Note: Descriptions are shown in the official language in which they were submitted.
CA 02654139 2009-02-17
FLUID SAMPLE COLLECTION SYSTEM AND METHOD
FIELD OF THE INVENTION
[0001] The present invention relates generally to sample collection
systems and,
more particularly, to a sample collection system for collecting and
transporting a fluid
sample.
BACKGROUND OF THE INVENTION
[0002] Fluid samples are often collected for diagnostics testing
including, by way
of example, "drugs of abuse" testing, hormone replacement therapy, other
diagnostics
and clinical testing including HIV screening, environmental sampling,
veterinarian
sample collection and other similar applications. The substances collected are
varied
and include, for example, bodily fluids such as saliva, blood, urine, surface
moisture
from any type of surface including exterior body surfaces, or any other type
of fluid that
is typically subjected to diagnostics testing.
[0003] It is a common practice to use a swab of absorbent material as a
collection medium. The swab is often mounted on an end of a collection stick
and is
supplied in a kit that also contains a container or vial into which the swab
and sample
are placed for purposes of transportation or analysis. The vial may contain a
buffer
solution into which the swab with the sample is submerged.
[0004] To collect a sample, the swab is brought into contact with the
fluid sample
to be collected to transfer the sample to the swab. The swab is then placed in
the vial
and submerged in the buffer solution. There are many variations in the
subsequent
sample collecting process depending on how the collected sample is to be
analyzed.
For example, in some applications, the sample is analyzed contemporaneously
with it
CA 02654139 2015-06-30
- 2 -
being collected, and that process may be carried out in the vial or a
contiguous
container. However, in other applications, the vial with the collected sample
is sealed
and sent to a different location for subsequent analysis.
[0005] With known sample collection systems in which a collected sample
is to
be shipped to a location for analysis, it is known to seal the swab and the
collection
stick in the vial for transportation to the location of analysis. At the
location of analysis,
a technician typically uses the collection stick to compress the swab against
the bottom
of the vial to extract or express the sample from the absorbent swab into the
buffer
solution. The swab is then removed from the vial and may be discarded. A
pipette or
other instrument is then introduced into the vial to remove a mixture of the
fluid sample
and buffer solution from the vial for analysis.
[0006] However, it will be appreciated that if the swab is not
sufficiently
compressed in the vial by the technician to express the sample from the swab,
the
integrity of the analysis may be compromised. Moreover, requiring the
technician
performing the analysis to extract the sample from the swab generally
increases the
cost and complexity of the analysis process and also risks contamination of
the sample
while the collection stick is being handled.
[0007] A sample collection system has recently been developed that
overcomes
many of the drawbacks and shortcomings of known prior systems for collecting a
sample from an absorbent. That sample collection system, owned by the common
assignee and fully described in U.S. Patent No. 7,915,032, includes a vial and
a
collection stick. The vial has a wall defining a closed end and an open end
and may be
CA 02654139 2015-06-30
- 3 -
configured to contain an optional buffer solution within the vial. A cap is
provided to
selectively seal with the open end of the vial.
[0008] The collection stick has an elongated handle portion and an
absorbent
head portion detachably connected to the handle portion. The absorbent head
portion
is configured to absorb and retain the fluid sample within the absorbent head
portion
prior to insertion of the collection stick within the vial.
[0009] The vial wall and the absorbent head portion are configured to
permit
insertion of the collection stick into the vial so that the absorbent head
portion is
compressed against the closed end of the vial to express the fluid sample from
the
absorbent head portion and mix with a buffer solution within the vial if the
buffer solution
is present within the vial. The elongated handle portion is detached from the
absorbent
head portion and removed from the vial while the absorbent head portion is
retained in
a generally compressed state within the vial.
[0010] While the sample collection system described in U.S. Patent No.
7,915,032 overcomes many of the drawbacks and shortcomings of known prior
systems
for collecting a sample from an absorbent, there is a continuing need for a
sample
collection system that simplifies the extraction of a fluid sample from an
absorbent for
subsequent analysis of the sample. There is also a continuing need for a
sample
collection system that assures proper mixing of a fluid sample and buffer
solution within
a vial for accurate analysis of the sample, with minimal loss or contamination
of the
sample during the collection process.
CA 02654139 2015-06-30
- 4 -
SUMMARY OF THE INVENTION
[0011] The present invention overcomes the foregoing and other
shortcomings
and drawbacks of sample collection systems heretofore known for use in
collecting and
transporting fluid samples for analysis. While the invention will be described
in
connection with certain embodiments, it will be understood that the invention
is not
limited to these embodiments. On the contrary, the invention includes all
possible
alternatives, modifications and equivalents.
