Note: Descriptions are shown in the official language in which they were submitted.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
ANTIMICROBIAL COATINGS
PRIOR APPLICATION INFORMATION
The present application claims the benefit of US Provisional Application
60/824,479, filed September 5, 2006, US Provisional Patent Application
60/939,698, filed
May 23, 2007 and US Provisional Application 60/940,428, filed May 28, 2007.
BACKGROUND OF THE INVENTION
Raw poultry products can serve as a source of human pathogens such as
Salmonella and Campylobacter that may cross-contaminate other foods. When
appropriate rearing and shipping practices are followed, most poultry
contamination by
these organisms occurs during or after slaughter and processing (Slader et
al., 2002;
Zhao et al., 2001). Carcass washing with approved antimicrobials (AMs) has had
limited
success because many microorganisms are physically hidden in the feather
follicles and
skin folds which protect them from the action of AMs (Mehyar et al., 2005;
Schneider et
al., 2002; Wang et al., 1997; Xiong et al., 1998). Furthermore, increased line
speed
reduces the antimicrobial contact time with target microorganisms, and the
moisture on
chicken skin surface can act as a diluent, reducing antimicrobial
effectiveness (Oyarzabal
et al., 2004). An alternative approach to extending the contact time would be
increasing
the effectiveness of AMs. To obtain improved effectiveness without changing
process
speeds in the plant, edible gels containing AMs could be sprayed on chicken
surfaces. In
theory, the agents would gradually diffuse from the gels or coating material
into skin
irregularities and if applied early (after defeathering), provide increased
contact time with
target microorganisms and yield improved effectiveness. Most food-related
antimicrobial
coatings have been tested only for their quantitative antimicrobial
effectiveness (Janes et
al., 2002; Natrajan and Sheldon, 2000a,b; Siragusa and Dickson, 1992). No
report has
been found which relates the antimicrobial activity of the coatings to their
surface
properties or absorption into contaminated foods. Studying these physio-
chemical
properties will help in determining the minimum quantities of AMs required to
eliminate
pathogens from foods using methods which have beneficial economic and
environmental
consequences.
Chicken skin consists of two layers, the upper layer called the epidermis and
the
lower layer called the dermis (Lucas and Stettenheim, 1972). The epidermis is
divided into
the Stratum corneum (cuticle) and Stratum germinativum. The cuticle of the
epidermis
consists of waxy material which covers the skin surface, whereas the lower
region is
composed of cell layers that can be differentiated to become a part of the
cuticle in
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
2
response to damage. Scalding at high temperature removes the cuticle layer
from the skin
which will affect skin adhesiveness characteristics (Lucas and Stettenheim,
1972). Indeed,
a thinner cuticle layer increases skin hydrophilicity and makes microbial
contamination
more likely whereby organisms may be deposited within the skin and its folds
(Suderman
and Cunningham, 1980). The contact angle of a liquid drop on a smooth surface
has been
used to characterize the surface energies of solids (Choi and Han, 2002; Han
and
Krochta, 1999). In this study, this surface chemistry has been used to measure
the
adhesion force of coatings to the skin. In addition, it is also known that the
contact angle of
a liquid drop is affected by the extent of roughness of the target surface,
and such effects
could be substantial on a rough surface like chicken skin. The determination
of contact
angles can be used to explain solid surface properties in terms of both
surface energy and
roughness (Han and Krochta, 2001). The dermal layer of chicken skin contains
collagen
which readily absorbs water from the skin surface and swells, causing changes
in skin
microtopography (Thomas and McMeekin, 1982). Liquid absorption rate and
maximum
absorptiveness can be measured to reflect how fast and how much of an applied
liquid
penetrates and is absorbed by the skin.
Consumer interest in unprocessed foods preserved with natural ingredients has
significantly increased recently (Cagri et al., 2004; Debeaufort et al.,
1998). Development
of edible films and coatings which have comparable properties with synthetic
preservative
ingredients is an approach taken to satisfy this interest (Mehyar and Han,
2004). Both
starch and alginate have been shown to be structurally compatible with
alkaline and acidic
agents (Siragusa and Dickson, 1992; Ratnayake et al., 2002). The goal of the
present
work was to model the effectiveness of trisodium phosphate (TSP) and acidified
sodium
chlorite (ASC) in pea starch (PS) and alginate coatings, when applied to
broiler carcasses
during processing for their ability to reduce surface contamination by
Salmonella. Since
current standards require that carcasses should be free of any residual
additives before
shipping from the processing plant, the effect of these chemical applications
on skin pH
and persistence of coatings on the chicken skin were also determined,
targeting 60 min for
completion of carcass chilling and neutralization of the additives.
Hydrogel is a network of hydrophilic polymer chains which are able to hold up
water but are kept from dissolution by either physical or chemical cross-
links. There has
been an increasing interest in physically cross-linked hydrogel, in lieu of
chemically cross-
linked hydrogel, which may involve the use of toxic agents. Several physical
interactions
have been exploited in the design of hydrogel, such as electrostatic
attraction (Bodmeier
and Wang, 1993, J Pharmaceut Sci 82: 191-194; Bodmeier et al., 1989,
Pharmaceut Res
6: 413-417; Doria-Serrano et al., 2001 Biomacromolecules 2: 568-574; Grant et
al, 1973,
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
3
FEBS Lett 32: 195-198; Seely and Hart, 1974, Macromolecules 7: 706-701; Ortega
and
Perez-Mateos, 1998, J Chem Technol Biotechnol 73: 7-12), hydrogen bonding
(Durrani
and Donald, 1995, Polym Gels Networks 3: 1-27; Goodfellow and Wilson, 1990,
Biopolymers 30: 1183-1189; Ring et al., 1987, Carbohydr Res 162: 277-293; Liu
and Han,
2005, J Food Sci 70: E31-E36), and antigen-antibody binding (Miyata et al.,
1999,
Macromolecules 32: 2082-2084). Basically, it is required that polymers possess
an
abundance of functional groups (e.g. -OH, -COO", -NH, -SH) to achieve inter-
and intra-
molecular interactions in the formation of hydrogel.
As a major storage polysaccharide in plants, starch is a compound of amylose
and
amylopectin, with its composition depending on the plant origin. Amylose is a
nearly linear
polymer of ^-1,4 anhydroglucose units, with molecular weight of 105-106
(Durrani and
Donald, 1995; Galliard and Bowler, 1987 in Starch: Properties and Potential
(Galliard, ed;
John Wiley and Sons: New York, p 57-78)). In contrast, amylopectin is a highly
branched
polymer consisting of short a-1,4 chains linked by a-1,6 glucosidic branching
points
occurring every 25-30 glucose units, with molecular weight of 107-109 (Durrani
and
Donald, 1995; Galliard and Bowler, 1987). When heated in water at 60 C or
above, starch
granules gelatinize, characterized by granular swelling, amylose exudation and
disruption
of long-order crystalline structure (Liu, 2005 in Innovations in Food
Packaging (J.H. Han
ed., Academic Press: New York, p318-337)). Suspension of gelatinized starch
starts
gelling upon cooling as a result of inter- and intra-molecular hydrogen
bonding of amylose
and linear branches on amylopectin (Goodfellow and Wilson, 1990; Liu and Han,
2005).
Macroscopically, starch gel is a three-dimensional network constructed mainly
by spring-
like strands of polymeric chains (Ring et al., 1987).
Alginate in a form of free acid or sodium salt is a collective term for a
family of
polysaccharide prepared mostly from brown algae (Smidsrod and Grasdalen, 1984,
Hydrobiologia 116-117: 19-28). Chemically, alginate is a mixture of poly(P-D-
mannuronate), poly((x-L-guluronate), and poly(R-D-mannuronate (X-L-
guluronate), with its
exact composition depending on algal source. Similar to starch gel, alginate
gel features a
3-D network structure (Ahearne et al., 2005, J R Soc Interface 2: 455-463;
Doria-Serrano
et al., 2001; Decho, 1999, Carbohydr Res 315: 330-333; Walkenstrom et al.,
2003, Food
Hydrocol 17: 593-603). However, alginate forms hydrogel by polymeric chains
interacting
with Ca2+ and other divalent and trivalent metal ions (Donati et al., 2005,
Biomacromolecules 6: 1031-1040; Rees and Samuel, 1967, J Chem Soc C Organic
22:
2295-2298), according to the so-called "egg-box" model (Grant et al., 1973).
As a result of
ionic interaction, the presence of di- or multivalent cations enable the
formation of junction
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
4
zones between helical chains of guluronic blocks, those of mannuronic blocks,
and those
of mannuronic-guluronic blocks (Donati et al., 2005).
In addition to many other biomedical applications such as enzyme
immobilization
(Ortega and Perez-Mateos, 1998) and tissue engineering (Ahearne et al., 2005;
Li et al.,
2005, Biomaterials 26: 3919-3928), hydrogel is useful for drug release
(Rajaonarivony et
al., 1993, J Pharmaceut Sci 82: 912-917; Bodmeier and Wang, 1993). Drug
release from
hydrogel occurs mainly due to gel swelling, which can be controlled by the
formulation
chemistry of polymeric network (e.g., functional groups, degree of cross-
linking) and by
the environmental conditions (e.g., pH, temperature, ionic strength, etc.)
(Peppas et al.,
2000, Annu Rev Biomed Eng 2: 9-29). The swelling of hydrogel in water permits
the
entrapped drug to diffuse throughout the entire network and release from the
gel. The
release rate is primarily determined the degree of swelling (Prokop et al.,
2002, Adv Polym
Sci 160: 119-173).
Due to its ability to sustain the release of antimicrobials, hydrogel has
become a
potent carrier of antimicrobials in the meat and poultry industries (Natrajan
and Sheldon,
2000, J Food Prot 63: 1189-1196; Natrajan and Sheldon, 2000, J Food Prot 63:
1268-
1272). Herein, the swelling and rheological properties of starch and alginate
hydrogels in
physiological saline and the release of antimicrobials from the hydrogels to
the saline
solution, which simulates the fluidic condition on the surfaces of chicken
skin, pork and
beef.
Quality of fresh poultry offered at retail depends greatly on the
microbiological
quality of fresh eviscerated chicken (Mehyar and others 2005). Most research
has been
concerned with the contamination of chicken carcasses and poultry products by
Salmonella or Campylobacter which are predominant pathogens, and Pseudomonas
which are the major psychotropic spoilage bacteria of refrigerated poultry
products (Smith
and others 2005a; Mehyar and others 2005; Uyttendaele and others 2006). A
Belgian
survey in 2001, as an example, showed that 18% of chicken fillets and 35% of
chicken
carcasses were contaminated by Campylobacter, and this number has remained at
a high
level (Uyttendaele and others 2006). Campylobacter numbers on poultry are much
higher
than that of Salmonella, which are estimated to be 102 - 107 and 1- 102
cfu/bird,
respectively (Jorgensen and others 2002; Zhao and others 2001). Poultry
processing lines
operate at high-speed, often processing over 150 bird/min. At this high speed
poultry meat
is very vulnerable to cross-contamination. Consequently, much effort is spent
to maintain
good sanitation during processing, and these efforts involve optimization of
specific unit
operating procedures, and adoption of good manufacturing practice (GMP) and
HACCP-
based quality systems.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
Various processing methods are used to reduce levels of undesired
microorganisms on broiler carcasses in poultry processing lines. Among them,
one of the
important unit processes is washing using an inside-outside bird washer before
immersion
or air chilling (Smith and others 2005a; 2005b). Recently, immersion at 75 -
80 C before
cold water immersion chilling (Corry and others 2007), extended immersion time
(24 h) in
cold chlorine water (Cason and others 2006), and the use of large amounts of
cold water
during immersion chilling (Northcutt and others 2006) have been tried to
reduce poultry
carcass contamination. However, hot water immersion and day-long cold
immersion, or
the use of a large quantity of water are not commercially feasible processes
although
these approaches reduced the numbers of some pathogens. It appears that after
washing
followed by chilling, there is no unit process in use which can satisfactorily
remove
pathogens or spoilage microorganisms from poultry carcasses.
