Note: Descriptions are shown in the official language in which they were submitted.
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
1
CONTROL OF PARTICLE SIZE OF
ARAGONITE PRECIPITATED CALCIUM CARBONATE
[Technical Field]
The present invention is related to a method for manufacturing
aragonite-type precipitated calcium carbonate according to the
homogeneous precipitation reaction between calcium hydroxide
and aqueous solution of sodium carbonate. Particularly, the
present invention is related to a method for manufacturing
aragonite particles having various sizes by making the
suspension of calcium hydroxide having various sizes by
changing the solid-liquid ratio of the hydration reaction of
caustic lime, and using sodium carbonate and sodium hydroxide.
[Background Art]
Precipitated calcium carbonate is an inorganic material which
is not soluble in pure water, has a proper specific gravity,
and has properties such as a high whiteness, non-
combustibility, etc. It is applicable extensively as inorganic
filler in various industrial areas such as rubber, paint,
plastic, paper, cosmetics, and toothpaste industries.
Particularly, aragonite-type precipitated calcium
carbonate is needle shaped and has a very large aspect ratio
(ratio of the size with respect to the length of crystals). It
may be substituted as a new functional in organic material
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
2
that can grant the mechanical functionality as well as optical
functionality since its strength may be enhanced, its
whiteness may be improved, and its opaqueness may be
controlled owing to the complicated surface structure of the
needle shape when it is used as a filler of rubbers, plastics,
and coating materials, or an industrial material for pigments
for paper.
Since the above-described aragonite may have been applied
to many more areas by controlling its particle sizes or aspect
ratios of the needle shape, the technology of controlling its
particle sizes has been developed continuously.
The present invention is devised in order to solve the
problems with prior art.
[Summary of the Invention]
Additional features and advantages of the invention will
be set forth in the description which follows, and in part
will be apparent from the description, or may be learned by
practice of the invention. The objectives and other advantages
of the invention will be realized and attained by the process
particularly pointed out in the written description and claims
hereof, as well as the appended drawings.
The present invention is to provide a new method for
manufacturing aragonite with controlled particle sizes and
aspect ratios using the suspension of calcium hydroxide
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
3
manufactured by controlling the solid-liquid ratios and
changing the initial reaction temperature in the step of
hydration of CaO, which is the caustic lime component
manufactured by the calcination of limestone.
Another objective of the present invention_to provide a
new method for manufacturing calcium hydroxide having various
particle sizes.
It is still another object of the present invention to
provide a method of manufacture of highly functional and high-
value-added aragonite-type precipitated calcium carbonate by
improving the aspect ratio and production yield through the
manufacture of precipitated calcium carbonate by using the
aqueous solution reaction of sodium carbonate.
The method for manufacturing aragonite according to the
present invention to solve the above-described pr_oblems is a
new method enabling the control of various sizes comprising
the steps of manufacturing the caustic lime by calcination of
limestone; manufacturing the suspension of calcium hydroxide
through the hydration reaction while changing the solid-liquid
ratio and the initial reaction temperature of water using the
solid-liquid ratio of caustic lime to water of 1 to 30 or
greater parts by weight, preferably, 1 to 30 - 70 parts by
weight; manufacturing aragonite-type sedimentary calcium
carbonate through the homogeneous precipitation reaction of
the aqueous solution of sodium carbonate by adding sodium
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
4
hydroxide and sodium carbonate to the above suspension of
calcium hydroxide; and obtaining aragonite by washing,
filtering, and drying the above aragonite-type sedimentary
calcium carbonate.
The above method for manufacturing aragonite-type
sedimentary calcium carbonate is characterized by comprising
the steps of manufacturing the suspension in which calcium
hydroxide is dispersed in distilled water; stabilization in
which the aqueous solution of sodium hydroxide is added to the
suspension of calcium hydroxide produced during the above step
of manufacture of the suspension; and mixing so that the
suspension of calcium carbonate having a constant
concentration is formed by supplying the aqueous solution of
sodium carbonate at a fixed rate while mixing the above
suspension of calcium hydroxide.
