Note: Descriptions are shown in the official language in which they were submitted.
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
BARIUM SULFATE PRODUCT
FIELD OF THE INVENTION
[0001] The present invention relates generally to a barium sulfate product and
to
processes for making the product from barite ore containing barium sulfate
crystals and gangue
materials. The barium sulfate product can be used in the production of paints,
rubbers and drilling
fluids for oil and gas exploration and is suitably of medical grade and can be
employed in various
other applications, including as a component of pharmaceutical, veterinary and
cosmetic.
compositions, particularly where a barium sulfate product having a "whiter"
appcarance is desircd.
BACKGROUND OF THE INVENTION
[0002] Barium sulfate is a radiopaque medium widely used as an X-ray contrast
agent in
medical examination of the gastrointestinal tract. Generally, for a
gastrointestinal examination,
the subject undergoing examination drinks a suspension of barium sulfate in
water, by itself, or in
combination with a carbon dioxide producing agent, such as BAROS effervescent
granules
(Mallinclcrodt, Saint Louis, MO). The barium sulfate used is a fine, white,
odorless, tasteless
bulky powder that is practically insoluble in water, in organic solvents and
in acidic and alkaline
solutions. It does, however, exhibit some solubility in hot concentrated
sulfuric acid. This
extremely inert quality of barium sulfate makes it ideal as a radiopaque
medium, as it is not
absorbable by an intact mucosa and is therefore considered safe to administer
for radiological use.
100031 One method used to produce medical grade barium sulfate, sometimes
referred to
as the Mallinckrodt process, involves dissolving finely divided barite ore
into solution by means
of an acid and then precipitating the barium ions from solution as barium
sulfate using sulfuric
acid. Because of the inertness of barium sulfate, this is a very difficult and
expensive procedure.
Often the barium sulfate precipitate formed still contains many impurities and
must be subjected
to a number of additional purification steps including acid leaching. In
addition, such
precipitation processes generally yield a barium sulfate product largely
comprised of particles of a
size much less than about 1 micron. For use as a contrast agent in
preparations for examination of
the gastrointestinal tract, particularly examination of the upper
gastrointestinal tract, it is often
preferred that medical barium sulfate particles be larger than those typically
produced by
precipitation techniques.
[0004] Stone, U.S. Patent No. 4,119,700, discloses an alternative process for
producing a
medical barium sulfate product from barite ore that does not require
dissolution for purification,
= 1
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
but instead isolates crystals of substantially pure barium sulfate present in
the ore. The process
includes grinding the naturally occurring barite ore containing barium sulfate
crystals and gangue
minerals to form a granular ore material, passing this granular ore through a
high intensity wet
magnetic separator to remove magnetic particles, and subjecting the non-
magnetic fraction
containing barium sulfate and low specific gravity minerals to gravity
separation to separate a
substantially pure barium sulfate fraction. The process further includes
subjecting this barium
sulfate fraction to further grinding to achieve the desired particle size
distribution (e.g., on the
order of about 1 micron), leaching the finely divided barium sulfate fraction
with a mineral acid
such as sulfuric or hydrochloric acid and washing the leached product with
water to neutralize the
leaching'acid.
[0005] Although the teachings of Stone are useful and address some of the
problems
attendant production of medical barium sulfate by precipitation techniques,
the product is not
always satisfactory. Sometimes barium sulfate produced in this fashion has a
less than desirable
off-white or gray coloration. The gray coloration is believed to be
attributable to impurities such
as silica and other persistent gangue materials from the barite ore.
Furthermore, during
examination and investigation of the gastrointestinal tract, medical barium
sulfate preparations are
typically suspended in water for ingestion by the patient. The persistent
gangue materials present
in the barium sulfate component of the preparation may separate and form an
undesirable off-
white or gray float material in the suspension to be ingested by the subject
undergoing
examination.
[00061 Accordingly, a need persists for more effective techniques of making a
medical
barium sulfate product from barite ore having improved appearance (e.g., a
more consistent
"whiter" color) and other properties that make it more suitable for use as an
X-ray contrast agent
in preparations for examination of the gastrointestinal tract as well as in
other applications
requiring a cleaner, whiter barium sulfate product.
SUMMARY OF THE INVENTION
[00071 Briefly, therefore, the present invention is directed to a process for
making a
barium sulfate product from barite ore containing barium sulfate particles and
gangue materials.
The process comprises mixing barium sulfate-containing particles obtained from
barite ore with a
fluidizing agent in a liquid medium to form a treated mixture comprising
barium sulfate-
containing particles and fluidized gangue materials released from the barium
sulfate-containing
2
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
particles. The fluidized gangue materials are separated from the treated
mixture and the barium
sulfate-containing particles are dried to produce the barium sulfate product.
