Note: Descriptions are shown in the official language in which they were submitted.
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
1
CERVIMETRY CONTROL APPARATUS
BACKGROUND OF THE INVENTION
During the later stages of pregnancy, the cervix typically undergoes numerous
physical changes which provide increased safety and ease with which the fetus
can be
delivered. Particularly, the cervical canal tissue softens and increases in
pliability,
and subsequently, the diameter of the cervical canal begins to increase.
Eventually,
the dilation of the cervix is completed, allowing for the unobstructed passage
of the
fetus.
Cervical diameter is monitored throughout labor and is instrumental in
diagnosing such conditions as dysfunctional or arrested labor, to determine
whether
labor augmentation or a cesarean section should be performed, as well as to
establish
whether or when various pharmaceutical agents should be administered. Physical
examination of the cervical diameter is generally performed by inserting two
fingers
into the vagina and up to the cervix. Upon reaching the cervix, the fingers
are spread
apart to determine the approximate dilated diameter. While an obstetrician may
be
fairly experienced in performing a manual cervical diameter measurement, the
accuracy of such a measurement can be highly subjective and can further vary
depending on the particular experience, judgment, and even finger size of the
attending physician. Considering the importance of the cervical dilation
measurement
in assessing labor progression, it is crucial to provide dilation information
that is
precise as well as reproducible among different healthcare providers or
physicians.
Given the subjectivity and probability of inaccurate or imprecise dilation
measurements, it would be desirable to provide for the precise and accurate
attainment of cervical dilation measurements on a repeat basis during the
course of
labor. In addition, it would be desirable to provide for ease of monitoring
and control
of cervical dilation to assist a physician throughout labor management.
SUMMARY OF THE INVENTION
The present invention advantageously provides a method and system for the
accurate and precise measuring of cervical dilation during labor, as well as a
method
and system for performing cervical dilation. The medical device of the present
invention may include an elongate body defining a proximal end and a distal
end,
with the elongate body further including an inflation lumen. An expandable
element
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
2
may be coupled to the elongate body in fluid communication with the inflation
lumen,
and an array of movable elements may be circumferentially disposed about the
elongate body, with the array of movable elements being movably coupled to the
elongate body by a plurality of wires. The medical device may also include a
measurement mechanism able to determine a radial spacing of the array of
movable
elements, where the measurement mechanism can include a tension ring coupled
to
the plurality of wires. In addition, a dilation indicator can be provided in
communication with the measurement mechanism, while at least one pressure
sensor
may be coupled to at least one of the array of movable elements. Moreover, a
distal
pressure sensor can be coupled to the distal end of the elongate body, with
the medical
device also providing a control element in communication with the at least one
pressure sensor and the distal pressure sensor. The medical device can also
include an
inflation source in fluid communication with the expandable element, as well
as an
exhaust valve in fluid communication with the expandable element. Furthermore,
the
medical device may include a camera as well as a lighting element coupled to
the
distal end of the elongate body, thereby providing visual feedback to aid in
the
positioning of the device.
The control element of the present invention may further provide for
monitoring and controlling the operation of the medical device. The control
element
may be in communication with the one or more sensors disposed on the medical
device, as well as being in communication for control and/or monitoring of the
additional components of the medical device, such the camera, lighting source,
inflation source, or the like. The control element may provide a display or
other
indication elements for conveying information, such as pressure, size, etc. to
a
physician during a particular procedure. Moreover, the control element may
provide
for additional patient safety by having an automatic alarm and/or shut down
process
in response to measurements and/or conditions that differ from a pre-
determined set
of parameters.
In an alternative embodiment, the present invention also provides a cervical
dilation sensor to aid in the manual, two-finger approach commonly employed.
The
cervical dilation sensor may include a first rod, a second rod, and a sensor
housing.
The first and second rods may be rotatably and pivotably coupled to the sensor
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
3
housing, as to freely move about the housing in at least two planes of motion.
