Note: Descriptions are shown in the official language in which they were submitted.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
1
CLEANING IMPLEMENT WITH ERODIBLE FOAM SUBSTRATE AND CONTROLLED
RELEASE SYSTEM OF ACTIVE AGENT
FIELD OF THE INVENTION
The present invention relates to a cleaning implement. More specifically, the
present
invention relates to a cleaning implement containing an erodible foam
substrate, such as a
melamine foam substrate.
BACKGROUND OF THE INVENTION
Use of erodible foam, such as melamine-formaldehyde resin foam, referred to
herein as
melamine foam, and phenolic foam in hard surface cleaning is well known.
Indeed, cleaning
implements of cut/molded melamine foam are popular for removing soils and/or
stains from hard
surfaces. Melamine foams are currently marketed in some countries under the
tradename of Mr.
Clean Magic Eraser~'4.
Melamine foams show excellent soil and/or stain removal performance in
cleaning hard
surfaces, when wetted with an appropriate solvent, such as tap water, and is
brought into contact
with and used to wipe a soiled surface. By "wipe", "wiped" or "wiping" it is
meant wiping,
swiping, rubbing or the like so as to exert manual force upon a surface to be
cleaned. Although
melamine foam is generally quite effective in removing soils and/or stains
from hard surfaces,
consumers still may find it is difficult to remove certain kind of tough
stains with melamine foam
even though extra rubbing force is applied. For example, common adhesive resin-
like or semi-
solid denatured oil stains from food, colored stains, such as tea, coffee,
fruit juice, grass, and
carotenoid stains, permanent marker and ink, mold and mildew, fungus, etc. are
often difficult to
remove with a plain melamine foam.
To improve the cleaning performance of a sponge, such as melamine foam over
certain
type of tough stains, one may use a sponge together with a detergent
composition. Sponge and
detergent can be provided either separately in a kit or the sponge may be
impregnated with
detergents. However, consumers may still find it inconvenient to apply a
detergent composition
and then scrub. A sponge impregnated with an active agent tends to release the
active agent too
quickly, leading to a significant loss of the active agent after the first
several uses. Thus, reduced
cleaning properties are observed as the active agent is used up. Also, when an
active agent
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
2
releases very quickly in the first or second use, the high level of active
agent may require extra
rinsing.
Thus, the need exists for an improved cleaning implement which is able to
clean tough
stains, provide a controlled-release of an active agent and is convenient for
use.
SUMMARY OF THE INVENTION
The present invention encompasses a cleaning implement containing an erodible
foam
substrate and a controlled-release system including an active agent selected
from the group
consisting of a surfactant, a bleaching agent, a limescale reducing agent, a
biocide, a solvent and
a mixture thereof.
The present invention further encompasses a method of cleaning a hard surface
with a
cleaning implement herein.
It has now been surprisingly found that by combining a controlled-release
system with an
active agent and an erodible foam substrate, the cleaning performance of the
cleaning implement
on tough hard surface stains is significantly improved. At the same time, the
lifetime of the active
agent in the cleaning implement is increased and improved cleaning performance
is provided
over a longer period of time.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view of an embodiment of the cleaning implement herein
with an
erodible foam substrate and a second substrate;
Fig 2 is a perspective view of an embodiment of the cleaning implement herein
with three
substrate layers in a oblique rectangular prism-like shape;
Fig 3 is a perspective view of an embodiment of the cleaning implement herein
with a
second substrate and two layers of semi-permeable films; and
Fig. 4 is an exploded view of the cleaning implement shown in Fig. 3.
DETAILED DESCRIPTION OF THE INVENTION
All percentages, ratios and proportions herein are "by-weight", all
temperatures herein are
in degree Celsius ( C), unless otherwise indicated.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
3
As used herein, "average particle size" means the average particle size by
volume of a
given particle material determined by conventional analytical techniques such
as, microscopic
determination.
Cleaning implement
The cleaning implement herein is an article of manufacture of any suitable
shape and/or
size and/or volume suitable for cleaning, i.e., removing spots and/or stains
from hard surfaces.
The cleaning implement herein includes an erodible foam substrate and a
controlled release
system with an active agent therein. "Erodible foam" herein means foam which
crumbles into
small particles and peels off by friction. Useful erodible foam herein
includes, but is not limited
to melamine foam, phenolic foam, etc. In one embodiment herein, the erodible
foam is open-cell
foam having a density in the range of from about 5 to about 1000 kg/m3, or
from about 6 to about
500 kg/m3 or from about 7 to about 300 kg/m3 and a BET surface area in the
range of from about
0.1 to about 50 m2/g, preferably from about 0.5 to about 20 m2/g, determined
according to DIN
66131. As used herein, "open-cell foam" means foam in which at least 50%, or
from about 60%
to about 100%, or from about 65% to about 99.9% of all the lamellae are open,
determined
according to DIN ISO 4590. Said cells can be shaped, e.g. like channels and
can have an average
pore diameter (number-average) in the range from about 1 m to about 1 mm, or
from about 50
m to about 500 m, determined via evaluation of micrographs of sections.
In another embodiment herein, from about 5% to 20%, or from about 10% to 15%
by
weight of the active agent contained in the cleaning implement presents in the
cleaning
implement in free form to ensure that active agent is available in the first
several uses when
active agent in the controlled release system may not yet be sufficiently
released. As used in
contrast to the controlled release system, "active agent in free form" means
that the active agent is
supplied to the cleaning implement in its neat form whose release from the
cleaning implement is
not controlled or sustained on purpose.
Suitable shapes of the cleaning implements herein may be selected from the
group
consisting of a cubic shape, a rectangular shape, a pyramidal shape, a
cylindrical shape, a conic
shape, an oblique rectangular prism shape, a cuboid shape, a tetrahedron
shape, a sphere shape, a
globular shape, and an ellipsoid shape. "Oblique rectangular prism shape"
herein means a
voluminous body having six walls, wherein three pairs of parallel and equally
shaped and sized
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
4
walls exist and wherein one pair of walls are in the shape of a parallelogram
and the remaining
two pairs of walls are of rectangular shape.
The cleaning implement may have any thickness and volume appropriate for its
intended
use. The erodible foam substrate may have a thickness of less than about 30
mm, or from about 2
mm to about 15 mm, or from about 5 mm to about 10 mm and has a total volume of
from about
50 cm3 to about 600 cm3, or from about 80 cm3 to about 300 cm3, or from about
150 cm3 to about
275 cm3. Wherein the cleaning implement contains one or more additional
substrates, each
additional substrate may have any thickness and volume appropriate for its
intended use. In one
embodiment herein, each additional substrate has a thickness of less than
about 30 mm, or from
about 2 mm to about 15 mm, or from about 5 mm to about 10 mm. "Thickness"
means the length
in mm of the side having the smallest extension compared to other sides of the
substrate (the
height of the substrate). Where the cleaning implement is based on an
irregular shape and/or the
extension of the thickness of the substrate varies, it is sufficient that at
least once the thickness of
the substrate extends over the thickness herein.