[0012] In accordance with the principles of the present invention, a
sample
collection system or kit is provided including a vial, a plunger and a fluid
sample
collection device having an absorbent for absorbing and retaining a fluid
sample
therein. The vial has a wall defining a closed end and an open end and may be
configured to contain an optional buffer solution within the vial. A cap is
provided to
selectively seal with the open end of the vial.
[0013] According to one aspect of the present invention, the plunger
includes an
elongated handle portion and a plunger head portion that is detachably
connected to
the handle portion. During the sample collection process, the saturated
absorbent is
placed into the vial and may be at least partially submerged in the optional
buffer
solution. The user thereafter advances the plunger toward the closed end of
the vial so
that the absorbent is compressed against the closed end of the vial. The
compression
of the absorbent against the closed end of the vial causes the sample to
express from
the absorbent and mix with the buffer solution. Following compression of the
CA 02654139 2009-02-17
- 5 -
absorbent, the handle portion is removed from the vial while the plunger head
portion is
retained within the vial with the absorbent in a generally compressed state.
[0014] According to another aspect of the present invention, the plunger
includes
a splash guard that is supported by the handle portion of the plunger. The
splash guard
is configured to minimize fluid movement into a space defined between the
splash
guard and the open end of the vial while the absorbent is being compressed
against the
closed end of the vial. The splash guard minimizes splashing of the buffer and
sample
mixture as the absorbent is being compressed to express the sample from the
absorbent. The splash guard reduces the potential loss of the collected sample
through
the open end of the vial and also minimizes potential contamination of the
sample
during the sample collection process.
[0015] The above and other objects and advantages of the present
invention
shall be made apparent from the accompanying drawings and the description
thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
[0016] The accompanying drawings, which are incorporated in and
constitute a
part of this specification, illustrate embodiments of the invention and,
together with a
general description of the invention given above, and the detailed description
of the
embodiments given below, serve to explain the principles of the invention.
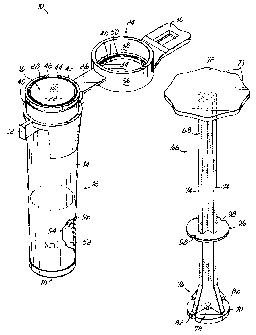
[0017] Fig. 1 is a perspective view of a vial and a plunger that may be
used in a
fluid sample collection system or kit in accordance with one embodiment of the
present
invention;
[0018] Figs. 2A-2C are perspective views of an exemplary fluid sample
collection
device having a handle and an absorbent detachably connected to the handle
that may
CA 02654139 2009-02-17
- 6 -
be used in combination with the vial and plunger shown in Fig. 1 as part of
the sample
collection system or kit;
[0019] Figs. 3A-3F are partial cross-sectional views illustrating an
exemplary
sample collection system or kit and various steps for using the vial and
plunger of Fig. 1
and the fluid collection device of Figs. 2A-2C for collecting a fluid sample
and making it
available for analysis;
[0020] Figs. 4A-4B are partial cross-sectional views illustrating various
alternative
steps for using the sample collection system or kit of the present invention;
[0021] Fig. 5 is a schematic block diagram illustrating various steps for
obtaining,
expressing and extracting a sample using the sample collection system and kit
of the
present invention according to one embodiment; and
[0022] Fig. 6 is a schematic block diagram illustrating various steps for
obtaining,
expressing and extracting a sample using the sample collection system and kit
of the
present invention according to an alternative embodiment.
DETAILED DESCRIPTION OF THE INVENTION
[0023] Referring now to the figures, a fluid sample collection system or
kit 10
according to one embodiment of the present invention is shown for collecting
and
transporting a fluid sample for diagnostics testing. By way of example, the
fluid sample
may comprise saliva, blood, urine, surface moisture from any type of surface
including
an exterior body surface, or any other type of fluid that is typically
subjected to
diagnostics testing. For example, the sample collection kit 10 can be used in
applications such as "drugs of abuse" testing, hormone replacement therapy,
other
CA 02654139 2009-02-17
- 7 -
diagnostics and clinical testing including HIV screening, environmental
sampling,
veterinarian sample collection and other similar applications.
[0024] As shown in Fig. 1, the sample collection kit 10 includes a vial 12
having a
vial wall 14 that defines an open end 16, a closed end 18, and an interior
surface 20
extending between the open and closed ends 16, 18 of the vial to form a vial
cavity 22.
In one embodiment, a cap 24 is connected to the vial 12 by means of a flexible
hinge
strap or tab 26. As will be described in greater detail below, the cap 24 is
configured to
form a generally fluid-tight seal with an annular lip 28 provided at the open
end 16 of
the vial 12 when the cap 24 is closed over the open end 16 during
transportation of the
vial 12 to and from the collection site as will be described in greater detail
below.
