Note: Descriptions are shown in the official language in which they were submitted.
CA 02634144 2008-06-27 =
1
=
NOVEL FISH PATHOGEN
Technical field of the invention
The present invention relates to a novel fish pathogen. In particular the
invention
provides a fish virus, which when injected intraperitoneally or
intramuscularly into
fish, has the ability to introduce the symptoms and disease of Cardiomyopathy
Syndrome (CMS). Further the invention pertains to vaccines and therapeutics
developed on the basis of this pathogen.
Background of the invention
Heart and skeletal muscle inflammation (HSMI) is emerging as a significant
disease problem and the Norwegian Food Safety Authority has recommended that
it should be classified and handled as a group B disease. The infectious
nature of
HSMI has been indicated, and the first isolation of the virus was previously
reported. By electron microscopy virus-like particles of approximately 70 nm
were
observed. Most outbreaks are reported in fish transferred to seawater 5-9
months
earlier. Cardiac lesions are characterized by myocardial degeneration and
infiltration of mononuclear cells in both the spongious and compact layers of
the
ventricle. Lesions are also regularly observed in the red skeletal muscle
localized
along the lateral line of the fish, sometimes extended to adjacent white
fibres.
Cardiomyopathy syndrome (CMS) is a related disease affecting primarily large
Atlantic salmon in the second year in seawater close to harvest, and therefore
the
economic impact is significant. Affected fish may suddenly die without showing
signs of disease, or may show symptoms such as abnormal swimming behaviour
and anorexia. CMS is diagnosed on the basis of histopathology, showing severe
inflammation and degeneration of spongious myocardium in the atrium and
ventricle. A possible secondary effect of circulatory disturbance is
rnultifocal liver
necrosis which is commonly observed. Whereas the cause of CMS is still
unknown,
infectious aetiology has been hypothesized and debated, but not yet proven or
rejected. Both intracellular inclusions and virus-like particles have been
observed,
but reports are not consistent as apparent from a review provided in Kongtorp
et
al. 2005: in 1997 Grotmol et al. reported the presence of nodavirus-like
particles
in endothelial cells in the heart of Atlantic salmon diagnosed as suffering
from
CMS. The particles had a diameter of 25 nm. However, the assumed virus was
never isolated, and infection trials were never conducted to confirm the
aetiology
of the disease. In 2005 Hodneland et al. described the isolation and
CA 02634144 2008-12-10
2
characterization of a togavirus, salmonid alphavirus 3 (SAV3) from rainbow
trout
and Atlantic salmon diagnosed with Pancreatic disease or CMS. This Norwegian
subtype of the salmonid alphaviruses is the causative agent of pancreatic
disease
in Norwegian waters but any involvement of this pathogen in the ethiology of
CMS
has not been confirmed. Most recently, as documented in a report of November
2007, challenge experiments conducted by the Institute of Aquaculture,
University
of Stirling, Scotland failed to establish whether CMS is in fact infectious:
in these
experiments only one of 25 Atlantic salmon developed symptoms of CMS when
exposed to cell extracts from kidney and heart from a CMS outbreak. The
authors
discuss that CMS outbreaks in farmed salmon may be a production problem
resulting from fish growing too fast (Report prepared for the Scottish
Aquaculture
Research Forum by Institute for Aquaculture, University of Stirling, 2007).
Consequently, there is a need to first establish whether CMS has viral or
bacterial
aetiology and, if this is the case, to isolate and characterise the causative
agent.
Secondly the economical impact which CMS infections may have in the fish
farming industry provides the impetus to develop tools for detecting and
diagnosing CMS infections and therapeutics for prophylaxis and treatment of
CMS
infections.
Summary of the invention
The present invention is based on the finding that Cardiomyopathy Syndrome has
a viral aetiology, and accordingly, an object of the present invention relates
to a
fish Virus, which when infecting a fish causes the symptoms and/or disease
and/or
histopathological lesions of CMS.
In particular, It is an object of the present invention to provide a virus
which may
be useful in the prevention of outbreaks of CMS and/or in the treatment of the
disease.
Thus, one aspect of the invention relates to a fish virus, which when injected
intraperitcineally or intramuscularly in doses of 0.1 - 0.2 ml/fish of an
infected GF-
1 cell homogenate prepared from 1 passage of the virus, has the ability to
introduce the symptoms and disease of Cardiomyopathy Syndrome (CMS) and
wherein:
i) said virus is the strain deposited under the Budapest Treaty with
the
European Collection of Cell Culture (ECACC), Health Protection Agency,
Porton Down, Salisbury, Wiltshire (UK), SP4 OJG UK on the 29 March
2007 under accession number 07032902 or a strain with related
genotypic and/or phenotypic characteristics, and/or
CA 02634144 2008-12-10
3
ii) said virus reacts with immune serum from rabbit raised against the
strain deposited under ECACC accession number 07032902.
Another aspect of the present invention relates to a cell line comprising the
fish
virus as defined above.
Yet another aspect of the present invention is to provide a vaccine comprising
a
virus as defined above, or a component or part of said virus.
Further aspects of the invention provide a feed comprising a vaccine according
to
the invention, an antiserum or an isolated antibody which selectively binds to
a
virus as defined above or to a component or part of said virus.
In yet a further aspect the invention provides a method of isolating or
producing a
virus as defined above, said method comprising
i) producing a homogenate of a tissue from a fish suffering from/showing the
symptoms of Cardiomyopathy Syndrome (CMS);
ii) inoculating a cell culture of a suitable cell line;
iii) isolating virus particles from said cell and/or from the medium in which
the
cell line is cultured.
Also the invention provides a virus as defined above or a part of said virus
for use
in medicine/veterinary medicine.
In a related aspect the invention pertains to a virus as defined above or a
part of
said virus for use in prevention of Cardiomyopathy Syndrome (CMS) in fish
and/or
for reducing the viral load in a fish and/or for reducing the incidence of CMS
in a
population of fish and/or for treatment of Cardiomyopathy Syndrome (CMS) in
fish
and/or for reducing the incidence and/or severity of lesions caused by
Cardiomyopathy Syndrome Virus in fish.
A further aspect relates to the use of the virus as defined above or a part of
said
virus for the manufacture of a medicament for prevention of Cardiomyopathy
Syndrome (CMS) in fish and/or for reducing the viral load in a fish and or for
reducing the incidence of Cardiomyopathy Syndrome in a population of fish
and/or
for treatment of Cardiomyopathy Syndrome in fish, and/or for reducing the
incidence and/or severity of lesions caused by Cardiomyopathy Syndrome Virus
in
fish.
Finally, still further aspects of the invention provide a method for
prevention of
Cardiomyopathy Syndrome in fish and/or for reducing the viral load in a fish
and
or for reducing the incidence of Cardiomyopathy Syndrome in a population of
fish
= 35 and/or for treatment of Cardiomyopathy Syndrome in fish, and/or for
reducing the
incidence and/or severity of lesions caused by Cardiomyopathy Syndrome Virus
in
CA 02634144 2008-06-27
4
fish, said method comprising administering to the fish a vaccine according to
the
invention.
Brief description of the figures
Figure 1 shows histological changes in the heart (He) and skeletal muscle (Mu)
during a challenge study with HSMIV in Atlantic salmon. Histological changes
are
scored at different time post challenge,
Figure 2 shows histological changes in the heart (He) and skeletal muscle (Mu)
over time in cohabitant Atlantic salmon residing in the same tank as the fish
injected with HSMIV,
Figure 3 shows histological changes in the heart (He) and skeletal muscle (Mu)
during a challenge study with CMSV in Atlantic salmon. Histological changes
are
scored at different time post challenge,
Figure 4 shows histological changes in the heart (He) and skeletal muscle (Mu)
over time in cohabitant Atlantic salmon residing in the same tank as the fish
injected with CMS,
Figure 5 shows stained tissue sections of heart from CMS virus infected fish
at 6
weeks post infection; A: Ventricle; no or infrequent inflammatory changes are
seen in the epicard. B. Ventricle; focal to multi-focal inflammation is seen
in the
myocard. C and D: Atrium showing focal inflammation. Circles mark inflammatory
loci.
Figure 6 shows stained tissue sections of heart from HSMI virus infected fish
at 7
weeks post infection; A and B: Ventricle; inflammation in the epicard extends
into
the compact layer of the ventricle as marked by arrow in (A). C and D: Atrium
¨
no inflammation seen.
Figure 6 C and D: Atrium from HSMI virus infected fish.
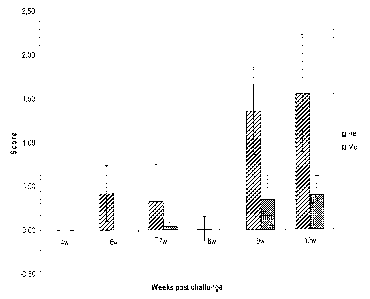
Figure 7: Graph illustrating the prevalence of lesions in the atrium after
CSMV
challenge of fish which have been vaccinated with a CMS vaccine according to
the
invention, compared with CMSV challenge of PBS controls and fish having
received
an HSMI vaccine
Figure 8: Graph illustrating the prevalence of lesions in the ventricle after
CSMV
challenge of fish which have been vaccinated with a CMS vaccine according to
the
invention, compared with CMSV challenge of PBS controls and fish having
received
an HSMI vaccine
CA 02634144 2008-06-27
Figure 9: Electron micrograph of cells infected with virus according to the
invention, showing spherical structures- having an approximate diameter of 70
nm.
The present invention will now be described in more detail in the following.
5
Detailed description of the invention
Definitions
Prior to discussing the present invention in further details, the following
terms and
conventions will first be defined:
The term "genotypic characteristics" refers broadly to the composition of one
or
more parts of an individual's genonne which contributes to determining a
specific
trait of the individual. In the context of the present invention, genomic
characteristics of the virus may be assessed by RT-PCR using species specific
primers.
The term "phenotypic characteristics" refers equally broadly to one or more
observable properties of an organism that are produced by the inherited
genotype
or by interaction of the inherited genotype of the individual with transmitted
epigenetic factors, and/or non-hereditary environmental variation or factors.
In
the context of the present invention, the term "related phenotypic
characteristics"
includes any of the following characteristics: size, shape, density, pH
stability,
temperature stability, chloroform sensitivity and haemaglutination.
