Note: Descriptions are shown in the official language in which they were submitted.
03/25/09 _. 44ED 15:59 FAY 416 362 0823 RIDOUT & MAYBEE Z004
CA 02657312 2008-10-28
- 1 -
5PECIr1 CATiON
NOVEL COMPOUND HAVING [1FFINITY TO AMYLOCD
TECHNICAI. FIE:I..ll
[OO01.]
Thc present iriventa.on relatcs to a eompound for use
in di.agnosis of cerebral degenerative di..<,ease. Moz=e
specifical..ly, the inventiori re_l.aLes to a c;ompound usefu]
for amyloid detcc.tion at lesion sites in diagnosis of
Alzheimer's disease and other diseases with amyloid
accumulation.
BACKGROIJND AR'P
[0002]
1 Di.sease5 with the onset of deposition of a.f..ibrous
proLein called am.yloid in vaxiou.s organs or t_issucz~s in
bociies are generally rcf..erred to as amyloi.dosis, A
feature c.onunon to amyloidos:is is that Lhe librous protein
called amyl.oici which is enriched with the D-sheet
stru.ct_urc is dcpositPd at vsrious.o.r_gans systcmically o.r,
at sitcs topi.c:ally so that furictional abnor.maliti.es are
triggercd in the o.rgans or tissues.
[0003]
Alzhcimer's disease (herci,nafter rcferrcd to as AD),
which is a typical amyloid.osis disea:3e, i_s known as a
disPaSe c,ausing dementia. This disease is lethal with
progressive deposiLion of amyloid in brain, and thus is
said Lo be a disease thar. causes concern in society
03i25i09 ~9ED 16:00 FAX 416 362 0823 RIDOLiT & MAYBEE
CA 02657312 2008-10-28 005
- 2 -
compa.r.ed with other am.yloidosis diseases. In recent
years, the number o.f,. AD patie!nts is rapidly incrcasing in
deve.l,oped countries w,iLh agirig societies, thereby causi_ng
a social proble.rn.
[0oU9]
From the pat_hohistol.ogical viewpoint, AD is
char_acterizec3 by thrce pat.hological findings in brain,
namely development of scnilc plaques, forma-Cion of
neiirofi.brilJ.ary tangles, and extensive ncuronal loss.
The senile plaque has a struc.turc composed rnainl.y of
amyloid, and is said to appear at the carliest stage of
AD onset ancl thus is pathologically found in brain about
1.0 or more years before appe.arancye of clinical symptoms.
[00051
AD is diagnosed by carrying out various evaluations
of cognitive Functions (for example, I-Idsegawa scale,
ADAS-JCog and MMSE) in auxiliary combinai_ion with imaging
diaqnosis such as CT and MR.r. 1-lowevc~r, the methor,i bascd
on such evaluations o:E coqn.itive funcrtion.s is low in
diagnostic serisitivity at the early stage of the onset,
an.d is furthermore problematic in that diagnostic resuJt:s
are susceptib]..e to inbor_n cognitive functio.ns of
i.ridiv;.dual s. At present, it is practically impossiblc Lo
cdtabJ.ish a defLnitc diagiicsis of AD while an AD pat.ient
i5 sti.ll alive, because the dctinite diagnosis requires a
biopsy of a lesi,Qn (Noii-Fat.ent Document 1).
[000~]
Meanwhile, a report tells that amyloid consLitutinq
03/25/09 FVED 16:00 FAX 416 362 0823 RII10LiT & MAYBEE
Z006
CA 02657312 2008-10-28
- 3 -
seni] e plaques is an aggrcgat.c of arnyloid , pro-t_ein
(hereinafter referred to as AJ3) . Also, numerous reports
tell that the A~ aggregat:e forms aP-sheet structuze that
causc , ncrve ce11 toxicity. Basec~ on. Lhcse findings, the
so-called "Alnyloid Cascade Hypothcsis" is proposed, which
suggests that cerebral deposition of A(3 f:rigger.s the
downstream phen.oinena, namc:ly, formation c:C'
netlrofibrillary I-:anglea and neu,r.onal losJ (Non-Patcnt
C)ocumc;iit 2) 10 [000'7]
Tiased on these facts, at.tcmpts have recently been
made to detect AD in vivo u.!~ing a compound having nigh
affinity with amyloid Lis a mark~.r.
Many of 5uch probes for imaging diagnoses of
cerc.b:ral arnyloid arc hydrophobic low-mo1ccular compounds
t.hat. are high in atfinity witti amyloid and high in
cercbral t.ransf.erabi..lity anca are labeled with various
r..adioac_L-ive species such as 11C, 1"F and t"I. For example,
rcports tel]. 1C or rad;_oact.;.ve halogel; labeled forms ot"
compounds including various thioflavin deri,vdtives suc.h
as 6-iodo-2-[4'--(N,N-dimet.hylz7iino)r>heny,l.]benzothi.a.zolc
(hcreinafter referred to as '1'ZDM) and 6-hyd.r.oxy-2-[4' =_(N-
Fnethylamino)pheriyl]benzothiazole (hereinaftcr_ referred to
as 6-UH-BTA--1) (Pat.cnt. Document 1, Non-Patent Document
3); st.ilbene compounds such as (E)-4-methylamino-4'=-
hydroxy.5tilbene (herei.nafte.r referred L-o as SB-13) and
(r} -~1-dimet),ylami.rio-4' iodostilbene (here-inafter referred
to as m-l-SB) (Par.ent. nocument 2, Non-Patent nocument 4,
03/25i09 WED 1_6:00 FAX 416 362 0823 RII)OUT & MAYBEE 1~j007
.__
CA 02657312 2008-10-28
- / _
Nori-Patent Document 5); benzoxazoli derivatives :,uch as
6-iodo-2- [ 4' - (N,N-di.methy~.amino) phcrlyl] benzoxazole
(hereinafter referred -tlo a;; IBOX) and 6-[2-
(fiuoro) cthoxy] -2-[2- (2-dimetl-i.ylaminot.hi.azo1 M5-
yI)ethenyl]benzoxazolr (Non-Patc~-nt Document 6, Non-(?atent
Document 7), DDNP clerivativef, such c~s 2-(1-{6=-[(2-
i"luo.roethyl ) (me Lhy7 ) amin.o 1 --2-
napht.hyl}ethyli.dene)m,3lononitrile (hereinafter referred
to as FDDNP) (Pa.t:ent Documcnt 4, Non-Patent DacumFnt 8) ;
1C and imidazopyridine deri.vati.ves such as 6-iodo-2- [4' -
(N,N-dimethylamino)phenyl]imidazo[1,2-a}pyrid.ine
(hereinafter referred to as IMPY) (Patent uocumcrit 3,
Non-k'a'tent DocumPnt 9). Further, some of these probes
for imaging diagnosis have been studied on human im3ging
and have been repor-ted to show a significant ac:CumulaLlon
in AD patient's brain compared with normal persons (Non-
Patcnt DoCUment 10, Nori-Pat;:n't Dooument 11).
[00O8]
[Patcrit Document 1] JY-=T-2004-506723
[Patcnt Document 2] JP-T-2005-504055
[PaLent Document 31 JP-T-2005-512945
[Patent DUcument 4] JP-T-2002-523383
[Non-Natent. Document 1.] J. A. Hardy & G. A. Higgins,
"A'_zhei_mer's Disca.ce: The Amyloid Cascade Hypothesis.",
Scierice, 1992, 256, p.184-185
[Non-k?aten.t Document. 2] G. McKhann et al., "Cli.nical
diagnosis of Alzheimer's d_-sease: Report of the NINCDS-
ADRDA Work Group under thc auspices of Department of
03/25/09 WED 16:01 FAX 416 362 0823 RIllOUT & MAYBEE
. 008
CA 02657312 2008-10-28
Heal-Lh and IIunian Services Task Force on Al.zhei,rner'
DiseaSe.", Neu.r.ology, 1984, 34, p.939-944
[Non-?aten.t. Document 3] Z.-P. Zhuang et al.,
"Radioa-ociinaLed St.yrylbenzcnes and Thi-flflavins as Probes
for Amyloid Aggregates.", J_ Med. Chem., 2001, 44,
p.1905-1,914
[Non-Paten-t: Docutrent 4] Masahiro Ono et; al., "11C-labt_].ed
stilbene der.i.vatives as AR-aqgregate-spccific PET i..rnagin.g
agents for Alzheimer's disease.", Nuclear MF;dicine and.
Biology, 2003, 30, p.565-577
[Non-Patent Document 51 H. F. Kung c_'t a1., "Novel
Sti.lbenc.s as Probes for arnyl.oid plaqu.es.", J. American
Chemical aociety, 2001, 123, p.12740-12741
[Non-Pa.tent Doc.ume.n.t 6] Vi.i-Ping Zhuang et al., "IBOX(2-
(4'-dimethylaminophenyJ.)-6- i.ndobensoxazole): a ligand
f.:<jr imayinq amyloi.d plaques in the brain.", Nuclear
Mcclicinn and P~iology, 2003.., 2~3, p.887-894
[Nori-Patent Dor._ument 7] Fur-wmoto Y et al.,"[11C] Br-227 :
A Ne.w )~C-Labeled 2-Fathenylbenzoxaz.ole Derivative for
Amyloid-P Plaques l:rnaging.", Buropcari Journal of Nuclear
Medic:i ne azid MoJ,ecular Imaging, 2005, 32, Su.p. 1, P7S9
[NUn-Patent Document 8] E-ric D, Aqdeppa et al., "2-
Dialkylamino-6-=Acylm,~ilononitri.l.c Sub5j:iLutCd NaphLhalenes
(DDN2 Analogs) : Novel Diagnostic and Therapeutic Too.l;, in
hiz.hc.imer's Disease.", Molccular. Irnaqi-ng and Biology,
2003, 5, p.40!1-417
[Nan-Pat:ent Document 9] Zhi-Ping Zhuanq e't a.l..,
"Structure-Activity Relationship of lrnidazo ['1,-~R-
03/25/09 WED 16:01 FAX 416 362 0823 RII)OUT & MAYBEE
z009
CA 02657312 2008-10-28
- 6 -
alpyridines as Ligands for Deetecting (3-Arryloi.d Pl.aqucs in
the }3rain.", J. Med. Chem, 2003, 46, p.237-243
[Non-Patent Docurnent. 10] W. E. K1unk et al., "Imaging
bra.icr amyloid. in Alzheurner' s discase w~,th Pittsburgh
Compound.-B.", 1~nn. Neurol., 2004, 55, p.306-319
[Non-Patent Docurnent 11j Nicolaa P. L. G. Verhoeff et
al., "In-Vivo Tmaging of Alzhei_mer Di.seasc ~-Amyloid With
[11C]SB-13 PET.", Ame.r.ican Journal of Geriatric
Psychiat;ry, 2.004, 1.2, p.584-595
DISCL.USURI: OF THE INVI~;NTION
PROBLEMS TO DN:. SOLVED BY '1'HE INVENTION
~0009J
ns described above, var:i.ous compounds are disclosed.
as probes for im.aginq diagnosis for amyl.oid, aiid
researched for cli.nical application.
Lxperiments in no .r.rnal mice show that [l'sa:J -labeled
T%DM, T.SOX anci m-I-SE are all tran.sferred. into brain 2
minut_es after adrninistration. However, t-hese compounds
arc insufficient in clearance from normal ti.5sues, and
tend, to ac-cumulate g.r.aciually in brain as time passes
after adrninistration (JY=-T-2005-512945; ihi-Pa,rig Zhuang
et al., Nuclear Medicine anci Biology, 2001, 28, p.887-
894; H. F. Kung ct al., J. Am. Chem, Soc., 2001, 7.23,
p.1.2'740-12741) . Pdhen the clearance from normal t:_ssues
is insufficient, a problcm arises in that sufficient
contrast cannot be obtaine:d at. .3rnyloi.d accumula-tion sites.
ii
[ C] -=1abc:~led St3-13 shows ri clcasancc from normal tissues
iri experiments i_n rats, however, it cannot be said that
03/25/09 {NED 16:01 FAX 416 362 0823 RIDOLiT & MAYBEE 010
_
CA 02657312 2008-10-28
'7
the clearance is suf.f.icie.ntl.y fast (Masahiro Ono c-L a1.,
Nucicar Mcdicinc and 13i.c)loqy, 2003, 30, p.565-571) .
[0U10]
Meanwh,ile, iL is revealed that compounds having an
imidazopyridine skeleton .`,~llch as IMPY have a property of
t.r,.airsfcrring to brain and ac~cumul.aCing at_ amyloid a:ELer
administration, and a.l.5o hav~. an cxr..elicnt properLy of
rapid clearance .frorn normal ti.ssues ur like the above-
clescr..ibed compounds, as a result ot experiments using
[L`'I ]-l.abelcd. compounds. However, IMPY i.s a cornpound
positive iri reverse mutation t.est. In order to use this
compound as a. probc for imaging d.i..agnosis, sufficient
care must be taken about dosage and administration manncr.
(Int.ernational Publication W003/106439 pamphl.et)
FDUNP ie also reported r_o be positive in r.cverse
mutatiora test. (International Publ:Lcation W003/1.06439
pamp.hlet)
[0011]
A prcferablc probe l:argeti.rig amyloid for imagi.ng
diagnosis would be a compound that is excellerit in
affinity with amyloid and sufi'ici.ently .rapid ir7 clearance
fr..orn norrnal t~ 5sue5 like IMP`C but is suppressed in
toxi.clity such as mutaqeriicit.y. However, no compound wiCh
such pr:operties ha.s been. disclosed.
[00i2J
'T'he present invention has becn made under 5uch
circurnstarices, and aams at providing a c,:ompouricl thaL. has
affi.nity wi_th amyloid, exh'_b.iLs sufficienCiy rapid
03/25/09 WED 16:01 FAX 416 362 0823 RIDOUT & MAYBEE Ca011
CA 02657312 2008-10-28
- f3 -
ciearancc from normal tis.sues arrcl is suppressed in
toxici.t_y such as muta.geri.icity, and also providing a
d:iagnoSL-ic agent for R).:_heimc:r''S d.isease.
MrANS L'()1Z SOLV ING THE PRObLEM S
(001.'7
The inventors have founci that a grour.r of compounds
satisfying the above.-described .requirements can be
obt.ained from a C,ompound with an imidazopyridin.e-phcnyl
skeleton having a carbon htom to which hydroxyl c{.r.oup is
autach.ed, and Lhus have cornpleted the present invcnt.ion.
[00141
Specifically, the present. invention relates to a
cornpound repr. esentcd by the following .f. ormula (1) :
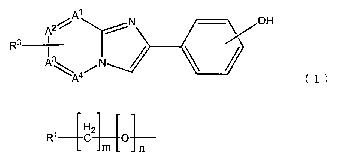
[0015j
A N jOH
RJ
A~A4/N ` (1)
1.5
or a sal.t thcr.c_of, and a low-toxic diagnostic agent. for
111zhcimer's disease compr..ising a compound represerited by
the above formu.la (1) or a salt t.hPreof.
[0Q1.67
In the formula ( 7. ), R3 is a group represcnt_ed by
H
R' C2 O
m n
03/25/09 WED 16:02 FAX 416 362 0823
RIDOUT & MAYBEE 0012
CA 02657312 2008-10-28
- ~~ -
R1 is a radioactive h.ologen 5ubsti,tuent, rn is an integer
of 0 to I a.nd n is 0 or 1. As 1_he radioactive halogen,
various el.ements can be uscd, prcferab]..y a halogen
selectcd from the group consisti.ng of "F, `'Br, `ljl, '."L,
]`''`T and 1j1I, an.d more prefc.r.-ably a halogen selected from
the group consistinq of 1bF, 111I and i"I. In the formula
(1) , when ri = 0, in = 0 i:o 4 is preferable, and when ri - 1,
m= 1. t=o 4 is prcferable.
[0017]
A1, A2, I~j and A' independently represent a ca.rbc7n or
na.t:rogcn, and i.t is nec:essary that at least one of these
represents a carbon. Pr..e.CerabJ.y, 3 or rnorc ol A', 112, As
and A' repre5ent carbons, anc! mox-e preferably, all of
uhem represent c.z.r.bons. In the formu].a (1), Rj binds to
1.5 a carbon represFnted by A1, A`, A3 or A~. When A AAj
and A' respectivcJ.y represent a carbon which is not bound
t.o R3, a hydrogpn atom bind:i.ng thcrer_o is to bc
unsub5Lit.ut.c_c3. A hydroxy.l_ group indicated in the .forrnula
(1) may bind l_o any of the carbons constitut.i.ng the
phenyl. skelctan thereof, but it i5 pre.f,c.rablc thaz the
hydroxyl group binds to a cart~on at 4'-position of the
phenyl skc].el_on. A binding site for R3 may be any of A',
A'', A3 and A4 a:1 long as it. i.s a car..bon, but is prefcrably
a carbon represented by A3, th~IL is, a car.bon at 6-
position.
[001II1
Accordi.rig to another aspr~ct of l:he -present inverition,
a compourict rcprese.nted by --he following forxttula (2)
03/25/09 WED 16:02 FAX 416 362 0823 RII)OUT & MAYBEE 013
CA 02657312 2008-10-28
- 10 -
[0019j
As ~A ~N OH
R4
A ~Aa1-1 N ( 2 )
[00201
or a salt thereof is prov,i.ded.
[0021)
In the formula (2), Fe" is a. group represcnted by
4H
mC)n
Rz CZ
man integer of. -U to 4, n is 0 or 1, and when m- n
0, R' is a non-radioac.t:ive halogen substituent, ni.t:ro
1.0 sub5titucnt, trial.kylstannyl subst.iLuent having from 3 Lo
12 carbon atoms or t.ripheny.Lstannyl.. substi.tuent, arid when
7n~0 and/or n,4-0, it is a non-ra.r.lioactive halogen
substituent, mothanesulfonyloxy substi.t.uent,
tr.i.lluoromethanesulfony.l..c)x.y subSLituent or aromatic
sulfonyloxy ,subSLituent. As the non-:radioacl:ive halogen
subst:ituent, a hal..ogen capa.ble of beinq a targi~-t of
nuelcophilic 5ubstitu~ion reactions using a radioactive
fluorine or a.ha'logcn capable of bcirig a target of
isotopc exchange reactions wa.th a radioactive i_odine cari
be u:.ed, and preferably iodine, broma,ne or chlorine can
be u!~ed. As tho t_rial}r.ylst.ann.yl substituent, vZ.ri_ous
substituents can be used, and preferably trirnethylstanny,l..
03/25/O~J WED 16:02 FAX 416 362 0823 RIDOUT & MAYBEE 10014
CA 02657312 2008-10-28
11
substituent and tribut~ylstannyl su.bstituent can be uFed.
In the formula (2), wh.en n= 0, m- 0 to 4 is prcferab).e:,
and when ri = 1, m= 1 to 4 is preferable.
[0022]
A, AE, A~ and A8 iridepcridently represent a carbon or
nit_rogcn, and it is necessary that at least oiie of t_hese
represents a carbon. L'rcferably, 2 or more of Ay, A", !L7
anct At; represent_ carbons, and irLore preferably, a11, of
them rep .r. esent cfirbons. In thc formula (2), R4 binds to
a carbon rchresented by A5, A6, A' or Ag . When A5, A6, A'
and Aa respectively r_cpr.esent a carbon which is not bound
to R4, a hydrogen atom binding theret.o is to bY
unsubstituted. A hydroxy]. group indicated in the Lormul.a
(2) may t)ind to any of the car.bons corlstitut?.ng thc
phenyl skcl.eLori th<-reof, bu':- it i.s preferable thsL the
hydroxyl group binds t;a a carbon at 4' -posi l-ion of the
phenyl skelctori. A binding site for.. t~' may be any of Ar,
A~ , A' and AB a.s long as i t is a carbon, but i. s prefcr, ably
a carbon represen.ted by A', that is, a carbon 6-
position.