The erodible foam substrate may be a commercially-available melamine foam
substrate,
e.g., BasotectTm from BASF. Preparation of inelamine foam is known in the art
and described, for
example in WO 2006/017298. Melamine foam typically has a pore size of from
about 20 m to
about 500 m, or from about 50 m to about 200 m. A bead or powder controlled
release system
can be loaded into the melamine foam substrate by dry-spraying. In such a
case, the melamine
foam herein may have a bigger pore size than that of the bead or powder, so as
to facilitate the
penetration of the controlled release system into the melamine foam substrate.
According to
another embodiment herein, the erodible foam substrate is a phenolic foam
substrate.
The cleaning implement herein may include one or more additional substrates,
such as a
second erodible foam substrate or substrates of a material different from the
erodible foam
substrate. Such additional substrate(s) may be attached directly to the first
erodible foam substrate
or to another additional substrate(s). Fig. 1 shows a cleaning implement (1)
with a melamine
foam substrate (2) and a second substrate (3) attached to the melamine foam
substrate (2) by an
adhesive attachment (4). The additional substrate may perform a function
different from the first
erodible foam substrate, for instance to serve as an absorbency substrate, a
wiping substrate, a
supporting substrate, a scrubbing substrate or a handle substrate. Where the
additional substrate is
designed as a handle substrate, controlled release system will be loaded into
the first erodible
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
foam substrate and expelled from the first erodible foam substrate in use.
Hand contact with the
active agent can be minimized by holding only the handle substrate.
Preferably, an indicia, such
as a different color, a marking, a word, etc. is included to guide a user to
hold the handle substrate
and contact the surface to be cleaned with the first erodible foam substrate.
Where the additional substrate(s) is not an erodible foam substrate, the
additional
substrate can be made from a cellulose foam sponge, a naturally occurring
sponge or a nonwoven,
or even a foam of a polymer comprising a monomer selected from the group
consisting of a
urethane, a propylene, an ethylene, a vinyl acetate, an ester, an acrylate, an
ether and a mixture
thereof, such as polyurethane, polypropylene, polyethylene, polyvinyl acetate,
polyester,
polyurethane-ether, polyurethane-ester, polyethylene-vinylacetate,
polyethylene-methacrylate, etc.
The second substrate may be a hydrophilic ester polyurethane foam, such as
CellulexTm from
Foamex L.P., capable of absorbing liquids, but do not swell appreciably. See
US Pat. No.
6,756,416.
The additional substrate may be more hydrophobic than the melamine foam and
used as a
handle substrate. Exemplary hydrophobic substrates include closed-cell foam of
a polymer having
a monomer selected from the group consisting of a urethane, a propylene, an
ethylene, a
butadiene, a styrene, vinyl acetate, a silicon, an ester, an acrylate, an
ether, cellulose acetate,
styrene, silicon, natural latex, rubber, vinylchloride, fluoroethylene and a
mixture thereof,
available as PlastazoteTm, EvazoteTm, SupazoteTm, PropazoteTm from Zotefoams
plc (Croydon,
UK) and FR, FM, CN or SD foam grade made with a significant fraction of
hydrophobic
polymer/materials.
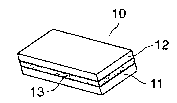
Fig. 2 shows a cleaning implement (10) with three layers of substrate in a
sandwiched
configuration having an oblique rectangular prism shape, wherein at least one
of the two outside
substrates (11) and (12) is a melamine foam substrate. The middle substrate
(13) is a liquid-
impermeable substrate and the controlled release system is contained in one of
substrates (11) and
(12). For example, the controlled release system containing an active agent
may be loaded only
into substrate (12), and the middle substrate (13) is a liquid-impermeable
substrate. In this case,
active agent is released only from substrate (12), substrate (11) can be used
as a handle substrate.
Materials useful as a liquid-impermeable substrate are known, such as the
hydrophobic foam
substrates described above or high barrier hydrophobic nonwoven or plastic
film, such as
polyethylene, polypropylene, polyamide, polyester, TeflonTm, etc.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
6
Where the cleaning implement herein includes more than one substrate or a semi-
permeable film, the erodible foam substrate, the semi-permeable film and the
additional
substrate(s) may be attached by any attachment suitable for joining the
substrates and films. The
attachment may be either permanent (wherein the two substrates cannot be
separated without
inflicting substantial damage to the substrates) or temporary (wherein the two
substrates may be
separated without inflicting substantial damage to the substrates) as desired.
Suitable permanent
attachments include permanent adhesive, foam flame lamination, sewing or
needle-punching the
substrates and/or films together, and a combination thereof. The substrates or
films can also be
joined together by a permanent adhesive. Useful adhesives include vinylic
emulsions, such as
those based on vinyl acetate or other vinyl esters, such as homopolymers and
copolymers of
ethylene and/or acrylic monomers (vinyl acrylics); homopolymers or copolymers
of acrylic
emulsions; a cross-linked adhesive including those created by including a
reactive co-monomer
(e.g., a monomer containing carboxyl, hydroxyl, epoxy, amide, isocyanate, etc.
functionality)
which are capable of cross-linking the polymer themselves (e.g. carboxyl
groups reacting with
hydroxyl, epoxy or isocyanate groups) or by reaction with an external cross-
linker (e.g. urea-
formaldehyde resin, isocyanates, polyols, epoxides, amines and metal salts,
especially zinc). The
adhesives herein can also include limited quantities of tackifying resins to
improve adhesion,
such as the addition of hydrogenated rosin ester tackifier to vinyl
acetate/ethylene copolymer
latex. See also the adhesive compositions in U.S. Pat. No. 5,969,025.
Adhesives can be applied
by, for example, spray coating to give a discontinuous attachment, curtain
coating, roll coating,
slot coating or lick coating to give a continuous attachment.
A suitable temporary attachment includes a weak adhesive, such as low peel
force
adhesive, repositionable adhesive, such as "PSA" (Pressure Sensitive Adhesive)
having
permanent tacks (some also called softgel or hydrogel adhesive, such as
DispomeltTM available
from National Starch); a hook-and-loop fastening system (e.g. VelcoTM); a
water-based, water-
soluble coating or adhesive; an interlocking substrate shape that provides
stability and an
interlocking fit, and a combination thereof.
Referring to Fig. 1, the controlled release system has an active agent in the
adhesive
attachment (4). In another embodiment, the adhesive attachment (4) is liquid-
impermeable and
the controlled release system with an active agent is contained in the
melamine foam substrate
(2). In this case, active agent is released only from substrate (2), thus,
second substrate (3) can be
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
7
used as a handle substrate. Useful liquid-impermeable adhesive materials
include PM17 and LA
hotmelt from Savare (Milano, Italy), PropelTm, SolarCureTM, OptimeltTm,
ClarityTM, Fullback'
hotmelts from Fuller (Minnesota, USA), Fulaprene, Bondseal solvent adhesive
from Fuller, and
RakollTm, AirSperseTM, LiquiLocTM, CasemateTM, water-based adhesives from
Fuller, etc.
