Note: Descriptions are shown in the official language in which they were submitted.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
1
LIVE ATTENUATED SALMONELLA VACCINE
Field of the invention
[0001] The present invention relates to attenuated
bacterial mutants, in particular attenuated Salmonella
enterica mutants, and to a live attenuated vaccine
comprising same.
State of the art
[0002] Salmonellae are Gram-negative, facultative
anaerobic, motile, non-lactose fermenting rods belonging
to the family Enterobacteriaceae. Salmonellae are usually
transmitted to humans by the consumption of contaminated
foods and cause Salmonellosis.
[0003] Salmonellae have been isolated from many animal
species including, cows, chickens, turkeys, sheep, pigs,
dogs, cats, horses, donkeys, seals, lizards and snakes.
10004] 95% of the important Salmonella pathogens belong
to Salmonella enterica, with S. enterica serovar
Typhimurium (S. Typhimurium) and S. enterica serovar
Enteritidis (S. Enteritidis) as the most common serovars.
[0005] Salmonella infections are a serious medical and
veterinary problem world-wide and cause concern in the
food industry. Contaminated food can not be readily
identified.
[00067 Control of Salmonellosis is important, to avoid
potentially lethal human infections and considerable
economic losses for the animal husbandry industry.
[0007] The ubiquitous presence of Salmonella in nature
complicates the control of the disease just by detection
and eradication of infected animals.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
2
[0008] Several control strategies based on the
principles of competitive exclusion and vaccination have
been tested to control the infection of e.g. poultry.
[0009] Vaccination of farm animals is often considered
as the most effective way to prevent zoonoses caused by
Salmonella.
[0010] Whole-cell killed vaccines and subunit vaccines
are used in the prevention of Salmonella infections in
animals and in humans with variable results. Inactivated
vaccines in general provide poor protection against
Salmonellosis.
[0011] Live attenuated Salmonella vaccines are
potentially superior to inactivated preparations owing to:
(i) their ability to induce cell-mediated immunity in
addition to antibody responses; (ii) oral delivery with no
risk of needle contamination; (iii) effectiveness after
single-dose administration; (iv) induction of immune
responses at multiple mucosal sites; (v) low production
cost; and (vi) their possible use as carriers for the
delivery of recombinant antigens to the immune system.
[0012] Few live attenuated Salmonella vaccines are
actually on the market because results with attenuated
mutant strains have not always been good.
[0013] For example, poultry has been vaccinated with
aro- or cAMP mutants, yet many chicks died shortly after
vaccination. As the infection by Salmonella often occurs
very early, vaccination of very young chicks is crucial.
However, at this age these are very sensitive to
Salmonella, possibly due to the immaturity of their immune
system. In addition to poor protection of the vaccinated
chicks, a prolonged excretion of the vaccine strains was
often observed.
[0014] Vaccination of birds with the Megan Vacl strain,
that carries deletions in the cya and crp genes (US
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
3
Patents 5, 389, 368; US 5, 855, 879; US 5, 855, 880) results in
a reduction of the number of birds from which a virulent
Salmonella challenge strain can be isolated 7 days after
challenge. However, it does not provide a full protection
(http://www.meganhealth.com/meganvac.html).
[0015] There is thus still a need for improved live
attenuated Salmonella vaccine strains, and for improved
live attenuated vaccine strains of bacteria infecting
veterinary species in general.
[0016] Information in literature on the influence of
guaB mutations on the virulence of Salmonella and other
bacteria is sparse.
[0017] McFarland and Stocker (1987, Microbial
pathogenesis 3:129-141) reported on the reduced virulence
of guaA and guaB TnlO insertion mutants of S. Typhimurium
and S. dublin in BALB/c mice. At high dosage (2.5x107 cfu
for S. Typhimurium and 104 cfu for S. dublin), however,
these authors reported a high lethality, resulting from
the multiplication of the auxotrophic strain.
[0018] Wang et al. (2001, Infection and Immunity
69:4734-4741) reported on pre-clinical trials with a
vaccine against typhoid fever in humans. The vaccine of
Wang et al. comprised a OguaBA mutant of Salmonella typhi.
This attenuated strain, however, showed a significant
residual virulence in mice.
[0019] International patent application WO 99/58146 and
US Patent 6,190,669 disclose Salmonella typhi vaccine
strains harboring a AguaBA mutation, which are used as a
live vector to deliver foreign antigens.
Aims of the invention
[0020] An object of the present invention is to provide
attenuated Salmonella enterica strains.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
4
[0021] Another object of the present invention is to
provide a live attenuated vaccine against Salmonellosis
and methods of prevention based thereon.
[0022] Yet another object of the present invention is
to provide live attenuated strains which are useful as
live vector and as DNA-mediated vaccines expressing
foreign antigens. Such strains are highly suitable for the
development of vaccines including polyvalent vaccines.
[0023] Still another object of this invention is to
provide a method to achieve a S. enterica deletion mutant
for use in a live attenuated vaccine.
[0024] Yet a further object of this invention is to
provide the same materials and methods for the preparation
of attenuated strains of bacteria infecting veterinary
specie, poultry more in particular.
[0025] The general aim is to improve food safety and
animal health.
Sununary of the invention
[0026] A first aspect of the invention relates to an
attenuated Salmonella enterica mutant strain which is
incapable of forming de novo guanine nucleotides, and
wherein said mutant contains a deletion mutation in the
guaB gene.
[0027] The principle as demonstrated here for S.
enterica can be applied to any organism that can use the
guanine nucleotide as an intermediary. An aspect of the
invention therefore relates to an attenuated mutant strain
of a bacterium infecting veterinary species that contains
a deletion mutation in the guaB gene. The term "bacterium
infecting veterinary species" in the context of the
invention refers in particular to bacteria that are
pathogenic to veterinary species, and which can be
attenuated by a deletion mutation in the guaB gene. The
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
bacterium infecting veterinary species may be a Gram-
negative bacterium. Preferred are Gram-negative bacteria
for poultry such as Salmonella, Pasteurella, Escherichia
coli, etc. Most preferred are Salmonella enterica and
5 (pathogenic) E. coli. By "pathogenic to" is meant that the
bacterium, if not attenuated, is capable of causing an
infectious disease in the veterinary species.