The
sensor housing may include one or more sensors coupled to the first and second
rods
as to measure the relative movement of the two rods, while the cervical
dilation
sensor may also include a control monitor in communication with the one or
more
sensors in the sensor housing for displaying and monitoring information
provided by
the sensors.
BRIEF DESCRIPTION OF THE DRAWINGS
A more complete understanding of the present invention, and the attendant
advantages and features thereof, will be more readily understood by reference
to the
following detailed description when considered in conjunction with the
accompanying
drawings wherein:
FIG. 1 is an illustration of an embodiment of a medical device in accordance
with the present invention;
FIG. 2 is a side view of a distal end of the medical device of FIG. 1;
FIG. 3 is a cross-sectional view of a distal end of the medical device of FIG.
1;
FIG. 4 is an additional cross-sectional view of the medical device of FIG. 1;
FIG. 5 is a cross-sectional view of an embodiment of a dilation indicator in
accordance with the present invention;
FIG. 6 is an illustration of an embodiment of a control element in accordance
with the present invention;
FIG. 7 is an illustration of a distal end of a medical device in a deflated
state in
accordance with the present invention;
FIG. 8 is an illustration of a distal end of a medical device in an inflated
state
in accordance with the present invention;
FIG. 9 is a flow chart of an embodiment of a method of use of a medical
device of the present invention;
FIG. 10 is a perspective illustration of an embodiment of a cervical dilation
sensor in accordance with the present invention;
FIG. 11 is a side view of the cervical dilation sensor of FIG. 10;
FIG. 12 is an additional illustration of the cervical dilation sensor of FIG.
10;
FIG. 13 is yet another depiction of the cervical dilation sensor of FIG. 10.
FIG. 14 shows an embodiment of a cervical dilation sensor coupled to a hand;
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
4
FIG. 15 depicts an embodiment of a cervical dilation sensor within a glove;
FIG. 16 illustrates an additional embodiment of a cervical dilation sensor
coupled to a hand;
FIG. 17 shows an embodiment of a calibration element for use with a cervical
dilation sensor in accordance with the present invention; and
FIG. 18 is a flow chart of an embodiment of a method of use of a cervical
dilation sensor in accordance with the present invention;
DETAILED DESCRIPTION OF THE INVENTION
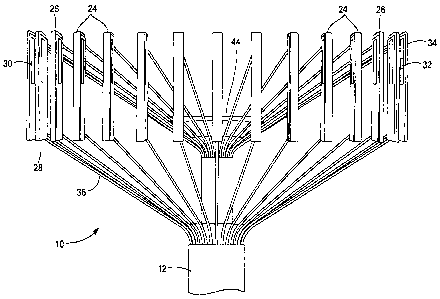
As shown in FIG. 1, the present invention provides a medical device 10 for
measuring and performing cervical dilation. The medical device 10 includes an
elongate body 12 defining a proximal end 14 and a distal end 16. The medical
device
may further include a dilation indicator 18 coupled to the proximal end 14 of
the
elongate body 12 that is capable of providing a visual indicator of the
dilation
measurement made by the medical device 10, as well as a control element 20 and
an
inflation source 22, which will be discussed in more detail below.
Now referring to FIG. 2, the medical device 10 may further include an array of
movable elements 24 disposed circumferentially about an axis of the elongate
body
12, where the array of movable elements 24 is located in proximity to the
distal end
16 of the elongate body 12. The array of movable elements 24 are movable in a
radial
direction as to expand and contact with the tissue of the cervix when
positioned for
measurement of cervical dilation. Moreover, the array of movable elements 24
may
be retracted upon completion of the desired measurement to ease the withdrawal
of
the medical device 10 from the patient. Each movable element may define an
upper
portion 26 and a lower portion 28. In addition, each movable element may
define a
channe130 such that one or more pressure sensors 32 may be mounted or
otherwise
positionable within the channel 30 of the movable element. Moreover, an outer
cushion 34 may be coupled to an outer surface of each movable element, where
the
outer cushion 34 may be constructed from a gel-like material or other suitable
padding. The array of movable elements 24 may further be movably coupled to
the
elongate body 12 of the medical device 10 by a plurality of wires 36 coupled
to the
upper and lower portions of the movable elements 24, where the plurality of
wires 36
further extend through a length of the elongate body 12.