"Phenotypic characteristics" further include the ability to induce symptoms or
clinical signs of CMS as described in the present application. "Phenotypic
characteristics" also include the ability of the virus to introduce such
clinical signs
in a laboratory challenge experiment when a homogenate from a cell culture or
a
tissue infected with the CMS virus is injected in Atlantic Salmon (Salmo Salar
L.)
as described herein.
The term "essentially free of other viral or microbial material" as used
herein
refers to a preparation of a virus according to the invention, wherein the
virus has
been removed from its natural genetic milieu, and is thus largely free of
other
extraneous or unwanted viral or microbial material. Thus, when substantially
free
of other viral or microbial material, the virus of the invention is in a
preparation
containing at the most 10% by titre or volume of other viral or microbial
material
(lower percentages of other viral or microbial material are preferred, e.g. at
the
most 8% by titre or volume, at the most 6% by titre or volume, at the most 5%
by titre or volume, at the most 4% by titre or volume, at the most 3% by titre
or
CA 02634144 2008-06-27
6
volume, at the most 2% by titre or volume, at the most 1% by titre or volume,
at
the mOst 0.5% by titre or volume, at the most 0.1% by titre or volume, or at
the
most 0.05% by titre or volume).
The term "substantially free of other viral or microbial material" as used
herein
refers to a preparation of a virus according to the invention, wherein the
virus is
in a preparation in which other viral or microbial material cannot be detected
using conventional techniques like seeding on TSA or cystein heart agar spread
plates, seeding in cell cultures known to support the growth of known fish
viruses
like Pancreas Disease Virus, Infectious Salmon Anemia virus and Infectious
pancreatic Necrosis virus and PCR with primers designed against known
sequences from fish pathogens. Further, it is to be understood that when
"substantially free of other viral or microbial material" the virus of the
invention is
in a form wherein it may be used for therapeutic purposes.
It is to be understood that providing virus according to the invention in
preparations being essentially or substantially free of other viral or
microbial
material does not exclude the possibility of combining such preparations with
other viral or microbial material, such as in the polyvalent vaccines
disclosed
herein.
The term "component or part of said virus' refers to a component or part of
the
nucleic acid core of the virus or of the surrounding protein coat. In the
context of
the present invention, it is preferred that the said component or part
possesses
antigenicity. Antigenicity may imply a capacity of the component or part of
said
virus to induce an immune response,that is to be recognized by and interact
with
an immunologically specific antibody or T-cell receptor; or a capacity to
produce
immunity.
Aspects and embodiments of the invention
Virus
A first aspect of the present invention pertains to a virus which is the
causative
agent of Cardiomyopathy Syndrome. In particular the invention provides a fish
virus, which when injected intraperitoneally or intramuscularly in doses of
0.1 -
0.2 ml/fish of an infected GF-1 cell homogenate prepared from 1st passage of
the
virus, has the ability to introduce the symptoms and disease of Cardiomyopathy
Syndrome (CMS) and wherein:
i) said virus is the strain deposited under the Budapest Treaty with
the
European Collection of Cell Culture (ECACC), Health Protection
CA 02634144 2015-03-18
7
Agency, Porton Down, Salisbury, Wiltshire (UK), SP4 OJG UK on the
29 March 2007 under the accession number 07032902 or a strain with
related genotypic and/or phenotypic characteristics, and/or
ii) said virus reacts with immune serum from rabbit raised against
the
deposited strain under ECACC accession number 07032902.
Particular embodiments of the invention pertain to virus being obtainable from
said deposited isolates.. As the skilled person will realize, replication of
viral
genomes may be accompanied by very high mutation rates. In RNA viruses this is
due to the lack of proofreading activity of RNA virus polymerases, which leads
to a
constant generation of new genetic variants e.g. during virus propagation.
Also, it
is known that different constellations of mutations may be associated with a
similar biological behaviour of the virus. Accordingly, the invention includes
viruses which are obtainable from the strain deposited under the Budapest
Treaty
with the European Collection of Cell Culture (ECACC), Health Protection
Agency,
Porton Down, Salisbury, Wiltshire (UK), SP4 OJG UK on the 29 March 2007 under
accession number 07032909, such as by passage of the virus on a cell line or
by
genetic manipulation. As illustrated in the present application, a useful cell
line for
the purpose of culturing the virus according to the invention is a GF-1 cell
line;
GF-1 cells are described in US patent 6,436,702, and have been deposited in
the
ATCC under deposit no. PTA-859. Genetic manipulation comprises for instance
introduction of substitutions and/or deletions of single or multiple nucleic
acid
residues.
In relation to the present invention the ability of the virus to introduce the
symptoms and disease of CMS symptoms was demonstrated when a homogenate
from a cell culture infected with the CMS virus was injected in Atlantic
Salmon
(Salmo Salar L.). As the skilled person will acknowledge the virus of the
invention
is likely to cause similar symptoms after having been injected into or after
having
infected fish from species within the salmonidae family. In particular the
virus is
likely to cause similar symptoms and disease in fish from the salmo subfamily
of
the salmonidae.
Specifically, the invention provides a virus in relation to which it is
characteristic
that said symptoms are seen when the cell homogenate is injected in a dose of
0.1 ml on each side of the fish in the lateral muscle.
Further, the symptoms may be seen in at least 20% of individuals in a
population
of fish, such as in at least 30%, at least 40%, at least 50%, at least 60 k,
at least
70%, at least 80%, at least 90% or in at least 95% of individuals in a
population
of fish.
CA 02634144 2008-06-27
8
In particular, the symptoms may appear 6 weeks post challenge or later, such
as
7 weeks post challenge or later, 8 weeks post challenge or later, 9 weeks post
challenge or later or 10 weeks post challenge or later.
In particular embodiments relating to the invention, the virus has the ability
of
introducing:
I)_ Inflammatory changes in areas of the spongious myocardium in the atrium
and/or the ventricle; and/or
ii) Abnormal swimming behaviour and/or anorexia; and/or
iii) Multifocal liver necrosis.
While some variation in the location and severity of the symptoms is to be
expected, the inflammatory changes in areas of the spongious myocardium in the
atrium and/or the ventricle may appear as foci of degeneration and
infiltration of
inflammatory cells, including lymphocytes and/or macrophages, and/or as areas
of necrosis, said areas optionally extending along fibres, the foci ranging in
number from 1->5 per viewed field.
The virus according to the invention may have the ability of introducing one
or
more symptoms selected from the group consisting of: skin haemorrhages, raised
scales, exopthalamos, ascites, fibrinous casts over the liver capsule, blood
or
blood clots filling the pericardial cavity, ruptures in the cardiac atrial
wall, dilation
of the cardiac atrium, compression of the cardiac ventricle, inflammation of
the
epi- and endocardium, liver lesions including multifocal to anastomosing
necrosis
of hepatocytes and fibrinous coating of the capsule, congestion of the spleen
and/or the gills.
When isolated using rate-zonal density gradient centrifugation on a sucrose
gradient, the virus according to the invention localized in a fraction having
a
refractive index between 1,3950 and 1,4000 and a density in between 1,160
g/cm3 and 1,175 g/cm3, and/or in a fraction having a refractive index between
1,365 and 1,75 and a density in between 1,090g/cm3 and 1,110g/cm3.
The fact that the virus according to the invention is localized in two
separate
fractions is likely due to the fact that the virus adheres to larger
particles/cell
debris during the process. Therefore, a part of the virus isolate will be
located in
the top part of the gradient. It is contemplated that the true density of the
virus is
in the range of 1,16-1,17 g/cm3 when analysed on a sucrose gradient.
For the purpose of analysing the virus according to the invention the rate-
zonal
density gradient centrifugation was performed using as starting material the
supernatant from a culture of GF-1 cells infected with the virus. The
supernatant
was centrifuged at 17700xg for 30 min, re-suspended in 4 ml culture media and
CA 02634144 2008-06-27
9
frozen at -80 C. The supernatant was spun at 100000xg for 3h and the pellet
was
re-suspended in PBS and frozen at -80 C.
The pellet material was diluted in PBS and loaded on to a sucrose density
gradient
consisting of: 6 ml 36% sucrose in PBS w/v, 6 ml 42% sucrose in PBS w/v, 6 ml
48% sucrose in PBS w/v, 6 rril 54% sucrose in PBS w/v, and 6 ml 60% sucrose in
PBS w/v which was subsequently spun at 100000xg at 4 C for 16h. Fractions were
collected in 2 ml aliquots from the bottom of the tube, and the refractive
index
was measured in each fraction.
Initial studies suggested that the virus according to the invention has a
diameter
of 60-100 nm, such as a diameter of 60-70 nm, a diameter of 65-75 nm, a
diameter of 70-80 nm, a diameter of 75-85 nm, a diameter of 80-90 nm, a
diameter of 85-95 nm or a diameter of 90-100 nm.
Inspection of electron micrographs (EM) of infected cell cultures suggested,
that
the virus according to the invention is a spherical virus. Further the EM
analyses
indicated that the virus according to the invention has an approximate
diameter of
70 nm. By "an approximate diameter of 70 nm" is to be understood a diameter in
the range of 65-75 nm, such as in the range of 67-75 nm, 67-73 nm, 65-73 nm,
or such as 67-73 nm.
Analyses by PCR on reverse transcribed RNA from the virus according to the
invention has indicated that the virus has genotypic characteristics which are
different from those of the salmonid alphaviruses, infectious pancreatic
necrosis
virus (IPNV), and nodavirus, including grouper nervous necrosis virus,
In particular, this was shown in studies where 1,7pg RNA isolated from the
virus
according to the invention was reverse transcribed using random hexamers as
primers.
PCR was performed on the reverse transcribed products using the following
primers:
Primers specific for salmonid alphavirus:
forward primer - CGTCACTTTCACCAGCGACTCCCAGACG (SEQ ID NO: 1)
reverse primer - GGATCCATTCGGATGTGGCGTTGCTATGG (SEQ ID NO: 2)
Primers specific for IPNV:
Forward primer - GTCCGGTGTAGACATCAAAG (SEQ ID NO: 3)
Reverse primer - TGCAGTTCCTCGTCCATCC (SEQ ID NO: 4);
Primers specific for Grouper nervous necrosis virus (Noda-virus):
.35 Forward primer - GGATTTGGACGTGGGACCAA (SEQ ID NO: 5)
Reverse primer - CGGATGACCCGGTTAGTTTTC (SEQ ID NO: 6);
CA 02634144 2015-03-18
The analysis is performed using in a standard PCR reaction, comprising heating
of
the sample to 95 C for 5 min, followed by 30 cycles of denaturing at 95 C for
30
sec, annealing at 50 C for 30 sec, and elongation at 72 C for 60 sec, followed
by
a single step of heating to 72 C for 10 min. While a detailed description of
the RT-
5 PCR methodology is provided in example 7 in the present application, it will
be
within the capacity of the skilled person to conduct such analyses using
general
knowledge on the requirements for buffers, polymerases, primers, nucleotide
mixtures and the like.