[0028] The mutants of the invention fail to express a
functional GuaB gene product. In other words the guaB gene
function is impaired, whereby an auxotrophic attenuated
strain is obtained.
[0029] The mutant strain of the invention, carrying a
deletion mutation in the guaB gene, can not grow on
Minimal A medium, unless this medium is supplemented with
0.3mM guanine, xanthine, guanosine or xanthosine.
[0030] The present invention in particular aims to
provide attenuated S. Enteritidis and S. Typhimurium
strains.
[0031] Preferably the deletion mutation in the guaB
gene is introduced into parent strain S. Enteritidis phage
type 4 strain 76Sa88 or into parent strain S. Typhimurium
1491S96.
[0032] One of the attenuated S. enterica strains
obtained according to the invention is a S. Enteritidis
strain SM69 having the deposit number LMG P-21641. Another
example is the S. Typhimurium strain SM86 having the
deposit number LMG P-21646.
[0033] The attenuated mutant strains of the invention
are highly suitable for use in a live attenuated vaccine
including a polyvalent or multivalent vaccine. The
attenuated mutant strain of the invention may encode and
express a foreign antigen and may as such be used as a
live vector and/or as a DNA-mediated vacci~n.e.
[0034] A second aspect of the invention concerns a
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
6
pharmaceutical composition or a vaccine for immunizing a
veterinary species against a bacterial infection (e.g.
Salmonellosis caused by Salmonellae) comprising:
- a pharmaceutically effective or an immunizing amount
of a mutant strain according to the invention, which is
incapable of forming de novo guanine nucleotides due to a
deletion mutation in the guaB gene; and
- a pharmaceutically acceptable carrier or diluent.
Preferred compositions are those comprising a Salmonella
enterica mutant strain according to the invention.
[0035] The attenuated strain of the invention may
transfer DNA encoding a foreign antigen in a eukaryotic
cell. This foreign antigen may be encoded by and expressed
from a plasmid comprised by the attenuated mutant strain
of the invention.
[0036] In general about 102 cfu to about 1010 cfu,
preferably about 105 cfu to about 1010 cfu is administered
(examples of a pharmaceutically effective or an immunizing
amount) . An immunizing dose varies according to the route
of administration. Those skilled in the art may find that
the effective dose for a vaccine administered parenterally
may be smaller than a similar vaccine which is
administered via drinking water, and the like.
[0037] The attenuated strains of the invention, and
pharmaceutical compositions or vaccines comprising same,
are highly suitable for immunizing an animal or veterinary
species against a bacterial infection (e.g. Salmonellosis)
and possibly other diseases (in the case of a multivalent
vaccine).
[0038] A further aspect of the invention therefore
concerns a method of immunizing animals, preferably
veterinary species, more preferably poultry such as
chicken against an infection by a bacterium (e.g.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
7
Salmonellosis caused by Salmonellae), said method
comprising the step of:
administering to the animal or veterinary species in need
thereof an immunizing amount of an attenuated mutant
strain of the invention and/or of a vaccine comprising
same, whereby a protective immune response is then invoked
in the animal or veterinary species.
[0039] Examples of veterinary species to be immunized
against Salmonellosis: poultry, small or heavy livestock
such as chicken, turkey, ducks, quails, guinea fowl, pigs,
sheep, young calves, cattle etc.
[0040] In general an appropriate dose is administered,
to these animals, preferably via the oral, nasal or
parenteral route.
[0041] A last aspect of the invention relates to a
mutant strain of the invention for use as a medicament
(e.g. for use in a vaccine) . Yet another aspect of the
invention relates to the use of an attenuated mutant
strain of the invention for the preparation of a
medicament, such as a vaccine, for the prevention (and/or
treatment) of a disease caused by a pathogen (the
infecting bacterium) such as Salmonellosis. Examples of
animals or veterinary species to be treated and
recommended doses are given above.
Detailed description of the invention
[0042] It was surprisingly found that a deletion
mutation in the guaB gene can lead to attenuated
Salmonella enterica strains with significantly reduced
virulence and capable of inducing an immune response in a
livestock animal. Such deletion would attenuate any
organism that can use the guanine nucleotide as an
intermediary.
[0043] The term "gene" as used herein refers to the
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
8
coding sequence and its regulatory sequences such as
promoter and termination signals.
[0044] The deletion mutant according to the invention
is one in which the purine metabolic pathway enzyme IMP
dehydrogenase (encoded by guaB) is inactivated.
[0045] Such inactivation may be obtained via a deletion
by which the guaB gene function is impaired, leading to a
null-function (no functional gene product formed) of the
affected gene(s). A person skilled in the art knows how to
obtain such mutants and a simple test can tell whether the
guaB gene function is impaired. The mutant strain which
fails to express a functional guaB gene product cannot
grow on Minimal A medium, unless this medium is
supplemented with (e.g. 0.3mM) guanine, xanthine,
guanosine or xanthosine.
[0046] The invention aims to provide, amongst others,
attenuated S. Enteritidis and S. Typhimurium strains since
these are the most common S. enterica serovars.
[0047] The present invention provides attenuated
strains. The invention provides amongst others attenuated
Salmonella enterica strains for use, inter alia, as live
attenuated vaccines against Salmonellosis, as live vector
and/or as DNA-mediated vaccines expressing foreign
antigens. As used herein, a"forei.gn antigen" means an
antigen foreign to Salmonella.
[0048] Live vector vaccines, also called "carrier
vaccines" and "live antigen delivery systems", comprise an
exciting and versatile area of vaccinology (Levine et al,
1990, Microecol. Ther. 19:23-32). In this approach, a live
viral or bacterial vaccine is modified so that it
expresses protective foreign antigens of another
microorganism, and delivers those antigens to the immune
system, thereby stimulating a protective immune response.
Live bacterial vectors that are being promulgated include,
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
9
among others, attenuated Salmonella.
