Note: Descriptions are shown in the official language in which they were submitted.
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
COMPOSITION AND USE OF PHYTO-PERCOLATE FOR
TREATMENT OF DISEASE
FIELD OF INVENTION
This invention relates generally to methods and compositions for treating
human
diseases, disorders, and conditions using a preparation of a phyto-percolate
isolated from a
= complex mixture of fresh water algae and other microorganisms.
BACKGROUND OF INVENTION
Enzymes have a very important use within biochemical cycles in the human body.
The majority of acute and chronic diseases create an inflammatory process that
results in
the destruction of surrounding tissue. This tissue debris becomes toxic and
further hinders
the processes of detoxification, elimination and defense by way of free
radical oxidation.
Proteolytic enzymes are responsible for the body's detoxification processes.
As humans
age and chronic disease processes progress, a deficiency of the proteolytic
enzymes that
carry out the body's waste detoxification processes may be experienced. This
enzymatic
deficiency aids in the production of a chronic hyper-inflammatory state, and
the disease
process becomes much more complex.
Enzymes are the catalysts that control and direct all metabolic processes.
Without
adequate enzymes in the body, chaos reigns and the immune system and other
metabolic
processes become less efficient, making tissue repair slow and poorly
replicated.
Proteolytic enzymes, or proteases, are enzymes capable of breaking down
proteins by
cleaving peptide bonds. They are produced and utilized by every living
organism on Earth
for protection, nutrient breakdown and assimilation, and waste removal. Many
degenerative diseases stem from proteolytic enzyme deficiencies, leading to
the inadequate
removal of carcinogenic wastes from the body.
It is believed that the immune system, which helps protect us from diseases
including cancer, cardiovascular disease, and other immune deficient or
deregulated
disorders, can become ineffective because of advanced disease state or age.
Immune
deficiency caused by disease state or advancing age can impair benefits
received from the
use of therapeutic drugs that may be taken for the treatment of these various
disorders.
Therapeutic drugs may lose their effectiveness in a compromised immune system
as a
disease state progresses due to metabolic dysfunction or poor therapeutic drug
assimilation.
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
With advancing age, humans experience an increasing accumulation of
environmental influences that are believed to have toxic effects on the, human
body. An
observed effect associated with aging is a less accurate tissue repair
process, possibly an
expression of DNA mutations caused by environmental factors. Because of these
alterations, foreign antigens in the way of microbes, and environmental toxins
such as
radiation and chemical compounds through foods, Water, and air, are allowed to
increasingly invade the human organism. These environmental toxins are
introduced
primarily through the mucous membranes of the intestinal tract, upper
respiratory tracts
and lungs.
Human genes, which are made up of double-strands of DNA, are the directors of
tissue repair. It is believed that through advancing age and contact with the
surrounding
destructive elements, the expression of such DNA may become less and less
accurate
because of replication errors and mutations, thus creating very different
functional end
products of repair when compared to a younger individual.
Impaired immune protection and regulation, it is believed, allows an
increasing
amount of toxic environmental components to invade the cells of our bodies.
These toxic
components express destructive patterns of oxidation by way of free radical
activity, thus
rendering important metabolic processes to function inadequately. Because of
biochemical
cellular destruction, dead, fractionated cellular components are created,
adding to the toxic
manifestations. White cells, which are an important part of the immune system,
congregate at the sites of tissue destruction in an effort to slow the process
down. A
chemical reaction that takes place at the site causes inflammation that
further increases the
destructive pattern. This pattern of tissue destruction, secondary to foreign
antigen
invasion and the associated white cell activity, can create an ongoing
autoimmune
hyperactive inflammatory state and an increasing amount of toxic tissue
destruction and
debris. Because of the increased inefficiency of tissue repair and the ever
presence of
surrounding environmental influences, human metabolic processes become less
and less
efficient with age.
The inner lining of the blood vessels, particularly the arteries, can be
affected by
this destructive pattern. Because many environmental contaminants are
introduced into
human bodies through the intestinal tract and lungs, they spread through the
body by way
of the vascular bed, thus coming first in contact with the inner lining of the
blood vessels.
2
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
This ongoing contact in the inner lining of the arteries with toxic free
radicals results in the
destructive oxidative process. This maintains an ongoing inflammatory state
that includes
cell break down and scar tissue formation in the form of sclerotic plaques.
These plaques
are made up of fibrous tissue, cholesterol, calcium deposits and necrotic
tissue (broken
down cellular components). Increasing arterial restriction and blood
thickening due to
pathological fibrin diminishes blood flow and alters oxygen and nutrient
distribution to
vital organs. This gradually increasing cellular starvation affects the
functions of the brain,
heart, kidneys, muscles, joints and other vital systems.
It is believed that accelerated DNA mutations and errors in replication,
increased
oxidation, inflammation, dysregulated white cell activity, and tissue
destruction are the
results of a gradual progression of contact with environmental forces,
including pathogenic
microbials. The amount of contact depends on lifestyle and individual health
care. Some
illnesses either originate from excessive free radical oxidation destruction
at the body's
cellular level, or cause a great increase in free radical oxidation
destruction. Therefore,
when the body's own metabolic and healing processes are unable to cope with
the excess
of toxic waste products, a cycle of ongoing inflammation and disease is
created that
interferes with the body's normal immune activity and tissue repair. Tissue
destruction
also activates the body's coagulation, or blood-clotting, mechanism,
generating a barrage
of intra-vascular thrombi, or blood clots, and blood-thickening fibrin, that
can precipitate
strokes, heart attacks, pulmonary emboli, kidney damage, and phlebitis.
Oxidative free radical activity becomes rampant because of the action of the
involving white cells attempting to control the initial cause of the
destruction. The resulting
pathological agents secondary to this influence of white cell activity create
an ongoing
destructive pattern upon local surrounding tissue, the endothelial cells that
line the vascular
bed, and the epithelial cells lining the intestinal tract. Not only is there
destructive activity
upon the above-mentioned tissues but also there is oxidative breakdown or
pathological
activation of the coagulation factors. This includes pathologically activated
fibrinogen to
produce a soluble fibrin that, unlike insoluble fibrin, which is an important
component of
the normal blood-clotting mechanism, cannot be cross-linked and is
pathological, or
harmful to the body. This soluble fibrin not only negatively influences
general capillary
circulation but also kidney filtration, oxygen exchange within the alveoli of
the lungs, and
3
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
oxygenation of brain tissue. It not only thickens the blood, but is in itself
an oxidative free
radical, and contributes to the degenerative oxidation process.
Much of the expressed symptomatology from the production of soluble fibrin is
caused by gram-negative bacteria, mycoplasma and Candida albicans, which are
allowed
to flourish in the immune-compromised environment created by excess wastes and
fibrin,
and is related to the cellular destruction and by-products of ongoing free
radical activity.
Fibrinolytic activity, or the process of breaking down fibrin, along with the
eradication of
the foreign pathological agents by other therapeutic interventions, can lead
to increasingly
effective immune system and white cell activity, and will greatly accelerate
the healing
process.
Most cancer processes liberate hydrogen peroxide, which acts as a free radical
oxidative agent. In addition to hydrogen peroxide, the effects of cancer
growth and
chemotherapy produce excess soluble fibrin products as a response to these
abnormal and
destructive processes. The fibrin is produced as part of the body's natural
reaction to tissue
damage, which also occurs normally at the site of a superficial wound.
