Note: Descriptions are shown in the official language in which they were submitted.
CA 02645161 2008-09-09
WO 2007/106709 PCT/US2007/063555
1
METHOD FOR ELECTROLYTIC PRODUCTION AND REFINING OF METALS
Field of Invention
The present invention relates to a method for electrolylic production and
refining of metals having a high melting point at above about 1000 C,
particularly silicon.,
Background Technology
There is a growing demand for high purity metals, particularly high purity
silicon of solar grade and electronic grade. Solar grade. Silicon feedstock
for
solar cells has been based on scrap/rejects from electronic grade silicon from
the semiconductor industry. Electronic grade silicon is produced by
production of s.ilane from metallurgical silicon and gas phase reduction of
silane to silicon. This process is, however, very costly. In addition the
availability of scraplrejects from the semiconductor industry is now loo small
to supply the fast growing solar cell market.
From f_IS patent No. 3,219,561 it is known a method for producing refined
silicon and germanium by passing a direct current between an anode in
contact with a molten salt bath containing a fluoride and an oxide of silicon
or
germanium and a cathode in contact with another molten salt ba[h containing
of fluoride where the baths are separated by a molten alloy of silicon or
germanium and another metal to reduce the oxide of silicon or germanium to
silicon or germanium and deposit it on the cathode. In this electrolylic
process
the silicon or germanium are deposited as a solid on the cathode. The solid
metal has to be removed from the cathode and has to be crushed and treated
by acids in order to remove irnprrri[ies trapped in the metal deposited on the
cathode.
SUBSTITUTE SHEET (RULE 26)
CA 02645161 2008-09-09
2
WO 2007/106709 PCT/US2007/063555
In US patent No. 3,254,010 there is disclosed another method for refining
impure silicon or germanium where a current is passed between a cathode
and an anode through a molten salt electrolyte containing a fluoride, where
the anode is made from impure silicon or germanium or alloys of impure
silicon or germanium with more noble metals than silicon or germanium to
deport on the cathode refined silicon or germanium. Also in this process solid
refined silicon or solid refined germanium are deposited on the cathode. The
electrolyte is preferably cryolite. The process of US patent No. 3,254,010
thus
io has the same drawbacks as the method of US patent No. 3,219,561.
Finally, for metals having relatively low melting points such as aluminium,
electrolytic refining is a conventional process and is described in US patent
No.1,534,318. In this patent it is described a process for electrolytic
refining
of aluminium where there is established a lower layer of molten metal
containing aluminium as an anode, an upper layer or of molten aluminium as
cathode and an intermediate layer of molten electrolyte of a greater density
than the molten aluminium, which electrolyte is essentially fluorides and
substantially free from chloride. Current is passed from the anode metal
through the electrolyte to the aluminium cathode where aluminium is removed
from the anode metal and deposited in the molten state at the cathode. The
molten electrolyte contains aluminium and sodium fluorides and between 20
and 60% of fluoride of an alkali earth metal having an atomic weight greater
than 80, for example barium fluoride.
The above process disclosed in US patent No. 1534318 can, however, not be
used for electrolytic refining of metals having a high melting point of above
1000 C since a lot of fluoride vapour would form in the fluoride-based molten
electrolyte at such high temperatures destroying the properties of the
electrolyte.
There is therefore a need for a process whereby high purity, high melting
.o metals such as silicon can be refined by an electrolytic refining process.