EI'I'F=S UF THE INVENTION
[(iU23]
In accordance with thc present invention, a c.ompound
that.has dlfinity with amyloaci and i!:; sufficiently fast
in clearance froin normal tissues and suppressed i.n
Loxici.Ly such as muLageniõuzi.ty can be orLaincd as well as
a diagnostic agent for Alz.l-ieimc-r' s di scase wi.-l.h low
toxicit.y_
.
03/25/0.91. WED 16:03 FAX 416 362 0823 RIDOUT & MAYBEE f]015
CA 02657312 2008-10-28
- 12 -
BEST MODE FOR CARRYING THE INVENTION
[0024]
Hereinafter, a method for syathesis of a precursor
compound for a radioactive halogen-labeled c:ompound
-:ir,cording to an. aspect ol thc present i.nventi.on will, be
dcsc:ribed, Laking Lhe case of 6-t.ributy.ls tannyl.-V2- [ 4' -
hyd..r.oxyphcnylJimidazo[l,2-a.lpyrid.i,ne.
U0251
Fi.r:,t, 4' -hydroxyacetophcnorie is zllowed to react
with cupric bromide to prepare 2-b.r_-orno-4' -
hyciroxyacetophcraorie (Fig_ 1, Step 1) ;[ri this ;instance,
the rPaction r._an bc c.onducteci in accordance with ordinary
me'L'hods, for example, tlie method described in a
].i'teratLire, King, L. Car.=ro1.1 anci Ostrum, G. Kenneth,
1.5 Journal of Organ..i.c Chemisl_ry, 1964, 29 (l.2) , p.3459-3461.
[0026]
Then, 2-brom.o-4'-hydroxyacctophenone as pr.eparcd
above i.5 allowed to react witti 2-amirio-S-b..r.omopyri.dine to
prepare 6-bromo-2- ( 4' -t'iyciroxyprieny.l.. ) imiclazo [ l, 2-
G]pyridi..ne (Fi.g. 1, Step 2). '.t'his step can be donc-
acco.rciing to the foliowing procedure.
(U027].
First, 2-brorno-4' -hyriroxyacetophenon(D arid 2-amino-5-=
bromopyridine are di.8solvcd in an inactive sol,vent such
as acetonitri_.1e, and. are al..l.owed to react wit.h. each other
at a ref1.ux temperature tor 2 to 6 hours to produce 6
-
brc~rno-2- (4' -hydroxyphenyl) :i,midazo [l, 2-alpyrid.ine
hydr_obromide salt as whiLe precipitates. 'Phe solvent
03/25/09 WED 1.6..:03 FAX 416 362 0823 RIDOUT & MAYBEE C~016
..
CA 02657312 2008-10-28
- 13 -
used in this instance may be acetonitrile or another
solvc:nt thaL is usually ernployed in a similar reaction,
for examp7.e, metha.nol and acetonc. The reaction
tempcrature may be a temperature a.l-l.owing refluxirig, fo.,r.
example, 90 C when the solvent is acetonit..r.i.le. The
amoutZt of the solvent to be used may he an amount
sufficient to effect- Lhe rcac_tion, howevcr-, it should be
noted tha t:: if the solvent: i_=, t.oo fnuch, 'i. t wil J- become
diffic.ult to obtai-n pr'ecip.i.t-ntes o:r." reacti.on pro&.lcts.
F'or example, when 2-brc>mo-4' -hydroxyacetophenone in an
a.mourit corresponding to 10 rune7. is used for t.he reaction,
the drnount of a solvent to be used can be about 40 to 50
mL.
[002.8]
Next, the r,eactiori solution is filtered to recover
th.c precipitates. The whiCe pr.rcipitat:es are suspended
in a niixed solution of mct.hanol/water (1.1). 'T'hen, an
aqueou5 saturated solution of sodium hyd.r.ogencar,bonatc is
adfled thereto in a very excessivc amount relati.ve to the
susK)Pnded precipitates to release 6-bromo-2- ( 4' -
hydroxyprienyl ) irnidazo ( l, 2-a1 pyridirle as precipit;ates.
The newiy generated precipitate5 arc lilt.cr-ed to recover
6-br(Dmo-2-(4'-hyd.roxyph.cnyl.)imi.dazo[1,2-a]pyrir~i.nF as t.he
t:argct compound in l':his step ( Fiy . l. , Ste.p 2) . The
amount of the mixcd soluti.on of water/rnethanoi is not
spccifically limited a~. lorig as it is sulficierit to
effect: the react;,an. However, it should be noted that if
the amount of the rtiixed solution is too much,
03/25/09 WED 16:03 FAX 416 362 0823 RIDOUT & MAYBEE Z017
CA 02657312 2008-10-28
- 14 -
pr..ecipitat:ion of produ.cLs wi].,1 be hi..ndered. For cxample,
whcn 2-bromo-4' -hydroxya.cetophenc5ne in ian amount
corresponding to 10 mmol is u:~ed, the mixed solution of
water/methanol may be uscd. in an amount of about 40 to
100 mL. The amount of sodium hydrogc_ricarbonaLe is riot
specifir:ally 1irriited as long as it is very excessive
relative to t.hc above-dc:5cribed precipitates as .r..eaction
substrates. For examplc, when the reacti..c}n is effected
under the dbove-dcscribed conciition5, the amount of an
aqueous saturated solution of sodium hydrogencarbonatc to
be added to the react.:ion solut~:Lon can be about 25mL.
[0029]
Then, the 6-bromo-2-(4'-hydr.oxyphenyl.)imidzzo[1,2-
a]pyridi.rle prepared above is suffi.c,iently dried,
dissUlved i:n dioxane, and after triethylam.i.nP is added,
bis (tribut'_yltin) and a cat:a.Lyt.ic amount ot tetrakis-
triphenylph.o.,phine palladium are added. This reaction
mixture is heated at about 90 C and reac-t::ed for about 24
hours, arrd then a solvcnt is distilled off and a
ch.r.ornatoclraphic puzification is performed to obtain 6-
tributylstann.yl-2-[4`=-hydroxyphenyl];i.midazo[1,2-
a]pyrid.irle as the target compourid (Fig. 1, Step 3) . '!'he
amourYt of bis (tri.butyltin) 'Lo be used is an amount
satisfying a conditi.on wher_c~ it is excessive relative to
the reac.tion substrat.c, specifically, i't is px-efer.ably
about 1.5 times :i..n rnolar r=atio relativ~ to the 6-b.r, orno-2-
(4'-hyd.roxyphenyl)imid.azo[].,?..-a]pyr_i..dine as Lhe rc:acrion
substratc.
03i25i09 WED 16:04 FAX 416 362 0823 RIDOUT & MAYBEE 1~j018
CA 02657312 2008-10-28
[0030]
When a compound wiLh a substituFrtL at t.tie 6-position
being a t:rialky].sLannyl, sub5tituent other thari
tri.buLylstarinyl substitucnt is obtained, various
bis (trialkylt:in) s that fit purpo,ses can be t_Lsed in.,tead
of bis (t.ributy]..t_in) in. Step 3. For cxarnple, when a
compound having a trimethyl.,~t_annyl substituent as a
;,ubstituerit at the 6-po. ,ition is synthesized, a reaction
similar to Lhe above can bc-i perfo.rmed in Step 3 using
bi,s (trimcthylt,in)
[0031]
Tn order to obtain a compound with a subst.i.t.uent a.'t
the 6-posi t;ion bci.rig a non-.r-adioactive halogen
substituent, the compound obtained in Step 2 pc.r se may
bc used as a compnund havzng brom.i.ne as the halogFn
sube>tituent, and for compou:nds having iluo.r..ine, chlorine
and i.odinc as the halogen subsLitucnt at the 6-position,
the same reaction as in Step 2 may be perLormed except
usi.n.g 2-am:i..no-5-f_].uoropyrid:ine, 2-aInino-5-c`.hloropyridine
and 2-arnino-5-iodopyr_i.dine respectivcly instead of 2-
amino-5-.b.romcpyri.dine in. Step 2.
[00321
Tn order t:o obtain a compound wi.th a substituent at
the 6-position being at:tachcd C_hereto via oxygen atom, 2-
amino-5-hydroxypyridinc instead. of 2-arnino-5-
bromopyridi.rie may he reacted to synthesi Te 2- (4' -
hydroxyphenyl )-6-h.yc]roxyim.idazo [ J., 2-a] py..r. iclinc, and a
bromide compotind having a:lubstit.uPnt dcsired to be
03/25/09 WED 16:04 FAX 416 362 0823 RIDOLTT & MAYBEE iO019
CA 02657312 2008-10-28
- 16 --
i.nLrodu.ced may be reacted ther.ewith in the p.r.esencc of a
base. For examp,l.e, in o,r.der to obLain a compound having
a 3-Lluo.ropropoxy substituent at the 6-position, 2-(4'-
hydroxyphenyl,.)--6-hyd.r.oxyimidaz.o[1,2-alpyridine can be
reacted with 1-bromo-3-fl.uoropropane in the presence of
potati;,,ium carbonate.
[0033]
Further, in or_der to obt;ain a compound with a
sub5tituent af_ the 6-=position being ai:t.ached -rhereto via
1.0 an alkyl cliairi, thc following operations can be performed.
For exampie, fo.r, a compou.nci with a subst.i.t_uent at Lhe 6-
posit.ion bcing a 3' -b:romopropyl group, 2- (4' -
hydroxyp]=ienyl) -6--bromoimidazo [ 1, 2-a) pyrid.irie obtained in
Stcp 2 may be rea.cted with a]].yltribut_yltin, arid
converted to 6-al].y1.-=2- (4' -'nydroxyphenyl) imida2o [1, 2-
a]pyridinc. '!'hen, i.t is sub]ec'Led to hydroboronation and
oxidation reactions so as to bc c;onvertc;d to 2- ( 4' -
hydroxyphen.yl)-6-(3'Jhydroxypx-opyl)imi.dazo[1,2.-a]pyri..dine.
~'urthcr.more, bromination of the hydroxyl. group by
tet.rabrom.omethanc TrLay be pFrlormed iri the presence of
triphenylphosphine.
Compounds .r. epresented by t.h,c. abovc formula (1) in
wh.ich A` among A1, A`, A'j and A 4 is a nit-r=ogen, and
compounds represcnL_ed by the above formula (2) i,ri which
A among AAA and AF ia a nitrogen can be produced
in accordance with the above method except usi.ng 2-arr.lino-
5-bromopyrimidi_nc. insteac3 of 2-amino-5-bromopyridinc iri
step 2 of Fig. 1.
03i25/Ofl WED 16:04 FAX 416 362 0823 RIDOUT & MAYBEE 020
CA 02657312 2008-10-28
1. I -
Compounds representecl by th.c abovc formula (1) in
which A2 and A 4 among Al, A`, A' and A' are nitrogcns, and.
cornpounds represented by 'the above formula (2) in which
A5 and A5 arnv~rg A~', l~6, A7 ~nci Ae az'e riitrogeris c.an be
produced in accordance with the above rnethod except using
6-am:ino-3-b.l-omo-1,2,4-Lriazi.nc: instcad. of 2-amino-5-
bromopyridinc i.n Stcp 2 of E'iq. 1.
[0034]
Hereinafter, a method for production of a
radioactivc: halogen-labelcd c:ompound according to another
aspect of the prescnt invent_ion will bc described by
taking the ca5e of 2- [ 4' -hyciroxyptienyl ]-6-
[1`3I]iodoimidazo[1,2-a]pyridinc.
[0035]
For t.he production ol' 2- [4' --hydror.yphenyl] -=6-
[123I].ic)doimi.da2o[1,2-a]pyrid.ine, a [1-3I]sodiurn iodide
soluti.on to .bc served for labeling is first obtaincd. A
f 1`3I J radioactivP iodinc can bc obtaincd by, for example,
a known rnethod iri whic.h a xenon gas is used aa a target
and exposed. to proton bombardment. This [123I] radioacti.ve
iodine is made i..rrLO [''''I'1 sodium iodidc solution by using
known riLethod;., and used for the labeling.
[~U36]
Therr, the labeling precursor 6-t.ributyl.stannyl. ==2-
[4'-hyd.roxyphcnyl]imidc).zo[]_,2-a]pyridine is dissolved in
an. inert organic solvcnt., and the [''',7_} sodium iodi,r.ie
solut:ion, an acid ancl an oxidizing aqcnL arc added.
t-.hereto and allowcd to rcar,.L to ob-Lain 2- [7' -
03/25/09 WED 16:04 FAX 416 362 0823 RIDOUT & MAYBEE U021
CA 02657312 2008-10-28
- 18 -
hydroxyphenyl ]-6- [II'I ] iodoimidazo [ 1, 2-aj py.r. idine as a
targc_L compound. A,a Lhe in.erc organic sol.vPnL dissalving
the precursor c.ompou.nd, variou:~ solvcn.Ls having no
rea.rLivity with the labeling precu.r.5or and t_he
[12~I] sodium i,odide ca.n he uc-cd, and prererabl=y methanol
can be used.
[003-7]
As the acid, may be used various ories, and
pre:ferably hydrochloric ac.id.
The oxidizing agcnt is not particula..r=1y limited as
long as it can effect the oxidation of iodine in Lhe
=r_eaction soiuti.on, and is p:=eferabl,y hydrogen peroxide or
pc.r,.acetic acid. The ainount of r_he oxidizing agent to be
addcd may bc ari amount sufficient to oxidize iodine in
the reaction solution_
[0038)
A compaund labeleci with a radioactive halogen other
than iacline can be synthesized by labe7.i.rig a labeling
precursor that fi.ts a purpose of synthesi..s with a
radioactive halogon t_hat fits the purpose. For example,
in order to synthesize 6- [18F] .f..l.uoropropaxy-2- ( 4' -
hydroxyphenyl ) imidazo [ 1, 2=-a] pyri.dine, the labcl=ing
precizrsor .?. - ( 4' -h,ydroxyphcn.yl ) -6- ( 3' -p-
toluenPsulfonyloxypropoxy) imi.dazo [7., 2~-a] pyri.diiie can be
2J reacted with ['r'.F] fluoride ion in 'the presence of a phase
transfer c1talyst arid potassium ca,r.bonate.
[0039]
The diagnostic agcpt according to the present
03i25i09 IVED 16:05 FAX 416 362 0823 RIDOUT & MAYBEE 022
CA 02657312 2008-10-28
invention can bc: prepared as a solution which comprises
tl-ie present radioacL-ive ha.logen-l-abeled compound blended
in water, a. physio,lc)gical saline sol.ution or a Ringer' s
Solution optionally adjust.pd to an appropr-iate pi-!, like
other COmmOnly-knOwn radioact.ive diagnostic agents. In
Lhis i.nstance, conccntr-ation of the present compound
should be adjuSLed to not more than the concci-itration at
which stabili-ty of the present cornpound is ensured.
Donage of the prc:.,ent compoucid is not specifically
limitcd as long as it is suf:fici-ent to obtain an imagc of
dist..r-ibution of an adininiste,.r..c,-d agent. For example, in
case of iodi.nc-123-labcled compounds and tluorine-18-
1abeled compounds, about 50 to 600 MBq per adult body of
60 kg wei..ght can. be admini,stered i-ntravenously or locally.
Distribution of admiriistered agents can be imaged by
kriown mc:t:hods. rar example, iod.i-ne-123-labeled. compound5
cari be i-maged by a SPECT apparatus while fluorine-18-
labeled compounds can be imaqed by aE'E'T' appa.r.aLus.
FXAML'LE
f0040]
Hercin.alter, the preserlL invcntion is desc.ribNd
below -i-n more detail by way of Exampl,es, Comparative
Examples and Rcferencc l',~amples. Howev~:r, these Examples
riever limit the scope of the present invcntion.
In t_he following 3^,xamplcs, the narr.tes of -t--he
individual compounds u.sed in the expc.r,-iment are defined
as showri in Table 1.
[O0n1]
03/25/09 1NED 16:05 FAX 416 362 0823 RIDOUT & MAYBEE [7j023
CA 02657312 2008-10-28
- 20 -
Te.blce 1: Names ot compounds used for ev,~luatiUn .in );::arilplcs
_.... ..._ ...,... ..:.. ~- .. _
cC7filpounCl.
Coinmon natrie
namc
Compcyllnd 1 6-brorrio-'L- ( 4 ' -hydroxyplienyl) imid 1zo 1 - ~ , 7 . -
a ] y.r.i..d:inc
Ccmpound 2, 2- ( 41-hydro:;ypheriyl )-6-ic)dc)imidazo [ 1, 2-al pyr. idi.ne
----- . _ ....._. ..,- ,. ,__ ....
cornpollnd 3 C'y.,( 3' -f1.uoropr_opo.:y) -2- ( 1' -
h drox hcn 1) im.-i.rlazo [ l, 7-l
y_ yp Y.... JpYricii.na_
Compouric.'. 4 `'- (4' -hydroxyphcnyl )-6-iodoim i,rlazo f 1, ?. -
a]rimi.d.i.r;F
_...,.. _..,..._ ... W,- .,., . .
Corupotrnd 5 -2- ( 4 ' -hytiro.x ypherly1 ) -r; inr.loimidazo [ ! . , 2-
a.] F>yridin
Cc.rmpound 6 ~ 1I J-2- ( 9' -hydrox_yphr-~ nyl )-6- i.ocloimid~~ o [ 1, 2-
aJ f~yridine
C:cmpourid 7 6-bromo-2- (4' -hydr i:)xyphcnyl) .i.midazc [1 , 7-
~a] razi..ne
-õ_ ..... __....._ ,.. .._ -....,.
Compexind 8 2- (4' -hydroxyphcnyl )-F,-iodoiin'i c3azo I. l, 2-a] pyrazl.nc
C;ompounrl g (!1' -Pcydrox,yph (:nyl )-F;- i adoimicJta?.o [ 1, 2-
,]' rimid:i_rlE
_._:_ ..... _... ..._ ...,,._
~:e ....:..
[ I J-2- ( 4' -hyd re~xyph_nyl. )-f~-iodo.i.o',i.dazo [. 1, 2-
COmf)qclnd 10
apy.r.izinc
,
Conlpourld 11 [i _I]_~,_(,1'-hydroxyf~}ic:nyl)-8-ic:iioimirlu;:o[1,2-
a J yride
~_......_ ...._ ._. _...,,.... _.
[0047_]
F,xamplc i 1: Synrhesis of;= 6-tribuLylstannyl-2-(4'_
1-1 y droxyph.enyl)imidazo[1,2-?]pyridi.ne
[0043]
50 rnL of eChyl acc:Late wz5 addcd 't_o 28.1.7 g
(corresponding to 126 nunoi) of cupric bromide to obtain a
suspension, to which a soluCion of 8.18 g(corr_espondiny
to 60,0 rnmol) ol V -hydroxyacet-ophenorl.e in a rnixed
solution of 50 mL ot ethyl aceta.'1,e and 50 mL of
chlo.r..afc)rm was added. Thcn., the rc-suitinq ntixturP was
refluxed. After 5 hot_Lrs, the reaction soJ,ution was
e~ooled down to room t.errtperature and. filtcrc:d. The
resulting filtrate wa..s conccnLratcd under reducod
pressure. The residue was dissolved in etY>.yl aceL-ate ar>.d
subjected Lo decolorinq operatlon with addition of active
03i25/09 WED 16:05 FAX 416 362 0823 RIDOUT & MAYBEE U024
CA 02657312 2008-10-28
- ~:1 -
charcoal. Then, the .r.esulting solution was filtered and
concentrated. The resulting crude product was purified
by flash sili.c:a gel column ch.rcamatogr.aphy (elution
sol.vent: chloroform/methanol = 20/1) , and .r.ecrystaJ.lized
from ethyl acetatF/petrolcurr. ether, to obtain 7.25 g
(corresponding to 33.7 mmol) of 2-brome-/1'-
hydr_oxyacetopherLone (r'ig. 1, Step 1).
