Note: Descriptions are shown in the official language in which they were submitted.
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
PREVENTION AND TREATMENT OF OSTEOCIiONDROSIS
IN ANIMALS AND HUMANS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This is a continuation in part of International Application
PCTIUS2006/021505,
with an international filing date of June 2, 2006, which claims the benefit
under 35 USC
119(e) of U.S. Provisional Application No. 60/687,653, filed June 2, 2005, all
of which are
incorporated herein by reference in their entirety.
BACKGROUND OF THE INVENTION
[0002] Lameness is a major cause of culling and death in female pigs of
breeding age,
affecting over 20 million animals annually. At least 3 to 10% of young growing
swine die or
are culled due to lameness. Osteochondrosis (OC) is a major factor in this
lameness, causing
economic losses potentially exceeding $200 million in the United States alone.
[0003] OC is a non-infectious disease of cartilage affecting young growing
animals and
humans. OC is characterized by abnormal development of articular cartilages of
the joints
and in the growth plates of the bones, with associated changes in bone
development.
Lameness occurs when OC changes cause pain and/or interfere with normal
skeletal function.
[0004] OC is the major cause of lameness in swine. It has been reported that
20 to 80
percent or more of growing pigs are affected by. OC. OC severe enough to cause
lameness is
observed in 5 to 10 percent of horses and large breed dogs, and in 1 of 40
humans. OC is
also reported in young growing cattle, especially bulls, and in sheep. OC is
not common in
cats but has been reported.
[0005] In humans, OC primarily afflicts adolescents, an age group that is very
physically
active and has bones which are still growing. The disease is more common among
boys than
girls. In children between the ages of 10 to 15, the disease frequently
appears at the elbow,
1
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
knee, or foot joints. Afflicted humans experience tenderness, swelling, and
pain at the
affected joints which worsens with activity.
[0006] Among the more common forms of OC in human children are: Freiberg's
disease,
which occurs in the head of the metatarsals of the feet in children between
the ages of 12-15;
Legg-Calve-Perthes' disease, which occurs in the hip in children between the
ages of 6 to 9;
Osgood-Schlatter disease, which occurs in the tibial tubercle apo.physis at
the insertion of the
patellar tendon in the knee in children between the ages of 10 to 15; Panner's
disease, which
occurs in the capitellum of the distal humerus at the elbow in children
between the ages of 5-
10; and Sinding-Larsen-Johannson disease, which occurs at the inferior pole of
the patella in
the knee in children between the of ages 10-15. Animal correlates of each
human OC
condition are observed, with species and breed related "predilection sites".
In particular,
different specific joints are more likely to be affected in a given species or
breed; for instance
there is a tendency for "elbow dysplasia" to develop in German Shepherds.
[0007] Thus, improved methods for the prevention and treatment of OC would be
of great
economic value in the livestock industry, would reduce animal suffering, and
would help
alleviate the painful joint discomfort and loss of function and mobility
experienced by
humans suffering from this disease.
[0008] Furthermore, phosphate pollution resulting from excess phosphorus in
animal feed
is an increasing problem. Such phosphorus can potentially contaminate ground
water. There
is a need to provide animal feed with reduced phosphorus content to reduce
ground water
contamination. Reduction in phosphorus use would assist animal producers in
complying
with nutrient control regulations.
[0009] In addition, other factors that lead to lameness are: (1) lesions in
the cartilage and
subarticular bone; (2) necrosis of the joint surface and subarticular bone,
which can
measured by the number of infarcts (necrotic tissue caused by the obstruction
of the local
blood supply); (3) growth plate widening, which can lead to hyperplasia,
necrosis and
hemorrhage ; and (4) articular cartilage damage, which can be evidenced by
reduced
concentrations of glycosaminoglycans (GAGs), hydroxyproline, and other
biomolecules that
are related to or components of proteoglycans or collagen.
2
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0010] It would be highly advantageous if one could provide a reduced
phosphorus
animal feed that would simultaneously facilitate the prevention and treatment
of OC and
lameness.
BRIEF SUMMARY OF THE PREFERRED EMBODIMENTS OF THE INVENTION
[0011] The inventors have made the unexpected discovery that the
administration of
boron containing compounds is effective in preventing and treating
osteochondrosis in
animals. In one embodiment, this invention provides an animal feed containing
supplemental
boron. Animal feeds contain plant material. Boron is a required element for
plant growth.
As such all plants and hence all plant material in animal feeds contain some
boron, e.g. 10-20
ppm boron in corn/soybean feed (unless the boron as been extracted). The
animal feeds of
the present invention contain supplemental boron in addition to the boron
naturally present in
the animal feed from the plant material. The supplemental boron is supplied as
a boron-
containing compound, as plant material with elevated boron levels or as
microorganisms such
as yeast with elevated boron levels. Among the boron-containing compounds that
may be
used in the practice of the present invention are sodium borate and boric acid
as typical boron
sources. However, the invention is not limited to these forms of boron. Also
included are
other inorganic forms of boron such as calcium borate, as well as, organic
boron compounds
and complexes that dissociate or are metabolized in the body to release boron
as borate or
boric acid. Among the inorganic forms are sodium borate, boric acid, calcium
borate,
magnesium borate, halogen containing borate, ammonium borate, potassium
borate, iron and
magnesium containing borate, tantalum borate, beryllium borate, iron and
nickel containing
borate, carbonate containing borate, sodium and calcium containing borate,
arsenate
containing borate, calcium and rare earth containing borate, sulphate
containing borate,
magnesium and calcium containing borate, manganese borate, aluminum borate,
calcium and
strontium containing borate, phosphate containing borate, tin borate,
strontium borate, zinc
borate, calcium borosilicate, sodium borosilicate, aluminum borosilicate,
calcium and rare
earth containing borosilicate, lead borosilicate, barium borosilicate, lithium
borosilicate, and
sodium fluoroborate. Among the organic forms are complexes and compounds
formed by
boron, usually as boric acid, with fructose, sorbitol, mannitol, xylitol,
sorbose, threonine,
methionine, modified starches, hydrolyzed starches, oxidized starches, non-
modified
starches, dextrins, amidated sugars, glucosamine, mannosamine, esters of
glycerol fatty acids,
3
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
salicylate complexes, salts of bisoxalato acid, calcium borbsucrose, alcohols,
alcohol amines,
sugar acids, saccharic acid, gluconic acid, aminated sugar acids, and calcium
borogluconate.
In this embodiment, the supplemental boron containing compounds are typically
included in
animal feed at concentrations providing about 1 to about 500 ppm stipplemental
elemental
boron. In other embodiments, the boron containing compounds are typically
included in
animal feed at concentrations providing about 1 to about 150 ppm supplemental
elemental
boron. In yet another embodiment, the supplemental boron containing compounds
are
typically included in animal feed at concentrations providing about 50 ppm or
about 25 to 50
ppm supplemental elemental boron. Among the animals that would benefit from
the animal
feed are pigs, horses, mules, donkeys, cattle, sheep, goats, llamas, dogs, and
cats.
[0012] In a further unexpected discovery, the inventors have determined that
the addition
of supplemental boron to animal feed allows for the reduction in phosphorus
content of the
animal feed. Thus, in another embodiment, the invention provides an improved
animal feed
containing supplemental boron-containing compounds and reduced phosphorus
content. In
such an embodiment, the supplemental boron containing compound can be sodium
borate or
boric acid. However, the invention is not limited to these forms of
supplemental boron.
Other inorganic forms of boron such as calcium borate, as well as, organic
boron compounds
and complexes that dissociate or are metabolized in the body to release boron
as borate or
boric acid can be used as well. Among the inorganic forms are sodium borate,
boric acid,
calcium borate, magnesium borate, halogen containing borate, ammonium borate,
potassium
borate, iron and magnesium containing borate, tantalum borate, beryllium
borate, iron and
nickel containing borate, carbonate containing borate, sodium and calcium
containing borate,
arsenate containing borate, calcium and rare earth containing borate, sulphate
containing
borate, magnesium and calcium containing borate, manganese borate, aluminum
borate,
calcium and strontium containing borate, phosphate containing borate, tin
borate, strontium
borate, zinc borate,. calcium borosilicate, sodium borosilicate, aluminum
borosilicate,
calcium and rare earth containing borosilicate, lead borosilicate, barium
borosilicate, lithium
borosilicate, and sodium fluoroborate. Among these organic forms are complexes
and
compounds formed by boron, usually as boric acid, with fructose, sorbitol,
mannitol, xylitol,
sorbose, threonine, methionine, modified starches, hydrolyzed starches,
oxidized starches,
non-modified starches, dextrins, amidated sugars, glucosamine, mannosamine,
esters of
glycerol fatty acids, salicylate complexes, salts of bisoxalato acid, calcium
borosucrose,
4
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
alcohols, alcohol amines, sugar acids, saccharic acid, gluconic acid, aminated
sugar acids,
and calcium borogluconate. The boron can be combined with talc in a ratio of
boron
containing compound to talc of approximately 5:1, 6:1, 7:1, 8:1, 9:1, 10:1,
11:1, 12:1, 13:1,
14:1. 15:1, 16:1, 17:1, 18:1, 19:1, 20:1; 21:1; 22:1, 23:1; 24:1 or 25:1 prior
to addition to the
animal feed. The supplemental boron containing compounds are included in the
animal feed
at about 1 to about 500, about 1 to about 150 or about 50 ppm or about 25 to
50 ppm
supplemental boron and the total phosphorus content is reduced by at least 3%
as compared
to a comparable animal feed without supplemental boron. Generally, the animal
feed is
supplemented with boron at concentrations ranging from about 5 to about 150
ppm. The
animal feed is suitable for pigs, horses, mules, donkeys, cattle, sheep,
goats, llamas, dogs, and
cats among other animals.
[0013] In another embodiment, the invention provides a method of decreasing
the amount
of phosphorus excreted by an animal. In this embodiment, animals are fed a
diet of an
improved animal feed composition containing about 1 to about 500, about 1 to
about 150 or
about 50 ppm or about 25 to 50 ppm supplemental boron supplied as boron
containing
compounds, plant material with elevated boron levels, yeast or other
microorganisms with
elevated boron levels in which the animal feed composition has at least a 3%
reduction in
phosphorus as compared to a comparable animal feed without supplemental boron.
Generally, the animal feed contains supplemental boron at concentrations
ranging from 5-150
ppm. In such an embodiment, the supplemental boron containing compound can be
sodium
borate or boric acid can be used. However, other inorganic forms of boron such
as calcium
borate, as well as, organic boron compounds and complexes that dissociate or
are
metabolized in the body to release boron as borate or boric acid can be used.
