal wall implants are
often susceptible to misalignment with relation to the
spine. A misalignment with respect to the spine will
offset the magnetic interaction between the tongue/soft
palate/uvula implant and the posterior pharyngeal wall
implant. If the rectangular device is attached only on
top part using sutures, then the magnetic force from
the tongue base will swing laterally and misalign the
back-wall plate.
Figs. 34A and 34B show a way to help stabilize the
posterior pharyngeal wall implant 54 into a position
that, while not hindering the natural movement of the
posterior wall, provides enough stiffness to the
posterior pharyngeal wall implant to prevent the
pendulum-like motion. In other words, the implant 54
allows for posterior-anterior motion for the normal
functioning of the posterior pharyngeal wall, while
preventing lateral motion that would cause the tissue
pocket to tear or re-open.
As shown in Figs. 34A and 34B, the implant 54
includes a support brace 180 secured to the posterior
(tissue facing) f side of the implant 54. The support brace
180 is thin and combines the shape of a cross and the
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 36 -
shape of a trident. The support brace 180 includes a
vertical axis 181, with a hole 183 on each of the caudal
and cranial ends for suturing the cranial and caudal ends
of the implant 54. The support brace 180 includes a
horizontal component 185 with two handles 187 raised at
an angle between 90 and 180 from each end. Each of the
handles 187 contains a hole 189 for suturing the support
brace 180 to the posterior pharyngeal wall tissue.
The posterior pharyngeal wall implant support is
desirably made of a material that is elastic in its
posterior-anterior movement while rigid with regard to
lateral movement and twisting about the vertical axis of
the support. Such materials include titanium,
biocompatible plastics, as well as other biocompatible
materials.
Fig. 35 shows an alternative design for the magnetic
posterior pharyngeal wall implant. The posterior
pharyngeal wall implant 184 is circular, with the.
attachment holes 186 placed in the center. The circular
design is sutured into place over the spine at the center
of the circle.
Assuming that the tongue implant is collinear with
the spine, then the circular magnetic pharyngeal wall
implant is attached at its center over the spine. The
circular shape favors perfect alignment without any
additional anchoring or correction. If the circular shape
is attached at the center, then it has a self-centered
geometry, as seen in Fig. 35.
2. Implants for the Tongue
Fig. 36 shows an implant 170 adapted for
implantation in a tongue. The implant 170 provides
preferential flexibility that takes into account the
shape and movement of the tongue. The implant 170
includes flexible cross members 172 that extend along the
long (longitudinal) axis that are thicker than (and thus
CA 02630742 2008-05-22
WO 2007/062120 PCT/US2006/045203
- 37 -
less flexible than) the cross members 174 that extend
along the short (transverse) axis. The design of this
implant 170 promotes longitudinal stiffening and
discourages the implant from folding in on itself. The
thinner cross members 174 running across the narrower
areas of the implant 170 allow for flexibility which
closely mimics the movements of the tongue during normal
oral activities. This embodiment of the invention has the
advantage of combining implant stability with increased
tolerance in the patient.
The implant 170 has other features described above
to impart stability and comfort while implanted. For
example, the implant 170 also includes integrated
fixation tabs 176 that extend outward from the magnetic
discs 178 to engage adjacent tissue and provide enhanced
fixation and stabilization.The implant also includes
holes 180 for tissue in-growth or the placement of a
tissue in-growth promoting material or bio-adhesive, as
previously described.
Although the disclosure hereof is detailed and exact
to enable those skilled in the art to practice the
invention, the physical embodiments herein disclosed
merely exemplify the invention which may be embodied in
other specific structures. While the preferred embodiment
has been described, the details may be changed without
departing from the technical features of the invention.