Since CaO used in the present invention reacts with water
readily in the air, and therefore, it is difficult to secure
CaO having proper characteristics, CaO is dehydrated at 700 C
for about 1 hour before the hydration reaction of CaO in the
present invention. In the step of manufacturing calcium
hydroxide having various particle sizes according to the
present invention, the ratio of mixing CaO and water is
changed, an aqueous solution having the solid-liquid ratio of
1 20 - 70 preferably is filled into a reactor, and the
hydration reaction is progressed by varying the initial
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
temperature of water for the hydration reaction to be 10 - 90 C
In the present invention, the solid-liquid ratio, i.e.,
the ratio of mixing water and CaO, is not greatly limited, but
it is preferable that the ratio of Ca0 to water is 1 to about
5 20 - 60. If the ratio is less than 20, it is not desirable in
that there is no big difference in particle sizes of the
suspension of calcium hydroxide produced although the initial
reaction temperature is changed; and if it is greater than 60,
it is not recommended since there are problems in productivity.
Also, the initial reaction temperature refers to the
temperature raised when there is water only in the reactor,
which is controlled to be 10 - 90- Cfor the reaction. If it is
below 10 C it is not desirable in that the particle sizes of
calcium hydroxide are not changed greatly even if the solid-
liquid ratio is changed; and if it is higher than 90 C; it is
not desirable both in that the particle size is rather reduced,
and it is difficult to control the heat of reaction.
As to the reactor for the hydration of CaO of the present
invention, a reactor filled with water and equipped with a
thermostatic water bath for maintaining a constant temperature,
an impeller for mixing, and a thermometer for measuring the
temperature is used.
Further, hydrated calcium hydroxide is transferred to a
manufacturing device for aragonite-type precipitated calcium
carbonate and reacted. The manufacturing device for aragonite-
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
6
type sedimentary calcium carbonate according to the present
invention is comprised of a reactor in which the suspension of
calcium hydroxide and the aqueous solution of sodium carbonate
are mixed; a thermostatic water bath filled with water and
equipped with the above reactor and a temperature control
means so that the inside of the reactor is maintained at a
constant temperature; a supplying part inserted into the above
reactor; an injector of sodium carbonate installed at the pipe
extended outside of the reactor from the supplying part so
that the amount of supply of the aqueous solution of sodium
carbonate is controlled by a flowmeter; a rotating axis
installed in such a way that it penetrates the cover of the
above reactor and rotates; a stirring wing installed at the
inner cross-section of the above rotating axis; and a stirrer
equipped with a motor installed at the outer cross-section of
the above rotating axis for mixing the suspension of calcium
hydroxide and carbon dioxide. In the reactor for manufacturing
the above aragonite, the following steps for manufacturing
calcium carbonate are performed: the transfer of the
suspension of calcium hydroxide manufactured to the above
reactor for manufacturing calcium hydroxide to the reactor;
stabilization by supplying the aqueous solution of sodium
hydroxide to the above suspension; mixing and stirring by
supplying the aqueous solution of sodium carbonate at a
constant rate; and washing, filtering, and drying.
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
7
Here, in the above step of stabilization, 0.1 - 0.2 moles
of the suspension of calcium hydroxide and 0.1 - 2.0 moles of
the aqueous solution of sodium hydroxide are mixed. And in the
above step of stirring, 0.1 - 1.5 moles of the aqueous
solution of sodium carbonate is added to the suspension of
calcium hydroxide at a rate of 1 10 mL/minute and reacted
maintaining the temperature at 10 ~ 80 C. The above numbers of
moles of calcium hydroxide and sodium hydroxide are within the
range facilitating the manufacture of single-phase aragonite
in the present invention. And if the rate of the addition of
aqueous solution of sodium carbonate is lower than the above
rate of addition, the rate of reaction may be too slow; and if
it is higher than the above rate of addition, it may be
difficult to manufacture aragonite having homogeneous physical
properties since the reaction heat is too high. It is
preferable to use alcohols to wash the above manufactured
aragonite.
[Brief Description of the Drawings]
The accompanying drawings, which are incorporated in and
constitute a part of this specification, illustrate
embodiments of the invention and, together with the
description, serve to explain the purpose, advantages, and
principles of the invention.
In the drawings,
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
8
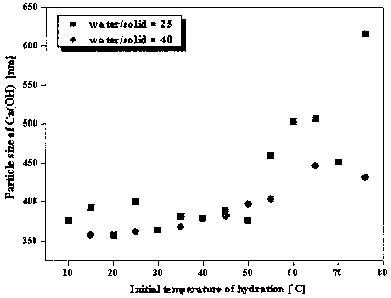
Figure 1 shows particle sizes of calcium hydroxide
according to changes in the initial reaction temperature and
the solid-liquid ratio of the hydration reaction;
Figure 2 shows particle sizes of calcium hydroxide and
subsequent particle sizes of aragonite-type precipitated
calcium carbonate according to the initial reaction
temperature;
F'igure 3 shows the SEM photograph of aragonite-type
precipitated calcium carbonate; and
Figure 4 shows the yields of aragonite-type precipitated
calcium carbonate according to the initial reaction
temperature of hydration.