[0025] The strap or tab 26 allows the cap 24 to move between an open
position
as shown in Figs. 1 and 3A-3C, wherein the cavity 22 within the vial 12 is
accessible
through the open end 16, and a closed position as shown in Fig. 3E, wherein
the cap
24 forms a generally fluid-tight seal with the annular lip 28. An optional
locking tab 30
(Figs. 1 and 3A-3F) may be provided extending outwardly from the cap 24 to
engage a
locking post 32 provided on the vial 12 when the cap 24 is closed to
releasably lock the
cap 24 in the closed position as shown in Fig. 3E.
[0026] In one embodiment, the cap 24 includes a top wall 34 and a skirt
wall 36
depending from the top wall 34. An inner surface 38 of the skirt wall 36 is
provided with
a contour (see Fig. 1) that is configured to generally form a seal with a
contoured outer
surface 40 (see Fig. 1) provided on the annular lip 28 when the cap 24 is
closed as
shown in Fig. 3E.
CA 02654139 2015-06-30
- 8 -
[0027] The vial 12 may include an annular recess 42 (Fig. 1) provided in
the
annular lip 28 to define an annular inner lip 44 and an annular outer lip 46.
The cap 24
may include a pair annular sealing flanges 48 and 50 that depend from the top
wall 34.
The first sealing flange 48 is configured to be received within the recess 42
formed in
the lip 28 of the vial 12 when the cap 24 is closed over the open end 16 and
generally
form a seal with the inner lip 44. The second sealing flange 50 is configured
to be
received within the vial body adjacent the inner lip 44 and generally form a
seal with an
inner surface of the inner lip 44 when the cap 24 is closed over the open end
16. The
sealing structure is intended to provide leak-proof characteristics at higher
levels of
internal pressure so that liquid samples contained within the vial 12 may be
transported
by air.
[0028] The exemplary embodiment of the sealing structure provided on the
vial
12 is fully described in U.S. Patent No. 8,083,094, owned by the common
assignee and
to which the reader is referred. It will be appreciated that other sealing
structures are
possible as well without.
[0029] The vial 12, cap 24 and strap or tab 26 may be made of plastic or
other
suitable material and may be integrally molded as a single component during a
molding
operation as will be understood by those of ordinary skill in the art. For
example, the
vial 12, cap 24 and strap or tab 26 may be made of polypropylene,
polyethylene,
polystyrene or any other suitable FDA approved material.
[0030] One example of a suitable molding process, which example is not
meant
to be limiting, is a conventional injection molding process that is disclosed
in U.S.
Patent No. RE 37,676. More particularly, as discussed in this patent, molten
plastic
CA 02654139 2015-06-30
- 9 -
may be injected through a sprue gate with about fifteen tons of pressure so as
to form
the product (at the same time, a press may be used to apply about fifteen tons
of
pressure to a mold). The injected product may be allowed to cool for about six
seconds
while the temperature thereof drops from about 550 F to about 100 -120 F. The
specific temperature to which the product is formed and the time, prior to
opening the
mold, may be dependent on numerous factors including the type of plastic, and
size
and type of product but should be cool enough so that the plastic will retain
its shape,
and hot enough so that the plastic is not fully set. Water may be circulated
through
water channels in the mold in order to accelerate the cooling of the product.
[00311 In another embodiment, the product may be ejected from the mold
using
any conventional design known in the art that completely removes the product
from the
mold without incurring damage thereto. For example, a jet of air may drive an
air
poppet through its housing until it contacts the product and pushes it from
the mold.
When the air poppet is projected into the mold cavity, air currents that drive
the air
poppet may further assist in ejecting the product. The air poppet may be
provided with
an angled surface that will contact the product in a flat manner so as not to
mark the
product. In another example, a mechanical pusher may be employed to contact
the
product and push it out of the mold. In a further example, an ejector sleeve
may be
employed to push the product out of the mold. In a still further example, a
robot
mechanism may be employed to remove the product. Additional plastic may then
be
injected into the mold to repeat the cycle.
CA 02654139 2015-06-30
,
- 10 -
[0032] Other patents disclosing a mold/molding process, which examples
are not
meant to be limiting, include U.S. Patent No. 4,783,056, U.S. Patent No.
4,812,116 and
U.S. Patent No. 6,303,064.
[0033] In one embodiment, the vial 12 is partially filled with a buffer
solution 52
up to the level of a fill line (not shown) and the cap 24 is thereafter
engaged over the
vial open end 16 to seal the vial 12 prior to use of the collection system or
kit 10. The
optional fill line (not shown) may be printed, molded or otherwise marked or
formed on
the vial wall 14 to indicate the desired level of buffer solution 52 within
the vial 12.
