Note: Descriptions are shown in the official language in which they were submitted.
CA 02652419 2014-08-15
CA 2652419
IMPROVEMENTS IN METHODS AND DEVICES TO CURB APPETITE
AND/OR REDUCE FOOD INTAKE
FIELD OF THE INVENTION
[001] The invention is in the field of medical devices and methods related
to curbing appetite and/or
reducing food intake.
BACKGROUND OF THE INVENTION
[002] Obesity, defined as a body mass index (BMI) of greater than 30, is a
major health concern in
the United States and other countries; it has been estimated that one in three
Americans and more than
300 million people world-wide are obese. Complications of obesity include many
serious and life-
threatening diseases including hypertension, diabetes, coronary artery
disease, stroke, congestive heart
failure, pulmonary insufficiency, multiple orthopedic problems, various
cancers and a markedly
decreased life expectancy. Intentional weight loss, however, can improve many
of these medical
complications associated with obesity.
[003] While weight loss can improve many of the medical complications
associated with obesity, its
management as a health concern has proven troublesome. A variety of approaches
including dietaty
methods, psychotherapy, behavior modification, and pharmacotherapy have each
met with some success
but as a whole failed to effectively control the rapid growth in the incidence
and severity of obesity seen
in the United States. The severity of problems associated with obesity also
has led to the development of
several drastic surgical procedures. One such procedure physically reduces the
size of the stomach so
that a person cannot consume as much food as was previously possible. These
stomach reduction
surgeries had limited early success, but now it is known that the stomach can
stretch back to a larger
volume over time, limiting the achievement of sustained weight loss in many
individuals. Another
drastic surgical procedure induces the malabsorption of food by reducing the
absorptive surface of the
gastrointestinal (GI) tract, generally via by-passing portions of the small
intestine. This gastric by-pass
procedure further has been combined with stomach reduction surgery. While
these described surgical
procedures can be effective to induce a reduction in food intake and/or
overall weight loss in some, the
surgical procedures are highly invasive and cause undue pain and discomfort.
Further, the described
procedures may result in numerous life-threatening postoperative
complications. These surgical
procedures are also expensive, difficult to reverse, and place a large burden
on the national health care
system.
1
CA 02652419 2015-07-17
CA 2652419
[004] Non-surgical approaches for the treatment of obesity also have been
developed. For example,
one non-surgical endoscopic approach to treating obesity includes the
placement of a gastric balloon
within the stomach. The gastric balloon fills a portion of the stomach,
providing the patient with a
feeling of fullness, thereby reducing food intake. This approach has yet to be
convincingly shown to be
successful, and a number of problems are associated with the gastric balloon
device, however, including
poor patient tolerance and complications due to rupture and/or migration of
the balloon. Other non-
surgical devices designed to induce weight loss limit the absorption of
nutrients in the small intestine by
funneling food from the stomach into a tube found within the small intestine
so that the food is not fully
digested or absorbed within the small intestine. While this type of device may
be somewhat effective at
limiting the absorption of consumed food, there is still room for a variety of
improvements in non-
surgical devices designed to induce weight loss and/or a reduction in food
intake.
10051 An understanding of biological events that contribute to the creation
of satiety signals
provides an opportunity to develop "smart" nonsurgical devices that can
trigger such events. The
amount of food that individuals consume is largely dependent on biological
signals between the gut and
the brain. Specifically, hormonal signals from the gut to the brain are
correlated with both the onset and
cessation of food intake. While increased levels of hormones such as ghrelin,
motilin and agouti-related
peptide are involved in the promotion of appetite and the onset of food
intake, increased levels of a
number of other hormones are involved in the cessation of food intake.
10061 Various biologic events contribute to the physiologic cessation of
food intake. Generally, as a
meal is consumed, the ingested food and by-products of digestion interact with
an array of receptors
along the GI tract to create satiety signals. Satiety signals communicate to
the brain that an adequate
amount of food has been consumed and that an organism should stop eating.
Specifically, GI tract
chemoreceptors respond to products of digestion (such as sugars, fatty acids,
amino acids and peptides)
while stretch and mechanoreceptors in the stomach and proximal small intestine
respond to the physical
presence of consumed foods. Chemoreceptors respond to the products of
digestion by causing the
release of hormones or other molecular signals. These released hormones and/or
other molecular signals
can stimulate nerve fibers to send satiety signals to the brain. The arrival
of these signals in the brain can
trigger a variety of neural pathways that can reduce food intake. The released
hormones and/or other
molecular signals can also travel to the brain themselves to help create
signals of satiety. Stretch and
mechanoreceptors generally send satiety signals to the brain through
stimulation of nerve fibers in the
periphery that signal the brain.
2
CA 02652419 2015-07-17
CA 2652419
SUMMARY
[007] The present disclosure provides methods and devices that help to
reduce food intake by
providing non-surgical devices and methods that trigger the aforementioned
biological events that
contribute to the creation of satiety signals.
[008] The claimed invention relates to a small intestinal insert
comprising: an elongated member
including a proximal end; a distal end; at least one angled portion between
the proximal end and the
distal end, the angled portion corresponding to at least one angled target
site within the small intestine;
and at least one flow reduction element supported by the elongated member, the
flow reduction element
configured to reduce the flow rate of chime in the small intestine; wherein at
least a portion of the insert
is formed of a biodegradable material; and the elongated member is configured
initially to sit stably
within the targeted site, and then, following degradation of the biodegradable
material, configured to
destabilize such that it becomes unseated from the target site.
[008A] The claimed invention also relates to a small intestinal insert
comprising: an elongated
member including a proximal end; a distal end; at least one angled portion
between the proximal end
and the distal end, the angled portion corresponding to at least one angled
target site within the small
intestine, and wherein the at least one angled target site within the small
intestine is in the duodenum;
and a neurological stimulator, supported by the elongated member.
1008B1 The claimed invention also relates to a small intestinal insert
comprising: an elongated
member including a proximal end; a distal end; at least one angled portion
between the proximal end
and the distal end, the angled portion corresponding to at least one angled
target site within the small
intestine; one or more releasable reservoirs containing one or more bioactive
materials, the one or more
reservoirs supported by the elongated member; and an active drug release
mechanism supported by the
elongated member, the active drug release mechanism and the one or more
releasable reservoirs in
operable communication with each other; wherein the angled portion comprises a
shape memory alloy
portion and a biodegradable portion.
3
CA 02652419 2015-07-17
CA 2652419
10091 The invention relates to a device to be inserted in the small
intestine, which may be referred to
generally as a small-intestinal insert. First to be summarized are embodiments
that include a
biodegradable material. Embodiments of the insert include an elongated or
central member with a
proximal end, a distal end, at least one angled portion between the proximal
end and the distal end, the
angled portion corresponding to at least one angled target site within the
small intestine, and at least a
portion of the insert formed of a biodegradable material. Embodiments may
include an elongated
member configured initially to sit stably within the targeted site, and then,
following degradation of the
biodegradable material, configured to destabilize such that it becomes
unseated from the target site, and
can then be eliminated from the body through the intestinal tract.
[010] In some embodiments, the angled portion includes biodegradable
material. In some
embodiments, the angled portion includes a shape memory material. In some of
these embodiments, the
shape memory material includes any of a shape memory alloy or a biodegradable
shape memory
polymer. In other embodiments, the angled portion includes both a shape memory
alloy portion and a
biodegradable portion. In some of these latter embodiments, the biodegradable
portion, upon
degradation, is configured to facilitate the destabilization and elimination
of the shape memory alloy
portion. In some of these
3a
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
embodiments, the shape memory alloy portion and the biodegradable portion are
joined at a
junction, the junction being configured to degrade as the biodegradable
portion degrades.
[00111 In some embodiments, the angled target site in the small intestine is
in the
duodenum. Further, in some embodiments, the angled target site in the duodenum
includes
two angles, and the insert has two angles corresponding to two angles of the
duodenum.
[0012] In some embodiments of the small intestinal insert, the device includes
at least one
flow reduction element supported by the elongated member, the flow reduction
element being
configured to reduce the flow rate of chyme in the small intestine. In some of
these
embodiments, the flow reduction element is formed at least in part from a
biodegradable
material. In some embodiments, the flow reduction element includes any of a
rib, a net, a
sleeve, a basket, a centrally mounted baffle, a peripherally mounted baffle, a
foam-like
material, or a fan. In embodiments of the flow reduction elements that include
a foam-like
material, the foam-like material may include any of an open cell foam, a
closed cell foam, or a
hydrogel. In some of these embodiments, the foam-like material includes a
bioactive material
incorporated thereinto. And in some of these embodiments, the foam-like
material is
biodegradable, and the bioactive material is released upon degradation of the
foam-like
material. In other embodiments, the flow reduction element includes at least
one releasable
reservoir of one or more bioactive materials.
[0013] In some embodiments with flow reduction elements, the elements reduce
the rate of
the chyme flow sufficiently to alter its biochemical profile. And in some of
these
embodiments, the biochemical profile is altered sufficiently to cause the
generation of a
hormonal signal of satiety.
[0014] In some embodiments, the physical dimension of the insert is such the
insert
distends a portion of the small intestine when the insert is seated therein,
and the distension is
sufficient to cause stretch receptors or other neurons of the small intestine
to generate a satiety
signal in response thereto. The physical dimensions or features of the insert
that cause
distension include any of length, width, volume, density, weight, porosity, or
surface
properties.
[0015] Some embodiments of the insert include one or more releasable
reservoirs
containing one or more bioactive materials, the one or more reservoirs
supported either
directly or indirectly by the elongated member, and further include an active
drug release
mechanism also supported either directly or indirectly by the elongated
member, the active
drug release mechanism and the one or more releasable reservoirs being in
operable
communication with each other.
4 =
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
100161 Some embodiments of the insert include a pump as a part of the active
drug release
mechanism for delivering a bioactive material, the pump being supported by the
elongate
member and coupled to the one or more releaseable reservoirs. Embodiments of
the pump
may include any of an osmotic pump, an electrically-driven mechanical pump, a
piezoelectric
pump, a flow-driven pump, or a peristaltic-action driven pump. Some of these
embodiments
further include an energy storage element configured to provide energy to the
pump. And in
some of these embodiments, the pump is controlled by a remote device.
100171 In other embodiments, the insert may further include an electronic
emitter or
neurostimulator configured to apply an electrical potential to a site in any
of the small
intestine or stomach, the emitter supported by the elongate member. In some of
these
embodiments, the the emitter, upon activation, stimulates a neuronal response
that contributes
to a signal of satiety. In some embodiments, the device further may include an
energy storage
element or apparatus configured to provide energy to the pump, and in some
embodiments,
the electronic emitter may be controlled by a remote device.
10018] Some embodiments of the device may further include an anchoring member
engaged to the proximal end of the elongated member, the anchoring member
beubg
configured to contribute to the stabilization of the device in the targeted
site. In some of these
anchored embodiments, the anchoring member resides in the stomach when the
elongate
member is seated in the target site within the small intestine.
[00191 Second to be summarized are embodiments of a small intestinal insert
that include a
neurological stimulator to electrically stimulate nerves in the small
intestine, such nerves
engaged in the generation of signals of satiety. This aspect of the invention
relates to a small
intestinal insert that includes an elongated member including a proximal end,
a distal end, at
least one angled portion between the proximal end and the distal end, the
angled portion
corresponding to at least one angled target site within the small intestine,
wherein the at least
one angled portion of the insert corresponds to at least one angled target
site within the small
intestine, and a neurological stimulator, supported by the elongate member.
[0020] In some of these embodiments, the insert includes a portion formed from
a
biodegradable material. Some of these embodiments are configured initially to
sit stably
within the targeted site, and then, following degradation of the biodegradable
material, they
are configured to destabilize such that it becomes unseated from the target
site, and may be
eliminated from the body by way of the intestinal tract.
100211 In some embodiments, the neurological stimulator is adapted to
stimulate one or
more nerves of the small intestine sufficiently to generate one or more
signals of satiety. In
some of these embodiments, the insert further includes an energy storage
element configured
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
to provide energy to the neurological stimulator. And in some of these
embodiments, the
neurological stimulator is controlled by a remote device.
[0022] In some embodiments of the insert, the angled target site in the small
intestine is in
the duodenum. And in some embodiments the angled target site in the duodenum
includes
two angles, the insert having two angles corresponding to two angles of the
duodenum.
[0023] In some embodiments, the insert includes at least one flow reduction
element, the
element configured to reduce the flow rate of chyme in the small intestine.
And in some of
these embodiments, the flow reduction element is formed at least in part from
a biodegradable
material.
[0024] In some embodiments of the insert, at least a portion of the insert is
formed of a
biodegradable material, the elongated member configured initially to sit
stably within the
targeted site, and then, following degradation of the biodegradable material,
configured to
destabilize such that it becomes unseated from the target site. In some of
these embodiments,
the angled portion of the insert includes biodegradable material. In still
other embodiments,
the insert may further include an anchoring member engaged to the proximal end
of the
elongated member, the anchoring member configured to contribute to the
stabilization of the
device in the targeted site.
[0025] Third to be summarized are embodiments of a small intestinal insert
that include
one or more releasable reservoirs containing one or more bioactive materials
and an active
drug release mechanism coupled to the reservoirs for conveying bioactive
materials to the
intraduodenal site. This aspect of the invention relates to a small intestinal
insert that includes
an elongated member including a proximal end, a distal end, at least one
angled portion
between the proximal end and the distal end, the angled portion corresponding
to at least one
angled target site within the small intestine, wherein the at least one angled
portion of the
insert corresponds to at least one angled target site within the small
intestine, and one or more
releasable reservoirs containing one or more bioactive materials, the one or
more reservoirs
supported by the elongated member; and an active drug release mechanism
supported by the
elongated member, the active drug release mechanism and the one or more
releasable
reservoirs in operable communication with each other.