Among the
inorganic forms are sodium borate, boric acid, calcium borate, magnesium
borate, halogen
borate, ammonium borate, potassium borate, iron and magnesium containing
borate, tantalum
borate, beryllium borate, iron and nickel containing borate, carbonate
containing borate,
sodium and calcium containing borate, arsenate containing borate, calcium and
rare earth
containing borate, sulphate containing borate, magnesium and calcium
containing borate,
manganese borate, aluminum borate, calcium and strontium containing borate,
phosphate
containing borate, tin borate, strontium borate, zinc borate, calcium
borosilicate, sodium
borosilicate, aluminum borosilicate, calcium and rare earth containing
borosilicate, lead
borosilicate, barium borosilicate, lithium borosilicate, and sodium
fluoroborate. Among the
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
organic forms are complexes and compounds formed by boron, usually as boric
acid, with
fructose, sorbitol, mannitol, xylitol, sorbose, threonine, methionine,
modified starches,
hydrolyzed starches, oxidized starches, non-modified starches, dextrins,
amidated sugars,
glucosamine, mannosamine, esters of glycerol fatty acids, salicylate
complexes, salts of
bisoxalato acid, calcium borosucrose, alcohols, alcohol amines, sugar acids,
saccharic acid,
gluconic acid, aminated sugar acids, and calcium borogluconate. The method is
suitable for
use with pigs, horses, mules, donkeys, cattle, sheep, goats, llamas, dogs, and
cats among
other animals.
[0014] An additional embodiment provides a method of increasing the efficiency
of
absorption of phosphorus in animals. In this embodiment, animals are fed a
diet of an
improved animal feed composition containing about 1 to about 500, about 1 to
about 150 or
about 50 ppm or about 25 to 50 ppm supplemental boron wherein the phosphorus
absorption
is improved by at least a 3% as compared to a comparable animal feed without
supplemental
boron. In such an embodiment, the supplemental boron containing compound can
be sodium
borate or boric acid. However, other inorganic forms of boron such as calcium
borate, as
well as, organic boron compounds and complexes that dissociate or are
metabolized in the
body to release boron as borate or boric acid can be used. Among the inorganic
forms are
sodium borate, boric acid, calcium borate, magnesium borate, halogen
containing borate,
ammonium borate, potassium borate, iron and magnesium containing borate,
tantalum borate,
beryllium borate, iron and nickel containing borate, carbonate containing
borate, sodium and
calcium containing borate, arsenate containing borate, calcium and rare earth
containing
borate, sulphate containing borate, magnesium and calcium containing borate,
manganese
borate, aluminum borate, calcium and strontium containing borate, phosphate
containing
borate, tin borate, strontium borate, zinc borate, calcium borosilicate,
sodium borosilicate,
aluminum borosilicate, calcium and rare earth containing borosilicate, lead
borosilicate,
barium borosilicate, lithium borosilicate, and sodium fluoroborate. Among the
organic forms
are complexes and compounds formed by boron, usually as boric acid, with
fructose, sorbitol,
mannitol, xylitol, sorbose, threonine, methionine, modified starches,
hydrolyzed starches,
oxidized starches, non-modified starches, dextrins, amidated sugars,
glucosamine,
mannosamine, esters of glycerol fatty acids, salicylate complexes, salts of
bisoxalato acid,
calcium borosucrose, alcohols, alcohol amines, sugar acids, saccharic acid,
gluconic acid,
6
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
aminated sugar acids, and calcium borogluconate. The method is suitable for
use with pigs,
horses, mules, donkeys, cattle, sheep, goats, llamas, dogs, and cats among
other animals.
[00151 In yet an additional embodiment, this invention provides a method of
reducing
environmental phosphorus pollution from an animal farm. In this embodiment,
animals are
fed a diet of an improved animal feed composition containing 1 to about 500,
about 1 to
about 150 or about 50 ppm or about 25 to 50 ppm supplemental boron containing
compounds
whereby the phosphorus efflux is reduced by at least 3% as compared to that of
a comparable
animal feed without supplemental boron. In such an embodiment, the
supplemental boron
containing compound can be sodium borate or boric acid. However, inorganic
forms of
boron such as calcium borate, as well as, organic boron compounds and
complexes that
dissociate or are metabolized in the body to release boron as borate or boric
acid can be used.
Among the inorganic forms are sodium borate, boric acid, calcium borate,
magnesium borate,
halogen containing borate, ammonium borate, potassium borate, iron and
magnesium
containing borate, tantalum borate, beryllium borate, iron and nickel
containing borate,
carbonate containing borate, sodium and calcium containing borate, arsenate
containing
borate, calcium and rare earth containing borate, sulphate containing borate,
magnesium and
calcium containing borate, manganese borate, aluminum borate, calcium and
strontium
containing borate, phosphate containing borate, tin borate, strontium borate,
zinc borate,
calcium borosilicate, sodium borosilicate, aluminum borosilicate, calcium and
rare earth
containing borosilicate, lead borosilicate, barium borosilicate, lithium
borosilicate, and
sodium fluoroborate. Among the organic forms are complexes and compounds
formed by
boron, usually as boric acid, with fructose, sorbitol, mannitol, xylitol,
sorbose, threonine,
methionine, modified starches, hydrolyzed starches, oxidized starches, non-
modified.
starches, dextrins, amidated sugars, glucosamine, mannosamine, esters of
glycerol fatty acids,
salicylate complexes, salts of bisoxalato acid, calcium borosucrose, alcohols,
alcohol amines,
sugar acids, saccharic acid, gluconic acid, aminated sugar acids, and calcium
borogluconate.
The method is suitable for use with pigs, horses, mules, donkeys, cattle,
sheep, goats, llamas,
dogs, and cats among other animals.
[00161 In a further embodiment, the invention also provides a method of
treating or
preventing OC by administering a therapeutically effective amount of a boron
containing
compound to a mammal in need of such treatment. In such an embodiment, the
boron
7
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
containing compound can be sodium borate or boric acid. However, the invention
can be
used with other inorganic forms of boron such as calcium borate, as well as,
organic boron
compounds and complexes that dissociate or are metabolized in the body to
release boron as
borate or boric acid can be used. Among the inorganic forms are sodium borate,
boric acid,
calcium borate, magnesium borate, halogen containing borate, ammonium borate,
potassium
borate, iron and magnesium containing borate, tantalum borate, beryllium
borate, iron and
nickel containing borate, carbonate containing borate, sodium and calcium
containing borate,
arsenate containing borate, calcium and rare earth containing borate, sulphate
containing
borate, magnesium and calcium containing borate, manganese borate, aluminum
borate,
calcium and strontium containing borate, phosphate containing borate, tin
borate, strontium
borate, zinc borate, calcium borosilicate, sodium borosilicate, aluminum
borosilicate, calcium
and rare earth containing borosilicate, lead borosilicate, barium
borosilicate, lithium
borosilicate, and sodium fluoroborate. Among the organic forms are complexes
and
'compounds formed by boron, usually as boric acid, with fructose, sorbitol,
mannitol, xylitol,
sorbose, threonine, methionine, modified starches, hydrolyzed starches,
oxidized starches,
non-modified starches, dextrins, amidated sugars, glucosamine, mannosamine,
esters of
glycerol fatty acids, salicylate complexes, salts of bisoxalato acid, calcium
borosucrose,
alcohols, alcohol amines, sugar acids, saccharic acid, gluconic acid, aminated
sugar acids,
and calcium borogluconate. The mammal to be treated can be a human or an
animal. The
supplemental boron-containing compounds can be administered prior to the
appearance of
symptoms of osteochondrosis as a preventive measure. Among the animals that
can benefit
from this invention are pigs, horses, mules, donkeys, cattle, sheep, goats,
llamas, dogs, and
cats.
[00171. In a further unexpected discovery, the inventors have determined that
the addition
of supplemental boron to animal feed allows for the reduction in the incidence
and extent of
lesions in the cartilage and subarticular bone of animals. Thus, in this
embodiment, the
invention provides a method for reducing the incidence and extent of lesions
in the cartilage
and subarticular bone of animals. In this embodiment, the supplemental boron
containing
compounds are typically included in animal feed at concentrations providing
about 1 to about
500 ppm supplemental elemental boron. In other embodiments, the boron
containing
compounds are typically included in animal feed at concentrations providing
about 1 to about
150 ppm supplemental elemental boron. In yet another embodiment, the
supplemental boron
8
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
containing compounds are typically included in animal feed at concentrations
providing
about 50 ppm or about 25 to 50 ppm supplemental elemental boron. In such an
embodiment,
the feed could also be in the form of a liquid. In such an embodiment, the
supplemental
boron containing compound can be sodium borate or boric acid can be used.
However, other
inorganic forms of boron such as calcium borate, as well as, organic boron
compounds and
complexes that dissociate or are metabolized in the body to release boron as
borate or boric
acid can be used. Among the inorganic forms are sodium borate, boric acid,
calcium borate,
magnesium borate, halogen borate, ammonium borate, potassium borate, iron and
magnesium
containing borate, tantalum borate, beryllium borate, iron and nickel
containing borate,
carbonate containing borate, sodium and calcium containing borate, arsenate
containing
borate, calcium and rare earth containing borate, sulphate containing borate,
magnesium and
calcium containing borate, manganese borate, aluminum borate, calcium and
strontium
containing borate, phosphate containing borate, tin borate, strontium borate,
zinc borate,
calcium borosilicate, sodium borosilicate, aluminum borosilicate, calcium and
rare earth
containing borosilicate, lead borosilicate, barium borosilicate, lithium
borosilicate, and
sodium fluoroborate. Among the organic forms are complexes and compounds
formed by
boron, usually as boric acid, with fructose, sorbitol, mannitol, xylitol,
sorbose, threonine,
methionine, modified starches, hydrolyzed starches, oxidized starches, non-
modified
starches, dextrins, amidated sugars, glucosamine, mannosamine, esters of
glycerol fatty acids,
salicylate complexes, salts of bisoxalato acid, calcium borosucrose, alcohols,
alcohol amines,
sugar acids, saccharic acid, gluconic acid, aminated sugar acids, and calcium
borogluconate.
Among the animals that would benefit from the animal feed are pigs, horses,
mules, donkeys,
cattle, sheep, goats, llamas, dogs, cats, as well as humans.
[0018] In a further unexpected discovery, the inventors have determined that
the addition
of supplemental boron to animal feed allows for the prevention of necrosis of
the joint
surface and subarticular bone as measured by the number of infarcts (necrotic
tissue caused
by the obstruction of the local blood supply). Thus, in this embodiment, the
invention
provides a method for preventing necrosis of the joint surface and
subarticular bone as
measured by the number of infarcts (necrotic tissue caused by the obstruction
of the local
blood supply). In this embodiment, the supplemental boron containing compounds
are
typically included in animal feed at concentrations providing about 1 to about
500 ppm
supplemental elemental boron. In other embodiments, the boron containing
compounds are
9
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
typically included in animal feed at concentrations providing about 1 to about
150 ppm
supplemental elemental boron. In yet another embodiment, the supplemental
boron
containing compounds are typically included in animal feed at concentrations
providing
about 50 ppm or about 25 to 50 ppm supplemental elemental boron. In such an
embodiment,
the feed could also be in the form of a liquid. In such an embodiment, the
supplemental
boron containing compound can be sodium borate or boric acid can be used.
