Note: Descriptions are shown in the official language in which they were submitted.
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
AUTOMATED ANALYSIS OF COMPLEX MATRICES
USING MASS SPECTROMETER
CROSS REFERENCE TO RELATED APPLICATIONS
AND PRIORITY CLAIM
[0001] This application claims the priority of United States
Provisional patent application
serial No. 60/742,910, filed 7 December 2005 and entitled Automated Analysis
of Complex Matrices
Using Mass Spectrometer and United States patent application serial No.
11/567,281, filed
6 December 2006 and entitled Automated Analysis of Complex Matrices Using Mass
Spectrometer.
INTRODUCTION
[0002] The invention relates to mass analyzers.
100031 It is sometimes desired to analyze simultaneously a large
number of analytes
contained in a complex matrix of substances. This can be useful, for example,
in forensic,
environmental, metabolic, and food, drug, and beverage studies.
[0004] One method of conducting such analyses has included the use of
chromatographic
devices such as liquid chromatographic (LC) columns used in combination with
mass spectrometers,
as for example in combination liquid-chromatography - recursive mass
spectroscopy (LC-MS/MS)
mass analyzers. Typically in such analyses a chromatographic device causes the
analyte matrix to be
released or otherwise provided to the mass spectrometer in a distributed
manner, such that various
analytes are provided to the mass spectrometer over various periods of time.
Multiple reaction
monitoring (MRM) and/or other recursive or distributed-analysis techniques can
be employed to
analyze the analytes as they are received by the mass spectrometer.
[0005] MRM techniques involve multiple scannings by the mass
spectrometer. Typically the
multiple scannings are adapted, as for example by configuring the mass
spectrometer to provide
suitable electromagnetic fields, for the detection of ions of varying mass-
charge (m/z) ratios as they
are released by the chromatographic device over time. Because of the varying
m/z ratios to be
analyzed, it is desirable for the mass spectrometer to be configured so as to
provide suitable
conditions for the release and detection of the corresponding ions during the
time periods at which
they are released by the chromatographic device.
100061 Using prior art systems, it has been necessary, in order to
obtain the most efficient
possible MRM analyses, for a user of the mass analyzer to provide to the mass
spectrometer a long
and difficult series of commands in an attempt to manually (i.e., non-
automatically) configure the
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
-2-
mass spectrometer for optimal analysis of the various analytes present in the
matrix. Such input
requirements are error-prone, undesirably time-consuming, and tend to result
in inefficient use of
mass analyzers.
SUMMARY
[0007] The invention provides improved systems, apparatus, methods,
and programming
useful for the automated analysis of compounds, and particularly of complex
substance matrices,
using mass spectrometers. Systems, apparatus, methods, and programming
according to the invention
provide for the automatic determination by a controller of a mass spectrometer
of an analysis
operation to be implemented using the mass spectrometer, the analysis
operation adapted specifically
for analysis of one or more substances based contained within a compound based
on identification of
the compound and/or substances provided by a user of the spectrometer, and a
database or other
library of information concerning suitable processes or process steps for
analyzing substances.
[0008] For example, in one embodiment a user is enabled to provide an
identifier, such as a
name or other unique means of specification, to the controller, for use by the
controller in accessing a
data base or other information library and automatically determining an
optimal duty cycle for each of
a plurality of analytes contained in a compound comprising a plurality of
substances, and determining
command signals suitable for configuring the mass analyzer to implement such
duty cycles. The duty
cycles may be implemented, for example, on a recursive mass analyzer such as a
Multiple Reaction
Monitoring (MRM) or Enhanced Product Ion (EPI) mass spectrometer.
[0009] Additional aspects of the present invention will be apparent
in view of the description
which follows.
BRIEF DESCRIPTION OF THE FIGURES
[0010] The invention is illustrated in the figures of the accompanying
drawings, which are
meant to be exemplary and not limiting, and in which like references are
intended to refer to like or
corresponding parts. Those skilled in the relevant art(s) will understand that
the drawings are for
illustrative purposes only, and are not intended to limit the scope of the
teachings herein in any way.
[0011] Figures 1 and 2 are schematic diagrams of mass analyzers
suitable for use in
implementing the invention.
[0012] Figure 3 is a schematic diagram of a controller suitable for
use in implementing the
invention.
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
-3-
[0013] Figure 4 is a schematic diagram of a process suitable for use
in implementing the
invention.
