Note: Descriptions are shown in the official language in which they were submitted.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
2-N-{5-[[4-[2-(METHYL-2-PYRIDINYLAMINO) ETHOXYI
PHENYLIMETHYLI-2 4-THIAZOLIDINEDIONEI BUTANEDIOIC ACID,
METHODS OF PREPARATION AND COMPOSITIONS WITH ROSIGLITAZONE
MALEATE
Throughout this application various publications,
published patent applications, and patents are referenced.
The disclosures of these documents in their entireties are
hereby incorporated by reference into this application in
order to more fully describe the state of the art to which
this invention pertains.
Field of the invention
The present invention relates to a rosiglitazone maleate
impurity and its use as a reference marker and reference
standard.
Background of the Invention
Rosiglitazone is an oral antidiabetic agent which acts
primarily by increasing insulin sensitivity. Chemically,
rosiglitazone maleate is ( )-5-[[4-[2-(methyl- 2-
pyridinylamino) ethoxy] phenyl] methyl]-2,4-
thiazolidinedione, (Z)-2-butenedioate (1:1) with a
molecular weight of 473.52 (357.44 free base). The molecule
has a single chiral center and is present as a racemate.
Due to rapid interconversion, the enantiomers are
functionally indistinguishable. (Physicians' Desk Reference
(electronic version), Thomson Micromedex, Greenwood
Village, Colorado (Edition expires [12/05])).
The structural formula of Rosiglitazone Maleate is:
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 2 -
S 2H
I I )==o (co
O O CO2H
C:r'NH
Rosiglitazone Maleate is marketed in the iJnited States
under the trade name AVANDIA in 2 mg, 4 mg, and 8 mg
tablets. In addition, Rosiglitazone Maleate in combination
with Metformin HC1 under the trade name AVANDAMET in
tablets with the following dosages: 1 mg/ 500 mg; 2 mg/ 500
mg; 4 mg/ 500 mg; 2 mg/ 1000 mg; 4 mg/ 1000 mg.
Rosiglitazone and its maleate salt are disclosed in the
following United States patents, hereby incorporated by
reference: 5,002,953; 5,741,803; 6,288,095.
Pharmaceutical products are regulated in most countries by
governmental agencies. For example, the U.S. Food & Drug
Administration (FDA) generally requires an applicant to
show safety and efficacy of the pharmaceutical product
during the approval/ review phase and requires monitoring
of the safety of the drug post-approval.
In order to satisfy safety concerns, the regulatory
agencies generally require a manufacturing specification
that sets the maximum amount of each identified impurity as
well as the maximum amount for all remaining unidentified
impurities. In addition, stability testing is performed on
the pharmaceutical composition to insure that it does not
substantially change over time.
Described herein is an impurity associated with
rosiglitazone maleate, along with methods and formulations
for minimizing the impurity.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 3 -
Susmnary of the Invention
The subject invention provides an isolated compound of the
formula :
s
~=0
N CO2H
0
>--1
HO2C
The subject invention also provides a composition
comprising a compound of the formula:
s
~=O
N C02H
O
>-/ HO2C
wherein the composition is free of rosiglitazone maleate.
The subject invention also provides a composition
comprising a compound having the formula(I):
s
)P--O
N CO2H
0
(I)
HO2C
in an amount of at least 0.2% based on the weight of the
composition, and a carrier.
The subject invention also provides a pharmaceutical
composition comprising a granulate of a mixture of:
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 4 -
a) rosiglitazone maleate;
b) at least one pharmaceutically acceptable carrier;
and
c) less than 0.1% of the compound of the formula (I):
S
N CO2H
(I)
>--~
HOzC
based, on the combined weight of the compound of the
formula (I) and rosiglitazone maleate.
The subject invention also provides a sealed package
comprising:
a pharmaceutical composition comprising rosiglitazone
maleate and at least one pharmaceutically acceptable
carrier; and
a desiccant.
The subject invention also provides a process for preparing
a pharmaceutical composition comprising rosiglitazone
maleate and a pharmaceutically acceptable carrier, wherein
the pharmaceutical composition comprises less than 1.2% of
2-N-{5-[[4-[2-(methyl-2-pyridinylamino)
ethoxy]phenyl]methyl]-2,4-thiazolidinedione} -butanedioic
acid, based on the combined weight of 2-N-{5-[[4-[2-
(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-2,4-
thiazolidinedione} -butanedioic acid and rosiglitazone
maleate,
comprising:
a. obtaining rosiglitazone maleate drug substance;
b. determining the total amount of 2-N-{5-[[4-[2-
(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-
2,4-thiazolidinedione} -butanedioic acid present
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 5 -
in the rosiglitazone maleate drug substance; and
c. including the rosiglitazone maleate ' drug
substance in the preparation of the
pharmaceutical composition only if the drug
substance is determined to have less than 0.10%
by weight of 2-N-{5-[[4-[2-(methyl-2-
pyridinylamino) ethoxy]phenyl]methyl]-2,4-
thiazolidinedione} -butanedioic acid.
