Note: Descriptions are shown in the official language in which they were submitted.
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
ENZYME-MICROBE SYNERGY
RELATED APPLICATIONS
[0001] This application claims the benefit of priority to U.S.
provisional patent application serial no. 60/796,635, filed May 1, 2006, which
is incorporated by reference herein.
GOVERNMENT RIGHTS
[0002] The United States Government may have certain rights in
the present invention as research relevant to its development was funded by
National Institute of Standards and Technology (NIST) contract number
io 60NANB1 D0064 and by Department of Energy contract number DE-FG02-
02ER15350.
BACKGROUND
[0003] Cellulosic biomass represents an inexpensive and readily
available resource which may be fermented to produce ethanol or other
is products. Among bioconversion products, interest in ethanol is high because
it may be produced as a renewable domestic fuel that could offer benefits in
terms of sustainability, security and rural economic development.
[0004] As bioconversion processes strive to become economically
competitive with petroleum fuel technologies, they face challenges associated
20 with the hydrolysis of cellulose into simple sugars. Process steps
associated
with overcoming the recalcitrance of cellulosic biomass are generally the most
costly and have the greatest potential for R&D-driven improvement. Cellulose
recalcitrance is typically overcome by acid pretreatment followed by
enzymatic breakdown of the pretreated cellulose by cellulase enzymes. Cost
25 estimates for cellulase enzymes currently range from $0.30 - 0.50/gallon of
ethanol. With the cost of enzymes acting as a limiting factor in the
production
of ethanol from cellulosic biomass, any means of reducing the amount of
cellulase necessary for ethanol production will remain an important
commercial goal. Accordingly, efforts have in the past been devoted to
-~-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
understanding the mode of action of multi-component cellulase enzyme
systems, and to improving their effectiveness.
[0005] Enzymatic hydrolysis can be mediated by cellulase enzymes
acting in the absence of cells, by cellulases acting in the presence of cells
but
with no cell-enzyme attachment, or by cellulases attached to cells. In the
latter case, hydrolysis is mediated by ternary cellulose-enzyme-microbe (CEM)
complexes rather than binary cellulose-enzyme (CE) complexes.
[0006] It is known that cells of aerobic microorganisms do not
adhere (or only weakly adhere) to cellulose. Thus, the main agent of cellulose
lo hydrolysis in aerobic systems is cellulase enzyme bound to cellulose to
form a
binary CE complex. These CE complexes feature discretely-acting
functionally-distinct proteins. By contrast, most anaerobic microorganisms
adhere to cellulose, and the main agent of cellulose hydrolysis is a ternary
CEM complex, in many but not all cases involving "cellulosome", wherein
ls multiple functionally-distinct proteins act in concert.
[0007] The phenomenon of synergy between components of CE
complexes, whereby the rate realized by two or more components in
combination is greater than the sum of the rates observed when the
components act separately, has been observed and evaluated in the
20 literature. However, "enzyme-microbe" synergy of CEM complexes has not
been previously evaluated and quantified.
SUMMARY
[0008] The instrumentalities reported herein advance the art by
providing a method for reducing the amount of enzyme necessary to achieve
25 hydrolysis of a given amount of biomass, or alternatively increasing the
amount of biomass that may be hydrolyzed by a given amount of enzyme.
[0009] In one embodiment, a method of utilizing a reduced cellulase
load to hydrolyze a cellulosic substrate, includes: determining an amount of
purified cellulase necessary to substantially hydrolyze a quantity of
cellulosic
30 substrate in a period of time; reducing the amount of purified cellulase by
a
factor of between 2 and 5 to determine a reduced cellulase load; and
-2-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
introducing to the cellulosic substrate a microorganism expressing cell-bound
cellulase in a concentration equal to the reduced cellulase load under
suitable
conditions and for said period of time sufficient to allow substantial
hydrolysis
of the cellulosic substrate.
[0010] In one embodiment, a method of utilizing a reduced cellulase
load to hydrolyze a cellulosic substrate, includes: determining an amount of
purified cellulase necessary to substantially hydrolyze a quantity of
cellulosic
substrate in a period of time; reducing the amount of purified cellulase by a
factor of between 2 and 5 to determine a reduced cellulase load; and
io introducing a fermentation agent that has been engineered to express cell-
bound cellulase to the cellulosic substrate under suitable conditions and for
said period of time sufficient to allow substantial hydrolysis and
fermentation
of the cellulosic substrate, wherein the cell-bound cellulase is present in a
concentration equal to the reduced cellulase load.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] FIG. 1 shows cellulose utilization by a microbe, where
hydrolysis is mediated by both CEM and CE complexes.
