Note: Descriptions are shown in the official language in which they were submitted.
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
PROCESSING ASPHALTENE-CONTAINING TAILINGS
FIELD
This disclosure relates to the recovery of energy, materials or both from
asphaltene-containing tailings, such as asphaltene-containing tailings
generated during
oil sand processing.
BACKGROUND
Asphaltenes are high molecular weight hydrocarbons having a chemical
structure that can include stacks of condensed aromatic rings. Due to their
high
molecular weight, asphaltenes can be found within the least volatile fraction
after
distillation of crude oil. Asphaltenes also can be found in oil sand along
with minerals
and other hydrocarbons. Among the other hydrocarbons, oil sand can include
lignite
and other low-rank coal phases.
Oil sand can be processed to recover hydrocarbons for upgrading into more
valuable products, such as oil. Asphaltenes, however, do not behave in the
same
manner as other hydrocarbons in oil sand, so the same processes typically
cannot be
used to upgrade them. Thus, in certain conventional processes for recovering
hydrocarbons from oil sand, the asphaltenes most often are separated along
with the
minerals, lignite and water into a tailings stream. Without further
processing, the
asphaltene-containing tailings can be damaging to the environment. Disposal of
the
asphaltene-containing tailings also can waste potentially valuable energy and
materials.
SUMMARY
Disclosed herein are embodiments of a method and a system for recovering
energy, materials or both from asphaltene-containing tailings, such as
asphaltene-
containing tailings from a process for recovering.hydrocarbons from oil sand.
Embodiments of the method can include a flotation separation and a hydrophobic
agglomeration separation. In some embodiments, coarse materials are separated
from
the asphaltene-containing tailings prior to further processing. This can be
-1-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
accomplished, for example, by subjecting the asphaltene-containing tailings to
a
cyclone separation, such as a gas-sparged hydrocyclone separation. The coarse
materials can be removed with an underflow from the cyclone separation.
The flotation separation can include, for example, introducing gas into the
asphaltene-containing tailings such that asphaltenes in the asphaltene-
containing tailings
rise with bubbles of the gas to form an asphaltene-rich froth over an
asphaltene-depleted
aqueous phase. The asphaltene-rich froth can include water, asphaltenes, any
remaining
solvent from previous processing and any naturally floatable or flotation
activated
mineral species, including lignite. The asphaltene-depleted aqueous phase can
include
water and non-floatable minerals. After the flotation separation, a thickening
process
can be used to convert the asphaltene-rich froth into an asphaltene-rich
slurry. In some
embodiments, heat energy is recovered from water removed from the asphaltene-
rich
froth or the asphaltene-rich slurry. Water and the contained heat energy also
can be
recovered from the asphaltene-depleted aqueous phase.
The asphaltene-rich froth or asphaltene-rich slurry can be separated into a
heavy
mineral concentrate and a light tailings, such as by a gravity separation
process. The
heavy mineral concentrate can include minerals targeted for recovery. These
minerals
can include, for example, oxygen-containing minerals, such as Group 4B metal
oxides,
particularly titania, zirconia, iron oxide-titania minerals (e.g., ilmenite),
and
combinations thereof. The heavy mineral concentrate also can include minerals
to be
excluded from waste generated by the overall process, such as sulfur-
containing
minerals (e.g., pyrite, marcasite, base metal sulfides, etc.). The light
tailings can
include water, asphaltenes, lignite and solvent. In some embodiments, a coarse
lignite
phase also is separated from the asphaltene-rich froth or asphaltene-rich
slurry. This
separ-ation can be accomplished, for example, by physical processing using a
size
separation such as screening, by a gravity separation such as a hydrocyclone
or by
solvent extraction to partially or fully dissolve the asphaltenes, leaving the
non-soluble
coal and lignite hydrocarbons or by any combination thereof.
A hydrophobic agglomeration separation can be performed on the light tailings.
This separation can include, for example, dispersing a hydrophobic
agglomeration agent
-2-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
within the light tailings to form droplets. The droplets can agglomerate with
the
asphaltenes to form asphaltene-containing particles, which can be separated as
an
asphaltene concentrate. In some embodiments, the asphaltene-containing
particles are
separated by gravity separation, filtration or both. The hydrophobic
agglomeration
agent can comprise diesel, a fuel oil, a surfactant, or a combination or
derivative
thereof. Dispersants and modifiers also can be added. Some embodiments include
shear mixing or ultrasonic attrition prior to hydrophobic agglomeration. In
addition,
some embodiments include introducing an oxidizing agent, a causticizing agent,
both or
a mixture thereof into the light tailings before or while dispersing the
hydrophobic
agglomeration agent. Furthermore, some embodiments include separating the
asphaltenes from one or more lignite phases.
In some disclosed einbodiments, solvent is recovered with the asphaltene
concentrate. In oil sand processing, this can be useful to reduce the need for
near
complete solvent recovery after separation of asphaltenes from other
hydrocarbons. For
example, some embodiments of the disclosed method include providing a bitumen
froth
comprising bitumen, asphaltenes, inorganic solids and water. For example, the
bitumen
froth can comprise between about 20% and about 80% bitumen, between about 10%
and about 75% water, between about 5% and about 45% inorganic solids and
between
about 1% and about 25% asphaltenes. This bitumen froth then can be mixed with
a
paraffinic hydrocarbon solvent to form a mixture. The paraffinic hydrocarbon
solvent
can have a chain length between about 5 and about 8 carbons. In some
embodiments,
the paraffinic hydrocarbon solvent comprises about 50% by weight pentane and
about
50% by weight hexane. Adding the paraffinic hydrocarbon solvent causes
precipitation
of the asphaltenes. The resulting mixture then can be separated into a dilute
bitumen
product and a residue, with the dilute bitumen product comprising bitumen and
paraffmic hydrocarbon solvent and having a lower concentration of precipitated
asphaltenes, inorganic solids and water than the mixture. Next, between
greater than
0% and about 95% of the remaining paraffinic hydrocarbon solvent present in
the
residue can be recovered in a solvent recovery unit. The solvent recovery unit
can
produce a tailings stream comprising water, inorganic solids, precipitated
asphaltenes
-3-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
and non-recovered paraffinic hydrocarbon solvent. The precipitated asphaltenes
and the
non-recovered paraffinic hydrocarbon solvent then can be separated from the
tailings
stream, such as by flotation, gravity separation, hydrophobic agglomeration,
or a
combination thereof. Since the tailings stream that exits the solvent recovery
unit is
subjected to further processing, the solvent recovery process used within the
solvent
recovery unit can be less complete and less expensive than stream stripping.