[0049] An object of the invention is to provide
attenuated strains, like attenuated S. enterica strains
for use in a live vaccine, possibly a polyvalent or
multivalent live vaccine.
[0050] One of the objects of the invention is therefore
to provide a vaccine against e.g. Salmonellosis
comprising:
- a pharmaceutically effective or an immunizing amount
of a mutant of the invention (e.g. a Salmonella enterica
mutant) which is incapable of forming de novo guanine
nucleotides, wherein said mutant contains a deletion
mutation in the guaB gene; and
- a pharmaceutically acceptable carrier or diluent.
[0051] Another object of the invention is to provide a
live vector vaccine comprising:
- a pharmaceutically effective or an immunizing amount
of a mutant of the invention (e.g. a Salmonella enterica
mutant), which is incapable of forming de novo guanine
nucleotides, wherein said mutant contains a mutation in
the guaB gene, and wherein said mutant encodes and
expresses a foreign antigen; and
- a pharmaceutically acceptable carrier or diluent.
[0052] The particular foreign antigen employed in the
(S. enterica) live vector is not critical to the present
invention.
[0053] Still another object of the invention is to
provide a DNA-mediated vaccine comprising:
- a pharmaceutically effective amount or an immunizing
amount of a mutant of the invention (e.g. a Salmonella
enterica mutant), which is incapable of forming de novo
guanine nucleotides, wherein said mutant contains a
mutation in the guaB gene; wherein said mutant contains a
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
plasmid which encodes and expresses in a eukaryotic cell,
a foreign antigen; and
- a pharmaceutically acceptable carrier or diluent.
[0054] Details as to the construction and use of DNA-
5 mediated vaccines can be found in U.S. patent 5,877,159,
which is incorporated by reference herein in its entirety.
Again, the particular foreign antigen employed in the DNA-
mediated vaccine is not critical to the present invention.
[0055] The decision whether to express the foreign
10 antigen in e.g. S. enterica (using a prokaryotic promoter
in a live vector vaccine) or in the cells invaded by e.g.
S. enterica (using a eukaryotic promoter in a DNA-mediated
vaccine) may be based upon which vaccine construction for
that particular antigen gives the best immune response in
animal studies or in clinical trials, and/or, if the
glycosylation of an antigen is essential for its
protective immunogenicity, and/or, if the correct tertiary
conformation of an antigen is achieved better with one
form of expression than the other (US Patent 5,783,196).
[0056] In the vaccines of the present invention, the
pharmaceutically effective amount or the immunizing amount
of the mutants of the present invention to be administered
will vary depending on the age, weight and sex of the
subject. By an "immunizing amount" as used herein is in
fact meant an amount that is able to induce an immune
response in the animal that receives the pharmaceutical
composition/vaccine. The immune response invoked may be a
humoral, mucosal, local and/or a cellular immune response.
[0057] The particular pharmaceutically acceptable
carrier or diluent employed is not critical to the present
invention, and are conventional in the art. Examples of
diluents include: buffer for buffering against gastric
acid in the stomach, such as citrate buffer (pH 7.0)
containing sucrose, bicarbonate buffer (pH 7.0) alone, or
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
11
bicarbonate buffer (pH 7.0) containing ascorbic acid,
lactose, and optionally aspartame. Examples of carriers
include: proteins, e.g., as found in skimmed milk; sugars;
e.g. sucrose; or polyvinylpyrrolidone.
5[0058] The deletion mutants according to the invention
have been created via standard homologous recombination
techniques, whereby part of the guaB gene, for instance
part of the guaB coding sequence, in a first step is
replaced by a resistance gene and flanking FRT sites.
[0059] Preferably, in a second step, said resistance
gene is removed by recombination between the two FRT
sites. One FRT site and the priming sites Pl and P2 remain
by the molecular mechanism of the recombination removing
the antibiotics resistance gene according to Datsenko and
Wanner (2000) (see for instance Figure 4).
[0060] A particular example of the invention relates
for instance to guaB deletion mutants of S. Enteritidis
that comprise a mutated guaB gene or coding sequence
comprising SEQ ID NO: 12.
Short description of the drawings
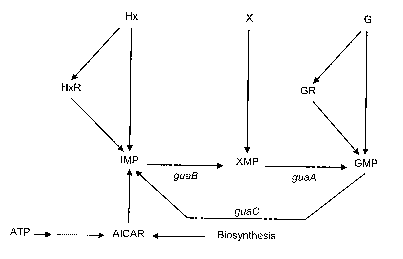
[0061] Figure 1 is a schematic representation of the
biosynthetic pathway of guanosine monophosphate (adapted
from Zalkin and Nygaard, 1996, in "Escherichia coli and
Salmonella, Cellular and Molecular Biology, Second
edition", 1996 F.C. Neidhardt ed. ASM Press, Washinggton
D.C., Vol.1, Ch. 34:561-579). AICAR: 51-phosphoribosyl-4-
carboxamide-5-aminoimidazole; ATP: adenosine triphosphate;
G: guanine; GMP: guanosine monophosphate; GR: guanosine;
Hx: hypoxanthine; HxR: hypoxanthine riboside (inosine);
IMP: Inosine monophosphate; X: Xanthine, XMP: Xanthosine
monophosphate; guaA: GMP synthetase, guaB: IMP
dehydrogenase; guaC: GMP reductase.
[0062] Figure 2 represents contig 1294 of the S.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
12
Enteritidis genome (SEQ ID NO: 10). The ATG initiation
codon and TGA termination codon of the guaB gene are in
bold.
[0063] Figure 3 represents the sequence of the AguaB
fragment of S. Enteritidis cloned in pUC18 (SEQ ID NO:
11). The primers that were used are indicated by
horizontal arrows. The fragment generated with primers
GuaB6-GuaB7 was cloned in pUCl8. The ATG initiation and
TGA termination codon of the guaB gene and the CCCGGG SmaI
restriction site are indicated in bold.
