Note: Descriptions are shown in the official language in which they were submitted.
CA 02646650 2008-09-18
SOLUBLE ADENYLATE CYCLASE INHIBITORS
The present invention relates to inhibitors of soluble adenylate cyclase,
production thereof and use thereof for the production of a medicinal product
for
contraception.
A large number of modern methods of contraception are currently available for
women, but very few methods are available for male fertility control (condom
and sterilization). Development of reliable new means of male fertility
control is
urgently needed. The infertility brought about by a "male pill" should be
reversible and should be just as effective as the existing methods available
to
women. Infertility should be developed relatively quickly and should persist
for
as long as possible. Such a method of contraception should have no side-
effects, and the preparations may be non-hormonal as well as hormonal. A
possible starting point is regulation of the activity of an enzyme that plays
an
important role in fertilization of the ovum: soluble adenylate cyclase (sAC).
This
enzyme is mainly expressed in the testicular stem cells and is present in
mature
sperm.
In 1999, the authors Levin and Buck succeeded in purifying and cloning an
isoform of sAC from rat testes (Proc. Natl. Acad. Sci. USA 96 (1): 79-84).
The recombinant rat enzyme can be stimulated by bicarbonate. It was
demonstrated, using antibodies, that the catalytic domain of the enzyme is
localized in testes, sperm, kidneys and the choroid plexus. These disclosures
are the subject of application WO01/85753, which was granted in the USA
(US6544768).
WO01/21829 (Conti et al.) claims isolated polynucleotide sequences that code
for the human isoform of sAC, isolated sAC polypeptides and test systems,
using which it is possible to identify substances that inhibit the activity of
sAC.
The possibility of using these substances to achieve a reversible reduction in
the motile sperm count, and their use as a means of controlling male
fertility, is
CA 02646650 2008-09-18
-2-
disclosed.
John Herr's group demonstrated the isolation and characterization of the human
isoform of sAC from sperm. WO 02/20745 claims, in addition to nucleic acids
that code for sAC, also test systems for identifying substances that modulate
the expression or the activity of human sAC. Such compounds might, for
example, selectively inhibit the activity of sAC, as a consequence of which
the
sperm would lose the capacity to fertilize an ovum. These sAC inhibitors might
therefore serve as medicinal products for non-hormonal contraception.
However, the sAC inhibitors that are already known display specific problems:
catechol estrogens (T. Braun, Proc Soc Exp Biol Med 1990, 194(1): 58ff) and
gossypol (KL Olgiati, Arch Biochem Biophys 1984, 231(2): 411ff) are inherently
toxic, whereas adenosine analogs only have a very weak inhibitory action (MA
Brown and ER Casillas, J Androl 1984, 5:361ff). The inhibitors of recombinant
human sAC described by Zippin et al. are somewhat more potent (IC50 < 10 pM)
(JH Zippin et al., J Cell Biol 2004, 164(4): 527ff).
In order to provide a means for male fertility control, there is an increasing
demand for substances that lead to infertility quickly, reversibly and
successfully.
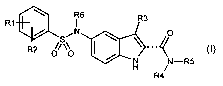
This problem is solved by the provision of the compounds of general Formula I,
R6 R3
R1 I
SiN ~ O
I ~
R2
O/\\O /
H j -R5
R4 (I)
where the following notation is used:
R' hydrogen, halogen, CF3, C3-C6-cycloaikyl, which is optionally
multiply saturated and optionally multiply substituted, or
the group Cl-C6-alkyl, Cl-C6-aryl, CrC6-acyl, halo-Cl-C6-alkyl, Cl-
C6-aIkyl-Cj-C6-aIkyl, C,-C6-aIkyl-C,-C6-acyl, C,-C6-acyl-C,-C6-acyl,
CA 02646650 2008-09-18
-3-
C1-C6-alkyl-Cj-C6-aryl, C,-C6-aryl-Cj-C6-alkyl or CF3, in which C,-
C6-alkyl, C,-C6-aryl, C,-C6-acyl, halo-C,-C6-alkyl, C,-C6-alkyl-C,-
C6-alkyl, C,-C6-alkyl-C,-C6-acyl, C,-C6-acyl-C,-C6-acyl, C,-C6-alkyl-
C,-C6-aryl or C1-C6-aryl-C,-C6-alkyl can optionally be interrupted
singly or multiply, identically or differently by oxygen, sulfur or
nitrogen, or
the group sulfonyl-Cl-C6-alkyl, sulfonamide, or cyano,
R 2 halogen, CF3, C3-C6-cycloalkyl, which is optionally multiply
saturated and optionally multiply substituted, or
the group Cl-C6-alkyl, Cl-C6-aryl, C,-C6-acyl, halo-C,-C6-alkyl, Cl-
C6-alkyl-Cl-C6-alkyl, C,-C6-alkyl-Cj-C6-acyl, CI-C6-acyl-C,-C6-acyl,
C,-C6-alkyl-C,-C6-aryl, Cl-C6-aryl-C1-C6-alkyl or CF3, in which Cl-
C6-alkyl, Cl-C6-aryl, C,-C6-acyl, halo-Cl-C6-alkyl, C,-C6-alkyl-Cl-
C6-alkyl, CI-C6-alkyl-CI-C6-acyl, CI-C6-acyl-Cj-C6-acyl, C,-C6-alkyl-
CI-C6-aryl or C1-C6-aryl-Cj-C6-alkyl can optionally be interrupted
singly or multiply, identically or differently by oxygen, sulfur or
nitrogen, or
the group sulfonyl-Cl-C6-alkyl, sulfonamide, or cyano,
R3 C6-C12-aryl, which can optionally be substituted singly or multiply,
identically or differently with halogen, Cl-C6-alkyl or CI-C6-acyl,
which can optionally be singly or multiply substituted,
or can be substituted with C,-C6-alkoxy, hydroxy, cyano, C02-(Cl-
C6-alkyl), N-(Cl-C6-alkyl)2, CO-NR4R5 or with CF3,
C5-C12-heteroaryl, which can optionally be substituted singly or
multiply, identically or differently with halogen, C,-C6-alkyl, C,-C6-
acyl, C,-Cs-alkoxy, hydroxy, cyano, C02-(C,-C6-alkyl), N-(Cl-C6-
alkyl)2, CO-NR4R5 or with CF3 or
C3-C6-cycloalkyl, which can optionally be substituted singly or
multiply, identically or differently with halogen, CF3, hydroxy, cyano,
C02-P-C6-alkyl), C,-C6-alkyl, C,-C6-acyl, N-(Cl-C6-alkyl)2, CO-
NR4R5 or C,-C6-alkoxy,
CA 02646650 2008-09-18
-4-
R4 hydrogen, C3-C6-cycloalkyl, which is optionally substituted singly or
multiply, identically or differently with Cl-Cs-alkyl, Cl-C6-acyl, Cl-
C6-alkoxy or CF3,
C6-C12-aryl, which is optionally substituted singly or multiply,
identically or differently with halogen, with C,-C6-alkyl, CI-C6-acyl,
Cl-C6-alkoxy, N-Cj-C6-alkyl-Cj-C6-alkyl, CF3 or cyano, or
C5-C12-heteroaryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C6-alkyl, C,-C6-acyl, C,-
C6-alkoxy, N-Cl-C6-alkyl-C,-C6-alkyl, CF3 or cyano, or
C,-C6-alkyl, which can be substituted arbitrarily,
R5 hydrogen, C,-C6-alkyl-C3-C6-cycloalkyl, which is optionally
substituted singly or multiply, identically or differently with C1-C6-
alkyl, Cl-C6-acyl, C,-C6-alkoxy or CF3,
C3-C6-cycloalkyl, which is optionally substituted singly or multiply,
identically or differently with C,-C6-alkyl, C,-C6-acyl, Cl-C6-alkoxy
or CF3,
Cs-C12-aryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C6-alkyl, Cl-C6-acyl, C,-
C6-alkoxy, N-C,-C6-alkyl-C,-C6-alkyl, CF3 or cyano, or
C5-C12-heteroaryl, which is optionally substituted singly or multiply,
identically or differently with halogen, C,-C6-alkyl, Cl-C6-acyl, C,-
C6-alkoxy, N-C,-C6-alkyl-C,-C6-aIkyl, CF3 or cyano, or
Cl-C6-alkyl, which can be substituted arbitrarily, and
R4 and R5 together form a 5-8-membered ring, which can contain further
heteroatoms, and
R6 the group C,-C6-alkyl, C,-C6-acyl, C,-C6-alkylcyclo-C3-C6-alkyl,
C,-C6-alkyl-C6-C,2-aryl, in which Cl-C6-alkyl, Cl-C6-acyl, C,-C6-
alkylcyclo-C3-C6-alkyl, Cj-C6-aIkyI-C6-C12-aryl can optionally be
substituted singly or multiply, identically or differently by hydroxy,
CA 02646650 2008-09-18
-5-
methoxy, ethoxy, iso-propoxy, chlorine, bromine, fluorine, cyano,
methylsulfonyl or aminosulfonyl,
as well as their isomers, diastereomers, enantiomers and salts, which overcome
the known drawbacks and display improved properties, i.e. display good
efficacy, good solubility and stability.
