Note: Descriptions are shown in the official language in which they were submitted.
CA 02638521 2008-11-03
- 1 -
METHOD OF SELECTIVELY DISSOLVING MINERALS
FROM A CARNALLITE OR SYLVENITE SOURCE
The present invention relates to potassium chloride production and more
particularly to
potassium chloride production from a camallite source.
As is known in the fertilizer art, camallite is a valuable compound in view of
the fact that it
contains potassium chloride, which is valuable to various industries and in
particular, to the
fertilizer industry. Carnallite is described by the formula KC1-MgC12 -6H20.
Currently, when potassium chloride ore is mined, it must undergo significant
unit operations for
upgrading, which is costly and significantly increases the price of this
commodity. For example,
a typical mine is at least a kilometre deep and is of the shaft variety.
Accordingly, this involves a
tremendous amount of expenditure in terms of the drilling of the shaft and
additionally involves
specialized tunnelling to accommodate work areas. Generally speaking, tunnels
in these types of
mines can exceed six kilometres in length and take inordinate amounts of time
to drill. Once
mined, the material must then be crushed, ground and deslimed as initial unit
operations.
Typically, this source of potassium chloride is affected by unacceptably high
levels of salt
(sodium chloride) contamination, which makes it un-saleable. In order to
diminish the quantity
of sodium chloride present, the mined product must undergo flotation to remove
the excessive
sodium chloride. Once this is done, the product must then be dried and sized
with further
processing in terms of compaction and crystallization. One of the problems
with the latter stages
of processing is the storage aspect. Storing the potassium chloride for
lengthy periods of time is
problematic, since the product is inherently hygroscopic. This results in
coagulation and
agglomeration of the crystals in to lumps.
Even with the degree of flotation that is typically employed to produce a
commercial grade of
potassium chloride fertilizer, the existing product in the marketplace is
typically impure and has
occluded impurities as well as a significant degree of magnesium chloride and
sodium chloride
contamination.
40251583.1
CA 02638521 2008-11-03
- 2 -
In order to attempt to circumvent the limitations in conventional potassium
chloride production,
solution mining has been employed.
Solution mining is a widely known mining engineering technique and has been
used extensively
to extract evaporite values from subterranean formations for many years.
The intrinsic value of the evaporites is realized in the fact that contained
potassium is the
progenitor for potash production. The necessity of potash for crop production,
animal feed inter
alia is well known. The value of potash has increased and now approximates
that of crude oil.
The escalating price of potash is based on unprecedented pressure currently
experienced by
farmers for greater and greater food production. Demand has increased prices.
In light of the foregoing, production has increased absent concomitant
improvements in the
existing solution mining techniques.
The techniques for solution mining currently followed involve the formation of
a cavern into
which water is injected as a solvent. This in and of itself is fine, however,
volume control of the
cavern is often uncontrolled and this results, depending on tectonics, in
eventual subsidence of
the formation. This is exacerbated by the fact that the formation pressure is
not maintained
during growth of the cavern. Accordingly, the mine is productive though with
environmental
consequences.
Perhaps one of the most significant limitations with existing techniques is
the issue concerning
tailings. By present methods, the tailings can be significant, require special
handling and occupy
large areas for storage.
It would be desirable to realize the benefits of solution mining in a
carnallite deposit also having
sylvinite contained therein without the limitations of existing methodology.
As is demonstrative of the existing limitations of the art, current process
engineering of the
potassium chloride results in a product that is at best 95% pure potassium
chloride.
This is what the fertilizer market, generally made up of the golf course
industry and agriculture
air-seeding industries, must accept. The particles that are produced with
current technology are
40251583.1
= . CA 02638521 2008-11-03
- 3 -
non-uniform and are plagued with dust problems from disintegrating granules.
There can be as
much as 30% discrepancy in the size of the particles in any one sample. This
makes it extremely
difficult to provide for a consistent, uniform application of the product. As
will be readily
apparent, since the size fluctuation exists in application, there can be
significant variations in
concentration from one area to the next. This makes for wastage of the product
and also can
result in over-fertilization in some areas and under-fertilization in adjacent
areas.