[0012] FIG. 2 shows enzymatic hydrolysis of cellulose during a
Simultaneous Saccharification and Fermentation (SSF) reaction involving a
CE complex.
[0013] FIG. 3 illustrates changes in pH and product concentration
over time for the experiment depicted in FIG. 1.
[0014] FIG. 4 illustrates changes in pH and product concentration
over time for the experiment depicted in FIG. 2.
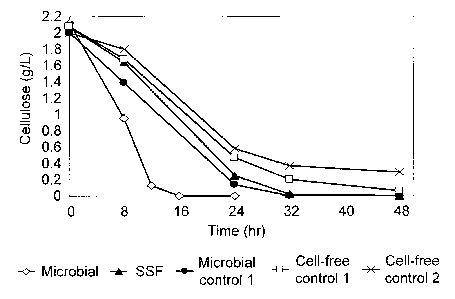
[0015] FIG. 5 shows cellulose concentration curves for the microbial
and SSF experiments depicted in FIGS. 1 and 2, as well as control
experiments.
[0016] FIG. 6 shows cellulose hydrolysis and product accumulation
for cell-free control 1.
-3-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
[0017] FIG. 7 shows cellulose hydrolysis and product accumulation
for continuous SSF of Avicel by T. thermosaccharolyticum in the presence of
0.064 g/L purified C. thermocellum cellulosome.
[0018] FIG. 8 shows cellulose hydrolysis and product accumulation
for continuous SSF of Avicel by T. thermosaccharolyticum in the presence of
0.052 g/L purified C. thermocellum cellulosome.
DETAILED DESCRIPTION
[0019] Synergy involving enhancement of the effectiveness of
cellulase by virtue of expression on the surface of a microbial cell as
io compared to when the cellulase is not cell-bound is disclosed herein. Such
"enzyme-microbe" synergy was quantitatively evaluated, and was shown to
give rise to a substantial increase in the effectiveness of cellulase enzymes.
[0020] The studies were carried out on Clostridium thermocellum,
which is an anaerobic, thermophilic bacterium that exhibits one of the highest
is rates of cellulose utilization (hydrolysis and fermentation) among
described
microorganisms. C. thermocellum produces a cellulase complex, or
"cellulosome", with a substantial fraction of the cellulosome bound to the
cell
surface under most culture conditions. Hydrolysis of microcrystalline
cellulose
(Avicel) was analyzed in batch and continuous cultures for the following two
20 systems:
(a) Microbial hydrolysis involving growth of C. thermocellum cultures in the
absence of added cellulase, in which hydrolysis is mediated by both
CEM and CE complexes.
(b) Enzymatic hydrolysis by purified C. thermocellum cellulosome with
25 fermentation of hydrolysis products by the non-cellulolytic thermophilic
anaerobe Thermoanaerobacterium thermosaccharolyticum. In this
SSF process, hydrolysis is mediated by CE complexes only.
[0021] Specific rates of cellulose hydrolysis were found to be about
2 to 5-fold higher for growing cultures of Clostridium thermocellum as
30 compared with purified cellulase preparations from the same organism. Thus,
accelerated reactions occur when C. thermocellum cellulase is presented on
-4-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
the surface of a cellulose-adhered cell as compared to when the C.
thermocellum cellulase acts without cell attachment.
[0022] From an applied science perspective, this 2 to 5-fold
synergistic effect is significant in the context of strategies that decrease
the
cost of enzymatic hydrolysis. In particular, quantification of enzyme-microbe
synergy provides an indication that higher hydrolysis rates may be achieved in
process configurations in which cellulose is hydrolyzed by adherent
cellulolytic
microbes as compared to processes in which hydrolysis is carried out by
cellulase enzymes in the absence of cellulolytic microbes.
[0023] The presence of a cellulose-adherent cellulolytic microbe
may increase hydrolysis rates by lowering the local concentration of
inhibitory
hydrolysis products through fermentation. For example, cellobiose, and to a
lesser extent glucose, are known to inhibit the C. thermocellum cellulosome.
Additionally, adherent microorganisms may be rewarded from an evolutionary
perspective, since organisms with improved substrate access (higher
concentration of substrate at the cell surface and/or less loss of substrate
to
the bulk medium) would presumably grow faster and thus have a selective
advantage.