For
example, flotation using an inert gas phase, gravity separation, vacuum
stripping, or a
combination thereof, can be used as the solvent recovery process in the
solvent recovery
unit. In some embodiments, the tailings stream exits the solvent recovery unit
at a
temperature between about 20 C and about 65 C.
Some disclosed embodiments include separating sulfur-containing minerals
from the heavy mineral concentrate. This separation can include, for example,
attritioning the heavy mineral concentrate to disagglomerate, scrub or clean
the sulfur-
containing minerals' surfaces. Similar to the separation of asphaltenes, the
separation
of sulfur-containing minerals can be achieved by flotation. Gas bubbles can be
introduced into the heavy mineral concentrate such that the sulfur-containing
minerals
rise with the gas bubbles to form a sulfur-rich froth over a sulfur-depleted
aqueous
phase. Thereafter, the sulfur-containing minerals can be recovered from the
sulfur-rich
froth, or a sulfur-rich slurry formed from the sulfur-rich froth, and oxygen-
containing
minerals, such as titania, zirconia, ilmenite, gangue minerals (e.g., garnet
and
staurolite), and combinations thereof, can be recovered from the sulfur-
depleted
aqueous phase.
A variety of reagents can be used to facilitate the separations included in
embodiments of the disclosed method. For example, frother and collector
reagents can
be used with each flotation separation. These reagents can be introduced prior
to the
introduction of gas bubbles. In the flotation separation performed on the
asphaltene-
containing tailings, the frother reagent can comprise an aliphatic alcohol, a
cyclic
alcohol, a phenol, an alkoxy paraffin, a polyglycol, or a combination or
derivative
thereof. The collector reagent used with this separation can comprise a fuel
oil, sodium
oleate, a fatty acid, a xanthate, an alkyl sulfuric salt, a dithiophosphate,
an amine, or a
-4-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
combination or derivative thereof. In the flotation separation performed on
the heavy
mineral concentrate, the frother reagent can comprise an aliphatic alcohol, a
cyclic
alcohol, a phenol, an alkoxy paraffm, a polyglycol, or a combination or
derivative
th.ereof. The collector reagent used with this separation can comprise a fuel
oil, sodium
oleate, a fatty acid, a xanthate, an alkyl sulfuric salt, a dithiophosphate,
an amine, or a
combination or derivative thereof. Reagents also can be used in conjunction
with the
separation of the asphaltene-rich froth or the asphaltene-rich slurry into the
heavy
mineral concentrate and the light tailings. These reagents can comprise, for
example, a
dispersant, a modifier, a surfactant, or a combination or derivative thereof.
In some
embodiments, the dispersant comprises a silicate, a phosphate, a citrate, a
lignin
sulfonate, or a combination or derivative thereof.
Embodiments of the disclosed system can include a flotation apparatus for
separating the asphaltene-containing tailings into the asphaltene-rich froth
and the
asphaltene-depleted aqueous phase, a gravity separation apparatus for
separating the
asphaltene-rich froth, or the asphaltene-rich slurry formed from the
asphaltene-rich
froth, into the heavy mineral concentrate and the light tailings, and a
hydrophobic
agglomeration mixing apparatus for dispersing the hydrophobic agglomeration
agent
within the light tailings. These and other embodiments also can include a
hydrophobic
agglomeration settling apparatus for separating the asphaltene concentrate
from the light
tailings. To separate coarse materials from the asphaltene-containing tailings
before the
asphaltene-containing tailings enter the flotation apparatus, some embodiments
also
include a cyclone separation apparatus.
In addition to a flotation apparatus configured to receive the asphaltene-
containing tailings, some embodiments of the disclosed system include a
flotation
apparatus configured to separate the heavy mineral concentrate into the sulfur-
rich froth
and the sulfur-depleted aqueous phase, which can, for example, contain gangue
minerals such as garnet and staurolite. One or both of the separation
apparatuses can be
associated with a thickening apparatus. For example, the flotation apparatus
that
receives the asphaltene-containing tailings can be connected to a thickening
apparatus
configured to thicken the asphaltene-rich froth to form the asphaltene-rich
slurry.
-5-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Many of the devices used in embodiments of the disclosed system separate
water from other materials. Some embodiments include one or more conduits for
recycling this water. For example, some embodiinents include a conduit for
recycling
water that exits one or more of the flotation apparatuses.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic diagram representing embodiments of a method and a
system for recovering energy, materials or both from asphaltene-containing
tailings.
FIG. 2 is a schematic diagram representing embodiments of a method and a
system for recovering energy, materials or both from asphaltene-containing
tailings
including a separation before flotation of the asphaltene-containing tailings.
DETAILED DESCRIPTION
Unless otherwise explained, all technical and scientific terms used herein
have
the same meaning as commonly understood by one of ordinary skill in the art to
which
this disclosure belongs. The singular terms "a," "an," and "the" include
plural referents
unless the context clearly indicates otherwise. Similarly, the word "or" is
intended to
include "and" unless the context clearly indicates otherwise. The term
"includes"
means "comprises." The method steps described herein, such as the separation
steps
and the mixing steps, can be partial, substantial or complete unless indicated
otherwise.
All percentages recited herein are dry weight percentages unless indicated
otherwise.
As used herein, the term "heavy minerals" refers to minerals having a greater
molecular weight than other minerals in a given stream or batch.
As used herein, the term "lignite" refers to all low-rank coal that may be
present
in oil sand, including lignite and subbittuninous coal:. This coal may, for
example, have
a moisture content greater than about 20%.
As used herein, the term "coarse materials" refers to material particles
having a
greater size than other material particles in a given stream or batch, such as
a size
sufficient to allow the coarse materials to be separated in an underflow
exiting a cyclone
separation process.
-6-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Disclosed herein are embodiments of a method and a system for recovering
energy and/or materials from asphaltene-containing tailings. Asphaltene-
containing
tailings often are generated as a byproduct of oil sand processing. One
example of oil
sand processing can be found in U.S. Patent No. 6,007,709, which is
incorporated
herein by reference. Oil sand processing can include a flotation separation
resulting in
the formation of a froth comprising hydrocarbons, certain minerals and
entrained sand.
For example, the froth can include about 60% bitumen, about 25% water, about
10%
inorganic solids and about 8% asphaltenes. Typical ranges for the
concentration of
bitumen in the froth are between about 20% and about 80% and between about 40%
and
about 70%. Typical ranges for the concentration of water in the frotli are
between about
10% and about 75% and between about 15% and about 40%. Typical ranges for the
concentration of inorganic solids in the froth are between about 5% and about
45% and
between about 5% and about 20%. Typical ranges for the concentration of
asphaltenes
in the froth are between about 1% and about 25% and between about 5% and about
15%.
