Note: Descriptions are shown in the official language in which they were submitted.
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
Systems and Methods for Providing Contact Lenses to Consumers
RELATED APPLICATIONS
[0001] The present application claims the benefit under 35 U.S.C.
119(e) of U.S. Provisional Patent Application No. 60/820,432 filed 7/26/2006
titled
"Contact Lens Package," which application is hereby incorporated by reference
in
its entirety.
BACKGROUND
[0002] Contact lenses are small lenses that are worn directly on the eye
and are widely used to correct vision variations. Contact lenses have
traditionally
been either rigid (hard) or soft. One important consideration in wearing
contact
is lenses is eye health, another is convenience. Contact lenses are placed
directly on
the eye. Consequently, it is important that the contact lenses and their
packaging
not introduce any unwanted contamination into the eye.
[0003] The more commonly used soft contact lenses are designed to be
used for a specified number of days, and are then to be replaced. For example,
many contact lenses are to be used for only one day after which they are to be
disposed of. This frequent replacement of soft contact lenses is highly
desirable
because it is difficult for a patient to sterilize or otherwise adequately
clean the
inevitable contamination that builds up on the lenses over time.
[0004] Another consideration in the use and manufacture of contact
lenses is convenience. Contact lenses that are intended to be replaced more
often
may not be as convenient to the user because of the frequent need to buy or
otherwise replace the contact lenses. In addition, the secondary packaging of
contact lenses is often difficult to understand. Traditionally the contact
lenses are
provided in a box which may contain multiple 'blister packages' of contact
lenses.
Although for some contact lens wearers only one level or strength of contact
lenses
1
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
is required, many users require a different strength for each eye which may
necessitate the ordering of two different boxes of contact lenses.
[0005] Additionally, when contact lenses are purchased online or over
the phone, the secondary packaging of the contact lens does not include the
patient's prescription information. This may be problematic, especially if the
patient
requires different strength contacts for each eye because the patient may not
always remember which contact strength corresponds to each eye. This problem
may be compounded if the user does not remember to open a new box of contact
lenses before disposing of the previous set of lenses. It may be difficult for
the
user to determine which of two boxes of contact lenses is intended for a
specific
eye, especially since the user may have significant visual impairment.
BRIEF DESCRIPTION OF THE DRAWINGS
i5 [0006] The accompanying drawings illustrate various embodiments of the
present system and method and are a part of the specification. The illustrated
embodiments are merely examples of the present system and method and do not
limit the scope thereof.
[0007] FIG. 1A is a front perspective view of a traditional secondary
contact lens package, according to the teachings of the prior art.
[0008] FIG. 1 B is a side perspective view of a traditional secondary
contact lens package, according to the teachings of the prior art.
[0009] FIG. 2A is a front perspective view of a traditional secondary
contact lens package, according to the teachings of the prior art.
[0010] FIG. 2B is a side perspective view of a traditional secondary
contact lens package, according to the teachings of the prior art.
[0011] FIG. 3 is a perspective view of a primary contact lens blister
package according to one embodiment.
[0012] FIG. 4 is a perspective view of another primary contact lens
package according to one embodiment.
2
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
[0013] FIG. 5 is a perspective view of a contact lens dispensing unit
according to one embodiment.
[0014] FIG. 6 is a perspective view of a secondary contact lens package
containing a six months supply of contact lenses pre-loaded into contact lens
dispensing units according to one exemplary embodiment.
[0015] FIG. 7 is a perspective view of another secondary contact lens
package according to one exemplary embodiment.
[0016] FIG. 8 is a flow-chart of an exemplary method.
[0017] FIG. 9 illustrates the packaging and flow of a contact lens
according to one exemplary embodiment of a contact lens packaging system.
[0018] Throughout the drawings, identical reference numbers designate
similar, but not necessarily identical, elements.
DETAILED DESCRIPTION
[0019] The present specification describes systems and methods for
providing contact lenses to users. According to one exemplary embodiment, the
secondary contact lens package provided by the manufacturer includes the
patient's prescription information such as the patient's name, the prescribing
doctor's name and phone number, usage information, and other information
related
specifically to the patient's prescription. Additionally, the present system
and
method includes a method of doing business in which contact lenses may be
stored by a contact lens provider and supplied the consumer in an efficient
and
economical on-demand manner.