The compounds according to the invention inhibit soluble adenylate cyclase and
so prevent capacitation of the sperm and thus provide male fertility control.
Alkyl means in each case a linear or branched alkyl residue, such as methyl,
ethyl, propyl, isopropyl, butyl, isobutyl, sec. butyl, tert. butyl, pentyl,
isopentyl
and hexyl.
Alkoxy means in each case a linear or branched alkoxy residue, such as
methoxy-, ethoxy-, n-propoxy-, iso-propoxy-, n-butoxy-, sec-butoxy-, iso-
butoxy-,
tert. butyloxy-, pentoxy-, iso-pentoxy- and hexoxy-.
Acyl means in each case a linear or branched residue, such as formyl, acetyl,
propionyl, butyroyl, iso-butyroyl, valeroyl and benzoyl.
Cycloalkyl means monocyclic alkyl rings such as cyclopropyl, cyclobutyl,
cyclopentyl and cyclohexyl.
The cycloalkyl residues can contain one or more heteroatoms, such as oxygen,
sulfur and/or nitrogen, instead of the carbon atoms. Such heterocycloalkyls
with
3 to 6 ring atoms are preferred. The ring systems, in which optionally one or
more possible double bonds can be contained in the ring, mean for example
cycloalkenyls such as cyclopropenyl, cyclobutenyl, cyclopentenyl,
cyclopentadienyl, cyclohexenyt, cycloheptenyl, where the coupling can take
place both on the double bond and on the single bonds.
Halogen means in each case fluorine, chlorine, bromine or iodine.
CA 02646650 2008-09-18
-6-
In each case the aryl residue comprises 6-12 carbon atoms and can for
example be benzocondensed. The following may be mentioned as examples:
phenyl, tropyl, cyclooctadienyl, indenyl, naphthyl, biphenyl, florenyl,
anthracenyl
etc.
In each case the heteroaryl residue comprises 5-16 ring atoms and can contain
one or more, identical or different heteroatoms, such as oxygen, sulfur or
nitrogen in the ring instead of carbon, and can be mono-, bi- or tricyclic and
can
additionally be benzocondensed in each case.
The following may be mentioned as exampies:
thienyl, furanyl, pyrrolyl, oxazolyl, imidazolyl, pyrazolyi, isoxazolyi,
isothiazolyl,
oxadiazolyl, triazolyl, thiadiazolyl, etc. and benzo derivatives thereof, e.g.
benzofuranyl, benzothienyl, benzooxazolyl, benzimidazolyl, indazolyl, indolyl,
isoindolyl, etc; or pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl, etc. and
benzo
derivatives thereof, e.g. quinolyl, isoquinolyl, etc; or azozinyl,
indolizinyl, purinyl,
etc. and benzo derivatives thereof; or quinolinyl, isoquinolinyl, cinnolinyl,
phthalazinyl, quinazolinyl, quinoxalinyl, naphthyridinyl, pteridinyl,
carbazolyl,
acridinyl, phenazinyl, phenothiazinyl, phenoxazinyl, xanthenyl, oxepinyl, etc.
The heteroaryl residue can be benzocondensed in each case. For example, the
following may be mentioned as 5-ring heteroaromatics: thiophene, furan,
oxazole, thiazole, imidazole, pyrazole and benzo derivatives thereof and as 6-
ring-heteroaromatics pyridine, pyrimidine, triazine, quinoline, isoquinoline
and
benzo derivatives.
Heteroatoms is to be taken to mean oxygen, nitrogen or sulfur atoms.
If an acid function is present, the physiologically compatible salts of
organic and
inorganic bases are suitable as salts, such as the readily soluble alkali and
alkaline-earth salts and N-methyl-glucamine, dimethyl-glucamine, ethyl-
glucamine, lysine, 1,6-hexadiamine, ethanolamine, glucosamine, sarcosine,
CA 02646650 2008-09-18
-7-
serinol, tris-hydroxymethylaminomethane, aminopropanediol, Sovak base, 1-
amino-2,3,4-butanetriol.
If a basic function is present, the physiologically compatible salts of
organic and
inorganic acids are suitable, such as hydrochloric acid, sulfuric acid,
phosphoric
acid, citric acid, tartaric acid etc.
Compounds of general Formula I in which the symbols have the following
meanings are especially preferred,
R' hydrogen, halogen, CF3, C3-C6-cycloalkyl, or the group Cl-C6-alkyl,
C,-C6-aryl, C,-C6-acyl, halo-Cl-C6-alkyl, Cl-C6-alkyl-C,-C6-alkyl,
C,-C6-alkyl-C,-C6-acyl, C,-C6-acyl-C,-C6-acyi, C,-C6-alkyl-C,-C6-
aryl, C,-C6-aryI-C,-C6-aIkyl or CF3, in which Cl-C6-alkyl, CI-C6-aryl,
C,-C6-acyl, halo-C,-C6-alkyl, C,-C6-aIkyl-Cj-C6-aIkyl, Cl-C6-alkyl-
Cti-C6-acyl, C,-C6-acyl-C,-C6-acyl, C,-C6-aIkyl-C,-C6-aryI or C,-C6-
aryl-Cl-C6-alkyl can optionally be interrupted singly or multiply,
identically or differently by oxygen, sulfur or nitrogen, or
the group sulfonyl-C,-C6-alkyl, sulfonamide, or cyano,
R2 halogen, CF3, C3-C6-cycloalkyl, or the group C,-C6-alkyl, C1-C6-
aryl, Cl-C6-acyl, halo-Cl-C6-alkyl, C,-Cs-alkyl-C,-C6-alkyl, Cl-C6-
alkyl-C,-C6-acyl, Cl-C6-acyl-Cl-C6-acyl, CI-C6-alkyl-Cl-C6-aryl, C,-
C6-aryl-C,-C6-alkyl or CF3, in which C,-C6-alkyl, C,-C6-aryl, C1-C6-
acyl, halo-C,-C6-alkyl, C1-C6-aIkyl-Cj-C6-alkyl, C,-C6-aIkyl-Cj-C6-
acyl, C1-C6-acyl-C,-C6-acyl, Cj-C6-alkyl-Cj-C6-aryl or C1-C6-aryI-C,-
C6-alkyl can optionally be interrupted singly or multiply, identically
or differently by oxygen, sulfur or nitrogen, or
the group sulfonyl-Cl-C6-alkyl, sulfonamide, or cyano,
R3 C6-C12-aryl, which can optionally be substituted singly or multiply,
identically or differently with halogen, Cl-C6-alkyl, Cl-C3-acyl, C,-
C3-alkoxy, cyano, hydroxy, N-(CH3)2, C02-(Cl-C3-alkyl), CO-NR4R5
CA 02646650 2008-09-18
-8-
or CF3,
C5-C12-heteroaryl, which can optionally be substituted singly or
multiply, identically or differently with chlorine and/or fluorine, with
CI-C6-alkyl, Cl-C3-acyl, Cl-C3-alkoxy, cyano, hydroxy, N-(CH3)2,
C02-(Cl-C3-alkyl), CO-NR4R5 or with CF3,
C3-C6-cycloalkyl, which can optionally be substituted singly or
multiply, identically or differently with chlorine and/or fluorine, CF3,
cyano, Cl-C3-alkyl, CI-C3-acyl, hydroxy, N-(CH3)2, C02-(CI-C3-
alkyl), CO-NR4R5 or Cl-C3-alkoxy,
R4 hydrogen, C3-C6-cycloalkyl, which is optionally substituted singly or
multiply, identically or differently with Cl-C3-alkyl, CI-C3-acyl, Cl-
C3-alkoxy or CF3,
C6-C12-aryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C3-alkyl, Cl-C3-acyl, C,-
C3-alkoxy, N-C,-C3-alkyl-C,-C3-alkyl, CF3 or cyano, or