To separate the asphaltenes from the hydrocarbons targeted for recovery, the
froth can be mixed with a solvent and subjected to one or more settling
stages. The
solvent can be, for example, a paraffinic hydrocarbon solvent, such as a
paraffinic
hydrocarbon solvent having a chain length between about 5 and about 8 carbons.
In a
specific example, the solvent comprises about 50% by weight pentane and about
50%
by weight hexane. The solvent used to precipitate the asphaltenes typically is
toxic and
would be harmful to the environment if included in a waste stream. Therefore,
the
solvent often is separated from the other waste materials and recycled.
Separation of
the solvent can occur, for example, in a tailings solvent recovery unit
(TSRU).
Conventionally, the tailings that exit the TSRU are disposed of as a waste
product.
The disclosed method and system can be used to recover additional value from
asphaltene-containing tailings, such as asphaltene-containing tailings that
exit a TSRU
within a process for recovering hydrocarbons from oil sand. This value can
result, for
example, from the recovery of energy and/or materials, such as asphaltenes,
sulfur-
containing minerals, oxygen-containing minerals and any solvent not removed in
the
-7-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
TSRU. The recovered asphaltenes can be at least partially upgraded into useful
oil,
such as by the Taciuk kiln process (as shown, for example, in U.S. Patent No.
6,589,417, which is incorporated herein by reference) or by non-Taciuk
pyrolysis (as
shown, for example, in U.S. Patent No. 5,961,786, which is incorporated herein
by
reference). Valuable minerals that can be recovered from asphaltene-containing
tailings
include, for example, oxygen-containing niinerals, such as Group 4B metal
oxides,
particularly titania, zirconia, iron oxide-titania minerals (e.g., ilmenite)
and
combinations thereof. In addition to recovering energy and/or materials, the
disclosed
method and system have the potential to reduce the adverse environmental
consequences associated with direct disposal of asphaltene-containing
tailings.
The disclosed method and system also can reduce costs associated with solvent
removal in the TSRU. Conventionally, steam stripping is used to remove the
solvent.
Steam stripping does not always result in a near complete separation of the
solvent and
it can be expensive due to the energy demands. Steam is required not only for
stripping
the volatile organic phase, but also for preheating the TSRU tailings and the
stripping
medium. Incorporating a separation process downstream from the TSRU has the
potential to significantly reduce the need for a near complete separation of
the solvent in
the TSRU. For example, in embodiments of the disclosed method, the
tailings_that exit
the TSRU may contain some solvent. This solvent can be removed with the
asphaltenes
by the various separations, such as flotation and/or hydrophobic agglomeration
separations. By eliminating the need for a near complete separation of the
solvent in the
TSRU, it is possible to use a less expensive solvent recovery process in the
TSRU, such
as vacuum stripping or column flotation under an inert gas (such as nitrogen)
blanket.
These processes can result in a solvent recovery, for example, between greater
than 0%
and 99.9%, such as between greater than, 0% and about 99% or between greater
than 0%
and about 95%. In comparison to steam stripping, these processes typically
require
significantly less heat and can be carried out at ambient temperatures. For
example, the
tailings that exit the TSRU can have a temperature between about 20 C and
about 85
C, such as between about 20 C and about 65 C or between about 20 C and
about 55
C.
-8-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Several different types of separations can be used in embodiments of the
disclosed method, including cyclone separation (e.g., gas-sparged
llydrocyclone
separation), flotation separation, gravity separation, hydrophobic
agglomeration
separation, and combinations thereof. hi some implementations, the separations
are
customized to the special characteristics of the asphaltene-containing
tailings being
processed. The separations also can be customized to the processing scheme.
For
example, the separations can be modified to accommodate continuous, batch or
semi-
batch processing.
Cyclone separation can be used, for example, to remove coarse material from
the asphaltene-containing tailings prior to further processing. Separating
coarse
materials at this stage may facilitate improved operation of downstream
equipment.
Cyclone separation can include inducing or facilitating spinning of the
asphaltene-
containing tailings in a conical vessel. The resulting centrifugal force
causes some
materials suspended in the tailings to collect in an underflow. When performed
on
TSRU tailings from a process for the recovery of hydrocarbons from oil sand,
the
underflow exiting the cyclone separator is likely to include coarse minerals
and heavy
minerals and some water. The coarse minerals can be separated from the water,
for
example, by gravity settling. The water then can be recycled back into the
process. The
overflow can be routed to a holding tank for further processing.
Like other cyclone separation processes, gas-sparged hydrocyclone separation
typically includes the application of centrifugal force. Gas-sparged
hydrocyclone
separation, however, also includes introducing fine gas bubbles into the
asphaltene-
containing tailings while centrifugal force is being applied. For example, the
bubbles
can be introduced through fme holes in the walls of a conical vessel in which
the
asphaltene-containing tailings are spun. Introducing these bubbles further
promotes
separation by the flotation principles discussed below. The gas can be, for
example, air
or another inert gas.
As mentioned above, flotation often is used in processes for recovering
hydrocarbons from oil sand. Flotation also can be used to separate asphaltenes
and
certain target minerals from other materials in asphaltene-containing
tailings. The
-9-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
target minerals can include valuable minerals, such as titania, ilmenite and
zirconia, as
well as minerals that may be harmful to the environment, such as sulfur-
containing
minerals. Flotation can be conducted over one or more than one separate
stages. For
example, some embodiments include a rougher stage to effect an initial or
rough
separation targeting high recovery, a scavenger stage to scavenge any
remaining
asphaltenes or target minerals and a cleaner stage to clean any one of the
rougher or
scavenger stage products of asphaltene or target minerals to higher purity.
Each
successive stage can be configured and optimized to the recovery of
diminishing
concentrations of asphaltenes and target minerals. Recirculation, recycle or
re-
treatment of some streams and products also can be included.
In some disclosed embodiments, separation by flotation includes introducing
gas, such as air or nitrogen, into the asphaltene-containing tailings.
Reagents also can
be introduced, as discussed, to achieve one or more desired results. These
reagents can
include, for example, frother reagents. Some embodiments include the use of a
frother
reagent selected to promote the formation of stable bubbles, such as stable
bubbles that
attract asphaltenes and/or the target minerals. Useful frother reagents
include, for
example, aliphatic alcohols, cyclic alcohols, phenols, alkoxy paraffins,
polyglycols and
combinations and derivatives thereof. In some embodiments, the frother
reagents have
a polar group, such as a hydroxyl polar group, a carboxyl polar group, a
carbonyl polar
group, an amino polar group, a sulfo polar group, or a combination thereof.