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
While the array of movable elements 24 may be extended and retracted by
manipulating the plurality of wires 36, an actuating mechanism may be provided
to
facilitate movement of the array of movable elements 24 from a retracted
position to
an extended position, and vice versa. The actuating mechanism may include a
spring
mechanism, a telescoping element, or, alternatively, the medical device 10 may
include an expandable element 38, such as a balloon. Now referring to FIG. 3,
the
medical device 10 of the present invention may further include the expandable
element 38 coupled to or.otherwise disposed on the elongate body 12 at or near
the
distal end 16 of the elongate body 12. The expandable element 38 may be
configured
in a myriad of shapes, including a toroidal configuration in which the
expandable
element 38 defines a ring-like, "0" shape. Moreover, an inflation lumen 40 can
be
included in fluid communication with the expandable element 38, where the
inflation
lumen 40 is disposed within and traverses a substantial length of the elongate
body
12.
The medical device 10 of the present invention may include additional
features providing safety, ease of use, and the like. For example, the medical
device
may include a protective sheath 42 encasing at least a portion of the distal
end 16
of the elongate body 12. The sheath 42 may include one or more layers of
various
materials to provide a water-tight seal around the medical device, as well as
adding to
patient comfort by having additional padding and/or a lubricious coating to
ease
positioning of the device. For example, a first layer may completely enclose
the
medical device to ensure the device is not exposed to external fluids or
objects. A
second layer may be placed over the first layer as a protective layer which is
removable by a physician or operator after each use, thereby providing a
sterile layer
and the possibility for re-use of the medical device. A third layer may be
provided
over the second layer and include a lubricious property allowing for smooth
insertion,
operation, and removal of the device.
Furthermore, a distal pad 44 may be coupled to the elongate body 12 at or near
the distal end 16, where the distal pad 44 may be contoured or shaped to
conform to
the curvature of the head of a baby. In addition, a distal pressure sensor 46
may be
coupled to the distal pad 44 to aid in monitoring the positioning of the
medical device
10 and for determining contact with the cervix or with the baby. The distal
pad 44
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
6
and distal pressure sensor 46 may provide feedback to a physician and aid in
the axial
positioning of the medical device 10 upon insertion into a patient.
Furthermore, a
camera 45 and a lighting element 47 may also be coupled to the distal portion
of the
medical device. The camera 45 may be a miniaturized instrument or pin-hole
camera
as commonly employed in endoscopic surgical procedures, while the lighting
element
47 may include a diode, fiber optic, or other illumination mechanism as is
known in
the art. The camera 45 and lighting element 47 may provide visual feedback to
a
physician to further aid in maneuvering and positioning the medical device
when in
use.
As shown in FIG. 4, the elongate body 12 may define a plurality of wire
lumens 48 for slideably receiving a portion of each of the plurality of wires
36
coupled to the array of movable elements 24. Each wire of the plurality of
wires 36
may be slideably positioned within each of the plurality of wire lumens 48 as
to slide
freely with little friction, thereby facilitating the movement of the array of
movable
elements 24 when the medical device 10 is in use. The wires 36 may have
sufficient
length as to extend through the entire length of the respective wire lumens
48, and
may further extend out of the proximal end 14 of the elongate body 12.