An attractive antimicrobial procedure would be one where a nonthermal
treatment
was used to reduce the number of microorganisms just prior to or during the
packaging
process. Such nonthermal treatments may include combinations of modified
atmosphere
packaging (MAP) with antagonistic cultures, electron beam irradiation, high
pressure
processing, or antimicrobial packaging/coating (Han 2007). MAP of pre-cooked
chicken
meats inhibited spoilage microorganisms (i.e., Pseudomonas, yeast and molds)
compared
to air packaging (Patsias and others 2006). Electron beam treatment also
reduced the
number of Escherichia coli 0157:H7 in chicken meat products and has the
potential to
control other pathogens (Black and Jaczynski 2006).
Edible coatings are produced from edible biopolymers and food-grade additives.
Film-forming biopolymers can be selected from proteins, polysaccharides
(carbohydrates
and gums), or lipids (Gennadios and others 1997). Various antimicrobial agents
may be
incorporated into edible coating materials to produce antimicrobial coating
systems, as
they allow a slow migration of the antimicrobial agents from the coating
materials and
extend the shelf-life of coated foods. Common edible antimicrobial agents
include organic
acids (e.g., acetic acid, and fatty acids), phenolics (e.g., benzoic acids and
cinnamaldehyde), bacteriocins (e.g., nisin, lacticin and others), enzymes
(e.g., lysozyme
and glucose oxidase), monoglycerides (e.g., monolaurin and monocaprin), and
various
plant extracts from herbs and spices (Han 2003; 2005).
A variety of antimicrobial coating systems have been applied to chicken
carcasses
and poultry meat products. Starch and calcium alginate gels incorporating
trisodium
phosphate and acidified sodium chlorite, respectively, effectively inhibited
an inoculated
Salmonella cocktail on chicken wings (Mehyar and others 2007). Nisin was mixed
with
protein and carbohydrate coating materials and reduced the number of
Salmonella and
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
6
Listeria on chicken meats (Janes and others 2002; Natrajan and Sheldon 2000a,
b).
Edible polymers in coating materials carrying active agents increased the
viscosity of
coating materials. The agents extended the contact time of incorporated agents
when
placed against chicken surfaces, and consequently improved the antimicrobial
efficiency
of the coating systems against pathogenic and spoilage microorganisms.
Among available antimicrobial agents, oils of plant or spice extracts are
attractive
since they are natural ingredients (which require no or a reduced label
declaration), are
accepted by consumers (Cagri and others 2004; Debeaufort and others 1998; Han
2003,
2005) and they can be extracted easily from herbs, spices and aromatic plants
by solvents
or steam distillation. Many of these essential oils contain antimicrobial as
well as
antioxidant activity. Examples include rosemary, clove, thyme, oregano and
basil oils, plus
horseradish and mustard extracts. They are mostly phenolics or terpenes while
the latter
two contain isothiocyanates (Burt 2004; Holley and Patel 2005).
Thyme oil mainly contains thymol, p-cymene and carvacrol, which demonstrate
antimicrobial and antioxidant activities (Kaloustian and others 2005; Sasso
and others
2006; Youdim and others 2002). Thyme oil has been reported to inhibit the
growth of
Escherichia coli 0157:H7, Salmonella spp., Staphylococcus aureus, Listeria
monocytogenes, Penicillium spp. and many other bacteria (Friedman and others
2006;
Smith and others 2001; Sasso and others 2006; Singh and others 2003; Suhr and
Nielsen
2003). The antimicrobial activity of thyme oil was adversely affected by food
composition,
especially lipid content (Singh and others 2003; Smith and others 2001).
SUMMARY OF THE INVENTION
According to a first aspect of the invention, there is provided an
antibacterial
coating comprising a hydrophilic polymer and a hydrophilic water soluble
antimicrobial.
According to a second aspect of the invention, there is provided a method of
protecting a perishable food surface from microbial contamination comprising:
providing an antibacterial coating comprising a hydrophilic polymer and a
hydrophilic water soluble antimicrobial; and
applying the antimicrobial coating to the perishable food surface wherein the
concentration of the hydrophilic polymer is such that it forms a solution that
is viscous
during coating and forms a gel during drying.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1. Application of coatings to chicken drumettes: A) 3.5 % (w/v) pea
starch
(PS) containing 10 % (w/v) trisodium phosphate (TSP): B) 1%(w/v) alginate
containing
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
7
1200 ppm acidified sodium chlorite (ASC). Solution (a) contained 1(w/v) %
CaCI2 plus
ASC; solution (b) contained 1%(w/v) sodium alginate.
Figure 2. Effect of inclusion of commercial AMs in polymeric coatings on
survival of
inoculated Salmonella on chicken skin during storage at 4 C for 5 d. TSP =
trisodium
phosphate, ASC = acidified sodium chlorite, PS = pea starch. Columns with
different
letters at the same sampling time are significantly (P <_ 0.05) different.
Figure 3. Surface pH of chicken drumettes dipped in 10 %(w/v) trisodium
phosphate (TSP) and 1200 ppm acidified sodium chlorite (ASC) with and without
inclusion
in 3.5 % (w/v) pea starch (PS) or 1.0 % (w/v) calcium alginate (Algn),
respectively during
storage at 4 C.
Figure 4. Effect of antimicrobial pea starch (PS+TSP) coating viscosity
(prepared
with different concentrations of PS) on the initial contact angle of coating
drops applied to
the chicken skin surface.
Figure 5. Effect of pea starch (PS) concentration change in the antimicrobial
pea
starch (PS+TSP) coatings on the initial contact angle of the coating drops on
the chicken
skin surface.
Figure 6 Schematic assembly used for preparing calcium alginate gel
Figure 7 Dimensionless mass of solids (Ms/Mso) in starch gels (a) and aiginate
gels
(b) as a function of time (t) of immersion in saline solution, with fitted
curves based on
Fikian diffusion
Figure 8 Dimensionless mass of water (M,N/Mo) in starch gels (a) and alginate
gels
(b) as a function of time (t) of immersion in saline solution, with fitted
curves based on
Fikian diffusion
Figure 9 Concentration of antimicrobials (C) released from PS+TSP and ALG+ASC
gels into the saline solution, as a function of immersion time (t), with
fitted curves based on
Fikian diffusion
Figure 10 Dimensionless storage moduli (G'/G'o) for starch gels (a) and
alginate
gels (b) as a function of time (t) of immersion in saline solution
Figure 11 Dimensionless solids content (SC/SCo) of starch gels (a) and
alginate
gels (b) as a function of time (t) of immersion in saline solution
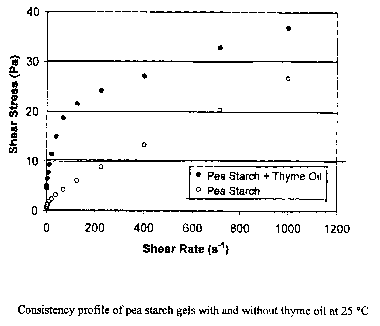
Figure 12 Consistency profile of pea starch gels with and without thyme oil at
25 C.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Unless defined otherwise, all technical and scientific terms used herein have
the
same meaning as commonly understood by one of ordinary skill in the art to
which the
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
8
invention belongs. Although any methods and materials similar or equivalent to
those
described herein can be used in the practice or testing of the present
invention, the
preferred methods and materials are now described. All publications mentioned
hereunder
are incorporated herein by reference.
Described herein is an antibacterial coating comprising a hydrophilic polymer
and a
hydrophilic water soluble antimicrobial.
In some embodiments, as discussed below, the concentration of the hydrophilic
polymer is such that it forms a solution that is viscous during coating and
forms a gel
during drying.
In a preferred embodiment, the antimicrobial coating comprises 0.1-10% or 0.1-
5%
hydrophilic polymer and 0.1-25% or 0.5-25% or 1-25% antimicrobial.
The hydrophilic polymer may be selected from the group consisting of
microcrystalline cellulose, (pre-)gelatinized starch, modified starch,
dextrin, maltodextrin,
pectin, iota-carrageenan, lambda-carrageenan, gum arabic, gum acacia, gum
ghatti, guar
gum, xanthan gum, gellan gum, pullulan and combinations thereof. As discussed
below, in
a preferred embodiment, the hydrophilic polymer is pea starch.
As will be apparent to one of skill in the art, in the instant invention, the
polymer
allows for the slow release of antimicrobials (or sanitizers) which in turn
extends the
effective antimicrobial period. Thus, the polymer provides sustained delivery
of
antimicrobial agents using gel type coating materials consisting of edible
polymers. In a
preferred embodiment, the antimicrobial coating is used for covering
perishable food
surfaces, thereby protecting the foods from contamination by environmental
microbial
hazards, and also eliminating microorganisms which may have previously existed
on the
food surfaces.
It is of note that animal carcasses are one example of a perishable food
surface.
However the antimicrobial coating may be used for any perishable solid foods
or any
foods which are susceptible to surface contamination during processing through
cross-
contamination. In addition to meat products, these include any solid foods
which are
subject to reprocessing or post-processing such as shredding, slicing,
cutting, grinding
and the like. These include for example but by no means limited to cheeses,
fruits,
vegetables, and any frozen/refrigerated foods.
In some embodiments, as discussed below, the concentration of the hydrophilic
polymer is such that it forms a solution that is viscous during coating and
forms a gel
during drying.
In a preferred embodiment, the antimicrobial coating comprises 0.1-10% or 0.1-
5%
hydrophilic polymer and 0.1-25% antimicrobial.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
9
As discussed below, in some embodiments, the antibacterial is preferably
thymol,
carvacrol, linalool, geraniol, thujanol, terpineol or a combination thereof.
As will be
appreciated by one of skill in the art, many natural oils are high in thymol
and pinene, for
example but by no means limited to thyme oil, rosemary oil, clove oil, basil
oil, mint oil,
Eucalyptus oil, tea tree oil and oregano oil. In the examples discussed below,
thyme oil is
used but it is to be understood that any suitable source of thymol, carvacrol,
linalool,
geraniol, thujanol-4, and/or terpineol may be used within the invention. In
other
embodiments, the antimicrobial is trisodium phosphate (TSP), acidified sodium
chlorite or
another such suitable antimicrobial known in the art as discussed herein.
As discussed below, it is believed that the thymol, carvacrol, iinalool,
geraniol,
thujanol, and terpineol in thyme oil enhances the intermolecular interaction
of the polymer,
for example, high-amylose pea starch, resulting in a film solution which has
much higher
yield stress.
In the present study, thyme oil was incorporated into a polymer, for example,
high-
amylose pea starch gel and applied on chicken breast meats pre-inoculated with
spoilage
or pathogenic microoranisms. The objective was to characterize: (1) the
rheological
characteristics of the starch-based coating material with and without thyme
oil; and (2) the
antimicrobial effectiveness of thyme oil in a starch-based coating material
against food
borne pathogens and spoilage bacteria on chicken meat. The goal of this
project was to
determine whether the formation of an antimicrobial coating containing thyme
oil applied to
chicken carcasses would be suitable to reduce the effects of contamination by
a high-
speed poultry line, enhance the safety of poultry products and extend their
shelf-life.
Fresh chickens are processed at plants using high-speed processing lines which
are vulnerable to rapid cross-contamination of large amounts of product.
Antimicrobial
coating on chicken carcasses may reduce the effects of this contamination
during
processing and improve product shelf-life and safety. Thyme oil, a natural
antimicrobial
flavor, was mixed at 0.5% (v/v) with a pre-gelatinized pea starch coating
solution. The
coating solution was spread on chicken breast meat after inoculation with
Salmonella
Typhimurium plus S. Heidelberg, and also Campylobacter jejuni, Listeria
monocytogenes,
or Pseudominas aeruginosa. After inoculation at 6 log cfu/g, the chicken meats
were
packaged in plastic bags and stored at 4 C. During 12 d storage, total aerobic
bacteria,
lactic acid bacteria and inoculated organisms were counted at 4 d intervals.
Thyme oil
treatments reduced the viability of Salmonella as well as the growth of
Listeria and
Pseudomonas by 2 log cfu/g, and appeared to eliminate inoculated Campylobacter
during
storage. The addition of thyme oil increased the viscosity of the pre-
gelatinized pea starch
solution, but these effects may be minimized by the use of a suitable washer
pressure at
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
application. The results suggested that thyme oil inclusion in an edible
starch coating may
be a satisfactory delivery system to enhance the safety of processed fresh
meat.