[0026] In some of these embodiments, the insert includes a portion formed from
a
biodegradable material. Some of these embodiments are configured initially to
sit stably
within the targeted site, and then, following degradation of the biodegradable
material, they
are configured to destabilize such that it becomes unseated from the target
site, and may be
eliminated from the body by way of the intestinal tract. In some of these
embodiments, it is
the angled portion of the device that includes the biodegradable material. '
6
=
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
[0027] Embodiments of the insert may include a drug release mechanism that may
include
any of an osmotic pump, an electrically-driven mechanical pump, a flow-driven
pump, a
peristaltic-action driven pump, or a piezoelectric pump. Embodiments may
further include an
energy storage element configured to provide energy to the pump. In some
embodiments, the
active drug release mechanism is controlled by a remote device. In some of
these
embodiments, the bioactive materials released by the active drug release
mechanism are
sufficient to generate a signal of satiety.
[0028] In some of these embodiments, the angled target site in the small
intestine is in the
duodenum. And in some of these embodiments, the angled target site in the
duodenum
comprises two angles, the insert having two angles corresponding to two angles
of the
duodenum.
[0029] In some embodiments of the insert, the angled portion comprises a shape
memory
portion. In other embodiments, the angled portion includes a shape memory
alloy portion and
a biodegradable portion. Some embodiments of the insert further include an
electronic emitter
configured to apply an electrical potential to a site in the small intestine,
the site generating a
neuronal response that contributes to a signal of satiety.
[0030] The invention further relates to methods of generating satiety in a
subject by
inserting an intraduodenal inserted device into a subject. The first methods
to be summarized
are those that use embodiments of the devise, as described above, which
include a
biodegradable material. Embodiments used in this method include an elongated
member with
a proximal end, a distal end, at least one angled portion between the proximal
end and the
distal end, the angled portion corresponding to at least one angled target
site within the small
intestine, and at least a portion of the insert formed of a biodegradable
material, the elongated
member configured initially to sit stably within the targeted site, and then,
following
degradation of the biodegradable material, configured to destabilize such that
it becomes
unseated from the target site. The method of using this device includes
generating one or
more signals of satiety due to one or more effects of any of the presence of
the insert or by an
active intervention by the insert.
[0031] The method may further include biodegrading the biodegradable material
of the
insert, unseating the devise from the target site, and eliminating it from the
body. In some
embodiments of the method, wherein the insert includes a portion with a shape
memory alloy,
biodegrading the biodegradable material facilitates elimination of the shape
memory alloy
portion.
[0032] In some embodiments of the method, where the insert further comprises
chyme
flow reduction elements, the method further includes slowing the passage of
chyme with the
7
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
flow reduction elements. In some of these embodiments, slowing the passage of
chyme
changes the biochemical profile of the chyme. And in some of these
embodiments, changing
the biochemical profile of the chyme activates cells in the intestine such as
chemoreceptors,
the chemoreceptors generating a neuronal signal or secreting a bioactive
material in response
thereto.
[0033] In some embodiments of the method, generating a satiety signal includes
stretch-
sensitive neurons of the intestine responding to distension of at least a
portion of the
duodenum due to the presence of the insert. In some embodiments of the method,
generating a
satiety signal includes cells of the intestine secreting one or more bioactive
materials in
response to the presence of the insert.
[0034] In some embodiments of the method, where the insert further comprises
bioactive
materials in releasable reservoirs, an active intervention by the insert
includes the insert
releasing one or more bioactive materials. In some of these embodiments,
releasing one or
more bioactive materials includes effluxing or eluting from the reservoir. In
other
embodiments, where the insert further includes a pump in operable connection
with the
releasable reservoirs, and releasing one or more bioactive materials includes
pumping the
materials from the reservoir. In such embodiments, the pumping may be from any
of an
osmotic pump, an electrically-driven pump, a piezoelectric structure, a flow-
driven pump, or a
peristalsis-driven pump.
[0035] In some embodiments, where the bioactive materials are included within
a portion
of the device comprising biodegradable materials, and the bioactive materials
are released
upon degradation of the biodegradable material. In some embodiments, the
biodegradable
materials are included in one or more flow reduction elements of the insert
that include a
foam-like material, such as an open cell foam, a closed cell foam, or a
hydrogel.
[0036] In some embodiments where the insert further includes a neurological
stimulator
supported by the elongated member, the active intervention includes
stimulating one or more
nerves of the duodenum with the stimulator.
[0037] The invention further relates to methods, the second to be described,
of generating
satiety in a subject by positioning an embodiment of intraduodenal inserted
device that
includes a neurological stimulator into a subject. The insert embodiment
includes an
elongated member including a proximal end, a distal end, at least one angled
portion between
the proximal end and the distal end, the angled portion corresponding to at
least one angled
target site within the small intestine, and a neurological stimulator,
supported by the elongated
member; the method including stimulating nerves of the duodenum with the
neurological
stimulator. The method may include, more specifically, stretch-sensitive
neurons of the
8
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
intestine, which responding to the distending presence of the insert by
sending a signal of
satiety.
[0038] The method may further include slowing the passage of chyme with the
flow
reduction elements, such slowing of chyme flow contributing to further
generation of satiety
signals. The method may further include endocrine cells of the intestine
responding to the
neurological stimulator by any of direct or neurally-mediated pathways, the
response
including secreting one or more hormones.
[0039] Where the insert includes bioactive materials in releasable reservoirs,
the method
may further include the insert releasing one or more bioactive materials. In
some
embodiments, the insert includes biodegradable materials, and the method
further includes
biodegrading the biodegradable material of the insert and eliminating the
insert from the
body.
[0040] The invention further relates to a third set of methods of generating
satiety in a
subject by positioning an embodiment of intraduodenal inserted device that
includes one or
more releasable reservoirs containing one or more bioactive materials and an
active drug
release mechanism. The insert embodiment includes an elongated member
including a
proximal end, a distal end, at least one angled portion between the proximal
end and the distal
end, the angled portion corresponding to at least one angled target site
within the small
intestine, and one or more releasable reservoirs containing one or more
bioactive materials,
the one or more reservoirs supported by the elongated member; and an active
drug release
mechanism supported by the elongated member, the active drug release mechanism
and the
one or more releasable reservoirs in operable communication with each other. A
method of
using this embodiment includes releasing the one or more bioactive agents into
the
duodenum.
[0041] In some embodiments, releasing one or more bioactive materials includes
pumping
from the reservoir. Pumps included in the embodiment of the insert may include
any of an
osmotic pump, an electrically-driven pump, a piezoelectric structure, a flow-
driven pump, or a
peristalsis-driven pump.
[0042] Embodiments of the method may further include slowing the passage of
chyme
with the flow reduction elements, such slowing of chyme flow contributing to
further
generation of satiety signals. Embodiments of the method may further include
stretch-
sensitive neurons of the intestine responding to the physical presence of the
insert.
Embodiments of the method may still further include endocrine cells of the
intestine secreting
one or more hormones in response to any of the physical presence of the insert
in response to
the bioactive agents released by the active drug release mechanism. The
embodiments of the
9
CA 02652419 2008-11-14
WO 2007/139920 PCT/US2007/012462
method may still further include biodegrading the biodegradable material of
the insert and
eliminating the insert from the body.
'
BRIEF DESCRIPTION OF THE DRAWINGS
[0043] Figure 1 is a general drawing of the stomach and duodenum of the small
intestine.
[0044] Figure 2 depicts several exemplary mechanisms through which satiety
signals may
be generated.
[0045] Figure 3 is a perspective view of one embodiment of a duodenal/small
intestinal
insert in accordance with the present invention positioned inside the stomach
and small
intestine.
[0046] Figure 4 is a partial section view of a central tube illustrating
attached flow
reduction elements and a central lumen.
[0047] Figure 5 is a partial section view of a central tube illustrating
eccentrically attached
flow reduction elements and a central lumen.
[0048] Figure 6 is a perspective view of an alternative embodiment showing an
elongated
member and illustrating attached flow reduction elements.
[0049] Figure 7 is a perspective section view of a central tube and an
anchoring member.
[0050] Figure 8 is a perspective view of an alternative embodiment of a
central tube and
an anchoring member.
[0051] Figure 9 is a section view of a central tube of the present invention
that may lodge
in the small intestine for a period of time without any anchoring to the
stomach or pylorus.
[0052] Figure 10 illustrates a central tube attached to an expandable sleeve,
the
expandable sleeve allowing expansion of particular segments of the central
tube to form flow
reduction elements.
[0053] Figure 11 illustrates an expandable sleeve in a collapsed configuration
for insertion
into the small intestine.
100541 Figure 12 illustrates one mechanism for keeping flow reduction elements
formed
with an expandable sleeve in a desired expanded configuration.
[0055] Figure 13 is a flow diagram depicting the intestinal insert's role
in contributing to
the generation of one or more signals of satiety.
[0056] Figure 14 is perspective view of the duodenum.
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
[0057] Figure 15 depicts a side view of the duodenum, showing the folds of
rugae that
form the periphery of the inner space within which embodiments of the insert
device are
positioned.
[0058] Figure 16 depicts an embodiment of the insert with flow reduction
elements in the
form of a simple coil.
[0059] Figure 17 depicts an embodiment of the insert with flow reduction
elements in the
form of a spine with ribs.
[0060] Figure 18 depicts an embodiment of the insert with flow reduction
elements in the
form of a spine with nets.
[0061] Figure 19 depicts an embodiment of the insert with flow reduction
elements in the
form of a sleeve.
[0062] Figure 20 depicts an embodiment of the insert with flow reduction
elements in the
form of closed mesh baskets, and further showing pig-tail proximal and distal
ends.
[0063] Figure 21 depicts an embodiment of the insert with flow reduction
elements in the
form of centrally-mounted outwardly-extending baffles, and further showing pig-
tail proximal
and distal ends.
[0064] Figure 22 depicts an embodiment of the insert with flow reduction
elements in the
form of peripherally-mounted inwardly-extending baffles, and further showing
pig-tail
proximal and distal ends.
[0065] Figure 23 depicts an embodiment of the insert with flow reduction
elements in the
form of a foam-like bodies, and further showing pig-tail proximal and distal
ends.
[0066] Figure 24 depicts an embodiment of the insert with flow reduction
elements in the
form of a porous nutrient weeping stent.
100671 Figure 25 depicts an embodiment of the insert with flow reduction
elements in the
form of a centrally-mounted fans, and further showing pig-tail proximal and
distal ends.
[0068] Figure 26 depicts an embodiment of the insert with bioactive material
in reservoirs
that passively elute.
[0069] Figure 27 depicts an embodiment of the insert with a bioactive material-
loaded
osmotic pump.
=
[0070] Figure 28 depicts an embodiment of the insert with a bioactive material
loaded
reservoir coupled to an electrically driven pump, energy storage unit, and an
external control.
11
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
[0071] Figure 29 depicts an embodiment of the insert with electrodes for local
neurostimulation, an energy storage unit, and an external control.
[0072] Figure 30 depicts an embodiment of the central member of an insert that
includes
biodegradable elements and shape memory elements. Figure 30A shows the central
member
in an intact configuration; Figure 30B shows the central member after
biodegradation.
[0073] Figure 31 depicts an embodiment of the central member of an insert that
includes a
biodegradable shape memory polymeric material. Figure 31A shows the central
member in
an intact configuration; Figure 31B shows the central member after
biodegradation.
DETAILED DESCRIPTION
Embodiments of the Device In Situ
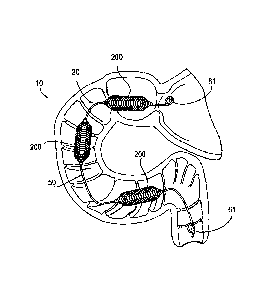
[0074] Figure 1 provides a view=of the human gastrointestinal (GI) tract,
including the
stomach 4 and duodenum of the small intestine 10. Important features are the
esophagus 2,
stomach 4, antrum 7, pylorus 8, pyloric valve 11, duodenum 10, jejunum 12 and
ampulla of
Vater (or hepatopancreatic ampulla) 13, which is formed by the union of the
pancreatic duct
and the common bile duct. Functionally, the esophagus 2 begins at the nose or
mouth at its
superior end and ends at the stomach 4 at its inferior end. The stomach 4
encloses a chamber
which is characterized, in part, by the esophageal-gastric juncture 6 (an
opening for the
esophagus 2) and the antrum-pyloric juncture 5 (a passageway between the
antrurn 7 through
the pylorus 8 to the duodenum 10 of the small intestine). The pylorus 8
controls the discharge
of contents of the stomach 4 through a sphincter muscle, the pyloric valve 11,
which allows
the pylorus 8 to open wide enough to pass sufficiently-digested stomach
contents (i.e., objects
of about one cubic centimeter or less). These gastric contents, after passing
into the duodenum
10, continue into the jejunum 12 and on into the ileum (not shown). The
duodenum 10,
jejunum 12 and ileum make up what is known as the small intestine. However
these
individual portions of the alimentary canal are sometimes individually
referred to as the small
intestine. In the context of this invention the small intestine can refer to
all or part of the
duodenum, jejunum and/or ileum. The ampulla of Vater 13, which provides bile
and
pancreatic fluids that aid in digestion is shown as a small protrusion on the
medial wall of the
duodenum 10.
