Note: Descriptions are shown in the official language in which they were submitted.
CA 02645103 2008-09-08
DESCRIPTION
METHOD OF REMOVING/CONCENTRATING METAL-FOG-FORMING METAL
PRESENT IN MOLTEN SALT, APPARATUS THEREFOR, AND PROCESS AND
APPARATUS FOR PRODUCING Ti OR Ti ALLOY BY USE OF THEM
TECHNICAL FIELD
[0001]
The present invention relates to a method of
removing/concentrating a metal-fog-forming metal, for example
Ca or Na, dissolved in a molten salt containing such a
metal-fog-forming metal as a constituent thereof, the method
comprising removing the metal-fog-forming metal from the molten
salt and transferring the same to other metal-fog-forming metal
containing molten salt to increase the concentration thereof,
and to an apparatus therefor, as well as to a process for producing
Ti or a Ti alloy by use of the above method in carrying out
the reduction treatment of a TiCl4-based metal chloride mixture
with Ca to produce Ti or a Ti alloy, and to an apparatus therefor.
BACKGROUND ART
[0002]
Such metals as Ti, Zr, Ta, Hf and V are useful metals
respectively having desirable characteristics but can be hardly
refined using such a conventional reducing agent as C or Al.
It is necessary to separate those metals from co-existent
congeners and f rom impurities and, therefore, they are generally
produced by refining through a number of steps such as solvent
extraction, roasting and chlorination, followed by conversion
to oxides or chlorides, further followed by reduction thereof
with a strong reducing agent such as Mg, Al, Na or Ca.
[0003]
Reducing agents such as Ca, Na and Al and, further, Li,
by nature, are themselves soluble in metal chlorides (e.g. Ca
is soluble in CaC12) and, on the occasion of dissolution, they
produce a foggy matter called "metal fog". Such metals are
referred to herein as "metal-fog-forming metals".
CA 02645103 2008-09-08
2
[0004]
These metal-fog-forming metals are refined from raw
material ores through various refining treatments to give their
respective pure forms for use in various fields of application,
including the use of such reducing agents as mentioned above.
On the other hand, the chlorides and fluorides, among others,
of these metals are often used also in the form of molten salts
either alone or in multi-component systems containing another
salt or other salts and, in particular, they are widely used
as industrial electrolytic baths in molten salt electrolysis.
[0005]
Further, concerning the utilization of these
metal-fog-forming metals, the technology which uses Ca as a
reducing agent in producing Ti from TiC14 has been proposed.
A conventional commercial process of producing Ti which can
utilize such a metal-fog-forming metal is the Kroll process
according to which TiC14 is reduced with Mg.
[0006]
In the Kroll process for commercial production of Ti,
metallic titanium is produced via a reduction step and a vacuum
separation step. In the reduction step, TiC14 in liquid form,
fed to a reaction vessel from above, is reduced by molten Mg
to form particles of metallic Ti, which successively move
downward to give spongy metallic Ti. In the vacuum separation
step, the unreacted portion of Mg and the by-product MgC12 are
removed from the spongy metallic Ti occurring in the reaction
vessel.
[0007]
In the production of metallic Ti by the Kroll process,
itispossible to producehigh-purity products. Since, however,
it is a batch-wise process, the production costs add up and
product prices become very high. One of the causes of increased
production costs is a difficulty in increasing the feeding rate
of TiC14.
[0008]
Several possible reasons therefor are conceivable. One
CA 02645103 2008-09-08
3
is that, at a high TiC14 feeding rate exceeding a certain limit,
the TiCl4 fed from above onto that portion of MgC12 which has
not yet moved downward but remains on the liquid surface is
partly discharged out of the reaction vessel in the form of
unreacted TiC14 gas and insufficiently reduced TiC13 gas, among
others, and the TiCl4 utilization efficiency decreases
accordingly.
[0009]
Further, in the Kroll process, the reaction occurs only
in the vicinity of the molten Mg liquid surface in the reaction
vessel, hence the heat release area is narrow. Therefore,
cooling cannot keep up with such high-rate feeding of TiC14,
which is another major reason for the TiC14 feeding rate being
restricted.
[0010]
Furthermore, the Ti powder formed moves downward in an
aggregated state due to wettability (viscosity) of the molten
Mg and, during moving downward, grains thereof are sintered
and grown due to the heat which the high-temperature melt harbors,
making it difficult to recover them out of the reaction vessel.
Therefore, the metallic Ti production cannot be performed in
a continuous manner; hence productivity is impaired.
[0011]
As for Ti production processes other than the Kroll process,
United States Patent No. 2, 205, 854 describes that Ca, forexample,
can be used instead of Mg as an agent for reducing TiC14. And,
United States Patent No. 4,820,339 describes a process for
producing Ti by utilizing reduction reaction with Ca which
comprises maintaining a CaC12-based molten salt in a reaction
vessel, feeding a metallic Ca powder to the molten salt from
above to allow the Ca to be dissolved in the molten salt while
feeding TiC14 gas from below to thereby cause the molten Ca
to react with the TiC14 in the CaC12-based molten salt.
[0012]
However, the process described in the above-cited United
States Patent No. 4, 820, 339 cannot serve asa commercialprocess
CA 02645103 2008-09-08
4
of producing Ti since the metallic Ca powder to be used as the
reducing agent is very expensive and, when this is purchased,
the production cost will become higher as compared with the
Kroll process. In addition, Ca, which is strongly reactive,
is very difficult to handle; this fact is also major factor
inhibiting the commercial Ti production by reduction with Ca.
[0013]
As a further process of producing Ti, United States Patent
No. 2,845,386 describes the so-called Olson process in which
Ti02 is directly reduced with Ca, not via TiClq. This process
is a kind of direct oxide reduction process. However, it is
necessary to use expensive high-purity Ti02 in this process.
[0014]
On the other hand, the present inventors, who considered
it necessary, for establishing a commercial process for
producing Ti by reduction with Ca, to supplement Ca consumed
in the reduction reaction to the molten salt in an economical
manner, proposed, in Japanese Patent Application Publication
Nos. 2005-133195 and 2005-133196, a process, namely the
so-called "OYIK process", according to which Ca formed upon
electrolysis of molten CaC12 is utilized and this Ca is recycled.
[0015]
The process described in the above-cited Japanese Patent
Application Publication No. 2005-133195 comprises introducing
a Ca-rich molten CaC12 supplemented with Ca formed by
electrolysis into a reaction vessel for use in formation of
Ti particles by reduction with Ca, and the process described
in Japanese Patent Application Publication No. 2005-133196
further comprises using an alloy electrode (e.g. Mg-Ca alloy
electrode) as the cathode for effectively suppressing the back
reaction in association with the electrolysis.
DISCLOUSRE OF THE INVENTION
[0016]
As mentioned above, a number of research and development
have so far been made in search of Ti production processes other
CA 02645103 2008-09-08
than the Kroll process. In the OYIK process proposed by the
present inventors, in particular, Ca in the molten salt is
consumed as the reduction reaction of TiC14 proceeds and, when
the resulting molten salt is electrolyzed, Ca is formed in the
molten salt, and the reuse of the thus-obtained Ca for the
reduction reaction makes it unnecessary to supplement Ca from
the outside and further makes it unnecessary to take out Ca
alone, so that the economical efficiency is improved.
[0017]
Therefore, the present inventors attempted to develop
a metallic Ti or Ti alloy production process whose basic
constitution is based on the OYIK process and which can be carried
out efficiently and stably on a commercial scale and made
investigations on all the production steps in that process.
[0018]
It is an object of the present invention, which has been
made in view of the fact that such metal-fog-forming metals
as Ca and Na are widely used as constituents in various molten
salts, typically industrial electrolytic baths, to provide a
method of removing/concentrating a metal-fog-forming metal in
a molten salt according to which a metal-fog-forming metal (Ca)
can be removed from a molten salt composition comprising a molten
salt (e.g. CaCl2) containing a metal-fog-forming metal such
as Ca or Na as a constituent thereof, with the metal-fog-forming
metal (Ca) dissolved therein, and, at the same time, the
thus-removed metal-fog-forming metal can be transferred to
another molten salt composition (molten salt composition
containing the metal-fog-forming metal as a constituent
thereof ), as well as an apparatus for use in carrying out such
method.
[0019]
It is a further object of the present invention to provide
a Ti or Ti alloy production process according to which the
reduction of TiCl4 or another metal chloride and, further, the
formation of Ca by molten salt electrolysis are effected
efficiently in the production of metallic Ti or a Ti alloy by
CA 02645103 2008-09-08
6
reduction of TiC14 or the other metal chloride with Ca formed
by electrolysis of molten CaC12 and which can be performed stably
on a commercial scale, as well as a production apparatus suited
for that process.
[0020]
The present invention has been made to accomplish the
obj ects mentioned above and is now described in its three aspects,
namely"l.method of removing/concentrating a metal-fog-forming
metal in a molten salt, and apparatus therefor", "2.process
for producing Ti or a Ti alloy which includes a Ca recovery
step, and apparatus therefor" and "3.process for producing Ti
or a Ti alloy which includes a Ca removing/concentrating step,
and apparatus therefor".
[0021]
1. Method of removing/concentrating a metal-fog-forming
metal in a molten salt, and apparatus therefor
The present inventors made investigations concerning the
case that the metal-fog-forming metal is Ca. As a result, it
was found that when the molten salt (CaC12) is held in a treatment
vessel (hereinafter, referred to as "vessel A") and a
Ca-containing molten a11oy(a molten Mg-Ca alloy) isheldthereon
in contact with the molten salt and a voltage below the
decomposition voltage for CaC12 is applied so that the electrode
plate on the molten alloy side may serve as a negative (-)
electrode and the electrode plate on the molten salt side as
a positive (+) electrode, the Ca dissolved in the molten salt
can be rapidly absorbed into the molten alloy, thus being
removed.
[0022]
When, on the contrary, a voltage below the decomposition
voltage for CaC12 is applied so that the electrode plate on
the molten alloy side may serve as a positive (+) electrode
and the electrode plate on the molten salt side as a negative
(-) electrode (hereinafter such vessel is referred to as "vessel
B"), the Ca is transferred from the molten alloy side to the
molten salt side.