The subject invention also provides a process for
validating a batch of a pharmaceutical composition
containing rosiglitazone maleate and at least one
pharmaceutically acceptable carrier for distribution
comprising:
a. determining the total amount of 2-N-{5-[[4-[2-
(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-
2,4-thiazolidinedione) -butanedioic acid in a
sample of the batch after stability testing; and
b. validating the batch for distribution only if the
sample of the batch is determined in step a) to
contain less than 1.2% by weight of 2-N-{5-[[4-
[2-(methyl-2-pyridinylamino)
ethoxy]phenyl]methyl]-2,4-thiazolidinedione) -
butanedioic acid.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 6 -
Detailed Description of the invention
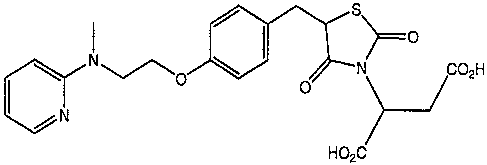
This invention provides an isolated compound (Compound 1)
of the following structural formula:
S
~ 1 ~--O
cc.N COzH
>-J HO2C
The chemical name of this compound is:
2-N-{5-[[4-[2-(methyl-2-
pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione}
-butanedioic acid.
The subject invention provides a isolated compound of the
formula:
S
~=O
N C02H
I O
HO2C
The subject invention also provides a composition
comprising a compound of the formula:
S
~=O
N CO2H
I 0
HO2C
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 7 -
wherein the composition is free of rosiglitazone maleate.
The subject invention also provides a composition
comprising a compound having the formula(I):
S
)==o
N CO2H
0 >---/, .
(I)
HO2C
in an amount. of at least 0.2% based on the weight of the
composition, and a carrier.
In an embodiment, the composition comprises at least 1.5%
of the compound of the formula (I), based on the weight of
the composition.
In another embodiment, the composition comprises at least
50% of the compound of the formula (I), based on the weight
of the composition.
In another embodiment, the composition comprises from more
than 0 to 99.8% by weight of rosiglitazone maleate, based
on the combined weight of the compound of the formula (I)
and the rosiglitazone maleate.
In another embodiment, the composition comprises from more
than 0 to 98.5% by weight of rosiglitazone maleate, based
on the combined weight of the compound of the formula (I)
and the rosiglitazone maleate.
In another embodiment, the composition is a pharmaceutical
composition and the carrier is a pharmaceutically
acceptable carrier.
In an embodiment of the pharmaceutical composition, the
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 8 -
compound of formula (I) is present in an amount between
0.20% and 5.0% based on the combined weight of the compound
of formula (I) and rosiglitazone maleate in the
composition.
By 0.20% to 5.0% it is meant that all hundredth, tenth and
integer unit amounts within the range are specifically
disclosed as part of the invention. Thus, 0.25%, 0.30%,
0.35% ... 4.95% unit amounts are included as embodiments
of this invention.
In another embodiment of the pharmaceutical composition,
the compound of formula (I) is present in an amount between
0.20% and 3.0% based on the combined weight of the compound
of formula (I) and rosiglitazone maleate in the
composition.
By 0.20% to 3.0% it is meant that all hundredth, tenth and
integer unit amounts within the range are specifically
disclosed as part of the invention. Thus, 0.25%, 0.30%,
0.35% ... 2.95% unit amounts are included, as embodiments
of this invention.
In another embodiment of the pharmaceutical composition,
the compound of formula (I) is present in an amount between
0.25% and 2.0% based on the combined weight of the compound
of formula (I) and rosiglitazone maleate in the
composition.
By 0.25% to 2.0% it is meant that all hundredth, tenth and
integer unit amounts within the range are specifically
disclosed as part of the invention. Thus, 0.30%, 0.35%,
0.40% ... 1.95% unit amounts are included as embodiments
of this invention.
In another embodiment of the pharmaceutical composition,
the compound of formula (I) is present in an amount between
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 9 -
0.70% and 2.0% based on the combined weight of the compound
of formula (I) and rosiglitazone maleate in the
composition.
By 0.70% to 2.0% it is meant that all hundredth, tenth and
integer unit amounts within the range are specifically
disclosed as part of the invention. Thus, 0.75%, 0.80%,
0.85% ... 1.95% unit amounts are included as embodiments
of this invention.
In another embodiment of the pharmaceutical composition,
the compound of formula (I) is present in an amount between
1.10% and 2.0% based on the combined weight of the compound
of formula (I) and rosiglitazone maleate in the
composition.
By 1.10% to 2.0% it is meant that all hundredth, tenth and
integer unit amounts within the range are specifically
disclosed as part of the invention. Thus, 1.15%, 1.20%,
1.25% ... 1.95% unit amounts are included as embodiments
of this invention.
The subject invention also provides a pharmaceutical
composition comprising a granulate of a mixture of:
a) rosiglitazone maleate;
b) at least one pharmaceutically acceptable carrier;
and
c) less than 0.1% of the compound of the formula ( I):
S
)-0
\ N\~\O \ N CO2H
O
(I)
HOZC
based on the combined weight of the compound of the
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 10 -
formula (I) and rosiglitazone maleate.
In an embodiment of the pharmaceutical composition, the
compound of formula (I) is present in an amount less than
0.1% based on the combined weight of the compound and
rosiglitazone maleate.
In another embodiment of the pharmaceutical composition,
the granulate is a product of wet granulation in an aqueous
solution.
In another embodiment of the pharmaceutical composition,
the granulate is a milled granulate.
In another embodiment, the pharmaceutical composition
further comprises at least one disintegrant and at least
one binder.