[0024] A list of cellulolytic anaerobes that mediate cellulose
2o hydrolysis primarily via CEM complexes is presented in Table 1.
Table 1. Cellulolytic Anaerobes
Clostridium straminosolvens
Acetivibrio cellulolyticus
Clostridium cellulolyticum
Clostridium stercorarium subs. stercorarium
Clostridium stercorarium subs. thermolacticum
Clostridium stercorarium subs. leptospartum
Clostridium hungatei
Caldicellulosiruptor kristjanssonii
Clostridium phytofermentans
Clostridium thermocellum
Ruminococcus albus
-5-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
Ruminococcus flavefaciens
Butyrivibrio fibrisolvens
Caldicellulosiruptor saccharolyticum
Eubacterium cellulosolvens
Fervidobacterium cellulosolvens
Fibrobacter succinogenes
Spirochaeta thermophila
Thermotoga neapolitana
Neocallimastix sp. (anaerobic cellulolytic fungi)
io Pyromyces sp. (anaerobic cellulolytic fungi)
[0025] The benefits of enzyme-microbe synergy may be exploited
by utilizing any of the anaerobic hosts shown in Table 1 for hydrolysis of a
cellulosic substrate. Further, it may be advantageous to alter the hosts in
Table 1(e.g., via genetic engineering or selection following an evolutionary
challenge) to have improved product producing properties (e.g., titer, yield)
while retaining enzyme-microbe synergy.
[0026] Alternatively, a fermentation agent that is not naturally
cellulolytic may be engineered to express cellulase from one of the organisms
in Table 1. Such a recombinant organism may express a cellulosome on the
cell surface, and the resulting organism may form CEM complexes and
achieve higher rates of cellulose hydrolysis as a result of enzyme-microbe
synergy. Recombinant organisms that express tethered cellulase enzymes,
and methods of producing such organisms, are disclosed for example in U.S.
Patent Application No. 60/867,018, which is expressly incorporated herein by
reference. Exemplary fermentation agents that may be engineered to express
cellulase are listed in Table 2.
Table 2. Fermentation agents that may be engineered to express cellulase
Saccharomyces cerevisiea
Zymomonas mobilis
3o Escherichia coli
Klebsiella oxytoca
-6-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
Clostridium acetobutylicum
Schizosaccharomyces pombe
Candida albicans
Kluyveromyces lactis
Pichia pastoris
Pichia stipitis
Yarrowia lipolytica
Hansenula polymorpha
Phaffia rhodozyma
lo Candida utilis
Arxula adeninivorans
Debaryomyces hansenii
Debaryomyces polymorphus
Schwanniomyces occidentalis
[0027] Cellulosic substrates that may be hydrolyzed according to
the present instrumentalities include, but are not limited to, grasses, such
as
switch grass, cord grass, rye grass, reed canary grass, miscanthus, or a
combination thereof; sugar-processing residues, such as but not limited to
sugar cane bagasse; agricultural wastes, such as but not limited to rice
straw,
2o rice hulls, barley straw, corn cobs, wheat straw, canola straw, oat straw,
oat
hulls, and corn fiber; stover, such as but not limited to soybean stover, corn
stover; and forestry wastes, such as but not limited to recycled wood pulp
fiber, sawdust, hardwood, softwood, or any combination thereof; ruminant
digestion products; municipal wastes; paper mill effluent; newspaper;
cardboard; and combinations of any of the above mentioned substrates.
Examples of hardwoods considered for ethanol production may include
willow, maple, oak, walnut, eucalyptus, elm, birch, buckeye, beech, and ash.
Examples of softwoods considered for ethanol production may include
southern yellow pine, fir, cedar, cypress, hemlock, larch, pine, and spruce.
or
combinations thereof.
-7-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
EXAMPLE 1
MICROBIALLY-MEDIATED VERSUS ISOLATED-ENZYME-MEDIATED
CELLULOSE HYDROLYSIS
[0028] Microbially-mediated cellulose hydrolysis by Clostridium
thermocellum was systematically compared to enzymatically-mediated
hydrolysis carried out by purified C. thermocellum cellulosomes in the
presence and absence of Thermoanaerobacterium thermosaccharolyticum, a
non-cellulolytic thermophile capable of utilizing soluble products of
cellulose
hydrolysis. Ternary CEM complexes are present in the case of microbially-
io mediated hydrolysis whereas cellulose hydrolysis occurs exclusively due to
the action of binary CE complexes in the case of enzymatically-mediated
hydrolysis.