CA 02645103 2008-09-08
7
[0023]
It was further found that when the molten alloy as a common
constituent for the above vessel A and vessel B is connected
to be integrated and a voltage (voltage below the decomposition
voltage for CaC12) is applied so that the electrode plate on
the molten salt side in vessel A may serve as a positive (+)
electrode and the electrode plate on the molten salt side in
vessel B as a negative (-) electrode, the removal (absorption
into the molten alloy) of the Ca dissolved in the molten salt
in vessel A and the transfer of the Ca from the molten alloy
side to the molten salt side in vessel B can be effected
simultaneously. Namely, it was revealed that the removal of
the Ca dissolved in the molten salt on one side and the enrichment
of the Ca dissolved in the molten salt on the other side can
be caused to proceed simultaneously and rapidly by appropriate
voltage application.
[0024]
And, it has been confirmed that when such a method as
mentioned above is applied to the above-mentioned OYIK process
proposed by the present inventors, it is possible to allow the
formation of Ca by electrolysis to proceed efficiently while
suppressing a so-called back reaction, which otherwise readily
occurs in the step of electrolysis of a molten CaC12, and ,
further, to effectively enhance the efficiency of the reduction
reaction of TiClq (formation of Ti particles by reduction of
TiC14 with Ca) as well.
[0025]
In applying this method to the OYIK process, all the
production steps of the process have been reexamined so as to
carry out the operations efficiently and stable on a commercial
scale and, as a result, a markedly improved and advanced
modification of the OYIK process has been newly developed; the
modification can be regarded as a new development of the OYIK
process and may be referred to, for example, as "OYIK-II
process".
[0026]
CA 02645103 2008-09-08
8
The method ofremoving/concentration a metal-fog-forming
metal in molten salt and the apparatus therefor according to
the present invention have been developed based on such f indings
and re-examination results as mentioned above and respectively
have constitutional features (1) and (2) described below.
[0027]
(1) A method of removing/concentrating a metal-fog-forming
metal in molten salt, the method comprising: holding a molten
salt in a metal-fog-forming metal concentrating and removing
regions, being apart from each other, inside a metal-fog-forming
metal removing/concentrating vessel, the molten salt being a
moltensalt mixture consisted ofoneor more ofinetal-fog-forming
metal containing molten salts, the metal-fog-forming metal
being dissolved therein; holding a metal-fog-forming metal
containing molten alloy in contact with the molten salt held
in each of the metal-fog-forming metal concentrating and
removing regions; and applying a voltage below a decomposition
voltage for the metal-fog-forming-metalcontaining molten salt
so that an electrode plate on the molten salt side within the
metal-fog-formingmetal removing region may serve as a positive
(+) electrode against the molten salt side within the
metal-fog-forming metal concentrating region, thereby causing
the metal-fog-forming metal dissolved in the molten salt within
the metal-fog-forming metal removing region to be absorbed into
the molten alloy, resulting in the decrease in concentration
thereof and, at the same time, causing the metal-fog-forming
metal dissolved in themolten saltwithinthe metal-fog-forming
metal concentrating region to be highly concentrated, resulting
in the increase in concentration thereof.
[0028]
The "metal-fog-forming metal" so referred to herein is
a metal strong in reducing power, solubleitself incorresponding
metal chloride (e.g. Ca being soluble in CaC12) and capable
of forming a fog called a metal fog on the occasion of dissolution,
such as Ca, Li, Na or Al.
[0029]
CA 02645103 2008-09-08
9
The term "a metal-fog-forming-metal containing molten
salt" as used herein refers to the molten salt in which a
metal-fog-forming metal is contained as a constituent thereof,
for example a molten CaC12 or a molten NaCl. "A molten salt
mixture consisted of one or more of inetal-fog-forming metal
containing molten salts", should mean, in the case of the
metal-fog-forming metal being Ca, forinstance, either a molten
CaC12 alone or a molten salt mixture comprising a molten CaC12
and CaF2 or the like that is added for lowering the melting
point and adjusting the viscosity, for instance.
[0030]
The "a metal-fog-forming metal containing molten alloy"
is an alloy in a molten state with the metal-fog-forming metal
being contained therein as a constituent thereof and, in the
case of the metal-fog-forming metal being Ca, for instance,
it indicates a molten Mg-Ca alloy, a molten Pb-Ca alloy or the
like.
[0031]
The electrodes between which a voltage is to be applied
are designated as "negative (-) electrode" and "positive (+)
electrode", as so referred to hereinabove, for avoiding the
confusion thereof with the terms "anode" and "cathode", which
are used on the premise that a salt bath (herein, molten salt)
is electrolyzed.
[0032]
The method ofremoving/concentrating a metal-fog-forming
metal according to the present invention as described above
under (1) can be carried out in a mode of embodiment in which
the metal-fog-forming metal is Ca and the metal-fog-forming
metal containing molten salt is a Ca-containing molten salt
and, further, in a desirable mode of embodiment in which the
metal-fog-forming metal is Ca and the metal-fog-forming metal
containing molten salt is CaC12. The "Ca-containing molten
salt" just mentioned above refers to CaC12 or CaF2r for instance.
[0033]
When, in the above mode of embodiment, the applied voltage
CA 02645103 2008-09-08
is lower than 3.2 V, it is possible to cause Ca to be dissolved
in molten alloy without causing decomposition of CaClZ while
controlling the voltage to be applied at specific numerical
value levels.
[0034]
(2) An apparatus for removing/concentrating a
metal-fog-forming metal in molten salt, comprising a
metal-fog-forming metal removing/concentrating vessel having
a for metal-fog-forming metal concentrating and removing
regions and a holding region for molten alloy, wherein the
concentrating region is for holding a molten salt mixture
consisted of one or more of inetal-fog-forming metal containing
molten salts, the molten salt mixture having an enriched
metal-fog-forming metal that is dissolved therein, and wherein
the removing region is for holding a metal-fog-forming metal
containing molten salt that has a rarified metal-fog-forming
metal as being dissolved therein as a result of application
of a voltage below the decomposition voltage for the
metal-fog-forming metal containing molten salt via an electrode
plate so that it may serve as a positive (+) electrode against
the molten salt side within the metal-fog-forming metal
concentrating region, and wherein the holding region is for
holding a metal-fog-forming metal containing molten alloy in
contact with the molten salt held in the metal-fog-forming metal
concentrating and removing regions.
[0035]
In the metal-fog-forming metal removing/concentrating
apparatus according to the present invention as described above
under (2), a mode of embodiment is possible in which the
metal-fog-forming metal is Ca and the metal-fog-forming-metal
containing molten salt is a Ca-containing molten salt and,
further, a mode of embodiment is possible in which the
metal-fog-forming metal is Ca and the metal-fog-forming metal
containing molten salt is CaCl2, in the apparatus as described
above under (2).
[0036]
CA 02645103 2008-09-08
11
The method ofremoving/concentratingametal-fog-forming
metal in a molten salt according to the present invention makes
it possible to remove the metal-fog-forming metal, for example
Ca or Na, that is dissolved in one portion of a molten salt
mixture consisted of one or more of metal-fog-forming metal
containing molten salts therefrom and transfer the same to the
othe portion of molten salt mixture to increase the concentration
of the metal-fog-forming metal therein, both portions of molten
salt mixture being respectively in contact with a
metal-fog-forming metal containing alloy. This method can be
carried out easily and properly using the apparatus according
to the present invention.
[0037]
2. Process for producing Ti or a Ti alloy which includes
a Ca recovery step, and apparatus therefor
For producing Ti or a Ti alloy in stable operation on
a commercial scale, it is important to cause the reduction
reaction of TiCl4 or other metal chlorides and the formation
of Ca by electrolysis of the molten salt to proceed efficiently
and, for stabilizing the operation simultaneously, it is
important to increase the concentration of Ca in a
CaC12-containing molten salt to be fed to the reaction vessel
for reducing TiC14, to retard concentration fluctuations, and
to remove (recover) Ca in the molten salt to be discharged out
of the reaction vessel and to be introduced into the electrolytic
cell. Further, for enabling Ti production on a commercial scale,
it is necessary to increase the rate of feeding of Ca to the
reaction vessel (in another word, to continuously treat a large
amount of a CaC12-containing molten salt in the electrolysis
step).
[0038]
If the Ca concentration in the molten salt fed to the
reaction vessel is too low, an unreacted TiC14 gas will be
discharged out of the vessel. Furthermore, gases of such
titanium subchlorides as TiC13 and TiC12 may be formed and
dissolved in the molten salt to react with the Ca formed by
CA 02645103 2008-09-08
12
electrolysis in the electrolytic cell to form Ti, which will
in turn precipitate out on the negative electrode surface and
possibly cause operational troubles. Further, it is feared,
among others, that TiC, which causes contamination of Ti by
C, may be generated.
[0039]
On the other hand, if the Ca concentration in the molten
salt is excessively high, a large amount of Ca may be contained
in the molten salt extracted from the reaction vessel; that
Ca will evaporate in the separation step, causing a loss.
[0040]
Further, when the molten salt after separation of Ti in
the separation step is returned to the electrolytic cell, a
so-called back reaction, namely the reaction of the Ca in the
molten salt with the chlorine formed by electrolysis, may occur
andlowerthe currentefficiency;thehigherthe Ca concentration
in the molten salt is, the more significant the decrease in
current efficiency is. Furthermore, the temperature
uniformityin the molten salt (salt bath) within the electrolytic
cell will be disturbed by the heat of reaction as resulting
from the back reaction, possibly causing troubles in salt bath
temperature control.
[0041]
Therefore, the present inventors made various
investigations in an attempt to inhibit the Ca concentration
in the molten salt to be fed to the reaction vessel from
fluctuating, to maintain that concentration at high levels,
and further to suppress the back reaction by rapidly recovering
Ca, namely removing Ca, in the molten salt to be fed to the
electrolytic cell.
[0042]
As a result, it was found that it is possible to cause
the Ca dissolved in the molten salt to be rapidly absorbed into
the molten alloy and to be recovered by keeping the molten salt
to be fed to the electrolytic cell in contact with a Ca-containing
molten alloy (molten Mg-Ca alloy) and applying a voltage below
CA 02645103 2008-09-08
13
the decomposition voltage for CaClZ so that the electrode bar
on the molten alloy side may serve as a negative (-) electrode
and the electrode bar on the molten salt side as a positive
(+) electrode. This means makes it possible to lower the Ca
concentration in the molten salt fed to the electrolytic cell
and to suppress the back reaction on the occasion of electrolysis
of the molten salt, thereby allowing the formation of Ca
efficiently.
