Note: Descriptions are shown in the official language in which they were submitted.
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
COMBINATION PREPARATIONS COMPRISING BIFEPRUNOX AND L-DOPA
INDEX page
Title of the invention 1
Index 1
Summary: technical field of the invention 1
Background of the invention 2
Detailed description of the invention 5
Definitions 7
Examples 10
Example 1: Pharmacological methods 10
Example 2: Pharmacological test results 12
Example 3: Pharmaceutical preparations 14
Legends to the Figures 1 - 8 16
References 17
Claims 20
Abstract 21
Figures 1 - 8 21
SUMMARY: TECHNICAL FIELD OF THE INVENTION
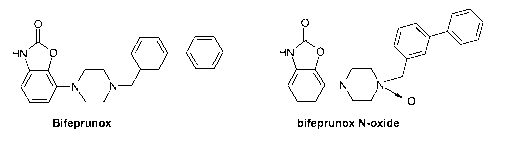
The invention concerns the use of a combination preparation of bifeprunox or
its N-oxide, or
pharmacologically acceptable salts of those compounds:
O O
HN )~ O N NP ~/ _ HN )~
O N N
t ~ ~
- \-/ O
Bifeprunox bifeprunox N-oxide
and L-DOPA, for simultaneous, separate or sequential use in the treatment of
disorders
requiring recovery of dopaminergic function, in particular Parkinson's disease
and restless leg
syndrome.
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
BACKGROUND OF THE INVENTION
Constant tremors in hands and legs, body movements that gradually become
stiffer, slower and
weaker, and mask-like facial expressions, are symptoms that have been observed
throughout
the history of mankind. In 1817 James Parkinson described this cluster of
symptoms as
`paralysis agitans', and shortly thereafter the disease was named after the
physician who first
described it in detail. The pathological cause of Parkinson's disease involves
destruction of
nerve cells in the substantia nigra, the part of the brain involved with
muscle movements. Loss
of around 80% of striatal dopamine in Parkinson's disease results in cardinal
symptoms of
akinesia, rigidity and bradykinesia (Hornykiewicz, 1966). Patients have
problems initiating
movement and exhibit postural instability and loss of coordination.
Current Parkinson's disease pharmacotherapy is based on recovery of
dopaminergic function
(Blandini, 2000; LIed6, 2000). Dopamine does not cross the blood brain barrier
and cannot
therefore be used to treat Parkinson's disease, its immediate precursor, L-
DOPA (the
levorotatory enantiomer of 3,4-dihydroxyphenylalanine, also referred to as
levodopa) is used
instead, because it penetrates the brain where it is decarboxylated to
dopamine. But levodopa is
decarboxylated in peripheral tissues too. Thus only a small portion of
administered levodopa is
transported to the brain. Carbidopa inhibits decarboxylation of peripheral
levodopa but cannot
itself cross the blood brain barrier, and has no effect on the metabolism of
levodopa in the brain.
The combination of carbidopa and levodopa is considered to be the most
effective treatment for
symptoms of Parkinson's disease. Nevertheless, certain limitations become
apparent within two
to five years of initiating therapy. As the disease progresses, the benefit
from each dose
becomes shorter ("the wearing off effect") and some patients fluctuate
unpredictably between
mobility and immobility ("the on-off effect"). "On" periods are usually
associated with high
plasma levodopa concentrations and often include abnormal involuntary
movements, i.e.,
dyskinesias. "Off" periods have been correlated with low plasma levodopa and
bradykinetic
episodes (Jankovic, 1993; Rascol, 2000). This has prompted clinicians to delay
the initiation of
L-DOPA treatment by prior treatment with dopaminergic agonists.
However, the use of full dopamine receptor agonists such as apomorphine,
bromocryptine, lisuride, pergolide, pramipexol or ropinirole, also has its
limitations: They prime
for dyskinesias, induce psychotic-like symptoms including hallucinations,
orthostatic
hypotension, somnolence, and other side-effects (Lozano, 1998; Bennett, 1999).
It has been
suggested that this could be overcome by using partial dopamine D2/3 receptor
agonists (i.e.
compounds that do not maximally stimulate dopamine D2/3 receptors) (Jenner
2002). Such
compounds would hypothetically be capable of stimulating dopamine D2/3
receptors when the
dopaminergic tone is low, while being able to counteract excessive stimulation
of the dopamine
2
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
D2 receptor when the dopaminergic tone is high, thereby resulting in
"stabilisation" of
dopaminergic transmission in the brain (Jenner, 2002).
5-HT,A receptor agonists may ameliorate the induction of dyskinesia since the
5-HT,A receptor
agonist tandospirone reduced dyskinesia in L-DOPA treated Parkinson's disease
patients
(Kannari, 2002) and haloperidol-induced extrapyramidal side effects in
primates (Christoffersen,
1998). More recently it has been suggested that sarizotan, a 5-HT,A receptor
agonist and
dopamine receptor ligand, could ameliorate dyskinetic symptoms (Olanow, 2004;
Bara-Jimenez,
2005; Bibbiani, 2001). The presence of 5-HT,A receptor agonist could be
beneficial to the
therapeutic effects of a partial D2,3 receptor agonist (Johnston, 2003).
Recently, different combination preparations containing L-DOPA and one or more
other
enzyme inhibitors have been introduced. Well known are the combinations L-
DOPA/carbidopa
(e.g. Sinemet ), L-DOPA/benserazide (e.g. Madopar ) and L-
DOPA/carbidopa/entacapone (e.g.
Stalevo , (Jost, 2005)). More recently, catecholamine-O-methyltransferase
(COMT) inhibitors
such as tolcapone and entacapone have been proposed as adjunctive therapy to L-
DOPA.
These compounds extend the plasma half-life of L-DOPA, without significantly
increasing Cmax.