[0008] In accordance with one prefen-ed embodinient, the process for making a
barium
sulfate p'roduct from barite ore containing barium sulfate particles and
gangue materials comprises
contacting barite ore particles with a leaching acid to leach acid-soluble
impurities and produce a
slurry comprising leached barium sulfate-containing particles having a reduced
concentration of
acid-soluble impurities followed by washing the leached barium sulfate-
containing particles with
water. The washed barium sulfate-containing particles are mixed with a
fluidizing agent in a
liquid medium to form a treated mixture comprising barium sulfate-containing
particles and
fluidized gangue materials released from the barium sulfate-containing
particles. The fluidized
gangue materials are separated from the treated mixture and the barium sulfate-
containing
particles are dried to produce the barium sulfate product.
[0009] Other features of this invention will be in part apparent and in part
pointed out
hereinafter.
BRIEF DESCRIPTION OF THE DRAWING
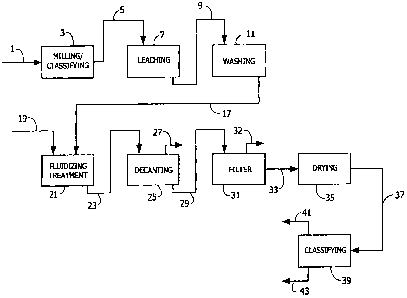
[0010] Fig. 1 is a schematic diagram of a process for making a barium sulfate
product
from baritc ore containing barium sulfate and gangue materials in accordance
with the present
invention.
DETAILED DESCRIPTION OF THE INVENTION
[0011.] In accordance witli the present invention, improved processes for
producing
barium sulfate products, which are particularly useful as X-ray contrast
agents in gastrointestinal
tract examinations, have been devised. More specifically, the use of
particular fluidizing agents or
fluidizers has been discovered as a useful means of reducing the concentration
of undesired
gangue materials in medical grade barium sulfate products obtained from
naturally occurring
barite ore that may otherwise undermine the appearance and other desired
properties of the
product.
[0012] The use of a fluidizing agent in accordance with the present invention
is readily
integrated into known processes for the production of barium sulfate products
from barite ore that
do not require dissolution and precipitation for purification of the barium
sulfate product, but
instead isolate substantially pure barium sulfate crystals present in the
barite ore. Such processes,
including that described by Stone in U.S. Patent No. 4,119,700, the entire
disclosure of which is
3
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
incorporated herein by reference, are generally known in the art and typically
include milling (e.g.,
crushing and grinding) the barite ore, classifying the milled barium sulfate-
containing particles to
the desired particle size, contacting the barium sulfate-containing particles
with an acid to leach
and remove acid-soluble impurities and washing and drying the barium sulfate
product. In the
practice of the present invention, these process operations may be conducted
in a batch, semi-
continuous, or continuous mode. The operations may be suitably carried out
using a variety of
apparatus and process techniques well-known to those skilled in the art and in
some instances may
be omitted or combined with other operations without departing from the scope
of the present
invention.
[0013] Generally, the process for isolating substantially pure barium sulfate
crystals
present in the barite ore is modifed in accordance with the present invention
by mixing barium
sulfate-containing particles obtained directly from barite ore (i.e., without
requiring precipitation
of barium sulfate) with a fluidizing agent in a liquid medium to form a
treated mixture comprising
barium sulfate-containing particles and gangue materials fluidized or released
from the barium
sulfate-containing particles. The fluidized gangue materials are typically
present in the treated
mixture as part of a float layer. The fluidized gangue materials are separated
from the treated
mixture and thereafter the barium sulfate-containing particles are dried to
produce the barium
sulfate product. As will be described in greater detail below, treating barium
sulfate-containing
particles obtained.from barite ore with a fluidizer in a liquid medium to
release and separate color-
forming gangue materials may be integrated in various ways into a process for
isolating
substantially pure barium sulfate crystals present in the barite ore.
[0014] In addition to crystals of substantially pure barium sulfate, naturally
occurring
barite ore or barytes typically contains many gangue materials, such as large
pieces of iron oxides
or hydrated iron oxides, iron carbonate, quartz or silica crystals along with
aluminum and
chromium compound impuritics. The iron compounds and the silica may sometimes
be associated
together (i.e., as nodules or relatively large size pieces of iron silicates).
The types of gangue
materials and their proportions can vary greatly in relation to the barium
sulfatc content of the ore,
it being appreciated that the lower the amount of barium sulfate present in
the ore, the greater
amount of ore that will have to be processed to obtain a given amount of
barium sulfate product.
An exemplary barite ore analysis given by Stone in U.S. Patent No. 4,119,700
revealed the
following components: BaSO4 74.4%; FeCO3 18.6%; Si02 5.6%; and other
miscellaneous
gangue materials 1.4%. Generally the oxide and carbonate based impurities
present in the ore are
removed by acid treatment leaving behind non-acid soluble gangue materials.
Regardless of the
4
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
exact barite ore composition, it is the silica and other gangue materials
present in the ore that
might otherwise persist in the barium sulfate product and are believed to
cause unwanted
coloration of the product and the undesirable float material in barium sulfate
suspensions
administered for gastrointestinal tract examinations.