The present invention provides for a uniform pellet product, which has a high
degree of
hardness, resistance to hygroscopicity, can be stored for lengthy periods and
allows the user to
apply the product in a consistent and even concentration while avoiding the
dusting problems,
which contribute to product waste.
In view of the limitations noted in the prior art, the present invention
overcomes the limitations
and provides an improved potassium chloride product as well as a method for
producing the
same.
One object of the invention is to provide an improved formulation for
potassium chloride
fertilizer pellets and a more robust and inexpensive method of producing such
pellets.
Yet another object of one embodiment of the present invention is to provide a
potassium
chloride granule having a purity of 96.5% and 99% and a binder content of 4.5%
and 1%.
As a preface, it is known that ore consists of carnallite and sylvinite and
this is a
mixture of sylvite (KC1, or potassium chloride) and halite (NaC1, or sodium
chloride. The remainder consists of insoluble clays.
Camallite dissolves rapidly from the mixture with the water having a
temperature
of between 20C and 100C with a more desirable range comprising between 40C
and 60C. This dissolution step allows for effective dissolution of potassium
chloride from the sylvinite in situ. With maintenance of the temperature,
sodium
chloride is precipitated while the potassium chloride dissolves to produce a
high
density brine which sinks to the bottom of the cavern (discussed herein
after). In
this manner, the bottom of the cavern may be managed to concentrate potassium
40251583.1
, = . .
CA 02638521 2008-11-03 - 4 -
chloride brine and settle fine salt and clay to leave the salt crystals in the
cavern.
Alternatively, the elevation of the cavern can be adjusted to settle out fine
salt and
from the production brine.
And the aspect of one embodiment of the present invention is to provide a
method of
separating potassium minerals from sodium minerals from a formation containing
sylvinite
and/or carnallite, comprising the steps of:
- providing a subterranean formation containing sylvinite
and/or carnallite;
- introducing water at a temperature of between 20C and 100C
into contact with the
formation to generate a cavern and dissolve the sylvinite;
maintaining an undersaturated concentration of magnesium chloride in the
cavern
to enable selective dissolution of potassium compounds from sodium compounds;
- forming, through passive cooling, in the cavern, a
concentration gradient of
crystallized sylvinite at the base of the cavern and a solution of camallite;
and
- removing the camallite solution from the cavern.
By practicing the methodology set forth herein, the result is a potassium
chloride product, which
has less than 1% sodium salt as an occluded impurity with significantly
reduced dust levels,
compared to competing products.
It has been found that the beneficial consolidation of the potassium chloride,
the resistance to
hygroscopicity and propensity to dust can be controlled if the pellets are
granulated, using
specific binders in a pan-granulation environment. The process involves the
use of gluten
extracted from grains. Suitable grains may be selected from raw rice, barley,
wheat, etc. The
important factor is to have gluten as the binding agent. It has been also
discovered that the
concentration range of 1% to 4.5% of the gluten is particularly effective for
granulation of the
potassium chloride.
40251583.1
CA 02638521 2008-11-03
- 5 -
A still further aspect of one embodiment of the present invention is to
promote a method of
separating potassium minerals from sodium minerals in situ from a subterranean
formation
containing sylvinite and/or carnallite, comprising the steps of:
- providing a subterranean formation containing sylvinite and/or
carnallite;
forming a brine solution cavern within the formation by injection of water
into the
formation;
- maintaining the water temperature to at least 40C to precipitate
sodium chloride
and dissolve potassium chloride from the sylvinite and/or carnallite;
- maintaining an undersaturated concentration of magnesium chloride in
the cavern
to enable selective dissolution of potassium compounds from sodium compounds;
- forming, through passive cooling, in the cavern, a concentration
gradient of
crystallized sylvenite at the base of the cavern and a solution of carnallite;
and
- removing the carnallite solution from the cavern
Regarding product formulation, as an example, the potassium chloride product
does not absorb
moisture below the 60% relative humidity factor. This is ideal for most
conditions under which
the product would be granulated. In terms of moisture content, it is obvious
that granulated
product must be exposed to some moisture in order to induce nucleation and
accordingly, the so-
called greenballs can typically contain between 5% to 10% by weight moisture.