(00d4]
2_ 1,5 g(cor'respond.i.ng to 7Ø 0 nunol) of 2-bro.no-V-
hydroxyacetophcnone and 1.74 g(co.r..respondi.ng to 10.0
mmo.l.. ) of 2-amino-5-h.r.0rnopyr_ i.d.irie were dissolved in 50 mL
of ac.etona.tr.ile. The resulting solutiori was refluxed iri
an oi.1. bath at 105 C for 6 hou.r.:5. AftEt: Lhe completion
of i:he rcact_ion, tbp reaction solut;i.on was c:ooled down to
z-oom tcmperatur, e, and p.r.~E;c:ipitates werc :filter_c d and
rcc-overed. The p.rccipitat.cs were washed wi.th
acetonitrilo arid dried under reduced pressure. The
resultirrg crude crystals s were su:,pended in a mi-xed
Solutiori of 20 rnL of wat_er and 20 mL of rrretha.nol. Th.cn,
about 25 mL of a saturatc:d sodiu.m hydrogc.ricarbonate
solution was arided thereto, an.d the mixture was sonicated
for 5 minutes usin.g an ulL-:rasonic washing machine.
Preci.pitatcs were fi..1t_ered and recovered fr..orn thc
resulting mixture, sufficiently washed with water, and
23 dried urider reduced pressure, to obtain 2.41 g
( c.orresponding c.o 8.32 111Mo7.) of 6-br..orno-2- (4' -
hyd.r.oxyphcnyl)imi.dazo[1,2-a]py.r.idine (Fig. 1, Step 2) .
[0045]
03/25/09 WED 16:06 FAX 416 362 0823 RIDOUT & MAYBEE Rj025
CA 02657312 2008-10-28
- 22 -
138 mq ((,orresponding to 0.476 nur:ol) of 6-bromo,2-
(4'-hydroxyphcnyl)imidazo[1,2-a]pyridine was dissolved in
20 mL of dioxane, and 2 mL of tri.ethylamir:e was added
thcreLO. Then, 360 }1L (corresponding Lo 0.713 nmol) of
bis (t.ributylti.n) and 20 nig (at a catalyt=ic amourit) of
tP'Lrakis--triphcn.ylphosphi.ne palladium were added.. After
thc reaction rnixture was stirred at 90 C for 22 hou.r.s,
r:he solvent was distilled off under reduced pressure.
The residue was pur..-ified by preparative TLC (elution
solvent: hcxarre/ethyl ac.etatc = 1/4). E'urthcr, Lhe
resulting crude product was puri,fied by recycle
hreparative HPLC (HPLC alapa:ratus; LC-908 (under trade
name; manufactured by Japan lkrialytical Indust.ry Ca,,
LLd. ) ; c.olumn: two of JAIGEL 21[ (under t..r.ade name;
mantrfac.ture(i by Japan Analyt._ical Ind:aSLry Co., Ltd, )
connccted together; m.obile phase: chloroform), to obtain
47 mg (corresporiding to 94.9 lzmol ) of' 6-tributyts tanny.7.-
2-(4'-hydroxyphen.yl)imidazc-)[1,2-alpyridin.e (Fig. 1, Step
3).
[004 61
`Phe NMH :neasuremen't resillds of the resulting 6T
Lributylstannyl-2-(4'-ttydroxyphenyl)imidazo[7.,2-
a]pyrid.ir:e (internal standard: t_etramettiylsilane) ar(~,
shown below.
[0o9'r~
NMR apparatus einpioyed.: JNM-.rCE'-500 (manufactured by
,7apan Electron Optics Laboratory Co., Ltd. (JEOL))
LH-NMR (solvcnt: chlorotorrn-dl, resonance frequency: 500
03/25/09 4VED 16:06 FAX 416 362 0823 RIDOLiT & MAYBEE Z026
CA 02657312 2008-10-28
- 23 -
MHz): o 13.01 7.94 (:n, 1H) 7.71-7.67 (m, 2H), 7,70-7.67
(m, 111) , 7. 64-7. 60 (.m, 1H) , 7.20-7 . 11 (m, 1H) , 6. 89-6. 85
(rri, 2I=1) , 1 . 62 -1 . 46 (m, 6H) , 1.. 3 4 (se'xt, J = 7. 3 Iiz, ESH) ,
1.18-1 .03 (m, 6-i) , 0.90 (L, J- 7.3 1:-Iz, 9H)
[0048]
13C-NMR (solvent:: chloroform-dl., resonance fr.equency: 125
MHz): b 157.85, 145.11, 144.72, 131.90, 1.29.93, 1.2"/.62,
121.02, 122.59, 1-16.11.4, 116.09, 106,19, 28.96, 27.27,
7.3.62, 9.81.
[0O~9]
Example 1-2: syntr.esis of [iLI]-2-(4'-hyd7gxyphcnyl)-6-
-iodoimidazo [1, 2-a] pyra.dine
[0050~
To 53 1iL of asol.ul:ion of 6-tr.i.butylstannyl-2 - (4' -
hyclx=oxyphenyl)imidazo[1,2-a]py.ridine in methariol
(concentration: l.. rng/mL) , 75 1zL of 1 tnol./L hyd.r.ochlor.ic
~
acid, [ z~'1lsodium i.odidc of 136 M13q (40 1rL in volume) and
10 L of 100 (w/v) hydrogen peroxide were a.dded. AfCer
th.o mixed solution was 1.eft to .:,r.and at S0 C for 10
minutcs, the soluti.on was subjected to I-IPLC unde:c' the
fol-lowinq conditions, to oLtain [i`5I] -2- (4' -
hydroxyphenyJ.)-6-iodhimida.zo[1,2-a]pyrid.ine fra.ction..
[0057]
HPLC conditions:
Column: Phenomenex Luna C18 (-L=rade name; manufactured by
Phcnurnenex Co.; size: 4,6 x 150 mm)
Mobi.J..e phasit:: 0.:1 i trif_l..tzoroacetic acid/acetonitr.ile =
20/80 to 0/100 (17 rninut.es,, lin.ear gradient)
03/25/09 WED 16:06 FAX 416 362 0823 RIDOUT & MAYBEE Z027
CA 02657312 2008-10-28
- 24 -
FJ.ow ratc ; 1.0 m.T.~/rniri.
DetccLor: Ultraviolet visiblc; absorptiosriete.r (Detection
wavelength: 282 nm) and radi-oace'-ivity countc.r-
(manu.f.-actured. by raytest: type S':i'EFFI)
[0052]
ITLl of wa.ter was addect to the fraction. The
resulting solution was passc.ci through a rcvcr. sed phase
column (tra.dc: name: Sep-Pak (registered Lrademark, Wat(t'-r.s
InveS Linents Limited) Light C:18 (;artridges manufactured by
10 Waters: Lhe packed amount oI' the packinq agent: 130 mg)
so that the column adsorbs and collcct., [1"5I]-2,^(/I1 -
hydroxyphenyl )-6-iodoimidazo [-1, 2-a] pyridi..ne. The c.olumn
was rinscd with 1. rriL of watc~r, and then 1 mJ,, of etharial
was passed, therethrough to c;.1,uLe ['z5T] -z- (9' -
hydro;:yphenyl )--6-iodoima-dazo [ 1, .7.-=,a] pyrida-rie . The amount
of rada.aactivi-ty of the nbtaincd. c:ompound was 37 - 5 MBq at
the end of synthesis. Fu.r,ther, the TLC analysis was
conducLed under the followinq conditions, and as a resulL,
ttie radiochemical purity or the compound was 96 . 5 , .
[005 "']
'I'LC analysis conditions;
'!'LC plate: RP-:1.8F254 (trade name; manufartured by Merck &
Co., Inc.)
Mobile phase: Methanol/water = 20/1.
Detector: Bio-.imaging Andlyzer_, BAS-2500 (ty.pe: BAS-2500
manufactured by PUJIFILM Corporation)
03/25/09 WED 16:07 FAX 416 362 0823 RIDOUT & MAYBEE Z028
CA 02657312 2008-10-28
- 2, 5 -
[0054J
1~,-;...
E,xaniple T.-3 : Synthes. ~s of [-1 } -2- ( 4 ` -hydroxy~henyl
iodoirnidazo[l,Z-a]pyr,.idine
[0055]
To '70 ))t-, of a so.l.ution of 6-LributyJ.:5tannyl-2- ('1' -
hydroxyphenyl)i-midazo[1,2-a]pyridi,ne in mcthariol
(concent:r,a~ion: 1, rny/mL) , 75-100 pL of 1 mol/L
hydrochlor.ic_ acid, [123I]sodium iodidc of 236-454 MBq (15w
120 u"L in volume) and 7.5-10 )aL of 1 mmol./L sodiu.m iodide
solution and 10-15 pL of 10`1i (w/v)hydrogen pe.roxide were
addcd. Aftc,r., t.he mixcd solution. was heated at 50 C for
10 minutes, the solution was subjected to I-1P:I,C under the
same conditions as in Examp,l.e 1-2, to obtain [ 113I j-2- ( 4' -
hyd.r.'oxyphenyl)-6-iodoimidazo[1,2-a]pyri.dine as a rracti-on.
[0056;
10 ml of water was added to the fracti,on. The
re5ultincl solution was passed Lhrouqh a reversed phase
column (tradc name: Scp-=Pak (rcq.i,~;tered trademark, Waters
Ir:vest.menLs Limited) Light C18 Cartridges manufactured by
Wat~rS: the pac);cd arriount of the packing aqcnt: 130 mg)
:~o that the column adsorbs and collects [ 23I] -2- ( 4' -
hydroxyphenyl)-6--iodoimidak;o[1,2-alpyridi,ne. The column
was rinscd. with 1 mL of water, and then 1 ml., of diethyl
ether.. was pas5ed therctl~~rough, to elutc [123I ]-2,- (4' -
hydroxyph(-nyl)-6-.:iodoimid.a::o[1,2-a]pyridine. The amount
of radioactivity of the obtained compound was 21-180 MBq
at: Lhe cnd of synthesis, r'urther, Lhe TT,C; anaiysis was
canductcd under the same conditions as in Exampl e I-2,
03/25/09 IVED 16:07 FAX 416 362 0823 RIDOUT & MAYBEE Z029
CA 02657312 2008-10-28
2 6 -
and as a result, Lhe radi.ochemica.l purity of the cotnpound.
was 99 . 5~, .
[005'Ij
Example I-~1: Synthesis of 6-bromo--2-(4'-
]ly_Q.r. oxyphcnvl ) imida.zo [ 1, 'L-a] pyridine
[00581
50 rnL of cthyl accta te was added to 28 . 17 q
(corresponding to 126 mmol) of cupric brorziide to obt=ain a
suspensi.on, to which a solul:ion of 8.18 g(corresponding
1.0 to 60 _ 0 mntol ) of Q' -hyd..r.oxyacctophenonc .i,n a mixed
solution of 50 mL of et.hyl acetate and 50 mL of
chioro:form was added. Then, the r.psulting rnixturc was
refluxed. After 5 hours, t:.he reaction solution was
cooled down to roorn tempcr.atuie and filtercd. The
resulting filtratc was conccnLrated urider rcdl~ced
pressure. The residuc was tJissolved in eLhyl acetate and
~~ubjected to decoloring operation. with addition o.f, active
charcoal. Thcn, the rest:tlt:ing solution was filtcred and
c_oncantrated. 'i'he resulting crudc: product was purified
by flash silica gel columri chromat:ography (elution
so].vent: chloroforrn/methanol = 20/1), and recrystallized
from ethyl acetate/petroleu.m zther to obtain 7.25 q
(c.orresponding to 33.7 mmol.) of 2-bromo-4'-
hydroxyacet ophenonc ([?i g. 2, S t_ep 1).
[00s9]
2.1.5 g(cor.responding to 10-0 mmol) of 2-bromo-4'-
hydroxyac.et.ophPrione and 1."/4 g(corre:spondinc3 to 10.0
mmol) of.. 2-amino--5-bromopy:ridine werc dissolved in 50 mL
03/25/09 WED 16:07 FAX 416 362 0823 RIDOUT & MAYBEE CN30
CA 02657312 2008-10-28
- i7 -
of acetonitrile. Tl.ze resulti.ng solution was r_elluxcd in
an oil bath at 1G5 C for 6 hou..r..s. Aftcr. Lhe completion
of the reaction, Lhe reaction solution was cooled down to
room temperature, and precipitates were f.iltered and
recovcred. The precipitates were washed with
a.c.etonit.r.ile and dried undcx- reduced pressurc. The
resulting crude crystals wer--e .,uspend.ed in a mz.xed
so7 uLion of 20 mL of water and 20 mL o.f, methano7.. Then,
about 25 n1L of a aturated sodium h.ydrogoncar.=bonate
soluLion was added thereto, ar:d the mixl.ure was sonicated
for 5 minutes us.irYg an ult.r..aõonic. wa!~lYing machine.
Precipitates were ,f.i.lLered and recovcr<.:d from the
resulting mixture, su.f.ficiently washed w.ith watcr, and
dried under reduced p.re5sure, to obtain 2.41 g
(corre5ponding to 8.32 mmol) of 6-bromo-2-(4'-
hydroxyphenyl ) imida zo [ x, 2-a] py:r-idine ( f~'ig . 2, Step 2).
[0060)
The NMR measu.rement resul.i;s of the resulting 6-
b.r-orno^2- (4' -hydroxypher:yl) imidazo [1, 2-a] pyridi.ne
(internal staridard: dirnethylsulfoxicle) jre snown below_
[0061]
NMF, apparatus ernp'loycd: JNM-ECF'-500 (manufactured by
Japan E1.ec:tron Optics ):-,aborato .r. y Co., Ltd. ( Ji,?UL) )
LH-NMR (solvent: dimethy].sultoxide-dE;, resonancc
frequency: 500 MI17.) : 0 9.54 (br. s, 1H), 8_ 83-8. 81.. (in,
1.13) , 9.17 (s, 11I) , 7. %9-7 ."]4 (m, 21-1) ,!. 51 (d, J-- 9.6 I-lz,
11-I) , 7.30 (dd, J- 9.6, :L.8 Hz, 111) , 6.86-6.81 (m, 2H) .
[0062]
03/25/09 WED 16:08 FAX 416 362 0823 RIDOUT & MAYBEE 2031
CA 02657312 2008-10-28
- ~:8 -
13C-NMH (solvent: dimcthylsul.f_oxide-d6, resonance
frequency: 125 MHz) : 6 158_15, 146.40, 143.79, 127.82,
127.67, 127.14, 125.01, 117.87, 116.1.5, 108.60, 106.05.
[0063]
L',xample I-5: Synthcsis of 2.- (4' -hydroxypheny.l ) 6-
iodoirriidazo [1, 2-aJpyridine
[00641
50 znL of ethyl acetate was added to 28.1'7 q
( co.r. respon.ding to 7.26 aYimol) of cupr_ i.c, bromide to obtain a
suspension, to which a solution of 8.18 g(corresponding
to 60.0 m.mo1) of 4'=-hydroxyacetophcnone in a mixed
solution of 50 mL of cthyl acctaLe and 50 mL of
chior_oform was added. 'Then, t.hc resulti..ng mixture was
reL'luxcd_ After 5 hours, Lhe reacl_ion sol.uLion was
cool,ed down. t.o room temperat.ure and filtercd. The
resulti.ng filtr_3Ce was concent.ratect under reduced
pressure. '1'he res.i.due was dissolvcd in ethyl acetate and
subjecLed to d.ecoloring operation with addition of active
charcoal. Thcn, the r_c.sultinq 5olution was fi.ltered and
concentrated. The resulLing crude product was purif.~ ed
by flash silica gel column chromat_c>graphy (elution
solvent: chloroform/methanol - 20/1), and rec.r.ystal].i.zed
fron ethyl.. acetate/petrol_euin ether, to obtain 7.25 g
(c:o.r.r.espondi.ng to 33.7 mmo].) of 2-brosno-4' -w
hydroxyacet.ophenonc (Fig. 3, Step 1).
[00651
44.1 rng (corresponding to 2.0 mmol) of 2-bromo-4' -
hydroxyacetophcnone and 44'3 mg (corresponding to 2_0
03/25/09 WED 16:08 FAX 416 362 0823 RIDOUT & MAYBEE 032
CA 02657312 2008-10-28
- 29 -
mrnol) of 2-amino-5-iodopyridine were dis5olved in 15 mL
of riretonitrile. The resulting solut:ion was refluxed in
an oil bath at 110 C fo.,r. 5 h.ours. Aftcr t.he completion
o,f. 'the rcac_tion, t,he reaction soluti,ori was cooled down to
room tempe.r.ature, and precipitaLes were filtered and
recovered. 'I'Iie preci.piLates were washed with
acetonitrile anci dried ur.ider reduced pres5ure. Tr1r
rce>ulting crude cry5tals wcrP suspcnded in a rnixed
solution of 10 citL of war_er and .lU mL of rriethanol. Thcn,
about 10 mL of a saturated sodium hydroqencarbonate
solul:ion was added thereto, and the mixture was son.i.cr3ted
for 5 minutes using an ultrasonic washing machirle.
PrecipiLates were filt.cf.,red -and re:covercd from the
resulting mixtu.r.e, suffici.eritiy wa5hed with water, and
ciried under reduced p.r.-essurc, to obtain 526 nig
(corresponding tc) 1.56 mmoi) of 2=- ( 4' -h,ydroxyphenyl) -6-
iod.oimidazo(1, 2-a)pyrictine (t-'ig. 3, Step 2),
[0066]
'I'he NMR measuremcnt resul.ts of th.e resulting 2- (4' -
hydroxyphenyl) -FS-iodoimi.daz.o [1, 2-a]pyridine (a..nternal.
stariddrd: dirnethylsulfoxidc:) are shown be.l ow.
,0067}
NMR apparatus ernploycd JNM-ECP -500 (manufactured by
Japan Elcctr-on Opti.(.s Laborhtory Co., Ltd. (JEOL))
1H-NMR (so7,vent: di.meLhylsulfoxide-d6, resonancc
frequc_ncy: 500 MHz) c 5 3.86-8.8~ (m, 1H) , 8. 1,1 (s, 1H) ,
7 . "lt3-7 . l4 (m, 2H), 7 . 4U-'7 .:3.5 (m, 2II) , 6. 86-6. 82 (m., 2H).
[0068]
03/25/09 WED 16:08 FAX 416 362 0823 RIDOUT & MAYBEE ~ 033
CA 02657312 2008-10-28
:3U
13C:-NMR (solvent: dimethyl..sulfoxide-d6, resonance
rrcquetlcy: 125 MIIz) - 5 158.08, 145.87, 143.87, 132.48,
7.31.72, 127.67, 124.99, 118.14, 116.14, 108.02, 75.85.
[0069j
Example I-6: Synthesis of 2-- (4' -hyd.r,axyphenyZ) -6-
iocioimidazo [:1. , 2-a] py. a.mictinc:
[00v0]
50 mL of ethyl acetate wa !~, addcd. t.o 28. 7.7 g
(corresponding to 126 mmol) of cupric bromide to cbtai.n a
suspension, to which a solution of 8.18 g(corre.-,ponding
to 60.0 mmol) of 4'-hydroxyaceLophcnone in a mixed
solution o.f. 50 mL of eLhyl aceLate and 50 m.T,,, of
chloroform was a(ided. Thei1, the resulta,ng mixture was
rcf..7_uxed. After 5 hours, the reaction mixLUre was cooled
dcwn to room temperature and filtercd. The resulti.ng
filLrate was concentrated urider reduced pressur.e. The
residue was disso_l..ved in ethyl acet:.ate and subjccted to
decoloring operation wiLh add?i:ien of activc charcoa.l.
