Note: Descriptions are shown in the official language in which they were submitted.
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
1
DESCRIPTION
MEDICINE EJECTION DEVICE
TECHNICAL FIELD
The present invention relates to a medicine
ejection device,for ejecting a medicine to be inhaled
by a user.
BACKGROUND ART
The medicine ejection devices disclosed in
International Publication No. W095/01137 and
International Publication No. W002/04043 are bringing
to realization treatments of users who utilize
information databases including electronic medical
charts in combination. Such medicine ejection devices
have a memory unit for storing personal information on
users including information on their medical charts and
prescriptions. These medicine ejection devices also
are portable terminals also serving as inhalers to have
medicines inhaled by their users. They also have a
spraying control unit which controls inhalers according
to each user's inhalation profile and sprays a medicin'e
so that he or she can inhale the medicine in accordance
with information from the prescription.
Within such a medicine ejection device, there
usually is a passage over which atomized medicine is
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
2
carried (hereinafter in this specification referred to
as an "air flow path"),from the point where the
medicine in the device until it is administered into
the user's body from the portion ejecting a medicine
within the device. In this process, part of the
ejected medicine may conceivably stick to the internal
wall face of the air flow path. Leaving it intact
might invite multiplication of various germs on the
internal wall of the air flow path where the medicine
has stuck, and would be undesirable from the sanitary
point of view. Thus, conventional medicine ejection
devices involve an'unsolved problem that, because the
internal wall of the air flow path in the device is
smeared when the patient,inhales, the member itself
constituting the air flow path should be taken out and
washed or replaced with a new member.
An object of the present invention, attempted in
view of the unsolved problem noted above, is to provide
a medicine ejection device which permits ready
remedying of contamination of the air flow path in the
medicine ejection device.
DISCLOSURE OF THE INVENTION
In view of the problem noted above, a medicine
ejection device according to the invention is a
medicine ejection device which-ejects a medicine to be
inhaled by a user via a suction port, comprising:
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
3
a flow path member linked to the suction port and
capable of forming an air flow path in which the
medicine is guided by,the inhalation by the user to the
suction port, and
an internal wall face constituting member which
is intended for formation of the internal wall of the
air flow path and allows detachable fitting in the flow
path member.
Also in view of the problem noted above, another
medicine ejection device according to the invention is
a medicine ejection device which ejects a medicine to
be inhaled by a user via an suction port, comprising:
a flow path member linked to the suction port and
capable of forming an air flow path in which the
medicine is guided by the inhalation by the user to the
suctio, n port,
a medicine ejection portion which ejects the
medicine to the air flow path formed in the flow path
member, and
a removing unit for removing the medicine
delivered from the medicine ejection portion and having
stuck to the internal wall of the air flow path.
In a medicine ejection device according to the
invention, as it is provided with a detachable internal
wall face constituting member which can.form a new
internal wall face within the air flow path, the stuck
medicine can be easily removed if the user detaches the
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
4
internal wall face constituting member after inhalation.
As a result, the air flow path can be kept clean.
In another medicine ejection device according to
the invention,, as it permits removal of the medicine
stuck within the air flow path after the use of the
medicine ejection device, the air flow path within the
device can be kept sanitary. Also, the trouble of
washing or replacing a member forming the air flow path
every time can be reduced. .
Further features of the present invention will
become apparent from the following description of
exemplary embodiments with reference to the attached
drawings.
BRIEF DESCRIPTION OF THE DRAWING
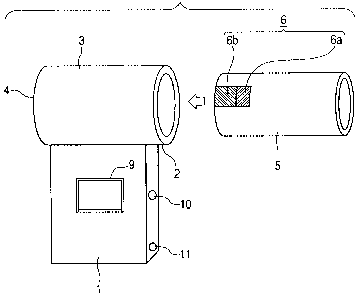
Fig. 1 shows an external view of an inhaler in.
one embodiment of the present invention;
Fig. 2 is a block diagram illustrating the
electrical configuration of the inhaler shown in Fig.
1;
Fig. 3 is a flow chart showing the operation of
the inhaler;
Fig. 4 shows a perspective view of an inhaler in
a second embodiment of the present invention;
Fig. 5 shows a section of the inhaler shown in
Fig. 4;
Fig. 6 shows a modified version of the inhaler
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
shown in Fig. 4, illustrating a section of the inhaler
in which a film 12 is disposed to the area of a suction
port 2;
Fig. 7 shows a perspective view of an inhaler in
5 a third embodiment of the present invention;
Fig. 8 shows a sectioh of the inhaler shown in,
Fig. ,7;
Fig. 9 illustrates the inhaler shown in Fig. 7 as
viewed from an air inlet 4 in a state in which a sheet
15 is folded back;
Fig. 10 shoias a section of the vicinities of an
air flow path in an inhaler in a fourth embodiment of
the present invention;
Fig. 11 shows a modified version of the inhaler
shown in Fig. 10;
Fig. 12 shows a perspective view of an inhaler in
a fifth embodiment of the present invention;
Fig. 13 illustrates the inhaler shown in Fig. 12
as viewed from the suction port 2 side;
Fig. 14 shows an enlarged view of the save
position for a wiper 25 shown in Fig. 13; and
Fig. 15 is a block diagram illustratingthe
electrical configuration of the inhaler shown in Fig.