However, other
inorganic forms of boron such as calcium borate, as well as, organic boron
compounds and
complexes that dissociate or are metabolized in the body to release boron as
borate or boric
acid can be used. Among the inorganic forms are sodium borate, boric acid,
calcium borate,
magnesium borate, halogen borate, ammonium borate, potassium borate, iron and
magnesium
containing borate, tantalum borate, beryllium borate, iron and nickel
containing borate,
carbonate containing borate, sodium and calcium containing borate, arsenate
containing
borate, calcium and rare earth containing borate, sulphate containing borate,
magnesium and
calcium containing borate, manganese borate, aluminum borate, calcium and
strontium
containing borate, phosphate containing borate, tin borate, strontium borate,
zinc borate,
calcium borosilicate, sodium borosilicate, aluminum borosilicate, calcium and
rare earth
containing borosilicate, lead borosilicate, barium borosilicate, lithium
borosilicate, and
sodium fluoroborate. Among the organic forms are complexes and compounds
formed by
boron, usually as boric acid, with fructose, sorbitol, mannitol, xylitol,
sorbose, threonine,
methionine, modified starches, hydrolyzed starches, oxidized starches, non-
modified
starches, dextrins, amidated sugars, glucosamine, mannosamine, esters of
glycerol fatty acids,
salicylate complexes, salts of bisoxalato acid, calcium borosucrose, alcohols,
alcohol amines,
sugar acids, saccharic acid, gluconic acid, aminated sugar acids, and calcium
borogluconate.
Among the animals that would benefit from the animal feed are pigs, horses,
mules, donkeys,
cattle, sheep, goats, llamas, dogs, cats, as well as humans.
[0019] In a further unexpected discovery, the inventors have determined that
the addition
of supplemental boron to animal feed allows for the reduction of hyperplasia,
necrosis and
hemorrhage. Thus, in this embodiment, the invention provides a method for
reducing
hyperplasia, necrosis and hemorrhage. In this embodiment, the supplemental
boron
containing compounds are typically included in animal feed at concentrations
providing
about 1 to about 500 ppm supplemental elemental boron. In other embodiments,
the boron
containing compounds are typically included in animal feed at concentrations
providing
CA 02651540 2008-11-03
WO 2007/142684
PCT/US2006/046687
about 1 to about 150 ppm supplemental elemental boron. In yet another
embodiment, the
supplemental boron containing compounds are typically included in animal feed
at
concentrations providing about 50 ppm or about 25 to 50 ppm supplemental
elemental boron.
In such an embodiment, the feed could also be in the form of a liquid. In such
an
embodiment, the supplemental boron containing compound can be sodium borate or
boric
acid can be used. However, other inorganic forms of boron such as calcium
borate, as well
as, organic boron compounds and complexes that dissociate or are metabolized
in the body to
release boron as borate or boric acid can be used. Among the inorganic forms
are sodium
borate, boric acid, calcium borate,'magnesium borate, halogen borate, ammonium
borate,
potassium borate, iron and magnesium containing borate, tantalum borate,
beryllium borate,
iron and nickel containing borate, carbonate containing borate, sodium and
calcium
containing borate, arsenate containing borate, calcium and rare earth
containing borate,
sulphate containing borate, magnesium and calcium containing borate, manganese
borate,
aluminum borate, calcium and strontium containing borate, phosphate containing
borate, tin
borate, strontium borate, zinc borate, calcium borosilicate, sodium
borosilicate, aluminum
borosilicate, calcium and rare earth containing borosilicate, lead
borosilicate, barium
borosilicate, lithium borosilicate, and sodium fluoroborate. Among the organic
forms are
complexes and compounds formed by boron, usually as boric acid, with fructose,
sorbitol,
mannitol, xylitol, sorbose, threonine, methionine, modified starches,
hydrolyzed starches,
oxidized starches, non-modified starches, dextrins, amidated sugars,
glucosamine,
mannosamine, esters of glycerol fatty acids, salicylate complexes, salts of
bisoxalato acid,
calcium borosucrose, alcohols, alcohol amines, sugar acids, saccharic acid,
gluconic acid,
aminated sugar acids, and calcium borogluconate. Among the aniinals that would
benefit
from the animal feed are pigs, horses, mules, donkeys, cattle, sheep, goats,
llamas, dogs, cats,
as well as humans.
[0020] In a further unexpected discovery, the inventors have determined that
the addition
of supplemental boron to animal feed allows for the reduction of dysplasia
(abnormal
development and or abnormal structure) in cartilage, growth plate and bone as
measured by
the growth plate width (a wide growth plate being representative of abnormal
growth and
improper ossification). Thus, in this embodiment, the invention provides a
method for
reducing dysplasia as measured by the growth plate width; (a wide growth plate
being
representative of abnormal growth and improper ossification). In this
embodiment, the
11
-Mu~;aÃmÃp'jqg
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
supplemental boron containing compounds are typically included in animal feed
at
concentrations providing about 1 to about 500 ppm supplemental elemental
boron. In other
embodiments, the boron containing compounds are typically included in animal
feed at
concentrations providing about 1 to about 150 ppm supplemental elemental
boron. In yet
another embodiment, the supplemental boron containing compounds are typically
included in
animal feed at concentrations providing about 50 ppm or about 25 to 50 ppm
supplemental
elemental boron. In such an embodiment, the feed could also be in the form of
a liquid. In
such an embodiment, the supplemental boron containing compound can be sodium
borate or
boric acid can be used. However, other inorganic forms of boron such as
calcium borate, as
well as, organic boron compounds and complexes that dissociate or are
metabolized in the
body to release boron as borate or boric acid can be used. Among the inorganic
forms are
sodium borate, boric acid, calcium borate, magnesium borate, halogen borate,
ammonium
borate, potassium borate, iron and magnesium containing borate, tantalum
borate, beryllium
borate, iron and nickel containing borate, carbonate containing borate, sodium
and calcium
containing borate, arsenate containing borate, calcium and rare earth
containing borate,
sulphate containing borate, magnesium and calcium containing borate, manganese
borate,
aluminum borate, calcium and strontium containing borate, phosphate containing
borate, tin
borate, strontium borate, zinc borate, calcium borosilicate, sodium
borosilicate, aluminum
borosilicate, calcium and rare earth containing borosilicate, lead
borosilicate, barium
borosilicate, lithium borosilicate, and sodium fluoroborate. Among the organic
forms are
complexes and compounds formed by boron, usually as boric acid, with fructose,
sorbitol,
mannitol, xylitol, sorbose, threonine, methionine, modified starches,
hydrolyzed starches,
oxidized starches, non-modified starches, dextrins, amidated sugars,
glucosamine,
mannosamine, esters of glycerol fatty acids, salicylate complexes, salts of
bisoxalato acid,
calcium borosucrose, alcohols, alcohol amines, sugar acids, saccharic acid,
gluconic acid,
aminated sugar acids, and calcium borogluconate. Among the animals that would
benefit
from the animal feed are pigs, horses, mules, donkeys, cattle, sheep, goats,
llamas, dogs, cats,
as well as humans.
[0021] In a further unexpected discovery, the inventors have determined that
the addition
of supplemental boron to animal feed allows for the reduction of articular
cartilage damage as
evidenced by higher concentrations, typical of healthy tissue, of
glycosaminoglycans
(GAGs), hydroxyproline, and other biomolecules that are related to or
components of
12
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
proteoglycans or collagen. Thus, in this embodiment, the invention provides a
method of
reducing articular cartilage damage as evidenced by higher concentrations,
typical of healthy
tissue, of glycosaminoglycans (GAGs), hydroxyproline, and other biomolecules
that are
related to or components of proteoglycans or collagen. In this embodiment, the
supplemental
boron containing compounds are typically included in animal feed at
concentrations
providing about 1 to about 500 ppm supplemental elemental boron. In other
embodiments,
the boron containing compounds are typically included in animal feed at
concentrations
providing about I to about 150 ppm supplemental elemental boron. In yet
another
embodiment, the supplemental boron containing compounds are typically included
in animal
feed at concentrations providing about 50 ppm or about 25 to 50 ppm
supplemental elemental
boron. ,In such an embodiment, the feed could also be in the form of a liquid.
In such an
embodiment, the supplemental boron containing compound can be sodium borate or
boric
acid can be used. However, other inorganic forms of boron such as calcium
borate, as well
as, organic boron compounds and complexes that dissociate or are metabolized
in the body to
release boron as borate or boric acid can be used. Among the inorganic forms
are sodium
borate, boric acid, calcium borate, magnesium borate, halogen borate, ammonium
borate,
potassium borate, iron and magnesium containing borate, tantalum borate,
beryllium borate,
iron and nickel containing borate, carbonate containing borate, sodium and
calcium
containing borate, arsenate containing borate, calcium and rare earth
containing borate,
sulphate containing borate, magnesium and calcium containing borate, manganese
borate,
aluminum borate, calcium and strontium containing borate, phosphate containing
borate, tin
borate, strontium borate, zinc borate, calcium borosilicate, sodium
borosilicate, aluminum
borosilicate, calcium and rare earth containing borosilicate, lead
borosilicate, barium
borosilicate, lithium borosilicate, and sodium fluoroborate. Among the organic
forms are
complexes and compounds formed by boron, usually as boric acid, with fructose,
sorbitol,
mannitol, xylitol, sorbose, threonine, methionine, modified starches,
hydrolyzed starches,
oxidized starches, non-modified starches, dextrins, amidated sugars,
glucosamine,
mannosamine, esters of glycerol fatty acids, salicylate complexes, salts of
bisoxalato acid,
calcium borosucrose, alcohols, alcohol amines, sugar acids, saccharic acid,
gluconic acid,
aminated sugar acids, and calcium borogluconate. Among the animals that would
benefit
from the animal feed are pigs, horses, mules, donkeys, cattle, sheep, goats,
llamas, dogs, cats,
as well as humans.
13
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0022] In another embodiment, the invention provides a method of decreasing
the amount
of pre-weaning mortality in animals. In another embodiment, the invention
provides a
method of improving reproductive rates of animals by increasing the rate of
return to estrus
and conception rates. In these embodiments, previously pregnant, pregnant,
nursing and/or
lactating animals are fed a diet of increased boron. The diet may contain
about I to about
500, about 1 to about 150 or about 50 ppm or about 25 to 50 ppm supplemental
boron
containing compounds. The boron may be provided in improved animal feed
composition or
in milk or water. Generally, the milk, water or animal feed contains
supplemental boron at
concentrations ranging from 5-150 ppm. In such embodiments, the supplemental
boron
containing compound can be sodium borate or boric acid can be used. However,
other
inorganic forms of boron such as calcium borate, as well as, organic boron
compounds and
complexes that dissociate or. are metabolized in the body to release boron as
borate or boric
acid can be used. Among the inorganic forms are sodium borate, boric acid,
calcium borate,
magnesium borate, halogen borate, ammoniurn borate, potassium borate, iron and
magnesium
containing borate, tantalum borate, beryllium borate, iron and nickel
containing borate,
carbonate containing borate, sodium and calcium containing borate, arsenate
containing
borate, calcium and rare earth containing borate, sulphate containing borate,
magnesium and
calcium containing borate, manganese borate, aluminum borate, calcium and
strontium
containing borate, phosphate containing borate, tin borate, strontium borate,
zinc borate,
calcium borosilicate, sodium borosilicate, aluminum borosilicate, calcium and
rare earth
containing borosilicate, lead borosilicate, barium borosilicate, lithium
borosilicate, and
sodium fluoroborate. Among the organic forms are complexes and compound formed
by
boron, usually as boric acid, with fructose, sorbitol, mannitol, xylitol,
sorbose, threonine,
methionine, modified starches, hydrolyzed starches, oxidized starches, non-
modified
starches, dextrins, amidated sugars, glucosamine, mannosamine, esters of
glycerol fatty acids,
salicylate complexes, salts of bisoxalato acid, calcium borosucrose, alcohols,
alcohol amines,
sugar acids, saccharic acid, gluconic acid, aminated sugar acids, and calcium
borogluconate.