[00151 The fluidizing agent combined with the barium sulfate-containing
particles
obtained from the barite ore is selected so as to be capable of fluidizing or
releasing at least a
portion of the gangue materials present and should otherwise be relatively
inert and non-harmful
should it persist to any significant extent in the barium sulfate product
intended for ingestion as
part of a gastrointestinal tract examination preparation. The fluidizing agent
may be in solid or
liquid forn-- (e.g., dissolved in solution) and is dispersible or at least
partially soluble, preferably
substantially soluble, in the liquid medium in which the barium sulfate-
containing particles are
treated with the fluidizing agent. Preferably, an aqueous liquid medium
comprising water is
employed.
[0016] In accordance with the present invention, various fluidizing agents
have been
identified that effectively reduce the concentration of gangue materials in
barium sulfate-
containing particles obtained from barite ore, particularly silica impurities.
These suitable
fluidizing agents include natural gums, synthetic and semi-synthetic gums or
polymers,
biopolymers, chelating agents, salts of polyacid monomers, inorganic salts,
and mixtures thereof.
[00171 Polymeric fluidizers include natural gums, synthetic and semi-synthetic
gums or
polymers and biopolymers. Examples of suitable natural gums include
carrageenan, alginate,
arabic gum, arabic gum treated with NaOH, pectin, and the like, and mixtures
thereof. Examples
of suitable synthetic and semi-synthetic gums or polymers include low
viscosity
carboxymethylcelluloses, copolymers of ethylene and either maleic anhydride or
maleic acid, such
as EMA 31, EMA 31 Na+, EMA 21, EMA 21 Na+, copolymers of methyl vinyl ether
and maleic
anhydride such as GANTREZ Na+ (GAF Corporation, New York, NY), and the like,
and
mixtures thereof. Examples of suitable biopolymers include heparin,
chondroitin sulfate, and the
like, and.mixtures thereof.
[00181 Other fluidizing agents include chelating agents, salts of polyacid
monomers
(e.g., salts of polycarboxylates), and inorganic salts. As used herein,
polyacid monomers are
polycarboxylic acid compounds having 2 to 5 -COOH groups. As used herein,
salts are
phannaceutically acceptable salts. Suitable pharmaceutically acceptable salts
include alkali metals
(e.g., sodium and potassium) alkaline earth metals (e.g., calcium and
magnesium) and organic
bases (e.g. meglumine). Examples of suitable chelating agents include salts of
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
polyaminocarboxylic acids, such as ethylenediaminetetraacetic acid (EDTA),
cyclohexane-trans-
I,2-diaminetetraacetic acid (CDTA), diethylenetriaminepentaacetic acid, and
the like, other
chelating agents such as lactic acid, N-p-ethanolamine-N,N-diacetic acid
(e.g., disodium N-(3-
ethanolamine-N,N-diacetate), and the like, and mixtures thereof. Examples of
suitable salts of
polyacid monomers include alkali metal and alkaline earth metal salts of
citric acid (e.g., sodium
citrate), nitrilotriacetic acid (e.g., trisodium nitrilotriacetate), nitrilo-
trimethylene triphosphoric
acid (e.g., trisodium nitrilo-trimethylene triphosphite), inositol
hexaphosphoric acid, N-[3-
ethanolamine-N,N-diacetic acid (e.g., disodium N-[3-ethanolamine-N,N-
diacetate), and the like,
and mixtures thereof. Suitable inorganic salts include, for example, sodium
hexametaphosphate,
sodium pyrophosphate, sodium tripolyphosphate, and the like, and mixtures
thereof. In
accordance with one preferred embodiment, the fluidizing agent comprises an
alkali metal or
alkaline earth metal polycarboxylate salt. Suitable polycarboxylate salts
include salts of
dicarboxylic acids such as oxalic acid, malonic acid, succinic acid, glutaric
acid, adipic acid, and
the like, and mixtures thereof, as well as salts of tricarboxylic acids,
including, but not limited to,
citric acid. Preferably, the polycarboxylate salt includes a hydroxyl
substituent. Preferred
polycarboxylate salts having a hydroxyl group include salts of citric acid,
isocitric acid, tartaric
acid, and malic acid.
[00191 Examples of particularly suitable fluidizing agents include EMA 21 Na+,
GANTREZ Na+, arabic gum treated with NaOH, sodium citrate, trisodium
nitrilotriacetate,
trisodium nitrilo-trimethylene triphosphite, sodium hexametaphosphate, sodium
pyrophosphate,
sodium tripolyphosphate, and inositol hexaphosphorate.
[0020] In one preferred embodiment, the fluidizing agent comprises sodium
pyrophosphate.