Thyme oil reduced C. jejuni viability below detectable levels, significantly
inhibited
the growth of S. enterica serovars as well as L. monocytogenes, and delayed
the growth
of P. aeruginosa on chicken breast meats. Pea starch coating was used as a
delivery
vehicle for thyme oil and also served as a viscosity enhancer to extend the
contact of
thyme oil with the chicken meat surface. This study has shown that thyme oil
either alone
or in a gelatinized pea starch coating was effective in delaying growth of
spoilage and
pathogenic bacteria on chicken meat surfaces during refrigerated storage.
These
treatments were effective in essentially eliminating large numbers of C.
jejuni from the
chicken meat and significantly reduced the viability of S. Typhimurium. The
pea starch
coating may be a useful vehicle for application of natural antimicrobials to
control
undesirable organisms on chicken carcasses.
Antimicrobial Effectiveness, Drumette Weight and Surface pH Changes
PS+TSP and alginate+ASC coatings on chicken appeared clear, continuous and
homogenous (Figure 1). Alginate+ASC coating imparted a pale yellowish color to
the
drumettes while the PS+TSP coating did not induce any noticeable visual
changes. Figure
2 shows the reduction in Salmonella on drumettes over 120 h at 4 C. PS not
only
maintained the antimicrobial activity of TSP longer but also increased its
antimicrobial
activity compared to the TSP treatment without PS. Because of the viscosity of
PS, the
TSP + PS solution has longer contact time to chicken surface compared to the
TSP
solution without PS. This extended contact time increased the effectiveness of
TSP.
Enhanced antimicrobial activity was also exhibited in the alginate+ASC
coating. Coatings
with TSP and ASC had significantly (P <_ 0.05) greater antimicrobial activity
than the
corresponding solutions without polymers after 24 h. AMs in aqueous solution
and in
antimicrobial-free coatings were unable to cause > 1.0 log cfu/g reductions.
Most (88 %) of the PS+TSP coating containing TSP appeared to drip from the
skin
within 1 h (Table 1), whereas the coating without TSP was better retained on
the surface
for 24h. This suggests that TSP may have reduced the viscosity of the PS
coatings and
accelerated its drip from the skin, which could have occurred as a result of
starch
degradation under alkaline conditions (BeMiller 1965). Calcium alginate
coatings with and
without ASC were more stable throughout incubation. Initial weight gains of
7.9 and 6.9 %
during alginate treatments were also greater than that of untreated controls
(water) at the
end of the tests (Table 1). It was suggested that the acidic nature (pH 5.0)
of ASC
increased the viscosity of the alginate matrix by enhanced charging of calcium
ions and
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
11
protonation of carboxyl groups (King 1982). Under these conditions calcium
ions can more
readily form bridges with the negatively-charged alginate matrix and the
repulsion between
protonated carboxyl groups of alginate is lowered, which promotes the
formation of cross-
linked networks (King 1982).
Figure 3 shows that TSP increased and ASC decreased the initial pH of the
chicken skin. Although AMs in solution caused significant (P <_ 0.05) initial
changes in the
skin pH, the effects were transient and did not last more than 24 h. TSP and
ASC in
coatings significantly changed the surface pH which was maintained up to 120 h
and 72 h,
respectively (Figure 3). Gelatinized starch is soluble in aqueous environments
(Ratnayake
et al., 2002). It slowly dissolves within the pores and follicles of the skin
and ostensibly
releases TSP into skin, which improves its antimicrobial action. The alginate
matrix
seemed to be more stable but chlorous acid (HCfO2) which is formed by sodium
chlorite
acidification during ASC formulation, may gradually diffuse inside the matrix.
As it reaches
the higher pH of the skin, chlorous acid is dissolved into the skin structure
(King 1982;
Oyarzabal et al., 2004; Schneider et al., 2002). From the results of this
study, it is shown
that the PS and alginate coatings can prolong the exposure of surface bacteria
to the TSP
and ASC at high and low pH, respectively, thereby interfering with cell
metabolic activity
(Siragusa and Dickson, 1992).
Coating Absorptiveness
Both the rate and amount in absorption of PS+TSP and alginate+ASC coatings to
the skin depended on the polymer content of the coatings (Table 2 and 3). At
concentrations > 3.5 % PS and > 0.5 % alginate, the absorptiveness was
significantly (P <_
0.05) reduced during 60 min possibly because the polymers are hydrophilic. At
the lowest
PS concentration (0.5 %), the amount of coating absorbed by the skin was
higher than
that of water (Table 2) because the polymers are diluted and do not exist as a
separate
layer on the skin. At low concentration, the hydrophilic polymers adhered on
the chicken
skin and increased the water absorptiveness. In addition, these values are
comparable to
the amounts of absorbed water during commercial immersion chilling for 30 min
(Thomas
and McMeekin, 1984). Retention of residual polymers inside skin crevices,
folds and
follicles which would not be removed by surface wiping may have contribution
to extra
weight gain. PS+TSP coatings were absorbed quicker than alginate+ASC coating
as
indicated by the higher absorption rate values (i.e., the slope of the
absorption curve) in
Table 3. Both the rate and quantity of PS absorbed was higher compared to
alginate at
concentrations that exerted antimicrobial effectiveness (3.5 % and 1.0 %,
respectively)
(Table 2 and 3). This may explain the higher and more prolonged (120 h)
antimicrobial
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
12
effectiveness of the PS+TSP coating compared to the alginate+ASC coating
(Figure 2).
This may also explain the greater antimicrobial activity of TSP in aqueous
media against
Salmonella on chicken skin (Mehyar et al., 2005). In addition, gelatinized PS
at low
viscosity may more easily fill skin follicles and pores, bringing TSP directly
in contact with
more surface bacteria that may have been protected by irregularities in skin
surface
topography. Alginate+ASC exhibited higher antimicrobial activity than ASC
alone only at <_
72 h of treatment (Figure 2B). This could have been due to the method of its
application
when the skin was first dipped in calcium chloride solution with ASC followed
by dipping in
an aqueous solution of sodium alginate. The formation of an ASC gradient in
the alginate
coating may have occurred which altered the amount of ASC exposed to targeted
bacteria.
Coating Adhesion and Skin Wetting Properties
Although the contact angle technique was successfully used to determine the
critical surface energy of solids such as coated paper surfaces using probe
liquids (Han
and Krochta 2001), the method was less successful on chicken skin. None of the
probe
solutions formed drops on the skin regardless of their surface tension values
which
indicates that other factors beside surface energy, such as surface roughness,
affected
the initial contact angle. Nonetheless, measurements of initial contact angle
were
successfully used to determine adhesion of liquid materials to food surfaces
(Michalski et
al., 1997). In the present tests, the formation of discrete drops by the
PS+TSP coating
solution allowed contact angle measurement. However, stable drops with
measurable
angles were unobtainable from alginate+ASC coatings. Due to low viscosity
calcium
chloride and sodium alginate solutions diffused over the skin and yielded a
thin film. This
means that the contact angle method was not available to measure the surface
energy of
chicken skins. However, this indicates that any hydrophilic coating layer can
adhere on the
surface of chicken and form a film structure with surface covering.
PS+TSP coating at low viscosity (below 0.37 N s m"z) linearly affected the
contact
angle. At higher viscosity PS+TSP formed a gel at room temperature and the
contact
angle was no longer dependent on the viscosity (Figure 4). When the
concentration of
polymers is very high in the coating solution, the coating solution turns into
what is
effectively a gel. It is hard to use this gelled coating solution for the
coating process. To
obtain better coating, the coating solution should be a viscous solution when
it is coated
on the surface, and form a gel as it dries. Therefore, the coating solution
should contain
polymers at the concentration lower than the gelation concentration for
coating process.
The effect of PS concentrations on the contact angle as an indicator of
coating
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
13
adhesiveness to the skin is shown in Figure 5. In general, increasing the PS
concentration
increased coating adhesion to the skin. At a low concentration of PS (< 0.5 %)
the
measurement of the contact angle was not possible, but between 0.5 and 1.5 %
PS, the
contact angle increased with concentration. At PS levels ranging from 1.5 %
through 3.5
%, the contact angle was not affected (P > 0.05). At 4.0 %, the contact angle
increased to
70 , whereas at higher concentrations the solutions began to gelatinize to
form a soft
solid, which invalidated estimation of adhesion by contact angle measurement.
Several
factors could influence the changes in the initial contact angles shown in
Figures 4 and 5.
Skin roughness was believed to be responsible for generating unstable liquid
drops of the
PS+TSP coating solution at low PS concentrations (< 0.5 %). Under these
conditions the
drops were quickly absorbed and disappeared in the skin. Increasing the PS
concentration
from 0.5 % to 1.5 % increased the coating viscosity from 0.004 to 0.37 N S m-
2, which
resulted in proportional increases in the initial contact angle.
The increase in viscosity gave the coating drops the strength to overcome the
effects of skin roughness and become stabilized on the surface. At 1.5 % to
3.5 % PS the
initial contact angle was not affected by the increases in viscosity (from
0.37 to 1.0 N s m
Z) and the resulting contact angle could account for the difference in the
surface energies
between the skin and the coating solution. In order for the probe solutions to
accurately
measure critical surface energy of the skin, they should have a viscosity in
the range of
0.37 to 1.0 N s m"z. At high levels of PS (> 4.0 %) the solutions started to
gelatinize and
the initial contact angle measured was independent of the surface energy
difference.
Overall, the adhesion of the coating to the skin depended on PS concentration
and
solution viscosity. As will be appreciated by one of skill in the art, just a
reduction of
polymer concentration can decrease viscosity. The polymer concentration is the
main
factor to control the viscosity of the antimicrobial coating layer and
effectiveness of the
antimicrobial activity.
Stabilizing TSP and ASC in PS and alginate coatings, respectively, enhanced
their
antimicrobial activity against Salmonella on chicken skin. PS+TSP caused
significant
reductions of the bacterial numbers for longer periods than alginate+ASC. This
could have
been caused by several factors including: distribution of the AMs within the
coatings;
prolonged effects of the treatments on skin pH; coating absorptiveness; and
coating
adhesion to the skin. Although PS+TSP was more effective, it was less stable
on the skin.
The coating tended to drip from the skin but also absorbed quicker than the
alginate+ASC
coating. Since they had transient (<_ 60 min) stability on the skin surface,
but had good skin
adhesion, with low absorption and significant antimicrobial activity, 5 - 15 %
TSP in
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
14
coatings of 1 - 5 % (w/v) PS may be of industrial value in applications to
reduce numbers
of Salmonella on poultry skin.
Solids loss and water uptake
Hydrogels in contact with solution lose solids and take up water (Figs.7 and
8).
After immersion in the saline solution for 3 hr, hydrogels lost 40% or more of
the initial
solids while absorbing more water at the same time. The solids loss and water
uptake
were largely a process of Fickian diffusion, as shown by good fittings.
However, the
noticeable scattering of data points about the Fickian curves may imply the
concurrent gel
erosion and swelling in an oscillatory manner (Makino et al., 1996, Colloids
Surf B
Biointerfaces 8: 93-100). The presence of TSP in starch gel aggravated the
loss of solids
(Fig.7a), whereas ASC made little difference in the solids loss of alginate
gels (Fig.7b). In
contrast, the presence of TSP or ASC in the gel substantially affected the
degree of water
uptake (Fig.8). For example, gels with antimicrobials absorbed about 45% more
water
than those without antimicrobials after 3-hr immersion in the saline solution.
Due to their
high charge density, phosphate anions also tend to structure water by hydrogen
bonding
(Jane, 1993, Starch/Starke 45: 161-166), and facilitate the water uptake of
starch gel. It is
likely that those electrolytes by electrostatic interactions open up the cross-
linked gel
structures, which become more accessible to water molecules. Meanwhile, more
solids
would be lost in a more open gel structure, since it imposes less hindrance
for small
molecules (e.g. antimicrobials) and/or dangling clusters to leach out.
