Note: Descriptions are shown in the official language in which they were submitted.
,
, .
. ,
CA 02657072 2009-03-05
WASTE WATER TREATMENT METHOD
BACKGROUND
Water, especially inth
t
ewestertUilited States and other arid regions,
is a valuable resource. Many oil and natural gas production operations
generate, in
addition to the desired hydrocarbon products, large quantities of waste water,
referred to as "produced water". Produced water is typically contaminated with
significant concentrations of chemicals and substances requiring that it be
disposed
of or treated before it can be reused or discharged to the environment.
Produced
water includes natural contaminants that come from the subsurface environment,
such as hydrocarbons from the oil- or gas-bearing strata and inorganic salts.
Produced water may also include man-made contaminants, such as drilling mud,
"frac flow back water" that includes spent fracturing fluids including
polymers and
inorganic cross-linking agents, polymer breaking agents, friction reduction
chemicals, and artificial lubricants. These contaminants are injected into the
wells
as part of the drilling and production processes and recovered as contaminants
in the
produced water.
Commonly encountered non-natural contaminants in produced water,
and their sources, are discussed below.
From high-viscosity fracturing operations¨gellants in the form of
polymers with hydroxyl groups, such as guar gum or modified guar-based
polymers;
cross-linking agents including borate-based cross-linkers; non-emulsifiers;
and
sulfate-based gel breakers in the form of oxidizing agents such as ammonium
persulfate.
From drilling fluid treatments¨acids and caustics such as soda ash, calcium
carbonate, sodium hydroxide and magnesium hydroxide; bactericides; defoamers;
emulsifiers; filtrate reducers; shale control inhibitors; deicers including
methanol
and thinners and dispersants.
From slickwater fracturing operations¨viscosity reducing agents
such as polymers of acrylamide.
Because of the very wide range of contaminant species as well as the
different quality of produced water from different sources, efforts to create
a cost
effective treatment system that can treat or recycle the spectrum of possible
CA 02657072 2009-03-05
produced water streams have little success. For example, while reverse osmosis
is
effective in treating many of the expected contaminants in produced water, it
is not
very effective in removing methanol and it may be fouled by even trace amounts
of
acrylamide.
As another example, there have been many attempts to reclaim
produced water and reuse it as fracturing feed water, commonly referred to as
"frac
water." Frac water is a term that refers to water suitable for use in the
creation of
fracturing (frac) gels which are used in hydraulic fracturing operations. Frac
gels
are created by combining frac water with a polymer, such as guar gum, and in
some
applications a cross-linker, typically borate-based, to form a fluid that gels
upon
hydration of the polymer. Several chemical additives generally will be added
to the
frac gel to form a treatment fluid specifically designed for the anticipated
wellbore,
reservoir and operating conditions.
However, some waste water streams are unsuitable for use as frac
water in that they require excessive amounts of polymer or more to generate
the
high-viscosity frac gel. For example, trace amounts of spent friction reducers
in the
stream inhibit the added polymer from gelling. Because it can be difficult to
prevent
produced water streams from different sources from being co-mingled, this
typically
results in all produced water from a well field being made unsuitable for
recycling as
frac water.
An additional problem occurs when the produced water is also
contaminated with methanol and it is desirable to discharge the water to the
environment. One way to treat produced water to the extent necessary to
discharge
the water to the environment, is through filtration techniques such as ultra
filtration
and reverse osmosis. However, methanol will pass through nearly any available
membrane filtration technology.
Yet another problem occurs when the produced water is also
contaminated with boron, such as from the use of borate-based cross-linking
agents,
and it is desirable to discharge the water to the environment. One way to
treat
produced water with boron is referred to as the HERO process in which the pH
is
raised up to at least about 11 prior to treatment with reverse osmosis,
resulting in the
boron being rejected with the reverse osmosis reject brine. However, raising
the pH
has several undesirable attributes. First, there is increased scaling within
the reverse
osmosis system increasing the maintenance costs of the system. Second, the pH
2
CA 02657072 2009-03-05
must then be reduced before the treated water may be discharged to the
environment. Third, the cost of the chemicals to raise the pH coupled with the
cost
of immediately thereafter lowering the pH and the cost of disposal of the
precipitated salts resulting from the lowering of the pH make the HERO
process
very expensive.
SUMMARY
Systems and methods have been developed for reclaiming water
contaminated with the expected range of contaminants typically associated with
produced water, including water contaminated with slick water, methanol and
boron.
The system includes anaerobically digesting the contaminated water, followed
by
aerating the water to enhance biological digestion. After aeration, the water
is
separated using a flotation operation that effectively removes the spent
friction
reducing agents and allows the treated water to be reclaimed and reused as
fracturing
water, even though it retains levels of contaminants, including boron and
methanol,
that would prevent its discharge to the environment under existing standards.
The
treated water may further be treated by removing the methanol via biological
digestion in a bioreactor, separating a majority of the contaminants from the
water
by reverse osmosis and removing the boron that passes through the reverse
osmosis
system with a boron-removing ion exchange resin.
In part, this disclosure describes a method for generating fracturing
water from produced water. The method includes transferring produced water
contaminated with slick water, methanol and boron into an anaerobic pond and
holding the produced water in the anaerobic pond for at least a first mean
residence
time. The method further includes transferring anaerobic pond effluent to an
aeration pond and aerating the anaerobic pond effluent in the aeration pond
for a
second mean residence time. After aeration, the method includes transferring
aeration pond effluent from the aeration pond to a dissolved air flotation
treatment
system and floating the aeration pond effluent with the dissolved air
flotation
treatment system to generate a floated aqueous effluent and a separated solids
effluent. The method further includes biologically digesting the floated
aqueous
effluent in a bioreactor until a desired concentration of methanol is
obtained. Then,
the bioreactor effluent is transferred from the bioreactor to a reverse
osmosis system
and contaminants are separated from bioreactor effluent with the reverse
osmosis
3
CA 02657072 2016-04-04
system, wherein the reverse osmosis system passes at least some boron in its
permeate. Boron is removed from the reverse osmosis permeate via a boron-
selective removal process to obtain a desired level of boron in the reverse
osmosis
permeate.
