Note: Descriptions are shown in the official language in which they were submitted.
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
A REINFORCED ABSORBABLE MULTILAYERED HEMOSTATIC WOUND
DRESSING AND METHOD OF MAKING
FIELD OF THE INVENTION
The present invention relates to a reinforced absorbable multilayered
hemostatic
wound dressing and method of making.
BACKGROUND OF THE INVENTION
The control of bleeding as well as sealing of air and various bodily fluids is
essential and critical in surgical procedures to minimize blood loss, to seal
tissue and
organ structures, to reduce post-surgical complications, and to shorten the
duration of
the surgery in the operating room.
In an effort to provide dressings with enhanced hemostatic and tissue sealing
and adhering properties, therapeutic agents, including, but not limited to,
thrombin,
fibrin and fibrinogen have been combined with dressing carriers or substrates,
including
gelatin-based carriers, polysaccharide-based carriers, glycolic acid or lactic
acid-based
carriers and a collagen matrix. Examples of such dressings are disclosed in
USP
6,762,336, USP 6,733,774 and PCT publication WO 2004/064878 Al.
Due to its biodegradability and its bactericidal, tissue sealing, tissue
repairing,
drug delivering and hemostatic properties, it is desirable to utilize
cellulose that has
been oxidized to contain carboxylic acid moieties, hereinafter referred to as
carboxylic-
oxidized cellulose, as a topical dressing in a variety of surgical procedures,
including
neurosurgery, abdominal surgery, cardiovascular surgery, thoracic surgery,
head and
neck surgery, pelvic surgery and skin and subcutaneous tissue procedures.
However, when carboxylic-oxidized cellulose is utilized in combination with
thrombin and/or fibrinogen, the acidic moieties that may be present in the
cellulose
denature the activity of the thrombin and/or fibrinogen. Therefore, it is
desirable to
shield the and/or fibrinogen from such acid moieties to maintain their
hemostatic
activities.
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
As used herein, the term "nonwoven fabric" includes, but is not limited to,
bonded fabrics, fonned fabrics, or engineered fabrics, that are manufactured
by
processes other than , weaving or knitting. More specifically, the term
"nonwoven
fabric" refers to a porous, textile-like material, usually in flat sheet form,
composed
primarily or entirely of staple fibers assembled in a web, sheet or batt. The
structure of
the nonwoven fabric is based on the arrangement of, for example, staple fibers
that are
typically arranged more or less randomly. The tensile, stress-strain and
tactile
properties of the nonwoven fabric ordinarily stem from fiber to fiber friction
created by
entanglement and reinforcement of, for example, staple fibers, and/or from
adhesive,
chemical or physical bonding. Notwithstanding, the raw materials used to
manufacture
the nonwoven fabric may be yarns, scrims, netting, or filaments made by
processes that
include, weaving or knitting.
SUMMARY OF THE INVENTION
The present invention is directed to a reinforced absorbable multilayered
hemostatic wound dressing comprising a first absorbable nonwoven fabric
reinforced
by one or more second absorbable woven or knitted fabric, and thrombin and/or
fibrinogen, and method of making. More particularly, the first absorbable
nonwoven
fabric comprises fibers comprising aliphatic polyester polymers, copolymers,
or blends
thereof; while the second absorbable woven or knitted fabric comprises
oxidized
regenerated cellulose fibers.
BRIEF DESCRIPTION OF THE DRAWING
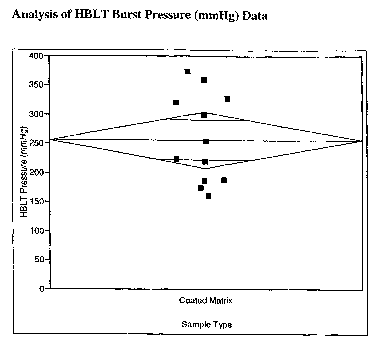
The figure shows the pressure required to disrupt/burst the seal formed
between
the tissue and the hemostatic wound dressing.