Using standard PCR conditions and temperatures as described above it was not
10 possible to detect any significant product formation in any of the tests.
Accordingly, the CMS virus as provided herein does not contain a ribonucleic
acid
species capable of forming significant amounts of a product with any one of
the
PCR primer pairs selected from the group consisting of:
i) a forward primer having a nucleotide sequence as set forth in SEQ ID NO: 1
and a reverse primer having a nucleotide sequence as set forth in SEQ ID
NO: 2;
ii) a forward primer having a nucleotide sequence as set forth in SEQ ID NO:
3 and a reverse primer having a nucleotide sequence as set forth in SEQ ID
NO: 4; and
iii) a forward primer having a nucleotide sequence as set forth in SEQ ID NO:
5 and a reverse primer having a nucleotide sequence as set forth in SEQ ID
NO: 6;
when analysed in a standard PCR reaction on reverse transcribed RNA isolated
from the fish virus, comprising heating of the sample to 95 C for 5 min,
followed
by 30 cycles of denaturing at 95 C for 30 sec, annealing at 50 C for 30 sec,
and
elongation at 72 C for 60 sec, followed by a single step of heating to 72 C
for 10
min. As illustrated in example 6 of the present application, studies have been
conducted in order to assess the ability of the virus according to the
invention to
grow in cultures of various cell lines which are commonly used to support
growth
of known viral fish pathogens. As demonstrated, the fish virus according to
the
invention, when provided in a tissue homogenate from an infected fish, is
incapable of growing in any cell line from the group consisting of: BF-2
(Bluegill
fry, caudal trunk, available from LCG Promochem as ATCC Number: CCL-91T'),
BB (Brown bullhead, connective tissue and muscle, available from LCG
Promochem as ATCC Number: CCL-59'), RTG-2 (Rainbow trout, gonadal tissue,
available from LCG Promochem as ATCC Number: CCL-55Tm), SHK-1 (salmon
head, kidney, established by Dannevig et al. as reported in 1995 and commonly
available in research laboratories), CHSE-214 (Chinook salmon, embryo,
available
CA 02634144 2008-06-27 -
11
from LCG Promochem as ATCC Number: CRL-1681Tm), CCO (Channel catfish,
ovary, available from LCG Promochem as ATCC Number: CRL-2772T), CHH-1
(Chum salmon, heart fibroblast, available from LCG Promochem as ATCC
Number: CRL-16801M) and FHM (Fat head minnow, epithelial like, available from
LCG Promochem as ATCC Number: CCL-42-"^).
As mentioned in the example most of these cell lines are permissive for one or
more known viral fish pathogens, providing further indication that the virus
according to the invention has phenotypic characteristics which are different
from
those of Infectious Pancreatic Necrosis Virus (IPNV), Viral Haemorrhagic
Syndrome Virus (VHSV), Infectious Haematopoietic Necrosis Virus (IHNV),
Channel Catfish Virus (CCV), Spring Viremia of Carp (SVCV), Infectious salmon
Anemia Virus, Pancreatic Disease Virus (PDV) and Catfish Tumour Virus (CTV).
In a further embodiment the virus according to the invention is inactivated by
incubation with 10% v/v chloroform for 30 minutes at 20 C with continuous
shaking. The sensitivity towards chloroform treatment indicates the presence
of
an envelope containing essential lipids.
In yet a further embodiment the fish virus according to the invention is
inactivated by incubation at 70 C for 30 minutes while it is insensitive to
incubation at 60 C for 30 minutes.
In other embodiments the culture of the fish virus according to the invention
is
insensitive to incubation with 1001.1.g/m1 DNA synthesis inhibitor
bromodeoxyuridine (BrdU). In these embodiments the insensitivity to DNA
synthesis inhibitors indicates that the virus according to the invention is an
RNA
virus.
In still other embodiments the fish virus according to the invention is
inactivated
after incubation 4 hours with pH 3.0 at 15 C, whereas pH 5.0 has no effect
compared with pH 7.2.
It is within the scope of the present invention to provide an isolate of the
virus
described above. The virus of the invention may thus be in an isolated form
and/or substantially or essentially free of other viral or microbial material.
It is further within the scope of the invention to provide a virus as
described _
above which is either attenuated or inactivated. Several approaches for
obtaining
attenuated virus are available to the skilled person. An attenuated strain of
a virus
may be generated for instance by passing the virus through cell culture a
number
of times, or deleting or mutating a gene involved in its replication pathway.
Inactivation of the virus may be obtained by chemical or physical means.
Chemical inactivation can be carried out by treatment of the viruses by for
=
CA 02634144 2015-03-18
12
example, but not limited to, treatment with enzymes, with formaldehyde, 8-
propiolactone or ethyleneimine or a derivative thereof, with organic solvent
(e.g.
halogenated hydrocarbon) and/or detergent, e.g. Triton or Tween .
Physiological
inactivation can advantageously be carried out by subjecting the viruses to
energy-rich radiation, such as UV light, gamma irradiation or X-rays. If
necessary,
the inactivating agent can be neutralized with thiosulphate. If required, the
pH is
subsequently returned to about pH 7.
Infected cell line
A second aspect of the invention provides a cell line comprising the fish
virus as
defined in above. Several cell lines may be used for propagation of the virus.
In
preferred embodiments, however, the cell line is derived from Epinephelus
cob ides.
In particular embodiments the cell line is a GF-1 cell line; GF-1 cells are
described
in US patent 6,436,702, and have been deposited in the ATCC under deposit no.
PTA-859. Another example of a useful cell line is the ASL cell line derived
from
Atlantic salmon liver. Cells were isolated from Atlantic salmon (SaImo salar)
liver
by a two-step collagenase perfusion procedure according to Dannevig and Berg
(1985). The cells have been sub-cultured more than 20 times.
Vaccine
Another aspect of the invention pertains to a vaccine comprising a virus as
defined
above, or a component or part of said virus.
The skilled person will realise that the content of a vaccine preparation
varies
depending on the intended use of the vaccine. In presently preferred
embodiments of the invention, such as the test vaccine which is used in the
clinical trial illustrated in Example 10 herein very low amounts of antigen
are
used. As illustrated in the example, good protection against histopathological
changes typical for CMS are shown in fish vaccinated with CMS vaccine
containing
for instance 0.016 mg antigen/dosage or 0.16 mg antigen/ml of vaccine
formulation. According to these embodiments the vaccine comprises an amount of
antigen, which is in the range of 0.05-1.0 mg/ml, such as from 0.15 to 0.5
mg/ml, from 0.15 - 0,4 mg/ml, from 0.15 - 0,3 mg/ml or such as from 0.1 to 0.5
mg/ml, from 0.1 - 0,4 mg/ml, from 0.1 - 0,3 mg/ml, from 0.1 - 0,25 mg/ml or
such as from 0.1 - 0.2 mg/ml. The vaccine may be for administration in dosages
CA 02634144 2015-03-18
13
of 0.005-0.5 mg/individual, preferably from 0.01-0.05 mg/individual, or such
as
from 0.01-0.02 mg/individual.
In a final product approved for commercial use in aquaculture, the preferred
amount of antigen may be somewhat higher. According to embodiments
pertaining to such products the preferred amount of antigen may be in the
range
of 0.5-2.0 mg/ml, such as from 0.5 to 1.5 mg/ml, from 0.5 - 1,3 mg/ml, from
0.6
- 1,5 mg/ml or 0.7-1.5 mg/ml, such as from 0.8 to 1.5 mg/ml, from 0.9 - 1,5
mg/ml, from 0.7 - 1,4 mg/ml, from 0.7-1.3 mg/ml, from 0.7-1.2 mg/ml, such as
from 0.8 to 1.4 mg/ml, from 0.8 - 1,3 mg/ml, from 0.8 - 1,2 mg/ml or from 0.9-
1.4 mg/ml, such as from 0.9 to 1.3 mg/ml, from 0.9 - 1,2 mg/ml. For such
embodiments a most preferred amount of antigen of 1 mg/ml is presently
contemplated. Additional embodiments provide a vaccine comprising an amount of
antigen corresponding to a TCID50 of 105-1010 per dosage, such as an amount of
antigen corresponding to a TCID50 of 106-109 per dosage, an amount of antigen
corresponding to a TCID50 of 107-109 per dosage, such as an amount of antigen
corresponding to a TCID50 of 5x107-5x108 per dosage, such as an amount of
antigen corresponding to a TCID50 of 8x107-4x108 per dosage, such as an amount
of antigen corresponding to a TCID50 of 1x108-4x108 per dosage, such as an
amount of antigen corresponding to a TCID50 of 1x108-3x108 per dosage, such as
an amount of antigen corresponding to a TCID50 of 1-2x108 or such as an amount
of antigen corresponding to a TCID50 of 1.5x108 per dosage.
The vaccine may be in the form of a suspension of the virus or it may be
lyophilized. In a lyophilized vaccine it may be useful to add one or more
stabilizers. Suitable stabilizers are for example carbohydrates such as
sorbitol,
mannitol, starch, sucrose, dextran; protein containing agents such as bovine
serum or skimmed milk; and buffers such as alkali metal phosphates.
The vaccine according to the invention may further be in a formulation
comprising
an adjuvant. Examples of adjuvants frequently used in fish and shellfish
farming
are muramyldipeptides, lipopolysaccharides, several glucans and glycans,
mineral
oil, MontanideTM and Carbopol . An extensive overview of adjuvants suitable
for
fish and shellfish vaccines is given in the review paper by Jan Raa (1996).
The vaccine of the invention may further comprise a suitable pharmaceutical
carrier. In a currently preferred embodiment the vaccine is formulated as an
emulsion of water in oil. The vaccine may also comprise a so-called "vehicle".