Numerical indicia (not shown) may be provided on the vial wall 14 to indicate
the
volume of buffer solution 52 contained within the vial 12. The vial 12 may be
made of a
sufficiently translucent material so that the level of buffer solution 52
within the vial 12
may be readily observed through the vial wall 14. Alternatively, the vial 12
may be
generally opaque and/or made of a resin providing ultraviolet (UV) protection
to prevent
change to the fluid sample within the vial 12 due to light exposure.
[0034] In one embodiment as shown in Fig. 2A-2C, a fluid sample
collection
device 54 is provided in the collection kit 10 to permit a user to obtain a
fluid sample.
One suitable fluid collection device 54 for use in the collection kit 10 is
fully described in
U.S. Patent No. 6, 440,087, to which the reader is referred. Briefly, the
sample
collection device 54 includes an absorbent pad 56 that is detachably connected
to a
handle 58. The absorbent pad 56 is comprised of any material that is suitable
for
collection of a fluid sample, including cellulose fiber such as paper, cotton,
nylon or
polyester absorbent pads. A plastic guard or shield 60 is provided adjacent a
bottom or
lower surface of the absorbent pad 56. The plastic guard or shield 60 may be a
CA 02654139 2015-06-30
-11 -
separate component that is mounted in the handle 58 as shown in Figs. 2A-2C
or,
alternatively, the plastic guard or shield 60 may be integrally molded with
the handle 58.
A separator shaft 62 is slidably mounted on the handle 58 for detaching a
forward
portion 64 of the absorbent pad 56 from the handle 58 once the fluid sample
has been
obtained by the user as will be described in greater detail below. It will be
appreciated
that other types of fluid sample collection devices are possible as well
without departing
from the spirit and scope of the present invention. Alternatively, it is
contemplated that
the fluid sample collection device may simply comprise a wad, pad, swab, plug
or other
suitable mass of absorbent material that is inserted into the vial 12 with a
tong or the
like after the sample has been obtained by the user.
[0035] In one exemplary process of obtaining a sample of saliva using the
sample collection device 54, the absorbent pad 56 is placed in a donor's mouth
for a
time sufficient for the absorbent pad 56 to absorb or collect a sample of the
saliva.
Thereafter, as shown in Fig. 2C, the separator shaft 62 is pushed forwardly by
the
user's thumb in the direction of the absorbent pad 56 so that the forward
portion 64 of
the absorbent pad 56 tears away from a rear portion 65 of the absorbent pad 56
that is
captured within the handle 58. The separator shaft 62 is then pulled
rearwardly by the
user's thumb to reposition the separator shaft 62 within the handle 58.
[0036] With the saturated absorbent portion 64 now resting on the plastic
shield
60, the user may insert the plastic shield 60 and absorbent portion 64 into
the open end
16 of the vial 12 and gently shake the handle 58 so that the absorbent portion
64 is
CA 02654139 2009-02-17
- 12 -
shaken off the shield 60 and into the buffer solution 52 as shown in Fig. 3A.
Alternatively, the user may separate the saturated absorbent portion 64 from
the shield
60 prior to inserting the absorbent portion 64 into the vial 12. In this
instance, the shield
60 of the sample collection device 54 is not inserted into the open end 16 of
the vial 12
during the sample collection process. In other embodiments, a saturated wad,
pad,
swab, plug or other suitable mass of absorbent material containing the fluid
sample is
inserted into the vial 12 with a tong or the like after the sample has been
obtained by
the user.
[0037] As shown in Figs. 2B and 2C, the absorbent pad 56 may include a
colored
vegetable dye 63 provided on a portion of the pad 56 that will migrate along
the pad in a
direction toward the rear portion 65 as the fluid sample, such as saliva, is
absorbed by
the pad 56. As shown in Figs. 2A-2C and Fig. 3A, the separator shaft 62
includes an
observation window 67 through which the dye, now dissolved in the saliva, will
be
visible as the dye migrates toward the rear portion 65 of the absorbent pad
56. The dye
63 and observation window 67 are used to confirm that a sufficient quantity of
saliva
has been collected in the pad 56 prior to separation of the detachable forward
portion
64 of the pad from the handle 58.
[0038] In one embodiment, as shown in Figs. 1 and 3B-3E, the sample
collection
kit 10 includes a plunger 66 having an elongated handle portion 68 and a
plunger head
portion 70 detachably connected to the handle portion 68. A handle or thumb
tab 72 is
provided at one end of the handle portion 68 remote from the plunger head
portion 70.