DETAILED DESCRIPTION OF THE INVENTION
The multilayered dressings described herein provide and maintain effective
hemostasis when applied to a wound requiring hemostasis. Effective hemostasis,
as
used herein, is the ability to control and/or abate capillary, venous, or
arteriole bleeding
within an effective time, as recognized by those skilled in the art of
hemostasis. Further
2
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
indications of effective hemostasis may be provided by governmental regulatory
standards and the like.
In certain embodiments, multilayered dressings of the present invention are
effective in providing and maintaining hemostasis in cases of severe or brisk
bleeding.
As used herein, severe bleeding is meant to include those cases of bleeding
where a
relatively high volume of blood is lost at a relatively high rate. Examples of
severe
bleeding include, without limitation, bleeding due to arterial puncture, liver
resection,
blunt liver trauma, blunt spleen trauma, aortic aneurysm, bleeding from
patients with
over-anticoagulation, or bleeding from patients with coagulopathies, such as
hemophilia.
The reinforced absorbable multilayered dressing generally comprises a
nonwoven fabric and one or more reinforcement fabric. The reinforcement fabric
provides a backing to which the nonwoven fabric may be attached, either
directly or
indirectly, wherein thrombin and/or fibrinogen are substantially homogeneously
dispersed throughout the nonwoven fabric and/or are disposed on the surface of
the
nonwoven fabric. The reinforcement fabric provides strength to the dressing
sufficient
to permit the user of the dressing to place and manipulate the dressing on or
within a
wound or directly onto tissue of a patient requiring hemostasis, or tissue
sealing and
adhering.
In addition to serving as a carrier for the thrombin and/or fibrinogen, the
nonwoven fabric also serves to shield the thrombin and/or fibrinogen from
acidic
moieties that may be present in the reinforcement fabric, such as is the case
where
carboxylic-oxidized cellulose is used as the reinforcement fabric.
The nonwoven fabric functions as the first absorbable nonwoven fabric of the
reinforced absorbable multilayered dressing described herein. The first
absorbable
nonwoven fabric is comprised of fibers comprising aliphatic polyester
polymers,
copolymers, or blends thereof. The aliphatic polyesters are typically
synthesized in a
ring opening polymerization of monomers including, but not limited to, lactic
acid,
3
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
lactide (including L-, D-, meso and D, L mixtures), glycolic acid, glycolide,
E-
caprolactone, p-dioxanone (1,4-dioxan-2- one), and trimethylene carbonate (1,3-
dioxan-
2-one).
Preferably, the first absorbable nonwoven fabric comprises a copolymer of
glycolide and lactide, in an amount ranging from about 70 to 95% by molar
basis of
glycolide and the remainder lactide.
Preferably, the nonwoven fabric is made by processes other than, weaving or
knitting. For example, the nonwoven fabric may be prepared from yarn, scrims,
netting
or filaments that have been made by processes that include, weaving or
knitting. The
yarn, scrims, netting and/or filaments are crimped to enhance entanglement
with each
other and attachment to the second absorbable woven or knitted fabric. Such
crimped
yarn, scrims, netting and/or filaments may then be cut into staple that is
long enough to
entangle. The staple may be between about 0.1 and 2.5 inches long, preferably
between
about 0.5 and 1.75 inches, and most preferably between about 1.0 and 1.3
inches. The
staple may be carded to create a nonwoven batt, which may be then
needlepunched or
calendared into the first absorbable nonwoven fabric. Additionally, the staple
may be
kinked or piled.
Other methods known for the production of nonwoven fabrics may be utilized
and include such processes as air laying, wet forming and stitch bonding. Such
procedures are generally discussed in the Encyclopedia of Polymer Science and
Engineering, Vol. 10, pp. 204-253 (1987) and Introduction to Nonwovens by
Albin
Turbank (Tappi Press, Atlanta GA 1999), both incorporated herein in their
entirety by
reference.