Antimicrobial release
As shown in Fig. 9, the release of antimicrobials from hydrogels into the
saline
solution followed Fikian diffusion. All R-squared values for the non-linear
fitting were
greater than 0.95. The apparent diffusivity for TSP in starch gel was 2.72X10-
9 mz/s, much
lower than the apparent diffusivity of the solids (10.3x10-9 mZ/s) but close
to the water
diffusivity (2.88x10-9 m2/s). Similarly, the apparent diffusivity for ASC
(6.58X10-9 m2/s) in
alginate gel was lower than the apparent diffusivity of the solids (9.22X10-9
m2/s) but fairly
close to the water diffusivity (5.21 X 10-9 m2/s). On this basis, the
antimicrobials were most
likely unattached to polymer chains in the gel, but rather liberated in the
water phase.
Therefore, the release of antimicrobials TSP and ASC resulted from the osmotic
pressure,
rather than dissolution of solids. Due to higher solids content of the starch
gel compared to
the alginate gel, the denser gel structure imposes a greater block for the
antimicrobial to
get out (or water to get in), resulting in a slower release rate of TSP.
Therefore, the
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
PS+TSP gel would be of particular interest to applications where sustained
release of the
antimicrobial agent is needed.
Storage modulus of hydrogel
The dimensionless storage modulus (G'/G'o) of hydrogel in the saline solution
decreased with immersion time in a trend of exponential decay (Fig.10).
Substantielly
decreased solids content (Fig.11) due to both solids loss (Fig.7) and water
uptake (Fig.8)
was largely responsible for the softening of gels as the immersion prolonged.
Since the
solids content of PS+TSP gel decreased faster than that of the PS-TSP gel
(Fig.11a), it is
not unexpected that storage modulus of the PS+TSP gel decreased faster than
that of the
PS-TSP gel (Fig.10a). However, the ALG+ASC gel showed significantly slower
modulus
reduction than the ALG-ASC gel (Fig.10b), even though both gels had little
difference in
the change in dimensionless solids content with time (Fig.11 b). The
stabilization effect of
ASC on the alginate gel presumably results from the immobilization of Caz+ in
the gel by
citrate from ASC. Otherwise CaZ+ would be prone to ion exchange with Na+ in
the saline
solution, as in the ALG-ASC gel.
The presence of antimicrobials substantially influenced the rheological
properties
of hydrogels by accelerating solids loss and water gain. Since the release of
antimicrobials
was slower than the loss of total solids in the gel, and antimicrobiais and
water had the
same level of diffusivity, it is suggested that the release of antimicrobial
TSP in starch gel
or ASC in alginate gel is largely controlled by osmotic-pressure-induced gel
swelling
(water in and ions out), rather than dissolution of polymer chains in the gel
structure. This
work implies that water diffusivity in hydrogel could be used as a monitor of
drug release
when the drug is known not to strongly interact with polymer chains in the
hydrogel. There
are two main mechanisms of release (1) diffusion and (2) erosion. Most gels
may have
either one or a combination of these two mechanisms. Diffusion is the release
of active
agent from the matrix gels through diffusion, and erosion means that the
release is caused
by the degradation of the matrix gels. Since all biodegradable polymers will
be eroded
eventually, the mechanism of early stage release is important to control the
release rate
so as to maximize effectiveness.
Antimicrobial Coatings
A 100 ml dispersion of 3.5 % (w/v) pea starch was prepared in cold water. The
mixture was heated to boiling with mixing and held for 5 min to complete
starch
gelatinization. The solution was then cooled to room temperature and trisodium
phosphate
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
16
(TSP) was added (10 % w/v), mixed and homogenized by a Powergen-700 for 5 s at
20000 rpm. This yielded PS+TSP coating solution.
Calcium alginate coating (alginate+ASC) consisted of two solutions of 100 ml
each.
Solution (a) was 1%(w/v) calcium chloride in acidified sodium chlorite (ASC,
1200 ppm)
prepared by mixing equal portions of the acid and salt parts of Sanova
provided by Alcide
Corp. This solution was used within 30 min as recommended by Alcide Corp.
Solution (b)
contained 1%(w/v) sodium alginate dissolved in water and mixed. Coatings free
of AMs
were prepared following the same procedures but without TSP addition to PS and
without
ASC addition to alginate. PS+TSP solutions containing 0.5, 1.5, 2.0, 3.5, 4.0
or 4.8 %
(w/v) PS, and alginate+ASC with 0.5, 1.0 or 1.5 % (w/v) alginate were prepared
as
outlined above. These solutions were used for absorptiveness, initial contact
angle and
viscosity measurements.
Chicken Treatment
Unchilled chicken thighs and drumettes (Mehyar et al., 2005) were obtained
from a
local processing plant immediately after slaughtering and used within 30 min
after their
arrival. The warm thighs were used for contact angle tests. The drumettes were
inoculated
with an ampicillin-resistant Salmonella cocktail. Bacterial cultures used to
inoculate
drumettes were: Salmonella entericia serovars Typhimurium (# 02-8425 and # 02-
8421)
and Heidelberg (# 271). The three strains were grown separately in tryptic soy
broth (TSB)
for 24 h at 37 C. Cultures were standardized to an OD600 of 0.80 using sterile
TSB to yield
about 9 log cfu/mi and were combined in equal portions. Inoculations were
performed by
dipping drumettes in triplicate into 300 ml bacterial suspension containing 7
log cfu/ml for
s 15 sec. The drumettes were hung for 10 min to allow bacterial attachment
before being
dipped for 0.25 min in one of the following solutions: (1) TSP (10% w/v); (2)
ASC (1200
ppm); (3) PS+TSP coating; (4) calcium chloride in ASC (solution a) then dipped
in sodium
alginate solution (solution b) to form the alginate+ASC coating; (5) coatings
of 3.5 %(w/v)
PS without AMs; or (6) 1 % (w/v) calcium alginate without AMs. Drumettes were
weighed
before and directly after dipping using a digital balance ( 0.00005 g). The
drumettes were
hung inside a covered glass chamber with 85 % relative humidity and incubated
at 4 C for
120 h. Samples were withdrawn in triplicate for testing after 1, 24, 72 and
120 h
incubation.
Changes in Drumette pH, Weight and Viable Salmonella after Coating
At each sampling day, the surface pH of the coated drumettes was measured at
three different locations using a pH meter equipped with an Isfet surface
probe and their
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
17
average values were recorded. Drumettes were then weighed and their skins were
excised and placed in stomacher bags with buffered peptone water (10 g
peptone, 5 g
NaCI, 3.5 g NaZHPO4, 1.5 g KH2PO4 per liter) and homogenized for 3 min to
prepare 10"'
homogenates. The homogenates were then serially diluted and plated on pre-
poured XLD
agar containing 100 ppm ampicillin. Salmonella were counted after 24 h at 35
C.
Logarithmic reductions were determined by calculating the differences in
Salmonella
numbers between the control and the treated samples.
Coating Absorptiveness
The method of Han and Krochta (1999) was modified to measure the coating
absorption into chicken skin. A plastic ring specimen holder with four screws,
similar to
that used by Han and Krochta (1999), was used to fix skin samples. Skins of
unchilled
chicken thighs were excised and used within 10 min. The outer surface of the
skin was
placed between the base and the ring (diameter 5.8 cm) facing upward in the
holder and
the ring was secured with screws. The holder with the skin was then weighed
(Wo) and 5
mi of the PS+TSP coating solution, or 2.5 ml of 1%(w/v) caicium chloride in
ASC
(solution a) and 2.5 ml of solution b were applied on the top of the skin.
Nine samples
were prepared for each coating and the holding units were placed on a flat
plate at room
temperature to allow the skin samples to absorb the coating solutions. Samples
were
withdrawn in triplicate at 10, 30 and 60 min after application. Absorption was
terminated by
wiping away the excess coating solutions which remained on the skin surface
with a tissue
at each sampling time. The weights of the apparatus holding the skin were
recorded
before (Wwet) and after drying (Wdry). The absorptiveness (% At) was defined
as:
% At =(Wwet - Wdry)/(Wo - We) x 100
where We is the weight of an empty apparatus without skin.
Contact Angle and Skin Wetting Properties
The initial contact angles for the various probe liquids and the coating
solutions on
the skin were used to determine critical surface energy of skin and absorption
profile of
coating solutions, respectively. Fresh, unchilled chicken thighs were used and
their
surfaces were wiped by a dry tissue to remove any residual water. The thighs
were cut on
one side lengthwise to the bone with a razor blade and a portion of the skin
and flesh was
removed from the thigh. For testing, the specimens were placed on a rack with
adjustable
height, and attached to the rack using plastic putty. A digital microscope (10
X
magnification) was aimed horizontally to observe the cut chicken surface.
Drops of 10 pL
of the probe liquids or coating solutions were placed on the skin surface
using a
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
18
microsyringe and the side images of the liquid drops were recorded by a
computer after
confirming the horizontal level position of samples. In order to account for
any asymmetry
of the image caused from improper leveling, the contact angles of both sides
of each liquid
drop were measured and the average values were recorded. All measurements were
done
inside a closed chamber equipped with an electric fan to circulate the
internal air which
was equilibrated to 85 % relative humidity with a saturated solution of zinc
sulfate. The
probe liquids used were HPLC grade water, glycerol, ethylene glycol and
dimethyl
sulfoxide. In order to study the effect of PS viscosity on the contact angle,
the dynamic
viscosity of PS+TSP solutions with different PS concentrations was determined
using a
rheometer. The instrument was operated with parallel plate geometry (plate
diameter = 20
mm and gap = 1 mm). Samples were placed in the apparatus and allowed to
equilibrate at
25 C prior to analysis. Measurements were conducted at 3 Pa shear stress and
1 Hz
frequency. The relationships between the initial contact angle and PS
concentration of
PS+TSP coating solution, and between the initial contact angle and the PS+TSP
coating
solution viscosity were determined.
Flow properties of starch-based coating solution
Figure 12 shows the shear stress-strain curve of the pea starch coating
solution
with and without thyme oil. From this figure the consistency index and power
law flow
behavior index were calculated, and these results are summarized in Table 4.
The
consistency of the gelatinized pea starch coating solution was affected
significantly by the
presence of thyme oil, which caused increased viscosity at low shear rate
range. The
addition of thyme oil decreased the power law flow behavior index and made the
starch
gel more viscous and pseudoplastic.
Figure 12 shows that both starch coating solutions, regardless of thyme oil
addition, exhibited shear-thinning pseudoplastic behavior below 100 s-1 of
shear rate.
However, above 100 s-1, the pseudoplastic characteristics were converted to
Newtonian
behavior, specifically Bingham flow. Starch solutions possess intermolecular
interactions
and form elastic starch gels when the deformation is not significant, such as
occurred
below 100 s-1 of shear. However, above this critical shear, the intermolecular
interaction
of starch gels could not be maintained and were converted from an elastic gel
to a viscous
solution. The corresponding critical shear stresses of 100 s-1 shear rate were
approximately 20 Pa and 5 Pa for pea starch with and without thyme oil,
respectively.
Yield stresses (the Y-intercept of Bingham) were 22.4903 Pa and 5.3486 Pa for
pea starch
solutions with and without thyme oil, respectively, which reflects the
dramatic increase in
the yield stress of the starch solutions caused by thyme oil addition. This
result implies
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
19
that thyme oil enhanced the intermolecular interaction of starch, perhaps by
the formation
of starch (amylose)-lipid complexes. Han and others (2006) found that the
addition of
beeswax to gelatinized pea starch did not change the starch structure and
related
characteristics until 30% (w/w) of beeswax had been added to the starch gel.
Therefore,
the changes in visco-elastic properties of pea starch gels by 5% thyme oil are
remarkable.