In another embodiment of the pharmaceutical composition,
the disintegrant is croscarmellose sodium, and the binder
is povidone-.
In another embodiment, the pharmaceutical composition
further comprises a biguanide antidiabetic compound.
In another embodiment of the pharmaceutical composition,
the biguanide antidiabetic compound is a pharmaceutically
acceptable salt of metformin.
In another embodiment of the pharmaceutical composition,
the pharmaceutically acceptable salt of metformin is
metformin hydrochloride.
In another embodiment, the pharmaceutical composition
further comprises a sulfonyl urea antidiabetic compound.
In another embodiment of the pharmaceutical composition,
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 11 -
the sulfonyl urea antidiabetic compound is glimepride or
glipizide.
In another embodiment, the pharmaceutical composition is in
the form of a tablet.
In another embodiment of the pharmaceutical composition,
the tablet comprises a tablet core, the tablet core
comprising, by weight, between 1% and 4% rosiglitazone
maleate, 6% croscarmellose sodium, 2% povidone, between
0.5% and 1% magnesium stearate and between 87% and 90%
filler, the filler consisting of lactose and
microcrystalline cellulose.
In another embodiment of the pharmaceutical composition,
the tablet comprises a tablet core, the tablet core
comprising, by weight, between 75% and 76% metformin HC1,
between 0.2% and 1% rosiglitazone maleate, between 5% and
6% povidone, between 1% and 2% croscarmellose sodium,
between 4% and 5% starch, and between 0.5% and 1%
magnesium stearate.
The subject invention also provides a sealed package
comprising the composition described herein or the
pharmaceutical composition described herein.
In an embodiment, the sealed package further comprises a
desiccant.
In another embodiment of the sealed package, the desiccant
is silica gel.
In another embodiment, the sealed package is a HDPE bottle.
In another embodiment of the sealed package, a net increase
of the total content of a compound of the formula (I):
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 12 -
S
~=o
\ N \~~O \ N CO2H
0 >--1
(I)
H02C
based on the combined weight of the compound of the
formula (I) and rosiglitazone maleate in the
pharmaceutical composition, is 0.7% or less after
storage of the sealed package at 75% relative humidity
and 40 C for 3 months.
In another embodiment, the sealed package comprises the
pharmaceutical composition described herein, wherein the
pharmaceutical composition contains less than 1.0% of a
compound of the formula (I):
s
)9-0
\ N \~~O \ N CO2H
0
N
(I)
HOZC
based on the combined weight of the compound of the
formula (I) and rosiglitazone maleate in the
pharmaceutical composition, after storage of the
sealed package at 75% relative humidity and 40 C for 3
months.
The subject invention also provides a sealed package
comprising:
a pharmaceutical composition comprising rosiglitazone
maleate and at least one pharmaceutically acceptable
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 13 -
carrier; and
a desiccant.
In an embodiment of the sealed package, the pharmaceutical
composition further comprises a compound of the formula
(I) :
S
N CO2H
0
N
M
HO2C
in an amount of 1.0% or below, based on the combined
weight of the compound of the formula (I) and
rosiglitazone maleate in the pharmaceutical
composition.
In another embodiment of the sealed package, the compound
of the formula (I):
S
~
N~/\O N COZH
O
N
~I)
HO2C
is present in the pharmaceutical composition in an
amount of less than 0.1%, based on the combined weight
of the compound of the formula (I) and rosiglitazone
maleate.
In another embodiment of the sealed package, the
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 14 -
pharmaceutical composition contains less than 1.0% of a
compound of the formula (I):
S
~=O
\ N \~\O \ N CO2H
0
N
(I) >--1
HOZC
based on the combined weight of the compound of the
formula (I) and rosiglitazone maleate in the
pharmaceutical composition, after storage of the
sealed package at 75% relative humidity and 40 C for 3
months.
In another embodiment of the sealed package, a net increase
of the total content of a compound of the formula (I):
~ S
~ `=O
\ N ~\O \ N CO2H
O
N
(I)
HO2C
based on the combined weight of the compound of the
formula (I) and rosiglitazone maleate in the
pharmaceutical composition, is 0.7% or less after
storage of the sealed package at 75% relative humidity
and 40 C for 3 months.
In another embodiment of the sealed package, the
pharmaceutical composition further comprises at least one
disintegrant and at least one binder.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 15 -
In another embodiment of the sealed package, the
disintegrant is croscarmellose sodium, and the binder is
povidone.
In another embodiment of the sealed package, the
pharmaceutical composition further comprises a biguanide
antidiabetic compound.
In another embodiment of the sealed package, the biguanide
antidiabetic compound is a pharmaceutically acceptable salt
of metformin.
In another embodiment of the sealed package, the
pharmaceutically acceptable salt of metformin is metformin
HC1.
In another embodiment of the sealed package, the
pharmaceutical composition further comprises a sulfonyl
urea antidiabetic compound.
In another embodiment of the sealed package, the sulfonyl
urea antidiabetic compound is glimepride or glipizide.
In another embodiment of the sealed package, the
pharmaceutical composition is in the form of a tablet.
In another embodiment of the sealed package, the tablet
comprises a tablet core, the tablet core comprising, by
weight, between 1% and 4% rosiglitazone maleate, 6%
croscarmellose sodium, 2% povidone, between 0.5% and 1%
magnesium stearate and between 87% and 90% filler, the
filler consisting of lactose and microcrystalline
cellulose.