The thickness of the nonwoven fabric may range from about 0.25 to 2 mm. The
basis weight of the nonwoven fabric ranges from about 0.01 to 0.2 g/in2;
preferably
from about 0.03 to 0.1 g/in2; and most preferably from about 0.04 to 0.08
g/in2. The
weight percent of first absorbable nonwoven fabric may range from about 5 to
50
4
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
percent, based upon the total weight of the reinforced absorbable multilayered
dressing
having thrombin and/or fibrinogen.
The second absorbable woven or knitted fabric functions as the reinforcement
fabric and comprises oxidized polysaccharides, in particular oxidized
cellulose and the
neutralized derivatives thereof. For example, the cellulose may be carboxylic-
oxidized
or aldehyde-oxidized cellulose. More preferably, oxidized regenerated
polysaccharides
including, but without limitation, oxidized regenerated cellulose may be used
to prepare
the second absorbable woven or knitted fabric. Regenerated cellulose is
preferred due to
its higher degree of uniformity versus cellulose that has not been
regenerated.
Regenerated cellulose and a detailed description of how to make oxidized
regenerated
cellulose are set forth in USP 3,364,200, USP 5,180,398 and USP 4,626,253, the
contents each of which is hereby incorporated by reference as if set forth in
its entirety.
Examples of fabrics that may be utilized as the reinforcement fabric include,
but
are not limited to, Interceed absorbable adhesion barrier, Surgicel
absorbable
hemostat; Surgicel Nu-Knit absorbable hemostat; and Surgicel Fibrillar
absorbable
hemostat; each available from Johnson & Johnson Wound Management Worldwide or
Gynecare Worldwide, each a division of Ethicon, Inc., Somerville, New Jersey.
The reinforcement fabric utilized in the present invention may be woven or
knitted, provided that the fabric possesses the physical properties necessary
for use in
contemplated applications. Such fabrics, for example, are described in USP
4,626,253,
USP 5,002,551 and USP 5,007,916, the contents of which are hereby incorporated
by
reference herein as if set forth in its entirety. In preferred embodiments,
the
reinforcement fabric is a warp knitted tricot fabric constructed of bright
rayon yarn that
is subsequently oxidized to include carboxyl or aldehyde moieties in amounts
effective
to provide the fabrics with biodegradability.
In an alternative embodiment, the reinforcement fabric comprises fibers
comprised of aliphatic polyester polymers, copolymers, or blends thereof alone
or in
combination with oxidized polysaccharide fibers.
5
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
The second absorbable woven or knitted fabric preferably comprises oxidized
regenerated cellulose and may have a basis weight ranging from about 0.001 to
0.2
g/in2, preferably in the range of about 0.01 to 0.1 g/in2, and most preferably
in the range
of about 0.04 to 0.07 g/in2.
The first absorbable nonwoven fabric is attached to the second absorbable
woven or knitted fabric, either directly or indirectly. For example, the
nonwoven fabric
may be incorporated into the second absorbable woven or knitted fabric via
needlepunching, calendaring, embossing or hydroentanglement, or chemical or
thermal
bonding. The staple of the first absorbable nonwoven fabric may be entangled
with
each other and imbedded in the second absorbable woven or knitted fabric. More
particularly, for methods other than chemical or thermal bonding, the first
absorbable
nonwoven fabric may be attached to the second absorbable woven or knitted
fabric such
that at least about 1% of the staple of the first absorbable nonwoven fabric
are exposed
on the other side of the second absorbable woven or knitted fabric, preferably
about 10-
20% and preferably no greater than about 50%. This ensures that the first
absorbable
nonwoven fabric and the second absorbable woven or knitted fabric remain
joined and
do not delaminate under normal handling conditions. The reinforced absorbable
multilayered fabric is uniform such that substantially none of the second
absorbable
woven or knitted fabric is visibly devoid of coverage by the first absorbable
nonwoven
fabric.