The medical device 10 of the present invention may further include a
measurement mechanism for monitoring and / or quantifying the movement of the
array of movable elements 24 when the medical device 10 is in use. For
example, as
shown in the FIG. 5 illustration of a cross-section of the dilation indicator
18, the
medical device 10 may include a tension ring 50 coupled to the plurality of
wires 36
such that the tension ring 50 moves as the wires 36 extend and retract in
response to
the movement of the array of movable elements 24. The tension ring 50 may
further
be slideably coupled to the dilation indicator 18, where the dilation
indicator 18
conveys a dilation measurement in response to the relative motion of the
tension ring
50, the plurality of wires 36, and thus, the array of movable elements 24. The
dilation
indicator 18 may include predetermined values calculated from the movement of
the
tension ring 50 as to eliminate the need for a physician to do any calculating
to
determine the dilation measurement. ,
Again referring to FIG. 1, in an exemplary system, the proximal end 14 of the
medical device 10 of the present invention is coupled to the control element
20,
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
7
through which a physician may monitor and/or control the various components of
the
medical device 10. The control element 20 may be in communication with any of
the
numerous sensors provided on the medical device 10, and may further be in
communication with additional components of the medical device 10, such as the
inflation source 22, the camera 45, and/or the lighting element 47. In an
exemplary
embodiment, the control element 20 may include a console that may be wrist-
mounted to ease the overall use of the medical device 10.
The control element 20 may include a display, such as an LCD screen or the
like, as well as other visual, audio, or tactile indicators to convey
information
regarding the various operating characteristics and conditions of the medical
device
to an operator or physician, including dilation measurements, dilation
pressure
exerted by the medical device, inflation pressure, etc. The control element 20
may
further include one or more control actuators, such as push-buttons, switches,
a touch-
screen, or the like, to enable a physician to provide input to the control
element in
order to manipulate and/or control a particular component or function of the
medical
device 10.
In addition, the control element 20 may include a processor component and an
electronic storage medium (not shown) for storing patient information,
measurements
and/or procedural information obtained during use of the medical device. The
control
element 20 may provide calculations and graphical illustrations including, but
not
limited to, a display of air pressure versus time, air pressure versus
diameter, upper
and lower limits of expansion pressure, etc. The control element 20 may
further
contain date/time information for measurements, the physician or nurse
performing
the procedure, and the like. Moreover, the control element 20 may be able to
communicate such recorded information to other devices and/or systems in the
hospital environment through the use of portable media and/or wireless
technologies
as is known in the art.
In an exemplary embodiment, as shown in FIG. 6, the control element may
include a housing including an visual display 52, a plurality of LEDs 54, and
a
plurality of control actuators 56 (shown as push buttons) which may be in
communication with the camera 45, light 47, and/or the inflation source 22, as
previously described. In addition, the housing of the control element 20 may
be
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
8
mounted on the wrist of a physician through one or more coupling elements 58.
Moreover, to further ease use, the visual display 52 may be movably coupled to
the
housing so that a physician may change the viewing angle or orientation of the
screen
during use of the medical device. A particular use of the control element 20
during a
cervical procedure with the medical device is described further below.
The inflation source 22 of the medical device may be coupled to the inflation
lumen 40 at the proximal end 14 of the elongate body 12, where the inflation
source
22 is able to provide a fluid or gas into the inflation lumen 40 for
subsequent delivery
to the expandable element 38. Examples of a suitable inflation source 22 may
include
manual pumps, powered pumps, or the like. The inflation source may be either
separate from the control element, as shown in FIG. 1, or integral with the
control
element 20 as shown in FIG. 6. Moreover, an exhaust valve 60 may be in fluid
communication with both the inflation source 22 as well as the inflation lumen
40 for
subsequent control of the release of fluid from the medical device 10, and may
further
be in communication with and/or controlled by the control element 20.
Referring now to FIGS. 7 and 8, in an exemplary use of the medical device 10
of the present invention, a precise dilation measurement may be performed
during the
various stages of labor. The medical device 10, in a deflated state, may be
positioned
such that the distal end 16 of the elongate body 12 is in proximity to the
dilated region
of the cervix 54. Proper positioning can be aided by feedback provided by the
distal
pressure sensor 46 when contacting the cervix or the head 56 of the baby, as
well as
monitoring the visual feedback from the camera 45. The sensor feedback as well
as
images obtained by the camera 45 may be displayed on the LCD screen of the
control
element. Upon proper positioning, the array of movable elements 24 may be
extended to contact the tissue of the cervix 54, for example, by actuating the
inflation
source 22 to inflate the expandable element 38. As the expandable element 38
is
inflated and subsequently expands, the array of movable elements 24 located
around
the periphery of the expandable element 38 will move outward in a radial
direction,
while lengths of the plurality of wires 36 will be drawn further into the
respective
plurality of wire lumens 48. As the array of movable elements 24 is coupled to
the
plurality of wires 36, which are further coupled to the tension ring 50, the
expandable
element 38 will expand outward uniformly from the elongate body 12.