The method is suitable for use with pigs, horses, mules, donkeys, cattle,
sheep, goats, llamas,
dogs, and cats among other animals.
[0023] In an additional embodiment, the boron-containing compounds are added
to
drinking water, mineral or vitamin supplements, in a milk formulation, or
other food products
for the treatment and prevention of OC and/or reduction in pre-weaning
mortality.
14
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0024] In an additional embodiment, this invention provides a boron-talc
composition
where the ratio of boron-containing compound to talc is approximately 5:1,
6:1, 7:1; 8:1, 9:1,
10:1, 11:1, 12:1, 13:1, 14:1, 15:1, 16:1, 17:1, 18:1, 19:1, 20:1; 21:1, 22:1,
23:1, 24:1 or 25:1.
In such an embodiment, the boron is a boron containing compound which can be
sodium
borate or boric acid. However, the invention is not limited to these forms of
supplemental
boron. Other inorganic forms of boron such as calcium borate, as well as,
organic boron
compounds and complexes that dissociate or are metabolized in the body to
release boron as
borate or boric acid can be used as well. Among the inorganic forms are sodium
borate, boric
acid, calcium borate, magnesium borate, halogen containing borate, ammonium
borate,
potassium borate, iron and magnesium containing borate, tantalum borate,
beryllium borate,
iron and nickel containing borate, carbonate containing borate, sodium and
calcium
containing borate, arsenate containing borate, calcium and rare earth
containing borate,
sulphate containing borate, magnesium and calcium containing borate, manganese
borate,
aluminum borate, calcium and strontium containing borate, phosphate containing
borate, tin
borate, strontium borate, zinc borate,. calcium borosilicate, sodium
borosilicate, aluminum
borosilicate, calcium and rare earth containing borosilicate, lead
borosilicate, barium '
borosilicate, lithium borosilicate, and sodium fluoroborate. Among these
organic forms are
complexes and compounds formed by boron, usually as boric acid, with fructose,
sorbitol,
mannitol, xylitol, sorbose, threonine, methionine, modified starches,
hydrolyzed starches,
oxidized starches, non-modified starches, dextrins, amidated sugars,
glucosamine,
mannosamine, esters of -glycerol fatty acids, salicylate complexes, salts of
bisoxalato acid,
calcium borosucrose, alcohols, alcohol amines, sugar acids, saccharic acid,
gluconic acid,
aminated sugar acids, and calcium borogluconate.
[0025] Talc is available for use in the present exemplary embodiments from a
variety of
commercial sources. For example, Luzenac America is a supplier of talc.
Examples of talc
products from Luzenac America include: E-Z Flow 40, E-Z- Flow MB, E-Z Flow MT,
E-Z
Flow RM, and E-Z Flow VT.
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
BRIEF DESCRIPTION OF THE DRAWINGS
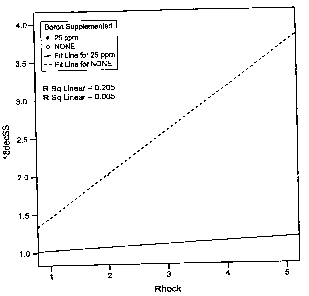
[0026] Figure 1 is a graph indicating a reduction in the occurrence of
osteochondrosis
with supplemental boron treatment.
[0027] Figure 2 is a graph showing the association between increasing
osteochondrosis
scores in the pig right hock with increasing soundness scores among pigs not
receiving
supplemental boron.
[0028] Figure 3 is a graph showing the effect of supplemental boron treatment
in the
reduction of soundness scores associated with early growth.
[0029] Figure 4 and 5 are graphs which show that administration of 3-NPB along
with
boron resulted in a prevalence and severity of gross joint pathology similar
to that observed
in unsupplemented pigs.
DETAILED DESCRIPTION OF THE INVENTION
Introduction
[0030] Boron has long been known to be an essential plant nutrient, but a role
for boron
in human physiology has only recently been appreciated. The present inventors
have
discovered a beneficial effect of boron supplementation of animal and human
diets. In
particular, although previous studies had shown that boron containing
compounds could
alleviate the bone disease, osteoporosis, the present inventors have
discovered that boron
containing compounds also alleviate a disease of the joints and growth plate
cartilages,
osteochondrosis (OC).
[0031] Osteoporosis is a disease in which bones become fragile and become
increasingly
likely to break as the disease progresses. Osteoporosis, or porous bone, is
characterized by
low bone mass and structural deterioration of bone tissue, which leads to bone
fragility and
an increased susceptibility to fractures of the hip, spine, and wrist. Thus,
osteoporosis is a
disease that specifically strikes the bone, generally after full 'and normal
development. Also,
because of its progressive nature, osteoporosis is a disease that most
commonly manifests
itself in older individuals. One out of every two women and one in four men
over the age of
50 will have an osteoporosis-related fracture in their lifetimes.
16
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0032] In contrast, osteochondrosis is a generalized skeletal disease of
growing animals
and results from a disturbance in the articular and growth plate cartilages.
The bone is only
secondarily affected. As a consequence, dyschondroplasia is technically a more
correct term
to describe this condition. A further condition, osteochondrosis dissecans,
results in the
chipping, fracturing and/or fragmentation of the articular surface.
Osteochondrosis dissecans
is thought to be caused by an underlying weakness in the cartilage caused by
an
osteochondrotic lesion. Lesions are characterized by focal impaired
endochondondral
ossification, resulting in areas of retained cartilage extending into the
subchondral bone. See
R. John Wardale and Victor C. Duance. Journal of Cell Science 107, 47-59
(1994).
[0033] Because of the differences in the pathobiology of OC and osteoporosis -
the two
diseases affect different aspects of the bone system and different age groups -
one would not
expect that a treatment that alleviates osteoporosis, a degenerative disease
of the bones of the
elderly, would help in the treatment of OC, a disease of the cartilage in
joints of the young.
The inventors have found that surprisingly, boron containing compounds are
useful agents in
the prevention and treatment of OC.
Role of boron in the behavior of cartilage
[0034] , The extracellular matrix (ECM) of the articular cartilage provides
cushioning
between opposing bone surfaces at a joint in a mammalian limb. Synovial fluid
is the fluid
contained in joints. Synovial membranes line the joints, bursae, and tendon
sheaths. The
function of the synovial fluid is to lubricate the joint space and transport
nutrients to the
articular cartilage. The articular cartilage provides a low friction point of
contact for the
smooth flexing operation of the joints and also a cushioning function at
joints, by absorbing
the impact of shocks transmitted through the bones and supporting the weight
of the animal.
The cartilage is composed of a variety of components including proteoglycan
and a collagen
network in an aqueous environment.
[0035] Proteoglycans play a role in maintaining the cushioning seen at joints.
The
cartilage ECM is illustrated as a network of the collagen fibers which
interlocks with and is
interlocked by proteoglycan. The proteoglycan is a flexible gel-like material
and the collagen
forms a mesh-network that holds the proteoglycan in place. The proteoglycan
provides
compressive strength while tensile strength is provided by the collagen
network.
17
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
Proteoglycan in articular and growth plate cartilage contains large amounts of
sulfated
glycosaminoglycans (GAG) that have a strong negative charge. At physiologic
pH, these
negatively charged GAG molecules draw sodium ions and water into the ECM of
the
cartilage, causing the proteoglycan to "inflate". The inflated proteoglycan
provides buoyant
pressure to resist compression, thus protecting the collagen network and
underlying structures
from compression damage. Better cushioning and thus greater compression
resistance is
provided by a fully hydrated proteoglycan complement and a fully extended and
taut collagen
network.
[0036] Without limiting this invention to any particular mechanism nor being
bound by
theory, one potential model for how boron functions in OC is the "standing
hypothesis". In
this hypothesis, boron functions by crosslinking the proteoglycan into the
extracellular
matrix. One postulated mechanism for how this occurs is that boron provides
for three-
dimensional boroester crosslinking of carbohydrate, proteoglycan,
glycoprotein, glycolipid,
lipid, protein, and amino acid structures. In the case of extracellular
membrane structures
such as cartilage and neural tissue, this would include proteoglycans such as
aggrecan (the
large aggregating proteoglycan of cartilage), complex proteins such as
collagen in its various
forms and types, and associated proteins such as cartilage link protein. The
crosslinking of
the proteoglycan stabilizes and unifies the matrix, allowing for better
distribution of
compressive forces and prevention of proteoglycan loss, which would decrease
the
cushioning ability of the synovial membrane. In contrast, boron functions to
prevent
osteoporosis by increasing the plasma levels of hydroxylated steroids. See
U.S. Patent No.
4,849,220. Thus, one would not have predicted that boron would have an effect
in treating a
disease of the cartilage, such as OC, which has a totally different etiology
from osteoporosis.
In osteoporosis, the bone itself is directly affected. In OC, the cartilage is
affected.
Etiologyand pathology of OC
[0037] Although the precise cause of OC is not yet known, a number of
mechanisms for
the progression of this disease have been suggested. The influence of
compressive forces in
producing damage to the growing and transitioning cartilage appears to be a
major factor.
Studies in pigs have suggested that focal changes in blood supply during
normal epiphyseal
growth is central to the pathogenesis of osteochondrosis. Cartilage canals are
temporary
blood vessel-containing structures within growing cartilage. The canals
gradually regress
18
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
with age during the process of chondrification, wherein the blood vessels
contained within
the canals are replaced with cartilage. Formation of the lesions associated
with
osteochondrosis has been associated with the premature chondrification and
regression of
these canals. In particular, the premature disruption of the blood supply
results in necrosis of
the cartilage canal distal to the point of interruption. See Ytrehus et al.
Bone 35: 1294-1306
(2004). Thus, it is not surprising that severe clinical osteochondrosis
appears most
commonly in fast-growing animals with rapid weight gain. See Wardale and
Duance Journal
of Cell Science 107: 47-59 (1994).
[0038] It has also been demonstrated in humans and dogs that in
osteochondrosis, the
proteoglycan of cartilage is resorbed by the action of matrix
metalloproteinase-3 (MMP-3)
derived from synovial membrane cells and chondrocytes. See Shinmei et al.
1991; Okada et
al. 1992; Mehraban et al. 1994. Loss of proteoglycan from the extracellular
matrix of the
cartilage would lead to a decreased capacity of the cartilage to absorb and
cushion
compressive forces.
Role of Boron in OC
[0039] In order to further probe the contribution of boron deficiency in OC,
and to test
whether boron functions in OC through quadrivalent crosslinking, 3-
nitrophenylboronic acid
(3-NPB) was administered to animals. 3-NPB blocks crosslinks by binding to
sites normally
occupied by boric acid or borate. The results are described in Example 5. 3-
NPB treated
animals had increased lameness and clinical manifestations of OC. The increase
in lameness
could be prevented by supplementing the diet with boron. These experiments
show that OC
is directly correlated to boron levels in pigs, horses, cattle, and dogs.
Boron compounds for the treatment of OC in Animals
[0040] Given the widespread occurrence of OC in livestock and in particular,
pigs, this
invention discloses a safe and effective means of preventing and treating OC
by providing for
animal feed to be supplemented with boron containing compounds. It will be
appreciated by
one of skill in the art that animal feeds, derived at least in part from plant
materials, will
contain basal levels of boron since boron is a required element for plant
growth. For
instance, typical alfalfa contains about 37 ppm boron. Thus, the term
supplemental boron as
used herein refers to exogenously added boron that supplements the basal
levels of boron
19
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
already present in commonly used animal feeds. When the term boron is used in
this
disclosure, it can denote both elemental boron and boron containing compounds.