Thyme oil contains mostly phenolic compounds that have very small molecular
weight
compared to those of beeswax. It is hypothesized that the small hydrophobic
molecules
can be incorporated within the amylose helix much easier than macromolecular
lipids, and
consequently form a high-degree amylose-lipid complex. For the practical
application of a
thyme oil-starch coating for poultry processing, it is suggested that an
inside-outside bird
washer be used. The washer would spray the starch coating solution at both
high pressure
and high speed feeding rate. Therefore, within the practical operating range
of feeding,
which will be definitely over 100 s-1 shear rate, the thyme oil-starch
solution will behave as
a Bingham fluid. A minimum 22.49 Pa of pressure is required for the bird
washer to initiate
the flow of the starch coating containing thyme oil. The higher yield stress
produces a
thicker coating weight, Since the yield stress of the coating solution
increased 5 times after
thyme oil addition, theoretically on a smooth surface hanging vertically
(e.g., chicken
carcass on an overhead conveyor), the thickness of the coating containing
thyme oil will
be 5 times greater than that of a starch coating without thyme oil. Therefore,
understanding the effects of yield stress upon coating viscosity is critical
to optimize
coating application and uniformity. After washing, chicken carcasses are warm
and the
antimicrobial coating solution can be sprayed at ambient processing room
temperature.
Microbial viability on Salmonella-inoculated chicken
Application of the starch coating to chicken cubes had little effect on the
numbers
of total organisms, the lactic acid bacteria present, and the viability of
inoculated
(ampicillin resistant) Salmonella during 12 d storage at 4 C (Table 5).
Numbers of total
organisms (psychrotrophs) and lactic acid bacteria increased similarly in the
presence or
absence of the starch coating. MRS agar is a non-selective enriched medium and
Salmonella were able to form colonies on this agar. Salmonella numbers
decreased by
about 1 log cfu/g during refrigerated storage in treatments with and without
the starch
coating. Inclusion of thyme oil in the coating delayed the growth of
psychrotrophs until day
4 and the lactic acid bacteria until after day 8. Thyme oil inclusion in the
coating had a
significant negative effect on Salmonella viability with recoveries being 2
log cfu/g lower at
day 4 and this reduction was increased to 3 log cfu/g at days 8 and 12.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
Microbial viability on Campylobacter-inoculated chicken
As with the previously reported trial (Table 5), the starch coating had
essentially no
effect on the growth of psychrotrophs and lactic acid bacteria during storage
of the chicken
meat at 4 C for 12 d (Table 6). However, addition of starch coating containing
thyme oil
significantly reduced the extent of both psychrotrophic and lactic acid
bacterial growth by 2
and 3 log cfu/g at days 8 and 12, respectively. Direct addition of thyme oil
as a water
emulsion without the coating caused a similar delay in psychrotrophic
bacterial growth, but
had a greater initial inhibitory effect on the lactic acid bacteria. These
latter recovered by
day 8 to reach about the same numbers as were present on chicken coated with
starch
containing thyme oil. These latter levels were 2 to 3 log cfu/g less than in
treatments
where thyme oil was not used. Campylobacter were absent from the chicken meat
used in
this study, and following inoculation their numbers were relatively stable
during storage at
4 C. A very slight reduction in Campylobacter viability was noted in response
to starch
coating at day 12, but use of thyme oil alone or use of thyme oil following
its incorporation
into the starch coating caused an immediate reduction in Campylobacter
viability to below
detectable levels, and this inhibitory or lethal effect was maintained for the
remainder of
the study (Table 6).
Microbial viability on Listeria-inoculated chicken
As noted in Tables 5 and 6, psychrotrophic and lactic acid bacteria naturally
present on uninoculated chicken grew rapidly and reached 7 to 8 log cfu/g by
12 d of
storage at 4 C (Tables 7 and 8). There was little difference in bacterial
recoveries
(psychrotrophs, lactic acid bacteria or inoculated L. monocytogenes) among the
media
used when starch-coated chicken (with or without L. monocytogenes inoculation)
was
stored at 4 C for 12 d. L. monocytogenes was able to grow on the MRS medium
used for
lactic acid bacteria recovery, and contributed to the number of colonies
recovered as lactic
acid bacteria.
The extent of bacterial growth on BHI and MRS agars was reduced in treatments
containing thyme oil, and inhibition caused by direct addition of thyme oil
was only slightly
greater than that caused by the thyme oil-starch coating (Table 7). The
inhibitory effects
were not as great as noted with Campylobacter (Table 6).
L. monocytogenes was not recovered on Listeria selective agar from
uninoculated
chicken during storage, but following its inoculation the organism increased
one log cfu/g
during storage. In addition, growth of L. monocytogenes was unaffected by the
presence
of the starch coating as noted with Salmonella and Campylobacter. Thyme oil
alone or
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
21
when incorporated into the starch coating was inhibitory to L. monocytogenes
(on Listeria
agar) to about the same extent (> 1 log cfu/g reduction) by 12 d storage.
Microbial viability on Pseudomonas-inoculated chicken
The microbial growth profile on chicken inoculated with P. aeroginosa as
monitored
on BHI and MRS agars (Table 8) did not differ from results obtained with the
other
inoculated organisms when thyme oil was not used (Tables 5, 6 and 7). In
addition, the
pea starch coating did not further alter bacterial recoveries on these media
or
Pseudomonas agar during storage at 4 C. Thyme oil along or when incorporated
in the
pea starch coating significantly delayed the growth of bacteria on chicken
monitored with
all three media. These differences were from one to 2 log cfu/g and were noted
at 12 d of
storage (Table 8), however, there was no significant difference in
effectiveness of thyme
oil action before or after incorporation in the starch coating.
Antimicrobial effectiveness of thyme oil
Thyme oil has been shown to one of several potently antimicrobial essential
oils
during tests against a range of spoilage and pathogenic bacteria. Its major
component,
thymol, was as effective as eugenol and carvacrol against most of the
pathogens tested in
the present study (Burt 2004). Generally, essential oils are more effective
against Gram
positive bacteria, but Gram negative bacteria can be vulnerable (Burt 2004;
Holley and
Patel 2005). In the present work delayed growth of aerobic psychrotrophs and
lactic acid
bacteria was not unexpected. Inhibition of L. monocytogenes growth and
reduction in
Salmonella viability in the presence of thyme oil reported here are consistent
with the
results from other studies where different substrates and temperatures of
incubation were
used (Burt 2004). The delayed growth of P. aeroginosa reported here is a
positive finding
since'Pseudomonas frequently show resistance to essential oil treatment
(Holley and
Patel 2005), however, it is likely that during longer storage P. aeroginosa
would recover
from the inhibitory effects of thyme oil exposure. One of the more important
observations
made here was the drastic reductions in numbers of C. jejuni which occurred
immediately
upon exposure to thyme oil alone or to the starch-thyme oil coating, which was
sustained
during 12 d storage. Surprisingly little work is reported in the literature
concerning C. jejuni
inhibition by thyme oil. In a study by Friedman and others (2002) thyme oil
was found to
be as effective as cinnamaidehyde, eugenol, carvacrol, citral, geranol, and
benzaldehyde
against C. jejuni in a microplate assay.
In the C. jejuni and L. monocytogenes tests reported here where thyme oil was
directly added to the chicken meat surface, a more immediate inhibitory effect
was found
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
22
against the lactic acid bacteria, however, this difference was not evident at
12 d storage.
In P. aeruginosa tests the starch-thyme oil coating initially showed a greater
inhibitory
effect but this difference was resolved by day 8 of storage. In a separate
test it was found
that Salmonella, L. monocytogenes, and P. aeruginosa were able to form small
colonies
on MRS agar. Thus, lactic acid bacterial recoveries may have been over-
estimated to
some extent. However, this observation does not affect the overall conclusions
from the
study.
Statistical Analysis
Data obtained were the average values of three replicates for treatments. Each
treatment was conducted twice in separate experiments. The statistical
analytical system
was used to compare means of the replicates at each sampling time. A
significance level
of 5 % was used for all analyses. Linear regression analysis for absorption
rate was
conducted using the data analysis option of a spread sheet for the absorption
curves
(weight vs. time).
Preparation of starch and alginate hydrogels
3 grams of pea starch (PS, 37% amylose, Nutri-Pea Ltd., Portage-la-Prairie,
MB)
was dispersed in 100 ml cold water. The dispersion was heated to boiling with
mixing and
held for 5 min when starch granules were almost fully gelatinized. The
solution was then
cooled to room temperature (23 C), and 10 grams of trisodium phosphate (TSP,
Sigma
Chemical Co., St. Louis, MO) was added in, followed by homogenization with a
Powergen-
700 (Fisher Scientific International Inc., Whitby, ON) for 5 s at 20,000 rpm.
The solution
was then poured into two 200 ml beakers, with 50 ml solution in each beaker,
and left
overnight at room temperature to allow the stabilization of gel structure. PS
hydrogel
without TSP was also prepared and used as control.
Two solutions were used to prepare calcium alginate (ALG) hydrogel. Solution
(a)
was an acidified sodium chlorite (ASC) solution containing 1% w/v of calcium
chloride
(CaCl2, Sigma Chemical Co., St. Louis, MO). The ASC solution was prepared by
mixing
equal portions of citric acid solution (900 ppm) and sodium chlorite solution
(1100 ppm)
(Sanova, Alcide Corp., Redmond, WA), and was used within 30 min after
preparation.
Solution (b) contained 0.5% w/v sodium alginate (Product No.180947, CAS 9005-
38-3,
Sigma Chemical Co., St. Louis, MO) dissolved in water at room temperature.
Calcium alginate gel was prepared using a plastic assembly consisting of a
mold
and two fixative rings (Fig.6). A piece of CaCI2 permeable membrane (Dialysis
Tubing,
Fisher Brand regenerated cellulose, Fisher Scientific, Nepean, ON) was first
attached onto
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
23
the mold by one fixative ring. Solution (b) of 50m1 was poured into the mold,
and the mold
was covered by another piece of the membrane, which was fixed onto the mold
using the
other ring. Two assemblies containing the sodium alginate solution were then
immersed in
solution (a) of 500 ml and taken out after 24h. Self-standing calcium alginate
gels
containing ASC were obtained after the removal of the rings and membranes.
Calcium
alginate gels without ASC were also prepared by the same procedure except that
solution
(a) used was a pure CaC12 solution with the same concentration of 1% w/v.
Rheological properties of hydrogels in air and in saline
Freshly prepared hydrogel was cut into a cylinder using a plastic borer with a
height of 10 mm and internal diameter of 20 mm. The gel cylinder was then
sliced into
specimens with a thickness of 5 mm by a sharp blade. Rheological analysis was
carried
out using a controlled stress rheometer (AR-1000, TA Instruments Inc., New
Castle, DE)
with 20-mm parallel plate geometry. After a specimen was centered on the base
platen,
the upper platen was programmed to move down at a decelerating speed until it
came in
contact with the specimen in order to avoid any pre-loading deformation.
Oscillatory stress
sweeps from 0.1 Pa to 10 Pa at a frequency of 1 Hz were done at a temperature
of 25 C
to determine the linear viscoelastic range for hydrogels in air. Since both
gels exhibited
linear elastic regions at stress below 2 Pa, a stress of 1 Pa was chosen for
the following
time-sweep experiments.
A physiological saline was prepared by dissolving 0.8 g of sodium chloride
(Sigma
Chemical Co., St. Louis, MO) in 100 ml tap water, followed by adjusting pH to
6.8. After a
gel specimen was centered in a bath (height 30 mm, inner diameter 34 mm, outer
diameter 64 mm) glued to the base platen, a saline solution double the
specimen's volume
was filled inside the bath while the upper plate came in contact with the
specimen.
Oscillatory time sweeps (1 Pa at 1 Hz) were run for all specimens in saline. A
series of
dynamic storage moduli (G) as a function of immersion time (t) were obtained
from the
control software.
Determination of TSP and ASC concentrations in saline
After the specimen was loaded, saline solution of 0.5 ml was withdrawn
periodically and collected in a vial. The samples, diluted 1000 fold, were
analyzed by an
ion chromatography system (Dionex Corporation, Sunnyvale, CA). The injection
volume
and flow rate were maintained at 50 NI and 1 ml/min, respectively, throughout
the analysis.
External standards (0, 5, 25, 50, 75 and 100 Ng/mI) for both phosphate and
chlorite anions
were used for calibration. NaOH solution of 30 mM was used as eluent for all
samples.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
24
The concentration of antimicrobial (C) in the saline was determined by the
peak area for
the elution which was calculated by Chromeleon Chromatography Management
Systems
(Dionex Corporation, Sunnyvale, CA). The concentration of ASC was determined
by
subtracting the peak area for chloride anions in the pre-load saline solution
from the peak
area for both chloride and chlorite anions in the samples containing ASC,
since both
chlorite anions from ASC and chloride anions from NaCI eluted at the same time
(3 min).