In one embodiment, the handle or thumb tab 72 has a generally disk shape and
extends radially outwardly from the handle portion 68. The handle or thumb tab
72 may
CA 02654139 2015-06-30
- 13 -
have a series of circumferential indentations 73 so that the handle or thumb
tab 72 is
designed to be easily grasped by a user's or lab technician's fingers to
facilitate
insertion of the plunger 66 into the vial 12 and separation of the handle
portion 68 from
the plunger head portion 70 as described in greater detail below. The handle
portion 68
includes a plurality of longitudinally extending ribs 74 that add rigidity to
the plunger 66.
The ribs 74 flare outwardly near the end 76 of the handle portion 68 to
maintain the'
orientation of the plunger head portion 70 while the plunger 66 is inserted
within the vial
cavity 22 toward the closed end 18 of the vial 12 as will be described in
greater detail
below.
[0039] In one embodiment, a frangible member 78, such as a pin, is
connected
between the plunger head portion 70 and the end 76 of the handle portion 68.
The
frangible member 78 is configured to break or separate upon twisting of the
handle
portion 68 relative to the plunger head portion 70 so as to permit detachment
of the
handle portion 68 from the plunger head portion 70 after the plunger 66 has
been
sufficiently inserted into the vial 12 to express the collected fluid sample
from the
absorbent 64 so that the sample will mix with the buffer solution 52 as will
be described
in detail below. The plunger head portion 70 compresses the absorbent 64
against the
closed end 18 of the vial 12 as the plunger 66 is advanced within the vial
cavity 22.
[0040] In one embodiment, the plunger head portion 70 has a generally
disk
shape as shown in Fig. 1 and remains in the vial 12 following detachment and
removal
of the handle portion 68 as shown in Fig. 3E. It will be appreciated that
other shapes of
the plunger head portion 70 suitable for compressing the absorbent 64 are
possible as
well. The handle portion 68, plunger head portion 70 and frangible member 78
may be
CA 02654139 2015-06-30
- 14 -
made of plastic or other suitable material and integrally molded as a single
component
during a molding operation as will be understood by those of ordinary skill in
the art.
For example, the plunger 66 may be made of polypropylene, polyethylene,
polystyrene
or any other suitable FDA approved material.
[0041] Alternatively, the handle portion 68 and the plunger head portion
70 may
be manufactured separately and then connected together through a detachable
connection. For example, a mechanical interlock (not shown) may be provided
between the end 76 of the handle portion 68 and the plunger head portion 70 so
that
the handle portion 68 is detachably connected to the plunger head portion 70.
Accordingly, it will be appreciated that other methods of detachably
connecting the
handle portion 68 to the plunger head portion 70 are possible as well without
departing
from the spirit and scope of the present invention.
[0042] The plunger head portion 70 may have a diameter that is generally
the
same as the inner diameter of the vial 12 generally proximate the closed end
18 so that
the plunger head portion 70 frictionally engages the interior surface 20 of
the vial 12
generally proximate the closed end 18 when the plunger 66 has been
sufficiently
inserted into the vial 12 to express the fluid sample from the absorbent
portion 64 as
described in detail below.
[0043] During the sample collection process as shown in Fig. 3B, the
absorbent
portion 64 is at least partially submerged in the buffer solution 52 and may
contact the
closed end 18. As the plunger head portion 70 is advanced toward the closed
end 18
of the vial 12 via the handle portion 68 of the plunger 66, the absorbent
portion 64 is
compressed against the closed end 18 of the vial 12 as shown in Figs. 3C-3D.
The
CA 02654139 2015-06-30
- 15 -
compression of the absorbent portion 64 against the closed end 18 of the vial
12
causes the saliva sample to express from the absorbent portion 64 and mix with
the
buffer solution 52. At the position shown in Fig. 3D, the plunger head portion
70 may
frictionally engage with the interior surface 20 of the vial 12 and the
absorbent portion
64 is in a generally compressed state.
[0044] The plunger head portion 70 may have one or more openings 82 that
allow a mixture 84 of the fluid sample and the buffer solution to migrate from
the bottom
side of the plunger head portion 70 (i.e., the space between the closed end 18
of the
vial 12 and the plunger head portion 70) to the opposite upper side as shown
in Fig. 3D.
For example, as shown Fig. 1, the openings 82 may comprise two peripheral
cutouts or
slots formed in the plunger head portion 70. It will be appreciated that other
configurations of openings 82 are possible as well.
[0045] Following compression of the absorbent portion 64 with the closed
end 18
of the vial 12, the handle portion 68 is then twisted in either direction, as
represented by
arrow 88 in Fig. 3D, to rotate the handle portion 68 about an axial centerline
90. The
plunger head portion 70 is prevented from rotating with the handle portion 68
due to its
frictional engagement with the vial wall 14. The twisting force applied to the
handle
portion 68 causes the frangible member 78 to break and thus separate the
handle
portion 68 from the plunger head portion 70.