'1hen, the resultinq soluticn was fiJ.tc.red and
cc5ncent.rated. 'Phe re.;u.lLing crude product was purif.i.ed
by flash silic.a gcl column chromatography (elution
solvent: chloroform/methanol = 20/1) , arid rec,rystalJ.i_zed
fr.om ethyl.. acetZte /petro.].eum ether, to obLain 7.25 q
(corresponding to 33. 7 mm.o-_) of 2- bromo-4' -
hyd.roYyacctophenone (Fig. 4, Stcp 1).
[0U71]
646 mg (corresponding to 3.0 mmol) of 2-bromo-9'-
hydr,oxyacetophcnone and 668 mg (corresponding 'to 3.0
03/25/09 WED 16:08 FAX 416 362 0823 RIDOUT & MAYBEE 034
CA 02657312 2008-10-28
- 31 -
mmol) of 2-amino-5-a.odopyrimidine wcre dissolved ir.i 20 mL
of acetoriitrilc. The resulting solution was refluxed irr
an oil ba.tli at 1:1.0 C for 8 hours. T=ltter the completion
of the reaction, tl-ie reaction solution was cooled dowr t.o
room t:emperature, and precipiLates were filtered and
recover.ed. The precipitates wcr..p washed with
acctonitril,.e and dried und.ei: reduced pressure. The
resulting crude crysta.~;r were suspended irs a mixed
so.ilation of 10 m7., of watc,r., and 10 mL of methanol. Then,
about 15 mr,, of a sat.urated ,.,odium hydrogenc.:arbonate
solution was added thereto, arrd the mixture was sonicated
for 3 nLinutcs using an ultrasorric washirig m.achine.
Precipitat'_es wcre filtered and r.er.overcd from thQ
r=esult.i_ng mix-i_.ure, sufficiently washeci with water, and
dx-ied under reduced pres5ure, to obtain.'137 mg
(corresponding to 2.19 mmol) of 2-(4'-hydroxypher:yl)-6W
iodoimida2.oj1,2-aJZ~yrimidi.ne (Fig. 4, Step 2)
[0072]
The NMR mcasuremcnt. resul.t..:~ of the resulting 2- (4' -
hydr,oxyphanyl) -6-i.odoimidaz.o [1, 2-a] pyrimidine (internal
st:andard: dimcthylfo.rmamide) are shown below.
[0073]
NMR appar=atus c;.niploycd: JNM-I'C;P-500 (manufactured by
Japan Elcctron Optics LaboraLory C:o., Ltci. (JEOL) )
1H-NMR (solvent: di.methylforrnami(jce-d/, r_esonancc:
frequency: 500 MHz): n 9.80 (br. s, 1H), 9.35 (d, J = 2_31
Hz, 1 H ) , 8.60 ( d , J - 2. 3 Hz, 1 H ) , 8 _ 2 3 ( s , 1 H ) , 7 _ 9 ~ -
7 . 9 0 (m, 2 K ) , 6. 98-6. 94 (rn, 2 .[t)
03/25/09 WED 16:09 FAX 416 362 0823 RIDOUT & MAYBEE Z035
CA 02657312 2008-10-28
- 32 -
[C07~]
13C-NMR (solvent: ditnethyliormami.de-d7, resonance
frequency: 125 MHz): b 158.87, 154.00, 2.47.18, 1.46.77,
139.07, 127_68, 124.50, 115.85, 106.10, 73.46.
[UU75]
Example I-- 7: S nthesis of 6-- (3' - fluoropro or, ).,... ~2- (4'
õ`L
hydroxyphenyl)imidazo[1,2-a]pyridin.e
[0 0 7 6
~
31.11 g(corresporrding -to 178.88 mmo1.) of 2-bromo-3w-
hydroxypyridine was dissol.vard in 95.8 mL of
diinethylsulfoxide, an.d 89.9 mL (cor.respond.i.ng to 89.9
mmol.) of I mcl/L sodium methoxide-metha.nol so1..t,iLion was
added [_hereto. Thcn, the reaction soluti,.on was heatcd to
90 C to disLill c7ff inetlianol. After the rcaC_Cion
solution was cooled down to 5 C or lower, 29.2 g
(corresponding to 205.62 mmol) o,f, rnethyl. iodid.c: was added,
and then stirred at. r-oom temperature for 1`1 hours. A:ELer
t.rs.e completion of the reacti..on, the reaction so.l.,uLion was
poured into ice water and ext.r.acted twice wi.~.:h chlaroforrn.
The combined chloroform layer was washed with ]..
sodiu.m hydr,oxide solution, wash.e:d twice with a saturated
;,odium chloride solution, and dried over. anhydrous sodium
sulfatc_. Af_tPr thc 5olvent was d.i_sLillccl off under
re:duced pressure, 20.74 g(corresponciing Lo 11Ø31 mmol)
of 2-bromo-3-methoxyp,yr,idinc was obtained (Fig. 5, Step
1)
[0077]
83 tnL of conc_ sulfuric ac.i.d was coolcd down to -5cC,
03/25/09 WED 16:09 FAX 416 362 0823 RIDOLiT & MAYBEE 10036
CA 02657312 2008-10-28
- ~3 -
and 83 mL of 90 1.- nitr,ir_ acid was carcfully added thc,reto.
Subsequc.ntly, 20.69 g(correspondirig to 110.04 mmol) of
2-hromo-3-rnethoxypyridine was ca.r..efuil,y added thGreto.
After the rear_tion rnixture was sti:r.-red in an icc bath for
5 rainutes, thc rnixture was sti..rred at r-ooni tPrnperature
for- 10 minutes, and then was heated to 55 C and further
stirred for an hour. Aft.cr., the rcac:l:ion solution. was
cooled to room r.empera=t.ure, the reaction solution was
poured 1i.t_t_le by little i..nt..o crushed ice to gcnerate
prcc.ipitate:s. The precipi.taLes were filte.red and washcd
with water, arid then dried over pho5phorous pentoxide
urider reduced pr.pssurc, to obtain 17.41 g(corresponding
to 74."/1 mmol.) of 2-bromo-3=-riiethoxy-6-nit.r.opyridi.ne (Fig.
5, Step 2).
1,5 [0078]
17.36 g(corrPspondi.ng to 74.50 mmol) of 2--bromo-3-
mcthoxy-6-nitropyridine was dits5olved in 520 mL of
ethanol, and 1 1. 63 g( 50's wet ) of 1.0 ?; pal lactium-carbon
was add.ed thereto under argon aLitlosphere. To thc mixture,
38.4 mL of hydrazine monohy(irate was added dropwise.
After the reaction mir,ture was refluxed for 45 minutes,
the reacti on solution was cooled down tc) room Lempc .r. 3ture .
Then, after palladium-carbon was lilter,e:d off, ehe
residue was washcd with ettiariol, arid tl-ie washi,ngs wc.r..e
combined with. the fi_l,trate. The corrrbined solution was
concPntrated under reduc.Pd pressure. Then, 4U2 mI., of
water and 38 mL of conc- aqucous ammonia were added to
r,h.c conccnt:rate, arid the resulting mixturc was Extracted
03/25/09 WED 16:09 FAX 416 362 0823 RIDOUT & MAYBEE 10037
CA 02657312 2008-10-28
- 34 -
c.1.ght times with chloroforiYt. The c:ombincd c~hloroform
layer was dried over anhydrous sodium sulfate and
concentrated undc.r reduced pressu.r.e= The x=esulting crud.e
product was d.istillcd under r.educed pressure Lo obtain
8.14 g (corresponding to 65.57 minol) of 2-ami..rro-S-
methoxypyridi,.ne (Fig. 5, Step 3)
[0079]
13.50 g(corr.espond.i.ng to 59.66 mm.ol) of 4`-
benzoyloxyacetophcnone was dissolved in 1100 ml of
methanol, and 34.52 g(corr.e!spondarrg to 71.59 m.mol) of
tet.ra-n-butylamm.onium tribromide was added thereto. The
rnixtu.re was stirreci overni.ght at room tertrperature, anc:l
wa; distilled off under reduced pres5ure to remove the
solvent. The residue was dissolved in ethyl ac`etatc and
15: washed. Lwicc with water and.then washed with an aqueous
saturated sodium chloride solution_ Aftcr the cthyl
acetatc~ laycr was dried over anhydrous sodium si.rlfatc and
conc_entrated under redue,ed pressure, -Lhe resulting r.rudc
product was purified by silica gel columri chromaL-oqraphy
(c7.iation solvcnt: hexane/methylene chloride = 1/;1..) , to
obtain 13.38 g (corresponding to 43.84 crunol) of 4' -
benzoyloxy-2--bromoar.etoph.er..ene (Fig. 5, Step 4) [O0a0]
13.33 g(corresponding tc) 43.68 nrnol) of 4' -
benzoyloxy--2-bromoacetophenonc and 5.67 g(corresponding
to 45.67 mmo7.) of 2-ataino-5-meth.oxypyridine were
dissolved in 481 mZ., of ethiznol. '1'he resultin.g solution
was refluxed for 2 hours. Y~fter the reaction solution
03/25/09 WED 16:10 FAX 416 362 0823 RIDOLtT & MAYBEE Z038
CA 02657312 2008-10-28
]J
wa.s cooled, 6.64 g(corresponding to 79.09 mmol ) of
sodium hydrogencarboriate was added t.hereto. 'r'he
resulting reaction mixture was furthcr refluxed fcr 4
hours. Aft-er the e.ornplet.ion of the reaction, the 5olvent
was coricent.rateci under reduced pressure. The resu].t:ing
residue was dissolved iri chlorof.orm and uhen washed with
watcr.. After the c.hloroform layer was dried over
anhydrous sodium sulfate, the solvent was disti].led off.
The resul.,t=ing c.r_ude product was purified by silica gel
column ch.r..-ornatography (elution solvcnt-_: chlos=oform./ethyl
acetate = 20/1), to obtain 10.20 g(correspond.ing to
30 . 8'l mmol ) of 2- (9' -k7enzoy].oxyphcnyl) -6-
methoxyimidazo[1,2-a]pyrieline (Fig. 5, St-ep 5).
[0081]
] 5 4.90 g (corresponding to 1.4.83 mrno"l) of 2- (4' -
benzoyloxypheny.7.. ) -6-mcthoxy:im;.dazo [ .1 , 2-a] pyridinc Chat
was sulficient'ly ciried to rrmove moisture was dissolved
in 245 mL of chloroforrn and cooled down to -"15 C. To
this solution, a solution of 12.62 m.L (corresponding to
133.48 mrno.l. ) of bo.ron tribromide in 134 mL of
dichloromethane was added d.r,opwise. After the
tcmperature of t_t-se rc~sulting solution was raiscd 'to room
temperature, the solution was stirred fo.r, 1-/ hours.
After the coltipicti.ori of the, reaction, Che .r.c:actiorl
solution was cooled with ice and supplcmented with 668 m7,
of mPLhanol, and. furthcr stirred at room temperature for
3 hours. i'he rcaction mixture was then concentrated
under reduced pr.es=surc. Thc resul..t.ing crude product was
03/25/09 WED 16:10 FAX 416 362 0823 RIDOUT & MAYBEE 039
CA 02657312 2008-10-28
36 -
supplementcd with 290 ml, of chloroform and 29 m't.., of
methanol to obtain slurry, and then prctcipitatcs were
filterc:d and recover-ed. The precipitatcs recovered were
washed with ch.loroform ar:d tt-ien d.ried undcr reduced
pressurc, Lo obtain 3.00 g(c,orresponding to 13.28 mmol)
of 2--(4'-hydroxyphen.yl)-6-hydroxyimidazo[1,2-a]pyridine
(Fig. 5, Step 6).
[0082j
560 mg (corre5ponding ta 2.~ 8 .m.mol ) o.f. 2- ( 4' -
hydroxyphenyl)-6-hyd.r.oxyimidayo[1,2-alpyridi.n.e was
dissolvcd in 21 mL of N,N--di.methylformami.de, and 1.37 g
(corrc-.Spondi_ra.g to 9.90 mmol) of potassium carbonatt; and
349 mg (corresponding to 2./18 m:rnol) o.f. 1-bromo-3-
fluoropropa.rle were added thereto. The salution was
sf'=irrccl at room temperature for 24 hours. The reaction
solution was concentrated u.inder r_cduced pressurt-, and
th n supplemented with 1.0 mL of chloroform and 10 mL of
methanol. to obtain s)..urry. 'T'he slurry was filtered and
fi.l..tratc was coricent.rated. 't'lie resultinq crude product
was purified by silica gel column chromatography (elution
5olvent.: ch].orofor-m./methanol = 20/1), ta obtain 151 mg
( (7orresponding to 0.527 pmcl ) of 6- ( 3' -:['luoropropoxy) -2-
(4' --hyd.r_oxyphenyl) imi.dac,o [1., 2-a]py.r.idine (Fig. 5, St.cp
L0083]
The NMR measurcmen-t rcsult,s of the resulting 6- ( 3' -
fluoropropoxy) --2- (4' -)-iydroxyphenyl ) imidazo [1, 2-ajpyr:idinc
(internal standard: diineth.ylsulfoxide) are shown below.
L0084]
03/25/09 WED 16:10 FAX 416 362 0823 RIDOUT & MAYBEE 040
CA 02657312 2008-10-28
- 37 -
NMR apparat_u5 employed: JN[K--GSX-270 (manufactured by
Japan Electron. Optics Laboratory C:o. , Ltd. (JF;O.L) )
1H-NMR (solvent: dimcthylsu1Foxidc-d6; resonance
frequ.ency: 270 M~Iz) .. a 9.52 (s, 1H) , 8.22 (d, J = 2.2 I-lz,
1H), 8.08 (s, 1H), 7.75-7.65 (m, 21I), 7.44 (d, J 9.6 Hz,
1 1I ) , 6 . 99 (dd, J = 9 . 6 , 2 _ 2 xz, 1 H ) , 6. 85--6. 75 (m, 2t1) ,
d. 62 (dt, `Jxr - 47.0 Hz, J= 6.0 Hz, 2II) , 4.05 (t, J=
6. 0 Hz, 2H) , 2. 13 (ciquint, 3J}.rr: - 25. 9 Hz, J = 6. 0 11 7õ 2H)
[0085]
Example 1-8: Syrithesis of 6-bromo-2- ( 4' -
~.roxyphen~rl ) imidazo f 1, 2-a] pyri,midine
[0086]
50 rnh of ethyl acetatc was addcd to 28.1.7
(corresponding to 1.26 mmol) ol cupric bromide Lo obtain a
suspension, to which a solution of 8.1.8 g(correspon.d.ing
tc) 60.0 mrnol ) of 4' -hyd .r.oxyacetophenonc i, n a mixed
solution of 50 mL of ethyl ac.-etate arid 50 .mL, or
chloroform was adciecl. TherL, the .r_esulting mixture was
ref_]uxed. nLter 5 hours, the reaction mixl;ure was coolcd
down to room tempcr_at:_ure and riltc.red. The resulting
filtrate was concentrated undcr reduced pressur-e. The
reriduc was dissolved in ethyl ~-jcetatc and svbjected to
decoloring operation wit.h radditiori of active charcoal _
Then, the resultinq solution was filtered and
'S corrcentrated. '1'1'le resul.t=ing crude product wa.:i purified
by flash silica gel column chromatography (eluLion
solvent._ chloroform/nic:thanol = 20/1), and rec_rysta.llized.
from ethy.l. acetate/petroleurn etrler, to obtai.n 7.25 g
03/25/09 WED 16:11 FAX 416 362 0823 RIDOUT & MAYBEE 041
CA 02657312 2008-10-28
- 3B -
(co.rrespond.i..ng to 33.7 mm.ol) of 2-hromo-4'-
hydroxyacetophenone (Fig. 6, Step 1).
[00a71
'748 mg (corresponding to 3.5 mmol) of 2-bromo-4'-
hydror.yacetophc.none arici 605 .ng (corresponding to 3.5
mmcl) of 2-amino-5-br_omopyr-i.mi..dine were dissolved in 30
rnL of acetonitrile. The r-cslzlting soli~t.ion was refluxed
i..n ari oil bath at 110 C for 5 hours. After the
completion of i;he reaction, the reaction solution was
cooled down to room tcmpe.r,ature, and precipitates were
filtered and.recpvered. The precipitates were washed
w.ith acetonitrile and dri..cci urider reduced pressure. The
resul-ti,.ng crude crystals wcre su5pended in a mi..xed
solution of 10 rnL of wate.r, ancl 15 mL of mothanol _ 'I'hen,
about 10 mT., of a saturated sodi.um hydrogencarbonate
soluti.ort was added thcrei:p, and the mirtUre was soni.caLed
for 5 minutes using an ultra.son.ic. washing machine.
Precipi.tates were fiJ.te:r.ed and recovered from the
resulta-ng rnix.ture, suffi c_ieritly washcd with water, and
dried undCr reduced pressure. The resulting crude
product was rccrysl.allized from N,N-dime.thylfo.r.mamide, to
obtain 289 mg (co..r..r.esponding to 0.997 mrnol) of 6-br'orno-2-
(41-hydroxyphenyl)imidazo[I.,.2-a]pyrimid.ine (Fig. 6, StPp
2) -
[00881
'I'he NMR measu.rem.ezlC results of l:he result i ng 6-
b.r- omo- 2- ( 4' -hyd.roxyphenyl ) im..i..dazv [ 1, 2-a ) pyrimidit7e
(interna1 standard: dimethylsulfoxide) are shown below.
03/25/09 WED 16:11 FAX 416 362 0823 RIDOUT & MAYBEE 042
CA 02657312 2008-10-28
- =,9 -
[0089j
NMIt apparatus employed: JNM-ECP-500 (manufactured by
Japan Elcctron Opt.iOs T.AabUratory Co., LLd. (JEOL))
I 1 I-NNiI? (5olvent: dirrLethylsulfoxide-d6, resona.nce
frequency: 500 MHz) : ~S 9.56 (br. s, 1H), 9.21 (d, J = 2.5
Hz, 1H), 8.46 (d, J= 2.5 f1z, 1.H), 8.09 (s, 1H), 7.79-
7-75 (rn, 2H) , 6.83-6.79 (m, 21T)-
[00901
13C-NMR (solvcnt: d,i..rnethylsulFoxide-d6, resona.nce.
frcgu.en.cy: 12S MHz): 5 158.63, 1.49.99, 147.68, 146.88,
134.78, 127.93, 124.52õ 116.23, 106.83, 103.94.
[009'.1,1
Examp].c I_ 9: Synthesis o.f,. 6--fluoio-2- (4' -
hydroxyphenyl ) imidazo [ 1, 2-a lpvz'idine
[0092]
70 ml., of e'thyl acetate was added to 40.0 g
(c.orresponding to 179 nunol) of cupric bromide -t:o obtain a.
suspension, Lo which a solurion of .1.1._6 g (corresponding
to 85.3 minol) of 4' -hyd.r_axyacetophenone in a mixed
solution of. 70 n1L of ethyl acetate and 70 mL of
chloroform was ad.c3ed. Then, the resulting inixture was
refluxcd. After 5.5 hours, the rcacti-on mixture wa.,
coo.l-ed down to roorn temporature and filtered. The
resultir:g filtrate was concentr_ated under reduc;ed
pressure= The residue was dissolved in ethyl. ac.etate and
subjected Lo decolo.r, i.ng operatiori wiLh addition of active
charcoal. Then, the resulting soluLion was filtered and
concentrated. The resul-ting crud.c product wa5 purified
03/25/09 WED 16:11 FAX 416 362 0823 RIDOUT & MAYBEE U043
CA 02657312 2008-10-28
by fl.ash si..l.,a,ca gc1 column chromatography (elution
solvent: chloroform/methanol = 20/1), and recrystallized
fr.om etllyl aCP.i.at'-e/petrOl(;urn Ot;hOr, l;O c)]:)tain 10.2 g
(corresponding to 47.3 mmol) of 2-bromo-4'-
hyd.r.oxyaceLophenone (Fig. 7, Step 1).