In another embodiment of the sealed package, the tablet
comprises a tablet core, the tablet core comprising, by
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 16 -
weight, between 75% and 76% metformin HC1, between 0.2%
and 1% rosiglitazone maleate, between 5% and 6% povidone,
between 1% and 2% croscarmellose sodium, between 4% and 5%
starch, and between 0.5% and 1% magnesium stearate.
In another embodiment of the sealed package, the desiccant
is silica gel.
In another embodiment of the sealed package, the
pharmaceutical composition is prepared by wet granulation
with water.
In another embodiment of the sealed package, the sealed
package is a HDPE bottle.
The subject invention also provides a process for making
the pharmaceutical composition described herein, comprising
a) granulating in the presence of water a mixture of
rosiglitazone maleate, croscarmellose sodium, povidone
and a filler to obtain a granualte;
b) drying the granulate;
c) milling the granulate to obtain a milled granulate;
d) mixing the milled granulate with magnesium stearate
to obtain a final blend; and
e) compressing the final blend into a tablet core.
In an embodiment of the process, steps c) - e) are
performed in an atmosphere having a relative humidity of
30% or less.
The subject invention also provides another process for
making the pharmaceutical composition described herein,
comprising
a) granulating in the presence of an aqueous povidone
solution a mixture comprising rosiglitazone maleate
and metformin to obtain a granulate;
b) drying the granulate;
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 17 -
c) milling the granulate to obtain a milled granulate;
d) mixing the milled granulate with croscarmellose
sodium and starch to obtain an first blend;
e) mixing the first blend with magnesium stearate to
obtain a final blend; and
f) compressing the final blend into a tablet core.
In an embodiment of the process, steps c) - f) are
performed in an atmosphere having a relative humidity of
30% or less.
In another embodiment, the process further comprises
coating the tablet core with a film coating.
The subject invention also provides another process for
preparing the pharmaceutical composition described herein
or the sealed package described herein, comprising:
a. obtaining rosiglitazone maleate drug substance;
b. determining the total amount of 2-N-{5-[[4-[2-
(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-
2,4-thiazolidinedione} -butanedioic acid present
in the rosiglitazone maleate drug substance; and
c. including the rosiglitazone maleate drug
substance in the preparation of the
pharmaceutical composition only if the drug
substance is determined to have less than 0.10%
by weight of 2-N-{5-[[4-[2-(methyl-2-
pyridinylamino) ethoxy]phenyl]methyl]-2,4-
thiazolidinedione} -butanedioic acid.
In an embodiment of the process, the pharmaceutical
composition is prepared by wet granulation in an aqueous
solution.
The subject invention also provides process for preparing a
pharmaceutical composition comprising rosiglitazone maleate
and a pharmaceutically acceptable carrier, wherein the
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 18 -
pharmaceutical composition comprises less than 1.2% of 2-N-
{5-[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-
2,4-thiazolidinedione} -butanedioic acid, based on the
combined weight of 2-N-{5-[[4-[2-(methyl-2-pyridinylamino)
ethoxy]phenyl]methyl]-2,4-thiazolidinedione} -butanedioic
acid and rosiglitazone maleate,
comprising:
a. obtaining rosiglitazone maleate drug substance;
b. determining the total amount of 2-N-{5-[[4-[2-
(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-
2,4-thiazolidinedione} -butanedioic acid present
in the rosiglitazone maleate drug substance; and
c. including the rosiglitazone maleate drug
substance in the preparation of the
pharmaceutical composition only if the drug
substance is determined to have less than 0.10%
by weight of 2-N-{5-[[4-[2-(methyl-2-
pyridinylamino) ethoxy]phenyl]methyl]-2,4-
thiazolidinedione} -butanedioic acid.
In an embodiment of the process, the pharmaceutical
composition is prepared by wet granulation in an aqueous
solution.
The subject invention also provides a process for
validating a batch of a pharmaceutical composition
containing rosiglitazone maleate and at least one
pharmaceutically acceptable carrier for distribution
comprising:
a. determining the total amount of 2-N-{5-[[4-[2-
(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-
2,4-thiazolidinedione} -butanedioic acid in a
sample of the batch after stability testing; and
b. validating the batch for distribution only if the
sample of the batch is determined in step a) to
contain less than 1.2% by weight of 2-N-{5-[[4-
[2-(methyl-2-pyridinylamino)
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 19 -
ethoxy]phenyl]methyl]-2,4-thiazolidinedione} -
butanedioic acid.
In an embodiment of the process, the batch is validated for
distribution only if the sample of the batch is determined
in step a) to contain less than 1.0% by weight of 2-N-{5-
[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-
2,4-thiazolidinedione} -butanedioic acid.
In another embodiment of the process, the batch is
validated for distribution only if the sample of the batch
is determined in step a) to contain less than 0.5% by
weight of 2-N-{5-[[4-[2-(methyl-2-pyridinylamino)
ethoxy]phenyl]methyl]-2,4-thiazolidinedione} -butanedioic
acid.
The subject invention also provides a process for making
the isolated compound described herein, comprising:
a. contacting rosiglitazone maleate with water at
a temperature of 40-100 C, and
b. isolating the compound from the reaction mixture
of step a).
In an embodiment of the method, the compound is isolated
from the mixture via chromatography.
In another embodiment of the method, the compound is
isolated from the mixture via recrystallization.