[0043]
On the other hand, it was found that it is effective,
for inhibiting the fluctuation of and maintaining at high levels
the Ca concentration in the molten salt to be fed to the reaction
vessel, to dispose a regulating vessel provided with a Ca supply
source between the electrolytic cell and reaction vessel, and
to introduce the molten salt into the regulating vessel to render
the Ca concentration constant, the molten salt being increased
in Ca concentration owing to the formation of Ca by electrolysis,
and thereafter to use the same for reduction. By doing so,
it becomes possible to maintain the Ca concentration in the
molten salt always at a constant high level and allow the
reduction reaction to proceed efficiently. Itwasalso revealed
that the molten alloy increased in Ca concentration as a result
of absorption of Ca therein by voltage application to the molten
salt can be used as a Ca supply source for the regulating vessel.
[0044]
Furthermore, the present inventors made detailed
investigations concerning the configuration of an electrolytic
cell vessel of a main electrolytic cell, the configuration of
electrodes, electrolysis conditions, and a distance between
the electrodes, among others, and, as a result, found that when
the molten salt is electrolyzed while causing the same to flow
in one direction in the vicinity of the cathode surface and
the molten salt enhanced in Ca concentration is recovered on
the outlet side of the electrolytic cell, it is possible to
suppress the back reaction and maintain the current efficiency
at a high level and, at the same time, effectively take out
CA 02645103 2008-09-08
14
the Ca-enriched molten salt alone and, further, continuously
treat the CaC12-containing molten salt in large quantity and
increase the feeding rate of Ca to the reaction vessel.
[0045]
The Ti or Ti alloy production process including the Ca
recovery step according to the present invention and the
apparatus therefor have been developed based on the above
findings and have constitutions/features as shown in (3) and
(4), respectively.
[0046]
(3) A process for producing Ti or a Ti alloy, comprising:
a reduction step in which a CaC12-containing molten salt with
Ca dissolved therein is held in a reaction vessel and the Ca
in the molten salt is allowed to react with a TiCl4-based metal
chloride to form Ti particles or Ti alloy particles in said
molten salt; a separation step in which said Ti particles or
Ti alloy particles are separated from the molten salt within
said reaction vessel or outside the reaction vessel; an
electrolysis step in which the molten salt taken out of said
reaction vessel is electrolyzed to form Ca to thereby increase
the Ca concentration in the molten salt; a return step in which
the Ca formed by said electrolysis is introduced, either alone
or together with the molten salt, into said reaction vessel;
and a Ca recovery step in which while the molten salt separated
in said separation step and to be sent to said electrolysis
step is kept in contact with a Ca- and Mg-containing molten
alloy, a voltage below the decomposition voltage for CaCl2 is
applied so that the electrode bar on the molten alloy side may
serve as a negative (-) electrode and the electrode bar on the
molten salt side as a positive (+) electrode to thereby cause
the Ca dissolved in the molten salt to be absorbed into the
molten alloy, and the molten salt decreased in Ca concentration
is sent to the electrolysis step.
[0047]
The "CaC12-containing molten salt" so referred to herein
is either molten CaC12 alone or a molten salt mixture consisted
CA 02645103 2008-09-08
of a molten CaC12 and CaF2 etc to be added for lowering a melting
point and adjusting viscosity thereof, among others. It is
sometimes referred to as a "molten salt" or a "molten CaC12"
for short.
[0048]
The "TiC14-based metal chloride" refers to TiC12 alone
or a mixture of TiCl4 and other metal chlorides, the metals
as constituent being intended for alloy elements in a Ti alloy,
for example V, Al and/or Cr. Since the other metal chlorides
are also reduced by Ca simultaneously with the reduction of
TiCl4, a Ti alloy can be produced by using such a TiC14-based
mixed metal chloride as raw material.
[0049]
When, in the Ti or Ti alloy production process described
above under (3), the applied voltage is lower than 3.2 V, it
is possible to control its applied voltage at specific numerical
value levels and thereby cause the Ca dissolved in CaC12 to
be rapidly absorbed into the molten alloy without allowing the
decomposition of CaC12.
[0050]
When, in the Ti or Ti alloy production process described
above under (3), the molten salt increased in Ca concentration
in an electrolysis step is introduced into a regulating vessel
provided with a Ca supply source and the molten salt is thus
brought into contact with the Ca supply source and thereby
rendered constant in Ca concentration and thereafter sent to
the reduction step, it becomes possible to maintain the Ca
concentration in the molten salt introduced into the reaction
vessel always at a constant high level to thereby allow the
reduction reaction to proceed efficiently.
[0051]
When, in the production process described above under
(3), the molten alloy increased in Ca concentration as a result
of absorption of Ca in the Ca recovery step is used as the Ca
supply source or part of it for the regulating vessel, it becomes
possible to effectively utilize the portion of Ca that has been
CA 02645103 2008-09-08
16
removed for suppressing the back reaction, which is desirable.
[0052]
(4) An apparatus for producing Ti or a Ti alloy, comprising:
a reaction vessel for holding a CaC12- containing molten salt
with Ca being dissolved therein and reacting a metal chloride
containing TiC14, the TiCl4 being to be fed into said molten
salt, with the Ca to cause the formation of Ti particles or
Ti alloy particles therein; separation means for separating
the Ti particles or Ti alloy particles formed in said molten
salt therefrom; an electrolytic cell which holds a left molten
salt after separation of said Ti particles or Ti alloy particles
and is equipped with an anode and a cathode for carrying out
electrolysis in the left molten salt to form Ca on the cathode
side; return means for introducing the Ca formed by said
electrolysis, either alone or together with an electrolyte
molten salt, into the reaction vessel; and Ca recovery means
for applying, while keeping the left molten salt separated by
the separation means and to be fed to the electrolytic cell
in contact with a Ca- and Mg-containing molten alloy, a voltage
below the decomposition voltage for CaC12 so that the electrode
bar on the molten alloy side may serve as a negative (-) electrode
and the electrode bar on the molten salt side as a positive
(+) electrode to thereby cause the Ca dissolved in the molten
salt to be absorbed into the molten alloy, thus decreasing in
Ca concentration thereof, and for sending the treated molten
salt having a lowered Ca concentration to the electrolytic
cell.
[0053]
When the Ti or Ti alloy production apparatus described
above under (4) further comprises a regulating vessel provided
with a Ca supply source and intended for introducing molten
salt in an electrolytic cell thereinto and bringing the same
into contact with the Ca supply source to thereby render the
Ca concentration in the molten salt constant and, thereafter,
feedingthat molten salt into the reaction vessel, the apparatus
can be suitably used for carrying out the production process
CA 02645103 2008-09-08
17
described above under (3).
[0054]
In accordance with the Ti or Ti alloy production process
comprising the Ca recovery step of the present invention, it
is possible to rapidly recover the Ca dissolved in molten salt
and thus suppress back reaction on the occasion of electrolysis
of the molten salt to thereby enhance the efficiency of Ca
formation. Furthermore, the process can contribute not only
to increase the Ca concentration in the molten salt to be sent
to the reduction step, simultaneously with the Ca recovery,
and to enhance the efficiency of Ca formation, but also to enhance
the efficiency of the TiC14 reduction reaction.
[0055]
Furthermore, the process makes it possible to inhibit
the fluctuation of and to maintain at a high level the Ca
concentration in the molten salt to be fed to the reaction vessel
by using the regulating vessel provided with a Ca supply source
and thus carry out the TiC14 reduction reaction efficiently
and, further, continuously treat the CaC12-containing molten
salt in large quantity in the electrolysis step and thereby
increase the feeding rate of Ca to the reaction vessel.
[0056]
3. Process for producing Ti or a Ti alloy which includes
a Ca removing/concentrating step, and apparatus therefor
Furthermore, the present inventors made various
investigations to inhibit the fluctuation of and maintain at
a high level the Ca concentration in the molten salt to be fed
into the reaction vessel, at the same time, to rapidly recover
and remove the Ca in the molten salt to be fed to the electrolytic
cell therefrom to thereby suppress the back reaction so that
the operation can be carried out in an efficient and stable
manner on a commercial scale.
[0057]
As a result, it was revealed that when, in view of the
fact that upon application of a voltage below the decomposition
voltage for CaC12 so that the electrode bar on the molten alloy
CA 02645103 2008-09-08
18
side may serve as a positive (+) electrode and the electrode
bar on the molten salt side as a negative (-) electrode, Ca
is transferred from the molten alloy side to the molten salt
side, the molten alloy is incorporated into an integrated
structure as a common structural element and, while the molten
salt is kept in contact with this molten alloy and a voltage
(voltage below the decomposition voltage for CaC12) is applied
so that the electrode bar on the side of first portion of the
molten salt may serve as a positive (+) electrode and the
electrode bar on the side of second portion of the molten salt
being as a negative (-) electrode, it becomes possible to
simultaneously effect the absorption of the Ca dissolved in
that one molten salt side into the molten alloy and the transfer
of Ca from the molten alloy side to the other molten salt side.
[0058]
Namely, it was revealed that the removal of the Ca dissolved
in first portion of the molten salt and the increase in the
concentration of Ca dissolved in second portion of the molten
salt can be simultaneously and rapidly realized by applying
a predetermined voltage.
[0059]
On the other hand, it was found that, for inhibiting the
fluctuation of and maintaining at a high level the Ca
concentration in the molten salt to be fed to the reaction vessel,
it is effective to dispose, between the electrolytic cell and
reaction vessel, a regulating vessel provided with a Ca supply
source and introduce the molten salt increased in Ca
concentration owing to the formation of Ca by electrolysis into
the regulating vessel to thereby render the Ca concentration
thereof constant and, thereafter, use the same for reduction.
[0060]
By doing so, it becomes possible to always maintain the
Ca concentration in the molten salt at a constant high level
and allow the reduction reaction to proceed efficiently. It
was also found that the molten alloy increased in Ca
concentration as a result of Ca absorption caused by voltage
CA 02645103 2008-09-08
19
application to the molten salt can be used as the Ca supply
source for the regulating vessel.
[0061]
Furthermore, the present inventors found that by
electrolyzing the molten salt while causing the same to flow
in one direction in the vicinity of the cathode surface and
by recovering the molten salt increased in Ca concentration
on the outlet side of the electrolytic cell, it becomes possible
to suppress thebackreaction and maintain the currentefficiency
at a high level and, at the same time, it becomes possible to
effectively take out the Ca-enriched molten salt alone and,
further, continuously treat the CaC12-containing molten salt
in large quantity and thereby increase the feeding rate of Ca
to the reaction vessel.
[0062]
The Ti or Ti alloy production process comprising the Ca
removing/concent rating step according to the present invention
and the apparatus therefor have been developed based on such
findings and have constitutions/features shown in (5) and (6)
below.