[0046] As shown in Fig. 3E, the handle portion 68 is then removed from
the vial
12 and may be discarded. The plunger head portion 70 is retained within the
vial 12
CA 02654139 2009-02-17
- 16 -
with the absorbent portion 64 in a generally compressed state. The mixture 84
of the
fluid sample and the buffer solution is present in the vial 12 above the
plunger head
portion 70.
[0047] Thereafter, as shown in Fig. 3E, the cap 24 is sealingly engaged
with the
vial 12 and the vial 12 with the mixture 84 of the fluid sample and buffer
solution is then
transported to a location where an analysis of the saliva sample is to be
performed. At
the location of the analysis, as shown in Fig. 3F, the cap 24 is unlocked and
opened,
and a pipette 91 can be inserted into the vial 12 to collect the mixture 84 of
the fluid
sample and buffer solution for analysis.
[0048] It will be appreciated that other types of mechanical
interferences known
to those of ordinary skill in the art are possible as well to retain the
plunger head portion
70 within the vial 12 while permitting detachment of the handle portion 68
without
departing from the spirit and scope of the present invention.
[0049] As shown in Figs. 1 and 3A-3F, a projection 92 may be provided
spaced
from the closed end 18 of the vial 12 and extending inwardly from the interior
surface
20 of the vial wall 14. In one embodiment, the projection 92 comprises a
continuous
annular rib having a contour that defines an inner diameter at the rib that is
slightly
smaller than the diameter of the plunger head portion 70. As the plunger head
portion
70 is urged toward the closed end 18 of the vial 12 to compress the absorbent
portion
64, the plunger head portion 70 engages and rides over the rib. As this
occurs, an
audible "click" and/or a tactile indication is provided to the user to
indicate that the
plunger 66 has been sufficiently inserted into the vial 12 to express the
fluid sample
from the absorbent portion 64. Following receipt of this audible and/or
tactile indication,
CA 02654139 2009-02-17
- 17 -
the user then twists and removes the handle portion 68 and the plunger head
portion 70
is retained in the vial 12 with the absorbent portion 64 in a generally
compressed state.
It will be appreciated that other configurations of projections capable of
providing an
audible and/or tactile indication to a user are possible as well without
departing from the
spirit and scope of the present invention.
[0050] During the sample collection process, it is desirable to minimize
splashing
of the buffer and sample mixture 84 as the absorbent portion 64 is being
compressed
against the closed end 18 of the vial 12 using the plunger 66. This reduces
the
potential loss of the collected sample through the open end 16 of the vial 12
and also
minimizes potential contamination of the sample during the sample collection
process.
According to one aspect of the present invention as shown in Figs. 1 and 3B-
3E, the
plunger 66 includes a splash guard 96 that is supported by the handle portion
68 at a
position located between the handle or thumb tab 72 and the plunger head
portion 70.
[0051] In one embodiment, the splash guard 96 has a generally disk shape
and
extends adjacent the wall 14 of the vial 12 to minimize fluid movement into a
space
defined between the splash guard 96 and the open end 16 of the vial 12 while
the
absorbent portion 64 is being compressed against the closed end 18 of the vial
12. The
splash guard 96 may have a diameter that is less than an inner diameter of the
vial 12
generally proximate a mid-portion of the vial 12. In this way, the splash
guard 96 does
not frictionally engage the wall 14 of the vial 12 but does stabilize the
plunger 66 as it is
advanced toward the closed end 18 of the vial 12 to minimize wobbling of the
plunger
66. Alternatively, the splash guard 96 may be dimensioned so as to
frictionally engage
the wall 14 of the vial 12 generally proximate the mid-portion of the vial 12.
CA 02654139 2009-02-17
- 18 -
[0052] It is contemplated that the splash guard 96 may be generally solid
or
continuous, such as a general disk shape or, alternatively, the splash guard
96 may be
discontinuous. By way of example, the splash guard 96 may comprise a disk
having
openings therein, a plurality of pie-shaped segments located on the same or
different
planes defining openings therebetween, a mesh-like structure or a spiral
member
without limitation. It is contemplated that the splash guard 96 may comprise
any
structure that is suitably configured to minimize fluid movement into the
space defined
between the splash guard 96 and the open end 16 of the vial 12 while the
absorbent
portion 64 is being compressed against the closed end 18 of the vial 12. The
splash
guard 96 may be integrally formed with the plunger 66 or, alternatively, may
be formed
separately therefrom and thereafter connected to or engaged with the handle
portion 68
of the plunger 66.