C5-C12-heteroaryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C3-alkyl, Cl-C3-acyl, Cl-
C3-alkoxy, N-Cj-C3-aIkyI-C1-C3-alkyl, CF3 or cyano, or
Cl-C6-alkyl, which can be substituted arbitrarily,
R5 hydrogen, CI-C6-alkyl-C3-C6-cycloalkyl, which is optionally
substituted singly or multiply, identically or differently with C,-C6-
alkyl, C,-C6-acyl, C,-Cs-alkoxy or CF3,
C3-C6-cycloalkyl, which is optionally substituted singly or multiply,
identically or differently with C,-C3-alkyl, CI-C3-acyl, CI-C3-alkoxy
or CF3,
C6-C12-aryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C3-alkyl, C,-C3-acyl, Cl-
C3-alkoxy, N-Cj-C3-alkyl-Cj-C3-alkyl, CF3 or cyano, or
C5-C12-heteroaryl, which is optionally substituted singly or multiply,
identically or differently with halogen, with C,-C3-alkyl, Cl-C3-acyl,
CI-C3-alkoxy, N-C,-C3-alkyl-Cj-C3-aIkyl, CF3 or cyano, or
CA 02646650 2008-09-18
-9-
Cl-C6-alkyl, which can be substituted arbitrarily,
R4 and R5 together form a 5-8-membered ring, which can contain further
heteroatoms, and
R6 the group C,-C6-alkyl, C1-C6-alkylcyclo-C3-C6-alkyl, C,-C6-alkyl-
C6-C,2-aryl, in which C,-C6-alkyl, C,-C6-alkylcyclo-C3-C6-alkyl,
C,-C6-alkyl-C6-C,2-aryl can optionally be substituted singly or
multiply, identically or differently by hydroxy, methoxy, ethoxy, iso-
propoxy, chlorine, bromine, fluorine, cyano, methylsulfonyl or
aminosulfonyl,
as well as their isomers, diastereomers, enantiomers and salts.
Compounds of general Formula I in which the symbols have the following
meanings are also preferred,
R' hydrogen,
R2 C3-C6-cycloalkyl, CI-C6-afkyl, CF3, cyano, bromine, or the group
-OCF3, -S02-CH3,
R3 C6-C,2-aryl, which can optionally be substituted singly or multiply,
identically or differently with halogen, Cl-C6-alkyl, Cl-C3-acyl, C,-
C3-alkoxy, cyano, hydroxy, N-(CH3)2, C02-(Cl-C3-alkyl), CO-NR4R5
or CF3,
C5-C12-heteroaryl, which can optionally be substituted singly or
multiply, identically or differently with chlorine and/or fluorine, with
C,-C6-alkyl, CI-C3-acyl, CI-C3-alkoxy, cyano, hydroxy, N-(CH3)2,
C02-(C,-C3-alkyl), CO-NR4R5 or with CF3,
C3-C6-cycloalkyl, which can optionally be substituted singly or
multiply, identically or differently with chlorine and/or fluorine, CF3,
CA 02646650 2008-09-18
-10-
cyano, C,-C3-alkyl, CI-C3-acyl, hydroxy, N-(CH3)2, CO2-(Cl-C3-
alkyl), CO-NR4R5 or Cl-C3-alkoxy,
R4 hydrogen,
R5 hydrogen, C,-C6-alkyl-C3-C6-cycloalkyl, which is optionally
substituted singly or multiply, identically or differently with C,-C6-
alkyl, CI-C6-acyl, Cl-C6-alkoxy or CF3,
C3-C6-cycloalkyl, which is optionally substituted singly or multiply,
identically or differently with Cl-C3-alkyl, Cl-C3-acyl, C,-C3-alkoxy
or CF3,
C6-C12-aryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C3-alkyl, C,-C3-acyl, Cl-
C3-alkoxy, N-C,-C3-alkyl-C,-C3-alkyl, CF3 or cyano, or
C5-C12-heteroaryl, which is optionally substituted singly or multiply,
identically or differently with halogen, CI-C3-alkyl, C,-C3-acyl, C,-
C3-alkoxy, N-C,-C3-alkyl-C,-C3-alkyl, CF3 or cyano, or
CI-C6-alkyl, which can be substituted arbitrarily,
R6 the group Cl-C4-alkyl, CH2-cyclo-C3-C6-alkyl, CH2-C6-C12-aryl, in
which Cl-C4-alkyl, CH2-cyclo-C3-C6-alkyl, CH2-C6-C12-aryl can
optionally be substituted singly or multiply, identically or differently
by hydroxy, methoxy, chlorine, fluorine, cyano or aminosulfonyl,
as well as their isomers, diastereomers, enantiomers and salts.
Compounds of general Formula I in which the symbols have the following
meanings are also preferred,
R' hydrogen,
R2 C3-C6-cycloalkyl, CI-C6-alkyl, CF3, cyano, bromine, or the group
CA 02646650 2008-09-18
-11-
-OCF3, -S02-CH3 in the para-position,
R3 C6-C12-aryl, which can optionally be substituted singly or doubly,
identically or differently with halogen, Cl-C3-alkyl, acetyl, methoxy,
ethoxy, cyano, hydroxy, N-(CH3)2, C02-(C,-C3-alkyl), CO-NHR5 or
CF3,
C5-C12-heteroaryl, which can optionally be substituted singly or
doubly, identically or differently with chlorine and/or fluorine, with
CI-C3-alkyl, acetyl, methoxy, ethoxy, cyano, hydroxy, N-(CH3)2,
C02-(C1-C3-alkyl), CO-NHR5 or with CF3,
C3-C6-cycloalkyl,
R4 hydrogen,
R5 hydrogen, C1-C6-alkyl-C3-C6-cycloalkyl, which is optionally
substituted singly or multiply, identically or differently with C,-C6-
alkyl, C,-C6-acyl, C,-C6-alkoxy or CF3,
C3-C6-cycloalkyl, which is optionally substituted singly or multiply,
identically or differently with Cl-C3-alkyl, C,-C3-acyl, C,-C3-alkoxy
or CF3,
C6-C12-aryl, which is optionally substituted singly or multiply,
identically or differently with halogen, Cl-C3-alkyl, CI-C3-acyl, Cl-
C3-alkoxy, N-Cj-C3-alkyl-Cj-C3-alkyl, CF3 or cyano, or
C5-C12-heteroaryl, which is optionally substituted singly or multiply,
identically or differently with halogen, C,-C3-alkyl, C,-C3-acyl, C,-
C3-alkoxy, N-C,-C3-alkyl-C,-C3-alkyl, CF3 or cyano, or
Cl-C6-alkyl, which can be substituted arbitrarily,
R6 the group C,-C4-alkyl, CH2-cyclo-C3-C6-alkyl, CH2-C6-C12-aryl, in
which C,-C4-alkyl, CH2-cyclo-C3-C6-alkyl, CH2-Cs-C,2-aryl can
optionally be substituted singly or multiply, identically or differently
by hydroxy, methoxy, chlorine, fluorine, cyano or aminosulfonyl,
CA 02646650 2008-09-18
-12-
as well as their isomers, diastereomers, enantiomers and salts.
Compounds of general Formula I in which the symbols have the following
meanings are also preferred,
R' hydrogen,
R2 tertiary butyl, iso-propyl, iso-butyl, sec. butyl, cyano, bromine, or
the group -O-CF3, -S02-CH3 in the para-position,
R3 the group
\ ~CH I \ C H3 I / 3 CH3
( \ I \ ( \ ~CFF
OH O~CH CH3
3
o__===cI c' cl ci R4 hydrogen,
CA 02646650 2008-09-18
-13-
R5 hydrogen or the group -(CH2)R,-N-(CH3)2, -(CH2)2-CH3, -(CH2)2-
NH-COCH3, -(CH2)-CHCH3-OH, -(CH2) 2-O-CH3,
-(CH2)2-OH, -CHCH3-CH2-OH, where m = 1-3,
I \ N~ \ N
N /
I ~ H
CH3
(N)
O
O O O O O O N
OO
R6 methyl, ethyl, propyl, 2-methoxyethyl, -CH2-CF3-, -(CH2)2-CF3 and
benzyl,
as well as their isomers, diastereomers, enantiomers and salts.