A
vehicle is a device to which the antigen adheres, without being covalently
bound
to it. Such vehicles are i.a. biodegradable nano/micro-particles or -capsules
of
PLGA (poly-lactide-co-glycolic acid), alginate or chitosan, liposomes,
niosomes,
micelles, multiple emulsions and macrosols, all known in the art. A special
form of
CA 02634144 2015-03-18
14
such a vehicle, in which the antigen is partially embedded in the vehicle, is
the so-
called ISCOM (European patents EP 109.942, EP 180.564 and EP 242.380).
In addition, the vaccine may comprise one or more suitable surface-active
compounds or emulsifiers, e.g. Cremophore , Tween and Span . Also
adjuvants such as interleukin, CpG and glycoproteins may be used.
It is to be understood that the vaccine may further be in a formulation
comprising
an antigen from a bacterial source, an antigenic material obtained from a
viral
source other than the fish virus as defined above, an antigenic material
obtained
from a parasitical source, and/or an antigenic material obtained from a fungal
source. Polyvalent vaccines containing antigens from typical fish pathogens
other
than CMS virus are well known in the art and are already commercially
available.
In addition, representative isolates of relevant fish pathogens are available
from
various sources.
In particular embodiments of the invention said antigen from a bacterial
source is
selected from the group consisting of: live, attenuated or killed bacteria of
the
species Piscirickettsias sp. Aeromonas sp., Vibrio sp., Listonelia sp.,
Monte/la
viscosa, Photobacterium damsela, Flavobacterium sp., Yersinia sp.,
Renibacterium
sp., Streptococcus sp., Lactococcus sp., Leuconostoc sp., Bifidobacterium sp.,
Pediococcus sp., Brevibacterium sp., Edwarsiella sp., Franc/se/la sp.,
Pseudomonas sp., Cytophaga sp., Nocardia sp., Mycobacerium sp., parts or
subunits of these bacteria, and any combination hereof.
Isolates of such bactera are available, e.g. from LGC Promochem/American Type
Culture Collection ATCC repository and distribution center (ATCC) including
strains
of A. salmonicida (ATCC 33658), V. salmonicida (ATCC 43839), V. anguillarum
serotype 01(ATCC 43305) and 02(ATCC 19264), and Monte/la viscosa (ATCC
BAA-105). In addition, cultures of Piscirickettsias salmonis have been
deposited in
the European Collection of Cell Culture (ECACC), Health Protection Agency,
Porton
Down, Salisbury, Wiltshire (UK), SP4 OJG UK on the 9 June 2006 under the
following accession numbers: 06050901, 06050902, 06050903 and 07032110.
Other specific embodiments pertain to a vaccine, wherein said antigenic
material
obtained from a viral source other than the fish virus as defined above is
from a
virus selected from the group consisting of: Viral Hemorrhagic Septicemia
Virus
(VHSV), Viral Hemorrhagic Septicemia Virus (VHSV), Infectious Hematopoietic
Necrosis virus (IHNV), Infectious Pancreatic Necrosis Virus (IPNV), Spring
Viremia
of Carp (SVC), Channel Catfish Virus (CCV), Infectious Salmon Anaemia virus
(ISAV), pancreatic disease virus (SPDV), Iridovirus, and heart and skeletal
muscle
inflammation virus (HSMIV), parts or subunits of any one of these viruses, and
CA 02634144 2008-06-27-
combinations hereof. Representative species of such viruses are available to
the
skilled artisan, for instance from the following deposits: infectious
pancreatic
necrosis virus (IPNV, ATCC VR-1318, country of origin: unknown), Viral
Hemorrhagic Septicemia Virus (VHSV, ATCC VR-1389, country of origin:
5 Denmark); Infectious Hematopoietic Necrosis virus (IHNV, ATCC VR-1392,
country of origin: USA)); Pancreatic Necrosis Virus; Spring Viremia of Carp
(SVC,
ATCC VR-1390, country of origin: Denmark); Channel Catfish Virus (CCV) (ATCC
VR-665; country of origin: USA); Infectious Salmon Anaemia (ISA) virus (ATCC
VR-1554, country of origin: Canada).
10 Patent deposits have previously been made by the present applicant of the
following viral species: Heart and Skeletal Muscle Infection Virus (HSMIV,
patent
deposit nr ECACC 04050401, country of origin: Norway).
In more specific embodiments, said antigenic material obtained from a viral
source other than the fish virus as defined above is from the group consisting
of:
15 Glycoprotein of Viral Hemorrhagic Septicemia Virus (VHSV), nucleoprotein of
Viral
Hemorrhagic Septicemia Virus (VHSV), glycoprotein of Infectious Hematopoietic
Necrosis virus (IHNV), nucleoprotein structural proteins of Infectious
Pancreatic
Necrosis Virus (IPNV), G protein of Spring Viremia of Carp (SVC), and a
membrane-associated protein, tegunnin or capsid protein or glycoprotein, of
Channel Catfish Virus (CCV), antigenic fragments of any of one of these
proteins
and combinations hereof.
In other embodiments said antigenic material from a parasitic source is from a
source selected from the group consisting of Lepeophtheirus Sp., Caligus Sp.,
and
Ichthyophthirius Sp, parts of any one of these parasites, and combinations
hereof.
In yet other embodiments said antigenic material is from a fungal source
selected
from the group consisting of Saprolegnia Sp., Branchiomyces sanguinis,
Branchiomyces demigrans and Icthyophonus hoferi.
The vaccine according to the invention may in particular be formulated for
administration to a fin fish. More specifically the vaccine may be
(formulated) for
administration to a telostei. The teleostei include, but are not limited to
salmonids, basses, breams, cods, snappers, flatfish, catfish, yellowtails and
tilapias.=
In a presently preferred embodiment the vaccine is formulated for
administration
to Atlantic salmon (Salmo Salar L.), Rainbow trout (Oncorhynchus mykiss)
and/or
Coho salmon (Oncorhychus kisutch)
In further embodiments of the invention the vaccine is formulated for
administration by a route selected from the group consisting of: Bath,
immersion,
intraperitoneal injection, intramuscular injection and oral administration.
CA 02634144 2008-06-27
16
Optionally, the vaccine would be administered to young fish in the fresh-water
stage.
Feed comprising vaccine
In a further aspect, the present invention provides a feed comprising the
vaccine
according to the invention, said feed may for example be pelleted or extruded
feed.
Antiserum/antibody
Another aspect of the invention provides an antiserum or an isolated antibody
which selectively binds to a virus as defined above or to a component or part
of
said virus. Antisera are conventionally obtained for instance by immunising a
laboratory animal with the appropriate antigenic determinant. Once the
concentration of antibodies in serum from the animal reaches a desired level,
the
animal is bled. The Immune serum obtained should contain antibodies produced
in
response to the immunogenic stimulus.
Likewise, techniques for the preparation of antibodies are known to the
skilled
person. The techniques include the traditional hybridoma technology and
alternative techniques such as mRNA display, ribosome display, phage display
and
covalent display.
In some embodiments of the invention the isolated antibody comprises a marker,
e.g. a radiolabel, a fluorescent tag, a chemiluminescent label or an enzyme.
The
antibody may be a polyclonal or monoclonal antibody.
In the context of the present invention immune sera and/or isolated antibodies
raised against the deposited virus strain provided according to the invention
will
be useful in identifying virus which are identical to the deposited strain or
which
are obtainable from said strain. Also, the immune sera and/or isolated
antibodies
will be effective in identifying virus having phenotypic or genotypic strains
which
are related to those of the said deposited strain.
Method of isolating virus
Another aspect of the invention provides a method of isolating or producing a
virus as defined above, said method comprising
iv) producing a homogenate of a tissue from a fish suffering from/showing the
symptoms of Cardiomyopathy Syndrome (CMS);
v) inoculating a cell of a suitable cell strain;
CA 02634144 2008-06-27
17
vi) isolating virus particles from said cell and/or from the medium in which
the
cell is cultured.
The virus according to the invention may be grown on a cell culture such as
the as
culture of GF-1 cells. The viruses thus grown may be harvested by collecting
the
cell culture fluids and/or cells. The virus yield can be promoted by
techniques that
improve liberation of virus from the grown substrate, e.g. sonication.
Medical use
An additional aspect of the invention provides a virus as defined above or a
part of
said virus for use in medicine/veterinary medicine.
Provided in a further aspect of the invention is a virus as defined above or a
part
of said virus for use in prevention of Cardiomyopathy Syndrome (CMS) in fish
and/or for reducing the viral load in a fish and or for reducing the incidence
of
CMS in a population of fish and/or for treatment of Cardiomyopathy Syndrome
(CMS) in fish and/or for reducing the incidence and/or severity of lesions
caused
by Cardiomyopathy Syndrome Virus in fish.
Provided in yet a further aspect is use of the virus as defined above or parts
of
said virus for the manufacture of a medicament for prevention of
Cardiomyopathy
Syndrome (CMS) in fish and/or for reducing the viral load in a fish and or for
reducing the incidence of Cardiomyopathy Syndrome in a population of fish
and/or
for treatment of Cardiomyopathy Syndrome in fish and/or for reducing the
incidence and/or severity of lesions caused by Cardiomyopathy Syndrome Virus
in
fish.
Preventive/therapeutic method
Also provided in relation to the present invention is a method for prevention
of
Cardiomyopathy Syndrome in fish and/or for reducing the viral load in a fish
and
or for reducing the incidence of Cardiomyopathy Syndrome in a population of
fish
and/or for treatment of Cardiomyopathy Syndrome in fish and/or for reducing
the
incidence and/or severity of lesions caused by Cardiomyopathy Syndrome Virus
in
fish, said method comprising administering to the fish a vaccine as described
in
details above.
Throughout the present specification the word "comprise", or variations such
as
"comprises" or "comprising", will be understood to imply the inclusion of a
stated
element, integer or step, or group of elements, integers or steps, but not the
exclusion of any other element, integer or step, or group of elements,
integers or
steps.
CA 02634144 2008-06-27
18
With respect to the above description of the various aspects of the present
invention and of the specific embodiments of these aspects it should be
understood that any feature and characteristic described or mentioned above in
connection with one aspect and/or one embodiment of an aspect of the invention
also apply by analogy to any or all other aspects and/or embodiments of the
invention described.