[00211 In accordance with an especially preferred embodiment, the fluidizing
agent
comprises sodium citrate. Sodium citrate has been found to be especially
effective in fluidizing or
releasing gangue materials, particularly silica, from barium sulfate-
containing particles obtained
from barite ore. Sodium citrate is also often included as a component of
conventional
gastrointestinal tract examination preparations to produce a free-flowing X-
ray contrast
composition. Accordingly, the persistence of some amount of the sodium citrate
used as a
fluidizer in the barium sulfate product may provide an additional benefit.
[0022] For purpose of illustration of some embodiments of the present
invention, a
process of making a barium sulfate product from barite ore containing barium
sulfate and gangue
materials will be described with reference to Fig. 1. Fig. 1 is a schematic
diagram of a process for
6
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
making a barium sulfate product from barite ore including using a fluidizing
agent to reduce the
concentration of undesired gangue matcrials. In the embodiment shown in Fig.
1, barium sulfate-
containing particles are treated with a fluidizing agent in a liquid medium
after the particles are
subjected to an acid leaching operation and following neutralization of the
leached material.
[0023] Barite ore 1 containing barium sulfate and gangue materials, as
obtained from the
mine, is subjected to a milling and classifying operation 3 to attain barium
sulfate-containing ore
particles 5 of the desired size distribution. The barite ore can, for example,
be subjected to an initial
crushing with care being taken to keep the production of fine particles to a
minimum. This can be
done by means of a jaw crusher followed by a gyratory crusher or other means
known in the art.
These coarse ore particles may then subjected to grinding, for instance in a
ball mill, rod mill or
hammer mill, to further reduce their size and produce a granular ore, again
with proper care taken to
avoid excessive production of fines. After the particles are crushed and
ground, the particles are then
classified by size to separate the fine particles and over-sized particles
from the barium sulfate-
containing ore particles of thc dcsircd size. Generally, the barium sulfate-
containing ore particles
subjected to further processing have a particle size distribution that
provides efficient acid leaching
and allows recovery of a final barium sulfate product having the desired
particle size distribution.
However, because the final barium sulfate product is typically subjected to a
further milling and
classifying operation, the particle size distribution of the barium sulfate-
containing ore particles
subjected to further processing is not narrowly critical.
[0024] The milled and classified barium sulfate-containing ore particles 5 are
next
subjected to an acid leaching operation 7 to reduce the concentration of any
acid-soluble gangue
materials or impurities, such as acid-soluble barium salts and oxides of iron
and/or manganese, in
order to meet applicablc USP specifications. The acid leaching operation
generally comprises
contacting the barium sulfate-containing ore particles with a leaching acid in
a suitable vessel(s) to
leach acid-soluble impurities from the particles and produce a slurry 9
comprising leached barium
sulfate-containing particles having a reduced concentration of acid-soluble
impurities.
[0025] Preferably, the leaching acid comprises a mineral acid. Examples of
suitable
mineral acids are sulfuric acid and hydrochloric acid. The strength or
concentration of the
leaching acid used in the leaching operation may vary depending on the
composition of the
incoming barite ore and the desired composition of the final barium sulfate
product. Generally,
higher concentrations of leaching acid are required as the concentration of
acid-soluble impurities
in the barite ore and/or the desired purity of the barium sulfate product with
respect to acid-soluble
impuritics increase. Typically, suitable results are obtained in the leaching
operation by
7
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
contacting the barium sulfate-containing ore particles with sulfuric acid or
hydrochloric acid at a
concentration sufficient to produce a leaching mixture or slurry having a pH
of less than about 3,
more preferably less than about 2.
[0026] The leaching operation may be carried out in a single stage leaching
system in
which a slurry comprising barium sulfate-containing ore particles and leaching
acid is mixed in a
slowly rotating drum. The leaching drum may be adapted to tilt at an angle to
drain off or decant
and recover the leaching acid after completion of the leaching operation. To
reduce acid
consumption, the leaching operation can be carried out in multiple stages, for
example, a counter-
current three-stage leaching operation. In an example of such an operation,
the barium sulfate-
containing ore particles pass from the first-stage through the sccond and
third-stage and are
contacted with leaching acid of increasing concentration. That is, fresh
leaching acid is
introduced into the final or third-stage and is recovered and passed
countercurrent to the barium
sulfate-containing ore particles through the second and then first-stages.
[0027] The resulting slurry 9 of leached barium sulfate-containing particles
is washed with
water to at least partially neutralize the leaching acid retained in the
slurry. The wash water can be tap
water or purified water, such as distilled or deionized, water. As illustrated
in Fig. 1, the slurry of
leached barium sulfate-containing particles 9 is washed with water in a
washing operation 11 to
produce a washed slurry 17 comprising leached barium sulfate-containing
particles. Like the leaching
operation, washing of the leached slurry can be conducted in one or several
stages until the desired pH
is attained. If washing is conducted in multiple stages, different purity wash
water may optionally be
used in different stages. For example, the leached barium sulfate-containing
particles may be washed
with water in the drum or other vessel used in the leaching operation by
introducing wash water into
the vessel and allowing the water and acid mixture to overflow from the drum.