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
9
The inflation source 22 may continue to inflate the expandable element 38
until the movable elements 24 of the medical device 10 come into contact with
the
dilated or undilated cervix 54. Such contact can be indicated and monitored
through
information provided by the pressure sensors 32 coupled to the movable
elements 24,
which, again, may be relayed to a physician or operator through the control
element.
In particular, the control element may provide a visual indicator of the
pressure being
exerted on the cervical tissue by the expansion of the medical device as well
as the
overall dilation measurements of the cervix. Furthermore, the control element
20 may
include an algorithm or computational ability to determine if the pressure
sensor
feedback indicates a substantially uniform circular state. That is to say,
that the
pressure measurements from each of the pressure sensors 32 disposed about the
movable elements 24 are approximately the same. When the desired inflation
level or
diameter has been attained as indicated by pressure sensor measurements or
from the
dilation indicator, the inflation source 22 may be deactivated, or,
alternatively, the
exhaust valve 60 may be triggered to prevent additional fluid from entering
the
expandable element 38. Each of these events may be triggered and/or controlled
by
actuator elements included on the housing of the control element.
Once appropriately inflated, the measuring mechanism and the dilation
indicator 18 can provide the dilation measurement as indicated by the distance
the
plurality of wires 36, and thus the tension ring 50, traveled in reaching the
expanded
state. As previously stated, the dilation indicator 18 can directly correlate
the distance
traveled by the wires 36, and thus, the measured expansion of the movable
elements
24, to an accurate and precise dilation measurement.
Upon completion of the desired measurement, the movable elements 24 are
retracted towards the elongate body 12, i.e., by deflating the expandable
element 38
by opening the exhaust valve 60, upon which the movable elements 24 will
retract to
a closed position for the removal of the medical device 10 from the patient.
Both the
tension ring 50 and the plurality of wires 36 may be biased towards a closed,
retracted
position, such that when the expandable element 38 is not under positive
inflation
pressure, the medical device 10 retains a closed, retracted state.
Furthermore, as
described above, the medical device 10 may include an outer sheath 42 which,
if used,
may be removed and replaced for subsequent uses of the medical device 10,
thereby
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
providing a re-usable device while maintaining the sterility of the medical
environment.
Referring to FIG. 8, in an alternative use of the medical device 10 of the
present invention, the distal portion of the medical device 10 may be employed
to
produce a safe and uniform cervical dilation where a desired dilated condition
has not
yet occurred or otherwise been achieved. The medical device 10 may be
positioned
proximate to a region of an undilated cervix and the array of movable elements
24 of
the medical device 10 may be expanded to contact the cervical tissue 54.
Similar to
obtaining a dilation measurement as described above, the distal pad, pressure
sensors
or camera may provide feedback to a physician or operator to aid in the axial
positioning of the device. Through monitoring information from any of the
aforementioned components, through the control element for instance, the
medical
device may traverse the length of the cervix while reducing the likelihood of
accidentally perforating the uterus, which may occur with the use of
conventional
devices. "
Upon initiating the desired contact, the array of movable elements 24 may
then be extended further, for example, through a controlled inflation of the
expandable element 38, in order to provide a desired rate of expansion, and
thus,
dilation. Alternatively, the array of movable elements may be actuated to
extend
outward through pressure or force applied through the plurality of wires 36,
or by
other actuating mechanisms as known in the art. At any point during the
dilation
procedure, information may be provided regarding the amount of force being
applied
to the cervical tissue via the one or more pressure sensors 32 coupled to the
array of
movable elements 24, as well as the radial spacing of the array of movable
elements.
