Note: Descriptions are shown in the official language in which they were submitted.
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
1
MENUFACTURE METHOD OF SINGLE PHASE ARAGONITE PRECIPITATED
CALCIUM CARBONATE BY CONTROLLING CALCIUM ION
[Technical Field]
The present invention is related to the methods of manufacture
of single-phase aragonite by controlling calcium ions,
particularly, to the methods of manufacture of single-phase
aragonite in solution process by controlling the rate of
elution of calcium ions in the suspension of calcium hydroxide
by adding the highly concentrated aqueous solution of sodium
hydroxide to the suspension of calcium hydroxide and adding
the aqueous solution of sodium carbonate to the above mixed
aqueous'solution at a constant rate.
[Background Art]
There are three representative polymorphs of calcium
carbonate: calcite which is stable at a room temperature is
cubic or spindle shaped, meta-stable aragonite is navicular or
needle shaped, and unstable vaterite is spherical mostly.
Among them, aragonite is of needle shape having a very large
aspect ratio, and is expected to be a new functional inorganic
material that can grant mechanical and optical functionalities
in that it becomes possible to enhance strength, to improve
whiteness, and to control opaqueness owing to the complicated
surface structure of its needle shape when it is used as an
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
2
industrial raw material such as the filler for rubber, plastic,
paints, or the pigment for paper, etc. Particularly, the 0.05
/cm down-shaped needle shape can increase oil absorption owing
to the increase in the specific surface area, and the needle
shape havirig the largest diameter of 50 - 60 M has a superior
impact resistance. Therefore, it is expected to have an effect
of increasing strength if aragonite is mixed with a
conformation control thermoplastic resin or a polypropylene
resin as filler.
However, aragonite is in the meta-stable phase existing stably
in the temperature range of below 75K under atmosphere, and is
precipitated at a temperature higher than 60 - 80 C due to a
faster rate of crystallization in the range of high
temperature compared to that of calcite. And it may be
synthesized at a low-temperature owing to the affects of pH of
the reaction solution, ions to be added, concentration of
calcium hydroxide, etc., but it is difficult to synthesize
single-phase aragonite.
Disclosed in Korean Laid-Open Patent No. 2005-0110118 is a
method of manufacture of aragonite by adding the aqueous
solution of sodium hydroxide to control the concentration of
calcium ions in the suspension of calcium hydroxide and
supplying the aqueous solution of sodium carbonate at a
constant rate. But it is practically difficult to manufacture
single-phase aragonite having a large aspect ratio since the
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
3
aqueous solution of sodium hydroxide having the concentration
lower than that of the suspension of calcium hydroxide is used
in the above method of manufacture.
[Disclosure]
[Technical problem]
Accordingly, it is an object of the present invention to
provide methods of manufacture of single-phase aragonite by
adding the highly concentrated aqueous solution of sodium
hydroxide in order to control the concentration of calcium
ions in the suspension of calcium hydroxide.
The present invention is devised in order to solve the above-
described problems, and it is an object of the present
invention to provide methods of manufacture of single-phase
aragonite by controlling calcium ions. The present invention
is characterized by adding the suspension of sodium hydroxide
to the suspension of calcium hydroxide to control calcium ions,
and it is an object of the present invention to provide more
highly functional and high-value-added aragonite by improving
the single phase, aspect ratio, and production yield of
aragonite by using solution process by the homogeneous
precipitation reaction of sodium carbonate with the above
suspension.
[Technical solution]
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
4
As a result of repeating studies in order to manufacture
single-phase aragonite, the inventors of the present invention
found out that the concentration of calcium ions in the early
reaction stage should be lower than the solubility in water in
order to manufacture single-phase aragonite. They also learned
that it was very effective to increase the concentration of
the aqueous solution of sodium hydroxide in order to lower the
concentration of calcium ions in the early reaction stage, and
completed the present invention from the above findings.
Accordingly, the present invention is related to the methods
of manufacture of single-phase aragonite by controlling
calcium ions, particularly, to the methods of manufacture of
single-phase aragonite in the aqueous solution process by
controlling the rate of elution of calcium ions in the
suspension of calcium hydroxide by adding the highly
concentrated aqueous solution of sodium hydroxide to the
suspension of calcium hydroxide and adding the aqueous
solution of sodium carbonate to the above mixed aqueous
solution at a constant rate.
