Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 332
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 332
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1
SYSTEMS AND METHODS FOR
OBTA.INING, STORING, PROCESSING AND UTILIZING
IMMUNOLOGIC AND OTHER INFORMATION OF AN INDIVIDUAL OR
POPULATION
CROSS-REFERENCE TO RELATED APPLICATIONS: .
This application is a continuation-in-part of, and hereby fully'incorporates
herein by reference,
United States Utility Patent Application.Serial No. 11/255,161, filed. on
October 18, 2005 ("the
Immunologic Informatics Patent"), which was published on September 28, 2006 as
United States
Patent Application Publication No. 2006/0218010 Al. Additionally, this
application claims the
benefit of United States Provisional Patent Application Serial"No.
60/796,266,=filed on April 27,
2006.
TECHNICAL FIELD:
The present invention relates to individualized health care, immunology and
medical inforrnatics,
and more particularly to systems and methods for acquiring, storing,
processing and utilizing
immunologic and other iniormation of individuals and populations.in various
applications.
BACKGROUND OF THE INVENTION:
Personalized medicine is considered by many to be the wave of the future. A
personalized
medicine approach seeks to identify whether a given individual needs a given
treatment or
intervention prior to adtininis'tering. it, rather than relying on "standards"
representing an average
person in a group or population.
This approach is based on the well known fact that some individuals in a
demographic
. , . population have naturally low or naturally high =values which are not
best measured against a
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
2
statistical mean for the demographic population, but against that individual's
own measured
history: For example, vaccines are a immunologic prophylactic whose frequency
and dose is determined
at the population level. Vaccines are approved for routine human use by
regulatory agencies
from different countries where the vaccines are to be applied, such as,
for.example, the U.S. the
Food and Drug Administration (FDA). After approval, population-wide
recommendations for
use are made by various medical agencies, such as the Advisory Committee on
Immunization
Practices (ACIP), whose members represent experts in.the vaccine field. The
ACIP is a U.S.
committee, to assist and advise the Secretary of Health and Human Services, as
well as the.
Centers for Disease Control and Prevention (CDC), on how to best implement
vaccination
strategies to prevent disease. Written recommendations are developed with
immunization
schedules that are published and updated as needed for both pediatric and
adult populations.
From these recommendations, certain vaccines are, mandated=for school entry
and -government-
sporisored programs.
Although such maiidated schedules are the norm, the need for them varies
across populations.
They *represent an a priori approach that does not take into account
individual specifics.
Immunity to disease wanes over time, but may be maintained'at low levels or
recalled, through.
immunologic memory, upon subsequent exposure.to the corresponding infectious
agent or. cross-
reactive antigens in the environment. For inactivated or subunit-based T cell-
dependent
vaccines, however, protective immunity may not last beyond 10 years.
For example, the protective responses to diphtheria, tetanus, and pertussis
vaccines (DTaP, Td)
have been shown to be absent after about 10 years, which is why Td (tetanus
and diphtheria)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
3
boosters are recommended every 10 years. For Tcell-independent vaccines, such
as
prieumococcal and meningococcal polysaccharides, there is no immunologic
memory; and
i.mmunity-may be gone in only 3 to 5 years. The result of these facts is that
vaccinating everyone
according to a standard protocol cari often result in either under-vaccinating
or over-vaccinating
in various individual cases.
Result of Over-vaccinating: Type III Hypersensitivity Reactions
Thus, while generalizations about the timing for boosters, whether at 3 or 5
or 10 years,'
represents one approach to the problem of maintaining long=term immunity,
other problems can
arise if,the duration of imrriunity does not follow- the expected pattern. For
example, it is well=
known that Tetanus boosters for adults (such as; for example, those
administered in emergency'
.rooms to prevent tetanus after someone steps on a rusty nail),, often lead to
local adverse '
reactions at the injection site; particularly if the. last booster was not too
many years earlier.
Because it may be difficult to determine when the last immunization was
received for tetanus,
health care providers tend to err on the side of caution by boosting.
While this general boosterapproach may readilyprevent tetanus, the possibility
of high levels of
circulating antibodiesmay lead-to an Arthus reaction, which is a local type
IIl'hypersensitivity
reaction due to the-development of immune complexes composed of IgG antibodies
and the
vaccine antigen. The immune complexes activate complement which binds to
complement receptors on the mast cells to cause the release of granules and.
increased vascular permeability.
This can ultimately lead to 'tissue damage. In an extreme case, a more
generalized or systemic
reaction can occur,where immune complexes are deposited in the kidneys and
joints, leading to
ai-thritis and glomerulonephritis. Subsequent,cellular immune responses and
tissue damage with'
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
4
respect to the glomerulus can lead to permanent 16ss.of kidney function. -The
CDC has recently.
noted that certain vaccines *produce increased rates of local or systemic
reactions in certain
recipients when administered too frequently, and that such reactions are
thought to result from
the- formation of antigen-antibody complexes (Centers for Disease Control and
Prevention,
General Recommendations on immunization: Recommendations of the Advisory
Coinmittee on
Immunization Practices and the Amer'icari Academy of Family Physicians.
11I1ViWR 2002;
51(No. RR-2):1-36):
A solution for the prevention of such a type III hypersensitivity
problem=resulting from over-_
vaccination would be to assess 'a person's immune status with respect to the
offending antigen,=
and rnake an existential determination of when to optirnally. administer the
vaccine booster: For
example, concerning vaccinations for interriationally adopted children
of=unknown immune
status, the CDC states: "If avoiding unnecessary injections is desired;
judic.ious use of serologic
testing might be helpful in determining which inununizations are needed."
Regarding DTaP
vaccinations specifically, the CDC also states:, "If a revaccination approach
is adopted and a
severe local reaction occurs, serologic testing for specific IgG antibody to
tetanus and diphtheria
toxins can be measured before administering additional doses.?' (see pages 20
and 21 of the
CDC 2002 reference cited above.) In this way, serologic testing could be used
to determine =
whether an antibody level is low enough to warrant further boosting of the
immune system for a
specific antigen, minimizing the risk of adverse reactions from over-
vaccinations.
Result, of Under-vaccinating: Increased Suseeptibility to Infection
Certain individuals'may be genetically predisposed to infections.as a result
of a compromised
irnmune system. For example, there are people that have been identified to be
at greater risk =of
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
meningococcal disease due to late-stage complement deficiency, since
complement usually
mediates aiitibody-dependent killing of meningococci. Others have been shown-
to be
. ~ . .
susceptible to a variety of diseases (e.g:, leprosy, salmonellosis,
Pseudomonas aeruginosa
infections, Yersinia infections, Listeria monocytogenes infections,
streptococcal diseases,
tuberculosis, Lyme disease, Chlamydia trachomatis infections,
Helicobacterpylori infections,
HIV disease, and various other viral infections) that appear to be correlated
to a different HLA .
haplotype. Still others have been shown to have increased susceptibility
to.certain diseases (e.g.,
Haemophilus influenzae type B meningitis in Eskimos, Apaches, and Navajos)
because their
immune systems respond with a less effective antibody repertoire based on
variable-region,gene
haplotypes. A.solution to this susceptibility problem would be to screen
people for the
appropriate'biologic or genetic markers and vaccinate accordingly.
Vaccinations would help to
enhance the aompromised immune systems with lugher levels of specific
antibodies that could
enable other immune mechanisms (e.g., opsonophagocytosis instead ofcomplement-
mediated
lysis), overcome low antibody avidity with greater antibody numbers, or alter
the relative
balance of antibody repertoires. In addition, continuous serologic testing
(e.g., annually) of the
immune status would allow for optimum timing of vaccinations to counter the
relentless waning
of iminunity over time while still avoiding the potential problems of over
imniunizing.
Determination of the immune status of individuals to, for example, vaccine-
preventable- diseases
requires an assay system that can detect antibodies that may-be present at
very low levels, -
especially when natural or vaccine exposure may have been many years
previouslyAn addition,
such an assay system could be used more generally to.assess an individual's
immune competence
at different stages.of that individual's life, a$ well as to also measure the
vaccine status of
individuals with varying special needs and requirements (e.g., military
personnel or travelers).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
6
What is thus iieeded in the art is a system and method for measuring and
processing
immunologic information of individuals and populations through various points
iii-time of their
lives so as to better track each individual's immune status' and make
appropriate diagnostic, .
prophylactic and therapeutic -recommendations.
.What is further needed in the art .is a supporting structure to conveniently
store the resultsof such
screenings for easy access and processing, for data mining purposes as well as
for use in a
variety of commercial, research and governrnental applications where. a
kriowledge of the
immunological indicia of customers, subjects and citizens can create
efficiencies and
optimiza.tions, as well'as allow for the exploitation of commercial
'opportunities and improve the
quality of life. '
SUMMARY OF T.HE INVENTION:
A system and method for assessing the immunological status of one or more
individuals in a=
patient population is presented. The method includes establishing a'database
comprising a
plurality of records of inforinatiori each representative of the immune status
6f an individual in
the population; each of said records including (1) current information from
one or more assays,
for the presence of a biochemical, and (2) individual specific information
comprising one or
more of said. individual's medical history, said individual's doctors'
observations and historical,
demographic, lifestyle, and familial information relating to said individual.
The method further
includes processing the information in said database to find trends or
patterns relating to the
immune status of individuals in said patient= population; and using the said
trends or patterns as
part of a health care related decision making process. In exerimplary
embodiments of the present '
invention, processing the information in the database includes generating a
list of correlations
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
7
between variables or fields in the database, and for each correlation in t:he-
list =gerierating a set of -
hypotheses that may explain said correlation. In exemplary embodiments of the
present
invention, as to each hypothesis in the set, automatically refuting,:
supporting or stating that there
is insufficient data to analyze said hypothesis by further processing of the
database; and reporting
the correlations, their associated hypotheses and the refutation, support, or
determination of
insufficient data to refute or support, to a user.
BRIEF DESCRIPTION OF THE DRAWINGS:
Section I Figures
Fig. 1 depicts a generalized exemplary process flow according to exemplary
embodiments -of the
present invention;
Fig. 2 depicts an exemplary system overview according to exemplary embodiments
'of the
present invention;
Fig. 2A depicts an alternate'exemplary system overview according to exemplary
embodiments of
the present invention;
Fig. 2B depicts yet another alternate exemplary systerri overview according to
exemplary
embodiments of the present invention;
Figs. 3 and 4 depict various exemplary configurations for assaying a patient
sample according to
an exemplary embodiment of the present invention;
Fig. 5 depicts a detailed system diagram according to an exemplary
embodirrient of the present
invention;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
8
Fig. 5A depicts a detailed system diagram according to an alternate exemplary.
embodiment= of
the present.invention;
Fig. 5B depicts exemplary process flow for automimmune disease screening and
follow-up
according to an exemplary embodiment of the present=invention;
Fig. 5C is an exemplary bar graph depicting CMV prevalance by age group in
Australia;
. Fig. 5D illustrates mechanisms of downregulation or attenuation of immune
response;
Section lI Figures
Fig. 6 depicts exemplary assay results in an exemplary database according to
the present .
invention;
Fig. 7 depicts exemplary diagnostic module recommendation types according to
an exemplary
embodiirient of the present irivention;
Fig. 8 illustrates an exemplary perceptron network which impleinents'a rule
for a normal
individual using as inputs the results of an exemplary menigicoccal diagnostic
panel;
Fig. 8A=illustrates the exemplary perceptron network of Fig. 8 implementing a
sirnilar.rule for an
abnormal individual;
Fig. 9 depicts an XML representation of the exemplary perceptron networks of
Figs. 8 and 8A;
Fig. 10 depicts an exemplarysymbology for diagnostic.goals which can be used
to articulate
diagnostic goals in an exemplary embodiment of the present invention;
Fig. 11 illustrates exemplary diagnostic goals using the symbology of Fig. 10;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
9
Fig. 12 illustrates an exemplary database schema for patient information
according to- an
exemplary =embodiment of the present invention;
Fig. 13 illustrates an exemplary database schema for visit information
according to an exemplary
embodiment of the present invention;=
Fig. 14 illustrates an exemplary database schema for test results according to
an exemplary
embodirnent of the present invention;
Fig. 15 depicts exemplary patient age intervals used in an exemplary database
according to an
exemplary embodiment of the present invention;
Fig. 16 is a.plot of an exemplary female antibody comparison over a number of
years according
to an exemplaryembodiment of the present invention.
Fig. 17 is a plot of an exemplary comparison of two individual females, one
vaccinated and one
not vaccinated, according to an exemplary embodiment of the present invention;
Fig. 18 is a plot of exemplary antibody levels in a compliment-deficient
individual according=to
an exemplary embodiment of the present invention;
=Fig. 19 is a plot of exemplary antibody levels in a healthy individiual
according to an exemplary
embodiment of the present invention;
Fig. 19A= is an example SQL query according to an exemplary embodiment of the
present
invention; and
Fig. 19B is a table illustrating the correlation among antibody levels in an
exemplary female
population according to an exemplary embodiment of the present invention;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
Figs. 20 through 20F illustrate.exemplary data mining results obtained -from'
operating on ari
exemplary database according to an exemplary embodiment of the present
invention;
Figs. 20G1 through 20G41 illustrate additional exemplary data mining results.
obtained from
operating on an expanded version of an exemplary database according to an
exemplary
embodiment of the present invention;
Fig. 21A.illustrates an exemplary pattern detection process flow according to
an exemplary
embodiment of the present invention;
Fig. 21B illustrates an exemplary pattern detection process flow with
hypothesis generation
according to an exemplary embodiment of the present invention; '
Fig. 21 C illustrates an exemplary automatic pattern detection process flow
according to- an
exemplary embodiment of the present invention;
Section III Figures
Fig. 22 is a process flow diagram for use in a healthcare management
'embodiment according to
the present invention;
Fig. 23 is a subset of the process flow depicted in Fig. 22;
Fig. 24 is an alternative process flow chart for healthcare management
according to the
exemplary embodiment of the present invention;
Fig. 24A is a more detailed process flow chart similar to that of Fig 22;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
11
Fig. 25 is.an alternative process flow chart for managing healthcare according
the=exemplary
embodimerit of the present invention;
Fig. 25A is the process flow chart of Fig. 25 with an additional optional
element;.
Fig. 26 is an alternative prooess flow chart for managing healthcare according
to the exemplary
embodiment of the present invention;
Fig. 26A is an alternative version of the process flow of Fig. 26 with greater
detail;
Fig. 27 is a process flow chart for cervical cancer prevention according to
the exemplary
embodiment of the present invention;
Fig. 28 is a process flow chart for managing the care of women of childbearing
age according to
the exemplary einbodiment of the present invention;
Fig. 29 is a process flow chart for an exemplary "Vaccine-O=Mat" application
according to an
exemplary embodiment of the present invention;
Fig. 29A is a system diagram of entities involved in the vaccine distribution
application
according. to an exemplary embodiment of the present invention;
Fig. 29B illustrates the necessary connectivity for the vaccine distribution
application illustrated
in Fig: 29A;
Fig129C is the connectivity displayed in that Fig. 29B recastby use of an
interapplication
connectivity provider according to an exemplary embodiment'of the present
invention;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
12
Fig. 30 is an exemplary flow chart for use in a life insurance optimizatioin
applicatiori according
to an exemplary embodiment of the present invention;
Fig. 31 is an exemplary process flow chart for use in an immunosenescence
management
application according to an exemplary embodiment of the present invention;. =.
Fig. 32 is an exemplary process flow chart for a disaster management
application according to an
exemplary embodiment of the present invention;
Fig. 33 is an alternative process flow chart for the psychological. aspects of
disaster response for
a disaster response application according to an exemplary embodiment of the
present inverition;
Fig. 34 depicts exemplary process flow in an immunogenicity discovery
application according to
an exemplary embodiment of the present invention;
Tig. 35 illustrates components of an exemplary two-sided market application
according to an-
exemplary embodiment of the present invention; and
Fig. 36 illustrates components of an exemplary drug hypersensitivity two-sided
market
application according to an exemplary embodiment of the present invention.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
13
Table of Contents
Page
SECTION I EXEMPLARY ASSAY PANELS
.:............................................................. 25
A. COLLEGE'STUDENT DIAGNOSTIC PANELS ................................:....:..
33
1. Meningococcal Diagnostic Panel ......
.........................................
............... 33
2. Sexually Transmitted biseases Assay. Panel.................................
.............:.. 36.
B. Pers'istent Immunity Induced by Childhood Vaccines
.............................. 37
C. ADULT DIAGNOSTIC PANELS ..............
:.......................................
.......... 39
D. Measurement of Immunity Induced By Vaccines for Military Personnel... 39
E. lmmunoScore Measurement of Vaccine-Induced Immunity for Travelers 43
F. ImmunoScore Measurement of Immunity in Health Care Workers ....:..... 45
1.1. Immunocompromised Health-Care Workers .................... * 66
5. ImmunoScore Analyses and Bioterrorism .................................
........ 71
6. ImmunoScore Analyses for Infection'and Chronic Disease
..................... 86
.7. Th 1-Th2 Paradigm
............:..........:.......................................................
.... 93
2. 7.1 Thl-Th2 Based Diagnostic Panel
......................................... 97
3. Autoimmunity/Inflammatory Disease ..........
............................... : 108
9. ImmunoScore Diagnostic Panel and Preventive Therapy for
Autoimmune Disease ..........:.. ..- 118
..................................................
10: 10. ImmunoScore Diagnostic Panel: Aging, Longevity, Cancer and
Human Cytomegalovirus
...........:....................:............................ 145
SECTION II EXEMPLARY IMMUNOSCORE SYSTEM DATABASES :............:........172
A. General Overview .....:......... 172
...............................................................,,,,
B. Exemplary Illustrative Database
....................,......................................... 180
1. Overall Description
........................................................ :............. 180
2. Impact of Data Minirig ..........:
.................................................... 185
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
14
Table of Contents
(continued)
Page
. . ~ . . . .
3. Diagnostic Module .................................
........................................ 1 . 88
11.. Overview .. .. .. ......... ...... .... ..... ......
....................................... 188
3.2. Perceptron algorithms
...................................................... 191
3.3... Alternate-algorithmic approaches ...........................
............. 195
3:3.1. Additional input data ..............:................:.....:..:.....
196
3.3'.2. ' Decision rule algorithms ..........................................
197
4. Data Mining Module ...................................................
................. 197
.4.1. Overview ............................................ 197
..............................
4.2. Sample Data..:.........
..:..:......:.....:.......:........:............... ..:... 198
4.3. Exemplary Use of the Patent Event Database ........ :...... ... 201.
C. Exemplary Canadian Immigrant Project Database Used To Illustrate Data
Mining and Hypothesis Generation................
.................. ........................ 203
D. Data -Mining - Analyses and
Conclusions................................................. 212
-1. Linear regression analysis - correlation coefficients .................:.
212
2.. Geometric mean values
................................................:...:......... 213
3. Percent support between
variables...................:......................... 215
4. Possible Conclusions..............................................
......:...:......... 216
5. Expansion of database
.....:.......................................:..........:....... 219
6. Distribution of geometric means according to age.:................... 220
7. .'Focus on CMV and Tetanus
.....................:.......:....:.:...........::...... 221
8. Simulation: Sampling over time ......................................
. . . . . .......... 222
E. Pattern Detection and Hypothesis Generation
.............:.......................223
SECTION III USES OF IMMUNOSCORE INFORMATION IN VARIOUS ~
COMMERCIAL, RESEARCH AND GOVERNMENTAL CONTEXTS ..... 236
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
Table of Contents
(continued)
PWe
A. Health Insurance Underwriting ad Management - Healthy Credits
Exchange ......... . ...............................
.................................................:. 236
B. Health Care/Health Insurance Credit Exchance
.............................:...... 248
C. Veterans Health. Care Management (Variant of Health Care) ................
252
D. Socialized Medicine Management
..........................:.............................. 253
' E. Supplemental Insurance (AFLAC Model) ........................ * 254
F. ImmunoScore and the Weliness Industry
...............................:...:.......... 256
G. Women of Childbearing Age/Screeriing of Pregnant Women .................
260
H. Vaccine-o-Mat/Vaccine Distribution Network
....:.................................:.. 262
1. ~ Consumer Accessibility to Immunologic Information
............................:. 266
J. Immunoscore Connectivity Via lnterapplication Translator/Data Integrator267
K. Immunologic Informatics Based Life Insurance Underwriting ................
268
L: Diagnosing and Managing lmmunosenescence in the Elderly ............... 271
M. Frozen Storage of Naive Immune Cells (IRP Considerations) ........:......
283
N. Vaccine Use Outcome/Design ..........................
................:................. 285
0. Research Services
..............................................................:................
.. 286
P. Immigration Consulting
...........................................:...............................
286
Q. Disaster Survivors: Immunizations, Recovery, Prognosis and Treatment290
R. Monitor Adoptive Immunotherapy/Transplants ........
.......................... :.... 293
S. Elective Surgery
......................................................:..:.:..:.....:.........:
...... 293
T. Services to Charitable Foundations Promoting Immunological Well Being294
U. Discovery of Unwanted Immunogenicity of Therapeutics ..........:...........
295
V. Two-Sided Market Applications
.....:.........:.............................................. 298 *
W. Drug Hypersensitivity
..................................:............................... .........
311
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
16
Table of Contents
(continued)
Page
X. Health Care Transparency and Competition
....:..........................:......... 321
1. Consistent high quafitY ............................................ .
:....:................ 322
2. Lower cost - follows from high quality. Higher quality is often
naturally less expensive. Providers improve quality by honing'their'
organizational processes to becorrie more efficient and effective -
to avoid.error and to do things right the first time . .........
::.............. 322
3. Available to all = for ethical, political, systemic, and business.
reasons, health care must be available to everyone . .................. 322
4. Single model every provider in the system must compete to offer
the best product at the- best price ................................
................
5. Shaped by market forces - the consumer market has tlie sustained '
systemic power to bring consumers more for less . .................:... 322
6. Practical - the solution must arise from.present realities. .:......:.. 322
7. Progressive - dramatic change can not occur all. at once. .......... 322
8. Self-reinforcing - as any part of the health care system moves
toward a new reality, that movement must allow and encourage
other parts to move forward as well .
........................................... 322
Y. User Acces's Via Data Networks and On-line Advertising ...::....... ... 332
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
17
Applicant notes that the TOC is defective, especially as regards Section I.
Applicant reserves tlie
rught to amend the Specification to correct the TOC via Pieliminary Amendment.
DETAILED DESCRIPTION OF THE INVENTION:
General Overview
In what follows, systems and methods of the.present invention will be often
referred to as the
"ImmunoScore" system, method and/or database, as the case may be. "ImmunoScore
" is a
trademark and/or service mark currently envisioned by the assignee hereof to
be utilized in
connection with exemplary embodiments of the present invention. '
The present invention is directed to the collection, processing, and use of
immunologic
information. Immunologic information is to be.understood in a broad sense,
including any
information which may be useful as an indicator of any immunological function
of a mammalian
body. More specifically, the present invention includes acquiring information
that is indicative
of the immune status of an individual, processing that information, storing
the raw information as
well as the outputs from the processing stage, and of that information at
various times and in
various ways to recommend various actions such as prophylactid or fu.rther
diagnostic
interventions, or abstention froin action, for individual or population. The
present.invention
exploits a number of advances in technology as well as advances in how people
think about
medical treatment. In exemplary embodiments of the present invention, a number
of
immunological or immunological related (in a broad sense) assays can be
administered to an
individual. Using modern technology such as; for example, the M1M Analyzer
marketed by BioVerisTm Corporation of Gaithersburg, Maryland, one can run a
large number of assays, such
as, for example 20, 40 or 60, and obtain results therefroin in a relatively
short period of time. .
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
18
Moreover, these assay results can be stored in a memory, either locally or at
one or more central
servers or in associated databases, and can be operated upon by various
algorith&s or rules
which can generate information as to that individual's immune status as well
as
recommendations for further augmenting that immune status or taking further
action in response
to the information acquired, from the assays and their processing. This
information can be used
in a variety of commercial, research, and healthcare contexts. Thus a variety
of business
methods or opportunities can be created or facilitated using the information
obtained according
to the methods of the present invention.
The present invention is described in three distinct sections. The first
section describes the.
scientific background and motivation for creating various assay,panels to be
administered,
singularly or in combination with other assay panels, to different individuals
in different
populations at different times in each individual's life cycle. This
diseussion culminates in
suggested or exemplary assay super panels which can be administered in various
contexts to
various individuals.
A second section describes how information obtained from results of the
administered assays can
be stored, processed, and utilized. This discussion comprises, inter alia, a
description of an
exemplary database in which (i) results from numerous assays.canbe stored
along with (ii)
individual-specific information and (iii) the outputs of"various algorithms
which operate upon the
assay results of that individual. This section also presents an exemplary
database upon which
immunologic data mining was performed according to the techniques of the
present invention,
and summarizes interesting and illustrative results form that exercise.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
19
In a third and fmal section, a variety of business and commercial methods are
described in which
information from the assay panels as stored in the database anand further
processed can be used to
increase business efficiencies, create new markets and opportunities, and/or
provide useful tools
for research and development.
Before describing each of these three areas in detail, a brief overview of a
generalized method
and system according to exemplary embodinients of the present invention is
presented with
reference to Figs. 1, 2, 2A and 2B.
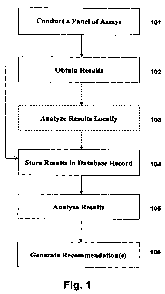
Fig. 1 depicts an exemplary process flow according to an exemplary embodiment
'of the present
invention. Beginning at 101, an assay or panel of assays can be conducted on a
biol'ogical
sample, e.g., blood, urine, eto., which has been taken from an individual.
Such individual can
.simple be an individual or he or she can be a member of a population or sub-
population whose '
immunologic informatics are of use to some entity or enterprise. For example,
the individual
could be an insured of a health insurance company that is using the techniques
of the present
invention to efficiently manage the healthcare of its insureds so as to
fninimize costs. Or,.
alternatively, such an individual could be an immigrant whose vaccination
history is unknown
.but whose immune status is of interest to his new country's irnmmigration
service: Such
exemplary'embodiments are described more fially below in Section III.
In Fig. 1, at 102 the results of the assay or assays conducted at 101 can be
obtained, and at 103
there can be an optional step of analyzing the assay results locally. In
exemplary embodiments
of the present invention assays can be conducted and read in a variety of
assay readiing devices.
There are many assays available using known techniques. Some of them are more
sophisticated
and some less sophisticated. In exemplary embodiments of the preserit
invention, an assay
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
reading device can, for example, obtain results at'102, store those results
and analyze them
locally, for example, in a processor communicably connected to the assay
reading device.
Alternatively, if only raw assay results are obtained from a less
sophisticated technology, those
results can, for example, be sent over a data network and stored in a database
record. This is
illustrated at 104. At 105, the results can be analyzed by accessing the
particular record
associated with the particular individual to whom the assay panel oi panels
were administered at
a given time. Such analysis can involve a variety of algorithms ranging from a
simplistic look at
quantity of antibodies per defined unit of blood or other bodily fluid, or it
can also, for example,
include a complex analysis where a variety of assay results are input and
combined in linear and
non-linear ways to produce some metric of immunologic significance. Such
algorithms are
described more fully below in Section II. Finally, 'at 106, based on the
results of the above described analysis, recommendations can be generated.
Such recommendations can include, for
example; that the individual obtain one or more vaccines, that the iridividual
be administered
prophylactic therapies to boost his or her immune system, or that the
individual be administered
gene therapy to correct the genetic defect which places him or her at risk of
communicating a
certain disease or condition, to name a few.
In general, in many exemplary embodiments according to- the present invention
process flow will
be equivalent to or substantially similar to the process flow depicted in
Fig..1. In each of those
exemplary embodiments, one or more panels of assays can be conducted with
respect to one or
more individuals. Results can be -obtained, stored and analyzed, and based on
such analysis,
recommendations for action (or inaction, such=as, for example, in cases of
over-vaccination, as
described above) can be recommended.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
21
Fig. 2 is an exemplary generalized system diagram which correlates to the
generalized rnethod
depicted in-Fig. 1. With reference to Fig. 2, there can be seen a number of
assaydevices 201.
These assay devices include one or more assay panels which have been conducted
with respect
to an individual or individuals and for which results have been obtained. The
results obtained
from the assay devices can, as described in connection with the generalized
method in Fig. 1, be
locally analyzed at each assay device, provided that such assay device has a
data processor and
memory and the results can be stored locally at the, assay device.
Alternatively, the assay-device
results can, for example, be communicated over a data network 202 to a central
processor 204
and stored in a central database 203. The central processor 204 can access the
records which it
hasreceived and analyze them by implementing a number of analytic algorithms
as described
more fully below.
Central processor 204, based on its analysis, can generate recommendations
based on decision
trees and'criteria embedded in the various analytic algorithms it implements.
These
recommendations can be displayed locally at the central processor at display
205 and caft there
be printed in a tangible medium for distribution to interested persons.
Alternatively, the -central.'
processor 204 can, for example, send the results of its analysis over a data
network to various
users who can access the results at user terminafs 210.
Fig. 2A presents an alternative generalized system diagram similar to Fig. 2.
However; as can be
seen in Fig. 2A, there is an additional database, the business rules database
220, communicably.
connected to central processor 204. In such an exemplary system the central
processor can,
implement algorithms to operate on stored assay data which can, for example,
also take as inputs
various business rules in generating a decision regarding a recommendation.
For example, as
described more fully below in Section III, an exemplary embodiment of the
present invention
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
22
can be utilized to help a health insurance underwriter manage its populatiori
of insureds: There .
can, for example, be an asuiual or semi-annual requirement of all insureds to
have assays for
various immunological compoiients. conducted on their blood or other bodily
fluids. After
analysis of the results of such assays, an insurance company can determine
whether a particular=
insured is susceptible to one or more given diseases or o=ther ailments which
would result irn
increased expenditures for medical treatment. The insurance company could then
decide if it
was not more economical to require the insured to undergo certain prophylactic
treatmeints, such
as, for example, vaccines or inimune system boosting therapies, etc., where
the cost of such
prophylactic therapies is less than, as determined by some user determined
factor, the expected
exposure for medical care =if the insured contracts one ormore of the diseases
or -ailments to
which he or she is susceptible.
In=such context, there would need to be a number of business rules where such
user.defined
quantities, threshold-levels, cost functions or metrics, figures of inerit,
expected risks, etc.; can be
input and articulated or incorporated in a number of rules. Such rules can
then be taken into
account by the central.processor 'in implementing algorithms which take as
inputs data from
business rules database 220 as well as a primary ImmunoScore database 203.
Fig. 2B presents an alternative generalized system diagram similar to Figs. 2A
and 2B.
However, as can be seen in Fig. 2B, there is shown yet another additional
database, a hypothesis
and rules database 250, communicably connected to central processor 204. Iri
such an exemplary
system a central processor can, for example, implement data mining algorithms
to operate on
stored immunologic and background data to find a set of correlations.
Such.data mining =
. algorithms can for example, be used to corroborate known or expected
relationships, such as, for
example, a correlation in antibody levels for ineasles, mumps and rubella in
=persons born in the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
23
United States after 1960, where the three vacciines were given -
simultaneously. In fact, an
interesting follow-up would be to track if the rates of antibody levels for
each of these three
diseases change in the individual at a similar or a different rate, and if
different, determine why.
Alternatively, for example, such data mining algorithms can be used to find
counter-intuitive, or
generally unknowns connections between variables or fields in the database.-
In either case, once a set of correlations is obtained, intelligence in an
exemplary system can be
used to automaticaIly generate=a set of hypotheses to explain such
correlations (o'r, if kn(jwn, any
follow-up data related thereto, as described above) and proceed to test the
viability of each .
hypothesis using the data in the.database. Or, alternatively, such
intelligence -can inform a user =
that additional data is needed to vet a hypothesis.
This process is explained more fully in Section II below.
Further, using such correlations, an exeinplary system can, for example, also
take as inputs '
various business rules in generating a decisiori regarding a recommendation.
For example, as =
described more fully below in Section III, an exemplary embodiment of the-
present invention
can be utilized to help a health insurance underwriter manage=its population
of insureds.: There
can, for example, be an annual or semi-annual requirement of all iinsureds to
have assays for =
various immunological components conducted on their blood or other. bodily
fluids. After
analysis of the results of such assays, an insurance company can determine
whether a particular
insured is susceptible to one or more given diseases or other ailments which
would result in
increased expenditures for. medical treatment. The insurance compariy could
then, decide if it
was not more economical to require the insured to undergo certain prophylactic
treatments, such
as, for example, vaccines or immune system boosting therapies, etc., where the
cost of such
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
24
prophylactic therapies is less than, as determined by some user determined
factor, the expected-
exposure for medical care if the insured contracts one or more of the diseases
or ailinents to
which he or she is susceptible.
In such context, there would need to be a number of business rules where such
user defined
quantities, threshold levels, cost functions or metrics, figures of merit,
expected risks, etc.; can be
input and articulated or incorporated in a number of rules. Such rules can
then be taken into
account by the central processor in implementing algorithms which take as
inputs data from
business rules database 220 as well as a primary ImmunoScore database 203.
Given the generalized exemplary method of Fig: 1 and the gerieralized
exemplary systems of
Figs. 2, 2A'and 2B, what is next described are a number of exemplary assay
panels which can be
administered to an individual or members of a population according to
exemplary embodiments
of the present invention. The scientific background behind the various
exemplary assay panel, as '
well as which segments of the general population such panels are best
administered to, are also
described.
SECTION I EXEMPLARY ASSAY PANELS
The present invention is, inter alia, concemed with assessing the "protective
immune status" or
"immunologic status" of an individual or population. A "protective immune
status" is -
understood to be represented by an array of detectable components (phenotypic
and/or
genotypic) of an immune system (adaptive and/or innate) that comprise its
protective capacity
against harmful substances and/or cells (such as, for example, microorganisms
or cancer). Such
components can, for example, consist of genes as well as-gene products. Genes
can include, for '
example, those which encode immunologic receptors (such as, for example, toll-
like receptors
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
("TLR"s) and chemoattiractant receptors) as well as effector molecules (such
as, for example,
cytokines and chemokines) which may also, for example, exist as genetic
polymorphisms
capable of deleterious and/or beneficial effects. Gene products can include,
for example,
antibodies, complements, cytokines, chemokines, chemoattractant receptors,
TLRs, lectins, and
other immune-related ligands. Harmful substances can consist of, for example,
chemicals,and/or
toxins originating from the environment, microorganisms, or one's self. .
Once diagnostic information is acquired from an individual regarding his or
her immune status,.
this information can be, for example, added to a system database. Such a
database ca.ricontain,
for example, not only the results of ImmunoScore diagnostic testing but a wide
variety of
demographic data and patierit history information as well.' Siich a system
database can, for -
example, be used to, record adverse events occurring coincident with
immunizations: Such
information can be invaluable to, for example, the ACIP for making
recommendations regarding
immunization scheduling, as well as help discover unsuspected patterns and
correlations relevant
to immune status and immune response. .
ImmunoScore diagnostic testing can be, for example, tailored to meet an
individual's specific
immunization status needs. In addition, each individual can, for example,
receive their own -
personal ImmunoScore card that they could carry with them to health care
office visits, and the
database information can be easily transferable in,the ever-increasingly
likely event that they
change physicians or other primary health care providers. Additionally,
ImmunoScore data,
analysis of such data and relevant database information can, for example, be
stored as part of a
person's totality of health information and medical records, in electronic
formats such as, for
example, entries in electronic health information databases, or computer -
chips embedded in, for
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
26
For economy =of description, most of the references cited herein are provided
in full citation in
Appendix A. to the Immunologic Informatics Patent. Throughout the text
citation=s are made to-
author and year of publication alone.
One component of TmmunoScore data can be, for exarinple, the raw as'well
as.processed.results
of diagnostic tests or assays relating to immune status, as described below.
ImmunoScore
diagnostic testing is envisioned to be done on a small assay device or testing
instrument that.can
be located, for example, in a doctor's office. The testing can be done, for
example, with a
sample of an individual's whole blood, plasma, serum, saliva, milk, semen,
tears, or urine. In the
case of blood, for example, the sample- can be obtained by a finger prick,
heel stick, ear stick,
other skin prick, capillary draw, venous draw, or an arterial draw. The
instrument =can, for
example, take assay panels and the patient sample. Patient information can
also be input. The
resulting information can be, for example, displayed to a user, printed,
stored in a removal
medium, -stored in the instrument, and/or transmitted (wired or wireless) to
other devices such as
via an intranet, a VPN or the Internet, for example.
Numerous systems and methods have been developed for the detection and
quantitation of analytes- of interest in biochemical and biological substances
that can be used; for example, in
such an instrument. Such methods and systems which are capable of measuring
trace amounts_of
microorganisms, pharmaceuticals, hormones, viruses, antibodies, nucleic acids
and other proteins
can be'of great value to researchers and clinicians.
A substantial body of art has been developed based upon well knowri binding
reactions, such as,
for example, antigen-antibody reactions,, nucleic acid hybridization
techniques, and protein-
ligand systems. The high degree of specificity in many biochemical and
biological binding
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
27
systems has led to many assay methods and systems of value in r-esearch and
diagnostics.
Typically, the existence of an analyte of interest is indicated by the
presence or absence of an
observable "label" attached to one or more of the binding materials. Of
particular interest are
labels which can be made to luminesce through photochemical, chemical, and/or
electrochemical.
means. "Photciluminescence" is the process whereby=a material is induced to
luminesce-when it '
absorbs electromagnetic radiation. Fluorescence and phosphorescence are types
of
photoluminescence. "Chemiluminescent" processes entail the creation of
luminescent species by
chemical transfer of energy. "Electrochemiluminescence" entails creation of
luminescerit
species electrochemically.
Electrochemiluminescent (ECL) assay techniques are an improvement over
chemiluminescent
techniques. They can, for example, provide a sensitive and precise measurement
of the presence
and concentration of an analyte of interest. In such techniques, the
iricubated-sample is exposed
to a=voltammetric working electrode in order to trigger luminescence. In the
proper chemical
environment, such electrochemilurninescence. is triggered by a voltage
impressed on the working
electrode at a particular time and in a particular manner. The light produced
by the label is
measured and indicates the presence or quantity of the analyte. For a fuller
description of such -ECL techniques, exemplary reference is made to US patents
5,221;605; 5,705;402; 6,140,138;
6,325,973; and 6,451,225. The disclosures of the aforesaid patents are hereby
incorporated
herein by reference.
Amplification techniques for nucleic acids may be combined with the above
assay techniques.
For example, US patent 6;048,687 discloses how NASBA can be combined with an
ECL
,technique; and US patent 6,174,709 discloses how PCR can be combined with an
ECL
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
28
technique. The disclosures of the aforesaid patents are also hereby
incorporated herein by
reference.
An assay instrument can, for example, be, or be similar. to, the BioVeris
Corporation M1R or
M1M instruments with an added sample processing front end. Aspects of these
iinstruments are
-disclosed in pending US patent application numbers 10/600,165 and 10/841,569,
each under
common.assignment herewith. The disclosures of these patent applications are
hereby
incorporated herein by reference.
In exemplary embodiments of the present invention,'an assay instrument can
include, for
example, amplification techniques such as PCR 'or NASBA. In exemplary
embodiments.of the
present invention, the instrument can use fluorescence, chemiluminescence, or
ECL assay
techniques. 'In exemplary embodiments, multiple measurements can be done
simultaneously; in
other exemplary embodiments of the present invention, multiple measurements
can be done
sequentially. In exemplary embodiments of the present invention, an assay
instrument can; for
example, contain self-test and/or self-calibration components.
In exemplary embodiments of the present invention, a sample can be added to an
assay panel,
and the combination then inserted into the test instrument, as shown in' Fig.
3. In alternate
exemplary embodiments, the sample and assay panel can be separately inserted
into the test
instruinent, as shown, for example, in Fig. 4.
As described below, entries to an exemplary master IrrimunoStore database can
be, for example,
coded so as to protect patient confidentiality. A patient could; however, be
able to learn from
their physician in real time, for example, which vaccines he or she rni=ght
need to ensure
protection from vaccine-preventable illnesses. The physician can, for examule.
offer'the vaccine.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
29
or other therapy, during the same visit, or shortly thereafter. Any possible
adverse effects from
any delivered vaccinations could be subsequently entered into an TmmunoScore
database and
that information could be shared with the ACIP or-other agencies or bodies, as
described more
fully below.
The actual assays can be performed, for.example, based upon the needs of the
individual or
individuals being examined. Age, occupation, travel plans, immigration status,
military status,=
and previous health status can all be =considered prior'to initiation of
ImmuiioScore =diagnostic
analyses= in exemplary embodiments.' In exemplary embodiments of the present'
invention, the '
following exemplary broad categories can, for . example, be utilized as focal
points for test
panels:
Entry to. primary school.
College entry.
Age 19-49 years.
Age 50-64 years.
Age > 65 years.
Health-care professionals.
Military personnel:
recruits and officer accessions;
alert forces;
individualized according to ocaupational or personal needs; and
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
veterans.
Travelers.
Imm.igrants:
Individuals with identifiable health risks, (not necessarily exclusively); =
a. Complement-deficient.iridividuals (e.g: meningococcal disease
susceptibility);
b. Genetically identified (e.g. HLA haplotype, sepsis susceptibility) disease-
susceptible individuals;
c. Manriose-binding le.ctin-deficient individuals;
.d. Hepatitis'B vaccine poor/non-responders;'and
e. Ethnic groups and others known to respond poorly to polysaccharide,
conjugate, or other vaccines.
Although the health effects may be just as great; coverage levels for
immunizations in adults are
not as high as those achieved in children. Barriers to adult immunization can
include, for
example, not knowing that-inununiza.tions are needed, misconceptions about
vaccines, and lack. -
of recommendations from health care providers. Adding an IrnmunoScore based
diagriostic
component to routine physical examinations in adults could easily point out
where
immunizations are needed, for example. Just as importantly, it could, for
exarriple, point out
exactly which individuals would not need to be unnecessarily boosted if their
serum antibody
levels proved to be sufficient for-anyparticular vaccine. The developmentand
acceptance of an
ImmuinoScore vaccine diagnostic surveillance system can not only aid in
increasing vaccine
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
31
coverage, but can also, for example, add increased surveillance of the level
of immune response
and duration of protection thereof for a wide variety of recommended vaccines.
There are still great risks posed to the population by vaccine-preventable
diseases. The risks
..posed by failure to immunize were vividly illustrated by the measles
outbreak.that began in
1989, which led to 43,000 cases and over 100 deaths, mainly among children in
the United
States. Despite better efforts at record-keeping for immunizations, for
example, the development
of the Adult and Adolescent Clinic Assessment Software Application (ACASA) to
facilitate
obtaining immunization data on adults and adolescents, adult compliance with
vaccination
protocols generally remains unsatisfactory. One way to demonstrate the need
for vaccination in
adults is to demonstrate a low antibody titer in an individual and'present the
need to boost the
antibody titer using assigned correlates of protection from vaccine-
preventable diseases. If an
antibody titer to a specific agent is determined to not meet the recognized
level of a correlate of
protection by an exemplary ImmunoScore analysis, and that titer is easily
boosted by
vaccination, then that individual would likely be more easily convinced of the
need for protective
immunization. Not only would this diagnostic tool prove extremely beneficial
to the individual,'
but data added to an ImmunoScore database can be collected regarding
immu.rie.correlates of
protection for very large populations: Demographic assessments can also be
compil'ed from the
database, leading to,, foir example, new discoveries regarding possible age-
related or ethnic
responses to immunizations and/or other immune status issues.
In exemplary embodiments of the present inventiori, targeted panels of immune
status assays can
be defined. Such exemplary targeted panels, can be organized, for example,
into two broad
.groups, college students and adults.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
32
For college students, three exemplary panels were'defined: (a) a Meningococcaf
il.iagnostic.
panel, (b) a panel designed- to measure the residual immunity induced by
childhood vaccines, and
(c) a panel directed to measuring immunity from common sexually transmitted
diseases.
For. the general adult population, exemplary panels were defined directed to
the following groups
or categories: (a) military personnel, (b) .travelers, (c) adults-general
immune status, (d) health
care workers, (e) bioterrorism, (t) chronic disease, (g) Th-1-Th2,diagnostic
panel, and (h)
immigrants and internationally adopted children.
Using these basic panels of assays as building blocks, in exemplaiy
enibodiments of the present
invention, aggregations of.one or more panels, with variations thereto as may
be desired, can be
defined for various purposes.* These'can be referred to, for exaxnple, as
ImmunoScore
"superpanels." For example, a primary scliool panel can be defined, to be
administered upon a= '
child's entry into grammar school. Such a panel .could inchide; for example, a
persistenr
immunity to childhood vaccines assay panel. : Similarly; a middle school
student superpanel 'can
be defined as well. Such a superpanel can include, for example, a persistent
immunity to
childhood vaccines p'anel.and a sexually transmitted disease panel. Another
exemplary
superpanel could be defined for women of childbearing years. Such an
=exemplary superpanel
can include, for example, a newly defined women of childbearing years panel, a
persistent
immunity induced'by childhood vaccines panel, and a sexually transrriitted
disease panel.
COLLEGE STUDENT DIAGNOSTIC PANELS
9. = Meningococcal Diagnostic= Panel
In exemplary embodiments of the.present invention, the following tests can be
included*in a
meningococcal diagnostic panel:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
33
1. Antibod.y (Ig) to (4 tests):
= Group A Meningococcal Polysaccliaride (GAMP) =
= Group C Meningococcal Polysaccharide (GCMP).
= Group Y Meningococcal Polysaccharide (GYMP)
= Group*VJ-135 Meningococcal Polysaccharide'(GWMP)
2. Antibody (IgM) to Group B Meningococcal Polysaccharide (+GBMP) (1 test)
3. Serum levels of complement components (7 -tests):
= C5
= C6
= C7
= C8.
: C9
= Properdin
= MBL
4. Measurement of gerietic polymorphisms (5 tests):
= FcyRIIa receptor
= IL-1
= =IL-1R-
= IL-6
= IL-10
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
34
In exemplary embodiments of the present invention, results from an exemplary
meningococcal
diagnostic panel can be analyzed as follows:
= Serum Ig levels for vaccine-preventable serogroups (A, C, Y, and W-135)
of.N.
meningitidis can be assessed. An Ig level exceeding 2.0 g/mL of all four
serogroups
would be presumptive of protection in an otherwise healthy individual. There
would
be no immediate recommendation for meningococcal vacciiiation in these
individuals.
= If deficiencies were to be revealed in ainy of an individual's complement
components
assayed, or if any unfavorable genetic polymorphisms were shown to exist, then
an Ig
level of >5.0 g/mL for the vaccine-preventable serogroups could be desirable
iri
these individuals. If these individuals had Ig levels exceeding 5.0 g/mL for
all four
serogroups, no vaccination would be recommended. If, however, the level of
antibody to any of the four serogroups were to be below 5.0 }ig%mL,-then a'
vaccination could be recommended.
= Once individuals were shown to have complement deficiencies, or unfavorable
genetic polymorphisms; they could be, for example, "flagged" for future
monitoring.
These are individuals at greatest risk for meningococcal infections, so serum
antibody
levels are very important in this group. Initially, they could be monitored
every 3-5
years for serum Ig to, the vaccine-preventable meningococcal serogroup
capsular
antigens.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
As yet there is limited information available regarding the persistence of
serum -Ig to
the N. -meizingitidis group polysaccharides. As, the.system database.grows;
more
infonnation regarding antibody persisteince can become available and analysts
can,
for example, have a better idea as to when to recommend retesting- and,
perhaps,
revaccination of individuals more susceptible to meningococcal disease.
= It has previously been demonstrated that repeated vaccination with the
capsular
polysaccharide from Group C organisms promotes immune hyporesponsiveness
(Richmond, et al. 2000, Jokhdar,. et al. 2004). This is'a 'red flag to.
overuse of the
vaccination protocol. Currently, immunocompromised individuals are
recommended=
for repeated immunizations every 3-5 years. In exemplary embodiments of the .=
present invention, the ImmunoScore meningococcal diagnostic panel can prevent
over-immunization with the polysaccharide forrnulation by first measuring
immune
status vis-a-vis menigococcal disease prior to simply vaccinating following a
stan dard
schedule..Immuniza.tion with Group C conjugate vaccine overcomes the
hyporesponsiveness, but is not yet approved in the- United States.
10. Sexually Transmitted Diseases Assay, Panel
In exemplary embodiments .of the present invention, the following tests can,
for example, be
used for Immun.oScore measurement of immunity to* STDs:'
Antibodies to Chlamydia - IgG, IgA, and IgM (3)
Antibodies to HSV - IgG to HSV-1 and HSV-2 (2)
DNA analyses of HPV types - particular emphasis on high-risk
Antibody to N. gonorrhoeae (1)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
36
T-cell related response to T. pallidum
Antibody to HIV
T=ce11 related response to HIV
Antibodies to GBS serotypes (at least 3)
Measurement of Thl/Th2 cytokines (many as current evolving definitions)
= Currently; there are no vacciines available for any of these STDs. Until
this situation'is
ameliorated, the objective of an ILnmunoScore STD diagnostic panel would thus
be'to
recommend treatment. The IiiiinuinoScore database can .gerierate correlate of
protection
information for all diseases. As vaccines are developed, ImmiunoScore
diagnoses can be
designed, to examine aritibody and other related immune responses to vaccine
components.
11. Persistent 'Immunity Induced by. Childhood Vaccines
In exemplary embodiments accordirig to the'present invention, the folloviring
tests.for
measurement of immunity to childhood vaccines can, be included in an exemplary
ImrnunoScore
panel directed to college students, or in other exemplary embodiments, to
adults in =general:
Antibody to HBs (1)
Antibody to diphtheria toxin (1)
Antibody to'tetanus ,toxin (1) =
Pertusis antibodies (4):
Antibody to pertussis toxin (PT)
Antibody to pertactin (PRN)
Antibody to filamentous hemagglutinin (FHA)
Antibody to fimbriae
Antibody to PRP (Hib)'(1)
Antibodies to poliovirus serotypes Pl, P2, and P3 (3)
Antibody to measles (1)
Antibody to mumps (1)
Antibody to rubella (1)
Antibody to varicella (1)
Antibody to pneumococcal serotypes (7)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
37
Given the above-described tests for persistent immunity induced by childhood
vacciries, in
exemplary embodiments of the present invention, the following exemplary
analyses and
recommendations can, for example, be made:
= For Hepatitis B, post-vaccination titers of anti-HBs IgG of 10 mIU or
greater correlate
with the induction of T cell helper responses that mediate the memory of B
cells (Plotkin,
2001). An antibody titer below 10 mILT would indicate need for vaccination -
one
booster dose ifpreviously vaccinated, or a course of three doseg if
unvaccinated.
= The current indication for partial protection from diphtheria disease is an
anti-diplitheria
toxin antibody concentration between 0.01 and 0.1 IU/mL. Protection is
considered to be
complete above 0.1 IU/mL (Plotkin, 2002). In exemplary embodiments ImmunoScore
diagnostics can recommend a booster dose if antibody concentration were to
fall below
0.1 IU/mL. The ImmunoScore database can shed further light in the future as to
the true
protective level of anti-diphtheria toxin antibody.
= The current indication for partial protection from tetanus 'disease is an
anti-tetanus toxin
antibody concentration between 0.01 and 0.1 IU/mL. 'Protection is considered
to be
complete above 0.1 IU/mL (Plotkin, 2002). InununoScore diagnostics can
recommend a
booster dose if antibody concentration were to fall below 0.1 IU/mL. The
ImmunoScore
database can shed further light in the future as to the true protective level
of anti-tetanus
toxin antibody.
= One of the most controversial areas within the subject of correlates of
imniunity is
pertussis vaccine. Two separate trials conducted in Sweden indicate that
pertussis toxin
can protect on= its own (Trollfors, et al. 1995). One trial suggests=that the=
addition of
filamentous hemagglutinin (FHA) is helpful, two trials suggest that pertactin
augments
the efficacy of PT and one trial suggests that agglutinogens add
efficacy.beyond those of
PT, FHA, and pertactin (Plotkin, et al. 1997). The problem. is that the
vaccines do not
resernble each other in quantity of antigens, and reliance can be placed only
on
demonstrated efficacy in the field. The role of IrnmunoScore diagnostics for
this
population can, for example, best be served in data acquisition and
correlation to
incidence of disease. There is not yet an adult pertussis vaccine, but
development
proceeds along those lines. The ImmunoScore diagnostic application can be
beneficial in
exemplary embodiments to ACIP for vaccine recommendations. Testing four
components for pertussis disease would lend weight to the accuinulated data.
= Individuals vaccinated with Hib conjugate vaccines are con'sidered to be
protected with
an IgG level > 0.15 g/mL (Goldblatt, et al. 1999). Booster vaccination can be
recommended if an individual's antibody titer fell below that level.
= Individual that receive oral polio vaccine (OPV) -are protected by both
serum and
secretory antibodies. Inactivated polio vaccine (IPV) recinients are denendent
nrimarilv
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
38
on seruin aritibody for protection against infection and disease (Onorato, et
a1..1991).
Neutralizing antibody assays are.currently used to assess protective Ig levels
(titer > 1.:8).
The format of these assays would necessarily need to be updated to be.included
in the
ImmunoScore analyses. Currently, the duration of protection froni IPV
vaccination is -not .
known, and ImmunoScore database analyses. could, for example, lend strength to
the
current knowledge levels.
= Serum antibody levels > 120 mIU are considered to be completely protective
against
measles infection (Plotkin, 2001). Vaccination can be considered for
individuals whose ~
antibody titers fall below this level.
= Protection against mumps disease is currently assessed with neutralization
assays: Like
the polio vaccine, the assay format would need updating for ImmunoScore
diagnosis.
= Protection against rubella disease is currently assessed with neutralization
assays. Like.
the polio and measles vaccine, the assay format would need updating for
ImmunoScore -
diagnosis.
= Protection against varicella disease is currently assessed with
neutralization assays. The .
assay- format would need updating for ImmunoScore diagnosis.
= Little is known about the correlate of protection for pnueniococcal anti-
capsular. :
polysaccharide antibodies. It is likely that the protective IgG. range would
fall between
0.15 and 1.0 g/mL, except for serotype 14, against which more antibody is-
necessary=
(Plotkin, 2001). An ImmunoScore diagnostic recommendation can, for,example,
initially
be for a boost if antibody levels fell below 1.0 g/mL,, and then the database
analyses can
be able to shape future recommendations.
ADULT DIAGNOSTIC PANELS -
12. Measurernent of Immunity Induced By*Vaccines for Military Personnel
Iri exemplary embodiments of the present invention military personnel can be
administered. the following diagnostic panels:
1. College Student ImmunoScore Panels consisting of:
= Meningococcal Diagnostic Panel;
=' 'Sexually Transmitted Disease Diagnostic Panel;
= Persistent Immunity Induced by Childhood Vaccine'Diagnostic Panel; and
as described above; and. in addition
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
39
2. -Military personnel can have specific vaccination needs as outlined in
Table =3 below
depending on their assignments and type of deployment. Specific branches of
the service may
also have specific vaccination -needs arid permutatioris of the. basic
diagnostic panels. :Thus, in
exemplary embodiments, military personnel can be administered one or more of
the following
tests:
TABLE 3
Vaccine Diagnostic Panels Exclusive to the Military:
Vaccine Aintibody Marker
Adenovirus 4& 7 Neutralizing antibody.
Anthrax PA
Cholera LPS IgG
Plague. . Fraction I Capsular Antigen
Smallpox Neutralizing antibody
Lyme disease OspA
In addition to the analytes listed above as "exclusive to the military, an
ImniunoScore diagnostic.
panel can, for example, be extremely flexible at adding new diagriostic tests
for vaccines under
development.
Analysis of results/recommendations for use of ImmunoScore diagnostic panel
data for analytes
for specific military applications can, in= exemplary embodiments of the
present invention,
include the following:
= Adenovirus-vaccine is not currently given to military recruits, but
infection with
adenovirus remains a concern. Development and use of adenovirus vaccines are
likely in'
'the future and an exemplary ImmunoScore diagno=stic application can require
an updated
assay format over the currently accepted neutralizing antibody assay.=
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
= Currently, serological correlates of protection tb inhalation anthrax are
being developed
in animal models. ImmunoScore diagnostics canjor example, measure level of
serum
IgG to protective antigen (PA) and the ImmunoScore database can, for example,
thus
build serologic correlates of protection in humans.
= Immunity to cholera is currently not completely understood. However,
ImmunoScore
diagnostics can focus first on levels of anti-LPS IgG, and further attempt to
build
meaning into the database correlates of protection.
= The need for a new vaccine for pneumonic plague is evident givein the
limited efficacy of
the current cellular vaccines, which consist of either the killed virulent
195/P or live
EV76 strains. While an efficient and safe live cellular vaccine has not been
identified
yet, there is an effort to develop alternative subunit vaccines based on
various antigens,
including the Fl and V antigens. ImmunoScore diagnostics can, for example,
monitor
serum antibody levels to current plague vaccine components and be able to
adapt to any
new vaccine configurations. ImmunoScore database can, for example, compile
ixnmune
response data and be correlate the relevant antibody levels to levels of
protection.
= Immune memory after smallpox vaccination is a valuable benchmark for
understanding
the kinetics and longevity of B cell memory in the absence of re-exposure to
antigen,
since immunization of the U.S. population was stopped in 1972 and smallpox
disease was
declared eradicated worldwide in 1980. Immune memory to smallpox is a useful
benchmark both for understanding the longevity and the stability of iinmune
memory in=
the absence of re-stimulation. Circulating antibody persists for over'50
years.
ImmunoScore diagnostics can, in exemplary embodiments of the present
invention,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
41
measuie antibody to smallpox. Correlates of protection can, for example, be
generated
-from analyses of the ImmunoScore database.
='The human protective response to vaccination against Lyine disease is purely
a serum-
mediated antibody response. Individuals are generally considered protected
with
antibody levels against OspA greater than 1100 IU. Subjects with less antibody
titers less
than 1100 would, in exemplary embodiments of the present invention, be
recbmmended
to have a booster vaccination.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
42
13. lmmunoScore Measurement of Vaccine-induced immunity for Travelers
In exemplary embodiments of the present invention,,an ImmunoScore traveler's
assay panel can,
for example, include the following:
Antibody to HAV (1)
Antibody to HBs (1)
Antibody to Japanese Encephalitis (1)
Antibody to rabies (1)
other rabies related cytokine assays (as necessary)
Antibody to Typhoid fever (1)
Antibody to yellow fever (1)
Antibody to dipbtberia toxin (1)
Antibody to tetanus toxin (1)
Pertusis antibodies (4): -
Antibody to pertussis toxin (PT)
Antibody to pertactin (PRN)=
Antibody to filamentous hemagglutinin (FIiA)
Antibody to fimbriae
Antibodies to poliovirus serotypes P1, P2, and P3 (3)
Antibody to measles (1)
Antibody to mumps (1)
Antibody to rubella (1)
In exemplary embodiments of the present invention, recommendations for use of
a Immunoscore
diagnostic panel for analytes specific to travelers can include all of the.
uses of the results of the =
Meningococcal Diagnostic Pane) tests, as described above. Additionally, the
followirig
recommendations/aonclusions can be implemented:
= The protective level, of antibody to hepatitis A has been established to'be
approximately
mIU/mL if that concentration is maintained for a two month period, although
some
individuals may be protected at much lower concentrations (Conrad and Lemon,
1987).
An individual that had less than 10 m1U/mL of antibody to hepatitis A can be
recommended for vaccination.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
43
at Current analyses of antibody levels to Japanese Encephalitis consist of
neutralizatioii
assays. These assays would need to be refined for ImmunoScore
diagnostic'applieatiori"s..
In exemplary embodiments of the present invention, an ImmunoScore database can
catalog aintibody levels in anticipation of establishing future serologic
correlates of
protection.
= The most important immune response to rabies vaccines is antib=ody to the G
envelope.
protein (Wicktor, et al. 1973), and= passively administered antibody is part
of standard
treatment to neutralize cell-free virus before it attaches to the axon of a
neuron (Plotkin,
2000). Because passive antibody alone is poorly effective unless supplemented
by active
vaccination, CD4+ and CD8+ cell responses are probably also important to
protection,
but whether these critical responses'relate to cytotoxic T lymphocyte
fixnction, interferon
synthesis or other cytokines is unknown (Hemachudha, et al. 1999). ImmunoScore
''diagnostic assays can include at the very least antibody levels to G
envelope protein and
can also measure relevant cytokines to assess serological correlates of
protection,
== Protection against typhoid fever might be'best achieved by a vaccine that=
stimulates. IgG,
antibody to Vi capsular polysaccharide in serum, IgG antibody to 0 antigen in
serum, and.
cell-mediated immune responses (Tackett, et al. 2004). ImrnunoScore diagnostic
analysis
caii thus, in exemplary embodiments of the present invention, focus on
antibody to Vi capsular polysaccharide. IrnmunoScore data analyses can create
necessary correlates of
protection against typhoid fever disease.
= Protection against yellow fever appears.to correlate with antibody titers
above 0.7 IU.