DETAILED DESCRIPTION OF VARIOUS EMBODIMENTS
[0014] Figures 1 and 2 are schematic diagrams of mass analyzers
suitable for use in
implementing the invention. Mass analyzer(s) 100 comprise ion or compound
source(s) (hereinafter
"ion source(s)" or "IS(s)") 12, mass spectrometer(s) ("MS(s)") 14, and
controller(s) 54. The example
shown in Figure 1 is more general than that shown in Figure 2, and illustrates
the concept that any
combination of an ion source(s), mass spectrometer(s), and controller of any
type(s) adaptable for the
purposes disclosed herein may be used in implementing the invention; and
illustrates general
conceptual relationships of the components of mass analyzer 100 to each other.
For example, ion
source 12 provides analyte ions to mass spectrometer 14 for analysis, while
controller 54 controls the
operation of mass spectrometer 14 and optionally ion source 12. For brevity,
the term ion source can
apply generally to each and/or all of the various components of the sample
introduction system,
including, for example, those used in liquid sample handling, liquid
chromatography and the ionizing
systems described below. In various embodiments, the ionization (ionizer) part
of the ion source 12,
in which ions are generated, can be a separate component associated with the
mass spectrometer 14 or
there can be a mass spectrometer interface where the ions are generated by
ionization methods
generally known in the art.
[0015] In the more specific embodiment shown in Figure 2, mass
analyzer 100 comprises a
liquid chromatography - recursive mass spectrometry (LC-MS/MS) mass analyzer
110. LC-MS/MS
mass analyzer 110 comprises an ion source 12 in the form of liquid
chromatograph 212 and ionizer
218 (ionspray as shown) for generating ions, and mass spectrometer 14
comprising triple quadrupole
mass spectrometer 214. Examples of such systems include the API 3000TM and API
4000TM LC-
MS/MS system marketed by MDS Sciex; however, those skilled in the relevant
arts will appreciate
that the invention can be applied to any suitably-controlled system comprising
MS, MS/MS or other
multi-MS capabilities (e.g., 3D traps or time-of-flight (TOF) analyzers).
[0016] As will be understood by those skilled in the relevant arts,
liquid chromatography is
an analytical chromatographic technique used to separate ions dissolved in
solvent(s), and is one way
in which, for example, multiple substances within a given compound can be
introduced to the MS
interface to be ionized, and thus ions of varying miz ratios provided to a
mass spectrometer over a
period of time, in a distributed manner. When for example a sample solution
comprising the targeted
analytes is introduced via sample injector 213 to solvent 215 provided by pump
216 and placed in
contact with suitable second solid or liquid phase reaction agent(s) in column
217, reactions may be
caused which have the effect of separating analytes of interest from other
substances. By making use
of different transit times required for the reaction products, including the
target analytes, to pass
CA 02631218 2008-05-28
WO 2007/065266 PCT/CA2006/001999
-4-
through the column 217, analytes of interest, which typically comprise ions of
varying m/z ratios, may
be introduced to a mass spectrometer 14, 214 in a distributed manner over a
range of times.
[0017] As will be further understood by those skilled in the relevant
arts, a wide variety of
ion sources including the ionizers 218, such as an ionspray, and LC columns
are suitable for use in
implementing the invention described herein. Preferred ion sources are those
which separate analytes
within the test matrix in such a way that the analytes or analyte ions are
provided to the mass
spectrometer 14 in a distributed manner, i.e., over a range of times, so as to
facilitate recursive mass
analyses by mass spectrometer 14 using MRM or other suitable techniques. LC
columns represent
only one type of source for introducing analyte solution to be ionized that is
currently available and
suitable for use in implementing the invention. Others are now commercially
available, and will
doubtless hereafter be developed. Analytes may be introduced to the mass
spectrometer 14 by means
other than LC; for example, analytes may be separated based on a variety of
selective extraction or
derivatization techniques, and presented in solution form to the mass
spectrometer 14 without the
benefit of further LC separation. Another example exists commercially as
analytes are crystallized
with or without a matrix and introduced to the mass spectrometer 14 for
ionization as is the case with
matrix-assisted-laser-desorption-ionization (MALDI), or other surface
ionization applications.
[0018] Mass spectrometer 14 in Figure 2 comprises a triple quad mass
spectrometer device
214 which includes tandem quadrupole ion guide 250. Ions provided by ion
source 12, 212 pass into
mass spectrometer 14, 214 through deferentially-pumped region 220, and from
there through skimmer
240 into a first collimating quadrupole=QO. In order to further accommodate
desired manipulation of
ions provided by ion source 12, 212, collimating quadrupole QO can for example
be located in a
chamber 16 maintained at a pressure around 10<sup>-2</sup> torr.