In part, this disclosure describes a system for treating water
contaminated with methanol and boron. The system includes: an anaerobic
digestor
that receives the water and holds at least a portion of the water under
anaerobic
conditions; an aerator that aerates the water; a flotation separator that
separates
contaminants from the water to produce a reclaimed water stream suitable for
use as
fracturing water; at least one bioreactor that biologically digests methanol
in the
water until a desired concentration of methanol is obtained; a boron-selective
removal system that removes boron from the water until a desired concentration
of
boron is obtained; and at least one filtration system that removes
contaminants from
the water until a desired concentration of contaminants other than boron and
methanol is obtained.
In part, this disclosure describes a method for removing
contaminants from produced water including boron, methanol and contaminants
that
inhibit the gelling of fracturing fluid. The method includes anaerobically
digesting
the produced water containing the contaminants for a first period of time and
after
anaerobically digesting the produced water, aerating the produced water for a
second
period of time. After aerating the produced water, the produced water is
treated by a
dissolved air flotation system and the effluent of the dissolved air flotation
system is
filtered to generate a filtered water containing concentrations of boron and
methanol,
but that is suitable for use as a fracturing water in that it does not require
excessively
increased amounts of gellant to create the high-viscosity frac gel. The method
further provides for biologically digesting the filtered water, thereby
reducing the
concentration of methanol in the filtered water and separating contaminants
from the
filtered water using reverse osmosis, in which the reverse osmosis passes at
least
some undesirable concentration boron in its permeate. The boron is removed
from
the reverse osmosis permeate via a boron removing ion exchange resin.
In accordance with an aspect of the invention, a method for
generating fracturing water from produced water is provided. The method
comprises:
4
CA 02657072 2016-04-04
transferring produced water contaminated with slick water, methanol
and boron into an anaerobic pond;
holding the produced water in the anaerobic pond for at least a first
mean residence time;
transferring anaerobic pond effluent to an aeration pond;
aerating the anaerobic pond effluent in the aeration pond for a second
mean residence time;
transferring aeration pond effluent from the aeration pond to a
solid/liquid flocculation separator; and
separating the aeration pond effluent with the solid/liquid flocculation
separator to generate an aqueous effluent separated from a solids effluent;
biologically digesting the aqueous effluent in a bioreactor until a
desired concentration of methanol is obtained;
transferring bioreactor effluent from the bioreactor to a reverse
osmosis system;
separating contaminants from bioreactor effluent with the reverse
osmosis system, the reverse osmosis system passing at least some boron in its
permeate; and
removing the boron from the reverse osmosis permeate via a boron-selective
removal process to obtain a desired level of boron in the reverse osmosis
permeate.
In accordance with another aspect of the invention, a method for generating
fracturing water from produced water is provided. The method comprises:
transferring produced water contaminated with slick water, methanol
and boron into an anaerobic pond;
holding the produced water in the anaerobic pond for at least a first
mean residence time;
transferring anaerobic pond effluent to an aeration pond;
aerating the anaerobic pond effluent in the aeration pond for a second
mean residence time;
transferring aeration pond effluent from the aeration pond to a
solid/liquid flocculation clarification treatment system;
floating the aeration pond effluent with the solid/liquid flocculation
clarification
treatment system to generate a floated aqueous effluent and a floc effluent;
4a
CA 02657072 2016-04-04
biologically digesting the floated aqueous effluent in a bioreactor
until a desired concentration of methanol is obtained;
transferring bioreactor effluent from the bioreactor to a first reverse
osmosis system;
separating contaminants from bioreactor effluent with the first reverse
osmosis system, the first reverse osmosis system passing at least some boron
in its
permeate;
transferring reject from the first reverse osmosis system to an
electrocoagulation system;
neutralizing electrostatic charges of contaminants suspended in the
reject of the first reverse osmosis system to remove or destroy some of the
suspended contaminants with the electrocoagulation system to generate an
electrocoagulation effluent containing at least some boron;
transferring at least some solids from the electrocoagulation system to
an injection well;
transferring the electrocoagulation effluent and the first osmosis
system permeate to a second reverse osmosis system;
separating contaminants from the electrocoagulation effluent with the
second reverse osmosis system, the second reverse osmosis system passing at
least
some boron in its permeate;
transferring the second reverse osmosis permeate to the boron-
selective treatment system; and
removing the boron from the first reverse osmosis permeate and the
electrocoagulation effluent via a boron-selective removal process to obtain a
desired level of
boron in the first reverse osmosis permeate and the electrocoagulation
effluent.
In accordance with another aspect of the invention, a method for
generating fracturing water from produced water is provided. The method
comprises:
transferring produced water contaminated with slick water, methanol
and boron into an anaerobic pond;
holding the produced water in the anaerobic pond for at least a first
mean residence time;
transferring anaerobic pond effluent to an aeration pond;
4b
CA 02657072 2016-04-04
aerating the anaerobic pond effluent in the aeration pond for a second
mean residence time;
transferring aeration pond effluent from the aeration pond to a
solid/liquid separator; and
separating the aeration pond effluent with the solid/liquid separator to
generate an aqueous effluent separated from a solids effluent;
biologically digesting the aqueous effluent in a bioreactor until a
desired concentration of methanol is obtained;
transferring bioreactor effluent from the bioreactor to a reverse
osmosis system;
separating contaminants from bioreactor effluent with the reverse osmosis
system, the reverse osmosis system passing at least some boron in its
permeate;
transferring reject from the reverse osmosis system to an
electrocoagulation system;
neutralizing electrostatic charges of contaminants suspended in the
reject of the reverse osmosis system to remove or destroy some of the
suspended
contaminants with the electrocoagulation system to generate an
electrocoagulation
effluent containing at least some boron;
transferring at least some solids from the electrocoagulation system to
an injection well;
transferring the electrocoagulation effluent to a boron-selective
treatment system;
transferring the reverse osmosis permeate to the boron-selective
treatment system; and
removing the boron from the reverse osmosis permeate and the
electrocoagulation effluent via a boron-selective removal process to obtain a
desired
level of boron in the reverse osmosis permeate and the electrocoagulation
effluent.
In accordance with a further aspect, a system for treating water
contaminated with methanol and boron is provided. The system comprises:
an anaerobic digester that receives the water and holds at least a
portion of the water under anaerobic conditions;
an aerator that aerates the water;
4c
CA 02657072 2016-04-04
a solid/liquid flocculation separator that separates contaminants from
the water to produce a reclaimed water stream suitable for use as fracturing
water;
at least one bioreactor that biologically digests methanol in the water
until a desired concentration of methanol is obtained;
a boron-selective removal system that removes boron from the water
until a desired concentration of boron is obtained; and
at least one filtration system that removes contaminants from the water until
a
desired concentration of contaminants other than boron and methanol is
obtained.