ImmunoScore diagiiostic analysis can recommend that an individual with
antibody titer'. =
below 0.7 IU be boosted.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
44
14. ImmunoScore Measurement of Immunity in Health Care Workers
Because of their contact with patients and/or infective material from
patients, many health-care
workers (e.g. physicians, nurses, emergency medical personnel, dental
professiorials and
students, medical and nursing students, laboratory technicians, hospital
volunteers, and administrative staff) are at risk for exposure to'and possible
transmission of vaccine-preventable
diseases. Maintenance of immunity is therefore an essential part of prevention
and infection
control programs for health-care workers. Optimal use of immunizing agents
safeguards the
health of workers and protects patients from becoming infected through
exposure to infected
workers. Consistent immunization programs 'could substantially reduce *both
the number of
susceptible.health-care ,ivorkers in hospitals and health departments and the
attendant risks for
transmission of vaccine-preventable diseases to other workers and patients. In
exemplary
embodiments of the present invention; the judicious application of ImmunoScore
diagnostics to
the needs of health-care workers can assure that these individuals will be
appropriately
immunized and protected from both becoming infected and spreading infection.
The Centers for
Disease Control (CDC) has recommended various immunizing agerits for health-
care workers.
Any medical facility or health department that provides direct patient care is
encouraged to
formulate a comprehensive immunization policy for all health-care workers. The
American
Hospital Association has endorsed the concept of immunization programs for
both hospital
personnel and patients (AHA, 1992). The use of ImmunoScore diagnostic
capability coupled
with rigorous immunization programs can assist in.the decline of nosocomial
infections.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
There are diseases for which the CDC strongly recommends vaccination for
health-care workers.
These include Hepatitis B, influenza, measles, mumps, rubella, varicella-
zostei, and tuberculosis.
There are other diseases that vaccination may be indicated for; these include
Hepatitis A,
meningococcal disease, Typhoid, and smallpox; Finally, for some health-care
workers, there
may be a recommendation for tetanus, diphtheria, pertussis, and pneumococcal
disease.
Hepatitis B virus (HBV) infection is the major infectious health hazard for
health-care personnel.
Data indicate that 5-10% of HBV-infected workers become chronically infected.
Individuals
with chronic HBV infection are at risk for chroriic liver disease and are
potentially infectious
throughout their lifetimes. The risk of acquiring HBV infection from
occupational exposures is dependent on the frequency of percutaneous and
permucosal exposures to blood or body fluids
containing blood. Depending on the tasks he or she performs, any health-care
or public safety
worker may be at high risk for HBV exposure. Workers performing tasks
involving exposure to
blood or blood-containing body fluids should be vaccinated. For public safety
workers whose
exposure to blood is infrequent, timely post-exposure prophylaxis may be
considered, rather than
routine pre-exposure vaccination.
Pre-vaccination serologic screening for prior infection is not currently
indicated for persons
being vaccinated because of occupational risk. Post-vaccination testing for
antibody to hepatitis
B surface antigen response is indicated for health-care workers who have blood
or patierit contact
and are at ongoing risk for injuries with sharp instruments or needlesticks.
Knowledge of
antibody response aids in determining appropriate post-exposure prophylaxis.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
46
Vaccine-induced antibodies to HBV decline ~gradually over time, and <60% of
persons who =
initially respond to vaccination will- lose detectable antibodies over
12`years (Stevens, et al.
1992). Studies among adults have demonstrated that, despite declining serum
levels of antiliody;
vaccine-induced immunity continues to prevent clinical disease or
detectable=viremic HBV
infection (Hadler, et al. 1992). Therefore, booster doses are not considered
necessary. Periodic
serologic testing=to monitor antibody concentrations after completion of the
three-dose series =is
currently not recommended. An obvious'advantage of the ImmunoScore dia,gnostic
panel would'
be that periodic monitoring 6f immune status could be correlated with any
outbreak of disease in
health-care workers. The availability of an ImmunoScore database can be
exceedingly. beneficial
to the health=care workers in the care settings. The possible need for booster
doses can be
assessed as additional data become available.
-Asymptomatic HBV infections. have been detected in vaccinated individuals by
means of
serologic testing for antibody to hepatitis B core antigen. However; these
infecti,ons also provide
lasting immunity and are not associated with HBV-related chronic liver
disease.
During community influenza outbreaks, admitting patients infected with
influenza to hospitals
has led to nosocomial transmission of the disease (Balkovic, et al. 1980),
including transrijissibn
from staff to patients.: Transinission of influenza among medical staff causes
abseinteeism- and
considerable disruption of health care. In addition, influenza outbreaks have~
caused morbidity
and mortality in nursing homes. Because there. is a recommendation= for an
annual influenza.
vaccination for health-care workers, it is unlikely that there would be an
ImmunoScore
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
47
diagnostic application for flu. The only potential heirewould be to=correlate
vaccination and-
protection in a multitude of individuals working in the health-care field.
Nosocomial measles transmission has been documented in the offices of private
physicians, in
emergency rooms, and on hospital wards. 'Although only 3:5% of all cases of
measles reported
during 1985-1989 occurred in medical settings, the risk for measles infection
in medical
personnel is estimated to be thirteen fold that for the general
population.(Watkins, et al.'1987;
Atkinson, et al. 1991). Of the 3,659 measles cases reported during 1992-1995,
the setting of
transmission was known for 2,735; 385 (13.9%) of these' cases occurred in
medical settings
(CDC, 1997). Although birth before 1957 is corisidered acceptable evidence of
measles immunity, serologic studies of hospital woikers indicate that 5-9
oo'of those born before 1957 are
not immune to measles (Smith, et al. 1990). Duririg 1985.-1.992, 27% of all
measles cases among
.health-care workers occurred in individuals born before.1957 '(CDC, 1997).
Measles vaccination
is contraindicated in pregnant and immunocompromised individuals, including
HIV-infected
persons who have eviclence of severe immunosuppression. Measles is also
contr:aindicated
following recent administration of immune globulin.
.In recent years, a substantial proportion of reported mumps has occurred
among unvaccinated..
adolescents and young adults on college campuses and in the workplace-(.Cochi,
et al. 198.8;
Kaplan, et al. 1988). Outbreaks of mumps in highly vaccinated populatioins
have been attributed
to primary vaccine failure. During recent years, the overall incidence of
mumps has fluctuated
only minimally, but an increasing proportion of cases have been reported in
individuals aged >.
15 years (CDC, 1995). The CDC states that progranis to ensure that medical
personnel are
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
48
immune to mumps are prudent and are easily lik~ed with measles and rubella
control prograiris
(CDC, 1997). Mumps vaccination is contraindicated in pregnant and
immunocompromised -
individuals.
Nosocomial rubella outbreaks involving both health-care workers and patients
have 'been
reported (Greaves, et al. 1982)., Although vaccination has decreased the
overall risk for rubella
transmission in all age groups in the United Sta,tes by > 95%, the potential
for transmission in
hospital and similar settings persists because 10-15% of young adults are
still susceptible (Bart,
et al. 1985). Although not as infectious as measles, rubella can be
transmitted effecti'velyby
both males and females. Transmission can occur whenever many. susceptible
individuals
congregate in one place. Aggressive rubella vaccination of susceptible men and
women with
-trivalent MMR vaccine can eliminate rubella transmission. Persons born before
1957 are
generally considered to be immune to rubella. However, findings of
seroepidemiologic studies
indicate that about 6% of health-care workers (including iridividuais born
before 1957) do not
have detectable rubella antibody (CDC, 1997). Rubella vaccinatiori is
contraindicated in
pregnant and immunocompromised individuals.
For all the infectious disease above=covered by the MMR vaccination, an
ImmunoScore
diagnostic panel would be a useful application' for health-care workers. =
These=vaccines are
available as monovalent or trivalent combinations. Lack of protective levels
of antibody to ariy
one of the components could be ameliorated by vaccination. In addition, health-
care workers
would again provide a large population to add an exemplary' Imn=iunoScore
database.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
49
Nosocomial transmission of varicella zoster.virus '(VZV) is well recognized.
Sources for
ndsocomial exposure'of patients and staff have inch.ided patients, hospital
staff, and visitors who
are infected with either varicella (chickenpox) or zoster (shingles). In
hospitals, airborne '
transmission of VZV from persons who had varicella or zoster to susceptible
=persons who had no
direct contact with the index case patient has occurred. Although all
susceptible hospitaliaed.
adults are at risk: pregnant women, premature infants born to susceptible
mothers, .infants born at
< 28 weeks' gestation or who weigh < 1,000 giams regardless of matemal immune
status, and
immunocompromised persons 'of all ages (including persons who are undergoing
immunosuppressive therapy, have malignant disease, or are immunodeficient).
Strategies for managing clusters of VZV infections in hospitals inchide:
= isolating.patients -who have varicella and otlier susceptible patients who
are
exposed to VZV;
= controlling air flow;
= using rapid serologic testing to 'determine susceptibility;
=Turloughing exposed susceptible personnel or screening .these. persons daily
for
skin lesions; fever, and systemic symptoms; and
= temporarily reassigning varicella-susceptible personnel to locations xemote
from
patient=care areas. A reliable history of chickenpox is a valid- measure of
VZV inimunity. = Serologic tests have been
used to assess the accuracy of reported histories of chickenpox. Among adults,
97-99% of
individuals with a positive history of varicella are seropositive. In
addition; the majority of
adults with negative o'r uncertain histories are seropositive (range 71-93%).
Persons who do not
have a history of varicella, or whose history is uncertain can be considered
susceptible,-and can
be tested serologically by ImmunoScore diagnostic methodology to determine
their immune
status. In health-care institutions, seiologic screening of personnel who have
a negative or
uncertain history of varicella is likely, to be cost effective fCDC, 1996).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
500
If susceptible health-care workers are exposed to varicella, they are
potentially infective 10-21-
days after exposure. They must be furloughed during this period, usually at
substantial cost:
Administration of varicella zoster immune globulin (VZIG) after exposure can
be costly. VZIG
does not necessarily prevent varicella, and may prolong the incubation period
by-a week or more,
thus extending the time during which personnel should not work.
Varicella virus vaccine protects approximately 70=90% of recipients against
infection and 95 10 ,
of recipients against severe disease for at least 7-10 years.after
vaccination. . Significant
protection is long-lasting. Breakthrough infections have occurred among
vaccinees after.
exposure to natural varicella virus. Estimates of vaccine efficacy and
persistence in vaccinees
are based on research conducted before widespread use of varicella vaccine
began to influence =
the prevalence of natural VZV infection. Therefore, the extent to which
boosting.from exposure
to natural virus increases the protection provided by vaccination remains
unclear. '.Whether
longer term immunity may wane as the circulation of natural VZV decreases also
is unknown.
The CDC recommends that vaccination should be considered for unvaccinated
health-care
workers who lack documented immuriity if they are exposed to varicella.
Howeveir, because the
effectiveness of post-exposure vaccination is unknown, individuals vaccinated
after an exposure
should be managed in the manner recommended for unvaccinated persons. Here;
again, the
ImmurioScore diagnostic assay for varicella would be a valuable assessment
tool prior to
initiation of vaccination of individuals uncertain of their immune status or
disease history.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
51
In the United States, Bacille Calmette-Gudrin (BCG) vaccine has not been
recommended for
.general'use's because the population risk for infection with Mycobacterium
tuberculosis (TB) is'
low and the protective efficacy of BCG vaccine uncertain. The immune response
to BCG vaccine also interferes with the use of the tuberculin skin test to
detect.M. tuberculosis infection
(CDC, 1996): TB prevention and control efforts are focused on interrupting
transmission from
patients who have active infectious TB, skin testing those at high risk for
TB, and administering
preventive therapy when appropriate. However, in certain situations; BCG-
vaccination may
contribute to the prevention and control of TB when other strategies are
inadequate.
The fundamental strategies for the prevention and control of TB include:
==Early detection and effective treatment of patients with active
cornmunicable TB.
= Preventative therapy for infected persons. Identifying and treating persons
who
are infected with M. tuberculosis can prevent the progression of latent
infection to=.
active infectious disease.
= Prevention of institutional transmission. The transmission of TB is a
recognized
risk- in health-care settings 'and is of particular concern in settings where
HIV
infected individuals work, volunteer, visit, or 'receive care. Effective TB
infection-control programs should be implemented in health-care facilities and
other institutional settings (e.g. shelters for homeless persons
and:correctional
facilities).
In a few geographic areas of the United States, increased risks for TB
transrnissioii in health-care
facilities (compared with risks observed in health-care facilities "in other
parts, of the United
States) bccur together with an elevated prevalence among TB patients -of M
tuberculosis strains
that are resistant to both isoniazid and rifampin. Even.in such situatipns,
coinprehensive'
application of infection control practices should be the primary strategy used
to protect health-
care workers from infection with M. tuberculosis. BCG vaccination of health-
care workers
should not be used as a primary TB control strategy because: =. the protective
efficacy of the vaccine in health-care workers is uncertain;
=. even if BCG vaccination is effective for a particular health-care worker,
other
persons in the health-care facility (e.g. patients; visitors and other health-
care .
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
52
workers) are not protected against possible exposure to and infection with
drug-
resistant strains of M. tuberculosis; and
= BCG vaccination may complicate preventive therapy because of difficulties
in.
distinguishing tuberculin skin test responses caused by infection with M.
tuberculosis from those, caused by the immune response to vaccination.
Hepatitis C virus (HCV) is the etiologic agent in most cases of parenterally
transmitted non-A,
non-B hepatitis in the United States. CDC estimates that the'annual number of
newly acquired
HCV infections has ranged from 180,000 in 1984 to 28,000 in 1995. Of these,
and estimated 2-
4% occuned among health-care personnel who were occupationally exposed to
bleed. 'At least
85% of individuals who contract HCV infection become chronically infected, and-
chronic
hepatitis develops in ari average of 70% of all HCV-infected individuals
(Shakil, et al. 1995).
Serologic enzyme immunoassays licensed -for the detection of antibody to HVD
have evolved
since their introduction in 1990 and a third version is now available
which'detects anti-HCV in >
95% of patients with HCV infection. These assays do not detect anti-HCV in
all:infected
individuals and do not distinguish among acute, chronic, or resolved
infection. In 80-90% of
HCV-infected individuals, seroconversion occurs an average of 10-12 weeks
after. exposure to
HCV: These screening assays also yield a high proportion (up to 5001o) of
falsely positive tests
when they are used iri populations with a low prevalence of HCV infection
(CDC, = 1991).
Based on seroepidemiological surveys, the core and the non-structuraf 3 (NS3)
proteins of
hepatitis C virus are thought to be two of the most immunogenic proteins of
HCV (Chiba, et al.
1991). A majority of HCV-infected immuno-competent individuals develop HCV
core
antibodies (Chen, et al. 1995). However, there seems to be low or no HCV
antibodies -early in
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
53
acute infection (Chen, et al. 1995.). The major IgG subclasses of antibodies
to HCV. core are
IgG I and IgG3 (Sallberg, et a.l. 1992).
In the absence of effective prophylaxis, individuals who have been exposed to
HCV may benefit
from knowing their infection status so they can seek evaluation for chronic
liver disease and
treatment. IG or antiviral agents are not recommended for past-exposure
proph.ylaxisof hepatitis
C. No vaccine =against hepatitis C is available. Health-care institutions
should consider
implementing policies and procedures to monitor health-care workers for HCV
infection after
percutaneous or permucosal exposures to blood. The CDC recommends, at minimum,
that such
policies. should include:
= For the source, baseline serologic testing for anti-HCV;
= For the person exposed to an anti-HCV positive source, baseline and follow-
up
(e.g. six months) serologic testing for anti-HCV and alanine aminotransferase
activity;
= Confirmation by supplemental anti-HCV testing of all anti-HCV results
reported
-as repeatedly reactive by EIA;
= Education of health-care workers about the risk for and prevention of
occupational transmission of all blood borne pathogens, including hepatitis-C,
using iip-to-date and accurate information.(CDC, 1997). =
There are otheir diseases for which immunizations of health-care workers are
or=may,be
indicated. Diseases are included in this category for one=of the following
reasons;
= Nosocomial transmission occurs, but health-care workers are inot at
increased risk
as a result of occupational exposure (e.g. hepatitis. A), =
= Occupational risk may be high, but protection via active or passive
immunization
is not available (i.e. pertussis), or .. , .
= Vaccines are.available but are not routinely recommended for all health-care
workers or are recommended only in certain gituations (i.e. vaccinia and
meningococcal vaccines).
-Occupational exposure generally does not increase health-care worker's risk
for hepatitis A virus
(HAV) infection. When proper infection control practices are followed,
nosocomial HAV
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
54
transmission is rare. Outbreaks caused by transrriission of HAV to neonatal
interisive care unit..
staff by infants infected thiough transfused blood have occas'ionally been
observed (Rosenblum,
et al. 1991). Transmission of HAV.from= adult patients to health-care workers
is usually
associated with fecal incontinence in the patients. However, most patients
hospitalized virith
hepatitis A are'admitted after onset of jauniiice, when they are beyond the
point of peak
infectivity (Goodman, 1985). Serologic surveys among many types of health-care
workers have
not identified an elevated'prevalence'of HAV infection compared with
other.accupatiorial
populations.
Two specific prophylactic measures are available for protection against
hepatitis A-
administratiori of immune globulin (IG) and hepatitis A vaccine. When
administered withiri two'.
weeks after an exposure, IG is > 85% effective in preventirig.hepatitis. There
are two inactivated
hepatitis A vaccines currently available in the United States. - The duration
of clinical protection
has not yet been established. An InnnunoScore database built from surveillance
of health-care
workers can thus be instrumental in the determination of the duration of
clinical protection of =
each of these vaccines.
Nosocomial transmission of Neisseria menfngitidis is uncornmon. In rare
instances, direct
contact with respiratbry secretions of infected persons (e.g. during mouth to
mouth resuscitation)
has resulted in transmission from patients with meningococcemia or
meningococcal meningitis
to health-care workers. Although meningococcal respiratory infections are
rare; health-care
workers may be at increased risk for meningococcal infection if exposed to N.
meningitidis-
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
infected patients with active productive coughs. Health-care workers can
decrease the risk for* .
infection by adhering to precautions to prevent exposure to respiratory
droplets.
Post-exposure prophylaxis is advised for individuals who have had intensive,
unprotected contact
with infected patients (e.g. intubating, =resuscitating, or olosely exarnining
the oropharynx of
patients).. Antimicrobial prophylaxis can eradicate carriage of N.
Meningitidis and.prevent
infections in individuals who have uriprotected-exposure to patients-with
meningococcal
infections. =
Although useful for controlling outbreaks of serogroup C meningococcal
disease, administration
of quadrivalerit A, C, Y, W-135 meiiingococcal polysaccharide.vaccines is of
little benefit for
-post-exposure prophylaxis. The. serogroups A and C vaccines, which have
demonstrated
estimated efficacies of 85-100% in older cliildren and adults, are useful for
control of epidernics.
The decision to implement mass vaccination to prevent serogroup C
meningococcal disease =
depends on whether the occurrence of more than one case of the disease
represents an outbreak
or an unusual clustering of endemic meningococcal disease. Surveillance for
serogroup C
disease and calculation of attack rates can be used to identify outbreaks and
determine whether,
use of meningococcal vaccine is warranted. The meningococcal diagnostic panel
of the
ImmunoScore diagnostic application=would be a useful tool to monitor health-
care workers, and
to also identify health-care workers at increased risk for meningococcal
disease.
Pertussis is highly contagious. Secondary attack rates among susceptible
hotisehdld :contacts
exceed 80% (Mortimer, 1990). Transmission occurs by direct contact with
respiratory secretions
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
56
or large aerosol droplets from the respiratory tract of iinfected persons. The
incubation period is
generally 7-10 days. The period of communicability starts with the onset of
the catarrhal stage'
and extends into the paroxysmal stage. Vaccinated adolescents and adults,
whose immunity
wanes 5-10 years after the last dose of vaccine (usually administered at age 4-
6 years), are. an
important source of pertussis infection for susceptible infants. The disease
can be transmitted
from adult patients to close contacts, especially unvaccinated children. 'Such
transmission may
occur in households and hospitals.
Transmission of pertussis in hospital settings has been documented in several
reports (Christie, et
al. 1995; Kurt, et al. 1972; Valenti, et al. 1980). Transmission has occurred
from a hospital
visitor, from hospital staff to patients, and from patients to hospital staff.
Although of limited
size, documented outbreaks were costly and disruptive. In each outbreak,
larger .riumbers of staff
were evaluated for cough illness and required nasopharyngeal cultures,
serologic tests,
prophylactic antibiotics, and exclusion from work..
During outbreaks that occur in hospitals, the risk for contracting pertussis
among patients or staff .
is often difficult to quantify because exposure is not well defined. Serologic
studies conducted=
among hospital staff during two outbreaks indicate that exposure to pertussis
is much more =
frequent than the attack' rates of clinical disease indicate (Mortimer, 1990;
Christie, et al. 1995;
Kurt, et al. 1972; Valenti, et al. 1980). Seroprevalence of pertussis
agglutinating-antibociies
correlated with the degree of patient contact and was highest among pediatric
house staff (82%)
and ward nurses (71%), and lowest among nurses with administrative.
responsibilities (35%) (Linnemann, et al. 1975).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
57
Prevention= of pertussis transmission in health-care settings involves
diagnosis and- early
treatment of clinical cases, respiratory isolation of infectious patients who
are hospitaiized, -
exclusion from work of staff who are infectious, and post-exposure
prophylaxis. Early diagnosis
of pertussis, before secondary transmission occurs, is difficult because the
disease is highly
-communicable during the catarrhal stage, when symptoms are still
non=specific. Pertussis should be one of the differential diagnoses for any
patient with an acute cough illness of >.7 days
duration without another apparent cause, particularly if characterized.by
paroxysms of coughing,
post-tussive vomiting, whoop, or apnea (CDC, 1997). Health-care settings would
be the ideal
placement for ImmunoScore diagnostic assays for pertussis. Periodic
measurement of the level
of pertussis antibody in health-care workers could become part of routine
screening to protect
both the health-care worker and the patient populations:
One acellular pertussis vaccine is immunogenic in adults, but does not
increase risk for adverse
events when administered with tetanus and diphtheria (Td) toxoids, as compared
with the
administration of Td alone (Edwards, et al. 1993). If acellular pertussis
vaccines are licensed for
use in adults in the future, booster doses of adult formulations of acellular
pertussis vaccines may
be recommended to prevent the occurrence arid spread of the disease in adults,
including=health-
care workers. However, acellular pertussis vaccines combined with diphtheria
and tetanus
toxoids (DTaP) will need to be reformulated for use in adults, because all
infant formulations
contaiin more diphtheria toxoid than is recommended for individuals aged > 7
years.
Recommendations regarding routine vaccination of adults will require
additional studies (e.g.
studies of the 'incidence, severity, and cost of pertussis among adults;
studies of the efficacy and
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
58
safety of adult formulations of.DTaP; and studies of the effectiveness and
cost-effectiveness of a
strategy of adult vaccination, particularly for health-care workers). Even
prior to =such.a
reconunendation by the ACIP, -an ImmunoScore diagnostic assay for pertussis
and 'the patient
database can be a valuable tool for evaluating the need and the effectiveness
of the.vaccine '
application.
The incidence of typhoid fever declined steadily in the United States from
1900 to 1960 and has
remained at a low level. During 1985-1994, the average number of cases
reported annually was.
441 (CDC, 1997). Nearly three quarters of patients infected with Salmonella
typhi reported
foreign travel during the 30 days before onset of symptoms. During this ten
year period, several
.cases of laboratory acquired typhoid fever were reported among microbiology
laboratory
workers, only one of whom had been vaccinated. S. typhi and other enteric
pathogens may be
nosocomially transmitted via the hands of personnel who are infected.
Generally, personal
hygiene, particularly hand washing before and after all patient contacts, will
minimize -risk for
transmitting enteric pathogens to patients. If health-care wor=kers;coritract
an acute diarrheal.
illness accompanied by fever, cramps, or bloody stools, they are likely to be.
excreting large
numbers of infective organisms in their feces. Excluding these workers from
care of patients
until the illness has been evaluated and treated will prevent trainsmission. -
Workers in
microbiology laboratories who frequently work with S. typhi should be
vaccinated with any one,
of the three typhoid vaccines distributed in the United States. InimunoScore
diagnostics.would
be able to monitor the immune status of vacciriated individuals.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
59
Smallpox,is a highly contagious infection caused by the DNA virus variola, a
member of the
genus Orthopoxvirus. As recently as 1967, millions of smallpox cases per year
were reported in
Asia and Africa. Smallpox is spread most efficiently in droplets "or aerosols
from the oropharynx
of infected individuals. Smallpox also can be spread by direct contact with
iinfected lesions or
with clothing or bed linens contaminated with the virus. After the incubation
period of 7'to 17
days, the period of infectivity begins as an exanthema and rash characterized
by maculae
progressing to papules, vesicles, and pustules 'all in the same stage,
developing first,on the face
and extremities. Patieiits remain contagious until the scabs have been. shed.
Most patients are
sick enough during the prodromal period to be confined to bed by the time the
rash develops.
For, this reason, household contacts, hospital workers, and other health-care
professionals are the
most likely'individuals to develop secondary cases.
.Case fatality rates.of 30% or higher were observed during epidemics of
smallpox.. In the- absence
of pre-existing'irnmunity, a favorable prognosis isless-likely for infants,.
the.elderly and pregnaint
women. Immunodeficiency,'whether.from immunosuppressive therapy or-from human
immunodeficiency viriis (HIV) infection, is likely to have a negative impact
on prognosis.
Secondary bacterial infections of the-skin, eyes and respiratory tract can
develop and lead to =
septicemia and disseminated bacterial disease: Laryngeal=lesions can lead to
edema and airway
obstruction.. Encephalitis also may corriplicate smallpox.
After an extensive worldwide eradication program,'the last non-laboratory case
of smallpox
occurred in 1977 in Somalia. In 1972, routine smallpox immunization was
discontinued in the =
United States, and since 1983, vaccine production has been halted. Stockpiled
vaccine has been
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
601
used only. for laboratory researchers working on orthopoxviruses.- In recent
years, there has been
a concern that smallpox virus stocks may be in the hands=of bioterrorists, and
thi's'concern has
been heightened by the:terrorist attack on the World Trade Center and the
Pentagon on
September 11, 2001. Because most of the population is considered to be non-
inamune, there is
debate as to whether smallpox immunization should be resumed.
Protection from infection was provided in the past.liy immunizing all children
beginning at one
year of age. An individual's concentration of neutralizing antibodies declines
signific.antly over
a 5 to 10, year period, and people who were immunized as infants or children
before 1972 are
unlikely to remain fully protected against -disease, but protection against
death afforded by
antibodies and cell-mediated immunity may persist for 30 years (AAC, 2003):
There are current concerns regarding smallpox. Stocks.of smallpox virus
were.retained in
government run laboratories of the United States and former Soviet.Union.
=There- are reports
that, before the dissolution of the Soviet Union, smallpox was being developed
as a weapon of
biological warfare (Henderson, et al. 1999). In addition, decreasing financial
support for Russian.
government laboratories in recent years led to concern that ttie virus and the
6xpertise to .
propagate a large amount of smallpox virus may have fallen into iion-Russian
hands. The
rapidity with which smallpox could spread in the U.S. population has led=to
concern that this
agent would present a particularly potent threat if it were to be used as
an'agent of bioterrorism .
(O'To61e, 1999; Meltzei=, et al. 2001).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
61
Immunization causes a local infection that is pruritic and uncomfortable.
Fever, malaise, and
regional lymphadenitis often occur about a week after immunization. The site
of immunization
develops a papule that matures =into .a pustule and then a scab that separates
by about the third
week after immunization. Re-immunization typically causes a milder lesion that
develops more
quickly. Occasionally, satellite or distant pustules develop when a vacciiie
recipient scratches
the pustule and auto inoculates the virus at another site.
A major reason. not to initiate universal immunization in the absence of
actual cases of smallpox
besides the limited availability of vaccine is the risk of serious
complications of immunization.
Severe complications of immunization include death, post-vaccinal
encephalitis, progressive
vaccinia, eczema vaccinatum, generalized rash, and accidental inoculation to
the face, eye or
other sites. Smallpox vaccine has been known for decades to produce
significant adverse effects,
especially with immunocompromised individuals. In patients with'chronic skin
conditions,
smallpox vaccine can cause a severe, so.metimes fatal dermatologic involvement
termed eczema
vaccinatum. The list of conditioris that place patients at risk of eczeina
vaccinatum is long and=
includes most disorders that disrupt epidermal integrity. Atopic dermatitis is
the most common.
disorder associated with severe eczema vaccinatum, and people with this
disorder may be
susceptible even if the skin disorder is in remission. Even unimmunized
susceptible individuals
can have such reactions if the virus spreads to them from those who have been
immunized.
Smallpox vaccine is not recommended for people with eczema= or other
exfoliative 'skin
disorders, for pregnant women, or for people with immunodeficiencies, whether
primary or
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
62
secondary. Atopic dermatitis, a genetically based inuziune abnormality, occurs
withiri the first
five years of life and affects 15% of the population.
Before its discontinuation, universal smallpox immunizationwas recommended in
the United
States for children 1 to 2 years of age. Re-immunization was recommended every
5 years and
-annually for people working in endemic areas. The current recommendation =for
=those
individuals at high risk because of occupational exposure is immunization
every 3 years. -People :
with multiple immunizations =during childhood probably have =longer-lasting
immunity, but the
degree of protection for those immunized before 1972 is unknown.
In the event of a known bioterrorist release of the smallpox- virus, vaccine
would be administered.
to exposed individuals. If vaccine is given within 3 to=4 days of exposure,
immunity can develop-
before'the disease= occurs, and this would be expected to prevent or
ameliorated the severity of
the disease. Post-exposure immunization is recommended foir persons who have
had face-to-
face, household contact with or have been in proximity to a person who has
active smallpox skin
lesions, persons who have been involved in the care of such an individual, and
persons exposed =
in any way to laboratory specimens or bedding from an infected patient. Such a
plan (referred to
as a "ring vaccination" program) would allow the most eÃfective=iise of
available stocks of
vaccine while =exposing a minimal number of individuals to=the risks of
immunization.
Variola virus as an agerit of bioterrorism has been'discussed widely,.but-the
difficulty of
introducing the virus into the population and the limited effects of doing so
have,persuaded most
public health authorities that the chances of a smallpox outbreak are very
sriiall. Because of the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
63
known adverse effects of smallpox immunization, the large number of
immunocompiomised
people in the population, and the currently limited supplics of vaccine. and
IG, all *stockpiled.
vaccine is considered an investigational agent and is available for use by
public health authorities
only.
=The major proposed strategies for smallpox immunization in the' face of,a
bioterrorism threat:
include mass immunization, voluntary irnmuriization; and ring vaccination.or
"surveillance and
containment " The proponents of mass immunization claim it to be the strategy
that would most
effectively prevent spread of disease. They also postulate that a bioterrorist
would be unlikely to
introduce variola into a well immunized population. Those who favor voluntary
immunization
feel that each individual should be allowed to weigh the pros-and'cons of
irnmunization and act
according to his or her own analysis (Bicknell, 2002). Unfortunately, much of
the population is
not fariniliar with the problems and complications of vaccinia immunization.
T,he.ring
vaccination strategy is siupported by the American Academy of Pediatrics
(AAP), =which
consideis this option to be the best approach at present (AAP, 2002).
The AAP supports the current CDC recommendation of the strategy known as ring
vaccinati=on,
also referred to as surveillance and containment. Using this'approach, if
smallpox were =
introduced in an act of terrorism, infected patients would be isolated.
Contacts of infected
individuals as well as their contacts would then be identified and immuniz-ed
by specially trained
teams 'of health-care professionals. This strategy can control a localized
outbreak with minimal
exposure of vulnerable populations to the complications of immunization. The
ring strategy'is
based on the knowledge that vaccination can prevent or.ameliorate disease
severity if given
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
64
within 3 to 4 days of initial exposure and can decrease symptoms if,given
withiri the first week of
exposure.
Immunizing and monitoring a ring of people around each infected individual and
his or her
contacts would help protect those at the greatest risk of contracting the
disease and form a.buffer
of immune individuals to prevent the spread of disease: The AAP supports the
opinion of tlie .
ACIP that it is desirable to have patients with smallpox cared for by persons
who have been
immunized. Thus, national, state-based, and local teams of health-care
professionals who
already have been immunized will be trained in all aspects of smallpox
investigation=and care
and will be available to go immediately to the site of a suspected or proven
smallpox case. With
teams available in every state, approximately 10,000 to 20,000 carefully
screened individuals
will receive smallpox vaccination. For health-care workers, it will be
necessary to monitor their
immune status with regards to smallpox immunizations. This can be a function
of the.
ImmunoScore diagnostic panel. Realth-care,professionals and military personnel
can provide.a
sound basis for the accumulated database regarding the persistence of
immuanityto smallpox
infection.
Health-care workers are not at greater risk for diphtheria, tetanus, and
pneumococcal disease '
than the general population. ACIP recommends that all adults be protected.
against diphtheria
and tetanus, and recommends pneumococcal vaccination of all persons aged > 65
years and 6f
younger individuals who have certain medical 'conditions. Thus, in exemplary
embodiments of
the present invention, an ImmunoScore diagnostic evaluation can be used
to.assess an individual.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
health-care worker's immune status with regards to these, or other, as may be
appropriate,
infectious diseases.
14.1. Immunocompromised Health=Care Workers
A physician inust assess the degree to which an individual health-care worker
is
immunocompromised. Severe immunosuppression can be the, result of congenital
immunodeficiency; HIV infection; leukemia; lymphoma; generalized mali=gnancy;
or therapy with alkylating reagents, antimetabolites, radiation, or large
amounts of corticosteroids. All
persons affected by some of these conditions are severely immunocompromised,
whereas for
other conditions (e.g. HIV infection), disease progression or treatment stage
determines the
degiee of immunocompromise. A determination that a health-care worker is
severely
immunocompromised ultimately must be made by his or her physician.
Immunocompromised
=health-care workers and their physicians should consider the risk for
exposure fo a vaccine-
preventable disease together with the risks and benefits=of vaccination.
The exact amount of systemically absorbed corticosteroids and the duration of
administration
needed to suppress the immune system of an otherwise healthy individual are
not well defined.
Most experts agree that steroid therapy usually does not contraindioate
administration of live
virus vaccines such as MMR and it=s component vaccines when therapy is a)
short term L.14
days) low to moderate dose; b) low to moderate dose administered daily or on
alternate days; c)'
long-term alternate day treatment with short-acting preparations; d)
maintenance physiologic
doses (replacement therapy); or e) administered topically (skin or eyes) by
aerosol, or by intra- =
articular, bursal, or tendon injection. Although the immunosuppressive effects
of steroid =
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
66
treatment vary, many clinicians consider a steroid dose that is equivalent to
or greater than a
prednisone- dose of 20 mg per day sufficiently immunosuppressive to cause
concern about the
safety of administering.live virus vaccines. Persons who have received
systemic corticosteroids
in excess of this- dose daily or on alternate days for an interval for > 14
days*should avoid
vaccination with MMR and its component vaccines for at least one month after
cessation of
steroid therapy. Individuals who have received prolonged or-extensive=topical,
aerosol; or other
local corticosteroid therapy should not receive IvIMR or its component
vaccines, and varicella
vaccine for at least one month after one month after cessation of therapy.
Persons who have a.
disease that, in itself, suppresses the immune response and who are also
receiving either systemic
or locally administered corticosteroids generally should not receive IvIMR,
its component
vaccines, or varicella vaccine. The use of ImmunoScore diagnostic analyses.and
database for
immunocompromised health-care workers can be used for assessing these workers
and in
monitoring them following corticosteroid therapy for levels of immune
response.
In general, symptomatic HIV-infected individuals have suboptimal immunologic,
responses to
vaccines. The.response to both live and killed antigens may decrease as the
disease progresses
(Vardinon, et al. 1990). Administration of higher doses of vaccine or more
frequent boosters to
HIV-infected persons may be considered. However, because'neither the initial
immune response'
to higher doses of vaccine nor the persistence of antibody in HIV-infected
patients has been
systematically evaluated, recommendations cannot be made at this time (CDC,
1997).
Limited studies of MMR immunization in both asymptomatic and symptomatic HIV-
infected
patients who did not have evidence of severe immunosuppression documented no -
serious or
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
67
unusual adverse events after=vaccination (Onorato, et al. 1992). HIV-infected
persons are at
increased risk for severe complications if infected with measles. Therefore,
MMR vaccine.is
recommended for all asymptomatic HIV-infected health-care workers wbo do not
have evidence
of severe immunosuppression. Administration of MMR to HIV-infected health-care
professionals who are asymptomatic but do not have evidence of severe
immunosuppression
because of a) a case of progressive measles pneumonia'has been reported after
administration of
MMR vaccine to a person with AIDS and severe immunosuppression, b)=the
incidence of
measles in the United States is currently very Iow, c) vacciination-related
morbidity has been
reported in severely immunocompromised persons who were not HIV-infected, =and
d) a
diminished antibody response to measles vaccination occurs amorig severely
immunocompromised H1V-infected individuals.
Recommendations of the CDC (1997)
Recommendations for administration of vaccines and other immunobiological
agents to health-
care professiorials are organized in three broad disease categories:
= those for which active immunization is strongly recommended because of
special
risks for health-care workers (i.e. hepatitis=B, influenza, measles, mumps,
rubella,
and varicella); . = those for which- active and/or passive immunizations of
health-care workers may
be indicated.in certain circumstances.(i:e. tuberculosis, hepatitis A
meningococcal
disease, typhoid fever, and vaccinia) or in the fuiture (i.e. pertussis); and
= those for which the immunization of all adults is recommended (i.e. tetanus,
diphtheria, and pneumococcal disease).
Immunization is strongly recommended for hepatitis B, influenza, measles,
mumps, rubella, and
varicella. In exemplary embodiments of the present invention, an ImmunoScore
diagnostic panel
can be in place in health-care settings for the routine monitoring of health-
care professionals.
=S,uch an ImmunoScore database can combine information obtained from iminune
status of
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
68
health-care workers with tha't of other segments of the population. The panel
cari be* a valuable.
tool for the health-care industry and hopefully reduce the burden ,of vaccine-
preventable
nosocomial illnesses. There are other diseases for which vaccination should be
considered; those
include tuberculosis (pretty much as a last resort), hepatitis A, pertussis,
meningococcal disease,
typhoid fever, and vaccinia. Other vaccine=preventable diseases for which
protectioin should be
maintained include tetanus, diphtheria; and pneumococcal disease. 'Levels of
antibodies can be
monitored periodically by ImmunoScore diagnostic immune status assays. In
addition,.the overall immune health could be measured ini.t'ially using the
meningococcal diagnostic panel. A
typhoid fever antibody assay could also be developed for health-care
professionals. In addition,
a hepatitis C antibody assay can also need -to be established. There is as yet
no vaccine for
hepatitis C, but an HCV infection presents a risk of nosocomial,infections:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
699
Table 7: Recommended Immunizing Agents for'Health Care Workers
'Vaccine Schedule
=
Hepatitis B ' '. 3 doses
Influenza Annual =
Measles, Mumps, Rubella- = 1 dose .
Varicella ' = =. 2 doses Tuberculosis BCG 1 does (in hi risk settin s
He atitis A '= 2 doses =
Meningococcal disease 1 dose..
Pertussis .1 d'ose (needs reformulation '
Typhoid 1 dose, boost 2 yrs.
Vaccinia = . 1 dose, boost 10 yrs.
Tetanus, diphtheria (Td) ' 1 dose; boost 10 yrs.
Pneumococcal polysaccharide I dose, boost>5 yrs. in ' risk settin s
= The only analyte specific for health care workers is tuberculosis. There is
no easily
measurable correlate of immunity to tuberculosis.. Delayed type
hypersensitivity, as
measured by the tuberculin test; is tiot' a measure of res'istance, because
both reactivation'
and superinfection may occur in tuberculin-positive_ subjects'(Plotkin, 2001).
Altliough
current experimental vaccinology is investigating the potential of.proteins
and capsule of
Mycobacterium tuberculosis, the majority opinion is still that antibodies are
irrelevant to
protection (McAdam, 1997). Thus, in exemplary embodiments of the present
invention,
ImmunoScore diagnostic analysis.can include measurement of as yet undetennined
cellular components important to controlling TB infection.'
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1-5. ImmunoScore Arialyses and Bioterrorism
Before the anthrax attacks of 2001, the threat of bioterrorism was based
primarily on the events
surrounding the sarin nerve agent attacks in Japan in 1995 and the biological
weapons production
and stockpiling programs of the former Soviet Union and Iraq. Now that
teriorists haveused the
United States Postal Service to disseminate anthrax spores contained
in,letters,.the threat of
lethal bioterrorism has become a reality (Darling, et.al. 2002).
In June of 1999, the Centers for Disease Control and. Prevention (CDC) and a
multidisciplinary
panel of experts formed a strategic workgroup to outline steps to strengthen
the US public health
irifrastructure and health-care capacity to protect against bioterrorism (CDC,
2000). They stated
that the public health infrastructure inust be prepared to prevent illness and
injury that would
result from biological and chemical terrorism, especially a covert terrorist
attack.
In the past, most planning for emergency response to terrorism has been
concerned with overt
attacks (e.g. bombings). Chemical terrorism acts are likely to be overt
because the effects of chemioal agents absorbed through inhalation or by
adsorption through'the skin or mucous
membranes are usually immediate and obvious. Such attacks elicit iminediate
response from
police, .fire, and EMS personnel.
In contrast, attacks withbiological agents are more likely to be covert. They
present different
challenges and require an additional dimension of emergency planning that
involves the public
healtli infrastructure. Covert dissemination of a biological agent in a public
place will not have
an immediate impact because of the delay between exposure a.nd the onset of
illness.,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
71
Consequently, the first casualties of a covert attack probably will be
identified by pliysicians,or
other primary health-care providers.
Potential biblogical and chemical agents are numerous, and the public health
infrastructure must
be equipped to-quickly resolve crises that would arise from a biological.or
chemical- attack.
However, to bestprotect the public, the preparedness efforts must be focused
on agents thav.
might have the greatest irnpaat on U.S. health and security, especially
agen'ts tliat are highly
contagious or that can be engineered for widespread dissemination via small-
particle aerosois.
Early detection =requires increased biological and chemical terrori sm
awareness ariiong front-iine
health-care providers because they are in the best position to report
suspicious illnesses and
injuries. Also; early* detectiori will require improved communication systems
between those-
providers and public health officials. - In addition, state and local health-
care agencies must have
enhanced capacity to investigate unusual events and unexplained illnesses, and
diagnostic
laboratories must be equipped to identify' biological and chemical agents that
are rarely seeri in
the United States. Fundamental to these efforts is comprehensive, integrated
tiiaining designed to
ensure core competency in public health preparedness and the highest levels of
scientific
expertise among local, state,and federal partners. ImmunoScore diagnostic
analyses can be an
integral part of preparation of events of bioterrorism.
The CDC has outlined the following steps for preparation for terrorist.attacks
using. biological
agents: ' = Enhance epidemiologic capacity to detect and respond to biological
attacks.
= Snpply diagnostic reageints to state and local public health agencies. '
= Establish communication programs to erisure delivery of accurate
inforrnatiori.
= Enhance bioterrorism-related education and training for health-caie
professionals.
= Prepare educational rriaterials that will 'inform and reassure the public
during and after
a biological attack. = Stockpile appropriate vaccines and drugs.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
72
=. Establish molecular surveillance.for microbial strains, including unusual
or drug-
resistant strains.
= Support the development of diagnostic tests.
= Encourage research on antiviral drugs and vaccines.
The planning group assembled by the CDC categorized biological agents
according to their.
perceived level of threat. The first of these are Category A agents. These
high-priority agents
include organisms that pose a risk to national security because they:
= can be easily disseminated or transmitted person-to-person;
= cause high mortality, with potential for major public health impact;
= might cause public panic and social disruption; and
"require special action for public health preparedness.
Category A agents, include:
= Variola major (smallpox)
= Bacillus anthracis (anthrax)
= Yersiniapestis (plague)
= Clostridium botulinum toxin (botulism)
= Francfsella tularensis (tularaemia)=
= filoviruses: o Ebola hemorrhagic fever
o Marburg hemorrhagic fever
= arenaviruses:
o Lassa (Lassa fever)
o Junin (Argentine hemorrhagic fever) and related viruses - -
It would be difficult to create a more ` perfect" biological weapon than
Bacillus anthracis, the,
causative agent of anthrax. Infection, usually by spores, is introduced
througli scratches or.
abrasions of the skin, inhalation, eating insufficiently cooked infected meat,
or by the bites of
flies. Anthrax spores may remain stable for decades or can be produced,
weaponized, and
delivered as a wet or dry= aerosol cloud.=
The bioterrorism related inhalational .anthrax cases that occurred during the
fall of 2001
presented in a predictable manner with a few, exceptions. Nearly all patients
initially developed
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
73
fatigue and malaise followed by minimal or non-productive cough. They soon
developed fever,.
chills, nausea, vomiting, and drenching sweats. This progressed to chest pain
and dyspnea.
Bacillus anthracis is detectable by Gram stain of the blood, blood culture on
routine media, and
by ELISA, but often not until later in the course of the illness.
Approximately 50% of the cases
are accompanied by hemorrhagic meningitis, and therefore organisms may also be
identified in
cerebrospinal fluid (Bush, et al. 2001). Only vegetative encapsulated bacilli
are present during
infection. Spores are not found within the body unless the bacilli are exposed
to ambient air.
Toxin production parallels the appearance of bacilli in the blood, and tests
are available to.
rapidly detect the toxin. With the appearance of symptoms, the white blood
cell count becomes
elevated and remains so until death. The primary cause of morbidity and
mortality is believed to
be the extreme toxin load generated by the organism.
More than 30,000 patients have been taking ciprofloxacin or doxycycline as
post-exposure
prophylaxis since the.b'ioterrorism incidents of October 2001 (CDC, 2001). If
confirmed that
anthrax has been used as a biological weapon, antibiotics should be continued
for at least 60 days
in all exposed individuals, and patieints should be closely followed after
antibiotics are
discontinued. Military doctrine also requires that service members begin
active immunization.
with anthrax vaccine while taking post-exposure antibiotics. Anthrax vaccine
is not currently
available for use by the general public. In response to the bioterrorism
events of 2001, however,
CDC offered anthrax vaccine as part of an investigational new drug,(IND)
protocol. This Was
necessary because anthrax vaccine was never licensed for use as a post-
exposure treatment
(Michigan Dept. Public Health, 1978). The CDC had no recommendation as to
whether patients
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
74
should or should not receive the vaccine; this led to considerable confusion
and consternation
among the public. Consequently, few patients chose to receive the vaccine.
On discontinuation of antibiotics, patients should be closely observed. If
clinical signs of
anthrax develop, empiric therapy for.anthrax is indicated, pending etiologic
diagnosis.
Optimally, patients should have medical care available from a fixed facility
with intensive care.
capabilities and readily available access to infectious 'disease consultants.
ImmunoScore =
diagnostic assays could become integral to diagnosis and treatment of anthrax
patients. The.
database information would be valuable at helping to determine the serological
correlate of
protection for aritlirax vaccine.
Smallpox is caused by the Variola virus. There are no non-human reservoirs for
smallpox and
. no human carriers. The disease has survived throughout history through
continual person-to-
person person transmission. Smallpox was probably responsible for more than
100. million deaths
during the 20th century alone:
Smallpox.is perhaps the most feared of potential biological warfare agents.
Researchers estimate
.that vaccinated individuals retain immunity for approximately.10 y,ears,
although in selected
populations this may continue past 20 years (Henderson'and Moss, 1999).
Therefore, most of
the population of the United States is probably susceptible to smallpox:
Vaccines are in'short
supply; however, the Federal government has entered into contracts to rectify
this. Finally,'
because few physicians are familiar with the clinical presentation of
smallpox, recognizing ari
outbreak may be problematic.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
The smallpox virion is readily transmitted from,person to person by way of
respiratory particles.
Virions can also remain viable on fomites for up to. one week. The virus
initially replicates in
respiratory tract epithelial cells then migrates to regional lymph nodes. From
there, a massive
asymptomatic viremia ensues three to four days later and may result in focal
infections involving
lymphoid,tissues, skin, intestines, lungs, kidneys, or braiii (Henderson, =et
al. 1999). Initial
symptoms resemble anacute viral illness. Following an incubation period of
approximately.12 days, a second viremia, lasting two to five days; results-in
high fevers,. malaise, headaclie,
backache, rigors, and vomiting. The patient may develop delirium. A rash
typically develops -
within 48 hours, beginning in the mouth, and heralds the onset of viral
shedding. Tlie rash
rapidly spreads to the hands and forearms followed by the legs and trunk. The
rash becomes
distinctive when the lesions become pustular. Viral shedding and secondary
infection cases may
occur from the onset of rash until scabs have separated (Henderson; et al. -
1999).- Death usually
occurs late in the first week or during the second week of the illness. and is
caused by the toxemia -
induced, by the overwhelming viremia.
During the vesicular stage, the rash may resemble cliickenpox. There are two
important
distinctions, however. First, the rash of smallpox develops =syrichronously,
in contrast to ttie=
asynchronous development observed with varicella. Second, the rash of
'smallpox is
concentrated on the face and extremities, as opposed to on the trunk as occurs
in.chickenpox
(Henderson, 1999)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
76
Initial diagnosis will likely be clinical, based= on the characteristic rash.
Diseases with similar
skin manifestations must be considered in the differential diagnosis,
including cutaneous lues
(syphilis), meningococcemia, acute leukemia, or drug toxicity. Laboratory
confirmation is
extremely important, as a single case of smallpox must be treated as an
interimational public
health emergency. Smallpox can be confirmed through clinical presentation and
identification of
the virion particles on electron microscopy of vesicular fluid, although this
only confirms
presence of an orthopox virus. Further classification of the orthopox virus
requires cell'culture
or growth on chorioallantoic egg membrane. Immuno5core diagnostic analysis can
be used-to.
identify'levels of smallpox antibody in sera of individuals. In addition;
ImmunoSc6re database
analyses could be performed on larger numbers of individuals'to track the
longevity of serum
antibody to' smallpox.
Yersinia pestis, a gram-negative bacillus, has tormented mankind throughout
history.' The
Byzantine Empire recorded a sixth century pandemic, and the Black Death killed
millions of
people throughout.140` century Europe. The most recent pandemic originated in
China and
spread worldwide at the turn of the 20'' century.
.Plague is a zoonosis with a rodent host and a flea vector. The vector is not
essential, however,= =
and direct host-to-host transmission cari occur by way of an infectious
aerosol. A bite from an
infected flea causes an infection in the =lymphatic system leading to the
bubonic form of the
disease. Inhalation of aerosolized bacillus, preferred for deliberate
dissemination, results in a
primary pulmonary infection, known as pneumonio plague. The disease is rapidly
fatal in the
absence of prompt antibiotic treatment and may result in, secondary aontagion
spread.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
77
Modern efforts to weaponize Y. pestis were begun by the Japanese during World=
War II, but
dissemination attempts were met with limited success. Infected fleas were bred
by the billions
and then released over northern Chinese cities that had not previously
recorded plague casualties.
Epidemics subsequently occurred and plague has remained endemic in the region
since
(Williams and Wallace, 1989). The United States dismissed plague as a
potential weapon
because of its persistence in the environment and friendly casualties after
ari attack.. The former
Soviet Union's extensive biological warfare program, however, reportedly
included, dry,
antibiotic-resistant, environnientally stable form,s of the plague organism
that could be
disseminated as ari aerosol (Alibek, 1999).
Skin penetration or direct ingestion of fewer than ten Y. pestis organisms can
induce an infection
. in humans. The clinical course will vary substantially with the route of
exposure. = If plague were '
used as a biological.weapon, the most likely exposure would be via inhalation.
Pneumonic
plague presents without buboes and may progress rapidly if vegetative
organisms with
previously developed antiphagocytic capsules and Yersinia outer-membrane
protein (Yop) antigens' have been inhaled as an aerosol (Poland, 1989). Most
patients develop=a productive
cough with blood-tinged sputum within 24 hours of the onset of symptoms. This
is an important
diagnostic clue that should lead one to oonsider bioterrorism if many
previously well patients
present with this sign (Cavanaugh, et al. 1982).
Serum antibody to Fraction I capsular antigen, as measured by the passive
hemagglutination (PHA) test, is correlated with resistance to Y. pestis
iin.fectiori in experimental animals. A
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
78
comparable correlation between PHA titer and immunity probably occurs iri
hunians {CDC,
1982). Plague vaccine that was protective against bubonic plague is no longer
available. At any
rate, it did not protect against aerosol infection in test iimodels
(Ehrenkranz and Meyer, 1955). A
vaccine for pneumonic plague is under development, but that is not a guarantee
-of siiccess.
ImmunoScore diagnostic analysis should be a willing partner for analyses of
any vaccines under :
development for combating plague.
The causative agent of tularemia, Francisella tularensis, is a small aerobic,
non-motile, gram-
negative, cocco-bacillus. Tularemia is a zoonotic disease that humans may
acquire after skin or
mucous membrane contact with tissues or body fluids of infected animals, or
from bites of
infected ticks,'deerflies, or mosquitoes. Less cominonly, inhalation of
contaminated dusts or
ingestion of contaminated foods or water may produce clinical disease.
Respiratory exposure by
aerosol would typically cause typhoidal or pneumonic tularemia. F. tularensis
remains viable for
weeks in water, soil, ca.rcasses,.hides, and for years in frozen ineat.
Resistant for months to
temperatures of freezing and below, it is easily killed by heat and
disinfectants (Evans and
Friedlander, 1997).
Francisella tularensis was weaponized by the United States in the 1950s and
1960s during the
U.S. offensive biowarfare program. Other countries are suspected to have
weaponized this
agent. This organism could potentially be stabilized for weaponization by an
adversary and
produced in a wet or dried form for delivery against U.S. forces or as a
weapon of terror..
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
79
Onset of disease is usually acute and*occurs after an incubation period that
ranges from 1 to 21
days. In humans, as few as 10 to 50 organisms may cause disease if inhaled or
injected
intradermally (McCrumb, et al. 1957). All ages are susceptible, and recovery
is generally
followed by permanent immunity.
.
Typhoidal tularemia occurs mainly after inhalation of infectious aerosols, but
can also occur affter.
intradeimal or gastrointestinal challenge. F. tularensis wbuld most likely, be
delivered as an
aerosol if used as a weapon and would primarily cause typhoidal tularemia that
manifests as
fevet, prostration, and weight loss. Pneumonia may be severe and fulminant.
Respirato ,ry
symptoms and.a cough (productive or non-productive) may also be present. Case
fatality rates
may be greater than the 1=3 % seen with appropriately treated -natural
disease.: Case fatality rates
are approximately 35% in untreated naturally acquired typhoidal cases
(Darling, et al. 2002).
.Similar to many bacterial and viral diseases, early symptoms of exposure to
F. tularensis are
fairly generic and nonspecific, making differential diagnosis difficult.
At present, a live vaccine strain (LVS) tularemia vaccine is under IND status
in a protocol at the
U.S. Army Medical Research Institute. of Infectious Diseases (USAIvIRIID), and
is available =
only for at-risk U.S. military personnel. .It is administered by*
scarification. Despite the -
increased"risk of a bioterror threat felt after September'11, further vaccine
development.for
tularemia remains slow. 'The projected date of a new licensed vaccine in the
United States is not
until 2009 (Nierengarten and Lutwick, 2002). There is some confusion over
which arm'of the
immune system should be targeted. New lots of LVS produced in the United
States show
immunogenicity in human volunteers, producing both brisk cell-mediated and
humoral immune
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
responses. InimunoScore diagnostic analysis can be applied in this -setting'to
monitor=the
response.to. vaccines in clinical trials and follow the duration of the immune
response. In.
addition, cellular cozxiponents of the immune system can also be tracked, for
example, through
compilation of information added to an ImmunoScore database.
The viral hemorrhagic fevers are caused by a diverse group of RNA viruses in
four separate
familie's: Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae. All have
lipid'envelopes,
liinited geographic ranges, are highly infectious by way of the aerosol route
{except Denga.e),
and are believed to have animal reservoirs with arthropod vectors. Terrorist
groups have
attempted to weaponize agents from this class (Carus, 2001). Each disease is
characterized by its
own unique characteristics, but all have a final common pathway of diffuse
hemorrhage and
bleeding diathesis.
Yellow fever and dengue (Flaviviridae) are probably the archetypical diseases
of this group, but
are not considered significant biological warfare threat agents.
Hantavirus.(Bunyaviridae) is
enzootic in rodents. West Africa's Lassa fever and Argentine, Bolivian,
Brazilian, and
Veneiuelan hemorrhagic fevers (Arenaviridae) are also enzootic in rodents
within their
respective areas. The most publicized viral hemorrhagic fevers are the Ebola
and Marburg
(Filoviridae) viruses. These viruses produce grotesquely lethal diseases. The
reservoir and
natural transmission of Ebola and Marburg are unknown but they are readily
trarismissible by
infected blood and tissue. Aerosols may be formed'naturally when.infectious
body fluids are
expelled or in the case of hantavirus when rodent feces and urine are
resuspended by movement
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
81
in the area. Laboratory cultu'res can yield sufficient concentrations of
organi'sms to provide a
credible terrorist weapon if disseminated as an aerosol (Darling, et al.
2002).
In a bioterrorism scenario, aerosol dissemination would result in many
patients who shared a
common location approximately three to eight days before presentation.
Specific disease
identification currently requires ELISA detection of antiviral IgM antibbdies
or-direct culture of
the viral agent from blood or tissue samples. During.the clinical course of
each of the- diseases,
hepatocellular enzymes are often elevated. 'Appropriate precautions should be
observed' in-
collection, handling, and processing of diagnostic samples, which should be
sent to a Leve1.D :.
laboratory that currently exist only at the CDC or USAMRIM. The only likely
application of=
ImmunoScore for hemorrhagic fever viruses would be in analyses of-vaccines in
development
and the possible placement with the CDC or USAMRIID for diagnoses.
Clostridium botulinum is a gram-positive, spore-forming anaerobic-
bacillus'found in soil around
the world. Botulism is the syndrome caused by botulinum toxin prodizced by
this bacterium:
Cases have historically been categorized according to transmission as food-
borne' illness (from
ingestion of the toxin in home-canned goods, poorly heated= vegetables, or
meats), wound
botulism (secondary to soil-contaminated wounds, drug abuse, and C-section
deliveries), and
infantile illness (from ingestion of spores) (Arnon, et al. 2001).
Botulinum toxin is =one of the most= toxic substances= known (Middlebrook
and==Franz; 1998)..
Seven distinct types of toxin exist, identified'by antigenicity and- referred
to as types A-G.
Botulinum toxin could be used to sabotage food supplies, although a more
likely scenario would
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
82
involve dissemination as an aerosol. During the Gulf War, Iraq produced
20,000'L of botulinum
toxin, 12,000 L of which were used in field testing and to fill warheads
(Zilinskas, 1997).
Despite the efforts to produce an effective botulinum. toxin weapon, most
authorities agree that it
is unlikely this toxin could 'ever be effectively deployed as a weapon of mass
destruction.
Aerosol delivery over a battlefield or a defined geographic region populated
by civilians would
require a precisely orchestrated effort: ' Large quantities'of toxin would
have to be delivered to
the area at the optimum time because -botulinum toxin quickly degrades in the
environment..
Even municipal water reservoirs are most likely safe from contamination by
terrorist actions
because literally ton quantities would be necessary because of the effects of
dilution. -
In the emergency setting the diagnosis of botulism'intoxication will be
clinical. An influx of
patients with descending muscle paralysis and bulbar fuidings may herald a
bioterrorist event or
a natural- outbreak of food-borne botulism. No routine lalioratory tests will
aid in the diagnosis.
The toxin may be detected by assays of seruni or gastric contents.
Three different antitoxin preparations are available in the United States.
Arititoxin may -prevent
progression or shorten the course of the illness. A pentavalent toxoid of
Clostridium botulinum
toxin types A, B, C, D, and E is available as an IND product for pre-exposure
prophylaxis. The.
currently recommended primary series of 0, 2, and 12 weeks, followed by a 1-
year booster,
induces immunity in greater than 90% of vaccinees after one year.(Darling,
et*al. 2002): In
exemplary eniibodiments, ImmunoScore analyses can be useful in examining
response to.the
prophylactic vaccine as well as in following the duration of protection.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
83
The Category B agents are the second highest priority and they include those
agents that:
=, are moderately easy to disseminate;
= cause moderate morbidity and low mortality; and
= require specific enhancements of CDC's diagnostic capacity and enhanced
disease
surveillance
Category B agents include:
= Coxiella burnetti (Q fever)
= Brucella species
= Burkholderia mallei
= alphaviruses
= ricin toxin from Ricinus communis (castor beans)
= epsilon toxin of Clostridium perfringens
= Staphylococcus enterotoxin B
= Salmonella species
= Shigella dysenteriae
= Escherichia coli 0157:H7
= Vibrio cholerae =
= Cryptosporidium parvum
The Category C agents are the third highest priprity and they include.
emerging pathogens that
could be engineered for mass dissemination in the future because of:
= availability.;
= ease of production and dissemination; and
= potential for high morbidity and mortality and major health impact.
Category C agents include:
= Nipah virus
= hantaviruses
= tickborne hemorrhagic fever viruses
= tickbome encephalitis viruses
= yellow fever
= multidrug-resistant tuberculosis
Preparedness for Category C agents requires ongoing research to improve
disease detection,
diagnosis, treatment, and prevention. Knowing in advance which newly
erimergent patkiogens
might be employed by terrorists is not possible; therefore linking
bioterrorism preparedness
efforts with ongoing disease surveillance and outbreak response activities is
imperative.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
84
Although once considered unlikely, bioterrorism is now a reality in the United
States =since the
anthrax cases began appearing in the fali. of 2001. Intelligence sources
indicate there are many
countries and terrorist organizations that either possess biological weapons
or are attempting to.
procure them. =In the future it is likely.that we will experience additional
acts of bioterrorism.
