Note: Descriptions are shown in the official language in which they were submitted.
CA 02643070 2008-08-20
SPECIFICATION
NOVEL 1,2,3,4-TETRAHYDROQUINOXALINE DERIVATIVE HAVING
GLUCOCORTICOID RECEPTOR BINDING ACTIVITY
Technical Field
The present invention relates to a novel
1,2,3,4-tetrahydroquinoxaline derivative or a salt
thereof, which is useful as a pharmaceutical. The
derivative has a glucocorticoid receptor binding
activity and is useful as a glucocorticoid receptor
modulator having a nonsteroidal structure (a
glucocorticoid receptor agonist and/or a
glucocorticoid receptor antagonist).
Background Art
A glucocorticoid receptor is a 94 kDa ligand-
activated intracellular transcriptional factor that
is a member of the nuclear receptor superfamily. This
receptor is a mediator of glucocorticoid action which
effects the metabolism of carbohydrates, proteins,
fats and the like, the suppression of the immune or
inflammatory responses, the activatation of the
central nervous system, the regulation of the
cardiovascular function and the basal and stress-
related homeostasis and the like.
As glucocorticoid action-related diseases, metabolic
disorders such as diabetes and obesity, inflammatory
diseases such as arthritis, enteritis and chronic
1
CA 02643070 2008-08-20
s-
obstructive pulmonary diseases, autoimmune diseases
such as connective tissue diseases, allergic diseases
such as asthma, atopic dermatitis, allergic rhinitis
and conjunctivitis, central nervous system diseases
such as psychiatric disorders, Alzheimer's disease
and drug use disorders, cardiovascular diseases such
as hypertension, hypercalcemia, hyperinsulinemia and
hyperlipidemia, homeostasis-related diseases causing
an abnormality of neuro-immune-endocrine balance,
glaucoma and the like are known. (SOUGOU RINSYOU,
54(7), 1951-2076 (2005), JP-A-2002-193955.)
Therefore, a compound having a glucocorticoid
receptor binding activity is considered to be useful
as a preventive and/or therapeutic agent for these
diseases.
As such a compound having a glucocorticoid
receptor binding activity, glucocorticoid receptor
agonists synthesized in the living body such as
cortisol and corticosterone, synthetic glucocorticoid
receptor agonists such as dexamethasone, prednisone
and prednisilone, non-selective
glucocorticoid
receptor antagonists such as RU486 and the like are
known. (JP-A-2002-193955)
On the other hand, compounds having a 1,2,3,4-
tetrahydroquinoxaline structure are disclosed in WO
04/099192 and JP-A-5-148243 and the like.
The
compounds disclosed in WO 04/099192 are protein
thyrosine phosphatase inhibitors essentially having a
carboxylic group. On the other hand, a large number
2
CA 02643070 2008-08-20
of compounds having 1,2,3,4-tetrahydroquinoxaline
structure are disclosed as anti-virus agents in JP-A-
5-148243. However, the present compound has not been
specifically disclosed in any of patents.
Disclosure of the Invention
Problems to be solved
It is a very interesting subject to study
synthesis of a novel 1,2,3,4-tetrahydroquinoxaline
derivative and to find a pharmacological action of
the derivative.
Means of Solving Problems
The present inventors conducted studies about
the synthesis of 1,2,3,4-
tetrahydroquinoxaline
derivatives having a novel chemical structure, and
succeeded in producing a large number of novel
compounds. Further, the present inventors studied the
pharmacological actions of the derivatives and as a
result, they found that the derivatives have a
glucocorticoid receptor binding activity and are
useful as a pharmaceutical, and thus the present
invention has been completed.
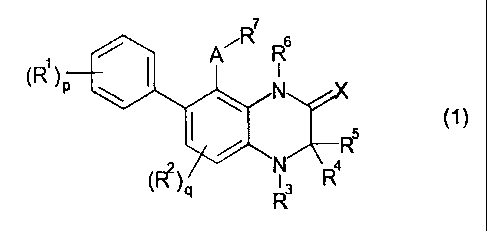
That is, the present invention relates to a
compound represented by the following general formula
(1) or a salt thereof (hereinafter referred to as
"the present compound") and a pharmaceutical
composition containing the same. Further, a preferred
invention in its pharmaceutical use relates to a
3
CA 02643070 2008-08-20
glucocorticoid receptor modulator, and its target
diseases are glucocorticoid receptor-
related
diseases, that is, metabolic disorders such as
diabetes and obesity, inflammatory diseases such as
arthritis, enteritis and chronic obstructive
pulmonary diseases, autoimmune diseases such as
connective tissue diseases, allergic diseases such as
asthma, atopic dermatitis, allergic rhinitis and
conjunctivitis, central nervous system diseases such
as psychiatric disorders, Alzheimer's disease and
drug use disorders, cardiovascular diseases such as
hypertension, hypercalcemia, hyperinsulinemia and
hyperlipidemia, homeostasis-related diseases causing
an abnormality of neuro-immune-endocrine balance,
glaucoma and the like. A particularly preferred
invention is an invention relating to a preventive or
a therapeutic agent for these diseases.
m7
6
A RI
(R1)p
1\1.,,X
(1)
______________________________________ R5
N µ4
(=e),
[wherein RI. represents a halogen atom, a lower alkyl
group which may have at least a substituent, a lower
cycloalkyl group which may have at least a
substituent, an aryl group which may have at least a
substituent, a heterocyclic group which may have at
least a substituent, a hydroxy group, an ester of a
4
CA 02643070 2008-08-20
hydroxy group, a lower alkoxy group which may have at
least a substituent, a lower cycloalkyloxy group
which may have at least a substituent, an aryloxy
group which may have at least a substituent, a
heterocyclic oxy group which may have at least a
substituent, a mercapto group, an ester of a mercapto
group, a lower alkylthio group which may have at
least a substituent, a lower cycloalkylthio group
which may have at least a substituent, an arylthio
group which may have at least a substituent, a
heterocyclic thio group which may have at least a
substituent, an amino group, a lower alkylamino group
which may have at least a substituent, a lower
cycloalkylamino group which may have at least a
substituent, an arylamino group which may have at
least a substituent, a heterocyclic amino group which
may have at least a substituent, an amide of an amino
group, an amide of a lower alkylamino group which may
have at least a substituent, an amide of a lower
cycloalkylamino group which may have at least a
substituent, an amide of an arylamino group which may
have at least a substituent, an amide of a
heterocyclic amino group which may have at least a
substituent, a formyl group, a lower alkylcarbonyl
group which may have at least a substituent, a lower
cycloalkylcarbonyl group which may have at least a
substituent, an arylcarbonyl group which may have at
least a substituent, a heterocyclic carbonyl group
which may have at least a substituent, a carboxy
CA 02643070 2008-08-20
group, an ester of a carboxy group, an amide of a
carboxy group, a lower alkylsulfonyl group which may
have at least a substituent, a lower
cycloalkylsulfonyl group which may have at least a
substituent, an arylsulfonyl group which may have at
least a substituent, a heterocyclic sulfonyl group
which may have at least a substituent, a sulfonic
acid group, an ester of a sulfonic acid group, an
amide of a sulfonic acid group, a nitro group or a
cyano group;
p represents an integer of 0 to 5;
in the case where p is 2 to 5, each R1 may be
the same or different;
R2 represents a halogen atom, a lower alkyl
group which may have at least a substituent, a
hydroxy group, an ester of a hydroxy group or a lower
alkoxy group which may have at least a substituent;
q represents an integer of 0 to 2;
in the case where q is 2, each R2 may be the
same or different;
R3 represents a hydrogen atom, a lower alkyl
group which may have at least a substituent, a lower
alkenyl group which may have at least a substituent,
a lower alkylcarbonyl group which may have at least a
substituent, a lower alkenylcarbonyl group which may
have at least a substituent or an arylcarbonyl group
which may have at least a substituent;
R4 and R5 may be the same or different and
represent a hydrogen atom, a halogen atom, a lower
6
CA 02643070 2008-08-20
alkyl group which may have at least a substituent, a
lower alkenyl group which may have at least a
substituent, a lower alkynyl group which may have at
least a substituent, a lower cycloalkyl group which
may have at least a substituent, an aryl group which
may have at least a substituent or a heterocyclic
group which may have at least a substituent;
R4 and R5 may be combined together to form a 3-
to 8-membered lower cycloalkane ring which may have
at least a substituent;
R6 represents a hydrogen atom, a lower alkyl
group which may have at least a substituent, a lower
alkenyl group which may have at least a substituent,
a lower alkynyl group which may have at least a
substituent, a lower cycloalkyl group which may have
at least a substituent, an aryl group which may have
at least a substituent or a heterocyclic group which
may have at least a substituent;
A represents a lower alkylene group which may
have at least a substituent;
R7 represents OR8, NR8R9, SR8, S(0)R8 or S(0)2R8;
R8 and R9 may be the same or different and
represent a hydrogen atom, a lower alkyl group which
may have at least a substituent, a lower alkenyl
group which may have at least a substituent, a lower
alkynyl group which may have at least a substituent,
a lower cycloalkyl group which may have at least a
substituent, an aryl group which may have at least a
substituent, a heterocyclic group which may have at
7
CA 02643070 2008-08-20
least a substituent, a formyl group, a lower
alkylcarbonyl group which may have at least a
substituent, a lower alkenylcarbonyl group which may
have at least a substituent, a lower alkynylcarbonyl
group which may have at least a substituent, a lower
cycloalkylcarbonyl group which may have at least a
substituent, an arylcarbonyl group which may have at
least a substituent, a heterocyclic carbonyl group
which may have at least a substituent, a carboxy
group, a lower alkoxycarbonyl group which may have at
least a substituent, a lower alkenyloxycarbonyl group
which may have at least a substituent, a lower
alkynyloxycarbonyl group which may have at least a
substituent, a lower cycloalkyloxycarbonyl group
which may have at least a substituent, an
aryloxycarbonyl group which may have at least a
substituent, a heterocyclic oxycarbonyl group which
may have at least a substituent, a lower
alkylsulfonyl group which may have at least a
substituent, a lower alkenylsulfonyl group which may
have at least a substituent, a lower alkynylsulfonyl
group which may have at least a substituent, a lower
cycloalkylsulfonyl group which may have at least a
substituent, an arylsulfonyl group which may have at
least a substituent, a heterocyclic sulfonyl group
which may have at least a substituent, an
aminocarbonyl group, a lower alkylaminocarbonyl group
which may have at least a substituent, a lower
alkenylaminocarbonyl group which may have at least a
8
CA 02643070 2008-08-20
substituent, a lower alkynylaminocarbonyl group which
may have at least a substituent, a lower
cycloalkylaminocarbonyl group which may have at least
a substituent, an arylaminocarbonyl group which may
have at least a substituent or a heterocyclic
aminocarbonyl group which may have at least a
substituent;
in the case where R7 is NR8R9, R8 and R9 may be
combined together to form a 3- to 8-membered
nitrogen-containing heterocyclic ring which may have
at least a substituent; and
X represents 0 or S. Hereinafter the same shall
apply.]
Advantage of the Invention
The present invention provides a 1,2,3,4-
tetrahydroquinoxaline derivative or a salt thereof,
which is useful as a pharmaceutical. The present
compound has an excellent glucocorticoid receptor
binding activity and is useful as a glucocorticoid
receptor modulator. In
particular, the present
compound is useful as a preventive or therapeutic
agent for glucocorticoid action related diseases,
that is, metabolic disorders such as diabetes and
obesity, inflammatory diseases such as arthritis,
enteritis and chronic obstructive pulmonary diseases,
autoimmune diseases such as connective tissue
diseases, allergic diseases such as asthma, atopic
dermatitis, allergic rhinitis and conjunctivitis,
9
CA 02643070 2008-08-20
. .
central nervous system diseases such as psychiatric
disorders, Alzheimer's disease and drug use
disorders, cardiovascular diseases such
as
hypertension, hypercalcemia, hyperinsulinemia and
hyperlipidemia, homeostasis-related diseases causing
an abnormality of neuro-immune-endocrine balance,
glaucoma and the like.
Best Mode for Carrying Out the Invention
Hereinafter, definitions of terms and phrases
(atoms, groups, rings and the like) to be used in
this specification will be described in detail.
The "halogen atom" refers to a fluorine,
chlorine, bromine or iodine atom.
The "lower alkyl group" refers to a straight
chain or branched alkyl group having 1 to 8 carbon
atoms. Specific examples thereof include methyl,
ethyl, n-propyl, n-butyl, n-pentyl, n-hexyl, n-
heptyl, n-octyl, isopropyl, isobutyl, sec-butyl,
tert-butyl and isopentyl groups and the like.
The "lower alkenyl group" refers to a straight
chain or branched alkenyl group having 2 to 8 carbon
atoms. Specific examples thereof include vinyl,
propenyl, butenyl, pentenyl, hexenyl, heptenyl,
octenyl, isopropenyl, 2-methyl-l-propenyl and 2-
methy1-2-butenyl groups and the like.
The "lower alkynyl group" refers to a straight
chain or branched alkynyl group having 2 to 8 carbon
atoms. Specific examples thereof include ethynyl,
CA 02643070 2008-08-20
propynyl, butynyl, pentynyl, hexynyl, heptynyl,
octynyl, isobutynyl and isopentynyl groups and the
like.
The "lower cycloalkyl group" refers to a
cycloalkyl group having 3 to 8 carbon atoms. Specific
examples thereof include cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl
groups.
The "lower cycloalkane ring" refers to a
cycloalkane ring having 3 to 8 carbon atoms. Specific
examples thereof include cyclopropane, cyclobutane,
cyclopentane, cyclohexane, cycloheptane and
cyclooctane rings.
The "aryl group" refers to a residue formed by
removing one hydrogen atom from a monocyclic aromatic
hydrocarbon group, or bicyclic or tricyclic condensed
polycyclic aromatic hydrocarbon having 6 to 14 carbon
atoms. Further, A
residue formed by removing one
hydrogen atom from bicyclic or tricyclic condensed
polycyclic hydrocarbon having 6 to 14 carbon atoms is
also included in the scope of the "aryl group".
Specific examples thereof include phenyl, naphthyl,
anthryl, phenanthryl and fluorenyl groups and the
like.
The "heterocyclic group" refers to a residue
formed by removing one hydrogen atom from a saturated
or unsaturated monocyclic heterocyclic ring, or a
bicyclic or tricyclic condensed polycyclic
heterocyclic ring having one or a plurality of
11
CA 02643070 2008-08-20
heteroatoms selected from a nitrogen atom, an oxygen
atom and a sulfur atom in the ring.
Specific examples of the saturated monocyclic
heterocyclic ring include pyrrolidine, pyrazolidine,
imidazolidine, triazolidine, piperidine,
hexahydropyridazine, hexahydropyrimidine, piperazine,
homopiperidine and homopiperazine rings and the like
having a nitrogen atom in the ring, tetrahydrofuran
and tetrahydropyran rings and the like having an
oxygen atom in the ring, tetrahydrothiophene and
tetrahydrothiopyran rings and the like having a
sulfur atom in the ring, oxazolidine, isoxazolidine
and morpholine rings and the like having a nitrogen
atom and an oxygen atom in the ring, and
thiazolidine, isothiazolidine and thiomorpholine
rings and the like having a nitrogen atom and a
sulfur atom in the ring.
Further, such a saturated monocyclic
heterocyclic ring can be condensed with a benzene
ring or the like to form a bicyclic or tricyclic
condensed polycyclic heterocyclic ring such as a
dihydroindole, dihydroindazole, dihydrobenzimidazole,
tetrahydroquinoline,
tetrahydroisoquinoline,
tetrahydrocinnoline,
tetrahydrophthalazine,
tetrahydroquinazoline,
tetrahydroquinoxaline,
dihydrobenzofuran, dihydroisobenzofuran, chromane,
isochromane,
dihydrobenzothiophene,
dihydroisobenzothiophene, thiochromane,
isothiochromane,
dihydrobenzoxazole,
12
CA 02643070 2008-08-20
dihydrobenzisoxazole,
dihydrobenzoxazine,
dihydrobenzothiazole,
dihydrobenzisothiazole,
dihydrobenzothiazine, xanthene, 4a-carbazole and
perimidine rings and the like.
Specific examples of the unsaturated monocyclic
heterocyclic ring include dihydropyrrole, pyrrole,
dihydropyrazole, pyrazole,
dihydroimidazole,
imidazole, dihydrotriazole, triazole,
tetrahydropyridine, dihydropyridine, pyridine,
tetrahydropyridazine, dihydropyridazine, pyridazine,
tetrahydropyrimidine, dihydropyrimidine, pyrimidine,
tetrahydropyrazine, dihydropyrazine and pyrazine
rings and the like having a nitrogen atom in the
ring, dihydrofuran, furan, dihydropyran and pyran
rings and the like having an oxygen atom in the ring,
dihydrothiophene, thiophene, dihydrothiopyran and
thiopyran rings and the like having a sulfur atom in
the ring, dihydrooxazole, oxazole, dihydroisoxazole,
isoxazole, dihydrooxazine and oxazine rings and the
like having a nitrogen atom and an oxygen atom in the
ring, dihydrothiazole, thiazole, dihydroisothiazole,
isothiazole, dihydrothiazine and thiazine rings and
the like having a nitrogen atom and a sulfur atom in
the ring.
Further, such an unsaturated monocyclic
heterocyclic ring can be condensed with a benzene
ring or the like to form a bicyclic or tricyclic
condensed polycyclic heterocyclic ring such as an
indole, indazole, benzimidazole, benzotriazole,
13
CA 02643070 2008-08-20
,
dihydroquinoline, quinoline,
dihydroisoquinoline,
isoquinoline, phenanthridine,
dihydrocinnoline,
cinnoline, dihydrophthalazine,
phthalazine,
dihydroquinazoline, quinazoline, dihydroquinoxaline,
quinoxaline, benzofuran, isobenzofuran, chromene,
isochromene, benzothiophene,
isobenzothiophene,
thiochromene, isothiochromene,
benzoxazole,
benzisoxazole, benzoxazine,
benzothiazole,
benzisothiazole, benzothiazine,
phenoxanthin,
carbazole, P-carboline, phenanthridine, acridine,
phenanthroline, phenazine, phenothiazine
or
phenoxazine rings and the like.
The "lower alkoxy group" refers to a group
formed by replacing the hydrogen atom of a hydroxy
group with a lower alkyl group. Specific examples
thereof include methoxy, ethoxy, n-propoxy, n-butoxy,
n-pentoxy, n-hexyloxy, n-heptyloxy,
n-octyloxy,
isopropoxy, isobutoxy, sec-butoxy, tert-butoxy and
isopentoxy groups and the like.
The "lower alkenyloxy group" refers to a group
formed by replacing the hydrogen atom of a hydroxy
group with a lower alkenyl group.
Specific examples
thereof include vinyloxy, propenyloxy, butenyloxy,
pentenyloxy, hexenyloxy, heptenyloxy, octenyloxy,
isopropenyloxy, 2-methyl-1-propenyloxy and 2-methyl-
2-butenyloxy groups and the like.
The "lower alkynyloxy group" refers to a group
formed by replacing the hydrogen atom of a hydroxy
group with a lower alkynyl group. Specific examples
14
CA 02643070 2008-08-20
A
thereof include ethynyloxy, propynyloxy, butynyloxy,
pentynyloxy, hexynyloxy, heptynyloxy, octynyloxy,
isobutynyloxy and isopentynyloxy groups and the like.
The "lower cycloalkyloxy group" refers to a
group formed by replacing the hydrogen atom of a
hydroxy group with a lower cycloalkyl group. Specific
examples thereof include
cyclopropyloxy,
cyclobutyloxy, cyclopentyloxy,
cyclohexyloxy,
cycloheptyloxy and cyclooctyloxy groups.
The "aryloxy group" refers to a group formed by
replacing the hydrogen atom of a hydroxy group with
an aryl group. Specific examples thereof include
phenoxy, naphthoxy, anthryloxy and phenanthryloxy
groups and the like.
The "heterocyclic oxy group" refers to a group
formed by replacing the hydrogen atom of a hydroxy
group with a heterocyclic group.
The "lower alkylthio group" refers to a group
formed by replacing the hydrogen atom of a mercapto
group with a lower alkyl group. Specific examples
thereof include methylthio, ethylthio, n-propylthio,
n-butylthio, n-pentylthio, n-hexylthio, n-heptylthio,
n-octylthio, isopropylthio, isobutylthio,
sec-
butylthio, tert-butylthio and isopentylthio groups
and the like.
The "lower cycloalkylthio group" refers to a
group formed by replacing the hydrogen atom of a
mercapto group with a lower cycloalkyl group.
Specific examples thereof include cyclopropylthio,
CA 02643070 2008-08-20
. .
cyclobutylthio, cyclopentylthio,
cyclohexylthio,
cycloheptylthio and cyclooctylthio groups.
The "arylthio group" refers to a group formed
by replacing the hydrogen atom of a mercapto group
with an aryl group. Specific examples thereof include
phenylthio, naphthylthio, anthrylthio
and
phenanthrylthio groups and the like.
The "heterocyclic thio group" refers to a group
formed by replacing the hydrogen atom of a mercapto
group with a heterocyclic group.
The "lower alkylamino group" refers to a group
formed by replacing one or both of the hydrogen atoms
of an amino group with a lower alkyl group. Specific
examples thereof include methylamino, ethylamino,
propylamino, dimethylamino, diethylamino
and
ethyl(methyl)amino groups and the like.
The "lower cycloalkylamino group" refers to a
group formed by replacing one or both of the hydrogen
atoms of an amino group with a lower cycloalkyl
group, or a group formed by replacing one of the
hydrogen atoms of an amino group with a lower
cycloalkyl group and the other hydrogen atom with a
lower alkyl group, a lower alkenyl group or a lower
alkynyl group. Specific examples thereof include
cyclopropylamino, cyclobutylamino, cyclopentylamino,
cyclohexylamino, cycloheptylamino, cyclooctylamino,
dicyclohexylamino,
cyclohexyl(methyl)amino,
cyclohexyl(vinyl)amino and cyclohexyl(ethynyl)amino
groups and the like.
16
CA 02643070 2008-08-20
.. .
The "arylamino group" refers to a group formed
by replacing one or both of the hydrogen atoms of an
amino group with an aryl group, or a group formed by
replacing one of the hydrogen atoms of an amino group
with an aryl group and the other hydrogen atom with a
lower alkyl group, a lower alkenyl group, a lower
alkynyl group or a lower cycloalkyl group. Specific
examples thereof include phenylamino, naphthylamino,
anthrylamino, phenanthrylamino,
diphenylamino,
methyl(phenyl)amino,
ethyl(phenyl)amino,
phenyl(vinyl)amino, ethynyl(phenyl)amino
and
cyclohexyl(phenyl)amino groups and the like.
The "heterocyclic amino group" refers to a
group formed by replacing one or both of the hydrogen
atoms of an amino group with a heterocyclic group, or
a group formed by replacing one of the hydrogen atoms
of an amino group with a heterocyclic group and the
other hydrogen atom with a lower alkyl group, a lower
alkenyl group, a lower alkynyl group, a lower
cycloalkyl group or an aryl group.
The "lower alkylcarbonyl group" refers to a
group formed by replacing the hydrogen atom of a
formyl group with a lower alkyl group.
Specific
examples thereof include
methylcarbonyl,
ethylcarbonyl, n-propylcarbonyl, n-butylcarbonyl, n-
pentylcarbonyl, n-hexylcarbonyl, n-heptylcarbonyl, n-
octylcarbonyl, isopropylcarbonyl, isobutylcarbonyl,
sec-butylcarbonyl, tert-butylcarbonyl
and
isopentylcarbonyl groups and the like.
17
CA 02643070 2008-08-20
, .
The "lower alkenylcarbonyl group" refers to a
group formed by replacing the hydrogen atom of a
formyl group with a lower alkenyl group. Specific
examples thereof include
vinylcarbonyl,
propenylcarbonyl, butenylcarbonyl, pentenylcarbonyl,
hexenylcarbonyl, heptenylcarbonyl, octenylcarbonyl,
isopropenylcarbonyl, 2-methyl-l-propenylcarbonyl and
2-methyl-2-butenylcarbonyl groups and the like.
The "lower alkynylcarbonyl group" refers to a
group formed by replacing the hydrogen atom of a
formyl group with a lower alkynyl group. Specific
examples thereof include
ethynylcarbonyl,
propynylcarbonyl, butynylcarbonyl, pentynylcarbonyl,
hexynylcarbonyl, heptynylcarbonyl, octynylcarbonyl,
isobutynylcarbonyl and isopentynylcarbonyl groups and
the like.
The "lower cycloalkylcarbonyl group" refers to
a group formed by replacing the hydrogen atom of a
formyl group with a lower cycloalkyl group. Specific
examples thereof include
cyclopropylcarbonyl,
cyclobutylcarbonyl,
cyclopentylcarbonyl,
cyclohexylcarbonyl, cycloheptylcarbonyl
and
cyclooctylcarbonyl groups.
The "arylcarbonyl group" refers to a group
formed by replacing the hydrogen atom of a formyl
group with an aryl group. Specific examples thereof
include phenylcarbonyl,
naphthylcarbonyl,
anthrylcarbonyl and phenanthrylcarbonyl groups and
the like.
18
CA 02643070 2008-08-20
The "heterocyclic carbonyl group" refers to a
group formed by replacing the hydrogen atom of a
formyl group with a heterocyclic group.
The "lower alkoxycarbonyl group" refers to a
group formed by replacing the hydrogen atom of a
formyl group with a lower alkoxy group. Specific
examples thereof include
methoxycarbonyl,
ethoxycarbonyl, n-propoxycarbonyl, n-butoxycarbonyl,
n-pentoxycarbonyl, n-hexyloxycarbonyl, n-
heptyloxycarbonyl, n-
octyloxycarbonyl,
isopropoxycarbonyl, isobutoxycarbonyl, sec-
butoxycarbonyl, tert-butoxycarbonyl and
isopentoxycarbonyl groups and the like.
The "lower alkenyloxycarbonyl group" refers to
a group formed by replacing the hydrogen atom of a
formyl group with a lower alkenyloxy group. Specific
examples thereof include
vinyloxycarbonyl,
propenyloxycarbonyl,
butenyloxycarbonyl,
pentenyloxycarbonyl,
hexenyloxycarbonyl,
heptenyloxycarbonyl,
octenyloxycarbonyl,
isopropenyloxycarbonyl, 2-methyl-1-
propenyloxycarbonyl and 2-methyl-2-butenyloxycarbonyl
groups and the like.
The "lower alkynyloxycarbonyl group" refers to
a group formed by replacing the hydrogen atom of a
formyl group with a lower alkynyloxy group. Specific
examples thereof include
ethynyloxycarbonyl,
propynyloxycarbonyl,
butynyloxycarbonyl,
pentynyloxycarbonyl,
hexynyloxycarbonyl,
19
CA 02643070 2008-08-20
heptynyloxycarbonyl,
octynyloxycarbonyl,
isobutynyloxycarbonyl and
isopentynyloxycarbonyl
groups and the like.
The "lower cycloalkyloxycarbonyl group" refers
to a group formed by replacing the hydrogen atom of a
formyl group with a lower cycloalkyloxy group.
Specific examples thereof include
cyclopropyloxycarbonyl,
cyclobutyloxycarbonyl,
cyclopentyloxycarbonyl,
cyclohexyloxycarbonyl,
cycloheptyloxycarbonyl and
cyclooctyloxycarbonyl
groups.
The "aryloxycarbonyl group" refers to a group
formed by replacing the hydrogen atom of a formyl
group with an aryloxy group. Specific examples
thereof include phenoxycarbonyl, naphthoxycarbonyl,
anthryloxycarbonyl and phenanthryloxycarbonyl groups
and the like.
The "heterocyclic oxycarbonyl group" refers to
a group formed by replacing the hydrogen atom of a
formyl group with a heterocyclic oxy group.
The "lower alkylaminocarbonyl group" refers to
a group formed by replacing the hydrogen atom of a
formyl group with a lower alkylamino group. Specific
examples thereof include
methylaminocarbonyl,
ethylaminocarbonyl,
propylaminocarbonyl,
dimethylaminocarbonyl,
diethylaminocarbonyl and
ethylmethylaminocarbonyl groups and the like.
The "lower alkenylaminocarbonyl group" refers
to a group formed by replacing the hydrogen atom of a
CA 02643070 2008-08-20
formyl group with a lower alkenylamino group.
Specific examples thereof include vinylaminocarbonyl,
propenylaminocarbonyl,
butenylaminocarbonyl,
pentenylaminocarbonyl,
hexenylaminocarbonyl,
heptenylaminocarbonyl,
octenylaminocarbonyl,
isopropenylaminocarbonyl, 2-methyl-1-
propenylaminocarbonyl, 2-methy1-2-
butenylaminocarbonyl, divinylaminocarbonyl and
methyl(vinyl)aminocarbonyl groups and the like.
The "lower alkynylaminocarbonyl group" refers
to a group formed by replacing the hydrogen atom of a
formyl group with a lower alkynylamino group.
Specific examples thereof include
ethynylaminocarbonyl,
propynylaminocarbonyl,
butynylaminocarbonyl,
pentynylaminocarbonyl,
hexynylaminocarbonyl,
heptynylaminocarbonyl,
octynylaminocarbonyl,
isobutynylaminocarbonyl,
isopentynylaminocarbonyl,
diethynylaminocarbonyl,
ethynyl(methyl)aminocarbonyl and
ethynyl(vinyl)aminocarbonyl groups and the like.
The "lower cycloalkylaminocarbonyl group"
refers to a group formed by replacing the hydrogen
atom of a formyl group with a lower cycloalkylamino
group. Specific examples thereof include
cyclopropylaminocarbonyl,
cyclobutylaminocarbonyl,
cyclopentylaminocarbonyl,
cyclohexylaminocarbonyl,
cycloheptylaminocarbonyl,
cyclooctylaminocarbonyl,
dicyclohexylaminocarbonyl,
cyclohexyl(methyl)aminocarbonyl,
21
CA 02643070 2008-08-20
cyclohexyl(vinyl)aminocarbonyl and
cyclohexyl(ethynyl)aminocarbonyl groups and the like.
The "arylaminocarbonyl group" refers to a group
formed by replacing the hydrogen atom of a formyl
group with an arylamino group. Specific examples
thereof include
phenylaminocarbonyl,
naphthylaminocarbonyl,
anthrylaminocarbonyl,
phenanthrylaminocarbonyl,
diphenylaminocarbonyl,
methylphenylaminocarbonyl
ethylphenylaminocarbonyl,
phenyl(vinyl)aminocarbonyl,
ethynyl(phenyl)aminocarbonyl and
cyclohexyl(phenyl)aminocarbonyl groups and the like.
The "heterocyclic aminocarbonyl group" refers
to a group formed by replacing the hydrogen atom of a
formyl group with a heterocyclic amino group.
The "lower alkylsulfonyl group" refers to a
group formed by replacing the hydroxy group of a
sulfonic acid group with a lower alkyl group.
Specific examples thereof include methylsulfonyl,
ethylsulfonyl, n-propylsulfonyl, n-butylsulfonyl, n-
pentylsulfonyl, n-hexylsulfonyl, n-heptylsulfonyl, n-
octylsulfonyl, isopropylsulfonyl, isobutylsulfonyl,
sec-butylsulfonyl, tert-butylsulfonyl and
isopentylsulfonyl groups and the like.
The "lower alkenylsulfonyl group" refers to a
group formed by replacing the hydroxy group of a
sulfonic acid group with a lower alkenyl group.
Specific examples thereof include vinylsulfonyl,
propenylsulfonyl, butenylsulfonyl, pentenylsulfonyl,
22
CA 02643070 2008-08-20
hexenylsulfonyl, heptenylsulfonyl, octenylsulfonyl,
isopropenylsulfonyl, 2-methyl-1-propenylsulfonyl and
2-methyl-2-butenylsulfonyl groups and the like.
The "lower alkynylsulfonyl group" refers to a
group formed by replacing the hydroxy group of a
sulfonic acid group with a lower alkynyl group.
Specific examples thereof include ethynylsulfonyl,
propynylsulfonyl, butynylsulfonyl, pentynylsulfonyl,
hexynylsulfonyl, heptynylsulfonyl, octynylsulfonyl,
isobutynylsulfonyl and isopentynylsulfonyl groups and
the like.
The "lower cycloalkylsulfonyl group" refers to
a group formed by replacing the hydroxy group of a
sulfonic acid group with a lower cycloalkyl group.
Specific examples thereof include
cyclopropylsulfonyl,
cyclobutylsulfonyl,
cyclopentylsulfonyl,
cyclohexylsulfonyl,
cycloheptylsulfonyl and cyclooctylsulfonyl groups.
The "arylsulfonyl group" refers to a group formed by
replacing the hydroxy group of a sulfonic acid group
with an aryl group. Specific examples thereof include
phenylsulfonyl, naphthylsulfonyl, anthrylsulfonyl and
phenanthrylsulfonyl groups and the like.
The "heterocyclic sulfonyl group" refers to a
group formed by replacing the hydroxy group of a
sulfonic acid group with a heterocyclic group.
The "3- to 8-membered nitrogen-containing
heterocyclic ring" refers to a saturated monocyclic
heterocyclic ring containing one or two nitrogen
23
CA 02643070 2008-08-20
atoms in the ring. Specific examples thereof include
aziridine, azetidine, pyrrolidine, piperidine,
imidazolidine, pyrazolidine, piperazine and
morpholine rings and the like.
The "lower alkylene group" refers to a straight
chain or branched alkylene group having 1 to 8 carbon
atoms. Specific examples thereof include methylene,
ethylene, trimethylene,
tetramethylene,
pentamethylene, hexamethylene,
heptamethylene,
octamethylene, methylmethylene and ethylmethylene
groups and the like.
The "ester of a hydroxy group" refers to an
ester formed from a hydroxy group and a carboxylic
acid and/or a group represented by -OCO-R.
Herein, R represents a hydrogen atom, a lower
alkyl group which may have at least a substituent, a
lower alkenyl group which may have at least a
substituent, a lower alkynyl group which may have at
least a substituent, a lower cycloalkyl group which
may have at least a substituent, an aryl group which
may have at least a substituent, a heterocyclic group
which may have at least a substituent, a lower alkoxy
group which may have at least a substituent, a lower
alkenyloxy group which may have at least a
substituent, a lower alkynyloxy group which may have
at least a substituent, a lower cycloalkyloxy group
which may have at least a substituent, an aryloxy
group which may have at least a substituent, a
heterocyclic oxy group which may have at least a
24
CA 02643070 2008-08-20
substituent, an amino group, a lower alkylamino group
which may have at least a substituent, a lower
cycloalkylamino group which may have at least a
substituent, an arylamino group which may have at
least a substituent or a heterocyclic amino group
which may have at least a substituent. R is the same
as below.
The "ester of a mercapto group" refers to a
thioester formed from a mercapto group and a
carboxylic acid and/or a group represented by -SCO-R.
Herein, R is the same as the above.
The "amide of an amino group" refers to an
amide formed from an amino group and a carboxylic
acid and/or a group represented by -NHCO-R. Herein, R
is the same as the above.
The "amide of a lower alkylamino group" refers
to an amide formed from a lower alkylamino group and
a carboxylic acid and/or a group represented by -
NR'CO-R. Herein, R' represents a lower alkyl group
which may have at least a substituent, and R is the
same as the above.
The "amide of a lower cycloalkylamino group"
refers to an amide formed from a lower
cycloalkylamino group and a carboxylic acid and/or a
group represented by -NR"CO-R. Herein, R"
represents a lower cycloalkyl group which may have at
least a substituent, and R is the same as the above.
The "amide of an arylamino group" refers to an
amide formed from an arylamino group and a carboxylic
CA 02643070 2008-08-20
acid and/or a group represented by -NR'"CO-R.
Herein, R"' represents an aryl group which may have
at least a substituent, and R is the same as the
above.
The "amide of a heterocyclic amino group"
refers to an amide formed from a heterocyclic amino
group and a carboxylic acid and/or a group
represented by -NR""CO-R. Herein, R'"' represents
a heterocyclic group which may have at least a
substituent, and R is the same for the above.
The "carboxylic acid" refers to a saturated
aliphatic carboxylic acid, an unsaturated aliphatic
carboxylic acid, an aryl carboxylic acid, a
heterocyclic carboxylic acid or the like represented
by RaCOOH (Ra represents a hydrogen atom, a lower
alkyl group which may have at least a substituent, a
lower alkenyl group which may have at least a
substituent, a lower alkynyl group which may have at
least a substituent, a lower cycloalkyl group which
may have at least a substituent, an aryl group which
may have at least a substituent, a heterocyclic group
which may have at least a substituent and the like).
Specific examples thereof include saturated aliphatic
carboxylic acids such as formic acid, acetic acid,
propionic acid, butyric acid, isobutyric acid,
valeric acid, isovaleric acid, pivalic acid,
cyclopropanecarboxylic acid, cyclobutanecarboxylic
acid, cyclopentanecarboxylic acid and
cyclohexanecarboxylic acid; unsaturated aliphatic
26
CA 02643070 2008-08-20
carboxylic acids such as acrylic acid, propionic
acid, crotonic acid, cinnamic acid,
cyclopentenecarboxylic acid and cyclohexenecarboxylic
acid; aryl carboxylic acids such as benzoic acid,
phthalic acid, isophthalic acid, terephthalic acid,
naphthoic acid and toluic acid; heterocyclic
carboxylic acids such as furancarboxylic acid,
thiophenecarboxylic acid, nicotinic acid and
isonicotinic acid; and the like.
The "ester of a carboxy group" refers to an
ester formed from a carboxy group and an alcohol or a
phenol.
The "ester of a sulfonic acid group" refers to
an ester formed from a sulfonic acid group and an
alcohol or a phenol.
The "alcohol" refers to a saturated aliphatic
hydroxy compound, an unsaturated aliphatic hydroxy
compound, a heterocyclic hydroxyl compound or the
like represented by RbOH (Rb represents a lower alkyl
group which may have at least a substituent, a lower
alkenyl group which may have at least a substituent,
a lower alkynyl group which may have at least a
subustituent, a lower cycloalkyl group which may have
at least a substituent, a heterocyclic group which
may have at least a substituent or the like).
Specific examples thereof include saturated aliphatic
hydroxy compounds such as methanol, ethanol,
propanol, butanol, isopropanol, cyclopropanol,
cyclobutanol, cyclopentanol, cyclohexanol,
27
CA 02643070 2008-08-20
benzylalcohol and phenethylalcohol; unsaturated
aliphatic hydroxy compounds such as vinyl alcohol,
allylalcohol, propagylalcohol, cyclopentenol and
cyclohexenol; heterocyclic hydroxy compounds such as
hydroxypiperidine and hydroxytetrahydropyran.
The "phenol" refers to an aryl hydroxy
compound, a heterocyclic hydroxyl compound or the
like represented by RcOH (Rc represents an aryl group
which may have at least a substituent, a heterocyclic
group which may have at least a substituent or the
like). Specific examples thereof include aryl
hydroxyl compounds such as phenol, naphthol, anthrol
and phenanthrol; heterocyclic hydroxyl compounds such
as hydroxypyridine, hydroxyfuran and hydroxythiophen.
The "amide of a carboxy group" refers to an
acid amide formed from a carboxy group and an amine.
The "amide of a sulfonic acid group" refers to
an acid amide formed from a sulfonic acid group and
an amine.
The "amine" refers to ammonia, a saturated
aliphatic amine compound, an unsaturated aliphatic
amine compound, an aryl amine compound, a
heterocyclic amine compound, a saturated cyclic amine
compound or the like represented by HNRdRe (Rd and Re
may be the same or different and represent a hydrogen
atom, a lower alkyl group which may have at least a
substituent, a lower alkenyl group which may have at
least a substituent, a lower alkynyl group which may
have at least a substituent, a lower cycloalkyl group
28
CA 02643070 2008-08-20
which may have at least a substituent, an aryl group
which may have at least a substituent, a heterocyclic
group or the like, or Rd and Re may be combined
together to form a saturated cyclic amine). Specific
examples thereof include ammonia; saturated aliphatic
amine compounds such as methylamine, ethylamine,
propylamine, pentylamine, dimethylamine,
diethylamine, ethylmethylamine,
cyclopropylamine,
cyclobutylamine, cyclopentylamine, cyclohexylamine,
benzylamine and phenetylamine; unsaturated aliphatic
amine compounds such as allylamine and propagylamine;
aryl amine compounds such as phenylamine,
naphthylamine, anthrylamine,
phenanthrylamine,
diphenylamine, methylphenylamine and
ethylphenylamine; heterocyclic amine compounds such
as furylamine, thienylamine,
pyrrolidylamine,
pyridylamine, quinolylamine and methylpyridylamine;
saturated cyclic amine compounds such as aziridine,
azetidine, pyrrolidine, piperidine and 4-
methylpiperidine.
The "lower alkyl group which may have at least
a substituent", "lower alkenyl group which may have
at least a substituent", "lower alkynyl group which
may have at least a substituent", "lower alkoxy group
which may have at least a substituent", "lower
alkyltio group which may have at least a
substituent", "lower alkylamino group which may have
at least a substituent", "lower alkylcarbonyl group
which may have at least a substituent", "lower
29
CA 02643070 2008-08-20
.. ..
alkenylcarbonyl group which may have at least a
substituent", "lower alkynylcarbonyl group which may
have at least a substituent", "lower alkoxycarbonyl
group which may have at least a substituent", "lower
alkenyloxycarbonyl group which may have at least a
substituent", "lower alkynyloxycarbonyl group which
may have at least a substituent", "lower
alkylaminocarbonyl group which may have at least a
substituent", "lower alkenylaminocarbonyl group which
may have at least a substituent", "lower
alkynylaminocarbonyl group which may have at least a
substituent", "lower alkylsulfonyloxy group which may
have at least a substituent",
"lower
alkenylsulfonyloxy group which may have at least a
substituent", "lower alkynylsulfonyloxy group which
may have at least a substituent" and "amide of lower
alkylamino group which may have at least a
substituent" refer to a "lower alkyl group", a "lower
alkenyl group", a "lower alkynyl group", a "lower
alkoxy group", a "lower alkylthio group", a "lower
alkylamino group", a "lower alkylcarbonyl group", a
"lower alkenylcarbonyl group",
a "lower
alkynylcarbonyl group", a "lower alkoxycarbonyl
group", a "lower alkenyloxycarbonyl group", a "lower
alkynyloxycarbonyl group", a
"lower
alkylaminocarbonyl group", a
"lower
alkenylaminocarbonyl group", a
"lower
alkynylaminocarbonyl group", a "lower alkylsulfonyl
group", a "lower alkenylsulfonyl group", a "lower
CA 02643070 2008-08-20
alkynylsulfonyl group" and an "amide of th lower
alkylamino group" which may have one or a plurality
of substituents selected from the following al group,
respectively.
[al group]
A halogen atom, a lower cycloalkyl group, an
aryl group, a heterocyclic group, a hydroxy group, an
ester of a hydroxy group, a lower alkoxy group, a
lower alkoxy group substituted by a halogen atom, a
lower alkenyloxy group, a lower alkynyloxy group, a
lower cycloalkyloxy group, an aryloxy group, a
heterocyclic oxy group, a mercapto group, an ester of
a mercapto group, a lower alkylthio group, a lower
alkenylthio group, a lower alkynylthio group, a lower
cycloalkylthio group, an arylthio group, a
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
heterocyclic amino group, a formyl group, a lower
alkylcarbonyl group, a lower alkenylcarbonyl group, a
lower alkynylcarbonyl group, a lower
cycloalkylcarbonyl group, an arylcarbonyl group, a
heterocyclic carbonyl group, a carboxy group, an
ester of a carboxy group, an amide of a carboxy
group, a lower alkylsulfinyl group, an arylsulfinyl
31
CA 02643070 2008-08-20
÷
. .
group, a lower alkylsulfonyl group, a lower
cycloalkylsulfonyl group, an arylsulfonyl group, a
heterocyclic sulfonyl group, a sulfinic acid group,
an ester of a sulfinic acid group, an amide of a
sulfinic acid group, a sulfonic acid group, an ester
of a sulfonic acid group, an amide of a sulfonic acid
group, a nitro group and a cyano group.
The "lower cycloalkyl group which may have at
least a substituent", "aryl group which may have at
least a substituent", "heterocyclic group which may
have at least a substituent", "lower cycloalkyloxy
group which may have at least a substituent",
"aryloxy group which may have at least a
substituent", "heterocyclic oxy group which may have
at least a substituent", "lower cycloalkylthio group
which may have at least a substituent", "arylthio
group which may have at least a substituent",
"heterocyclic thio group which may have at least a
substituent", "lower cycloalkylamino group which may
have at least a substituent", "arylamino group which
may have at least a substituent", "heterocyclic amino
group which may have at least a substituent", "lower
cycloalkylcarbonyl group which may have at least a
substituent", "arylcarbonyl group which may have at
least a substituent", "heterocyclic carbonyl group
which may have at least a substituent", "lower
cycloalkyloxycarbonyl group which may have at least a
substituent", "aryloxycarbonyl group which may have
at least a substituent", "heterocyclic oxycarbonyl
32
CA 02643070 2008-08-20
group which may have at least a substituent", "lower
cycloalkylaminocarbonyl group which may have at least
a substituent", "arylaminocarbonyl group which may
have at least a substituent", "heterocyclic
aminocarbonyl group which may have at least a
substituent", "lower cycloalkylsulfonyl group which
may have at least a substituent", "arylsulfonyl group
which may have at least a substituent", "heterocyclic
sulfonyl group which may have at least a
substituent", "amide of lower cycloalkylamino group
which may have at least a substituent", "amide of
arylamino group which may have at least a
substituent" and "amide of heterocyclic amino group
which may have at least a substituent" refer to a
"lower cycloalkyl group", an "aryl group", a
"heterocyclic group", a "lower cycloalkyloxy group",
an "aryloxy group", a "heterocyclic oxy group", a
"lower cycloalkylthio group", an "arylthio group", a
"heterocyclic thio group", a "lower cycloalkylamino
group", an "arylamino group", a "heterocyclic amino
group", a "lower cycloalkylcarbonyl group", an
"arylcarbonyl group", a "heterocyclic carbonyl
group", an "lower cycloalkyloxycarbonyl group", an
"aryloxycarbonyl group", a "heterocyclic oxycarbonyl
group", a "lower cycloalkylaminocarbonyl group", an
"arylaminocarbonyl group", a "heterocyclic
aminocarbonyl group", a "lower cycloalkylsulfonyl
group", an "arylsulfonyl group", a "heterocyclic
sulfonyl group", an "amide of lower cycloalkylamino
33
CA 02643070 2008-08-20
group", an "amide of arylamino group" and an "amide
of heterocyclic amino group" which may have one or a
plurality of substituents selected from the following
pl group, respectively.
[13' group]
A halogen atom, a lower alkyl group, a lower
alkyl group substituted by a halogen atom, a lower
alkenyl group, a lower alkynyl group, a lower
cycloalkyl group, an aryl group, a heterocyclic
group, a hydroxy group, an ester of a hydroxy group,
a lower alkoxy group, a lower alkoxy group
substituted by a halogen atom, a lower alkenyloxy
group, a lower alkynyloxy group, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a mercapto group, an ester of a mercapto
group, a lower alkylthio group, a lower alkenylthio
group, a lower alkynylthio group, a lower
cycloalkylthio group, an arylthio group, a
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
heterocyclic amino group, a formyl group, a lower
alkylcarbonyl group, a lower alkenylcarbonyl group, a
lower alkynylcarbonyl group, a lower
cycloalkylcarbonyl group, an arylcarbonyl group, a
heterocyclic carbonyl group, a carboxy group, an
34
CA 02643070 2008-08-20
ester of a carboxy group, an amide of a carboxy
group, a lower alkylsulfinyl group, an arylsulfinyl
group, a lower alkylsulfonyl group, a lower
cycloalkylsulfonyl group, an arylsulfonyl group, a
heterocyclic sulfonyl group, a sulfinic acid group,
an ester of a sulfinic acid group, an amide of a
sulfinic acid group, a sulfonic acid group, an ester
of a sulfonic acid group, an amide of a sulfonic acid
group, a nitro group, a cyano group, a lower
alkylaminocarbonyloxy group and an
arylaminocarbonyloxy group.
The term "a plurality of groups" as used herein
means that each group may be the same or different
and the number of groups is preferably 2. Further, a
hydrogen atom and a halogen atom are also included in
the concept of the "group".
The "glucocorticoid receptor modulator" as used
herein refers to a modulator that exhibits a
pharmaceutical action by binding to glucocorticoid
receptor. Examples thereof include glucocorticoid
receptor agonists, glucocorticoid receptor
antagonists and the like.
The "salt" of the present compound is not
particularly limited as long as it is a
pharmaceutically acceptable salt, and examples
thereof include salts with an inorganic acid such as
hydrochloric acid, hydrobromic acid, hydroiodic acid,
nitric acid, sulfuric acid or phosphoric acid; salts
with an organic acid such as acetic acid, fumalic
CA 02643070 2008-08-20
acid, maleic acid, succinic acid, citric acid,
tartaric acid, adipic acid, gluconic acid,
glucoheptonic acid, glucuronic acid, terephthalic
acid, methanesulfonic acid, lactic acid, hippuric
acid, 1,2-ethanedisulfonic acid, isethionic acid,
lactobionic acid, oleic acid, pamoic acid,
polygalacturonic acid, stearic acid, tannic acid,
trifluoromethanesulfonic acid, benzenesulfonic acid,
p-toluenesulfonic acid, lauryl sulfate ester, methyl
sulfate, naphthalenesulfonic acid or sulfosalicylic
acid; quaternary ammonium salts with methyl bromide,
methyl iodide or the like; salts with a halogen ion
such as a bromine ion, a chlorine ion or an iodine
ion; salts with an alkali metal such as lithium,
sodium or potassium; salts with an alkaline earth
metal such as calcium or magnesium; salts with a
metal such as iron or zinc; salts with ammonia; salts
with an organic amine such as triethylenediamine, 2-
aminoethanol, 2,2-iminobis(ethanol), 1-deoxy-1-
(methylamino)-2-D-sorbitol, 2-amino-2-
(hydroxymethyl)-1,3-propanediol, procaine Or N,N-
bis(phenylmethyl)-1,2-ethanediamine; and the like.
In the case where there are geometrical isomers
or optical isomers in the present compound, these
isomers are also included in the scope of the present
invention.
Further, the present compound may be in the
form of a hydrate or a solvate.
In the case where there is proton tautomerism
36
CA 02643070 2008-08-20
, .
.,
in the present compound, the tautomeric isomers
thereof are also included in the present invention.
In the case where there are crystalline
polymorphisms in the present compound, the
crystalline polymorphisms thereof are also included
in the present invention.
(a) Preferred examples of the present compound
include compounds in which the respective groups are
groups as defined below and salts thereof in the
compounds represented by the general formula (1) and
salts thereof.
In the general formula (1),
(al) R1 represents a halogen atom, a lower alkyl
group, a lower cycloalkyl group, an aryl group, a
heterocyclic group, a hydroxy group, an ester of a
hydroxy group, a lower alkoxy group, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a mercapto group, an ester of a mercapto
group, a lower alkylthio group, a
lower
cycloalkylthio group, an arylthio group,
a
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
heterocyclic amino group, a lower alkylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
37
CA 02643070 2008-08-20
carboxy group, an ester of a carboxy group, an amide
of a carboxy group, a lower alkylsulfonyl group, a
lower cycloalkylsulfonyl group, an arylsulfonyl
group, a heterocyclic sulfonyl group, a sulfonic acid
group, an ester of a sulfonic acid group, an amide of
a sulfonic acid group, a nitro group or a cyano
group;
in the case where Rl is a lower alkyl group, a
lower alkoxy group, a lower alkylthio group, a lower
alkylamino group, an amide of a lower alkylamino
group, a lower alkylcarbonyl group or a lower
alkylsulfonyl group, the lower alkyl group, lower
alkoxy group, lower alkylthio group, lower alkylamino
group, amide of a lower alkylamino group, lower
alkylcarbonyl group or lower alkylsulfonyl group may
have one or a plurality of groups selected from a
halogen atom, a lower cycloalkyl group, an aryl
group, a heterocyclic group, a hydroxy group, an
ester of a hydroxy group, a lower alkoxy group, a
lower alkoxy group substituted with a halogen atom, a
lower cycloalkyloxy group, an aryloxy group, a
heterocyclic oxy group, a lower alkylthio group, a
lower cycloalkylthio group, an arylthio group, a
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
38
CA 02643070 2008-08-20
-
. .
heterocyclic amino group, a lower alkylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
carboxy group, an ester of a carboxy group, an amide
of a carboxy group, a lower alkylsulfonyl group, a
lower cycloalkylsulfonyl group, an arylsulfonyl
group, a heterocyclic sulfonyl group, a sulfonic acid
group, an ester of a sulfonic acid group, an amide of
a sulfonic acid group, a nitro group and a cyano
group as substituents;
in the case where R1 is a lower cycloalkyl
group, an aryl group, a heterocyclic group, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a lower cycloalkylthio group, an arylthio
group, a heterocyclic thio group, a lower
cycloalkylamino group, an arylamino group, a
heterocyclic amino group, an amide of a lower
cycloalkylamino group, an amide of an arylamino
group, an amide of a heterocyclic amino group, a
lower cycloalkylcarbonyl group, an arylcarbonyl
group, a heterocyclic carbonyl group, a lower
cycloalkylsulfonyl group, an arylsulfonyl group or a
heterocyclic sulfonyl group, the lower cycloalkyl
group, aryl group, heterocyclic group, lower
cycloalkyloxy group, aryloxy group, heterocyclic oxy
group, lower cycloalkylthio group, arylthio group,
heterocyclic thio group, lower cycloalkylamino group,
arylamino group, heterocyclic amino group, amide of a
lower cycloalkylamino group, amide of an arylamino
39
CA 02643070 2008-08-20
group, amide of a heterocyclic amino group, lower
cycloalkylcarbonyl group, arylcarbonyl group,
heterocyclic carbonyl group, lower cycloalkylsulfonyl
group, arylsulfonyl group or heterocyclic sulfonyl
group may have one or a plurality of groups selected
from a halogen atom, a lower alkyl group, a lower
alkyl group substituted with a halogen atom, a
hydroxy group, an ester of a hydroxy group, a lower
alkoxy group, a lower alkoxy group substituted with a
halogen atom, a lower alkylthio group, an amino
group, a lower alkylamino group, an amide of an amino
group, an amide of a lower alkylamino group, a lower
alkylcarbonyl group, a carboxy group, an ester of a
carboxy group, an amide of a carboxy group, a lower
alkylsulfonyl group, a sulfonic acid group, an ester
of a sulfonic acid group, an amide of a sulfonic acid
group, a nitro group and a cyano group as
substituents; and/or
(a2) p represents an integer of 0 to 3;
in the case where p is 2 or 3, each R1 may be
the same or different; and/or
(a3) R2 represents a halogen atom, a lower alkyl
group, a hydroxy group or a lower alkoxy group;
and/or
(a4) q represents an integer of 0 to 2;
in the case where q is 2, each R2 may be the
same or different;
and/or
(a5) R3 represents a hydrogen atom, a lower
CA 02643070 2008-08-20
. .
,
alkyl group, a lower alkenyl group, a lower
alkylcarbonyl group, a lower alkenylcarbonyl group or
an arylcarbonyl group;
in the case where R3 is a lower alkyl group or a
lower alkylcarbonyl group, the lower alkyl group or
lower alkylcarbonyl group may have one or a plurality
of groups selected from a halogen atom and an aryl
group as substituents;
in the case where R3 is an arylcarbonyl group,
the arylcarbonyl group may have one or a plurality of
groups selected from a halogen atom, a lower alkyl
group, a lower alkyl group substituted with a halogen
atom, a lower alkoxy group and a lower alkoxy group
substituted with a halogen atom as substituents;
and/or
(a6) R4 and R5 may be the same or different and
represent a hydrogen atom, a halogen atom, a lower
alkyl group, a lower cycloalkyl group, aryl or a
heterocyclic group;
in the case where R4 or R5 is a lower alkyl
group, the lower alkyl group may have one or a
plurality of groups selected from a halogen atom, a
lower cycloalkyl group, an aryl group, a heterocyclic
group, a hydroxy group, an ester of a hydroxy group,
a lower alkoxy group, a lower alkoxy group
substituted with a halogen atom, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a lower alkylthio group, a lower
cycloalkylthio group, an arylthio group,
a
41
CA 02643070 2008-08-20
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
heterocyclic amino group, a lower alkylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
carboxy group, an ester of a carboxy group, an amide
of a carboxy group, a nitro group and a cyano group
as substituents;
in the case where R4 or R5 is a lower cycloalkyl
group, aryl or a heterocyclic group, the lower
cycloalkyl group, aryl or heterocyclic group may have
one or a plurality of groups selected from a halogen
atom, a lower alkyl group, a lower alkyl group
substituted with a halogen atom, a hydroxy group, an
ester of a hydroxy group, a lower alkoxy group, a
lower alkoxy group substituted with a halogen atom, a
lower alkylthio group, an amino group, a lower
alkylamino group, an amide of an amino group, an
amide of a lower alkylamino group, a lower
alkylcarbonyl group, a carboxy group, an ester of a
carboxy group, an amide of a carboxy group, a lower
alkylsulfonyl group, a sulfonic acid group, an ester
of a sulfonic acid group, an amide of a sulfonic acid
group, a nitro group and a cyano group as
substituents;
42
CA 02643070 2008-08-20
= .
. ,
R4 and R5 may be combined together to form a 3-
to 8-membered lower cycloalkane ring; and/or
(a7) R6 represents a hydrogen atom, a lower
alkyl group, a lower alkenyl group, a lower alkynyl
group or a lower cycloalkyl group;
in the case where R6 is a lower alkyl group, a
lower alkenyl group, a lower alkynyl group or a lower
cycloalkyl group, the lower alkyl group, lower
alkenyl group, lower alkynyl group or lower
cycloalkyl group may have one or a plurality of
groups selected from a halogen atom and an aryl group
as substituents; and/or
(a8) A represents a lower alkylene group which
may be substituted with a hydroxy group or a halogen
atom; and/or
(a9) R7 represents OR8, NR8R9 or SR8;
R8 and R9 may be the same or different and
represent a hydrogen atom, a lower alkyl group, a
lower alkenyl group, a lower alkynyl group, a lower
cycloalkyl group, an aryl group, a heterocyclic
group, a formyl group, a lower alkylcarbonyl group, a
lower alkenylcarbonyl group, a lower alkynylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
carboxy group, a lower alkoxycarbonyl group, a lower
alkenyloxycarbonyl group, a lower alkynyloxycarbonyl
group, a lower cycloalkyloxycarbonyl group, an
aryloxycarbonyl group, a heterocyclic oxycarbonyl
group, a lower alkylsulfonyl group, a lower
43
CA 02643070 2008-08-20
, .
, .
alkenylsulfonyl group, a lower alkynylsulfonyl group,
a lower cycloalkylsulfonyl group, an arylsulfonyl
group, a heterocyclic sulfonyl group,
an
aminocarbonyl group, a lower alkylaminocarbonyl
group, a lower alkenylaminocarbonyl group, a lower
alkynylaminocarbonyl group, a
lower
cycloalkylaminocarbonyl group, an arylaminocarbonyl
group or a heterocyclic aminocarbonyl group;
in the case where R8 or R9 is a lower alkyl
group, a lower alkenyl group, a lower alkynyl group,
a lower alkylcarbonyl group, a lower alkenylcarbonyl
group, a lower alkynylcarbonyl group, a lower
alkoxycarbonyl group, a lower alkenyloxycarbonyl
group, a lower alkynyloxycarbonyl group, a lower
alkylsulfonyl group, a lower alkenylsulfonyl group, a
lower alkynylsulfonyl group, a
lower
alkylaminocarbonyl group, a
lower
alkenylaminocarbonyl group or a
lower
alkynylaminocarbonyl group, the lower alkyl group,
lower alkenyl group, lower alkynyl group, lower
alkylcarbonyl group, lower alkenylcarbonyl group,
lower alkynylcarbonyl group, lower alkoxycarbonyl
group, lower alkenyloxycarbonyl group,
lower
alkynyloxycarbonyl group, lower alkylsulfonyl group,
lower alkenylsulfonyl group, lower alkynylsulfonyl
group, lower alkylaminocarbonyl group,
lower
alkenylaminocarbonyl group or
lower
alkynylaminocarbonyl group may have one Or a
plurality of groups selected from a halogen atom, a
44
CA 02643070 2008-08-20
lower cycloalkyl group, an aryl group, a heterocyclic
group, a hydroxy group, an ester of a hydroxy group,
a lower alkoxy group, a lower alkoxy group
substituted with a halogen atom, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a lower alkylthio group, a lower
cycloalkylthio group, an arylthio group, a
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
heterocyclic amino group, a lower alkylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
carboxy group, an ester of a carboxy group, an amide
of a carboxy group, a lower alkylsulfonyl group, a
lower cycloalkylsulfonyl group, an arylsulfonyl
group, a heterocyclic sulfonyl group, a sulfonic acid
group, an ester of a sulfonic acid group, an amide of
a sulfonic acid group, a nitro group and a cyano
group as substituents;
in the case where R8 or R9 is a lower cycloalkyl
group, an aryl group, a heterocyclic group, a lower
cycloalkylcarbonyl group, an arylcarbonyl group, a
heterocyclic carbonyl group, a lower
cycloalkyloxycarbonyl group, an aryloxycarbonyl
group, a heterocyclic oxycarbonyl group, a lower
CA 02643070 2008-08-20
. .
. .
cycloalkylsulfonyl group, an arylsulfonyl group, a
heterocyclic sulfonyl group, a
lower
cycloalkylaminocarbonyl group, an arylaminocarbonyl
group or a heterocyclic aminocarbonyl group, the
lower cycloalkyl group, aryl group, heterocyclic
group, lower cycloalkylcarbonyl group, arylcarbonyl
group, heterocyclic carbonyl group,
lower
cycloalkyloxycarbonyl group, aryloxycarbonyl group,
heterocyclic oxycarbonyl group,
lower
cycloalkylsulfonyl group,
arylsulfonyl group,
heterocyclic sulfonyl group,
lower
cycloalkylaminocarbonyl group,
arylaminocarbonyl
group or heterocyclic aminocarbonyl group may have
one or a plurality of groups selected from a halogen
atom, a lower alkyl group, a lower alkyl group
substituted with a halogen atom, a lower alkyl group
substituted with a hydroxy group, a lower alkenyl
group, a lower alkynyl group, a lower cycloalkyl
group, an aryl group, a heterocyclic group, a hydroxy
group, an ester of a hydroxy group, a lower alkoxy
group, a lower alkoxy group substituted with a
halogen atom, a lower alkenyloxy group, a lower
alkynyloxy group, a lower cycloalkyloxy group, an
aryloxy group, a heterocyclic oxy group, a lower
alkylthio group, a lower cycloalkylthio group, an
arylthio group, a heterocyclic thio group, an amino
group, a lower alkylamino group, a
lower
cycloalkylamino group, an arylamino group, a
heterocyclic amino group, an amide of an amino group,
46
CA 02643070 2008-08-20
an amide of a lower alkylamino group, an amide of a
lower cycloalkylamino group, an amide of an arylamino
group, an amide of a heterocyclic amino group, a
lower alkylcarbonyl group, a lower cycloalkylcarbonyl
group, an arylcarbonyl group, a heterocyclic carbonyl
group, a carboxy group, an ester of a carboxy group,
an amide of a carboxy group, a lower alkylsulfonyl
group, a lower cycloalkylsulfonyl group, an
arylsulfonyl group, a heterocyclic sulfonyl group, a
sulfonic acid group, an ester of a sulfonic acid
group, an amide of a sulfonic acid group, a nitro
group and a cyano group as substituents;
in the case where R7 is NR8R9, R8 and R9 may be
combined together to form a 5- or 6-membered
nitrogen-containing heterocyclic ring; and/or
(a10) X represents 0 or S.
That is, in the compounds represented by the
general formula (1), preferred examples include
compounds that comprises one or a combination of two
or more selected from the above (al), (a2), (a3),
(a4), (a5), (a6), (a7), (a8), (a9) and (a10), and
salts thereof.
(b) More preferred examples of the present
compound include compounds in which the respective
groups are groups as defined below and salts thereof
in the compounds represented by the general formula
(1) and salts thereof.
47
CA 02643070 2008-08-20
In the general formula (1),
(bl) R1 represents a halogen atom, a lower alkyl
group, a hydroxy group, an ester of a hydroxy group,
a lower alkoxy group, a lower alkylthio group, an
amino group, a lower alkylamino group, an amide of an
amino group, an amide of a lower alkylamino group, a
lower alkylcarbonyl group, a carboxy group, an ester
of a carboxy group, an amide of a carboxy group, a
lower alkylsulfonyl group, a nitro group or a cyano
group;
in the case where Rl is a lower alkyl group, a
lower alkoxy group, a lower alkylthio group, a lower
alkylamino group, an amide of a lower alkylamino
group, a lower alkylcarbonyl group or a lower
alkylsulfonyl group, the lower alkyl group, lower
alkoxy group, lower alkylthio group, lower alkylamino
group, amide of a lower alkylamino group, lower
alkylcarbonyl group or lower alkylsulfonyl group may
have one or a plurality of groups selected from a
halogen atom, a lower cycloalkyl group, an aryl
group, a heterocyclic group, a hydroxy group, an
ester of a hydroxy group, a lower alkoxy group, a
lower alkoxy group substituted with a halogen atom, a
lower cycloalkyloxy group, an aryloxy group, a
heterocyclic oxy group, a lower alkylthio group, a
lower cycloalkylthio group, an arylthio group, a
heterocyclic thio group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group, a heterocyclic amino group, an amide
48
CA 02643070 2008-08-20
of an amino group, an amide of a lower alkylamino
group, an amide of a lower cycloalkylamino group, an
amide of an arylamino group, an amide of a
heterocyclic amino group, a lower alkylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
carboxy group, an ester of a carboxy group, an amide
of a carboxy group, a lower alkylsulfonyl group, a
lower cycloalkylsulfonyl group, an arylsulfonyl
group, a heterocyclic sulfonyl group, a sulfonic acid
group, an ester of a sulfonic acid group, an amide of
a sulfonic acid group, a nitro group and a cyano
group as substituents; and/or
(b2) p represents an integer of 0 to 3;
in the case where p is 2 or 3, each R1 may be
the same or different; and/or
(b3) q represents 0; and/or
(b4) R3 represents a hydrogen atom, a lower
alkyl group, a lower alkenyl group, a lower
alkylcarbonyl group, a lower alkenylcarbonyl group or
an arylcarbonyl group;
in the case where R3 is a lower alkyl group, the
lower alkyl group may have one or a plurality of aryl
groups as substituents;
in the case where R3 is an arylcarbonyl group,
the arylcarbonyl group may have one or a plurality of
groups selected from a halogen atom and a lower alkyl
group as substituents; and/or
(b5) R4 and R5 may be the same or different and
49
CA 02643070 2008-08-20
represent a hydrogen atom, a halogen atom, a lower
alkyl group, a lower cycloalkyl group, aryl or a
heterocyclic group;
in the case where R4 or R5 is a lower alkyl
group, the lower alkyl group may have one or a
plurality of groups selected from a halogen atom, a
hydroxy group, an ester of a hydroxy group, a lower
alkoxy group, a lower alkoxy group substituted with a
halogen atom, a lower alkylthio group, an amino
group, a lower alkylamino group, an amide of an amino
group, an amide of a lower alkylamino group, a lower
alkylcarbonyl group, a carboxy group, an ester of a
carboxy group, an amide of a carboxy group, a nitro
group and a cyano group as substituents;
in the case where R4 or R5 is a lower cycloalkyl
group, aryl or a heterocyclic group, the lower
cycloalkyl group, aryl or heterocyclic group may have
one or a plurality of groups selected from a halogen
atom, a lower alkyl group, a lower alkyl group
substituted with a halogen atom, a hydroxy group, an
ester of a hydroxy group, a lower alkoxy group, a
lower alkoxy group substituted with a halogen atom, a
lower alkylthio group, an amino group, a lower
alkylamino group, an amide of an amino group, an
amide of a lower alkylamino group, a lower
alkylcarbonyl group, a carboxy group, an ester of a
carboxy group, an amide of a carboxy group, a lower
alkylsulfonyl group, a nitro group and a cyano group
as substituents;
CA 02643070 2008-08-20
. .