In accordance with another aspect of the invention, a method for
removing contaminants from produced water including boron, methanol and
contaminants that inhibit the gelling of fracturing fluid when the produced
water is
used as fracturing water, is provided. The method comprises:
anaerobically digesting the produced water containing the
contaminants for a first period of time;
after anaerobically digesting the produced water, aerating the
produced water for a second period of time;
after aerating the produced water, treating the produced water in a
solid/liquid flocculation separator;
filtering effluent of the solid/liquid flocculation separator to generate
a filtered water containing concentrations of boron and methanol;
biologically digesting the filtered water, thereby reducing the
concentration of methanol in the filtered water;
separating contaminants from the filtered water using reverse
osmosis, the reverse osmosis passing boron in its permeate and reject; and
electrocoagulating the reject, the electrocoagulation effluent passing
boron in its effluent;
removing the boron from the reverse osmosis permeate and the
electrocoagulation effluent via a boron removing ion exchange resin.
These and various other features as well as advantages will be
apparent from a reading of the following detailed description and a review of
the
associated drawings. Additional features are set forth in the description
which
follows, and in part will be apparent from the description, or may be learned
by
4d
CA 02657072 2009-03-05
practice of the described embodiments. The benefits and features will be
realized
and attained by the structure particularly pointed out in the written
description and
claims hereof as well as the appended drawings.
It is to be understood that both the foregoing general description and
the following detailed description are exemplary and explanatory and are
intended to
provide further explanation of the invention as claimed.
BRIEF DESCRIPTION OF THE DRAWINGS
The following drawing figures, which form a part of this application,
are illustrative of embodiments systems and methods described below and are
not
meant to limit the scope of the invention in any manner, which scope shall be
based
on the claims appended hereto.
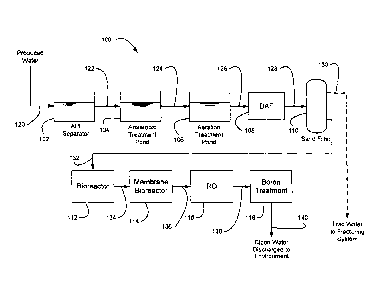
FIG. 1 illustrates an embodiment of a system for treating
contaminated water;
FIG. 2 illustrates an embodiment of a system for treating
contaminated water;
FIG. 3 illustrates an embodiment of a system for treating
contaminated water;
FIG. 4 illustrates an embodiment of a system for treating
contaminated water; and
FIG. 5 illustrates an embodiment of a system for treating
contaminated water.
DETAILED DESCRIPTION
Unless otherwise indicated, all numbers expressing quantities of
ingredients, properties such as molecular weight, concentrations, reaction
conditions, temperatures, and so forth used in the specification and claims
are to be
understood as being modified in all instances by the term "about."
Accordingly,
unless indicated to the contrary, the numerical parameters set forth in the
following
specification and attached claims are approximations that may vary depending
upon
the desired properties sought to be obtained. At the very least, and not as an
attempt
to limit the application of the doctrine of equivalents to the scope of the
claims, each
numerical parameter should at least be construed in the light of the number of
reported significant digits and by applying ordinary rounding techniques.
5
CA 02657072 2009-03-05
The term "residence time" refers to the average length of time that a
fluid or particle spends within a process vessel or in contact with a
catalyst. For the
purposes of this discussion, the mean residence time of a vessel is defined by
dividing the volume of liquid in a vessel (e.g., volume in cubic feet) by the
volumetric flow rate of the liquid (e.g., in cubic feet per second).
The term "floating" as used herein refer to treating a liquid with a
flotation operation to separate solid or liquid particles from a liquid phase.
There are
several types of flotation operations that are well known in the art including
dissolved-air flotation (DAF), air flotation and vacuum flotation.
Fracturing gel or "frac gel" refers to a high-viscosity gel fluid mix
for use in fracturing a subterranean formation. The term "fracturing gel" will
be
used herein to refer to a fluid having a viscosity greater than about 100
centipoise
when injected into the subsurface for the purpose of fracturing the subsurface
formations. The term "Fracturing water," as discussed above, refers to the
water to
which the gellant is added in order to create the fracturing gel. For the
purposes of
this disclosure, however, a water is suitable for use as fracturing water if
it can be
mixed with an economical amount of guar gum, relative to other clean water
supplies, to create a frac gel. That is, a water is not suitable for use as
fracturing
water if it requires significantly more polymer (in order to achieve target
properties
of the frac gel) than other sources of water readily available. Thus, for the
purposes
of this disclosure, a water is considered suitable for use as fracturing water
only if it
can be mixed with an amount of polymer (e.g., guar gum, guar gum derivatives,
or
other commonly applied gelling agent in the fracturing industry, that will
create a
frac gel) to create a frac gel having a stable viscosity greater than about 50
centipoise at the injection temperature, and the amount of gellinwagent
required is
no more than about 10% greater than that amount required to create the same
viscosity using an equivalently salty water, i.e., distilled water mixed with
an
equivalent amount of salt content as the purported fracturing water.
Slick water, on the other hand, refers to a relatively low viscosity
aqueous fluid used also for fracturing a subterranean formation. The term
"slick
water" as used herein further refers to low viscosity (i.e., a viscosity less
than that
used for frac gels) fluid to which friction reduction agents have been added
to
modify the flow characteristics of the fluid. For example, slick water is
often
created by adding a small amount of polymer to water in order to change the
flow
6
CA 02657072 2009-03-05
characteristics of the resulting aqueous mixture. Such friction reduction
agents
include, but are not limited to, polyvinyl polymers, polymethacrylamides,
cellulose
ethers, polysaccharides, lignosulfonates, and ammonium, alkali metal, and
alkaline
earth salts thereof. Specific examples of typical water soluble polymers are
acrylic
acid-acrylamide copolymers, acrylic acid-methacrylamide copolymers,
polyacrylamides, partially hydrolyzed polyacrylamides, partially hydrolyzed
polymethacrylam ides, polyvinyl alcohol, polyvinly acetate,
polyalkyleneoxides,
carboxycelluloses, carboxyalkylhydroxyethyl celluloses, hydroxyethylcellulose,
galactomannans (e.g., guar gum), substituted galactomannans (e.g.,
hydroxypropyl
guar, carboxymethyl hydroxypropyl guar, and carboxymethyl guar),
heteropolysaccharides obtained by the fermentation of starch-derived sugar
(e.g.,
xanthan gum), and ammonium and alkali metal salts thereof. Preferred water-
soluble
polymers include hydroxyethyl cellulose, starch, scleroglucan, galactomannans,
and
substituted galactomannans. For example, copolymers of acrylamides are
disclosed
as good friction reduces in U.S. Pat. No. 3,254,719 and U.S. Pat. No.