All patent and non-patent references cited in the present application,-are
hereby
incorporated by reference in their entirety.
The invention will now be described in further details in the following non-
limiting
examples.
Examples
Example 1: Challenge study comparing HSMI and CMS
Heart tissue from Atlantic salmon experiencing a clinical outbreak of HSMI was
collected and homogenized with quarts sand in a porcelain mortar, centrifuged
at
3000 xg, the supernatant passed through a 45mm syringe filter ((sartorius
17829) and inoculated into GF-1 cell cultures. After 7-10 days, cytoplasmic
vacuoles were observed in infected cells. Lysed and filtrated material from
these
infected cells was further used to infect fresh cell cultures and similar
vacuolization was observed.
Inflamed heart tissue was collected from a clinical outbreak of CMS and
treated as
described above. Clarified heart tissue homogenate was inoculated onto GE-1
cell
cultures resulting in cytoplasmic vacuoles in infected cells, with morphology
similar to that of HSMI infected cells. Lysed and filtrated material from
these
infected cells was further used to infect fresh cell cultures and similar
vacuolization was observed.
As both diseases are usually observed in seawater, the clinical trial was
conducted
in seawater. A challenge study was conducted using a "CMS viral isolate"
material.
The lysed and filtered material described above was used for intramuscular
injection of fish in seawater. Samples of heart and red muscle tissue were
collected at defined intervals to follow the development of the disease. The
intention was to examine whether material cultured from fish with a CMS or
HSMI
diagnosis would produce similar or different clinical signs in Atlantic
salmon. This
CA 02634144 2008-06-27
=
19
would provide information as to whether CMS and HSMI may be caused by the
same or different viral agents.
Objectives
The main objective of this pilot challenge study was to gain knowledge of the
development of HSMI and CMS in seawater, and to compare these disease entities
in terms of development of histopathological changes in internal organs.
Schedule
The major dates were as follows:
i Task Activity Experimental
1 week
Start of Transfer of fish to Week -6
I experiment experimental cell
1 Seawater Transfer of fish to seawater Week 0
'
Itransfer
Challenge Tank 1: HSMIV challenge by Week 0
I and control i.m. injection of 50 fish + 20 1
1 sampling cohabitants.
Tank 2: "CMSV" challenge by 1
i.m. injection of 50 fish + 20 1
cohabitants.
Sampling of 10 control fish
Sampling Blood and tissue sampling of Week
6-8 fish at indicated time 2,4,6,7,8,9,10
1
points from both tanks.
I End of End of experiment Week 10
1
experiment
,
Materials and methods
Challenge material
The challenge isolates were:
CA 02634144 2008-06-27
- HSMIV 1st passage stock of ALV-702/03, 06.04.04 grown in GF-1 cells, and
- CMSV 1st passage stock from HSMI 1002.06 grown in GF-1 cells.
Environment:
5 The fish were smoltified in one tank at 15 C with a 24 hour daylight regime.
After
transfer to seawater the fish were held at 12 C.
Challenge
Tank groups tagging challenge isolate/
amount added
1 50 fish i.m. injected none HSMIV-1
1st passage stock of
ALV-702/03
0.1 ml injected on
each side of the fish
in the lateral muscle
tissue beneath the
dorsal fin
20 cohabitant fish Adipose fin none
2 50 fish i.m. injected none HSMIV-2 ("CMSV91st
passage stock
0.1 ml injected on
each side of the fish
in the lateral muscle
tissue beneath the
dorsal fin
20 cohabitant fish Adipose fin none
Intramuscular (i.m.) injection challenge: The fish_were anesthetized and 0.1
ml
was injected on each side (0.2ml in total) of the fish in the lateral muscle
tissue
beneath the dorsal fin. Cohabitant fish were marked by clipping the adipose
fin
and the cohabitants are transferred to the tanks on day of challenge, 20 fish
in
each of the tanks.
Sampling
CA 02634144 2008-06-27
21
Week 33 35
37 39 40 41 42 43
Weeks after challenge 0 2 4 6 7 8 9 /0
Challenged fish HSMIV-1 6 6 6 8 8 8 7
(tank 1)
Cohabitants tank 1 6 6 8
Challenged fish HSMIV-2 6 6 6 8 8 8 7
(tank 2)
Cohabitants tank 2 6 6 8
Unchallenged control fish 10 -
Samples
The samples (# given above) for histological examination were collected from
all
fish of each time point and treated as given below.
Sample Sampled in Post-
fixation treatment
Heart tissue Formalin -filled
Processed for embedding by
Red muscle containers standard dehydration
Liver methods, embedded in
paraffin, sectioned and
stained with hematoxylin and
eosin. Examination was
carried out using a Zeiss light
microscope.
Assessment criteria
The assessment of histological changes in HSMI challenged fish was performed
on
the basis of the criteria given below and the scoring was marked on a visual
analog scale (VAS).
CA 02634144 2008-06-27
22
Inflammation Pathological description
score for HSMI
infected fish
0 No pathological changes observed.
1 Mild pathological changes characterized by a limited number
(countable) of mononuclear inflammatory cells infiltrating the
epicardium, not extending into the compact layer of the
ventricle. The infiltration of cells is multifocal to diffuse and
can involve parts of or the entire epicardium available for
assessment.
2 Moderate pathological changes consisting of high number
(uncountable) of inflammatory cells in the epicardium and
extending into the compact layer of the heart. The changes
in the compact layer can be multifocal or diffuse and typically
orient along small blood vessels. A few focal changes can
also be seen in the spongious layer.
3 Severe pathological changes characterized by intense
infiltration of inflammatory cells in the epicardium, extending
into the compact layer, typically with a diffuse distribution
pattern and involving the spongius layer in a multifocal
pattern. Degeneration and or necrosis of muscle fibers are
seen. Atrium can also be involved with inflammatory changes
comparable to what is seen in the ventricle.
The criteria listed above were of no use for the evaluation of the changes
seen in
CMS infected fish. A new score scheme was prepared and the scoring and grading
are given below.
CA 02634144 2008-06-27
23
Inflammation Pathological description
score for CMS
challenged
fish
0 No pathological changes observed.
1 Inflammatory changes are seen in the atrium primarily and
these appear as foci of degeneration and infiltration of
inflammatory cells. The foci are limited in number, 2-3,
limited in size and involve a few muscle fibres. A few foci,
similar to what is seen in the atrium, can also be observed in
the ventricle, typically in the spongious part.
2 Inflammatory changes are seen in the atrium and in the
ventricle and these appear as multiple foci of degeneration
and infiltration of inflammatory cells. The foci are more
numerous (>5 in the atrium and > 3 in the ventricle), involve
larger areas of the atrium and the ventricle and several
muscle fibres are involved in the changes observed.
3 Inflammatory changes are seen in the atrium and in the
ventricle and these appear at this stage as multiple larger
foci of degeneration and necrosis, accompanied with
infiltration of inflammatory cells, typically macrophages. The
foci are several in the atrium, in areas confluent and larger
areas of degeneration/necrosis are found in the ventricle,
and extend along fibres and can involve several fibres.
Results
HSMI challenged fish
The first histological changes typical of HSMI were seen by week 6 in injected
fish
(4 of 6 examined) with an average score of 0.25 ( 95% conf limits of 0.32)
indicating variation between fish. There was no increase seen by 7w and a
slight
CA 02634144 2008-06-27
24
decline by week 8. A marked increase in number of fish showing changes were
found by week 9 and the average score increased to 1,1 ( 95% conf limits of
0,5). The same average score was found by week 10.
Changes were also found in the red muscle, in 2 fish by week 7, no fish at 8w,
5
fish by week 9 and in 6 fish (of 7 sampled) at 10w. The changes in the red
muscle
tissue were mild, characterized by infiltration of inflammatory cells,
typically
around small vessel and in the intermuscular tissue accompanied by a few
degenerate muscle cells.
The findings are summarized in figure 1 of the present application.
Histological changes were observed in the cohabitant group (HSMI) by week 10,
and the average score in this group was 1,26 ( 95% conf limits of 0,32). One
fish
was also found with minor changes in the heart by week 8, but the remaining 5
fish at this time point were scored negative. The summary of the findings in
the
cohabitants at 6, 8 and 10 weeks (when sampling was performed) is given in
figure 2.
It is interesting to note that the average score for the cohabitant fish are
as high
as in the injected ones and fish also exhibited typical changes in red muscle
tissue, 1 of 6 by 6 weeks, and 3 of 8 by 10 weeks.
CMS challenged fish
The histological changes seen in the heart of fish challenged with CMS
inoculum
deviated markedly from the HSMI infected fish. The scoring scheme developed
for
the HSMI fish was of no use to assess the changes observed in this group and
for
this reason a new scoring scheme was established.
The first indications of pathological changes in the CMS group were seen at
6weeks post challenge, where 4 of 6 fish exhibited varying degree of
histonnorphological changes. In 4 of the fish, the atrium was included in the
specimens provided for assessment. By 7 weeks, all fish (8/8) had changes in
the
heart, and in only 2 fish the atrium was available for evaluation, meaning
that the
changes observed were found also in the ventricle. At 8 weeks, 8/8 fish were
positive (4 of 8 came with atrium present in the sections). By 9 and 10 weeks,
all
fish showed morphological changes, 4 of 4 had atrium present at 9 weeks, while
5
of 7 had atrium present at 10weeks. The findings are summarized in figure 3
and
below.
CA 02634144 2008-06-27
Note that the skeletal muscle is not included in the figures as no fish
infected with
the CMS inoculurn were found with changes in this organ, at any of the time
points examined.
Fish were found positive for typical CMS changes in the cohabitant group by 6
5 weeks, however with very low scores (2 of 6 fish were positive; 3 of 6 with
atrium
present in sections). By 8 weeks, 1 fish was found positive and by 10 weeks, 3
of
7 were found positive, at both time points the changes observed were minor
(below 0.5 on the visual analogue scale).
Representative histological sections of heart tissue from individuals infected
with
10 CMS and HSMI are presented in figures 5 and 6, respectively. As illustrated
in
figure 5, CMS infection primarily involves the atrium recognised as focal
inflammation with associated degeneration and necrosis of muscle cells. Focal
inflammation is also seen in the ventricle with degeneration and necrosis of
muscle cells and accompanying inflammatory changes. At later stages the
15 inflammatory changes also involve macrophages.