100751 Embodiments of the inventive device include two basic forms. Some
embodiments
of the intestinal insert are stabilized in the intestine by way of an
anchoring member that
resides in the stomach and is too large to be swept through the pylorus. Other
embodiments
reside stably in the intestine not by virtue of a separate anchoring member in
the stomach, but
rather by virtue of the device as a whole fitting into the small intestine
with angled portions
12
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
that fit or correspond with angled portions of the intestine, and the device
further having a
sufficient structural integrity that it resists being moved distally because
the distal location
does not physically accommodate the shape of the device. Aspects of the device
that are
adapted to provide anchorless stabilization at a target site in the intestine
include physical
dimensions of length and width, as well as angles of the device, all of which
complement the
target portion of intestine. In other embodiments, stabilizing features in the
intestine may
include expanded portions of the device in the duodenal bulb, which is larger
than the more
distal portion of the duodenum, and which thereby effectively prevents distal
movement (as in
Figure 18, for example). Other stabilizing or anchoring elements may include
any of hooks,
barbs, or protrusions on the device that engage the wall of the intestine.
[0076] Some embodiments of the device and associated methods of using the
device are
directed toward reducing the rate of food transit through the intestine by
physical mechanisms
of intervening in the rate of food transit. In other aspects, embodiments of
the invention act by
eliciting satiety signals by way of physiological mechanisms, or,
alternatively, by directly
providing satiety signals through bioactive materials or agents, or by
neuronal stimulation,
thereby reducing food intake behaviorally. Some embodiments of the device are
directed
toward medical purposes broader than satiety and digestive physiology alone,
although the
satiety and food consumption functionalities of embodiments of the device and
method will
be described herein in greater detail. In some aspects, embodiments of the
device may
contribute to slowing food transit and/or reducing food intake by the satiety
signals generated
by the intestine in direct response to the mere physical presence of the
device. Such signals
could, for example, be mediated by stretch-responsive neurons or
mechanoreceptors in the
intestinal wall. In other embodiments, satiety signals could be mediated by
hormones that are
responsive to physical presence of material in the intestine, or which are
secondarily
responsive to mechano-receptors. In other embodiments, the slowing of food or
the increased
residency time, and the consequent change in the chemical environment of the
intestine, may
elicit responses from chemoreceptors residing in the intestine to signal
either neurally or
hormonally in such a way that has a net effect of signaling satiety.
[0077] In still other embodiments of the invention, the device may convey
bioactive
material or agents that are released over time within the intestine, the
bioactive agents
conveying a net signal of satiety. In some embodiments, the bioactive agents
with a net satiety
signaling effect are passively released from sites such as coatings, depots,
or reservoirs within
the device. Bio active materials or agents have been described in detail
above, but briefly and
in broad aspect may include any of hormones, drugs, or cells. In some
embodiments,
bioactive agents may be held in osmotic pumps and released by osmotic drive.
Release
mechanisms such as osmotic pumps provide a level of control and predictability
to bioactive
13
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
agent release, but the mechanism remains relatively passive and without means
of
intervention. Other embodiments of the invention, however, may include more
active
mechanisms for bioactive agents release or delivery, as could be provided by
electrically
driven pumps, or by piezoelectric elements that allow or promote the release
stored bioactive
agents in response to applied current. Such devices may include power storage
elements, or
may be provided power by external sources by wired or wireless approaches.
100781 In still other embodiments of the invention, the device may include
electrodes or
conductive elements that provide electrical stimulation to nerves in the
intestine, such
resulting neural activity contributing to a net effect of signaling satiety to
the brain. In some
embodiments, satiety-related neuronal activity may further be mediated by
endocrine
mechanisms. As in embodiments of the invention with powered mechanisms for
bioactive
agent release, embodiments with electrical capability may include power
storage devices, or
be enabled to receive energy conveyed from external sourcei.
[0079] In other aspects of the invention, embodiments of the inserted device,
with or
without an anchor, may provide a platform for bioactive agent delivery, neural
stimulus
delivery, or radiation therapy delivery, for medical purposes more broad than
inducing satiety,
or intervening in food transit. For the delivery of some bioactive agents,
there may be
considerable advantage associated with local delivery of an agent to an
intestinal site. Such
advantages may include localization of dosing, lack of exposure to stomach
acid as occurs in
oral delivery, or diminished exposure to the metabolic machinery of the liver
and kidney that
iv. drug delivery, or any form of systemic delivery faces. Further,
embodiments of the device
may accommodate multiple drugs, in some embodiments the release of such
multiple drugs
may be independently controlled.
Digestive System Context of Invention
[0080] The description now addresses the digestive system, the digestive
process, and
aspects of the endocrinology and neurophysiology of satiety as they relate to
embodiments of
the invention. The adult duodenum is about 20-25 cm long and is the shortest,
widest, and
most predictably placed part of the small intestine. The duodenum forms an
elongated C-
shaped configuration that lies between the level of the first and third lumbar
vertebrae in the
supine position. Susan Standring (ed.), Gray's Anatomy, 39th Ed., 1163-64
(2005), provides a
standard reference. Returning to Figure 1 for reference and further detail of
aspects of the
digestive system, the first part of the duodenum, often referred to as the
duodenal bulb 10a, is
about 5 cm long and starts as a continuation of the duodenal end of the
pylorus 8. This first
part of the duodenum passes superiorly, posteriorly and laterally for 5 cm
before curving
sharply inferiorly into the superior duodenal flexure 465, which marks the end
of the first part
14
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
of the duodenum. The second part of the duodenum, often called the vertical
duodenum 10b,
is about 8-10 cm long. It starts at the superior duodenal flexure 465 and runs
inferiorly in a
gentle curve towards the third lumbar vertebral body. Here, it turns sharply
medially into the
inferior duodenal flexure 475 which marks its junction with the third part of
the duodenum.
The third part of the duodenum, often called the horizontal duodenum 10c,
starts at the
inferior duodenal flexure and is about 10 cm long. It runs from the right side
of the lower
border of the third lumbar vertebra, angled slightly superiorly, across to the
left and ends in
continuity with the fourth part of the duodenum in front of the abdominal
aorta. The fourth
part of the duodenum is about 2.5 cm in length; it starts just to the left of
the aorta and runs
superiorly and laterally to the level of the upper border of the second lumbar
vertebra. It then
turns antero-inferiorly at the duodenojejunal flexure and is continuous with
the jejunum.
' Some embodiments of the present invention take advantage of this
predictable configuration
of the small intestine to provide duodenal/small intestinal implants that do
not require
anchoring within the pylorus or stomach, as described more fully below.
100811 The digestive process starts when consumed foods are mixed with saliva
and
enzymes in the mouth. Once food is swallowed, digestion continues in the
esophagus and in
the stomach, where the food is combined with acids and additional enzymes to
liquefy it. The
food resides in the stomach for a time and then passes into the duodenum of
the small
intestine to be intermixed with bile and pancreatic juice. Mixture of the
consumed food with
bile and pancreatic juice makes the nutrients contained therein available for
absorption by the
villi and microvilli of the small intestine and by other absorptive organs of
the body.
[00821 The presence of partially digested food within the stomach and small
intestine
initiates a cascade of biological signals that create satiety signals and
contribute to the
cessation of food intake. One such satiety signal is initiated by the release
of cholecystokinin
(CCK). Cells of the small intestine release CCK in response to the presence of
digested foods,
and in particular, in response to dietary fat, fatty acids, small peptides,
and amino acids.
Elevated levels of CCK reduce meal size and duration and may do so through a
number of
different mechanisms. For example, CCK may act on CCK-A receptors in the liver
and within
the central nervous system to induce satiety signals. CCK stimulates vagal
afferent fibers in
both the liver and the pylorus that project to the nucleus tractus solitarius,
an area of the brain
that communicates with the hypothalamus to centrally regulate food intake and
feeding
behavior. CCK also stimulates the release of enzymes from the pancreas and
gall bladder and
inhibits gastric emptying. Because CCK is a potent inhibitor of gastric
emptying, some of its
effects on limiting food intake may be mediated by the retention of food in
the stomach.
= 15
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
100831 Cells of the small intestine (particularly L cells) also release
glucagon-like peptide
1 (GLP-1) and oxyntomodulin (OXM) in response to nutrient signals of
digestion. Elevated
levels of GLP-1 and OXM are associated with satiety signals and the cessation
of food intake.
These hormones may signal satiety by activating receptors on afferent vagal
nerves in the
liver and/or the GI tract and/or by inhibiting gastric emptying.
[0084] Pancreatic peptide (PP) is released in proportion to the number of
calories ingested,
and in response to gastric distension. Elevated levels of PP have been shown
to reduce food
intake and body weight. PP may exert some of its anorectic effects via vagal
afferent
pathways to the brainstem, as well as through more local effects, such as by
suppression of
gastric ghrelin production.
[00851 Peptide YY3-36 (PYY3-36) is another biological signal whose peripheral
release may
be correlated with reduced food intake and/or the cessation of eating.
Specifically, low levels
of PYY3_36 have been correlated with obesity while its administration
decreases caloric intake
and subjective hunger scores. Intravenous administration of PYY3-36 may reduce
food intake
through its effects of suppressing ghrelin expression, delaying gastric
emptying, delaying
various secretion from the pancreas and stomach and increasing the absorption
of fluids and
electrolytes from the ileum after a meal.
[00861 Insulin and leptin are two additional biological signals that regulate
satiety and
eating behavior. Through parasympathetic innervation, beta Cells of the
endocrine pancreas
release insulin in response to circulating nutrients such as glucose and amino
acids, and in
response to the presence of GLP-1 and gastric inhibitory peptide (GIP).
Insulin stimulates
leptin production from adipose tissue via increased glucose metabolism.
Increased insulin
levels in the brain leads to a reduction in food intake. Elevated leptin
levels also decrease food
intake and induce weight loss. Insulin and leptin have also been implicated in
the regulation
of energy expenditure since their administration induces greater weight loss
than can be
explained by reduction in food intake alone. Both insulin and leptin act
within the central
nervous system to inhibit food intake and to increase energy expenditure, most
likely by
activating the sympathetic nervous system. Insulin's effects to decrease food
intake also
involve interactions with several hypothalamic neuropeptides that are also
involved in the
regulation of feeding behavior such as, by way of example, NPY and
melanocortin ligands.
[00871 Other hormones or biological signals that are involved in the
suppression or
inhibition of food intake include, by way of example, GIP (secreted from
intestinal endocrine
K cells after glucose administration or ingestion of high carbohydrate meals;
enterostatin
(produced in response to dietary fat; amylin (co-secreted with insulin from
pancreatic beta
16
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
cells); glucagon, gastrin-releasing peptide (GRP), somatostatin, neurotensin,
bombesin,
calcitonin, calcitonin gene-related peptide, neuromedin U (NMU), and ketones.
[0088] In relation to embodiments of the present invention, when the passage
of partially
digested food or chyme is partially impeded within the duodenum of the small
intestine and
the flow rate through this area is reduced (or to express the same phenomenon
in another way,
as residency time is increased), the emptying of the stomach and the duodenum
will occur
more slowly. This slowing, by itself, may create extended feelings of satiety
and thus lead to a
decrease in food intake (due to the longer retention time of food in the
stomach). The slowing
of the passage of food also provides more time for the partially digested food
to interact with
chemoreceptors, stretch receptors, and mechanoreceptors along the GI tract so
that
stimulation of satiety signals may be increased and/or prolonged, which may,
in turn, lead to a
reduction in food intake during an eating period and/or longer periods between
food intake.
[0089] In addition to keeping partially-digested food within the small
intestine for an
extended period of time, the methods and devices of the present invention may
also enhance
and/or prolong the release of satiety signals by releasing signals into the
small intestine
themselves. For example, in some embodiments, the methods and devices of the
present
invention may release nutrient products of digestion to stimulate
chemoreceptors to cause the
release of hormones and/or other molecular signals that contribute to the
creation of satiety
signals. In another embodiment, the methods and devices of the present
invention may exert a
small amount of pressure on the walls of the GI tract to stimulate stretch
and/or
mechanoreceptors to generate and send satiety signals to the brain. In another
embodiment,
the methods and devices of the present invention may release signals, such as,
by way of
example, nutrient by-products of digestion of food, to stimulate
chemoreceptors as described
above and may exert a small amount of pressure on the walls of the small
intestine as
described above to contribute to the generation of satiety signals.
Device with Flow Reduction Elements, and embodiments with an anchoring member
[0090] The methods and devices of the present invention may contribute to
weight loss and
the treatment of obesity by covering portions of the walls of the small
intestine, thus blocking
some nutrient uptake and/or interrupting or reducing the intermixing of the
digestive fluids. In
some embodiments, the methods and devices of the present invention may further
include a
central tube which funnels a portion of the consumed food through the small
intestine without
being fully digested or absorbed. In these manners, the methods and devices of
the present
invention may inhibit the absorption of partially digested food materials. The
partially
digested food materials are then passed to the large intestine for elimination
with limited
caloric absorption by the body.
17
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
[0091] Figure 2 depicts several exemplary non-limiting mechanisms through
which satiety
signals may be generated. In this Figure 2, a by-product of digestion, such as
a fatty acid or
other protein, stimulates an L-cell of the small intestine to release CCK
locally and into the
circulation. CCK released locally may stimulate vagal afferent nerve fibers in
the area to
generate satiety signals to the central nervous system (CNS). CCK that enters
the circulation
may travel to the liver to stimulate vagal afferent nerve fibers in the liver
to generate satiety
signals to the CNS. CCK in the circulation may travel to the gall bladder and
pancreas to
upregulate the digestion-related activities of these organs. CCK in the
circulation also may
travel to the CNS itself to contribute to the creation of a satiety signal.
Once satiety signals are
received and integrated within the CNS, the CNS may trigger physiological
effects that serve
to contribute to a feeling of fullness and/or the cessation, slowing or
reduction of food intake.
[0092] Turning now to embodiments of the invention, Figure 3 shows an
exemplary small
intestinal insert 20 made in accordance with the present invention that may
contribute to the
creation of satiety signals. The insert 20 is positioned in the stomach 4 and
small intestine 10.
