Note: Descriptions are shown in the official language in which they were submitted.
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 1 -
CALCIUM ENRICHMENT COMPOSITIONS METHOD OF
PRODUCTION THEREOF AND USE
FIELD OF THE INVENTION
This invention relates to calcium containing compositions, process for their
production and their use. More particularly, this invention is directed to
stable calcium-
containing compositions for use as food and beverage additives.
BACKGROUND OF THE INVENTION
Mineral and vitamin supplements are often used to fortify the composition of
food and beverages, both for human and veterinary use. For example, US
4,772,467 to
Pak et al, discloses the use of citrate based calcium sources for increasing
the
bioavailability of the calcium. US Patent No. 4,786,518 to Nakel et al.,
describes
nutritional supplements comprising iron-sugar complexes. US Patent 4,992,282
to
Mehansho et al. describes stable nutritional vitamin and mineral supplemented
beverages.
It is known that the recommended daily allowance (RDA) of calcium, for
example, is around 1200 mg per day for an adult. Most of the dietary calcium
in a
western diet is from cow milk and other dairy products. The calcium content of
cow
milk is typically in the range of 900 ¨ 1100 mg per liter, such that one liter
almost
provides the RDA. Cow milk substitutes such as soy milk or rice milk provide
much
less calcium than cow milk and almost all the Calcium is added artificially.
Calcium supplements find wide applications as food and beverage supplements.
They are used, inter alia, to compensate calcium loss from the human body, as
is
exhibited in osteoporosis. For example, US Patent No. 4,994,283 to Mehansho et
al.
discloses iron-calcium mineral supplements with enhanced bioavailability. US
Patent
No. 5,445,837 to Burkes et al., discloses as sweetener supplement fortified
with a
concentrated bioavalible calcium source and process for making them. US Patent
No.
5,486,506 to Andon discloses a concentrated bioavalible calcium source. US
Patent No.
6,828,130 to Chatterjee et al., discloses methods for production of gluconate
salts. US
CA 02645269 2013-09-26
- 2 -
Patent No. 6,887,897 to Walsdorf, Sr., et al. discloses calcium glutarate
supplements and
phosphorus binders.
Numerous other relevant patents in the art of food and beverage supplements
include: US 4,214,996; 4,351,735; 4,551,342; 4,737,375; 4,851,221, 4,895,980,
4,985,593; 5,204,134; 5,213,134; 5,213,838; 5,219,889; 5,928,691; 6,287,607;,
6,248,376
and 6,599,544.
Buddemeyer et al., disclose phosphate containing compositions for use as
additives to milk in US 6,248,376 and US 6,599,544.
SUMMARY OF THE INVENTION
The present invention is directed to edible calcium comprising compositions
that
are stable in food and beverages. The calcium comprising compositions are
stable in
beverages, or in their concentrates, and may not separate out of the liquid
phase even
under long storage periods. The calcium comprising composition of the present
invention
may be palatable and may not affect the organoleptic properties of the
beverage or
beverage concentrate to which it is introduced and thus can serve as an
effective calcium
supplement (fortifier) for beverages and solid food.
Thus, in one aspect the present invention provides a dry calcium rich
composition
comprising:
(i) at lease one source of calcium;
(ii) at least one source of metal that is an alkaline metal other than
calcium or an
alkali metal; and
(iii) at least one source of citrate;
wherein the composition has a bulk density of less than 0.4-0.5 g/cm3,
comprises
at least 15% (wt/wt) calcium and at least 66% (wt/wt) of citrate on dry weight
basis.
More preferably the composition comprises 15% to 20% (wt/wt) calcium and at
least
71% (wt/wt) citrate on a dry basis.
The composition may further comprise 7-10% crystalline water. It may further
comprise stabilizers, coloring agents or emulsifiers.
In particular, the calcium rich composition of the invention may be used to
enrich
beverages with calcium, especially in milk, milk-like beverages and naturally
or
CA 02645269 2013-09-26
- 3 -
artificially fortified protein containing beverages. It may be either soluble
in the beverage
or exist as a suspended addition. In some embodiments, the calcium enriched
composition introduced into a beverage is stable for a period of at least 10
to 70 days
wherein less than 5% (wt/wt) of the composition sediments out of the beverage.