[0020] Further details of the present exemplary systems and methods will
be provided below with reference to the figures. In the following description,
for
purposes of explanation, numerous specific details are set forth in order to
provide
a thorough understanding of the present systems and methods. It will be
apparent,
however, that the present apparatus, systems and methods may be practiced
without these specific details. Reference in the specification to "an
embodiment,"
3
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
"an example" or similar language means that a particular feature, structure,
or
characteristic described in connection with the embodiment or example is
included
in at least that one embodiment, but not necessarily in other embodiments. The
various instances of the phrase "in one embodiment" or similar phrases in
various
places in the specification are not necessarily all referring to the same
embodiment.
[0021] As used herein and in the appended claims, the terms "primary
package," "primary packaging," or "primary case" will be used to refer to the
packaging in which a contact lens is placed during the manufacturing
processes.
This package is traditionally a`blister' package but other primary packages
are also
used. Similarly, the term "secondary case," "secondary contact lens case" and
"secondary package" shall be used interchangeably and shall be interpreted
broadly as including any container or packaging that a lens or supply of
lenses may
be placed in subsequent to being placed into a primary package. Secondary
s5 packaging includes any box or package that a supply of contact lenses may
be
placed in by the manufacturer for delivery to the lens wearer, a distributor,
or to
another destination.
[0022] As used herein and in the appended claims, the terms
"manufacturer" or "distributor" will be used to refer to a third party
provider of
contacts lenses who provide contact lenses to individuals based on
prescriptions.
The terms "manufacturer" or "distributor" may refer to any business,
individual, or
company that sells or distributes contact lenses, excluding optometrists,
ophthalmologists or others who have in-person contact with the patient.
[0023] Turning now to the figures, FIGS. 1A and 1 B illustrate a front
perspective view and a side perspective view, respectively, of a typical prior
art
secondary contact lens package. Similarly, FIGS. 2A and 2B show a front
perspective view and a back perspective view, respectively, of an alternative
prior
art secondary contact lens package.
[0024] As mentioned previously, the traditional secondary contact lens
packages (100) provided by manufactures such as those illustrated in FIGS. 1A
4
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
through 2B fail to notify or otherwise provide the user with prescription
information.
Consequently, a user may have difficulty identifying which box or strength of
lens is
intended for a particular eye. This difficulty is exacerbated by the fact that
contact
lenses are typically packaged according to the power or strength of the
lenses. In
practice, a user who does not readily recall their prescription will likely
receive two
boxes of lenses with no information indicating which box contains lenses for
the
right eye and which contains lenses for the left; leaving the patient to
decide for
themselves.
[0025] As shown in FIGS. 1A-1 B, traditional secondary contact lens
packages (100) provided by manufactures often include a strength or power
indicator (110). They also may include a brand-name (105) and a bar-code
(115).
Secondary packages may be constructed from any number of materials including,
but in no way limited to, cardboard, plastic, or paper. Furthermore, secondary
packages may include, according to various exemplary embodiments, re-closable
mating fasteners (120) such as a tab and slot fastening device, Velcro, and/or
a
resealable adhesive. Typically, secondary packages contain from one to two
hundred contact lenses depending on the size of the package and the type of
the
contact lenses.
[0026] According to one exemplary embodiment, the present system
includes one or more primary contact les packages contained within a secondary
package. According to this exemplary embodiment, the secondary package
includes prescription information specific to an intended patient. As used in
the
present specification, and in the appended claims, the term "prescription
information" is meant to be understood as including information specific to a
contact lens receiving patient such as a prescribing doctor's information, a
phone
number of a prescribing doctor, or personal patient information not
traditionally
included on secondary contact lens packages. Various components of the present
system will be described in detail below with reference to FIGS. 3 through 9.