[0029] Batch culture of C. thermoce/lum and relevant controls.
Five ml of a Clostridium thermocellum (ATCC 27405) stock culture was
inoculated via syringe into 100 ml MTC medium containing 2 g/L Avicel
PH105 (FMC Corp., Philadelphia, PA) and 10 g/L MOPs buffer (initial pH 7.6)
in triplicate 200 mi sealed serum vials (Bellco Biotechnology, Vineland, NJ)
under a N2 atmosphere. Cultures were incubated at 60 C in a temperature
controlled water bath with rotary shaking at 200 rpm. Once 2 g/L Avicel was
consumed, as determined by visual inspection, supplemental Avicel was
added via syringe as a 40 g/L sterile suspension to a concentration of 2 g/L,
pH was adjusted to 7.6 by addition of 4M NaOH, and the gas phase was
replaced by flushing with filter-sterilized N2.
[0030] Microbial cellulose utilization data are taken with the initial
(time zero) data point just after supplemental Avicel addition. Microbial
control 1 was carried out as above except that a sterilized 1 M sodium azide
solution was added to a final concentration of 38.5 mM in conjunction with
supplemental Avicel addition. Addition of azide as specified above resulted in
cessation of fermentation as indicated by constant concentrations of
fermentation products over time as determined by HPLC.
[0031] Cellulosome preparation and purification. Cellulosome
used for batch SSF experiments was obtained from batch cultures of C.
-8-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
thermocellum grown in MTC medium in a 200 ml flask with Avicel as the
growth substrate at an initial concentration of 4 g/L. Cellulosome for
continuous SSF experiments was obtained from steady-state continuous
cultures of C. thermocellum grown in MTC medium at a dilution rate (flow
rate/fermentor working volume) of 0.052 hr' and feed cellulose concentration
of 4 g/L. Cellulosome was purified from the supernatant of the culture broth
by affinity digestion. Purified cellulosome preparations used for batch and
continuous SSF experiments contained approximately 1.2 g/L cellulase with a
specific activity of 2.8 IU/mg cellulase in Tris buffer (50mM, with 10mM
CaC12,
io pH 6.8). The concentration of soluble hydrolysis products in the purified
cellulosome preparation was verified by HPLC to be sufficiently small (< 0.002
g/L) so as not to complicate the interpretation of SSF experiments.
[0032] Batch SSF and relevant enzyme controls. Five ml of a
Thermoanaerobacter thermosaccharolyticum (ATCC 31960) stock culture was
inoculated into 100 ml MTC medium containing 2 g/L Avicel PH105 and 2 g/L
cellobiose in triplicate 200 ml serum vials under a N2 atmosphere. Cultures
were incubated at 60 C in a temperature controlled water bath with rotary
shaking at 200 rpm. Once cellobiose was consumed, as determined by HPLC,
pH was adjusted to 7.6, and a purified cellulosome preparation (above) was
filter sterilized (Millex-GV, 0.22um pore size, Millipore, Billerica, MA) and
added to the culture via syringe to a final concentration of 100 mg/L. SSF
data were taken with the initial (time zero) data point just after cellulase
addition. Cell-free control 1 was carried out in the presence of 2 g/L Avicel
and 100 mg/L purified cellulosome as above except that no fermenting
organism was present. Cell-free control 2 was carried out as for cell-free
control 1 except that a sterilized 1 M sodium azide solution was added to a
final concentration of 38.5 mM.
[0033] Continuous culture. A modified 1 L fermentor (Applikon,
Dependable Instruments, Foster City, CA, modified by NDS) with an overflow
sidearm (i.d. 0.38") and 0.5 L working volume was used for both microbial
fermentation by C. thermocellum and for SSF carried out in continuous mode.
pH was controlled at 6.8 by a Delta V process control system (New England
-9-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
Controls Inc., Mansfield, MA) with addition of 4M NaOH, the fermentor was
stirred at 250 rpm, and temperature was controlled at 60 C by circulating hot
water through the fermentor jacket. MTC medium containing 4 g/L Avicel
PH105 was fed by a peristaltic pump to achieve the desired residence times.