One method of making the multilayered fabric described herein is by the
following process. Absorbable polymer fibers, having a denier per fiber of
about 1 to 4,
may be consolidated to about 80 to 120 denier multifilament yarn and then to
about 800
to 1200 denier yarns, thermally crimped and then cut to a staple having a
length
between about 0.75 and 1.5 inch. The staple may be fed into a multiroller dry
lay
carding machine one or more times and carded into a uniform nonwoven batt,
while
humidity is controlled between about 20-60% at a room temperature of 15 to 24
C. For
example, the uniform nonwoven batt may be made using a single cylinder roller-
top
card, having a main cylinder covered by alternate rollers and stripper rolls,
where the
6
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
batt is doffed from the surface of the cylinder by a doffer roller and
deposited on a
collector roll. The batt may be further processed via needlepunching or any
other
means such as calendaring. Thereafter, the first absorbable nonwoven fabric
may be
attached to the second absorbable woven or knitted fabric by various
techniques such as
needlepunching. The reinforced absorbable multilayered fabric may then be
scoured by
washing in an appropriate solvent and dried under mild conditions for 10-30
minutes.
It is desirable to control process parameters such as staple length, opening
of the
staple, staple feed rate, and relative humidity. For example, the consolidated
yarns may
have from about 5 to 50 crimps per inch and preferably from about 10 to 30
crimps per
inch. Efficient cutting of the crimped yarns is desirable, as any long and
incompletely
cut staple tends to stick on the carding machine and cause pilling. A
preferred range of
the staple length is from about 0.75 to 1.5 inches, and preferably from about
1.0 to 1.3
inches.
To optimize uniformity and minimize the build-up of static electricity, the
relative humidity may be controlled during batt processing, preferably during
carding to
form the uniform nonwoven batt. Preferably, the nonwoven batt is processed
using a
dry lay carding process at a relative humidity of at least about 20% at a room
temperature of about 15 to 24 C. More preferably, the nonwoven batt is
processed at a
relative humidity of from about 40% to 60%.
The multilayered fabric is scoured using solvents suitable to dissolve any
spin
finish. Solvents include, but are not limited to, isopropyl alcohol, hexane,
ethyl acetate,
and methylene chloride. The multilayered fabric is then dried under conditions
to
provide sufficient drying while minimizing shrinkage.
The reinforced absorbable multilayered fabric may have an average thickness of
between about 0.5 and 3.0 mm, preferably between about 1.00 and 2.5 mm, and
most
preferably between about 1.2 and 2.0 mm. The reported thickness is dependent
upon
the method of thickness measurement. Preferred methods are the ASTM methods
(ASTM D5729-97 and ASTM D1777-64) conventionally used for the textile industry
in
7
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
general and non-woven in particular. Such methods can be slightly modified and
appropriately adopted in the present case as described below. The basis weight
of the
reinforced absorbable multilayered fabric is between about 0.05 and 0.25
g/in2,
preferably between about 0.08 and 0.2 g/in2, and most preferably between about
0.1 and
0.18 g/in2. The reinforced absorbable multilayered fabric is uniform such that
there is
no more than about 10% variation (relative standard deviation of the mean) in
the basis
weight or thickness across each square inch.
The thrombin and/or fibrinogen may be animal derived, preferably human, or
may be recombinant. The thrombin activity on the multilayered dressing may be
in the
range of about 20 to 500 IU/cm2, preferably about 20 to 200 IU/cm2, and most
preferably about 50 to 200 IU/cm2. The fibrinogen activity on the multilayered
dressing
may be in the range of about 2 to 15 mg/cm2, preferably about 3 to 10 mg/cm2,
and
most preferably about 4 to 7 mg/cm2.
The basis weight of the multilayered dressing having the thrombin and/or
fibrinogen powders is between 0.1 and 1.0 g/in2, preferably between 0.1 and
0.5 g/in2,
and most preferably between 0.1 and 0.3 g/in2. The multilayered dressing
having the
thrombin and/or fibrinogen may be sterilized, for example, by radiation,
preferably by
electron beam radiation.