CA 02645161 2008-09-09
3
WO 2007/106709 PCT/US2007/063555
Description of the Invention
It is an object of the present invention to provide an electrolytic method for
production and refining of metals of high melting points above the about
1000 C, particularly silicon, where the refined metal is in molten state.
The present invention thus relates to an electrolytic method for production
and
of refining of metals having a melting point above about 1000 C, particularly
silicon, said method being characterized in that it:
(a) provides to a first electrolytic cell, an upper molten electrolyte layer
1o comprising a first oxide-based electrolyte containing an oxide of the metal
to
be refined, wherein the first electrolyte is in a molten state and has a
melting
point below the operating temperature of the process, an anode positioned in
the upper molten electrolytic layer, and a lower molten alloy layer comprising
an alloy of the metal to be refined and at least one metal more noble than the
is metal to be refined, said alloy constituting a cathode in the first
electrolytic
cell, said first electrolyte having a density less than the density of the
alloy;
(b) adds a raw material to said upper molten electrolyte layer, the raw
material comprising a metal oxide of the metal to be refined;
(c) passes a direct current through the anode to the cathode for reducing
20 the metal oxide to produce an alloy having a higher concentration of the
metal
to be refined;
(d) transfers the alloy of the lower molten alloy layer of the first
electrolytic
cell to a second electrolytic cell so as to provide a lower molten alloy layer
comprising the alloy to a second electrolytic cell, said alloy constituting an
25 anode in the second electrolytic cell;
(e) provides to the second electrolytic cell an upper molten metal layer
comprising a metal of the same metal as the metal to be refined, said upper
molten metal layer constituting a cathode, and an intermediate molten
electrolyte layer comprising a second oxide-based electrolyte containing an
30 oxide of the metal to be refined, where the second electrolyte is in a
molten
CA 02645161 2008-09-09
4
WO 2007/106709 PCT/US2007/063555
state and has a melting point below the operating temperature of the process,
said second electrolyte having a density between the density of the upper
molten metal layer and lower molten alloy layer; and
(f) passes a direct electric current through the anode to the cathode of the
second electrolytic cell whereby the metal to be refined moves from the anode
alloy to the upper molten metal layer.
Using the two electrolytic cells of the present invention, the first cell
produces
an alloy from the raw material and the second cell refines the alloy to
produce
a metal.
j o In the first cell, direct current passes through the anode, the first
electrolyte
and the cathode alloy to produce an alloy having a higher concentration of the
metal to be refined in the alloy layer from the raw material.
In the second cell, direct current passes through the anode alloy, the second
electrolyte and the metal to refine the alloy to the metal.
The two cells can also be operated independent of one another. Thus, the
method of the present invention can be defined as a two-step process. The
first step is producing an alloy from raw material in one electrolytic cell;
and
the second step is refining an alloy to make a metal.
The alloy is preferably transferred from the first electrolytic cell to the
second
no electrolytic cell in fluid state, but the alloy may also be tapped from the
first
electrolytic cell, solidified and supplied to the second electrolytic cell in
solid
state.
Broadly, the method to electrolytically produce an alloy comprising a first
and
second metal, in accordance with the present invention characterized in that
it:
(a) provides to a first electrolytic cell, an upper molten electrolyte layer
comprising a first oxide-based electrolyte containing an oxide of the first
metal
wherein the first electrolyte is in a molten state and has a melting point
below
the operating temperature of the process, an anode positioned in the upper
CA 02645161 2008-09-09
WO 2007/106709 PCT/US2007/063555
molten electrolytic layer, and a lower molten alloy layer comprising an alloy
of
the first metal and the second metal, wherein the second metal is more noble
than the first metal, said alloy constituting a cathode in the first
electrolytic cell,
said first electrol~rfre having a density less than the density of the alloy;
s (b) adds a raw material to said upper molten electrolyte layer, the raw
material comprising a metal oxide of the first metal; and
(c) passes a direct current from the anode to the cathode alloy to in the
first electrolytic cell to produce an alloy having a higher concentration of
the
first metal.
in The raw material is any conventional source of metal oxide containing the
metal to be refined, or the first metal, for example, quartz for silicon or
rutile
for titanium.
