Note: Descriptions are shown in the official language in which they were submitted.
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
NANOSTRUCTURED COMPOSITIONS HAVING ANTIBACTERIAL,
ANTI-FUNGAL, ANTI-YEAST, AND/OR ANTI-VIRAL PROPERTIES
FIELD OF THE INVENTION
The invention is directed to nanostructured compositions having antibacterial,
anti-
fungal, anti-yeast, and/or anti-viral properties. The compositions are useful
as drug delivery
carriers for one or more active agents or, in the absence of an active agent,
in methods where
such compositions are desirable.
BACKGROUND
A. Background Regarding the Use of Antibacterial
Agents in Drug Dosage Forms and Consumer Use Products
Microbial preservatives are added to nonsterile dosage forms to protect them
from
microbiological growth or from microorganisms that are introduced
inadvertently during or
subsequent to the manufacturing process. In the case of sterile articles used
in multi-dose
containers, antimicrobial preservatives are added to inhibit the growth of
microorganisms that
may be introduced by repeatedly withdrawing individual doses.
All useful antimicrobial agents are toxic substances. See USP 26, General
Chapters,
Section 51, "Antimicrobial Effectiveness Testing." For maximum protection of
patients, the
concentration of the preservative shown to be effective in the final prepared
packaged
product should be below a level that may be toxic to humans.
U.S. Food and Drug Administration guidelines require that antimicrobial
effectiveness, whether inherent in the product (i. e. , for an antibiotic
agent) or whether
produced because of the addition of an antimicrobial agent, must be
demonstrated for all
injections packaged in multiple-dose containers or for other products
containing antimicrobial
preservatives. Antimicrobial effectiveness must be demonstrated for multiple-
dose topical
and oral dosage forms, and for other dosage forms such as ophthalmic, otic,
nasal, irrigation,
and dialysis fluids. See USP 26, General Chapters, Section 51, "Antimicrobial
Effectiveness
Testing."
The addition of an antimicrobial agent to a therapeutic dosage form can be
undesirable, as such compounds can be toxic and they can have undesirable
interactions with
the primary active agent to be delivered. In addition, the use of
microbiocides can promote
1
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
the generation of drug resistant bacteria, drug resistant yeast, drug
resistant fungi, etc. This
has been observed with the wide spread use of antibacterial lotions, soaps,
cleaning products,
etc.
Antibiotic resistance among bacteria has increased in recent years, and
concerns have
been raised that cross resistance might develop in bacteria or other
microorganisms due to
exposure to antibiotics or biocides. Rutala, W. A., "APIC Guideline for
Selection and Use of
Disinfectants," American J., 24:313-342 (1996); Russell et al., "Do
Antiseptics and
Disinfectants Select for Antibiotic Resistance?" J. of Medicinal Microbiology,
48:613-615
(1999). More effective disinfectants can be extremely irritant and toxic,
resulting in health
complications such as contact dermatitis and mucous membrane irritation among
personnel.
Hansen, K.S., "Occupational Dermatoses in Hospital Cleaning Women," Contact
Dermatitis,
9:343-351 (1983); Beauchamp et al., "A Critical Review of the Toxicology of
Glutaraldehyde, Critical Reviews in Toxicology, 22:143-174 (1992). Thus, there
is a
continuing need for effective and safe biocidal agents for topical and surface
disinfection and
microorganisms change and resistant strains develop.
B. Background Regarding Fungal, Bacterial,
Yeast, and Viral Opportunistic Organisms
The USP specifies testing for effectiveness in killing E. colf, P. aeruginosa,
and S.
aureus, plus acting to control and limit the growth of C. albicans, and A.
niger, to determine
the antimicrobial effectiveness of a dosage form. See USP 26 Section 51,
General Chapters,
"Antimicrobial Effectiveness Testing." These particular microorganisms are
representative
of the most problematic and troublesome bacteria, fungi, and yeast.
Aspergillus niger is a fungus and one of the most common species of the genus
Aspergillus. It causes black mold on certain types of fruit and vegetables,
and is a common
contaminant of food.
C. albicans is the major fungal pathogen of humans. Infections can be
localized, such
as vaginal infections and oral infections, which cause a considerable degree
of discomfort. In
some patient groups, whose defense system is severely compromised (prematurely
born
infants, leukemics and burn patients), the yeast can turn into a deadly
pathogen causing
systemic infections - up to 50% of the patients infected die as a result. The
incidence of such
infections is increasing rapidly, especially in hospitalized patients. In New
Zealand, such
2
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
infections are now ten times more frequent than they were 15 years ago.
Moreover, the
reservoir of anti-Candida. drugs is very limited, and these agents'can have
severe side effects.
E. coli is an ubiquitous bacteria, with a large number of known strains. The
particularly nasty E. coli strain E. coli 0157:H7 is a member of the EHEC -
enterohemorrhagic E. coll group. Enterohemorrhagic means an intestinally-
related organism
which causes hemorrhaging - and therefore, loss of blood. E. coli 0157:H7
produces a toxin,
called Shiga-like toxin (SLT) or Vero toxin. The toxin is a protein which
causes severe
damage to intestinal epithelial cells. This condition is particularly
dangerous to small
children, and may be lethal, as children are too small to tolerate significant
blood and fluid
loss. In some cases another syndrome is involved which is called hemolytic
uremic
syndrome (HUS), which is characterized by kidney failure and loss of red blood
cells.
Approximately 5% to 10% of children progress to this stage of disease. In
severe cases, the
disease can cause permanent kidney damage. The presence of this bacterium can
also be very
dangerous to the elderly or infirm. There can be a combination of HUS and
other factors
which involve the blood system, which can be lethal to the elderly in 50% of
the cases. There
is evidence that E. coli 0157:H7 is becoming more prevalent.
Pseudo zonas aeruginosa is a Gram-negative bacterium that is noted for its
environmental versatility, ability to cause disease in particularly
susceptible individuals, and
its resistance to antibiotics. The most serious complication of cystic
fibrosis is respiratory
tract infection by the ubiquitous bacterium Pseudomonas aeruginosa. Cancer and
burn
patients also commonly suffer serious infections by this organism, as do
certain other
individuals with immune systems deficiencies. Unlike many environmental
bacteria, P.
aeruginosa has a remarkable capacity to cause disease in susceptible hosts. It
has the ability
to adapt to and thrive in many ecological niches, from water and soil to plant
and animal
tissues. The bacterium is capable of utilizing a wide range of organic
compounds as food
sources, thus giving it an exceptional ability to colonize ecological niches
where nutrients are
limited. P. aeruginosa can produce a number of toxic proteins which not only
cause
extensive tissue damage, but also interfere with the human immune system's
defense
mechanisms. These proteins range from potent toxins that enter and kill host
cells at or near
the site of colonization to degradative enzymes that permanently disrupt the
cell membranes
and connective tissues in various organs.
Staphylococcus aureus is a leading cause of soft tissue infections, as well as
toxic
shock syndrome (TSS) and scalded skin syndrome. The pathogenic effects of
Staph are
3
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
mainly associated with the toxins it produces. Most of these toxins are
produced in the
stationary phase of the bacterial growth curve. In fact, it is not uncommon
for an infected site
to contain no viable Staph cells. The S. aureus enterotoxin causes quick onset
food poisoning
which can lead to cramps and severe vomiting. Infection can be traced to
contaminated
meats which have not been fully cooked. These microbes also secrete
leukocidin, a toxin
which destroys white blood cells and leads to the formation of pus and acne.
Particularly, S.
aureus has been found to be the causative agent in such ailments as pneumonia,
meningitis,
boils, arthritis, and osteomyelitis (chronic bone infection). Most S. aureus
are penicillin
resistant, but vancomycin and nafcillin are known to be effective against most
strains.
The molds such as Aspergillus fumigatus are filamentous fizngi. They are
especially
prevalent growing on the nonliving organic materials in the soil. They
disperse their non-
sexual spores called conidia in the air. Most Aspergilli are harmless to
humans and A.
fumigatus in particular is harmless to humans whose immune system has not been
compromised by disease, drug therapy or genetic conditions. Exposure to A.
fumigatus can
cause an allergic response in sensitive individuals. More importantly, A.
fumigatus is an
opportunistic pathogen of bone marrow transplant patients, AIDS patients, and
other immune
compromised individuals.
Aspergillusfumigatus is the most common mold causing infection worldwide. The
first infection described in man, an aspergilloma, was reported in Edinburgh
in 1842 and
many cases of invasive disease in non-immunocompromised patients have been
reported.
These cases and more recent epidemiological data emphasize that A. fumigatus
is a primary,
albeit rare, pathogen of man. Allergic disease due to Aspergillus was first
described in
London in 1952 and the first invasive (and fatal) infection in an
immunocompromised patient
was described in 1953 in the British Medical Journal in a patient from
Gloucester.
The frequency of invasive disease has risen approximately 14-fold over the 12
years
to 1992, as judged after death in unselected autopsies. Invasive aspergillosis
has overtaken
candidiasis as the most frequent fiun.gal pathogen detected post mortem in
tertiary care
hospitals in Europe. Thus 4% of all patients dying had invasive aspergillosis,
compared with
about 2% with invasive candidiasis. Patients at risk based on the disease
frequency include
those with chronic granulomatous disease (25-40%), lung transplant recipients
(17-26%),
allogeneic bone marrow transplant patients (4-30%), neutropenic patients with
leukemia (5-
25%), heart transplant recipients (2-13%), pancreas transplant recipients (1-
4%), renal
transplant patients in Europe and the USA (- 1%) and in India (-10%), and
patients with
4
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
AIDS, multiple myeloma and severe combined immunodeficiency (-4%). Over
500,000
transplants are performed annually in the world. Acute leukemia affects about
3/100,000 of
the population and on average each patient receives 3 cycles of chemotherapy,
with each
cycle defining a major risk period. Similar incidence is observed for high
grade lymphoma
patients who are also at high risk of invasive aspergillosis. In the
industrialized nations alone
these treatment protocols generate about 250,000 periods of major risk per
year. AIDS cases
are predicted to exceed 40 million by the end of the year 2000 which would
result in about
1.4 million cases of invasive aspergillosis, although in developing countries
most patients
will not live long enough to get this disease.
