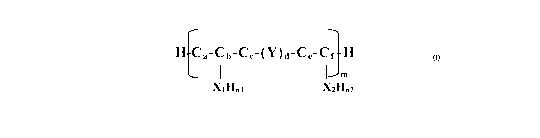Note: Descriptions are shown in the official language in which they were submitted.
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
1
FORMULATIONS WITH FERULOYL GLYCERIDES
AND METHODS OF PREPARATION
CROSS-REFERENCE TO RELATED APPLICATIONS
[01] This application claims priority to U.S. Provisional Application No.
60/817,537, filed
on June 29, 2006, and entitled "Formulations with Feruloyl Glycerides and
Methods of
Preparation, which is hereby incorporated herein by reference in its entirety.
FIELD OF THE INVENTION
[02] The present invention relates to feruloyl glycerides, their method of
preparation, and
their use in personal care consumer product applications.
BACKGROUND OF THE INVENTION
[03] Feruloyl-substituted and coumaryl-substituted acylglycerols, their method
of
preparation through the transesterfication of a triglyceride and a ferulic or
coumaric
ester, and the use of these compounds as sunscreen ingredients is taught in
U.S. Patent
No. 6,346,236, which incorporated herein by reference in its entirety.
SUMMARY OF THE INVENTION
[04] Feruloyl glycerides have been discovered and are the subject matter of
pending patent
applications. See U.S. Provisional Application Serial No. 60/723,209, filed
October 3,
2005 and entitled "Accelerated Feruloylation of Vegetable Oils," which is
incorporated
herein by reference, and U.S. Patent Application Serial No. 11/425,094, [Atty.
Docket
No. 1396-00601], filed June 19, 2006, and entitled "Compositions Comprising A
UV-
Absorbing Chromophore," and U.S. Patent Application Serial No. 11/425,096,
[Atty.
Docket No. 1396-00602], filed June 19, 2006, and entitled "Methods of Making
Compositions Comprising A UV-Absorbing Chromophore," all of which are
incorporated by reference herein in their entireties.
[05] It has been discovered that these compounds provide a springboard into a
broad
spectrum of formulating applications within the personal care and over-the-
counter
(OTC) product categories. Unlike traditional ingredients, the feruloyl
glycerides of the
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
2
present invention have multi-functional properties, and as a result provide a
multitude
of unexpected benefits and superior characteristics to skin, hair, bath,
dental and OTC
products.
[06] Disclosed herein products for topical application comprising a chemical
composition
comprising a linker agent and a compound comprising at least one UV-absorbing
chromophore, wherein the linker agent is characterized by the general formula:
H Ca-Cb-Cc-(Y)d-Ce-Cf H
I I m
X'1 Hn1 X'2Hn2
wherein Xi and X2 are the same or different, and at least one of Xi or X2 is a
functional
group that bonds with the compound comprising at least one UV-absorbing
chromophore, and b+f > 2, Y comprises an 0, N, or S that is substituted or
unsubstituted, each a, b, c, e and f is > 0 and a+b+c+e+f _2, d is 0 or 1, nl
and n2
represent the number of hydrogen atoms required to complete the undesignated
valencies, and m ranges from 1 to about 100 and each individual m unit may be
the
same or different, and a topically acceptable agent that is different from the
linker agent
and the compound comprising at least one UV-absorbing chromophore.
[07] Also disclosed herein are products for topical application comprising
formulations
comprising a fat soluble composition comprising a mono or diacylglycerol
esterified
with a plant-derived functional group comprising an aromatic species, an
unsaturated
isoprenoid, an unsaturated terpenoid, a hindered hydroxy-substituted cinnamic
acid, an
unhindered hydroxy-substituted cinnamic acid or combinations thereof, and a
topically
acceptable agent that is different from the fat soluble composition.
[08] Also disclosed herein are products for topical application comprising
formulations
comprising a fat-soluble composition, comprising a glycerol esterified with a
plant-
derived functional group comprising maleanilic acid, homovanillic acid, folic
acid,
crocetin, coumaric acid, caffeic acid, ferulic acid, sinapic acid (sinapinic
acid),
derivatives thereof or combinations thereof, wherein the esterified glycerol
includes at
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
3
least two plant-derived functional groups, and a topically acceptable agent
that is
different from fat-soluble composition.