The refining method of the present invention can use alloy made from a
different process than the first step of the present invention.
is Broadly, the method to electrolytically refine the alloy to the metal in
accordance with the present invention is characterized in that it:
(a) provides to a second electrolytic cell an upper molten metal layer
comprising a metal of the same metal as the metal to be refined, said upper
molten metal layer constituting a cathode, a lower molten alloy layer
20 comprising an alloy of the metal to be refined and at least one metal more
noble than the metal to be refined said lower layer constituting an anode, and
an intermediate molten electrolyte layer comprising a second oxide-based
electrolyte containing an oxide of the metal to be refined where the second
electrolyte is in molten state and has a melting point below the operating
25 temperature of the process, said second electrolyte having a density
between
the density of the upper molten metal layer and lower molten alloy layer; and
(b) passes a direct electric current from the anode alloy through the
second electrolyte to the cathode whereby the metal to be refined is moved
from the alloy and deposited in molten state at the cathode.
CA 02645161 2008-09-09
6
WO 2007/106709 PCT/US2007/063555
The metal to be produced and refined is, in addition to silicon, titanium and
scandium.
In the refining process, both the alloy as well as a less pure metal of the
metal
to be refined can be added to the alloy layer. For example, metallurgical
s grade silicon can be added to the alloy layer, thereby becoming refined.
One of the unique aspects of the present invention is that a variety of raw
material can be used in the first cell. Normal carbothermic production of
metal puts constraints on the type of raw material used and introduces into
the
metal impurities especially through the carbon source. Any particulate form of
to raw material can be added to the first cell and the impurities from the
carbon
source are eliminated since no carbon source is necessary. This means that
the alloy can be purer than conventional alloys and assists in the refining
process of the present invention.
As noted, the alloy used in the refining need not be the alloy made in
15 accordance with the present invention.
When the process is first started, the alloy layer can comprise an alloy of
the
metal to be refined and a metal or metals more noble than the metal to be
refined, called the second metal, or the second metal, alone. During the
running of the process, the alloy itself will form as the metal to be refined
or
20 the first metal moves into the alloy layer.
The lower molten alloy layer comprising the alloy of the metal to be refined
or
the first metal and at least one metal more noble than the metal to be refined
or the second metal must have a composition that meets the following
requirements:
2s - a density greater than the density of the molten first and second
electrolyte; and
- a melting point close to or below the melting point of the metal to be
refined, so that it is molten and can flow at the operating temperatures of
the method of the invention.
CA 02645161 2008-09-09
7
WO 2007/106709 PCT/US2007/063555
Particularly for the refining of silicon the lower molten alloy layer may for
example consist of Si-Cu alloy, FeSi alloy or Cu-Fe-Si alloy. These alloys
have melting points well below the melting point of silicon and accordingly
also below the melting temperature of the first and second electrolyte.
The first oxide-based electrolyte must have a composition that meets the
following requirements:
must have a density at the operating temperature, which is less than the
density of the lower alloy layer of the alloy containing the metal to be
refined;
io - must have a melting point below the operating temperature;
- must have solubility for ions of the metal to be refined;
- the main constituents of the oxide-base electrolyte must be less noble than
the metal to be refined; and
- must contain an oxide of the metal to be refined, for example, Si02 for
silicon.
The second oxide-based electrolyte must have a composition that meets the
requirements of the first oxide-based electrolyte, and it must have a density
at
the operating temperature which is greater than the density of the metal to be
refined.
The oxide-based electrolytes further have the advantages that oxides are non-
toxic and have low vapour pressures. Another advantage is that used oxide-
based electrolytes are non-toxic and do not have to be deposited as special
waste. The non-toxic nature of the electrolytes is true except for those which
contain barium oxide, because barium oxide is considered toxic.