The insert 20 has a proximal portion 30 and a distal portion 40, and a central
tube 50 that
extends from the proximal portion 30 to the distal portion 40. One or more
flow reduction
elements 200 that are sized to fit within the small intestine 10 may be
attached to the central
tube 50. While not required, the portion of the central tube 50 near the
ampulla of Vater 13
generally will not include a flow reduction element 200 so that the
introduction of bile and
pancreatic fluid into the small intestine is not impeded.
[0093] In some embodiments, the central tube 50 has an anchoring member 100
near its
proximal end 52, with the anchoring member 100 securing the proximal end 52 of
the central
tube 50 in the antrum 7 of the stomach. The anchoring member 100 is sized so
that it will not
pass through the pylorus 8. In this way, embodiments of the present invention
including an
anchoring member anchor the flow reduction elements 200 within the small
intestine. In some
embodiments, the anchoring member may be established by one or more inflatable
balloons
102 that when inflated are larger than the pylorus 8. The inflatable balloons
102 may be
deflated for delivery into the stomach and then inflated inside the stomach.
The inflatable
balloons 102 may also be deflated for later removal using endoscopic
techniques.
[0094] As will be described in further detail below, embodiments of flow
reduction
elements 200 may assume many configurations, and may vary further with regard
to physical
features such as composition, nature of the surface, and porosity of the bulk
material. Some
further exemplary embodiments of flow reduction elements 200 are depicted in
Figures 16 ¨
25. In some embodiments, as depicted in Figure 16, the central tube or member,
also referred
to as an elongated member, may, itself, be configured into a form that reduces
chyme flow in
18
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
the duodenum. A functional property that embodiments of flow reduction
elements have in
common is that they slow the transit of digesting food without blocking it,
and within
clinically appropriate guidelines. The process of slowing the transit rate may
also have effects
on the composition of the digesting food material, such as varying its
biochemical profile with
regard to the nutritional compounds being metabolized. Chemical receptors and
nerves of the
duodenum are sensitive to the biochemical profile of metabolites within the
chyme, and
participate in the coordination of physiology of digestion and satiety and
hunger, accordingly.
As such, by altering the flow rate and hence, the biochemical profile of
chyme, embodiments
of the inventive small intestinal insert contribute to the generation of
signals associated with
satiety. Flow reduction elements may further effect the composition of the
digesting food
material by the mixing action the flow reduction elements may provide.
[0095] The length of the central tube 50 may be established depending on the
therapeutic
result desired. For example, the central tube 50 and the one or more attached
flow reduction
elements 200 may extend into a portion of or through the entire duodenum 10.
On some
patients the central tube 50 and the one or more attached flow reduction
elements 200 may
extend past the duodenum 10 and into the jejunum 12. It is anticipated that
differing lengths
of central tubes and differing numbers and configurations of the flow
reduction elements may
be used by a physician to treat various body types and metabolic demands. In
one example, if
a patient is 20% overweight, a physician might select a length of central tube
50 with attached
flow reduction elements 200 that permit absorption of only 80% of the
nutritional potential of
a typical daily intake of calories. This reduction of caloric intake over time
could lead to an
appropriate amount of weight loss in the patient.
[0096] Figure 4 shows an embodiment of the invention with a central tube 50
that includes
an outer wall 54 and an inner wall 56 that define an interior space 58. The
interior space 58
forms an inner lumen 59 that may be continuous from the proximal end 52 of the
central tube
50 to just short of the distal end 53 of the central tube 50. The distal end
53 of the central tube
50 is sealed at a point 55 so that fluid introduced into the central tube 50
does not leak out
distally into the small intestine. In some embodiments a valve 90 may be
located substantially
at the proximal end of the inner lumen 59. The valve 90 may be a self sealing
valve that has a
septum 92 that may be accessed by a needle or blunt tip tube for introduction
of fluid into the
inner lumen 59. The valve 90 also may be accessed so that the fluid inside the
inner lumen 59
of the central tube 50 may be aspirated for removal. It is to be understood
that the valve type
is not limited to a septum type valve only, and that other types of mechanical
valves may also
be used in place of the septum valve described. Particular embodiments of the
present
invention are adapted to accept fluids in this manner so that the devices of
the present
19
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
invention may be implanted in a deflated configuration and later expanded into
an inflated
configuration.
[00971 As shown in Figure 4 and as mentioned above, one or more flow reduction
elements 200 may be attached to the central tube 50. In some embodiments the
diameter of
each flow reduction element 200 may be concentric with the axis of the central
tube 50. In the
embodiment depicted in Figure 4, each flow reduction element 200 has an outer
wall 210, an
inner wall 212, and an inner space 214. At or near its proximally-oriented
surface 220 and
also at or near its distally-oriented surface 222, each flow reduction element
200 may be
attached to the central tube 50 with the inner space 214 of the flow reduction
element 200 in
fluid communication with the lumen 59 of the central tube 50, such that the
inner space 214
surrounds the outer wall 54 of the central tube 50. Each flow reduction
element 200 may be
attached to the central tube 50 by, for example, adhesives, heat bonding,
mechanical restraint
or other suitable methods.
10098] As also depicted in Figure 4, the central tube 50 may be formed with
plural
inlet/exit ports 216 that are located inside respective flow reduction
elements 200. More
specifically, each port 216 is formed completely through the central tube wall
51 to establish a
pathway for fluid communication between the inner lumen 59 of the central tube
50 and the
inner space 214 of the respective flow reduction elements 200. Consequently,
the inner lumen
59 of the central tube 50 may be used to introduce fluid into the inner spaces
214 of the flow
reduction elements 200 and to inflate the flow reduction elements 200 from a
collapsed =
configuration, in which insertion and removal of the flow reduction elements
200 is
facilitated, to an inflated configuration shown in Figure. 4, in which
resistance to food
passage is increased to induce satiety. Thus, as suggested earlier, the flow
reduction element
or elements 200 in this embodiment act as balloons that may be deflated and
collapsed around
the central tube 50 for introduction into the small intestine and then
inflated to the desired
diameter once in position.
[00991 Embodiments of the flow reduction elements 200 may assume other forms,
such as
coils, ribs, fans, baffles, either peripherally-mounted or centrally-mounted,
as well as sleeves,
mesh cages or baskets. Embodiments such as these are described further, below,
in the section
entitled "Further embodiments of the invention", which also includes
description of
embodiments with biodegradable components, active biomaterial release
mechanisms, and
nerve stimulation features, and as depicted in Figures 15 ¨ 31.
[00100] In some embodiments, individual flow reduction elements 200 of the
present
invention may be elastic balloons or inelastic balloons. When an elastic
balloon material is
used to establish a flow reduction element 200, the flow reduction element 200
inflates to a
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
diameter that is dependent on the volume of fluid introduced into the inner
space of the flow
reduction element. This embodiment permits adjustment of the balloon size as
determined by
the physician. If the balloon is too small, for instance, additional fluid
could be introduced to
enlarge the balloon diameter. Alternatively, if the balloon is too large,
additional fluid could
be removed to shrink the balloon diameter. It is understood that an alternate
embodiment
consisting of an inelastic balloon that inflates to a diameter that is
independent of a yolume of
fluid introduced into its inner space is also included within the present
invention. The
diameter of this type of balloon is fixed when manufactured and does not
permit in situ
adjustment of the balloon size. However, this type of balloon prevents
possible over inflation
and rupture if too much fluid is introduced into the balloon.
[00101] The flow reduction elements 200 shown in Figure 4 have the shape of a
round
sphere. However, other shapes are contemplated and any shape that effectively
functions to
inhibit the passage of partially digested food in the small intestine is
acceptable in accordance
with the present invention. It is understood that the ability of the small
intestinal insert to
remain within the small intestine may be affected by the shape, orientation
and tautness of the
flow reduction elements 200. For example alternate shapes such as ovoid,
elliptical, elongated
ellipse and even irregular non-geometrical shapes could be used in accordance
with the
present invention.
[00102] Figure 5 illustrates an alternative embodiment of the present
invention in which
one or more flow reduction elements 300 are eccentrically attached to a
central tube 350. In
this embodiment the axis or diameter of the flow reduction element or elements
300 is not
concentric with the axis of the central tube. The outer wall 302 of the flow
reduction element
is attached to the side of an outer wall 354 of the central tube 350. An inner
space 314 of each
flow reduction element 300 is eccentric relative to the axis of the central
tube 350 and is in
fluid communication with an inner lumen 359 of the central tube 350 through a
respective
opening 316. As was the case with the embodiment shown in Figure 4, in the
embodiment
shown in Figure 5 the inner lumen 359 may be used to introduce and remove
fluid into the
inner space 314 of the flow reduction element 300 to move the flow reduction
element 300
between inflated and deflated configurations.
[001031 In some embodiments of the present invention, the flow reduction
elements 300
may be inflated with a fluid, including a liquid and/or a gas. In some
embodiments, the gas
may be, for example, air, nitrogen or carbon dioxide. In another embodiment a
liquid may be,
for example, water or water mixed with other solutions. Any appropriate
inflation medium
may be modified to deliver bioactive materials or other signals that may
diffuse from the
insert of the present invention into the small intestine to trigger biological
signals of satiety.
21
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
When bioactive materials are delivered through an inflation medium, the
central tube and/or
flow reduction elements should be permeable to the bioactive materials.
Porosity may be
adjusted to control the diffusion rate of the bioactive materials.
[00104] When inflating the flow reduction elements of the present invention,
it may be
important for the physician to monitor the flow reduction element 300 location
in the small
intestine and the diameter of the flow reduction element relative to the
diameter of the small
intestine. For this purpose, the flow reduction element may be inflated with a
radiopaque fluid
that is visible on X-ray. When the flow reduction element contains radiopaque
fluid, a
physician may non-invasively visualize the size and placement of the flow
reduction
element(s) from outside the patient's body. This knowledge enables the
physician to adjust the
size and/or placement of the flow reduction element(s). Likewise radiopaque
marker bands
218 as shown in Figure 5 may be placed around the central tube to facilitate
visualization of
the central tube's location in the small intestine. The radiopaque marker
bands 218 may be
placed at predetermined intervals so that the distance inside the small
intestine may be used as
depth markers and may be measured from outside of the body.
[00105] The central tube and flow reduction elements of the present invention
may be
flexible. In some embodiments, they may be constructed of a polymeric material
that may be
easily formed or extruded and delivered with the aid of an endoscope by known
techniques. A
central tube 50 that is soft and flexible will contour to the anatomy of the
gastrointestinal tract
and provide *less irritation of the stomach and intestinal lining.
[00106] Figure 6 shows an alternative embodiment of the invention with flow
reduction
elements that are generally self-expanding, and do not necessarily include a
central lumen.
These embodiments include a central shaft 450 around which flow reduction
elements are
concentrically attached 400 and/or are eccentrically attached 410. The
elements 400 and 410
may be attached to the central shaft 450 by, for example, heat fusing,
adhesives or other
suitable methods as known in the art. These flow reduction elements 400 may be
made from
material that may be folded or collapsed to a first volume suitable for
insertion with the aid of
an endoscope and then may self expand to a second volume suitable for
restricting the flow of
partially digested food according to the present invention. These flow
reduction elements may
be made from materials, or materials may be configured so as to take the form
of such as, by
way of example, a sponge, a foam, a hydrogel, or springs that may be compacted
into a small
volume and then self expand to a pre-determined shape and volume when
unrestricted. Gel-
or sponge-based embodiments may include open cell or closed cell forms. In
addition to
having features that allow such gel- or sponge-based embodiments to be
collapsible and
expandable for deployment, such embodiments typically have a high surface area
which is
22
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
beneficial in embodiments that may include bioactive agents, and may further
be conducive
for purposes of biodegradability. Another foam-related embodiment is described
below in the
section entitled "Further embodiments of the invention", and depicted in
Figure 23. Because
the flow reduction elements self expand, the need for an inflation system is
eliminated and
this embodiment represents a simple mechanical design. These flow reduction
elements may
also be impregnated with bioactive materials or other signals that may trigger
biological
signals of satiety.
[001071 The central shaft 450 of an embodiment such as that depicted in Figure
6 may be
solid and without an inner lumen or inner space. In another embodiment the
central shaft 450
may include a passageway for consumed food so that the food may pass through
the small
intestine without being fully absorbed.
[001081 Turning now to various anchoring members that may be used in
accordance with
the present invention, Figure 7 depicts one such member. In Figure 7, the
central tube 50 has
an anchoring member 100 near its proximal end 52. As stated earlier, the
anchoring member
100 may be established by one or more inflatable balloons 102. These balloons
102 may be
eccentrically attached to the central tube at point 104 near the proximal end
52 of the central
tube 50. These balloons may be formed in many shapes and are not limited to
the spherical
shape shown. The central tube may be formed with an opening 116 for each
respective
balloon 102 so that a pathway for fluid communication is established between
the inner lumen =
59 of the central tube 50 and the inner space of each balloon 106. The inner
lumen 59 is used
to introduce fluid into the inner space of the balloon 106 and inflate the
balloon 102 from a
first volume in a collapsed state to a second volume or inflated state.
[00109] When the one or more balloons 102 of the anchoring member 100 are
fully inflated,
they secure the proximal end of the central tube 52 within the antrum of the
stomach. The one
or more inflatable balloons 102 have a combined cross sectional diameter
greater than the
diameter of the pyloric valve to prevent migration across the pylorus. The
inflatable balloons
102 may be inflated and deflated by adding or removing fluid from the central
tube inner
lumen 59. The inflatable balloons 102 may be connected to the same central
tube inner lumen
59 as the one or more flow reduction elements attached to the central tube and
may be inflated
simultaneously with the flow reduction elements. The central tube 50 may also
have more
than one inner lumen so that the inflatable balloons 102 and individual one or
more flow
reduction elements may be inflated and deflated independently as well.