[0019] Upstream from rod set Q1 within chamber 18, in the embodiment
shown, is a short
collimating rod set 22. Collimating rod set 22 can for example be used to
focus ions of selected m/z
ratios prior to their being introduced to rod set Ql.
[0020] Mass spectrometer 214 can further comprise downstream chamber
18, comprising
triple rod sets Q1, Q2 and Q3, with Q2 being indicated within an interior
subsidiary chamber 20.
Chamber 18 can be maintained at a pressure of approximately 10<sup>-5</sup> torr,
while the subsidiary
chamber 20 is supplied with nitrogen or argon gas as indicated at 21 for
effecting collision-induced
dissociation (CID). In currently-commercialized embodiments, chamber 20 is
typically maintained at
a pressure of around 10<sup>-2</sup> torr.
[0021] The various chambers 16, 18, 20 can be connected in known
manner to suitable
pumps, as indicated at 21, 24, 25 and 26. Commonly, for example,
differentially-pumped region 12
can be connected to a roughing pump, which can serve to back up higher
performance pumps
connected to the pump connections 25 and 26.
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
-5-
.
100221 Rod sets Q1 and Q3 can be operated in various modes, including
a mass-resolving
mode, to select ions of particular m/z ratios. Selected ions pass through into
Q2 and may be subjected
to CID and/or other desired reaction. The resulting product ions and any
remaining precursor ions
may then be passed through into mass-resolving rod set Q3 and recorded by
detector 28.
[0023] Thus, as will be understood by those skilled in the relevant arts,
ions provided by ion
source 12, 212 can be controlled by the various components of tandem
quadrupole ion guide 250 in
order to provide the ions to mass resolver Q3 in desired sequences. As
described herein, for example,
ions of various m/z ratios can be provided to Q3 at desired times and in
desired sequences by suitably
controlling gas pressures in the various chambers or devices 16, 18, 20, 220,
240, and/or by suitably
controlling voltages applied across the electrodes of rod sets QO, Ql, Q2, Q3
and 22. As further
described herein, control signals suitable for controlling such gas pressures
and voltages can be
provided by controller 54.
[0024] As will be understood by those skilled in the relevant arts,
mass spectrometers of any
configurations or capabilities compatible with the purposes described herein
are suitable for use in
implementing the invention. Thus quadrupole linear ion trap (QTrap), tandem
quadrupole time-of-
flight (QqT0F), and other varieties of known mass spectrometers, and many
types of mass
spectrometers which may be developed in the future, are suitable for use in
implementing the
invention. Of particular advantage in some instances, as will occur to those
skilled in the relevant arts
once they have been made familiar with this disclosure, are mass spectrometers
compatible with
Multiple Reaction Monitoring (MRM) and Enhanced Product Ion (EPI) analysis.
[0025] Mass analyzer 100, 110 further comprises controller 54, which
is adapted for
receiving, storing, and otherwise processing data signals acquired from or
otherwise provided by user-
controlled input device(s) and/or by mass analyzer 100, 110; and for executing
suitable algorithms to
determine, and for providing command signals adapted for the control of
operations performed by
mass analyzer 100, 110 in accordance with such signals. For example,
controller 54 is adapted for
interpreting and providing signals useful for controlling voltages and
pressures applied by and
maintained within mass spectrometer 14, 214, and optionally for controlling
ion source 12, 212.
Controller 54 further provides a user interface suitable for controlling the
mass analyzer 100, 110, and
its components, and thus can include input/output devices suitable for
accepting from the user and
implementing commands suitable for analyzing substances.
[0026] In particular, controller 54 is adapted for receiving, from an
input source 62, signals
representing identifier(s) identifying one or more substances, using the
identifier(s) to automatically
access a data set comprising analysis parameters associated with the
identifier, and, using the accessed
data, automatically determining and providing to the mass spectrometer 14, 214
a set of command
signals for use by a mass spectrometer in analyzing the substance(s).
Controller 54 may further be
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
-6-
adapted for processing data acquired by mass spectrometer 14, 214 in response
to the provided
command signals, and for using such acquired data in determining command
signals for use by the
mass spectrometer in further analyzing the substance(s). For example,
controller 54 can be adapted to
store data acquired from mass spectrometer 14, 214 representing substances
identified by mass
analyzer 100, and/or to process such data for output to a user in human-
interpretable form such as a
printed or displayed graph or plot.