[0064] Figure 4 represents the nucleotide sequence of
the S. Enteritidis PCR fragment, which includes the guaB
deletion, obtained after sequencing, using primer GuaB10
(SEQ ID NO: 12) . The PCR fragment was amplified with
primers GuaB6-GuaB7, using total genomic DNA of the mutant
SM20. The remaining FRT site is indicated in bold italic
and the P1 and P2 primers by arrows (.Datsenko and Wanner,
2000, PNAS 97:6640-6645). The ATG initiation and TGA
termination codon of the guaB gene are indicated in bold.
[0065] Figure 5 represents the guaB gene of S.
Typhimurium LT2, section 117 of 220 of the complete genome
(SEQ ID NO: 13). The ATG initiation codon and TGA
termination codon of the guaB gene are in bold.
[0066] Figures 6-7 represent the deposit receipts of
SM69 and SM86 respectively.
[0067] The invention will be described in further
details in the following examples and embodiments by
reference to the enclosed drawings. Particular embodiments
and examples are not in any way intended to limit the
scope of the invention as claimed.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
13
Examples
Example 1: auxotrophic mutation affects the guaB gene
[0068] An auxotrophic insertion mutant of a wild type
S. Enteritidis was obtained via insertion mutagenesis.
Only when supplemented with 0.3 mM guanine, xanthine,
guanosine or xanthosine could the mutant strain grow on
Minimal A medium.
[0069] These data strongly suggest that the auxotrophic
mutation of the strain affects the guaB gene, encoding the
enzyme IMP dehydrogenase (EC 1.1.1.205). This enzyme
converts inosine-5'-monophosphate (IMP) into xanthosine
monophosphate (XMP) as indicated in Figure 1.
[0070] An insertion mutant can revert, thereby
restoring the pathogenicity of the strain. This limits its
applicability in a live attenuated vaccine. In that aspect
deletion mutants are preferred. guaB deletion mutants of
S. Enteritidis and S. Typhimurium were therefore created
and tested. The guaB genes of both serovars are given in
Figures 2 and 5.
Example 2: guaB deletion mutants
Construction of guaB deletion mutants
[0071] guaB deletion mutants were created according to
the method for generating deletion mutations in the genome
of Escherichia coli K12 (Datsenko and Wanner, 2000, PNAS
97:6640-5, incorporated by reference herein).
[0072] This method relies on homologous recombination,
mediated by the bacteriophage X Red recombinase system, of
a linear DNA fragment generated by PCR.
[0073] The guaB sequence is hereby substituted by an
antibiotic resistance gene. This resistance gene is
flanked by FRT sites (FLP recognition target sites) and
can be excised from the genome by site-specific
recombination, mediated by the FLP recombinase.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
14
[0074] Overlap PCR (Ho et al., 1989, Gene 77:51-59) was
applied to construct a linear fragment. The principle
relies on the use of two primer sets, one upstream pair
(GuaB3 -GuaB4 ; GuaB3 : 5' GGCTGCGATT GGCGAGGTAG TA 31, SEQ
ID NO 2; GuaB4: 5' GGTGATCCCG GGCGTCAAAC GTCAGGGCTT CTTTA
3', SEQ ID NO 3) and one downstream pair (GuaB5-GuaB2;
GuaB5: 5' TTGACGCCCG GGATCACCAA AGAGTCCCCG AACTA 31, SEQ
ID NO 4; GuaB2: 5' CGTTCAGGCG CAACAGGCCG TTGT 3', SEQ ID
NO 1) of the guaB gene.
[0075] Both sets contain primers (GuaB4, GuaB5) that
are partially complementary and to which a SmaI
restriction site was added.
[0076] After annealing of the resulting complementary
sequences and chain elongation, PCR with the outward
primers (GuaB6-GuaB7; GuaB6: 5' GCAACAACTC CTGCTGGTTA 3',
SEQ ID NO 5; GuaB7: 5' AGACCGAGGA TCACTTTATC 3', SEQ ID NO
6) generated a fragment with a 6 basepair Smal site
replacing an 861 basepair internal segment of the guaB
coding sequence. This OguaB fragment was cloned in the
vector pUC18 (see Figure 3).
[0077] The chloramphenicol resistance gene- (cat) with
its flanking FRT sequences was amplified using the primers
P1 (5' GTGTAGGCTG GAGCTGCTTC 3', SEQ ID NO 8) and P2 (5'
CATATGAATA TCCTCCTTAG 3', SEQ ID NO 9) (Datsenko and
Wanner, 2000) and plasmid pKD3 DNA (Datsenko and Wanner,
2000) as a template.
[0078] This PCR fragment was ligated in the SmaI site
of the cloned OguaB fragment. The desired fragment was
generated using nested primers (GuaB6-GuaB7).
[0079] The resulting PCR fragment was electroporated
into S. Enteritidis phage type 4 strain 76Sa88 (a clinical
isolate from a turkey, obtained from the Veterinary and
Agrochemical Research Centre, Groeselenberg 99, B-1180
Ukkel, Belgium) harboring the temperature sensitive
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
replication plasmid pKD46, encoding the bacteriophage
Lambda Red recombinase system.
[0080] The chloramphenicol resistant transformants were
tested on Minimal A medium and on Minimal A medium
5 supplemented with 0.3 mM guanine. The oguaB::catFRT
mutants were confirmed by PCR using the following primer
combinations: GuaB6-GuaB7, GuaB6-P2, GuaB7-Pl and Pl-P2.
[0081] The S. Enteritidis oguaB::catFRT mutant (SM12)
was transformed with the temperature sensitive replication
10 plasmid pCP20 by electroporation. The plasmid pCP20
encodes the FLP recombinase, which recognizes the FRT-
sites, to remove the cat gene. The resulting strain was
named SM20.
[0082] The PCR fragment in which the deletion is
15 located was obtained using total genomic DNA of the mutant
SM20 and the primer combination GuaB6-GuaB7 (see Figure
4).
[0083] The aguaB mutation was confirmed by sequencing
of this fragment, using the primer GuaBlO (5' AGGAAGTTTG
AGAGGATAA 3', S E Q ID NO 7).
[0084] The sequences of all above-mentioned primers are
given in Table 1.
[0085] To avoid the presence of possible additional
mutations, caused by the expression of the Red recombinase
system, an isogenic strain was constructed.