Thus, they decrease the duration of wearing-off but tend to increase the
intensity of peak-dose
side effects including peak dose dyskinesias. Tolcapone appears to induce
significant liver
toxicity in a small percentage of patients. Another strategy aimed at slowing
down the
metabolism of dopamine is the use of monoamine oxidase-B (MAO-B) inhibitors in
combination
with L-DOPA. The administration of MAO inhibitors, however, is associated with
a number of
debilitating side effects that limit their use. These effects include, for
example, nausea,
dizziness, lightheadedness, fainting, abdominal pain, confusion,
hallucinations, dry mouth, vivid
dreams, dyskinesias, and headache. Characteristic for combination preparations
is that they
exist in many different dose combinations, because during the course of the
disease usually
higher doses of L-DOPA are necessary to keep the symptoms under control.
Combination
preparations in the form of tablets containing fixed amounts of drugs are easy
to use, but
simultaneously also offer limited flexibility. An illustration of the fact
that fixed combinations are
not universally useful is e.g. the use of the selective MAO-B inhibitor
selegiline in the treatment
of Parkinson's disease. In the early stage of the disease, selegiline may be
given as
monotherapy: the compound will slow down the metabolism of endogenous dopamine
enough
to keep the symptoms within tolerable limits. In later stages of the disease,
the use of L-DOPA
can become necessary. When the efficacy of L-DOPA starts to wear, usually the
first solution to
that problem is the use of a decarboxylase inhibitor like carbidopa (see
above), and when also
that gets insufficient, co-therapy with selegiline will restore L-DOPA's
efficacy by reducing the
breakdown of the dopamine generated from the L-DOPA. Thus, in practice L-DOPA
and
3
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
selegiline are administered in separate preparations which may be given
simultaneously or
sequentially.
Victims seriously afflicted with Restless Leg Syndrome (RLS; also known as
Ekbom's
syndrome), are virtually unable to remain seated or even to stand still.
Activities that require
maintaining motor rest and limited cognitive stimulation, such as
transportation (car, plane, train,
etc.) or attending longer meetings, lectures, movies or other performances,
become difficult if
not impossible. Tortured by these sensations which become more severe at
night, RLS patients
find sleep to be virtually impossible, adding to the diminishing quality of
their lives. The urge to
move, which increases over periods of rest, can be completely dissipated by
movement, such
as walking. However, once movement ceases, symptoms return with increased
intensity. If an
RLS patient is forced to lie still, symptoms will continue to build like a
loaded spring and,
eventually, the legs will involuntary move, relieving symptoms immediately.
Rhythmic or semi-
rhythmic movements of the legs are observed if the patient attempts to remain
laying down
(Pollmacher, 1993). These movements are referred to as dyskinesias-while-awake
(DWA)
(Hening, 1986) or more commonly, periodic limb movements while awake (PLMW).
Clinically,
RLS is indicated when four diagnostic criteria are met: (1) a sensation of an
urge to move the
limbs (usually the legs); (2) motor restlessness to reduce sensations; (3)
when at rest,
symptoms return or worsen; and (4) marked circadian variation in occurrence or
severity of RLS
symptoms; that is, symptoms worsen in the evening and at night (Allen, 2001).
Current treatments for RLS are varied and plagued with undesirable side
effects.
Therapies have included the administration of dopamine agonists, other
dopaminergic agents,
benzodiazepines, opiates and anti-convulsants. In cases where RLS results from
a secondary
condition, such as pregnancy, end-stage renal disease, erythropoietin
treatment, or iron
deficiency, removing the condition, such as giving birth or treating with
traditional iron
supplementation, can reduce or eliminate symptoms in at least some cases
(Allen, 2001).
However, RLS resulting from non-secondary conditions ("idiopathic" RLS),
presents a greater
treatment challenge. Dopaminergic agents such as levodopa generally provide
effective initial
treatment, but with continued use, tolerance and symptom augmentation occur in
about 80% of
RLS patients (Allen, 1996); this complication is also common for dopamine
agonists (Earley,
1996). The other alternatives, benzodiazepines, opiates and anti-convulsants
are not as
uniformly effective as the dopaminergic agents (Chesson, 1999; Hening, 1999).
Despite changes
in their treatment regimes, 15-20% of patients find that all medications are
inadequate because
of adverse effects and limited treatment benefit
4
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
O O
HN )~ O N NP ~/ - HN )~
O N N
~ ~
b~/ - \-/ O
Bifeprunox bifeprunox N-oxide
DU 127090, 7-[4-([1,1'-biphenyl]-3-ylmethyl)-1-piperazinyl]-2(3H)-
benzoxazolone, now know as
bifeprunox, binds to dopamine D2-like receptors and 5-HT,A receptors; it is a
partial agonist at
dopamine D2,3 receptors and also a partial agonist at serotonin 5-HT,A
receptors (WO 97/36893;
Van Vliet, 2000; Feenstra, 2001, 2002; Hesselink, 2003ab; Mealy, 2004). In WO
2007/023141 it
was disclosed that in vivo the N-oxide of bifeprunox is rapidly converted to
the parent
compound, thus functioning as `prodrug'.
DETAILED DESCRIPTION OF THE INVENTION
The goal of the present invention was to develop a treatment as effective as L-
DOPA, but
without its side effects: In particular without its characteristic "on-off
effect", causing
dyskinesias during "on"-periods, and bradykinetic episodes during "off"-
periods.
Surprisingly, in studies in MPTP-treated marmosets, an animal model with
predictive value for
Parkinson's disease, it was found that combined treatment with L-DOPA and
bifeprunox
reduced peak locomotor activity as observed after L-DOPA alone, such that
hyperactivity was
not observed. The duration of activity ("on"-time) following L-DOPA was
increased by co-
administration of bifeprunox.
