Note: Descriptions are shown in the official language in which they were submitted.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
Novel dual NK2/NK3-antagonists, pharmaceutical compositions
comprising them, and processes for their preparation.
Description
The present invention relates to novel dual NK2/NK3-antagonists and also to
pharmaceutical compositions comprising these compounds. Furthermore, the
invention relates to processes for the preparation of the novel dual NK2/NK3-
antagonists.
Neurokinins (NKs) also kown as tachykinins, include the naturally-occurring
neuropeptides substance P, neurokinin A and neurokinin B. The tachykinins act
as
agonists of receptors occurring in larger mammals and humans, such as the
neurokinin-1 receptor, the NK-2 receptor and the NK-3 receptor. Artificially
prepared
compounds which are antagonistic to tachykinin receptors are usually
classified
according to their relative ability to bind to one or more of the
aforementioned three
receptor subtypes. In the physiological process the tachykinins play e.g. an
important
part in the transmission of pain, emesis, neurogenic inflammation, bladder
inflammation, inflammatory joint diseases or asthmatic complaints.
A review article about NK receptor antagonists was recently published by
Gerspacher (Progress in Medical Chemistry, 2005, Vol. 43, 49 to 103) giving an
overview about recent developments on selective (NK1- or NK2- or NK3-receptor
antagonists) and on combined (NK1/NK2-; NK1/NK2/NK3-; and NK2/NK3-) receptor
antagonists.
The class of combined NK2/NK3-receptor antagonists appears to be limited by
two approaches from GSK and Sanofi. GSK preferred a stepwise modification of
the
structure of the NK3 selective antagonist talnetant, by the introduction of a
variety of
substituents at the meta-position of the quinoline moiety of the molecule. A
highly
effective compound is the following:
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
2
CH3
O N-H
NO-
N
Sanofi-Syntheselabo describes in WO 2002/094821 (published November 28,
2002) a series of piperidine-carboxamide derivatives of the following cyclic,
non-linear
general structure:
O~
N N N
O
N(CH3)2 0 CI
CI
Sanofi-Syntheselabo reports a potent NK2-receptor affinity with a Ki value of
0.04 nM and a potent NK3-receptor affinity with a Ki value of 0.04 nM.
However, compounds with a linear core having either a selective NK3-receptor
affinity or a combined NK2-/NK3-receptor affinity have not been reported so
far.
It was therefore an object of the present invention to provide novel active
compounds with a linear core having properties antagonistic to NK2- and/or NK3
tachykinin receptors. These compounds appear particularly suitable for the
treatment
of a variety of disorders.
Surprisingly, it has now been found that a group of novel linear core-
compounds
is distinguished by properties antagonistic to tachykinin receptors, in
particular to NK2
and/or NK3-receptors. Accordingly, the group of compounds according to the
invention
appears particularly suitable for the treatment of peripheral disorders in
which
tachykinins, in particular neurokinin A and/or neurokinin B, participate as
transfer
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
3
agents, for example for the treatment and/or prophylaxis of any pathology
where either
neurokinin A and/or NK2-receptors, or neurokinin B and/or NK3-receptors, or
both
neurokinin A and neurokinin B and/or NK2 and NK3-receptors are involved.
In more detail, the compounds of the present invention appear particularly
suitable for the treatment and/or prophylaxis of pathologies of the
respiratory,
gastrointestinal, urinary, immune and cardiovascular systems and of the
central
nervous system as well as pain, migraine, inflammation, nausea and vomiting,
and skin
diseases.
In even more detail, the compounds of the present invention appear
particularly
suitable for the treatment and/or prophylaxis of pathologies of respiratory
diseases, in
particular asthma, chronic obstructive pulmonary disease, chronic obstructive
bronchitis, bronchitis, cough, and rhinitis; skin diseases, in particular
inflammatory skin
reactions, allergic skin reactions, and psoriasis; arthropathy diseases, in
particular
arthtitis, vasculitides and systemic lupus erythematosus; functional or
inflammatory
disorders in the gastrointestinal tract, in particular pseudomembranous
colitis, gastritis,
acute and chronic pancreatitis, ulcerative colitis, Crohn's disease and
diarrhoe; bladder
diseases such as cystitis and interstitial cystitis; cardiovascular diseases
such as
hypertension, treatment of cancer especially melanomas, gliomas, small-cell
and large-
cell lung cancers, diseases of the immune system, bipolar disorders; migraine;
pain,
anxiety, depression, cognitive disorders, stress-related somatic disorders,
psychosis, in
particular schizophrenia, mania, schizoaffective disorder and panic disorders.
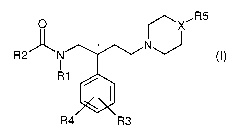
The subject of the present invention are compounds of general formula I,
0 R5
* N~ I
R2 N
I
R1
R4 R3
wherein
R1 is selected from the group consisting of: alkyl and cycloalkyl;
R2 is selected from the group consisting of: alkyl, cycloalkyl, aryl,
alkylenearyl, alkenylenearyl, heteroaryl, and heterocyclic ring;
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
4
R3 and R4 are independently selected from the group consisting of: hydrogen,
halogen, hydroxyl, cyano, and carboxyalkyl;
X is selected from the group consisting of: CR6 and nitrogen;
R5 is selected from the group consisting of: alkyl optionally substituted with
(CO)mNR9R10, cycloalkyl optionally substituted with (CO)m NR9R1O,
and NR7R8;
R6 is selected from the group consisting of: hydrogen, alkyl, cycloalkyl, and
(CO)mNR9R10;
R7 and R8 are independently selected from the group consisting of: alkyl,
cycloalkyl, aryl, alkylenearyl, alkyleneoxyalkyl, COalkyl, COaryl, or
wherein R7 and R8 form together a 5- to 7-membered ring optionally
containing an additional heteroatome, wherein such ring may be
substituted by CONR9R10, and wherein in a 6-membered ring, none of
the ring atoms is replaced by carbonyl;
R9 and R10 are independently selected from the group consisting of: hydrogen,
alkyl, cycloalkyl, aryl, alkylenearyl, alkyleneoxyalkyl, or wherein R9 and
R10 form together a 5- to 7-membered ring optionally containing an
additional heteroatome;
m is selected from 0 or 1;
and physiologically compatible acid addition salts of compounds of Formula I.
Furthermore, another subject of the present invention are pharmaceutical
compositions comprising the compounds of Formula I.
Still another subject of the present invention are processes for the
preparation
of the compounds of Formula I.
Where in the compounds of Formula I or in other compounds described within
the scope of the present invention substituents are or contain alkyl and/or
alkylene,
these may each be straight-chain or branched and possess from 1 to 20 carbon
atoms,
preferably from 1 to 10 carbon atoms, more preferably from 1 to 7 carbon atoms
and
even more preferably from 1 to 4 carbon atoms. The most preferred straight-
chain
alkyl and/or alkylene group is methyl and/or methylene, respectively. The most
preferred branched alkyl and/or alkylene group is isopropyl and/or
isopropylene,
respectively.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
Where in the compounds of Formula I or in other compounds described within
the scope of the present invention substituents are or contain cycloalkyl
and/or
cycloalkylene, these may possess from 1 to 20 carbon atoms, preferably from 1
to 10
carbon atoms, more preferably from 1 to 7 carbon atoms and even more
preferably
5 from 1 to 4 carbon atoms. The most preferred cycloalkyl and/or cycloalkylene
groups
are cyclopentyl and cyclohexyl and/or cyclopentylene and cyclohexylene,
respectively.
Where in the compounds of Formula I or in other compounds described within
the scope of the present invention substituents are or contain alkenyl and/or
alkenylene, these may each be straight-chain or branched and possess from 2 to
20
carbon atoms, preferably from 2 to 10 carbon atoms, more preferably from 2 to
7
carbon atoms and even more preferably from 2 to 4 carbon atoms. The most
preferred
alkenyl and/or alkenylene group is ethenyl and/or ethenylene, respectively.
Where substituents in compounds of Formula I are or contain halogen, fluorine,
chlorine or bromine are suitable. Chlorine is preferred. Where substituents in
compounds of Formula I are or contain carboxyalkyl, -OC(O)alkyl or C(O)Oalkyl
are
suitable, OC(O)alkyl is preferred.
Where substituents in compounds of Formula I are or contain aryl and/or
arylene, monocyclic, bicyclic, tricyclic and tetracyclic aromatic ring systems
are
suitable. Phenyl is preferred.
Where substituents in compounds of Formula I are or contain heteroaryl and/or
heteroarylene, monocyclic, bicyclic, tricyclic and tetracyclic aromatic ring
systems
containing at least one heteroatom such as nitrogen are suitable.
Where substituents in compounds of Formula I are or contain heterocyclic
rings,
monocyclic, bicyclic, tricyclic and tetracyclic non-aromatic ring systems
containing at
least one, if not two or three or even four heteroatoms such as nitrogen
and/or sulfur
and/or oxygen are suitable. A monocyclic ring system is preferred. Nitrogen
and/or
oxygen are preferred as heteratoms.
Where R7 and R8 and/or R9 and R10 form together a 5- to 7-membered ring
and wherein in a particular embodiment, each of these rings independently
optionally
contain an additional heteroatom, such heteroatom may be selected from
nitrogen,
oxygen and sulphur, preferably oxygen.
Where in the compounds of Formula I or in other compounds described within
the scope of the present invention are or contain alkyl and/or alkylene, alkyl
and/or
alkylene, aryl and/or arylene, heteroaryl and/or heteroarylene, heterocyclic
rings, all
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
6
these substituents may be further substituted by any of: alkyl, alkenyl,
hydroxyl, SH,
carbonylalkyl, carboxyalkyl, carbonylaryl, carboxyaryl, carbonylheteroaryl,
carboxyheteroyaryl, carbonylheterocyclic ring, carboxyheterocyclic ring,
halogen,
cyano, oxyalkyl, oxyalkenyl, aryl, heteroaryl, NOz, SO2R11, and SO3R11.
In a preferred embodiment of the present invention, in compounds of Formula I,
R1 is methyl.
In another preferred embodiment of the present invention, R3 and R4 are
independently selected from the group consisting of: hydrogen, fluoro, chloro,
preferably hydrogen or chloro.
In third preferred embodiment of the present invention, in compounds of
Formula I X is CR6, R5 is NR7R8, and R6 is (CO),rNR9R10 with m =1.
In another preferred embodiment of the present invention, in compounds X is N,
R5 is cycloalkyl substituted with (CO)mNR9R10 and m =1.
In a fourth preferred embodiment of the present invention, in compounds of
Formula I, R7 and R8 form together a 6-membered ring or R7 and R8 form
together a
6-membered ring substituted by CONR9R10.
In a fifth preferred embodiment of the present invention, in compounds of
Formula I R9 and R10 are both methyl, or R9 and R10 form together a 6-membered
ring, or R9 and R10 form together a 5-membered ring substituted by carbonyl.
In another preferred embodiment of the present invention, in compounds of
Formula I R2 is selected from the group consisting of: Cl to C20 alkyl; C3 to
C20
cycloalkyl; C2 to C20 alkenyl;
R11
(HzC) t R12 (HzC) t Y, (~ ~ Hz)t
I
Q \ ~V
R15 R13 R16 Q' V=V
R14
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
7
R18
z
R1
R19 R19
O
z z
R18 O
., I \ F ' ., I \
O \
x and
o 'F / z z
wherein each of R11 to R16 are independently selected from the group
consisting of: hydrogen, fluoro, chloro, bromo, hydroxyl, alkoxy, cyano,
N(H)C(O)Oalkyl, aminoalkyl, dialkylamino, OCF3, CF3, carboxyalkyl, S(O)2NH2,
phenyl,
alkyl, and cycloalkyl;
wherein each of R18 and R19 are independently selected from the group
consisting of:
hydrogen, cyano and aryl;
wherein t is 0 or 1;
wherein each Q is independently selected from the group consisting of: CR1 1
and N;
wherein Y is selected from the group consisting of: CH, N and NO;
wherein Z is selected from the group consisting of: C-benzyl, NH, N-benzyl, N-
alkyl, 0
and S;
wherein each V is independently selected from the group consisting of: N and
CR17;
and;
wherein R17 is selected from the group consisting of: hydrogen, alkyl,
cycloalkyl, aryl,
and thioalkyl.
In another preferred embodiment of the present invention, R5 is selected from
the group consisting of:
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
8
N(CH3)Z
O
--N ; - N ; --- N % ; and ---N
Particular embodiments of the present invention are the following compounds:
1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid methylamide; 1'-[4-
(Cyclohexanecarbonyl-
methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(4-fluoro-benzoyl)-methyl-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; Acetic acid 4-{[2-(3,4-
dichloro-
phenyl)-4-(4'-dimethylcarbamoyl-[1,4']bi-piperidinyl-1'-yl)-butyl]-methyl-
carbamoyl}-
phenyl ester; 1'-{3-(3,4-Dichloro-phenyl)-4-[(4-hydroxy-benzoyl)-methyl-amino]-
butyl}-
[1,4']bi-piperidinyl-4'-carboxylic acid dimethylamide; Acetic acid 2-{[2-(3,4-
dichloro-
phenyl)-4-(4'-dimethylcarbamoyl-[1,4']bi-piperidinyl-1'-yl)-butyl]-methyl-
carbamoyl}-
phenyl ester; 1'-[4-[(3-Chloro-4-fluoro-benzoyl)-methyl-amino]-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[(3,5-difluoro-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-[4-[(5-Chloro-2-fluoro-benzoyl)-methyl-amino]-3-(3,4-
dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-
(3,4-Dichloro-
phenyl)-4-[methyl-(naphthalene-l-carbonyl-3-cyano)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-hydroxy-
benzoyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[(2,4-difluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3,4-difluoro-
benzoyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[(2,5-difluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2,3,4-
trifluoro-
benzoyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
1'-{3-(3,4-
Dichloro-phenyl)-4-[methyl-(1-oxy-pyridine-4-carbonyl)-amino]-butyl}-
[1,4']bipiperidinyl-
4'-carboxylic acid dimethylamide; 1'-[4-[(6-Chloro-pyridine-3-carbonyl)-methyl-
amino]-3-
3 0 (3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-{3-
(3,4-Dichloro-phenyl)-4-[methyl-(pyridine-3-carbonyl)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
9
carboxylic acid dimethylamide; 1'-[4-[(3-Benzyl-2-methylsulfanyl-3H-imidazole-
4-
carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(4-oxo-2-phenyl-4H-
chromene-3-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide;
1'-[4-(Cyclopropanecarbonyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bi-
piperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-(Cyclopentanecarbonyl-
methyl-
amino)-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-methylamino-benzoyl)-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; N-[2-(3,4-Dichloro-
phenyl)-4-(4'-
dimethylcarbamoyl-[1,4']bipiperidinyl-1'-yl)-butyl]-N-methyl-phthalamic acid;
1'-{3-(3,4-
Dichloro-phenyl)-4-[(4-methoxy-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-[(Biphenyl-4-carbonyl)-methyl-amino]-3-
(3,4-
dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
1'-{3-(3,4-
Dichloro-phenyl)-4-[(3,3-diphenyl-propionyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-(3-(3,4-Dichloro-phenyl)-4-{[3-(4-hydroxy-
phenyl)-
propionyl]-methyl-amino}-butyl)-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-
{3-(3,4-Dichloro-phenyl)-4-[methyl-(1-methyl-1 H-pyrrole-2-carbonyl)-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-
[(furan-2-carbonyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(naphthalene-2-carbonyl)-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(2-Biphenyl-4-yl-
acetyl)-
methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-[4-{[3-(4-Chloro-phenyl)-acryloyl]-methyl-amino}-3-(3,4-
dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-
(3,4-Dichloro-
phenyl)-4-[methyl-(1 H-pyrrole-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-
4'-carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(furan-2-carbonyl)-methyl-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[methyl-(thiophene-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(thiophene-3-carbonyl)-
amino]-
3 0 butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[(1 H-indole-3-carbonyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-1 H-indol-3-yl-acetyl)-methyl-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[(1 H-indole-5-carbonyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(pyrazine-2-carbonyl)-
amino]-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[methyl-(pyridine-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(pyridine-4-carbonyl)-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
5 [methyl-(4-oxo-4H-chromene-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(4-sulfamoyl-
benzoyl)-
amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(4-
Chloro-3-
sulfamoyl-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-1 H-imidazol-
4-yl-
10 acetyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-{3-
(3,4-Dichloro-phenyl)-4-[methyl-(2-pyridin-2-yl-acetyl)-amino]-butyl}-
[1,4']bipiperidinyl-
4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-
pyridin-3-yl-
acetyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[methyl-(2-pyridin-4-yl-acetyl)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-[(1-Acetyl-piperidine-4-carbonyl)-methyl-
amino]-3-
(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-{3-
(3,4-Dichloro-phenyl)-4-[methyl-(tetrahydro-pyran-4-carbonyl)-amino]-butyl}-
[1,4']bi-
piperidinyl-4'-carboxylic acid dimethylamide; (4-{[2-(3,4-Dichloro-phenyl)-4-
(4'-
dimethylcarbamoyl-[1,4']bipiperidinyl-1'-yl)-butyl]-methyl-carbamoyl}-phenyl)-
carbamic
acid tert-butyl ester; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3-{trifluoromethyl-
methoxy}-
benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide;
1'-(3-(3,4-Dichloro-phenyl)-4-{[2-(2,4-di{trifluoromethyl}-phenyl)-acetyl]-
methyl-amino}-
butyl)-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-(3-(3,4-
Dichloro-phenyl)-4-
{[2-(2,6-dihydroxy-pyrimidin-4-yl)-acetyl]-methyl-amino}-butyl)-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 4-{[2-(3,4-Dichloro-phenyl)-4-(4'-
dimethylcarbamoyl-
[1,4']bipiperidinyl-1'-yl)-butyl]-methyl-carbamoyl}-piperidine-1-carboxylic
acid tert-butyl
ester; 1'-{3-(3,4-Dichloro-phenyl)-4-[(1 H-imidazole-4-carbonyl)-methyl-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; (1-{[2-(3,4-Dichloro-
phenyl)-4-(4'-
dimethylcarbamoyl-[1,4']bipiperidinyl-1'-yl)-butyl]-methyl-carbamoyl}-2-phenyl-
ethyl)-
3 0 carbamic acid tert-butyl ester; [2-(3,4-Dichloro-phenyl)-4-(4'-
dimethylcarbamoyl-
[1,4']bipiperidinyl-1'-yl)-butyl]-methyl-carbamic acid tert-butyl ester; 1'-{3-
(3,4-Dichloro-
phenyl)-4-[(furazan-3-carbonyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2,2-difluoro-
benzo[1,3]dioxole-5-
carbonyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-
3 5 {3-(3,4-Dichloro-phenyl)-4-[methyl-(1 H-pyrrole-3-carbonyl)-amino]-butyl}-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
11
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-(3-(3,4-Dichloro-
phenyl)-4-{[3-(4-
fluoro-phenyl)-5-methyl-isoxazole-4-carbo-nyl]-methyl-amino}-butyl)-[1,4']
bipiperid inyl-
4'-carboxylic acid dimethylamide; 1'-(3-(3,4-Dichloro-phenyl)-4-{[5-(4-methoxy-
phenyl)-
oxazole-4-carbonyl]-methyl-amino}-butyl)-[1,4']bipiperidinyl-4'-carboxylic
acid dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(5-methyl-l-phenyl-1 H-
[1,2,3]triazole-4-
carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
1'-[4-
[(Benzofuran-5-carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperi-
dinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-
(5-methyl-
benzo[b]thiophene-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-[4-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]-3-(3,4-
dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(2-
Bromo-
benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-fluoro-benzoyl)-methyl-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[3-(3,4-
Dichloro-phenyl)-4-
(methyl-pentafluorobenzoyl-amino)-butyl]-[1,4']bipiperidinyl-4'-carboxylic
acid dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2,6-difluoro-benzoyl)-methyl-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(2,4-Dichloro-
benzoyl)-methyl-
amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-[4-[(2,6-Dichloro-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-
[methyl-(2-trifluoromethyl-benzoyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-methyl-benzoyl)-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(3-
fluoro-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-[4-[(3-Chloro-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bi-
piperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(3,4-Dichloro-benzoyl)-
methyl-
amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3-methoxy-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-
3 0 [methyl-(3-trifluoromethyl-benzoyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid di-
methylamide; 1'-[4-[(4-Chloro-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(4-
methoxy-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(4-trifluoromethyl-benzoyl)-
amino]-butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(4-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
12
methyl-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2,2-dimethyl-propionyl)-methyl-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[3-(3,4-Dichloro-
phenyl)-4-
(methyl-phenylacetyl-amino)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide;
1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-phenyl-cyclopropanecarbonyl)-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(4-Cyano-benzoyl)-
methyl-
amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethyl-
amide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(naphthalene-1-carbonyl)-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(3-Cyano-
naphthalene-l-
carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-[4-(Benzoyl-methyl-amino)-3-phenyl-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid ethyl-methyl-amide; N-{2-(3,4-
Dichloro-
phenyl)-4-[4-(1-dimethylcarbamoyl-cyclohexyl)-piperazin-l-yl]-butyl}-N-methyl-
benz-
amide; 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-2-
carboxylic acid dimethylamide; 1-[4-[(3-Cyano-naphthalene-l-carbonyl)-methyl-
amino]-
3-(3,4-dichloro-phenyl)-butyl]-4-pyrrolidin-1-yl-piperidine-4-carboxylic acid
dimethyl-
amide; 1-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-4-pyrrolidin-
l-yl-
piperidine-4-carboxylic acid dimethylamide; N-[4-[4-(Cyclopropylmethyl-
propionyl-
amino)-piperidin-1-yl]-2-(3,4-dichloro-phenyl)-butyl]-N-methyl-benzamide; N-{2-
(3,4-
Dich loro-phenyl)-4-[4-( isopropyl-propionyl-a mi no)-piperid i n-1-yl]-butyl}-
N-methyl-
benzamide; N-{2-(3,4-Dichloro-phenyl)-4-[4-(phenyl-propionyl-amino)-piperidin-
1-yl]-
butyl}-N-methyl-benzamide; N-[4-[4-(Butyl-propionyl-amino)-piperidin-1-yl]-2-
(3,4-
dichloro-phenyl)-butyl]-N-methyl-benzamide; N-[4-[4-(Butyl-
cyclopropanecarbonyl-
amino)-piperidin-1-yl]-2-(3,4-dichloro-phenyl)-butyl]-N-methyl-benzamide; N-[4-
[4-
(Butyl-cyclohexanecarbonyl-amino)-piperidin-1-yl]-2-(3,4-dichloro-phenyl)-
butyl]-N-
methyl-benzamide; N-[4-[4-(Benzoyl-butyl-amino)-piperidin-1-yl]-2-(3,4-
dichloro-
phenyl)-butyl]-N-methyl-benzamide; N-(2-(3,4-Dichloro-phenyl)-4-{4-[(4-methoxy-
butyl)-
propionyl-amino]-piperidin-1-yl}-butyl)-N-methyl-benzamide; N-[4-{4-
3 0 [Cyclopropanecarbonyl-(4-methoxy-butyl)-amino]-piperidin-1 -yl}-2-(3,4-
dichloro-
phenyl)-butyl]-N-methyl-benzamide; N-[4-{4-[Cyclohexanecarbonyl-(4-methoxy-
butyl)-
amino]-piperidin-l-yl}-2-(3,4-dichloro-phenyl)-butyl]-N-methyl-benzamide; N-[4-
{4-
[Benzoyl-(4-methoxy-butyl)-amino]-piperidin-l-yl}-2-(3,4-dichloro-phenyl)-
butyl]-N-
methyl-benzamide; N-[4-{4-[cyclohexyl(propionyl)amino]piperidin-1-yl}-2-(3,4-
dichlorophenyl)butyl]-N-methylbenzamide; N-[4-{4-
[cyclohexyl(cyclopropylcarbonyl)-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
13
amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-methylbenzamide; N-[4-{4-
[cyclo-
hexyl(cyclohexylcarbonyl)amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-
methyl-
benzamide; N-[4-{4-[benzoyl(cyclohexyl)amino]piperidin-1-yl}-2-(3,4-
dichlorophenyl)-
butyl]-N-methylbenzamide; N-[2-(3,4-dichlorophenyl)-4-{4-[(1-methylpiperidin-4-
yl)-
(propionyl)amino]piperidin-l-yl}butyl]-N-methylbenzamide; N-[4-{4-
[(cyclopropyl-
carbonyl)(1-methylpiperidin-4-yl)amino]piperid in-1 -yl}-2-(3,4-
dichlorophenyl)butyl]-N-
methylbenzamide; N-[4-{4-[(cyclohexylcarbonyl)(1-methylpiperidin-4-
yl)amino]piperidin-
1-yl}-2-(3,4-dichlorophenyl)butyl]-N-methylbenzamide; N-{1-[4-
[benzoyl(methyl)amino]-
3-(3,4-dichlorophenyl)butyl]piperidin-4-yl}-N-(1-methylpiperidin-4-
yl)benzamide; N-{2-
(3,4-Dichloro-phenyl)-4-[4'-(pyrrolidine-1 -carbonyl)-[1,4']bipiperidinyl-1'-
yl]-butyl}-N-
methyl-benzamide; 1-[4-[(3-Cyano-naphthalene-1-carbonyl)-methyl-amino]-3-(3,4-
dichloro-phenyl)-butyl]-4-(2-oxo-pyrrolidin-1-yl)-piperidine-4-carboxylic acid
dimethyl-
amide; 3-Cyano-naphthalene-1 -carboxylic acid {2-(3,4-dichloro-phenyl)-4-[4'-
(piperidine-1 -carbonyl)-[1,4']bipiperidinyl-1'-yl]-butyl}-methyl-amide; 1'-[4-
(Benzoyl-
methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic
acid di-
propylamide; 1-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-4-
morpholin
-4-yl-piperidine-4-carboxylic acid dimethylamide; 1-[4-(Benzoyl-methyl-amino)-
3-(3,4-
dichloro-phenyl)-butyl]-4-pyrrolidin-l-yl-piperidine-4-carboxylic acid
isopropyl-methyl-
amide; N-{2-(3,4-Dichloro-phenyl)-4-[4-(piperidine-1-carbonyl)-4-pyrrolidin-l-
yl-
2 0 piperidin-l-yl]-butyl}-N-methyl-benzamide; 1-[4-(Benzoyl-methyl-amino)-3-
(3,4-
dichloro-phenyl)-butyl]-4-pyrrolidin-l-yl-piperidine-4-carboxylic acid
diethylamide; N-{2-
(3,4-Dichloro-phenyl)-4-[4-(morpholine-4-carbonyl)-4-pyrrolid in-l-yl-
piperidin-l-yl]-
butyl}-N-methyl-benzamide; and physiologically compatible acid addition salts
of these
compounds.