In order to manufacture single-phase aragonite with a high
yield, which is an object of the present invention, it is
preferable to use the aqueous solution of sodium hydroxide
having a higher concentration than that of calcium hydroxide
contained in the suspension, more preferably, to add 3 to 10
mol/L of the aqueous solution of sodium hydroxide with respect
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
to 0.2 to 3 mol/L of the suspension of calcium hydroxide. And
it is possible to manufacture single-phase aragonite having a
large aspect ratio by inserting the aqueous solution of sodium
carbonate to the mixed solution of the suspension of calcium
5 hydroxide and the aqueous solution of sodium hydroxide at a
low rate.
Therefore, the present invention provides methods of
manufacture of single-phase aragonite through the control of
calcium ions comprising the steps of:
a) manufacturing 0.2 to 3 mol/L suspension of calcium
hydroxide in which calcium hydroxide is dispersed in distilled
water;
b) manufacturing the mixed solution of the suspension of
calcium hydroxide and the aqueous solution of sodium hydroxide
by adding 3 to 10 mol/L aqueous solution of sodium hydroxide
to the above suspension of calcium hydroxide; and
c) manufacturing single-phase aragonite by inserting the
aqueous solution of sodium carbonate at a constant rate while
stirring the above mixed solution.
It is another object of the present invention to provide
needle-shaped single-phase aragonite having a large aspect
ratio as it is manufactured according to the above method of
manufacture.
The present invention is illustrated in more detail below:
The flow chart of manufacturing single-phase aragonite
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
6
according to the present invention is shown in Figure 1, and
equipment for manufacturing single-phase aragonite according
to the present invention is shown in Figure 2.
As shown in Figure 1, the processes of manufacturing single-
phase aragonite according to the present invention are
comprised of the steps of manufacturing the suspension of
calcium hydroxide; manufacturing the mixed solution of the
suspension of calcium hydroxide and the aqueous solution of
sodium hydroxide by mixing the aqueous solution of sodium
hydroxide with the above suspension of calcium hydroxide;
reacting the aqueous solution by inserting the aqueous
solution of sodium carbonate while heating and stirring the
above mixed solution; and washing, filtering, and drying.
Also, the equipment for the manufacturing of aragonite-type
precipitated calcium carbonate according to the present
invention is comprised of a reactor (1) in which the
suspension of calcium hydroxide and the aqueous solution of
sodium carbonate are mixed and stirred; a thermostatic water
bath (2) filled with water and equipped with the above reactor
and a temperature controlling means so that the inside of the
reactor is maintained at a constant temperature; an injection
container (7) of the aqueous solution of sodium carbonate
connected to a supplying unit inserted into the iriside of the
above reactor through a pipe extended outside of the reactor
from the supplying unit so that the amount of supply of the
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
7
aqueous solution of sodium carbonate is controlled by means of
a flowmeter (8); a stirrer (6) comprised of a rotation axis
installed rotatably penetrating the cover of the above reactor
and a motor at the outer end of the above rotation axis so
that the suspension of calcium hydroxide and carbon dioxide
are mixed; and a pH measuring device (4) to which a pH
electrode (3) and a thermometer (5) installed inside of the
above reactor are connected.
The methods of manufacture of single-phase aragonite according
to the present invention are illustrated more concretely as
follows:
As described in the above, it is necessary to control the
concentration of calcium ions in the early stage of the
reaction in order to manufacture single-phase aragonite. The
aqueous solution of NaOH is added for such control of the
concentration. This is to lower the initial concentration of
Ca2+ ions by reducing the solubility of calcium hydroxide by
the common ion effect. The ions existing in the entire
reaction system before adding NaOH include Ca2+, OH-, Na+, and
C032-, where OH- ion becomes a common ion if NaOH is added. If
a common ion exists in a solution, the initial concentration
of Ca'+ ion is reduced as the ionic product becomes greater
than the solubility product and the solubility is reduced.