The boron
containing compounds useful for the practice of this invention may include any
suitable
organic or inorganic boron containing compounds, including boron containing
minerals.
Among the preferred forms of boron are sodium borate and boric acid. Other
useful
inorganic forms of boron include calcium borate. One of skill in the art will
recognize that
other inorganic forms of boron that may be used in this invention include
borates with:
magnesium, halogen, ammonium, potassium, iron and magnesium, tantalum,
beryllium and
nickel, carbonate, sodium and calcium, arsenate, calcium and rare earth,
sulphate, magnesium
and calcium, manganese, aluminum, calcium and strontium, phosphate, tin, zinc,
and
strontium. Other forms include: borosilicates or silicoborates with calcium,
sodium,
aluminum, calcium and rare earth, lead, barium, lithium, and fluoroborate with
sodium.
Natural inorganic boron containing compounds are known to skilled artisans by
various
mineral names such as borax, colemanite, hydroboracite, kernite, ulexite,
datolite, danburite,
szaibelyite, suanite, inderite, sassolite, inyoite, probertite, howlite,
ezcurrite, kurnakovite,
meyerhofferite, priceite, nobleite, and searlesite to name but a few such
designations. A
listing of inorganic borate compounds and minerals can be found in Supplement
to Mellor's
Comprehensive Treatise on Inorganic and Theoretical Chemistry, Volume V Boron,
by
Joseph William Mellor, Longman Group Limited, London, 1980.
[0041] Examples of organic boron-containing compounds are well known to those
of
skill in the art. Examples of such organic boron-containing compounds are
found in U.S.
Patent Nos. 4,312,989, 4,499,082, and 5,312,816 all of which are hereby
incorporated by
reference. Among the forms of organic boron that would be useful in the
practice of this
invention are organic boron complexes such as boron threonine, boron
methionine, and boron
ascorbate, as well as boron complexed with other amino acids. These amino
acids can
include the 20 common amino acids that are specified by the genetic code, as
well as variant
and modified amino acids which are not encoded by the genetic code. These are
examples of
organic forms of boron that are rapidly metabolized to release borate or boric
acid. Other
useful forms of organic boron are boron carbohydrate complexes such as those
disclosed in
U.S. Patent No. 5,962,049. Among the carbohydrates that form useful complexes
with boron
include saccharides such as fructose, sorbitol, mannitol, xylitol, and
sorbose. A
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
commercially available form of boron complexed with fructose is Fruitex BTM
available from
FutureCeuticals and described in US Patent No. 5,962,049.
[0042] Other organic forms of boron that can be used in the practice of this
invention
include: borated modified starches (such as hydrolyzed or oxidized starches),
borate non-
modified starches, borated dextrins, borated amidated sugars (such as
glucosamine or
mannosamine), borate esters of glycerol fatty acids, borate-salicylate
complexes, salts of
bisoxalato borate (such as sodium or potassium salts), calcium borosucrose,
borate esters (
such as (RO) 3B), alcohol amine borate esters, and borate complexes with sugar
acids (such
as saccharic acid and gluconic acid), and borate complexes with aminated sugar
acids. One
particularly desirable sugar acid to use in this invention is calcium
borogluconate. Yet
another form of boron are anion exchange resins which can be boronated. One
such resin
which can be boronated is AmberliteTM
j0043] It will be appreciated by one of skill in the art that when a
particular boron
containing compound is-described herein, it is intended that all possible
solvates,
pharmaceutically acceptable salts, esters, amides, complexes, chelates,
stereoisomers,
geometric isomers, crystalline or amorphous forms, metabolites, metabolic
precursors or
prodrugs of the compound are also separately described by a chemical
structural formula or
chemical name. Furthermore, if any of the boron containing compounds described
herein
contain stereochemistry, all enantiomeric and diastereomeric forms of the
compound are
intended. Thus, when applicable, boron containing compounds may occur as
racemates,
racemic mixtures and as individual diastereomers, or enantiomers with all
isomeric forms
being included. A racemate or racemic mixture does not necessarily imply a
50:50 mixture
of stereoisomers.
[0044] Furthermore, it will be appreciated by one of skill in the art that the
borates of the
present invention will encompass many different grades, including those that
are FDA and
non-FDA approved. Thus, among the grades of borates that can be used in the
practice of
this invention are: pharmaceutical or formulary grade, nuclear grade,
fertilizer grade,
industrial grade, pesticidal grade, and special quality (SQ) grade.
[0045] Suitable ranges for use of the boron containing compounds includes the
supplementation of boron in animal feed from about 1 to about 500 ppm above
that naturally
21
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
present in the animal feed. Another suitable range for supplementation is
about 1 to about
150 ppm. As shown in Figures 1, 3 and 4, the inventors have found that
supplemental boron
at 25 ppm to 50 ppm provides a significant reduction in the occurrence of OC
in pigs.
Accordingly, in one embodiment, this invention provides an animal feed
composition that is
supplemented with 25 ppm to 50 ppm boron containing compounds. In one
embodiment, the
supplemental boron containing compound is sodium borate. In another
embodiment, the
supplemental boron containing compound is boric acid. It will be clear to one
of skill in the
art that other concentrations of boron may be used depending on the severity
of the disease or
animal to be treated. Furthermore, it will be clear to one of skill in the art
that other
supplemental boron containing compounds may also be used in the practice of
this invention.
[00461 The boron described herein may be combined with talc. The boron-
containing
compound to talc ratio may be approximately 5:1, 6:1, 7:1, 8:1, 9:1, 10:1,
11:1, 12:1, 13:1,
14:1, 15:1, 16:1, 17:1, 18:1, 19:1, 20:1; 21:1, 22:1, 23:1, 24:1 or 25:1.
Incorporation of supnlemental boron into animal feeds
[0047] A variety of methods are known in the art for the production of animal
feeds.
These various methods can be adapted to allow inclusion of supplemental boron
into the feed
in amounts that will have a beneficial effect on OC when fed to animals.
[0048] For example, supplemental boron in the amounts disclosed above can be
incorporated into animal feed compositions such as those described in U.S.
Patent No.
3,946,109. Alternatively, a variety of other feed compositions are
commercially available
from suppliers such as Purina, ADM, Land O'Lakes, and Moorman's. Supplemental
boron
can be mixed into a composition of choice using for instance, the mixing
methods disclosed
in U.S. Patent No. 4,189,240. The composition containing supplemental boron
can be used to
form animal feed food blocks such as those disclosed in U.S. Patent No.
5,120,565.
Alternatively, supplemental boron can be incorporated into an animal feed
composition
which is formed by methods such as spray drying as disclosed in U.S. Patent
No. 4,777,240.
The citation of these patents is solely to illustrate various methods
available in the art for
incorporating supplemental boron into an animal feed product and is not meant
to limit the
practice of the invention to the use of any one or more of these methods.
22
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0049] Other boron sources that can be incorporated into animal feeds to
practice this
invention include yeast preparations that are high in boron. It is already
common practice to
incorporate yeast into animal feeds. Hence, it would be fairly straightforward
to include
yeast with elevated boron levels in animal feed. Alternatively, crops that
have been grown in
soils with elevated boron levels can be harvested specifically for the purpose
of serving as an
enhanced boron source that can be incorporated into animal feeds. Such
elevated boron
levels may be naturally in the soil, may result from boron pollution, or may
be added to the
soil by fertilization or other means. Alternatively, supplemental boron
containing compounds
can be added to supplements, base mixes, and premixes that also contain
vitamins and
minerals. Such supplements, mixes, or premixes are typically added at an
amount to
constitute 0.5% to 30% of the final animal feed composition. In such an
embodiment, the
elemental boron concentration would be much higher (from about 3 times to 200
times
higher) prior to dilution in the animal feed to result in a supplemental boron
equivalent of I -
500 ppm over a total daily ration.
[0050] Another alternative is to supplement animal feeds with foods, such as
alfalfa,
grapes, or coffee grounds, which are naturally high in boron content.
Additionally, these and
other foods can be manipulated to contain higher levels of boron by growth
under elevated
boron conditions as described above or by means of transgenic plant technology
or other
recombinant methods.
[0051] In a further embodiment, supplemental boron containing compounds can be
provided as dietary supplements that can be directly hand-fed or "top-dressed"
onto an
animal feed. Such an embodiment could be in a formulation that contains other
nutrients,
excipients, or flavors. As an example, an equine nutrient supplement
containing
supplemental boron and other vitamins and minerals could be fed to a horse
with a small
spoon or cup or in the form of a bar or pellet. Alternatively, the supplement
could be placed
on top of or mixed in the animal's feed.
[0052] Such boron can be supplied to animal feeds as a boron-talc composition.
The
ratio of boron-containing compound to talc in the boron-talc composition can
be
approximately 5:1, 6:1, 7:1, 8:1, 9:1, 10:1, 11:1, 12:1, 13:1, 14:1, 15:1,
16:1, 17:1, 18:1, 19:1,
20:1; 21:1, 22:1, 23:1, 24:1 or 25:1
23
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0053] Should it be necessary, boron levels can be precisely determined by a
variety of
methods known in the art. U.S. Patent Publication 20040020840 and the patents
disclosed
therein describe a number of such methods.
Reduction ofphosphorus content of animal feeds and improvement of pre-
weaningmortality
[0054] Phosphate pollution resulting from excess phosphorus in animal feed is
an
increasing problem. For example, approximately 70% of the phosphorus in a
typical
corn/soybean meal diet is unavailable to pigs, according to the National
Research Council's
1998 Nutrient Requirements for Swine. This unavailable phosphorus ends up
being excreted
in manure. The high phosphate content of swine manure contributes to the
environmental
pollution associated with pig farming. Reducing the amount of excreted
nutrients,
particularly phosphorus, in swine production systems is an environmental
priority and an
important economic issue facing the swine industry. Thus, a means to increase
the
bioavailability of phosphorus in feed ingredients used to formulate swine
rations would be
desirable. The inventors have found that inclusion of supplemental boron in
pig feed results
in an increased absorption and utilization of the phosphorus present in pig
diets.
Supplemental boron promotes the efficient incorporation of phosphate into the
calcium
phosphate (hydroxyapatite) of bones. This effect is expected to be true in
other animals as
well.
[0055] By increasing the efficiency of absorption and utilization of
phosphorus from
animal diets such as pig diets, the inventors have found that the amount of
phosphorus in
typical pig feed formulations can be reduced. These results are shown in
Example 4. The
increased utilization of phosphorus from pig diets coupled with the reduction
in the starting
amount of phosphorus in pig feed can be expected to contribute to a reduction
of phosphate
pollution that results from pig farming. Thus, the inclusion of supplemental
boron in animal
feed as taught by this invention will not only contribute to the prevention
and treatment of
OC, it will also contribute to pollution reduction. While the foregoing
discussion has focused
on pigs, this invention is not limited to the reduction of phosphorus from pig
feed
exclusively. Rather, one of skill in the art will recognize that reduction of
phosphorus use is
applicable to all animals.