[0027] As will be understood by those skilled in the relevant arts,
controller 54 can comprise
any data-acquisition and processing system(s) or device(s) suitable for use in
accomplishing the
purposes described herein. Controller 54 can comprise, for example, a suitably-
programmed or -
programmable general- or special-purpose computer or computer chip, or other
automatic data
processing equipment, with associated programming and data acquisition, input,
output,
communications, and control devices. In particular, controller 54 preferably
comprises or is linked to
or otherwise associated with suitable volatile and/or persistent memory(s).
[0028] Accordingly, controller 54 can comprise one or more automatic
data processing chips
adapted for automatic and/or interactive control by appropriately-coded
structured programming,
including one or more application and operating system programs, and any
necessary or desirable
volatile or persistent storage media. As will be understood by those of
ordinary skill in the relevant
arts, a wide variety of processors, programming languages, data acquisition,
and control devices
suitable for implementing the invention are now available commercially, and
will doubtless hereafter
be developed.
[0029] Examples of suitable controllers, comprising suitable
processors, memories, input and
output devices, and programming are those incorporated in the API 3000TM or
API 4000TM LC-
MS/MS systems available through MDS Sciex of Ontario, Canada.
[0030] As will be understood by those skilled in the relevant arts,
an automated mass
analyzer is any mass analysis device adapted to perform one or more operations
useful in or required
for mass analysis of target substances without a requirement for specific user
command inputs.
Combinations of controllers 54 adapted for such purposes with mass analyzers
100, 110 are examples
of automated mass analyzers.
[0031] An example of a controller architecture suitable for use in
implementing the invention
is shown in Figure 3. In the embodiment shown in Figure 3, controller 54
comprises one or more
processors 56 and associated volatile memory 58, persistent memory 60, input
device(s) 62, and
output device(s) 64. Controller 54 is communicatively linked to mass
spectrometer 12, 214 and to ion
source 12, 212 (including various individual components thereof), in order to
obtain data signals there
from and to provide command signals thereto, as described herein. Controller
54 may further be
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
communicatively linked to one or more remote data bases 66 via a
communications network 76 such
as a wired or wireless public or private network, such as the Internet or a
local or wide-area network.
[0032] As will be understood by those skilled in the relevant arts,
memories 58, 60, input and
output devices 62, 62, and communications interface 66 can comprise any
suitable devices or
components, including for example optical and magnetic ROMs and RAMs,
keyboards, pointing
devices, display screens, printers, wireless devices, and modems. A wide
variety of suitable devices
and components are now commercially available, and will doubtless hereafter be
developed.
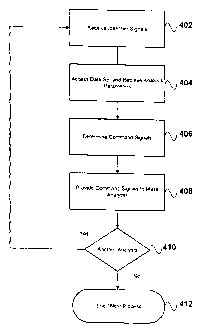
[0033] Figure 4 is a schematic diagram of a process suitable for use
in implementing the
invention. Process 400 illustrated in Figure 4 is suitable for implementation
using, for example, mass
analysers 100, 110 such as those shown in Figures 1 and 2, under the control
of controllers as shown
in Figure 3, executing programs implementing suitably-programmed algorithms;
and is described
below as if so implemented. It is to be understood, however, that, as
described herein, process
illustrated 400 in Figure 4 can be implemented using a wide variety of system
and component
configurations, including a wide variety of programming techniques.
[0034] At 402 controller 54 acquires or otherwise receives from an input
source signals
representing identifier(s) identifying one or more compounds or substances to
be analysed. For
example, a user of a mass analyzer 100, 110 uses a suitably-programmed and/or
controlled input
device 62 such as a keyboard, pointing device, and / or interactive screen
display to enter data
representing an accepted compound or substance name, or abbreviation thereof,
or other unique
identifier. The input device provides signals representing the entered data to
the processor(s) 56. For
example, the user can enter data identifying one or more chemicals, biological
products such as a
clinical test or forensic samples, or nutritional substances such as a foods
or beverages by name, or by
other coded reference such as index or reference number(s).