With the foregoing general description of the invention, reference will now be
made to the
accompanying drawings, illustrating preferred embodiments.
Figure 1 is a schematic illustration of the overall extraction process of the
invention according to
one embodiment;
Figure 2 is a schematic illustration of a salt cavern;
Figure 3 is a schematic illustration of a multi cavern system;
40251583.1
CA 02638521 2008-11-03
- 6 -
Figure 4 is a detailed process flow diagram illustrating the process according
to a further
embodiment; and
Figure 5 is schematic illustration of a granulation process employed to
synthesize the potassium
chloride granules.
It will be noted that throughout the appended drawings, like features are
identified by like
reference numerals.
Referring to Figure 1, shown is the overall schematic illustration of the
extraction phase of the
potassium chloride. The origin of potassium chloride is a carnallite ore
source from a
subterranean formation, designated by numeral 10. The ore source may be mined
in a shaft mine
as noted herein previously but preferably by solution mining. Water from an
external source (not
shown) or from a water-bearing formation 12 may be used to solubilize the
carnallite or
otherwise render the carnallite fluid so that the same can be pumped by pump
13 to a purification
stage, which stage may comprise crystallizers 14. In the example, a grouping
in series of
crystallizers 14 are shown; however, it will be apparent to those skilled in
the art that the same
may be arranged in parallel or may include any number of crystallizers,
depending upon the
intended load of the overall circuit. The crystallizers are useful to purify
the potassium chloride,
which is essentially impure when it is introduced into the crystallizers. The
potassium chloride
contains magnesium chloride as an impurity as well as sodium chloride and by
making use of the
crystallization circuit, the KC1 is progressively purified without risk of
significant amounts of
sodium chloride or magnesium chloride being occluded within the potassium
chloride.
Waste brine from the purification process is pumped by a pump 15 to a
subterranean formation,
denoted by numeral 16. This offers an opportunity for the brine to be re-
introduced into an
environment which is not detrimentally affected by spent brines.
The potassium chloride is then dried with a conventional dryer 17 and
subsequently forwarded to
the granulation phase noted in Figure 5.
As noted previously, the ore body upon dissolution with water comprises a
cavern 18, shown in
Figure 2 and produces a brine having the composition as follows: between 8%
and 23% MgC125
40251583.1
, . .
CA 02638521 2008-11-03 - 7 -
12% to 25% or more KC1 and less than 5% NaCl. As is illustrated in Figure 2,
the cavern 18 has
a solubilized carnallite layer 20 and a crystallized sylvinite layer 22. A
distinct advantage in the
process is that the underground process selectively dissolves the magnesium
chloride and
potassium chloride minerals into a brine and does not significantly dissolve
the sodium chloride,
which is at the top and the bottom of the formation. The brine is evaporated
to magnesium
chloride saturation at 120C and then cooled in the evaporative crystallizers
noted herein
previously to the natural equilibrium of potassium chloride at the
temperature. A product of 0.15
t of potassium chloride can be produced for every ton of feed brine. The high
grade potassium
chloride has an impurity of magnesium chloride residual. To remove this, it is
best to transform
the magnesium chloride into magnesium carbonate with soda ash and centrifuge
the magnesium
carbonate and potassium chloride solids as final product. In this manner, the
feedstock of the
potassium chloride for the granulation phase becomes ideal.
Figure 3 illustrates a further embodiment of the present invention where a
multiple cavern
system is employed. In the embodiment shown, a central cavern 18 is networked
in fluid
communication with a series of peripherally arranged subsidiary caverns 18'.
In this manner, the
caverns can continue to dissolve the mineral and extraction from a larger area
becomes
progressively greater, eventually mining the entire area.