[0063]
(5) A process for producing Ti or a Ti alloy, comprising:
a reduction step in which a CaC12-containing molten salt with
Ca dissolved therein is held in a reaction vessel and the Ca
in the molten salt is allowed to react with a TiC14-based metal
chloride to form Ti particles or Ti alloy particles in said
molten salt; a separation step in which said Ti particles or
Ti alloy particles are separated from the molten salt inside
or outside said reaction vessel; an electrolysis step in which
the molten salt taken out of said reaction vessel is electrolyzed
to form Ca, thereby increasing the Ca concentration in the molten
salt; a return step in which the Ca formed by said electrolysis
is introduced, either alone or together with the molten salt,
into said reaction vessel;and a Ca removing/concentratingstep
in which a voltage below the decomposition voltage for CaC12
is applied so that the electrode plate on the molten salt side
CA 02645103 2008-09-08
within a Ca-removing region serving to hold the molten salt
separated in said separation step and to be sent to said
electrolysis step may serve as a positive (+) electrode against
the electrode plate on the molten salt side within a
Ca-concentrating region, apart from the Ca-removing region,
serving to hold the molten salt to be sent to said reduction
step, and the molten salt reduced in Ca concentration in the
Ca-removing region is sent to the electrolysis step and the
molten salt increased in Ca concentration in the
Ca-concentrating region is sent to the reduction step.
[0064]
The terms "CaC12-containing molten salt" and "TiCl4-based
metal chloride" are as exactly as defined in the above "2: Ti
or Ti alloy production process comprising a Ca recovery step".
[0065]
When, in the Ti or Ti alloy production process described
above under (5), the applied voltage is lower than 3.2 V, it
is possible to control the applied voltage at a specific
numerical value level and cause rapid absorption of the Ca
dissolved in CaC12 into the molten alloy without allowing
decomposition ofCaC12. Further, when the molten salt increased
in Ca concentration in the electrolysis step is introduced into
the regulating vessel provided with a Ca supply source and
brought into contact with the Ca supply source to render the
Ca concentration in the molten salt constant and, thereafter,
the resulting molten salt is sent to the reduction step, it
enables the Ca concentration in the molten salt to be introduced
into the reaction vessel to be maintained always at a constant
high level, thus allowing the reduction reaction to proceed
efficiently.
[0066]
(6) An apparatus for producing Ti or a Ti alloy, comprising:
a reaction vessel for holding a CaC12-containing molten salt
with Ca dissolved therein and reacting a TiCl4-based metal
chloride fed into the molten salt with said Ca to cause the
formation of Ti particles or Ti alloy particles therein;
CA 02645103 2008-09-08
21
separation means for separating the Ti particles or Ti alloy
particles formed in said molten salt from the molten salt; an
electrolytic cell which holds the molten salt after separation
of said Ti particles or Ti alloy particles therefrom and is
equipped with an anode and a cathode for carrying out
electrolysis in said molten salt to form Ca on the cathode side;
a return means for introducing the Ca formed by said electrolysis,
either alone or together with a treated molten salt, into said
reaction vessel; and a Ca removing/concentrating unit which
comprises: (a) a Ca-removing region for holding the molten salt
separated in said separation step and to be fed to said
electrolysis step; and (b) a Ca-concentrating region, apart f rom
the Ca-removing region, serving to hold the molten salt to be
sent to said reduction step and which serves to apply a voltage
below a decomposition voltage for CaC12 so that the electrode
plate on the molten salt side in the Ca-removing region may
serve as a positive (+) electrode against the electrode plate
on the molten salt side in the Ca-concentrating region and,
further, serves to send the molten salt thus decreased in Ca
concentration in the Ca-removing region to the electrolysis
step and the molten salt thus increased in Ca concentration
in the Ca-concentrating region to the reduction step.
[0067]
When the Ti or Ti alloy production apparatus described
above under (6) further comprises a regulating vessel provided
with a Ca supply source and intended for introducing molten
salt in the electrolytic cell thereinto and bringing the same
into contact with the Ca supply source to thereby render the
Ca concentration in the molten salt constant and, thereafter,
feeding that molten salt into the reaction vessel, the apparatus
can be suitably used for carrying out the production process
described above under (5).
[0068]
In accordance with the Ti or Ti alloy production process
comprising a Ca removing/concentrating step according to the
present invention, it is possible to rapidly remove the Ca
CA 02645103 2008-09-08
22
dissolved in the molten salt therefrom and thus suppress the
back reaction on the occasion of electrolysis of the molten
salt to thereby enhance the efficiency of Ca formation.
Furthermore, the process can contribute not only to increase
the Ca concentration in the molten salt to be sent to the reduction
step, simultaneously with the Ca removal, and enhance the
efficiency of Ca formation but also to enhance the efficiency
of the TiC14 reduction reaction.
j0069]
Furthermore, the process makes it possible to inhibit
the fluctuation of and maintain at a high level the Ca
concentration in the molten salt to be fed to the reaction vessel
by using the regulating vessel provided with a Ca supply source
and thus carry out the TiCl4 reduction reaction efficiently
and, further, continuously treat the CaC12-containing molten
salt in large quantity in the electrolysis step to thereby
increase the feeding rate of Ca to the reaction vessel.
BRIEF DESCRITPION OF THE DRAWINGS
[0070]
Fig. 1 is a schematic representation of an example of
the principal constitution/feature of an apparatus to be used
in carrying out the method of removing/concentrating a
metal-fog-forming metal inamolten saltaccordingtothe present
invention.
Fig. 2 is a schematic representation of the relation between
the voltage applied between molten Mg-Ca alloy and molten CaC12
and the current flowing between both electrodes. Fig. 2 (a)
is a representation for the case where Ca is not dissolved in
CaC12 (before addition of Ca) , and Fig. 2 (b) is a representation
for the case where Ca is dissolved in CaC12 (after addition
of Ca).
Fig. 3 is a schematic representation of an example of
the principal constitution/feature of an apparatus to be used
in carrying out the method of recovering (i.e. method of
removing) a metal-fog-forming metal in a molten salt according
CA 02645103 2008-09-08
23
to the present invention.
Fig. 4 is a schematic representation of an example of
the constitution/feature of an apparatus to be used in carrying
out the Ti or Ti alloy production process including a Ca recovery
step according to the present invention.
Fig. 5 is a schematic representation of another example
of the constitution/feature of an apparatus to be used in
carrying out the Ti or Ti alloy production process including
a Ca recovery step according to the present invention.
Fig. 6 is a schematic representation of the
constitution/feature of an apparatus to be used in carrying
out the Ti or Ti alloy production process including a Ca
removing/concentrating step according to the present
invention.
Fig. 7 is a schematic representation of another example
of the constitution/feature of an apparatus to be used in
carrying out the Ti or Ti alloy production process including
a Ca removing/concentrating step according to the present
invention.
BEST MODES FOR CARRYING OUT THE INVENTION
[0071]
In the following, the subject matters of the following
three aspects of the present invention are described in more
detail: "l: method of removing/concentrating a
metal-fog-forming metal in a molten salt, and apparatus
therefor", "2: process for producing Ti or a Ti alloy which
includes a Ca recovery step, and apparatus therefor" and "3:
process for producing Ti or a Ti alloy which includes a Ca
removing/concentratingstep, and apparatus therefor". The case
where a metal-fog-forming metal is Ca is exemplified in the
following.
[0072]
1. Method of removing/concentrating a metal-fog-forming
metal in a molten salt, and apparatus therefor
Fig. 1 is a schematic representation of an example of
CA 02645103 2008-09-08
24
the principal constitution/feature of an apparatus to be used
in carrying out the method of removing/concentrating a
metal-fog-forming metal in molten salt according to the present
invention. The constitution/feature shown is the same as that
of a Ca removing/concentrating unit 28 in Fig. 6 or Fig. 7,
which is to be hereinafter referred to and in which the same
reference numerals are used. Further, in Fig. 1, Ca denotes
a metal-fog-forming metal, a molten CaC12 denotes a molten salt
mixture consisted of one or more of metal-fog-forming metal
containing molten salts, and a molten Mg-Ca alloy denotes a
metal-fog-forming metal containing molten alloy, and these
designations are used in the description which follows.
[0073]
As shown in Fig. 1, this apparatus has a Ca
removing/concentration vessel 28a, and a molten CaC12 is held
in this vessel 28a in a condition separated into a
Ca-concentrating region 29 and a Ca-removing region 30 by a
partition wall 31, and a molten Mg-Ca alloy 8 is held thereon
in contact with each portion of the molten salt held in the
Ca-concentrating region 29 and Ca-removing region 30.
[0074]
Further, on the bottom of the Ca removing/concentrating
vessel 28a, there is disposed an electrode plate 33 for applying
a voltage below a decomposition voltage for CaC12 so that the
molten salt side in the Ca-removing region 30 may be on the
positive (+) electrode side against the molten salt side in
the Ca-concentrating region29. Generally, an electrode plate
34 is disposed so that the electrode plate on the molten salt
side in the Ca-concentrating region 29 may serve as a negative
(-) electrode, as shown in the f igure . In the example shown,
the Ca-concentrating region 29 and Ca-removing region 30 are
separated from each other by the partition wall 31 but the
invention is not limited to this constitution/feature. Thus,
for example, a constitution/feature such that both regions are
apart from each other by the use of two independent, detachable
cells may also be employed.
CA 02645103 2008-09-08
[0075]
In carrying out the method of removing/concentrating Ca,
the Ca being a metal-fog-forming metal, in a molten salt using
the apparatus shown in Fig. 1, a molten CaC12 as molten salt
with Ca dissolved therein is first held in the Ca-concentrating
region 29 in the Ca removing/concentrating vessel 28a as well
as in the Ca-removing region 30 separated f rom the concentrating
region 29 by the partition wall 31. Further, a molten Mg-Ca
alloy 8 is held onto the molten salt in both regions 29 and
in contact with both portions of the molten salt.
[0076]
Then, a voltage below the decomposition voltage for CaC12
is applied so that the electrode plate 33 on the molten salt
side in the Ca-removing region 30 may serve as a positive (+)
electrode against the electrode plate 34 on the molten salt
side in the Ca-concentrating region 29.
[0077]
Upon this voltage application, the molten Mg-Ca alloy
8 existing in the adjacent surface of contact with the molten
salt in the Ca-removing region 30 functions as a negative (-)
electrode relative to the molten salt side (+ electrode side)
in the Ca-removing region 30 and, therefore, the dissolved Ca
transfers to the molten Mg-Ca alloy 8 side and is absorbed
thereinto, as shown by the arrows in Fig. 1. As a result, the
dissolved Ca in the Ca-removing region 30 is removed therefrom
and the Ca concentration in the Mg-Ca alloy 8 increases.