Controlled release s, s~
The controlled release system herein contains an active agent and is in
communication
with the erodible foam substrate. The controlled release system can be
impregnated or dispersed
inside the erodible foam substrate, applied onto the attachment attaching the
substrates and/or
applied in-between a semi-permeable film and a second film attached to the
erodible foam
substrate. Exemplary controlled release systems useful herein include those
having a polymer
matrix, a microcapsule, a particulate porous carrier, a complexing agent,
and/or a semi-permeable
film. The controlled release system herein can be a liquid, a gel, a paste, a
bead, a powder or a
film, etc. and can be applied to the cleaning implement by any conventional
means, such as
dipping, spraying, dousing, coating, etc. Where the controlled release system
is a bead or powder,
the bead or powder may be deposited or dry-sprayed onto the substrate while
vibrating the
substrate to permit a better penetration of the bead or powder inside the
substrate. Preferably, the
average particle size of the bead or powder is less than the average pore
diameter of the melamine
foam substrate, i.e. the average particle size of the controlled release
system is from about 1 m
to about 400 m, or from about 10 m to about 100 m, or from about 10 m to
about 30 m.
Where the controlled release system comprises a semi-permeable film, it can be
attached to the
substrate by an adhesive.
The controlled release system may further comprise a plasticizer, a tackifier,
solid
organic or mineral filler, and/or a preserving agent. These materials may
provide some further
advantages, such as binding the substrates or films together, facilitating the
immobilization of
the controlled release system inside the substrate, further controlling the
release of the active
agent, etc.
Suitable plasticizers include citric acid esters, such as
acetyltributylcitrate and
triethylcitrate, low molecular weight polyesters, polyethers, liquid rosin
esters (e.g. ForalynTM
5020F), aromatic sulphonamides, phthalates, benzoates, sucrose esters,
diacetin, polyfunctional
alcohols derivatives, adipates, tartrates, sebacates, esters of phosphoric
acid, fatty acids and
diacids, fatty alcohols and diols, epoxidized vegetable oils and mixtures
thereof.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
8
Tackifiers are used in hot-melt adhesives to improve adhesive properties and
are in
general organic compounds with polycyclic structures. Tackifiers are
thermoplastic materials,
stable at about 200 C, amorphous glassy at room temperature, and have a Tg
higher than about
50 C, or from about 80 C to about 125 C and a molecular weight of from about
500 Daltons to
about 2000 Daltons. The tackifier may be a rosin or its derivative which is
solid at room
temperature, such as alpha-methylstyrene copolymer available as KristalexTm
from Hercules with
a mean molecular weight of about 1200 When present, the tackifier will
represent from about 2%
to about 60%, or from about 5% to about 40% by weight of the controlled
release system.
Suitable solid organic or mineral fillers can be oxides, chlorides, sulfates,
phosphates,
carbonates of Mg, Mn, Ba, Ca, W, Zr, Ti, Si, Mo, in particular Ti02, Si02 and
A1203. Further
suitable materials are water-insoluble sodium polymetaphosphate, hydrated
alumina, dicalcium
orthophosphate dihydrate, calcium pyrophosphate, tricalcium phosphate, calcium
polymetaphosphate.
The amount of the controlled release system in the cleaning implement may vary
widely
due to a variety of factors including capacity of the erodible foam and any
additional substrate(s)
to absorb the controlled release system, viscosity of the controlled release
system, etc. In one
embodiment herein, the cleaning implement contains from about 0.1-500 parts,
or from about
0.5-100 parts, or from about 1-50 parts by weight of the controlled release
system per 100 parts
by weight of the erodible foam substrate.
Polymer matrix
In one embodiment herein, the controlled release system comprises a polymer
matrix and
an active agent. The active agent is absorbed or dissolved in the polymer
matrix or chemically
linked to the polymer matrix. Weight ratio of the polymer matrix to the active
agent is from about
19:1 to about 1:19, or from about 3:2 to 2:3. The controlled release system
comprising polymer
matrix and active agent may be prepared by any known process for manufacturing
thermoplastic
polymeric compositions and will typically comprise steps of melting the
polymer, homogenously
blending the plasticizer and/or tackifier, if present, and the active agent to
form a homogenous
mass which is then cooled to give the controlled release system. Preferred
polymer matrix has
low melt temperature and viscosity and therefore is suitable for use as hot
melts. The controlled
release system may also be prepared using a polymer solution. Typical steps
include dissolving
the polymer, tackifier and/or plasticizer, if present and active agent in an
effective solvent,
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
9
heating, if necessary to prepare a solution or a gel and eliminating the
solvent by evaporation.
Alternatively, the controlled release system may be prepared in the form of an
aqueous emulsion
or dispersion.
The active agent can be released from the controlled release system via
migration, water
activation before use, degradation, such as hydrolysis of the weak chemical
bond linking the
active agent and the polymer matrix, and/or mechanical erosion of the polymer
matrix by friction.
Polymers useful as the polymer matrix herein include thermoplastic hydrophilic
polymers, water-
soluble polymers, water-swellable polymers, polymers erodible by mechanical
friction, polymers
comprising at least one primary and/or secondary amine group, nanoparticle
polymers comprising
cationic monomers. "Water-soluble polymers" herein refers to polymers whose
solubility in
water (pH is 7, 25 C) is more than 1.5 g/1, preferably more than 2 g/l. "Water-
swellable
polymers" herein refers to polymers having a water uptake at 20 C of at least
10% by weight, or
at least 20% by weight, measured according to ISO 8361.
Polymers useful herein can be those having a monomer selected from the group
consisting
of an olefin, an acrylic acid, an acrylate, an ether, a vinyl alcohol, a vinyl
pyrrolidone, a urethane,
a siloxane, a saccharide, an ethylene imine, an amide, an ester, and/or a
vinyl acetate and may
have a molecular weight (Mn) in the range from 500 to 1,000,000 g/mol, or from
1,500 to
500,000 g/mol, or from 2,000 to 200,000 g/mol, or up to 20,000 g/mol,
determined, for example,
by gel permeation chromatography (GPC).