4,152,274,
which disclosures are hereby incorporated herein by reference. An example of
an
acrylamide-based friction reducer includes that sold under the product name
FRW-
14 by BJ SERVICES COMPANY. Others are well known in the art.
It should be noted that both fracturing fluids and slick water may
include other compounds such as demulsifiers, corrosion inhibitors, friction
reducers, clay stabilizers, scale inhibitors, biocides, breaker aids, mutual
solvents,
alcohols, surfactants, anti-foam agents, defoamers, viscosity stabilizers,
iron control
agents, diverters, emulsifiers, foamers, oxygen scavengers, pH control agents,
and
buffers, and the like.
When referring to concentrations of contaminants in water or to
water properties such as pH and viscosity, unless otherwise stated the
concentration
refers to the concentration of a sample properly taken and analyzed according
to
standard Environmental Protection Agency (EPA) procedures using the
appropriate
standard test method or, where no approved method is available, commonly
accepted methods may be used. For example, for Oil and Grease the test method
identified as 1664A is an approved method. In the event two or more accepted
methods provide results that indicate two different conditions as described
herein,
the condition should be considered to have been met (e.g., a condition that
must be
7
CA 02657072 2009-03-05
"above pH of about 7.0" and one accepted method results a pH of 6.5 and
another in
pH of 7.2, the water should be considered to be within the definition of
"about 7.0").
FIG. 1 illustrates an embodiment of a system for treating
contaminated water. The contaminated water may be produced water 120 generated
by oil field operations or waste water from some other industrial or
residential
source. The system 100 is illustrated and discussed below as a continuous flow
system. However, in an alternative embodiment some or all of the processes of
the
system 100 may be operated as batch processes.
In the embodiment shown, the contaminated water is produced water
120 generated from oil, gas or other subsurface extraction operations. In an
embodiment, the produced water is contaminated with methanol and boron derived
either from natural sources in the subsurface or added as part of the
extraction
operations.
In an embodiment, the system of FIG. 1 is anticipated to receive
produced water having at least about 7,000 milligrams per liter (mg/I) of
total
dissolved solids (TDS), at least about 10 mg/1 of boron, and at least about
500 mg/1
methanol, although the system could be used to treat less contaminated water
as
well. Furthermore, as discussed in greater detail below, the effluent of the
system
100 is desired to contain less than about 500 mg/I of TDS, less than about 2
mg/1
boron, and less than about 1 mg/1 methanol. Preferably, the system 100 can
accept
any produced water of any quality. In testing, waste water, including produced
water with the following ranges of contaminant as provided in Table 1
concentrations, were treated.
TABLE 1
Parameter Range
TDS @180 C., mg/1 up to at least 8830
TSS @ 105 C., mg/I up to at least 141
Turbidity, NTU up to at least 239
TOC, mg/I up to at least 1130
COD, mg/I up to at least 5750
BOD, mg/1 up to at least 1820
pH up to at least 7.21
Iron, mg/1 up to at least 0.3
Chloride, mg/I up to at least 4310
Potassium, mg/I up to at least 59.2
Calcium, mg/I up to at least 78.5
Magnesium, mg/I up to at least 9.1
8
. .
CA 02657072 2009-03-05
Sodium, mg/1 up to at least 2750
Sulfate, mg/1 up to at least 26
Carbonate, mg/I ND as CO 3
Bicarbonate, mg/I up to at least 459 as HCO 3
Boron, mg/I up to at least 11.6
Methanol, mg/I up to at least 610
In an embodiment, the produced water 120 may also be contaminated
with slick water and thus may contain friction reducers such as acrylam ides.
Such
contaminants are relevant in that they are hard to remove, foul many treatment
operations such as reverse osmosis systems, and inhibit the formation of
fracturing
gels if the contaminant exists in sufficient concentration in fracturing
water.
The system 100 is designed in anticipation that the produced water
120 is likely to contain these contaminants at all times or intermittently.
The system 100 receives the produced water 120 and may
temporarily store it, such as in a holding tank, before beginning active
treatment.
The produced water 120 may be received via truck, pipeline, surface flow or
any
other suitable method. Produced waters 120 from different sources may also be
received and co-mingled immediately or independently treated until the
anaerobic
treatment stage discussed below. As the system is adapted to treat any type of
expected contaminant, this is an advantage over other systems that are
tailored to
specific water qualities from specific wells or sources.
The produced water 120 may be treated with a gravity separator, such
as an API separator as shown, to remove immiscible phases of oil and grease.
Gravity separation is well known and any suitable gravity separation system,
e.g.,
API separator design, gunbarrel separator or gravity clarifier, may be used.
The aqueous separator effluent 122 then is transferred to the
anaerobic treatment system 104 for anaerobic digestion of contaminants. In an
embodiment, an anaerobic pond may be used as the anaerobic treatment system or
as
part of the anaerobic treatment system 104. Anaerobic ponds are known in the
art
and refer to a deep pond that maintains anaerobic conditions at depth, except
for a
shallow (typically less than about 2 feet) surface zone. In an embodiment,
some
oxygen may be added to water contained in the anaerobic pond through spray
evaporation and ambient contact with air, as long as very little dissolved
oxygen is
achieved below 2 feet of depth to ensure that the conditions at depth remain
9
CA 02657072 2009-03-05
anaerobic. In an embodiment, other than mixing incidental to the mixing of the
effluent 112 with the contents of the anaerobic treatment system 104 vessel,
no
additional mixing or aeration is provided by the operators.