The concentration of TSP was determined directly by the peak area for
phosphate anions
in the samples. The concentration of antimicrobial after 12-hr immersion in
the saline
solution was taken as the equilibrium concentration (C.).
Solids loss and water uptake during gel swelling
A freshly prepared gel sample (diameter 20 mm and thickness 5 mm) was weighed
before (M;) and after (Md) drying at 105 C to constant weight (about 5 hr).
The initial solids
content (SCo) of the fresh gel was determined as MdIM;. A gel sample, after
weighing (Mo),
was immersed in a saline bath (the sample size, the inner diameter of the
bath, the
concentration and amount of the saline solution same as those used in the
rheological
determination) for a period of time up to 8 hr. The swollen gel was then
weighed (MsW),
followed by drying at 105 C to constant weight (Ms). The amount of solids (Mo)
and water
(M,,,,o) in the pre-swelling gel were MoSCo and Mo(1-SCo), respectively. The
amount of
solids (Ms) and water (Mw) in the post-swelling gel were MS and Msw M5,
respectively. The
solids content (SC) of the swollen gel was M/MS,. All samples are duplicated.
Apparent diffusivities of solids, water and antimicrobials
Solids loss, water uptake and antimicrobial release were all assumed to follow
Fikian diffusion. A simple form of the solution (Schwartzberg and Chao, 1982,
Food
Technol 36: 73-86) is:
X-X~ q;Dt
=CiEXp(- Z ) (1)
Xo - X. R
where X can be the amount of solids (Ms) or water (M,N), or the concentration
of
antimicrobial released (C), and the subscripts 0 and oo stand for at zero and
infinite time,
respectively. The constants c, and q, are correlated. For infinite
cylinder qt = 4(a + 1)(a - c, ) l c, l a , where the stripping factor ^= 2
(the volume of
saline solution divided by the volume of gel). R is the radius of gel sample
(10mm), and t
the immersion time. The apparent diffusivity (D) of solids, water and
antimicrobial were
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
obtained by 3-parameter non-linear fitting of Ms/MSo - t, MWIM o- t, and CIC. -
t, based on
Equation 1.
Air-chilled fresh chicken breast meats were obtained from a local poultry
processing plant (Dunn-Rite, Winnipeg, Manitoba, Canada) about 4 h before the
experiment. The meats were cut into 2 cm x 2 cm cubes (10 g 1 g) with a
knife
disinfected in 70% ethanol. Starch extracted from Canadian yellow field peas
(Pisum
sativum L. Miranda) by a conventional wet milling process was supplied by
Nutri-Pea Ltd.
(Portage-La-Prairie, Manitoba, Canada). Pea starch is a C-type starch
containing 37 -
40% amylose. One gram of phosphatidyl choline (Fisher Scientific, Nepean,
Ontario,
Canada) was dissolved in 15 mL of thyme oil (Sigma Chemicals Co., St. Louis,
MO) and
stored at 4 C until used.
Ampicillin resistant Salmonella entericia serovars (i.e., Typhimurium and
Heidelberg) and Campylobacterjejuni were obtained from R. Ahmed, Canadian
Centre for
Human and Animal Health (Winnipeg, Manitoba, Canada). Listeria monocytogenes
and
Pseudomonas aeruginosa were obtained from the culture collections of the
Department of
Food Science and the Department of Microbiology, respectively, at the
University of
Manitoba (Winnipeg, Manitoba, Canada).
Consistency profile of starch coating solution
Fully gelatinized 2.5 % (w/v) aqueous starch solution (prepared by boiling 20
min)
containing 1.25% (w/v) glycerol was mixed with 5% (v/v) thyme oil at room
temperature,
and its consistency was determined using a rheometer (AR1000, TA Instruments,
New
Castle, DE). The volume of samples was 0.99 mL. Operating conditions of the
rheometer
were 25 C using a 60 mm diameter 1 angle steel cone. Initial shear rate was
1.275 s-1
and was ramped to 1000 s-1. Shear rate was increased by steady state flow mode
with a
logarithmic ramp pattern. Consistency index and fluid behavior index were
calculated
using the power law equation by parameter estimate of regression analysis.
Each
treatment was tested in triplicate.
Bacterial inoculum preparation
All bacterial cultures were maintained in BHI (brain heart infusion) broth and
enumerated on BHI agar (Difco Division, Becton Dickinson Co., Sparks, MD)
after
incubation at 35 C for 24 to 48 h. For Campylobacter culture BHI agar and
broth media
were used with 0.5% (w/v) yeast extract and 10% (w/v) laked horse blood (Oxoid
Ltd.,
Nepean, Ontario, Canada), and were incubated at 35 C under microaerophilic
conditions
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
26
created by the CampyPak Plus system (Becton Dickinson Co., Cockeysville, MD)
for 48 h.
Bacterial culture broth was centrifuged at 3000g for 15 min at 10 C (Sorvall
RC2-B
Refrigerated Centrifuge, Du Pont, Newtown, CT). The sedimented culture pellet
was
suspended in 0.85% sterile saline solution to wash and was recentrifuged. The
pellet was
diluted to yield an optical density of 0.80 at 600 nm and the live bacterial
population was
determined using a spiral plating unit (Autoplate 4000, Spiral Biotech,
Bethesda, MD). The
equivalent number of bacteria for 0.8 optical density units was 109 cfu/mL.
The two
Salmonella cultures were mixed at equal numbers of cells to obtain a cocktail
of S.
Typhimurium and S. Heidelberg.
Antimicrobial pea starch coating
Pea starch suspension was prepared by mixing 25 g pea starch and 12.5 g
glycerol (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada) in 1 L sterile
cold distilled
water. This suspension was boiled for 20 min with agitation to gelatinize pea
starch, and
cooied in a water bath at 50 C. The thyme oil and phosphatidyl choline
mixture was
blended into the pea starch coating solution to give a 5% (v/v) concentration
and stirred for
min.
Inoculation of chicken meat
Chicken meat cubes (approximately 2 kg) were placed in a sterile aluminum tray
and 2 L of inoculum containing 106 cfu/mL of each of the test organisms and
the
Salmonella cocktail were separately poured on the chicken cubes. The tray was
shaken 2
to 3 times during 15 min exposure to allow the meat to adsorb bacteria, then
the excess
liquid was drained. The inoculated meats were dried for 5 min in the tray. One
quarter of
the inoculated cubes (approximately 0.5 kg) were enclosed in a high-barrier
plastic bag
(Deli*1, WinPak, Winnipeg, Manitoba, Canada) composed of nyfon/ethylene vinyl
alcohol/polyethylene, and heat-sealed. The film was 75 pm thick with an oxygen
transmission rate of 2.3 cm3 m-z d"' at 23 C, and water vapor transmission
rate of 7.8 g m-2
d" at 37.8 C and 98% relative humidity. The second quarter of the inoculated
cubes was
transferred onto a sterile tray and 1 L of pea starch coating solution was
poured onto the
cubes. After shaking for 1 to 2 min, the excess starch solution was drained.
The coated
cubes were dried for 1 h in the tray, and each cube was packaged in the high-
barrier
plastic bag. The third quarter of inoculated cubes was placed in a sterile
tray, and I L of
pea starch coating solution containing 5% thyme oil was poured on the chicken
cubes.
The last quarter of inoculated chicken cubes was mixed with 1 L sterile water
containing
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
27
5% thyme oil. Both thyme oil treatments were mixed, dried and packaged as
described
earlier. Chicken meats without inoculation and coating were packaged as
control samples
(i.e., no treatment). All samples were stored at 4 C.
Viable numbers of bacteria
At 0, 4, 8 and 12 d of storage after inoculation, three bags per treatment
were
opened aseptically and 90 mL of 0.1 % peptone water was added. This bag was
placed in
a stomacher and pummeled for 1 min. After appropriate serial dilutions, the
samples were
plated on agar media using the spiral plating unit, and incubated. All plates
were counted
in duplicate from each sample (total 6 analyses per treatment). Types of agar
media used
and incubation conditions used for inoculated bacteria were:
Total aerobes: BHI agar at 35 C for 24 h
Lactic acid bacteria: MRS agar (Difco) at 32 C for 48 h
Salmonella: XLD agar (Difco) containing 100 ppm ampicillin (Sigma-Aldrich) at
35 C for
24 h
Campylobacter: Karmali agar (Oxoid Ltd.) containing a growth supplement (Oxoid
SR 139)
at 35 C for 48 h under microaerophilic conditions
Listeria: Listeria selective agar (Oxford selective fomulation, Oxoid Ltd.) at
35 C for 24 h
Pseudomonas: Pseudomonas agar (Oxoid Ltd.) with a supplement (Oxoid SR 103) at
35
C for 24 h
While the preferred embodiments of the invention have been described above, it
will be recognized and understood that various modifications may be made
therein, and
the appended claims are intended to cover all such modifications which may
fall within the
spirit and scope of the invention.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
28
REFERENCES
BeMiller, J. N. 1965. Alkaline degradation of starch. Pages 521-532 in Starch:
Chemistry and Technology. R. L. Whistler and E. F. Paschall, ed. Academic
Press, New
York.
Cagri, A., Z. Ustunol, and E. T. Ryser. 2004. Antimicrobial edible films and
coatings. J. Food Prot. 67:833-848.
Choi, W. S., and J. H. Han. 2002. Film-forming mechanism and heat denaturation
effect on the physical and chemical properties of pea-protein-isolate edible
films. J. Food
Sci. 67:1399-1406.
Debeaufort, F., J. Quezada-Gallo, and A. Voilley. 1998. Edible films and
coatings:
tomorrow's packaging: A review. Crit. Rev. Food Sci. Nutr. 38:299-313.
Han, J. H., and J. M. Krochta. 2001. Physical properties and oil absorption of
whey-
protein-coated paper. J. Food Sci. 66:294-229.
Han, J. H., and J. M. Krochta. 1999. Wetting properties and water vapor
permeability of whey-protein-coated paper. Trans. ASAE. 42:1375-1382.
Janes, M. E., S. Kooshesh, and M. G. Johnson. 2002. Control of Listeria
monocytogenes on the surface of refrigerated, ready-to-eat chicken coated with
edible
zein film coatings containing nisin and/or calcium propionate. J. Food Sci.
67:2754-2757.
King, A. H. 1982. Brown seaweed extracts (alginates). Pages 115-188 in Food
Hydrocolloids. M. Glicksman, ed. CRC Press, Boca Raton, FL.
Lucas, A. M., and P. R. S. Stettenheim. 1972. Microscopic structure of skin
and
derivatives. Pages 485-636 in Avian Anatomy Integument. A. M. Lucas and P. R.
S.
Stettenheim, ed. Agricultural Research Service, USDA, Washington, D.C.
Mehyar, G. F., G. Blank, J. H. Han, A. Hydamaka, and R. A. Holley. 2005.
Effectiveness of trisodium phosphate, lactic acid and commercial
antimicrobials against
pathogenic bacteria on chicken skin. Food Prot. Trends. 25:351-362.
Mehyar, G. F., and J. H. Han. 2004. Physical and mechanical properties of high
amylose rice and pea starch films as affected by relative humidity and
plasticizer. J. Food
Sci. 69:E449-E454.
Michalski, M., S. Desobry, and J. Hardy. 1997. Food materials adhesion: a
review.
Crit. Rev. Food Sci. Nutr. 37:591-619.
Natrajan, N., and B. W. Sheldon. 2000a. Efficacy of nisin-coated polymer films
to
inactivate Salmonella Typhimurium on fresh broiler skin. J. Food Prot. 63:1189-
1196.
Natrajan, N., and B. W. Sheldon. 2000b. Inhibition of Salmonella on poultry
skin
using protein- and polysaccharide-based films containing a nisin formulation.
J. Food Prot.
63:1268-1272.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
29
Oyarzabal, O. A. C., C. Hawk, S. F. Bilgili, C. C. Warf, and G. K. Kemp. 2004.