Suitable thermoplastic hydrophilic polymers are disclosed in WO 99/64077,
WO 99/64505 and EP 1193289. Particularly preferred thermoplastic hydrophilic
polymers are
thermoplastic ethylene-vinyl acetate copolymer (e.g. ElvaxTm 250 from Dupont,
a random
ethylene-vinyl acetate copolymer resin with 28% by weight of vinyl acetate and
72% by weight of
ethylene), thermoplastic poly-ether-amide block copolymers (e.g. PebaxTm
MH1657 from
Atofina, a polyethylene oxide-block co-poly-s-caprolactam polymer, each block
has Mw of about
1500 g/mol. Melting point: 204 C according to ASTM D3418), thermoplastic
polyester block
copolymers (e.g. HytrelTm 8171 from Dupont, a hydrophilic
butylenes/polyethylene glycol
phthalate block copolyester), thermoplastic polyurethanes, typically non-
reactive polyurethanes
(e.g. EstaneTm 5170 from Noveon, a polyethylene glycol block polyurethane) and
mixtures
thereof.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
Suitable water-soluble or water-swellable polymers can be polyethylene glycols
(polyethylene oxide) which are solid at room temperature (e.g. PluriolTm E9000
having Mw of
about 9000 g/mol and PluriolTm E400 having Mw of about 400 g/mol), polyvinyl
pyrrolidone,
polyvinyl alcohol and partially hydrolyzed polyvinyl acetate, polyacrylamide,
polysaccharide,
such as agar, dextran, guatti gum, acacia, guar, starch, etc., polynucleotide,
polypeptide
(polyglutamic), polyacrylate, crosslinked polyacrylate, physically cross-
linked polyethylene
oxide, such as AquacalkTm from Sumitomo, polyalkyleneglycol-vinylacetate graft
copolymers,
copolymers of (meth)acrylic acid and (meth)acrylates, such as C1_1o alkyl
esters of (meth)acrylic
acid and copolymers of (meth)acrylic acid and ethylenic unsaturated
dicarboxylic acids, such as
fumaric acid, itaconic acid, and particularly maleic acid.
Suitable erodible polymers can be waxes. Examples of waxes are natural waxes,
such as
paraffin wax, microcrystalline wax, bio-wax, such as lanolin, candellila,
carnauba, mineral wax,
such as montane wax, and synthetic wax, such as polyethylene wax with an
average molecular
weight (Mn) in the range of 500 to 20,000 g/mol, and polypropylene wax with an
average
molecular weight (Mn) in the range of 500 to 20,000 g/mol.
Suitable nanoparticle polymers comprising cationic monomers may contain from
about
0.1% to about 50%, or from about 1% to about 10% by weight of cationic
monomers and having
a particle size of from about 100 nm to about 50 m. The cationic monomers
useful herein
comprise a cationic unit. A cationic unit is understood to mean a moiety which
when
incorporated into the structure of the polymeric particle is capable of
maintaining cationic charge
within the pH range of from about 2 to about 8. The cationic unit is not
required to be
protonated at every pH value within the range of about 2 to about 8. Suitable
cationic monomers
include dimethylamino alkyl acrylates, especially dimethylaminoethyl
methacrylate, vinyl
pyrrolidones, vinyl imidazoyls, vinyl ethers having dialkyl amino groups,
vinyl pyridines, alkyl
acrylamides, dialkyl acrylamides, dialkylamino alkyl acrylamide, and amino
alkyl acylamides.
Suitable polymers comprising at least one primary and/or secondary amine group
can be a
linear homo-, co-polymer and optionally branched, grafted and/or cross-linked
polymer.
Preferably, these polymers comprise more than 10 amino groups and have a
number average
molecular weight (Mn) ranging from about 150 to about 2.10E6, or from about
600 to about
40,000. Examples of such polymers include polyethylene imine commercially
available as
LupasolTm from BASF, polyvinyl amine, polyvinyl amine-vinyl alcohol copolymer,
polyvinyl
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
11
amine-vinyl formamide copolymer, polyamino acid (L-lysine/lauric acid in a
molar ratio of 10:1,
L-lysine/aminocaproic acid/adipic acid in a molar ratio of 5: 5:1, L-
lysine/aminocaproic
acid/ethylhexanoic acid in a molar ratio of 5:3:1), polylysine-cocaprolactam,
polylysine
hydrobromide, cross-linked polylysine, amino substituted polyvinyl alcohol.
In one embodiment herein, the polymer matrix is a polyalkyleneglycol-
vinylacetate graft
copolymer having an average molecular weight (Mn) of from 3,000 to 100,000
g/mol. Examples
of the polyalkyleneglycol-vinylacetate graft copolymer can be made from a base
polymer (A)
selected from:
(Al) polyethylene glycols, which may be capped with one or two C1_25 alkyl
groups, or
uncapped polyethylene glycols, having an average molecular weight Mn in the
range of from
1,500 to 20,000 g/mol, or from 2,500 to 15,000 g/mol;
(A2) copolymers of ethylene oxide and propylene oxide and/or butylene oxide
with an
ethylene oxide content of at least 50% by weight, capped with one or two C1_25
alkyl groups, or
uncapped, having an average molecular weight Mn in the range of from 1,500 to
20,000 g/mol, or
from 2,500 to 15,000 g/mol;
(A3) chain extended products obtainable by conversion of polyethylene glycols
(Al) or
copolymers of ethylene oxide and propylene oxide and/or butylene oxide (A2)
with C2_12
dicarboxylic acids or C2_12 dicarboxylic acid (m)ethyl esters or C6_18
diisocyanates. Particularly
preferably, chain extended products (A3) have an average molecular weight Mn
in the range of
from 2,500 to 25,000 g/mol.
To graft any of the base polymers (Al) to (A3), vinyl esters, such as vinyl
acetate or vinyl
propionate are preferred. In one embodiment herein, vinyl esters are the sole
monomer for
grafting. In another embodiment herein, 1 to 50 mol% of vinyl ester is
replaced by (meth)acrylic
acid.
Microcapsule
In one embodiment herein, the controlled release system includes a
microcapsule
encapsulating an active agent. The microcapsule can be any ruptureable capsule
containing an
active agent therein or capsule which is controllably penetrable by the active
agent encapsulated
therein. The rupture strength of the microcapsules should be within a range
that can endure
handling and spraying without rupturing and yet break by an external force of
friction or
moisture.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
12
Shell of the microcapsules can be made from a wide variety of materials known
in the art.
Suitable microcapsule shell materials and/or methods for making microcapsule
are disclosed in,
e.g., U.S. Patent Nos. 2,800,458; 3,159,585; 3,516,846; 3,516,941; 3,533,958;
3,697,437;
3,778,383; 3,888,689; 3,965,033; 3,996,156; 4,010,038; 4,016,098; 4,087,376;
4,089,802;
4,100,103; 4,251,386; 4,269,729; 4,303,548; 4,460,722; 4,610,927; and
5,591,146; UK Patent
Nos. 1,156,725; 1,483,542; 2,006,709; 2,041,319; 2,048,206 and 2,062,570; and
Benita, Simon
(ed.), Microencapsulation: Methods And Industrial Applications (Marcel Dekker,
Inc. 1996).
Microcapsule herein has an average size of from about 0.1 m to about 1,000
m, or from
1 m to about 500 m, or from about 10 m to about 100 m. The active agent is
contained in
the microcapsule at a level of from about 1% to about 99%, or from about 10%
to about 95%, or
from about 30% to about 90%, by weight of the microcapsule.