However, at the
site of cancer growth, fibrin coats cancer cells, tragically insulating them
from destruction
by the body's immune system. These coagulation mechanisms, stimulated by the
oxidative
damage associated with chronic illness, the damaging effects of chemotherapy,
and the
nature of abnormal cancer growth, all lead to further damage. Chronic
illnesses such as
cancer produce an acceleration of disseminated intravascular coagulation,
causing not only
a build-up of soluble fibrin but also of small intravascular thrombi, or clots
that float
around the vascular bed acting as emboli that obstruct circulation. The use of
a fibrinolytic
agent, along with any other therapeutic regime, will increase immune
regulation and the
effectiveness of white cell activity, improve capillary circulation and
nutrient flow to the
body's organs, aid in eliminating toxins, and enhance the benefits of other
therapeutic
agents. In addition, fibrinolytic agents will reduce the amount of free
radical soluble fibrin
that accelerates degenerative oxidation, and can increase the body's immune
effectiveness
in combating cancer growth.
In vivo laboratory monitoring of disease processes has supported the
observations
that improved cellular function and efficiency come with less oxidative, free
radical
activity, improved cellular nutrition, enhanced immune activity and white cell
function and
improved oxygenation.
4
CA 02631773 2013-11-22
SUMMARY OF THE INVENTION
In one aspect, the invention provides a method for treating or preventing a
disorder
in a mammal (e.g., human, dog, cat, horse, etc.) by administering to the
mammal a
therapeutically effective amount of phyto-percolate or derivative thereof.
In useful embodiments, the phyto-percolate derivative is a protein having a
molecular weight of about 67.5 kDa, a protein having a molecular weight of
about 21.0
kDa, or a polysaccharide. In another embodiment, the phyto-percolate
derivative has
fibrinolytic enzymatic activity. The phyto-percolate derivative may be
isolated from the
phyto-percolate or it may be produced by any appropriate method known in the
art.
Suitable methods for producing the phyto-percolate derivative include, for
example,
recombinantly expressing the derivative (e.g., protein) by a microorganism and
synthetically producing a derivative (i.e., chemical (cell-free) synthesis).
The recombinant
microorganism may be one or more of the species present in ATCC Deposit #PTA-
5863
(filed March 17, 2004), or it may be any other appropriate specie.
In particular embodiments a particular dosage is between about 1 and about 8
ounces per day of the phyto-percolate. Particularly noted is a dosage of about
1 to about 4
ounces per day. Preferably, the phyto-percolate that is administered to the
human contains
between about 10 ppm and about 150 ppm of a phyto-percolate derivative. In
another
useful embodiment, a therapeutically effective amount of one or more of the
derivatives is
administered to the human. Preferably, the mammal is administered between
about 1 mg
and 1000 mg of the derivative per day. Suitable methods for administration of
the phyto-
percolate include oral administration. Suitable methods for administration of
a phyto-
percolate derivative (e.g., an isolated derivative) include, for example,
oral, topical, rectal,
or vaginal administration as well as intravenous, intramuscular, and
subcutaneous
injection.
Another aspect of this invention is directed to a method of treating an
overweight
condition or obesity comprising administering to the mammal . (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating type I
and II
diabetes comprising administering to the mammal (e.g., human) a
therapeutically effective
amount of a phyto-percolate or derivative thereof.
5
4190042 vi
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
Another aspect of this invention is directed to a method for treating an
inflammatory disorder comprising administering to the mammal (e.g., human) a
therapeutically effective amount of a phyto-percolate or derivative thereof.
It is believed
that the phyto-percolate and derivatives have broad spectrum anti-inflammatory
properties
and therefore may be used to reduce or prevent inflammation in a wide range of
diseases
and disorders.
Another aspect of this invention is directed to a method for treating a
stomach
disorder comprising administering to the mammal (e.g., human) a
therapeutically effective
amount of a phyto-percolate or derivative thereof. Stomach disorders amenable
to
treatment with the phyto-percolate and/or derivatives thereof include, for
example, a
stomach ulcer and gastric reflux disease.
In another aspect of this invention, the phyto-percolate or derivatives may be
used
= to alleviate side-effects of another primary therapy. For example, the
phyto-percolate may
be administered to reduce the oxidative stress, chemotherapy-induced nausea,
chemotherapy-induced liver damage, appetite suppression, hair loss, fingernail
and toenail
loss and discoloration that result from anti-AIDS therapy and anti-cancer
therapy (e.g.,
chemotherapy and radiation therapy).
In another aspect of this invention, the phyto-percolate or derivatives may be
used
to reduce the recovery time in mammals (e.g., humans and horses) after periods
of stress
(e.g., exercise). In a related aspect, the phyto-percolate or derivatives are
administered in
order to restore physical energy and mental acuity following periods of
stress.
In another aspect of this invention, the phyto-percolate or derivates may also
be
administered topically directly to the eye (e.g., in the form of eye drops) to
treat lesions of
the corned, dry eyes, and similar ocular disorders.
Another aspect of this invention is directed to a method for treating
conditions or
disorders associated with infectious disease (e.g., a viral infection)
comprising
administering to the mammal (e.g., human) a therapeutically effective amount
of a phyto-
percolate or derivative thereof. Infectious disease may be the cause of many
of the above
and below listed diseases such as pneumonia, all viruses, acariosis, acne,
adenovirus,
AIDS, amebiasis, anthrax, athlete's food, babesiosis, bartonellosis, Bell's
palsy, botulism,
candidiasis, carbuncles, Chaga's disease, chicken pox, Chlamydia,
coccidiomycosis,
coronavirus, cryptococcosis, cytomegalovitus, Dengue fever, echovirus,
erysipelas,
6
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
furuncle, gangrene, Guillan-Barre syndrome, hepatitis, impetigo, influenza,
leucopenia,
Lyme's disease, malaria, martolditis, measles, mumps, mycobacterium, mycosis,
parasites,
pediculosis, P.I.D. pyodermia, rabies, rubella, salmonella, salpingitis,
septicemia, shingles,
sinusitis, syphilis, tetanus, Tindi Cruzi and warts.
Another aspect of this invention is directed to a method for treating diseases
related
to the heart, blood vessels, renal, liver, and endocrine system comprising
administering a
therapeutically effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating a
vasospasm
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating heart
failure
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating cardiac
hypertrophy comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
dysregulated
blood pressure comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating angina
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
peripheral
vascular disease comprising administering to a mammal (e.g., human) a
therapeutically
effective amount ofa phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating cerebral
diseases and diseases related to the central nervous system that are vascular
in origin
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating neuro-
degeneration comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
7
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
Another aspect of this invention is directed to a method for treating
Alzheimer's
disease comprising administering to a mammal (e.g., human) a therapeutically
effective
amount of a compound of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
depression
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-perco late or derivative thereof.
Another aspect of this invention is directed to a method for treating
addiction,
including drug detoxification and/or substance abuse including nicotine,
cocaine and
alcohol abuse comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
attention deficit
disorder and attention deficit hyperactivity disorder comprising administering
to a mammal
(e.g., human) a therapeutically effective amount of a compound of a phyto-
percolate or
derivative thereof.
Another aspect of this invention is directed to a method for treating sleep
disorders
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating seasonal
affective disorder comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
environmental
and food allergies comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
conditions
related to pain or nocioception comprising administering to a mammal (e.g.,
human) a
therapeutically effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating migraine
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a compound of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
disorders
related to disruption of circadian rhythms including jet lag comprising
administering to a
=
8
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
mammal (e.g., human) a therapeutically effective amount of a phyto-percolate
or derivative
thereof.
Another aspect of this invention is directed to a method for treating diseases
related
to abnormal gastrointestinal motility, secretion, and/or function comprising
administering
to a mammal (e.g., human) a therapeutically effective amount of a phyto-
percolate or
derivative thereof.
Another aspect of this invention is directed to a method for treating diarrhea
and/or
incontinence comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating a
gastric ulcer
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-perco late or derivative thereof.