[0027] Referring now to FIG. 3, there is shown a typical prior art primary
contact lens package (300) which is formed in two parts. As illustrated, the
primary
5
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
contact lens package (300) includes a blister pack member (310) which is
sealed
by a membrane (320) forming a lid on the package (300) and which may be peeled
away to release a contact lens (325) contained therein. The primary contact
lens
package (300) illustrated in FIG. 3 is shown with the membrane (320) peeled
away
to expose the contact lens (325). Typically, the blister pack member (310)
includes
a preformed blister pack having a profiled recess (315) formed therein which
provides a recess in which a lens (325) may be placed. According to
traditional
forming methods, the blister pack member (310) is typically injection molded
and
the contact lens package (300) is completed with the coupling of a sealing
membrane (320) which mates with a flange (330) to create a sterile seal.
During
packaging, the contact lens (325) is immersed in a solution (335) which keeps
the
lens (325) hydrated until it is removed from the pack (300).
[0028] FIG. 4 is a top perspective view of an alternative primary
packaging for contact lenses that may be included in the present exemplary
system. As illustrated in FIG. 4, the exemplary contact lens package (400)
includes a center substrate (425) having a top sheet member (430) coupled to
the
top surface of the substrate (425). The top sheet member (430) may be coupled
to
the top surface of the substrate (425) by a secure but detachable connection
such
that the top sheet member (430) can be separated from the substrate (425) with
a
constant and relatively low pulling force. Additionally, the top sheet member
(430)
is coupled to the top surface of the substrate (425) sufficient to allow the
exemplary
contact lens package (400) to be autoclaved. Further, FIG. 4 shows that the
top
sheet member (430) may contain various words and/or images including, but in
no
way limited to a brand name (415), a design (420), and/or information about
the
contact (410), for example, that it is for the left or right eye, and
instructions for use.
According to one exemplary embodiment, contact lenses in their primary
packaging
may be pre-loaded into one or more contact lens dispensers, as shown in FIG.
5,
before being packaged in secondary packaging.
[0029] FIG. 5 depicts a contact lens dispenser (500) that may be
included in the secondary packaging as provided by the manufacturer, according
to
6
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
one exemplary embodiment. As illustrated, the contact lens dispenser (500) may
include a base member (510) and a hinged lid (515) coupled to the base member.
When loaded into the dispenser (500) of the present exemplary embodiment, the
primary contact lens packaging (520) is suitably flat, allowing each primary
contact
lens package containing one contact lens to be independently slid out from the
container. As the top primary contact lens package (520) is removed, the
dispenser may, according to one exemplary embodiment, be configured to raise
the level of the remaining packages allowing access to the subsequent package.
The dispenser (500) may also include a retention device (525) configured to
secure
the primary contact lens packages (520) to the dispenser. Particularly,
according
to one exemplary embodiment, the retention device (525) can be configured to
hold
the primary packages (520) in place when the exemplary contact lens dispenser
(500) is shaken or held upside-down.
[0030] The dispenser may also be configured to separably house the
contact lenses and their respective primary contact lens packaging (520)
intended
for use in the left and right eyes of a patient. In some embodiments the
primary
packaging (520) may contain a label such as an "L" or "R" in order to allow
the user
to easily differentiate between the lenses. In other embodiments the primary
contact lens packaging (520) may be one color for the lenses intended for use
on
the left eye, and a different color for the lenses intended for use on the
right eye. In
yet another exemplary embodiment, the primary contact lens packaging (520)
containing a contact lens intended to be worn on a first eye of the patient is
a first
color such as blue, and the primary contact lens packaging (520) containing a
contact lens to be worn on a second eye is a second color such as white.
[0031] As mentioned previously, any type of primary contact lens
packages, including those illustrated in FIGS. 3, 4, and 5, can be included in
the
present exemplary secondary contact lens package including prescription
information. FIG. 6 illustrates a perspective view of a secondary contact lens
package (600), according to one exemplary embodiment. As illustrated in FIG.
6,
the present exemplary secondary contact lens package (600) includes
prescription
7
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
information (640) specific to an intended patient. As discussed above, the
prescription information (640) may include information such as the patient's
name,
the prescribing doctor, the doctor's phone number, usage instructions, a
prescription number, or other information specifically related to the
patient's
prescription. In the present exemplary embodiment, the prescription
information
(640) may be printed directly on the secondary packaging. Alternatively, the
prescription information (640) may be affixed to the secondary contact lens
package (600) by any number of methods including, but in no way limited to,
printing the prescription information onto an adhesive label and attaching the
label
to the secondary contact lens package (600) before the secondary contact lens
package (600) is shipped to the patient.