The frother
reagents can be introduced at a concentration selected to promote the
formation of
stable bubbles, such as stable bubbles that attract asphaltenes and/or the
target minerals.
For example, the frother reagents can be introduced at a concentration between
about 5
ppm and about 100 ppm, such as between about 15 ppm and about 35 ppm.
Some embodiments also include the use of collector reagents selected to
increase the hydrophobicity (i.e., the contact angle) of the asphaltenes
and/or the target
minerals. Useful collector reagents include fuel oils, sodium oleate, fatty
acids,
xanthates, alkyl sulfuric salts, dithiophosphates, amines and combinations and
derivatives thereof. The collector reagents can be anionic or cationic. The
collector
reagents can be introduced at a concentration selected to increase the
hydrophobicity of
-10-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
the asphaltenes and/or the target minerals. For example, the collector
reagents can be
introduced at a concentration between about 5 ppm and about 500 ppm, such as
between about 25 ppm and about 50 ppm.
In addition to frother reagents and collector reagents, some embodiments
include the use of modifiers, such as depressants, dispersants, regulators,
and activators.
Depressants can be used, for example, to surface coat certain minerals to
prevent
hydrophobicity and floating of these minerals. Depressants can be used in
conjunction
with collector reagents to selectively float target minerals. This process can
be used, for
example, to separate particles within the asphaltene-containing tailings.
Regulators can
be used, for example, to control the pH of the asphaltene-containing tailings.
Activators
can be used, for example, to promote interaction between the collector reagent
and the
asphaltenes and/or the target minerals.
During flotation, the asphaltenes and the target minerals attach to and rise
with
the gas bubbles to form an asphaltene-rich froth while other materials remain
in the
aqueous solution. This occurs because the asphaltenes and target minerals,
either
naturally or by action of a collector reagent, are hydrophobic. The minerals
that remain
in the aqueous solution are those minerals that, either naturally or by action
of a
depressant, are hydrophilic. In addition to asphaltenes and target minerals,
the
asphaltene-rich froth may include naturally floatable minerals, minerals
entrained in the
asphaltenes and residual solvent. After the flotation process, the remaining
aqueous
phase can be routed to recycle for heat and water recovery or disposal and the
asphaltene-rich froth can be routed to further processing.
After flotation to separate asphaltenes and/or target minerals from other
materials in the asphaltene-containing tailings, the resulting asphaltene-rich
froth can be
thickened, such as by the removal of at least a portion of the contained gas
phase. The
thickening process also can include the removal of at least a portion of the
water.
Thickening can be performed, for example, using a dewatering cyclone or a
conventional dewatering, clarifying, thickening and/or filtration process
resulting in a
clarified water overflow and an underflow. Excess water can be recovered with
the
- 11 -
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
overflow. The underflow can take the form of an asphaltene-rich slurry or an
asphaltene-rich filter cake, which can be routed to further processing.
Some disclosed embodiments include one or more gravity separation processes.
Gravity separation can be used, for example, to separate the asphaltene-rich
froth or the
asphaltene-rich slurry into a light tailings and a heavy mineral concentrate.
If the
gravity separation follows another separation step, such as a flotation
separation, the
heavy mineral concentrate may include a high percentage of the minerals
targeted for
recovery as well as unwanted minerals to be rejected. Reagents can be added to
enhance the separation of the two phases. Attrition scrubbing also can be used
to clean
the mineral surfaces thereby enhancing the separation. Useful reagents for use
in
connection with a gravity separation process for separating the asphaltene-
rich froth or
the asphaltene-rich slurry into the light tailings and the heavy mineral
concentrate
include, for example, dispersants, surfactants and solvents. These reagents
facilitate the
separation, for example, by surface charge alteration and dispersion. In some
embodinlents, the dispersant comprises a silicate, a phosphate, a citrate, a
lignin
sulfonate, or a combination or derivative thereof. Flotation and gravity
separation can
be combined into one process step, such as an air-sparged hydrocyclone
flotation step
(as shown, for example, in U.S. Patent No. 4,838,434, which is incorporated
herein by
reference).
To recover an asphaltene concentrate, some embodiments include a hydrophobic
agglomeration separation, which also may be referred to as a hydrophobic
flocculation
separation, an oil agglomeration separation or an oil flocculation separation.
One
example of such as separation is shown in U.S. Patent No. 5,162,050, which is
incorporated herein by reference. This separation can be performed, for
example, on
the light tailings that exit the gravity separation, on the asphaltene-rich
froth that exits
the flotation separation or on the asphaltene-rich slurry that exits the
thickening step.
Hydrophobic agglonleration generally involves the use of a hydrophobic
agglomeration
agent that flocculates small particles of the material to be separated into
larger flocs.
The selectivity arises from differences in the surface properties of the
materials in the
solution, particularly differences in hydrophobicity. Typically, the
hydrophobic
-12-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
agglomeration agent is introduced into the solution and then is dispersed to
form
droplets. The hydrophobic agglomeration agent also can be introduced and
dispersed
simultaneously. The droplets agglomerate with some materials and leave other
materials in the solution. Dispersing the hydrophobic agglomeration agent to
form
droplets can be accomplished, for example, by agitating the solution or
spraying the
hydrophobic agglomeration agent through a nozzle. Once agglomeration has
occurred,
the large flocs including the material to be separated can be removed from the
solution,
such as by settling or filtration.
Hydrophobic agglomeration is used in some disclosed embodiments to separate
asphaltenes. For example, hydrophobic agglomeration can follow a flotation
separation
or a gravity separation. Hydrophobic agglomeration often is performed as a
final
separation before recovery of an asphaltene concentrate because it allows for
the rapid
separation of asphaltenes from water. Hydrophobic agglomeration also can have
a high
degree of selectivity, which allows for the recovery of a relatively pure
asphaltene
concentrate. After it is formed, the asphaltene concentrate can be upgraded
into more
valuable hydrocarbon products or burned, for example, as a feed stock for a
gasifier.
Any minerals in the remaining solution also can be recovered. In some
embodiments,
the remaining solution is combined with previously separated minerals, such as
a heavy
mineral concentrate that exits a gravity separation.
The hydrophobic aggloineration process can be configured to maximize the
selective recovery of asphaltenes. For example, a hydrophobic agglomeration
agent can
be selected that selectively agglomerates with asphaltenes, while leaving
other materials
in the solution. In some embodiments, the hydrophobic agglomeration agent
comprises
diesel, a fuel oil, a surfactant, or a combination or derivative thereof. The
hydrophobic
agglomeration agent can be introduced at a concentration selected to separate
asphaltenes from other components in the solution. For example, the
hydrophobic
agglomeration agent can be introduced at a concentration between about 5,000
ppm and
about 15,000 ppm, such as between about 10,000 ppm and about 12,000 ppm.