[00110] Figure 8 illustrates another embodiment of the invention, wherein an
anchoring
member 100 of the present invention is deployed in the antrum 7. In this
embodiment, a
central tube 50 is attached to an inverted umbrella skeleton 160. This
skeleton 160 has a ring
23
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
162 that surrounds the central tube 50 and is supported by struts. In the
depicted embodiment
the ring 162 is supported by three struts 164, 165, and 166, however more or
fewer struts may
be successfully employed. In the embodiment depicted in Figure 8, the struts
are joined
together at the central tube 50 at point 167 and attached to the ring 162 at
points 170,171 and
172. The ring 162 of this anchor configuration may be made from, by way of
example,
flexible plastic material or flexible wire and has a diameter significantly
larger than the
diameter of the pyloric valve. This umbrella skeleton 160 may be collapsed
around the central
tube 50 for insertion into the stomach with the aid of an endoscope. As the
device is released
from the endoscope, the umbrella skeleton 160 may spring out and assume a
configuration
similar to that shown in Figure 8. The struts 164, 165 and 166 may be made
from, by way of
example, plastic, metal or from plastic covered metal. The edge of the ring
which is in contact
with the antrum walls 163, may be constructed to assist in securing the
umbrella ring 162 to
the walls of the antrum. In some embodiments, the surface may be roughened to
increase
surface friction or the wall may have protrusions or barbs that physically
attach to the
stomach lining.
Device without an anchoring member
[00111] Figure 9 shows a central tube or elongated member 50 of the present
invention that
may lodge and remain in the small intestine for a period of time without any
anchoring to the
stomach or pylorus. Embodiments of the present invention that can lodge and
remain within
the small intestine for a period of time without any anchoring to the stomach
or pylorus do so
by (i) adopting a central tube with appropriately placed angles that mimic the
contours of the
small intestine; and (ii) flow reduction elements of an appropriate diameter
that help to hold
the intestinal insert in place. In one embodiment, while not required, these
flow reduction
elements can have an abrasive surface or anchoring barbs that can help them
adhere to the
walls of the small intestine.
[001121 In Figure 9, the first three parts of the duodenum, including the
duodenal bulb WA,
the vertical duodenum 10B, and the horizontal duodenum 10C are depicted. The
flow
reduction elements of the depicted embodiment have been removed for clarity.
Distal to the
pylorus 8 and immediately after entering the duodenum 10, the central tube 50
may assume a
sharp bend of radius p between the duodenal bulb 10A and the vertical duodenum
10B, and a
sharp bend of radius a between the vertical duodenum 10B and horizontal
duodenum 10C. In
some embodiments the radius p and the radius a may be between about 45 degrees
and about
110 degrees. In another embodiment the radius i3 and the radius a may be
between about 60
degrees and about 100 degrees such that the central tube 50 bends to follow or
correspond to
the inner lumen of the duodenum 10 at these locations that contain predictably
configured
24
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
bends. In another embodiment the radius p and the radius a may be about 80
degrees. While
most embodiments of the present invention will include lengths that require
adoption of angle
and angle a, shorter devices adopting one or the other are also included
within the scope of
the present invention. In these described embodiments of the present
invention, it may be
advantageous that the central tube 50 be flexible enough to conform to the
sharp angulations
of the small intestine to avoid kinking. One or more flow reduction elements
with a diameter
about equal to that of the small intestine are also included along the length
of the central tube
50. In some embodiments, this diameter is about 3 cm; in other embodiments
this diameter is
about 4 cm.
[00113] To stabilize an intestinal insert in situ without the need for an
anchoring element,
the central tube or elongated member 50 may be pre-formed with a configuration
that
conforms to the duodenal angulations prior to insertion in the body. This
embodiment of the
present invention may be constrained in a straight configuration by a
stiffening rod 110 placed
down the inner lumen 59 of the central tube 50 as shown. This stiffening rod
110 may be
placed into a separate lumen designed to house this stiffening rod or may be
imbedded in the
wall of the central tube 50. Upon insertion into the patient with the aid of
an endoscope, when
the central tube 50 reaches the location of the sharp bends in the duodenum
10, the stiffening
rod 110 may be withdrawn, thereby allowing the central tube 50 to assume a pre-
formed
shape.
[001141 In another embodiment that stabilizes in situ without an anchoring
member, the
central tube or elongated member 50 may have a shape memory alloy wire
embedded inside
the central tube wall 51 or residing in the inner lumen 59. This shape memory
alloy wire has a
pre-set bend configuration with a radius p and a radius a that matches or
corresponds to the
bend configuration of the duodenum and is positioned in the central tube 50 at
the
corresponding location. Upon insertion into the patient with the aid of an
endoscope, when
the central tube 50 reaches the location of the sharp bend in the duodenum 10
and the shape
memory alloy wire reaches a pre-set transition temperature equal to body
temperature or
about 37 C., the wire assumes the programmed shape and forces the central
tube 50 and the
central tube wall 51 to assume the same shape.
1001151 In another embodiment, the central tube or elongate member 50 may have
a spring
embedded inside the central tube wall 51 or inner lumen 59. This spring could
be pre-shaped
to the anatomy of the wall of the small intestine. The spring is held straight
during delivery
and conforms to the small intestine anatomy after release, and such shape
enables the device
to remain in place. The shape enables the device to remain in place. In one
embodiment, due
to its configuration that matches or corresponds to the predictable placement
and
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
configuration of the small intestine, the device can remain in place for a
period of time within
the small intestine without anchoring to the stomach or pylorus of the
stomach.
[001161 While the present embodiments of the present invention can remain in
the small
intestine for a period of time without anchoring to the stomach or pylorus,
they are not
intended to remain indefmitely. In some embodiments, the inserts are
endoscopically removed
after a predetermined period of time. In other embodiments, the inserts may be
formed of one
or more biodegradable materials that are eventually degraded and eliminated
from the body.
The rate of biodegradability of any embodiment of the inventive device may be
adjusted by
varying the biodegradable aspects of the embodiment, thus allowing for a
manufacturing
route to control the residency time in the intestinal tract to a clinically
appropriate level.
Biodegradable composition may be varied in qualitative terms, by varying the
composition of
the materials. Biodegradability of devices may also be varied in quantitative
terms, for
examply by varying the quantity of material at a location vulnerable to
biodegradation. For
example, varying the thickness of a junction designed for biodegradable
vulnerability may be
varied in thickness.
[001171 Biodegradable aspects of embodiments of the invention are described
further
below; all embodiments described herein, and all embodiments as depicted in
Figures 3 ¨ 12,
and 16 ¨ 31 may have portions that include biodegradable materials, both
within the central
tube or member, also referred to synonymously as elongate member 50 and/or any
of the
various embodiments of the chyme flow reduction elements 200. In the
description that
ensues, some embodiments are used as specifically illustrative examples that
are formed
wholly or in part from biodegradable materials, but, as stated, all
embodiments may include
biodegradable materials, even when not specifically identified as such,
including
embodiments with and without an anchoring member.
Deployment of Inserts and Flow Reduction Elements
100118] The description now turns to considerations related to deployment of
the inventive
insert, some embodiments of which include flow reduction elements. Flow
reduction elements
are referenced in a generic sense with the label 200, but some exemplary
embodiments make
use of different label numbers, for their particular features. Figure 10
illustrates an
embodiment of the present invention where flow reduction elements may be
created through
the expansion of portions of an expandable sleeve; this embodiment will be
used in the
context of describing an example of how to deploy a device with flow reduction
elements. In
the embodiment depicted in Figure 10, a central tube 50 is attached to an
expandable sleeve
508 at the expandable sleeve's distal end 510 near the distal portion of a
duodenal/small
intestinal insert of the present invention. In a delivery configuration of the
depicted
26
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
embodiment, the opposite proximal end of the central tube 50 is attached to a
detachable
extension tube 520 that may lock onto a proximal portion of the central tube
50 when the flow
reduction elements 530 are expanded (post delivery). One non-limiting method
of detachable
attachment is the use of one or more screws 504, whereby the extension tube
520 screws into
the central tube 50. The central tube 50 may be pre-formed to have a
configuration that
conforms to the anatomy of the duodenum 10 shown in Figure 1. A central tube
50 so
described would force the expandable sleeve 508 to assume the configuration of
the central
tube 50. The central tube 50 may be constructed, merely by way of example, of
wire, spring,
superelastic or shape memory alloys, hollow steel tubing or plastic polymers.
In some
embodiments a stiffening rod or guide wire 110 may also be inserted through
the lumen of
central tube 50.
[00119] The expandable sleeve 508 herein described is designed to expand at
predefined
segments to allow the formation of flow reduction elements 530. In some
embodiments, the
non-expanded segments 532 of expandable sleeve 508 may be coated with a
polymer to
prevent their expansion. In another embodiment, the flow reduction elements
530 may be
covered with a flexible polymer to prevent partially digested food from
entering the flow
reduction elements 530. In another embodiment, a stiffening rod or guide wire
110 may be
inserted through the lumen of central tube 50 to straighten the central tube
50 when the device =
is delivered into the duodenum.
[00120] The expandable sleeve 508 may, merely by way of example be configured
as any
one or more of a knit, a weave, a mesh or a braid that may be formed, merely
by way of
example from any one or more of a metal, a wire, a ribbon, a plastic polymer
or a
biodegradable material.
[00121] Figure 11 illustrates the expandable sleeve 508 consisting of flow
reduction
elements 530 in a collapsed configuration for insertion into the small
intestine. In this
configuration a force A is applied to the expandable sleeve 508 to collapse
the flow reduction
elements 530. The collapsed form may be restrained by a constraining mechanism
such as,
merely by way of example, a sheath or a tightly wound string, or by applying
sustained
traction on the proximal end of the expandable sleeve 508. Figure 11 also
shows portions of
the central tube that will remain unexpanded 532, a detachable extension tube
520 and a
guidewire 110.
[00122] The expansion of the flow reduction elements 530 in the embodiments
depicted in
' Figures 10 and 11 may occur passively or actively. One example of passive
expansion may
be the removal of a constraining mechanism to allow the flow reduction
elements 530 to
expand to an original expanded state. Another non-limiting mechanism can be to
release
27
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
traction on the proximal end of an expandable sleeve 508 to allow the flow
reduction
elements 530 to expand to an original expanded state.
[00123] The flow reduction elements 530 of the embodiments depicted in Figures
10 and
11 can expand in a distal to proximal fashion, a proximal to distal fashion or
in a central
fashion depending on their relative position in relation to, in some
embodiments, motion of
the expandable sleeve 508 and the central tube 50 to one another. For example,
if the
proximal end of the flow reduction element lumen is held in the duodenal bulb
and the central
tube 50 is pulled back, the distal end of the flow reduction element lumen may
expand first.
Expansion in this direction may be advantageous because the position of the
proximal end of
the flow reduction element lumen remains in the duodenal bulb.
[00124] Figure 12 illustrates some embodiments of the present invention that
may lock the
proximal end of the expandable sleeve 508 to the central tube 50 at a position
to keep the flow
reduction elements in a desired expanded configuration. Traction on the
extension tube 520
retracts central tube 50 until wedge 52 engages the proximal end of the
expandable sleeve
508. The central tube 50 may have multiple ratchet-like wedges that may lock
the expandable
sleeve 508 at different degrees of expansion. The extension tube may be
unscrewed from the
central tube 50 after deployment of the device and expansion of the expandable
sleeve 508.
Biodegradable Features
[00125] While the present embodiments of the present invention may remain in
the small
intestine for a period of time, they are not intended to remain indefinitely.
In some
embodiments, the inserts are endoscopically removed after a predetermined
period of time. In
other embodiments, the inserts may be formed or partially-formed of one or
more
biodegradable materials that are eventually degraded and eliminated from the
body. In some
embodiments, the device may include some material that is biodegradable and
some material
that is not biodegradable. In some embodiments that include non-biodegradable
materials, the
degradation of the biodegradable portions of the device may facilitate the
breakdown and
eventual elimination of the non biodegradable portions.
[00126] Biodegradable is used in a broad sense, so as to include the any type
of material
breakdown or disintegration of any type that may occur in a biological
environment, such
environment being defined primarily by the biological host, but also by any
microorganisms
within the host. Other terms that biodegradability broadly embraces include
bioabsorbability
and bioerodibility. Biodegradation, per embodiments of the invention, may
occur, for
example, by dissolution, by effects of pH, such as action of acids, by
hydrolytic mechanisms,
by hydration, by digestive or enzyme-catalyzed effects such as cleavage, or by
physical
effects of bodily or muscular movement. An example of biodegradation is
provided by the
28
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
hydrolysis, dissolution, or reaction to pH, or enzymatic lysis that results in
a scission of the
polymer backbone of an inserted device. Microorganisms such as those that
reside in the
intestine, may eat or digest polymers, and also initiate a mechanical,
chemical, or enzymatic
aging. The biodegradable materials of embodiments of the invention are also
biologically
compatible, as well as are breakdown products of biodegradable materials, as
included in
embodiments of the present invention. Biodegradable materials may include
organic and
inorganic compounds. Some representative inorganic compounds are described
below in the
section related to "device features to accommodate bioactive agents"; in this
section, a
description of biodegradable polymers is provided for inclusion as embodiments
of the
present invention.
[00127] As mentioned above, some embodiments of the invention may include a
resilient
shape holding portion, and in some embodiments, a shape memory portion that
supports the
maintenance of an advantageous configuration of the device, particularly with
regard to
maintenance of angles alpha and beta of the inventive C-shaped duodenal insert
device.
Metals as well as some polymers are capable of resiliently holding a shape.
Shape memory
materials include metal alloys as well as biodegradable polymers. Shape memory
alloy
elements of the device are not biodegradable, but these alloy structural
elements may be
combined or joined with polymeric elements that are biodegradable, and upon
such
degradation, the alloy elements are released in a form that allows their
elimination. Such
embodiments are depicted in Figures 30A and 30B, as described below. Other
embodiments
or the invention may include biodegradable shape memory polymeric elements.