12, including a stepping motor 27.
BEST MODE FOR CARRYING OUT THE INVENTION
A mode for carrying out the invention embodied in
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
6
a medicine ejection device will be described with
reference to an inhaler, an example made portable for
its user. Fig. 1 shows the configuration'of the
inhaler in one embodiment of the present invention.
Fig. 2 is a block diagram illustrating the electrical
configuration of the inhaler shown in Fig. 1. The
inhaler has a configuration in which a flow path member
3 and a suction port (mouthpiece) 2, to be fitted to
the mouth or nose of the user for inhalation, are -
fitted to a device body 1. At one end of the flow path
member 3, an opening (air inlet .4) is provided to
generate an air flow in the flow path member 3 when the
user inhales from the suction port 2. The other end of
the flow path member 3 is linked to the suction port 2.
When the user inhales, an air flow is generated from
the air inlet 4 toward the suction port 2. Then,
ejecting a medicine into the air flow path in the flow
path member 3 causes the medicine to be carried toward
the suction port 2 and then into the user's mouth.
To be noted here, the "flow path member" in this
specification means a member which constitutes,the air
flow path, which is the passage of the medicine from
the point where the medicine is ejected within the
medicine ejection device to the suction port. Thus, a
space formed in the flow path member constitutes the
"air flow path".
However, part of the medicine ejected into the
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
7
air flow path is feared to remain stuck to the internal
wall of the air flow path. If the flow path.member 3
and the suction port 2 are made detachable from the
device body, the member can be washed every time.
However, this inhaler has a mechanism which remov,es any
medicine stuck to the internal wall of the air flow
path,, and accordingly. the flow path member 3 need not
'be washed every time. The material to constitute the
flow path member 3 is subject to no particular
limitation, but can be selected as desired according to
the specification of the medicine ejection device.
Typical examples include plastic, metallic or rubber
members. Incidentally, the flow path member and the
suction port can be configured either as mutually
linkable separate members or as an integrated body.
A controller 8 shown in Fig. 2, disposed within
the device body 1, is a CPU which controls the
operations of the whole inhaler including the driving
of an ejection head 6a. When a signal of detection of
inhalation is output from a pressure sensor 7 for
detecting a negative pressure generated in the air flow
path by the user's inhalation, the controller 8
transmits a drive signal to the ejec.tion head 6a to
start the delivery of the medicine in synchronism with
the detection signal. In addition to these, a display
unit 9 to enable the user to visually recognize any
desired information on the operation of the inhaler,
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
8
ejection conditions and the like may also be disposed.
Refereince numeral 10 denotes a power switch and 11, a
power indicator LED which is lit when power supply to
the inhaler is on.
According to the invention, the medicine ejection
portion (ejection head) has an ejection pressure
generating element of any desired type. Conceivable
examples of the ejection pressure generating element
included,an electrothermal transducer which provides
thermal energy to the medicine or an electromechanical
transducer which provides mechanical energy to it.
Typical examples of the medicine ejecting method
include a method by which the medicine is given thermal
energy by an electrothermal transducer and'ejected from
-15 the ejection nozzle (thermal jet system) and another
method by which the medicine is ejected from the
ejection nozzle by using the vibratory pressure of an
electromechanical transducer (e.g. piezoelectric
element) which.gives mechanical energy to the medicine.
The ejecting method can be selected according to the
type of the medicine and other factors.
When the thermal jet system is used, the bore of
the ejection nozzle, the calorific value of thermal
pulse used for ejection, and the size precision and
reproducibility of a micro-heater as the electrothermal
transducer can be increased for each individual liquid
ejection unit. For this reason, it is possible to
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
9
achieve a narrow distribution of drip diameters. The
production cost of the head is low, and this enhances,
the applicability to small units which require frequent
headreplacement. Therefore, especially where the
medicine ejection device is required to be portable and
handy, a thermal jet ejection device can be used.
In this invention, the ejection head may be
configured either as a medicine ejection cartridge
integrated with a reservoir for accommodating the
medicine or as a separate item from the reservoir.