[00931
439 m.q (corresponding t.o 2.0 rcunol) of 2-bromo-4' -
hydroxyacetophenone and 224 mg (corresponding to 2.0
mmol) of 2-arnino-5-fluoropyridine were dissolved in 20 mL
of acetonitrile. The .r.esult.i..ng 5olution was refluxed i,.n
an oil bath at 17.0 C for 5 hours. Aftcr the completion
of Lhe reaction, the rQacLicn solution wa.:, cocled down to
room temperature, and p.recipitaLes were filtered and
recovered. The prccipi.t.atc:s were washed with
:1.5 acetonitrile and dried undcr .r_educed pressurc. '1'he
resulting crude crystal:, were suspcnclecl in a mixcd
solution of 8 rnL of water and 8 rnL of inethanol. Then,
about 10 rnL of a sat.u.rh-t:ed sodium hydrogencarbonate
soJ.ut_ion was added thereto, and thc_ mixture was sonicated
for 5 rninutes using 3ri ultraeoni_c washing machi.ne.
Precipitates were filtered cind recovcrcd :Fr=orn the
resu7,ting mixture, sufficie:ztly washed with water, and
dried under reduced pressu.r.~,, r-o obtain 302 mg
(c;orrespond.i.ng to 1.32 mmo.l..) of 6-fluoro-2-- (4' -
hyclrUxypheny]. ).i,mida2o [1, 2-aJpyridine (Fig. 7, Step 2).
[00941
The NMR m.casurement r.csul.t., of the resulting 6-
iluoro-2~-(4'-hyd.r..oxyphenyl)imidazo[1,2-a]pyr.idine
03/25/09 WED 16:12 FAX 416 362 0823 RIDOUT & MAYBEE ~ 044
CA 02657312 2008-10-28
- 4:1 -
(internal staridard: dirneth.ylsulfoxi.de) are shown below.
[0095]
NMR apparaLus employed.: JNM-ECP-,500 (manufactured by
Japan Elec,f_.r.on Optics Laboratory Co., Ltd. (JEOL) )
1 H-NMR (so.l.vent: dirnethylsul:Loxide-d6, resoriancc
frPque:n.cy: 500 MHz) : 6 9. 45 (1-)r, s, 1H) , 8. 65 (ddct, 3JrIF =
4_ 6 I=lz, J = 2. 5, 0_ 7 Hz, 1H) , 8. 16-8 . 15 (in, 7. 75-
7. 69 (m, 2H), 7.56-'7. 51 (m., 1H) ,-7.2=3 (ddd, 'J,,r = 8.4 xz.,
J- 9. 9, 2. 5 Hz, 1H) , 6.82-6. "16 (in, 2 1-1)
[0095]
~` jC-NMR ( solvent : (ii..met.hylsul.foxide-=d6, resonance
frequency: 125 MF-lz) : 6 157.8:2, 1.52. 81. (d, '-Jc~- = 232.3 Hz) ,
1 4 6 . 5 8 , 142 - 92, 127 . 3j, 124 . 99, 1.17 . 19 (d, 3Jc.,E, = 9. 6 11 z)
,
1.1,6_40 (d, ;lJr,E - 25.9 Hz), 115_89, 1..13.66 (cl, `J~t = 41.8
Hz), 109.48.
[0097]
19F-NMR (solvent: dirnet_hylsu.l,.f-oxide-d6, resonance
freque-nC,y: 470 c5 141.9:3 (br. s).
[0098].
Exarnple I--10: Synt3zcs.i.s of 2-- (4' -hydroxyphenyl) -6-
nitroimidazo [ 1, 2-a] pyriclir~e
[0099]
70 mL of ethyl acetate w,3;~ added t.o 40.0 g
(corresponcLirig Lo 179 rnmo-1.) of cupric bromide to obtain a
suspension, to which a;;c.~].ut.i on. of 11.6 g(corresponding
to 85.3 zYanol) of 4'-hyd.roxyacctophcnone in a mixed
soluLion of 70 rnL of ethyl acctatc a.nd 70 m7., of
chloroform was added. Then, the resulting mixture was
03/25/09 WED 16:12 FAX 416 362 0823 RII)OUT & MAYBEE 045
CA 02657312 2008-10-28
- 42 -
refluxed. Af..tcr 5.5 hours, Lhe reaCtion mixture was
cooled down to rooin t;c;:m.pe.rat'are and filtered. The
resu].L-ing .fi]=t,r.atc was conce:ztrated undPr reduced
pressure. The residue was di..ssolvcd in ethyl acetate and
suh]e(:'LP,d Lo decoJ.or.i..n.g opcration with addition of active
cha:rc:oal. the resulting solution was filtered and
concentrated. The resulting crude product was puritied
by flash silica gel column chrotriaLogr-aphy (elu.t_ion
solvcnt: chloroform/methanol - 20/1), and recrystallizcd
trom ethyl acetate/petroleum ether, to obtain 10.2 g
(correspondi,n.g to 47.3 mm.o1,.) of 2=-Y)r_orncy-4' -
hydroxyacetophenone (Fig. 8, Step :l).
[0100]
432 mg (cc)rresporrding tc 2.0 ntrnol} of 2-bromo-4'-
hydroxyacetophenonc and 279 mg (cnr...esponding to 2.0
mmol) of 2 =amino-5-nitropyridine wcrc dissolved in 20 mL
ol acetonitrile. The ..result.iriy solution was ref_.luxed in
an oa...l bath at 110 C for 6 hou.r.s. nfLer the complotion
of the reaction, the reaction solution was cooled down to
room -ternperature, and precipitat.cs were Liltered and
recovered. The p.r..ec=ipiLaLeS were washed with
aceton.i.t-.:rile and dried under reduced pr_essure. The
resulting crudc c.rystal.;s were suspended in a mixe,d
soluLion of 8 mL of water and 8 tnL of methanol.. 'I'heri,
a.bout 8 mL of a;aturat.ed soditim hydrogenc.arbonat.c
solution was added thcz-cto, and Lhe mixture was sonicated
for 3 miriuLes using an ult.r.usonic; washing machin.c.
Precipitatcs were filtered and recover=ed from the
03/25/09 WED 16:13 FAX 416 362 0823 RIDOUT & MAYBEE 002
CA 02657312 2008-10-28
- 43 -
resultinq mixture, sufficiently washcd wi.,-l;h water, and
cl.ried under rcdu.ced pressure, to obtain. 7.48 mg
(corresponding to 0.580 mmal ) of 2- (4' -hydr.. oxyphenyl )-6-
riitioimidazo[1.,2-a]pyridinc (Fig. 8, Step 2).
[ 010 .]. ]
The NMR mea6urement results of the result;.ng 2-(4'-
hydroxyphenyl) -6-n.it.roimidazo [1, 2-a J pyriciine (internal
standard: dirnettiylsulfoxi.de) are shown below.
[C107..]
NMR apparatus employed: JNM-ECP-50G (manufacturcd by
Japan Elect.r..ori Optics Laboratory Co. , Lt_d. (JEO7,,) )
1H-NMR (solvent: di..rrtethylsulfo%:ide-d6, resonance
frequency: 500 MHz) : 0 9. /n-9.72 (m, 1H), 9.59 (br. .s,
1H) , 8.39 (s, 1I-]) , 7. 8"7 (dd, ,.i = 9.9, 7. 3 Hz, 7.It),-/ .79-
7.74 (m, 21-T) , 7. 65-7. 67. (rn, 'lIl) , 6. 8,1-6.80 (m, 2H) =
[ 01031
"C-NMR (solvcnt: dimethylsu.lfoxiõdc_-ci6, resonance
f.r.equency: 125 MIIz) : 0 158.47, 148.51., 1~5.25, 136.63,
127.93, 127.81, 124.06, 118.92, 116.09, 1.15.92, 7..10.37.
[n104]
Example II-1 : Synthcs3,s of 6-tr, ~butylstann_y1-2- (4' -
hydroxyphenyl)imidazo(1.,2-a]pyri.midine
[U105]
50 mi, of ethyl ac.etat.e was addecl to 28 _ 1'7 g
(corresponding to 1.26 mmol) ol cupri..c: bromide to obtain a
suspcnsion, to which a solution of 0.18 g(corrG;)pondin.g
to 60,C) rnrnol ) of n' -hydroxyacotcphenone in a mixed
solution of 50 mL of cthyl acetaLe and 50 mL o.f..
03/25/09 WED 16:14 FAX 416 362 0823 RIDOUT & MAYBEE 003
CA 02657312 2008-10-28
- 44 -
chloro:Eor_'tn was added. Then, thC resulti.ng rn:ixf:_ur'e was
ref:lux.ed. After 5 hours, I.he reaction solution was
cooled dowrr to room temperature and filtered. The
resulting filtrat.e was corice.:rLrated under r_cduced
pressure. The residu.c was di_ssolved in ethy]. acetaLe arrd
subjectcd. to decoloring operation wiLh acidition of ac-tive
cha.rcoal. Then, the ,r.esulLing solution was filtered and
c.oncentrated. '1'he resulting crude product was purified
by fla5h silica gc1. column chromatography (elution
solvent: c:hloroform/mcthan.ol = 20/1), and recrystaJ.J.ized
from ethyl acctaee/petroleum ether, to obtairi 7.25 q
(corrc:>ponciing to 33.7 rcmol) of 2-bromo-4' -
hydroxyacctophenone (ri.g_ 10, Step 1)
[01061
'748 mg (corresponding to 3.48 mrrtol) of 2-hromo-4' -
hydroxyacetophcnorle and 605 mg (corresponding to 3.48
mrnol) of 5=-bromo-2-aminopyri.mi.di.ne were d,i.ssolved i_n 30
mL of acetonitrile. Th.e resulting solution was refluxed
in an oil bath at 110 C for 5 hours. After the
c:omplet:Lon of the reaction, the reacti.ori solutiori was
cooled down to room tem.perature, arrd precz,pitates were
iiitered and recovered. The precipitates wc.re washed
wiLh acctoriitrilc arici dried under reduced pressu .r. e. The
resulting crude crystals we__c 5uspended in a mir.cd
2S soltrtion of 1.0 mL of wat:er and. 15 mL of meLhanol. '1'hen,
about 10 mL of, a saturated sodium hydrogcncarbonatc.
solutlOrl was added thereto, and the rTtixtLlr~3 was son.ic;ated
for 5 minutes usi.ng an ult.rasonic washing ma.chine.
03/25/09 WED 16:14 FAX 416 362 0823 RII)OLiT & MAYBEE 0004
CA 02657312 2008-10-28
PrF~:ipiLates were fi"ltere.d and recovcrcd from thc.
resultinc.l m.i..xLure, !~ufLicienLly washed with water, and
dried under reduced pressure. The obtained solid was
:r.ec-rystallized from N,N-dimet]~.yJ.forrt:arT~ide, to obtain 289
S mg (c.or.r.espo.riding to 1.00 mmol) of 6-bromo-2-(4'-
hydrouyphenyl)imi,dazo[1,2-a]pyrimidine (I'i.g. 10, SCep 2)).
[0107]
75.4 rng (corresponding to 0.260 mmol) of 6-bromo-2-
(4' -hydroxyphcnyJ. ) 1Irl1d`dzCU [l, 2-a] pyrim.i..dirie was dissolved
1,0 in 10.0 m1r of dioxane, and 2.0 :riL of triethy.l.amine was
acidcd. thereto. Thcn, 0.20 mL (correspon(iing to 0.39
mmol) of bis(tributylt.in) rircd 20.1 mg (at a catalytic
amount) of tct.rakis-triphenyl,pY7osphine palladium were
added thereto. After the .r.c~~c.t:ion mixture was stirrcd at
15 90 C fo.r 10 hours, the soivent wos distillcd ofl under
rcduc:ed pressure. The residue was purified by flash
ci'lica gcl.. Column chromaLography (elution solvent:
hexanc/ethyl acetate = 1/1), to obtain 24_0 rng
(cor.r..csponcding to 0.048 mmol) of 6-tributylsLannyl-2-(4'-
20 hydroxyphenyl)im.idazo[1,2-a]pyrimidine (Fig. 10, Step 3).
[01.08]
T he NMR measu..rement resul,.=L of the x.-esultinq Fi-
t.ributylstannyl-2- (4' -hydroxyphcnyl) imidazo [1, 2-
a]py:r.imidinc (inLernal standaid: tet..r.amethylail..a.ne) are
25 shown below.
[0109]
NMlZ apparatus employed: JNM-ECP-500 (manufactured. by
Japan E].ecLron Opti.cs Laboratory Co., 1:.,t:d. (JEOI,) )
03/25/09 WED 16:14 FAX 416 362 0823 RII)Ot1T & MAYBEE 005
CA 02657312 2008-10-28
- 4:6 -
1H-NMR (solvent: chloroform-dl, re,sonancc f.r.equency: .500
MHz) :r5 8.41.. (s, 1H), 8.23 (s, 1H) , 7.80 (d., J- 8.7 Hz,
2H) , 7. 63 (s, 1H.) , G. 93 (d, J = 8 .7 Hz, 2I t) , 1.5-7= 1 . 51 (m,
6H) , 1.37-] .23 (nl, 6H) , 1.16-1 . 12 (m, 6H) , U. E38 (d, J
7. 3 H-. , 9 H)
[0110]
Exam~l.n I I-2: Synthesi..s of 6- tributylstann.y7..-2~- ( 4' -
hydroxypt,t:nyl ) irnidazo [ 1, 2.-a ]p.yrazine
[0'_11]
50 rnL of eth..y.l.. ac..eLate was added to 2a . 17 q
(corresponding to 126 mmol) o-f cupric bromide to obtain ~i
suspensi.on, to which a solution o.f. 6_.18 g (corresponding
t.o 60.0 nunol) of 4' -hydroxyac_etophenonc in a mixed
solution of 50 m7_, of ethyl acctate and 50 mL, of
chloroforrn was addcd. Then, the .r.c_sultind mixture was
refluxed. Alter 5 hours, the rcaction solutiorr was
cooJ..?d down t.o room temperat.ure and .f:i.ltered. The
resulting filt_rate wa5 roncentrate.d under reduced
pressure. The residue was di,.s;olved in eL'hy1 acetate and
;,ubjected l;o decolo.ririg operation with addition of ac:Live
chzarcoal. Tl,.eri, the .resillting solution was filtered cand
concentratcd. 'rhe resul..t.inc crudc pr'OdL1Ct was purified
by fla5h silica gel colum.n chromatog.r_aphy (c.l.u_ion
solvent: chloroform./mPLhano.L - 20/1), ar.Id recrystallized
from eLhyl acctate/pet.r.oleum ethcr., Lo obtain "7.25 g
(corresponding to 33./ mmol) of 2-bromo-4'-
hydroxyacetopherione ( Fig. 11, St.ep 1).
[0112]
03/25/09 WED 16:15 FAX 416 362 0823 RII)OUT & MAYBEE ~ 006
CA 02657312 2008-10-28
- !l7 -
9.66 g(cc>rresponding to 21.6 mmol) of 2-bromo-4'-
hydroxyac.etophenonc and 2.53 g(cor.r,e5ponding to 14.5
mmol) of 5-brcmo-2-aminopyrazine werc disso.lved in 100 rrtL
of accton.itril.e. '!'he resulC:ing solution was refluxed in
an oil bath, at 110 C for 3.5 hour-s. After the com}?.l.cti.on
of the reaction, the reaction solution was cooled down to
room temperature, and. precipitates were filtcred. ari.d
recovered. precipitates were washed with
acetonitrile and dried ulider reduc:ed pressure. 'I'he
resulting c.rudc crystal.s were suspendcd in a mixed
solution of 10 mL of water znd 10 niL of inethan.ol.. '1'l-ren,
about 20 mL of a 5a[:urated sodium hydrogencarbonate
solution was added thercto, and the mixture was sonicatcd
for. 10 minutes u.sirlg an uitrasoni.c: washing machine.
1.5 Precip.i.tat:es werc filtered and recovcr_ed from the
resulting mixture, sufficierlt.ly washed wi.tti water, and
dried under reduced pressure, to obtain 1.32 g
( corresponding to 4.55 trunol; of 6-bromo-2- ( 4' -
hydroxyphenyl) imidazo [a., 2--a] pyrazinc (Fig. 11, Step 2).
[07.1.3]
1.00 g(r.orrespondin.g t.o 3.,15 mmol.) of 6-br.omo-2-
(4'-riydroxyphenyl)imidazo[1,2-a]pyrazinc was dissol,ved in
50.0 m'.[, of dioxane, and 20.3 mI, c?r triethy,l..amine was
added ttlereto. Then, 4.5 mL (corresponding to 5.18 mmol)
of bis(t.r.ibutyltin.) and 239 mg (at: a catalytic amount) of
tct:.rakis-triphc.rlylphosphi.ne palladium were addcd thereto.
Af_1.-.er the rcacj:ion mixture was stirred aL' 90 C for 24
hours, t.he solvent was distilled otf under reduced
03/25/09 WED 16:15 FAX 416 362 0823 RII)OUT & MAYBEE ~ 007
CA 02657312 2008-10-28
- 48 -
pressure. The residue was pu.r_ified by flash silica gel
c;olurnn c:h..romatoqrd.phy (elution solvenL: hexane/ethyl
acetate = 1/1), to obl=.aiii ?14 m.q (corresponding to 0.628
m.mol ) of 6-tr,.i.butylstannyl-2- (~' -
hydroxyphenyl)imidazo[1,2-a]pyrazi.nt: (t'i.cJ_ 17., 5tep 3)
[0114]
':f'tie NMR measurement results of the resulting 6-
tributylstanny'l-2-(4'-hydroxyphenyl)imidazo[1,2-
a]pyrazi,.zie (i.rlte,.r.nal standard: tctramcthy].silanc) are
shown below.
[0115J
NMR apparatus employed: JNM-ECP-500 (manuf,acti;r-eci by
Jspari Electron Optics Laboratory Co., Ltd. (JT;C,OT.,) )
lII-NMR (sol..ven.t- chloroform-d1, resonance frequency: 500
3.5 MHz): 6 9.21 (s, lI-1), 7.95 (s, 1.I=1), 7_79 (d, J= 8."7 Hz,
2I-1) , 7.77 (s, 1hi) , 6.91 (d, J = 8.7 Hz, 211) , 1.70W1. 55 (rn,
6H), 1. 38-1 .31 (m, 6I-1) , 7. . 7..8-:.1.. 7.5 (m, 6H), 0.89 (d, J
7.3 IIz, 9H)
[. 0 l. l. 61
13C-NMR (solvent: ch'loroforiYL-dl, resonance fr_equericy: 500
MHz); a 157.3, 146.4, 143.5, 140.3 128.0, 124.9, 123.6,
116.1, 106.9, 29-0, 2-7.3, 13.7, 10Ø
[0117]
Example 11-3; Synthesis of ;~-(4'-hydroxyphenyl)-6-
iodoi-midazo[1,2-a]pyrazine
[0118]
314 mg (co.r.responding to 0.628 mmol) of 6-
tributylstannyl-2-[4'-hydro:xyphenyl]imidazc[1,2-
03/25/09 WED 16:15 FAX 416 362 0823 RII)OUT & MAYBEE 1~J008
CA 02657312 2008-10-28
- 49 -
a]pyrazine obLairied. from Example 11-2 was dissolved in
5.0 ntL of flic3al.oromethane, Lo whi.ch 114 mg (eorre5ponda.,n.g
to 0.942 mmol) of iodi,nc dissolved in 5.0 cnL of
dichlo,rorr.Gethane was added. rPhe reaction mixture was
stirred aL- the te.rnpcrature of 0 C for 1.0 mi,.nutes and at
roortI temperature for 30 hours . Then, a satu.rated aqueous
sodium hydrogencarbonatP sol.uti.on and. a saturated aqueous
sodium thiosulfate solixti..on wcre added thereto.