[09] Also disclosed herein products for topical application comprising
formulations
comprising a chemical composition comprising a linker agent and a compound
comprising at least one UV absorbing chromophore, wherein the linker agent is
characterized by the general formula:
X3 Xa
I I
H Ca-Cb-Cc-(Y)d-Ce-Cf H
I I m
X'1 Hn1 X'2Hn2
wherein Xi and X2 are the same or different at least one of Xi or X2 is a
functional group
that bonds with the compound comprising at least one UV-absorbing chromophore,
and
b+f > 2, X3 and X4 are the same or different and X3, X4 or both is a
hydrophobic moiety,
Y comprises an 0, N, or S that is substituted or unsubstituted, each a, b, c,
e and f is > 0
and a+b+c+e+f _2, d is 0 or 1, nl and n2 represent the number of hydrogen
atoms
required to complete the undesignated valencies, and m ranges from 1 to about
100 and
each individual m unit may be the same or different, and a topically
acceptable agent that
is different from the linker agent and the compound comprising at least one UV-
absorbing
chromophore.
[10] Also disclosed herein are products for topical application comprising
formulations
comprising a chemical composition comprising at least two compounds having the
general formula:
X3 Xa
I I
H Ca-Cb-Cc-(Y)d-Ce-Cf H
I I m
X'1 Hn1 X'2Hn2
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
4
wherein Xi and Xz are different at least one of Xi or Xz is a functional group
that bonds
with a compound comprising at least one UV-absorbing chromophore, and b+f > 2,
X3
and X4 are the same or different and X3, X4 or both is a hydrophobic moiety, Y
comprises
an 0, N, or S that is substituted or unsubstituted, each a, b, c, e and f is >
0 and
a+b+c+e+f _2, d is 0 or 1, nl and n2 represent the number of hydrogen atoms
required to
complete the undesignated valencies, m ranges from 1 to about 100 and each
individual m
unit may be the same or different, and wherein the compound comprising at
least one
UV-absorbing chromophore comprises a phytochemical which further comprises an
aromatic species, an unsaturated isoprenoid, an unsaturated terpenoid, a
hindered
hydroxy-substituted cinnamic acid, an unhindered hydroxy-substituted cinnamic
acid or
combinations thereof, and a topically acceptable agent that is different from
the chemical
composition comprising at least two compounds.
[11] The foregoing has outlined rather broadly the features and technical
advantages of the
present invention in order that the detailed description of the invention that
follows may
be better understood. Additional features and advantages of the invention will
be
described hereinafter that form the subject of the claims of the invention. It
should be
appreciated by those skilled in the art that the conception and the specific
embodiments
disclosed may be readily utilized as a basis for modifying or designing other
structures
for carrying out the same purposes of the present invention. It should also be
realized
by those skilled in the art that such equivalent constructions do not depart
from the
spirit and scope of the invention as set forth in the appended claims.
DETAILED DESCRIPTION OF EMBODIMENTS
[12] The formulations of the present invention comprise compounds that are
described in
U.S. Patent Application Serial No. 11/425,094, [Atty. Docket No. 1396-00601],
filed
June 19, 2006, and entitled "Compositions Comprising A UV-Absorbing
Chromophore," and U.S. Patent Application Serial No. 11/425,096, [Atty. Docket
No.
1396-00602], filed June 19, 2006, and entitled "Methods of Making Compositions
Comprising A UV-Absorbing Chromophore," both of which are incorporated by
reference herein in their entireties.
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
[13] Described below are several product applications for formulations in
accordance with
the present invention. Each application has been made and tested in blind
coded
consumer panel samples. These tests demonstrate superior aesthetic and
consumer
product characteristics arising from the compounds of the present invention.
The
product applications for using the feruloyl glycerides in the present
invention are not
limited to examples cited herein. Rather, these examples are evidence of the
unique
multi-functional benefits that are provided with the use of these naturally
derived mono,
di and triglycerides.