Compounds of general Formula I in which the symbols have the following
meanings are also preferred,
R' hydrogen,
CA 02646650 2008-09-18
-14-
R2 tertiary butyl, iso-propyl, iso-butyl, sec. butyl, cyano, bromine, or
the group -O-CF3, -S02-CH3 in the para-position,
R3 the group
O F N
1
Q1~ CH3
CH3
R4 hydrogen,
R5 hydrogen or the group, -(CH2)-CHCH3-OH,
-(CH2) 2-O-CH3, -CHCH3-CH2-OH,
(N) I
I / O Q
~ (")6
O N O O O Q
R6 methyl, ethyl, propyl, 2-methoxyethyl, -CH2-CF3-, -(CH2)2-CF3 and
benzyl,
as well as their isomers, diastereomers, enantiomers and salts.
The following compounds according to the present invention are quite
especially
preferred:
1. 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-2-
carboxylic
acid-(tetrahydropyran-4-yl )amide
CA 02646650 2008-09-18
-15-
2. 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-2-
carboxylic
acid (2-morpholin-4-ylethyl)amide
3. ( )-5-[(4-tert-butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-2-
carboxylic acid-(2-hydroxy-1-methylethyl)amide
4. ( )-5-[(4-tert-butylphenyfsuffonyl)methylamino]-3-phenyf-1 H-indole-2-
carboxylic acid-(2-hydroxypropyl)amide
5. 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-2-
carboxylic
acid-(pyridin-4-yl)amide
6. 5-[(4-tert-butyfphenylsulfonyl)benzylaminoj-3-phenyl-1 H-indole-2-
carboxylic
acid-(tetrahydropyran-4-yl)amide
7. 5-[(4-tert-butylphenylsulfonyl)benzylamino]-3-phenyl-1 H-indole-2-
carboxylic
acid (2-morpholin-4-yiethyl)amide
8. ( )-5-[(4-tert-butyfphenyfsuffonyl)benzylamino]-3-phenyl-1 H-indole-2-
carboxylic acid-(2-hydroxy-1-methyfethyf)amide
9. ( )-5-[(4-tert-butylphenylsulfonyl)benzylamino]-3-phenyl-1 H-indole-2-
carboxylic acid-(2-hydroxypropyl)amide
10. 5-[(4-tert-butyfphenyisulfonyl)-(2-methoxyethyl)amino]-3-phenyl-1 H-indole-
2-carboxylic acid-(tetrahydropyran-4-yl)amide
11. 5-[(4-tert-butylphenylsulfonyl)-(2-methoxyethyl)aminoj-3-phenyl-1 H-indole-
2-carboxylic acid (2-morpholin-4-yiethyl)amide
12. ( )-5-[(4-tert-butylphenylsulfonyl)-(2-methoxyethyl)amino]-3-phenyl-1 H-
indofe-2-carboxyiic acid-(2-hydroxy-l-methylethyl)amide
13. ( )-5-[(4-tert-butylphenylsuffonyl)-(2-methoxyethyl)aminoj-3-phenyl-1 H-
indole-2-carboxylic acid-(2-hydroxypropyl)amide
14. 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-(3-fluorophenyl)-1 H-indole-
2-
carboxylic acid-(tetrahydropyran-4-yl)amide
15. 5-[(4-tert-butyfphenyfsulfonyl)methyfamino]-3-(3-fluorophenyl)-1 H-indole-
2-
carboxylic acid (2-morphofin-4-yiethyl)amide
16. 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-(3-methoxyphenyl)-1 H-indofe-
2-carboxylic acid-(tetrahydropyran-4-y!)amide
17. 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-(3-methoxyphenyl)-1 H-indole-
2-carboxylic acid (2-morpholin-4-ylethyl)amide
CA 02646650 2008-09-18
-16-
The invention also provides a process for preparing the compounds of general
Formula I according to the present invention, which process is characterized
in
that a compound of Formula 11,
1-:ZZ R6 R3
R1
SiN O
R2 //\\ ):):N O O 5 o-R7
(II)
where R1, R2, R3 and R6 are each as defined above and R' may be hydrogen or
C,-C6-alkyl preferably is hydrogen, methyl or ethyl, is reacted with an amine
of
general Formula II1
R5
/
HN
\
R4 (III)
where R4 and R5 are each as defined above, according to methods known to
one skilled in the art, and any required protective groups are subsequently
cleaved.
When R' is hydrogen, the reaction may initially be effected by the activation
of
the acid function, for example by initially converting the carboxylic acid of
general Formula 11 in the presence of a tertiary amine, as for example
triethylamine, with isobutyl chloroformate into the mixed anhydride. The
reaction
of the mixed anhydride with the alkali metal salt of the corresponding amine
is
effected in an inert solvent or solvent mixture, such as for example
tetrahydrofuran, dimethoxyethane, dimethylformamide,
hexamethylphosphoramide, at temperatures between -20 C and +60 C,
preferably at 0 C to 30 C.
A further possibility consists in activating the carboxylic acid of general
Formula
11 by means of reagents such as for example HOBt or HATU. The reaction of
the acid with HATU, for example, is carried out in an inert solvent such as
for
example DMF in the presence of the corresponding amine of general Formula
CA 02646650 2008-09-18
-17-
III and a tertiary amine such as for example ethyldiisopropylamine at
temperatures between -50 and +60 C, preferably at 0 C to 30 C.
When R6 is C,-C6-alkyl, it is also possible for example to carry out a direct
amidolysis of the ester with the corresponding amine with or without
assistance
of aluminum trialkyl reagents, preferably aluminum trimethyl.
The compounds of general Formula II which serve as starting materials are
obtainable for example by proceeding in a conventional manner to reduce the
nitro group in the known indole esters IV
4
o2H ~ /~ \ o
N o-R7
H (IV)
where R7 is Cl-C6-alkyl, preferably methyl or ethyl, in a hydrogen atmosphere
or
hydrogen source such as for example ammonium formate in the presence of a
palladium catalyst to the amino function and then to react this amine with a
halide of general Formula V
R1
/ .,Hal
R2 // \\
O O M,
where R' and R2 are each as defined above and Hal represents halogen,
preferably fluoride, chloride or bromide, in the presence of a base such as
for
example pyridine, diisopropylethylamine, triethylamine or potassium carbonate
to form the compounds of general Formula VI
11-~
R1 H
~N O
R2 O// \\ I \ ~
O /
H O-R7
(VI).
CA 02646650 2008-09-18
-18-
The esters of general Formula VI are then halogenated in the 3-position for
examples by means of iodine, NBI, NBS or else CuBr2 to obtain the compounds
of general Formula VII
R1 H Br
iN O
R2 // \\ I
O O /
H 0-R7
where R1, R2 and R' are each as defined above.
These esters are subsequently reacted in the presence of a base such as for
example diisopropylethylamine, potassium carbonate or caesium carbonate in
acetone or tetrahydrofuran with the halide of general Formula VIII
,Hal
R6 (Vi 11),
where R6 is as defined above and Hal represents halogen, preferably iodide,
chloride or bromide, to form the compounds of general Formula IX
R6 Br
R1 1
SiN O
R2 // \\
O O
H O-R7
(IX).
The esters of general Formula IX are then converted in the 3-position, in a Pd-
catalyzed reaction with boronic acid derivatives of general Formula X
R3 "IB(oH)2
~X1
where R3 is as defined above, if appropriate after detachment of required
protective groups in R6, if appropriate followed by a saponification, for
example
with sodium hydroxide solution, into the compounds of general Formula II
CA 02646650 2008-09-18
-19-
R6 R3
R1 I
iN O
R2 // \\ I \
O O
H O-R7
(II).