PrecipiLates were f.il.te.r.ed and recovered, washed with
water and ethyl acetate in this order, and dried undcr
reduced pressure, to obtain 131 rng (corresponding t_o
0.369 rrirno]. ) of 7--(4' -hyd.r..oxyphcnyl) -6-iodoim.idazo [ 7., 2-
a]pyrazine (Fig. 12, Step 1).
[0119]
1.5 The NMR measurement results of the resulting 2-(4'-
hydroxyphcny7. )-6-iodoi.mi.d.azo [ 1, 2-a] pyraziire (iriLernal
5'tandard: tetramethylsilane) are shown below.
[0120]
NMR apparatus employed: JNM-rC;V-500 (manufaCtured by
Japan Electron Optics Laboratory Co., Ltd. (JEOL))
'H-NMIt (solvent: dime'Lhylforrnamide-d'/, resonance
frcqucncy: 500 MHz): 5 9.89 (s, 1H), 9.01 (s, 1H), 8.82
(s, 1H) , 8.42 (s, 1H), 7.92 (d, J = 8.7 f:-iz, 7H) , 0.93 (d,
J = 8.7 Hz, 2H) -
03/25/09 WED 16:16 FAX 416 362 0823 RII)OUT & MAYBEE la009
CA 02657312 2008-10-28
- 50 -
[0121]
Exarriple 1I~-4 : Synthesis of 8-tributylstannyl-2-( 4' -
hydroxyphcnyl)imidazo[1,2-a]pyridine
[0122]
50 mt.,, of cthyl acetate was added to 28 .17 g
( corresponding t_o 126 mmoJ.. ) of cupric bromide ~() obta i.n a
,;uspcnsion, to which a solution of 8.18 q (corresponding
to 60.0 mmol) of 4' -hydroxyacet.ophenone in a rnixed
solutiori of 50 m.r..., of ethyl acetate and 50 mL of
c;hl.o.r,ofor.m wa.s addcd. Thcn, tl-ie resulting rnixt_ure was
refluxed. After 5 hours, t=:ht~ reac:t;_on so]ut.i.on was
cooled down to room temperature and filtered. The
resulting fi.l,tLate was conccnt.ratcd under .r.ed.uccd
pressure. The residue was dissolved in ethyl acetate and
5ubjected 'to decoloring operation with addition ot' activc
cha.rcoal. Then, Lhe resulLinq solution was filtered and
concentr_atcd. The rC5ul.ti.ng c.r.ude produGl-_ was purified
r~y flash silica gel column chromatography (elution
solvcnt: cti].o.r,ofortrl/rriel:hanol = 20/1) , and recrystallized
from ethyl acetate/petroleu7. cther, to obta.i.n -7.25 g
(corr,esponding to 33.7 iiunol) of 2-bromo-q'-
hydrox,yacct.ophcnone~. ( r.='i g_ 13, Step 1) .
[0123]
d32 mg (corresponding to 2.01. mmol) of 2-br'orno-9'-
hydroxyacetophenone and 318 mg (corresponding to 2_01
mmol) of 3-bromo-2-ami.nopyr:i.d.irze were dissolved in 20 mL
af acetonitrile. The resu7..ti.ng soluLion was refluY.ed. 1n
an oil bath at 110 C for 6 1-.ours. AlLer Lhe complction
03/25/09 WED 16:16 FAX 416 362 0823 RII>OUT & MAYBEE 1?1010
CA 02657312 2008-10-28
- `>1 -
of the reaction, the r.tact.ion solution was c~ool.ed down to
rc>om temperature, and precipitates wcrc filtered and
recovered. The preca-pitatcs were washed with
a.ce-l,qnitri..l.P and dried under reduced pressure. The
resulting crude crystals were suspended in a mixed
solution of 8 mL of wa~e.r.= ~xnd 8 m.L of inethanol. Then,
about S m.;L of a saturated sodium hydrogencarbonate
soiution was added thereto, and the mi.xture was soni.catcd
for 5 minutcs using an ultrasonic washing machine.
Precipitates were filtered and recovered froni the
result,i.ng rrl:~xtu,re, suf,f,.i.ca-cnt].y washed w.i.t_h. watcr-, a.rid
dried und.cr r_cduce.d pr_cs.surc, t.o obtain 368 mg
(corresponding to 1.27 mmol) of 8-bromo-2- (4' -
hydroxyphcnyl. ) im.idaz.o [. 1. ,2 a] pyriciine (Fig. 13, Step 2).
[01241
"75.2 mq (corresponding to 0.260 inmol) of 8-bromo-2-
(4' -hydroxyphenyl) imidazo [:1., 2-a]pyridirie was di5solved i-.n
10.0 m,r, cf dioxane, and 2.0 mL of tricthylamirie was added
thcreto. Theri, 0.20 mL (corresponding to 0.39 mrrlol) of
bis(tributyltin) arid 20 - 1 mcj (at a catalytic amount) of
tctraki,;,==.,rriphenyiphosphine palladium were added thereto.
After the react.ion m.i..xture was stirred at 90 C for 11
hours, a solvcnt was d.i.sta.7.].ed off urider reduced pressure.
'T'he residue was pu.r..i.t,icd by flash silica gel column
cl-iromaLography (clution soJ..vent- hexane/ethyl acct.ate
1/1), to oht:aiir 62.5 mg (corresponding to 0.125 mmol) of
0-lõ~ik~utylst~-~nnyl-.^_- (~' -hyri, nxyprrnrl ) i.m.i.dazo (1, ~
a]pyridine (Fig. 13, Step 3).
03/25/09 WED 16:16 FAX 416 362 0823 RII)OUT & MAYBEE [a011
CA 02657312 2008-10-28
- .'52 -
[01.25]
The NMR measuremerit resul.t.s of the resulting 8-
tributylstannyJ.-2-(4'-hydroxyphenyl)imidazo[1.,2-
a]pyridine (internal Sta.ndard: tctramethylsilane) are
shown below.
[01261
NMR appar_a.tus cm.ploycd: JNM-ECP-500 (manufactured by
Japan Electron Optics Laboratory Co., Ltd. (JEOL))
1H-NMR (solvent: chlorotorin-dl, resonance frequency: 500
MHr.,) : 5 8.01 (c9, J= 6.4 Hz, ],H) , 7.87 (d, J- 8.7 Hx,
2H), 7.70 (s, 1H), 7. 1-7 (d, J= 6.4 Hz, 1H), 6. 87 (d, J
8 . 7 Hz, 21=I) , 6 . 68-6. 66 (m, 1H) , 1 . 69-1 . 56 ( m , 611) , 1 _ 38--
1. 30 (rn, 6H) , 1.28-1 . 16 (m, 6H) , 0. E38 (t, J= 7.3 r{z., 91-1)
[C11.27]
I'C-NMR (so.l..vent: (~hloro.Corm-dl, resonance frequency: 500
MHz) : 8 145.2, 141.0, 139.2, 132.4, 131.8, 12'7.7, 127.3,
125.0, 115.4, 112.2, 106.4, 29.2, 27.4, 13.7, 10.2.
[0128]
Example 11-5:Synthesis of [:1.'zI] -2- (4' -hydroxy~Yienyl} -6-
iodoimidazo[,1,2-n]pyrimidine
[0129]
To 100 pL of a solution of 6--tributylstannyl-2.- (4' -
hyd.r.oxypl'lenyl ) imidazo [ 1, 2-a_ pyrimidi,ne :irr rnethanoi (at a
concentration of 1. mg/mL), :L00 L of 2 mol/L hydrochloric
ac~.d, [1`'3I] sodium iodide of 621.. Msc1 (150 11L in volumc),
20 uL of 1. rrrnol/L sodium iodide .solu.tion arid 20 uL of 10%
(w/v) hydrogen peroxide wer,c--- added. 1Lrter the mixed
solution. was ]:ieated at 50 C for 1.0 miriuLes, the .sol.ution
03/25/09 WED 16:16 FAX 416 362 0823 RII)OUT & MAYBEE 0012
CA 02657312 2008-10-28
- 53 -
was subjected ~o Hf?:L,C under the same conditions as
described in I xam.ple 1-2, to obLain [1`':I )-2- ( 4' -
hyd.roxyphenyl)-6-iodoimidazo[1,2-,2]pyri.m..i.dine as a
fraction.
[01301
The same operation as trEe prcceding paragraph was
pcrformed to obtain
[ 123 I],-."' . -( 4' -hydrox.ypheriyl.. ) --6-i.od.oi.rni.dazo [ I. , 2-al
pyri..mi.di.ne
(the amount of reagents to be added: 150 uL of a solution
of 6-tributylstannyl-2-(4'-r.ydroxyphenyl)-6-
iodoimida2o[1,2-a]pyrimidine. in methanol (concentration:
1 mq/mL), 75 iaTõ o.f 2 mo.],/:f., ] yd.r.och.lor:ic acid, [123I]sodium
:i odide of 48'7 MBq (150 ~iL ir. volume) , 20 iaL of 1 nunol/L
5odium iodide solution and _30 ja.L of 10 , (w/v) hydrogen
1.5 pe.rox.ide).
[0137.]
Two f.ract_ioTls obLained by the operations of the Cwo
preceding paragraphs wcre m.i,xc~d, and 10 ml of water was
added 1:herel.o. The resulting solution was pa6SeCi through
a reversed phase column (t,rzi(ie riame: Se.p-Pak (regi.stered
trademark, Waters Investments Limitcd) .T.dght C8
Cartridges manufac=rurPd by Vqaters: the packed. amount of
-the packing agent: 130 rng) so that the column adsorbs arld
col.lects [1,23j] -2- ( 4' -h.yd.rc)xyphenyl) -6-iodoimid.azo [ 1, 2 -
2.,5 a]pyrimidine. The c.olumn, wa.s rinsed with 1 mL of water,
and then 1 m:L, of diethylether was passed the.ret:hrough to
elute [ 1?311 -2,- ( 4' w,hydroxyph(Bnyl )-6-iodoim.idezo [ 1, 2-
a]pyridinc. The arnounL of radioactivity oF the obtained
03/25/09 WED 16:17 FAX 416 362 0823 RIDOUT & MAYBEE 013
CA 02657312 2008-10-28
- 54 -
compound was E7 M13c3 at the end of synthcsis. Further,
Lhe Ta.,C analysis was conductcd under the follow.i.n.g
conditions, and as a resuit, t_he radiochomical purity of
thc compound was 92.5".
[0132]
TLC analysis c:c>nd.i,t.a.ons:
TLC plat.c: Silica Gel 60 F251, (l_rade n.ame; ma.nufacturcd by
MPrC`k & Co. , .Cric. )
Mobile phase: chloroform/methanol/e_r=iel-hylami.ne - 100/1/2
Uet'Pct:or: Rit:a SL'ar (trade namc; manufactured by ra,ytest)
[0133]
Example 11-6: Synthesis of [123I ]-2- ( 4' -hydroxypherlyl) -6-
i.odoimidazo [1, :3]pyrazine
[0134]
To 100 uL of a solution of 6-tributylstannyl-2-(4'-
hydroxyphcnyl)imidazo[.1,2-alhyraz:i.ne ].ri mee.hanol
(concentration: 1 mg/mL), 75 ui, of 2 mol/L hydroch],o.r.ic
acid, ["='I ] sodi.=um i=odi..dc of 4 69 Ml3q (100 L in volume ),
}.i.L, of .l mmol/L :;odi-t,im iodide solution and 20 uL of 100
20 (w/v) h=ydrogcn peroxide we.r-e' added. ASter the mixed
sol..utiori was heated at .50 C for 10 minutes, th.c sol.ut-i.on.
was subjected to fIPLC under the same Conc3il_ions as
dcscr,.i.bed in Example 1-2, tc> obtain [ t"I] -2- ( 4' -
hyd.roxyphenyl) --6-iodoimidazo [ 1, 3] pyrazine as a. f.r,3c,t=ion.
[01.35.1
10 m.l. of water was added to the fraction. The
resulting soluti.on. wa.s pa5sf~d tilrough a reversed phase
col.umn (Lrade name; Sep-Pak (reqister.cd trhdernark, Waters
03/25/09 WED 16:17 FAX 416 362 0823 RIDOUT & MAYBEE ~ 014
CA 02657312 2008-10-28
- 55 -
Invest:ments Limited) Light c;8 Cart.ridges manufactur.ed by
WaL.er5: thc packed amount-_ o.f, the packing agent: 130 mg)
so that the colurtrn adsorbs and collects [``-i:r. ] -2- (4' -
hyd.rox.yphcnyl) -6-iodoimidazo [1, 2-a] pyrazine. The (_c) 1.u?nn
was rinsed with 1 m.T,., of water, and then 1 inL of diet.hyl
ether was passed therethrough t;o elu.tc ["'-"I] -2- (4' -
hydroxyphenyl)-5-iodc)_Lm;,da?o[7.,2-a]pyrazine. The amount
of radioactivity of tl=ie obtai_ncd compound was 133 MBq at
the end of syn.thesi.s. Further, the TLC analysis was
conducted under the same condit:i.ons a5 de5c.r.a,bed in
Example 11-5, and as a result, the raciiochemical purity
of the compound wa5 99 _ O9(s _
[0.136]
Example 11-7: Synthesis of ( -L`'I ] -2- ( 4' -hydroxyphen.,y]. ) -8 _
iodoimidazo[1,2-a]pyridine
[01371
To 70 uL of a solution of 8-tributy1.st.a3-Inyl.-2- (4' -=
hyd.r.Uxypl,ienyl)irnidazo[1,2-aJpyrazine in methanol
(concentration: 1, rng/rn:C,), 50 l., of 2 mol/L hydrochloric
acid, [i`'I] sodium iodidc of 454 M1:3q (100 pL in volume) ,
20 l,rT., of 1 itirnol/L sodiurn iodide solution and 20 }pI., of 109,
(w/v) hydrogen. pe:.roxide were added. After the mixcd
solution was heated at 50 C lor 10 minutes, the solu.ti.on
wa.5 subjected to HPLC under the samc con.di.tions as
described in .L=;xam.pl.e 1:-2, t.o ob'tain [123I] -2- (4' -
hydroxyphenyl)-8-iodoimidaro[1,2.-.a]pyridine as a fraction.
(0133]
10 ml of water was added to the fraction. 'I'he
03/25/09 WED 16:17 FAX 416 362 0823 RIDOUT & MAYBEE 015
CA 02657312 2008-10-28
- 56 -
resulting solut,i.on was passed through a.r..evcrsed phase
column (trade na.me: Sep-Pak (registzred t.rademark, Wat:ers
Inve..t'.ments Limited) :I:.,i..ght C13 Cartr.i.dqes manufactured by
Waters: the packe(I amount of t;:he packing agenC: 130 m.q)
so that the column adsorbs and collects [123j] -2- ( 4' -
hyd.roxyphenyl )-8-iotloi-midazo [ 1, 2-a] py.r. id.i..ne . The coluli n
was rinsed wi,th 1 mL ot water, and. then 1 mL of diethyl
ethc.r was passed therel.h..r..ouqh to elute j12ja; ] -2- (4' -
hydrox.yphenyl )-8-iodoimidazo [ 1, 2--a] pyr, i.din.c. The amount
of radioac_L-ivi.ty of the obtairied compout-id was 185 MBq at
the end of synthesis. F"ur.t-he.r-, the TLC analysis was
conducted under the samc conditions as descrik~ed in.
Example 11-5, and as a re5ult, the radiochemical purity
of the compound was 91.7%.
[01391
Reference Example 1: Synt_Yic=Sis of [ss T]-TMPY
[0140]
[ leSI]-IMPY was prepared i-n accordance with the
r,... -~
following steps for use in C:omparative Example
(Comparative Example 1-6) ror evaluation on 1,ogY,,,t;,,,o1.
[0-141]
In accordance with the lit_erature (Zhi-Ying Zhuang
et al., J. Med. Chem, 2003, 46, p.237-243), 6-
LribuLyls'Lannyl-2- [ 4' - (N, N-
dimethyl,,am.ino) phenyl,li.midazo []., 2-a] pyri.di-ne was
synthesized, and dissolved in me'L-hanol (concentration:
7.mg/mL). To 53 uL of the resulting solution, -/S pL of 1
mo1/L liycirochlex'ic acid, 20 L of [i`SI] sodium iodide of
03/25/09 WED 16:18 FAX 416 362 0823 RIIIOUT & MAYBEE ~016
CA 02657312 2008-10-28
- :57 -
13.5 MBq, an(3 10 uL of 7..01~ (w/v) hydrogen peroxide were
added. After the mixed solu~1--.i.on was left to stand at
50 C for 10 minutes, L'.hc solution was subjecLed to HPLC
unde.r, the'same (-onditions as dcscribed in l:-,xamp'le 1-2, to
obtain [121I]-IMPY fractiorr.
[01421
m1 of water was addcd. to the l.raction. The
resu.7.ting solution was passed L_.hr.ouqh a reversed phase
column (C_radc name: Sep Pak (registered trademark, Waters
10 Investments Lim.itcd) Light C18 C;a.rt.r..i_dqes manufacturc_d by
Water,; the packed amourit_ of thc packing agerit _ 1.30 mq),
so that thc column adsorbs and collects L-he [125T] -TMPY.
The column was rinsed with 1 mL of water, and th.e~n 1 mL
of et.hanol was passed therethrot,rgh, to clut.e ["'I]-IMPY.
The obtained radioactivity was 2.6 MBq at the prid. of
syrithesis_ i'u..rther, the TLC analysis was conducted under
the same conditions as described in Example 1-2, and as a
result, the radinr..hemi.caJ. purity of the compound was
9E.01s.
[0143]
Rcfercncc Examplc 2: Synthesis of [1=ja: ]-IMi.'Y
[0144J
["".T.]-IMPY was prepared iri ac.c_orciarice with the
following steps for use in C:ompa.rat.i.vc Examples
(Coinparative Example 1:-7) for c~va.luations on accumulation
in brairi.
[0145]
In accordance with the liteYaLure (Zhi-Ping Zhuang
03/25/09 WED 16:18 FAX 416 362 0823 RIDOUT & MAYBEE 017
CA 02657312 2008-10-28
- ~)6 -
et al., J. Med. Chem, 2003, 46, p.237-243), 6-
tribu-t,yl.stannyl-2- [ 4' -- (N, N-
di,rnethylamino)pheny.l.]imidazo[1,2-a]pyridine was
synt.hes.i.z.ed, and dis5o],vcd in methano]. (concentration:
1mg/mL). To 53 pL of the resulting solution, 100 U:[., of 1
rriol /1.., hydrochloric acid, 20-50 ]-iL of [ :I ] sod.ium iodide
of 190-240 MBq, 10 }j.L, of a 1 nuTLol/L soda.um. iodide
solution and 10 pL., of 10`a (w/v) hydrogen peroxide were
added. After the mixed soluLiori was _l_eft to stand aL
50 C; for 10 minutes, the sol.uti.on was subjected to HPLC
under t:he same conditions as described in :F,,mample 1-2, to
obtain [1~'1]-IMPY frar.ti..on.
[0146]
10 ml of water was added to the fracti-on._ The
resulting solution was passed through a.reversed pha5c
colurnn (l_rade :na.me: Sep-.k'a]c (.registercd trademark, Waters
Investments Limited) Light C18 Cartridges manufactured by
Glate:rs; the pa.cked amount of th.c packing agent: 130 mcI)
~
so that th.e column adsorbs and collects the [1.` I]-IMPY.
The col.umn was rinsed with I mL of water, and then '1 niL
of ethanol was passed LhereL.hrough, to elute [12":f]-='1MPY.