It should
be noted that "stable" relates to the fact that the calcium enriched
composition remains
within the liquid phase substantially without sedimenting out. By
"substantially without"
it is meant that less than 5% of the composition is precipitated. Remaining
within the
liquid means at least one of remaining suspended, remaining dissolved and
remaining
bound to a suspended solid or liquid.
In some embodiments, the calcium source is calcium hydroxide, calcium oxide,
calcium carbonate, calcium propionate, calcium gluconate, calcium citrate,
calcium
stearate, calcium fumarate, or calcium glycerophosphate.
In some embodiments, the citrate is citric acid, citric acid monohydrate,
citric acid
mono-, di- or tri-sodium salt, citric acid mono-, di- or tri-potassium salt or
ammonium
citrate.
In some embodiments, the at least one metal source is a source of sodium, a
source of potassium, a source of magnesium or their mixtures. In some
embodiments, the
potassium source is selected from potassium hydroxide, potassium citrate,
potassium
carbonate, potassium bicarbonate or their mixtures. In some embodiments, the
magnesium source is selected from magnesium oxide, magnesium hydroxide,
magnesium
carbonate, magnesium citrate or their mixtures. In some embodiments, the
sodium source
is selected from sodium hydroxide, sodium citrate, sodium carbonate, sodium
bicarbonate
or their mixtures.
Preferably, the calcium enriched dry composition of the present invention
comprises a molar ratio of 3-4.5 citrate; 4-6 calcium; and 2-3 of at least one
metal source.
Such a preferred composition may comprise: (i) a molar ratio of citrate 3-4.5:
calcium 4-
6: potassium 2-3: and magnesium 0-1; (ii) a molar ratio of citrate 4:
potassium 2: calcium
5; (iii) a molar ratio of citrate 3-4.5: calcium 4-6: potassium 2-3: and
sodium 0-1.
In another aspect, the invention provides a method for producing a dried
calcium-
rich composition comprising:
CA 02645269 2013-09-26
- 4 -
(i) mixing at least one source of citrate with at least one source of calcium
and at least
one metal source selected from:
a) an alkaline earth metal source; and
b) an alkali metal source;
in a molar ratio of at least three moles citrate, at least five moles of
calcium and at least
two moles of the at least one metal source so as to produce an organic calcium
solution;
and
(ii) drying the organic calcium solution so as to produce the dried calcium-
rich
composition, wherein said composition comprises at least 66% citrate on a dry
weight
basis, and wherein said composition comprises at least 15% calcium on a dry
weight
basis.
In other aspects, the invention provides a food or nutritional product
comprising
the calcium enriched composition. The nutritional product may be a beverage or
beverage
concentrate comprising the calcium enriched composition. In particular
embodiments, the
beverages are milk based beverages that may be fortified with proteins,
vitamins,
minerals or their mixtures. Non-limiting examples of beverages are selected
from soy
milk, cow milk, camel milk, goat milk, or their mixtures. Such beverages may
further
comprise additional edible supplements selected from cocoa, vanilla, fruit or
vegetable
concentrates or flavorings.
BRIEF DESCRIPTION OF THE DRAWINGS
In order to understand the invention and to see how it may be carried out in
practice, embodiments will now be described, by way of non-limiting example
only, with
reference to the accompanying drawings, in which:
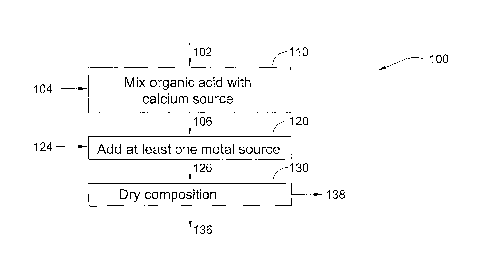
Fig. 1 is a simplified flowchart illustrating a process for producing a dried
calcium-rich composition according to a preferred embodiment of the present
invention;
Fig. 2 is a simplified flowchart illustrating further details of one
embodiment of
step 110 of Fig. I;
Fig 3 is a simplified flowchart illustrating a process for producing a dried
calcium-rich composition according to a preferred embodiment of the present
invention;
CA 02645269 2013-09-26
- 4a -
Fig. 4 is a simplified flowchart illustrating a process for supplementing a
food or
beverage with the dried calcium-rich composition according to a preferred
embodiment
of the present invention; and
Fig. 5 is another simplified flowchart illustrating a process for
supplementing a
food or beverage with the dried calcium-rich composition according to a
preferred
embodiment of the present invention.