The CDC category A agerits represent'the greatest challenge because they have
the potentiaT-to
cause grave harm to the medical and public health systems of agiven
population. Thus; it is
imperative that plans be developed now to 'deal with the consequences of an
intentionalvelease
of any one or more of these pathogens (Darling, et al. 2002):
In exemplary embodiments of the present inventicin, an IrnmunoScore diagnostic
platform can. be
. constructed so as to be able to .grow with the needs of bioterror agent
analyses. As new agents '
arise, diagnostic testing can available to test for immune responses to such
agents as well 'as any
vaccines that have been or will be= developed.;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
16. ImmunoScore Analyses for Infection and Chronic Qisease
Chronic diseases take a huge toll. In the=United States, more than'70% of all
deaths a=e due to
one or more chronic diseases, and more than 90 million people suffer daily.
Even diseases not.
typically associated with pathogens may have underlying infectious causes. Of
the eight millioxi
new cases of cancer in the'world each year, one milliori are attributable to a
known infectiotis
agent.
The infectious origins of some chronic diseases.have been known for decades.
These include
tuberculosis, syphilis, leprosy, and a number of parasitic diseases. - Only
more recently has it
been realized that coronary artery disease, diabetes mellitus, cancer, and
neurological disorders
can have an infectious etiology, either as a cause or a co=factor.
Koch's postulates for distinguishing a pathogenic from an adventitious microbe
were formulated
in 1884. Koch stated for an organism to be pathogenic, it needed to fulfill
the following criteria:
= the organism is regularly found in the lesions of the disease
=, the organism can be isolated in pure culture on artificial media
= inoculation of this culture produces a similar disease in experimental
animals .
= the organism can be recovered from the lesions of these newly infected
animals
Koch's postulates=are indeed relevant for acute infections, but there is-a
problem using them
when considering chronic or long-term symptoms. With chronic illness, the
requirements to
culture microorganisrns and demonstrate infectivity may have become obsolete.
Microbes do
not use. one single strategy to disable their hosts for the long term. Several
of the long=term
strategies include:
= induction of autoimmunity (Group A Streptococcus and heart valve disease)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
86
= persistent or repeated infection (HIV)
= Non-obvious connection to chronic disease (Helicobacter pylori and gastric
ulcers)
Helicobacterpylori is a gram-negative bacterium that causes a lifelong
infection in over half of
the world's human population. Without specific antimicrobial treatment,
all.infected individuals
exhibit chronic gastric inflammation, and a small percentage will develop
peptic ulcers and
gastric adenocarcinoma or mucosa associated lymphoid tissue lymphoma. In
response to*
infection, the host launches a vigorous imniune response, including the
mucosal infiltration of
neutrophils, lymphocytes, and macrophages. This immune response is
insufficient for clearance
of the bacterium, suggesting the H. pylori is capable of evading host immune
responses.
Infection with H. pylori induces apoptosis in macrophages, disrupts phagosome
maturation,'and
disrupts cytokine signaling. Induction of macrophage apoptosis may represent a
mechanism by,
which H. pylori usurps the host inunune response to establish a chronic
infection in humans.
Cardiovascular disease for all causes accounts for 29% of all deaths worldwide
(behind only
infectious and parasitic diseases). Deaths from cardiovascular disease are
often premature, and millions of non-fatal events result in disability.
Atherosclerosis, a major component'of
cardiovascular disease, has been considered a public health problem of
industrialized countries.
Many individuals with atherosclerosis lack identifiable traditional risk
factors (smoking, diet and
exercise, hypercholesterolemia, hypertension, diabetes, and genetic factors).
Atherogenic
processes resemble many aspects of chronic inflammation, a response that may
be promoted by
microorganisms. Both Chlamydiapneumoniae and cytomegalovirus (CivIV) are
widely
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
87
distributed; can infect blood vessel walls, and exhibit persistence, latency,
and-recurrence of
infection.
There are several lines of evidence associating C pneumoniae. infection with
atlierosclerosis.
These lines of evidence include:
= seroepidemiologic studies- ,
= direct detection of bacterial components in atherosclerotic lesions
= isolation -of viable organisms from coronary and carotid athe'romatous
tissue -
= in vitro and animal experiments
Cross-sectional and prospective studies have correlated seroprevalence with
myocardial
infarction, chronic coronary heart disease, or stroke:
More than 38 studies have reported a positive association between antibodies
to C. pneumoniae
and atherosclerotic disease. However, more than 50% of adults have been
*infected with C.
pneumoniae at least once. The strongest evidence associating. C. pneumoniae
and atherosclerotic
cardiovascular disease has been detection of bacterial components in
atherosclerotic lesions.
Historic findings do not, however, establish a causal role for C. pneumoniae
in atherogenesis.
Studies have linked cytomegalovirus to three arterial diseases - primary
atherosclerosis, post-
angioplasty restenosis, and post-transplantation arteriosclerosis.
Seroepidemiology has relied on
single measures of viral IgG antibodies which only indicate previous exposure.
Like C.=.
pneumoniae, the=worldwide ubiquity of lifelong, latent CMV infections could
mask or falsely
highlight causality.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
88
Other microbes may be associated with cardiovascular disease. Several reports
have suggested.a
relationship between chroriic oral infections (e.g. periodontitis) and
cardiovascular disease.
These oral pathogens include Porphyromonas gingivalis, Bacteroidesforsythus,
Campylobacter
rectus, Fusobacterium nucleatum, Treponema spp., Prevotella spp., and
Streptococcus sanguis:
Raised H. pylori and Herpes Simplex Virus (HSV). antibody levels have also
been a'ssociated.
with cardiovascular disease. Mycobacterial disease shares interesting
connections.to heart
disease. Not, only is tuberculosis the, only microorganism to depend: on
cholesterol for its
pathogenesis, but CDC maps for cardiovascular disease bear a striking
similarity to those of
State and regional tuberculosis, cases. Present day markers suggested as
indicators for heart
disease susceptibility. such as C-reactive protein (CRP), interleukin-6, and
homocysteine are all
similarly elevated in tuberculosis..
Group A streptococci are important human pathogens which cause a variety of
pyrogenic
infections that can be mild (e.g, pharyngitis, impetigo) to extremely severe
(cellulites,
necrotizing fasciitis, septicemia, pneumonia and streptococcal toxic shock
syndrome).
Molecular mimicry between streptococcal and heart components has been proposed
as the
-triggering factor leadiing to autoimmunity in rheumatic heart disease.
Medically sigriificant complications of Herpes Simplex Virus (HSV) are rare,
but= constitute a
significant burden, given the high rates of HSV seropositivity in. the
population. HSV ocular
infection is the leading cause.of infectious corneal blindness in the United
States. HSV-1
shedding is associated with reduced hospital survival iri
patients'receivingassisted ventilation in .
-intensive care units. Following productive infection by HSV at the site of
inoculation, the virus
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
89
spreads to and enters sensory neurons, where it establishes a latent
infection. Latent infection
forms a reservoir of virus for recurrent infection, disease, and transmission
to otlier'individuals.
HSV-1 is usually associated with primary infections of the orofacial area and
latent infections of
the trigeminal ganglion. HSV-2 is usually associated with genital infections
and latent infection
iri sacral ganglia.
Human papillomavirus (HPV) is one of the most common causes of sexually
transmitted disease
in both men and women world wide. HPV is associated with a variety of clinical
conditions that
range from innocuous lesions to cancer. Most HPV infections are benign=-
plantar and palmar
warts, common warts, and flat warts. Strains that target the face make skin
cancer more likely.
Other strains that grow primarily in the lining of the mouth produce small
elevated nodules that
can develop into fatal squamous cell cancers. Cervical cancer is the tlhird
most common cancer
in women in the United States.. The magnitude of the association between HPV
and cervical
squamous cell carcinoma is higher than that for the association between
smoking and lung
cancer. HPV has been implicated in 99.7% of cervical squamous cell cancer
cases world wide.
Pseudomonas aeruginosa is classified as an opportunistic pathogen, primarily
infecting
individuals who are immunocompromised, such as patients with cancer or AIDS.
Cystic fibrosis
(CF) almost always leads to chronic airway infection with P. aeruginosa:
Despite advances =in
antibiotic therapy, after chronic infection, rapid deterioration in lung
function occurs, increasing
morbidity and mortality: Chronic P. aeruginosa airway infections remain the
primary cause of
morbidity and mortality in the CF population. Young children with CF may be.
infected as -early
as 6 months of age and P. aeruginosa becomes chronic in the first decade of
life with pulmonary
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
exacerbations'increasing in frequency. A pulmonary infection with P.
aeruginosa is characterized by a strong recruitment of neutrophils and
significant inflammation ih the lung
parenchyma, which results 'in extensive damage to the lung tissue through the
action'of
neutrophil enzymes and oxidants.
Tuberculosis.has been declared a. global emergency. Pulmonary TB is .the
second leading cause
of mortality from infectious disease world wide, with 8 million new cases=and
2 millioin deaths
due to TB each year. There is an urgent need *for rapid, cost-effective, and
accurate methods for
tfie diagnosis of TB. A serologic test is attractive because it would be
relatively rapid and would '
not require sputum expectoration. Challenges for the development of effective
serologic tests
include:
= the need to discriminate active disease from latent- infection
= to avoid cross-reactivity with M. bovis BCG or mycobacteria other than M.
tuberculosis .
= to perform consistently with genetically and immunologically diverse
populations.
Lyme disease is a troubling chronic infection. Infection of humans by Borrelfa
burgodorferi
results in a spectruin of clinical illnesses.. Earliest symptoms may include a
typical or atypical
rash, followed by flu-like illness. As the disease progresses, other
rieurologic and musculoskeletal symptoms and signs may develop. The
pathophysiology of the.chronic
symptoms is not well understood, with hypotheses rangirig from persisting
infection to
autoimmunity to a combination of the two. The diagnosis of chronic Lyme
disease has:been
made difficult because of several factors. The multi-symptom complex
consisting of fatigue,
musculoskeletal pains and neurocognitive dysfunction cannot be distinguished
from disorders
that have been tertried fibromyalgia, chronic fatigue and Gulf War syndrome.
Laboratory testing
has not been reliable, including cultures; antibody studies'(ELISA, Western
blot) and PCR-DNA
tests.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
91
Malaria, which had been eliminated or effectively suppressed in many parts of
the world, is
undergoing a resurgence. Malaria is estimated to cause up to 400 million
clinical cases and 2
million deaths each year. Many of the=clinical manifestations of malaria
(including acute febrile
illness, anemia, cerebral malaria, and hypoglycemia) are mediated in part by
overproduction of
pro-inflammatory cytokines such as tumor necrosis factor (TNF-a); interleukin-
1 (IL-1) and
gamma interferon (TFN-y):
Hepatitis C virus (HCV) is a small RNA virus that chronicaliy infects 170-350
million people
world wide. Of those acutely infected, only 15% recover, while the remaining
85% succumb to
chronic hepatitis. Up to 20% of the individuals with chronic hepatitis C
progress to cirrhosis and,
these patients are at greater risk of developing hepatocellular.carcinoma.
Extensive studies have
been carried out in the past decade in order to find immunodominant HCV
peptides and there are
many peptides capable of inducing cellular immune responses. None of these,
however; has
proven to be clinically effective in preventing HCV disease. Although
interferon and other
agents are effective for eliminating HCV in certain patients, they are too
expensive for the
majority of HCV patients in most countries. There is an urgent need to
determine
immunodominant peptides useful for the development of effective and low-priced
vaccines. In
addition, there is aIso a need to develop a simple and low-priced diagnostic
tool for HCV since
the currently available kit is also expensive for the majority of people not
living in developed
countries.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
92
Epstein-Barr Virus (EBV) is a B-lymphotropic human herpesvirus, and like other
herpesviruses,
establishes =a lifelong presence in the host. The virus infects the vast
majority of the world's.
adult population and is well known for its association with a broad spectrum
of benign and malignant diseases including:
= . infectious mononucleosis
= Burkitt's lymphoma
= nasopharyngeal carcinoma
= B-cell lymphoma in immunocornpromised individuals.
Respiratory tract infections caused by viruses, Chlamydia, and Mycoplasma have
been
implicated in the pathogenesis of asthma. Viruses have been demonstrated to
be.associated with
asthma epidemiologically in at least tWo ways. During irifancy, certain
viruses have been
implicated as potentially being responsible for the inception of the asthmatic
phenotype. In
patients with established asthma, viral upper respiratory tract infections
play a significant role iri
producing acute exacerbations of airway obstruction that tnay result in
frequent= outpatient visits
or hospitalizations. Recent attention has focused on Chlamydia and Mycoplasma
as potential
contributors to both exacerbations and the severity of chronic asthma in terms
of loss of lung
function or medication requirements.
Various microorganisms are implicated in the initiation and/or progression of
chronic illnesses.=
There are other effects of carriage of these inicroorganisms on-the immune
system (e:g.
cytokines, cellular responses; effector molecules).. 'Monitoring of antibody
responses and plasma
cytokine levels merit serious consideration for ImmunoScore diagnostic
analyses= in exemplary
embodiments of the present invention.
17. Th1-Th2 Paradigm
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
93
Lymphocytes are the effector cells of acquired (or adaptive) immunity,
originating as bone
marrow stem cells that undergo hematopoiesis. A portion of these lympho:cytes
migrate to the
thymus to undergo further differeritiation and maturatioii to become T cells,
which can be
divided into subsets based on physical markers or surface receptors (e.g.,
CD4; CD8, and either
a,o or y8 T cell receptor), representing a generally irreversible genetic
commitment (for review,
see Kidd P, 2003; Pier GB et al., 2004). Other subsets have been defined by
functional
properties that may be environmentally altered; e.g., expression of different
cytokines, which are :
chemicals used for cell-to-cell communication. It was originally determined in
mice that there
are two T helper cell subsets, Thl and Th2, based on two distinct'cytokine
profiles that resulted-
in=the overall regulation bf an immune response (Mosmann TR et al., 1986;
Mosmann TR.,
Coffman RL, 1989). For example, Fig. 41 (from Harber M et al., 2000) shows
some of the,
complex interactions between the polarized Thl and Th2 responses.
-It is clear from this.Thl-Th2 paradigm that the cytokines secreted by the Th
cells will feedback
and reinforce the particular clonal phenotype from which they
origiinated'(e.g., IL-4 for Thl vs.
IFN-y for Th2), as well as suppress the alternate phenotype, resulting in
crossregulation.
The same Th1-Th2 paradigm from mice has been applied to humans (Romagnaru S,
1991; Del
Prete GF et al., 1991) to also explain the'immunologic aspect of disease
(Lucey DR et'al., 1996;
Romagirnani S et al., 1997; Romagnani S, 1997). Fig. 4J (from Harber M et al:,
2000) shows the
complex balance between Thl and Th2 cells as dictated by the Thl-= paradigni
regarding
disease.
The Thl cell (with its associated cytokin,es: INF-y, TNF-a, IL-2, IL-12) is
biased towards the
cell-mediated side of immunity, effective against intracellular parasites, and
'its downregulation
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
94
of Th2 can provide relief from allergic reactions due to IgE; but detrimerital
effects niay result in
autoimmunity and graft rejection. On the other hand, the =Th2 cell (with its
associated cytokines: =
IL-4, IL-5,11-6, IL-10, IL-13) favors humoral (antibody) immunity, providing
an effective.
correlate of protection for most vaccines, and its downregulation of Thi can
result in some
benefit of tolerance to prevent cellular autoimmune reactions; but certain
harmful characteristics
related to IgE-based allergies and autoimmunity may result.
The simplicity of the mouse system, however; hasnot translated well to humans.
The ciear'i'hl-:
Th2 polarization in mice with- discrete cytokine profiles has given way to a
more flexible
continuum of responses in humans, where the functionality of Th cells -may be
more variable and
not necessarily. locked into a single type for subsequent generations (Kelso
A, Groves P; 1997;
Kelso A et al., 1999; Doyle AG et al., 1999; Fitzpatrick DR at al., 1999).
This flexibility in
human Th subsets and complexity of Th cell interactions have led some
researchers to question
.the Th1-Th2 paradigm and the difficulty to generalize for all situations
(Kelso A, 1995;
Kunzendorf U et al., 1998; Biaze ME et al., 2003; Sheikh A, Strachan DP, 2004;
Chaouat-G et
al., 2004). Nonetheless, the Thl-Th2 paradigm has provided valuable insight
into the natuie and
treatment/prevention of infectious diseases and immunologic disorders (e.g.,
aller.gies and
autoimmunity).
=Fig. 4K (from Harber M et al., 2000) shows a inore comprehensive picture of
irnmune regulation
with additional cell types. From this figure, one can see'additional T
regulator cells which
contribute to the paradigin by providing suppressor functions (e.g.,'NKT,
CD45RB'0,
CD4+CD25+), including'some that are antigen-specific (e.g., Th3; Trl), thereby
preventing
autoimmune diseases. In addition, others have identified a nonpolarized
effector T cell, TFH
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
(follicular helper T cell), that specifically provides help for the antibody-
procluciing B
lymphocytes (Mackay CR; 2000; Schaerli P et a1., 2001; Chtanova T et al.,
2004).
Cytokine secretion and regulatory functions are not restricted to just
lymphocytes or lymphoid
cells, but these activities are also provided by and impact myeloid cells
(also originating from
stem cells througli hematopoiesis), includirig neutrophils, eosinophils,
basophils, mast cells,
dendritic cells, monocytes, and macrophages.
Figs. 4L (left and right) (from Kidd P, 2003) show more of these interactions
(left panel) as well
as differentiation among different cell types (right panel), including
antibody-producing B. cells,
antigen presenting cells (APC), and natural killer cells (NK).
Studies have shown that macrophage activation,may occur in two different
states (classicaf vs.
alternative) that operate in parallel to the Thl-Th2 paradigm, resulting in
pro= vs. anti-
inflammatory responses (Birk RW et al., 2001), as well, as regulation of
endocytosis/antigen
uptake through decreased vs. increased marinose receptor expression
(1Vlontaner LJ et al., 1999).
During the effector phase of an immune response, T cells and other effector
cells find their way
into specific tissue where needed and interact with each other in spatial, and
temporal patterns by
way of secreted chemokines (chemotactic cytokines) and chemokine receptors
expressed on their
surfaces. T cells interact with eosinophils, mast cells, and basophils during
allergic reactions, or
with macrophages a nd neutrophils for delayed-type hypersensitivity reactions
(Sallusto F et al.,
2000).
Specific disease states have been identified that are associated with, and
possibly result from, an
imbalance of the immune regulatory process already described. The predominance
=of a
particular phenotype (Thl vs. Th2), or polarization towards one extreme, may
determine the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
96
presentation and/or severity of disease (for reviews, see Lucey DR et al:,
'1996' Harber M et al.,
2000; Kidd. P, 2003). Atopy (familial allergy) in humans was shown to be
characterized by a
Th2 profile in whole blood cell culture, where high levels 'of IL-4 and low
levels of IFN-y were
observed for CD4+ T cells, but the Th2 deviation in'atopic asthma showed high
levels of=IFN-y
for CD8+ T cells (Magnan AO et al., 2000). IL-4 was used therapeutically to
amelioiate the
clinical disease in mice that were experimentally giveri an autoinimune
disease, allergic
.encephalomyelitis, switching the Thl cells to Th2 cells (Racke MK et
al.,.1=994). It-is now clear
that the application of exogenous cytokines can be used to push the Th status
in either direction,
ehabling the development of potential therapeutic applications. (Lucey DR et
al:, 1996; Harber M'
et, al.; 2000; Kidd P, 2003; Sun QL, Ran W, 2004).
Th1-Th2 Based Diagnostic Panel
In orde'r to diagnose or predict an immunologic disease and/or provide therapy
or prophylaxis,
.the Th polarization status must be determined; this should also be applied to-
measure -
susceptibility to infectious and neoplastic diseases. Th status is'measurable
in terms of cytokine
profiles'(House RV, 1999; Harber M et al., 2000; House RV, 2001); chemokine /
chemoattractant receptors (Sallusto et al., 1998; Syrbe U et al., 1999;
Sallusto'et al., 2000;
Kaplan AP, 2001; Cosmi L et al., 2001),. specific effector cell products
(Venge P et al, 1999;
Venge.P, 2004), or gene expression prof les (Rogge L;=2002). Table 8 below-
shows how the
cytokines and chemokine/chemoattractant receptors can, for example, be aligned
within the Th1
Th2 pa"radigm for an exemplary diagnostic panel according to an-exemplary
embodimerit of the
present invention.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
97
Th1 Th2
Cytokines Rece tors. Cytokines Rece tors
INF-y CC;RS IL-4 CGR3
TNF-a CXCR3 tL-5. CCR4.
IL-2 CCRI IL-6- : CCR8
IL-12 IL-10 CRTh2
IL-13
Table 8
There are 4 major ways to measure cytokine profiles (House RV, 2001):
bioassays,
irnmurioassays, molecular biological techniques, and flow cytometry.
=Bioassays require living
material to induce proliferation, maintain viability, stimulate'migration,
induce a secondary
fiinction, or inhibit a function. Immunoassays are.commonly the enzyme-linked
immunosorbent
assay (ELISA) or the radioimmunoassay (RIA); the ELISA is most often used,
being acolorimetric antibody-based assay. Molecular biological.methods usually
employ the
polymerase chain reaction (PCR), or reverse transcriptase PCR (RT-PCR) to
measure the mRNA
representing a particular cytokine. -Flow cytometry is used to detect and
quantify, cells that 'are
stained with fluorescent anti-cytokine antibodies.
In addition, combinations of these assays can used for improved results
concerning a particixlar
application (House RV, 2001); e.g., RT-PCR ELISA, where the RT-PCR
amplifies'the message
and the ELISA detects the result; in sitti hybridization, where genetic
material is detected with
labeled antibodies; ELISPOT assay,'where cytokines are detected from single
cells by ELISA
and molecular methodology; and cytokine immunotrapping assay, a capture ELISA.
wher~e
cytokine antibodies are used to capture cytokines expressed from isolated
cells:for analysis.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
98
Over 60 cherriokine receptors have been identified (Pier GB et al., 2004);
but. only a few are
preferentially expressed by, specific Th clones (Sallusto et al., 2000) as
indicated in a previous
table. These receptors may appear as cell surface-bound and in soluble forms.
Bioassays and
immunoassays can measure soluble receptors, but flow cytometry and in situ
hybridization
would be more appropriate for surface-bound receptors (House RV, 2001).
Effector cells, such as eosinophils, release different cytotoxic products upon
activation during
allergic inflammation (Venge P, 2004). Some products include eosinophil
cationic protein
(ECP), eosinophil peroxidase (EPO), and eosinophil protein X eosinophil-
derived neurotoxin
(EPX/EDN). ECP and EPO are most cell-specific fdr eo'sinophils, while EPX/EDN
is also.
produced by neutrophils. Table 10 following (from Venge P, 2004) shows
examples ofsecretory
products that can used as markers for other- inflammatory cells:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
99
Effector cells, such.as eosinophils, release= different cytotoxic products
upon activation during allergic inflammation (Venge P, 2004). Sonie =
products include eosinophil cationic protein (ECP), eosinophil
peroxidase (EPO), and eosinophil protein X eosinophil-derived
neurotoxin (EPX/EDN). ECP and EPO are most cell-specific for
eosinophils,.while EPX/EDN is also produced by neutrophils. Table 10
following (froni Venge P, 2004) shows examples of secretory products
that can used as inarkers for other inflammatory cells:
Table 10: Inflamatory cells and some of their secretory products that may be
used as. =
markers of their activity and turnover
Eosinophils
Eosinophil cationic protein (ECP)*
Eosinophil peroxidase (EPO)*
Eosinophil protein X/eosinophil derived neutrotoxin
(EPX/EDN)
Mast cells
Tryptase*
Neutrophils
Bastase
Human neutrophil lipocalin (HNL)*
Lactoferrin
Myeloperoxidase (MPO)
Manacytes/macrophages
Lysozyme
Interleukin (IL)-6
T-Iymphocytes
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
100
SIL2r
Endothelial cells
E-selectin`
Unique.and cell-specific riiarkers are marked with asterisk.
Table 10
ECP may be measured in serum, plasma, sputum, or saliva as an indicator of
eosinophil
granulocyte activity and turnover in the'allergic or asthmatic patient (Venge
P,et.al., 1999; Bjork
A et al., 2000; Venge P, 2004). EPX/EDN may'be measured in urine as another
noninvasi,ve
way of monitoring eosinophil-related allergic inflammation (Venge P, 2004).
Elevated urine
levels of EPX/EDN have been shown in atopic dermatitis (Breuer K et al., 2001)
and have also
'been predictive of asthma development in children (Oymar K; 2001).
As an alternative to rimeasuring cytokines, receptors, and'other immunolrigic
products, the gene
expression of these substarices can also be evaluated; deriving gene
expression profiles' to
correlate with the Thl-Th2 paradigm (Rogge L, 2002). Oligonucleotide
microarrays have been
used to assess human gene expression with a transcript level display capacity
of 6000 human
genes. From purified and stimulated Thl , and Th2 cells, 215 genes were
found.to be differentially
expressed at a 95% confidence level (Rogge L, 2002). These results were also
confirmed by RT-
PCR for 28 out of 29 genes. Infectious and Neoplastic Diseases
In the event of a microbial. or cancerous attack, the type of immune response
will usually dictate
the'outcome. It is generally considered that a Thl cell-mediated response
would be-desirabl.e
against viruses, intracellular bacteria, fungi, parasites, and cancer, while a
Th2 humoral response
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
101
might work better for most mucosal and extracellular bacterial infections;
however, this is really
an over-simplification for a complex area fraught with conflicting scenarios
(for review, see
Lucey DR, 1996). For example, humoral antibody responses are often established
as
measurements of potency or correlates of protection for vaccines, even against
'viruses, such as-
poliovirus (Fox JP, 1984; Salk J, 1984; Sutter RW. et al., 1995), and
intracellular bacteria; such as
Salmonella typhi (Klugman KP et al.; 1996; Tacket CO et al., 2004). It is
clear that 2 distinct
mechanisms of protection (humoral vs. cell-mediated) can occur against the
same disease (Kaul
D, Ogra PL, 1998; Tacket CO et al., 2004). Due to the complexity of
pathogenesis, with
different stages of infection and transmission, it is likely that a balance of
Thl and Th2 is
required to enable either part to play.a role as needed. Nonetheless, it
appears that a simple Thl-
Th2 pa:radigm does apply to certain organisms, such as Mycobacterium
tuberculosis, during a
natural infection (Kidd P, 2003). Epidemiological studies*have shown that Thl-
mediated (IFN-y,
IL-12) protection is essential for protection against tuberculosis, and Tli2
predominance leads to
severe disease that is often fatal (Newport MJ et al., -1996; Lienhardt C et
al., 2002). It is
probable that people who are predisposed to only one side of the Th1-Th2
paradigm would be at
a disadvantage in terms of options available in response to disease. For a
detailed review of
infectious and neoplastic diseases in relation to Thl and Th2 profiles, see
Lucey DR et al., 1996.
Thl-Th2 and Immunologic Diseases (AllergylAtopy and
Autoimmunityilnflammatory Disease)
Early Innate Modulation of Thl or Th2 Cells
The nature of the immune resporise is first influenced by the specific signals
that are involved in
the early recruitment of immune components to the site of inflammation
(Cookson; 2004). As
different pattern-recognition receptors can signal through different pathways,
different pathogens
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
102
or antigens can induce different =immune responses (Palaniyar, et al. 2002). -
Second,.the nature of
the local immune response might also be strongly influenced by tissue-specific
facors, and it has
been suggested that the epithelial cells, in general, tend to initiate Th2
rather then Thl-type
responses (Matzinger, 2002). In addition, there is evidence that dendritic
cells from airways
encourage Th2=cell development by default (Stumbles, et al. 1998), and that
the induction of Th2
or Th1 type responses by dendritic cells depends on the stimiulus with which
they are activated
.(Mazzoni and Segal, 2004).
.The perception that specific early signals induced by different infectioris
(or damage by different
proteins or other entities) might modify the nature. of the subsequent immune
response has
implications for the Th1=Th2 paradigm of atopic disease. One important issue
is the timing of
establishment of the Th2-cell bias: on the one hand, Thl- or Th2-cell
responses to allergens
might be .fixed at the time of first exposure in early childhood, and the bias
might be
subsequently manipulated by bacterial and other adjuvants. On the other hand,
Thi- or Th2-cell
responses might develop as a consequence of activation of particular patter-
recognition receptors
by particular pattern-associated molecular patterns (PAMPs) that are present
in aller=gens
(Cookson, 2004).
Allergy/Atopy
Allergy or atopy (familial allergy) usually involves Th2 predominance,
particularly related to
IgE antibodies which attach to basophils and mast cells and cause the release
of mediators such=
as histamine, leukotrienes, and prostaglandins (Kidd P, 2003). Injection of
purified allergens.
(e.g., grass pollen) has been used successfully for immunotherapy towards
aller=gies (Bousquet et
al., 1991) by reducing Th2 (IL-4) cytokines (Secrist H et al., 1993) and
increasing Thl (IL-12)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
103
cytokines (Hamid QA et al., 1997). The scientific evidence generally supports
the idea that
all=ergies correlate with detectable Th2-dominant coriditions that can be
treated with Th2-directed
immunotherapy.
Any model of the immunology of asthma and atopic dermatitis (eczema) has to -
take into 'account
the observatiori that both diseases have increased i-n prevalence duriing the
past 'centiuy. Asthma'
prevalence has= been linked to increasing hygiene standards and the
progressive westernization of
lifestyles in many countries, and a protective effect against asthma of
microbial exposure in early
childhood has been suggested by the "hygiene hypothesis" -(Strachan, -l 989).
This hypothesis
argues that early childhood exposure to infections inhibit the tendency to
develop allergic
disease. As a consequence, children with westernized.lifestyles, protected as
they are from the
infectioi.us burdens of early life that are common in.the developing world,
suffer an increased risk.=
of developing allergic disease.= There is now strong evidence.indicating that
microbial exposure
is important for protection against asthma, although the-nature of the
microbial protective effect
is still unknown (Cookson, 2004).=
Several theories have= been put forward to' explain the association between
asthma and= hygiene:= =
The theory of immune deviation suggests that atopic asthma is initiated
shortly after birth, when
the naive immune system is first confronted with potentially aller=genic
airborne antigens (Holt,
et al. 1999). It is suggested that the initial phase of allergen exposure
results in =
\compartmentalization -of immunological memory .into either Thl or Th2 cell
phenotypes in non-
atopic and atopic individuals, respectively. Microbial exposure in infancy
encourages a milieu in
which initail allergen exposures produce benign Thl 'cell responses. In the
absence of such
exposure, Th2 cell responses predominate, and can be followed by chronic Th2
cell driven,
inflammation in the airways (Holt, et al. 1999). This raises the possiblity
that rnanipulation of
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
104
the immune system in early life could result in persistent Thl or Th2 type
responses. If this is'.
the case, vaccination to induce Thl cell responses might be effective against
asthma and other
allergic disorders (Holt, 1994).= As an alternative to the immune deviation
theory, it has been
proposed that lack of normal microbial exposure leads to reduced activity of
regulatory T cells -
rather than Th2 cell deviation (Romagnani; 2004).
Asthma is an inflammatory conditiori; both atopic and nonatopic, that is
generally. Th2 (IL-4)
dominant (Larche M, 2003). Asthma has now'reached epidemic proportions, with
more than
10% of children being affected in many westernized societies (Cookson, 2004).
Allergen
injections have been used effectively as immunotherapy in IgE-mediated disease
(Abramson MJ
et al., 1995).
Studies of carididate genes have identified genes that might be involved in
asthrna susceptibilityy,'.
many of which exert their effects in the mucosa. For example, IL-13
polymorphism influences
mucus production as well as -serum IgE levels thiough a receptor encoded by
the polymarphic
IL-4R (Ober, et al. 2000). FCERIB variants modify the activity of FcERI on
mast cells,-possibly
by modulating the level of'expression of the receptor on the cell surface
(Donnadieu, et al. 2003).
A receptor expressed by T, cells for the key mast cell signalling factor
prostanoid DP has also
been reported to be associated with asthma (Oguma, et al. 2004). These
findings =indicate that
the role of mast cells in epithelial inflammation might also be a potential
target.in asthma
therapy.
Other asthma susceptibility genes include the pattern-recognition receptors of
the innate immune
system, which are expressed by dendritic cells and other cells, aind
recognize'specific microbial
.components and activate innate immune responses (Cookson, 2004). Polymorphism
in CD14,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
105
Toll-like receptor 2 (TLR2), nucleotide-binding oligomerization domain 2
(NOD2, or
alternatively CARD 15), and T-cell immunoglobulin domain and mucin domain 1
have all been
shown to influence asthma susceptibility (Baldini, et al. 1999; Eder, et al.
2004; Kabesch, et= al.
2003; Mclntire, et al. 2003), indicating that these genes might be important
in providing the link
between microbial exposure and reduced susceptibility to asthma (Cookson,
2004). TLR10,
which responds to an unknown ligand, has recently been associated with asthma
(Lazarus, et al.
2004). However, none of these studies has tested for IgE responses to
particular allergens, so
systemic studies of pattern-recognition receptor activation in asthma are now
needed. (Cookson;
2004).
Other recognized effects are from tumor-necrosis factor (Moffatt and Cookson,
1997), which
encbdes a potent pro-inflammatory cytokine that is released by many cells,
including airway
epithelial cells and transforming growth factor-p-(Pulleyn, et al. 2001),
which is an important
local regulator of epithelial inflammation.
Atopie dermatitis (eczema) can involve a mixture of Thl and Th2 states,
depending on the type
or stage of disease. The acute disease is usually Th2 (IL-4), while the
chronic disease may'show
more Thl (IL-12) cytokines (Singh VK et al., 1999). Further studies indicate
that the initial
phase of disease is Th2, while Thl may appear later (Bohm I, Bauer R,.1997).
For more detailed reviews and applications concerning Allergy/Atopy, see:
Lucey DR et al.,
1996; Kidd P, 2003.
Although the current emphasis in understanding asthma and atopic. dermatitis
is now moving
from involvement of distant adaptive immune responses to local response's at
epithelial-cell
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
106
surfaces, it is probable that a fitll.understanding of'these diseases will
also depend on studies that
include commensal bacteria.
Current understanding of the hygiene hypothesis. rests on the suggestion that
microbial
stimulation during early life is essential for the normal development,of the
immune system= and
to achieve the correct cytokine balance (Rook and.Standf6rd,19.98): However,
the,evidence described earlier indicated that, damage to the epithelium is
probably, the initiating event:in atopic disease, and=the Thl- or Th2-cell
bias of subsequent iriflammation might be secondary to the
nature of the damage (Cookson, 2004).
Alternative mechanisms for bacterial products to modify the risk of atopic
diseases include the
enhancement of an effective airway barrier.by the induction of mucus
production through IL-13
stimulation (Kuperman, et al. 2002), or the induction of sufficient polyclonal
IgA or IgE to
pro'vide nonspecific protection against allergens. Additionally, a protective
role by'=
microorganisms might follow the acquisition of distinct commensal or symbiotic
organisms.
Once an individual's commensal microflora is established in the first year of
life, it remains
relatively stable (Hooper and Gordon,. 2001).. =Substantial differences have
been observed in the:
intestinal microflora between neighboring countires with= a different
prevalence of atopic disease
(Sepp; et al. 1997), and between atopic and non-atopic children living in each
-of these countries
(Bjorksten, et al. 1999). As.commensal and symbiotic organisms actively
manipulate host
imriiunity and the activity of other bacteria, it should be considered that
interactions among
commensal bacteria, pathogens and the host might contribute to the increase
and prevalence of -
asthma and atopic dermatitis (Cookson, 2004).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1107
18. Autoirrimunity/Inflarrmmatory Disease
Rbeumatoid arthritis (RA) is an autoimmune disease with apparent Th cell
involvement.
Activated.T-helper cells are found in the inflammato.ry filtrates, arid T cell-
directed therapies
have provided some clinical benefit (Schulze-Koops H, Kalden JR, 2001). It
appears to be'Thl-
driven (IFN-y); but there may be a Th2 (IL=4, IL-10) component at the
early=stages of disease
(Gerli R et al., 2002). It is interestingto riote that pregriancy, which seems
to have=a Th2 bias,
appears to ameliorate the progression of RA, providing indirect evidence of
the role of Thl in .
RA (Da Silva JA, Spector TD, 1992). Schulze-Koops and Kalden suggest that
several of the
current anti-RA drugs work by altering Th1/Th2 balance. But evidence for t.his-
is indirect and
comes mostly from non-clinical settings. Schulze-Koops and Kalden concede that
it may be
overly simplistic to remold the RA data to make it fit the Thl/Th2 hypothesis.
They admit it is
possible Thl is subject to simple guilt-by-association with RA, rather than
being a major
mechanism driving the disease (Schulze-Koops and Kalden, 2001): ImniunoScore
analyses
would further the understanding of the relationship between RA and the Thl/Th2
paradigm.
Multiple sclerosis (MS) is an autoimmu.rie disease that appears to,be Thl-
driven (IL-12, IFN-y),
with some conflicting data (Kidd P, 2003); this may be a complication of the
role of regulatoiy
cells (Tr) secreting cytokines (IL-10) to normally downregulate the Thl cells
(Bettelli=E et al.,
1998). Defining the factors that initiate and perpetuate the ongoing
pathogenesis, as well as=
designing- treatment strategies for this disease, have been complicated by
absence of an
identifiable causative agent, diversity of co-existing CNS lesion stages (ie,
acute, chronic active,
chrbnic inactive, remyelinating, gliotic plaque), an unpredicatble relapsing-
remitting clinical
course early in the disease, lack of a direct correlation of clinical symptoms
to the occurence of
=new white matter lesions, and the absence of a naturally occurring animal
form,of the disease
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
108
(Jordan, et al. 1999). One group tried T cell receptor peptide therapy on MS
patients. Of the less
than 200 patients studied, 50-90 percent supposedly showed immunological
response to
vaccination and as much as 35 percent had some degree of favorable clinical
response
(Vandenbark, et al. 2001).
Type 1 diabetes is an autoimmune disease that may be Thl dominant. Data
available thus far in
human diseases favor a prevalent Thl lymphokine profile in target organs.of
patients with organ-
specific autoimmunity. Adjuvant therapy with BCG injections seems to benefit
patients and
nonobese diabetic mice by raising Th2 (IL-4) cytokine levels (Singh VK et al.,
1999). Hbwever;
administration of Th2.cells to nonobese diabetic mice can worsen the disease;
if the recipient
mice are immunocompromised (Pakala, et al. 1997).
In sununary, for three major autoimmune diseases - RA, MS; and type' 1
diabetes - a Thl -
dominance has not been well enough established to rationalize balancing
intervention. On both
pragmatic and theoretical grounds there is real possibility of making the
patient. sicker through
efforts to intervene with Th@ cells or Th2 cytokines (Kidd, 2003). The
ImmunoScore
diagnostic panel would be invaluable in assessing the relationship of Thl/Th2
cytokine levels in
relationship to these disease conditions..
Miscarriage might be the result of an autoimmune response to the fetus during
pregnancy,
where the normally Th2 (IL-3, IL-4, IL-10) dominance during pregnancy has
shifted to a Thl
state (IL-2, IFN-y, TNF-a), allowing the maternal cell-mediated response to
be.directed towards
the pateinal antigens of the fetus (Chaouat G et al., 2004). While the
simplicity of the Thl-Th2 =
paradigm applied to pregnancy is being questioned, particularly in terms of
potential=therapy and '=
the inability to generalize across all individuals, there may still be a Th2
bias for normal
pregnancies (Chaouat G et al., 2004).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
109
Systemic lupus erythematosus is a chronic, recurrent, potentially fatal
multisystem
inflammatory disorder that typically shows anti-nuclear and other
autoantibodies, with elevated
Thl (IL-2, IFN-y) and Th2 (IL-4) cytokines (Kidd P, 2003). Patients with
arthritis have =higher
Thl cytokine levels, while those with CNS involvement have higher Th2 cytokine
levels (Chang
DM et al., 2002).
It is possible that the association of genetic polymbrphism (Chang DM et al.,
2002), along with
disease stage and presentation, all work together to affect the Thl-
Th2'pattern. Complete
complement components C4A and C4B deficiencies have been identified and.
studied cTinically
(Yang, et al. 2004a). All but one of the coinplete C4-deficierit subjects
experienced symptoms
related to immune complex clearance disorders such as SLE, a lupus-like
disease, -or
=glomerulonephritis (Yu, et al.. 2003). The human-C4 locus is remarkably
complex. Among
different individuals in a population, two to seven (possibly eight) C4 genes-
may, be present in a
diploid genome, leading to a 3- to 5- fold variation in plasma C4
protein:concentrations and the.
presence of multiple allotypes (Yang, et al. 2003). Considering the ioles of
C4A and C4B in
immunoclearance, memory, and effector functions of the.humoral immune
response, it is not
unexpected that a deficiency of C4A or C4B is frequently associated with
infectious and/or
autoimmune diseases (Yang, et al. 2004b). An elucidation of the molecular
basis of complete
C4A and C4B deficiencies may help in designing a comprehensive screening
strategy to
determine the prevalence of C4A and C4B mutations in autoimniurie, infectious,
and kidney
diseases (Yang, et al. 2004b).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
110
In humans, outside of major histocompatibility complex (MHC) class II, genetic
polymorphisms
or=defects in genes involved in antigen uptake and/or process and in immune
complex clearance
such as complement, FCGR2A and FCGR3A have been identified to contribute to
SLE
susceptibility (Wakeland, et al. 2001). Recently, programmed cell death gene
1(PDCDIT) which
regulates B cell activation has been identified as an autoimmunity candidate
gene iri the mouse
(Nishimura, et al. 1999), and a single-nucleotide polymorphism (SNP) in a
putative RUNXl
binding site in the promoter of human PDCD1 gene has been inplicated as a risk
allele for SLE
(Prokunina, et al. 2002).
Fibrotic disease, involving tissue fibrosis (scarring), is the result of a Th1-
Th2 imbalance during
wound healing in response to chronic=inflammation, and is responsible for an
estimated 45% 6f
U.S. deaths (Wynn TA, 2004). In this case, the wound healing Th2 (IL-4, IL-5,
IL-13) response,=
opposing the initial Thl (IFN-y, IL-12) regenerative inflammatory response, is
continuous and
leads to excessive tissue remodeling (permanent scar tissue). While the Th2
wound healing is
necessary for long-term survival from an injury, persistent healing, in
response to a chronic Thl
stimulus, might end in fibrotic tissue causing major organ failure and death
(Wynn TA, 2004).
For more detailed reviews and applications concerning
Autoimmunity/Int2ammatory Disease,
see: Lucey DR et al., 1996; Kidd P, 2003.
8. lmmunoScore Analyses for Immigrants and Internationally=Adopted
Children
The current U.S. Immigration and Naturalizatiori law has vaccination
requirements for the
following vaccine-preventable diseases:
= = Measles
= Mumps
= Rubella
= Polio
= Tetanus
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
111
= Diphtlieria
= Pertussis
i,. Haemophilus influenzae type B (Hib)
= Hepatitis B
= Varicella.
= Pneumococcal disease
= Influenza
Vaccination of Internationally Adopted Children
The ability of a clinician- to determine that an individual is protected from
vaccine-preventable
.disease on the basis of their country of origin and their personal medical
records alone is limited.
Currently, only written docuirientation should be accepted as evidence of
prior vaccination.
Although vaccines with inadequate potency have been produced in other
countries, the majority
of vaccines used worldwide are produced with adequate control standards and
are potent.: Data
are inconclusive regarding the extent to which an internationally adopted
child's immuirization
record reflects the level of the child's protection from vaccine-preventable
diseases. = For
example, a record might indicate administration of Measles, Mumps, and Rubella
(MMR)
-vaccine when only single antigen measles vaccine was administered: A study
of,children
adopted from China, Russia, and Eastern Europe determined that only 39% of
childreri with
documentation of>3 doses of DTP had protective levels of diphtheria and
tetanus antitoxin
(Hostetter; et al., 1998). Rather than rely on records and memories that may
be less than
'satisfactory, the ImmunoPrint diagnostic assay system would be a highly
practical tool to test the
specific antibody levels of individuals entering the country or children being
adopted fronm other
lands.
The CDC states that dcises of measles-containing vaccine administered prior to
the first birthday. =
should not be counted as part of the series (CDC, 2002). - They also state
that serological testing
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
112
for IgG antibody to MMR vaccine viruses can be considered if the individual
lacks the
appi=opriate- paperwork. A child whose record indicates receipt of measles or
measles-rubella
vaccine at.age > 1 year and who has protective antibody levels against measles
and rubella
should receive a"single dose of IVIMR ,as age appropriate to ensure protection
against mumps.
Regarding poliovirus vaccine, the CDC suggests that the "simplest approach" is
to revaocinate .
immigrants with IPV according to the U.S. schedule (CDC, 2002). They also
state that children
appropriately vaccinated with-three doses of oral polio vaccine (OPV) in
economically
developing countries might have suboptimal seroconv*ersion. Currently,
serologic testing for
neutralizing antibody to poliovirus types 1, 2, and 3 can be obtained
cornmercially and at.certain
state health'department laboratories. Incorporation of poliovirus assays into
ImmunoPrint
diagnostics would enable immigration authorities to screen individuals for
seroconversion to
poliovirus types 1, 2, and 3. Recommended immunization boosters could then be
followed
. through with in timely fashion.
Vaccination providers can re-vaccinate a child with DTaP vaccine without
regard to recorded
doses; however, one concern regarding this approach is that data indicate
increased rates of local
adverse reactions after the fourth and fifth doses of DTP or DTaP.' If a re-
vaccination approach
is adopted and a severe local reaction occurs, serologic testing for specific
IgG antibody to
tetanus and diphtheria toxins can be measured by ImmunoPrint analyses before
administering .
additional doses. Protective concentration indicates that further doses are
unnecessary and
subsequent vaccination should occur as age-appropriate. There is, as yet, no
serologic correlate
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
113
of protection for pertussis. The lack of a serologic correlate of protection
is one area where
application of the ImmunoPrint database would be of great value.
Because the number of vaccinations needed for protection from Haemophilus
influenzae-type B
(Hib) disease decreases with age and adverse events are'rare, age-appropriate
vacciiiations for
immigrants should be provided (CDC, 2002). Hib vaccination is not routinely
recommended -for
children over 5 years of age. -
The ACIP recommends serologic testing for hepatitis B surface antigen (Hl3sAg)
for'
international adoptees (CDC, 2002). Children determined to be HBsAg positive
should be
moiiitored for the development of liver disease. Household members -of HBsAg-
positive
-children should be vaccinated.. The current recommendation from the ACIP
states that a child
whose records indicate receipt of > 3 doses of vaccine can be considered
protected and additional-
doses of vaccine are not needed if > I d6ses were administered at > 6 months
of age. Those who
have received < 3 doses should complete the series at the recommended
intervals (CDC, 2002).
This rather complicated recommendation depending on accurate record-keeping
could be
replaced with ImmunoPrint diagnostic testing. A positive anti-HBsAg IgG
antibody would be
indicative of protection in these individuals.
Varicella vaccine is not administered in the majority of countries. The ACIP
recommends that a
child who lacks a reliable medical history regarding prior varicella disease
should be vaccinated
as age-appropriate (CDC, I996). A well-timed ImmunoScore diagnostic assay can,
in exemplary,
embodiments of the present invention, remove speculation from the vaccination
protocol.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
114
Pneumococcal conjugate and pneumococcal polysaccharide vaccines are not
administered in the .
majority of countries. The CDC recommends that vaccines should be
administered.as age- =
appropriate or as indicated by the presence of underlying medical conditions
(CDC, 2002). .
ImmunoPrint diagnostic analysis could be used to point out the need for
vaccination in
immigrating individuals.
Each country may have needs for assessing the immune status of immigrants that
may not
necessarily coincide with the U.S. requirements as previously outlined. In
addition, there may be
other needs inside or outside the U.S., dictated by. a particular
investigation at a particular -site.
'For example, Greenaway et al. (2004) have embarked on a mission to assess the
immune status
of immigrants iii the Montreal area of Canada, with initial emphasis on 5
different.infectibus
-agents: hepatitis A, measles, mumps, rubella, and varicella (Greenaway CA,
Boivin JF, Dongier
..P, Miller MA, Schwartzman K. Susceptibility to vaccine-preventable diseases
in newly arrived
immigrants. Abstract G-538, pp 254-5, In 44t' ICAAC Abstracts 2004
[Interscience Conference
on Antimicrobial Agents and Chemotherapy, Washington, DC, Oct 30 -Nov 2,
2004]: American,
Society for Microbiology, Washington, DC.). Subsequent studies may.expand to
include tetanus
and diphtheria, as well as -other agents listed for -routine immunizations of
Canadians, which are
essentially identical to those listed for the U.S. Note that hepatitis A, in
the above study, does
not represent an infectious agent designated for routine immunization in
Canada or the U.S.; but
it is listed for selective iinmunization where people have been identified to
be at greateriiisk of
disease. Likewise, diagnostic panels may be expanded where appropriate to
represent additional,
infectious agerits that are listed for selected immunization.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
115
In exemplary embodiemtns of the present invention diagnostic subpanels can be
=developed to
accommodate the needs for different researchers where there is an interest to
follow up on
particular infectious agents in a region where there may be new or ongoing
outbreaks of disease.
The influx of immigrants.that are. unprotected against vaccine preventable
diseases (VPD) has
already been shown to contribute the increased incidence of disease. For
example, varicella and
rubella vaccines are not routinely administered in many countries, and this
has therefore resulted
in an over representation of immigrants in outbreaks of varicella and rubella
in areas where these
vaceines already exist. Studies have shown that in Canada, for example,
immigrants are more
likely to be susceptible to varicella, rubella, and mumps than North
Americans. In addition;
they have stated, "Adult immigrants may benefit from targeted vaccination
programs but given
the geographic variation in susceptibility to VPD, this must be taken into
consideration when
=developing these programs." For this scenario, ImmunoScore diagnostic panels
can prove
invaluable to identify the target according to the individually assessed
immune status.
Tuberculosis (TB) is another disease that may be of considerable importance to
monitor, not
necessarily for immune status, but for active infection, particiilarly in
immigrant populations: It
=is estimated that one third of the global population is infected with =TB.
Due to improved-
laboratory services during the 1990s, there has been a resumption of an
overall.decline in U.S.
cases of TB. Nonetheless, the CDC states, "TB continues to pose substantial
social, public
health, and economic costs." (Centers for Disease Control and Prevention.
National plan for
reliable tuberculosis laboratory services using a systems approach:
recommendations from CDC.
and the Association of Public Health Laboratories Task Force on Tuberculosis
Laboratory
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
116
Services. MMWR 2005;54[No. RR-6]:1-12). This 2005 CDC report indicates that
the U.S.
spends nearly $1 billion annually on TB-related costs, with 9-14 million
people having latent TB
infections and 15,000 new cases reported in 2003. -The CDC also states, "to
eliminate TB in the
United States, further improvements are needed.in laboratory services to
support TB treattnent,=
prevention,.and control." As a result, "TB control is now entering a new phase
in=the United
States, a transition from low incidence to elimination." (Centers for Disease
Control and
Prevention. Progressing toward tuberculosis elimination in low-incidence areas
of the United
States: recommendations of the- Advisory Council for the Elimination of
Tuberculosis. IvI1ViWR
2002;51 [No. RR-5]:1-16). An ImmunoScore diagnostic panel containing TB, for -
example; could
be utilized in this regard in the U.S.
The BCG vaccine, currently licensed for TB, is not recommended for routine use
in the U.S.
because of questionable efficacy; however, there are other countries that
routinely use this
vaccine. The United Kingdom, iri 2005, announced that, after 50 years, it is
dropping its school
TB vaccination program for young teenagers, in favor of targeting infants in
ethnic populations
that are at greater risk (Celia Hall, Medical Editor, Telegraph Group Limited,
July 7, 2005). For
example, they have indicated that the. case rate in whites is 3.6 per 100,000,
while the rate in
Africans is 279.8 per 100,000, and the rate in Indian,.Pakistani, and
Bangladeshi people is 126.7.
New immigrants from countries with high TB incidence would also be targeted
for vaccinations.
It is possible that a diagnostic panel which includes TB. would prove useful
for=screening these=
populations.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
117
As demonstrated in the UK, there is a.greater incidence of TB in irnmigrants
froin certain regions '
of the world. It would be therefore useful to add a TB diagnostic to immigrant
panels previously
described. For example, a TB diagnostic could be included in the subpanel
proposed for Canada,
or used as a separate diagnostic, as a follow-up to the Greenaway et al.
study: It is possible to
use specific antibody detection=to distinguish active TB infections from-non-
active or non-:TB
(Tong M et al. 2005. J Immunol Methods: 301:154-63). In this case, specific TB
antigens,
particularly those of a carbohydrate niature, may be selected for inclusion in
the proposed
diagnostic panels to identify people with active TB infections in need of
treatment.
10. . ImmunoScore Diagnostic Panel and Preventive Therapy for Autoimmune
Disease
Antibodies directed against.self antigens and tissues are known -as
autoantibodies. These
autoantibodies can be expressed years or decades before the. autoimmunity
causes clinical
disease. The clinical disease arises when.the.autoantibodies-cause so much
damage that the.afflicted individuals begin to show'syrimptoms: For example,
antibodies attacking self nuclear
airntigens cause glomeruloneprhtis and vasculitis as symptoms of systemic
lupus erythematosus;
and antibodies to myelin basic protein and myeling oligodendiocyte
glycoprotein eventually
cause brain invasion by CD4+ T cells in multiple sclerosis. ImmunoScore
technology may one
day allow physicians to screen a healthy person's blood for autoantibodies
years prior to causing
disease, and thereby, enable a physician to recommend a course of treatment to
delay, or even
perhaps prevent, the manifestation of clinical=disease.
There has already been rnuch time and money invested in researching and
developing the
necessary tools to screen an individual's genetic makeup to see if a patient
might be at risk for
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
118
autoimmune disease. Most chronic diseases, however; arise from a complex
interplay between
environmental influences and multiple genes that each'mdke a small
contributiori to the course of
the disease. Many factors apparently play an as yet completely undefined role
in the expression
of Guillain-Barre syndrome. Elements that have been proposed to be of
importance in the course
of this disease==include mannose-binding lectin polymorphisms; expression of
apolipoprotein E
(apo-E) isoform; levels of cytokines IFN-y, IL-6, and TNF-a; levels-of serum
proteins including
apo A-IV, haptoglobin, transthyretin, and fibrinogen; antibodies to GDla/Gd1B
complex; LM-1,
and GM1; HLA haplotype; and even exposure to vaccine antigens. Detection of
susceptible
genes would not necessarily reveal when (or even if) an individual would be
stricken with an
autoimmune disease. Detection of specific antibodies with the ImmunoScore
Autoimmune
Disease detection panel would signal that a= disease-causing process was
already underway.
=Fig. 5B depicts exemplary process flow in which one or more exemplary
ImmunoScore
autoimmune panels can be used. With refemce thereto, at 5B 10 healthy patients
can, for
example, be screened at regular intervals for antibodies or other markers
known to be indicative
of autoirnmune disease (as described below) at, for example, their physician's
office. Wonien
and patients with a family history of autoinunune disease can, for example, be
scheduled for =
more regular ImmunoScore Autoimmune Disease diagnostic screenings. The
patient's HLA
type can also, for example, be a useful input to the appropriate diagnostic
detenmination. At
5B20, by implementing regular screenings, an individual patient's rise and
fall of relevant
antibody populations coitld be followed and stored in an ImmunoScore Database
(sometimes
referred to herein as an= "InununoScoreKeeper"). If it happened, such as at
5B30, that a patient's
antibody levels were not a cause for concern, that patient's data could be
maintained in the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
119
autoimmune disease. Most chronic diseases, however, arise from a complex
interplay betweeri .
erivironmental influences and multiple genes that each make a small
contribution to the course of
the disease. Many factors apparently play an as yet completely undefined role
in the exprtssion
of Guillain-Barre syndrome. Elements that hav.e been proposed to be of
importance 'in the course
of this disease include mannose-binding lectin polymorphisms; expression of
apolipoprotein E
~apo-E) isoform; levels of cytokines IFN-'y, IL-6, and TNF-a; levels of serum
proteins including
apo A-IV, haptoglobin,. transthyretin; and fibrinogen; antibodies to GD1a/Gd1B
complex, LM-1,
and GM 1; HLA haplotype; and even exposure. to vaccine antigens. Detection of
susceptible'
genes would ndt necessarily reveal.when (or even if) an individual would be
strickeri with aii
autoimmune disease.. Detection of specific antibodies with the IminunoScore
Autoimmune
Disease detection panel would signal that a.disease-causing pro.cess was
already underway. Fig. 5B depicts exerriplary process flow in which one or
rnore=exemplary ImmunoScore
autoimmune panels can be used. With refernce thereto, at'5B'10 healthy
patients ~can, for
example, be screened at regular intervals'for antibodies or other markers
known to be indicative
of autoimmune disease (as described below) at, for example, their physician's
office. Women
and patients with a family history of autoimmune disease can, for example, be
scheduled for
more regular ImmunoScore Autoimmune Disease diagnostic screenings. The
patient's HLA type'can also, for example, be a usefiil input to the
appropriate diagnostic determination. At
5B20, by implementing regular screenings, an individual patient's rise and
fall of relevant
antibody populations could be followed and stored in an ImmunoScore Database
(sometimes referred to herein as an "ImmunoScoreKeeper"). If it liappened,
such as at 5B30; that a patierit's.
antibody levels were not a cause for concem, that patient's data could be
maintained in the -
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
120
database and referred to by researchers as well as that patient's physician(s)
at subsequent visits.
In addition; this information might be of use to insuraice =providers and
health maintenance
organizations for statistical analyses. At 5B35, if the patient has'increased
antibody levels, but
no sign of clinical disease, a'therapeutic course of action involving
treatment with appropriate
pharmaceuticals, or perhaps other suggested therapies including dietary
modifications or similar
recommendations can be implemented. In addition, the patient can be regularly
screened for any
change in the antibody or marker levels, as shown at.5B40. Such an
early=indication of
autoirnmune disease might be helpful to the insurance industry and the
treatment prioi to chronic,
disease would be more cost-effective to both the patient and the insurance
provider. Therapies
considered experimental can, foz example, be monitored by researchers with
regular
ImmunoScore Autoimmune Disease diagnostic measurements during the course of
the.
-experimental treatment(s). Efficacy of drugs or behavior modifications could
thereby be
monitored before the disease outbreaks became full blown. The presence
of=predictive
= antibodies woiild not mean that a. patient would get defin.itely sick, but
would ;give a pex=~centage
risk of autoimmune disease developing over some years or months. Currently,
there is a paucity
of effective treatments for autoimmune disease conditions, but this should not
stand in the way of
ImmunoScore Diagnostic screening, which can, for example, accelerate the
development of-such
treatments.
Thus, in exemplary embodiments of the present invention, by gathering data
over a large patient
population, over a large interval of time, and processing the da.ta and making
available the data
and any analyses thereof, as shown at 5B45, useful information can be provided
to
Insurers/HMOs 5B50, researchers'5B55 and Clinicians'5B57.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
121
Cell therapy, pioneered for the treatment of malignancies in the form of bone
marrow
transplantation, has subsequently been tested and successfully employed in
autoiminune
diseases. Autologous hemopoietic stem cell transplantation (1-iSCT) has become
a curative
option for conditions with very poor prognosis such as severe fornis of
scleroderma; multiple
sclerosis and lupius, in which targeted therapies have little or'no effect
(Dazzi, et al. 2007).
Regulatory T cells, found abnormal in several autoimmune diseases, have beexi
proposed as
central to achieve long-term remissions.. Mesenchymal stem cells of bone
marrow origiri have
more recently been shown not only to be able to differentiate into multiple
tissues; but also .to
exert a potent antiproliferative effect that results in the inhibition of
immune r.esponses an
prolonged survival of hematopoietic stem cells.' At=the current tiune,-all.of
these potential
resources clearly need to be iizvestigated at the preclinical level but
support a great deal of
enthusiasm for cell therapy of autoimmune diseases (Dazzi,'et al. 2007).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
122
Autoimmune Disease
Autoimmune diseases ar'ise frorri an overactive immutie r=esponse of the body
against
substances and tissues normally present in the body:. The many diseases called
autoimmune are
either systemic (involving inany body organs) or organ specific. The immune
system may-=
initiate an attack on self because the normal controlling rnechanisms of the
immune =system are
impaired,.because a host response to ain extrinsic irnmunogen, such'as a
virus, fails to distinguish*
between normal tissue and the object=of the attack (cross-reactivity); or
because immunogenic
tissues, normally hidden from the immune system, are made visible to, the
immune systein (loss
of tolerance). Any or all of these mechanisms may occur in human autoimmune
disease
(Lockshin, 2001). Differences among authors among their defirutions of
autoimmunity cause
published lists of autoimmune diseases to differ:
Women tend to be affected rriore often by autoimmune 'disorders = nearly 79%
of
autoimmune disease patients in the U.S. are women, most- frequently during the
childbeariag
years. Table I lists the striking female-to-male ratios in some autoimmune
diseases
(http://www.aarda.or~). The reasons for the sex bias in autoimmune diseases
are unclear but=
may include such factors as sex-related differences in immune responsiveness,
response to
infection, sex steroid effects and sex-linked genetic factors (Whitacre; et
al. 1999). In human,
autoimmune illness, the term "ferimale predominance" refers to sex differences
.of inciderice, not
severity. Severity differences are slight or nonexistent (Weyand, et al.