The crude mortality from invasive aspergillosis is around 85% and falls to
around
50% if treated. The new drugs in trial (voriconazole, etc.) may reduce the
mortality slightly
(-r10%), but patients in trials tend to do better than those treated in
clinical practice. In
addition to invasive disease, Aspergillus causes a number of other diseases in
man. These
include aspergilloma ("colonization" of existing pulmonary cavities),
sinusitis in normal
people, allergic bronchopulmonary and sinus infections, keratitis (which
usually leads to
blindness in that eye and is common in the developing world) and postoperative
infections in
immunocompetent patients. Aspergilloma numbers are set to rise dramatically
due to the
increasing incidence of tuberculosis and such aspergilloma cases are
notoriously difficult to
treat. Cavities of 2 cm or larger after tuberculosis subsequently develop
aspergillomas in 15-
20% of patients (in the UK). The 5 year survival of patients with
aspergillomas is about
40%. Allergic bronchopulmonary aspergillosis occurs in patients with cystic
fibrosis and
asthmatics (an increasing number) causing pulmonary fibrosis and death usually
within 10
years of diagnosis.
Aspergillus flavus is the etiologic agent in a wide range of infections
including
mycotoxicoses owing to aflotoxins, hypersensitivity pneumonitis, otitis,
sinusitis, and
invasive disease. Some reports suggest the disease process may be potentiated
by aflotoxins,
particularly in the immunocompromised/neutropenic host. The organism is
extremely
angioinvasive with resultant necrosis and infarction.
C. Prior Art Drug Delivery Techniques
Ease of active pharmaceutical agent delivery is a key issue facing
pharmaceutical
companies that develop and commercialize therapeutic products. An active agent
that is
readily soluble in water, for example, is not difficult to formulate into a
suitable dosage form.
5
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
However, formulating poorly water-soluble active agent into suitable dosage
forms poses a
significant challenge. This is because the human body is a water based system;
thus, as a
condition of producing therapeutic activity, a drug must dissolve following
administration.
Some poorly water-soluble active agents are never commercialized because they
cannot be effectively solubilized, and therefore fail to exhibit acceptable in
vivo therapeutic
activity. Alternatively, the quantity of poorly water-soluble active agent
required to be
administered to achieve an acceptable level of therapeutic activity may be too
great, given the
poor water solubility of the agent, and result in unacceptable toxicity. Even
if an active agent
is formulated into a liquid, wherein the active agent is solubilized in a
solvent, such dosage
forms sometimes perform sub-optimally. For example, such dosage forms may have
unpredictable properties or induce undesirable side effects.
Prior art methods exist for enhancing active agent solubility. For example,
the
particle size of the active agent can be reduced, thereby increasing the
exposed surface area
of the active agent, resulting in increased dissolution rate and greater water
solubility. One
prior method for particle size reduction is wet milling. This method requires
grinding of an
active agent with beads made of hard glass, ceramic, porcelain, zirconium
oxide, zirconium
silicate, polymeric resin, or other suitable substance in a media in which the
active agent is
poorly soluble, such as water. The active agent is physically converted into
much smaller
particles that remain suspended in the grinding media. The resultant micron-
or nanometer-
sized active agent particles can then be isolated from the grinding media by
methods such as
filtration or centrifugation, and formulated into an appropriate dosage form.
See U.S. Patent
Nos. 5,145,684; 5,518,187; 5,862,999; and 5,718,388. The media in which the
active agent is
ground typically contains one or more compounds that function as a surface
stabilizer for the
active agent. The surface stabilizers adsorb to the surface of the active
agent and act as a
steric barrier to active agent particle size growth.
Conventional wet milling techniques therefore produce a "bi-phasic" system in
which
the stabilized active agent nanoparticles are suspended in the aqueous media.
The
nanoparticulate drug delivery technology commercialized by Elan Drug Delivery
(King of
Prussia, PA) under the trade name NanoCrystal technology, and SkyePharma,
plc's
Insoluble Drug Delivery (IDD~) technology exemplify such wet milling
techniques.
However, wet milling of active agent has drawbacks, principally being the cost
of the
process. The added cost for formulating a poorly water-soluble active agent
into a
6
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
nanoparticulate composition utilizing wet milling can be prohibitive.
Additionally,
amorphous compounds are not amenable to wet milling techniques.
Other known methods of making nanoparticulate active agent compositions
include
precipitation, homogenization, and super critical fluid methods.
Microprecipitation is a
method of preparing stable dispersions of poorly soluble active agents. Such a
method
comprises dissolving an active agent in a solvent followed by precipitating
the active agent
out of solution. Homogenization is a technique that does not use milling or
grinding media.
Active agent in a liquid media constitutes a process stream propelled into a
process zone,
which in a Microfluidizer (Microfluidics, Inc.) is called the Interaction
Chamber. The
geometry of the interaction chamber produces powerful forces of sheer,
impaction, and
cavitation which are responsible for particle size reduction. U.S. Patent No.
5,510,118 refers
to a bi-phasic process using a Microfluidizer resulting in nanoparticulate
active agent
particles. Finally, supercritical fluid methods of making nanoparticulate
active agent
compositions comprise dissolving an active agent in a solution. The solution
and a
supercritical fluid are then co-introduced into a particle formation vessel.
The temperature
and pressure are controlled, such that dispersion and extraction of the
vehicle occur
substantially simultaneously by the action of the supercritical fluid.
Examples of known
supercritical methods of making nanoparticles include International Patent
Application No.
WO 97/144407 and U.S. Patent No. 6,406,718.
There is a need in the art for drug delivery dosage forms having inherent
antibacterial,
anti-yeast, anti-fungal, and/or anti-viral properties. In addition, there is a
need for
compositions having such inherent antibacterial, anti-yeast, anti-fungal,
and/or anti-viral
properties, and which can be used in the absence of an active agent. The
present invention
satisfies these needs.
SUMMARY
The invention is directed to nanostructured compositions having antimicrobial,
anti-
fungal, anti-yeast, and/or anti-viral properties. The compositions comprise at
least one oil, at
least one surfactant, at least one solvent, and water. Additionally, the
compositions can
comprise an active agent. The active agent can be useful, for example, as a
pharmaceutical,
diagnostic, or cosmetic.
7
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Another aspect of the invention is directed to pharmaceutical compositions
comprising a nanostructured composition according to the invention, as well as
one or more
desired pharmaceutically acceptable carriers and/or desired excipients which
may include
viscosity modifiers, colors, flavoring agents, fragrances, etc.
. One aspect of the invention is directed to a unique active pharmaceutical
ingredient
nano-structured formulation, which comprises (1) a micelle component, (2) a
hydro-alcoholic
component, i.e., a mixture of water and water-miscible solvent, (3) an oil-in-
water emulsion
droplet component, and optionally (4) a solid particle component of an active
agent. Any or
all of these components may comprise a desired active pharmaceutical,
diagnostic, cosmetic,
or other active ingredient. Thus the active agent may be in solution, as
denoted in
components 1 to 3, or it may be in precipitated suspension form, as is the
case in component
4.
In one embodiment, when the composition is applied to the skin, the
solubilized form
travels across the skin and into.deeper dermal layers, such as into the
dermis. The other
components, such as the micelles, oil fraction, and/or the particulate drug
may typically
position themselves towards the Stratum corneum of the skin layer. Depending
on various
physical and chemical properties, certain compounds may position themselves in
different
layers of epidermis and dermis, while others might permeate directly across
the skin.
This composite formulation avoids having to incorporate chemical permeation
enhancers that are otherwise necessary to induce transdermal permeation of the
active
pharmaceutical ingredient.
Another aspect of the invention is directed to a method for preparing the
nanostructured compositions of the invention comprising: (a) combining a
mixture of at least
one oil, at least one solvent, and at least one surfactant (also referred to
as a surface stabilizer)
to form an emulsion base, (b) adding water to the emulsion base, and (c)
homogenizing or
vigorously stirring the mixture. If an active agent is to be utilized in the
composition, an
exemplary method of making the composition comprises: (a) adding the active
agent to a
mixture of oil, solvent, and surfactant or stabilizer to form an emulsion
base, wherein the
active agent is soluble in either or both of oil and solvent, but is not
soluble in water, (b)
adding water to the emulsion base, and (c) homogenizing or vigorously stirring
the mixture.
Finally, methods of using the compositions of the invention to treat subjects
in need,
or as a broad spectrum antimicrobial composition for topical or surface
disinfection, are also
encompassed by the invention.
8
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Both the foregoing general description and the following brief description of
the
drawings and detailed description are exemplary and explanatory and are
intended to provide
further explanation of the invention as claimed. Other objects, advantages,
and novel features
will be readily apparent to those skilled in the art from the following
detailed description of
the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
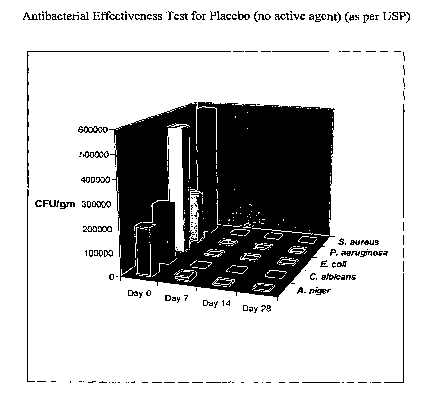
Figure 1 shows the results of antibacterial effectiveness testing for a
placebo composition
(i.e., lacking an active agent) of the invention conducted according to the
protocol specified
in USP 25, <51>, pp. 1869-1871 (testing with E. coli (ATCC 8739), P.
aeruginosa (ATCC
9027), S. aureus (ATCC 6538), C. albicans (ATCC 10231), and A. niger (ATCC
16404)).
Figure 2 shows the results of antibacterial effectiveness testing for a
placebo composition
(i.e., lacking an active agent) of the invention conducted according the
protocol specified in
USP 25, <51>, pp. 1869-1871, but with additional microorganisms (P. aeruginosa
ATCC
13388), P. aeruginosa (ATCC 25619) A. flavus, and A. fumigatus).