Another aspect of this invention is directed to a method for treating
irritable bowel
syndrome comprising administering to a mammal (e.g., human) a therapeutically
effective
amount of a phyto-percolate or derivative thereof
Another aspect of this invention is directed to a method for treating
inflammatory
bowel disease comprising administering to a mammal (e.g., human) a
therapeutically
. effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating nausea
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating sexual
dysfunction comprising administering to a mammal (e.g., human) a
therapeutically
effective amount of a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for altering
fertility
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate or derivative thereof.
Another aspect of this invention is directed to a method for treating
conditions or
disorders associated with the immune system comprising administering to a
mammal (e.g.,
human) a therapeutically effective amount of a phyto-percolate. Immune system
deficiency may be the cause of many of the above and below listed diseases
such as cancer,
9
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
emphysema, encephalitis, environmental sensitivity, erysipelas, food poisoning
and
Reynaud's disease.
Another aspect of this invention is directed to a method for treating
conditions or
disorders associated with hormonal imbalances comprising administering to a
mammal
(e.g., human) a therapeutically effective amount of a phyto-percolate.
Hormonal
imbalances may be the cause of many of the above and below listed diseases
such as acne,
Addison's disease, endometriosis, Grave's disease, osteoporosis, menstrual and
menopausal regulation, glucose, and other metabolic regulation. In this
regards, the phyto-
percolate and derivatives may be used to improve the general health and
overall function of
metabolic organs like the kidney, liver, and pancreas. It is believed that the
phyto-
percolate and derivatives improve the efficiency of those organs and increases
their
metabolic and endocrine functions.
Another aspect of this invention is directed to a method for treating
conditions or
disorders associated with neurological deficiencies comprising administering
to a mammal
(e.g., human) a therapeutically effective amount of a phyto-percolate.
Neurological
deficiencies may be the cause of many of the above and below listed diseases
such as Lou
Gehrig's disease, chronic pain, Huntingdon's Chorea, diabetic neuropathy,
multiple
sclerosis, Myasthenia Gravis, Parkinson's disease, poliomyelitis, senile
dementia,
nigrostriatal degeneration, stroke, tardive dyskinesia and tinnitus.
Another aspect of this invention is directed to a method for treating
respiratory
diseases comprising administering to a mammal (e.g., human) a therapeutically
effective
amount of a phyto-percolate.
Another aspect of this invention is directed to a method for treating asthma
comprising administering to a mammal (e.g., human) a therapeutically effective
amount of
a phyto-percolate.
Another aspect of this invention is directed to a method for treating diseases
related
to abnormal hormone release and utilization comprising administering to a
mammal (e.g.,
human) a therapeutically effective amount of a phyto-percolate.
Another aspect of this invention is directed to a method for treating abnormal
insulin release and utilization. comprising administering to a mammal (e.g.,
human) a
therapeutically effective amount of a compound of a phyto-percolate.
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
Another aspect of this invention is directed to a method for treating skin
lesions and
disorders.
In addition to the "direct" effect of the phyto-percolate of this invention
there are
diseases/conditions wherein subjects with said diseases/conditions will
benefit from the
associated weight loss, and metabolic and immune system regulation, such as
insulin
resistance with impaired glucose tolerance, Type II Diabetes, hypertension,
hyperlipidemia, cardiovascular disease, gall stones, certain cancers, sleep
apnea, etc.
resulting from use of phyto-percolate.
In a further illustrative embodiment a method of making the inventive phyto-
percolate is disclosed. The phyto-percolate is prepared by cultivating a
mixture of
freshwater algae and bacteria that is augmented by a nutrient blend that is
related to the
production of fibrinolytic enzymes, proteins, and other molecules, forming a
fortified algae
culture. Added to this fortified algal and bacterial culture is purified fresh
water that has
been purified by reverse osmosis, distillation and/or deionization. The
cultdre is percolated
with said purified fresh water and nutrient blend for a predetermined time
forming a phyto-
percolate that is fibrinolytic and proteinaceous in nature. The phyto-
percolate is decanted
from the fortified algal and bacterial culture and processed. Suitable methods
of
processing the phyto-percolate include filtration, centrifugation,
lyophilization,
purification, dilution, and other methods. The filtering of the decanted phyto-
percolate in
one particular embodiment is by micro-filtration where the micro-filtration
removes
particles larger than about 0.22pm.
In another aspect, this invention provides a substantially pure compound
isolated
from a phyto-percolate. In a preferred embodiment, the compound is isolated
from the
percolate produced by culturing the microorganisms of ATCC Deposit #PTA-5863
or
other appropriate species as described herein. In another embodiment, the
compound is a
protein having a molecular weight of about 67.5 IcDa.
In a related aspect, the invention provides a pharmaceutical formulation
comprising
a substantially pure compound isolated from a phyto-percolate and a
pharmaceutically
acceptable excipient.
The term "inflammatory disorder" encompasses a variety of conditions including
conditions related to a hyperactive immune system, chronic inflammation, and
autoimmune disorders. Inflammatory disorders include, for example, acne
vulgaris; acute
11
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
febrile neutrophilic dermatosis; acute respiratory distress syndrome;
Addison's disease;
adrenocortical insufficiency; adrenogenital ayndrome; allergic conjunctivitis;
allergic
rhinitis; allergic intraocular inflammatory diseases, ANCA-associated small-
vessel
vasculitis; angioedema; ankylosing spondylitis; aphthous stomatitis;
arthritis, asthma;
atherosclerosis; atopic dermatitis; autoimmune disease; autoimmune hemolytic
anemia;
autoimmune hepatitis; Behcet's disease; Bell's palsy; berylliosis; balanitis
circumscripta
' plasmacellularis; balanoposthitis; bronchial asthma; bullous herpetiformis
dermatitis;
bullous pemphigoid; carditis; celiac disease; cerebral ischaemia; chronic
obstructive
pulmonary disease; cirrhosis; Cogan's syndrome; contact dermatitis; COPD;
Crohn's
disease; Cushing's syndrome; dermatomyositis; diabetes mellitus; discoid lupus
erythematosus; eczema (e.g., asteatotic eczema, dyshidrotic eczema, vesicular
palmoplantar eczema); eosinophilic fascitis; epicondylitis; erythema annulare
centrifugum;
erythema dyschromicum perstans; erythema multiforme; erythema nodosum;
exfoliative
dermatitis; fibromyalgia; focal glomerulosclerosis; giant cell arteritis;
gout; gouty arthritis;
graft-versus-host disease; granuloma annulare; hand eczema; Henoch-Schonlein
purpura;
herpes gestationis; hirsutism; hypersensitivity drug reactions; idiopathic
cerato-scleritis;
idiopathic pulmonary fibrosis; idiopathic thrombocytopenic purpura; inflamed
prostate;
inflammatory bowel or gastrointestinal disorders, inflammatory dermatoses; =
juvenile
rheumatoid arthritis; laryngeal edema; lichen nitidus; lichen planus; lichen
sclerosus et
atrophicus; lichen simplex chronicus; lichen spinulosus; Loeffler's syndrome;
lupus
nephritis; lupus vulgaris; lymphomatous tracheobronchitis; macular edema;
multiple
sclerosis; musculoskeletal and connective tissue disorder; myasthenia gravis;
myositis;
nummular dermatitis; obstructive- pulmonary disease; ocular inflammation;
organ
transplant rejection; osteoarthritis; pancreatitis; pemphigoid gestationis;
pemphigus
vulgaris; polyarteritis nodosa; polymyalgia rheumatica; primary adrenocortical
insufficiency; primary billiary cirrhosis; pruritus scroti; pruriti s/i n fl
ammati on, psoriasis;
psoriatic arthritis; Reiter's disease; relapsing polychondritis; pyoderma
gangrenosum;
rheumatic carditis; rheumatic fever; rheumatoid arthritis; rosacea. caused by
sarcoidosis;
rosacea caused by scleroderma; rosacea caused by Sweet's syndrome; rosacea
caused by
systemic lupus erythematosus; rosacea caused by urticaria; rosacea caused by
zoster-
associated pain; sarcoidosis; scleroderma; segmental glomerulosclerosis;
septic shock
syndrome; serum sickness; shoulder tendinitis or bursitis; Sjogren's syndrome;
Still's
12
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
disease; stroke-induced brain cell death; Sweet's disease; systemic
dermatomyositis;
systemic lupus erythematosus; systemiC sclerosis; Takayasu's arteritis;
temporal arteritis;
thyroiditis; toxic epidermal necrolysis; tuberculosis; type-1 diabetes;
ulcerative colitis;
uveitis; vasculitis; and Wegener's granulomatosis.