R4 and R5 may be combined together to form a 3-
to 8-membered lower cycloalkane ring; and/or
(b6) R6 represents a hydrogen atom, a lower
alkyl group, a lower alkenyl group, a lower alkynyl
group or a lower cycloalkyl group;
in the case where R6 is a lower alkyl group, a
lower alkenyl group, a lower alkynyl group or a lower
cycloalkyl group, the lower alkyl group, lower
alkenyl group, lower alkynyl group or lower
cycloalkyl group may have one or a plurality of
groups selected from a halogen atom and an aryl group
as substituents; and/or
(b7) A represents a lower alkylene group;
and/or
(b8) R7 represents OR8, NR8R9 or SR8;
R8 and R9 may be the same or different and
represent a hydrogen atom, a lower alkyl group, a
lower alkenyl group, a lower alkynyl group, a lower
cycloalkyl group, an aryl group, a heterocyclic
group, a formyl group, a lower alkylcarbonyl group, a
lower alkenylcarbonyl group, a lower alkynylcarbonyl
group, a lower cycloalkylcarbonyl group, an
arylcarbonyl group, a heterocyclic carbonyl group, a
carboxy group, a lower alkoxycarbonyl group, a lower
alkenyloxycarbonyl group, a lower alkynyloxycarbonyl
group, a lower cycloalkyloxycarbonyl group, an
aryloxycarbonyl group, a heterocyclic oxycarbonyl
group, a lower alkylsulfonyl group, a lower
alkenylsulfonyl group, a lower alkynylsulfonyl group,
51
CA 02643070 2008-08-20
. .
. ,
a lower cycloalkylsulfonyl group, an arylsulfonyl
group, a heterocyclic sulfonyl group,
an
aminocarbonyl group, a lower alkylaminocarbonyl
group, a lower alkenylaminocarbonyl group, a lower
alkynylaminocarbonyl group, a
lower
cycloalkylaminocarbonyl group, an arylaminocarbonyl
group or a heterocyclic aminocarbonyl group;
in the case where R8 or R9 is a lower alkyl
group, a lower alkenyl group, a lower alkynyl group,
a lower alkylcarbonyl group, a lower alkenylcarbonyl
group, a lower alkynylcarbonyl group, a lower
alkoxycarbonyl group, a lower alkenyloxycarbonyl
group, a lower alkynyloxycarbonyl group, a lower
alkylsulfonyl group, a lower alkenylsulfonyl group, a
lower alkynylsulfonyl group, a
lower
alkylaminocarbonyl group, a
lower
alkenylaminocarbonyl group Or a
lower
alkynylaminocarbonyl group, the lower alkyl group,
lower alkenyl group, lower alkynyl group, lower
alkylcarbonyl group, lower alkenylcarbonyl group,
lower alkynylcarbonyl group, lower alkoxycarbonyl
group, lower alkenyloxycarbonyl group,
lower
alkynyloxycarbonyl group, lower alkylsulfonyl group,
lower alkenylsulfonyl group, lower alkynylsulfonyl
group, lower alkylaminocarbonyl group,
lower
alkenylaminocarbonyl group or
lower
alkynylaminocarbonyl group may have one or a
plurality of groups selected from a halogen atom, a
lower cycloalkyl group, an aryl group, a heterocyclic
52
CA 02643070 2008-08-20
.,
. .
group, a hydroxy group, an ester of a hydroxy group,
a lower alkoxy group, a lower alkoxy group
substituted with a halogen atom, a lower alkylthio
group, an amino group, a lower alkylamino group, an
amide of an amino group, an amide of a lower
alkylamino group, a lower alkylcarbonyl group, a
carboxy group, an ester of a carboxy group, an amide
of a carboxy group, a lower alkylsulfonyl group, a
nitro group and a cyano group as substituents;
in the case where R8 or R9 is a lower cycloalkyl
group, an aryl group, a heterocyclic group, a lower
cycloalkylcarbonyl group, an arylcarbonyl group, a
heterocyclic carbonyl group, a
lower
cycloalkyloxycarbonyl group, an aryloxycarbonyl
group, a heterocyclic oxycarbonyl group, a lower
cycloalkylsulfonyl group, an arylsulfonyl group, a
heterocyclic sulfonyl group, a
lower
cycloalkylaminocarbonyl group, an arylaminocarbonyl
group or a heterocyclic aminocarbonyl group, the
lower cycloalkyl group, aryl group, heterocyclic
group, lower cycloalkylcarbonyl group, arylcarbonyl
group, heterocyclic carbonyl group,
lower
cycloalkyloxycarbonyl group, aryloxycarbonyl group,
heterocyclic oxycarbonyl group,
lower
cycloalkylsulfonyl group,
arylsulfonyl group,
heterocyclic sulfonyl group,
lower
cycloalkylaminocarbonyl group,
arylaminocarbonyl
group or heterocyclic aminocarbonyl group may have
one or a plurality of groups selected from a halogen
53
CA 02643070 2008-08-20
. ,
. .
atom, a lower alkyl group, a lower alkyl group
substituted with a halogen atom, a lower alkyl group
substituted with a hydroxy group, a lower alkenyl
group, a lower alkynyl group, a lower cycloalkyl
group, an aryl group, a heterocyclic group, a hydroxy
group, an ester of a hydroxy group, a lower alkoxy
group, a lower alkoxy group substituted with a
halogen atom, a lower alkenyloxy group, a lower
alkynyloxy group, a lower alkylthio group, an amino
group, a lower alkylamino group, an amide of an amino
group, an amide of a lower alkylamino group, a lower
alkylcarbonyl group, a carboxy group, an ester of a
carboxy group, an amide of a carboxy group, a lower
alkylsulfonyl group, a nitro group and a cyano group
as substituents;
in the case where R7 is NR8R9, R8 and R9 may be
combined together to form a 5- or 6-membered
nitrogen-containing heterocyclic ring; and/or
(b9) X represents 0.
That is, in the compounds represented by the
general formula (1), more preferred examples include
compounds that comprises one or a combination of two
or more selected from the above (bl), (b2), (b3),
(b4), (b5), (b6), (b7), (b8) and (b9), and salts
thereof.
(c) Further more preferred examples of the
present compound include compounds in which the
54
CA 02643070 2008-08-20
. .
, .
respective groups are groups as defined below and
salts thereof in the compounds represented by the
general formula (1) and salts thereof.
In the general formula (1),
(cl) R1 represents a halogen atom, a lower alkyl
group, a hydroxy group, an ester of a hydroxy group,
a lower alkoxy group, a lower alkylthio group, an
amino group, an amide of an amino group, an amide of
a lower alkylamino group, a lower alkylcarbonyl
group, a carboxy group, an ester of a carboxy group,
a nitro group or a cyano group;
in the case where Rl is a lower alkyl group or a
lower alkoxy group, the lower alkyl group or lower
alkoxy group may have one or a plurality of groups
selected from a halogen atom, a hydroxy group and a
lower alkoxy group as substituents; and/or
(c2) p represents 1, 2 or 3;
in the case where p is 2 or 3, each R1 may be
the same or different; and/or
(c3) q represents 0; and/or
(c4) R3 represents a hydrogen atom; and/or
(c5) R4 and R5 may be the same or different and
represent a lower alkyl group; and/or
(c6) R6 represents a hydrogen atom, a lower
alkyl group or a lower alkenyl group; and/or
(c7) A represents a lower alkylene group;
and/or
(c8) R7 represents OR8 or NR8R9;
R8 and R9 may be the same or different and
CA 02643070 2008-08-20
represent a hydrogen atom, an aryl group, an
arylcarbonyl group or a heterocyclic carbonyl group;
in the case where R8 or R9 is an aryl group, an
arylcarbonyl group or a heterocyclic carbonyl group,
the aryl group, arylcarbonyl group or heterocyclic
carbonyl group may have one or a plurality of groups
selected from a halogen atom, a lower alkyl group, a
lower alkyl group substituted with at least a halogen
atom, a lower alkyl group substituted with at least a
hydroxy group, a lower alkenyl group, an aryl group,
a lower alkoxy group, a lower alkylcarbonyl group, an
ester of a carboxy group, a nitro group and a cyano
group as substituents; and/or
(c9) X represents 0.
That is, in the compounds represented by the
general formula (1), further more preferred examples
include compounds that comprises one or a combination
of two or more selected from the above (cl), (c2),
(c3), (c4), (c5), (c6), (c7), (c8) and (c9), and
salts thereof.
(d) Further more preferred examples of the
present compound include compounds in which the
respective groups are groups as defined below and
salts thereof in the compounds represented by the
general formula (1) and salts thereof.
In the general formula (1),
(d1) Rl represents a halogen atom, a hydroxy
56
CA 02643070 2008-08-20
group, an ester of a hydroxy group, a lower alkoxy
group, an amide of an amino group or an amide of a
lower alkylamino group; and/or
(d2) p represents 2 or 3, in this case, each R1
may be the same or different; and/or
(d3) q represents 0; and/or
(d4) R3 represents a hydrogen atom; and/or
(d5) R4 and R5 may be the same or different and
represent a lower alkyl group; and/or
(d6) R6 represents a lower alkyl group; and/or
(d7) A represents a lower alkylene group;
and/or
(d8) R7 represents OR8 or NR8R9;
R8 represents an aryl group, an arylcarbonyl
group or a heterocyclic carbonyl group, in this case,
the aryl group, arylcarbonyl group or heterocyclic
carbonyl group may have one or a plurality of groups
selected from a halogen atom, a lower alkyl group, a
lower alkyl group substituted with at least a halogen
atom, a lower alkyl group substituted with at least a
hydroxy group, a lower alkenyl group, an aryl group,
a lower alkoxy group, a lower alkylcarbonyl group, an
ester of a carboxy group, a nitro group and a cyano
group as substituents;
R9 represents a hydrogen atom; and/or
(d9) X represents 0.
That is, in the compounds represented by the
general formula (1), further more preferred examples
include compounds that comprises one or a combination
57
CA 02643070 2008-08-20
,
of two or more selected from the above (dl), (d2),
(d3), (d4), (d5), (d6), (d7), (d8) and (d9), and
salts thereof.
(e) Preferred examples of R1 in the present
compound include compounds that satisfy the following
requirement and salts thereof.
A compound or a salt thereof wherein in the
general formula (1), R1 represents a halogen atom, a
hydroxy group, an ester of a hydroxy group, a lower
alkoxy group, an amide of an amino group or an amide
of a lower alkylamino group, and satisfies the
requirement of the above (a), (b) and/or (c).
(f) Preferred examples of R4, R5 and R6 in the
present compound include compounds that satisfy the
following requirement and salts thereof.
A compound or a salt thereof wherein in the
general formula (1), R4, R5 and R6 each represent a
methyl group, and satisfies the requirement of the
above (a), (b),(c) and/or (d).
(g) Preferred examples of R8 in the present
compound include compounds that satisfy the following
requirement and salts thereof.
A compound or a salt thereof wherein in the
general formula (1), R8 represents an aryl group, an
arylcarbonyl group or a heterocyclic carbonyl group,
and the aryl group represents a phenyl group, the
58
CA 02643070 2008-08-20
arylcarbonyl group represents a phenylcarbonyl group,
and/or the heterocyclic carbonyl group represents a
thiophenecarbonyl group, and satisfies the
requirement of the above (a), (b), (c) and/or (d).
(h) Preferred examples of A in the present
compound include compounds that satisfy the following
requirement and salts thereof.
A compound or a salt thereof wherein in the
general formula (1), A represent a methylene group,
and satisfies the requirement of the above (a), (b),
(c) and/or (d).
(i) Preferred examples of which Rl is an ester
ofa hydroxyl group in the present compound include
compounds that satisfy the following requirement and
salts thereof.
In the R1 of the general formula (1), the ester
of a hydroxy group represents -000- in which the
Ral
represents a hydrogen atom, a lower alkyl group
which may have at least a substituent, a lower
alkenyl group which may have at least a substituent,
a lower alkynyl group which may have at least a
substituent, a lower cycloalkyl group which may have
at least a substituent, an aryl group which may have
at least a substituent, a heterocyclic group which
may have at least a substituent, a lower alkoxy group
which may have at least a substituent, a lower
alkenyloxy group which may have at least a
59
CA 02643070 2008-08-20
, .
substituent, a lower alkynyloxy group which may have
at least a substituent, a lower cycloalkyloxy group
which may have at least a substituent, an aryloxy
group which may have at least a substituent, a
heterocyclic oxy group which may have at least a
substituent, an amino group, a lower alkylamino group
which may have at least a substituent, a lower
cycloalkylamino group which may have at least a
substituent, an arylamino group which may have at
least a substituent or a heterocyclic amino group
which may have at least a substituent,
more preferred examples, the Rai represents a
hydrogen atom, a lower alkyl group, a lower alkenyl
group, a lower cycloalkyl group, an aryl group, a
heterocyclic group, a lower alkoxy group, a lower
alkenyloxy group, a lower cycloalkyloxy group, an
aryloxy group, a heterocyclic oxy group, an amino
group, a lower alkylamino group, a lower
cycloalkylamino group, an arylamino group or a
heterocyclic amino group;
in the case where Rai is a lower alkyl group, a
lower alkenyl group, a lower alkoxy group, a lower
alkenyloxy group or a lower alkylamino group, the
lower alkyl group, lower alkenyl group, lower alkoxy
group, lower alkenyloxy group or lower alkylamino
group may have one or a plurality of groups selected
from a halogen atom, an aryl group, a heterocyclic
group, a hydroxy group, an ester of a hydroxy group,
an amino group, a lower alkylamino group, a carboxy
CA 02643070 2008-08-20
. ,
. .
group and an ester of a carboxy group as
substituents; and
in the case where Ral is a lower cycloalkyl
group, an aryl group, a heterocyclic group, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a lower cycloalkylamino group, an
arylamino group or a heterocyclic amino group, the
lower cycloalkyl group, aryl group, heterocyclic
group, lower cycloalkyloxy group, aryloxy group,
heterocyclic oxy group, lower cycloalkylamino group,
arylamino group or heterocyclic amino group may have
one or a plurality of groups selected from a halogen
atom, a lower alkyl group, a lower alkyl group
substituted with at least a halogen atom, a hydroxy
group, an ester of a hydroxy group, a lower alkoxy
group, a mercapto group, a lower alkylthio group, a
formyl group, a lower alkylcarbonyl group, a carboxy
group, an ester of a carboxy group, a nitro group and
a cyano group as substituents,
further more preferred examples, the Ral
represents a lower alkyl group, a lower alkenyl
group, a lower cycloalkyl group, an aryl group, a
heterocyclic group, a lower alkoxy group, an aryloxy
group, a lower alkylamino group, a
lower
cycloalkylamino group, an arylamino group or a
heterocyclic amino group;
in the case where Ral is a lower alkyl group,
the lower alkyl group may have one or a plurality of
groups selected from an aryl group and a lower
61
CA 02643070 2008-08-20
..
alkylamino group as substituents;
in the case where Rai is an aryl group, the aryl
group may have one or a plurality of groups selected
from a halogen atom, a lower alkyl group, a lower
alkyl group substituted with at least a halogen atom,
an ester of a hydroxy group, a lower alkoxy group, a
lower alkylthio group, a lower alkylcarbonyl group,
an ester of a carboxy group and a nitro group as
substituents;
in the case where Rai is a heterocyclic group,
the heterocyclic group may have one or a plurality of
groups selected from a halogen atom, a lower alkyl
group, a hydroxy group and a lower alkoxy group as
substituents;
in the case where Rai is a lower alkylamino
group, the lower alkylamino group may have one or a
plurality of groups selected from an aryl group, a
heterocyclic group and an ester of a carboxy group as
substituents; and
in the case where Rai is an arylamino group, the
arylamino group may have one or a plurality of groups
selected from a halogen atom, a lower alkyl group and
a lower alkoxy group as substituents, and satisfies
the requirement of the above (a), (b), (c) (d) and/or
(e).
(j) Preferred examples of which R1 is an amide
ofan amino group in the present compound include
compounds that satisfy the following requirement and
62
CA 02643070 2008-08-20
. .
. .
salts thereof.
In the Rl of the general formula (1), the amide
of an amino group represents -NHCO- Rbl, in which the
Rbl represents a hydrogen atom, a lower alkyl group
which may have at least a substituent, a lower
alkenyl group which may have at least a substituent,
a lower alkynyl group which may have at least a
substituent, a lower cycloalkyl group which may have
at least a substituent, an aryl group which may have
at least a substituent, a heterocyclic group which
may have at least a substituent, a lower alkoxy group
which may have at least a substituent, a lower
alkenyloxy group which may have at least a
substituent, a lower alkynyloxy group which may have
at least a substituent, a lower cycloalkyloxy group
which may have at least a substituent, an aryloxy
group which may have at least a substituent, a
heterocyclic oxy group which may have at least a
substituent, an amino group, a lower alkylamino group
which may have at least a substituent, a lower
cycloalkylamino group which may have at least a
substituent, an arylamino group which may have at
least a substituent or a heterocyclic amino group
which may have at least a substituent,
more preferred examples, Rbl represents a
hydrogen atom, a lower alkyl group, a lower alkenyl
group, a lower cycloalkyl group, an aryl group, a
heterocyclic group, a lower alkoxy group, a lower
alkenyloxy group, a lower cycloalkyloxy group, an
63
CA 02643070 2008-08-20
aryloxy group, a heterocyclic oxy group, an amino
group, a lower alkylamino group, a lower
cycloalkylamino group, an arylamino group or a
heterocyclic amino group;
in the case where Rbi is a lower alkyl group, a
lower alkenyl group, a lower alkoxy group, a lower
alkenyloxy group or a lower alkylamino group, the
lower alkyl group, lower alkenyl group, lower alkoxy
group, lower alkenyloxy group or lower alkylamino
group may have one or a plurality of groups selected
from a halogen atom, an aryl group, a heterocyclic
group, a hydroxy group, an ester of a hydroxy group,
an amino group, a lower alkylamino group, a carboxy
group and an ester of a carboxy group as
substituents; and
in the case where Rbi is a lower cycloalkyl
group, an aryl group, a heterocyclic group, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a lower cycloalkylamino group, an
arylamino group or a heterocyclic amino group, the
lower cycloalkyl group, aryl group, heterocyclic
group, lower cycloalkyloxy group, aryloxy group,
heterocyclic oxy group, lower cycloalkylamino group,
arylamino group or heterocyclic amino group may have
one or a plurality of groups selected from a halogen
atom, a lower alkyl group, a lower alkyl group
substituted with at least a halogen atom, a hydroxy
group, an ester of a hydroxy group, a lower alkoxy
group, a mercapto group, a lower alkylthio group, a
64
CA 02643070 2008-08-20
formyl group, a lower alkylcarbonyl group, a carboxy
group, an ester of a carboxy group, a nitro group and
a cyano group as substituents,
further more preferred examples, the Rbl
represents a lower alkyl group, an aryl group, a
heterocyclic group, an aryloxy group, a lower
alkylamino group or an arylamino group;
in the case where Rbl is a lower alkyl group,
the lower alkyl group may have one or a plurality of
amino groups as substituents;
in the case where Rbl is an aryl group, the aryl
group may have one or a plurality of groups selected
from a halogen atom, a lower alkyl group, a lower
alkyl group substituted with at least a halogen atom,
an ester of a hydroxy group, a lower alkoxy group, a
lower alkylthio group, a lower alkylcarbonyl group,
an ester of a carboxy group and a nitro group as
substituents;
in the case where R131 is a heterocyclic group,
the heterocyclic group may have one or a plurality of
groups selected from a halogen atom, a lower alkyl
group, a hydroxy group and a lower alkoxy group as
substituents; and
in the case where Rbl is a lower alkylamino
group, the lower alkylamino group may have one or a
plurality of aryl groups as substituents, and
satisfies the requirement of the above (a), (b), (c)
(d) and/or (e).
CA 02643070 2008-08-20
(k) Preferred examples of which R1 is an amide
ofa lower alkylamino group in the present compound
include compounds that satisfy the following
requirement and salts thereof.
In the R1 of the general formula (1), the amide
of a lower alkylamino group represents -NRc1C0 -Rc2, in
which the Rcl represents a lower alkyl group which may
have at least a substituent, and the Rc2 represents a
hydrogen atom, a lower alkyl group which may have at
least a substituent, a lower alkenyl group which may
have at least a substituent, a lower alkynyl group
which may have at least a substituent, a lower
cycloalkyl group which may have at least a
substituent, an aryl group which may have at least a
substituent, a heterocyclic group which may have at
least a substituent, a lower alkoxy group which may
have at least a substituent, a lower alkenyloxy group
which may have at least a substituent, a lower
alkynyloxy group which may have at least a
substituent, a lower cycloalkyloxy group which may
have at least a substituent, an aryloxy group which
may have at least a substituent, a heterocyclic oxy
group which may have at least a substituent, an amino
group, a lower alkylamino group which may have at
least a substituent, a lower cycloalkylamino group
which may have at least a substituent, an arylamino
group which may have at least a substituent or a
heterocyclic amino group which may have at least a
substituent,
66
CA 02643070 2008-08-20
more preferred examples, the Rd l represents a
lower alkyl group, and the Rc2 represents a hydrogen
atom, a lower alkyl group, a lower alkenyl group, a
lower cycloalkyl group, an aryl group, a heterocyclic
group, a lower alkoxy group, a lower alkenyloxy
group, a lower cycloalkyloxy group, an aryloxy group,
a heterocyclic oxy group, an amino group, a lower
alkylamino group, a lower cycloalkylamino group, an
arylamino group or a heterocyclic amino group;
in the case where Rc2 is a lower alkyl group, a
lower alkenyl group, a lower alkoxy group, a lower
alkenyloxy group or a lower alkylamino group, the
lower alkyl group, lower alkenyl group, lower alkoxy
group, lower alkenyloxy group or lower alkylamino
group may have one or a plurality of groups selected
from a halogen atom, an aryl group, a heterocyclic
group, a hydroxy group, an ester of a hydroxy group,
an amino group, a lower alkylamino group, a carboxy
group and an ester of a carboxy group as
substituents; and
in the case where Rc2 is a lower cycloalkyl
group, an aryl group, a heterocyclic group, a lower
cycloalkyloxy group, an aryloxy group, a heterocyclic
oxy group, a lower cycloalkylamino group, an
arylamino group or a heterocyclic amino group, the
lower cycloalkyl group, aryl group, heterocyclic
group, lower cycloalkyloxy group, aryloxy group,
heterocyclic oxy group, lower cycloalkylamino group,
arylamino group or heterocyclic amino group may have
67
CA 02643070 2008-08-20
one or a plurality of groups selected from a halogen
atom, a lower alkyl group, a lower alkyl group
substituted with at least a halogen atom, a hydroxy
group, an ester of a hydroxy group, a lower alkoxy
group, a mercapto group, a lower alkylthio group, a
formyl group, a lower alkylcarbonyl group, a carboxy
group, an ester of a carboxy group, a nitro group and
a cyano group as substituents,
further more preferred examples, the
represents a lower alkyl group, and the Rc2 represents
a lower alkyl group, an aryl group or a heterocyclic
group;
in the case where Rc2 is a lower alkyl group,
the lower alkyl group may have one or a plurality of
amino groups as substituents;
in the case where Rc2 is an aryl group, the aryl
group may have one or a plurality of groups selected
from a halogen atom, a lower alkyl group, a lower
alkyl group substituted with at least a halogen atom,
an ester of a hydroxy group, a lower alkoxy group, a
lower alkylthio group, a lower alkylcarbonyl group,
an ester of a carboxy group and a nitro group as
substituents;
in the case where Rc2 is a heterocyclic group,
the heterocyclic group may have one or a plurality of
groups selected from a halogen atom, a lower alkyl
group, a hydroxy group and a lower alkoxy group as
substituents; and
in the case where Rc2 is a lower alkylamino
68
CA 02643070 2008-08-20
. ,
group, the lower alkylamino group may have one or a
plurality of aryl groups as substituents, and
satisfies the requirement of the above (a), (b), (c)
(d) and/or (e).
(1) Particularly preferred specific examples of
the present compound include the following compounds
and salts thereof. A compound or a salt thereof
selected from
7-[2-Methoxy-4-(2-methylbenzoyloxy)pheny1]-8-(4-
methoxybenzoyloxymethyl)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(5-Fluoro-2-methoxypheny1)-8-(4-
methylbenzoyloxymethyl)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(4-Fluoro-2-methoxypheny1)-8-(5-methylthiophen-2-
ylcarbonyloxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Bromothiophen-2-ylcarbonyloxymethyl)-7-(4-
fluoro-2-methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-(4-Fluoro-2-methoxypheny1)-8-(2-methy1-5-
nitrophenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(5-Chloro-2-methoxypheny1)-8-[2-(2-
hydroxyethyl)phenoxymethy1]-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Chloro-2-methylphenoxymethyl)-7-(4-fluoro-2-
methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
6 9
CA 02643070 2008-08-20
4
quinoxalin-2-one
7-(4-Fluoro-2-methoxypheny1)-8-(2-methoxy-5-
nitrophenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(2-Allylphenoxymethyl)-7-(4-fluoro-2-
methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(4-Fluoro-2-methoxypheny1)-8-(2-methoxy-5-
methylphenylaminomethyl)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-(5-Chloro-2-methoxypheny1)-8-(5-fluoro-2-
methylphenylaminomethyl)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-(5-Chloro-2-methoxypheny1)-8-(2-
isopropylphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-(4-Fluoro-2-methoxypheny1)-8-(2-
methoxyphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-Benzoyloxymethy1-7-(5-fluoro-2-methoxypheny1)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-(5-Fluoro-2-methoxypheny1)-8-phenoxymethy1-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-(5-Fluoro-2-methoxypheny1)-8-phenylaminomethyl-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
1-Ethy1-7-(5-fluoro-2-methoxypheny1)-8-(4-
methylbenzoyloxymethyl)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one
1-(Propen-3-y1)-7-(5-fluoro-2-methoxypheny1)-8-(4-
7 0
CA 02643070 2008-08-20
. .
. ,
methylbenzoyloxymethyl)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-[2-Methoxy-4-(2-methylbenzoyloxy)pheny1]-8-(4-
methoxybenzoyloxymethyl)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-[2-Methoxy-4-(2-methylbenzoyloxy)pheny1]-8-(2-
methoxy-5-nitrophenoxymethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(3-Fluorobenzoyloxymethyl)-7-[2-methoxy-4-(2-
methylbenzoyloxy)pheny1]-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-(2-Chloropheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-
methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-
methylthiopheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(4-Fluoro-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(5-Chloro-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-methoxy-5-
trifluoromethylpheny1)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
71
CA 02643070 2008-08-20
7-(6-Fluoro-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-methoxy-5-
nitropheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(5-Benzoyloxy-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(2-Methoxyphenylaminomethyl)-7-(2-methoxy-5-
trifluoromethylpheny1)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-(4-Amino-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(5-
hydroxymethy1-2-methoxypheny1)-1,3,3-trimethyl-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(4-hydroxy-2-
methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(4-Hydroxy-2-methoxypheny1)-8-(5-methylthiophen-2-
ylcarbonyloxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(2-
methylbenzoyloxy)pheny1]-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-[4-(2-Chlorobenzoyloxy)-2-methoxypheny1]-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
7 2
CA 02643070 2008-08-20
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[4-(furan-3-
ylcarbonyloxy)-2-methoxypheny1]-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-
(pyridin-4-ylcarbonylamino)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one
7-[4-(2-Chlorobenzoylamino)-2-methoxypheny1]-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(4-
methoxybenzoyloxy)pheny1]-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-(4-Acryloyloxy-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-methoxy-4-
methoxycarbonyloxypheny1)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-methoxy-4-
phenoxycarbonyloxypheny1)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-methoxy-4-
phenoxycarbonylaminopheny1)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-[4-(2-Fluorobenzoyloxy)-2-methoxypheny1]-8-(5-
methylthiophen-2-ylcarbonyloxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(3-
7 3
CA 02643070 2008-08-20
methoxycarbonylbenzoyloxy)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(2-
methylpyridin-3-ylcarbonyloxy)pheny1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-[4-(2-Acetoxybenzoyloxy)-2-methoxypheny1]-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(2-
methylthiobenzoyloxy)pheny1]-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(6-
methylpyridin-3-ylcarbonyloxy)pheny1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-
(oxazol-4-ylcarbonyloxy)pheny1]-1,3,3-trimethyl-3,4-
dihydro-1H-quinoxalin-2-one
7-[4-(3-Acetylbenzoyloxy)-2-methoxypheny1]-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-[4-(3-Chlorothiophen-2-ylcarbonyloxy)-2-
methoxypheny1]-8-(5-fluoro-2-methylphenoxymethyl)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(2-
methoxypyridin-3-ylcarbonyloxy)pheny1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-[2-Methoxy-4-(2-methylthiobenzoyloxy)pheny1]-8-(5-
methylthiophen-2-ylcarbonyloxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
74
CA 02643070 2008-08-20
= ,
7-[4-(N-Acetyl-N-methylamino)-2-methoxypheny1]-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-
(pyridin-3-ylaminocarbonyloxy)pheny1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-(2-Methoxy-4-phenylaminocarbonyloxypheny1)-8-(5-
methylthiophen-2-ylcarbonyloxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-
(morpholin-4-ylcarbonyloxy)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one
7-(4-Dimethylaminocarbonyloxy-2-methoxypheny1)-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-(4-Hydroxy-2-methoxypheny1)-8-(2-
methoxyphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-(4-Butyryloxy-2-methoxypheny1)-8-(2-
methoxyphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
7-[2-Methoxy-4-(2-methylpyridin-3-
ylcarbonyloxy)pheny1]-8-(2-methoxyphenylaminomethyl)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
8-(2-Methoxyphenylaminomethyl)-7-[2-methoxy-4-
(thiazol-4-ylcarbonyloxy)pheny1]-1,3,3-trimethyl-3,4-
dihydro-1H-quinoxalin-2-one
8-[N-(5-Fluoro-2-methylpheny1)-N-(9-
fluorenylmethoxycarbonyl)aminomethy1]-7-(4-hydroxy-2-
7 5
CA 02643070 2008-08-20
methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-[2-Methoxy-4-(2-methylbenzoyloxy)pheny1]-8-(2-
methoxy-5-nitrophenoxymethyl)-3,3-dimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-
methylpheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(4-Benzoyloxy-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-(4-Benzoyloxy-2-methoxypheny1)-8-(5-methylthiophen-
2-ylcarbonyloxymethyl)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one
7-[4-(Furan-2-ylcarbonyloxy)-2-methoxypheny1]-8-(5-
methylthiophen-2-ylcarbonyloxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-[2-Methoxy-4-(2-methoxybenzoyloxy)pheny1]-8-(5-
methylthiophen-2-ylcarbonyloxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-[2-Methoxy-4-(3-methoxycarbonylbenzoyloxy)pheny1]-
8-(5-methylthiophen-2-ylcarbonyloxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
7-[2-Methoxy-4-(3-methylfuran-2-
ylcarbonyloxy)pheny1]-8-(5-methylthiophen-2-
ylcarbonyloxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
7-[4-(3-Benzylureido)-2-methoxypheny1]-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
7 6
CA 02643070 2008-08-20
,
. .
quinoxalin-2-one
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(3-
phenylureido)pheny1]-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one
8-(2-Methoxyphenylaminomethyl)-7-[2-methoxy-4-
(pyridine-3-ylcarbonyloxy)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one
7-[2-Methoxy-4-(2-methoxybenzoyloxy)pheny1]-8-(2-
methoxyphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
8-(2-Methoxyphenylaminomethyl)-7-[2-methoxy-4-
(thiophen-3-ylcarbonyloxy)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one, and
7-[2-Methoxy-4-(2-methylbenzoyloxy)pheny1]-8-(2-
methoxyphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one
The present compound can be synthesized
according to the following procedures. The individual
concrete preparation procedures are explained in
details in the following examples, [preparation
examples]. These examples are intended to make the
present invention more clearly understandable, and do
not limit the scope of the present invention. The Hal
shown in the following synthetic routes represents a
halogen atom, MOM represents methoxymethtyl group and
Fmoc represents 9-fluorenylmethoxycarbonyl group.
The present compound (I)-(a) (the compound that
77
CA 02643070 2008-08-20
A is methylene group, X is 0 in the general formula
(1)) can be synthesized according to the synthetic
route 1. Namely, the compound (I)-(a) can be given by
the reaction of the present compound (I)-(b) (the
compound that A is methylene group, X is 0, R6 is H in
the general formula (1)) with a corresponding halide
(II) in an organic solvent such as N, N-
dimethylformamide (hereinafter referred to as DMF),
tetrahydrofuran (hereinafter referred to as THF),
1,4-dioxane, methylene dichloride in the presence of
a base such as cesium carbonate, potassium carbonate
at 0 C to 50 C for 1 hour to 24 hours.
FiEli-R6
(R1)p
(II) (Ri)p 1:27 Rs
NOR5 40 NOR5
(Fe), R (R2), 1, R4
R3
M- (b) (I)- (a)
Synthetic Route 1
The present compound (I)-(b) (the compound that
A is methylene group, X is 0, R6 is H, R7 is OR8, NR8R9
or SR8 in the general formula (1)) can be synthesized
according to the synthetic route 2. Namely, the
compound (I)-(b) can be given by the reaction of the
compound (III) with a corresponding alcohol,
carboxylic acid, phenol, amine, thiol, thiophenol and
the like (IV) in an organic solvent such as DMF, THE,
ethanol in the presence of a base such as potassium
carbonate, sodium hydride at 0 C to 100 C for 1 hour
78
CA 02643070 2008-08-20
to 48 hours.
CI R7
(RI )p H (R 1)p
NO (IV)
11101 X)R5
(R2), 1 R4 (R2), " R4
R3 R3
(III) (I) - ( b )
Synthetic Route 2
The present compound (I)-(c) (the compound that
A is methylene group, X is 0, R7 is OR8 in the general
formula (1)) can be synthesized according to the
synthetic route 3. Namely, the compound (I)-(c) can
be given by the reaction of the compound (V) with a
corresponding halide (VI) in an organic solvent such
as DMF, THF, methylene dichloride in the presence of
a base such as triethylamine, potassium carbonate at
0 C to 50 C for 1 hour to 48 hours.
IR8
Hal
(1R1)p
(VI) (R,
0101 XDR5 101 5
(R2), 13 R4
(R2), 13 R4
(I)- (c)
Synthetic Route 3
The compound (III) and (V) can be synthesized
according to the synthetic route 4. Namely, the
compound (IX) can be given by the reaction of the
compound (VII) with a corresponding boronic acid or
its ester (VIII) in a solvent such as DMF, 1,4-
79
CA 02643070 2008-08-20
dioxane, ethanol, toluene, water and in the presence
of a base such as cesium carbonate, sodium carbonate,
sodium hydrogen carbonate, tripotassium phosphate and
a catalyst such as bis(triphenylphosphine)palladium
(II) dichloride,
tetrakis(triphenylphosphine)palladium (0) at 50 C to
120 C for 1 hour to 48 hours. The compound (V) can be
given by the treatment of the compound (IX) in an
organic solvent such as diethylether, THE' in the
presence of a reductive agent such as lithium
aluminium hydride at -30 C to room temperature for 1
hour to 24 hours. The compound (III) can be given by
the treatment of the compound (V) with
methanesulfonyl chloride in an organic solvent such
as methylene dichloride, THE' in the presence of a
base such as triethylamine, diisopropylethylamine
(hereinafter referred to as DIEA) at 0 C to room
temperature for 30 minutes to 12 hours.
=
0R1), ill
.2m
= cR is = =
NM (R1),
Br 010 Dr,
(132)q Y Fe (R2), N 4
1 R
R3 R3
(VII) (IX)
= CI
r
(131)p (R1)p R5 so 45
, (R2), 13 Fe
(V) (III)
CA 02643070 2008-08-20
Synthetic Route 4
The compound (VII) can be synthesized according
to the synthetic route 5. Namely, the compound (XI)
can be given by the treatment of the compound (X) in
an organic solvent such as methanol, ethanol, DMF in
the presence of a reductive agent such as tin(II)
chloride, ferric(II) chloride at 50 C to 120 C for 1
hour to 12 hours. The compound (XII) can be given by
the treatment of the compound (XI) with an
acetylation agnet such as acetyl chloride, acetic
anhydride in an organic solvent such as methylene
dichloride, THF in the presence of a base such as
triethylamine, DIEA at 0 C to 50 C for 1 hour to 12
hours. The compound (XIII) can be given by the
treatment of the compound (XII) with nitric acid in a
solvent such as water in the presence of an acid such
as sulfuric acid at -20 C to room temperature for 30
minutes to 12 hours. The compound (XIV) can be given
by the treatment of the compound (XIII) in an organic
solvent such as methanol in the presence of an acid
such as boron trifluoride ether complex at 50 C to
the temperature under reflux for 1 hour to 12 hours.
The compound (XVI) can be given by the reaction of
the compound (XIV) with a corresponding halide (XV)
in the presence of a base such as cesium carbonate,
potassium carbonate at 50 C to 120 C for 1 hour to
120 hours. The compound (VII) can be given by the
treatment of the compound (XVI) in an organic solvent
81
CA 02643070 2008-08-20
such as methanol, ethanol, DMF in the presence of a
reductive agent such as tin(II) chloride, ferric(II)
chloride at 50 C to 120 C for 1 hour to 12 hours.
I I I I
= = = = = = = =
Br s ---.- Br 0 __... Br 0 _ Br ,... la
0 1,020
.K
= Nt
(R2), (R2), (R2), H (Fe)q H
(X) (XI) (XII) (XIII)
R4 R5
Hal (CEt
I 1 1
= =
= = =
= 0
H
(XV)
--.- Br Es 0 ________________ Br, y
40 5 _ _ _ ... Br,
R R
i?Et
N 4
R3
R 0
(XIV) (XVI) (VII)
Synthetic Route 5
The present compound (I)-(a) (the compound that
A is methylene group, X is 0 in the general formula
(1)) can be also synthesized according to the
synthetic route 6. Namely, the compound (I)-(a) can
be given by the reaction of the compound (XVII) with
a corresponding boronic acid or its ester (VIII) in a
solvent such as DMF, 1,4-dioxane, ethanol, toluene,
water and in the presence of a base such as cesium
carbonate, sodium carbonate, sodium hydrogen
carbonate, tripotassium phosphate and a catalyst such
as bis(triphenylphosphine)palladium (II) dichloride,
tetrakis(triphenylphosphine)palladium (0) at 50 C to
120 C for 1 hour to 48 hours.
82
CA 02643070 2008-08-20
µ,
- ,
=
etc.
(R1)P 40
_7
B--CR .7
R6 1 R6
1 CR (R 1)p Olt 1
Br 1.1 yO5 (VIII)
0 xDR,
(R2)q
13 R (R2), N 4
1R
R R3
(XVIM M-(a)
Synthetic Route 6
Moreover, the present compound (I)-(a) (the
compound that A is methylene group, X is 0 in the
general formula (1)) can be also synthesized
according to the synthetic route 7. Namely, the
compound (I)-(a) can be given by the reaction of the
compound (XVIII) with a corresponding halide (XIX) in
a solvent such as DMF, 1,4-dioxane, ethanol, toluene,
water and in the presence of a base such as cesium
carbonate, sodium = carbonate, sodium hydrogen
carbonate, tripotassium phosphate and a catalyst such
as bis(triphenylphosphine)palladium (II) dichloride,
tetrakis(triphenylphosphine)palladium (0) at 50 C to
120 C for 1 hour to 48 hours.
,..
= --CH
B
6,2 01 etc.
(R1), 410
7
. 6 . I
CR IR'R6
Fo,BI so 7x0R51
OM (R1)P S0/10 Nr1R5
13 Fe (R2), 1 Fe
R R3
(XVIII ) M - ( a )
Synthetic Route 7
83
CA 02643070 2008-08-20
The compound (XVII) and (XVIII) can be
synthesized according to the synthetic route 8.
Namely, the compound (XX) can be given by the
treatment of the compound (VII) in an organic solvent
such as diethylether, THF in the presence of a
reductive agent such as lithium aluminium hydride at
0 C to 50 C for 1 hour to 24 hours. The compound (XXI)
can be given by the reaction of the compound (XX)
with a corresponding halide (II) in an organic
solvent such as DMF, THE, 1,4-dioxane, methylene
dichloride in the presence of a base such as cesium
carbonate, potassium carbonate at 0 C to 50 C for 1
hour to 24 hours. The compound (XXII) can be given by
the treatment of the compound (XXI) with
methanesulfonyl chloride in an organic solvent such
as methylene dichloride, THE in the presence of a
base such as triethylamine, DIEA at 0 C to room
temperature for 30 minutes to 12 hours. The compound
(XVII) can be given by the reaction of the compound
(XXII) with a corresponding alcohol, carboxylic acid,
phenol, amine, thiol, thiophenol (IV) in an organic
solvent such as DMF, THE, ethanol in the presence of
a base such as potassium carbonate, sodium hydride at
0 C to 100 C for 1 hour to 48 hours. The compound
(XVIII) can be given by the reaction of the compound
(XVII) with a corresponding diboron (XXIII) or borane
(XXIV) in a solvent such as dimethylsulfoxide, DMF,
1,4-dioxane in the presence of a base such as
84
CA 02643070 2008-08-20
. 1 = '
potassium acetate, triethylamine and a catalyst such
as
[1,1'-
bis(diphenylphosphino)ferrocene]palladium(II)dichlori
de, bis(triphenylphosphine)palladium (II) dichloride
at 50 C to 120 C for 10 minutes to 48 hours.
I = ,R6
HaI
0 0 . R6
H I
H (II) Br 401
Br is ,r05 _._ Br si .0R5
---'"
(R2)q 13 R4 (R2), 13 R4
"---aR
(R2)q 13 R R R
R
OM POO WO
7
M CR M
CI 6 H-R
6
R -7 6-6 B-I
.7
R6 FO CR = FO, etc.
Br 0 "r
CR
R
i 1 1
I
(IV) Br 40 xo, woo (XXIV)
R5
- cR,B 40 N r5
(W), Fe
e (R2)q 13 R4
(R2)q
13 F
F R
e
R
(XXII) (XVII)
(XVIII)
B .
etc.
Synthetic Route 8
The present compound (I)-(d) (the compound that
A is methylene group, X is 0, one of Rl is OR1c), RI is
a lower alkyl group which may have at least a
substituent, a lower alkylcarbonyl group which may
have at least a substituent, an arylcarbonyl group
which may have at least a substituent, a heterocyclic
carbonyl group which may have at least a substituent,
a lower alkoxycarbonyl group which may have at least
a substituent or an aryloxycarbonyl group which may
have at least a substituent, and the like in the
general formula (1)) can be synthesized according to
. CA 02643070 2008-08-20
.
i,
the synthetic route 9. Namely, the compound (I)-(d)
can be given by the reaction of the present compound
(I)-(e) (the compound that A is methylene group, X is
0, one of Rl is OH in the general formula (1)) with a
corresponding halide (XXV) in an organic solvent such
as THF, methylene dichloride, DMF in the presence of
a base such as triethylamine, DIEA, potassium
carbonate at 0 C to 100 C for 1 hour to 24 hours.
.7
eal Hal
1
(R1),-1WI R6 (XXV) R: e* R6
I
(R )p_,
la
(R2), 13 IR4 (W), 13 IR'
R R
(I)- (e) 0)-(d)
Synthetic Route 9
The present compound (I)-(f) (the compound that
A is methylene group, X is 0, one of the R1 is OCOR11,
Ril is a lower alkyl group which may have at least a
substituent, an aryl group which may have at least a
substituent or a heterocyclic group which may have at
least a substituent, and the like in the general
formula (1)) can be synthesized according to the
synthetic route 10. Namely, the compound (I)-(f) can
be given by the reaction of the present compound (I)-
(e) (the compound that A is methylene group, X is 0,
one of R1 is OH in the general formula (1)) with a
corresponding carboxylic acid (XXVI) in an organic
solvent such as DMF, methylene dichloride in the
presence of a condensation agent such as N,N'-
86
. CA 02643070 2008-08-20
4 =
dicyclohexylcarbodiimide (hereinafter referred to as
DCC), 0-(7-azabenzotriazol-1-y1)-N,N,N,N-tetramethyl
uroniumhexafluorophosphate (hereinafter referred to
as HATU) and a base such as DIEA at room temperature
to 50 C for 1 hour to 3 days.
Rily
.7 6
0
(XXVI) R(6
101 CR5
(F6,1 S 11c5 11 (RI)p_1111
(R2), R4 (R2), R4
R3 R3
(I) - ( e ) (I) - ( f )
Synthetic Route 10
The present compound (I)-(g) (the compound that
A is methylene group, X is 0, one of the R1 is
OCONR12R13, R12 and R13 may be the same or different and
are a lower alkyl group which may have at least a
substituent, an aryl group which may have at least a
substituent, and the like in the general formula (1))
can be synthesized according to the synthetic route
11. Namely, the compound (I)-(g) can be given by the
reaction of the present compound (I)-(e) (the
compound that A is methylene group, X is 0, one of
the R1 is OH in the general formula (1)) with 1,1'-
carbonyldiimidazole (hereinafter referred to as CDI)
in an organic solvent such as methylene dichloride,
THE at room temperature to 50 C for 30 minutes to 12
hours followed by the reaction with a corresponding
amine (XXVII).
87
CA 02643070 2008-08-20
, =
Ri2-Ny
sob,-7 R6
'7 R6
CD I
(R40
(R14-1
(Fe),
R1)+1 (R2), 13 R4
000/10
M-(e) M-(g)
Synthetic Route 11
The present compound (I)-(e) (the compound that
A is methylene group, X is 0, one of the Rl is OH in
the general formula (1)) can be synthesized according
to the synthetic route 12. Namely, the present
compound (I)-(h) can be given by the reaction of the
compound (XVII) with a corresponding boronic acid or
its ester (XXVIII) in a solvent such as DMF, 1,4-
dioxane, ethanol, toluene, water in the presence of a
base such as cesium carbonate, sodium carbonate,
sodium hydrogen carbonate, tripotassium phosphate and
a catalyst such as bis(triphenylphosphine)palladium
(II)
dichloride,
tetrakis(triphenylphosphine)palladium (0) at 50 C to
120 C for 1 hour to 48 hours. The compound (I)-(e)
can be given by the treatment of the compound (I)-(h)
in an organic solvent such as 1,4-dioxane, methylene
dichloride in the presence of an acid such as
hydrogen chloride, trifluoroacetic acid at 0 C to
50 C for 1 hour to 24 hours.
88
CA 02643070 2008-08-20
= I
--0
B1
etc
KM
Igak, KM
W 6
.7 y obi
eabi
(R
(R1)pi BC:IR
)p_i
.71R6
R1'
(R2
Br 010 OR5
(XXVIII )03 (W)p_,N")74
R (R2), Ire
=
(1R2), 13 R4
(XVII ) (I) - ( h )
(I) - ( e )
Synthetic Route 12
Further, the present compound (I)-(e) (the
compound that A is methylene group, X is 0, one of
the R1 is OH in the general formula (1)) can be also
synthesized according to the synthetic route 13.
Namely, the compound (XXIX) can be given by the
reaction of the compound (VII) with a corresponding
boronic acid or its ester (XXVIII) in a solvent such
as DMF, 1,4-dioxane, ethanol, toluene, water in the
presence of a base such as cesium carbonate, sodium
carbonate, sodium hydrogen carbonate, tripotassium
phosphate and a catalyst such as
bis(triphenylphosphine)palladium (II)
dichloride,
tetrakis(triphenylphosphine)palladium (0) at 50 C to
120 C for 1 hour to 48 hours. The compound (XXX) can
be given by the treatment of the compound (XXIX) in
an organic solvent such as diethylether, THF in the
presence of a reductive agent such as lithium
aluminium hydride at -30 C to room temperature for 1
hour to 24 hours. The compound (XXXI) can be given by
the treatment of the compound (XXX) with
methanesulfonyl chloride in an organic solvent such
89
CA 02643070 2008-08-20
ft
as methylene dichloride, THF in the presence of a
base such as triethylamine, DIEA at 0 C to room
temperature for 30 minutes to 12 hours. The present
compound (I)-(i) can be given by the reaction of the
compound (XXXI) with a corresponding alcohol,
carboxylic acid, phenol, amine, thiol, thiophenol and
the like (IV) in an organic solvent such as DMF, THF,
ethanol in the presence of a base such as potassium
carbonate, sodium hydride at 0 C to 100 C for 1 hour
to 48 hours. The present compound (I)-(h) can be
given by the reaction of the compound (I)-(i) with a
corresponding halide (II) in an organic solvent such
as DMF, THF, 1,4-dioxane, methylene dichloride in the
presence of a base such as cesium carbonate,
potassium carbonate at 0 C to 50 C for 1 hour to 24
hours. The compound (I)-(e) can be given by the
treatment of the compound (I)-(h) in an organic
solvent such as 1,4-dioxane, methylene dichloride in
the presence of an acid such as hydrogen chloride,
trifluoroacetic acid at 0 C to 50 C for 1 hour to 24
hours.
CA 02643070 2008-08-20
'
B . B
('=F2 6-1 = B6,¨ , o',)\¨ etc.
NA14
1
eal
I 2CR KM
I I KM
I
= = (R1)p_119
B eau, = = Sabi
Br =
I
H (XXVIII )CR H
VI H
010 x:
(R),1111" (R1),
40 1
0 45
(R2), 1, Fe (Fe), 1, R A
4 , 1
R4
3
(VII) (XXIX ) (XXX )
KM KM
I I
.7
= H-R7 = Ha I ,Fe
H (IV) 1 H 01)
1 so Nr5 _,.. (R)610 0
(R )p_ill CI 4, --
(R2), 13 R4 (R2), 13 R4
R R
(XXXI ) (I) - ( i)
(114 = _7
.7
*obi
R6 RI'
I
-.- (R1)P-14) is NrR5
(R1)p.:91111
Is 4,
(R2)q 13 R4 (R2), 13 R4
R
R
(I) - ( h ) (I) - ( e )
Synthetic Route 13
The present compound (I)-(j) (the compound that
A is methylene group, X is 0, one of the R1 is OR", R7
is NHR8, R" is a lower alkyl group which may have at
least a substituent, a lower alkylcarbonyl group
which may have at least a substituent, an
arylcarbonyl group which may have at least a
substituent, a heterocyclic carbonyl group which may
have at least a substituent, a lower alkoxycarbonyl
group which may have at least a substituent, an
aryloxycarbonyl group which may have at least a
substituent, and the like in the general formula
(1)), the present compound (I)-(k) (the compound that
A is methylene group, X is 0, one of the R1 is 00OR11,
91
CA 02643070 2008-08-20
. 6
R7 is NHR8, R11 is a lower alkyl group which may have
at least a substituent, an aryl group which may have
at least a substituent, a heterocyclic group which
may have at least a substituent, and the like in the
general formula (1)) and the present compound (I)-(1)
(the compound that A is methylene group, X is 0, one
of the Rl is 000NR12R13, R7 is NHR8, R12 and R1-3 may be
the same or different and are a lower alkyl group
which may have at least a substituent, an aryl group
which may have at least a substituent, and the like
in the general formula (1)) can be synthesized
according to the synthetic route 14. Namely, the
compound (I)-(j), (I)-(k) and (I)-(1) can be given by
the reaction of the present compound (I)-(m) (the
compound that A is methylene group, X is 0, one of
the Rl is OH, R7 is NR8(Fmoc) in the general formula
(1)) with a corresponding halide (XXV), a carboxylic
acid (XXVI) or an amine (XXVII) according to the
method of synthetic route 9, 10 or 11 respectively,
followed by the treatment in an organic solvent such
as DMF, methylene dichloride in the presence of a
base such as piperidine at 0 C to 50 C for 5 minutes
to 24 hours.
92
CA 02643070 2008-08-20
R8
,,R10
Hal
R10 .1) R6
(XXV)
(R1)p_i
iS rR5
,,
(I) - ( j )
R8
=
R18
¨Rroc 0
11, (XXVI)
RI'
R6
(R1)_1 =
(R1)p_1W *
0õ3 ,
0, ,
(,)-(m) (,)-( k )
R13
R12r\Y) R8
seah
CDI R6
13\ (R1)0_16"
N-I
IR4
(XXVII)
(I) - )
Synthetic Route 14
The present compound (I)-(m) (the compound that
A is methylene group, X is 0, one of the R1 is OH, R7
is NR8(Fmoc) in the general formula (1)) can be
synthesized according to the synthetic route 15.
Namely, the compound (XXXIII) can be given by the
reaction of the compound (XXII) with a corresponding
amine (XXXII) in an organic solvent such as DMF, THE,
ethanol in the presence of a base such as potassium
carbonate, sodium hydride at 0 C to 100 C for 1 hour
to 48 hours. The present compound (I)-(o) can be given
by the reaction of the compound (XXXIII) with a
corresponding boronic acid or its ester (XXVIII) in a
93
CA 02643070 2008-08-20
. . = =
solvent such as DMF, 1,4-dioxane, ethanol, toluene,
water in the presence of a base such as cesium
carbonate, sodium carbonate, sodium
hydrogen
carbonate, tripotassium phosphate and a catalyst such
as bis(triphenylphosphine)palladium (II) dichloride,
tetrakis(triphenylphosphine)palladium (0) at 50 C to
120 C for 1 hour to 48 hours. The present compound
(I)-(n) can be given by the reaction of the compound
(I)-(o) with 9-fluorenylmethoxycarbonyl chloride in a
solvent such as 1,4-dioxane, water in the presence of
a base such as sodium hydrogen carbonate at 0 C to
50 C for 1 hour to 24 hours. The compound (I)-(m) can
be given by the treatment of the compound (I)-(n) in
an organic solvent such as 1,4-dioxane, methylene
dichloride in the presence of an acid such as
hydrogen chloride, trifluoroacetic acid at 0 C to
50 C for 1 hour to 24 hours.
. B --..,,-0
B-
Km
1
%al
NCM
R6
CI 6 W A 1
R R)
' e
1 , VI B
HP 6 (igi
I R I
R6
Br0 so 0 (XXXII) Br 1 (XXVIII )CR
1
,
R6 (R1)111, 4
54
(R2), 1, Fe
R (R2), 1 Fe (R2),
13 R4
R3
R
(XXII) (XXXIII) (I) -
( o )
WA R8 R8
1 1 1
.al --FRDC obi FRDC
(R,mp
NI-----
136 IR6
1
la N, 45
(Ri)p,RIP
III) 4
(R2)q ,3 Fe (R2)q N 4
13 R
R R
(I) - ( n ) 0) - ( m )
94
CA 02643070 2008-08-20
,
Synthetic Route 15
The present compound (I)-(p) (the compound that
A is methylene group, X is 0, one of the Rl is OR1 , R7
is OR8, Rl is a lower alkyl group which may have at
least a substituent, a lower alkylcarbonyl group
which may have at least a substituent, an
arylcarbonyl group which may have at least a
substituent, a heterocyclic carbonyl group which may
have at least a substituent, a lower alkoxycarbonyl
group which may have at least a substituent, an
aryloxycarbonyl group which may have at least a
substituent, and the like in the general formula (1))
can be synthesized according to the synthetic route
16. Namely, the compound (XXXIV) can be given by the
treatment of the compound (XXX) in an organic solvent
such as 1,4-dioxane, methylene dichloride in the
presence of an acid such as hydrogen chloride,
trifluoroacetic acid at 0 C to 50 C for 1 hour to 24
hours. The compound (XXXV) can be given by the
reaction of the compound (XXXIV) with a corresponding
halide (XXV) in an organic solvent such as THF,
methylene dichloride, DMF in the presence of a base
such as triethylamine, DIEA, potassium carbonate at
0 C to 100 C for 1 hour to 24 hours. The compound (I)-
(q) can be given by the reaction of the compound
(XXXV) with a corresponding halide (VI) in an organic
solvent such as DMF, THF, methylene dichloride in the
CA 02643070 2008-08-20
Synthetic Route 15
The present compound (I)-(p) (the compound that
A is methylene group, X is 0, one of the R1 is OR10, R7
is OR8, Rl is a lower alkyl group which may have at
least a substituent, a lower alkylcarbonyl group
which may have at least a substituent, an
arylcarbonyl group which may have at least a
substituent, a heterocyclic carbonyl group which may
have at least a substituent, a lower alkoxycarbonyl
group which may have at least a substituent, an
aryloxycarbonyl group which may have at least a
substituent, and the like in the general formula (1))
can be synthesized according to the synthetic route
16. Namely, the compound (XXXIV) can be given by the
treatment of the compound (XXX) in an organic solvent
such as 1,4-dioxane, methylene dichloride in the
presence of an acid such as hydrogen chloride,
trifluoroacetic acid at 000 to 50 C for 1 hour to 24
hours. The compound (XXXV) can be given by the
reaction of the compound (XXXIV) with a corresponding
halide (XXV) in an organic solvent such as THF,
methylene dichloride, DMF in the presence of a base
such as triethylamine, DIEA, potassium carbonate at
0 C to 100 C for 1 hour to 24 hours. The compound (I)-
(q) can be given by the reaction of the compound
(XXXV) with a corresponding halide (VI) in an organic
solvent such as DMF, THF, methylene dichloride in the
CA 02643070 2008-08-20
conjunctivitis, central nervous system diseases such
as psychiatric disorders, Alzheimer's disease and
drug use disorders, cardiovascular diseases such as
hypertension, hypercalcemia, hyperinsulinemia and
hyperlipidemia, homeostasis-related diseases causing
an abnormality of neuro-immune-endocrine balance,
glaucoma, comprising administering to a patient a
therapeutically effective amount of the present
compound or a salt thereof.
In order to find the usefulness of the present
compound as a pharmaceutical, by using a
glucocorticoid receptor competitor assay kit, a
glucocorticoid receptor competitor assay was carried
out by a fluorescence polarization method. As a
result, the present compound showed an excellent
glucocorticoid receptor binding activity.
Incidentally, the glucocorticoid receptor is
associated with the occurrence of various diseases as
described above, therefore, the present compound
having an excellent binding activity to the
glucocorticoid receptor is useful as a glucocorticoid
receptor modulator.
A detailed explanation of this matter will be
described in the section of "Pharmacological Test" in
Examples described below.
The present compound can be administered either
orally or parenterally. Examples of the dosage form
97
CA 02643070 2008-08-20
include a tablet, a capsule, a granule, a powder, an
injection, an eye drop and the like. Such a
preparation can be prepared using a commonly used
technique.
For example, an oral preparation such as a
tablet, a capsule, a granule or a powder can be
prepared by optionally adding a necessary amount of
an excipient such as lactose, mannitol, starch,
crystalline cellulose, light silicic anhydride,
calcium carbonate or calcium hydrogen phosphate; a
lubricant such as stearic acid, magnesium stearate or
talc; a binder such as starch, hydroxypropyl
cellulose, hydroxypropylmethyl cellulose or
polyvinylpyrrolidone; a disintegrant such as
carboxymethyl cellulose, low-
substituted
hydroxypropylmethyl cellulose or calcium citrate; a
coating agent such as hydroxypropylmethyl cellulose,
macrogol or a silicone resin; a stabilizer such as
ethyl p-hydroxybenzoate Or benzyl alcohol; a
corrigent such as a sweetener, a sour agent or a
flavor, or the like.
A parenteral preparation such as an injection
or an eye drop can be prepared by optionally adding a
necessary amount of a tonicity agent such as sodium
chloride, concentrated glycerin, propylene glycol,
polyethylene glycol, potassium chloride, sorbitol or
mannitol; a buffer such as sodium phosphate, sodium
hydrogen phosphate, sodium acetate, citric acid,
glacial acetic acid or trometamol; a surfactant such
98
CA 02643070 2008-08-20
. .
as polyoxyethylene sorbitan monoolate, polyoxy 40
stearate or polyoxyethylene hydrogenated castor oil
60; a stabilizer such as sodium citrate or sodium
edetate; a preservative such as benzalkonium
chloride, paraben, benzothonium chloride,
p-
hydroxybenzoate ester, sodium benzoate
or
chlorobutanol; a pH adjusting agent such as
hydrochloric acid, citric acid, phosphoric acid,
glacial acetic acid, sodium hydroxide, sodium
carbonate or sodium hydrogen carbonate; a soothing
agent such as benzyl alcohol, or the like.
The dose of the present compound can be
appropriately selected depending on the symptoms,
age, dosage form or the like.
For example, in the
case of an oral preparation, it can be administered
in an amount of generally 0.01 to 1000 mg, preferably
1 to 100 mg per day in a single dose or several
divided doses. Further, in the case of an eye drop, a
preparation containing the present compound at a
concentration of generally 0.0001% to 10% (w/v),
preferably 0.01% to 5% (w/v) can be administered in a
single dose or several divided doses.
Hereinafter, Production Examples of the present
compound, Preparation Examples and results of
Pharmacological Test will be described. However,
these examples are described for the purpose of
understanding the present invention better and are
not meant to limit the scope of the present
invention.
99
CA 02643070 2013-08-02
, 25088-306
Fmoc in the chemical structure in Production
= Examples represents 9-fluorenylmethoxycarbonyl group.
[Production Example]
Reference Example 1
= 7-Bromo-8-methoxycarbony1-3,3-dimethy1-3,4-dihydro-
= 1H-quinoxalin-2-one (Reference Compound No.1)
=
Methyl 5-amino-2-bromobenzoate (Reference Compound
No.1-(1))
Methyl 2-bromo-5-nitrobenzoate (25.3 g, ,97.3
mmol) was dissolved in anhydrous methanol (500 mL),
Tin (II) chloride (93.3 g, 487 mmol) was added
thereto, and then the reaction mixture was refluxed
=
for 2 hours. The reaction mixture waS cooled down,
ethyl acetate (500 mL) and water (100 .mL) were added .
thereto, the mixture was neutralized with 4N aqueous
= sodium hydroxide solution, and then filtered on
= celite. The filtrate was concentrated under reduced
pressure, ethyl acetate (200 mL) was added thereto,
and then the mixture was Washed with saturated
aqueous sodium hydrogen carbonate solution (200 mL, 2
times), water (200 mL), and saturated brine (200 mL)
successively. The organic layer was dried over
= anhydrous magnesium sulfate and the Solvent was
removed under reduced pressure to give the titled
reference compound (21.0 g) as a pale yellow oil.
= (Yield 94%)
*Trade mark
1 0 0
CA 02643070 2008-08-20
=
0 0 1, H-NMR (400 MHz, DMSO-
d0
Br si 6 3.80 (s, 3H), 5.55 (s,
2H), 6.63 (dd, J = 8.8, 2.
NH 2
8 Hz, 1H), 6.94 (d, J = 2.
8 Hz, 1H), 7.29 (d, J = 8.
8 Hz, 1H)
Methyl 5-acetylamino-2-bromobenzoate
(Reference
Compound No.1-(2))
Methyl 5-amino-2-bromobenzoate
(Reference
Compound No.1-(1), 21.0 g, 91.2 mmol) was dissolved in
anhydrous dichloromethane (450 mL), triethylamine
(19.0 mL, 137 mmol) and acethyl chloride (13.0 mL,
182 mmol) were added dropwise over 30 minutes
successively, and then the mixture was stirred at 0 C
for 2 hours. The reaction mixture was washed with
water (200 mL, 2 times), saturated aqueous sodium
hydrogen carbonate solution (200 mL, 2 times), and
saturated brine (200 mL) successively, dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was filtered with hexane - ethyl acetate (20 : 1) to
give the titled reference compound (24.2 g) as a pale
yellow solid. (Yield 98%)
siBr 0 0, 1H-NMR (400 MHz, DMSO-d6)
6 2.06 (s, 3H), 3.86 (s,
0 3H), 7.63-7.66 (m, 2H), 8.
07 (s, 1H), 10.25 (s, 1H)
Methyl
3-acetylamino-6-bromo-2-nitrobenzoate
101
. CA 02643070 2008-08-20
,
. .
(Reference Compound No.1-(3))
To conc. sulfuric acid (150 mL), methyl 5-
acetylamino-2-bromobenzoate (Reference Compound No.1-
(2). 18.5 g, 68.1mmol) was added at 0 C portionwise,
and conc. nitric acid (150 mL) was added dropwise
thereto over 1 hour. The reaction mixture was stirred
for 30 minutes, poured into iced water (1 L), and
then extracted with ethyl acetate (500 mL, 2 times).