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 5 -
DETAILED DESCRIPTION OF EMBODIMENTS
The present invention is thus directed to calcium comprising compositions,
which are stable in food and beverages and in food and beverage supplements.
Preferably the compositions are suitable for use in milk, soy milk and other
"milk-like",
"milk-containing", protein containing beverages or their mixtures. Despite the
large
number of supplements currently available and known in the art, many of them
are
unstable and precipitate out of solution over time. The products of the
present invention
may be used to meet the demand in the market for stable sources of calcium,
which are
suitable for adding to foods and beverages. The products of the present
invention are
used as supplements and do not affect the organoleptic properties or the taste
of the food
or beverage to which they are added. The calcium products of the present
invention are
both stable and do not typically precipitate during the storage of the
food/beverage even
after storage periods of about 70-80 days.
The present invention relates in particular to stable dried compositions of
organic calcium, in the form of calcium citrate with at least one other
additional metal,
to methods for the preparation of these compositions and their use as calcium
supplements. The products of this invention may be used either directly for
enhancing
uptake of calcium or as an additive in various food and beverages to fortify
these food
products with calcium. The compositions are stable in beverages and in food,
to which
they added.
The compositions of the present invention exhibit high bioavailability. The
compositions of the present invention are stable in sterilization and
pasteurization
processes known in the art of food and beverage processing. The composition of
the
present invention does not require the co-addition of hydrocolloids in order
to retain the
calcium in a stable suspension.
Reference is now made to Fig. 1, which is a simplified flowchart 100
illustrating
a process for producing a dried calcium-rich composition according to a
preferred
embodiment of the present invention.
In a first mixing step 110, an organic acid solution 102, being a citrate
solution
is mixed with a calcium source 104. The citrate solution which is citric acid
or its salts,
is typically in a concentration range of 0.1 to 0. 5 M. Non limiting sources
of calcium
according to the opresent invention are selected from the group of calcium
hydroxide,
calcium oxide, calcium carbonate, calcium propionate, calcium gluconate,
calcium
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 6 -
citrate, calcium stearate, calcium fumarate, calcium glycerophosphate. The
calcium
source 104 is provided to produce a solution with molar ratio of Calcium to
Citrate
typically in the range of 1.1 ¨ 1.3. Typically this step is performed in a
standard mixed
vessel well known in the art. This mixing step 110 typically takes up to 30
minutes. In
this step 110, the vessel is typically cooled to a set temperature below 25 C.
Cooling
jackets known in the art, may be employed on a large scale, or the vessel may
be at least
partially immersed in a water bath on the small scale, as is known in the art.
In the
present invention different chillers were used (such as CH1OTR nameplate
number
30089, Unique, Nehalim, Israel or CC230, Huber High Precision
Thermoregulation,
Offenburg, Geimany)
Typically, the citrate solution 102 is obtained commercially. Alternatively,
it
may be prepared in situ as is exemplified in Fig. 2 herein below.
Typically, solution 106 comprises 4 to 25 % total dissolved solids (TDS). In
some embodiments, there are 5 to 10 % TDS in solution.
Solution 106 and/or solution 126 (described hereinbelow) typically has a pH
value in the range of 4.5 to 12, and more preferably from 5 to 10.