Particularly preferred are the following compounds:
1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid methylamide; 1'-[4-
(Cyclohexanecarbonyl-
3 0 methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(4-fluoro-benzoyl)-methyl-amino]-
butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; Acetic acid 4-{[2-(3,4-
dichloro-
phenyl)-4-(4'-dimethylcarbamoyl-[1,4']bi-piperidinyl-1'-yl)-butyl]-methyl-
carbamoyl}-
phenyl ester; 1'-{3-(3,4-Dichloro-phenyl)-4-[(4-hydroxy-benzoyl)-methyl-amino]-
butyl}-
[1,4']bi-piperidinyl-4'-carboxylic acid dimethylamide; Acetic acid 2-{[2-(3,4-
dichloro-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
14
phenyl)-4-(4'-dimethylcarbamoyl-[1,4']bi-piperidinyl-1'-yl)-butyl]-methyl-
carbamoyl}-
phenyl ester; 1'-[4-[(3-Chloro-4-fluoro-benzoyl)-methyl-amino]-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[(3,5-difluoro-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-[4-[(5-Chloro-2-fluoro-benzoyl)-methyl-amino]-3-(3,4-
dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-
(3,4-Dichloro-
phenyl)-4-[methyl-(naphthalene-1-carbonyl-3-cyano)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-hydroxy-
benzoyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[(2,4-difluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3,4-difluoro-
benzoyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[(2,5-difluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2,3,4-
trifluoro-
benzoyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
1'-[4-[(6-
Chloro-pyridine-3-carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-
[methyl-(pyridine-3-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-[4-(Cyclopentanecarbonyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[methyl-(2-methylamino-benzoyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; N-[2-(3,4-Dichloro-phenyl)-4-(4'-dimethylcarbamoyl-
[1,4']bipiperidinyl-
1'-yl)-butyl]-N-methyl-phthalamic acid; 1'-{3-(3,4-Dichloro-phenyl)-4-[(4-
methoxy-
benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-(3-
(3,4-Dichloro-phenyl)-4-{[3-(4-hydroxy-phenyl)-propionyl]-methyl-amino}-butyl)-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-
[methyl-(1-methyl-1 H-pyrrole-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(furan-2-carbonyl)-methyl-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
3 0 [methyl-(naphthalene-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(1 H-pyrrole-2-carbonyl)-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[(furan-2-carbonyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(thiophene-2-carbonyl)-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
[methyl-(thiophene-3-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(1 H-indole-5-carbonyl)-methyl-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[methyl-(pyridine-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
5 dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(pyridine-4-carbonyl)-
amino]-
butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[methyl-(4-oxo-4H-chromene-2-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(4-sulfamoyl-
benzoyl)-
amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(4-
Chloro-3-
10 sulfamoyl-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-
pyridin-2-yl-
acetyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[methyl-(2-pyridin-3-yl-acetyl)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-
pyridin-4-yl-
15 acetyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
1'-{3-(3,4-
Dichloro-phenyl)-4-[methyl-(tetrahydro-pyran-4-carbonyl)-amino]-butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; (4-{[2-(3,4-Dichloro-
phenyl)-4-(4'-
dimethylcarbamoyl-[1,4']bipiperidinyl-1'-yl)-butyl]-methyl-carbamoyl}-phenyl)-
carbamic
acid tert-butyl ester; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3-{trifluoromethyl-
methoxy}-
benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-(3-
(3,4-Dichloro-phenyl)-4-{[2-(2,4-di{trifluoromethyl}-phenyl)-acetyl]-methyl-
amino}-butyl)-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(2,2-
difluoro-benzo[1,3]dioxole-5-carbonyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(5-methyl-
1-
phenyl-1 H-[1,2,3]triazole-4-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-[4-[(Benzofuran-5-carbonyl)-methyl-amino]-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(2-Bromo-
benzoyl)-
methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-fluoro-benzoyl)-methyl-amino]-
butyl}-
3 0 [1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(2,6-
difluoro-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-[4-[(2,4-Dichloro-benzoyl)-methyl-amino]-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-phenyl)-4-
[methyl-(2-trifluoromethyl-benzoyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-methyl-benzoyl)-amino]-
butyl}-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
16
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(3-
fluoro-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-[4-[(3-Chloro-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(3-
methoxy-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(3-trifluoromethyl-
benzoyl)-
amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-
Dichloro-
phenyl)-4-[(4-methyl-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-
carboxylic acid
dimethylamide; 1'-[4-[(4-Cyano-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(3-Cyano-
naphthalene-l-
carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-
carboxylic
acid dimethylamide; 1'-[4-(Benzoyl-methyl-amino)-3-phenyl-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid ethyl-methyl-amide; N-{2-(3,4-
Dichloro-
phenyl)-4-[4-(1-dimethylcarbamoyl-cyclohexyl)-piperazin-1-yl]-butyl}-N-methyl-
benzamide; 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-2-carboxylic acid dimethylamide; 1-[4-[(3-Cyano-
naphthalene-1-
carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-4-pyrrolidin-1-yl-
piperidine-4-
carboxylic acid dimethylamide; 1-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-
butyl]-4-pyrrolidin-1-yl-piperidine-4-carboxylic acid dimethylamide; and
physiologically
compatible acid addition salts of these compounds.
Most preferred compounds of the present invention are the following:
1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-(Cyclohexanecarbonyl-methyl-amino)-3-(3,4-
dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
1'-{3-(3,4-
Dichloro-phenyl)-4-[(4-fluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; Acetic acid 4-{[2-(3,4-dichloro-phenyl)-4-(4'-
dimethylcarbamoyl-[1,4']bi-piperidinyl-1'-yl)-butyl]-methyl-carbamoyl}-phenyl
ester; 1'-
3 0 {3-(3,4-Dichloro-phenyl)-4-[(4-hydroxy-benzoyl)-methyl-amino]-butyl}-
[1,4']bi-
piperidinyl-4'-carboxylic acid dimethylamide; Acetic acid 2-{[2-(3,4-dichloro-
phenyl)-4-
(4'-dimethylcarbamoyl-[1,4']bi-piperidinyl-1'-yl)-butyl]-methyl-carbamoyl}-
phenyl ester;
1'-[4-[(3-Chloro-4-fluoro-benzoyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-
phenyl)-4-[(3,5-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
17
difluoro-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-[4-[(5-Chloro-2-fluoro-benzoyl)-methyl-amino]-3-(3,4-
dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-{3-
(3,4-Dichloro-
phenyl)-4-[methyl-(naphthalene-1-carbonyl-3-cyano)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(2-hydroxy-
benzoyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[(2,4-difluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3,4-difluoro-
benzoyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[(2,5-difluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-[4-[(6-Chloro-pyridine-3-carbonyl)-methyl-
amino]-3-
(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-[4-
(Cyclopentanecarbonyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-
4'-carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(furan-2-
carbonyl)-
methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[methyl-(pyridine-2-carbonyl)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(2-
pyridin-2-yl-
acetyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-
{3-(3,4-
Dichloro-phenyl)-4-[methyl-(2-pyridin-3-yl-acetyl)-amino]-butyl}-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[(3-
{trifluoromethyl-
methoxy}-benzoyl)-methyl-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid
dimethylamide; 1'-{3-(3,4-Dichloro-phenyl)-4-[methyl-(5-methyl-1-phenyl-1H-
[1,2,3]triazole-4-carbonyl)-amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic
acid
dimethylamide; 1'-[4-[(Benzofuran-5-carbonyl)-methyl-amino]-3-(3,4-dichloro-
phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide; 1'-[4-[(3-Cyano-
naphthalene-
1-carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-[1,4']bipiperidinyl-
4'-carboxylic
acid dimethylamide; 1-[4-[(3-Cyano-naphthalene-1-carbonyl)-methyl-amino]-3-
(3,4-
dichloro-phenyl)-butyl]-4-pyrrolidin-1-yl-piperidine-4-carboxylic acid
dimethylamide; and
physiologically compatible acid addition salts of these compounds.
The compounds of Formula I and their acid addition salts may be prepared by
reacting a compound of the general Formula II
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
18
O
* i0
R2 N II
R1 /
R4 R3
wherein R1 to R4 have the above meanings, with a compound of formula III
r^~ R5 HNJ III
wherein R5 has the above meaning, to result in a compound of general formula I
which
is optionally converted into its physiologically compatible acid addition
salt.
Alternatively, the compounds of Formula I and their acid addition salts may be
prepared by reacting a compound of the general Formula III
X R5
r
HNJ III
wherein R5 has the above meaning, with a compound of general formula IV
O
AIkyIO N IV
R1
R4 R3
wherein R1, R3 and R4 have the above meanings, to give a compound of the
general
formula V,
O ^X~ R5
* Nr~
AlkylO N V
R1 /
R4 R3
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
19
The compound of general formula V is then hydrolyzed in an acidic medium to
give a compound of general formula VI,
^ R5
H~N Nrj
I VI
R1 /
R4 R3
wherein R1 and R3 to R5 have the above meanings. The compound of general
formula VI is then reacted with a compound of formula VII
O
A VII
R2 CI
wherein R2 has the above meaning, to result in a compound of general formula I
which
is optionally converted into its physiologically compatible acid addition
salt.
Alternatively, the compounds of Formula I and their acid addition salts may be
prepared by reacting a compound of the general Formula X
O
R2 N X
T\11
R15 R4 R3
wherein Q is selected from the group consisting of: halogen, preferably, bromo
or iodo;
and methylsulfonyl; with a compound of formula III
rll"~11 R5
HNJ III
to result in a compound of general formula I which is optionally converted
into its
physiologically compatible acid addition salt.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
The compounds of Formula II can be prepared by reacting a compound of
formula VII .....
* OH
HN
R1 / I VIII
R4 R3
5 with a compound of formula VII
O
A VII
R2 CI
to give a compound of formula IX
O
R2 N OH
I
R1 IX
R4 R3
The compound of formula IX is then oxidized to give a compound of formula II.
The compounds of Formula III are well-known and can be prepared as
disclosed in any of J. Med. Chem. 1964, p. 619 (van de Westeringh);
W002/094821
(Sanofi); J. Org. Chem. 1980, 45, p. 3671 (J. T. Tai); J. Med. Chem. 1974, 9,
p. 424 (B.
Devaux); J. Med. Chem. 2002, 45, p. 3972 (J. T. Albert). A reaction scheme for
the
preparation of compounds of formula II is giving in example 37.
The compounds of Formula IV can be prepared in a similar manner as
suggested for compounds of Formula II, by selecting an appropriate R2.
The compounds of Formula VII are known per se or can be prepared by the
person skilled in the art from known compounds in known manner.
The compounds of Formula X can be prepared by reacting a compound of the
general Formula IX
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
21
O
R2 N OH
R1 / I IX
R4 R3
with HHalogen, preferably HBr or HI to deliver compounds of Formula X wherein
Q is a
halogen, preferably bromo or iodo, or, alternatively, with
methanesulfonylchloride to
deliver compounds of Formula X wherein Q is methylsulfonyl
O
R2 N X
R1
R4 R3
The compounds of Formula I may be isolated from the reaction mixture and
purified in known manner. Acid addition salts may be converted into the free
bases in
conventional manner, and these may if desired be converted in known manner
into
physiologically compatible acid addition salts. Physiologically compatible
salts of
compounds of Formula I are their conventional salts with inorganic acids, for
example
sulphuric acid, phosphoric acids or hydrohalic acids, preferably hydrochloric
acid, or
with organic acids, for example lower aliphatic monocarboxylic, dicarboxylic
or
tricarboxylic acids such as maleic acid, fumaric acid, lactic acid, tartaric
acid, citric acid,
or with sulphonic acids, for example lower alkanesulphonic acids such as
methanesulphonic acid or trifluoromethanesulphonic acid, or benzenesulphonic
acids
optionally substituted in the benzene ring by halogen or lower alkyl, such as
p-
toluenesulphonic acid.
The compounds of Formula I contain in the y-position to the ring nitrogen atom
in the 4-position of the piperidine or piperazine ring, respectively, an
asymmetrical
carbon atom, namely the carbon atom *C bearing the phenyl ring substituted by
R3 and
R4. Hence, the compounds of Formula I may be present in several stereoisomeric
forms. The present invention covers both the mixtures of optical isomers and
the
isomerically pure compounds of Formula I. Preferred are compounds of Formula I
in
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
22
which the carbon atom *C bearing the phenyl ring substituted by R3 and R4 is
in the S-
configuration. If mixtures of optical isomers of the starting compound, for
example of
the compounds of Formula II or the compounds of Formula IV, are used in the
synthesis of the compounds of Formula I, the compounds of Formula I are also
obtained in the form of mixtures of optical isomers. Departing from
stereochemically
uniform forms of the starting compound, stereochemically uniform compounds of
Formula I can also be obtained. The stereochemically uniform compounds of
Formula
I can be obtained from the mixtures of optical isomers in known manner, for
example
by chromatographic separation on chiral separating materials or by reaction
with
suitable optically active acids, for example tartaric acid or 10-
camphorsulphonic acid,
and subsequent separation into their optically active antipodes by fractional
crystallisation of the diastereomeric salts obtained.
The compounds of Formula I and their acid addition salts have properties which
are antagonistic to tachykinin receptors and are therefore suitable for the
treatment of
pathological conditions in larger mammals, particularly humans, in which
tachykinins
are involved as transfer agents. The group of compounds according to the
invention is
distinguished by a particularly beneficial action profile which is
characterised by a high
selectivity to NK2 and/or NK3-receptors. Furthermore, the group of compounds
according to the invention is distinguished by good compatibility even over
prolonged
periods of administration, and by comparatively good oral availability. Owing
to their
action profile, the compounds of Formula I are suitable in particular for
inhibiting
processes in which tachykinins, such as neurokinin A, which bind to NK2-
receptors,
and/or neurokinin B, which which to NK3-receptors are involved. Owing to the
action
which is advantageously directed at the peripheral region, the compounds of
Formula I
are suitable in particular for the treatment and/or prophylaxis of any
pathology where
either neurokinin A and/or NK2-receptors, or neurokinin B and/or NK3-
receptors, or
both neurokinin A and neurokinin B and/or NK2 and NK3-receptors are involved.
Some compounds of the present invention and in particularly those, wherein R2
is a
cyano-substituted naphthalene ring system are also suitable for inhibiting
processes in
which tachykinins, such as substance P, which bind to NK1-receptors, are
involved.
Owing to the action which is advantageously directed at the peripheral region,
compounds of Formula I wherein R2 is a cyano-substituted naphthalene ring
system,
are suitable in particular for the treatment and/or prophylaxis of any
pathology where
substance P and/or NK1-receptors, or neurokinin A and/or NK2-receptors, or
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
23
neurokinin B and/or NK3-receptors, or any combination of two or all three
substance P,
neurokinin A and neurokinin B and/or NK1, NK2 and NK3-receptors are involved.
In more detail, the compounds of the present invention appear particularly
suitable for the treatment and/or prophylaxis of pathologies of the
respiratory,
gastrointestinal, urinary, immune and cardiovascular system and of the central
nervous
system as well as pain, migraine, inflammation, nausea and vomiting, and skin
diseases.
In even more detail, the compounds of the present invention appear
particularly
suitable for the treatment and/or prophylaxis of pathologies of respiratory
diseases, in
particular asthma, chronic obstructive pulmonary disease, chronic obstructive
bronchitis, bronchitis, cough, and rhinitis; skin diseases, in particular
inflammatory skin
reactions, allergic skin reactions, and psoriasis; arthropathy diseases, in
particular
arthtitis, vasculitides and systemic lupus erythematosus; functional or
inflammatory
disorders in the gastrointestinal tract, in particular pseudomembranous
colitis, gastritis,
acute and chronic pancreatitis, ulcerative colitis, Crohn's disease and
diarrhoe; bladder
diseases such as cystitis and interstitial cystitis; cardiovascular diseases
such as
hypertension, treatment of cancer especially melanomas, gliomas, small-cell
and large-
cell lung cancers, diseases of the immune system, bipolar disorders; migraine;
pain,
anxiety, depression, cognitive disorders, stress-related somatic disorders,
psychosis, in
particular schizophrenia, mania, schizoaffective disorder and panic disorders.