1998).
Many, but not all; autoimmune diseases primarily affect women. In'humans,
severity of
.illness does not differ between men and women. Men and women respond
similarly to infection
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
123
and vaccination, wh.ich suggests that the intrinsic differences in immune
response between the
sexes do not account for differences in disease frequency. In autoimmune-like
illnesses caused
by recognized environmental agents, sex discrepancy is usually explained by
differences in
exposure. Endogenous horrriones are not a likely explanation for sex
discrepancy; hormones
could have an effect if the effect is a threshold rather than quantitative. X
and Y chromosomal
differences have not been studied in depth: Other possibilities to explain sex
discrepancy include
chronobiologic. difference and various other biologies, such and pregnancy and
menstruation, in
which men differ from women (Lockshin, 2006)..
If infections (as yet unidentified) or toxins induce 'autoimmune disease,
exposure
differences- remain as plausible explanations for the sex differences. Gonadal
hormones, if they
play a role, likely do so through a threshold or permissive mechanism rather
than through
quantitative 'immunomodulation. Differences related to X inactivation,
imprinting, X or Y
chromosome genetic modulators, and intrauterine 'influences xemain as
alternate explanations for =
sex differences of incidence. The -epidemiology of the sex-discrepant
autoimmune diseases
(afflicting preferentially young females) suggests that an explanation for sex
discrepancy lies in
differential exposure, vulnerable periods, or=thresholds (Lockshin,,2001).
For T-cell niediated autoinimune diseases, the presence of -serum antibodies
can predate
the onset of disease, and be predictive of the development of clinical
symptoms..
Autoimmune diseases
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
124
= Acute disseminated encephalomyelitis (ADEM) - Acute disseminated
encephalornyelitis
(ADEM) is a monophasic autoimmune demyelinating disease of the central
nervotts system
that typically follows a febrile infection or =a vaccination. Children are
predominantly .
affected. A plethora of=viral and. bacterial pathogens and a number of
vaceinations have-been
associated =with ADEM. Experimental animal studies indicate that both
primary.aiid
secondary autoimmune responses contribute to central nervous -system
inflammation and
subsequent demyelination. The clinical diagnosis of ADEM is strongly
suggested. by a close
tempoiral relationship between an infectious incident or an immunizatioii aind
the onset of
leukoencephalopathic neurological symptoms'(Menge, et al. ~005).
= Addison's disease - about 7 in 10 cases are due to'an autoimmune disease. In
auto-inimurie
Addison's disease, antibodies are made which -attach to cells in the adrenal
cortex. These
destroy the cells which make cortisol and aldosterone: Patients
with*autoimmune Addison's
disease (AAD) are prone tb develop other autoimmurie . manifestations. An
increased
prevalence of celiac disease (CD) has recently beeri demonstrated in Norkhern
Europeari
patients with AAD. In.patients with AAD there is a high prevalence of both=CD
and IgA .
deficiency. Consequeintly, it is important to screen, for CD with tissue
transghitarninase =.
autoantibodies of the IgA class and for IgA levels (Betterle, et al. 2006).
=' Ankylosing spondylitis = is A chronic, progressive autoimmune disease
characterized by
arthritis, inflamination, and eventual immobility of a number ofjoints. The
disease-usually
involves the spine and surrounding :spinal structures and usually begins
between the ages of
fifteen and thirty-five years and affects young white males three times as
frequently as
females. Some patients of this debilitating disease have antibodies to the
bacterium
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
125
Klelisiella. This micro-organism apparently has some antigenicity which.
causes the body to
make antibodies which in turn attack the lower spiin.e and cause ankylbsing
spondylitis.
= Antiphospholipid antibody syndromie (APS) - affects blood=clotting process-
causes
blood clots to form in veins and/or arteries. There are ftee primary classes
of antibodies
associated with the antiphospholipid antibody syndrome: i)
anticardiolipin;antibodies,;2) the:
lupus anticoagulant and 3) antibodies directed against=specific molecules
including a
molecule known as beta-2=glycoprotein 1. (University of Illinois -
Urbana/Champaign. Carle
Cancer Center. Hematology. Resource Page http://www-=
admin.med. uiuc. edu/hematol ogy/PtAPS.htm).
= Aplastic anem'ia - rriay be caused by autoimmune attack on the bone marrow,
slowing or.'
shutting down productioYi of new blood cells. Some acquired aplastic anemia
(AA) results
from immune-mediated destruction of hematopoietic stern cells. Cytokine gene
polymorphisms are implicated in controlling=.cytokine production and
iricreasing the
susceptibility to some autoimmune diseases (Gidvani, et'al. 2007).
= Autoimmune hepatitis. -- caused by attack on'the= liver by body's
immune'system. The
disease is usually quite serious and, if not treated, gets worse over time:
"It's usually chronic,
meaning it can last for years, and can lead to cirrhosis (scarring and
hardening) of the liver
and eventually liver failure. Autoimmune hepatitis is classified as -either
type I or II. Type I.
is the most common forni in North America.:It occurs at any age and=is more
common among
women than men: About half of those with type -I.have other autoimrrrnune,
disorders; such as
type 1 diabetes, proliferative glomerulonephritis, tliyroiditis, Graves'
disease, Sjogren's
syndrome, autoimmune anemia, and ulcerative colitis. Type II autoimmune
hepatitis is less
common, typically affecting girls ages 2 to 14, although adults can have it
too (National.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
126
Institute of Diabetes and Digestive and Kidiney Diseases, NIH.
http://digestive.niddk.nih.gov/ddiseases/pubs/autoimmunehep/index.htnn ).
= Coeliac disease = characterized by chronic inflammation of the proximal
portion of the small
intestine. When people with celiac disease eat foods or use products
containing gluten, their
immune system responds by damaging the small intestine. The villi lining the
small intestine
are damaged or destroyed (National Institute of Diabetes and Digestive and
Kidney Diseases,
NIH. http://digestive.niddk.nih.gov/ddiseases/pubs/celiacn.
= Crohn's disease - form of inflammatory bowel disease characterized by
chronic
inflammation of the intestinal tract - major symptoms include abdominal pain
and diarrhea.
The new treatrim.ent is an antibody designed to disable interleukin-12 (IL-
12), an immune
system protein involved in inflammation. People with Crohn's produce exuess IL-
12.
Previous studies conducted by the NIAID linked IL-12 to the cascade of immurie
system
events that leads to the debilitating symptoms of Crohn's disease (NIH News
http=//digestive.niddk.nih. gov/ddiseases/pubs/crohns/index.htm).
= Diabetes mellitus - characterized by a deficiency or absence of insulin
production (Type I) -
often the consequence of autoimmune attack on the insulin-prodticing beta
cells in the islets
of Langerhans of the pancreas. LADA (latent Autoimmune Diabetes of Adulthood)
or
type 1.5 diabetes - a slowly progressive form of type 1 diabetes mellitus.
Patients are often
diagnosed as type II diabetes, but have positive paincreatic islet antibodies,
especially to
glutamic acid decarboxylase (GAD). They do not inunediately require insulin
for treatment,
are often not overweight, and have little or no resistance to insulin (Johns
Hopkins
Autoimmune Disease Research Center.
http://autoimmune.pathology jhmi.edu/diseases.cfin?systemID=3&DiseaselD=23).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1127
= Goodpasture's syndrome - characterized by rapid destruction of the kidneys
and
-hemorrhaging of the lungs through autoimmune reaction against an antigen
found in both
organs. Because the early,symptoms are characteristically vague, and because
of the
tendency of the disease to undergo very rapid progression, it is common for
the true
diagnosis to be reached at a relatiyely late stage. Tests for anti- glomerular
basement'
. membrane antibodies (GBM) antibodies in the blood can be very useful. They
should always
combined with tests for antibodies to neutrophil cytoplasmic aintigens (ANCA),
as the two
types of autoantibody can occur together. Kidneybiopsy is almost always
necessary. It is
ofteii the quickest way to-secure a diagnosis, and-even if the diagnosis looks
certain, it may
give valuable information about the likely effect of treatment (Yang, et al.
2007).
= Grave's disease - most common form. of hyperthyroidism - caused by anti-
thyroid
antibodies that have the effect of stimulating the thyroid into overproduction
of thyroid
hormone. Grave's disease is an autoimmune disorder in which antibodies to the
thyrotropin
receptor result in constitutive activation of the'receptor and increased
levels of thyroid
hormone. Thl and Th2-like cytokines are also involved'in the pathogenesis'of
Graves'
disease (Molnar, 2007).
= Guillain-Barr6 syndrome (GBS) -an acquired immune-mediated inflammatory
disorder of
the peripheral nervous system - also called acute inflammatory demyelinating
polyrieuropathy, acute idiopathic polyradiculoneuritis, acute idopathic
polyneuritis, and
's ascending paralysis. When Guillain-Barrd is preceded by. a viral or.
bacterial
Landry
infection, it is possible that the virus, has changed the. nature of cells in
the nervous system= so'.
that the immune system treats them as foreign cells. It is also possible that
the virus makes
the immune system itself less discriminating abotxt.what cells it recognizes
as its own,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
128
allowing some of the immune cells, such as certain kinds of lymphocytes and
macrophages,
to attack the myelin. Sensitized T lymphocytes cooperate with B'lymphocytes to
produce
= r
antibodies against components of the myelin sheath and may contribute to
destruction.of the
myelin.'Scientists are investigating these and other possibilities to find why
the immune
system goes awry in Guillain-Barre syndrome and other autoimmune diseases: The
cause and
course of Guillain-Barrd syndrome is an active area of neurological
investigation,
incorporating the cooperative efforts of neurological scientists,
immunologists, and
virologists (National Institute of Neurological Disorders'and Stroke.
http://www.ninds.nih.gov/disorders/gbs/gbs.htm).
= Hashimoto's disease - common form of hypothyroidism.- characterized by
initial
inflammation of the thyroid, and later dysfunction and =goiter - there are
several characteristic
antibodies (e.g., anti-thyroglobulin). The qiuest contiiiues for the
identification of
susceptibility genes for autoimmune thyroiditis. In addition to the classical
major
histocompatibility complex class II genes and cytotoxic T cell antigen-4, new
studies have
appeared on CD40 the protein tyrosine phosphatase-22. Too much iodine
increases the
incidence of Hashimoto's thyroiditis, perhaps by augmenting the antigenicity
of
thyroglobulin. T regulatory cells, Toll-like receptors and presentation of
lipid antigens by.
CD1 molecules are new areas of basic immunological investigation that have
been applied to
autoimmune thyroiditis (Caturegli, et al. 2007).
= Idiopathic thrombocytopenic purpura - an autoimmune disease where the body
produces
anti-platelet antibodies resulting in a low platelet count. = Immune
thrombocytopenic purpura
is classified as primary or as secondary to an underlying disorder and as
acute (of six months
or less in duration) or chronic. In one study thirty-seven patients with
idiopathic
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1129
thrombocytopenic purpura (IT?) were treated with, a standard Helicobacter
pylori eradication
regimen irrespective of H. pylorf infection. Their results indicate that
platelet recovery results
from the disappearance= of H. pylori itself, and is mediated, in part,
through= suppression of
anti-platelet autoantibody production (Asahi, et al. 2006).
Multiple sclerosis (MS) - disorder of the central nervous system characterized
by decreased
nerve function due to myelin loss and secondary= axonal damage. Analysis of
antibodies
against myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein
(MBP) in
patients with a clinically isolated syndrome is a rapid, inexpensive, and
precise method for
the prediction of early conversion to'clinically defiinite multiple sclerosis
(Berger, et al. -
2003).
= Myasthenia gravis - a disorder-of neuromuscular transmission=leading to
fluctuating
weakness and fatigue - weakness is caused by antibodies that block
acetylcholine receptors,
that is anti-acetylchoiine-receptor (anti-AChR) antibody at the neuromuscular
junction..
Immunosuppresive treatment has been shown to be effective in MG patients.
(Tsinzerling, et
al. 2007).
= Opsoclonus myoclonus syndrome (OMS) - a neurological disorder - result of
autoinimune
attack on the nervous system - symptoms include opsoclonus; myoclonus,
=ataxia, intention
tremor dysphasia, dysarthria, mutism, hypotonia, lethargy, irritability, or
malaise. Despite
circumstantial evidence that opsoolonus-myoclonus (OM) is often imiriune
mediated, no
specific autoantigen has been identified. A groupfound frequent aind
heterogeneous
immunity to neuronal autoantigens without a single specific antibody marker of
OM
(Bataller, et al. 2003).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
130
= Optic neuritis - inflammation of the optic nerve that may cause a complete
or paxtial loss of
vision. Patients with optic neuritis had more anti-myelin basic protein and
anti-proteolipid
protein antibodies than did control subjects <Sellebjerg, et al. 199$). Since
high
concentrations of GQ1b gangliosides are known to be present in the human optic
nerve and
anti-GQ1b antibodies can crossthe blood-brain barrier, the optic disc oedeina
in [a] patient
could represent an anti-GQlb IgM complement mediated inflammatory
derimyelination (Chan,
2003):
= Ord's thyroiditis - similar to Hashimoto's disease, except the thyroid is
reduced -in size.
= Pemphigus - an autoimmune disorder that causes blistering and raw sores on
skin and
mucous membranes. Patients'with pemphigus develop antibodies that bind to the
keratinocyte cell surface, the site of prirnary pathology (Payne, et al.
2005).
= Pernicious aneinia - autoimmune disorder characterized by anemia due to
maladsorption of -
vitamin B12. Today, the best tests for diagnosing pernicious anemia are the
vitamin B12
level, folic-acid level, methylmalonic (MMA) level, and antibody tests for
antibodies to
intrinsic factor and parietal cells. Levels of MNIA are elevated in both serum
and urine-before
levels of vitamin B 12 become abnormally' decreased or symptoms of deficiency
appear: The
blood test for MMA is considered superior to the urine measurement (Greenwood
and
Sentry, 2007).
= Primary biliary cirrhosis - an organ-specific autoimmune disease that
predominantly
affects women and is characterized by chronic, progressive destruction .of
small intrahepatic
bile ducts with portal inflammation and ultimately 'fibrosis, leading to liver
failure in the
absence of treatment. The serologic hallmark of PBC is the presence of auto-
antibodies to
mitochondria, especially to the E2 component ofthe pyruvate dehydrogenase -
cornplex
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
131
(PDC). Current theories on the pathogenesis of PBC favor the hypothesis that
the disease
develops as a result of an inappropriate immune response following stimulation
by an
environmental or infectious agent. The pathogenetic mechanism is believed to
be caused by
a defect-in immunologic tolerance, resulting in the activation and expansion
of self-antigen
specific T and B lymphocyte clones and the production of circulating
autoantibodies in
addition to a myriad of cytokines and other inflammatory mediatoxs. This leads
to
ductulopenia and persistent cholestasis, by developing end-stage hepatic-cell
failure
(Reshetnyak, 2006).
= Rheumatoid arthritis - a heterogeneous autoimmune disorder wherein the
immune system
attacks the bone joints. In asymptoinatic patients and in patients with
undifferentiated
arthritis, the presence of anti-cyclic citrullinated peptide (CCP) antibodies
is a predictor of
progression to RA (Van Gaalen, et al. 2000). Other antibody reactivities
include those
against heat-shock proteins (Hsp65, Hsp90, DnaJ, and BiP), heterogeneous
nuclear RNPs
A2/B1 and D, annexin V, calpastatin, type'Il collagen, glucose-6-phosphate
isomerase (GPI),
elongation factor, and human cartilage.gp39 (Van Boekel, et a1. 2002).
= Reiter's syndrome - autoimmune attack on various body systems in response to
a bacterial
infection and the body's confusion over the HLA-B27 marker. Other polymorphic
. determinants of 1VIHC class I antigens might play a critical role in the
pathogenesis of
Reiter's syndrome (Shimamoto, et al. 2000).
= Sjogren's syndrome - autoimmune disorder in which immune cells attaclk and
destroy the
exocrine glands that produce tears and saliva. Antibody self-reactivities
include anti-fodrine and anti-salivary duct antibodies, rheumatoid factor,
especially of the IgA isotype, and anti-
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
132
nuclear antibodies, mostnotably anti-Ro/SSA and anti-La/SSB antibodies
(Youinou, et al..
2005).
= Systemic lupus erythematosus - a chronic autoimmune disease wherein the
immune. system
becomes hyperactive and attacks normal tissue - this results in inflammation-
an b'rings about
symptoms.. About 90% of people who have lupus are young women in their.late
teens to 30s,
but children (mostly girls) and older "men and women can also be affected. The
number and
variety of antibodies that can appear in lupus are greater thain those in any
other disorder.
These antibodies, which are the underlying physiologic problem in lupus; along
with othe'r
unknown factors, determine which symptoms develop. However, the levels of
these
antibodies may not always be proportional to the person's symptoms. Laboratory
tests can
help doctbrs confirm the diagnosis. A blvod test can detect antinuclear
'antibodies, which are .
present in alrnost all people who have lupus (
htta: //www.merck. com/mmhe/sec05/ch068/ch068b. html).
= Temporal arteritis - also known as giant cell arteritis -'inflammation of
blood vessels --
most commonly the large and medium arteries of the head. Aiiticardiolipin
antibody levels
predict flares and ielapses in patients with giant-cell (temporal) arteritis
(Kerleau, et al.
1994).
= Warm- autoimmuine hemolytic anemia - characterized by IgM attack against"red
blood. =
cells. In the warm antibody type, the'autoantibodies attach to and -destroy
red blood cells at
temperatures equal, to or in excess of normal body temperature. One study
showed that the
peak incidence of AIHA was in the first 4 years of life. No sex predominance
was noted.
Warm AIHA was the most common type of acquired immune hemolytic anemia; it
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
133
comprised 64 of the 100 patients, whereas 26 patients showed a cold AIHA
(Vaglio, et al.
2007).
= Wegener's granulomatosis - form of vasculitis that affects the lungs,
kidneys and other
organs. It is,a type ofvasculitic syndromes that all feature the presence for
an abnonual type
of circulating antibody termed ANCAs (antineutrophil.cytoplasmic antibodies)
and affect
small and medium-sized blood vessels (Aries, et al. 2007).
Diseases suspected to be linked to autoimmunity,
'
= Alopecia universalis - suspected autoimmune disease in which the body's
white blood cells
attack hair and result in total baldness. Its association with other
autoimmune disorders
-renders an autoimmune pathogenesis very likely, the targeted antigen being
private to the hair
follicle (Seifert, et al. Blood. 2005).
=' Behiot's disease - multi-system condition where the'immune system produces
inflammation
in bodily tissues, primarily causing vasculitis. Behcet's is one of the few
forms of vasculitis
in which there is a known genetic predisposition. The presence of the gene HLA-
B51 is a
risk factor for this disease. However, it must be emphasized that presence of
the gene in and:
of itself is not enough to cause Behcet's: many people possess the =gene, but
relatively few
develop Behcet's (httn://vasculitis.med.jhu edu/ty,pesof/behcets html).
= Chagas disease - believed in the chronic phase to be result frcim homology
of a
Trypanosoma cruzi antigen to body tissue - resulting in a delayed autoimmune
reacticin
leading to Chagasic cardiopathy (cardiomegaly), volvulus or constipation, and
ultimately,
death. Chagas disease afflicts about 30% of the 20 million people infected
with T. cruzi in
. South America (Marin-Neto, et al. 2007).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
134.
= Chronic fatigue syndrome - a disorder whose prunary symptom is usually
intense fatigue -.
likely has multiple causes - some maintain that autoimmune damage'to the brain
stem is the
principal mechanism in a significant subset of cases. Chronic fatigue syndrome
is a disorder
characterised by prolonged fatigue and debility and is mostly associated with
post-infection
sequelae although ongoing infection isunproven. Inzmunological aberration.is
likely and this
may prove to be associated with an expanding group of vasoactive neuropeptides
in the
context of molecular mimicry and inappropriate immunological memory.
Vasoactive
neuropeptides including vasoactive intestinal peptide (VIP) and pituitary
adenylate activating
polypeptide (PACAP) belong to the secretin/glucagon superfamily and act as
hormones,
neurotransmitters, immune modulators and neurotrophes. They are readily
catalysed to
smaller peptide fragments by antibody hydrolysis: They and their binding sites
are
immunogenic and are known to.be associated with a range of autoimmune
conditions.
Vasoactive neuropeptides are widely distributed in the body particularly in
the central,
autonomic and peripheral nervous systems and have been. identified in the
gut,, adrenal =gland,
reproductive organs, vasculature, blood cells and other tissues. All
documexited symptoms of
CFS are explained by vasoactive neuropeptide compromise, namely fatigue and
nervous.
=system dysfunction through impaired acetylcholine activity, myalgia through
nitric oxide and
endogenous opio'id dysfunction, chemical sensitivity through peroxynitrite and
adenosin.e=
dysfunction, and immunological disturbance, through changes in immune
modulation.
Perverse immunological memory established against these substances or their
receptors may
be the reason for the protracted nature of this condition (Staines, 2004).
= Dysautonomia - malfunction of the autonomic nervous system ~- including such
disorders as
posturai orthostatic tachycardia syndrome. Post-viral autoimmune damage
appears to be a
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
135
frequent cause.. Acute autonomic neuropathy can affect pazasympathetic,
sympathetic, and
enteric nerves or neurons and is associated with antibodies to =ganglionic
nicotinic=
acetylcliolirie receptors. These antibodies appear to be causative based on a
rabbit.
imrriunization model and serum transfer studies from patients and animals
(Etienrie and.
Weimer, 2006).
= Endometriosis - common medical.condition wherein the tissue lining the
uterus is found
outside the uterus, typically affecting other organs in the pelvis - can lead
to serious -health
problems, primarily pain and infertility. Endometriosis and polycystic ovary
syndrome
(PCOS) are associated with higher values of anti-FSH-immunoglobulin .(Ig)A,
anti-V 14D=
IgA, and endometriosis with anti-V 14D=IgG. These data sug=gest, that anti-FSH-
IgA could be.'
a marker of ovarian disorders that cause infertility (Haller, et'al. 2005).
='Hidradenitis suppurativa - rare skin disease in which apocrine sweat glands
become
severely inflamed. A reductiori in the percentage of NK cells over time and a
loviwer
monocyte response to triggering by bacterial components is observed in
patients with
hidradenitis suppurativa. Further research is needed to clarify if these
changes are connected
to an autoimmune niechanisrin in the pathogenesis of hidradenitis suppurativa
(Giamarellos-
Bourboulis, et al. 2007).
= Interstitial cystitis - a urinary bladder disease characterized by any of
the following
symptoins - pelvic pain, urinary frequency, urgency, pain with sexual
intercourse, and pain
' with urination. Interstitial cystitis (IC) has been deemed by some authors
as a local
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
136
manifestation of a systemic disease, particularly one of the autoimmune
disorders (Porru, et
al. 2005).
= Lyme disease - caused by Borrelia burgdorferi - characterized by
'intermittent bouts of
arthritis with severe joint pain and swelling. Some patients may develop
chronic
neurological complaints months to years after infection including shooting
pains, numbness
or tingling of hands or feet, and problems with concentration and short term
memory.
Although the causative agent of Lyme'disease is definitively known to be the
tick-borne
spirochete, Borrelia burgdorferi, the etiology of chronic joint inflammation
that ensues in a
subset of patients remains less well understood. PeTsisterice of arthritis
after apparent
eradication of the spirochete suggests an autoimmune reaction downstream of
the original
bacterial infection. Research has identified cytokeratin 10, present in
synovial microvascular
endothelium, as- a target ligand and a putative autoantigen in chronic,
antibiotic treatment-
resistant Lyme arthritis. Furthermore,*there is cross-reactivity between
cytokeratin 10 and a
prominent B. burgdorferi Ag, outer surface protein A. Release of the self
protein in the
context of inflammation-induced tissue injury and the resulting in situ
response to it could set
in motion a feed-forward loop, which amplifies the inflammatory process,
thereby rendering
it chronic and self-perpetuating, even in the absence of the inciting pathogen
(Ghosh, et al.
2006).
= Neuromyotonia - spontaneous muscular activity resulting from repetitive
motor unit action
potentials of peripheral origin. It develops as a resuit of both acquired *and
hereditary
diseases. The acquired form is more frequent and is usually caused by
antibodies against
neuromuscular junction. About 40% of patients with acquired neuromyotonia will
have
detectable, voltage-gated potassium-channel antibodies. Clinical,
electrophysiological and
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
137
immunological measurements are important in definizig the phenotype of
neuromyotonia, and
other, milder forms of peripheral nerve hyperexcitability (Maddison, 2006).
= Psoriasis - a skin disorder in which rapidly-multiplying skin cells produce
itchy, scaly =
inflamed patches on the skin. Psoriasis is a common chronic inflammatory skin.
disease, the ;
study of which might also be of considerable value to.the understanding of
other
inflammatory and autoinunune-type.diseases, such as rheumatoid arthritis,
inflammatory
bowel disease, multiple sclerosis and diabetes mellitus. There is clear
evidence that,T cells
and dendritic cells have a central role in psoriasis (Boyman, et al. 2007).
= Sarcoidosis - disease.wherein granulotnas can form anywhere in the body, but
particularly in
the lungs. Various autoimmune diseases have=been reported to occur in patients
with
sarcoidosis (Talkahashi, et al. 2006). Iii one study The prevalence of
Chlamydophila
pneumoniae-specific antibodies in bronchoalveolar lavage-fluids was
significantly higher in
sarcoidosis patients for IgG and IgA (IgG: 74.4%; IgA: 46.2%) compared to
controls (IgG:
14.7%; IgA: 14.7%) (Gaede, et al. 2002).
= Schizophrenia - mental disease characterized by impairments in the
perception or
expression of reality and by significarit social or occupational dysfitnction.
Initial
investigations of the possible interaction between schizophrenia and the
immiine system.
began in the early 1900s and have proceeded in a rather halt'vng fashiori
because of the
methodological challenges faced by investigators. However, a-confluence of
recent data
suggests that activation of the inflammatory response system, the cellular
immune system,
and the humoral immune system may be present in some patients with -
schizophrenia. 7Some
of the inost compelling data support the hypothesis that minor levels of
iinmune activation
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
138
may be associated with acute psychotic exacerbations. However, a second body
of evidence
suggests that some individuals with schizophrenia may have chronic,'evolving
autoimmune
processes (Rapaport and Deirahim, 2001).
= Scleroderma - chronic disease oharacterized by excessive deposits of
collagen. =Progressive
systemic scleroderma can be fatal - the. local type of the disease. is not -
serious:
Aiitoantibodies, to centromeric proteins are commonly found in .sera of
limited scleroderma
and other rheumatic disease patients. Utilizing samples frorn 263 anti-
centromere
immunofluorescence positive patients, 93.5% were found to have anti-CENP-A
reactivity*
and 95.4% had anti-CENP-B reactivity by ELISA. Very few patierit samples
exclusively ~
targeted CENP-A (2.7%) or. CENP-B (4.2 Jo) (Akbarali, et al. 2006).
= Ulcerative colitis - an inflanunatory disease of the bowel that usually
affects the distal eind=
of the large intestine and rectum. . Complerrient activation observed in
relation to epithelium=
bound IgGl in ulcerative. colitis indicates that the surface epithelium is*
subjected to
immunological attack by an autoiminune reaction. These luminal deposits
regularly contain
terminal cytotoxic complement, and often also C3b as a sign of persistent
activation.
Comparison of ideintical twins, discordant with regard to ulcerative colitis,
suggests that the
markedly skewed local IgG 1 response 'seeii in this IBD entity may be
genetically determined
(Brandtzaeg, et al: 2006). One study shows that in these models, IL-23 is
esseiitial for
manifestation of chronic intestinal inflammation (Yen, et al. 2006).
= Vitiligo - spontaneous loss of pigment from areas'of skin = anti-melanocyte
antibodies have
been detected in some cases of vitiligo. Mannose binding lectin (MBL) is a
calciurri
dependent lectin that causes predisposition to infections and autoimmune
Aiseases. One =study
aimed to examine the presence of any association between MBL2,gene variants
and vitiligo.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
139
Results suggested that codon 54 polyniorphism in the MBL2 gene may play a role
in
susceptibility to vitiligo (Onay, et al. 2007).
Ratios
= oimnILine Disease
Hashimoto's disease/hypothyroiditis 50:1
Systemic lupus erythematosis 9:1
Sjogren's syndrome 9:1
Antiphospholipid syndrome = 9:1
Primary biliary cirrhosis . 9:1
Mixed connective tissue disease 8:1
Chronic active hepatitis 8:1
Grave's disease/hyperthyroiditis 7:1
Rheumatoid arthritis 4:1.
Scleroderma * . 3:1
Myasthenia gravis 2:1
Multiple sclerosis 2:1
Chronic idiopathic thrombocytopenic purpura = 2:1
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
140
Exemplary ImmunoScore Assays for Autoimmunity Panel
Panels to examine levels/exposure in individuals:
= Antibodies
= Cytokines
= Autoimmunity susceptibility genes
= Environmental factors leading to autoimmunity (obtained by
polling/surveillance)
In exemplary embodiments of the present invention, IxnmunoScoze assays for
autoimnaunity
can include dozens of screening tests run on individual patients from "cradle
to grave" Ideally,
individuals would 'first be screened at the time of entry to: the public
school =system to obtain
baseline measurements. Those individuals- with ImmunoScores of concern or
familial history of
autoimmune disease can be screened more frequently than other individuals as
they aged. At the
time of college entry/high school graduation, it would be more imperative to
screen everyone for an adult baseliine ImmunoScore for autoinunune disease
predictability. The.timing of the
screening can, for example, thereby precede development of most autoimmune
diseases in'
susceptible patients.
An exemplary autoimmune baseline.screening panel follows. -This screening can
be performed on the young adult population. Females, particularly with a
family histpry of
autoimmune disease should be screened more regularly than males. Preginant
females tould
routinely be screened during pregnancy to monitor'any possible onset of
autoimmune symptoms.:
Males could again be screened at the 50 year old checkup as a means of
gathering another
appropriate time-point to their personal IminunoScore record.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
141
In exemplary embodiments of the present invention, some or all of the
following assays can be
included in an ImrnunoScore Autoimmune Screening/Diagnostic Panel: 1. Antibody
Assays
= anti-myelin oligodendrocyte glycoprotein (MOG) antibody
= anti-measles virus antibodies
= anti-21-hydroxylase antibody
= anti-adrenal cortex antibody ,
= anti-Klebsiella antibodies
= anti-oardiolipin antibody
= anti-lupus anticoagulant antibody
= anti-beta-2-glycoprotein antibody
= anti-hematopoietic precursor cell antibodies
= anti-soluble liver antigen antibody
= anti-RO/SSA antibody
= anti-endomysial antibody (AEA)
= anti-tissue transglutaminase (anti-tTG)
= anti-Saccharomyces cerevisiae antibody (ASCA)
~ anti-neutrophil antibody (pANCA)
= anti-porin protein C of E. coli antibody (anti-OmpC)
= = anti-glutamic acid decarboxylase antibody (GADA).
o particularly anti-65 kDa isoform =
= anti-protein tyrosine phosphatase-like molecule antibody (IA-2A)
= anti-glomerular basement membrane (GBM) antibody
= anti-neutrophil cytoplasmic antigens (ANCA)
= anti-GDla/GDlb complex antibody
= anti-LMI antibody
= anti-GM1 antibody
= anti-thyroglobulin antibody
= anti-nuclear antibodies (ANA)
o lupus anticoagulant (LA) antibody
o anti-phospholipid (aPL)
o anti-SS/A antibody
o anti-SS/B antibody
o anti-Sm antibody
o anti-RNP antibody
o anti-Jol antibody.
o anti-Scl-70 antibody
o anti-dsDNA antibody.
o anti-Centromere B antibody
=o anti-Histone antibody .
= anti-alphalIbbeta 3.IgM
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
142
= 'anti-acetylcholine receptor (anti-AChR) antibody
= anti-muscle-specific tyrosine'kinase (MuSK) antibody
= anti-neuroleukin antibody
=. anti-gliadin antibody .
= anti-C V 2, antibody :
= anti-GQ 1 b.IgG
= anti-GQ 1 b IgM
= anti-thyroid peroxidase antibody
= keratinocyte cell-surface antibodies
o anti-BP 180 (bullous pemphigoid antigen 2)
o anti-BP 230 (bullous pemphigoid antigen 1)
= anti-intrinsic factor antibody
= anti-parietal cell antibodies
= anti-mitochondrial antibodies
o in particular, anti-E2 component of pyruvate dehydrogenase complex(PDC)
antibody
= anti-cyclic citrullinated peptide (CCP) antibody
= anti-heat shock protein (HSP) 65.antibody
= anti-HSP 90 aintibody
= anti=DnaJ antibody
~ anti-BiP antibody
= anti-heterogeneous nuclear RNP A2/B1 antibody
=. = anti-heterogeneous nuclear RNP D antibody
= anti-annexin V. antibody
= ariti-calpastatin antibody
= anti-type II collagen antibody
= ainti-glucose-6-phosphate (GPI) antibody
= anti-elongation factor -
= anti-human cartilage gp39 antibody
= anti-Chlamydia antibodies
= anti-La/SSB antibody
: anti-fodrine antibody
= anti-salivary duct antibodies
= anti-Red Blood Cell (RBC) IgM
= anti-neutrophil cytoplasm.ic antibodies
= anti-thyroid microsomal antibody (ATMA).
= anti-smooth muscle antibody (SMA)
= anti-mitochondrial antibody (AMA)=
= anti-extractable nuclear antigens (ENA) antibody
= anti-actin antibody (AAA)
= anti-hair follicle antibodies
o anti-anagen,matrix antibody
.o anti-cuticle antibody
o anti-cortex keratinocytes antibody
o anti-melanocyte nuclear antigen
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
143
= anti-human dermal microvascular endothelial 'cells (HDMEC) antibodies
o- anti-81 kDa HDMEC 'antigen, in particular
ypanosoma cruzi antibodies
= anti-Tr
= . anti-oleic acid IgM . .
= anti-paimitic acid IgM
= anti-myristic acid IgM
= anti-azelaic acid IgM
= anti-malondialdehyde IgM
= anti-aceylcholine IgM
~ anti-S-farnesyl-L-cysteine IgM
: anti-ganglioziic nicotinic acetylcholine receptor antibody.
= anti-follicle-stimulating hormone (FSH) IgA
= anti-V 14D 1gA
= anti-V 14D IgG
= anti-cytoskeleton-associated protein 4/p63 (CKA4/p63)-specific antibody
+ anti-cytokeratin 10 antibody
:
= anti-Voltage-Gated Potassium Channels (',V.GKCs).antibodies=
= anti-Chlamydia pneumoniae antibodies
= anti-human cytomegalovirus (CMV) antibodies '
=' anti-To.xoplasma gondii antibodies
= anti-CENP-A antibody
=.. anti-CENP-B antibody
2. Cytokine Assays
= Iriterleukin-1 a (IL-1a)
= . l~L-1(i
= IL-2
= IL-4
= IL-5
= IL=6
= IL-7
= IL-8
= = YL-10
= IL-12
= IL--13
= IL-15
= IL-18
= . Interferon a (IFN-a)*
. TFN_y
= TNF-a
= =G-CSF
= MCP-1
= MIP-1 a
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
144
= MIP-1 ~
= MIP-3 a
= MIP-3 ~
= EGF
. VEGF
= TNFRII
= EGFR
3: Toll-like Receptor (TLR) genetic variants
= TLR 2
= TLR 3
= TLR4'
=' TLR 7
= TLR 8
= TLR 9
4. HLA Haplotype screening
= HLA A24
= HLA B8
= HLA B 18
= HLAB27-
=- HLAB51
= HLA B60
= HLA B62
= HLA DR2 '
= HLA DR3
= HLA DR4
= 'HLA DR5
=. = HLA DR7
5. Protein isoforms/ genetic polymorphisms/ serum protein levels
= Apolipoprotein E isoforms
o apoE2
o apoE3
o apo E4
= Serum Apolipoprotein A-N level
= Mannose-binding lectin (MBL) polymorphism
= Serum Haptoglobin level
= SerumTransthyretin level
= ' Serum. Fibrinogen level
= Serum Vitamin B12 level
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
145
Serum Folic acid level . .
19. 10. ImmunoScore Diagnostic Panel: Aging, Longevity; Cancer and Human- -
Cytomegalovirus
Old age is accompanied by an increased incidence of infection and poorer
responses to
vaccination. A progressive decline in the integrity ofthe immune system is one
of the
physiologic changes during mammalian aging. Perhaps the most profound clinical
impact of age',
on the immune system concerns the response of the elderly to
vaccination.(Pawelec, 2005). An
immune risk phenotype (IRP) was described wherein individuals possessed high
CD8 and low
CD4 numbers and poor proliferative response (Wikby, et a1.-2005).
Characteristics of the IRP
are listed in Table I (Vasto, et al. 2007). The IRP consists of a cluster of
these parameters, not
each parameter individually.' Which are the most important and which
additional factors are
involved remains to be determined.
Lifelong and chronic antigenic load may represent the major driving force for
immunosenescence, which impacts on humazi lifespan by reducing the number of
virgin antigen-
non experienced T cells, and results in their replacement by expanded. clones
of antigen-
experienced effector and inemory T cells which display a late differentiation
phenotype.
Gradually, the T cell population shifts to a lower ratio. of naive cells to
memory cells, ihe thymus
releases fewer naYve T cells with age and those T cells remaining, especially
the CD8+ subset,
also show increased oligoclonality with age. Presumably, the repertoire of
cells available to
respond to antigenic challerige from previously encountered pathogens shrinks.
In addition,
older organisms often are overrun by memory.cells that carry a single type of
T cell'receptor, i.e.
the clonal expansion referred to.above. Therefore, the memory cells from old
individuals might recognize a limited set of antigens despite being plentiful
in number, and in addition, are likely to
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
146
show various -degrees of dysfunctionality. Mariy of the clonal expansions
filling the individual's
immune_system seem to result from previous infections by persistent viruses,
especially CiViV (Ouyang, et al. 2003b),:but also, to a lesser extent EBV
(Ouyang, et al. 2003a) and possibly other
herpes viruses (Vasto, et al. 2007). A high number of CD8} cells are found to
be specific for a
single CMV epitope (Pawelec, et al. 2005; Pawelec, et al. 2004). In humans,
the, accumulation of
CMV-specific T cells has been observed to reduce T cell immunity'toward EBV
infection (Khan,
et al. 2004) and influenza vaccination (Trzonkowski,'et al. 2003). Functional
analyses
peiformed with T cells from nonagenarians demonstrated that they were
characterized by
decreased functional capacity when compared with similar cells isolated from
middle aged
individuals (Hadrup, et al. 2006). = This suggests' that increased numbers of
CMV-specific T.cells
could be the result of a compensatory mechanism enablirig control of CMV
despite lower
functional capacity (Hadrup, et al. 2006). The biology of CMV infection in
humans cari be
conceptualized as.an evolutionary "negotiated" balance between viral
mechanisms of.
pathogenesis, persistence; and imrimune evasion aiid the host cellular immune
response
(Sylwester, et al. 2005).
One of the immunodominant viral antigens recognized by CMV-specif c CD8" T
cells is derived from the 65-kDa phosphoprotein (pp65). Samples from-
octogenarian and nonagenarian
populations revealed that a large number of CD8+CD28` cells were specific for
the pp65 antigen.
These. findings imply a co-dominant role of CMV as a cause for a compromised
immunity in old
age (Vasto, et al. 2007Y. A second immunodominant.antigen is the IE-1 antigen.
Epitope
specificity and immunodominance of CD8 T cells against IE-i and pp65 are
comparable in acute=
=infection and long-term memory often with. marked focusing of responses that
are probably
established very early on. However, the kinetics of CD8 T cell responses for
these antigens
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
147
expressed at opposite ends of the replicative cycle of the virus reflect the
different modes of
antigen presentation, which probably depend on levels of viral activity
occurring over the
lifetime of the host (Khan, et al. 2007). Other studies have suggested an
extraordiriary
complexity=of CMV-speeific T cell responses to chronic infection (Sylwester,
et al. 2005). This
complexity complicates efforts to understand the basis of the CMV -immune
balarice and, in
clinical practice, to deterniine the thresholds that define the boundary
between controlled vs:.
progressive CMV infection iri immunocompromised subjects and between normal
and excessive
CMV-specific immunity in the =elderly (Sylwester, et al. 2005).
There are a suggested sequence of stages for IRP individuals. that begin with
the acquisition of
CMV infection in earlier life, followed by generation of,CD8+CD28' cclis to
control persistent
CMV infection, and eventually the development of an IRP. Recently, a group of
rare individuals'
was discovered who moved out of the IRP category by a process of immu.ne
suppression,
including increases in IL-6 and IL-10 and decreases in the number of
CD3+CD$+CD28' cells
(Wikby, et al. 2006).
There are two theories regarding the evolution of senescence - mutation
accumulation and
antagonistic pleiotropy. The mutation accumulation theory of senescence
postulates that there
are numerous loci subject to mutation to deleterious*alleles, whose effects on
survival or other
components of fitness are restricted to narrow bands of ages (Rose, 1991).
'The equilibrium
frequencies of such'deleterious alleles will be higher the later in life ii
which they act
(Charlesworth, 1994). The alternative path involves antagonistic pleiotropy,
according to which '
genes that increase early performance are likely to become established in a
population even if
they have adverse effects on later performance (Williams, 1957; Rose, 1991):
Antagonistic,
nleiotronv was oriainallv defined as meaning onnosite effects of the same
allele at different ages
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
148
(Williams, 1957). Antagonistic pleiotropy in evolutionary theory iisually
refeis to opposite
effects of a geinotype on fecundity and survival. The existence of trade-offs
between these two.,
components of Aarwinian fitness was proposed to explain- the evolution of
senescence and the
maintenance, *ia the creation of the heterozygous advaiantage, of polymorphism
at loci involved
in the determination of bothAraits (Kirkwood and Rose, 1991). In a later
model, antagonistic
pleiotropy involved, instead, relative survival values of a genotype at
different ages' (Toupance,
et al. 1998). The two theories are not mutually exclusive, and modeling
exercises have examined.
the validity of each (Charlesworth and Hughes, 1996).
An example of antagonistic pleiotropy would be the high expression of
testosterone in a male
gorilla, which could lead to increased aggression and strength that would
allow the male to
become dominant and mate more frequently, but may eventually lead to a
shortened lifespan due,
to increased atherosclerosis. Recent studies at the molecular level have
suggested-that cellular
senescence may be antagonistically pleiotropic because it prevents
tumorigenesis; but also
contributes to organismic aging (Troen, 2003).
In one study, it was suggested that cellular. senescence was antagonistically
pleiotropic,
protecting from cancer early in life, bufipromoting carcinogeriesis in aged
organisms (Krtolica,-et
al. 2001). Another study (Hughes, et al. 2002) found the AP.(antagonistic
pleiotropy) model is
consistent with the existence of a few genes with individually large effects
on late-life iitness,
whereas the MA (mutation accutnulation) process should lead to the maintenance
of may
deleterious alleles at interrnediate frequencies within populations and these
alleles can have
individiually small effects on late-life performance and health: Current
methods of identifying
aging -genes (such as mutation studies and quantitative'trait locus-mapping
experiments) are most
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
:149
effective in finding alleles of large effect, and even well designed.studies
will probably miss.
genes with.small effects. Novel approaches are needed to find such .genes.
Cancer rates also increase sharply with age in both sexes, and the majority of
cases of canceir
occur in patients over the age of 65. Tumor progression is a complex process
that depends on
interactions between tumor cells and host cells. The inflammatory aspeet of
tlie liost response is
of particular inteiest beeause it includes the release of pro-infJ.ammatory-
cytokines, some of
which may promote tumor growth and hence influence survival.. Some kinds of
solid tumors are '
likely affected by regulatory cytokine genotypes. 'In particular, pro-
inflammatory genotypes
characterized by a low IL-10 or a high IL-6 producer seem to be associated
with a worse clinical
outcome (Caruso, et al. 2004). On the other hand, recent evidence has linked
IL-10 and IL-6
cytokine polymorphisms to longevity. In fact, individuals who are genetically
predisposed to
produce high levels of IL-6 have a reduced capacity to reach the extreme
limits of human life,
whereas the high IL-10 producer genotype is increased among centenarians
(Caruso, et al. 2004).
The -opposite effect of IL-6 and IL-10 in cancer and longevity is intriguing.
'Inflammatory
genotypes may be both friends and enemies. The immune system has evolved to
control
pathogens, therefore pro-inflammatory responses are likely to be
evolutionarily.programmed to
resist fatal infections, and a high IL-6 oT a low IL-10 production is
associated with increased
resistance to pathogens. However, decreased level of IL-6 or increased level
of IL-10 might
better.oontrol inflammatory responses. and cancer= development. These
c6nditions'rnight result in
an increased chance of long life, survival in an environment with reduced
pathogen loads
(Caruso, et al. 2004).
Most tumor supressor genes can be classified as either caretakers or
gatekeepers (Kinzler and
Voglestein, 1997). Caretaker tumor suppressor genesprevent=cancer by
protecting thegenorne
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
15W
from mutations. They generally act by preventing DNA damage or optimizing DNA
repair. In
addition to preventing cancer, genes that help maintain genomic integrity also
prevent or retard
the development of other aging, phenotypes and age-related pathologies (Hasty,
et al. 2003).
Gatekeeper tumor suppressors, by contrast, prevent cancer by acting on intact
'cells - specifically,
mitotic cells that are at risk for neoplastic transformation. Gatekeepers can
virtually, eliminate
potential cancer cells by inducing programmed cell death (apoptosis).
Alternatively, they can
prevent potential cancer cells from proliferating by inducing permanent
withdrawal from the cell
cycle (cellular senescence).. Although little is known about how cells choose
between apoptotic
and senescence responses, there is little doubt that both responses are
crucial for suppressing
cancer (Campisi, 2001;* Green and Evan, 2002).
Increasing evidence suggests that the rise in cancer with age results from a
synergy between the
accumulation of mutations and age-related, pro-oncogenic changes in the tissue
milieu. Most
age related cancers derive from epithelial cells. Epithelial tissues are
supported by a stroma,
which is composed-of extracellular matrix and several cell types. One age-
related change that
occurs in epithelial tissues is the accumulation of senescent cells. Cellular
senescence is a pot6nt
tumor suppressive mechariism that irreversibly arrests proliferation in
response to damage or*
stirnuli'that put cells at risk for neoplastic transformation. Senescent cells
secrete factors that can
disrupt tissue architecture and stimulate neighboring cells to proliferate.
The suggestion has
been made that senescent cells can create a tissue environment that synergizes
with oncogenic
mutations to promote the progression of age-related cancers (Krtolica and
Carnpisi, 2003).. The
recenfevidence indicates that cellular senescence may be an example of
evolutionary
antagonistic pleiotropy.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1511
A major difference between microbial pathogens and tumors as potential vaccine
targets is that
cancer cells are derived from the- host, arid most of their macromolecules
'are normal -self
antigens present in normal cells. To take advantage of the immune system's
specificity, antigens
must be found that clearly mark the cancer cells as different -from host -
cells. An area generating
much interest is the possibility of overcorizing mechanisms that downregulate
or, attenuate the
imrnune response, as is depicted in Fig. SD (Berzofsky, et al. 2004b). With
refemce thereto, Fig.-
5D illustrates negative regulation of tumor immunosurveillance'and antitumor
immune.
responses. Fig. 5D(A) depicts CD4CD25!`T regulatory cells, induced by peptide
presented by
class II MHC molecules in the presence of IL-2, may inhibit induction of
effector-CD4* or CD8+
T cells by.a contact=dependent mechanism, possibly involving cell surface
and/or secreted-TGF- (3, .and Fig. 5D(B) illustrates- how CD4+ NKT cells may
be -iriduced by tumor glycolipid
presented by CDId to secrete IL-13, which stimulates Gr-1+CD11- b+myeloid
cells to produce
TGF-A, which inhibits induction of CD8+'CTLs mediating tumor
irnmunosurveillance. TGF-0
may also inhibit CD4+ T cells (not shown). Blockade of other mechanisms can
improve
imrnunosurveillance and the response to vacciries: Other suppressor or
negative:regulatory cells
have been describecl in other contexts, but not as well study in the eontext
of cancer (Berzofsky,
, et al.). , Such mechanisms may have evolved to, reduce inflanimation and
immunopathology or to
prevent autoinnmunity. Tumors have co-opted these mechanisms to evade
immunosurveillance.
Thus, it has been postulated that the excess of dysfunctional CD8 T cells is
indirectly
immunosuppresive by' filling the "immunolo.gic space" and shrinking'the T-cell
irepertoire for
new antigens, as well as directly suppresive via cytokine secretion. It is
associated with the IRP
predioting two and four year mortality in longitudinal studies of very old
people. It is
'hypothesized that deletion of such accumulations. of dysfunctional cells
would be beneficial'to
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1522
the individual. It may be possible to distinguish functional CMV-specific
cells (which are
essential to maintain immunosurveillance) from dysfunctioinal= ones by their
expression of certain =
surface molecules. This, coupled with methods directed at reinvigorating the
thymus (siuch as,
for example, the use of interleukin 7), and targeting CMV by pharmacologic and
'
immunotherapeutic interventions might result in the immunorejuvenation
sufficient to take
elderly individuals out of the risls category and thereby extend healthy
longevity (Pawelec, et.al.
2006).. Animal models suggest that IL-7 improves immune reconstitution through
increasing
thymic output and, perhaps more importantly, through antigen-independent
homeostatic 'driven
proliferation in the periphery (Sasson, et al. 2006). A study in old Rhesus
rn.acaques showed that
treatment of the elderly with IL-7 may provide an effective therapy to
iniprove the immune
system (Aspinall, et al. 2007).
In rural Gambians, the season of birth strongly predicts adult mortality.
Those born during the
-harvest season have longer life spans than do those born during the hungry
season, and the
deaths associated with infectious diseases suggest permanent early-life
influences on immunity
(Ngom, et al. 2004). One group studied thymic size and output in Gambian
infants born in-either
the hungry or the harvest season by measuring signal-joint T cell receptor-
rearrangement circles
(sjTRECs) at birth and at 8 weeks of age. They found that by 8 weeks of age,
those born in the=
hungry season had significantly lower sjTREC counts (indicating poor immune
fun.ction)'than=
did those born in the harvest season. These results correlated directly with
lower ELISA
measurements of IL-7 in mothers' breast milk (Ngom, et al. .2004). This
researcli group
speculated that these data show a plausible pathway linking external -season
insults to mothers
with thymic development in their infants, which suggests possible implications
for long term
programming of immunity.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
153
ImmunoScore Measurements and Applications. Thus, there is a balance between
viral
mechanisms of pathogenesis, persistence, and inunune evasion and the host
cellular iminune ,
response. The immunologic basis of this balance has not been'completely
characterized. The
nature aind threshold of CMV=specific T cell responses required for long-term
CMV containment
yet remain to be defined. This information would facilitate identification of
highly susceptible
individuals and provide a specific target for immunotherapeutic approaches
designed to
establish, maintain, or restore immunologic protection (Sylwester, et al.
2005).. There seem to be
clinical consequences to an overly robust CMV-specific T cell response. An
obvious
prerequisite for a better understanding of what constitutes insufficient or
excessive CMV-
specific T cell immunity is the ability to evaluate the overall CMV-specific T-
ceil response in,
infected individuals. Future longitudinal studies would benefit frorn
combining data on viral
reactivation and primary infection with immunological monitoring ~Hadrup, et
al. 2006): The
ImmunoScore diagnostic and database systems would provide just such ari
opportunity for data.
collection and monitoring longitudinal data collection. -
Although CMV seropositivity appears to be one of the driving forces for
induction of CDg T cell
clonality, this is not currently detectable in the middle-age population
(Hadrup, et al. 2006). The
influence of CMV on clonality only becomes relevant at a detectable level in
the elderly.
Superior detection capabilities available through the ImmunoScore technology
might lead to
earlier detection of possible immune depletion as individuals pass through
middle age.
ImmunoScore technology by its riature :of compiling individual patient data
would offer the
opportunity for longitudinal design of research studies. The longitudinal
design is a superior
alternative to the cross-sectional inethod for conducting ageing iesearch, but-
it has seldom been
used due to extensive costs as studies are currently conducted. The
ImmunoScore svstem would
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1154
naturally build a longitudinal component into patient care at no increased
initial cost. The
database would yield important insights into ageing and all its implications
at a lower= cost and
dramatically improve healthcare.
Qtiestions have been raised concerning CMV infection and its relationship to
the IRP (Vasto, et
al. 2007). Uncertainties that require clarification are:' Is=there an
immunogenetic component
influencing the IRP phenotype that might explain the different degree of CMV
clonal. expansion
vs. non-IRP phenotype? May this difference depend on social and/or
environmental.factors?
Might the genetic or environmental component affect the degree of clonal
expansion of CMV in
IRP individuals? What can be. the main cause of death in IRP? Can IRP
selection be predictive
in young as well as in old 'individuals? Is it possible to revert/prevent
accumulation of CMV- '
specific ce11s?
These are all questions that can, in exemplary embodiments, of the present
invention, be
addressed by the application of ImmunoScore'diaginostic and database
technologies.
Irnmunogenetic components can, for exainple, be monitored using unique
technology designed
to investigate single nucieotide polyinorphisms (SNPs) rapidly and those data
could be stored in
the IIrnmunoScore central database. Additionally, social and environmental
factors can be part of
the ImmunoScore demographic data collected at routine patien't visits to their
physicians. The
accumulation of these data on the=ImmunoScore database. would yield potential
relationships
regarding environmeiital and social factors to the IRP.
Careful monitoring of the ImmunoScore database would shed more light onto
environmental :
and/or genetic factors contributing to the clonal expansion of CIvfV T cells
in IRP individuals
and the non-IRP individuals.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
155
As the ImmunoScore data collection system is envisaged as a cradle=to-grave
system of
healthcare, the cause of death in IRP individuals can be collected and
collated. Preliminary
indications are that IRP selection is likely to be predictive in the young as
well as in the very
elderly. The InununoScore cradle-to-grave philosophy of patient data tracking
can be invaluable
in assessing these issues. Additionaly, prevention/reversion of the
accumulation of CMV-
specific T cells would seem an issue of paramount importance. Preliminary
studies in'an'imal.
models regarding judicious use of IL-7 have been promising. 'ImmunoScore can,
for example,
track treatments and even shed light on when such treatments should commence
in the life of the.
afflicted individuals.
CMV Vaccine and Vaccines Against Chronic Viral Infections and Cancer. In a
recent
review of priorities for vaccine development, CMV was ranked in the highest of
five tiers by the
Institute of Medicine in the United States as a potentially cost-saving
vaccine tar=get (Stratton, et
al. 2000). In general, CNiV is acquired earlier in life in developing
countries and among the
lower socioeconomic strata of the developed countries (Stagno and Cloud, 1-
990). Recently, the
seroepidemiology of CMV was examined in Australia (Seale, et al. 2006).. The
pattern of age-
specific seroprevalence of CMV antibody, as provided in'Fig. 5C, closely
matched the pattern
found from analysis of the exemplary CIP database described in Section II,
below. Indeed, a
review of CMV seroprevalence studies conducted around the world revealed that
residents of
developing countries have higher rates of CMV seropositivity than those of
developed countries
(Enright and Prober, 2004). The potential benefits of a CMV vaccine would
incltzde reduced
transmision to pregnant women and less CMV.disease due to primary infection or
reactivation in.
=organ transplant recipients and the immunosuppressed (Griffiths, et al.
2000).
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
156
ItIs possible that the development of a vaccine that is effective against
viruses that cause chronic
infection may require consideration of a paradigm different than those
previously used for
organisms causing acute infection (Berzofsky et al. 2004). In most ca'ses of
chronic viral
irifection,'the immune response to the natural infection is not sufficient to
eradicate that
infection. The. challenge for the 215t century is to apply the latest
fundamental knowledge in
molecular biology, virology, and immunology to developing vaccines that are
more effective at
eliciting immunity than the natural infections and consequently, effective
against chronic viral
and other infectious diseases in addition to. cancer, which do not fit the
classic paradigm.
ImmurioScore diagnostic and database tracking would be invaluable in analyzing
the.efficacy of
a CMV vaccine, as well as vaccines developed against HN, hepatitis C virus-
(HCV:), human
papilloma virus (HPV) and Epstein-Barr virus (EBV), among others.
As prophylaxis against acute infectious diseases, vaccines have been among the
most cost-
effective agents, saving many millions of lives. However, for treatinent of
chronic infections and
cancer, vaccines have yet to. achieve, widespread success. Increased
understanding of the
immune system has raised riew hope of harnessing the exquisite specificity of
the immune
system to attack cancer (Berzofsky, et al. 2004b). In exemplary embodiments of
the present '
inventiori exemplary IrnmunoScore diagnostic panels and database systems can
add corisiderably
to this knowledge base andcan, for example, assist in intelligent'vaccine
design and monitoring,
of the efficacy of the vaccines as they are developed. =
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
157
o
CD4:CD8 ratio < 1 ,
Poor T cell proliferative responses to mitogens
Increased CD8+CD28- and CD8+CD57i celis
Low B cell count
CMV seropositivity
Clonal expansion of CD8 cells carrying receptors for CMV
High proportion of dysfunctional cells amongst-the CMV-speeific CD8 cells C.
EXEMPLARY IMMUNOSCORE SUPERPANELS
1. Middle Schooi Student ImmunoPrint Super Diagnostic Panel
In exemplary erribodiments of the present inveintion, a middle school
superpanelcan, for
example, comprise the following exemplary panels:
1.1 'Persistent7mmunity lnduced by Childhood Vaccines
This panel is described above in section.A3:
1.2 Sexually.Transmitted Disease (STD) Diagnostic Panel
For children entering middle school (grades six through eight) a baseline
determination for
antibody levels to STDs is advisable. Recommended tests for ImmunoPrint
measurement of
immunity to STDs:
= Antibodies to Chiamydia - IgG, IgA, and IgM (3)
= Antibodies to HSV - IgG. to HSV-1 and HSV-2 (2)
= DNA analyses of HPV types - particular emphasis on high-risk
= Antibody to N.. gonorrhoeae = (1)
= Antibody to T. pallidum (1)
= T-cell related response to T. pallidum
= Antibody to HIV
= T-cell related response to HIV
=' Antibodies to GBS serotypes (at least 3)
==Measurement of Thl/Th2=cytokines (niany as current evolving definitions)
= Antibodies to organisms that cause,Urinary Tract Infection{UTIs)=
o Escherichia coli
o Staphylococcus saprophyticus
o Proteus tnirahili.c
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1158
o Klebsiella pneumoniae
o Enterococcus species
o Pseudomonas aeruginosa
Currently, there are no vaccines available for any of these STDs, with the
exception of the Merck
HPV vaccine. ' Until this situation is ameliorated as to a particular vaccine
preventable disease,
an ImmunoScore STD diagnostic panel would thus be to recommend treatments,
track
immunological response or provide other analyses, and not be used to recommend
a vaccine or
track the persistence of immunity conferred by it. Thus, in exemplary
embodiments of the
present invention an exemplary ImmunoScore database can, for example, generate
correlates of
protection information for all disease-causing organisms. As vaccines are
developed,
ImmunoScore diagnoses could, for example, be designed to examirie antibody and
other related
immune responses to vaccine components.
= Chlamydia trachomatis infection is the most commonly reported sexually
transmitted
disease in the United States, with the highest rates among adolescent females
and young
women. Because up to 70% of chlamydiai infections in women are asymptomatic,.
routine screenung and treatment of infected persons is essential to prevent
pelvic
inflammatory disease, infertility, ectopic pregnancy, and perinatal
infections. The third
U.S. Preventive Services Task Force (USPSTF) recommends that primary care
physicians routinely screen all women whether or not they are.pregnant if
they:
o Are sexually active and aged 25 or younger.
o Have more than one sexual partner, regardless of age.
o Have had an STD in the past, regardle=ss of age.
o Do not use condoms consistently and correctly, regardless of age.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
159
According to studies reviewed by the third USPSTF:
o The cost of screening women who are not pregnant and who are at risk for
chlamydial infection may be less than the cost of treating Chlamydia and its
-complications.
o Screening patients at greatest risk is more cost effective than screening
all.
patients.
o DNA or RNA amplification tests are more sensitive than culture.
A low cost diagnostic test for Chlamydia infection or immune response to a
Chlamydia
vaccine would be a welcome addition to immune. status determination by -
ImmunoPrint
diagnostic testing.
= Herpes simplex virus type 2 (HSV-2) is the primary cause of genital herpes,
a common
sexually transmitted disease with at least 40 to 60 million infected
individuals in the U.' S. Medically serious complication's of HSV are rare but
constitute a significant burden,
given the high rates of HSV seropositivity in the popula.tion. Many
prophylactic and
therapeutic vaccination approaches have been explored for the prevention or
treatnient of
HSV infection. Infection induces both humoral and T-cell immunity. Vaccine.
candidates for HSV-2 infection include subunit vaccines, killed and live
attenuated virus
vaccines, and viral DNA vaccines.