[14] Before offering detailed formula examples of product applications wherein
superior
consumer benefits have been realized through the use of feruloyl glycerides,
it is worth
a few sentences to review the multi-functional attributes that this material
has exhibited.
[15] The feruloyl glycerides used in accordance with the present invention are
lipophilic in
nature, with a unique fingerprint of saturated and unsaturated esterified
fatty acids,
feruloyl esters and hydroxyl groups. As such, the feruloyl glycerides of the
present
invention are miscible with many other oil-like substances, have a high kb
(kauri
butanol) value and solvent power, and have excellent skin and hair absorption
and/or
penetration characteristics. The feruloyl glycerides of the present invention
provide the
skin with superior moisture barrier properties, add light reflective or
radiance
characteristics to dull, dry skin, absorb both Ultra Violet A and B
wavelengths of
sunlight, offer the skin superior antioxidant protection at various levels
within the
epidermis, and improve the emollient and dry feel characteristics of the skin.
The
feruloyl glycerides of the present invention protect colored hair from sun
bleaching (i.e.
elimination of the color producing chromophore in oxidized dye, such as
paraphenylenediamine, plus peroxide), and add conditioning benefits and light
reflection to hair. The feruloyl glycerides also help prevent the photodamage
that causes
all hair types to develop undersirable combing properties after UV exposure.
This
damage is frequently observed in the cuticle (outer skin of hair fiber) of the
hair fiber.
The prevention of photodamage to gray hair swatches can be observed in combing
force
of treated and control hair tresses, contact angle measurements, and
preserving
tryptophan, a key amino acid that decomposes on exposure to UV radiation. The
feruloyl glycerides of the present invention protect lips, skin and hair
against
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
6
environmental damage, and create a synergistic effect with many other cosmetic
and
over-the-counter ("OTC") ingredients.
[16] In the above paragraph, we indicated that the feruloyl glycerides have
excellent solvent
characteristics. This solvency is shown in the following phospholipid vitamin
complex
lotions. 1) A clear solution of 10% cholesterol powder in 90% feruloyl soy
glyceride, 2)
A set of clear solutions of 10% Vitamins E, A, D3, F (gamma linolenic acid)
and 90%
feruloyl soy glycerides 3) A set of clear solutions comprising 10% Uniqema's
"ArlasilkTM Phospholipid EFA [ Linoleamidopropyl PG-Dimonium Chloride
Phosphate] and 90% feruloyl soy glycerides, 4) A series of solutions
containing OTC
sunscreens Avobenzone (3%), Benzophenone (5%), Octyl salicylate (4%), Octyl
methoxycinnamate (7.5%), Octocrylene (10%) and q.s (quotient sufficient to
make
100%) with feruloyl soy glycerides. All solutions remained stable for more
than 3
months at 75F.
[17] Manufacturing Procedure: (for all of the formulations listed below)
[18] In general, add Phase A components to main batch tank. Mix using a high
shear mixer
(e.g. a Lightning Mixer No. 4). Sprinkle thickener (if any) into vortex,
created by a
mixer. Mix until particles are dissolved or dispersed, while heating to 55 C.
The
feruloyl glyceride in the following examples comprised a family of feruloyl
glycerides
made from soy. It will be recognized that any feruloyl glyceride derived from
any
source or in any manner is encompassed within this invention. Feruloyl
glyceride can
be added to the oil phase ingredients in phase B, in a suitably sized premix
vessel,
heated and/or mixed until dissolved and/or dispersed, and then added to the
main batch
(phase A) with mixing.
[19] Heat Phase B to 55 C, with mixing. When particles are dissolved, add
Phase B to Phase
A with vigorous mixing.
[20] Turn on slow sweep and cool batch to 40 C, add other Phases.
[21] Sample to Quality Control for review and approval at 25 C.
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
7
[22] Feruloyl glyceride is an oil based component and has an oily feel at room
temperature.