The obl_ained radiohc,l.ivi_icy was 47-56 M13q at the Cn.d of
synt.hesa..:, _ Furth.e.r., the TLC analysis was conducted under
the same conditions as descr-ibed in Example 1-2, and as a
result, the radiochernical puriLy of the conapound was
98.04.
03/25/09 WED 16:18 FAX 416 362 0823 RIDOUT & MAYBEE 018
CA 02657312 2008-10-28
- 59 -
[.0147]
Exarnp.l..es I-11, to 1-14, Corqparative T?,xamples I-1 Lo .T.-5:
M.casurement of amyloid affini-Cy
A..ffinity of the p.r.esenL- compounds with amyloid was
exa?nined by the following in vitrv binding tests.
[014II]
(1) Apl,_1o (Peptide InstiLUte, INC.) was dissoJ.ved in
phosphaLe buffer (pEi 7.4) and shaken at 37 C for 62-72
hcu.r-s, to obt:ai.n a suspension of aggregaLed A~
(concentrata.on: 1 rng/mL equivalent, hereinafter referred
tc ~:a,, amyloid suspcnsion in these Exarnpl_es )
[0149]
(2) ILc,cording 'Lo the method descr.ibed in a
l..i,terat.ure (Naiki,, H., eC al., Laboratory .l:nvestigation
7d, p.374-383 (1996)), the amyloid suspension wa.;
subjected to qua.l.a.tative expr:.ri,ment. based ori f.],uorescence
spectrophoLometric mEthoc4 u.sing Thioflavin T
(manufactured by Fluka) to confirm t_hat the aggregated A~
obtained in (1) was amyloid (rnea5urement conditions:
excitation wavelength of 446 rrm, znd emisSiori wavelength
of 490 [01..50]
(3) Accord,ing to the meL;hod dcscribed in a
literature (Wang, Y., eL al_,. J. Labeled Compounds
Radicpharrriaceut. 44, S239 (2001) ) , [121I] 2- (3' -=i.odo-4' -
arninophen.y.l.)benzothiazole (hereinafter referred to as
[""T]3'-I-IITA-0) was prepared from a l-abel,i..ng precursor
2- ( 4' -aminophernyl ) bcnzothia2,ole, and dissolved iri ethariol _
03/25/09 WED 16:19 FAX 416 362 0823 RIDOUT & MAYBEE 019
CA 02657312 2008-10-28
- 60 - 51 ~`] sodivm iodidF., of 12-7:1. MBq (10-30 pL in volumc) was
used for the 1~`'
producLion, to obtain [ T]3'-I-b'!'A-0 of 1-
22 MBq at the er,d of synthesis. As Congo Hcd, Thicaf.].avin
1 and 6-met.hyl-2-[4'-(N,N-
dimethylamino)phcnyl]benzot.hiazole (hereirraf:ter refer-rcd
Lo as 6-Me-BTA-2), corrimcrcially available reagents were
weighed and used as l.i-)cy were.
[0151]
( 4 ) 2- ( 3' -Iodo-4' -am.a.nophenyl ) benzothia::o7..c
(herPina.f-ter referred to as 3'-I-BTA-O) and. IMPY were
synthesized according to the methUds described an a
litnrat.urc (Wang. Y-, ot al., J. Labelled Compounds
Radiopharmaceur. 44, S239 (2001)) and a 1iLeratuz.-e
(Zhuang, Z. P-, et al., J. med. Crem. ~6, 237 (2003))
rPshec:ti.vcly.
[0152j
(5) Samples in which [IIII]3'-I-BTAWO, each compound
for evaluation and amyloid wer(t dissolved in a 0.1
bovine serum albvmin-contairYYrig phosphate buffer (pf-I 7.4)
at final concentrations showrr in Table 2 were prepared.
The .r.esu.lting samples were placed in ear.Yf we..l.l (about 0.3
mL in volume) of.a 96-well microplat-.e.
03/25/09 WED 16:19 FAX 416 362 0823 RIDOtiT & MAYBEE Z020
CA 02657312 2008-10-28
_ ~ 1 .,..
[01,31
Tabl~ >; Linal conGentrat.i.ons oP each compourid in ssampl~ :,<~:1.ution,
L'oncentrat:.i.on oL [1 1] 3' -I-
Experim_nt. C'~mpori.r:d for
e.valu zL L. i, on Lt)rnpoLlnd Fqr 2TA-0 Amyloid
evaluat;:i on c:oncorrLration
ComparzLivc 3'-r-BT11-0
~f31Trf~].C' I-1
CcmtF3ar..ative
C:on.go R~d
~'.xampic 1-2
CvrnFaarativc_
i'h i of;lavin T
t~-xantplc 1-3
Compirar,.ivP Eacn
Examp le I 4 concentx~~1_ion of
Comparal,; i ve 0.0U.1, 0,01, 40C1 pmol/L '1
IMPY U.1, 11 10, lUU,
1000 rirnv.i./L
E x~t mpl,e Compound 1
1 - .1.1..
Exnmp].Y
C U(fl},?6Und 2
.1-12
Gxamulc
C OrnpvUnd ~
1-13
---- - ....... ......_._ p _,..,._
Examplc Com our,c9 4
:L-14
[0154]
(6) The microplat_e fi.lled with tlie sample solutions
was Sh.akcn at a yiven rate (400 rpm) at 22 C: for 3 hours.
Thcn, each samplc solution was filtered t=hrouqh a glass
fibE~.r filter (trade name: Mu1ut;1.sc1.-cen""-FC, manufactured
by Millipore) , to scparate ttte [1LI] 3' -I-13'T'A-0 attached
I 0 to amyloid from the [1251] 3' -I.-BTA-0 free from amyioid.
[0155~
(%) `1:'he glass fiber filter used for thc filtratiori
of cach sample 5ol.ution was washcd with a 0.1 ~. bovine
serum alburitiny-containing phosphate buffer (pH 7.4) (0.5
mL x 5), and radioactivity of the glass fibe.r. filter was
measured wit.h an autoweli ganuna system (manufact;urc;d by
03/25/09 WED 16:19 FAX 416 362 0823 RIDOUT & MAYBEE Z021
CA 02657312 2008-10-28
- 62 -
Aloka, Type; AF,C;-301b) . T'he radioactivity was used as
the radioactivity levcl of each sanplc solution at.tached
to amylo.id for calcu..lating an inhibition ratio
(hereinafter, A denotes tt-ie .radi.oactivity level irl. a
sarriple with zero (0) c:oncent.r-ation of each, compound lor
evaluation, and B cienotes the radioactivity levcl in a
sample with 0.001 nmol/T., or highcr coricentrati.on of each
compound for evaluation)
[01,56]
1.,0 (8) Separately, a solution c_on.taining 1.5 umol/L of
6-Me-BTA-2, 400 pmol/L of [1'SI~ 3' -I-B!'i~-0 and 1,amol/L of
Ap 1..40 in a 0.1 % bovine serum albumin-containi-ng
phosphate buffer (pi:f 7.1) was prepared and subj ected to
trhe same procedu.,re.s as desc:ri..bcd above in (6) and (7) to
measure a radi..oactivity level. The measured
radioactivity level was dcfined as the background
radioactivity level, and useci in the calculation of the
inhibit,ioa ratio (her-einafter referred to as BG) -
[01!57]
(9) Usirig the radioacta,vity levels mcasured above in
(7) anci (8), the inhibition ratio was deternlined by thc
followi.ng formula (1).
[07.58]
B -13(.~
.t.nhibition Rati.o = = x100 (~) (1)
;I - BG
[0-'-59]
A graph in whic.h values convertc:;d by probit
transformation from the obtained inhl.bition ratios were
03/25/09 WED 16:19 FAX 416 362 0823 RIDOUf & MAYBEE 022
CA 02657312 2008-10-28
- 63 -
plotted relative to 7..ogariLhms of concentrati..ons of
compounds for evaluation was prepared to obtain arr
approxi.mate st:r..a.iqht line by t^Yic least sguare rnethod.
Using Lhe line, t:hc concentration of eacti compound for.
~ evaluation was determined, at which thc radioactivity
J.e-vel is half of l.he. tevel or. the sample free fr.om each
compourid for evaluation, and was defined as a 50 %
inh:iYyi.t.i_on concentration of each compound (herei.nafter
r.cfcrred to as IC,o;, vaJ..uc) . Using the value as an
indicator, af,finity of each compourzd. for evaluation with
arnyloid (aggregated ARi...no) wa;, evaluated_
[01601
i:CSOr; value of each compourrd for evaluation is shown
iri TabJ,c 3. Compounds 1 to all Showcd IC.yo., values of
lcss than 100 and had higher affinity with arrtyl.oid
(aggrega,t.ed A(31-4o) thari Congo Red and Thioflavin T. The
.r,esu.lts show that Compounds ]. Lo 4 have good affiniLy
wiLh c1my,1oid (aggregated AO1-,;o) . In partl.cular, Compound
1 had higher aff'iniL-y with amyloid (agg.r.egat.cd A~1..,4o)
than .3' -I-BTA-0 arid 6-Mc-BTA-=2 and had the affinity
comparable Co 1:MP.Y,'.
03/25/09 WED 16:20 FAX 416 362 0823 RIDOUT & MAYBEE U023
CA 02657312 2008-10-28
[0161]
I'ahlc 3: ].C:,,n, va:Lui;s of i_he preaent- cornpoundõ
Lxperimr;n l_ C:o.T3pounct 1or. valuc~
evaluation (ri.mol/L)
-.., _....... _... Comparative Exampla -I 13T11-0
10.1
Coiypa r=at ive F::ailrple Cor.gp 1ZECi >1000
7:-2
...,. -..._. -
c.omparative F'xainple?
>1000
I-3
- ......._. _.,.,. ..__ ..... _,,..,,, .
C:c,cr~~~i::ative TxBr[p1~ 6_Me-13TA-2 25. q
I-4
c, .,..,-'
prn~>az.~ativc Ex ~rn~~le :IMPY 9 U
1-5 _ ._. --,..._ ,.. ,
Examplo I-1 1. CCr[ipound 1 4.4
- ., ... - ....,:,.,.,
F xanlp].e 1-12 Compounc,i 2 26.U
..,,.._ .. ....,_ ....... ......
Exampl.e I-13 Cam~x,ur d. 3 5~ .9
..,.,.. ...... -- ,._-_ _...
F;xa.mple I-14 Compoti.r7d 1 54.1
[.01.62]
Example_I-15, CxampleTT-8 to II-10, Comparative r;x.arnple
1-6: Measurement_ of partition coefficienf_bascd on the
octanol exl'_:-raction method
[01.63]
Partitiori c_oF.f....f.i-cienr-s based on the octariol
extraction method (hereinafter_ referred ~o as loqPo,_taõol)
were measured, which are generally knowri a;; a.n indicator
of permeability of c,ompounds througYi. the blood-brain
barrier (hereina.f,.tcr referred 'to as BnB).
[0164]
A die-zhyl ether solution of Compound 5 prepared iri.
Exarnple 1-2 (Example 1-15), a diethyl ether eolutaon of
Compound 9 prcparecl in Example 11-5 (Example II-8) , a
diel_hyl.. ethcr solution of. Compound 10 prepared in Example
I1-6 (Example II-9), a da,.ethyl ether solution of Compound
11 prepared in Example TI-7 (Example 11-10) and a diethyl
03/25/09 WED 16:20 FAX 416 362 0823 RIDOUT & MAYBEE U024
CA 02657312 2008-10-28
-
6 5
ethF.r. soluCion of [""I ]-IM.PY prepa rC.d in Reference
Example 1(Comparat-i.ve Example 1-6) were each diluted
with 10 mg/mL ascorbic acid-containing physiological
saline solution, and adjusted to r-adioac;t?ve
coriccntration of 20-30 MBq/m:C,- 10 4L cach of thc
prepared sample sol.ution wa:, respecta.vely adcied to 2 inL
of octanol,, further, 2 mL of. 10 mmol/?-, phosphate buffer
(pH 7,4) was adcled, and ;t.a-rred for 30 seconds. AfteY-
r.hc mixture was centr.i.fuqed with a low-spPi~.,d centrilugc
(2000 r.p.m x 60 TCtin. ), the octanol layer and the water
layer wer.e; sampied each in an amount of 1 m:rõ and
5ubjccted to rneasu.r-emeriL of radioactivity count with an
autowell gamma systern (Type: ARC--30113, manutact_u.r,(!!d by
Aloka) . Using the obtained radiQactivity courit,
was calculated in accordancc with the equation
(2).
[U165]
Rauiioac'tivity count of octanot layerl
lo9 Poccanat = loBio (Radioa.ctiviCy count of water layer 1 (2)
[01.6h~
The resulL., are shown in Table 4. Compound 5 sh.owed
a value of 1. 6, and ["~'I ]-IMPY sh.owed a
loqPo` õc,1 value c,:F 2.1. It is krtown t.hat. compounds
permeable to 5 5 show a value between 1 and 3
(Dot_iglas E:). Dischino et alõ J. Nucl. Med., (1983), 24,
p.1U30-1038) . Thus, it is im.p].i.ed that both cornpounds
have a h}il~ pcrmeability comparable to IMPY.
03/25/09 WED 16:20 FAX 416 362 0823 RIDOUT & MAYBEE 025
CA 02657312 2008-10-28
- Ei6 -
f.0167]
Tah7.e 9: 109P,,tano, valUe of thn present ro:r.npound
H;xperimerif: C:oiipound loqp~. ,n~:, Value
Comparative 125
FY7tti.ple I-G I I.)'_IMI?Y 2.1
... ~,,,,,. ....--- ...:,.,,._ ..,_...
Examp] e I-15 Compound 5 1.6
Gxampl.e II-8 Compourid 9 1.7
Fxarnp1F 11-9 Compouri.d. 10 2, 3
... ...,... ...,.,. ....,_ .._..
E,xample I:1;-10 C~rr.lpourid 11 3. U
[016F3j
Exanipl.e I-16, Camparative Exa.mple I-7: Mcasurement; of
transferability into brain. and clearance
[0169]
Using Compourid. 6, a time c:our_se change of
radioactive accumulation iri bra..i_n of male Wi;;ta..r rats. ( 7-
week old) was measured.
[0170]
0.05 mL (20-30 MBq/mL in radioactive concentration)
of a solution of Compound 6(Exa.mpl.c 1-16) in a 10 mg/mL
ascorbic acid- containing physiological saline soli~tion
and 0.05 mL (20-30 MBq/mL in rada.oactive concentration)
of a solution of [12I! ]-:1:MPY ( Comparative F: xample I-7 )
prepared abovc in ReferPnce ~,xample 2 in a 10 mg/mL
a.SCorbic acid-containi..nd physiological salinc solution
we.re injected uridc,r thiopental anestricsia into the tail
vein of respective Wistar rats (7-week o1d). The rats
were sacrificed by bleeding _rom abdominal artery, and
brairis were r.cmoved and subjcctcd to measurement of
radioactivity (hereiriaft:e .r. rF fcrrcd to as A in t.hi.s
Example) with an aut,owcl.l garttma system (Type: ARC-:30a13,
ina_iu:tactured k)y A1 oka ) and further sub j cc~ted to
03/25/09 WED 16:21 FAX 416 362 0823 RIDOUT & MAYBEE IN26
CA 02657312 2008-10-28
- 67 -
measurement of mass of braa.ns 2, 5, 30 an.d 60 min.utes
after the i.njecL:ion. A15o, radioactivity (hereinafter
referred to as B in this Example) of 0.05 mL of a 1000-
fold diluted solution of the ,i-njeC.-Led solution was
rnea.sured in the seme manner as above. Usi-ng these
mea5u.rement results, r,.adioact.i.ve accumulation per unil;
weight of brairi ( ;ID/g) at the re:;pective t-i.me points was
cal..culated in accordarlcc with thc followi.ng forfnuia (5)
Two cani.mals were used for both Example I-16 and
Conrparal:i..vc Example 1-7 at the respective time poi,nts.
[0171]
% tDl A _...... ...
br x] 000 x h~ ui weiglrl x 100 ( 5)
[01-72]
The result;, arc shown in T'able S. As sl-iown in Tab1e
5, Compourrd 6 showed a accumulation corriparablc to 123I-
IMP'Y at. t,he time point of t.wo minutes after the inject:ion,
and then showed a tendency to .r-apidly clear away in 60
minutes. Ttzesc results suggcst that Com.pound 6 possesses
ex(-e]._lcn.t transf"ernbi:lit.y to brairi. and rapid clea.r-ancc
from bfain like "L3I-IMPY.
[01'73]
I'ablc 5: Radioacti ve' aecumulatiotl i r) k>:ra.i-n of Compounc[ 6af l,c: r
Ini.-rdvenous injor_r.ion (rats) _
hadicactive accumlll.Jt.IUl1 per unit
Compoun(i _ weiqht ( oTD/r )
_..:,. ..._ _
Ilftcr AP.ter Aftor ACler
_
_....,...._.. -. _,..---
C'Umparative 121
I-IMPY 1.07 0.99 0.20 0.08
E::r~zn 1.e I-/
_.__..,......... ._ ....... .
1: :aznpl r-. ConvDound F, 0.96 0.69 0,16 0. 04
I -16 ` ....,.,,....--
[01"79j
03/25/09 WED 16:21 FAX 416 362 0823 RIDOUT & MAYBEE 027
CA 02657312 2008-10-28
Exa~lE: I-1"7: Confirmation of imagi_nq of am 1oi.,d in b.ra.in
[0175]
'.t'he tollowing experitrierzt was carried out in order to
examine whetheiz amyloi.d in brain cari be imagcd by the
compound of the present invnntion.
[01-7h1
(1) (manufactur'ed by Peptide IrY:,;titute, 'I:NC. )
was dissolved in phosphate br_cffer (pH 11.4) and shaken at
3`/ C for 72 hours, to Ubtain a suspension of aggregzted
11~, (AR concentration: :1. mg/riiL ecluivaler,t, hereinafter
refer:r.c.d to as arnyloid stlspcnaion :i.n this Example)
[0177]
(2) 25 L (corresponding to 25 }ig) of the amy.l.oid
suspensiQn was injer-i_e.d into ar7 amyqdaloid nuc.lcus on orre
side of a male Wisttar. rat (7--weFk old) 1~s a control, 25
1aL of a phosphate blaf.f.cred phys.i.ol.oclical saline solution
(pH 7.4) was injected into an amygdaloid nucleus on the
other- si.dc of the rat. The rat:5 were exaYnined 1. day
after the injeCt=i.on of the anyloid suspensa.on and 'Lhe
phosphate buffered phy5iological ~3J..inc solution (p1-1 7.4).
[O1"78]
(3) Compound 6 was dissolvcd in a 10 rcrg/mi,, ascorbic
acid-containing physiological sa.line so1uL'ion to obtain a
sample soluoi.o.n (32 MBq/m1:, i.n radioactivity
conc:entrat.ion). This solution was injected into the rat
through the t.a:i l. vein (dosage - 0.5 mL, dosed
r.adioactivity: 1:6 M3c{ equival.ent).
[0-.,=7q]
03/25/09 WED 16:21 FAX 416 362 0823 RIDOUT & MAYBEE ~028
CA 02657312 2008-10-28
- 69 -
(4) Brain was r_emovcd. 60 minutes after the injcction
to prcpare a brair: slice of 10 1-im in thickncss with a
microtome (type, CM30505, cnanufactur.cd by :[.,rICA) . The
brairi slice was exposed to an imaging plate for 20 hours,
and therl image analy5.i..s was c;arried out by use of a Bic-
imaging Analyzer (t;ype: Bn5-15U0; manufactured by
FUJIFILM Corporation).