The functional disorders in the gastrointestinal tract which can be treated by
the
compounds according to the invention include in particular the disorders of
the lower
intestinal tracts known under the name "irritable bowel syndrome" (= IBS).
Typical
symptoms for the diagnosis of IBS are described, for example, in W.G. Thompson
et
al., Gastroenterology International 2 (1989) 92-95 or in W.G. Thompson et al.,
GUT
45/II (1999) 1143-1147, and are generally known among experts by the term
"Rome
Criteria". The essential symptoms of IBS accordingly include pains in the
lower
abdomen, which appear to be due to hypersensitivity of the visceral afferent
nervous
system, and anomalies in bowel movement, such as constipation, diarrhoea or
alternating constipation and diarrhoea. Further inflammatory disorders in the
gastrointestinal tract which can be beneficially influenced by the group of
compounds
according to the invention are for example the inflammatory disorders in the
small
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
24
intestine and large intestine regions usually covered by the term
"inflammatory bowel
disease" (= IBD), for example ulcerative colitis or Crohn's disease. Owing to
their
action mechanism, the compounds according to the invention furthermore appear
suitable for the treatment of other disorders in which tachykinins and in
particular
neurokinin A are involved as transfer agents. These disorders include for
example
neurogenic inflammation, inflammatory joint diseases such as rheumatic
arthritis,
asthmatic complaints, allergic disorders, disorders of immune regulation,
bladder
inflammation or also functional dyspepsia.
Another advantage of the compounds of the present invention is the synergistic
effect between the NK2- and NK3-profile.
Another advantage of the compounds of the present invention is their very
balanced combined NK2- and NK3-profile.
Description of the pharmacological test methods
The example numbers given for the compounds of Formula I used as test
substances in the pharmacological tests given below relate to the preparation
examples described below.
1. Determination of the binding potency of the test substances to NK1 -
receptors in
vitro
The affinity of test compounds for NK1-receptors can be determined by
measuring the ability of the test compound to displace a radiolabeled ligand
from its
specific binding site. The tests were performed at Solvay Pharmaceuticals,
Weesp, The
Netherlands.
The radiolabel used in this assay is [3H]-Substance P. Receptors were obtained
from membrane preparations of CHO-cells (Chinese Hamster Ovary cells), in
which the
human NK1-receptor was stably expressed. Membranes were incubated with [3H]-
Substance P in the absence or presence of test compounds at different
concentrations,
diluted in a buffer system. Separation of bound radioactivity from free
radioactivity was
done by filtration through glass fiber filters (Packard GF/B) with several
washings with
ice-cold buffer solution. Bound radioactivity was measured with a liquid
scintillation
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
counter (total binding). Unspecific binding was determined by incubation with
an
excess (1 pmol/1) of unlabeled Substance P.
Specific binding is obtained by subtraction of the unspecific from the total
binding.
5 Radioactivity of the specific binding was plotted against the concentration
of the
test compound and IC50 values, i.e. the concentration of test compound by
which 50%
of the radioligand is displaced, were calculated. The inhibition constant (Ki)
was
calculated according to the Cheng-Prusoff equation, and listed as its negative
logarithmic value (pKi). The pKi value describes the potency of a test
compound to bind
10 to a receptor.
Table 1: Binding potency of the test substances to NK1-receptors in vitro
Example No. 1 4 8 9 10 11 14 15 24
pKi 7,1 7,2 7,4 7,6 7,1 8,2 7,0 7,6 7,1
Example No. 49 55 56 65 70 80 82 83 89
pKi 7,0 7,0 7,2 7,1 7,2 7,1 7,1 7,1 7,0
Example No. 92 93 98 101 102 103 105 108 116
pKi 7,0 8,1 8,3 8,7 8,7 7,3 7,6 7,9 7,1
Example No. 125 126 127
pKi 7,9 8,2 7,1
For the compounds of all examples, the affinity to human NK1-receptors was
determined in each case by at least three measurements of the test substances
in
concentration series of 10-6 to 10-10 mol/l. The average result of all
measurements is
listed above. The compounds of Example No. 1, 4, 8 to 11, 14, 15, 24, 49, 55,
56, 65,
70, 80, 82, 83, 89, 92, 93, 98, 101 to 103, 105, 108, 116, and 125 to 127
exhibited pKi
values of at least 7.0 in this test model. The compounds of Example No. 11,
93, 98,
101, 102 and 126 exhibited pKi values of at least 8Ø
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
26
2. Determination of the binding potency of the test substances to NK2-
receptors in
vitro
The affinity of test compounds for NK2-receptors can be determined by
measuring the ability of the test compound to displace a radiolabeled ligand
from its
specific binding site.
The radiolabel used in this assay is [3H]-SR 48968 (saredutant). Receptors
were obtained from membrane preparations of CHO-cells (Chinese Hamster Ovary
cells), in which the human NK2-receptor was stably expressed. Membranes were
incubated with [3H]-saredutant in the absence or presence of test compounds at
different concentrations, diluted in a buffer system. Separation of bound
radioactivity
from free radioactivity was done by filtration through glass fiber filters
(Packard GF/B)
with several washings with ice-cold buffer solution. Bound radioactivity was
measured
with a liquid scintillation counter (total binding). Unspecific binding was
determined by
incubation with an excess (0.1 pmol/1) of unlabeled saredutant.
Specific binding is obtained by subtraction of the unspecific from the total
binding.
Radioactivity of the specific binding was plotted against the concentration of
the
test compound and IC50 values, i.e. the concentration of test compound by
which 50%
of the radioligand is displaced, were calculated. The inhibition constant (Ki)
was
calculated according to the Cheng-Prusoff equation, and listed as its negative
logarithmic value (pKi). The pKi value describes the potency of a test
compound to bind
to a receptor.
Table 2: Binding potency of the test substances to NK2-receptors in vitro
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
27
Example No. 1 2 3 4 5 6 7 8 9
pKi 9,8 9,4 8,8 9,6 9,0 9,2 8,7 9,1 9,2
Example No. 10 11 12 13 14 15 16 17 18
pKi 8,9 8,7 8,7 9,0 9,1 9,2 9,0 7,5 8,9
Example No. 19 20 21 22 23 24 25 26 27
pKi 8,5 7,6 7,9 7,8 8,7 8,3 8,3 8,3 7,8
Example No. 28 29 30 31 32 33 34 35 36
pKi 7,7 8,6 8,6 8,4 7,8 7,1 7,8 8,5 8,8
Example No. 37 38 39 40 41 42 43 44 45
pKi 8,8 8,8 7,7 7,9 8,5 7,8 9,6 8,3 8,6
Example No. 46 47 49 50 51 52 53 55 56
pKi 8,2 8,5 8,0 7,4 7,1 7,8 7,8 8,2 7,6
Example No. 58 59 61 63 64 65 66 67 68
pKi 7,0 7,0 7,4 8,4 7,9 7,6 7,8 8,2 8,7
Example No. 69 70 71 72 73 74 75 76 77
pKi 7,0 7,6 8,3 8,5 7,9 8,4 8,3 7,8 8,5
Example No. 78 79 80 81 82 83 84 85 86
pKi 8,8 8,7 8,7 7,9 8,6 8,3 7,9 7,9 7,9
Example No. 87 89 90 91 92 93 94 95 96
pKi 8,1 7,9 7,9 8,1 7,7 7,8 8,5 9,0 8,7
Example No. 97 98 99 100 101 102 103 104 105
pKi 8,7 8,3 9,2 8,5 9,4 8,3 8,7 8,7 8,7
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
28
Example No. 108 116 124 125 126 127
pKi 8,4 8,3 9,0 7,3 8,0 8,7
For the compounds of Example No. 1 to 47, 49 to 53, 55, 56, 58, 59, 61, 63 to
104 and 124 to 127, the affinity to human NK2-receptors was determined in each
case
by at least three measurements of the test substances in concentration series
of 10-6
to 10-10 mol/l. The average result of all measurements is listed above. All
the
aforementioned test substances exhibited pKi values of at least 7.0 in this
test model.
The compounds of Example No. 1 to 16, 18, 19, 23 to 26, 29 to 31, 35 to 38,
41, 43 to
47, 49, 55, 63, 67, 68, 71, 72, 74, 75, 77 to 80, 82, 83, 87, 91, 94 to 105,
108, 116, 124,
126 and 127 exhibited pKi values of at least 8Ø The compounds of Example No.
1, 2,
4 to 6, 8, 9, 13 to 16, 95, 104 and 124 exhibited pKi values of at least 9Ø
3. Determination of the binding potency of the test substances to NK3-
receptors in
vitro
The affinity of test compounds for NK3-receptors can be determined by
measuring the ability of the test compound to displace a radiolabeled ligand
from its
specific binding site.
The radiolabel used in this assay is [3H]-SR 142801 (osanetant). Receptors
were obtained from membrane preparations of CHO-cells (Chinese Hamster Ovary
cells), in which the human NK3-receptor was stably expressed. Membranes were
incubated with [3H]-osanetant in the absence or presence of test compounds at
different concentrations, diluted in a buffer system. Separation of bound
radioactivity
from free radioactivity was done by filtration through glass fiber filters
(Packard GF/B)
with several washings with ice-cold buffer solution. Bound radioactivity was
measured
with a liquid scintillation counter (total binding). Unspecific binding was
determined by
incubation with an excess (1 pmol/1) of unlabeled osanetant.
Specific binding is obtained by subtraction of the unspecific from the total
binding.
Radioactivity of the specific binding was plotted against the concentration of
the
test compound and IC50 values, i.e. the concentration of test compound by
which 50%
of the radioligand is displaced, were calculated. The inhibition constant (Ki)
was
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
29
calculated according to the Cheng-Prusoff equation, and listed as its negative
logarithmic value (pKi) The pKi value describes the potency of a test compound
to bind
to a receptor.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
Table 3: Binding potency of the test substances to NK3-receptors in vitro
Example No. 1 3 4 5 6 7 9 10 11
pKi 8,6 8,2 8,2 8,3 8,7 8,0 8,4 8,0 7,9
Example No. 12 13 14 15 16 18 19 20 21
pKi 7,9 7,4 8,5 8,4 7,8 8,3 7,5 7,0 7,2
Example No. 22 23 24 25 26 28 29 30 31
pKi 7,3 8,0 7,0 7,2 7,3 7,0 7,3 7,7 7,4
5
Example No. 32 33 35 36 37 38 39 40 41
pKi 7,4 7,5 7,0 8,3 7,7 7,6 7,5 7,2 7,8
Example No. 43 44 45 46 47 49 50 51 52
pKi 8,3 7,5 7,9 7,5 7,7 8,1 8,0 8,2 7,9
Example No. 53 54 55 56 57 60 63 64 65
pKi 8,5 8,2 8,4 8,3 7,2 7,2 7,7 7,1 7,8
Example No. 66 67 68 69 70 71 72 73 74
pKi 7,7 8,3 8,0 7,3 7,2 7,4 7,4 7,4 7,6
Example No. 75 76 77 78 79 80 81 82 83
pKi 7,4 7,3 7,1 7,6 7,7 7,1 7,1 7,6 8,5
Example No. 84 85 86 87 89 90 91 92 95
pKi 7,4 7,3 7,2 7,4 7,8 7,1 7,8 7,0 7,7
Example No. 97 98 99 101 102 103 104 124 125
pKi 7,1 7,2 7,2 7,7 7,0 7,0 7,7 7,8 7,3
Example No. 126
pKi 7,4
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
31
For the compounds of Example No. 1, 3 to 7, 9 to 16, 18, 19, 21 to 23, 25, 26,
29 to 33, 36 to 41, 43 to 47, 49 to 57, 60, 63 to 87, 89 to 91, 95, 97 to 104,
and 124 to
126 the affinity to human NK3-receptors was determined in each case by at
least three
measurements of the test substances in concentration series of 10-6 to 10-10
mol/l.
The average result of all measurements is listed above. All the aforementioned
test
substances exhibited pKi values of at least 7.0 in this test model. The
compounds of
Example No. 1, 3 to 10, 14, 15, 18, 23, 36, 43, 49 to 51, 53 to 56, 67, 68 and
83
exhibited pKi values of at least 8Ø
4. Functional cellular tests of the NK1-, NK2- and NK3-antagonism
Functional cellular tests of the antagonistic effect of the compounds of the
present invention on the human tachykinin receptors were performed in CHO
cells
expressing the recombinant human NK1, NK2 or NK3-receptor. In these tests the
inhibition of ligand-induced increase in mobilization of intracellular calcium
and
inhibition of ligand-induced phosphorylation of mitogen activated protein
kinase
(MAPK) were determined, which can be used as a measure of functional activity
of
tachykinin-antagonists. Additionally, the antagonistic properties of reference
compounds on the different tachykinin receptors were characterized for
comparison.
The effects of test compounds were assessed using Chinese hamster ovary
(CHO) fibroblast cells, stably expressing cloned human NK1, NK2 or NK3-
receptors.
The NK receptor is coupled to Gq. The activation of the Gq protein by ligand
binding to
the receptor leads to a mobilization of intracellular calcium and
phosphorylation of
MAPK. Both systems were used to determine functional effects of the test
compounds.
Ca2+ measurements using FLIPR for NK1 and NK2 activity
For tests, cells were seeded 24 hours prior to the experiment into black 96-
well
microplates. The cell density was 2.2x104 cells/well. All steps were done
under sterile
conditions. In order to observe changes in intracellular calcium levels, cells
were
loaded with a calcium-sensitive dye. This dye (FLUO-4, from Molecular Probes)
excites at 488 nm, and emits in the 500 nm to 560 nm range, only if a complex
with
calcium is formed. For the dye loading the growth-medium was aspirated out of
the
well without disturbing the confluent cell layer and 100 pl loading medium
(HBSS, 4 pM
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
32
FLUO-4, 0.005% (w/v) pluronic acid, 2.5mM probenecid, 20 mM HEPES, pH 7.4) was
dispensed into each well using an automatic pipettor system (Multidrop,
Labsystems).
Pluronic acid was added to increase dye solubility and dye uptake into the
cells,
whereas probenecid, an anion exchange inhibitor, was added to the loading
medium to
increase dye retention in the cells. The cells were incubated in a 5% CO2
incubator at
37 C for 40 minutes. After dye loading, the cells were washed three times
with wash-
buffer (HBSS, 2.5 mM probenecid, 20 mM HEPES, pH 7.4) to reduce basal
fluorescence. In the last washing step the buffer was aspirated and replaced
with
100 pl washing buffer. For the antagonism screening mode 50 pl of the compound
(final concentration ranges from 10 pM to 1.4 nM) were applied 7 min prior to
addition
of substance P (final concentration:10-$ M; NK1 agonist) or NKA (final
concentration:
10-' M; NK2 agonist). The FLIPR setup parameters were set to 0.4 sec exposure
length, filter 1, 50 pl fluid addition, pipettor height at 125 pl, dispense
speed 40 pl/sec
without mixing. Maximal fluorescence changes were obtained using the statistic
function of the FLIPR software, and data plotted using GraphPad Prism 4. All
points
were expressed as a percentage inhibition of the control agonist. IC50 values
were
determined using sigmoidal dose-response curve fitting. Antagonist potencies
(pA2)
values were calculated using equation:
pA2 = -log (IC50/(1 + [L] / EC50)),
in which the IC50 of the test compound was obtained from concentration-effect
relationships, [L] is the concentration of the agonist (substance P for NK1
test, NKA for
NK2 test, NKb for NK3 test), and the EC50 is the potency of the agonist at the
respective human cloned NK receptor (EC50 substance P: 10-9.6 M; EC5o NKA: 10-
$-$ M,
EC5o NKB: 10-$-$ M). The results are summarized in tables 4 and 5:
Table 4: pA2 data for NK1:
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
33
Example No. 1 8 9 10 11 13 14 15 23
pA2 8,1 7,1 8,0 7,1 7,9 7,5 7,3 7,2 8,9
Example No. 93 95 96 98 99 101 102 103 104
pA2 8,1 7,2 7,5 8,8 7,3 7,2 8,7 7,5 7,7
Example No. 105 108 116
pA2 7,5 7,5 7,5
Table 5: pA2 data for NK2
Example No. 1 2 3 4 5 6 7 8 9
pA2 9,7 9,3 8,7 8,6 8,5 8,8 8,6 8,6 8,6
Example No. 10 11 12 13 14 15 16 17 18
pA2 8,4 8,4 8,3 8,3 8,2 8,3 8,3 7,8 8,5
Example No. 19 23 36 37 43 51 52 55 67
pA2 9,3 8,9 9,3 8,8 9,2 7,4 8,4 8,7 7,6
Example No. 68 83 93 94 95 96 97 98 99
pA2 8,0 7,8 8,4 8,8 8,5 8,9 9,6 9,3 9,6
Example No. 101 102 103 104 105 108 116
pA2 10,0 7,4 8,7 7,8 10,1 9,0 8,9
Ca2+ measurements using aeguorin for NK3 activity
NK3 antagonism was measured in a cell line expressing the human NK3
receptor and mitochondrially targeted apoaequorin. In this system, cells
expressing
apoaequorin are incubated with coelenterazine, which is the chromophore co-
factor of
aequorin. Upon incubation of the cells with senktide, a potent agonist on NK3,
intracellular calcium concentration increases. Traces of free calcium lead to
a
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
34
concentration dependent activation of the catalytic activity of aequorin,
which oxidizes
coelenterazine and yields apoaequorin, coelenteramide, C02 and light
(Amax=469nm).
Once the photon has been emitted, the complex must dissociate and the
apoaequorin
must recombine with the co-factor before the complex can emit another photon.
Thus,
in this system, measurements of luminescence (light emission) following
senktide
addition reflects an increase in intracellular calcium due to activation of
NK3 receptors.
For the test, cells were grown to confluency and harvested with complexon
(5mM EDTA in PBS). Cells were centrifugated and resuspended in DMEM/F-12
(nutrient mixture according to Ham) without phenolred, supplemented with 10%
FCS to
a density of 5x106 cells/ml. In order to load the cells, coelenterazine was
added to a
final concentration of 5pM and cells were stirred at room temperature for 4
hours.
Loaded cells were diluted in DMEM/F12 without phenolred and supplements to a
density of 2.8x105 cells/ml, pre-heated to 37 C, and stirred for 60min at room
temperature. For the antagonism screening mode 15pl of the cell suspension
were
added to 85pl of the compound (final concentration ranges from 4.5nM to 10pM)
in
white 96-well plates. After an incubation time of 20 min 50pl senktide (final
concentration of 5x10-8M) were applied and chemoluminescence was measured
immediately for 20 seconds using the Microlumat (Berthold). All points were
expressed
as a percentage inhibition of the control agonist. IC50 values were determined
using
sigmoidal dose-response curve fitting of GraphPad Prism 4. Antagonist
potencies
(pA2) values were calculated using equation:
pA2 = pIC50 + log [(L / EC50)-1],
in which the pIC50 is the negative logarithm of the IC50 value of the test
compound
that was obtained from concentration-effect relationships, L is the
concentration of
senktide and EC50 its potency at the human cloned NK3 receptor (EC50 senktide:
10-
8.8M).
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
Table 6: pA2 data for NK3
Example No. 1 2 3 4 5 6 7 8 9
pA2 8,6 7,5 8,2 8,2 8,5 8,7 8,1 8,4 8,0
Example No. 10 11 12 13 14 15 16 17 18
pA2 8,3 8,1 7,9 8,0 8,1 8,1 7,9 6,9 8,0
Example No. 19 23 36 37 43 51 53 55 67
pA2 7,3 7,9 7,2 7,5 7,5 7,5 7,3 8,1 7,8
5
Example No. 97 101 102 103 104 105
pA2 7,1 7,9 7,4 7,3 7,8 7,0
5. Determination of functional NK1-receptor antagonism of test compounds in
tissue isolated from guinea pigs.
The NK1-antagonistic action of test compounds was tested in aortic ring
preparations isolated from guinea pigs. The preparations were kept in an
oxygenated
nutrient solution kept at 37 C. For measuring contraction of the circular
vascular
muscle, the preparations were fixed to a hook and connected to force
displacement
transducers. Contractions/relaxations were recorded on a pen recorder. The
preparations were given a medium tonus by addition of phenylephrine.
The NK1-receptors were stimulated with the NK1-receptor agonist Substance
P, causing a relaxation of the muscular tone. Before (predrug) and after the
administration of the test compound such relaxations were measured and
quantified in
percent of the predrug relaxation. 2-3 concentrations of the test compound
were
applied showing the inhibition of the receptor stimulation concentration
dependently.
The concentration of half-maximal inhibition (IC50) and its negative logarithm
pIC50= -
log (IC50) was calculated. The pIC50 value indicates the inhibitory potency of
the test
compounds to the NK1-receptor.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
36
Table 7: Functional NK1 -antagonism of the test substances on isolated guinea
pig tissue
Example No. 11
pIC50 8,26
6. Determination of functional NK2-receptor antagonism of test compounds in
tissue isolated from guinea pigs.
The NK2 antagonistic action of test compounds was tested in gall bladder
preparations isolated from guinea pigs. The preparations were kept in an
oxygenated
nutrient solution kept at 37 C. For measuring contraction of the gall bladder
muscle, the
preparations were fixed to a hook and connected to force displacement
transducers.
Contractions were recorded on a pen recorder.