The organic layer was washed with water (1 L, 2
times), saturated aqueous sodium hydrogen carbonate
solution (1 L ), and saturated brine (1 L)
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled reference compound (13.4 g) as a
yellow solid. (Yield 62%)
0 Ci 1H-NMR (400 MHz, DMSO-d6)
Br0 NO2 6 2.05 (s, 3H), 3.87 (s,
3H), 7.55 (d, J = 8.8 Hz,
NH 1H), 8.02 (d, J = 8.8 Hz,
1H), 10.48 (s, 1H)
(DiL'
Methyl 3-amino-6-bromo-2-nitrobenzoate
(Reference
Compound No.1-(4))
Methyl
3-acetylamino-6-bromo-2-nitrobenzoate
(Reference Compound No.1-(3), 13.4 g, 42.2 mmol) was
dissolved in methanol (240 mL), boron trifluoride
diethyl etherate complex (24.0 mL, 190 mmol) was
102
CA 02643070 2008-08-20
. .
added thereto, and then the mixture was refluxed for
2.5 hours. After the reaction mixture was neutralized
with sodium hydrogen carbonate (48 g), the mixture
was concentrated under reduced pressure. After ethyl
acetate (500 mL) and water (700 mL) were added therto
and the mixture was partitioned, the ethyl acetate
layer was washed with water (700 mL) and saturated
brine (700 mL) successively, dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure to give the titled reference
compound (11.6 g) as an orange solid. (Yield 100%)
0 10 1H-NMR (500 MHz, CDC13)
Br NO2
6 3.98 (s, 3H), 6.15 (br
la
s, 2H), 6.78 (d, J = 9.2 H
NH 2 z, 1H), 7.48 (d, J - 9.2 H
z, 1H)
Methyl 6-bromo-3-[(2-ethoxycarbonyl)propan-2-
yl]amino-2-nitrobenzoate (Reference Compound No.1-
(5) )
The mixture of methyl 3-amino-6-bromo-2-
nitrobenzoate (Reference Compound No.1-(4) , 11.6 g,
42.0 mmol), ethyl 2-bromoisobutyrate (60.4 mL, 412
mmol), potassium iodide (7.76 g, 46.2 mmol) and
cesium carbonate (56.1 g, 172 mmol) was stirred at
85 C for 4 days. After the mixture was cooled down,
ethyl acetate (500 mL) and water (500 mL) were added
therto, the mixture was partitioned, and then the
water layer was extracted with ethyl acetate (300
mL). The organic layer was combined, washed with
103
CA 02643070 2008-08-20
=
water (1 L, 2 times) and saturated brine (1 L)
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled reference compound (5.08 g) as an
orange oil. (Yield 31%)
0 0, 1H-NMR (400 MHz, CDC13)
Br si NO2 6 1.22 (t, J = 7.1 Hz, 3H
), 1.65 (s, 6H), 3.98 (s,
N(C) 3H), 4.20 (d, J = 7.1 Hz,
H o 2H), 6.56 (d, J = 9.4 Hz,
1H), 7.49 (d, J = 9.4 Hz,
1H), 8.31 (s, 1H)
7-Bromo-8-methoxycarbony1-3,3-dimethy1-3,4-dihydro-
1H-quinoxalin-2-one (Reference Compound No.1)
Methyl 6-bromo-3-[(2-ethoxycarbonyl)propan-2-
yl]amino-2-nitrobenzoate (Reference Compound No.1-
(5), 105 mg, 0.26 mmol) was dissolved in anhydrous
ethanol (4.5 mL), tin (II) chloride (247 mg, 1.30
mmol) was added thereto, and then the reaction
mixture was refluxed for 5 hours. After the reaction
mixture was cooled down, ethyl acetate (25 mL) was
added thereto, the mixture was neutralized with
aqueous sodium hydrogen carbonate solution, and then
filtered on celite. After the filtrate was
partitioned, the water layer was extracted with ethyl
acetate (10 mL, 2 times), the combined organic layer
was washed with water (50 mL, 2 times) and saturated
104
CA 02643070 2008-08-20
4 =
brine (50 mL) successively, dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled reference compound
(56.3 mg) as a pale yellow solid. (Yield 70%)
1H-NMR (400 MHz, CDC13)
0 0 6 1.39 (s, 6H), 3.86 (s,
Br is NO
1H), 3.98 (s, 3H), 6.62 (d
, J = 8.5 Hz, 1H), 7.13 (d
, J - 8.5 Hz, 1H), 8.89 (s
, 1H)
Reference Example 2
8-Methoxycarbony1-7-(2-methoxypheny1)-3,3-dimethyl-
3,4-dihydro-1H-quinoxalin-2-one (Reference Compound
No. 2-1)
A mixture of 7-bromo-8-methoxycarbony1-3,3-
dimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference
Compound No.1, 203 mg, 0.64 mmol),
2-
methoxyphenylboronic acid (196 mg, 1.28 mmol),
cessium carbonate (629 mg, 1.92 mmol) and
bis(triphenylphosphine)pallladium dichloride
(II)
(45.8 mg, 0.06 mmol) was suspended with anhydrous
N,N-dimethylformamide (3 ml) and stirred at 80 C for
2 days. After the mixture was cooled down, ethyl
acetate (30 mL) and water (30 mL) were added thereto
and the mixture was partitioned. The organic layer
was washed with water (30 mL) and saturated brine (30
mL) successively, dried over anhydrous magnesium
105
CA 02643070 2008-08-20
41
sulfate, and then the solvent was removed under
reduced pressure. The obtained residue was purified
by silica gel column chromatography (hexane-ethyl
acetate) to give the titled reference compound (116
mg) as a pale yellow amorphous product. (Yield 31%)
H-NMR (400 MHz, DMSO-dd
0 0 5 1.56 (s, 6H), 3.50 (s,
a&
NO 3H), 3.72 (s, 3H), 3.84 (s
, 1H), 6.81 (d, J = 8.1 Hz
O , 1H), 6.86 (d, J = 8.1 Hz
, 1H), 6.86 (dd, J = 7.7,
0.9 Hz, 1H), 7.01 (td, J =
7.7, 0.9 Hz, 1H), 7.22 (d
d, J = 7.7, 1.7 Hz, 1H), 7
.28 (td, J = 7.7, 1.7 Hz,
1H), 9.56 (s, 1H)
Using any compounds among Reference Compounds No.1,
18-3, 18-5 and available compounds, the following
Reference Compounds (No.2-2-2-5) were obtained by a
method similar to that of Reference Compound No.2-1.
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-dd
)-8-methoxycarbony1-3,3-dim 6 1.27 (s, 6H), 3.50 (s, 3
ethyl-3,4-dihydro-1H-quinox
H), 3.65 (s, 3H), 6.41 (s,
alin-2-one (Reference Compo
1H), 6.74 (d, J = 8.2 Hz, 1
und No.2-2)
H), 6.78 (td, J = 8.5, 2.4
Hz, 1H), 6.87 (dd, J = 11.3
F 0 0
, 2.4 Hz, 1H), 6.89 (d, J
N,0 8.2 Hz, 1H), 7.14 (dd, J =
8.5, 7.0 Hz, 1H), 9.48 (s,
N.< 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-dd
)-8-methoxycarbony1-3,3-dim 6 1.27 (s, 6H), 3.50 (s, 3
ethyl-3,4-dihydro-1H-quinox
H), 3.61 (s, 3H), 6.46 (s,
alin-2-one (Reference Compo
1H), 6.78 (d, J = 8.2 Hz, 1
und 2-3)
H), 6.90 (d, J = 8.2 Hz, 1H
), 6.94 (dd, J = 8.8, 4.7 H
106
CA 02643070 2008-08-20
= =
z, 1H), 7.00 (dd, J = 9.3,
40 0 0 3.2 Hz, 1H), 7.08 (td, J =
8.8, 3.2 Hz, 1H), 9.49 (s,
ta6
NO 1H)
N
8-Methoxycarbony1-7-(2-meth 1H-NMR (500 MHz, CDC13)
oxy-4-methoxymethoxyphenyl 6 1.42 (br s, 6H), 3.52 (s
)-3,3-dimethy1-3,4-dihydro-
, 3H), 3.54 (s, 3H), 3.70 (
1H-quinoxalin-2-one (Refere
s, 3H), 3.81 (br s, 1H), 5.
nce Compound No.2-4)
21 (s, 2H), 6.57 (d, J - 2.
1 Hz, 1H), 6.69 (dd, J = 8.
20.õ0 0 0
2, 2.1 Hz, 1H), 6.79 (d, J
NO = 8.2 Hz, 1H), 6.82 (d, J
=
8.2 Hz, 1H), 7.12 (d, J =
N 8.2 Hz, 1H), 9.51 (s, 1H)
7-(4-Benzoyloxy-2-methoxyph 1H-NMR (400 MHz, CDC13)
eny1)-8-methoxycarbony1-3, 6 1.41 (br s, 3H), 1.48 (b
3-dimethy1-3,4-dihydro-1H-q
r s, 3H), 3.56 (s, 3H), 3.7
uinoxalin-2-one (Reference
2 (s, 3H), 3.86 (s, 1H), 6.
Compound No.2-5)
76 (d, J = 2.3 Hz, 1H), 6.8
010
o ash" 00 2 (d, J = 8.2 Hz, 1H),
6.87
(d, J = 8.2 Hz, 1H), 6.89
(dd, J = 8.1, 2.3 Hz, 1H),
0 VI NO 7.25 (d, J = 8.1 Hz, 1H),
7
1.0 N)< .52-7.56 (m, 2H), 7.66
(tt,
J = 7.4, 1.4 Hz, 1H), 8.23
(dd, J = 8.2, 1.4 Hz, 2H),
9.60 (s, 1H)
Reference Example 3
8-Hydroxymethy1-7-(2-methoxypheny1)-3,3-dimethyl-3,4-
dihydro-1H-quinoxalin-2-one (Reference Compound No.3-
1)
Lithium aluminium hydride (26.0 mg, 0.64 mmol)
was suspended in anhydrous tetrahydrofuran (0.5 mL)
under nitrogen atmosphere. An
anhydrous
tetrahydrofuran solution (1.5 mL) of
8-
methoxycarbony1-7-(2-methoxypheny1)-3,3-dimethyl-3,4-
dihydro-1H-quinoxalin-2-one (Reference Compound No.2-
107
CA 02643070 2008-08-20
1, 108 mg, 0.32 mmol) was added thereto at 0 C, and
stirred for 3 hours at the same temperature. After
ethyl acetate (3 mL) and water (3 mL) were added
dropwise successively, ethyl acetate (30 mL), water
(30 mL) and 1N aqueous hydro chloride solution (5 mL)
were added thereto, and the mixture was partitioned.
After the water layer was extracted with ethyl
acetate (20 mL), the organic layer was combined. The
organic layer was washed with water (50 mL, 2 times)
and saturated brine (50 mL) successively, dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was purified by silica gel column chromatography
(hexane-ethyl acetate) to give the titled reference
compound (61.0 mg) as a pale yellow amorphous
product. (Yield 61%)
OH H-NMR (400 MHz, DMSO-d6)
010
N 6 1.39 (s, 3H), 1.50 (s,
3H), 3.77 (s, 3H), 4.44 (d
N , J = 6.3 Hz, 2H), 6.69 (d
, J = 8.0 Hz, 1H), 6.75 (d
, J = 8.0 Hz, 1H), 6.97 (d
, J = 8.2 Hz, 1H), 7.04 (t
d, J = 7.4, 1.1 Hz, 1H), 7
.18 (dd, J = 7.4, 1.8 Hz,
1H), 7.34 (ddd, J = 8.2, 7
.4, 1.8 Hz, 1H), 8.57 (br
s, 1H)
Using any compounds among Reference Compounds No.2-
2-2-5, the following Reference Compounds (No.3-2-3-5)
were obtained by a method similar to that of
Reference Compound No.3-1.
108
CA 02643070 2008-08-20
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-dd
)-8-hydroxymethy1-3,3-dimet 6 1.24 (s, 3H), 1.27 (s, 3
hy1-3,4-dihydro-1H-quinoxal
H), 3.70 (s, 3H), 4.18 (dd,
in-2-one (Reference Compoun
J = 12.9, 5.0 Hz, 1H), 4.4
d No.3-2)
4 (dd, J = 12.9, 5.0 Hz, 1H
F 010 OH
H
id& ,0 ), 5.29 (t,
J = 5.0 Hz, 1H)
N
, 6.07 (s, 1H), 6.57 (d, J
= 8.1 Hz, 1H), 6.66 (d, J =
0 IW N 8.1 Hz, 1H),
6.79 (td, J -
H 8.4, 2.5 Hz, 1H), 6.94 (dd
, J = 11.5, 2.5 Hz, 1H), 7.
12 (dd, J = 8.4, 7.1 Hz, 1H
), 9.24 (s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSC-d6)
)-8-hydroxymethy1-3,3-dimet 6 1.25 (s, 3H), 1.28 (s, 3
hy1-3,4-dihydro-1H-quinoxal
H), 3.67 (s, 3H), 4.20 (dd,
in-2-one (Reference Compoun
J = 12.8, 5.3 Hz, 1H), 4.4
d No.3-3)
6 (dd, J = 12.8, 5.3 Hz, 1H
F
), 5.30 (t, J = 5.3 Hz, 1H)
010 OH
H
N,0 , 6.11 (s, 1H), 6.61 (d, J
= 8.1 Hz, 1H), 6.67 (d, J =
8.1 Hz, 1H), 6.97 (dd, J =
0 W N 9.0, 3.2 Hz,
1H), 7.03 (dd
H , J = 8.9, 4.8 Hz, 1H), 7.1
4 (td, J = 8.9, 3.2 Hz, 1H)
, 9.25 (s, 1H)
8-Hydroxymethy1-7-(2-methox 1H-NMR (400 MHz, CDC13)
y-4-methoxymethoxypheny1)- 3 61.38 (s, 3H), 1.49 (s, 3H
,3-dimethy1-3,4-dihydro-1H-
), 2.13 (t, J = 6.9 Hz, 1H)
quinoxalin-2-one (Reference
, 3.53 (s, 3H), 3.75 (s, 4H
Compound No.3-4)
), 4.45 (d, J = 6.9 Hz, 2H)
00 I. OH
H , 5.22 (s, 2H), 6.67 (d, J
N0 = 2.7 Hz,
1H), 6.67 (d, J =
id& ,
8.1 Hz, 1H), 6.72 (dd, J -
ICo IW N 8.0, 2.7 Hz,
1H), 6.72 (d,
H J = 8.0 Hz, 1H), 7.07 (d,
J = 8.1 Hz, 1H), 8.57 (s, 1
H)
7-(4-Hydroxy-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-hydroxymethy1-3,3-dime 6 1.39 (s, 3H), 1.49 (s, 3
thy1-3,4-dihydro-1H-quinoxa
H), 2.07 (t, J = 6.5 Hz, 1H
lin-2-one (Reference Compou
), 3.74 (s, 4H), 4.45 (d, J
nd No.3-5)
= 6.5 Hz, 2H), 4.95 (s, 1H
), 6.48 (dd, J = 7.8, 2.3 H
z, 1H), 6.50 (d, J - 2.3 Hz
, 1H), 6.67 (d, J = 8.1 Hz,
1H), 6.72 (d, J = 8.1 Hz,
109
CA 02643070 2008-08-20
,
..,
HO . OH 1H), 7.01 (d, J = 7.8 Hz, 1
H H), 8.56 (s, 1H)
I.
NO
,0
NX
H
Reference Example 4
8-Chloromethy1-7-(4-fluoro-2-methoxypheny1)-3,3-
dimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference
Compound No.4-1)
7-(4-Fluoro-2-methoxypheny1)-8-hydroxymethyl-
3,3-dimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference Compound No.3-2, 70.0 mg, 0.21mmol) was
dissolved in the mixed solvent of anhydrous
dichloromethane (1 mL) and anhydrous tetrahydrofuran
(1.5 mL) , and triethylamine (35 p L, 0.25 mmol) and
methanesulfonyl chloride (18 p L, 0.23 mmol) were
added thereto successively. The reaction mixture was
stirred at room temperature overnight. Ethyl acetate
(30 mL) and water (30 mL) were added to the reaction
mixture and partitioned. The organic layer was washed
with water (30 mL) and saturated brine (30 mL)
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled reference compound (49.5 mg) as a
pale yellow solid. (Yield 68%)
110
CA 02643070 2008-08-20
F CI
1H-NMR (400 MHz, DMSO-dd
so
id& N,10 5 1.19 (s, 3H), 1.30 (s,
3H), 3.70 (s, 3H), 4.29 (d
N , J = 11.7 Hz, 1H), 4.91 (
d, J = 11.7 Hz, 1H), 6.18
(s, 1H), 6.59 (d, J = 8.1
Hz, 1H), 6.74 (d, J = 8.1
Hz, 1H), 6.82 (td, J - 8.4
, 2.4 Hz, 1H), 6.97 (dd, J
= 11.5, 2.4 Hz, 1H), 7.14
(dd, J = 8.4, 7.1 Hz, 1H)
, 9.77 (s, 1H)
Using any compounds among Reference Compounds No.3-3
and 3-4, the following Reference Compounds (No.4-2
and 4-3) were obtained by a method similar to that of
Reference Compound No.4-1.
8-Chloromethy1-7-(5-fluoro- 1H-NMR (400 MHz, DMSO-d6)
2-methoxypheny1)-3,3-dimeth 6 1.19 (s,
) 1.30 (s, 3
y1-3,4-dihydro-1H-quinoxali
H), 3.67 (s, 3H), 4.31 (d,
n-2-one (Reference Compound
J = 11.7 Hz, 1H), 4.95 (d,
No.4-2)
J = 11.7 Hz, 1H), 6.22 (s,
1H), 6.63 (d, J = 8.1 Hz, 1
CI
id& N,0 H), 6.75 (d, J = 8.1 Hz, 1H
), 6.99 (dd, J = 9.0, 3.2 H
z, 1H), 7.07 (dd, J = 8.9,
N 4.6 Hz, 1H), 7.19 (td, J =
8.9, 3.2 Hz, 1H), 9.80 (s,
1H)
8-Chloromethy1-7-(2-methox 1H-NMR (400 MHz, CDC13)
y-4-methoxymethoxypheny1)-3 6 1.44 (s, 3H), 1.45 (s, 3
,3-dimethy1-3,4-dihydro-1H-
H), 3.53 (s, 3H), 3.74 (s,
quinoxalin-2-one (Reference
4H), 4.42 (s, 2H), 5.22 (s,
Compound No.4-3)
2H), 6.66 (d, J = 2.3 Hz,
00
1H), 6.70 (d, J = 8.1 Hz, 1
id&
NO H), 6.70 (dd, J = 8.3, 2.3
Hz, 1H), 6.77 (d, J = 8.1 H
N z, 1H), 7.11 (d, J = 8.3 Hz
, 1H), 7.85 (br s, 1H)
Reference Example 5
1 1 1
CA 02643070 2008-08-20
7-Bromo-8-hydroxymethy1-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.5)
Litium aluminium hydride (38.5 mg, 1.01 mmol)
was suspended in anhydrous tetrahydrofuran (0.5 mL)
under nitrogen atmosphere. An anhydrous
tetrahydrofuran solution (1.5 mL) of 7-bromo-8-
methoxycarbony1-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.1, 101 mg,
0.323 mmol) was added dropwise thereto at 0 C, and
stirred for 1 hour at the same temperature. Ethyl
acetate (10 mL), water (10 mL), and 1N aqueous
hydrochloride solution (2 mL) were added thereto
successively and the mixture was partitioned. The
organic layer was washed with saturated brine (10
mL), dried over anhydrous magnesium sulfate, and then
the solvent was removed under reduced pressure. The
obtained residue was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled reference compound (67.4 mg) as an orange
amorphous product. (Yield 74%)
OH H-NMR (400 MHz, CDC13)
Br is N,0 6 1.39 (s, 6H), 3.18 (br
s, 1H), 3.75 (s, 1H), 4.99
1\r< (d, J = 9.5
Hz, 2H), 6.51
(d, J = 8.3 Hz, 1H), 7.07
(d, J = 8.3 Hz, 1H), 9.40
(s, 1H)
Reference Example 6
7-Bromo-8-hydroxymethy1-1,3,3-trimethy1-3,4-dihydro-
1 1 2
CA 02643070 2008-08-20
1H-quinoxalin-2-one (Reference Compound No.6)
A mixture of 7-bromo-8-hydroxymethy1-3,3-
dimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.5, 62.7 mg, 0.220 mmol), methyl iodide
(68.6p L, 1.10 mmol), and cessium carbonate (180 mg,
0.552 mmol) was suspended in anhydrous N,N-
dimethylformamide (1 mL) and stirred at room
temperature for 2.5 hours. Ethyl acetate (10 mL) and
water (10 mL) were added to the reaction mixture and
partitioned. The organic layer was washed with
saturated brine (10 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled reference compound
(45.5 mg) as an orange amorphous product. (Yield 69%)
OH H-NMR (400 MHz, CDC13)
Br O
6 1.31 (s, 6H), 3.56 (s,
i N
3H), 3.77 (br s, 1H), 4.73
s
1\r< (d, J = 7.1
Hz, 2H), 6.57
(d, J = 8.4 Hz, 1H), 7.17
(d, J = 8.4 Hz, 1H)
Reference Example 7
7-Bromo-8-chloromethy1-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one (Reference Compound No.7)
7-Bromo-8-hydroxymethy1-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Reference Compound No.6,
37.5 mg, 0.125 mmol I ) was dissolved in anhydrous
dichloromethane (1 mL), and triethylamine (20.9 p L,
1 1 3
CA 02643070 2008-08-20
0.150 mmol) and methanesulfonyl chloride (10.7 p L,
0.138 mmol) were added thereto successively. The
reaction mixture was stirred at room temperature
overnight. Ethyl acetate (10 mL) and water (10 mL)
were added to the reaction mixture and partitioned.
The organic layer was washed with saturated brine (10
mL), dried over anhydrous magnesium sulfate, and then
the solvent was removed under reduced pressure. The
obtained residue was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled reference compound (28.7 mg) as an orange
amorphous product. (Yield 72%)
CI 1H-NMR (400 MHz, CDC13)
Br 410NO 6 1.30 (s, 6H), 3.55 (s,
3H), 3.76 (br s, 1H), 4.76
Nr< (s, 2H), 6.61
(d, J = 8.4
Hz, 1H), 7.23 (d, J = 8.4
Hz, 1H)
Reference Example 8
7-Bromo-8-(5-fluoro-2-methylphenoxymethyl)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.8-1)
A mixture of 7-bromo-8-chloromethy1-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.7, 801mg, 2.52 mmol), 5-fluoro-2-
methylphenol (330 p L, 3.02 mmol), and potassium
carbonate (524 mg, 3.79 mmol) was suspended in
anhydrous N,N-dimethylformamide (10 mL) and stirred
at 80 C overnight. After cooling down, ethyl acetate
114
CA 02643070 2008-08-20
,.
(80 mL) and water (50 mL) were added to the reaction
mixture and partitioned. The organic layer was washed
with saturated brine (50 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled reference compound
(945 mg) as a colorless solid. (Yield 92%)
F 1 40 H-NMR (400 MHz, CDC13)
6 1.24 (s, 6H), 2.13 (s,
3H), 3.41 (s, 3H), 3.78 (b
0 r s, 1H), 5.16 (s, 2H), 6.
I
Br40 NO54-6.57 (m, 1H), 6.58 (d,
J = 9.5 Hz, 1H), 6.62 (d,
Nr< J = 8.5 Hz, 1H), 7.05 (t,
H J - 7.6 Hz, 1H), 7.23 (d,
J = 8.5 Hz, 1H)
Using any compounds among Reference Compounds No.7
and available compounds, the following Reference
Compounds (No.8-2-8-4) were obtained by a method
similar to that of Reference Compound No.8-1.
7-Bromo-8-(2-methylphenoxym 1H-NMR (400 MHz, CDC13)
ethyl)-1,3,3-trimethy1-3,4- 6 1.23 (s, 6H), 2.20 (s, 3
dihydro-1H-quinoxalin-2-one
H), 3.43 (s, 3H), 3.76 (br
(Reference Compound No.8-2
s, 1H), 5.18 (s, 2H), 6.61
)
(d, J - 8.4 Hz, 1H), 6.83 (
010 d, J = 8.1 Hz, 1H), 6.86 (t
, J = 7.2 Hz, 1H), 7.12-7.1
6 (m, 1H), 7.23 (d, J = 8.4
0
I Hz, 1H), 7.26-7.27 (m, 1H)
Br 40 NO
I\1<
H
7-Bromo-8-(2-methoxyphenyla 1H-NMR (400 MHz, CDC13)
1 1 5
CA 02643070 2008-08-20
minomethyl)-1,3,3-trimethy 6 1.29 (s, 6H), 3.50 (s, 3
1-3,4-dihydro-1H-quinoxali
H), 3.74 (s, 1H), 3.84 (s,
n-2-one (Reference Compound
3H), 4.30 (d, J = 5.6 Hz, 2
No.8-3)
H), 4.73 (t, J = 5.6 Hz, 1H
0 ), 6.57 (d, J = 8.3 Hz, 1H)
, 6.67 (dd, J = 7.8, 1.5 Hz
, 1H), 6.72 (td, J = 7.8, 1
NH .5 Hz, 1H), 6.80 (dd, J = 7
Br NO .8, 1.5 Hz, 1H), 6.89 (td,
J = 7.8, 1.5 Hz, 1H), 7.21
Nr< (d, J = 8.3 Hz, 1H)
7-Bromo-8-(5-fluoro-2-methy 1H-NMR (500 MHz, CDC13)
lphenylaminomethyl)-1,3,3-t 6 1.30 (s, 6H), 2.11 (s, 3
rimethy1-3,4-dihydro-1H-qui
H), 3.47 (s, 3H), 3.78 (s,
noxalin-2-one (Reference Co
1H), 4.12 (br s, 1H), 4.30
mpound No.8-4)
(d, J = 5.5 Hz, 2H), 6.35-6
F
.40 (m, 2H), 6.60 (d, J = 8
.6 Hz, 1H), 6.98 (t, J = 7.
2 Hz, 1H), 7.22 (d, J = 8.6
NH Hz, 1H)
Br Nio N,0
Reference Example No.9
7-Bromo-8-methoxycarbony1-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Reference Compound No.9)
A mixture of 7-bromo-8-methoxycarbony1-3,3-
dimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.1, 102 mg, 0.326 mmol), methyl iodide
(100 p L, 1.60 mmol), and cessium carbonate (272 mg,
0.835 mmol) was suspended in anhydrous N,N-
dimethylformamide (5 mL) and stirred at room
temperature for 2 hours. Ethyl acetate (25 mL) and
water (25 mL) were added to the reaction mixture and
partitioned. The organic layer was washed with
saturated brine (20 mL), dried over anhydrous
1 1 6
CA 02643070 2008-08-20
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled reference compound
(86.0 mg) as a pale yellow solid. (Yield 83%)
H-NMR (500 MHz, CDC13)
0 0 6 1.34 (s, 6H),
3.27 (s,
Br 40 3H), 3.85 (br
s, 1H), 3.95
(s, 3H), 6.64 (d, J = 8.2
1\r< Hz, 1H), 7.15
(d, J = 8.2
Hz, 1H)
Reference Example 10
7-(5-Chloro-2-methoxypheny1)-8-methoxycarbony1-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.10-1)
A mixture of 7-bromo-8-methoxycarbony1-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.9, 3.75 g, 11.5 mmol), 5-chloro-2-
methoxyphenylboronic acid (2.57 g, 13.8 mmol),
cessium carbonate (7.49 g, 23.0 mmol) and
tetrakis(triphenylphosphine)pallladium (0) (1.33 g,
1.16 mmol) was suspended in anhydrous N,N-
dimethylformamide (70 ml) and stirred at 80 C
overnight under argon atmosphere. After cooling down,
ethyl acetate (300mL), diethylether (150 mL) and
water (400 mL) were added and partitioned. The
organic layer was washed with water (250 mL) and
saturated brine (150 mL) successively, dried over
anhydrous magnesium sulfate, and then the solvent was
1 1 7
CA 02643070 2008-08-20
removed under reduced pressure. The obtained residue
was purified by silica gel column chromatography
(hexane-ethyl acetate) to give the titled reference
compound (3.84 g) as a colorless amorphous product.
(Yield 86%)
CI H-NMR (400 MHz, CDC13)
00 6 1.39 (s, 3H), 1.41 (s,
id& N,0 3H), 3.21 (s, 3H), 3.61 (s
, 3H), 3.72 (s, 3H), 3.91
O N (s, 1H), 6.80 (d, J = 8.1
Hz, 1H), 6.82 (d, J = 8.6
Hz, 1H), 6.87 (d, J = 8.1
Hz, 1H), 7.14 (d, J = 2.5
Hz, 1H), 7.25 (dd, J = 8.6
, 2.5 Hz, 1H)
Using any compounds among Reference Compounds No.9
and available compounds, the following Reference
Compound (No.10-2) was obtained by a method similar
to that of Reference Compound No.10-1.
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-methoxycarbony1-1,3,3-t ö 1.21 (s, 3H), 1.25 (s, 3
rimethy1-3,4-dihydro-1H-qui
H), 3.02 (s, 3H), 3.54 (s,
noxalin-2-one (Reference Co
3H), 3.67 (s, 3H), 6.45 (s
mpound No.10-2)
, 1H), 6.76 (td, J = 8.3, 2
.5 Hz, 1H), 6.79 (d, J = 8.
F 0 0
3 Hz, 1H), 6.90 (dd, J = 11
N,0 .4, 2.5 Hz,
1H), 6.91 (d, J
= 8.3 Hz, 1H), 7.04 (dd, J
231 N = 8.3, 7.1 Hz, 1H)
Reference Example 11
9-Chloro-2 , 2 , 4-trimethy1-1 , 4-dihydro-2 H -6-oxa-1 , 4-
diazachrysen-3 , 5-dione (Reference Compound No.11-1)
118
CA 02643070 2008-08-20
7-(5-Chloro-2-methoxypheny1)-8-methoxycarbonY1-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference Compound No.10-1, 3.81 g, 9.80 mmol) was
dissolved in anhydrous dichloromethane (30 mL), boron
tribromide (7.62 g, 30.4 mmol) was added thereto at -
78 C, and then stirred at room temperature for 1
hour. The reaction mixture was poured into ice water
(500 mL), ethyl acetate (500 mL) was added thereto
and partitioned. The organic layer was washed with
saturated brine (200 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
dissolved in N,N-dimethylformamide (50 mL), 60%
sodium hydride (23.1 mg, 0.578 mmol) was added
thereto, and then stirred at 70 C overnight. 60%
sodium hydride (31.2 mg, 0.780 mmol) was added more
thereto and stirred at 80 C overnight. After cooling
down, ethyl acetate (200 mL), diethylether (200 mL)
and water (300 mL) were added and partitioned. The
organic layer was washed with water (200 mL) and
saturated brine (200 mL) successively, dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was filtered with ethyl acetate/hexane (1/4, 30 mL)
to give the titled reference compound (2.04 g) as a
pale yellow solid. (Yield 61%)
1 1 9
CA 02643070 2008-08-20
0 0 1 H-NMR (400 MHz, CDC13)
N,,0 6 1.40 (s, 6H), 3.33 (s,
CI 3H), 4.25 (br s, 1H), 7.17
V< (d, J = 8.5
Hz, 1H), 7.25
(d, J = 8.4 Hz, 1H), 7.34
(dd, J = 8.4, 2.4 Hz, 1H)
, 7.68 (d, J = 8.5 Hz, 1H)
, 7.89 (d, J = 2.4 Hz, 1H)
Using Reference Compound No.10-2, the following
Reference Compound (No.11-2) was obtained by a method
similar to that of Reference Compound No.11-1.
8-Fluoro-2 , 2 , 4-trimethyl-
1H-NMR (400 MHz, DMSO-d6)
6 1.23 (s, 6H), 3.15 (s,
1, 4-dihydro-2 H -6-oxa-1, 4-
3H), 6.97 (s, 1H), 7.23 (t
diazachrysen-3 , 5-dione (R d, J = 8.9, 2.6 Hz, 1H), 7
eference Compound No.11-2) .32 (dd, J = 8.3, 2.6 Hz,
F 0 0 1H), 7.34 (d, J = 8.5 Hz,
1H), 7.97 (d, J = 8.5 Hz,
NO 1H), 8.23 (dd, J = 8.9, 6.
V< 1 Hz, 1H)
Reference Example 12
7-(5-Chloro-2-hydroxypheny1)-8-hydroxymethy1-1,3,3-
trimethy1-3,4-dihydro-1H-guinoxalin-2-one (Reference
Compound No.12-1)
Lithium aluminium hydride (442 mg, 11.7 mmol)
was suspended in anhydrous tetrahydrofuran (10 mL)
under nitrogen atmosphere. An anhydrous
tetrahydrofuran solution (40 mL) of 9-chloro-2,2,4-
trimethy1-1,4-dihydro-2 H -6-oxa-1,4-diazachrysen-3,5-
dione (Reference Compound No.11-1, 1.99 g, 5.81 mmol)
was added dropwise thereto at -10 C and stirred for
120
CA 02643070 2008-08-20
minutes at the same temperature. After ethyl
acetate (1 mL) and water (1 mL) were added thereto
successively, ethyl acetate (300 mL) and saturated
brine (300 mL) were added and partitioned. The
organic layer was washed with saturated brine (150
mL), dried over anhydrous magnesium sulfate, and then
the solvent was removed under reduced pressure. The
obtained residue was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled reference compound (1.38 g) as a pale yellow
solid. (Yield 69%)
CI 1H-NMR (400 MHz, CDC13)
SiOH
I
ilah 1\10 5 1.24 (s, 3H), 1.45 (s, 3
H), 2.25 (br s, 1H), 3.62
(s, 3H), 3.85 (s, 1H), 4.4
OH qp NXZ 6 (d, J = 11.5 Hz, 1H), 4.
H 57 (dd, J = 11.5, 3.8 Hz,
1H), 5.92 (br s, 1H), 6.78
(d, J = 7.8 Hz, 1H), 6.84
(d, J = 7.8 Hz, 1H), 6.96
(d, J = 8.4 Hz, 1H), 7.14
(d, J = 2.4 Hz, 1H), 7.26
(dd, J = 8.4, 2.4 Hz, 1H)
Using Reference Compound No.11-2, the following
Reference Compound (No.12-2) was obtained by a method
similar to that of Reference Compound No.12-1.
7-(4-fluoro2-hydroxyphenyl 1H-NMR (400 MHz, DMSO-dd
)-8-hydroxymethy1-1,3,3-tri 6 1.17 (s, 6H), 3.32 (s, 3
methyl-3,4-dihydro-1H-quino
H), 4.12-4.69 (m, 3H), 6.02
xalin-2-one (Reference Comp
ound No.12-2) (s, 1H),
6.60-6.70 (m, 2H)
, 6.68 (d, J = 8.1 Hz, 1H),
6.74 (d, J = 8.1 Hz, 1H),
7.12 (t, J = 7.8 Hz, 1H), 9
.76 (s, 1H)
121
CA 02643070 2008-08-20
F OH
dab N,0
OH Rip N
Reference Example 13
7-(5-Chloro-2-methoxypheny1)-8-hydroxymethy1-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.13-1)
A mixture of 7-(5-chloro-2-hydroxypheny1)-8-
hydroxymethy1-1,3,3-trimethyl-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.12-1, 1.36 g,
3.92 mmol), methyl iodide (244 p L, 3.92 mmol), and
potassium carbonate (1.08 g, 7.81 mmol) was suspended
in anhydrous N,N-dimethylformamide (20 mL) and
stirred at 50 C for 1 hour. After cooling down, ethyl
acetate (70 mL), diethylether (70 mL), and water (150
mL) were added and partitioned. The organic layer was
washed with water (100 mL) and saturated brine (50
mL) successively, dried over anhydrous magnesium
sulfate, and then the solvent was removed under
reduced pressure. The obtained residue was purified
by silica gel column chromatography (hexane-ethyl
acetate) to give the titled reference compound (1.36
g) as a pale yellow amorphous product. (Yield 96%)
122
CA 02643070 2008-08-20
CI 1 H-NMR (400 MHz, CDC13)
el OH
I
N ,C) 5 1.21 (s, 3H), 1.47 (s,
3H), 2.79 (dd, J = 9.0, 3.
3 Hz, 1H), 3.65 (s, 3H), 3
0 IW N .77 (s, 1H), 3.78 (s, 3H),
H 4.35 (dd, J = 12.5, 3.3 H
z, 1H), 4.45 (dd, J = 12.5
, 9.0 Hz, 1H), 6.73 (d, J
= 7.9 Hz, 1H), 6.78 (d, J
= 7.9 Hz, 1H), 6.95 (d, J
= 8.8 Hz, 1H), 7.17 (d, J
= 2.6 Hz, 1H), 7.34 (dd, J
= 8.8, 2.6 Hz, 1H)
Using Reference Compound No.12-2, the following
Reference Compound (No.13-2) was obtained by a method
similar to that of Reference Compound No.13-1.
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-dd
)-8-hydroxymethy1-1,3,3-tri 6 1.12 (s, 3H), 1.23 (s, 3
methyl-3,4-dihydro-1H-quino
H), 3.36 (s, 3H), 3.72 (s,
xalin-2-one (Reference Comp
3H), 4.17 (d, J = 11.9 Hz,
ound No.13-2)
1H), 4.47 (dd, J = 11.9, 5.
F 410OH
I 0 Hz, 1H), 4.60 (t, J = 5.0
NO Hz, 1H), 6.04 (s, 1H), 6.6
7 (d, J = 7.9 Hz, 1H), 6.73
0 IW N (d, J = 7.9 Hz, 1H), 6.80
H (td, J = 8.4, 2.4 Hz, 1H),
6.94 (dd, J = 11.6, 2.4 Hz,
1H), 7.20 (dd, J = 8.4, 7.
0 Hz, 1H)
Reference Example 14
7-(5-Chloro-2-methoxypheny1)-8-chloromethy1-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Reference
Compound No.14-1)
7-(5-Chloro-2-methoxypheny1)-8-hydroxymethyl-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference Compound No.13-1, 1.34 g, 3.71 mmol) was
123
CA 02643070 2008-08-20
,
dissolved in anhydrous dichloromethane (19 mL), and
triethylamine (621 p L, 4.46 mmol) and methanesulfonyl
chloride (316 p L, 4.08 mmol) were added thereto
successively. The reaction mixture was stirred at
room temperature overnight. Ethyl acetate (200 mL)
was added to the reaction mixture, washed with water
(200 mL), dried over anhydrous magnesium sulfate, and
then the solvent was removed under reduced pressure.
The obtained residue was purified by silica gel
column chromatography (hexane-ethyl acetate) to give
the titled reference compound (1.14 g) as a colorless
amorphous product. (Yield 81%)
CI 'H-NMR (500 MHz, CDC13)
leiCI
I
id& NO 6 1.27 (s, 3H), 1.41 (s,
3H), 3.52 (s, 3H), 3.74 (s
, 3H), 3.80 (s, 1H), 4.45
0 IW N (d, J = 12.5 Hz, 1H), 4.66
H (d, J = 12.5 Hz, 1H), 6.7
(d, J - 8.1 Hz, 1H), 6.8
1 (d, J = 8.1 Hz, 1H), 6.8
8 (d, J = 8.9 Hz, 1H), 7.2
4 (d, J - 2.7 Hz, 1H), 7.3
2 (dd, J = 8.9, 2.7 Hz, 1H
)
Using Reference Compound No.13-2, the following
Reference Compound (No.14-2) was obtained by a method
similar to that of Reference Compound No.14-1.
8-Chloromethy1-7-(4-fluor 1H-NMR (400 MHz, DMSO-d6)
o-2-methoxypheny1)-1,3,3-t 6 1.11 (s, 3H), 1.24 (s, 3H
rimethy1-3,4-dihydro-1H-gu
), 3.36 (s, 3H), 3.74 (s, 3H
inoxalin-2-one (Reference
), 4.44 (d, J = 12.8 Hz, 1H)
Compound No.14-2)
, 4.77 (d, J - 12.8 Hz, 1H),
6.24 (s, 1H), 6.74 (d, J =
124
CA 02643070 2008-08-20
F 0 CI 8.1 Hz, 1H),
6.84 (d, J = 8.
I 1 Hz, 1H),
6.85 (td, J = 8.4
40 NO , 2.5 Hz,
1H), 6.99 (dd, J =
(-_)
N 11.3, 2.5
Hz, 1H), 7.21 (dd
, J = 8.4, 7.1 Hz, 1H)
H
Reference Example 15
4-Benzoyloxyanisole (Reference Compound No.15-1)
4-Hydoxyanisole (1.25 g, 10.1 mmol) was
dissolved in anhydrous dichloromethane (10 mL), and
triethylamine (4.25 mL, 30.5 mmol) and benzoyl
chloride (1.40 mL, 12.1 mmol) were added thereto
successively. The reaction mixture was stirred at
room temperature for 4 hours. Chloroform (50 mL) and
water (50 mL) were added to the reaction mixture and
partitioned. The organic layer was washed with
saturated brine (50 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
filtered with hexane (50 mL) to give the titled
reference compound (2.24 g) as a colorless solid.
(Yield 98%)
1 H-NMR (400 MHz, DMSO-d6)
010 6 3.78 (s,
3H), 7.01 (d, J
= 9.1 Hz, 2H), 7.21 (d, J
0 0 = 9.1 Hz, 2H),
7.61 (t, J
= 7.8 Hz, 2H), 7.75 (t, J
010 = 7.8 Hz, 11-
I), 8.13 (d, J
= 7.8 Hz, 2H)
0
Using Reference Compound No.16-3, the following
125
CA 02643070 2008-08-20
Reference Compound (No.15-2) was obtained by a method
similar to that of Reference Compound No.15-1.
5-Benzoyloxy-2-iodoanisole 1H-NMR (400 MHz, CDC13)
(Reference Compound No.1 6 3.89 (s, 3H), 6.65 (dd,
5-2)
J = 8.5, 2.4 Hz, 1H), 6.73
(d, J = 2.4 Hz, 1H), 7.5
010 0 0-7.54 (m, 2H), 7.63-7.68
(m, 1H), 7.80 (d, J = 8.5
0 40
I Hz, 1H), 8.18-8.21 (m, 2H)
Reference Example 16
2-Bromo-4-chloro-5-fluoroaisole (Reference Compound
No. 16-1)
A mixture of 4-chloro-3-fluoroanisole (124 p L,
1.00 mmol) and N-bromosuccinimide was dissolved in
mixed solvent of anhydrous N,N-dimethylformamide (0.5
mL) and anhydrous dichloromethane (1 mL), and stirred
at 40 C for 3 days. After cooling down, chloroform
(30 mL) and water (30 mL) were added and partitioned.
The water layer was extracted with chloroform (30 mL,
2 times). The combined organic layer was washed with
saturated brine (30 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled reference compound
(195 mg) as a colorless solid. (Yield 82%)
1 2 6
CA 02643070 2008-08-20
CI 1H-NMR (500 MHz, DMSO-dd
F 40 6 3.88 (s, 3H), 7.32 (d,
J = 11.3 Hz, 1H), 7.87 (d,
Br J = 8.2 Hz, 1H)
Using any compounds among Reference Compounds No.15-1
and available compounds, the following Reference
Compounds (No.16-2-16-3) were obtained by a method
similar to that of Reference Compound No.16-1.
4-Benzoyloxy-2-bromoanisol 1H-NMR (400 MHz, DMSO-d6)
e (Reference Compound No.1 a 3.88 (s, 3H), 7.19 (d,
6-2)
J = 9.0 Hz, 1H), 7.32 (dd,
410J = 9.0, 2.8 Hz, 1H), 7.5
9-7.63 (m, 3H), 7.75 (tt,
J = 7.8, 1.5 Hz, 1H), 8.12
0 0 (dt, J = 7.8, 1.5 Hz, 2H)
401 Br
2'00
5-Hydroxy-2-iodoanisole (R 1H-NMR (400 MHz, CDC13)
eference Compound No.16-3) 6 3.85 (s, 3H), 4.82 (s,
HO is
1H), 6.25 (dd, J = 8.4, 2.
7 Hz, 1H), 6.40 (d, J = 2.
I 7 Hz, 1H), 7.56 (d, J = 8.
0 4 Hz, 1H)
Reference Example 17
5-Chloro-4-fluoro-2-methoxyphenylboronic acid
(Reference Compound No.17)
2-Bromo-4-chloro-5-fluoroanisole (Reference
Compound No.16-1, 239 mg, 1.00 mmol) was dissolved in
mixed solvent of anhydrous toluene (2 mL) and
anhydrous tetrahydrofuran (0.5 mL), 1.6M hexane
solution of n-butyl lithium (750 p L, 1.20 mmol) was
1 2 7
= . CA 02643070 2008-10-10
25088-306
added thereto at -40 C, and then the reaction mixture
was stirred at the same temperature for 30 minutes.
Triisopropyl boronic acid (277 p L, 1.20 mmol) was
added dropwise to the reaction mixture, warmed to -
20 C over 10 minutes, and then 2N aqueous HC1
solution (1 mL) was added. After the reaction mixture
was stirred at room temperature for 20 minutes, ethyl
acetate (20 mL) and water (20 mL) were added and
partitioned. The organic layer was washed with
saturated brine (20 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure to give the titled reference
compound (181 mg) as a colorless solid. (Yield 89%)
CI H-NMR (400 MHz, DMSO-d0
F
B _OH 6 3.81 (s, 3H), 7.10 (d,
J = 12.2 Hz, 1H), 7.57 (d,
OH J = 9.5 Hz, 1H), 7.86 (br
O S, 1H), 8.45 (br s, 1H)
Reference Example 18
5-Nitro-2-methoxyphenylboronic acid (Reference Compound
No. 18-1)
A mixture of 2-Bromo-4-nitroanisole (100 mg,
0.431 mmol), bis (neopentylglycolate) diborane (151 mg,
0.668 mmol), potassium acetate (129 mg, 1.31 mmol),
and [ 1, l' -bis (diphenylphosphino) ferrocene] palladium (
)dichloride dichloromethane complex (1 : 1) (35.5 mg,
0.0435 mmol) was suspended in dimethylsulfoxide (3
mL), and the reaction mixture was stirred at 80 C for
minutes under microwave. After cooling down, ethyl
1 2 8
CA 02643070 2008-08-20
acetate (30 mL) and water (30 mL) were added to the
reaction mixture and partitioned. The organic layer
was washed with saturated brine (30 mL), dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure to give the titled
reference compound (72.5 mg) as a yellow solid.
(Yield 85%)
0 1H-NMR (500 MHz, DMSO-dd
'N*0
6 3.89 (s, 3H), 7.35 (d,
J = 9.4 Hz, 1H), 8.12 (d,
B.OH J = 2.7 Hz, 1H), 8.34 (dd,
J = 9.4, 2.7 Hz, 1H)
,O OH
Using any compounds among Reference Compounds No.15-
2, 16-2, 23 and available compounds, the following
Reference Compounds (No.18-2-18-7) were obtained by a
method similar to that of Reference Compound No.18-1.
2-(5,5-Dimethyl[1, 3, 2]clio H-NMR (500 MHz, DMSO-dd
xaborinan-2-y1)-5-nitroanis 6 0.98 (s, 6H), 3.47 (s, 4
ole (Reference Compound No. H), 3.86 (s, 3H), 7.67 (d,
18-2) J = 1.9 Hz,
1H), 7.69 (d, J
= 8.0 Hz, 1H), 7.77 (dd, J
0
-N+ = 8.0, 1.9 Hz, 1H)
0' 401
4 -Benz oyl oxy- 2 -me t hox ypheny 1H-NMR (500 MHz, CDC13)
lboronic acid (Reference Co 3.93 (s,
3H), 5.63 (s, 2
mpound No.18-3)
H), 6.83 (d, J = 2.1 Hz, 1H
), 6.91 (dd, J = 8.0, 2.1 H
z, 1H), 7.53 (t, J = 7.9 Hz
, 2H), 7.64-7.67 (m, 1H),
7.91 (d, J = 8.0 Hz, 1H), 8
.20-8.22 (m, 2H)
129
CA 02643070 2008-08-20
SO ,..,
0 IW B0H
,0 OH
5-Benzoyloxy-2-methoxypheny 1H-NMR (400 MHz, CDC13)
lboronic acid (Reference Co 6 3.94 (s, 3H), 5.92 (s, 2
mpound No.18-4)
H), 6.96 (d, J = 8.9 Hz, 1H
S), 7.30 (dd, J = 8.9, 3.0 H
z, 1H), 7.51 (t, J = 7.8 Hz
, 2H), 7.63 (tt, J = 7.8, 1
0 0 .5 Hz, 1H), 7.66 (d, J = 3.
0 Hz, 1H), 8.20 (dd, J = 7.
8, 1.5 Hz, 2H)
S B0H
,O OH
2-Methoxy-4-methoxymethoxyp 1H-NMR (400 MHz, CDC13)
henylboronic acid (Referenc 6 3.49 (s, 3H), 3.90 (s, 3
e Compound No.18-5)
H), 5.21 (s, 2H), 5.58 (s,
0,.,0 40 2H), 6.60 (d, J = 2.0 Hz, 1
B.OH H), 6.70 (dd, J = 8.2, 2.0
Hz, 1H), 7.75 (d, J = 8.2 H
,O OH z, 1H)
2-Methoxy-4-nitrophenylboro 1H-NMR (500 MHz, CDC13)
nic acid (Reference Compoun 6 4.03 (s, 3H), 5.68 (s, 2
d No.18-6)
H), 7.75 (d, J = 1.8 Hz, 1H
0 ), 7.89 (dd, J - 8.1, 1.8 H
,W- z, 1H), 8.03 (d, J = 8.1 Hz
0- S, 1H)
B0H
,O OH
1 H-NMR (400 MHz, CDC13)
2-(5, 5-Dimethyl[1, 3, 2] di
oxaborinan -2-y1)-4-nitroan 6 1.06 (s, 6H), 3.81 (s, 4
isole (Reference Compound H), 3.94 (s, 3H), 6.91 (d,
No.18-7) J = 9.2 Hz, 1H), 8.26 (dd,
J = 9.2, 3.0 Hz, 1H), 8.55
0--N-0 (d, J = 3.0 Hz, 1H)
11101 B.0,
,O 6,\
Reference Example 19
1 3 0
CA 02643070 2008-08-20
5-Cyano-2-trifluoromethylsulfonyloxyanisole
(Reference Compound No.19-1)
A mixture of 5-cyano-2-hydroxyanisole (600 mg,
4.02 mmol) and triethylamine (1.40 mL, 10.0 mmol) was
dissolved in anhydrous tetrahydrofuran (20 mL) under
argon atomosphere. Trifluoromethanesulfonyl chloride
(642 p L, 6.03 mmol) were added thereto at -10 C, and
stirred at the same temperature for 1 hour. Ethyl
acetate (100 mL) and water (100 mL) were added to the
reaction mixture and partitioned. The organic layer
was washed with saturated brine (50 mL), dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was purified by silica gel column chromatography
(hexane-ethyl acetate) to give the titled reference
compound (979 mg) as a colorless solid. (Yield 87%)
NC 1H-NMR (400 MHz, DMSO-dd
= 0õ0
:S' F 6 3.97 (s, 3H), 7.61 (dd,
0 J = 8.4, 1.9 Hz, 1H), 7.7
2 (d, J = 8.4 Hz, 1H), 7.9
2 (d, J = 1.9 Hz, 1H)
Using available compounds, the following Reference
Compounds (No.19-2-19-4) were obtained by a method
similar to that of Reference Compound No.19-1.
5-Acetyl-2-trifluoromethyls 1H-NMR (400 MHz, CDC13)
ulfonyloxyanisole (Referenc 5 2.62 (s, 3H), 3.99 (s, 3
e Compound No.19-2)
H), 7.32 (d, J = 8.5 Hz, 1H
), 7.57 (dd, J - 8.5, 2.0 H
z, 1H), 7.66 (d, J = 2.0 Hz
, 1H)
1 3 1
CA 02643070 2008-08-20
0
0 õO
:S F
0
5-Methyl-2-trifluoromethyls 1H-NMR (400 MHz, CDC13)
ulfonyloxyanisole (Referenc 6 2.37 (s, 3H), 3.89 (s, 3
e Compound No.19-3)
H), 6.75-6.77 (m, 1H), 6.83
0õ0 (d, J = 1.7
Hz, 1H), 7.08
0 F (d, J = 8.3 Hz, 1H)
,0
4-Methoxycarbony1-2-trifluo 1H-NMR (400 MHz, CDC13)
romethylsulfonyloxyanisole 6 3.91 (s, 3H), 3.98 (s, 3
(Reference Compound No.19-4
H), 7.07 (d, J - 8.6 Hz, 1H
), 7.89 (d, J = 2.1 Hz, 1H)
0 , 8.05 (dd, J = 8.6, 2.1 Hz
0
, 1H)
0õ0
F
Reference Example 20
5-Cyano-2-(4 , 4 , 5, 5-tetramethyl[1, 3, 2]dioxaborolan-
2-yl)anisole (Reference Compound No.20-1)
A mixture of 5-cyano-2-
trifluoromethylsulfonyloxyanisole (Reference Compound
No.19-1, 200 mg, 0.711 mmol), bis(pinacolato)diboron
(200 mg, 0.788 mmol), potassium acetate (213 mg, 2.17
mmol), and [1,1'-bis(diphenylphosphino)ferrocene]
(20.0 mg, 0.0361 mmol), and [1,1'-
bis(diphenylphosphino)ferrocene]palladium( II
)dichloride dichloromethane complex (1 : 1) (29.4 mg,
0.0360 mmol) was suspended in 1,4-dioxane (4 mL), and
the reaction mixture was stirred at 80 C overnight.
After cooling down, ethyl acetate (100 mL) and water
1 3 2
CA 02643070 2008-08-20
(100 mL) were added and partitioned. The organic
layer was washed with saturated brine (50 mL), dried
over anhydrous magnesium sulfate, and then the
solvent was removed under reduced pressure. The
obtained residue was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled reference compound (123 mg) as a colorless
solid. (Yield 67%)
NC H-NMR (500 MHz, CDC13)
DO 6 1.36 (s, 12H), 3.86 (s,
/ 3H), 7.06 (s, 1H), 7.23 (d
0 0 , J = 7.5 Hz, 1H), 7.72 (d
, J = 7.5 Hz, 1H)
Using Reference Compound No.19-4, the following
Reference Compound (No.20-2) was obtained by a method
similar to that of Reference Compound No.20-1.
H-NMR (500 MHz, CDC13)
4 -Methoxycarbonyl- 2 - (4 , 4
6 1.36 (s, 12H), 3.88 (s,
, 5, 5-tetramethyl [1, 3 , 2]
3H), 3.89 (s, 3H), 6.88 (
dioxaborolan -2-yl)anisole
d, J = 8.8 Hz, 1H), 8.10 (
(Reference Compound No.2
dd, J - 8.8, 2.4 Hz, 1H),
0-2)
8.34 (d, J = 2.4 Hz, 1H)
00
010 0
,0
Reference Example 21
7-(5 , 5-Dimethyl[1 , 3 , 2]dioxaborinan-2-y1)-8-(5-
fluoro-2-methylphenoxymethyl)-1,3,3-trimethy1-3,4-
133
CA 02643070 2008-08-20
dihydro-1H-quinoxalin-2-one(ReferenceCompound21)
A mixture of 7-bromo-8-(5-
fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.8-1, 98.7 mg,
0.242 mmol), bis(neopentyl glycolate)diboron (170 mg,
0.753 mmol), potassium acetate (112 mg, 1.14 mmol),
and [1,1'-bis(diphenylphosphino)ferrocene]palladium(II
)dichloride dichloromethane complex (1 : 1) (20.7 mg,
0.0253 mmol) was suspended in dimethylsulfoxide (2
mL), and the reaction mixture was stirred at 80 C for
15 minutes under microwave. After cooling down, ethyl
acetate (15 mL) and water (15 mL) were added to the
reaction mixture and partitioned. The organic layer
was washed with saturated brine (15 mL), dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was purified by silica gel column chromatography (1st
: hexane-ethyl acetate, 2nd : chloroform). The
obtained residue was filtered with hexane (5 ml) to
give the titled reference compound (70.2 mg) as a
colorless solid. (Yield 65%)
F H-NMR (400 MHz, CDC13)
6 0.95 (s, 6H), 1.25 (s,
6H), 2.08 (s, 3H), 3.38 (s
o 0
, 3H), 3.64 (s, 4H), 3.86
(s, 1H), 5.36 (s, 2H), 6.5
c) -B
2 (td, J = 8.3, 2.4 Hz, 1H
1\(< ), 6.59 (dd, J = 11.2, 2.4
Hz, 1H), 6.69 (d, J = 7.8
Hz, 1H), 7.01 (t, J = 7.6
Hz, 1H), 7.47 (d, J = 7.8
Hz, 1H)
1 3 4
CA 02643070 2008-08-20
,
Reference Example 22
8-(5-Fluoro-2-methylphenoxymethyl)-7-(4,4,5,5-
tetramethyl[1,3,2]dioxaborolan-2-y1)-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one (Reference Compound
No. 22)
A mixture of 7-
bromo-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.8-1, 101 mg,
0.248 mmol), bis(pinacolato)diboron (156 mg, 0.614
mmol), potassium acetate (75.5 mg, 0.769 mmol), 1,1'-
bis(diphenylphosphino)ferrocene (7.2 mg, 0.013 mmol),
and [1,1'-bis(diphenylphosphino)ferrocene]palladium(H
)dichloride dichloromethane complex (1 : 1) (10.7 mg,
0.0131 mmol) was suspended in 1,4-dioxane (2 mL), and
the reaction mixture was stirred at 80 C overnight.
After cooling down, ethyl acetate (15 mL) and water
(15 mL) were added and partitioned. The organic layer
was washed with saturated brine (15 mL), dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was purified by silica gel column chromatography (1st
: hexane-ethyl acetate, 2nd : chloroform-methanol) to
give the titled reference compound (87.9 mg) as a
colorless amorphous product. (Yield 78%)
1 3 5
CA 02643070 2008-08-20
F 1H-NMR (500 MHz, CDC13)
6 1.22 (s, 12H), 1.25 (s,
6H), 2.07 (s, 3H), 3.41 (
0 s, 3H), 3.92
(s, 1H), 5.37
-B N..0 (s, 2H), 6.50-
6.57 (m, 1H
0 40), 6.64 (dd,
J = 11.0, 2.4
1\(< Hz, 1H), 6.70
(d, J - 7.6
Hz, 1H), 6.94-7.05 (m, 1H
), 7.53 (d, J - 7.9 Hz, 1H
Reference Example 23
2-Bromo-5-methoxymethoxyanisole (Reference Compound
No. 23)
A mixture of 4-bromo-3-methoxyphenol (500 mg,
2.46 mmol), chlorodimethylether (281p L, 3.70 mmol),
and potassium carbonate (850 mg, 6.15 mmol) was
suspended in anhydrous N,N-dimethylformamide (8 mL)
and stirred at 50 C for 1 hour. After cooling down,
the reaction mixture was diluted with ethyl acetate
(150 mL). The mixture was washed with water (150 mL)
and saturated brine (50 mL) successively, dried over
anhydrous magnesium sulfate, and then the solvent was
removed under reduced pressure. The obtained residue
was purified by silica gel column chromatography
(hexane-ethyl acetate) to give the titled reference
compound (421 mg) as a coloreless oil. (Yield 69%)
00 1
H-NMR (500 MHz, CDC13)
6 3.48 (s, 3H), 3.87 (s, 3
Br H), 5.16 (s,
2H), 6.56 (dd
0 J = 8.8, 2.7
Hz, 1H), 6.
63 (d, J = 2.7 Hz, 1H), 7.
40 (d, J = 8.8 Hz, 1H)
1 3 6
CA 02643070 2008-08-20
,
Reference Example 24
8-Hydroxymethy1-7-[2-methoxy-4-(2-
methylbenzoyloxy)pheny1]-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.24)
7-(4-Hydroxy-2-methoxypheny1)-8-hydroxymethyl-
3,3-dimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference Compound No.3-5, 430 mg. 1.31 mmol) was
dissolved in tetrahydrofuran (10 mL) , and
triethylamine (365 p L. 2.62 mmol) and 2-methylbenzoyl
chloride (222 p L, 1.70 mmol) were added thereto
successively. After the reaction mixture was stirred
at the same temperature for 80 minutes, the reaction
mixture was diluted with ethyl acetate (200 mL). The
mixture was washed with water (100 mL) and saturated
brine (100 mL) successively, dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled reference compound
(362 mg) as a colorless solid. (Yield 62%)
1 H-NMR (400 MHz, CDC13)
010 0 OH 6 1.40 (s, 3H), 1.50 (s,
H 3H), 2.06 (t, J = 4.8 Hz,
0 el NO 1H), 2.70 (s, 3H), 3.78 (s
0 IW N , 1H), 3.79 (s, 3H), 4.49
(d, J = 4.8 Hz, 2H), 6.70
H
(d, J = 8.1 Hz, 1H), 6.76
(d, J = 8.1 Hz, 1H), 6.84
(d, J = 2.2 Hz, 1H), 6.90
(dd, J = 8.2, 2.2 Hz, 1H),
7.22 (d, J = 8.2 Hz, 1H),
7.34 (d, J = 7.6 Hz, 1H),
7.35 (t, J = 7.6 Hz, 1H),
137
CA 02643070 2008-08-20
,
7.49-7.53 (m, 1H), 8.19 (
d, J = 7.6 Hz, 1H), 8.59 (
s, 1H)
Reference Example 25
5-Bromothiophene-2-carbonylchloride
(Reference
Compound No.25)
A mixture of 5-bromothiophene-2-carboxylic acid
(300 mg, 1.45 mmol), thionyl chloride (423 p L, 5.80
mmol), and N,N-dimethylformamide (1 drop) was
dissolved in chloroform (3 mL) , and refluxed for 1
hour. After cooling down, the reaction mixture was
concentrated under reduced pressure to give the
titled reference compound (324 mg) as a pale yellow
solid. (Yield 99%)
CI 1H-NMR (400 MHz, CDC13)
0
l 6
7.19 (d, J = 4.2 Hz, 1H)
s
Br , 7.74 (d, J = 4.2 Hz, 1H)
[Examples]
Example 1
8-Benzoyloxymethy1-7-(2-methoxypheny1)-3,3-dimethyl-
3,4-dihydro-1H-quinoxalin-2-one (Compound No.1-1)
8-Hydroxymethy1-7-(2-methoxypheny1)-3,3-
dimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference
Compound No.3-1, 54.2 mg, 0.17 mmol) was dissolved in
tetrahydrofuran (1.5 mL) and
triethylamine (73.0 p L
, 0.52 mmol) and benzoyl chloride (30.0 p L, 0.26
mmol) were added thereto successively. The reaction
mixture was stirred at room temperature for 24 hours.
138
CA 02643070 2008-08-20
Ethyl acetate (30 mL) and water (30 mL) were added to
the reaction mixture and partitioned. The organic
layer was washed with water (30 mL) and saturated
brine (30 mL) successively, dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
filtered with ethyl acetate to give the titled
compound (54.1 mg) as a colorless solid. (Yield 76%)
H-NMR (500 MHz, DMSO-d6)
0 41$ 6 1.25 (s, 3H), 1.30 (s,
3H), 3.60 (s, 3H), 4.97 (d
010 0
.4& ,0 , J = 12.5 Hz, 1H), 5.19
N (
d, J = 12.5 Hz, 1H), 6.20
(s, 1H), 6.66 (d, J = 7.9
N Hz, 1H), 6.80 (d, J - 7.9
Hz, 1H), 6.94 (td, J = 7.5
, 1.0 Hz, 1H), 7.03 (d, J
= 8.2 Hz, 1H), 7.10 (dd, J
= 7.5, 1.7 Hz, 1H), 7.31
(ddd, J - 8.2, 7.5, 1.7 Hz
, 1H), 7.48 (t, J = 7.8 Hz
, 2H), 7.61 (t, J = 7.8 Hz
, 1H), 7.81 (d, J = 7.8 Hz
, 2H), 9.90 (s, 1H)
Using any compounds among Reference Compounds No.3-2,
3-3, 24, and 25, the following Compounds (No.1-2-1-7)
were obtained by a method similar to that of Compound
No.1-1.
8-Benzoyloxymethy1-7-(4-flu 1H-NMR (400 MHz, DMSO-d6)
oro-2-methoxypheny1)-3,3-di 5 1.24 (s, 3H), 1.30 (s, 3
methyl-3,4-dihydro-1H-quino
H), 3.60 (s, 3H), 4.96 (d,
xalin-2-one (Compound No.1-
2) J = 12.4 Hz, 1H), 5.17 (d,
J = 12.4 Hz, 1H), 6.19 (s,
1H), 6.64 (d, J = 8.1 Hz, 1
H), 6.74 (td, J = 8.4, 2.5
1 3 9
CA 02643070 2008-08-20
Hz, 1H), 6.78 (d, J = 8.1 H
0 0 z, 1H), 6.93 (dd, J = 11.5,
2.5 Hz, 1H), 7.11 (dd, J =
F 0
8.4, 7.1 Hz, 11-1), 7.48 (t,
J - 7.8 Hz, 2H), 7.62 (t,
NO J = 7.8 Hz, 1H), 7.81 (d, J
O N = 7.8 Hz, 2H), 9.92 (s, 1H
8-Benzoyloxymethy1-7-(5-flu 1H-NMR (400 MHz, DMSO-d6)
oro-2-methoxypheny1)-3,3-di 6 1.23 (s, 3H), 1.30 (s, 3
methyl-3,4-dihydro-1H-quino
H), 3.58 (s, 3H), 4.98 (d,
xalin-2-one (Compound No.1-
J = 12.5 Hz, 1H), 5.21 (d,
3)
J = 12.5 Hz, 1H), 6.23 (s,
0 010 1H), 6.68 (d, J = 8.1 Hz, 1
H), 6.79 (d, J = 8.1 Hz, 1H
), 6.96 (dd, J = 9.0, 3.2 H
410 0
id& 11,40 z, 1H), 7.02 (dd, J = 8.9,
4.6 Hz, 1H), 7.13 (td, J
8.9, 3.2 Hz, 1H), 7.48 (t,
N J = 7.4 Hz, 2H), 7.62 (t, J
= 7.4 Hz, 1H), 7.81 (d, J
= 7.4 Hz, 2H), 9.94 (s, 1H)
7-[2-Methoxy-4-(2-methylben 1H-NMR (400 MHz, CDC13)
zoyloxy)pheny1]-8-(4-methox 5 1.46 (s, 6H), 2.71 (s, 3
ybenzoyloxymethyl)-3,3-dime
H), 3.69 (s, 3H), 3.80 (s,
thy1-3,4-dihydro-1H-quinoxa
1H), 3.85 (s, 3H), 5.02 (d,
lin-2-one (Compound No.1-4)
J - 12.8 Hz, 1H), 5.33 (d,
010 0
J = 12.8 Hz, 1H), 6.74 (d,
00 0 0
0 J = 8.2 Hz, 1H), 6.82 (d,
J = 2.2 Hz, 1H), 6.83 (d, J
= 8.2 Hz, 1H), 6.89 (d, J
OSHN0 - 9.0 Hz, 2H), 6.90 (dd, J
- 8.1, 2.2 Hz, 1H), 7.26 (d
,0 N , J - 8.1 Hz, 1H), 7.35 (t,
J = 8.0 Hz, 1H), 7.36 (d,
J = 8.0 Hz, 1H), 7.48-7.52
(m, 1H), 7.93 (d, J = 9.0 H
z, 2H), 8.19 (d, J = 8.0 Hz
, 1H), 8.65 (s, 1H)
7-[2-Methoxy-4-(2-methylben 1H-NMR (400 MHz, CDC13)
zoyloxy)pheny1]-8-(4-methyl 6 1.45 (s, 6H), 2.39 (s, 3
benzoyloxymethyl)-3,3-dimet
H), 2.71 (s, 3H), 3.68 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.81 (s, 1H), 5.03 (d,
in-2-one (Compound No.1-5)
J = 12.8 Hz, 1H), 5.34 (d,
J = 12.8 Hz, 1H), 6.75 (d,
J = 8.2 Hz, 1H), 6.82 (d,
J = 2.0 Hz, 1H), 6.83 (d, J
= 8.2 Hz, 1H), 6.90 (dd, J
140
CA 02643070 2008-08-20
<
0 ill
= 8.2, 2.0 Hz, 1H), 7.21 (
d, J = 8.2 Hz, 2H), 7.26 (d
010 0 0
, J = 8.2 Hz, 1H), 7.34 (d,
J = 7.9 Hz, 1H), 7.35 (t,
H J = 7.9 Hz, 1H), 7.49-7.52
0 el NO
(m, 1H), 7.87 (d, J = 8.2 H
0 =N
z, 2H), 8.19 (d, J - 7.9 Hz
, 1H), 8.59 (s, 11-I)
H
8-(3-Fluorobenzoyloxymethyl 1H-NMR (500 MHz, CDC13)
)-7-[2-methoxy-4-(2-methylb 6 1.46 (s, 3H), 1.46 (s, 3
enzoyloxy)pheny1]-3,3-dimet
H), 2.71 (s, 3H), 3.69 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.82 (s, 1H), 5.07 (d,
in-2-one (Compound No.1-6)
J = 12.7 Hz, 1H), 5.35 (d,
0 010 F
J = 12.7 Hz, 1H), 6.76 (d,
J = 8.2 Hz, 1H), 6.83 (d,
010 0
J = 2.1 Hz, 1H), 6.83 (d, J
= 8.2 Hz, 1H), 6.91 (dd, J
H
0 lei0N,0
= 8.2, 2.1 Hz, 1H), 7.23-7
.29 (m, 2H), 7.33-7.37 (m,
0 IW N
2H), 7.40 (td, J = 7.8, 5.6
H Hz, 1H), 7.49-7.52 (m, 1H)
, 7.63-7.66 (m, 1H), 7.77 (
d, J = 7.8 Hz, 1H), 8.19 (d
, J - 7.6 Hz, 1H), 8.49 (s,
1H)
8-(5-Bromothiophen-2-ylcarb 1H-NMR (400 MHz, CDC13)
onyloxymethyl)-7-[2-methox 6 1.45 (s, 3H), 1.46 (s, 3H
y-4-(2-methylbenzoyloxy)phe ), 2.71 (s, 3H), 3.71 (s, 3
ny1]-3,3-dimethy1-3,4-dihyd H), 3.81 (s, 1H), 5.01 (d,
ro-1H-quinoxalin-2-one (Corn J = 12.7 Hz, 1H), 5.30 (d,
pound No.1-7)
J = 12.7 Hz, 1H), 6.75 (d,
J = 8.1 Hz, 1H), 6.82 (d, J
010 0 0 j-----Br
S = 8.1 Hz, 1H), 6.83 (d, J
- 2.2 Hz, 1H), 6.91 (dd, J
0
= 8.1, 2.2 Hz, 1H), 7.06 (d
ft, J - 3.9 Hz, 1H), 7.25 (d,
0 010 id& 0
0 IW N
J = 7.7 Hz, 1H), 7.35 (t, J
J = 8.1 Hz, 1H), 7.34 (d,
H - 7.7 Hz, 1H), 7.49-7.52 (
m, 1H), 7.52 (d, J = 3.9 Hz
, 1H), 8.19 (d, J = 7.7 Hz,
1H), 8.47 (s, 1H)
Example 2
7-(5-Fluoro-2-methoxypheny1)-8-(5-methylthiophen-2-
ylcarbonyloxymethyl)-3,3-dimethy1-3,4-dihydro-1H-
1 4 1
CA 02643070 2008-08-20
quinoxalin-2-one (Compound No.2-1)
8-Chloromethy1-7-(5-fluoro-2-methoxypheny1)-
3,3-dimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Reference Compound No.4-2, 50.9 mg, 0.14 mmol), 5-
methy1-2-thiophenecarboxylyc acid (62.5 mg, 0.44
mmol), and potassium carbonate (79.9 mg, 0.58 mmol)
were suspended in anhydrous N,N-dimethylformamide
(1.5 mL) and stirred at 80 C for 4.5 hours. Ethyl
acetate (30 mL) and water (30 mL) were added to the
reaction mixture and partitioned. The organic layer
was washed with water (30 mL) and saturated brine (30
mL) successively, dried over anhydrous magnesium
sulfate, and then the solvent was removed under
reduced pressure. The obtained residue was purified
by silica gel column chromatography (hexane-ethyl
acetate) to give the titled compound (55.0 mg) as a
colorless solid. (Yield 85%)
H-NMR (400 MHz, DMSO-dd
6 1.23 (s, 3H), 1.30 (s,
0
N 3H), 2.47 (s,
3H), 3.60 (s
, 3H), 4.90 (d, J = 12.5 H
z, 1H), 5.17 (d, J - 12.5
o N Hz, 1H), 6.22
(s, 1H), 6.6
7 (d, J = 8.1 Hz, 1H), 6.7
8 (d, J = 8.1 Hz, 1H), 6.8
8 (d, J = 3.7 Hz, 1H), 6.9
4 (dd, J - 9.0, 3.2 Hz, 1H
), 7.02 (dd, J = 8.9, 4.6
Hz, 1H), 7.14 (td, J = 8.9
, 3.2 Hz, 1H), 7.46 (d, J
= 3.7 Hz, 1H), 9.86 (s, 1H
Using any compounds among Reference Compounds No.4-
1 4 2
CA 02643070 2008-08-20
1-4-3, 14-1, 14-2, and available compounds, the
following Compounds (No.2-2-2-19) were obtained by a
method similar to that of Compound No.2-1.