The air porosity of the multilayered dressing having the thrombin and/or
fibrinogen powders ranges from about 50-250 cm3/sec/cm2, preferably between 50-
150
cm3/sec/cm2, and most preferably 50-100 cm3/sec/cm2.
When the reinforced absorbable multilayered dressing is used internally, about
50 to 75% of its mass is absorbed after about 2 weeks. The percent of mass
loss may be
measured by using a rat implantation model. Here the dressing is inserted into
the rat
by first making a midline incision (approximately 4 cm) in the skin over the
lumbosacral vertebral column of a rat. The skin is then separated from the
underlying
connective tissue, bilaterally, to expose the superficial gluteal muscles. An
incision is
then made in the dorso-lateral fascia, which is located above the gluteal
muscles and
8
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
directly adjacent to the vertebral column. Using blunt dissection, a small
pocket is
created between the fascia and the gluteal muscle lateral to the incision. The
multilayered dressing is placed in the gluteal pocket. The fascia is then
sutured in
place. After two weeks, the rat is euthenized and the multilayered dressing is
explanted
to determine the percent mass loss over the two week period.
The first absorbable nonwoven fabric retains solid thrombin and/or solid
fibrinogen powder without separation and with minimal loss of the powder from
its
surface. Thrombin and/or fibrinogen containing solutions are separately
lyophilized.
The lyophilized materials are then ground into powders using a superfine mill
or a
cooled blade mill. The powders are weighed and suspended together in a carrier
fluid
in which the proteins are not soluble. A preferred carrier fluid is a
perfluorinated
hydrocarbon, including but not limited to HFE-7000, HFE-7100, HFE-7300 and PF-
5060 (commercially available from 3M of Minnesota). Any other carrier fluid in
which
the proteins do not dissolve may be used, such as alcohols, ethers or other
organic
fluids. The suspension is thoroughly mixed and applied to the first absorbable
nonwoven fabric via conventional means such as wet, dry or electrostatic
spraying, dip
coating, painting, or sprinkling, while maintaining a room temperature of
about 60 to 75
degrees F and relative humidity of about 10 to 45%. The multilayered dressing
is then
dried at ambient room temperature and packaged in a suitable moisture barrier
container. The multilayered dressing having the thrombin and/or fibrinogen
contains
no more than 25% moisture, preferably no more than 15% moisture, and most
preferably no more than 5% moisture.
The amount of thrombin and/or fibrinogen powder applied to the nonwoven
fabric is sufficient to cover its surface such that no area is visibly devoid
of coverage.
The powder may sit mostly on top of the nonwoven fabric or may penetrate into
the
nonwoven fabric as far as the surface of the second absorbable woven or
knitted fabric.
However, the bulk of the powder does not contact the second absorbable woven
or
knitted fabric, and no more than trace amounts of the powders penetrate to the
underside of the second absorbable woven or knitted fabric.
9
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
As a surgical dressing, the multilayered dressing described herein may be used
as an adjunct to primary wound closure devices, such as arterial closure
devices,
staples, and sutures, to seal potential leaks of gasses, liquids, or solids as
well as to
provide hemostasis. For example, the multilayered dressing may be utilized to
seal air
from tissue or fluids from organs and tissues, including but not limited to,
bile, lymph,
cerebrospinal fluids, gastrointestinal fluids, interstitial fluids and urine.
The multilayered dressing described herein has additional medical applications
and may be used for a variety of clinical functions, including but not limited
to tissue
reienforcement and buttressing, i.e., for gastrointestinal or vascular
anastomoses,
approximation, i.e., to connect anastomoses that are difficult to perform
(i.e. under
tension), and tension releasing. The dressing may additionally promote and
possibly
enhance the natural tissue healing process in all the above events. This
dressing can be
used internally in many types of surgery, including, but not limited to,
cardiovascular,
peripheral-vascular, cardio-thoracic, gynecological, neuro- and general
surgery. The
dressing may also be used to attach medical devices (e.g. meshes, clips and
films) to
tissues, tissue to tissue, or medical device to medical device.