[0086] The 4guaB::catFRT mutation of the mutant SM12
was transduced by bacteriophage P22 HT int- (Davis, R.W.,
Botstein D. and Roth, J.R. (1980) In Advanced Bacterial
Genetics, A manual for genetic engineering. Cold Spring
Harbor Laboratory, Cold Spring Harbor, N. Y. ) to wild type
S. Enteritidis 76Sa88. The cat gene was removed using the
plasmid pCP20. The resulting strain was called SM69
(deposit number LMG P-21641).
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
16
[0087] A LguaB mutant of S. Typhimurium strain 1491S96
was constructed using the same procedure and the same
primers. The resulting strains S. Typhimurium
LlguaB: : catFRT and S. Typhimurium aguaB were named SM9 and
SM19 respectively. SM86 (having the deposit number LMG P-
21646) is the isogenic strain obtained therefrom, after
transduction by bacteriophage P22 HT int- lysate from
strain SM9 and excision of the cat gene using the plasmid
pCP20.
[0088] The LguaB mutants SM19, SM20, SM86 and SM69 are
sensitive to bacteriophage P22. This proves the presence
of intact lipopolysaccharides (LPS).
Virulence and protection tests with the guaB deletion
mutant SM20 in mice
[00891 The virulence of the mutant SM20 in mice was
tested by oral infection of 6-8 week old female BALB/c
mice in two independent experiments. These were performed
as described above. The wild type strain S. Enteritidis
76Sa88 was tested in parallel as a positive control. The
S. Enteritidis 76Sa88 LaroA mutant SM50 was included in
the experiment as a vaccine control. This mutant carries a
precise deletion of the complete aroA coding sequence and
was constructed by the method of Datsenko and Wanner
(2000).
[0090] The complete data are given in Tables 2 and 3.
These results demonstrate that the AguaB mutant SM20 is
strongly attenuated in mice but still shows some residual
pathogenicity when administered at this high dose. Oral
immunization with the mutant induces protective immunity
against infection by a high dose of the corresponding
pathogenic wild type S. Enteritidis strain 76Sa88. The
protection is at least equal to the protection conferred
by the S. Enteritidis L1aroA mutant SM50.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
17
Virulence and protection tests with the isogenic guaB
deletion mutants SM69 and SM86 in mice
[0091] The virulence of the mutants SM69 and SM86 in
mice was tested by oral infection of 6-8 week old female
BALB/c mice. These were performed as described above. The
wild type strains S. Enteritidis 76Sa88 and S. Typhimurium
1491S96 were tested in parallel as positive controls.
[0092] The complete data are given in Tables 4-7. These
results demonstrate that the oguaB mutants SM69 and SM86
are strongly attenuated in mice but still show some
residual pathogenicity when administered at this high
dose. Oral immunization with the mutants induces
protective immunity against infection by a high dose of
the corresponding pathogenic wild type strain.
Safety evaluation of the S. Enteritidis dguaB deletion
mutant SM69 in one-day-old chickens by the intratracheal
and oral gavage routes
[0093] The objective of this study was to evaluate the
safety of S. Enteritidis aguaB mutant strain SM69 master
seed in one-day-old chickens. Mortality was used as a
primary parameter for the determination of safety.
[0094] Chicks at one day of age were leg-banded and
randomly placed in each of the four treatment groups
(Group 1: SM69-IT, group 2: SM69-OG, group 3: PBS-IT and
Group 4: PBS-OG). After the master seed inoculation, the
birds from groups 1 and 2 were placed in one isolator and
those of groups 3 and 4 in another isolator.
[0095] Chickens in groups 1 and 2 were inoculated with
the SM69 master seed by the intratracheal (IT) route or
oral gavage (OG) route, respectively, with an actual titer
of 1.28 x 108 cfu/0.2 ml per bird. Chickens in groups 3 and
4 were administered with 0.2 ml PBS (phosphate buffered
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
18
saline) per bird by the intratracheal or oral gavage,
respectively.
[0096] Following the inoculation of SM69 or PBS, chick
mortality was observed daily until 38 days post
inoculation. Table 8 summarizes the results of mortality
for all 4 groups. In group 1, one bird died during the
inoculation due to inoculation trauma. Two birds died at 2
days post inoculation (DPI). Three birds died from day 3
to day 13 (at 3, 5 and 13 DPI respectively) . A total of 6
birds thus died in group 1. In group 2, two birds died in
total. One died during inoculation due to inoculation
trauma and one died at day 5 post inoculation. No birds
died in the PBS treated groups either by the intratracheal
or oral gavage route.
[0097] This study indicates that the S. Enteritidis
LguaB mutant strain SM69 is not safe when administered at
1.28 x 108 cfu per bird at one day of age by the
intratracheal or oral gavage route.
Safety evaluation of the S. Enteritidis aguaB deletion
mutant SM69 in 2-week old chickens by the intratracheal
and oral gavage routes
[0098] Safety of the S. Enteritidis aguaB mutant strain
SM69 was then evaluated in 2 week-old specific pathogen
free (SPF) chickens by the intratracheal and oral gavage
routes. Mortality was used as a primary criterion and body
weight as a secondary criterion for the determination of
safety.
[0099] Birds at 2 weeks of age were leg-banded and
randomly placed in each of the four treatment groups:
SM69-IT, SM69-OG, Poulvac ST-IT and PBS-IT. Ten birds in
group 1 were inoculated with SM69 by the intratracheal
route; ten birds in group 2 were inoculated with SM69 by
oral gavage; ten birds in group 3 were inoculated with a
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
19
S. Typhimurium AroA- vaccine (Poulvac ST) by the
intratracheal route; and five birds in group 4 were
inoculated with PBS by the intratracheal route.
[0100] Chickens in groups 1 and 2 were inoculated with
SM69 master seed by the intratracheal or oral gavage
route, respectively, with the actual titer of 2.304 x 108
cfu/0.2 ml per bird. Chickens in group 3 were administered
with Poulvac ST by the intratracheal route with 2.19 x 108
cfu/0.2 ml per bird. Chickens in group 4 were administered
by the intratracheal route with 0.2 ml PBS per bird.