In an addition step 120, at least one metal source 124 selected from an
alkaline
earth metal source and an alkali metal source is added to calcium citrate
solution 106 to
form a calcium metal citrate solution 126. This may be performed in the same
or
different vessel to that of step 110. The at least one metal source is
selected from at least
one potassium source, at least one magnesium source, at least one sodium
source or
their mixtures. Non-limiting examples of the potassium source are potassium
hydroxide,
potassium citrate, potassium carbonate and potassium bicarbonate. Typically
the
potassium salt is added in to produce the molar ratio of potassium to citrate
in a range
0.6 ¨ 0.8. Non-limiting examples of the magnesium source are magnesium oxide,
magnesium hydroxide magnesium citrate, magnesium carbonate. Typically the
magnesium salt is added in suitable concentration to produce the molar ratio
of
Magnesium and Citrate in range 0.1 to 0.25.
In the drying step 130, solution 126 is dried and liquid 138 is removed there
from to faun a dry calcium metal citrate composition 136. Step 130 typically
drying
solution 126 into a powder using a spray drying or freeze drying process in a
dryer APV
PSD52 (APV Nordic Anhydro, Silkeborg, Denmark) using the inlet air with
temperature from 190 up to 350 C as is known in the art. Excess liquid 138 is
removed
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 7 -
from the solution until a solid phase forms. The solid phase may be in the
form of a
powder, flakes, granules or other solid form. The resultant composition 136
may then
be suitably stored and/or packaged (not shown). The resultant composition
typically has
a bulk density of less than 0.6 g/cm3, more typically, less than 0.5 g/cm3.
Dry calcium metal citrate composition 136 typically has a composition as is
shown in Table 1. It should be noted that all the examples herein of the
composition
produced comprises 7-10% adsorbed (crystalline) water.
Table 1: Typical Composition of a Calcium Metal Citrate Composition on a Dry
Weight Basis*
COMPONENT RELATIVE MOLAR PERCENT OF DRY COMPOSITION
RATIO [WT/WT Vo]
CITRATE 1 63-75
CALCIUM 0.8 -2 13-18
POTASSIUM 0.4-1 6-10
MAGNESIUM 0.03 ¨ 0.3 0.5 ¨ 1.5
* it should be noted that the "dry weight" was calculated excluding up to 10%
adsorbed
water in the product
Reference is now made to Fig. 2, which is a simplified flowchart 200
illustrating
further details of one embodiment of step 110 of Fig. 1.
In a mixing step 210, water 202, such as deionized water, is mixed with a
citrate
containing solid or solution 204. Citrate containing solid or solution 204 is
selected
from citric acid (Sigma Aldrich Corporation, St. Louis, Missouri, USA cat.
Number
C0759, C7129 or cat. Number C0706, C1909), sodium citrate (Sigma Aldrich
Corporation, St. Louis, Missouri, USA cat. Number C0759, C7129 or cat. Number
S4641) and potassium citrate (Sigma Aldrich Corporation, St. Louis, Missouri,
USA
cat. Number C0759, C7129 or cat. Number C8385). Typically suitable amount of
citric
acid or a citrate salt is added to provide a concentration of citrate ions in
range up to 0.5
moles per liter of water. Water 202 and citrate containing solid or solution
204 are
typically mixed at room temperature in any kind of well known in the art mixed
vessel.
This mixing step 210 typically takes up to 1 hour to provide a full
dissolution. In this
step 210, the vessel is typically cooled to a set temperature ranging from 5-
25 C.
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 8 -
Cooling jackets known in the art, may be employed on a large scale, or the
vessel may
be at least partially immersed in a water bath on the small scale, as is known
in the art.
The resultant citrate solution 206, typically comprises 0.1 -0.5 mol per liter
of
citrate.
In an addition step 220, a suitable source of calcium 224 is added to the
citrate
solution to form an organic calcium citrate solution 226. Typically this step
is
performed in a standard well known in the art mixed vessel. This step 220
typically
takes up to 30 minutes while mixed. In this step 110, the vessel is typically
cooled to a
set temperature ranging below 25 C. Cooling jackets known in the art, may be
employed on a large scale, or the vessel may be at least partially immersed in
a water
bath on the small scale, as is known in the art.