At early time points post infection HSMI infection (figure 6) involves
inflammatory
changes (lymphocytic) in the epicard. Changes are extending along vessels of
the
compact layer to involve larger parts over time (diffuse inflammation) while
changes in the atrium are infrequently seen.
The findings are summarised in figure 4 and in the comments/summary below.
Comments/summary
The findings in the study can be summarised as follows;
1) HSMI- and CMS-challenge result in two different categories/entities of
histological changes in the heart tissue:
a. HSMI changes are initially found in the epicardium of the ventricle,
develop in intensity and extend into underlying muscle tissue
involving the spongious part of the ventricle and extend also to the
atrium at late stages.
b. CMS changes are initially found in the atrium but can occur
simultaneously in the ventricle. The changes seem to develop more
rapidly though in the atrium. Epicardial changes (inflammatory
changes) are absent or insignificant in CMS challenged fish.
c. The characteristics of the inflammatory cells involved in the changes
observed are also different, typically lymphocytic in HSMI and
CA 02634144 2008-06-27
26
involving more macrophage-like cells in CMS, particularly at late
stages of disease.
2) The HSMIV and CMSV spread to other fish (cohabitants) over a lOweek
period, however the findings in this study are that HSMIV spread more
readily by cohabitation.
Example 2: Temperature stability and lipid solvent sensitivity test of HSMI
and
CMS cell culture supernatants.
Intention:
The aim was to determine the lipid solvent sensitivity and temperature
stability of
HSMI and CMS cell culture supernatants produced in GF-1 cells.
Materials:
Cell cultures and cell culture medium:
= Cell cultures: 25cm2 cell culture flasks with 4.4 x 104 GF-1 cells (p31)
per
CM2 .
= Growth medium: L-15 supplemented with 10 /0 FBS, 1% L-glutamine and
0.1% Gentamicin
Virus controls:
= Infectious Pancreas Necrosis Virus (IPNV)
= Pancreas Disease virus (PD)
= Relevant cell lines
Both virus controls were diluted in growth medium to a concentration of 8 x
105 viable virus per ml.
Chemicals: Chloroform MERCK 1.02445.1000, K35964445.
Test materials:
= HSMI supernatant: HSMI 1004.06, ALV-702 1p, 20.09.06.
= CMS supernatant: HSMI 1004.06, "CMS" 1p, 20.09.06.
CA 02634144 2008-06-27
27
Methods:
Chloroform sensitivity test
Chloroform was added to the two virus controls, the test materials and control
growth medium to a final concentration of 10% (v/v), and shaken for 30 minutes
at room temperature (ca 20 C). After centrifugation at 2000 x g for 10
minutes,
two cell cultures per sample were inoculated with 150p1 supernatant. The cell
cultures were incubated at 15 C for 19 days.
Temperature stability
400 pl aliquots of virus controls and test materials were heated for 30
minutes at
50, 60 and 70 C and then cooled immediately by inoculation of 150p1 sample
into
two cell cultures per sample. The cell cultures were incubated at 15 C for 19
days.
The same positive controls as for the chloroform test were used.
Results:
Both HSMI and CMS samples were inactivated with chloroform and 30 min
treatment at 70 C, whereas temperatures up to 60 C had no effect on the
development of CPE/vacuoles after inoculation of GF-1 cell cultures.
PD virus was inactivated both with chloroform and 30 min treatment at 50 C.
IPNV resisted chloroform and 30 min treatment at temperatures up to 70 C, but
the infection seemed to be slightly delayed at the highest temperatures.
Temperature stability and chloroform sensitivity of test material and control
virus.
Test Result / days after
inoculation
article
+ ctr 50 C 60 C 70 C Chloroform
(20 C)
HSMIV + 11 days + 11 days + 11 days - 19 days - 19 days
Pi Pi pi pi Pi
CMSV + 7 days + 7 days + 7 days - 19 days - 19 days
Pi Pi Pi Pi Pi
PD virus + 6 days - 19 days - 19 days - 19 days - 19 days
Pi Pi Pi Pi Pi
CA 02634144 2008-06-27
28
IPNV + 4 days + 4 days + 4 days + 6 days + 4 days
PI PI PI pi Pi
L-15 N/A N/A N/A N/A - 19 days
Pi
- Conclusions:
= Both HSMI and CMS samples were inactivated with chloroform and 30 min
treatment at 70 C, whereas temperatures up to 60 C had no effect on the
development of CPE/vacuoles after inoculation of GF-1 cell cultures.
= For HSMI virus, the results confirm the findings in the previous patent
application N020065765/W005121325.
Example 3: Sensitivity to DNA synthesis inhibitor
The purpose is to examine if the use of DNA synthesis inhibitor
bronnodeoxyuridine (BrdU) inhibits HSMIV and CMS infections in cell lines (GF-
1),
and thus indicate whether the viruses are RNA or DNA viruses.
BrdU is a synthetic thymidine analog that gets incorporated into a cell's DNA
when
the cell is dividing (during the S phase of the cell cycle).
Negative control: IPNV (double stranded RNA virus) infected BF-2 cells.
Positive control: Irido virus (DNA virus) infected BF-2 cells.
BrdU
No of flasks Virus Cells Temp
(ug/ml)
2 GF-1 15 C 0
2 GF-1 15 C 100
2 HSMIV GF-1 15 C , 0
2 HSMIV GE-1 15 C 100
2 CMS GF-1 15 C 0
2 CMS GF-1 15 C 100
2 BF-2 15 + 25 C 0
2 BF-2 15 +25 C 100
2 IPNV BF-2 15 C 0
2 IPNV BF-2 15 C 100
CA 02634144 2008-06-27
29
2 Irido(RSIV) BF-2 25 C 0
2 Irido(RSIV) BF-2 25 C 100
GE-1 cells, p30: 5 x 104 cells/cm2
BF-2 cells, p25: 8 x 104cells/cm2
Both cell lines were grown in L-15 supplemented with 10% FBS, 1% L-glutamine
and 0.1% Gentamicin in 25 cm2 flasks at 15 C or 25 C
Virus added pr 25 cm2 flask
IPNV: 400 PFU/flask.
HSMIV: HSMI 1004.06 ALV702 lp 20/9-06. 150 ul/flask.
CMS: Isolate 1004.06 "CMS" lp 20/9-06. 150 ul/flask.
Irido virus: diluted homogenate (1:1000) 90 ul/flask was added.
Results
Virus Cells Temp BrdU Day Day Day Day Day
4 7 10 14 21
- GF-1 15 C no - - -
- GE-1 15 C yes -
HSMIV GF-1 15 C no - + + ++ +++
HSMIV GF-1 15 C yes - + + + ++
CMS GF-1 15 C no - + + ++ +++
CMS = GF-1 15 C yes - + + + ++
- BF-2 15 + 25 C no - - - -
- BF-2 15 + 25 C no - - -
- BF-2 15 + 25 C yes - -
-
IPNV BF-2 15 C no ++ +++
+++ na na
IPNV BF-2 15 C yes ++ +++ +++ na na
Irido(RSIV) BF-2 25 C no - + +++ na na
Irido(RSIV) BF-2 25 C yes - - - - -
+=weak infection +++=fully developed infection
CA 02634144 2015-03-18
Conclusions / findings:
IPNV (RNA virus) infection was not inhibited by BrdU.
Irido (DNA virus) infection was inhibited by BrdU.
5 HSMI virus infection in early stages (up to 10 days) was not inhibited by
BrdU.
Beyond 14 days of incubation, more vacuoles were observed in flasks without
BrdU. This is possibly due to inhibited cell division when BrdU is present,
resulting
in inhibited virus propagation.
10 CMS virus infection in early stages (up to 10 days) was not inhibited by
BrdU.
Beyond 14 days, more vacuoles were observed in flasks without BrdU This is
possibly due to inhibited cell division when BrdU is present, resulting in
inhibited
virus propagation.
15 The DNA synthesis inhibitor BrdU also affected the cells. At late
timepoints BF-2
cells showed morphological changes due to the BrdU in the medium. GF-1 cells
also responended to the BrdU in the medium. GF-1 cells did not divide, but
grew
large and streched. Some cells had detached. This was observed in both
uninfected and infected cell cultures.
Example 4: pH stability test of HSMIV and CMSV infected cell culture
supernatants
Intention
The aim was to determine the pH stability of HSMI and CMS virus infected cell
culture supernatants produced on GF-1 cells.
Materials
Virus controls
= IPNV: ALPHARMA AS, IPNV control (095), (-80 C), 30.05.-03.
= PD virus: ALV-405, p6, 19.02.-07.
= Relevant cell lines
Both virus controls were diluted to 5 x 105 TCID50/m1 in L-15.
Test materials
= HSMI supernatant: HSMI 1004.06, ALV-702 1p, 20.09.06.
= CMS supernatant: HSMI 1004.06, "CMS" lp, 20.09.06.
CA 02634144 2008-06-27
31
= GF-1 cell cultures: 25cm2 cell culture flasks with 4.5 x 104 GF-1 cells
(p43)
per cm2
= Growth medium: L-15 (Sigma L-5520, lot 086K2414) with 10% FBS
(Invitrogen 10101-145, lot 3105387 S, 1% L-glutamine (Sigma G-7513,Iot
66K2435) and 0.1% Gentamicin (Sigma G-1397, lot 66K2433)
Methods
The pH in L-15 was adjusted to 5.0 and 3.0 by addition of 1.0M HCI, and the pH
adjusted L-15 solutions were sterile filtrated (0.20pm). 350p1 test substances
or
control virus were incubated in 5m1 of the respective L-15 solutions in
addition to
normal L-15 (pH 7.2) for 4 hours at 15 C. After incubation, the pH was
adjusted
to 7.2 with 1.0M NaOH before 2.5m1 of each solution were inoculated into two
parallel 25cm2 cell cultures in 5m1 growth medium. All flasks were incubated
at
C for three weeks and microscopically examined twice per week.
15 Results
Both HSMI and CMS virus were inactivated after incubation 4 hours with pH 3.0
at
15 C, whereas pH 5.0 had no effect compared with pH 7.2 on the development of
CPE/vacuoles after inoculation of GF-1 cell cultures.
PD virus was not completely inactivated at pH 3.0 or 5.0, but the infection
was
significantly delayed in flasks inoculated with pH 5.0 treated virus, and even
more
delayed in the flasks inoculated with the pH 3.0 treated virus.