Biodegradable shape memory polymers have been described in various
publications,
including U.S. Patents US Patents US 6,160,084, US 6,281,262, US 6,388,043, US
6,720,402,
and US Published Applications US 20050075405A1, US 20030055198A1, US
20040015187A1, US 20040110285A1, US 20050245719A1, and US 20060142794A1.
Embodiments of the invention may include any one or more of such shape memory
materials,
and further, suCh materials may be joined together in various ways as depicted
in Figures
31A and 31B.
[00128] A variety of natural, synthetic, and biosynthetic polymers are
biologically
degradable and may be included as materials that comprise embodiments of the
intestinal
insert device. A polymer based on the C-C backbone tends to be
nonbiodegradable, whereas
hetero atom-containing polymer backbones confer biodegradability.
Biodegradability may be
engineered into polymers by the judicious addition of chemical linkages such
as anhydride,
ester, or amide bonds, among others. The mechanism for degradation is by
hydrolysis or
enzymatic cleavage resulting in a scission of the polymer backbone.
Microorganisms, such as
29
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
those that reside in the intestine, may eat or digest polymers, and also
initiate a mechanical,
chemical, or enzymatic aging.
[00129] Biodegradable polymers with hydrolyzable chemical bonds are
appropriate as
materials for a biodegradable intestinal insert. In addition to being
biocompatible, the material
should meet other criteria, for example, being processable, sterilizable, and
capable of
controlled stability or degradation in response to biological conditions. The
degradation
products often define the biocompatibility of a polymer, not necessarily the
polymer itself.
Poly(esters) based on polylactide (PLA), polyglycolide (PGA), polycaprolactone
(PCL), and
their copolymers have been extensively employed as biomaterials. Degradation
of these
materials yields the corresponding hydroxy acids, making them safe for in vivo
use.
[00130] Other biodegradable polymers include poly(hydroxyalkanoate)s of the
PHB-PHV
class, additional poly(ester)s, and natural polymers, particularly, modified
poly(saccharide)s,
e.g., starch, cellulose, and chitosan. Chitosan is derived from chitin, and is
the second most
abundant natural polymer in the world after cellulose. Upon deacetylation, it
yields the novel
biomaterial Chitosan, which upon further hydrolysis yields an extremely low
molecular
weight oligosaccharide. Chitosan is biocompatible, antibacterial and
environmentally friendly
polyelectrolyte, thus appropriate for medical devices and as material for
controlled release in
drug delivery.
[00131] Poly(ethylene oxide), PEO, a polymer with the repeat structural unit -
CH2CH20-,
has applications in drug delivery. The material known as poly(ethylene
glycol), PEG, is in
fact PEO but has in addition hydroxyl groups at each end of the molecule. In
contrast to high
molecular weight PEO, in which the degree of polymerization, n, might range
from 103 to 105,
the range used most frequently for biomaterials is generally from 12 to 200,
that is PEG 600
to PEG 9000, though grades up to 20,000 are commercially available. Key
properties that
make poly(ethylene oxide) attractive as a biomaterial are biocompatibility,
hydrophilicity, and
versatility. The simple, water-soluble, linear polymer may be modified by
chemical
interaction to form water-insoluble but water-swellable hydrogels retaining
the desirable
properties associated with the ethylene oxide part of the structure.
[00132] Multiblock copolymers of poly(ethylene oxide) (PEO) and poly(butylene
terephthalate) (PBT) may also be appropriate for intestinally inserted
devices. These materials
are subject to both hydrolysis (via ester bonds) and oxidation (via ether
bonds). Degradation
rate is influenced by PEO molecular weight and content. Additionally, the
copolymer with the
highest water uptake degrades most rapidly.
[00133] A widely used nondegradable polymer is ethylene-vinyl acetate
copolymer. This
copolymer has excellent biocompatibility, physical stability, biological
inertness, and
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
processability. In drug delivery application these copolymers usually contain
30-50 weight
percent vinyl acetate. Ethylene-vinyl acetate copolymer membrane acts as the
rate-limiting
barrier for the diffusion of the drug. In the Type II class of degradable
polymers, the
conversion of the hydrophobic substituents to hydrophilic side groups is a
first step in the
degradation process. The tyrosine-derived polycarbonate poly(DTE-co-DT
carbonate), may,
for example, be an appropriate material for a biodegradable intestinal insert.
The material may
be made with the pendant group via the tyrosine as either an ethyl ester (DTE)
or free
carboxylate (DT). Through alteration of the ratio of DTE to DT, the material's
= hydrophobic/hydrophilic balance and rate of in vivo degradation may be
manipulated.
[00134] Water-swellable polymer networks may function as hydrogels at one end
or as
superabsorbers at the other extreme. Hydrogels are characterized by the
pronounced affinity
of their chemical structures for aqueous solutions in which they swell rather
than dissolve.
Such polymeric networks may range from being mildly absorbing, typically
retaining 30 wt.
% of water within their structure, to superabsorbing, where they retain many
times their
weight of aqueous fluids. Several synthetic strategies have been proposed to
prepare
absorbent polymers including: polyelectrolyte(s) subjected to covalent cross-
linking,
associative polymers consisting of hydrophilic and hydrophobic components
("effective"
cross-links through hydrogen bonding), and physically interpenetrating polymer
networks
yielding absorbent polymers of high mechanical strength. These approaches are
not mutually
exclusive, and materials may include composite gels that are critically
reliant on the balance
between polymer-polymer and polymer-solvent interactions under various stimuli
including
changes in temperature, pH, ionic strength, solvent, concentration, pressure,
stress, light
intensity, and electric or magnetic fields.
Bioactive Materials
[00135] As previously stated, in some embodiments, the central tube and/or
flow reduction
elements of the invention may be adapted to release bioactive materials or
bioactive agents
that trigger biological satiety signals; . In some embodiments, the one or
more of the flow
reduction elements and/or central tube may be a porous and malleable solid
designed to
release a signal into the gastrointestinal (GI) tract over time. In some
embodiments, nutrient
products of digestion are released from the one or more flow reduction
elements 200 and/or
central tube or elongate member 50 to trigger chemoreceptors within the GI
tract to release
molecular signals involved in transmitting and/or creating satiety signals.
[00136] The description now turns to a consideration of release of bioactive
materials from
the device in furtherance of reducing appetite or slowing food absorption or
intake. The term
"bioactive material(s)" refers to any organic, inorganic, or living agent that
is biologically
31
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
active or relevant; the term has been extensively described in U.S.
Application No.
11/300,283, and will described here only briefly. For example, a bioactive
material may be a
protein, a polypeptide, a polysaccharide (e.g. heparin), an oligosaccharide, a
mono- or
disaccharide, a lipd, an organometallic compound, or an inorganic compound, an
antimicrobial agent (including antibacterial and anti-fungal agents), an anti-
viral agent, anti-
tumor agent, immunogenic agent. It may include a living or senescent cell, a
stem cell, a
bacterium, a virus, or any part thereof. It may include a biologically active
molecule such as a
hormone, a growth factor, a growth factor-producing virus, a growth factor
inhibitor, a growth
factor receptor, an anti-inflammatory agent, an antimetabolite, or a complete
or partial
functional insense or antisense gene. It may also include a man-made particle
or material that
carries a biologically relevant or active material. A bioactive material also
may be a by-
product of digestion or an agent that alters the pH of its surrounding
environment.
[001371 Bioactive materials also may include drugs such as chemical or
biological
compounds that can have a therapeutic effect on a biological organism.
Bioactive materials
also may include precursor materials that exhibit the relevant biological
activity after being
metabolized, broken-down (e.g. cleaving molecular components), or otherwise
processed and
modified within the body. Combinations, blends, or other preparations of any
of the foregoing
examples may be made and still be considered bioactive materials within the
intended
meaning herein. Aspects of the present invention directed toward bioactive
materials may
include any or all of the foregoing examples.
1001381 Examples of bioactive materials included with the present invention
include
hormones and other compounds that convey satiety promoting signals. Bioactive
materials of
the present invention may also include other naturally-occurring or
synthesized peptide,
protein, and steroid hormones. Bioactive agents further may include anti-tumor
agents,
antimicrobial agents, such as antibiotics: cephalosporins: aminoglycosides:;
macrolides:
tetracyclines, chemotherapeutic agents, sulfonamides, urinary tract
antiseptics, anaerobic
infection antibiotics, drugs for tuberculosis, drugs for leprosy, antifimgal
agents, antiviral
agents, chemotherapeutic agents for amebiasis, anti-helminthiasis agents, anti-
inflammatory
agents, anti-gout agents, centrally acting analgesics, thyroid drugs,
including those used in
adjunctive therapy, and those used as anti-thyroid agents, viral surface
antigens or parts of
viruses, bacterial surface antigens or parts of bacteria, surface antigens of
parasites causing
disease or portions of parasites, immunoglobulins, antitoxins, and antigens
that elicit an
immune response, such as disease-associated antigens, or bioactive agents such
as hormones,
enzymes or clotting factors:
32
CA 02652419 2014-08-15
CA 2652419
Device Features to Accommodate Bioactive Agents for Delivery
[00139] The central tube 20 and/or flow reduction elements 200 of the present
invention may have
bioactive materials adhered to their surface (through dip-coating, spray-
coating, sputter-coating and a
variety of other techniques known to those of skill in the art) or included in
reservoirs or depots
accessible to the surface, or may be manufactured so that the materials making
up the intestinal insert
include and diffuse such bioactive materials. The central tube and/or flow
reduction elements of the
present invention that diffuse bioactive materials, may be created by a number
of different procedures
that are referenced in U.S. Application Serial No. 11/300,283 of Bininoeller,
filed on December 15,
2005 and published as U.S. Publication 2006/0178691 on.August 10, 2006,
including references to U.S.
Pat. No. 5,019,400 to Gombotz etal. U.S. Pat. No. 6,685,957 to Bezemer etal.
and U.S. Pat. No.
6,685,957.
[00140] When a hydrophobic bioactive material, such as a steroid hormone is
incorporated by the
above-described method, at least one hydrophobic antioxidant may be present.
Hydrophobic
antioxidants which may be employed include, tocopherols (such as a.-
tocopherol, fl-tocopherol, y-
tocopherol, 8-tocopherol, epsilon.-tocopherol, zetartocopherol, zeta2-
tocopherol, and eta-tocopherol)
and 1-ascorbic acid 6-palmitate. Such hydrophobic antioxidants may retard the
degradation of the
copolymer and retard the release of the bioactive material.
[00141] When a loaded polymer made according to the above-referenced technique
includes a
hydrophilic bioactive material, the loaded polymer may also include, in
addition to a hydrophobic
antioxidant, a hydrophobic molecule such as, by way of example, cholesterol,
ergosterol, lithocholic
acid, cholic acid, dinosterol, betuline, or oleanolic acid, which may serve to
retard the release rate of the
agent from the copolymer. Such hydrophobic molecules prevent water penetration
into the loaded
polymer, but do not compromise the degradability of the polymer matrix.
Further, such molecules may
decrease the polymer matrix diffusion coefficient for the bioactive material
to be released and therby
provide for a more sustained release of a bioactive material from the polymer
matrix.
[00142] Methods of dispersing bioactive materials into polymers and the role
of lyophilization to
include thermoprotectants have been provided in U.S. 2006/0178691.
[00143] Non-limiting examples of polymers that may be used in accordance with
the present
invention, particularly with regard to accommodating and releasing bioactive
agents, include
polyurethanes, polyesterurethanes, silicone, fluoropolymers, ethylene vinyl
acetate, polyethylene,
33
CA 02652419 2014-08-15
CA 2652419
polypropylene, polycarbonates, trimethylenecarbonate, polyphosphazene,
polyhydroxybutyrate,
polyhydroxyvalerate, polydioxanone, polyiminocarbonates, polyorthoesters,
ethylene vinyl alcohol
copolymer, L-polylactide, D,L-polylactide, polyglycolide, polycaprolactone,
copolymers of lactide and
glycol ide, polymethylmethacrylate, poly(n-butyl)methacrylate, polyacrylates,
polymethaerylates,
elastomers, and mixtures thereof. Representative elastomers that may also be
used include, by way of
example, a thermoplastic elastomer material available under the trade name "C-
FLEX" from Concept
Polymer Technologies of Largo, Fla., polyether-amide thermoplastic elastomer,
fluoroelastomers,
fluorosilicone elastomer, sytrene-butadiene rubber, butadiene-styrene rubber,
polyisoprene, neoprene
(polychloroprene), ethylene-propylene elastomer, chloro-sulfonated
polyethylene elastomer, butyl
rubber, polysulfide elastomer, polyaciylate elastomer, nitrile, rubber,
polyester, styrene, ethylene,
propylene, butadiene and isoprene, polyester thermoplastic elastomer, and
mixtures thereof.
[00144] One of skill in the art can determine the amount or concentration of
bioactive material(s) to
include on the surface or within the material of the intestinal inserts of the
present invention depending
on particular treatment objectives and desired release profiles, as described
in U.S. 2006/0178691.
[00145] In some embodiments, the intestinal inserts of the present invention,
or portions thereof, may
include a topcoat or barrier to slow the diffusion or release of bioactive
materials. Typically, the barrier
should be biocompatible (i.e., its presence does not elicit an adverse
response from the body), and may
have a thickness ranging from about 50 angstroms to about 20,000 angstroms. In
some embodiments the
barrier may include a polymer provided over the polymer that diffuses
bioactive materials.
[00146] In some embodiments, a barrier of the present invention comprises
inorganic materials, which
have been detailed in U.S. 2006/0178691. Further detailed in that application
are several methods that
may be used to deposit a barrier over the inserts of the present invention.