At completion of the
washing operation, the vessel can be tilted at an angle to drain off or decant
the wash water and
recover the leached and washed barium sulfate-containing particles.
Alternatively, the slurry of
leached barium sulfate-containing particles may be transferred to a scparatc
vessel for the washing
operation. Regardless of how the washing operation is conducted, washing is
preferably continued to
substantially neutralize and increase the pH of the leached material to at
least about 6, more preferably
to from about 6 to about 7.
[0028] In accordance with the embodiment of the present invention illustrated
in Fig. 1,
the washed barium sulfate-containing particles of slurry 17 are subjected to a
fluidizing or
peptizing treatment 21 in which the particles are mixed with an appropriate
quantity of fluidizing
agent 19'in a liquid medium to fluidize or release gangue materials from the
barium sulfate-
8
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
containing particles. The particular construction and configuration of the
equipment used to mix
the washed barium sulfate-containing particles and the fluidizing agent is not
critical in the
practice of the present invention. The apparatus used may comprise a suitable
vessel, preferably
equipped with an agitation device (e.g., a stirred tank). Preferably, an
aqueous liquid medium
comprising water is utilizcd in the fluidizing treatment.
[00291 In general, the amount of fluidizing agent added to the barium sulfate-
containing
particles varies depending on the composition of the incoming barite ore used,
the desired purity
of the barium sulfate product, and the concentra.tion of the barium sulfate-
containing particles in
the treated mixture. Generally, as the concentration of gangue materials in
the barite ore
increases, more fluidizing agent may be required. Similarly, if a lower
concentration of gangue
materials is required in the barium sulfate product, it may be necessary to
use more fluidizing
agent. Although higher or lower concentrations may be employed, particularly
depending on the
effectiveness of the specific fluidizing agent used, suitable results are
generally achieved when the
concentration of the barium sulfate-containing particles is from about 15% to
about 65% by
weight in the treated mixture and the fluidizing agent used to treat the
barium sulfatc-containing
particles is added in a proportion of at least about 0.03% by weight of the
treatcd mixture,
preferably from about 0.05% to about 5% by weight, more preferably from at
least about 0.05% to
about 0.5% by weight.
[00301 The liquid medium in which the washed barium sulfate-containing
particles are
mixed with the fluidizing agent is suitably maintained at typical
temperatures, for example, from
at least about 5 C to about 50 C, and preferably at ambient temperatures of
from at least about
15 C to about 30 C. The fluidizing treatment is allowed to proceed for a time
sufficient to
thoroughly mix the barium sulfate-containing particles and the fluidizing
agent in the liquid
medium and to allow the fluidizing agent to release at least a portion of the
gangue materials
present in the particles to be treated. Typically, the mixture of the barium
sulfate-containing
particles and the fluidizing agent is contacted for a period sufficient to
achieve the desired results,
such as,'for example, for a period of at least about 15 minutes, and
preferably at least about 60
minutes.
[00311 The fluidizing treatment produces a treated mixture 23 comprising
barium
sulfate-containing particles and fluidized gangue materials released from the
barium sulfate-
containing particles. The fluidized gangue materials include primarily silica,
and other optional
iinpurities such as aluminum, chromium, and iron compounds. Upon conclusion of
the fluidizing
treatment, the fluidized gangue materials are separated from the treated
mixture. The equipment
9
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
and techniques used to separate the fluidized gangue materials from the
treated mixture can be any
conventional cquipment or technique known in the art, and one skilled in the
art would be readily
able to select a separation operation appropriate in view of overall process
considerations. For
example, the fluidized gangue materials may be suitably separated from the
treated mixture by
decantation, filtration, centrifugation or even a combination of such
operations. Typically, at least
a portion of the gangue materials released from the barium sulfate-containing
particles utilizing
fluidizing agents in accordance with the present invention are present in a
float layer in the treated
mixture. Accordingly, separation of the fluidized ganguc materials from the
treated mixture often
includes decantation of the float layer as a viable means of separating at
least a portion of the
fluidized gangue materials.
[0032] In the embodiment shown in Fig. 1, the treated mixture 23 comprising
barium
sulfate-containing particles and fluidized gangue materials is first subjected
to a decantation
operation 25 to remove such a float layer 27 from the treated mixture and form
a decanted treated
mixture 29 that is then subjected to a filtration operation 31 to further
remove fluidized gangue
materials and other impurities. Decantation of the float layer containing
gangue materials released
from the barium sulfate-containing particles prior to filtration of the
decanted treated mixture
reduces the risk of premature blinding of the filtration device.
[0033] The particular construction and configuration of the equipment used to
decant the
float layer 27 from the treated mixture 23 is not critical in the practice of
the present invention.