Example 1.
Poly (glycolide-co-lactide) (PGL, 90/10 mol/mol) was melt-spun into fiber. A
80 denier multifilament yarn was consolidated into a 800 denier consolidated
yam. The
consolidated yarn was crimped at approximately 110 C. The crimped yarn was
cut into
staple having a length of about 1.25" in length. 20 g of the crimped staple
was
accurately weighed and laid out uniformly on the feed conveyor belt of a multi-
roller
carding machine. The environmental conditions (temp: 21 C /55% RH) were
controlled. The staple was then carded to create a nonwoven batt. The batt was
removed from the pick-up roller and cut into 4 equal parts. These were re-fed
into the
carder perpendicular to the collection direction. After this second pass the
batt was
weighed (19.8 g: 99% fabric yield) and then compacted into a felt. The compact
felt
was precisely laid onto an ORC fabric and firmly attached via 2 passes in the
needlepunching equipment. The multilayered fabric was trimmed and scoured in 3
discrete isopropyl alcohol baths to remove spin finish and any machine oils.
The
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
scoured multilayered fabric was dried in an oven at 70 C for 30 minutes,
cooled and
weighed.
18.93 g of BAC-2 [(Omrix Biopharmaceuticals, Inc.) specific activity (by
Clauss) 0.3g/g] and 1.89 g of thrombin-containing powder (also from Omrix
Biopharmaceuticals, Inc.) were mixed thoroughly with about 420 ml of HFE-7000.
The
slurry was sprayed through a nozzle onto the multilayered fabric weighing
about 12g
and sized to 8" x 12". The multilayered hemostatic wound dressing was air
dried for
about 30 minutes. The environmental conditions were maintained at 24 C /45%
RH
throughout the process. The multilayered hemostatic wound dressing was cut
into
appropriate sizes and packed in a tray. The tray is specifically designed such
that the
clearance between the top and the bottom of the tray is slightly less than the
overall
thickness of the dressing to ensure minimized motion of the dressing during
shipping
and handling, to prevent the coated powder from dislodging during transit..
The tray is
further packaged in a foil pouch, which is thermally sealed with dessicants as
needed.
The dressing was stored at 2-8 C until needed.
The "thickness" of the multilayered fabric/dressing was measured as described
herein. The measurement tools were:
(1) Mitutoyo Absolute gauge Model number ID-C125EB [Code number-- 543-
452B]. The 1" diameter foot was used on the gauge.
(2) A magnetic holder was used to lock in place and set the caliper up to the
die
platen.
(3) Two metal plates - 2.75" x 2" x 0.60", weighing between 40.8g to
41.5g [combined total of -82.18g].
The multilayered fabric/dressing was placed on a platen surface that is a
smooth and
machined surface. The two metal plates were placed on top of each other on the
multilayered fabric/dressing and gently pressed at their corners to make sure
the
multilayered fabric/dressing is flat. The gauge foot was placed onto the top
of the metal
plates and was then re-lifted and re-placed, at which time a reading was made.
11
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
Example 2
In general, anesthetized pigs were dissected to expose the abdominal aorta. A
biopsy punch was used to remove a 4 mm section of the aorta. The blood was
allowed
to flow freely, and the dressing to be tested was quickly applied to the wound
site while
aspirating any excessive pooling blood. Manual pressure was applied to hold
the
dressing to the wound site for 3 minutes. At the end of the three-minute
period,
pressure was removed. The test was considered a "pass" if the dressing adhered
well to
the wound and achieved full hemostasis with no re-bleeding.