The calcium source may be selected from, but is not limited to, calcium
citrate;
calcium oxide, calcium hydroxide and calcium carbonate (of Sigma Aldrich
Corporation, St. Louis, Missouri, USA cat. Numbers C2178, C4830, C7887, or
Fluka
Buchs, Switzerland, cat. number 21118, cat. number 21120). The calcium source
is
provided to produce a molar ratio of calcium to citrate ion in range 1.1 -1.3
in solution
226.
Reference is now made to Fig 3, which is a simplified flowchart 300
illustrating
a process for producing a dried calcium-rich composition according to a
preferred
embodiment of the present invention.
In a mixing step 310, citric acid 304 (Gadot Biochemical Industries, Ltd.,
Haifa,
Israel) is mixed in water 302, typically deionized water so as to produce a
citric acid
solution 306 of 0.1 to 0.5 moles per liter.
In a metal addition step 320, at least one source of metal 324 is added to the
citric acid solution to form a metal rich citric acid solution 326. Typically,
at least one
source of potassium is added. The potassium source is selected from, but not
limited to
potassium hydroxide, potassium citrate, potassium carbonate and potassium
bicarbonate
(Sigma Aldrich Corporation, St. Louis, Missouri, USA Cat. Numbers P9144,
P5833,
P4379, P6037, C8385, P5958, P1767, 60025, 60028). Typically the potassium salt
is
added in to produce the molar ratio of potassium to citrate in a range 0.6 ¨
0.8. In some
embodiments, Sodium was used instead of potassium. In some embodiments,
ammonium was used instead of potassium or sodium.
=
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 9 -
In some embodiments, the magnesium source is selected from, but not limited
to, magnesium oxide, magnesium hydroxide, magnesium citrate, magnesium
carbonate,
(Sigma Aldrich Corporation, St. Louis, Missouri, USA Cat. Numbers M7179,
M5671,
M5421, M8511, 30.77-2 , M7861, and Merck & Co, Inc, Whitehouse Station, NJ ,
USA Cat. Numbers 105904)). Typically the magnesium salt is added in a suitable
concentration to produce the molar ratio of magnesium and citrate in range 0.1
to 0.25.
In some embodiments, no magnesium is added.
In some embodiments, step 330 is performed before step 320. Other variations
on the flowcharts of Figs. 1-3 also fall within the scope of the present
invention.
In a second addition step 330, a calcium source 334 is added to solution 326
so
as to form a calcium metal citrate solution 336.
The calcium source 334 may be selected from, but is not limited to, calcium
citrate; calcium oxide, calcium hydroxide and calcium carbonate (Sigma Aldrich
Corporation, St. Louis, Missouri, USA cat. Numbers C2178, C4830, C7887, or
Fluka
Buchs, Switzerland, cat. number 21118, cat. number 21120). The calcium source
is
provided to produce solution 336 with molar ratio of Calcium to Citrate in
range 1.1 ¨
1.3. The process conditions in this step and in step 330 may be similar to,
identical to or
different from those of step 120 of Fig. 1.
In a drying step 340, solution 336 is dried, liquid 348 is removed there from
to
form a dry calcium metal citrate composition 346. Step 340 typically dries
solution 336
into a powder using a spray drying or freeze drying process in a dryer (APV
PSD52,
APV Nordic Anhydro, Silkeborg, Denmark) using the inlet air with temperature
from
190 up to 350 Degrees Celsius as is known in the art. Excess liquid 348 is
removed
from the solution until a solid phase forms. The solid phase may be in the
form of a
powder, flakes, granules or other solid form. The resultant composition 346
may then
be suitably stored and/or packaged (not shown). Typically, the composition
obtained
has a bulk density of less than 0.5 g/cm3.
Reference is now made to Fig. 4, which is a simplified flowchart 400
illustrating
a process for supplementing a food or beverage with the dried calcium-rich
composition
according to a preferred embodiment of the present invention.