[0078]
On the other hand, the molten Mg-Ca alloy in the adjacent
surface of contact with the molten salt in the Ca-concentrating
region 29 functions as a positive (+) electrode relative to
themolten salt side (- electrode side) in the Ca-concentrating
region 29. Therefore, the Ca in the molten Mg-Ca alloy 8
transfers to the molten salt side in the Ca-concentrating region
29 and the Ca concentration in the Ca-concentrating region 29
rises, namely that concentration is increased.
[0079]
CA 02645103 2008-09-08
26
In this manner, by applying a voltage below the
decomposition voltage for CaC12 so that the electrode plate
on the molten salt side in the Ca-removing region 30 may serve
as a positive (+) electrode against the electrode plate on the
molten salt side in the Ca-concentrating region 29, it becomes
possible to remove the dissolved Ca in the Ca-removing region
30 therefrom and at the same time increase the dissolved Ca
concentration in the Ca-concentrating region 29. In addition,
when an apparatus having the main structural elements shown
in Fig. l is used, these simultaneous treatments can be carried
out with ease using a very facile apparatus in terms of both
the configuration and constitution/feature.
[0080]
That the voltage to be applied should be lower than the
decomposition voltage for CaC12 is to avoid the formation of
Ca in case of the decomposition of CaC12.
As for the electrodes for the voltage application
mentioned above, it is recommended that iron or like metals
be used as the negative (-) electrode and a graphite electrode
or like insoluble electrodes as the positive (+) electrode.
[0081]
In a practical mode of operation, it is possible to control
the operation while judging the degrees of the above-mentioned
removal and concentration of the dissolved Ca, based on the
"limiting current" which is observable as a result of such Ca
transfer from the molten salt side (+ electrode side) to the
molten alloy side (- electrode side), as mentioned below, and
which can be regarded as the measure (indicator).
[0082]
Fig. 2 is a schematic representation of the relation
between applied voltage and flowing current, the voltage being
applied between molten Mg-Ca alloy and molten CaC12, the current
flowing between both electrodes, which is obtained based on
the results of an experimental investigation made by the present
inventors. Fig. 2 (a) is for the case where Ca is not dissolved
in CaC12 (before addition of Ca), and Fig. 2 (b) is for the
CA 02645103 2008-09-08
27
case where Ca is dissolved therein (after addition of Ca).
[0083]
When Ca is not dissolved, no current flows at all even
if increasing the applied voltage, as shown (cf. Fig. 2(a)).
On the contrary, after the addition of Ca, a benign electric
current begins to flow just when a slight voltage is applied
and, thereafter, upon increasing the voltage, an almost constant
current flows until the applied voltage comes close to the
decomposition voltage Vb (3.2 V) for CaC12 (such current is
called "limiting current") . When the voltage is further
increased,CaClziselectrolyzed and therefore the current value
rapidly increases (Fig. 2 (b)).
[0084]
The limiting current mentioned above is observed as a
result of the transfer of Ca from the molten salt side
(+(positive) electrode side) to the molten alloy side
(-(negative) electrode side) (i.e. absorption of the Ca,
dissolved in CaC12, into the molten alloy) , and the intensity
thereof depends on the concentration of Ca dissolved in CaC12
and the limiting current value decreases with the decrease in
Ca concentration. According to the investigation results
obtained by the present inventors, the Ca concentration was
about 0.01 % by mass when the limiting current value was 0. 14
A/cm2 .
[0085]
Therefore, it is also possible, for example, to carry
out the operation in such a manner that the voltage to be applied
be set at a level below the decomposition voltage for CaCl2
and the treatment be finished at the time that the limiting
current value, which gradually decreases, reaches a
predetermined current density, although the mode of operation
may vary depending on the intended use of the molten salt obtained
by the method of removing/concentration a metal-fog-forming
metal in a molten salt as mentioned above.
[0086]
Since the limiting current value decreases as the Ca
CA 02645103 2008-09-08
28
concentration in the molten salt decreases, as mentioned above,
it is desirable, for rapidly decreasing the Ca concentration
and thereby enhancing the Ca removal ef f iciency, that the contact
area between molten salt and molten alloy in the Ca-removing
region be broadened. Since the same amount of electric current
as that flowing in the Ca-removing region flows also in the
Ca-concentrating region, it is desirable that the
above-mentioned contact area in the Ca-concentrating region
be also widened in the same manner as above to lower the
resistance.
[0087]
The case where "the metal-fog-forming metal is Ca and
the metal-fog-forming metal containing molten salt is a
Ca-containing molten salt" and, further, the case where "the
metal-fog-forming metal is Ca and the metal-fog-forming metal
containing molten salt is CaCl2" are desirable modes of
embodiment of the process according to the present invention.
[0088]
In either of these modes of embodiment, the
metal-fog-forming metal is limited to Ca because the production
of metallic Ti by Ca reduction of TiCl4 through an intermediary
molten salt is thought to be one of the promising fields from
the viewpointof metal-fog-forming metal utilization; further,
the molten salt can also be restricted to CaClz which is
relatively inexpensive and easy to handle.
[0089]
In the process according to the present invention, Ca
can be removed while "the applied voltage is maintained at a
level lower than 3.2 V (namely, voltage below the decomposition
voltage for CaCl2) ". By this measure, it becomes possible to
control the applied voltage at specific numerical value levels
and cause Ca to be rapidly absorbed into the molten alloy by
making a potential difference between the electrode plate on
the molten salt side and the electrode plate on the molten alloy
side, without allowing decomposition of CaC12.
[0090]
CA 02645103 2008-09-08
29
On that occasion, even when the applied voltage is slight,
the Ca removing effect can be retained and, therefore, the lower
limit thereto is not particularly specified. For effective
Ca removal, however, it is preferable that the applied voltage
be not lower than 0.01 V.
[0091]
The apparatus for removing/concentrating a
metal-fog-forming metal in a molten salt according to the present
invention has such main structural elements as shown in Fig.
1 as above andmakes it possible to carry out the above-mentioned
method of removing/concentrating a metal-fog-forming metal
according to the present invention in an easy and proper manner.
[0092]
Fig. 3 is a schematic representation of an example of
the principal constitution/feature of an apparatus to be used
in carrying out the method of recovering (i.e. method of
removing) a metal-fog-forming metal in molten salt according
to the present invention. The constitution/feature shown in
Fig. 3 is the same as that of Ca recovery means 5 shown in Fig.
4 and Fig. 5, which is to be hereinafter referred to and in
which the same reference numerals are used. In Fig. 3, like
in the above-mentioned case shown in Fig. 1, the
metal-fog-forming metal is represented by Ca, so is the molten
salt mixture consisted of one or more of inetal-fog-forming metal
containing molten salts by a molten CaC12, and the
metal-fog-forming metal containing molten alloy by a molten
Mg-Ca alloy, and these designations are used in the description
which follows.
[0093]
As shown in Fig. 3, the Ca recovery means 5 comprises
a Ca recovery vessel 6 and, within this recovery vessel 6, there
is held a molten CaC12 7 and, thereupon, a molten Mg-Ca alloy
8 is held in contact with the molten salt 7. An electrode bar
9 inserted in the molten salt 7 constitutes a positive (+)
electrode and an electrode bar 10 inserted in the molten Mg-Ca
alloy 8 constitutes a negative (-) electrode.
CA 02645103 2008-09-08
[0094]
In recovering the metal-fog-forming metal Ca using the
Ca recovery means 5 shown in Fig. 3, a molten CaC12 with Ca
dissolved therein is first held in a Ca recovery vessel 6.
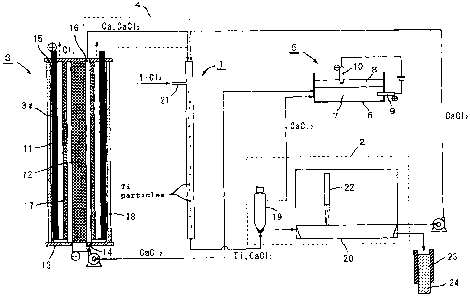
Further, the molten Mg-Ca alloy 8 is held onto the thus-held
molten salt 7 to be in contact with the molten salt 7.
[0095]
Then, a voltage below the decomposition voltage for CaC12
is applied so that the electrode bar 10 inserted in the molten
Mg-Ca alloy 8 may serve as a negative (-) electrode and the
electrode bar 9 inserted in the molten salt 7 as a positive
(+) electrode. By this voltage application, that portion of
the molten Mg-Ca alloy 8 present in the adjacent surface of
contact with the molten salt 7 within the Ca recovery vessel
6 functions as a negative (-) electrode relative to the molten
salt side (+ electrode side) within the Ca-removing vessel 6
and, therefore, the Ca dissolved therein transfers to the molten
Mg-Ca alloy 8 side, as indicated by the arrows in Fig. 3, and
is absorbed thereinto. As a result, the dissolved Ca in the
Ca-removing vessel 6 is recovered (removed).
[0096]
An application example of the method of
removing/concentrating a metal-fog-forming metal according to
the present invention as shown hereinabove in Fig. 1 is described
below under "3: Ti or Ti alloy production process including
a Ca removing/ concent rating step, and apparatus therefor". An
application example of the method of removing a
metal-fog-forming metal according to the present invention as
shown hereinabove in Fig. 3 is described below under "2: Ti
or Ti alloy production process including a Ca recovery step,
and apparatus therefor".
[0097]
2. Ti or Ti alloy production process including a Ca recovery
step, and apparatus therefor
Fig. 4 is a schematic representation of an example of
the constitution/feature of an apparatus to be used in carrying
CA 02645103 2008-09-08
31
out the Ti or Ti alloy production process including a Ca recovery
step according to the present invention. The figure shows the
case where TiC14 alone is used as the raw material.
[0098]
As shown in Fig. 4, this apparatus comprises: a reaction
vessel 1 for holding a CaC1z-containing molten salt with Ca
dissolved therein and for reacting TiC14 fed into said molten
salt with Ca to form Ti particles; a separation means 2 for
separating said Ti particles formed in the molten salt therefrom;
an electrolytic cell 3 for electrolyzing the molten salt after
separation of said Ti particles therefrom to form Ca on the
cathode side; a return means 4 for introducing the Ca formed
upon electrolysis into the reaction vessel 1; and a Ca recovery
means 5 for removing the Ca dissolved in the molten salt separated
in the separation means and to be fed to the electrolytic cell.