The NK2-receptors were stimulated with the NK2-receptor agonist neurokinin A,
causing a contraction of the muscle. Before (predrug) and after the
administration of
the test compound such contractions were measured and quantified in percent of
the
predrug contraction. 2-3 concentrations of the test compound were applied
showing the
inhibition of the receptor stimulation concentration dependently. The
concentration of
half-maximal inhibition (IC50) and its negative logarithm pIC50= -log (IC50)
was
calculated. The pIC50 value indicates the inhibitory potency of the test
compounds to
the NK2-receptor.
Table 8: NK-2-receptor-antagonistic effectiveness of the test substances of
Formula I on the guinea pig gall bladder in vitro
Example No. 1 2 3 4 6 10 11 36 43
pA2 8,17 8,96 8,96 8,86 8,88 8,78 8,03 9,05 10,41
7. Determination of functional NK3-receptor antagonism of test compounds in
tissue isolated from guinea pigs.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
37
The NK3-antagonistic action of test compounds was tested in ileal preparations
isolated from guinea pigs. The preparations were kept in an oxygenated
nutrient
solution kept at 37 C. For measuring contraction of the longitudinal muscle of
the
ileum, the preparations were fixed to a hook and connected to force
displacement
transducers. Contractions were recorded on a pen recorder.
The NK3-receptors were stimulated with the NK3-receptor agonist [MePhe']-
neurokinin B, causing a contraction of the muscle. Before (predrug) and after
the
administration of the test compound such contractions were measured and
quantified
in percent of the predrug contraction. 2-3 concentrations of the test compound
were
applied showing the inhibition of the receptor stimulation concentration
dependently.
The concentration of half-maximal inhibition (IC50) and its negative logarithm
pIC50= -
log (IC50) was calculated. The pIC50 value indicates the inhibitory potency of
the test
compounds to the NK3-receptor.
Table 9: Functional NK3-antagonism of the test substances on isolated guinea
pig tissue
Example No. 1 3 4
pA2 8,4 8,5 8,0
8. Determination of the NK3-receptor-antagonistic effectiveness of the test
substances in vivo (reduction of body temperature in gerbils with senktide-
induced hypothermia)
NK3 agonists reduce the body temperature of gerbils. The senktide-induced
hypothermia can be antagonized by administering compounds with NK3-
antagonistic
properties. Measuring the level of hypothermia is indicative for the degree of
activity of
the test compounds. The own effect of the tested compound is assessed in the
same
experiment to exclude an additional hyperthermic effect.
Male gerbils with a body weight between 60 to 90 g are housed in groups under
normal day-night rhythm and under constant environmental temperature (room
temperature: 21 2 C) with a constant relative humidity level (50 10%). Water
and food
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
38
are freely available. Reference agonist: Senktide 0.03 mg/kg sc. Antagonists:
see
example compounds in table X.
Per series of experiments, one condition is always the vehicle/vehicle group
to
determine the normal body temperature and one condition is always the
vehicle/senktide
group to determine the senktide induced hypothermia (=100%). The animals are
weighted and marked 60 minutes prior to the agonist administration. For oral
(po)
testings, the test compounds are administered at t = -60 minutes. For
parenteral or
subcutaneous (i por sc, resp.) administration, at t = -30 minutes. At t = 0
minutes the
agonist Senktide is administered (sc). At t = 15 minutes the temperature is
measured
orally, and registered with a 0.1 C accuracy after a 10 second reading. This
way,
animals are measured every 60 sec.
Using the Dunett's test, the vehicle/vehicle group is used as reference for
the
analysis of the own effect; whereas the vehicle/senktide group is used for
comparison for
the interaction test (i.e. example compounds/senktide groups).
Table 10: Inhibition of temperature increase at t = 60 minutes after oral (po)
administration
Example No. Dose: 30 mg/kg Dose: 10 mg/kg
1 54% 37%
Table 11: Inhibition of temperature increase at a dose of 10 mg/kg after
parenteral
(ip) administration
Example No. t = 15 minutes after t = 30 minutes after
administration administration
4 54% 68%
6 - 75%
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
39
9. Determination of the NK-3-receptor-antagonistic effectiveness of the test
substances in vivo (reduction of blood pressure in guinea-pigs with senktide-
induced hypertension)
NK3 agonists increase the blood pressure in guinea pigs. The senktide-
induced hypertension can be antagonized by administering compounds with NK3-
antagonistic properties. Measuring the level of hypertension is indicative for
the degree
of activity of the test compounds. The own effect of the tested compound is
assessed
in the same experiment to exclude an additional hypertensional effect.
Male Dunkin Hartley guinea pigs with a body weight between 450 and 550 g
were anesthetized with Ketamin (200 mg/kg i.m.; Aescoket 10%) and the left
carotid
artery and left jugular vein were cannulated for blood pressure measurement
and drug
application, respectively. The animals were allowed to breathe spontaneously.
Blood pressure was measured with a strain gauge transducer connected to a
computer via an amplifier.
After hemodynamic stabilization two subsequent injections of the NK3-receptor
agonist senktide (0.4 pg/kg i.v.; 0.5 ml/kg) were given with an interval of 15
minutes
and the peak increase in mean arterial blood pressure of each injection
determined.
The mean value of the increase in blood pressure served as the pre-drug
hypertensive
effect of senktide. Five minutes thereafter the test compound was given as an
infusion
over 10 min (0.1 ml/min), immediately followed by an injection of 0.4 pg/kg
senktide
and the peak increase in mean arterial blood pressure determined. Up to 5
cycles of
additive test compound dosages were applied and the hypertensive effect of
senktide
determined after each dose. The effect of the test compound on the peak rise
in blood
pressure induced by senktide is expressed as percentage relative to the pre-
drug
value. ID50 values (dose that produces a 50% inhibition of the senktide
response) were
calculated from the dose response curve. The effect of the vehicle on the
senktide
induced pressure response was determined on regular base.
For oral administration, vehicle (1% methylcellulose) or test compound was
administered in a dose volume of 5 ml/kg. Forty five minutes thereafter,
anesthesia
was induced and the carotid artery and jugular vein were cannulated. After a
10
minutes period for hemodynamic stabilization, two subsequent injections of the
NK3-
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
receptor agonist senktide (0.4 pg/kg i.v.; 0.5 ml/kg) were given with an
interval of 15
minutes and the peak increase in mean arterial blood pressure of each
injection
determined. The first senktide injection was approximately 80 to 90 minutes
post
dosing. The mean value of the increase in blood pressure served as the
hypertensive
5 effect of senktide in that animal. The mean hypertensive effect of senktide
following
vehicle treatment served as the control value (100%). The effect of each test
compound dose on the peak rise in blood pressure induced by senktide is
expressed
as percentage relative to this control value and averaged per dose group. ID50
values
(dose that produces a 50% inhibition of the senktide response) were calculated
from
10 the dose response curve.
Senktide (0.8 pg/ml) was dissolved in saline. For intravenous administration,
the test compounds were dissolved in 40% hydroxypropyl-[3-cyclodextrine (HPCD)
containing 10% DMSO, diluted with saline and administered intravenously in
cumulative dose ranges. A vehicle group of animals received the corresponding
15 HPCD/DMSO solutions instead of the test compound. For oral administration,
the test
compound was suspended in 1% methylcellulose. A vehicle group of animals
received
the corresponding HPCD/DMSO solutions instead of the test compound.
20 Table 12: Inhibition of blood pressure as ID50-value after intravenous (iv)
and oral
(po) administration
Example No. ID50 (iv) ID50 (po)
[mg/kg] [mg/kg]
1 0,2557 6,3796
2 1,9809 -
4 0,7564 2,3313
6 0,157 -
11 1,0554 10
13 0,5013 < 10
14 0,5168 6,811
15 0,3732 -
18 1,0108 -
19 0,3902 -
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
41
10. Determination of the NK1- and NK2-receptor-antagonistic effectiveness of
the
test substances in vivo
The NK1- and NK2-antagonistic activities of the test substances were
investigated in anaesthetised guinea pigs in each case after intravenous (=
i.v.) and
oral (= p.o.) administration in vivo. With the present test model it is
possible to detect
both NK2-antagonistic effects in three different organ systems (respiratory
tracts, colon
and circulation) and NK1-antagonistic effects (rapid drop in blood pressure)
in an
animal simultaneously.
Pirbright-White guinea pigs of a body weight of 500-700 g were anaesthetised
with ketamine/xylazine (67/13 mg/kg subcutaneously, initial dose, further
doses
administered as required). The animals were provided with an intravenous
catheter in
order to administer the substance and an intra-arterial catheter to measure
the blood
pressure. The animals were artificially ventilated via a tracheal cannula and
the
respiratory pressure was recorded by means of a pressure transducer. A balloon
was
introduced into the distal colon of the animals for manometric recording of
colon motility
by means of a pressure transducer. Blood pressure, heart rate, respiratory
pressure
and colonic pressure were measured continuously for each animal and plotted on
a
recorder and by means of a digital data-processing system. Neurokinin A (=
NKA; 200
pmol/animal) was administered i.v. as a bolus as a test stimulus to stimulate
the NK1-
and the NK2-receptors. NKA injection resulted in an increase in respiratory
pressure
(bronchoconstriction) and colonic pressure, and in a biphasic drop in blood
pressure.
The first phase of hypotension (= phase of maximum hypotension within the
first
minute after administration of NKA) is mediated via NK-1 receptors, since they
can be
blocked completely by specific NK-1 receptor antagonists. The second phase of
delayed hypotension (= phase of maximum hypotension after 2-5 min.) on the
other
hand is mediated via NK2-receptors, since they can be blocked by specific NK2-
receptor antagonists. The doses of the test substances are given as ED50
values
which each result in a response to the NKA test stimulus which is reduced to
50% of
the initial value, as characteristic variables for the individual measurement
parameters
bronchoconstriction, colonic pressure and change in blood pressure mediated by
NK1
or NK2.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
42
The antagonistic effects of the test substances were first investigated in
cumulative form, the time of the NKA test stimulus being 1 min after the
administration
of the respective doses of the test substances had ended. These ED50 values
obtained from cumulative dose effect curves are plotted in table 13.
Table 13: NK1- and NK2-receptor-antagonistic potency of the test substances of
Formula I on guinea pigs in vivo after intravenous administration
ED50 iv [pmol/kg] after 1 min (cumulative)
Example NK1 NK2 NK2 NK2
No. (early (late (broncho- (colonic motility)
hypotension) hypotension) constriction)
1 > 0,46 0,044 0,033 < 0,003
3 > 1,462 0,397 0,221 0,07
4 > 1,462 0,04 0,058 0,053
6 > 1,462 0,04 0,06 0,096
8 > 1,462 1,113 0,731 0,294
9 > 1,462 0,108 0,058 0,053
11 0,433 0,212 0,052 0,085
14 > 1,462 0,247 0,26 0,245
> 1,462 0,1 0,075 0,066
16 > 1,462 0,34 0,38 0,132
18 > 1,462 0,181 0,18 0,068
19 > 1,462 0,209 0,093 0,028
10 The measured values plotted in table 14 above show, inter alia, that the
compounds of Example No. 11 after cumulative administration i.v. (detection of
the
antagonism 1 min. after the administration of test substance had ended) caused
a
marked NK-1-receptor-antagonistic activity on the early hypotension. The data
show
further that compounds of all Example No. caused a marked NK2-receptor-
antagonistic
15 activity of colon motility, late drop in blood pressure and respiratory
resistance.
In order additionally to detect the variation over time of the antagonistic
effects
of the test substances, the action of the NKA test stimulus was determined at
different
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
43
times (1, 30, 60, 90, 120, 150 and 180 min.) after oral administration of the
test
substances. The antagonistic effects of the test substances were then
determined as
"area under the curve" ("AUC") over the investigation period after
administration of the
test substances (1 - 180 min after administration) and the ED50 values after
oral
administration obtained therefrom were plotted in table 14.
Table 14: NK1- and NK-2-receptor-antagonistic potency of the test substances
of
Formula I on guinea pigs in vivo after oral administration
ED5o AUC1_18o min oral [pmol/kg]
Example NK1 NK2 NK2 NK2
compound (early (late (broncho- (colonic motility)
hypotension) hypotension) constriction)
1 > 3,2 2,236 0,457 1,08
4 >10 12 < 3,2 < 3,2
8 > 3,2 > 3,2 > 3,2 > 3,2
11 23,027 2,509 2,213 11,228
The compounds according to the invention, in particular the compounds of
Example No. 1, 4, 8 and 11 as shown in table 15, are furthermore active orally
on the
NK2. Example 11 was also active as NK1 receptor antagonists.
The compounds of Formula I may be administered in conventional
pharmaceutical preparations. The doses to be used may vary individually and
will
naturally vary according to the type of condition to be treated and the
substance used.
In general, however, medicinal forms with an active substance content of 0.2
to 200
mg, in particular 1 to 50 mg, active substance per individual dose are
suitable for
administration to humans and larger mammals. The compounds may be contained
according to the invention, together with conventional pharmaceutical
auxiliaries and/or
carriers, in solid or liquid pharmaceutical preparations. Examples of solid
preparations
are preparations which can be administered orally, such as tablets, coated
tablets,
capsules, powders or granules, or alternatively suppositories. These
preparations may
contain conventional pharmaceutical inorganic and/or organic carriers, such as
talcum,
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
44
lactose or starch, in addition to conventional pharmaceutical auxiliaries, for
example
lubricants or tablet disintegrating agents. Liquid preparations such as
suspensions or
emulsions of the active substances may contain the usual diluents such as
water, oils
and/or suspension agents such as polyethylene glycols and the like. Other
auxiliaries
may additionally be added, such as preservatives, taste correctives and the
like.
The active substances may be mixed and formulated with the pharmaceutical
auxiliaries and/or carriers in known manner. For the production of solid
medicament
forms, the active substances may for example be mixed with the auxiliaries
and/or
carriers in conventional manner and may be wet or dry granulated. The granules
or
powder can be poured directly into capsules or be pressed into tablet cores in
conventional manner. These may be coated in known manner if desired.
The following examples are intended to explain the invention further, without
limiting its scope.
The LC-MS data (API) were obtained by the following conditions:
Instrument- Description: API 100 Single Quad, PE Applied Biosystems
PE 200 BI LC-Pump, PE Applied Biosystems
Gilson XL 215 Autosampler, Gilson Inc.
Sedex 75 Lightscattering-Detector, S.E.D.E.R.E
Column: XTerra MS C18, 2.5 um, 50 x 4.6 mm
Guard-Column: XTerra MS C18, 3.5 um, 20 x 3.9 mm
Solvent A: 0.01 M NH4ac pH 5.0 + 5 % Acetonitrile
Solvent B: Acetonitrile
Gradientprofile: 100 % A ----10min --->95 % B
2min isocratic 95 % B
100 % A <---0.5min---- 95 % B
100 % A 2.5min isocratic
Flow: 1.0 ml / min
Wavelength: 225 nm
The LC-MS data (ZQ) were obtained by the following conditions:
Instrument- Description: ZQ Single Quad, Waters / Micromass
Alliance HT 2795, Waters
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
PL-ELS 1000 Lightscattering-Detector, Polymer Labs
Column: XTerra MS C18, 2.5 um, 50 x 4.6 mm
Guard-Column: XTerra MS C18, 3.5 um, 10 x 2.1 mm
Solvent A: 0.01 M NH4ac pH 5.0 + 5 % Acetonitrile
5 Solvent B: Acetonitrile
Gradientprofile: 100 % A 1 min isocratic
100%A ---- 6min---> 95%B
1 min isocratic 95 % B
100 % A <---1 min---- 95 % B
10 100 % A 2min isocratic
Flow: 1.0 ml / min
Wavelength: 205-350 nm
15 Example 1: Synthesis of N4(2S)-2-(3,4-dichlorophenyl)-4-oxobutyll-N-methyl-
benzamide as starting material for the preparation of example compounds 1, 2
and 99
\
p
N
O
O
cl cl
In a solution of 15 ml (0.172 mol) of oxalyl chloride in 200 ml of methylene
20 chloride cooled to -60/-70 C under nitrogen were added in 75 minutes
dropwise in
order to maintain the temperature at -60/-70 C 30 ml of DMSO in 100 ml
methylene
chloride. The reaction mixture was stirred for 20 minutes at -70 C and
subsequently a
suspension of 31 g (0.088 mol) of N-[2-(3,4-Dichloro-phenyl)-4-hydroxy-butyl]-
N-
methyl-benzamide in 200 ml of methylene chloride were added in 60 minutes in a
way
25 to maintain the temperature of the reaction mixture at -60 C. As this
temperature were
added dropwise 90 ml of triethylamine in 40 ml methylene chloride. The mixture
was
let to come back to room temperature slowly and allowed to stay for 15 hours.
The
reaction mixture was concentrated in vacuum and the residue was dissolved in a
mixture of 200 ml toluene and 200 ml ethyl acetate. The organic phase was
washed by
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
46
100 ml of a saturated solution of NaCI in water and 4 times with 100 ml water
each.
The recovered organic phase was dried on sodium sulphate and concentrated in
vacuum to deliver 32.6 g of a glassy material.
17.15 g of the compound were put in suspension in 10 ml methylene chloride
under stirring at room temperature. To the suspension were added 50 ml of n-
hexane
and the mixture was put at 45 C giving a slurry. To the slurry were added 40
ml of n-
hexane and the mixture was let to cool down to room temperature. The obtained
suspension was filtered off to give a solid which was washed three times with
10 ml n-
hexane. After dissolution in methylene chloride and subsequent concentration
to
dryness 15 g of a solid (melting point: 78-79 C) were obtained: Overall yield:
90%
Example 2: Synthesis of 1'-[(3S)-4-[benzoyl(methyl)aminol-3-(3,4-
dichlorophenyl)-
butyll-N,N-dimethyl-1,4'-bipiperidine-4'-carboxamide (example compound 1)
\ -
N
O
N ~
~O
CI CI \
To a suspension of 15 g (0.0428 mol) of the aldehyde from example 1, 14.9 g
(0.0540 mol) of [1,4']Bipiperidinyl-4'-carboxylic acid dimethylamide
hydrochloride and
5.3 g sodium acetate in 800 ml of THF at room temperature under stirring were
added
5 ml of acetic acid and the mixture was stirred for 5 hours at room
temperature. To the
mixture were added portion wise 18.5 g (0.0876 mol) of sodium triacetoxy-
borohydride
and the mixture was further stirred at room temperature for 15 hours. The
mixture was
concentrated to dryness in vacuum and re-dissolved in 300 ml ethyl acetate, 50
ml of
MTBE and 6 g of KOH dissolved in 100 ml of water. The organic phase was washed
with sodium hydrogenocarbonate until pH 5-6 and 3 times with 100 ml of water.
The
organic solution was dried on sodium sulphate and concentrated to dryness to
give 27
g of the raw base as a foam. 17 g of raw base were dissolved in 30 ml of
methylene
chloride at room temperature to which 13 ml of 5N HCI in isopropanol were
added and
a solution was obtained. To the solution were added 500 ml of MTBE and a solid
appeared. The suspension was heated to 50 C and cooled down. The solid was
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
47
recovered by filtration, washed 3 times with 100 ml of MTBE. The solid was
dissolved
in 100 ml methanol and concentrated to dryness to give 16.2 g of the compound
identified as dihydrochloride in elemental analyses.
Yield: 90%.
Optical rotation: -16.3 (c=1 % in methanol).
Raw base purified by dissolution in water in the presence of concentrated HCI
gives after elimination of insoluble by- products by extraction with MTBE and
basification by means of KOH of the water phase and subsequent extraction of
the
base using methylene chloride a pure base as a yellowish compound with a 90%-
yield.
Optical rotation; -17.8 (c= 1% in methanol).
'HNMR(as base) (500 MHz, CDC13, 30 C) 6: 7.40-7.25 (m, 4.6H), 7.25-7.0 (m,
2.7H),
6.9, 6.7 (2xbs, 0.7H), 3.82 (bs, 0.7H), 3.55 (dd, 0.7H), 3.5-3.25 (m, 4.4 H),
3.2 (bs, 0.7
H), 3.0 (m, 2H), 2.95-2.90 (2xs, 3H), 2.8-2.5 (m, 4.5H), 2.4-1.6 (m, 12H), 1.5
(s, 4H),
1,4 (s, 2H).
13CNMR (125MHz, CDC13, 30 C) 6: 173.5, 171.6, 143.0, 136.4., 132.4, 130.5,
128.4,
126.8, 66.9, 57.1, 56.1, 53.3, 51.7, 47.3, 41.7, 38.7, 37.7, 33.6, 30.9, 26.9,
25.2.
LC-MS: M+1 (monoisotope) 573.
Retention time: 7.90 min (API); 5.38 min (ZQ).
Example 3: Synthesis of 1'-[(3S)-4-[benzoyl(methyl)aminol-3-(3,4-
dichlorophenyl)-
butyll-N-methyl-1,4'-bipiperidine-4'-carboxamide (example compound 2)
\
N
O
N 'ND
O
-N
CI CI H
To a suspension of 15 g (0.0428 mol) of the aldehyde from example 1, 14.1 g
(0.0540 mol) of [1,4']Bipiperidinyl-4'-carboxylic acid methylamide
hydrochloride and, 5.3
g sodium acetate in 800 ml of THF at room temperature under stirring were
added 5 ml
of acetic acid and the mixture was stirred for 5 hours at room temperature. To
the
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
48
mixture were added portion wise 18.5 g (0.0876 mol) of sodium triacetoxyboro-
hydride
and the mixture was further stirred at room temperature for 15 hours. The
mixture was
concentrated to dryness in vacuum and re-dissolved in 300 ml ethyl acetate, 50
ml of
MTBE and 6 g of KOH dissolved in 100 ml of water. The organic phase was washed
with sodium hydrogenocarbonate until pH 5-6 and 3 times with 100 ml of water.