Figure 3 shows the results of antibacterial effectiveness testing for
estradiol compositions of
the invention conducted according to the protocol specified in USP 25, <51>,
pp. 1869-1871
(testing with E. coli (ATCC 8739), P. aeruginosa (ATCC 9027), S. aureus (ATCC
6538), C.
albicans (ATCC 10231), andA. niger (ATCC 16404)).
Figure 4 shows the results of antibacterial effectiveness testing for
testosterone compositions
of the invention conducted according to the protocol specified in USP 25,
<51>, pp. 1869-
1871 (testing with E. coli (ATCC 8739), P. aeruginosa (ATCC 9027), S. aureus
(ATCC
6538), C. albzcans (ATCC 10231), andA. niger (ATCC 16404)).
Figure 5 shows the estradiol particle size in a nanostructured composition
following
homogenization.
Figure 6 shows the estradiol particle size in a nanostructured composition
following the
Silverson Method.
9
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Figure 7 shows the testosterone particle size in a nanostructured composition
following
homogenization.
DETAILED DESCRIPTION
A. Overview of the Invention
The invention is directed to nanostructured compositions that surprisingly
have
antimicrobial, anti-fungal, anti-yeast, and/or anti-viral properties. The
nanostructured
compositions of the invention comprise at least one solvent, at least one oil,
at least one
surface stabilizer (also referred to as a surfactant), and aqueous medium. The
compositions
additionally may comprise oine or more active agents, which may be dissolved
or dispersed in
any one of the oil, solvent, or water. The active agent can be useful, for
example, as a
pharmaceutical or cosmetic. No external antibacterial agent or preservative is
required to be
added to the compositions of the invention to impart the antimicrobial, anti-
yeast, anti-fungal,
and/or anti-viral properties.
The compositions of the invention meet the Antimicrobial Effectiveness Testing
criteria as described in the United States Pharmacopoeia (USP - General
Chapters, Section
51). This is demonstrated by the testing described in Example 4 below, in
which various
microbes were cultured in the presence of a composition according to the
invention (testing
with E. coli (ATCC 8739), P. aeruginosa (ATCC 9027), S. aureus (ATCC 6538), C.
albicans
(ATCC 10231), A. niger (ATCC 16404), P. aeruginosa (ATCC 13388), P. aeruginosa
(ATCC 25619) A. flavus, and A. fumigatus). The results of the testing are also
shown in
Figure 1, which graphically shows the dramatic antimicrobial properties of the
compositions
of the invention.
The standard USP testing requires evaluation in five microorganisms:
Aspergillus
niger (ATCC 16404), Candida albicans (ATCC 10231), Escherichia coli (ATCC
8739),
Pseudomonas aeruginosa (ATCC 9027) and Staphylococcus aureus (ATCC 6538).
Additional strains were used to confirm the anti-microbial effectiveness in A.
flavus, A.
fumigatus, P aeruginosa (ATCC 25619) and P. aeruginosa (ATCC 13388). As shown
in
Figure 2, which graphically shows the results of this testing, antimicrobial
effectiveness in
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
these additional strains confirm beyond doubt that the composition vehicle
itself is
antimicrobial.
Moreover, the incorporation of an active agent does not compromise the
antimicrobial
effectiveness of the compositions of the invention. Specifically, as shown in
Examples 3 and
4, compositions of the invention comprising estradiol and testosterone also
exhibited
significant antimicrobial effects, and satisfied the USP testing requirements
for demonstrating
antimicrobial activity. See also Figures 3 and 4.
The compositions of the invention are particularly useful in products used for
topical
treatment of infections, wound healing, etc., as in addition to the
pharmacologic properties of
the active agent in such compositions, the vehicle itself acts as a
microbicidal agent. Such a
composition possibly induces synergistic action and reduces the possibility of
development of
drug resistance microorganisms. Moreover, such a composition may enable the
use of lower
doses of the active agent.
At present, various types of cosmetic and pharmaceutical compositions require
the
addition of an antimicrobial agent to retard microbial growth. Choosing the
right
antimicrobial agent can be challenging due to potential interactions between
the active agent
and the antimicrobial agent. Moreover, the antimicrobial agent can be toxic
and it can induce
adverse reactions in patients.
In one embodiment of the invention, the compositions comprise an active agent,
and
the active agent is selected from the group consisting of, but not limited to
fenofibrate,
estradiol, alendronic acid, acyclovir, paclitaxel, and cyclosporine.
In another embodiment, the oil is selected from the group consisting of, but
not
limited to, almond oil (sweet), apricot seed oil, borage oil, canola oil,
coconut oil, corn oil,
cotton seed oil, fish oil, jojoba bean oil, lard oil, linseed oil (boiled),
Macadamia nut oil,
medium chain triglycerides, mineral oil, olive oil, peanut oil, safflower oil,
sesame oil,
soybean oil, squalene, sunflower seed oil, tricaprylin (1,2,3-trioctanoyl
glycerol), and wheat
germ oil.
In one embodiment, the solvent is selected from the group consisting of, but
not
limited to isopropyl myristate, triacetin, N-methyl pyrrolidinone, aliphatic
and aromatic
alcohols, polyethylene glycols, and propylene glycol. Other examples of useful
solvents are
long-chain alcohols. Ethyl alcohol and benzyl alcohol are yet other examples
of alcohols that
may be used in the present invention.
11
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
In yet another embodiment, the stabilizer or surfactant is selected from the
group
consisting of, but not limited to, sorbitan esters, glycerol esters,
polyethylene glycol esters,
block polymers, acrylic polymers (such as Pernuleno'), ethoxylated fatty
esters (such as
Cremophoro RH-40), ethoxylated alcohols (such as Brij ), ethoxylated fatty
acids (such as
Tween 20), monoglycerides, silicon based surfactants,.and polysorbates. In a
further
embodiment, the sorbitan ester stabilizer is Span or Arlacel ; the glycerol
ester is glycerin
monostearate; the polyethylene glycol ester is polyethylene glycol stearate;
the block polymer
is a Pluronic ; the acrylic polymer is Pemulen ; the ethoxylated fatty ester
is Cremophor
RH-40; the ethoxylated alcohol is Brij ; the ethoxylated fatty acid is Tween
20, or a
combination thereof.
In another embodiment, a homogenizing step is performed via a high-pressure
system
at 1,000 to 40,000 psi.
In one embodiment, the active agent particles, droplets comprising active
agent, or a
combination thereof have a mean particle size of less than about 10 microns.
In other
embodiments of the invention, the active agent particles, droplets comprising
active agent, or
a combination thereof have a mean particle size of less than about 9 microns,
less than about
8 microns, less than about 7 microns, less than about 6 microns, less than
about 5 microns,
less than about 4 microns, less than about 3 microns, less than about 2900 nm,
less than about
2800 nm, less than about 2700 nm, less than about 2600 nm, less than about
2500 nm, less
than about 2400 nm, less than about 2300 nm, less than about 2200 nm, less
than about 2100
rim, less than about 2000 nm, less than about 1900 nm, less than about 1800
nm, less than
about 1700 nm, less than about 1600 nm, less than about 1500 nm, less than
about 1400 rim,
less than about 1300 nm, less than about 1200 rim, less than about 1100 nm,
less than about
1000 nm, less than about 900 nm, less than about 800 nm, less than about 700
nm, less than
about 600 nm, less than about 500 nm, less than about 400 nm, less than about
300 nm, less
than about 200 nm, or less than about 100 nm, less than about 90 nm, less than
about 80 nm,
less than about 70 nm, less than about 60 nm, less than about 50 nm, less than
about 40 nm,
less than about 30 nm, less than about 20 nm, or less than about 10 nm. In one
embodiment,
the active agent particles, droplets comprising active agent, or a combination
thereof have a
mean particle size of less than about 3 microns in diameter.
Three methods of making the compositions of the invention are described. The
first
method does not require the presence of an active agent. In this method, at
least one oil, at
12
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
least one solvent, and at least one surfactant are combined to form an
emulsion base, water is
added to the emulsion base, and (c) the mixture is homogenized or vigorously
stirred.
If an active agent is to be utilized in the composition, an exemplary method
of making
the composition cornprises: (a) adding the active agent to a mixture of oil,
solvent, and
surfactant or stabilizer to form an emulsion base, wherein the active agent is
soluble in either
or both of oil and solvent, but is not soluble in water, (b) adding water to
the emulsion base,
and (c) homogenizing or vigorously stirring the mixture.
Two specific methods for making active agent compositions of the invention are
described. In the first method ("Route I"), active agent is milled (i.e.,
homogenized or
vigorously stirred to reduce the particle size of the active agent) in an
emulsion base. This
method requires that the active agent is poorly soluble or insoluble in all
phases of the oil
phase/lipophilic phase and the water or buffer. In the second method ("Route
Il"),
simultaneous milling (i.e., homogenizing or vigorously stirring to reduce the
particle size of
the active agent) and precipitation of the active agent in an emulsion base is
observed. The
second method requires that the active agent is soluble or partially soluble
in one or more
phases of the emulsion base; i.e., that the active agent is soluble in an oil,
solvent, or water or
buffer.
One benefit of the methods of making the compositions of the invention as
compared
to prior art methods, such as wet milling, is that the methods are applicable
to water-soluble
active agent as well as poorly water soluble active agent. Another benefit of
the methods of
the invention is that they do not require grinding media or specialized
grinding process or
equipments. The use of such grinding media can add cost and complexity to a
particle size
reduction process for an active agent. Yet another benefit of the methods of
the invention is
that it can be used to process amorphous agents.