Particulate porous carrier
In one embodiment herein, the controlled release system contains a particulate
porous
carrier, and the active agent is releasably loaded into the pores of the
carrier. The particulate
porous carrier absorbs the active agent and then releases it either over an
extended period of time
or as a result of external pressure, moisture, etc. The particulate porous
carrier is typically
selected from silica, silicate, clay, metal oxides, zeolite, sodalite, chitin
micro bead,
carboxyalkylcellulose, starch, sugar, porous carbon, and/or their derivatives.
Preferably, the
particulate porous carrier is Zeolite A having a primary particle size of from
about 0.1 m to
about 10 m.
A typical method for loading an active agent into a particulate porous carrier
includes the
steps of spraying an active agent or a solution of an active agent onto the
particulate porous
carrier followed by stirring the solid mixture or suspension to obtain the
active agent-loaded
particulate porous carrier. Alternatively, one may deposit the particulate
porous carrier onto the
substrate and then spray the active agent or a solution of the active agent
onto the substrate. The
weight ratio of the particulate porous carrier to the active agent is from
about 100: 1 to 1: 1, or
from about 100: 20 to 100: 60.
Complexing agent
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
13
In one embodiment herein, the controlled release system comprises a complexing
agent
binding an active agent. Useful complexing agent can be cyclodextrin.
Both natural or chemically modified cyclodextrin, such as a-, (3-, and y-
cyclodextrin,
glucosyl-a-cyclodextrin, maltosyl-a-cyclodextrin, glucosyl-(3-cyclodextrin,
and maltosyl-(3-
cyclodextrin can be used herein. Polymerized cyclodextrin may also be used and
may form a
complex with the active agent. Suitable solubility of the cyclodextrin/active
agent complex in
water is from about 0.1 g to about 100 g, or from about 0.5 g to 20 g, or from
about lg to 5 g of
complexes per 100 g water at 25 C. In one embodiment herein, the cyclodextrin
is a(3-
cyclodextrin having a water solubility of about 1.8 g to about 2 g per 100 g
water at 25 C. Same
method for loading an active agent into a particulate porous carrier as
described above can be
used for binding cyclodextrin with an active agent. In one embodiment herein,
the molar ratio of
cyclodextrin to the active agent is from about 20:1 to about 1:1 mol
equivalent, or from about
10:3 to about 10:8 mol equivalent.
Semi-permeable film
The controlled release system may contain a first semi-permeable film
attaching to the
erodible foam substrate, a second film of a semi-permeable film or a liquid-
impermeable film
attaching to a second substrate, and an active agent applied in-between the
first semi-permeable
film and the second film. Active agent can be used in its neat form, or in a
form of any of the
controlled release system herein. Fig. 3 and Fig. 4 show a cleaning implement
(20) with a
melamine foam substrate (21), a second substrate (22), and two layers of semi-
permeable film
(23) laminated together and attached to the melamine foam substrate (21) and
the second
substrate (22) separately. An active agent (24) is applied in-between the two
senii-permeable
films (23).
Suitable senii-permeable films herein include flexible liquid-impermeable
films having
open pores, such as polyolephin films based on polyethylene and polypropylene,
polyester,
polyamide, polyester-ether copolymer, polyaniide-ether, TeflonTm films, etc.
These films
typically have a basis weight of 1-250 g/m2, or 2-60 g/m2. Semi-permeable
films are
commercially available from Clopay, RKW, Mitsui, Tacolin, 3M, Dupont,
International plastic,
etc. Pore size and pore density (number of pores per square meter of films)
can be adjusted to
tailor the release kinetics of the active agent through the pores. Typically,
the pore size is from
about 100 m to about 10 mm, or from about 0.5 mm to about 2 mm, and the pore
density is from
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
14
about 100 pores/m2 to about 500,000 pores/m2, or from about 3000 pores/m2 to
about 30,000
pores/m2. Microporous film is generally defined by their water vapor
permeability (WVTR) as
measured, for instance by PERMATRAN-WTM Mode1398 from Mocon (e.g.: ASTM
Standard E-
398). Suitable microporous film has a WVTR of from about 100 to about 25,000
g/m2/day, or
from about 2000 to about 6,000 g/m2/day. Other suitable semi-permeable film is
high liquid-
barrier nonwoven containing a high fraction of fibers made of hydrophobic
material. Typical high
liquid-barrier nonwoven has a basis weight of 1-500 g/m2, or from 10-150 g/m2,
or from 40-80
g/m2. Preferable high liquid-barrier nonwoven is made of 100% of polypropylene
fibers and
formed by spunbond (S), meltblown (M), and combinations thereof, such as SMS,
SMMS, etc.
High liquid-barrier nonwoven is commercially available from BBA, PGI,
Freudenberg, Alsthom,
Jacob holm, etc.
Active agent
The cleaning implement herein contains an active agent selected among a
surfactant, a
bleaching agent, a limescale reducing agent, a biocide, a solvent and a
mixture thereof.
Surfactants can be nonionic, anionic, cationic, amphoteric and/or a
zwitterionic surfactant.
Suitable nonionic surfactants include alkoxylated fatty alcohol having the
formula of
RO(EO)e(PO)pH, where R is a hydrocarbon chain of from 2 to 24 carbon atoms, EO
is ethylene
oxide and PO is propylene oxide, e and p respectively epresenting the average
degree of
ethoxylation and propoxylation, are independently from 0 to 24, or R is a
straight alkyl chain
having from 6 to 22 carbon atoms, e is 5-12 and p is 0. Suitable cationic
surfactants herein
include derivatives of quaternary ammonium, phosphonium, imidazolium and
sulfonium
compounds. Preferred cationic surfactants herein are trimethyl quaternary
ammonium
compounds. Suitable amphoteric surfactants herein include amine oxides,
betaine or ammonium
sulfate or ammonium carboxylate, having the following formula R1R2R3NO,
R1R2R3NR4SO4 or
R1R2R3NR4CO2 wherein each of Rl, R2 and R3 is independently a saturated
substituted or
unsubstituted, linear or branched alkyl groups of from 1 to 30, or from 8 to
18 carbon atoms,
except for R4 which preferably contain 3 saturated carbons. Preferred amine
oxides herein are for
instance natural blend C8-Clo amine oxides, and C12-C16 amine oxides, such as
cetyl dimethyl
amine oxide. Preferred betaine herein is cocamidopropyl betaine and
lauramidopropyl betaine.