The
organic solution was dried on sodium sulphate and concentrated to dryness to
give 27
g of the raw base as an foam. 17 g of raw base were dissolved in 30 ml of
methylene
chloride at room temperature to which 13 ml of 5N HCI in isopropanol were
added and
a solution was obtained. To the solution were added 500 ml of MTBE and a solid
appeared. The suspension was heated to 50 C and cooled down. The solid was
recovered by filtration, washed 3 times with 100 ml of MTBE. The solid was
dissolved
in 100 ml methanol and concentrated to dryness to give 16.2 g of the compound
identified as dihydrochloride in elemental analyses.
Yield: 100%.
'HNMR(as base) (500 MHz, CDC13) b: 7.45-6.70. (m, 9H), 3.84 (bs, 0.7H), 3.55-
2.8 (m,
4.3H), 2.78-2.75 (2xs, 3H), 2.7-2.5 (m, 3H), 2.4-1.6 (m, 14H), 1.5 (s, 4H),
1,4 (s, 2H).
13CNMR (125MHz, CDC13, 30 C) 6: 176.9, 171.6, 143.0, 136.4., 132.4, 130.5,
128.3,
126.7, 64.6, 57.2, 56.0, 53.4, 50.8, 47.3, 41.7, 38.7, 34.2, 30.4 29.5, 27.2,
25.8, 24.9
LC-MS: M+1: 559.
Retention time: 6.98 (API)
Example 4: Synthesis of 1-f(3S)-4-fbenzoyl(methyl)aminol-3-(3,4-
dichlorophenyl)-
butyll-N,N-dimethyl-4-pyrrolidin-l-ylpiperidine-4-carboxamide (example
compound 99);
\
N
O
N NrJ\
O
-N
CI CI
Here: synthesis of N,N-dimethyl-4-pyrrolidin-l-ylpiperidine-4-carboxamide as
starting
material
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
49
O
HN N
I
C ~
V
Ethyl 1-benzyl-4-[(4-chlorobutanoyl)aminolpiperidine-4-carboxylate
O
N
NH
O
CI
78.3 g (0.298 mol) ethyl 4-amino-l-benzylpiperidine-4-carboxylate and 50 ml
pyridine (0.6 mol, 2 eq) were dissolved in 1 L CH2CI2. The mixture was cooled
to 5 C
and 0.37 ml chlorobutyryl chloride (0.33 mol, 1.1 eq) dissolved in 200 ml
CH2CI2 was
added drop wise. The mixture was stirred over the weekend at ambient
temperature.
800 ml of a saturated solution of NaHCO3 were added and the organic layer was
separated. The water layer was extracted with CH2CI2 (2x 500 ml). The organic
layer
was washed with NaHCO3 (2x 800 ml) and evaporated to dryness. The mixture was
purified over Silica (eluent: CH2CI2/MeOH, 100/0 to 97/3 to 95/5, v/v).
Yield: 113 g (- quantitative yield) of a yellow semi solid.
Ethyl 1-benzyl-4-(2-oxopyrrolidin-1-yl)piperidine-4-carboxylate
N p/~
O
~
12 g sodium hydride (290 mmol, 1.2 eq) were suspended in 600 ml THF. To
this suspension was added a gel of 88.5 g of ethyl 1-benzyl-4-[(4-
chlorobutanoyl)-
amino]-piperidine-4-carboxylate (240 mmol, 1 eq) in 200 ml THF. The mixture
was
stirred overnight at room temperature. NMR analysis revealed 60-70%
conversion. 5
g of extra sodium hydride (120 mmol, 0.5 eq) and 9 g (60 mmol, 0.3 eq.) sodium
iodide
were added and the mixture was stirred for another day at room temperature.
NMR
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
analysis revealed now complete conversion. The reaction was quenched with 1 L
of
water. The mixture was extracted with ethyl acetate (3x 1 L). The organic
layer was
washed with 500 ml of a saturated solution of sodium chloride in water. The
combined
water layers were extracted with dichloromethane (2x 1 L). The combined
organic
5 layers were evaporated to dryness. The crude mixture was further purified
over Silica
(eluent: CH2CI2/MeOH, 100/0 to 95/5, v/v) yielding 68.4 g of the target
compound (207
mmol, 67%) as light yellow solid.
1-Benzyl-N,N-dimethyl-4-(2-oxopyrrolid in-1 -yl)piperidine-4-carboxamide
o
N~
N I
O
~
65 g (200 mmol, 1 eq) of ethyl 1-benzyl-4-(2-oxopyrrolidin-1 -yl)piperidine-4-
carboxylate was dissolved in 600 ml THF and 400 ml 1 M NaOH was added. The
mixture was stirred at reflux overnight. TLC analysis revealed not complete
conversion. 100 ml 1 M extra NaOH was added and the mixture was stirred at
reflux for
another 2.5 h. The mixture was cooled to ambient temperature and diluted with
500 ml
of water. The mixture was extracted with CH2CI2 (2x 500 ml). The water layer
was
cooled and neutralized with concentrated HCI (ca. 20 ml). The mixture was
concentrated in vacuum to dryness.
This crude mixture was suspended in 1 L dichloromethane). Dimethylamine
HCI salt (21.9 g, 270 mmol, 1.35 eq), TBTU (86.7g, 270 mmol, 1.35 eq) and
triethyl
amine (138 ml, 1.0 mol, 5 eq) were added. The mixture was stirred overnight at
room
temperature. The mixture was diluted with CH2CI2 (500 ml) and washed with
water
(500 ml), NaHCO3 (500 mL), water and a saturated solution of sodium chloride
in water
(250 ml). The mixture was evaporated to dryness. The crude mixture was
purified
over Silica (eluent: CH2CI2/MeOH, 95/5, v/v).
Yield: 30.8 g of 1-Benzyl-N,N-dimethyl-4-(2-oxopyrrolidin-l-yl)piperidine-4-
carboxamide
(93.5 mmol, 52%) as light yellow solid.
N,N-Dimethyl-4-(2-oxopyrrolidin-1 -yl)piperidine-4-carboxamide
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
51
0
I
HC~.. N
O Benzyl-N,N-dimethyl-4-(2-oxopyrrolidin-1-yl)piperidine-4-carboxamide (29 g,
88
mmol) was dissolved in tert.-Butanol / water (600 ml, 9/1, v/v). Pd-C (6 g,
10%, wet)
was added and the mixture was stirred in hydrogen atmosphere (5 bar) at 50 C
for 16
h. NMR analysis revealed complete conversion. The Pd-C was removed by
filtration
over celite. The celite crop was washed with tert.-Butanol / water (100 mL,
9/1, v/v).
The volatiles were removed by evaporation in vacuum. The mixture was further
purified over Silica (eluent: CH2CI2/ 3 N ammonia in methanol, 95/5 to 90/10,
v/v). The
appropriate fractions were pooled and concentrated to dryness.
Yield: 18 g (75 mmol, 86%) as white solid.
N.N-Dimethyl-4-pyrrolidin-1-ylpiperidine-4-carboxamide
0
~
HN N
I
To the suspension of 1 g (4.2 mmol) N,N-dimethyl-4-(2-oxopyrrolidin-1-yl)-
piperidine-4-carboxamide in the 50 ml absolute tetrahydrofuran 1.8 ml Redal
(sodium-
bis-(2-methoxyethoxy)-dihydroaluminate) (3.5M/toluene, 6.3 mmol) was drop wise
added. The resulting solution was vigorously stirred for 4h at the room
temperature,
then reaction was quenched by the addition of 20 g NaF followed by 5 ml of 80%
aqueous tetrahydrofuran solution. The resulting slurry was stirred for another
hour,
then solids were filtered off, gently washed with 2x50 ml tetrahydrofuran and
organic
phase was evaporated under reduced pressure. Crude amine was further purified
by
column chromatography over silica gel (acetonitrile 6.25 : aqueous ammonia 25%
1)
delivering pale yellow oil with slowly crystallizes on standing (330 mg, 35%).
Melting point: 150 C.
Synthesis of 1-f(3S)-4-fbenzoyl(methyl)aminol-3-(3,4-dichlorophenyl)-butyll-
N,N-
dimethyl-4-pyrrolidin-l-ylpiperidine-4-carboxamide (example compound 99)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
52
\ N
p
O N N
O
-N
CI CI
To a suspension of 150 mg (0.0428 mmol) of the aldehyde from example 1, 96
mg (0.0428 mmol) of N,N-dimethyl-4-pyrrolidin-1-ylpiperidine-4-carboxamide and
0,5 g
sodium acetate in 21 ml of THF at room temperature under stirring were added
0.05 ml
of acetic acid and the mixture was stirred for 5 hours at room temperature. To
the
mixture were added portion wise 0.13 g (0.062 mmol) of sodium
triacetoxyborohydride
and the mixture was further stirred at room temperature for 15 hours. The
mixture was
concentrated to dryness in vacuum and re-dissolved in 6 ml ethyl acetate, 10
ml of
MTBE and 0.12 g of KOH dissolved in 5 ml of water. The organic phase was
washed
with sodium hydrogenocarbonate until pH 5-6 and 3 times with 10 ml of water.
The
organic solution was dried on sodium sulphate and concentrated to dryness.
Crude
amine was further purified by column chromatography over silica gel (ethyl
acetate 10:
triethylamine 1; Rf=0.35) delivering 109 mg of a colourless amorphous foam
(45%).
'HNMR (500 MHz, CDC13) 6: 7.45-7.25 (m, 4.5H), 7.25-7.0 (m, 3H), 6.9, 6.75
(2xbs,
0.6H), 3.85 (bm, 0.6H), 3.51 (bdd, J=12.5, 10.1 Hz, 1H), 3.5-2.75 (m, 9H), 2.7
(bs, 2H),
2.6 (bs, 4H), 2.4-1.7 (m, 9H), 1.65 (bs, 4H). ).
13CNMR (125MHz, CDC13) 6: 173.3, 171.5, 142.9, 136.4, 130.5, 130.2, 129.5,
128.4,
127.3, 126.8, 63.2, 56.1, 55.8, 53.3, 51.8, 51.3, 45.0, 42.5, 41.7, 38.8,
37.9, 30.6, 28.0,
24.1.
LC-MS: M+1: 559.
Retention time: 5.44 min (API).
Example 5: Synthesis of (2R)-1'-f(3S)-4-fbenzoyl(methyl)aminol-3-(3,4-dichloro-
phenyl)butyll-N,N-dimethyl-1,4'-bipiperidine-2-carboxamide (example compound
100 in
RS-configuration)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
53
N
O
i 7N C i
ci
ci
1 g of (7.75 mmol) L-pipecolic acid and 1.6 g (8.0 mmol) N-Boc-piperidin-4-one
were dissolved in 20 ml dichloromethane. To this solution 0.48 ml acetic acid
was
added and after 30 min., 3.27 g (2 eq) of sodium triacetoxyborohydride were
added.
The resulting suspension was stirred for 24 h at room temperature. The
reaction
mixture was diluted with 30 ml of a saturated solution of sodium chloride in
water and
the product was extracted with 6 x 20 ml dichloromethane. The organic phase
was
dried over Na2SO4 and evaporated under reduced pressure to yield 2.4 g of
[1,4']-
bipiperidinyl-2,1'-dicarboxylic acid 1'-tert-butyl ester.
2.4 g (7.7 mmol) of [1,4']-bipiperidinyl-2,1'-dicarboxylic acid 1'-tert-butyl
ester
were dissolved in 20 ml dichloromethane and 8 ml (2M/THF, 16 mmol)
dimethylamine,
1.75 ml (16 mmol) N-methyl-morpholine, 2.1 g (16 mmol) N-
hydroxymethylbenzotriazole were added. Finally 3 g (16 mmol) of EDC *HCI
(ethyl-n'-
(3-dimethylaminopropyl)-carbodiimide HCI salt) were added and reaction mixture
was
stirred for 20h at room temperature. The reaction mixture was diluted with 50
ml ethyl
acetate and washed with 2 x 20 ml 10% K2C03/water solution. The organic phase
was
separated, dried over anhydrous sodium sulphate and evaporated under reduced
pressure. The crude amide was purified by column chromatography over silica
gel
(ethyl acetate 2: ethanol 1, Rf=0.45) yielding 1.4 g colorless solid 2-
dimethylcarbamoyl-
[1,4']-bipiperidinyl-1'-carboxylic acid tert-butyl ester (53%).
[a]22p= +47 , c=1, MeOH.
1.4 g (4.13 mmol) 2-dimethylcarbamoyl-[1,4']-bipiperidinyl-1'-carboxylic acid
tert-butyl ester were dissolved in 1 ml dichloromethane and then 10 ml 6M
HCI/isopropanol were added. The reaction mixture was stirred at room
temperature for
3 h in which time, product crystallized in the reaction medium. The suspension
was
diluted with 50 ml MTBE, allowed to stir 30 more minutes, then filtered and
washed
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
54
with MTBE. The solid was dried under high vacuum for 1 h to remove traces of
volatile
materials yielding 1.17 g white powder [1,4']-bipiperidinyl-2-carboxylic acid
dimethylamide (91%).
To a suspension of 150 mg of the aldehyde from example 1 (0.0428 mmol), 135
mg (0.0428 mmol) of [1,4']-bipiperidinyl-2-carboxylic acid dimethylamide and
0,5 g
sodium acetate in 21 ml of THF at room temperature under stirring were added
0.05 ml
of acetic acid and the mixture was stirred for 5 hours at room temperature. To
the
mixture were added portion wise 0.13 g (0.062 mmol) of sodium
triacetoxyborohydride
and the mixture was further stirred at room temperature for 15 hours. The
mixture was
concentrated to dryness in vacuum and re-dissolved in 6 ml ethyl acetate, 10
ml of
MTBE and 0.12 g of KOH dissolved in 5 ml of water. The organic phase was
washed
with sodium hydrogenocarbonate until pH 5-6 and 3 times with 10 ml of water.
The
organic solution was dried on sodium sulphate and concentrated to dryness.
Crude
amine was further purified by column chromatography over silica gel (ethyl
acetate 10:
methanol 1: triethyl amine 1; Rf=0.35) delivering 121 mg of a colourless
amorphous
foam (49%).
[a]22p= -46.5 : c=1, CHC13.
'HNMR (500 MHz, CDC13) 6: 7.45-7.3 (m, 4.5H), 7.25-7.0 (m, 2.8H), 6.9, 6.75
(2xbs,
0.65H), 3.85 (bm, 0.65H), 3.6-3.4 (m, 1H), 3.55 (dd, J=13.1, 9.5 Hz, 1H), 3.25
(bs, 3H),
3.0 (m, 2H), 2.9 (s, 3H), 2.75 (m, 2H), 2.6 (bs, 2H), 2.4 (m, 1 H), 2.2 (dt,
J=11.0, 2.4 Hz,
1 H), 2.18 (m, 1 H), 2.0-1.3 (m, 14H), 1.25 (m, 1 H).
13CNMR (125MHz, CDC13) 6: 173.1, 171.6, 142.9, 136.4, 132.7, 132.5, 130.5,
130.2,
129.5, 128.4, 127.2, 126.7, 63.9, 58.7, 55.9, 53.8, 53.6, 53.4, 45.5, 42.3,
41.4, 38.8,
36.8, 36.2, 34.5, 33.5, 30.8, 30.3, 28.9, 25.8, 24.3, 23.9.
LC-MS: M+1=573.
Retention time: 4.73 min (API).
Example 6: Synthesis of (2S)-1'-[(3S)-4-[benzoyl(methyl)aminol-3-(3,4-dichloro-
phenyl)-butyll-N,N-dimethyl-1,4'-bipiperidine-2-carboxamide (example compound
100
in SS-configuration)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
N` J
O Y
i N l
~
ci
ci
See procedure for example 5. Instead of L-pipecolic acid, D-pipecolic acid is
used yielding to 1.267 g of white crystalline amine salt (2S)-1'-[(3S)-4-
5 [benzoyl(methyl)amino]-3-(3,4-dichloro-phenyl)butyl]-N,N-dimethyl-1,4'-
bipiperidine-2-
carboxamide and after the consequent steps including purification (ethyl
acetate 10:
methanol 1: triethyl amine 1; Rf=0.35) to 69 mg colorless amorphous foam
(28%).
[a]22p= -16.2 , c=1, CHC13.
'HNMR (500 MHz, CDC13) 6: 7.45-7.3 (m, 4.5H), 7.25-7.0 (m, 2.8H), 6.9, 6.75
(2xbs,
10 0.65H), 3.85 (bm, 0.65H), 3.6-3.4 (m, 1H), 3.55 (m, 1H), 3.23 (bs, 3H), 3.0
(m, 2H),
2.95 (s, 3H), 2.68 (m, 2H), 2.4 (m, 1 H), 2.2 (dt, J=11.0, 2.4 Hz, 1 H), 2.18
(m, 1 H), 2.0-
1.3 (m, 14H), 1.25 (m, 1 H).
13CNMR (125MHz, CDC13) 6: 173.1, 171.7, 142.8, 136.0, 132.5, 130.6, 130.2,
129.5,
128.4, 127.2, 126.7, 63.8, 58.7, 56.0, 54.1, 53.4, 53.2, 45.5, 42.5, 41.5,
38.9, 36.8,
15 36.2, 34.5, 33.7, 30.7, 30.2, 28.9, 25.7, 24.2, 23.8.
LC-MS: M+1 = 573.
Retention time: 4.73 min (API).
20 Example 7: Synthesis of 1'-[(3S)-4-[benzoyl(methyl)aminol-3-(3,4-dichloro-
phenyl)-
butyll-N,N-dimethyl-1,4'-bipiperidine-2-carboxamide (example compound 100)
^ 'N
O IrJY
e i N O i~ ~
ci
ci
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
56
See procedure for example 5. Instead of L-pipecolic acid or D-pipecolic acid,
(D-L)-pipecolic acid is used yielding to 1.267 g of white crystalline amine
salt (2)-1'-
[(3S)-4-[benzoyl(methyl)amino]-3-(3,4-dichloro-phenyl)butyl]-N,N-dimethyl-1,4'-
bi-
piperidine-2-carboxamide and after the consequent steps including purification
(ethyl
acetate 10: methanol 1: triethyl amine 1; Rf=0.35) to 69 mg colorless
amorphous foam
(28%).
[a]22p= -16.2 , c=1, CHC13.
'HNMR (500 MHz, CDC13) 6: 7.45-7.3 (m, 4.5H), 7.25-7.0 (m, 2.8H), 6.9, 6.75
(2xbs,
0.65H), 3.85 (bm, 0.65H), 3.6-3.4 (m, 1H), 3.55 (m, 1H), 3.23 (bs, 3H), 3.0
(m, 2H),
2.95 (s, 3H), 2.68 (m, 2H), 2.4 (m, 1 H), 2.2 (dt, J=11.0, 2.4 Hz, 1 H), 2.18
(m, 1 H), 2.0-
1.3 (m, 14H), 1.25 (m, 1 H).
13CNMR (125MHz, CDC13) 6: 173.1, 171.7, 142.8, 136.0, 132.5, 130.6, 130.2,
129.5,
128.4, 127.2, 126.7, 63.8, 58.7, 56.0, 54.1, 53.4, 53.2, 45.5, 42.5, 41.5,
38.9, 36.8,
36.2, 34.5, 33.7, 30.7, 30.2, 28.9, 25.7, 24.2, 23.8.
LC-MS: M+1=573.
Retention time: 4.73 min (API).
Example 8: Synthesis of N-f(2S)-2-(3,4-dichlorophenyl)-4-(4-{1-
f(dimethylamino)-
carbonyllcyclohexyl}piperazin-l-yl)butyll-N-methylbenzamide (example compound
96);
/ \
\
N
O
\
N ~N
O N"
CI CI I
Here: synthesis of 1-(4-benzylpiperazin-l-yl)-N,N-dimethylcyclo-
hexanecarboxamide
as starting material
C /
N /-\ N
0 i/
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
57
7.9 g (0,0262 mol) of 1-(4-benzylpiperazin-1-yl)-cyclohexanecarboxamide
(known from W00058292) in 120 ml of anhydrous THF was added drop wise at room
temperature under stirring to a suspension of 2.83 g (0.071 mol) of sodium
hydride
60% in oil in 120 ml of anhydrous THF under nitrogen. The mixture was then
heated to
60 C for 2 hours under stirring. After cooling down of the reaction mixture to
room
temperature, a solution of 6.33 g of methyl iodide in 80 ml of anhydrous DMF
was
added at room temperature and the reaction mixture was further stirred for 5
days. The
reaction mixture was then poured on 500 g of iced water and the product
extracted with
500 ml MTBE. The organic layer was recovered and washed with 500 ml of a
saturated solution on sodium chloride in water. The organic phase was then
dried on
sodium sulphate and concentrated to dryness. The HPLC-MS shows the presence of
the expected compound as well as of its monomethylated analogue.
The mixture was then separated by column chromatography on silicagel with n-
hexane: 1/ ethyl acetate 9 to deliver 3.2 g of the expected compound (yield:
38%).
Here: synthesis of N,N-dimethyl-l-piperazin-l-ylcyclohexane-carboxamide as
starting
material
H NN
0 N~
1
3.2 g (0.0097 mol) of the 1-(4-benzylpiperazin-1-yl)-N,N-dimethylcyclo-
hexanecarboxamide was dissolved in 100 ml ethanol and 0.7 ml of acetyl
chloride were
added. Subsequently 1 g of 10% palladium on charcoal was added and the
suspension was hydrogenated at 3 bars at room temperature for 15 hours. The
catalyst was separated by filtration and washed with 25 ml ethanol. The
solvent was
then distilled off to deliver 2.6 g of N,N-dimethyl-l-piperazin-1-
ylcyclohexane-
carboxamide as a crystalline hydrochloride (yield: 97%).