Hemostatic
Thrombin Fibrinogen Performance-
Activity Activity Porcine Aortic
Sample ID (IU/cm2) (m cm) Punch
1 -50 4.86 Pass
2 -50 6.23 Pass
3 -50 5.36 Pass
4 -50 5.49 Pass
5 -50 6.19 Pass
6 -50 7.80 Pass
7 -50 7.90 Pass
8 -50 6.77 Pass
9 -50 6.97 Pass
10 -50 3.31 Fail
11 -50 5.99 Pass
12 -50 5.89 Pass
13 -50 8.52 Pass
14 -50 7.11 Pass
-50 11.07 Fail
16 -50 12.47 Pass
17 -50 8.43 Pass
18 -50 11.77 Fail
19 -50 8.61 Fail
-50 8.70 Pass
21 -50 8.52 Fail
22 -50 6.50 Pass
23 -50 6.68 Pass
24 -50 9.13 Fail
-50 7.68 Pass
26 ~50 6.59 Pass
27 -50 7.03 Pass
28 -50 7.55 Pass
12
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
29 -50 6.85 Pass
30 -50 5.0-10.0*** pass
31 -50 5.0-10.0*** pass
32 -50 5.0-10.0*** pass
33 -50 5.0-10.0*** pass
34 -50 5.0-10.0*** pass
35 -50 5.0-10.0*** pass
36 -50 5.0-10.0*** pass
37 -50 5.0-10.0*** fail*
38 -50 5.0-10.0*** fail*
39 -50 5.0-10.0*** pass
40 -50 5.0-10.0*** pass
41 -50 5.5 - 7.5 pass
42 -50 5.5 - 7.5 pass
43 -50 5.5 - 7.5 fail*
44 -50 5.5 - 7.5 fail*
45 -50 5.5 - 7.5 fail**
46 -50 5.5 - 7.5 pass
47 -50 5.5 - 7.5 pass
48 -50 5.5 - 7.5 pass
*Failure occurred due to inadequate aspiration of pooling blood at the
puncture site
** Failure occurred due to inadequate aspiration of pooling blood at the
puncture site as a result of suction
hose failure
*** Targeted range during production
All animals were euthenized after conclusion of the test, except for Sample ID
46 and
47, which survived for at least 2 weeks post surgery.
Example 3
Non-woven PGL fabric with ORC reinforcement fabric.
Poly (glycolide-co-lactide) (PGL, 90/10 mol/mol) was melt-spun into fiber. The
fiber was cut into small staple and then carded to create a very fine nonwoven
fabric of
about 1.25 millimeters thick and had a density of about 98.1 mg/cc. The
nonwoven
fabric was then needle punched into a knitted carboxylic-oxidized regenerated
cellulose
fabric, available from Ethicon, Inc., under the tradename Interceed , to
secure the
nonwoven fabric to the ORC fabric. The final construct comprised about 60
weight
percent of the nonwoven fibers.
Example 4
13
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
Analysis of adhesive/sealant properties of samples coated with fibrinogen and
thrombin
The material described in Example 3 was coated with dry particles consisting
mostly of
fibrinogen (7 to 8 mg/cm2) and thrombin (501U/cm2), and then tested using a
Hydraulic
Burst Leak Test (HBLT). Samples were cut into circular pieces of 3/4 inch
diameter.
The samples were placed onto a tissue substrate derived from bovine
pericardium with
a hole in the center of the tissue. The pierced tissue substrate was placed
over an
airtight chamber into which saline was pumped. The pressure required to
disrupt/burst
the seal formed between the tissue and the sample was measured (see Figure 1).
Samples without protein coating do not adhere to the tissue.