In a mixing step 410, a dried calcium metal citrate composition 402 is mixed
with a food or beverage 404 to foi il]. calcium enriched beverage/food 416.
Composition
402 may be similar or identical to composition 136 or composition 346. The
food may
CA 02645269 2013-09-26
- 10 -
be in a liquid or solid state. The food may be exemplified by, but not limited
to a cheese,
yoghurt, cream, spread, cereal, or chocolate.
The beverage may be a natural based beverage or a non-natural based beverage.
Non-limiting examples of beverages according to the present invention are
fruit or
vegetable based beverages, milk-based beverages, that may further comprise
flavoring
additives such as proteins, minerals or vitamins. Thus these may be milk,
milkshake,
nectars, or chocolate milk. The milk may be selected from, but not limited to,
soy milk,
reconstituted milk formula, goat milk, sheep milk, camel milk, substitute
milk, cow milk,
oat milk and human milk, or may be any mixture thereof, or beverages based on
them.
The ratio of composition 402 added to a liquid food/beverage 404 is typically
suitable to provide a Calcium concentration up to 1.5 RDA of Calcium per
liter. Calcium
enriched beverage/food 416 typically comprises 1200 mg/1 calcium.
Alternatively, calcium enriched beverage/food 404 is in a solid form and is
mixed
with composition 402 in any kind of well known in the art mixed vessels for
about 15
minutes or till the homogeneous dispersion is obtained.
In an optional storage step 420, a liquid calcium enriched beverage/food 416
is
stored for a period of few months at ambient conditions or during
refrigeration. The
properties of stored calcium enriched beverage/food 426 are compared to those
of
calcium enriched beverage/food 416. Typically, the composition is stable in
the liquid
and less than 10 % of the calcium precipitates out of the liquid. In
accordance with the
stability of the added calcium fortifying composition, the initial calcium
concentration
introduced into the food/beverage 426 is maintained, where it preferably
comprises at
least 1200 mg/L of calcium.
In an optional dilution step 430, stored calcium enriched beverage/food 426 is
diluted with water 434 to form a ready-to-use calcium enriched beverage/food
product
436. For example, beverage/food 426 may be in a concentrated form such as a
milk
powder, baby milk liquid/solid formula, cream or concentrate, which may be
diluted for
use with water according to the relevant ratio or instructions provided
therewith.
In some other embodiments, food product 404 is pre-dried and is reconstituted
in
step 430. Non-limiting examples are dried mashed potatoes, dried packet soups,
milk
powder, meat, yeast and protein extracts and "heat and eat" meals.
CA 02645269 2013-09-26
- 10a -
Reference is now made to Fig. 5, which is a simplified flowchart 500
illustrating a
process for supplementing a food or beverage with the dried calcium-rich
composition
according to a preferred embodiment of the present invention.
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 11 -
In a mixing step 510, a dried calcium rich composition 502 is mixed with water
504 to form a wet calcium rich composition 516.
Composition 502 may be similar or identical to composition 136 or composition
346. Step 510 is performed in a any kind of well known in the art mixing
vessel until an
homogeneous suspension is obtained.
Typically about 100 g of composition 502 is added per liter of water such that
the wet calcium rich composition comprises about 15 g calcium/1.
In an addition step 520, wet composition 516 is added to food/beverage 524 to
form calcium-enriched food/beverage 526. The calcium-enriched food/beverage
typically comprises 1200mg calcium/1.
Step 520 is performed in any kind of well known in the art mixing for about 15
min or until the homogeneous suspension obtained.
In an optional storage step 530, calcium-enriched food/beverage 526 is stored
for a period of at least a few month at ambient conditions or at
refrigeration. The
properties of stored calcium enriched beverage/food 536 are compared to those
of
calcium enriched beverage/food 526. Typically, less than 10 % of the calcium
precipitates out of the liquid. In some embodiments less than 5 % of the
calcium
precipitates out of the liquid such that stored beverage/food 536 comprises at
least 1200
mg/1 calcium.