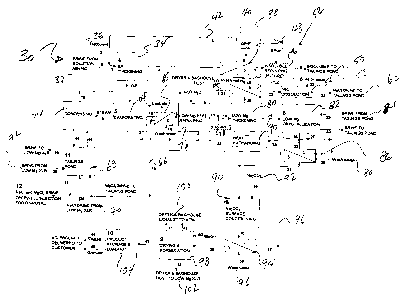
Referring now to Figure 4, shown is a detailed flow diagram for the process
described generally
above. Numeral 30 denotes the overall process. Brine 32 from the solution
mining discussed
above is introduced into a thickener 34 for thickening. A flocculent 36 may be
included to
augment the thickening process. Once thickened, the material is transported to
filter 38 for
filtration into a liquid and solid phase. Wash water 40 may be added to the
liquid phase and the
subsequently washed liquid reintroduced by a circulation loop 42 to the
introduction of thickener
34. The solid material 44 may be optionally introduced into a re pulping
device. This is an
optional operation as indicated in chain line denoted by number 46. In the
event that the
insoluble re pulping stage is included, brine 48 may be introduced into the re
pulping operation.
In any event, insolubles from the solid filtered phase may be introduced into
tailing pond, the
global operation being denoted by numeral 50.
40251583.1
CA 02638521 2012-02-17
= - 8 -
As a further alternative, the insolubles could be transported with a vehicle
directly to the pond.
The transportation step being indicated by numeral 52.
Returning to the filtration of a solid 44, at least a portion may be
introduced into a further filter
54 together with wash water. The so washed solids are then subjected to a
sodium chloride
dissolution step 56 into which brine or water 58 may be introduced into the
operation with
subsequent discharge of the sodium chloride brine to a tailings pond, the
latter step being
denoted by numeral 60.
The liquid phase from filter 54, the liquid being denoted by numeral 62 will
be discussed
hereinafter in greater detail.
Returning to the thickening stage, denoted with thickener 34 at least a
portion of the material
may be introduced into an evaporating stage and particularly an evaporator 64
with the
subsequently increased concentration material being filtered in filter 65. The
liquid from the
filter 65, may be passed on via line 66 to a magnesium chloride brine tailings
pond, denoted by
numeral 68. To the pond may be added additional brine from the sodium chloride
evolved from
the process. Additionally, further brine from the low magnesium
crystallization phase of 76 may
also be added to the tailings pond at this point. This addition is denoted by
numeral 72. A
condensing operation 74 may be included to receive material from the tailings
pond 68 for
condensing with hot water from condensing operation being introduced into a
magnesium feed re
pulping stage 76 a liquid 62 from filter 54 may then be combined with the
magnesium feed re
pulping material and optionally flocculent 78 into a magnesium thickening
operation using
thickener 80. The thickened magnesium containing material is then introduced
to a crystalli7er
82 to which may be introduced brine from the tailings pond, the introduction
being denoted by
numeral 84. The crystallized material then is subjected to filtration phase
using filter 86 to
which wash water 88 may be added. The separated liquid then may be passed into
a heat
exchanger 90 and subsequently returned to the magnesium feed re pulping stage
denoted by
numeral 76.
40248143.1
=
CA 02638521 2008-11-03 - 9 -
Solvents from filter 86 which solids contain residual magnesium chloride on
the surface of the
potassium chloride crystals are introduced into a sodium bicarbonate surface
conditioning
operation, denoted by numeral 90 to which sodium bicarbonate is added at 92.
This operation assists in converting the magnesium chloride to a non reactive
form of
magnesium carbonate. This is an important step in the procedure since it
contributes to the
stability of the final potassium chloride product. As is known, magnesium
chloride is
significantly hygroscopic and will absorb moisture from the air when the
relative humidity
exceeds 30%. By providing this operation, Applicant ensures that the
contaminated potassium
chloride crystals are "conditioned" so that the hygroscopic issue is
alleviated. Once conditioned,
the solution is passed through a filter 94 where the liquid is returned via
line 96 to evaporator 64.