Suitable anionic surfactants include alkyl diphenyl ether sulphonate and alkyl
carboxylate. Other
suitable anionic surfactants herein include water soluble salts or acids of
the formula ROSO3M
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
wherein R is preferably a Clo-C24 hydrocarbyl, or C12-C18 alkyl or
hydroxyalkyl, and M is H or a
cation, such as sodium, potassium, lithium, or ammonium or substituted
ammonium. Other
suitable anionic surfactants include soap salts, C9-C20 linear
alkylbenzenesulfonates, C8-C22
primary or secondary alkylsulfonates, sulfonated polycarboxylic acids, C8-C24
alkylpolyglycolethersulfates (containing up to 10 moles of ethylene oxide);
alkyl ester sulfonates,
sulfates of alkylpolysaccharides, alkyl polyethoxy carboxylates, such as those
of the formula
RO(CH2CH2O)kCH2COO-M+ wherein R is a C8-C22 alkyl, k is an integer from 0 to
10, and M is a
soluble salt-forniing cation. Resin acids and hydrogenated resin acids are
also suitable. Further
examples are given in "Surface Active Agents and Detergents" (Vol. I and II by
Schwartz, Perry
and Berch). A variety of such surfactants are also generally disclosed in U.S.
Patent 3,929,678.
Bleaching agent herein is selected from a hydrogen peroxide source, a
preformed
peroxycarboxylic acid, a hypohalite bleach source and a mixture thereof.
Hydrogen peroxide
sources herein include persulfate, dipersulphate, persulfuric acid,
percarbonate, perborate, metal
peroxide, perphosphate, persilicate, urea peroxyhydrate and a mixture thereof.
Preformed
peroxycarboxylic acids herein include those containing one, two or more peroxy
groups, and can
be aliphatic or aromatic. When the organic percarboxylic acid is aliphatic,
the unsubstituted acid
suitably has the linear formula: HO-O-C(O)-(CH2)õ-Y, wherein Y is H, CH3,
CH2C1, COOH or
C(O)OOH; n is an integer of 1-20. Branched analogs are also acceptable. When
the organic
percarboxylic acid is aromatic, the unsubstituted acid suitably has formula:
HO-O-C(O)-C6H4-Y
wherein Y is hydrogen, alkyl, alkyhalogen, halogen, -COOH or -C(O)OOH.
Monoperoxycarboxylic acids useful as oxygen bleach herein are further
illustrated by alkyl
percarboxylic acids and aryl percarboxylic acids such as peroxybenzoic acid
and ring-substituted
peroxybenzoic acids, e.g., peroxy-a-naphthoic acid; aliphatic, substituted
aliphatic and arylalkyl
monoperoxy acids such as peroxylauric acid, peroxystearic acid, and N,N-
phthaloylaminoperoxycaproic acid (PAP); and 6-octylamino-6-oxo-peroxyhexanoic
acid.
Peracids can be used in acid form or any suitable salt with a bleach-stable
cation. Suitable
hypohalite bleaching agents herein include those that form positive halide
ions and/or hypohalite
ions, and bleaching agents that are organic based sources of halides, such as
chloroisocyanurates.
Suitable hypohalite bleaching agents herein include alkali metal and alkaline
earth metal
hypochlorite, hypobromite, hypoiodite, chlorinated trisodium phosphate
dodecahydrate,
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
16
potassium and sodium dichloroisocyanurates, potassium and sodium
trichlorocyanurates, N-
chloroimides, N-chloroamides, N-chloroamines and chlorohydantoins.
Limescale reducing agents herein include, but are not limited to acids and
chelating
agents. Exemplary acids useful herein include hydrochloric acid, phosphoric
acid, sulfuric acid,
sulfamic acid, acetic acid, hydroxyacetic acid, citric acid, benzoic acid,
tartaric acid, formic acid
and mixtures thereof. A mixture of organic and inorganic acid is preferred.
Chelating agents
useful herein can include, but are not limited to, carboxylates, phosphates,
phosphonates,
polyfunctionally-substituted aromatic compounds, polyamines, biodegradable
compounds, the
alkali metal, ammonium or substituted ammonium salts or complexes of these
chelating agents,
and mixtures thereof. Further examples of suitable chelating agents and levels
of use are
described in U.S. Pat. Nos. 3,812,044; 4,704,233; 5,292,446; 5,445,747;
5,531,915; 5,545,352;
5,576,282; 5,641,739; 5,703,031; 5,705,464; 5,710,115; 5,710,115; 5,712,242;
5,721,205;
5,728,671; 5,747,440; 5,780,419; 5,879,409; 5,929,010; 5,929,018; 5,958,866;
5,965,514;
5,972,038; 6,172,021; and 6,503,876.
Biocide means any known ingredient having the ability of reducing or even
eliminating by
killing or removing the micro-organisms existing on a surface, such as those
described in US
6,613,728. Biocide useful herein includes a quaternary surface active
compound, a guanidine, an
alcohol, a glycerol, a phenolic compound, a heavy metal salt, an inorganic and
organic acid, a
halogen, a halogen-containing compound, a dye, an essential oil, an oxidizing
compound, an
adsorbent, a fungicide, an algaecide and a mixture thereof. Exemplary
quaternary surface active
compounds include benzalkonium chloride, benzethonium chloride, cetyl
pyridinium chloride,
sodium tetradecyl sulfate, sichlorobenzalkonium chloride, methylbenzethonium
chloride, cetyl
dimethyl ethyl ammonium bromide. Exemplary guanidines include chlorohexidine
hydrochloride,
chlorohexidine gluconate, dodecylguanidine hydrochloride,
polyhexmethylenebiguanidine
hydrochloride, and 6-acetoxy-2,4-dimethylmetadioxane. Exemplary alcohols
include methanol,
ethanol, propanol, isopropanol, etc. Exemplary phenolic compounds include
cresol, resolcinols
and related compounds, phenol; substituted phenols--cresols, meta-
cresylacetate, creosote,
quaiacol, resorcinol, hexylresorcinol, pyrogallol, thymol, thymol iodide,
picric acid, chlorinated
phenols--dichlorophene, hexachlorophene, tars. Exemplary halogens and halogen-
containing
compounds include iodine and iodoform. Exemplary oxidizing agents include
peroxide, sodium
perporate, potassium permanganate, zinc permanganate, potassium chlorate.
Exemplary heavy
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
17
metal salts include mercuric chloride, miscellaneous ionizable mercuric salts,
organic mercurials,
silver nitrate, silver lactate, silver picrate, silver proteins, silver
halides, zinc oxide, zinc stearate,
copper sulfate and organic tin derivatives. Exemplary dyes include azo dyes,
acridene dyes,
fluorescein dyes, phenolphthalein dyes and triphenylmethane dyes. Exemplary
inorganic and
organic acids include hydrochloric acid, sulfuric acid, nitric acid, citric
acid, sorbic acid, acetic
acid, boric acid, formic acid, maleic acid, adipic acid, lactic acid, malic
acid, malonic acid,
glycolic acid, and mixtures thereof. Exemplary essential oils are thyme oil,
clove oil, cinnamon
oil, geranium oil, eucalyptus oil, peppermint oil, citronella oil, ajowan oil,
mint oil or mixtures
thereof. Other useful biocide herein includes furan derivatives,
nitrofurantoin, sulfur, sulfur
dioxide, ichthamol, chrysarobin, anthralin, betanaphthol, balsams, volatile
oils, chlorophyl.