IPNV was not affected by treatment al pH 3.0 or 5Ø
All negative flasks remained negative throughout the observation period.
Microscopic observation.
pH Flask Result / day of conclusive recording
Neg. ctr. PDV IPNV CMSV HSMIV
A - 21 d.pi. + 4 d.pi. + 4 d.pi. + 10 d.pi. +
10 d.pi.
7.2
- 21 d.pi. + 4 d.pi. + 4 d.pi. + 10 d.pi. + 10 d.pi.
A - 21 d.pi. + 7 d.pi. + 4 d.pi. + 10 d.pi. +
10 d.pi.
5.0
- 21 d.pi. + 7 d.pi. + 4 d.pi. + 10 d.pi. + 10 d.pi.
CA 02634144 2015-03-18
32
3.0 A - 21 d.pi. + 10 d.pi. + 4 d.pi. - 21 d.pi. -
21 d.pi.
3.0 B - 21 d.pi. + 13 d.pi, + 4 d.pi. - 21 d.pi. -
21 d.pi.
Conclusion
= Both HSMI and CMS virus were inactivated after a 4 hour treatment with
pH 3.0 at 15 C, whereas pH 5.0 had no effect on the development of the
cytopathogen effect (CPE) after inoculation of GF-1 cell cultures.
Example 5: Test of hemagglutination and hemabsorption, HSMI and CMS virus
A test for hemagglutination and hemabsorption was performed with HSMI and
CMS as a part of the characterization of the HSMI og CMS agents.
Hemagglutination:
Virus inoculates:
- HSMI Alv 702 1004.06
- Isolate "CMS" 1004.06
- Positive control: Infectious Salmon Anemia Virus (ISAV)
- Negative control: Supernatant from GF-1 cell culture
Atlantic salmon (SaImo salar ¨ appr. 1kg fish) red blood cells (RBC):
suspension
of washed (3 x in PBS) erythrocytes (0.5%) in PBS.
The test was performed in 96 well plates (Nunc 268152)
Final concentration RBC: 0.25%
Total final volume per well: 200p1
Incubated at 15 C for 1 hour.
Virus 1:1 1:2 1:4 1:8 1:16 1:32 1:64 Negative Negative
dilution control control
' 1 2 3 4 5 6 7 11 12
HSMI(p1) - A - - - ' - - - - - -
HSMI(p1) B - - - - - - - -
CMS(p1) C - - - - - - - -
CMS(p1) - - -
CA 02634144 2015-03-18
33
ISAV(p3) E + + +
ISAV(p3) F + + +
Neg.homog. G -
Neg.homog. H -
hemagglutination, - - no hemagglutination
Hemabsorption:
Infected cell cultures:
- HSMI p2 cultured in GF-1
- CMS p2 cultured in GF-1
- Negative control: Un-infected GE-1
Cytopathogen effect (CPE) was distinct in infected cell cultures.
Medium was removed and the cells were washed x 3 in PBS. Subsequently, 5 ml
RBC (0.5%) was added to the flasks and incubated for 1 hour at 15 C.
The flasks were inspected under a microscope, and no hemabsorption was
visible.
Example 6: Test of the CMS virus stock for contamination with other virus
The different cell lines were grown according to the literature and infected
with
100p1 CMS virus 1. passage stock. The cultures were observed for 28 days and
appearance of cytopathogen effect was recorded. If a cytopathogen effect was
observed, the culture was termed positive, if not the culture was termed
negative.
Tests in cells sensitive to viruses pathogenic for salmonids
Cell line ATCC Viruses the cell line is Result
Source number permissive for
BF-2 CCL-91 Infectious Pancreatic Necrosis Virus negative
Bluegill fry, Viral Haemorrhagic Syndrome Virus
caudal trunk Infectious Haematopoietic Necrosis
Virus (IHNV)
CA 02634144 2008-06-27
34
BB CCL-59 Infectious Pancreatic Necrosis negative
Brown bullhead, Virus (IPNV)
Connective Channel Catfish Virus (CCV)
tissue and
muscle
RTG-2, CCL-55 Infectious Pancreatic Necrosis negative
Rainbow trout, Virus (IPNV)
gonadal tissue Viral Haemorrhagic Syndrome
Virus VHSV)
Infectious Haematopoietic Necrosis
Virus (IHNV)
Spring Viremia of Carp Virus
(SVCV)
SHK-1, Infectious Salmon Anemia Virus negative
Salmon head
kidney
CHSE-214 CRL-1681 Infectious Pancreatic Necrosis negative
Chinook Virus (IPNV)
salmon, Pancreatic Disease Virus (PD)
embryo
CCO CRL-2772 Channel Catfish Virus (CCV) negative
Channel catfish,
ovary
CHH-1 CRL-1680 negative
Chum salmon,
heart fibroblast
FHM ECACC nr: Infectious Pancreatic Necrosis negative
Fat head - 88102401 Virus (IPN)
minnow Catfish Tumour Virus (CTV)
Epithelial like
GE-1 Heart and Skeletal Muscle positive
Grouper Infection Virus (HSMIV)
fin Cardiomyopathy Syndrome
CA 02634144 2015-03-18
Virus (CMSV)
- ______
The results indicate that the CMS virus stock which is provided herein is free
from
contaminating virus of any of the species: Infectious Pancreatic Necrosis
Virus
(IPNV), Viral Haemorrhagic Syndrome Virus VHSV), Infectious Haematopoietic
5 Necrosis Virus (IHNV), Channel Catfish Virus (CCV), Spring Viremia of Carp
Virus
(SVCV), Infectious Salmon Anemia Virus, Pancreatic Disease Virus (PD), Catfish
Tumour Virus (CT'!), The results further indicate that the virus has
phenotypic
characteristics which are different from the characteristics of any of these
viruses.
10 Example 7: PCR-analyses of the CMS virus stock using species specific
primers
Virus isolates:
4,5x10E04 GF-1 cells/cm2 was transferred to 2 x 175cm2 cell flasks and 1 x
75cm2
flask. Both 175cm2 flasks were inoculated with 500p1 homogenate from fish with
a
15 CMS diagnosis.
The CMSV positive flasks were freeze/thawed in 2 cycles 13 days post
infection. At
this point, the positive flasks showed severe CPE, while the negative control
did
not deviate from the normal morphology. The cell lysate was pulled through a
23g
20 and then a 25g syringe, before being sterile filtered (0,201Jm). The ysate
was
then dispensed in 4,5 ml tubes and frozen at -80 C.
Positive controls:
Infectious Pancreatic Necrosis Virus (IPNV) and Grouper Nervous Necrosis Virus
(GNNV) from cell culture, Salmonid Alphavirus 3 E2 gene, plasmid mini prep.
The
25 GNNV was used as a positive control for nodaviruses. The primers used in
the PCR
reaction will also recognise other nodaviruses like Viral Nervous Necrosis
virus
(VNNV) from cod.
RNA isolation: RNA was isolated using TriZol procedure in accordance with
protocol (Invitrogen).
RT-reaction:
The following reactions were set up:
- 6p1 RNA template (1,7pg CMSV RNA 202,74ng IPNV RNA or 753,18ng
GNNV RNA)
- lpl Random Hexamers (50ng/p1)
CA 02634144 2015-03-18
36
- 1p1 Annealing Buffer
The reaction were incubated at 65 C for 5min, and then on ice for at least a
minute.
To the reactions was then added:
- 10p1 2x First-Strand Reaction Mix
- 2p1 SuperScript' III/RNaseOUT Enzyme Mix
The reactions were then incubated at:
- 25 C - 10min
- 50 C - 50min
- 85 C - 5min
PCR reaction:
- 15p1G0TaqTm Master Mix
- 2x1p1 primer fwd/rev (25pM per primer)
- 5p1cDNA templat (from a 1:10 dilution)
- 2p1dH20
- 1p1 GoTaq pol. enzyme
The reactions went through the following temperature cycle:
- 95 C - 5min
- 95 C - 30sek
- 50 C - 30sek 30 cycles
- 72 C - 1min
- 72 C - 10
PCR setup and results:
PCR Temp Primer sequence Target PCR test
Tube late size result
(bp)
1 CMS CGTCACTTTCACCAGCGACTCCCAGACG 305 negative
(SEQ ID NO: 1)
GGATCCATTCGGATGTGGCGTTGCTATG
G (SEQ ID NO: 2)
2 CMS CTGCGGTGTAGACATCAAAG (SEQ ID 222 negative
NO: 3)
TGCAGTTCCTCGTCCATCC (SEQ ID NO:
CA 02634144 2008-06-27
37
4)
3 CMS GGATTTGGACGTGGGACCAA (SEQ ID 891 negative
NO: 5)
CGGATGACCCGGTTAGTTTTC (SEQ ID
NO: 6)
4 none CGTCACTTTCACCAGCGACTCCCAGACG 305 negative
(SEQ ID NO: 1)
GGATCCATTCGGATGTGGCGTTGCTATG
G (SEQ ID NO: 2)
none CTGCGGTGTAGACATCAAAG (SEQ ID 222 negative
NO: 3)
TGCAGTTCCTCGTCCATCC (SEQ ID NO:
4)
6 SAV3 CGTCACTTTCACCAGCGACTCCCAGACG 305 positive
(SEQ ID NO: 1)
GGATCCATTCGGATGTGGCGTTGCTATG
G (SEQ ID NO: 2)
7 IPNV CTGCGGTGTAGACATCAAAG (SEQ ID 222 positive
NO: 3)
TGCAGTTCCTCGTCCATCC (SEQ ID NO:
4)
8 GNNV GGATTTGGACGTGGGACCAA (SEQ ID 891 positive
NO: 5)
CGGATGACCCGGTTAGTTTTC (SEQ ID
NO: 6)
Conclusion: All CMS samples and the no template controls were negative in the
PCR-tests, while the positive controls were positive. The SAV specific primers
5 (SEQ ID Nos: 1 and 2) recognise all known types of salmonid alphavisru
(SAV),
the GNNV specific primers (SEQ ID Nos: 5 and 6) recognise most VNN, and the
IPNV specific primers recognise most of the IPNV serotypes. These tests
indicate
that the CMS virus isolates are not contaminated by IPNV, nodavirus or
salmonid
alphavirus. The results further suggest that the CMS virus does not have close
CA 02634144 2015-03-18
38
resemblance to any of these viruses and that it has genotypic characteristics
which are different from the genotypic characteristics of any of these
viruses.