Hydrophobic agglomeration is facilitated in some embodiments by the addition
of one or more oxidizing agents, such as oxygen, or a chemical oxidizing
agent, such as
-13-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
a peroxide, a hydroxide, a permanganate, Fenton's reagent, or a combination or
derivative thereof. The oxidizing agent, if used, can be added in an amount
that
facilitates the desired result, such as an amount ranging from about 3,500 ppm
to about
10,000 ppm or an amount ranging from about 5,000 ppm to about 7,500 ppm.
Oxidizing agents can be used, for example, to oxidize the surfaces of minerals
to be
separated from the asphaltenes. This may improve selectivity by reducing or
substantially eliminating hydrophobic compounds attaclied to these surfaces.
For
example, in some embodiments, oxidation is used to convert and substantially
eliminate
residual collector reagent adhered to the minerals during a previous flotation
separation.
Oxidation also may be useful to eliminate hydrophobic materials that naturally
adhere
to the surfaces of certain minerals, such as pyrite. Other reagents that may
be used in
connection with the hydrophobic agglomeration separation include dispersant
reagents,
modifying reagents, and causticizing agents. Examples of potentially useful
causticizing agents include sodium hydroxide, potassium hydroxide, quicklime
and
combinations thereof.
In addition to separations directed to the recovery of asphaltenes, some
embodiments include separations directed to the recovery of certain materials,
such as
lignite-type materials, sulfur-containing minerals and/or oxygen-containing
minerals,
particularly sulfide minerals and/or oxide minerals. In embodiments in which
solvent
exits the TSRU with the asphaltenes, it may be useful to perform at least some
mineral
recovery upstream from the TSRU. This can be useful, for example, to retain a
combined solvent/asphaltene stream with minimum inorganic compounds. In some
embodiments, a heavy mineral concentrate is separated from the asphaltene-
containing
tailings, such as by gravity separation, and subjected to further processing.
Further
processing can begin with an attritioning step, which can include shear
attritioning,
scrubbing or cycloning. Attritioning, like oxidation, can be useful to clean
the mineral
surfaces, such as to remove residual collector reagent adhered to the minerals
during a
previous flotation separation. The attritioning can involve subjecting the
minerals to a
high sliear environment either in an attrition scrubber or attrition mill
where the surfaces
can rub together in an autogenous cleaning action.
-14-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Some embodiments include one or inore steps for separating sulfur-containing
minerals from other minerals to be recovered. Although they typically have
little or no
commercial value, sulfur-containing minerals can be separated with other
target
minerals to prevent their inclusion in tailings exiting the overall process.
This reduces
the environmental impact of tailings disposal because sulfur-containing
minerals (e.g.,
pyrite, marcasite, etc.) tend to oxidize when stored in a tailings pond. The
separation of
sulfur-containing minerals from other minerals, particularly oxygen-containing
minerals, can be accomplished, for example, by flotation. Frotlier and
collector
reagents can be used to facilitate the separation. Useful frother reagents
include, for
example, aliphatic alcohols, cyclic alcohols, phenols, alkoxy paraffins,
polyglycols, and
combinations and derivatives thereof. In some embodiments, the frother
reagents have
a polar group, such as a hydroxyl, a carboxyl, a carbonyl, an amino or a sulfo
polar
group, or a combination thereof. The frother reagents can be introduced at a
concentration selected to promote the formation of stable bubbles that attract
sulfur-
containing minerals. For example, the frother reagents can be introduced at a
concentration between about 5 ppm and about 100 ppm, such as between about 10
ppm
and about 25 ppm. Useful collector reagents include fuel oils, sodium oleate,
fatty
acids, xanthates, alkyl sulfuric salts, dithiophosphates, amines or
combinations or
derivatives thereof. The collector reagents can be anionic or cationic. The
collector
reagents can be introduced at a concentration selected to increase the
hydrophobicity of
the sulfur-containing minerals. For example, the collector reagents can be
introduced at
a concentration between about 5 ppm and about 100 ppm, such as between about
25
ppm and about 50 ppm.
The introduction of gas bubbles, such as air bubbles, then can result in the
formation of a sulfur-rich froth over a sulfur-depleted aqueous phase. Solid--
sulfur-
containing minerals can be recovered from the sulfur-rich forth and stockpiled
as a solid
waste product or subjected to further processing to create a saleable product.
The
sulfur-depleted aqueous phase can have a high concentration of the minerals
targeted
for recovery. These minerals can include, for example, oxygen-containing
minerals,
such as Group 4B metal oxides, particularly titania, ilmenite and zirconia,
which have
-15-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
significant value. The recovered minerals can be sold as commodities or
upgraded by
further purification and/or chemical modification. Recovered titania, for
example, can
be used to produce a pigment (as shown, for example, in U.S. Patent No.
6,375,923,
which is incorporated herein by reference).
Embodiments of the disclosed method and system can be used to recover energy
as well as asphaltenes, solvent and minerals. Asphaltene-containing tailings
often have
excess heat energy relative to the ambient environment because solvent
recovery in
processes for recovering hydrocarbons from oil sand typically includes steam
stripping.
In some disclosed embodiments, aqueous tailings streams are produced by
several
different separation steps. Heat can be recovered from each of these aqueous
tailings
streams. The aqueous tailings streams also can be consolidated and subjected
to a
unified energy recovery process. For example, the consolidated tailings can be
passed
though a single heat exchanger. The heat exchanger can be used, for example,
to heat
water in the TSRU prior to its conversion into steam.
In addition to the primary unit operations, such as the unit operations
described
above, embodiments of the disclosed method and system can include secondary
unit
operations, such as pumps, plenums and regulators.
Some embodinients of the disclosed method and system for recovering energy
and/or materials from asphaltene-containing tailings are described with
reference to the
figures in the following subsections.