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(4-methylbenzoyloxymeth 6 1.23 (s, 3H), 1.30 (s, 3
y1)-3,3-dimethy1-3,4-dihydr
H), 2.35 (s, 3H), 3.58 (s,
o-1H-quinoxalin-2-one (Comp
3H), 4.95 (d, J = 12.5 Hz,
ound No.2-2)
1H), 5.19 (d, J = 12.5 Hz,
0 41$ 1H), 6.23 (s, 1H), 6.67 (d,
J = 8.1 Hz, 1H), 6.78 (d,
F J = 8.1 Hz, 1H), 6.95 (dd,
,o
H
.46 N,0 J --= 9.0, 3.2 Hz, 1H), 7.01
(dd, J = 9.0, 4.8 Hz, 1H),
7.13 (td, J = 9.0, 3.2 Hz,
IW N 1H), 7.27 (d, J = 8.1 Hz, 2
H H), 7.70 (d, J = 8.1 Hz, 2H
), 9.92 (s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-d6)
)-8-(3-methylbenzoyloxymeth 6 1.23 (s, 3H), 1.31 (s, 3H
y1)-3,3-dimethy1-3,4-dihydr
), 2.32 (s, 3H), 3.59 (s, 3
o-1H-quinoxalin-2-one (Comp
H), 4.97 (d, J = 12.5 Hz, 1
ound No.2-3)
H), 5.21 (d, J = 12.5 Hz, 1
0 Oil H), 6.23 (s, 1H), 6.68 (d,
J = 8.1 Hz, 1H), 6.79 (d, J
F = 8.1 Hz,
1H), 6.96 (dd, J
010 0
H
id& N,0 d = 9.0, 3.2 Hz, 1H), 7.02 (
d, J = 8.8, 4.5 Hz, 1H), 7
.13 (td, J = 8.8, 3.2 Hz, 1
0 IW N H), 7.36 (t, J = 7.8 Hz, 1H
H ), 7.43 (d, J = 7.8 Hz, 1H)
, 7.60-7.61 (m, 2H), 9.94 (
s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2-methylbenzoyloxymeth 6 1.45 (s, 6H), 2.55 (s, 3H
y1)-3,3-dimethy1-3,4-dihydr
), 3.65 (s, 3H), 3.82 (s, 1
o-1H-quinoxalin-2-one (Comp
H), 4.99 (d, J = 12.8 Hz, 1
ound No.2-4)
H), 5.32 (d, J = 12.8 Hz, 1
0 010 H), 6.74 (d, J = 8.1 Hz, 1H
), 6.80 (d, J = 8.1 Hz, 1H)
F
, 6.86 (dd, J = 8.8, 4.4 Hz
!o
H
,0 , 1H), 6.95 (dd, J - 8.8, 3
N
.2 Hz, 1H), 7.04 (td, J = 8
.8, 3.2 Hz, 1H), 7.20-7.23
IW N (m, 2H), 7.37-7.41 (m, 1H),
H 7.84 (dd, J
= 8.3, 1.4 Hz,
1 4 3
CA 02643070 2008-08-20
1H), 8.51 (s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-d6)
)-8-(thiophen-2-ylcarbonylo 61.23 (s, 3H), 1.30 (s, 3H
xymethyl)-3,3-dimethy1-3,4-
), 3.60 (s, 3H), 4.93 (d, J
dihydro-1H-quinoxalin-2-one
= 12.5 Hz, 1H), 5.20 (d, J
(Compound No.2-5)
= 12.5 Hz, 1H), 6.22 (s, 1
H), 6.67 (d, J = 8.2 Hz, 1H
), 6.79 (d, J = 8.2 Hz, 1H)
010 0
, 6.95 (dd, J = 9.1, 3.2 Hz
, 1H), 7.03 (dd, J = 8.9, 4
NO .6 Hz, 1H), 7.14 (td, J = 8
O N .9, 3.2 Hz, 1H), 7.17 (dd,
J = 4.9, 3.7 Hz, 1H), 7.65
(dd, J = 3.7, 1.3 Hz, 1H),
7.90 (dd, J = 4.9, 1.3 Hz,
1H), 9.89 (s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(4-methoxybenzoyloxymet 6 1.23 (s, 3H), 1.30 (s, 3
hyl)-3,3-dimethy1-3,4-dihyd
H), 3.58 (s, 3H), 3.81 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 4.94 (d, J = 12.6 Hz,
pound No.2-6)
1H), 5.18 (d, J = 12.6 Hz,
0 0,
1H), 6.22 (s, 1H), 6.67 (d,
0 J = 8.1 Hz, 1H), 6.78 (d,
J = 8.1 Hz, 1H), 6.95 (dd,
411 0
N,10 J = 9.0, 3.2 Hz, 1H), 7.00
(d, J = 9.0 Hz, 2H), 7.00-7
.03 (m, 1H), 7.13 (td, J =
O N 8.6, 3.2 Hz, 1H), 7.76 (d,
J = 9.0 Hz, 2H), 9.90 (s, 1
H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(4-methoxybenzoyloxymet 6 1.20 (s, 3H), 1.42 (s, 3
hyl)-1,3,3-trimethy1-3,4-di
H), 3.47 (s, 3H), 3.74 (s,
hydro-1H-quinoxalin-2-one (
3H), 3.79 (s, 1H), 3.83 (s,
Compound No.2-7)
3H), 5.14 (d, J - 13.4 Hz,
010 0,
1H), 5.28 (d, J = 13.4 Hz,
0 1H), 6.63-6.68 (m, 1H), 6.
64 (d, J = 9.3 Hz, 1H), 6.7
F 0 6 (d, J = 7.8 Hz, 1H), 6.84
(d, J = 9.0 Hz, 2H), 6.82-
id& N,0
6.86 (m, 1H), 7.21 (t, J =
N 7.5 Hz, 1H), 7.78 (d, J = 9
.0 Hz, 2H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(thiophen-2-ylcarbonylo 6 1.20 (s, 3H), 1.42 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 3.46 (s, 3H), 3.75 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.79 (s, 1H), 5.14 (d,
one (Compound No.2-8)
J = 13.3 Hz, 1H), 5.28 (d,
144
CA 02643070 2008-08-20
J = 13.3 Hz. 1H), 6.65-6.6
o 9 (m, 2H),
6.76 (d, J = 8.1
Hz, 1H), 6.85 (d, J = 8.1
F 0 Hz, 1H),
7.04 (dd, J = 5.0,
3.8 Hz, 1H), 7.20 (dd, J =-
id&
NO
8.5, 6.5 Hz, 1H), 7.50 (dd
N , J = 5.0, 1.2 Hz, 1H), 7.6
3 (dd, J = 3.8, 1.2 Hz, 1H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-d6)
)-8-(5-methylthiophen-2-ylc 6 1.02 (s, 3H), 1.24 (s, 3
arbonyloxymethyl)-1,3,3-tri
H), 2.46 (s, 3H), 3.30 (s,
methyl-3,4-dihydro-1H-quino
3H), 3.72 (s, 3H), 5.04 (d,
xalin-2-one (Compound No.2-
J = 13.4 Hz, 1H), 5.21 (d,
9)
J = 13.4 Hz, 1H), 6.22 (s,
1H), 6.76 (d, J = 7.9 Hz,
1H), 6.78 (td, J = 8.4, 2.4
F 0 Hz, 1H), 6.83
(d, J = 7.9
Hz, 1H), 6.87 (d, J = 3.7 H
gd&
NO z, 1H), 6.96
(dd, J = 11.3,
N 2.4 Hz, 1H),
7.23 (dd, J =
8.4, 7.2 Hz, 1H), 7.38 (d,
J = 3.7 Hz, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(4-methoxybenzoyloxymet 6 1.02 (s, 3H), 1.25 (s, 3
hyl)-1,3,3-trimethy1-3,4-di
H), 3.32 (s, 3H), 3.71 (s,
hydro-1H-quinoxalin-2-one (
3H), 3.80 (s, 3H), 5.09 (d,
Compound No.2-10)
J = 13.4 Hz, 1H), 5.25 (d,
00 0,
J = 13.4 Hz, 1H), 6.28 (s,
0 1H), 6.81 (d,
J = 8.1 Hz,
CI 1H), 6.84
(d, J = 8.1 Hz, 1
010 0
id& N,0 H), 6.96 (dt,
J = 9.0, 2.5
Hz, 2H), 7.07 (d, J = 8.9 H
z, 1H), 7.26 (d, J = 2.7 Hz
N , 1H), 7.36 (dd, J = 8.9, 2
.7 Hz, 1H), 7.66 (dt, J = 9
.0, 2.5 Hz, 2H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(thiophen-2-ylcarbonylo 6 1.23 (s, 3H), 1.42 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 3.45 (s, 3H), 3.75 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.82 (s, 1H), 5.14 (d,
one (Compound No.2-11)
J = 13.3 Hz, 1H), 5.30 (d,
J = 13.3 Hz, 1H), 6.77 (d,
CI J = 8.1 Hz,
1H), 6.83-6.87
N
410 0 0
4s, , (m, 1H), 6.86 (d, J = 8.1
Hz, 1H), 7.04 (dd, J = 5.0,
3.7 Hz, 1H), 7.23-7.29 (m,
N2H), 7.51 (dd, J = 5.0, 1.
2 Hz, 1H), 7.63 (dd, J = 3.
7, 1.2 Hz, 1H)
1 4 5
CA 02643070 2008-08-20
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-methylthiophen-2-ylc 6 1.24 (s, 3H), 1.41 (s, 3
arbonyloxymethyl)-1,3,3-tri
H), 2.48 (s, 3H), 3.44 (s,
methyl-3,4-dihydro-1H-quino
3H), 3.75 (s, 3H), 3.81 (s,
xalin-2-one (Compound No.2-
1H), 5.09 (d, J = 13.2 Hz,
12)
1H), 5.27 (d, J = 13.2 Hz,
1H), 6.70 (d, J = 3.8 Hz,
Cly"; 1H), 6.76 (d, J - 8.0 Hz, 1
CI
0
ti& N,0 H), 6.83-6.87 (m, 1H), 6.86
(d, J = 8.0 Hz, 1H), 7.23-
7.26 (m, 2H), 7.43 (d, J
S
N 3.8 Hz, 1H)
7-(2-Methoxy-4-methoxymetho 1H-NMR (500 MHz, CDC13)
xypheny1)-8-(5-methylthioph 6 1.44 (s, 6H), 2.51 (s, 3
en-2-ylcarbonyloxymethyl)-3
H), 3.53 (s, 3H), 3.68 (s,
,3-dimethy1-3,4-dihydro-1H-
3H), 3.76 (s, 1H), 4.98 (d,
quinoxalin-2-one (Compound
J = 12.5 Hz, 1H), 5.22 (d,
No.2-13)
J = 6.7 Hz, 1H), 5.23 (d,
J - 6.7 Hz, 1H), 5.26 (d, J
= 12.5 Hz, 1H), 6.64 (d, J
0,0 0= 2.3 Hz,
1H), 6.71 (dd, J
= 8.2, 2.3 Hz, 1H), 6.72 (
001 d, J = 8.1 Hz, 1H), 6.74 (d
o N , J = 3.7
Hz, 1H), 6.79 (d,
J = 8.1 Hz, 1H), 7.11 (d,
J = 8.2 Hz, 1H), 7.58 (d, J
= 3.7 Hz, 1H), 8.48 (s, 1H
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-chlorothiophen-2-ylc 6 1.25 (s, 3H), 1.42 (s, 3
arbonyloxymethyl)-1,3,3-tri
H), 3.43 (s, 3H), 3.75 (s,
methyl-3,4-dihydro-1H-quino
3H), 3.83 (s, 1H), 5.12 (d,
xalin-2-one (Compound No.2-
J = 13.1 Hz, 1H), 5.28 (d,
14)
J = 13.1 Hz, 1H), 6.77 (d,
J = 8.3 Hz, 1H), 6.85 (d,
0
J = 8.3 Hz, 1H), 6.86 (d, J
0
N,0 = 7.6 Hz, 1H), 6.87 (d, J
Olt
= 4.0 Hz, 1H), 7.22-7.27 (m
, 2H), 7.40 (d, J = 4.0 Hz,
N 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-ethylthiophen-2-ylca 6 1.25 (s, 3H), 1.29 (t, J
rbonyloxymethyl)-1,3,3-trim
= 7.6 Hz, 3H), 1.41 (s, 3H
ethyl-3,4-dihydro-1H-quinox
), 2.83 (q, J - 7.6 Hz, 2H)
alin-2-one (Compound No.2-1
, 3.44 (s, 3H), 3.75 (s, 3H
1 4 6
CA 02643070 2008-08-20
5) ), 3.81 (s, 1H), 5.09 (d, J
= 13.2 Hz, 1H), 5.28 (d, J
I \
0 = 13.2 Hz,
1H), 6.74 (d, J
CI = 3.7 Hz, 1H), 6.76 (d, J
410 0
= 8.1 Hz, 1H), 6.84 (d, J =
9.5 Hz, 1H), 6.86 (d, J =
8.1 Hz, 1H), 7.23-7.26 (m,
N 2H), 7.46 (d, J = 3.7 Hz, 1
H)
8-(5-Bromothiophen-2-ylcarb 1H-NMR (400 MHz, CDC13)
onyloxymethyl)-7-(4-fluoro- 6 1.23 (s, 3H), 1.42 (s, 3
2-methoxypheny1)-1,3,3-trim
H), 3.44 (s, 3H), 3.75 (s,
ethyl-3,4-dihydro-1H-quinox
3H), 3.80 (s, 1H), 5.11 (d,
alin-2-one (Compound No.2-1
J = 13.2 Hz, 1H), 5.27 (d,
6)
J = 13.2 Hz, 1H), 6.64-6.7
0 (m, 2H), 6.76 (d, J = 8.1
0
Hz, 1H), 6.84 (d, J = 8.1
F 0 Hz, 1H),
7.01 (d, J - 3.9 H
z, 1H), 7.17-7.21 (m, 1H),
id& N,0 7.37 (d, J = 3.9 Hz, 1H)
N
8-(4-Chlorobenzoyloxymethyl 1H-NMR (500 MHz, CDC13)
)-7-(5-chloro-2-methoxyphen 6 1.23 (s, 3H), 1.41 (s, 3
y1)-1,3,3-trimethy1-3,4-dih
H), 3.44 (s, 3H), 3.74 (s,
ydro-1H-quinoxalin-2-one (C
3H), 3.82 (s, 1H), 5.16 (d,
ompound No.2-17)
J = 13.3 Hz, 1H), 5.34 (d,
0 010 CI
J = 13.3 Hz, 1H), 6.77 (d,
J = 8.0 Hz, 1H), 6.83 (d,
CI J = 9.4 Hz,
1H), 6.87 (d, J
010 0
14& N,0 = 8.0 Hz,
1H), 7.22-7.26 (
m, 2H), 7.35 (d, J = 8.9 Hz
, 2H), 7.75 (d, J = 8.9 Hz,
N 2H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-methoxyfuran-2-ylcar 6 1.25 (s, 3H),
1.40 (s, 3
bonyloxymethyl)-1,3,3-trime
H), 3.43 (s, 3H), 3.75 (s,
thy1-3,4-dihydro-1H-quinoxa
3H), 3.80 (s, 1H), 3.90 (s,
lin-2-one (Compound No.2-18
3H), 5.07 (d, J = 13.3 Hz,
1H), 5.23 (d, J = 13.3 Hz,
1H), 5.26 (d, J = 3.7 Hz,
1H), 6.75 (d, J = 7.9 Hz, 1
H), 6.82-6.75 (m, 1H), 6.84
(d, J = 7.9 Hz, 1H), 6.93
(d, J = 3.7 Hz, 1H), 7.24-7
.26 (m, 2H)
147
CA 02643070 2008-08-20
CI
So
N
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-dd
)-8-(5-methylthiophen-2-ylc 6 1.24 (s, 3H), 1.30 (s, 3
arbonyloxymethyl)-3,3-dimet
H), 2.48 (s, 3H), 3.63 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 488 (d, J = 12.5 Hz,
in-2-one (Compound No.2-19)
1H), 5..13 (d, J = 12.5 Hz,
1H), 6.19 (s, 1H), 6.63 (d,
J = 8.1 Hz, 1H), 6.77 (td,
F 0
ea& NO J = 8.4, 2.4
Hz, 1H), 6.78
(d, J = 8.1 Hz, 1H), 6.89
(d, J = 3.8 Hz, 1H), 6.94 (
N dd, J = 11.5, 2.4 Hz, 1H),
7.10 (dd, J = 8.4, 7.1 Hz,
1H), 7.47 (d, J = 3.8 Hz, 1
H), 9.84 (s, 1H)
Example 3
7-(4-Fluoro-2-methoxypheny1)-8-phenoxymethy1-3,3-
dimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No.3-1)
A mixture of 8-chloromethy1-7-(4-fluoro-2-
methoxypheny1)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.4-1, 47.1 mg,
0.14 mmol), phenol (37.5 mg, 0.40 mmol), and
potassium carbonate (73.0 mg, 0.53 mmol) was
suspended in anhydrous N,N-dimethylformamide (1.5 mL)
and stirred at 80 C for 19 hours. After cooling down,
ethyl acetate (30 mL) and water (30 mL) were added to
the reaction mixture and partitioned. The organic
layer was washed with water (30 mL) and saturated
brine (30 mL) successively, dried over anhydrous
148
CA 02643070 2008-08-20
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled compound (35.7 mg)
as a pale yellow solid. (Yield 67%)
H-NMR (400 MHz, DMSO-dd
1110 6 1.18 (s, 3H), 1.27 (s,
3H), 3.68 (s, 3H), 4.62 (d
F 0
SN,0 , J = 11.5 Hz, 1H), 5.11 (
d, J = 11.5 Hz, 1H), 6.14
(s, 1H), 6.63 (d, J = 8.1
O N Hz, 1H), 6.74 (d, J = 8.1
Hz, 1H), 6.71-6.77 (m, 3H)
, 6.87 (t, J = 7.3 Hz, 1H)
, 6.93 (dd, J = 11.4, 2.6
Hz, 1H), 7.12 (dd, J = 8.3
, 7.1 Hz, 1H), 7.19 (dd, J
= 8.5, 7.3 Hz, 2H), 9.50
(s, 1H)
Using any compounds among Reference Compounds No.4-2,
14-1, 14-2, and available compounds, the following
Compounds (No.3-2-3-19) were obtained by a method
similar to that of Compound No.3-1.
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-phenoxymethy1-3,3-dimet 6 1.19 (s, 3H), 1.28 (s, 3
hy1-3,4-dihydro-1H-quinoxal
H), 3.65 (s, 3H), 4.62 (d,
in-2-one (Compound No.3-2)
J = 11.5 Hz, 1H), 5.15 (d,
410 J - 11.5 Hz, 1H), 6.18 (s,
1H), 6.67 (d, J = 8.1 Hz, 1
H), 6.73 (d, J = 8.8 Hz, 2H
010 0
), 6.75 (d, J = 8.1 Hz, 1H)
, 6.87 (t, J = 7.3 Hz, 1H),
6.94 (dd, J = 9.3, 3.2 Hz,
N 1H), 7.01 (dd, J = 8.9, 4.
9 Hz, 1H), 7.11 (td, J = 8.
9, 3.2 Hz, 1H), 7.19 (dd, J
= 8.8, 7.3 Hz, 2H), 9.56 (
1 4 9
CA 02643070 2008-08-20
,
s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-d0
)-8-(3-fluorophenoxymethyl 6 1.19 (s, 3H), 1.28 (s, 3
)-3,3-dimethy1-3,4-dihydro-
H), 3.64 (s, 3H), 4.63 (d,
1H-quinoxalin-2-one (Compou
J = 11.6 Hz, 1H), 5.15 (d,
nd No.3-3)
J = 11.6 Hz, 1H), 6.19 (s,
010 F
1H), 6.56-6.59 (m, 21-1), 6.6
7 (d, J = 8.2 Hz, 1H), 6.6
F 8-6.72 (m, 1H), 6.76 (d, J
ft
00 0
id& N,0 = 8.2 Hz, 1H), 6.93 (dd, J
= 9.2, 3.1 Hz, 1H), 7.01 (d
d, J = 8.8, 4.7 Hz, 1H), 7.
0 IW N 11 (td, J = 8.8, 3.1 Hz, 1H
H ), 7.19-7.24 (m, 1H), 9.66
(s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(4-methylphenoxymethyl 6 1.18 (s, 3H), 1.28 (s, 3
)-3,3-dimethy1-3,4-dihydro-
H), 2.18 (s, 3H), 3.64 (s,
1H-quinoxalin-2-one (Compou
3H), 4.58 (d, J = 11.7 Hz,
nd No.3-4)
1H), 5.12 (d, J = 11.7 Hz,
1H), 6.17 (s, 1H), 6.61 (d,
F 0
J = 8.7 Hz, 2H), 6.66 (d,
J - 8.1 Hz, 1H), 6.74 (d, J
= 8.1 Hz, 1H), 6.93 (dd, J
010 0
H
id& N,0 d = 9.3, 3.2 Hz, 1H), 6.98 (
, J = 8.7 Hz, 2H), 7.01 (d
d, J = 9.0, 4.9 Hz, 1H), 7.
0 IW N 11 (td, J = 9.0, 3.2 Hz, 1H
H ), 9.48 (s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(3-methylphenoxymethyl 6 1.18 (s, 3H), 1.28 (s, 3
)-3,3-dimethy1-3,4-dihydro-
H), 2.19 (s, 3H), 3.66 (s,
1H-quinoxalin-2-one (Compou
3H), 4.61 (d, J = 11.9 Hz,
nd No.3-5)
1H), 5.16 (d, J = 11.9 Hz,
F 010 1H), 6.17 (s, 1H), 6.50-6.6
0 (m, 1H), 6.52 (s, 1H), 6.
65-6.78 (m, 1H), 6.66 (d, J
410 0
H
id& N,0 = = 8.1 Hz, 1H), 6.74 (d, J
8.1 Hz, 1H), 6.94 (dd, J
= 9.2, 3.2 Hz, 1H), 7.01-7.
0 IW N 08 (m, 2H), 7.13 (td, J - 8
H .7, 3.2 Hz, 1H), 9.49 (s, 1
H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(2-methylphenoxymethyl 6 1.17 (s, 3H), 1.29 (s, 3
)-3,3-dimethy1-3,4-dihydro-
H), 2.02 (s, 3H), 3.66 (s,
1H-quinoxalin-2-one (Compou
3H), 4.67 (d, J = 11.7 Hz,
nd No.3-6)
1H), 5.17 (d, J = 11.7 Hz,
1 5 0
CA 02643070 2008-08-20
1H), 6.19 (s, 1H), 6.61 (d,
100J = 7.8 Hz, 1H), 6.68 (d,
J = 8.1 Hz, 1H), 6.74-6.78
010 0
id& (m, 1H), 6.76 (d, J = 8.1 H
Hz, 1H), 6.96 (dd, J = 9.0,
3.2 Hz, 1H), 6.99-7.09 (m,
N 3H), 7.13 (td, J = 8.7, 3.2
Hz, 1H), 9.61 (s, 1H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d0
)-8-(2-methyl-5-nitrophenox 6 0.53 (s, 3H), 1.18 (s, 3
ymethyl)-1,3,3-trimethy1-3,
H), 2.09 (s, 3H), 3.37 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.83 (s, 3H), 4.89 (d,
ne (Compound No.3-7)
J = 14.2 Hz, 1H), 5.47 (d,
0 J = 14.2 Hz,
1H), 6.13 (s,
0'-N 40 1H), 6.78 (d, J = 8.1 Hz,
1H), 6.82 (d, J = 8.1 Hz, 1
H), 6.92 (td, J = 8.4, 2.4
F 0
id& N,0 Hz, 1H), 6.97 (d, J = 2.2 H
z, 1H), 7.03 (dd, J = 11.5,
2.4 Hz, 1H), 7.29 (d, J =
N 8.2 Hz, 1H), 7.42 (dd, J =
8.4, 7.0 Hz, 1H), 7.60 (dd,
J = 8.2, 2.2 Hz, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d0
)-8-(2-methyl-5-nitrophenox 6 0.54 (s, 3H), 1.18 (s, 3
ymethyl)-1,3,3-trimethy1-3,
H), 2.09 (s, 3H), 3.37 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.81 (s, 3H), 4.88 (d,
ne (Compound No.3-8)
J = 14.2 Hz, 1H), 5.48 (d,
0 J = 14.2 Hz,
1H), 6.19 (s,
0'-N 40 1H), 6.79 (d, J = 8.1 Hz,
1H), 6.86 (d, J = 8.1 Hz, 1
H), 6.99 (d, J = 2.4 Hz, 1H
010 0
id& N,0 ), 7.13 (d, J 9.7 Hz, 1H)
, 7.30 (d, J = 8.2 Hz, 1H),
7.43 (dd, J = 9.7, 2.4 Hz,
N1H), 7.43 (d, J = 2.3 Hz,
1H), 7.61 (dd, J = 8.2, 2.3
Hz, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-d6)
)-8-(2,5-dimethylphenoxymet 6 0.80 (s, 3H), 1.16 (s, 3
hyl)-1,3,3-trimethy1-3,4-di
H), 1.91 (s, 3H), 2.10 (s,
hydro-1H-quinoxalin-2-one (
3H), 3.32 (s, 3H), 3.80 (s,
Compound No.3-9)
3H), 4.72 (d, J = 13.8 Hz,
1H), 5.19 (d, J = 13.8 Hz,
1H), 6.13 (s, 1H), 6.16 (s
, 1H), 6.49 (d, J - 7.6 Hz,
1H), 6.79 (d, J = 7.9 Hz,
1H), 6.82 (d, J = 7.9 Hz, 1
1 5 1
CA 02643070 2008-08-20
H), 6.86 (d, J = 7.6 Hz, 1H
4101 ), 7.14 (d,
J = 9.0 Hz, 1H)
CI
, 7.31 (d, J = 2.7 Hz, 1H),
010 0
NO 7.42 (d, J - 9.0, 2.7 Hz,
1H)
N
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-[2-(2-hydroxyethyl)phen 6 1.13 (s, 3H), 1.39 (s, 3
oxymethy1]-1,3,3-trimethyl-
H), 2.01 (t, J = 6.0 Hz, 1H
3,4-dihydro-1H-quinoxalin-
), 2.67-2.71 (m, 1H), 2.76-
2-one (Compound No.3-10)
2.80 (m, 1H), 3.42 (s, 3H),
410 3.68-3.72
(m, 2H), 3.79 (s
, 4H), 4.83 (d, J = 12.8 Hz
CI OH
, 1H), 5.14 (d, J = 12.8 Hz
010 0
, 1H), 6.47 (d, J - 7.7 Hz,
1H), 6.76 (d, J = 8.1 Hz,
1H), 6.81 (t, J = 7.7 Hz, 1
N H), 6.89 (d, J - 8.1 Hz, 1H
), 6.89 (d, J = 8.7 Hz, 1H)
, 7.03 (t, J = 7.7 Hz, 1H),
7.08 (d, J - 7.7 Hz, 1H),
7.28 (d, J = 2.7 Hz, 1H), 7
.30 (d, J = 8.7, 2.7 Hz, 1H
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(3-trifluoromethylpheno 6 0.90 (s, 3H), 1.29 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 3.51 (s, 3H), 3.73 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 3.79 (s, 3H), 4.84 (d,
one (Compound No.3-11)
J = 13.3 Hz, 1H), 5.23 (d,
F F J = 13.3 Hz, 1H), 6.71 (br
1110s, 1H), 6.72 (d, J = 8.3 H
z, 1H), 6.78 (dd, J = 8.1,
CI 2.4 Hz, 1H), 6.86-6.88 (m,
0
010
N 1H), 6.87 (d, J - 8.3 Hz, 1
H), 7.06 (d, J = 7.6 Hz, 1H
), 7.21 (t, J = 8.1 Hz, 1H)
N , 7.27-7.30 (m, 2H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(4-phenylphenoxymethyl 6 1.02 (s, 3H), 1.30 (s, 3
)-1,3,3-trimethy1-3,4-dihyd
H), 3.51 (s, 3H), 3.74 (s,
ro-1H-quinoxalin-2-one (Com
1H), 3.80 (s, 3H), 4.82 (d,
pound No.3-12)
J - 12.9 Hz, 1H), 5.18 (d,
J = 12.9 Hz, 1H), 6.65 (d,
J = 8.7 Hz, 2H), 6.72 (d,
J = 7.8 Hz, 1H), 6.87 (d, J
152
CA 02643070 2008-08-20
,
S= 7.8 Hz, 1H), 6.88 (d, J
= 8.7 Hz, 1H), 7.26-7.39 (m
, 7H), 7.44-7.49 (m, 2H)
CI S
lei 0
I
id& N,0
, 0 IW N
H
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2-methoxyphenoxymethyl 6 1.06 (s, 3H), 1.30 (s, 3
)-1,3,3-trimethy1-3,4-dihyd
H), 3.52 (s, 3H), 3.72 (s,
ro-1H-quinoxalin-2-one (Corn
1H), 3.74 (s, 3H), 3.77 (s,
pound No.3-13)
3H), 4.80 (d, J = 12.7 Hz,
1H), 5.21 (d, J = 12.7 Hz,
410
CI 0
3 Hz, 1H), 6.65-6.69 (m, 1H
1H), 6.40 (dd, J = 8.0, 1.
0 ), 6.71 (d, J = 8.0 Hz, 1H)
I I
IN NO
, 6.75-6.80 (m, 2H), 6.83 (
d, J = 8.0 Hz, 1H), 6.85 (d
,0
NX
, J = 8.8 Hz, 1H), 7.25-7.3
H 0 (m, 2H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(3-methoxycarbonylpheno 6 0.89 (s, 3H), 1.34 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 3.50 (s, 3H), 3.73 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 3.78 (s, 3H), 3.90 (s,
one (Compound No.3-14)
3H), 4.82 (d, J = 13.0 Hz,
0
1H), 5.23 (d, J - 13.0 Hz,
CI S ICY
1H), 6.72 (d, J = 8.0 Hz,
1H), 6.84 (ddd, J = 8.0, 2.
5, 1.2 Hz, 1H), 6.85 (d, J
0
410
I
N ,0 = 8.7 Hz, 1H), 6.86 (d, J =
8.0 Hz, 1H), 7.14 (dd, J =
2.5, 1.2 Hz, 1H), 7.18 (t,
0 IW N
J = 8.0 Hz, 1H), 7.27 (dd,
H J = 8.7, 2.6 Hz, 1H), 7.37
(d, J - 2.6 Hz, 1H), 7.51
(dt, J = 8.0, 1.2 Hz, 1H)
8-(5-Chloro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-(4-fluoro-2-metho 6 0.90 (s, 3H), 1.32 (s, 3
xypheny1)-1,3,3-trimethy1-3
H), 2.02 (s, 3H), 3.46 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.72 (s, 1H), 3.82 (s,
one (Compound No.3-15)
3H), 4.80 (d, J = 13.6 Hz,
1H), 5.21 (d, J = 13.6 Hz,
1H), 6.26 (d, J = 1.8 Hz,
1H), 6.68 (dd, J - 7.9, 1.8
1 5 3
CA 02643070 2008-08-20
CI 10Hz, 1H), 6.71-6.73 (m, 1H)
, 6.72 (d, J = 8.1 Hz, 1H),
6.77 (td, J = 8.2, 2.4 Hz,
F 0
SN,0 1H), 6.86 (d,
J = 8.1 Hz,
1H), 6.89 (d, J = 7.9 Hz, 1
H), 7.30 (dd, J = 8.2, 6.7
S
N Hz, 1H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, DMSO-d6)
)-8-(2-methoxy-5-nitropheno 6 0.70 (s, 3H), 1.15 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 3.36 (s, 3H), 3.78 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.79 (s, 3H), 4.81 (d,
one (Compound No.3-16)
J = 13.6 Hz, 1H), 5.39 (d,
0
J = 13.6 Hz, 1H), 6.11 (s,
,
1H), 6.78 (s, 2H), 6.84 (t
0N1 40
d, J = 8.4, 2.5 Hz, 1H), 6.
98 (d, J = 11.5, 2.5 Hz, 1H
N,0
, 7.10 (d, J = 2.7 Hz, 1H),
F 0 ), 7.04 (d,
J = 9.0 Hz, 1H)
7.34 (dd, J = 8.4, 7.1 Hz,
N1H), 7.76 (dd, J = 9.0, 2.
7 Hz, 1H)
8-(2-Allylphenoxymethyl)-7- 1H-NMR (500 MHz, CDC13)
(4-fluoro-2-methoxypheny1)- 6 1.05 (s, 3H), 1.33 (s, 3
1,3,3-trimethy1-3,4-dihydr
H), 3.19 (dd, J = 15.4, 6.7
o-1H-quinoxalin-2-one (Comp
Hz, 1H), 3.26 (dd, J = 15.
ound No.3-17)
4, 6.7 Hz, 1H), 3.44 (s, 3H
NIO ), 3.73 (s, 1H), 3.79 (s, 3
H), 4.79 (d, J = 12.8 Hz, 1
H), 4.95-4.99 (m, 2H), 5.14
F 0
id& , (d, J = 12.8 Hz, 1H), 5.87
N0
(ddt, J = 16.8, 10.4, 6.7
Hz, 1H), 6.40 (dd, J = 7.7,
N 1.2 Hz, 1H),
6.68 (dd, J =
10.4, 2.5 Hz, 1H), 6.72 (d
d, J - 8.2, 2.5 Hz, 1H), 6.
73 (d, J = 7.9 Hz, 1H), 6.7
7 (td, J = 7.7, 1.3 Hz, 1H)
, 6.85 (d, J = 7.9 Hz, 1H),
6.97 (td, J = 7.7, 1.2 Hz,
1H), 7.03 (dd, J = 7.7, 1.
3 Hz, 1H), 7.25 (dd, J = 8.
2, 7.0 Hz, 1H)
8-(3-Acetylphenoxymethyl)- 1H-NMR (500 MHz, DMSO-d6)
7-(4-fluoro-2-methoxyphenyl 6 0.78 (s, 3H), 1.12 (s, 3
)-1,3,3-trimethy1-3,4-dihyd
H), 2.46 (s, 3H), 3.35 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 3.78 (s, 3H), 4.78 (d,
pound No.3-18)
J = 13.1 Hz, 1H), 5.22 (d,
154
CA 02643070 2008-08-20
0 J = 13.1 Hz,
1H), 6.11 (s,
1H), 6.78 (s, 2H), 6.81 (t
40 d, J = 8.3, 2.5 Hz, 1H), 6.
82 (dd, J = 8.2, 2.6 Hz, 1H
F . 0
id& ,0 ), 6.97 (dd, J - 11.3, 2.5
I
Hz, 1H), 7.02-7.03 (m, 1H)
N ,
7.27 (t, J = 8.2 Hz, 1H),
0 IW N 7.35 (dd, J = 8.3, 7.0 Hz,
1H), 7.41-7.43 (m, 1H)
H
8-(3-Cyanophenoxymethyl)-7- 1H-NMR (500 MHz, DMSO-dd
(4-fluoro-2-methoxypheny1)- 6 0.90 (s, 3H), 1.04 (s, 3
1,3,3-trimethy1-3,4-dihydr
H), 3.33 (s, 3H), 3.79 (s,
o-1H-quinoxalin-2-one (Comp
3H), 4.83 (d, J = 13.4 Hz,
ound No.3-19)
1H), 5.17 (d, J = 13.4 Hz,
ON
1H), 6.15 (s, 1H), 6.77-6.8
2 (m, 3H), 6.89-6.91 (m, 1H
), 6.94-6.95 (m, 1H), 6.98
F . 0
I
4& N,0 (dd, J =
11.3, 2.4 Hz, 1H),
7.24-7.28 (m, 2H), 7.32-7.
35 (m, 1H)
20 W N
H
Example 4
7-(5-Fluoro-2-methoxypheny1)-8-(4-
methylphenylaminomethyl)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Compound No.4-1)
A mixture of 8-chloromethy1-7-(5-fluoro-2-
methoxypheny1)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.4-2, 50.7 mg,
0.15 mmol), 4-methylaniline (19.3 mg, 0.18 mmol), and
potassium carbonate (60.6 mg, 0.44 mmol) was
suspended in anhydrous N,N-dimethylformamide (1 mL)
and stirred at 80 C overnight. After cooling down,
ethyl acetate (30 mL) and water (30 mL) were added
and partitioned. The organic layer was washed with
water (30 mL) and saturated brine (30 mL)
155
CA 02643070 2008-08-20
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled compound (48.2 mg) as a pale yellow
solid. (Yield 80%)
1H-NMR (400 MHz, CDC13)
NIO 5 1.38 (s, 3H), 1.49 (s,
3H), 2.25 (s, 3H), 3.65 (s
F , 4H), 3.78 (s, 1H), 3.84
010 NH
H
id&
NO (dd,
J = 12.7 Hz, 1H), 4.07
(dd, J = 10.9, 7.0 Hz, 1H
), 6.59 (d, J = 8.1 Hz, 2H
0 W N.< ), 6.70 (d, J = 8.1 Hz, 1H
H ), 6.75 (dd, J = 9.5, 3.9
Hz, 1H), 6.75 (d, J = 8.1
Hz, 1H), 6.89-6.98 (m, 2H)
, 7.00 (d, J = 8.1 Hz, 2H)
, 8.96 (s, 1H)
Using any compounds among Reference Compounds No.4-2,
14-1, 14-2, and available compounds, the following
Compounds (No.4-2-4-11) were obtained by a method
similar to that of Compound No.4-1.
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, DMSO-d6)
)-8-phenylaminomethy1-3,3-d 6 1.21 (s, 3H), 1.26 (s, 3
imethy1-3,4-dihydro-1H-guin
H), 3.67 (s, 3H), 3.84 (dd,
oxalin-2-one (Compound No.
J = 13.0, 4.9 Hz, 1H), 4.0
4-2)
(dd, J = 13.0, 4.9 Hz, 1H
F 010 ), 5.52 (t, J = 4.9 Hz, 1H)
, 6.13 (s, 1H), 6.55 (d, J
= 8.6 Hz, 2H), 6.56 (t, J =
010 NH
H
410 N,...0 8 8.2 Hz, 1H), 6.66 (d, J =
.1 Hz, 1H), 6.71 (d, J = 8
.1 Hz, 1H), 6.99-7.04 (m, 4
0 N H), 7.11 (td, J = 8.6, 3.3
H Hz, 1H), 9.28 (s, 1H)
156
CA 02643070 2008-08-20
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(3-methylphenylaminomet 6 1.38 (s, 3H), 1.49 (s, 3
hyl)-3,3-dimethy1-3,4-dihyd
H), 2.27 (s, 3H), 3.66 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 3.72 (d, J = 11.9 Hz,
pound No.4-3)
1H), 3.79 (s, 1H), 3.86 (d,
J = 11.9 Hz, 1H), 4.06-4.1
2 (m, 1H), 6.48 (d, J = 7.6
Hz, 1H), 6.49 (s, 1H), 6.6
N
NH
id& ,0 3 (d, J = 7.6 Hz, 1H), 6.70
(d, J = 8.2 Hz, 1H), 6.73-
6.79 (m, 1H), 6.75 (d, J =
O N 8.2 Hz, 1H), 6.91 (dd, J =
8.7, 3.2 Hz, 1H), 6.96 (td,
J = 8.7, 3.2 Hz, 1H), 7.07
(t, J = 7.6 Hz, 1H), 8.85
(s, 1H)
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(2-methylphenylaminomet 6 1.38 (s, 3H), 1.50 (s, 3
hyl)-3,3-dimethy1-3,4-dihyd
H), 2.15 (s, 3H), 3.58 (d,
ro-1H-quinoxalin-2-one (Corn
J = 5.2 Hz, 1H), 3.61 (s, 3
pound No.4-4)
H), 3.80 (br s, 1H), 3.91 (
410 d, J = 12.2 Hz, 1H), 4.08-4
.11 (m, 1H), 6.64 (d, J = 7
.9 Hz, 1H), 6.72 (d, J = 7.
010
N
NH
id&NO9 Hz, 1H), 6.76-6.79 (m, 3H
), 6.92 (dd, J = 8.7, 3.2 H
z, 1H), 6.97 (td, J = 8.4,
N 3.2 Hz, 1H), 7.09 (d, J = 7
.3 Hz, 1H), 7.12 (t, J = 7.
9 Hz, 1H), 8.86 (s, 1H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2-methoxy-5-methylphen 6 1.18 (s, 3H), 1.43 (s, 3
ylaminomethyl)-1,3,3-trimet
H), 2.17 (s, 3H), 3.47 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.70 (s, 3H), 3.73 (s,
in-2-one (Compound No.4-5)
1H), 3.78 (s, 3H), 4.07 (b
0 r s, 2H), 4.44 (br s, 1H),
6.14 (d, J = 1.5 Hz, 1H), 6
.36 (dd, J = 8.1, 1.5 Hz, 1
F 410 NH
id& N,0 H), 6.55 (d, J = 8.1 Hz, 1H
), 6.62-6.71 (m, 2H), 6.69
(d, J = 7.9 Hz, 1H), 6.77 (
N d, J = 7.9 Hz, 1H), 7.12 (d
d, J = 8.3, 6.8 Hz, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(2-methoxy-5-methylphen 6 1.18 (s, 3H), 1.43 (s, 3
ylaminomethyl)-1,3,3-trimet
H), 2.17 (s, 3H), 3.48 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.71 (s, 3H), 3.75 (s,
in-2-one (Compound No.4-6)
1H), 3.77 (s, 3H), 4.10-4.
157
CA 02643070 2008-08-20
14 (m, 2H), 4.42-4.44 (m, 1
H), 6.14 (d, J = 1.5 Hz, 1H
0 ), 6.36 (dd, J = 8.0, 1.5 H
410 NH
N 0 z, 1H), 6.55 (d, J = 8.0 Hz
, 1H), 6.69 (d, J = 8.0 Hz,
0
1H), 6.78 (d, J = 8.0 Hz,
1H), 6.82 (d, J = 8.9 Hz, 1
H), 7.17 (d, J = 2.8 Hz, 1H
), 7.25 (dd, J = 8.9, 2.8 H
z, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylpheny 6 1.22 (s, 3H), 1.40 (s, 3
laminomethyl)-1,3,3-trimeth
H), 1.85 (s, 3H), 3.42 (s,
y1-3,4-dihydro-1H-quinoxali
3H), 3.73-3.80 (m, 2H), 3.7
n-2-one (Compound No.4-7)
7 (s, 3H), 4.10-4.22 (m, 2H
F 410
), 6.03 (d, J = 11.5, 2.5 H
z, 1H), 6.24 (td, J = 8.3,
CI 2.5, 1H), 6.72 (d, J = 8.1
010 NH
N,0 Hz, 1H), 6.77 (d, J = 8.1 H
z, 1H), 6.84-6.88 (m, 1H),
6.87 (d, J = 8.8 Hz, 1H), 7
N .18 (d, J = 2.7 Hz, 1H), 7.
29 (d, J = 8.8, 2.7 Hz, 11-I)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(2-methoxyphenylaminome 5 1.21 (s, 3H), 1.42 (s, 3
thyl)-1,3,3-trimethy1-3,4-d
H), 3.46 (s, 3H), 3.74 (s,
ihydro-1H-quinoxalin-2-one
3H), 3.75 (s, 1H), 3.77 (s,
(Compound No.4-8)
3H), 4.11-4.14 (m, 2H), 4.
11043 (br s, 1H), 6.32 (dd, J
= 7.8, 1.5 Hz, 1H), 6.57 (t
0 d, J = 7.8, 1.5 Hz, 1H), 6.
010 NH
N,0 66 (dd, J = 7.8, 1.5 Hz, 1H
), 6.69 (d, J = 8.0 Hz, 1H)
, 6.72 (td, J = 7.8, 1.5 Hz
O N , 1H), 6.78 (d, J = 8.0 Hz,
1H), 6.82 (d, J = 8.9 Hz,
1H), 7.16 (d, J = 2.7 Hz, 1
H), 7.24 (dd, J = 8.9, 2.7
Hz, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2,5-dimethylphenylamin 6 1.20 (s, 3H), 1.43 (s, 3
omethyl)-1,3,3-trimethy1-3,
H), 1.88 (s, 3H), 2.21 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.46 (s, 3H), 3.69 (s,
ne (Compound No.4-9)
1H), 3.77 (s, 3H), 4.10-4.
19 (m, 2H), 6.19 (s, 1H), 6
.40 (d, J = 7.3 Hz, 1H), 6.
71 (d, J = 7.8 Hz, 1H), 6.8
0 (d, J = 7.8 Hz, 1H), 6.8
2-6.85 (m, 1H), 6.86 (d, J
158
CA 02643070 2008-08-20
11101 = 8.8 Hz, 1H), 7.20 (d, J
2.5 Hz, 1H), 7.27 (dd, J =
CI 8.8, 2.5 Hz, 1H)
NH
=
N,0
N
7-(5-Chloro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2-isopropylphenylamino 6 1.21 (d, J = 6.8 Hz, 3H)
methyl)-1,3,3-trimethy1-3,
, 1.15 (d, J = 6.8 Hz, 3H),
4-dihydro-1H-quinoxalin-2-o
1.26 (s, 3H), 1.41 (s, 3H)
ne (Compound No.4-10)
, 2.49-2.56 (m, 1H), 3.45 (
010 s, 3H), 3.76 (s, 3H), 3.80
(s, 1H), 3.81 (br s, 1H), 4
CI .15-4.22 (m, 2H), 6.43 (dd,
NH
.4& N,0 J = 7.4, 1.3 Hz, 1H), 6.68
(td, J = 7.4, 1.3 Hz, 1H),
6.72 (d, J = 7.9 Hz, 1H),
6.81 (d, J = 7.9 Hz, 1H), 6
.85 (d, J = 8.7 Hz, 1H), 7.
01 (td, J = 7.4, 1.3 Hz, 1H
), 7.06 (dd, J = 7.4, 1.3 H
z, 1H), 7.20 (d, J = 2.7 Hz
, 1H), 7.26 (d, J = 8.7, 2.
7 Hz, 1H)
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(2-methoxyphenylaminome 6 1.18 (s, 3H), 1.42 (s, 3
thyl)-1,3,3-trimethy1-3,4-d
H), 3.46 (s, 3H), 3.73 (s,
ihydro-1H-quinoxalin-2-one
3H), 3.76 (s, 1H), 3.77 (s,
(Compound No.4-11)
3H), 4.10 (d, J = 5.2 Hz,
010
0 2H), 4.45 (t, J = 5.2 Hz, 1
H), 6.32 (dd, J = 7.7, 1.4
Hz, 1H), 6.57 (td, J = 7.7,
FS NH
SN,0 1.4 Hz, 1H),
6.62-6.69 (m,
3H), 6.69 (d, J - 7.9 Hz,
1H), 6.73 (td, J = 7.7, 1.4
O N Hz, 1H), 6.78
(d, J = 7.9
Hz, 1H), 7.12 (dd, J = 8.4,
6.9 Hz, 1H)
Example 5
8-Benzoyloxymethy17-(5-fluoro-2-methoxypheny1)-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No. 5-1)
159
CA 02643070 2008-08-20
A mixture of 8-benzoyloxymethy1-7-(5-fluoro-2-
methoxypheny1)-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Compound No.1-3, 42.9 mg, 0.099
mmol), methyliodide (30.7 p L, 0.49 mmol), and cessium
carbonate (89.0 mg, 0.27 mmol) was suspended in
anhydrous N,N-dimethylformamide (1 mL) and stirred at
room temperature for 1.5 hours. The reaction mixture
was diluted with ethyl acetate (100 mL). The mixture
was washed with water (100 mL) and saturated brine
(50 mL) successively, dried over anhydrous magnesium
sulfate, and then the solvent was removed under
reduced pressure. The obtained residue was purified
by silica gel column chromatography (hexane-ethyl
acetate) to give the titled compound (35.0 mg) as a
colorless amorphous product. (Yield 79%)
1 H-NMR (400 MHz, CDC13)
0 010 6 1.21 (s, 3H), 1.42 (s,
F 3H), 3.47 (s, 3H), 3.72 (s
0100
I
a& N,0 , 3H), 3.82 (br s, 1H), 5.
20 (d, J = 13.3 Hz, 1H), 5
.36 (d, J = 13.3 Hz, 1H),
,0 IW N 6.77 (d, J = 8.1 Hz, 1H),
H 6.83 (dd, J = 8.8, 4.4 Hz,
1H), 6.88 (d, J = 8.1 Hz,
1H), 6.96-7.02 (m, 2H), 7
.36 (t, J = 7.7 Hz, 2H), 7
.49-7.53 (m, 1H), 7.81-7.8
4 (m, 2H)
Using any compounds among Reference Compounds No.1-
4-1-7, 2-1-2-6, 2-13, 3-1-3-6, 4-1-4-4, 22, and
available compounds, the following Compounds (No.5-
2-5-25) were obtained by a method similar to that of
160
CA 02643070 2008-08-20
Compound No.5-1.
7-(5-Fluoro-2-methoxypheny 1H-NMR (500 MHz, DMSO-dd
1)-8-(5-methylthiophen-2-y 6 1.02 (s, 3H), 1.25 (s, 3H
lcarbonyloxymethyl)-1,3,3-
), 2.45 (s, 3H), 3.31 (s, 3H
trimethy1-3,4-dihydro-1H-q
), 3.69 (s, 3H), 5.08 (d, J
uinoxalin-2-one (Compound
= 13.4 Hz, 1H), 5.24 (d, J =
No.5-2)
13.4 Hz, 1H), 6.26 (s, 1H),
6.80 (d, J = 7.9 Hz, 1H), 6
.84 (d, J = 7.9 Hz, 1H), 6.8
0
N 6 (d, J = 3.7 Hz, 1H), 7.05
(dd, J = 8.8, 4.6 Hz, 1H), 7
.07 (dd, J = 8.9, 3.3 Hz, 1H
O N ), 7.16 (td, J = 8.8, 3.3 Hz
, 1H), 7.38 (d, J = 3.7 Hz,
1H)
7-(5-Fluoro-2-methoxypheny 1H-NMR (500 MHz, DMSO-dd
1)-8-(4-methylbenzoyloxyme 6 1.01 (s, 3H), 1.25 (s, 3H
thyl)-1,3,3-trimethy1-3,4-
), 2.34 (s, 3H), 3.30 (s, 3H
dihydro-1H-quinoxalin-2-on
), 3.69 (s, 3H), 5.13 (d, J
e (Compound No.5-3)
= 13.4 Hz, 1H), 5.28 (d, J =
0 01013.4 Hz, 1H), 6.27 (s, 1H),
6.82 (d, J = 7.9 Hz, 1H), 6
.84 (d, J = 7.9 Hz, 1H), 7.0
0
N 4 (dd, J = 8.9, 4.6 Hz, 1H),
7.09 (dd, J - 9.0, 3.2 Hz,
1H), 7.15 (td, J = 8.9, 3.2
O N Hz, 1H), 7.24 (d, J = 8.2 Hz
, 2H), 7.60 (d, J - 8.2 Hz,
2H)
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(3-methylbenzoyloxyme 6 1.23 (s, 3H), 1.43 (s, 3H)
thyl)-1,3,3-trimethy1-3,4-
, 2.34 (s, 3H), 3.46 (s, 3H)
dihydro-1H-quinoxalin-2-on
, 3.73 (s, 3H), 3.82 (s, 1H)
e (Compound No.5-4)
, 5.18 (d, J = 13.4 Hz, 1H),
0 el 5.35 (d, J =
13.4 Hz, 1H),
6.77 (d, J = 8.1 Hz, 1H), 6.
83 (dd, J = 8.7, 4.5 Hz, 1H)
010 0
N , 6.88 (d, J - 8.1 Hz, 1H),
6.96-7.02 (m, 2H), 7.23-7.32
(m, 2H), 7.62-7.64 (m, 2H)
N
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(2-methylbenzoyloxyme 51.26 (s, 3H), 1.42 (s, 3H)
thyl)-1,3,3-trimethy1-3,4-
, 2.48 (s, 3H), 3.46 (s, 3H)
dihydro-1H-quinoxalin-2-on
, 3.71 (s, 3H), 3.82 (s, 1H)
1 6 1
CA 02643070 2008-08-20
, .
e (Compound No.5-5) , 5.13 (d, J - 13.3 Hz, 1H),
5.34 (d, J = 13.3 Hz, 1H),
0 01$6.77 (d, J = 8.0 Hz, 1H), 6.
F 83-6.88 (m, 1H), 6.87 (d, J
010 0
,
40 N......,o.,0 = 8.0 Hz, 1H), 6.98-7.01 (m,
1H), 6.99 (d, J = 8.1 Hz, 1
H), 7.13-7.19 (m, 2H), 7.35
0 N (td, J = 8.6, 1.5 Hz, 1H), 7
.66 (dd, J = 8.0, 1.2 Hz, 1H
H
)
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(thiophen-2-ylcarbony 6 1.20 (s, 3H), 1.42 (s, 3H)
loxymethyl)-1,3,3-trimethy
, 3.46 (s, 3H), 3.74 (s, 3H)
1-3,4-dihydro-1H-quinoxali
, 3.82 (s, 1H), 5.17 (d, J =
n-2-one (Compound No.5-6)
13.3 Hz, 1H), 5.33 (d, J =
13.3 Hz, 1H), 6.77 (d, J = 8
F 01,1-; .0 Hz, 1H), 6.85 (dd, J = 8.
I
S 0
N ,C) 6, 4.4 Hz, 1H), 6.87 (d, J =
8.0 Hz, 1H), 6.98-7.05 (m,
3H), 7.50 (dd, J = 4.9, 1.2
0 IW N Hz, 1H), 7.63 (dd, J = 3.6,
1.2 Hz, 1H)
H
7-(5-Fluoro-2-methoxypheny 1H-NMR (500 MHz, DMSO-dd
1)-8-(4-methoxybenzoyloxym 6 1.02 (s, 3H), 1.25 (s, 3H
ethyl)-1,3,3-trimethy1-3,
), 3.32 (s, 3H), 3.69 (s, 3H
4-dihydro-1H-quinoxalin-2-
), 3.80 (s, 3H), 5.11 (d, J
one (Compound No.5-7)
= 13.3 Hz, 1H), 5.26 (d, J =
S13.3 Hz, 1H), 6.26 (s, 1H),
0
6.81 (d, J = 8.2 Hz, 1H), 6
F .84 (d, J = 8.2 Hz, 1H), 6.9
010 0
,
N ,0 5 (d, J = 8.7 Hz, 2H), 7.04
(dd, J = 8.8, 4.7 Hz, 1H), 7
.09 (dd, J = 9.2, 3.1 Hz, 1H
0 IW N ), 7.15 (td, J = 8.8, 3.1 Hz
H , 1H), 7.66 (d, J = 8.7 Hz,
2H)
7-(4-Fluoro-2-methoxypheny 1H-NMR (400 MHz, DMSO-dd
1)-8-phenoxymethy1-1,3,3-t 6 0.87 (s, 3H), 1.09 (s, 3H
rimethy1-3,4-dihydro-1H-qu
), 3.32 (s, 3H), 3.79 (s, 3H
inoxalin-2-one (Compound N
), 4.71 (d, J - 13.1 Hz, 1H)
o.5-8)
, 5.10 (d, J - 13.1 Hz, 1H),
6.11 (s, 1H), 6.53-6.56 (m,
2H), 6.76 (d, J = 8.1 Hz, 1
H), 6.78-6.83 (m, 2H), 6.81
(td, J = 8.3, 2.4 Hz, 1H), 6
.98 (dd, J = 11.5, 2.4 Hz, 1
H), 7.11 (dd, J = 8.5, 7.3 H
z, 2H), 7.28 (dd, J = 8.4, 7
162
CA 02643070 2008-08-20
.0 Hz, 1H)
F 0
id&
NO
,0 N
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-phenoxymethy1-1,3,3-t 6 1.00 (s, 3H), 1.31 (s, 3H
rimethy1-3,4-dihydro-1H-qu
), 3.49 (s, 3H), 3.72 (br s,
inoxalin-2-one (Compound N
1H), 3.77 (s, 3H), 4.80 (d,
o.5-9)
J = 12.9 Hz, 1H), 5.15 (d,
010 J = 12.9 Hz, 1H), 6.57-6.59
(m, 2H), 6.71 (d, J = 7.9 Hz
, 1H), 6.79-6.83 (m, 1H), 6.
Olt 0
id& N,0 86 (dd, J = 8.9, 4.5 Hz, 1H)
, 6.86 (d, J = 7.9 Hz, 1H),
6.99-7.13 (m, 4H)
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, DMSO-dd
1)-8-(3-fluorophenoxymethy 6 0.95 (s, 3H), 1.06 (s, 3H
1)-1,3,3-trimethy1-3,4-dih
), 3.30 (s, 3H), 3.74 (s, 3H
ydro-1H-quinoxalin-2-one (
), 4.79 (d, J = 12.6 Hz, 1H)
Compound No.5-10)
, 5.13 (d, J = 12.6 Hz, 1H),
F
6.19 (s, 1H), 6.37-6.43 (m,
2H), 6.63 (td, J = 8.4, 2.0
Hz, 1H), 6.80 (d, J = 8.1 H
010 0
z
id& N,0 , 1H), 6.82 (d, J = 8.1 Hz,
1H), 7.05-7.18 (m, 4H)
N
7-(5-Fluoro-2-methoxypheny 1H-NMR (500 MHz, DMSO-dd
1)-8-(4-methylphenoxymethy 5 0.94 (s, 3H), 1.10 (s, 3H
1)-1,3,3-trimethy1-3,4-dih
), 2.13 (s, 3H), 3.32 (s, 3H
ydro-1H-quinoxalin-2-one (
), 3.74 (s, 3H), 4.69 (d, J
Compound No.5-11)
- 13.1 Hz, 1H), 5.07 (d, J =
13.1 Hz, 1H), 6.15 (s, 1H),
4016.46 (d, J = 8.4 Hz, 2H), 6
.78 (d, J - 8.4 Hz, 1H), 6.8
0 (d, J = 8.4 Hz, 1H), 6.92
010 0
id& N,0 (d, J = 8.4 Hz, 2H), 7.06 (d
d, J = 8.8, 4.7 Hz, 1H), 7.1
0 (dd, J = 9.0, 3.2 Hz, 1H),
N 7.15 (td, J = 8.8, 3.2 Hz,
1H)
1 6 3
CA 02643070 2008-08-20
,
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, DMSO-d6)
1)-8-(3-methylphenoxymethy 6 0.92 (s, 3H), 1.11 (s, 3H
1)-1,3,3-trimethy1-3,4-dih
), 2.14 (s, 3H), 3.33 (s, 3H
ydro-1H-quinoxalin-2-one (
), 3.75 (s, 3H), 4.72 (d, J
Compound No.5-12)
= 12.7 Hz, 1H), 5.11 (d, J =
410
12.7 Hz, 1H), 6.15 (s, 1H),
6.32-6.39 (m, 2H), 6.62 (d,
J = 7.3 Hz, 1H), 6.79 (d, J
410 0
id& N,0
= 8.1 Hz, 1H), 6.81 (d, J =
8.1 Hz, 1H), 6.99 (t, J = 7
.8 Hz, 1H), 7.05-7.18 (m, 3H
N
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, DMSO-dd
1)-8-(2-methylphenoxymethy 6 0.85 (s, 3H), 1.11 (s, 3H
1)-1,3,3-trimethy1-3,4-dih
), 1.96 (s, 3H), 3.29 (s, 3H
ydro-1H-quinoxalin-2-one (
), 3.78 (s, 3H), 4.80 (d, J
Compound No.5-13)
= 13.3 Hz, 1H), 5.17 (d, J =
13.3 Hz, 1H), 6.17 (s, 1H),
6.38 (d, J = 7.8 Hz, 1H), 6
.69 (t, J = 7.1 Hz, 1H), 6.7
410 0
id& N,0 9 (d, J = 8.1 Hz, 1H), 6.83
(d, J = 8.1 Hz, 1H), 6.94 (t
, J = 7.8 Hz, 1H), 7.00 (d,
O N
J = 7.1 Hz, 1H), 7.10 (dd, J
= 9.0, 4.6 Hz, 1H), 7.13-7.
23 (m, 2H)
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-phenylaminomethy1-1,3 6 1.18 (s, 3H), 1.44 (s, 3H
,3-trimethy1-3,4-dihydro-1
), 3.50 (s, 3H), 3.76 (br s,
H-quinoxalin-2-one (Compou
1H), 3.80 (s, 3H), 4.02 (d,
nd No.5-14)
J = 12.0 Hz, 1H), 4.03 (br
410 s, 1H), 4.16 (d, J = 12.0 Hz
, 1H), 6.34 (d, J = 7.6 Hz,
2H), 6.62 (t, J = 7.3 Hz, 1H
NH
id& N,0
), 6.69 (d, J = 7.9 Hz, 1H),
6.79 (d, J - 7.9 Hz, 1H), 6
.86 (dd, J = 9.0, 4.4 Hz, 1H
O N
), 6.90 (dd, J = 8.5, 3.2 Hz
, 1H), 6.96-7.01 (m, 1H), 7.
03-7.07 (m, 2H)
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(4-methylphenylaminom 6 1.18 (s, 3H), 1.44 (s, 3H
ethyl)-1,3,3-trimethy1-3,
), 2.17 (s, 3H), 3.51 (s, 3H
4-dihydro-1H-quinoxalin-2-
), 3.75 (s, 1H), 3.80 (s, 3H
one (Compound No.5-15)
), 3.90 (br s, 1H), 3.98 (d,
J - 12.5 Hz, 1H), 4.13 (d,
J - 12.5 Hz, 1H), 6.27 (d, J
164
CA 02643070 2008-08-20
, *
- 8.3 Hz, 2H), 6.69 (d, J =
7.8 Hz, 1H), 6.78 (d, J = 7
1110 .8 Hz, 1H), 6.83-6.92 (m, 4H
F ), 6.95-7.02 (m, 1H)
NH
0
I
40 N,....0
,0 N.<
H
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(3-methylphenylaminom 6 1.18 (s, 3H), 1.45 (s, 3H
ethyl)-1,3,3-trimethy1-3,
), 2.19 (s, 3H), 3.51 (s, 3H
4-dihydro-1H-quinoxalin-2-
), 3.75 (s, 1H), 3.80 (s, 3H
one (Compound No.5-16)
), 3.95-4.04 (m, 2H), 4.10-4
ON .19 (m, 1H), 6.16 (d, J = 7.