The subject invention also provides a method for increasing
insulin sensitivity in a human subject comprising
administering to the human subject the pharmaceutical
composition described herein.
The subject invention also provides use of the composition
described herein or the pharmaceutical composition
described herein for manufacturing a medicament for
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 20 -
increasing insulin sensitivity in a human subject.
The subject invention also provides a the pharmaceutical
composition described herein for increasing insulin
sensitivity in a human subject.
One of the methods for determining the level of Compound 1
in rosiglitazone maleate drug substance:
(a) Measuring by HPLC the area under a peak corresponding
to rosiglitazone in a reference standard at a known level;
(b) Measuring by HPLC the area under a peak corresponding
to Compound 1 in a sample comprising rosiglitazone maleate
and Compound 1;
(c) Determining the amount of Compound 1 in the sample by
comparing the area of Step (a) to the area of Step (b) and
taking into account the relative response factor between
Compound 1 and rosiglitazone.
As used herein, "drug substance" or "DS" refers to the
active ingredient in a drug product, which provides
pharmacological activity or other direct effect in the
diagnosis, cure, mitigation, treatment, or prevention of
disease, or to affect the structure or any function of the
body of man or animals.
As used herein, "drug product" or "DP" refers to the
finished dosage form containing the drug substance as well
as at least one pharmaceutically acceptable carrier.
As used herein, a"sealed" package, container or coating
substantially prevents the atmosphere from coming in
contact with the contents of the package or container or
with the material coated by the coating.
As used herein, a "HDPE" bottle refers to a bottle
constructed of high density polyethylene plastic.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 21 -
Impurities can be present in the active pharmaceutical
ingredient (API) or in the pharmaceutical dosage form, for
example, in tablets. In the case of impurities found in the
pharmaceutical dosage form and not in the corresponding
API, it is possible that the excipients in the dosage form
cause the degradation of rosiglitazone maleate and thereby
bring about the presence of impurities. It is possible that
these impurities only come into being after time and are
found as a result of long term or accelerated stability
studies.
Generally, side products, by-products, and adjunct reagents
(collectively "impurities") are defined spectroscopically
and or with another physical method, and then associated
with a peak position, such as that in a chromatogram or a
spot on a TLC plate. (Strobel, H.A.; Heineman, W. R.,
Chemical Instrumentation: A systematic Approach, 3rd ed.
(Wiley & Sons: New York 1989) p. 953.) Thereafter, the
impurity can be identified by its position in the
chromatogram where the position in a chromatogram is
conventionally measured in minutes between injection of the
sample on the column and elution of the particular
component through the detector.
The retention time can vary based upon the condition of the
instrumentation, as well as many other factors. To mitigate
the effects such variations have upon accurate
identification of an impurity, practitioners use the
"Relative retention time," (RRT) to identify impurities.
(Strobel p. 922) The RRT of an impurity is defined as the
retention time of the impurity divided by the retention
time of a reference marker. It may be advantageous to
select a compound other than the API that is added to the
mixture in an amount sufficiently large to be detectable
and sufficiently low as not to saturate the column and to
use that compound as the reference marker for determination
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 22 -
of the RRT.
A reference standard is a compound in a relatively pure
state, used to quantify the amount of the compound in an
unknown mixture. A reference standard can be used as either
an external standard or as an internal standard.
An external standard is employed when a solution of a known
concentration of the reference standard and an unknown
mixture are analyzed using the same technique. The amount
of the compound in the mixture can be determined by
comparing the magnitude of the detector responses.
An internal standard is employed when an unknown mixture
contains a detectable -amount of the reference standard
compound without addition of the reference standard. The
amount of internal standard is determined by preparing at
least two samples by adding known and differing amounts of
the internal standard. The proportion of the detector
response due to the reference standard present in the
mixture can be determined by plotting the detector response
attained for each of the samples against the amount of the
reference standard added to each of the samples and
extrapolating the plot to zero.
The compositions may be prepared as medicaments to be
administered orally, parenterally, rectally or
transdermally. Suitable forms for oral administration
include tablets, compressed or coated pills, dragees,
sachets, hard or soft gelatin capsules, sublingual
tablets, syrups and suspensions; for parenteral
administration the invention provides ampoules or vials
that include an aqueous or non-aqueous solution or
emulsion; for rectal administration there are provided
suppositories with hydrophilic or hydrophobic vehicles;
and for topical application as ointments and transdermal
delivery there are provided suitable delivery systems as
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 23 -
known in the art.
Specific examples of pharmaceutical acceptable carriers
and excipients that may be used to formulate oral dosage
forms of the present invention are described, e.g., in
U.S. Pat. No. 6,126,968 to Peskiri et al., issued Oct. 3,
2000. Techniques and compositions for making dosage forms
useful in the present invention are described-in the
following references: 7 Modern Pharmaceutics, Chapters 9
and 10 (Banker & Rhodes, Editors, 1979); Pharmaceut-ical
Dosage Forms: Tablets (Lieberman et al., 1981); Ansel,
Introduction to Pharmaceutical Dosage Forms 2nd Edition
(1976); Remington's Pharmaceutical Sciences, 17th ed.
(Mack Publishing Company, Easton, Pa., 1985); Advances in
Pharmaceutical Sciences (David Ganderton, Trevor Jones,
Eds., 1992); Advances in Pharmaceutical Sciences Vol 7.