The subject matter of the invention are combination preparations of bifeprunox
or its N-oxide, or
pharmacologically acceptable salts, hydrates and solvates thereof, and L-DOPA
and, optionally,
a decarboxylase inhibitor and/or, optionally, a COMT-inhibitor, and/or,
optionally, a MAO-B
inhibitor, for simultaneous, separate or sequential use in therapy of
disorders requiring recovery
of dopaminergic function, in particular Parkinson's disease and `Restless Leg
syndrome'.
The invention relates to the use of bifeprunox or its N-oxide, a true
`prodrug', in cases in which a
L-DOPA induces dyskinesias, or can be anticipated to induce dyskinesias. In
such cases, the
specific pharmacological activities of the compound, viz., partial agonism on
dopamine-D2 and
5
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
dopamine-D3 receptors, as well as full agonism on serotonin 5-HT,A receptors,
result in a
blockade of the dyskinesias without reducing the therapeutic effect of L-DOPA.
The present invention relates to pharmaceutical formulations, comprising:
(i) bifeprunox, its N-oxide, or pharmacologically acceptable salts, hydrates
and solvates
thereof, and:
(ii) L-DOPA, in admixture with a pharmaceutically acceptable adjuvant, diluent
or carrier.
A further aspect of the present invention relates to kits of parts comprising:
(i) a vessel containing bifeprunox, its N-oxide, or pharmacologically
acceptable salts,
hydrates, and solvates thereof, optionally in admixture with a
pharmaceutically
acceptable adjuvant, diluent or carrier, and:
(ii) a vessel containing L-DOPA, optionally in admixture with a
pharmaceutically acceptable
adjuvant, diluent or carrier, and:
(iii) instructions for the sequential, separate or simultaneous administration
of bifeprunox
and the L-DOPA, to a patient in need thereof.
According to a further aspect of the invention, there is provided a method of
making a kit of
parts as defined herein, which method comprises bringing a component (i), as
defined above,
into association with a component (ii), as defined above, thus rendering the
two components
suitable for administration in conjunction with each other. Bringing the two
components into
association with each other, includes that components (i) and (ii) may be:
(i) provided as separate formulations (i.e. independently of one another),
which are
subsequently brought together for use in conjunction with each other in
combination
therapy; or
(ii) packaged and presented together as separate components of a "combination
pack" for
use in conjunction with each other in combination therapy.
Yet another aspect of the invention relates to methods for treatment of a
patient suffering from,
or susceptible to, a condition in which recovery of dopaminergic function is
required or desired,
which method comprises administering to the patient a therapeutically
effective total amount of:
(i) bifeprunox, its N-oxide, or pharmacologically acceptable salts, hydrates
and solvates
6
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
thereof, optionally in admixture with a pharmaceutically acceptable adjuvant,
diluent or
carrier; in conjunction with:
(ii) L-DOPA, optionally in admixture with a pharmaceutically acceptable
adjuvant, diluent or
carrier.
Still another aspect of the invention relates to the use of pharmaceutical
formulations,
comprising:
(i) bifeprunox, its N-oxide, or pharmacologically acceptable salts, hydrates
and solvates
thereof, and:
(ii) L-DOPA, in admixture with a pharmaceutically acceptable adjuvant, diluent
or carrier, in
the manufacture of a medicament for the treatment of a condition in which
recovery of
dopaminergic function is required or desired.
DEFINITIONS
Examples of decarboxylase inbitors are: carbidopa and benserazide. Examples of
catechol-
amine-O-methyl transferase (COMT) inhibitors are: entacapone, nitecapone and
tolcapone,
and monoamine oxidase-B (MAO-B) inhibitors include: deprenyl, (-)-deprenyl
(selegiline),
desmethyldeprenyl, N-propargyl-l-(R)-aminoindan (rasagaline), phenelzine
(nardil), tranyl-
cypromine (parnate), CGP3466, furazolidone, isocarboxazid, pargyline,
methyclothiazide and
procarbazine
To provide a more concise description, some of the quantitative expressions
given
herein are not qualified with the term "about". It is understood that whether
the term "about" is
used explicitly or not, every quantity given herein is meant to refer to the
actual given value, and
it is also meant to refer to the approximation to such given value that would
reasonably be
inferred based on the ordinary skill in the art, including approximations due
to the experimental
and/or measurement conditions for such given value.
Throughout the description and the claims of this specification the word
"comprise" and
variations of the word, such as "comprising" and "comprises" is not intended
to exclude other
additives, components, integers or steps.
The term "composition" as used herein encompasses a product comprising
specified
ingredients in predetermined amounts or proportions, as well as any product
that results,
directly or indirectly, from combining specified ingredients in specified
amounts. In relation to
pharmaceutical compositions, this term encompasses a product comprising one or
more active
7
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
ingredients, and an optional carrier comprising inert ingredients, as well as
any product that
results, directly or indirectly, from combination, complexation or aggregation
of any two or more
of the ingredients, or from dissociation of one or more of the ingredients, or
from other types of
reactions or interactions of one or more of the ingredients. In general,
pharmaceutical
compositions are prepared by uniformly and intimately bringing the active
ingredient into
association with a liquid carrier or a finely divided solid carrier or both,
and then, if necessary,
shaping the product into the desired formulation. The pharmaceutical
composition includes
enough of the active object compound to produce the desired effect upon the
progress or
condition of diseases. Accordingly, the pharmaceutical compositions of the
present invention
encompass any composition made by admixing a compound of the present invention
and a
pharmaceutically acceptable carrier. By "pharmaceutically acceptable" it is
meant the carrier,
diluent or excipient must be compatible with the other ingredients of the
formulation and not
deleterious to the recipient thereof.