[0101] After inoculation, the birds from treatment
groups 1 and 2 were placed in one isolator and those from
groups 3 and 4 in another isolator.
[0102] Following inoculations, mortality was observed
daily until 21 days post-inoculation. Body weight of all
birds was also recorded at the end of the study period (21
days) Poulvac ST and PBS were used as intratracheal
procedure controls.
[0103] During the 21-day observation period, one bird
in the SM69 intratracheal treatment group (group 1) died
from an infected yolk sac. No mortality was associated
with SM69 inoculation, indicating that the SM69 strain is
safe at the titer tested, 2.304 x 108 cfu per bird by the
intratracheal and oral gavage routes. As expected, no
death was observed either in the Poulvac ST treated birds
at the titer of 2.19 x 108 cfu per bird or in the PBS
treated birds, indicating that the study was valid (Table
9) .
[0104] Body weight was compared amongst groups in an
analysis of variance (ANOVA) model with body weight as the
dependent variable and treatment included as an
independent variable. Group comparisons were made using
Tukey's test for multiple comparisons. The level of
significance was set a p <0.05. The study was considered
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
valid because the control chickens (PBS control group)
remained healthy and free of clinical signs of diseases or
mortality throughout the study.
[0105] There were no significant differences in the
5 final body weight in chickens administered with SM69 by
the intratracheal or oral gavage inoculation, Poulvac ST,
or PBS (Table 5). Even though no baseline was established
of the birds in each group at one day of age, it was
unlikely that there was a significant difference in the
10 initial body weight amongst the four groups since the
birds were randomly placed into each of the 4 treatment
groups.
I0106] Since no mortality was attributable to the
inoculation of SM69, it can be concluded that the S.
15 Enteritidis aguaB mutant strain, SM69, is safe when
administered at the tested titer of 2.304 x 108 cfu/0.2 ml
dose per 2-week-old bird, by either the intratracheal or
oral gavage inoculation route. SM69 inoculations had no
effect on the final body weight of the birds, bird weight
20 being the second safety parameter evaluated here.
[0107] A deposit has been made according to the
Budapest Treaty at the BCCM/LMG Culture Collection,
Laboratorium voor Microbiologie, K.L. Ledeganckstraat 35,
B-9000 Gent (Belgium) for the following micro-organisms:
Salmonella Enteritidis SM69 under deposit number LMG P-
21641 (deposit date: 9 August, 2002) and S. Typhimurium
SM86 under deposit number LMG P-21646 (deposit date: 28
August, 2002). The deposits have been made in the name of
Prof. J.-P. Hernalsteens, previous address: Vrije
Universiteit Brussel, Laboratorium Genetische Virologie,
Paardenstraat 65, B-1640 Sint-Genesius-Rhode, current
address: Vrije Universiteit Brussel, Onderzoeksgroep
Genetische Virologie, Pleinlaan 2, B-1050 Brussels,
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
21
Belgium.
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
22
~ M
H
U zJ
U U
CJ U
c~ U U
C7 H
CH7 on U to
rl . . . .
H ~C H U rq cn cn cn
P U ~ ~ U ~ U L7
H H
0 LU') U rG LU7 H ~ UU' U
U L7 C7 H U U CD U U
so C7 L7 L~7 H U ~ ~ U
LU7 H C U 7 ~ U ~ L7 ~ U' r
LU7 ~ U U U CU7 U U H
U U H ~ ~ U
a) +
~ U 0 0 U U ~ ~ LH7 U
a) 07 . .
V L/2 IP Ul Ln LIl Lf7 Id1 l.fl Ln t(1
fi
a)
m ~ t o
S-i f4 fYl m W Pa PQ P 1 Pq
-rj a a, a,
x--
P4
0
~
~ A
H
~
.~ a
r~ w
[-~ tll .-1 N tVl d+ LIl ~D I~ 00 Ol
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
23
a) r= r= A
4J
0 0 0 0 0
R4 Ri o o Ra
~
4.) w 41 =-~ -rI 0 U) .0 ul ul
U
41 4J m [~A cll fd 4~-I (~11 Lo tU1l
,u ~ ~ a) 'J., =~~-I a)
~ -~ ~ ~ ~ ~ -~ ~ -i =~
-
O Zj N ro 'z3 rd
'r-I 41
41 0 0 Q ~ ~ 0 0 0
a)
~
d 4-I ~
O co cj) rn
ro C)~
bi ro ~ \ co \ \
~
.ri ~
r-I
~
~> r--I Ln H m cYl co
~ \ \
N ~ ~ ~ 0 1-01 rn o cn cn
N ~4
a) 00 co 00 a~ OC) OC) co
P4 ~ ~ ~ o 0 0
,~ r-i r-I
ae x x W ae x x
U AO W ~ ~n ~ ~ d~ ~ rn
si 4-1
to N N U) O \ rl N ,-1
y-I ,~
O W o U o
u) u)
U CL) U] co U1
c0 CD
rd rz~ (d FC
U lc/)0
PA9 ~ 4 ~ 4
-I
=~-
pq p ~ ~ '~ ~
rl ro -r-I -Cf
,u .u =~ -U -~
rl U -!-)
~ ~ I rl 0 ~-I -ri 0
N ~ 4-) Uz] .I-~ cx
l)
p -r1 W W ~ W W ~
p4
~ ~ ~ ~ = ~
b) ~ bl
d~) r-1 rl GI r-I r-I d
~ 0 ~ 0 u) ~ 0 0 r-i
m
~ 41 -u ~-! = 1! -U .u ~I -1-I
=ri r, r, 4-) zs r-I 0 .u ~
> 0 0 G -ri 0 0 ;:i -~
U C) 0 -P U U 0 .I-u
U -r-I U =H
= N N i-I N 4J ~-I
N N N ~ T N
rl rl ' -I, =-I -rl 5:11 -U
N 43 -ri ~ 4-1 -U -rl i
r-I c~ =r-l v W r~ -~i U W
bi w U ~ cn U
a) 0 m N 0 co
ID4 Q. ~ ~
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
24
~ ~
~ u a~ U -,I .u
~
U '0
Un u~ IS,
N,~' N 0 m m [a A
~
R~ 5 O a
e) a) r-I U
0 U 4j t71 ~ I '-1
~ a) co
A in .~u
4J N ~ Ul r~1 4) ~ 4a
4I ctS U ~5l ,.SZ: rLf
0 m cA 4 4J
'O d~-1 4) -i 4) 4) 4) .u 0 =~ 0 =~ 4 ~ -H a) di
m Q 0 U -u m x A ~
0
ro
N+ .