(David Ganderton, Trevor Jones, James McGinity, Eds.,
1995); Aqueous Polymeric Coatings for Pharmaceutical
Dosage Forms (Drugs and the Pharmaceutical Sciences,
Series 36 (James McGinity, Ed., 1989); Pharmaceutical
Particulate Carriers: Therapeutic Applications: Drugs and
the Pharmaceutical Sciences, Vol 61 (Alain Rolland, Ed.,
1993); Drug Delivery to the Gastrointestinal Tract (Ellis
Horwood Books in the Biological Sciences. Series in
Pharmaceutical Technology; J. G. Hardy, S. S. Davis, Clive
G. Wilson, Eds.); Modern Pharmaceutics Drugs and the
Pharmaceutical Sciences, Vol 40 (Gilbert S. Banker,
Christopher T. Rhodes, Eds.).
Tablets may contain suitable binders, lubricants,
disintegrating agents, coloring agents, flavoring agents,
flow-inducing agents, and melting agents. For instance,
for oral administration in the dosage unit form of a
tablet or capsule, the active drug component can be
combined with an oral, non-toxic, pharmaceutically
acceptable, inert carrier such as lactose, gelatin, agar,
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 24 -
starch, sucrose, glucose, methyl cellulose, dicalcium
phosphate, calcium sulfate, mannitol, sorbitol,
microcrystalline cellulose and the like. Suitable binders
include starch, gelatin, natural sugars such as glucose or
beta-lactose, corn starch, natural and synthetic gums such
as acacia, tragacanth, or sodium alginate, povidone,
carboxymethylcellulose, polyethylene glycol, waxes, and
the like. Lubricants used in these dosage forms include
sodium oleate, sodium stearate, sodium benzoate, sodium
acetate, sodium chloride, stearic acid, sodium stearyl
fumarate, talc and the like. Disintegrators include,
without limitation, starch, methyl cellulose, agar,
bentonite, xanthan gum, croscarmellose sodium, sodium
starch glycolate and the like.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 25 -
Experimental Details
Example 1 - Isolation of Compound 1 by Preparative HPLC
1g of rosiglitazone maleate was mixed with 1g of water in a
reaction vessel with screw cap. The mixture was kept at 700
C for 5 days. To this mixture, 24 mL of methanol: water
solution of trifluoroacetic acid (with a pH of about 2.8)
(3:2) were added. The rosiglitazone base concentration of
the solution was 40mg/mL.
Preparative HPLC was performed to the solution using the
following conditions:
Column & Packing: Semi-preparative, Luna 54 C18(2).
250x10 mm.
Column Temperature: Ambient
Mobile Phase: Solution A - 100% Water. Solution B -
50:50 Water:Acetonitrile.
Elution proceeded according to the following schedule:
Time (min) % Solution A % Solution B
0 75 25
50 50
45 0 100
48 0 100
50 75 25
52 75 25
20 Flow Rate: 7.OmL/min
Detector: W at 245nm, 10mm flow cell path length
Injection Volume: 200 L
Injector Wash Solution: Solution B.
The peaks of interest eluted at approximately 33 minutes.
Separated fractions of Compound 1 were analyzed by HPLC.
The relevant fractions were concentrated in vacuo using
rotor evaporator and lyophilized. The obtained yield was
about 8mg.. The obtained compound was determined to be
Compound 1.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 26 -
13
6 12 4 S
8 16
2 O
3 7 O 9 11 O 15 N C
O2H
4 CON ~
5 HOzC ,
The compound was analyzed using NMR and the following
5 spectra were attained:
Atom # 1H C
1 - 157.89
2 6.66 105.89
3 7.51 137.45
4 6.57 111.52
5 8.08 147.26
6 3.07 37.03
7 3.90 48.52
8 4.11 65.33
9 - 157.56
10 6.87 114.39
11 7.15 130.29
12 - 128.27
13 3.39,3.00 36.55
14 4.99 50.66
- 173.22
16 - 170.37
NH - -
1' - 171.20
2' 5.09 50.66
3' 3.05, 2.65 33.02
4' - 168.98
MH+ MS = 473 mass units. This data and theNMR analysis
confirmed that Compound 1 was attained.
4
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 27 -
Example 2 - Isolation of Compound 1 by Crystallization
1.3g of rosiglitazone maleate was mixed with 1.3g of water
in a reaction vessel with screw cap. The mixture was kept
at 700 C for 5 days. 4 ml of methanol: water solution of
trifluoroacetic acid with a pH of about 2.8 (3:2) were
added to the mixture.
The sample was stored at 2-8 C for 5 days. Crystals of
rosiglitazone maleate enriched with Compound 1 precipitated
spontaneously from the sample. These crystals were vacuum-
filtered and washed with cold water and methanol. These
crude crystals were then dissolved in hot isopropyl alcohol
and cooled in an ice bath until crystals of Compound 1
appeared. The crystals were vacuum-filtered then washed
with cold isopropyl alcohol, methanol and dried to yield 40
mg. Purity: about 97-98%.