.The term "substantially pure," when referring to a protein or other
derivative of the
phyto-percolate, means the state of a substance that has been separated from
the other
components of the phyto-percolate. Typically, a substantially pure derivative
is at least
80%, by weight, free from the proteins and other organic molecules of the
phyto-percolate.
Preferably, the substantially pure derivative is at least 90%, 95%, or 99%, by
weight, free
from those organic molecules. A substantially pure protein derivative may be
obtained, for
example, by extracting it from a source other than the phyto-percolate. A
protein
derivative, for example, may be recombinantly expressed in another
microorganism or in a
cell-free translation system.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other features and advantages of the present invention will
be
more fully understood from the following detailed description of illustrative
embodiments,
taken in conjunction with the accompanying drawing in which:
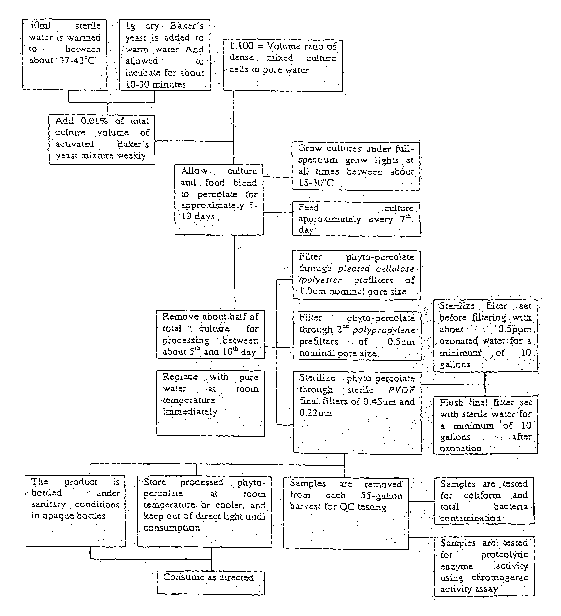
FIGURE 1 is a flow chart showing a method of preparing a phyto-percolate;
.FIGURE 2 is an HPLC chromatogram of the diluted phyto-percolate;
FIGURE 3 is an FTIR spectrum of the diluted phyto-percolate; and
FIGURE 4 is a [11-1]-NMR spectrum of the diluted phyto-percolate.
DETAILED DESCRIPTION
The present invention provides a phyto-percolate that has therapeutic and
other
beneficial properties when administered to humans and other animals. Without
being
bound by any theory, it is believed that at least one of the therapeutically
active agents in
the phyto-percolate is an enzyme. Methods for preparing the phyto-percolate
are also
provided. Detailed embodiments of the present invention are disclosed herein,
however, it
is to be understood that the disclosed embodiments are merely exemplary of the
invention,
which may be embodied in various forms. Therefore, specific functional details
disclosed
13
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
=
herein are not to be interpreted as limiting, but merely as a basis for the
claims and as a
representative basis for teaching one skilled in the art to variously employ
the present
invention in virtually any appropriately detailed embodiment.
Phyto-Percolate Production
According to the invention, a phyto-percolate is derived from a culture
comprised
of freshwater algae, moss, bacteria, actinomycetes, and fungi. It is believed
that the culture
is comprised of at least one or more of the; following genera:
Acinetobacter Liefsonia Staphylococcus
Aerococcus Micrococcus Stenotrophomonas
Aquaspirillium Oedocladium Stichococcus
Bacillus Phyllobacterium Streptomyces
Brevibacterium Pseudomonas Ulothrix
Caseobacter Ralstonia Variovorax
Chlorella Rhizobium Weeksella
Clavibacter Rhodococcus Xanthomonas
Corynebacterium Riemerella
Dermacoccus Shingomonas
Particular note is made of the genera Aquaspirillum, Bacillus, Pseudomonas,
Ralstonia, Stenotrophomonas, Stichococcus, and Ulothrix. Without being bound
by any
theory, it is believed that these genera are the most abundant organism in
each culture and
may be the primary procuders of the phyto-percolate derivatives. A deposit of
a culture
resulting in a phyto-percolate of the present invention has been placed in the
American
Type Culture Collection, of Manassas, VA., as Deposit #: PTA-5863.
In particular embodiments, a heterotrophic rotifer species exists in the
cultures, as
well as bacteria that have been identified as Stenotrophomonas rnaltophilia,
Ralstonia
pickettii, Ralstonia paucula, Acinetobacter genospecies 11, Acinetobacter
junii, Leilsonia
aquatica, Riemerella anatipestifer, Variovorax paradoxus, and Streptomyces
griseorubens
. Without being bound to any particular theory, it is believed that these
bacteria may
produce proteolytic enzymes that are contributors to the effectiveness of the
phyto-
percolate.
14
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
A method of producing phyto-percolate is depicted in FIGURE 1. Phyto-percolate
cultures of approximately 100-200 ml of dense algal cells in approximately 2.5
gal, or
approximately 10 liters, of reverse-osmosis purified sterile water are fed
about 1 milliliter
(ml) per week of liquid extract of live active yeast, or Baker's yeast,
Saccharomyces
cerevisiae, which has been prepared from 1.0g dry active yeast added to 50m1
warm water,
at between about 37 and about 43 C. The mixture is allowed to incubate for 10-
30
minutes, or until it slightly foams. The cultures are fed in either 1.0 ml
weekly doses, or
0.5m1 twice-weekly doses. It is contemplated within the scope of the invention
that other
yeast cultures may be used. It is further contemplated that other organic
nutrients or
substrates known in the art may be used such as glucose or proteose, or other
algal growth
media prepared from inorganic nutrients, supplements, and/or vitamins.
In one embodiment, the cultures are grown under full-spectrum grow lights at
about
25 C, and produce a final unadjusted pH of between about 6.2 to about 7 that
fluctuates.
The cultures are grown in clear glass fishbowl containers having a volume of
approximately 2.5 gal with semi-transparent plastic lids, with the exception
of about a
3mm hole in the lid for gas exchange. It is contemplated within the scope of
the invention
that other culture containers, ingredients, conditions and methods known in
the art may be
used that allow the cells to grow in a manner in which the phyto-percolate
derivatives are
expressed. Such methods may include larger batch, semi-continuous, continuous
or other
type culture systems including bireactors or photoreactors, may or may not
include
aeration or agitation, may or may not include solid, liquid, semi-solid or
other form of
growth media or substrate, may or may not include the above particular
conditions of
temperature, contact time or area, or light intensity.
In this particular embodiment the cultures are harvested weekly or bi-weekly,
between the 5rd and 10th day after feeding, by drawing off the top 1.25 gal of
phyto-
percolate from each 2.5 gal culture. This is referred to as the "raw phyto-
percolate." The
algal or other cells and yeast food forming the phyto-percolate culture remain
in the bottom
of the culture container substantially undisturbed while the phyto-percolate
is decanted.