=. Human papillomaviruses (HPV) are small double-stranded DNA viruses that are
responsible for pathological conditions ranging from benign skin warts to
invasive
cervical carcinomas. Cervical cancer is the second leading cause of cancer
death among
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
160
women.worldwide, and more than 99% of cervical cancers contain HPV,
particularly the
high-risk HRP type,16 (HPV-16). Two HPV oncoproteins, E6 arid E7, are
consistently'
expressed in HPV-associated cancer cells and are responsible for their
malignant
transformation. These'oncogenic proteins represent ideal target antigens for
developing
vaccines and immunotherapeutic strategies against HPV-associated neoplasms.
More
than 10,000 American women a year are diagnosed with cancer or precancerous
cells
caused by HPV, and 3,700 of them will. die. Eighty times that number will die
worldwide. An effective vaccine could prevent nearly all of those deaths. The
CDC is
currently considering an HPV vaccine for all children aged 12 years. A
positive
recommendation by the ACIP could start states thinking of requiring.the
vaccine for entry
into rniddle school.
= Neisseria gonorrhoeae, the causative agent or gonorrhea, is one of the most
common
sexually transmitted pathogens worldwide. Although a robust inflamrnatory
response
ensues during symptomatic infection, no apparent protective immunity
is'.developed
following infeation, as shown in a male human challenge.study and by the -high
incidence
of recidivism among patients attending sexually transmitted disease clinics.
The search
for a vaccine against gonorrhea has been largely disappointing. In human
vaccine trials,
partially lysed gonococci, purified pilin, and purified porin were shown to be
immunogenic, but all failed to elicit protection upon subsequent natural
exposure. The
lack of protective immunity is likely due, in part, to the capacity of many
gonococcal
surface antigens to undergo high-frequency phase and antigenic variation.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
161
= Individuals infected with Treponema pallidum subsp. pallidum develop
specific immune
respon'ses that are able to clear millions of treponemes from sites of primary
and
secondary syphilis. Despite the fact that humans develop robust immune
responses
against 77 pallidum, they can be infected multiple times. The'response is a T-
cell
mediated delayed-type hypersensitivity response in which T cells infiltrate
syphilitic
lesions and activate macrophages to phagocytose antibody-opsonized
treporiemes. How
treponemes from heterologous isolates can evade the recall response of a
previously
infected individual is unknown. Data from animal studies suggest that both
antibodies,
and T cells play a role in protection but neither'alone prevents infection. It
is possible
that antigenic diversity of T. pallidum 'accounts for the= lack of
heterologous .protection.
The T. pallidum repeat protein K(TpiK) is a strong candidate for a treponemal
factor
involved in immune evasion: Epitope mapping studies revealed that, during
experimental
infection, T cells are directed to the conserved regions of TprK, while the
antibodies are
directed to the variable regions:
...A safe, effective prophylactic human immunodeficiency virus (HIV) vaccine
is urgently
needed to curb the current AIDS epidemic. There are currently 40 million
individuals in
the world infected with HIV, and nearly 16,000 new infections occur worldwide-
each
day. Effective HIV-1 vaccines must be-capable of protecting immunized
individuals
from infection with a broad array of diverse viral variants. Attempts to
develop a safe
and effective AIDS vaccine have been slowed, in part, by the difficulty in
clearly
defining specific immune responses that can prevent infection and- limit
disease=
progression. This is in part due to the poor immunogenicity of the envelope
glycoprotein,,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
162
the tremendous variability of the virus, its ability to evade and impair the
host's immune
systerri, and its ability to persist by integrating into the host's immune
system, and its
ability to persist by -integrating into the host's genome of -a number of
different -cell types.
It is generally believed that an effective HIV-1 vaccine must be capable of
inducing
neutralizing antibodies as well as strong cell-mediated immune responses in
outbred
populations.
= Group B Streptococci.(GBS) emerged dramatically in the 1970s as the leading
cause of
neonatal infection and as an important cause of maternal uterine infection.
The burden
from GBS disease in elderly persons has-also increased. In 1996, the fnst
national '
consensus guidelines were released. Since then, there has beeri a 70%
reduction in early-
onset neonatal GBS infectioYi. In 2002, new national guidelines were released
reconunending:
o solely a screen-based prevention strategy
o a new algorithm for patients with penicillin allergy
o more specific practices in certain clinical scenarios
Yet clinical issues remain, including implementation of new diagnostic
techniques,
management of preterm rupture of membranes, use of alternative antib'iotic
approaches,
improvement of compliance, prevention of low birth weight infants, emergence
of
resistant organisms, and vaccine iievelopment.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1631
= Urinary tract infections (UTIs) are a leading cause of morbidity, and
mortality and health
care expenditures in persons of all ages. Sexually active young women are
disproportionately affected, but several. other populations, including elderly
persons and
those undergoing genitourinary instrumentation and catheteriza.tion, are also
at risk.
UTIs are the leading cause of gram-negative bacteremia (Orenstein and Wbng,
1999).
= Lymphocytes are the effector cells of acquired immunity. There are two T
helper
subsets, Th1 and Th2, based on two distinct cytokine profiles that resulted in
the overall
regulation of the immune response. The Thi cell (with its associated
cytokines:lNF-y,
TNF-a, IL-2, IL-12) is biased towards the cell-mediated side of immuility,
effective
against intracellular parasites, and its down regulation of Th2 can provide
relief from
allergic reactions due to IgE; but detrimental effects may result in
autoimmunity and graft
rejection. On the other hand, the Th2 cell (with its associated cytokines IL-
4, IL-5, IL-6,
IL-10, IL-13) favors humoral immunity, providing an effective correlate of
protection for
most vaccines, and its down regulation of Thl can result in some benefit of
tolerance to
prevent cellular autoimmune reactions; but certain harmful characteristics
i=elated to IgE-
based allergies and autoimmunity may result. In order to diagnose or predict
an
inununologic disease and/or provide therapyor prophylaxis, the Th polarization
status
must be determined; this should also be applied to measure susceptibility to
infectious
and neoplastic diseases. Th status is measurable in terms of cytokine
profiles,
chemokine/chemoattractant receptors, specific effector cell products, or=gene
expression
profiles. An exemplary diagnostic panel is described in the table below:
Th1 Th2
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
164
Cytokines Receptors Cytokines Receptors
!NF-y- CCRS IL-4 CCR3
TNF-a CXCR3 IL-5 CCR4
IL-2 CCRI IL-6 CCR8
'IL-12 IL-10 CRTh2 '
IL-13
2. .Exemplary lmmunoScore Diagnostic Panels for Women of Cliild-Bearirig
Years
Adult immuniza.tion rates have fallen short of natianal goals partly because
of misconceptions
about the safety and benefits of current vaccines. The danger of
misconceptions, is magnified
during pregnancy when concerned physicians are hesitant to administer vaccines
and patients are
reluctant to r.eceive them. Routine vaccines that are generally safe to
administer during
pregnancy include diphtheria, tetanus, influenza, and hepatitis B. Other
vaccines, such as
meningococcal and rabies, may be considered. Vaccines that are
contraindicated, 'because of the
theoretical risk of fetal transmission, include measles, rriumps and rubella;
vari oella; and BCG.
A number of other-vaccines have not yet been adequately studied; therefore;
theoretic risks of
vacciriation must be weighed against the risks of disease to mother and fetus.
The administration of vaccines during pregnancy poses a number of concerns to
physicians and
patients about the risk of transmitting a virus to a developing fetus. This
risk is primarily
theoretical. No evidence exists of risk from vaccinating pregnant women
with'inactivated virus
or bacterial vaccines or toxo.ids (CDC, 2002). Physicians should consider
vaccinating pre.gnant*
women. on the basis of the risks of vaccination versus the benefits of
ptotection in each particular'
-sitaation, regardless of-whether live or inactivated vaccines are used (Sur,
et al. 2003).
Generally, live-virus vaccines are contraindicated for pregnant women because
of the theoretical
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
165
risk of transmission of vaccine virus to the fetus. The following table
summari;aes
recommendations for vaccines commonly administered and their indication for
use during
pregnancy.
Table'11: Immunizations During Pregnancy
Considered safe ' Contraindicated Special
if . during recomm
otherwise endation
indicated pregnancy or s pertain:
safety
not established
Tetanus and BCG*. Anthrax
diphtheria
toxoids
(Td)
Hepatitis B Measles* . Hepatitis A
Influenza Mumps* Japanese
encepha
litis
Meningococcal Rubella*. Pneumococcal '
Rabies Varicella* Polio (IPV)
Typhoid
Vaccinia*
Yellow fever*
Live, attenuated vaccine
Women in their second and third trimesters*of pregnancy have an increased risk
of influenza-
related complications including pneumonia and a four-fold risk of
hospitalization (Neuzil, et al.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
166
1998). The CDC has recommended that women who will be in the second or third
triniester
during influenza season and all pregnant women with additional high-risk
medical conditions
should receive vaccination in the fall. Despite publication of these
guidelines, rates of
vaccination among high-risk patients remain low (Silverman and Greif,
2001;'Schrag, et al..
2003). Many possible explanations exist for this discrepancy, including
vaccine unavailability,
logistical concerns, poor reimbursement, fear of side effects, and lack of
adequate patient or.=
physician education (Wallis, et al. 2004).
A number of maternal conditions were perceived as potential contraindications
to=influenza =
vaccination during pregnancy. The most common of these were the first
trimester, history of=
preterm labor, history of intrauterine fetal demise,.and pregnancy induced
hypertension; none of.
these are listed by the CDC as contraindications (Wallis, et al. 2004).
According to= this group, -
another potentially significant obstacle to influenza vaccination during
pregnancy was physician
reinibursement. Several responders remarked that reimbursement from insurance
companies
played a part in whether they stocked the vaccine in their offices and whether
it was administered
to pregnant patients. Although they acknowledged the indications for the
vaccine, some
obstetricians stated that insurance plans have refused reimbursement for
vaccination because
they were not the patient's primary care provider for this "preventive"
service. Although
patients may still be instructed to obtain vaccination elsewhere, this
additional obstacle to
recommended obstetrical care may result in lower immunization rates. These
authors concluded
by stating that further research is needed to determine effective methods of
increasing
vaccination rates in this high-risk population.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
167
Cytomegalovirus (CMV) is found universally throughout all geographic
loc.ations and
socioeconomic groups, and infects between 50-80 fo.of adults-in the United
States by 40 years of
age. CMV is also the virus most frequently transmitted to a developing child
before birth. The
incidence of primary CMV infection in pregnant women in the U.S. varies froin
1-3 fo. Healthy
pregnant women are not at special risk for disease from CMV infection. When
infected with
CMV, most women have no symptoms and very few have a disease resembling
mononucleosis.
It is.their unborn babies that may be at risk for.congenital CMV disease. CMV-
remains the most
important cause of congeriital viral infection in the U.S. For infants who are
infected by their'
mothers before birth, two potential problems exist:
1. Generalized infection may accur in the infant, and symptoms may range from
moderate enlargement of the liver and, spleen (with jaundice) to fatal
illness. With
supportive treatment most infants with CMV disease usually survive. However;
from 80-90%.will have complications within the first few- years of life that
may
include hearing loss, vision impairment,- and varying degrees of inental ', .
retardation. 2. Another 5-10% of infants who are infected but without symptoms
at birth will
subsequently have varying *degrees of hearing and mental or coordination
problems.
However, these risks appear to be almost exclusively associated with women who
previously
have not been infected with CMV and who are'having their first infection
during pregnancy.
There appears to be little risk of CMV-related complications for women who
have been infected
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
168
at least six months prior to conception. The current recommendations from the
CDC for
pregnant women with regard to CMV infection are:
1. Throughout the pregnancy, practice good personal hygiene, especially
hand washing with soap and water, after contact with diapers or oral
secretions (particularly with a child who is in day care):
2. Women who develop a mononucleosis-like illness during pregnancy
should be evaluated for CMV infection and' counseled about the possible
risks to the unborn child.
3. Laboratory testing for antibody to. CMV can be performed to determine if
a woman already had a CMV irifection:
4. Recovery of CMV from the cervix or =urine of women at or before the
time of delivery does not warrant a cesarean section.
-5. The demonstrated benefits of breast-feeding outweigh the minimal risk of =
acquiring CMV infection from the breast-feeding mother.
6. There is no need to either screen for CNIV or exclude CMV-excreting
children from schools or institutions because the virus is frequently found
in many healthy children and adults.
Recently,. it was found that hyperimmune globulin therapy in pregnant women
was associated
with a significantly lower risk of congenital CMV disease (Nigro, et al.
2005). This group
concluded that treatrnent of pregnant women with CMV-specific hyperimrnune
globulin is sage,
and their findings suggested that it may be effective in the treatment and
prevention of congenital
CMV infection.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
169
Specific ImmunoScore diagnostic panel recommendations must take into account
the woman of
child-bearing years status with regard to pregnancy. Ideally, an ImmunoScore
screening of a
young women prior to child-bearing years would give an appropriate "baseline"
reading of that
individual. In this instance, for example, a positive serologic test for CIv1V
would be an '
indication that C1VIV-like illness during pregnancy would not.be a cause of
concern regarding
transmission to. that mother's infant during a pregnancy later in that woman's
life.
Clearly, women of child-bearing years that are not pregnant, or not planning
to get pregnant in
the six months following ImmunoScore screening would have different
reconvnendations than
pregnant women. An ideal location and time for ImmunoScore diagnostic
screening women of
child-bearing years vaould be during their annual recommended visit to the
OB/GYN. An early
baseline could be achieved for each patient and the Specialist could make use
of the specific
recommendations without confusion as to which immunizations would be
appropriate. It is very
important to assure immunity to the components of the measles-mumps-rubella
vaccine prior to
pregnancy and the ImmunoScore service would enable that assurance.
Accordingly, in exemplary embodiments of the present invention a Women=of
Child-Bearing
Years ImmunoScore superpanel can be defined as follows.
2.1 Recommended tests for ImmunoScore Measurement of -Immunity:
= Antibody to Cytomegalovirus (1)
o History of CMV infection needs to be captured to complete ImmunoScore
database and add relevance to pregnancy..
= Pregnancy test (1)
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
170
A pregnancy test is critical to making the correct decisions regarding
administration of vaccines
to women of this age -group. There are, of course, other considerations here,
but the status of the
woman in question regarding pregnancy must be resolved in order to make
accurate therapeutic
decisions. In addition to CMV antibody, the physician(s) of women of child
bearing years need
to be aware of the recommendations of the -CDC regarding immunizing pregnant
women and the
risks of immunization vs. the risks of foregoing immunizations. In-addition,
physicians should
be aware that following appropriate inununization protocols and assuring a
competent immune
status is extremely iinportant for women of child-bearing years.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
171
-2:2 Persistent Immunity Snduced by Childhood Vaccines Ibiagnostic Panel
Described above:
2.3 Sexually Transmitted Disease-(STD) Diaqnostic Panel
Described above.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
172;
SECTION [l EXEMPLARY IMMUNOSCORE. SYSTEM DATABASES
.GENERAL OVERVIEW*
In exemplary embodiments of the present invention the results of immunologic
and other assays '
of an individual together with additional medical, lifestyle, environmenta.l
and other
demographic- information can be collected at the same time as, or dexived
from, the collected
data, and can, for example, be stored in a system database. Such a database
can,. for example,
serve as an electronic record of immune status and other data over a period-of
time, both for
individuals as well as for populations or sub-populations, as described below.
Additionally, for
example, such a database can be augmented with information regarding diagnoses
received,
treatments administered, pharmaceuticals prescribed, costs of medical services
perforned,
insurance re-imbursements, metrics as to the efficacy of treat.inents and/or
pharmaceuticals
administered, as well other relevant information to facilitate evaluation of
the efficacy and
efficiency of medical services rendered, as described more fully below.
Thus, for example, for each run of an exemplary ImmunoScore assay within an
exemplary
system, various categories of data can be collected. Data can, for example, be
stored in an
electronic database using standard techniques as are known in the art. An
example of data which
can be stored and the manner in which it can be stored is next described. It
'is understood that this
example is riot intended to preclude the, storage of additional collected or
derived data as'may
prove usefial for the purposes of trending, data mining, evaluation or
diagnostic improvement, as
described below, or as may be needed in or useful to any of the exemplary
applications described
in Section III below.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
173
For each assay an exemplary system can record a.unique assay ID, which can
incorporate, for
example, among other information, an identifier for the assay.instrument: This
ID can=be unique
over the universe of instruments, ensuring that when data is aggregated into a
central system no
two assayresult records will liave-the same identifier. A possible
implementation of this
functionality is given, for example, by Microsoft's use of the GUID (Globally
Unique 1dentif er),
a 16 byte identifier generated by a computer and guaranteed to be unique
across all computers.
Each record can include the time and date that the assay was performed, stored
to a time
resolution of, for example, one second.' As is known, there are a variety of
standard means of
storing time and date information in a database: One simple means is, for
example, to record the
number of seconds from an'arbitrary start time, such as; for example, January,
1", 1900 at
midnight.
Each record can, for example, also include an indication ofthe. location
wliere.the sample. was. prooessed. This cari include, for example, an
identifier.of the instrument used, as well.as real-
world location information, such as, for'example, -the name and address of
the, facility where the
instrument has been.installed.
,.The aforementioned exemplary fields comprise' identification information
which is important to
maintain for all samples. In addition, information about the sannple and
patient can be.stored'in
the database as well. Patient information can, for example, be stored in a
form which is separate
from the bulk of the data, and referenced by a data link. Patient.
information, which caian include,
for example, name, social security number, birth date or other information
(such as is described
below in detail), can be maintained with emphasis on security standards
are.known in the art.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
174
The storage of identifiable individual patient information, in a separate
virtual location from the
remaining data can help to maintain such a high level of security.
In exemplary embodiments of the present inventiori, :a system can, for
each.assay result, alao
stoie an identifier indicating exactly which assay was performed on.the
sample.' This can
indicate not only the analyte to be determined, but also information regarding
the production of
the reagents used in the assay. This information can be used to distinguish.
between, and,
compensate for, for example, lot-to-lot variations in assay manufacture. It
can also allow for.
converting different assays for the same analyte into a normalized value, so
that trends across
geography as well as time can be obtained.
The measurement of an immune response to a particular disease or other analyte
can involve the
collection of a large quantity of low level data generated by an instrumerit.
For an ECL instrument, for example, an instrument can measure the light
emitted from~ the.
eiectrochemiluminescence over some time period as well as other information
such as voltages
and currents used to induce. the electrochemiluminescence and the temperature
near the
electrodes through which the electrical energy is delivered to drive the
electrochemiluminescent
reaction. From this "raw data" and possibly instrument calibration
information, a single number,
for example, can be computed to represeint an ECL signal for that measurement.
Additional
information can be computed from the raw data and instrument calibration
information that
indicates the quality of the ECL signal, for example, whether the instrument
was operating in an
appropriate environmental condition, whether sample was present, or
whethei'the instrument was
operating as expected. The raw data and such' derived data can, for example;
be stored in an
exemplary ImmunoScore system 'database. In general the size of the storage
"required for this
raw data can varv denendina uvon the resolution at which the data is captured.
It is possible that
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1175
a"finer-grained resolution, resulting in a larger data storage requirement,
will yield more useful
analysis for some assays rather than others. Storage of both the raw data and
the derived values
can be done, for example; using industry-standard methods for the persistence
of floatirig point
numbers. For example, four (4) bytes of storage, yielding approximately six
(6) significant
digits, can be used for each stored value.
The quantity of greatest interest in an assay is the concentration of the
analyte under evaluation.
This concentration can be determined by bonverting a-computed ECL signal to a
concentration.
This conversion can be done, for example, by backfitting the ECL signal
through a calibration
curve that relates ECL signal to analyte concentration. In general, sucli a
calibration curve can
vary from assay to assay, and can change over time for a given assay as that
assay is refined.
Calibration curves enable both interpolation and extrapolation of ECL signal
measurements for'
samples with known analyte concentrations for ECL signal measurements of
samples of
unknown amounts of analyte. The form of the mathematical functions used in a
curve fit can, for
example, make assumptions regarding the continuity and/or smoothness of the
underlying
relation such as through interpolating the measurements with functions such as
piecewise
constant, piecewise linear, cubic spline, or- for example, by throughfitting
all the data with linear,
quadratic, cubic, or quartic polynomials. For overconstrained systems,
parameters can be
computed by minixnizing an error function such as, for example, least squares
(e.g., Press et al.
1992) or total least squares (e.g.; Van Huffel et al. 1991). The form of the
mathematical function
may make assumptions about the assay mechanism, such as a one site saturation,
two site
saturation, one site saturation with nonspecific binding, two site saturations
with nonspecific
binding, a sigmoidal dose response curve with or without a variable slope, one-
site competition,
two-site competition, or a four-parameter logistic. Generation of a
calibration curve entails
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
176
selecting the form of the mathematical function and then fitting the
parameters of the.function
with measurements. The measurements can, for example, be done on the test
instnunent or can
be done in whole or in part elsewhere (e.g.; at the place the assay is
manufacturud). The
measurements'can either perfectly constrain or over-constrain the mathematical
function. As
noted, for overconstrained systeins, model parameters can be computed by
m.iiiimizing an error
function such as least squares.
In exemplary einbodiments of the present invention, for each analyte the form
of the
mathematical function or model (stored, for example, as an index into a table
of known models),
the computed model parameters, as well as the data used to compute the model
parameters; can
be associated with each measurement of the analyte. To reduce. the amount of
redundant
information stored in the database, the association for each measurement can
be a link to the
calibration data rather than the calibration data itself. Instniments can-be
re-calibrated at any
tiine, such as, for example, on a weekly basis or with every measurement. The=
quality of the
calibration can also be assessed, for example, through the running ofoontrols
or.by computing
the residual error from an overconstrained curve fit.
Thus, a calculated concentration can be stored by the system. This can be, in
exemplary
embodiments of the present invention, the primary input to analysis
recommendation algorithrris .
employed by the remainder of the system. It is noted that not all assays will
result in a.`
quantitative concentration. For example, some assays, due to the shape of
their calibration curve,
may yield two different concentiations for the same measured signal. Such
assays are said to
"hook." In such cases the most an exemplary system-can store is an indicator
that the'measured
concentration is above a certain level, the lower of the two returned
calculated values. Other
assays, for various reasons, may return only qualitative results rather than
true quantitative
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
177
resuits. In all cases, a system database can be capable of storing and
retrieving the -result. For this
reason, in exemplary embodiments of the present invention, the result of an
assay can be stored
not as a simple floating point nuxnber, but as a complex object which can take
into =aceount the
various scenarios described above. Such an object can have; for example,
several fields of its
own.
A compressed version of the database can, in exemplary embodiments. of the
present invention,
consist of only the initial ID information, patient ID information, test ID
information, and the
calculated concentration of analyte. This is a minimal set of data which can
prove productive for
data mining and trending analysis, as detailed below. The additional data
described herein cari,
for example, be used to enhance the value of this analysis.
Algorithms encoded or implemented or implemented in an exeinplary system can
be used, for.
example, to determine a recommendation for action. This recommendation can be
based upon- a
calculated concentration of, for example, antibody response. Other
inforrriation can also be
considered, including, for.example; the results of other assays upon the same
sample within a
given assay panel.
Regardless of the means of determining the recommended action, as described
above, -a fmal
recommendation can be stored in the database. A system database can, for
example, also store
the "reasoning" behind the recommendation, allowing a human to later query the
database to
determine why a given course of action was recommended. Given that the nuniber
of
recommended courses of action can be broad, these actions can be categorized
and encoded. For
example, a recommendation to administer.a particular vaccination may be
encoded with one byte
-to indicate "give vaccination" and two additional bytes to indicate the
particular vaccination that
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
178
is warranted. -A field for comments can also be*included, to allow the capture
of the system's
reasoning = in this case, an explanation of how algorithms and rules were
applied to determine
the stated conclusion:
A system database according to an exemplary embodiment of the present
invention can be
. implemented, for example, as a shared resource spread over multiple computer
platforms.. For
purposes of trending and analysis, it may be necessary to accumulate the data
from a large .
number of systems into a central repository as depicted in Figs. 2, or, for
example, in tlie oase of
having only decentralized information, by using a mechanism or process to
locate and query the
distributed sources. The individual databases can therefore require the
capability to link up with
a defined central database and upload their contents. This can occur ori a
periodic basis, or as
may be triggered by a user of the system. Additionally, there can be multiple
central servers, so
that a given enterprise may choose to aggregate their data at any level:
Unique IDs associated
with sample and panel records can serve to allow for the combination of data
froni disparate
sources without data "collision."
The linkage between local databases and a central database can be implemented,
for example,
across* a local area network (LAN), a private data network, a.VPN; an intranet
or across the
Internet. It is also possible to link databases on a periodic basis using
physical media; such as
CD-ROMs. Similarly, various users such as, for example, health care providers,
individuals,
insurance executives, consumers of research services,= health care management
personnel, etc.,
can access an exemplary system via a web based interface across a local area
network (LAN), a=
private data network, a VPN, an intranet or across the Internet.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
179
Once data has-been accumulated into a central repository, a separate system
can be used to
perform data mining and data trending analysis upon the stored data. There are
many valuable
sorts of analyses which cari be, performed on the accumulated data in an
exemplary system
according to the.present invention.
Given that each data record can, for example, be identified with a particular
individual or patient
and a particular time and date, it becomes possible to perform trending
analysis of a patient's:(or
a population's) ImmunoScore profile over time. In many cases an individual's
absolute
measured value of an analyte is not as important as the trending of that value
over a time. Some
individuals may have naturally low or naturally higli values which are not
bestmeasured against
a statistical mean for their demographic population, but rather against that
individual's own
measured history.
As described above, each patient can, for example, also be placed'within
certain demographic
categories. It can be useful to'compare a patient's measured ImmunoScore
profile against the
corresponding profile for the demographic groups to which he or she belongs.
Deviatiori 'from =
the measured means for a demographic slice of the population can prove more
meaningful than-
can a comparison to a total threshold. Thus, in exemplary embodiments of the
preserit invention,
collected data can be used to continually modify the demographic. profile
averages known to the .
system, taking care to not pollute the system with outlying data points. For
example, it may
prove useful to produce separate ImmunoScore demographic profiles for patients
who are known
to have experienced vaccinations versus those for whom there is no known
iminunization record.
Alternatively, as is described below in Section III, such an immunization
record can be inferred
and reconstructed, as in the provision of ImmunoScore services to national
immigration services
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
180
Trending information in a demographic profile, for example, can. also be
usefl.il. For example,.
tracking an indication of a typical person (e.g., mean, median, or mode), or
an indication of the
spread amongst people (e.g., standard deviation, interquartile range, or
range) over-time can
enable an exemplary system to assess the relationship between immune status'
indicia and
external factors, such as, for example, seasonal effects. Eating habits,
sleeping habits, time
aboard ship, etc. can be found to affect 'immune status in groups where these
external factors are -
partially controllable (such as, for example, in military personnel).
Comparing=inunune -status
indicators of differing demographic profiles can have important
epidemiological significance.'
Finally, it is expected that the collection of ImrriunoScore data from a large
number of
individuals and/or populations can eventually lead to the improvenient of
diagnostic tests, thus
forming a feedback loop. These improved diagnostic tests can then, for
example, be deployed to -
field instruments, resulting in more accurate measurements and diagnoses: Such
exemplary
embodiments having feedback loops can be implemented, for example, with
respect to particular
populations or demographic-groups, such as, for example, the military, college
students,*
immigrants or any other group or combination thereof as described above.
EXEMPLARY ILLUSTRAT/VE DATABASE
1. Overall Description
To illustrate the systems and methods of the present invention, a database
system was
constructed to serve as a testbed'for the exercise of the business models
described below. Such
an exemplary database system was used to demonstrate the tools and techniques
that might be
used in a full scale system according to the present invention. Accordingly, a
large data set was
constructed using statistical techniques. The data was produced according to
match existing
knowledge about the distribution of immune response values among the,general
population.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
181
The exemplary database system has two primary components. These two components
represent
the algorithmically interesting sections that can be, for, example, present in
a full-scale
operational system according to an embodiment of the present iiivention. Such
a full system
could, for example, contain other modules as well,. along the lines of
industry staridard large
scale database systems. Such an exemplary system is depicted in Fig. 5 and is'
next generally
'described.
. With reference to Fig. 5, an exemplary system architecture can be
constructed. The exeiriplary
system architecture can be, for example, divided iinto two sub-systems, one
relatively local to
"point of care" or locations where the individuals or patients whose immune
status is to be =
analyzed are located. The other.subsystem can be in a central location where
complex data '
mining and analysis can occur. Thus, with'reference to Fig. 5, an upper
portion of the figure
contains components which can be located at the point of care and a lower
portion of the figure
.contains components which can be, for example, located at a system central
location. *The point
of care is divided from the central location in the figure, by a double=dotted
and dashed line for
ease of identification. =
With reference to the point of care sub-system, there can be one or,more
Instruments 505 which
are devices which can read immunologic assays. Tnstruments 505 yield Assay
Results '506.
Assay Results 506, along with Doctor's Observations' 503, Patient History 502
and Demographic
Information 501 regarding the individual or patient can all be stored in Local
PatientEvent
Database 510. Database 510 can be , for example, an online transaction
processing database:
Because the point of care sub-system is generally directed to gerierating a
recommendation in a
relatively short time, there are two pathways to Diagnostic Module 515.
Diagnostic Module 51'S
applies algoiithmic rules to the assay results to determine a proper course of
treatment or action
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
182
based on current readings and optionally on past histo;y. Thus, there is a
flow of information
from Assay Results 506 to Diagnostic Module 515. Alternatively, Diagnostic
lviodule'S15 canõ
implement algorithms having other inputs besides the current Assay
Results,506, such as, for
example, Demographic Information 501, Patient History 502, and Doctor's
Observations 503
(understood to include any observations by any health care provider, or the
like, in a geiieral
sense) which can be stored in Local PatientEvent Database'510. Thus, in Fig.
5, there is an
arrow labeled "optional" running from Local PatientEvent Database 510 to
Diagnostic Module
5.15. 'Regardless of which source of information Diagnostic Module 515 draws'
upon,. it oan, for .
example, output the patient action recommendation 516 as indicated.
Returning to the central location=sub-system of Fig. 5, a connection exists
between Local.
PatientEvent Database 510 and a Central PatientEvent Database 520. This
connects the two.sub-=
systems. It is contemplated that at regular intervals data from Local
PatientEvent Database 510
can be uploaded to Central PatientEvent Database 520. Moreover, although the
central location
sub-system could be miurored in a number of distributed central location
subsysteins, the point of .
care sub-system is contemplated to take data from numerous instruments and in
fact have
numerous local patient event databases in those locales. In short, the point
of care sub-system is
found wherever potential customers or patients are found. It is noted that
there can be a myriad
of such locations, given the various and sundry applications and business
models that exemplary
embodiments of the present, invention contemplate. Examples of such
application's are described
more fully in Section III, below. Therefore; there could be a great number of
local patient event
databases all of which feed into Central PatientEvent Database 520. None of
these additional
point of care sub-systems are shown in Fig. 5, for reasons of ease of
illustration. .
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1183
Retuming again to Central PatientEvent Database 520, it is noted that this
database cari, for
example, also be an online.transaction processing. database or OLTP. It is
contemplated that this
database can, for example, periodically load data to an online analytic
processing database, or
OLAP in the form of PatientEvent Database 530. PatientEvent Database 530 oari
be, for.
example, adapted to provide inputs to complicated algorithms dealing with data
mining and
pattern detection, as next described.
PatientEvent Database 530 can, for example, reside on a central server and
utilize a data
warehouse approach. There can be a variety of connections to PatientEvent
Database 530 such=
as, for example, a Query Module 531, a Data Mining Module'532 and a Pattern
Detection
Module 533. Query Module 531 can be, for example, an interface by which a user
can
interactively search for information in database 530. Query Module 531 can
also access Central
PatientEvent Database 520 implement a variety of operations on the data there
as well. Data
Mining Module 532 can be an interface by which a user can interactively use
OLAP tools.to
fmds trends and summaries in the stored data: Finally, Pattern Detection
Module 533 can be a
program module which can be used to automatically search for patterns or'other
"hidden"
correlations between various data points in a database.' It is contemplated
that in exemplary
embodiments of the present invention Pattem Detection Module 533 can regularly
sort through
all of the stored data looking for patterns using various algorithms. Some of
such algorithms
can, for example, articulate some hunch or a correlative assumption provided
by a panel of
immunological experts for which.they do not have hard data. Pattern Detection
Module 533 is
thus an important feature in exemplary embodiments of the present invention.
Additional
exemplary databases which Patter Detection Module 533 can utilize are
described below in
connection with Fig. 5A.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
184
The exemplary system depicted in Fig. 5 will next be described iri greater
detail. A first module
of interest is termed Diagnostic Module 515. The function of this software
module is to input a
set of assay results 506 obtained through measurements by instruments 505, and
to make one or
more recommendations 516 based upon the analysis of assay results 506.
Diagnostic iViodule
515 can be designed in such a way that additional assay panels can be slotted
into an existing
system as they are developed. Some exemplary algorithms used to make
recommendations as a
function of assay results are described in more detail below, including
descriptions both of
algorithms used in the exemplary database as well as additional algorithms
that could be
implemented in various exemplary embodiments of the present invention.
Diagnostic Module 515 can rest upon a Local Database 510 containing Assay
Results 506
obtained from Instruments 505. These results are peitinent to an individual
patient. Local
Database 510 can-also, for example, contain background medical history 502 for
that patient,
demographic information 501 pertinent to the patient,'and a summary of other
medical
observations 503 made by medical professionals or persons fulfilling a similar
function.. Local
Database 510 can also, for example, contain statistical, information obtained
from a larger central
database, as described below..
A second exemplary module of interest is Data Mining Module 532. Whereas
Diagnostic
Module 515 is intended for the analysis of a particulai. individual's data at
a particular point in
time, Data Mining module 532 can; for example, look at a broader, range of
data collected from
many individuals over a range, or interval, of time.. Through analysis af this
collected data a
system can, for example, be used to support various business methods and other
applications by
deducing trends and patterns within an immunologicallandscape. A particular
result could be fed
back into the Diagnostic Module's algorithms, improving their effectiveness bv
nroviding
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
185
additional specificity with regard to an individual's background, possibly in
terms of background
or demographic information such as, for example, gender, racial background,
geographic origin,
lifestyle, economic circumstances social circumstances, or age.
As can be seen from Fig. 5, while the 'Diagnostic Module's functionalities are
primarily local in
nature and patient-specific, the Data Mining Module's functionalities are
primarily central, and
system-wide. As noted, .this. structure is reflected'in the division of Fig. 5
into two zones, the '.
"Point of Care" zone, shown at the top of the figure, and the "Central
Location" zorie, shown at
the bottom of the figure.
Data. Mining Module 532 depends upon the existence of a large ceritral
database containing
records from a wide variety of individuals over a long span of time. Thus, the
local databases
described above.can, for example, exist in a federated state with the central
database, uploading'
their information on a regular basis, where this information can, for example,
be integrated into
the full system:
2. - Impact of Data Mining
Patterns can be detected within the data in an exemplary database which are
related to
demographic and other non-immunologic information such as, for example,
gender, age,.
ethnioity, geographic origin, employment, etc. These patterns =may not be
obvious until large -
numbers of individuals are assessed, using a computer that can be by nature
much more efficient,
unbias ed, and precise in pattern recognition.
From such patterns, new correlates can, for example, =can be established, and
old correlates can
be changed. For=example, in immunization related applications, it may be
proposed, based on
previous data, that a serum antibody concentration of 2 micrograms per ml
should be used to
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
186
represent a threshold of protection against meningococcal disease, so that
anyone with less
antibody would be recommended for inununization. Subsequent and coritinued
analysis,
however, may show that this threshold value should be reduced or raised for
given-individuals,
depending on, for example, age or ethnic background, or some other undefined
parameter. In
turn, an ethnicity evaluation could lead to the discovery.of a specific
biological or genetic
marker. For example, the functional activity of Haemophilus influenzae type b
(Hib) antibodies
may vary with different individuals, where the same antibody concentration may
not possess the
same level of bacteriocidal activity due to differences in antibody avidity.
For example,=
regarding age, Hib polysaccharides were -shown to be poorly immunogenic in
children less than
2 years of age (Granoff DM, 1985, J Pediatr 107:330-36). Similarly, regarding
ethnicity it has
been shown from previous studies that Eskimos and Apaches are more
=susceptible to Hib
rneningitis because they possess a less effective antibody repertoire to the
Hib polysaccharide -
capsule, based on the presence or absence of certain variable region
genes'used in the production
of the polysaccharide-specific antibodies. Additionally, variations in host
factors can lead to significant differences in the immune response
to vaccines, which can also be discerned by data mining. For example, late-
stage complement..
deficiency may have no impact on antibody production, but would certainly
reduce the
effectiveness of those antibodies in killing bacteria, thereby lowering their
activity. In such case,
the antibody tlireshold for protection may need to be raised in order to
achieve the same level of
protection in this subpopulation. .
As previously- described for Hib, 'the capacity for protective antibody
production is the direct
result of variable region gene haplotypes. In this case, ethnic iiifferences
were first =observed as a
gross marker, but the presence of specific genes was later determined to be
responsible. In a.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
187
similar but different manner; HLA haplotypes have also been coirelated with
the. susceptibility. to
certain infections, as well as the unresponsiveness to certain vaccines. For
example, certain
HLA antigens appear to be correlated with chronic, hepatitis B virus (I1BV)
infections. and HBV
vaccine nonresponsiveness. In such'cases, in exemplary embodiments, of
the'present'invention,
subpopulations can be identified, initially by ethnicity, then later by.
genetics, to evo.lve a more
specific and appropriate diagnostic outcome.
Another example of the influence of ethnicity on responsiveness to treatment
is the case of
NitroMed's BiDi1TM, which was approved by the.U.S. FDA in 2005 for the
treatment of heart
failure in.African Americans. BiDiITM is an orally administered; nitric oxide-
enhancing drug that
was shown to have, clearly different effects ori blacks versus whites in
clinical trials, where the
"differences may be related to environmental, social, lifestyle, or genetic
factors or to interactions among all of these."
(see,http://www.fda.gov/fdac/fe;itures/2005/505 BiDil.html).
In exemplary embodiments of the present invention, data mining can, for
example, be used to
observe and identify these kinds of effects and correlations, and then be
later used to determine
the specific underlying mechanisms.
Data mining can also be used, for example, to change or reverse previously
held dogma(s)
concerning long-term protection'from vaccination. For example, immunity
resulting from the
smallpox vaccine, used extensively duriing the previous century, was
originally thought to last for '
less than a decade. Recent analyses however, have shown that "more -than 90%
of volunteers
vaccinated 25-75 years ago still maintain substahtial humoral or cellular
imniunity (or both)
against vaccinia, the virus used to vaccinate against smallpox." (Hammarlund E
et al., 2003,=
Nature Medicine 9:1131-37). The same study further showed that "Antiviral
antibody responses
remained stable between 1-75 years after vaccination, whereas antiviral T-
cell. responses
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
188
declined slowly, with a half-life of 8-15 years." While it is not clear what
level and combination
of responses is. required for protection, the authors concluded that "the
morbidity and mortality
associated with an intentional smallpox outbreak would be substantially
reduced because of pre-
existing immunity in a large number of previously vaccinated individuals."
This is exactly the
type of information that could be obtained tbrough data mining over time on
large populations,
as contemplated in exemplary embodiments of the present invention.
As noted above, an exemplary system similar to that depicted in Fig. 5 was
built using standard
software development tools an.d packages. The algorithms were encoded using
the XML data
description language. The engine for executing the.algorithms was built using
the Java
prog'rarnming language. An Oracle database was used for data storage and data
mining querying.
Excel spreadsheets were used for data construction and analysis. Details of
the construction are
given below.
3. Diagnostic Module
3.1. Overview
Diagnostic Module 515 forms the heart of an exemplary ImmunoScore decision
system. At a
basic level, the diagnostic module exists to provide relevant information
and/orto suggest
courses of recommended action (for various purposes, depending upori the
application; see
Section III below) based upon an individual's immune status, as measured by
instrumentation or
obtairied from elsewhere, in combination with other supporting data. There are
many different
ways that such a determination could be made. Next described are some
exemplary algorithms
that were used in the example system as well as other exemplary decision
support algorithms
-which could be implerriented using the same techniques.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
189
Oine essential function of a diagnostic module can be, for example, to assist,
a medical or other.
professional in making decisions regarding which actions to take with a
specific individual,
making use of data regarding that person's immune status. As*noted, iin
exemplary embodiments
of the pre'sent invention, an individual's immune status can be determined
by'conducting, a panel
of assays, each, of which assays can produce'an element of data. For purposes
of the example
database, information presumed to be. obtainable through such assays is
surnmarized in Fig. 6. It =
should be noted that in practice some, of this information may not yet be
obtainable, although it is
expected that assays could be developed along the lines of existing tests in
order to complete this
spectrum.
In addition to immune status infor.mation obtained from assays, a-diagnostic
inodule can maice
use of other information specific to the patient being exannined: This
infoririation falls into two
'principal categories: demographic information, such as, for example, age and
gender, and patient
medical history. Most demographic information can be'simply expressed in a
database. Patient
medical history is more problematic, although there are many existing
healthcare database
systems which do this adequately. The difficulty with patient medical history,
however, is-in
devising algorithms which can make use of this qualitative data. It is -
expected that particular'care
can be taken to use algorithmic techniques which have proven adept in dealing
with iriconsistent
or unreliable data, such as, for example, neural networks, described in
greater detail below. This
is due to the iriherent unreliability of self-reported-medical history data,
along with the historic
problems found in the transfer of inedical records. If a system with built-in
reliability checks is
implemented, then it can be possible to rely more strongly upon
historical.data.
Thus, the exemplary system described below can store both deniographic and
past medical
history information for individual patients, but does not make use of these
factors in performing
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
190
diagnostic assessments or recommendations of courses of action: However,.the
algorithms
irriplemented can easily be. extended into these realms once more information
becomes available.
The output of Diagnostic Module 515 can be, for example, a series of
recommendations. A
recommendation is simply defined as any discernible bit of data which might be
of interest to a'
medical professional, health care or life insurer, medical services analyst,
researcher or otlier user -
of the present invention in deterrnining a given course of action. In #he case
of a patient's
immune status, a common recommendation could be, for example, to recommend a
particular
vaccination, to conclude whether the individual is in an overall sense
healthy,,to.conclude that .
certain potential hypotheses need further data to be fully explored, to tag
the individual as being
potentially immunosenescent, or to grant a health insurance credit or debit
relative to a health
insurance policy or. HMO membership fee.. Or, for example,'a recommendation
not to-vaccinate,=
to reduce the over-vaccination of the populace. A summary of soine. exemplary
types
recommendations that can be offered by an exemplary Diagriostic IViodule are
provided in Fig. 7.
In exemplary embodiments of the present invention a Diagnostic Module can be-
.capable of
producing a set bf recomrnendations for each analysis.: For example, it might
recommend that
both vaccizie V be administered and that the individual be retested in three
weeks to monitor his
or her response to such vaccine. Tor each recommendation, an -exemplary
Diagnostic Module
can, for exarnple,.also'provide a confidence level; which is a measure of the
system's support for
any given conclusion. A user can take this confidence level into account when
deciding upon a
course of action. A course of action with a low confidence level but a high
I'inancial cost, for
example, could be delayed until additional data could be gathered to more
strongly support the
course of action.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
1191
In exemplary embodiments of the present invention a Diagnostic Module can, for
example, be
constructed in a manner to allow the deployment of many different algorithms
within its basic
shell. For the exemplary system, an algorithmic approach based upon
perceptrons was used. This
approach is detailed below. Additionally described are alternative algorithmic
approaches, each
of which has different strengths and weaknesses. It is noted that some of
these approaches are
realistically infeasible until such time as large-scale data collection of
immune status informatics
becomes available.
3:2. Perceptron algorithnis
A perceptron is a simple neural network, a computer science representation
based upon an a.nalogy, with the operation of human neurons. Perceptrons were
invented by Frank Rosenblatt in
1957, and have been used in artificial intelligence research siince that time.
A perceptron is
simplistic, but adequate for the computation of algorithmic diagnostic results
within the
exemplary system of the invention. More importantly, there is a clear
progression.between
perceptrons and more sophisticated artificial intelligence techniques, which
may be of use in
more complex embodiments of the invention.
An example of a perceptron is given in Figs. 8 and 8A. These networks
encode.the decision
making process for the running of a Meningococcal Diagnostic Panel, as
de'scribed above. There
are seventeen inputs to the algorithm, one for each of the measurements that
can be taken in an
exemplary meningococcal assaypanel. Five inputs are for the meningococcal
serogroups, seven
for the complement components, and five for the genetic poymorphisms. There
are two output
recommendations from this panel Rl 810 (or in Fig. 8A, R2 810) and R3 840.
R1/R2 is a
recoznmendation'to vaccinate an individual 'with a meningococcal vaccine. R3
840 is a
recommendation to monitor the individual on a stricter interval schedule than
normal_ because
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
192
tlie individual may be more susceptible to this condition than the average
individual in'the
populace. Figs. 8 and 8A depict the same prerceptron; with different values
for the various
nodes upon firing.
With reference to Fig. 8, serum IgG levels for vaccine-preventable serogroups
(A, C, W-135, and
Y) of Neisseria Meningitis can be assessed. As seen in the fifffi input to Rl,
the panel also has a
built-in facility to measure and consider serogroup B, but there is no
currently available vaccine
or clearly known th'reshold of protection for this serogroup, so it was left
blank. A serum IgG
level exceeding 2.0 ug/mL for all' four serogroups would be presumptive of
protection in an
otherwise healthy individual, i.e., an individual'(i) found not deficient in
serum levels of
measured complement components, and (ii) having no deleterious genetic
polymorphisms.as
indicated in the CC Test 820 and Genetic Polymorphism Test 830. There' would
be no
immediate recommendation for =meningococcal vaccinatioin= for these
individuals.
The following is a d'escription of rule execution flow for the exemplary
perceptron of Figs. 8 and
8A.
Rl - Recommend Vaccination. With reference to Fig. 8;If the CC Test 820 and
the Genetic
Poly Test 830 show the person is normal, both of them will fire, giving a
minimai total bf 2.0 at
R3. Then no contribution at R] from R3, and. if any of the serogourps is
deficient, Rl will lie at
least-.=1.0 and'R1 will fire. If the CC Test 820 or the Genetic Poly Test 830
show that the person
is not normal, R3 840=will fire,.giving a base total of -4Ø Nothing will be
contributed from the
R.3= conclusion as even if the inputs to Rl 810 from the four"serogroup assays
are all 1.0,{all
deficient), this added to -4.0 = 0, which.is <1.0, and Rl' needs to be >= 1.0
to fire:= Thus Fig. 8
only operates as to normal individuals vis-a-vis the CC and Genetic Poly
tests.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
193
R3 - Recommend Flagging: If the total at R3 840 is less than 2.0,
the.individual is not normal,
R3 fires and the recommendation will be to.flag this individual for
monitoring.
Fig. 8A is similar to Fig. 8, except that.it applies a different recommend
vaccination rule, R2 at
810, for a different immunological context. The perceptron is- modified as to
values, but the
nodes are identical:
R2 - Recommend Vaccination. With reference to Fig. 8A, if deficiencies were.
to be revealed '
in any of an individual's complement components, or if any unfavorable -
genetic polyinorphisms
were shown to exist, then it is likely that a serum IgG level of > 5.0 ug/mL
(not the >.2.0 UG;
level as in the rule of Fig. 8) for the vaccine-preventable serogroups would
be desirable in these
individualsAf these. individuals had IgG levels exceeding' 5.0 ug/mL for all
four serogroups, no
vaccination would be recommended. If the level of antibody to ariy of the four
serogroups were
to be below 5.0 ug/mL, then a vaccination would be recommended. If the CC -
Test or the.
Genetic Poly Test show the person is not.normal, one of them, will fire,
giving a minimal total of
at R2. Then, all that is required'is for one of tlie serogroups to be
deficient.(i:e., < 5.0 ug/;nl)
in order for the recommendation at R2 to evaluate to true.
R3 - Recommend Flagging. If the CC Test and the Genetic Poly Test show the
person is
norrnal, both of them will fire, giving a minimal total of 2Ø If the total
is less than 2:0, R3 fires,
they are not normal and the recommendation will be to flag this individual
for=monitoring.,
Because al perceptrons operate on the data in parallel, an abnormal individual
can, for example,
be captured in the perceptron of Fig: 8A and can thus receive no vaccination
recommendation
from the perceptron of Fig. 8..
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
194
A perceptron operates through software by simulating -the "firing" of nodes
based upon
numerical conditions being met. As each node fares, it can contribute to the
firing of other nodes,
in some cases positively and in some cases in an inhibitory fashion. The
network as a whole has
completed execution when the rightmost nodes, representing diagnostic
recommendations, have
either fired or have come to rest.
The perceptrons in the exemplary system were ericoded manually based upon
existing
knowledge of diagnostic recommendations in use today. Each perceptron can be
represented
either graphically, as in Fig. 8, or textually, as in Fig. 9. Fig. 9 is thus a
textual representation of
.the perceptron network using a language called XML, or eXtensible Markup
Language. In the
exemplary these XIVSL files can be deployed to the diagnostic module as
discrete packets'. An
exemplary Diagnostic Module connected to an instrument, or bank of
instruments, could, for
example, be configured with only those perceptron algorithms required for that
site.
In addition, updated versions of these algorithms could be deployed as the
algorithms are
improved over time in a continuous process of system learning or iteration.
Thus, in exemplary
embodiments of the present invention, knowledge gained through use of the data
mining module,
detailed below, can be fed back into the individual diagnostic modules, thus
improving the
accuracy of the entire system. For example, it may be deduced through data
mining of an
exemplary database that the level of antibody activity which is a strong
indication of the'need for
vaccination is lower in men than in women. A new perceptron algorithm could
then be deployed,
for example, including the gender of the patient as a new input node, with a
link to the
vaccination reconunendation node.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
195
More subtly, a perceptron can include within it a series of weightswhich can;
for example,
correspond=to the importance of each bit of evidence to the recommendation
procedure. Over
time these weights can be continually adjusted and redeployed to reflect
increased understanding'
of the role of each of the immunological factors being measur=ed.
3.3. Alternate algorithmic approaches
There are a number of alternate algorithmic approaches which can be used
within a Diagnostic.
Module. Each has varying strengths and weaknesses. An exemplary system can,
for exa.niple;
include a combination of these approaches in order to come up with the most
complete
recommendation for a course of action.
The process'of evaluating algorithmic approaches involves a=corisideration of
the -goals which are
to be met. A Diagnostic Module can, for example, be configured to optimiize
for any one of a
number of different criteria. Possible goals can include, for example;
optimizing the welfare of
the patient, minimizing costs for the patient related to the diseasein
question, minimizing overall
patient healthcare costs, and minimizing life insurance costs. The decision
algorithm used in the
diagnostic module can thus vary depending on how these goals are prioritized.
A key difference between a system according to the present invention and
existing systems is the
use of an iiidividual's immune status information and associated data as
inputs to the decision.
procedure. This allows the system to provide more tailored and individualized
recommendations
instead of relying upon aggregate statistical measures. A second key
difPerence is the
introduction of historical patient immune status and other data. It is
possible, for example, that a
given individual's antibody level is below some computed norm, but is in fact
high in relation to
that individual's past results. This might conventionall.y:be, - or example,
a contraindication for
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
196
vaccination, a.recommendation which would not be made if the individual's
immune status were
only to be compared to the population standards.
Using the exemplary symbology laid out in Fig.l0, various diagnosticgoals as
shown in'Fig.l l
can be summarized.
-3.3.1. Additional input'data
This section describes additional data which could be incorporated into a
diagnostic module, iri
exemplary embodiemtns of the present invention.
As noted above, historical inimune status information can be a useful
addition. liasing a
recommendation solely upon an individual's status at the current point in time
is an adequate
approach, but it risks making incorrect recommendations for those patients who
do not fall
within the average range of the population at lar;e. A simple extension to the
system would be=to
move-away from absolute measures of, for example, antibody level and antibody
activity level,
and to substitute instead relative measures based upon the percent change
in,these-values since
the last historical measurement, or in comparison to the individual's
historical averages. The
same decision procedures could be applied, but retooled so that a decision
rule such as "the'level
is greater than 30" becomes "the level, is greater than 15% above the
patient's baseline". In order
for this to occur, an exemplary system can either maintain a central record of
the patient's
immune status over time, or provide means to allow ttie portable storage and
transfer of this
historical record, perhaps under the patient's control. Various forms
of="sinartcard" or electronic
storage technologies as are known could be used for this purpose.
A second type of additional input data relates to demographic information.
Current decision
procedures do little to distinguish treatment recommendations based upon an
individual's age,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
197
gender or racial background, although it is known that these factors have a
considerabie effect on
the interpretation of inunune status information. Thus, an exemplary system
could make use of
such demographic information, customizing the diagnostic algorithms to take
into account
observed patterns. Additional research would be required to deduce these
patteriis in the.
population as a whole in order to make reasonable modifications to the
decision procedures.
3.3.2. Decision rule algorithms
A clear successor to the perceptron approach could be to extend the system to
full neural
networks. The distinction between perceptrons and more complex neural networks
is the
incorporation into the latter of feedback links from later nodes to earlier
nodes in the netwtirk.
This not only increases the complexity of the algorithms which can be
implemented, but allows =
for algorithms which improve over time through a learning mechanism. Neural
networks are a
well-established domain of artificial research. The primary impediment to
neural networks is that
they are difficult to construct by hand. A typical neural network is instead
evolved through #he
use of training algorithms. These training algorithms require as input a set
of training data. In an
exemplary embodiment of the present invention, the training data could consist
of immune status
data from a large population of people coupled with data about the eventual
onset of diseases in
that population. Were such a database to exist, neural networks could be
consl.ructed which could
predict the onset of disease based upon features in an individual's immune
status information.
An advantage to using neural networks is that they could be a simple drop-in
replacement to the
current Diagnostic Module in terms of inputs and outputs.
4. -Data Mining Module
4.1. Overview
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
198
The Data Mining Module is the large-scale component of exemplary systems
according to the
present invention. As noted above, while the Diagnostic Module focuses upon
obtaining results
specific to a particular individual, the Data Mining Module can be, for
example, designed to
examine trends in large data* sets assembled for many individuals and with
many readings per
individual. This capability is necessary to support business models in which
iriformation is
deduced about inunune status patterns, as well'as to improve the"functionality
of the.Diagnostic
Module over time.
As noted, an exemplary system was constructed using an Oracle. database
server. The .schema for
the database system is given in Figs. 12 through 14. The schema used is termed
a`star schema',
which is a database layout optimized-for online analytical"processing. This is
a standard concept
in data mining. More information about the data storage is given below.
4.2. Sample Data
The sample database was intended to represent actual immune status information
which could be
collected from a large population over a large span of time. The test
measurements contained
within the database are randomly generated within the constraints detailed
below. =
The exemplary database contains three distinct sorts of information.
The first block of information is individual immune status information. As an
example; the
individual is assumed to be a patient in some healthcare context. The schema
for the patient
information table is given in Fig. 12. To summarize, the database contains
information on the
patient'=s birthdate, gender, racial background and geographic.location. All
of this information
.can potentially be used for data mining efforts related to immune status. The
database also
contains other information strictly for identification purposes, such as name
and ID.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
199
In the exemplary database, patient information was randomly generated. Gender
was split
evenly, and geographic placement was divided among four test cities. Racial
backgrounds were
assigned to match latest U.S. census figures available.
The second block of information is patient visit information. A schema for the
patient visit
information table is given in Fig. 13. To summarize, this information covers,
data that could, for
example, be collected by a physician at the time of a patient's visit. There
can be multiple visit,
information records for*each patient. The majority of this information covers
various symptoms
present in the patient at the time of the visit. This information can be used
within the Diagnostic
Module, above, as part of an algorithm which takes into account diagnostic
information other
than the immune status assay results. This information can also be used- in
data mining to
discover correlations between physical symptoms, immune status indicator
levels, and
subsequent onset of disease. The visit information section of the database is
also used to store
recommendations from the Diagnostic Module.,
In the exemplary database, symptomatic information was assigned randomly. The
example
Diagnostic Module did not make use of symptomatic information:
The third block of inforrnation is the actual results of immune status assays.
In the exemplary
database there are 48 distinct simulated measured quantities, altliough this
can be expanded, for *
exani.ple, to any reasonable number-in a straightforward manner. The schema
for this data block
is given in Fig. 14.
In the exemplary database, assay test results are generated with care. The
distribution of
antibody levels are randomly generated based upon a log-normal
distributiori'with an average of
S0 micrograms per milliliter, as is'consistent'with measured antibody levels
in practice. These
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
200
values aire used. as initial baseline levels for the patieints in the
database. New values =are then
entered to simulate readings taken at set time intervals in the exemplary
patients' lives, as
indicated in Fig. 15. At each age, the antibody'levels were perturbed using a
srnall=normal
distribution; to simulate variation in the populatioii over time. Results*are
biased to match the
observed behavior of antibody activity in populations as they age, as shown in
Fig. 16. All data :
in Fig: 16 is from simulated vaccinated patients.
Half of the sample population was treated as if they had received a
standard.vaccination schedule
at age 5; -the other half was left uritreated'. Antibody levels were adjusted
to suit, as shown in Fig.
17. In addition, a subset of patients were given 'arti.ficially lowered
complement levels and =
antibody activity levels with no change- to the* measured antibody =levels,
simulating the effect of
complement-deficient patients on the data mining procedure. This is shown in
Figs. 18 and 19.
The intent behind this production of sample data was to produce a
population.with interesting
chacacteristics that could be highlighted in the data mining module.'Although
the exact features
used may not be strictly representative of the population.as a whole, they
represent the type of
correlation that a system such as this could detect within real patieiit data.
It could easily be
imagined, for example, that individuals of a particular racial background
might naturally have
elevated levels of a particular antibody. -The system being described could be
used to deduce= that
fact, which may have implications for the immunological care that such
individuals would
receive.
It is noted that all assay results; such as antibody levels, such as, for
example, "Gcmp AVG" in
Fig. 16, may be measured and quantified as-units (U) per volume (e.g., rnl),
where U may be
=defined as some arbitrary unit of a particular assay for the purpose
ofrelative comparisons. ' In
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
201
addition, U may be replaced by a more precise measurement of mass, such as
inicro=grams, where
possible and appropriate. Antibody activity, such as, for exainple, "Gcmp AVG"
=in Fi=g. 16,
refers to the functional activity of an antibody, which may consist of, but
not necessarily be
restricted to, bactericidal or bacterial killing properties. In these specific
examples, assay. results
from individuals may be processed for statistical purposes in the evaluation
of a population, as in
Fig. 16, where individuals may be averaged (AVG) by appropriate statistical
formulas. . Where
statistical processing assumes a normal distribution, geometric means may be
used to average the.
results from different individuals, thereby requiring a log transformation of
data sets, since it is
generally found that only the log values of immune responses will follow a
normal distribution.
42. Exemplary Use of the Patent Event Database
In exemplary embodiments of the present invention, a database used for data
mining can, for
example, be accessed in three different modes, as indicated in Fig. 5. =
A first mode can be, for example, an interactive query mode. A user can
interactively search for
results in the database. Typically queries might include the retrieval of a
single individual's
immune status over time, or the comparison of two such individuals, as shown
in Fig. 19.
Queries can be submitted, for example, using either a.graphical query tool or
th.rough the use of
Structured Query Language (SQL), a compute'r language for the querying of
databases. An
exemplary SQL query is shown in Fig. 19A. Both of these methods of access are
well-kriown in
the industry. With reference to Fig. 5, a user can use the query mode via
Query Module 531.
A second exemplary mode is the use of Online Analytical Processing tools, or
OLAP tools; to
find patterns within the database. A simple example of this is the production
of aggregate
statistics for subpopulations within the whole. In Fig. 19=B, for example, a
query for correlation
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
202
coefficients to GCMP levels=is restricted to female patients. A siinilar
query=might look at only
patients from a distinct geographical area or racial background. Correlation
statistics can also- be
generated, to test hypotheses about possible causal=links among measured
antibodies, between
antibody measurements and physical symptoms, dr correlations between any of
these =and
demographic information. The utility of such a tool depends directly on the
quantity. and quality :
of data that is input into the system. For the exemplary system, trends that
were deliberately=,
introduced into the sample data can be "discovered", but other correlations
are simply a function
of randoin noise. In a real system, a variety of interesting patterns can be
deduced. For the
exemplary database, standard OLAP tools were used. With refereince to Fig. 5,
a user. can use the
data mining mode via Data Mining Module 532.
A third exemplary mode that is anticipated is the construction of a pattern
'detection module.
'Tliis can, for example, comprise software programmed to sift through the
accumulated immune
status and other data and search for patterns that might not be evident to a
human observer. it is
generally true that there are statistically significant patterns in the
underlying data which are too
subtle or too complex for simple detection schemes. Such an automated
detection systern -can,
for example, rely upon one or more of the artificial intelligence pattern
recognition techniques as
described above and in the standard literature.. In exemplary embodiments of
the present
irivention both neural networks and genetic algorithms can, for example, be
used to perform this
task. With reference to Fig. 5, a user can use the pattern detection mode via
Pattern Detection
Module 533.