Within the context of this application, the term `combination preparation'
comprises
both true combinations, meaning bifeprunox and other medicaments physically
combined in one
preparation such as a tablet or injection fluid, as well as `kit-of-parts',
comprising bifeprunox
and L-DOPA in separate dosage forms, together with instructions for use,
optionally with further
means for facilitating compliance with the administration of the component
compounds, e.g.
label or drawings. With true combinations, the pharmacotherapy by definition
is simultaneous.
The contents of `kit-of-parts', can be administered either simultaneously or
at different time
intervals. Therapy being either concomitant or sequential will be dependant on
the
characteristics of the other medicaments used, characteristics like onset and
duration of action,
plasma levels, clearance, etc., as well as on the disease, its stage, and
characteristics of the
individual patient.
The dose of the composition to be administered will depend on the relevant
indication,
the age, weight and sex of the patient and may be determined by a physician.
The dosage will
preferably be in the range of from 0.01 mg/kg to 10 mg/kg. The typical daily
dose of the active
ingredients varies within a wide range and will depend on various factors such
as the relevant
indication, the route of administration, the age, weight and sex of the
patient and may be
determined by a physician. In general, oral and parenteral dosages will be in
the range of 0.1 to
1,000 mg per day of total active ingredients.
The term "therapeutically effective amount" as used herein refers to an amount
of a
therapeutic agent to treat a condition treatable by administrating a
composition of the invention.
That amount is the amount sufficient to exhibit a detectable therapeutic or
ameliorative
8
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
response in a tissue system, animal or human. The effect may include, for
example, treating the
conditions listed herein. The precise effective amount for a subject will
depend upon the
subject's size and health, the nature and extent of the condition being
treated,
recommendations of the treating physician (researcher, veterinarian, medical
doctor or other
clinician), and the therapeutics, or combination of therapeutics, selected for
administration.
Thus, it is not useful to specify an exact effective amount in advance.
The term "pharmaceutically acceptable salt" refers to those salts that are,
within the
scope of sound medical judgment, suitable for use in contact with the tissues
of humans and
lower animals without undue toxicity, irritation, allergic response, and the
like, and are
commensurate with a reasonable benefit/risk ratio. Pharmaceutically acceptable
salts are well-
known in the art. They can be prepared in situ when finally isolating and
purifying the
compounds of the invention, or separately by reacting them with
pharmaceutically acceptable
non-toxic bases or acids, including inorganic or organic bases and inorganic
or organic acids.
Pharmaceutically acceptable salts may be obtained using standard procedures
well known in
the art, for example by mixing a compound of the present invention with a
suitable acid, for
instance an inorganic acid or an organic acid.
"Administration in conjunction with", includes that respective formulations
comprising
bifeprunox and L-DOPA are administered, sequentially, separately and/or
simultaneously, over
the course of treatment of the relevant condition, which condition may be
acute or chronic.
Preferably, the term includes that the two formulations are administered
(optionally repeatedly)
sufficiently closely in time for there to be a beneficial effect for the
patient, that is greater, over
the course of the treatment of the relevant condition, than if either of the
two formulations are
administered (optionally repeatedly) alone, in the absence of the other
formulation, over the
same course of treatment. Determination of whether a combination provides a
greater beneficial
effect in respect of, and over the course of treatment of, a particular
condition, will depend upon
the condition to be treated or prevented, but may be achieved routinely by the
person skilled in
the art. Thus, the term "in conjunction with" includes that one or other of
the two formulations
may be administered (optionally repeatedly) prior to, after, and/or at the
same time as,
administration with the other component. When used in this context, the terms
"administered
simultaneously" and "administered at the same time as" include that individual
doses of
bifeprunox and L-DOPA are administered within 48 hours, e.g. 24 hours, 18
hours, 12 hours, 6
hours, 3 hours, 2 hours, 1 hour or 30 minutes of each other.
The term "treatment" as used herein refers to any treatment of a mammalian,
preferably
human condition or disease, and includes: (1) inhibiting the disease or
condition, i.e., arresting
9
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
its development, (2) relieving the disease or condition, i.e., causing the
condition to regress, or
(3) stopping the symptoms of the disease.
As used herein, the term "medical therapy" intendeds to include prophylactic,
diagnostic and therapeutic regimens carried out in vivo or ex vivo on humans
or other
mammals.
The term "subject" as used herein, refers to an animal, preferably a mammal,
most
preferably a human, who has been the object of treatment, observation or
experiment.
EXAMPLES
Treatment with the neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-
tetrahydropyridine) leads to
depletion of dopamine in the caudate-putamen and `parkinsonian-like' behaviour
in non-human
and human primates (Lange, 1992; Langston, 1984; Langston, 1986).
EXAMPLE 1: Interaction between bifeprunox and L-DOPA
Animals: The study employed common marmosets (Callithrix jacchus) ( n=6, aged
between 3
to 5 years; 5 female and 1 male) previously treated with MPTP (2 mg/kg sc
daily) for 5 days and
which exhibited stable motor deficits. These animals were L-DOPA responsive
and not test drug
naive. They had not been experimentally primed for dyskinesia although some
dyskinesia was
present.
Drug treatment: Bifeprunox mesylate was suspended in 10% sucrose solution and
administered orally by gavage in a volume of 2.0 ml/kg. L-DOPA: L-3,4-
Dihydroxyphenylalanine
(L-dopa) methylester (Sigma Chemical Co, UK) was dissolved in 10% sucrose
solution and
administered orally by gavage in a volume of 2.0 ml/kg. Carbidopa: (Merck,
Sharp and Dohme,
UK) was administered orally as a suspension in 10% sucrose solution in a
volume of 2.0 ml/kg.