[Description of the Preferred Embodiments of the Invention]
Hereinafter, the present invention is illustrated in
terms of a few preferred embodiments of the present invention
as follows:
<Preferred Embodiment 1>
Changes in the diameter of calcium hydroxide according to the
conditions for hydration reaction
The hydration reaction was performed under two conditions of
1 : 25 and 1 : 40 for the rate-liquid ratio of CaO to water in
order to have the initial temperature of the inside of the
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
9
reactor constant in the temperature range of 10 - 75 C The
reaction time was for 30 minutes, and the rate of stirring was
maintained to be constant at 400 rpm.
Since CaO, used as a sample in the hydration reaction,
reacted with water readily even in the air, it was dehydrated
at 700 C for 1 hour right before the hydration reaction. The
particle size of calcium hydroxide produced after the
hydration reaction was measured by using ELS-8000 (Otsuka
Electronics Company), and the results of the measurement were
shown in Figure 1. As seen in Figure 1, the particle size was
smaller when the solid-liquid ratio was 1. 40 than when it
was 1 : 25, and the particle size was changed greatly as the
initial reaction temperature was increased, i.e., at a
temperature higher than 50'C; thus enabling the manufacture of
calcium hydroxide having various diameters.
<Preferred Embodiment 2>
Affect of the particle size of calcium hydroxide on the size
of aragonite
The 1.5-mole suspension of calcium hydroxide having various
part.icle sizes manufactured in Preferred Embodiment 1 shown in
Figure 2 was mixed with the 1.0-mole aqueous solution of
sodium hydroxide, and 1.5-mole aqueous solution of sodium
carbonate was added to the above mixture at a rate of 3
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
mL/minute and reacted at 55 C for 2 hours. The relationship
between the particle size of aragonite and that of calcium
hydroxide was shown in Figure 2, and the purity of single-
phase aragonite was measured by using SEM (Leo 1455VP)
5 photograph shown in Figure 3 and x-ray diffraction pattern.
The results of the measurement are shown in figure 4.
As shown in Figure 3, single-phase needle-shaped
aragonite sedimentary calcium carbonate was obtained
irrespective to its diameter, i.e., with any diameter, and the
10 yield of aragonite with calcite suppressed was shown to be 90%
or greater as shown in Figure 4.
As shown in Figure 2, the diameter of aragonite was
increased as the diameter of calcium hydroxide was increased,
and it was possible to obtain large-sized aragonite particles
when the initial reaction temperature was 50 C or higher during
the hydration reaction for manufacturing calcium hydroxide.
The reasons for this seemed to be that the particle size of
calcium hydroxide was maximized if the initial temperature of
hydration reaction was 50 C or higher, and the particle size of
the product was increased greatly if the particles of calcium
hydroxide were produced by sodium carbonate since the rate of
elution of Caz+ in the particles was lowered as the specific
surface area was reduced according to the particle size and
the rate of growth of aragonite was fast.
[Industrial Applicability]
CA 02635023 2008-06-25
WO 2007/078016 PCT/KR2005/004688
11
In the hydration reaction of CaO according to the present
invention, calcium hydroxide having various sizes was obtained
unexpectedly by controlling the initial reaction temperature.
Particularly, provided in the present invention is a new
method for manufacturing large- and various-particle-sized
needle-shaped aragonite-type calcium carbonate by the
homogeneous precipitation reaction of the particles of calcium
hydroxide in the aqueous solution of sodium carbonate as the
particle size of calcium hydroxide was increased at
temperatures higher than 50 C
Accordingly, a simple, economic, new, and reproducible
method for manufacturing aragonite, through highly functional
and high-value-added aragonite-type sedimentary calcium
carbonate may be manufactured by increasing its aspect ratio
and yield of production, is provided in the present invention.
It will be apparent to those skilled in the art that
various modifications and variations can be made in the
disclosed process and product without departing from the scope
or spirit of the invention. Other embodiments of the invention
will be apparent to those skilled in the art from
consideration of the specification and practice of the
invention disclosed herein. It is intended that the
specification and examples be considered as exemplary only,
with a true scope and spirit of the invention being indicated
by the following claims.