Nitride barrier coatings, such
as, by way of example, titanium nitride, titanium carbonitride, chromium
nitride, titanium aluminum
nitride, and zirconium nitride may be deposited on the inserts of the present
invention at relatively low
temperatures by cathodic arc vacuum deposition. Such a method may be chosen
where bioactive
materials included within an insert of the present invention are temperature-
sensitive. Further detailed in
that application are methods for producing films of pure metals and alloys.
[00147] In some embodiments, it is contemplated that the barrier will contain
mostly inorganic
material. However, other embodiments may include barriers with a mixture of
34
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
organic and inorganic materials or barriers of all organic materials. Some
organic compounds
that may be used in accordance with the present invention include, by way of
example,
polyacrylonitrile, polyvinylidene chloride, nylon 6-6, perfiuoropolymers,
polyethylene
terephthalate, polyethylene 2,6-napthalene dicarboxylate, and.polycarbonate.
Generally, the
solubility of the drug in the material of the barrier is less than the
solubility of the drug in its
polymer carrier. Also, generally, the diffusivity of the drug in the material
of the barrier is
lower than the diffusivity of the drug in its polymer carrier. The some
embodiments, the
barrier may be biodegradable. Appropriate biodegradable materials that may be
used to create
a barrier include, by way of example, calcium phosphates such as, by way of
example,
hydroxyapatite, carbonated hydroxyapatite, tricalcium phosphate, p -tricalcium
phosphate,
octacalcium phosphate, amorphous calcium phosphate, and calcium
orthophosphate. Certain
calcium salts such as calcium phosphate (plaster of Paris) may also be used.
The
biodegradability of the barrier may act as an additional mechanism for
controlling drug
release from the underlying first layer.
Active Control of Bioactive Material Release
1001481 Some embodiments of the device and methods provide a more active,
i.e., a more
controlled, or metered method of delivering bioactive agents, in contrast to
the more passive
diffusion of drug from surfaces or depots. These approaches are also more
amenable to
handling the delivery of multiple-drug release. Embodiments of the inventive
devise may
include a pump to dispense one or more bioactive agents from a reservoir or
depot. Pumps
may include electrically-driven pumps 72 mechanical pumps, piezo-electric
devices that
control pores, for example, or pumps may be osmotically-driven pumps 71. The
osmotic
pump delivery is relatively passive in that it does not require energy input,
but it is
controllable, predictable, and calibratable. Osmotic pumps typically are
driven or urged via
pH difference or concentration gradients. Release of bioactive materials may
be controlled by
external control devices, such as by an electronic signaling device either
user-controlled or a
programmable pacing/signaling device. Examples of devices that embody these
active
approaches to the delivery of bioactive materials or agents are described
further below, and
are depicted in Figures 26 ¨ 28.
1001491 There are advantages to a drug delivery site within the intestinal
lumen that may,
for example advantageously be applied to the delivery of bioactive agents in a
broader array
than just drugs specific to modulating digestion or appetite. Such other
agents may include
chemotherapeutic agents, or radioactive particles for anti-cancer therapy.
Another type of
bioactive material that may benefit from local delivery may include cells,
such as stem cells
or activated immune cells, for cellular therapy of the intestine. Advantages
of the intra-
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
duodenal site of release may include proximity to target sites, taking
advantage of specific
chemical recovery receptors in the intestine, and minimizing systemic
metabolism of drugs
that occurs during the passage of the drug through such organs as the liver
and kidney that
occurs when drugs are delivered intravenously or orally.
1001501 In addition to delivering bioactive materials to the small intestine
that may reduce
food intake, the methods and devices of the present invention may be used to
deliver other
bioactive materials normally taken orally as well. The release of bioactive
materials directly
into the small intestine may be advantageous because many bioactive materials,
including
many drugs that are generally taken orally, are degraded by the harsh
conditions of the
stomach before they may reach the small intestine to be absorbed. For this
reason, many
bioactive materials are coated with layers of protective materials. By
releasing bioactive
materials, including drugs, directly into the small intestine, coatings to
protect the bioactive
materials may not be required. This lack of required protective coatings may
be beneficial for
patients because less unnecessary substances are introduced into their
systems, and it is
further beneficial as a process step reduction and cost reduction measure.
[00151] In another aspect of the invention which takes a more active
interventional role,
embodiments of the device may include an electronic emitter configured to
apply an electrical
potential to tissue in the stomach or duodenum. This electrical potential will
trigger neuron-
= receptors and/or mechano-receptors, and/or osmo-receptors, and/or chemo
receptors to send
satiety signals to the brain. Exemplary embodiments of the device such as
these are described
further below, and depicted in Figure 29. The role of embodiments of the
intestinal insert and
methods associated with its use are more generally considered in the context
of Figure 13, as
detailed in the following section.
Further Exemplary Embodiments of the Invention
[00152] Figure 13 is a schematic flow diagram of the various ways in which
embodiments
of the device engage the physiology of the host subject, and intervene in ways
to generate a
sense of satiety that ultimately reduces food intake. Embodiments of the
inventive device
intervene in the physiology of digestion and satiation by two broad
approaches, each of which
mimic or exploit the natural mechanisms of satiety. Embodiments may engage the
physiology
of the host subject by (1) their mere physical presence having effects, and/or
(2) they may
intervene more directly or actively by the direct provision of bioactive
agents or direct neural
stimulation. Figure 13 and this associated description are provided as a
simplified theoretical
framework for understanding the invention; it is not intended to be complete
in all detail;
various interactions, dotted lines, and blurring of distinctions are omitted
for sake of
simplicity.
36
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
[00153] First, the mere physical presence of a device has two main effects, it
has
distensional effects and, if it has distinct flow reduction elements, it
impedes the flow of
chyme. Each of these two broad effects is dependent on the dimensions of the
device and its
flow reduction system, if the latter is present. First, then, the presence of
the device distends
the duodenum, and such distension may be neurally-sensed or detected, as for
example, by
stretch-sensitive neurons in the duodenum. Accordingly, any physical
dimension, aspect, or
feature, such as, by way of example, any of length, width, total volume,
overall conformation
or topography, density, weight, or surface properties may affect distension,
or may be
neurally detected in some way. Secondly, with regard to physically impeding
the flow of
chyme, this impeding process may alter the biochemical profile of digesting
chyme, and
chemoreceptors in the duodenum sense that profile as being more fully
digested. It may also
be that there is neural recognition more specifically of longer chyme
residency time, as
information separate from the altered biochemical profile per se; an effect
such as that also
then may be related to neural detection of distension. Neuronal pathways are
indeed
stimulated by distension, and neuroelectric signals and/or neuropeptides and
neurotransmitters
may be released for local or more distant sites of action. Joining neural
feedback are chemical
signals, both from the metabolite profile per se, and by the secretion of
hormones such as
CCK. Neural and chemical responses emanate to the central nervous system and
other organs
which, in sum, indicate that enough has been eaten, and satiation is achieved.
In further
response, the central nervous system supports a cessation of eating and
digestive processes
slow.
[00154] Second, with further reference to Figure 13, embodiments of the device
may
intervene in a more active manner, beyond that which is provoked by mere
physical presence.
Embodiments of the device may assertively provide (1) bioactive agents and/or
(2) provide
electrical stimulation of nerves which then engage the physiology of satiety
and digestion in
the much the same manner, or through the same physiological pathways described
above. In
sum, a variety of effects of the presence of the device in the duodenum result
in biochemical
effects or signals (such as hormonal responses, and/or biochemical profile of
metabolites both
within the intestine and in the blood stream) and neural activity involving
electrical signals,
all of which converge physiologically to result in "satiety", with its
complement of sensed
satiety, sensed or perceived appetite, psychological correlates, and
behavioral and habitual
responses.
[00155] Embodiments of the invention, a small intestinal insert, typically
include an
elongated member including at least one angled portion and at least one flow
reduction
element, for slowing the passage of chyme (or, stated in other terms,
increasing the residency
time of chyme) in the duodenum, although some embodiments of the device do not
37
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
necessarily include a flow reduction element (as illustrated in Figures 26 -
28), and in some
embodiments, the central or elongated member itself may be configured to
reduce flow
(Figure 16, for example). These embodiments typically do have one or two
angled portions
that correspond to angled target portions of the duodenum. The configuration
of the angled
portions of the insert, including the flow reduction elements, is such that
the device resides
stably in the duodenum for a period of time. Embodiments of the insert may
include
adaptations that contribute to the generation of one or more physiological
signals of satiety.
Embodiments of the insert may include otherfeatures, such as the inclusion of
biodegradable
portions, a neurological stimulator, and one or more releasable reservoirs of
bioactive
materials that can be actively released by a bioactive material release
mechanism
1001561 Residency time of embodiments of the insert within the targeted angled
site within
the duodenum will vary according to the configuration of the embodiment and
accOrding to
the particulars of the biodegradable materials that comprise portions of the
device.
Degradation of the device by biological processes is typically what causes
release or
unseating, or disengagement of the device from the target site, and
elimination of the device
through the intestinal tract. It may be understood therefore, that the device
may be configured
initially to sit or be seated in the targeted angled portion of the small
intestine, and then,
following a period of residency and through the effects of biodegradation,
then configured to
be unseated from the target site, and eliminated from the body by way of
defecation.
Biodegradability is feature of some polymers, and may be included in polymeric
portions of
any embodiment described herein and/or as illustrated in Figures 3 ¨ 12, and
16 ¨ 29.
Biodegradation is a feature explicitly depicted in the embodiments shown in
Figures 30 and
31.
[00157] Embodiments of the device elicit physiological signals of satiety
typically through
hormonal or neurological pathways. In some embodiments, the pathways are
stimulated by
the physical presence of the device, including the sum total of a central
member and flow
reduction elements, whose collective dimensions, either length, width, or
total volume, or
surface properties, are such that neuronal elements of the intestine, such as
mechanoreceptors
or stretch receptors, sense the presence of material which is interpreted as
the presence of
partially digested food, and therefore stimulate neuronal messages to the
central nervous
system that are interpreted as food satiation. In other embodiments, the
satiety signal may be
hormonal. Flow reduction elements slow the passage of chyme being processed in
the
duodenum, the biochemical profile of the food breakdown products is altered,
and
chemo receptors in the duodenum respond to the altered biochemical profile in
a manner that
conveys satiety to the central nervous system and other portions of the
digestive system.
38
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
[00158] In still other embodiments, the device includes reservoirs of
bioactive materials that
may be released, either by passive or active mechanisms. In the embodiments,
the satiety
signals are provided directly by the device, not by the endocrine pathways of
the insert's host.
Embodiments of the device may include material reservoirs of any type,
including, for
example, drug coatings that elute passively, or in concert with degradation of
a host coating
material, and some embodiments include reservoirs that are coupled with pumps.
Such pumps
may be mechanical, harnessing for example, biological energy conveyed by
peristalsis, or
electrical energy, or mechanical energy. Some embodiments may include osmotic
pumps,
which do not require input of electrical energy, but instead tap into the
stored energy of
osmotic gradients. Embodiments that are dependent on electrical energy for
release by a pump
typically include an energy storage device, such as a battery or a capacitor.
Some of the
powered embodiments include, as part of a larger system, a remote stimulator
that can control
the action of the pump. In some embodiments, the device may provide direct
neural
stimulation, through electrodes that stimulate local nerves in the duodenum,
which convey a
sensation of satiety to the central nervous system. As with pumps, devices
that include neural
stimulation features, may also include energy storage devices and external
on/off or variable
power control devices that communicate either by direct wired connection or
wirelessly, as
for example through radiofrequency signals.
[00159] Figure 14 provides a view of a portion of the human gastrointestinal
tract that
focuses on the duodenum of the small intestine 10, starting at the antrum-
pyloric juncture 5,
and extending to the entrance of the jejunum 12. Shown are the ampulla of
Vater 13, the site
of the entrance of the hepatopancreatic duct 15, which is formed by the union
of the
pancreatic duct (from the pancreas 9) and the common bile duct from the liver.
The pylorus 8
controls the discharge of contents of the stomach through a sphincter muscle,
the pyloric
valve 11, which allows the pylorus 8 to open wide enough to pass sufficiently-
digested
stomach contents. These gastric contents, after passing into the duodenum 10,
continue into
the jejunum 12 and on into the ileum. The duodenum 10, jejunum 12 and ileum
make up what
is known as the small intestine, however the individual portions of the
alimentary canal are
also commonly referred to as the small intestine. In the context of this
invention the small
intestine can refer to all or part of the duodenum, jejunum and/or ileum.
Figure 15 provides a
flattened planar view of the duodenum 10, including the rugae 19, or inner-
folding lining
portion of the duodenum that form the periphery of the inner space within
which
embodiments of the insert device are positioned. Also depicted are the pylorus
8, the pyloric
valve 11, the duodenal bulb 10A, the vertical duodenum 10B, and the horizontal
duodenum
10C, the ampulla of Vater 13, and the initial portion of the jejunum 12. This
figure provides a
39
CA 02652419 2008-11-14
WO 2007/139920 PCT/US2007/012462
visual background for Figures 16 ¨ 29 that follow, each of which depicts an
embodiment of
the inventive inserted device seated within the targeted site of the duodenum.
[00160] Figure 16A depicts an embodiment of the insert 20 with a central tube
or member
50 in the form of a simple coil like a telephone cord; in this embodiment the
flow reduction
elements 200A may be understood as the individual coiled elements or segments
of the
extended central member 50. Figure 16B shows a detail of a proximal or distal
end portion of
the device that takes the form of a pig-tail 61, as it would emerge from a
device deployment
tube 620. Pig-tail end portion embodiments may provide utility and advantage
during
deployment, as well as a stabilizing and non-irritating end-point when the
insert is seated in
the target site.
[00161] Figure 17A depicts an embodiment of the insert 20 with a central tube
or member
50 in the form of a C-shaped spine, similar to the embodiment depicted in
Figures 3 and 9,
with flow reduction elements 200 in the form of ribs attached to the spine.