[0099]
The Ca recovery means 5 shown by way of example in Fig.
4 comprises its essential constituents as shown, and the molten
salt 7 separated in the above-mentioned separation means 2 is
introduced into the Ca recovery vessel 6. Thereon is held a
Ca- and Mg-containing molten alloy 8 (also referred to as "molten
Mg-Ca alloy" or as "molten alloy" for short). The electrode
bar 9 inserted in the molten salt 7 constitutes a positive (+)
electrode and the electrode bar 10 inserted in the molten Mg-Ca
alloy 8 constitutes a negative (-) electrode.
[0100]
The electrolytic cell 3 comprises a piping-like
(cylindrical) electrolytic cell vessel 3a elongated in one
direction and intended for holding a CaC12-containing molten
salt, and likewise, cylindrical anode 11 and a round
column-shaped cathode 12, each disposed within the electrolytic
cell vessel 3a along the length-wise direction of the vessel
3a. One end (bottom plate 13), in a lengthwise direction, of
the electrolytic cell vessel 3a is equipped with a molten salt
supply port 14, and the other end (cover plate 15) is equipped
with a molten salt extraction port 16. The surface of the anode
CA 02645103 2008-09-08
32
11 and the surface of the cathode 12 are disposed substantially
vertically in a facing relation with each other and, further,
a partition wall 17 is disposed between the anode 11 and cathode
12 so as to inhibit the Ca formed by electrolysis of the molten
salt from passing therethrough. A cooling device 18 is provided
surrounding the outside surface of the anode 11.
[0101]
In the apparatus shown in Fig. 4, a decanter type centrifuge
(high-temperature decanter) 19 and a separating vessel 20 are
used as a separation means 2.
[0102]
In carrying out a Ti or Ti alloy production process
according to the present invention using the apparatus shown
in Fig. 4, the molten salt fed from the electrolytic cell 3
via a return means 4 is first held in a reaction vessel 1 and
the TiClq fed through a TiC14 supply port 21 is caused to react
with the Ca in the molten salt to form Ti particles in the molten
salt. Thus, the "reduction step" is carried out.
[0103]
In this reduction step, the molten salt held in the reaction
vessel 1 is not at rest but is gradually moving downward from
the upper part of the reaction vessel 1 toward the bottom and,
during the downward movement, TiC14 as raw material is reduced
by the Ca in the molten salt to form Ti particles. In cases
where a mixed metal chloride comprising TiC14 and at least one
of other metal chlorides (e.g. chloride of V, Al, Cr, etc.)
is used as the raw material, the other metal chlorides are also
reduced by Ca, as mentioned above, and, therefore, by
preliminarily adding predetermined amounts of such metal
chlorides to TiClq, it becomes possible to formTi alloyparticles
and finally produce a Ti alloy.
[0104]
The Ti particles formed in the reduction step are separated
from the molten salt in the "separation step".
When an appropriate reaction vessel is used, the
separation of the Ti particles from the molten salt can be
CA 02645103 2008-09-08
33
realized also within the reaction vessel but, in this case,
the process is carried outbatch-wise. Therefore, forenhancing
productivity, it is recommended, for example, that the molten
salt with Ca dissolved therein be continuously fed using a
reaction vessel of the type shown in Fig. 4 and the formed Ti
particles be extracted out of the reaction vessel and separated
from the molten salt outside the vessel.
[0105]
In the separation step, when the apparatus shown in Fig.
4 is used, the Ti particles are first separated and recovered
in the high-temperature decanter 19 and then the molten salt
adhering to the Ti particles is removed in the separating vessel
20.
[0106]
The decanter type centrifuge is a centrifuge of the type
such that a suspended substance is caused to settle by
centrifugation by rotating a rotary cylinder at a high speed
and enables high-speed treatment and has high dehydration
performance. A type allowing high-temperature treatment has
also already been developed and can be used as the
high-temperature decanter 19 in this separation step.
[0107]
The Ti particles taken out of the high-temperature
decanter 19 are heated and melted by plasma emitted from a plasma
torch 22 in the separation vessel 20, and the melt is cast into
a mold 23 to give a Ti ingot 24.
[0108]
On the other hand, the molten salt separated from Ti
particles (suchmoltensalthereinafter referred to as"adherent
molten salt") may possibly contain Ti particles. Therefore,
returning of this adherent molten salt to the electrolysis step
may possibly cause problems; it is therefore desirable that
it be returned to the reaction vessel 1, as indicated in Fig.
4. In addition, a certain amount of Ca remains in the adherent
molten salt, so that it is reasonable to return it to the react.ion
vessel 1 from the viewpoint of effective Ca utilization as well.
CA 02645103 2008-09-08
34
[0109]
The molten salt reduced in Ca concentration as separated
in said high-temperature decanter 19 is sent to the "Ca recovery
step". Namely, said molten salt is introduced into the Ca
recovery vessel 6 and kept in contact with the molten Mg-Ca
alloy 8 and a voltage is applied so that the electrode bar on
the molten alloy side may serve as a negative (-) electrode
and the electrode bar on the molten salt side as a positive
(+) electrode. The applied voltage on that occasion is lower
than the decomposition voltage for CaC12. It becomes possible
thereby to cause the Ca dissolved in CaC12 to be rapidly absorbed
into the molten alloy without allowing decomposition of CaC12
and rapidly send the molten salt decreased in Ca concentration
to the electrolysis step. Since the Ca concentration in the
molten salt is reasonably lowered, the back reaction is
suppressed.
[0110]
As for the electrodes for the above-mentioned voltage
application, it is recommended that iron or a like metal be
used as the negative (-) electrode and a graphite electrode
or a like insoluble electrode as the positive (+) electrode.
[0111]
As shown in Fig. 2, the limiting current results from
the transfer of Ca from the molten salt side (+ electrode side)
to the molten alloy side (- electrode side) and the intensity
thereof depends on the concentration of Ca dissolved in CaC12,
and the limiting current decreases as the Ca concentration
decreases. According to the investigation results obtained
by the present inventors, the Ca concentration was about 0. 01 0
by mass when the limiting current density was 0.14 A/cm2.
[0112]
Since the limiting current becomes small as the Ca
concentration in the molten salt decreases, asmentioned above,
it is desirable, for rapidly decreasing the Ca concentration
and thereby enhancing the Ca removal (recovery) efficiency,
to use a large-sized Ca recovery vessel so that the contact
CA 02645103 2008-09-08
area between molten salt 7 and molten Mg-Ca alloy 8 may be
broadened.
[0113]
By removing Ca by selecting "the voltage applied at a
level lower than 3.2 V (namely, voltage below the decomposition
voltage for CaC12)" in the Ti or Ti alloy production process
according to the present invention, it becomes possible to
control the applied voltage at specific numerical value levels
and cause Ca to be rapidly absorbed into the molten alloy by
implementing a potential difference between the electrode bar
on the molten salt side and the electrode bar on the molten
alloy side, without allowing decomposition of CaCl2. Since,
even when the applied voltage is benign, the voltage application
produces a Ca-removing effect, the lower limit to the applied
voltageisnot particularlyspecified. For effective Ca removal,
however, it is desirable that the applied voltage be not lower
than 0.01 V.
[0114]
The molten salt reduced in Ca concentration in the Ca
recovery step is sent to the "electrolysis step" and electrolyzed
to form Ca, whereupon the Ca concentration in the molten salt
is increased.
[0115]
Thus, as shown in Fig. 4, the molten salt is first fed
into and held in the space between the cathode 12 and partition
wall 17 in the electrolytic cell 3. Since the electrolytic
cell 3 has a shape elongated in one direction (in the example
shown, apiping-like(cylindrical)shape elongated in a vertical
direction), it is possible to provide the molten salt in the
vicinity of the surface of the cathode 12 with a flow rate in
one direction and cause the molten salt to flow in one direction
in the vicinity of the surface of the cathode 12 by continuously
or intermittently feeding the molten salt from one end of the
electrolytic cell 3 to the space between the anode 11 and cathode
12. The feeding of the molten salt is generally carried out
continuously. Depending on the subsequent step and/or other
CA 02645103 2008-09-08
36
factors, the feeding may be carried out intermittently, namely
the feeding of the molten salt may be temporarily halted and
then resumed.
[0116]
Then, the molten salt is electrolyzed. While the molten
salt is allowed to flow in one direction in the vicinity of
the surface of the cathode 12, the molten salt is electrolyzed
to form Ca on the cathode surface. Since the electrolytic cell
3 has a shape elongated in one direction and, further, in the
example shown in Fig. 4, the distance between the anode 11 and
cathode 12 is set to be relatively short so that the electrolysis
voltage may be suppressed to a low level, it is possible to
effectively draw out only the molten salt enriched in Ca while
inhibiting the mixing of the Ca-rarified molten salt in the
vicinity of the molten salt supply port 14 with the Ca-enriched
molten salt in the vicinity of the molten salt extraction port
16 by electrolysis.
[0117]
While the extraction system employed in the electrolytic
cell shown by way of example in Fig. 4 is such that CaC12 is
fed into the electrolytic cell 3 from below the cell 3 and taken
out at the top, a converse system such that CaC12 is fed from
above the electrolytic cell 3 and extracted at the bottom can
also be employed.
[0118]
In the electric cell used in this process, the anode surface
and cathode surface are disposed substantially vertically in
a facing relation with each other while the molten salt in the
vicinity of the cathode surface is given a flow rate in one
direction and, therefore, the direction of flow of the molten
salt is vertical and the chlorine gas generated on the anode
side readily floats up to the surface and can be recovered with
ease.
[0119]
In carrying out the electrolysis of the molten salt using
this electrolytic cell, a large amount of the molten salt is
CA 02645103 2008-09-08
37
treated continuously, so that it is preferable to effectively
carry out heat removal in the electrolytic cell. More
specifically, it is desirable, forexample, that a cooling device
be disposed in the central portion of the cathode for removing
the heat of reaction from inside the cathode. A tube-type heat
exchanger, for instance, is suited for use as the cooling device.
[0120]
When a cooling device (heat exchanger) is disposed on
the anode side as well, the heat removal efficiency is further
enhanced. In the electrolytic cell shown in Fig. 4, the cooling
device 18 disposed so as to surround the anode 11 is an example
of such cooling device.
[0121]
The Ca formed upon electrolysis in the electrolysis step
is introduced, either alone or together with the molten salt,
into the reaction vessel via the "return step".
[0122]
When the apparatus shown in Fig. 4 is used, the molten
salt increased in Ca concentration in the electrolytic cell
is obtained and the Ca is introduced, together with the molten
salt, into the reaction vessel via the return step.