For Route I, an active agent is first suspended in a mixture of a non-miscible
liquid,
such as an oil, solvent, and water or buffer to form an emulsion base,
followed by
homogenization or vigorous stirring of the emulsion base. Nanoparticles can be
produced
with reciprocating syringe instrumentation, continuous flow instrumentation,
or high speed
mixing equipment. High velocity homogenization or vigorous stirring, producing
forces of
high shear and cavitation, are preferred. High shear processes are preferred
as low shear
processes can result in larger active agent particle sizes. The resultant
composition is a
composite mixture of active agent suspended in the emulsion droplet (nano-
emulsion
fraction) and sterically stabilized microcrystalline and/or nanocrystalline
active agent in the
13
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
media. This tri-phasic system comprises particulate drug, oil, and water or
buffer. The
resultant micro/nano-particulate active agent has an effective average
particle size of less than
about 3 microns. Smaller particulate active agent can also be obtained, as
described below.
The active agent can be precipitated out from the oil droplets by adding more
of the
non-miscible liquid. The precipitated active agent typically has an effective
average particle
size of less than about 3 microns. If desired, the active agent particles can
be prevented from
aggregating or clumping together by incorporating a surfactant or emulsifier,
i.e., a "surface
stabilizer."
Route II is utilized for an active agent that is soluble in at least one part
of the
emulsion base, such as the solvent. For Route II, an active agent is dissolved
in a mixture of
oil, solvent, and stabilizer to form an emulsion pre-mix. The active agent
remains in soluble
form if water or buffer is not added to the mixture. Upon the addition of
water or buffer and
the application of shear forces, the active agent is precipitated into
micro/nanoparticles
having an effective average particle size of less than about 3 microns.
Nanoparticles can be
produced with reciprocating syringe instrumentation, continuous flow
instrumentation, or
high speed mixing equipment. High energy input, through high velocity
homogenization or
vigorous stirring, is a preferred process. The high energy processes reduce
the size of the
emulsion droplets, thereby exposing a large surface area to the surrounding
aqueous
environment. High shear processes are preferred, as low shear processes can
result in larger
particle sizes. This is followed by precipitation of nanoparticulate active
agent previously
embedded in the emulsion base. The end product comprises active agent in
solution and
particulate suspension, both distributed between the solvent, oil, and water
or buffer. In one
embodiment, nanoparticulate active agent has at least one surface stabilizer
associated with
the surface thereof.
Examples of active agent that are poorly water soluble in water but soluble in
another
liquid include estradiol, which is soluble in ethanol, and fenofibrate, which
is freely soluble
in 1-methyl-2-pyrrolidone or N-methyl-pyrrolidinone [NMP], slightly soluble in
oil and
stabilizer, and insoluble in water.
If desired, the water miscible oil droplets and active agent nanoparticles
prepared
using Route I or Route II can be filtered through either a 0.2 or 0.45 micron
filter. Larger oil
droplets and/or active agent particles can be created by simply increasing the
water content,
decreasing the oil-stabilizer-solvent content, or changing the shear in
forming the oil droplets.
14
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
For the emulsion base used as an antimicrobial composition, or used in Route I
or
Route II as a drug delivery vehicle, the preferred ratio of
oil:stabilizer:solvent is about 23:
about 5: about 4, respectively, on a weight-to-weight basis. The preferred
ratio of the oil
comprising phase to water or buffer is about 2: about 1, respectively. In
other embodiments
of the invention, the oil may be present at about 5% to about 50% (w/w); the
solvent may be
present at about 0.5% to about 10% (w/w); the stabilizer or surfactant may be
present at about
0.5% to about 10% (w/w), the water may be present at about 20% to about 80 %
(w/w), or
any combination thereof.
B. Compositions of the Invention
The methods of the invention can produce several different types of
compositions. A
first composition comprises: (1) at least one oil; (2) at least one solvent;
(3) at least one
surfactant or surface stabilizer; and (4) water. This composition exhibits
broad spectrum
antimicrobial activity and, therefore, the composition can be used as a
general purpose
disinfectant.
Other types of compositions according to the invention comprise at least one
active
agent. For example, a second composition can comprise: (1) nanoparticulate
active agent
having associated with the surface thereof at least one surface stabilizer;
(2) water or a buffer;
(3) an emulsion pre-mix or oil phase or lipophilic phase comprising at least
one oil and
optionally at least one solvent; and optionally (4) microparticulate active
agent. The
microparticulate active agent can be the same as or different from the
nanoparticulate active
agent. The particulate active agent can be present in the water or buffer,
oil, solvent, or a
combination thereof. Such a composition is made utilizing Route I.
A third composition comprises: (1) nanoparticulate active agent having
associated
with the surface thereof at least one surface stabilizer; (2) water or buffer;
and (3) an
emulsion pre-mix or oil phase or lipophilic phase comprising at least one oil,
optionally at
least one solvent, and solubilized active agent. The composition may
additionally comprise
microparticulate active agent. The solubilized active agent can be present in
the water or
buffer, oil, solvent, or a combination thereof. In addition, nanoparticulate
active agent can be
present in the water or buffer, oil, solvent, or a combination thereof. Such a
composition is
made utilizing Route II. In a further embodiment of the invention, the
solubilized active
agent can be precipitated out from the emulsion droplets. The precipitated
active agent
preferably has an effective average particle size of less than about 3
microns.
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The tri-phasic compositions of the invention are beneficial for several
reasons. First,
formulations resulting from the Route II method comprise both solid and
solubilized forms of
the same active agent. This enables a resultant pharmaceutical formulation to
provide both
immediate release and controlled release of the component active agent,
providing for fast
onset of activity combined with prolonged activity of the active agent.
Moreover, when formulated for topical application to the skin, in a cream or
lotion for
example, the solid active agent nanoparticles may provide an immediate local
therapeutic
effect at the skin surface, while the solubilized active agent within the
emulsion base crosses
the skin/cell barrier allowing the active agent to enter the body's system.
That is, the
solubilized active agent crosses the skin rapidly and penetrates into deeper
layers, whereas
the solid part does not permeate into deeper skin layers, but acts as local
depot and as a
reservoir for supplying drug into deeper layers. Hence, a formulation
comprising both active
agent nanoparticles and solubilized active agent can provide local and
systemic therapeutic
effects, which are particularly beneficial for transdermal dosage forms.
The different components of the two types of compositions described above can
be
separated and, if desired, used independently.
This invention permits many different types of active agents to be formulated
into
emulsion-based formulations. Examples of such active agents include, but are
not limited to,
acyclovir, cyclosporine, estradiol, fenofibrate, ceterizine, nicotine,
naltrexone, and alendronic
acid.
1. Active Agent Nanoparticles
The solid active agent nanoparticles can be separated from the aqueous
suspension
media and/or the emulsion globules, for instance, by filtration or
centrifugation. This
provides a convenient method of obtaining nanoparticles of a poorly water-
soluble or water-
insoluble active agent. Furthermore, when a surface stabilizer is included in
the particle size
reduction process, it prevents the active agent nanoparticles from aggregating
and, therefore,
the active agent nanoparticles are stabilized at a nanoparticulate size. If
desired, the active
agent nanoparticles can then be formulated into any suitable dosage form.
Active agent
nanoparticles can be made using food grade, USP or NF grade materials suitable
for human
use applications.
Exemplary dosage forms include, but are not limited to, liquid dispersions,
oral
suspensions, tablets, gels, aerosols, ointments, creams, capsules, dry
powders,
16
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
multiparticulates, sprinkles, sachets, lozenges, and syrups. Moreover, the
dosage forms of the
invention may be solid dosage forms, liquid dosage forms, semi-liquid dosage
forms,
immediate release formulations, modified release formulations, controlled
release
formulations, fast melt formulations, lyophilized formulations, delayed
release formulations,
extended release formulations, pulsatile release formulations, mixed immediate
release and
controlled release formulations, or any combination thereof. The compositions
of the
invention may be formulated for delivery via any suitable method, such as for
parenteral
injection (i.e., intravenous, intramuscular, or subcutaneous), oral
administration in solid,
liquid, or aerosol form, vaginal, nasal, rectal, ocular, otic, local (powders,
ointments or
drops), buccal, intracisternal, intraperitoneal, or topical administration,
and the like.
Since the active agent nanoparticles have an effective average particle size
of less
than about 3 microns, the particles typically are more readily able to move
across absorption
barriers, such as skin, as compared to microcrystalline active agent.
Similarly, the small
active agent particle size enables passage through blood/tissue and
blood/tumor barriers of
various organs.
17
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
2. Emulsion Globules Comprising Active Agent
Nanoparticles and/or Solubilized Active Agent
The emulsion globules comprising solubilized active agent, active agent
nanoparticles, or a combination thereof can also be isolated, if desired, from
the surrounding
aqueous or buffer phase and used in therapeutic dosage fonns. The emulsion
globules can be
made using food grade, USP or NF grade materials suitable for human use
applications.
Nanoparticulate oil globules comprising solubilized active agent, and methods
of making the
same, are described in U.S. Patent No. 5,629,021 ("the `021 patent"), which is
incorporated
herein by reference. The emulsion globules of the invention typically
comprise: (1) at least
one oil; (2) at least one solvent; (3) at least one surface stabilizer or
surfactant; and optionally
(4) solubilized active agent, particulate active agent, or a combination
thereof. Emulsion
globules comprising solubilized active agent, particulate active agent, or a
combination
thereof can be isolated, if desired, for example, by filtration. Emulsion
globules comprising
solubilized active agent are particularly suitable vehicles for transporting
active agent across
the skin barrier and into the blood. Hence, globules comprising solubilized
active agent offer
a systemic way to administer active agent to an individual.
In general, the emulsion globules comprising solubilized active agent, active
agent
nanoparticles, or a combination thereof have diameters of about 10 to about
1000 nm, and
comprise a significant quantity of active agent, with a mean a diameter of
less than about 1
micron preferred, and with the smallest globules filterable through a 0.2
micron filter, such as
is typically used for microbiological purification. The range of active agent
concentration in
the globules can be from about 1% to about 50%. The emulsion globules can be
stored at
between about 20 to about 40 C. In one embodiment of the invention, at least
about 50%, at
least about 60%, at least about 70%, at least about 80%, or at least about 90%
of the globules
in the preparation have diameters of less than about 1 micron, less than about
900 nm, less
than about 800 nm, less than about 700 nm, less than about 600 nm, less than
about 500 nrn,
less than about 400 nm, less than about 300 nm, less than about 200 nm, or
less than about
100 nrn.