Figure 17B shows
the central member 50 and tips of ribs 200 emerging from a deployment tube
620. Some
embodiments of the rib-formed flow reduction elements 200 may be spring-like
and
outwardly biased, the elements reducing flow by their presence, but also, and
advantageously,
stimulating the wall of the duodenum 10, thereby contributing to the
generation of a satiety
=
signal, and further, contributing to stabilization of the insert as it resides
within the targeted
and angled site of the duodenum. In the latter regard, the duodenal bulb
portion 10A bulges
out to a wider radius than the more distal portion of the duodenum, and thus,
an expansive
element in this site provides a particularly effective stabilization site.
[00162] Figure 18 depicts an embodiment of the insert 20 with flow reduction
elements in
the form of a spine with nets. This embodiment may be considered similar to
that shown in
Figure 17, but with a net, filter, or mesh deployed between expandable ribs.
The expandable
ribs provide benefits as described above; the netting provides leverage in
terms of reducing
the flow of chyme being processed through the duodenum 10. By use of mesh of
varying pore
size in the flow reduction elements 200, the device may be provided in
variations that slow
the flow rate to varying degree. Further, the mesh elements may be formed of
materials of
varying properties, such as varied hydrophilicity or hydrophobicity, which may
have effects
on chyme flow rate. Further still, the mesh may provide a site advantageous by
virtue of its
high surface area for the adsorption of bioactive materials, which may then
passively elute or
desorb during the period that the insert 20 resides in the duodenum.
[00163] Figure 19 depicts an embodiment of the insert 20 with flow reduction
elements in
the form of a distally-closed sleeve that is secured by a proximal portion 30A
in the form of a
proximally-open ring-like end cap. The sleeve may have pores of various
dimensions,
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
providing leakage of various degrees, and generally provide the features
ascribed to the nets
of the embodiment depicted in Figure 18.
[00164] Figure 20A depicts an embodiment of the insert 20 with flow reduction
elements
200 in the form of closed mesh baskets along a central member 50 and
contiguous with it, and
further showing pig-tail proximal and distal ends 61. Figure 208 shows the
device emerging
from a deployment tube 620, and expanding on emergence. Embodiments of the
mesh baskets
are flexible and expandable, the mesh may be of varying dimension and
composition.
Typically, the basket portions themselves do not form angles, but the
interconnecting central
portion 50 may form and resiliently hold predetermined angles. The composition
of the
baskets and the central portion may be identical and continuous, or the
compositions may
vary from each other. The interconnecting central portion 50, in particular,
may further have
shape-memory features, as provided either by shape memory alloys or shape
memory
polymers. The polymeric materials comprising the baskets 200 and/or the
central member 50,
whether resiliently shape-holding, or shape-memory capable, may further be
biodegradable.
[00165] Figure 21 depicts an embodiment of the insert 20 with flow reduction
elements 200
in the form of centrally-mounted outwardly-extending baffles, and further
showing pig-tail
proximal and distal ends 61. The baffles are mounted at spatial intervals on a
central member
50, that may include angled portions that are maintained by resiliently-shaped
or memory-
shaped materials, as described in the context of the embodiment shown in
Figure 20.
[00166] Figure 22 depicts an embodiment of the insert 20 with flow reduction
elements 200
in the form of peripherally-mounted inwardly-extending baffles, and further
showing pig-tail
proximal and distal ends 61. The baffles are mounted at spatial intervals on a
hollow central
member 50 that may include angled or curved portions that are maintained by
resiliently-
shaped or memory-shaped materials, as described in the context of the
embodiment shown in
Figures 20 and 21. This embodiment differs generally from many others depicted
by virtue
of it hollow body aspect. The device 20 is open both at its proximal portion
30 and distal
portion 40. This hollow form may confer some particular advantages with regard
to
contributing to the generation of satiety signals. The form of the device is
space-filling within
the duodenum 10, and accordingly may be particularly well suited for the
stimulation of
mechanically-responsive or stretch-responsive nerves within the duodenal wall,
the
stimulation of such nerves generally providing a sensation of satiety. The
device 20 in this
form may also have a force distribution advantage over those with a more
centrally-disposed
central member 50, as seen in most other depicted embodiments. Further, the
configuration of
the surface of the device 20 may vary; it may, for example be solid or housing-
like, ir
substantially housing-like, but with holes or openings (not shown), or it may
be cage-like or
41
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
mesh-like (not shown). Each of these forms may have particular advantages.
Further, with a
more substantial physical presence, a housing-like device provides a physical
platform for
attachment or mounting of drug reservoirs, as well as pumps and circuitry, as
depicted in
another embodiment in Figures 27 and 28. Further, such a housing-like form may
provide an
appropriate mounting for neurally-stimulating electrodes, as depicted in an
embodiment
shown in Figure 29. This particular embodiment may be understood as being
particularly
amenable to capturing kinetic energy from the body in the form of peristaltic
movement with
the baffles, particularly when they have a spring bias. Such capturing of
energy may have
benefits with regard to slowing the flow of chyme, while reducing likelihood
of clogging or
creating pockets of chyme that become isolated and stalled with regard to the
main flow.
[00167] Figure 23 depicts an embodiment of the insert 20 with flow reduction
elements 200
in the form of a foam-like bodies, and further showing pig-tail proximal and
distal ends 61.
This embodiment, in broad aspect, is similar to the embodiment shown in Figure
6. Foam-
like flow reduction elements 200 are compressible and expandable, and are
thereby amenable
to deployment into a target zone through narrow tubes or scopes. The foam-like
materials
may be of a closed-cell form or an open-cell form, or they may be hydrogels.
Such foam-like
materials serve the flow reduction function well because they are bulky,
compliant, and tend
to be space-filling. They also provide a high amount of surface area, which is
advantageous
for adsorption of bioactive agents, as provided by embodiments of the
invention, that may
then be passively desorbed during the residency period within the duodenum.
Such foam or
sponge-like materials may also be wholly or partially biodegradable. The
biodegradability
generally serves the purpose of providing for a limited residency time, as
well as being a way
in which to disperse bioactive agents incorporated or adsorbed onto the
material.
[00168] Figure 24 depicts an embodiment of the insert 20 with a flow reduction
element
200 in the form of a porous nutrient-weeping stent, open both at the proximal
end 30 and the
distal end 40. This embodiment differs generally from that depicted in Figure
19 for having
greater integrity in form; in contrast, the embodiment of Figure 19 is
referred to an open
sleeve, and has no particular structural form. The nutrient-weeping stent
embodiment depicted
here has structural integrity and is more assertively space-filling. The
material typically takes
the form of a mesh, as with other types of intraluminal stents, and may be
formed from
polymeric and/or metal strands. The mesh is typically open enough to provide a
weeping of
liquefied nutrient-rich portions of processing chyme 18, while maintaining the
bulk flow of
chyme within the channel enclosed by the stent.
[001691 Figure 25 depicts an embodiment of the insert 20 with flow reduction
elements 200
in the form of a centrally-mounted fans or blades, mounted at spatial
intervals on a central
42
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
member 50, and further showing pig-tail proximal and distal ends 61. Such fan
blades 200
may be rotatable, with variable degrees of resistance to rotation, including
minimal resistance.
To the extent that such flow reduction embodiments 200 do rotate in accordance
with the flow
of processing chyme, such movement may be beneficial in similar ways to those
described in
the embodiment depicted in Figure 22, in that mixing of chyme may be a useful
process as an
adjunct to reducing flow rate.
[00170] Figure 26 depicts an embodiment of the insert 20 with bioactive
material in
reservoirs or depots, or layered, or adsorbed, or incorporated on the central-
or elongated
member SO, from which the bioactive agent may passively elute into the
duodenum 10. This
embodiment emphasizes the bioactive material or agent delivery aspect of the
devise, and it is
shown without any particularly formed flow reduction element other than its
own physical
dimension, however, it should be understood that a drug eluting central member
50 such as
this may be combined with any of the various flow reduction elements 200
depicted in other
figures.
[00171] Figure 27 depicts an embodiment of the insert 20 with a bioactive
material-loaded
osmotic pump 71, as supported by central or elongate member 50. Osmotic pumps
are well
known in the art, as provided, for example, by Alza Corporation (Cupertino,
CA). The
actuation of an osmotic pump may occur in various ways; for example, water
driven by a
chemical potential crosses an osmotic membrane and enters a salt chamber. The
increased
volume in the salt chamber forces an expansion membrane to deflect into a drug
reservoir. As
the expansion membrane pushes into the reservoir, the drug is dispensed via
one or more
outlet ports.
1001721 Figure 28 depicts an embodiment of the insert 20 with a bioactive
material loaded
reservoir 73 coupled to an electrically driven pump 72, energy storage and
dispensing unit 75,
all such components being supported by central or elongate member 50, as well
as external
control 77. As in Figures 26 and 27, the presently depicted embodiment
emphasizes the
delivery of a bioactive agent or material to the targeted duodenal site. Any
of these
embodiments could include more than one drug. The difference between this
bioactive agent
dispensing embodiment and those of Figures 26 and 27 is that they are
relatively passive,
running on a predetermined time course as determined by the particulars of
bioactive agent
release mechanism. In the embodiment depicted in Figure 28, however, the
release of the
bioactive material is under active control of a pump, and the pump may be
further under the
control of an external control that communicates to the pump either by an
implanted wire, or,
as depicted here, by wireless communication, as for example by radiofrequency
transmission.
A device may include more than one such unit, or a single unit may include
More than one
43
CA 02652419 2008-11-14
WO 2007/139920
PCT/US2007/012462
reservoir and pump, thus more than one bioactive agent may be delivered
independently from
a single device 20.
[00173] Figure 29 depicts an embodiment of the inventive insert 20 with
electrodes 78 for
local neurostimulation, an energy storage and delivery unit 75, such as a
battery or capacitor,
and an external controller 77, all such components being supported either
directly or
indirectly by the central or elongate member 50. This embodiment is
illustrated in such a way
so as to focus on neuronal stimulation, but as explained above in reference to
drug-eluting
devices, the neural stimulatory features of the device may be combined with
any of the
various flow reduction elements 200. In the present embodiment, the electrodes
may be
advantageously positioned at sites where nerves are known to reside.
Electrodes may target
more than one nerve to stimulate, or may target a nerve at more than one
point.
[00174] Figure 30 depicts an embodiment of the central member 50 of an insert
20 that
includes biodegradable elements and shape memory elements. Figure 30A shows
the central
member in an intact configuration with angles a and p apparent; Figure 30B
shows the
central member after biodegradation has begun. At a later point, the central
member will
deteriorate further, lose its integrity and conformation, and the angles a and
p will disappear
as the defining arms of the C-shape device disappear. As such, this represents
an
embodiments of device that is configured first to sit within a targeted site
in the intestine, and
then following residency and a period of biodegradation, the device is
configured to become
unseated from the target site, such unseating due to the loss of the initial
conforming
correspondence to the target site. In the exemplary embodiment depicted here,
an insert
device 20 is formed from a combination of curved shape-memory alloy portions
22 and
relatively straight biodegradable polymer portions. The metal portions 22 and
polymer
portions 24 are joined together segmentally to create an insert of full
dimension and with a
complete an angle or curve as desired, such as angles of radius a and radius
13 depicted in
Figure 9. The metal portions have expanded portions at either end to provide a
more
substantial joining surface, and to protect the host subject from injury or
irritation from
sharps, as the metal elements are loosed upon biological degradation of the
member 20 as a
whole. Metal and polymer portions may be joined together in other ways to
complete an
angled device, as would be familiar to those skilled in the art.
[00175] Figure 31 depicts an embodiment of the central member 50 of an insert
that
includes a biodegradable polymeric material; biodegradable polymers have been
described
extensively, above. Figure 31A shows the central member in an intact
configuration; Figure
31B shows the central member after biodegradation has begun. At a later point,
the central
member 50 will further disintegrate, and angles of radius a and radius (3 will
no longer hold
=
44
CA 02652419 2014-08-15
CA 2652419
their form. In some embodiments, the polymeric material may be capable of
resiliently holding an angle,
such as angles of radius a and radius II depicted in Figure 9, and in other
embodiments, the polymer
may be of a type capable of holding a shape memory, as described above.
Terms anti Conventions
[00176] Unless defined otherwise, all technical terms used herein have the
same meanings as
commonly understood by one of ordinary skill in the art to which this
invention belongs. Various
conventions and terms have also been described in the related U.S.
2006/0178691. Specific methods,
devices, and materials are described in this application, but any methods and
materials similar or
equivalent to those described herein can be used in the practice of the
present invention.
1001771 While embodiments of the inventive device and method have been
described in some detail
and by way of exemplaiy illustrations, such illustration is for purposes of
clarity of understanding only,
and is not intended to be limiting. Various terms have been used in the
description to convey an
understanding of the invention; it will be understood that the meaning of
these various terms extends to
common linguistic or grammatical variations or forms thereof. It will also be
understood that when
terminology referring to devices, equipment, or drugs has used trade names,
brand names, or common
names, that these names are provided as contemporary examples, and the
invention is not limited by
such literal scope. Terminology that is introduced at a later date that may be
reasonably understood as a
derivative of a contemporary term or designating of a subset of objects
embraced by a contemporary
term will be understood as having been described by the now contemporary
terminology. Further, while
some theoretical considerations have been advanced in furtherance of providing
an understanding, for
example, of the various ways that embodiments of the invention engage the
physiology of satiety, the
claims to the invention are not bound by such theory. Moreover, any one or
more features of any
embodiment of the invention can be combined with any one or more other
features of any other
embodiment of the invention, without departing from the scope of the
invention. Still further, it should
be understood that the invention is not limited to the embodiments that have
been set forth for purposes
of exemplification, but is to be defined only by a fair reading of claims that
are appended to the patent
application, including the full range of equivalency to which each element
thereof is entitled.