Figure 3 shows changes in the solubility of calcium hydroxide
according to the concentration of the aqueous solution of
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
8
sodium hydroxide in 1.5 M suspension of calcium hydroxide. The
solubilities of calcium hydroxide according to temperature in
the state that NaOH is not added are shown in terms of dotted
lines in order to compare solubilities when NaOH is added and
not added. As shown in Figure 3, it is seen that the
solubility of calcium hydroxide is reduced as the
concentration of the aqueous solution of sodium hydroxide in
the suspension of calcium hydroxide is increased, which leads
to lowering of the concentration of calcium ions, that are the
main reaction products, in the reaction solution, and
supersaturation, which is greatly affected by the
concentration of the reaction products, is also lowered.
Therefore, in the present invention, the aqueous solution of
sodium hydroxide having a higher concentration than that of
the suspension of calcium hydroxide is used, more concretely,
3 to 10 mol/L aqueous solution of sodium hydroxide with
respect to 0.2 to 3 mol/L suspension of calcium hydroxide. If
the concentration of the aqueous solution of sodium hydroxide
is less than 3 mol/L, it is failed to manufacture single-phase
aragonite since calcite is produced; and if it exceeds 10
mol/L, it is not economical since the concentration of calcium
ions is reduced exceedingly and the reaction time becomes too
long. It is preferable that the concentration of the above
aqueous solution of sodium hydroxide is 5 to 8 mol/L, at which
pure aragonite having no calcite practically may be
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
9
manufactured. Having no calcite practically means that no
calcite peaks are shown in the x-ray diffraction pattern of
aragonite obtained through washing, filtering, and drying
aragonite produced in the above step c). If it is expressed in
terms of a numerical value, it means that the content of
calcite is less than about 0.01%.
The mixed solution of the suspension of calcium hydroxide and
the aqueous solution of sodium hydroxide is heated to 60 to
80 'Q and the aqueous solution of sodium carbonate is inserted
to the mixed solution while stirring it. It is preferable that
the concentration of the aqueous solution of sodium carbonate
is 0.1 to 1 mol/L, more preferably, 0.1 to 0.5 mol/L, and the
rate of insert is 1 to 5 ml/minute. If the concentration of
sodium carbonate is too low or the rate of insert is too low,
the reaction may not be progressed well since it takes too
long to react or the nucleus is not produced; and if the
concentration of sodium carbonate is higher than the above or
the rate of insert is higher than the above, the size of
crystals is small and it is difficult to manufacture single-
phase aragonite since the rate of producing the nucleus is
increased.
Also, it is preferable that the above reaction temperature is
60 to 80 - Q preferably, 75 to 80 C since the production of
aragonite is more favorable at a high temperature compared to
the production of calcite. That is, if the reaction
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
temperature is lower than 60 ~ it is difficult to manufacture
single-phase aragonite due to the production of calcite, and
the reaction system may become unstable due to the evaporation
of moisture during the reaction if the reaction temperature is
5 higher than 80'C
[Brief Description of the Drawings]
A more complete appreciation of this invention, and many of
the attendant advantages thereof, will be readily apparent as
10 the same becomes better understood by reference to the
following detailed description when considered in conjunction
with the accompanying drawings, wherein:
Figure 1 is a flow chart of manufacturing aragonite according
to the present invention;
Figure 2 shows equipment for manufacturing aragonite according
to the present invention;
Figure 3 shows changes in the solubility of calcium hydroxide
according to each concentration of the aqueous solution of
sodium hydroxide according to the present invention;
Figure 4 shows the x-ray diffraction pattern of aragonite
manufactured according to each reaction time in 3 M aqueous
solution of sodium hydroxide according to the present
invention;
Figure 5 shows production yields of aragonite manufactured
according to each reaction time in 3 M aqueous solution of
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
11
sodium hydroxide according to the present invention;
Figure 6 shows the x-ray diffraction pattern of aragonite
manufactured according to each reaction time in 5 M aqueous
solution of sodium hydroxide according to the present
invention;
Figure 7 shows production yields of aragonite manufactured
according to each reaction time in 5 M aqueous solution of
sodium hydroxide according to the present invention;
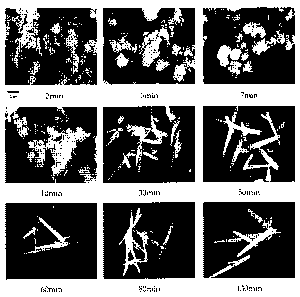
Figure 8 shows the electron micrographs of aragonite
manufactured according to each reaction time in 5 M aqueous
solution of sodium hydroxide according to the present
invention; and
Figure 9 shows the x-ray diffraction pattern of aragonite
manufactured according to each concentration of the aqueous
solution of sodium hydroxide according to the present
invention.