Biocides useful herein also include fungicides and algaecides which act
against molds and
mildew. Removal of algae and fungi from hard surfaces is difficult. Moreover,
fungi and algae
reappear promptly if not completely removed or inhibited. Suitable fungicides
and algaecides
include metal salts, such as zinc sulfate, zinc acetate, zinc bromide, zinc
chloride, zinc iodide,
zinc nitrate, zinc bromate and zinc chlorate, cooper halide, copper sulfate,
organic tin derivatives,
water-insoluble or partially water-soluble fungicides and algaecides, such as
diiodomethyl p-tolyl
sulfone, N-(trichloromethyl thio) phthalimide, N,N-dimethyl-N'-phenyl N'-
(fluorodichloromethyl
thio) sulphamide, 2-(thiocyanomethylthio) benzothiazole / methylene
bis(thiocyanate), 3-iodo-2-
propynyl butyl carbamate, etc., all available from ALDRICH chemical. Above
biocides are
optionally mixed with concentrated acids, such as acetic acid, formic,
propionic, n-butanoic, n-
pentanoic, trimethylacetic, n-hexanoic, lactic, methoxyacetic, cyanoacetic,
chloroacetic, citric,
partaric, etc.
Active agent can be a solvent having a good dissolving ability for greasy
stains. Solvents
useful herein include those which are at least partially water-miscible, such
as alcohols, ethers,
such as diethylene glycol diethylether, diethylene glycol dimethylether,
propylene glycol
dimethylether, propylene glycol monomethylether, propylene glycol
monoethylether, propylene
glycol monopropylether, propylene glycol monobutylether, ethylene glycol
monobutylether,
dipropylene glycol monomethylether, dipropylene glycol monopropyl ether,
dipropylene glycol
monobutyl ether, diethyleneglycol monobutylether, lower esters of
monoalkylethers of ethylene
glycol or propylene glycol, such as propylene glycol monomethyl ether acetate,
N-methyl
pyrolidone and tetrahydrofuran. Mixtures of several solvents can also be used.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
18
Packaging means
The cleaning implement herein may be combined in an article of manufacture
with a
packaging means known for packaging cleaning implements. Particularly suitable
packaging
means herein can be paper bags, plastic bags, cartons, carton boxes, flow
wraps, plastic wraps,
and paper wraps, and the like and combinations thereof.
Method of cleaning a hard surface
In another embodiment, the present invention encompasses method of cleaning a
hard
surface with a cleaning implement herein. In yet another embodiment, the
present invention
encompasses a method of cleaning a hard surface by bringing a cleaning
implement herein into
contact with said hard surface. "Cleaning" means removing spots and/or stains
from hard
surfaces.
Suitable hard surfaces herein are tiles, walls, floors, sanitary fittings such
as sinks,
showers, shower curtains, wash basins, WCs, household appliances including,
but not limited to,
refrigerators, freezers, washing machines, automatic dryers, ovens, microwave
ovens,
dishwashers and so on.
The methods of cleaning a hard surface herein may additionally include the
step of
wetting said cleaning implement with an appropriate solvent, preferably tap
water, prior to
bringing said cleaning implement into contact with said hard surface.
The present invention is further illustrated by the following examples. In all
the following
examples, the erodible foam substrate is an open-cell foam having an open-cell
factor of 99.6%
according to DIN ISO 4590, a density of 10.0 kg/m3 determined according to EN
ISO 845, an
average pore diameter of 210 m determined via evaluation of micrographs of
sections and a
BET surface area of 6.4 m2/g determined according to DIN 66131. The open-cell
foam is
commercially available as BasotecTM melamine foam from BASF. All the melamine
foam
substrate and any additional substrate(s), if present, have a cuboid shape
with a length of about
125 mm and a width of about 65 mm.
Examples 1-8
A controlled release system comprising a low melting hotmelt polymer matrix
and an
active agent is prepared by mixing the compositions shown in below Table 1 in
a high-shear
hotmelt blender (TM 20 twin screw extruder from Maris, Torino, Italy). All the
percentages in the
table are by weight based on the total weight of the controlled release
system. About 1.5 g of the
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
19
controlled release system is extruded onto the surface of a melamine foam
substrate having a
thickness of about 23 mm and weighted about 1.85g in a series of separate
lanes at about 70 C by
a slot-coating nozzle (EP-1 1 from Nordson Germany or an LH-3 laboratory
coater/laminator from
Acumeter USA). After applying the melted controlled release system onto the
melamine foam
substrate, a second substrate of an open-cell polyurethane foam (SweetaneTM
series by Recticel)
having a thickness of about 6 mm is adhered to the melamine foam substrate
along the surface
coated with the controlled release system by a liquid-impermeable polyamide
hotmelt adhesive
(FullbackTM from Fuller). After the adherence of the two substrates, the
substates are squeezed
for a few times or heated to a higher temperature to facilitate the
penetration of the controlled
release system into the melamine foam substrate and polyurethane foam
substrate. Cleaning
implements having a controlled release system located at the interface of a
melamine foam
substrate and an open-cell polyurethane foam substrate are obtained.