Feruloyl glyceride is not water soluble, and it can act as a solvent for
ingredients that
are difficult to dissolve in water. In some embodiments, it may be desirable
to suspend
feruloyl glyceride withn a matrix of emulsion or gel. In some embodiments, it
may be
desirable to obtain an emulsion comprising feruloyl glyceride using a suitable
emulsifier. In some embodiments, it may be desirable to incorporate such an
emulsion
in a final product that has a desired viscosity, and to achieve such a desired
viscosity, a
suitable viscosity modifier may be used. An emulsifier and/or viscosity
modifier and/or
surfactant can be a topically acceptable agent that is different from the
linker agent or
the compound comprising at least one UV-absorbing chromophore.
[23] The pH of the final product comprising feruloyl glyceride can be any
acceptable pH that
is compatible with the purpose of the final product. For example, but not by
way of
limitation, when the final product is to be applied to the skin or hair, the
pH range can
be about 2.5 to about 9Ø For example, but not by way of limitation, when the
final
product is a shampoo, the pH range can be about 6.0 to about 8Ø For example,
but not
by way of limitation, when the final product is a moisture skin care product,
the pH
range can be about 4.0 to about 6Ø For example, but not by way of
limitation, when
the final product is an exfoliant and/or hair conditioner, the pH range can be
about 2.0
to about 4Ø The final product can be hypoallergenic.
[24] Example 1
[25] Phospholipid Vitamin Complex Lotion
[26] Phase Wt. % Ingredient
A 69.5 Water
A 2.0 Hydroxyethylcellulose (a viscosity modifier)
A 5.0 Glycerin (a skin emollient)
A 2.8 Ascorbic Acid (Vitamin C) (an antioxidant)
A 0.1 Phytonadione (Vitamin K) (an antioxidant)
A 0.1 Biotin (Vitamin B7) (an antioxidant)
B 3.0 Stearyl Alcohol (a stabilizer and for thickening the oil
phase)
B 2.0 Glyceryl Stearate (a stabilizer and for thickening the oil
phase)
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
8
B 0.5 Squalane (a skin emollient)
B 0.5 Olive Oil Amidopropyl Phosphatidyl PG-Dimonium
Chloride (an emulsifier, also comprising what issometimes called
an essential fatty acid ("EFA") of a phospholipid
B 3.0 Tocopherol Acetate (Vitamin E)
B 0.5 Retinyl Palmitate (Vitamin A)
B 0.5 Cholecaliferol (Vitamin D3 )
B 0.5 Linoleic Acid (Vitamin F)
B 2.0 PEG-40 Stearate (an emulsifier)
B 5.0 Feruloyl Glyceride
B 0.1 Ubiquinone 50 (Coenzyme Q10) (an antioxidant)
B 1.0 Cyclomethicone (a smoothing agent and a component that
provides water resistance so final product does not
undesirably wash off skin easily)
B 0.9 Steareth-20 (an emulsifier)
C 0.2 Fragrance
D 0.3 Water
D 0.3 Phenoxyethanol (a preservative)
D 0.2 Methylparaben (a preservative)
100.00
[27] It is well known that certain vitamins and amino acids are referred to as
"essential" in
nutritional products, since the body does not make these materials and one
must include
them in one's diet. In topically applied products they are equally important.
Both
Vitamin E and C are well known for their antioxidant properties within the
skin and
also for the fact that they work together in maintaining a natural defense
against
radicals. There is a significant amount of scientific evidence that the
combination of
Vitamin E and C significantly reduced the sunburn reaction to UVb irradiation.
There is
also evidence of the benefit of applying Vitamin D3 to the skin. The feruloyl
glycerides
of the present invention are excellent solvents for cholesterol and its
derivatives which
are excellent skin conditioning agents. Vitamin D3 is easily solubilized as
well as other
antioxidants, such as Coenzyme Q-10 [ubiquinone] or Superoxide Dismutase. If
desired, a chelating agent, e.g., ethylenediamine tetraacetic acid ("EDTA"),
can be
added in the above example, and a corresponding amount of water can be reduced
if a
chelating agent (often in solution) is added.
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
9
[28] In pre-sun and post-sun applications during a weekend, panelists treating
one arm with
the cream described above and a second arm with a similar control cream
without the
feruloyl glycerides of the present invention. When compared side by side, the
arms
treated with the feruloyl glycerides of the present invention had far less
sunburn and
blistering versus the control arm. All panelists verified that they had spent
a minimum
of 4 total hours in the sun on Saturday and Sunday.