When, however, use is made of an electrolytic cell having
a constitution/feature such that the Ca formed upon
electrolyzing the molten salt can be recovered as such, namely
as Ca alone (including, however, the condition such that a slight
amount of the molten salt is admixed in the Ca) , it is possible
to employ, in the Ti or Ti production process according to the
present invention, a mode of embodiment such that the Ca formed
by electrolysis is introduced, while being dissolved in a molten
salt, into the reaction vessel.
[0123]
Thus, in such mode of embodiment, the molten salt is not
utilized as a transfer medium for Ca in the return step but
the Ca formed is transferred as such to a site in the vicinity
of the reaction vessel and dissolved there in a separately
prepared molten salt and then introduced into the reaction
CA 02645103 2008-09-08
38
vessel; a reduction in transfer cost can be then expected.
[0124]
It is further possible to employ a mode of embodiment
such that when a reaction vessel which makes it possible to
feed the Ca thus formed into the reaction vessel for reaction
with TiC14 is used, the Ca alone is introduced into the reaction
vessel.
[0125]
Fig. 5 is a schematic representation of another example
of the constitution/feature of an apparatus to be used in
carrying out the Ti or Ti alloy production process including
a Ca recovery step according to the present invention.
This apparatus is a modification to the apparatus shown
in Fig. 4 that is made by further providing a regulating vessel
25 for introducing the molten salt in the electrolytic cell
3 thereinto and bringing the same into contact with a Ca supply
source to render the Ca concentration in the molten salt constant
and, thereafter, feeding the resulting molten salt to the
reaction vessel 1.
[0126]
The production process shown in Fig. 5 is a modification
of the Ti or Ti alloy production process according to the present
invention and includes the step of "introducing the molten salt
increased in Ca concentration in the electrolysis step into
a regulating vessel provided with a Ca supply source and bringing
the molten salt with the Ca supply source to thereby render
the Ca concentration in the molten salt constant and feeding
the resulting molten salt to the reduction step".
[0127]
By using the apparatus shown in Fig. 5, it becomes possible
to introduce the Ca-enriched molten salt taken out of the
electrolytic cell 3 into the regulating vessel 25 and brining
the same into contact with the Ca supply source 26 to render
the Ca concentration in the molten salt 27 constant and then
feed the resulting molten salt into the reaction vessel 1. The
process is thus a modification in which the treatment in the
CA 02645103 2008-09-08
39
regulating vessel 25 is incorporated in the return step.
[0128]
The Ca concentration in the molten salt enriched in Ca
in the electrolysis step varies with certain changes in
electrolysis conditions in the electrolyticcell3. Therefore,
when the molten salt subjected to electrolysis treatment in
the electrolytic cell 3 is directly introduced into the reaction
vessel 1, the Ca concentration is not always maintained at a
constant level and, therefore, the formation of titanium
subchlorides and decreases in current efficiency due to the
back reaction, among others, may occur, as mentioned hereinabove,
and, in some instances, the TiCl4reduction reaction efficiency
may be lowered and/or the operation may become difficult to
carry out stably.
[0129]
Therefore, the molten salt increased in Ca concentration
by using the electrolytic cell 3 in the electrolysis step is
introduced into the regulating vessel 25 provided with a Ca
supply source 26 and brought into contact with the Ca supply
source 26 and thereby rendered constant in Ca concentration;
the resulting molten salt can be used for reducing TiC14 in
the reduction step.
[0130]
The flow rate of the adherent molten salt separated from
Ti particles in the separation vessel 20 is very low as compared
with the flow rate of the molten salt introduced from the
electrolytic cell 3 into the reaction vessel 1 via the regulating
vessel 25, so that the adherent molten salt may be returned
directly to the reaction vessel 1, as mentioned above. It is
preferable, however, to once introduce it into the regulating
vessel 25 and, after rendering the Ca concentration constant,
introduce the same into the reaction vessel 1, as shown in Fig.
3.
[0131]
Usable as the Ca supply source 26 are molten metallic
Ca and molten alloys containing Ca at relatively high content
CA 02645103 2008-09-08
levels, such as molten Mg-Ca alloy.
Thus, molten metallic Ca or a molten Mg-Ca alloy, for
instance, is caused to float on the molten salt 27 increased
in Ca concentration and introduced into the regulating vessel
25 and such Ca supply source 26 and the molten salt 27 are kept
in contact with each other. By doing so, if the Ca concentration
in the molten salt 27 is lower than the saturation solubility
thereof, Ca is supplied from the Ca supply source 26 to the
molten salt 27 and, in this manner, the Ca concentration can
be maintained at a level in the vicinity of the saturation
solubility.
[0132]
In case the Ca concentration in the molten salt 27 is
at its saturation solubility and the precipitated metallic Ca
coexist therein, the metallic Ca floats up to the surface and
is separated in the regulating vessel 25 owing to the specific
gravity difference and, thus, the Ca concentration can be
maintained at a level in the vicinity of the saturation
solubility. Furthermore, by controlling the temperature of
the molten salt 27 on the occasion of extraction from the
regulating vessel 25 to a constant level, it becomes possible
to control the Ca concentration at a constant level in the
vicinity of the saturation solubility at that temperature.
[0133]
Therefore, by providing the regulating vessel 25 and
introducing thereinto the molten salt taken out of the
electrolytic cell 3, irrespective of whether the Ca
concentration in the molten salt enriched in Ca in the
electrolytic cell 3 is at or below the saturation solubility,
it becomes possible to feed the molten salt whose Ca
concentration is at a constant level in the vicinity of the
saturation solubility thereof to the reaction vessel 1 and allow
the TiCl4reduction reaction to proceed efficiently and, thus,
carry out the operation stably.
[0134]
If, however, the electrolysis in the electrolytic cell
CA 02645103 2008-09-08
41
3 is carried out to an extent such that the Ca concentration
exceeds the saturation solubility, metallic Ca may precipitate
out within the electrolytic cell 3, possibly causing such a
trouble as electrolytic cell obstruction. Therefore, in
increasing the Ca concentration in the electrolytic cell 3,
it is preferable to carry out the operation in a manner such
that the electrolysis is carried out under control so that the
Ca concentration may be reasonably high but just short of the
saturation solubility, and the molten salt high in Ca
concentration but lower than the saturation solubility is
introduced into the regulating vessel25and brought into contact
with the Ca supply source 26 to thereby adjust the Ca
concentration to a constant level in the vicinity of the
saturation solubility.
[0135]
The production process according to the present invention
is preferably one in which the Ca supply source in the regulating
vessel shown in Fig. 5 is specified in manner such that "the
molten alloy increased in Ca concentration as a result of
absorption of Ca in the Ca recovery step is used as the whole
Ca supply source or part of it in the regulating vessel".
[0136]
Thus, as shown in Fig. 5, the molten alloy 8 increased
in Ca concentration as a result of absorption of Ca in the Ca
recovery step (a Ca recovery means 5) is transferred to the
regulating vessel 25 for use as the Ca supply source 26. The
whole Ca supply source 26 may be covered by the molten alloy
transferred from the Ca recovery step or the molten alloy may
be used as part of the Ca supply source 26 when insufficient
in quantity. In either case, the Ca removed from the molten
salt separated in the high-temperature decanter 19 and to be
sent to the electrolysis step so as to suppress the back reaction
can be utilized efficiently.
[0137]
The Ti or Ti alloy production apparatus according to the
present invention is an apparatus to be used in carrying out
CA 02645103 2008-09-08
42
the Ti or Ti alloy production process including such a Ca recovery
step as mentioned above and the constitution/feature thereof
is as schematically shown in Fig. 4. The functions of the
respective structural elements are the same as mentioned above
and, when this apparatus is used, the Ti or Ti alloy production
process (including the mode of embodiment la) according to the
present invention can be properly carried out.
[0138]
3. Process for producing Ti or a Ti alloy which includes
a Ca removing/concentrating step, and apparatus therefor
Fig. 6 is a schematic representation of the
constitution/feature of an apparatus to be used in carrying
out the Ti or Ti alloy production process including a Ca
removing/concentratingstep according to the present invention.
Here, too, the case of using TiCl4 alone as raw material is
described.
[0139]
This apparatus is a modification to the apparatus shown
in Fig. 4 that is made by providing a Ca removing/concentrating
apparatus instead of the Ca recovery means and changing the
transfer route of the molten salt accordingly.
Thus, as shown in Fig. 6, the apparatus comprises: a
reaction vessel 1 for holding a CaC12-containing molten salt
with Ca dissolved therein and allowing TiC14 fed into the molten
salt to react with the Ca to form Ti particles; a separation
means 2 for separating the Ti particles formed in the molten
salt from the molten salt; an electrolytic cell 3 for
electrolyzing the molten salt after separation of the Ti
particles to form Ca on the cathode side; a return means 4 for
introducing the Ca formed by electrolysis into the reaction
vessel 1; and a Ca removing/concentrating unit 28 for removing
the Ca dissolved in the molten salt that is separated by the
separation means (high-temperature decanter) and is to be fed
to the electrolytic cell 3, and for simultaneously increasing
the concentration of Ca dissolved in the molten salt separated
by a separation means (a separating vessel) and to be introduced
CA 02645103 2008-09-08
43
into the reaction vessel 1.
[0140]
The Ca removing/concentrating unit 28 whose main parts
are shown in the figure comprises a Ca removing/concentrating
vessel 28a, wherein a molten CaC12 is held in the vessel 28a
in a condition separated into a Ca-concentrating region 29 and
a Ca-removing region 30 by a partition wall 31 and, thereon,
a molten Mg-Ca alloy 8 is held in contact with either portion
of the molten salt that are present in the Ca-concentrating
region 29 and Ca-removing region 30.
[0141]
Further, at the bottom of the Ca-removing region 30, there
is provided an electrode plate 33 for applying a voltage below
the decomposition voltage for CaC12 so that it may serve as
a positive (+) electrode against an electrode plate 34 on the
molten salt side in the Ca-concentrating region 29. Although,
in the example shown, the Ca-concentrating region 29 and
Ca-removing region 30 are separated from each other by the
partition wall 31, the constitution/feature thereof is not
limited to this. Thus, for example, both regions may be
separated from each other by use of two independent, detachable
vessels.
[0142]
In carrying out the Ti or Ti alloy production process
described above under (2) using the apparatus shown in Fig.