1: reactor 2 : thermostatic water bath
3 : pH electrode 4 : pH measuring device
5 : thermometer 6 : stirrer
7 : injection container 8 : flowmeter
( Mode for invention ]
The present invention is illustrated in terms of a few
preferred ernbodiments of the invention below:
2 5
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
12
[Preferred Embodiment]
The equipment for the manufacture of aragonite used in the
present invention is a 1-liter heat-resistant glass reactor
having a length of 300 mm and a diameter of 150 mm equipped with
a jacket and connected to a thermostatic water bath. The
thermostatic water bath is equipped with in order to control
the temperature of the suspension of calcium hydroxide and a
pH measuring device and a thermometer are equipped with inside
of the suspension in order to measure the changes in pH and
reaction temperature of the suspension of calcium hydroxide.
The suspension is dispersed in distilled water, a stirrer
equipped with a 4-cm-long impeller is used in order to mix the
reaction solution and to maintain a homogeneous reaction, and
a flowmeter that can inject sodium carbonate at a rate of 3
mL/mminute is used in order to control the rate of insert of
sodium carbonate. Aragonite thus manufactured is investigated
through x-ray diffraction analysis and electron microscopy
after it is washed, filtered, and dried.
[Preferred Embodiment 1]
The aqueous solution reaction is performed at 75 C for 100
minutes by adding 3 M aqueous solution of sodium hydroxide to
1.5 M suspension of calcium hydroxide mixing, and further
adding 0.5 M aqueous solution of sodium carbonate at a rate of
3 mL/minute.
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
13
As seen in the x-ray diffraction pattern and production yields
of aragonite according to the reaction time shown in Figures 4
and 5, it is seen that calcite is not produced any further but
only aragonite is produced after 2 minutes.
[Preferred Embodiment 2]
Figure 6 shows the x-ray diffraction pattern of the aqueous
reaction performed at 75 C for 130 minutes by adding 5 M
aqueous solution of sodium hydroxide and further adding 0.5 M
aqueous solution of sodium carbonate at a rate of 3 mL/minute,
and Figures 7 and 8 show the production yields and electron
micrographs of aragonite. As shown in Figure 6, it is seen
that no calcite is produced, but only aragonite is produced
purely. Particularly, at the reaction time of 130 minutes,
almost no calcium hydroxide remains, and the content of
aragonite is about 98%. It is also seen in the micrographs
shown in Figure 8 that single-phase aragonite having the
aspect ratio of about 20 is manufactured.
[Preferred Embodiment 3]
Figure 9 is the x-ray diffraction pattern of the case that the
concentration of the aqueous solution of sodium hydroxide is
changed up to 0- 5 mol/L. It is seen that the production
yields of aragonite are increased as the concentration of the
aqueous solution of sodium hydroxide is increased, and single-
CA 02633808 2008-06-18
WO 2007/078018 PCT/KR2005/004691
14
phase aragonite having no calcite at all may be manufactured
by adding 5 M aqueous solution of sodium hydroxide.
[Industrial Applicability]
As described in the above, the method of manufacture of
single-phase aragonite according to the present invention is a
method of manufacturing aragonite by adding the highly
concentrated aqueous solution of sodium hydroxide into the
suspension of calcium hydroxide. This method is advantageous
in that it is possible to manufacture highly functional high-
value-added single-phase aragonite having no calcite produced
with a high yield by controlling calcium ions.
While certain present preferred embodiments of the invention
have been shown and described, it is to be distinctly
understood that the invention is not limited thereto but may
be otherwise variously embodied and practiced within the scope
of the following claims.