Example 5. Poly (glycolide-co-lactide) (PGL, 90/10 mol/mol) was melt-spun into
fiber. A 80 denier multifilament yarn was consolidated into a 800 denier
consolidated
yarn. The consolidated yarn was crimped at approximately 110 C. The crimped
yarn
was cut into staple having a length of about 1.25" in length. 44 g of the
crimped staple
was accurately weighed after conditioning the yarn for about 30 minutes in a
high
humidity environment (>55% RH). The yarn was laid out uniformly on the feed
conveyor belt of a multi-roller carding machine. The feed time (5minutes) was
accurately controlled to within 30-45 seconds. The environmental conditions
(temp:
21 C /25% RH) were recorded. Static bars were employed near the 2nd Randomiser
roller as well as near the steel pick up roller and were turned on during the
run to
minimize the detrimental impact of static generation on the uniformity and
yield of the
resulting batt. The staple was then carded to create a nonwoven batt. Two
vacuum
inlets were strategically placed near the two edges of the 2nd Randomizer
roller to
control the width of the ensuing batt. The batt was removed from the pick-up
roller and
weighed (41g: 91% yield). The uniform batt was precisely laid onto an ORC
fabric
and firmly attached via a single pass in the needlepunching equipment. The
needle
penetration depth was controlled at 12 mm. The multilayered fabric was trimmed
and
scoured on a rack (along with other similarly produced sheets) suspended in a
tank
containing isopropyl alcohol to remove spin finish and any machine oils. The
scoured
14
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
multilayered fabric (matrix sheet) was calendered to remove excess solvent and
dried in
an oven at 70 C for app. 30 minutes, cooled and weighed.
Example 6.
The matrix sheet as described has an off-white/beige color on both sides. One
side may
be described as the non-woven side where as the other side as the knitted
fabric side.
For certain application, it may be vital to identify the non-woven versus
knitted surfaces
of the matrix. Under difficult environmental conditions, the similarity in
color and
texture (to some extent) makes it difficult to identify one side from the
other. Several
means were employed to impart sidedness to the matrix sheet, which enables the
observer to distinguish the 2 sides apart. These means include physical
(stitching/knitting, braiding, pleating, etc), thermo-mechanical (heat, heat
embossing;
laser etching; etc) and chromic (use of a dye) means may be employed to
achieve
sidedness. The following examples describe some of the means:
6a) The matrix sheet was modified on the knitted fabric side by attaching a
lmm wide 4
inch long braided tape of the polyglactin 910 fiber. The tapes although
successful in
imparting sidedness add to the amount of the longer resorbing Polyglactin 910.
6b) A web made of dyed nylon fiber was placed under the knitted fabric and the
non-
woven batt during the needle-punching step. The web is secured to the knitted
fabric
side due to the needling process. The web affords excellent sidedness and if
available
in an absorbable material, could be used to make completely resorbable,
implantable
matrix sheets. The web (mesh) can be secured similarly on the non-woven side.
Other
means of securing the web may be thermo-mechanical in nature. Inclusion of
such a
web can be for the reason of mechanical enforcement as well. In such cases the
web
could be secured on either side or even between the two layers. Such a
reinforced
structure may have multiple applications.
6c) The small amount of Polyglactin 910 that resides on the knitted fabric
side (due to
the needle-punching step) of the matrix sheet can be thermally modified to
create
sidedness. This can include heating under pressure such that a shiny film of
Polyglactin
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
910 is formed. Other options include heat embossing a discernible pattern.
Both
approaches achieve sidedness but may result in thermal degradation of the
polymer/construct
6d). The knitted ORC fabric, prior to the needle-punching step is pleated
(vertical or
horizontal pleats). The pleats are stabilized by using heat and pressure. The
pleated
fabric is then used in place of the regular fabric for the rest of the process
as described
in Example 5. The resulting matrix sheet has distinct stripes that achieve the
sidedness.
6 e) Dyed Polyglactin 910 creates matrix sheet that is colored on the non-
woven side
and off-white/beige on the other. This construct achieves sidedness. A dye can
be used
similarly by employing a dyed suture thread etc. on the knitted side. The
suture
(braided into a tape or used as is) may be sewed in or thermally bonded.
16
CA 02649081 2008-10-10
WO 2007/117237 PCT/US2006/013282
While the examples demonstrate certain embodiments of the invention, they are
not to be interpreted as limiting the scope of the invention, but rather as
contributing to
a complete description of the invention. All reinforcement fabrics described
in the
examples below are the nonsterile materials of the corresponding commercial
products
referred by their tradenames.
17