As such, through the monitoring of sensor feedback information, the dilating
force
applied to the array of movable elements either through the plurality of wires
36 or by
the expandable element 38 may be appropriately adjusted in order to achieve
the
desired dilation without unnecessarily damaging the cervical tissue.
Additionally, the
spacing of the array may be monitored to achieve a desired dilated state.
Through the
monitoring and manipulation of the operating characteristics of the medical
device,
including the rate of extension of the array, the pressure between the medical
device
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
11
and the tissue, and/or the distance traveled and thus the radial spacing of
the array, a
precise and accurate dilation may be induced.
The above-described dilation may be performed for obstetrical uses, for
example, in cervical "ripening" to assist in the induction of labor in cases
of poorly
dilated or effaced cervices. In addition, pre-operative dilation may be
performed
using the medical device of the present invention in cases of uterine
curettage for
failed pregnancy, miscarriage, or retained products of conception. Moreover,
the
medical device may be used for gynecological purposes of cervical dilation in
cases
of curettage of the endocervix or endometrium, elective termination of
pregnancy,
diagnostic and operative hysteroscopy, thermal endometrial ablation
techniques, as
well as treatment of cervical stenosis.
While it has been discussed that the control element may provide a variety of
information from the numerous sensors and other components to a physician or
operator during the above-mentioned procedures, the control element may
further
provide for enhanced safety during use by including pre-determined
circumstances
and/or threshold values for safe operation, irrespective of whether the aim is
to take
measurements or to cause dilation. Now referring to FIG. 9, for example, the
medical
device may be positioned initially in the cervix, while values obtained from
the distal
pressure sensor are monitored. Should a particular pressure be experienced
that
exceeds a pre-determined safe operating pressure that could damage the baby or
surrounding tissue, the control element 20 may provide an audio, visual, or
tactile
alarm to the operator to withdraw the device. Upon proper positioning, the
inflation
source 22 may be actuated. Should a pressure in the inflation lumen, RPM, or
other
indicator of the operation of the inflation source 22 differ from expected
values, the
control element 20 may again cause an alarm and/or automatically terminate
operation of the inflation source 22. Similarly, during use of the medical
device 10, if
the pressure being exerted on the cervical tissue during expansion is greater
than
desired, or should the diameter of the expanded portion of the medical device
appear
out of the desired range, the control element 20 may convey these
circumstances
through visual, audio or tactile indicators and/or initiate a shut-down
sequence of
events, which may include deflation, retracting the array or movable elements,
etc.
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
12
Now referring to FIGS. 10-13, in an alternative embodiment of the present
invention, a cervical dilation measurement device 100 is provided to aid in
the
manual, two-finger approach of measuring cervical dilation. The measurement
device
100 may include a first extension element 102, a second extension element 104,
and a
base element 106. The first and second extension elements 102,104 may be
rotatably
and pivotably coupled to the base element 106, as to freely move about the
housing in
at least two planes of motion. The base element 106 may include a dilation
indication
mechanism to measure the distance between and/or the relative movement of the
two
extension elements. The dilation indication mechanism may include one or more
sensors coupled to or otherwise in communication with the first and second
extension
elements 102,104. Sensors suitable for monitoring the movement of the first
and
second extension elements 102,104 may include sensors mechanically coupled to
the
extension elements capable of measuring their displacement or movement
directly,
including but not limited to torque or strain gauges, or may alternatively
include
sensors positioned in the tips of the first and second extension elements that
can
monitor distance between the two tips via radiofrequency, optical energy, or
the like.
A third sensor may be incorporated, in the base element 106 for example, to
provide
increased accuracy and precision through triangulation methods. The
measurement
device 100 may also include the control element 20, as previously described
and
illustrated in FIG. 1, in communication with the base element 106 and one or
more
sensors for displaying and monitoring information provided by the sensors.