For SSF experiments, an additional peristaltic pump was used to deliver
purified cellulosome, stored at 4 C, in 50mM Tris buffer (pH 6.8). The
compositions of MTC medium and the concentration of Avicel used for SSF
experiments were adjusted to provide the same concentrations as those used
in C. thermocellum fermentation experiments (e.g., final concentration of 4
g/L
lo Avicel). SSF experiments were initiated by inoculating 50 ml of a late-
exponential phase culture of T. thermosaccharolyticum into MTC medium
containing 4 g/L Avicel supplemented with 2 g/L cellobiose. Once growth was
evident, cellulase addition commenced. Samples used to calculate steady-
state values for continuous fermentations were taken at intervals of at least
one residence.
[0034] Measurement of residual cellulose and fermentation
products. Residual cellulose was determined by quantitative saccharification.
Concentrations of sugars (cellobiose, glucose) and fermentation products
(lactic acid, acetic acid and ethanol) were analyzed by HPLC using a Bio-Rad
2o HPX-87H column operated at 55 C with 0.01 % (v/v) H2SO4 as effluent and a
refractive index detector. Oligomer sugars were analyzed according to the
modified NREL post-hydrolysis procedure reported by Ehrman, C.I., M.E.
Himmel. Biotechnology Techniques, 8(2): 99 (1994).
[0035] Measurement of protein, cellulosome, and cellulase
activity. The protein content in supernatant samples was determined with
bovine serum albumin as the standard in accordance with the Bradford
protein assay (Bradford, M.M. Anal. Biochem. 72, 24 (1976)). Protein content
in the pellet was measured using the pellet protein assay described previously
by Zhang et al. (Zhang, Y.-H. P, L.R. Lynd. J. Bacteriol. 187, 99 (2005)).
Supernatant and pellet cellulosome concentrations were determined by an
ELISA method reported by Dubois, M., K. Gilles, J.K. Hamilton, P.A. Rebers,
F. Smith. Nature. 168, 167 (1951). Avicelase activity in supernatant and
pellet
-10-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
samples was measured at 60 C, using the method of Zhang (2005), based on
soluble sugar production as determined by the phenol-sulfuric acid method of
Dubois (1951). Results are expressed in terms of International Units (IU) = 1
pmole glucose equivalent/L/min.
[0036] Batch Results. In batch culture under controlled conditions,
microbial hydrolysis required 16 hours for complete hydrolysis of 2 g
cellulose/L during which the cellulosome and cell protein concentrations
roughly doubled from 48 to 98 mg/L and 125 to 264 mg/L respectively (FIG. 1).
As shown in FIG. 2, SSF using purified cellulosome in the presence of T.
lo thermosaccharolyticum (enzymatic hydrolysis) required 32 hours for complete
hydrolysis, during which the cellulosome concentration was 100 mg/L and the
cell protein concentration increased from 160 to 260 mg/L.
[0037] The conditions under which microbial hydrolysis and SSF
occurred were similar. Product concentrations and pH versus time for the
reactions of FIGS. 1 and 2 are shown in FIGS. 3 and 4, respectively. The
products monitored were ethanol (Eth), acetate (Act), cellobiose (CB) and
glucose equivalents (Glu Eqv). Hydrolysis products (total soluble glucans, or
glucose equivalents) in the growth medium were < 0.02 g/L at all times for
both microbial hydrolysis and SSF, two orders of magnitude less than
concentrations at which 50% inhibition is generally observed. As shown in
Table 3, the cellulosome specific activity was quite similar for both
microbial
and enzymatic hydrolysis and remained nearly constant throughout the
experiment.
Table 3. Comparison of enzyme activity in C. thermocellum batch culture,
SSF and relevant enzyme control
Cellulosome in Avicelase activity Specific activity
phase 2 (IU/L) (IU/mg cellulosome)
(mg/L)
Begin* End Begin End Begin End
C. thermocellum 48 5 98 5 136 9 265 7 2.88 0.11 2.72 0.08
SSF 102 6 100 5 303 11 268 6 2.97 0.06 2.68 0.07
Enzyme Control 95 8 100 6 280 9 259 8 2.95 0.18 2.59 0.08
-11-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
* Beginning of phase 2 (FIGS. 1 and 2, Ohr- 48hr) of C. thermocellum batch
culture and SSF,
referring to 0 hr.
# End of phase 2 (FIGS. 1 and 2, Ohr- 48hr), referring to 16 hr for C.
thermocellum batch and
32 hr for SSF and enzyme control.