For the present invention, and especially for silicon, the following oxide
based
electrolytes are suitable:
CA 02645161 2008-09-09
WO 2007/106709 8 PCT/US2007/063555
CaO-SiO2, preferably containing 40-75 wt % Si02
- CaO-MgO-SiO2 having a MgO content of up to 40%
- CaO-A1203-SiO2 having a A1203 content up to 50%
- Al203-CaO-SiO2-TiO2
s - BaO-S102, preferably containing 25-60 wt % Si02
- BaO-TiO2- Si02, preferably about 10-50 wt % BaO, about 10-50 wt % Ti02
and about 10-50 wt % Si02
- CaO-TiO2-Si02, preferably about 10-50 wt % CaO, about 10-50 wt % Ti02
and about 10-50 wt % Si02
1 o - MgO-TiO2-SiO2, preferably about 10-50 wt % MgO, about 10-50 wt % Ti02
and about 10-50 wt % Si02
- A1203-CaO-MgO-SiO2, and
- CaO-MgO-SiO2-TiO2
In addition halides, particularly alkali and alkaline earth fluorides, may be
15 added to the oxide-based electrolytes in order to modify the viscosity,
density,
melting point and electric conductivity of the electrolytes. The amount of
halides added to the oxide-based electrolytes is preferably below 20 wt % and
more preferably below 7 wt %.
Particularly for silicon, the oxide-based electrolytes should have a density
ao above about 2.57 g/cm3 which is the density of molten silicon at the
melting
point of silicon, and below about 3.37 g/cm3 if 75% FeSi is used as alloy and
below about 5.5 g/cm3 if 50% FeSi is used as alloy. For silicon the oxide-
based electrolytes must have a melting point close to or below the melting
point of silicon which is 1414 C.
CA 02645161 2008-09-09
9
WO 2007/106709 PCT/US2007/063555
A particular suitable oxide-based electrolyte for silicon is a CaO-Si02
electrolyte containing 40-75% Si02. This electrolyte has a density of between
about 2.5 g/cm3 and about 2.7 g/cm3 and has a high solubility of Si-ions, low
solubility of Si and low volatility at an operating temperature above the
melting
point of silicon.
The first and second electrolyte can have the same composition or they can
be different. The second electrolyte must have a density in the molten state
such that it forms the intermediate molten electrolyte layer and positions
itself
between the upper molten metal layer and the lower molten alloy layer. The
io first electrolyte is not so constrained. The first electrolyte must have a
density
in the molten state such that it floats on top of the lower molten alloy
layer, i.e.
has a density less than the molten alloy. However, the first electrolyte need
not have a density in the molten state that is greater than metal in the
molten
state.
is Either the production of the alloy or the refining method of the present
invention can be performed in suitable conventional vessels that have a heat
resistant refractory lining such as alumina, magnesia silicon nitride, silicon
carbide or graphite. The side walls of the vessel may favourably be provided
with conventional cooling systems, such as evaporation cooled elements in
20 order to create a freeze lining on the inside of the side walls of the
vessels.
In the present invention, when the method entails simultaneously producing
and refining where separate vessels are employed, they may be in fluid
communication with each other, such as through a pipe in the side wall of both
vessels. The port for the pipe in both side walls must be positioned below the
25 level of the bottom molten alloy layer, in other words, the top of the
molten
alloy layer should be above the level of the ports for the pipe which provides
fluid communication between the vessels. In such an arrangement, one
vessel acts as the first electrolytic cell to produce the alloy and the other
vessel acts as the second electrolytic cell for refining.
CA 02645161 2011-04-29
Preferably, a single vessel is used for simultaneously making the alloy and
refining the metal, wherein the vessel has been divided into the first
electrolytic
cell and the second electrolytic cell and the two cells are in fluid
communication
with each other through the alloy layer. Such an arrangement is shown in U.S.
5 Patent No. 3,219,561, issued November 23, 1965.
In the arrangements for the simultaneous making of the alloy and refining the
metal, the two electrolytes are separate from each other and do not
contaminate each other.
In either arrangement, the anodes and the cathodes are connected to a direct
current source in a conventional way in order to supply direct current for the
method.