The decanted material is then processed as desired. The volume of the
container is then
optionally returned to original volume. Conveniently this is accomplished with
reverse-
osmosis purified water at approximately room temperature, about 25 C. It is
contemplated
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
within the scope of the invention that other culture and harvest systems,
timetables
volumes and methods may be used that result in phyto-percolate derivatives.
Without being bound by any particular theory, it is believed the patterns of
harvest
and feeding affect enzyme production. It is believed that more frequent
smaller feedings
such as 0.5m1 twice-weekly may stimulate greater enzyme production than single
large
amount feedings such as 2m1 bi-weekly, while discouraging contamination with
undesirable bacteria and rotifer colonization. Since enzyme systems are highly
dynamic
and are directly affected by the immediate surroundings, the suggestion is
supported that a
food blend such as a liquid extract of active Baker's yeast increases the
active proteolytic
enzymes in the phyto-percolate culture compared with other foods or nutrient
blends.
The peaks of enzyme concentration in the percolate over the course of several
weeks are mapped under various feeding regimens, and serve to dictate the
optimal date for
harvests. According to the invention, the enzyme concentration is analyzed in
the cultures
and processed phyto-percolate to detect any negative effects of regular
harvesting on the
algal cultures over time, and is combined with data on the effects of
environmental and
stress factors such as dark/light, starvation, and/or changes in temperature
or pH, which
may stimulate or discourage enzyme production. Methods for analyzing these
parameters
include the isolation and homogenization of select cultures to eliminate all
variables
besides those being tested, and include monitoring of chlorophyll, total
protein and enzyme
activity, utilizing spectro-photometric methods, to measure the health and
enzyme activity
of the cultures over the course of an isolated-variable experiment.
In this particular embodiment the method for analyzing proteolytic activity is
a
typical chromogenic assay using Chromogenix substrate from DiaPharma, S-2251:
chromogenic substrate for plasmin and streptokinase-activated plasminogen.
Chromogenic
substrates are peptides that react with proteolytic enzymes and proportionally
change color
as the substrate is lysed by the enzymes. The color change may be measured
spectro-
photometrically over time and is proportional to the proteolytic activity. The
synthetic
chromogenic assay substrates are designed to have enzyme binding selectivity
similar to
that of the enzyme's natural substrate. It is believed that the enzymes
present in phyto-
percolate are selective for substrates including fractionated proteins and
fibrin. It is
contemplated within the scope of the invention that other methods for
analyzing proteolytic
activity and phyto-percolate derivatives may be used.
16
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
Enzyme activity for samples of described phyto-percolate currently ranges from
15-
50 mU/mL of plasmin-like activity, when phyto-percolate is prepared as
described. These
values have been found to correlate with clinical observations of reduced
pathological
fibrin in humans orally consuming phyto-percolate. Methods for evaluating in
vivo effects
of phyto-percolate include peripheral blood observations on wet and dry blood
smears,
diagnostic and/or analytical blood tests, and various clinical observations
and
measurements such as body weight. Reductions in excess pathological fibrin and
platelet
aggregation have been observed, which are secondary to inflammation and tissue
destruction. Changes in white blood cell mobility and, number have also been
observed.
Anti-inflammatory effects of phyto-percolate in vivo have also been monitored
with
independent blood laboratory studies focusing on chronic inflammatory activity
and hyper-
coagulant states.
In an alternative embodiment, the phyto-percolate may be produced using a
continuous culture format in which the phyto-percolate is substantially
continuously
removed from the culture and the lost volume is replaced with fresh culture
media and/or
nutrients. Further, the phyto-percolate may be produced using a bioreactor
that is suitable
for production on a larger scale than the batch culture method described
above.
Phyto-Percolate Filtration
= = After harvest of the phyto-percolate from the cultures, the decanted fluid
is filtered
through a series of depth prefilters and sterile membrane filters made of low-
protein
binding materials. Examples of suitable final sterilizing filters are provided
by Millipore
Corp. Durapore brand filters, made of PVDF material. These have been shown to
protect
the enzyme concentration, and provide a final sterile filtration level of
about 0.22 microns,
as well as being chemically inert to ozonated water. Ozonated water is used
for sterilizing
the filter system, as it does not leave a damaging residue like chlorine.
All filters are 10" cartridge membrane or depth filters of various chemically-
inert
materials. The prefilters are housed in cartridge filter housings made of
styrene-
acrylonitrile (SAN). The final filters are housed in polypropylene (PP)
housings with
Kynar fittings. The material is harvested and filtered using Tygon tubing,
peristaltic pumps
and 55 gallon containers or other containers that have been pre-sterilized
with zonated
water.
17
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
The phyto-percolate passes through a filtration regimen comprised of two pre-
filters in SAN housings of pore size 11.1m (nominal), made of pleated
cellulose/polyester.
Examples of these filters are manufactured by Cole-Parmer, Vernon Hills,
Illinois, USA,
catalog number EW-29830-20. It is contemplated within the scope of the
invention that
other filters know in the art may be used in this step as pre-filters, that
are chemically inert.
The phyto-percolate is again filtered using a second stage pre-filter made of
polypropylene in a polypropylene housing, with a nominal pore size of about
0.5um. In
one illustrative embodiment, this finishing filter is manufactured by
Millipore Corporation,
Bedford, MA., Durapore brand, Catalog # D00501S01. It is contemplated within
the
scope of the invention that other filters known in the art may be used in this
step as second
pre-filters, that are chemically inert.
The phyto-percolate is then passed through a pre-sterilized final filter that
sterile-
filters the phyto-percolate and removes all traces of bacteria, yeast, mold,
algae and other
particle contaminants_ According to the invention, a final filter set consists
of sterile
membrane filters in PP housing having progressively smaller pore sizes of
0.45um and
0.22 p.m (absolute). These finishing filters' membranes are made of
hydrophilic extremely-
low protein-binding PVDF. In one illustrative embodiment, these finishing
filters are
manufactured by Millipore Corporation, Durapore brand, Catalog tt's CVHIO1TPE
and
CVDIO1TPE. It is contemplated within the scope of the invention that other
filters know in
the art may be used that are inert to the phyto-percolate derivatives and
processing and
sanitizing materials including zonated water. It is also contemplated within
the scope of
the invention that other methods of processing may be used.
Filtration by size exclusion removes approximately >99.9% of contaminants such
as bacteria, yeast and mold spores, and algal cells. It is also believed to
preserve
enzymatic activity if filter materials are made of low-protein-binding,
chemically-inert
materials. The resulting liquid, the phyto-percolate, is substantially
comprised of water,
active enzymes, proteins and sugars. The phyto-percolate, after passing
through the
= finishing filter is then usefully stored in sealed sterile 55 gal HDPE
drums at between 21
= and 27 C until bottling. Samples are taken from each batch immediately
after filtering to
test for enzyme efficacy and contamination and for standardization. It is
contemplated
within the scope of the invention that other methods of sampling and testing
may be used.
The acceptable values for fibrinolytic enzyme efficacy to be administered p.o.
are observed
18
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
in the phyto-percolate as between 0 and 50 milli-units of plasmin-like
activity; however
higher levels may provide greater therapeutic benefit. It is believed that
this filtered phyto-
percolate contains approximately 50 ppm of the 67.5 kDa protein (see below).
The phyto-percolate is processed and bottled under sanitary conditions known
in
the art using ozone sterilization. It is believed that this step avoids enzyme
degradation
associated with the use of chlorine or heat sterilization because ozone leaves
no residue if
left to dissipate, or if followed by a rinse of sterile water. It is
contemplated within the
scope of the invention that other methods of filtration and sanitization known
in the art
may be used that are not unreasonably degrading of the enzymatic or other
activity. The
phyto-percolate is usefully packaged in opaque UV-protectant bottles and
shipped with
cold packs to reduce product degradation. It is contemplated within the scope
of the
invention that other methods of packing, bottling, storing, and transporting
may be used.