O
ro .'~u ~ r~
N co m ~
N A 'd 11- w rn ~
.,~
ro ~
^,~ cd
'u > l0 I.n Ln tn
''q 'ri ~ ~. ---l
N N 41 k.0 o rn Ln
4-) N
~
0
0 m m w
rr1 P4 o 0 0
r-I H H
~ t O n W u xi ~i uxi
.u 0 .U = = =
rn m uo
,=~ -~ -~ -~ -~
4J r-t -~ -~
ro t3' ~ == ~ ~ m
-~ a) q -ri o 00 00 CO
U H rd rd .r.~ W rd W ~S W r0
V (d {-1 tJl Co Ul U]
cd
U Ul C) O 0 ~ ~ ~l ~
N
U
=,-I
r4
co co
yW 0 0
0 r-I `-I
U) x x
Ea Ln ~
0 =
N i.n
(D
~ m m
rl r-I -ri
(d
U 0 r r:~ -H a -a o
rl ~-1 l!1 I N
a) .. a) .. .. a) X n)
cY1 (d ~> r-I ~> ri 4) rl -1-) Co
-~F-i t 0 W ~C W W
-U ~ -u 0
r 1 U tti r6 -U ri$ _0 U -IJ 0 ~
Q U ~- ~5l ~1 ~` U ~ I
cd (f aJ 4) 0 w 0 r0 0 = bl
H > U] r, U r, 0 > U ~ 4 tf) <
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
(D ro
~ ~ ~
U N (Ii
.{-)
U) N cl~
M
ID4 u u t~l O ~ ~ m r4
44 r~ ,-Ei a) O
0 4) a) U 4) =H
N rl r~ ~ ~I r[S ~ C)
N zS 4) (1) (t) a1
N w a) .u m 4-I
U1 'Zi Q -~iU tti !a 0 -~
4-I
0
~ OD ~
e~r RS H
N
td N C)
A 'd \ orn rn ,-f
~
rd
,~ N N f')
M
> 4-1 \ \ \ \
O c-1 N
a N
C) C) C)
N H H ra
a~ W x x x
Ln Ln
o = = =
0
U
q) tn Ul U1
-rl -r-I =r-I
L12
-r-I -ri -~-i -r-I
r=
S"+ ~> r-I 4) N 4)
r-I t~f ni .U riS c1~ W cd
.0 .u 4) 0 110 ~o ~10
U tR ~ L) ~ l- l- U) r--
0 0
rA r~
m x x
~ ~ rn
0
,~ Q \ \ N r-I
U
~'i -rl =-I
-rl _S4 -114
i~ rl I ri ri
-i-} U
0 0 rz: =ri o =ri o
U ri ~-1 Ln -I N
m Qi ~> H '`~ r-I N r-1 4-) U1 .!-1 U)
9 ~ -rl 0 -r-i 0 ~ 0 F-: ~
tll rI =~t 41 ~4 -u ~4 -r-t ~-4 W FC W ~
r-I U td ri$ -Ii ro -U L) -I, O c~
0 i4 t71 5:i i51 r~ U ~ ~I
Rf co 41 (L) 0 () 0 r73 0 RS bl
H ct) r, v r, v > U U) -4 U1 4
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
26
4J ~
0 0 0
~ ~
~ w H
,~ ~ ~
~
~ o + a) 0 0
N =u 44 U
44 'Zj 0
0 uQ O O
~ 41 ~-UI P. O (~d ,~ ~ a
ro
m ~ ~ a H ~
44 m u~
0 ~ 0
u a) rd
N ~ Z3 H I ~ Ifl ~ Lfl
~ U) c~n ~ ~ .u u~i c~n ~ c~n rd
~ a)
44
w
0 '
.u : 0 4 OD H
~r (d 00 rti ~ ~ oo
W b1 A'd ~ orn oo
U
=~ > cY) Ln ro ~ Ln
>
~ ~ eN O U! > O N
4.3 m
a) O O
H r-I t~ O O
H r-I
O ` I 4) Ul H
r-I
0 (Yi
3 .~ U) q) m a)
-i P-1 ~
U 3 u u OD
41
ro
m N U ~4 ko
Ell N.
~
cl)
U ri rd rTi m 't~ RS
U)
4-3 4-3 4J
~ ~ U U]
4)
U
w x
a) m U 0 t~
N
ty)
~4 ~ 0 0 a ~
~I :4 .u .u ril rd u)
o o ~ 4 -~
CY)
=u U 0 rd
u u ~
0
-~ ~
o q)
.u
~ C) -ri .u .u -u a) -~ =ri -u ~4 .u r:q
N (d rd -1 ~,' (d (6 U ~ Rf
w ~4 t)l cn w A U p tj) r~ ;~i
cd q .u a) 0 = rti (d .u Q) o = b)
H H m f~ Q ~n H > m ~ v ta <
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
27
Q0 `tS
r.