[0180]
(5) After the completion of the image analysis using
the Bio---imaging Analyzer, pathological staining wiLh
':1'hioflavin T was carr.ied ouL to perform imaging by use cf
a fluorescence microscope (type: '11',2000-U model;
manufactured by NIKON Corpora'tion; exciLat-_iQn wavelerrgth:
400-440 nm; detectLion wavelength: 470 nm)_ Thus, iL was
confirmed that amy].oid was deposit.ed oil thc slice (Fig.
9b).
[01~.31]
Fig. 9 shows images by autoradiogram and Thioflava.n.
T stair:ing of the brain slice of Lh.e rat to whir:h amyloid
was injected intracerebrally. As shown in Fig. 9, a
jnarked accumulation of radio,activity was observed in Lhe
a.mygdaloid nuc_1cus on the si.dc t.o which the amyloid
susperisiorr was injected_ From the rFsult of Thiofl_a.v.in T
st:a.i.n.i_nc~ in the s:it.e where rndioactivity acr.umulated, it
was confirmed that amyloid, w s presen'L ir: the
accumulation site. On rl,e other harld, no siqnificant
accumulaLic3n of radioactivity was observed iõn the
amygdaloid nucleus on the side to which thc physiological
03/25/09 WED 16:21 FAX 416 362 0823 RIDOUT & MAYBEE 029
CA 02657312 2008-10-28
- 70 -
saline solu'Lion was injected, compared with thc other
sites.
These r_esults suggest that Compound 6 pUSsesses a
propzrty of accumulating on intr.acerebral amyloi..d, and a
S c:apability of imaging a.ntrace.rebral amyloid.
[0182,]
r',xam le I-1.8 to 1-20: Reverse mutat_aon test
f 0'13:3]
:(:n order to examine mutager,.icity of Compounds 1, 2
1D and 4, rcverse mutation t.est. using Salnronella typhimur:i,uzn
'l:'A98 and Tn1..00 (hereinafter refcrred to as Ames te,rt) was
coneductcd.
[0184]
The L-est was conducted w:ithout addition of S9mix and
13 with addition of S9rnix. Dimethyl~-,u.lfoxide was usod as a
negative control. A positive control was 2--(2-furyl)-3-
(5-nitro-2=-furyl) acrylam.i.dc wn case S9mix was not, ad.ded,
and 2-aminoanOh,racene in casc S9 mix was add.cd.
[0185]
20 The arnourit of each samp] c to be added to the test
plate wa.s 7 dosages (gPom.et.r'_c raCia 4) with the rtraximum
dosc being 5000 Ezg/plate. After a sample 'to be examined
and a strai,n (TA98 or TA1,00), or a sarrrplc t.o be examined,
S9rn:i.x and the strain were mixed together, the mixturc was
25 multilayered using soft agar on a medium of a test plate,
and theri iric:ubatcd at 37 C for 48 hours. Judgment was
made by counting the number of .r.everse muLaLion c:o.lon.ies
on the plate aftcr the incubation, and wherr 1.he numbcr of
03/25/09 1M1'ED 16:22 FAX 416 362 0823 RIDOUT & MAYBEE 16030
CA 02657312 2008-10-28
- -11 -
reverse mutatiori colonie;s was not less than two time.s the
riumber Y.n negati.ve coritrol an.d showed concentration-
dependent in.crease, mutagenicit_y was determined. to be
positive.
[0186]
The results are shown in Tab].c 6. 'I'i.,e numbers of
reverse mutatio.n colonies of ttie r,cspecLivc strairis in
the group treatcd with Compoi_inds 1, 2 and 4 were, .l.ess
than two times the number i.n the group treated with ttie
negative control, regardless of addition of S9mix and. the
addi.tion amourtt of a sample to be examined. F..r.om the
aforementioned resulLs, it is j dged that Compounds 1, 2
and 4 are negative in the Ames t.cst and have no
mutageni.city.
(0187]
I' ct>le 6: kc su1T:; Ames test
Mi~f::;igenicity
Compot)nc.3 Withoutac9.d;iot With ,fc.3c:I:i.L.iort of
S9rnix o9ffliX
... ~--- ,,.. __,..
TA98 '1'A7.00 Tn98 TA100
=.... -...... _..,.,._. _
E:xEi rnple Compound
:1-.1.II 1 Ne'y=~tivi: Negativc Nc?gar,:i ve Ncgativc
.,_. ^....,..., J ~
Examplc Comp~~und
I-19 7 Negativ_ Ni_g1G:i.vr~ Ncgativo Na Livr.
......:...... __.. ......_. ._.,.,ti, ..
1;r ample C'ompouric3
Ni:yaLi.vc, NcgaT.ivi; Negative Ncgat;=ive
1.-2u 4
[018 8]
hxam le 11-11, 11-12, Comparar_i..ve Example II-1:
.
.y _
Measurement of tr.ansferabilit into brain and clearance
[0189]
U~ing the Compounds 10 and 11, a time course change
of radioac.t_ive accumulation in brain of male Wistar rats
(7--week old) was measu.red.
03/25/09 WED 16:22 FAX 416 362 0823 RIDOUT & MAYBEE ~031
CA 02657312 2008-10-28
- '72 -
[0190]
0.05 mL (20-31 Ml3q/m.L in radioactivity
concentration) of a solution of Compound 10 (Example II-
11), Compound 1.1 (Example I1-12) and [1'`3I]-IMPY
(Comparative fSxample II-1) prepared in the above
Reference Example 2 respectively i.n a 10 rng/mL ascorbic
hci-d-conCa,i..ning physiological saline solution wer-e
injected unde:,r. thiopent.al anestlzesia into the tail vein
of respective W:i.star rata. 'I'he rats were sacrificed by
bJ.eedina from abdomirial arte3fy, and brains were removed
arid su.bjected to measurement of mas5 of brains and
further subjected to cneasuremenL of radioactt:i.vi.t.y
(hereinafter referred to as A in this ExamplE ) with a
5in,7-1,c channel analyzer (detector type: SP-20
manufactured by OHYO KOKH,N KOGYO Co., I.,td.) 2, 5, 30 and
60 minutes after rhc injection. Al.so, radioac.tlvity
(hereinafte.r referred 'to as B in this Exarnpl.e) of the
rest of the whole body was measured an. the same mar:ner as
above. lJsing these measur.,ement result;s, ra.dioactive
ac.Cilmulation per unit wcight of br3in (%ID/g) at r_he
respective t;imc points were calculated iri accordance wi-th
!;he following forrrnlla ( 6) .
Three animals were used for experiment at Lhe.
respective t_imc points.
~0191]
%ID A - x 100 .,. (6)
B x brain iveighC
[0192]
03/25/09 WED 16:22 FAX 416 362 0823 RIDOUT & MAYBEE Z032
CA 02657312 2008-10-28
- 73 -
'f.'he results are stiown in Tab1C: 7. As shown in Table
7, Compounds 10 and 11 showed a.signi,ficant radioactive
accumulation like 123I,:CMPY at the l.i.me poi,.nt of two
m.a.nutes after the injection, ancl t.hen showed a tendency
to rapidly clcar away in 60 minutcs. 'a:'hese results
suggc,-st that Compounds 10 and 1:1. possess excellent
t.r_ansfe.ra.bility to brai.n. and rapid c.l.earance ,f.rom brain
like ''aI-IMS?Y.
[0193]
1_0 T~il>>.e. 7: Radioactivc acCUmulat].ofl in brain a.[ the prperi.t
comphi,rd aiter i.r,Cravcnou:; .i..rl-jcctio.n (rats)
,..,_ -...,... _.. _..,._ ,,., , ..:..
R~~di.oactive~ ~a:c.umulation per unit
Compourid _ ,._weicfY t:. (%ID/q)
After After ACI:er Aft Øc
2 rnin.
~.. __.....,,..... ............... , ..,,.
Compound 1Q 0.62 0.33 0.08 U.02
l:a:-11
........__ ......... .._...._ ...,..._.. .... ,._..
L,x,mp7.e Compound 17. 0.65 0-!13 0.09 0.03
L.I.-:12
..,.... ,.,.,.. -_......... ...,,_
Com.~, arati.v~
Dx<~mpl~ I-II~YY 1.19 0.97 0.23 0.09
Ff
[0.1.94]
T',xample II~13~ ex vivo autoradioqram of_ Compourid 10 using
rats of ar+:tyloid ixljected model
:1.5 [0"195]
(1) AP1._42 (nianu.f.zctured by Poptide Institute, Inc.)
was dissoLvcd in phosphate buffer (pH 7.4) and shaken at
37 C; for 72 hours, to obtain 1 ing/mL of a suspension of
agg?.cgat.ed A(3 (hereinafter refe.rrcd to as amyloi.d
20 suspension in thi.s .Exampie)
[01961
(2) 2_5 ~i.L (corresponding to 2S pg) of the amyioid
suspension was injected into an amygdaloid nucleus on one
of a male Wisl-_ar rat (7-week old). As a control,
side
03/25/09 WED 16:23 FAX 416 362 0823 RIDOUT & MAYBEE ~ 033
CA 02657312 2008-10-28
- 4 -
2.5 L of, a pl-iosphate bka.Lfered physi,ological saline
solution (pH `7.4) was injected inta an amygdal.oid nucleu5
on the other side of the rat. Thc rats were exaruined 1
day after the injection of Lt-ac amyloid suspension and the
phosphaLe buffered physiological saline solution (pH 7.4) .
[0"19"'!]
(3) Compound 10 was dis-so]..ved in a 10 mg/mL ascorbic
ac..i..d-containing phy5iological saline solu-Lion to obtain a
sample solutiori (31 MBq/mL iri radi,oactivir_y concenLration
i.n the sample solutiori). This solution was injected
under r_hi.opcntal anesthesia i.nto the rat through the tail
veii7 (dosage: 0.5 znl,, dosed .r-adioactiv_i.ty: 15 MBq
equivalent).
{U1~8]
7.5 (4) Brai,n was removed 60 minuLeS after the injection
to preparc a brain sla..ce of 10 prn }..n thickness with a
rn.ic.r.otome (type: CM3050S, manufactured by LEICA). The
brain slice was cxposed L-o an imaqing plate for 20 hours,
and then image analysis was carried ouL by usc of a Bio-
imaging Analyzer ('Gype: I3AS-2500; manufactured by
FUJIFILM Corporation).
(019C3]
(5) After the completion of the image analysi:a using
the Bio-imaging Analyzer, pathclog.1cal staining with
'I'hioflavin T was carried out t_o perform imaging by use of
a fluorescenc-e microscope (ma.nufactured by NIKON
Corporation; type: TE2000-11 model; excitation wavelength;
400-440 nm; detec.tior.i wavel.ength: 470 nm) , Thus, it was
03/25/09 WED 16:23 FAX 416 362 0823 RIDOUT & MAYBEE U034
CA 02657312 2008-10-28
- 75 -
confirmed that arrtyl,oid was deposited on rh.c slicc (Fig.
14b) _
[0200]
E'ig. 14 shows images by autoradiogram and Thiofl.avirr
T sk:a.ining of the brain slice of the r.at to which amyloid
was injectcd intr_acerebrally. As shown in I?ig. 14, a
ina.r. ked accumulation of radioacLivi_ty wa;> observed in 'the
amygdaloid r:ucleus ori the si:dc to which the amyloid
suspension was irijccted. On the c~thnr hand, no
5igni..ficant accumulation of radioactivity was observed in
the amygdaloi..d nucleus on the sidc to which the
physiolog;,.cal saline solution was injected, compared with
the other sites. Ori th~- autoradiog.r.am, little
accsmti].ation of radioactivity was observed at 5ites other
than the site Lo which antyloi.d was injected.. From the
result of Th.i..o.flavin T stai.ni.ng, i-t wa;, confirmed Lha.t
amyloid was present iri the site where. radioactivity
aucumulated (Fig. 14b) . These results suggest that
Compound 10 possesses a propc.r_t.,y of accumulati_ng on
intrac.e.r.Fbral amyloid an.d. a capability of imaging
intracerebral amyloid.
[0201]
Examplc II-11: ex vz.vo autoradioyram of Compound ,7.1. usinq
rats of amyloid irrjected model
[0202]
The sartLe operation a, :i n Example I1-13 was conducted
except using a solution (radioactive concentration of :30.
Mbq/rnT, in a saniple solution) of CotYipound ]_1. in a 10 mg/m'L
03/25/09 WED 16:23 FAX 416 362 0823 RIDOUT & MAYBEE 035
CA 02657312 2008-10-28
_ -7 6
ascorbic acid soluL;..on as a sarnple sol.ution.
[02031
Fig. 15 shows imagcs by autoradiogram and 'I'bioflav.i,n
'.I' staini.ng of the brain slice of t.hc rat to which amyloi.d
was i.njected intracere.bral..ly. As showri in Fig. 15, a
marked accumulation of radioactiviLy was observed in. the
amygdaloid nucleras on the .side to which the amyloid
suspcnsion was injected. From the result of 'i'hioflavin T
staining, it was confirrned that arnyl.oid was present in
the si_tc where radioactivity accumul.a.ted (Fig. 1.5b). On
the othei~ lian.d, no signi.ficant accumulation of,
radioactivity was observed iri the amygda.l..oid nucleus Un
the side to which the physio].ogic_a7. ;,aline solution was
in.jected, compared with the othc.r sites. 'I'h.esc results
1.5 suggest that_ C.ompound 11 pc)sscsses a proper.ty of
accumulaL.irig on intracerc-.br. a].. amyloid, and a capabili Cy
of :imaging i.ntracerebral amyloid.
[0204]
Exam le II-,15: Reverse mutati.on tcst
...,.
20 102051,
In order to examine nrutageni-city of CoizLpvund 8,
reverse m.utation test usi,ng Sa1mc,?ne11a (_yph.i.muriunl TA98
and TA7..00 (hereinafter rcferred to as nmes test) was
conducted.
[02061
The test: was conducted, w~.thout addition of S9mix and
with addition of S9mix. Dimcth.ylsulfoxide was used as a
negati.ve control. A posi.t.ive control was 2-- (2-f..uryl) -3-
03/25/09 WED 16:24 FAX 416 362 0823 RIDOUT & MAYBEE 0036
CA 02657312 2008-10-28
- 7 -
( 5-nitro-2-furyl. ) acrylamide .i.n case S9mix was not added,
and 2-aminoanthracene i_n casc S9mix was addcd.
[0207]
The amount of Compound 8 to be added to the test
plate was 7 dosages (geomctric ratio 3) with Lhe maximum
dose being 5000 ug/p.l.ate. A:Ft.er Corrtpound 8 and a strain
(TA98 or TA10U), or Cornpound 8, S9mix and the sLraz.n were
rnixed togeCher, the in.ixture was multi.layere.d usincl sofL
agar on a medium of a test plate, and then incubated at-_
37 <.; for 48 hou.r, s. Judgment was rnade by c.ounLing the
number of reverse mutcation colonies on the plate after
the incubation, and wheri the number of r..cverse mut.ati,on
colonies was not less t;han two Limes the number in
negative c.ontrol and showeci c:oncentration-d.epcndent
increase, rnut:agcnicity was det.crmined. 'Lo he positive.
[02U8]
The results are shown in Tab1e S. '1'hc numbers of
revrrsc mutation colonies of the respcctive strains in
the group treated with Compoun.d 8 were 1e5s than two
t:i.mes the number in t1,.c qroup treat_ed with the negative
controi, regardless of acfdiLi.o.n. of S9mix and Che addition
amount af a sa.mple to be examined. On Lhe other hand, a
marked. incrcase in tYie number of revelse mutation
co,lonies was obser-ved in the group treated with the
positive eontrol,. From the aforc:ment.ioneci results, it is
judged that Compound Q is nec-ative in the AmeS test and
has rio mutagenicity.
[025g]
03/25/09 WED 16:24 FAX 416 362 0823 RIDOUT & MAYBEE U037
CA 02657312 2008-10-28
- 78 -
T@b3.e 8: F\esultS o.Y ILmG:~ tcsi:
Mur.lgenicity
Compound Witho,at add..i. L:ion of With 6adclition oi
S ym.:i. c S' 9mi x
.._ ,,,.
TA1U0 TA96 TA100
.. _.. _., .,- -.....,.
F;xample
Compound f3 Ncgat:i.ve Nc,qative Negative Negativc_
INDUS'.['RIAL APf?LICABII,ITY
[0210~
'I.'hc compo~in.d.s and di..aqnostic agents of the presE'nt.
invention can be util.i<.ed in the field of d.iagnoscic-z..
BRIEF Dr:;:,SCRIFTION Q.:r THE DRnw:rNGS
[02:11]
~'ig. J. is a scheme of synthYSi,s of 6-
tributylsL-ann,yl-2-(4'-hyd.roxyphenyl)imi.dazo[1,2-
a]py.ridine.
Fig. 2 is a schcme of syrit:hesi_s of 6-bromo=.--2- (4' -
hydr_oxyphenyl)im.i.da.zo[1,2-a]pyridi.nc.
Fig. 3 is a sc.heme of syrithesis of 2-(4'-
hyd,r_oxyphcnyl ) -6-iodo.i.mi.dazo [ 1, 2-a] pyi-idi.ne .
Fig. 4 i5 a scheme of synt:hesi.s of 2-(4'-
hydr.oxyphenyl)-6-iodoirnidazo[1,2-a]pyrimidine.
Fig. 5 is a sc.liemc: of syrithesis of 6- (3' -
fluoropropc~xy) -~-?õN (4' -hydroxyphen.yu. ) .i.m.i..d.azo [1, 2-a] pyridine.
Fig. 6 i.s a.scheme of 5yn~.hl,si.5 of 6-bromo-2- (4' --
hyclroxyph(z-n.y1) imidazo [ 1, 2-a] pyrimidine .
Fig. '7 is a scheme of synthesis oF 6-.f. l.uoro-2- ( 4' -
hydroxyphPnyl. ) imi.dazo [1, 2-a]pyri.di.ne.
Fig. 8 i_s a scheme of :;yrithcsis of 2-(4'-
hydr.oxypher.iyl..)-6-nitroimidazo[1,2-a.]py.r.idine.
Fig. 9(a) is an au.t.oradiogram ol the brai..n 51.icc
03/25/09 WED 16:24 FAX 416 362 0823 RIDOUT & MAYBEE 10038
CA 02657312 2008-10-28
- i~ ...
after the injection of Compound 6, and Fig. 9 (u) is a
fluor.,cscenL microscropic image of the Thiofl,avin 'r stairied
(a magnification of the 5a_te ta wl-iich 'the amyloid
sample
suspension was inject;cd).
Fig. 10 is a scheme of synthesis of 6-
tributylstannyl-2-(4'-hydroxyphen.yl)irru_d.azo[1,2-
a.]pyril7iidinC .
Fig. .1_1 is a scheme of 5ynthesi5 of 6-
-Cributylstannyl-2- (4'-hydroxyphenyl) im.i..dazo [1, 2.==
a]pyrazine.
Fig. 12 i.: a scheme of synthesis of 2- ( 4' -
hy(Jr.oxyphenyl) -6-iodo.imidazo [1, 2--a.]py.razine.
Fig. 13 is a scheme of syn.t.hcsis of 8-
tributylstan.nyl-2-(4`-hydroxyphrnyl)imidazo[1,2-
a]pyrid.i.ne.
Fig. 14 (a) is an autoradiograM of t-he brain slice
after the injecti-on of Compourid 10, and Fig. 1~ (b) is a
fluorescent microscopic image of the Thioflavin T stained
sample (a magnification of the site r.o whi.c-h, the amyloid
suspensiori wa5 .injected).
t'.,g. 15(a) is an auLorad_:og.r.a.m of the brain slice
after th.c injection of Compound 11, and Fig. 15(b) is a
fluorescent microscop3_c image of the Tha.o.f,-.l.,)vi.n T stained
samplP (a magnification of t_he si.tE: to which the amyloid
suspension was injecaed).