Domperidone: (2.0 mg/kg po, Sigma UK) was dissolved in 10% sucrose and
administered 60
min prior to bifeprunox in a volume of 2.0 ml/kg. Bifeprunox (4 or 8 mg/kg po
free base) or its
vehicle (10% sucrose) was administered alone and in combination with L-DOPA
(7.5 mg/kg po
free base, given in combination with carbidopa, 12.5 mg/kg po) to MPTP-treated
marmosets
(n=6). Animals were treated with bifeprunox or its vehicle 90 min prior to
administration of L-
DOPA or its vehicle administration. Carbidopa was administered 30 minutes
prior to
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
administration of L-DOPA. Domperidone was administered 60 min prior to
bifeprunox
administration.
Behavioral Studies: In all studies, animals were acclimatised to behavioural
study cages for 1
hour prior to treatment. The animals were placed individually into activity
cages (50 x 60 x 70
cm) fitted with a clear perspex door to allow clear visibility for
observation. Each cage was
equipped with 8 horizontally orientated infrared photocell emitters and their
corresponding
detectors arranged so as to permit maximum assessment of movement. Locomotor
activity was
assessed as the number of light beam interruptions caused by movement of the
animals
accumulated in 10-minute intervals for up to 7 hours. The animals were allowed
a 60-minute
acclimatisation period in the activity cages during which baseline activity
was assessed, before
drug administration. 'On' Threshold was defined as 3 times baseline activity
in MPTP-treated
marmosets. Hyperactivity was defined as 3 times normal activity in naive
marmosets. `On' time
was the period of time in minutes that activity was above the `On' Threshold.
Animals were
treated with bifeprunox, vehicle and/or L-DOPA. Following drug treatment,
animals were
assessed for locomotor activity and disability as described below for up to 6
h following drug
administration. The animals used in the study were in the automated activity
cages for no
longer than 8 h on any study day, and were allowed at least 3 days recovery
period between
drug treatments to ensure appropriate animal welfare consideration. The
locomotor activity,
motor disability and presence of dyskinesia were assessed as follows:
Locomotor Activity: In all studies the animals were acclimatised to
behavioural study cages for
1 hour prior to bifeprunox treatment. Animals were then treated with
appropriate drugs.
Following treatment, animals were assessed for antiparkinsonian activity for
up to 6 h using
automated activity cages and by the rating of disability by an observer
blinded to the treatment.
Locomotor 'On-time' was defined as the period of time the animals showed
activity above 3 x
baseline locomotor activity or a locomotor activity of 100 which ever was
greater.
Disability and dyskinesia scores: Disability and dyskinesia was assessed
before, and during
the last 10 minutes in every 30 min period after bifeprunox administration for
up to 6 hours
following drug treatment. The following disability rating scales were used:
Rating of disability: The disability of animals was scored as follows;
alertness (normal 0,
sleepy 2); reaction (normal 0, reduced 1, slow 2, absent 3); checking
movements (present 0,
reduced 1, absent 2); attention and eye movements (normal 0, abnormal 1);
posture (normal 0,
11
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
abnormal trunk +1, abnormal limbs +1, abnormal tail +1, or grossly abnormal
4); balance/co-
ordination (normal 0, impaired 1, unstable 2, spontaneous falls 3);
vocalisation (normal 0,
reduced 1, absent 2); motility (normal 0, bradykinesia/hyperkinesia 1,
akinesia/hyperkinesia 2).
Sedation was noted if present. Disability 'On-time' was defined as the period
of time the animals
showed a disability score of 6 or less ('On threshold').
Data and Statistical Analysis: Data are presented as median locomotor activity
(counts/30
min) or disability score (score over 10 min/30 min) over the 7 hour period of
the experiment, or
as total locomotor activity (counts/420 min) or disability (total score over
420min). Data were
analysed by Kruskall Wallis ANOVA and post-hoc Man-Whitney test where
appropriate.
Analyses were corrected for multiple comparisons using the formula:
Acceptable probability =a/number of comparisons where a=5%
A separate group of animals from those used in the study were treated with
vehicle and were
used for statistical comparison.
EXAMPLE 2: Effects of bifeprunox on L-DOPA induced reversal of motor
disabilities
Locomotor Activity: L-DOPA (7.5 mg/kg po) plus carbidopa (12.5 mg/kg po 30 min
prior to L-
DOPA) increased locomotor activity compared to vehicle administration (Fig 1,
2, 3 and 4)
immediately after administration. Peak activity (median 1627, range 832-2289
counts per 30
min) was reached between 60 and 180 min after administration of L-DOPA. The
median
duration of activity (On-Time) was 180 min/420 min (Fig 4). Total locomotor
activity and
locomotor 'on-time' was greater than vehicle-treated animals (p<0.05, Mann
Whitney).
Bifeprunox (4 mg/kg po) increased locomotor activity compared to vehicle
administration (Fig 1
and 3) immediately after administration. Peak activity (median 685, range 512-
1967 counts per
min) was reached between 60 and 180 min after administration. The median
duration of
activity (On-Time) was 240min/420 min (Fig 4). Total locomotor activity and
locomotor 'On-
30 Time' were greater than in vehicle-treated animals (p<0.05, Mann Whitney).
Bifeprunox (8 mg/kg po) increased locomotor activity compared to vehicle
administration (Fig 2
and 3) immediately after administration. Peak activity (median 1110, range 669-
2058 counts
per 30 min) was reached between 60 and 300 min after administration. The
median duration of
activity (On-Time) was 345min/420 min was greater than vehicle treatment (Fig
4). Total
locomotor activity was greater than vehicle-treated animals (p<0.05, Mann
Whitney).