Example 3 - HPLC Analysis of Compound 1 Present in
Rosiglitazone Maleate DS
Column & Packing: Zorbax Eclipse XDB C8, 5 , 150 x 4.6 mm
Column. Temperature: 30 C
Mobile Phase: Buffer at pH 2.8 (20mM sodium dihydrogen
phosphate and 10mM sodium pentanesulfonate solution):
acetonitrile (80/20)
Flow Rate: 1.5mL/min
Detector: UV at 245nm, 10mm flow cell path length
Injection Volume: 20 L
Standard Solution preparation:
Rosiglitazone maleate analytical standard was dissolved in
diluent to make a solution with a concentration of
approximately 0.2 g/mL in terms of rosiglitazone.
Sample preparation:
20mg of the rosiglitazone maleate raw material was weighed
into a 25m1 volumetric flask and dissolved in diluent.
The standard and samples were injected and the peak due to
Compound 1 was monitored and calculated in the relevant
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 28 -
sample. The peak which corresponded to Compound 1 was found
to elute at approximately a Relative Retention Time of 0.7,
when compared to the rosiglitazone maleate peak.
Example 4 - HPLC Method for Determining Presence of
Compound 1 in Formulation of Rosiglitazone Maleate Tablets
or in Combined Formulation of Rosiglitazone Maleate and
Metformin HC1 Tablets
Column & Packing: Zorbax Eclipse XDB C8, 54, 150 x 4.6 mm
Column Temperature: 30 C
Mobile Phase: Solution A - 20mM sodium dihydrogen phosphate
and
10mM sodium pentanesulfonate solution at pH 2.8.
Solution B - 75% Solution A and 25% acetonitrile.
Elution proceeded according to the following schedule:
Time (min) Solution A % Solution B %
0 50 50
15 0 100
22 0 100
24 50 50
28 50 50
Flow Rate: 1.5mL/min
Detector: W at 245nm, 10mm flow cell path length
Injection Volume: 15 L
Standard Solution
Rosiglitazone maleate analytical standard was diluted with
diluent to a concentration of 0.1 g/mL.
Sample Solution Preparation
Tablets were dissolved and diluted based on the declared
strengths.
The standard and samples were injected and the peaks due to
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 29 -
Compound 1 were monitored and calculated in the relevant
sample.
The quantifiable limit of this method was determined to be
0.1% based on the combined weight of Compound 1 and
rosiglitazone.
Example 5a - Preparation of Tablets Comprising
Rosiglitazone Maleate and Metformin
Tablets were prepared using the excipients in Table 1.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 30 -
Table 1
Metformin HC1/ Metformin HC1/ Metformin HCl/
Rosiglitazone Rosiglitazone
MATERIAL Maleate Maleate Rosiglitazone
500/1 & 1000/2 500/2 & 1000/4 Maleate
500/4 mg
mg mg
Part I %W/W %W/W %W/W
Metformin HC1 75.76 75.6 75.0
Povidone USP(PVP 3.03 3.03 3.01
K-30)
Part II
Microcrystalline 2.88 2.87 2.86
Cellulose NF
Colloidal 0.03 0.03 0.03
Silicon Dioxide
Part III
Rosiglitazone 0.20 0.4 0.80
Maleate
Microcrystalline 2.88 2.87 2.86
Cellulose NF
Part IV
Microcrystalline 0.30 0.30 0.30
Cellulose NF
Granulation
Solution :
Povidone USP
(PVP K-90 ) in 2.73 2.72 2.71
hot purified
water
Part V
Croscarmellose 1.59 1.59 1.58
Sodium NF-
Microcrystalline 5.30 5.3 5.27
Cellulose NF-
Starch NF 4.39 4.34 4.37
Part VI
Magnesium 0.91 0.91 0.90
Stearate NF
Total (Cores) 100.0 100.0 100.0
Film coating (%
calculated as 3.0 3.0 3.0
per core weight)
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 31 -
1. Metformin HC1 & Povidone (PVP K-30) of Part I were
transferred to a high shear mixer and mixed.
2. Microcrystalline cellulose and Colloidal Silicon Dioxide
of part II were screened and mixed in a Y-cone mixer with
Rosiglitazone and Microcrystalline cellulose of Part III
and with Microcrystalline cellulose of part IV.
3. The mixture from Step 2 was transferred to the high
shear mixer from Step 1 and mixed.
4. Povidone (PVP K-90) solution in hot purified water was
added to the high shear mixer from Step 3 & was mixed to
attain a wet granulate.
5. The granulate from Step 4 was dried in a Fluid Bed
Dryer.
Steps 6 to 10 which follow were performed in an atmosphere
with lower than 30% relative humidity.
E. The dried granulate was milled and transferred to a Flow
bin or Y-cone.
7. Components of part V were screened through a sieve,
transferred to Flow bin or Y-Cone from Step 6, and mixed.
8. Magnesium stearate was screened through a sieve,
transferred to Flow bin or Y-cone from Step 7, and mixed to
get the final blend.
9. The final blend from Step 8 was compressed into cores in
a tableting machine to yield tablet cores of the
appropriate dosages.
10. The cores were coated using OPADRY , which consists of
HPMC2910/HYPROMELLOSE 6 cP, Titanium dioxide, Macrogol /
PEG 400, Iron Oxide yellow/ iron oxide red; using a coating
machine.
35
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 32 -
Example 5b - Preparation of Tablets Comprising
Rosiglitazone Maleate
Tablets were prepared using the excipients below.