[29] In the above example, it is estimated that the SPF in the control was
about 2-4, and that
the SPF of the product containing feruloyl glycerides in accordance with the
invention
was about 7-10.
[30] Example 2
[31] Water Resistant Sunscreen with Feruloyl Glycerides
[32] Phase Wt. % Ingredient
A 51.40 Deionized Water
A 1.50 Butylene Glycol (an emollient)
A 0.20 Carbomer 940 (a visocity modifier)
B 7.50 Octinoxate (a sunscreen)
B 6.00 Oxybenzone (a sunscreen)
B 5.00 Octisalate (a sunscreen)
B 10.00 Octocrylene (a sunscreen)
B 8.00 Feruloyl Glyceride
B 1.50 PEG 40 Stearate (an emulsifier)
B 0.50 Steareth 20 (an emulsifier)
C 5.00 Deionized Water
C 0.30 Triethanolamine (a component that acts to
neutralize Carbomer 940)
D 2.00 Acrylates/C12-22 Alkylmethacrylate Copolymer
(a component that provides water resistance
so final product does not undesirably wash
off skin easily)
E 0.60 Propylene Glycol and/or
lodopropynyl Butylcarbamate (Liquid Germal Plus)
(preservataives)
E 0.50 Phenoxyethanol and/or
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
Isobutylparaben and/or Isopropylparaben and/or
Butylparaben (Liquapar Optima) (preservatives)
[33] The feruloyl glycerides of the present invention are especially well
suited to solubilizing
sunscreens. Many of these sunscreen ingredients are known to form a powder
after skin
application as an emulsion and drying. This crystal formation causes a loss in
UVb
protection as the sunscreens simply flake off after drying. The presence of
feruloyl
glycerides solubilize all oil soluble sunscreens and create a low melting
eutectic which
insures that the sunscreens remain fluid, are absorbed into the skin and will
not powder
on dry down. This provides products with longer lasting sun protection.
[34] In side-by-side comparison testing, the formulation containing feruloyl
glycerides
provided longer lasting sun protection than the same formulation that did not
contain
feruloyl glycerides.
[35] Example 3
[36] Lasting Color Hair Conditioner with Feruloyl Glycerides
[37] Phase Wt.% Ingredient
A 80.0 Deionized Water
A 0.5 Carbomer 940 (a viscosity modifier)
B 0.2 Isostearamidopropyl Ethydimonium Ethosulfate (a hair
conditioner
B 0.3 Soyamidopropyl Ehydimonium Ethosulfate (a hair
conditioner)
B 3.0 Feruloyl Glycerides
B 2.0 Polyquatemium 11 (a hair conditioner)
B 1.5 PEG 100 Stearate (an emulsifier)
B 1.0 Self Emulsifying Glyceryl MonoStearate (an emulsifier)
C 0.7 Triethanolamine a component that acts to
neutralize Carbomer 940)
C 10.0 Deionized Water
C 0.5 Imidazolidinyl Urea (a preservative)
[38] The feruloyl glycerides of the present invention add excellent
conditioning properties to
hair conditioner formulations. They are readily absorbed into porous hair
which has
been treated with chemical services (hair coloring, bleaching, permanent
waves,
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
11
straightening, etc.). After dry down, the hair exhibits excellent shine and
luster,
manageability and feels silky smooth. The feruloyl glycerides of the present
invention
are excellent additives for enhancing the long lasting hair colors that are
perceived in
permanent dyes. They do this by protecting the synthetic hair color from air
oxidation
and absorb both UVa and UVb ultraviolet rays which damage the color and
eliminate
the color producing chromophore.