By varying different parameters of Route I and Route II, the size and
integrity of such
globules can be modified. Hence, the stability of globules comprising
dissolved active agent
can be altered to enable the release of active agent, either as a solution or
precipitate. This is
a microreservoir-dissolution-controlled system, where the drug solids acts as
depot and, as
the solubilized fraction is depleted, more drug is drawn into solution form
the particulate
18
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
depot. Thus, the emulsion globules comprising solubilized active agent enable
controlled
active agent release over time.
The small size of the emulsion globules comprising solubilized active agent,
active
agent nanoparticles, or a combination thereof and their compatibility with
tissue render them
applicable to numerous uses. For example, the emulsion globules are useful as
topical drug
delivery vehicles as they enable rapid dermal penetration. The globules are
also exceptionally
versatile in that the active agent utilized can be any active agent that is
suspendable or
dissolvable in any of the water or buffer, oil, or solvent. These properties
allow this system
to be used with active agents that are difficult to formulate for use in other
delivery systems.
In addition, the emulsion globules comprising solubilized active agent, active
agent
nanoparticles, or a combination thereof can be diluted with aqueous solutions
without
stability loss. This enables the use of high active agent concentration, i.e.
up to about 30%,
products which can be diluted for use as necessary. The concentration of
active agent,
however, depends on the solubility of the actual drug and the amount of
solvent used to
dissolve it.
In one embodiment of the invention, the emulsion globules comprise as an
active
agent estradiol, acyclovir, or testosterone and are formulated into a dosage
form for
transdermal delivery.
C. Components of the Methods and Compositions of the Invention
1. Active Pharmaceutical Ingredient
a. Properties
Any suitable active agent may be employed in the compositions and methods of
the
invention. For an active agent to be utilized in the Route I method, the
active agent must be
poorly soluble, or insoluble, in all phases of the milling (i.e.,
homogenization or vigorously
stirring) system, including water and the solvent and oil to be used in the
method. For an
active agent to be utilized in Route II, the active agent must be poorly water
soluble, or water
insoluble, but soluble in at least one phase of the emulsion base, such as the
oil or solvent and
stabilizer or stabilizer solution.
By "poorly water-soluble" or "water insoluble" it is meant that the active
agent has a
solubility in water of less than about than about 20 mg/mL, less than about 10
mg/mL, less
than about 1 mg/mL, less than about 0.1 mg/mL, less than about 0.01 mg/mL, or
less than
about 0.001 mg/mL at ambient temperature and pressure and at about pH 7.
19
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The active agent to be used in the methods of the invention, and present in
the
compositions of the invention, can be amorphous, semi-amorphous, crystalline,
semi-
crystalline, or a mixture thereof.
b. Active Agent Particle Size
As used herein, active agent particle size is determined on the basis of the
weight
average particle size as measured by conventional techniques well known to
those skilled in
the art, such as sedimentation field flow fractionation, photon correlation
spectroscopy, laser
diffraction, or disk centrifugation.
As used herein, "nanoparticulate active agent" refers to active agent having
an
effective average particle size of less than about 3 microns.
"microcrystalline active agent"
refers to active agent having an effective average particle size of greater
than about 3
microns.
As used herein, an "effective average particle size" for an active agent is
the size
below which about 50% of the active agent particles fall. Thus, if the
effective average
particle size is less than about 3 microns, at least 50% of the active agent
particles in the
composition have a size of less than about 3 microns. In other embodiments of
the invention,
the effective average particle size of the active agent particles in the
compositions of the
invention can be less than about 2900 nm, less than about 2800 nm, less than
about 2700 nm,
less than about 2600 nm, less than about 2500 nm, less than about 2400 mn,
less than about
2300 nm, less than about 2200 mn, less than about 2100 nm, less than about
2000 nm, less
than about 1900 nm, less than about 1800 mu, less than about 1700 nm, less
than about 1600
nm, less than about 1500 nm, less than about 1400 mn, less than about 1300 mn,
less than
about 1200 nm, less than about 1100 nm, less than about 1000 mn, less than
about 900 nm,
less than about 800 nm, less than about 700 nm, less than about 600 nm, less
than about 500
nm, less than about 400 nm, less than about 300 nm, less than about 200 nm, or
less than
about 100 nm.
In other embodiments of the invention, at least about 60%, at least about 70%,
at least
about 80%, at least about 90%, at least about 95%, or at least about 99% of
the active agent
particles have a size less than the effective average, i.e., less than about 3
microns, less than
about 2900 nm, less than about 2800 mn, etc.
c. Exemplary Active Agents
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Any suitable active agent may be used in the methods and compositions of the
invention. Examples of classes of useful active agent include, but are not
limited to,
therapeutic and diagnostic agents, pigments, paints, inks, dyes, photographic
materials,
cosmetic ingredients, etc.
Amphiphile-type active agents may be incorporated into the present
formulations.
That is, drugs or therapeutic compounds that can be ionized and are soluble in
polar or non-
polar solvents may be incorporated in the formulations of the present
invention. Such
compounds are soluble, therefore, both in oil and aqueous environments
(amphiphiles).
Examples of such compounds include nicotine and ceterizine_
Hydrophilic active agents also may be incorporated into a formulation of the
present
invention. Such compounds include, but are not limited to naltrexone
hydrochloride,
alendronic acid, and ceterizine dihydrochloride.
The active agent may be a hormone, such as testosterone, progesterone, and
estrogen.
Other hormones include: (1) Amine-derived hormones, such as catecholamines,
adrenaline
(or epinephrine), dopamine, noradrenaline (or norepinephrine), tryptophan
derivatives,
melatonin (N-acetyl-5-methoxytryptamine), serotonin (5-HT), tyrosine
derivatives, thyroxine
(T4), triiodothyronine (T3); (2) peptide hormones, such as antimullerian
hormone (AMH,
also mullerian inhibiting factor or hormone), adiponectin (also Acrp30),
adrenocorticotropic
hormone (ACTH, also corticotropin), angiotensinogen and angiotensin,
antidiuretic hormone
(ADH, also vasopressin, arginine vasopressin, AVP), atrial-natriuretic peptide
(ANP, also
atriopeptin), Calcitonin, cholecystokinin (CCK), corticotropin-releasing
hormone (CRH),
erythropoietin (EPO), follicle-stimulating hormone (FSH), gastrin, glucagons,
gonadotropin-
releasing hormone (GnRH), growth hormone-releasing hormone (GHRH), human
chorionic
gonadotropin (hCG), growth hormone (GH or hGH), insulin, insulin-like growth
factor (IGF,
also somatomedin), leptin, luteinizing hormone (LH), melanocyte stimulating
hormone (MSH
or a-MSH), neuropeptide Y, oxytocin, parathyroid hormone (PTH), prolactin
(PRL), relaxin,
rennin, secretin, somatostatin, thrombopoietin, thyroid-stimulating hormone
(TSH),
thyrotropin-releasing hormone (TRH); (3) steroid hormones, such as
glucocorticoids, cortisol,
mineralocorticoids, aldosterone, sex steroids, androgens, testosterone,
dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS),
androstenedione, dihydrotestosterone (DHT), Estrogens, estradiol,
Progestagens,
progesterone, Progestins, (4) sterol hormones, such as vitamin D derivatives
and calcitriol,
21
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
(5) lipid and phospholipid hormones (eicosanoids) such as prostaglandins,
leukotrienes,
prostacyclin, and thromboxane.
In one embodiment of the invention, therefore, the active agent is estradiol,
fenofibrate, acyclovir, alendronic acid, or testosterone. Specific examples of
active agents
that may be utilized in the methods of the invention include, but are not
limited to, insulin,
calcitonin, calcitonin gene regulating protein, atrial natriuretic protein,
betaserori,
erythropoietin, alpha interferon, beta interferon, gamma interferon,
somatropin, somatotropin,
somastostatin, insulin-like growth factor, luteinizing hormone releasing
hormone, factor VIII,
interleukins, interleukin analogues, hematological agents, anticoagulants,
hematopoietic
agents, hemostatics, thrombolytic agents, endocrine agents, antidiabetic
agents, antithyroid
agents, beta-adrenoceptor blocking agents, growth hormones, growth hormone
releasing
hormone, sex hormones, thyroid agents, parathyroid calcitonin,
bisphosphonates, uterine-
active agents, cardiovascular agents, antiarrhythmic agents, anti-anginal
agents, anti-
hypertensive agents, vasodilators, agents used in treatment of heart
disorders, cardiac
inotropic agents, renal agents, genitourinary agents, antidiuretic agents,
respiratory agents,
antihistamines, cough suppressants, parasympathomimetics, sympathomimetics,
xanthines,
central nervous system agents, analgesics, anesthetics, anti-emetic agents,
anorexiants,
antidepressants, anti-migraine agents, antiepileptics, dopaminergics,
anticholinergics,
antiparkinsonian agents, muscle relaxants, narcotic antagonists, sedatives,
stimulants,
treatments for attention deficit disorder, methylphenidate, fluoxamine,
bisolperol, tacrolimus,
sacrolimus, cyclosporine, gastrointestinal agents, systemic anti-infectives,
agents used in the
treatment of AIDS, anthelmintics, antimycobacterial agents, immunologic
agents, vaccines,
hormones; dermatological agents including, anti-inflammatory agents, elastase
inhibitors,
antimuscarinic agents, lipid regulating agents, blood products, blood
substitutes,
antineoplastic agents including, leuprolide acetate, chemotherapy agents,
oncology therapies,
nutrients, nutritional agents, chelating agents, interleukin-2, IL-1 ra,
heparin, hirudin, colony
stimulating factors, tissue plasminogen activator, oxytocin, nitroglycerine,
diltiazem,
clonidine, nifedipine, verapamil, isosorbide-5-mononitrate, organic nitrates,
diuretics,
desmopressin, vasopressin, expectorants, mucolytics, fentanyl, sufentanil,
butorphanol,
buprenorphine, levorphanol, morphine, hydromorphone, hydrocodone, oxymorphone,
methadone, lidocaine, bupivacaine, diclofenac, naproxen, paverin, scopolamine,
ondansetron,
domperidone, metoclopramide, sumatriptan, ergot alkaloids, benzodiazepines,
phenothiozines, prostaglandins antibiotics, anti-viral agents, anti-fungals,
22
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
immunosuppressants, anti-allergic agents, astringents, corticosteroids
fluorouracil,
bleomycin, vincristine, or deferoxamine. The active agent may be useful in the
treatment of
wound-healing.