Now referring to FIGS. 14-16, the measurement device 100 of the present
invention may also include one or more lateral sensors 108,108' positionable
about
the sides of the first and second fingers used in the manual cervical dilation
measurement technique. The lateral sensors 108,108' may provide pressure
feedback
information when in contact with the cervix that may assist a physician in
making a
measurement while avoiding or minimizing cervical distension. As such, the
reduced
likelihood of cervical distension increases the ability to provide an accurate
and
precise dilation measurement. The lateral sensors 108,108' may include one or
more
thin film pressure sensors, as known in the art, to minimize the increase in
width or
thickness of the device, thereby providing ease of use and reducing discomfort
of the
patient, and may further be placed in communication with the control element
20.
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
13
The measurement device 100 of the present invention may also include one or
more finger-tip pressure sensors 110,110' positionable about the tips of the
first and
second fingers used in the manual cervical dilation measurement technique. The
finger-tip pressure sensors 110,110' may indicate pressure feedback
information via
the control element 20 upon contact with the head of the baby. In addition to
providing feedback information to prevent excess pressure on the head of the
baby,
upon recognition that the finger tips are indeed contacting the head of the
baby, a
marker or other measurement indicator may be used to gauge the position and
descent
of the baby, as described below.
Historically, practitioners have used the ischial spine as the index point (0
station) for a determination of fetal descent, and assigned an arbitrary
number in
centimeters above and below the ischial spine. More specifically, "station"
refers to
the level of the presenting fetal part in the birth canal as described in
relationship to
the ischial spines, which are halfway between the pelvic inlet and the pelvic
outlet.
When the lowermost portion of the fetal presenting part is at the level of the
ischial
spine, it is designated as being at zero (0) station. In the past, the long
axis of the
birth canal has been arbitrarily divided into segments for a determination of
the
position of the baby. Thus, as the presenting fetal part descends from the
inlet toward
the pelvic outlet, the typical designation is -5, -4, -3, -2, -1, 0 station,
+1, +2, +3, +4,
+5. Using this method, the degree of accuracy (in centimeters) is difficult to
achieve
clinically. In practice, physicians may generally make an educated guess about
the
station of the presenting part of the baby, since after the "0" point (0
station), the
baby's head covers the ischial spine point and eliminates the ability to
measure and
reproduce distance caudal to this point. Contrary to the typical method
employed,
where accuracy and precision may be difficult to maintain, the feedback from
the
finger-tip sensors may provide an indication of contact with the head of the
baby.
Upon such indication, a marking or other descent indicator 112 on the portion
of the
hand of the physician external to the genitalia may be used to provide an
accurate and
precise measurement of the location and descent of the baby. Measurements over
the
course of labor indicate rates of progression which are practical, relatively
easier to
standardize and explainable to the patient or other practitioners. This
approach of
measurement is termed "Advancement".
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
14
In an exemplary use, the measurement device 100 is coupled to the hand of a
physician, with the first extension element 102 being paired to a first
finger, the
second extension element 104 being paired to a second finger, and the base
element
106 being positioned in between the first and second fingers. Moreover, where
the
lateral sensors 108,108' or finger-tip sensors 110,110' are included, the
sensors will
be positioned about the sides and tips of the fingers, respectively, as
described above.
The coupling may be achieved through the integration of the measurement device
100
with a glove 114, or through direct adhesion of the various components to the
fingers
themselves. Additionally, the cervical dilation measurement device 100 may
include
two cap elements 116,116' positionable about the fingertips, with the first
and second
extension elements 102,104 extending from the cap elements 116,116' and
towards
the base element 106, and with the lateral and finger-tip sensors coupled to
the cap
elements in the appropriate positions. Any wires or other communicative
elements
connecting the sensors to the control element 20 may be routed through the
glove or
positioned down the back of the hand as needed to provide connectivity while
preventing interference with the use of the device. Alternatively, the various
sensors
may communicate with the control element 20 wirelessly as known in the art.