When direct current is passed through the electrolytic cell or cells, the
metal to
be refined, for example, silicon in the alloy enters the second oxide-based
electrolyte together with ions of any impurities in the alloy that is
electrochemically less noble than silicon. Since silicon is the noblest
element of
the second electrolyte, silicon ions will be reduced at the cathode and will
form
molten pure silicon, which is collected in the molten silicon cathode. Thus
impurities more noble than silicon are trapped in the alloy layer while
impurities
less noble than silicon are trapped in the second electrolyte.
The refining method of the present invention can be carried out both as a
batch
process and as a continuous process.
When the refining method is carried out as a batch process, alloy is added to
the alloy layer continuously or intermittently. Eventually the electrolytes
and the
alloy will become too high in impurities. The process is then stopped and the
electrolytes and the remaining part of the alloy are removed form the cell.
New
alloy and new oxide-based electrolytes are added together with a start cathode
of the metal to be refined, whereafter electric current is again passed
through
the electrolytic cell.
CA 02645161 2008-09-09
11
WO 2007/106709 PCT/US2007/063555
When the two separate cells are used, a first for production of the alloy and
a
second for refining, the alloy from the second cell which is depleted of the
metal to be refined, is intermittently tapped and added to the first
electrolytic
cell.
s When the refining method of the present invention is carried out as a
continuous process, there are arranged means for continuous or intermittent
supply of alloy, means for continuous or intermittent removal of oxide-based
electrolytes and means for continuous or intermittent supply of fresh oxide-
based electrolytes. Finally there are arranged means for continuous or
1o intermittent tapping of refined metal from the upper molten metal layer.
The
reason for removal of alloy is that the alloy will, during electrolysis get an
increased content of impurity elements more noble than the metal to be
refined. Also, during electrolysis the electrolytes will get an increased
content
of elements less noble than the metal to be refined, and to reduce this
content
is of impurity elements, part of the electrolytes are removed and may after
purification be returned to the electrolyte layers in the cell or be
deposited.
In a similar manner, the method for both making the alloy and refining the
metal can be carried out as either a batch or a continuous process.
By the present invention it is thus provided a simple cost effective method
for
?o obtaining a pure form of metals, especially, silicon. Low cost alloys of
the
metal to be refined and a metal more noble than the metal to be refined can
be used as the alloy. For silicon, silicon alloys such as FeSi alloys and Cu-
Si
alloys can be used as alloy. Such alloys can be produced in accordance with
the present invention or in any conventional manner using any conventional
zs means.
Short description of the drawings
Figure 1 shows a schematic view of [he refining method according to the
invention;
Figure 2 shows a schematic view of the method for making the alloy and
30 refining the metal according to the invention; and
CA 02645161 2008-09-09
12
WO 2007/106709 PCT/US2007/063555
Figure 3 shows a schematic of a method for producing the alloy.
Detailed description of the Invention
In figure 1 there it is shown a schematic view of an electrolytic cell for
carrying
out the method of the present invention for refining of silicon. The
electrolytic
s cell comprises a vessel 1 having a refractory layer 2. In the electrolytic
cell
there is a lower layer 3 of an alloy of silicon and a metal more noble than
silicon such as a Cu-Si alloy that acts as an anode in the electrolytic cell.
Above the lower anode layer 3 there is an oxide-based electrolyte 4 having a
density lower than the density of the anode alloy 3 and a higher density than
j o molten silicon. A suitable electrolyte 4 is a mixture of 50 % by weight of
CaO
and 50 % by weight of Si02. On the top of the electrolyte layer 4 there is a
layer 5 of pure silicon metal acting as a cathode. The anode 4 and the
cathode 5 are, via contacts 6 and 7 respectively, connected to a direct
current
source (not shown) for conducting current to the electrolytic cell. When
direct
15 current is passed through the electrolytic cell, silicon in the anode alloy
3
enters the oxide-based electrolyte 4 together with ions of any impurities in
the
anode alloy 3 that is electrochemically less noble than silicon. Since silicon
is
the noblest element of the electrolyte 4 silicon ions will be reduced at the
cathode 5 and will form molten pure silicon, which is collected in the molten
20 silicon cathode 5. Thus impurities more noble than silicon are trapped in
the
anode layer 3 while impurities less noble than silicon are trapped in the
electrolyte 4. Pure refined silicon is from time to time tapped from the
molten
cathode layer 5. Additional solid or molten anode alloy or solid or molten
unrefined grade of the metal to be refined is continuously or intermittently
supplied to the molten anode layer 3 through an anode alloy supply channel
8.