[0032] According to the present exemplary system and method, the
inclusion of prescription information (640) on the secondary contact lens
package
(600) increases the level of safety experienced by a patient when using
contact
lenses. First, the inclusion of personal prescription information (640) allows
a
patient to be assured that the secondary contact lens package (600) containing
contact lenses is specifically for them and contains contact lenses with the
proper
specifications. Second, the inclusion of a patient's personal prescription
information (640) including a prescribing doctor's name and/or contact
information
will provide a patient with a convenient reference should questions arise.
[0033] Continuing again with FIG. 6, the present exemplary secondary
contact lens package (600) also includes according to one exemplary
embodiment,
a left contact lens power indicator (625) and a right contact lens power
indicator
(630). Also included may be a brand name (650), a bar-code (635) and other
information regarding the contact lenses, the manufacturer, and the like.
According
to one exemplary embodiment, the primary contact lens packages (400) may be
disposed within the secondary contact lens package (600) of the present
embodiment. The primary contact lens packages (400) may be contained
individually within the secondary contact lens package (600) or they may be
disposed inside contact lens dispensers (500) prior to insertion into the
secondary
8
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
package. Additionally, the primary contact lens packages (400) may be
contained
within separate bins or portions of the secondary contact lens package (600)
indicative of which eye of the patient the contact lenses contained in the
primary
contact lens packages (400) are intended for.
[0034] As illustrated in FIG. 6, the secondary contact lens package (600)
of the present exemplary embodiment includes a bottom member (610). The
bottom member is preferably made from a substantially resilient and form
conserving material such as, by way of example only, cardboard or plastic. The
bottom member may be configured to hold any number of contact lenses housed in
primary contact lens packaging. The bottom member (610) may be enclosed by a
plastic covering (645) such as a heat shrink wrap, vacuum sealed plastic, or
the
like, according to one exemplary embodiment. This plastic (645) may be
substantially clear and configured to allow a clear view of the contents of
the
secondary contact lens package (600).
[0035] FIG. 7 is a perspective view of another exemplary embodiment of
a secondary contact lens package. As illustrated in FIG. 7, the secondary
contact
lens package (600) includes prescription information (640). As discussed above
the prescription information (640), may include information such as the
patient's
name, the prescribing doctor's name, the prescribing doctor's phone number,
usage instructions, a prescription number, or other information related to the
prescription. Again, the prescription information (640) may be printed
directly on
the secondary contact lens packaging (600). Alternatively, the prescription
information may be printed by the manufacturer or distributor onto an adhesive
label and attached to the secondary contact lens package (600) before the
package is shipped to the patient.
[0036] The exemplary embodiment of a secondary contact lens package
(600) illustrated in FIG. 7 also includes a left contact lens power indicator
(625) and
a right contact lens power indicator (630). Additionally, the markings
disposed on
the outer surface of the secondary contact lens package (600) may also
include,
but are in no way limited to, a brand name (650), a bar-code (635), and/or
other
9
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
information regarding the contact lenses, the manufacturer, etc. Additionally,
similar to the exemplary embodiment illustrated in FIG. 6, the primary contact
lens
packages (400) disposed within the secondary contact lens package (600) may be
located inside contact lens dispensers (500). Alternatively, the primary
contact
lens packages (400) may be placed directly within the secondary contact lens
package (600) without any dispensers (500).
[0037] The secondary contact lens package (600) of the exemplary
embodiment illustrated in FIG. 7 may be made from any number of structural
materials including, but in no way limited to, cardboard, plastic, structural
paper, or
any other substantially resilient material suitable for the manufacture of
containers.
According to one exemplary embodiment, the secondary contact lens package
(600) illustrated in FIG. 7 is a box structure made from a single sheet of
cardboard,
formed so as to be able to contain between 1 and several hundred primary
contact
lens packages. The secondary contact lens package of the present exemplary
embodiment also includes a mating fastener such as a tab and slot fastener
(615)
configured such that after the package (600) is initially opened, the user is
able to
re-close the package (600) in order to store the unused contact lenses.