24
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0056] The formation of most bones of the axial skeleton begins with the
formation of a
cartilage model which is calcified and remodeled into bone that is mineralized
with calcium
and phosphate. Supplemental boron enhances the efficiency of this process. The
postulated
mechanism by which supplemental boron enhances the efficiency of the process
is through
stabilization of the extracellular matrix, although there may be other
mechanisms.
[0057] Supplemental boron improves the efficiency of cartilage
transformation/bone
mineralization which improves the structural integrity of bone and bone
mineralization
characteristics. Calcium is added to diets at a level that promotes sufficient
bone strength.
The level of calcium that promotes optimum bone strength also paradoxically
inhibits the
intestinal absorption of phosphorus. Phosphorus absorption is also more
efficient when
dietary phosphorus level is reduced. The inventors have discovered that
addition of
supplemental boron to animal feed promotes bone mineralization and permits a
proportional
3 to 5 % reduction of both calcium and phosphorus in the animal feed while
maintaining bone
strength.
[0058] The 1998 NRC report on "Nutrient Requirements of Swine", data from
which is
shown below in Table 1, indicates that typical requirements for calcium and
phosphorus will
vary during the lifetime of a pig. At earlier stages, when the bones of the
skeleton are still
undergoing development, greater amounts of calcium and phosphorus are needed
to support
increased bone growth. The requirements for calcium and phosphorus decrease as
a pig
matures and bone development is completed. Although the data presented below
are for pigs,
similar trends in requirements for calcium and phosphorus are observed during
the life cycle
of other animals.
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
Table 1
Growing Pigs (NRC, 1998)
Body Weight (kg) 3-5 5-10 10-20 20-50 50-80 80-120
Calcium (%) 0.90 0.80 0.70 0.60 0.50 0.45
Phosphorus, total 010 0.70 0.65 0.60 0.50 0.45 0.40
Sows
Calcium % 0.75
Phosphorus, total !0 0.60
[0059] The inclusion of supplemental boron in the diet of various animals
allows the
levels of calcium and phosphorus to be reduced by at least 3% throughout the
life cycle of
animals. Thus, while the ratio of calcium to phosphorus is generally kept
constant at each
weight range indicated in Table 1, the absolute amounts of calcium and
phosphorus can be
lowered by at least 3% due to the addition of supplemental boron containing
compounds.
[0060] Thus, in another embodiment, this invention provides an animal feed
containing
supplemental boron with a reduced level of phosphorus. In one embodiment, the
supplemental boron is preferably provided at a concentration of about I to
about 500 ppm
elemental boron and the phosphorus level is reduced by 3 to 5% as compared to
comparable
animal feed without supplemental boron. The calcium level is generally reduced
comparably
to the phosphorus level. However, it will be recognized that if the
supplemental boron
containing compound is supplied as a calcium salt, such as calcium borate or
calcium
borogluconate, levels of calcium in the animal feed can also be
correspondingly reduced.
One such compound, calcium borogluconate, is already in use to treat
hypocalcemia in cattle,
sheep, and goats. In another embodiment, the supplemental boron concentration
is
preferably about 1 about 150 ppm and the phosphorus level is reduced by 3 to
5% as
compared to comparable animal feed without supplemental boron. In yet another
embodiment, the supplemental boron concentration is preferably about 25 ppm to
50 ppm and
the phosphorus level is reduced by 3 to 5% as compared to comparable animal
feed without
supplemental boron.
[0061] In another embodiment, the invention provides a method of decreasing
the amount
of pre-weaning mortality by animals. In this embodiment, pregnant, nursing or
lactating
animals are fed a diet of an improved animal feed composition containing about
1 to about
500, about 1 to about 150 or about 50 ppm or about 25 to 50 ppm supplemental
boron
26
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
containing compounds in which the animal feed composition has at least a 3%
reduction in
phosphorus as compared to a comparable animal feed without supplemental boron.
Generally, the animal feed contains supplemental boron at concentrations
ranging from 5-150
ppm. In such an embodiment, the supplemental boron containing compound can be
sodium
borate or boric acid can be used or other inorganic forms of boron as
described herein.
OC in humans
[0062] Osteochondrosis with its various manifestations has been found to be
strikingly
similar in six species of animals in which it has been reported. This has
prompted experts to
assert that it would be expected that osteochondrosis in humans would have the
same
etiology, pathogenesis, and pathology as has been observed in animals. See
Olsson, S.E. and
Reiland, S. 1978. The nature of osteochondrosis in animals - summary and
conclusions with
comparative aspects on osteochondrosis dissecans in man. Acta Radiologica
Supplement No.
358:299-306.
[0063] Osteochondrosis in humans is defined in Dorland's Medical Dictionary as
follows: a disease of the growth or ossification centers in children that
begins as a
degeneration or necrosis followed by regeneration or recalcification. Also
called epiphyseal
ischemic necrosis (q.v.), it may affect (1) the calcaneus (os calcis), a
condition sometimes
called apophysitis; (2) the capitular epiphysis (head) of the femur, a
condition known as
Legg-Calvd-Perthes disease, Perthes disease, Waldenstrom's disease, coxa
plana, and
pseudocoxalgia; (3) the ilium; (4) the lunate (semilunar) bone, known as
Kienbock's disease;
(5) head of the second metatarsal bone, known as Freiberg's infraction; (6)
the navicular
(tarsal scaphoid); (7) the tuberosity of the tibia, called Osgood-Schlatter
disease and
Schlatter's disease; (8) the vertebrae, called Scheuermann's disease or
kyphosis, juvenile
kyphosis, vertebral epiphysitis, and kyphosis dorsalis juvenilis; (9) the
capitellum of the
humerus, called Panner's disease.
[0064] The locations, of the joints affected in human children can be
contrasted with
regions affected in the pig. In the pig, osteochondrosis can be located in the
following areas,
listed in descending orde'r of severity of lesions: 1. Articular-epiphyseal
lesions: stifle,
elbow, lumbar synovial intervertebral joints, hock, shoulder, and hip, 2.
Growth plate lesions:
distal ulna, distal femur, costochondral junction, femoral head, humeral head,
ischiatic
27
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
tuberosity, and thoracolumbar vertebrae, 3. Epiphysiolysis and apophysiolysis
lesions:
glenoid cavity, ischiatic tuberosity, capital femoral epiphysis, vertebral
epiphyses, anconeal
process, and distal ulnar epiphysis.
[0065] Because of the similarity of the disease in pigs and humans, another
embodiment
of the invention is the treatment and prevention of OC in humans. Boron
compounds can be
administered to patients suffering from OC. The boron containing compounds
useful for the
practice of this invention may include any suitable organic, inorganic, or
mineral boron
containing compounds. Among the preferred forms of boron are sodium borate and
boric
acid. Other useful inorganic forms of boron include calcium borate. Examples
of organic
boron-containing compounds are well known to those of skill in the art.
Examples of such
organic boron-containing compounds are found in U.S. Patent Nos. 4,312,989,
4,499,082,
and 5,312,816. Dosages that may fmd use in humans include 1-13 ppm.
Formulations of boron for use in humans
[0066] Described below are administration methods that are useful for humans.
One
particularly useful administration method is the provision of boron as mineral
or vitamin
supplements, for example, in food or pill format. However, it will be
appreciated that many
of the methods disclosed below, while especially applicable to humans, can
also be used for
the administration of boron to animals as well.
[0067] One especially useful form of administration for the boron containing
compounds
of the present invention is as a mineral supplement with vitamins that can be
taken orally as a
pill or added to food. Multi-vitamin and mineral supplements are useful in the
maintenance
and improvement of health by insuring adequate intake of micronutrients that
are needed for
disease prevention and to compensate for nutritional deficiencies that result
from factors as
inadequate dietary intake of essential nutrients. Vitamin and mineral
preparations are
commonly administered as general nutritional supplements or to treat specific
medical
conditions. Accordingly, the supplemental boron containing compounds of the
present
invention can be administered as mineral supplements with vitamins such as
vitamin A,
vitamin C, vitamin D, vitamin E, vitamin K, vitamin B 1, vitamin B2,
niacinamide, vitarnin
B6, vitamin B12, biotin, pantothenic acid, carnitine, silicon, molybdenum,
germanium iron,
phosphorus, iodine, magnesium, zinc, selenium, copper, chromium, potassium,
choline,
28
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
lycopene, and co-enzyme Q-10. Examples of mineral supplement formulations to
which
supplemental boron containing compounds can be added can be found in U.S.
Patent Nos.
4,752,479, 5,869,084, and 6,361,800. Such supplements containing the boron
compounds of
the present invention can be administered as chewable vitamin pills, or as
supplements that
can be added to beverages, or as supplements that can be added to foods.
[0068] In practicing the method of the present invention, the boron compounds
may be
administered per se or as components of a pharmaceutically acceptable
composition. When
used in medicine, the form of the supplemental boron compounds should be both
pharmacologically and pharmaceutically acceptable.
[0069] Thus, the present invention may be practiced with the boron compounds
being
provided in pharmaceutical formulations, both for veterinary and for human
medical use,
comprising the active agent (the boron compound) together with one or more
pharmaceutically acceptable carriers thereof and optionally any other
therapeutic ingredients.
The carrier(s) must be pharmaceutically acceptable in the sense of being
compatible with the
other ingredients of the formulation and not unsuitably deleterious to the
recipient thereof.
The active agent is provided in an amount effective to achieve the desired
pharmacological
effect, as described above, and in a quantity appropriate to achieve the
desired daily dose.
[0070] The formulations include those suitable for oral, rectal, topical,
nasal, ophthalmic,
or parenteral (including subcutaneous, intramuscular, and intravenous)
administration.
Formulations suitable for parenteral administration are preferred.
[0071] The formulations may conveniently be presented in unit dosage form and
may be
prepared by any of the methods well known in the art of pharmacy. All methods
include the
step of bringing the active compound into association with a carrier which
constitutes one or
more accessory ingredients. In general, the formulations may be prepared by
uniformly and
intimately bringing the active compounds into association with a liquid
carrier, a finely
divided solid carrier, or both, and then, if necessary, shaping the product
into desired
formulations.
[0072] Formulations of the present invention suitable for oral administration
may be
presented as discrete units such as capsules, cachets, tablets, or lozenges,
each containing a
predetermined amount of the active ingredient as a powder or in the form of
granules; or as a
29
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
suspension in an aqueous liquor or a non-aqueous liquid, such as a syrup, an
elixir, an
emulsion, or a draught.
[0073] A tablet may be made by compression or molding, optionally with one or
more
accessory ingredients. Compressed tablets may be prepared by compressing in a
suitable
machine, with the active compound being in a free-flowing form such as a
powder or
granules which optionally is mixed with a binder, disintegrant, lubricant,
inert diluent, surface
active agent, or discharging agent. Molded tablets comprised of a mixture of
the powdered
active compound with a suitable carrier may be made by molding in a suitable
machine.
[0074] One desirable formulation of the composition for administration is in a
powdered
form for dissolution or dilution with water or another suitable beverage or
liquid before use.
Alternatively, the composition can be contained in a ready to use form as part
of a fortified
beverage in liquid form. Also, boron containing compounds can be added to milk
replacers.
The composition can also be contained in a pudding with a custard or flan like
texture or in
the form of a bar suitable for ready consumption.