Asphaltene-Containing Tailings
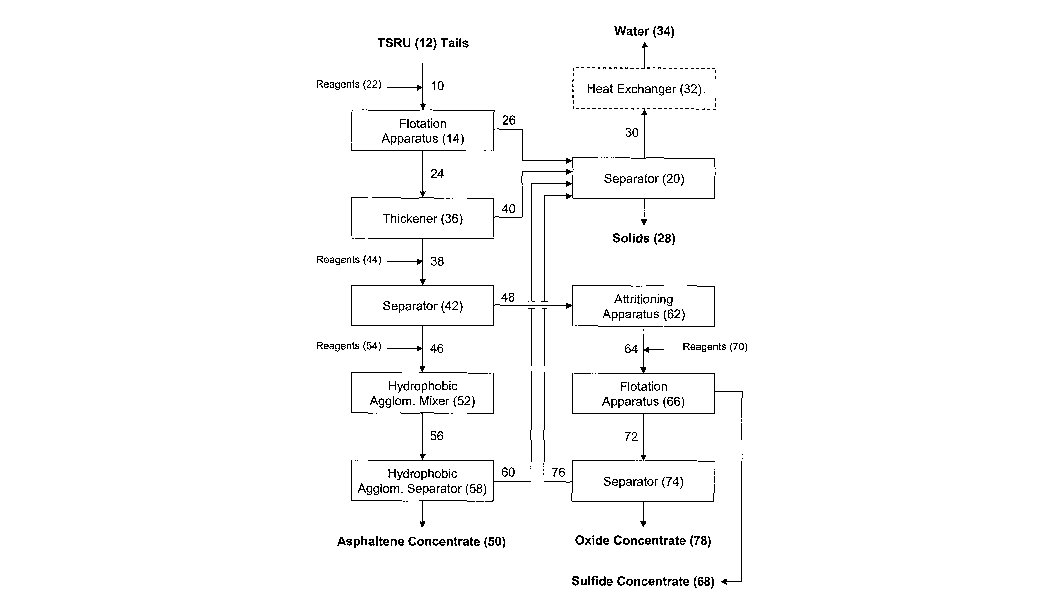
In some disclosed embodiments, asphaltene-containing tailings 10 originate in
a
TSRU 12 unit operation. The asphaltene-containing tailings 10 that exit the
TSRU 12
can be routed directly into a flotation apparatus 14, as shown in FIG. 1.
Alternatively,
as shown in FIG. 2, the asphaltene-containing tailings 10 can be routed
through a
separator 16, such as a cyclone separator, before entering the flotation
apparatus 14.
The separator 16 can be useful, for example, to separate coarse or heavy
materials from
the asphaltene-containing tailings 10 before the asphaltene-containing
tailings 10 enter
the flotation apparatus 14. The underflow 18 containing the coarse or heavy
materials
-16-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
can exit the separator 16 and be routed to a separator 20, which is described
in greater
detail below. The overflow 21 can be routed to the flotation apparatus 14.
The flotation apparatus 14 can be used to separate asphaltenes and target
minerals from other materials in the asphaltene-containing tailings 10. The
floatation
apparatus 14 can include a single floatation cell or multiple flotation cells,
such as
staged flotation cells configured as roughing, cleaning and/or scavenging
cells.
Reagents, indicated as 22 in FIGS. 1 and 2, can be added prior to or during
the flotation
process to facilitate the process as desired. The reagents 22 can include, for
example, a
frother reagent, a collector reagent, a modifier, or a combination thereof. In
some
embodiments, the reagents 22 include sodium hydroxide, a fuel oil, a glycol
frother, or a
combination or derivative thereof.
The flotation process within the flotation apparatus 14 can include
introducing
gas into the asphaltene-containing tailings 10. The flotation apparatus 14
can, for
example, include a conventional agitated tank cell or a gas or mechanically
stirred
column cell. The solution can be mechanically agitated to promote the
formation of
bubbles of the gas and to promote interaction between the bubbles and the
asphaltenes
and/or the target minerals. In some embodiments, agitation is created by a
mechanically-driven member located near the bottom of a vessel. The gas
bubbles can
be introduced via a gas conduit between a pressurized source and one or more
openings
within the vessel. In some embodiments, the gas is introduced near the
mechanically-
driven member so that the strong agitation readily distributes the bubbles
throughout the
asphaltene-containing tailings 10. The gas bubbles also can be introduced
through a
nozzle or though a perforated conduit. Typically, the gas is air, although in
some
embodiments it can be an inert gas such as nitrogen.
During the flotation process within the flotation apparatus 14, the
asphaltenes
and/or the target minerals in the asphaltene-containing tailings 10 rise with
the gas
bubbles to form an asphaltene-rich froth 24 over an asphaltene-depleted
aqueous phase
26. The asphaltene-depleted aqueous phase 26, which typically includes water
and non-
floatable minerals, can be routed to the separator 20, where it can be
separated into
-17-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
solids 28 and water 30. The separator 20 can be any separator capable of
separating
solids from water. In some embodiments, the separator 20 is a cyclone or a
thickener.
The solids 28 exiting the separator 20 can include minerals that were not
targeted for removal with the asphaltenes during the flotation process within
the
flotation apparatus 14. In some embodiments, the solids 28 mainly comprising
inorganic materials (e.g., silica sand), are disposed of as a waste material.
To reduce the
adverse environmental impact associated with disposal of the solids 28, some
disclosed
embodiments include the separate removal of potentially hannful materials from
the
asphaltene-containing tailings 10. For example, in some embodiments, sulfur-
containing minerals, which can be damaging to the environment, are targeted
for
separation during the flotation process within the flotation apparatus 14 so
as to
minimize their concentration in the solids 28. The sulfur-containing minerals
can be
targeted, for example, by using a collector reagent that increases the
hydrophobicity of
the sulfur-containing minerals. By removing sulfur-containing minerals with
the
asphaltene-rich froth 24 exiting the flotation apparatus 14, the concentration
of sulfur-
containing minerals in the solids 28 can be reduced, for example, to between
about
0.05% and about 0.8%, such as between about 0.1% and about 0.5% or between
about
0.2% and about 0.3%.
If the asphaltene-containing tailings 10 exit the TSRU 12 at an elevated
temperature, the water 30 exiting the separator 20 is likely to contain excess
heat energy
relative to the ambient environment. In some embodiments, the water 30 is
routed back
to the TSRU 12 to be converted into steam or to an alternative part of the
process for
reuse. The water 30 also optionally can be routed through a heat exchanger 32.
Heat
from the heat exchanger 32 can be used, for example, to partially heat water
before it is
converted into steam for use in the TSRU 12. The water 34 that exits the heat
exchanger 32 can be recycled for use in other unit operations of the oil sand
recovery
processes.
After exiting the flotation apparatus 14, the asphaltene-rich froth 24 can be
routed to a thickener 36. The thickener 36 can be configured to thicken the
asphaltene-
rich froth 24 into an asphaltene-rich thickener underflow slurry 38. The
thickener 36
-18-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
can operate, for example, by removing gas and water from the asphaltene-rich
froth 24.
Removed water 40 can be routed to the separator 20 to be separated and
recycled with
the asphaltene-depleted aqueous phase 26. The asphaltene-rich thickener
underflow
slurry 38 can be routed to a separator 42, such as a gravity separator, for
further
processing.