Example 1
1840 ml of deionized water were placed in a 5 L beaker and the temperature
kept in the range of 5-25 C. While stirring, 66.5g citric acid was added
followed by
2.5g of MgO. Thereafter, 23.3gr CaO were added. Finally, 13 g KOH was added.
The
mixture was well stirred, dried (as described with reference to the flowcharts
hereinabove). The dry product obtained had properties in the range of those
described in
Table 1 hereinabove.
Example 2
920 ml of deionized water were placed in a 5 L beaker and the temperature kept
in the range of 5-25 C. While stirring, 36.5 g citric acid was added followed
by 13.6g of
CaO. Finally, 6.5 g KOH were added. The mixture was well stirred and dried (as
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 12 -
described with reference to the flowcharts hereinabove). The dry product
obtained had
properties in the range of those described in Table 1 hereinabove.
Example 3
920 ml of deionized water were placed in a 5 L beaker and the temperature kept
in the range of 5-25 C. While stirring, 66.5g citric acid was added followed
by 2.5g of
MgO. Thereafter were added 23.3gr CaO. Finally, there were added 13 g,r KOH.
The
mixture was well stirred, dried (as described with reference to the flowcharts
hereinabove). The dry product obtained had properties in the range of those
described in
Table 1 hereinabove.
Example 4
To 1000 ml of cow milk 3 % fat (Tnuva, Rehovot, Israel), 6.7 grams of
composition, prepared as described in Example 1, was added while stirring with
well
known in the art laboratory magnetic stirrer. The initial concentration of
calcium was
tested and found 2230 mg/liter. After storing for 6 days at 4 Degrees Celsius,
the
concentration of Calcium in the upper layer was tested again and found to be
2225
mg/liter.
Example 5
To 1000 ml of natural soy milk (Alpro N.V., Wevelgem, Belgium), 6.7 g of
composition (prepared as described in example 1) was added while stirred with
a
laboratory magnetic stirrer to form an enriched soy milk. The initial
concentration of
calcium in the enriched soy milk was tested and found 1327 mg/liter. After
storing for 6
days at 4 Degrees Celsius, the concentration of calcium of the enriched soy
milk was
tested again and found 1325 mg/liter
Example 6
1840 ml of deionized water were placed in a 5 L beaker and the temperature
kept in the range of 5-25 C. While stirring, 66.5g citric acid were added
followed by
2.5g of MgO. Thereafter were added 23.3gr CaO. Finally, there were added 7.7
gr
NaOH. The mixture was well stirred, dried (as described with reference to the
flowcharts hereinabove).
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 13 -
Example 7
To 1000 ml of natural soy milk (Alpro N.Y., Wevelgem, Belgium) 6.7 g of
composition (prepared as described in Example 6 hereinabove) was added under
stirring
with a laboratory magnetic stirrer so as to form enriched soy milk. The
initial
concentration of calcium was tested in the enriched soy milk and found to be
1305
mg/liter. After storing for 6 days at 4 Degrees Celsius, the concentration of
Calcium in
the enriched soy milk was tested again and found to be 1306 mg/liter
Example 8
To 1000 ml of natural soy milk (Alpro N.Y., Wevelgem, Belgium), 6.7 g of
composition (prepared as described in Example 1 hereinabove) was added under
stirring
with a laboratory magnetic stirrer to form enriched soy milk. In addition, 0.2
g of Cappa
Karragenan (Sigma Aldrich Corporation, St. Louis, Missouri,USA cat. Number
C1263)
was added. The initial concentration of calcium in the enriched soy milk was
tested and
found to be 1396.7 mg/liter. After storing for 6 days at 4 Degrees Celsius,
the
concentration of calcium in the enriched soy milk was tested again and found
1401.1
mg/liter
Example 9
To 1000 ml of natural soy milk (Alpro N.Y., Wevelgem, Belgium) 6.7 g of
composition (prepared as described in Example 1 hereinabove) was added while
stirred
with a laboratory magnetic stirrer to form enriched soy milk. The enriched soy
milk was
passed through homogenizer (APV PSD52, APV Nordic Anhydro, Silkeborg,
Denmark). Thereafter, the initial concentration of calcium was tested and
found 1446.6
mg/liter. After storing for 6 days at 4 Degrees Celsius, the concentration of
calcium of
the enriched soy milk was tested again and found 1432.3 mg/liter
Example 10
The material was prepared as described in Example 1 hereinabove, but the
aqueous suspension was stored for 10 hours prior to the drying step.