3 Hz, 1H), 6.17 (s, 1H), 6.4
F 4 (d, J = 7.3 Hz, 1H), 6.69
00 NH
I
ed& N,0 (d, J = 8.1 Hz, 1H), 6.79 (d
, J = 8.1 Hz, 1H), 6.84-7.01
,C$ IW N.< (m, 4H)
H
7-(5-Fluoro-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(2-methylphenylaminom 6 1.21 (s, 3H), 1.43 (s, 3H
ethyl)-1,3,3-trimethy1-3,
), 1.92 (s, 3H), 3.45 (s, 3H
4-dihydro-1H-quinoxalin-2-
), 3.73 (s, 1H), 3.75 (s, 3H
one (Compound No.5-17)
), 3.78 (s, 1H), 4.18-4.19 (
00 m, 2H), 6.36 (d, J = 7.8 Hz,
1H), 6.58 (t, J - 7.1 Hz, 1
F
H), 6.71 (d, J = 7.9 Hz, 1H)
00 NH
id& NI.0 , 6.82 (d, J = 7.9 Hz, 1H),
6.86 (dd, J = 8.9, 4.5 Hz, 1
0
H), 6.92-7.03 (m, 4H)
IW N
H
1-Ethyl-7-(5-fluoro-2-meth 1H-NMR (500 MHz, DMSO-d0
oxypheny1)-8-(4-methylbenz 5 0.96 (s, 3H), 1.01 (t, J
oyloxymethyl)-3,3-dimethy =
7.0 Hz, 3H), 1.27 (s, 3H),
1-3,4-dihydro-1H-quinoxali
2.33 (s, 3H), 3.62 (dq, J =
n-2-one (Compound No.5-18)
7.0 Hz, 1H), 3.69 (s, 3H),
0 Oil 4.29 (dq, J = 7.0 Hz, 11-1), 5
.10 (d, J = 13.4 Hz, 1H), 5.
F
17 (d, J - 13.4 Hz, 1H), 6.2
50 r
40 N,....0 3 (s, 1H), 6.82 (d, J = 7.9
Hz, 1H), 6.85 (d, J = 7.9 Hz
, 1H), 7.05 (dd, J = 8.9, 4.
0 N 6 Hz, 1H), 7.10 (dd, J - 9.0
H , 3.2 Hz, 1H), 7.16 (td, J =
165
CA 02643070 2008-08-20
8.9, 3.2 Hz, 1H), 7.24 (d,
J = 8.2 Hz, 2H), 7.59 (d, J
= 8.2 Hz, 2H)
1-(Propen-3-y1)-7-(5-fluor 1H-NMR (400 MHz, DMSO-dd
o-2-methoxypheny1)-8-(4-me 6 1.03 (s, 3H), 1.27 (s, 3H
thylbenzoyloxymethyl)-3,3-
), 2.34 (s, 3H), 3.64 (s, 3H
dimethy1-3,4-dihydro-1H-qu
), 4.27 (dd, J - 16.7, 5.4 H
inoxalin-2-one (Compound N
z, 1H), 4.73-4.80 (m, 1H), 5
o.5-19)
.04 (dd, J = 10.6, 1.6 Hz, 1
0 010H), 5.08 (d, J = 13.7 Hz, 1H
), 5.10 (dd, J = 17.3, 1.6 H
z, 1H), 5.18 (d, J - 13.7 Hz
010 0
Ny? , 1H), 5.70 (ddt, J - 17.3,
10.6, 5.4 Hz, 1H), 6.31 (s,
1H), 6.81 (d, J = 8.1 Hz, 1H
,0 N)< ), 6.86 (d, J = 8.1 Hz, 1H), -'
7.02 (dd, J = 9.0, 4.6 Hz,
1H), 7.04 (dd, J = 9.2, 3.2
Hz, 1H), 7.13 (td, J = 9.0,
3.2 Hz, 1H), 7.25 (d, J = 8.
0 Hz, 2H), 7.60 (d, J = 8.0
Hz, 2H)
7-(2-Methoxy-4-methoxymeth 1H-NMR (400 MHz, CDC13)
oxypheny1)-8-(5-methylthio 6 1.20 (s, 3H), 1.42 (s, 3H
phen-2-ylcarbonyloxymethyl
), 2.47 (s, 3H), 3.44 (s, 3H
)-1,3,3-trimethy1-3,4-dihy
), 3.51 (s, 3H), 3.75 (s, 4H
dro-1H-quinoxalin-2-one (C
), 5.13 (d, J = 13.4 Hz, 1H)
ompound No.5-20)
, 5.18 (d, J = 6.8 Hz, 1H),
5.21 (d, J = 6.8 Hz, 1H), 5.
0
29 (d, J - 13.4 Hz, 1H), 6.6
0 3 (d, J - 2.4 Hz, 1H), 6.66
(dd, J = 8.3, 2.4 Hz, 1H), 6
id& 1\10 .69 (d, J - 3.6 Hz, 1H), 6.7
N 4 (d, J = 8.1 Hz, 1H), 6.86
(d, J - 8.1 Hz, 1H), 7.17 (d
, J = 8.3 Hz, 1H), 7.43 (d,
J = 3.6 Hz, 1H)
7-[2-Methoxy-4-(2-methylbe 1H-NMR (500 MHz, CDC13)
nzoyloxy)pheny1]-8-(4-meth 5 1.20 (s, 3H), 1.43 (s, 3H
oxybenzoyloxymethyl)-1,3,
), 2.70 (s, 3H), 3.48 (s, 3H
3-trimethy1-3,4-dihydro-1
), 3.77 (s, 3H), 3.80 (s, 1H
H-quinoxalin-2-one (Compou
), 3.83 (s, 3H), 5.20 (d, J
nd No.5-21)
= 13.4 Hz, 1H), 5.35 (d, J =
13.4 Hz, 1H), 6.77 (d, J =
7.9 Hz, 1H), 6.80 (d, J = 2.
1 Hz, 1H), 6.84 (dd, J = 8.2
, 2.1 Hz, 1H), 6.84 (d, J =
8.7 Hz, 2H), 6.91 (d, J - 7.
9 Hz, 1H), 7.32 (d, J = 8.2
166
CA 02643070 2008-08-20
aim 0 (Hz, 1H), 7.32-7.36 (m, 2H),
7.50 t .3-1
= 7 Hz 1H) 7.
o q1PI 80 (c1, 8.71z, 2H), 8.17
410 0 0 (d, J = 7.9 Hz, 1H)
0 Si Ny?
N)
7-[2-Methoxy-4-(2-methylbe 1H-NMR (400 MHz, CDC13)
nzoyloxy)pheny1]-8-(2-meth 5 0.72 (s, 3H), 1.34 (s, 3H
oxy-5-nitrophenoxymethyl)-
), 2.70 (s, 3H), 3.54 (s, 3H
1,3,3-trimethy1-3,4-dihydr
), 3.69 (s, 1H), 3.85 (s, 3H
o-1H-quinoxalin-2-one (Corn
), 3.86 (s, 3H), 5.02 (d, J
pound No.5-22)
= 13.8 Hz, 1H), 5.47 (d, J =
0 13.8 Hz, 1H), 6.72 (d, J =
44+ 7.9 Hz, 1H), 6.76 (d, J - 8.
0" 010 8 Hz, 1H), 6.86 (d, J = 2.2
Hz, 1H), 6.92 (d, J - 7.9 Hz
Olt 0 0 , 1H), 6.97 (dd, J = 8.3, 2.
2 Hz, 1H), 7.15 (d, J - 2.6
0 410 gd& NO Hz, 1H), 7.34 (d, J = 7.6 Hz
N , 1H), 7.35 (t, J = 7.6 Hz,
1H), 7.48-7.52 (m, 1H), 7.53
(d, J = 8.3 Hz, 1H), 7.74 (
dd, J = 8.8, 2.6 Hz, 1H), 8.
19 (d, J = 7.6 Hz, 1H)
7-[2-Methoxy-4-(2-methylbe 11-1-NMR (400 MHz, CDC13)
nzoyloxy)pheny1]-8-(4-meth 6 1.20 (s, 3H), 1.43 (s, 3H
ylbenzoyloxymethyl)-1,3,3-
), 2.36 (s, 3H), 2.69 (s, 3H
trimethy1-3,4-dihydro-1H-q
), 3.47 (s, 3H), 3.76 (s, 3H
uinoxalin-2-one (Compound
), 3.80 (s, 1H), 5.19 (d, J
No.5-23)
= 13.2 Hz, 1H), 5.36 (d, J =
13.2 Hz, 1H), 6.77 (d, J =
o 8.1 Hz, 1H), 6.80 (d, J - 2.
2 Hz, 1H), 6.84 (dd, J = 8.2
40 0 0 , 2.2 Hz, 1H), 6.91 (d, J =
0 1.1 Ny? 8.1 Hz, 1H), 7.16 (d, J - 8.
1 Hz, 2H), 7.32 (d, J = 8.2
,0
N)<- Hz, 1H), 7.33 (d, J - 7.7 Hz
, 1H), 7.34 (t, J = 7.7 Hz,
1H), 7.48-7.52 (m, 1H), 7.74
(d, J = 8.1 Hz, 2H), 8.17 (
d, J = 7.7 Hz, 1H)
8-(3-Fluorobenzoyloxymethy 11-1-NMR (500 MHz, CDC13)
1)-7-[2-methoxy-4-(2-methy 6 1.23 (s, 3H), 1.44 (s, 3H
lbenzoyloxy)pheny11-1,3,3-
), 2.69 (s, 3H), 3.46 (s, 3H
trimethy1-3,4-dihydro-1H-q
), 3.77 (s, 3H), 3.82 (s, 1H
uinoxalin-2-one (Compound
), 5.23 (d, J - 13.3 Hz, 1H)
No.5-24)
167
CA 02643070 2008-08-20
, 5.39 (d, J = 13.3 Hz, 1H),
6.79 (d, J = 8.2 Hz, 1H), 6
0 00
F .80 (d, J =
2.4 Hz, 1H), 6.8
010 0 0 3 (dd, J = 8.2,
2.4 Hz, 1H),
0 le 6.92 (d, J =
8.2 Hz, 1H), 7
.19-7.23 (m, 1H), 7.29 (d, J
O N = 8.2 Hz,
1H), 7.32-7.37 (m
, 3H), 7.48-7.53 (m, 2H), 7.
64 (dt, J = 7.9, 1.2 Hz, 1H)
, 8.17 (d, J = 7.6 Hz, 1H)
8-(5-Bromothiophen-2-ylcar 1H-NMR (500MHz, CDC13)
bonyloxymethyl)-7-[2-metho 6 1.23 (s, 3H), 1.43 (s, 3H
xy-4-(2-methylbenzoyloxy)p
), 2.70 (s, 3H), 3.45 (s, 3H
heny11-1,3,3-trimethyl-3,
), 3.78 (s, 3H), 3.81 (s, 1H
4-dihydro-1H-quinoxalin-2-
), 5.17 (d, J = 13.3 Hz, 1H)
one (Compound No.5-25)
, 5.34 (d, J = 13.3 Hz, 1H),
6.78 (d, J = 7.9 Hz, 1H), 6
.81 (d, J = 2.1 Hz, 1H), 6.8
010 0 010 0 5 (dd, J = 7.9,
2.1 Hz, 1H),
6.91 (d, J = 7.9 Hz, 1H), 7
0 ti& N,0 .01 (d, J =
4.0 Hz, 1H), 7.3
O N 0 (d, J -
7.9 Hz, 1H), 7.33-
7.36 (m, 2H), 7.38 (d, J = 4
.0 Hz, 1H), 7.50 (td, J = 7.
7, 1.4 Hz, 1H), 8.18 (d, J =
7.7 Hz, 1H)
Example 6
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-methoxy-4-
trifluoromethoxypheny1)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one (Compound No.6-1)
A mixture of 7-bromo-
8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.8-1, 49.7 mg,
0.122 mmol), 2-methoxy-4-
trifluoromethoxyphenylboronic acid (58.1 mg, 0.246
mmol), cessium carbonate (119 mg, 0.36 mmol) and
bis(triphenylphosphin)palladium (II) dichloride (12.0
mg, 0.0171 mmol) was suspended in anhydrous N,N-
dimethylformamide (0.5 ml) and stirred at 80 C for 2
168
CA 02643070 2008-08-20
hours under argon atomosphere. After cooling down,
ethyl acetate (100mL) and water (100 mL) was added
and partitioned. The organic layer was washed with
saturated brine (50 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled compound (52.7 mg)
as a colorless amorphous product. (Yield 58%)
F 1H-NMR (500 MHz, CDC13)
6 0.98 (s, 3H), 1.27 (s,
3H), 2.00 (s, 3H), 3.46 (s
0 , 3H), 3.74 (s, 1H), 3.83
FF 0/0
id& NO (s, 3H), 4.81 (d, J = 13.4
Hz, 1H), 5.17 (d, J = 13.
N 4 Hz, 1H), 6.05 (dd, J = 1
1.3, 2.4 Hz, 1H), 6.40 (td
, J = 8.4, 2.4 Hz, 1H), 6.
73 (d, J = 7.9 Hz, 1H), 6.
82 (d, J = 1.8 Hz, 1H), 6.
86 (d, J = 7.9 Hz, 1H), 6.
89-6.92 (m, 2H), 7.31 (d,
J = 8.2 Hz, 1H)
Using any compounds among Reference Compounds No.8-
1-8-4, 17, 18-1-18-5, 20-1, 20-2, and available
compounds, the following Compounds (No.6-2-6-34 and
6-37-6-43) were obtained by a method similar to that
of Compound No.6-1. The following Compounds (No.6-35
and 6-36) were obtained by a method similar to that
of Reference Compound No.20-1 using any compounds
among Reference Compounds No.19-2, 19-3, and
available compounds followed by a method similar to
that of Compound No.6-1 using Reference Compound
169
CA 02643070 2008-08-20
No.8-1 and available compounds.
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2-methylphenoxymethyl 6 1.00 (s, 3H), 1.33 (s, 3
)-1,3,3-trimethy1-3,4-dihyd
H), 2.07 (s, 3H), 3.44 (s,
ro-1H-quinoxalin-2-one (Com
3H), 3.72 (br s, 1H), 3.80
pound No.6-2)
(s, 3H), 4.80 (d, J = 12.9
Hz, 1H), 5.16 (d, J = 12.9
Hz, 1H), 6.33 (d, J = 8.1 H
z, 1H), 6.68-6.75 (m, 3H),
0
id& N,0 6.72 (d, J =
8.1 Hz, 1H), 6
F
.85 (d, J = 8.1 Hz, 1H), 6.
93 (t, J = 7.9 Hz, 1H), 7.0
1 (d, J = 7.9 Hz, 1H), 7.2
4-7.28 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-fluoropheny1)- 6 1.06 (s, 3H), 1.32 (s, 3
1,3,3-trimethy1-3,4-dihydr
H), 2.02 (s, 3H), 3.45 (s,
o-1H-quinoxalin-2-one (Comp
3H), 3.80 (s, 1H), 4.87 (br
ound No.6-3)
s, 1H), 5.17 (br s, 1H), 6
F
.10 (dd, J = 11.0, 2.4 Hz,
1H), 6.43 (td, J = 8.3, 2.4
Hz, 1H), 6.77 (d, J = 8.1
010 0
Hz, 1H), 6.88-7.00 (m, 1H),
6.94 (d, J = 8.1 Hz, 1H),
7.12-7.19 (m, 1H), 7.22 (td
F RIP N , J = 7.4,
1.2 Hz, 1H), 7.3
1-7.45 (m, 2H)
7-(2-Chloropheny1)-8-(5-flu 1H-NMR (400 MHz, CDC13)
oro-2-methylphenoxymethyl)- 6 1.09 (s, 3H), 1.34 (s, 3
1,3,3-trimethy1-3,4-dihydr
H), 2.03 (s, 3H), 3.43 (s,
o-1H-quinoxalin-2-one (Comp
3H), 3.80 (s, 1H), 4.71 (d,
ound No.6-4)
J = 12.5 Hz, 1H), 5.18 (d,
F
J = 12.5 Hz, 1H), 6.14 (dd
, J = 11.0, 2.4 Hz, 1H), 6.
43 (td, J = 8.3, 2.4 Hz, 1H
010 0
), 6.77 (d, J = 8.0 Hz, 1H)
, 6.86 (d, J = 8.0 Hz, 1H),
6.91-6.95 (m, 1H), 7.28-7.
Cl N 33 (m, 2H),
7.39-7.43 (m, 1
H), 7.45-7.49 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methylpheny1)-
6 1.20 (s, 3H), 1.28 (s, 3
1,3,3-trimethy1-3,4-dihydr
H), 2.04 (s, 3H), 2.13 (s,
o-1H-quinoxalin-2-one (Comp
3H), 3.42 (s, 3H), 3.76 (s,
ound No.6-5)
1H), 4.74 (d, J = 12.0 Hz,
1 7 0
CA 02643070 2008-08-20
F 1H), 4.94
(d, J = 12.0 Hz,
1H), 6.14 (dd, J = 11.0, 2
.4 Hz, 1H), 6.44 (td, J = 8
O .3, 2.4 Hz, 1H), 6.76 (d, J
= 8.1 Hz, 1H), 6.84 (d, J
= 8.1 Hz, 1H), 6.94 (t, J =
7.6 Hz, 1H), 7.16-7.29 (m,
1\r< 4H)
7-(2-Ethylpheny1)-8-(5-fluo 1H-NMR (500 MHz, CDC13)
ro-2-methylphenoxymethyl)-1 6 1.06 (t, J = 7.6 Hz, 3H)
,3,3-trimethy1-3,4-dihydro-
, 1.21 (s, 3H), 1.30 (s, 3H
1H-quinoxalin-2-one (Compou
), 2.04 (s, 3H), 2.42-2.55
nd No.6-6)
(m, 2H), 3.40 (s, 3H), 3.76
F
(s, 1H), 4.72 (d, J = 11.9
Hz, 1H), 4.90 (d, J = 11.9
Hz, 1H), 6.14 (dd, J = 11.
O 0, 2.4 Hz, 1H), 6.45 (td, J
101 = 8.2, 2.4
Hz, 1H), 6.76 (
d, J = 7.9 Hz, 1H), 6.85 (d
, J = 7.9 Hz, 1H), 6.95 (t,
J = 7.3 Hz, 1H), 7.17-7.24
(m, 2H), 7.28-7.32 (m, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-trifluoromethy 6 1.29 (s, 3H), 1.32 (s, 3
lpheny1)-1,3,3-trimethy1-3,
H), 2.06 (s, 3H), 3.37 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.83 (s, 1H), 4.49 (d,
ne (Compound No.6-7)
J = 11.6 Hz, 1H), 4.88 (d,
F
J = 11.6 Hz, 1H), 6.21 (dd
, J = 10.9, 2.5 Hz, 1H), 6.
48 (td, J = 8.3, 2.5 Hz, 1H
O), 6.76 (d, J = 8.1 Hz, 1H)
101 , 6.86 (d, J
= 8.1 Hz, 1H),
6.98 (t, J = 7.5 Hz, 1H),
F F F N<7.43-7.52
(m, 3H), 7.73 (d,
J = 7.3 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxyphenyl 6 0.93 (s, 3H), 1.28 (s, 3
)-1,3,3-trimethy1-3,4-dihyd
H), 2.01 (s, 3H), 3.47 (s,
ro-1H-quinoxalin-2-one (Com
3H), 3.71 (s, 1H), 3.83 (s,
pound No.6-8)
3H), 4.85 (d, J - 13.7 Hz,
F
1H), 5.23 (d, J = 13.7 Hz,
1H), 6.05 (dd, J = 11.2, 2
.4 Hz, 1H), 6.38 (td, J = 8
0
.3, 2.4 Hz, 1H), 6.72 (d, J
= 8.0 Hz, 1H), 6.88 (td, J
= 7.6, 0.7 Hz, 1H), 6.91 (
N d, J - 8.0
Hz, 1H), 6.99 (d
d, J = 8.3, 0.7 Hz, 1H), 7.
171
CA 02643070 2008-08-20
v
06 (td, J = 7.5, 1.1 Hz, 1H
), 7.30-7.33 (m, 1H), 7.35-
7.40 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-trifluorometho 5 1.03 (s, 3H), 1.37 (s, 3
xypheny1)-1,3,3-trimethy1-3
H), 2.02 (s, 3H), 3.42 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.81 (s, 1H), 4.70 (d,
one (Compound No.6-9)
J = 12.9 Hz, 1H), 5.18 (d,
F
J = 12.9 Hz, 1H), 6.09 (dd
, J = 11.0, 2.4 Hz, 11-1), 6.
43 (td, J = 8.3, 2.4 Hz, 1H
0
), 6.77 (d, J = 8.1 Hz, 1H)
, 6.90 (d, J = 8.1 Hz, 1H),
6.93 (t, J = 7.5 Hz, 1H),
FKO RIP N 7.34-7.52 (m, 4H)
F-/
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methylthiophen 6 1.09 (s, 3H), 1.33 (s, 3
y1)-1,3,3-trimethy1-3,4-dih
H), 2.03 (s, 3H), 2.38 (s,
ydro-1H-quinoxalin-2-one (C
3H), 3.43 (s, 3H), 3.78 (s,
ompound No.6-10)
1H), 4.68 (d, J = 12.5 Hz,
F
1H), 5.20 (d, J = 12.5 Hz,
1H), 6.18 (dd, J = 11.2, 2
.4 Hz, 1H), 6.43 (td, J = 8
0
.3, 2.4 Hz, 1H), 6.77 (d, J
= 8.1 Hz, 1H), 6.87 (d, J
= 8.1 Hz, 1H), 6.91-6.95 (m
,s 41! N ,
1H), 7.15-7.29 (m, 3H), 7
.33-7.37 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxycarbony 6 1.25 (s, 3H), 1.28 (s, 3
lpheny1)-1,3,3-trimethy1-3,
H), 2.06 (s, 3H), 3.42 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.62 (s, 3H), 3.76 (s,
ne (Compound No.6-11)
1H), 4.69 (d, J = 12.0 Hz,
F
1H), 4.97 (d, J = 12.0 Hz,
1H), 6.19 (dd, J = 10.9, 2
.3 Hz, 1H), 6.46 (td, J = 8
0.3, 2.3 Hz, 1H), 6.73 (d, J
1.1 N,,0 =
8.1 Hz, 1H), 6.78 (d, J
= 8.1 Hz, 1H), 6.96 (t, J =
O 0 1\(<
7.7 Hz, 1H), 7.39-7.42 (m,
2H), 7.47-7.49 (m, 1H), 7.
91 (dd, J = 7.9, 1.5 Hz, 1H
7-(4-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 0.96 (s, 3H), 1.27 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.01 (s, 3H), 3.46 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.72 (s, 1H), 3.81 (s,
1 7 2
CA 02643070 2008-08-20
one (Compound No.6-12) 3H), 4.81 (d, J = 13.4 Hz,
F 1H), 5.17
(d, J = 13.4 Hz,
1H), 6.05 (dd, J = 11.0, 2
.5 Hz, 1H), 6.40 (td, J = 8
0
id& N,0 .3, 2.5 Hz, 1H), 6.71-6.73
(m, 1H), 6.72 (d, J = 7.9 H
F
z, 1H), 6.76 (td, J = 7.9,
N 2.4 Hz, 1H),
6.86 (d, J = 7
O .9 Hz, 1H),
6.91 (t, J = 7.
9 Hz, 1H), 7.24-7.27 (m, 1H
7-(5-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 1.01 (s, 3H), 1.29 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.01 (s, 3H), 3.44 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.75 (s, 1H), 3.79 (s,
one (Compound No.6-13)
3H), 4.82 (d, J = 13.3 Hz,
F
1H), 5.18 (d, J = 13.3 Hz,
1H), 6.10 (dd, J = 11.2, 2
.4 Hz, 1H), 6.41 (td, J = 8
010 0
.3, 2.4 Hz, 1H), 6.73 (d, J
= 8.1 Hz, 1H), 6.88 (d, J
= 8.1 Hz, 1H), 6.88-6.94 (m
, 2H), 7.02-7.08 (m, 2H)
7-(4-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 0.96 (s, 3H), 1.28 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.01 (s, 3H), 3.45 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.73 (s, 1H), 3.82 (s,
one (Compound No.6-14)
3H), 4.80 (d, J = 13.4 Hz,
F
1H), 5.17 (d, J - 13.4 Hz,
1H), 6.05 (dd, J = 11.3, 2
.4 Hz, 1H), 6.40 (td, J = 8
CI 0
.0, 2.4 Hz, 1H), 6.72 (d, J
= 7.9 Hz, 1H), 6.85 (d, J
id& N,0
- 7.9 Hz, 1H), 6.91 (t, J =
O N 8.0 Hz, 1H),
6.97 (d, J =
1.8 Hz, 1H), 7.05 (dd, J =
8.1, 1.8 Hz, 1H), 7.24 (d,
J = 8.1 Hz, 1H)
7-(5-Chloro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 1.05 (s, 3H), 1.28 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.02 (s, 3H), 3.44 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.76 (s, 1H), 3.80 (s,
one (Compound No.6-15)
3H), 4.80 (d, J = 13.1 Hz,
1H), 5.14 (d, J = 13.1 Hz,
1H), 6.11 (dd, J = 11.3, 2
.4 Hz, 1H), 6.42 (td, J - 8
.2, 2.4 Hz, 1H), 6.73 (d, J
= 7.9 Hz, 1H), 6.87 (d, J
173
CA 02643070 2008-08-20
i
F
= 7.9 Hz, 1H), 6.90 (d, J =
0
8.6 Hz, 1H), 6.92 (d, J =
8.6 Hz, 1H), 7.29-7.30 (m,
CI
S 0
I
N ,0 1H), 7.32 (d, J = 2.7 Hz, 1
H)
0 IW N
H
7-(2,4-Dimethoxypheny1)-8-( 1H-NMR (500 MHz, CDC13)
5-fluoro-2-methylphenoxymet 6 0.90 (s, 3H), 1.28 (s, 3
hyl)-1,3,3-trimethy1-3,4-di
H), 2.01 (s, 3H), 3.47 (s,
hydro-1H-quinoxalin-2-one (
3H), 3.68 (s, 1H), 3.81 (s,
Compound No.6-16)
3H), 3.87 (s, 3H), 4.86 (d
F 40
, J = 13.6 Hz, 1H), 5.22 (d
, J = 13.6 Hz, 1H), 6.04 (d
d, J = 11.2, 2.4 Hz, 1H), 6
0 0
I .38 (td, J = 8.4, 2.4 Hz, 1
010
H), 6.56 (d, J = 2.3 Hz, 1H
N ,0
), 6.60 (dd, J = 8.2, 2.3 H
0 IW N
z, 1H), 6.70 (d, J = 7.9 Hz
H , 1H), 6.87-6.90 (m, 1H), 6
.88 (d, J = 7.9 Hz, 1H), 7.
23 (d, J = 8.2 Hz, 1H)
7-(2,5-Dimethoxypheny1)-8-( 1H-NMR (400 MHz, CDC13)
5-fluoro-2-methylphenoxymet 6 0.91 (s, 3H), 1.31 (s, 3
hyl)-1,3,3-trimethy1-3,4-di
H), 2.01 (s, 3H), 3.47 (s,
hydro-1H-quinoxalin-2-one (
3H), 3.72 (s, 1H), 3.76 (s,
Compound No.6-17)
3H), 3.81 (s, 3H), 4.87 (d
F 40
, J = 13.7 Hz, 1H), 5.25 (d
, J = 13.7 Hz, 1H), 6.09 (d
0 d, J = 11.2, 2.4 Hz, 1H), 6
0
010
I
N ,0 .39 (td, J = 8.3, 2.4 Hz, 1
H), 6.72 (d, J = 8.1 Hz, 1H
0
), 6.87-6.92 (m, 5H)
IW N
H
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-5-meth 6 0.93 (s, 3H), 1.29 (s, 3
ylpheny1)-1,3,3-trimethy1-3
H), 2.01 (s, 3H), 2.33 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.47 (s, 3H), 3.70 (br
one (Compound No.6-18)
s, 1H), 3.79 (s, 3H), 4.84
(d, J = 13.7 Hz, 1H), 5.22
(d, J = 13.7 Hz, 1H), 6.08
(dd, J = 11.2, 2.4 Hz, 1H)
, 6.39 (td, J = 8.3, 2.4 Hz
, 1H), 6.72 (d, J = 8.0 Hz,
1H), 6.87-6.91 (m, 2H), 6.
90 (d, J = 8.0 Hz, 1H), 7.1
174
CA 02643070 2008-08-20
1
F * 3 (d, J = 2.3 Hz, 1H), 7.16
(dd, J = 8.3, 2.3 Hz, 1H)
010 0
Lo
0 IW N
H
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-(2-methoxy-5-trif 6 1.09 (s, 3H), 1.28 (s, 3
luoromethylpheny1)-1,3,3-tr
H), 1.99 (s, 3H), 3.45 (s,
imethy1-3,4-dihydro-1H-quin
3H), 3.78 (s, 1H), 3.88 (s,
oxalin-2-one (Compound No.
3H), 4.77 (d, J = 12.8 Hz,
6-19)
1H), 5.11 (d, J = 12.8 Hz,
F 010
1H), 6.09 (dd, J = 11.0, 2
F F F
.4 Hz, 1H), 6.42 (td, J = 8
.4, 2.4 Hz, 1H), 6.76 (d, J
410 0
I
N ,0 = 7.9 Hz, 1H), 6.89 (d, J
= 7.9 Hz, 1H), 6.90-6.94 (m
, 1H), 7.04 (d, J = 8.9 Hz,
0 SN 1H), 7.56 (d, J - 2.1 Hz,
H 1H), 7.62 (dd, J = 8.9, 2.1
Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(5-isopropyl-2-me 5 0.88 (s, 3H), 1.26 (d, J
thoxypheny1)-1,3,3-trimethy
= 6.9 Hz, 3H), 1.26 (d, J
1-3,4-dihydro-1H-quinoxali
= 6.9 Hz, 3H), 1.30 (s, 3H)
n-2-one (Compound No.6-20)
, 2.01 (s, 3H), 2.91 (septe
F le
t, J = 6.9 Hz, 1H), 3.49 (s
, 3H), 3.70 (s, 1H), 3.81 (
s, 3H), 4.86 (d, J = 13.8 H
010 0
I
NOz, 1H), 5.25 (d, J = 13.8 H
z, 1H), 6.04 (dd, J = 11.2,
2.4 Hz, 1H), 6.37 (td, J =
0 IW N
8.3, 2.4 Hz, 1H), 6.72 (d,
H J = 8.1 Hz, 1H), 6.86-6.90
(m, 1H), 6.92 (d, J = 8.3
Hz, 1H), 6.92 (d, J = 8.1 H
z, 1H), 7.19 (d, J = 2.4 Hz
, 1H), 7.23 (dd, J = 8.3, 2
.4 Hz, 1H)
7-(5-tert-Butyl-2-methoxyph 1H-NMR (400 MHz, CDC13)
eny1)-8-(5-fluoro-2-methylp 6 0.84 (s, 3H), 1.31 (s, 3
henoxymethyl)-1,3,3-trimeth
H), 1.34 (s, 9H), 2.01 (s,
y1-3,4-dihydro-1H-quinoxali
3H), 3.50 (s, 3H), 3.69 (s,
n-2-one (Compound No.6-21)
1H), 3.81 (s, 3H), 4.87 (d
, J = 13.9 Hz, 1H), 5.27 (d
, J = 13.9 Hz, 1H), 6.01 (d
1 7 5
CA 02643070 2008-08-20
,
F
d, J = 11.2, 2.4 Hz, 1H), 6
.36 (td, J = 8.3, 2.4 Hz, 1
H), 6.73 (d, J = 7.9 Hz, 1H
I
010 0
N,0 ), 6.85-6.90 (m, 1H), 6.92
(d, J = 7.9 Hz, 1H), 6.92 (
d, J = 8.5 Hz, 1H), 7.36 (d
0 IW N , J = 2.4 Hz, 1H), 7.39 (dd
, J = 8.5, 2.4 Hz, 1H)
H
7-(3-Fluoro-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 0.96 (s, 3H), 1.37 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.00 (s, 3H), 3.44 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.66 (d, J = 1.7 Hz, 3
one (Compound No.6-22)
H), 3.78 (br s, 1H), 4.80 (
F 40
d, J = 13.3 Hz, 1H), 5.28 (
d, J = 13.3 Hz, 1H), 6.09 (
dd, J = 11.1, 2.4 Hz, 1H),
010 0
I 6.41 (td, J = 8.3, 2.4 Hz,
F 11,0
1H), 6.75 (d, J = 8.1 Hz, 1
il& ,
H), 6.91 (t, J = 7.6 Hz, 1H
0 IW N ), 6.91 (d, J = 8.1 Hz, 1H)
H , 7.07-7.17 (m, 3H)
7-(6-Fluoro-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 1.06 (s, 3H), 1.17 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.02 (s, 3H), 3.46 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.75 (br s, 1H), 3.82
one (Compound No.6-23)
(s, 3H), 5.02 (s, 2H), 6.15
F 40
(dd, J = 11.3, 2.5 Hz, 1H)
, 6.39 (td, J = 8.3, 2.5 Hz
, 1H), 6.73 (d, J = 8.1 Hz,
F 0
op
I 1H), 6.78-6.83 (m, 2H), 6.
88-6.91 (m, 1H), 6.91 (d, J
N,0
= 8.1 Hz, 1H), 7.31 (td, J
0 IW N = 8.3, 6.6 Hz, 1H)
H
7-(3,5-Difluoro-2-methoxyph 1H-NMR (400 MHz, CDC13)
eny1)-8-(5-fluoro-2-methylp ö 1.03 (s, 3H), 1.37 (s, 3
henoxymethyl)-1,3,3-trimeth
H), 2.00 (s, 3H), 3.42 (s,
y1-3,4-dihydro-1H-quinoxali
3H), 3.59 (s, 3H), 3.83 (s,
n-2-one (Compound No.6-24)
1H), 4.76 (d, J = 13.2 Hz,
F 0
1H), 5.24 (d, J = 13.2 Hz,
1H), 6.15 (dd, J = 11.0, 2
F .4 Hz, 1H), 6.44 (td, J = 8
0100
I .3, 2.4 Hz, 1H), 6.76 (d, J
F l&
= 7.8 Hz, 1H), 6.87-6.95 (
i N,10
N m, 4H)
0 IW
H
176
CA 02643070 2008-08-20
7-(3,4-Difluoropheny1)-8-( 1H-NMR (500 MHz, CDC13)
5-fluoro-2-methylphenoxymet 6 1.26 (s, 6H), 2.13 (s, 3
hyl)-1,3,3-trimethy1-3,4-di
H), 3.45 (s, 3H), 3.80 (br
hydro-1H-quinoxalin-2-one (
s, 1H), 4.86 (s, 2H), 6.22
Compound No.6-25)
(dd, J = 11.0, 2.4 Hz, 1H),
F
6.51 (td, J = 8.2, 2.4 Hz,
1H), 6.78 (d, J = 8.1 Hz,
1H), 6.92 (d, J = 8.1 Hz, 1
F 0 H), 7.01 (t,
J = 7.5 Hz, 1H
ro ), 7.11-7.18
(m, 2H), 7.24-
N< 7.29 (m, 1H)
7-(5-Chloro-4-fluoro-2-meth 1H-NMR (500 MHz, CDC13)
oxypheny1)-8-(5-fluoro-2-me 6 1.08 (s, 3H), 1.27 (s, 3
thylphenoxymethyl)-1,3,3-tr
H), 2.02 (s, 3H), 3.43 (s,
imethy1-3,4-dihydro-1H-quin
3H), 3.77 (s, 1H), 3.80 (s,
oxalin-2-one (Compound No.
3H), 4.76 (d, J = 13.0 Hz,
6-26)
1H), 5.10 (d, J = 13.0 Hz,
F
1H), 6.12 (dd, J = 11.0, 2
.4 Hz, 1H), 6.44 (td, J = 7
CI .9, 2.4 Hz,
1H), 6.73 (d, J
F 0 = 8.1 Hz,
1H), 6.79 (d, J
= 10.7 Hz, 1H), 6.84 (d, J
id& N,0
= 8.1 Hz, 1H), 6.94 (t, J =
O N 7.9 Hz, 1H),
7.33 (d, J =
8.2 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-5-nitr 6 1.18 (s, 3H), 1.27 (s, 3
opheny1)-1,3,3-trimethy1-3,
H), 2.01 (s, 3H), 3.43 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.84 (s, 1H), 3.93 (s,
ne (Compound No.6-27)
3H), 4.73 (d, J = 12.7 Hz,
1H), 5.05 (d, J = 12.7 Hz,
0'N-t0 F 1H), 6.12
(dd, J = 11.0, 2
.4 Hz, 1H), 6.44 (td, J = 8
0
.3, 2.4 Hz, 1H), 6.78 (d, J
010
= 8.0 Hz, 1H), 6.88 (d, J
= 8.0 Hz, 1H), 6.91-6.95 (m
O N , 1H), 7.04
(d, J = 9.0 Hz,
1H), 8.21 (d, J = 2.8 Hz,
1H), 8.27 (dd, J = 9.0, 2.8
Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-nitr
6 1.05 (s, 3H), 1.31 (s, 3
opheny1)-1,3,3-trimethy1-3,
H), 1.99 (s, 3H), 3.45 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 3.85 (s, 1H), 3.93 (s,
ne (Compound No.6-28)
3H), 4.75 (d, J = 13.2 Hz,
1H), 5.15 (d, J - 13.2 Hz,
1 7 7
CA 02643070 2008-08-20
F 1H), 6.05
(dd, J - 11.0, 2
.4 Hz, 1H), 6.43 (td, J = 8
.3, 2.4 Hz, 1H), 6.76 (d, J
0 = 8.1 Hz,
1H), 6.86 (d, J
a 010 NO
= 8.1 Hz, 1H), 6.92 (t, J =
7.6 Hz, 1H), 7.47 (d, J =
N 8.2 Hz, 1H), 7.83 (d, J = 2
.2 Hz, 1H), 7.93 (dd, J = 8
.2, 2.2 Hz, 1H)
7-(5-Cyano-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 1.13 (s, 3H), 1.27 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.02 (s, 3H), 3.43 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.82 (s, 1H), 3.88 (s,
one (Compound No.6-29)
3H), 4.72 (d, J = 12.8 Hz,
F
1H), 5.06 (d, J = 12.8 Hz,
1H), 6.10 (dd, J = 11.0, 2
ON .4 Hz, 1H),
6.45 (td, J = 8
0
.3, 2.4 Hz, 1H), 6.76 (d, J
= 8.1 Hz, 1H), 6.84 (d, J
= 8.1 Hz, 1H), 6.95 (t, J =
N 7.2 Hz, 1H), 7.02 (d, J =
8.5 Hz, 1H), 7.59 (d, J = 2
.2 Hz, 1H), 7.66 (dd, J = 8
.5, 2.2 Hz, 1H)
7-(5-Acetyl-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 1.03 (s, 3H), 1.29 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.00 (s, 3H), 2.58 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.46 (s, 3H), 3.77 (s,
one (Compound No.6-30)
1H), 3.90 (s, 3H), 4.79 (d
F
, J = 13.2 Hz, 1H), 5.14 (d
0 , J = 13.2
Hz, 1H), 6.07 (d
d, J = 11.1, 2.5 Hz, 1H), 6
410 0
Asith .41 (td, J = 8.3, 2.5 Hz, 1
H), 6.76 (d, J = 8.1 Hz, 1H
), 6.88-6.93 (m, 1H), 6.89
N (d, J = 8.1 Hz, 1H), 7.03 (
d, J = 8.8 Hz, 1H), 7.92 (d
, J = 2.3 Hz, 1H), 8.03 (dd
, J = 8.8, 2.3 Hz, 1H)
7-(4-Benzoyloxy-2-methoxyph 1H-NMR (400 MHz, CDC13)
eny1)-8-(5-fluoro-2-methylp 6 0.95 (s, 3H), 1.28 (s, 3
henoxymethyl)-1,3,3-trimeth
H), 2.03 (s, 3H), 3.47 (s,
y1-3,4-dihydro-1H-quinoxali
3H), 3.73 (br s, 1H), 3.84
n-2-one (Compound No.6-31)
(s, 3H), 4.89 (d, J = 13.7
Hz, 1H), 5.23 (d, J = 13.7
Hz, 1H), 6.09 (dd, J = 11.0
, 2.4 Hz, 1H), 6.40 (td, J
, 8.3, 2.4 Hz, 1H), 6.73 (d
, J = 8.1 Hz, 1H), 6.89-6.9
1 7 8
CA 02643070 2008-08-20
;
F 6
(m, 4H), 7.37 (d, J = 8.1
Hz, 1H), 7.52-7.56 (m, 2H)
So 0 , 7.64-7.69 (m, 1H),
8.22-8
.25 (m, 2H)
0 010 N,0
,0 N
7-(5-Benzoyloxy-2-methoxyph Ili-NKR (500 MHz, CDC13)
eny1)-8-(5-fluoro-2-methylp 6 1.03 (s, 3H), 1.25 (s, 3
henoxymethyl)-1,3,3-trimeth
H), 2.01 (s, 3H), 3.45 (s,
y1-3,4-dihydro-1H-quinoxali
3H), 3.77 (s, 1H), 3.84 (s,
n-2-one (Compound No.6-32)
3H), 4.90 (d, J = 13.4 Hz,
0 F
1H), 5.20 (d, J = 13.4 Hz,
1H), 6.17 (dd, J = 11.3, 2
0 0
0 410 .4
Hz, 1H), 6.41 (td, J = 8
.2, 2.4 Hz, 1H), 6.73 (d, J
= 8.1 Hz, 1H), 6.90 (t, J
= 7.3 Hz, 1H), 6.96 (d, J =
N 8.1 Hz, 1H), 7.02 (d, J =
8.8 Hz, 1H), 7.19 (d, J = 3
.0 Hz, 1H), 7.23 (dd, J = 8
.8, 3.0 Hz, 1H), 7.51 (t, J
= 7.8 Hz, 2H), 7.64 (t, J
= 7.8 Hz, 1H), 8.19 (d, J =
7.8 Hz, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-(5-hydroxy-2-meth 6 0.93 (s, 3H), 1.29 (s, 3
oxypheny1)-1,3,3-trimethyl-
H), 2.01 (s, 3H), 3.45 (s,
3,4-dihydro-1H-quinoxalin-
3H), 3.73 (s, 1H), 3.75 (s,
2-one (Compound No.6-33)
3H), 4.87 (d, J = 13.7 Hz,
F
1H), 5.01 (s, 1H), 5.23 (d
, J = 13.7 Hz, 1H), 6.10 (d
OH d, J = 11.0, 2.4 Hz,
1H), 6
010 0
14& N,0 .39 (td, J = 8.4, 2.4 Hz, 1
H), 6.71 (d, J = 7.9 Hz, 1H
), 6.83-6.86 (m, 3H), 6.89
N (d, J = 7.9 Hz, 1H), 6.90 (
t, J = 7.3 Hz, 1H)
7-(4-Cyano-2-methoxyphenyl 1H-NMR (500 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 1.03 (s, 3H), 1.30 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 1.99 (s, 3H), 3.45 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.81 (s, 1H), 3.86 (s,
one (Compound No.6-34)
3H), 4.75 (d, J = 13.1 Hz,
1H), 5.14 (d, J = 13.1 Hz,
1H), 6.04 (dd, J = 11.0, 2
.4 Hz, 1H), 6.43 (td, J = 8
.2, 2.4 Hz, 1H), 6.75 (d, J
1 7 9
CA 02643070 2008-08-20
,
F
= 7.9 Hz, 1H), 6.84 (d, J
*
= 7.9 Hz, 1H), 6.91-6.94 (m
, 1H), 7.20 (d, J = 1.5 Hz,
0 I.
I
1H), 7.35 (dd, J = 7.6, 1.
NC
Hz, 1H), 7.40 (d, J = 7.6
N,0 Hz, 1H)
0 IW N
H
7-(4-Acetyl-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylpheno 6 0.96 (s, 3H), 1.31 (s, 3
xymethyl)-1,3,3-trimethy1-3
H), 2.00 (s, 3H), 2.66 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.46 (s, 3H), 3.77 (s,
one (Compound No.6-35)
1H), 3.90 (s, 3H), 4.79 (d
F 10
, J = 13.5 Hz, 1H), 5.20 (d
, J = 13.5 Hz, 1H), 6.03 (d
0 d, J = 11.1, 2.4 Hz, 1H), 6
010 0
I
N,0 .40 (td, J = 8.2, 2.4 Hz, 1
H), 6.74 (d, J = 8.1 Hz, 1H
), 6.89-6.92 (m, 1H), 6.89
0 IW N.< (d,
J = 8.1 Hz, 1H), 7.41 (
H d,
J - 7.8 Hz, 1H), 7.60 (d
, J = 1.6 Hz, 1H), 7.64 (dd
, J = 7.8, 1.6 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-(2-methoxy-4-meth 6 0.90 (s, 3H), 1.28 (s, 3
ylpheny1)-1,3,3-trimethy1-3
H), 2.01 (s, 3H), 2.43 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 3.46 (s, 3H), 3.68 (s,
one (Compound No.6-36)
1H), 3.81 (s, 3H), 4.86 (d
F 40
, J = 13.7 Hz, 1H), 5.23 (d
, J = 13.7 Hz, 1H), 6.05 (d
d, J = 11.3, 2.4 Hz, 1H), 6
010 0
I
id& N,0 .38
(td, J = 8.2, 2.4 Hz, 1
H), 6.71 (d, J = 8.1 Hz, 1H
), 6.80 (s, 1H), 6.87-6.90
0 IW N.< (m,
2H), 6.89 (d, J - 8.1 H
H z,
1H), 7.20 (d, J = 7.6 Hz
, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-5-meth 6 1.08 (s, 3H), 1.24 (s, 3
oxycarbonylpheny1)-1,3,3-tr
H), 2.01 (s, 3H), 3.45 (s,
imethy1-3,4-dihydro-1H-quin
3H), 3.81 (s, 1H), 3.88 (s,
oxalin-2-one (Compound No.
3H), 3.89 (s, 3H), 4.80 (d
6-37)
, J = 13.1 Hz, 1H), 5.11 (d
, J = 13.1 Hz, 1H), 6.09 (d
d, J = 11.1, 2.4 Hz, 1H), 6
.41 (td, J = 8.3, 2.4 Hz, 1
H), 6.75 (d, J = 7.8 Hz, 1H
), 6.89-6.92 (m, 1H), 6.90
180
CA 02643070 2008-08-20
F (d, J = 7.8
Hz, 11-I), 7.01 (
d, J = 8.5 Hz, 1H), 7.99 (d
0 0 , J = 2.2
Hz, 1H), 8.07 (dd
010 0
, J = 8.5, 2.2 Hz, 1H)
NO
8-(2-Methoxyphenylaminometh 1H-NMR (400 MHz, CDC13)
y1)-7-(2-methoxy-5-trifluor 6 1.22 (s, 3H), 1.43 (s, 3
omethylpheny1)-1,3,3-trimet
H), 3.47 (s, 3H), 3.71 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.78 (s, 1H), 3.84 (s,
in-2-one (Compound No.6-38)
3H), 4.09-4.11 (m, 2H), 4.
39 (br s, 1H), 6.30 (dd, J
F F F = 7.8, 1.5
Hz, 1H), 6.57 (t
d, J = 7.8, 1.5 Hz, 1H), 6.
NH
011
N,0 65 (dd, J =
8.1, 1.5 Hz, 1H
), 6.70-6.74 (m, 1H), 6.71
(d, J = 8.1 Hz, 1H), 6.80 (
N d, J = 8.1 Hz, 1H), 6.96 (d
, J = 8.7 Hz, 1H), 7.45 (d,
J = 2.0 Hz, 1H), 7.56 (dd,
J = 8.7, 2.0 Hz, 1H)
7-(2-Methoxy-4-methoxymetho 1H-NMR (500 MHz, CDC13)
xypheny1)-8-(2-methoxypheny ö 1.16 (s, 3H), 1.42 (s, 3
laminomethyl)-1,3,3-trimeth
H), 3.46 (s, 3H), 3.50 (s,
y1-3,4-dihydro-1H-quinoxali
3H), 3.70 (s, 1H), 3.73 (s,
n-2-one (Compound No.6-39)
3H), 3.77 (s, 3H), 4.13 (d
010
0 , J = 5.3 Hz, 2H), 4.52 (t,
J = 5.3 Hz, 1H), 5.19 (s,
2H), 6.34 (dd, J = 7.6, 1.5
70,0 NH
Hz, 1H), 6.56 (td, J = 7.6
N,0 , 1.5 Hz,
1H), 6.61 (d, J =
2.4 Hz, 1H), 6.65-6.67 (m,
N 2H), 6.68 (d,
J = 7.9 Hz,
1H), 6.72 (td, J = 7.6, 1.5
Hz, 1H), 6.80 (d, J = 7.9
Hz, 1H), 7.07 (d, J = 8.2 H
z, 1H)
7-(2-Methoxy-5-methylphenyl 1H-NMR (400 MHz, CDC13)
)-8-(2-methoxyphenylaminome 5 1.19 (s, 3H), 1.42 (s, 3
thyl)-1,3,3-trimethy1-3,4-d
H), 2.29 (s, 3H), 3.47 (s,
ihydro-1H-quinoxalin-2-one
3H), 3.71 (s, 1H), 3.73 (s,
(Compound No.6-40)
3H), 3.76 (s, 3H), 4.13 (b
r s, 2H), 4.52 (br s, 1H),
6.34 (dd, J = 7.8, 1.4 Hz,
1H), 6.56 (td, J = 7.8, 1.4
Hz, 1H), 6.66 (dd, J = 7.8
181
CA 02643070 2008-08-20
;
, 1.4 Hz, 1H), 6.69 (d, J =
8.1 Hz, 1H), 6.72 (td, J =
0
7.8, 1.4 Hz, 1H), 6.80 (d,
010 NH
d& , J - 8.2 Hz, 1H), 6.82 (d,
J = 8.1 Hz, 1H), 6.99 (d, J
N0
- 2.0 Hz, 1H), 7.09 (dd, J
O N = 8.2, 2.0 Hz, 1H)
8-(5-Fluoro-2-methylphenyla 1H-NMR (400 MHz, CDC13)
minomethyl)-7-(2-methoxy-4- 5 1.17 (s, 3H), 1.40 (s, 3
methoxymethoxypheny1)-1,3,
H), 1.85 (s, 3H), 3.42 (s,
3-trimethy1-3,4-dihydro-1H-
3H), 3.51 (s, 3H), 3.73 (s,
quinoxalin-2-one (Compound
1H), 3.77 (s, 3H), 3.83 (b
No.6-41)
r s, 1H), 4.13-4.23 (m, 2H)
, 5.20 (s, 2H), 6.03 (dd, J
F
= 11.7, 2.5 Hz, 1H), 6.22
(td, J = 8.4, 2.5 Hz, 1H),
6.65 (d, J = 2.3 Hz, 1H), 6
NH.70 (d, J = 7.8 Hz, 1H), 6.
71 (dd, J = 8.3, 2.3 Hz, 1H
id& N,0
), 6.81-6.85 (m, 1H), 6.83
O N (d,
J - 7.8 Hz, 1H), 7.11 (
d, J = 8.3 Hz, 1H)
7-(2-Methoxy-5-methoxycarbo 1H-NMR (400 MHz, CDC13)
nylpheny1)-8-(2-methoxyphen 6 1.20 (s, 3H), 1.43 (s, 3
ylaminomethyl)-1,3,3-trimet
H), 3.47 (s, 3H), 3.70 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.76 (s, 1H), 3.85 (s,
in-2-one (Compound No.6-42)
3H), 3.88 (s, 3H), 4.09 (d
, J - 5.3 Hz, 2H), 4.41 (t,
0 o 010 J -
5,3 Hz, 1H), 6.31 (dd,
0
J = 7.7, 1.4 Hz, 1H), 6.56
010 NH
SN,0
(td, J = 7.7, 1.4 Hz, 1H),
6.64 (dd, J = 7.7, 1.5 Hz,
1H), 6.71 (d, J = 8.0 Hz,
N 1H), 6.71 (td, J = 7.7, 1.5
Hz, 1H), 6.81 (d, J = 8.0
Hz, 1H), 6.93 (d, J = 8.5 H
z, 1H), 7.88 (d, J = 2.2 Hz
, 1H), 8.01 (dd, J = 8.5, 2
.2 Hz, 1H)
7-(2-Methoxy-5-nitrophenyl 1H-NMR (500 MHz, CDC13)
)-8-(2-methoxyphenylaminome 6 1.25 (s, 3H), 1.43 (s, 3
thyl)-1,3,3-trimethy1-3,4-d
H), 3.47 (s, 3H), 3.70 (s,
ihydro-1H-quinoxalin-2-one
3H), 3.82 (s, 1H), 3.89 (s,
(Compound No.6-43)
3H), 4.06 (d, J = 14.1 Hz,
1H), 4.13 (d, J = 14.1 Hz,
1H), 4.30 (br s, 1H), 6.28
(dd, J = 7.8, 1.4 Hz, 1H),
1 8 2
CA 02643070 2008-08-20
6.57 (td, J = 7.8, 1.4 Hz,
1H), 6.64 (dd, J = 7.8, 1.
C 10
0 4 Hz, 1H),
6.70 (td, J = 7.
NH
8, 1.4 Hz, 1H), 6.73 (d, J
= 7.9 Hz, 1H), 6.79 (d, J
7.9 Hz, 1H), 6.94 (d, J=
o N 9.2 Hz, 1H),
8.09 (d, J = 2
.7 Hz, 1H), 8.20 (dd, J = 9
.2, 2.7 Hz, 1H)
Example 7
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-nitropheny1)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.7-1)
A mixture of 7-(5,5-
dimethyl[1,3,2]dioxaborinan-2-y1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.21, 68.2 mg,
0.155 mmol), 2-nitro-1-iodobenzene (79.0 mg, 0.317
mmol), sodium hydrogen carbonate (41.5 mg, 0.494
mmol) and tetrakis(triphenylphosphine)palladium (0)
(19.0 mg, 0.0164 mmol) was suspended in mixed solvent
of anhydrous N,N-dimethylformamide (0.5 ml) and water
(0.5 mL), and stirred at 120 C for 30 minutes by
irradiated microwave. After cooling down, ethyl
acetate (15 mL) and water (15 mL) were added and
partitioned. The organic layer was washed with
saturated brine (15 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled compound (42.6 mg)
as a yellow solid. (Yield 61%)
1 8 3
CA 02643070 2008-08-20
F 1H-NMR (400 MHz, CDC13)
6 1.30 (s, 6H), 2.09 (s,
3H), 3.42 (s, 3H), 3.84 (s
010 0
, 1H), 4.62 (d, J - 11.5 H
z, 1H), 4.93 (d, J = 11.5
Hz, 1H), 6.24 (dd, J = 10.
OO N 7, 2.4 Hz, 1H), 6.49 (td,
J = 8.3, 2.4 Hz, 1H), 6.75
(d, J = 8.0 Hz, 1H), 6.78
(d, J = 8.0 Hz, 1H), 6.99
(t, J = 7.4 Hz, 1H), 7.4
6-7.57 (m, 3H), 7.92 (dd,
J = 8.1, 0.7 Hz, 1H)
Using any compounds among Reference Compounds No.22
and available compounds, the following Compound
(No.7-2) was obtained by a method similar to that of
Compound No.7-1.
7-(2-Cyanopheny1)-8-(5-fluo 1H-NMR (400 MHz, CDC13)
ro-2-methylphenoxymethyl)-1 6 1.15 (s, 3H), 1.36 (s, 3
,3,3-trimethy1-3,4-dihydro-
H), 2.04 (s, 3H), 3.45 (s,
1H-quinoxalin-2-one (Compou
3H), 3.88 (s, 1H), 4.72 (d,
nd No.7-2)
J - 12.4 Hz, 1H), 5.21 (d,
F
J = 12.4 Hz, 1H), 6.13 (dd
, J = 11.0, 2.4 Hz, 1H), 6.
46 (td, J = 8.3, 2.4 Hz, 1H
010 0
), 6.80 (d, J = 8.1 Hz, 1H)
, 6.94 (d, J - 8.1 Hz, 1H),
6.96 (t, J = 7.6 Hz, 1H),
CN N 7.45 (td, J - 7.7, 1.4
Hz,
1H), 7.54 (dd, J = 7.7, 0.9
Hz, 1H), 7.61 (td, J = 7.7
, 1.4 Hz, 1H), 7.75 (dd, J
- 7.7, 0.9 Hz, 1H)
Example 8
7-(5-Amino-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Compound No.8-1)
1 8 4
CA 02643070 2008-08-20
\
A mixture of 8-(5-
fluoro-2-
methylphenoxymethyl)-7-(2-methoxy-5-nitropheny1)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.6-27, 18.8 mg, 0.0392 mmol) and tin
chloride (II)
(23.0 mg, 0.121 mmol) was suspended in
mixed solvent of anhydrous N,N-dimethylformamide
(0.25 ml) and anhydrous ethanol (0.5 mL), and stirred
at 80 C overnight. After cooling down, the reaction
mixture was diluted with ethyl acetate (10 mL) and
saturated aqueous sodium hydrogen carbonate solution
was added thereto until the pH became 9. After the
precipitate was filtered, the filtrate was washed
with water (50 mL) and saturated brine (50 mL)
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled compound (8.2 mg) as a brown solid.
(Yield 47%)
F H-NMR (400 MHz, CDC13)
6 0.89 (s, 3H), 1.31 (s,
NH2 3H),
2.01 (s, 3H), 3.46 (s
010 0
id& NO , 3H), 3.72 (s, 3H), 3.81
(s, 1H), 4.88 (d, J = 13.7
Hz, 1H), 5.26 (d, J - 13.
N 7
Hz, 1H), 6.11 (dd, J = 1
1.4, 2.5 Hz, 1H), 6.39 (td
, J = 8.3, 2.5 Hz, 1H), 6.
70-6.73 (m, 3H), 6.82 (d,
J = 8.8 Hz, 1H), 6.89 (t,
J = 7.2 Hz, 1H), 6.90 (d,
J = 7.8 Hz, 1H)
185
CA 02643070 2008-08-20
Using Compound No.6-28, the following Compound (No.8-
2) was obtained by a method similar to that of
Compound No.8-1.
7-(4-Amino-2-methoxyphenyl 1H-NMR (400 MHz, CDC13)
)-8-(5-fluoro-2-methylphen 6 0.87 (s, 3H), 1.28 (s, 3H
oxymethyl)-1,3,3-trimethy
), 2.01 (s, 3H), 3.47 (s, 3H
1-3,4-dihydro-1H-quinoxali
), 3.64 (s, 1H), 3.78 (s, 3H
n-2-one (Compound No.8-2)
), 4.89 (d, J = 13.7 Hz, 1H)
F
, 5.23 (d, J = 13.7 Hz, 1H),
6.05 (dd, J = 11.2, 2.4 Hz,
1H), 6.33 (d, J = 2.2 Hz, 1
=0
N,0 H), 6.37
(td, J = 8.4, 2.4 H
H2 N
z, 1H), 6.40 (dd, J - 8.0, 2
.2 Hz, 1H), 6.68 (d, J = 8.1
N Hz, 1H),
6.88 (d, J = 8.1 H
z, 1H), 6.88 (t, J - 7.6 Hz,
1H), 7.10 (d, J = 8.0 Hz, 1
H)
Example No.9
8-(5-Fluoro-2-methylphenoxymethyl)-7-(5-
hydroxymethy1-2-methoxypheny1)-1,3,3-trimethyl-3,4-
dihydro-1H-quinoxalin-2-one (Compound No. 9-1)
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-
methoxy-5-methoxycarbonylpheny1)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Compound No.6-37, 50.2
mg, 0.102 mmol) was dissolved in anhydrous
tetrahydrofuran (0.5 ml), and lithium aluminum
hydride (6.8 mg, 0.18 mmol) was added thereto at 0 C.
After the reaction mixture was stirred at same
temperature for 30 minutes, ethyl acetate (1 mL) and
water (1 mL) were added thereto successively.
Moreover ethyl acetate (10 mL) and water (10 mL) were
added and partitioned. The organic layer was washed
186
CA 02643070 2008-08-20
with saturated brine (10 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled compound (29.8 mg)
as a pale yellow solid. (Yield 47%)
F 1H-NMR (500 MHz, CDC13)
HO 6 0.97 (s, 31-1), 1.29 (s,
3H), 2.01 (s, 3H), 3.47 (s
410 0
, 3H), 3.80 (br s, 1H), 3.
83 (s, 3H), 4.66 (s, 2H),
4.81 (d, J = 13.3 Hz, 1H),
O N 5.20 (d, J = 13.3 Hz, 1H)
, 6.07 (dd, J = 11.3, 2.4
Hz, 1H), 6.39 (td, J = 8.4
, 2.4 Hz, 1H), 6.73 (d, J
= 7.9 Hz, 1H), 6.89-6.91 (
m, 1H), 6.91 (d, J = 7.9 H
z, 1H), 6.97 (d, J = 8.5 H
z, 1H), 7.32 (d, J = 2.2 H
z, 1H), 7.37 (dd, J = 8.5,
2.2 Hz, 1H)
Using Compound No.6-42, the following Compound (No.9-2
) was obtained by a method similar to that of Compound
No.9-1.
7-(5-hydroxymethy1-2-metho 1H-NMR (400 MHz, CDC13)
xypheny1)-8-(2-methoxyphen 6 1.21 (s, 3H), 1.42 (s, 3H
ylaminomethyl)-1,3,3-trime
), 3.47 (s, 3H), 3.73 (s, 4H
thy1-3,4-dihydro-1H-quinox
), 3.79 (s, 3H), 4.07 (d, J
alin-2-one (Compound No.9-
= 13.7 Hz, 1H), 4.13 (d, J =
2)
13.7 Hz, 1H), 4.47 (br s, 1
H), 4.61 (d, J = 5.9 Hz, 2H)
, 6.32 (dd, J = 7.8, 1.5 Hz,
1H), 6.56 (td, J = 7.8, 1.5
Hz, 1H), 6.67 (dd, J - 7.8,
1.4 Hz, 1H), 6.70 (d, J = 7
.8 Hz, 1H), 6.73 (td, J = 7.
1 8 7
CA 02643070 2008-08-20
HO 110 8, 1.4Hz,
1H), 6.82 (d, J =
7.8 Hz, 1H), 6.89 (d, J = 8.
0 3 Hz, 1H),
7.19 (d, J = 2.2
NH
f4s N,0 Hz, 1H), 7.29
(dd, J = 8.3,
010
2.2 Hz, 1H)
0 N
Example 10
7-(5-Carboxy-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Compound No.10-1)
8-(5-Fluoro-2-methylphenoxymethyl)-7-(2-
methoxy-5-methoxycarbonylpheny1)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Compound No.6-37, 50.5
mg, 0.103 mmol) was dissolved in mixed solvent of
tetrahydrofuran (1 mL) and methanol (1 mL), and 4N
aqueous sodium hydroxide solution (0.5 mL) was added
thereto and stirred at room temperature overnight.
Ethyl acetate (15 mL) and 0.25N aqueous HC1 solution
(20 mL) were added to the reaction mixture and
partitioned. The organic layer was washed with
saturated brine (15 mL), dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate). The obtained materials were filtered
with chloroform (3 mL) to give the titled compound
(20.3 mg) as a pale yellow solid. (Yield 42%)
188
CA 02643070 2008-08-20
, .
F 0 1H-NMR (400 MHz, DMSO-d0
HO 0 6 0.83 (s, 3H), 1.11 (s,
3H), 1.92 (s, 3H), 3.32 (s
010 0
id& NI.0 , 3H), 3.86 (s, 3H), 4.75
(d, J = 13.7 Hz, 1H), 5.20
(d, J = 13.7 Hz, 1H), 6.1
0 IW N 5-6.17 (m, 2H), 6.49 (t, J
H = 7.7 Hz, 1H), 6.83 (s, 2
H), 6.99 (t, J = 7.7 Hz, 1
H), 7.19 (d, J - 8.4 Hz, 1
H), 7.82 (s, 1H), 7.96 (d,
J = 8.4 Hz, 1H), 12.6 (br
s, 1H)
Using Compound No.6-42, the following Compound
(No.10-2) was obtained by a method similar to that of
Compound No.10-1.
7-(5-Carboxy-2-methoxypheny 1H-NMR (400 MHz, DMSO-d6)
1)-8-(2-methoxyphenylaminom 6 0.99 (s, 3H), 1.23 (s, 3
ethyl)-1,3,3-trimethy1-3,4-
H), 3.31 (s, 3H), 3.64 (s,
dihydro-1H-quinoxalin-2-one
3H), 3.83 (s, 3H), 4.05 (d,
(Compound No.10-2)
J = 4.9 Hz, 2H), 4.44 (t,
J = 4.9 Hz, 1H), 6.15 (s, 1
HO 0 10 H), 6.23 (d, J = 7.8 Hz, 1H
0
), 6.46 (t, J = 7.8 Hz, 1H)
410 NH
I
id&
NO , 6.57 (t, J = 7.8 Hz, 1H),
6.67 (d, J - 7.8 Hz, 1H),
6.73 (d, J = 8.0 Hz, 1H), 6
0 IW N .78 (d, J = 8.0 Hz, 1H), 7.
H 16 (d, J -- 8.7 Hz, 1H), 7.6
9 (d, J = 2.2 Hz, 1H), 7.93
(dd, J = 8.7, 2.2 Hz, 1H),
12.60 (br s, 1H)
Example 11
8-(5-Fluoro-2-methylphenoxymethyl)-7-(4-hydroxy-2-
methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Compound No.11)
7-(4-Benzoyloxy-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
189
CA 02643070 2008-08-20
quinoxalin-2-one (Compound No.6-31, 422 mg, 0.761
mmol) was dissolved in mixed solvent of methanol (2
mL) and tetrahydrofuran (2 mL), and 4N aqueous sodium
hydroxide solution (0.761 mL, 3.04 mmoL) was added
thereto. After the reaction mixture was stirred at
room temperature for 40 minutes, water (100 mL) and
1N aqueous HC1 solution (4 mL) were added thereto.
After the mixture was extracted with ethyl acetate
(100 mL), the organic layer was washed with water
(100 mL) and saturated brine (50 mL) successively,
dried over anhydrous magnesium sulfate, and then the
solvent was removed under reduced pressure. The
obtained residue was filtered with a mixed solvent of
hexane (10 mL) and ethyl acetate (10 mL) to give the
titled compound (292 mg) as a colorless solid. (Yield
85%)
F H-NMR (400 MHz, CDC13)
6 0.91 (s, 3H), 1.28 (s,
3H), 2.02 (s, 3H), 3.47 (s
HO 0
, 3H), 3.68 (br s, 1H), 3.
80 (s, 3H), 4.85 (d, J = 1
3.4 Hz, 1H), 4.92 (s, 1H),
N 5.21 (d, J = 13.4 Hz, 1H)
, 6.05 (dd, J = 11.4, 2.5
Hz, 1H), 6.38 (td, J - 8.4
, 2.5 Hz, 1H), 6.50-6.53 (
m, 2H), 6.70 (d, J - 8.1 H
z, 1H), 6.87 (d, J = 8.1 H
z, 1H), 6.87-6.91 (m, 1H),
7.16 (d, J = 8.3 Hz, 1H)
Example 12
7-(2-Methoxy-4-methoxymethoxypheny1)-8-[N-(2-
methoxypheny1)-N-(9-
190
CA 02643070 2008-08-20
fluorenylmethoxycarbonyl)aminomethy1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No.12-1)
7-(2-Methoxy-4-methoxymethoxypheny1)-8-(2-
methoxyphenylaminomethyl)-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Compound No.6-39, 104
mg, 0.212 mmol) and sodium hydrogen carbonate (22.0
mg, 0.262 mmol) were dissolved in mixed solvent of
1,4-dioxane (1.5 mL) and water (1 mL), and 9-
fluorenylmethoxycarbonyl chloride (60.3 mg, 0.233
mmol) was added thereto. After the reaction mixture
was stirred at room temperature for 30 minutes, the
mixture was diluted with ethyl acetate (50 mL). The
mixture was washed with water (50 mL) and saturated
brine (50 mL) successively, dried over anhydrous
magnesium sulfate, and then the solvent was removed
under reduced pressure. The obtained residue was
purified by silica gel column chromatography (hexane-
ethyl acetate) to give the titled compound (149 mg)
as a colorless amorphous product. (Yield 99%)
010 H-NMR (400 MHz, CDC13)
1.28 (s, 3H), 1.38 (s,
0
3H), 3.34-3.89 (m, 16H), 4
C1,0 N¨Fmoc .36-4.63 (m, 1H), 4.97-5.0
NO 2 (m, 1H), 5.17 (s, 1H), 5
.46-5.65 (m, 1H), 6.36-7.3
0 3 (m, 15H), 7.64-7.67 (m,
1\r<
2H)
Using any compounds among Compounds No.6-41 and
available compounds, the following Compound (No.12-2)
1 9 1
CA 02643070 2008-08-20
was obtained by a method similar to that of Compound
No.12-1.