Effects of postchill application of acidified sodium chlorite to control
Campylobacter spp.
and Escherichia coli on commercial broiler carcasses. J. Food Prot. 67:2288-
2291.
Ratnayake, W. S., R. Hoover, and T. Warkentin. 2002. Pea starch: composition,
structure and properties - a review. Starch. 54:217-234.
Schneider, K. R., G. Kere-Kemp, and M. L. Aldrich. 2002. Acidified sodium
chlorite
antimicrobial treatment of air chilled broiler carcasses. Dairy Food Environ.
Sanit. 22:102-
108.
Siragusa, G. R., and J. S. Dickson. 1992. Inhibition of Listeria monocytogenes
on
beef tissue by application of organic acids immobilized in calcium alginate
gel. J. Food Sci.
57:293-296.
Slader, J., G. Domingue, F. Jorgensen, K. McAlpine, R. J. Owen, F. J. Bolton,
and
T. J. Humphrey. 2002. Impact of transport crate reuse and of catching and
processing on
Campylobacter and Salmonella contamination of broiler chicken. Appi. Environ.
Microbiol.
68:713-719.
Suderman, D. R., and F. E. Cunningham. 1980. Factors affecting adhesion of
coating to poultry skin, effect of age, method of chilling, and scald
temperature on poultry
skin ultrastructure. J. Food Sci. 45:444-449.
Thomas, C. J., and T. A. McMeekin. 1982. Effect of water immersion on the
microtopography of the skin of chicken carcasses. J. Sci. Food Agric. 33:549-
554.
Thomas, C. J., and T. A. McMeekin. 1984. Effect of water uptake by poultry
tissues
on contamination by bacteria during immersion in bacterial suspensions. J Food
Prot.
47:398-402.
Wang, W., Y. Li, M. F. Slavik, and H. Xiong. 1997. Trisodium phosphate and
cetylpyridinium chloride spraying on chicken to reduce attached Salmonella
typhimurium.
J. Food Prot. 60:992-994.
Xiong, H., Y. Li, M. F. Slavik, and J. T. Walker. 1998. Spraying chicken skin
with
selected chemicals to reduce attached Salmonella typhimurium. J. Food Prot.
61:272-275.
Zhao, C., Ge Beilei, J. Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D.
Wagner,
and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli and
Salmonella
serovars in retail chicken, turkey, pork and beef from the greater Washington,
DC area.
Appl. Environ. Microbiol. 67:5431-5436.
Bodmeier R, Wang JJ. Microencapsulation of drugs with aqueous colloidal
polymer
dispersions. J Pharmaceut Sci 1993;82(2):191-194.
Bodmeier R, Chen HG, Paeratakul O. A novel-approach to the oral delivery of
micro-particles or nanoparticies. Pharmaceut Res 1989;6(5):413-417.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
Doria-Serrano MC, Ruiz-Trevino FA, Rios-Arciga C, Hernandez-Esparza M,
Santiago P. Physical characteristics of poly(vinyl alcohol) and calcium
alginate hydrogels
for the immobilization of activated sludge. Biomacromolecules 2001;2(2):568-
574.
Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. Biological interactions
between polysaccharides and divalent cations: the egg box model. FEBS Left
1973;32(1):195-198.
Seely GR, Hart RL. The binding of alkaline earth metal ions to alginate.
Macromolecules 1974;7(5):706-710
Ortega NBMD, Perez-Mateos M. Stabilisation of ^-glucosidase entrapped in
alginate and polyacrylamide gels towards thermal and proteolytic deactivation.
J Chem
Technol Biotechnol 1998;73(1):7-12.
Durrani CM, Donald AM. Physical characterisation of amylopectin gels. Polym
Gels
Networks 1995;3(1):1-27.
Goodfellow BJ, Wilson RH. A Fourier transform infrared study of the gelation
of
amylose and amylopectin. Biopolymers 1990;30:1183-1189.
Ring SG, Colonna P, I'Anson KJ, Kalichevsky MT, Miles MJ, Morris VJ, Orford
PD.
The gelation and crystallisation of amylopectin. Carbohydr Res 1987;162(2):277-
293.
Liu Z, Han JH. Film-forming characteristics of starches. J Food Sci
2005;70(1):E31-E36.
Miyata T, Asami N, Uragami T. Preparation of an antigen-sensitive hydrogel
using
antigen-antibody bindings. Macromolecules 1999;32(6):2082-2084
Galliard T, Bowler P. Morphology and composition of starch. In: Galliard T,
editor.
Starch: Properties and Potential. New York: John Wiley & Son, 1987. p. 55-78.
Liu Z. Edible films and coatings from starches. In: Han JH, editor.
Innovations in Food
Packaging. New York: Academic Press, 2005. p. 318-337.
Smidsrrad 0, Grasdalen H. Polyelectrolytes from seaweeds. Hydrobiologia
1984;116-117:19-28
Ahearne M, Yang Y, El Haj AJ, Then KY, Liu KK. Characterizing the viscoelastic
properties of thin hydrogel-based constructs for tissue engineering
applications. J R Soc
Interface 2005;2(5):455-463.
Decho AW. Imaging an alginate polymer gel matrix using atomic force
microscopy.
Carbohydr Res 1999;315(3-4):330-333
Walkenstrom P, Kidman S, Hermansson AM, Rasmussen PB, Hoegh L.
Microstructure and rheological behaviour of alginate/pectin mixed gels. Food
Hydrocol
2003;17(5):593-603.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
31
Donati I, Holtan S, Morch YA, Borgogna M, Dentini M, Skjak-Braek G. New
hypothesis on the role of alternating sequences in calcium-alginate gels.
Biomacromolecules 2005;6(2):1031-1040.
Rees DA, Samuel JWB. Structure of alginic acid. VI. Minor features and
structural
variations. J Chem Soc C Organic 1967;22:2295-2298.
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds
for bone tissue engineering. Biomaterials 2005;26(18):3919-3928
Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development
of a new drug carrier made from alginate. J Pharmaceut Sci 1993;82(9):912-917.
Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical
foundations and structural design of hydrogels in medicine and biology. Annu
Rev Biomed
Eng 2000;2:9-29.
Prokop A, Kozlov E, Carlesso G, Davidson JM. Hydrogel-based colloidal
polymeric
system for protein and drug delivery: physical and chemical characterization,
permeability
control and applications. Adv Polym Sci 2002;160:119-173.
Schwartzberg HG, Chao RY. Solute diffusivities in leaching processes. Food
Technol 1982;36(2):73-86
Makino K, Idenuma R, Ohshima H. A model for erosion kinetics of a hydrogel
matrix. Colloids Surf B Biointerfaces 1996;8(1-2):93-100.
Jane J. Mechanism of starch gelatinization in neutral salt solutions.
Starch/Starke
1993;45(5):161-166.
Black JL, Jaczynski J. 2006. Temperature effect on inactivation kinetics of
Escherichia coli 0157:H7 by electron beam in ground beef, chicken breast meat,
and trout
fillets. J Food Sci 71(6): M221-227
Burt S. 2004. Essential oils: their antibacterial properties and potential
applications
in foods - a review. Int J Food Microbiol 94: 223-253
Cason JA, Berrang ME, Smith DP. 2006. Recovery of bacteria from broiler
carcasses rinsed zero and twenty-four hours after immersion chilling. Poultry
Sci 85(2):
333-336
Corry JEL, James SJ, Purnell G, Barbedo-Pinto CS, Chochois Y, Howell M, James
C. 2007. Surface pasteurization of chicken carcasses using hot water. J Food
Eng 79(3):
913-919
Friedman M, Henika PR, Levin CE, Mandrell RE. 2006. Antimicrobial wine
formulations active against the foodborne pathogens Escherichia coli 0157:H7
and
Salmonella enterica. J Food Sci 71(7): M245-251
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
32
Friedman M, Henika PR, Mandrell RE. 2002. Bactericidal activities of plant
essential oils and some of their constituents against Campylobacterjejuni, E.
coli, Listeria
monocytogenes and Salmonella Typhimurium. J Food Prot 65(10): 1545-1560
Gennadios A, Hanna MA, Kurth LB. 1997. Application of edible coatings on
meats,
poultry and seafoods: a review. Lebensm Wiss u Technol 30(4): 337-350
Han JH. 2007. Packaging for nonthermal food processing: future. In: Packaging
for
Nonthermal Processing of Foods (Han JH, ed.). Blackwell Publishing
Professionals, Ames,
IA. p. 213-226
Han JH, Seo GH, Park IM, Kim GN, Lee DS. 2006. Physical and mechanical
properties of pea starch edible films containing beeswax emulsions. J Food Sci
71(5):
E290-296
Han, J.H. 2005. Antimicrobial packaging systems. In: Innovations in Food
Packaging (Han JH, ed.). Elsevier Academic Press, Oxford, UK. p. 80-107
Han, J.H. 2003. Antimicrobial food packaging. In: Novel Food Packaging
Techniques (Ahvenainen R, ed.). Woodhead Publishing Ltd., Cambridge, UK. p. 50-
70
Holley RA, Patel D. 2005. Improvement in shelf-life and safety of perishable
foods
by plant essential oils and smoke antimicrobials. Food Microbiol 22: 273-292
Jorgensen F, Bailey R, Williams S, Henderson P, Wareing DRA, Bolton FJ, Frost
JA, Ward L, Humphrey TL. 2002. Prevalence and numbers of Salmonella and
Campylobacter spp. on raw, whole chicken in relation to sampling methods. Int
J Food
Microbiol 76: 151-164
Kaloustian J, Abou L, Mikail C, Amiot MJ, Portugal H. 2005. Southern French
thyme oils: chromatographic study of chemotypes. J Sci Food Agric 85(14): 2437-
2444
Mehyar GF, Han JH, Holley RA, Blank G, Hydamaka AW. 2007. Suitability of pea
starch and calcium alginate as antimicrobial coatings on chicken skin. Poultry
Sci 87(2):
386-393
Min SC, Harris LJ, Han JH, Krochta JM. 2005. Listeria monocytogenes inhibition
by whey protein films and coatings incorporating lysozyme. J Food Prot 68(11):
2317-2325
Northcutt JK, Cason JA, Smith DP, Buhr RJ, Fletcher DL. 2006. Broiler carcass
bacterial counts after immersion chilling using either a low or high volume of
water. Poultry
Sci 85(10): 1802-1806
Patsias A, Chouliara I, Badeka A, Savvaidis IN, Kontominas MG. 2006. Shelf-
life of
a chilled precooked chicken product stored in air and under modified
atmospheres:
microbiological, chemical, sensory attributes. Food Microbiol 23(5): 423-429
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
33
Sasso M, Culici M, Brega PC, Guffanti EE, Mucci M. 2006. Thymol: inhibitory
activity on Escherichia coli and Staphylococcus aureus adhesion to human
vaginal celis. J
Essential Oil Res 18(4): 455-461
Singh A, Singh RK, Bhunia AK, Singh N. 2003. Efficacy of plant essential oils
as
antimicrobial agents against Listeria monocytogenes in hotdogs. Lebensm Wissen
u
Technol 36(8): 787-794
Smith DP, Cason JA, Berrang ME. 2005a. Effect of fecal contamination and cross-
contamination on numbers of coliform, Esherichia coli, Campylobacter, and
Salmonella on
immersion-chilled broiler carcasses. J Food Prot 68(7): 1340-1345
Smith DP, Northcutt JK, Musgrove MT. 2005b. Microbiology of contaminated or
visibly clean broiler carcasses processed with an inside-outside bird washer.