Exemplary Methods
[0038] Referring now to FIG. 8, a flowchart illustrating an exemplary
method of providing contact lenses to consumers is shown. According to the
exemplary method, the secondary contact lens cases provided to consumers
include personalized prescription information. As illustrated in FIG. 8, the
exemplary method (800) includes manufacturing (step 801) contact lenses having
different powers and geometries. The contact lenses are then packaged (step
802)
in primary packaging. Once packaged, the contact lenses can then be grouped
(step 803) according to the statistically most common combinations of power
and
geometry prescribed. These most common combinations can then be packaged
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
together in a secondary packaging step (step 804). Once packaged, the various
combinations can then be held in stock until they are printed and shipped
(step
805) to the consumer. Further details of the present exemplary method will be
provided below.
[0039] As mentioned above, the initial step in the exemplary method
includes manufacturing the contact lenses including different powers and
geometries (step 801). According to one exemplary embodiment, the
manufacturing of the contact lenses may be performed according to any known
lens manufacturing methods including, but in no way limited to, spin-casting,
lathe
cutting, or cast molding. According to the present exemplary embodiment, the
manufacture of various powers and geometries may be performed in batches for
each power and geometry, for efficiency.
[0040] Once the contact lenses are manufactured (step 801), the contact
lenses are packaged in primary packages (step 802). While the manufactured
contact lenses may be stored in any number of primary contact lens packages,
according to one exemplary embodiment, the contact lenses are packaged in one
of the exemplary primary contact lens packages illustrated in FIGS. 3 and 4.
[0041] Once packaged in a primary contact lens package (step 802) the
primary contact lens packages are grouped according to the statistically most
common combinations of power and geometry prescribed by optometrists.
Particularly, according to on exemplary embodiment, it is possible to assemble
the
most frequently prescribed combinations of power and geometry and thereby have
sufficient combinations to satisfy the demand of over 98% of the population.
This
allows a provider to stock and readily locate a left and right contact lens
supply for
a prescribed contact lens combination. Research suggests that as little as one
thousand combinations may be adequate to serve 98 percent of the contact lens
wearing population. For example, more than 95,000 people may have a
prescription for -3.75 for their right eye and -3.25 for their left. The
manufacture
may need to only manufacture and stock a relatively small percentage of the
total
11
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
possible combinations in order to fulfill a very high percentage of the orders
for
contact lenses.
[0042] Once the desired lens combinations have been manufactured,
packaged in primary contact lens packages, and grouped according to desired
combinations, the combinations can be packaged in secondary contact lens cases
(step 804). As mentioned previously, the groups of primary contact lens
packages
can be packaged in any number of secondary contact lens cases, including those
illustrated in FIGS. 6 and 7.
[0043] The groupings of contact lenses contained in secondary contact
lens packages can be kept as stock by a manufacturer or distributor until a
request
for the combination is received (step 805). According to one exemplary
embodiment, once a request for a specified combination is received, including
a
patient's prescription information, the secondary contact lens package can be
marked and shipped. The marking of the secondary contact lens case can be
marked with the patient specific prescription information using any number of
marking methods including, but in no way limited to, printing methods to print
the
information directly on the secondary contact lens box such as laser printing
or dot-
matrix printing, the application of a label or other adhesive substrate
containing the
prescription information, or other similar marking means. Alternatively, the
prescription information may be imparted onto the secondary contact lens case
during manufacture if a specialized combination of lenses is manufactured for
a
patient.
[0044] The present exemplary system and method allows the
manufacture/distributor of contact lenses to increase the convenience of
purchasing and wearing contact lenses for the patients. According to the
present
exemplary system and method, the patient no longer purchases and receives two
separate packages when ordering a supply of contact lenses for both eyes. This
exemplary method can also decrease costs for the manufacturers. Particularly,
shipping both sets of contact lenses in a single box may significantly
decrease
shipping costs. Manufacturers have not heretofore employed the present method
12
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
because it was thought to be too difficult to maintain the thousands of
different
combinations necessary to serve the entire population.