8-[N-(5-Fluoro-2-methylphen 1H-NMR (400 MHz, CDC13)
y1)-N-(9-fluorenylmethoxyca 6 1.26 (s, 3H), 1.41 (s, 3
rbonyl)aminomethy1]-7-(2-me
H), 1.87 (s, 3H), 3.36-4.27
thoxy-4-methoxymethoxypheny
(m, 13H), 4.66-5.21 (m, 4H
1)-1,3,3-trimethy1-3,4-dihy
), 6.07-7.64 (m, 16H)
dro-1H-quinoxalin-2-one (Co
mpound No.12-2)
FO
20,0 I. N¨Fmoc
I
id& 1\1,.0
0 IW N
H
Example 13
7-(4-Hydroxy-2-methoxypheny1)-8-[N-(2-methoxypheny1)-
N-(9-fluorenylmethoxycarbonyl)aminomethy1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No.13-1)
7-(2-Methoxy-4-methoxymethoxypheny1)-8-[N-(2-
methoxypheny1)-N-(9-
fluorenylmethoxycarbonyl)aminomethy1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No.12-1, 137 mg, 0.192 mmol) was dissolved in 1,4-
dioxane (1 mL) and 4N hydrochloride/1,4-dioxane
solution (144 p L, 0.576 mmol) was added thereto.
After the reaction mixture was stirred at room
temperature for 2 hours, the mixture was diluted with
ethyl acetate (100 mL). The mixture was washed with
water (100 mL) and saturated brine (50 mL)
1 9 2
CA 02643070 2008-08-20
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled compound (71.6 mg) as a colorless
solid. (Yield 56%)
0 1H-NMR (400 MHz, CDC13)
1.27 (s, 3H), 1.38 (s, 3
H), 3.32 (s, 3H), 3.49-4.1
HO =
N¨Fmoc
5 (m, 10H), 4.39-4.59 (m,
id& NO 1H), 5.23-5.90 (m, 2H), 6.
29-7.33 (m, 15H), 7.62-7.6
N 6 (m, 2H)
Using Compounds No.5-20 and 12-2, the following
Compounds (No.13-2 and 13-3) were obtained by a
method similar to that of Compound No.13-1.
7-(4-Hydroxy-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(5-methylthiophen-2-y1 6 1.20 (s, 3H), 1.42 (s, 3
carbonyloxymethyl)-1,3,3-tr
H), 2.47 (s, 3H), 3.45 (s,
imethy1-3,4-dihydro-1H-quin
3H), 3.73 (s, 3H), 3.76 (s,
oxalin-2-one (Compound No.1
1H), 5.14 (d, J = 13.3 Hz,
3-2)
1H), 5.17 (s, 1H), 5.27 (d
, J - 13.3 Hz, 1H), 6.42 (d
d, J = 8.2, 2.3 Hz, 1H), 6.
HO I. 0
46 (d, J = 2.3 Hz, 1H), 6.6
9 (d, J = 3.9 Hz, 1H), 6.74
d&NO (d, J = 8.0
Hz, 1H), 6.86
N (d, J = 8.0
Hz, 1H), 7.11 (
d, J = 8.2 Hz, 1H), 7.45 (d
, J = 3.9 Hz, 1H)
8-[N-(5-Fluoro-2-methylphen 1H-NMR (400 MHz, CDC13)
y1)-N-(9-fluorenylmethoxyca 6 1.26 (s, 3H), 1.40 (s, 3
rbonyl)aminomethy1]-7-(4-hy
H), 1.89 (s, 3H), 3.33-4.54
droxy-2-methoxypheny1)-1,3,
3-trimethy1-3,4-dihydro-1H-
(m, 10H), 5.15-5.58 (m, 3H
), 6.04-7.64 (m, 16H)
quinoxalin-2-one (Compound
193
CA 02643070 2008-08-20
No. 13-3)
F
HO * N¨Fmoc
rLo
41) ,0 N
Example 14
7-(4-Butyryloxy-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Compound No.14-1)
8-(5-Fluoro-2-methylphenoxymethyl)-7-(4-
hydroxy-2-methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one (Compound No.11, 25 mg, 0.055
mmol) was dissolved in tetrahydrofuran (1 mL), and
triethylamine (20 p L, 0.14 mmol) and butyryl chloride
(7.6 p L, 0.073 mmol) were added successively. After
the reaction mixture was stirred at room temperature
for 1 hour, the mixture was purified by silica gel
column chromatography (hexane-ethyl acetate) to give
the titled compound (27 mg) as a colorless amorphous
product. (Yield 92%)
F H-NMR (500 MHz, CDC13)
6 0.93 (s, 3H), 1.07 (t,
J - 7.3 Hz, 3H), 1.27 (s,
.r0 0
3H), 1.78-1.86 (m, 2H), 2.
0 11) NO 01 (s, 3H), 2.58 (t, J = 7
.3 Hz, 2H), 3.46 (s, 3H),
N 3.71 (s, 1H), 3.81 (s, 3H)
, 4.85 (d, J = 13.6 Hz, 1H
), 5.20 (d, J = 13.6 Hz, 1
H), 6.06 (dd, J = 11.3, 2.
194
CA 02643070 2008-08-20
-,
4 Hz, 1H), 6.39 (td, J = 8
.2, 2.4 Hz, 1H), 6.71 (d,
J = 8.1 Hz, 1H), 6.75 (d,
J = 2.3 Hz, 1H), 6.81 (dd,
J = 8.2, 2.3 Hz, 1H), 6.8
8-6.91 (m, 1H), 6.88 (d, J
= 8.1 Hz, 1H), 7.30 (d, J
= 8.2 Hz, 1H)
Using any compounds among Compounds No.8-2, 11, 13-2,
and available compounds, the following Compounds
(No.14-2-14-62) were obtained by a method similar to
that of Compound No.14-1.
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, DMSO-d6)
methyl)-7-[2-methoxy-4-(2-m 6 0.80 (s, 3H), 1.07 (s, 3
ethylbenzoyloxy)pheny1]-1,3
H), 1.94 (s, 3H), 2.62 (s,
,3-trimethy1-3,4-dihydro-1
3H), 3.34 (s, 3H), 3.82 (s,
H-quinoxalin-2-one (Compoun
3H), 4.88 (d, J = 13.9 Hz,
d No.14-2)
1H), 5.25 (d, J = 13.9 Hz,
F 40
1H), 6.15 (dd, J = 11.6, 2
010.4 Hz, 1H), 6.15 (s, 1H), 6
I .50 (td, J = 8.5, 2.4 Hz, 1
0 40 0
H), 6.82 (d, J = 8.1 Hz, 1H
o a& NO ),
6.86 (d, J = 8.1 Hz, 1H)
, 7.00 (dd, J = 8.1, 2.1 Hz
0 IW N ,
1H), 7.00-7.02 (m, 1H), 7
H .13 (d, J = 2.1 Hz, 1H), 7.
34 (d, J = 8.1 Hz, 1H), 7.4
2 (d, J = 7.5 Hz, 1H), 7.43
(d, J = 7.5 Hz, 1H), 7.59
(td, J = 7.5, 1.4 Hz, 1H),
8.11-8.13 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, DMSO-d6)
methyl)-7-[2-methoxy-4-(2-m 6 0.80 (s, 3H), 1.07 (s, 3
ethoxybenzoyloxy)pheny1]-1,
H), 1.93 (s, 3H), 3.33 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.82 (s, 3H), 3.89 (s,
H-quinoxalin-2-one (Compoun
3H), 4.87 (d, J = 13.8 Hz,
d No.14-3)
1H), 5.24 (d, J = 13.8 Hz,
1H), 6.14 (dd, J = 11.2, 2
.6 Hz, 1H), 6.15 (s, 1H), 6
.50 (td, J = 8.4, 2.6 Hz, 1
H), 6.81 (d, J = 8.1 Hz, 1H
), 6.85 (d, J = 8.1 Hz, 1H)
, 6.95 (dd, J = 8.2, 2.2 Hz
195
CA 02643070 2008-08-20
F 1H), 6.98-
7.02 (m, 1H), 7
.05 (d, J = 2.2 Hz, 1H), 7.
12 (td, J = 7.4, 0.8 Hz, 1H
010 (--)0
0
O 410
gi& N,0 ), 7.25 (dd,
J = 8.7, 0.8 H
z, 1H), 7.33 (d, J = 8.2 Hz
, 1H), 7.66 (ddd, J - 8.7,
o N 7.4,
1.7 Hz, 1H), 7.95 (dd,
J = 7.4, 1.7 Hz, 1H)
7-[4-(2-Chlorobenzoyloxy)- 1H-NMR (500 MHz, DMSO-d6)
2-methoxypheny1]-8-(5-fluor 6 0.80 (s, 3H), 1.08 (s, 3
o-2-methylphenoxymethyl)-1,
H), 1.93 (s, 3H), 3.34 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.83 (s, 3H), 4.87 (d,
H-quinoxalin-2-one (Compoun
J - 13.9 Hz, 1H), 5.24 (d,
d No.14-4)
J - 13.9 Hz, 1H), 6.16 (dd
F 010
, J = 11.6, 2.4 Hz, 1H), 6.
16 (s, 1H), 6.50 (td, J = 8
Cl
.2, 2.4 Hz, 1H), 6.82 (d, J
o 0
gi& N,0 = 7.9 Hz,
1H), 6.86 (d, J
O 010
= 7.9 Hz, 1H), 6.98-7.03 (m
, 2H), 7.14 (d, J = 2.1 Hz,
O N 1H), 7.36
(d, J = 8.1 Hz,
1H), 7.56-7.60 (m, 1H), 7.6
9-7.70 (m, 2H), 8.13-8.15 (
m, 1H)
7-[4-(3-Chlorobenzoyloxy)- 1H-NMR (400 MHz, DMSO-dd
2-methoxypheny1]-8-(5-fluor 6 0.81 (s, 3H), 1.08 (s, 3
o-2-methylphenoxymethyl)-1,
H), 1.93 (s, 3H), 3.33 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.81 (s, 3H), 4.86 (d,
H-quinoxalin-2-one (Compoun
J = 13.8 Hz, 1H), 5.24 (d,
d No.14-5)
J = 13.8 Hz, 1H), 6.15 (dd
F
CI , J = 12.0,
2.5 Hz, 1H), 6.
16 (s, 1H), 6.51 (td, J = 8
010.4, 2.5 Hz, 1H), 6.82 (d, J
0 0 = 8.1 Hz,
1H), 6.86 (d, J
1
O N - 8.1
Hz, 1H), 6.99-7.03 (m
, 1H), 7.02 (dd, J = 8.0, 2
O N .2 Hz, 1H),
7.17 (d, J = 2.
2 Hz, 1H), 7.35 (d, J = 8.0
Hz, 1H), 7.68 (t, J = 8.0
Hz, 1H), 7.86 (ddd, J = 8.0
, 2.2, 1.0 Hz, 1H), 8.10-8.
13 (m, 1H), 8.14-8.18 (m, 1
H)
7-[4-(4-Chlorobenzoyloxy)- 1H-NMR (400 MHz, DMSO-d6)
2-methoxypheny1]-8-(5-fluor 6 0.86 (s, 3H), 1.07 (s, 3
o-2-methylphenoxymethyl)-1,
H), 1.93 (s, 3H), 3.33 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.81 (s, 3H), 4.87 (d,
H-quinoxalin-2-one (Compoun
J = 13.7 Hz, 1H), 5.24 (d,
d No.14-6)
196
CA 02643070 2008-08-20
F J = 13.7
Hz, 1H), 6.15 (dd
, J = 11.1, 2.6 Hz, 1H), 6.
CI I. 0 16 (s, 1H),
6.51 (td, J = 8
0
0/0 N,0 2.6 Hz,
1H), 6.81 (d, J
8.1 Hz, 1H), 6.86 (d, J
0 Olt
.1 Hz, 1H), 6.99-7.02 (m
,0, 1H), 7.01 (dd, J = 8.2, 2
N" .2 Hz, 1H), 7.14 (d, J = 2.
2 Hz, 1H), 7.34 (d, J = 8.2
Hz, 1H), 7.71 (d, J = 8.8
Hz, 2H), 8.16 (d, J = 8.8 H
z, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-t 6 0.94 (s, 3H), 1.27 (s, 3
rifluoromethylbenzoyloxy)ph
H), 2.02 (s, 3H), 3.47 (s,
eny1]-1,3,3-trimethy1-3,4-d
3H), 3.73 (s, 1H), 3.85 (s,
ihydro-1H-quinoxalin-2-one
3H), 4.89 (d, J = 13.7 Hz,
(Compound No.14-7)
1H), 5.23 (d, J = 13.7 Hz,
FF
F 410
1H), 6.08 (dd, J = 11.1, 2
.4 Hz, 1H), 6.40 (td, J - 8
0
SF
O 410 F .3, 2.4 Hz,
1H), 6.73 (d, J
0
N,0 = 8.1 Hz,
1H), 6.88-6.92 (
m, 2H), 6.90 (d, J = 8.1 Hz
, 1H), 6.98 (dd, J = 8.2, 2
,o RIP N .2 Hz, 1H),
7.37 (d, J - 8.
2 Hz, 1H), 7.71-7.73 (m, 2H
), 7.85-7.87 (m, 1H), 8.01-
8.04 (m, 1H)
8-(5-Fluoro-2-methylphenoxy H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr 6 0.97 (s, 3H), 1.27 (s, 3
idin-2-ylcarbonyloxy)phenyl
H), 2.02 (s, 3H), 3.47 (s,
]-1,3,3-trimethy1-3,4-dihyd
3H), 3.73 (s, 1H), 3.83 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 4.87 (d, J = 13.4 Hz,
pound No.14-8) F
1H), 5.21 (d, J = 13.4 Hz,
140 1H), 6.09
(dd, J = 11.3, 2
.4 Hz, 1H), 6.40 (td, J = 8
.2, 2.4 Hz, 1H), 6.74 (d, J
0
- 7.9 Hz, 1H), 6.89-6.92 (
0 id& N,0 m, 1H),
6.93 (d, J = 7.9 Hz
, 1H), 6.94 (d, J = 2.1 Hz,
,o RIP N 1H), 7.00
(dd, J = 8.2, 2.
1 Hz, 1H), 7.37 (d, J = 8.2
Hz, 1H), 7.59 (ddd, J = 7.
8, 4.8, 1.1 Hz, 1H), 7.95 (
td, J = 7.8, 1.8 Hz, 1H), 8
.31 (dt, J = 7.8, 1.1 Hz, 1
H), 8.87 (ddd, J = 4.8, 1.8
, 1.1 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr
1 9 7
CA 02643070 2008-08-20
idin-3-ylcarbonyloxy)phenyl 6 0.96 (s, 3H), 1.28 (s, 3
]-1,3,3-trimethy1-3,4-dihyd
H), 2.03 (s, 3H), 3.47 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 3.74 (s, 1H), 3.85 (s,
pound No.14-9)
3H), 4.88 (d, J = 13.5 Hz,
F 410
1H), 5.23 (d, J = 13.5 Hz,
1H), 6.09 (dd, J = 11.2, 2
.5 Hz, 1H), 6.41 (td, J = 8
0 0 .2, 2.5 Hz,
1H), 6.74 (d, J
0 to& N,0 = 8.1 Hz,
1H), 6.90-6.93 (
m, 1H), 6.90 (d, J = 2.3 Hz
O N , 1H), 6.92
(d, J = 8.1 Hz,
1H), 6.96 (dd, J = 8.2, 2.
3 Hz, 1H), 7.38 (d, J = 8.2
Hz, 1H), 7.50 (ddd, J = 8.
0, 4.8, 0.9 Hz, 1H), 8.49 (
dt, J = 8.0, 2.0 Hz, 1H), 8
.88 (dd, J = 4.8, 2.0 Hz, 1
H), 9.43 (dd, J = 2.0, 0.9
Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr 5 0.96 (s, 3H), 1.28 (s, 3
idin-4-ylcarbonyloxy)phenyl
H), 2.03 (s, 3H), 3.47 (s,
1-1,3,3-trimethy1-3,4-dihyd
3H), 3.75 (s, 1H), 3.84 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 4.87 (d, J = 13.5 Hz,
pound No.14-10)
1H), 5.22 (d, J = 13.5 Hz,
F 010
1H), 6.09 (dd, J = 11.2, 2
.4 Hz, 1H), 6.41 (td, J = 8
N;
0 .3, 2.4 Hz,
1H), 6.74 (d, J
0 410
id& N,0 = = 7.8 Hz, 1H), 6.89 (d, J
2.3 Hz, 1H), 6.90-6.93 (m
, 1H), 6.92 (d, J = 7.8 Hz,
N 1H), 6.95 (dd, J = 8.1, 2.
3 Hz, 1H), 7.38 (d, J = 8.1
Hz, 1H), 8.04 (dd, J = 4.3
, 1.6 Hz, 2H), 8.89 (dd, J
= 4.3, 1.6 Hz, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[4-(furan-3-ylcar 6 0.95 (s, 3H), 1.27 (s, 3
bonyloxy)-2-methoxypheny11-
H), 2.02 (s, 3H), 3.47 (s,
1,3,3-trimethy1-3,4-dihydr
3H), 3.73 (s, 1H), 3.83 (s,
o-1H-quinoxalin-2-one (Comp
3H), 4.87 (d, J = 13.4 Hz,
ound No.14-11)
1H), 5.22 (d, J = 13.4 Hz,
1H), 6.08 (dd, J = 11.0, 2
.4 Hz, 1H), 6.40 (td, J = 8
.2, 2.4 Hz, 1H), 6.73 (d, J
= 8.2 Hz, 1H), 6.85 (d, J
= 2.1 Hz, 1H), 6.89-6.92 (m
, 4H), 7.34 (d, J = 8.2 Hz,
1H), 7.53 (t, J = 1.7 Hz, 1
1 9 8
CA 02643070 2008-08-20
F H), 8.23 (dd, J = 1.7, 0.6
Hz, 1H)
0
0 010 1\1,,0
,0 a& N
7-(4-Cyclohexylcarbonyloxy- 1H-NMR (400 MHz, CDC13)
2-methoxypheny1)-8-(5-fluor 6 0.93 (s, 3H), 1.27 (s, 3
o-2-methylphenoxymethyl)-1,
H), 1.31-1.44 (m, 3H), 1.6
3,3-trimethy1-3,4-dihydro-1
0-1.73 (m, 3H), 1.83-1.87 (
H-quinoxalin-2-one (Compoun
m, 2H), 2.01 (s, 3H), 2.08-
d No.14-12)
2.11 (m, 2H), 2.59 (tt, J =
F
11.2, 3.7 Hz, 1H), 3.46 (s
, 3H), 3.71 (s, 1H), 3.81 (
s, 3H), 4.85 (d, J = 13.6 H
(21).(0 0 z, 1H),
5.21 (d, J = 13.6 H
0 01$ NO z, 1H),
6.05 (dd, J = 11.1,
2.5 Hz, 1H), 6.39 (td, J =
O N 8.3, 2.5
Hz, 1H), 6.71 (d,
J = 8.2 Hz, 1H), 6.73 (d,
J = 2.2 Hz, 1H), 6.79 (dd,
J = 8.2, 2.2 Hz, 1H), 6.88
(d, J = 8.1 Hz, 1H), 6.87-6
.91 (m, 1H), 7.30 (d, J = 8
.1 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-phen 6 0.93 (s, 3H),
1.26 (s, 3
ylacetoxypheny1)-1,3,3-trim
H), 2.00 (s, 3H), 3.45 (s,
ethyl-3,4-dihydro-1H-quinox
3H), 3.71 (s, 1H), 3.79 (s,
alin-2-one (Compound No.14-
3H), 3.90 (s, 2H), 4.82 (d
13)
, J = 13.7 Hz, 1H), 5.19 (d
F
, J = 13.7 Hz, 1H), 6.04 (d
d, J - 11.2, 2.4 Hz, 1H), 6
.38 (td, J = 8.3, 2.4 Hz, 1
0
010 0 410 0
No 7,6.0 (d, J = 8.2
Hz, 1H
(d, J = 2.2 Hz, 1H)
, 6.79 (dd, J = 8.2, 2.2 Hz
0 N , 1H), 6.86 (d, J = 8.2 Hz,
1H), 6.88-6.91 (m, 1H), 7.
29 (d, J = 8.2 Hz, 1H), 7.3
0-7.43 (m, 5H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(3-p 6 0.94 (s, 3H), 1.26 (s, 3
henylpropionyloxy)pheny1]-1
H), 2.01 (s, 3H), 2.93 (t,
,3,3-trimethy1-3,4-dihydro-
J = 7.6 Hz, 2H), 3.11 (t, J
1H-quinoxalin-2-one (Compou
= 7.6 Hz, 2H), 3.46 (s, 3H
nd No.14-14)
199
CA 02643070 2008-08-20
F ), 3.71 (s, 1H), 3.78 (s, 3
H), 4.84 (d, J = 13.7 Hz, 1
4100 010 0 H), 5.19 (d,
J = 13.7 Hz, 1
H), 6.05 (dd, J = 11.3, 2.4
Hz, 1H), 6.39 (td, J = 8.2
0 N,0 , 2.4 Hz, 1H), 6.62 (d,
J =
N2.4 Hz, 1H), 6.71 (d, J =
7.9 Hz, 1H), 6.74 (dd, J =
8.1, 2.4 Hz, 1H), 6.87 (d,
J = 7.9 Hz, 1H), 6.88-6.91
(m, 1H), 7.24-7.36 (m, 6H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, DMSO-d6)
methyl)-7-[4-(furan-2-ylcar 6 0.81 (s, 3H), 1.07 (s, 3
bonyloxy)-2-methoxypheny11-
H), 1.93 (s, 3H), 3.33 (s,
1,3,3-trimethy1-3,4-dihydr
3H), 3.80 (s, 3H), 4.86 (d,
o-1H-quinoxalin-2-one (Comp
J = 14.0 Hz, 1H), 5.23 (d,
ound No.14-15)
J = 14.0 Hz, 1H), 6.15 (dd
F
, J - 11.1, 2.4 Hz, 1H), 6.
15 (s, 1H), 6.50 (td, J = 8
TILy.3. 2.4 Hz, 1H), 6.81 (d, J
0 0 = 8.1 Hz, 1H), 6.82 (dd,
J
0 .4& NO = 3.7, 1.7
Hz, 1H), 6.85 (
d, J = 8.1 Hz, 1H), 6.97 (d
N d, J = 8.3, 2.1 Hz, 1H), 6.
99-7.02 (m, 1H), 7.10 (d, J
= 2.1 Hz, 1H), 7.33 (d, J
= 8.3 Hz, 1H), 7.59 (dd, J
= 3.7, 0.8 Hz, 1H), 8.13 (d
d, J = 1.7, 0.8 Hz, 1H)
7-(4-Acetoxy-2-methoxypheny 1H-NMR (400 MHz, DMSO-d6)
1)-8-(5-fluoro-2-methylphen 6 0.86 (s, 3H), 1.07 (s, 3
oxymethyl)-1,3,3-trimethyl-
H), 1.92 (s, 3H), 2.29 (s,
3,4-dihydro-1H-quinoxalin-
3H), 3.32 (s, 3H), 3.78 (s,
2-one (Compound No.14-16)
3H), 4.83 (d, J = 14.0 Hz,
F
1H), 5.21 (d, J = 14.0 Hz,
1H), 6.12 (dd, J = 11.5, 2
.5 Hz, 1H), 6.14 (s, 1H), 6
0 0
.49 (td, J = 8.3, 2.5 Hz, 1
0 id&
NO H), 6.79 (d,
J - 8.0 Hz, 1H
), 6.82 (d, J = 8.0 Hz, 1H)
N , 6.83 (dd, J = 8.2, 2.2 Hz
, 1H), 6.93 (d, J = 2.2 Hz,
1H), 6.98-7.01 (m, 1H), 7.
27 (d, J = 8.2 Hz, 1H)
7-(4-Benzoylamino-2-methoxy 1H-NMR (400 MHz, CDC13)
phenyl)-8-(5-fluoro-2-methy 6 0.92 (s, 3H), 1.29 (s, 3
lphenoxymethyl)-1,3,3-trime
H), 2.02 (s, 3H), 3.48 (s,
thy1-3,4-dihydro-1H-quinoxa
3H), 3.72 (s, 1H), 3.89 (s,
lin-2-one (Compound No.14-1
3H), 4.87 (d, J = 13.6 Hz,
7)
200
CA 02643070 2008-08-20
F 1H), 5.25
(d, J = 13.6 Hz,
1H), 6.06 (dd, J = 11.2, 2
010 kl 00 0 .4 Hz, 1H),
6.39 (td, J = 8
.3, 2.4 Hz, 1H), 6.73 (d, J
= 8.1 Hz, 1H), 6.88-6.91 (
0
NO m, 1H), 6.90
(d, J = 8.1 Hz
o RIP N , 1H), 7.07 (dd, J = 8.1, 2
.0 Hz, 1H), 7.31 (d, J = 8.
1 Hz, 1H), 7.50-7.61 (m, 3H
), 7.77 (d, J = 2.0 Hz, 1H)
, 7.89-7.92 (m, 31-1)
7-(4-Acetylamino-2-methoxyp 1H-NMR (400 MHz, CDC13)
heny1)-8-(5-fluoro-2-methyl 6 0.91 (s, 3H), 1.29 (s, 3
phenoxymethyl)-1,3,3-trimet
H), 2.01 (s, 3H), 2.22 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.46 (s, 3H), 3.70 (s,
in-2-one (Compound No.14-18
1H), 3.84 (s, 3H), 4.84 (d
, J = 13.4 Hz, 1H), 5.22 (d
F
, J = 13.4 Hz, 1H), 6.04 (d
d, J = 11.2, 2.5 Hz, 1H), 6
.38 (td, J = 8.2, 2.5 Hz, 1
N 0
H), 6.71 (d, J = 8.1 Hz, 1H
0 id& r10 ), 6.87 (d,
J = 8.1 Hz, 1H)
, 6.87-6.91 (m, 1H), 6.92 (
N dd, J = 8.2, 1.8 Hz, 1H), 7
.24 (d, J = 8.2 Hz, 1H), 7.
58 (d, J = 1.8 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr 6 0.92 (s, 3H), 1.29 (s, 3
idin-2-ylcarbonylamino)phen
H), 2.02 (s, 3H), 3.48 (s,
y11-1,3,3-trimethy1-3,4-dih
3H), 3.71 (s, 1H), 3.91 (s,
ydro-1H-quinoxalin-2-one (C
3H), 4.88 (d, J = 13.7 Hz,
ompound No.14-19)
1H), 5.25 (d, J = 13.7 Hz,
F 010
1H), 6.07 (dd, J = 11.2, 2
.4 Hz, 1H), 6.38 (td, J = 8
N
" .2, 2.4 Hz,
1H), 6.73 (d, J
0 = 8.1 Hz, 1H), 6.87-6.91
410
I (
0 id& NO m, 1H), 6.92
(d, J = 8.1 Hz
, 1H), 7.25 (dd, J = 8.2, 2
o N .2 Hz,
1H), 7.33 (d, J = 8.
2 Hz, 1H), 7.52 (ddd, J = 7
.7, 4.7, 1.1 Hz, 1H), 7.85
(d, J = 2.2 Hz, 1H), 7.94 (
td, J = 7.7, 1.7 Hz, 1H), 8
.32 (dt, J = 7.7, 1.1 Hz, 1
H), 8.65 (ddd, J = 4.7, 1.7
, 1.1 Hz, 1H), 10.16 (s, 1H
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr 6 0.94 (s, 3H), 1.30 (s, 3
idin-3-ylcarbonylamino)phen
201
CA 02643070 2008-08-20
y1]-1,3,3-trimethy1-3,4-dih H), 2.02 (s, 3H), 3.47 (s,
ydro-1H-quinoxalin-2-one (C 3H), 3.73 (s, 1H), 3.89 (s,
ompound No.14-20) 3H), 4.86
(d, J = 13.6 Hz,
F 0/0 1H), 5.24
(d, J = 13.6 Hz,
1H), 6.07 (dd, J = 11.2, 2
.4 Hz, 1H), 6.39 (td, J = 8
I H
0 .3, 2.4 Hz,
1H), 6.73 (d, J
= 8.1 Hz, 1H), 6.89-6.92 (
0 NO m, 1H), 6.90
(d, J = 8.1 Hz
N , 1H), 7.10 (dd, J = 8.1, 1
.9 Hz, 1H), 7.33 (d, J - 8.
1 Hz, 1H), 7.49 (ddd, J = 7
.9, 4.8, 1.5 Hz, 1H), 7.71
(d, J = 1.9 Hz, 1H), 7.91 (
s, 1H), 8.24 (dt, J = 7.9,
1.5 Hz, 1H), 8.82 (dd, J =
4.8, 1.5 Hz, 1H), 9.13 (d,
J = 1.5 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr 6 0.94 (s, 3H), 1.30 (s, 3
idin-4-ylcarbonylamino)phen
H), 2.02 (s, 3H), 3.47 (s,
y1]-1,3,3-trimethy1-3,4-dih
3H), 3.73 (s, 1H), 3.89 (s,
ydro-1H-quinoxalin-2-one (C
3H), 4.85 (d, J = 13.6 Hz,
ompound No.14-21)
1H), 5.23 (d, J = 13.6 Hz,
F 010
1H), 6.06 (dd, J = 11.2, 2
.4 Hz, 1H), 6.40 (td, J = 8
.2, 2.4 Hz, 1H), 6.73 (d, J
0 010
N,0 = 8.1 Hz,
1H), 6.90 (d, J
= 8.1 Hz, 1H), 6.89-6.92 (m
, 1H), 7.09 (dd, J = 8.3, 2
N .1 Hz, 1H),
7.33 (d, J = 8.
3 Hz, 1H), 7.71 (d, J = 2.1
Hz, 11-1), 7.74 (dd, J = 4.4
Hz, 2H), 7.95 (s, 1H), 8.8
4 (dd, J = 4.4 Hz, 2H)
7-[4-(2-Chlorobenzoylamino 1H-NMR (400 MHz, CDC13)
)-2-methoxypheny1]-8-(5-flu 6 0.92 (s, 3H), 1.29 (s, 3H
oro-2-methylphenoxymethyl)- ), 2.02 (s, 3H), 3.47 (s, 3
1,3,3-trimethy1-3,4-dihydr H), 3.71 (s, 1H), 3.89 (s,
o-1H-quinoxalin-2-one (Comp 3H), 4.87 (d, J = 14.0 Hz,
ound No.14-22) 1H), 5.25 (d,
J = 14.0 Hz,
F 010 1H), 6.07
(dd, J = 10.9, 2.
4 Hz, 1H), 6.39 (td, J = 8.
CI 4, 2.4 Hz,
1H), 6.72 (d, J
N410 0 = 8.1 Hz,
1H), 6.88-6.92 (m
, 1H), 6.90 (d, J = 8.1 Hz,
0 1H), 7.06
(dd, J = 8.2, 1.
N 9 Hz, 1H),
7.31 (d, J = 8.2
Hz, 1H), 7.39-7.52 (m, 2H)
, 7.43 (td, J - 7.6, 2.0 Hz
2 0 2
CA 02643070 2008-08-20
.,
, 1H), 7.74 (d, J = 1.9 Hz,
1H), 7.81 (dd, J = 7.6, 2.
0 Hz, 1H), 7.97 (br s, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(4-m 6 0.92 (s, 3H),
1.29 (s, 3
ethoxybenzoylamino)pheny1]-
H), 2.02 (s, 3H), 3.47 (s,
1,3,3-trimethy1-3,4-dihydr
3H), 3.71 (s, 1H), 3.88 (s,
o-1H-quinoxalin-2-one (Comp
3H), 3.89 (s, 3H), 4.87 (d
ound No.14-23)
, J = 13.8 Hz, 1H), 5.24 (d
F 410
, J = 13.8 Hz, 1H), 6.06 (d
0 d, J = 11.2, 2.4 Hz, 1H), 6
00
H .38 (td, J = 8.2, 2.4 Hz, 1
N010 0 H), 6.72 (d, J = 8.0 Hz, 1H
I
0 N ,0 ), 6.88-6.91 (m, 1H), 6.90
(d, J = 8.0 Hz, 1H), 7.01 (
0 10 N d, J = 8.8 Hz, 2H), 7.04 (d
H d, J = 8.1, 2.1 Hz, 1H), 7.
30 (d, J = 8.1 Hz, 1H), 7.7
6 (d, J = 2.1 Hz, 1H), 7.83
(s, 1H), 7.87 (d, J = 8.8
Hz, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-(4-isobutyryloxy- 6 0.93 (s, 3H), 1.27 (s, 3
2-methoxypheny1)-1,3,3-trim
H), 1.35 (d, J = 7.0 Hz, 6H
ethyl-3,4-dihydro-1H-quinox
), 2.01 (s, 3H), 2.84 (sept
alin-2-one (Compound No.14-
, J = 7.0 Hz, 1H), 3.46 (s,
24)
3H), 3.71 (s, 1H), 3.82 (s
F 40
, 3H), 4.85 (d, J = 13.7 Hz
, 1H), 5.21 (d, J = 13.7 Hz
, 1H), 6.06 (dd, J = 11.0,
lei 0 2.4 Hz, 1H), 6.39 (td, J =
I
0 8.4, 2.4 Hz, 1H), 6.71 (d,
J = 8.1 Hz, 1H), 6.74 (d, J
0 IW N =
2.2 Hz, 1H), 6.80 (dd, J
H = 8.2, 2.2 Hz, 1H), 6.88-6
.91 (m, 1H), 6.89 (d, J = 8
.1 Hz, 1H), 7.31 (d, J = 8.
2 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(thi 6 0.95 (s, 3H), 1.27 (s, 3
ophen-3-ylcarbonyloxy)pheny
H), 2.02 (s, 3H), 3.47 (s,
1]-1,3,3-trimethy1-3,4-dihy
3H), 3.72 (s, 1H), 3.83 (s,
dro-1H-quinoxalin-2-one (Co
3H), 4.88 (d, J = 13.4 Hz,
mpound No.14-25)
1H), 5.23 (d, J = 13.4 Hz,
1H), 6.08 (dd, J = 11.3, 2
.4 Hz, 1H), 6.40 (td, J = 8
.2, 2.4 Hz, 1H), 6.73 (d, J
= 7.9 Hz, 1H), 6.88 (d, J
= 2.1 Hz, 1H), 6.89-6.92 (m
203
CA 02643070 2008-08-20
õ
F is , 1H), 6.91 (d, J = 7.9 Hz,
1H), 6.93 (dd, J = 8.3, 2.
1 Hz, 1H), 7.35 (d, J = 8.3
Sa)ro 0
Hz, 1H), 7.41 (dd, J = 5.0
I , 3.1 Hz, 1H), 7.69 (dd, J
0 el NO =
5.0, 1.2 Hz, 1H), 8.35 (d
0 IW N d, J = 3.1, 1.2 Hz, 1H)
H
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-piva 6 0.93 (s, 3H), 1.27 (s, 3
loyloxypheny1)-1,3,3-trimet
H), 1.39 (s, 9H), 2.01 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.46 (s, 3H), 3.71 (br
in-2-one (Compound No.14-26
s, 1H), 3.82 (s, 3H), 4.85
)
(d, J = 13.7 Hz, 1H), 5.21
F 40
(d, J = 13.7 Hz, 1H), 6.06
(dd, J = 11.2, 2.4 Hz, 1H)
, 6.39 (td, J = 8.3, 2.4 Hz
0
I , 1H), 6.71 (d, J = 8.1 Hz,
0 Is N,..0
1H), 6.72 (d, J = 2.1 Hz,
1H), 6.79 (dd, J = 8.3, 2.1
0 N
Hz, 1H), 6.87-6.92 (m, 1H)
H , 6.88 (d, J = 8.1 Hz, 1H),
7.31 (d, J = 8.3 Hz, 1H)
7-[4-(2-Fluorobenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-fluor 6 0.95 (s, 3H), 1.27 (s, 3
o-2-methylphenoxymethyl)-1,
H), 2.02 (s, 3H), 3.47 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.73 (br s, 1H), 3.84
H-quinoxalin-2-one (Compoun
(s, 3H), 4.88 (d, J = 13.7
d No.14-27)
Hz, 1H), 5.23 (d, J = 13.7
Hz, 1H), 6.08 (dd, J = 11.0
F
F 0
, 2.4 Hz, 1H), 6.40 (td, J
= 8.2, 2.4 Hz, 1H), 6.73 (d
0
0 010 0
I
ti& N,0 , J = 8.1 Hz, 1H), 6.89-6.9
2 (m, 1H), 6.91 (d, J = 2.2
Hz, 1H), 6.92 (d, J = 8.1
0 IW N
Hz, 1H), 6.97 (dd, J = 8.2,
H 2.2 Hz, 1H), 7.22-7.26 (m,
1H), 7.31 (td, J = 7.6, 0.
9 Hz, 1H), 7.37 (d, J = 8.2
Hz, 1H), 7.61-7.65 (m, 1H)
, 8.14 (td, J = 7.6, 1.8 Hz
, 1H)
7-[4-(3-Fluorobenzoyloxy)- 1H-NMR (400 MHz, CDC13)
2-methoxypheny1]-8-(5-fluor 5 0.96 (s, 3H), 1.28 (s, 3
o-2-methylphenoxymethyl)-1,
H), 2.03 (s, 3H), 3.48 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.64-3.85 (m, 1H), 3.8
H-quinoxalin-2-one (Compoun
4 (s, 3H), 4.88 (d, J = 13.
d No.14-28)
6 Hz, 1H), 5.23 (d, J = 13.
2 0 4
CA 02643070 2008-08-20
..
F Is 6 Hz, 1H), 6.09 (dd, J = 11
F .2, 2.4 Hz, 1H), 6.41 (td,
J = 8.3, 2.4 Hz, 1H), 6.74
010 0 0 (d, J = 8.0 Hz, 1H), 6.88-6
I .94 (m, 1H), 6.89 (d, J = 2
0 el NO .4 Hz, 1H), 6.92 (d, J - 8.
0 IW N 0 Hz, 1H), 6.95 (dd, J = 8.
3, 2.4 Hz, 1H), 7.37 (tdd,
H J = 8.2, 2.7, 1.2 Hz, 1H),
7.37 (d, J = 8.3 Hz, 1H), 7
.52 (td, J = 8.2, 5.5 Hz, 1
H), 7.90-7.93 (m, 1H), 8.0
2-8.05 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(3-m 6 0.96 (s, 3H), 1.28 (s, 3
ethoxybenzoyloxy)pheny1]-1,
H), 2.03 (s, 3H), 3.48 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.73 (s, 1H), 3.84 (s,
H-quinoxalin-2-one (Compoun
3H), 3.91 (s, 3H), 4.89 (d
d No.14-29)
, J = 13.6 Hz, 1H), 5.24 (d
F 40
0 , J = 13.6 Hz, 1H), 6.09 (d
d, J = 11.2, 2.4 Hz, 1H), 6
.40 (td, J = 8.3, 2.4 Hz, 1
410 0 0 H), 6.74 (d, J = 8.1 Hz, 1H
I
0 010 id& N,0 ), 6.89-6.96 (m, 1H), 6.89
(d, J = 2.3 Hz, 1H), 6.93 (
0 IW N d, J = 8.1 Hz, 1H), 6.95 (d
H d, J = 8.2, 2.3 Hz, 1H), 7.
21 (dt, J = 8.1, 1.3 Hz, 1
H), 7.37 (d, J = 8.2 Hz, 1H
), 7.45 (t, J = 8.1 Hz, 1H)
, 7.73-7.74 (m, 1H), 7.83-7
.85 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(4-m 5 0.94 (s, 3H),
1.28 (s, 3
ethoxybenzoyloxy)pheny1]-1,
H), 2.03 (s, 3H), 3.47 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.73 (s, 1H), 3.83 (s,
H-quinoxalin-2-one (Compoun
3H), 3.91 (s, 3H), 4.89 (d
d No.14-30)
, J = 13.6 Hz, 1H), 5.23 (d
F 410
, J = 13.6 Hz, 1H), 6.09 (d
0 00
d, J = 11.2, 2.4 Hz, 1H), 6
,O
0
.40 (td, J = 8.3, 2.4 Hz, 1
0 010 0
I
H), 6.73 (d, J = 8.1 Hz, 1H
NO
), 6.88-6.95 (m, 1H), 6.88
0 110 (d, J = 2.2 Hz, 1H), 6.92 (
N' d, J = 8.1 Hz, 1H), 6.93 (d
H d, J - 8.2, 2.2 Hz, 1H), 7.
01 (dt, J = 9.5, 2.4 Hz, 2H
), 7.36 (d, J = 8.2 Hz, 1H)
, 8.18 (dt, J = 9.5, 2.4 Hz
2 0 5
CA 02643070 2008-08-20
, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(4-n 6 0.97 (s, 3H),
1.28 (s, 3
itrobenzoyloxy)pheny1]-1,3,
H), 2.03 (s, 3H), 3.47 (s,
3-trimethy1-3,4-dihydro-1H-
3H), 3.75 (s, 1H), 3.85 (s,
quinoxalin-2-one (Compound
3H), 4.87 (d, J = 13.4 Hz,
No.14-31)
1H), 5.22 (d, J = 13.4 Hz,
0 F 1H), 6.09
(dd, J = 11.2, 2
-N .4 Hz, 1H),
6.41 (td, J = 8
0" 4/0 0 .3, 2.4 Hz, 1H),
6.74 (d, J
0
0 411
N 0
= 8.1 Hz, 1H), 6.90 (d, J
= 2.4 Hz, 1H), 6.91-6.93 (m
1H), 6.92 (d, J = 8.1 Hz,
110 N 1H), 6.96
(dd, J = 8.0, 2.
4 Hz, 1H), 7.39 (d, J = 8.0
Hz, 1H), 8.37-8.43 (m, 4H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-prop 6 0.94 (s, 3H), 1.27 (s, 3
ionyloxypheny1)-1,3,3-trime
H), 1.30 (t, J = 7.6 Hz, 3H
thy1-3,4-dihydro-1H-quinoxa
), 2.01 (s, 3H), 2.63 (q, J
lin-2-one (Compound No.14-3
= 7.6 Hz, 2H), 3.46 (s, 3H
2)
), 3.71 (s, 1H), 3.81 (s, 3
F
H), 4.85 (d, J = 13.7 Hz, 1
H), 5.20 (d, J = 13.7 Hz, 1
H), 6.06 (dd, J = 11.1, 2.4
0 Hz, 1H),
6.39 (td, J = 8.3
0 ti& NO , 2.4 Hz, 1H), 6.71
(d, J =
8.0 Hz, 1H), 6.75 (d, J =
N 2.1 Hz, 1H), 6.81 (dd, J =
8.1, 2.1 Hz, 1H), 6.88-6.92
(m, 1H), 6.89 (d, J = 8.0
Hz, 1H), 7.30 (d, J = 8.1 H
z, 1H)
7-(4-Acryloyloxy-2-methoxyp 1H-NMR (500 MHz, CDC13)
heny1)-8-(5-fluoro-2-methyl 6 0.94 (s, 3H), 1.27 (s, 3
phenoxymethyl)-1,3,3-trimet
H), 2.02 (s, 3H), 3.46 (s,
hy1-3,4-dihydro-1H-quinoxal
3H), 3.72 (s, 1H), 3.82 (s,
in-2-one (Compound No.14-33
3H), 4.86 (d, J = 13.6 Hz,
1H), 5.21 (d, J = 13.6 Hz,
F
1H), 6.05 (dd, J = 10.5, 1
.2 Hz, 1H), 6.07 (dd, J = 1
1.2, 2.4 Hz, 1H), 6.35 (dd,
7)f0 010 0 J = 17.3,
10.5 Hz, 1H), 6.
0 id& NO 39 (td, J = 8.4, 2.4
Hz, 1H
), 6.65 (dd, J = 17.3, 1.2
N Hz, 1H), 6.72 (d, J = 8.1 H
z, 1H), 6.80 (d, J = 2.1 Hz
, 1H), 6.89 (dd, J = 8.1, 2
.1 Hz, 1H), 6.89-6.91 (m, 1
206
CA 02643070 2008-08-20
H), 6.89 (d, J = 8.1 Hz, 1H
), 7.32 (d, J = 8.1 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(thi 6 0.95 (s, 3H), 1.27 (s, 3
ophen-2-ylcarbonyloxy)pheny
H), 2.02 (s, 3H), 3.47 (s,
1]-1,3,3-trimethy1-3,4-dihy
3H), 3.73 (s, 1H), 3.84 (s,
dro-1H-quinoxalin-2-one (Co
3H), 4.87 (d, J = 13.6 Hz,
mpound No.14-34)
1H), 5.22 (d, J = 13.6 Hz,
F
1H), 6.08 (dd, J = 11.3, 2
.4 Hz, 1H), 6.40 (td, J = 8
.2, 2.4 Hz, 1H), 6.73 (d, J
0
- 8.1 Hz, 1H), 6.89-6.90 (
0 010 NO m, 1H), 6.90
(d, J = 2.1 Hz
140 , 1H), 6.91 (d, J = 8.1 Hz,
1H), 6.95 (dd, J = 8.1, 2.
1 Hz, 1H), 7.19-7.21 (m, 1H
), 7.35 (d, J = 8.1 Hz, 1H)
, 7.70 (d, J = 4.9 Hz, 1H),
8.01 (d, J = 3.7 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-meth 6 0.94 (s, 3H), 1.27 (s, 3
oxycarbonyloxypheny1)-1,3,
H), 2.01 (s, 3H), 3.46 (s,
3-trimethy1-3,4-dihydro-1H-
3H), 3.73 (s, 1H), 3.82 (s,
quinoxalin-2-one (Compound
3H), 3.94 (s, 3H), 4.84 (d
No.14-35)
, J = 13.5 Hz, 1H), 5.20 (d
F
, J = 13.5 Hz, 1H), 6.05 (d
d, J = 11.2, 2.4 Hz, 1H), 6
.39 (td, J = 8.3, 2.4 Hz, 1
Oy0 0 H), 6.72 (d, J = 8.1
Hz, 1H
0 N,0 ), 6.84 (d,
J = 2.2 Hz, 1H)
, 6.87-6.92 (m, 3H), 7.31 (
N d, J = 8.3 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-phen 6 0.96 (s, 3H),
1.27 (s, 3
oxycarbonyloxypheny1)-1,3,
H), 2.01 (s, 3H), 3.46 (s,
3-trimethy1-3,4-dihydro-1H-
3H), 3.73 (s, 1H), 3.84 (s,
quinoxalin-2-one (Compound
3H), 4.85 (d, J - 13.6 Hz,
No.14-36)
1H), 5.20 (d, J = 13.6 Hz,
F
1H), 6.06 (dd, J = 11.2, 2
.4 Hz, 1H), 6.39 (td, J = 8
.3, 2.4 Hz, 1H), 6.73 (d, J
00 0010 = 7.9 Hz,
1H), 6.89 (d, J
010 0 N 0
= 7.9 Hz, 1H), 6.88-6.92 (m
, 1H), 6.95 (d, J = 2.3 Hz,
0 N 1H), 7.00 (dd, J = 8.2, 2.
3 Hz, 1H), 7.28-7.32 (m, 3H
), 7.34 (d, J = 8.2 Hz, 1H)
, 7.41-7.46 (m, 2H)
2 0 7
CA 02643070 2008-08-20
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-(2-methoxy-4-phen 5 0.92 (s, 3H), 1.29 (s, 3
oxycarbonylaminopheny1)-1,3
H), 2.02 (s, 3H), 3.47 (s,
,3-trimethy1-3,4-dihydro-1
3H), 3.71 (s, 1H), 3.83 (s,
H-quinoxalin-2-one (Compoun
3H), 4.85 (d, J = 13.7 Hz,
d No.14-37)
1H), 5.22 (d, J = 13.7 Hz,
F
1H), 6.05 (dd, J = 11.2, 2
.4 Hz, 1H), 6.39 (td, J = 8
.3, 2.4 Hz, 1H), 6.71 (d, J
OrN 0 = 8.1 Hz,
1H), 6.89 (d, J
0 NO = 8.1 Hz,
1H), 6.89-6.91 (m
, 2H), 7.10 (br s, 1H), 7.2
1101 N 1 (dd, J =
8.5, 1.2 Hz, 2H)
, 7.25-7.28 (m, 2H), 7.40-7
.44 (m, 2H), 7.52 (br s, 1H
7-[4-(2-Chlorobenzoyloxy)- 1H-NMR (400 MHz, CDC13)
2-methoxypheny1]-8-(5-methy 6 1.21 (s, 3H), 1.42 (s, 3
lthiophen-2-ylcarbonyloxyme
H), 2.47 (s, 3H), 3.46 (s,
thyl)-1,3,3-trimethy1-3,4-d
3H), 3.78 (s, 4H), 5.13 (d,
ihydro-1H-quinoxalin-2-one
J = 13.2 Hz, 1H), 5.31 (d,
(Compound No.14-38)
J = 13.2 Hz, 1H), 6.70 (d,
J = 3.7 Hz, 1H), 6.77 (d,
CI
J = 8.0 Hz, 1H), 6.85 (d, J
0 410 0 = 2.3 Hz,
1H), 6.89 (dd, J
= 8.2, 2.3 Hz, 1H), 6.89 (
0 110 NO d, J = 8.0
Hz, 1H), 7.33 (d
,0 N , J = 8.2
Hz, 1H), 7.39-7.4
3 (m, 1H), 7.45 (d, J = 3.7
Hz, 1H), 7.49-7.56 (m, 2H)
, 8.07 (ddd, J = 7.8, 1.7,
0.5 Hz, 1H)
7-(4-Benzoyloxy-2-methoxyph 1H-NMR (500 MHz, CDC13)
eny1)-8-(5-methylthiophen- 6 1.22 (s, 3H), 1.42 (s, 3
2-ylcarbonyloxymethyl)-1,3,
H), 2.47 (s, 3H), 3.46 (s,
3-trimethy1-3,4-dihydro-1H-
3H), 3.77 (s, 3H), 3.79 (s,
quinoxalin-2-one (Compound
1H), 5.14 (d, J = 13.3 Hz,
No.14-39)
1H), 5.31 (d, J = 13.3 Hz,
I \ 1H), 6.70
(d, J = 3.7 Hz,
0
1H), 6.77 (d, J - 7.9 Hz, 1
010 0 0 H), 6.83
(d, J = 2.3 Hz, 1H
), 6.86 (dd, J = 8.1, 2.3 H
0 010 z, 1H),
6.91 (d, J = 7.9 Hz
,o RIP N , 1H), 7.32
(d, J = 8.1 Hz,
1H), 7.45 (d, J = 3.7 Hz,
1H), 7.53 (t, J = 7.8 Hz, 2
H), 7.66 (t, J = 7.8 Hz, 1H
), 8.22 (d, J = 7.8 Hz, 2H)
7-[2-Methoxy-4-(pyridin-4-y 1H-NMR (500 MHz, CDC13)
2 0 8
CA 02643070 2008-08-20
lcarbonyloxy)pheny1]-8-(5 m
- 6 1.22 (s, 3H), 1.42 (s, 3
ethylthiophen-2-ylcarbonylo
H), 2.47 (s, 3H), 3.46 (s,
xymethyl)-1,3,3-trimethy1-3
3H), 3.77 (s, 3H), 3.82 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 5.13 (d, J = 13.3 Hz,
one (Compound No.14-40)
1H), 5.30 (d, J = 13.3 Hz,
1H), 6.70 (d, J = 3.8 Hz,
01\ 1H), 6.78
(d, J = 7.9 Hz, 1
0 H), 6.82 (d,
J = 2.4 Hz, 1H
0 410 NO
), 6.87 (dd, J = 8.2, 2.4 H
z, 1H), 6.90 (d, J = 7.9 Hz
O N , 1H), 7.34
(d, J = 8.2 Hz,
1H), 7.45 (d, J = 3.8 Hz,
1H), 8.02 (dd, J = 4.4, 1.6
Hz, 2H), 8.88 (dd, J = 4.4
, 1.6 Hz, 2H)
7-[4-(Furan-2-ylcarbonyloxy 1H-NMR (500 MHz, CDC13)
)-2-methoxypheny1]-8-(5-met 6 1.22 (s, 3H), 1.42 (s, 3
hylthiophen-2-ylcarbonyloxy
H), 2.47 (s, 3H), 3.45 (s,
methyl)-1,3,3-trimethy1-3,
3H), 3.76 (s, 3H), 3.79 (s,
4-dihydro-1H-quinoxalin-2-o
1H), 5.11 (d, J = 13.4 Hz,
ne (Compound No.14-41)
1H), 5.30 (d, J - 13.4 Hz,
0
1H), 6.61 (d, J = 3.5 Hz,
1H), 6.70 (dd, J = 3.8 Hz,
Or0 0 1H), 6.76
(d, J - 7.9 Hz, 1
0 410 H), 6.82 (d,
J = 2.3 Hz, 1H
0 ), 6.86 (dd,
J = 8.2, 2.3 H
N z, 1H), 6.89
(d, J = 7.9 Hz
O , 1H), 7.31
(d, J = 8.2 Hz,
1H), 7.40 (dd, J = 3.5, 0.
9 Hz, 1H), 7.44 (d, J - 3.8
Hz, 1H), 7.69 (dd, J = 1.7
, 0.9 Hz, 1H)
7-[2-Methoxy-4-(thiophen-3- 1H-NMR (500 MHz, CDC13)
ylcarbonyloxy)pheny1]-8-(5- 6 1.22 (s, 3H), 1.42 (s, 3
methylthiophen-2-ylcarbonyl
H), 2.47 (s, 3H), 3.46 (s,
oxymethyl)-1,3,3-trimethyl-
3H), 3.76 (s, 3H), 3.77 (s,
3,4-dihydro-1H-quinoxalin-
1H), 5.13 (d, J = 13.4 Hz,
2-one (Compound No.14-42)
1H), 5.30 (d, J = 13.4 Hz,
1H), 6.70 (d, J = 3.6 Hz,
0
1H), 6.77 (d, J = 7.9 Hz, 1
fk)ro 0 H), 6.81 (d,
J = 2.1 Hz, 1H
), 6.84 (dd, J = 8.3, 2.1 H
0 101 z, 1H), 6.90
(d, J = 7.9 Hz
N , 1H), 7.31 (d, J = 8.3 Hz,
1H), 7.40 (dd, J = 5.3, 3.
1 Hz, 1H), 7.45 (d, J - 3.6
Hz, 1H), 7.68 (dd, J - 5.3
, 0.9 Hz, 1H), 8.33 (dd, J
= 3.1, 0.9 Hz, 1H)
2 0 9
CA 02643070 2008-08-20
7-[4-(Furan-3-ylcarbonyloxy 1H-NMR (500 MHz, CDC13)
)-2-methoxypheny1]-8-(5-met 6 1.22 (s, 3H), 1.42 (s, 3
hylthiophen-2-ylcarbonyloxy
H), 2.47 (s, 3H), 3.46 (s,
methyl)-1,3,3-trimethy1-3,
3H), 3.76 (s, 3H), 3.79 (s,
4-dihydro-1H-quinoxalin-2-o
1H), 5.12 (d, J = 13.3 Hz,
ne (Compound No.14-43)
1H), 5.30 (d, J = 13.3 Hz,
1H), 6.70 (d, J = 3.7 Hz,
00
1H), 6.77 (d, J = 7.9 Hz, 1
CC:lyo 0 H), 6.79 (d, J = 2.1 Hz, 1H
), 6.82 (dd, J - 8.1, 2.1 H
0 el z, 1H), 6.89-6.90 (m, 1H),
N 6.90 (d, J = 7.9 Hz, 1H), 7
O .30 (d, J = 8.1 Hz, 1H), 7.
45 (d, J - 3.7 Hz, 1H), 7.5
1-7.52 (m, 1H), 8.21-8.22 (
m, 1H)
7-[4-(2-Fluorobenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-methy 6 1.21 (s, 3H), 1.42 (s, 3
lthiophen-2-ylcarbonyloxyme
H), 2.47 (s, 3H), 3.46 (s,
thyl)-1,3,3-trimethy1-3,4-d
3H), 3.77 (s, 3H), 3.80 (s,
ihydro-1H-quinoxalin-2-one
1H), 5.13 (d, J = 13.3 Hz,
(Compound No.14-44)
1H), 5.31 (d, J = 13.3 Hz,
0
1H), 6.70 (d, J = 3.7 Hz,
1H), 6.77 (d, J = 8.1 Hz, 1
010 0 40 0 H), 6.84 (d, J = 2.4 Hz, 1H
), 6.88 (dd, J = 8.5, 2.4 H
0 z, 1H), 6.90 (d, J = 8.1 Hz
O N , 1H), 7.21-7.26 (m, 1H), 7
.30 (t, J = 7.6 Hz, 1H), 7.
32 (d, J = 8.5 Hz, 1H), 7.4
(d, J = 3.7 Hz, 1H), 7.6
0-7.64 (m, 1H), 8.12 (td, J
= 7.6, 1.7 Hz, 1H)
7-[4-(4-Chlorobenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-methy 6 1.22 (s, 3H), 1.42 (s, 3
lthiophen-2-ylcarbonyloxyme
H), 2.47 (s, 3H), 3.46 (s,
thyl)-1,3,3-trimethy1-3,4-d
3H), 3.77 (s, 3H), 3.80 (s,
ihydro-1H-quinoxalin-2-one
1H), 5.13 (d, J = 13.4 Hz,
(Compound No.14-45)
1H), 5.30 (d, J - 13.4 Hz,
1H), 6.70 (d, J = 3.7 Hz,
CI =
1H), 6.77 (d, J = 7.9 Hz, 1
0 00 0 H), 6.81 (d, J = 2.2 Hz, 1H
), 6.85 (dd, J - 8.2, 2.2 H
0 z, 1H), 6.90 (d, J = 7.9 Hz
, 1H), 7.32 (d, J = 8.2 Hz,
1H), 7.45 (d, J = 3.7 Hz,
1H), 7.51 (d, J = 8.6 Hz, 2
H), 8.15 (d, J = 8.6 Hz, 2H
2 1 0
CA 02643070 2008-08-20
7-[2-Methoxy-4-(2-methylben 1H-NMR (400 MHz, CDC13)
zoyloxy)pheny1]-8-(5-methyl ö 1.21 (s, 3H), 1.42 (s, 3
thiophen-2-ylcarbonyloxymet
H), 2.47 (s, 3H), 2.70 (s,
hyl)-1,3,3-trimethy1-3,4-di
3H), 3.46 (s, 3H), 3.77 (s,
hydro-1H-quinoxalin-2-one (
3H), 3.80 (s, 1H), 5.14 (d
Compound No.14-46)
, J = 13.3 Hz, 1H), 5.31 (d
, J = 13.3 Hz, 1H), 6.70 (d
, J = 3.7 Hz, 1H), 6.77 (d,
010 0010 0 J = 8.1 Hz,
1H), 6.81 (d,
J = 2.2 Hz, 1H), 6.85 (dd,
0 .4& N,0 J = 8.2, 2.2 Hz, 1H), 6.90
O N (d, J - 8.1 Hz, 1H), 7.32-7
.36 (m, 2H), 7.33 (d, J = 8
.2 Hz, 1H), 7.45 (d, J = 3.
7 Hz, 1H), 7.50 (td, J = 7.
9, 1.5 Hz, 1H), 8.18 (d, J
= 7.9 Hz, 1H)
7-[2-Methoxy-4-(pyridin-3-y 1H-NMR (400 MHz, CDC13)
lcarbonyloxy)pheny1]-8-(5 m
--- 6 1.22 (s, 3H), 1.42 (s, 3
ethylthiophen-2-ylcarbonylo
H), 2.48 (s, 3H), 3.46 (s,
xymethyl)-1,3,3-trimethy1-3
3H), 3.78 (s, 3H), 3.81 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 5.13 (d, J = 13.3 Hz,
one (Compound No.14-47)
1H), 5.30 (d, J = 13.3 Hz,
1H), 6.70 (d, J = 3.6 Hz,
0
1H), 6.78 (d, J = 8.1 Hz, 1
So 0
H), 6.83 (d, J = 2.2 Hz, 1H
0
), 6.87 (dd, J = 8.2, 2.2 H
0 NO z, 1H), 6.91 (d, J = 8.1 Hz
O N , 1H), 7.34 (d, J - 8.2 Hz,
1H), 7.45 (d, J = 3.6 Hz,
1H), 7.49 (ddd, J = 8.0, 4.
9, 0.9 Hz, 1H), 8.47 (dt, J
= 8.0, 1.9 Hz, 1H), 8.87 (
dd, J = 4.9, 1.9 Hz, 1H), 9
.42 (dd, J = 1.9, 0.9 Hz, 1
H)
7-[2-Methoxy-4-(2-methoxybe 1H-NMR (500 MHz, CDC13)
nzoyloxy)pheny1]-8-(5-methy 6, 1.21 (s, 3H), 1.42 (s, 3
lthiophen-2-ylcarbonyloxyme
H), 2.47 (s, 3H), 3.46 (s,
thyl)-1,3,3-trimethy1-3,4-d
3H), 3.77 (s, 3H), 3.79 (s,
ihydro-1H-quinoxalin-2-one
1H), 3.96 (s, 3H), 5.13 (d
(Compound No.14-48)
, J = 13.4 Hz, 1H), 5.31 (d
, J - 13.4 Hz, 1H), 6.70 (d
, J = 3.7 Hz, 1H), 6.77 (d,
J = 7.9 Hz, 1H), 6.84 (d,
J = 2.2 Hz, 1H), 6.87 (dd,
J - 8.2, 2.2 Hz, 1H), 6.90
(d, J = 7.9 Hz, 1H), 7.05-7
.08 (m, 2H), 7.31 (d, J = 8
2 1 1
CA 02643070 2008-08-20
.2 Hz, 1H), 7.45 (d, J = 3.
010 0,0 I S\ 7 Hz, 1H), 7.56 (ddd, J = 8
.7, 6.9, 1.8 Hz, 1H), 8.04
0
00/0 0 (dd, J = 7.8, 1.8 Hz, 1H)
id& N,0
,0 N
7-(4-Butyryloxy-2-methoxyph 1H-NMR (500 MHz, CDC13)
eny1)-8-(5-methylthiophen- 6 1.06 (t, J = 7.5 Hz, 3H)
2-ylcarbonyloxymethyl)-1,3,
, 1.20 (s, 3H), 1.42 (s, 3H
3-trimethy1-3,4-dihydro-1H-
), 1.80 (qt, J = 7.5, 7.4 H
quinoxalin-2-one (Compound
z, 2H), 2.47 (s, 3H), 2.56
No.14-49)
(t, J = 7.4 Hz, 2H), 3.44 (
s, 3H), 3.74 (s, 3H), 3.78
0
(s, 1H), 5.10 (d, J = 13.4
0 Hz, 1H), 5.28 (d, J = 13.4
Hz, 1H), 6.68 (d, J = 2.1 H
0 id& N,0 z, 1H), 6.69 (d, J - 3.7 Hz
,0 N , 1H), 6.72 (dd, J = 8.1, 2
.1 Hz, 1H), 6.75 (d, J = 8.
1 Hz, 1H), 6.87 (d, J = 8.1
Hz, 1H), 7.26 (d, J = 8.1
Hz, 1H), 7.43 (d, J = 3.7 H
z, 1H)
7-(4-Isobutyryloxy-2-methox 1H-NMR (500 MHz, CDC13)
ypheny1)-8-(5-methylthiophe 6 1.20 (s, 3H), 1.33 (d, J
n-2-ylcarbonyloxymethyl)-1,
= 7.0 Hz, 6H), 1.42 (s, 3H
3,3-trimethy1-3,4-dihydro-1
), 2.47 (s, 3H), 2.79-2.84
H-quinoxalin-2-one (Compoun
(m, 1H), 3.45 (s, 3H), 3.75
d No.14-50)
(s, 3H), 3.78 (s, 1H), 5.1
0 (d, J = 13.4 Hz, 1H), 5.2
0
8 (d, J - 13.4 Hz, 1H), 6.6
.r() 0 8 (d, J = 2.1 Hz, 1H), 6.69
(d, J = 3.7 Hz, 1H), 6.72
0 id& NO (dd, J = 8.2, 2.1 Hz, 1H),
,O 'pp N 6.75 (d, J = 7.9 Hz, 1H), 6
.87 (d, J = 7.9 Hz, 1H), 7.
26 (d, J = 8.2 Hz, 1H), 7.4
3 (d, J = 3.7 Hz, 1H)
7-(2-Methoxy-4-phenylacetox 1H-NMR (500 MHz, CDC13)
ypheny1)-8-(5-methylthiophe 6 1.20 (s, 3H), 1.41 (s, 3
n-2-ylcarbonyloxymethyl)-1,
H), 2.46 (s, 3H), 3.44 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.72 (s, 3H), 3.77 (s,
H-quinoxalin-2-one (Compoun
1H), 3.88 (s, 2H), 5.08 (d
d No.14-51)
, J - 13.3 Hz, 1H), 5.27 (d
= J = 13.3 Hz, 1H), 6.67 (d
= J = 2.3 Hz, 1H), 6.68 (d,
J = 3.7 Hz, 1H), 6.71 (dd,
2 1 2
CA 02643070 2008-08-20
, .
,
J = 8.1, 2.3 Hz, 1H), 6.74
0-1---s
(d, J = 8.1 Hz, 1H), 6.85
(d, J = 8.1 Hz, 1H), 7.24 (
0 0 d, J = 8.1 Hz, 1H), 7.29-7.