The anaerobic treatment system 104 treats the water by anaerobic
conversion of organic wastes into carbon dioxide,. methane, other gaseous end
products, alcohols possibly including methanol, and organic acids. Inorganic
wastes
may also be anaerobically converted. Some separation will occur in the
anaerobic
treatment system 104 due to precipitation of converted contaminants as well as
via
settling. In operation, it was noted that anaerobic digestion served at least
two
beneficial purposes. First, it typically reduced chemical oxygen demand (COD)
by
30% or more and usually by at least 50%. However, it notably did not reduce
biological oxygen demand (BOD) by very much. Second, anaerobic digestion
reduced the ratio of COD to BOD from the initial value (typically around 3:1)
to 2:1
or less.
In an embodiment, the water is treated in the anaerobic treatment
system 104 based on residence time. A mean residence time of at least about 50
days has been found to be effective. Larger mean residence times are also
effective.
In an alternative embodiment, an alternative benchmark or combination of
benchmarks may be used to determine if sufficient treatment has occurred, such
as a
targeted COD reduction relative to the inlet amount (e.g., at least about 15%
reduction, or at least about 30% or at least about 50% reduction criteria) or
threshold
COD to BOD ratio being achieved. A combination benchmark may include a
minimum of 50 days residence time and any other benchmark such as COD
concentration.
Effluent 124 from the anaerobic treatment system 104 is transferred
to an aeration system 106, which may also be referred to as an aerator 106.
The
aeration system 106 actively aerates the water to allow the biological
digestion of
contaminants in the water over time. In an embodiment, the aeration system 106
treats the water for a mean residence time of at least about 5 days with mean
residence times of 5 to 10 days being one treatment target. During treatment,
the
dissolved oxygen of the system is monitored and the aeration is adjusted to
maintain
a dissolved oxygen concentration above at least 50% of the solubility limit of
oxygen in water at the aeration system 106 temperature, preferable above 75%
of the
solubility limit and more preferable above 90% of the solubility limit.
However, the
- -
CA 02657072 2009-03-05
target dissolved oxygen concentration used may be balanced against the cost of
providing the aeration and current throughput needs of the system.
In an embodiment, no supplemental nutrients for bioremediation are
added in the aeration treatment step. The amount of aeration may be controlled
based on measurement of dissolved oxygen of the water in the aeration system
106.
Aeration may also be controlled based on the effectiveness of the flotation
treatment
and water quality of the flotation treatment effluent 128. Submerged
combustion
heaters, or other heat sources, may be used to raise water temperature as
desired,
such as in the winter to prevent water freezing if the aerator 106 is an
outdoor pond.
In addition to biological digestion, it is believed that some oxidation
or other aerobic conversion of some contaminants occurs in the aeration system
106.
In an embodiment, a benchmarks to determine proper aeration may include a
minimum residence time at a specific rate of aeration and temperature, a
reduction
of BOD to below a target threshold (e.g., less than about 1300 mg/1, or more
preferably less than about 1000 mg/1), a reduction of sulfate to below 10 mg/1
sulfate, a reduction of 50-75% of the input concentration of sulfate in the
anaerobic
treatment system effluent 124 , and/or a reduction in barium to less than 1
mg/l.
However, as mentioned above, sufficient aeration is primarily indicated by the
effectiveness of the flotation treatment and water quality of the flotation
treatment
effluent 128.
In the aeration system 106, aerobic digestion of trace metals occurs
helping to clarify these compounds and serves many beneficial functions.
First,
aerobic digestion of trace metals occurs helping to clarify these compounds.
This
was evidenced by analyses of sludge taken with insufficient aeration and
sufficient
aeration showing that insufficient aeration resulted in leachable barium
(determined
by the TCLP analysis) being found in the sludge whereas, under conditions of
proper aeration, leachable barium was reduced below the detection limit.
Experimental data suggest that the aeration step does reduce the COD
and BOD of the water being treated, but, without being bound to any particular
theory, the aeration step also appears to cause a change in the nature of the
COD
which increases the effectiveness of the flotation system 108 in removing
contaminants. This was evidenced by experiments in which insufficiently
aerated
effluent from the aeration system 106 was transmitted to the flotation system
108
and it was found that the flotation system's ability to coagulate and separate
11
_
CA 02657072 2009-03-05
contaminants was drastically reduced. Notably, another effect of insufficient
aeration observed during testing was that the resulting COD that was passed by
the
DAF 108 fouled the bioreactor 112. Proper aeration eliminated this fouling.
Without being limited to a particular theory, it is believed that the COD in
produced
water contaminated with frac flow back water is at least in part due to long
chain
acrylamide polymers, fragments of frac gel and other stimulation chemicals,
that can
be floated out in the DAF, but only after conversion by the digestion
operations 104,
106.
In an embodiment, an aeration pond is used as the aeration system
106. Aeration ponds are known in the art. An aeration pond is typically a
large,
shallow earthen basin provided with some means for actively aerating the water
contained in the pond. Types of active aeration using air include sprayers
that spray
the water into the air and forced air injection via diffusers submerged in the
pond
attached to floating aerators. Many other aeration means are known in the art;
any
suitable means for aerating the water may be used.
Aeration system effluent 126 is transferred, with heat as needed for
proper operation, to a flotation separator 108. The flotation separator 108
separates
solid particles from the aqueous phase by introducing fine gas bubbles into
the
aqueous phase. The bubbles attach to the particulate matter and the buoyant
force of
the combination is great enough to cause the particle to rise to the surface
and
subsequently be skimmed off or otherwise mechanically separated from the
aqueous
phase.
Flotation separators 108 are well known in the art. In experiments, a
dissolved air flotation (DAF) separator was used to float and separate
particulates
from the aqueous phase; however, there is no reason to believe that other
flotation
separators, such as air flotation or vacuum flotation systems, may not also be
effective. In embodiments that utilize a DAF separator, any suitable DAF
design,
now known or later developed may be utilized. For example, a three vessel DAF
in
which coagulant is added in the first vessel, the flocculent is added in the
second
vessel and the third vessel is the actual flotation chamber in which air is
added and
separation occurs.
Furthermore, any DAF additives may be used as determined to be
experimentally suitable in increasing the effectiveness of the DAF separator
in
removing contaminants. Commercially available coagulants were used to assist
the
12
CA 02657072 2009-03-05
coagulation and increase the performance of the DAF. In an embodiment, Ashland
Chargepac 55 with a dose rate between 100 and 200 ppm was used as the
coagulant
and flocculent polymer was mixed from Ciba Magnafloc 336 and then diluted to a
final dose rate of 2 to 7.5 ppm. Preferably, the DAF separator is operated
above 35
degrees F. and more preferably at about 55 degrees F. In an embodiment, the
DAF
separator is operated as necessary to obtain an effluent 128 with an NTU level
of
less than about 10 NTU.