6, the procedures in the "reduction step", "separation step",
"electrolysis step" and "return step" are fundamentally the
same as in the above-mentioned case of using the apparatus shown
in Fig. 4. In the same manner as in that case, a Ti alloy can
be produced as a final product when TiC14-based mixed metal
chlorides are used as the raw material.
[0143]
The difference from the use of the apparatus shown in
Fig. 4 lies in the destination of transfer of the molten salt
separated from Ti particles in the separation step and the
treatment at that destination. Thus, the molten salt decreased
CA 02645103 2008-09-08
44
in Ca concentration as being separated in the high-temperature
decanter 19 is sent to the Ca-removing region 30 in the Ca
removing/concentrating vessel 28a provided in the Ca
removing/concentrating unit 28 via Route La, as shown in Fig.
6, while the adherent molten salt separated from Ti particles
in the separation vessel 22 is sent to the Ca-concentrating
region 29 in the Ca removing/concentrating vessel 28a via Route
Lb.
[0144]
Here, a voltage below the decomposition voltage for CaC12
is applied via the electrode plate 33 and electrode plate 34
so that the electrode plate disposed on the molten salt side
in the Ca-removing region 30 may serve as a positive (+) electrode
against the electrode plate 34 disposed on the molten salt side
in the Ca-concentrating region 29.
[0145]
This voltage application causes a molten Mg-Ca alloy 32
existing in the vicinity of the contact surface with the molten
salt in the Ca-removing region 30 to function as a negative
(-) electrode relative to the molten salt side (+ electrode
side) in the Ca-removing region 30, so that the dissolved Ca
transfers to the molten Mg-Ca alloy 32 side, as indicated by
the arrows given in the Ca removing/concentrating vessel 28a
in Fig. 6, and absorbed into the alloy. As a result, the
dissolved Ca in the Ca-removing region 30 is removed and the
Ca concentration in the Mg-Ca alloy 32 increases.
[0146]
On the other hand, the molten Mg-Ca alloy 32 in the vicinity
of the contact surface with the molten salt in the
Ca-concentrating region29functionsasa positive (+) electrode
relative to the molten salt side (- electrode side) in the
Ca-concentrating region 29. Therefore, the Ca in the molten
Mg-Ca alloy 32 transfers to the molten salt side in the
Ca-concentrating region 29, so that the Ca concentration in
the Ca-concentrating region 29 increases.
[0147]
CA 02645103 2008-09-08
In this way, by applying a voltage below the decomposition
voltage for CaC12 to the electrode plate 33 in the Ca
removing/concentrating vessel 28a, it becomes possible to
remove the dissolved Ca in the Ca-removing region 30 and, at
the same time, increase the dissolved Ca concentration in the
Ca-concentrating region 29. In addition, by using the Ca
removing/concentrating unit 28 whose principal
constitution/feature is shown in Fig. 6, it becomes possible
to carry out these simultaneous treatments with ease using such
an apparatus that is very facile in configuration and
constitution/feature.
[0148]
That the voltage to be applied is selected at a level
lower than the decomposition voltage for CaC12 is to avoid the
formation of Ca in case of decomposition of CaC12.
As for the electrodes for the voltage application
mentioned above, it is recommended that iron or a like metal
be used as the negative (-) electrode and a graphite electrode
or a like insoluble electrode as the positive (+) electrode,
like in the case of the electrode bars to be mounted on the
above-mentioned Ca recovery vessel 6 (shown in Fig. 4 and Fig.
5).
[0149]
In the Ca removing/concentrating unit 28, the Ca removal
and concentration treatments are carried out simultaneously
in the manner mentioned above, and the Ca dissolved in the molten
salt in the Ca-removing region 30 is removed and the Ca
concentration in the molten salt in the Ca-concentrating region
29 increases.
[0150]
Route Lc disposed between Route La and Route Lb is the
route for taking a balance of the amounts of the portion of
the molten salt in the Ca-removing region 30 and those in the
Ca-concentrating region 29. Thus, since the amounts of the
molten salt separated in the high-temperature decanter 19 is
overwhelmingly larger than those of the adherent molten salt
CA 02645103 2008-09-08
46
separated in the separation vessel 22, Route La and Route Lb
themselves are not able to take a balance of the amounts of
the molten salt in the Ca-removing region 30 and those in the
Ca-concentrating region 29, with a result that it is not possible
anymore to continuously carry out the Ca removal and
concentration treatments in the Ca removing/concentrating unit
28. Therefore, part of the molten salt separated in the
high-temperature decanter 19 is sent, via Route Lc, to the
Ca-concentrating region 29 so that the treatments mentioned
above may be carried out continuously.
[0151]
The molten salt deprived of Ca in the Ca
removing/concentrating unit 28 is sent to the "electrolysis
step". Since that molten salt has been deprived of Ca, a
so-called back reaction, namely the reaction of Ca in the molten
salt with chlorine formed by electrolysis, is suppressed and
the Ca formation by electrolysis can be executed efficiently.
[0152]
The molten salt in the Ca-concentrating region 29 is
returned to the reduction step. Since the remaining Ca in the
adherent molten salt has been concentrated and has an increased
Ca concentration, it is effective in enhancing the efficiency
of the TiC14 reduction reaction.
[0153]
The Ca formed by electrolysis in the electrolysis step
is introduced, either alone or together with the molten salt,
into the reaction vessel via the "return step".
[0154]
In the Ti or Ti alloy production process according to
the present invention, it is desirable that, in the Ca
removing/concentrating unit 28, the dissolved Ca in the
Ca-removing region 30 be removed and, at the same time, the
concentration of the dissolved Ca in the Ca-concentrating region
29 be increased at an "applied voltage lower than 3.2 V (namely,
voltage below the decomposition voltage for CaC12) ". The lower
limit to the applied voltage is not particularly specified.
CA 02645103 2008-09-08
47
For effective Ca removal, however, it is preferable that the
applied voltage be not lower than 0.01 V.
[0155]
Fig. 7 is a schematic representation of another example
of the constitution/feature of an apparatus to be used in
carrying out the Ti or Ti alloy production process including
a Ca removing/concentrating step according to the present
invention.
This apparatus is a modification to the above-mentioned
apparatus shown in Fig. 6 that is made by further providing
a regulating vessel 25 for introducing thereinto the molten
salt in the electrolytic cell 3 and bringing the same into contact
with a Ca supply source to thereby render the Ca concentration
in that molten salt constant, and for thereafter feeding the
resulting molten salt into the reaction vessel 1.
[0156]
The Ti or Ti alloy production process according to the
present invention is desirably one in which "the molten salt
increased in Ca concentration in the electrolysis step is
introduced into a regulating vessel provided with a Ca supply
source and the molten salt is brought into contact with the
Ca supply source to render the Ca concentration in the molten
salt constant, and thereafter, the resulting molten salt is
sent to the reduction step".
[0157]
The Ti or Ti alloy production apparatus according to the
present invention is an apparatus to be used in carrying out
the above-mentioned Ti or Ti alloy production process including
such a Ca removing/concentrating step, and the
constitution/feature thereof and the functions of the
respective structural elements are as schematically shown in
Fig. 6. When this apparatus is used, the Ti or Ti alloy
production process including such a Ca removing/concentrating
step according to the present invention can be properly carried
out.
[0158]
CA 02645103 2008-09-08
48
In carrying out the above-mentioned "2: Ti or Ti alloy
production process including a Ca recovery step" and "3: Ti
or Ti alloy production process including a Ca
removing/concentrating step", it is possible to employ a mode
of embodiment such that a chlorination step is added and the
formed TiCl4 is used as a raw material for the Ti formation
reaction within the reaction vessel.
[0159]
More specifically, chlorine (C12) is generated as
byproduct on the anode side upon electrolysis of the molten
salt in the above-mentioned electrolysis step, and this C12r
when allowed to react with titanium oxide (Ti02), gives TiC19.
Therefore, the C12 formed on the anode side with the progress
of electrolysis of the molten salt is caused to react with a
titanium ore to form TiC14 and this TiCl4, after purification
by distillation, is used as the raw material in the production
of Ti or a Ti alloy.
[0160]
In the case of Ti alloy production, the Clz formed on
the anode side is caused to react with a mixture of Ti02 and
metal oxide (s) because at least one metal is added as an alloy
element, to give a metal chloride mixture including TiC14, which
can be used as the raw material.
[0161]
By employing such a mode of embodiment, it becomes possible
to effectively utilize the by-product C12 obtained in the
electrolysis of the molten salt and recycle the ClZ in the
production process.
INDUSTRIAL APPLICABILITY
[0162]
The method of removing/concentratingametal-fog-forming
metal in a molten salt according to the present invention can
remove the metal-fog-forming metal dissolved in a molten salt
mixture consisted of one or more of metal-fog-forming metal
containing molten salts from one portion of the molten salt
CA 02645103 2008-09-08
49
mixture and transfer the same to the other portion of the molten
salt mixture for increasing the concentration thereof in that
mixture. This method can be carried out easily and properly
using the apparatus according to the present invention.
[0163]
Therefore, the method of removing/concentrating a
metal-fog-forming metal in a molten salt and the apparatus
therefor can be expected to be utilized as one of means for
treating a molten salt in various industrial fields in which
metal-fog-forming metal containing molten salts, the
metal-fog-forming metal being such as Ca or Na, are handled.
In particular,they can be effectively utilized in the production
of Ti by Ca reduction.
[0164]
The Ti or Ti alloy production process according to the
present invention makes it possible to remove (recovery) the
Ca dissolved in the molten salt to be fed to the electrolytic
cell and thereby aim at enhancing the Ca formation efficiency
in electrolyzing the molten salt. Further, the process can
contribute not only to increase the Ca concentration in the
molten salt to be fed to the reaction vessel, simultaneously
with the Ca removal (recovery), while allowing to enhance the
efficiency of the Ca formation, but also to enhance the
efficiency of the TiClq reduction reaction and, when a regulating
vessel is used, the process can make it possible to inhibit
the fluctuation of and maintain at a high level the Ca
concentration in the molten salt to be fed into the reaction
vessel. Furthermore, the process makes it possible to
continuously treat a large quantity of a CaC12-containing molten
salt and increase the feeding rate of Ca to the reaction vessel
and thereby it becomes possible to efficiently carry out the
Ca formation in electrolysis of the molten salt and the reduction
of TiC14 and carry out the operation stably on a commercial
scale.
[0165]
Theref ore, the Ti or Ti alloy production process according
CA 02645103 2008-09-08
to the present invention and the production apparatus according
to the present invention which makes it possible to carry out
that process easily and properly can be effectively utilized
in the production of Ti or a Ti alloy by reduction with Ca.