[39] In one test, hair swatches were colored with one of two commercially
available
permanent hair coloring products for medium ash brown. Some hair swatches were
then wrapped in foil, while other hair swatches were either treated with a
conditioner
that contained feruloyl glycerides in accordance with the above formulation,
or a
control formulation that did not contain feruloyl glycerides. The non-foiled
hair
swatches were then exposed to northern sunlight for 32 hours and then compared
to the
hair swatches that had been foiled. The hair swatches that had been treated
with a
conditioner that contained feruloyl glycerides in accordance with the above
formulation
were much closer in color to the foiled hair swatches than the control
formulation that
did not contain feruloyl glycerides.
[40] Example 4
[41] Lip Protector/Lip Stick
[42] Phase Wt. % Ingredient
A 49 Castor Oil (a diluent)
A 10 Feruloyl Glycerides
A 8 Beeswax (a wax component to build stick structure)
A 4 Carnauba Wax (a wax component to build stick structure)
A 5 Candelilla Wax(a wax component to build stick structure)
A 1 Ozokerite Wax(a wax component to build stick structure)
A 10 Isopropyl Myristate (an emollient)
A 5 Dimethicone (a silicone to provide shine)
A 8 Color Grind of Pigments and
Castor Oil (an optional component if color is desired)
[43] A major site of sun damage and premature aging is the lip area. The
feruloyl glycerides
of the present invention show excellent compatibility with lipstick
ingredients and
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
12
provide emolliency, absorption, environmental protection (sun and wind
damage), and
prevention of transdermal moisture loss.
[44] In one test, lips were treated with feruloyl glycerides in accordance
with the above
formulation, and separately treated at a different time with a control
formulation that did
not contain feruloyl glycerides. Lips that had been treated with feruloyl
glycerides in
accordance with the above formulation felt better than lips that had been
treated with
the control formulation that did not contain feruloyl glycerides.
[45] Example 5
[46] Extra Moisturizing, Anti-Aging Shave Cream
[47] Phase Wt. % Ingredient
A 6.00 Stearic Acid (for soap formation in combination
with Triethanolamine)
A 1.00 Coconut Acid (for soap formation in combination
with Triethanolamine)
A 1.00 Lauryeth 4 (an emulsifier)
A 2.00 Mineral Oil (an emollient)
A 4.00 Feruloyl Glycerides
A 3.00 Isostearyl Neopentanoate (an emollient)
B 60.0 Demineralized Water
B 5.0 Glycerin (an emollient)
B 8.0 Ammonium Lauryl Sulfate (a surfactant)
B 2.0 Cocamidopropyl Betaine (a surfactant)
B 2.0 Acrylates/Octylacrylamide Copolymer (a viscosity
modifier)
B 4.8 Triethanolamine (neutralizes stearic acid and
coconut acid and for forms a soap with each)
C 1.0 Propylene Glycol and/or
Methylparaben and/or Propylparaben
(preservatives)
D 0.2 Fragrance
100
[48] Some skin specialists have viewed daily shaving as an exercise in daily
exfoliation of
the skin. When looked at shaving in this manner, one is not only removing the
daily
beard, but is also eliminating much of the stratum comeum cells on the skin's
surface.
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
13
This exposes the new skin surface to unprotected UVb and UVa exposure everyday
after shaving. The presence of feruloyl glycerides of the present invention in
a shaving
preparation insures some skin absorption of this powerful antioxidant at the
beginning
of each day, thereby reducing environmental damage to the skin.
[49] Panelists preferred the above shaving formulation containing feruloyl
glycerides over a
shaving control formulation that did not contain feruloyl glycerides.
[50] Example 6
[51] Skin Lightener Formulations
[52] The feruloyl glycerides of the present invention may be used in skin
lightener
formulations that carry other active ingredients into the epidermis and thus
slow the
melanin formation process in age spots. A formulation can be prepared using
the
formulation of Example 2, adding 1.5% Kojic acid, and reducing the amount of
water a
corresponding amount, i.e., reduce the amount of water by 1.5%. Kojic acid can
be part
of Phase B. Kojic acid can penetrate the epidermis and help reduce the
production of
melanin.
[53] In one test, a formulation containing feruloyl glycerides was applied to
one area of skin
having age spots, and a control formulation not containing feruloyl glycerides
was
applied to another area of skin having age spots. Panelists preferred the
formulation
containing feruloyl glycerides over the control formulation. Viewing the skin
areas
after 28 days, 56 days and 84 days showed that skin areas treated with the
formulation
containing feruloyl glycerides were lighter in color than the skin areas that
had been
treated with the control formulation.