In the embodiment shown, the aqueous effluent 128 of the flotation
separator 108 is further clarified by passing the effluent .128 through a
filtration
system 110. Additionally, the effluent 128 may be monitored, such as via a
turbidity
meter, conductivity sensor or other monitoring device. If the observed level
does
not meet the desired level of treatment, the effluent 128 may be recycled to
an
earlier treatment operation. Furthermore, at any point after the aerobic
digestion, a
biocide may be introduced to eliminate microbes and promote removal of same,
such as in the DAF separator 108 or the filtration system 110 or prior to
shipment to
a frac system.
In the embodiment shown, a sand filter, nominally effective as a 10
micron filter, was used as the filtration system 110 to achieve a turbidity of
less than
about 5 NTU and preferably less than about 1 NTU. Other filtration designs may
also be used. Effluent 128 from the DAF separator 108 may be feed via gravity
through the filters 110 to a lift station that transfers water to one or more
intermediate surge tanks. In order to achieve the desired level of treatment,
one or
more separate filters may be utilized in series or in parallel. In an
embodiment, each
sand filter may be equipped with a sight glass to show the operator how much
head
is developing in the filter and also with an inline turbidity meter to
directly measure
filter performance. When-the feed water level in the filter reaches the high
tank
level switch a backwash cycle may be initiated by a programmable logic
controller ,
(PLC) that monitors operation of the filters or the system as whole. The back
wash
cycle may also be triggered manually or based on the readings of the turbidity
meter.
Back wash water and overflow from the sand filter inlet may be recycled to any
prior treatment operation as desired by the operator.
The effluent 130, 132 of the filtration system 110 is suitable for use
as a fracturing water even though in experiments it still contained
significant
concentrations of COD, total organic carbon (TOC), TDS, and biological oxygen
13
- õ
CA 02657072 2009-03-05
demand (BUD). Its use as a fracturing water was evidenced by the ability to
gel
sufficiently when combined with polymers to create a high-viscosity fracturing
gel.
Without being bound to a particular theory, it is believed that trace amounts
of the
friction reducers from slick water impair the gelling reaction. These friction
reducers are also very difficult to remove using either anaerobic or aerobic
treatment
alone and also difficult to remove without the use of flotation. Indeed, it is
believed
the combination of anaerobic, aerobic and flotation treatment operations is
the most
effective way of reclaiming produced water that is unsuitable for use as
fracturing
water and convert it into water that is suitable for use as a fracturing
water.
Typical and target values of contaminant concentrations for
fracturing water 130, 132 obtained from the system 100 are provided below in
Table
2.
TABLE 2
Parameter Range Target
TDS @ 180 C., mg/1 9,000-16,000 <10,000
TSS @ 105 C., mg/I 0-100 <75
Turbidity, NTU 0-5 <1
TOC, mg/1 400-800 <700
COD, mg/1 1000-3000 <2000
BOD, mg/1 500-1500 <1000
pH 6.5-8 7-7.5
Iron, mg/1 1-10 '<5
Chloride, mg/1 5,000-10,000 <6,000
Potassium, mg/I 100-500 <300
Calcium, mg/I 50-250 <150
Magnesium, mg/I 10-100 <25
Sodium, mg/1 2000-5000 <3000
Sulfate, mg/1 40-200 <50
Carbonate, mg/1 0-100 <25
Bicarbonate, mg/1 100-1200 <800
Boron, mg/ 0-20 <15
In the embodiment shown in FIG. 1, in addition to generating water
suitable for reuse as fracturing water, additional treatment operations are
provided
that treat the produced water to a quality sufficiently clean for discharge to
the
environment. Thus, depending on the need for frac water, the system 100 may be
operated so that more or less frac water 130 is produced from the produced
water
120 stream. Any surplus of unused frac water 130 may then be treated by the
14
CA 02657072 2009-03-05
remaining portions of the system 100 to a water quality that allows the water
to be
discharged to the environment.
Treatment of the frac water 130, 132 to a quality suitable for discharge to
the
environment requires that the system 100 address methanol and boron=
concentrations. Methanol is often a contaminant in produced water. In
addition,
anaerobic digestion may produce methanol from the digestion of guar gels.
Testing
has shown that in the system shown in FIG. 1 while some methanol reduction
(e.g.,
at the top of the pond) may occur under certain conditions during the
anaerobic
treatment operation, methanol may be treated significantly during the aeration
treatment. However, the aeration system 106 as described can not be depended
upon
to sufficiently treat all of the methanol in the produced water. This
variability may
be due to lack of nutrients, composition of the particular inlet produced
water being
treated or the ambient weather conditions under which the aeration treatment
is
being operated.
In the embodiment shown, the system 100 further provides for the
effluent 132 of the filtration operation to be transferred to one or more
bioreactors
112, 114 for the biological digestion of the effluent 132. Biological
digestion of the
effluent 132 drastically reduces the concentration of the methanol in the
water. In an
embodiment, the biological digestion of the effluent 132 is performed for a
duration
sufficient to reduce the methanol to below the target discharge limit or
alternatively
to a level at which the methanol can no longer be detected.
In the embodiment shown, two stages of biological digestion are
performed. First, a bioreactor 112 may be used to perform the majority of the
biological digestion. In an embodiment, the bioreactor may be an enclosed
vessel,
such as a steel tank with internal epoxy coating and standard tank roof with
appropriate vents. Coarse bubble diffusers may be mounted on the bottom of the
tank with air supplied by compressors. The bioreactor 112 may or may not be
heated as needed to maintain a healthy biological environment for digestion.
Additionally, nutrients may be added, such as gaseous ammonia for nitrogen and
phosphoric acid for phosphorous, as necessary. In an embodiment, a residence
time
may be chosen so that methanol is completely eliminated or reduced to a
desired
concentration in the bioreactor 112. The design and operation of bioreactors
are
well known in the art and any suitable design may be utilized as part of this
operation.