Fig. 5A illustrates an alternative exemplary system architecture to that of
Fig. 5. Fig. SA has a
few additions, namely, Hypothesis D'atabase 560 and Rules Database 565. Each
of-these
databases can be used, for example, when pal.tern detection module 533
discovers a correlatiori
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
203
betweeri database variables. = When that occurs, a list of such correlations
.can, for exaniple, be .
reported to a human expert or group of experts for review. Or, for example, an
intelligent system
can attempt to recognize the characteristics of such a correlation and
associate possible
hypotheses to explain it. These can be generated, for example, from a
Hypotliesis Database .5'60,
and the rules b.y which a given correlation can, for example, be mapped to one
or more
hypotheses can be stored, for example, in a Rules Database 565. In such
exemplary
embodiments, once a set of hypotheses =is generated, an exemplary system
itself can go back and
mine the data to either rule out,. corroborate, or confirm that'there is
insufficient data to either'
confirm or rule out, each hypothesis in the set. In the latter case the system
can recommend that
further information'be collected, such as, for example, via additional assay
panels known to the
system, lab tests, additional patient history items, etc. This process is
described in greater detail .
below.
EXEMPLARY CANADIAN IMMIGRANT PROJECT DATABASE USED TO
ILLUSTRATE DATA MINING AND' HYPOTHESIS GENERAT/OW
Appendix A contains selections (i.e. an initial.set of records) from
an,exemplarydatabase which
was used to illustrate various data mining functionalities according
to'exemplary embodiments of
the present invention. The database was created from data obtained iri
interviews with and by= performing tests on blood obtained from a number of
inewly arrived immigrants to. Canada under
the auspices of Dr. Chris Greenaway (Assistant Professor in the Department of
Medicine, McGill
University, and a staff physician in the Departments of Microbiology and
Internal Medicine, Sir
Mortimer B. Davis Jewish General Hospital, Mcintreal, Quebec). As can be seen
from the initial -
pages of the database, there are eintries for assay results for each of
measles, mumps, varicella,
rubella, hepatitis A, tetanus, diphtheria, cytomegalovirus, hepatitis B,
hepatitis C, as well as other
factors such as age, gender, region/country of origin, socioeconomic status,
etc.. In the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
204
descriptions that follow, this database will sometimes be referred to as the
CIP database (for
Canadian Immigration Project). The entire CIP database has approximately 1500
records.
Originally the database did not contain assay results for tetanus, diphtheria,
cytomegalovirus,
hepatitis B, hepatitis C, which were added later.
The database now contains, specifically, the following data:
Immunological Tests:
Hepatitis A
Measles (two different manufacturers for diagnostic testing)
Mumps
Rubella
Varicella
Tetanus
Diphtheria
Cytomegalovirus
Hepatitis B
Hepatitis C
HistoricaVDemographic Data:
Region of Origin, being one of:
Sub-Saharan Africa
Latin America and South America
Caribbean
-Europe
Eastern. Europe
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
205
South Asia
Southeast Asia
North Africa/Middle East
Demographic information: Date recruited
Gender
Age (all participants -were adults > 18 years of age)
Whether the interview was taken through an interpreter
Country of origin, being one of:
India
Bangladesh
SriLanka
Pakistan
Morocco
Vietnam
Congo
Other
Date moved to Canada
Citizenship status, being one of Refugee claimant.
Refugee
Immigrant.
Other .
Pregnancy
History of vaccine-preventable diseases
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
206
Participant had written vaccination record?
Participant's residence in home country had indoor toilet/no indoor toilet
If indoor toilet,
Flush?
Other?
Participant's residence in home countty-had outdoor toilet/no outdoor'toilet '
If outdoor toilet,
Outhouse?
Covered pit latrine?
Other?
Participant's residence in home country water supply
Tap inside?
Tap outside?
Closed well?
Public stand pipe?
Bottle?
Pump?
River?
Pump earth system?
Tap inside and closed well?
Other?
University education?
Participant's residence in home country degree of crowding (number of
individuals/room)?
Participant's residence in home,country had electricity/no electricity?
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
207
The CIP database could be augmented to facilitate a broader scope of data'
mining. In such
embodiments results of the following assays could be added: Tuberculosis,
Avian (H5N1) flu,
Pandemic flu (not necessarily H5N1), Chronic infectious diseases other than
CMV
EBV, Herpes/Type?, Zoster outbreak/varicella antibody level following
outbreak?, HPV, HN
HTLV I, Helicobacter pylori, Lyme disease, Tularemia, Parasite infections,
Malaria,
Strongyloides, Hantavirus, Leishmaniasis, Toxoplasmosis (particularly among
pregnant women),.
Antibody levels to other infectious diseases currently on vaccination
schedules: Hib,.
Pneumococcal (conjugate vs. PS vaccines); Meningococcal (conjugate vs. PS
vaccines);
Poliovirus; Traveler's vaccines; Japanese encephalitis; Cholera; Yellow fever;
Military-specific
vaccines: Anthrax, Smallpox, Plague, Rabies; Other infectious agents not
currently vaccinated
for, including: Staphylococcus aureus, Moraxella catarrhalis.
In exemplary embodiments of the present invention, the following non-
immunologic data can,
for example, also be obtained and storedin an individual's exemplary
Immunoscore database
record:
Environmental considerations
Zip/Postal code
Rural/Urban home environment
Working environment
many interactions with many people
few interactions with few people
Interactions with types of people at honie/work:
adults
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
208
children/age of children.
interactions with local travelers
interactions with global travelers
Commute to work
public transport/drive?
duration?
crowded/stressful?
Power source
proximity to power lines
type of fuel
proximity to power plant
Water
. source
well
city
nature of treatment
Nutrition
Diet =
high/low fat
meat/vegetable intake
Common food infections
Salmonella
Cholera
Hepatitis A
Typhoid'
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
209
Alcohol consumption/volume
Vitamin supplements
Fitness
Regular exerciselsedentary.
Cardiac/blood pressure assessment
History of smoking
Second hand smoking
School(s) attended
Day school/boarding school
Crowding at school?
Work environment
high/low/interinediate stress
.job satisfaction
occupation
work described as physical/mental/combinat'ion?
safety considerations at work?
infectious organisms present
nosocomial infections a concern?
chemical agents?
Air quality
home
work
Animal exposure
pets
-work
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
210
farm
lab
leisure
wooded environment?
horseback riding?
Family/personal history:
Chronic disease/nature?
Cancer/type?
Heart disease
Diabetes
Known immunodeficiency
Asthma
Kidney disease
Liver disease
Lung disease
Allergies/type?
Mental illness
Back problems
Joiiit pain/injury?
Chronic fatigue
Osteoporosis
Arthritis
Epilepsy
Education level
highest grade achieved
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
2111
Education type
public/private?
education environment
crowding?
stress
quality of school (measured objectively, of course)
Military service?
Nature of deployment
Service branch?
Rank
It is ianderstood that an exemplary database according to the present
invention can contain
records for various individuals from different countries and locales, being
managed under
various health care systems, and that various types of assays can be used to
obtain assay results.
Thus, in exemplary embodiments of the present invention, the data stored in
the dataliase can, for
exaniple, be normalized to some database wide standard defined for each data
field used iri the
database, or, for example, can be stored in its original form and any
algorithm that seeks to
access data first performs normalizing of the vari.ous records which' are
input to.that algorithm. It,
is for the purposes of such normalizing that information regarding assay
manitfacturer, type,'and
curve that maps an OD or other assay raw result to lUs Qf an antibody or other
measured
biochemical needs, in general, to be stored in the database.
Next described are the results of data mining and hypothesis generation
studies performed on the
exemplaryClP database. These examples illustrate methods and techniques that-
can be used in
exemplary embodiments of the present invention.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
212
DATA=MININ.G - ANALYSES AND CONCLUSIONS
In exemplary embodiments of the present invention, immunologic information
stored in an
exemplary database (such as, for example, the UP database, described aliove)
can, for exampie,
be analyzed in various ways and related to other variables in the=database.
Three useful
examples of such analysis can, for example; include: (1) linear regression
analysis on two
variables to determine whether a positive.or a negative.correlation exists;
(2) compari'son of
geometric mean immune values (obtained; for example, as antibody
concentration, optical
density, etc.) for both genders by geographical regions; and=(3) percentage of
positive or negative
suppoirt within a population for one variable with respect to another.
Examples of such analyses
are described below using data from the CIP database.
5. Linear regression analysis correlation coefficients
In exemplary embodiments of the present invention, tables of correlation
coefficients (r) can, for
example, be generated when comparing one parEicular imrnunologic variable
(such as, for
example, varicella antibody=optical density) against other disease-related
immune ineasurements,
either for both genders together or separately. For example, Fig. 20 presents
the correlation
coefficients between Varicella OD =and various other variables in the CIP
database. Fig. 20 '.-
presents three tables. 'The top table is the correlation of Varicella OD with
each of nine other
variables from the CIP database for all persons in the CIP database. The
second and third tables
present this infornlation segregating males and females. For=example, in Fig.
20, r values have
been highlighted by shading when they are either > 0.05 or <-0.05, as a means
of readily
identifying pattems of relatedness (where I r('_ 0.05 is considered as
"related").
With reference to Fig. 20, Varicella optical density is obviously highly
positively correlated with
Varicella titration dilution, inasmuch as one is calculated from the other,
but other relationships'
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
2213
also appear, although somewhat less pronounced. If the =genders are separated,
then the
relationships appear even more strongly, as expected, since scatter is thus
reduced. From the
tables presented in Fig. 20, Measles and Mumps immunity (Dade assay) appear to
be slightly
correlated with Varicella immunity.
The r values in the tables can also, for example, be graphed in such a way so
as to better
visualize any condition patterns, as is shown, for example, in Fig. 20A.'
.Again, the Measles and
Mumps relationship to Varicella stands out above the others (not considering
the Varicella
titration dilution data, which is obviously correlated to Varicelia OD).
6. Geometric mean values
In exemplary embodiments of the present invention, immune data can, for
example, be
statistically analyzed for the purpose of characterizing populations of
different geographical
regions, as well as for comparing results across genders. Such mean=values can
thus be
graphically compared by'gender and region to visualize population dynamics.
For example, the
geometric means of Rubella antibody concentrations for different regions can
be graphically
analyzed by gender, as shown in Fig. 20B. With reference thereto, a trend'can
be seen where.
males have higher antibody levels than females across all populations in the
database. It is also
apparent that persons from Southeast Asia show a lower antibody level relative
to the other
regions in this study. To help facilitate this assessment, dotted lines were
drawn on Fi=g. 20B to
indicate the mean of the means (geometric) from all of the populations
(excluding Southeast
Asia) separately for males and females. The arrows above the bars for the
Southeast Asia data.
show the difference between the mean values for Southeast Asia compared with
such mean of
'the means for all other 'regions. It would thus appear that Southeast Asia
has a lower immune
profile for Rubella. This can, for example, be explained as the effect of (i)
no specific Rubella
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
2214
vaccine program; and (ii) a possibly a lower exposure rate compared with the
rest of the world,
making Southeast Asians more susceptible to this disease when traveling to
other =geographic.
regions.
This finding highlights one of the many potential uses of the present
invention. ' As described
below in Section III, exemplary embodiments of the present invention can be
directed to health
insurance. underwriting. Here, for example, knowledge of the fact that
Southeast- Asians tend to -
be vulnerable to. Rubella would indicate'that such persons, as a condition of
maintaining insured
status under a health plan or HMO, could be required to obtain Rubella
vaccination.
In a similar manner, the geometric means of Hep A units in the CIP database
(which are
inversely proportional to antibody, concentrations and derived from
immunoassays), were plotted
in Fig. 20C for different geographical regions, again separately for each
gender. In.this=case, it .
appears that there is no significant difference between males and females
across all populations
except one, Eastern Europe. Also, once again; Southeast Asia appears to be
different from the
other regions, where the Hep A antibodies are' lower, as shown by higher assay
units which; as
noted, are inversely related to'antibody concentration. : In addition, persons
from Eastern Europe
are also seen as being generally lower in antibodies, and the Eastern European
females (dotted*
bars) are seen as having= particularly lower antibodies than the males. Again,
in Fig. 20C a dotted
line has been drawn to represent the miean of the means (geometric) from all
of the populations,
excluding Southeast Asia and East Europe, but combining males and females.
Another dotted
line has been. drawn to represent the mean of .the= means from the excluded
populations,. except
for the Eastern European females, which are noticeably higher in units (and
thus lower in
antibodies). The arrows in Fig. -20C highlight the 'differences'between (i)
the*overall population
mean of means and the mean for Southease Asians and=Eastern Euronean male~-
and fiil tl,P
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
215
overall population mean of means and the unit levels for Eastem European
females. Overall,
there appears to be less Hep A reactivity for Southeast Asia and Eastern
Europe when compared
with other regions; this is especially so among East European females: This
may; for .example,
iridicate no -vaccination and possibly less exposure, with greater disease
susceptibility: Thus,
from a health insurance/health management perspective, an adult female from
Eastern- Europe
should have a Hep A vaccination.
7. Percent support between variables
'In exemplary embodiments of the present invention, the percentage of a
population that
demonstrates a'positive or negative relationship for one variable-with respect
to'another variable
can, for example, be deterinined and graphically analyzed. For example, using
data from the CYP
database,'Ruliella antibody levels were measured in females from China, and
the results were '*
grouped according to immune. status: immune support (protective- high antibody
level), low level
support (equivocal antibody level), or susceptible suppoit (non-iminune.
antibody level).` The
percentage of each of these groups that supports an association with another
immune variable,
either positively or negatively, for various different diseases was then
plotted in Fig. 20D.- It is
apparent that there is no significant difference in support for Rubella with
Hep A(non-reactive.
or reactive) or with Varicella (positive); once again, in Fig. 20D dotted
lines have been -drawn -to
help visualize that the Rubella immune levels show no clear trend from immune
to lower
immunity to siusceptible in these specific cases ofother diseases. However; as
regards Mumps,
there is a clear trend for Rubella immune support when compared with Mumps.
The arrow
shows that there is a greater percentage of Rubella immune 'support for
positive mumps, =f.e.,
immune response for Rubella is correlated with that for Mumps:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
216
The immune support of Mumps for other immune variables can, for example, also
be used to
compare different geographical regions, as is shown, for example, in Fig. 20E.
In Fig. 20E, only
the positive and negative'Mumps support groups are plotted (leaving out the
equivocal "low
level support" group) for each of Eastern Europe and Sub Saharan Africa with
respect to Hep A
= non-reactive, measles = positive, and Rubella = immune. Dotted lines
have'been drawn to
illustrate that there is no difference in Mumps immune support for Hep A= non-
reactive in both
regions, but the arrows show that there is a difference for Measles = positive
in Eastern Europe
only, and a difference for Rubella = immune in both regions. Thus, a higher
percentage 'of
Mumps immunity is seen with Measles immunity in East Europe, and with Rubella
immunity in
both East Europe and Sub Saharan Africa.
In exemplary embodiments of the present invention, immune support can also be
related to -other
variables that do not measure immune status, such as, for example, education.
An example of
such a correlation analysis is shown in Fig. 20F. In this example, the
positive and negative
Mumps support groups are plotted for Southeast Asia and East Europe
with'respect to university
attendance. From these results it appears that for Southeast Asia, a higher
percentage of negative
Mumps immune support occurs when there=is less university attendance, and
in.the expected
reciprocal way, a higher percentage of positive Mumps immune support occurs
with university*
attendance. For Eastern Europe, however, there is no relationship seen between
Mumps immune
support and university attendance.
8. Possible Conciusions '
The data mining examples described above demonstrate the usefulness, in
exemplary
=embodiments of the present invention, of an analysis of relationships among
different variables,
both immunologic and otherwise in an unbiased mathematical manner. Regression
analysis can,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
217
for example, be performed to just look for correlations. at random, but the
relationships may be
weak and difficult to see. Additionally, for example, population means can be
used to detect
broad population differerices or similarities: Also, percentage support
analysis'betweendiffer~ent
variables can, for example, allow for a greater focus on specific
relationships between different
immune status results and other factors that may affect them.
The examples described above point towards interesting correlations, some of
which can be
explained based on known immunization 'practices; and others which may, for
example, indicate
previously unforeseen relationships involving exposure to disease. For
example, in countries
where MMR (Measles, Mumps, Rubella) vacciri.es are administered, one might
expect to see a
clear correlation of immunity for all three diseases; but this would usually
occur only in' .
developed countries such as the U.S., Canada, and parts of Europe. Also, in
some cases; there
may only be single immunizations for Measles. The imrriigrant populations used
in the examples
*discussed above, however, were most likely not immunized for the diseases
under analysis, and
thus most of the observed immunity would be due to environmental= exposure to
the infectious
agents of disease, or possibly some other agents or substances that cross-
react with these disease
agents.
Due to socioeconomic conditions in these regions, it is possible that exposure
to one disease
might also indicate exposure to others, particularly in crowded areas, or
areas where diseases are
known to be endemic. It is therefore not surprising to see positive
correlations between Mumps
and Measles or Rubella,. as seen in the China and Eastern Europe data. In
certain circumstances;
however, the disease exposure may be so prevalent (>90% of population) that
there would be, no
way to establish correlations to other factors since everyone has it; this
might be the case, for
example, for Mumpq support with Measles in Sub Saharan Africa asshown in Fig.
20E.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
218
Iricreased immunity for Mumps in Southeast Asia for those who attend a
university could be the
result of these more fortunate people being allowed greater access to
vaocines, or, for example, it
could be due to greater disease. exposure in crowded dormitories. No
difference in Mumps for
university attendance in Eastern Europe might mean that there is greater
disease incidence, or,
for example, that there is greater university attendance, since both- positive
and negative support:
percentages are high. No difference in Rubella support for Hep A reactivity or
positive Varicella
in China may be, for example, the result of higher disease prevalence and
exposure overall. A
trend towards higher Rubella antibodies in males for all regions might
indicate an unforeseeri
gender preference that could warrant further epidemiological studies in
relation.=genetic
polymorphism if this is not the result.of broad cultural practices regarding
vaccinations or-
disease exposure. The significantly lower Hep A antibody levels (higher assay
units) only for .
females in Eastern Europe may, for example, might indicate a cultural
phenomenon for further
study.
These examples merely scratch the surface of what can be explored in terms of
epidemiology,
immunity, socioeconomics; and genetic polymorphism in exemplary embodiments of
the present
invention. Such exemplary analyses, can be used, for example, to design more
focused studies.
on specific areas of interest or, for example, to test specific relationships
that are only1inted at in
the beginning. It is also useful to remember that the data in these examples
only represent
immigrants eritering Canada; it may therefore be important, in exemplary
embodiments of the
present invention to collect more samples and expand the database to other
population segments,
and/or to follow the same persons through time taking samples of each
participant annually for
an extended period of time.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
219
As can be seen from the above description, in exemplary embodiinents of the
present invention,
once a list of correlations has been obtained by analysis of a given set of
records in an exernplary
database, either humans or intelligent systeins are needed=to postulate
explanatory hypothesis,
which can then be verified, or at least can be attempted to be verified,
excluded oi~ determined as
inconclusive.
9. Expansion of database
The database was expanded, since the previous items analyzed and discussed in
sections
1-through 4 above, to include' additional immunolbgic values that cover the
following.infectious
diseases or infectious agents: Cytomegalovirus (CMV), Tetanus, Diphtheria,
Hepatitis A, and
1Iepatitis B.' CMV was of particular interest because of the prevailing
scientific iiteratuie
supporting its role in the development of immunosenescence: during natural
human aging.
Tetanus and Diphtheria also appeared to be good candidate- markers for
following=the immune
status regarding vaccines that are commonly received in childhood and often as
adults
(particularly Tetanus). The possibility was anticipated that we might see an=
inverse correlation
between CMV and other immune markers such as Tetanus. In fact such'
correlations have been.
observed, which will be demonstrated in the following sections (6 and 7).
-As in section 1, tables of correlation coefficients have been constructed to'
include the
new data. Fig. 20G1 represents the correlation of CMV with the other immune
factors;. To be
more selective, we raised the highlighting (color) threshold to + or - 0.1. At
this level, South
Asia was the only region that showed no overall correlation for CMV, and males
generally
showed more correlations than females in other parts- of the world. Looking at
the graphic
representation in- Fig. 20G2, Fig. 20G3,' and Fig. 2094, there appears to be
more negative
correlations overall in SE Asia for Dinhtheria and Heu B. and.more bositive
correlation in
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
220
Eastern Europe for Tetanus. These tables and graphs, however, do not take into
account
differences that may relate to aging, which is the subject of the following
sections.
10. Distribution of geometric means according to agc
Next the'effects of age were looked at globally.as to effect on the geometric
mean
iminune values in order to observe any gross patterns, particularly regarding
CMV, Tetanus, and
Diphtheria, which are respectively represented in=Figs. 20G5; 2066, and 20G7.
As a result, an-
inverse correlation between CMV and Tetanus antibody levels was discovered.
Fig. 20G5 shows'
that CMV gradually increases .with age, while Tetanus sharply declines in Fig.
20G6, and
Diphtheria showed no clear change in Fig. 20G7. This correlation can; for
example, be an -
indication of an immunosuppressive effect of CMV infection on the immune
status regardirig
Tetanus (hypothesis associated with correlation). Since Tetainus and
Diphtheria vaccines are
usually given in'combination, this would imply a specific interaction for
Tetanus. -
While it may be normal for immunity to wane with age, the decline for Tetanus
appeared to be
more evident than what was observed for others. *Based on the step-like rise
observed for CMV,
where the ages in years can be grouped in 3 categories (18-35, 36-55, 56-80),
the age range was
split into three groups for analysis of any trend in geometric means for those
groups.= = With this
approach, all showed mostly a rise in immunity with age'(as seen in Figs.
20G8, 20G10, -20G11,
20G12, 20G13, 20G14; 20G15, 20G16, 20G17, and 20G18), except for Hep B(Fig.-
20'G19 and
Fig. 20G20), which may also be=inversely correlated with CMV. Tetanus, of
course, agaiii
showed a decline, as depicted in Fig. 20G9. It is noted that Fig. 20G17 does
show a rise for Hep.
A, even though the means are declining in the graph, -because .the low assay
values actually
translate into high immune values. Also, Fig. 20G18 shows mostly a flat
response for Rubella,
with a possible convergence of the genders as they age.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
221
11. Focus on CMV and Tetanus
Based on the above. described correlations, it became clear that CMV and
Tetanus needed
closer scrutiny. As described above in section 2, the -geometric mean immune
values for
males and females by geographic region were next looked at. Comparing CMV in
Fig.
20G21 with Tetanus in Fig. 20G22,- it is apparent that the pattern of bar
heights from each
graph is generally opposite in configuration; i. e., up in one becomes down in
the other,
and vice versa - another demonstration of the inverse correlation on a global
scale. For example, Latin America and East Europe have the highest Tetanus
levels, and the lowest
CMV levels. In addition, the males are higher for Tetanus and lower for C1VIV
when -
compared with the, females. In contrast, the patterns for Diphtlieria in Fig.
20G23 and.
Hep C in Fig. 20G24 more closely resemble CMV (except for Eastem Europe).
The females in Sub Saharan Africa appeaied to have notably higher Tetanus
responses
than the males, so it was decided to focus more on regions in Africa (North
versus Sub Saharan),
along with their age groups.- In this case, the-age range was split into two
categories (18-35 and
36-80) in order to maintain higher numbers of people per =group (which
declines rapidly in the=
older age range). Comparing CMV in Fig. 20G25 with Tetanus in Fig. 20G26, the
females of .
Sub Saharan Africa showed dramatically higher Tetanus levels, regardless of
age, along with
high CMV levels, but the Tetanus level- dropped for North Afriea as they
became older, while
CMV increased. One might suspect that vaccine intervention could cause this
effect for Tetanus,
which is what we believe occurred in Sub Saharan Africa. For example, it is
known that WHO
has supported campaigns against neonatal Tetanus in Sub Saharan Africa by
immunizing
women. Fig. 20G27 also shows an increase in Diphtheria levels as the
population aged, which
' might be expected if they were vaccinated with Tetanus and Diphtheria in
combination.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
222
As in section 3, the percent support between immune variables was
investigated. First,
examining the Tetanus and Diphtheria reactive support for gender, Fig. 20G28
shows that
Tetanus has greater support in females than males of Sub Saharan Africa, but
Diphtheria shows
no difference in Fig. 20G29; all as expected.For CMV reactive support, the
confidence level
was not high enough from the database to -determine the values for Sub
Saharan. Africa. ~ Fi=gs.
' 20G30 and 20G3 1, however, do show greater CMV reactive support respectively
for Tetanus in
South Asia and Diphtheria in South and SE Asia. Figs. 20G32 and 20G33 show, in
several
regions, that Tetanus and Diphtheria respectively provide=higher support for
each other in -=
females than males. Finally,.university attendance may have less reactive
support from CMV
(Fig. 20G34), but more reactive support from: Tetanus'(Fig. 20G35)
andDiphtheria (Fig. 20G36).
It should be noted that one needs to consult tables of total numbers and
percentages (Fig. 20G37)
for the occurrence of these reactive thresholds in the different populations,
as discussed above in
section 4, in order to be sure that high suliport is not just a result of high
occurrence in a=
population.
12. Simulation: Sampling over time
'One should.be able to measure immune variables for individuals over time;
where the
visits to the clinic or office. may occur after lengthy tinie inteivals,
allowing for trend analyses =
that might aid in predicting the status of individuals over many years. This
was simulated by =trending the data for populations over different age ranges.
For example; using 10 year intervals,
it was estimated that the CMV levels would increase by 50 OD units, Tetanus
would decrease by
0.05 OD unit, and Varicella would increase by,0.1' OD unit. The global
population was then split
into two age groups, 18-35 and 36-76. All the individuals would have their OD
units
incremented by their respective amounts every 10 years; with the population
moving in time
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
223
from one age group to the next. This would lead to the young group
disappearing as the
individuals-aged, while the older individuals would be removed on the other
end (past age 76) as
they would be expected to naturally die off. The data were -simulated in this
way to account for a.
total of four visits, where the first visit represents the oiiginal data from
the database.
Fig. 20G38 shows the CNIV response moving from the young group to the
oldergroup,
increasing with time for-each visit. Fig. 20G39 shows the Tetanus.response
decreasing over time ' -
for each visit. Fig. 20G40 shows the Varicella response increasing with each
visit. = Finally, Hep
A was looked at as an example of a response that inight cha:nge upon leaving
the original
environment as an immigrant. In this case, one might predict that the
immigrant population from
the-developing world, arriving in a developed country (Canada), would-no
longer be exposed to
Hep A, thereby leading to a reduction in Hep A in the older age group, whicli
is really a lack of
increase in Hep A as the younger individuals age. For this study, remember
that the high Hep A
values represent low antibody levels; so, higher bars would represent the
maintenance of low
Hep.A, which is exactly what is shown in Fig. 20G41, where nothing was
added.(or subtracted)
to the values as they aged. -
PA TTERN DETECTION AND HYPOTHESIS.GENERAT{ON
Fig; 21A illustrates an exemplary process flow for pattern detection
accordiilg to exemplary
embodiments of the.present 'invention. With reference thereto, at 21 A01
patient information
attributes can be collected and then grouped together into separate logical
groupings. The:
following table illustrates such an exemplary grouping.
Lo ical rou Attributes Example
Patient Infonnation Patient's informationthat never changes '
e.g. Gender, Birth Date
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
224
Current medical Visit date, female patient is pregnant or not at the
information time of visit, patient is takin medication or not etc.
Geo a h Patient's country of origin, region of ori '
Immune Status Optical density of various diseases like HepA,
Rubella etc and also the immune interpretation =i.e..
Positive, negative or susceptible for a disease. =
Environmental Patients education level, Type of toilet, water supply,
conditions average people in house hold, number of rooms in
house hold; type of water su i etc.
Patients medical history Has patient been hospitalized before? If the patient
had diseases like measles, mumps etc and at what
a e, patient has vaccine record.
Miscellaneous Information that does not fall into any of the above
Next, at 21A05, the logical groups can be prioritized in an order in which
they are to be
correlated. For example, one could choose the highest priority logicaTgroups
that you want to
find correlations between (e.g. Immune Status v. Geography of the patient).
This can be done,
for example, at 21A15, for all the logical groups. At 21A20, correlations can
be sought. This
can be done, for exa.mple, as follows:
1. obtain the percentage of people in the same geographical regions that are
immune, not
immune or susceptible to diseases.=
2. try to fmd a region where the patient population has variation in immunity
status towards a
disease. The reason for this is that if 89 % of the people are Immune to Mumps
in, say, N.
America, this means that there is not,enough data for people who are not
irnmune to Mumps
for evaluation.. Whereas in South East Asia 67% are immune to Rubella,
therefore there is a
large percentage of the population (33%) that are either susceptible to
Rubella or not
inunune. Thus when there is difference in immune status in population in the
same region the
remaining data can be explored to attempt to determine the cause.
3. Try to evaluate the above results by next logical group (i.e. patient
information - does the
immunity status of a region differ by gender?). Obtain the percentage of
population by
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
225
region and.gender that are immune, not immune or susceptible to the disease.
Ifthere is a.
major variation in the percentage of male - female population for saiime
region that are
immune or not immune, then there is a discrepancy and the other data can then
be explored to attempt to determine a cause.
4. ' Use a data.mining tool to find the correlations of the next logical group
(i. e., for example
environmental conditions on the patients within the same region and =gender
and same
immune status). S. Obtain the geometric means of the optical density of the
various diseases by geography and
gender. This can determine if there is a difference in the.antibody level
between genders living in the same geographical regions. After seeking-
correlations at 21A20, if a correlation- -
is found at 21 A25, it can. be reporte at 21 A27.. The process can continue
until all groups, have
been searched, and process flow ends, =at 21A50.
In exemplary embodiments of the present inveintion, Oracle Data Miner can
be.used, for
example, as a tool for fin' ding patterns in a database. -
Using this tool, for example, there are different ways 6f finding correlations
in thedata. Association Rules :
Oracle data miner uses Apriori Algorithm to find these association rules.
AprioriAlgorithm Details
Oracle Data Miner calculates the following two properties of association
rules:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
226
- Support: Support of an associating pattern is the percentage of task-
relevant data
transactions for which the data is true.
IfA-=>B.
Support (A => B) = Number of tuples containingLboth A and B
Total number of tuples
- Confidence: Confidence is defined as the measure of certainty or
trustworthiness
associated with each discovered pattern.
IfA=>B
Confidence (A => B) = Number of tuples containingboth A. and B
Number of tuples containing A
Associations can be calculated in 3 steps:
1. Find all combinations of items, called frequent itemsets, whose support is -
greater
than minimum support.
2. Decide the xninimum support and minimum confideince required for choosing
the
rules. As the data set under consideration was srriall=we kept the minimum
support
= 0.1 and minimum confidence as 0.1 so that we do not miss any 'data that
might
have any inverse co relation or strong co relation.
3. Use the frequent 'iteinsets to generate the desired rules. Rules that
satisfy both
minimum support threshold and minimum confidence threshold are called strong
rules. Reading the confidence and support get the rules that are correlated.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
227
For. example, association rules generated for Chinese females. who are immune.
to
rubella.
Some exemplary rules that can be generated can be, for example:
Rules Corifidence`; $u r
If Hep A Non-Reactive then Rubella Immune
if Hep A Reactive then Rubella Immune = '=" `".:1:`0:0000;~ 0 74
If Measles = Negative then Rubella Immune
~. If Measles = Positive then Rubella Immune t~~~a1~QOU00 Q,8 ~$
~ n ~.:=;~-=~r :
If Varicella = Positive then Rubella Immune ~~~~ ~1;~000~~. ~ =
Conclusions can be derived, for example, from the rules generated by data
miner.
Thus, Rule I means that 25 % of the Chinese females who are immune to Rubella
.are Hep A non reactive. The trustworthiness of this statement is 100%.
= Regressian :
Regression creates predictive inodels. The difference=between regTession and
classification
is that regression deals with numerical/continuous target attributes, whereas
olassification
deals with discrete/categorical target attributes..In other words, if the
target attribute
contains continuous (floating=point) values, a regression technique is
required. If the target
attribute contains categorical (string or discrete integer) values, a
classification ~ technique
is called for.
The most common form cif regression is Iiriear regression, in which a line
that best fits the
data is calculated; that is, the line that minimizes the. average distance of
all the points
from the line.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
228
This line becomes a predictive model when the.value of the dependent variable
is not
known; its value is predicted by the point on the .1ine that corresponds to
the values of the
independent variables for that record. Oracle Data iVMining- provides both
linear and non-
liriear regression models.
Algorithm options: Support Vector Machines (SVM)
Support Vector Machine (SVNI) ,is a classification and regression piediction
tool that uses
machine learning theory to maximize predictive accuracy while
automatically..avoiding
over fit of the data.
= Geometric Meant
; .Y1.~2Y3 ...
The geometric mean of a set of positive data is defined as the e root of the
product.of all
the members of the set, where n is the number of members.
Another way to calculate the geometric mean, which may aid 'in statistical
analyses, is to
-define it as the antilog of the mean of the log values for a set of riumbers.
Exemplary Data Mining Algorithm
Using the CIP. database, the following exemplary algorithiii was performed:
1. The logical groups were:prioritized so that the immune status (immune assay
results) could be shown according to . geography, followed by Gender. This is
shown in all examples of the data mining (regression analysis, geometric
means,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
229
and percent support). All other logical -groups could be examined later for
possible relationships that might help explain the observed correiations.
2. Regress'ion analyses were performed between all immune variables at each
geographic location, and by gender. Varying cut-offs -could be set to detect
patterns of correlations from tabulated correlation coefficients. For example,
r
values were highlighted in the table where they were >0.05 or <-0.05, is
described
above in connection with Fig. 20.. This resulted a possible association of
Varicella with Measles and Mumps. These r values were also rgraphed to
facilitate any visualization, as demonstrated.
3. Geometric means of immune assay results were calculated for all geographic
regions, and by gender. Graphic analyses were performed, as demonstrated, to
detect differences or similarities between regions, as well as -gender. For
example, there appeared to be a gender difference globally for Rubella
immunity
in favor of males, and a lower immunity overall in Southeast Asia. Hep A
showed this gender difference only for East Europe, with lower overall
reactivity
in both Southeast Asia and East Europe. .
4. Setting the confidence at =100% for different immune subsets of a disease, -
different geographical regions were examined for the percent support of the
association with other variables. For example, in each immune subset of
Rubella
(immune; low level, susceptible) for Chinese females, the percent support was
determined for each of the other disease immune variables. The graph (Fig.
20D)
shows that there is a greater association of Rubella immune support for
peysitive
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
230
Mumps, when compared with Rubella low level or susceptible support. ' In
another graph (Fig. 20E), positive and negative Mumps support was associated
with other diseases in different geographical locations. In this case; there
was a
greater percent positive Mumps support for Measles aiid Rubella in both East
Europe and Sub Saharan Africa. . :.
5: To enhance the chances of seeing meaningful associations, regions where,
there
was a lower incidence of inunune status result (e.g., <80%) were looked at, so
that
associations were not just based on the fact that everyone has a particular
status:
For example, if 95% of a population has a particular status, then that status
could
likely be associated with anytliing; however, as: noted above, since there is
67 ~'0
inununity for Rubella in.Southeast Asia, then there was enough non-immunity to
allow some possibility of detecting meaningful associations. .
6. Other.logical groups were. -then now be examined for other possible
associations
and explanations of previous assbciations. For example, an association was =
graphically demonstrated between positiveMumps support and university
attendance in Southeast Asia (Fig. 20F).
Fig. 21B depicts the exemplary pattern detection process flow of Fig. 21A with
additional expert
systein functionalities. Thus, at 21B60, for each'correlation, the hypothesis
database-can be
searched for possible explanations of the given correlation. In general, this
can be done, =for
example, by using a Rules Database and Hypothesis'as shown in Fig. 5A (and
Fig. 2B) to map
correlations to hypotheses according to defined rules. Once a set of
hypotheses is'generated, ior =
=example; at 21B65 each hypothesis can be tested; to the extent possible,
authomatically, using
. , . .
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
231
data in the system database. Finally, at 21 B67, a report ean be generated
which lists the
generated hypothesis.and states, based on system data, if that.hypothesisis
corroborated, ruled'
out, or inconclusive, as next described.
Thus, in exemplary embodiments of the present invention, a Hypothesis
Database. (and
associated Rules Database) can function as a repository for expert knowledge.
= When .
correlations are discovered by the sysfem; these databases can be consulted to
provide possible
explanations as to why certairi correlations may exist. A Rules Database -can,
for example, map
- as a function of its conditions on attributes, such as , for example, the
database variables
involved in the -correlation - correlations to hypothesis already stored in a
Hypot}iesis Database.
For example, a possible sequence may occur as follows:
1. Database Searched.
. 2. . Correlation found between Rubella and Varicella where Antibody levels
are directly
proportional. .
3. Consult Hypotheses Database for possible explanations.
4. Possible explanations:
a. Cross Reactivity (when exposed to one disease, build up resistance to the
other)
b. Multiple disease vaccinations; and
c. Patient living in an a.rea where risk of exposure is great.
5. System can automatically seek to verify whether each hypothesis generated
by the
system, using the Rules Database and Hypothesis Database, as above, is valid.
For example, the database records for the individuals involved in the
correiation can be .
checked for (i) vaccinations for either or:both of Rubella and Varicella, and
for (ii) living and/or
socioeconomic conditions conducive to exposure.
Next, the hypothesis and the support/nonsupport/non-conclusiveness of each
hypothesis
can be reported to humans, -as shown in 21B67 of Fig. 21B. G. Afterreceiving a
report, each cdrrelation can be analyzed by a human to determine if a
new hypothesis should be added and fed backinto the Hypotheses Database; or if
aii existing,
hypothesis is operative infi he given context:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
232
An example of this process can be illustrated with reference to Fig. 20B,
which presents levels of
Rubella antibody concentration across various regions using data form the CIP
database. First,.
the data was grouped=by gender and region to determine if any trends were
discovered.
As noted above, -it was discovered that the females in Southeast Asia had
especially low levels of
Rubella antibodies. Upon furtlier, lower level, geographic analysis it was
found that the
individuals from China were the ones with low levels.
Possible hypotlieses for this occurrence are, for example:
1.The females tested were never vaccinated for Rubella.
2. The females tested were not exposed to Rubella via the general populace.
As above, data already in the system can be used to examine the validity of
each of these
hypotheses.
In this way future correlations can, for example, be analyzed by the system
itself to suggest
possible reasons as to why trends or patterns have emerged. .
Exemplary Automatic Pattern Detection Module
Fig. 21C depicts exemplary process flow for an exemplary automated pattern
detection module,
according to an exemplary embodiment of the present invention. With general
reference thereto,
the following exemplary process can be implemented in exemplary embodiments of
the present
invention for such a module:
1. Prepare data for data mining. Most data mining algorithms require data to
be suitably =
transformed in order to produce good results. Some common data transformations
are:
binning, normalization, missing value imputation, and outlier removal. In
exemplary
embodiments of the present invention, techniques used for transforming the
data can be,
for example, selected based on attribute data type, attribute value range,
attribute
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
233
cardinality, and percentage of missing values for an attribute or a record.
.(21 C01, 21 C03,
21 C05)
2. Group the attributes of the data into different logical groups like patient
current immune
status, patient history, environmental conditions they lived. in, geography,
:etc. (21 C07)
3. As this is a data centri c data mining system for diseases, a focal point
is to get the disease
immune status relativity. The attribute importance of each attribute cari be
found to'rank.
them in an ascending order to determine which attributes- effect patient's.
immune status
to a particular disease. Attribute Importance ranks the predictive
attributes.by eiiniinating
redundant, irrelevant, or uriinformative attributes and. identifying those
predictor. -
attributes that may have the most influence in making predictions. (21 C07)
Example For rubella interpretation attribute the following was found:.
Attribute Importance Order
RUBELLA ANTIBODY LEVEL I
'
MEASLES OPD ZEUS 2 =
ELECTRICITY = 3
PT GENDER . = 4
4. For each disease immune status (21C15) find a correlation with all,possible
combinations
of identified set of attributes found in Step 3. (21C1-B), (21C20) =.'
For example, a correlation between Rubella interpretation and Rubella antibody
level and
gender was found.
5. For each correlation a threshold can be with . the help of a (human) domain
expert
(2100).
6. Compare the correlation found by the data ininer with the thresholds set.
Verify the
combination of attributes resulting correlation with the disease immunity
status vvith
acceptable threshold with the hypotheses database. If such=relation already
exists r=emove=
this combination from further investigation. ' =
7. If the correlation can not be explained by existing hypotheses, 'analyze
this attribute
combination further for each attribute's contribution to the correlation'of
the whole=set of
attributes with disease immune status.
8. Derive association rules for the correlated attribute set found from Step
7. '.
9: 'Check rules with the discovered set of rules for its existence. ,
10. Analyze the Rules for determining the patterns in the data set using line
or curve fitting.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
234
11. Report discovered pattern and verify with existing hypotheses database.
For the exemplary analyses of the CIP database described above, whe're data
was received in*the
form of an Excel spreadsheet,'and data mining was accomplished using Oracle
software, the
following process was utilized. Similar processes can be implemented in
exemplary.
embodiments of the present invention.,
Data Mining Steps .
Data' Preparation:
1. The data is received in xis format.
2. The data then -needs'to be scrutinized for each column and modiFed.*
Example: some columns have data like ">250". That needs to be changed to -
some number greater than 250 (Scientist discretion) since the data needs to be
-imported into the database as a nurriber and ">250" is not a number. . 3. All
the data is checked for valid values in the xls sheet before importing into
the
database. "
4. Save the data.xis file as data.csv file (Comma separated file)*. . .
5. Create the table using the data feceived using the script createtable.sql.
.6. Now import-the data from the xls sheet into the.new-table using the ,
Immnoscore_Mar2.007.ct1 file. .
Association Rules: . :
Association Rules provide the ability to show relationships that exist, in'the
data.
To find the association rules between the attributes of the,data use the
Oracle.-
-Data Miner (ODM).
E.g. Get association rules for everyone that has a Measles Interp.retation
which-
is positive : . ,
1. Create a view of records that have the attribute value "measles
interpretation" as
"positive". . . . . .
2. Next go to Oracle Data'Miner.
3.. Click on Models > Association Rules > Build.
4. . Name your Model and. Click Next to continue.
5. Specify the location of the data used to build the model..
= Schema: Select'the schema containing the input table.
0 Input table: Select the table or view to use.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
235
. Records per Case: Select Single Record per case. (As each patient
record is 1 record in your view).
Click Next to continue.
6. ODM supports Apriori Algorithm to build Association Rules. You can.change
the
defaults.
Minimum Support: A real number between 0-1.Ask the scientist for details. *
Minimum Confidence: A real number between 0-1.Ask the scientist for details.
Limit Number of Attributes in Each Rule: Number between 2-100.
After specifying the values Click Next to continue.
7. Select data preparation if any is required. Click Next to continue.
8. Choose the attribute to include in your model. Click Next to continue
9. Click Finish to queue your mining activity.
10. Once the mining activity is executed without error, get the rules based on
Rule
'Length Ascending, Support Descending and Confidence Descending.
11. Export the rules to an xls sheets.
Regression:
Regression Models provide the ability to predict numerical attributes about
data
"entities._ Steps: .
1. Create an Oracle View from the main table with all the numerical fields
that you
want in your regression model.
2. Click on ODM. = '
3.. Click Model > Regression >Build
4. Name your Model. Click Next to continue.
5. Specify the location of the data used to build the model.
Schema: Select the schema containing the input table: .
Input table: Select the table or view to use.
Records per Case: Select Single Record per case. (As each patient
record is 1 record in your view).
Click Next to continue.
G. ODM uses the Support Vector Machine algorithm for regression. Change the.
values of the defaults by asking the scientists. Currently defaults given by
ODM'
are used.
.7. Click Next to Continue. 8. Select Automatic Preparation option for your
Model. Click Next to continue.
9., Select the attribute you want to predict. Click Next to continue.
10. Select all the attributes that must be in your model. Click Next to
continue.
11. Click Finish to queue the mining task on the server. 12. Once the task is
done export the results to an xls sheet.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
236
SECTION 11! USES OF IMMUNOSCORE INFORMATION IN
VARIOUS COMMERCIAL, RESEARCH AND
GOVERNMENTAL CONTEXTS
In exemplary embodiinents of the present invention, IrninunoScore information
(including, for example, results of assay panels, individual history and
records of health,
care visits and treatments administered or undergone) processed in an
exemplary system
,and stored in, an exemplary database can be used in a variety of commer=cial,
research and-
governmental applications. These uses can range from optimizing the liealth
care costs of
a medical insurance underwriter to facilitating.inununogenicity studies for a
pharmaceutical manufacturer, or, for example, to- tracking the incoming and
subsequent =
immune status'of immigrants.' In what follows, descriptioiis of several
exemplary
business methods which leverage or exploit the use of ImmunoScore informatics
are.
presented. A.. HEALTH INSURANCE UNDERWRITING AND MANAGEMENT
In exemplary erribodiments of the present invention, systems and methods
according to =
the present invention can be used, for example, to optimize the business of
health insurers
as well as healthcare providers, who are essentially self insurers. In
general; a health
insurance underwriter or a health insurance provider has a population
of.individuals,-
generally called insureds or plan members, whose medical 'care costs are i-
eimbursed or
paid for directly by the healthcare insurer or the healthcare plan. - in such -
6ontexts, it is
useful to monitor the health of the population of insureds or plan members,
especially
those who are older and in those yeais, generally, for example,'stazting at
age 60, when
individuals begin to encounter greater health and medical problems.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
237
In exemplary embodiments of the present inventibn, each plan =member or
insured, or, for
example, each plan member or insured above a certain age, can be assayed, and
the
results can be used to=determine whether any prophylactic therapy should be
administered
to these individuals. Sometimes the decision is as simple as identiying
vaccine
preventable diseases for which the individual does not have sufficient-levels
of
antibodies. In that case, the prophylactic therapy would be the administration
of the
vaccine in question. More complicateddecisions could include identification'of
diseases,
oT of biochemical markers therefor, that an insured or plan member is
susceptible.to that
do not have a direct and economical prophylactic= therapy. In that case,
there.can be, for
example, a more complex algorithtn which decides, given,(i) assay results and
(ii) the
relative= costs of assuming the risk that the insured will contract the
disease verus the
costs of prophylactic therapies to prevent the disease or diseases irnplicated
what to do .
Such algorithms could, for example, be implemented in a system such as is
depicted in
Fig. 2A, where, for example, in addition to database 203 where the results of
assays
conducted on individuals. are stored, there can also be a business rules
database 220
which can also supply inputs.to a central processor 204 which implements sucli
analysis
and algorithms. The inputs to such algorithms can then be, for example, not
just 'assay =
results, medical history and demographic information, but also a'set of
business rules
allowing a decision to be made or facilitated, taking into account the
relative costs arid
benefits of administering prophylactic therapies. Such benefits to be
considered, .can, for
example, be those inuririg to the individual as well as those inuring to the
niembers of the
health care plan as a whole, or, those which seek to maximize profits or
e#ficiencies. In
'exemplaryeni:bodiments of the present=invention.such a healthcare insurance
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
238
optimization method could be implemented as is illustrated in process flow
diagrams
Figs. 22 and 23.
As can be envisioned from the CIP database, it'appears that the level of anti-
Rubella
antibody is unifonnly lower in those individuals from SE Asia. Rubella is a
generally a
mild, self-resolving infection except in pregnant females, in which instance
there are
undue complications to the newborn, known as Chronic Rubella Syndrome (CRS).
In an
immigrant population such as the one documented in the CIP database, if women
of
child-bearing age from SE Asia were demonstrated to be susceptible to Rubella
infection,
health care authorities, as well as those underwriting insurance policies
would be well
made aware of such information. Not only are those worrien more at risk during
pregnancy, but this particular immigrant population would be more likely to
infect-native
Canadians of child-bearing age (assuming that their own antibody levels had
waned).
The general health of the population, therefore, would be well-served making
sure that
these individuals were appropriately vaccinated to avoid Rubella infection and
possible
complications to child-bearing women. These data reveal that Canadian
authorities (and
by extension, those in the United States) could, for example, be well served
and fiscally
responsible in the long run by testing and immunizing the immigrant population
against
Rubella and other vaccine-preventable diseases.
Fig. 22 depicts an exemplary process flow for a health care management
application.
With reference thereto, at 2201 an insured's immune status can be examined,
for example
by conducting one or more assays or panels of assays such as, for example,
those that are
described above. At 2202, for example, the results of those assays can be used
to identify
diseases that the insured is susceptible to;.and moreover, the risk of
contraction of each
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
239
disease for that individual can be calculated: At 2203, prophylactic therapies
that.could
prevent, each identified disease can be identifiied, and at 2204, for each
identified disease
a decision can be made by calculating the expected costs of treatment (such
as, for
example, liy taking the kinown costs =of tteatrrient multiplied by
the'probability'of
contraction) and the costs of associated prophylactic therapies. Finally, at
2205,
prophylactic therapies that cost less than the expected costs of treatment can
be required
for the insured as a condition of maintaining his .or her insurance coverage
or membership
in the health plan, and at 2206, for those prophylactic therapies whose costs
are greater
than expected treatment costs, they can also be required and the insured's
premium
increased.
Fig. 23 depicts a particular subset of the process flow illustrated in Fig. 22
where ttie'
prophylactic therapies are simple and the aihnents identified are vaccine
preventable
diseases. Beginning at 2301, an insured or plan member's immune status is
examined by
conducting one or more assays or panels of assays such as those described
above. At
2302, vaccine preventable diseases that the insured is susceptible to are
identified based
on an analysis of the results of the immune status from 2301. At 2303, the
insured can
be, fox example, required to obtain vaccines=for the identified vaccine
preventable
, diseases. At 2304, follow-up examiriations of the insured's immune status
post-
vaccination can be made, again by conducting one or more assays or panels of
assays,;
and these results can also be, stored in the database. At 2305, the follow-up=
examination
results can be used to evaluate the efficacy of any administered=vaccines to
provide the
necessary immunity to the identified diseases for this'individual. When
extended to an
entire population; such as, for example, the insureds 'of a health insurance
company or the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
240
members of a health plan, this can, for example,-provide a means of evaluating
the '
efficacy of vaccines in. an aging population. This can also be very useful in
the =context of
measuring and dealing with immunosenescense, as described below.
Next described are a number of process flow charts which illustrate exemplary
process
flow accordingto various embodiments of the present invention applied to
healthcare
management applications. Fig. 24 is an alternative process flow to. that
depicted in Fig.
22, is concemed with adjusting an insurance premium or an HMO participation
fee for an. .
individual based upon identificatioxi of,potential diseasts that an individual
is susceptible
to using ImmunoScore diagnostics..
The context of Fig. 24 could arise, for eacample, in the context of an
insurance company
or HMO requiring an annual ImmurioScore -diagnostic panel as a condition of -
maintaining insurance coverage or participation under a healthcare plan.' Such
annual.
requirement would be akin to the annual information questiorinaires that
automobile
insurance companies require of all of their insureds wherein an insured
must,.state if he
has had any *serious health problems, if he'has beeri involved in any
accidents; or if other
out of the ordinary events have occurred. With reference .to Fig. 24= at
2401,. the
individual's immune status 'can be examined and at 2402, based upon the
results of such ,
examination, all diseases that the individual is susceptible to can be
identified. 2405 is a
decision tree which is applied to each disease identified at 2402. Thus, at
,2405, for each=
disease a decision is made as to whether a prophylactic therapy is available.
If no, flow
terminates at 2410 where the insured's preinium is adjusted upwards, to
account for the
additional risk the insurance company is taking in continuing to cover this
individual. If,
at 2405 there is a prophylactic therapy available then the flow moves to 2406
where it is
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
241
detezmined whether-to administer or approve the prophylactic.therapy.= Based
upon this
decision, the premium can also be adjusted.
Fig. 24A is a more detailed version of the analyses described in connection
with Figs. 22
and 24. With reference to Fig.. 24A, at 24AOI the immune status of an
individual can be'
examined, and at 24A02 the initial total cost can be set to zero. 24A02
through 24A35
are then applied in a:loop which cycles over all of the diseases tested for in
the
examination at 24A01. Such identified diseases can be, for example, those
indicated by
analyzing the results of assays conducted and other data associated with the
individual or
various populations to which he/she belongs, as described above. For each
potential
'disease, at 24A05 it can be determined whether the individual is susceptible
or not based
upon the assay results. If no, process flow can terminate as to that disease
at 24A20 and -
no incrementation of cost occurs. If yes, flow moves to 24A10 where it is
determined
whether a prophylactic therapy exists. If a prophylactic therapy does not
exist, at 24A30
the total cost is incremented by the cost of treatment. If it does exist, at
24A05 it can be '
deterniined whether the treatment cost from the disease is greater than the
cost of the
prophylactic therapy. If no, then at 24A35 the prophylactic therapy can be
offered to be
reimbursedup to the treatment cost and the total cost.can be incremented by
the treatment
cost. If yes, then at 24A25 the individual is required to take the
prophylactic therapyand
the*total cost can be incremented by the prophylactic therapy's cost. After
looping
through all of the potentially relevant diseases, at 24A50 the piemium can be
adjusted
based upon the total cost. The computation of total cost and prophylactic
therapy cost at
both the disease specific level and the over all levels can be given by the
following rules:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
242
Disease specific: Computation of TC: P(CDIIS and not PT) * C(TICD and not PT
and iS)
Coinputation of PT Cost: P(CDIIS and PT) * C(TICD.and PT and IS)
Overall disease-related healthcare costs:
TC =I P(CDijnot PT and IS) * C(TiICDi and PT'and IS) + C(PT) (in all diseases)
PT =7 P(CDiinot PT and IS) * C(TiICDi and not PT and IS)
The various exemplary implementations of healthcare management described above
have
considered each disease individually. Fig. 25 addresses a more complicated
situation
where all of the potential diseases are identified and all prophylactic
therapies available
for all of the identified diseases are also identified in all possible
combinations of
'diseases arid prophylactic therapies are analyzed using a cost=
benefit.approach. Thus;
with reference to Fig. 25, at 2501 a panel of assays can be conducted. At
2502, based
upon the results of such assays all diseases the individuals are.susceptible
to aie
identified. At 2505 all prophylactic therapies' which are available for each
of the
identified diseases can also be identified, and at 2510 a cost 'benefit
analysis of all
po'ssible combinations of prophylactic therapies and diseases can be,= for
exainple,
undertakeri usirig business rules. This functionality represents a much more-
complex
level of analysis in order to implement as it is necessary to first define all
possible
combinations of diseases and prophylactic therapies. For exarriple, if the
individual is
susceptible to five diseases and a prophylactic therapy exists for each'of
them but these
propbylactic therapies vary widely in cost, it can be, for example, useful to
.a healthcare
manager or a healthcare insurance underwriter to know whether it may be more
economical to only, administer some, of the identified prophylactic therapies
and run the
risk of the individual contracting the diseases for which prophylactic
therapies are not
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
243
administered. For each of the possible combinations a cost in terms of cost of
administering the prophylactic therapy and expected cost of treatment without
the therapy
is assessed and at 2515 one, or more therapies can be approved and%r the
insured's
premium or the individual's insurance premium adjusted.
It is understood that in the description of the various possible algorithms
which can be*
used in an ImmunoScore analysis for healthcare management that the term
individual,
insured, and.healthcare plan participant are functionally equivalent. While
some
algorithms are expressed in terms of health insurance context that can easily
the same
analysis represented by them can be applied to HMO management or management of
other healthcare plans. As will be described below, the same techniques can be
applied
where the entire population is covered under a healthcare plan, such as, for
example, in. a
socialized medicine jurisdiction. Alternatively, the same techiiiques can be
applied
where a large population of some mutual affinity is covered by a single
healthcare plan
such as, for example, United States Veterans whose healthcare is provided by
the U.S.
Veteran's Administration. Thus, it is understood that any particular algorithm
or method
described in one context also applies to any other.
Fig. 25A is identical to Fig.'25 except that it offers an additional option.
At 25A20, if in
fact the minimum cost, which is simply the total cost of the least costly
permutation at
25A10, is, for example, too great for underwriting limits or healthcare
management.
criteria at 25A20, the participant can, for example, be canceled from the
plan.
Fig. 26 depicts.an exemplary process flow for use in healthcare management
applications.
Fig. 26 is not concerned with dollar costs but rather cost in terms of quality
of life. Such
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
244
an analysis.would be useful where dollar cost is less important than quality
of life, such
as, for example, in exemplary embodiments where a supplemental insurance
company
insures a minimum quality of life and undertakes to provide for whatever
healthcare cost
are necessary to maintain that qiiality of life. Additionally, a socialized
niedicine:
jurisdiction, for example, could have a minimum quality of life which it
seeks=to provide
to each citizen as a basic human right which that jurisdiction sees as all its
citizens as
having. With reference to Fig. 26, at 2601, an immune status of an individual
can be
examinedand the quality of life can be=.set to zero. For.the purposes of Fig.
26, a higher quality of life score translates to a higher quality of life. At
2602 all diseases to which
the individual is susceptible are identified and a decrease in QOL score can,
for examp'le, .
be assigined to each disease. The scoring data (i.e., a map of identified
health scenarios to
some QOL metric) can, for example, be stored in a business rules database such-
as is
depicted in Fig. 2A. Such a decrease in quality of life score can be, for
example, a
measure of unexpected pain and suffering, a measure of how many sick days are
generally associated with it, orJor example, whether the sick days are at
home, taken at
the hospital, or taken while still at work, aixd finally whether surgery is
involved. At
2605, all prophylactic therapies which'are =available for all of the
identified diseases at
2602 can also be ideintified. At 2610 for each identified disease and each
possible
combination of identified diseases (assuming that the individual could
contract more than
one disease, eitlier simultaneously or in succession) the probability of
contracting the
.disease can be computed and from that probability an associated expected
decrease in
quality of life can be, for example, computed. As provided in Fig. 26, an,
exemplary
formula which can be used 'in this context:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
245
E(QOLDEC) = Prob(Disease) * AQOL;
QOL = QOL - E(QOLDEC)
At 2615 an increase in quality of life can be assessed for each identified
disease or
combination of identified diseases for which either prophylactic therapies or
therapeutic
therapies exist. Thus, in exemplary embodiments of the present invention,. the
quality of
life score can be incremented by looping through each disease and adding the
expected
increase in quality of life associated with either (i) providing a
prophylactic therapy or.(ii) '
a therapeutic measure to mitigate the loss and quality of life due to
contracting the
disease. For example, not every disease for which there is a prophylactic
therapy can be
totally obviated. Some diseases to which individuals are susceptible can be
mitigated but
not prevented by prophylactic therapies. For example, when people feel they
onset of a.
cold they often take echinacea. Echinacea tends to lower the amount of time
one is
symptomatic but rarely totally prevents contracting the cold. Alternatively,
.if a
prophylactic therapy completely obviates the individual from contracting the
disease then
the E(QOLin,) should exactly equal the E(QOLdec). If the prophylactic
therapy,happens,
for example, to bestow other benefits besides preventing the disease,-tlien
the expected
increase in the QOL associated with undergoing the prophy,lactic therapy would
exceed
the E(QOL&~). Similar computations would apply to= various possibilities. At
the end of
process flow in Fig. 26 a net quality of life figure can thus be computed.
Fig. 26A is a more detailed process flow for the example illustrated in Fig.
26. At 2-6A01
immune status can be examined and at 26A02 the quality of life can be set to=
zero. At
26A10 the probability of contracting a disease given the immune status
obtained in at =
26A01 can, for example, be computed. At 26A20 the probability of contracting
the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
246
disease given the inunune status can be multiplied by a "badness" score: At
26A30 this
product can be added to the quality af life score. 26Ai0 through 26A35 can
then:be
repeated for each disease for which susceptibility could be examined, given
the assays
administered at 26A01. In this exemplary process flow a better quality of life
is.'
associated with a lower number which is the opposite convention of that
adopted in the process flow of Fig. 26. It is for this reason that a"badness '
score =is assigned to each
disease and a expected "badness" is added.to the quality of life at 26A30.
Additionally,
at 26A15, all possible physical therapies for the identified disease (it is
noted that 26A1'S and 26A35 are within the for-each-disease loop as well) =can
be generated and. mitigation ~
scores can be assigned for each physical therapy or combination ttiereof
'At26A35, the
mitigation score can be, for example, subtracted from the quality" of life
score and once
flow is looped from 26A10 through 26A35,for each. disease, at 26A40 a total
quality of
life score can, for example, be output: Using this total quality of life
score, at 26A-50 the
best set of prophylactic therapies in terms of higher quality of life can be
offered to the
individual with the stated quality of life improvement.
It is noted that in the schema of Figs. 26A a badness score is associated with
each
coritracted identified disease. An exemplary badness scoring system is
presented in the
upper right of Fig. 26A and comprises, for example, +1 for a home sick day,
+10 for a
hospital sick day, :+'/z for a work sick day, and +100 for a'surgery.
Accordingly,, the
quality of life score would dramatically decrease if the individual was found
to
susceptible to a number of diseases each of which required surgery if
contracted=.
Fig. 27 is a final healthcare management exemplary proce'ss'flow chart. Fig.