[54] Example 7
[55] Anti-Aging Stick Protector
[56] The feruloyl glycerides of the present invention may be incorporated into
an anti-aging
stick protector to carry peptides into the skin, calling for maximum
production of
collagen and elastin proteins and minimizing premature degradation of these
skin
tightening support proteins. Anti-Aging formulations may protect the skin from
UVa
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
14
irradiation from normal sunlight, may alter the skin's moisture retentive
characteristics,
may add to the skin luminescence, may prevent the excess formation of melanin
containing age spots, and may prevent the formation of radicals from UVb and
UVa,
which then react with other skin ingredients to alter its natural flexibility
and tightness.
The manufacturing process can involve addition of the liquids to the main
batch kettle
with mixing. Heat from a jacket kettle can be turned on and the solid
ingredients can be
added one at a time, until the complete system is melted and solubilized
and/or fully
dispersed. The hot liquid can then poured in suitable stick molds and allowed
to cool
and solidify.
Anti-Aging Stick
Phase % Ingredient
A 40.25% Castor oil (a diluent)
A 20% Feruloyl Soy Glycerides
A 10% Isopropyl Myristate (an emulsifier)
A 9.0 Octycrylene (a sunscreen)
A 3.0 Avobenzone (a sunscreen)
A 7.0 Octyl methoxycinnamate (a sunscreen)
A 5.0 Candelilla Wax (a was to build a stick structure)
A 1. 0 Carnauba Wax (a was to build a stick structure)
A 0.75 Ozokerite Wax (a was to build a stick structure)
A 4.0 Beezwax (a was to build a stick structure)
100%
[57] Panelists preferred the formulation containing feruloyl glycerides over a
control
formulation that did not contain feruloyl glycerides. This product is
particularly useful
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
to protect skin around the eyes, the skin of the nose, and other portions of
the face from
sunlight.
[58] Example 8
[59] Dental Cleanser
[60] The feruloyl glycerides of the present invention may be incorporated into
a dental
cleanser to solubilize dental stains and to flush them away, for example, as
part of a
dental paste or mouthwash.
Dental Gel
Phase % Ingredients
A 1.0 Carboxymethyl cellulose (a thickener)
A 3.0 Feruloyl Soy Glycerides
A 60 Sorbitol (a diluent and sweetener)
A 11.4 Water
A 5 Sodium Saccahrin (a sweetener)
A 8 Sodium Silicate (a thickener)
A 10 Sodium Silicate (an abrasive, with larger
particles than the sodium silicate used as a
thickener)
A 1.0 Flavor
A 0.6 Sodium Lauryl Sulfate (dental grade cleanser,
.e.g, a dental grade cleanser without a soapy
taste)
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
16
[61] Panelists preferred the formulation containing feruloyl glycerides over a
control
formulation that did not contain feruloyl glycerides.
[62] Example 9
[63] Co-Solvent for Fragrances
[64] The feruloyl glycerides of the present invention may be used as co-
solvent in fragrance
compounding. When creating fragrances, perfumers draw on thousands of scented
and
fixing compounds which complex with other ingredients within the perfume
formulation to achieve the ultimate fragrance interpretation that is desired.
The science
of perfumery is thousands of years old and normally requires an apprenticeship
of 5
years or more with a Master Perfumer to receive accreditation by the American
Society
of Perfumers. The basic construction of fragrances includes 3 divisions within
the
fragrance. These are referred to as top, middle and bottom notes. The top
notes are
light, delicate and quickly volatize at room temperature. The middle notes
have more
body and require more heat and energy to lift from the skin. The bottom notes
of a
fragrance form its foundation. They are the heavy lasting notes. Holding the
top,
middle and bottom notes together involves the science of fixatives. A well-
balanced
fragrance normally presents itself as a well-balanced bouquet with strong
foundation
notes, middle statements and sparkles of unexpected top notes. While this is
generally
true, the opposite is often an unexpected pleasure as in the classic ChannelTM
No. 5
fragrance, which is mostly sparkling top notes, with moderate to slight base
notes.