2. Oils
For both the methods of Route I and Route II and the compositions of the
invention,
any suitable oil can be used. Exemplary oils that can be used include, for
example, vegetable
oils, nut oils, fish oils, lard oil, mineral oils, squalane, tricaprylin, and
mixtures thereof.
Specific examples of oils that may be used include, but are not limited to,
almond oil (sweet),
apricot seed oil, borage oil, canola oil, coconut oil, corn oil, cotton seed
oil, fish oil, jojoba
bean oil, lard oil, linseed oil (boiled), Macadamia nut oil, medium chain
triglycerides, mineral
oil, olive oil, peanut oil, safflower oil, sesame oil, soybean oil, squalene,
sunflower seed oil,
tricaprylin (1,2,3-trioctanoyl glycerol), wheat germ oil, and mixtures
thereof.
3. Surface Stabilizers or Surfactants
The compositions of the invention also comprise at least-one surface
stabilizer or
surfactant. When the compositions additionally comprise a nanoparticulate
active agent, the
surface stabilizer used in the methods and compositions of the invention
associates with, or
adsorbs to, the surface of the nanoparticulate active agent, but does not
covalently bind to the
active agent. The surface stabilizer is preferably soluble in water. One or
more surface
stabilizers may be used in the compositions and methods of the invention. As
used herein,
the terms "stabilizer", "surface stabilizer", and "surfactant" are used
interchangeably.
Any suitable nonionic or ionic surfactant may be utilized in the compositions
of the
invention, including anionic, cationic, and zwitterionic surfactants.
Exemplary stabilizers or
surfactants that may be used in the compositions of the invention lacking an
active agent, and
in active agent comprising compositions of the invention, include but are not
limited to, non-
phospholipid surfactants, such as the Tween (polyoxyethylene derivatives of
sorbitan fatty
acid esters) family of surfactants (i.e., Tween 20, Tween 60, and Tween
80), nonphenol
polyethylene glycol ethers, sorbitan esters (such as Span and Arlacel ),
glycerol esters (such
as glycerin monostearate), polyethylene glycol esters (such as polyethylene
glycol stearate),
block polymers (such as Pluronics'll), acrylic polymers (such as Pemulen ),
ethoxylated fatty
esters (such as Cremophor RH-40), ethoxylated alcohols (such as Brij ),
ethoxylated fatty
23
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
acids, monoglycerides, silicon based surfactants, polysorbates, and Tergitol
NP-40
(Poly(oxy-1,2-ethanediyl), a -(4-nonylphenol)-.omega.-hydroxy, branched
[molecular weight
average 1980]), and Tergitol NP-70 (a mixed surfactant--AQ=70%).
4. Solvents
Any suitable solvent can be used in the methods and compositions of the
invention.
Exemplary solvents include, but are not limited, to isopropyl myristate,
triacetin, N-methyl
pyrrolidinone, long-chain alcohols, polyethylene glycols, propylene glycol,
diethyleneglycol
monoethyl ether, and long- and short-chain alcohols, such as ethanol. Other
short chain
alcohols and/or amides may be used, as well as aliphatic and aromatic
alcohols, such as
benzyl alchohol. Mixtures of solvents can also be used in the compositions and
methods of
the invention.
5. Water or Buffer
If the methods and/or compositions of the invention use or comprise water or a
buffer,
the aqueous solution is preferably a physiologically compatible solution such
as water or
phosphate buffered saline.
6. Other Ingredients
A number of other materials may be added to the compositions of the invention.
Volatile oils, such as volatile flavor oils, can be used in lieu of some of
the oil or can be
added in addition to the primary oil. Exemplary volatile oils or fragrances
that can be utilized
in the invention include, but are not limited to, balm oil, bay oil, bergamot
oil, cedarwood oil,
cherry oil, cinnamon oil, clove oil, origanum oil, and peppermint oil. A
coloring agent, such
as a food coloring agent can also be used. Exemplary food colors that can be
utilized in the
compositions of the invention include, but are not limited to, green, yellow,
red, and blue.
The food colors utilized are food grade materials (McCormick), although
materials from
other sources can be substituted. In addition, a flavoring extract can be used
in the methods
and compositions of the invention. Exemplary flavored extracts include, but
are not limited
to, pure anise extract (73% alcohol), imitation banana extract (40% ethanol),
imitation cherry
extract (24% ethanol), chocolate extract (23% ethanol), pure lemon extract
(84% ethanol),
pure orange extract (80% ethanol), pure peppermint extract (89% ethanol),
imitation
24
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
pineapple extract (42% ethanol), imitation rum extract (35% ethanol),
imitation strawberry
extract (30% ethanol), and pure or imitation vanilla extract (35% ethanol).
Typically, the
extracts utilized are food grade materials (McCormick), although materials
from other
sources can be substituted.
D. Methods of Using the Compositions_of the Invention
The compositions of the invention are useful as a broad spectrum antimicrobial
agent.
They can be used for topical or surface disinfection, for example, of
biological and non-
biological surfaces. The compositions can be used to disinfect surfaces likely
to be exposed
to microbes, such as cooking surfaces, eating surfaces, any surface in a
hospital, areas
exposed to young children, etc. Biological surfaces include skin, hair, mucous
membranes,
wound surfaces, insect bites, etc.
The compositions can also be used as delivery vehicles for an active agent,
such as a
drug or cosmetic. The compositions avoid the requirement of adding a
antimicrobial agent to
retard microbial growth. When formulated into a dosage form for administration
to a
mammal, such as a human, the compositions of the invention can be administered
to a subject
via any conventional means. The compositions can be useful in the formulation
of active
agents intended to treat wounds and would surfaces. Some of these active
agents are not
compatible with typical antimicrobial agents. Therefore, a vehicle that is
inherently
antimicrobial may be an ideal delivery system.
As used herein, the term "subject" is used to mean an animal, preferably a
mammal,
including a human or non-human. The terms patient and subject may be used
interchangeably. In addition, the compositions of the invention can be
formulated into any
suitable dosage form, such as liquid dispersions, oral suspensions, tablets,
gels, aerosols,
ointments, creams, capsules, dry powders, multiparticulates, sprinkles,
sachets, lozenges, and
syrups. Moreover, the dosage forms of the invention may be solid dosage forms,
liquid
dosage forms, semi-liquid dosage forms, immediate release formulations,
modified release
formulations, controlled release formulations, fast melt formulations,
lyophilized
formulations, delayed release formulations, extended release formulations,
pulsatile release
formulations, mixed immediate release and controlled release formulations, or
any
combination thereof.
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The compositions of the invention may also comprise adjuvants such as
preserving,
wetting, emulsifying, and dispensing agents. Prolonged absorption of the
injectable
pharmaceutical form can be brought about by the use of agents delaying
absorption, such as
aluminum monostearate and gelatin.
Liquid dosage forms include pharmaceutically acceptable emulsions, solutions,
suspensions, syrups, and elixirs. In addition to the active agent, the liquid
dosage forms may
comprise inert diluents commonly used in the art, such as water or other
solvents, solubilizing
agents, and emulsifiers. Exemplary emulsifiers are ethyl alcohol, isopropyl
alcohol, ethyl
carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propyleneglycol,
1,3-
butyleneglycol, dimethylformamide, oils, such as cottonseed oil, groundnut
oil, corn germ
oil, olive oil, castor oil, and sesame oil, glycerol, tetrahydrofurfuryl
alcohol,
polyethyleneglycols, fatty acid esters of sorbitan, or mixtures of these
substances, and the
like. Besides such inert diluents, the composition can also include adjuvants,
such as wetting
agents, emulsifying and suspending agents, sweetening, flavoring, and
perfuming agents.
"Therapeutically effective amount" as used herein with respect to an active
agent
dosage shall mean that dosage that provides the specific pharmacological
response for which
the active agent is administered in a significant number of subjects in need
of such treatment.
It is emphasized that "therapeutically effective amount," administered to a
particular subject
in a particular instance may not be effective for 100% of patients treated for
a specific
disease, and will not always be effective in treating the diseases described
herein, even
though such dosage is deemed a"therapeutically effective amount" by those
skilled in the art.
One of ordinary skill will appreciate that effective amounts of an active
agent can be
determined empirically and can be employed in pure form or, where such forms
exist, in
pharmaceutically acceptable salt, ester, or prodrug form. Actual dosage levels
of an active
agent in the compositions of the invention may be varied to obtain an amount
of the active
agent that is effective to obtain a desired therapeutic response for a
particular composition
and method of administration. The selected dosage level therefore depends upon
the desired
therapeutic effect, the route of administration, the potency of the
administered active agent,
the desired duration of treatment, and other factors.
Dosage unit compositions may contain such amounts of such submultiples thereof
as
may be used to make up the daily dose. It will be understood, however, that
the specific dose
level for any particular patient will depend upon a variety of factors: the
type and degree of
the cellular or physiological response to be achieved; activity of the
specific agent or
26
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
composition employed; the specific agents or composition employed; the age,
body weight,
general health, sex, and diet of the patient; the time of administration,
route of administration,
and rate of excretion of the agent; the duration of the treatment; drugs used
in combination or
coincidental with the specific agent; and like factors well known in the
medical arts.
The following examples are given to illustrate the present invention. It
should be
understood, however, that the invention is not to be limited to the specific
conditions or
details described in these examples. Throughout the specification, any and all
references to a
publicly available document, including a U.S. patent, are specifically
incorporated by
reference.
EXAMPLES
Example 1
The purpose of this example was to describe preparation of an exemplary
nanostructured composition according to the invention.
Ethyl alcohol, soybean oil, and Polysorbate 80 were mixed together. Water was
then
added to this mixture, and the resulting composition was mixed well using a
paddle stirrer.