Melting point: 230-1 C.
Synthesis of N-f(2S)-2-(3,4-dichlorophenyl)-4-(4-{1-f(dimethylamino)-
carbonyllcyclohexyl}piperazin-l-yl)butyll-N-methylbenzamide (example compound
96);
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
58
N
O
\
N ~N
O N
cl OI I
To a suspension of 150 mg of the aldehyde from example 1 (0.0428 mmol), 130
mg (0.0428 mmol) of N,N-dimethyl-1-piperazin-1-ylcyclohexane-carboxamide
hydrochloride and 0,5 g sodium acetate in 21 ml of THF at room temperature
under
stirring were added 0.05 ml of acetic acid and the mixture was stirred for 5
hours at
room temperature. To the mixture were added portion wise 0.13 g (0.062 mmol)
of
sodium triacetoxyborohydride and the mixture was further stirred at room
temperature
for 15 hours. The mixture was concentrated to dryness in vacuum and re-
dissolved in
6 ml ethyl acetate, 10 ml of MTBE and 0.12 g of KOH dissolved in 5 ml of
water. The
organic phase was washed with sodium hydrogenocarbonate until pH 5-6 and 3
times
with 10 ml of water. The organic solution was dried on sodium sulphate and
concentrated to dryness. Crude amine was further purified by column
chromatography
over silica gel (ethyl acetate 10: methanol 1: triethyl amine 1; Rf=0.35)
delivering 260
mg of a colourless amorphous foam (85%) as dihydrochloride.
'HNMR(as hydrochloride) (500 MHz, CD3 OD) 6: 7.65-6.95 (m, 8H), 3.82 (m, 2H),
3.80-3.5 (m, 3H), 3.2-2.9 (m, 15H), 2.8 (m, 2H), 2.5-1.1(m, 12H).
13CNMR (125MHz, CD3 OD ) 6: 174.1, 142.7, 137.3., 133.8, 132,5, 132.1, 129.7,
129.1, 128.1, 127.7, 58.2, 56.0, 53.7, 51.7, 47.3, 42.1, 39.2, 28.5, 27.1,
25.1
LC-MS: M+1(monoisotope): 585.
Retention time: 5.22 min (API).
Example 9: Synthesis of 1-f(3S)-4-fbenzoyl(methyl)aminol-3-(3,4-
dichlorophenyl)butyll-
4-cyclohexyl-N,N-dimethyl-piperidine-4-carboxamide (example compound 95)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
59
N
O
N
N /
CI O
CI
g (13.25 mmol) 4-phenyl-4-piperidinecarboxylic acid tosylate salt was
dissolved in 50 ml of dichloromethane, then 3.75 ml (27 mmol) triethylamine
and 3.1 g
5 (14 mmol) bis- tert-butyloxycarbonate were added. The reaction mixture was
stirred for
16 h, then diluted with 100 ml of ethyl acetate and washed with lx 50 ml 10%
aqueous
acetic acid and with 3x 50 ml of a saturated solution of sodium chloride in
water. The
organic phase was separated, dried over anhydrous sodium sulphate and
evaporated
under reduced pressure yielding 3.97 g white crystals of N-Boc-4-phenyl-4-
piperidinecarboxylic acid (98%).
13 mmol N-Boc-4-phenyl-4-piperidinecarboxylic acid was dissolved in 100 ml
ethanol and hydrogenated with hydrogen (6 bars) over 5%-Rh/AI20s at 60 C for
20 h.
After filtration and evaporation of solvent, pure crystalline N-Boc-4-
cyclohexyl-4-
piperidinecarboxylic acid was isolated 4.0 g (100%).
Melting point: 156 C.
1 g (3.2 mmol) of N-Boc-4-cyclohexyl-4-piperidine-carboxylic acid was
dissolved in 20 ml of dichloromethane and one drop of dimethylformamide was
added.
To the resulted solution 0.29 ml (1.05 eq) oxalyl chloride was added and
solution
stirred for 30 min. 3.2 ml of dimethylamine (2M/THF, 2 eq) was added and
mixture was
stirred for 30 more minutes. Solution was diluted with 50 ml ethyl acetate and
washed
with 2x 20 ml aqueous 10% potassium carbonate. The organic phase was dried
over
anhydrous sodium sulphate and evaporated under reduced pressure. Crude amide
was purified by column chromatography over silica gel (dichloromethane 20:
acetone 1,
Rf=0.3) yielding to 217 mg of N-Boc-4-cyclohexyl-4-piperidine-carboxylic acid
dimethylamide as a colorless oil (20%).
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
217 mg (0.64 mmol) N-Boc-4-cyclohexyl-4-piperidine-carboxylic acid dimethyl-
amide was dissolved in 0.5 ml of dichloromethane and then 2 ml 6M
HCI/isopropanol
were added. The reaction mixture was stirred at room temperature for 3 h in
which
time product crystallized in the reaction medium. The suspension was diluted
with 20
5 ml MTBE, allowed to stir 30 more minutes and then filtered and washed with
MTBE.
The solid was dried under high vacuum for 1 h to remove traces of volatile
materials
yielding 157 mg 4-cyclohexyl-4-piperidinecarboxylic acid dimethylamide
hydrochloride
(90%) as a white powder.
10 To a suspension of 95 mg (0.027 mmol) of the aldehyde from example 1, 75 mg
(0.0428 mmol) of 4-cyclohexyl-4-piperidinecarboxylic acid dimethylamide
hydrochloride
and 0,3 g sodium acetate in 20 ml of THF at room temperature under stirring
were
added 0.03 ml of acetic acid and the mixture was stirred for 5 hours at room
temperature. To the mixture were added portion wise 0.1 g (0.045 mmol) of
sodium
15 triacetoxyborohydride and the mixture was further stirred at room
temperature for 15
hours. The mixture was concentrated to dryness in vacuum and re-dissolved in 6
ml
ethyl acetate, 10 ml of MTBE and 0.12 g of KOH dissolved in 5 ml of water. The
organic phase was washed with sodium hydrogenocarbonate until pH 5-6 and 3
times
with 10 ml of water. The organic solution was dried on sodium sulphate and
20 concentrated to dryness. Crude amine was further purified by column
chromatography
over silica gel (ethyl acetate 10: triethylamine 1; Rf=0.35) delivering 102 mg
of a
colourless amorphous foam (66%).
'HNMR (500 MHz, CDC13) 6: 7.45-7.3 (m, 4.5H), 7.25-7.0 (m, 2.7H), 6.9, 6.75
(2xbs,
25 0.65H), 3.8 (m, 0.5H), 3.6-3.4 (m, 1.5H), 3.15 (m, 0.6H), 3.0 (s, 6H), 2.9-
2.5 (m, 4H),
2.2 (m, 0.6H), 2.1 (bd, J=12.5 Hz, 2H), 2.05-1.8 (m, 4H), 1.78 (bd, J=11.6 Hz,
2H), 1.6,
(m, 6H), 1.3-1.0 (m, 5H).
13CNMR (125MHz, CDC13) 6: 173.6, 170.5, 143.0, 135.4, 131.4, 129.6, 129.5,
129.2,
128.4, 127.3, 126.5, 126.2, 125.7, 55.4, 52.3, 51.1, 50.6, 49.5, 42.7, 41.5,
40.6, 37.7,
30 32.5, 29.7, 27.1, 26.2, 25.5.
LC-MS: M+1: 572.
Retention time: 5.57 min (API).
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
61
Example 11: Synthesis of N-{(2S)-2-(3,4-dichlorophenyl)-4-f4-pyrrolidin-l-yl-4-
(pyrrolidin-l-ylcarbonyl)piperidin-l-yllbutyl}-N-methylbenzamide (example
compound
103)
N
O ~
N N
O
N
cl CI ~
Here: synthesis of 1-benzyl-4-pyrrolidin-l-yl-4-(pyrrolidin-l-
ylcarbonyl)piperidine as
starting material
CNL?
O No
2.34 ml (0.0132 mol) of N-benzylpiperidin-4-one, 4.4 ml (0.0527 mol) of
pyrrolidine, 1.6 ml (0.02 mol) of chloroform and 38 mg of n-benzyl
triethylammonium
chloride were stirred at 5 C. To the stirred mixture and by maintaining the
temperature
at 5 C, was added drop wise a solution of 2.6 g of sodium hydroxide in 5 ml of
water.
After 15 hours of stirring at 5 C, 50 ml of water were added and the organic
material
was extracted 3 times with 10 ml of methylene chloride. The organic phase was
separated and washed twice with 30 ml of water. The organic phase was then
separated, dried on sodium sulphate and concentrated to dryness delivering
3.426 g of
an oily material showing the presence in NMR and MS of the expected compound.
Purification: 3 g of the mixture were separated in MPLC on 112 g of Si02 with
n-hexane-etyl ester 1:1 as eluant mixture delivering 1.94 g of the expected
compound
as white solid.
(according to Lai, J. Org. Chem 1980, p. 3671).
Here: synthesis of 4-pyrrolidin-l-yl-4-(pyrrolidin-l-ylcarbonyl)-piperidine as
starting
material
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
62
HN
N
O No
1.9 g (0.00556 mol) of 1-benzyl-4-pyrrolidin-1-yl-4-(pyrrolidin-1-ylcarbonyl)-
piperidine was dissolved in 200 ml of ethanol at 30 C and hydrogenated in the
presence of 4 spatules of palladium hydroxide 20% on charcoal (moisture 60% )
at 4
bars for 6 hours at 40 C. The catalyst was recovered by filtration and the
solution
concentrated to dryness delivering the expected de-benzylated compound which
was
identified in NMR and MS and used without further purification.
Synthesis of N-{(2S)-2-(3,4-dichlorophenyl)-4-f4-pyrrolidin-l-yl-4-(pyrrolidin-
1-ylcarbo-
nyl)piperidin-l-yllbutyl}-N-methylbenzamide (example compound 103);
N
O ~
N N
_ O
N
CI CI
To a suspension of 180 mg (0.51 mmol) of the aldehyde from example 1, 180
mg (0.51 mmol) of 4-pyrrolidin-1-yl-4-(pyrrolidin-1-ylcarbonyl)-piperidine and
0,3 g
sodium acetate in 20 ml of THF at room temperature under stirring were added
0.03 ml
of acetic acid and the mixture was stirred for 5 hours at room temperature. To
the
mixture were added portion wise 0.2 g (0.09 mmol) of sodium triacetoxyboro-
hydride
and the mixture was further stirred at room temperature for 15 hours. The
mixture was
concentrated to dryness in vacuum and re-dissolved in 6 ml ethyl acetate, 10
ml of
MTBE and 0.2 g of KOH dissolved in 5 ml of water. The organic phase was washed
with sodium hydrogenocarbonate until pH 5-6 and 3 times with 10 ml of water.
The
organic solution was dried on sodium sulphate and concentrated to dryness.
Crude
amine was further purified by column chromatography over silica gel (ethyl
acetate 10:
triethylamine 1; Rf=0.35) delivering 236 mg of a colourless amorphous foam
(79%).
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
63
'HNMR(as base) (500 MHz, CDC13) 6: 7.45-6.70 (m, 8H), 3.85 (m, 2H), 3.6-3.4
(m,
4H), 3.3-2.5 (m, 10H), 2.35-1.9 (m, 8H), 1.78 (s, 4H), 1,67 (s, 4H).
13CNMR (125MHz, CDC13 ) 6: 172.4, 171.6, 143.0, 136.5, 132,4, 130.5, 129.4,
128.3,
127.3, 126.7, 62.9, 56.1, 53.3, 51.7, 51.1, 47.5, 45.1, 41.7, 38.7, 27.9,
27.4, 24.0, 23.2
LC-MS: M+1 (monoisotope): 585.
Retention time: 5.85 min (API).
Example 12: Synthesis of 3-cyano-N-{(2S)-2-(3,4-dichlorophenyl)-4-[4-
pyrrolidin-1-yl-
4-(pyrrolidin-1-ylcarbonyl)piperidin-1-yllbutyl}-N-methyl-1-naphthamide
(example
compound 102)
~N
/
b-
0 \ N N N
O
CI ci ~
Here: synthesis of 3-Cyano-naphthalene-l-carboxylic acid [2S-(3,4-dichloro-
phenyl)-4-
oxo-butvll-methvl-amide as starting material
o
O
CN CI
cl
12.2 g DMSO in 100 ml dichloromethane are added drop wise to 7.3 g oxalyl
chloride in 100 ml dichloromethane unter nitrogen at - 70 C unter stirring.
The
resulting mixture was stirred for another 15 minutes before 20 g of [2S-(3,4-
dichloro-
phenyl)-4-hydroxy-butyl]-methyl-carbamic acid tert-butyl ester in 200 ml
dichloromethane were added. The mixture was stirred at - 70 C for one hour
before
40.3 ml of triethylamine in 50 ml dichloromethane were added drop wise. The
solution
was stirred at - 70 C for 15 minutes and then allowed to warm up to room
temperature.
The solvent was removed and the residue was dissolved in 300 ml of toluene and
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
64
200 ml of ethyl acetate. The resulting solution was washed six times with 200
ml of a
saturated solution of NaCI in water, dried over sodium sulfate and
concentrated to
dryness to deliver 19.7 g of 3-Cyano-naphthalene-l-carboxylic acid [2S-(3,4-
dichloro-
phenyl)-4-oxo-butyl]-methyl-amide.
Synthesis of 3-cyano-N-{(2S)-2-(3,4-dichlorophenyl)-4-[4-pyrrolidin-l-yl-4-
(pyrrolidin-l-
ylcarbonyl)piperidin-l-yllbutyl}-N-methyl-l-naphthamide (example compound
102);
253.8 mg (0.0006 mol) of 3-Cyano-naphthalene-l-carboxylic acid [2S-(3,4-
dichloro-phenyl)-4-oxo-butyl]-methyl-amide, 150 mg (0.006 mol) of 4-pyrrolidin-
1-yl-4-
(pyrrolidin-1-ylcarbonyl)-piperidine and 72 mg ( 0.0012 mol) of acetic acid
were
dissolved in 20 ml of methylene chloride and stirred for 30 minutes at room
temperature. Subsequently, 163.7 mg of sodium triacetoxyborohydride was added
and
the mixture stirred at room temperature for 3 hours. The reaction mixture was
then
diluted with 40m1 of ethyl acetate and the resulting phase washed twice with
50 ml a
10% aqueous solution of potassium carbonate and once with 50 ml of a saturated
sodium chloride water solution. The organic phase was then separated and
concentrated to dryness to deliver 280 mg of the title base as an amorphous
solid.
'HNMR (500 MHz, CDC13, ) 6: 8.24 (0.35H), 8.19(0.65H), 7.97-6.50) (m, 8H), 4.4
(bs,
0.65H), 3.85 (bs , 2H), 3.7-3.25 (m, 3.35H), 3.2 (s,m, 1 H), 3.0-1.9 (m, 10H),
1.8 (s, 4H),
1.68 (s, 4H).
13CNMR (125MHz, CDC13 ) 6: 172.4, 172.3, 142.5, 136.5, 134.7, 132.4, 131.0,
130.6,
130.5, 130.1, 128.3, 127.5, 126.7, 125.2, 123.9, 118.4,109.0, 63.0, 57.1,
56.0, 53.3,
51.7, 51.1, 47.5, 45.1, 45.0, 41.7, 38.7, 37.7, 33.6, 31.6, 27.9, 27.4, 24.0
LC-MS: M+1 (monoisotope): 573.
Retention time: 7.90 min (API).
Example 13: Synthesis of N-{(2S)-2-(3,4-dichlorophenyl)-4-[4'-(piperidin-l-
ylcarbonyl)-
1,4'-bipiperidin-1'-yllbutyl}-N-methylbenzamide (example compound 104)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
\
N
O
N
N O
CI CI V
Here: synthesis of 1'-benzyl-4'-(piperidin-l-ylcarbonyl)-1,4'-bipiperidine as
starting
material
N
N
0 No
5
1'-benzyl-4'-(piperidin-1-ylcarbonyl)-1,4'-bipiperidine was obtained by
condensation of 2.34 ml (0.00132 mol) of N-benzylpiperidin-4-one, 5.3 ml
(0.00536
mol) of piperidine, 1.6 ml ( 0.002 mol) of chloroform and 38 mg of
10 benzyltriethylammonium chloride. A raw mixture of 4.445 g of an oily
material was
delivered (yield: 90%), 2 g of which were subsequently purified in preparative
HPLC
with a gradient acetonitrile/water containing formic acid in 2 runs to deliver
678 mg of
the product as diformiate after lyophilisation of the fractions containing the
pure
expected compound.
Here: synthesis of 4'-(piperidin-l-ylcarbonyl)-1,4'-bipiperidine as starting
material
HN
N
O No
576 mg of 1'-benzyl-4'-(piperidin-1-ylcarbonyl)-1,4'-bipiperidine as
diformiate
was transformed into dihydrochloride, dissolved in 50 ml ethanol and
hydrogenated at
40 C for 4 days in presence of 50 mg of 10% palladium on charcoal. The
catalyst was
separated by filtration and washed 3 times with 50 ml ethanol. The organic
phases
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
66
were reunited and concentrated to dryness to deliver 4'-(piperidin-1-
ylcarbonyl)-1,4'-
bipiperidine as a solid dihydrochloride.
Synthesis of N-{(2S)-2-(3,4-dichlorophenyl)-4-f4'-(piperidin-1-ylcarbonyl)-
1,4'-
bipiperidin-1'-yllbutyl}-N-methylbenzamide (example compound 104)
/ \
\
N
O
N
N O
CI CI ~
127 mg (0.00036 mol) of the aldehyde from example 1, 160 mg (0.0036 mol) 4'-
(piperidin-1-ylcarbonyl)-1,4'-bipiperidine as a solid dihydrochloride and 35
mg (0.0006
mol) of acetic acid were dissolved in 20 ml of methylene chloride and stirred
for 30
minutes at room temperature. Subsequently, 85 mg of sodium
triacetoxyborohydride
was added and the mixture stirred at room temperature for 3 hours. The
reaction
mixture was then diluted with 20m1 of ethyl acetate and the resulting phase
washed
twice with 50 ml a 10% aqueous solution of potassium carbonate and once with
50 ml
of a saturated sodium chloride water solution. The organic phase was then
separated
and concentrated to dryness to deliver 206 mg of the title base as a
colourless foam in
form of the hydrochloride (quantitative yield).
'HNMR(as hydrochloride) (500 MHz, CDC13 OD) 6: 7.65-6.95 (m, 8H), 3.8 -3.55
(m,
8H), 3.35-3.25 (2xs, 3H), 3.2-2.4 (m, 13H), 2.35-2.15 (m, 2H), 1.95 ( s, 4H )
1.7 (s, 2H),
1,62 (s, 4H).
13CNMR (125MHz, CD3 OD ) 6: 174.1, 142.7, 137.3., 133.8, 132,5, 132.1, 129.7,
129.1, 128.1, 127.7, 58.2, 56.0, 53.7, 51.7, 47.3, 42.1, 39.2, 28.5, 27.1,
25.1
LC-MS: M+1 (monoisotope): 613.
Retention time: 6.14 min (API).
Example 13: Synthesis of N-{(2S)-2-(3,4-dichlorophenyl)-4-f4'-(pyrrolidin-l-
ylcarbonyl)-
1,4'-bipiperidin-1'-yllbutyl}-N-methylbenzamide (example compound 124)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
67
ON
N
N
O
CI CI
~
Here: synthesis of 1'-benzyl-1,4'-bipiperidine-4'-carbox-amide as starting
material
N I \
N /
N
0
4,5 g (52.8 mmol) of piperidine, 5 g (26.4 mmol) N-benzyl-piperi-din-4-one,
9,5
g (3 eq) of magnesium sulphate and 2.3 ml of N-dimethylacetamide were mixed
together and then 2.6 ml (1 eq) of 2-cyano-2-hydroxy-propane were added. The
resulting suspension was stirred over 48 h at 55 C whereupon pasty suspension
solidifies. Crude product was mixed with 100 ml water and 100 ml ethyl
acetate.
Organic phase was washed with water (2 x 50 ml), dried over sodium sulphate
and
evaporated yielding 7 g crude aminonitrile.
1.3 g (3,6 mmol) of the resulted aminonitrile was dissolved in 15 ml 90% wt.
sulfuric acid and heated for 10 minutes at 100 C. The resulting solution was
poured on
ice and then basicified to pH 9 with sodium hydroxide. Crude amine was
extracted with
ethyl acetate (3 x 30 ml), organic phase washed one time with a saturated
solution of
sodium chloride in water, dried over sodium sulphate and evaporated. Yield 1 g
(92%)
light yellow crystals of 1'-benzyl-1,4'-bipiperidine-4'-carboxamide.