Thereafter, the
material was dried as described with reference to the flowcharts hereinabove.
The dry
product obtained had properties in the range of those described in Table 1
hereinabove.
CA 02645269 2008-09-09
WO 2007/107999 PCT/1L2007/000375
- 14 -
Example 11
To 1000 ml of natural soy milk (Alpro N.Y., Wevelgem, Belgium) 6.7 g of
composition (prepared as described in Example 10) was added under stirring
with a the
art laboratory magnetic stin-er and an enriched soy milk product was formed.
The initial
concentration of calcium in the product was tested and found 1267 mg/liter.
After
storing for six days at 4 Degrees Celsius, the concentration of calcium in the
product
was tested again and found 1266 mg/liter
Example 12
To 1000 ml of natural soy milk (Alpro N.Y., Wevelgem, Belgium) 6.7 g of
composition (prepared as described in Example 1 hereinabove) was added under
stirring
employing a laboratory magnetic stirrer to form a calcium-enriched
composition. The
calcium-enriched composition underwent ultra-high temperature (UHT) treatment
(4
sec at 140 C) as is known in the art. The initial concentration of calcium in
the calcium-
enriched composition was tested and found 905 mg/liter. After storing for 7
days at 4
Degrees Celsius, the concentration of calcium in the calcium-enriched
composition was
tested again and found 906 mg/liter. After storing for another 7 days at 4
Degrees
Celsius, the concentration of calcium in the calcium-enriched composition was
tested
again and found 905.5 mg/liter. After retention of 70 days at 4 Degrees
Celsius, the
concentration of calcium in the calcium-enriched composition was tested again
and
found 905 mg/liter.
Example 13
1840 ml of deionized water were placed in a 5 L beaker and the temperature
kept in the range of 5-25 C. While stirring, 66.5g citric acid were added
followed by
2.5g of MgO. Thereafter were added 23.3g CaO. Finally, there were added 8.12
gr
NH4OH. The mixture was well stirred and dried (as described with reference to
the
flowcharts hereinabove). The dry product obtained had properties in the range
of those
described in Table 1 hereinabove.
Example 14
=
CA 02645269 2008-09-09
WO 2007/107999
PCT/1L2007/000375
- 15 -
_
To 1000 ml of natural soy milk (Alpro N.Y., Wevelgem, Belgium) 6.7 g of
composition (prepared as described in Example 13) was added under stirring,
employing a laboratory magnetic stirrer, to form enriched soy milk. The
initial
concentration of calcium in the enriched soy milk was tested and found 1332
mg/liter.
After storing for 6 days at 4 Degrees Celsius, the concentration of calcium in
the
enriched soy milk was tested again and found 1343 mg/liter.
Example 15
To 1000 ml of milk based cocoa beverage ( Machlevot Yutveta, Israel) 2 g of
composition (prepared as described in Example 1) was added under stirring,
employing
a laboratory magnetic stirrer, to form Calcium enriched beverage. The initial
concentration of calcium in the enriched beverage was tested and found 1402
mg/liter.
After storing for 6 days at 4 Degrees Celsius, the concentration of calcium in
the
enriched beverage was tested again and found 1390 mg/liter.
It is appreciated that certain features of the invention, which are, for
clarity,
described in the context of separate embodiments, may also be provided in
combination
in a single embodiment. Conversely, various features of the invention, which
are, for
brevity, described in the context of a single embodiment, may also be provided
separately or in any suitable sub-combination.
Although the invention has been described in conjunction with specific
embodiments thereof, it is evident that many alternatives, modifications and
variations
will be apparent to those skilled in the art.