27= addresses
the newly discovered HPV vaccine which is 100% effective in preventing
cervical cancer.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
247
in women. The question. is who should receive the vaccine and when should.they
be
tested. From the point.of view of society as a whole, perhaps everybody who
has not
contracted HPV should be vaccinated to prevent them from ever contracting it
and thus
preveiat the females amongst -thern, and females in contact with the males
amongst them,.
from ever contracting cervical cancer. Of course, this has a greater cost than
simply
vaccinating women prior to their exposure to HPV. . Therefore, the decision as
to who
receives the HPV vaccine-will often depend upon who is managing the healthcare
of the
population in questiori. This will be described in connection with the
final'decision.at
2715.
With reference to Fig. 27, beginning at 2701, an assay panel containing an HPV
assay :
can, for example, be conducted relative.to one or more individuals. 'At 2705
it can -be
determined whether.that individual is seronegative or seropositive to the HPV
virus. If
seronegative, the individual has not yet contracted HPV and flow moves to
2710, where
the decision as to the individual's gender is made. If the individual. is not
seronegative,
he is seropositive.to HPV,.then flow can terminate at 2706 and any
therapeutic,treatments
that are available can be administered. Continuing at 2710, if the individual
is a female
flow terminates at 2711 and the HPV vaccine is always administered. Whether
the
healthcare manager is an insurance company, an HMO, a socialized medicine
jurisdiction
or a large scale healthcare.managemerit entity such as the'Veteran'.s
Adm.inistiation; any.
female whose healthcare is being rnanaged should be vaccinatedto prevent any
healthcare expenditure in-treatment expenditure for cervical=cancer. However,
what ,
about males? The only utility, derived from vaccinating males is that females
in -sexual
contact with them will not contract HPV. If those females are managed by a
different
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
248
healthcare entity there is little utility in protecting "our" men. If those -
females are
protected in the same healthcare management entity, then there is utility in
protecting
them. Alternatively, even.if the females are not provided healthcare or
healthcare
insurance under a given plan, a government regulating that plan may see a
social benefit
in wiping out cervical cancer, or at least those cervical cancers attenuated
to HPV,'which
are the vast majority of them. Accordingly, given all of these concerns, at
2715, the HPV
vaccine can be administered if the utility value of the prophylactic effect is
.greater than
the cost of treatment, which is simply the cost of the vaccine. The utility
value will, 'as
noted above, be a complicated function of number -of factors, most=prominent
of which
being who is responsible (fmancially, politically or morally) for the
healthcare of the
females that this male may come in contact with.
B. HEALTH CARElHEALTH INSURANCE CREDIT EXCHANCE
The applications that have been described thus far relating to heathcare
management all
assume that in the cost benefit analysis, additional costs can be passed to an
insured, or,
for example, if too high, the insured or member of a health plan (such as an
HiViO) can be
canceled. While this may maximize profits for the health plan or the health
insurance
company in the short run, it can result in the= dissatisfied insureds and
eventually loss of a
certain percentage of the insured base of individuals. Loss of customers is
never a gaod
thing, even if under certain analyses they are unprofitable customers. = One
way of solving
this problem is instead of passing costs through to consumers, i.e., to
insureds or health
plan members, is to set up a means by which they can procure credits in years
when they
are predominately healthy and use those, credits when not costs but - debits -
are assessed
against them as per the exemplary analyses are described above in connection
with Figs.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
249
22 through.27. Thus, in exemplary embodiments of the present invention, a
health'care
provider, a health, care insurance coxnpany, or other financial intermediary
in conjunction=
with the health insurance provider or health care provider, such as an HMO,
can set up a
health insurance credit txahange. Such an exchange can operate in a
similar'fashion as.
' do those government programs which have rules against excessive energy use:
o.r.
excessive pollution derived from an'entity's activities. An entity which is a
polluter, or
an "excessive" user of energy or a natural resourse such as water, for
example, can.
purchase credits from other, individuals or entities who have a low energy
use, low water
use or are low polluters. In this fashion, those individuals or entities who
exceed.a
certain threshold of some desirable metric, such as; for example, low energy
use, low
water. use, or other "green" factors, can purchase, negotiate, trade or
otherwise procure=
credits from those who are below such threshold so as to avoid fines or
negative
consequences from violating the.enviYonmental or natural resource use
standards.
Thus, in the health care context there are always someindividuals who are more
sicic than
others. Individuals do riot know whether they will be in the underwriting bin
of more =
sickly than average or less sickly than average. Insurance compariies try to
spread the
risk of the more sickly amongst a larger population which obviously
includes*those who
are less sickly,,and charge an essentially average health insurance premium-
to everyone.
However,.as underwriting becomes more granular, using exemplary embodiments of
the
present invention, it can be predicted, even decades in advance whether a
particular'
individual is more or less likely to contract a disease, such as for example,
autoimmune
diseases as described above. For example, as described above in Section, I,
certain
autoimmune diseases have markers which are harbingers of their eventual
symtomology
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
250
7-10 years in advance. Thus, using exemplary embodiments= of the.present
invention,
health care plans,.health care administrators and health care insurers will be
able to divide
the population into many more bins of insureds= and associate with each of
them a more
accurate health insurance premium cost. This can cause'those in the more risky
bins to
have a much greater insurance cost. One way of ameliorating this is to
encourage people
to join health care plans early in their lives when they-are healthy and
before even the
onset.of eventual disease emerges, such as via a marker or predictor in an
Immunoscore
assay result marker context? In so doing, people who are healthy can receive
credits =
which they can bank within the system or buy, sell or trade. If regular
Immunoscore
audits of individuals reveal that someone is moving from a less risky bin into
a more
risky bin, and a cost would be -added to their health insurance premium (i.e.,
a debit),
instead of paying an extra premium they can.procure a credit througli a health
care credit
exchange either from their own account which they banked in earlier Years or
from other
healthy peoples' accounts which are presently available for eacchange. -
Thus, in exemplary embodiments of the present invention, an insurance company
could,
by setting up and maintaining such a healthcare credit exchange, retain. more
customers '
as well as encourage customers to join its ranks of insureds early on in their
lives so as to
be able to bank for the future and/or sell credits for being healthy. By
acting as
intermediary, an exemplary system can make a market for such health care
credits, and
not have to wait fo'r a'particular debit holder to fiind a particular oredit
holder willing to=
exchange. Acting in some ways as a securities market maker, an Immunoscore
based
third party can'buy credits and sell debits.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
251
Thus, in exemplary embodiments of the present invention, insureds can thus be
induced
to pay higher premiums when they are younger and more healthy which would
therefore
give them extra protection against being assessed debits later on should they
become sick.
This result's in a net flow of capital to the health insurers, or the HMOs,
because they can
charge higher premiums than the "true" or correct "premium" with the full
consent of the
insured in exchange for allowing and facilitating participation in the health
care credits
exchange. On the other hand, they can also retain more customers because
people who
are subject to debits as a result of more granular analyses of their overall
health via
Immunoscore diagnostics can simply use credits they have accumulated earlier
in their
lives or procure credits from other insureds which would ultimately be cheaper
for them.
than having to find substandard coverage. Additionally, the insurance company
is not
faced with canceling bad insureds and then having to spend client development
money to
procure new "good" insureds, rather, it can more or less retain its insured
base as well as
generate additional profits from the maintenance of the healthcare credit
exchange.
Further, if a healthcare management entity sets up a health care credit
exchange it can, in
exemplary embodiments of the present invention, require immunoscore
diagnostics, such.
as set forth in Section 1 above, at various significant life points in each
insureds lifetime.
This can have the effect of positive feedback in the amount of data that an
immunoscore
database has available and thus, an improvement and greater accuracy and
predictive
value of the algorithms of the immunoscore analysis can provide to the
insurer. = Over the
course of the time an insurer will tend to make more money and have more
accurate
predictive models than its competitors who do not use such an Irninunoscore
system.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
252
Finally, ImmunoScore databases lend themselves to storing health care credit
and debit
information as part of an individual's record, making it nearly seamless to
create
algorithms=to track such credits/debits and manage the exchange. After all,
. ImmunoScore. is the tool being used to generate the very'granularity that
assigns the =
credits/debits and makes the entire business possible.
C. = VETERANS HEALTH CARE MANAGEMENT (VARIANT OF HEALTH
CARE)
A special instance of health care management relates to, veteran's care. In
the United
States, the Veterans Health Administration (VHA) provides a-broad spectrum..of
inedical;
surgical, and rehabilitative care to its customers. Individuals that qualify
for veterans
healthcare services include, for example; returning Active Duty, National
Guard and
Reserve service members of Operation= Enduring Freedom (OEF) and Operation
Iraqi
Freedom (OIF). The vision statement of the VHA, states that it needs to be a
comprehensive, integrated healthcare. system that provides excellence in
health care
value, excellence in service as defiined by its customers, and excellence in
education and
research, and needs to be an organization characterized, by exceptional
accountability and
by being an employer of choice.
In exemplary embodiments of the present invention, veterans, with their
special
requirements based on service, can be well served by ImmunoScore diagnostics
and data
management. As previously described in Section=I, soldiers have very=specific
vaccination requirements.based on their deployment and area of eXpertise.
ImmunoScore
diagnostic panels can be tailored to the needs and context of'the individual
soldier based
upon-his or her previous exposure to immunization and also to different
irifectious agents
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
253
depending on the relevant theater of deployment. In addition to immune
r.esponse to
infectious agents,. veterans are likely candidates for measurement of immune
system
perturbations induced by, for example, Post Traumatic Stress Disorder (PTSID);
exposure
to unique chemical agents (e:g:, Agent Orange), Gulf War Syndrome,
and"recovery from
injuries sustained in service.
As described above in connection with the CIP database, linear regression
analysis of a
patient database could yield valuable infonnation pertinent to appropriate
treatment of
veterans after their years of service: Those analyses displayed possible
correlations
between, for example, measles and mumps. immunity and immunity to varicella
infecti6n,
for example. Any possible associatioris between service locale and. adverse
agents could
be documented and analyzed by an exemplary ImmunoScore data mining process in
similar fashion.
'The VA Research and Development program (The Office of Research and
Development)
aspires to lead the Veterans Health Administration in providing unequaled
health care
value to veterans. = The ImmunoScore technology can help contain healthcare
costs for
veterans by monitoring and analyzing=immunologic inforxnation.
D. SOCIALIZED MEDICINE MANAGEMENT
A socialized medicine jurisdiction is essentially a health care providei or
insurer for 'an
entire population: Thus, the health care management applications of
ImmunoScore -.
described above can also be implemented in a socialized medicine jurisdiction.
Countries
with socialized medicine, such as the UK, New Zealand, and particularly
Canada, present
opportunities to stress preventive medicine for the good of the populace
(i.e., by
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
254
maximizing QOL for a given health care budget) and the advantages of lower
cost
healthcare as represented by ImmunoScore managed healthcare. These governments
could be provided with healthcare management services via an implementation of
the
InununoScore system.
The CIP database discussed in Section II above, has revealed the utility of
ari exemplary
ImmunoScore database for a country with an immigrant population.: There has
been
much concern regarding outbreaks of mumps in the United States and Europe.
This
disease has clearly been 'shown to spread from contact with travelers (CDC,
2006). The
CIP database indicates a degree of relatedness between patients that had
antibodies to
=both Rubella and Mumps. If this type of analysis were to be.extended to
geographic .
regions and associated with specific genders, a government that supported
socialized
medicine could, for example, be very much in favor of assuring that an
immigrant
population was properly immunized, for the protection of that immigrant
population, as
welI as the native population.
E. SUPPLEMENTAL INSURANCE (AFLAC MODEL)
AFLAC is the leading provider of supplemental insurance, which provides help
with
expenses not covered by an individual's major medical plan. The company is the
number
one provider of guaranteed-renewable insurance in the United States and Japan.
Its
products provide protection to more than 40 million people and go beyond the
traditional
insurance by directly paying claimants with cash benefits.
With the cost of health care rising, the challenge for most employers is to
satisfy the
specialized needs of each employee without having to fund expensive new plans.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
255
AFLAC provides products including, for example, the following: Accideint
Disability;
Short Term; Disability; Cancer Benefit; Hospital Indemnity.
ImmunoScore diagnostic testing and database storage can provide information
for use iri
just such supplemental insurance programs. ImmunoScore cain, for example,,
provide an
individual with immune status testing that could be monitored over time and
'offer the.
peace of mind that would come.from knowing that that patient had a "healthy"
iminune
system. In addition, an insurer would be better able to underwrite premiums
for
supplemental health insurance with a sounder understanding of the patient's
health status.
Additionally, in exemplary embodiments of.the preseint invention, a
"immunological
insurarice plan" could be offered. Such a plan could provide all
imrriunological
monitoring and therapeutics to each insured for a fixed annual premium and
=guarantee a
certain defined quality of life to each insured. Such a plan could utilize one
or more of
the health care management processes described above.
To be able to effectively underwrite such supplemental insurance, supplemental
insurance finns need to be aware of relatedness between immune parameters as
revealed
by database analyses. For instance, the CIP database revealed tendencies 'for
Hepatitis A
antibody to be present in individuals from 'certain geographic regions.
Supplemental -
insurance coverage could benefit from insuring that travelers to these
=regians were
assured of their own immune system's ability to combat Hepatitis A infections
in regions
where the disease is'endenuc. Or, for example, the CIP databme. revealed a
possible
suspension of Tetanus immunity amongst individuals reactive to CMV. In
exemplary
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
256
embodiments of the present invention, a health insurer (whether supplemental
or primary)
would take special care to take such a factor into account:
F. tMMUNOSCORE AND THE WELLNESS INDUSTRY
In 1994, the U.S. Congress laid the groundwork for the Wellness Industry by
passing the
Dietary Supplement Health and Education Act (DSIiEA). This Act set new
standards'for
the manufacturing, testing and marketing of nutritional products. Products
that. meet
strict government standards earn the title of nutraceuticals. Blurring the.
line between
conventional foods and drugs, nutraceuticals are defined as foods or parts of
food that
confer health or medicinal value, including.the prevention and treatment of
disease.
The Food Policy Institute (http://www.foodpolicyinstitute.org) has defined
drivers of
nutraceutical industry growth. The nutraceutical market was once viewed as
largely a
counter-culture "back to nature" phenomenon, but is now buoyed by a-n.u.rnber
of solid
fundamentals.
Changing consumer demographics. Americans are living longer and emphasizing
the
importance of quality of life in their later years. As the baby boomers
approach ages
where personal health becomes more paramount, the demand for mechanisms for
conveying health will grow.
Increasing ethnic diversrfication. The mainstream U.S. nutraceuticals industry
is a
relatively new phenoinenon. However, the use of foods, herbals, and other
natural
products to convey health and medicinal values has a long history of
acceptance by many .
of the world's cultures.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
257
Paradigm shift in personal health. Americans are taking more responsibility
for their
.personal health, embracing the concept of health maintenance and wellness.
Thus, the
paradigm is shifting away from disease'treatment and towards disease
prevention.
Dissatisfaction with Western healthcare. Americans are becoming more reticent
about
accepting the side effects of synthetic drugs and remedies. Similarly, rising
healthcare
costs are encburaging Americans to explore alternatives to traditional
orthodox medicine.' Increasing acceptance of alternative healthcare practices.
There 'is a growing. acceptance
among Americans of alternative or cornplementary therapies and wellness
modalities.
Recent years have witnessed increased use, for example, of chiropractic care,
vitamin
therapy, aromatherapy, meditation and relaxation techniques, and-aciupuncture.
Increased uriderstanding and awareness of diet-disease, relationships. Many of
the
leading causes of premature death iri the U.S..are diet-related. Exarnples
include heart
disease, diabetes, and many types of cancer. The USDA estimates that diet-
related
disease and death costs the U.S. in excess of $250-billion each year.
The Food Policy Institute goes on to state that the nutraceutical industry is
facing
'challenges.
= Few farmers are producing herbals and other botanical inputs (due to
limited market knowledge, technical requirements and other obstacles.
= Limited access to finance and capital constrains industry development and
expansion.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
258
= Ambiguous regulatory framework for ensuring product standardization
and efficacy.
= Regtilatory restrictions on marketing products via health claims impede=
retail efforts. ..
= Raw material supply issues (consistency of quality and availability) for
botanical manufacturers.
= Limited endorsement by traditional healthcare practitioners.
= Consumers can not differentiate between high and low quality pioducts
and are not sufficiently educated to make informed decisions about proper
product use.
IniinunoScore diagnoses and database could provide the answers to these
challenges.
Individuals and populations could be studied with respect to the-e -fficacy.
of a
nutraceutical diet. ImmunoScore would either pave the way for more -growth in
curtain
nutraceuticals, or perhaps point out the sale of "snake oil." Individual
products, or
product lines could be endorsed as valid by ImmunoScore measurements.
The Wellness Industry is expected to grow. The Wellness Industry includes the -
coiicept
of `wellness insurance" to lower health care costs to individuals. This may
provide yet
another opportunity to leverage ImmunoScore testing and data storage into the
insurance
industry.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
259
In addition, workplace wellness as a concept has been used extensively in
recent years by
management in business and industry, health professionals, fitness experts,
and others.
Well-designed and administered programs deliver positive outcomes for
employers as
well as employees. Because'healthy employees cost less than
employees'suffering from
illness, ImmunoScore can be a part of employee insurance offered by employers
wanting
the best and most affordable health care for their employees.
Analyses of the CIP database have shown the development of ppsitive and
negative
relationships for one variable with respect to another variable (for example,
Rubella
antibody and Hepatitis A antibody levels as' is illustrated in Fig. 20D, and
for example,'
Mumps, antibody vis-a-vis Hep A, Measles and Rubells, as shown in Fig. 20E).
This
type of analysis could be extended to other variables regarding " weliness."
For example,
fitness measurements could be incorporated (body mass- index, cardiac
function, etc) into
an overall immune fitness relationship.
Virtual PhysicalsTM - Incorporate ImmunoScore Diagnostic and Database
The Virtual PhysicalTM is a comprehensive diagnostic screening procedure that
uses state-
of-the-art technology to take a global look at a patient's body and identify a
variety of
conditions at early stages where intervention can be most helpful. A Virtual
Physicalr'" niay also be viewed as an integral component of a holistic,
behavioral medicine program,
where the body, and one's diet, exercise, and lifestyle habits=are viewed as a
whole,- -
determining where problems may exist and where changes might be required.
The Virtual Physical'sTM early detection capability can uncover asymptomatic
and often
life-threatening diseases generally not detectable by physical exam or
standard screening
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
260
tests. This allows the management of disease in early stages; where medical
therapy and
treatment options.are typically less costly, less invasive and more effective.
Virtual Physical'sTM comprehensive scan of an iindividual's body is
significantly more
detailed than an X-ray. It covers: (a) the heart and arteries, identifying
near microscopic
amounts. of plaque; (b) the lungs at the air cell level showirig the earliest
stages of smoke
damage, emphysema, or lung cancer; (c) the spine, evaluating for osteoporosis;-
disc
disease and other back problems; (d) internal organs for detection of tumors,
stones =and
cysts of all sizes; (e) aneurysms in the abdominal and chest cavities; (f)
thyroid and
parathyroid disease; (g) joint disease; and (li) uterine, ovarian, and
prostate disease.
In the interest of determining a patient's "totality of health," ImnnunoScore
screening
could accompany a Virtual PhysicalTM=to add an immune health component to the
virtual
screening. It is possible that insurance will cover a Virtual. PhysicalTM in
the, future, and
ImmunoScore testing and data storage could be incorporated into the patierit's
records
that could be transferred to the pat'ient's primary care physician or
specialist.:
G. WOMEN OF CHILDBEARING AGE/SCREENING OF PREGNANT
WOMEN
A superpanel for women of childbearing age was described above in Section I.
With light thereof, ImmunoScore diagnostic tests and database storage
availability in the
offices of obstetricians would greatly enable appiopriate immunization of
pregnant
women as well as find correiates of prenatal interest. In addition to
screening pregnant
women for their immune status regarding vaccine preventable diseases,
ImmunoScore
diagnoses and data management could also be of value in determining the immune
status
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
261
of pregnant women regarding, for example, group B streptococcal infection,
cytomegalovirus (CMV) infection, and other infectious diseases that may
adversely effect
the newborn, yet are treatable prenatally. Early onset GBS infection has been
the leading
cause of death attributable to infection in newborn infants for over three
decades, with
over 6,000 cases a year in the United States (Vallejo, et al. 1994).
Antibiotics have been
used to good effect to prevent newborn GBS infection. There is also promising
preliminary data on an effective intervention to prevent CMV infection in
newborns in
pregnant women that has been published recently (Nigro, et al. 2005). All
these
treatments can be more advantageously administered using ImmunoScore
technology.
Fig. 28. depicts and exemplary process flow for managing the immune status of
women
of child-bearing age. Beginning at 2801 the immune status of a women of child-
bearing
ag,e is examined. At 2810 the vaccine preventable diseases that the wman is
susceptible
to are identified as well as the woman CMV infection status and pregnancy
status. At
2820 these three variables are used to generate healthcare recommendations,.
as follows.
If the woman has not been infected with CMV and is not pregnant she is advised
to
obtain immunizations for the identified vaccine preventable diseases. If she
is an insured
under a healthcare insurance plan, or her healthcare is provided by an HMV or
socialized
medicine entity she can be, for exaniple, required to obtairi these
immunizations to save
future treatment costs as well as to serve the utility of having a healthy
population. If 'she
has not been infected with CMV but is pregnant she can be informed of extra
precautions
regarding CMV status and pregnancy. Moreover, no immunization with attenuated
vaccines is recommended or should be performed, however, other immunizations
should
be recommended based upon current CDC guidelines. If the woman is seropositive
to
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
262
CMV and not pregnant she can be advised oirequired, as the case may be, to
obtaiin
immunizations for the identified vaccine preventable disease. Finally, if she
seropositive
for CMV and pregnant no extra precautions should be taken regarding the CMV
status
unless there is an active prirriary infection. Moreover, no attenuated vaccine
'should be= .
recommended or administered. However, other immunizations can be recommended
or
required based upon current CDC guidelines. At 2830 a=follow-up -examination
of the
women's immune status post-vaccination can be conducted, and if she is not
pregnant the
information can simply be stored in a system database. If she is pregnant a
post-natal
follow-up can be recommended or required, as the case may be, comprising
IviIvIR
vaccination to the mother and monitoring of CMV status of the child. Firially,
at 2840;. .-
based.upon the post-vaccination'follow-up at 2830 the efficacy of the
administered =
vaccines can be evaluated as to -whether they.provide the necessary immunity
to- the'.
identified vaccine preventable diseases identified at 281-0. .. '
The CIP clearly points out the need for antibody ineasurements in women of
child-
bearing years. The obv'ious antibody to be examined is that for Rubella, in
which.the
women of SE Asia were shown to have levels below average. Other important
antibodies
in'wornen of child bearing years are, of course, those to group B
Streptococcal or=ganisms
and others that.effect fetal development or those associated with neonatal
illnesses.' From
an insurance and public health perspective, these are extremely
important'issues.
H. VACC/NEEO-MATIVACC%NE DISTRIBUTION NETWORK
In exemplary embodiments of the present invent ion, ImmunoScore technologies
can be
used to facilitate the easy dispensing of vaccines to the public as well as
giving the public
access to their immunologic information.'.Therefore, in exemplary embodiments
of the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
263
present invention a business analogous to the "Fotomat" photograph fiiiisliing
stores,
once located in malls and strip malls across America, can be created. For
purposes of the
present description, this exemplary embodiment of the present invention can be
called
"Vaccine-o-Mat". Vaccine-o-Mats can be located in small buildings in corners
of malls
and strip malls, as concessions in large chain stores such as Target or Wa1-
Mart, or in
appropriate markets they can be located almost anywhere and oine day be as
ubiquitous as
Starbucks Coffee centers. At a Vaccine-o-Mat a member of the public can have
his'
immune status checked and can receive any vaccines that he may be deficient
in. -If an
individual steps on a rusty nail and doesn't remember the last time.he had a
tetanus
booster he can- simply drive to the nearest Vaccine-o-Mat,'have a panel of
assays
containing tetanus and any related compliments as conducted and determine then
and
there whether he needs a vaccine. What makes the Vaccine-o-Mat business
possible is
instruments which can process large numbers of assays in a relatively =short
period of
time, as noted above. One of such instruments is the M1M analyzer currently
marketed
by BioVeris Corporation of Gaithersburg, Maryland, the assignee hereof.
Fig. 29 depicts an exemplary process flow for use at a Vaccine-o-Mat. At 2901,
the
customer's immune status is examined for vaccine preventable diseases and
related
immunologic information. It is furtlier contemplated that a particular
custorner may want
to have his bodily fluids assayed for a wide variety of immunologic tests and
not have:
them restricted to vaccine preventable diseases. Therefore 2901 need not to be
strictly
directed towards vaccine preventable diseases. At 2910, within 90 minutes the
assay
results can be processed to generate recommendations for appropriate vaccines.
This
functionality depends upon, as noted above, instruments which can process a
large
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
264
nurriber of assays in a relatively short arnount.of time. This concept allows -
for partnering -
with large chain stores. or malls where customers could make their first stop
at the
Vaccine-o-Mat have their blood tested. They could then continue shopping and
then
return at the end of their shopping excursion to receive any necessary
vaccines and report
regarding their immune status. At 2920'appropriate.vaccines can be
administered'to the
customer on site, and at.2930.the customer can be provided with a printout of
the assay .
results the updated vaccination record and his or her database record from the
ImmunoScore database along with in.structions. on how to access that
information in-the
future. Finally, at 2940 all of the additionally required customer
informatiorr resulting =
from that particiular visit is stored. in the database for future reference.
..One of the benefits of the ImmunoScore technology is the ability to link
diagnostic
testing of the inunune system with rapid delivery of inedication at the point
of care
(ideally, during the course of an office visit): ' Thus;`in eXemplary
embodiments of the
present invention a vaccine distribution rietwork can be set up, for example,
to link
vaccine manufacturers to physicians offices - or other authorized vaccine
dispensing
personnel -- equipped- with diagnostic facilities. Vaccine distribution can
also, for
example, become part of the ImmunoScore database tracking specific
manufacturer's lot
numbers to points of sale. This can be importa.rit in getting timely
information
incorporated into the Vaccine Adverse Event Reporting System (VAERS). `.
Fig. 29A depicts exemplary envisioned interactions between various parties
according to
an exemplary embodiment of the present invention directed towards vaccine
distribution. '.
Information =gathered to an exemplary ImmunoScore database can, for example;
be
shared with the various agencies responsible for dictating vaccination
decisio.ris.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
265
Unsiuspected or unknown relationships regarding inuriune health or. function
can be, for
. . r . = . . '
example, "fished". or "mined" from a system database, using appropriate
queries and
analysis.. In addition, in exemplary embodiinents of the present invention,
suspected
adverse =events from vaccination could be addressed and'acknowledged
or*dismissed,.
based upon information gleaned from the system database.
With reference to Fig. 29A, various entities and institutions which can,.for
example, be
involved in vaccine'distribution or vaccine distribution network are
depicted.= They=
include any vaccine manufacturers 29A05 who through vaccine sales provide
vaccines to
physicians or healthcare providers 29A10. =The physicians or healthcare
providers 29A10
also receive diagnostic testing kits and research services, such as, for
example,
ImmunoScore vaccine diagnostic panels 29A01. The government 29A15 has a
variety of
roles in a vaccine distributioii network, including subsidizing or providing
economic
incentives to create or build a supply of vaccines by a transfer of furids to,
or via tax
incentives to, vaccine manufacturers 29A05. The government can further
subsidize or
fund HMOs 29A25 and in this context the Veteran's Administration, described
above=can
be considered.one of them. Additionally, the govenunent 29A15 can mandate
vaccine
benefits to certain.segments of the population and those can be provided by
HMO- 29A25=
or equivalent. Finally, the government 29A15 can itself access personalized
immune
statu=s data as to individuals or populations or sub-populations 29AI2 for a
variety of
research or health management purposes. The CDC and ACIP 29A50 can receive
input
from Physicians/Healthcare Providers 29A10'as=well as from a vaccine status
database
29A30. Vaccine status database 29A30 can be generated from an Immunization
Registry
29A40 set up by the CDC, ACIP or other, similar institutions or bodies to
maintain
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
266
immunization records for the population so'as to better know who should be
vaccinated.
.Figs. 29B and 29C, described below illustrate improving connectivity between
entities
and organizations who could access and utilize ImmunoScore information in this
context,
allowing the benefits of InununoScore to be ubiquitously available.
l. CONSUMER ACCESSIBILITY TO IMMUNOLOGIC INFORMATION
Americans are playing a risky game of sexual roulette, according to a new poll
that found .
only 39 percent of respondents alway's ask a new lover if they are infected
with HIV. The
poll, taken by Zogby for MSNBC.com also found that 73 percent of respondents
were
involved in a monogamous relationship, 66 percent of those surveyed had had
-unprotected sex while under the influence of alcohol. While.39 percent of
respondents
said they always asked whether a new partner is infected with HIV or other
sexually
transmitted diseases, 31 percent said they never discuss the touchy issue with
a new
partner. Moreover, the survey found that 15 percent of Americans had paid for
sex, 35
percent of respondents said they had been with between one and five sexual
partners, and
19 percent said they had had more than 25 partners.
In exemplary embodiments of the present invention this "risky business" can be
ameliorated. Accordingly, at the Vaccine-o-Mat described above, =individuals
can have
their immune status tested by conducting, for example, an STD assay panel, as
described
in Section I above, which can then be shown to potential sexual partners to
fully disclose
the immunologic risks that may be involved in any proposed liason. For
example, a
couple can stop at a Vaccine-o-Mat near a romantic restaurant of their-choice.
They can
have the assays conducted and go off to dine. If things are =going well, by
the time their
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
267
coffee has. arrived they can obtain each otlier's immune status and be off ~
either alone or
together -depending upon the ImmunoScore results. .
Alternatively, for example, someone worried by past promiscuities can
routinely procure
his or her immune status at the local Vaccine-o-Mat in 90 minutes, and put any
worries tb
rest, or at= least know what they are facing. J. ,. IMMUNOSCORE CONNECTIVITY
VIA INTERAPPLICATION .
TRANSLATOR/DATA INTEGRATOR
In many exemplary embodiments according to the present invention, the power of
an ImrnunoScore diagnosis and database lies in the interaction of the database
with many different organizations, as shown in Fig. 29B.. Use of a web
services interconnector,to
provide this connectivity is illustrated iu'Fig. 29C, next described. The CDC,
the =
government '(or governments, for that matter), health-maintenance,
organizations, vaccine.
ma.nufacturers, and physicians would all be able to interact with the database
and each .
other to make the best possible decisions regarding the health and welfare of
the
citizenry.
With reference to Figs. 29B and 29C, a number of entities and organizations
who could
access and iitilize ImmunScore information are showin. Fig. 29B shows a
complicated
information exchange structure wherein each entity involved has to set up a
separate .
communications line or pathway to each of the other entities in the network.
This can'
easily be remedied, as shown in Fig. 29C, by utilization of an
Interapplication
Connectivity Provider 29C50 which cari interconnect the various individual and
.sometimes proprietary computer systems, computer networks, databases, and
applications
of each of the individual entities participating in the vaccine
distribution/creation network
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
268
so that they- can talk to each other. This technology is often referred to as
interappl'ication
connectivity or interapplication translation. One example of such a
iriterapplication
connectivity provider is the.IBM, in particular the IBM Web Services Centers
Of -
.Excellence. Additionally, Eriterprise Computing service'companies, such asjor
example, EDS also provide products which link different and disparate
computing
platforms so that they can exchange data and information in an efficient
manner.
The CIP database has only scratched the surface of what can be captured and
shared by a
large ImmunoScore database, but important information can be =gleaned from
this
database, such as it is, of use to government'sources, patients, physicians,
and insurers.
Demographic information regarding crowding and sanitary facilities have been
shown to
correlate to degrees of protection to vaccine-preventable diseases in the
populations examined. If the database were to also include information
regarding themovement of
patients (for instance), much useful information could be shared among these
concerned
groups. .
K. IMMUNOLOGIC INFORMAT/CS BASED LIFE INSURANCE
UNDERWRITING
In the exemplary embodiments of the present invention ImmunoScore data can be
used to
optimize the underwriting of life insurance. Additionally, assuming that
regulatory=
restrictions are not preclusive, IinrnunoScore data can be used by companies
which
provide both life and health insurance to the same clientele. =The use of
IminunoScore
technology for these purposes is depicted in the exemplary process flow chart
of Fig.-30.
With reference to Fig. 30, at 3001 an individual's immune status can be
examined and
any diseases to which he or she is susceptible identified. At 3015, by
accessing Business
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
269
Rules Database 3010, the probability of death of the individual =given the
immune status
identified at 3001 can be computed. At 3016 the cost of insuring that
individual, based
on the probability of.death of years to death calculated iri at 3015 can be
computed and
premiums can be set at 3020. It is noted that the term "death" appearing in
Fig. 30 is a
shorthand for "years remaining until death." =
Additionally, at 3002=all combinations of possible prophylactic therapies
canbe
generated given the immune status obtained at 3001. From these combinations,
at 3005,.
the probability of time (generally in years) to death given the -immune status
and the
various combinations of prophylactic therapies can be computed. Such
computation, at.
3005; exchanges data with Business Rule Database 3010. Tor convenience, two
Business
Rules Databases 3010 are depicted n Fig. 30; in exemplary embodiments of the
present
invention there could be one or many Business Rules Databases each devoted to
a
specific informationaY domain. In the depicted exezriplary embodiment of Fig.
=30 they
could most likely be combined inasmuch as they are providing iriformation
which allows
a system to compute the probable time to death given an irnmune status:
However, the,
Business Rules Database on the right side of the figure may require more
complex
information to also factor in the available set of possible
preventive=therapies for each
=identified disease. =
At 3016, the outputs of 3015 and 3005 are input to allow the exemplarysystem
to
compute the cost of insuring the given individual.. At 3021 the system can
select the two
or three best sets of prophylactic therapies from the inforrniation generated
at 3002, and at,
3025 it can offer these prophylactic therapies to the client with a proviso
that the life
...n w.,....o ...=omi..rt. o.at ~+ zn~n ir. o{~carnca rif =Far,'FArITA' in
YlTn7l'hVIAf:t1C. tbP.r'.iT11P.C Y:MIld hP. =
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
270
lower by (x) if the client chooses to undertake the prophylactic'therapies.
Alternatively,.
at 3030 =it may be in an insurance company's interest to pay for the
prophylactic
therapies, i.e., offering them to the insured for free, if the cost of the
prophylactic
therapies is less than'thepresent value of the expected savings to the Iife-
insuiance
companies by the insured having the prophylactic therapies perform. This can
be
expressed, for example, as:
PT cost < PV{(death benefit)*[(Prob (deathi no IS, no PT) - Peob (deathl IS,,
PT)]}
Thus, if at 3030 such an offer is made, any premium adjustment at 3020 can be
diminished or completely reduced. The function of 3030 is to iincrease the
profits to the
life insurance company by not only identifying the premiurn at which it would
charge the
insured but also, based on the immune status data obtained during the
underwriting
process (or during an annual audit process), to identify prophylactic
treatments that could =
be offered to increase the time to death for the =same individual thus
allowing the
insurance company to continue to earn the return on the cumulative premiums
prior to.
having to pay the death benefit to the survivors. =
It is also noted that at 3021 where the 2-3 best sets of prophylactic
therapies are found the
term best is really a= function of how much the probable time to death is
increased. .
Finally, the availability of probable time to death given a certain immune
status and
certain prophylactic therapy can be computed'using the following equation as
noted in
Fig. 30: Prob (deathlIS and PT) _
P(CDIPT and IS) * P(DICD and IS) +
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
271
P(not CDIPT and IS) * P(Djnot CD and PT IS)
When offering prophylactic therapies to an insured, unique opportunities arise
for
insurance companies providing both life and health. A healthier insured l'ives
longer and
uses less health care, resulting in twofold savings for an insurer.. Because
such a life
. . . ~
insurance company also approves health care expenditures, there is no red tape
or
customer effort spent on securing approval for any offered or recommended
prophylactic
therapies. Thus, in such contexts, the real world optimizations can actually
converge on
the theoretical optimizations calculated by an ImmunoScore analysis as
depicted in Fig.
30. This can, in exemplary embodiments, increase QOL for insureds and profits
for the insurers, as well as hopefully.
Patient commonalities, as revealed by analyses of the CIP data.base, could be
visualized.
For example, if a population immigrating from Eastern Europe were shown, in
general, to
have lower protection against a specific disease or diseases, that information
could, for
example, be of interest to health/life insurance companies.
L; DIAGNOSING AND MANAGING IMMUNOSENESCENCE /N THE
ELDERLY
Human aging is associated with progressive decline in immune functions and
increased
frequency. of infections. Morbidity and mortality due to infectious disease is
=greater in
the elderly than in the young, at least partly because of age-associated
decreased immune
competence, which renders individuals more susceptible to pathogens (Pawelec,
et al.
2005). A decline in immune function is a hallmark of aging that affects the
ability to
resist influenza and respond to. vaccination. An accumulation of dysfunctional
T cells
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
272
may be detrimental under conditions of chronic antigenic stress (chronic-
infection, ',
cancer, autoimmunity).. The most important changes occur in T-cell immunity,
and are
manifested particularly as altered clonal expans.ion of cells of limited
antigen specificity
(Fulop, et al. 2005). This is most marked in the CD8+ T=cell subset, which
displays a.
decrease in, both responsiveness and n'ormal function. Normally, CDB+ T oells
appear to '
be 'strongly associated with cytolytic 'activity, either.by direct killing of
antigen-bearing
target.cells by granule-mediated exocytosis or Fas-mediated'cytotoxic
mechanisms.. In
addition, it is suggested tliat antigen-activated CD8+ T lymphocytes can
eliminate or
control viral infection by secretion of antiviral cytokines; sucli as =g'amma
interferon (IFiN;
y) and tumor necrosis factor alpha (TNF-a). IFN-y production by -CD8+ =T cells
can have .=
both local and systemic consequences, whereas cytotoxins such as perforin are
cytolytic.-
for the cells that come in direct contact with the cytolytic T lymphocytes
(CTL):
The output of the T cell pool is governed by output from the thymus' and not
by .
replication (Aspinall and Andrew, 2000). As thymic T cell production
dirninishes with .
age, -a decline in contribution made by. thymic emigrants to the naive T cell
pool occurs
(Mackall, et al. 1995). Diminution in tlie size of the naive T cell pool is a
common
finding with aging, and is a.consequence of reduced thymic output (Kurashima,
et =al.
1995). Thymic atrophy is thought to.result from a failure of the thymic
microenvironment to support tlhymiopoiesis in old age and recent evidence
suggests that a
decline in interleukin-7 (IL-7) expression may limit thymocyte development by
restricting combinations of survival, proliferation and rearrangement of the
beta chairn of =
the T cell receptor (Andrew and .Aspinall', 2002). Therapeutic intervention
with IL-7 and
derivatives has been shown to reverse thymic atrophy in old animals and also
lead to
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
273
improved immune function compared with age and sex matched control animals .
(Aspinall, 2005).
The CD8+ T cell repertoire becomes less diverse in old age due to reduced
thymic output
and the accumulation of clonally expanded memory CD8+ T cells as a consequence
of
prolonged antigenic stimulation. Clonally expanded T cells are usually CD84-
and show
an increased'incidence with age, so far it seems that clonal expansion is not
due to
malignancy but may follow antigen stimulation (Aspinall, 2005). It has been
suggested.
that repeated or persistent infections with viruses such as influenza,
cytomegalovirus
(CMV), and Epstein-Barr virus (EBV) may drive responses that result in large T
cell
=clones. Longitudinal studies=suggest that a set of immune parameters
including highpercentages of peripheral CDB+ CD28- 'CD57+ T cells, Iow CD4+
and B cell counts, and
poor T cell proliferative responses to mitogens is associated with,decreased
remaining
longevity in the free-living very elderly (>85 years) (Ouyang, et al. 2003).
CMV
seropositivity is closely associated with increases in the size of the CD57+
CDB+ T cell
pool, which is thought to represent a highly differentiated population of late
inemory
cells. Furthermore, CMV seropositivity is associated with increases in CD8
count in old
age and has been documented to have negative influences on immune parameters
in the
very elderly. A group concluded that the "obsession" of a large fraction of
the entire CD8t T cell subset with one single viral epitope may contribute to
the increased incidence of infectious disease in the elderly by shrinking the
T cell repertoire for
responses to other antigeris (Ouyang, et al. 2003). Like CMV, EBV manages to
persist
for the lifetime of the infected host. During chronic asymptomatic infection
in healthy
individuals, EBV resides in memory T cells (Babcock, et aI. 1998). Expansion
of
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
274
peripheral CD8+ CD28- T cells in response to chronic EBV infection has been
linked to
rheumatoid arthritis (Klatt, et al. 2005).. The clinical consequences of these
changes are
as yet not well defined, exc.ept for their extremely important negative impact
on defense
against infections. Considering the public health consequences of
decreased'immune
competence in old age, strategies for immune response modulation are desirable
to
decrease the health burden for the. elderly and improve their quality of life.
(Fulop, et a1. ,
2005).
Features of successful aging have been associated with well-preserved immune
function.
while poor survival is predicted by high CTL counts, low nuinbers of B cells
'and poor =
responses by T cells to polyclonal stimulation. The .phenomenon of
repli'cative
sensescence lias been associated with these changes and relates to a fnite
number of
doublings (25-30 cycles) after which cell cycle arrest occurs. 'In CTLs, this
growth arrest
is associated with increased production of.several pro-inflammatory cytokines,
resistance
to apoptosis and loss of the co-stimulatory rnolecule, CD28, required for
optimal
stimulation of CTLs. In older adults, greater than 50% of CTLs fail to
express. CD28 and '
these cells are resistant to' apoptosis.
The loss of CD28-eatpressioii due to replicative senescence.has been
associated with a
number of the= adverse effects of aging on inunune function. Although the
frequency of
influenza virus-specific CTLs does not appreciably change with age, the
decline in.CTL=
activity.against influenza may be due to a loss of antigen-specific
proliferation and/or
diminished lytic activity. Noimal loss of CD28 expression during CTL
activation and the
potential for these cells to undergo activation-induced cell death, may be-
confused with
the loss of CD28 with replicative senescence and resistance of CTLs to
anontosis. .
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
275
Furthermore, the role of cytokines (such as IL-2, IL-7, and IL-i5) in
preventing
activation-induced cell. death and age-related changes in the production of
these cytokines
create a complex array of interactions that may.confound the interpretation of
in vitro,
experiments. Understanding=the=complexity will provide an opportunity to
optimize the.
CTL response to vaccination by manipulating C'TLs.that retain their
replicative capacity
in response to appropriate antigenic stimuli. Currently, influenza vaccination
of elderly individuals is recommended worldwide... A
recent study looked retrospectively 'at influenza vaccine efficacy in
individuals aged'65
years or older (Jefferson, et al. 2005). They found that in homes for elderly
iindividuals;
that vaccines were not signif cantly effective against= influenza, influenza-
like illne-ss, or
pneumonia. More encouragingly, vaccine performance was improved for admissions
to
the hospital for influenza or pneumonia, respiratory diseases, and cardiac,
disease
(Jefferson, et al. 2005). This group concluded that the usefulness of
influenza and
pneumococcal vaccines was modest. On -the same day the Jefferson report was
published
online, the American ivledical Directors Association ieleased a special
announc~ement -
regarding the Jefferson study and influenza vaccine recommendations for the
elderly
(http://www.amda.com/newsroom/092205. vaccines.htm). While not
disagreeing=with
the tenets of the study, they continued to recommend for vaccination of the
elderly -
because inf uenza vaccination is effective at preventing severe illness,
secondary
complications, and 'deaths. They also reiterated that the CDC recommends
influenza -
vaccination for people age 65 years -and over and for all persons in long-term
-care
facilities (htW://www.amda.com/newsroom/092205 vaccines.htm). Both groups
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
276
concluded that better influenza vaccines that offer.more protection in older
persons are
desirable and a high priority of influenza researchers.
The threat of pandeinic influenza has increased with the direct transmission
of highly
pathogenic avian H5N1 viruses to humans. Continued reliance in killed virus or
subunit
vaccines will leave adults at significantly higher risk of illness, disability
and death in the
event of an influenza pandemic. Research that increases our understanding of
how
immunosenescence affects the cell-mediated response to influenza and vaccine
responsiveness is critical to the development of effective pandemic influenza
vaccines for
older people. In the absence of influenza vaccines that target these defects,
an influenza
=pandemic will have a significant impact on older people and.quickly overwhelm
the .
health care system.
The CDC has recently (August 8, 2005) stated that the effectiveness of
inactivated
influenza vaccine depends primarily on the age and the immunocompetence of the
vaccine recipient and the degree of similarity between the viruses in the
vaccine and
those in circulation. When the vaccine and circulating viruses are
antigenically similar,
influenza vaccine prevents influenza illness among approximately 70-90% of
healthy
adults aged < 65 years. Children aged > 6 months can develop protective levels
of anti-
influenza antibody against specific influenza viuus. strains after
vaccination, althougl~ the
antibody response among children at high risk for influenza-related
complications might
be lower than among healthy children. In addition, no efficacy was
demonstrated among
chiidren who had received only one dose of influenza vaccine, illustrating the
importance
of administering two doses of vaccine to previously unvaccinated children aged
<9 years.
Older persons and persons with certain chronic diseases might develop lower
post-
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
277
vaccination antibody titers than healthy young adults and thus remain
susceptible to
influenza infection and influenza-related upper respiratory tract illness
(http://www.cdc.&ov/flu/professionals/vaccination/efficacy.htrn). While
current vaccines
are cost-saving, new influenza vaccines will likely be needed to avoid the
crisis
anticipated in health care related to the general aging of the population.
Another component to the aging immune system is the relationship between
"inriate
immunity and inflainmation. During evolution the human was set to live 40 or
"50 years;
today, liowever, the immune system must remain active for a much longer time.
This
very long activity leads to a chronic inflainmation that slowly but inexorably
damages
one or several organs. This is a typical phenomenon linked tci aging and it is
considered
the major risk factor for age-related=chronic diseases. Alzheirner's disease,
atherosclerosis, diabetes, sarcopenia, and cancer to name several; all have an
important
inflammatory component, though disease progression seems also dependent on the
genetic background of iridividuals (Licastro, et al. 2005). Inflammatory -
genotypes are an
important and necessary part of the normal host response to pathogens in
early.life, btit
the overproduction of inflammatory molecules might also cause immune-related
inflammatory diseases and eventual death later (Licastro, et al. 2005).
Most age-related diseases have complex etiology and pathogenic mechanisms. The
clinical diagnosis and therapy of these diseases requires a multidisciplinary
approach
with progressively increased costs. A body of experimental and clinical
evidence suggest
that the'irrimune system is implicated, with a variable degree of importance,
in almost all
age-related or associated diseases. Both innate and'the clonotypic immune
system are
usually involved in the pathogenesis of these chronic diseases (Caruso, et al.
2004;
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
278
Pawelec, et al. 2002). Several functional markers of the immune system may be
used
either as markers of successful aging or conversely as.markers of unsuccessful
aging. A=
combination of high CD8} and low CD4 and poor T cell proliferation has beeii =
associated with higher inortality in very old subjects (Caruso, et al. 2004).
Old men =
carrying an anti-inflammatory IL-10 high-producer genotype or a pro-
inflamniatory IL-2
low-producer genotype show the lowest values of CD8+-cells (Caruso, et al.
2004). Thi's
study, however, did not do a functional assessment of T cells.
In a mouse model looking at T cell subset patterns, researchers found that= a
composite
combination of subset values was a significant predictor of longevity among
genetically
heterogeneous mice, with a strength of association higher in older mice than
among the
young (Miller and Chrisp, 2002). Developing useful biomarkers of aging has
proven to
be remarkable difficult, in'part because many age-sensitive variables tested
as candidate
biomarkers are sensitive to genetic and nongenetic influences other than
aging. Any =
individual assay, for exainple a, test of a specific T cell subset in a single
blood sample, is
likely to hav.e a good deal of uncertainty, but the combination of results
from related tests
may increase the signal-to-noise ratio. and thus provide stronger predictive
power than
any single assay by itself (Miller and Chrisp, 2002). In humans, ImmunoScore
testing
would help build the models of T cell subset patterns. Possible courses of
therapy would
then be ideally tailored to meet the needs of the individual and not.a "best-
guess, one size
fits all" course of treatnient: . =
Clearly, the population aged > 65 years would be better served by ImmunoScore
diagnostics rather than the current state of affairs. A blanket
recornm.endation for an'
influenza or pneumococcal vaccination for the entire elderlv nonulatiori ma.v
nnt he in thP.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
279
best interest of an individual being immunized. IrnmunoScore diagnostic tests
could, for
example, first reveal levels of protective antibody to vaccine-preventable
diseases. Of
particular interest would be antibody levels against inflitenza, pneumococcal
infection,
tetanus, diphtheria, pertussis, hepatitis, varicella, CMV, and EBV. Just as
important as
determination of antibody levels in elderly patient sera, ImmunoScore
diagnostic tests
could reveal the status of cellular components of the immune system. The
proportion of =
naive/committed T and B cells would.be crucial for further recommendations by
the
attending medical staff. As therapeutic interventions are developed for
dealing with
immunosenescence, the ImmunoScore diagnostic information regarding individuals
and
compiled database inforrnation will shed valuable light onto the effects of
treatments on =
the immune system. As the populafion. ages, strategies for immune response
modulation
are desirable to decrease the health burden for the elderly and improve their
quality of
life.
A preliminary immune risk phenotype (IRP) has been developed from longitudinal
studies of the eldeirly (Wikby, et al. 2005). Immune system measurements
consisted of
determinations of T-cell subsets, plasma IL-6, IL-2 responsiveness to
conconavalin A,
and CMV and EBV serology. Regression analyses. indicated that ttie IRP and
cognitive
impairment together predicted 58% of observed deaths. This type of analysis
would be a
valuable adjunct to assessing.insurance premiums.
The following table captures exemplary desirable analytes to. monitor in the
population as
individuals age. A database storing the results of such assays could ensure
that agiven
individual'.s analyte levels could be tracked over time rather than merely
captured as a
snanchnt~
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
280
Alteration Anal te
CD45RO cells *
CD95 cells
CD28 expression
CD152 expression killer cell lectin-like receptor Gl
apoptosis of CD8 cells
apoptosis of CD4 cells
lFN-y production
IL-2 production
telomere lengths
telomerase induction
0 DNA damage .
DNA repair
stress resistance and heat-shock protein expression
Table 1: Alterations in the T-cell compartme'nt'with age
Thus, in exemplary embodiinents.of the present invention an Imrnunosenescence
supperpanel can be defined, comprising the following panels:
Meningococcal Diagnostic Panel; ~ Persistent Immunity Induced by Childhood
Vaccines; and *
Immunosenescence Diagnostic Panel The first two panels are defmed in Sections
IA1 arid IA3 of the Immunologic Information
Patent, and an Immunosenesence panel can, for example, be defmed as follows.
Human aging is associated with progressive decline in immune functions and
increased
'frequency of infections. A decline in immune function is a hallmark of aging
that affects
the ability to resist influenza and respond to vaccination. The most important-
changes
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
2811
occur in T cell immunity: An accumulation of d.ysfunctional T cells may be
detrimental
under conditions of chronic antigenic stress .(chronic infection, cancer,
autoimmunity).
Exemplary Alterations in T-cell compartment to monitor: =
Typical Analyte
Alteration
Increased CD45RO cells
Increased CD95 =cells
Decreased CD 28 ex ression
Increased CD 152 ex ression
Increased Killer cell lectin-like rece tor Gl.,
Decreased A o tosis of CD8 cells
Increased A o tosis of CD4 cells
Decreased IFN- production
Decreased IL-2 roduction
Decreased ' Telomere len
Decreased Telomerase induction
Increased DNA=dama e
Decreased DNA re gair
Decreased = Stress resistance and heat-shock protein
expression Other analytes of particular interest in an inununosenescence assay
panel can, for
example, include:
= Antibody to CMV
= Antibody to EBV
= Antibody to influenza
= Antibody to pneumococcal disease.
= Antibody to pertussis
= Antibody to tetanus
= Antibody to diphtheria
= Plasma levels of IL-6
= Th1/Th2 components as described below:..
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
282
Rece tors C~`y kines ece to
INF-y CCR5 IL-4 CCR3
TNF-a CXCR3 IL-5 CCR4
IL-2 CCR1 IL-6 CCR8
IL-12 IL-10 CRTh2
LL-13
Fig. 31 depicts an exemplary process flow for managing immunosenescent
individuals,
either in a health care provider or a health care'insurer context.
In exemplary embodiments of the present invention immunosenescense in an
individual
can be managed using the process exemplary flow depicted in Fig. 31. With
reference =
thereto,. at 3101. an elderly individual's immune status can be examined. This
can be
accomplished by conducting one or more assay panels as described above in
Section I.
At 3110, the va.ccine preventable diseases that the elderly individual is
susceptible to-can
be identified at the same time the iridividuals CMV infection status together
with other relevant markers of an immune system competence can also be
determined. At 3120 .
vaccine and/or other healthcare recommendations can be made based upon the
immune.
status examined at 3 I 01. Additionally, a separate T cell compartment can be
assessed. =
3130 the individual can be immunized for vaccine preventable disease based
upon his or
her immune system's ability to response to vaccination. Using the ImmunoScore
data,
the individual can be classified as either (1) immunocompetent (2) immuno-
deficient or
(3) somewhere in between immunocompetent or immuno deficient. At 3130 an
immuno-
competent individual can be vaccinated as recominended by current ACIP
recomrnendations. An immuno-deficient individual would need to be managed
using
different measures than routine vaccination. Such measures could include, for
example,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
283
adoptive transfer of a coinpartment of T ob B= cells or extraordinary
hygiene'measur.es..
The individuals who falls somewhere between immuno-competence and inununo-
deficiency need some kind of hybrid health management between standard
vaccination
and immunoadjuvant tlierapies sucli as adoptive transfer of T or B cells
and.extraordinaty
hygiene measures. At 3140, the elderly individual's. immune status.can be
followed-up
post vaccination or post treatment and= these results stored in the system
database. At
3150 this information =can be used to. evaluate the efficacy of the.
vaccinatiori or other
therapies as to their abilities to prov.ide'the necessary immunity to the
identified diseases.
M. FROZEN STORAGE OF NAIVE IMMUNE CELLS (IRP
CONSIDERATIONS)
. ,
As previously described,'the immune risk phenotype (IRP) is an emerging
concept -
predicting mortality based on CIvIV seTopositivity (Pawelec, et al. 2005).
This group
maintained that the manner in which CM'V and the host immune system interact
is critical'
in determining the IRP and is hence predictive of mortality. The consequences,
of IIt.P is
early expression of immunosenescence. Immunosenescence leads to: a) decreased
T- and
B-cell responses to foreign antigen; b) increased responses to self antigens;
c) increased.,
rriorbidity and mortality to infectious disease; and d) decreased response to
vaccine ;
antigens.
Greater elucidation of the IRP and its consequences is to be expected in the
future.
Genetic screening at a very early age could lie predictive of immune health at
a much
more advanced age. The =ImmunoScore diagnostic screen could be perforrned from
a.heel
stick done at birth, and a child's baseline immune status could almost
instantaneously be
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
284
generated. Pre-natal screening tests could also be developed in the.future as
an
immunodiagnostic tool.*
Concerned parents may wish to store their child's cord blood as a source of
hematopoietic progenitor cells that could be -stored (at a cost to the parents
or the
insurer's) for that child for treatment of developing IRP symptoms rriuch
laterin= life.
Umbilical cord blood (UCB) is currently used as a source of these
hematopoietic '=
progenitor cells as an alternative to the bone marrow or peripheral blood for
treatment of
several onco-hematological diseases (Adami, et al. 2005).
On April 18, 2005 the Institute of Medicine (I M) issued a report recommending
that a
new cord-blood coordinating center - similar to the existiiig National Marrow
Donor
Program - be set up to ensure a standardized and interconnected national
system to cost-
effectively store and distribute these cells.
ImmunoScore diagnostics shows the need for storing cord blood.
Another application for InimunoScore diagnostics is to link storage
and.analysis of naive
cells of the immune system (innate or'adaptive), as next d.escribed.-.
T cells currently used for adoptive immunotherapy trials are 'selected for
their capacity to
produce high levels of IFN--y_ and for their ability to efficiently and
specifically lyse .
relevant target cells. (Dudley and Rosenbur=g,' 2003; Yee, et al. 2002).
However, it.was
'found that CD8+ T cells that acquire complete effector piroperties and
exhibit increased
anti-tumor activity in vitro are less effective at triggering tuiiior
regressions arid cures in
vivo (Gattinoni, et al. 2005). Wliile the pragressive acquisition of terminal
efI'ector
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
285
properties is characterized by pronounced in vitro tumor killing, in vivo
T'cell activation,
proliferation, and survival are progressively impaired. These findings suggest
that the
current methodology for selecting T cells for transfer is inadequate
(Gattinoni, et al.
2005). It is clear that new solutions are needed to generate more effective
anti-tumor T
cells for the development of experimental human adoptive transfer-based
therapies.
The indication is that storage of naive T and B cells is important for
individuals who will
become immunocompromised later in life, whether those cells come from that
individual
or from another source. Naive cells would also not necessarily be isolated
from cord
blood, but could also be isolated from bone marrow or peripheral blood. In
addition,
-screening methods can be used to characterize those immune. cells regarding
cell surface
characteristics and cytokine expression. Here too, ImmunoScore can be used to
a distinct
advantage. .
N. VACCINE USE OUTCOME/DES/GN
Currently, what the public considers vaccines are designed as a prophylactic
means to
avoid illness caused by infectious disease. - In practice, agents used to
promote an
immune response as a therapeutic course of action for cancer or immunotherapy
have
also been ternied "vaccines." It is the intent of the ImmunoScore design to
beable to
monitor changes in an individual's immune =system in relation to a
prophylactic or
.. . .
therapeutic vaccine and enable the individual patient and his physician to
make the best
possible decisions regarding the patient's immune system health regarding
prophylactic,
vaccination, therapeutic vaccination, or other therapeutic treatment in
attempt to "shift"
the immune system of that patient. In addition, the ImmunoScore database will
compile
important population data regarding demographics and population genetics.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
286
0. RESEARCH SERVICES
In exemplary embodiments of the present invention ImmunoScore technologies can
be
used to provide research services, such as, for example, -for clinical trials
iri the following
areas:
1. vaccines;
2. ' . transplants;
3. adoptive immunotherapy;
4. population modeling; and.
5. government applications.
P. IMMIGRATION CONSULTING
Testing the immigrant poptilation for vaccine=preventable diseases is another
embodiment of the invention: Governments are very interested in the
irrimunizatiow..
status of individuals and families immigrating into their countries. The
invention can
rapidly provide the results.of assays to governmental authorities for all
required
immunizations. There would be no need to rely on paperwork - a diagnostic
exarnmation
would yield more suitable data regarding immune status. The current
vaccination =
requirements for immigration into the United States are for measles, mumps,
rubella, polio, tetanus, diphtheria, pertussis, influenza, hepatitis B and any
other vaccinations
recommended by the Advisory Committee -for Immunization Practices (ACIP).
Current
.recommendations of the ACIP also include varicella, Haemophilus influenzae
type B,
and pneumococcal vaccines. The current law requires all iiidividuals applying
for status
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
287
as a lawful permanent resident (either by applying for an immigrant visa
abroad or for
adjustment of status in the United States) to establish that they have been
vaccinated.
Nonimmigrant (temporary) visa applicants are not required to comply with'the
vaccination r.equirements as a condition of visa issuance, but musfcomply if
they apply
for adjustment of status at a later date (Immigration and Naturalizatiori
Services, 2001).
One or more 'exemplary ImrnunoScore diagno'stic panels could, for example; be
provided '='
to INS or other immigration authorities as a means'to determine the iniinune
status of
immigrants. In practice, ImmunoScore diagnostic testing can be more cost-
effective than=
a paper record trail and more likely, to be reliable=as an accurate assessment
of immune :
-status of individuals relocatirig to the United States.
Additionally, the Institute of Medicine (IOM) has concluded that the United
States
quarantine system is in need of a strategic overhaul. The IOM reports that the
United
States once had 55 federal quarantitne stations, -but the perception that
microbial threats
had been controlled led to dismantling of most of the system in the 1970s.
However,
nearly 40 new infectious diseases have been identified since 1973, and
bioterrorism has.
become a serious concern. The 25 stations that will make up the expanded
quarantine
station system now receive more than 75 niillion international travelers a
year, according
to IOM reports. The stations screen travelers, refugees, immigrants, animals,
and cargo
for-disease agents shortly before' and during their arrival. However, the
quarantine system
relies on a much broadet network that includes local public health agencies;
hospitals,
customs agents, agricultural inspectors, and others, the IOM -said.
The IOM recommended the following:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
288
The quarantine stations, the CDC, and the DGMQ (called the quarantine core)
should
lead the effort by developing a national strategic plan with unifoim
principles and'
outcomes. The quarantine core should shift its main focus from inspecting
people -and -
cargo at ports to leading the activities of the overall quarantine system.
The.strategic plan
should help participating government agencies and other,groups in the
system:to-
pri oritize activities and focus resources on the greatest risks.
The quarantine core should work with partners in the quarantine network to
define or
redefine each group's roles, authority, and communication channels.