Example 8: Electron micrograph
Cells were grown in slide flasks and infected with CMS virus according to the
invention. The cells were washed with PBS and fixed with 2.5% glutaraldehyde
in
0.1M sodium cacodylate buffer. They were further post-fixed with 2% osmium
tetroxide and 1.5% potassium ferrocyanide in 0.1M sodium cacodylate buffer,
followed by staining with 1.5% uranyl acetate in distilled water. Upon
dehydration
though increasing alcohol concentrations the specimens were embedded in Epon
TM
plastic resin. Ultrathin sections were cut and post-stained with 0.2% lead
citrate.
Sections were examined in a PhilipsTM CM 100 at 80 kv.
Results: Examination of the electron micrographs revealed the presence of
spherical virus-like particles structures having a diameter of approximately
70 nm.
Example 9: CMSV cultivation in cell factory (CF-1) and subsequent isolation of
virus
In order to achieve a high virus titer, putative CMSV was cultivated in GF-1
cells in a cell factory (CF-10 + CF-1). A CMS positive tissue homogenate was
used as inoculum.
Since isolation of CMSV after the 2nd passage has been difficult to identify
(no
CPE), a homogenate was chosen as inoculum in the cell factory (p1). Further
cultivation after the isolation (p2) procedure would then hopefully yield the
expected CPE in GF-1 cells (vacuolization).
Experimental and Results
Homogenate: The homogenate was made of 4 hearts, Atlantic salmon - Salmo
salar - diagnosed with CMS, each weighing approx. 10g. The salmons were all
localized to Vindsvik, Rogaland, Norway.
Bulbus arteriosus was removed and the remaining atrium and ventricle was cut
in
to smaller pieces with a sterile scalpel. The tissue was then crushed, in
combination with quartz sand and 80m1 L-15 media, using a pestle. This step
was
followed by centrifugation at 2000xg for 10min, and filtration of the
supernatant
(0,2 pm).
CA 02634144 2008-06-27
39
Growth in cell factory:
Odpi:
GF-1 cells were trypsinated and transferred to one CF-1 and one CF-10 in a
concentration of 4,5E4 cells/cm2. 9 ml of CMSV homogenate was added to the
cell
suspension upon transfer.
7dpi:
Examination revealed developing vacuoles and slightly toxic effect.
15dpi:
No changes.
37dpi:
At this point, CPE could be observed in almost all cells, and cell culture
supernatant was harvested from both CF-1 and CF-10 (approx. 2L).
Rate-zonal density gradient:
The supernatant was centrifuged at 17700xg in a JA-10 rotor for 30 min. The
pellet was re-suspended in 4 ml L-15 media and frozen at -80 C.
Refractive index of the supernatant was measured and found to be 1,3360, the
equivalent to a density of 1,0060g/cm3. This step was performed because the
media in a GF-1 cell culture tend to be fairly viscous.
The supernatant was spun at 100000xg for 3h in a JS 24.38 rotor, which was
capable of spinning a volume of 6x38m1 at a time. For every round in the
centrifuge the 6 pellets were re-suspended in a'total of 150p1 PBS and frozen
at -
80 C.
Sucrose density gradients:
- 6m136% sucrose in PBS w/v
- 6m142% sucrose in PBS w/v
- 6m148% sucrose in PBS w/v
- 6m154% sucrose in PBS w/v
- 6m160% sucrose in PBS w/v
After layering the different sucrose concentrations in the tube, it was
incubated at
4 C over night.
600p1 of pellet material (after ultra-centrifugation) was diluted to 5m1 in
PBS and
loaded on to the gradient, which subsequently was spun at 100000xg at 4 C for
16h. When the gradient was examined after run, a band was clearly visible in
the
CA 02634144 2008-06-27
denser part of the gradient. Also, two weaker bands were visible in the upper,
less
dense part of the gradient.
. Fractions were collected in 2 ml aliquots from the bottom of the tube, and
the
refractive index was measured in each fraction (Table 1).
5
Table 1:
Fraction Refractive Density CPE
index (g/cm3)
1 1,4132 1,2186 No
2 1,4113 1,2079 No
3 1,4073 1,1972 No
4 1,4015 1,1868 No
5 1,3964 1,1663 Yes
6 1,3925 1,1562 No
7 1,3899 1,1464 No
8 1,3876 1,1366 No
9 1,3859 1,1366 No
10 1,3841 1,1366 No
11 1,3822 1,1270 No
12 1,3806 1,1270 No
13 1,3788 1,1175 No
14 1,3724 1,1082 Yes
15 1,3588 1,0678 No
16 1,3474 1,0361 No
17 1,3434 1,0259 No
Each fraction was diluted to 35m1 PBS and then spun at 100000xg at 4 C for 3h.
10 Pelleted material was re-suspended in 150p1 PBS.
75p1 of each fraction was used as inoculums in respective 25cm2 cell flasks
with
4.5x10E04 GF-1 cells/cm2. Cells were incubated at 15 C and inspected
routinely.
The only flasks showing cells with CPE were the ones inoculated with fraction
5
and 14. The CPE was characterized by vacuolated cells, and was observed from 7
15 dpi with increasing intensity.
Conclusions/findings:
After removing most of the cellular debris from a cell culture supernatant (CF-
10,
with GF-1 inoculated with CMS positive material) by conventional
centrifugation,
high-speed centrifugation was applied in order to concentrate the viral load
in the
CA 02634144 2015-03-18
41
supernatant. After concentrating the material, a rate-zonal density gradient
centrifugation was performed. The resulting gradient showed two bands which,
in
a following cell culture experiment, gave a CPE with the usual characteristics
(both time and morphology) associated with CMS positive material in GF-1
cells.
Example 10: Preparation of antigen for vaccines and immunization of rabbits
CMS-material stock was prepared from homogenates of heart tissue from fish
diagnosed with CMS. The heart tissue (1,3 g) was homogenized in a mortar with
the addition of sand and was suspended in (3,2 ml) L-15 (Sigma L-5520, lot
64K22413) with 10% FBS (Sigma F-3885, lot 012K84132), 1% L-glutamine
(Sigma G-7513,Iot 24K2431) and 0.1% Gentamicin (Sigma G-1397, lot
113K2312). The resulting suspension was centrifuged for 10 minutes at 3000xg.
The supernatant was filtered through a 45 pm syringe filter (sartorius 17829)
and
was subsequently kept frozen at -80 C.
Antigen was prepared with the use of GE-1 cells (ATCC PTA-859) infected with
1st
passage stock of CMS-material stock. The cells were seeded at 4.5 x 104
cells/cm2
and were infected with 400 p.1 1st passage of CMS-material stock per 75 cm2.
The
cell cultures were incubated for 14-18 days at 15 C until a cytopathogen
effect
(CPE) was visible throughout the entire culture. The supernatant was removed
and collected in 50 ml vials and subsequently spun at 3000xg for 30 minutes,
The
supernatant was transferred into transparent centrifuge tubes and spun at
100000xg for two hours. The resulting pellet (A) was re-suspended in phosphate
buffered saline (PBS).
The cells were harvested by scraping with a rubber policeman and were
transferred to sample vials. The cells were then homogenised by passing the
material 5 times through a 21G syringe 5 times through a 23G syringe and,
finally, 4-5 times through a 25G. The homogenate was centrifuged for 30 min.
at
3000xg. The supernatant was transferred to centrifuge tubes (clear
transparent)
and was centrifuged for 2 hours at 100000 xg. The resulting pellet (B) was re-
suspended in PBS. The re-suspended pellets (A) and (B) were combined.
Inactivation was performed by adding formalin in relative amounts of 2000 ppm
(2 g/liter). The antigen was transferred to new tubes and incubated at room
temperature for 48 hours. Finally, the antigen was dialysed against PBS in a
cassette, cut off value being 10.000. PBS was changed three times, the antigen
being left overnight in the last change of PBS.
Protein Dilution mg/ml OD-
42
concentration Blank
was
determined
using the DC
protein assay
from BioRad:
Sample
CMS 4 0,767002 0,0305
16 1,056199 0,0105
CMS = 0.8 mg/ml
A water-in-oil fish vaccine was prepared containing a 1:5 dilution of the
antigen
prepared as described above per ml.
Clinical trial:
A clinical trial was run using ca 30 g Atlantic salmon post smolts. The trial
was run
in salt water at 12 C. Each fish was injected with a 0.1 ml dose of vaccine.
Six
weeks later the fish were challenge intramuscularly with 0.2 ml of 1st
passage.
Samples were collected at 6, 8, 10 and 12 weeks post challenge, and changes in
the atrium and ventricle of the heart was determined by histological analyses
using standard methods.
The results show that the fish vaccinated with the CMS vaccine is protected
against histopathological changes resembling CMS. Some fish become positive
for
histopathological changes when challenged with CMSV, but the changes disappear
10-12 weeks after challenge. This shows that a vaccine containing inactivated
CMS virus can protect against the development of CMS. There was no significant
reduction of histopathological changes in fish which had received a HSMI
vaccine
as compared to PBS controls.
The cardiomyopathy syndrome virus (CMS) has been deposited with the European
collection of cell cultures on March 29, 2007, and was granted accession
number
07032902.
CA 2634144 2017-07-27
43
References
1. Dannevig, B.H and Berg, T: Comp. Biochem.Physiol. Vol 82B, No 4, p683-688,
1985F.
2. Hodneland, K. et al.: Dis Aquat Organ. 2005 Sep 5;66(2):113-20 (Erratum in:
Dis Aquat Organ. 2005 Nov 9;67(1-2):181).
3. The aetiology and epidemiology of PD, HSMI and CMS in Scotland (Project
code: SARF015) November 2007, Report prepared for the Scottish Aquaculture
Research Forum by: Institute for Aquaculture, University of Stirling.
4. Kongtorp, R. T. et al.: Rapport. Cardiomyopathy syndrome (CMS): a
litterature
review, National Veterinary Institute, Norway, November 2005.
5. Jan Raa, 1996, Reviews in Fisheries Science 4(3): 229-228
6. Dannevig, B. H., et al.: Journal
CA 2634144 2017-07-27