For example, the equipment used may comprise a suitable vessel adapted to tilt
at an angle to pour
off the float layer or the vessel may be provided with a decantation port at a
suitable elevation
through which the float layer may be drawn off from the treated mixture. Still
further, decantation
of the float layer may be achieved in a vessel provided with suitable vacuum
means. The
decantation operation may be carried out in the same vessel in which the
fluidizing agent is mixed
with the barium sulfate-containing particles. In another embodiment, the
treated mixture 23 with
the float material may be transferred to another vessel in which the float
material is decanted from
the treated mixture. The decanting step may optionally be conducted in either
a single stage or
multiple stages. The water used in the fluidizing treatment and decanting
steps may be tap water
or purified water, such as distilled or deionized, water. In a multistage
process, different purity
water may be used in different stages, e.g. the final stage may optionally use
purified water.
[0034] In the embodiment shown in Fig. 1, the decanted treated mixture 29 is
subjected
to a filtration operation 31 to further remove any additional impurities and
produce a filtrate or
dryer feed stream 33 comprising barium sulfate-containing particles. The
filtration device is
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
constructed and configured to retain undesirable impurities larger than the
barium sulfate-
containing particles. In one embodiment, the decanted treated mixture may be
filtered through a
mesh filter such as a 325 mesh U.S. Standard Sieve Series screen or similar
device.
[00351 After separation of the fluidized gangue materials from the treated
mixture 23, the
barium sulfate-containing particles in the dryer feed stream 33 are subjected
to a drying operation
35 to produce a dried barium sulfate product 37, typically having a moisture
content of no greater
than about 2% by weight and containing particles ranging in size of from about
0.5 m to about
15 m, although smaller and larger particles may also be present. The
particular construction and
configuration of the equipment used for drying the barium sulfate-containing
particles is not
critical in the practice of the present invention and may comprise a steam
tube dryer or other
suitable industrial drying device.
100361 Once dried, the barium sulfate product 37 may be subjected to a further
milling
and classification operation 39 to separate and obtain one or more barium
sulfate products 41 and
43 having the desired particle size distribution. Alternately, the drying,
grinding, and classifying
operations may be performed simultaneously. In one embodiment, medical barium
sulfate product
particles intended for use in a pharmaceutical gastrointestinal examination
formulation have a
mean particle size of at least about 3 m, preferably from about 3 p.m to
about 4.5 m. In another
embodiment, medical barium sulfate product particles have a mean particle size
from about 8 m
to about 1 1 m.
100371 Although the process for making a barium sulfate product from barite
ore
illustrated in Fig. I shows a preferred embodiment including treatment of the
barium sulfate-
containing particles with a fluidizing agent after the acid leaching operation
and water washing to
neutralize the leached material, it should be recognized that the fluidizer
treatment may be
integrated into the process in other ways without departing from the scope of
the present
invention. The pH during the fluidizing treatment may impact the ability if
the fluidizing agent
to release gangue materials from the barium sulfate-containing particles. If
the fluidizing
treatment occurs prior to complete neutralization of the leached material, it
is preferable to choose
a fluidizer that is not susceptible to substantially complete protonation at
the given pH. For
example, in such an embodiment, a fluidizing agent comprising an alkali metal
or alkaline earth
metal polycarboxylate salt may be less preferred. Furthermore, a conventional
process for
producing a barium sulfate product from barite ore may be modified in
accordance with the
present invention by subjecting the dried product to a fluidizing treatment
including mixing
particles -of the barium sulfate product with a fluidizing agent in a liquid
medium (e.g., water) and
I1
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
thereafter separating fluidized gangue materials from the treated mixture and
drying the treated
product. However, this latter alternative embodiment is somewhat less
preferred as it would
require an additional drying operation and increase the overall operating cost
of the process.
.(00381 Moreover, it should be understood that the treatment of barium sulfate-
containing
particles with a fluidizer may be carried out multiple times (e.g., serially)
within the process
scheme to enhance removal of persistent gangue materials from the barium
sulfate product. For
example, a first treated mixture may be prepared by first mixing the barium
sulfate-containing
particles with a fluidizing agent and then mixing the treated barium sulfate-
containing particles
obtained with a second fluidizing agent to form a second treated mixture
comprising barium
sulfate-containing particles and additional fluidized gangue materials.
Fluidized gangue materials
(e.g., float layer) may be separated from the first treated mixture prior to
contacting the treated
barium sulfate-containing particles contained therein with the second
fluidizing agent and/or
fluidized gangue materials may be separated from the second treated mixture.
The fluidizing
agents used in each of the fluidizing treatments may be the same, or different
fluidizing agents
may be employed in the fluidizing treatments.