After some time of operation of the electrolytic cell, the anode layer will
have
an increased content of impurities of metals more noble than silicon and the
electrolyte will get an increased content of elements less noble than silicon.
0 The electrolytic cell must therefor be stopped and restarted with pure anode
alloy and new uncontaminated electrolyte.
CA 02645161 2008-09-09
WO 2007/106709 13 PCT/US2007/063555
In Figure 2, vessel 10 has refractory layer 11. Alloy layer 12 comprises the
alloy and electrolyte layers 13 contains the second electrolyte and
electrolyte
layer 14 contains the first electrolyte. Layer 15 is pure metal and acts as
cathode, Anode 16 and cathode 17 via conventional contacts are connected
to a direct current source, not shown. Wall 18 separates the two cells, the
first
electrolyte cell 19 and the second electrolytic cell 20. Alloy layer 12 flows
between the two cells under wall 18. In the first electrolytic cell 19, raw
material, e.g. quartz, Si02, is reduced electrolytically to metallic state
such as
silicon to increase the concentration of the metal to be refined in alloy
layer 12
i o and then in the second electrolytic cell 20, the metal to be refined, such
as
silicon alloy is moved from the anode layer through the second electrolyte
layer 13 to the pure metal layer 15. The alloy layer 12 fills the cells to a
level
above the lower edge of wall 18 and thereby separates the two electrolytes of
the two cells. The anode 16 is immersed in electrolyte layer 14 and cathode
is 17 is immersed in metal layer 15, but neither is in direct contact with
alloy
layer 12. The alloy layer 12 acts as a common electrode.
The metal to be refined and elements more noble than the metal to be refined
that are in the first electrolyte of electrolyte layer 14 precipitate at, and
alloy
with, the molten alloy.
20 Anode 16 can be either inert or consumable, such as, baked carbon or
graphite.
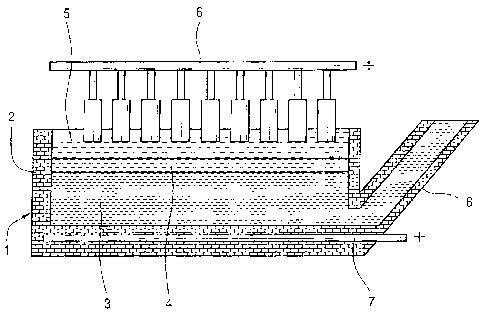
In Figure 3, in vessel 30, which was a graphite crucible, electrolyte layer 31
had a composition of 55 wt. % CaO and 45 wt. % Si02. Raw material of Si02,
quartz, was added frequently to layer 31 to maintain the electrolyte
25 composition and to provide a source of raw material to the process. A
voltage
of 4.5 V was applied between graphite anode 32 and cathode 33, to give a
cathode current density of approximately 1 A/cm2. The cell temperature was
held constant at 1650 C. The cell started with a liquid cathode 34 made of
copper. The first metal is silicon and the second metal is copper in this
cell.
CA 02645161 2008-09-09
WO 2007/106709 14 PCT/US2007/063555
As current flowed through the cell, silicon oxide ions are transported to the
cathode where they are reduced to silicon. After 12 hours of electrolysis, the
copper cathode contained about 20 wt. % Si, giving a current efficiency of
about 40%. Thus, the alloy was produced of SiCu.
s As can be seen, this cell started with pure second metal in the alloy layer
and
through the operation of the cell the alloy is formed in the alloy layer.