Alternatively, it would also be possible to convert the esters of general
Formula VII initially in the 3-position in a Pd-catalyzed reaction with the
boronic
acid derivatives of the general Formula X
,B(OH)2
R3 X ( )
,
where R3 is as defined above, into the compounds of general Formula XI
R1 H R3
SI~N ~ O
2 // \\ I \
O O /
H O-R7 (Xl)
where R', R2, R3 and R7 are each as defined above, and subsequently to carry
out the N-alkylation step in the presence of a base such as for example
diisopropylethylamine, potassium carbonate or caesium carbonate in acetone or
tetrahydrofuran and the halide of general Formula VIII
~Hal R6 (VIII),
where R6 is as defined above and Hal represents halogen, preferably iodide,
chloride or bromide, if appropriate after detachment of required protective
groups in R6, if appropriate followed by a saponification, for example with
sodium hydroxide solution, to form the compounds of general Formula 11.
The compounds according to the invention inhibit soluble adenylate cyclase,
and this is also the basis of their action for example in male fertility
control.
Adenylate cyclases are the effector molecules for one of the most-used signal-
CA 02646650 2008-09-18
-20-
transduction pathways, they synthesize the second messenger molecule cyclic
adenosine monophosphate (cAMP) from adenosine triphosphate (ATP) with
splitting-off of pyrophosphate (PP). cAMP mediates numerous cellular
responses to a large number of neurotransmitters and hormones. Soluble,
sperm-specific adenylate cyclase (sAC, human mRNA sequence
(GenBank) nm_018417, human gene ADCY X) is one out of ten adenylate
cyclases described in the human genome. sAC exhibits some specific
properties that distinguish it from the other adenylate cyclases. In contrast
to all
other adenylate cyclases, sAC is stimulated by the concentration of
bicarbonate
in the surrounding medium and not by G proteins. sAC does not possess any
transmembrane regions in its amino acid sequence, it cannot be inhibited by
forskolin, can be stimulated much more strongly by manganese than by
magnesium, and only displays slight sequence homologies to the other
adenylate cyclases (s 26% identity of the catalytic domains I and II of sAC
with
other adenylate cyclases at the amino acid level).
Specific, manganese-dependent activity of sAC was first described by T. Braun
et al. (1975, PNAS 73:1097ff) in rat testis and sperm. N. Okamura et al.
(1985,
J. Biol. Chem 260(17):9699ff) showed that the substance which stimulates the
activity of sAC in boar seminal fluid is bicarbonate. It was also shown that
AC
activity that can be stimulated by bicarbonate can only be detected in rat
testis
and sperm, but not in other tissues. sAC was purified from rat testis and
sequenced for the first time by the Buck and Levin group (J. Buck et al. 1999,
PNAS 96:79ff, WO 01/85753). The expected properties (e.g. capacity to be
stimulated by bicarbonate and magnesium) were confirmed on recombinantly
expressed protein (Y. Chen et al. 2000, Science 289:625ff).
Testis-specific and sperm-specific expression of the enzymes can be concluded
from data on the distribution of sAC mRNA and on sAC activity that can be
stimulated by bicarbonate (ML Sinclair et al. 2000, Mol Reprod Develop 56:6ff;
N Okamura et al. 1985, J. Biol. Chem 260(17):9699ff; J. Buck et al. 1999, PNAS
96:79ff). In the testis, sAC mRNA is only expressed in later stages, of the
gametes developing to sperm, but not in somatic cells (ML Sinclair et al.
2000,
CA 02646650 2008-09-18
-21-
Mol Reprod Develop 56:6ff).
There have been a number of pharmacological investigations into the function
of sAC in sperm in mammals. Before sperm can penetrate the zona pellucida of
the ovum and then fuse with the oolemma of the ovum, sperm must be
prepared for this functionality. This process, sperm capacitation, has been
thoroughly investigated. A capacitated sperm is characterized by an altered
pattern of movement and by the ability to go through the process of the
acrosome reaction (release of lytic enzymes which presumably serve for
penetration of the zona pellucida by the sperm) when suitably stimulated.
Sperm capacitation takes place in vivo and in vitro and among other things
independently of a raised bicarbonate concentration in the medium (PE Visconti
& GS Kopf (1998), Biol Reprod 59:1ff; E de Lamirande et al. 1997, Mol Hum
Reprod 3(3):175ff). Sperm capacitation can also be stimulated by adding
suitable membrane-passing cAMP analogs, e.g. db-cAMP, and an inhibitor that
prevents their degradation (e.g. IBMX). The presumed dependence of sperm
function on sAC was confirmed only recently by a genetic deletion model, a so-
called knock-out mouse (G Esposito et al. 2004, PNAS 101(9):2993ff). Male
mice lacking the gene for sAC exhibit spermatogenesis that proceeds normally,
but are infertile. The sperm have motility defects and are not capable of
fertilizing an egg. The animals did not display any other defects or abnormal
findings, which contradicts other hypothesized functions of sAC (JH Zippin et
al.
2003, FASEB 17:82ff)).
sAC has a unique sequence and only slight homology to other somatic
adenylate cyclases. It is the only adenylate cyclase in mammalian sperm and
the activity is essential for sperm motility and capacitation. Specific sAC
inhibitors accordingly represent an important possibility for controlling male
fertility.
The present invention therefore relates to medicinal products that contain at
least one of the compounds as claimed in claims 1-7.
CA 02646650 2008-09-18
-22-
The present invention also relates to the use of the compounds as claimed in
claims 1-7.
For use of the compounds according to the invention as medicinal products they
are converted to the form of a pharmaceutical preparation, which contains, in
addition to the active substance, pharmaceutical, organic or inorganic inert
vehicles that are suitable for enteral or parenteral application, for example
water, gelatin, gum arabic, lactose, starch, magnesium stearate, talc,
vegetable
oils, polyalkylene glycols etc. The pharmaceutical preparations can be in
solid
form, for example as tablets, dragees, suppositories, or capsules, or in
liquid
form, for example as solutions, suspensions or emulsions. If necessary they
also contain excipients, such as preservatives, stabilizers, wetting agents or
emulsifiers; salts for altering osmotic pressure or buffers. These
pharmaceutical
preparations are also the object of the present invention.
Injection solutions or suspensions, especially aqueous solutions of the active
compounds in polyhydroxyethoxylated castor oil, are particularly suitable for
parenteral application.
Surface-active excipients such as salts of bile acids or animal or vegetable
phospholipids, as well as mixtures thereof and liposomes or their
constituents,
can also be used as carrier systems.
In particular, tablets, dragees or capsules with talc and/or hydrocarbon
vehicles
or binders, for example lactose, maize starch or potato starch, are suitable
for
oral application. Application can also be in liquid form, for example as
juice, to
which a sweetener is added if required.
For vaginal application, for example suppositories are suitable and
conventional.
The present invention also relates to enteral, parenteral, vaginal and oral
application.
CA 02646650 2008-09-18
-23-
The dosage of the active substances can vary depending on the route of
administration, the patient's age and weight, the nature and severity of the
disease to be treated and similar factors. The daily dose is 0.5-1000 mg,
preferably 50-200 mg, and the dose can be a single dose that is to be
administered once, or can be divided into 2 or more daily doses.
The compounds according to the invention of general Formula I are, among
other things, excellent inhibitors of soluble adenylate cyclase. Inhibitors of
soluble adenylate cyclase lead to depression of the cAMP signal. The cAMP
level is decisive for control of the processes that play an important role in
cell
proliferation, cell differentiation and apoptosis. Diseases, e.g. cancer, in
which
depression of the cAMP level is decisive, can be modulated by inhibitors of
soluble adenylate cyclase. This modulation can have prophylactic and
therapeutic effects for patients suffering such a disease. At the present time
diseases which are, like cancer, associated with increased cell pro;`feration,
are
treated for example by radiotherapy and chemotherapy. These methods are
nonspecific and have a high potential for side-effects. The provision of new
substances, which act directly on particular target sites, is therefore
advantageous. The present invention relates to substances that modulate cAMP
production by the inhibition of soluble adenylate cyclase. For example,
abnormal cell proliferation can be reduced or prevented by regulation or
inhibition of cAMP production. Soluble adenylate cyclase can be inhibited by
the
use of the substances according to the invention, with a consequent reduction
in
cell proliferation. The present invention relates to medicinal products for
the
treatment of diseases that contain at least one compound according to general
Formula I, and medicinal products with suitable vehicles and excipients. The
diseases are characterized in that they are caused by disturbances of
metabolism of the second messenger cAMP.