[65] Within every fragrance the choice of solvent (or several co-solvents)
often plays a
critical role in the fragrances stability, application characteristics and
lasting power.
Typical fragrance ingredients (remember there are thousands to choose from)
include
terpineol, linalool, benzyl alcohol, benzaldehyde, ylang-ylang and so on. To
hold all of
these 25-100 ingredients together in a stable solution is no small task. Many
times the
perfumer feels that they have a stable solution but then there is a formation
of one
crystal, then another and quickly an avalanche of components falling from what
was a
clear solution.
[66] Diethyl phthalate (DEP), Dioctyl adipate (DOA), and Propylene Glycol are
three of the
most common solvents used in perfumery by perfumers. The feruloyl glycerides
of the
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
17
present invention bring a totally new dimension to the science of perfumery.
It is a
useful solvent that takes a broad variety of hydrophobic and some slightly
hydrophilic
materials into solution. The feruloyl glycerides of the present invention have
shown
possibilities of forming clathrate structures around fragrances and individual
components, which will enhance their stability, performance and resistance to
attack.
Being a partial triglyceride, fatty acid ester and ferulic acid ester we see
an unexpected
synergy of solvent properties, antioxidant characteristics, UVa & b absorbance
qualities, skin absorption characteristics, surface activity, and addition to
structural type
delivery. Fragrances can be made into alcohol solutions, gels, solids, etc.
They can be
positioned as providing health benefits, anti-aging benefits, environmental
protectants,
sun protection fragrances as well as many other characteristics. With some
hydroxyl
substitution and/or ethoxylation on the triglyceride moieties, the molecule
can solubilize
and/or emulsify hydrophilic materials.
[67] Panelists found that the formulation containing feruloyl glycerides
performed just as
well in terms of fragrance stability, application characteristics and lasting
power as a
control formulation that contained DEP instead of feruloyl glycerides. And, a
formulation containing DEP does not provide the same UV protection and anti-
aging
attributes as a formulation containing feruloyl glycerides.
[68] In accordance with the invention the amount of feruloyl glyceride (which
is a
combination of a linker agent and a compound comprising at least one UV-
absorbing
chromophore) in a product for topical application as described above can be
any
suitable range, but is typically about 10% by weight or less of the product.
In one
preferred embodiment, the amount of the combination of a linker agent and a
compound
comprising at least one UV-absorbing chromophore in a product for topical
application
is in the range of about 3% to 10% by weight. For a phospholipid vitamin
complex
lotion, the amount of a combination of a linker agent and a compound
comprising at
least one UV-absorbing chromophore is preferably about 5% by weight. For a
water
resistant sunscreen, the amount of a linker agent and a compound comprising at
least
one UV-absorbing chromophore is preferably about 8% by weight. For a hair
conditioner, the amount of a combination of a linker agent and a compound
comprising
at least one UV-absorbing chromophore is preferably about 3% by weight. For a
lip
CA 02656091 2008-12-22
WO 2008/003090 PCT/US2007/072557
18
protector and/or lip stick, the amount of a combination of a linker agent and
a
compound comprising at least one UV-absorbing chromophore is preferably about
10%
by weight. For a shave cream, the amount of a combination of a linker agent
and a
compound comprising at least one UV-absorbing chromophore is preferably about
4%
by weight. In each event the resultant combination incorporates topically
acceptable
agents other than the linker agent/chromophore such as for feruloyl glyceride
in an
amount of about 90% by weight or more. The resultant combination comprises a
synergistic mixture which is favored and efficacious relative to mixtures
which are
absent the linker agent/chromophore combination (e.g., feruloyl glyceride).
[69] The embodiments of the invention, and the invention itself, are now
described in such
full, clear, concise and exact terms to enable a person of ordinary skill in
the art to make
and use the invention. To particularly point out and distinctly claim the
subject matters
regarded as invention, the following claims conclude this specification. To
the extent
variations from the preferred embodiments fall within the limits of the
claims, they are
considered to be part of the invention, and claimed.