12
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
Pretreatment with bifeprunox (4 mg/kg po), 90 min prior to L-DOPA (7.5 mg/kg
po) plus
carbidopa (12.5 mg/kg po, 30 min prior to L-DOPA), increased locomotor
activity compared to
baseline (Fig 2 and 3) immediately after administration. Peak activity (median
1581, range 556-
2232 counts per 30 min) was reached between 60 to 390 min after
administration. The median
duration of activity (On-Time, 390min/420min, Fig 4) was slightly greater than
that seen after L-
DOPA alone and bifeprunox. Locomotor activity tended to increase compared to L-
DOPA alone
and bifeprunox (4 mg/kg po) alone (Figure 1, 2, 3 and 4).
Pretreatment with bifeprunox (8 mg/kg po), 90 min prior to L-DOPA (7.5 mg/kg
po) plus
carbidopa (12.5 mg/kg po 30 min prior to L-DOPA), increased locomotor activity
compared to
baseline (Fig 2 and 3) immediately after administration. Peak activity (median
1211, range 409-
1342 counts per 30 min) was reached between 60 and 360 min after
administration. The
median duration of activity (On-Time, 300min/420 min, Fig 4) was slightly
shorter than
bifeprunox alone. Total locomotor activity and duration of activity (On-time)
was not different
when compared to bifeprunox (8 mg/kg po) alone and L-DOPA (7.5 mg/kg po) plus
carbidopa
(12.5 mg/kg po 30 min prior to L-DOPA) alone (Figure 2, 3 and 4).
Disability: L-DOPA (7.5 mg/kg po) plus carbidopa (12.5 mg/kg po 30 min prior
to L-DOPA)
decreased disability scores compared to vehicle administration (Fig 5,6,7 and
8) immediately
after administration (p<0.05 compared to vehicle treated control, Mann
Whitney). Peak activity
(median score, 3; score range 2-4) was reached between 30 and 300 (median 75)
minutes after
administration of L-DOPA. The disability'On-Time'for L-DOPA alone was
120min/420min.
Bifeprunox (4 mg/kg po) decreased disability scores compared to vehicle
administration (Fig 5
and 7) immediately (30-60min) after administration (p<0.05 compared to vehicle-
treated control,
Mann Whitney). Peak scores (median score 3, score range 2-4) were reached
between 30 and
240 min after administration. Median duration of activity (On-Time) was
210min/420 min (Fig 8).
Bifeprunox (8 mg/kg po) decreased disability scores compared to vehicle
treatment immediately
after administration (Fig 6 and 7) (p<0.05 compared to vehicle treated
control, Mann Whitney).
Total decrease in disability was lower than vehicle-treated animals (Figure
7). Peak scores
(median 4, range 2-5) were reached between 30 and 150 min after
administration. Duration of
activity (On-Time) was 225min/420min was greater than vehicle treatment (Fig
8).
Pretreatment with bifeprunox (4 mg/kg po), 90 min prior to L-DOPA (7.5 mg/kg
po) plus
carbidopa (12.5 mg/kg po 30 min prior to L-DOPA), decreased disability scores
over the period
13
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
of the study. (Fig 5 and 7). Peak scores (median 3, range 2-4) were reached
between 60 and
240 min after administration. Duration of activity (On-Time) was
360min/420min. Duration of
activity tended to increase compared to bifeprunox alone (Fig 8).
Pretreatment with bifeprunox (8 mg/kg po), 90 min prior to L-DOPA (7.5 mg/kg
po) plus
carbidopa (12.5 mg/kg po 30 min prior to L-DOPA), decreased disability scores,
(Figure 6 and
7). Peak scores (median 3, range 2-5) were reached between 60 and 120 min
after
administration. Duration of activity (On-Time) was 165min/420min. Duration of
activity was not
different to either bifeprunox (8 mg/kg po) alone or L-DOPA (7.5 mg/kg po)
plus carbidopa (12.5
mg/kg po 30 min prior to L-DOPA)(Fig 8).
CONCLUSION: L-DOPA (7.5 mg/kg po) increased locomotor activity and decrease
disability
scores in MPTP-treated common marmosets. bifeprunox (4 and 8 mg/kg po) also
increased
locomotor activity and decrease disability scores. When given in combination
with L-DOPA,
bifeprunox (4 or 8 mg/kg po) tended to increase total locomotor activity and
locomotor'On-Time'
compared to L-DOPA alone.
EXAMPLE 3: PHARMACEUTICAL PREPARATIONS
Types of pharmaceutical compositions that may be used include, but are not
limited to, tablets,
chewable tablets, capsules (including microcapsules), solutions, parenteral
solutions, ointments
(creams and gels), suppositories, suspensions, and other types disclosed
herein or apparent to
a person skilled in the art from the specification and general knowledge in
the art. The
compositions are used for oral, intravenous, subcutaneous, tracheal,
bronchial, intranasal,
pulmonary, transdermal, buccal, rectal, parenteral or other ways to
administer. The
pharmaceutical formulation contains at least one preparation of the invention
in admixture with a
pharmaceutically acceptable adjuvant, diluent and/or carrier. The total amount
of active
ingredients suitably is in the range of from about 0.1 %(w/w) to about 95%
(w/w) of the
formulation, suitably from 0.5% to 50% (w/w) and preferably from 1% to 25%
(w/w). The molar
ratio between bifeprunox (or its N-oxide) and L-DOPA may be in the range of
from about 1000:1
to about 1:1000, suitably lies in the range of from 300:1 to 1:300, and
preferably from 50:1 to
1:50.
The preparations of the invention can be brought into forms suitable for
administration by
means of usual processes using auxillary substances such as liquid or solid,
powdered
ingredients, such as the pharmaceutically customary liquid or solid fillers
and extenders,
solvents, emulsifiers, lubricants, flavorings, colorings and/or buffer
substances. Frequently used
14
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
auxillary substances include magnesium carbonate, titanium dioxide, lactose,
saccharose,
sorbitol, mannitol and other sugars or sugar alcohols, talc, lactoprotein,
gelatin, starch,
amylopectin, cellulose and its derivatives, animal and vegetable oils such as
fish liver oil,
sunflower, groundnut or sesame oil, polyethylene glycol and solvents such as,
for example,
sterile water and mono- or polyhydric alcohols such as glycerol, as well as
with disintegrating
agents and lubricating agents such as magnesium stearate, calcium stearate,
sodium stearyl
fumarate and polyethylene glycol waxes. The mixture may then be processed into
granules or
pressed into tablets.
The active ingredients may be separately premixed with the other non-active
ingredients,
before being mixed to form a formulation. The active ingredients may also be
mixed with each
other, before being mixed with the non-active ingredients to form a
formulation.
Soft gelatine capsules may be prepared with capsules containing a mixture of
the active
ingredients of the invention, vegetable oil, fat, or other suitable vehicle
for soft gelatine capsules.
Hard gelatine capsules may contain granules of the active ingredients. Hard
gelatine capsules
may also contain the active ingredients together with solid powdered
ingredients such as
lactose, saccharose, sorbitol, mannitol, potato starch, corn starch,
amylopectin, cellulose
derivatives or gelatine. Dosage units for rectal administration may be
prepared (i) in the form of
suppositories that contain the active substance mixed with a neutral fat base;
(ii) in the form of a
gelatine rectal capsule that contains the active substance in a mixture with a
vegetable oil,
paraffin oil or other suitable vehicle for gelatine rectal capsules; (iii) in
the form of a ready-made
micro enema; or (iv) in the form of a dry micro enema formulation to be
reconstituted in a
suitable solvent just prior to administration.
Liquid preparations may be prepared in the form of syrups, elixirs,
concentrated drops or
suspensions, e.g. solutions or suspensions containing the active ingredients
and the remainder
consisting, for example, of sugar or sugar alcohols and a mixture of ethanol,
water, glycerol,
propylene glycol and polyethylene glycol. If desired, such liquid preparations
may contain
coloring agents, flavoring agents, preservatives, saccharine and carboxymethyl
cellulose or
other thickening agents. Liquid preparations may also be prepared in the form
of a dry powder,
reconstituted with a suitable solvent prior to use. Solutions for parenteral
administration may be
prepared as a solution of a formulation of the invention in a pharmaceutically
acceptable
solvent. These solutions may also contain stabilizing ingredients,
preservatives and/or buffering
ingredients. Solutions for parenteral administration may also be prepared as a
dry preparation,
reconstituted with a suitable solvent before use.
Also provided according to the present invention are formulations and `kits of
parts'
comprising one or more containers filled with one or more of the ingredients
of a pharmaceutical
composition of the invention, for use in medical therapy. Associated with such
container(s) can
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
be various written materials such as instructions for use, or a notice in the
form prescribed by a
governmental agency regulating the manufacture, use or sale of pharmaceuticals
products,
which notice reflects approval by the agency of manufacture, use, or sale for
human or
veterinary administration. The use of formulations of the present invention in
the manufacture of
medicaments for use in treating a condition in which recovery of dopaminergic
function is
required or desired, and methods of medical treatment or comprising the
administration of a
therapeutically effective total amount of at least one preparation of the
invention to a patient
suffering from, or susceptible to, a condition in which recovery of
dopaminergic function is
required or desired.
LEGENDS TO THE FIGURES 1-8
Figure 1: The effect of bifeprunox (4 mg/kg/ p.o.) alone or in combination
with L-DOPA (7.5
mg/kg p.o. on locomotor activity in MPTP treated common marmosets.
Figure 2: The effect of bifeprunox (8 mg/kg/ p.o.) alone or in combination
with L-DOPA (7.5
mg/kg p.o. on locomotor activity in MPTP treated common marmosets.
Figure 3: The effect of bifeprunox (4 a activity) over 7 hours in MPTP treated
common
marmosets. * p,0.05 compared to Vehicle-Vehicle (Mann Whitney).
Figure 4: The effect of bifeprunox (4 or 8 mg/kg p.o.) alone or in combination
with L-DOPA (7.5
mg/kg p.o.) on locomotor 'ON-Time' over 7 hours in MPTP treated common
marmosets. *
p<0.05 compared to Vehicle-Vehicle (Mann Whitney).
Figure 5: The effect of bifeprunox (4 mg/kg/ p.o.) alone or in combination
with L-DOPA (7.5
mg/kg p.o.) on disability score in MPTP treated common marmosets.
Figure 6: The effect of bifeprunox (8 mg/kg/ p.o.) alone or in combination
with L-DOPA (7.5
mg/kg p.o.) on disability score in MPTP treated common marmosets.
Figure 7: The effect of bifeprunox (4 or 8 mg/kg/ p.o.) alone or in
combination with L-DOPA (7.5
mg/kg p.o.) on total disability scores over 7 hours in MPTP treated common
marmosets. *
p<0.05 compared to Vehicle-Vehicle (Mann Whitney).
Figure 8: The effect of bifeprunox (4 or 8 mg/kg/ p.o.) alone or in
combination with L-DOPA (7.5
mg/kg p.o.) on total disability 'ON-Time' over 7 hours in MPTP treated common
marmosets. *
p<0.05 compared to Vehicle-Vehicle (Mann Whitney).
16
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
REFERENCES
Allen and Earley `Augmentation of the restless leg syndrome with
carbidopa/levodopa. Sleep
19: 205-213, 1996.
Allen and Earley, Restless leg syndrome: a review of clinical and
pathophysiologic features. J
Clin Neurophysiol 18: 128-147, 2001
Bara-Jimenez W et al,. 2005. Effects of serotonin 5-HTIA agonist in advanced
Parkinson's
disease. Movement Disorders 20: 932-936;
Bennett and Piercey, Pramipexole - a new dopamine agonist for the treatment of
Parkinson's
disease. J Neurol Sci 163: 25-31, 1999.