Table 1
1 2 3 4 5 6 7 8
Elvax' 250i 30%
PebaxTMMH16572 30%
Hytrel' 81713 30%
EstaneTM 51704 30%
PluriolTM EE90005 65% 65% 65% 65%
ForalynTM 5020F6 15% 15% 15%
KristalexTM F857 5%
acetyltributylcitrate 10%
triethylcitrate 15%
Diacetin 15% 15%
PluriolTM EE4005 20%
LutensolTM XL708 40%
LutensolTM XL 809 40%
LutensolTM XL 10010 40%
PlurafacTM LF 901 ii 35% 35% 15%
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
N,N-phthaloylaminoperoxy 35%
caproic acid
Sodium hypochlorite 35%
Cetyl pyridinium chloride
Propylene glycol n-butylether 20%
1. ethylene-vinyl acetate copolymer from DuPont
2. polyether-block amides polymer from Atofina Chemicals
3. polyether-polyester block copolymer from DuPont
4. thermoplastic elastic polyurethane from Noveon
5. polyethylene glycol from BASF
6. rosin ester tackifier from Eastman Chemical
7. a-methylstyrene copolymer hydrocarbon resin tackifier from Hercules
8. Clo alkyl polyethelene glycol ether with a degree of ethoxylation of 7 from
BASF
9. Clo alkyl polyethelene glycol ether with a degree of ethoxylation of 8 from
BASF
10. Clo alkyl polyethelene glycol ether with a degree of ethoxylation of 10
from BASF
11. nonionic straight chain primary oxyethylated alcohol from BASF
Examples 9-16
A liquid controlled release system comprising a polymer matrix and an active
agent is
prepared by mixing the compositions shown in Table 2. All the percentages in
Table 2 are by
weight based on the controlled release system. About 1 g of the liquid
controlled release system is
sprayed onto the surfaces of two melamine foam substrates each having a
thickness of about 10
mm using an A7A spray guns or AD handgun from Nordso. The melamine foam
substrates
weight about 0.8g each before applying the controlled-release system. Adhere
the controlled
release system-coated surfaces of the melamine foam substrates to a third
substrate of closed-cell
polypropylene foam (from Zotefoam, UK) having a thickness of about 10 mm in a
sandwiched
configuration. Said third substrate is adhered in-between the two melamine
foam substrates by a
polyamide hotmelt (commercially available as liquid-impermeable FullbackTm
hotmelt adhesive
from Fuller). The three-ply laminate is then manually compressed to allow the
penetration of the
controlled release system into the melamine foam substrates. The cleaning
implements thus
obtained are then air dried to evaporate water and ethanol.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
21
Table 2
9 10 11 12 13 14 15 16
PEG-polyvinyl acetate solutionl 70% 70% 70% 70%
MowiolTm 40-88 solution2 70% 70% 70% 70%
Alkyl polyethylene glycol ether 20%
Cetyl dimethyl amine oxide 10%
Nonionic straight chain primary 20%
oxyethylated alcohol
Sodium dodecyl benzene 10%
sulfonate3
N,N-phthaloylaminoperoxy 30% 30%
caproid acid
Sodium hypochlorite 30% 30%
Cetyl pyridinium chloride 30% 30%
1. A viscous solution mixture containing 70% by weight of polyethylene glycol
grafted with vinyl
acetate, 20% by weight of ethanol and 10% by weight of water
2. A viscous solution mixture available from Harlow Chemical Company Ltd
containing 60% by
weight of polyvinyl alcohol (88% of which is hydrolyzed), 10% by weight of
ethanol and 30% by
weight of water
3. NacconolTm available from Stepan
Examples 17-19
A controlled release system comprising beta-cyclodextrin particles having an
average
particle size of about 150 microns, water and an active agent is prepared by
mixing and stirring
compositions shown in Table 3 for four hours to give a suspension. The
suspension is then
sprayed onto the surface of a melamine foam substrate having a thickness of
about 14 mm and
weighting about 1.lg before loaded with the controlled-release system. The
melamine foam
substrate is then attached to a second melamine foam substrate of equal
thickness using
FullbackTm adhesive from Fuller. Cleaning implements are thus obtained.
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
22
Table 3
17 18 19
Beta-cyclodextrinl 1 g 1 g 1 g
Water 5ml 5m1 5ml
Nonionic surfactant2 0.25 g
N, N-phthaloylaminoperoxycaproic acid 0.25 g
Cetyl pyridinium chloride 0.25 g
1. Cavamax W7 from Wacker
2. Neodol 91-8, a C9_11 alkyl polyethylene glycol surfactant with an
ethoxylation degree of about
8 from Shell
Example 20
A particulate porous carrier mixture of 0.15 g Zeolite A and 0.15 g mesoporous
silica
ZSM-5 having a particle size of about 150 microns is deposited into one of the
two largest
surfaces of a melamine foam substrate having a thickness of about 14 mm and
weighting about
1.1g before loaded with the controlled-release system. A mixture of active
agent comprising
0.067 g nonionic C12_13 primary alcohol ethoxylate surfactant with an
ethoxylation of about 3
(NeodolTm 23-3 from Shell), 0.033g cocoamidopropyl betaine surfactant
(AmphosolTm from
Stepan) and 1 g water is then sprayed onto the zeolite/silica particles
deposited on the surface of
the melamine foam substrate. A second melamine foam substrate of about equal
thickness is then
adhered to the active agent-loaded surface of the first melamine foam
substrate by FullbackTm
adhesive. Cleaning implements with a controlled release system of particulate
porous carrier and
active agent located across the interface of the two melamine foam substrates
are thus obtained.
Examples 21-22
Same steps to Example 20 are repeated, except that the mixture of active agent
is 0.1 g
N,N-phthaloylaminoperoxycaproic acid dissolved in 1 g water (Example 21) and
0.1 g cetyl
pyridinium chloride dissolved in 1 g water (Example 22).
Example 23
A controlled release system is prepared by mixing 0.3 g polyethyleneimine
(LupasolTm
from BASF) and 0.15 g NacconolTm in 5 ml water to form a suspension. The
suspension is then
deposited into one of the two largest surfaces of a melamine foam substrate
having a thickness of
about 14 mm and weighting about 1.1g before loaded with the controlled-release
system. A
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
23
second melamine foam substrate of about equal thickness and weight is then
adhered to the
controlled release system-loaded surface of the first melamine foam. A
cleaning implement is
thus obtained.
Example 24
Active agent described in the above examples is sprayed at an amount of about
25 g/m2
onto a polyethylene film having a basis weight of 30 g/m2 from Tacolin or
Clopay. The active
agent-loaded polyethylene film is then laminated to another virgin
polyethylene film with
ultrasonic discontinuous bonding pattern followed by needle punching treatment
with 1 mm-
diameter needles at a pore density of 1500 pores/m2 (needle punching treatment
may be
conducted prior to ultrasonic lamination). The punched laminated polyethylene
films are then
laminated between two melamine foam substrates each having a thickness of 14
mm and
weighting about 1.1g before loaded with the controlled-release system.
Cleaning implements are
thus obtained.
Example 25
Same steps to Example 24 are taken except that polyethylene films are replaced
with
polypropylene nonwoven (100% PP Melten 65 g/m2 SMS from Tenotext).
Example 26
Same steps to Example 24 are taken except that one of the polyethylene films
is not
subject to needle-punching treatment and the polyethylene film laminates is
then laminated
between a melamine foam substrate having a thickness of about 23 mm and
weighting about 1.85
g before applying the controlled-release system and an open-cell polyurethane
foam substrate
(SweetaneTM series by RecticelTM) having a thickness of about 6 mm, wherein
the unpunched
film is attached to the polyurethane foam substrate.
Example 27
Same steps to Example 26 are taken except that the needle-punched polyethylene
film is
replaced with a microporous polyethylene film (HBBS 40 g/m2 MD stretched from
RKW AG).
All documents cited in the Detailed Description of the Invention are, in
relevant part,
incorporated herein by reference; the citation of any document is not to be
construed as an
admission that it is prior art with respect to the present invention. To the
extent that any
meaning or definition of a term in this document conflicts with any meaning or
definition of the
CA 02635974 2008-07-14
WO 2007/080553 PCT/IB2007/050101
24
same term in a document incorporated by reference, the meaning or definition
assigned to that
term in this document shall govern.
While particular embodiments herein have been illustrated and described, it
would be
obvious to those skilled in the art that various other changes and
modifications can be made
without departing from the spirit and scope of the invention. It is therefore
intended to cover in
the appended claims all such changes and modifications that are within the
scope of this
invention.