Int J Poultry
Sci 4(12): 955-958
Smith PA, Stewart J, Fyfe L. 2001. The potential application of plant
essential oils
as natural food preservations in soft cheese. Food Microbiol 18(4): 463-470
Suhr KI, Nielson PV. 2003. Antifungal activity of essential oils evaluated by
two
different application techniques against rye bread spoilage fungi. J Appl
Microbiol 94(4):
665-674
Uyttendaele M, Baert K, Ghafir Y, Daube G, De Zutter L, Herman L, Dierick K,
Pierard D, Dubois JJ, Horion B, Debevere J. 2006. Quantitative risk assessment
of
Campylobacter spp. in poultry based meat preparations as one of the factors to
support
the development of risk-based microbiological criteria in Belgium. Int J Food
Microbiol
111(2): 149-163
Youdim KA, Deans SG, Finlayson HJ. 2002. The antioxidant properties of thyme
(Thymus zygis L.) essential oil: an inhibitor of lipid peroxidation and a free
radical
scavenger. J Essential Oil Res 14(3): 210-215
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
34
Table 1. Weight changes' of chicken drumettes dipped in 10 % (w/v) trisodium
phosphate
(TSP) with or without 3%(w/v) pea starch (PS), or in 1200 ppm acidified sodium
chlorite
(ASC) with or without 1%(w/v) calcium alginate during storage at 4 C
Treatment % weight change during storage (means SD)
Oh lh 24h 72h 120h
PS+TSP 5.12 0.48 0.62 0.2 0.12 0.19 0.35 0.14 -0.68 0.13
PS 4.84 0.49 3.86 0.37 3.64 0.36 0.91 0.31 0.89 0.31
TSP 1.49 0.18 0.52 0.21 -0.89 0.19 ND 2 ND
Alginate+ASC 7.86 0.84 5.32 1.0 5.25 1.07 3.98 0.814 4.05 1.2
Calcium alginate 6.88 0.47 4.98 0.29 4.1 0.30 2.5 0.19 2.6 0.11
ASC 1.43 0.15 1.25 0.16 -0.55 0.13 ND ND
Water (control) 1.47 0.04 1.66 1.1 -1.93 0.60 ND ND
' Weight gained or lost/ initial weight x 100.
2 Not determined.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
Table 2. Percentage absorptiveness (% At)' of antimicrobial pea starch
(PS+TSP) and
calcium alginate (alginate+ASC) coatings containing different polymer
concentrations applied
to chicken skin and held at room temperature for s 60 min
Treatment Polymer concentration %At (means SD) after holding (min,)
(% w/v) 10 30 60
PS+TSP 0.5 2.40 t 0.61 a 4.51 t 0.36 a 5.81 0.66 a
3.5 1.98t0.23a 3.81 0.55 ab 4.73t0.49a
4.8 0.93 0.20 b 1.23 0.26 1.61 t0.30b
Alginate+ASC 0.5 0.98 f 0.26 b 1.15 0.15 1.21 t 0.65 b
1.0 0.62t0.15 0.75 0.15 0.92t0.30b
1.5 0.45t0.21 0.32t0.21d 0.51 t0.36b
Water (control) 0.0 1.8 0.20 a 2.7 0.36 b 4.8 0.96 a
5 a-c Means within the same column with common letters are not significantly
(P > 0.05)
different.
'%A t= (Wwet'Wdry)/(Wo We) X 100; Wwet and Wdry are weights of absorptiveness
apparatus
holding the skin before and after drying, respectively; Wo initial weight of
the skin; We weight
of the empty apparatus.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
36
Table 3. Linear regression analysis' of changes in weight of chicken skin
coated with
antimicrobial pea starch (PS+TSP) and calcium alginate (alginate+ASC) coatings
containing
different polymer concentrations with time
Treatment Polymer concentration Absorption rate Y-intercept R2
(% w/v) (g/min) (initial weight g)
PS+TSP 0.5 0.066 2.00 0.94
3.5 0.053 1.74 0.91
4.8 0.013 0.81 0.99
Alginate+ASC 0.5 0.005 0.96 0.86
1.0 0.006 0.56 0.79
1.5 0.001 0.46 0.51
Water (control) 0.0 0.060 1.10 0.98
equation: Y = ax + b; Y is weight of sample; x is time in min.; a is
absorption rate; b is initial
absorption.
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
37
t ~ ~
~ m
+ 0
CD
0
~ CD
0 CD
o ~.
0
~. V1 00 r.. bq
+ 1+ cn
cn o O
O 00 ~
00
OG 01 Q,
RR tA
~ O O (z Cl ~N
n w ~p (jq
cn.
0 0 ~ u
~ o ~e o
co'
~
cn
ON 0 CD
O 0
fD 0~0 ( J tz
0 ^I~ N U~q [=~
a, 00
'-r
`.
c~
N Vi
O N W
r'~ CD
¾,
1+ GO
~, I+
y W N ~ 0
.., ~
v N
o 0
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
38
Table 5 Effects of thyme oil treatments on the numbers (log cfu/g) of
Salmonella Typhimurium
and S. Heidelberg on chicken breast meat at 4 C.
Treatments Day 0 Day 4 Day 8 Day 12
Total aerobes
No treatment 3.1 0.2 4.6 0.1 6.1 0.2 6.6 0:3
Salmonella inoculation 4.7 0.0 5.0 0.1 6.6 0.5 7.1 0.1
Salmonella + Pea starch coating 4.8 0.05 5.2 0.3 7.0 0.4 7.5 0.1
Salmonella + Pea starch coating + 4.0 0.05 a 3.4 0.5 b 5.8 0.3 7.2 0.4
Thyme oil
Lactic acid bacteria
Notreatment 2.6 0.3 3.6 0.1 5.2 0.6 5.4 0.7
Salmonella inoculation 4.7 0.0 4.7 0.2 5.3 0.3 6.1 0.6
Salmonella + Pea starch coating 4.9 0.2 4.8 0.1 5.4 0.2 6.9 0.1
Salmonella + Pea starch coating + 3.9 0.1b 3.0 0.0 2.8 0.7c 5.8 0.4a
Thyme oil
Salmonella
Notreatment 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0
Salmonella inoculation 5.2 0.0 a 4.5 0.1 b 4.3 0.2 b 4.2 0.1
Salmonella + Pea starch coating 5.1 0.1 a 4.2 0.2 b 4.4 0.2 b 3.9 0.1
Salmonella + Pea starch coating + 4.3 0.4 a 2.9 0.2 b 2.0 1.7 b 2.2
0.4'
Thyme oil
Experiments with Salmonella + H20 + thyme oil treatment were not conducted.
Different
superscripts indicate a significant difference of values in rows (t-test, n 6,
p < 0.05).
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
39
Table 6 Effects of thyme oil treatments on the survival (log cfu/g) of
Campylobacter jejuni on
chicken breast meat at 4 C.
Treatments Day 0 Day 4 Day 8 Day 12'
Total aerobes
Notreatment 3.2 0.0 5.0 0.1 7.7 0.1 7.5 0.1
Campylobacter inoculation 3.4 0.2 4.9 0.1 6.9 0.1 7.2 0.3
Campylobacter + Pea starch coating 3.5 0.3 4.9 0.1 7.7 0.0 7.8 0.1
Campylobacter + H20 + Thyme oil ND 3.4 0.4 5.3 0.5 5.3 0.6
Campylobacter + Pea starch coating 2.0 0.0 2.4 0.1 5.0 0.05 5.7 1.0
+ Thyme oil
Lactic acid bacteria
Notreatment 2.4 0.1 4.3 0.0 6.3 0.2 6.9 0.0
Campylobacter inoculation 3.2 0.0 4.4 0.0 6.1 0.2 7.0 0.1
Campylobacter + Pea starch coating 3.4 0.2 4.2 0.1 6.3 0.1 7.5 0.2
Campylobacter + H20 + Thyme oil ND 2.0 0.0 2.3 0.0 4.1 0.5
Campylobacter + Pea starch coating ND ND 2.3 0.0 4.7 0.2
+ Thyme oil
Campylobacter jejuni
Notreatment 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0
Campylobacter inoculation 4.6 0.1 a 4.2 0.1 b 3.8 0.2 3.7 0.1 c
Campylobacter + Pea starch coating 4.2 0.5 a 3.4 0.1 b 4.6 0.3 a 2.5
0.1
Campylobacter + H20 + Thyme oil 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0
Campylobacter + Pea starch coating 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0
+ Thyme oil
Different superscripts indicate a significant difference of values in rows (t-
test, n = 6, p < 0.05).
ND stands for not detectable (< 100 cfu/g).
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
Table 7- Effects of thyme oil treatments on the growth (log cfu/g) of Listeria
monocytogenes on
chicken breast meat at 4 C.
Treatments Day 0 Day 4 Day 8 Day 12 '
Total aerobes
No treatment 3.0 0.6 4.6 0.1 6.8 0.1 7.7 0:0
Listeriainoculation 5.6 0.0 5.2 0.4 6.9 0.2 8.1 0.9
Listeria + Pea starch coating 4.7 0.1 6.1 0.1 7.2 0.1 8.3 0.0
Listeria + H20 + Thyme oil 4.0 0.4a 3.5 O.lb 5.3 0.6 5.1 0.6
Listeria + Pea starch coating + 4.5 0.3 5.1 0.1 5.9 0.8 6.8 0.5
Thyme oil
Lactic acid bacteria
No treatment 2.5 0.5 4.6 0.1 6.7 0.2 .7.6 0.1
Listeria inoculation 5.5 0.1 5.6 0.0 6.9 0.2 7.7 0.2
Listeria + Pea starch coating 4.8 0.1 5.8 0.2 7.0 0.4 7.8 0.1
Listeria + H20 + Thyme oil 3.9 0.4a 3.3 0.1b 3.9 0.6a 5.O O.lb
Listeria + Pea starch coating + 4.5 0.3 4.5 0.5 5.3 0.3 5.5 1.0
Thyme oil
Listeria monocytogenes
No treatment 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0
Listeria inoculation 5.5 0.1 5.5 0.0 5.9 0.1 6.4 0.2
Listeria + Pea starch coating 4.7 0.0 5.9 0.3 6.6 0.3 7.2 0.2
Listeria + H20 + Thyme oil 6.0 0.4a 3.1 0.0d 3.6 0.3c 5.1 0.0b
Listeria + Pea starch coating + 4.3 0.3 4.6 0.5 4.8 0.1 5.1 0.2
Thyme oil
Different superscripts indicate a significant difference of values in rows (t-
test, n 6, p < 0.05).
CA 02656397 2008-12-29
WO 2008/028278 PCT/CA2007/001547
41
Table 8 Effects of thyme oil treatments on the growth (log cfu/g) of
Pseudomonas aeruginosa on
chicken breast meat at 4 C.
Treatments Day 0 Day 4 Day 8 Day 12 '
Total aerobes
Notreatment 3.2 0.1 5.5 0.0 6.9 0.2 7.9 0.2
Pseudomonas inoculation 5.1 0.1 5.6 0.6 7.0 0.3 7.5 0.6
Pseudomonas + Pea starch coating 4.8 0.1 5.5 0.6 6.9 0.1 8.2 0.1
Pseudomonas + H20 + Thyme oil 4.2 0.0 4.6 0.5 4.9 0.6 6.8 0.4
Pseudomonas + Pea starch coati.ng 4.0 0.3 b 3.1 0.7 a 5.1 0.8 5.6
1.1
+ Thyme oil
Lactic acid bacteria
Notreatment 2.4 0.4 4.9 0.3 6.0 0.2 7.3 0.3
Pseudomonas inoculation 5.0 0.1 4.9 0.1 6.3 0.2 6.9 0.3
Pseudomonas + Pea starch coating 4.2 1.2 4.8 0.5 5.9 0.5 7.4 0.0
Pseudomonas + H20 + Thyme oil 4.1 0.1 4.3 0.5 4.6 0.5 5.9 0.5
Pseudomonas + Pea starch coating 3.9 0.3 b 2.7 0.8 a 4.5 0.7 b 5.1 0.9
b,c
+ Thyme oil
Pseudomonas aeruginosa
Notreatment 3.2 0.1 5.0 0.2 7.6 0.1 7.9 0.5
Pseudomonas inoculation 5.1 0.2 5.3 0.3 7.8 0.0 7.7 0.2
Pseudomonas + Pea starch coating 4.8 0.1 5.2 0.3 7.6 0.1 8.4 0.1
Pseudomonas + H20 + Thyme oil 4.1 0.0 4.5 0.5 6.0 0.6 6.8 0.1
Pseudomonas + Pea starch coating 4.0 0.2 b 2.8 0.9 a 5.6 1.9 6.0 1.0 c
+ Thyme oil
Different superscripts indicate a significant difference of values in rows (t-
test, n 6, p < 0.05).