MATERIAL Rosiglitazone Rosiglitazone Rosiglitazone
Maleate Tabs Maleate Tabs Maleate Tabs
(2 mg base) (4 mg base) (8 mg base)
Part I %W/W %W/W %W/W
Lactose Monohydrate NF 45.2 44.33 44.33
Croscarmellose Sodium 6.0 6.0 6.0
NF
Rosiglitazone Maleate 1.77 3.53 3.53
Povidone USP (PVP K-30) 2.0 2.0 2.0
Microcrystalline 44.36 43.47 43.47
Cellulose NF
Purified water USP as needed as needed as needed
Part II
Magnesium Stearate NF 0.67 0.67 0.67
Total (Cores) 100.0 100.0 100.0
Film coating(% 3.0 3.0 3.0
calculated as per core
weight)
Production method
1. The components of Part I were transferred to a high
shear mixer and mixed.
2. Purified water (granulation solution) was added to the
high shear mixer from Step 1 and mixed, and a wet granulate
was attained.
3. The granulate from Step 2 was dried in a Fluid Bed
Dryer.
Steps 4 to 7 which follow were performed in an atmosphere
with lower than 30% relative humidity.
4. The dried granulate was milled and transferred to a flow
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 33 -
bin or Y-cone and mixed.
5. Magnesium stearate was screened through a sieve,
transferred to the flow bin or Y-cone from Step 4 and mixed
to attain the final blend.
6. The final blend from Step 5 was compressed in to cores
in a tableting machine.
7. The cores were coated in a coating machine using Opadry
which consists of: HPMC2910/HYPROMELLOSE 6 cP, Titanium
dioxide, Macrogol / PEG 3350, Triacetin/Glycerol,
Triacetate, Talc, Iron Oxide yellow/ red/black, FD&C
Blue#2/Indigo Carmine Aluminum Lake.
Example 6 - Thermodegradation Study
Two batches of Rosiglitazone maleate drug substance were
analyzed for presence of Compound 1 after
thermodegradation, and after storage at RT, using the
method described in Example 4.
Also, drug product (500 mg metformin/ 4 mg rosiglitazone
base) prepared as in Example 5a made from two different
batches of the rosiglitazone maleate drug substance was
analyzed for presence of Compound 1= before and after
thermodegradation using the method described in Example 4.
The thermodegradation was performed by subjecting the
samples to 65 C heat and 100% relative humidity for 5 days.
The content of Compound 1 is listed in the table below. The
peaks were measured and the area percent (area of Compound
1 peak/ area of all rosiglitazone related peaks) of the
Compound 1 peak is listed below.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 34 -
Sample Content of Compound Content of Compound
1 without thermo- 1 after thermo-
degradation % (w/w) degradation % (w/w)
DS A 0.0 0.03
DS B 0.16 0.18
DP A 0.0 0.68
DP B 0.15 1.05
This Example shows that Compound 1 is formed to a greater
extent in DP than in the DS.
Example 7 - Compatibility Study
Rosiglitazone maleate was mixed with various excipients and
with metformin in the same proportions as present in the
500mg/4mg (base strength) metformin/ rosiglitazone maleate
tablets. 0.5 ml of water was added to each mixture, and the
samples were kept at 65 C for 4 days. The amount of
Compound 1 was determined in each of the samples using the
method in Example 4. The results are summarized in the
table below. The peaks were measured and the area percent
(area of Compound 1 peak/ area of all rosiglitazone related
peaks) of the Compound 1 peak is listed below.
Sample Area Percent
Rosiglitazone Maleate (alone) 0.9
PVP K90 1.3
PVP K30 1.5
Metformin HC1 0.2
Avicel 2.9
Croscarmellose 0.3
Starch 6.7
Mg Stearate 0.3
Placebo mix A 0.1
Placebo mix B 0.1
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 35 -
Placebo mix A refers to a mixture of excipients as
indicated in Example 5a, with the exception of
rosiglitazone maleate.
Placebo mix B refers to a mixture of excipients as
indicated in Example 5b, with the exception of
rosiglitazone maleate.
This Example indicates that the presence of moisture and
some excipients, especially starch, enhances the production
of Compound 1 in accelerated conditions.
Example 8 - Stability of Tablets
Tablets were prepared as described in Example 5. 100
tablets were packed into high density polyethylene (HDPE)
bottles=either with or without silica gel and were stored
at 75% RH and 40 C for 3 months. The tablets were then
tested for Compound 1. The results, in terms of percentage
of Compound 1 are listed in the table below. The percentage
of compound in all of the dosages in the table below before
the stability testing was less than 0.1%.
Tablets Stored without Stored with
silica gel silica gel
500/1 mg 0.7 0.4
1000/2 mg 0.6 0:4
500/2 mg 0.8 0.5
1000/4 mg 1.0 0.5
500/4 mg 1.3 0.7
It can be seen from this experiment that the absence of
water in pharmaceutical compositions comprising
rosiglitazone maleate enhances stability. Addition of
silica gel to packaging of pharmaceutical compositions
comprising rosiglitazone maleate reduces the formation of
Compound 1.
CA 02647829 2008-09-29
WO 2007/130064 PCT/US2006/017994
- 36 -
Example 9 Measurement of Content of Compound 1 in
AVANDAMET 1000/2 mg tablets
The amount of Compound 1 in commercially available
AVANDAMET tablets 1000/2 mg was analyzed using Example 4.
Compound 1 was present in an amount that was less than
quantifiable.