[0053] The splash guard 96 may have one or more openings 98 that allow
air
within the vial cavity 22 to migrate from the bottom side of the splash guard
96 (i.e., the
space between the upper level of the buffer solution 52 and the splash guard
96) to the
opposite upper side of the splash guard 96. This minimizes the build-up of air
pressure
below the splash guard 96 as the plunger 66 is advanced toward the closed end
18 of
the vial 12.
[0054] In one exemplary embodiment of the present invention, the vial 12
may
have a length of about 3.2 in. (about 8.1 cm) between the open and closed ends
16, 18
of the vial. The open end 16 of the vial 12 may have an inner diameter of
about 0.5 in.
(about 1.3 cm) and the closed end 18 may have an inner diameter of about 0.48
in.
(about 1.2 cm). The vial 12 may have an inner diameter of about 0.49 in.
(about 1.25
CA 02654139 2015-06-30
- 19 -
cm) generally proximate a mid-portion of the vial 12. The fill line (not
shown) may be
located about 0.7 in. (about 1.7 cm) above the closed end 18 of the vial 12.
The
annular rib 94 may be located about 0.38 in. (about 0.96 cm) above the closed
end 18
of the vial 12 and have an axial height of about 0.02 in. (about 0.005 cm) and
a radially
inward depth of about 0.008 in. (about 0.23 cm) from the interior surface 20
of the vial
wall 14. The annular rib 94 may define an inner diameter of about 0.465 in.
(about 1.18
cm) at the rib.
[0055] The plunger 66 may have length of about 3.2 in. (about 8.1 cm).
The
plunger head portion 70 may have a thickness of about 0.05 in. (about 0.12 cm)
and a
diameter of about 0.48 in. (about 1.2 cm). The frangible pin 80 may have a
length of
about 0.016 in. (about 0.43 cm). The splash guard 96 may have a diameter of
about
0.48 in. (about 1.2 cm) and a thickness of about 0.017 in. (about 0.43 cm.).
The splash
guard 96 may be located about 1.25 in. (about 3.2 cm) above the plunger head
portion
70. It will be appreciated that other dimensions and configurations of the
vial 12 and
plunger 66 are possible as well.
[0056] In one embodiment, the sealed and sterilized vial 12 and its
associated
buffer solution 52, the sterilized fluid sample collection device 54 and the
sterilized
plunger 66 may be packaged together in kit form for distribution and use. In
this
instance, as shown schematically in Fig. 5, the user obtains the sample at a
first
location using the sample collection device 54 as shown at step 100 and places
the
sample (e.g., the absorbent portion 64) into the open end 16 of the vial 12 as
shown at
step 102. Using the plunger 66 provided in the kit, the user compresses the
absorbent
CA 02654139 2009-02-17
- 20 -
portion 64 against the closed end 18 of the vial 12 as described in detail
above to
express the sample from the absorbent portion 64 and mix the sample with the
buffer
solution 52 as shown at step 104. After the plunger 66 has been removed from
the vial
12 by the user, the user then closes and optionally locks the vial 12 as shown
at step
106. At step 108, the closed vial 12, with the expressed sample contained
therein, is
then shipped to a second location, such as a laboratory that is remote from
the first
location of the user, for testing of the sample. During the testing procedure,
the vial 12
is opened at the laboratory as shown at step 110, and the sample is then
extracted
from vial 12 using the pipette 91 or other suitable device as shown at step
112.
[0057] Alternatively, it is contemplated that the sample collection
system or kit
distributed to the first location may include the sealed and sterilized vial
12 and its
associated buffer solution 52 and the sterilized fluid sample collection
device 54, but not
the plunger 66. In this embodiment, as shown in Figs. 4A and 6, the user
obtains the
sample at a first location using the sample collection device 54 as shown at
step 300
and places the sample (e.g., the absorbent portion 64) into the open end 16 of
the vial
12 as shown at step 302. As shown in Figs. 4B and 6, the user then closes and
optionally locks the vial 12 as shown at step 304. At step 306, the closed
vial 12, with
the saturated absorbent portion 64 and buffer solution contained therein, is
then
shipped to a second location, such as a laboratory that is remote from the
first location
of the user, for testing of the sample. During the testing procedure, the vial
12 is
opened at the laboratory as shown at step 308, and the laboratory technician
then uses
the plunger 66 to compress the absorbent portion 64 against the closed end 18
of the
vial 12 as described in detail above to express the sample from the absorbent
portion
1
CA 02654139 2009-02-17
,
-21-
64 and mix the sample with the buffer solution 52 as shown at step 310. The
sample is
then extracted from vial 12 using a pipette 91 or other suitable device as
shown at step
312.