The salt from the filtration is washed with water 97 and the solid
subsequently passed into a
drying and formulation operation, denoted by numeral 98. In this operation
dryer and bag house
exhaust are discharged to the atmosphere at 100 and the bag house and dry air
dust denoted by
numeral 102 are introduced into the magnesium crystallizer 82. The dried and
prepared product
is then either stored or packaged, the broad step being denoted by numeral
104.
Referring now to Figure 5, in the embodiment shown, the circuit is
representative of a ten ton per
hour circuit. Reference to numeral 106 denotes the introduction of potassium
chloride feedstock
initially in a size distribution of between -30 mesh and 100 mesh.
The potassium chloride may be introduced along with suitable binder material
108 set forth
herein previously. The feedstock and binder may be then introduced together
into a pulverizer
110 to pulverize the feedstock such that a product is produced having 90% -200
mesh. The
pulverizer 110 may be a classifying pulverizer or air sweep pulverizer or any
other suitable
pulverizer known by those skilled in the art. Once pulverized, the stream,
generally represented
by numeral 112, is introduced onto a pan granulator globally denoted by
numeral 114, which
includes scrapers 116 and 118. The disposition of the scrapers 116 and 118 can
be positioned to
establish the desired size distribution. Nucleating material 120 may be added
in an amount
between 0% and 3% by weight. The nucleating material may be in a size
distribution of -30 to
40251583.1
=
CA 02638521 2008-11-03 -10-
+100 mesh and assists in initiating the granulation process. Water 121 may be
added to augment
the moisture on the pan when required.
Once the granules are formed, they are dried in a dryer 122 which may be of
the air float carrier
type. Other suitable options are well within the purview of one skilled. The
dry granules are then
screened with screen for classification with granules in the size distribution
-14 to +30 mesh
either being recycled to pan 114 as indicated by 126 or alternatively sold as
product 126. The
oversized material, i.e. +4 mesh can be returned to pulverizer 110 as
indicated by 128. The
product is also screened to a size distribution of -4 to +14 mesh to result in
a product having at
least 95% by weight potassium chloride.
It will also be readily appreciated that the process is interruptible and
therefore can be custom
designed to produce granules having a variety of layers of material to produce
a host of valuable
granules. It will be clear to those skilled in the art that the process is
effective for producing a
number of different forms of fertilizer and has particular utility with
respect to the formation of
high grade fertilizer for use on golf courses, etc.
To this end, the particles may include a compound having reduced solubility
relative to the
fertilizer in order to facilitate a timed release of the fertilizer. Examples
of such material include
sulfur reducing agents, nitrogen fixing compounds, urea, etc. The dissipation
of the fertilizer
could also be timed with the growth cycle of the material receiving the
fertilizer or the
requirement thereof.
Depending upon material being granulated, a range of binders may be selected
for use.
Examples include lignosol, sugars, saturated salts and proteins, water,
calcium sulfate, sodium
sulfate, potassium chloride, dry glutens, wheat grains, barley grains, rice
grains and calcium
phosphate among others. The choice of the binder will depend on the desired
characteristics of
the granule and accordingly, the aforementioned binders are only exemplary.
With respect to the feedstock and binder, when the binder chosen contains a
higher moisture
content, i.e. 5% to 10% the use of additional moisture may not be necessary.
By making use of
the fine powder feedstock and progressive layering of the material, a solid
uninterrupted cross
40251583.1
CA 02638521 2012-02-17
- 11
section for the granule is obtained with homogeneously dispersed feed
throughout. This obviates
the inconsistencies in product content within the granules formulated using
existing technologies.
As a further very significant advantage, the magnesium chloride conversion to
magnesium
carbonate using the soda ash ensures that the surfaces of the potassium
chloride granules do not
absorb moisture. It has been found that storage life is interminable provided
the relative
humidity remains below 60%. This is in marked contrast to existing compactor
prepared
granules which have a short storage life, typically approximately three
months.
402481431