[0045] It is therefore -an exemplary element of the present exemplary
method to only stock a percentage of the possible contact lens combinations,
rather than stock the entire range of possible contact lens combinations. In
some
exemplary embodiments a manufacturer may only stock the combinations of
contact lenses necessary to serve approximately 98 percent of the population.
Research suggests that as little as one thousand combinations may be adequate
to
serve 98 percent of the contact lens wearing population.
Exemplary Systems
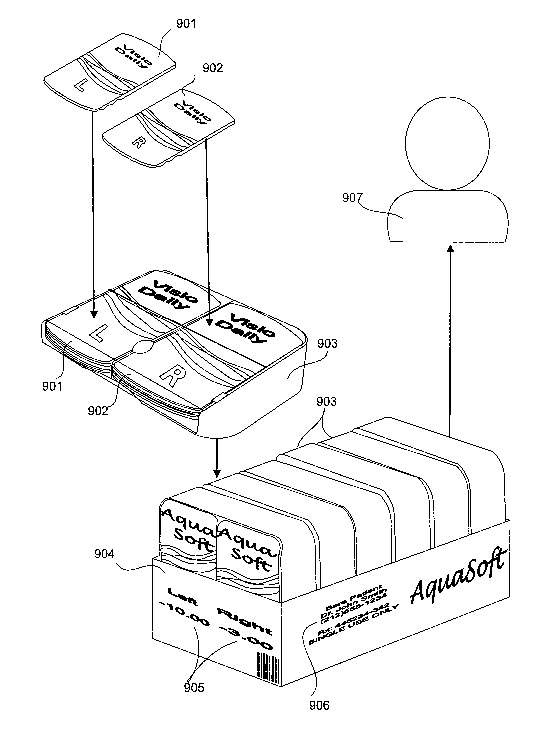
[0046] Referring now to FIG. 9, an exemplary system for providing
contact lenses to consumers according to the present exemplary systems and
methods is shown. The exemplary system illustrated in FIG. 9 may decrease the
costs for the manufacturers and increase the convenience of ordering and using
contact lenses for the end users. As shown, contact lenses are first
manufactured
and packaged in primary packaging (901, 902). The primary packaging may be
provided, according to one exemplary embodiment, with a label indicating which
eye the contact lens should be placed on. The contact lenses (901, 902) can
then
pre-loaded into dispensing units (903) if desired. According to one exemplary
embodiment, the dispensing units (903) may be configured to securely contain
fifteen left contacts (901) and fifteen right contacts (902) in separate
compartments.
This separation of the contact lenses may add to the overall convenience of
the
system. The dispensing units (903) may then be packaged in groups of six for
example, in a secondary contact lens package (904).
[0047] Although the secondary package (906) depicted contains six
dispensers (903), it is understood that any number of dispensers (903) may be
packaged together, including but not limited to one, three, six, or twelve.
The
secondary package (906) of the present embodiment also includes a label
containing prescription information. This label (906) may serve to reinforce
the
13
CA 02657572 2009-01-12
WO 2008/029293 PCT/IB2007/003681
idea that contact lenses are medical devices and should be treated
accordingly.
The label (906) may also provide the user with usage information and
information
about the prescribing doctor. Also located on the secondary package (904) are
labels (905) indicating the power of the contact lenses contained within the
package (904).
[0048] After being labeled, the secondary package may then be shipped
or otherwise delivered to the user (907). The effect of providing the user
with a
single package containing two powers of contact lenses pre-loaded into
dispensing
units may be to make the purchasing and use of contact lenses more convenient.
[0049] In conclusion, the present exemplary system, method, and
apparatus provide a more convenient and cost effective way to provide contact
lenses to consumers. The inclusion of prescription information on the
secondary
packaging, and contact lenses for both eyes pre-loaded into dispensers will
simplify
the purchasing and use of contact lenses. The costs of shipping the contact
lenses
will also be reduced.
[0050] The preceding description has been presented only to illustrate
and describe embodiments of the exemplary systems, methods, and apparatus. It
is not intended to be exhaustive or to limit the systems and methods to any
precise
form disclosed. Many modifications and variations are possible in light of the
above teaching.
14