The separator 42 can be used to separate the minerals removed with the
asphaltene-rich froth 24 from the asphaltenes. These minerals can include
minerals of
value to be recovered during later processing and minerals removed to avoid
their
inclusion in the solids 28. Separation at this separation stage is exemplified
by gravity
separation. Gravity separation can be accomplished using several different
techniques.
In some embodiments, the separator 42 is a shaking table. Shaking tables
typically
provide agitation that causes lighter materials to move greater distances than
heavier
materials. Ridges can be included on the surface of the table to further
inhibit
movement of the heavier materials while allowing movement of the lighter
materials.
Other suitable types of gravity separators include hydrocyclones, spiral
concentrators,
fluidized bed hydrosizers and centrifugal concentrators. Reagents, indicated
as 44 in
FIGS. 1 and 2, can be added to facilitate the separation.
The asphaltene-rich thickener underflow slurry 38, after exiting the
separator.42,
can be separated into a light tailings 46 and a heavy mineral concentrate 48.
These
streams can be subjected to further processing.
Light Tailings
The light tailings 46 that exit the separator 42 can be processed to recover
an
asphaltene concentrate 50. In some disclosed embodiments, hydrophobic
agglomeration is used to recover the asphaltene concentrate 50. For example,
the light
tailings 46 can be routed into a liydrophobic agglomeration mixer 52. Reagents
54 can
be added, including a hydrophobic agglomeration agent. The light tailings 46
and the
hydrophobic agglomeration agent can be mixed in the hydrophobic agglomeration
mixer 52 to disperse the hydrophobic agglomeration agent into droplets. These
droplets
then can agglomerate with the asphaltenes in the light tailings 46 to form
asphaltene-
-19-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
containing particles. In addition to the hydrophobic agglomeration agent, the
reagents
54 can include an oxidizing agent and/or a causticizing agent.
In some disclosed embodiments, the resulting mixture 56, including the
asphaltene-containing particles, is routed from the hydrophobic agglomeration
mixer 52
to a hydrophobic agglomeration separator 58. In other embodiments, mixing and
separating occur in the same device. Within the hydrophobic agglomeration
separator
58, the asphaltene-containing particles can be separated from a remainder 60,
such as by
settling or filtration. Filtration can be performed, for example, using a mesh
with an
average pore size between about 150 m and about 750 m, such as between about
250
m and about 500 m or between about 275 m and about 325 m. The remainder 60,
which can include water and any remaining mineral solids, can be routed to the
separator 20 for recycling or disposal.
Some embodiments of the disclosed method yield an asphaltene concentrate 50
with a relatively high degree of purity. For example, in some embodiments, the
asphaltene concentrate 50 includes between about 60% and about 95%
asphaltenes,
such as between about 70% and about 90% or between about 80% and about 90%.
After recovery, the asphaltene concentrate 50 can be sold as a commodity, such
as a
fuel, or subjected to further processing, such as to upgrade the asphaltene
concentrate
50 into oil or into gas through a gasification process.
Heavy Mineral Concentrate
The heavy mineral concentrate 48 that exits the separator 42 can be routed to
an
attritioning apparatus 62. The attritioning process within the attritioning
apparatus 62
can include grinding the heavy mineral concentrate 48 to disperse aggregates
and
remove any coatings that may interfere with subsequent processing. The
attritioning
apparatus 62 can be, for example, a high shear mixer, attrition scrubber or an
attrition
grinding mill.
After exiting the attritioning apparatus 62, the attritioned minerals 64 can
be
routed to a flotation apparatus 66 for separation. The flotation apparatus 66
can be
used, for example, to separate a sulfur-containing mineral concentrate 68 from
the
-20-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
attritioned minerals 64. Separating sulfur-containing minerals in a
concentrated form
can be useful to reduce the environmental impact of the waste materials
created by the
overall process. The flotation apparatus 66 can be configured for the
separation of
sulfur-containing minerals, for example, by selection of reagents 70. The
floatation
apparatus 66 can include a single floatation cell or multiple flotation cells,
such as
staged flotation cells configured as roughing, cleaning and/or scavenging
cells. As with
the reagents 22 used with the flotation apparatus 14, the reagents 70 can
include, for
example, a frother reagent and/or a collector reagent. In addition to frother
reagents and
collector reagents, the reagents 70 can include modifiers, such as
dispersants,
regulators, and activators.
The sulfur-containing mineral concentrate 68 can exit the flotation apparatus
66
with the froth. In some embodiments, the froth is thickened and any remaining
water is
removed to solidify the sulfur-containing mineral concentrate 68. Any
asphaltenes
removed from the sulfur-containing mineral concentrate 68 can mixed with the
light
tailings 46 described above. After separation of the sulfur-containing mineral
concentrate 68, the remaining sulfur-depleted aqueous phase 72 can include the
minerals targeted for recovery, such as commercially valuable minerals
included in the
oil sand from which the asphaltene-containing tailings 10 were derived. These
minerals
can include, for example, oxygen-containing minerals, such as Group 4B metal
oxides,
particularly titania, ilmenite and zirconia. In some embodiments, the sulfur-
depleted
aqueous phase 72 is routed to a separator 74 after exiting the flotation
apparatus 66.
Within the separator 74, a remainder 76 can be separated, leaving an oxygen-
containing
mineral concentrate 78. The remainder 76, which includes mostly water, can be
routed
to the separator 20 for recycling or disposal.
The oxygen-containing mineral concentrate 78 can be sold as a commodity or
subjected to further processing. Further processing can include refining into
specific
mineral types (e.g., ilmenite, leucoxene, anatase, rutile and zirconia). This
can be done,
for example, using conventional magnetic and electrostatic separations. These
and
other separation processes can be used to produce various grades of product,
including
ultra pure commercial grade concentrates. In some disclosed embodiments, an,
ilmenite
-21-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
mineral concentrate or other titania-containing mineral concentrate from the
oxygen-
containing mineral concentrate 78 is upgraded into pigment.
EXAMPLES
The following examples are provided to illustrate certain particular
embodiments of the disclosure. Additional embodiments not limited to the
particular
features described are consistent with the following examples.
Example 1
An initial flotation separation on TSRU tailings was carried out in a 3 meter
long column flotation cell. The flotation was conducted at a temperature of 70
to 75 C.
A glycol ester frother reagent was added at a concentration of 25 grams per
ton of
solids. After optimization of the flotation conditions, a high grade
concentrate (froth)
containing the asphaltenes and heavy minerals was produced. The silicate and
clay
non-targeted minerals were rejected to a tailings product. The grades of
various
minerals in the concentrate, tailings and feed streams are shown in Table 1,
along with
the percent recovery of the minerals in the concentrate and tailings. As shown
in Table
1, the mass reject to tailings was 33.5% of the total feed. Recoveries of the
targeted
asphaltenes and heavy minerals were high. In laboratory tests, the tailings
from the
flotation were successfully thickened using a commercial polymeric flocculant.