[0035] At 402 the user may also enter additional data identifying or
otherwise specifying
information relating to the manner in which the identified substance(s) are to
be made available to the
mass spectrometer 14 for analysis. For example, where a mass analyzer 100
comprises an LC column
212, and it is expected that an identified substance or compound will pass or
begin to pass from the
LC column to the mass spectrometer 14 at a given time, or within a given time
range, the user can
input data representing such time and/or time range, or coded reference to
such information. As
explained herein, such additional data can be used, for example, to further
improve the efficiency of
the analysis of the identified substance(s). Signals representing any such
data input by the user may
be provided to processor(s) 56 for use in controlling analysis of the
identified substance(s) by the
mass analyser 100. In applications which require the detection of hundreds or
even thousands of
substances in a sample, the efficiency of the mass analyser 100 may be
improved by monitoring only
for those compounds which are expected to pass from the LC column at specific
times during the
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
-8-
analysis. For example, in the analysis of apple products for pesticide
residues, there may be 400
compounds to be monitored. Each compound has a specific elution time from the
LC column. By
employing the techniques described herein, the user may simply provide
information about the
compounds to be analysed such as their mass, desired parent-daughter ion
transitions to be monitored,
and elution time or time window, and the system can automatically control the
analyser scan functions
during the analysis such that only the compounds expected at each time point
during the analysis
would be scanned for; thus at any given time during the analysis much fewer
than the total 400
compounds are being scanned for. Because the system can interpret the
information provided by the
user on a scan-by-scan basis, there is no need to construct a complicated
acquisition method to the
mass analyser 100 in advance of the analysis.
[0036] At 404 controller 54, using the identifier(s) received at 402,
automatically accesses
data comprising analysis parameters associated with the identifier(s). For
example, processor(s) 56
can, by causing the transmission of suitable command signals, without further
input from the user
beyond the identifiers received at 402, query one or more of memories /
databases or other data stores
58, 60, 66 to retrieve data associated with a compound associated with
identifiers received at a
process step 402, the compound comprising a plurality of substances and times
or ranges of times
over which those substances can be expected to be released or otherwise made
available to a mass
spectrometer 14 by an ionizer 218 as provided by the output from an LC column
214. In various
embodiments, the ionizer 218 can generate ions directly from the output of the
LC column in real time
or the output substance from the LC column can be deposited onto a MALDI plate
surface for future
ionization as known in the art. For example, complex compounds comprising
multiple known
substances suspended or dissolved in known solvents have been analyzed using
LC columns
comprising known reaction agents, and the output of substances from the LC
columns recorded as a
function of time. Thus for example data sets comprising identifiers such as
names representing
compounds, and/or substances, and the times and/or ranges of times (including
suitable tolerances for
variations) at which various ions are released from the LC column (sometimes
known as "retention
time") are known and can be used in accordance with the invention. An example
of an application of
this aspect of the invention is an on-demand analysis application for rapid
drug screening. In the case
where blood or urine sample is provided and requires urgent analysis for a
large number of possible
toxins or drugs of abuse, this invention provides the user with the ability to
simply supply a list of
desired analytes. The system can then automatically perform the appropriate
mass analyser 100
functions to detect the compounds by obtaining information about scanning
parameters, elution times,
desired identification ions, etc. from a database.
100371 At 408 controller 54, using data accessed at 404, provides
command signals
determined at 406 to mass analyzer 100, in order to configure the mass
analyzer for analysis of
substance(s) provided by ion source 14. In preferred embodiments command
signals thus provided
CA 02631218 2008-05-28
WO 2007/065266
PCT/CA2006/001999
-9-
cause mass analyzer to be configured accordingly, and are thus used by mass
analyzer 100 in
performing a desired analysis of the substance(s) and as desired in any
subsequent processing of data
obtained by the mass analyzer 100.
[0038] One of the advantages offered by the invention is improved
analysis of multiple
compounds and/or the analysis of relatively complex compounds. The invention
can be useful, for
example, in enabling controller 54 to cause the re-configuration of mass
analyzer 100 and/or any
components thereof in performing sequential analyses of multiple substances,
or in breaking down the
analysis of complex compounds into multiple steps, and re-configuring one or
more components of
mass analyzer 100 for efficient analysis during such analyses or steps, as for
example in LC/MS/MS
or other recursive spectroscopy. Furthermore, because the invention allows the
determination of
subsequent analysis based on the data provided by the user in specifying the
method, and/or accessed
at 404, the system may combine this data with the data collected by the mass
analyzer 100 on a per
scan basis to correct for deviations in LC performance which may result in
compounds of interest
eluting at times different from those specified, and/or to adjust the mass
analyser 100 duty cycle to
improve the signal-to-noise ratio for low intensity peaks, and/or any other
appropriate adjustment of
the mass analyser 100 parameters which my be indicated by the current or
previous scan data. Thus at
410 a determination is made by controller 54 as to whether any subsequent
analyses, analysis steps, or
mass analyser 100 adjustments are to be performed. If so, process 402 - 408 is
repeated as desired.