Lowering of the cAMP concentration by inhibition of soluble adenylate cyclase
can provide a means of modulation of sperm capacitation. The present
invention relates to the use of the substances according to the invention for
the
CA 02646650 2008-09-18
-24-
lowering and/or inhibition of male gamete fertility, mediated by the reduction
or
inhibition of soluble adenylate cyclase activity and accordingly of sperm
capacitation.
Fertilization of the ovum can be prevented by administering an effective
amount
of a substance that leads to inhibition of cAMP production. The present
invention also relates to the use of the compound of general Formula I for the
production of a medicinal product for non-hormonal contraception.
If the production of the starting compounds is not described, these are known
or
can be produced similarly to known compounds or methods described here. It is
also possible to carry out all the reactions described here in parallel
reactors or
using combinatorial techniques.
The mixtures of isomers can be separated into the enantiomers or E/Z isomers
by usual methods, for example crystallization, chromatography or salt
formation.
The salts are produced in the usual manner, by adding the equivalent amount or
an excess of a base or acid, which is in solution if necessary, to a solution
of the
compound of Formula 1, separating the precipitate or processing the solution
in
the usual way.
Production of the compounds according to the invention
The following examples explain the production of the compounds of general
Formula I according to the invention, without limiting the scope of the
claimed
compounds to these examples.
The compounds of general Formula I according to the invention can be
produced as described below.
Example 1: 5-[(4-tert-Butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-
2-carboxylic acid-(tetrahydropyran-4-yl)amide
CA 02646650 2008-09-18
-25-
/
~ I -
SiN ~ O
O O ~
\ N N-CO
H H
A solution in 0.75 ml of dimethylformamide of 45 mg of the acid prepared in
Example 1f) is admixed with 40.3 mg of N-[(dimethylamino)-1 H-1,2,3-
triazolo[4,5-b]pyridin-1 -ylmethylene]-N-methylmethanaminium hexafluoro-
phosphate N-oxide (HATU) and 9.84 mg of 4-aminotetrahydropyran. Then
18.0 NI of ethyldiisopropylamine are added dropwise at 0 C before stirring at
room temperature for 20 hours. This is followed by addition of 25 ml of water,
stirring for 30 minutes and filtering with suction. The residue thus obtained
is
purified by chromatography over silica gel with hexane/0-70% ethyl acetate to
obtain 49.7 mg of the title compound.
NMR (300 MHz, DMSO-d6): 8= 1.22 (2H), 1.26 (s, 9H), 1.65 (2H), 3.07 (3H),
3.32 (2H), 3.68 (2H), 3.89 (1 H), 6.91 (1 H), 6.99 (1 H), 7.26-7.33 (4H), 7.34-
7.41
(5H), 7.54 (2H), 11.85 (1 H).
The starting material for the title compound above is prepared as follows:
1 a) Ethyl 5-amino-1 H-indole-2-carboxylate
"2" o
¾--\
5 g of ethyl 5-nitro-1 H-indole-2-carboxylate are initially charged in 170 ml
of
methanol and 0.5 ml of water, admixed with 6.73 g of ammonium formate and
with 50 mg of palladium on carbon (10%) and refluxed at 90 C for 1 hour. This
is followed by filtration through Celite with suction and washing with warm
methanol. After solvent removal, the residue is admixed with 100 ml of water
and stirred for 10 minutes and the precipitated solid material is dried under
reduced pressure to obtain 4.12 g of the title compound.
CA 02646650 2008-09-18
-26-
NMR (300 MHz, DMSO-d6): 8 = 1.28 (3H), 4.25 (2H), 4.63 (2H), 6.62-6.68 (2H),
6.79 (1H), 7.12 (1H), 11.35 (1H).
1 b) Ethyl 5-(4-tert-butylphenylsulfonylamino)-1 H-indole-2-carboxylate
H
u Q
i <):)N
H o
A solution, in 195 mi of DMF, of 4.12 g of the amine prepared in Example 1 a)
is
admixed at 0 C with 5.18 ml of ethyidiisopropylamine and 4.69 g of 4-tert-
butylphenyisulfonyl chloride and stirred at room temperature for two hours.
The
solvent is removed under reduced pressure and the residue is purified by
chromatography over silica gel with hexane/0-80% ethyl acetate to obtain 7.56
g
of the title compound.
NMR (300 MHz, DMSO-d6): S= 1.20 (9H), 1.27 (3H), 4.27 (2H), 6.97-7.03 (2H),
7.25 (1H), 7.31 (1H), 7.48 (2H), 7.59 (2H), 9.93 (1H), 11.80 (1H).
1 c) Ethyl 3-bromo-5-(4-tert-butylphenylsulfonylamino)-1 H-indole-2-
carboxylate
H Br
O
OSO / ! \
A solution, in 217 ml of tetrahydrofuran, of 7.56 g of the sulfonamide
prepared in
Example 1 b) is admixed with 3.36 g of N-bromosuccinimide and stirred at room
temperature for 40 minutes. After diluting with 300 ml of ethyl acetate,
washing
once with 50 ml of water and twice with 50 ml of saturated sodium chloride
solution each time, the organic phase is dried over sodium sulfate. After
filtration
and concentrating under reduced pressure, the residue thus obtained is
recrystallized from hexane/ethyl acetate to obtain 8.11 g of the title
compound.
CA 02646650 2008-09-18
-27-
NMR (300 MHz, DMSO-d6): 8= 1.20 (9H), 1.30 (3H), 4.31 (2H), 7.08-7.15 (2H),
7.33 (1 H), 7.50 (2H), 7.60 (2H), 10.08 (1H), 12.16 (IH).
1 d) Ethyl 3-bromo-5-[(4-tert-butylphenylsulfonyl)methylamino]-1 H-indole-
2-carboxylate
I I o
o
N 0
H
A suspension, in 10 ml of acetone, of 1 g of the bromide prepared in Example
1 c) is admixed with 375 mg of potassium carbonate and 0.13 ml of methyl
iodide and stirred at room temperature for 24 hours. The mixture is diluted
with
300 ml of ethyl acetate and washed once with 50 ml of water and once with
50 ml of saturated sodium chloride solution. Drying over sodium sulfate and
filtration is followed by concentrating under reduced pressure. The residue
thus
obtained is purified by medium pressure chromatography over silica gel with
hexane/ethyl acetate in a ratio of 7:3 to obtain 670 mg of the title compound.
NMR (300 MHz, DMSO-d6): S= 1.27 (9H), 1.32 (3H), 3.14 (3H), 4.34 (2H), 6.92
(1 H), 7.08 (1 H), 7.39 (1 H), 7.41 (2H), 7.56 (2H), 12.33 (1 H).
1 e) Ethyl 5-[(4-tert-butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-
2-carboxylate
'I o
io
N
"
A solution, in a mixture of 25.5 ml of ethanol and 25.5 ml of toluene, of 666
mg
of the ester prepared in Example 1d) is admixed with 239 mg of phenylboronic
acid and 3.37 ml of 1 M aqueous sodium carbonate solution and also 160 mg of
lithium chloride. After addition of 125 mg of tetrakis(triphenylphosphine)-
palladium the reaction mixture is refluxed for 20 hours. After cooling to room
CA 02646650 2008-09-18
-28-
temperature, the reaction mixture is filtered through Celite with suction and
the
filter residue is washed with ethyl acetate. The organic phase thus obtained
is
washed with 10 ml of saturated sodium bicarbonate and saturated sodium
chloride solution and dried over sodium sulfate. After concentrating under
reduced pressure, the residue thus obtained is purified by chromatography over
silica gel with hexane/0-60% ethyl acetate to obtain 626 mg of the title
compound.
NMR (300 MHz, DMSO-d6): S= 1.13 (3H), 1.27 (9H), 3.06 (3H), 4.18 (2H), 6.90
(1 H), 7.07 (1 H), 7.25-7.7.36 (5H), 7.39 (2H), 7.43 (1 H), 7.55 (2H), 10.24
(1 H).