Clean,
hot supematant water was recovered from the flocculated tailings. This
illustrates one
example of heat and energy recovery.
-22-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Table 1: Data for Froth Flotation Separation of TSRU Tailings
Grade - %
Wt % A1203 Si02 Ti02 Zr02 Fe S C LOI*
Concentrate 66.5 6.7 15.5 6.9 1.9 3.8 5.7 40.9 59.9
Tailing 33.5 12.5 73.6 1.4 0.08 1.3 0.4 2.6 8.4
Feed 100 8.6 35.0 5.0 1.3 3.0 3.9 28.1 42.6
Recovery - %
Wt % A1203 Si02 Ti02 Zr02 Fe S C LOI*
Concentrate 66.5 51.5 29.4 91.0 98.0 85.6 97.0 96.8 93.4
Tailing 33.5 48.5 70.6 9.0 2.0 14.4 3.0 3.2 6.6
* = Loss on ignition
Example 2
The froth flotation concentrate from Example 1 was subjected to gravity
separation to obtain an asphaltene rich phase and a heavy or oxide mineral
rich phase.
Table 2 shows the experimental data for a single stage gravity separation
process. The
results can be further improved upon by using a series of gravity separators
with
roughing, cleaning and scavenging duties.
Table 2: Data for First Stage Gravity Separation of Froth Flotation
Concentrate
Grade - %
Wt % A1203 Si02 Ti02 Zr02 Fe S C LOI
Heavy 20.2 6.6 22.0 22.6 6.5 6.5 4.6 20.7 29.7
Concentrate
Asphaltene 79.8 5.8 13.0 3.6 0.5 3.4 5.9 51.5 71.8
Lights
Feed 100.0 6.0 14.8 7.5 1.7 4.0 5.6 45.2 63.3
Recovery - %
Wt % A1203 Si02 Ti02 ZrO2 Fe S C LOI
Heavy 20.2 22.4 30.0 61.1 75.2 32.5 16.6 9.3 9.5
Concentrate
Asphaltene 79.8 77.6 70.0 38.9 24.8 67.5 83.4 90.7 90.5
Lights
- 23 -
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Example 3
The heavy mineral concentrate from Example 2 was subjected to further
cleaning using a de-oiling step. This step included conditioning the heavy
mineral
concentrate in sodium hydroxide and hydrogen peroxide to clean the particle
surfaces
and prevent the particles from floating. A further flotation step was then
used to reduce
the asphaltene content and to separate the sulfide niinerals. The sulfide
minerals were
activated with copper sulfate. A xanthate-type bulk flotation collector also
was added.
After the flotation, the resultant froth contained the sulfide niinerals and
residual
hydrocarbons. This left a cleaner heavy mineral product as a flotation
tailing. The
grades of various niinerals in the asphaltene/sulfide concentrate, heavy
mineral product
and feed streams are shown in Table 3 along with the percent recovery of the
minerals
in the asphaltene/sulfide concentrate and heavy mineral product.
Table 3: Data for Froth Flotation Separation of Heavy Mineral Concentrate
Grade %
Wt % A1203 Si02 Ti02 Zr02 Fe S C LOI
Asphaltene/Sulfide 39.8 1.3 6.6 6.3 2.7 12.9 11.3 46.7 65.8
Concentrate
Heavy Mineral 60.1 10.1 32.2 33.4 9.0 2.3 0.2 3.5 5.8
Product
Feed 100 6.6 22 22.6 6.5 6.5 4.6 20.7 29.7
Recovery %
Wt % A12O3 Si02 TiO2 Zr02 Fe S C LOI
Asphaltene/Sulfide 39.9 7.8 11.9 11.1 16.5 79.0 97.8 89.8 88.2
Concentrate
Heavy Mineral 60.1 92.0 88.0 88.8 83.2 21.3 2.6 10.2 11.7
Product
-24-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
Example 4
The asphaltene lights from Example 2 were treated by oil agglomeration. The
results of this process are shown in Table 4. The oil agglomeration process
included
treating the wet asphaltene concentrate with a caustic additive. The resultant
slurry then
was subjected to ultrasonic conditioning for 30 minutes and nlixed with diesel
in a high-
speed mixer for 10 minutes. The resultant pulp then was screened with a 50
mesh (300
m). Slime passed through the mesh while the agglomerated asphaltenes were
captured
on the mesh. The agglomerated asphaltene was re-pulped with the high-speed
niixer
and re-screened to reject additional slime. The asphaltene product was found
to contain
15% inorganic solids with in excess of 95% carbon recovery to the asphaltene
concentrate. About 70% A1203, 76% Si02 and 36% S was rejected. The asphaltene
concentrate had a carbon content of about 63% and a loss on ignition of about
86%.
The heating value was about 12,000 Btu per pound. The asphaltene concentrate
also
was found to contain residual hydrocarbon solvent that could be recovered
during
further processing and converted to lower chain hydrocarbons. The asphaltene
concentrate provides a ready fuel source for energy or heat generation in oil
sand
processing.
Table 4: Oil Agglomeration of Asphaltenes
Grade (%)
Wt % A12O3 Si02 Ti02 Zr02 Fe S C LOI
Asphaltene 71.6 2.3 5.4 2.2 0.5 3.2 5.0 63.1 86
Slime I 21.4 16.3 52.4 2.8 0.1 2.4 8.9 5.2 24
Slime II 7.0 4.7 15.5 3.2 0.4 3.4 1.1 13.5 70
Feed 100 5.5 16.2 2.4 0.4 3.0 5.6 47.2 71.6
Distribution (%)
Wt % A12O3 SiO2 Ti02 Zr02 Fe S C LOI
Asphaltene 71.59 30.1 23.9 65.7 87.9 75.3 64.4 95.6 86.0
Slime I 21.43 618 69.4 25.0 5.3 16.9 34.2 2.4 7.2
Slime II 6.98 6.0 6.7 9.3 6.9 7.8 1.4 2.0 6.8
-25-
CA 02645450 2008-09-08
WO 2007/102819 PCT/US2006/008263
In view of the many possible embodiments to which the principles of the
disclosed invention may be applied, it should be recognized that the
illustrated
embodiments are only examples of the invention and should not be taken as
limiting the
scope of the invention. Rather, the scope of the invention is defined by the
following
claims. We therefore claim as our invention all that comes within the scope
and spirit
of these claims.
-26-