[0058] The sample collection kit 10 of the present invention
provides several
advantages over known sample collection systems. For example, splash guard 96
provided on the plunger 66 reduces the potential loss of the collected sample
through
the open end 16 of the vial 12 and also minimizes potential contamination of
the
sample during the sample collection process. Moreover, since the plunger 66
and the
sample collection device 54 are separate components, it is possible for the
user to
obtain and express the sample at the collection site if the plunger 66 is
provided to the
user in the kit 10. Alternatively, the user may simply obtain the sample using
the vial 12
and sample collection device 54 and return the collected sample to the
collection site
within the sealed and locked vial 12 so that the sample may be expressed using
a
plunger 66 at the location of analysis.
[0059] The sample collection kit 10 may be used for collecting
many different
types of fluid samples and therefore, the absorbent material and the buffer
solution may
vary depending on the needs and requirements of a particular application. For
example, while the absorbent material is shown as being a generally flat pad,
in other
embodiments, it may be hemispherical, straight-sided, fabricated from folded
or layered
material, etc. Thus, the size and/or shape of the absorbent material is
selected based
on the diagnostics testing requirements. For example, for collecting a saliva
sample,
the absorbent material may be a hydrophilic absorbent and selected so as to
absorb
i
CA 02654139 2009-02-17
- 22 -
and release fluids into the buffer solution for testing. In different
embodiments, the
hydrophilic absorbent may
- include extruded plastic material with a hollow cell
configuration, or
- be a material interacting with a buffer solution and/or fluid to
be tested, or
- be selected based in part on the desired interference between
the absorbent and a fluid, or
- be a material for preventing tetrahydrocannabinol (THC) from
binding with the absorbent, or
- include a polyurethane fiber material treated with a surfactant
for providing a wicking action, wherein a degree of wicking is
based on the amount of saliva or other fluid to be collected for
testing purposes, or
- be a material made from at least one of a cotton fiber, a
paperboard, plastic or other absorbent material.
[0060]
The type and/or amount of the buffer solution is selected to interact with a
fluid, for example, saliva, to be tested. Typically, the buffer solution
resists changes in
pH when small quantities of an acid or alkali are added to it. An acid is a
compound
that donates a hydrogen ion to another compound whereas an alkali is a
compound
that accepts a hydrogen ion. Thus, generally, there are two types of buffer
solutions:
an acidic buffer solution having a pH less than 7 and an alkaline buffer
solution with a
pH greater than 7.
CA 02654139 2009-02-17
- 23 -
[0061] In one embodiment, an acidic buffer solution is a solution for
resisting
changes in pH, for example, a solution that is made from a weak acid and a
corresponding salt, for example, sodium salt. In alternative embodiments, an
acidic
solution includes a mixture of ethanoic acid and sodium ethanoate. An acidic
buffer
solution containing equal molar concentrations of both the acid and the salt
has a pH of
approximately 4.76. The buffer solution can be changed by changing the ratio
of acid
to salt, or by choosing a different acid and one of its salts.
[0062] In another embodiment, an alkaline buffer solution is a solution
for
resisting changes in pH, for example, a solution that is made from a weak base
and a
corresponding salt. An alkaline solution may include a mixture of an ammonia
solution
and ammonium chloride solution. In this embodiment, an alkaline buffer
solution having
equal molar proportions of this base and acid has a pH of approximately 9.25.
In acidic
and alkaline solutions, the concentration of the mixture is independent of the
amount of
mixture as long as the concentration is the same.
[0063] In an alternative embodiment of the present invention, a buffer
solution 52
is not contained within the vial 12 when the fluid sample is introduced into
the vial 12.
In this alternative embodiment, the fluid sample collection device 54 may be
used to
collect a fluid sample as described in detail above. The plunger 66 is
inserted through
the open end 16 of the vial 12 and advanced within the vial cavity 22 toward
the closed
end 18. As the user continues to urge the plunger head portion 70 toward the
closed
end 18 via the handle portion 68 of the plunger 66, the absorbent portion 64
is
compressed against the closed end 18 of the vial 12. The compression of the
absorbent portion 64 against the closed end 18 of the vial 12 causes the fluid
sample to
CA 02654139 2015-06-30
-24 -
express from the absorbent portion 64 and flow through the openings 82 into
the vial
cavity 22 where it can be later analyzed at the analysis site. The plunger
head portion
70 is retained within the vial 12 with the absorbent portion 64 in a generally
compressed
state as described in detail above.
[0064] The present invention has been illustrated by description of
various
embodiments and while those embodiments have been described in considerable
detail, variations are possible. Additional advantages and modifications will
readily
appear to those skilled in the art. The invention in its broader aspects is
therefore not
limited to the specific details and illustrative examples shown and described.
The scope
of the claims should not be limited by the preferred embodiments set forth in
the
examples, but should be given the broadest interpretation consistent with the
description as a whole.