1 f) 5-[(4-tert-Butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-2-
carboxylic
acid
I
0
N OH
H
A mixture of 600 mg of the ester prepared in Example le) in 14.5 ml of
ethanol,
and 7.25 ml of ethanol is admixed with 905 mg of sodium hydroxide and stirred
at room temperature for 20 hours. The mixture is then diluted with 100 ml of
water and acidified with 5% of sulfuric acid. The precipitate is filtered off
and
dried to obtain 205 mg of the title compound which is further reacted without
further purification.
NMR (300 MHz, DMSO-d6): S= 1.26 (9H), 3.06 (3H), 6.88 (1H), 7.04 (1H),
7.24-7.35 (5H), 7.36-7.43 (3H), 7.55 (2H), 11.94 (1 H), 12.93 (1 H).
Example 2: 5-[(4-tert-Butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-
2-carboxylic acid-(2-morpholin-4-ylethyl)amide
CA 02646650 2008-09-18
-29-
/
~ I -
( N 0
o/\o 1
N N
H H
Example 1 is repeated with 45 mg of the acid of Example 1f) and 12.8 NI of
2-(morpholin-4-yl)ethylamine to obtain 36.2 mg of the title compound.
NMR (300 MHz, DMSO-d6): 8= 1.27 (9H), 2.18 (4H), 2.25 (2H), 3.06 (3H), 3.25
(2H), 3.38 (4H), 6.85 (1 H), 6.98 (1 H), 7.27-7.42 (9H), 7.55 (2H), 11.85 (1
H).
Example 3: ( )-5-[(4-tert-Butylphenylsulfonyl)methylamino]-3-phenyl-1 H-indole-
2-carboxylic acid-(2-hydroxy-l-methylethyl)amide
O/\O / I O OH
~ H N~
H
Example 1 is repeated with 45 mg of the acid of Example 1f) and 7.75 NI of
2-amino-l-propanol to obtain 42.6 mg of the title compound.
NMR (300 MHz, DMSO-d6): 8= 0.94 (3H), 1.26 (9H), 3.07 (3H), 3.13-3.30 (2H),
3.89 (1 H), 4.62 (1 H), 6.89 (1 H), 6.96 (1 H), 6.99 (1 H), 7.26-7.41 (8H),
7.54 (2H),
11.83 (1 H).
Example 4: ( )- 5-[(4-tert-Butylphenylsulfonyl)methylamino]-3-phenyl-1 H-
indole-
2-carboxylic acid-(2-hydroxypropyl)amide
N
0
pp / ( \ --OH
N N
H H
CA 02646650 2008-09-18
-30-
Example 1 is repeated with 45 mg of the acid of Example 1f) and 7.31 mg of
1 -amino-2-propanol to obtain 45.4 mg of the title compound.
NMR (300 MHz, DMSO-d6): 8= 0.91 (3H), 1.27 (9H), 3.00-3.17 (2H), 3.06 (3H),
3.58 (1 H), 4.59 (1 H), 6.87 (1 H), 6.99 (1 H), 7.15 (1 H), 7.26-7.41 (8H),
7.54 (2H),
11.84 (1 H).
Biological examples:
Example 1: sAC assay
In a suitable buffer system, soluble, sperm-specific adenylate cyclase
catalyzes
the conversion of adenosine triphosphate (ATP) to cyclic adenosine
monophosphate (cAMP) and pyrophosphate. Free cAMP generated in this way
is then used in a competitive detection technique, in which the binding of a
europium cryptate Eu[K]-labeled anti-cAMP antibody (anti-cAMP-Eu[K]-AB) to a
modified allophycocyanine-1 molecule labeled with cAMP molecules (cAMP-
XL665) is prevented. In the absence of exogenous cAMP, after excitation at
335 nm there is Fluorescence Resonance Energy Transfer (FRET) between the
anti-cAMP-Eu[K]-AB (FRET donor) and the cAMP-XL665 molecule (FRET
acceptor). This process is quantified, time-resolved, on the basis of the
emission of the FRET acceptor XL665 (665nm and 620nm). A decrease in
signal (measured as Well Ratio; calculated from the Formula:
[(E665nm/E620nm) x 10000] ) can be attributed to the presence of cAMP and
thus to the activity of sAC. First, 1.5 NI of the test substance (in 30% DMSO)
is
placed in each well of a 384-well test plate (polystyrene; 384, NV), and in
the
solvent controls only 30% DMSO. Then 10 ul of a dilute sAC enzyme solution is
applied (enzyme stock solution in 300 mM NaCl, 10% glycerol; pH 7.6; enzyme
intermediate and final dilution a) 1:10 and b) 1:2000 in each case in: 1.0 mM
MnC12; 0.2% BSA; 50 mM Tris pH 7.5 in H20). The enzyme reaction is started
by adding 5 NI of the ATP substrate solution (200 pM ATP in H20) and after
incubation (25 min at room temperature) stopped by adding 5pI of the stop
solution (200 pM EDTA in PBS). Finally the whole reaction is adjusted to a
total
volume of 91.5 NI by adding 70 pl PBS.
CA 02646650 2008-09-18
-31-
Next, 8 pl of detection solution 1 is placed in a well of the 384-well
measuring
plate (measuring plate: polystyrene; 384, SV - black; detection solution 1: 50
pl
cAMP-XL665; 950 pl reconstituted buffer; 2200 pl PBS; cAMP-XL665: prepared
by adding 5 ml H20 to the lyophilized product according to the instructions in
Cis
bio Kit: #62AMPPEC; storage: in aliquots at -80 C). Next, 3 pl from the 91.5
NI is
added to the corresponding well of the test plate. Finally, 8 NI of detection
solution 2 is added (detection solution 2: 50 pl anti-cAMP-Eu[K]-AB; 950 pl
reconstituted buffer; 2200 pl PBS; anti-cAMP-Eu[K]-AB: prepared according to
the instructions in Cis bio Kit: #62AMPPEC; storage: in aliquots at -80 C).
After further incubation for 90 min at room temperature, the HTRF result is
measured either on the Packard Discovery or with the RubiStar HTRF
measuring instrument (delay: 50 ps; integration time: 400 ps).
Example 2. Isolation of human sperm from ejaculates and capacitation
2.1. Isolation of the sperm
Human sperm from ejaculate are purified in a two-layer gradient system based
on colloidal silica particles (trade name: Percoll or ISolate).
Per ejaculate, 2.5 ml of pre-warmed lower layer ("90% ISolate lower layer",
from
Irvine) is placed in a 15 ml centrifuge tube (conical, plastic) and is
carefully
covered with 2.5 ml of pre-warmed upper layer ("50% ISolate upper layer", from
Irvine) and held at 37 C for < lh on a water bath. The gradient is carefully
covered with max. 3 ml of normal (with respect to sperm count, motility and
liquefaction) ejaculate. Sedimentation of the sperm is carried out at 1000 x g
for
min at room temperature. Using a glass capillary, the two layers are removed
25 by suction to just above the sperm pellet. For elutriation of the ISolate
gradients,
the sperm pellets, each resuspended in approx. 200 NI, are transferred to a
15 ml plastic tube with 12 ml mHTF medium (4 mM NaHCO3; 0.01% BSA; 37 C)
and the sperm are sedimented at 1000 x g for 20 min. The medium is removed
by suction to just above the pellet and adjusted with mHTF medium (4 mM
NaHCO3; 0.01 % BSA; 37 C) to 1000 pl. The sperm count is determined in a
Neubauer counter and for subsequent capacitation is adjusted if necessary to
4x106 sperm/150 pl with mHTF medium (4 mM NaHCO3; 0.01 % BSA; 37 C).
CA 02646650 2008-09-18
-32-
2.2. Capacitation
If the influence of test substances on the acrosome reaction is to be tested,
the
sperm must be preincubated with the test substances. This preincubation
(15 min in a heating cabinet at 37 C) is necessary to permit penetration of
the
test substances into the sperm before the start of capacitation, i.e. to
achieve
presaturation of the binding sites in the sperm, especially in the case of
substances that do not pass through the membrane easily. It is also necessary
because the increase in BSA concentration during capacitation due to the high
lipid binding of the BSA could lead to a decrease in the effective
concentration
of test substance in the sample.