100391 The barium sulfate product produced in accordance with the present
invention is
suitable for use in the production of paints, rubbers and drilling fluids for
oil and gas exploration,
but is particularly suited for use as a radiopaque X-ray contrast agent in
preparations administered
to patients undergoing medical examination of the gastrointestinal tract. The
barium sulfate
product of the present invention may be used in liquid formulations as well as
in dry formulations
that are constituted (e.g., suspended in water) prior to ingestion by the
patient. The quality and
particle size exhibited by the barium sulfate product of the present invention
has becn found to be
particularly useful in medical examinations of the upper gastrointestinal
tract because it provides
uniform coating of the gastrointestinal tract and the proper opacification
during X-ray
examination. In addition, the barium sulfate product of the present invention
has a desirable
substantially white coloration and is less susceptible to the formation of an
off-white or gray float
when suspended in water to form a barium sulfate suspension for ingestion by a
patient
undergoing gastrointestinal examination. This latter characteristic is
particularly advantageous in
gastrointestinal tract examination formulations that do not contain a
viscosity modifier to keep the
barium sulfate particles in suspension. Dry formulations typically do not
contain a viscosity
modifier. In the absence of a viscosity modifier, the appearance and severity
of the gray float is
often more pronounced upon suspension of a dry formulation in water.
Accordingly, the barium
12
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
sulfate product of the present invention is particularly useful as a
radiopaque X-ray contrast agent
in dry gastrointestinal tract examination formulations.
[0040] In addition to the barium sulfate product described herein,
pharmaceutical
preparations for use in gastrointestinal examinations may include other
additives known to those
skilled in the art to improve suspension properties, mucosal coating adhesion,
film thickness
propcrties and patient acceptance and tolerance. These include, for example,
stabilizing and
suspending agents to prevent settling, viscosity modifiers (e.g., certain
gums), fluidizing agents
such as sodium citrate and sodium tripolyphosphate, sweeteners, such as
sucrose and sorbitol, and
flavoring agents to improve palatability, water-soluble salts to prevent
foaming, and preservatives
and antiliacterial agents to extend product shelf-life.
[0041] In one embodiment, the barium sulfate product prepared in accordance
with the
present invention has a substantially reduced concentration of certain gangue
materials,
particularly silica impurities, which might otherwise persist and undermine
the desired appearance
(e.g., color) properties of the product. The quantity of undesired, color-
forming gangue materials
removed in accordance with the present invention relative to the incoming
baritc ore will vary
depending upon the composition of the ore, the selection of the fluidizing
agent and the manner in
which the fluidizing treatment(s) are carricd out. For example, by practicing
the techniques
described above, it is possible to remove at least about 80% by weight of the
silica and other
gangue materials from the barium sulfate starting material, more preferably at
least about 90%.
[0042] The following examples are simply intended to further illustrate and
explain the
present invention. The invention, therefore, should not be limited to any of
the details in these
examples.
EXAMPLE 1 Elemental Analysis of Raw Barite Ore, Barium Sulfate Product and
"Gray
Float"
[00431 Raw barite ore was processed conventionally (e.g., without fluidizer
treatment) to
yield a barium sulfate product. During processing, samples of washing liquid
and waste liquid
were collected. To determine the elemental components as well as relative
levels of each element
in the raw barite ore, barium sulfate product, washing liquid, waste liquid,
and "gray float",
samples derived from each were subjected to spectrographic analysis using
qualitative DC arc
emission techniques.
13
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
[0044] The results of the spectrographic analysis are shown in Table I. In
Table I,
measured levels of each element are defined qualitatively using abbreviations
defined below in
Table II.
[0045] In Table I, the sample denoted "Wash Water" was derived from a
collection of
wash liquid samples from the above-described production process. The collected
samples were
allowed to settle and formed a mixture comprising a clear liquid and solid
white powder. "Waste
Stream" denotes a sample derived from a collection of waste liquid from the
acid wash tank of the
above-described production process that contained large gray chunks of solid
material. The wet
insoluble powder and gray chunks were isolated from the collected wash liquid
and waste liquid
samples,-respectively, and dried. Samples denoted "Float #1" and "Float #2" in
Table I are "gray
float" samples comprising isolated gray float produced upon suspending pilot
formulations
containing the barium sulfate product in water. After suspension in water, the
gray float material
was isolated and allowed to settle to forrn a mixture comprising an insoluble
powder. The wet
insoluble powder was similarly removed and dried.
[0046] The four dried samples, as well as the raw barite ore and the barium
sulfate
product were subjected to analysis using DC arc emission techniques. Specimens
of each were
added to #44UC graphite electrodes. Blank electrodes were prepared in much the
same manner
for comparison. The blank electrodes and specimen-containing electrodes were
burned and
emission spectra collected using a Thermo Jarrell Ash AtomComp 2000 DC Arc
Spectrometer,
which utilizes a Charge Injection Device (CID) detector. The instrumental
parameters were as
follows: Gas Flush - 3.00 s (sheath gas 30% 02 in Argon); Preburn - 0.00 s;
Arc Current
Sequence - 25.0 s at 15 Amps; and Total Integration Time - 25.0 s.