The quantities of each component are shown below in Table 1.
TABLE 1
Ingredient Quantity
Ethyl Alcohol USP 8.8 gm
Polysorbate 80 NF 9.4 gm
Soybean oil USP 50.2 gm
Water USP 31.7 gm
The composition was then fed into a high-pressure homogenizer (APV Invensys,
model APV-1000) and the pressure tuned to 10,000 psi. The mixture was run
through the
homogenizer for 2 passes.
Alternatively, water can be added to the ethyl alcohol, oil, and Polysorbate
80 mixture
under high-speed stirring using a rotor-stator assembly mounted on Silverson
mixer (10,000
rpm for 15 minutes).
27
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The resulting composition can be used directly as a topical lotion or cream
having
antibacterial, anti-viral, anti-fungal, and/or anti-yeast properties, or the
composition can be
formulated into another suitable dosage form.
Example 2
The purpose of this example was to describe preparation of an exemplary
nanostructured composition according to the invention comprising the active
agent estradiol.
Estradiol is chemically described as estra-1,3,5(10)-triene-3,17beta -diol.
The
molecular formula of estradiol is C18H2402 and the structural formula is:
CH3
-=r" ~ .
OH
The molecular weight of estradiol is 272.39.
Current U.S. Food and Drug Administration (FDA) approved estradiol products
include oral pills (Estinyl , Estrace , Gynodiol , Oveon 35 ), transdermal
patches
(Climarat , Vivelle , Estraderm'8'), a vaginal ring (Estring"'), and a topical
emulsion
(Estxasorb ). The drug is used in hormone replacement therapy, to treat
moderate to severe
symptoms of hot flashes and night sweats associated with menopause and to
prevent
pregnancy.
Estradiol was dissolved in ethanol. The oil and Polysorbate 80 were added to
the
estradiol solution, water was added to the estradiol/ethyl
alcohol/oil/Polysorbate 80 mixture,
and the resulting composition was mixed well using a paddle stirrer. The
quantities of each
component are shown below in Table 2A.
TABLE2A
Ingredient Quantity
Estradiol 0.25
Ethyl Alcohol USP 8.8 gm
Polysorbate 80 NF 9.4 m
Soybean oil USP 50.2 gm
Water USP 31.7 m
28
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The composition was fed into a high-pressure homogenizer (APV Invensys, model
APV-1000) and the pressure was tuned to 10,000 psi. The mixture was run
through the
homogenizer for 2 passes. The particle size of the composition is detailed in
Table 2B below
and is further represented in Figure 5.
Table 2B
Mean ( m) Standard
Deviation m
0.0826 0.048
Alternatively, estradiol can be dissolved in ethyl alcohol, the oil and water
can be
added to the estradiol solution, and water can be added to the resulting
composition under
high-speed stirring using a rotor-stator assembly mounted on Silverson mixer
(10,000 rpm for
minutes).
The particle size of the composition according to the Silverson method is
detailed in Table
2C below and is further represented in Figure 6.
15 Table 2C
Mean (pm) Standard
Deviation m
1.204 0.930
The resulting composition can be used directly as an estradiol topical lotion
or cream
having antibacterial, anti-viral, anti-fungal, and/or anti-yeast properties,
or the composition
can be formulated into another suitable dosage form.
Example 3
The piurpose of this example was to describe preparation of an exemplary
nanostructured composition according to the invention comprising the active
agent
testosterone.
Testosterone USP is a white to practically white crystalline powder chemically
described as 17-beta hydroxyandrost-4-en-3-one.
29
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
H3.O H
OH
H3C H
H H
Testosterone
It has the chemical formula C19H2802 and a molecular weight of 288.42.
Testosterone is
commercially available, for example, in a injectable dosage forms, transdermal
gel
(AndroGel , Testim8'), transdermal delivery device (Androdermo, Testoderm ),
and a buccal
drug delivery system (Striant ). Testosterone is used, for example, in hormone
replacement
therapy.
Testosterone was dissolved in ethyl alcohol. Oil and Polysorbate 80 were added
to
the testosterone solution, and water was added to the resulting composition.
The resulting
composition was mixed well using a paddle stirrer. The quantities of each
component are
shown below in Table 3A.
TABLE3A
Ingredient Quantity
Testosterone 3.09 m
Ethyl Alcohol USP 8.8
Polysorbate 80 NF 9.4 gm
Soybean oil USP 50.2 gm
Water USP 31.7 gm
The testosterone/ethyl alcohol/oil/Polysorbate 80/water composition was fed
into a
high-pressure homogenizer (APV Invensys, model APV-1000) and the pressure was
tuned to
10,000 psi. The mixture was run through the homogenizer for 2 passes. The
particle size of
the composition is detailed in Table 3B below and is further represented in
Figure 7.
Table 3B
Mean ( m) Standard
Deviation (ttm)
1.052 0.507
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Alternatively, the testosterone can be dissolved in ethyl alcohol, and oil and
the
Polysorbate 80 can be added to the testosterone solution. Water can be added
to the
testosterone/ethyl alcohol/oil/Polysorbate 80 composition under high-speed
stirring using a
rotor-stator assembly mounted on Silverson mixer (10,000 rpm for 15 minutes).
The resulting composition can be used directly as a testosterone topical
lotion or
cream having antibacterial, anti-viral, anti-fungal, and/or anti-yeast
properties, or the
composition can be formulated into another suitable dosage form.
Examnle 4
The purpose of this example was to determine the effect of concentration of
excipients on the antimicrobial properties of the formulation. The composition
of Example I
was diluted with water by 10-fold and tested for antimicrobial effectiveness.
The quantities of each component are shown below in Table 4
TABLE 4
Ingredient Quantity
Ethyl Alcohol USP 0.88 gm
Polysorbate 80-NF 0.94 gm
Soybean oil USP 5.02 gm
Water USP 93.16
This formulation did not meet the criteria set forth when tested according to
USP
Antimicrobial Effectiveness Testing standards. The resulting composition
cannot be used
directly as a topical lotion or cream having antibacterial, anti-viral, anti-
fungal, and/or anti-
yeast properties, nor can the composition be formulated into another suitable
dosage form in
order to impart antimicrobial properties.
31
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Example 5
The purpose of this example was as yet another example to determine the effect
of
concentration of excipients on the antimicrobial properties of the
formulation. The
composition of Example 1 was diluted with water by 50-fold and tested for
antimicrobial
properties.
The quantities of each component are shown below in Table 4
TABLE 5
Ingredient Quantity
Eth 1 Alcohol USP 0.18 gm
Polysorbate 80 NF 0.19 gm
So bean oil USP 1.0 gm
Water USP 98.63 gm
This formulation did not meet the criteria set forth when tested according to
USP
Antimicrobial Effectiveness Testing standards. The resulting composition
cannot be used
directly as a topical lotion or cream having antibacterial, anti-viral, anti-
fungal, and/or anti-
yeast properties, nor can the composition be formulated into another suitable
dosage form in
order to impart antimicrobial properties.
Example 6
The purpose of this example was to describe preparation of an exemplary
nanostructured composition according to the invention.
Ethyl alcohol, soybean oil, and Pluronic F-68 were mixed together. Water was
then
added to this mixture, and the resulting composition was mixed well using a
paddle stirrer.
The quantities of each component are shown below in Table 6.
TABLE 6
ln redient Quantity
Eth l Alcohol USP 8.8 gm
Pluronic F-68 6.0 gm
So bean oil USP 50.2 gm
Water USP 35.0 m
32
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The composition was then fed into a high-pressure homogenizer (APV Invensys,
model APV-1000) and the pressure tuned to 10,000 psi. The mixture was run
through the
homogenizer for 2 passes.
Alternatively, water can be added to the ethyl alcohol, oil, and Pluronic F-68
mixture
under high-speed stirring using a rotor-stator assembly mounted on Silverson
mixer (10,000
rpm for 15 minutes).
The resulting composition can be used directly as a topical lotion or cream
having
antibacterial, anti-viral, anti-fungal, and/or anti-yeast properties, or the
composition can be
formulated into another suitable dosage form.
Example 7
The purpose of this example was to describe preparation of an exemplary
nanostructured composition according to the invention.
Ethyl alcohol, benzyl alcohol, isopropyl myristate, light mineral oil, and
Pluronic F-
68 were mixed together. Water was then added to this mixture, and the
resulting composition
was mixed well using a paddle stirrer. The quantities of each component are
shown below in
Table 7.
TABLE 7
Ingredient Quantity
Ethyl Alcohol USP 10.6
Benzyl Alcohol 5.3 gm
Pluronic F-68 7.4 gm
Iso ro l Myristate 5.3 gm
Light Mineral Oil 40.4
Water USP 30.9 gm
The composition was then fed into a high-pressure homogenizer (APV Invensys,
model APV- 1000) and the pressure tuned to 10,000 psi. The mixture was run
through the
homogenizer for 2 passes.
Alternatively, water can be added to the ethyl alcohol, oil, and Pluronic F-68
mixture
under high-speed stirring using a rotor-stator assembly mounted on Silverson
mixer (10,000
rpm for 15 minutes).
The resulting composition can be used directly as a topical lotion or cream
having
antibacterial, anti-viral, anti-fungal, and/or anti-yeast properties, or the
composition can be
formulated into another suitable dosage form.
33
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Example 8
The purpose of this example was to describe preparation of a nanostructured
composition that included propofol, a model active pharmaceutical ingredient,
and that did
not meet the USP Antimicrobial Effectiveness Testing standards.
Propofol, Ethyl alcohol, soybean oil, and Polysorbate 80 were mixed together.
Saline
was then added to this mixture, and the resulting composition was mixed well
using a paddle
stirrer. The quantities of each component are shown below in Table 8.
TABLE 8
Ingredient uanti
Propofol 1.0 gm
Ethyl Alcohol USP 0.5 gm
Polysorbate 80 NF 0.9
Soybean oil USP 4.5
Saline 93.1 gm
The composition was then fed into a high-pressure homogenizer (APV Invensys,
model APV-1000) and the pressure tuned to 10,000 psi. The mixture was run
through the
homogenizer for 2 passes.