[0038] Cellulose concentration is plofted vs time in FIG. 5 for
microbial hydrolysis (from FIG. 1), SSF (from FIG. 2) and for controls as
follows: microbial control 1, a C. thermocellum culture (100 mg/L cellulosome,
264 mg/L cell protein) with 38.5 mM sodium azide; cell-free control 1, 100
mg/L purified cellulosome with no fermenting organism; cell-free control 2, as
lo for cell-free control 1 with 38.5 mM sodium azide. The rate of hydrolysis
is
substantially higher for growing cells (microbial hydrolysis) than for
metabolically-inactive cells (microbial control 1), in spite of the fact that
the
cellulosome concentration is lower through most of the experiment for
microbial hydrolysis (see FIG. 1) than for microbial control 1 (100 mg/L
cellulosome). The lower rates of hydrolysis by metabolically-inactive cells
are
not primarily due to the affect of azide on the cellulosome, since the rates
of
cell-free hydrolysis observed in the presence and absence of azide are
similar.
[0039] FIG. 6 shows cellulose hydrolysis and product accumulation
for cell-free control 1. It can be observed that although concentrations of
2o hydrolysis products are an order of magnitude higher for cell-free control
1
than SSF (FIG. 6 compared to FIG. 2), the hydrolysis rates are similar.
Accumulation of hydrolysis products in the bulk fermentation broth is
therefore
not a plausible explanation for the marked difference between microbial
hydrolysis and SSF.
[0040] The batch results support a degree of enzyme-microbe
synergy, equal to the ratio of the cellulosome-normalized hydrolysis rates
observed for the microbial system divided by that for the enzymatic system, of
between 2.8 and 4.7 (Table 4). See Example 2 for further description of the
quantification of enzyme-microbe synergy.
[0041] Continuous Culture Results. Microbial hydrolysis and SSF
were also compared in steady-state continuous cultures. Mean values for
four or more steady-state data points are reported in Table 4, with cellulase
specific activities in Table 5. Microbial steady states 1 and 2 were obtained
at
-12-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
residence times (-u = fermentor volume/feed flow rate) of 6.8 and 9.8 hours,
respectively. For microbial steady state 1, 65.3% of the feed cellulose was
hydrolyzed in the presence of a total cellulosome concentration of 39 mg/L,
whereas 76.8% hydrolysis was achieved at 63 mg cellulosome/L for microbial
steady state 2. Steady-state continuous SSF mediated by purified C.
thermocellum cellulosome in conjunction with fermentation by T.
thermosaccharolyticum was carried out at conditions chosen to achieve
similar conversion and total cellulosome concentrations to those observed for
microbial cellulose utilization. For SSF steady state 1, comparable to
io microbial steady state 1, cellulose hydrolysis of 67% was observed at ti=
24.4
hr and added cellulosome at 52 mg/L. For SSF steady state 2, cellulose
hydrolysis of 75.3% was observed at r = 19.23 hr and 63 mg/L cellulosome.
The concentration of cellulose hydrolysis products was below detection limits
(2.5 mg/L) for both microbial and SSF steady states. Time course SSF data
are presented in FIG. 7 (SSF steady state 1) and FIG. 8 (SSF steady state 2).
The experiment was switched from continuous to batch at 168 hours to
prevent further accumulation of cellobiose; continuous feeding was reinitiated
at 184 hours. Cellulase specific activity was similar for microbial and SSF
steady states (Table 5).
2o Table 4. Continuous culture data and degree of synergy calculation.
Cellulose (g/L) Xa Cellulase (g/L) T Spec. Rate (hr') Synergy
Ca(g/L) C(g/L) Ep Et (hr) rEt rEP DSEM DSE;~,
Batch
Microbial hydrolysis
After 8 hours 2.1 0.95 0.55 - 0.057 8 2.52 - 4.69 -
Complete reaction2.1 0.0 1.0 - 0.073 16 1.80 - 2.78 -
SSF (enzymatic hydrolysis)
After 8 hours 2.07 1.64 0.21 - 0.10 8 0.54 -
Complete reaction 2.07 0.0 1.0 - 0.10 32 0.65 -
Continuous
Microbial hydrolysis
Steady state 1 4.68 1.63 0.653 0.028 0.039 6.80 11.5016.01 4.81 4.77
Steady state 2 4.66 1.08 0.768 0.029 0.046 9.80 7.94 10.66 2.80 2.84
SSF (enzymatic hydrolysis)
Steady state 1 4.53 1.50 0.67 0.037 0.052 24.4 2.39 3.36
Steady state 2 4.65 1.16 0.753 0.041 0.064 19.2 2.84 4.43
a(Co - C)/Co. All cellulose concentrations reported in terms of glucose
equivalent.