The quarantine system needs enhanced skills; more people, more training, more
space,.
and better use of technology to fulfill its evolving role. An example of
technology cited in
the news release was targeted use of passenger locator cards that could beused
on flights
to and from countries with 'disease outbreaks. The cards would log passenger
seat
numbers and contact information in a scannable format. This could simplify
tracking of
passengers potentially exposed to disease,'such as those who flew to the
United States
from Sierra Leone' in 2004 with a man who later died of Lassa ,fever.
The quarantine core must review its methodology periodically to ensure
stations are in
the best places.and appropriately staffed.
The quarantine core must have plans, capacity, resources, and "clear and
sufficient legal
authority" to respond quickly to surges in activity at one or more ports.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
289
The core must define and fund a research agenda to measure the effectiveness
of its
=procedures. The committee found that many routines at quarantine stations
are. based on
experience and tradit'ion and lack a scientific basis.
.
The.core must use scientifically'sound methods to measure the effectiveness
and quality.
of its operations, including assessing performance of critical functions
throughout the. -
system. It must also: address any shortfalls that come to light.
(http://www.nap.edu/books/030909951X/html).
ImmunoScore technology could be useful at such immigration port of entry
screening
=points. There is a need for global health that can not be understated. The
=cost of fa'ilure'
could be extremely high. There are people moving around the globe and among
the
states with clear health needs, and they are currently moving without the
ability of
government authorities to track them.
Additionally, ImmunoScore technologies can be used to discover links between
immunological phenomena. For example; from the results of Greenway (discussed
above
in Section I regarding the immigrant panel) a possible link between TB
infection'and
HepB prevalence in can be investigated by analyzing sera from an immigrant
population
for both active TB and HepB seropositivity. It is possible that more than one
co-infection
may be found in this manner: For example, in the following study, A high
prevalence of
hepatitis B virus infection among tuberculosis patients with and without HIY
in Rio de
Janeiro, Brazil, Blal CA et al Eur J Clin Microbiol Infect-Dis. 2005
Jan;24(1):41-3, such
a correlation vvas in fact found.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
290
The Blal study sought to=investigate the prevalence and exposure factors
associat=ed with
hepatitis B infection in= tuberculosis patients with and without HIV type 1 co-
infeotion,
the presence of hepatitis B virus serological markers was investigated in a
retrospective
study.. The seroprevalence of hepatitis B virus in patients with
tuberculosis.onlywas=
14.6%, and in tuberculosis patients co-infected with HIV it, increased to 35.8
l0. In
patients with H1V and tuberculosis co-infection, homosexuality constituted the
principat.
exposure factor,. while in tuberculosis patients without HIV, a gradual
increase in
hepatitis B virus seroprevalence was noted along with increasing age. These
results =
demonstrate that hepatitis B infection is highly prevalent in tuberculosis
patients in Brazil
and suggest that a vaccination program for the general population should be
considered in.=
order to prevent further hepatitis B infections.
Q. DISASTER SURV/VORS: IMMUNIZATIONS; REeOVERY, PROGNOSIS
AND TREATMENT
In exemplary embodiments of the present irivention, rapid response services to
disaster
survivors can be provided. -Fig. 32 depicts an exemplary process flow for such
an
application.
At 3201 a disaster survivors immune, status can be examined using one or more
ImmunoScore assay panels as described above in Section I. At 3210 the vaccine
=
preventable diseases to which the survivor is 'susceptible can be identified
and
simultaneously the' cellular component of his= or her immune'system can be
assessed to =
get an immediate post disaster baseline. At 3220 vaccination and healthcare
recommendations can be generated based upon antibody levels to the identified
to the
assay vaccine preventable diseases. At 3230 immunization can be carried out
and at
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
291
3240 follow-up examination of the survivor's imm.une status can be
administered and the
results stored in the system database. Further screening of T ce1T components
of the
inunune system is recommended for all survivors regardless of their
psychological state
. at the time in order to develop data regarding post-traumatic stress
disorder.. Finally, at -
3250 the efficacy of the vaccine and/or therapies can be evaluated as to their
abilit,y to:.
provide necessary immunity to the-identified diseases:
'There are many different possible responses of an individual to an event=
perceived as
potentially life-threatening. It is difficult to predict long-term'xesponses
to trauma based
on the acute response to a traumatic event. -If physiological risk factors are
important in
understanding how psychopathology develops, then InununoScore ineasurements
can
provide invaluable research information- and possibly identify treatments yet
to be
defmed. This could pave the way to personalized medicine. Fig: 33 illustrates
possible
responses to trauma.
With reference thereto, at 3301 'a Disaster Trauma occurs. There are two
pathways
leading from 3301; namely, Normal Respanse Factors 3305 and Pathological
Response
Factors 3303. A Normal Response Factors 3305 pathway from Disaster Trauma 3301
leads to Recovery *at'3310. However, Pathological Response Factors 3303 lead
an
individual from Disaster Trauma 3301 to Post-Traumatic Stress Disorder 3320.
It is the
job of healthcare personnel to put the individual on a Pathway to Recovery
3310. In
exemplary embodiments of the present inveintion ImmunoScore technologies can
be used
to' determitie possible therapies 3315, as well as to track immunological
correlates of
*PTSD to verify diagnosis and evaluate therapeutic efficacies.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
292
In the, immediate aftermath of a traumatic event, most people experience a
combination
-of the following symptoms: (a) difficulty sleeping, (b) difficulty
concentrating; (c)
irritability, (d) nightmares, (e) recurrent thoughts of the trauma, and (f)
distress at the
reminder of the traumatic event. The question in the determination of a
pathological
response,is when does the continuation of these "normal" responses become
pathological,
and have serious effect on the health of the individual's immune system?
There are different possible outcomes of trauma exposure. There is an
increased risk of:
(a) Post-Traumatic Stress Disorder (PTSD), (b) major depression, (c) panic di-
sorder, (d)
geneiralized anxiety disorder, (e) substance abuse, and (f) other somatic
symptoms or
expressions of physical illness including hypertension; asthma, and chronic
pain
syndromes. The differential outcomes may rely on different physiological
parameters.
Pre-existing cognitive factors may or may not be the cause, result, or
correlate of pre-
existing biological alterations, either or both setting the stage for an
extreme response to
the trauma. Clarifying the precise nature and biological correlates of
symptoms that
appear in the immediate aftermath of a trauizia,will assist in developing
models for
potential prophylactic interventions and early treatments. In this regard the
'
ImmunoScore diagnostic panel could initially be used in a research application
to track
immune system markers and relate them to specific conditions. As a system
database
evolves, ImmunoScore panels can, for example, be used as a guide to-
therapeutic
treat.tnent.
Individuals currently at the greatest risk for developing PTSD following
trauma are those
individuals with (a) a family history of psychopathology,. (b) a history of
childhood
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
293
abuse, (c) prior trauma exposure, and (d), the cognitive factors of lower IQ,
female =
gender, an poor social support. There is increased concordance for PTSD in
monozygotic twins compared with dizygotic twins lending support to the
=genetic pre-
=disposition argument of PTSD.
R.. MONITOR ADOPTIVE IMMUNOTHERAPY/TRANSP.LANTS
After adoptive transfer, several events must occur for T cells to cause the
regi=ession of
established tumors. T cells must be activated in vivo through antigen-specific
vaccination. They must then vigorously expand to levels capable of causing
.the.
destruction of significant tumor burdens. Finally, anti-tumor T cells must
survive long
enough to complete the eradication of all tumor cells=(Overwijk, et al. 2003).
It has been
found in an animal model that the progressive differentiation of T cells to a
terminal
differentiated effector stage results in a series of phenotypic and
fixnctional changes that
make them less "fit" to perform these functions.(Gattinoni, et al. 2005).
In patients under consideration for adoptive immunotherapy and/or
transplantation,
history and analyses of exposure to CMV, EBV, West Nile Virus, and viral
hepatitis'in
both the donor and recipient are crucial. ImnaunoScore diagnoses of both the
donor and
recipient would examine the immune history of both individuals.
S. ELECTIVE SURGERY
Many patients opt for elective surgery - plastic surgery, facial plastic
surgery,
derrnatology, cosmetic dentistry, vision, urology, and infertility among
others. Whenever
uridergoing surgery, there is a risk of nosocomial infection. Common
organisins that
cause nosocomial infections are Apergillus, Candida, Staphylococcus aureus,
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
294
Staphylococcus epidermidis, Pseudomonas aeruginosa, and Bordetella pertusis. '
Prior to
elective surgery, it would benefit the patient and the attending sur=geon to
know the ievel =
of antibody protectiorito these infectious agents. An ImmunoScore panel could
be
. tailored to meet these diagnostic needs and immunizations could be provided
to tliose
agents with available vaccine. In addition, following surgery patients could
be screene.d
for c reactive protein (CRP), tumor necrosis factor-alpha (TNF-(x), IL-6, and
soluble IL-2
receptor (sYL-2R) as possible early indicators of inflammation leading to
sepsis. It is
important to screen for a panel of analytes indicating sepsis, as one analyte
is oflen riot
enough to get a proper diagnosis.
T. SERVICES TO CHARITABLE'FOUNDA TIONS PROMOTING '
IMMUNOLOGICAL WELL BEING
Currently, the lack of accurate, affordable, and accessible diagnostio tests
significantly
impedes global health efforts. The Global Alliance for Vaccines and
Immuniza.tions.,
(GAVI) was created in 1999 to protect health and save. children's lives
throughout the
widespread use of modern vaccines. GAVI is' a partnership of governments,
.international
organizations, major philanthropists, research institutions, and the private
sector that
work together to: (a) improve access to sustainable inunuriization services,
(b) expand the'
safe use of all needed cost-effective vaccines, (c) accelerate research and
development
efforts for new vaccines needed in developing countries, (d) make
immuniza.tion
coverage a key indicator of development, (e) promote sustainability by
adequate
financing, and (f) reinforce global and national immunization.goals including
eradicating
polio, eliminating maternal and neonatal tetanus, reducing measles, and
increasing access
=
' to vitamin A.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
295
Underlying all health care tools - including therapeutic products, vaccines,
and other
preventative tools - are "platform" technologies that define and facilitate
their use. For
example, immunochromatography is a technology platform that has enabled the =
development of affordable, easy-to-use dipstick format diagnostic tools. - The
'
ImmunoScore diagnostic panel, a platform technology, can be used to great
advantage by
GAVI to improve global health efforts
GAVI issues requests for proposal (RFPs) to support research efforts to create
diagnostic
technology platforms and tolls that enable improved prevention, treatment, and
surveillance in developing country settings. The foundation issues RFPs to
support the
systemic evaluation of sets of genes, proteins, and cellular pathways to
determine their
potential role in contributing to the development of new vaccines,
diagnostics, and diugs
for GAVI's priority diseases and conditions. One area of concem is population
genetics
and how to design drugs and vaccines to discourage the emergence of resistance
and to
discover how genetics affects the efficacy of drugs and other interventions.
The
ImmunoScore database- would be an ideal tool for GAVI to use to evaluate
genetic
parameters and immune response to vaccines and drugs under =consideration. A
second
area is applied immunology. Here systematic approaches, such as that provided
by the
ImmunoScore technology, are needed to measure the human immune response to
guide
vaccine design and define biological signs that identify early or latent
infection.
U. DISCOVERY OF UNWANTED IMMUNOGENICITY OF THERAPEUTICS
There is potential of the human immune system to identify biological
therapeutic
products as foreign and mount an immune response. There are three main areas
of
concern with the production of antibodies against biological therapeutics in
humans:
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
2296
Safety - assurance of safety involves the assessment of whether antibodies
induced -could
have adverse clinical implications.
Efficacy - can be affected by.the presence of antibodies binding to the
product =and
reducing its potency.
Measurement of phamacokinetic/pharmacodynamic parameters= - the presence of
antibodies can alter these clinical parameters and=also interfere in the
assays used in their
assessment (Koren, et al. 2002).
The immunogenicity of therapeutic proteins can be influenced by riaany
factors, including
the genetic background of the patient, the type of disease, the type of
protein (human :or '
nonhuman), the presence of conjugates or fragments, the:route of
administration, dose
frequency, and duration of treatment (Schellekens, 2002). Manufacturing,
handling, and
storage can introduce contaminants, or alter the three dimensional structLUe
of the protein
via oxidation or aggregate formation. Various means have been suggested by
which
therapeutic proteins might be modified to reduce their imrnunogenicity,
including
PEGylation, site-specific mutagenesis, exon shuffling, and humaziization of
monoclonal
antibodies (Schellekens, 2002). In the future, it may be possible to predict
the
iriimunogenicity of new therapeutic proteins more accurately, using
specifically desi=gned '=
animal models, including nonhuman primates-and transgenic mice.
ImmunoScore diagnoses and database storage could be instrumental in the
development
of analytical techniques to monitor botli the drugs- and the patient
population. An
individual's tendency to mount an immune challenge to a protein therapeutic
could be
revealed'prior'to initiation of the treatment based upon the patient's
ImmunoScore
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
297
profile. In addition, once therapeutic treatment began, ImmunoScore diagnoses
and
database management could track a patient's immune response tio the drug. The
drug
manufacturers would be able to use the IminunoScore technology to conduct
clinical
trials arid also to select an appropriate population in which to test the.
drug. Based upon
ImmunoScore population data, the drug could be designated fcir use based upon=
the
genotype of the individual being treated.
Fig. 34 depicts an exemplary process flow for an ImmunoScore 'immunogenicity
study in
exemplary embodiments of the present invention. The exemplary study is
directed to
immunogenicity of therapeutic proteins.
With reference thereto, at 3401 a prospective patient's immune status can be
examined to
obtain a baseline ImmunoScore. At 3410 patients for whom treatment would not
be
advisable (based upon immune system profiling) can be identified, and
therapeutic
treatment for a patient group for which therapy is advisable can be initiated.
At 342.0
patients' further treatment and health care recommendations can be made, based
on
careful periodic monitoring of antibody levels to therapeutic proteins. In
addition,
cellular components of the immune system would warrant careful monitoring --
particularly in regard to the antigenic components of the therapeutic
compound. At 3430 .
patient data can be compiled for drugs in clinical trial. Populatiori data can
also be
compiled to assist in drug design. At 3440, follow-up examinations of
patients' immune
status post-treatment can be implemented and the results stored in a system
database.
Further screening of antibody levels and T-cell components.of immune system
can be
implemented for all patients. Finally, at 3450 the efficacy of therapies to
provide
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
298
necessary treatment to patients can be evaluated, and extent of undesirable
immunogenicity can be determined.
V. TWO-SIDED MARKET APPLICATIONS.
A two-sided market is a market wherein there are two sets (at least) of
customers that , in
effect, need each other. Each type of customer values the market more if tlie
other.type
of customer also buys the service. Businesses service such markets by acting
as
"matchmakers."
Examples of two-sided markets:
= computer operating systems
o software developers write applications
.0 computer users run applications
. video game console manufactuiers serve
o video game players
o video game designers '
= paymeint card companies
o consumers
0 merchants
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
299
These businesses all produce platforms that make matches between two or more
distinct =
groups of consumers.
The description of two-sided markets likely came about from payment card
companies
and legal ramifications of what may have been perceived as monopolistic
business
practices, but was actually the demonstration of two-sided marketing
practices. A.key
aspect to the business model for most of these industries involves the optimal
pricing
structure: the division of revenues between the two sides of the market that
gets both.
sides on=board. The need for pricing structure as well as pricing level
distinguishes
industries based on a two-sided market from the industries 'ordinarily studied
by
economists. In two-sided markets, the product may not exist=at all.if.the
business does
not get the pricing structure right. Currently, there is no appreciable market
for adult
vaccines, other than those for influenza and pneumococcus. ImmunoScore
diagnostics
can, for example, likely reveal lapses in protection for vaccine.preventable
cliseases, such'
as pertussis, tetanus, diphtheria, mumps, measles, and others. Dzagnostic
testing can thus
reveal a large marketing potential for vaccine manufacturers.
Both the ImmunoScore diagnostic application and the ImmunoScore database
management modules can be considered as two-sided marketing opportunities, in
that
none of the participants (patients, insurers, researchers, primary
oaxe=physicians, vaccine
manufacturers, or government entities) may necessarily be willing to enter
into a
beneficial marketing alliance without direction provided by the ImmunoScore
platforms,
as'illustrated in Fig. 35. ImmunoScore can act as a "matchmaker" for these
different
groups of consumers. An ImmunoScore diagnosticplatform can, for example, serve
to
link patients, physicians; and vaccine manufacturers and help to illustrate
the need for
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
300
continuing vaccine coverage in adults and children at risk. As an ImmunoScore
database
module grows from an= ImmunoScore diagnostic module, insurers, research groups
(both
academic and commercial), and groups responsible for vaccine decision-making
(ACIP
and AAP) and tracking (CDC and VAERS) can be able to take advantage of the
data=
generated from assessing the immune status of patients.
Network effects. A network effect arises when the value that one user=receives
from a
product increases with the number of other users of that product. It goes
without saying
that the vatue to governmental decision-making, insuring, and research
interests can
expand enormously with the increase in size of an ImmunoScore patient
database. Health
insurers can also be involved at ImnnunoScore diagnostic platform level.
Insurers that=
would be iiiterested in providing insuraince based upon an individual
patient's
ImmunoScore would benefit most from the ImmunoScore database platform. Most
network effects arise because a product tends to be two sided. ImmunoScore,
having
rimore than two interactive sides, would demonstrate large network effects
that should
benefit society as a whole, with better health for the population at large and
decreased
costs for the insurers. Information garnered from an ImmunoScore Database can
enable
the performance of vaccine researchers and the vaccine decision-makers in the
government tremendously.
Survey of Two-Sided Markets
= Diverse industries:
= credit cards
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
301
computer operating systems
. video games
= corporate bond trading
= resideritial real estate
Firms in these industries have adopted similar business models and pricing
strategies for
solving the problem they have in common - getting and keeping two sides of a
market =on
board. The intermediary helps customers complete a= transaction by providing a
platform.
The transactior.i occurs when both sides get together. Currently, there is a
real need to,get
adult patients and vaccine manufacturers together for the betterment of public
health.
ImmunoScore diagnostics will be an effective facilitator of this interaction,
with the =
medical insurance companies being a third beneficiary. The intermediaries
succeed in
the businesses by figuring out how a pricing stiucture that internalizes the
externalities
between the two sides. In the case of ImmunoScore Diagnostics, the
health=insurers
should be willing to pay for the diagnostic testing as well as the cost of
vaccination, as
those costs would be less than those to treat debilitating diseases otherwise
preventable=
by judicious use of vaccination.
A market is two-sided if at any point in time there are:
= two distinct groups of customers - with ImmunoScore diagnostic and:
ImmunoScore database platforms, there would be patients, vaccine
manufacturers, health insurers, vaccine researchers, and vaccine decision-
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
302
making organizations that would benefit from the two-sided ImmunoScore
platforms.
=. the value obtained by one kind of customer increases with the =number of
the other,kind of customers - as the'number of patients were added to the =
database, the database would increase in potential utility to researchers;
vaccine decision~makers, insurers, and vaccine.manufacturers. The more
the database grows, the better it would be for the patient population as
physicians would better be able to determine individual patients' inimune -
status based on knowledge. accumulated over the entire patient population.
= the intermediary is necessary for internalizing the externalities created by
one group for the other group - there is currently no real push for adults
or older children to have diagnostic screening related to=vaccine-
preventable diseases. As ImmunoScore data accumulate, there"should be
an added impetus for adult and adolescent vaccination coverage.
Researchers have examined the pricing and production strategy of a firm in a
two-sided
market. Consider the case in which both sides of the =market are buying
a"transaction"
and in which the seller incurs a marginal cost for consummating that
transaction. The
prices charged to the buyers and, sellers are two prices. The buyer's demand
depends
only on the price faced by the buyer and the seller's demand depends only on
tlie price
faced by the seller. The demands can be thought of, roughly speaking, as the
number of
buyers and sellers using the system. The transactions that'a seller engages
in; and its
benefits from those transactions, increase proportionally with the number of
buyers ori
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
303
the system. The same holds for an individual buyer. Total demand equals the
product of the two demands.= Thus, if there were 500 sellers and 100 buyers,
there would be 50,000
transactions. The assumption of a multiplicative. demand between the two sides
actually
understates the importance of the indirect network effects. It ignores the
fact that the
value each-side obtains from the other side increases with the number of
customers on.the
other side. In the cases of ImmunoScore diagnostics and an Irninu.noScore
database, the'.
benefit to all sides of the market could increase dramatically (presumably
something
more than a multiplicative effect) as the number of consumers grows. Feeding
information to the database can only assist patients, physicians, vaccine
decision=making
bodies, vaccine manufacturers, and health insurers.
None of the conditions for determining the price level or the price structure
in two-sided
markets corresponds to marginal revenue equaling marginal costs on either side
of the
market. Such conditions have no meaning in two-sided markets because there is
no way
to allocate the increases in revenues from changes in prices to one side or
the other.
Changes in prices result in more "transactions" from which each side jointly
benefits.
Business Models in Two-Sided Markets. Both sides need to be brought on board:
For
instance, there would be no demand by households for payment card is they
could not be
used anywhere, and no demand by retailers if no one had them to use:
Investment and
pricing strategies are key to getting both sides on board. Even with both
sides on board,
.businesses have to carefully balanc=e their two demands. They have to
consider how.
changes on one side of the market will impact the other side=of the market.
The need to
balance the needs of the various consumers will be of utmost importance to the
careful
development of ImmunoScore diagnostics and ImmunoScore database management as
=
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
304
two-sided rinarkets. Currently, patients, physicians, and vaccine
manufaaturers seem
painfully unaware of the need for diagnostic testing and boosting for vaccine-
preventable
diseases.
Getting both sides on board. One way to'get both sides'on board is to obtain a
critical
mass of users on one side of the market by giviing them the service for free
or even
paying them to take it. Another way to, solve the chicken-and-egg problem is
to invest in
one side of the market to lower the costs to consumers on that side of
participatirig irn the
market. Providing low prices or transfers to one side of the market helps
the=platform
solve the chicken-and-egg problem by encouraging the benefited group's
participation -
which in turn, due to network effects, encourages the non-benefited group's
participation.
Another effect of providing benefits to one side is that this assistance.can
discourage use
of competing two-sided firms. In the case of the ImmunoScore diagnostic and
database
platforms, initially the medical insurance industry ~would.likely bear the
burden of any
associated costs, but the benefit.tb this industry in increased wellness of
their clientele
should offset any up-front costs. In addition, to those patients who are seen
to have an
unfavorable ImmunoScore the supplemental insurance industry should be
available and
able to come in and insure those individuals with special needs.'
Pricing strategies and balancing interests. Firms in mature two-sided markets
still
have to devise and maintain an optimal pricing structure. In most observed.two-
sided
markets, companies seem to settle oin pricing structures that are heavily
skewed towards
one 'side of the market. = Certain customers on one side of the =market may be
extremely
valuable to customers on the other side of the market - "marquee buyers." In
the case of.
the ImmunoScore diagnostic and database-platforms, the "marquee buyeirs" could
be seen-
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
305
as large HMOs that would in truth benefit from having a healthier patient
population.
The existence of marquee buyers tends to reduce the price to all buyers and
iincrease it to
its sellers. Acceptance of ImmunoScore platforms by large insurance
organizations and =
gavernment agencies would enable "bringing on board" smaller insurance
agencies.. A
similar phenomenon occurs when certain customers are extremely loyal to the
two-sided
firm - perhaps because of long-term contracts or sunk-cost investments.
Multihoming. Most two-sided markets in the real world appear to have several
competing two-sided firms and at least one side appears to multihome.
Multihomir-g
affects both the price level and the pricing structure. Not surprisingly the
price level
tends to be lower with multihoming. The possibility of niultihoming may
encourage
firms to lower their prices on the side of the market in which multihoming
could occur.
By lowering their prices, firms discourage customers on that side' fiom
affiliating.with
other'two-sided firms. The firm can then charge more to customers on the other
side, for
whom fewer substitutes are available.
Two-Sided Markets and Social Welfare. A relatively small number of firms tend
to
compete in two-sided markets. That is because these markets have network
effects and
usually incur substantial fixed costs for getting one or both sides on board.
Largerfums
have advantages over smaller firms because larger'size delivers more.value - a
bigger.
network - to consumers. Firms in concentrated two-sided markets may have.
opportunities to earn supra-competitive profits = i.e. profits that exceed
those necessary to
attract capital to the industry after accounting for risk. Several factors
affect the extent to
which this can happen over time.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
306
.7. The extent to -which firms are competing to become established in a two-
sided market. This results in investment to. court customers, to provide
them with subsidies in the form of equipment, and to offer them low or
negative prices. Vaccine manufacturers and physicians offices might '
initially need to be coaxed into the, ImmunoScore diagnostic and database
markets, but should see the benefits as the structure =grows.
8: The extent. to which there are first mover.advantages in &t.ting either
side .
of the market on board then the competition #o: make these 'investments
should reduce the opportunities to earn significant supra-competitive
returns. Savvy Health Maintenance Organizations could be the first to
realize the benefits to their coverage that immunoScoie diagnostics and
database platforrns could provide, and as _such may be eag~r to get into this.
opportunity at the ground level. - The governmental organizations could
also be made to see the benefits of diagnosing and cataloging lapses -in
vaccine-preventable disease conditions.
The consequences- of having relatively few competitors in two-sided markets;
and the
existence of network effects, raise familiar issues concernirig the efficacy
of competitive'
markets and tlie possible roles for goverrunerit intervention. The pricing and
investment
strategies that firms in two-sided markets use to get both sides on board and
balance the
interests of both sides raise novel questions. These pricing and other
business strategies
are needed to solve a fundamental economic problem arising from the
interdependency of
demand on both sides of the market. In some cases, the product could not evenn
exist
without efforts to subsidize one side of the market or the other. In the case
of the
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
307
ImmunoScore platforms, the=patients would likely need to be.subsidized by
the_.
participation of health insurers.
Researchers have compared the pricing structure adopted by firms in two-sided
markets
-to the pricirig structure that would maximize social welfare. Interestingly,
they find that a
monopoly firm, a firm with competition, and a benevolent social planner would
adopt
similar pricing structures.. The precise relative prices would-differ
somewhat. They
found that the pricing structure adopted by the market is.not biased towards
one side.of
the*market or the other side of the market compared to the pricing structure
that'would be
adopted by the benevolent social planner. ImmunoScore 'diagnostic and database
platforms may be though. of as. a benevolent social plan: The wel=fare of the
patients is '.
paramount, and there would be additional benefits presented to vaccine
manufacturers,
research groups, and goverarnent organizations.
Two-sided markets are an increasingly important. part of the global economy.
Firms- that
provide platforms for multiple consuriier groups are a critical part of many
interrelated
segments of the computer industry. In most industrialized countries a large
fraction of
payments takes place through firms and associations that provide platforms for
merchants
and customers to exchange money. The increased importarice of the Internet for
household-to-household, business-to-household, and business-to-business
transactions
and the emergence of e-pay.systems on the Internet will increase the fraction
of payinents
going through commercial payment.platforms. ImmunoScore diagnostic and
database
platforms would help bring health care into the 215` century.' There is .a
tremendous need
for portability in health care record-keeping, and the ImmunoScore database
platform
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
308
would be instrumental in the.transfer of health care records from primary care
physician
to specialist.
Two-sided firms have to come up with the right price structure and the right
investinent
strategy for balancing the derriands of the customer groups they must.get and
keep=on
their platforms.
In many industries, platforms court two (or more) sides that use the platform
to inte-ract
with each other. The platform may charge interaction-independent fixed fees.
For
example, American Express charges yearly fees to cardholders. Iri the case of
video
.games, platforms charge game developers fees for development kits on top
of;oyalties
per copy sold, and they charge gamers for the console. In the case of the
ImmunoScore
database platform, it might be appropriate to charge academic and,commercial
research
grciups for use of the information captured by the database modules.
Managers devote considerable time and resources to figure out which side
should bear
the pricing burden, and commonly end up, making little money on orie side
(or.even using
this side as a loss-leader) and recouping their costs on the other side.
Marketing
managers for the ImmunoScore platforms will need t6 carefully balance many
consumers' needs and the applications of fees.
Pricing Principles for Two-Sided Platforms. Departures from standard business
strategies that result from the platform's internalization of the other side's
welfare (the
linkage between the two sides from the platform's viewpoint). This linkage is
nnost
apparent when the platform makes no or loses money on one side. A factor that
is
conducive to a high price on one side, to the extent that it raises the
platform's margin on
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
309
that side, tends also to call =for a low price on the other side as
atfiracting members on that
other side becomes more profitable. In the case of the ImmunoScore platforms,
it is
imperative to bring patients on board, but their participation might be
encouraged by the
duel factors of their curiosity as to their personal ImmunoScore-and also the
participation
of their insurer in the platform.
Platforms must perform a.balancing act with respect to their price structure.
as weIi as
other policy dimensions; quite generally, they encourage positive
externalities arid' =. =
discourage negative ones and to do so usually constrain one side to the
benefit of the
other: While asymmetric information and the concomitant rent extraction
conoerns keep the platform's price structure neutral, it is nonetheless'a
source of sub-bptimal trade.
among end-users. The platform has an incentive to cap or alter through a
subsidy the
price charged to buyers so -as to boost buyer's surplus and their willingness
to joiii the
platform. Then the platform behaves.pretty'miich like a-public
utility'commission-that .
addresses a market power problem by setting a price cap or by subsidizing some-
services
through a fund levied from other services: = '' ..
The rationale for constraining the price charged by the seller to the buyer
would vanish if
the industry were organized according to the vertical view: 'were the platform
not to deal. -
directly with buyers, the platform would warit.to provide sellers with the
maximal profit
in their relationship with buyers and therefore would grant them
maximalcommercial
' freedoni. It is only because. the platform can extract surplus on the buyer
side that it- is
willing to displease the seller side by constraining it.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
310
End-users often care not only about the price (that they pay to the platform
and to the
other s'ide), but also about the quality of the interaction. In health care,
the quality of the
physician-patient interaction assumes particular importance. An ImmunoScore
Diagnostic platform will help nurture the doctor-patient relationship and
focus on the
patient's "wellness" rather than strictly on "treatment"
While price regulation is complex or inefficient, the platform may still make
itself
attractive to one side of the market by encouraging competition on the other
side..
Competition on the other side brings prices closer to marginal cost, and the
volume of
interactions closer to the efficient volume; it also protects against the hold
up of one's -
specific investments. An ImmunoScore diagnostic platform could encourage
competition
among vaccine manufacturers on behalf of the patient population. The
manufacturers
should still realize greater sales, but their prices should remain competitive
for the
insurers and patient population. Accordingly, a two-sided platform benefits
from
allowing competition on a given side as it can at least partly recoup benetits
on the other
side.
Dynamics. To create a two-sided market, a "chicken or egg" problem has to be -
solved:
.to convince some buyers to adopt a certain intermediation platform, it is
necessary to=
convince first some sellers, but to convince the seliers, there must be some
.buyers on the
market. In most models, this problem is avoided by assuming the simultaneous.
arrival of
agents on the two market sides, in a rational expectations equilibrium.
However, there '
are circumstances in which one market side has to intervene before the other
one. The
most cited case is the one of videogame consoles which, to get customers, must
appear on
the market already equipped with a complete range oLgames and complementary
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
311
applications. There appears to be a growing need for the determination of a
patient's
immune status. There is a current outbieak of mumps disease in the Midwest in
individuals that have received two IvIMR immunizations. The incidence of
pertussis
continues to increase. Travel has now been- related to the 'spread of Severe
Acute
Respiratory Syndrome (SARS), influenza, measles, tuberculosis, and mumps.
'.The tirrie
is appropriate for the introduction of the measurement of the immune status of
individual
patients, and the tracking of information regarding each individual's
immune,status: '
ImmunciScore diagnostic and database platforms can tip the balance from a
display of
need to a mode of action going forward.
W. DRUG HYPERSENSITIVITY'
Incorporating Drug Hypersensitivity =into a Two-Sided Business Model
Adverse drug reactions are common. 'Identifying true drug allergy, however,
can be
challenging. Drug hypersensitivity is- a clinical diagnosis based upon
available. data..
Drug hypersensitivity is defined as an immune-mediated response to a drug
agent in a
sensitized patient. * Identifiable risk factors for drug hypersensitivity
reactions -include '
age, female gender, concurrent illnesses, and previous hypersensitivity to
related drugs.
Monitoring drug hypersensitivity in patients and* incorporating those data
into an
ImmunoScore database platform is ainother example of a two-sided market
opportunity
As with the other. examples of two-sided markets, the medical insurance
organizations
would likely initially cover.most of the expenditures to bring the other
market
components into the market that would be beneficial io all participants; Other
"sides" of the market would involve patients, physicians, researchers for both
the pharmaceutical
. , = '
industry and allergy specialists. .
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
312
The Gel and Coombs classification system describes the predominant immune..
mechanisms'that lead to clinical.symptoms of drug hypersensitivity (Table 1)..
This
classification system includes IgE-mediated Type I reactions, cytotoxic. Type
II reactions,
' Type III reactions involving the formation of immune complexes, and the
delayed, cell-
mediated Type IV reactions. However, some . drug hypersensitivity ireactions
are difficult
to classify because of a lack.of evidence supporting a predominant immunologic
mechanism. . These =include certain cutaneous drug reactions and specific=drug
hypersensitivity syndromes.
Diagnostic testing for these reactions remains somewhat problematic. The
current types
of tests and therapeutic considerations are for each of the four types of
hypersensitivity. =
reactions are described in Table 2 below. Confirination of suspected 'Type I
hypersensitivity reactions require the detection of antigen-specific IgE.-=
Currently; skin
testing is a useful, diagnostic piocedure for'reactivity to penicillin. With
other drug
agents, a negative skin test does not effectively rule out the presence of
specific IgE.
Further IgE test for other agents await development. The sensitivity of ECL
technology
as embodied in an exemplary ImmunoScore diagnostic platform can be a very
effective
tool to .enable researchers to better study IgE populations specific for drug
component
antigens. Currently, the diagnosis bf.drug hypersensitivity is usually based
upon clinical
judgrnent because'definitive, confirmatory drug-specific testing is often
difficult.
'Once the diagnosis has been established, appropriate documentation should be
included
in= the medical record specifying the causative drug and the riature of the
adverse -effect.
Immune-mediated drug hypersensitivity reactions typically pose a predictable,
more
serious health risk with re-exposu're to the drug.' In this application of the
technology, an
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
313
exemplary ImmunoScore database platform can, for=example, capture all
pertinent
inforination related to any adverse drug reaction. This would not only be of
benefit to the
patient, but also as data was accrued, pharmaceutical companies would benefit
from
statistical information gathered from mining the database. Real drug
hypersensitivity
would also be separated from reactions that may not be hypersensitivity.
Instead of
relying on patient recall and a faulty data collection system, an exemplary
ImmunoScore
database can only include documented case histories. Patient medications -can,
for
example, be tracked via an ImmunoScore database and real hypersensitivity can
be.
officially documented.
The most important drug-related risk factors for drug hypersensitivity concern
the
chemical properties and molecular weight of a'drug. Larger drugs with greater
structural
complex.ity are more likely to be immunogenic. Heterologous antisera,
streptokinase and
insulin are examples of complex antigens capable of eliciting hypersensitivity
reactions.
Another factor affecting -the frequency of hypersensitivity drug reactions is
the route of.
drug administration; topical, intramuscular, and intravenous administrations
are more
likely to cause hypersensitivity reactions. These effects are caused by the
efficiency of
antigen presentation in the skin, the adjuvant effects of repository drug
preparations, and
the high concentrations of circulating drug antigeri rapidly achieved with
intravenous'
therapy. Oral medications are less likely to result in drug hypersensitivity.
Most medications, because of their small molecular size; are unable to elicit
an immune
response independently. Drugs must 1'irst covalently bind to larger carrier
molecules such
as tissue or serum proteins to act as complete multivalent antigens. This
process is-called
haptenation, and the drugs act as haptens. The elicited immune response may be
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
314
humoral, with the production of specific antibodies, cellular, with the
generation of
specific T lymphocytes, or both. Frequently, the identity of the metabolites
is unknown,
making it impossible to develop accurate diagnostic tests for drug
allergy{Solensky,
2006).
A thorough history is an essential component of the evaluation of patients
with suspected .
drug allergies. The history helps guide the clinician in the choice of
diagnostic tests an.d=
the decision whether it is safe to reintroduce the medication. Typically,
years or decades
have passed since reactions occurred, and, as a result, these records' are
usually
unavailable at the time of consultation.
Patients labeled penicillin-allergic are more likely to be treated with more
expensive=and
broad-spectrum antibiotics, a practice that leads to the development and
spread of
multiple drug-resistant bacteria and higher direct and indirect health caie
costs. Ainong .
patients with a reported history of penicillin allergy, 80-90% have no
evidence of IgE
antibodies to penicillin on skiri testing and thius avoid penicillin
unnecessarily. The
discrepancy between claimed and real penicillin aller=gies probably results
froni several factors. The reaction may have been predictable or due to the
underlying=illness and =
hence may have been mislabeled as allergic from the onset. Another contributor
to the
discrepancy is the tendency of patients with type I penicillin allergy to lose
penicillin-
specific IgE antibodies over time. Insight into the immunochemistry of
penicillin has
allowed for the development of validated skin-test reagents to detect
penicillin-specific =
IgE.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
315
Together with penicillin, cephalosporins are the antibiotics most widely.us,ed
for treating
common infections, and like peniciliin, can cause immediate reactions.
Manifested
clinically by urticaria, angio-edema, ihinitis, brochospasm, and anaphylactic
shock, such
reactions are generally IgE-mediated and are among the most dangerous.
Although the
incidence of severe immediate reaotions to cephalosporins does not seem to be
much
different from that to penicillin, studies of cephalosporins as allergens are
not nearly as
numerous or thorough as those on penicillin, and very few have been dedicated
to the still
little known determinants responsible for allergic reactions..
Unpredictable adverse reactions to aspirin and NSAIDS fall into several major
categories.
Respiratory reactions occur in patients with underlying asthma, non-allergic
rhinitis, and
nasal polyposis. The preferred term for this disorder is aspirin-exacerbated
respiratory
disease (AERD). The reactions typically involve the entire respiratorytr=act,
with
symptoms of rhinitis, conjunctivitis, and bronchospasm. Patients who have'AERD
exhibit cross-reactivity with all- nori-steroidal anti-inflammatory drugs
=(iVSAIDS), but
they can tolerate cyclo-oxygenase 2 enzyme (COX-2) selective inhibitors. No in
vitro
tests to detect aspirin sensitivity exist, =and oral challenge reniains the
gold staridard
diagnostic test for AERD.
True hypersensitivity reactions to local anesthetics are unconunon and'
usually consist of
delayed contact dermatitis; anaphylaxis from local anesthetics occurs rarely
if ever. Most
'adverse reactions are vasovagal, psychogenic, toxic, or predictable side
effects of
epinephrine that is often used in combination with local anesthetics. Large-
scale studies
have found that, following full evaluation, virtually all patients with a
history of allergy
to local anesthetics are able to tolerate these drugs. Unfortunately, patients
who
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
316
experience any adverse reaction to local anesthetics are frequently labeled
allergic.and
told to avoid all "-caines" in the future. Because evaluation of these
patients irivariably
finds them able to receive a local anesthetic, such evaluation prevents them
from being
subjected to the increased risk of general anesthesia or, alternatively, to
pain from the
absence of anesthesia. Evaluation of patients with a supposed allergy to local
anesthetics
'is also important because it serves to alleviate- dentists' or physicians'
legal (malpractice- :
related) concerns regarding use of a drug to which-the patient is listed- as
being allergic.
Allergic drug reactions compose a small percentage of adverse drug reactions,
yet they
are commonly encountered in clinical practice, and physicians are taught to
routinely
question 'patients about these reactions du.ri.ng history. taking. Medical
history taking is
critical in the evaluation of antibiotic allergy and in distinguishing
allergic-reactions from-
other adverse reactions. This information is important; since over-diagnosis
of allergic
reactions can lead to unnecessary use of more costly antimicrobial agents and
may
promote the development of resistant= microorganisms. Whenever possible,
patients who
are being evaluated for possible antibiotic-allergy should be eneouraged to
provide all
medical records related to'previous adverse drug reactions.
Treatment. For drugs that are presumed to be mediated by IgE, drug
desensitization my
be performed if the implicated agent is required for treatment.
De'seinsitization involves
the administration of increasing amounts of the antibiotic slowly over'a
period of hours
until a therapeutic dose is reached. The mechanism by which clinical tolerance
is
achieved is unclear, but it is thought to involve antigen-specific mast-cell
desensitization:
Since mairitenance of a desensitized state requires the continuous preserice
of the drug,
desensitization must be iepeated if the antibiotic is required again later. :
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
317
For reactions that are not considered to be mediated by IgE, management
depends on the
cliniaafmanifestations of the previous reaction. For macropapular eruptions,
the
specialist may consider a graded drug challenge, which is equivalent to
provocation
testing: Initial starting doses are generally higher than those used for
desensitization; and
the interval between doses varies; ranging from hours to days or weeks. The
decision .
'whether to discontinue an antibiotic if a reaction occurs depends onthe
nature of the
reaction; bullous lesions or those involving mucous membranes warrant
withdrawal of
the drug, whereas it may' be reasonable to treat through milder reactions,
such as
maculopapular eruptions, with the use of antihistaxnines, corticosteroids, or
both as
-needed.
Cephalosporin in patients with penicillin allergy. Penicillins and
cephalosporins share
a(3-lactam ring structure, rnaking cross-reactivity a coticern. Whereas most
patients who
have a history of penicillin allergy will tolerate cephalosporins,
indiscriminate
administration cannot be recommended, especially for patients who have had
life-,
threatening reactions. For patients with a history of penicillin allergy who
iequire a
cephalosporin, treatment depends on whether the previous reaction was mediated
by I=gE.
If testing is positive and a cephalosporin is considered necessary, then
desensitization
should be performed with the use of the particular' cephalosporin chosen for
treatment.
Areas of Uncertainty. (Gruchalla and Pirmohamed, 2006) The mechanisms
underlying
antibiotic allergy have not been clearly elucidated. This understanding is
needed to facilitate the development of better diagnostic tools and drugs then
are less immunogenic:
Better understanding is needed of factors mediating individual susceptibility
to aller=gxc
reactions to antibiotics. * Some patients have reported adverse reactions to
many
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
318
chemically unrelated antibiotics. The existence of the so-called multiple drug
allergy
syndrome is controversial, and accepted diagnostic tests are needed to
document drug
allergy in these patients.
Recommendations. Patients who report a history of antibiotic allergy require=a
careful
assessment of the nature of the reaction to determine if the likelihood that
it was
immunologically mediated. For patients whose history suggests and IgE-mediated
reaction to penicillin, skin testing is indicated. If the test results are
negative, the (3=. .
lactam agent may be administered. If the test results are positive or testing
cannot be
done, the drug should be avoided or a desensitization procedure should
be=performed.
Immuno5core and Drug Hypersensitivity. Exemplary ImmunoScore diagnostic and.
ImmunoScore database platforms can be seen as examples of two-sided markets in
both
. , ..
the diagnoses of drug hypersensitivity as well as in the retentiori of an
individual patient's
drug hypersensitivity testing and records for future health care medication
decisions as
shown in Fig. 36. In such cases, it would be predicted that the health
maintenance
organizations and pharmaceutical manufacturers (seen at the base of the
diagram,
propping up the platform structure) would belong to the side(s) of the market
most=eager
to subsidize the bther partners. Patients, physicians, and allergy specialists
are natural
partners to exemplary ImmunoScore diagnostic and database platforms regarding
drug
hypersensitivity. Because the diagnoses of drug hypersensitivity-reactions are
in their
infancy from a scientific standpoint, research groups developing diagnostic
assays are
also natural customers for the two ImmunoScore platforms.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
319
Initial-patient histories should include a recording of all prescription and
non-prescription
drugs taken within the last month, including dates of administration and
dosage: = This is a
real example of the proposed utility of the ImmunoScore database platform,
wherein
patient medications could be tracked and also easil.y transferable from
primary care
physicians to specialists.
For the HMOs and other insurers, drug hypersensitivity diagnoses and
cataloging by
ImmunoScore are a natural marriage. There are dual.concerns in health care
regarding '
the expense of exotic antibiotics and the development of antibiotic-resistant
strains of
organisms. Real patient information regarding drug hypersensitivity (as
opposed to
patient recall and lirriited health records) would certainly be welcomed by
the medical
and insurance professions. '
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
320
Table 1- Gell and Coombs Classification of Drug Hypersensitivity Reactions
(Riedi and Casillas, 2003)
Immune reaction Mechanism Clinical manifestations Timing of
reactions
Type I (IgE Drug-IgE complex Urticaria, angioedema, = Minutes to hours
mediated) binding to mast cells bronchospasm, after=drug
with release of pruritus, vomiting, exposure
histamine, - =diarrhea, anapliylaxis'.
inflammatory
mediators
Type II Specific IgG or IgM Hemolytic anemia, Variable .
(cytotoxic) antibodies directed at neutropenia,
drug-hapten coated thrombocytopenia
cells ,
Type III (immune Tissue deposition of Serum s'ickness, fever; 1 to 3 weeks
complex) drug-antibody= rash, arthralgias,. after drug
complexes with- lymphoadenopathy, exposure
complement urticaria, -
activation and glomerulonephritis,
inflammation . vasculitis . .
Type IV (delayed, MHC presentation of Allergic contact 2 to 7 days a$er =
cell-mediated) drug molecules to T= 'dermatitis, . =, cutaneous. drug
cells with cytokine maculopapular drug : exposure
and iiiflammatory . rash ' mediator release
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
321
Table 2
Diagnostic Testing and Therapy for Drug Hypersensitivity (Solensky, 2005)
Immune reaction . Laboratory tests Therapeutic.considerations
Type I (IgE-mediated) Skin testing Discontinue drug
Radioallergosorbent test Consider epinephrine,
(RAST) antihistamines, systemic
Serum trypase corticosteroids,
bronchodilators = Inpatient moriitoring, if
severe =
Type II (cytotoxic) Direct or indirect Coombs' Discontinue =drug
test . Consider systeniic
corticosteroids
Transfusion in severe cases
Type III (immune Erythrocyte sedimentation Discontinue drug
complex) rate (ESR) Consider NSAIDS,
Complement studies antihistamines, or systemic
Antinuclear antibody, coricosteroids; or
antihistone antibody plasmapheresis, if severe
Tissue biopsy for
immunofluorescence
stiudies .
Type IV (delayed., cell- Patch testing Discontinue drug-
mediated) Lymphocyte proliferation Consider topical'
assay corticosteroids,
antihistamines; or systemic
corticosteroids, if severe
X. HEALTH CARE TRANSPARENCYAND CONIPET/T/ON Currently, health care in the
United States consumes $2 trillion per year. Out-of-pocket
costs'for those who have insurance have nearly tripled in the last six years,
and 46 million
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
322
Americans -are uninsured. Unpaid and unpayable health care bills account for
the
majority of all personal bankruptcies in the country. Eight criteria for
improving health
care can be articulated as:
1. Consistent high quality
2. Lower cost - follows from high quality.' Higher quality is often naturally
less
expensive. Providers improve quality by honing their organizational -processes
to
become more efficient and effective - to avoid error and to do things right
the first
time.
3. Available to all - for ethical, political, systemic, and business reasons,
health care must be available to everyone.
4.. Single model - every provider in the system must compete to offer the .
*best product at the best price.
5. . Shaped by market forces - the consumei- market has the sustained
systemic power to bring consumers more for less:
6. Practical - the solution must arise from present realities.
7. Progressive - dramatic change can not occur all at once.
8. Self-reinforcing - as any part of the health care system moves toward a *
new reality, that movement must allow and encourage other parts to move
forward as well.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
323
Competition thus far has failed to work the same wonders in health care that
it has in so
many other industries. In Redefining Health =Care: Creating Value-Based
Competition on
Results, Michael Porter and Elizabeth Teisberg argue that this is because
competition has
taken place at the wrong level and over the wrong goals. Further exacerbating
the
problem is the complete absence of feedback loops. Very little in health care
has a'real
price or a real measurable result. Competition in health care has consisted of
health
plans' and providers' attempts to push cost and risk of themselves and onto
each otlier or
onto employers - and now, onto the consumers. Consumers are not looking to'
embrace
an institution, but are looking for a solution for a particular problem. One
can envision a
world in which health care is organized mainly around products tailored to
particular
medical conditions. Such products can be delivered by medically integrated
practice
units made up of teams that work together on the same medical condition over
long
periods of time. In this particular vision, transparency drives quality.'
Health plaris could
steer patients toward the providers who offer the best results for the least
money.
Referring physicians could refuse to recommend any specialist or package with
quality
scores in the lower quintiles, for fear of being sued for malpractice
themselves. When
health.care providers compete at the level of the medical condition, on real
prices and real
results, feedback loops can become extremely compelling:, Offeriiig the
highest possible
quality at the lovirest possible price will no longer be voluntary, and health
plans will also
be forced to compete on the basis of real results and genuine customer service
at the
lowest price, rather than at their current modus operandi - which can include'
denying
coverage and shifting cost and risk to employers, consumers, and providers.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
324
Nev-w structures for public reporting of medical results are popping up on-
federal; state
and regional.levels. In many of these initiatives, process measures are
starting to -give
way to results measures. In a number of regions, new tiered payment models use
co-
payments and other means to encourage patierits to use the providers with 'the
lowest cost
and highest quality scores. Such models'also reward more efficient systems,
those that
beat their risk-adjusted cost targets, with higher reimbursements, and punish
those less
efficient providers with lower reimbursements.
The pieaes = transparency, integrated products, and true measurement - are
coming into
play in the health care marketplace. Once it becomes common for health care
providers
to post actual prices and actual results in standardized ways that produce
comparable
data, it is hard to see how consumers; insurance companies, and referring
physiciaris
would ever choose low quality at high prices.
In exemplary embodiments of the present invention, ItnmunoScore
diagnostics.and
database management can, for example,
= keep score not only of patient's immune data, but effectiveness of
treatments/vaccine '
= tie records of physician recommendations relating immune status to fiscal
responsibility and patient well-being
= provide data for insurers
= -provide data for providers
= provide data for consumers
Major decisions about health care in the U.S.*have traditionally been made.by
employers,
who determine for their employees which benefits and forms of coverage are
needed,.
what types of providers are included in the network, and which organizations
administer
the benefits. But this paternalistic approach effectively allowed the consumer
to be a
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
325
passive participant iri his' or.her own health care.. The consumer to this
point has'had no
economic incentive to seek the best care at the fairest price, or to -give up
unhealthy
habits. It has been written (Knott, et-a1. 2007) that new health care formats
and
competitors are gaining traction, with MinuteClinics and RediClinics - low
cost walk-in
health care'centers for common ailments at one end of the spectrum, and highly
personalized "concierge care" at the other. In additiori, companies that are
not traditional
health care players are leveraging their capabilities to create entirely new
offerings that.
enable and encourage the move toward health care consumerism. Fidelity, for
example,
is developing products and tools that expivit the emergirig health-wealth
intersection,
such as a calculator that helps predict out-of-pocket health care costs.
Standardized data
on cost, service, and outcomes, has the powe'r tb establish a new basis of
competition. Payers are also pushing for new payment mechanisms, such as pay-
for-performance;that
base reimbursement on outcomes or adherence to broadly accepted clinical
guidelines,
known as evidence based medicine.
To make competition and innovation among payers and suppliers possible,'an
exemplary
system could include the following:
= consumers who live healthy Iives* and plan for their future health care
-needs
= a fundamentally restructured supply side that provides consumers all the
information they need to make wise choices and is quick to respond to
changing consumer demands=. = new kinds of intermediaries to help align the
supply and demand sides and
help consumers navigate the complex system
Much of what is needed on the demand side is in place today or likely to
=emerge in the
near teim. Consumer-directed health plan (CDBP) enrollees offer an early
glimpse of
subtle changes in a retail market. = CDHP enrollees are more likely to be
aivvare of price
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
326
and quality differences in products and services and more likely to have seen
information
and shop around; they are more likely to ask for prices up front, more likely
to negotiate
prices, and more willing to trade convenience for lower prices. They are also
more likely
to plan ahead when making health care decisions and to invest dollars now to
=prevent
problems later.
The overall design of the ImmunoScore technology is one- in which preventative
medicine takes the forefront in treatment options. Vaccine status is the most
obvious
application, and patients lacking protective antibody levels can be
vaccinated. Other
levels of immune preparedness would also be similarly assessed and
preventative
measures could be undertaken prior to clinical manifestations of autoimmune
disease;
cancer, or immunosenescence. Similarly, evaluations of physicians and health
plans
could be readily facilitated using the ImmunoScore database. ImniunoScore can
be used
to discover and define fundamental relationships, such as, for example, (i)
optimal
Thl/'Th2 balances, or (ii) lack of any members for immunosenescense or
autoimmune
desease, that can serve as indicators of overall imune system harmony. Such
relationships can, for example, be quantified as one or more."ImmunoScores."
Patients
with healthy ImmunoScores would point to their primary care physicians and
their
insurers as providers of admirable health care practices. Prevention being
much more
cost-effective than treatment.would provide the best of all worlds to the
patients,
physicians and insurers. Physicians whose patients had consistently lower =.
ImmunoScores would raise a cautionary flag and.those doctors and practices
could be
scrutinized for provision of first rate health care (or something less). If
the records were
transparent, patients as consumers would use their dollars to pay for the best
po=ssible
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
327
health care -rather than pay for poor care at high cost. For example, in 2005,
Aetna began
testing tools that allow consumers to compare physicians on actual "cost, so
that they can
gauge their out-of-pocket expenses. WellPoint .has embarked on a pilot program
at the
suggestion of General Motors to provide complete comparative cost data for
hospitals on
"episodes of care." A niumber of employers are also finding success with
weliness
programs. Typical weilness programs feature free or low-cost health screenings
and
other sorts of preventive care.
It has been approved proposed that additioinal investments in health
information
technology and greater connectivity among providers will be needed to ease
sharing of
patient information and enable consumers to better manage their own health.
InununoScore database manag,ement can, for, example, serve as an excellent
tool to
address connectivity issues that patients, physicians, and insurers would
have. Thus,
'ImmunoScore technology proposes to be a new. intermediary in health care -
connectivity.
Patients would have more control over their own:health care decisions -
spending as well
as courses of treatment.
Public health and data collection. Iri public health, the current underlying
assumption
is that good data will lead to better decisions, which will result in enhanced
population
health: In practice, no necessary linear sequence exists from good data to
better health (AbouZahr,' et al. .2007). Various types of data are obtained at
different levels of the
health system, to be used by several. actors for many reasons. Providers
generate aiid use
iriforniatioin in the context of patients' care; managers need data to enhance
efficiency
and effectiveness; planners rely on statistics for operational decisions; and
policymakers,
use information for prioritization and resource allocation (AbouZahr, et'a1:
2007). There
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
328
are different data sources currently used to formulate,public policy - each
with
advantages and disadvantages:
= Routinely repor'ted'service data: Rotttine and adm.inistrative reports are
generated as a by-product of patient-provider interactions and health
facility functioning. 'Health facilities are a primary. source of data for
notifiable diseases and are at the heart of a country's surveillance and
response programs, although facility case reporting needsto be
complemented by active case seeking strategies to generate a complete
picture of epidemic risk. No matter hovri many data. elements are routinely
reported, information is inevitably tiiased by patterns of service use and'
non-use, and the extent or direction of bias is impossible to ascertaiii
withoi,it recourse to other sources of data. Services delivered (number of
immunizations, antenatal visits, outpatients seen, etc) do not'necessarily
equate to population need.. = Population based data. Mistrust of service-based-
statistics has fuelled
interest in household surveys that can generate unbiased' data for
populations as a whole rather than just the sections that use'available
health services. These household surveys have several disadvantages.
They need large investments in human and financial resources and
therefore are usually.fanded: externally, resulting in bias towards the
interests of donors or well-sponsored programs. They are . also tinie-
consuming and are undertaken only occasionally,.and generate results.
spanning a period, rather than the immediate past. Samples are rarely of
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
329
sufficient size to deliver nationally valid results. Growth in surveys to
generate health related data has been fuelled by their ability to deliver
statistics on child mortality, population coverage, and certain risk'factors..
In the past few years, scope for measurement of health status with
household surveys has greatly expanded owing to cheap and reliable
diagnostic tests that can be used in the research setting to =generate
population-based estimates of disease prevalence. But surveys are not as
effective for measurement of adult mortality, which is a relatively rare
event compared with child mortality.
IrnmunoScore would relieve much of the concern regarding public health and
data
collection. As stated above, there has been concern regarding bias in the
routinely
reported service data. As ImmunoScore grows in size and popular'usage,
concerns about
bias can be alleviated. Services delivered to any individual patient would be
based solely
upon the needs of that particular patient, and tailored to that individual
patient's
immunologic needs, with no regard for social stratum. Politicai justifications
for mis-
representation of data would be eliminated by the automatic and mechanical
:nature of the
data acquisition.
The tremendous requirement for human and financial resources for collecting
population
based data would also be alleviated by ImmunoScore technology. Data can be
collected
at hundreds of remote locations and transferred back to an exemplary
ImmunbScore
central database. There would be no need for third party human resources -
data
collection would occur at the hospital, clinic, or physician's office and
stored for future
use.
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
330
ImmunoScore Tracking of Medical Services (ImmunoScoreKeeping)
As has been described,=heath care processes are very complex, involving both
clinical
and administrative tasks, large volumes of data, and a Iarge number of
patients and
personnel. Health care processes are also very dynamic. As new processes are
initiated,.
changes in health care treatments, drugs, and protocols can invalidate current
methodologies, requiring reparative actions. ImmunoScoreKeeping`can, for
example,
capture all of such complicated dynamic componeints and provide. accurate
perfonnance
measurements ( `ImmunoScoreCards ') not only for individual patients, but also
as a
means of tracking relative efficiencies of other complex components of the
health care
system. =
For example, upon a visit to a prbvider using an ImmunoScore system, the
patient's data
can be captured by an "ImmunoScorekeeper." (an exemplary POC assay reader
connected to a system database, as described above). Not only demographic and
test
data; but also treatments/drugs prescribed, physician's ID number, and insurer
can-be
stored. Any additional testing or measurements (blood chemistry, X-rays,
physical
therapy, etc.) can be entered into the remote ImmunoScore data collection
system at, for .
example, the physician's office. A critical requirement for efficient
management of
health. care is the management of the quality of service. Appropriate control
of quality of
service leads to the creation of quality care services; these, in turn, can
fulfill patient =
satisfaction. . = Traditionally, health care services have been managed using
limited forms of-workflow.
Some examples of these are clinical and administrati=ve protocols. However,
these
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
331
protocols have remained'limited in their usefulness in part because developers
have rarely:
incorporated both" clinical and administrative activities into one
comprehensive care
protocol. This lack of integration hinders the delivery of care, as the
effectiveziess of =
protocols is often dependent on many administrative tasks being properly
executed at the
correct time.
Thus, in exemplary embodiinents of the present invention, ImmunoScoreKeeping
can
enable medical practices to provide better quality care at reduced costs. -
ImniunoScore
can, for example, maintain keep ImmunoScoreCards on:
+ individual patients=
+ physicians
= groups of physicians/managed care organizations
+ insurers
In addition, 'as vaccines, drugs, and therapies prescribed can all be
monitored and tracked,
an ImmunoScoreKeeper can also monitor the =efficacy of the vaccines, drugs,
and
treatments prescribed. As these data are compiled, they can be shared and
submitted to ".
appropriate oversight organizations (FDA, CDC, ACIP, Physician's
or.ganizations, drug
and vaccine manufacturers, etc.) to better enable these =groups to make clear
decisions
and/or recommendations. Such organizations would be consumers of ImmunoScore
data.
Thus, an ImmunoScoreKeeper can allow insurers to rate physicians and enable
their
customers (the patients) to make better informed decisions regarding their
choice of
CA 02650584 2008-10-27
WO 2007/127490 PCT/US2007/010576
332
physician: An ImmunoScoreKeeper can track effectiveness of treatments to
patient
=outcomes. Prescription drug and vaccine efficacies can be monitored not
only=in
population-based'samplings, but longitudinally in individual 'patients with
repeated
ImmunoScore diagnostic testing protocols: Physicians can thus monitor'the
efficiency of
the practice that they are associated with, and thereby make the best career
choices to
.advance their careers in the most efficient practices. Hospitals could be
measured for :.
effectiveness in patient care against other hospitals and groups of
physicians. Types of
hospital settings coi.uld be evaluated prospectively. Causes of nosocomial
infections
might be tracked, for exainple, to certain types of hospital environments.
Insurers can be
measured against common metrics and be forced to compcte for business via
accurate
ImmunoScoreKeeping.
In exemplary embodiments of the present invention, an ImmunoScoreKeeper can,
for
example, provide a means of integrated monitoring of individual patient
treatriment and
also administration of both physicians and insurers practices. The
ImmunoScareKeeper
can, for example, generate a numerical value for each component of the health
care
system upon wfiich real competition among providers and insurers could be
generated.
This competition would thereby provide substantial increases in health care
quality and
decreases in health care costs. ' . ==
Y. ' USER ACCESS V/A DATA NETWORKS AND ON-LINE ADVERTISING
In exemplary embodiments of the present invention, users can, for example,
access an
ImmunoScore Database via coinputer data networks. Such networks can include,
for
example, VPN's or the Internet. In exemplary embodiments of the present
invention, an
ImmunoScore database can be accessed, updated and queried via one or more web
page =
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 332
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 332
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: