Note: Descriptions are shown in the official language in which they were submitted.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-1-
Pyrazolopyrimidones
Field of application of the invention
The invention relates to pyrazolopyrimidone derivatives, which can be used in
the pharmaceutical
industry for the production of pharmaceutical compositions.
Known technical background
The US application US2004/0242596 contains pyrazolo[3,4-d]pyrimidones which
are said to be useful
as anti-cancer agents and to induce mitotic arrest.
Description of the invention
It has now been found, that the pyrazolo[1,5-c]pyrimidone derivatives, which
are described in greater
details below, differ from prior art compounds by unanticipated structural
features and have surprising
and particularly advantageous properties.
Thus, for example, the compounds according to this invention can act as
inhibitors of Eg5 kinesin.
In more detail, it has been unexpectedly found that these derivatives are
potent and highly efficacious
inhibitors of cellular (hyper)proliferation and/or cell-cycle specific
inducers of apoptosis in cancer cells.
Therefore, these compounds can be particular useful for treating
(hyper)proliferative diseases and/or
disorders responsive to the induction of apoptosis, notably cancer. By having
a cell-cycle specific
mode of action, these derivatives should have a higher therapeutic index
compared to standard
chemotherapeutic drugs targeting basic cellular processes like DNA replication
or interfering with basic
cellular molecules like DNA.
Thus, for example, the compounds according to this invention are expected to
be useful in targeted
cancer therapy.
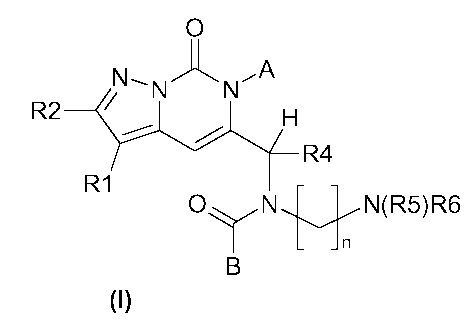
The invention thus relates to compounds of formula I
O
N_- N'J~ N'q
R2 ~ H
R4
R1
O\ /N nN(R5)R6
~"
B
in which
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-2-
R1 is hydrogen or halogen,
R2 is 1-4C-alkyl,
A is Aryl-1-4C-alkyl, in which
Aryl is phenyl, or R3- and R31-substituted phenyl, in which
R3 is 1-4C-alkyl, trifluoromethyl, 1-4C-alkoxy, hydroxyl, halogen, or
completely or predominantly
fluorine-substituted 1-4C-alkoxy,
R31 is hydrogen, halogen, 1-4C-alkyl or 1-4C-alkoxy,
R4 is hydrogen, 1-4C-alkyl, 3-7C-cycloalkyl or 3-7C-cycloalkyl-1-4C-alkyl,
R5 is hydrogen or 1-4C-alkyl,
R6 is hydrogen or 1-4C-alkyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is 1-4C-alkyl, trifluoromethyl, cyano, 1-4C-alkoxy, halogen, carboxyl, 1-4C-
alkylcarbonyl,
methylenedioxy, ethylenedioxy, 1-4C-alkoxy-1-4C-alkyl, hydroxy-1-4C-alkyl, or
completely or
predominantly fluorine-substituted 1-4C-alkoxy,
R71 is hydrogen, halogen, 1-4C-alkyl or 1-4C-alkoxy,
n is 2, 3, 4, 5 or 6,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
As used herein, "alkyl" alone or as part of another group refers to both
branched and straight chain
saturated aliphatic hydrocarbon groups having the specified numbers of carbon
atoms, such as for
example:
1-4C-Alkyl is a straight-chain or branched alkyl radical having 1 to 4 carbon
atoms. Examples are the
butyl, isobutyl, sec-butyl, tert-butyl, propyl, isopropyl, ethyl and methyl
radicals, of which propyl,
isopropyl, ethyl and methyl are more worthy to be mentioned.
Halogen within the meaning of the present invention is iodine or, in
particular, bromine, chlorine or
fluorine.
1-4C-Alkoxy represents radicals which, in addition to the oxygen atom, contain
a straight-chain or
branched alkyl radical having 1 to 4 carbon atoms. Examples which may be
mentioned are the butoxy,
isobutoxy, sec-butoxy, tert-butoxy, propoxy, isopropoxy, ethoxy and methoxy
radicals, of which
propoxy, isopropoxy, and, particularly, ethoxy and methoxy are more worthy to
be mentioned.
The term "cycloalkyl" alone or as part of another group refers to a monocyclic
saturated aliphatic
hydrocarbon group having the specified numbers of carbon atoms, such as for
example:
3-7C-Cycloalkyl stands for cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl
and cycloheptyl, of which
cyclopentyl, cyclobutyl and, in particular, cyclopropyl are more worthy to be
mentioned.
3-7C-Cycloalkyl-1-4C-alkyl stands for one of the abovementioned 1-4C-alkyl
radicals, which is
substituted by one of the abovementioned 3-7C-cycloalkyl radicals. Examples
which may be
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-3-
mentioned are the 3-7C-cycloalkylmethyl radicals, of which cyclopentylmethyl,
cyclobutylmethyl and,
in particular, cyclopropylmethyl are more worthy to be mentioned.
1-4C-Alkoxy-1-4C-alkyl represents one of the abovementioned 1-4C-alkyl
radicals, which is
substituted by one of the abovementioned 1-4C-alkoxy radicals. Examples which
may be mentioned
are the 2-isopropoxyethyl, 2-ethoxyethyl, 2-methoxyethyl and, in particular,
isopropoxymethyl,
ethoxymethyl and, in more particular, methoxymethyl radical.
Hydroxy-1-4C-alkyl represents one of the abovementioned 1-4C-alkyl radicals,
which is substituted by
a hydroxyl radical. Examples which may be mentioned are the 3-hydroxypropoxy,
2-hydroxyethyl and,
in particular, hydroxymethyl radical.
Completely or predominantly fluorine-substituted 1-4C-alkoxy is, for example,
the 2,2,3,3,3-
pentafluoropropoxy, the perfluoroethoxy, the 1,2,2-trifluoroethoxy and in
particular the 1,1,2,2-
tetrafluoroethoxy, the 2,2,2-trifluoroethoxy, the trifluoromethoxy and the
difluoromethoxy radical, of
which the trifluoromethoxy and the difluoromethoxy radicals are preferred.
"Predominantly" in this
connection means that more than half of the hydrogen atoms of the 1-4C-alkoxy
groups are replaced
by fluorine atoms.
1-4C-Alkylcarbonyl is a carbonyl group, to which one of the abovementioned 1-
4C-alkyl radicals is
bonded. An example is the acetyl radical (CH3CO-).
Aryl-1-4C-alkyl stands for one of the abovementioned 1-4C-alkyl radicals,
which is substituted by an
Aryl radical as defined below, such as e.g. the Aryl-methyl or 2-Aryl-ethyl
radical, of which the Aryl-
methyl radical is to be emphasized.
Aryl stands for phenyl, or R3- and R31-substituted phenyl.
Examples of the Aryl-1-4C-alkyl radical include the phenethyl and,
particularly, the benzyl radical,
each of which is optionally substituted by R3 and R31 on the phenyl moiety.
Suitable salts for compounds according to this invention - depending on
substitution - are all acid addi-
tion salts or all salts with bases. Particular mention may be made of the
pharmacologically and/or
pharmaceutically tolerable inorganic and organic acids and bases customarily
used in pharmacy.
Those suitable include, but are not limited to, water-insoluble and,
particularly, water-soluble acid
addition salts with acids such as, for example, hydrochloric acid, hydrobromic
acid, phosphoric acid,
nitric acid, sulphuric acid, acetic acid, citric acid, D-gluconic acid,
benzoic acid, 2-(4-hydroxybenzoyl)-
benzoic acid, butyric acid, sulphosalicylic acid, maleic acid, lauric acid,
malic acid, fumaric acid,
succinic acid, oxalic acid, tartaric acid, embonic acid, stearic acid,
toluenesulphonic acid,
phenylsulphonic acid, methanesulphonic acid or 3-hydroxy-2-naphthoic acid, the
acids being
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-4-
employed in salt preparation - depending on whether a mono- or polybasic acid
is concerned and
depending on which salt is desired - in an equimolar quantitative ratio or one
differing therefrom.
Preferred are the salts selected from hydrochlorides, mesylates, tartrates,
citrates, fumarates or
sulfates.
On the other hand, salts with bases are - depending on substitution - also
suitable. As examples of
salts with bases are mentioned the lithium, sodium, potassium, calcium,
aluminium, magnesium,
titanium, ammonium, meglumine or guanidinium salts, here, too, the bases being
employed in salt
preparation in an equimolar quantitative ratio or one differing therefrom.
Salts which are unsuitable for pharmaceutical uses but which can be employed,
for example, for the
isolation or purification of free compounds of formula I or their
pharmaceutically acceptable salts, are
also included.
Pharmaceutically unacceptable salts, which can be obtained, for example, as
process products during
the preparation of the compounds according to this invention on an industrial
scale, are converted into
pharmaceutically acceptable salts by processes known to the person skilled in
the art.
According to expert's knowledge the compounds of formula I according to this
invention as well as
their salts may contain, e.g. when isolated in crystalline form, varying
amounts of solvents. Included
within the scope of the invention are therefore all solvates and in particular
all hydrates of the
compounds of formula I according to this invention as well as all solvates and
in particular all hydrates
of the salts of the compounds of formula I according to this invention.
In one embodiment of this invention, salts of compounds of formula I include a
salt of a compound of
formula I with hydrochloric acid (hydrochloride).
The substituents R3 and R31 of compounds of formula I can be attached in the
ortho, meta or para
position with respect to the binding position in which the Aryl ring is bonded
to the 1-4C-alkyl group,
whereby emphasis is given to the attachment of R3 in the meta or para
position. In one embodiment
R31 is hydrogen.
The substituents R7 and R71 of compounds of formula I can be attached in the
ortho, meta or para
position with respect to the binding position in which the phenyl ring is
bonded to the carbonyl group,
whereby emphasis is given to the attachment of R7 in the meta or para
position. In one embodiment
R71 is hydrogen.
In the context of this invention, hyperproliferation and analogous terms are
used to describe aberrant /
dysregulated cellular growth, a hallmark of diseases like cancer. This
hyperproliferation might be
caused by single or multiple cellular / molecular alterations in respective
cells and can be, in context
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-5-
of a whole organism, of benign or malignant behaviour. Inhibition of cell
proliferation and analogous
terms is used herein to denote an ability of the compound to retard the growth
of and/or kill a cell
contacted with that compound as compared to cells not contacted with that
compound. Most
preferable this inhibition of cell proliferation is 100%, meaning that
proliferation of all cells is stopped
and/or cells undergo programmed cell death. In some preferred embodiments the
contacted cell is a
neoplastic cell. A neoplastic cell is defined as a cell with aberrant cell
proliferation and/or the potential
to metastasize to different tissues or organs. A benign neoplasia is described
by hyperproliferation of
cells, incapable of forming an aggressive, metastasizing tumor in-vivo. In
contrast, a malignant
neoplasia is described by cells with different cellular and biochemical
abnormalities, e.g. capable of
forming tumor metastasis. The aquired functional abnormalities of malignant
neoplastic cells (also
defined as "hallmarks of cancer") are replicative potential
("hyperproliferation"), self-sufficiency in
growth signals, insensitivity to anti-growth signals, evasion from apoptosis,
sustained angiogenesis
and tissue invasion and metastasis.
Inducer of apoptosis and analogous terms are used herein to identify a
compound which executes
programmed cell death in cells contacted with that compound. Apoptosis is
defined by complex
biochemical events within the contacted cell, such as the activation of
cystein specific proteinases
("caspases") and the fragmentation of chromatin. Induction of apoptosis in
cells contacted with the
compound might not necessarily be coupled with inhibition of cell
proliferation. Preferably, the
inhibition of cell proliferation and/or induction of apoptosis is specific to
cells with aberrant cell growth
(hyperproliferation). Thus, compared to cells with aberrant cell growth,
normal proliferating or arrested
cells are less sensitive or even insensitive to the proliferation inhibiting
or apoptosis inducing activity
of the compound. Finally, cytotoxic is used in a more general sense to
identify compounds which kill
cells by various mechanisms, including the induction of apoptosis / programmed
cell death in a cell
cycle dependent or cell-cycle independent manner.
Cell cycle specific and analogous terms are used herein to identify a compound
as inducing apoptosis
only in continously proliferating cells actively passing a specific phase of
the cell cycle, but not in
resting, non-dividing cells. Continously proliferating cells are typical for
diseases like cancer and
characterized by cells in all phases of the cell division cycle, namely in the
G ("gap") 1, S ("DNA
synthesis"), G2 and M ("mitosis") phase.
Compounds according to this invention worthy to be mentioned are those
compounds of formula I, in
which
R1 is hydrogen or halogen,
R2 is 1-2C-alkyl,
A is Aryl-1-2C-alkyl, in which
Aryl is phenyl, or R3- and R31-substituted phenyl, in which
R3 is 1-2C-alkyl, trifluoromethyl, 1-2C-alkoxy, hydroxyl, halogen, or
completely or predominantly
fluorine-substituted 1-2C-alkoxy,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-6-
R31 is hydrogen, halogen, 1-2C-alkyl or 1-2C-alkoxy,
R4 is hydrogen, 1-4C-alkyl, cyclopropyl or cyclopropyl-1-2C-alkyl,
R5 is hydrogen or 1-3C-alkyl,
R6 is hydrogen or 1-3C-alkyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is 1-2C-alkyl, trifluoromethyl, cyano, 1-2C-alkoxy, halogen, carboxyl, 1-2C-
alkylcarbonyl,
methylenedioxy, ethylenedioxy, 1-2C-alkoxy-1-2C-alkyl, hydroxy-1-2C-alkyl, or
completely or
predominantly fluorine-substituted 1-2C-alkoxy,
R71 is hydrogen, halogen, 1-2C-alkyl or 1-2C-alkoxy,
n is 2, 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention more worthy to be mentioned are those
compounds of formula
I, in which
R1 is hydrogen or halogen,
R2 is methyl or ethyl,
A is Arylmethyl, in which
Aryl is phenyl, or R3- and R31-substituted phenyl, in which
R3 is methyl, ethyl, trifluoromethyl, methoxy, ethoxy, hydroxyl, halogen,
difluoromethoxy or
trifluoromethoxy,
R31 is hydrogen, halogen, methyl, ethyl, methoxy or ethoxy,
R4 is methyl, ethyl, propyl, isopropyl, sec-butyl, cyclopropyl or
cyclopropylmethyl,
R5 is hydrogen, methyl, ethyl, propyl or isopropyl,
R6 is hydrogen, methyl, ethyl, propyl or isopropyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is methyl, ethyl, trifluoromethyl, cyano, methoxy, ethoxy, halogen,
carboxyl, acetyl,
methylenedioxy, ethylenedioxy, methoxymethyl, hydroxymethyl, difluoromethoxy
or
trifl uoromethoxy,
R71 is hydrogen, halogen, methyl, ethyl, methoxy or ethoxy,
n is 2, 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention in particular worthy to be mentioned are
those compounds of
formula I, in which
R1 is hydrogen, fluorine, chlorine or bromine,
R2 is methyl or ethyl,
A is Arylmethyl, in which
Aryl is phenyl, or R3- and R31-substituted phenyl, in which
R3 is methyl, ethyl, trifluoromethyl, methoxy, ethoxy, hydroxyl or halogen,
R31 is hydrogen, halogen, methyl or methoxy,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-7-
R4 is ethyl, propyl, isopropyl, sec-butyl, cyclopropyl or cyclopropylmethyl,
R5 is hydrogen, methyl, ethyl, propyl or isopropyl,
R6 is hydrogen, methyl, ethyl, propyl or isopropyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is methyl, ethyl, trifluoromethyl, cyano, methoxy, ethoxy, halogen,
carboxyl, methylenedioxy,
ethylenedioxy, methoxymethyl or hydroxymethyl,
R71 is hydrogen, halogen, methyl, ethyl or methoxy,
n is 2, 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention in more particular worthy to be
mentioned are those
compounds of formula I, in which
R1 is hydrogen, chlorine or bromine,
R2 is methyl or ethyl,
A is benzyl, halobenzyl (e.g. fluorobenzyl, chlorobenzyl or bromobenzyl),
methylbenzyl,
methylhalobenzyl, methoxybenzyl, hydroxybenzyl, dihalobenzyl (e.g.
dichlorobenzyl), or
dimethoxybenzyl,
R4 is ethyl, propyl, isopropyl, sec-butyl, cyclopropyl or cyclopropylmethyl,
R5 is hydrogen, methyl, ethyl, propyl or isopropyl,
R6 is hydrogen, methyl, ethyl, propyl or isopropyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is methyl, ethyl, trifluoromethyl, cyano, methoxy, ethoxy, halogen,
carboxyl, methylenedioxy,
ethylenedioxy, methoxymethyl or hydroxymethyl,
R71 is hydrogen, halogen, methyl or ethyl,
such as, for example,
B is phenyl, methylphenyl, ethylphenyl, halophenyl, methylhalophenyl,
(hydroxymethyl)phenyl,
methoxyphenyl, ethoxyphenyl, trifluoromethylphenyl,
halo(trifluoromethyl)phenyl, dihalophenyl,
methylenedioxyphenyl, ethylenedioxyphenyl, (methoxymethyl)phenyl,
methoxychlorophenyl,
carboxyphenyl or cyanophenyl,
n is 2, 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention in further more particular worthy to be
mentioned are those
compounds of formula I, in which
R1 is hydrogen, chlorine or bromine,
R2 is methyl or ethyl,
A is benzyl, halobenzyl (e.g. fluorobenzyl, chlorobenzyl or bromobenzyl),
methylbenzyl,
methoxybenzyl or hydroxybenzyl,
R4 is ethyl, propyl, isopropyl, sec-butyl or cyclopropyl,
either
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-8-
R5 and R6 are both hydrogen,
or
R5 is methyl, and
R6 is hydrogen,
or
R5 is ethyl, and
R6 is hydrogen,
or
R5 is propyl, and
R6 is hydrogen,
or
R5 is isopropyl, and
R6 is hydrogen,
or
R5 and R6 are both methyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is methyl, ethyl, trifluoromethyl, cyano, methoxy, ethoxy, halogen,
carboxyl, methylenedioxy,
ethylenedioxy, methoxymethyl or hydroxymethyl,
R71 is hydrogen, halogen, methyl or ethyl,
such as, for example,
B is phenyl, methylphenyl, ethylphenyl, halophenyl, dihalophenyl,
methylhalophenyl,
(hydroxymethyl)phenyl, halo(trifluoromethyl)phenyl, trifluoromethylphenyl,
methoxyphenyl,
methylenedioxyphenyl or cyanophenyl,
such as, for more detailed example,
B is phenyl, 4-methylphenyl, 3-methylphenyl, 4-chlorophenyl, 4-bromophenyl, 4-
fluorophenyl, 3-
fluoro-4-methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl, 4-
trifluoromethylphenyl, 3,4-
dichlorophenyl or 2,3-dichlorophenyl,
n is 2, 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention to be emphasized are those compounds of
formula I, in which
R1 is hydrogen, chlorine or bromine,
particularly
R1 is chlorine or bromine,
R2 is methyl,
A is benzyl, fluorobenzyl, chlorobenzyl, bromobenzyl, methylbenzyl,
methoxybenzyl or
hyd roxybenzyl,
in particular
A is benzyl or fluorobenzyl (e.g. 3-fluorobenzyl or 4-fluorobenzyl),
R4 is ethyl, propyl, isopropyl, sec-butyl or cyclopropyl,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-9-
either
R5 and R6 are both hydrogen,
or
R5 is methyl, and
R6 is hydrogen,
or
R5 is ethyl, and
R6 is hydrogen,
or
R5 and R6 are both methyl,
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is methyl, ethyl, trifluoromethyl, fluorine, chlorine or bromine,
R71 is hydrogen, methyl, fluorine, chlorine or bromine,
such as, for example,
B is phenyl, methylphenyl, trifluoromethylphenyl, methoxyphenyl, halophenyl,
dihalophenyl or
methylhalophenyl,
such as, for more detailed example,
B is phenyl, methylphenyl, trifluoromethylphenyl, methoxyphenyl, fluorophenyl,
chlorophenyl,
bromophenyl, dichlorophenyl, difluorophenyl, methylfluorophenyl,
methylchlorophenyl or
methylbromophenyl,
such as, for yet more detailed example,
B is phenyl, 4-methylphenyl, 3-methylphenyl, 4-chlorophenyl, 4-bromophenyl, 4-
fluorophenyl, 3-
fluoro-4-methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl or 3,4-
dichlorophenyl,
n is 2 or 3,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention to be more emphasized are those
compounds of formula I, in
which
R1 is chlorine or bromine,
R2 is methyl,
A is benzyl or fluorobenzyl (e.g. 3-fluorobenzyl or 4-fluorobenzyl),
R4 is ethyl, propyl, isopropyl, sec-butyl or cyclopropyl,
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl,
B is phenyl, methylphenyl, trifluoromethylphenyl, methoxyphenyl, halophenyl,
dihalophenyl or
methylhalophenyl,
such as, for example,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-10-
B is methylphenyl, trifluoromethylphenyl, methoxyphenyl, fluorophenyl,
chlorophenyl,
bromophenyl, dichlorophenyl, difluorophenyl, methylfluorophenyl,
methylchlorophenyl or
methylbromophenyl,
such as, for more detailed example,
B is 4-methylphenyl, 3-methylphenyl, 4-chlorophenyl, 4-bromophenyl, 4-
fluorophenyl, 3-fluoro-4-
methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl or 3,4-dichlorophenyl,
n is 2 or 3,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention to be in particular emphasized are those
compounds of
formula I, in which
R1 is chlorine or bromine,
R2 is methyl,
A is benzyl, 3-fluorobenzyl or 4-fluorobenzyl,
R4 is ethyl, propyl, isopropyl or cyclopropyl,
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl,
B is methylphenyl, trifluoromethylphenyl, methoxyphenyl, chlorophenyl,
dichlorophenyl,
bromophenyl, fluorophenyl or methylfluorophenyl,
such as, for example,
B is 4-methylphenyl, 3-methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl,
4-chlorophenyl,
4-bromophenyl, 4-fluorophenyl or 3-fluoro-4-methylphenyl,
n is 2 or 3,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
In a preferred embodiment, the invention relates to compounds of formula I
according to the invention,
in which
R1 is hydrogen, chlorine or bromine,
R2 is methyl,
A is benzyl or fluorobenzyl,
R4 is ethyl, propyl, isopropyl, sec-butyl or cyclopropyl,
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl,
B is phenyl, methylphenyl, trifluoromethylphenyl, methoxyphenyl, halophenyl,
dihalophenyl or
methylhalophenyl,
n is 2 or 3,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-11-
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention to be in more particular emphasized are
those compounds of
formula I, in which
R1 is chlorine or bromine,
R2 is methyl,
A is benzyl,
R4 is ethyl or isopropyl,
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl,
B is methylphenyl, chlorophenyl, trifluoromethylphenyl, methoxyphenyl,
bromophenyl or
fluorophenyl,
such as, for example,
B is 4-methylphenyl, 3-methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl,
4-chlorophenyl,
4-bromophenyl or 4-fluorophenyl,
n is 3,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention to be particularly emphasized are those
compounds of formula
I comprising one or more of the following:
R1 is chlorine or bromine;
R2 is methyl;
A is benzyl;
R4 is ethyl or isopropyl;
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl;
B is 4-methylphenyl, 3-methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl,
4-fluorophenyl, 4-
chlorophenyl, 4-bromophenyl or 3-fluoro-4-methylphenyl; and
n is 3;
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
Compounds according to this invention to be in further more particular
emphasized are those
compounds of formula I, in which
R1 is chlorine or bromine,
R2 is methyl,
A is benzyl,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-12-
R4 is isopropyl,
R5 and R6 are both hydrogen,
B is methylphenyl, chlorophenyl, trifluoromethylphenyl, methoxyphenyl,
bromophenyl or
fluorophenyl,
such as, for example,
B is 4-methylphenyl, 4-chlorophenyl or 4-bromophenyl,
n is 3,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
The invention also relates to compounds of formula I according to the
invention comprising one or
more of the following:
R1 is chlorine or bromine;
R2 is methyl;
A is benzyl;
R4 is isopropyl;
R5 and R6 are both hydrogen;
B is 4-methylphenyl, 4-chlorophenyl or 4-bromophenyl; and
n is 3;
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
In a further preferred embodiment, the invention relates to compounds of
formula I in which
R1 is hydrogen, chlorine, bromine or fluorine,
R2 is methyl,
A is benzyl,
R4 is ethyl, isopropyl, sec-butyl, cyclopropyl or cyclobutyl,
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl,
B is 4-methylphenyl, 3-methylphenyl, 4-fluorophenyl, 4-bromophenyl, 4-
chlorophenyl, 4-
methoxyphenyl, 4-trifluoromethylphenyl, 3-fluoro-4-methylphenyl, 2-fluoro-4-
methylphenyl, 3,4-
dichlorophenyl or 2,3-dichlorophenyl,
n is 2, 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
In a further preferred embodiment, the invention relates to compounds of
formula I according to the
invention, in which
R1 is hydrogen, chlorine or bromine,
R2 is methyl,
A is benzyl,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-13-
R4 is ethyl or isopropyl,
either
R5 and R6 are both hydrogen,
or
R5 and R6 are both methyl,
B is 4-methylphenyl, 3-methylphenyl, 4-fluorophenyl, 4-bromophenyl, 4-
chlorophenyl, 4-
methoxyphenyl,
n is 3,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
In a further preferred embodiment, the invention relates to compounds of
formula I in which
R1 is chlorine, bromine or fluorine,
R2 is methyl,
A is benzyl,
R4 is ethyl, isopropyl, sec-butyl or cyclopropyl,
R5 and R6 are both hydrogen,
B is 4-methylphenyl, 3-methylphenyl, 4-fluorophenyl, 4-bromophenyl, 4-
chlorophenyl, 4-
methoxyphenyl, 3-fluoro-4-methylphenyl, 2-fluoro-4-methylphenyl or 3,4-
dichlorophenyl,
n is 3 or 4,
and the salts, stereoisomers and the salts of the stereoisomers of these
compounds.
A special interest in the compounds according to this invention refers to
those compounds of formula I
which are included -within the scope of this invention- by one or, when
possible, by a combination of
more of the following special embodiments:
Another special embodiment (embodiment 1) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is hydrogen.
Another special embodiment (embodiment 2) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is chlorine.
Another special embodiment (embodiment 3) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is bromine.
Another special embodiment (embodiment 3a) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is fluorine.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-14-
Another special embodiment (embodiment 4) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R2 is methyl.
Another special embodiment (embodiment 5) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is chlorine, and R2 is methyl.
Another special embodiment (embodiment 6) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is bromine, and R2 is methyl.
A special embodiment (embodiment 7) of the compounds of formula I according to
this invention
refers to those compounds of formula I, in which
A is benzyl.
Another special embodiment (embodiment 8) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
A is fluorobenzyl, such as e.g. 3-fluorobenzyl or 4-fluorobenzyl.
Another special embodiment (embodiment 9) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
A is benzyl, halobenzyl (such as e.g. chlorobenzyl or bromobenzyl),
methylbenzyl,
hydroxybenzyl or methoxybenzyl.
Another special embodiment (embodiment 10) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R4 is ethyl.
Another special embodiment (embodiment 11) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R4 is isopropyl.
Another special embodiment (embodiment 11 a) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is chlorine, and R2 is methyl, and
R4 is isopropyl.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-15-
Another special embodiment (embodiment 11 b) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
A is benzyl, and
R4 is isopropyl.
Another special embodiment (embodiment 11c) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R1 is chlorine, and R2 is methyl, and
A is benzyl, and
R4 is isopropyl.
Another special embodiment (embodiment 12) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R4 is propyl.
Another special embodiment (embodiment 13) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R4 is cyclopropyl.
Another special embodiment (embodiment 13a) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R4 is cyclobutyl.
Another special embodiment (embodiment 14) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R4 is sec-butyl.
Another special embodiment (embodiment 15) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
n is 2.
Another special embodiment (embodiment 16) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
n is 3.
Another special embodiment (embodiment 17) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 and R6 are the same and selected from hydrogen, methyl and ethyl.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-16-
Another special embodiment (embodiment 18) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 is methyl, ethyl, propyl or isopropyl, and R6 is hydrogen.
Another special embodiment (embodiment 19) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 is methyl or ethyl, and R6 is hydrogen.
Another special embodiment (embodiment 20) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 and R6 are both hydrogen, and n is 2.
Another special embodiment (embodiment 21) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 and R6 are both methyl, and n is 2.
Another special embodiment (embodiment 22) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 and R6 are both hydrogen, and n is 3.
Another special embodiment (embodiment 23) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
R5 and R6 are both methyl, and n is 3.
Another special embodiment (embodiment 24) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
B is phenyl, or R7- and R71-substituted phenyl, in which
R7 is fluorine, chlorine, bromine, methyl, trifluoromethyl or ethyl,
R71 is hydrogen, fluorine, chlorine or methyl.
Another special embodiment (embodiment 25) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
B is phenyl, halophenyl, dihalophenyl, cyanophenyl,
halo(trifluoromethyl)phenyl,
(hydroxymethyl)phenyl, (methoxymethyl)phenyl, methoxyphenyl, ethoxyphenyl,
carboxyphenyl,
methylphenyl, ethylphenyl, methylenedioxyphenyl, ethylenedioxyphenyl,
methoxychlorophenyl,
methylhalophenyl or (trifluoromethyl)phenyl.
Another special embodiment (embodiment 26) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-17-
B is phenyl, methylphenyl, halophenyl (e.g. chlorophenyl or bromophenyl),
dihalophenyl (e.g.
dichlorophenyl), methylhalophenyl (e.g. methylfluorophenyl),
(hydroxymethyl)phenyl,
halo(trifluoromethyl)phenyl, methylenedioxyphenyl, (trifluoromethyl)phenyl,
methoxyphenyl or
cyanophenyl.
Another special embodiment (embodiment 27) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
B is methylphenyl, chlorophenyl, dichlorophenyl, bromophenyl, fluorophenyl,
(trifluoromethyl)phenyl, methoxyphenyl or methylfluorophenyl.
Another special embodiment (embodiment 28) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
B is 4-methylphenyl, 3-methylphenyl, 4-trifluoromethylphenyl, 4-methoxyphenyl,
4-fluorophenyl, 4-
chlorophenyl, 4-bromophenyl or 3-fluoro-4-methylphenyl.
Another special embodiment (embodiment 29) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
B is 4-methylphenyl, 4-chlorophenyl or 4-bromophenyl.
Another special embodiment (embodiment 30) of the compounds of formula I
according to this
invention refers to those compounds of formula I, in which
B is 4-methylphenyl.
Another special embodiment (embodiment 31) of the compounds of formula I
according to this
invention refers to those compounds which are from formula 1* as shown below.
Another special embodiment (embodiment 32) of the compounds of formula I
according to this
invention refers to those compounds which are from formula 1** as shown below.
A respective another special embodiment (embodiment 33 to 92) of the compounds
of formula I
according to this invention refers to those compounds of formula I, in which
R1 is bromine, R2 is methyl, and
A, R4, n, R5 and R6 have the respective meanings indicated in Table 1 given
below.
Table 1:
embodiment A R4 n R5 R6
33 benzyl ethyl 2 H H
34 3-fluorobenzyl ethyl 2 H H
35 4-fluorobenzyl ethyl 2 H H
36 benzyl isopropyl 2 H H
37 3-fluorobenzyl isopropyl 2 H H
38 4-fluorobenzyl isopropyl 2 H H
39 benzyl sec-butyl 2 H H
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-18-
embodiment A R4 n R5 R6
40 3-fluorobenzyl sec-butyl 2 H H
41 4-fluorobenzyl sec-butyl 2 H H
42 benzyl c cloprop I 2 H H
43 3-fluorobenzyl c cloprop I 2 H H
44 4-fluorobenzyl c cloprop I 2 H H
45 benzyl propyl 2 H H
46 3-fluorobenzyl propyl 2 H H
47 4-fluorobenzyl propyl 2 H H
48 benzyl ethyl 3 H H
49 3-fluorobenzyl ethyl 3 H H
50 4-fluorobenzyl ethyl 3 H H
51 benzyl isopropyl 3 H H
52 3-fluorobenzyl isopropyl 3 H H
53 4-fluorobenzyl isopropyl 3 H H
54 benzyl sec-butyl 3 H H
55 3-fluorobenzyl sec-butyl 3 H H
56 4-fluorobenzyl sec-butyl 3 H H
57 benzyl c cloprop I 3 H H
58 3-fluorobenzyl c cloprop I 3 H H
59 4-fluorobenzyl c cloprop I 3 H H
60 benzyl propyl 3 H H
61 3-fluorobenzyl propyl 3 H H
62 4-fluorobenzyl propyl 3 H H
63 benzyl ethyl 2 methyl methyl
64 3-fluorobenzyl ethyl 2 methyl methyl
65 4-fluorobenzyl ethyl 2 methyl methyl
66 benzyl iso ro I 2 methyl methyl
67 3-fluorobenzyl iso ro I 2 methyl methyl
68 4-fluorobenzyl iso ro I 2 methyl methyl
69 benzyl sec-butyl 2 methyl methyl
70 3-fluorobenzyl sec-butyl 2 methyl methyl
71 4-fluorobenzyl sec-butyl 2 methyl methyl
72 benzyl c clo ro I 2 methyl methyl
73 3-fluorobenzyl c clo ro I 2 methyl methyl
74 4-fluorobenzyl c clo ro I 2 methyl methyl
75 benzyl propyl 2 methyl methyl
76 3-fluorobenzyl propyl 2 methyl methyl
77 4-fluorobenzyl propyl 2 methyl methyl
78 benzyl ethyl 3 methyl methyl
79 3-fluorobenzyl ethyl 3 methyl methyl
80 4-fluorobenzyl ethyl 3 methyl methyl
81 benzyl iso ro I 3 methyl methyl
82 3-fluorobenzyl isopropyl 3 methyl methyl
83 4-fluorobenzyl isopropyl 3 methyl methyl
84 benzyl sec-butyl 3 methyl methyl
85 3-fluorobenzyl sec-butyl 3 methyl methyl
86 4-fluorobenzyl sec-butyl 3 methyl methyl
87 benzyl c cloprop I 3 methyl methyl
88 3-fluorobenzyl c cloprop I 3 methyl methyl
89 4-fluorobenzyl c cloprop I 3 methyl methyl
90 benzyl propyl 3 methyl methyl
91 3-fluorobenzyl prop I 3 methyl methyl
92 4-fluorobenzyl propyl 3 methyl methyl
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-19-
A respective another special embodiment (embodiment 93 to 152) of the
compounds of formula I
according to this invention refers to those compounds of formula I, in which
R1 is chlorine, R2 is methyl, and
A, R4, n, R5 and R6 have the respective meanings indicated in Table 2 given
below.
Table 2:
embodiment A R4 n R5 R6
93 benzyl ethyl 2 H H
94 3-fluorobenzyl ethyl 2 H H
95 4-fluorobenzyl ethyl 2 H H
96 benzyl isopropyl 2 H H
97 3-fluorobenzyl isopropyl 2 H H
98 4-fluorobenzyl isopropyl 2 H H
99 benzyl sec-butyl 2 H H
100 3-fluorobenzyl sec-butyl 2 H H
101 4-fluorobenzyl sec-butyl 2 H H
102 benzyl c cloprop I 2 H H
103 3-fluorobenzyl c cloprop I 2 H H
104 4-fluorobenzyl c cloprop I 2 H H
105 benzyl propyl 2 H H
106 3-fluorobenzyl propyl 2 H H
107 4-fluorobenzyl propyl 2 H H
108 benzyl ethyl 3 H H
109 3-fluorobenzyl ethyl 3 H H
110 4-fluorobenzyl ethyl 3 H H
111 benzyl iso ro I 3 H H
112 3-fluorobenzyl iso ro I 3 H H
113 4-fluorobenzyl iso ro I 3 H H
114 benzyl sec-butyl 3 H H
115 3-fluorobenzyl sec-butyl 3 H H
116 4-fluorobenzyl sec-butyl 3 H H
117 benzyl c clo ro I 3 H H
118 3-fluorobenzyl c clo ro I 3 H H
119 4-fluorobenzyl c clo ro I 3 H H
120 benzyl propyl 3 H H
121 3-fluorobenzyl propyl 3 H H
122 4-fluorobenzyl propyl 3 H H
123 benzyl ethyl 2 methyl methyl
124 3-fluorobenzyl ethyl 2 methyl methyl
125 4-fluorobenzyl ethyl 2 methyl methyl
126 benzyl isopropyl 2 methyl methyl
127 3-fluorobenzyl isopropyl 2 methyl methyl
128 4-fluorobenzyl isopropyl 2 methyl methyl
129 benzyl sec-butyl 2 methyl methyl
130 3-fluorobenzyl sec-butyl 2 methyl methyl
131 4-fluorobenzyl sec-butyl 2 methyl methyl
132 benzyl c cloprop I 2 methyl methyl
133 3-fluorobenzyl c cloprop I 2 methyl methyl
134 4-fluorobenzyl c cloprop I 2 methyl methyl
135 benzyl propyl 2 methyl methyl
136 3-fluorobenzyl propyl 2 methyl methyl
137 4-fluorobenzyl propyl 2 methyl methyl
138 benzyl ethyl 3 methyl methyl
139 3-fluorobenzyl ethyl 3 methyl methyl
140 4-fluorobenzyl ethyl 3 methyl methyl
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 20 -
embodiment A R4 n R5 R6
141 benzyl iso ro I 3 meth I methyl
142 3-fluorobenzyl iso ro I 3 meth I methyl
143 4-fluorobenzyl isopropyl 3 meth I methyl
144 benzyl sec-butyl 3 methyl methyl
145 3-fluorobenzyl sec-butyl 3 methyl methyl
146 4-fluorobenzyl sec-butyl 3 methyl methyl
147 benzyl c cloprop I 3 methyl methyl
148 3-fluorobenzyl c cloprop I 3 methyl methyl
149 4-fluorobenzyl c cloprop I 3 methyl methyl
150 benzyl propyl 3 methyl methyl
151 3-fluorobenzyl propyl 3 methyl methyl
152 4-fluorobenzyl propyl 3 methyl methyl
It is to be understood that the present invention includes any or all possible
combinations and subsets
of the special embodiments defined hereinabove.
The compounds of formula I, in which R4 is different from hydrogen, are chiral
compounds having a
chiral center in position 2'.
Numbering:
O
N A
N 7 N 6
R2 H
3 4 5 2, R4
R1
OY
N N(R5)R6
n
g
The invention includes all conceivable stereoisomers of the compounds of this
invention, like e.g.
diastereomers and enantiomers, in substantially pure form as well as in any
mixing ratio, including the
racemates, as well as the salts thereof.
In this connection, compounds of formula I worthy to be mentioned are those
which have, with respect
to the position 2', the same configuration as shown in formula I*:
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-21 -
O
NN'J~ N'4
R2 H
R4
*
R1
O\ /NJ 1/N(R5)R6
~" Mn
(11 B
In a preferred embodiment, the invention therefore relates to compounds of
formula I as described in
this application, wherein said compounds have the configuration as shown in
formula I*, and the salts
thereof.
If, for example, in compounds of formula I* R4 has the meaning methyl, ethyl,
propyl, isopropyl, sec-
butyl or cyclopropyl, then the configuration - according to the rules of Cahn,
Ingold and Prelog - is R
in the 2' position.
Further on, compounds of the formula I also worthy to be mentioned are those
which have, with
respect to the position 2', the same configuration as shown in formula I**:
0
NN'J~ N'4
R2 ~ R4
H
*
R1
O\ /N~ N(R5)R6
~" Mn
B
If, for example, in compounds of formula I** R4 has the meaning methyl, ethyl,
propyl, isopropyl, sec-
butyl or cyclopropyl, then the configuration - according to the rules of Cahn,
Ingold and Prelog - is S
in the position 2'.
In general, enantiomerically pure compounds of this invention may be prepared
according to art-
known processes, such as e.g. via asymmetric syntheses, for example by
preparation and separation
of appropriate diastereoisomeric compounds/intermediates, which can be
separated by known
methods (e.g. by chromatographic separation or (fractional) crystallization
from a suitable solvent), or
by using chiral synthons or chiral reagents; by chromatographic separation of
the corresponding
racemate on chiral separating columns; by means of diastereomeric salt
formation of the racemic
compounds with optically active acids (such as e.g. those mentioned below) or
bases, subsequent
resolution of the salts and release of the desired compound from the salt; by
derivatization of the
racemic compounds with chiral auxiliary reagents, subsequent diastereomer
separation and removal
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 22 -
of the chiral auxiliary group; by resolution via diastereomeric inclusion
compounds (e.g. complexes or
clathrates); by kinetic resolution of a racemate (e.g. by enzymatic
resolution); by enantioselective
(preferential) crystallization (or crystallization by entrainment) from a
conglomerate of
enantiomorphous crystals under suitable conditions; or by (fractional)
crystallization from a suitable
solvent in the presence of a chiral auxiliary.
Thus, e.g. one possible alternative for enatiomer separation may be carried
out at the stage of the
compounds of formula I or of the starting compounds having a protonatable
group, e.g. a free amino
group, such as starting compounds of formula II as defined later. Hereby,
separation of the
enantiomers may be carried out, for example, by means of salt formation of the
racemic compounds
of formula II with optically active acids, preferably carboxylic acids,
subsequent resolution of the salts
and release of the desired compound from the salt. Examples of optically
active carboxylic acids
which may be mentioned in this connection, without being restricted thereto,
are the enantiomeric
forms of mandelic acid, tartaric acid, O,O'-dibenzoyltartaric acid, camphoric
acid, quinic acid, glutamic
acid, pyroglutamic acid, malic acid, camphorsulfonic acid, 3-
bromocamphorsulfonic acid,
a-methoxyphenylacetic acid, a-methoxy-a-trifluoromethylphenylacetic acid or 2-
phenylpropionic acid
or the like.
Another possible alternative for enantiomer separation may be carried out by
chromatographic
separation of a racemic mixture of compounds of formula I or of starting
compounds thereof on a
chiral separating column, such as e.g. described in the following examples or
analogously or similarly
thereto, using the appropriate separation conditions.
The enantiomers having the formula 1* and the salts thereof are part of the
invention.
The enantiomers having the formula I** and the salts thereof are also part of
the invention.
Preference is given in this connection to those compounds of formula I which
have with respect to the
asymmetric -C(R4)H- atom (position 2') the same absolute configuration as the
compound (+)-N-(3-
amino-propyl)-N-[1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-methyl-benzamide hydrochloride having the specific optical rotation
[a]p20 =+ 231
(c=0.4535, CHCI3), as well as the salts thereof.
The invention therefore relates to compounds of formula I according to the
invention, in which R4 is
different from hydrogen, and which have with respect to the asymmetric -C(R4)H-
atom the same
absolute configuration as the compound (+)-N-(3-amino-propyl)-N-[1-(6-benzyl-3-
chloro-2-methyl-7-
oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl)-propyl]-4-methyl-benzamide
hydrochloride having the
specific optical rotation [a]p20= + 231 (c=0.4535, CHCI3), as well as the
salts thereof.
As exemplary compounds according to this invention the following compounds of
formula 1*,
in which
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-23-
R1 is bromine, and R2 is methyl,
and the salts thereof,
may be mentioned by means of the substituent meanings for A, R4, n, R5, R6 and
B in the Table 3
given below.
As further exemplary compounds according to this invention the following
compounds of formula 1*,
in which
R1 is chlorine, and R2 is methyl,
and the salts thereof,
may be mentioned by means of the substituent meanings for A, R4, n, R5, R6 and
B in the Table 3
given below.
As further exemplary compounds according to this invention the following
compounds of formula 1*,
in which
R1 is hydrogen, and R2 is methyl,
and the salts thereof,
may be mentioned by means of the substituent meanings for A, R4, n, R5, R6 and
B in the Table 3
given below.
As further exemplary compounds according to this invention the following
compounds of formula I**,
in which
R1 is bromine, and R2 is methyl,
and the salts thereof,
may be mentioned by means of the substituent meanings for A, R4, n, R5, R6 and
B in the Table 3
given below.
As further exemplary compounds according to this invention the following
compounds of formula I**,
in which
R1 is chlorine, and R2 is methyl,
and the salts thereof,
may be mentioned by means of the substituent meanings for A, R4, n, R5, R6 and
B in the Table 3
given below.
As further exemplary compounds according to this invention the following
compounds of formula I**,
in which
R1 is hydrogen, and R2 is methyl,
and the salts thereof,
may be mentioned by means of the substituent meanings for A, R4, n, R5, R6 and
B in the Table 3
given below.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-24-
Table 3:
A R4 n R5 R6 B
1) benzyl ethyl 2 H H 4-meth I hen I
2) benzyl ethyl 2 H H 4-bromophenyl
3) benzyl ethyl 2 H H 4-chlorophenyl
4) benzyl ethyl 2 H H 4-meth I-3-fluorophen I
5) benzyl ethyl 2 H H 4-fluorophenyl
6) benzyl ethyl 2 H H 4-trifluorometh Iphen I
7) benzyl ethyl 2 H H 4-methox phen I
8) benzyl ethyl 3 H H 4-meth Iphen I
9) benzyl ethyl 3 H H 4-bromophenyl
10) benzyl ethyl 3 H H 4-chlorophenyl
11) benzyl ethyl 3 H H 4-meth I-3-fluorophen I
12) benzyl ethyl 3 H H 4-fluorophenyl
13) benzyl ethyl 3 H H 4-trifluorometh Iphen I
14) benzyl ethyl 3 H H 4-methox phen I
15) benzyl ethyl 2 methyl methyl 4-meth Iphen I
16) benzyl ethyl 2 meth I methyl 4-bromophenyl
17) benzyl ethyl 2 methyl methyl 4-chlorophenyl
18) benzyl ethyl 2 methyl methyl 4-meth I-3-fluorophen I
19) benzyl ethyl 2 methyl methyl 4-fluorophenyl
20) benzyl ethyl 2 methyl methyl 4-trifluorometh Iphen I
21) benzyl ethyl 2 methyl methyl 4-methox hen I
22) benzyl ethyl 3 methyl methyl 4-meth I hen I
23) benzyl ethyl 3 methyl methyl 4-bromo hen I
24) benzyl ethyl 3 methyl methyl 4-chloro hen I
25) benzyl ethyl 3 meth I methyl 4-meth I-3-fluoro hen I
26) benzyl ethyl 3 methyl methyl 4-fluoro hen I
27) benzyl ethyl 3 methyl methyl 4-trifluorometh I hen I
28) benzyl ethyl 3 methyl methyl 4-methox hen I
29) 3-fluorobenzyl ethyl 2 H H 4-meth I hen I
30) 3-fluorobenzyl ethyl 2 H H 4-bromo hen I
31) 3-fluorobenzyl ethyl 2 H H 4-chloro hen I
32) 3-fluorobenzyl ethyl 2 H H 4-meth I-3-fluoro hen I
33) 3-fluorobenz I eth I 2 H H 4-fluoro hen I
34) 3-fluorobenzyl ethyl 2 H H 4-trifluorometh I hen I
35) 3-fluorobenzyl ethyl 2 H H 4-methox hen I
36) 3-fluorobenzyl ethyl 3 H H 4-meth I hen I
37) 3-fluorobenz I eth I 3 H H 4-bromo hen I
38) 3-fluorobenzyl ethyl 3 H H 4-chloro hen I
39) 3-fluorobenzyl ethyl 3 H H 4-meth I-3-fluoro hen I
40) 3-fluorobenzyl ethyl 3 H H 4-fluoro hen I
41) 3-fluorobenzyl ethyl 3 H H 4-trifluorometh Iphen I
42) 3-fluorobenzyl ethyl 3 H H 4-methox phen I
43) 3-fluorobenzyl ethyl 2 methyl methyl 4-meth Iphen I
44) 3-fluorobenzyl ethyl 2 methyl methyl 4-bromophenyl
45) 3-fluorobenzyl ethyl 2 methyl methyl 4-chlorophenyl
46) 3-fluorobenzyl ethyl 2 methyl methyl 4-meth I-3-fluorophen I
47) 3-fluorobenzyl ethyl 2 methyl methyl 4-fluorophenyl
48) 3-fluorobenzyl ethyl 2 methyl methyl 4-trifluorometh Iphen I
49) 3-fluorobenzyl ethyl 2 methyl methyl 4-methox phen I
50) 3-fluorobenzyl ethyl 3 methyl methyl 4-meth Iphen I
51) 3-fluorobenzyl ethyl 3 methyl methyl 4-bromophenyl
52) 3-fluorobenzyl ethyl 3 methyl methyl 4-chlorophenyl
53) 3-fluorobenzyl ethyl 3 methyl methyl 4-meth I-3-fluorophen I
54) 3-fluorobenz I ethyl 3 methyl methyl 4-fluorophenyl
55) 3-fluorobenzyl ethyl 3 methyl methyl 4-trifluorometh Iphen I
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-25-
A R4 n R5 R6 B
56) 3-fluorobenzyl ethyl 3 methyl methyl 4-methox hen I
57) 4-fluorobenzyl ethyl 2 H H 4-meth I hen I
58) 4-fluorobenzyl ethyl 2 H H 4-bromophenyl
59) 4-fluorobenzyl ethyl 2 H H 4-chlorophenyl
60) 4-fluorobenzyl ethyl 2 H H 4-meth I-3-fluorophen I
61) 4-fluorobenzyl ethyl 2 H H 4-fluorophenyl
62) 4-fluorobenzyl ethyl 2 H H 4-trifluorometh Iphen I
63) 4-fluorobenzyl ethyl 2 H H 4-methox phen I
64) 4-fluorobenzyl ethyl 3 H H 4-meth Iphen I
65) 4-fluorobenzyl ethyl 3 H H 4-bromophenyl
66) 4-fluorobenzyl ethyl 3 H H 4-chlorophenyl
67) 4-fluorobenzyl ethyl 3 H H 4-meth I-3-fluorophen I
68) 4-fluorobenzyl ethyl 3 H H 4-fluorophenyl
69) 4-fluorobenzyl ethyl 3 H H 4-trifluorometh Iphen I
70) 4-fluorobenzyl ethyl 3 H H 4-methox phen I
71) 4-fluorobenzyl ethyl 2 methyl methyl 4-meth Iphen I
72) 4-fluorobenzyl ethyl 2 methyl methyl 4-bromophenyl
73) 4-fluorobenzyl ethyl 2 methyl methyl 4-chlorophenyl
74) 4-fluorobenzyl ethyl 2 methyl methyl 4-meth I-3-fluorophen I
75) 4-fluorobenzyl ethyl 2 methyl methyl 4-fluorophenyl
76) 4-fluorobenzyl ethyl 2 methyl methyl 4-trifluorometh Iphen I
77) 4-fluorobenzyl ethyl 2 methyl methyl 4-methox phen I
78) 4-fluorobenzyl ethyl 3 methyl methyl 4-meth I hen I
79) 4-fluorobenzyl ethyl 3 methyl methyl 4-bromo hen I
80) 4-fluorobenzyl ethyl 3 methyl methyl 4-chloro hen I
81) 4-fluorobenzyl ethyl 3 methyl methyl 4-meth I-3-fluoro hen I
82) 4-fluorobenzyl ethyl 3 methyl methyl 4-fluoro hen I
83) 4-fluorobenzyl ethyl 3 methyl methyl 4-trifluorometh I hen I
84) 4-fluorobenzyl ethyl 3 methyl methyl 4-methox hen I
85) benzyl iso ro I 2 H H 4-meth I hen I
86) benzyl isoprop 1 2 H H 4-bromo hen I
87) benzyl iso ro I 2 H H 4-chloro hen I
88) benzyl iso ro I 2 H H 4-meth I-3-fluoro hen I
89) benzyl iso ro I 2 H H 4-fluoro hen I
90) benzyl iso ro I 2 H H 4-trifluorometh I hen I
91) benzyl iso ro I 2 H H 4-methox hen I
92) benzyl iso ro I 3 H H 4-meth I hen I
93) benzyl iso ro I 3 H H 4-bromo hen I
94) benzyl iso ro I 3 H H 4-chloro hen I
95) benzyl iso ro I 3 H H 4-meth I-3-fluoro hen I
96) benzyl isoprop 1 3 H H 4-fluoro hen I
97) benzyl iso ro I 3 H H 4-trifluorometh I hen I
98) benzyl isopropyl 3 H H 4-methox phen I
99) benzyl isopropyl 2 methyl methyl 4-meth Iphen I
100) benzyl isopropyl 2 methyl methyl 4-bromophenyl
101) benzyl isopropyl 2 methyl methyl 4-chlorophenyl
102) benzyl isopropyl 2 methyl methyl 4-meth I-3-fluorophen I
103) benzyl isopropyl 2 methyl methyl 4-fluorophenyl
104) benzyl isopropyl 2 methyl methyl 4-trifluorometh Iphen I
105) benzyl isopropyl 2 methyl methyl 4-methox phen I
106) benzyl isopropyl 3 methyl methyl 4-meth Iphen I
107) benzyl isopropyl 3 methyl methyl 4-bromophenyl
108) benzyl isopropyl 3 methyl methyl 4-chlorophenyl
109) benzyl isopropyl 3 methyl methyl 4-meth I-3-fluorophen I
110) benzyl isopropyl 3 methyl methyl 4-fluorophenyl
111) benzyl isopropyl 3 methyl methyl 4-trifluorometh Iphen I
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-26-
A R4 n R5 R6 B
112 benzyl iso ro I 3 methyl methyl 4-methox hen I
113 3-fluorobenzyl iso ro I 2 H H 4-meth I hen I
114 3-fluorobenzyl isopropyl 2 H H 4-bromophenyl
115 3-fluorobenzyl isopropyl 2 H H 4-chlorophenyl
116 3-fluorobenzyl isopropyl 2 H H 4-meth I-3-fluorophen I
117) 3-fluorobenzyl isopropyl 2 H H 4-fluorophenyl
118) 3-fluorobenzyl isopropyl 2 H H 4-trifluorometh Iphen I
119) 3-fluorobenzyl isopropyl 2 H H 4-methox phen I
120) 3-fluorobenzyl isopropyl 3 H H 4-meth Iphen I
121) 3-fluorobenzyl isopropyl 3 H H 4-bromophenyl
122) 3-fluorobenzyl isopropyl 3 H H 4-chlorophenyl
123) 3-fluorobenzyl isopropyl 3 H H 4-meth I-3-fluorophen I
124) 3-fluorobenzyl isopropyl 3 H H 4-fluorophenyl
125) 3-fluorobenzyl isopropyl 3 H H 4-trifluorometh Iphen I
126) 3-fluorobenzyl isopropyl 3 H H 4-methox phen I
127) 3-fluorobenzyl isopropyl 2 methyl methyl 4-meth Iphen I
128) 3-fluorobenzyl isopropyl 2 methyl methyl 4-bromophenyl
129) 3-fluorobenzyl isopropyl 2 methyl methyl 4-chlorophenyl
130) 3-fluorobenzyl isopropyl 2 methyl methyl 4-meth I-3-fluorophen I
131) 3-fluorobenzyl isopropyl 2 methyl methyl 4-fluorophenyl
132) 3-fluorobenzyl isopropyl 2 methyl methyl 4-trifluorometh Iphen I
133) 3-fluorobenzyl isopropyl 2 methyl methyl 4-methox phen I
134) 3-fluorobenz I iso ro I 3 methyl methyl 4-meth I hen I
135) 3-fluorobenzyl iso ro I 3 methyl methyl 4-bromo hen I
136) 3-fluorobenz I iso ro I 3 methyl methyl 4-chloro hen I
137) 3-fluorobenzyl iso ro I 3 methyl methyl 4-meth I-3-fluoro hen I
138) 3-fluorobenz I iso ro I 3 methyl methyl 4-fluoro hen I
139) 3-fluorobenzyl iso ro I 3 methyl methyl 4-trifluorometh I hen I
140) 3-fluorobenzyl iso ro I 3 methyl methyl 4-methox hen I
141) 4-fluorobenzyl iso ro I 2 H H 4-meth I hen I
142) 4-fluorobenzyl iso ro I 2 H H 4-bromo hen I
143) 4-fluorobenz I iso ro I 2 H H 4-chloro hen I
144) 4-fluorobenzyl iso ro I 2 H H 4-meth I-3-fluoro hen I
145) 4-fluorobenzyl iso ro I 2 H H 4-fluoro hen I
146) 4-fluorobenzyl iso ro I 2 H H 4-trifluorometh I hen I
147) 4-fluorobenz I iso ro I 2 H H 4-methox hen I
148) 4-fluorobenzyl iso ro I 3 H H 4-meth I hen I
149) 4-fluorobenzyl iso ro I 3 H H 4-bromo hen I
150) 4-fluorobenzyl iso ro I 3 H H 4-chloro hen I
151) 4-fluorobenz I iso ro I 3 H H 4-meth I-3-fluoro hen I
152) 4-fluorobenz I iso ro I 3 H H 4-fluoro hen I
153) 4-fluorobenzyl iso ro I 3 H H 4-trifluorometh I hen I
154) 4-fluorobenzyl isopropyl 3 H H 4-methox phen I
155) 4-fluorobenzyl isopropyl 2 meth I methyl 4-meth Iphen I
156) 4-fluorobenzyl isopropyl 2 methyl methyl 4-bromophenyl
157) 4-fluorobenzyl isopropyl 2 methyl methyl 4-chlorophenyl
158) 4-fluorobenzyl isopropyl 2 methyl methyl 4-meth I-3-fluorophen I
159) 4-fluorobenzyl isopropyl 2 methyl methyl 4-fluorophenyl
160) 4-fluorobenzyl isopropyl 2 methyl methyl 4-trifluorometh Iphen I
161) 4-fluorobenzyl isopropyl 2 methyl methyl 4-methox phen I
162) 4-fluorobenzyl isopropyl 3 methyl methyl 4-meth Iphen I
163) 4-fluorobenzyl isopropyl 3 methyl methyl 4-bromophenyl
164) 4-fluorobenzyl isopropyl 3 methyl methyl 4-chlorophenyl
165 4-fluorobenz I isopropyl 3 methyl methyl 4-meth I-3-fluorophen I
166) 4-fluorobenzyl isopropyl 3 methyl methyl 4-fluorophenyl
167) 4-fluorobenzyl isopropyl 3 methyl methyl 4-trifluorometh Iphen I
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-27-
A R4 n R5 R6 B
168) 4-fluorobenzyl iso ro I 3 methyl methyl 4-methox hen I
169) benzyl c clo ro I 2 H H 4-meth I hen I
170) benzyl c cloprop I 2 H H 4-bromophenyl
171) benzyl c cloprop I 2 H H 4-chlorophenyl
172) benzyl c cloprop I 2 H H 4-meth I-3-fluorophen I
173) benzyl c cloprop I 2 H H 4-fluorophenyl
174) benzyl c cloprop I 2 H H 4-trifluorometh Iphen I
175) benzyl c cloprop I 2 H H 4-methox phen I
176) benzyl c cloprop I 3 H H 4-meth Iphen I
177) benzyl c cloprop I 3 H H 4-bromophenyl
178) benzyl c cloprop I 3 H H 4-chlorophenyl
179) benzyl c cloprop I 3 H H 4-meth I-3-fluorophen I
180) benzyl c cloprop I 3 H H 4-fluorophenyl
181) benzyl c cloprop I 3 H H 4-trifluorometh Iphen I
182) benzyl c cloprop I 3 H H 4-methox phen I
183) benzyl c cloprop I 2 methyl methyl 4-meth Iphen I
184) benzyl c cloprop I 2 methyl methyl 4-bromophenyl
185) benzyl c cloprop I 2 methyl methyl 4-chlorophenyl
186) benzyl c cloprop I 2 methyl methyl 4-meth I-3-fluorophen I
187) benzyl c cloprop I 2 methyl methyl 4-fluorophenyl
188) benzyl c cloprop I 2 methyl methyl 4-trifluorometh Iphen I
189) benzyl c cloprop I 2 methyl methyl 4-methox phen I
190) benzyl c clo ro I 3 methyl methyl 4-meth I hen I
191) benzyl c clo ro I 3 methyl methyl 4-bromo hen I
192) benz I c clo ro I 3 methyl methyl 4-chloro hen I
193) benzyl c clo ro I 3 methyl methyl 4-meth I-3-fluoro hen I
194) benzyl c clo ro I 3 methyl methyl 4-fluoro hen I
195) benzyl c clo ro I 3 methyl methyl 4-trifluorometh I hen I
196) benzyl c clo ro I 3 methyl methyl 4-methox hen I
197) 3-fluorobenzyl c clo ro I 2 H H 4-meth I hen I
198) 3-fluorobenzyl c clo ro I 2 H H 4-bromo hen I
199) 3-fluorobenz I c clo ro I 2 H H 4-chloro hen I
200) 3-fluorobenzyl c clo ro I 2 H H 4-meth I-3-fluoro hen I
201) 3-fluorobenzyl c clo ro I 2 H H 4-fluoro hen I
202) 3-fluorobenz I c clo ro I 2 H H 4-trifluorometh I hen I
203) 3-fluorobenz I c clo ro I 2 H H 4-methox hen I
204) 3-fluorobenzyl c clo ro I 3 H H 4-meth I hen I
205) 3-fluorobenzyl c clo ro I 3 H H 4-bromo hen I
206) 3-fluorobenzyl c clo ro I 3 H H 4-chloro hen I
207) 3-fluorobenz I c clo ro I 3 H H 4-meth I-3-fluoro hen I
208) 3-fluorobenzyl c clo ro I 3 H H 4-fluoro hen I
209) 3-fluorobenzyl c clo ro I 3 H H 4-trifluorometh I hen I
210) 3-fluorobenzyl c cloprop I 3 H H 4-methox phen I
211) 3-fluorobenzyl c cloprop I 2 meth I methyl 4-meth Iphen I
212) 3-fluorobenzyl c cloprop I 2 methyl methyl 4-bromophenyl
213) 3-fluorobenzyl c cloprop I 2 methyl methyl 4-chlorophenyl
214) 3-fluorobenzyl c cloprop I 2 methyl methyl 4-meth I-3-fluorophen I
215 3-fluorobenzyl c cloprop I 2 methyl methyl 4-fluorophenyl
216 3-fluorobenzyl c cloprop I 2 methyl methyl 4-trifluorometh Iphen I
217) 3-fluorobenzyl c cloprop I 2 methyl methyl 4-methox phen I
218) 3-fluorobenzyl c cloprop I 3 methyl methyl 4-meth Iphen I
219) 3-fluorobenzyl c cloprop I 3 methyl methyl 4-bromophenyl
220) 3-fluorobenzyl c cloprop I 3 methyl methyl 4-chlorophenyl
221) 3-fluorobenz I c cloprop I 3 meth I methyl 4-meth I-3-fluorophen I
222) 3-fluorobenzyl c cloprop I 3 methyl methyl 4-fluorophenyl
223) 3-fluorobenzyl c cloprop I 3 methyl methyl 4-trifluorometh Iphen I
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 28 -
A R4 n R5 R6 B
224) 3-fluorobenzyl c clo ro I 3 methyl methyl 4-methox hen I
225) 4-fluorobenzyl c clo ro I 2 H H 4-meth I hen I
226) 4-fluorobenzyl c cloprop I 2 H H 4-bromophenyl
227) 4-fluorobenzyl c cloprop I 2 H H 4-chlorophenyl
228) 4-fluorobenzyl c cloprop I 2 H H 4-meth I-3-fluorophen I
229) 4-fluorobenzyl c cloprop I 2 H H 4-fluorophenyl
230) 4-fluorobenzyl c cloprop I 2 H H 4-trifluorometh Iphen I
231) 4-fluorobenzyl c cloprop I 2 H H 4-methox phen I
232) 4-fluorobenzyl c cloprop I 3 H H 4-meth Iphen I
233) 4-fluorobenzyl c cloprop I 3 H H 4-bromophenyl
234) 4-fluorobenzyl c cloprop I 3 H H 4-chlorophenyl
235) 4-fluorobenzyl c cloprop I 3 H H 4-meth I-3-fluorophen I
236) 4-fluorobenzyl c cloprop I 3 H H 4-fluorophenyl
237) 4-fluorobenzyl c cloprop I 3 H H 4-trifluorometh Iphen I
238) 4-fluorobenzyl c cloprop I 3 H H 4-methox phen I
239) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-meth Iphen I
240) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-bromophenyl
241) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-chlorophenyl
242) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-meth I-3-fluorophen I
243) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-fluorophenyl
244) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-trifluorometh Iphen I
245) 4-fluorobenzyl c cloprop I 2 methyl methyl 4-methox phen I
246) 4-fluorobenzyl c clo ro I 3 methyl methyl 4-meth I hen I
247) 4-fluorobenzyl cycloprop 1 3 meth I methyl 4-bromo hen I
248) 4-fluorobenzyl c clo ro I 3 methyl methyl 4-chloro hen I
249) 4-fluorobenzyl c clo ro I 3 methyl methyl 4-meth I-3-fluoro hen I
250) 4-fluorobenzyl c clo ro I 3 methyl methyl 4-fluoro hen I
251) 4-fluorobenzyl c clo ro I 3 methyl methyl 4-trifluorometh I hen I
252) 4-fluorobenzyl c clo ro I 3 methyl methyl 4-methox hen I
253) benzyl sec-butyl 2 H H 4-meth I hen I
254) benzyl sec-butyl 2 H H 4-bromo hen I
255) benzyl sec-butyl 2 H H 4-chloro hen I
256) benzyl sec-butyl 2 H H 4-meth I-3-fluoro hen I
257) benzyl sec-butyl 2 H H 4-fluoro hen I
258) benzyl sec-butyl 2 H H 4-trifluorometh I hen I
259) benzyl sec-butyl 2 H H 4-methox hen I
260) benzyl sec-butyl 3 H H 4-meth I hen I
261) benzyl sec-butyl 3 H H 4-bromo hen I
262) benzyl sec-butyl 3 H H 4-chloro hen I
263) benzyl sec-butyl 3 H H 4-meth I-3-fluoro hen I
264) benzyl sec-butyl 3 H H 4-fluoro hen I
265) benzyl sec-butyl 3 H H 4-trifluorometh I hen I
266) benzyl sec-butyl 3 H H 4-methox phen I
267) benzyl sec-butyl 2 methyl methyl 4-meth Iphen I
268) benzyl sec-butyl 2 methyl methyl 4-bromophenyl
269) benzyl sec-butyl 2 methyl methyl 4-chlorophenyl
270) benzyl sec-butyl 2 methyl methyl 4-meth I-3-fluorophen I
271) benzyl sec-butyl 2 methyl methyl 4-fluorophenyl
272) benzyl sec-butyl 2 meth yl methyl 4-trifluorometh Iphen I
273) benzyl sec-butyl 2 methyl methyl 4-methox phen I
274) benzyl sec-butyl 3 methyl methyl 4-meth Iphen I
275) benzyl sec-butyl 3 methyl methyl 4-bromophenyl
276) benzyl sec-butyl 3 methyl methyl 4-chlorophenyl
277) benzyl sec-butyl 3 meth I methyl 4-meth I-3-fluorophen I
278) benzyl sec-butyl 3 methyl methyl 4-fluorophenyl
279) benzyl sec-butyl 3 methyl methyl 4-trifluorometh Iphen I
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 29 -
A R4 n R5 R6 B
280) benzyl sec-butyl 3 methyl methyl 4-methox hen I
281) 3-fluorobenzyl sec-butyl 2 H H 4-meth I hen I
282) 3-fluorobenzyl sec-butyl 2 H H 4-bromophenyl
283) 3-fluorobenzyl sec-butyl 2 H H 4-chlorophenyl
284) 3-fluorobenzyl sec-butyl 2 H H 4-meth I-3-fluorophen I
285) 3-fluorobenzyl sec-butyl 2 H H 4-fluorophenyl
286) 3-fluorobenzyl sec-butyl 2 H H 4-trifluorometh Iphen I
287) 3-fluorobenzyl sec-butyl 2 H H 4-methox phen I
288) 3-fluorobenzyl sec-butyl 3 H H 4-meth Iphen I
289) 3-fluorobenzyl sec-butyl 3 H H 4-bromophenyl
290) 3-fluorobenzyl sec-butyl 3 H H 4-chlorophenyl
291) 3-fluorobenzyl sec-butyl 3 H H 4-meth I-3-fluorophen I
292) 3-fluorobenzyl sec-butyl 3 H H 4-fluorophenyl
293) 3-fluorobenzyl sec-butyl 3 H H 4-trifluorometh Iphen I
294) 3-fluorobenzyl sec-butyl 3 H H 4-methox phen I
295) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-meth Iphen I
296) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-bromophenyl
297) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-chlorophenyl
298) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-meth I-3-fluorophen I
299) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-fluorophenyl
300) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-trifluorometh Iphen I
301) 3-fluorobenzyl sec-butyl 2 methyl methyl 4-methox phen I
302) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-meth I hen I
303) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-bromo hen I
304) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-chloro hen I
305) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-meth I-3-fluoro hen I
306) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-fluoro hen I
307) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-trifluorometh I hen I
308) 3-fluorobenzyl sec-butyl 3 methyl methyl 4-methox hen I
309) 4-fluorobenzyl sec-butyl 2 H H 4-meth I hen I
310) 4-fluorobenzyl sec-butyl 2 H H 4-bromo hen I
311) 4-fluorobenzyl sec-butyl 2 H H 4-chloro hen I
312) 4-fluorobenzyl sec-butyl 2 H H 4-meth I-3-fluoro hen I
313) 4-fluorobenzyl sec-butyl 2 H H 4-fluoro hen I
314) 4-fluorobenz I sec-butyl 2 H H 4-trifluorometh I hen I
315) 4-fluorobenzyl sec-butyl 2 H H 4-methox hen I
316) 4-fluorobenzyl sec-butyl 3 H H 4-meth I hen I
317) 4-fluorobenzyl sec-butyl 3 H H 4-bromo hen I
318) 4-fluorobenzyl sec-butyl 3 H H 4-chloro hen I
319) 4-fluorobenz I sec-butyl 3 H H 4-meth I-3-fluoro hen I
320) 4-fluorobenzyl sec-butyl 3 H H 4-fluoro hen I
321) 4-fluorobenzyl sec-butyl 3 H H 4-trifluorometh I hen I
322) 4-fluorobenzyl sec-butyl 3 H H 4-methox phen I
323) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-meth Iphen I
324) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-bromophenyl
325) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-chlorophenyl
326) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-meth I-3-fluorophen I
327) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-fluorophenyl
328) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-trifluorometh Iphen I
329) 4-fluorobenzyl sec-butyl 2 methyl methyl 4-methox phen I
330) 4-fluorobenzyl sec-butyl 3 methyl methyl 4-meth Iphen I
331) 4-fluorobenzyl sec-butyl 3 methyl methyl 4-bromophenyl
332) 4-fluorobenzyl sec-butyl 3 methyl methyl 4-chlorophenyl
333) 4-fluorobenzyl sec-butyl 3 methyl methyl 4-meth I-3-fluorophen I
334) 4-fluorobenz I sec-butyl 3 methyl methyl 4-fluorophenyl
335) 4-fluorobenzyl sec-butyl 3 methyl methyl 4-trifluorometh Iphen I
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-30-
A R4 n R5 R6 B
336) 4-fluorobenz I sec-butyl 3 methyl methyl 4-methox hen I
The compounds according to the invention can be prepared e.g. as described as
follows and
according to the following specified reaction steps, or, particularly, in a
manner as described by way
of example in the following examples, or analogously or similarly thereto
according to preparation
procedures or synthesis strategies known to the person skilled in the art.
As shown in the synthesis route outlined in scheme 1 below, the trione
compound of formula IX, in
which R2 has the meanings given above (particularly, R2 is methyl), is
condensed and cyclized with
semicarbazide (HzN-C(O)-NHNHz*HCI) to give the corresponding
pyrazolopyrimidone compounds of
formula VIII. Said cyclocondensation reaction can be carried out as it is
habitual for the skilled person
or as described in the following examples or analogously or similarly thereto,
in the presence of a
suitable base (e.g. sodium carbonate) in a suitable solvent, for example
water, at elevated
temperature.
Compounds of formula IX, in which R2 has the meanings given above,
particularly methyl, are known
or can be prepared according to known procedures or as described in the
following examples, or
analogously or similarly thereto. Thus, e.g. compounds of formula IX can be
obtained from
corresponding compounds of formula X by alkaline hydrolysis
Compounds of formula X are known or can be obtained according to known
procedures.
Compounds of formula VIII are benzylated with compounds of formula A-X1, in
which A has the
meanings given above and Xl is a suitable leaving group (e.g. halogen or the
like), in a standard
manner or as described exemplarily in the following examples to give
corresponding compounds of
formula VII.
Compounds of formula VII, in which R2 and A have the meanings given above, are
halogenated using
a suitable halogenating reagent under appropriate conditions to obtain
corresponding compounds of
formula VI, in which R2 and A have the meanings given above and R1 is halogen,
in particular
fluorine or, in more particular, chlorine or bromine. Said halogenation
reaction can be carried out
according to customary procedures or as described in the following examples,
or analogously or
similarly thereto.
Thus, chlorination or bromination reaction can be carried out using an
appropriate electrophilic
chlorinating or brominating reagent, e.g. N-chlorosuccinimide (NCS) or N-
bromosuccinimide (e.g. NBS
/ AIBN), respectively, under conditions customary for the skilled person or as
described in the
following examples.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-31 -
Reaction scheme 1:
0 0 0
lt~ 'NH2
0 0 0 H2N H N NNH A-X1
A -~ - R2
R2 O CH3 R2 CH3 ~ / CH benzylation
3
H
(X) (IX) (VIII)
0 0
N- N~NA halogenation N- N~NA oxidation
R2 / ~ R2
/ ~
CH3 R1 CH3
H (VII) (VI)
O 0
N- 'J~ NNA R4-M ~ - NN,A oxidation
R2 / R2 r R4
H addition
R1 0 R1 OH
(V) (IV)
0 HZN, 1/N(R5)R6 0
R2 /\N NA Mn R2 N NA
R4 H
red u ctive R4
R1 O amination R1
HNN(R5)R6
(III) (II) i+. 1. B-C(O)-X2, benzoylation
2. When R1 = H, optionally
halogenation (e.g. bromination)
3. If necessary, removal of temporary
protecting groups
0
~ ,NN
R2 H
R4
R1
Oy N~ N(R5)R6
Mn
B
(I)
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-32-
Fluorination reaction may be carried out using a suitable electrophilic
fluorinating reagent, such as e.g.
1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis-
(tetrafluoroborate) (Selectfluor I) or 1-
methyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis-(tetrafluoroborate)
(Selectfluor II) or the like,
under appropriate conditions.
Using appropriate conditions, said halogenation reaction is carried out
largely regioselectively to
obtain halogenation on the pyrazole moiety of the pyrazolopyrimidone scaffold,
i.e. the 3-position of
the pyrazolopyrimidone scaffold.
The methyl group attached to the pyrimidone moiety of the pyrazolopyrimidone
of compounds of
formula VI, in which R1 is halogen and A and R2 have the meanings given above,
is oxidized under
suitable conditions to obtain corresponding compounds of formula V. Said
oxidation is carried out in a
manner habitual for the skilled person or as described in the following
examples using a suitable
oxidation reagent, e.g. selenium dioxide, to give largely regioselectively the
5-formyl derivative of
formula V.
Compounds of formula V, in which R1 is halogen and A and R2 have the meanings
given above, are
reacted in a nucleophilic addition reaction with compounds of formula R4-M, in
which R4 is different
from hydrogen and has the meanings given above and M is a suitable metal or
metal complex, to give
corresponding compounds of formula IV. Said nucleophilic addition reaction can
be obtained using
organomagnesium halides (Grignard reagents) of formula R4-Mg-X (X = Halogen)
with or without
further additives (e.g. LiCI or alkyl lithium reagents). Advantageously said
reaction is carried out in the
presence of alkyl lithium reagents, e.g. methyl lithium, forming in situ more
reactive
organomagnesium reagents, e.g. lithium trialkyl magnesiates such as R4Me2MgLi
from 2 equiv. LiMe
and 1 equiv. R4-Mg-X. The nucleophilic addition reaction can be carried out in
a manner customary
for the skilled person or as described in the following examples.
When compounds of formula V, in which R1 is bromine, are reacted with Grignard
reagents in said
nucleophilic addition reaction, debromination of the 3-bromo substituent can
be obtained depending on
the reaction conditions to give corresponding compounds of formula IV, in
which R1 is hydrogen. Said
debromination is performed as described in the following examples.
Alcohols of formula IV, in which R1 is hydrogen, fluorine or chlorine and A,
R2 and R4 have the
meanings given above, are converted into the corresponding ketones of formula
III by oxidation
reaction using an appropriate oxidation reagent, such as e.g. NMO (N-
methylmorpholine-N-oxide) /
TPAP (tetrapropylammonium perruthenate) or the like, under conditions known
for the skilled person
or as described in the following examples.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-33-
Compounds of formula III, in which R1 is hydrogen, fluorine or chlorine and A,
R2 and R4 have the
meanings given above, are subjected to a reductive amination reaction with
amines of formula H2N-
(CH2)n-N(R5)R6, in which n has the meanings indicated above and R5 and R6
stand for a suitable
temporary protective group PG1 or for the meanings given above with the
exception that R5 and R6
are not hydrogen, to afford corresponding compounds of formula II in a manner
which is habitual for
the skilled person or which is described in the following examples. In more
detail, in a first step of the
reductive amination reaction the reaction partners are reacted in the presence
of a suitable Lewis
acid, e.g. a suitable titanium reagent like titanium(IV)isopropoxide, to give
the corresponding imine
compounds, which are reduced in a second step, such as with the aid of a
suitable reducing reagent,
e.g. a suitable hydride like a borohydride (e.g. NaCNBH3, NaBH4 or Na(OAc)3BH)
or the like, Raney-
Ni or in the presence of hydrogen/transition metal catalyst (e.g. H2/Pd).
PG1 stands for a suitable temporary protective group for the amino group, for
example tert-
butoxycarbonyl, benzyloxycarbonyl or 4-methoxy-benzyloxycarbonyl or one of
those art-known
protective groups mentioned in "Protective Groups in Organic Synthesis" by T.
Greene and P. Wuts
(John Wiley & Sons, Inc. 1999, 3~d Ed.) or in "Protecting Groups (Thieme
Foundations Organic
Chemistry Series N Group" by P. Kocienski (Thieme Medical Publishers, 2000).
Compounds of formula II, in which R1 is hydrogen, fluorine or chlorine and A,
n, R2 and R4 have the
meanings given above and R5 and R6 stand for a suitable temporary protective
group PG1 as defined
above or for the meanings given above with the exception that R5 and R6 are
not hydrogen, are
benzoylated with compounds of formula B-C(O)-X2, in which B has the meanings
given above and X2
is a suitable leaving group (e.g. chlorine), to give corresponding compounds
of formula I.
Compounds of formula I, in which R1 is hydrogen and A, n, B, R2 and R4 have
the meanings given
above and R5 and R6 stand for a suitable temporary protective group PG1 as
defined above or for the
meanings given above with the exception that R5 and R6 are not hydrogen, can
be halogenated,
particularly brominated, using a suitable halogenating reagent (for
bromination e.g. NBS) under
appropriate conditions to obtain corresponding further compounds of formula I,
in which R1 is halogen.
Said halogenation reaction can be carried out according to customary
procedures, such as those
mentioned above, or as described in the following examples, or analogously or
similarly thereto.
Compounds of formula I, in which R1, A, n, B, R2 and R4 have the meanings
given above and R5 and
R6 stand for a suitable temporary protective group PG1 as defined above, are
deprotected by removal
of said protecting group PG1 in a manner known to the person skilled in the
art or as described in the
following examples to give corresponding deprotected compounds of formula I.
Compounds of formula I, in which R5 and R6 are 1-4C-alkyl, e.g. methyl, and in
which R1, A, n, B, R2
and R4 have the meanings given above, can be obtained by the methods described
above, starting
from compounds of formula I, in which R5 and R6 are 1-4C-alkyl, e.g. methyl,
and in which R1, A, n,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-34-
B, R2 and R4 have the meanings given above. Alternatively, compounds of
formula I, in which R5 and
R6 are 1-4C-alkyl, e.g. methyl, and in which R1, A, n, B, R2 and R4 have the
meanings given above,
can be obtained by alkylation, e.g. methylation, of compounds of formula I, in
which R5 and R6 are
hydrogen. Alternatively, these compounds of formula I, in which R5 and R6 are
1-4C-alkyl, e.g.
methyl, can be obtained by any other method that is known to the person
skilled in the art.
The invention therefore relates to a process for preparing a compound as
described in this application,
comprising at least one of the steps
(i) benzoylation of a compound of formula II, with the meanings of R1, R2, A,
R4, R5 and R6 as
indicated for the compounds according to the invention, or with at least one
of R5 and R6 being part of
a protective group,
O 0
N_ NNA N_ N'k NA
R2 H R2 H
/ R4 R4
R1 HNL N(R5)R6 R1 O N~ 1/N(R5)R6
Jn ~ Mn
B
(II) (I)
(ii) halogenation of a compound of formula I wherein R1 = H to give a compound
of formula I wherein
R1 = halogen, or
N_ N ~ N A N_ N N A
R2 / H R2 H
R4 / R4
R1 OyNMn ~ 1/N(R5)R6 R1 Oyn
NM~ l/ N(R5)R6
B B
(I) R1 = H (I) R1 = halogen
(iii) optionally the removal of protecting groups represented by at least one
of R5 and R6 as indicated
under (i).
Optionally, compounds of the formula I can be converted into their salts, or,
optionally, salts of the
compounds of the formula I can be converted into the free compounds.
Corresponding processes are
customary for the skilled person.
When one of the final steps or purification is carried out under the presence
of an inorganic or organic
acid (e.g. hydrochloric, trifluoroacetic, acetic or formic acid or the like),
the compounds of formula I
may be obtained - depending on their individual chemical nature and the
individual nature of the acid
used - as free base or containing said acid in an stoechiometric or non-
stoechiometric quantity. The
amount of the acid contained can be determined according to art-known
procedures, e.g. by titration.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-35-
It is moreover known to the person skilled in the art that if there are a
number of reactive centers on a
starting or intermediate compound it may be necessary to block one or more
reactive centers
temporarily by protective groups in order to allow a reaction to proceed
specifically at the desired
reaction center. A detailed description for the use of a large number of
proven protective groups is
found, for example, in "Protective Groups in Organic Synthesis" by T. Greene
and P. Wuts (John
Wiley & Sons, Inc. 1999, 3~d Ed.) or in "Protecting Groups (Thieme Foundations
Organic Chemistry
Series N Group" by P. Kocienski (Thieme Medical Publishers, 2000).
The substances according to the invention are isolated and purified in a
manner known per se, for
example by distilling off the solvent under reduced pressure and
recrystallizing the residue obtained
from a suitable solvent or subjecting it to one of the customary purification
methods, such as, for
example, column chromatography on a suitable support material.
Salts can be obtained by dissolving the free compound in a suitable solvent
(e.g. a ketone, such as
acetone, methyl ethyl ketone or methyl isobutyl ketone, an ether, such as
diethyl ether,
tetrahydrofuran or dioxane, a chlorinated hydrocarbon, such as methylene
chloride or chloroform, or a
low-molecular-weight aliphatic alcohol, such as methanol, ethanol or
isopropanol) which contains the
desired acid or base, or to which the desired acid or base is then added. The
salts can be obtained by
filtering, reprecipitating, precipitating with a nonsolvent for the addition
salt or by evaporating the
solvent. Salts obtained can be converted into the free compounds, which can in
turn be converted into
salts, by alkalization or by acidification. In this manner, pharmacologically
and/or pharmaceutically
unacceptable salts can be converted into pharmacologically and/or
pharmaceutically acceptable salts.
Suitably, the conversions mentioned in this invention can be carried out
analogously or similarly to
methods which are familiar per se to the person skilled in the art.
The person skilled in the art may know on the basis of his/her knowledge and
on the basis of those
synthesis routes, which are shown and described within the description of this
invention, how to find
other possible synthesis routes for compounds according to this invention. All
these other possible
synthesis routes are also part of this invention.
The present invention also relates to intermediates (including their salts,
stereosiomers as well as salts
of these stereoisomers), methods and processes useful in synthesizing
compounds according to this
invention.
Having described the invention in detail, the scope of the present invention
is not limited only to those
described characteristics or embodiments. As will be apparent to persons
skilled in the art,
modifications, analogies, variations, derivatizations, homologisations and
adaptations to the described
invention can be made on the base of art-known knowledge and/or, particularly,
on the base of the
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-36-
disclosure (e.g. the explicite, implicite or inherent disclosure) of the
present invention without
departing from the spirit and scope of this invention as defined by the
appended claims.
The following examples illustrate the invention in greater detail without
restricting it. Likewise, further
compounds according to this invention, of which the preparation is not
explicitly described, can be
prepared in an analogous or similar manner or in a manner familiar per se to
the person skilled in the
art using customary process techniques.
Any or all of the compounds of formula I according to the present invention
which are mentioned as
final compounds in the following examples, as well as the salts, stereoisomers
and salts of the
stereoisomers thereof, are a preferred subject of the present invention.
In the examples, m.p. stands for melting point, h for hour(s), min for
minutes, conc. for concentrated,
calc. for calculated, fnd. for found, EF for elemental formula, MS for mass
spectrometry, M for
molecular ion in mass spectrometry, TFA for trifluoroacetic acid, and other
abbreviations have their
meanings customary per se to the skilled person.
Further on, according to common practice in stereochemistry, the term "(RS)"
characterizes a
racemate comprising the one enantiomer having the configuration R and the
other enantiomer having
the configuration S; each of these enantiomers and their salts in pure form as
well as their mixtures
including the racemic mixtures is part of this invention.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-37-
Examples
Final compounds
1. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-4-methyl-benzamide hydrochloride
150 mg {3-[[1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(1-p-tolyl-
carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester (0.26 mmol) are solved
in 1 ml diethylether. 10
ml HCI in diethylether (ca. 4 N) are added and the suspension is stirred 1 h
at ambient temperature.
Afterwards the suspension is evaporated, the colorless solid is washed twice
with diethylether and
dried in vacuo. By this method 120 mg (91%) of a colorless solid are obtained.
M.p.: 235-250 C (decomposition).
MS: m/z (MH+) = 472.1.
2. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-propyl]-4-methyl-benzamide hydrochloride
150 mg {3-[[1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(1-
p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert butyl ester (0.26 mmol)
are solved in 1 ml
diethylether, 4 ml HCI in diethylether (ca. 4N) are added and the mixture is
stirred 1 h at ambient
temperature. Afterwards the suspension is evaporated, the colorless solid is
washed twice with
diethylether and dried in vacuo. By this method 135 mg (88%) of a colorless
solid are obtained.
M.p.: > 190 C (decomposition).
MS: m/z (MH+) = 550.0, 552Ø
3. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-3-methyl-benzamide; isolated
as
trifluoroacetate salt
0.144 g (1.0 eq) {3-[[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-(1-m-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester is dissolved in 8 mL
dichloromethane and treated with 2 mL trifluoro acetic acid. After stirring 2
hours at ambient
temperature the solvent is removed. Addition of diethylether results in a
white slurry that is filtered.
The precipitate is washed with diethylether and yields after drying 0.108 g
(73%) of the title compound
as a trifluoroacetate.
M.p. 162 C.
MS: m/z (MH+) = 520.1, 522.1.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-38-
4. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-fluoro-benzamide; isolated
as trifluoroacetate
salt
0.090 g (1.0 eq) (3-{[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(4-fluoro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester is dissolved
in 6 mL dichloromethane and treated with 1.5 mL trifluoro acetic acid. After
stirring 2 hours at ambient
temperature the solvent is removed. Addition of diethylether results in a
white slurry that is filtered.
The precipitate is washed with diethylether and yields after drying 0.068 g
(75%) of the title compound
as a trifluoroacetate.
M.p. 182 C.
MS: m/z (MH+) = 524.1, 526.1.
5. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methoxy-benzamide; isolated
as
trifluoroacetate salt
0.014 g (1.0 eq) (3-{[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(4-methoxy-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester is
dissolved in 0.8 mL dichloromethane and treated with 0.2 mL trifluoro acetic
acid. After stirring 2 hours
at ambient temperature the solvent is removed. Addition of diethylether
results in a white slurry that is
filtered. The precipitate is washed with diethylether and yields after drying
0.007 g (48%) of the title
compound as a trifluoroacetate.
M.p. 149 C.
MS: m/z (MH+) = 536.1, 538.1.
6. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-bromo-benzamide; isolated as
trifluoroacetate
salt
0.153 g (1.0 eq) (3-{[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(4-bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-
butyl ester is dissolved
in 10 mL dichloromethane and treated with 2.5 mL trifluoro acetic acid. After
stirring 2 hours at
ambient temperature the solvent is removed. Addition of diethylether results
in a white slurry that is
filtered. The precipitate is washed with diethylether and yields after drying
0.086 g (55%) of the title
compound as a trifluoroacetate.
M.p. 204-205 C (decomposition).
MS: m/z (MH+) = 584.0, 585.9, 587.9.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-39-
7. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-chloro-benzamide; isolated
as trifluoroacetate
salt
0.130 g (1.0 eq) (3-{[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(4-chloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester is dissolved
in 8 mL dichloromethane and treated with 2 mL trifluoro acetic acid. After
stirring 2 hours at ambient
temperature the solvent is removed. Addition of diethylether results in a
white slurry that is filtered.
The precipitate is washed with diethylether and yields after drying 0.075 g
(56%) of the title compound
as a trifluoroacetate.
M.p. 199-201 C (decomposition).
MS: m/z (MH+) = 540.0, 542.0, 544Ø
8. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide
hydrochloride
0.129 g (1.0 eq) {3-[[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester (compound A8) is
dissolved in 20 mL of a 1 M solution of hydrochloric acid in diethylether at
ambient temperature. After
stirring for 16 hours the precipitate is filtered off and washed with
diethylether. This yields after drying
0.083 g (71%) of the title compound as a hydrochloride salt.
M.p. 183 C.
MS: m/z (MH+) = 520.0, 522.1.
Separation of enantiomers:
8a. (+)-N-(3-Amino-propyl)-N-[1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide hydrochloride (assumed
(R)-isomer)
and
8b. (-)-N-(3-Amino-propyl)-N-[1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide hydrochloride (assumed
(S)-isomer)
1.79 g of racemic N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-
oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide
hydrochloride are separated into
its enantiomers by preparative HPLC (column: CHIRALPAK AD 10 pm, 250 x 20 mm;
mobile phase:
n-Heptane / ethanol / diethylamine 80:20:0.1 (v/v/v); flow rate: 30 ml/min;
detection: UV 230 nm;
temperature: 25 C).
The enantiomers elute at 7.49 min and 13.95 min. Both isomers are isolated by
solving in
dichloromethane and treatment with 4M solution of HCI in dioxane. After
stirring for 6 hours at ambient
temperature, the solvent is removed. Addition of diethylether results in a
white slurry that is filtered.
The precipitate is washed with diethylether and dried in vacuo.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 40 -
The HCI-salt of the first eluting enantiomer (ti = 7.49 min, > 99.5 % ee, 94 %
purity, 556 mg, example
8a) has an optical rotation of [a]p20 = +225 (c = 1.02, dichloromethane), a
melting point of 203-263
C (decomposition) and MS: m/z (MH+) = 519.8, 521.9.
The HCI-salt of the second eluting enantiomer (ti = 13.95 min, > 98 % ee, > 99
% purity, 870 mg,
example 8b) has an optical rotation of [a]p20 = -205 (c = 0.725,
dichloromethane), a melting point of
206-233 C (decomposition) and MS: m/z (MH+) = 520.1, 522Ø
The absolute stereochemistry of (+)-N-(3-Amino-propyl)-N-[1-(6-benzyl-3-chloro-
2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide
hydrochloride (8a) is
tentatively assigned to the enantiomer with the R-configuration (+)-N-(3-Amino-
propyl)-N-[(R)-1-(6-
benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl )-2-
methyl-propyl]-4-methyl-
benzamide hydrochloride. Consequently, the S-configuration is assigned to the
(-)-enantiomer.
Furthermore, a variety of salts of compound 8, 8a and 8b is prepared:
8c. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide phosphate
100 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) are
suspended in 1 mL ethanol
and heated at reflux until the amine is completely dissolved. 13.1 pL
phosphoric acid (85% in water,
1.0 eq) are added and the mixture is stirred for 5 minutes without heating
anymore. Evaporation of the
solvent and subsequent addition of diethylether yielded (after 30 min
ultrasonification) in a white slurry
that is filtered and washed with several portions of diethylether. The
colorless solid is dried in vacuo to
yield 63 mg (53%) of the title compound. There are slight impurities of
diethylether and
diethylammonium phosphate.
M.p.: foaming at 123 C, melting 160-205 C (decomposition).
MS: m/z (MH+) = 519.9, 522.1.
8d. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide semi fumarate
100 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) and 22.3 mg
of fumaric acid (1.0
eq) are suspended in 1 mL ethanol and heated at reflux for 2 minutes. The
mixture is stirred for 5
minutes without heating anymore. Evaporation of the solvent and subsequent
addition of diethylether
yielded in a white slurry that is filtered and washed with several portions of
diethylether. The colorless
solid is dried in vacuo to yield 61 mg (55%) of the title compound. There are
slight impurities of
diethylether and diethylammonium fumarate. The semi fumarate is elucidated by
NMR spectroscopy.
M.p.: sinter from 140 C on, melting 195-215 C (decomposition).
MS: m/z (MH+) = 519.9, 522.1.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-41 -
8e. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide sulfate
100 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) are
suspended in 1 mL ethanol
and heated at reflux until the amine is completely dissolved. 10.2 pL sulfuric
acid (1.0 eq) are added
and the mixture is stirred for 5 minutes without heating anymore. Evaporation
of the solvent and
subsequent addition of diethylether yielded in a white slurry (after 15 min
ultrasonification) that is
filtered and washed with several portions of diethylether. The colorless solid
is dried in vacuo to yield
73 mg (62%) of the title compound. There are slight impurities of diethylether
and diethylammonium
sulfate.
M.p.: foaming at 130 C, melting 198-209 C (decomposition).
MS: m/z (MH+) = 519.9, 522.1.
8f. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide sesqui citrate
100 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) and 37 mg
of citric acid (1.0 eq)
are suspended in 1 mL ethanol and heated at reflux for 2 minutes. The mixture
is stirred for 5 minutes
without heating anymore. Evaporation of the solvent and subsequent addition of
diethylether yielded
(after 15 min ultrasonification) in a white slurry that is filtered and washed
with several portions of
diethylether. The colorless solid is dried in vacuo to yield 85 mg (55%) of
the title compound. There
are slight impurities of diethylether and diethylammonium citrate. The sesqui
citrate is elucidated by
NMR spectroscopy.
M.p.: sinter from 110 C on, melting 160-191 C (decomposition).
MS: m/z (MH+) = 520.0, 522Ø
8g. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (D)-tartrate
100 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) and 29 mg
of D-tartaric acid (1.0
eq) are suspended in 1 mL ethanol and heated at reflux for 2 minutes. The
mixture is stirred for 5
minutes without heating anymore. Evaporation of the solvent and subsequent
addition of diethylether
yielded (after 20 min ultrasonification) in a white slurry that is filtered
and washed with several portions
of diethylether. The colorless solid is dried in vacuo to yield 98 mg (76%) of
the title compound. There
are slight impurities of diethylether and diethylammonium tartrate. The mono
tartrate is elucidated by
NMR spectroscopy.
M.p.: foaming at 100 C, melting 162-176 C (decomposition).
MS: m/z (MH+) = 519.9, 522Ø
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 42 -
8h. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide mesylate
100 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) are
suspended in 1 mL ethanol
and heated at reflux until the amine is completely dissolved. 12.5 pL methane
sulfonic acid (1.0 eq)
are added and the mixture is stirred for 5 minutes without heating anymore.
Evaporation of the solvent
and subsequent addition of diethylether yielded in a white slurry (after 15
min ultrasonification) that is
filtered and washed with several portions of diethylether. The colorless solid
is dried in vacuo to yield
63 mg (53%) of the title compound. There are slight impurities of diethylether
and diethylammonium
mesylate. The mono mesylate is elucidated by NMR spectroscopy.
M.p.: melting 150-214 C (decomposition).
MS: m/z (MH+) = 520.0, 522Ø
8i. N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide benzoate
50 mg of (-)-N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8b) are
suspended in 1 mL ethanol
and heated at reflux until the amine is completely dissolved. 11.8 mg benzoic
acid (1.0 eq) are added
and the mixture is stirred for 5 minutes without heating anymore. Evaporation
of the solvent and
subsequent addition of diethylether yielded in a white slurry (after 10 min
ultrasonification) that is
filtered and washed with several portions of diethylether. The colorless solid
is dried in vacuo to yield
29 mg (47%) of the title compound. There are slight impurities of diethylether
and diethylammonium
benzoate. The mono benzoate is elucidated by NMR spectroscopy.
M.p.: sinter from 145 C, melting 160-175 C (decomposition).
MS: m/z (MH+) = 520.0, 522Ø
8j. N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide mesylate
60 mg of (-)-N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8a) are
suspended in 1 mL ethanol
and heated at reflux until the amine is completely dissolved. 7.5 pL methane
sulfonic acid (1.0 eq) are
added and the mixture is stirred for 5 minutes without heating anymore.
Evaporation of the solvent
and subsequent addition of diethylether yielded in a white slurry (after 10
min ultrasonification) that is
filtered and washed with several portions of diethylether. The colorless solid
is dried in vacuo to yield
63 mg (89%) of the title compound. The mono mesylate is elucidated by NMR
spectroscopy.
M.p.: foaming at 125 C, melting 190-198 C (decomposition).
MS: m/z (MH+) = 519.9, 521.9.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-43-
8k. N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide fumarate
59 mg of (-)-N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8a) and 13.2 mg
of fumaric acid (1.0
eq) are suspended in 1 mL ethanol and heated at reflux for 2 minutes. The
mixture is stirred for 5
minutes without heating anymore. Evaporation of the solvent and subsequent
addition of diethylether
yielded in a white slurry that is filtered and washed with several portions of
diethylether. The colorless
solid is dried in vacuo to yield 73 mg (quant.) of the title compound. The
mono fumarate is elucidated
by NMR spectroscopy.
M.p.: sinter from 165 C on, melting 190-200 C (decomposition).
MS: m/z (MH+) = 520.0, 522Ø
81. N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide tosylate
58 mg of (-)-N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (example 8a) and 21.2 mg
of p-toluenesulfonic
acid monohydrate (1.0 eq) are suspended in 1 mL ethanol and heated at reflux
for 2 minutes. The
mixture is stirred for another 5 minutes without heating anymore. Evaporation
of the solvent and
subsequent addition of diethylether yielded in a white slurry that is filtered
and washed with several
portions of diethylether. The colorless solid is dried in vacuo to yield 65 mg
(85%) of the title
compound. The mono tosylate is elucidated by NMR spectroscopy.
M.p.: sinter from 160 C on, melting 170-175 C (decomposition).
MS: m/z (MH+) = 519.9, 522Ø
9. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chIoro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-propyl]-4-methyl-benzamide hydrochloride
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester (5.00 g, 8.2
mmol) is treated with
HCI/diethylether (4N, 100 mL), stirred for 1h at ambient temperature, the
solvents are evaporated and
the residue is washed with diethylether. 3.90 g (87 %) of the title compuond
are obtained as colorless
solid with m.p. 201-203 C.
MS: m/z (MH+) = 506.0, 508.1.
Separation of enantiomers:
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-44-
N-(3-Amino-propyl)-N-[(R)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-4-methyl-benzamide hydrochloride and
N-(3-Amino-propyl)-N-[(S)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-4-methyl-benzamide hydrochloride
The separation of the enantiomers of racemic N-(3-amino-propyl)-N-[(RS)-1-(6-
benzyl-3-chloro-2-
methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl)-propyl]-4-methyl-
benzamide hydrochloride is
performed by preparative chromatography: 250 x 20 mm CHIRALPAK AD-H 5pM, 0.7
ml/min of
ethanol + 0.1% diethylamine at 40 C:
The enantiomers elute at 6.59 and 15.38 min. Both enantiomers are isolated by
mixing with 1 N HCI in
diethylether and stirring in this mixture. The mixture is evaporated and the
residue is dissolved in a
mixture of water and acetonitrile and lyophilized.
The first eluting enantiomer (6.59 min, example 9a) has a MH+ of 506.1 (ee =
97.9%) and at Na
589nm at 20 C in CHCI3 (0.4535 g/100ml) [a]p20 = + 226 . Thus,
enantiomerically pure (+)-N-(3-
Amino-propyl)-N-[1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-methyl-benzamide hydrochloride has [a]p20 = + 231 (c=0.4535,
CHCI3).
The second eluting enantiomer (15.38 min, example 9b) has a MH+ of 506.0 (ee =
99.7%).
The absolute stereochemistry of the (+)-isomer (9a) is tentatively assigned to
the enantiomer with the
R-configuration. Consequently, the S-configuration is assigned to the (-)-
enantiomer (9b).
10. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-trifluoromethyl-benzamide
trifluoroacetate
81 mg of (3-{[(RS)-1-6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(4-trifluoromethyl-phenyl)-carbonyl]-amino}-propyl)-carbamic
acid tert-butyl ester
(compound A10) are dissolved in 4 mL dichloromethane and 1.5 mL
trifluoroacetic acid. After stirring
this mixture for 2 hours at ambient temperature the solvent is removed.
Addition of diethylether results
in a white slurry that is filtered. The precipitate is washed with
diethylether and dried in vacuo. By this
method 64 mg (78%) of the title compound are obtained as colorless solid.
M.p.: 218 C.
MS: m/z (MH+) = 574.0, 575.9.
Using similar procedures to those described herein above but with suitable
choice of the starting
materials, which are mentioned below, the following compounds of formula I
(especially in form of
their hydrochloride salts) may be prepared:
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-3-methyl-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-fluoro-benzamide
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-45-
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-methoxy-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-bromo-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-chloro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-4-trifluoromethyl-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-3-methyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-fluoro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-methoxy-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-bromo-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-chloro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-trifl uoromethyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-3-methyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-fluoro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-methoxy-benzam ide
N-(3-Am ino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-bromo-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-chloro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-propyl]-4-trifl uoromethyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-4-methyl-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-3-methyl-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-4-fluoro-benzamide
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-46-
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-4-methoxy-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-4-bromo-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-4-chloro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-4-trifluoromethyl-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-4-methyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-3-methyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-4-fluoro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-4-methoxy-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-4-bromo-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-4-chloro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-propyl]-4-trifl uoromethyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-4-methyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-3-methyl-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-4-fluoro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-4-methoxy-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-4-bromo-benzam ide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-4-chloro-benzamide
N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-
5-yl )-2-methyl-butyl]-4-trifl uoromethyl-benzam ide
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-47-
11. N-[(RS)-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
methyl-propyl]-N-(3-dimethylamino-propyl)-4-methyl-benzamide
A solution of 89 mg (1.0 eq) 6-Benzyl-3-chloro-5-[(RS)-1-(3-dimethylamino-
propylamino)-2-methyl-
propyl]-2-methyl-6H-pyrazolo[1,5-c]pyrimidin-7-one (compound A11), 87 L (3.0
eq) triethylamine and
33 L (1.2 eq) 4-methylbenzoyl chloride in 2 mL dichloromethane is stirred for
16 hours at ambient
temperature. The product is extracted between dichloromethane and a saturated
NaHCO3-solution,
the organic phase is dried over magnesium sulfate and the crude product is
purified by preparative
HPLC. 20 mg (17%) of the title compound is isolated by means of
lyophilization.
M.p.: sinter at 71-72 C and melting at 88-91 C.
MS: m/z (MH+) = 548.1, 550.2.
Using similar procedures to those described for Example 11 but with suitable
choice of the starting
materials, which are mentioned below, and the appropriate benzoic acid
derivatives the following
compounds of formula I may be prepared:
N-[(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-methyl-propyl]-N-(3-
dimethylamino-propyl)-3-methyl-benzamide
N-[(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-methyl-propyl]-N-(3-
dimethylamino-propyl)-4-fluoro-benzamide
N-[(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-methyl-propyl]-N-(3-
dimethylamino-propyl)-4-methoxy-benzamide
N-[(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-methyl-propyl]-N-(3-
dimethylamino-propyl)-4-bromo-benzamide
N-[(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-methyl-propyl]-N-(3-
dimethylamino-propyl)-4-chloro-benzamide
N-[(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-methyl-propyl]-N-(3-
dimethylamino-propyl)-4-trifluoromethyl-benzamide
12. N-(4-Amino-butyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide trifluoroacetate
126 mg of {4-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-butyl}-carbamic acid tert-butyl
ester (compound A12) are
dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic acid. After
stirring this mixture for 2 hours
at ambient temperature the solvent is removed. Addition of diethylether
results in a white slurry that is
filtered. The precipitate is washed with diethylether and dried in vacuo. By
this method 85 mg (66%) of
the title compound are obtained as colorless foam.
M.p.: 179-182 C.
MS: m/z (MH+) = 534.1, 536.1.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 48 -
13. N-(2-Amino-ethyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide
164 mg of {2-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-ethyl}-carbamic acid tert-butyl
ester (compound A13) are
dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic acid. After
stirring this mixture for 1 hour
at ambient temperature the solvent is removed. Addition of dichloromethane and
basification with
saturated aqueous NaHCO3-solution followed by extraction of the aqueous phase
with
dichloromethane results after drying over magnesium sulfate and evaporation of
the solvent in 145 mg
(quant.) of the title compound.
M.p.: 119-120 C.
MS: m/z (MH+) = 506.1, 507.9.
14. N-(2-Amino-ethyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide trifluoroacetate
125 mg of {2-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-ethyl}-carbamic acid tert-butyl
ester (compound A13) are
dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic acid. After
stirring this mixture for 2 hours
at ambient temperature the solvent is removed. Addition of diethylether
results in a white slurry that is
filtered. The precipitate is washed with diethylether and dried in vacuo. By
this method 70 mg (55%) of
the title compound are obtained as colorless solid.
M.p.: 173-174 C.
MS: m/z (MH+) = 506.0, 508Ø
15. N-[(RS)-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
methyl-propyl]-N-(2-dimethylamino-ethyl)-4-methyl-benzamide
A mixture of 70 mg N-(2-Amino-ethyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-
oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide (compound
13), 33 L formalin
and 52 L formic acid is heated for 8 hours at 100 C. After cooling, the
crude product is purified by
silica gel flash chromatography using a gradient of dichloromethane and
methanol from 100:0 to
90:10. This yielded 35 mg (46%) of the title compound as colorless solid.
M.p.: 94-95 C.
MS: m/z (MH+) = 534.0, 536Ø
16. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo
[1,5-c]pyrimidin-5-yl)-1-cyclobutyl-methyl]-4-methyl-benzamide
trifluoroacetate
159 mg of {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-1-
cyclobutyl-methyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-
butyl ester (compound A14)
are dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic acid. After
stirring this mixture for 1
hour at ambient temperature the solvent is removed. Addition of diethylether
results in a white slurry
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 49 -
that is filtered. The precipitate is washed with diethylether and dried in
vacuo. By this method 140 mg
(87%) of the title compound are obtained as colorless solid.
M.p.: sinter at 158-179 C and melting at 180-185 C.
MS: m/z (MH+) = 532.0, 534.1.
17. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-butyl]-4-methyl-benzamide
trifluoroacetate
60 mg of {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-butyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester (compound A15) are
dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic acid. After
stirring this mixture for 1.5
hours at ambient temperature the solvent is removed. Addition of diethylether
results in a white slurry
that is filtered. The precipitate is washed with diethylether and dried in
vacuo. By this method 43 mg
(70%) of the title compound are obtained as colorless solid.
M.p.: 179-182 C.
MS: m/z (MH+) = 534.0, 535.8.
18. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-1-cyclopropyl-methyl]-4-methyl-benzamide
hydrochloride
80 mg of {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-1-
cyclopropyl-methyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-
butyl ester (compound A16)
dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic acid. After
stirring this mixture for 2 hours
at ambient temperature the solvent is removed. The residue is dissolved in
dichloromethane and
treated with saturated aqueous NaHCO3-solution. Extraction of the aqueous
layer followed by drying
over MgSO4 results in the free base of the title compound. The solvent is
evaporated and the residue
is treated with 1M HCI in diethylether. The precipitate is filtered, washed
with diethylether and dried in
vacuo. This afforded 18 mg (26%) of the title compound as colorless solid.
M.p.: 156-158 C.
MS: m/z (MH+) = 518.0, 520.1.
19. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-1-cyclopropyl-methyl]-4-bromo-benzamide
trifluoroacetate
34 mg of (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-1-
cyclopropyl-methyl]-[1-(4-bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
(compound A17) are dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic
acid. After stirring
this mixture for 2 hours at ambient temperature the solvent is removed.
Addition of diethylether results
in a white slurry that is filtered. The precipitate is washed with
diethylether and dried in vacuo. By this
method 21 mg (60%) of the title compound are obtained as colorless solid.
M.p.: 178-180 C (decomposition).
MS: m/z (MH+) = 581.9, and also MH++2 and MH++4.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-50-
20. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-3-fluoro-4-methyl-benzamide
trifluoroacetate
104 mg of (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(3-fluoro-4-methyl-phenyl)-carbonyl]-amino}-propyl)-carbamic
acid tert-butyl ester
(compound A18) are dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic
acid. After stirring
this mixture for 1.5 hours at ambient temperature the solvent is removed.
Addition of diethylether
results in a white slurry that is filtered. The precipitate is washed with
diethylether and dried in vacuo.
By this method 61 mg (57%) of the title compound are obtained as pale yellow
solid.
M.p.: 140-158 C (decomposition).
MS: m/z (MH+) = 537.9, 540Ø
21. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-2-fluoro-4-methyl-benzamide
trifluoroacetate
83 mg of (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(2-fluoro-4-methyl-phenyl)-carbonyl]-amino}-propyl)-carbamic
acid tert-butyl ester
(compound A19) are dissolved in 4 mL dichloromethane and 1 mL trifluoroacetic
acid. After stirring
this mixture for 2 hours at ambient temperature the solvent is removed.
Addition of diethylether results
in a white slurry that is filtered. The precipitate is washed with
diethylether and dried in vacuo. By this
method 39 mg (46%) of the title compound are obtained as colorless powder.
M.p.: sinter 178-181 C and melting at 182-192 C.
MS: m/z (MH+) = 538.0, 539.9.
22. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-3,4-dichloro-benzamide
trifluoroacetate
88 mg of (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(3,4-dichloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
(compound A20) are dissolved in 2 mL dichloromethane and 0.5 mL
trifluoroacetic acid. After stirring
this mixture for 1 hour at ambient temperature the solvent is removed.
Addition of diethylether results
in a white slurry that is filtered. The precipitate is washed with
diethylether and dried in vacuo. By this
method 37 mg (41 %) of the title compound are obtained as colorless solid.
M.p.: 184-185 C.
MS: m/z (MH+) = 573.8, 575.9, 577.8.
23. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-2,3-dichloro-benzamide
trifluoroacetate
67 mg of (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-[1-(2,3-dichloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
(compound A21) are dissolved in 2 mL dichloromethane and 0.5 mL
trifluoroacetic acid. After stirring
this mixture for 1 hour at ambient temperature the solvent is removed.
Addition of diethylether results
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-51 -
in a white slurry that is filtered. The precipitate is washed with
diethylether and dried in vacuo. By this
method 31 mg (46%) of the title compound are obtained as colorless solid.
M.p.: 187-188 C.
MS: m/z (MH+) = 573.9, 576.0, 577.8.
24. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide
hydrochloride
87 mg of {3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-
methyl-propyl]-(4-methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl
ester (compound A22) are
dissolved in 4 M HCI in dioxane. After stirring this mixture for 3 hour at
ambient temperature the
solvent is removed. By this method 78 mg (quant.) of the title compound are
obtained as colorless
solid.
MS: m/z (MH+) = 563.9, 566Ø
25. N-(3-Amino-propyl)-N-[(RS)-1-(6-benzyl-3-fluoro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-methyl-propyl]-4-methyl-benzamide
hydrochloride
155 mg of {3-[[(RS)-1-(6-Benzyl-3-fluoro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester (compound A24) are
dissolved in 2 mL dioxane and treated with 4 M HCI in dioxane (3 mL). After
stirring this mixture for 3
hour at ambient temperature the solvent is removed. By this method 80 mg (57%)
of the title
compound are obtained as colorless solid.
MS: m/z (MH+) = 504Ø
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-52-
Starting materials:
Some of the compounds in this section have an optionally substituted benzoyl
group, which may be
designated in the systematic name as phenyl-carbonyl group or alternatively,
as a phenyl-methanoyl
group. A special substituted phenyl group in this connection is the m-tolyl
group (3-methyl-phenyl) or
the p-tolyl group (4-methyl-phenyl).
Al. {3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-
5-yl)-propyl]-
(4-methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
393 mg {3-[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propylamino]-
propyl}-carbamic acid tert-butyl ester (0.87 mmol) (compound B1) are solved in
5 ml dichlormethane,
afterwards 0.37 ml triethylamine (2.61 mmol) and 161 mg 4-Methyl-benzoyl
chloride (1.04 mmol) are
added and the reaction mixture is stirred 4 h at ambient temperature. The
product is extracted
between CHzCl2 and saturated NaHCO3-solution, the organic phase is dried over
sodium sulphate and
the crude product is purified by silica gel flash chromatography. By this
method 377 mg (77%) of a
colorless solid are obtained.
A2. {3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-(4-methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
220 mg {3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(1-p-
tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester (0.38 mmol)
(compound A1) are solved in
2ml dichloromethane. 70 mg N-Bromo-succinimide (0.39 mmol) are added and the
reaction mixture is
stirred 4 h at ambient temperature. The product is extracted between CHzCl2
and saturated NaHCO3-
solution. The organic layer is dried over sodium sulfate. The solvents are
evaporated and the crude
product is purified by silica gel flash chromatography. By this method 170 mg
( 69 %) of a colorless
solid are obtained.
M.p.: 95-97 C.
A3. {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-(1-m-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester
0.300 g (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester (compound
B3), 0.095 mL (1.2 eq) m-
toluoyl chloride and 0.248 mL (3.0 eq) triethylamine are dissolved in 3 mL
dichloromethane and stirred
for 48 hours at ambient temperature. After addition of 10 mL dichloromethane
and 10 mL saturated
aqueous sodium hydrogen carbonate solution the phases are separated and the
aqueous phase is
extracted with dichloromethane. The combined organic phase is dried over
magnesium sulfate and the
solvent is evaporated in vacuo. Purification of the crude product by silica
gel flash chromatography
using n-hexane and ethyl acetate in a mixture of 3:2 povides a mixture of
starting material and the title
compound. A second silica gel flash chromatography using a gradient of n-
hexane, ethyl acetate and
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-53-
acetic acid from 39:59:2 to 0:100:0 yields 0.151 g (41%) of the title compound
as a white solid.
Furthermore 0.105 g (35%) of the starting material is recovered.
M.p. 83.9 C.
MS: m/z (MH+) = 619.9, 621.9.
A4. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(4-fluoro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
0.300 g (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester (compound
B3), 0.085 mL (1.2 eq) 4-
fluorobenzoyl chloride and 0.248 mL (3.0 eq) triethylamine are dissolved in 3
mL dichloromethane and
stirred for 48 hours at ambient temperature. After addition of 10 mL
dichloromethane and 10 mL
saturated aqueous sodium hydrogen carbonate solution the phases are separated
and the aqueous
phase is extracted with dichloromethane. The combined organic phase is dried
over magnesium
sulfate and the solvent is evaporated in vacuo. Purification of the crude
product by silica gel flash
chromatography using gradient of n-hexane, ethyl acetate and acetic acid from
59:39:2 to 0:100:0
provides the title compound with some impurities and the pure starting
material (0.190 g, 63%). A
second silica gel flash chromatography using a gradient of n-hexane and ethyl
acetate from 100:0 to
50:50 yields 0.096 g (26%) of the title compound as a white solid.
M.p. 143.0 C (foam).
MS: m/z (MH+) = 623.9, 625.9.
A5. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-S-yl)-
2-methyl-propyl]-[1-(4-methoxy-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
0.300 g (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester (compound
B3), 0.122 g (1.2 eq) p-
anisoyl chloride and 0.248 mL (3.0 eq) triethylamine are dissolved in 3 mL
dichloromethane and
stirred for 48 hours at ambient temperature. After addition of 10 mL
dichloromethane and 10 mL
saturated aqueous sodium hydrogen carbonate solution the phases are separated
and the aqueous
phase is extracted with dichloromethane. The combined organic phase is dried
over magnesium
sulfate and the solvent is evaporated in vacuo. Purification of the crude
product by silica gel flash
chromatography using of n-hexane, ethyl acetate and acetic acid in a mixture
of 39:59:2 yields 0.018 g
(5%) of the title compound as a white solid.
M.p. 115.7 C (decomposition).
MS: m/z (MH+) = 635.9, 637.9.
A6. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-S-yl)-
2-methyl-propyl]-[1-(4-bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
0.300 g (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester (compound
B3), 0.158 mg (1.2 eq) p-
bromobenzoyl chloride and 0.248 mL (3.0 eq) triethylamine are dissolved in 3
mL dichloromethane
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-54-
and stirred for 48 hours at ambient temperature. After addition of 10 mL
dichloromethane and 10 mL
saturated aqueous sodium hydrogen carbonate solution the phases are separated
and the aqueous
phase is extracted with dichloromethane. The combined organic phase is dried
over magnesium
sulfate and the solvent is evaporated in vacuo. Purification of the crude
product by silica gel flash
chromatography using n-hexane and ethyl acetate in a mixture of 3:2 povides a
mixture of starting
material and the title compound. A second silica gel flash chromatography
using a gradient of n-
hexane, ethyl acetate and acetic acid from 39:59:2 to 0:100:0 yields 0.159 g
(39%) of the title
compound as a white solid. Furthermore 0.180 g (60%) of the starting material
are recovered.
M.p. 96 C (foam).
MS: m/z (MH+) = 683.8, 685.8, 687.8.
A7. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(4-chloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
0.300 g (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester (compound
B3), 0.092 mL (1.2 eq) 4-
chlorobenzoyl chloride and 0.248 mL (3.0 eq) triethylamine are dissolved in 3
mL dichloromethane
and stirred for 48 hours at ambient temperature. After addition of 10 mL
dichloromethane and 10 mL
saturated aqueous sodium hydrogen carbonate solution the phases are separated
and the aqueous
phase is extracted with dichloromethane. The combined organic phase is dried
over magnesium
sulfate and the solvent is evaporated in vacuo. Purification of the crude
product by silica gel flash
chromatography using n-hexane and ethyl acetate in a mixture of 3:2 povides a
mixture of starting
material and the title compound. A second silica gel flash chromatography
using a gradient of n-
hexane, ethyl acetate and acetic acid from 39:59:2 to 0:100:0 yields 0.160 g
(42%) of the title
compound as a white solid. Furthermore 0.170 g (57%) of the starting material
is recovered.
M.p. 93 C (foam).
MS: m/z (MH+) = 639.9, 641.8, MH++4.
A8. {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester
0.405 g (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester (compound
B3), 0.336 mL (1.2 eq) 4-
methylbenzoyl chloride and 0.128 mL (3.0 eq) triethylamine are dissolved in 4
mL dichloromethane
and stirred for 16 hours at ambient temperature. After addition of 10 mL
dichloromethane and 10 mL
saturated aqueous sodium hydrogen carbonate solution the phases are separated
and the aqueous
phase is extracted with dichloromethane. The combined organic phase is dried
over magnesium
sulfate and the solvent is evaporated in vacuo. Purification of the crude
product by silica gel flash
chromatography using a gradient of n-hexane, ethyl acetate and acetic acid
from 39:59:2 to 0:100:0
yield 0.145 g (29%) of the title compound as a white solid. Furthermore 0.259
g (64%) of the starting
material are recovered.
M.p. 92-94 C, sinter 82 C.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-55-
MS: m/z (MH+) = 619.9, MH++2.
A9. {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propyl]-(4-methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propylamino]-
propyl}-carbamic acid tert-butyl ester (4.00 g, 8.2 mmol) (compound B2) is
dissolved in
dichlormethane (40 mL), treated with triethylamine (3.6 mL, 24.6 mmol) and 4-
methylbenzoyl chloride
(1.30 mL, 9.8 mmol) and stirred 15 h at ambient temperature. It is diluted
with dichlormethane,
washed with aqueous NaHCO3-solution and the organic phase is dried. After
evaporation, the residue
is purified by chromatography. 4.5 g (90 %) of a colorless solid of m.p. 80-82
C is obtained.
A10. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(4-trifluoromethyl-phenyl)-carbonyl]-amino}-propyl)-
carbamic acid tert-
butyl ester
A solution of 200 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
(compound B3), 88 L
(1.5 eq) 4-(trifluoromethyl)-benzoyl chloride with catalytic amount of DMAP in
10 mL pyridine is
heated for 16 hours at 70 C. Evaporation of the pyridine followed by two
times coevaporation with
toluene results in the crude product. This material is purified by silica gel
flash chromatography using
a gradient of n-hexane and ethyl acetate from 100:0 to 50:50. By this method
102 mg (38%) of the title
compound are obtained as a colorless solid.
M.p.: 106-108 C.
MS: m/z (MH+) = 673.9, 675.8.
The following compounds may be prepared by benzoylation reaction of compound
B1 with the
appropriate benzoic acid derivative analogously or similarly as described for
compounds Al and A3 to
A9:
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(3-methyl-
benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(4-fluoro-
benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(4-methoxy-
benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(4-bromo-
benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(4-chloro-
benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-propyl]-(4-
trifluoromethyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-56-
The following compounds may be prepared by bromination of the appropriate
compounds mentioned
afore analogously or similarly as described for compound A2:
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(3-
methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
fluoro-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
methoxy-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
bromo-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
chloro-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
trifluoromethyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
The following compounds may be prepared by benzoylation reaction of compound
B2 with the
appropriate benzoic acid derivative analogously or similarly as described for
compounds Al and A3 to
A9:
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(3-
methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
fluoro-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
methoxy-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
bromo-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
chloro-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-propyl]-(4-
trifluoromethyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl ester
The following compounds may be prepared by benzoylation reaction of compound
B4 with the
appropriate benzoic acid derivative analogously or similarly as described for
compounds Al and A3 to
A9:
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-2-methyl-propyl]-(1-p-
tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-2-methyl-propyl]-(1-m-
tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester
(3-{[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-propyl]-[1-(4-
fluoro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl ester
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-57-
(3-{[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-2-methyl-propyl]-[1-(4-
methoxy-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl ester
(3-{[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-2-methyl-propyl]-[1-(4-
bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl ester
(3-{[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-2-methyl-propyl]-[1-(4-
chloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl ester
(3-{[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-yl
)-2-methyl-propyl]-[1-(4-
trifluoromethyl-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
The following compounds may be prepared by bromination of the appropriate
compounds mentioned
afore analogously or similarly as described for compound A2:
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propyl]-(1-m-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester
(3-{[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propyl]-[1-(4-fluoro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
(3-{[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propyl]-[1-(4-methoxy-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-
butyl ester
(3-{[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propyl]-[1-(4-bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
(3-{[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propyl]-[1-(4-chloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
(3-{[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl )-2-methyl-
propyl]-[1-(4-trifluoromethyl-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
The following compounds may be prepared by benzoylation reaction of compound
B5 with the
appropriate benzoic acid derivative analogously or similarly as described for
compounds Al and A3 to
A9:
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester
{3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-(1-m-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl ester
(3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-[1-(4-fluoro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
(3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-[1-(4-methoxy-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
(3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-[1-(4-bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-58-
(3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-[1-(4-chloro-phenyl)-carbonyl]-amino}-propyl)-carbamic acid tert-butyl
ester
(3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
butyl]-[1-(4-trifluoromethyl-phenyl)-carbonyl]-amino}-propyl)-carbamic acid
tert-butyl ester
All. 6-Benzyl-3-chloro-5-[(RS)-1-(3-dimethylamino-propylamino)-2-methyl-
propyl]-2-methyl-
6H-pyrazolo[1,5-c]pyrimidin-7-one
A suspension of 544 mg (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-
propanoyl)-6H-
pyrazolo[1,5-c]pyrimidin-7-one (compound C3) and 742 L N',N'-dimethylpropane-
1,3-diamine (2.5
eq) in 1.5 mL Ti(O'Pr)4 is heated for 16 hours at 50 C. This crude mixture
with the imine as main
product is dissolved in 5 mL glacial acetic acid and treated with 400 mg (6.6
eq) NaBH4. This mixture
is stirred for 16 hours at ambient temperature to give the crude mixture
containing the title compound.
The solution is treated with 2 M aqueous NaOH-solution to an pH of 12 and
extracted with 1-butanol.
Purification by silica gel flash chromatography using a gradient of
dichloromethane, methanol and
triethylamine from 89:9:2 to 79:19:2 resulted in 105 mg (15%) of the title
compound as a colorless oil.
MS: m/z (MH+) = 430.1, 432.2.
The following compounds may be prepared by reductive amination reaction of the
appropriate
compound Cl to C6 with N',N'-dimethyl-propane-1,3-diamine (Me2N-(CH2)3-NH2)
analogously or
similarly as described for compounds B1 to B3.
6-Benzyl-5-[(RS)-1-(3-dimethylamino-propylamino)-propyl]-2-methyl-6H-
pyrazolo[1,5-c]pyrimidin-7-
one
6-Benzyl-3-chloro-5-[(RS)-1-(3-d imethylamino-propylamino)-propyl]-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
6-Benzyl-5-[(RS)-1-(3-d imethylamino-propylamino)-2-methyl-propyl]-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
6-Benzyl-5-[(RS)-1-(3-d imethylamino-propylamino)-2-methyl-butyl]-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
6-Benzyl-3-chloro-5-[(RS)-1-(3-d imethylamino-propylamino)-2-methyl-butyl]-2-
methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-one
A12. {4-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-butyl}-carbamic acid tert-butyl
ester
A solution of 180 mg (1.0 eq) {4-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-butyl}-carbamic acid tert-butyl ester
(compound B6), 175 L
(1.5 eq) 4-methyl benzoyl chloride and 145 L (3.7 eq) triethylamine in 3.5 mL
dichloromethane is
stirred for 3 days at ambient temperature. After addition of 10 mL
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution, the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-59-
using a gradient of n-hexane and ethyl acetate from 100:0 to 50:50 yielded 127
mg (71%) of the title
compound as a colorless solid.
M.p.: 82-84 C.
MS: m/z (MNa+) = 656.2, and also MNa++2.
A13. {2-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-ethyl}-carbamic acid tert-butyl
ester
A solution of 200 mg (1.0 eq) {2-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-ethyl}-carbamic acid tert-butyl ester
(compound B7), 205 L
(3.8 eq) 4-methyl benzoyl chloride and 171 L (3.0 eq) triethylamine in 4 mL
dichloromethane is
stirred for 62 hours at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane, ethyl acetate and acetic acid from 39:59:2 to
0:100:0 yielded 125 mg
(50%) of the title compound as a colorless solid.
M.p.: 119-120 C.
MS: m/z (MH+) = 605.9, 608Ø
A14. {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
1-cyclobutyl-methyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-
butyl ester
A solution of 300 mg (1.0 eq) (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-1-cyclobutyl-methyl]-amino}-propyl)-carbamic acid tert-butyl
ester (compound B8),
115 L (1.5 eq) 4-methyl benzoyl chloride and 241 L (3.0 eq) triethylamine in
6 mL dichloromethane
is stirred for 2 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane and ethyl acetate from 100:0 to 50:50 yielded 194
mg (53%) of the title
compound as a colorless foam. Furthermore 76 mg (25%) of the starting material
is recovered.
M.p.: 108-111 C.
MS: m/z (MH+) = 631.8, 633.8.
A15. {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-butyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester
A solution of 260 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-
pyrazolo[1,5c]pyrimidin-5-yl)-2-methyl-butylamino]-propyl}-carbamic acid tert-
butyl ester (compound
B5), 100 L (1.5 eq) 4-methyl benzoyl chloride and 208 L (3.0 eq)
triethylamine in 5 mL
dichloromethane is stirred for 16 hours at ambient temperature. After addition
of 10 mL of
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-60-
dichloromethane and 10 mL saturated aqueous NaHCO3-solution the phases are
separated and the
aqueous phase is extracted three times with dichloromethane. The combined
organic phase is dried
over magnesium sulfate and the solvent is evaporated in vacuo. Purification of
the crude product by
silica gel flash chromatography using a gradient of n-hexane, ethyl acetate
and acetic acid from
39:59:2 to 0:100:0 yielded 81 mg (26%) of the title compound as a pale yellow
foam. Furthermore 147
mg (57%) of the starting material is recovered.
M.p.: 67-68 C.
MS: m/z (MH+) = 633.9, 635.8.
A16. {3-[[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
1-cyclopropyl-methyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-
butyl ester
A solution of 257 mg (1.0 eq) (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-1-cyclopropyl-methyl]-amino}-propyl)-carbamic acid tert-
butyl ester (compound B9),
135 L (2.0 eq) 4-methyl benzoyl chloride and 212 L (3.0 eq) triethylamine in
5 mL dichloromethane
is stirred for 3 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane, ethyl acetate and acetic acid from 39:59:2 to
0:100:0 yielded 90 mg
(29%) of the title compound as a colorless foam. Furthermore 73 mg (29%) of
the starting material is
recovered.
M.p.: 85-87 C.
MS: m/z (MH+) = 617.9, 619.9.
A17. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
1-cyclopropyl-methyl]-[1-(4-bromo-phenyl)-carbonyl]-amino}-propyl)-carbamic
acid tert-butyl
ester
A solution of 196 mg (1.0 eq) (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-1-cyclopropyl-methyl]-amino}-propyl)-carbamic acid tert-
butyl ester (compound B9),
105 mg (1.2 eq) 4-bromo benzoyl chloride and 166 L (3.0 eq) triethylamine in
4 mL dichloromethane
is stirred for 3 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane and ethyl acetate from 100:0 to 50:50 yielded 50
mg (18%) of the title
compound as a colorless foam.
M.p.: 120-122 C.
MS: m/z (MH+) = 681.8, 683.6, 685.4.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-61 -
A18. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(3-fluoro-4-methyl-phenyl)-carbonyl]-amino}-propyl)-
carbamic acid tert-
butyl ester
A solution of 330 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
(compound B3), 171 mg
(1.5 eq) 3-fluoro-4-methyl benzoyl chloride and 274 L (3.0 eq) triethylamine
in 8 mL dichloromethane
is stirred for 3 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane, ethyl acetate and acetic acid from 59:39:2 to
0:100:0 yielded 115 mg
(27%) of the title compound as a pale yellow foam. Furthermore 122 mg (37%) of
the starting material
is recovered.
M.p.: 88-91 C.
MS: m/z (MH+) = 637.8, 639.8.
A19. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(2-fluoro-4-methyl-phenyl)-carbonyl]-amino}-propyl)-
carbamic acid tert-
butyl ester
A solution of 277 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
(compound B3), 142 mg
(1.5 eq) 2-fluoro-4-methyl benzoyl chloride and 229 L (3.0 eq) triethylamine
in 7 mL dichloromethane
is stirred for 2 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane, ethyl acetate and acetic acid from 59:39:2 to
0:100:0 yielded 100 mg
(28%) of the title compound as a colorless foam. Furthermore 100 mg (36%) of
the starting material is
recovered.
M.p.: sinter at 100 C and melting at 110-112 C.
MS: m/z (MH+) = 637.9, 639.9.
A20. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(3,4-dichloro-phenyl)-carbonyl]-amino}-propyl)-carbamic
acid tert-butyl
ester
A solution of 259 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
(compound B3), 129 mg
(1.2 eq) 3,4-dichloro benzoyl chloride and 214 L (3.0 eq) triethylamine in 6
mL dichloromethane is
stirred for 3 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-62-
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane and ethyl acetate from 100:0 to 50:50 yielded 88
mg (22%) of the title
compound as a colorless foam.
M.p.: 104-107 C.
MS: m/z (MH+) = 673.6, 675.7, 677.8.
A21. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-[1-(2,3-dichloro-phenyl)-carbonyl]-amino}-propyl)-carbamic
acid tert-butyl
ester
A solution of 300 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
(compound B3), 143 mg
(1.2 eq) 2,3-dichloro benzoyl chloride and 249 L (3.0 eq) triethylamine in 7
mL dichloromethane is
stirred for 3 days at ambient temperature. After addition of 10 mL of
dichloromethane and 10 mL
saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is extracted
three times with dichloromethane. The combined organic phase is dried over
magnesium sulfate and
the solvent is evaporated in vacuo. Purification of the crude product by
silica gel flash chromatography
using a gradient of n-hexane, ethyl acetate and acetic acid from 39:59:2 to
0:100:0 yielded 67 mg
(17%) of the title compound as a colorless solid. Furthermore 164 mg (55%) of
the starting material is
recovered.
M.p.: 109-110 C.
MS: m/z (MH+) = 673.8, 676.0, 677.9.
A22. {3-[[(RS)-1-(6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-(4-methyl-benzoyl)-amino]-propyl}-carbamic acid tert-butyl
ester
0.10 g of {3-[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-
propylamino]-propyl}-carbamic acid tert-butyl ester (compound A23) are
dissolved in 3 mL DMF. After
adding 36.3 mg (1.2 eq) NBS at 0 C the mixture is stirred for 16 hours at
ambient temperature.
Addition of water followed by extraction of the aqueous phase with
dichloromethane yielded after
drying over magnesium sulfate and evaporation of the solvent the crude
product. The residue is
purified by flash chromatography using n-hexane / ethylacetate in a ratio of
2:1 and yielded 90 mg
(80%) of the title compound as colorless oil.
A23. {3-[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-
5-yl)-2-methyl-
propylamino]-propyl}-carbamic acid tert-butyl ester
A solution of 151 mg {3-[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidin-5-yl)-2-
methyl-propylamino]-propyl)-carbamic acid tert butyl ester (1.0 eq) (compound
B4), 55 mg (1.5 eq) p-
methyl benzoyl chloride and 132 L (3.0 eq) triethylamine in 5 mL
dichloromethane is stirred for 1
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-63-
days at ambient temperature and for 1 day at 40 C. After addition of 10 mL of
dichloromethane and
mL saturated aqueous NaHCO3-solution the phases are separated and the aqueous
phase is
extracted three times with dichloromethane. The combined organic phase is
dried over magnesium
sulfate and the solvent is evaporated in vacuo. Purification of the crude
product by silica gel flash
chromatography using a ratio of n-hexane, ethyl acetate and acetic acid
66:32:2 yielded 105 mg (80%)
of the title compound with impurities of acetic acid.
A24. {3-[[(RS)-1-(6-Benzyl-3-fluoro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propyl]-(1-p-tolyl-carbonyl)-amino]-propyl}-carbamic acid tert-butyl
ester
A solution of 155 mg (1.0 eq) {3-[(RS)-1-(6-Benzyl-3-fluoro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
(compound B10), 62 pL
(1.5 eq) p-tolyl benzoyl chloride and 137 L (3.0 eq) triethylamine in 6 mL
tetrahydrofurane is stirred
for 3 days at ambient temperature. The solvent is evaporated in vacuo.
Purification of the crude
product by silica gel flash chromatography using a ratio of n-hexane, ethyl
acetate and acetic acid
66:32:2 yielded 150 mg (78%) of the title compound with impurities of acetic
acid.
B1. {3-[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-
propylamino]-propyl}-carbamic acid tert-butyl ester
437 mg 6-Benzyl-2-methyl-5-propionyl-6H-pyrazolo[1,5-c]pyrimidin-7-one (1.48
mmol) (compound C1)
and 516 mg (3-Amino-propyl)-carbamic acid tert-butyl ester (2.96 mmol) are
dissolved in 4 ml THF.
1.9 ml Ti(O'Pr)4 (4.44 mmol) are added and the reaction mixture is stirred 14
h at ambient
temperature. Afterwards 4 ml ethanol and 412 mg NaCNBH3 (5.92 mmol) are added
and the mixture
is stirred 2 h at ambient temperature. After adding water the inorganic salts
are filtrated, washed with
ethanole and the filtrate is evaporated. The residue is suspended in
dichloromethane, dried over
sodium sulphate and filtrated. After removing the solvents the crude product
is purified by silica gel
flash chromatography. By this method 540 mg (80 %) of a colorless solid is
obtained.
M.p.: 72-74 C.
B2. {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
propylamino]-propyl}-carbamic acid tert-butyl ester
6-Benzyl-3-chloro-2-methyl-5-propionyl-6H-pyrazolo[1,5-c]pyrimidin-7-one (3.70
g, 11.2 mmol)
(compound C2) and N-Boc-1,3-diaminopropane (2.60 g, 14.6 mmol) are dissolved
in THF (28 mL),
treated with Ti(O'Pr)4 (6.40 g, 22.4 mmol) and stirred for 14h at ambient
temperature. Then ethanol
(28 mL) and NaCNBH3 (1.40 g, 22.4 mmol) are added and it is stirred for 2 h at
ambient temperature.
After adding of water it is filtered and the filtrate is evaporated. The
residue is purified by silica gel
flash chromatography. A colorless solid of m.p. 73-75 C is obtained.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-64-
B3. {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
1.10 g (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-propanoyl)-6H-
pyrazolo[1,5-c]pyrimidin-7-
one (compound C3) are dissolved in 15 ml tetrahydrofurane. 1.39 g (2.5 eq)
tert-butyl-N-(3-
aminopropyl)carbamate and 2.86 mL (3.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred for 72 hours at reflux. After cooling the mixture is treated with an
aqueous citric acid solution
and stirred for another 15 min at ambient temperature. Addition of
dichloromethane and filtration of
the mixture yield a two-phase system. The two phases are separated and the
aqueous phase is
extracted with dichloromethane. The combined organic phase is dried over
magnesium sulfate and the
solvent is removed in vacuo. This yields the crude mixture of the
corresponding imine.
This mixture of the crude imine is dissolved in 20 mL acetic acid. 0.61 g (5.0
eq) sodium borohydride
are added. The reaction mixture is stirred for 1 hour at ambient temperature.
After addition of water
the mixture is extracted with etyhl acetate. The combined organic phase is
once washed with
saturated aqueous sodium hydrogencarbonate solution. The organic phase is
dried over magnesium
sulfate and the solvent is evaporated in vacuo. The crude product is purified
by silica gel flash
chromatography using a gradient of n-hexane and ethyl acetate from 100:0 to
50:50. This yields 0.62
g (39%) of the title compound as a white foam.
M.p. 51-53 C (foam).
MS: m/z (MH+) = 501.9, 504Ø
B4. {3-[(RS)-1-(6-Benzyl-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidin-5-
yl)-2-methyl-
propylamino]-propyl}-carbamic acid tert-butyl ester
The title compound may be prepared by reductive amination reaction of compound
C4 with N-Boc-1,3-
diaminopropane analogously or similarly as described for compounds B1 to B3.
B5. {3-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-butylamino]-propyl}-carbamic acid tert-butyl ester
0.70 g(1.0 eq) of 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-butanoyl)-6H-
pyrazolo[1,5-c]pyrimidin-7-one
(compound C5) are dissolved in 40 mL tetrahydrofurane. 1.36 g (4.0 eq) tert-
butyl-N-(3-
aminopropyl)carbamate and 3.50 mL (6.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred in a sealed tube for 72 hours at reflux. After cooling, the mixture is
treated with an aqueous
solution of citric acid and stirred for another 15 min at ambient temperature.
Addition of
dichloromethane and filtration of the mixture yielded a two-phase system. The
phases are separated
and the aqueous phase is extracted with dichloromethane. The combined organic
phase is dried over
magnesium sulfate and the solvent is removed in vacuo. This yielded the crude
mixture of the
corresponding imine.
The mixture of the crude imine is dissolved in 12 mL acetic acid. 0.37 g (5.0
eq) sodium borohydride
are added and the mixture is stirred for 16 hours at ambient temperature. The
solvent is evaporated
and the residue is partitioned between saturated aqueous NaHCO3-solution and
dichloromethane.
After phase separation, the aqueous phase is extracted three times with
dichloromethane. The
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-65-
combined organic phase is dried over magnesium sulfate and the solvent is
evaporated in vacuo. The
crude product is purified by silica gel flash chromatography using a gradient
of n-hexane and ethyl
acetate from 100:0 to 50:50. This yielded 0.34 g (34%) of the title compound
as colorless foam.
M.p.: 64-65 C.
MS: m/z (MH+) = 516.0, 518Ø
B6. {4-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propylamino]-butyl}-carbamic acid tert-butyl ester
1.87 g (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-propanoyl)-6H-
pyrazolo[1,5-c]pyrimidin-7-
one (compound C3) are dissolved in 23 mL tetrahydrofurane. 2.56 g (2.5 eq)
tert-butyl-N-(3-
aminobutyl)carbamate and 4.86 mL (3.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred in a sealed tube for 72 hours at 80 C. After cooling, the mixture is
treated with an aqueous
solution of citric acid and stirred for another 15 min at ambient temperature.
Addition of
dichloromethane and filtration of the mixture yielded a two-phase system. The
phases are separated
and the aqueous phase is extracted with dichloromethane. The combined organic
phase is dried over
magnesium sulfate and the solvent is removed in vacuo. This yielded the crude
mixture of the
corresponding imine.
The mixture of the crude imine is dissolved in 35 mL acetic acid. 1.05 g (5.0
eq) sodium borohydride
are added and the mixture is stirred for 1 hour at ambient temperature. The
solvent is evaporated and
the residue is partitioned between saturated aqueous NaHCO3-solution and
dichloromethane. After
phase separation the aqueous phase is extracted three times with
dichloromethane. The combined
organic phase is dried over magnesium sulfate and the solvent is evaporated in
vacuo. The crude
product is purified by silica gel flash chromatography using a gradient of n-
hexane and ethyl acetate
from 100:0 to 50:50. This yielded 0.44 g(16%) of the title compound as
colorless foam.
M.p.: 63-65 C.
MS: m/z (MH+) = 516.0, 518Ø
B7. {2-[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propylamino]-ethyl}-carbamic acid tert-butyl ester
0.61 g (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-propanoyl)-6H-
pyrazolo[1,5-c]pyrimidin-7-
one (compound C3) are dissolved in 8 mL tetrahydrofurane. 0.71 g (2.5 eq) tert-
butyl-N-(3-
aminoethyl)carbamate and 1.58 mL (3.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred in a sealed tube for 72 hours at 80 C. After cooling, the mixture is
treated with an aqueous
solution of citric acid and stirred for another 15 min at ambient temperature.
Addition of
dichloromethane and filtration of the mixture yielded a two-phase system. The
phases are separated
and the aqueous phase is extracted with dichloromethane. The combined organic
phase is dried over
magnesium sulfate and the solvent is removed in vacuo. This yielded the crude
mixture of the
corresponding imine.
The mixture of the crude imine is dissolved in 11 mL acetic acid. 0.33 g (5.0
eq) sodium borohydride
are added and the mixture is stirred for 1 hour at ambient temperature. The
solvent is evaporated and
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-66-
the residue is partitioned between saturated aqueous NaHCO3-solution and
dichloromethane. After
phase separation, the aqueous phase is extracted three times with
dichloromethane. The combined
organic phase is dried over magnesium sulfate and the solvent is evaporated in
vacuo. The crude
product is purified by silica gel flash chromatography using a gradient of n-
hexane and ethyl acetate
from 100:0 to 50:50. This yielded 0.21 g (25%) of the title compound as
colorless foam.
M.p.: 73-75 C.
MS: m/z (MH+) = 487.9, 490Ø
B8. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
1-cyclobutyl-methyl]-amino}-propyl)-carbamic acid tert-butyl ester
0.63 g (1.0 eq) of 6-Benzyl-3-chloro-5-(1-cyclobutyl-carbonyl)-2-methyl-6H-
pyrazolo[1,5-c]pyrimidin-7-
one (compound C6) are dissolved in 4 mL tetrahydrofurane. 0.54 g (2.5 eq) tert-
butyl-N-(3-
aminopropyl)carbamate and 1.10 mL (3.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred in a sealed tube for 72 hours at 80 C. After cooling, the mixture is
treated with an aqueous
solution of citric acid and stirred for another 15 min at ambient temperature.
Addition of
dichloromethane and filtration of the mixture yielded a two-phase system. The
phases are separated
and the aqueous phase is extracted with dichloromethane. The combined organic
phase is dried over
magnesium sulfate and the solvent is removed in vacuo. This yielded the crude
mixture of the
corresponding imine.
This mixture of the crude imine is dissolved in 8 mL acetic acid. 0.23 g (5.0
eq) sodium borohydride is
added and the mixture is stirred for 1 hour at ambient temperature. The
solvent is evaporated and the
residue is partitioned between saturated aqueous NaHCO3-solution and
dichloromethane. After phase
separation, the aqueous phase is extracted three times with dichloromethane.
The combined organic
phase is dried over magnesium sulfate and the solvent is evaporated in vacuo.
The crude product is
purified by silica gel flash chromatography using a gradient of n-hexane and
ethyl acetate from 100:0
to 50:50. This yielded 0.50 g (79%) of the title compound as colorless foam.
M.p.: 73-75 C.
MS: m/z (MH+) = 514.0, 516Ø
B9. (3-{[(RS)-1-(6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
1-cyclopropyl-methyl]-amino}-propyl)-carbamic acid tert-butyl ester
1.81 g (1.0 eq) of 6-Benzyl-3-chloro-5-(1-cyclopropyl-carbonyl)-2-methyl-6H-
pyrazolo[1,5-c]pyrimidin-
7-one (compound C7) are dissolved in 20 mL tetrahydrofurane. 2.31 g (2.5 eq)
tert-butyl-N-(3-
aminopropyl)carbamate and 4.70 mL (3.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred in a sealed tube for 5 days at 80 C. After cooling, the mixture is
treated with an aqueous
solution of citric acid and stirred for another 15 min at ambient temperature.
Addition of
dichloromethane and filtration of the mixture yielded a two-phase system. The
phases are separated
and the aqueous phase is extracted with dichloromethane. The combined organic
phase is dried over
magnesium sulfate and the solvent is removed in vacuo. This yielded the crude
mixture of the
corresponding imine.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-67-
This mixture of the crude imine is dissolved in 30 mL acetic acid. 1.0 g (5.0
eq) sodium borohydride
are added and the mixture is stirred for 2 hours at ambient temperature. The
sovent is evaporated and
the residue is partitioned between saturated aqueous NaHCO3-solution and
dichloromethane. After
phase separation, the aqueous phase is extracted three times with
dichloromethane. The combined
organic phase is dried over magnesium sulfate and the solvent is evaporated in
vacuo. The crude
product is purified by silica gel flash chromatography using a gradient of n-
hexane and ethyl acetate
from 100:0 to 50:50. This yielded 0.26 g of a mixture containing the title
compound. This mixture was
used for the further synthetic pathway.
M.p.: 74-76 C.
MS: m/z (MH+) = 500.0, 502.0 (analytical data measured with recovered pure
starting material from
A16).
B10. {3-[(RS)-1-(6-Benzyl-3-fluoro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidin-5-yl)-
2-methyl-propylamino]-propyl}-carbamic acid tert-butyl ester
0.55 g (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-propanoyl)-6H-
pyrazolo[1,5-c]pyrimidin-7-
one (compound C8) are dissolved in 7 mL tetrahydrofurane. 0.74 g (2.5 eq) tert-
butyl-N-(3-
aminoetyl)carbamate and 1.51 mL (3.0 eq) titanium(IV)isopropoxide are added
and this mixture is
stirred in a sealed tube for 72 hours at 80 C. After cooling, the mixture is
treated with an aqueous
solution of citric acid and stirred for another 15 min at ambient temperature.
Addition of
dichloromethane and filtration of the mixture yielded a two-phase system. The
phases are separated
and the aqueous phase is extracted with dichloromethane. The combined organic
phase is dried over
magnesium sulfate and the solvent is removed in vacuo. This yielded the crude
mixture of the
corresponding imine.
The mixture of the crude imine is dissolved in 12 mL acetic acid. 0.33 g (5.0
eq) sodium borohydride
are added and the mixture is stirred for 90 minutes at ambient temperature.
The solvent is evaporated
and the residue is partitioned between saturated aqueous NaHCO3-solution and
dichloromethane.
After phase separation, the aqueous phase is extracted three times with
dichloromethane. The
combined organic phase is dried over magnesium sulfate and the solvent is
evaporated in vacuo. The
crude product is purified by silica gel flash chromatography using a
cyclohexane ethyl acetate in a 2:1
mixture. This yielded 0.16 g (19%) of the title compound as colorless solid.
Cl. 6-Benzyl-2-methyl-5-propionyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
770 mg 6-Benzyl-5-((RS)-1-hydroxy-propyl)-2-methyl-6H-pyrazolo[1,5-c]pyrimidin-
7-one (2.59 mmol)
(compound D1) are dissolved in 15 ml dichloromethane, 1.5 g molecular sieve (4
A, 500 mg/mmol
Substrat) and 1060 mg NMO ( 7.8 mmol) are added and the reaction mixture is
stirred 2 h at ambient
temperature. Afterwards TPAP ( 100 mg, 0.26 mmol) are added and the mixture is
stirred 14 h at
ambient temperature. The reaction mixture is filtrated over silica gel, washed
with etylacetate and
evaporated. The residue is purified by silica gel flash chromatography. By
this method 452 mg ( 59 %)
of a yellow solid are obtained. M.p.: 128-130 C.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-68-
C2. 6-Benzyl-3-chloro-2-methyl-5-propionyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
6-Benzyl-3-chloro-5-((RS)-1-hydroxy-propyl)-2-methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-one (6.00 g, 18.0
mmol) (compound D2) is dissolved in CHzCl2 (90 mL) and treated with molecular
sieve (4A, 9.00 g)
and NMO (7.30 g, 54.0 mmol) and stirred for 2h at ambient temperature. To the
mixture is added
TPAP (633 mg, 1.8 mmol) and it is stirred for 14 h at ambient temperature. The
mixture is filtered and
evaporated. The residue is purified by silica gel flash chromatography. The
title compound is obtained
as 3.80 g of a yellow solid with m.p. 128 C.
C3. 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-propanoyl)-6H-pyrazolo[1,5-
c]pyrimidin-7-one
1.61 g (1.0 eq) of 6-Benzyl-3-chloro-5-((RS)-1-hydroxy-2-methyl-propyl)-2-
methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-one (compound D3) are dissolved in 60 mL of dichloromethane at
room temperature. To
this mixture are added 7 g (1,5 g/mmol) of mol sieves 4 A and 2.53 g (4.0 eq)
N-methylmorpholine-N-
oxide. After 30 min stirring at ambient temperature 0.33 g (0.2 eq)
tetrapropylammonium perruthenate
is added. After 16 hours the reaction mixture is filtered through silica gel
and washed out with ethyl
acetate. The solvent is removed in vacuo. Flash chromatography using a
gradient of n-hexane and
ethyl acetate from 100:0 to 50:50 yields 1.09 g (68%) of the title compound as
a white solid.
M.p. 146.7 C.
MS: m/z (MH+) = 344.1, 346.1.
C4. 6-Benzyl-2-methyl-5-(2-methyl-propanoyl)-6H-pyrazolo[1,5-c]pyrimidin-7-one
The title compound may be prepared by oxidation of compound D4 analogously or
similarly as
described for compounds Cl to C3.
C5. 6-Benzyl-3-chloro-2-methyl-5-(2-methyl-butanoyl)-6H-pyrazolo[1,5-
c]pyrimidin-7-one
1.12 g (1.0 eq) of 6-Benzyl-3-chloro-5-((RS)-1-hydroxy-2-methyl-butyl)-2-
methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-one (compound D5) are dissolved in 50 mL of dichloromethane at
ambient temperature.
4.6 g (1.5 g/mmol) of mol sieves 4 A and 1.68 g (4.0 eq) N-methylmorpholine-N-
oxide are added to
this mixture. After 30 min stirring at ambient temperature 0.22 g (0.2 eq)
tetrapropylammonium
perruthenate are added. After 16 hours the reaction mixture is filtered
through silica gel using ethyl
acetate as eluent. The solvent is removed in vacuo. Flash chromatography using
a gradient of n-
hexane and ethyl acetate from 100:0 to 50:50 yielded 0.78 g (70%) of the title
compound as a
colorless solid.
M.p.: 122-123 C.
MS: m/z (MH+) = 358.1, 360.1.
C6. 6-Benzyl-3-chloro-5-(1-cyclobutyl-carbonyl)-2-methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-one
A suspension of 0.63 g (1.0 eq) of 6-Benzyl-3-chloro-5-((RS)-1-cyclobutyl-l-
hydroxy-methyl)-2-methyl-
6H-pyrazolo[1,5-c]pyrimidin-7-one (compound D6), 2,2,6,6-
tetramethylpiperidinyloxy (TEMPO) and
1.29 g iodobenzene diacetate in 4 mL dichloromethane is stirred for 2 days at
ambient temperature.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-69-
This mixture is treated with aqueous NazSzO3-solution (1M) and
dichloromethane. Phase separation
and extraction of the aqueous layer with dichloromethane resulted after drying
over magnesium
sulfate and evaporation of the solvent in vacuo in the crude product. Silica
gel flash chromatography
with a ratio of n-hexane and ethyl acetate 3:7 yielded 0.63 g (quant.) of the
title compound as a
colorless solid.
M.p.: 96-97 C.
MS: m/z (MH+) = 356.1, 358.1.
C7. 6-Benzyl-3-chloro-5-(1-cyclopropyl-carbonyl)-2-methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-
one
0.33 g (1.0 eq) of 6-Benzyl-3-chloro-5-(1-cyclopropyl-(RS)-1-hydroxy-methyl)-2-
methyl-6H-
pyrazolo[1,5-c]pyrimidin-7-one (compound D7) are dissolved in 15 mL of
dichloromethane at ambient
temperature. 1.43 g (1.5 g/mmol) of mol sieves 4 A and 0.51 g (4.0 eq) N-
methylmorpholine-N-oxide
are added to this mixture. After 30 min stirring at ambient temperature 67 mg
(0.2 eq)
tetrapropylammonium perruthenate are added. After 16 hours the reaction
mixture is filtered through
silica gel with ethyl acetate as eluent. The solvent is removed in vacuo.
Flash chromatography using a
gradient of n-hexane and ethyl acetate from 100:0 to 30:70 yielded 0.23 g
(68%) of the title compound
as a colorless solid.
M.p.: 151-152 C.
MS: m/z (MH+) = 342.2, 344.2.
C8. 6-Benzyl-3-fluoro-2-methyl-5-(2-methyl-propanoyl)-6H-pyrazolo[1,5-
c]pyrimidin-7-one
A suspension of 0.55 g (1.0 eq) of 6-Benzyl-3-fluoro-5-(1-hydroxy-2-methyl-
propyl)-2-methyl-6H-
pyrazolo[1,5-c]pyrimidin-7-one (compound D8), 55 mg (0.22 eq) 2,2,6,6-
tetramethylpiperidinyloxy
(TEMPO) and 1.21 g (2.2 eq) iodobenzene diacetate in 10 mL dichloromethane is
stirred for 3 days at
ambient temperature. After addition of 25 mg (0.1 eq) TEMPO the mixture is
stirred for another 2 days
at ambient temperature. This mixture is treated with aqueous NazS203-solution
(1 M) and
dichloromethane. Phase separation and extraction of the aqueous layer with
dichloromethane resulted
after drying over sodium sulfate and evaporation of the solvent in vacuo in
the crude product. Silica
gel flash chromatography with a ratio of cyclohexane and ethyl acetate of 3:2
yielded 0.55 g (quant.)
of the title compound as a yellow solid.
Dl. 6-Benzyl-5-((RS)-1-hydroxy-propyl)-2-methyl-6H-pyrazolo[1,5-c]pyrimidin-7-
one
To a solution of 7.2 ml MeLi (11.5 mmol, 1.6 M in Et20) in THF (20 ml), 2.3 ml
EtMgBr (6.9 mmol, 3 M
in Et20) are added at - 78 C. The solution is stirred 1 h at - 78 C.
1 g 6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidine-5-
carbaldehyde (2.89
mmol) (compound El) diluted in 4 ml DMPU and 10 ml THF are added dropwise
under argon
atmosphere. Afterwards the reaction mixture is stirred 2 h at - 78 C and 3 h
at -30 C. The reaction
mixture is quenched with aqueous NH4CI-solution and then warmed up to ambient
temperature. The
product is extracted between ethylacetate and water. The organic phase is
dried over sodium
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-70-
sulphate. After evaporating the solvents the crude product is purified by
silica gel flash
chromatography. By this method 352 mg (42%) of the title compound are
obtained. M.p.: 190-191 C.
D2. 6-Benzyl-3-chloro-5-((RS)-1-hydroxy-propyl)-2-methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-one
To a mixture of THF (4 mL) and MeLi (1.25 mL, 2.0 mmol, 1.6 M in Et20) are
added at -78 C EtMgBr
(0.48 mL, 1.2 mmol, 3.0 M in Et20), the solution is stirred 1 h at this
temperature and 6-benzyl-3-
chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidine-5-carbaldehyde
(compound E2) is added.
The mixture is stirred for 6h at - 70 C and then the mixture is hydrolyzed
with aqueous NH4CI solution
and stirred at ambient temperature. After adding water the mixture is
extracted with ethylacetate, the
organic phase is dried and evaporated. The residue is purified by silica gel
flash chromatography. 201
mg are obtained with a melting point of 80-82 C.
D3. 6-Benzyl-3-chloro-5-((RS)-1-hydroxy-2-methyl-propyl)-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
25 mL of methyllithium (2.0 eq, 1.6 M solution in diethylether) and 24 mL of
isopropylmagnesium
bromide (1.2 eq, -1M solution in tetrahydrofurane) are added to 200 mL dry
tetrahydrofurane at -
78 C. After one hour at -78 C 6g (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-7-oxo-
6,7-dihydro-
pyrazolo[1,5-c]pyrimidine-5-carbaldehyde (compound E2) is added. After
stirring this mixture for an
additional 2 hours, a saturated aqueous solution of ammonium chloride is
added. At ambient
temperature the mixture is extracted with ethyl acetate and the combined
organic phase is dried over
magnesium sulfate. After filtration the solvent is evaporated under vacuo. The
crude product is
crystallized from ethyl acetate. This yields 2.83 g of the title compound.
Flash chromatography of the
mother liquor using a gradient of n-hexane and ethyl acetate from 100:0 to
50:50 yields another 1.15 g
of the title compound (together 58% yield) as a white solid.
M.p. 72.9 C.
MS: m/z (MH+) = 345.9, 347.9.
D4. 6-Benzyl-5-((RS)-1-hydroxy-2-methyl-propyl)-2-methyl-6H-pyrazolo[1,5-
c]pyrimidin-7-
one
The title compound may be prepared from compound El analogously or similarly
as described for
compound Dl.
D5. 6-Benzyl-3-chloro-5-((RS)-1-hydroxy-2-methyl-butyl)-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
12.5 mL of methyllithium (2.0 eq, 1.6 M solution in diethylether) and 12 mL of
sec-butylmagnesium
bromide (1.2 eq, -1M solution in tetrahydrofurane) are added to 100 mL dry
tetrahydrofurane at -
78 C. After one hour at -78 C 3.0 g (1.0 eq) of 6-Benzyl-3-chloro-2-methyl-7-
oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidine-5-carbaldehyde (compound E2) are added. After
stirring this mixture for an
additional 2.5 hours, a saturated aqueous solution of ammonium chloride is
added. The mixture is
extracted with ethyl acetate and the combined organic phase is dried over
magnesium sulfate. After
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-71 -
filtration, the solvent is evaporated in vacuo. Silica gel flash
chromatography using a gradient of n-
hexane and ethyl acetate from 100:0 to 50:50 yielded 1.2 g (33%) of the title
compound as colorless
foam.
M.p.: 72-73 C.
MS: m/z (MH+) = 360.2, 362.2.
D6. 6-Benzyl-3-chloro-5-((RS)-1-cyclobutyl-l-hydroxy-methyl)-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
1.0 mL (3.0 eq) Cyclobutylbromide and 0.24 g (3.0 eq) magnesium (Grignard) are
dissolved in 20 mL
diethylether. The grignard reaction is started with iodine and heating of the
suspension to reflux. After
90 minutes at reflux a solution of 1.0 g (1.0 eq) of 6-Benzyl-3-chloro-2-
methyl-7-oxo-6,7-dihydro-
pyrazolo[1,5-c]pyrimidine-5-carbaldehyde (compound E2) in 15 mL THF is added
dropwise. After
stirring for 16 hours at ambient temperature the reaction is stopped with
ammonium chloride.
Extraction of the aqueous phase with ethyl acetate resulted after drying over
magnesium sulfate and
evaporation of the solvent in vacuo in the crude product. Silica gel flash
chromatography using a
gradient of n-hexane and ethyl acetate from 100:0 to 50:50 yielded 0.20 g
(17%) of the title compound
as a colorless solid.
M.p.: 188-193 C (decomposition).
MS: m/z (MH+) = 358.1, 360Ø
D7. 6-Benzyl-3-chloro-5-(1-cyclopropyl-(RS)-1-hydroxy-methyl)-2-methyl-6H-
pyrazolo[1,5-
c]pyrimidin-7-one
1.0 g(1.0 eq) of 6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-
c]pyrimidine-5-
carbaldehyde (compound E2) are dissolved in 30 mL THF and at 0 C 16.5 mL (2.5
eq, 0.5 M in THF)
cyclopropylmagnesiumbromide are added dropwise. After stirring for 3 days at
ambient temperature
the mixture is treated with a saturated aqueous solution of ammonium chloride.
Extraction of the water
phase with dichloromethane resulted after drying over magnesium sulfate and
evaporation of the
solvent in vacuo the crude product. Silica gel flash chromatography using a
gradient of n-hexane and
ethyl acetate from 100:0 to 50:50 yielded 0.56 g (49%) of the title compound
as a colorless solid.
M.p.: 119-120 C.
MS: m/z (MH+) = 344.1, 346.1.
D8. 6-Benzyl-3-fluoro-5-(1-hydroxy-2-methyl-propyl)-2-methyl-6H-pyrazolo[1,5-
c]pyrimidin-
7-one
3.2 mL of methyllithium (2.0 eq, 1.6 M solution in diethylether) and 3 mL of
isopropylmagnesium
bromide (1.2 eq, -1 M solution in tetrahydrofurane) are added to 25 mL dry
tetrahydrofurane at -78 C.
After one hour at -78 C 0.6 g (1.0 eq) of 6-Benzyl-3-fluoro-2-methyl-7-oxo-6,7-
dihydro-pyrazolo[1,5-
c]pyrimidine-5-carbaldehyde (compound E3) are added. After stirring this
mixture for an additional 3
hours the reaction is quenched by treatment with a 10% aqueous ammonium
chloride solution. The
mixture is extracted with diethylether and the combined organic phase is dried
over magnesium
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-72-
sulfate. After filtration, the solvent is evaporated in vacuo. Silica gel
flash chromatography using a
ratio of n-hexane and ethyl acetate of 1:1 yielded 0.23 g (27%) of the title
compound as slightly yellow
solid.
El. 6-Benzyl-3-bromo-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidine-5-
carbaldehyde
6-Benzyl-3-bromo-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one (24.0 g, 72.2
mmol) (compound F1)
are dissolved in 350 ml dioxane. 24 g selendioxide are added and the reaction
mixture is heated to
reflux for 12 h. Afterwards the reaction mixture is filtered, the residue is
washed with diethylether and
the filtrate is evaporated. By this method 22.7 g (90 %) of the title compound
are obtained. M.p.: 187-
189 C.
E2. 6-Benzyl-3-chloro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidine-5-
carbaidehyde
2.29 g (1.0 eq) of 6-Benzyl-3-chloro-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-
7-one (compound F2)
are dissolved in 40 mL dioxane and treated with 2.64 g (3.0 eq) selenium
dioxide. This mixture is
heated at reflux for 5 hours. After filtration off the inorganic salts, the
filtrate is evaporated in vacuo.
This residue is purified by silica gel flash chromatography using cyclohexane
and ethyl acetate in a
mixture of 2:1 to yield 2.10 g (88%) of the title compound. M.p. 187-188 C.
E3. 6-Benzyl-3-fluoro-2-methyl-7-oxo-6,7-dihydro-pyrazolo[1,5-c]pyrimidine-5-
carbaidehyde
A solution of 6.75 g(1.0 eq) of 6-Benzyl-3(4)-fluoro-2,5-dimethyl-6H-
pyrazolo[1,5-c]pyrimidin-7-one
(compound F3) in 125 mL dioxane is treated with 10.55 g (3.0 eq) selenium
dioxide. The mixture is
heated for 7 days at reflux. This mixture is filtered, the solution is
concentrated and washed three
times with diethylether. The mother liquor is purified using flash
chromatography (ethylacetate as
eluent). This resulted in 0.60 g (8%) of the title compound.
Fl. 6-Benzyl-3-bromo-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
32 g (126 mmol) of the 6-Benzyl-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
(compound G1) are
dissolved in 300 ml CCI4. 22.5 g (126 mmol) NBS and 2.1 g (12.6 mmol) AIBN are
added to the
solution, it is stirred at ambient temperature for 18 h. Afterwards the
solution is diluted with
dichloromethane and washed with saturated aqueous NaHCO3solution. The organic
phase is dried
over sodium sulphate and the solvents are evaporated. The crude product is
purified by silica gel flash
chromatography. By this method 24.1 g (58 %) of the title compound are
obtained.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-73-
F2. 6-Benzyl-3-chloro-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
2.83 g (1.0 eq) 6-Benzyl-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
(compound G1) are dissolved
in 35 mL tetrachloromethane and treated with 1.50 g (1.0 eq) N-
chlorosuccinimide for 5 hours at
reflux. The reaction mixture is diluted with dichloromethane and washed with
saturated aqueous
sodium hydrogen carbonate solution. Drying of the organic phase over sodium
sulfate and evaporation
of the solvent yields the crude product. The residue is purified by silica gel
flash chromatography using
cyclohexane and ethyl acetate in a mixture of 2:1 to yield 2.70 g (84%) of the
title compound.
M.p. 140-142 C.
F3. 6-Benzyl-3(4)-fluoro-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
14.7 g of 6-Benzyl-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one (1.0 eq,
compound G1) are
dissolved in 300 mL acetonitrile and treated at 0 C with 22.6 g Selectfluor
(1.1 eq). After stirring for 1
hour at this temperature, the solvent is removed in vacuo, the residue is
dissolved in dichloromethane
and neutralized with a saturated aqueous hydrogen carbonate solution. The
aqueous phase is
extracted with dichloromethane and the combined organic layer is dried over
magnesium sulfate. After
evaporation of the solvent, the oily residue is crystallized from
diethylether. The crystals are further
purified using flash chromatography (cyclohexane / ethylacetate = 1.5:1). This
yielded 2.67 g(17%) of
the title compound as a colorless solid.
G1. 6-Benzyl-2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
2.30 g (1.0 eq) 2,5-dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one (compound H1)
are dissolved in 35 mL
N,N-dimethylformamide (DMF). 2.20 g (1.1 eq) potassium carbonate and 1.9 mL
benzyl bromide are
added. The mixture is stirred at ambient temperature for 14 hours. After
addition of water the aqueous
phase is extracted with dichloromethane. The combined organic phase is dried
over sodium sulfate
and the solvent is evaporated in vacuo. The residue is washed with
diethylether and yield 3.35 g
(94%) of the title compound.
H1. 2,5-Dimethyl-6H-pyrazolo[1,5-c]pyrimidin-7-one
2.30 g (1.0 eq) semicarbazide hydrochloride are dissolved in 40 mL water.
After addition of 1.10 g (0.5
eq) of a saturated aqueous solution of sodium carbonate and 2.90 g (1.0 eq) of
heptane-2,4,6-trione
(compound 11), the mixture is heated for 1 hour at 100 C. Extraction of the
mixture with
dichloromethane yields after evaporation of the solvent in vacuo 2.6 g (79%)
of the title compound.
M.p. 205-208 C.
11. Heptane-2,4,6-trione
50.0 g 2,6-Dimethyl-y-pyrone are dissolved in 250 mL ethanol and treated with
50 mL of a 16 M
aqueous solution of sodium hydroxide. This mixture is heated for 5 hours at 60
C and for another 1
hour at 100 C. The residue is filtered off and washed with diethylether. This
residue is now dissolved
in water and put in 500 mL of a 3 M aqueous solution of hydrochloric acid. The
aqueous phase is
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-74-
extracted with diethylether. The combined organic phase is dried over sodium
sulfate and the solvent
is evaporated in vacuo. This yields 31.6 g (55%) of the title compound. M.p.
43-45 C.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-75-
Commercial utility
The compounds of formula I, 1* and I**, and their pharmacologically and/or
pharmaceutically
acceptable salts (= the compounds according to the present invention) have
valuable pharmacological
and/or pharmaceutical properties which can make them commercially applicable.
Thus, for example,
the compounds according to this invention can act as inhibitors of the mitotic
kinesin Eg5 and these
compounds are expected to be commercially applicable in the therapy of
diseases responsive to the
inhibition of this kinesin, such as e.g. those diseases mentioned below. Also,
for example, the
compounds according to this invention can display cell-cycle dependent, anti-
proliferative and/or
apoptosis inducing activity.
The mitotic kinesin Eg5 is an enzyme essential for the assembly and function
of the bipolar mitotic
spindle. Eg5 plays essential roles during all phases of mitosis. Drugs that
perturb mitosis have proven
clinically effective in the treatment of many cancers. Despite the diverse
array of essential spindle
proteins that could be exploited as targets for the discovery of novel cancer
therapies, all spindle-
targeted therapeutics in clinical use today act on only one protein, tubulin.
Surprisingly, kinesin Eg5
expression is most abundant in proliferating human tissues, whereas it is
absent from most postmitotic
cells, such as e.g. human central nervous system neurons, consistent with an
exclusive or almost
confined role for Eg5 in cell proliferation. In contrary to drugs that
directly interfere with microtubule
dynamic instability, Eg5 kinesin inhibitors are expected not to disrupt
microtubule-based cellular
processes, e.g. neuronal transport, that are unrelated to proliferation.
During mitosis, Eg5 is essentially
involved in organizing microtubules into a bipolar structure that forms the
mitotic spindle.
Experimental perturbation of Eg5 function causes a characteristic malformation
or dysfunction of the
mitotic spindle, frequently resulting in cell cycle arrest and cell death.
The compounds according to this invention can be used to modulate mitotic
spindle formation, thus
causing prolonged cell cycle arrest in mitosis, which is frequently followed
by apoptosis. By "modulate"
herein is meant altering mitotic spindle formation, including increasing and
decreasing spindle
formation. By "mitotic spindle formation" herein is meant organization of
microtubules into bipolar
structures by mitotic kinesins. By "dysfunction of the mitotic spindle" herein
is meant mitotic arrest and
monopolar spindle formation. "Malformation of the mitotic spindle" encompasses
the splaying of
mitotic spindle poles, or otherwise causing morphological perturbation of the
mitotic spindle.
Further on, these compounds can be useful in the treatment of benign or
malignant neoplasia.
A "neoplasia" is defined by cells displaying aberrant cell proliferation
and/or survival and/or a block in
differentiation. A "benign neoplasia" is described by hyperproliferation of
cells, incapable of forming an
aggressive, metastasizing tumor in-vivo. In contrast, a "malignant neoplasia"
is described by cells with
multiple cellular and biochemical abnormalities, capable of forming a systemic
disease, for example
forming tumor metastasis in distant organs.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-76-
Various diseases are caused aberrant cell proliferation ("hyperproliferation")
as well as evasion from
apoptosis. These diseases include e.g. benign hyperplasia like that of the
prostate ("BPH") or colon
epithelium, psoriasis, glomerulonephritis or osteoarthritis. Most importantly
these diseases include
malignant neoplasia commonly described as cancer and characterized by tumor
cells finally
metastasizing into distinct organs or tissues. Malignant neoplasia include
solid and hematological
tumors. Solid tumors are exemplified by tumors of the breast, bladder, bone,
brain, central and
peripheral nervous system, colon, endocrine glands (e.g. thyroid and adrenal
cortex), esophagus,
endometrium, germ cells, head and neck, kidney, liver, lung, larynx and
hypopharynx, mesothelioma,
sarcoma, ovary, pancreas, prostate, rectum, renal, small intestine, soft
tissue, testis, stomach, skin,
ureter, vagina and vulva. Malignant neoplasia include inherited cancers
exemplified by retinoblastoma
and Wilms tumor. In addition, malignant neoplasia include primary tumors in
said organs and
corresponding secondary tumors in distant organs ("tumor metastases").
Hematological tumors are
exemplified by aggressive and indolent forms of leukemia and lymphoma, namely
non-Hodgkins
disease, chronic and acute myeloid leukemia (CML / AML), acute lymphoblastic
leukemia (ALL),
Hodgkins disease, multiple myeloma and T-cell lymphoma. Also included are
myelodysplastic
syndrome, plasma cell neoplasia, paraneoplastic syndromes, cancers of unknown
primary site as well
as AIDS related malignancies.
The invention therefore relates to a use of the compounds according to the
invention in the
manufacture of pharmaceutical compositions, a method of treatment or a
combination according to
the invention, in which the cancer to be treated is selected from the group
consisting of
cancer of the breast, bladder, bone, brain, central and peripheral nervous
system, colon, endocrine
glands, esophagus, endometrium, germ cells, head and neck, kidney, liver,
lung, larynx and
hypopharynx, mesothelioma, sarcoma, ovary, pancreas, prostate, rectum, renal,
small intestine, soft
tissue, testis, stomach, skin, ureter, vagina and vulva;
inherited cancers, retinomblastoma and Wilms tumor;
leukemia, lymphoma, non-Hodgkins disease, chronic and acute myeloid leukaemia,
acute
lymphoblastic leukemia, Hodgkins disease, multiple myeloma and T-cell
lymphoma;
myelodysplastic syndrome, plasma cell neoplasia, paraneoplastic syndromes,
cancers of unknown
primary site and AIDS related malignancies.
It is to be noted that a cancer disease as well as a malignant neoplasia does
not necessarily require
the formation of metastases in distant organs. Certain tumors exert
devastating effects on the primary
organ itself through their aggressive growth properties. These can lead to the
destruction of the tissue
and organ structure finally resulting in failure of the assigned organ
function.
Neoplastic cell proliferation might affect normal cell behaviour and organ
function. For example the
formation of new blood vessels, a process described as neovascularization, is
induced by tumors or
tumor metastases. Compounds according to this invention can be commercially
applicable for the
treatment of pathophysiological relevant processes caused by benign or
neoplastic cell proliferation,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-77-
such as but not limited to neovascularization by unphysiological proliferation
of vascular endothelial
cells.
Drug resistance is of particular importance for the frequent failure of
standard cancer therapeutics.
This drug resistance is caused by various cellular and molelcular mechanisms
like overexpression of
drug efflux pumps or mutation within the cellular target protein. The
commercial applicability of the
compounds according to this invention is not limited to 1St line treatment of
patients. Patients with
resistance to defined cancer chemotherapeutics or target specific anti-cancer
drugs (2nd or 3~d line
treatment) can be also amenable for treatment with the compounds according to
this invention.
Due to their cellular anti-proliferative properties, compounds according to
the present invention may
be also commercially usable for treatment of diseases associated with cell
cycle and cell proliferation,
such as, besides cancer discussed above, for example, fibroproliferative and
differentiative disorders,
psoriasis, rheumatoid arthritis, atherosclerosis, hyperplasia, restenosis,
cardiac hypertrophy,
(auto)immune disorders, fungal disorders, bone diseases, or acute or chronic
inflammation.
Thus, the invention relates to compounds according to the invention for use in
the treatment of
diseases.
Compounds according to the present invention can be commercially applicable
for treatment,
prevention or amelioration of the diseases of benign and malignant behavior as
described before,
such as e.g. benign or malignant neoplasia, particularly cancer (such as e.g.
any of those cancer
diseases described above), especially a cancer that is susceptible to Eg5
inhibition.
In the context of their properties, functions and usabilities mentioned
herein, the compounds according
to the present invention are expected to be distinguished by valuable and
desirable effects related
therewith, such as e.g. by low toxicity, superior bioavailability in general
(such as e.g. good enteral
absorption), superior therapeutic window, absence of significant side effects,
and/or further beneficial
effects related with their therapeutic and pharmaceutical suitability.
The invention further includes a method for treating (hyper)proliferative
diseases and/or disorders
responsive to the induction of apoptosis, particularly those diseases,
disorders, conditions or illnesses
mentioned above, in mammals, including humans, suffering therefrom comprising
administering to
said mammals in need thereof a pharmacologically and/or pharmaceutically
active and therapeutically
effective and tolerable amount of one or more of the compounds according to
this invention.
The present invention further includes a method useful to modulate apoptosis
and/or aberrant cell
growth in the therapy of benign or malignant neoplastic diseases, such as e.g.
cancer, comprising
administering to a subject in need of such therapy a pharmacologically and/or
pharmaceutically active
and therapeutically effective and tolerable amount of one or more of the
compounds according to this
invention.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-78-
The invention further includes a method for modulating, particularly
inhibiting, Eg5 activity in cells
comprising administering a pharmacologically and/or pharmaceutically active
and therapeutically
effective and tolerable amount of one or more of the compounds according to
this invention to a
patient in need of such modulation, particularly inhibition.
The present invention further includes a method to modulate the mitotic
spindle, i.e., for example,
altering mitotic spindle formation, including decreasing spindle formation, or
increasing or decreasing
spindle pole separation causing malformation of the mitotic spindle poles,
comprising administering a
pharmacologically and/or pharmaceutically active and therapeutically effective
and tolerable amount
of one or more of the compounds according to this invention to a patient in
need of such modulation.
The present invention further includes a method to inhibit mitosis in cells
comprising administering a
pharmacologically and/or pharmaceutically active and therapeutically effective
and tolerable amount
of one or more of the compounds according to this invention to a patient in
need of such inhibition.
The present invention further includes a method for treating, preventing or
ameliorating diseases
and/or disorders associated with Eg5 kinesin activity, such as, for example,
(hyper)proliferative
diseases and/or disorders responsive to induction of apoptosis, for example,
benign neoplasia or
malignant neoplasia, e.g. cancer, in a mammal comprising administering a
pharmacologically and/or
pharmaceutically active and therapeutically effective and tolerable amount of
one or more compounds
according to the present invention to said mammal in need thereof.
The present invention further relates to the use of the compounds according to
this invention for the
production of pharmaceutical compositions which are employed for the
treatment, prophylaxis and/or
amelioration of one or more of the illnesses mentioned.
The present invention further relates to the use of the compounds according to
this invention for the
production of pharmaceutical compositions which can be used in the treatment,
prevention or
amelioration of (hyper)proliferative diseases of benign or malignant behaviour
and/or disorders
responsive to the induction of apoptosis in a mammal, such as, for example,
benign or malignant
neoplasia, e.g. cancer.
The present invention further relates to the use of the compounds according to
this invention for the
production of pharmaceutical compositions which can be used use in the
treatment, prevention or
amelioration of disorders responsive to arresting of aberrant cell growth
and/or induction of apoptosis.
The present invention further relates to the use of the compounds according to
this invention for the
production of pharmaceutical compositions for treating, preventing or
ameliorating benign or
malignant neoplasia, particularly cancer, such as e.g. any of those cancer
diseases described above.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-79-
The present invention further relates to pharmaceutical compositions
comprising one or more of the
compounds according to this invention and a pharmaceutically acceptable
carrier or diluent.
The present invention further relates to pharmaceutical compositions made by
combining one or more
of the compounds according to this invention and a pharmaceutically acceptable
carrier or diluent.
The present invention further relates to pharmaceutical compositions
comprising one or more of the
compounds according to this invention and pharmaceutically acceptable
auxiliaries and/or excipients.
The present invention also relates to pharmaceutical compositions for treating
(hyper)proliferative
diseases and/or disorders responsive to induction of apoptosis, which include
benign neoplasia and
malignant neoplasia, including cancer, comprising a compound according to this
invention.
The present invention further relates to combinations comprising one or more
of the compounds
according to this invention and pharmaceutically acceptable auxiliaries,
excipients and/or vehicles,
e.g. for treating, preventing or ameliorating benign or malignant neoplasia,
particularly cancer, such as
e.g. any of those cancer diseases described above.
The present invention further relates to a combination comprising a compound
according to this
invention and a pharmaceutically acceptable excipient, carrier and/or diluent,
e.g. for treating,
preventing or ameliorating benign or malignant neoplasia, particularly cancer,
such as e.g. any of
those cancer diseases described above.
The present invention further relates to a composition consisting essentially
of a therapeutically
effective and tolerable amount of one or more compounds according to this
invention together with the
usual pharmaceutically acceptable vehicles, diluents and/or excipients for use
in therapy, e.g. for
treating, preventing or ameliorating hyperproliferative diseases, such as e.g.
cancer, and/or disorders
responsive to induction of apoptosis.
The present invention further relates to compounds according to this invention
for use in therapy, such
as, for example, in the treatment, prevention or amelioration of
(hyper)proliferative diseases of benign
or malignant behaviour and/or disorders responsive to the induction of
apoptosis, such as e.g. those
diseases mentioned herein, particularly cancer.
The present invention further relates to compounds according to this invention
having anti-proliferative
and/or apoptosis inducing activity.
The present invention further relates to compounds according to this invention
having Eg5 inhibiting
properties.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-80-
The present invention further relates to pharmaceutical compositions according
to this invention
having Eg5 inhibiting properties.
The present invention further relates to pharmaceutical compositions according
to this invention
having anti-proliferative activity.
The present invention further relates to pharmaceutical compositions according
to this invention
having apoptosis inducing activity.
The invention further relates to the use of a pharmaceutical composition
comprising one or more of
the compounds according to this invention as sole active ingredient(s) and a
pharmaceutically
acceptable carrier or diluent in the manufacture of pharmaceutical products
for the treatment and/or
prophylaxis of the illnesses mentioned above.
Additionally, the invention relates to an article of manufacture, which
comprises packaging material
and a pharmaceutical agent contained within said packaging material, wherein
the pharmaceutical
agent is therapeutically effective inhibiting Eg5 and/or inhibiting cellular
(hyper)proliferation and/or
inducing apoptosis, ameliorating the symptoms of a Eg5 mediated disease and/or
a
(hyper)proliferative disease and/or a disorder responsive to the induction of
apoptosis, and wherein the
packaging material comprises a label or package insert which indicates that
the pharmaceutical agent
is useful for preventing or treating a Eg5 mediated disease and/or a
(hyper)proliferative disease and/or
a disorder responsive to the induction of apoptosis, and wherein said
pharmaceutical agent comprises
one or more compounds according to the invention. The packaging material,
label and package insert
otherwise parallel or resemble what is generally regarded as standard
packaging material, labels and
package inserts for pharmaceuticals having related utilities.
The pharmaceutical compositions according to this invention are prepared by
processes which are
known per se and familiar to the person skilled in the art. As pharmaceutical
compositions, the
compounds of the invention (= active compounds) are either employed as such,
or preferably in
combination with suitable pharmaceutical auxiliaries and/or excipients, e.g.
in the form of tablets,
coated tablets, dragees, pills, cachets, granules, capsules, caplets,
suppositories, patches (e.g. as
TTS), emulsions (such as e.g. micro-emulsions or lipid emulsions), suspensions
(such as e.g. nano
suspensions), gels, solubilisates or solutions (e.g. sterile solutions), or
encapsuled in liposomes or as
beta-cyclodextrine inclusion complexes or the like, the active compound
content advantageously
being between 0.1 and 95% and where, by the appropriate choice of the
auxiliaries and/or excipients,
a pharmaceutical administration form (e.g. a delayed release form or an
enteric form) exactly suited to
the active compound and/or to the desired onset of action can be achieved.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-81 -
The person skilled in the art is familiar with auxiliaries, vehicles,
excipients, diluents, carriers or
adjuvants which are suitable for the desired pharmaceutical formulations,
preparations or
compositions on account of his/her expert knowledge. In addition to solvents,
gel formers, ointment
bases and other active compound excipients, for example antioxidants,
dispersants, emulsifiers, pre-
servatives, solubilizers (such as e.g. polyoxyethylenglyceroltriricinoleat 35,
PEG 400, Tween 80,
Solutol HS15 or the like), colorants, complexing agents, permeation promoters,
stabilizers, fillers,
binders, thickeners, disintegrating agents, buffers, pH regulators (e.g. to
obtain neutral, alkaline or
acidic formulations), polymers, lubricants, coating agents, propellants,
tonicity adjusting agents,
surfactants, flavorings, sweeteners or dyes, can be used.
In particular, auxiliaries and/or excipients of a type appropriate to the
desired formulation and the
desired mode of administration are used.
The administration of the compounds, pharmaceutical compositions or
combinations according to the
invention may be performed in any of the generally accepted modes of
administration available in the
art. Illustrative examples of suitable modes of administration include
intravenous, oral, nasal,
parenteral, topical, transdermal and rectal delivery. Oral and intravenous
delivery are preferred.
For the treatment of dermatoses, the compounds of the invention can be in
particular administered in
the form of those pharmaceutical compositions which are suitable for topical
application. For the
production of the pharmaceutical compositions, the compounds of the invention
(= active compounds)
are preferably mixed with suitable pharmaceutical auxiliaries and further
processed to give suitable
pharmaceutical formulations. Suitable pharmaceutical formulations are, for
example, powders,
emulsions, suspensions, sprays, oils, ointments, fatty ointments, creams,
lotions, pastes, gels or
solutions.
The pharmaceutical compositions according to the invention can be prepared by
processes known per
se. The dosage of the compounds of the invention (= active compounds) is
carried out in the order of
magnitude customary for Eg5 inhibitors, inhibitors for cellular
(hyper)proliferation or apoptosis
inducers. Topical application forms (such as ointments) for the treatment of
dermatoses thus contain
the active compounds in a concentration of, for example, 0.1-99%. The
customary dose in the case of
systemic therapy (p.o.) may be between 0.03 and 60 mg/kg per day, (i. v.) may
be between 0.03 and
60 mg/kg/h. In another embodiment, the customary dose in the case of systemic
therapy (p.o.) is
between 0.3 and 30 mg/kg per day, (i. v.) is between 0.3 and 30 mg/kg/h.
The choice of the optimal dosage regime and duration of medication,
particularly the optimal dose and
manner of administration of the active compounds necessary in each case can be
determined by a
person skilled in the art on the basis of his/her expert knowledge.
Depending upon the particular disease, to be treated or prevented, additional
therapeutic active
agents, which are normally administered to treat or prevent that disease, may
optionally be
coadministered with the compounds according to this invention. As used herein,
additional therapeutic
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-82-
agents that are normally administered to treat or prevent a particular disease
are known as appropriate
for the disease being treated.
For example, compounds according to this invention may be combined with one or
more standard
therapeutic agents used for treatment of the diseases as mentioned before.
In one particular embodiment, compounds according to this invention may be
combined with one or
more art-known anti-cancer agents, such as e.g. with one or more
chemotherapeutic and/or target
specific anti-cancer agents as described below.
Examples of known chemotherapeutic anti-cancer agents frequently used in
combination therapy
include, but not are limited to (i) alkylating/carbamylating agents such as
Cyclophosphamid
(Endoxan ), Ifosfamid (Holoxan ), Thiotepa (Thiotepa Lederle ), Melphalan
(Alkeran ), or
chloroethylnitrosourea (BCNU); (ii) platinum derivatives like cis-platin
(Platinex BMS), oxaliplatin,
satraplatin or carboplatin (Cabroplat BMS); (iii) antimitotic agents /
tubulin inhibitors such as vinca
alkaloids (vincristine, vinblastine, vinorelbine), taxanes such as Paclitaxel
(Taxol ), Docetaxel
(Taxotere ) and analogs as well as new formulations and conjugates thereof,
epothilones such as
Epothilone B(Patupilone ), Azaepothilone (Ixabepilone ) or ZK-EPO, a fully
synthetic epothilone B
analog; (iv) topoisomerase inhibitors such as anthracyclines (exemplified by
Doxorubicin /
Adriblastin ), epipodophyllotoxines (examplified by Etoposide / Etopophos )
and camptothecin and
camptothecin analogs (exemplified by Irinotecan / Camptosar or Topotecan /
Hycamtin ); (v)
pyrimidine antagonists such as 5-fluorouracil (5-FU), Capecitabine (Xeloda ),
Arabinosylcytosine /
Cytarabin (Alexan ) or Gemcitabine (Gemzar ); (vi) purin antagonists such as 6-
mercaptopurine
(Puri-Nethol ), 6-thioguanine or fludarabine (Fludara ) and finally (vii)
folic acid antagonists such as
methotrexate (Farmitrexat ) or premetrexed (Alimta ).
Examples of target specific anti-cancer drug classes used in experimental or
standard cancer therapy
include but are not limited to (i) kinase inhibitors such as e.g. Imatinib
(Glivec ), ZD-1839 / Gefitinib
(Iressa ), Bay43-9006 (Sorafenib), SU11248 / Sunitinib (Sutent ) or OSI-774 /
Erlotinib (Tarceva );
(ii) proteasome inhibitors such as PS-341 / Bortezumib (Velcade ); (iii)
histone deacetylase inhibitors
like SAHA, PXD101, MS275, MGCD0103, Depsipeptide / FK228, NVP-LBH589, NVP-
LAQ824,
Valproic acid (VPA) and butyrates (iv) heat shock protein 90 inhibitors like
17-allylaminogeldanamycin
(17-AAG); (v) vascular targeting agents (VTAs) like combretastin A4 phosphate
or AVE8062 / AC7700
and anti-angiogenic drugs like the VEGF antibodies, such as Bevacizumab
(Avastin ), or KDR
tyrosine kinase inhibitors such as PTK787 / ZK222584 (Vatalanib); (vi)
monoclonal antibodies such as
Trastuzumab (Herceptin ) or Rituximab (MabThera / Rituxan ) or Alemtuzumab
(Campath ) or
Tositumab (Bexxar ) or C225/ Cetuximab (Erbitux ) or Avastin (see above) as
well as mutants and
conjugates of monoclonal antibodies, e.g. Gemtuzumab ozogamicin (Mylotarg ) or
Ibritumomab
tiuxetan (Zevalin ), and antibody fragments; (vii) oligonucleotide based
therapeutics like G-3139 /
Oblimersen (Genasense ); (viii) Toll-like receptor / TLR 9 agonists like
Promune , TLR 7 agonists
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-83-
like Imiquimod (Aldara ) or Isatoribine and analogues thereof, or TLR 7/8
agonists like Resiquimod as
well as immunostimulatory RNA as TLR 7/8 agonists; (ix) protease inhibitors
(x) hormonal therapeutics
such as anti-estrogens (e.g. Tamoxifen or Raloxifen), anti-androgens (e.g.
Flutamide or Casodex),
LHRH analogs (e.g. Leuprolide, Goserelin or Triptorelin) and aromatase
inhibitors.
Other known target specific anti-cancer agents which may be used for
combination therapy include
bleomycin, retinoids such as all-trans retinoic acid (ATRA), DNA
methyltransferase inhibitors such as
the 2-deoxycytidine derivative Decitabine (Dacogen ) and 5-azacytidine,
alanosine, cytokines such as
interleukin-2, interferons such as interferon a2 or interferon-y, death
receptor agonists, such as TRAIL,
DR4/5 agonistic antibodies, FasL and TNF-R agonists.
As exemplary anti-cancer agents, which may be useful in the combination
therapy according to the
present invention, any of the following drugs may be mentioned, without being
restricted thereto,
FU, actinomycin D, ABARELIX, ABCIXIMAB, ACLARUBICIN, ADAPALENE, ALEMTUZUMAB,
ALTRETAMINE, AMINOGLUTETHIMIDE, AMIPRILOSE, AMRUBICIN, ANASTROZOLE,
ANCITABINE, ARTEMISININ, AZATHIOPRINE, BASILIXIMAB, BENDAMUSTINE, BEVACIZUMAB,
BEXXAR, BICALUTAMIDE, BLEOMYCIN, BORTEZOMIB, BROXURIDINE, BUSULFAN, CAMPATH,
CAPECITABINE, CARBOPLATIN, CARBOQUONE, CARMUSTINE, CETRORELIX, CHLORAM-
BUCIL, CHLORMETHINE, CISPLATIN, CLADRIBINE, CLOMIFENE, CYCLOPHOSPHAMIDE,
DACARBAZINE, DACLIZUMAB, DACTINOMYCIN, DAUNORUBICIN, DECITABINE, DESLORELIN,
DEXRAZOXANE, DOCETAXEL, DOXIFLURIDINE, DOXORUBICIN, DROLOXIFENE,
DROSTANOLONE, EDELFOSINE, EFLORNITHINE, EMITEFUR, EPIRUBICIN, EPITIOSTANOL,
EPTAPLATIN, ERBITUX, ERLOTINIB, ESTRAMUSTINE, ETOPOSIDE, EXEMESTANE,
FADROZOLE, FINASTERIDE, FLOXURIDINE, FLUCYTOSINE, FLUDARABINE, FLUOROURACIL,
FLUTAMIDE, FORMESTANE, FOSCARNET, FOSFESTROL, FOTEMUSTINE, FULVESTRANT,
GEFITINIB, GENASENSE, GEMCITABINE, GLIVEC, GOSERELIN, GUSPERIMUS, HERCEPTIN,
IDARUBICIN, IDOXURIDINE, IFOSFAMIDE, IMATINIB, IMPROSULFAN, INFLIXIMAB,
IRINOTECAN, IXABEPILONE, LANREOTIDE, LETROZOLE, LEUPRORELIN, LOBAPLATIN,
LOMUSTINE, LUPROLIDE, MELPHALAN, MERCAPTOPURINE, METHOTREXATE, METUREDEPA,
MIBOPLATIN, MIFEPRISTONE, MILTEFOSINE, MIRIMOSTIM, MITOGUAZONE, MITOLACTOL,
MITOMYCIN, MITOXANTRONE, MIZORIBINE, MOTEXAFIN, MYLOTARG, NARTOGRASTIM,
NEBAZUMAB, NEDAPLATIN, NILUTAMIDE, NIMUSTINE, OCTREOTIDE, ORMELOXIFENE, OXALI-
PLATIN, PACLITAXEL, PALIVIZUMAB, PATUPILONE, PEGASPARGASE, PEGFILGRASTIM,
PEMETREXED, PENTETREOTIDE, PENTOSTATIN, PERFOSFAMIDE, PIPOSULFAN,
PIRARUBICIN, PLICAMYCIN, PREDNIMUSTINE, PROCARBAZINE, PROPAGERMANIUM,
PROSPIDIUM CHLORIDE, RALOXIFEN, RALTITREXED, RANIMUSTINE, RANPIRNASE,
RASBURICASE, RAZOXANE, RITUXIMAB, RIFAMPICIN, RITROSULFAN, ROMURTIDE,
RUBOXISTAURIN, SARGRAMOSTIM, SATRAPLATIN, SIROLIMUS, SOBUZOXANE, SORAFENIB,
SPIROMUSTINE, STREPTOZOCIN, SUNITINIB, TAMOXIFEN, TASONERMIN, TEGAFUR,
TEMOPORFIN, TEMOZOLOMIDE, TENIPOSIDE, TESTOLACTONE, THIOTEPA, THYMALFASIN,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-84-
TIAMIPRINE, TOPOTECAN, TOREMIFENE, TRAIL, TRASTUZUMAB, TREOSULFAN,
TRIAZIQUONE, TRIMETREXATE, TRIPTORELIN, TROFOSFAMIDE, UREDEPA, VALRUBICIN,
VATALANIB, VERTEPORFIN, VINBLASTINE, VINCRISTINE, VINDESINE, VINORELBINE,
VOROZOLE and ZEVALIN.
The anti-cancer agents mentioned herein above as combination partners of the
compounds according
to this invention are meant to include pharmaceutically acceptable derivatives
thereof, such as e.g.
their pharmaceutically acceptable salts.
The person skilled in the art is aware on the base of his/her expert knowledge
of the kind, total daily
dosage(s) and administration form(s) of the additional therapeutic agent(s)
coadministered. Said total
daily dosage(s) can vary within a wide range.
In practicing the present invention, the compounds according to this invention
may be administered in
combination therapy separately, sequentially, simultaneously, concurrently or
chronologically
staggered (such as e.g. as combined unit dosage forms, as separate unit dosage
forms, as adjacent
discrete unit dosage forms, as fixed or non-fixed combinations, as kit-of-
parts or as admixtures) with
one or more standard therapeutics (chemotherapeutic and/or target specific
anti-cancer agents), in
particular art-known anti-cancer agents, such as any of e.g. those mentioned
above.
In this context, the present invention further relates to a combination
comprising
a first active ingredient, which is at least one compound according to this
invention, and
a second active ingredient, which is at least one art-known anti-cancer agent,
such as e.g. one or
more of those mentioned herein above,
for separate, sequential, simultaneous, concurrent or chronologically
staggered use in therapy, such
as e.g. in therapy of any of those diseases mentioned herein.
The term "combination" according to this invention may be present as a fixed
combination, a non-fixed
combination or a kit-of-parts.
A "fixed combination" is defined as a combination wherein the said first
active ingredient and the said
second active ingredient are present together in one unit dosage or in a
single entity. One example of
a "fixed combination" is a pharmaceutical composition wherein the said first
active ingredient and the
said second active ingredient are present in admixture for simultaneous
administration, such as in a
formulation. Another example of a "fixed combination" is a pharmaceutical
combination wherein the
said first active ingredient and the said second active ingredient are present
in one unit without being
in admixture.
A "kit-of-parts" is defined as a combination wherein the said first active
ingredient and the said second
active ingredient are present in more than one unit. One example of a "kit-of-
parts" is a combination
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-85-
wherein the said first active ingredient and the said second active ingredient
are present separately.
The components of the may be administered separately, sequentially,
simultaneously, concurrently or
chronologically staggered.
The present invention further relates to a pharmaceutical composition
comprising
a first active ingredient, which is at least one compound according to this
invention, and
a second active ingredient, which is at least one art-known anti-cancer agent,
such as e.g. one or
more of those mentioned herein above, and, optionally,
a pharmaceutically acceptable carrier or diluent,
for separate, sequential, simultaneous, concurrent or chronologically
staggered use in therapy.
The present invention further relates to a combination product comprising
a.) at least one compound according to this invention formulated with a
pharmaceutically acceptable
carrier or diluent, and
b.) at least one art-known anti-cancer agent, such as e.g. one or more of
those mentioned herein
above, formulated with a pharmaceutically acceptable carrier or diluent.
The present invention further relates to a kit-of-parts comprising a
preparation of a first active
ingredient, which is a compound according to this invention, and a
pharmaceutically acceptable carrier
or diluent; a preparation of a second active ingredient, which is an art-known
anti-cancer agent, such
as one of those mentioned above, and a pharmaceutically acceptable carrier or
diluent; for
simultaneous, concurrent, sequential, separate or chronologically staggered
use in therapy. Optionally,
said kit comprises instructions for its use in therapy, e.g. to treat
(hyper)proliferative diseases and/or
disorders responsive to the induction of apoptosis, such as e.g. cancer, more
precisely, any of those
cancer diseases described above.
The present invention further relates to a combined preparation comprising at
least one compound
according to this invention and at least one art-known anti-cancer agent for
simultaneous, concurrent,
sequential or separate administration.
The present invention further relates to combinations, compositions,
formulations, preparations or kits
according to the present invention having Eg5 inhibitory activity and/or anti-
proliferative and/or
apoptosis inducing properties.
In addition, the present invention further relates to a method for treating in
combination therapy
(hyper)proliferative diseases and/or disorders responsive to the induction of
apoptosis, such as e.g.
cancer, in a patient comprising administering a combination, composition,
formulation, preparation or
kit as described herein to said patient in need thereof.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-86-
In addition, the present invention further relates to a method for treating
(hyper)proliferative diseases
of benign or malignant behaviour and/or disorders responsive to the induction
of apoptosis, such as
e.g. cancer, in a patient comprising administering in combination therapy
separately, simultaneously,
concurrently, sequentially or chronologically staggered a pharmaceutically
active and therapeutically
effective and tolerable amount of a pharmaceutical composition, which
comprises a compound
according to this invention and a pharmaceutically acceptable carrier or
diluent, and a
pharmaceutically active and therapeutically effective and tolerable amount of
one or more art-known
anti-cancer agents, such as e.g. one or more of those mentioned herein, to
said patient in need
thereof.
In further addition, the present invention relates to a method for treating,
preventing or ameliorating
(hyper)proliferative diseases and/or disorders responsive to induction of
apoptosis, such as, for
example, benign or malignant neoplasia, e.g. cancer, particularly any of those
cancer diseases
mentioned herein, in a patient comprising administering separately,
simultaneously, concurrently,
sequentially or chronologically staggered to said patient in need thereof an
amount of a first active
compound, which is a compound according to the present invention, and an
amount of at least one
second active compound, said at least one second active compound being a
standard therapeutic
agent, particularly at least one art-known anti-cancer agent, such as e.g. one
or more of those
chemotherapeutic and target-specific anti-cancer agents mentioned herein,
wherein the amounts of
the first active compound and said second active compound result in a
therapeutic effect.
In yet further addition, the present invention relates to a method for
treating, preventing or
ameliorating (hyper)proliferative diseases and/or disorders responsive to
induction of apoptosis, such
as e.g. benign or malignant neoplasia, e.g. cancer, particularly any of those
cancer diseases
mentioned herein, in a patient comprising administering a combination
according to the present
invention.
In addition, the present invention further relates to the use of a
composition, combination, formulation,
preparation or kit according to this invention in the manufacture of a
pharmaceutical product, such as
e.g. a commercial package or a medicament, for treating, preventing or
ameliorating
(hyper)proliferative diseases, such as e.g. cancer, and/or disorders
responsive to the induction of
apoptosis, particularly those diseases mentioned herein, such as e.g.
malignant or benign neoplasia.
The present invention further relates to a commercial package comprising one
or more compounds of
the present invention together with instructions for simultaneous, concurrent,
sequential or separate
use with one or more chemotherapeutic and/or target specific anti-cancer
agents, such as e.g. any of
those mentioned herein.
The present invention further relates to a commercial package consisting
essentially of one or more
compounds of the present invention as sole active ingredient together with
instructions for
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-87-
simultaneous, concurrent, sequential or separate use with one or more
chemotherapeutic and/or target
specific anti-cancer agents, such as e.g. any of those mentioned herein.
The present invention further relates to a commercial package comprising one
or more
chemotherapeutic and/or target specific anti-cancer agents, such as e.g. any
of those mentioned
herein, together with instructions for simultaneous, concurrent, sequential or
separate use with one or
more compounds according to the present invention.
The compositions, combinations, preparations, formulations, kits or packages
mentioned in the
context of the combination therapy according to this invention may also
include more than one of the
compounds according to this invention and/or more than one of the art-known
anti-cancer agents
mentioned.
The first and second active ingredient of a combination or kit-of-parts
according to this invention may
be provided as separate formulations (i.e. independently of one another),
which are subsequently
brought together for simultaneous, concurrent, sequential, separate or
chronologically staggered use
in combination therapy; or packaged and presented together as separate
components of a
combination pack for simultaneous, concurrent, sequential, separate or
chronologically staggered use
in combination therapy.
The type of pharmaceutical formulation of the first and second active
ingredient of a combination or
kit-of-parts according to this invention can be similar, i.e. both ingredients
are formulated in separate
tablets or capsules, or can be different, i.e. suited for different
administration forms, such as e.g. one
active ingredient is formulated as tablet or capsule and the other is
formulated for e.g. intravenous
ad m in istration.
The amounts of the first and second active ingredients of the combinations,
compositions or kits
according to this invention may together comprise a therapeutically effective
amount for the
treatment, prophylaxis or amelioration of a (hyper)proliferative diseases
and/or a disorder responsive
to the induction of apoptosis, particularly one of those diseases mentioned
herein, such as e.g.
malignant or benign neoplasia, especially cancer, like any of those cancer
diseases mentioned herein.
In addition, compounds according to the present invention can be used in the
pre- or post-surgical
treatment of cancer.
In further addition, compounds of the present invention can be used in
combination with radiation
therapy.
A combination according to this invention can refer to a composition
comprising both the compound(s)
according to this invention and the other active anti-cancer agent(s) in a
fixed combination (fixed unit
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-88-
dosage form), or a medicament pack comprising the two or more active
ingredients as discrete
separate dosage forms (non-fixed combination). In case of a medicament pack
comprising the two or
more active ingredients, the active ingredients are preferably packed into
blister cards which are
suited for improving compliance.
Each blister card preferably contains the medicaments to be taken on one day
of treatment. If the
medicaments are to be taken at different times of day, the medicaments can be
disposed in different
sections on the blister card according to the different ranges of times of day
at which the medicaments
are to be taken (for example morning and evening or morning, midday and
evening). The blister
cavities for the medicaments to be taken together at a particular time of day
are accommodated in the
respective range of times of day. The various times of day are, of course,
also put on the blister in a
clearly visible way. It is also possible, of course, for example to indicate a
period in which the
medicaments are to be taken, for example stating the times.
The daily sections may represent one line of the blister card, and the times
of day are then identified
in chronological sequence in this column.
Medicaments which must be taken together at a particular time of day are
placed together at the
appropriate time on the blister card, preferably a narrow distance apart,
allowing them to be pushed
out of the blister easily, and having the effect that removal of the dosage
form from the blister is not
forgotten.
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-89-
Biological Investigations
The ATPase activity of Eg5 kinesin motor domains (Cytoskeleton, cat. No. EG01)
can be used to
monitor the effects of modulating agents. The test compounds are dissolved as
10 mM solutions in
dimethylsulfoxide (DMSO). 2 lal of appropriate DMSO dilutions of the test
compounds are added to
each well of a 96 well flat bottom plate. Each compound dilution is tested as
triplicates. The reagents
are added and the final reaction of the standard assay contains 15 mM Pipes,
pH 6.8, 5.0 mM MgCl2,
0.5 mM KCI, 1 mM EGTA, 0.1 mg/ml BSA, 1 pM Paclitaxel, 250 nM preformed
microtubules
(Cytoskeleton, cat. No. MT001), 300 pM ATP, and Eg5 protein (50 ng) in a
reaction volume of 100 lal.
The controls include buffer wells with ATP and 2% DMSO. Reactions are started
by the addition of
ATP, incubated at room temperature for 30 min., and terminated by removing 20
lal of the reaction
volume and adding it to 80 lal of 1 M perchloric acid, followed by the
addition of 80 lal Malachite green
reagent. Malachite green reagent is prepared by mixing a solution of 4.2 g
ammonium molybdate in
100 ml 4 N HCI with a solution of 0.135 g Malachite green in 300 ml H20. The
reactions are incubated
for a further 20 min. and then read at 615 nm.
The corresponding IC50 values of the compounds for Eg5 inhibition are
determined from the
concentration-effect curves.
Representative inhibitory values [measured as -log IC50 (mol/1)] determined in
the aforementioned
assay follow from the following table A, in which the numbers of the compounds
correspond to the
numbers of the examples.
Table A
Inhibition of Eg5 activity
Compound -log IC50 [mol/1]
2 to 9, 9a, 10 and
The inhibitory values of these listed
11
compounds are all > 6.6
The anti-proliferative / cytotoxic activity of the compounds described herein
can be tested on
subclones of RKO human colon adenocarcinoma cells (Schmidt et al., Oncogene
19, 2423-2429;
2000) using the Alamar Blue cell viability assay (described in O'Brien et al.
Eur J Biochem 267, 5421-
5426, 2000). The compounds are dissolved as 10 mM solutions in DMSO and
subsequently diluted in
semi-logarithmic steps. DMSO dilutions are further diluted 1:100 into
Dulbecco's modified Eagle's
medium (DMEM) containing 10% fetal calf serum to a final concentration twice
as much as the final
concentration in the test. RKO subclones are seeded into 96 well flat bottom
plates at a density of
4000 cells per well in a volume of 50 lal per well. 24 hours after seeding the
50 lal each of the
compound dilutions in DMEM medium are added into each well of the 96 well
plate. Each compound
dilution is tested as triplicates. Wells containing untreated control cells
are filled with 50 lal DMEM
medium containing 1% DMSO. The cells are then incubated with the substances
for 72 hours at 37 C
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 90 -
in a humidified atmosphere containing 5% carbon dioxide. To determine the
viability of the cells, 10 lal
of an Alamar Blue solution (Biosource) are added and the fluorescence is
measured at an extinction of
544 nm and an emission of 590 nm. For the calculation of the cell viability
the emission value from
untreated cells is set as 100% viability and the emission rates of treated
cells are set in relation to the
values of untreated cells. Viabilities are expressed as % values. The Graphpad
Prism program is used
for the calculation of EC50 values for anti-proliferative / cytotoxic activity
out of the obtained dose-
response curves.
To determine the cell cycle specific mode of action, subclones of RKO colon
adenocarcinoma cells
(RKOp27 as described by Schmidt et al. in Oncogene 19, 2423-2429; 2000) are
seeded into 96 well
flat bottom plates at a density of 16000 cells per well in a volume of 50 lal
per well in DMEM growth
medium with 10% FCS containing 10 pM Ponasterone A. 24 hours after seeding the
50 lal each of the
compound dilutions in DMEM medium are added into each well of the 96-well
plate. Each compound
dilution is tested as triplicates. Wells containing untreated control cells
are filled with 50 lal DMEM
medium containing 1% DMSO. The cells are then incubated with the substances
for 72 hours at 37 C
in a humidified atmosphere containing 5% carbon dioxide. To determine the
viability of the cells, 10 lal
of an Alamar Blue solution (Biosource) are added and the fluorescence is
measured at an extinction of
544 nm and an emission of 590 nm. For the calculation of the cell viability
the emission value from
untreated cells is set as 100% viability and the emission rates of treated
cells are set in relation to the
values of untreated cells. Viabilities are expressed as % values. The Graphpad
Prism program is used
for the calculation of EC50 values out of the obtained dose-response curves.
Viability is compared of
proliferating cells grown in the absence of the inducer Ponasterone A, versus
viability of cells arrested
by the expression of ectopic p27Kipl induced by Ponasterone A.
Representative values for anti-proliferation / cytotoxicity [measured as -log
EC50 (mol/1)] determined in
the aforementioned assays follow from the following table B, in which the
numbers of the compounds
correspond to the numbers of the examples.
Table B
Anti-proliferative / cytotoxic activity on RKO colon cancer cells
Examples
-log EC50 [mol/1]
2, 3, 4, 5, 6, 7, 8, 8a, 9a, 13,
RKO p27 uninduced
14, 17, 18, 19, 20, 21, 22, 24
(proliferating) > 7.0
-log EC50 [mol/1]
RKO p27 uninduced
1, 9, 10, 11, 12, 15, 16, 23
(proliferating) < 7.0 but
> 6.0
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
-91 -
The value of -log EC50 [mol/1] RKO p27 induced (arrested) was below the
minimum determined by the
assay specification (<_ 5.0, <_ 5.5 or <_ 6.0).
The induction of apoptosis can be measured by using a Cell death detection
ELISA (Roche
Biochemicals, Mannheim, Germany). NCI-H460 non-small cell lung cancer cells
are seeded into 96
well flat bottom plates at a density of 10000 cells per well in a volume of 50
tal RPMI medium
(containing 10% fetal calf serum) per well. The compounds are dissolved as 10
mM solutions in
DMSO and subsequently diluted in semi-logarithmic steps. DMSO dilutions are
further diluted 1:100
into RPMI medium (containing 10% fetal calf serum) to a final concentration
twice as much as the
final concentration in the test. 24 hours after seeding the 50 tal each of the
compound dilutions in
RPMI medium are added into each well of the 96 Well plate. Each compound
dilution is tested at least
as duplicates. Wells containing untreated control cells are filled with 50 tal
RPMI medium containing
1% DMSO. The cells are then incubated with the substances for 24 hours at 37 C
in a humidified
atmosphere containing 5% carbon dioxide. As a positive control for the
induction of apoptosis, cells
are treated with 50 pM Cisplatin (Gry Pharmaceuticals, Kirchzarten, Germany).
Medium is then
removed and the cells are lysed in 200 tal lysis buffer. After centrifugation
as described by the
manufacturer, 10 tal of cell lysate is processed as described in the protocol.
The degree of apoptosis is
calculated as follows: The absorbance at 405 nm obtained with lysates from
cells treated with 50 pM
cisplatin is set as 100 cpu (cisplatin units), while an absorbance at 405 nm
of 0.0 is set as 0.0 cpu. The
degree of apoptosis is expressed as cpu in relation to the value of 100 cpu
reached with the lysates
obtained from cells treated with 50 pM cisplatin.
Experimental perturbation of Eg5 function causes a characteristic malformation
of the mitotic spindle,
which can be examined by confocal laser scanning microscopy. HeLa cervical
cancer cells are grown
overnight on glass cover slips (NuncTM Lab-TekTM Chamber Slides) in 1800 tal
DMEM medium
containing 10% fetal calf serum. The test compounds are dissolved as 10 mM
solutions in DMSO.
Appropriate DMSO dilutions of the test compounds are further diluted 1:20 into
DMEM medium
containing 10% fetal calf serum to a final concentration ten times as much as
the final concentration in
the test. 24 hours after seeding, 200 tal of the compound dilutions in DMEM
medium are added into
each well of the cover slip. As a control, 200 tal DMEM medium containing 5%
DMSO are added. 24
hours after incubation with the test compounds, the cells are washed with PBS,
and fixed with 3.7%
formaldehyde in H20 for 20 min. at 37 C. Subsequently, cells are washed with
PBS and incubated
with 0,1% Triton X-100 in a buffer containing 1.471 mM KH2PO4, 8.504 mM
NazHPO4, 137 mM NaCI,
1.325 mM CaCl2, 2.685 mM KCI, 0.542 mM MgCl2, pH 7.2 for 15 min. at room
temperature. For
saturation of non-specific binding, cells are incubated in 2% BSA/10% FCS in
PBS (= blocking buffer)
for 30 min. at room temperature prior to incubation with anti-alpha tubulin
monoclonal antibodies
(Sigma, #T5168; 1:1000), followed by Cy3-conjugated rabbit anti-mouse IgG
(H+L) antibody (Jackson
Immuno Research; 1:1000). All antibody incubations are performed for one hour
at 37 C in blocking
buffer, and cells are washed three times in PBS between different incubations.
DNA is counterstained
with Hoechst 33342 (0.1 tag/ml). Coverslips are mounted in Vectashield (Vector
Laboratories,
CA 02655267 2008-12-12
WO 2007/144384 PCT/EP2007/055846
- 92 -
Burlingame, CA) and examined with a Leica TCS SP2 confocal laser scanning
microscope fitted with
appropriate filters (Leica Microsystems, Bensheim, Germany).
Some of the compounds according to this invention may be efficacious against p-
glycoprotein
mediated multidrug-resistent tumour cell lines (e.g. HCT-15), that can be
measured as follows:
All cell lines used are cultured at standard conditions in a tissue culture
incubator at 37 C, 5% COz
and 95% humidity. At day 1, cells are detached with Trypsin / EDTA and
pelleted by centrifugation.
Cells are resuspended at the appropriate density in culture medium, seeded
into 96well microtiter
plates and incubated over night in a tissue culture incubator at 37 C, 5% COz
and 95% humidity.
Stock solution of all compounds to be tested are dissolved at 10mM in DMSO and
at day 2 added to
the microtiter plates in the desired dilutions. The final DMSO concentration
in the microtiter plates is
kept at 0.5 %. Control cells are treated with culture medium including a final
concentration of 0.5%
DMSO only. The microtiter plates are incubated with the compounds in a tissue
culture incubator at
37 C, 5% COz and 95% humidity for further 72 hours. To determine the viability
of the cells at day 5,
an Alamar Blue solution (Biosource) is added at 1/10 culture volume to the
microtiter plates. The cells
are incubated in a tissue culture incubator at 37 C, 5% COz and 95% humidity
for additional 1-6 hours
and the fluorescence is measured at an extinction of 544 nm and an emission of
590 nm. For the
calculation of the cell viability the emission value from untreated cells is
set as 100% viability and the
emission rates of treated cells are set in relation to the values of untreated
cells. Viabilities are
expressed as % values.
The Graphpad Prism program is used for the calculation of EC50 values out of
the obtained dose-
response curves.