This formulation did not meet the criteria set forth when tested according to
USP
Antimicrobial Effectiveness Testing standards.
Example 9
The purpose of this example was to determine the antimicrobial effects of a
composition prepared as described in Example 1.
Antimicrobial testing was conducted according to USP 25, <51>, pp. 1869-1871.
A
quantity of the composition prepared as described in Example 1 was challenged
with various
bacteria, yeast, or fungus: Aspergillus niger (fungus), Candida Albicans
(yeast), Escherichia
colf (bacteria), Pseudomonas aeruginosa (bacteria), Staphylococcus aureus
(bacteria),
Aspergillus fumigatus (fungus), and Aspergillus flavus (fungus).
Prior to the test, the surface of a suitable volume of solid agar medium was
inoculated
from a recently revived stock culture of each of the organisms shown below in
Table 4. The
quantity of inoculum of the composition prepared as in Example 1 for each test
is shown in
34
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
Table 4, below. The quantity of bacteria, fungus, or yeast was measured at the
time of
inoculation, at day 7, day 14, and at day 28. The culture conditions were
conducted
according to USP 25, <51>, pp. 1869-1871. The results of the tests are shown
in Table 4. In
addition, the results are graphically shown in Figure 1 and 2.
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
~'^~Qc~=~ o~ o A~ cn A~ tw~A~ on~
~--~ O q y o O~ O o O~ O O U
0~ Q~' N O^+V "Cl O"d TJ ~
00 U ~ Z V A V A Z O V I !~ Rdp.
L`t eA
.~ ..
C~ U U
(:N U .-O. d0 4, .~0. bA R.-O. OD a
vi o -~ o A0 an =a A`~ oo r s A~ on
C) N ^O,~ U y O c.a O r=O,~ U
O G O c~ d' "RC O ~ ~f' 'UR
V A VA ~.' Z V A
U bA a p d4 a~ b0 R
o~ o A`~ on~A~ ao aA~ ao
Q O O ~
O O Oi~ ~td p~oet'7 O~ ~Y 'RU ?
N U N f-+ V A e7-+ V A I.N.
'cl
ta
U 0 2 OA a~ bD a~ bA a
w o o ~ on'~A~ oo A~ oo=o ~
O r' '~! 'd O"' ~? C O
m 'U O
r"A
V A V d=
.~ p0 b ^CS 'LS ~
kn
-~f=i CS U O ~ ~~ p a~i ~~ p~'~. 0 N
A~lo o A Uo+ o~
v-d ob o vv
=~ ~ ~ ~ ~ z v n v n v n
eG O ~ Cp v +- Ur E-1
3õ" ~ bA R O CU a~ bA a
et ~Zi '~o U
o o A~ on'~A~ an A~ oq.o 0
U o~ o a~ " o o c~ o y o c
W ~F=+ o~ o cso-- ~ o~ ~ ao~ o ~
ao ,
r.~ as c a ,_.; o--~ er zs --+ d b o- v v
peLC~ '~ cv v w V V A 2
E" ..r ba v ~ ~U
o O
b q a~oi bA a a aD a E-+
~V o o en a
U O~ O N v U y U.-~i v N V V =x=
E'~ O O a O.'"' R R O R
O d" "C O -" 'LS
v~i v rn Z V n P.' Z V h~ Z VA w
y bA N d N
U c~ U
e.Hy+ ~ a'~ o A`r'o o~ A O o~ A O o~
O^'rY'C O^' d T! p
N V Z V A Z V A Z V A
w
Y K a bA
.~ U o ';"~+ a ~ a a
"' U er o
0 ao ew cn o
'.
0 C) o`~ o 0 0 0 0 0 ~ o 0
o na b o~
t~~' ~
~ C) .-~ .-~ ~-Ni N=-r .~. t~ c'~) I~i
.~ ~
V
ci = N
O
H R, " o A A A
36
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
The results dramatically demonstrate the surprising effectiveness of the
compositions
of the invention as antibacterial, antifungal, and anti-yeast agents. For all
but one tested
organism (A. niger), all organisms were eradicated after 7 days, and for A.
niger, the growth
of the organism was drastically diminished, with only 70 cfu/g measured on Day
28.
Moreover, the results demonstrate that the composition meets the USP 25
requirements for
Antimicrobial Effectiveness Test.
Example 10
The purpose of this example was to determine the antimicrobial effects of a
composition prepared as described in Example 2, to determine if the addition
of an active
agent such as estradiol affects the antimicrobial, anti-yeast, anti-fungal,
and/or anti-viral
properties of the compositions of the invention. This example also evaluates
the effect on
antimicrobial activity of varying the quantity of alcohol present in the
compositions of the
invention.
Antimicrobial testing was conducted according to USP 25, <51>, pp. 1869-1871.
A
quantity of the composition prepared as in Example 2 was challenged with
various bacteria,
yeast, or fungus: Aspergillus niger (fungus), Candida albicans (yeast),
Escherichia colf
(bacteria), Pseudomonas aeruginosa (bacteria), and Staphylococcus aureus
(bacteria).
Prior to the test, the surface of a suitable volume of solid agar medium was
inoculated
from a recently revived stock culture of each of the organisms shown below in
Table 5. The
quantity of inoculum of the composition prepared as described in Example 2 for
each test is
shown in Table 5, below. The quantity of bacteria, fungus, or yeast was
measured at the time
of inoculation, at day 14 (Table 5) and day 28 Table 6). The culture
conditions were
conducted according to USP 25, <51>, pp. 1869-1871. The results of the tests
are shown in
Tables 5 and 6. In addition, the results are graphically shown in Figure 3.
37
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
TABLE 5: Antimicrobial Effectiveness Test for Estradiol-Comprising Composition
Day 14 Results
Or anism A. niger C. albicans E. coli P. aeruginosa S. aureus
ATCC 16404 10231 8739 9027 6538
Strain #
Inoculum 200,000 cfu/mL 250,000 cfu/mL 550,000 cfu/mL 220,000 cfu/mL 580,000
cfu/mL
per mL
12roduct
% EtOH
7.7% 30,000 cfu/g None Detected None Detected None Detected None Detected
1 log reduction <10 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g
> 4 log reduction > 4 log reduction > 4 lo reduction > 4 lo reduction
7.3% 17,000 cfu/g None Detected None Detected None Detected None Detected
1 log reduction <10 efu/g <10 cfu/g <10 cfu/g <10 cfu/g
> 4 log reduction > 4 log reduction > 4 log reduction > 4 log reduction
7.4% 17,000 cfu/g None Detected None Detected None Detected None Detected
1 log reduction <10 cfu/g <10 cfu/g <10 cfu/g <10 cfulg
> 4 lo reduction > 4 log reduction > 4 log reduction > 4 log reduction
7.0% 13,000 cfu/g None Detected None Detected None Detected None Detected
I log reduction <10 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g
> 4 log reduction > 4 log reduction > 4 log reduction > 4 log reduction
TABLE 6: Antimicrobial Effectiveness Test for Estradiol-Comprising Composition
Day 28 Results
Organism A. niger C. albicans E. coli P. aeruginosa S. aureus
ATCC 16404 10231 8739 9027 6538
Strain #
Inoculum 200,000 cfu/mL 250,000 cfu/mL 550,000 cfu/mL 220,000 cfu/mL 580,000
cfu/mL
per mL
product
% EtOH
7,7"/u None Detected None Detected None Detected None Detected None Detected
<100 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g
>3 log reduction > 4 log reduction > 4 log reduction > 4 log reduction > 4 log
reduction
7,3% None Detected None Detected None Detected None Detected None Detected
<10 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g
> 4 log reduction > 4 log reduction > 4 log reduction > 4 log reduction > 4
log reduction
7,4% 180 cfu/g None Detected None Detected None Detected None Detected
3.2 log reduction <10 cfu/g <10 cfu/g <10 cfu/g <10 cfu/g
> 4 lo reduction > 4 log reduction > 4 log reduction > 4 lo reduction
7,0% 100,000 cfu/g None Detected 180 cfu/g None Detected None Detected
0.5 log reduction <10 cfu/g 3.6 log reduction <10 cfu/g <l 0 cfu/g
> 4 log reduction > 4 log reduction > 4 log reduction
Example 11
The purpose of this example was to determine the antimicrobial effects of a
composition prepared as described in Example 3, to determine if the addition
of an active
38
CA 02648360 2008-10-03
WO 2007/123790 PCT/US2007/008054
agent such as testosterone affects the antimicrobial, anti-yeast, anti-fungal,
and/or anti-viral
properties of the compositions of the invention.
Antimicrobial testing was conducted according to USP 25, <51>, pp. 1869-1871.
A
quantity of the composition prepared as in Example 2 was challenged with
various bacteria,
yeast, or fungus: Aspergillus niger (fungus), Candida albicans (yeast),
Escherichia colf
(bacteria), Pseudomonas aeruginosa (bacteria), and Staphylococcus aureus
(bacteria).
Prior to the test, the surface of a suitable volume of solid agar medium was
inoculated
from a recently revived stock culture of each of A. niger, C. albicans, E.
coli, P. aeruginosa,
and S. aureus. The quantity of bacteria, fungus, or yeast was measured at the
time of
inoculation and at day 7, 14, and 28. The culture conditions were conducted
according to
USP 25, <51>, pp. 1869-1871. The results, shown in Figure 4, dramatically
demonstrate the
surprising effectiveness of the compositions of the invention as
antibacterial, anti-fungal, and
anti-yeast agents when the compositions are used as drug delivery vehicles.
Surprisingly, the
presence of an active agent does not negate the antimicrobial effects of the
compositions of
the invention. Moreover, the results demonstrate that the composition meets
the USP 25
requirements for Antimicrobial Effectiveness Test.
It will be apparent to those skilled in the art that various modifications and
variations
can be made in the methods and compositions of the present invention without
departing
from the spirit or scope of the invention. Thus, it is intended that the
present invention cover
the modifications and variations of this invention provided they come within
the scope of the
appended claims and their equivalents.
39