The test substances are dissolved in DMSO and diluted with mHTF medium
(4 mM NaHCO3; 0.01 % BSA; 37 C), so that in the final 400-NI capacitation
sample the DMSO concentration is 0.5%. In each case 150 ul of sperm
suspension is added by pipette to 150 NI of the aforementioned temperature-
controlled solution of test substance, followed by preincubation at 37 C for
15 min. Capacitation of the sperm is started by adding 100 NI of mHTF medium
(88 mM NaHCO3; 4% BSA; 37 C). In the final 400-NI capacitation sample, the
concentration of sperm is 10x106/ml, the bicarbonate concentration is 4 mM and
the BSA concentration is 1%. Capacitation is carried out for 3 hours at 37 C
in
the heating cabinet.
For stopping capacitation, the samples (each of 400 pi) are each transferred
completely to a 15-m1 sample tube with 1.5 ml mHTF (4 mM NaHCO3; 37 C),
centrifuged for 5 min at 1000 x g and the supernatant is removed. This step
removes both the large amount of protein and the test substances.
Example 3. Flow cytometric determination of the acrosome reaction
3.1. lnitiation of the acrosome reaction by treatment with ionophore and
simultaneous CD46-FITC staining
The acrosome reaction (AR) of the sperm is triggered by binding of the sperm
to
the zona pellucida (ZP). This releases enzymes from the acrosome, enabling
the sperm to penetrate the ZP and reach the ovum. In the AR, there is partial
fusion, at the sperm, of the plasma membrane with the outer acrosomal
membrane (OAM). At the end the sperm head is still restricted by the inner
CA 02646650 2008-09-18
-33-
acrosomal membrane (IAM). The CD46 antigen is only detectable at the IAM.
In vitro the acrosome reaction can only be induced with a suitable
concentration
of the calcium ionophore A23187 on capacitated sperm, but not on
uncapacitated sperm or sperm for which capacitation was inhibited by test
substances. By means of the FITC-labeled anti-CD46 antibody (from
Pharmingen) to the IAM, the acrosome-reacted sperm can be differentiated
from the acrosome-intact sperm, in which the IAM is not exposed, in the flow
cytometer. With simultaneous staining of the sperm with the DNA stain ethidium
homodimer (EhD), which only stains cells that have defective DNA membranes,
i.e. are dead, it is possible to distinguish dead sperm from live sperm.
Because the ionophore dilutions for initiating the AR appear to be very
unstable
and must be mixed with the CD46-FITC solution for simultaneous staining, the
solutions cannot be prepared before the start of the test, but must be
prepared
during processing of the capacitation samples.
The sperm pellets are resuspended in the residue of the supernatant and are
diluted with 450 pI mHTF (4 mM NaHCO3; 0.01% BSA; 37 C) on a water bath
(37 C). 100 NI aliquots of the sperm suspensions are transferred by pipette to
prepared sample-FACS flow tubes (on the water bath). 150 NI of a solution with
ionophore and FITC-labeled anti-CD46 antibody is added by pipette to the
sperm. The final concentration is 800 nm ionophore and a 1:125 dilution of the
anti-CD46 antibody in mHTF (4 mM NaHCO3; 0.01% BSA; 37 C). The sperm
are incubated therein for 30 min, protected from the light, on a water bath at
37 C.
Incubation is stopped by adding 3.5 ml PBS [0.1% BSA] / sample, followed by
centrifugation for 5 min at 700 x g (room temperature) and then removal of the
supernatants with suction. After centrifugation, the samples are kept warm on
a
hot-plate until measurement.
3.2. EhD staining (for differentiation of the dead/live acrosome-reacted
sperm}
500 NI of freshly prepared EhD solution (150 nm EhD in PBS [w/o BSA]; 37 C)
is added to each of the sperm pellets after removal by suction. The samples
can
then be measured in the flow cytometer (BD FacsCalibur). Measurement is
CA 02646650 2008-09-18
-34-
performed at a laser excitation wavelength of 488 nm, detecting 10000 sperm
per measurement. Acrosome-reacted sperm are measured via CD46-FITC in
the FL-1 filter at 530 nm. Dead sperm are measured by means of EhD-DNA
staining in the FL-2 filter at 634 nm. The measurement channels are
correspondingly compensated relative to one another beforehand.
3.3 Evaluation
The sperm are selected as a very uniform cell population in an FSC-H (forward
scatter) versus SSC-H (sideward scatter) dot-blot. As two-color fluorescence
staining is used, evaluation is performed by quadrant analysis in an FL-1
(EhD;
X axis) vs. FL-2 (FITC-CD46, Y axis) dot-blot with the selected sperm
population
from the FSC vs. SSC dot-blot:
Quadrant in FL-1 Staining Analysis
vs. FL-2 dot-blot
Q1 = UL upper left only EhD dead, not acrosome-reacted
sperm
Q2 = UR upper right EhD and dead, acrosome-reacted sperm
FITC-CD46
Q3 = LL lower left unstained live, not acrosome-reacted
sperm
Q4 = LR lower right only live, acrosome-reacted sperm
FITC-CD46
To calculate the %-induced acrosome-reacted sperm (= "IAR[%]"), only the live
sperm from Q3 and Q4 are taken, and their total count is set equal to 100%.
IAR is then calculated as follows:
IAR[%]- LRx100
LL + LR
A proportion of the sperm undergo the acrosome reaction spontaneously
without addition of ionophore (= "SAR[%]"). Therefore a control measurement is
always performed on identically-treated sperm without addition of ionophore.
Calculation of SAR is similar to calculation of IAR. The acrosome reaction
actually induced by the ionophore (= "ARIC[%]") is calculated as the
difference:
ARIC = IAR - SAR.
For subsequent analysis of the influence of our inhibitors on sAC-mediated
capacitation (measured as the capacity of the sperm for the ionophore-induced
CA 02646650 2008-09-18
-35-
acrosome reaction), the percentage of acrosome-reacted sperm in the positive
capacitation control (= incubation with mHTF medium with 25 mM NaHCO3; 1%
BSA without test substances) is set = 100%. The capacity of the sperm to which
the test substances have been added, for the acrosome reaction, is stated
relative to this maximum acrosome reaction.
Materials used
mHTF = modif. human tubular fluid (from Irvine Scientific), Dulbeccos's
Phosphate-Buffered-Saline (from Gibco) (with Ca2+, Mg2+, 1 g/L D-glucose,
36 mg/L Na-pyruvate, w/o phenol red, w/o NaHCO3); bovine serum albumin,
Fraction V (from Fluka); dimethylsulfoxide (DMSO), anhydrous (from Merck);
sodium bicarbonate 7.5% solution (893mM) (from Irvine Scientific); Isolate-
Gradient (from Irvine Scientific); lonophore-A23187 free acid, (from
Calbiochem); Ethidium Homodimer (EhD) (from Molecular Probe), Mouse Anti
Human CD46:FITC (from Pharmingen).
References:
J. W. Carver-Ward, Human Reproduction Vol. 11, No. 9, pp: 1923 ff, 1996
High fertilization prediction by flow cytometric analysis of the CD46 antigen
on
the inner acrosomal membrane of spermatozoa
O. J. D'Cruz, G. G. Haas, Fertility and Sterility Vol. 65, No. 4, pp: 843 ff,
1996
Fluorescence-labeled fucolectins are superior markers for flow cytometric
quantitation of the sperm acrosome reaction
E. Nieschlag, H.M. Behre, Andrology, Springer Verlag 1996
CA 02646650 2008-09-18
-36-
Examples:
# R R IC50 (NM)
CH3 2.6
N p~ N
H H \O/
2
-CH2-CHCH3-OH CH3 1.3
~ I -
/\0 / I
N C
\ N
H H
4
OH Ohrtel 13
H
i
H H
~
HO
OH
4-OH-estradiol
oH~;,a 11
H
HO
H H
HO
2-OH-estradiol
It can be seen from the table that with respect to the inhibition of soluble
adenylate cyclase, expressed by the IC50 value, the compounds according to
the invention display approximately 10-fold greater activity than the known
catechol estrogens (OH-estradiols). The catechol estrogens are toxic,
therefore
the compounds according to the invention are far superior to the known
compounds. The compounds according to the invention are also approximately
10-fold more potent than the compounds presented by Zippin.