Phvto-Percolate Characterization
It is believed that the raw phyto-percolate, prior to filtration, is a complex
mixture
of macromolecules. It was expected that the filtration process described above
reduced the
molecular complexity of the phyto-percolate filtrate. We performed several
physico-
chemical tests to determine the composition of the filtrate. In each case, the
phyto-
percolate filtrate was lyophilized, redissolved in ddH20, and refiltered to
remove any
undissolved particulate matter.
A sample of the lyophilized phyto-percolate was subjected to isocratic reverse
phase HPLC, on a size-exclusion chromatography column (TSK-GEL Super SW
Series;
Tosoh Biosciences, Montgomeryville, PA), under non-denaturing conditions.
Proteins
were identified using a micro flow cell UV detector at 280 nm. As shown in
FIGURE 2, a
major protein species of 67.5 kDa was identified (retention time 18.747
minutes). The
67.5 kDa peak contributed about 90% of the total signal measured at 280 nm.
Also
=
detected were peaks at retention times of 21.544 minutes (21.0 kDa) and 23.957
minutes.
Analysis under denaturing and other conditions indicates that the 21.0 kDa
species is a
protein molecule and the 23.957 minute peak is primarily polysaccharide. The
major
components of the phyto-percolate (the 67.5 kDa protein, 21.0 kDa protein, and
the
polysaccharide identified at 23.957 minutes) are referred to herein as phyto-
percolate
19
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
derivatives and may contribute to the biological and therapeutic efficacy of
the phyto-
percolate.
Another sample of the lyophilized phyto-percolate was subjected to Fourier
Transform Infrared (FTIR) spectroscopy. The results are provided in FIGURE 3.
FIGURE 3 shows a spectrum that is characteristic of a dissolved protein
sample.
A third sample of the lyophilized phyto-percolate was used for [111]-NMR. The
NMR spectrum is provided in FIGURE 4. Here again, the results are consistent
with a
single protein species.
Weight Management Using Phyto-Percolate
Excessive weight has emerged as a prominent and growing health problem.
Greater than 61% of Americans over the age of 20 are overweight, 25% of whom
are
obese. Second only to tobacco use as the top underlying preventable cause of
death,
excessive weight is a major risk factor for developing diabetes, heart
disease, hypertension,
gallbladder disease, arthritis, lung diseases, and certain types of cancer.
Example 1: Rodent Model of Weight Loss
A 21 day weight loss study using twelve mature (12 month old) Sprague-Dawley
rats was performed. Each animal was orally administered 10 ml/kg of undiluted
and
unfiltered phyto-percolate (i.e., raw phyto-percolate) for 14 days, followed
by non-dosing
for 7 days. Each animal was weighted daily and observed for signs of toxicity.
As shown
in more detail in Table 1, the rats lost an average of 33 grams (6.3%) of body
weight over
the initial 14 day dosing period. They immediately began to regain lost body
weight upon
cessation of phyto-percolate administration. By the 21 day time point (7 days
of non-
dosing), the rats had lost an average of 25 grams (4.7%) of initial body
weight (i.e., gained
an average of 8 grams since phyto-percolate cessation).
The test animals were observed for adverse reactions immediately after each
dose
and at 4 and 24 hours subsequent. Daily observation for adverse reactions was
continued
during the 7 day non-dosing period. Specifically, clinical observations for
adverse
reactions were made for respiration, motor activity, convulsions, reflexes,
ocular signs,
salivation, piloerection, analgesia, muscle tone, gastrointestinal effects,
and skin/dermal
alterations. Gastrointestinal effects were the only observed adverse reaction.
Soft to loose
stool was observed in all test animals. No other adverse reaction was
observed.
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
Table 1: Individual Weight Loss Data
Test Pre-dosing 14 Day Weight Loss 21 Day
Weight Loss
Subject Weight (g) Weight (g) (% Initial Body Weight (g) (% Initial Body
Weight) Weight)
1 484 443 41 (8.5%) 453
31(6.4%)
2 482 461 21 (4.4%) 479 3 (0.6%)
3 549 521 28(5.1%) 531
18(3.3%)
4 536 499 37 (6.9%) 507 29
(5.4%) '
510 462 48 (9.4%) 468 42 (8.2%)
6 488 459 29 (5.9%) 465 23
(4.7%)
' 7 535 506 29 (5.4%) 514
21(3.9%)
8 586 558 28(4.8%) 562
24(4.1%)
9 569. 504 65(11.4%) 518
51 (9.0%)
522 492 30 (5.7%) 498 24(4.6%)
11 556 532 24(4.3%) 537
19(3.4%)
12 524 503 21(4.0%) 507 17
(3.2%) .. .
AVG 528.4 495.0 33.4 (6.3%) 503.3 25.1
(4.7%)
Example 2: Human Weight Loss and Glucose Control Study
A single-center, prospective, randomized, triple-masked, placebo-controlled
5 parallel-group-design pilot clinical trial of the phyto-percolate was
performed using two
different batches of the phyto-percolate. This trial was conducted in
accordance with FDA
regulations and under a protocol approved by an Institutional Review Board
(IRB).
Subjects: Primary inclusion criteria were men and women having a body mass
index (BMI) of 25-40 mfkg2, 18-70 years old (inclusive), and desirous of
losing weight.
10 Major exclusion criteria were moderate to severe co-morbid disease (e.g.,
cancer); history
of stroke, transient ischemic attack (TIA), or similar conditions;
uncontrolled hypertension,
insulin-dependent diabetes, renal disease, moderately severe cardiac disease,
lupus, alcohol
21
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
abuse, and current or recent use of certain medications including medications
and/or
supplements for weight loss, glucose management, or arthritis. Women were
excluded if
they were pregnant, nursing, or actively trying to become pregnant.
Protocol: Patients were assigned to self-administer one ounce of filtered
phyto-
percolate or placebo three times each day (t.i.d.) on an empty stomach at
least 30 minutes
before a meal. Subjects were asked to Participate in a reduced carbohydrate
diet and light
exercise program and complete a one-day-per-week Food Log and a daily Exercise
Log for
the duration of the clinical trial. Patients were evaluated during a baseline
examination and
then again at 2-week, 4-week, and 6-week visits. Evaluations included
measurement of
body weight, arm and waist circumference, and body fat measurements.
Glucose Control Study: At the baseline examination and at the 4-week and 6-
week
visits, patients' fasting (12 hour) blood glucose was measured and then their
blood glucose
was measured one hour after a glucose challenge (25 grams of jelly beans;
90.4%
carbohydrate). The difference between the glucose challenge reading and the
baseline
reading in a single visit is an indicator of the patient's ability to regulate
serum glucose
levels.
Test Materials: The patients in the treatment groups were assigned one of two
different lots (Batch 1 and Batch 2) of phyto-percolate prepared as described
above. The
placebo product was similar in appearance (color, viscosity, and odor) to the
diluted phyto-
percolate. All test materials were dispensed in unlabeled blue bottles with
instructions to
refrigerate after opening.
Enrollment: A total of 44 subjects were enrolled and randomized for this
trial. Ten
subjects completed the study on Batch 1 (Cohort 1) of the phyto-percolate and
twelve
subjects completed Batch 2 (Cohort 2). Seven subjects completed the placebo
phase of the
trial.
Results: There were no significant adverse events reported. Patients in the
treatment arms of the study reported greater energy and reduced hunger
compared to the
Placebo group. The remaining results are as follows:
After 2, 4, and 6 weeks of treatment with the diluted filtered phyto-
percolate, the
average percent total weight loss (above placebo) for all treated patients
(Cohorts 1 and 2;
n=22) 77.7%, 48.5%, and 68.1%, respectively. After six weeks of phyto-
percolate
22
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
treatment, Cohort 1 lost an average of 106% (9.03 lbs) and Cohort 2 lost an
average of
37% (6.01 lbs) more than the weight loss measured in the Placebo group (4.39
lbs).