I 34 N
410 0 010 0 (m, 1H), 7.37-7.47 (m, 5
,õ ,
H)
0 IW N
H
7-(4-Cyclohexylcarbonyloxy- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1)-8-(5-methy 6 1.20 (s, 3H), 1.29-1.39
lthiophen-2-ylcarbonyloxyme
(m, 3H), 1.42 (s, 3H), 1.5
thyl)-1,3,3-trimethy1-3,4-d
7-1.72 (m, 3H), 1.82-1.85 (
ihydro-1H-quinoxalin-2-one
m, 2H), 2.05-2.09 (m, 2H),
(Compound No.14-52)
2.47 (s, 3H), 2.55-2.60 (m,
1H), 3.41 (s, 3H), 3.74 (s
= 3H), 3.78 (s, 1H), 5.10 (
CLIf 0 d, J = 13.3 Hz, 1H), 5.28 (
I d, J = 13.3 Hz, 1H), 6.67 (
0 411 4& NO d, J = 2.2 Hz, 1H), 6.69 (d
0 IW N , J = 3.8 Hz, 1H), 6.71 (dd
, J - 8.1, 2.2 Hz, 1H), 6.7
H 5 (d, J = 8.1 Hz, 1H), 6.87
(d, J - 8.1 Hz, 1H) 7.25-7
.26 (m, 1H), 7.43 (d, J = 3
.8 Hz, 1H)
7-[2-Methoxy-4-(4-methoxybe 1H-NMR (400 MHz, CDC13)
nzoyloxy)pheny1]-8-(5-methy 6 1.22 (s, 3H), 1.42 (s, 3
lthiophen-2-ylcarbonyloxyme
H), 2.47 (s, 3H), 3.46 (s,
thyl)-1,3,3-trimethy1-3,4-d
3H), 3.76 (s, 3H), 3.78 (s,
ihydro-1H-quinoxalin-2-one
1H), 3.91 (s, 3H), 5.13 (d
(Compound No.14-53)
, J - 13.3 Hz, 1H), 5.31 (d
I \ , J = 13.3 Hz, 1H), 6.70 (d
,0 000
S , J - 3.7 Hz, 1H), 6.77 (d,
0 4/0 0
J - 8.1 Hz, 1H), 6.81 (d,
I J = 2.1 Hz, 1H), 6.84 (dd,
0 iii& 1\1,,0 j = 8.1, 2.1 Hz, 1H), 6.91
0 IW N (d, J = 8.1 Hz, 1H) 7.00 (d
, J = 8.9 Hz, 2H), 7.31 (d,
H
J = 8.1 Hz, 1H), 7.45 (d,
J = 3.7 Hz, 1H), 8.17 (d, J
= 8.9 Hz, 2H)
7-[2-Methoxy-4-(thiophen-2- 1H-NMR (500 MHz, CDC13)
ylcarbonyloxy)pheny1]-8-(5- 6 1.22 (s, 3H), 1.42 (s, 3
methylthiophen-2-ylcarbonyl
H), 2.47 (s, 3H), 3.47 (s,
oxymethyl)-1,3,3-trimethyl-
3H), 3.77 (s, 3H), 3.80 (s,
3,4-dihydro-1H-quinoxalin-
1H), 5.13 (d, J = 13.1 Hz,
2-one (Compound No.14-54)
1H), 5.30 (d, J = 13.1 Hz,
1H), 6.70 (d, J = 3.7 Hz,
1H), 6.77 (d, J = 7.9 Hz, 1
2 1 3
CA 02643070 2008-08-20
H), 6.83 (d, J = 2.1 Hz, 1H
), 6.86 (dd, J = 8.3, 2.1 H
z, 1H), 6.90 (d, J = 7.9 Hz
(111,0 0 , 1H) 7.20 (dd, J = 4.9, 3.
S 0 010 .4& 7 Hz, 1H), 7.31 (d, J = 8.3
N,0
Hz, 1H), 7.45 (d, J = 3.7
N Hz, 1H), 7.68 (dd, J = 4.9,
1.2 Hz, 1H), 8.00 (dd, J =
3.7, 1.2 Hz, 1H)
7-(2-Methoxy-4-methoxycarbo 1H-NMR (400 MHz, CDC13)
nyloxypheny1)-8-(5-methylth 6 1.21 (s, 3H), 1.41 (s, 3
iophen-2-ylcarbonyloxymethy
H), 2.47 (s, 3H), 3.45 (s,
1)-1,3,3-trimethy1-3,4-dihy
3H), 3.75 (s, 3H), 3.79 (s,
dro-1H-quinoxalin-2-one (Co
1H), 3.92 (s, 3H), 5.09 (d
mpound No.14-55)
,J = 13.3 Hz, 1H), 5.28 (d
,(11) , J = 13.3 Hz, 1H), 6.69 (d
0
, J = 3.8 Hz, 1H), 6.75 (d,
Oy0 0 J = 8.1 Hz, 1H), 6.77
(d,
J = 2.2 Hz, 1H), 6.82 (dd,
0 ti& N,0 J = 8.2, 2.2 Hz, 1H), 6.86
O N (d, J = 8.1 Hz, 1H), 7.27 (
d, J = 8.2 Hz, 1H), 7.43 (d
, J = 3.8 Hz, 1H)
7-(2-Methoxy-4-phenoxycarbo 1H-NMR (400 MHz, CDC13)
nyloxypheny1)-8-(5-methylth 6 1.21 (s, 3H), 1.41 (s, 3
iophen-2-ylcarbonyloxymethy
H), 2.47 (s, 3H), 3.45 (s,
1)-1,3,3-trimethy1-3,4-dihy
3H), 3.76 (s, 3H), 3.80 (s,
dro-1H-quinoxalin-2-one (Co
1H), 5.11 (d, J = 13.3 Hz,
mpound No.14-56)
1H), 5.29 (d, J = 13.3 Hz,
1H), 6.69 (d, J = 3.8 Hz,
0
1H), 6.76 (d, J = 8.1 Hz, 1
00 0 H), 6.87 (d, J = 8.1 Hz, 1H
), 6.89 (d, J = 2.2 Hz, 1H)
410 0 010 , 6.92 (dd, J = 8.2, 2.2 Hz
O N , 1H), 7.27-7.31 (m, 4H), 7
.40-7.45 (m, 3H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[4-(furan-2-ylcar 6 0.92 (s, 3H), 1.29 (s, 3
bonylamino)-2-methoxyphenyl
H), 2.02 (s, 3H), 3.47 (s,
1-1,3,3-trimethy1-3,4-dihyd
3H), 3.71 (s, 1H), 3.88 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 4.86 (d, J = 13.6 Hz,
pound No.14-57)
1H), 5.24 (d, J = 13.6 Hz,
1H), 6.06 (dd, J = 11.2, 2
.4 Hz, 1H), 6.38 (td, J = 8
.3, 2.4 Hz, 1H), 6.59 (dd,
J = 3.5, 1.8 Hz, 1H), 6.72
(d, J = 8.1 Hz, 1H), 6.87-6
.91 (m, 1H), 6.90 (d, J = 8
214
CA 02643070 2008-08-20
F .1 Hz, 1H), 7.07 (dd, J = 8
.3, 2.1 Hz, 1H), 7.27 (dd,
J = 3.5, 0.9 Hz, 1H), 7.30
0 N ilM 0 (d, J = 8.3 Hz, 1H), 7.55 (
dd, J = 1.8, 0.9 Hz, 1H), 7
0 W .4& NO .75 (d, J = 2.1 Hz, 1H), 8.
N 17 (s, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-m 6 0.92 (s, 3H), 1.29 (s, 3
ethylbenzoylamino)pheny1]-1
H), 2.02 (s, 3H), 2.55 (s,
,3,3-trimethy1-3,4-dihydro-
3H), 3.47 (s, 3H), 3.71 (s,
1H-quinoxalin-2-one (Compou
1H), 3.88 (s, 3H), 4.87 (d
nd No.14-58)
, J = 13.6 Hz, 1H), 5.24 (d
F
, J = 13.6 Hz, 1H), 6.07 (d
010 rl 010 0 d, J 11.2, 2.4
Hz, 1H), 6
.39 (td, J = 8.2, 2.4 Hz, 1
H), 6.72 (d, J = 7.9 Hz, 1H
0 N,0 ), 6.89-6.92 (m, 1H), 6.89
(d, J = 7.9 Hz, 1H), 7.02O (
N d, J = 7.3 Hz, 1H), 7.29 (t
, J = 7.6 Hz, 1H), 7.30 (d,
J = 7.6 Hz, 1H), 7.30 (d,
J = 7.3 Hz, 1H), 7.38-7.41
(m, 1H), 7.52 (d, J = 7.6 H
z, 1H), 7.55 (s, 1H), 7.72
(s, 1H)
7-[4-(2-Fluorobenzoylamino 1H-NMR (500 MHz, CDC13)
)-2-methoxypheny1]-8-(5-flu 5 0.92 (s, 3H), 1.29 (s, 3
oro-2-methylphenoxymethyl)-
H), 2.02 (s, 3H), 3.48 (s,
1,3,3-trimethy1-3,4-dihydr
3H), 3.71 (s, 1H), 3.89 (s,
o-1H-quinoxalin-2-one (Comp
3H), 4.87 (d, J = 13.7 Hz,
ound No.14-59)
1H), 5.24 (d, J = 13.7 Hz,
FF 010
1H), 6.07 (dd, J = 11.0, 2
.4 Hz, 1H), 6.39 (td, J = 8
i [
0 .2, 2.4 Hz, 1H), 6.72 (d, J
N
0 010
m
id& N,0 = 7.9 Hz,
1H), 6.88-6.91 (
, 1H), 6.90 (d, J = 7.9 Hz
, 1H), 7.10 (dd, J = 8.0, 2
O N .1 Hz, 1H), 7.22 (dd, J = 1
1.9, 7.9 Hz, 1H), 7.31 (d,
J = 8.0 Hz, 1H), 7.33-7.37
(m, 1H), 7.53-7.58 (m, 1H),
7.76 (d, J = 2.1 Hz, 1H),
8.19-8.22 (m, 1H), 8.55-8.
58 (m, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-m 5 0.89 (s, 3H),
1.30 (s, 3
215 1 5
CA 02643070 2008-08-20
1,3,3-trimethy1-3,4-dihydr H), 2.02 (s, 3H), 3.48 (s,
o-1H-quinoxalin-2-one (Comp 3H), 3.70 (s, 1H), 3.90 (s,
ound No.14-60) 3H), 4.10 (s, 3H), 4.89 (d
010 OF , J = 13.7
Hz, 1H), 5.27 (d
, J = 13.7 Hz, 1H), 6.06 (d
d, J - 11.3, 2.4 Hz, 1H), 6
ri0 .38 (td, J =
8.2, 2.4 Hz, 1
0 010
N,0 H), 6.72 (d,
J = 7.9 Hz, 1H
), 6.88-6.90 (m, 1H), 6.91
O N (d, J = 7.9 Hz, 1H), 7.00 (
dd, J = 8.0, 1.9 Hz, 1H), 7
.07 (d, J = 7.9 Hz, 1H), 7.
15-7.18 (m, 1H), 7.29 (d, J
= 8.0 Hz, 1H), 7.53 (ddd,
J = 8.6, 7.3, 1.7 Hz, 1H),
7.91 (d, J = 1.9 Hz, 1H), 8
.31 (dd, J = 7.3, 1.7 Hz, 1
H), 9.94 (s, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-t 6 0.92 (s, 3H), 1.29 (s, 3
rifluoromethylbenzoylamino)
H), 2.02 (s, 3H), 3.47 (s,
phenyl]-1,3,3-trimethy1-3,
3H), 3.71 (s, 1H), 3.88 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 4.88 (d, J = 13.4 Hz,
ne (Compound No.14-61)
1H), 5.24 (d, J = 13.4 Hz,
F
1H), 6.07 (dd, J = 11.3, 2
410 NI 010 0 .4 Hz, 1H),
6.39 (td, J = 8
.4, 2.4 Hz, 1H), 6.72 (d, J
- 7.9 Hz, 1H), 6.88-6.91 (
0 4s6 N,0 m, 1H), 6.89
(d, J = 7.9 Hz
FFF , 1H), 7.02
(dd, J = 8.0, 1
N .9 Hz, 1H),
7.30 (d, J = 8.
0 Hz, 11-1), 7.54 (s, 1H), 7.
61-7.64 (m, 1H), 7.66 (d, J
- 1.9 Hz, 1H), 7.68-7.71 (
m, 2H), 7.79 (d, J = 7.6 Hz
, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-n 6 0.93 (s, 3H), 1.29 (s, 3
itrobenzoylamino)pheny1]-1,
H), 2.03 (s, 3H), 3.48 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.73 (s, 1H), 3.88 (s,
H-quinoxalin-2-one (Compoun
3H), 4.88 (d, J = 13.7 Hz,
d No.14-62)
1H), 5.25 (d, J = 13.7 Hz,
1H), 6.08 (dd, J = 11.0, 2
.3 Hz, 1H), 6.40 (td, J = 8
.3, 2.3 Hz, 1H), 6.73 (d, J
- 8.1 Hz, 1H), 6.89-6.93 (
m, 1H), 6.90 (d, J = 8.1 Hz
, 1H), 7.04 (dd, J = 8.1, 1
.7 Hz, 1H), 7.31 (d, J - 8.
1 Hz, 1H), 7.62 (s, 1H), 7.
216
CA 02643070 2008-08-20
F 65-7.70 (m, 3H), 7.77 (t, J
= 7.6 Hz, 1H), 8.17 (d, J
010
= 8.0 Hz, 1H) F1\11 0
0 010 NO
0 '0
N
Example 15
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(3-
methoxycarbonylbenzoyloxy)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one (Compound No.15-1)
A mixture of 8-(5-fluoro-2-
methylphenoxymethyl)-7-(4-hydroxy-2-methoxypheny1)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.11, 25.3 mg, 0.0562 mmol), monomethyl
isophthalate (20.5 mg, 0.114 mmol), N,N-
diisopropylethylamine (38.8 p L, 0.223 mmol) and 0-(7-
azabenzotriazol-1-y1)-N,N,N,N-tetramethyluronium
hexafluorophosphate (43.4 mg, 0.114 mmol) was
dissolved in anhydrous N,N-dimethylformamide (0.5 mL)
and the reaction mixture was stirred at room
temperature overnight. The mixture was diluted with
ethyl acetate (15 mL). The mixture was washed with
water (15 mL) and saturated saturated brine (15 mL)
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled compound (22.0 mg) as a colorless
solid. (Yield 64%)
2 1 7
CA 02643070 2008-08-20
.õ
o
Co F 1H-NMR (500 MHz, CDC13)
6 0.96 (s, 3H), 1.28 (s,
3H), 2.03 (s, 3H), 3.47 (s
410 0010 0 , 3H), 3.73 (br s, 1H), 3.
0 id& r\l,,0 84 (s, 3H), 3.99 (s, 3H),
4.89 (d, J = 13.6 Hz, 1H),
O N 5.23 (d, J = 13.6 Hz, 1H)
, 6.10 (dd, J = 11.3, 2.4
Hz, 1H), 6.41 (td, J = 8.2
, 2.4 Hz, 1H), 6.74 (d, J
= 7.9 Hz, 1H), 6.90-6.93 (
m, 1H), 6.90 (d, J = 2.4 H
z, 1H), 6.92 (d, J = 7.9 H
z, 1H), 6.97 (dd, J = 8.2,
2.4 Hz, 1H), 7.38 (d, J =
8.2 Hz, 1H), 7.64 (t, J =
7.8 Hz, 1H), 8.33 (dt, J
= 7.8, 1.5 Hz, 1H), 8.41 (
dt, J = 7.8, 1.5 Hz, 1H),
8.89 (t, J = 1.5 Hz, 1H)
Using any compounds among Compounds No.8-2, 11, 13-2
and available compounds, the following Compounds
(No.15-2-15-32) were obtained by a method similar to
that of Compound No.15-1.
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-m 60.96 (s, 3H), 1.28 (s, 3H
ethylpyridin-3-ylcarbonylox
), 2.03 (s, 3H), 2.96 (s, 3
y)pheny1]-1,3,3-trimethy1-3
H), 3.47 (s, 3H), 3.74 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 3.85 (s, 3H), 4.88 (d,
one (Compound No.15-2)
J = 13.7 Hz, 1H), 5.23 (d,
010
J = 13.7 Hz, 1H), 6.09 (dd
F
Nõ , J = 11.2, 2.4 Hz, 1H), 6.
41 (td, J = 8.3, 2.4 Hz, 1H
0 0 ), 6.74 (d, J = 8.2 Hz, 1H)
0 NO , 6.87 (d, J = 2.2 Hz, 1H),
6.89-6.93 (m, 1H), 6.91 (d
N , J = 8.2 Hz, 1H), 6.94 (dd
, J = 8.2, 2.2 Hz, 1H), 7.3
3 (dd, J = 8.0, 4.8 Hz, 1H)
, 7.38 (d, J = 8.2 Hz, 1H),
8.47 (dd, J = 8.0, 2.0 Hz,
2 1 8
CA 02643070 2008-08-20
1H), 8.72 (dd, J = 4.8, 2.
0 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(3 m
--- 6 0.95 (s, 3H), 1.27 (s, 3
ethylfuran-2-ylcarbonyloxy)
H), 2.02 (s, 3H), 2.47 (s,
phenyl]-1,3,3-trimethy1-3,
3H), 3.47 (s, 3H), 3.72 (br
4-dihydro-1H-quinoxalin-2-o
s, 1H), 3.83 (s, 3H), 4.87
ne (Compound No.15-3)
(d, J = 13.6 Hz, 1H), 5.22
F
(d, J = 13.6 Hz, 1H), 6.08
(dd, J = 11.3, 2.5 Hz, 1H)
/ 1 , 6.39 (td,
J = 8.3, 2.5 Hz
0( 0
, 1H), 6.47 (d, J = 1.8 Hz,
0 id& N,0 1H), 6.73 (d,
J = 7.9 Hz,
1H), 6.88-6.92 (m, 1H), 6.8
O N 9 (d, J =
2.2 Hz, 1H), 6.91
(d, J = 7.9 Hz, 1H), 6.94
(dd, J = 8.2, 2.2 Hz, 1H),
7.35 (d, J = 8.2 Hz, 1H), 7
.56 (d, J = 1.8 Hz, 1H)
7-[4-(2-Acetoxybenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-fluor 6 0.96 (s, 3H) ,
1.27 (s, 3
o-2-methylphenoxymethyl)-1,
H), 2.02 (s, 3H), 2.34 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.47 (s, 3H), 3.73 (br
H-quinoxalin-2-one (Compoun
s, 1H), 3.83 (s, 3H), 4.87
d No.15-4)
(d, J = 13.4 Hz, 1H), 5.22
() F
(d, J = 13.4 Hz, 1H), 6.08
(dd, J = 11.3, 2.5 Hz, 1H)
C)0
, 6.40 (td, J = 8.3, 2.5 Hz
0
id& N,0 , 1H), 6.73
(d, J = 8.1 Hz,
1H), 6.83 (d, J = 2.4 Hz,
0
1H), 6.89-6.92 (m, 1H), 6.9
O N 0 (dd, J =
7.6, 2.4 Hz, 1H)
, 6.91 (d, J = 8.1 Hz, 1H),
7.20 (d, J = 7.5 Hz, 1H),
7.35 (d, J - 7.6 Hz, 1H), 7
.42 (t, J - 7.5 Hz, 1H), 7.
67 (td, J = 7.5, 1.6 Hz, 1H
), 8.26 (dd, J = 7.5, 1.6 H
z, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(thi 6 0.97 (s, 3H), 1.27 (s, 3
azol-4-ylcarbonyloxy)phenyl
H), 2.02 (s, 3H), 3.47 (s,
]-1,3,3-trimethy1-3,4-dihyd
3H), 3.75 (br s, 1H), 3.83
ro-1H-quinoxalin-2-one (Corn
(s, 3H), 4.87 (d, J = 13.4
pound No.15-5)
Hz, 1H), 5.21 (d, J = 13.4
Hz, 1H), 6.09 (dd, J = 11.3
, 2.4 Hz, 1H), 6.40 (td, J
= 8.4, 2.4 Hz, 1H), 6.73 (d
, J = 8.1 Hz, 1H), 6.89-6.9
219
CA 02643070 2008-08-20
F 2 (m, 1H),
6.92 (d, J - 8.1
Hz, 1H), 6.92 (d, J - 2.3
Hz, 1H), 6.98 (dd, J = 8.1,
0 2.3 Hz, 1H), 7.36 (d, J
8.1 Hz, 1H), 8.48 (d, J = 2
0 el .0 Hz, 1H),
8.96 (d, J = 2.
N 0 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-m 6 0.94 (s, 3H), 1.27 (s, 3
ethylthiobenzoyloxy)phenyl
H), 2.02 (s, 3H), 2.50 (s,
]-1,3,3-trimethy1-3,4-dihyd
3H), 3.47 (s, 3H), 3.72 (br
ro-1H-quinoxalin-2-one (Corn
s, 1H), 3.84 (s, 3H), 4.89
pound No.15-6)
(d, J = 13.4 Hz, 1H), 5.23
410
F
(dd, J - 11.0, 2.4 Hz, 1H)
(d, J - 13.4 Hz, 1H), 6.08
S
, 6.39 (td, J = 8.2, 2.4 Hz
O
0 Olt 0
,
id& N,0 1H), 6.73 (d, J = 7.8 Hz,
1H), 6.89-6.92 (m, 1H), 6.
90 (d, J = 2.4 Hz, 1H), 6.9
N 1 (d, J = 7.8 Hz, 1H), 6.96
(dd, J = 8.2, 2.4 Hz, 1H),
7.24-7.28 (m, 1H), 7.36 (d
, J = 8.2 Hz, 1H), 7.36 (d,
J = 7.9 Hz, 1H), 7.58 (t,
J = 7.9 Hz, 1H), 8.28 (dd,
J = 7.9, 1.7 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(5-m 6 0.95 (s, 3H), 1.27 (s, 3
ethylfuran-2-ylcarbonyloxy)
H), 2.02 (s, 3H), 2.46 (s,
phenyl]-1,3,3-trimethy1-3,
3H), 3.46 (s, 3H), 3.72 (br
4-dihydro-1H-quinoxalin-2-o
s, 1H), 3.82 (s, 3H), 4.87
ne (Compound No.15-7)
(d, J = 13.7 Hz, 1H), 5.21
F 010
(d, J = 13.7 Hz, 1H), 6.08
(dd, J = 11.2, 2.4 Hz, 1H)
, 6.23 (d, J = 3.4 Hz, 1H),
0 0 6.40 (td, J
= 8.3, 2.4 Hz,
0 id&
NO 1H), 6.73 (d, J = 8.0 Hz,
1H), 6.88 (t, J - 2.7 Hz, 1
O N H), 6.88-6.92 (m, 1H), 6.91
(d, J = 8.0 Hz, 1H), 6.93
(dd, J - 8.3, 2.7 Hz, 1H),
7.33 (d, J = 3.4 Hz, 1H), 7
.34 (d, J = 8.3 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(thi 6 0.97 (s, 3H), 1.28 (s, 3
azol-5-ylcarbonyloxy)phenyl
H), 2.02 (s, 3H), 3.47 (s,
]-1,3,3-trimethy1-3,4-dihyd
3H), 3.74 (br s, 1H), 3.84
ro-1H-quinoxalin-2-one (Com
220
CA 02643070 2008-08-20
pound No.15-8) (s, 3H), 4.86
(d, J = 13.4
F Hz, 1H), 5.21
(d, J = 13.4
Hz, 1H), 6.08 (dd, J = 11.0
, 2.5 Hz, 1H), 6.40 (td, J
0 = 8.4, 2.5
Hz, 1H), 6.73 (d
S /0
, J = 7.9 Hz, 1H), 6.89 (d,
0
0 al& N,0 J = 2.1 Hz,
1H), 6.90-6.92
N(m, 1H), 6.91 (d, J = 7.9
Hz, 1H), 6.95 (dd, J = 8.2,
2.1 Hz, 1H), 7.37 (d, J =
8.2 Hz, 1H), 8.72 (d, J = 0
.6 Hz, 1H), 9.07 (d, J = 0.
6 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(6-m 5 0.96 (s, 3H), 1.28 (s, 3
ethylpyridin-3-ylcarbonylox
H), 2.02 (s, 3H), 2.69 (s,
y)pheny1]-1,3,3-trimethy1-3
3H), 3.47 (s, 3H), 3.78 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 3.83 (s, 3H), 4.88 (d
one (Compound No.15-9)
, J = 13.4 Hz, 1H), 5.23 (d
F
, J = 13.4 Hz, 1H), 6.09 (d
d, J = 11.2, 2.4 Hz, 1H), 6
.40 (td, J = 8.3, 2.4 Hz, 1
0 0 H), 6.74
(d, J = 8.0 Hz, 1H
0 N ), 6.89 (d,
J = 2.3 Hz, 1H)
, 6.89-6.93 (m, 1H), 6.92 (
0 N d, J = 8.0 Hz, 1H), 6.95 (d
d, J = 8.2, 2.3 Hz, 1H), 7.
34 (d, J = 8.1 Hz, 1H), 7.3
7 (d, J = 8.2 Hz, 1H), 8.35
(dd, J = 8.1, 1.9 Hz, 1H),
9.30 (d, J = 1.9 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(pyr 5 0.--
(s, 3H), 1.28 (s, 3
imidin-5-ylcarbonyloxy)phen
H), 2.03 (s, 3H), 3.47 (s,
y11-1,3,3-trimethy1-3,4-dih
3H), 3.75 (s, 1H), 3.85 (s,
ydro-1H-quinoxalin-2-one (C
3H), 4.87 (d, J = 13.4 Hz,
ompound No.15-10)
1H), 5.21 (d, J = 13.4 Hz,
F 010
1H), 6.09 (dd, J = 11.2, 2
N, .4 Hz, 1H),
6.41 (td, J = 8
I .3, 2.4 Hz,
1H), 6.74 (d, J
0 = 8.1 Hz,
1H), 6.89 (d, J
0 id& N,0 = 2.2 Hz,
1H), 6.90-6.93 (m
, 1H), 6.91 (d, J = 8.1 Hz,
N 1H), 6.96 (dd, J = 8.3, 2.
2 Hz, 1H), 7.39 (d, J = 8.3
Hz, 1H), 9.47 (s, 1H), 9.4
8 (s, 2H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(oxa 6 0.96 (s, 3H), 1.27 (s, 3
zol-4-ylcarbonyloxy)phenyl
2 2 1
CA 02643070 2008-08-20
]-1,3,3-trimethy1-3,4-dihyd H), 2.02 (s, 3H), 3.47 (s,
ro-1H-quinoxalin-2-one (Corn 3H), 3.74 (s, 1H), 3.82 (s,
pound No.15-11) 3H), 4.86
(d, J = 13.5 Hz,
F 1H), 5.21
(d, J = 13.5 Hz,
1H), 6.08 (dd, J = 11.3, 2
.4 Hz, 1H), 6.40 (td, J = 8
0 .4, 2.4 Hz,
1H), 6.73 (d, J
= 8.1 Hz, 1H), 6.89 (d, J
0 010 .4&
NO = 2.2 Hz,
1H), 6.89-6.92 (m
O N , 1H), 6.91
(d, J = 8.1 Hz,
1H), 6.95 (dd, J = 8.2, 2.
2 Hz, 1H), 7.35 (d, J = 8.2
Hz, 1H), 8.04 (d, J = 1.0
Hz, 1H), 8.48 (d, J = 1.0 H
z, 1H)
7-[4-(4-Acetylbenzoyloxy)- H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-fluor 6 0.96 (s, 3H), 1.28 (s, 3
o-2-methylphenoxymethyl)-1,
H), 2.03 (s, 3H), 2.69 (s,
3,3-trimethy1-3,4-dihydro-1
3H), 3.47 (s, 3H), 3.84 (s,
H-quinoxalin-2-one (Compoun
3H), 4.88 (d, J = 13.6 Hz,
d No.15-12)
1H), 5.23 (d, J = 13.6 Hz,
F
0 1H), 6.09
(dd, J = 11.0, 2
.4 Hz, 1H), 6.40 (td, J = 8
010.4, 2.4 Hz, 1H), 6.74 (d, J
0 0 = 7.9 Hz, 1H), 6.90 (d, J
N 0
0 010
= 2.3 Hz, 1H), 6.90-6.93 (m
0 Nio , 1H), 6.92
(d, J = 7.9 Hz,
1H), 6.96 (dd, J = 8.2, 2.
3 Hz, 1H), 7.37 (d, J - 8.2
Hz, 1H), 8.10 (d, J = 8.2
Hz, 2H), 8.32 (d, J = 8.2 H
z, 2H)
7-[4-(3-Acetylbenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-fluor 6 0.96 (s, 3H), 1.28 (s, 3
o-2-methylphenoxymethyl)-1,
H), 2.03 (s, 3H), 2.67 (s,
3,3-trimethy1-3,4-dihydro-1
1H), 2.71 (s, 3H), 3.47 (s,
H-quinoxalin-2-one (Compoun
3H), 3.85 (s, 3H), 4.89 (d
d No.15-13)
, J = 13.5 Hz, 1H), 5.23 (d
0 F , J - 13.5
Hz, 1H), 6.09 (d
d, J = 11.5, 2.4 Hz, 1H), 6
.41 (td, J = 8.3, 2.4 Hz, 1
010 0 0 H), 6.74 (d,
J = 7.9 Hz, 1H
0 SNy? ), 6.90 (d,
J = 2.1 Hz, 1H)
, 6.90-6.93 (m, 1H), 6.93 (
d, J = 7.9 Hz, 1H), 6.97 (d
N)<-'
d, J = 8.1, 2.1 Hz, 1H), 7.
37 (d, J = 8.1 Hz, 1H), 7.6
7 (t, J = 7.8 Hz, 1H), 8.26
(d, J = 7.8 Hz, 1H), 8.43
(d, J = 7.8 Hz, 1H), 8.78 (
2 2 2
CA 02643070 2008-08-20
s, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(4-m 6 0.95 (s, 3H),
1.28 (s, 3
ethylpyridin-3-ylcarbonylox
H), 2.03 (s, 3H), 2.73 (s,
y)pheny1]-1,3,3-trimethy1-3
3H), 3.47 (s, 3H), 3.76 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 3.85 (s, 3H), 4.89 (d
one (Compound No.15-14)
, J = 13.4 Hz, 1H), 5.23 (d
, J = 13.4 Hz, 1H), 6.09 (d
F
d, J = 11.3, 2.4 Hz, 1H), 6
.40 (td, J = 8.3, 2.4 Hz, 1
0 0 H), 6.73 (d,
J = 7.9 Hz, 1H
0 a& 1\l,.0 ), 6.89 (d,
J = 2.2 Hz, 1H)
, 6.91 (d, J = 7.9 Hz, 1H),
O N 6.88-6.92
(m, 1H), 6.95 (d
d, J = 8.2, 2.2 Hz, 1H), 7.
28 (d, J = 5.2 Hz, 1H), 7.3
8 (d, J = 8.2 Hz, 1H), 8.66
(d, J = 5.2 Hz, 1H), 9.35
(s, 1H)
7-[4-(5-Bromofuran-2-ylcarb 1H-NMR (400 MHz, CDC13)
onyloxy)-2-methoxypheny1]- 6 0.96 (s, 3H), 1.27 (s, 3
8-(5-fluoro-2-methylphenoxy
H), 2.02 (s, 3H), 3.46 (s,
methyl)-1,3,3-trimethy1-3,
3H), 3.73 (s, 1H), 3.83 (s,
4-dihydro-1H-quinoxalin-2-o
3H), 4.86 (d, J = 13.7 Hz,
ne (Compound No.15-15)
1H), 5.20 (d, J = 13.7 Hz,
F
1H), 6.07 (dd, J = 11.2, 2
.4 Hz, 1H), 6.40 (td, J = 8
0 .3, 2.4 Hz, 1H), 6.57 (d, J
0
410 = 3.7 Hz, 1H), 6.73 (d, J
Br
0 N,0 = 8.1 Hz,
1H), 6.87 (d, J =
2.2 Hz, 1H), 6.88-6.92 (m,
0 N 1H), 6.90 (d, J = 8.1 Hz,
1H), 6.93 (dd, J = 8.3, 2.2
Hz, 1H), 7.35 (d, J = 8.3
Hz, 1H), 7.36 (d, J = 3.7 H
z, 1H)
7-[4-(5-Chlorothiophen-2-y1 1H-NMR (400 MHz, CDC13)
carbonyloxy)-2-methoxypheny 6 0.95 (s, 3H), 1.28 (s, 3
1]-8-(5-fluoro-2-methylphen
H), 2.02 (s, 3H), 3.47 (s,
oxymethyl)-1,3,3-trimethyl-
3H), 3.83 (s, 3H), 4.86 (d,
3,4-dihydro-1H-quinoxalin-
J = 13.5 Hz, 1H), 5.21 (d,
2-one (Compound No.15-16)
J = 13.5 Hz, 1H), 6.08 (dd
, J = 11.2, 2.4 Hz, 1H), 6.
40 (td, J = 8.3, 2.4 Hz, 1H
), 6.73 (d, J = 8.1 Hz, 1H)
, 6.87 (d, J = 2.2 Hz, 1H),
6.88-6.93 (m, 1H), 6.90 (d
, J - 8.1 Hz, 1H), 6.92 (dd
, J = 8.2, 2.2 Hz, 1H), 7.0
2 2 3
CA 02643070 2008-08-20
F 4 (d, J = 4.2 Hz, 1H), 7.35
(d, J = 8.2 Hz, 1H), 7.80
(d, J = 4.2 Hz, 1H)
CI
0
0 .4& N,0
,0 N
7-[4-(3-Chlorothiophen-2-y1 1H-NMR (400 MHz, CDC13)
carbonyloxy)-2-methoxypheny 6 0.95 (s, 3H), 1.28 (s, 3
1]-8-(5-fluoro-2-methylphen
H), 2.02 (s, 3H), 3.47 (s,
oxymethyl)-1,3,3-trimethyl-
3H), 3.84 (s, 3H), 4.86 (d,
3,4-dihydro-1H-quinoxalin-
J = 13.7 Hz, 1H), 5.22 (d,
2-one (Compound No.15-17)
J = 13.7 Hz, 1H), 6.08 (dd
F 410
, J = 11.1, 2.4 Hz, 1H), 6.
40 (td, J = 8.3, 2.4 Hz, 1H
6.73 (d, J = 8.1 Hz, 1H)
0
, 6.88-6.92 (m, 3H), 6.96 (
NO dd, J = 8.2, 2.2 Hz, 1H), 7 :< .12 (d, J
= 5.3 Hz, 1H), 7.
O 36 (d, J = 8.2 Hz, 1H), 7.6
1 (d, J = 5.3 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-(5 m
- 6 0.94 (s, 3H), 1.27 (s, 3
ethylthiophen-2-ylcarbonylo
H), 2.02 (s, 3H), 2.59 (s,
xy)pheny1]-1,3,3-trimethyl-
3H), 3.47 (s, 3H), 3.72 (s,
3,4-dihydro-1H-quinoxalin-
1H), 3.83 (s, 3H), 4.87 (d
2-one (Compound No.15-18)
, J = 13.6 Hz, 1H), 5.22 (d
F
, J = 13.6 Hz, 1H), 6.08 (d
d, J = 11.2, 2.4 Hz, 1H), 6
OyD1.39 (td, J = 8.4, 2.4 Hz, 1
0 H), 6.72 (d, J = 8.1 Hz, 1H
410
0 N,0 ), 6.86-6.92 (m, 4H), 6.93
(dd, J = 8.4, 2.6 Hz, 1H),
0 N 7.34 (d, J = 8.4 Hz, 1H), 7
.82 (d, J = 3.9 Hz, 1.1H)
7-[4-(3-Chloro-4-methylthio 1H-NMR (400 MHz, CDC13)
phen-2-ylcarbonyloxy)-2-met 6 0.94 (s, 3H), 1.28 (s, 3
hoxypheny1]-8-(5-fluoro-2-m
H), 2.02 (s, 3H), 2.30 (s,
ethylphenoxymethyl)-1,3,3-t
3H), 3.47 (s, 3H), 3.73 (s,
rimethy1-3,4-dihydro-1H-qui
1H), 3.84 (s, 3H), 4.87 (d
noxalin-2-one (Compound No.
, J = 13.7 Hz, 1H), 5.22 (d
15-19)
, J = 13.7 Hz, 1H), 6.07 (d
d, J = 11.0, 2.4 Hz, 1H), 6
.40 (td, J = 8.3, 2.4 Hz, 1
H), 6.73 (d, J = 8.1 Hz, 1H
), 6.88-6.92 (m, 1H), 6.90
(d, J = 8.1 Hz, 1H), 6.91 (
224
CA 02643070 2008-08-20
F010 d, J = 2.2
Hz, 1H), 6.95 (d
d, J = 8.3, 2.2 Hz, 1H), 7.
33 (s, 1H), 7.35 (d, J = 8.
0 010 0 3 Hz, 1H)
0 id& N,0
,0 N
7-[4-(2-Chloropyridin-4-ylc 1H-NMR (400 MHz, CDC13)
arbonyloxy)-2-methoxyphenyl 6 0.97 (s, 3H), 1.28 (s, 3
]-8-(5-fluoro-2-methylpheno
H), 2.02 (s, 3H), 3.47 (s,
xymethyl)-1,3,3-trimethy1-3
3H), 3.75 (s, 1H), 3.84 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 4.86 (d, J = 13.6 Hz,
one (Compound No.15-20)
1H), 5.21 (d, J = 13.6 Hz,
F 010
CI 1H), 6.08
(dd, J = 11.2, 2
.4 Hz, 1H), 6.41 (td, J = 8
.3, 2.4 Hz, 1H), 6.74 (d, J
0I. 0 = 8.1 Hz, 1H), 6.87 (d, J
0 N,0 = 2.2 Hz,
1H), 6.90-6.93 (m
, 1H), 6.91 (d, J = 8.1 Hz,
O N 1H), 6.94 (dd, J = 8.3, 2.
2 Hz, 1H), 7.38 (d, J = 8.3
Hz, 1H), 7.96 (dd, J = 5.1
, 1.3 Hz, 1H), 8.08 (dd, J
= 1.3, 0.7 Hz, 1H), 8.65 (d
d, J = 5.1, 0.7 Hz, 1H)
7-[4-(6-Chloropyridin-3-ylc 1H-NMR (500 MHz, CDC13)
arbonyloxy)-2-methoxyphenyl 6 0.97 (s, 3H), 1.28 (s, 3
]-8-(5-fluoro-2-methylpheno
H), 2.02 (s, 3H), 3.47 (s,
xymethyl)-1,3,3-trimethy1-3
3H), 3.74 (s, 1H), 3.84 (s,
,4-dihydro-1H-quinoxalin-2-
3H), 4.87 (d, J = 13.4 Hz,
one (Compound No.15-21)
1H), 5.22 (d, J = 13.4 Hz,
CIN F 010
1H), 6.09 (dd, J = 11.3, 2
.4 Hz, 1H), 6.41 (td, J = 8
.4, 2.4, 1H), 6.74 (d, J =
0 7.7 Hz, 1H),
6.88 (d, J = 2
0 NO .1 Hz, 1H),
6.90-6.93 (m, 1
H), 6.91 (d, J = 7.7 Hz, 1H
0 N ), 6.94 (dd,
J = 8.2, 2.1 H
z, 1H), 7.38 (d, J = 8.2 Hz
, 1H), 7.52 (dd, J = 8.3, 0
.6 Hz, 1H), 8.42 (dd, J = 8
.3, 2.4 Hz, 1H), 9.20 (dd,
J = 2.4, 0.6 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-m 6 0.95 (s, 3H),
1.27 (s, 3
ethoxypyridin-3-ylcarbonylo
H), 2.02 (s, 3H), 3.47 (s,
xy)pheny1]-1,3,3-trimethyl-
3H), 3.72 (s, 1H), 3.80 (s,
3,4-dihydro-1H-quinoxalin-
225
CA 02643070 2008-08-20
2-one (Compound No.15-22) 3H), 4.11 (s, 3H), 4.88 (d
F , J = 13.6
Hz, 1H), 5.22 (d
, J = 13.6 Hz, 1H), 6.08 (d
d, J = 11.3, 2.4 Hz, 1H), 6
0 0 .40 (td, J =
8.4, 2.4 Hz, 1
H), 6.73 (d, J = 8.1 Hz, 1H
0 .4&
NO ), 6.89-6.92 (m, 1H), 6.90
,0 41! N (d, J = 2.1 Hz, 1H), 6.91 (
d, J = 8.1 Hz, 1H), 6.94 (d
d, J = 8.2, 2.1 Hz, 1H), 7.
04 (dd, J = 7.6, 5.0 Hz, 1H
), 7.36 (d, J = 8.2 Hz, 1H)
, 8.40 (dd, J = 7.6, 2.1 Hz
, 1H), 8.41 (dd, J = 5.0, 2
.1 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (500 MHz, CDC13)
methyl)-7-[2-methoxy-4-(2-m 6 0.91 (s, 3H), 1.29 (s, 3
ethylthiobenzoylamino)pheny
H), 2.02 (s, 3H), 2.53 (s,
11-1,3,3-trimethy1-3,4-dihy
3H), 3.48 (s, 3H), 3.71 (s,
dro-1H-quinoxalin-2-one (Co
1H), 3.89 (s, 3H), 4.88 (d
mpound No.15-23)
, J = 13.8 Hz, 1H), 5.25 (d
F
, J = 13.8 Hz, 1H), 6.07 (d
d, J = 11.2, 2.5 Hz, 1H), 6
S
0 .39 (td, J =
8.3, 2.5, 1H),
0
N,0 6.72 (d, J = 7.9 Hz, 1H),
6.88-6.91 (m, 1H), 6.90 (d,
J = 7.9 Hz, 1H), 7.05 (dd,
,0 410 N J = 8.0, 2.1 Hz, 1H), 7.2
6-7.32 (m, 1H), 7.30 (d, J
= 8.0 Hz, 1H), 7.42 (dd, J
= 8.0, 1.4 Hz, 1H), 7.46 (t
d, J = 7.3, 1.4 Hz, 1H), 7.
76-7.78 (m, 2H), 8.45 (s, 1
H)
7-[2-Methoxy-4-(2-methylthi 1H-NMR (500 MHz, CDC13)
obenzoyloxy)pheny1]-8-(5-me 6 1.21 (s, 3H), 1.42 (s, 3
thylthiophen-2-ylcarbonylox
H), 2.47 (s, 3H), 2.49 (s,
ymethyl)-1,3,3-trimethy1-3,
3H), 3.46 (s, 3H), 3.74 (s,
4-dihydro-1H-quinoxalin-2-o
1H), 3.77 (s, 3H), 5.13 (d
ne (Compound No.15-24)
, J = 13.4 Hz, 1H), 5.31 (d
, J = 13.4 Hz, 1H), 6.70 (d
010 S , J = 3.8 Hz, 1H), 6.77 (d,
0010 0 J = 7.9 Hz, 1H), 6.84 (d,
J = 2.2 Hz, 1H), 6.87 (dd,
0 .4&N0 J = 8.1, 2.2 Hz, 1H),
6.90
,0 VIP N (d, J = 7.9
Hz, 1H), 7.23-7
.27 (m, 1H), 7.32 (d, J = 8
.1 Hz, 1H), 7.35 (d, J = 7.
9 Hz, 1H), 7.45 (d, J = 3.8
Hz, 1H), 8.33 (t, J = 7.9
226
CA 02643070 2008-08-20
- -
Hz, 1H), 8.26 (d, J = 7.9 H
z, 1H)
7-[2-Methoxy-4-(3-methoxyca 1H-NMR (500 MHz, CDC13)
rbonylbenzoyloxy)pheny1]-8- 6 1.22 (s, 3H), 1.42 (s, 3
(5-methylthiophen-2-ylcarbo
H), 2.47 (s, 3H), 3.46 (s,
nyloxymethyl)-1,3,3-trimeth
3H), 3.77 (s, 3H), 3.96 (s,
y1-3,4-dihydro-1H-quinoxali
1H), 3.99 (s, 3H), 5.14 (d
n-2-one (Compound No.15-25)
, J = 12.8 Hz, 1H), 5.31 (d
0 C)
, J = 12.8 Hz, 1H), 6.70 (d
, J = 3.7 Hz, 1H), 6.78 (d,
J = 7.9 Hz, 1H), 6.84 (d,
011 0 0J = 2.1 Hz, 1H), 6.88 (dd,
0 Olt
J = 8.3, 2.1 Hz, 1H), 6.91
N,0
(d, J = 7.9 Hz, 1H), 7.33 (
O N
d, J = 8.3 Hz, 1H), 7.46 (d
, J = 3.7 Hz, 1H), 7.63 (t,
J = 7.8 Hz, 1H), 8.33 (d,
J = 7.8 Hz, 1H), 8.40 (d, J
= 7.8 Hz, 1H), 8.87 (s, 1H
7-[2-Methoxy-4-(3-methylfur 1H-NMR (400 MHz, CDC13)
an-2-ylcarbonyloxy)pheny11- 6 1.21 (s, 3H), 1.42 (s, 3
8-(5-methylthiophen-2-ylcar
H), 2.46 (s, 3H), 2.47 (s,
bonyloxymethyl)-1,3,3-trime
3H), 3.45 (s, 3H), 3.76 (s,
thy1-3,4-dihydro-1H-quinoxa
3H), 3.79 (s, 1H), 5.12 (d
lin-2-one (Compound No.15-2
, J = 13.3 Hz, 1H), 5.30 (d
6)
,J = 13.3 Hz, 1H), 6.46 (d
, J = 1.6 Hz, 1H), 6.69 (d,
0
J = 3.7 Hz, 1H), 6.76 (d,
0 0
J = 8.1 Hz, 1H), 6.82 (d, J
- 2.2 Hz, 1H), 6.86 (dd, J
010
0 Nyp
= 8.3, 2.2 Hz, 1H), 6.90 (
N
d, J = 8.1 Hz, 1H), 7.31 (d
, J - 8.3 Hz, 1H), 7.44 (d,
J = 3.7 Hz, 1H), 7.56 (d,
J = 1.6 Hz, 1H)
7-[2-Methoxy-4-(thiazol-4-y 1H-NMR (400 MHz, CDC13)
lcarbonyloxy)pheny1]-8 (5 m
6 1.22 (s, 3H), 1.42 (s, 3
ethylthiophen-2-ylcarbonylo
H), 2.47 (s, 3H), 3.46 (s,
xymethyl)-1,3,3-trimethy1-3
3H), 3.76 (s, 3H), 3.80 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 5.11 (d, J = 13.4 Hz,
one (Compound No.15-27)
1H), 5.30 (d, J - 13.4 Hz,
1H), 6.70 (d, J = 3.6 Hz,
1H), 6.77 (d, J = 8.1 Hz, 1
H), 6.86 (d, J = 1.8 Hz, 1H
), 6.90 (dd, J = 8.1, 1.8 H
z, 1H), 6.90 (d, J = 8.1 Hz
, 1H), 7.32 (d, J = 8.1 Hz,
1H), 7.45 (d, J = 3.6 Hz,
2 2 7
CA 02643070 2008-08-20
1H), 8.46 (d, J = 2.0 Hz, 1
H), 8.95 (d, J = 2.0 Hz, 1H
P=N
0
S\YO0 010 id& N,0
,0 N
7-[2-Methoxy-4-(thiazol-5-y 1H-NMR (400 MHz, CDC13)
lcarbonyloxy)pheny1]-8-(5 m
6 1.22 (s, 3H), 1.42 (s, 3
ethylthiophen-2-ylcarbonylo
H), 2.48 (s, 3H), 3.46 (s,
xymethyl)-1,3,3-trimethy1-3
3H), 3.77 (s, 3H), 3.81 (s,
,4-dihydro-1H-quinoxalin-2-
1H), 5.12 (d, J = 13.2 Hz,
one (Compound No.15-28)
1H), 5.30 (d, J = 13.2 Hz,
1H), 6.71 (d, J = 3.8 Hz,
1H), 6.77 (d, J - 7.9 Hz, 1
)(0 0 H), 6.82 (d,
J = 2.2 Hz, 1H
S 4/0
), 6.87 (dd, J = 8.3, 2.2 H
0 ti& N,0 z, 1H), 6.90
(d, J = 7.9 Hz
O N , 1H), 7.33 (d, J = 8.3 Hz,
1H), 7.45 (d, J = 3.8 Hz,
1H), 8.71 (d, J = 0.6 Hz, 1
H), 9.06 (d, J = 0.6 Hz, 1H
7-[2-Methoxy-4-(2-methylpyr 1H-NMR (500 MHz, CDC13)
idin-3-ylcarbonyloxy)phenyl 6 1.22 (s, 3H), 1.42 (s, 3
]-8-(5-methylthiophen-2-ylc
H), 2.47 (s, 3H), 2.94 (s,
arbonyloxymethyl)-1,3,3-tri
3H), 3.46 (s, 3H), 3.77 (s,
methyl-3,4-dihydro-1H-quino
3H), 3.80 (s, 1H), 5.14 (d
xalin-2-one (Compound No.1
, J = 13.4 Hz, 1H), 5.30 (d
5-29)
,J = 13.4 Hz, 1H), 6.70 (d
, J = 3.8 Hz, 1H), 6.77 (d,
0
J = 8.1 Hz, 1H), 6.81 (d,
J = 2.1 Hz, 1H), 6.85 (dd,
0S0
J = 8.3, 2.1 Hz, 1H), 6.90
0 id& NO (d, J = 8.1
Hz, 1H), 7.31-7
O N .33 (m, 1H), 7.34 (d, J = 8
.3 Hz, 1H), 7.45 (d, J = 3.
8 Hz, 1H), 8.45 (dd, J = 7.
9, 1.8 Hz, 1H), 8.71 (dd, J
= 4.9, 1.8 Hz, 1H)
7-[4-(3-Acetylbenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(5-methy 5 1.22 (s, 3H), 1.42 (s, 3
lthiophen-2-ylcarbonyloxyme
H), 2.47 (s, 3H), 2.71 (s,
thyl)-1,3,3-trimethy1-3,4-d
3H), 3.47 (s, 3H), 3.78 (s,
ihydro-1H-quinoxalin-2-one
3H), 5.14 (d, J = 13.1 Hz,
(Compound No.15-30)
1H), 5.31 (d, J - 13.1 Hz,
1H), 6.70 (d, J = 3.7 Hz,
1H), 6.78 (d, J = 7.9 Hz, 1
228
CA 02643070 2008-08-20
O H), 6.84 (d,
J = 2.1 Hz, 1H
), 6.88 (dd, J = 8.1, 2.1 H
z, 1H), 6.91 (d, J = 7.9 Hz
Olt 0 0 , 1H) 7.34
(d, J = 8.1 Hz,
1H), 7.46 (d, J = 3.7 Hz, 1
0 010 id& NO H), 7.65 (t,
J = 7.8 Hz, 1H
N ), 8.25 (dt,
J = 7.8, 1.6 H
z, 1H), 8.41 (dt, J = 7.8,
1.6 Hz, 1H), 8.77 (t, J = 1
.6 Hz, 1H)
7-(4-tert-Butoxycarbonylami 1H-NMR (400 MHz, CDC13)
noacetylamino-2-methoxyphen 5 0.91 (s, 3H), 1.28 (s, 3
y1)-8-(5-fluoro-2-methylphe
H), 1.50 (s, 9H), 2.01 (s,
noxymethyl)-1,3,3-trimethy
3H), 3.46 (s, 3H), 3.70 (s,
1-3,4-dihydro-1H-quinoxali
1H), 3.84 (s, 3H), 3.95 (d
n-2-one (Compound No.15-31)
, J = 6.1 Hz, 2H), 4.83 (d,
F
J = 13.8 Hz, 1H), 5.20 (br
s, 1H), 5.21 (d, J = 13.8
40Hz, 1H), 6.04 (dd, J = 11.2
0N(N 0 , 2.4
Hz, 1H), 6.38 (td, J
H 8 NO = 8.3,
2.4 Hz, 1H), 6.71 (d
, J = 8.1 Hz,
1H), 6.87 (d,
0 410 N J = 8.1 Hz,
1H), 6.87-6.91
(m, 1H), 6.97 (dd, J = 7.5
, 1.9 Hz, 1H), 7.25 (d, J =
7.5 Hz, 1H), 7.54 (d, J =
1.9 Hz, 1H), 8.22 (br s, 1H
7-[4-(3-Chlorothiophen-2-y1 1H-NMR (500 MHz, CDC13)
carbonyloxy)-2-methoxypheny 6 1.21 (s, 3H), 1.42 (s, 3
1]-8-(5-methylthiophen-2-y1
H), 2.47 (s, 3H), 3.46 (s,
carbonyloxymethyl)-1,3,3-tr
3H), 3.77 (s, 3H), 3.78 (br
imethy1-3,4-dihydro-1H-quin
s, 1H), 5.12 (d, J = 13.4
oxalin-2-one (Compound No.1
Hz, 1H), 5.30 (d, J = 13.4
5-32)
Hz, 1H), 6.70 (d, J = 3.9 H
z, 1H), 6.77 (d, J = 7.9 Hz
r_14 -s
, 1H), 6.85 (dd, J = 9.2, 2
o .8 Hz, 1H), 6.88 (t, J = 2.
S 8 Hz, 1H),
6.89 (d, J = 7.9
0 id& 1\1,,0 Hz, 1H), 7.11
(d, J = 5.2
o N Hz, 1H), 7.31 (d, J = 9.2 H
z, 1H), 7.44 (d, J = 3.9 Hz
, 1H), 7.60 (d, J = 5.2 Hz,
1H)
Example 16
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy4-[N-
2 2 9
CA 02643070 2008-08-20
..
methyl-N-(pyridin-4-ylcarbonyl)amino]pheny1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound
No.16-1)
A mixture of 8-(5-
fluoro-2-
methylphenoxymethyl)-7-[2-methoxy-4-(pyridin-4-
ylcarbonylamino)pheny1]-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one (Compound No.14-21, 13.9 mg,
0.0251 mmol), cessium carbonate (41.7 mg, 0.128 mmol),
and methyl iodide (4.7 p L, 0.075 mmol) was suspended
in anhydrous N,N-dimethylformamide (0.5 ml) and
stirred for 3 hours at room temperature. The mixture
was diluted with ethyl acetate (10 mL). The mixture
was washed with water (10 mL) and saturated brine (10
mL) successively, dried over anhydrous magnesium
sulfate, and then the solvent was removed under
reduced pressure. The obtained residue was purified
by silica gel column chromatography (hexane-ethyl
acetate) to give the titled compound (4.6 mg) as a
yellow amorphous product. (Yield 32%)
F 010 1H-NMR (400 MHz, CDC13)
6 0.98 (s, 3H), 1.28 (s,
N
1 1 3H), 2.00 (s, 3H), 3.43 (s
0 1 , 3H), 3.55 (s, 3H), 3.61
0 010 il& NO (s, 3H), 3.76 (br s, 1H),
4.66 (d, J - 13.3 Hz, 1H),
20 IW N 5.08 (d, J = 13.3 Hz, 1H)
H , 6.00 (dd, J = 11.1, 2.4
Hz, 1H), 6.44 (td, J = 8.4
, 2.4 Hz, 1H), 6.71 (d, J
= 8.1 Hz, 1H), 6.78 (d, J
= 8.2 Hz, 1H), 6.80 (d, J
= 8.1 Hz, 1H), 6.92-6.95 (
m, 1H), 7.19 (d, J - 4.9 H
z, 2H), 7.20 (d, J - 8.2 H
2 3 0
CA 02643070 2008-08-20
z, 1H), 8.02 (s, 1H), 8.43
(d, J = 4.9 Hz, 2H)
Using any compounds among Compounds No.14-17-14-20
and available compounds, the following Compounds
(No.16-2-16-5) were obtained by a method similar to
that of Compound No.16-1.
7-[4-(N-Benzoyl-N-methylami 1H-NMR (400 MHz, DMSO-d6)
no)-2-methoxypheny1]-8-(5 f
-- 6 0.83 (s, 3H), 1.09 (s, 3
luoro-2-methylphenoxymethyl
H), 1.90 (s, 3H), 3.26 (s,
)-1,3,3-trimethy1-3,4-dihyd
3H), 3.45 (s, 3H), 3.58 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 4.64 (d, J = 13.3 Hz,
pound No.16-2)
1H), 5.06 (d, J = 13.3 Hz,
F
1H), 6.03 (dd, J = 11.5, 2
010 N 010 0 .4 Hz, 1H), 6.14
(s, 1H), 6
.54 (td, J = 8.4, 2.4 Hz, 1
H), 6.74 (d, J = 8.1 Hz, 1H
0S N,0 ), 6.78 (d,
J = 8.1 Hz, 1H)
, 6.83 (dd, J = 7.8, 2.0 Hz
N , 1H), 6.87
(d, J = 2.0 Hz,
1H), 7.01-7.04 (m, 1H), 7.
(d, J = 7.8 Hz, 1H), 7.1
1 (t, J = 7.2 Hz, 2H), 7.18
(t, J = 7.2 Hz, 1H), 7.28
(d, J - 7.2 Hz, 2H)
_
7-[4-(N-Acetyl-N-methylamin 1H-NMR (400 MHz, CDC13)
o)-2-methoxypheny1]-8-(5-fl 6 1.01 (s, 3H), 1.29 (s, 3
uoro-2-methylphenoxymethyl
H), 1.89 (s, 3H), 2.01 (s,
)-1,3,3-trimethy1-3,4-dihyd
3H), 3.31 (s, 3H), 3.48 (s,
ro-1H-quinoxalin-2-one (Corn
3H), 3.78 (br s, 1H), 3.82
pound No.16-3)
(s, 3H), 4.80 (d, J - 13.2
F
Hz, 1H), 5.17 (d, J = 13.2
Hz, 1H), 6.06 (dd, J = 11.
1, 2.5 Hz, 1H), 6.40 (td, J
0
= 8.3, 2.5 Hz, 1H), 6.75 (
0 id& N,0 d, J = 8.1 Hz, 1H),
6.77 (s
, 1H), 6.86 (dd, J = 8.0, 1
O N .8 Hz, 1H),
6.89 (d, J = 8.
1 Hz, 1H), 6.89-6.93 (m, 1H
), 7.32 (d, J = 8.0 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-[N-m 6 0.99 (s, 3H), 1.30 (s, 3
ethyl-N-(pyridin-2-ylcarbon
H), 2.00 (s, 3H), 3.40 (s,
yl)amino]pheny1]-1,3,3-trim
2 3 1
CA 02643070 2008-08-20
*
ethyl-3,4-dihydro-1H-quinox 3H), 3.59 (s, 3H), 3.62 (s,
alin-2-one (Compound No.16-
3H), 3.77 (br s, 1H), 4.62
4) (d, J = 13.1 Hz, 1H), 5.05
F 410
(d, J = 13.1 Hz, 1H), 5.97
(d, J = 11.0 Hz, 1H), 6.45
N
(t, J = 8.3 Hz, 1H), 6.62
N
0
(br s, 1H), 6.71 (d, J = 8.
1 Hz, 1H), 6.79 (br s, 1H),
0 id&
NO
6.82 (d, J = 8.1 Hz, 1H),
N
6.93-6.96 (m, 1H), 7.11 (br
s, 1H), 7.15 (d, J = 7.8 H
z, 1H), 7.46 (br s, 1H), 7.
54 (br s, 1H), 8.27 (br s,
1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[2-methoxy-4-[N-m 6 1.01 (s, 3H), 1.30 (s, 3
ethyl-N-(pyridin-3-ylcarbon
H), 2.00 (s, 3H), 3.40 (s,
yl)amino]pheny1]-1,3,3-trim
3H), 3.57 (s, 3H), 3.61 (s,
ethyl-3,4-dihydro-1H-quinox
3H), 3.76 (br s, 1H), 4.65
alin-2-one (Compound No.16-
5) (d, J = 13.1 Hz, 1H), 5.05
(d, J = 13.1 Hz, 1H), 6.01
F 010
(dd, J = 11.2, 2.4 Hz, 1H)
, 6.45 (td, J = 8.3, 2.4 Hz
I I
, 1H), 6.57 (d, J = 1.9 Hz,
0
1H), 6.71 (d, J = 7.9 Hz,
0NO 1H), 6.78 (dd, J = 8.0, 1.9
Hz, 1H), 6.81 (d, J = 7.9
Hz, 1H), 6.92-6.96 (m, 1H),
7.02 (dd, J = 7.8, 4.9 Hz,
1H), 7.20 (d, J = 8.0 Hz,
1H), 7.59 (dt, J = 7.8, 1.9
Hz, 1H), 8.45 (dd, J = 4.9
, 1.9 Hz, 1H), 8.62 (d, J =
1.9 Hz, 1H)
Example 17
7-[4-(3-Chlorophenylaminocarbonyloxy)-2-
methoxypheny1]-8-(5-fluoro-2-methylphenoxymethyl)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.17-1)
8-(5-Fluoro-2-methylphenoxymethyl)-7-(4-
hydroxy-2-methoxypheny1)-1,3,3-trimethy1-3,4-dihydro-
1H-quinoxalin-2-one (Compound No.11, 20.3 mg, 0.0451
2 3 2
CA 02643070 2008-08-20
mmol) was dissolved in anhydrous dichloromethane (0.5
mL), then triethylamine (13.6p L, 0.0977 mmol) and 3-
chlorophenyl isocyanate (6p L, 0.05 mmol) were added
to the mixture successively. After the reaction
mixture was stirred for 1 hour at room temperature,
the mixture was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled compound (22.4 mg) as a colorless solid.
(Yield 82%)
F 1H-NMR (500 MHz, CDC13)
6 0.94 (s, 3H), 1.27 (s,
3H), 2.02 (s, 3H), 3.47 (s
CI 40Ny0 01 , 3H), 3.72 (s, 1H), 3.83
020 010 N,0 (s, 3H), 4.87 (d, J = 13.7
Hz, 1H), 5.22 (d, J = 13.
Nr< 7 Hz, 1H), 6.07 (dd, J = 1
1.3, 2.4 Hz, 1H), 6.39 (td
, J = 8.2, 2.4 Hz, 1H), 6.
72 (d, J = 7.9 Hz, 1H), 6.
87 (d, J = 2.3 Hz, 1H), 6.
89-6.92 (m, 1H), 6.89 (d,
J = 7.9 Hz, 1H), 6.90 (dd,
J = 8.2, 2.3 Hz, 1H), 6.9
8 (br s, 1H), 7.11 (d, J =
7.6 Hz, 1H), 7.29 (d, J =
7.6 Hz, 1H), 7.31-7.32 (m
, 1H), 7.33 (d, J = 8.2 Hz
, 1H), 7.59 (br s, 1H)
Using any compounds among Compounds No.8-2, 11, 13-2,
and available compounds, the following Compounds
(No.17-2-17-17) were obtained by a method similar to
that of Compound No.17-1.
8-(5-Fluoro-2-methylphenoxym 1H-NMR (400 MHz, CDC13)
ethyl)-7-(2-methoxy-4-phenyl 6 0.93 (s, 3H), 1.27 (s,
aminocarbonyloxypheny1)-1,3,
233
CA 02643070 2008-08-20
3-trimethy1-3,4-dihydro-1H-q 3H), 2.02 (s, 3H), 3.47 (
uinoxalin-2-one (Compound No s, 3H), 3.72 (s, 1H), 3.8
.17-2) 3 (s, 3H), 4.87 (d, J = 13
F .7 Hz, 1H), 5.22 (d, J = 1
3.7 Hz, 1H), 6.07 (dd, J =
11.2, 2.4 Hz, 1H), 6.39 (
0
110 NO 010 0
.4& N,0 td, J = 8.3, 2.4 Hz, 1H),
6.72 (d, J = 8.0 Hz, 1H),
6.88 (d, J = 2.3 Hz, 1H),
N 6.89 (d, J - 8.0 Hz, 1H),
6.91 (dd, J = 8.2, 2.3 Hz,
1H), 7.00 (br s, 1H), 7.1
2-7.15 (m, 1H), 7.31-7.56
(m, 6H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (500 MHz, CDC13)
ethyl)-7-(2-methoxy-4-propyl 6 0.92 (s, 3H), 1.00 (t,
aminocarbonyloxypheny1)-1,3,
J = 7.5 Hz, 3H), 1.26 (s,
3-trimethy1-3,4-dihydro-1H-q
3H), 1.59-1.67 (m, 2H), 2
uinoxalin-2-one (Compound No
.01 (s, 3H), 3.25-3.29 (m
.17-3)
, 2H), 3.46 (s, 3H), 3.71
F
(s, 1H), 3.81 (s, 3H), 4.
86 (d, J = 13.7 Hz, 1H), 5
.07 (t, J - 6.1 Hz, 1H), 5
0/0 0 .21 (d, J = 13.7 Hz, 1H),
0 id&
NO 6.05 (dd, J = 11.2, 2.3 Hz
, 1H), 6.38 (td, J = 8.3,
O N 2.3 Hz, 1H), 6.71 (d, J =
8.1 Hz, 1H), 6.82 (d, J =
2.2 Hz, 1H), 6.84 (dd, J =
8.0, 2.2 Hz, 1H), 6.87-6
.91 (m, 1H), 6.88 (d, J
8.1 Hz, 1H), 7.28 (d, J =
8.0 Hz, 1H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (400 MHz, CDC13)
ethyl)-7-(4-isopropylaminoca ö 0.91 (s, 3H), 1.26-1.27
rbonyloxy-2-methoxypheny1)-1
(m, 6H), 1.26 (s, 3H), 2.
,3,3-trimethy1-3,4-dihydro-1
01 (s, 3H), 3.46 (s, 3H),
H-quinoxalin-2-one (Compound
3.71 (s, 1H), 3.82 (s, 3H
No.17-4)
), 3.88-3.97 (m, 1H), 4.8
F
6 (d, J = 13.7 Hz, 1H), 4.
88-4.90 (m, 1H), 5.22 (d,
J = 13.7 Hz, 1H), 6.05 (d
,Ny0 0
d, J - 11.2, 2.4 Hz, 1H),
0 010Q 6.38 (td, J = 8.3, 2.4 Hz,
1H), 6.71 (d, J = 8.1 Hz,
O N 1H), 6.82
(d, J = 2.1 Hz,
1H), 6.84 (dd, J - 8.3, 2
.1 Hz, 1H), 6.87-6.91 (m,
1H), 6.88 (d, J = 8.1 Hz,
1H), 7.28 (d, J = 8.3 Hz,
2 3 4
CA 02643070 2008-08-20
e-
1H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (500 MHz, CDC13)
ethyl)-7-[2-methoxy-4-(pyrid 6 0.95 (s, 3H), 1.27 (s,
in-3-ylaminocarbonyloxy)phen
3H), 2.02 (s, 3H), 3.47 (
y1]-1,3,3-trimethy1-3,4-dihy
s, 3H), 3.72 (s, 1H), 3.8
dro-1H-quinoxalin-2-one (Corn
3 (s, 3H), 4.86 (d, J = 13
pound No.17-5)
.4 Hz, 1H), 5.21 (d, J = 1
F
3.4 Hz, 1H), 6.07 (dd, J =
11.3, 2.4 Hz, 1H), 6.39 (
td, J = 8.4, 2.4 Hz, 1H),
NNC) 0
6.73 (d, J = 8.2 Hz, 1H),
0 NO 6.87-6.93 (m, 4H), 7.34 (
d, J = 8.0 Hz, 1H), 7.42-7
0 41! N .45 (m, 1H), 8.34 (br s, 1
H), 8.39 (d, J = 3.9 Hz, 1
H), 8.79 (s, 1H)
7-(4-Cyclohexylaminocarbonyl 1H-NMR (500 MHz, CDC13)
oxy-2-methoxypheny1)-8-(5-fl 6 0.93 (s, 3H), 1.27 (s,
uoro-2-methylphenoxymethyl)-
3H), 1.35-1.77 (m, 10H),
1,3,3-trimethy1-3,4-dihydro-
2.01 (s, 3H), 3.46 (s, 3H
1H-quinoxalin-2-one (Compoun
), 3.58-3.60 (m, 1H), 3.8
d No.17-6)
1 (s, 3H), 4.86 (d, J = 13
F
.7 Hz, 1H), 4.94 (d, J = 8
.2 Hz, 1H), 5.21 (d, J = 1
3.7 Hz, 1H), 6.05 (dd, J =
0
N 0
a 0
,s, N,0 t 11.0, 2.3 Hz, 1H), 6.38 (
d, J = 8.3, 2.3 Hz, 1H),
6.75 (d, J = 7.7 Hz, 1H),
,o RIP N 6.83-6.90 (m, 4H), 7.26-7
.29 (m, 1H)
8-(5-Fluoro-2-methylphenoxym H-NMR (500 MHz, CDC13)
ethyl)-7-(4-furfurylaminocar 6 0.92 (s, 3H), 1.27 (s,
bonyloxy-2-methoxypheny1)-1,
3H), 2.01 (s, 3H), 3.46 (
3,3-trimethy1-3,4-dihydro-1
s, 3H), 3.71 (s, 1H), 3.8
H-quinoxalin-2-one (Compound
1 (s, 3H), 4.48 (d, J = 5.