Here: synthesis of N,N-diallyl-1'-benzyl-1,4'-bipiperidine-4'-carboxamide as
starting
material
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
68
N
O
1,75 g (5,83 mmol) of 1'-benzyl-1,4'-bipiperidine-4'-carboxamide was dissolved
in 10 ml hexamethylphosphortriamide. To this solution 466 mg (2 eq) sodium
hydride
(60% in oil) was added portion-wise and the suspension was stirred at 60 C for
2
hours. Then the resulting solution was cooled to room temperature and 1 ml (2
eq)
allyl bromide was added over 6 hours via syringe pump. After 24 h reaction was
quenched by addition of 10% aqueous ammonium chloride (50 ml) and product
extracted with ethyl acetate. The organic phase dried over sodium sulphate and
evaporated. Crude compound was purified by column chromatography over silica
gel
(ethyl acetate 5: ethanol 1, Rf=0.3) yielding 1 g (45%) of desired N,N-diallyl-
1'-benzyl-
1,4'-bipiperidine-4'-carboxamide.
Here: synthesis of 1'-benzyl-4'-(2,5-dihydro-1H-pyrrol-1-ylcarbonyl)-1,4'-
bipiperidine as
starting material
N
O
N
a-N
1 g (2,6 mmol) N,N-diallyl-1'-benzyl-1,4'-bipiperi-dine-4'-carboxamide was
dissolved in 50 ml dichloromethane and to the resulted refluxed solution was
added
portion wise Grubbs-1 catalyst (1 g, 50 mol%) over 36 hours. The solvent was
evaporated and product was purified by column chromatography over silica gel
(ethyl
acetate, Rf= 0.35) yielding 640 mg (70%) of pure 1'-benzyl-4'-(2,5-dihydro-1H-
pyrrol-1-
ylcarbonyl)-1,4'-bipiperidine.
Here: synthesis of 4'-(pyrrolidin-l-ylcarbonyl)-1,4'-bipiperidine as starting
material
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
69
HN N
O
ID
0.64 g (1,81 mmol) of 1'-benzyl-4'-(2,5-dihydro-1 H-pyrrol-1-ylcarbonyl)-1,4'-
bipiperidine were dissolved in 70 ml ethanol and 200 mg of Pd(OH) 20% over
carbon
(60% moisture) were added. The compound was hydrogenated with hydrogen under 5
bar pressure. After 4 hours, the catalyst was removed by filtration and
solvent
evaporated under reduced pressure yielding 470 mg (99%) of blue-gray
crystalline
powder.
Melting point: 144 C.
Synthesis of N-{(2S)-2-(3,4-dichlorophenyl)-4-[4'-(pyrrolidin-l-ylcarbonyl)-
1,4'-bipiperi-
din-1'-yllbutyl}-N-methylbenzamide (example compound 124)
O
/ N-
N
N
O
CI CI
~
150 mg (0.005 mol) of the aldehyde from example 1, 120 mg (0.005 mol) 4'-
(pyrrolidin-1-ylcarbonyl)-1,4'-bipiperidine and 35 mg ( 0.0006 mol) of acetic
acid were
dissolved in 20 ml of methylene chloride and stirred for 30 minutes at room
temperature. Subsequently, 85 mg of sodium triacetoxyborohydride was added and
the mixture stirred at room temperature for 3 hours. The reaction mixture was
then
diluted with 20m1 of ethyl acetate and the resulting phase washed twice with
50 ml a
10% aqueous solution of potassium carbonate and once with 50 ml of a saturated
sodium chloride water solution. The organic phase was then separated and
concentrated to dryness to deliver the title compound. After purification, 171
mg of N-
{(2S)-2-(3,4-dichlorophenyl)-4-[4'-(pyrrolidin-l-ylcarbonyl)-1,4'-bipiperidin-
1'-yl]butyl}-N-
methylbenzamide (66%) were isolated.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
'HNMR (500 MHz, CDC13) 6: 7.45-7.3 (m, 4.6H), 7.25-7.0 (m, 2.8H), 6.9, 6.75
(2xbs,
0.65H), 4.1-3.7 (m, 2H), 3.6-3.4 (m, 3.65H), 3.15 (bs, 0.65H), 3.0 (bs, 1H),
2.9-2.6 (m,
5H), 2.46 (s, 4H), 2.3-1.85 (m, 8H), 1.75 (bs, 7H), 1.5 (m, 4H), 1.4 (m, 2H).
13CNMR (125MHz, CDC13) 6: 172.4, 171.6, 142.9, 136.4, 132.4, 130.5, 130.2,
129.4,
5 128.3, 127.2, 126.7, 66.4, 57.1, 56.1, 55.7, 53.3, 51.6, 51.1, 47.8, 47.2,
42.5, 41.6,
38.7, 33.6, 30.9, 27.4, 27.1, 26.9, 25.1, 23.2.
LC-MS: M+1=598.
Retention time: 5.82 min (API).
Example 14: Alternative synthesis of 1'-[(3S)-4-[benzoyl(methyl)aminol-3-(3,4-
dichlorophenyl)butyll-N-ethyl-N-methyl-1,4'-bipi-peridine-4'-carboxamide
(example
compound 95)
\ / ~
N
O
N N
O
N
cl cl
100 mg (0.158 mmol) of amide 1'-[4-(benzoyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid methylamide were
dissolved in 1 ml
absolute DMSO and then 21 mg (0.19 mmol) of potassium tert-butylate were added
under inert atmosphere. The resulting solution was stirred at room temperature
for 1 h,
then 25 mg (0.16 mmol) of ethyl iodide were added and the reaction mixture
stirred for
additional 24h at room temperature. The reaction mixture was diluted with 10
ml of
10% NH4CI water solution and product extracted with ethyl acetate (3x 10 ml).
The
organic phase was dried over Na2SO4 and evaporated under reduced pressure.
Crude
product was purified by column chromatography over silica gel (ethyl acetate
5:
methanol 1, Rf=0.25) yielding colorless foam (31 mg, 30%).
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
71
'HNMR (500 MHz, CDC13) 6: 7.3 (m, 4.5H), 7.25-7.0 (m, 3H), 6.9, 6.7 (2xbs,
0.6H), 3.9
(bs, 0.6H), 3.5 (dd, J=12.97, 9.92 Hz, 1 H), 3.4 (m, 3.5 H), 3.2 (bs, 1 H),
3.0-2.7 (m, 3H),
2.7 (s, 3H), 2.5 (bs, 4H), 2.4-1.6 (m, 10H), 1.5 (s, 4H), 1,4 (s, 2H), 1.1
(bs, 3H).
13CNMR (125MHz, CDC13) 6: 173.0, 172.0, 136.3, 130.6, 130.2, 129.5, 128.4,
127.2,
126.8, 66.4, 56.2, 55.9, 53.4, 51.7, 51.1, 50.6, 47.3, 44.6, 44.1, 42.5, 41.6,
41.1, 38.8,
36.0, 34.0, 33.5, 30.2, 29.7, 29.4, 26.9, 25.1, 11.7.
LC-MS: M+1=587.
Retention time: 5.59 min (API).
Example 14: synthesis of 4-({[(2S)-2-(3,4-dichlorophenyl)-4-{4'-
[(dimethylamino)-
carbonyll-1,4'-bipiperidin-1'-yl}butyll(methyl)amino}carbonyl)phenyl acetate
(example
compound 5)
0
0
N
O
N N
O
N
CI CI
Here: synthesis of tert-butyl [(2S)-2-(3,4-dichlorophenyl)-4-{4'-
[(dimethylamino)-
carbonyll-1,4'-bipiperidin-1'-yl}butyllmethyl carbamate as starting material
\ o~
N~
O ^
N NfJI
O
N
CI CI \
To a suspension of 4 g (0.0115 mol) [2-(3,4-Dichloro-phenyl)-4-oxo-butyl]-
methyl-carbamic acid tert-butyl ester, 4.35 g (0.0139 mol) of the
piperidinopiperidineketamide hydrochloride [1,4']Bipiperidinyl-4'-carboxylic
acid
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
72
dimethylamide and 2 g of sodium acetate in 200 ml of THF, 1.5 ml of acetic
acid were
added. The reaction mixture was stirred for 4 hours at room temperature. To
the
reaction mixture were the added portion-wise 4.88 g (0.0231 mol) of sodium
triacetoxyborohydride and the solution was stirred for 15 hours at room
temperature
and subsequently concentrated to dryness. The remaining material was dissolved
in
100 ml of MTBE and 5 g of potassium hydroxide in 50 ml of water. The separated
organic layer was then washed three times with 50 ml water, dried on sodium
sulphate
and concentrated to deliver 6.06 g of a foam which was used in the next step
without
further purification. Yield: 92%
Here: synthesis of 1'-[(3S)-3-(3,4-dichlorophenyl)-4-(methylamino)butyll-N,N-
dimethyl-
1,4'-bipiperidine-4'-carboxamide as starting material
\
NH
N N~
O
N
CI CI \
6 g (0.0105 mol) of [2-(3,4-Dichloro-phenyl)-4-(4'-dimethylcarbamoyl-[1,4']-
bipiperidinyl-1'-yl)-butyl]-methyl-carbamic acid tert-butyl ester were
dissolved in 10 ml
methylene chloride and 40 ml of of 5N solution of HCI in isopropanol (0.2 mol)
at room
temperature. A precipitate of 1'-[(3S)-3-(3,4-dichlorophenyl)-4-
(methylamino)butyl]-
N,N-dimethyl-1,4'-bipiperidine-4'-carboxamide as hydrochloride precipitated
and the
mixture was stirred for 15 hours till completeness of the reaction. The
mixture was
dropped in 150 ml MTBE and further stirred for 1 hour at room temperature. The
precipitate was separated by filtration, washed 3 times with 10 ml of MTBE and
the
obtained solid dried under vacuum at 60 C to deliver 4.1 g of 1'-[(3S)-3-(3,4-
dichlorophenyl)-4-(methylamino)butyl]-N,N-dimethyl-1,4'-bipiperidine-4'-
carboxamide
as trihydrochloride.
Yield: 67%.
Optical rotation: -2.0 (c=1% in methanol)
Here: synthesis of 4-({[(2S)-2-(3,4-dichlorophenyl)-4-{4'-[(-N,N-
dimethylamino)-
3 0 carbonv111,4'-bipiperidin-1'-vl}butvll(methvl)amino}carbonvl)phenvl
acetate
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
73
O
O-~
~
N
O
N N
O
N
CI CI ~
220 mg (0.00038 mol) of 1'-[(3S)-3-(3,4-dichlorophenyl)-4-(methylamino)butyl]-
N,N-
dimethyl-1,4'-bipiperidine-4'-carboxamide were dissolved in 20 ml of methylene
chloride in the presence of 200 pl of triethylamine under stirring at room
temperature.
To the reaction mixture, were added drop wise at room temperature, a solution
of 98
mg of 4-acetoxybenzoyl chloride in 20 ml of methylene chloride and
subsequently 200
pl of triethylamine were added. The reaction mixture was stirred for 15 hours
at room
temperature and concentrated in vacuum. The residue was dissolved in 50 ml of
ethyl
acetate and 30 ml of MTBE in the presence of 200 mg potassium hydroxide
dissolved
in 20 ml of water. The organic phase was separated and washed 4 times with 20
ml of
water. The organic layer was recovered, dried on sodium sulphate and
concentrated to
dryness to deliver 240 mg (quantitative yield) of 4-({[(2S)-2-(3,4-
dichlorophenyl)-4-{4'-
[(dimethylamino)carbonyl]-1,4'-bipiperidin-1'-
yl}butyl](methyl)amino}carbonyl)phenyl
acetate as a glassy material.
'HNMR(as base) (500 MHz, CDC13 ) 6: 7.45-7.1 (m, 7H), 7.0, 6.8 (2xbs, 0.7H),
3.82
(bs, 0.7H), 3.55 (dd, 1H), 3.5-3.2 (m, 4 H), 3.1 (bs, 0.7 H), 3.0 (m, 2H),
2.95-2.90 (2xs,
3H), 2.8-2.5 (m, 4.5H),2.3 (s, 3H) 2.25-1.6 (m, 12H), 1.5 (s, 4H), 1,4 (s,
2H).
13CNMR (125MHz, CDC13 ) 6: 170.7, 168.2, 150.7, 131.7., 121.5, 120.9, 66.1,
55.2,
56.1, 52.6, 51.7, 50.9, 50.4, 46.6, 40.9, 38.0, 36.9, 29.9, 26.2, 24.4, 20.4
LC-MS: M+1 (monoisotope): 631.
Retention time: 7.78 min (API).
Example 15: Synthesis of 1'-{(3S)-3-(3,4-dichlorophenyl)-4-[(4-hydroxybenzoyl)-
(methyl)aminolbutyl}-N,N-dimethyl-1,4'-bipiperidine-4'-carboxamide (example
compound 6)
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
74
OH
~
N
O ^
N NfJI
O
N
CI CI ~
170 mg (0.00027 mol) of 4-({[(2S)-2-(3,4-dichlorophenyl)-4-{4'-
[(dimethylamino)-
carbonyl]-1,4'-bipiperidin-1'-yl}butyl](methyl)amino}carbonyl)phenyl acetate
was dis-
solved in 30 ml of methanol in the presence of 300 mg of potassium hydroxide
at room
temperature and stirred for 20 hours. The solution was then concentrated to
dryness
and dissolved in 50 ml of ethyl acetate, 40 ml of MTBE and a solution of 2 g
of
ammonium chloride in 20 ml of water until a pH7 was reached. The organic phase
was
separated, washed 3 times with 20 ml of water, dried on sodium sulphate,
concentrated
under vacuum to deliver 102 mg of 1'-{(3S)-3-(3,4-dichlorophenyl)-4-[(4-
hydroxybenzoyl)(methyl)amino]-butyl}-N, N-dimethyl-1,4'-bipiperidine-4'-
carboxamide as
a glassy compound (yield: 64%).
'HNMR(as base) (500 MHz, CDC13) 6: 7.40-6.70 (m, 7H), (2xbs, 0.7H), 3.88 (bs,
0.7H),
3.45 (dd, 1 H), 3.5-3.25 (m, 4. H), 3.2 (bs, 0.7 H), 3.0 (m, 2H), 2.95-2.90
(2xs, 3H), 2.8-
2.5 (m, 4.5H), 2.4-1.6 (m, 12H), 1.5 (s, 4H), 1,4 (s, 2H).
13CNMR (125MHz, CDC13) 6: 173.5, 172.8,172.4, 160.1, 158.8, 132.9, 131.56,
131.4,
130.9, 129.8, 127.1, 70.6, 65.9, 55.6, 53.4, 51.7, 47.3, 38.7, 37.8, 31.9,
26.7, 26.0,
24.9
LC-MS: M+1 (monoisotope): 589.
Retention time:7.06 min (API).
Example 16: Synthesis of 1'-[(3S)-4-[benzoyl(methyl)aminol-3-phenylbutyll-N,N-
dimethyl-1,4'-bipiperidine-4'-carboxamide
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
N
O
N N
O
N
700 mg (0.00108 mol) of 1'-[4-(benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-
butyl]-[1,4']bipiperidinyl-4'-carboxylic acid methylamide dihydrochloride were
dissolved
5 in 100 ml ethanol at room temperature. To the solution were added 50 ml of
water and
560 mg of potassium hydroxide. The solution was hydrogenated for 5 days at
room
temperature in the presence of 5 spatules of 10% palladium on charcoal at 4
bars. The
solution was recovered after filtration and separation of the catalyst,
concentrated to
dryness and subsequently dissolved in 60 ml MTBE. The organic phase was washed
3
10 times with 10 ml of water, separated, dried on sodium sulphate and
concentrated to
dryness to deliver the title product as 405 mg of an oily material (Yield:
74%). 390 mg
of the base were dissolved in 2 ml of ethanol at 40 C and 330 pl of HCI 5N in
IPA were
added under stirring to give a solution to whom 20 ml of MTBE were added to
deliver a
precipitate. After heating of the solution to 50 C, the suspension was cooled
down to
15 room temperature. The precipitate was recovered by filtration, washed twice
with 10 ml
of methanol, dissolved in methanol the concentration to dryness of which
delivered 435
mg (98% yield) of a foamy colourless dihydrochloride.
'HNMR(as base) (500 MHz, CDC13) 6: 7.40-6.85 (m, 10H), 3.87 (bs, 0.7H), 3.58
(dd,
20 0.7H), 3.5-3.25 (m, 4.4 H), 3.1 (bs, 0.7 H), 2.96 (m, 2H), 2.95-2.85 (bs,
3H), 2.85-2.45
(m, 4.5H), 2.3-1.6 (m, 12H), 1.51 (s, 4H), 1,41 (s, 2H).
13CNMR (125MHz, CDC13) 6: 173.5, 171.5, 142.4, 136.8., 129.2, 128.7,128.5,
128.2,128.0, 126.7, 66.9, 57.6, 56.4, 53.4, 51.8, 47.3, 42.4, 38.5, 37.7,
33.6, 31.1,
26.9, 25.1
25 LC-MS: M+1 (monoisotope): 505.
Retention Time: 4.94 min (API).
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
76
Examples 17 to 36
Compounds 105 to 123 were obtained by reductive amination as outlined in
process (a). An aldehyde is reductive aminated with an amine to yield compound
of
Formula I, examples 105 to 123
R7
O NuR8
N
I N7 R8 ~ N N O
T II
CH ,
+ y ~
/ CH
-
CI HN O T
CI
CI
CI
II III I
Synthesis of N-f(2S)-4-{4-f(cyclopropylmethyl)(propionyl)-aminolpiperidin-l-
yl}-2-(3,4-
dichlorophenyl)butyll-N-methylbenzamide (example compound 105)
rA
N O
O N
CAN
CI
CI
To a mixture of 105 mg (0.3 mmol) of N-[2-(3,4-Dichloro-phenyl)-4-oxo-butyl]-N-
methyl-benzamide (compound II), 70 mg (0.33 mmol) of N-(cyclopropylmethyl)-N-
piperidin-4-ylpropanamide (compound III), and 37 mg (0.45 mmmol) sodium
acetate in
6 ml THF at room temperature under stirring were added in 0.03 ml (0.51 mmol)
acetic
acid. After 0.5 h stirring, 127 mg (0.6 mmol) of sodium triacetoxyborohydride
were
added to this mixture. After stirring for further15 h, the mixture was
concentrated under
vacuum to dryness and re-dissolved in 20 ml of dichloromethane. The organic
solution
was washed three times with potassium hydrogencarbonate and then dried on
sodium
sulfate. After filtration the organic phase was concentrated under vacuum to
dryness.
The crude product was purified by column chromatography on silica gel using
ethyl
acetate / ethanol as eluents (100/0 to 80/20 v/v) yielding 93 mg of a
colorless oil.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
77
The following compounds were prepared according to the described process of
reductive amination:
N-[(2S)-2-(3,4-dichlorophenyl)-4-{4-[isopropyl(propionyl)amino]piperidin-l-
yl}butyl]-N-methylbenzamide (compound example 106); N-[(2S)-2-(3,4-
dichlorophenyl)-
4-{4-[phenyl(propionyl)amino]piperidin-1-yl}butyl]-N-methylbenzamide (compound
example 107); N-[(2S)-4-{4-[butyl(propionyl)amino]piperidin-1-yl}-2-(3,4-
dichloro-
phenyl)butyl]-N-methylbenzamide (compound example 108); N-[(2S)-4-{4-[butyl-
(cyclopropylcarbonyl )am i no] piperid i n-1-yl}-2-(3,4-d ich lorophenyl
)butyl]-N-methyl-
benzamide (compound example 109); N-[(2S)-4-{4-[butyl(cyclohexylcarbonyl)-
amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-methylbenzamide (compound
example 110); N-[(2S)-4-{4-[benzoyl(butyl)amino]piperidin-1-yl}-2-(3,4-
dichloro-
phenyl)butyl]-N-methylbenzamide (compound example 111); N-[(2S)-2-(3,4-
dichlorophenyl)-4-{4-[(4-methoxybutyl)(propionyl)amino]piperidin-1-yl}butyl]-N-
methylbenzamide (compound example 112); N-[(2S)-4-{4-[(cyclopropylcarbonyl)(4-
methoxybutyl)amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-
methylbenzamide
(compound example 113); N-[(2S)-4-{4-[(cyclohexylcarbonyl)(4-methoxybutyl)-
amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-methylbenzamide (compound
example 114); N-[(2S)-4-{4-[benzoyl(4-methoxybutyl)amino]piperidin-1-yl}-2-
(3,4-
dichlorophenyl)butyl]-N-methylbenzamide (compound example 115); N-[(2S)-4-{4-
[cyclohexyl(propionyl)amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-
methyl-
benzamide (compound example 116); N-[(2S)-4-{4-
[cyclohexyl(cyclopropylcarbonyl)-
amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-methylbenzamide (compound
example 117); N-[(2S)-4-{4-[cyclohexyl(cyclohexylcarbonyl)amino]piperidin-1-
yl}-2-(3,4-
dichlorophenyl)butyl]-N-methylbenzamide (compound example 118); N-[(2S)-4-{4-
2 5 [benzoyl(cyclohexyl)amino]piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-
methyl-
benzamide (compound example 119); N-[(2S)-2-(3,4-dichlorophenyl)-4-{4-[(1-
methyl-
piperidin-4-yl)(propionyl)amino]piperidin-1-yl}butyl]-N-methylbenzamide
(compound
example 120); N-[(2S)-4-{4-[(cyclopropylcarbonyl)(1-methylpiperidin-4-
yl)amino]-
piperidin-1-yl}-2-(3,4-dichlorophenyl)butyl]-N-methylbenzamide (compound
example
121); N-[(2S)-4-{4-[(cyclohexylcarbonyl)(1-methylpiperidin-4-
yl)amino]piperidin-1-yl}-2-
(3,4-dichlorophenyl)butyl]-N-methylbenzamide (compound example 122); and N-{1-
[(3S)-4-[benzoyl(methyl)amino]-3-(3,4-dichlorophenyl)butyl]piperidin-4-yl}-N-
(1-
methylpiperidin-4-yl)benzamide (compound example 123).