= -
CA 02657072 2009-03-05
In the embodiment shown, a second, and optional, stage of combined
biological digestion and filtration is provided in which the effluent 134 of
the
bioreactor 112 is transferred to a membrane bioreactor (MBR) 114 as shown. The
MBR 114 provides additional biological digestion as well as removing by
filtration
some contaminants contributing to TOC concentrations in the water 134. Cleaned
water (permeate 136) is extracted through the membranes of the MBR 134. In an
embodiment, reject from the MBR 114 may be returned to the bioreactor 112 for
additional digestion or to any other prior treatment stage. Any suitable
membrane
bioreactor design may be utilized, for example a hollow fiber membrane
bioreactor
such as that sold by ZENON under the trademark ZEE WEED is suitable for use as
the MBR 114.
Permeate 136 of the MBR 114 is transferred to an RO system. In the
embodiment shown, a reverse osmosis (RO) system 116 is used to filter the
remaining TOC, TDS and other contaminants from the permeate 136 to a level
acceptable for discharge, except boron. RO systems 116 are well known in the
art
and any design, now known or later developed, may be utilized.
Notably, where the pH of the water is not raised, such as for the
purposes of precipitating out contaminants, in the prior operations such as is
necessary in the HERO process. In an embodiment, there may be some minor
reduction of pH in order to maintain the proper conditions within the
bioreactor.
This, however, does not cause the precipitation of any contaminants, but
rather
increases the solubility of some contaminants. The pH of the RO permeate 138
will
be dictated primarily by the pH of the produced water 120. Thus, the pH of the
RO
permeate 138 will generally be much lower than the permeate of the RO in a
HERO process. Preferably the RO permeate 138 in the system 100 will be less
than about 10.0, still yet less than about 9.0 and even more advantageously
less than
about 8.0 and greater than about 6.5.
By avoiding lime softening, the production of waste solids by the
system 100 is significantly lower in comparison. Other than solids derived
from the
original contaminants in the produced water feed, the major source of solids
generated as a result of the treatment operations is due the use of liquid
coagulant in
the DAF. This represents a significant cost savings over systems and processes
that
actively adjust the pH through chemical addition as part of the treatment.
16
CA 02657072 2009-03-05
However, because of the pH range at which the RO 116 is operated
as described above, boron will not be removed from ,the water by the RO system
116
in quantities sufficient to meet the desired discharge concentration. In
experimental
analyses, MBR effluent 136 contained roughly the same concentration of boron
as
the produced water 120. The RO system 116 is expected to pass a significant
portion of the boron in the stream¨a portion that is expected to be beyond the
limits
necessary to discharge the boron to the environment.
In the system 100 of FIG. 1, boron is removed from the RO permeate
138 by means of a boron-selective treatment system 118. In an embodiment, the
boron selective treatment system 118 is an ion-exchange resin adapted to
optimally
remove boron from an otherwise relatively clean aqueous stream. One example of
such a resin suitable for use in the systems described herein is that offered
by Dow
Chemical under the trade name of XUS-43594.00, now alternately referred to
under
the trade name BSR1, which is marketed as a uniform particle size weak base
anion
exchange resin for selective boron removal. Other boron-selected resins known
in
the art include the product MK-51 sold by SYBRON and S-108 sold by PUROLITE.
Other systems that are effective for removing boron may also be used, whether
now
known or later developed. In fact, because the RO permeate 138 is
substantially
clean except for the boron, any effective boron removal system may be used
without
worry of fouling or degradation due to other contaminants.
Effluent 140 of the boron-selective treatment system 118 will be of
sufficient quality to be discharged to the environment. Exemplified target
values of
contaminant concentrations for effluent 140 from an embodiment of the system
100
are provided below in Table 3. If, upon testing, the values are outside of the
target
ranges, the effluent 140 may be recycled to one of the treatment operations
until the
effluent 140 quality meets the discharge requirements.
TABLE 3
Parameter Range
TDS <500 mg/1
TOC <5 mg/I
Boron 1-2 mg/1
pH 6.5-9.0
Oil & Grease <10 mg/I
Radium 226 <60 mg/I
Chlorides <230 mg/I
17
CA 02657072 2009-03-05
Various waste streams other than the primary aqueous streams
discussed may be disposed in any suitable manner. For example, reject from the
RO
system 116 may be used as backwash for prior treatment systems, shipped to the
oilfield for use as frac water, returned to the treatment flow for
reprocessing and
further concentration or disposal via injection well. As a further example, in
embodiments using an ion-exchange resin for boron removal, the boron-laden
regenerate from the ion-exchange regeneration may be blended with RO reject
fluid
to neutralize the regenerate and injected in the disposal well.
In an embodiment, some or all of the operations of the treatment
system may be automated using process controllers, automated transfer pumps,
flow
control valves, sensors and other equipment as is known in the art.
The fracturing water 130 output of the system 100 may be stored in
holding tanks prior to transfer to a fracturing gel production system via
pipeline or
truck to a wellhead or other location where fracturing chemicals are added to
generate fracturing gel. Similarly, the boron-selective treatment system
effluent
may be discharged to a holding tank for confirmation testing prior to
discharge.
In another embodiment, the flotation separator 108 or DAF may be
replaced with a different type of solid/liquid separator 109 as illustrated in
FIG. 3.
In this embodiment, the aeration system effluent 126 may be transferred, with
heat
as needed for proper operation, to the separator 109. The separator 109
separates
solid particles from the aqueous phase. Depending on the chemical makeup of
the
aeration system effluent 126 being treated any of the typical separation
processes
may be used, including solids thickening, gravity separation, filtration, and
centrifugation.
In one embodiment, the solid/liquid separator 109 allows solid
particles to settle at the bottom of a tank where the solid particles are
removed. Any
suitable solid/liquid separation device and/or process may be utilized, such
as a
clarifier or a primary sedimentation tank. A clarifier separates solid
particles from
an aqueous phase by flocculation. Flocculation refers to the process by which
fine
particulates are caused to clump together into floc. The floc may then float
to the
top of the liquid, settle to the bottom of the liquid, or can be readily
filtered from the
liquid for separation.
18
CA 02657072 2009-03-05
Solid/liquid separators 109 are well known in the art. In experiments,
a DAF separator was used to separate particulates from the aqueous phase;
however,
depending on the quality of the aeration system effluent 126 the resulting
floc
sometimes was lighter than water and was removed from the top of the separator
109 and sometimes was heavier than water and was removed from the bottom of
the
separator 109. There is no reason to believe that other separator designs,
such as an
inclined plate clarifier, circular center feed clarifier or sedimentation tank
separator,
may not also be effective. In embodiments that utilize a separator, any
suitable
separator design, now known or later developed, may be utilized.