Bibbiani et al., 2001. Serotonin 5-HTlA agonist improves motor complications
in rodent and
primate parkinsonian models. Neurology 57: 1829-1834;
Blandini et al., `Functional changes of the basal ganglia circuitry in
Parkinson's disease',. Prog
Neurobiol 62, 63-88, 2000.
Chesson et al (1999) Practice parameters for the treatment of restless leg
syndrome and
periodic limb movement disorder. An American Academy of Sleep Medicine Report.
Standards of Practice Committee of the American Academy of Sleep Medicine.
Sleep 22:
961-968;
Christoffersen and Meltzer, 1998. Reversal of haloperidol-induced
extrapyramidal side effects in
cebus monkeys by 8-hydroxy-2-(di-n-propylamino)tetralin and its enantiomers.
Neuropsychopharmacology 18: 399-402).
Earley and Allen (1996) Pergolide and carbidopa/levodopa treatment of the
restless leg
syndrome and periodic leg movements in sleep in a consecutive series of
patients. Sleep
19: 801-810.
Feenstra et al., 2001, "New 1-aryl-4-(Biarylmethylene)piperazines as potential
atypical anti-
psychotic agents with mixed dopamine D2- receptor- and serotonin 5-HTIA
receptor
affinities", Bioorg. Med. Chem. Lett., 11, 2345-2349.
Feenstra et al., 2002, "New approaches in antipsychotics: design and synthesis
of ligands
binding to dopamine-D2 and serotonin 5-HTIA receptors, clinical candidates DU
127090
and SLV313", Drugs of the Future 2002, 27 (Suppl. A).
Hening et al., (1986) Dyskinesias while awake and periodic movements in sleep
in restless leg
syndrome: treatment with opioids. Neurology 36: 1363-6
Hening et al., (1999) The treatment of restless leg syndrome and periodic limb
movement
disorder. An American Academy of Sleep Medicine Review. Sleep 22: 970-999
17
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
Hesselink et al., 2003, DU 127090, SLV308 and SLV318: characterization of a
chemically
related class of partial dopamine agonists with varying degrees of 5-HTIA
agonism, Eur. J.
Neurol. 10: S1, 2151, 2003a
Hesselink et al., DU 127090, SLV308 and SLV318: characterization of a
chemically related
class of partial dopamine agonists with varying degrees of 5-HTIA agonism,
Eur. J. Neurol.
10: S 1, 2151, 2003b.
Hornykiewicz 0 (1966). Dopamine (3-hydroxytyramine) and brain function.
Pharmacol Reviews,
18, 925-964).
Jankovic, J.,'Natural course and limitations of levodopa therapy'. Neurology
43: S14-S17, 1993.
Jenner P. Pharmacology of dopamine agonists in the treatment of Parkinson's
disease.
Neurology 26: S1-8, 2002.
Johnston, L.C., et al., Association between Intrinsic Activity and the
Antiparkinsonian Effects of
a Novel Dopamine D2 Agonist series in the 1-methyl-4-phenyl-1,2,3,6-
terahydropyridine
Treated Primate Model of Parkinson's Disease. Eur. J. Neurol. 10: S1, 2158,
2003.
Jost, W.H. et al., `Efficacy and tolerability of Stalevo in patients with
Parkinson's disease
experiencing wearing-off, Aktuelle Neurologie, 32, Suppl. 6, S318-S325, 2005.
Kannari et al., Tandospirone citrate, a selective 5-HTIA agonist, alleviates L-
DOPA induced
dyskinesia in patients with Parkinson's disease. No To Shinkei 54: 133-137,
2002.
Lange K.W., et al. (1992). Terguride stimulates locomotor activity at 2 months
but not 10 months
after MPTP-treatment of common marmosets. Eur J of Pharmacology, 212, 247-52;
Langston and Irwin (1986). MPTP: Current concepts and controversies. Clin
Neuropharmacol 9,
485-507.
Langston et al,. (1984). MPTP-induced parkinsonism in humans and non-human
primates-
Clinical and experimental aspects. Acta Neurol Scand 70, 49-54).
Lledo, A., `Dopamine agonists: the treatment for Parkinson's disease in the
XXI century?
Parkinsonism Relat Disord 7, 51-58, 2000.
Lozano et al., New developments in understanding the etiology of Parkinson's
disease and in its
treatment. Curr Opin Neurobiol 8: 783-90, 1998.
Mealy, N.E., et al., 'Bifeprunox mesylate", Drugs of the Future, 29(9), 938,
2004.
Olanow et al, 2004, Multicenter, open label, trial of sarizotan in Parkinson
disease patients with
levodopa-indiced dyskinesias (the SPLENDID Study). Clin Neuropharmacol 27: 58-
62;
Pearce, et al., De Novo Administration of Ropinirole and Bromocriptine Induces
Less Dyskinesia
than L-DOPA in the MPTP-treated Common Marmoset. Mov Dis, Mar, 13(2), 234-41,
1998
18
CA 02654557 2008-12-05
WO 2007/144422 PCT/EP2007/055956
Pollmacher and Schulz, `Periodic leg movements (PLM): their relationship to
sleep stages.
Sleep 16: 572-577, 1993
Rascol et al., A five-year study of the incidence of dyskinesia in patients
with early Parkinson's
disease who were treated with ropinirole or levodopa. N Engl J Med 342: 1484-
1491, 2000
Van Vliet et al., 2000, "DU 127090: a highly potent, atypical dopamine
receptor ligand", Journal
of the European College of Neuropsychopharmacology (ECNP), 10(3), S294, 2000
WO 97/36893
WO 2007/023141
19