14
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
Table I
Qualitative Elemental Analysis of Barite Ore, Barium Sulfate Product and Other
Samples
Element Float #1 Float #2 Wash Waste Raw Barium
Water Stream Barite Ore Sulfate
Product
Al W-M W-M W-M S M W-M
B VF VF -- -- -- --
Ba P P P P P P
Ca M-S M-S M-S M-S M-S M-S
Cr VF F VF M -- --
Cu -- VF VF F F --
Fe W W W M-S M-S W
K F F F F F VF
Mg M-S S M-S V S V S M
Mn -- -- - F W
Na W-M M W-M W-M W W
P W-M -- -- -- -- --
-- --
Pb - -- -- X (very
faint
Si M-S M-S M-S S S M-S
Sn -- -- -- F -- --
Sr S S S S S S
Ti VF F -- F VF --
Table II
Abbreviations Designating Relative Levels of Elements in Samples of Table I
P Primary
VS Very Strong
S Strong
M-S Moderate to Strong
M Moderate
W-M Weak to Moderate
W Weak
F Faint
VF Very Faint
X Identification Uncertain
-- Element not detected
[00471 Elements not detected in any of these samples included: Ag, As, Au, Be,
Bi, Cd,
Co, Cs, Gd, Hg, Li, Mo, Ni, Pd, Pt, Rb, Sb, Ta, Tl, V, W, and Zn.
[0048] Based on the qualitative analysis presented in Table I, conventional
processing of
the barite ore resulted in the reduction, and in some cases the elimination,
of many of the impurity
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
elements in the barium sulfate product. However, substantial impurity levels,
especially of silicon
and calcium, persist in the barium sulfate product.
[0049] These results merited further quantitative investigation. The raw
barite ore,
barium sulfate product and the gray chunks isolated from the waste stream were
analyzed by
inductively coupled plasma-atomic emission spectroscopy (ICP-AES) to determine
concentrations
of elemental impurities in each sample. In preparation for analysis, a portion
of each sample was
fused with a sodium carbonate/boric acid blend in a muffle furnace and the
resulting melt was
dissolved using dilute hydrochloric acid. However, it is noted that the sample
preparation for
ICP-AES analysis was not completely successful in that a precipitate formed
during the
dissolution of the melt, although the melt itself appeared clear upon removal
from the muffle
furnace. The dissolved samples were filtered or decanted and the clear
filtrate or decantate
subjected to ICP-AES analysis using a Thermo Jarrell Ash Atom 25 Inductively
Coupled Plasma
Atomic Emission Spectrometer.
[0050] The results of the ICP-AES analysis are shown in Table III. Due to the
difficulty
in completely dissolving the samples subjected to ICP-AES, it is possible that
some of the analytes
were lost with the precipitates that fonned during sample preparation, which
would impact the
quantitative analysis and reproducibility of the results reported in Table
III. Nevertheless, the
trends observed during the ICP-AES analysis are consistent with and confirm
the qualitative
analysis results generated using DC arc cmission tcchniqucs.
Table III
Quantitative Analysis of Barite Ore, Barium Sulfate Product and Waste Stream
Element Waste Stream Raw Barite Ore Barium Sulfate
Product
Si 3000 gg/g 1000 ~tg/g 400 gg/g
Al 480 gg/g 140 30
Cr 130 gg/g <IO <10 gg/g
Ca 1400-1800 ~tg/g 1400-1800 gg/g 300 /
Fe 400 / 400 g/g 40 gg/g
[0051] As can be seen from the quantitative analysis, conventional processing
of the raw
barite ore without fluidizing treatment can reduce the concentrations of major
impurities such as
silicon, calcium, and iron. These elements appear in high concentration in the
waste stream and in
lower concentration in the barium sulfate product. However, it is difficult
using conventional
processing to adequately remove the elements, such as silicon, believed to
impair the appearance
16
CA 02634271 2008-06-12
WO 2007/070472 PCT/US2006/047217
of the barium sulfate product and cause "gray float" upon suspension of the
barium sulfate product
in water.
EXAMPLE 2 Fluidizing Treatment of Barium Sulfate Product to Remove "Gray
Float"
Impurities
[00521 A barium sulfate product (11 g) processed conventionally (e.g., without
fluidizer
treatment) from barite ore was mixed with water (500 mL) and sodium citrate (3
g) in a beaker.
The solution was stirred with a mechanical stirrer, and a "gray float" layer
formed above the
suspension. The suspension was passed through a 120 mesh (125 m) screen,
which readily
separated the "gray float" material. The filtrate, comprising suspended barium
sulfate particles
was substantially clear and free of the "gray float" material.
[0053] The above description of the preferred embodiments is intended only to
acquaint
others skilled in the art with the invention, its principles, and its
practical application, so that
others skilled in the art may adapt and apply the invention in its numerous
forms, as may be best
suited to the requirements of a particular use. The present invention,
therefore, is not limited to
the above embodiments, and may be variously modified.
[00541 With reference to the use of the word(s) "comprise" or "comprises" or
"comprising" in this specification (including the claims), Applicants note
that unless the context
requires otherwise, those words are used on the basis and clear understanding
that they are to be
interpreted inclusively, rather than exclusively, and that Applicants intend
each of those words to
be so interpreted in construing this specification (including the claims).
17