Example 37: Preparation of starting materials (compound III):
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
78
':To //~~ O
tert.butoxycarbonyl anhydride '
HN
tBOC N
Illd Illc
HZN' R7 reductive amination
R8 y 0 O
H
N.R7 CIARB N, R7
tBOC, N acylation tBOC'N
Illa Illb
deprotection
R7
Ny R8
HN O
III
Commercially available compound Illd is reacted with tertiary butoxycarbonyl
anhydride to deliver compound Illc which is then is transformed to
intermediate Illb
under conditions of reductive amination. Intermediate Illb is acylated to the
corresponding amide Illa. Amide Illa is then deprotected to yield intermediate
III.
Example 38: Preparation of N-(cyclopropylmethyl)-N-piperidin-4-ylpropanamide
rll~,
N
^
H N ~O `
To a mixture of 1 g (5 mmol) of tert-butyl 4-oxopiperidine-l-carboxylate
(compound Illc), 0.39 ml (4.5 mmol) of 1-cyclopropylmethanamine and 0.56 g
(6.8
mmmol) sodium acetate in 20 ml THF at room temperature under stirring were
added
0.44 ml (7.75 mmol) acetic acid. After 1 h stirring 1.93 g (9.1 mmol) of
sodium
triacetoxyborohydride were added to this mixture. After stirring for further
15 h the
mixture was concentrated under vacuum to dryness and re-dissolved in 50 ml of
ether.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
79
The organic solution was extracted three times with 30 ml of 0.1 n aqueous
HCI. The
combined water layers were alkalized by aqueous sodium hydroxide and then
extracted three times with 30 ml of ether. The combined organic phase was
dried on
sodium sulfate, filtered and concentrated to dryness under vacuum to give 1.1
g crude
tert-butyl 4-[(cyclopropylmethyl)amino]piperidine-l-carboxylate (Illb).
To a 5 C cold solution of 1.1 g (4.3 mmol) of crude crude tert-butyl 4-
[(cyclopropylmethyl)-amino]piperidine-l-carboxylate (compound Illb) and 1.13
ml (6.5
mmol) N-ethyl-diisopropylamine in 25 ml dichloromethane, 0.45 ml (5.2 mmol)
propanoyl chloride in 3 ml dichloromethane were added. After stirring for 2 h
the
mixture was evaporated and redissolved in 50 ml ether. The organic phase was
sequentially washed 2 times with 30 ml water, 2 times with 20 ml of 0.1 N
aqueous
sodium hydroxide, with 30 ml of 0.1 N aqueous hydrochloric acid, dried on
sodium
sulfate and concentrated to give 1.1 g of crude tert-butyl 4-
[(cyclopropylmethyl)(propionyl)amino]cyclohexane-carboxylate (compound Illa).
The solution of 1.1 g of tert-butyl 4-[(cyclopropylmethyl)(propionyl)amino]-
piperidine-l-carboxylate (compound Illa) in 20 ml 4M hydrogen chloride in
dioxane and
5 ml ethanol was stirred at room temperature for 15 h, then evaporated and re-
dissolved in 50 ml dichloromethane and sequentially washed 3 times with 20 ml
of
aqueous potassium carbonate (10 %), with 30 ml water, dried on sodium sulfate
and
concentrated under vacuum to yield 0.45 g of N-(cyclopropylmethyl)-N-piperidin-
4-
ylpropanamide (compound III) as colourless oil.
The preparation of the required series of 4-substituted piperidines (see
above)
was performed according to the described process.
The compounds of formula I listed in table 14 below were prepared according to
the process described in the above examples or according to processes
analogous
thereto.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
ormula I
= - -- - _
Ex:Hi M~ m
1 CH3 ""= CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
CH3 "\/ CI CI CR6 NR7R8 (CO)mNR9N10 CH3 H 1
CH3 CI CI CR6 NR7R8 (CO)mNR9N10 N~ CH3 CH3 1
~ 4 CH3 \/ F CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
1~ CH3 ococH, CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
6-_j CH3 --= \/ oH CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
H CCO-O
7 CH3 --- CI CI CR6 NR7R8 (CO)mNR9N10 N~ CH3 CH3 I
ci
d CH3 CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
9 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
CI
10 CH3 CI CI CR6 NR7R8 (CO)n,NR9N10 CH3 CH3 I
11 CH3 --- ~\/ CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
CN
HO
12 CH3 CI CI CR6 NRTR8 (CO),NR9N10 No CH3 CH3 I
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
81
13 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 -== CH3 CH3 1
14 CH3 _--~ CI Cf CR6 NR7R8 (CO)mNR9N10 ---r0 CH3 CH3 1
F
15 CHs Cl Cl CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
~^ F F
16 CH3 =-- CI CI CR6 NR7R8 (CO)mNR9N10 --- CH3 CH3 I
;õ! 1 7 CH3 \~-o CI CI CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 1
<<:..
CH3 \ N ~ CI Cl CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
Cl CI CR6 NR7R8 (CO)mNR9N10 HQ CHs CH3 I
(CeHs
CHa N11sC", CI CI CR6 NR7R8 (CO)mNR9N10 ---10 CH3 CH3 1
21 CH3 \ o ~ C{ Cl CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
CHs
22 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
23 CH3 CI Cl CR6 NR7R8 (CO)mNR9N10 =-- CH3 CH3 I
-LCN(N)
24 CH3 -- ~~ CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 i
HOOC
25 CH3 --- CI CI CR6 NR7R8 (CO)mNR9N10 -No CH3 CH3 1
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
82
~ 26 CH3 Cl Cl CR6 NR7R8 (CO)mNR9N10 ~ CH3 CH3 1
27 CH3 --- CI Cl CR6 NR7R8 (CO),,,NR9N10 CH3 CH3 1
28 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
29 CH3 aH Cl Cl CR6 NR7R8 (CO)mNR9N10 --- No CH3 CH3 1
CH3 CI CI CR6 NR7R8 (CO),nNR9N10 CH3 CH3 I
_- o
CH3 \01 Cl Cl CR6 NR7R8 (CO)mNR9N10 N~ CH3 CH3 1
CH3 CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
CH3 Cl Cl CR6 NR7R8 (CO)mNR9N10 --=No CH3 CH3 I
34' CH3 Cl Cl CR6 NR7R8 (CO)mNR9N10 ---<D CH3 CH3 I
35 CH3 -=- ~ I CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
36 CH3 CI CI CR6 NR7R8 (C%,NR9N10 -~ CH3 CH3 1
37 CH3 ~ 4I Cl Cl CR6 NR7R8 (CO)~,NR9N10 --No CH3 CH3 1
38 CH3 Cl Cl CR6 NR7R8 (CO),nNR9N10 ---No CH3 CH3 I
N
CH3 --= s Cl Cl CR6 NR7R8 (CO)mNR9N10 --- No CH3 CH3 1
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
83
40 CH3 5IIJGJ CI CI CR6 NR7R8 (CO),NR9N10 ---~ CH3 CH3 1
41 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
N
42 CH3 CC) CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
43 CH3 \/ CI CI CR6 NR7R8 (CO)R,NR9N10 --- CH3 CH3 1
44 CH3 \~N CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
CH3 O\/ CI CI CR6 NR7R8 (CO),nNR9N10 CH3 CH3 I
CH3 --- CI CI CR6 NR7R8 (CO)rt,NR9N10 CH3 CH3 1
CH3 CI CI CR6 NR7R8 (CO)mNR9N10 --No CH3 CH3 I
S(Oj~NFL,
1!s CH3 ~N CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
49 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 -0 CH3 CH3 1
50 CH3 \ N CI CI CR6 NR7R8 (CO),nNR9N10 ---No CH3 CH3 1
51 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 ---~ CH3 CH3 1
52 CH3 G+-aotoH, CI CI CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 1
53 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 ---No GH3 CH3 1
CH3 --- \/ N(HJCOO-leN_-buyl CI CI CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 I
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
84
CH3 \! OcF, CI CI CR6 NR7R8 (CO)mNR9N10 N0 CH3 CHs 1
CF~
56 CH3 \! cF, CI CI CR6 NR7R8 (CO)mNR9N10 " "0 CH3 CH3 1
,-- H
57 CH2 Cl Cl CR6 NR7R8 (CO),nNR9N10 CH3 CH3 I
OH
58 CH3 0-C(0)0.ten:netyl CI CI CR6 NR7R8 (CO),õNR9N10 ---~ CH3 CH3 1
59 CHa ... //--~ Cl CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
CH3 \! \! CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
N(H)C(O).teit: butyl (H)C(O)O.tetllbutyl
CH3 Cl CI CR6 NR7R8 (CO)mNR9N10 -""ND CH3 CH3 I
CH3 "y CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
F
J
6S CH3 --- \ / ~ Oo F CI CI CR6 NR7R8 (CO),,,NR9N10 CH3 CH3 1
64 CH3 ---" CI CI CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 1
65 CH3 N _ Cl Cl CR6 NR7R8 (CO)mNR9N10 """No CH3 CH3 1
\ ! F
Ale
66 CH3 CI Cl CR6 NR7R8 (CO),nNR9N10 ""-10 CH3 CH3 I
."./ ~
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
67 CH3 N-_N 1 i CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
68 CH3 ""- \/ o CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
69 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 """ CH3 CH3 1
70 CHs CI CI CR6 NR7R8 (CO)mNR9N10 -XD CH3 CH3 1
CF,
CH3 _B~ CI CI CR6 NR7R8 (CO),,,NR9N10 No CH3 CH3 1
\ /
F
CH3 b CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
CH3 - F\ F CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
rF
74'! CH3 F\ / CI CI CR6 NR7R8 (CO)mNR9N10 ---~ CH3 CH3 1
CI
75 CH3 --" \/ " CI CI CR6 NR7R8 (CO),nNR9N10 ---No CH3 CH3 1
76 CH3 cl\ / CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
a
CH CF~
77' I 3 --- ~/ CI CI CR6 NR7R8 (CO)mNR9N10 CH3 CH3 1
b
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
86
78 CH3 ... CI CI CR6 NR7R8 (CO),NR9N10 N~ CH3 CH3 1
79 CH3 CI CI CR6 NR7R8 (CO),nNR9N10 --- CH3 CH3 I
CI
80 CH3 CI Cl CR6 NR7R8 (CO)mNR9N10 CH3 CH3 I
81 CH3 -z7 Cl Cl CR6 NR7R8 (CO),nNR9N10 - No CH3 CH3 1
cl
CH3 -_- Cl Cl CR6 NR7R8 (CO),nNR9N10 CH3 CH3 1
CF~
CH3 Cl CI CR6 NR7R8 (CO),,NR9N10 --=No CH3 CH3 1
CH3 === Cl Cl CR6 NR7R8 (CO),NR9N10 N~ CH3 CH3 1
85 CH3 ~ 7 Onae CI Cl CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 1
86 CH3 --- 0cF3 Cl Cl CR6 NR7R8 (CO),nNR9N10 ---No CH3 CH3 1
87 CH3 -=- Cl Cl CR6 NR7R8 (CO),õNR9N10 --- CH3 CH3 1
88 CH3 CI Cl CR6 NR7R8 (CO)mNR9N10 --=No CH3 CH3 1
89 CH3 Cl CI CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 1
90 CH3 Cl Cl CR6 NR7R8 (CO)mNR9N10 ---No CH3 CH3 I
CH3 --- 0 cN Cl Cl CR6 NR7R8 (CO)mNR9N10 -==N~ CHs CH3 1
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
87
92 CHa CI CI CR6 NR7R8 (CO)mNR9N10 N~ CH3 CH3 1
93 CHa Cl CI CR6 NR7R8 (CO)mNR9N10 N\~ CH3 H 1
NC ~ ~
94 CHa H H CR6 NR7R8 (CO)mNR9N10 N~ CH3 CH3 1
95 CHa CI CI CR6 NR7R8 (CO)mNR9N10 No C2Hs CH3 1
CH3 --- ci ci N - - - - -
C N(CH''-
CH3 CI CI CR6 NR7R8 H
{ CH3 CI CI CR6 NR7R8 (CO)mNR9N10 10 J CH3 CHa 1
NG
99 CH3 CI CI CR6 NR7R8 (CO)mNR9NIO CH3 CHa 1
oN(GH>)=
100 CH3 --- CI Cl CR6 NR7R8 H - - -
101 CH3 CI CI CR6 --o (CO)mNR9NIO - CH3 CHa 1
102 CH3
Nc ~ i CI CI CR6 NR7R8 (CO)mNR9N10 N~ ~ 1
103:CH3 --- Cl CI CR6 NR7R8 (CO)mNR9N10 ---N\~ -N~ 1
104 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 ~ ~ 1
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
88
105, CHa --- CI CI CR6 NR7R8 H ,=--Q C(O)CZHS - - -
100 CHa -=- CI CI CR6 NR7R8 H i-propyl C(O)C2H5 - - -
107 CH3 CI CI CR6 NR7R8 H - 0 C(O)C2H5 - - -
108 CH3 CI CI CR6 NR7R8 H n-butyl C(O) C2H5 -
109 CHa CI CI CR6 NR7R8 (CO),,NR9N10 n-butyl C(O)cyclo- -
ro
110 CH3 CI CI CR6 NR7R8 CO ,nNR9N10 n-but I C(O)cyclo-
( ) y hex
117 CH3 CI CI CR6 NR7R8 (CO),,NR9N10 n-butyl C(O)phenyl - - -
I I'? CH3 --- CI CI CR6 NR7R8 (CO)mNR9N10 (CH2)4 C(O)ethyl - - -
OCH3
I 1, CH3 CI CI CR6 NR7R8 (CO)R,NR9N10 (CH2)4 C(O)cyclo- - -
OCHa ro
14 CH3 --- CI CI CR6 NR7R8 (CO)mNR9N10 (CH2)4 C(O)cycIo-
OCH3 hex I
115 CH3 -- CI CI CR6 NR7R8 (CO),õNR9N10 (CH2)4 C(O)phenyl - - -
OCH3
116. CH3 CI CI CR6 NR7R8 (CO)mNR9N10 Cyclo- hex C(O)ethyl
117 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 Cyclo- C(O)cyClo- - -
hex ro
118 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 Cyclo- C(O)cyclo- - -
hex hex
119 CHa --- ~ ~ CI CI CR6 NR7R8 (CO)mNR9N10 Cycfo- C(O)phenyl - - -
hex
120 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 ~j- C(O)ethyl - - -
I11 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 C(O)cyclo-
ro
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
89
122 CH3 -= CI CI CR6 NR7R8 (CO)mNR9N10 C(O)oyolo- - -
hexl
123 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 ~ C(O)phenyl - - -
124 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 N~ N~ 1
125 CH3 Nc ~ e Cl CI CR6 NR7R8 (CO)mNR9N10 N~ ~ 1
I CH3 CI CI CR6 NR7R8 (CO),nNR9N10
12+ CH3 --- CI CI CR6 NR7R8 (CO),nNR9N10 CH3 CH3 CH3 CH3 I
128 CH3 Cl Cl CR6 NR7R8 (CO),,,NR9N10 ~o ---IC0 1
t29 CH3 CI CI CR6 NR7R8 (CO)mNR9N10 ... C2H5 CZHS CH3
13Q CH3 CI CI CR6 NR7R8 (CO),nNR9N10 CH3 CHa 1
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
Table 16 contains analytical data from mass spectroscopy indicating the
retention
time in relation to the molecular weight observed.
Table 16: MS data and retention time
~~e~pte I~~r~en~ Rei t~~e ~1c~TV~e~ght~a~~t~
.....,.. ..,...... .., ................. ... ....... ...... .
........,...,......
..... . .. ....... .............:...
1 100 5,43 573,6048
3 100 5,74 579,6522
4 100 5,47 591, 5949
14 100 5,58 609,585
19 100 4,86 574, 5929
20 96,44 5,65 699,7872
21 92,45 5,77 717, 7334
22 100 5,23 537,5718
23 100 5,65 565,6254
24 100 5,59 602,6465
25 100 5,29 617,6138
26 99,43 5,93 657,6009
27 100 5,99 649, 7024
28 100 6,25 677,756
29 100 5,26 617,6574
30 100 5,42 576,6087
31 100 5,27 563,566
32 100 5,75 623,6646
33 100 6,06 663, 7292
34 100 5,87 634, 0877
35 100 5,36 562,5819
36 100 5,25 563,566
37 100 5,43 579,633
38 100 5,36 579,633
39 100 5,74 612,6417
40 100 5,52 626,6685
41 100 5,3 612, 6417
42 98,54 4,91 575,581
43 100 5,03 574,5929
44 100 4,84 574, 5929
45 100 5,27 641,6358
46 100 4,96 652,6837
47 100 5,13 687,1288
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
91
48 100 4,51 577,5968
49 100 5,06 588,6197
50 100 4,92 588,6197
51 100 4,85 588,6197
52 100 4,79 622,6771
53 99,7 5,01 581,6244
54 100 5,8 688,7359
55 100 5,9 657,6009
56 100 6,3 723,6258
57 100 4,59 621,6058
58 100 5,7 680,7565
59 98,64 4,7 563,57
60 100 6,57 792,8871
61 100 5,79 688,7359
62 100 5,1 565, 5422
63 100 5,79 653,594
64 100 5,06 562, 5819
65 100 5,7 672,6686
66 100 5,47 670,6775
67 100 5,52 668,7093
68 100 5,49 613,6258
69 100 6,05 643, 7196
70 100 6,22 709,599
71 100 5,71 652,5009
72 99,84 5,5 591,5949
73 100 5,95 663,5553
74 92,69 5,4 609,585
75 98,97 5,92 642,495
76 92,88 5,61 642,495
77 100 5,8 641,6019
78 100 5,56 587,6316
79 100 5,5 591,5949
80 100 5,63 608,0499
81 100 5,86 642,495
82 100 5,44 603,6306
83 100 5,77 641,6019
84 100 5,66 608,0499
85 100 5,41 603,6306
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
92
86 98,96 5,82 641,6019
87 100 5,6 587,6316
88 100 5,67 553,6144
89 100 5,57 587,6316
91 100 5,31 598,6149
92 100 5,7 623, 6646
Example 39:
Capsules containing 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
Capsules with the following composition per capsule were produced:
1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid dimethylamide 20 mg
Corn starch 60 mg
Lactose 300 mg
Ethyl acetate q.s.
The active substance, the corn starch and the lactose were processed into a
homogenous pasty mixture using ethyl acetate. The paste was ground and the
resulting
granules were placed on a suitable tray and dried at 45 C in order to remove
the solvent.
The dried granules were passed through a crusher and mixed in a mixer with the
further
following auxiliaries:
Talcum 5 mg
Magnesium stearate 5 mg
Corn starch 9 mg
and then poured into 400 mg capsules (= capsule size 0).
Example 40:
Capsules containing 1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-
butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid methylamide
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
93
Capsules with the following composition per capsule were produced:
1'-[4-(Benzoyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-
carboxylic acid methylamide 20 mg
Corn starch 60 mg
Lactose 300 mg
Ethyl acetate q.s.
The active substance, the corn starch and the lactose were processed into a
homogenous pasty mixture using ethyl acetate. The paste was ground and the
resulting
granules were placed on a suitable tray and dried at 45 C in order to remove
the solvent.
The dried granules were passed through a crusher and mixed in a mixer with the
further
following auxiliaries:
Talcum 5 mg
Magnesium stearate 5 mg
Corn starch 9 mg
and then poured into 400 mg capsules (= capsule size 0).
Example 41:
Capsules containing 1'-[4-(Cyclohexanecarbonyl-methyl-amino)-3-(3,4-dichloro-
phenyl)-butyl]-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
Capsules with the following composition per capsule were produced:
1'-[4-(Cyclohexanecarbonyl-methyl-amino)-3-(3,4-dichloro-phenyl)-butyl]-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide 20 mg
Corn starch 60 mg
Lactose 300 mg
Ethyl acetate q.s.
The active substance, the corn starch and the lactose were processed into a
homogenous pasty mixture using ethyl acetate. The paste was ground and the
resulting
granules were placed on a suitable tray and dried at 45 C in order to remove
the solvent.
CA 02641817 2008-08-01
WO 2007/088181 PCT/EP2007/050964
94
The dried granules were passed through a crusher and mixed in a mixer with the
further
following auxiliaries:
Talcum 5 mg
Magnesium stearate 5 mg
Corn starch 9 mg
and then poured into 400 mg capsules (= capsule size 0).
Example 42:
Capsules containing 1'-{3-(3,4-Dichloro-phenyl)-4-[(4-fluoro-benzoyl)-methyl-
amino]-butyl}-[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide;
Capsules with the following composition per capsule were produced:
1'-{3-(3,4-Dichloro-phenyl)-4-[(4-fluoro-benzoyl)-methyl-amino]-butyl}-
[1,4']bipiperidinyl-4'-carboxylic acid dimethylamide 20 mg
Corn starch 60 mg
Lactose 300 mg
Ethyl acetate q.s.
The active substance, the corn starch and the lactose were processed into a
homogenous pasty mixture using ethyl acetate. The paste was ground and the
resulting
granules were placed on a suitable tray and dried at 45 C in order to remove
the solvent.
The dried granules were passed through a crusher and mixed in a mixer with the
further
following auxiliaries:
Talcum 5 mg
Magnesium stearate 5 mg
Corn starch 9 mg
and then poured into 400 mg capsules (= capsule size 0).