-13-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
Table 5. Continuous culture of C. thermocellum and SSF
Conditions N Co C X D ET Eads Specific
(g/L) (g/L) (%) (h-') (g/L) (g/L) Activity
IU/mg
a. Microbial 6 4.68 0.234 94.5 0.051 0.067 0.028 2.55 0.09
0.05 0.1 1.4 0.004 0.002
b. Microbial 4 4.66 1.079 76.8 0.099 0.046 0.026 2.68 0.13
0.08 0.05 1.1 0.007 0.04
c. SSF 6 4.65 1.163 75 0.052 0.063 0.044 2.46 0.1
0.03 0.14 3.02 0.008 0.007
X - conversion of cellulose
ET - total cellulosome
Eads - adsorbed cellulosome
[0042] The continuous culture data support a degree of enzyme-
microbe synergy, equal to the ratio of the cellulosome-normalized hydrolysis
rates observed for the microbial system divided by that for the enzymatic
io system, of between 2.8 and 4.8 (Table 4). See Example 2 for further
description of the quantification of enzyme-microbe synergy.
[0043] For both batch and continuous culture experiments the C.
thermocellum cellulase complex is substantially more effective during
microbial hydrolysis as compared to SSF under the conditions examined.
Such enzyme-microbe synergy requires the presence of metabolically active
cellulolytic microbes, and is not explained by removal of hydrolysis products
from the bulk fermentation broth. The key apparent difference between
microbial hydrolysis and SSF appears to be that CEM complexes are present
during microbial hydrolysis whereas this is not the case for SSF.
EXAMPLE 2
QUANTIFICATION OF ENZYME-MICROBE SYNERGY
[0044] The cellulosome-specific hydrolysis rate on a cellulosome
basis ( r~ , g cellulose = g cellulosome-' = hr') may be calculated using:
E (Co - C)/-C
(1)
rc E
-14-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
where Co is the cellulose concentration in g/L either initially (for batch
reactions) or in the feed (for steady-state continuous reactions), C is the
fermentor cellulose concentration in g/L after time t (batch) or at steady-
state
(continuous), ti is the elapsed time (batch) or the residence time
(continuous),
and E is the average cellulosome concentration in g/L over the elapsed time
(batch) or for multiple steady-state points (continuous). The degree of
enzyme-microbe synergy, DSEM , may be calculated from the cellulosome-
specific hydrolysis rates observed for microbial hydrolysis and SSF using:
(r'')
C microbial
DSEM = E.
) (2)
(~C
SSF
[0045] The degree of synergy on a total cellulosome basis, DSEM , is
found by using the total cellulosome concentration, ET, in equation (1).
Alternatively, the degree of synergy on a pellet cellulosome basis, DSEM , is
found if the pellet cellulase concentration (Ep, potentially including both CE
and CEM complexes) is used.
[0046] Values for DSEM calculated from batch and continuous data
are presented in Table 4. DSEM based on batch data after eight hours is 4.69.
If DSEEM is calculated after complete cellulose hydrolysis is achieved, a
value
of 2.78 is obtained. In continuous culture, a DSEM value of 2.80 is obtained
based on microbial and SSF steady states 2, for which about 75% of the feed
cellulose is hydrolyzed. For microbial and SSF steady states 1, for which
about 66% hydrolysis is achieved, DSEM is equal to 4.81. Values for enzyme-
microbe synergy on a pellet cellulase basis, DSEn; , are quite similar to
DSEM values observed in continuous culture: 2.84 for microbial and SSF
steady states 2, and 4.77 for microbial and SSF steady states 1. Decreasing
-15-
CA 02651753 2008-10-31
WO 2007/136971 PCT/US2007/067954
synergy is seen with increasing extents of cellulose hydrolysis, and with
decreasing substrate to enzyme ratios, for both batch and continuous culture.
[0047] Changes may be made in the above methods and systems
without departing from the scope hereof. It should thus be noted that the
matter contained in the above description or shown in the accompanying
drawings should be interpreted as illustrative and not in a limiting sense.
The
following claims are intended to cover all generic and specific features
described herein, as well as all statements of the scope of the present
method,
io which, as a matter of language, might be said to fall there between.
-16-