[0075] A syrup may be made by adding the active compound to a concentrated
aqueous
solution of a sugar, for example sucrose, to which may also be added any
accessory
ingredient(s). Such accessory ingredient(s) may include flavorings, suitable
preservatives,
agents to retard crystallization of the sugar, and agents to increase the
solubility of any other
ingredient, such as a polyhydroxy alcohol, for example glycerol or sorbitol.
[0076] Formulations suitable for parenteral administration conveniently
comprise a sterile
aqueous preparation of the active compound, which preferably is isotonic with
the blood of
the recipient (e.g., physiological saline solution).
[0077] Nasal spray formulations comprise purified aqueous solutions of the
active
compound with preservative agents and isotonic agents. Such formulations
preferably are
adjusted to a pH and isotonic state compatible with the nasal mucous
meinbranes.
[0078] Formulations for rectal administration may be presented as a
suppository with a
suitable carrier such as cocoa butter, hydrogenated fats, or hydrogenated
fatty carboxylic
acids.
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0079] Topical formulations comprise the active compound dissolved or
suspended in
one or more media, such as mineral oil, petroleum, polyhydroxy alcohols, or
other bases used
for topical pharmaceutical formulations.
[0080] In addition to the aforementioned ingredients, the formulations of this
invention
may further include one or more accessory ingredient(s) selected from
diluents, buffers,
flavoring agents, binders, disintegrants, surface active agents, thickeners,
lubricants,
preservatives (including antioxidants), and the like.
[00811 The following examples further demonstrate several preferred
embodiments of
this invention. Vi7hile the examples illustrate the invention, they are not
intended to limit the
invention. The patents cited herein are incorporated by reference in their
entirety.
Example 1: Boron Supplementation and its Effects on OC-associated Lameness in
Swine
Materials and Methods
[0082] Three groups of 19 pigs, Duroc and Yorkshire pigs were randomly blocked
by
=breed, litter and weight. The basal diet consisted of commercial corn-soy
diet containing 10
ppm boron.
[0083] Test diet group B was fed a basal diet plus 25 mg/kg boron as sodium
borate
decahydrate (borax). Test diet group A was fed a basal diet plus 25 mg/kg
boron as sodium
borate decahydrate (borax) and 250 mg/kg ascorbic acid.
[0084] Pigs were weighed at the beginning of the study, 4 weeks later and
every 3 weeks
until the end of the study. Animals were scored for soundness on a 5-point
scale at each
weighing. (Five-point scale: 1= no soundness defects; 2= minor soundness
issues but still
sound enough for retention as breeding animal; 3=not sound 'enough for
retention for
breeding but still marketable; 4= unsound, likely to be rejected at slaughter;
5=severely lame,
requiring euthanasia for humane reasons.) Grading was done by the caretaker
and the
investigator at all weighing times. An experienced independent treatment-blind
rater also
31
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
evaluated the soundness of each animal and the ratings he provided were
compared with the
ratings of the caretaker and investigator by use of Cohen's Kappa test.
[0085] The animals were housed in a modern curtain sided barn with deep straw
bedding,
with ad libitum access to feed and water. Waterers and feeders were on a
concrete pad but the
remainder of the flooring was deep straw over a ground limestone and sand
base. Floor space
allowances exceeded the recommendations of the Guide for the Care and Use of
Agricultural
Animals in Agricultural Research and Teaching, First Revised Edition, 1999.
Federation of
Animal Science Societies. Savoy, IL.
[0086] Pigs in the study were fed a typical corn-soybean meal diet which
contained a
proprietary commercial supplement from the Moorman's company at the
manufacturer's
suggested inclusion rate. The basal diet (no boron supplementedy was analysed
and found to
contain boron at the rate of 10 ppm which is typical for a corn-soybean meal
based diet.
[0087] Animals were observed twice daily by the caretaker.
[0088] In mid October one pig was observed to be severely lame and was
euthanized and
necropsied. Three additional pigs, one lame Duroc, a sound York and a sound
Duroc
(littermate to the lame pig) were euthanized and necropsied for observational
purposes in
mid-November.
[0089] At the end of the study the pigs were transported to a laboratory where
they were
euthanized and necropsied. Six pigs in each of group B and group A were put on
the control
diet for 7 days at the end of the study.
[0090] At necropsy, samples of liver, heart, kidney, fat, skeletal muscle,
proximal tibia,
blood, and the rostral snout were retained and frozen at -40 C prior to
chemical analysis. All
joints of the axial skeleton were evaluated for the presence of gross lesions
of
osteochondrosis and graded on a 5 point scale. (5-point scale: 1= no gross
abnormalities;
2=minor imperfections in articular conformation or articular reddening present
but no
cartilage erosions; 3 = cartilage intact but surface irregularities of the
cartilage are present; 4=
fissuring or erosion of articular cartilage is obvious; 5= full thickness
cartilage lesions or
cartilage flaps, osteochondritis dissecans obviously present).
32
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0091] The proximal femur (femoral head) and articular surfaces and growth
plates
associated with the stifle, hock, shoulder, elbow and carpus were sectioned
with a band saw
and approx. 0.5 to 1.0 cro sections fixed in formalin. Bone sections were
decalcified with
formic acid/sodium citrate, embedded in paraffin and sectioned at 5 microns.
Sections were
deparaffinized according to standard procedures. Two sections were made from
each joint
and growth plate and stained with either hematoxylinleosin (H&E) or with
toluidine blue (pH
4)/fast green for evaluation of the articular cartilage, subchondral bone and
growth plate.
[0092] Mean joint lesion scores, growth rate and soundness scores by treatment
and by
factors boron and ascorbate were compared using an analysis of variance and t-
test as
appropriate. Soundness scores were dichotomised into binary categorical
variables for
lameness (soundness score>2) and for absence of defects (soundness<2), and the
binary
variables analysed by chi-square and logistic regression.
[0093] Tissue specimens stained with H&E were examined microscopically for the
presence of lesions of osteochondrosis. Toluidine Blue staining allowed
assessment of
retention or loss of proteoglycan from the extracellular matrix (ECM) of the
cartilage. Each
tissue section was scored by a treatment-blind board-certified pathologist.
[0094] Further, specific joints and structures are selected for more detailed
histomorphometric analysis. Both epiphyseal and growth plate specimens were
obtained
from boron treated and untreated animals.
[0095] The results show that boron supplementation can be effective in
reduction of the
incidence of osteochondrosis-associated lameness in growing swine. Animals
supplemented
with boron had healthier joints than those receiving the basal diet with no
supplemental boron
(Figure 1). Increasing soundness score (higher score=increasing lameness/leg
unsoundness)
is associated with increasing lameness in the pigs not receiving boron (Figure
2). Figure 3
illustrates the effect of early rapid growth (weight on 23 October) on
soundness scores at the
termination of the study (18 December). Pigs that did not receive boron and
grew rapidly
tended to develop leg unsoundness and lameness. Boron supplementation was
useful in
cartilage protection and prevention of lameness in rapidly growing swine,
whereas the
untreated group displayed a high prevalence of lameness and leg unsoundness
that was
clearly associated with the presence of cartilage damage typical of
osteochondrosis in swine.
33
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[0096] In further microscopic analysis of the cartilage, one hundred and forty-
one (141)
sections of articular cartilage and growth plate have been prepared by formate
decalcification
and staining with H&E and Toluidine blue. The toluidine blue (TBlue) stain
provided a
semi-quantitative measure of sulfated glycosaminoglycan (sGAG) content. The
consistently
higher TBlue staining intensity among the boron supplemented group suggested a
higher
sGAG content in cartilage from boron supplemented pigs. (This effect of boron
has been
confirmed by a sGAG analysis procedure.) Lesions were evaluated by an
experienced board
certified pathologist specializing in porcine tissues. Lesions are classified
into 2 factors.
One factor comprising presence or absence of necrosis, infarction, hemorrhage
or
eosinophilic matrix streaks provides a measure of the structural integrity of
the articular
surface, while another factor comprising hyperplasia and abnormal
differentiation provides a
measure of the condition of the growth plate associated with growth plate
widening. Growth
plate lesions associated with widening were found in 57% of tissues from the
unsupplemented group as compared with 19% of the tissues from pigs receiving
25 ppm
boron. Articular cartilage lesions (necrosis, hemorrhage, infarction, or
streaks) were found
in 21 Jo of tissues from unsupplemented pigs as compared with 4% of tissues
from pigs
receiving 25 ppm supplemental B. These data indicate that supplementation with
boron can
improve the structural integrity of the articular cartilage and the growth
plate.
[0097] Anecdotal evidence from aontinuing experimental use of boron added to
feed or
drinking water indicates a consistent and sustained positive response in swine
in a variety of
production settings and genetics. This evidence is described in Example 6.
Example 2: Glycosaminoglycan Study.
[0098] Boron nutrition is necessary to maintain the glycosaminoglycan
concentration of
cartilage at normal, healthy levels required for the cartilage to perform its
function of
resisting compression forces or to maintain the weight bearing ability of the
cartilage.
[0099] Proteoglycans, a major component of healthy cartilage, draw and hold
water
which allows them to bear weight. We have measured the major subcomponent of
proteoglycan, glycosaminoglyca.n (GAG) and have found that it is significantly
reduced in
non-boron supplemented pig cartilage tissue. Our data and the literature would
support the
34
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
statement that cartilage with low GAG levels fails to function much more
readily than
cartilage with higher levels of GAG.
[00100] Hock joints and elbow joints were obtained at necropsy from pigs in
two feeding
groups: one group having been fed a commercial ration supplemented with 50 mg
B/kg feed
and the other being fed only the standard commercial pig ration.
[00101] Cartilage plugs were harvested from the articular surfaces of 40 hock
and elbow
joints using a #5 (10.5 mm) cork borer. The samples were papain digested and
analyzed for
concentration of sulfated glycosaminoglycan (sGAG) using a spectrophotometric
microplate
analysis procedure adapted from Farndale, et al (1986), using a chondrotin
sulfate standard.
This method measures total sulfated glycosaminoglycan, most of which is
chondrotin sulfate
and keratan sulfate components of aggrecan. Aggrecan is the large aggregating
proteoglycan
of cartilage responsible for creating and maintaining the osmotic pressure of
cartilage.
Quanititation of sGAG concentration thus provides a measure of the weight-
bearing and anti-
friction properties of cartilage and is a measure of cartilage quality and
durability.
[00102] The sGAG concentration (dry matter basis) in the cartilage of the pigs
fed 50 ppm
boron was 11050 ng/g as compared with 5045 ng/g among the unsupplemented pigs.
This
difference was significarit at p<0.01. These data indicate that the
chondroprotective
properties of boron are mediated at least in part via mechanism that lead to
increased sGAG
levels in the cartilage.
Example 3: Reproductive effects in Female Swine.
[00103] It was observed that when sows were fed diets containing 50 ppm
supplemental
boron during the late gestation and early lactation period, piglet quality as
assessed by
uniformity, growth, and general thrift was improved, and pre-weaning piglet
mortality was
reduced. A preliminary pilot study confirmed these observations. Sows were fed
a standard
corn-soy diet. Half of the sows received an oral administration of a boron
supplement to
provide 1 mg boron per kg body weight. The other half did not receive any
supplementation.
Preliminary analysis of the data from the first 600 pigs indicated that the
provision of boron
to the gestating and lactating sows reduced pre-weaning mortality from 23% to
16% and
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
increased piglet weight at 12 days of age from 8.0 pounds to 8.5 pounds, as
compared to the
non-supplemented groups.