Furthermore, any additives may be used as determined to be
experimentally suitable in increasing the effectiveness of the eparator 109
in
removing contaminants. Commercially available coagulants may be utilized to
assist the coagulation and increase the performance of the solid/liquid
separator 109.
In an embodiment, the separator 109 is operated as necessary to obtain an
effluent
129 with an NTU level of less than about 10 NTU.
In the embodiment shown, the aqueous effluent 129 of the separator
109 is further clarified by passing the effluent 129 through a filtration
system 110.
Additionally, the effluent 129 may be monitored, such as via a turbidity
meter,
conductivity sensor or other monitoring device. If the observed level does not
meet
the desired level of treatment, the effluent 129 may be recycled to an earlier
treatment operation.
In one embodiment, the solids 127 from the solid/liquid separator 109
or the solids 125 from the flotation separator 108 are recycled back to the
anaerobic
treatment pond 104 as illustrated in FIGS. 2 through 5. Furthermore, at any
point
after the aerobic digestion, a biocide may be introduced to eliminate microbes
and
promote removal of same, such as in the solid/liquid separator 109 or the
filtration
system 110 or prior to shipment to a frac system. In another embodiment, the
solids
133 of the bioreactor 112 are recycled back to the anaerobic treatment pond
104 as
illustrated in FIGS. 2 through 5. In a further embodiment, the solids 135 from
the
MBR 114 are recycled back to the anaerobic treatment pond 104 as illustrated
in
FIGS. 2 through 5.
FIGS. 4 and 5 illustrate embodiments of water treatment systems
utilizing an electrocoagulation (EC) system in conjunction with the RO system
in
order to further decrease the amount of system concentrate that must be
disposed via
19
CA 02657072 2009-03-05
the injection well. FIGS. 4 and 5 differ in how in the EC system 115 is
integrated
with the RO system116. In FIG. 4, the EC system 115 treats all of the RO
reject
137, while in FIG. 5, the EC system 115 treats only a portion of the RO reject
from a
first RO system 116a which is then returned to the second RO system 116b for
further treatment.
In FIGS. 4 and 5, an injection well 117 is used to dispose of the
system waste system that cannot be recycled. In order to reduce the volume of
system waste being sent to the injection well 117, the electrocoagulation (EC)
system 115 is used to further concentrate the RO effluent 137. The EC system
115
is designed to remove metals, colloidal solids, particles, and soluble
inorganic
pollutants from an aqueous media by introducing highly charged polymeric metal
hydroxide species. These species neutralize the electrostatic charges on
suspended
solids and oil droplets to facilitate agglomeration or coagulation and
resultant
= separation from the aqueous phase. The treatment prompts the
precipitation of
certain metals and salts.
In one embodiment, the EC system 115 is made up of an electrolytic =
cell with at least one anode and cathode. When connected to an external power
source, the anode material will electrochemically corrode due to oxidation,
while the
cathode will be subjected to passivation. During electrolysis, the positive
side
undergoes anodic reactions, while on the negative side, cathodic reactions are
encountered. Consumable metal plates, such as iron or aluminum, may be
utilized
as sacrificial electrodes to continuously produce ions in the water. The
released ions
neutralize the charges of the particles and thereby initiate coagulation. The
released
ions remove undesirable contaminants either by chemical reaction and
precipitation,
or by causing the colloidal materials to coalesce, which can then be removed
by
flotation. In addition, as water containing colloidal particulates, oils, or
other
contaminants moves through the applied electric field, there may be
ionization,
electrolysis, hydrolysis, and free-radical formation which can alter the
physical and
chemical properties of water and contaminants. As a result, the reactive and
excited
state causes contaminants to be released from the water and destroyed or made
less
soluble. EC is a process known in the art and any suitable EC system or
technology,
now known or later developed, may be employed herein without departing from
the
scope of this disclosure.
_
CA 02657072 2009-03-05
The EC system 115 produces a treated EC effluent 139 and an EC
waste stream 141. In the embodiment illustrated in FIG. 4, the EC waste stream
141
is disposed via the injection well 117. Alternatively (not shown), the EC
waste
stream 141 may be recycled to one of the prior stages such as the anaerobic
treatment pond 104 or further concentrated using thermal processes (e.g.,
evaporation).
FIG. 5 illustrates an alternative embodiment in which the EC system
115 is used to process the first RU system 116a reject 137 so that the water
can then
be reprocessed through an additional RU system 116b at higher pressures
without
fouling. The permeate 150 from the second RU system 116b can be sent to the
boron removal as shown or, alternatively, directly used for industrial water
in
applications do not require a low concentration of boron. In an embodiment,
the
reject 150 from those additional RU steps is approaching the pressure limit of
RO.
In this embodiment, the resulting solids 141 separated from the
aqueous solution of the EC system 115 are fed into the injection well 117
and/or
recycled back into the anaerobic treatment pond 104.
Those skilled in the art will recognize that the methods and systems
of the present disclosure may be implemented in many manners and as such are
not
to be limited by the foregoing exemplary embodiments and examples. In other
words, functional elements being performed by a single or multiple components,
in
various combinations. In this regard, any number of the features of the
different
embodiments described herein may be combined into single or multiple
embodiments, and alternate embodiments having fewer than or more than all of
the
features herein described are possible.
While various embodiments have been described for purposes of this
disclosure, various changes and modifications may be made which are well
within
the scope of the present invention. For example, between one or more of the
treatment operations described herein, transfer pumps, surge tanks, control
valves,
heaters, and other equipment may be provided to assist the efficient operation
and
maintenance of the system and to provide for various contingencies such as
surges,
cleaning operations, recycling of flow, bypassing of operations, and low or
high
ambient temperatures. As a specific example, water being transferred between
any
two operations may be analyzed and recycled to a previous stage if certain
contaminant concentrations are out of a predetermined desired range.
Additionally,
21
CA 02657072 2016-04-04
if the system is operated as a continuous flow system, surge tanks and
overflow
capacity may be provided at different points within the system to allow for
the
system throughput to be managed as necessary to obtain the proper water
quality at
each stage of treatment.
The scope of the claims should not be limited by the preferred
embodiments set forth in the examples, but should be given the broadest
interpretation consistent with the description as a whole.
22