Table 2: Average Weight Loss
2-Week 4-Week 6-Week
Placebo
2.60 3.71 4.39
(n=7)
Cohort 1
5.71 6.81 9.03*
(n=10)
Cohort 2
3.71 4.43 6.01
(n=12)
= p <0.10 (unpaired Student's t-test)
Table 3: Frequency Distribution of Weight Loss in Individual
Patients at 6 Weeks
Weight Loss Placebo Cohort 1
(number of patients) (number of patients)
> +1 lb. 1
+1 lb. > patient > -1 lb. 1
-1 lb. > patient > -3 lb. 2
-3 lb. > patient > -5 lb. 2
-5 lb. > patient > -7 lb. 3 1
-7 lb. > patient > -9 lb. 3
-9 lb. > patient > -11 lb. 2
-11 lb. > patient > -13 lb.
-13 lb. > patient > -15 lb.
-15 lb. > patient > -17 lb. 1
=
-17 lb. > patient > -19 lb. .
23
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
< -19 lb. = -- .1*
* maximum weight loss was 28 lbs.
=
Table 4: Arm and Waist Circumference -
Difference Between Baseline and 6 Weeks
Placebo Cohort 1 Cohort 2
Arm 0.083" 0.41" * 0.13"
Waist 1.09" 2.08" ** 1.34"
* p < 0.042
** p < 0.21
Table 5: Body Composition ¨ Percent Body Fat:
Difference Between Baseline and 6 Weeks
Placebo Cohort 1 Cohort 2
Body Fat @ Baseline 39.1% 39.2% 39.0% *
Improvement in Body Fat (lbs) 2.11 6.03* 2.89
Improvement in Lean Mass (lbs) 0.16 0.79** 0.24
* p < 0.01
**p<0.15
=
Table 6: Frequency Distribution of Body Fat Loss in Individual
Patients at 6 Weeks
Weight Loss Placebo Cohort 1
(number of patients) (number of patients)
> +1 lb. 2
+1 lb. > patient > -1 lb. 2 1
-1 lb. > patient > -3 lb. 2
-3 lb. > patient > -5 lb. 2 2
=
24
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
-5 lb. > patient > -7 lb. 1 2
-7 lb. > patient > -9 lb.
-9 lb. > patient > -11 lb. 1
-11 lb. > patient > -13 lb.
-13 lb. > patient > -15 lb.
-15 lb. > patient > -17 lb. 1
-17 lb. > patient > -19 lb.
< -19 lb. 1
* maximum weight loss was 28 lbs.
=
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
Table 7: Serum Glucose Levels In Individual Patients In Cohort 1 (mg/di)
Baseline 4-Week 6-Week
Patient Fast Chal. Diff. Fast Chal. Diff. Fast Chal. Diff.
1 158 264 106 155 246 91 152 238 86
2 72 128 56 89 107 18 80 94 14
3 75 135 60 87. 130 '43 91 117 26
4 73 128 55 78 74 -4 76 80 4
. 5 105 151 46 104 127 23 103 125 22
6 139 210 71 129 198 69 126 181 55
7 145 ' 204 59 124 200 76 ' 132 195 63
8 85 122 37 74 159 85 83 133 50
9 91 143 52 91 125 34 92 121 29
78 119 41 92 99 7 88 98 10
Mean 58.3 44.2 35.9
n>126* 3 2 2
* values > 126 mg/di are indicative of diabetes.
Table 8: Group Mean Data For Glucose Tolerance Test (mg/di)
Baseline 4-Week 6-Week
Placebo 61.7 58.3 54.0
Improvement 3.4 (5.5%) 7.7
(12.3%)
Cohort 1 58.3 44.2 35.9
Improvement 14.1 (24.2%)
22.4 (39.6%)* '
Cohort 2 60.6 56.2 55.4
Improvement 4.2 (6.9%) 5.2 (8.6%) -
* p < 0.08
5
Conclusions: The weight loss, improvement in body fat, improvement in glucose
control, as well as energy and hunger categories over the course of this six-
week study for
26
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
those on the phyto-percolate was strong, particularly when compared to the
placebo group.
Cohort 1 lost about twice as much weight (1.5 lbs/week) as the placebo group
(0.78
lbs/week). Seven of the ten subjects in Cohort 1 lost seven pounds or more,
while none of
the seven in the placebo group lost that much weight. Correspondingly, a
significant
reduction in waist size was measured in Cohort 1.
Significant improvements also were measured in the glucose tolerance test.
Test
subjects demonstrated an average of 2.6x (156%) and 1.7x (69%) improved
glucose
control at 4 weeks and 6 weeks, respectively, when compared to the placebo
group.
Furthermore, 6 of the 22 test subjects met the clinically important criterion
of > 50%
control over baseline. Three of these six demonstrated complete control of the
glucose
challenge, defined as > 85% glucose control over baseline.
In Vitro Anti-inflammatory Effects: COX-2 Inhibtion
Cyclooxygenase-2 (COX-2) is a key regulator of the inflammatory cascade. COX-
2 inhibitors are believed to reduce inflammation by blocking prostaglandin
production. In
view of the adverse effects associated with mixed COX inhibitors (aspirin,
ibuprofen, and
naproxen) and the presently available COX-2-specific inhibitors (valdecoxib,
celecoxib,
rofecoxib), there is a need for improved anti-inflammatory therapies with
fewer side
effects.
Three separate preparations of the phyto-percolate were screened, using an in
vitro
assay, for COX-2 inhibition. Riendeau et al., Can. J. Physiol. Pharmacol. 75:
1088-1095,
1997; Warner et al., Proc. Natl. Acad. ScL USA 96: 7563-7568, 1999. Briefly,
this assay
measured to conversion of 0.3 1.1M arachidonic acid to PGE2 by human
recombinant insect
Sf21 cells expression human COX-2. The incubation buffer contained 100 mM Tris-
HCl
(pH 7.7), I mM glutathione, 1 jiM hematin, and 500 jiM phenol. PGE2 was
quantified
using an enzyme-linked immunoassay (ETA).
Sample 1 was a sample of diluted phyto-percolate concentrated approximately
100-
fold by drying under N2. Sample 2 was prepared by drying a 4800 ttl sample of
diluted
phyto-percolate under N2 and reconstituting it in 96 I of ddH20 just prior to
assay.
Sample 3 was prepared by lyophilizing a 4800 1 sample of diluted phyto-
percolate and
reconstituting it in 96 111 of ddH20 just prior to assay. The concentrations
of phyto-
percolate used, 100X, 10X, and IX, refer to 10 jil, 1 jil, and 0.1 I of
sample, respectively,
27
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
in a final assay volume of 100 111. Rofecoxib was used as a positive control
for COX-2
inhibition. Each sample was assayed in at least three concentrations and the
assays were
performed in duplicate.
Table 9: COX-2 Inhibition By Phyto-percolate
Sample Concentration % COX-2 Inhibition ICso
(individual assay values)
1 100X 29 (27, 30.9) > 100X
10X 11(9.2,13.4)
1X -4 (-9.0, 0.3)
2 100X 61(66.7, 56.1) 46.5X
10X 27 (23.7, 30.5)
1X 20 (13.3, 27.6)
3 100X 58 (63.9, 52.3) 61.9X
10X 24 (21.7, 26.0)
1X 18 (13.3, 23.1)
rofecoxib 1 p.1µ,1 88 (90.1, 85.6) 0.198
j.J.A4
0.3 p.M 55 (58.8, 51.8)
0.1 p.M 33 (34.7, 31.5)
0.03 i_tM 16 (22, 10.5)
0.01 12M 11(8.2, 14)
In Vivo Anti-inflammatory Effects: Carageenan-Induced Paw Edema
The carrageenan-induced paw edema assay was used as an in vivo indicator of
the
anti-inflammatory effects of the phyto-percolate. Carrageenan induces local
inflammation
and edema when injected into the paw pad of a rat (Di Rosa et al., 1971). The
development of paw edema is believed to be biphasic (Vinegar et al., 1969).