[00104] To test the effect of boron on sows and their litters, a trial was
established in a
large commercial swine operation during an outbreak of porcine viral
reproductive and
respiratory disease (PRRSV). Boron was administered orally to a group of 51
sows
individually housed in crates at the rate of 1 mg boron per pound BW per day
beginning 1
week prior to farrowing and continuing until the piglets were weaned at 14
days of age.
Their performance was compared to that of cohort of 50 sows of identical
genetics and
identical housing and husbandry conditions that did not receive boron. Boron
treatment was
supplied as a single daily dosing and was discontinued at weaning. All sows
were fed a
standard commercial sow diet.
[00105] There were no effects on litter size at birth or piglet birth weight.
Piglets raised by
sows consuming boron weighed 9.01 lbs at 12 days of age as compared with 8.32
lbs for the
piglets raised by control sows (p=0.06). Piglet mortality in the boron treated
group was
15.2% as compared with 20.3% among the controls (p=0.03). Sows that were fed
boron
returned to estrus an average of 1.6 days quicker than control sows (p=0.047).
Boron treated
sows were 1.2 times as likely as control sows to conceive (p=0.04). This
result would be
expected to have significant positive impact in a commercial pig raising
operation.
[00106] It is postulated that borate exerts its beneficial effects in OCD
prevention by
modulation and stabilization of the extracellular matrix (ECM). In tissues
like cartilage that
possess an abundant ECM consisting of proteoglycan and collagen, the main
effect of boron
is likely mediated by a change in the mechanic (material) properties of the
cartilage ECM.
However many other tissues with important functions do also possess ECM
components and
extracellular receptors, the structure of which may be stabilized by boron
cross-links which
improves their functionality in cell to cell signaling, receptor functions,
and adhesion
functions. It is postulated that the effect of borate on reproduction is
modulated by this sort
of mechanism.
36
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
Example 4: Effects of Boron on Phosphorus Digestibility and Excretion and Feed
Conversion.
[00107] A 28-day feeding trial was conducted in a large commercial farm
setting with 144
crossbred pigs of initial body weight of 24 kg. Pigs were randomly allocated
to 24 pens of 6
pigs per pen in a thermoneutral controlled environment barn with steel grid
flooring. Each
pen was equipped with a single-hole feeder. Water was available free-choice
from a nipple
drinker. Pigs were fed a commercial pig diet containing 0.5% phosphorus plus 0
or 50 mg/kg
Boron and a calcium level of either 0.5 or 0.65% in a 2 x 2 factorial design.
Feces were
collected on the last 3 days of the study from each pen and a pooled aliquot
was dried and
submitted for chemical analysis. Yttrium oxide was added to the diet at 0.05%
and served as
a marker for phosphorus digestibility. Pig growth and feed consumption was
measured at the
end of the study with the pen as the experimental unit. Data was analyzed for
effects of
boron and calcium by univariate and multivariate analysis of variance and t-
tests.
j00108] Supplemental boron increased average daily gain, improved feed
conversion ratio
and phosphorus digestibility, and reduced fecal phosphorus excretion per unit
of growth
(p<0.05). (Table 2). Daily feed intake was not significantly modified by boron
or calcium
level (p>.20). Decreasing calcium level improved feed conversion (p<0.05).
There were no
significant interactive effects of boron x calcium on feed conversion or
phosphorus excretion
(p>.25).
[00109] The digestibility and fecal excretion of phosphorus is of significant
concem to
animal agriculture. Phosphorus is a costly dietary ingredient and the
phosphorus in animal
wastes is a potential environmental pollutant. In the present study, the
phosphorus excretion
per unit of production was reduced by 15% by adding boron to the diet. Boron
also
produced a significant effect on overall feed conversion ratio. It is expected
that these effects
of boron would have a significant impact in reducing environmental pollution
as well as
reducing production costs in animal agriculture
Table 2. Effects of Boron Supplementation
37
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
No Added B 50 ppm Added B S.E. p
Feed Conversion
Ratio (feedlgain) 2.65 2.32 0.082 0.033
Phosphorus
Digestibility (%) 33.4 36.3 0.820 0.017
Phosphorus excretion in
Feces (g/Kg gain) 88.1 74.6 2.88 0.028
Average daily gain
(grams per day) 507 549 13.6 0.018
Avg. daily feed intake 1330 1270 45 0.21
N 12 12
Example 5: 3-NPB Studies
[00110] It was hypothesized that boron mediates its effect in the pig by
quadrivalent
crosslinking. 3-nitrophenylboronic acid (3-NPB) which is an avid blocker of
boron
crosslinks was administered orally to pigs of about 100 kg body weight at the
rates of 0 and 1
grams in combination with supplemental boron at the rates of 0 and 50 ppm in
feed. The 3-
NPB was administered for 10 days. The pigs were evaluated for lameness daily
and
euthanatized on day 13 and the joints and other organs were examined.
[00111] All of the pigs that received 3-NPB but no supplemental boron
developed clinical
manifestations of OCD within 10 days, but only one in 5 of the pigs receiving
supplemental
boron developed lameness when 3-NPB was provided (Table 3). A chi-square
analysis
indicated a significant (p<0.05) effect of 3-NPB in inducing lameness and a
significant effect
of supplemental boron in preventing the lameness induced by 3-NPB.
[001121 Examination of shoulder, stifle, hock and elbow joints showed that 3-
NPB treated
pigs with no supplemental boron had a higher prevalence of osteochondrosis
lesions and a
more intense severity of lesions than other treatment groups, with the lowest
prevalence of
lesions and the lowest severity among those pigs receiving supplemental boron
and no 3-
NPB. Administration of 3-NPB along with boron resulted in a prevalence and
severity of
gross joint pathology similar to that observed in unsupplemented pigs.
(Figures 4 and 5).
[00113] It was concluded that 3-NPB is acting as a competitive inhibitor of
borate.
38
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
[00114] It is generally considered that the pig is the archetypic model
species for
osteochondrosis in mammals (see Reiland S. Osteochondrosis in the pig. Acta
Radiol 1-118,
1975) The cascade of pathophysiologic events that culminate in clinical
manifestations of
osteochondrosis (OCD) in the pig are generally believed to be those events
that occur in the
other mammalian species that develop OCD, particularly the horse, dog,
ruminants and
humans. Since the pig is the model for OCD in other mammals and it has been
demonstrated
that boron is useful for prevention and treatment of OCD in'the pig, it
logically follows that
boron should have a similar effect in other mammals and the effect should be
mediated by a
similar biochemical mechanism.
[00115] Therefore, it was concluded that administration of 3-NPB in species
that are
known to be susceptible to OCD but at a low prevalence, specifically cattle,
horses, and dogs,
should produce cartilage lesions indicating presence of a boron receptor
moiety in cartilage.
[00116] Three healthy Holstein steer calves of average weight about 2501bs and
three
healthy Quarter Horse fillies of average weight of about 500 lbs were
administered 3-NPB at
the rate of 10 mg/kg body weight per day. The 3-NPB was given to the calves by
daily
intraperitoneal injection, while the horses were administered the daily dose
of 3-NPB mixed
in feed. All animals consumed a standard ration of commercial feed and free-
choice alfalfa-
grass mixed hay.
[001171 Lameness was first observed in the calves at day 7 of the 3-NPB
treatment. One
calf was euthanatized at treatment day 14 and the other two at treatment day
21. Severe OCD
lesions were visible in the hock and elbow joints of all calves with
increasing severity noted
with increasing time on 3-NPB.
[00118] Among the horses treated with ora13-NPB, one filly showed clear signs
of front
leg lameness during exercise on treatment day 10 and was euthanatized on day
14. The other
two horses were euthanatized on treatment day 28, at which time lameness was
visible during
exercise in one of the two horses. OCD lesions of varying degree were observed
in the
shoulder, elbow, fetlock and hock joints of all horses. Among the notable
lesions were a 1 cm
x 1 cm necrotic lesion of the cartilage was found on the proximal articular
surface of left
front P1 at day 14, a developing flap lesion on the distal tibia at day 28 and
in another horse,
39
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
profound thinning of cartilage and obvious cartilage wear lines were observed
in the left front
fetlock and left elbow at day 28.
[001191 In experiments with normal healthy crossbred hounds of age 10 weeks
(body
weight 8 kg), the administration of 3-NPB as a single daily dose of 10 mg per
kg body weight
resulted in visible foreleg lameness as early as 12 days. Necropsy revealed
gross lesions of
necrosis and hemorrhage in the distal ulnar growth plate. There was also
evidence of
cartilage erosion in the articular surfaces of the distal tibia and the
proximal ulna. The gross
lesions in the distal ulnar growth plates resembled the pathologic changes
associated with
osteochondrosis in swine.
100120] It is concluded that the pig is a suitable model for OCD in both
ruminant and non-
ruminant animals, including carnivores, and that boron receptor sites exist in
all mammalian
species, and that supplemental boron is expected to be an effective
preventative and remedy
in all mammalian species.
Table 3
3-NPB' Clinical Lameness Score * Level of Boron Supplementation
Crosstabulation
Count
Clinical Lameness Score
Level of Boron Visible
Supplementation Normal Lameness Total
0 3-NPB None 6 0 6
1 gram daily x 10 days 0 4 4
Total 6 4 10
50 ppm 3-NPB None 5 0 5
1 gram daily x 10 days 4 1 6
Total 9 1 10
Example 6: Use of boron in swine in field situations
[00121] Boron was added to the diet of sows at 1 mg/kg body weight as boric
acid. There
was no negative effect on reproduction or fertility. The sows appeared to have
normal estrus
activity and normal conception rate, with no negative effects on cyclic
activity or on pigs
born or on piglet viability. Three sows that were noticeably and seriously
lame became
perfectly sound. Among a group of 95 piglets the birth to weaning mortality
was 2 piglets.
CA 02651540 2008-11-03
WO 2007/142684 PCT/US2006/046687
The usual mortality for this farm was about 5 to 7%. These piglets remained on
boron 50 ppm
plus ascorbate 125ppm. One pig was euthanized and examined at a weight of
about 85
pounds. No abnormalities were observed in any of the joints. All bones of the
appendicular
skeleton were sectioned on the band saw. Bones had excellent mineralization
and the growth
plates were narrow and crisply demarcated, including the distal ulnar growth
plate which is
an early predilection site for OC-related abnormalities.
[00122] An Iowa farm treated a group of about 100 pigs with 50 ppm boron as
boric acid.
None of these pigs developed any signs of lameness or unsoundness. The farmer
reported
that these pigs are the most sound he has raised. The estimated previous
lameness/unsoundness rate was about 25 to 30%, and zero in the test group. The
pigs
demonstrated excellent growth rate. The absence of lameness and hock swelling
was
observed. Two pigs with hock swelling were euthanised from among the younger
and older
pigs not treated with boric acid. The younger pig of about 50 pounds body
weight showed
evidence of early OC changes in the hock. An older pig of about 250 pounds
body weight
with severe lameness in the right hock was euthanised. Severe advanced OCD was
observed
in the hock, and growth plate abnormalities were observed when the bones were
sectioned.
Culture of the hock joints was negative, ruling out bacterial infection and
indicating that OC
is the likely cause of lameness.
41