No.17-7)
8 Hz, 2H), 4.86 (d, J = 13
F
.7 Hz, 1H), 5.21 (d, J = 1
3.7 Hz, 1H), 5.39 (t, J =
5.8 Hz, 1H), 6.05 (dd, J =
0
0 NO
11.2, 2.4 Hz, 1H), 6.30-
0 N 0 6.32 (m, 1H), 6.36-6.40 (
,O 410 m, 2H), 6.71 (d, J = 7.9 H
O N
z, 1H), 6.82-6.90 (m, 4H)
, 7.28 (d, J = 8.0 Hz, 1H)
, 7.41 (d, J = 1.2 Hz, 1H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (500 MHz, CDC13)
ethyl)-7-[2-methoxy-4-(2-met 6 0.94 (s, 3H), 1.27 (s,
hoxyphenylaminocarbonyloxy)p
3H), 2.02 (s, 3H), 3.47 (
heny1]-1,3,3-trimethy1-3,4-d
235
CA 02643070 2008-08-20
ihydro-1H-quinoxalin-2-one ( s, 3H), 3.71 (s, 1H), 3.8
Compound No.17-8) 3 (s, 3H),
3.94 (s, 3H), 4
F .88 (d, J =
13.6 Hz, 1H),
5.22 (d, J = 13.6 Hz, 1H),
O 6.07 (dd, J = 11.3, 2.4 H
H
0
le NO 1411 0
id& N,0 z, 1H), 6.39
(td, J = 8.2,
2.4 Hz, 1H), 6.72 (d, J =
7.9 Hz, 1H), 6.88-6.93 (
m, 3H), 6.90 (d, J = 7.9 H
z, 1H), 6.92 (dd, J = 8.0,
2.3 Hz, 1H), 7.00 (t, J =
7.8 Hz, 1H), 7.07 (t, J =
7.8 Hz, 1H), 7.32 (d, J =
8.0 Hz, 1H), 7.62 (br s,
1H), 8.12 (br s, 1H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (400 MHz, CDC13)
ethyl)-7-[2-methoxy-4-(4-met 6 0.93 (s, 3H), 1.27 (s,
hylphenylaminocarbonyloxy)ph
3H), 2.02 (s, 3H), 2.34 (
eny1]-1,3,3-trimethy1-3,4-di
s, 3H), 3.47 (s, 3H), 3.7
hydro-1H-quinoxalin-2-one (C
2 (s, 1H), 3.83 (s, 3H), 4
ompound No.17-9)
.87 (d, J = 13.7 Hz, 1H),
F
5.22 (d, J = 13.7 Hz, 1H),
6.07 (dd, J = 11.2, 2.4 H
FNI1
z, 1H), 6.39 (td, J = 8.3,
0 0 2.4 Hz, 1H),
6.72 (d, J =
410 0 410
id& I\L.0 8.1 Hz, 1H),
6.87-6.92 (
m, 1H), 6.88 (d, J = 2.2 H
O z, 1H), 6.89
(d, J = 8.1 H
z, 1H), 6,91 (dd, J = 8.1,
2.2 Hz, 1H), 7.17 (d, J =
8.3 Hz, 2H), 7.32 (d, J =
8.1 Hz, 1H), 7.36 (d, J =
8.3 Hz, 2H)
7-(4-Ethoxycarbonylmethylami 1H-NMR (400 MHz, CDC13)
nocarbonyloxy-2-methoxypheny 6 0.93 (s, 3H), 1.26 (s,
1)-8-(5-fluoro-2-methylpheno
3H), 1.32 (t, J = 7.2 Hz,
xymethyl)-1,3,3-trimethy1-3,
3H), 2.01 (s, 3H), 3.46 (
4-dihydro-1H-quinoxalin-2-on
s, 3H), 3.71 (s, 1H), 3.8
e (Compound No.17-10)
1 (s, 3H), 4.08 (d, J = 5.
F
3 Hz, 2H), 4.27 (q, J = 7.
2 Hz, 2H), 4.86 (d, J = 13
0 H
.4 Hz, 1H), 5.21 (d, J = 1
ocNC) = 0
3.4 Hz, 1H), 5.58 (t, J =
0 010 5.3 Hz, 1H),
6.05 (dd, J =
11.2, 2.4 Hz, 1H), 6.38 (
,0
N td, J = 8.3, 2.4 Hz, 1H),
6.71 (d, J = 8.0 Hz, 1H),
6.82 (d, J = 2.3 Hz, 1H),
6.86 (dd, J - 8.1, 2.3 Hz,
1H), 6.87-6.91 (m, 1H),
2 3 6
CA 02643070 2008-08-20
6.88 (d, J = 8.0 Hz, 1H),
7.29 (d, J = 8.1 Hz, 1H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (400 MHz, CDC13)
ethyl)-7-[2-methoxy-4-[2-(2- 6 0.93 (s, 3H), 1.27 (s,
methylacryloyloxy)ethylamino
3H), 1.99 (s, 3H), 2.01 (
carbonyloxy]pheny1]-1,3,3-tr
s, 3H), 3.46 (s, 3H), 3.6
imethy1-3,4-dihydro-1H-quino
3 (q, J - 5.6 Hz, 2H), 3.7
xalin-2-one (Compound No.17-
1 (s, 1H), 3.81 (s, 3H), 4
11)
.34 (t, J = 5.6 Hz, 2H), 4
F
.86 (d, J = 13.7 Hz, 1H),
5.21 (d, J = 13.7 Hz, 1H),
0 H 5.36 (t, J =
5.6 Hz, 1H),
0 5.64 (s,
1H), 6.05 (dd, J
0 NI) N,0 = 11.4, 2.5
Hz, 1H), 6.18
(s, 1H), 6.38 (td, J = 8.
,0
N-K 2, 2.5 Hz, 1H), 6.71 (d, J
= 8.0 Hz, 1H), 6.81 (d, J
- 2.2 Hz, 1H), 6.84 (dd,
J = 8.2, 2.2 Hz, 1H), 6.8
7-6.91 (m, 1H), 6.88 (d,
J = 8.0 Hz, 1H), 7.29 (d,
J = 8.2 Hz, 1H)
7-(4-Benzylaminocarbonyloxy- 1H-NMR (400 MHz, CDC13)
2-methoxypheny1)-8-(5-fluor 5 0.92 (s, 3H), 1.26 (s,
o-2-methylphenoxymethyl)-1,3
3H), 2.01 (s, 3H), 3.46 (
,3-trimethy1-3,4-dihydro-1H-
s, 3H), 3.70 (s, 1H), 3.8
quinoxalin-2-one (Compound N
2 (s, 3H), 4.49 (d, J = 5.
o.17-12)
9 Hz, 2H), 4.86 (d, J = 13
.7 Hz, 1H), 5.21 (d, J = 1
3.7 Hz, 1H), 5.37 (t, J =
H 5.9 Hz, 1H),
6.05 (dd, J =
NyO F fai! 11.2, 2.4 Hz, 1H), 6.38
1401 (
0 NO
td, J = 8.3, 2.4 Hz, 1H),
6.71 (d, J = 8.0 Hz, 1H),
1.1 N 6.84-6.91 (m, 3H), 6.88 (
d, J = 8.0 Hz, 1H), 7.29 (
d, J = 8.3 Hz, 1H), 7.31-7
.39 (m, 5H)
7-[4-(3-Benzylureido)-2-meth 1H-NMR (400 MHz, CDC13)
oxypheny1]-8-(5-fluoro-2-met 6 0.90 (s, 3H), 1.28 (s,
hylphenoxymethyl)-1,3,3-trim
3H), 2.00 (s, 3H), 3.45 (
ethyl-3,4-dihydro-1H-quinoxa
s, 3H), 3.69 (s, 1H), 3.8
lin-2-one (Compound No.17-13
0 (s, 3H), 4.49 (d, J = 5.
8 Hz, 2H), 4.83 (d, J = 13
.7 Hz, 1H), 5.12 (t, J = 5
.8 Hz, 1H), 5.21 (d, J = 1
3.7 Hz, 1H), 6.03 (dd, J
11.2, 2.4 Hz, 1H), 6.37 (
td, J = 8.3, 2.4 Hz, 1H),
237
CA 02643070 2008-08-20
F 6.50 (s,
1H), 6.70 (d, J =
8.1 Hz, 1H), 6.76 (dd, J
= 8.2, 2.2 Hz, 1H), 6.86 (
H H
NN 0 d, J = 8.1
Hz, 1H), 6.86-6
.90 (m, 1H), 7.20 (d, J =
0 8.2 Hz, 1H),
7.28-7.35 (m
,0 RIP N , 5H), 7.33 (d, J = 2.2 Hz
, 1H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (400 MHz, CDC13)
ethyl)-7-[2-methoxy-4-(3-phe 6 0.91 (s, 3H), 1.29 (s,
nylureido)pheny1]-1,3,3-trim
3H), 2.02 (s, 3H), 3.47 (
ethyl-3,4-dihydro-1H-quinoxa
s, 3H), 3.70 (s, 1H), 3.8
lin-2-one (Compound No.17-14
4 (s, 3H), 4.85 (d, J = 14
.0 Hz, 1H), 5.22 (d, J = 1
F
4.0 Hz, 1H), 6.05 (dd, J =
11.2, 2.5 Hz, 1H), 6.38 (
H
N N 0 td, J = 8.2, 2.5 Hz, 1H),
6.62 (s, 1H), 6.71 (d, J =
410 0 40 N,0 8.0 Hz, 1H), 6.72 (s,
1H)
, 6.82 (dd, J = 8.2, 2.1 H
,o RIP N z, 1H), 6.87
(d, J = 8.0 H
z, 1H), 6.87-6.91 (m, 1H)
, 7.14-7.19 (m, 1H), 7.24
(d, J = 8.2 Hz, 1H), 7.3
7-7.40 (m, 4H), 7.39 (d,
J = 2.1 Hz, 1H)
7-(4-Isopropylaminocarbonylo 1H-NMR (500 MHz, CDC13)
xy-2-methoxypheny1)-8-(5-met 6 1.21 (s, 3H), 1.26 (d,
hylthiophen-2-ylcarbonyloxym
J = 6.1 Hz, 6H), 1.41 (s,
ethyl)-1,3,3-trimethy1-3,4-d
3H), 2.47 (s, 3H), 3.44 (
ihydro-1H-quinoxalin-2-one (
s, 3H), 3.74 (s, 3H), 3.7
Compound No.17-15)
8 (s, 1H), 3.89-3.93 (m,
1H), 4.87 (d, J = 8.0 Hz,
1H), 5.10 (d, J = 13.3 Hz,
y0 I. 0 1H), 5.29
(d, J = 13.3 Hz
, 1H), 6.69 (d, J = 3.9 Hz
0
NO , 1H), 6.73-
6.76 (m, 2H),
,0 410 N 6.75 (d, J =
8.1 Hz, 1H),
6.87 (d, J = 8.1 Hz, 1H),
7.23 (d, J = 7.6 Hz, 1H),
7.43 (d, J = 3.9 Hz, 1H)
7-(2-Methoxy-4-phenylaminoca 1H-NMR (500 MHz, CDC13)
rbonyloxypheny1)-8-(5-methyl 6 1.21 (s, 3H), 1.42 (s,
thiophen-2-ylcarbonyloxymeth
3H), 2.47 (s, 3H), 3.45 (
y1)-1,3,3-trimethy1-3,4-dihy
s, 3H), 3.75 (s, 3H), 3.7
dro-1H-quinoxalin-2-one (Corn
9 (s, 1H), 5.12 (d, J = 13
pound No.17-16)
.3 Hz, 1H), 5.29 (d, J = 1
3.3 Hz, 1H), 6.69 (d, J =
238
CA 02643070 2008-08-20
3.8 Hz, 1H), 6.76 (d, J =
7.9 Hz, 1H), 6.80 (d, J =
2.1 Hz, 1H), 6.82 (dd, J =
NO 0 7.9, 2.1 Hz,
1H), 6.88 (d
, J = 7.9 Hz, 1H), 6.97 (b
0 id& 1\10
r s, 1H), 7.13 (t, J = 7.5
O N Hz, 1H),
7.28 (d, J = 7.9
Hz, 1H), 7.36 (t, J = 7.5
Hz, 2H), 7.44 (d, J = 3.8
Hz, 1H), 7.47 (d, J = 7.5
Hz, 2H)
8-(5-Fluoro-2-methylphenoxym 1H-NMR (500 MHz, CDC13)
ethyl)-7-[2-methoxy-4-(3-pro 6 0.91 (s, 3H), 0.96 (t,
pylureido)pheny1]-1,3,3-trim
J = 7.2 Hz, 3H), 1.29 (s,
ethyl-3,4-dihydro-1H-quinoxa
3H), 1.58 (sextet, J = 7.
lin-2-one (Compound No.17-17
2 Hz, 2H), 2.01 (s, 3H), 3
.26 (td, J = 7.2, 5.7 Hz,
F
2H), 3.47 (s, 3H), 3.69 (
s, 1H), 3.83 (s, 3H), 4.7
H H 3 (t, J =
5.7 Hz, 1H), 4.8
NyN 0 5 (d, J =
13.6 Hz, 1H), 5.
0 1\1C) 22 (d, J =
13.6 Hz, 1H), 6
.04 (dd, J = 11.3, 2.4 Hz,
O N 1H), 6.32
(s, 1H), 6.38 (
td, J - 8.2, 2.4 Hz, 1H),
6.70 (d, J = 8.1 Hz, 1H),
6.77 (dd, J = 8.1, 2.0 Hz,
1H), 6.87 (d, J = 8.1 Hz,
1H), 6.88-6.90 (m, 1H),
7.22 (d, J = 8.1 Hz, 1H),
7.33 (d, J = 2.0 Hz, 1H)
Example 18
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-
(morpholin-4-ylcarbonyloxy)pheny1]-1,3,3-trimethyl-
3,4-dihydro-1H-quinoxalin-2-one (Compound No.18-1)
A mixture of 8-(5-fluoro-2-
methylphenoxymethyl)-7-(4-hydroxy-2-methoxypheny1)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.11, 153 mg, 0.340 mmol), 1 , 1 ' -
carbonyldiimiazole (95.6 mg, 0.590 mmol), and 4-
dimethylaminopyridine (5.2 mg, 0.043 mmol) was
239
CA 02643070 2008-08-20
dissolved in anhydrous tetrahydrofuran (3 mL) and
stirred for 1 hour at room temperature. After
morpholine (58.3 p L, 0.666 mmol) was added to the
reaction mixture and the mixture was stirred for 2
hour, the mixture was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled compound (61.2 mg) as a colorless solid.
(Yield 32%)
F 010 H-NMR (400 MHz, CDC13)
6 0.93 (s, 3H), 1.27 (s,
3H), 2.01 (s, 3H), 3.46 (s
LõNO 0 , 3H), 3.61 (br s, 2H), 3.
0 010 .4µ 72 (br s, 2H), 3.73 (br s,
1H), 3.75-3.79 (m, 4H), 3
0 N .82 (s, 3H), 4.85 (d, J =
13.7 Hz, 1H), 5.21 (d, J =
13.7 Hz, 1H), 6.06 (dd, J
= 11.2, 2.4 Hz, 1H), 6.39
(td, J = 8.3, 2.4 Hz, 1H)
, 6.71 (d, J = 8.0 Hz, 1H)
, 6.80 (d, J = 2.3 Hz, 1H)
, 6.83 (dd, J = 8.3, 2.3 H
z, 1H), 6.88-6.92 (m, 1H),
6.89 (d, J = 8.0 Hz, 1H),
7.30 (d, J = 8.3 Hz, 1H)
Using any compounds among Compounds No.11 and
available compounds, the following Compounds (No.18-2
and 18-3) were obtained by a method similar to that
of Compound No.18-1.
7-(4-Dimethylaminocarbonylo 1H-NMR (400 MHz, CDC13)
xy-2-methoxypheny1)-8-(5-fl 6 0.92 (s, 3H), 1.27 (s, 3
uoro-2-methylphenoxymethyl
H), 2.01 (s, 3H), 3.05 (s,
)-1,3,3-trimethy1-3,4-dihyd
3H), 3.14 (s, 3H), 3.46 (s,
ro-1H-quinoxalin-2-one (Com
3H), 3.71 (s, 1H), 3.81 (s
pound No.18-2)
, 3H), 4.86 (d, J - 13.7 Hz
2 4 0
CA 02643070 2008-08-20
F , 1H), 5.21 (d, J = 13.7 Hz
, 1H), 6.06 (dd, J = 11.2,
2.4 Hz, 1H), 6.38 (td, J =
NyO 0 8.3, 2.4 Hz, 1H), 6.71 (d,
J = 7.8 Hz, 1H), 6.80 (d, J
0 id& 1\10 = 2.2 Hz, 1H), 6.83 (dd, J
N = 8.2, 2.2 Hz, 1H), 6.87-6
.91 (m, 1H), 6.88 (d, J = 7
.8 Hz, 1H), 7.29 (d, J = 8.
2 Hz, 1H)
8-(5-Fluoro-2-methylphenoxy 1H-NMR (400 MHz, CDC13)
methyl)-7-[4-(4-hydroxypipe 5 0.93 (s, 3H), 1.27 (s, 3
ridin-1-ylcarbonyloxy)-2-me
H), 1.63-1.65 (m, 2H), 1.9
thoxypheny1)-1,3,3-trimethy
7-2.02 (m, 2H), 2.01 (s, 3H
1-3,4-dihydro-1H-quinoxali
), 3.30 (br s, 1H), 3.40 (b
n-2-one (Compound No.18-3)
r s, 1H), 3.46 (s, 3H), 3.7
F So (s, 1H), 3.82 (s, 3H), 3.
HO 96-4.01 (m, 2H), 4.04 (br s
, 1H), 4.86 (d, J = 13.7 Hz
Ny0 0 , 1H), 5.21 (d, J = 13.7 Hz
0 NO , 1H), 6.06 (dd, J = 11.1,
2.4 Hz, 1H), 6.38 (td, J =
,O 410 N 8.3, 2.4 Hz, 1H), 6.71 (d,
J = 8.1 Hz, 1H), 6.79 (d, J
= 2.2 Hz, 1H), 6.83 (dd, J
= 8.2, 2.2 Hz, 1H), 6.88-6
.91 (m, 1H), 6.89 (d, J = 8
.1 Hz, 1H), 7.29 (d, J = 8.
2 Hz, 1H)
Example 19
8-(5-Fluoro-2-methylphenoxymethyl)-7-[2-methoxy-4-(N-
methyl-N-phenylaminocarbonyloxy)pheny1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No. 19)
A mixture of 8-(5-fluoro-2-
methylphenoxymethyl)-7-(4-hydroxy-2-methoxypheny1)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.11, 25.4 mg, 0.0564 mmol) and N -methyl-
N -phenylcarbamoyl chloride (20.4 mg, 0.120 mmol) was
dissolved in pyridine (1 mL), and stirred for 2 hours
2 4 1
A CA 02643070 2008-08-20
at 100 C. The reaction mixture was concentrated, and
then the obtained residue was purified by silica gel
column chromatography (hexane-ethyl acetate) to give
the titled compound (28.7 mg) as a pale yellow
amorphous product. (Yield 87%)
F 1H-NMR (500 MHz, CDC13)
6 0.91 (s, 3H), 1.26 (s,
3H), 2.00 (s, 3H), 3.45 (s
0 0 , 3H), 3.46 (s, 3H), 3.70
110 0 410
N 0 ( s 1H),
3.80 (s, 3H), 4.8
3 (d, J = 13.6 Hz, 1H), 5.
0 N 19 (d, J = 13.6 Hz, 1H), 6
.03 (d, J = 8.6 Hz, 1H), 6
.37 (t, J = 8.6 Hz, 1H), 6
.70 (d, J = 7.9 Hz, 1H), 6
.86-6.90 (m, 3H), 6.87 (d,
J = 7.9 Hz, 1H), 7.27-7.3
0 (m, 2H), 7.38-7.47 (m, 4
H)
Example 20
7-(4-Aminoacetylamino-2-methoxypheny1)-8-(5-fluoro-2-
methylphenoxymethyl)-1,3,3-trimethy1-3,4-dihydro-1H-
quinoxalin-2-one hydrochloride (Compound No.20)
7-(4-tert-Butoxycarbonylaminoacetylamino-2-
methoxypheny1)-8-(5-fluoro-2-methylphenoxymethyl)-
1,3,3-trimethy1-3,4-dihydro-1H-quinoxalin-2-one
(Compound No.15-31, 10.5 mg, 0.0173 mmol) was
dissolved in 1,4-dioxane (0.2 mL), 4N 1,4-dioxane
solution of hydrochloride (41.3p L., 0.165 mmol) was
added thereto. After the reaction mixture was stirred
for 4 hours at room temperature, it was diluted with
hexane (10 mL). The precipitated solid was filtered
to give the titled compound (7.8 mg) as a pale yellow
2 4 2
CA 02643070 2008-08-20
solid. (Yield 83%)
F 1H-NMR (500 MHz, CDC13)
6 0.78 (s, 3H), 1.08 (s,
HCI H 3H), 1.92 (s,
3H), 3.33 (
0 s, 3H), 3.78
(s, 3H), 3.8
H2N-ri\I
0 NO
0-3.81 (m, 2H), 4.82 (d,
J = 14.3 Hz, 1H), 5.22 (d,
0 N J = 14.3 Hz,
1H), 6.10 (d
d, J = 11.6, 2.4 Hz, 1H),
6.13 (s, 1H), 6.49 (td, J
= 8.4, 2.4 Hz, 1H), 6.79 (
d, J = 8.2 Hz, 1H), 6.81 (
d, J = 8.2 Hz, 1H), 6.98-7
.01 (m, 1H), 7.24 (d, J =
8.2 Hz, 1H), 7.29 (dd, J =
8.2, 1.8 Hz, 1H), 7.43 (d
, J - 1.8 Hz, 1H), 8.16 (b
r s, 2H), 8.20 (br s, 1H),
10.66 (s, 1H)
Example 21
8-(2-Methoxyphenylaminomethyl)-7-[2-methoxy-4-
(pyridin-3-ylcarbonyloxy)pheny1]-1,3,3-trimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Compound No.21-1)
7-(4-Hydroxy-2-methoxypheny1)-8-[N-(2-
methoxypheny1)-N-(9-
fluorenylmethoxycarbonyl)aminomethy1]-1,3,3-
trimethy1-3,4-dihydro-1H-quinoxalin-2-one (Compound
No.13-1, 30.0 mg, 0.0448 mmol) was dissolved in mixed
solvent of tetrahydrofuran (1 mL) and dichloromethane
(1 mL), and triethylamine (25 p L, 0.18 mmol) and
nicotynoyl chloride hydrochloride (12.0 mg, 0.0674
mmol) were added successively. After the reaction
mixture was stirred for 40 minutes at room
temperature, it was purified by silica gel column
2 4 3
1
f CA 02643070 2008-08-20
chromatography (hexane-ethyl acetate). The obtained
colorless amorphous product was dissolved in N,N-
dimethylformamide (1 mL) and piperidine (50 p L) was
added thereto. After the reaction mixture was stirred
for 20 minutes at room temperature, it was diluted
with ethyl acetate (50 mL). The mixture was washed
with water (50 mL) and saturated brine (50 mL)
successively, dried over anhydrous magnesium sulfate,
and then the solvent was removed under reduced
pressure. The obtained residue was purified by silica
gel column chromatography (hexane-ethyl acetate) to
give the titled compound (13.0 mg) as a colorless
solid. (Yield 52%)
1 H-NMR (400 MHz, CDC13)
N, NIO 6 1.19 (s, 3H), 1.43 (s,
0
3H), 3.47 (s, 3H), 3.74 (s
0 I. NH
I , 3H), 3.75 (s, 1H), 3.80
0 ash NO (s, 3H), 4.16 (d, J = 5.5
Hz, 2H), 4.50 (t, J = 5.5
23 IW N Hz, 1H), 6.34 (dd, J = 7.8
H , 1.5 Hz, 1H), 6.57 (td, J
= 7.8, 1.5 Hz, 1H), 6.67
(dd, J = 7.8, 1.5 Hz, 1H),
6.71 (d, J - 7.9 Hz, 1H),
6.71-6.75 (m, 1H), 6.82 (
d, J = 2.2 Hz, 1H), 6.85 (
d, J = 7.9 Hz, 1H), 6.89 (
dd, J = 8.2, 2.2 Hz, 1H),
7.24 (d, J - 8.2 Hz, 1H),
7.49 (ddd, J = 8.0, 4.9, 0
.8 Hz, 1H), 8.46 (dt, J =
8.0, 2.0 Hz, 1H), 8.87 (dd
, J = 4.9, 2.0 Hz, 1H), 9.
41 (dd, J = 2.0, 0.8 Hz, 1
H)
Using any compounds among Compounds No.13-1, 13-3,
2 4 4
CA 02643070 2008-08-20
and available compounds, the following Compounds
(No.21-2-21-24) were obtained by a method similar to
that of Compound No.14-1, 15-1, 17-1 or 18-1 followed
by a method similar to that of Compound No.21-1.
7-[4-(Furan-2-ylcarbonyloxy 1H-NMR (400 MHz, CDC13)
)-2-methoxypheny1]-8-(2-met 6 1.18 (s, 3H), 1.43 (s, 3
hoxyphenylaminomethyl)-1,3,
H), 3.47 (s, 3H), 3.74 (s,
3-trimethy1-3,4-dihydro-1H-
3H), 3.76 (s, 1H), 3.79 (s,
quinoxalin-2-one (Compound
3H), 4.15 (s, 2H), 4.51 (b
No.21-2)
r s, 1H), 6.33 (dd, J = 7.8
410
0 , 1.5 Hz, 1H), 6.56 (td, J
= 7.8, 1.5 Hz, 1H), 6.61 (d
nf0 =
NH d, J = 3.6, 1.7 Hz, 1H), 6.
0
010 66 (dd, J = 7.8, 1.5 Hz, 1H
0
), 6.70 (d, J = 8.1 Hz, 1H)
, 6.72 (td, J - 7.8, 1.5 Hz
O N , 1H), 6.80 (d, J = 2.3 Hz,
1H), 6.84 (d, J = 8.1 Hz,
1H), 6.87 (dd, J = 8.2, 2.3
Hz, 1H), 7.21 (d, J - 8.2
Hz, 1H), 7.40 (dd, J = 3.6,
0.9 Hz, 1H), 7.69 (dd, J =
1.7, 0.9 Hz, 1H)
7-(4-Hydroxy-2-methoxypheny 1H-NMR (400 MHz, CDC13)
1)-8-(2-methoxyphenylaminom 6 1.16 (s, 3H), 1.42 (s, 3
ethyl)-1,3,3-trimethy1-3,4-
H), 3.46 (s, 3H), 3.70 (s,
dihydro-1H-quinoxalin-2-one
1H), 3.73 (s, 3H), 3.76 (s,
(Compound No.21-3)
3H), 4.11 (s, 2H), 4.52 (b
410
0 r s, 1H), 4.90 (s, 1H), 6.3
3 (dd, J = 7.7, 1.5 Hz, 1H)
, 6.43 (dd, J = 7.9, 2.3 Hz
HO NH
, 1H), 6.44 (d, J = 2.3 Hz,
1H), 6.56 (td, J = 7.7, 1.
ti& N,0
Hz, 1H), 6.66 (dd, J = 7.
N 7, 1.5 Hz, 1H), 6.68 (d, J
= 8.1 Hz, 1H), 6.73 (td, J
= 7.7, 1.5 Hz, 1H), 6.80 (d
, J = 8.1 Hz, 1H), 7.01 (d,
J = 7.9 Hz, 1H)
7-[4-(2-Chlorobenzoyloxy)- 1H-NMR (400 MHz, CDC13)
2-methoxypheny1]-8-(2-metho 6 1.18 (s, 3H), 1.43 (s, 3
xyphenylaminomethyl)-1,3,3-
H), 3.47 (s, 3H), 3.74 (s,
trimethy1-3,4-dihydro-1H-qu
3H), 3.80 (s, 3H), 4.16 (s,
inoxalin-2-one (Compound No
2H), 4.50 (br s, 1H), 6.33
.21-4)
2 4 5
CA 02643070 2008-08-20
401(d, J = 7.8 Hz, 1H), 6.56
(t, J - 7.8 Hz, 1H), 6.66 (
CIO d, J = 7.8 Hz, 1H), 6.70
(d
O 010 NH
N,0 , J = 7.9
Hz, 1H), 6.72 (t,
J = 7.8 Hz, 1H), 6.83 (d,
J - 2.2 Hz, 1H), 6.84 (d, J
A) N = 7.9 Hz, 1H), 6.91 (dd, J
= 8.2, 2.2 Hz, 1H), 7.23 (
d, J = 8.2 Hz, 1H), 7.39-7.
43 (m, 1H), 7.49-7.55 (m, 2
H), 8.06 (dd, J = 8.1, 1.2
Hz, 1H)
7-(4-Butyryloxy-2-methoxyph 1H-NMR (400 MHz, CDC13)
eny1)-8-(2-methoxyphenylami 6 1.06 (t, J - 7.4 Hz, 3H)
nomethyl)-1,3,3-trimethy1-3
, 1.17 (s, 3H), 1.42 (s, 3H
,4-dihydro-1H-quinoxalin-2-
), 1.75-1.85 (m, 2H), 2.55
one (Compound No.21-5)
(t, J = 7.4 Hz, 2H), 3.46 (
NIO0 s, 3H), 3.73
(s, 4H), 3.77
(s, 3H), 4.13 (br s, 2H), 4
.48 (br s, 1H), 6.31 (dd, J
010 NH
- 7.9, 1.5 Hz, 1H), 6.56 (
O 5N,0 td,
J = 7.9, 1.5 Hz, 1H), 6
.65 (dd, J = 7.9, 1.5 Hz, 1
O N H), 6.67 (d, J = 2.3 Hz, 1H
), 6.69 (d, J = 7.9 Hz, 1H)
, 6.70-6.73 (m, 1H), 6.74 (
dd, J = 8.1, 2.3 Hz, 1H), 6
.81 (d, J = 7.9 Hz, 1H), 7.
16 (d, J = 8.1 Hz, 1H)
7-[2-Methoxy-4-(2-methoxybe 1H-NMR (500 MHz, CDC13)
nzoyloxy)pheny1]-8-(2-metho 6 1.18 (s, 3H), 1.42 (s, 3
xyphenylaminomethyl)-1,3,3-
H), 3.47 (s, 3H), 3.74 (s,
trimethy1-3,4-dihydro-1H-qu
4H), 3.79 (s, 3H), 3.96 (s,
inoxalin-2-one (Compound No
21-6) 3H), 4.16
(br s, 2H), 4.51
.
(br s, 1H), 6.33 (dd, J =
00 NIO 7.8, 1.4 Hz,
1H), 6.56 (td,
J = 7.8, 1.4 Hz, 1H), 6.66
(dd, J = 7.8, 1.4 Hz, 1H),
O N0 6
Olt NH
id& , 6.70 (d, J = 7.9 Hz, 1H)
O ,
.72 (td, J = 7.8, 1.4 Hz,
1H), 6.83 (d, J = 2.1 Hz, 1
N H), 6.84 (d, J = 7.9 Hz, 1H
), 6.88 (dd, J - 8.1, 2.1 H
z, 1H), 7.05-7.08 (m, 1H),
7.05 (d, J = 7.6 Hz, 1H), 7
.21 (d, J = 8.1 Hz, 1H), 7.
54-7.58 (m, 1H), 8.03 (dd,
J = 7.9, 1.8 Hz, 1H)
7-(4-Isopropylaminocarbonyl 1H-NMR (400 MHz, CDC13)
oxy-2-methoxypheny1)-8-(2-m
2 4 6
CA 02643070 2008-08-20
ethoxyphenylaminomethyl)-1, 6 1.16 (s, 3H), 1.25 (d, J
3,3-trimethy1-3,4-dihydro-1
= 7.3 Hz, 6H), 1.41 (s, 3H
H-quinoxalin-2-one (Compoun
), 3.45 (s, 3H), 3.73 (s, 4
d No.21-7)
H), 3.77 (s, 3H), 3.88-3.95
4100 (m, 1H), 4.13 (br s, 2H),
4.51 (br s, 1H), 4.87 (d, J
= 7.6 Hz, 1H), 6.32 (dd, J
NH
,
= 7.8, 1.3 Hz, 1H), 6.55
Ny0 (
0 N 0
td, J = 7.8, 1.3 Hz, 1H), 6
.65 (dd, J = 7.8, 1.3 Hz, 1
0 N
H), 6.68 (d, J = 7.9 Hz, 1H
), 6.69-6.74 (m, 1H), 6.74
(d, J = 2.0 Hz, 1H), 6.77 (
dd, J = 8.1, 2.0 Hz, 1H), 6
.80 (d, J = 7.9 Hz, 1H), 7.
14 (d, J = 8.1 Hz, 1H)
8-(2-Methoxyphenylaminometh 1H-NMR (500 MHz, CDC13)
y1)-7-[2-methoxy-4-(thiophe 6 1.18 (s, 3H), 1.42 (s, 3
n-2-ylcarbonyloxy)pheny1]-1
H), 3.47 (s, 3H), 3.74 (s,
,3,3-trimethy1-3,4-dihydro-
4H), 3.79 (s, 3H), 4.15 (br
1H-quinoxalin-2-one (Compou
s, 2H), 4.50 (br s, 1H), 6
nd No.21-8)
.33 (dd, J = 7.8, 1.4 Hz, 1
410
0 H), 6.57 (td, J - 7.8, 1.4
Hz, 1H), 6.66 (dd, J = 7.8,
ay() NH 1.4 Hz, 1H), 6.70 (d, J =
8.1 Hz, 1H), 6.71-6.74 (m,
410
s
0 1H), 6.82
(d, J = 2.1 Hz, 1
H), 6.84 (d, J = 8.1 Hz, 1H
N ), 6.88 (dd, J = 8.2, 2.1 H
z, 1H), 7.19 (dd, J = 5.0,
3.7 Hz, 1H), 7.21 (d, J = 8
.2 Hz, 1H), 7.68 (dd, J - 5
.0, 1.2 Hz, 1H), 7.99 (dd,
J = 3.7, 1.2 Hz, 1H)
7-[2-Methoxy-4-(morpholin- 1H-NMR (500 MHz, CDC13)
4-ylcarbonyloxy)pheny1]-8-(
s 6 1.16 (s, 3H), 1.42 (s, 3
2-methoxyphenylaminomethyl
H), 3.45 (s, 3H), 3.59 (br
)-1,3,3-trimethy1-3,4-dihyd
s, 2H), 3.68 (br s, 2H), 3.
ro-1H-quinoxalin-2-one (Corn
73 (s, 3H), 3.75-3.77 (m, 5
pound No.21-9)
H), 3.78 (s, 3H), 4.12 (s,
410
0 2H), 4.52 (br s, 1H), 6.32
(dd, J = 7.7, 1.5 Hz, 1H),
6.56 (td, J = 7.7, 1.5 Hz,
NH
1H), 6.66 (dd, J = 7.7, 1.4
0 id& NO Hz, 1H), 6.69
(d, J = 7.9
Hz, 1H), 6.71 (d, J = 2.4 H
N z, 1H), 6.72 (td, J = 7.7,
1.4 Hz, 1H), 6.76 (dd, J =
8.2, 2.4 Hz, 1H), 6.82 (d,
2 4 7
CA 02643070 2008-08-20
J = 7.9 Hz, 1H), 7.16 (d, J
= 8.2 Hz, 1H)
7-[2-Methoxy-4-(4-methoxybe 1H-NMR (500 MHz, CDC13)
nzoyloxy)pheny1]-8-(2-metho 6 1.18 (s, 3H), 1.42 (s, 3
xyphenylaminomethyl)-1,3,3-
H), 3.47 (s, 3H), 3.74 (s,
trimethy1-3,4-dihydro-1H-qu
4H), 3.79 (s, 3H), 3.91 (s,
inoxalin-2-one (Compound No
3H), 4.16 (d, J = 5.6 Hz,
.21-10)
2H), 4.51 (t, J = 5.6 Hz, 1
Op H), 6.34 (dd,
J = 7.7, 1.4
0 010 0 Hz, 1H),
6.57 (td, J - 7.7,
1.4 Hz, 1H), 6.66 (dd, J =
0 010 NH 7.7, 1.4 Hz,
1H), 6.70 (d,
0 NO
J = 7.9 Hz, 1H), 6.73 (td,
J = 7.7, 1.4 Hz, 1H), 6.80
0 401 N (d, J = 2.1
Hz, 1H), 6.85
(d, J = 7.9 Hz, 1H), 6.86 (
dd, J = 8.1, 2.1 Hz, 1H), 7
.00 (d, J = 8.9 Hz, 2H), 7.
21 (d, J = 8.1 Hz, 1H), 8.1
6 (d, J = 8.9 Hz, 2H)
8-(2-Methoxyphenylaminometh 1H-NMR (400 MHz, CDC13)
y1)-7-[2-methoxy-4-(thiophe 6 1.18 (s, 3H), 1.42 (s, 3
n-3-ylcarbonyloxy)pheny1]-1
H), 3.47 (s, 3H), 3.74 (s,
,3,3-trimethy1-3,4-dihydro-
4H), 3.79 (s, 3H), 4.16 (s,
1H-quinoxalin-2-one (Compou
2H), 4.51 (br s, 1H), 6.33
nd No.21-11)
(dd, J = 7.8, 1.5 Hz, 1H),
o6.57 (td, J = 7.8, 1.5 Hz,
1H), 6.66 (dd, J = 7.8, 1.
D')f
Hz, 1H), 6.70 (d, J = 8.1
S,, 0 NH Hz, 1H),
6.73 (td, J = 7.8
0NO , 1.5 Hz, 1H), 6.79 (d, J =
2.2 Hz, 1H), 6.84 (d, J =
N 8.1 Hz, 1H), 6.85 (dd, J =
8.2, 2.2 Hz, 1H), 7.21 (d,
J = 8.2 Hz, 1H), 7.40 (dd,
J = 5.1, 3.0 Hz, 1H), 7.67
(dd, J = 5.1, 1.2 Hz, 1H),
8.32 (dd, J = 3.0, 1.2 Hz,
1H)
7-[2-Methoxy-4-(2-methylpyr 1H-NMR (500 MHz, CDC13)
idin-3-ylcarbonyloxy)phenyl 6 1.18 (s, 3H), 1.43 (s, 3H
]-8-(2-methoxyphenylaminome
), 2.94 (s, 3H), 3.46 (s, 3
thyl)-1,3,3-trimethy1-3,4-d
H), 3.74 (s, 4H), 3.80 (s,
ihydro-1H-quinoxalin-2-one
3H), 4.16-4.16 (m, 2H), 4.5
(Compound No.21-12)
1 (br s, 1H), 6.34 (dd, J =
7.9, 1.3 Hz, 1H), 6.57 (td
, J - 7.9, 1.3 Hz, 1H), 6.6
7 (dd, J = 7.9, 1.3 Hz, 1H)
, 6.71 (d, J = 7.9 Hz, 1H),
248
. . CA 02643070 2008-08-20
6.74 (td, J - 7.9, 1.3 Hz,
1H), 6.79 (d, J = 2.2 Hz,
N 410
0 1H), 6.84 (d, J = 7.9 Hz, 1
I
0 si NH H), 6.86 (dd, J = 8.1, 2.2
I Hz, 1H), 7.24 (d, J = 8.1 H
O4& N,0 z, 1H), 7.31 (dd, J = 7.8,
0 IW N 4.7 Hz, 1H), 8.44 (dd, J =
7.8, 1.8 Hz, 1H), 8.71 (dd,
H
J = 4.7, 1.8 Hz, 1H)
8-(2-Methoxyphenylaminometh 1H-NMR (400 MHz, CDC13)
yl ) -7- [2-methoxy-4- ( thiazo 51.19 (s, 3H), 1.43 (s, 3H
1-4-ylcarbonyloxy)pheny1]-1
), 3.47 (s, 3H), 3.74 (s, 3
,3,3-trimethy1-3,4-dihydro-
H), 3.77 (s, 1H), 3.79 (s,
1H-quinoxalin-2-one (Compou
3H), 4.15 (br s, 2H), 4.49
nd No.21-13)
(br s, 1H), 6.32 (dd, J = 7
401.8, 1.4 Hz, 1H), 6.57 (td,
J = 7.8, 1.4 Hz, 1H), 6.66
(dd, J = 7.8, 1.4 Hz, 1H),
NH 6.71 (d, J - 8.0 Hz, 1H), 6
I
O 5 NO .72 (td, J = 7.8, 1.4 Hz, 1
H), 6.84 (d, J = 8.0 Hz, 1H
0 IW N ), 6.86 (d, J = 2.2 Hz, 1H)
H , 6.92 (dd, J = 8.2, 2.2 Hz
, 1H), 7.23 (d, J = 8.2 Hz,
1H), 8.46 (d, J = 2.2 Hz,
1H), 8.95 (d, J = 2.2 Hz, 1
H)
7-(4-Cyclohexylcarbonyloxy- 1H-NMR (400 MHz, CDC13)
2-methoxypheny1)-8-(2-metho 6 1.17 (s, 3H), 1.25-1.39
xyphenylaminomethyl)-1,3,3-
(m, 4H), 1.42 (s, 3H), 1.5
trimethy1-3,4-dihydro-1H-qu
8-1.72 (m, 2H), 1.81-1.85 (
inoxalin-2-one (Compound No
m, 2H), 2.05-2.09 (m, 2H),
.21-14)
2.52-2.59 (m, 1H), 3.45 (s,
4103H), 3.73 (s, 4H), 3.77 (s
. , 3H), 4.14 (d, J = 3.4 Hz,
2H), 4.48 (br s, 1H), 6.31
CD)r0 NH (dd, J = 7.8, 1.5 Hz, 1H),
I
O 0
NO 6.55 (td, J = 7.8, 1.5 Hz,
1H), 6.65 (dd, J = 7.8, 1.
0 IW N 5 Hz, 1H), 6.65 (d, J = 2.2
H Hz, 1H), 6.68 (d, J = 7.9
Hz, 1H), 6.69-6.73 (m, 1H),
6.72 (dd, J = 8.1, 2.2 Hz,
1H), 6.81 (d, J = 7.9 Hz,
1H), 7.16 (d, J = 8.1 Hz, 1
H)
7-[2-Methoxy-4-(2-methylben 1H-NMR (400 MHz, CDC13)
zoyloxy)pheny1]-8-(2-methox 6 1.18 (s, 3H), 1.43 (s, 3
yphenylaminomethyl)-1,3,3-t
H), 2.70 (s, 3H), 3.47 (s,
rimethy1-3,4-dihydro-1H-qui
2 4 9
CA 02643070 2008-08-20
noxalin-2-one (Compound No. 3H), 3.74 (s, 4H), 3.80 (s,
21-15) 3H), 4.17 (d, J = 4.9 Hz,
2H), 4.52 (t, J = 4.9 Hz, 1
H), 6.34 (dd, J = 7.8, 1.4
Hz, 1H), 6.57 (td, J = 7.8,
010 0 NH 1.4 Hz, 1H),
6.67 (dd, J =
0
0 le 7.8, 1.4 Hz,
1H), 6.70 (d,
N
J - 8.0 Hz, 1H), 6.73 (td,
,o RIP N J = 7.8, 1.4
Hz, 1H), 6.79
(d, J = 2.1 Hz, 1H), 6.84
(d, J = 8.0 Hz, 1H), 6.86 (
dd, J = 8.1, 2.1 Hz, 1H), 7
.23 (d, J = 8.1 Hz, 1H), 7.
33 (d, J = 7.3 Hz, 1H), 7.3
4-7.36 (m, 1H), 7.48-7.52 (
m, 1H), 8.16-8.18 (m, 1H)
7-(2-Methoxy-4-phenylacetox 1H-NMR (400 MHz, CDC13)
ypheny1)-8-(2-methoxyphenyl 6 1.17 (s, 3H), 1.41 (s, 3
aminomethyl)-1,3,3-trimethy
H), 3.45 (s, 3H), 3.71 (s,
1-3,4-dihydro-1H-quinoxali
3H), 3.72 (s, 1H), 3.74 (s,
n-2-one (Compound No.21-16)
3H), 3.87 (s, 2H), 4.10-4.
S0 13 (m, 2H), 4.46 (br s, 1H)
, 6.30 (dd, J = 7.8, 1.6 Hz
, 1H), 6.55 (td, J = 7.8, 1
0 NH .6 Hz, 1H),
6.63-6.73 (m, 2
0 01 N,0 H), 6.64 (dd,
J - 7.8, 1.6
Hz, 1H), 6.68 (d, J = 7.8 H
0 N z, 1H), 6.71
(dd, J = 8.1,
2.2 Hz, 1H), 6.79 (d, J = 8
.1 Hz, 1H), 7.14 (d, J = 8.
1 Hz, 1H), 7.32-7.41 (m, 5H
7-[2-Methoxy-4-(3-methoxyca 1H-NMR (400 MHz, CDC13)
rbonylbenzoyloxy)pheny1]-8- 6 1 .19 (s, 3H), 1.43 (s, 3
(2-methoxyphenylaminomethyl
H), 3.47 (s, 3H), 3.75 (s,
)-1,3,3-trimethy1-3,4-dihyd
4H), 3.80 (s, 3H), 3.98 (s,
ro-1H-quinoxalin-2-one (Corn
3.H), 4.16 (s, 2H), 4.51 (
pound No.21-17)
br s, 1H), 6.34 (dd, J = 7.
8, 1.5 Hz, 1H), 6.57 (td, J
0
= 7.8, 1.5 Hz, 1H), 6.67
0 (
010 dd, J = 7.8,
1.5 Hz, 1H), 6
0
.71 (d, J = 8.1 Hz, 1H), 6.
010 0 NH 74 (td, J =
7.8, 1.5 Hz, 1H
0 010 id& N,0 ), 6.82 (d,
J = 2.3 Hz, 1H)
, 6.85 (d, J = 8.1 Hz, 1H),
o N 6.89 (dd, J
= 8.2, 2.3 Hz,
1H), 7.24 (d, J = 8.2 Hz,
1H), 7.63 (t, J = 7.8 Hz, 1
H), 8.32 (dt, J = 7.8, 1.6
Hz, 1H), 8.39 (dt, J = 7.8,
2 5 0
CA 02643070 2008-08-20
1.6 Hz, 1H), 8.86 (t, J =
1.6 Hz, 1H)
7-[4-(Furan-3-ylcarbonyloxy 1H-NMR (400 MHz, CDC13)
)-2-methoxypheny1]-8-(2-met 6 1.18 (s, 3H), 1.42 (s, 3
hoxyphenylaminomethyl)-1,3,
H), 3.46 (s, 3H), 3.74 (s,
3-trimethy1-3,4-dihydro-1H-
4H), 3.79 (s, 3H), 4.15 (s,
quinoxalin-2-one (Compound
2H), 4.50 (br s, 1H), 6.33
No.21-18)
(dd, J = 7.7, 1.5 Hz, 1H),
4106.56 (td, J = 7.7, 1.5 Hz,
0 1H), 6.66
(dd, J = 7.7, 1.
CC:1)(0 NH 5 Hz, 1H),
6.70 (d, J = 8.1
Hz, 1H), 6.73 (td, J = 7.7
0 el , 1.5 Hz,
1H), 6.77 (d, J =
,0 Rip N 2.0 Hz, 1H),
6.83 (dd, J =
8.2, 2.0 Hz, 1H), 6.84 (d,
J = 8.1 Hz, 1H), 6.88 (dd,
J = 1.8, 0.8 Hz, 1H), 7.21
(d, J = 8.2 Hz, 1H), 7.51
(t, J = 1.8 Hz, 1H), 8.21 (
dd, J = 1.8, 0.8 Hz, 1H)
7-[4-(3-Acetylbenzoyloxy)- 1H-NMR (500 MHz, CDC13)
2-methoxypheny1]-8-(2-metho 5 1. 19 (s, 3H), 1.43 (s, 3H
xyphenylaminomethyl)-1,3,3-
), 2.70 (s, 3H), 3.47 (s, 3
trimethy1-3,4-dihydro-1H-qu
H), 3.75 (s, 4H), 3.81 (s,
inoxalin-2-one (Compound No
3H), 4.16-4.17 (m, 2H), 4.5
.21-19)
1 (br s, 1H), 6.34 (dd, J =
0
7.8, 1.4 Hz, 1H), 6.57 (td
410 , J = 7.8, 1.4 Hz, 1H), 6.6
0 7 (dd, J =
7.8, 1.4 Hz, 1H)
010 0 NH , 6.71 (d, J
= 8.0 Hz, 1H),
0 SI 6.74 (td, J -
7.8, 1.4 Hz,
1H), 6.82 (d, J = 2.1 Hz,
1H), 6.85 (d, J = 8.0 Hz, 1
H), 6.89 (dd, J = 8.2, 2.1
Hz, 1H), 7.24 (d, J = 8.2 H
z, 1H), 7.66 (t, J = 7.8 Hz
, 1H), 8.25 (dt, J = 7.8, 1
.4 Hz, 1H), 8.41 (dt, J - 7
.8, 1.4 Hz, 1H), 8.77 (t, J
= 1.4 Hz, 1H)
7-[4-(3-Chlorothiophen-2-y1 1H-NMR (400 MHz, CDC13)
carbonyloxy)-2-methoxypheny 6 1.18 (s, 3H), 1.43 (s, 3H
1]-8-(2-methoxyphenylaminom
), 3.46 (s, 3H), 3.74 (s, 4
ethyl)-1,3,3-trimethy1-3,4-
H), 3.80 (s, 3H), 4.15 (br
dihydro-1H-quinoxalin-2-one
s, 2H), 4.50 (br s, 1H), 6.
(Compound No.21-20)
33 (dd, J = 7.8, 1.5 Hz, 1H
), 6.56 (td, J = 7.8, 1.5 H
z, 1H), 6.66 (dd, J = 7.8,
1.5 Hz, 1H), 6.70 (d, J = 7
2 5 1
CA 02643070 2008-08-20
.8 Hz, 1H), 6.73 (td, J = 7
NIO .8, 1.5 Hz, 1H), 6.83 (d, J
0 = 7.8 Hz,
1H), 6.83 (d, J
NH = 2.2 Hz, 1H), 6.88 (dd, J
= 8.2, 2.2 Hz, 1H), 7.11 (d
S 0 010 NO, J = 5.2 Hz, 1H),
7.21 (d,
O NJ = 8.2 Hz, 1H), 7.60 (d,
J = 5.2 Hz, 1H)
7-(2-Methoxy-4-methoxycarbo 1H-NMR (500 MHz, CDC13)
nyloxypheny1)-8-(2-methoxyp 6 1.18 (s, 3H), 1.42 (s, 3
henylaminomethyl)-1,3,3-tri
H), 3.46 (s, 3H), 3.72 (s,
methyl-3,4-dihydro-1H-quino
3H), 3.73 (s, 1H), 3.77 (s,
xalin-2-one (Compound No.2
3H), 3.92 (s, 3H), 4.14 (b
1-21)
r s, 2H), 4.46 (br s, 1H),
S0 6.31 (dd, J = 7.7, 1.4 Hz,
1H), 6.56 (td, J = 7.7, 1.4
Hz, 1H), 6.65 (dd, J = 7.7
Oy0 NH , 1.4 Hz,
1H), 6.69 (d, J =
0 id&
NO 8.0 Hz, 1H),
6.72 (td, J =
7.7, 1.4 Hz, 1H), 6.76 (d,
o N J = 2.3 Hz,
1H), 6.81 (d,
J = 8.0 Hz, 1H), 6.83 (dd,
J = 8.1, 2.3 Hz, 1H), 7.18
(d, J = 8.1 Hz, 1H)
8-(5-Fluoro-2-methylphenyla 1H-NMR (400 MHz, CDC13)
minomethyl)-7-[2-methoxy-4- 6 1.18 (s, 3H), 1.40 (s, 3
(2-methylbenzoyloxy)phenyl
H), 1.87 (s, 3H), 2.70 (s,
1-1,3,3-trimethy1-3,4-dihyd
3H), 3.43 (s, 3H), 3.77 (s,
ro-1H-quinoxalin-2-one (Corn
1H), 3.80-3.85 (m, 1H), 3.
pound No.21-22)
81 (s, 3H), 4.16-4.27 (m, 2
F
H), 6.03 (dd, J = 11.6, 2.5
Hz, 1H), 6.23 (td, J - 8.3
, 2.5 Hz, 1H), 6.72 (d, J =
010 0 NH 7.9 Hz, 1H),
6.82-6.86 (m,
0 010 NO 1H), 6.84 (d, J = 2.1
Hz,
1H), 6.87 (d, J = 7.9 Hz, 1
N H), 6.90 (dd, J = 8.2, 2.1
Hz, 1H), 7.26 (d, J = 8.2 H
z, 1H), 7.33 (d, J - 7.3 Hz
, 1H), 7.34 (t, J = 7.3 Hz,
1H), 7.48-7.52 (m, 1H), 8.
18 (d, J = 7.3 Hz, 1H)
7-[2-Methoxy-4-(3-methylfur 1H-NMR (400 MHz, CDC13)
an-2-ylcarbonyloxy)pheny11- 6 1.18 (s, 3H), 1.42 (s, 3
8-(2-methoxyphenylaminometh
H), 2.46 (s, 3H), 3.46 (s,
y1)-1,3,3-trimethy1-3,4-dih
3H), 3.73 (s, 4H), 3.79 (s,
ydro-1H-quinoxalin-2-one (C
3H), 4.15 (s, 2H), 4.49 (b
ompound No.21-23)
r s, 1H), 6.32 (dd, J = 7.7
252
CA 02643070 2008-08-20
NIO
0 , 1.4 Hz, 1H), 6.46 (d, J =
1.4 Hz, 1H), 6.56 (td, J =
7.7, 1.4 Hz, 1H), 6.66 (dd
0(Osi NH
, J = 7.7, 1.4 Hz, 1H), 6.7
0 (d, J = 8.0 Hz, 1H), 6.72
0
NO (td, J =
7.7, 1.4 Hz, 1H),
0 N6.81 (d, J = 2.2 Hz, 1H),
6.84 (d, J = 8.0 Hz, 1H), 6
.87 (dd, J = 8.2, 2.2 Hz, 1
H), 7.21 (d, J = 8.2 Hz, 1H
), 7.55 (d, J = 1.4 Hz, 1H)
8-(2-Methoxyphenylaminometh 1H-NMR (500 MHz, CDC13)
y1)-7-[2-methoxy-4-(pyridi 6 1.18 (s, 3H), 1.42 (s, 3
n-3-ylaminocarbonyloxy)phen
H), 3.46 (s, 3H), 3.74 (s,
y11-1,3,3-trimethy1-3,4-dih
4H), 3.79 (s, 3H), 4.15 (s,
ydro-1H-quinoxalin-2-one (C
2H), 4.49 (br s, 1H), 6.33
ompound No.21-24)
(dd, J = 7.7, 1.4 Hz, 1H),
NIO 0 6.57 (td, J = 7.7, 1.4 Hz,
1H), 6.66 (dd, J = 7.7, 1.
4 Hz, 1H), 6.70 (d, J = 7.9
NNO NH
Hz, 1H), 6.73 (td, J = 7.7
, 1.4 Hz, 1H), 6.79 (d, J =
2.1 Hz, 1H), 6.82 (d, J =
0 N 7.9 Hz, 1H), 6.84 (dd, J =
8.3, 2.1 Hz, 1H), 7.03 (s,
1H), 7.20 (d, J = 8.3 Hz, 1
H), 7.31 (dd, J = 8.1, 4.7
Hz, 1H), 8.05 (d, J = 8.1 H
z, 1H), 8.38 (dd, J = 4.7,
2.1 Hz, 1H), 8.59 (d, J = 2
.1 Hz, 1H)
Example 22
7-[2-Methoxy-4-(2-methylbenzoyloxy)pheny1]-8-(2-
methoxy-5-nitrophenoxymethyl)-3,3-dimethy1-3,4-
dihydro-1H-quinoxalin-2-one (Compound No.22)
A mixture of 8-hydroxymethy1-7-[2-methoxy-4-(2-
methylbenzoyloxy)pheny1]-3,3-dimethy1-3,4-dihydro-1H-
quinoxalin-2-one (Reference Compound No.23, 40.1 mg,
0.0898 mmol), 2-methoxy-5-nitrophenol (22.8 mg, 0.135
mmol), and tri n -butylphosphine (33.7 p L, 0.135
mmol) was dissolved in anhydrous tetrahydrofuran (1
2 5 3
. . CA 02643070 2008-08-20
mL), 1,1'-(azodicarbonyl)dipiperidine (34.0 mg, 0.135
mmol) was added thereto, and then the mixture was
stirred at room temperature. After 20 minutes, 2-
methoxy-5-nitrophenol (23.1 mg, 0.137 mmol), tri n -
butylphosphine (33.7 p L, 0.135 mmol), and 1 , 1' -
(azodicarbonyl)dipiperidine (33.9 mg, 0.134 mmol)
were added thereto, and after 80 minetes, 2-methoxy-
5-nitrophenol (22.9 mg, 0.135 mmol), tri- n -
butylphosphine (33.7 p L, 0.135 mmol) , and 1,1' -
(azodicarbonyl)dipiperidine (34.0 mg, 0.135 mmol)
were added further. The stir was stopped 3 hours
later and the reaction mixture was concentrated. The
obtained residue was purified by silica gel column
chromatography (hexane-ethyl acetate) to give the
titled compound (22.1 mg) as a pale yellow amorphous
product. (Yield 41%)
0 1H-NMR (500 MHz, CDC13)
AV+ 6 1.43 (s, 3H), 1.43 (s,
0" 0 3H), 2.71 (s, 3H), 3.78 (s
ICY , 1H), 3.79 (s, 3H), 4.06
010 0 010 0 (s, 3H), 4.95 (d, J = 11.9
H Hz, 1H), 5.13 (d, J = 11.
0 id& NO 9 Hz, 1H), 6.72 (d, J = 8.
0 IW 1 Hz, 1H), 6.82 (d, J = 8.
N 1 Hz, 1H), 6.87 (d, J = 2.
H 2 Hz, 1H), 6.94 (d, J = 9.
1 Hz, 1H), 6.97 (dd, J = 8
.2, 2.2 Hz, 1H), 7.33 (d,
J = 8.2 Hz, 1H), 7.34 (d,
J -. 7.6 Hz, 1H), 7.35 (t,
J = 7.6 Hz, 1H), 7.51 (td,
J = 7.6, 1.3 Hz, 1H), 7.5
6 (d, J - 2.5 Hz, 1H), 7.9
4 (dd, J = 9.1, 2.5 Hz, 1H
), 8.21 (dd, J = 7.6, 1.3
Hz, 1H), 9.11 (s, 1H)
2 5 4
= CA 02643070 2008-08-20
[Preparation Examples]
Hereinafter, typical preparation examples of the
present compound are shown.
1) Tablet (in 150 mg)
Present compound 1 mg
Lactose 100 mg
Cornstarch 40 mg
Carboxymethyl cellulose calcium 4.5 mg
Hydroxypropyl cellulose 4 mg
Magnesium stearate 0.5 mg
A tablet of the above-mentioned formulation is
coated with 3 mg of a coating agent (for example, a
conventional coating agent such as
hydroxypropylmethyl cellulose, macrogol or a silicone
resin), whereby an objective tablet can be obtained.
In addition, a desired tablet can be obtained by
appropriately changing the kind and/or amount of the
present compound and additives.
2) Capsule (in 150 mg)
Present compound 5 mg
Lactose 135 mg
Carboxymethyl cellulose calcium 4.5 mg
Hydroxypropyl cellulose 4 mg
Magnesium stearate 1.5 mg
A desired capsule can be obtained by
2 5 5
CA 02643070 2008-08-20
appropriately changing the kind and/or amount of the
present compound and additives.
3) Eye drop (in 100 mL)
Present compound 100 mg
Sodium chloride 900 mg
Polysorbate 80 500 mg
Sodium hydroxide q.s.
Hydrochloric acid q.s.
Sterile purified water q.s.
A desired eye drop can be obtained by
appropriately changing the kind and/or amount of the
present compound and additives.
[Pharmacological Test]
1. Evaluation Test for Binding Activity to
Glucocorticoid Receptor (hereinafter referred to as
"GR")
In order to evaluate a binding activity to GR, a
receptor competitor assay was carried out by a
fluorescence polarization method. In the assay, a GR
competitor assay kit (manufactured by Invitrogen, cat
No. P2816) was used, and a procedure was carried out
according to the protocol attached to the kit.
Hereinafter, the specific method will be described.
(Preparation of Reagents)
GR screening buffer: A buffer containing 10 mM
potassium phosphate (pH 7.4), 20 mM sodium molybdate
(Na2Mo04), 0.1 mM ethylene diamine tetraacetic acid
2 5 6
CA 02643070 2008-08-20
=
(EDTA), 5 mM dithiothreitol (DTT), 0.1 mM stabilizing
peptide and 2% dimethylsulfoxide was prepared.
4 x GS1 solution: FluormoneTM GS1, which is a
fluorescent glucocorticoid ligand, was diluted with
GR screening buffer, whereby a 4 nM solution was
prepared.
4 x GR solution: Recombinant human GR was
diluted with GR screening buffer, whereby a 16 nM
solution was prepared.
(Preparation of Test Compound Solution)
After a test compound was dissolved in
dimethylsulfoxide, the resulting solution was diluted
with GR screening buffer, whereby a 20 M test
compound solution was prepared.
(Test Method and Measurement Method)
1) The test compound solution was added in an
amount of 25 L into each well of a 96-well plate,
and then, 4 x GS1 solution and 4 x GR solution were
added in an amount of 12.5 L into each well,
respectively.
2) The plate was incubated in a dark place at
room temperature for 2 to 4 hours.
3) By using a multimode plate reader, AnalystTM
HT (manufactured by LJL Biosystems), fluorescence
polarization of each well was measured. As the blank,
a well containing GR screening buffer in place of the
test compound and 4 x GS1 solution was used.
4) The same procedure as that in the above 1) to
3) was carried out except that GR screening buffer
2 5 7
= CA 02643070 2008-08-20
was used in place of the test compound solution, and
the obtained result was taken as the negative
control.
5) The same procedure as that in the above 1) to
3) was carried out except that 2 mM dexamethasone was
used in place of the test compound solution, and the
obtained result was taken as the positive control.
(Calculation Equation of GR Binding Ratio)
A GR binding ratio (%) was calculated from the
following equation.
GR binding ratio (%) = 100 x [1 - (fluorescence
polarization of test compound solution - fluorescence
polarization of positive control solution) /
(fluorescence polarization of negative control
solution - fluorescence polarization of positive
control solution)]
(Test Results and Discussion)
As an example of the test results, the GR
binding ratios (%) of the test compounds (Compound 1-
4, Compound 2-2, Compound 2-9, Compound 2-16,
Compound 3-7, Compound 3-10, Compound 3-15, Compound
3-16, Compound 3-17, Compound 4-5, Compound 4-7,
Compound 4-10, Compound 4-11, Compound 5-1, Compound
5-9, Compound 5-14, Compound 5-18, Compound 5-19,
Compound 5-21, Compound 5-22, Compound 5-24, Compound
6-4, Compound 6-8, Compound 6-10, Compound 6-12,
Compound 6-15, Compound 6-19, Compound 6-23, Compound
6-27, Compound 6-32, Compound 6-38, Compound 8-2,
Compound 9-1, Compound 11, Compound 13-2, Compound
2 5 8
= CA 02643070 2008-08-20
=
14-2, Compound 14-4, Compound 14-11, Compound 14-21,
Compound 14-22, Compound 14-30, Compound 14-33,
= Compound 14-35, Compound 14-36, Compound 14-37,
Compound 14-44, Compound 15-1, Compound 15-2,
Compound 15-4, Compound 15-6, Compound 15-9, Compound
15-11, Compound 15-13, Compound 15-17, Compound 15-
22, Compound 15-24, Compound 16-3, Compound 17-5,
Compound 17-16, Compound 18-1, Compound 18-2,
Compound 21-3, Compound 21-5, Compound 21-12,
Compound 21-13, Compound 21-22, Compound 22) are
shown in Table I.
2 5 9
CA 02643070 2008-08-20
,
[Table I]
Test compound GR Binding ratio Test compound GR
Binding ratio
(%) (0/0)
Compound 1-4 91 Compound 13-2 91
Compound 2-2 99 Compound 14-2 100
Compound 2-9 99 Compound 14-4 100
Compound 2-16 100 Compound 14-11 100
Compound 3-7 100 Compound 14-21 100
Compound 3-10 100 Compound 14-22 100
Compound 3-15 100 Compound 14-30 100
Compound 3-16 100 Compound 14-33 100
Compound 3-17 100 Compound 14-35 93
Compound 4-5 100 Compound 14-36 88
Compound 4-7 100 Compound 14-37 93
Compound 4-10 100 Compound 14-44 100
Compound 4-11 100 Compound 15-1 100
Compound 5-1 , 100 Compound 15-2 100
Compound 5-9 100 Compound 15-4 98
Compound 5-14 100 Compound 15-6 100
Compound 5-18 100 Compound 15-9 100
Compound 5-19 100 Compound 15-11 100
Compound 5-21 100 Compound 15-13 100
Compound 5-22 99 Compound 15-17 95
Compound 5-24 , 100 Compound 15-22 100
Compound 6-4 98 Compound 15-24 94
Compound 6-8 98 Compound 16-3 86
Compound 6-10 100 Compound 17-5 91
Compound 6-12 100 Compound 17-16 88
Compound 6-15 100 Compound 18-1 100
Compound 6-19 100 Compound 18-2 99
Compound 6-23 98 Compound 21-3 100
Compound 6-27 100 Compound 21-5 98
Compound 6-32 89 Compound 21-12 100
Compound 6-38 100 Compound 21-13 94
Compound 8-2 100 Compound 21-22 100
Compound 9-1 100 Compound 22 100
Compound 11 100
As is apparent from Table I, the present
compound showed an excellent GR binding activity.
Accordingly, the present compound can be used as a GR
modulator, and is useful for a preventive or
therapeutic agent particularly for GR-related
2 6 0
CA 02643070 2008-08-20
diseases, that is, metabolic disorders, inflammatory
diseases, autoimmune diseases, allergic diseases,
central nervous system diseases, cardiovascular
diseases, homeostasis-related diseases, glaucoma and
the like.
Industrial Applicability
1,2,3,4-tetrahydroquinoxaline derivative or the
salt according to the present invention has a binding
activity to GR and is useful for GR modulator of
nonsteroidal compound.
,
2 6 1