Thus the invention provides, for example, for increased performance in the
execution of multiple scan
duty cycles in LC/MS/MS or other recursive analyses.
[0039] For example, a recursive mass analyzer such as an LC-MS/MS
device 110 can be
configured to perform a series of scanning by for example configuring the
device to provide suitable
electromagnetic fields in devices QO, Ql, Q2, Q3, and 22 of Figure 2, and/or
suitable pressures in
chambers or devices 16, 18, 20, 220, 240, at varying points in time, for the
optimal detection of ions
of varying mass-charge (m/z) ratios as they are released by a chromatographic
device. In particular,
the mass analyzer may be configured for the detection of ions of varying m/z
ratios during the time
periods at which they are released by the chromatographic device and made
available to the mass
analyzer.
[0040] An example of the usefulness of this invention in practice is
easily found in modern
routine analysis. For example, in the analysis of blood or urine samples for
drugs of abuse, the
analyst is challenged to find a method which can simultaneously detect the
presence of hundreds of
compounds in the blood or urine sample. In order to facilitate this analysis,
LC separation is used to
present the mass spectrometer with a liquid stream in which the analytes of
interest are generally
separated in time throughout the period of the analysis. Because the analytes
appear individually for a
limited time (peak width) in the LC stream, and because the detector must scan
for hundreds of
compounds individually in a looped sequence of MRM or other compound specific
scan functions, the
CA 02631218 2013-10-09
WO 2007/065266 PCT/CA2006/001999
-10-
number of data points available to characterize the presence of a compound in
the sample is limited by
the number of complete scan cycles which can be accomplished within the
typical peak width. For
typical triple-quad (QQQ) instrumentation, a scan cycle comprising the
individual selective scans for
400 compounds may take up to 20 seconds, depending on the instrument. For
typical peak widths of
10-30 seconds, it would be normal to expect only one or two data points per
peak. In extreme cases,
there is the likelihood that a compound would go undetected as the peak width
is smaller than the
scan cycle and thus occurs between subsequent scans, thereby eluting
undetected. With the
implementation of the invention described herein, the user can simply provide
the system with a list of
compound names and expected elution times or time windows. The system would
then automatically,
on a scan-by-scan basis, determine which compounds to scan for, thus
significantly reducing the
number of compounds which are being simultaneously scanned for at any given
time during the
analysis. Furthermore, the systems has the ability to adjust mass analyser 100
properties on a scan-
by-scan basis to perform subsequent MS/MS analysis for compound confirmation,
correct for
variations in LC performance, and improve signal-to-noise ratios of small
signals. This has the effect
of increasing the number of data points which represent the eluting analyte
peaks, and increasing the
instrument duty cycle with respect to each individual analyte. By employing
this invention, the data
generated by such a multi-compound screening method can be improved by
reducing the probability
of an undetected peak, improving the quality of peak area determination
(quantitation) and allowing
for an increase in the speed of analysis. Furthermore, the implementation of
large numbers of
analytes in a method becomes very simple for the user as all that is required
is a list of expected
elution times for the desired compounds which the system can interpret in
order to appropriately
control the detector during the analysis.
[00411 As noted above, process 400 illustrated in Figure 4 and other
processes described
herein are suitable for implementation using mass analysers such as those
shown in Figures 1 and 3,
and controllers as shown in Figure 3, executing suitably-programmed
algorithms. As will be
understood by those of ordinary skill in the relevant arts, such algorithms
can be coded or otherwise
programmed in a wide variety of ways to provide computer-readable and -
executable program codes,
including for example through the use of binary language or high-level
computer languages such as
FORTRAN, C# or any other suitable programming language. The implementation of
the code
may be approached in several different ways, and the code and information
storage necessary may be
implemented on either the instrument controller of the host computer.
00421 Except to the extent necessary or inherent in the processes
themselves, no particular
order to steps or stages of methods or processes described in this disclosure,
including the Figures, is
implied. In many cases the order of process steps may be varied without
changing the purpose,
effect, or import of the methods described.
CA 02 631218 2013-10-09
WO 2007/065266
PCT/CA2006/001999
-I 1-
10043j The section headings used herein are provided for organizational
purposes only, and
are not to be construed as limiting the subject matter described in any way.