The initial
phase is attributable to the local release of histamine and serotonin
(Crunkhon et al., 1971)
and the second phase is caused by prostaglandin release as a result of COX
activation. The
second phase is measured as an increase in paw volume and has been
demonstrated to be
responsive to steroidal and non-steroidal anti-inflammatory agents.
28
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
Groups of test subjects (n=6) received oral doses of either vehicle control
(water; 5
ml/kg), indomethacin (30 mg/kg), aspirin (100 mg/kg), unfiltered phyto-
percolate (10
ml/kg), or filtered phyto-percolate (10 ml/kg) 30 minutes prior to
intraplantar
administration of carrageenan (0.1 ml of a 1% solution). Paw volume was
measured at 0,
2, 4, 6, 8, and 20 hours after treatment using a plesthysmometer to measure
volume
displacement. Each treatment group is compared to control.
As shown in Table 10, the paw volume of the control animals and all treatment
groups nearly doubled in two hours and remained so. through the four hour time
point. By
six hours, paw volume was reduced by 30% and 50% in the groups administered
the
filtered and unfiltered phyto-percolate, respectively. This reduction in edema
was
significantly better than that observed for either the indomethacin or the
aspirin groups at
this time. Further, the reduction in edema measured for the two phyto-
percolate groups
was comparable to both the indomethacin and aspirin groups at the 8 hour and
20 hour
time points.
Table 10: In Vivo Anti-inflammatory Effects of Phyto-percolate
Mean paw volume (m1) + SD (% change from control)
Group 0 hours 2 hours 4 hours 6 hours 8 hours
20 hours
Control
1.24 + 0.17 2.18 + 0.24 2.17 + 0.27 2.12 + 0.15 2.05 + 0.08 1.85 + 0.08
Indomethacin 1.25 + 0.05 2.25 + 0.23 2.18 + 0.22 2.00 + 0.22 1.83 + 0.23 1.37
+ 0.10
(1%) (7%) (1%) (-12%) (-22%)
(-38%)
Aspirin
1.25 + 0.08 2.22 + 0.28 2.07 + 0.23 1.92 + 0.18 1.80 + 0.18 1.42 + 0.16
(1%) (4%) (-10%) (-20%) (-25%)
(-23%)
'Filtered
1.22 + 0.04 2.15 + 0.10 2.15 + 0.10 1.78 0.10 1.78 + 0.10 1.35 + 0.08
(-2%) (-2%) (-34%) (-27%)
(-30%)
Unfiltered 1.20 + 0.13 2.15 + 0.12 2.13 + 0.10 1.67 + 0.10 1.67 + 0.10 1.28
0.12
(-4%) (-3%) (-4%) (-45%) (-38%) (-37%) =
= Immunological Effects: Rodent Model of HIV Infection
29
CA 02631773 2008-06-02
WO 2007/065024 PCT/US2006/046320
The effect of treatment using the phyto-percolate was investigated using a rat
model of HIV infection. The HIV model used inoculates rats with seven (7) of
the nine (9)
HIV genes, making it a non-contagious model that develops full symptoms of HIV
by 9
months after inoculation, with a life expectancy of 12 months.
Some of the most devastating symptoms of HIV manifest themselves in the liver
and the immune system. Liver problems are frequent causes of illness and death
in people
with HIV infection. Throughout the study, liver function tests including AST,
ALT,
GGTP, bilirubin, and albumin were monitored in the treatment and control
groups. C-
reactive protein was assayed as an inflammatory marker. The immune response
was
monitored using IgG, IgA, and IgM levels which are known to decline during the
progression of AIDS.
For testing, serum was drawn by cardiac puncture for baseline (pre-
inoculation)
values. The treatment group received diluted phyto-percolate for their
drinking water,
which was allowed ad libitum, while the control group received filtered water.
Serum was
drawn by cardiac puncture, as above, every thirty (30) days until the
termination of the
study.
After 60 days of treatment with the diluted phyto-percolate, the treatment
group
had an average 30% increase in IgA levels, 50% increase in IgG levels, and a
40%
reduction in C-reactive protein (C-RP) levels, relative to the untreated group
(Table 11).
No significant differences in body weight, average daily food consumption, or
average
daily liquid consumption were detected between the groups.
Table 11: Serum Analysis From Rat HIV Study
Animal AST ALT Bilirubin C-RP IgG IgM IgA
Group (U/L) (U/L) (mg/dL) (mg/ml) (mg/dL) (mg/dL) (mg/dL)
Control
Base 117 70 0.07 3.41 57 27 18
1 Mo. 95 60 0.12 0.65 69 26 24
2 Mo. 122 67 0.12 0.93 120 26 24
HIV
CA 02631773 2008-06-02
WO 2007/065024
PCT/US2006/046320
Base 116 77 0.07 3.37 60 26 21
/Mo. 166 76 0.21 0.58 108 27 25
2 Mo. 139 81 0.13 0.56 167 23 38
Administration of Phyto-Percolate
The phyto-percolate dosage will vary with the severity of the disease, the
biochemical activity of the disease, and the age and weight of the subject.
The effects of
using the phyto-percolate will be measured using standard parameters known in
the art for
any such disease state.
In one embodiment, the phyto-percolate is orally administered as a liquid. As
described in several of the foregoing examples, the phyto-percolate is diluted
in filtered
water to about 50 ppm of the protein species of the 67.5 kDa peak measured by
HPLC and
UV detection (described above). However, depending upon the severity of
disease or
desired clinical outcome, the concentration of phyto-percolate (and hence the
dosage for
the protein species) may be altered. For example, the protein species may be
present in the
orally administered liquid in concentrations including about 100 ppm, 250 ppm,
500 ppm,
750 ppm, 1000 ppm, 1500 ppm, or more. It is also contemplated that the protein
fraction is
isolated from the phyto-percolate and formulated for parentera administration
(e.g.,
intravenous, intramuscular, and subcutaneous injection, topical, rectal or
vaginal
administration or other).
In an adult subject, the dosage of diluted phyto-percolate will vary from
about one
ounce per day, generally on an empty stomach, such as for maintenance and the
retardation
of aging, to about an ounce every hour, up to about 12 ounces per day, in a
hospitalized
burn or accident case, or during the chemotherapy infusion. The controlled
diabetic or
cardiovascular subject is generally treated at about two to three ounces of
phyto-percolate
per day. Dosing on an empty stomach is noted because of the potential for
interference on
phyto-percolate function from food-stimulated gastrointestinal activities. A
50-70 lb. child
is dosed at about three to four ounces per day, generally dosing on an empty
stomach,
during an acute infection. The greater the free radical oxidative tissue
destructive activity
caused by age or disease state, the greater the recommended dosage of the
phyto-percolate.
Without being bound to any particular theory, it is thought that the intake of
phyto-
31
CA 02631773 2016-09-28
percolate per day is more directly related to the severity of oxidative tissue
destruction than to
the weight of the subject.
It will be understood that various modifications may be made to the
embodiments disclosed
herein. Therefore, the above description should not be construed as limiting,
but merely as
exemplification of the various embodiments. Likewise it should be understood
that the
phyto-percolate can be used to enhance the well being and performance of
animals.
The foregoing has been a description of an illustrative embodiment of the
present
invention. While several illustrative details have been set forth, such are
only for the
purpose of explaining the present invention.
32