u
Id w ~ ~
zn a) ~
~
0 ~ m
w P-4
~ 41 o cn ~ j-'
Ul
44
c~d 0 m ,-r m a' o
t 5 m .u
t~n 4,dl 4-) a 10
c~d
~
c~d ~4 0 .~ rr-i
i (t 0 o
m ri
44 rn ^ N 41
O ~ ~ ~ ~ N ~
b
0
~ 0
~ i-n
Ln +n .U
C!1 ~ ~ Ln ~ N Y 'H
H Ua *f .I.) o cq 0
0
ON 14 Ei
H 0 ~
P4
4~ ~ a ~ W
~ 4-3 o w rd CD ` ~
~ ~ A u
0
~ v
rl)
w
H 4j
w >1
0 ~
~
~
~- ~
ri 0
~ 0 P4
N td ~
~
U q4
~ ri
0 ~4 41
o ~ vz ~ Lw
~4
0 0 r+ 0 ~
~ . ~ } ~4 r,
. i Cf
0
.ri co ~
N 03
.U
41 Id
ro ~ v
A rr-i
uO
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
28
>1
4 0
'd
U r-I
j U ~ o 0 o
1-1 H 0 4-) 4p-I w 4-4
U U
ri 5 o ril ~ o
r~i 41 ~ co 0 j ~>
N ~ 4-) ~ 0
~
O y1 ~ r-i Ul >1 Ul
m 3 ai a~ ~ ~ ~ a) 1d
~ k ~ p ~ c~ ~ ~
m rl cd
N -H 4-I r-+ td N 0 U-,1 a) ~11
.u ~i trl ~ rtf U m 0 P~ .u rn 0
rt
4-) H ya ~
0
~
o
41
U U cd N \ m ~ ~
'd
4-)
,-~ 0
`4 `d
U1 0 =~> N di I.n lfl ~-I
rl m 'J {l O ~ tn 0
~
0
a)
n n
a) m m U
-1 R+ ,-4 ,-t 'aC
.o-( ,~
5C
W x x w x ss a)
w 7Ui M M r, r U)
r-I r-
- N N
H A A] c-1 r-1
.u ~ O ~
(d r] ~ v
w CI~ w
0
N
.ri ~ a) ~
'Cl r-i
U) ~ H ~ H m H ~ ?C
Fi 4-3 v H U v] tq ~~-i t!) ~ Ul H ~ -1 U1 ~ ~ -I 0
r ~
a 0
-ri
~ o
o U O x
.r] o 00
4.3 0 q k i o
4J r-I ~
~ ~ u
o ~ x x 0
r
-ri
0 -~ -~ -~
-rl ~4 r= ~o
4-) OD Oo
~ cn
-,~ -~ ol -~I 0 =~-I 0
N
ri ~A
0 af ~ ro rd .u ro rd .u
cAd cd a..1 H ~ b) ~ O ~ (~ ) O
H > tn va H a ~ U a ~ U
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
29
N
,-I H
0 A
.{.1
N fd tn 04
0 rd
~
44
0 rl H
124
.~ ~/y N Ln Zj
1
%D A 0 0
N
td
.J-3 Ln
=rl ~ k 0 0
M
rd 0 0 r-i H 41
43 H r-I 1~1 41 fj
O 0 (o
~ ~ H rd
o -r-I
~
~ rn ~ rn
-~
~ ~ ~ rt ~ ~
ro ~ rn -~'u rn ~
r--q
~ o 41 ~4 41 , r-' ~
0 -~-I 0
-~
w
.,q 4-) o 0
b
.,i r-I 41
4.) N N ~:j ~
~ m ao U
O N O N (D O 0 ~
r-i x O X O I I -0
0
~
.1 N~l U) CI] 'rq
W ~ N
-ri = 4-1 = 4-1 (Yl pq
E1 r-I U ,-1 U W PW
0
tll rq
u-t U -r-i
0 H 0 H O -H ~
~ R,
~o uD cl~ ~ -r~ -i ~ ~
O -r-I
cn m w w ~ A
u 0 U ,-{ H
H 0 w H
L7 ri N fn da ~ N r-I ~ Q
rn rn U c~ .U ~ N N
N
u .M ~ 0 0 r0 ~ ~ ~ c~ ~
~
0) U U] Cf1 -I ~ I ~ I H U] ~ '~ t51
4-I =rl -r-I -rI -0 -I1 -1 1 StS >I, ~ I.
(d 14" 'zJ zf ~i p rd ~ -rl
C!] U -~-I -H 0 0 H O q H ,.Q H
-U U U
= f~' -~-1 -ri
00 0 ~-I 4 a) N
-ri Q) Q) ~> ~>
a1 4-) I1 .1J -rl =r-I
r I 0 -rl r, 41 1--)
A d) cd W W r~ rd
Rf 44 p t51 U1 ~51 m H
H .a-) = = a~ al a) pq H ~ a,
H m Cf) U'a 5:1 a, 0 a, H O Q
CA 02646421 2008-09-19
WO 2007/106956 PCT/BE2006/000019
rd
r-I
o 41
r[i =~ o 0 0 0
. . .
~ v] $ o 0 0 0
$
N
.~i
CFl (y' . . N N N 00
lD (a m M
0 O O O
4J
-fi H
.~ A
O
4J
O
.,-I
41
N ri
5
cd
rl
ro o -o o
i r-i
~ r-i tn
o o ~ H
ro ~ ~ ~4 r-, Ln
=
b) ~ cl)
4J
m ;j J'
0 H O H O
,q
4-) ~
-H
p r-q
a) F-
a' 51 N N
W o o -~I
ri N rl N O N 0 ()
~
{-I d{ O d{ O )C O ( 0
h 1 ~ r-1 ~ Ul ~
M
-, N U N U N U W ~
4-4 0
O O O ~ L1
z rl ,-I r-t Ln
`4 r. U E
O O m ~l
~ -rl U~ -rl
H L7 U H 0 rd H -t, ~4
~ orn a ~
rn r~i Fi r t r-I ~
4-) o a~n u
0 M cf) a H a ~ 8 0
O ~
H CJ H N M F I ~N
~ N N i 41
N
>
rn rn
~l stS O ~
M Q4
~
~ ~
a) 0 ~-I ~i CO .!J -H
44 ., { Ul iI] 0 ~-1 -1-} (CS >v (o rl
~ .ci -rl H ~-I -k .u ~ ~I riS Q)
Ul U 'zf '~ ~ C~ O H
O U) O
0) 0 O O N
=H N N (t) U ~
41 41 .0 ~ ~ -~
U > .u
N rd W f=7 UH (0
H
ai RS 0 aN Ca H L7 (~ x
E-1 H U] CIa I!) ~> 124 F-i W H o Q -k k