Subsequently, the physician may position the first and second fingers and the
cervical dilation measurement device 100 in proximity to the cervix. Upon
reaching
the desired location, the two fingers can be spread either into a "V" shape or
an "L"
shape, and the relative movement of the first and second extension elements
102,104
may be measured by the one or more sensors in the base element 106, with the
lateral
sensors 108,108' preventing cervical distension as previously described. As a
result,
the physician will not be required to make a subjective observation as to the
actual
cervical dilation, as the actual width between the spread fingers can be
accurately
assessed by the cervical dilation measurement device 100 and provided to the
physician through the control element 20. In addition, upon contacting the
head of the
baby with the finger-tip sensors, the descent indicator 112 may be referenced
to
determine the location of the baby.
While the method of measurement as described above may provide an
accurate and precise measurement of cervical dilation, it is realized that
different
physicians may have variations in both finger length and thickness which may
affect
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
the accuracy of the measured dilation. Now referring to FIG. 17, the present
invention may include a calibration element 120 for use with the measurement
device
100 to compensate for the variations in the finger dimensions of a physician.
The
calibration element 120 may include an object of known dimensions, thereby
providing a reference value from which the measurement device 100 may be
calibrated. For example, the measurement device 100 may be coupled or
otherwise
positioned about the hand of a physician or operator, with the fiust extension
element
102 being paired to a first finger, the second extension element 104 being
paired to a
second finger, and the base element 106 being positioned in between the two
fingers.
Subsequently, the first and second fingers may be extended such that an outer
portion
of the fust and second fingers contact a portion of the calibration element
120,
providing a "simulated" distance measurement. Upon contacting the calibration
element 120, the first and second fingers will be separated by a known
distance, and
the relative movement of the first and second extension elements 102,104 about
the
base element 106 can be appropriately modified to reflect an accurate and
precise
measurement. Such modification may include, for example, an algorithm or other
computational calculation taking into account the known, fixed dimensions of
the
calibration element 120, the known length of the first and second extension
elements
102,104, as well as the angle formed between them at the intersection with the
base
element 106_ The suggested calibration procedure may be performed a single
time for
each operator who may thereafter use the measurement device 100, and such
values
and calibration modifications may be stored in the control element 20 for ease
of
subsequent use without the need to re-calibrate the device. Alternatively, the
suggested calibration procedure may be performed prior to each dilation
measurement
to ensure accuracy and precision.
As discussed above, the control element 20 may be coupled to the
measurement device 100 similarly to that of the medical device 10 in order to
provide
a variety of information from the numerous sensors and other components of the
measurement device 100 to a physician or operator during the above-mentioned
procedures. Once again, the control element 20 may further provide for
enhanced
safety during use by including pre-determined circumstances and/or threshold
values
for safe operation, irrespective of whether the aim is to take measurements or
to cause
CA 02635712 2008-06-27
WO 2007/089319 PCT/US2006/045003
16
dilation. Now referring to FIG. 18, for example, the measurement device 100
may be
initially positioned in the cervix, while values obtained from the finger-tip
sensors
110, 110' are monitored. Should a particular pressure be experienced that
exceeds a
pre-determined safe operating pressure that could damage the baby or
surrounding
tissue, the control element 20 may provide an audio, visual, or tactile alarm
to the
operator to withdraw the device. Upon proper positioning, the operator or
physician
may expand their fingers. Subsequently, during use of the measurement device
100,
if the pressure being exerted on the cervical tissue during expansion is
greater than
desired as indicated or otherwise measured by lateral sensors 108, 108', or
should the
diameter of the expanded fingers as measured by the measurement device 100
appear
out of the desired range, the control element 20 may convey these
circumstances
through visual, audio or tactile indicators, upon which the physician or
operator may
close and retract their fingers and the accompanying measurement device 100.
It will be appreciated by persons skilled in the art that the present
invention is
not limited to what has been particularly shown and described herein above. In
addition, unless mention was made above to the contrary, it should be noted
that all of
the accompanying drawings are not to scale. A variety of modifications and
variations are possible in light of the above teachings without departing from
the ope
and spirit of the invention, which is limited only by the following claims.