Although the medicine ejection cartridge is integrated
with an internal wall face constituting member 5, to be
described in more detail afterwards in the illustration
in Fig. 1, usually a fitting portion which enables the
cartridge to be fitted to the device body 1, and the
ejection head is directed into the air flow path so
that it can eject the medicine into the air flow path.
The concept of the medicine in the context of the
invention is not limited to medicines which are
pharmaceutical compounds manifesting pharmacological
and physiological actions, but also covers, in addition
to pharmaceutical compounds, contents intended as
components for scenting and flavoring, dyes and
pigments. The medicine may be either liquid or powder.
The medicine for use in this invention is a
liquid medicine or a liquid medium containing a
medicine. The liquid medicine may contain any desired
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
additive. The state of the medicine in the liquid may
be dissolved, dispersed, emulsified, suspended or
slurried, more preferably uniformized in the liquid.
When a medicine liquid is to be used as the
5 medicine, it is preferable for the main medium of the
liquid to be water or an organic substance, and water
as the main medium is more preferable in view of the
circumstance where it is intended for administration to
a living body.
10 The mechanism for removing the medicine stuck
within the air flow path, which is a characteristic
feature of the invention, may be one or another of the
following propositions. One is the formation of a new
internal wall face over the internal wall face of the
air flow path to which a medicine remains stuck after
the medicine is ejected. Another is to dispose a
wiping unit to clean the internal wall face of the air
flow path to which a medicine remains stuck after the
medicine is ejected.
The internal wall face of the air flow path
should be sanitary because it comes into contact with
the air. flow which carries the medicine, and these
arrangements enable the medicine to be ejected into the
air flow path which will remain clean next time it is
used. Moreover, since the flow path member 3 need not
be replaced or detached and cleaned every time, they
are user-friehdly.
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
11
Specific embodiments will be described below.
(Embodiment 1)
As shown in Fig. 1, the inhaler in this
embodiment has the device body 1, the flow path member
3 and the mouthpiece 2 supported by the device body 1,
and the internal wall face constituting member 5
detachably fitted into the flow path member 3.
The internal wall face constituting member 5 is
formed to be detachable from the flow path member 3 and
the mouthpiece 2, and the mouthpiece 2 is formed to be
detachable from the device body 1. As at least a part
of the internal wall face constituting member 5 is made
of a flexible material, this member can'be easily
attached to or detached from the flow path member 3 and
the mouthpiece 2.
To be noted_here, the "internal wall face
constituting member" in this specification can be
anything that has an action capable of preventing the
medicine from directly sticking to the internal wall of
-20 the flow path member and, in particular where it is
configured to be detachable from the flow path member,
it means a member constituting the internal wall of the
air flow path when it is fitted to the flow path member.
Thus, as is evident from Fig. 1, while part of the
.25 surface of the flow path member 3 constitutes "the
internal wall of the air flow path" when the internal
wall face constituting member 5 is not fitted, when the
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
12
internal wall face constituting member 5 is fitted,
this member constitutes the internal wall of the air
flow path. Incidentally, though it is preferable for
the internal wall face constituting member to span the
whole internal wall face of the flow path member, it
need not fully cover the whole face depending on the
extent of the contamination, but may cover only a part.
-Using such an inhaler, the user may inhale the
medicine three times a day for instance. And the
internal wall face constituting member 5 is replaced on
every occasion of inhalation. Further, the flow path
member 3 and the mouthpiece 2 are supposed to be
replaced for use once a day for instance.
It is preferable for the material of the internal
.15 wall face constituting member 5 to be, though not
limited to, what can be easily attached to and detached
from the flow path member 3 and does not chemically
react with the medicine even if the medicine sticks to
it. Typically, it can conceivably be a plastic
material or a rubber material. A hollow cylindrical
member like the illustrated one would be easy to
fabricate by resin molding or otherwise.
Replacing the internal wall face constituting
member 5 on every occasion of inhalation would enable
.25 the medicine to be ejected to a clean air flow path
every time.
It is preferable for the ejection head 6a and the
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
13
reservoir 6b constituting the medicine ejection portion
to be configured integrally with the internal wall face
constituting member 5 as shown in Fig. 1. The reason
is that, where the ejection head and the reservoir are
discarded every time, the ejection head and other
replaceable elements can be removed in a single
procedure at the same time as the removal of the
internal wall face constituting member 5.
The shape of the air flow path to be formed
inside the flow path member 3 or the internal wall face
constituting member 5, though shown to be cylindrical
in Fig. 1, is not limited to this; but may be prismatic
or otherwise.
Fig..3 is a flow chart showing the operation of
the inhaler.
First, the user joins the flow path member 3 and
the mouthpiece 2 with the device body 1. Further, the
user joins the ejection head 6a and the internal wall
face constituting member 5 having the reservoir 6b
built into it with the mouthpiece 2.
The power switch is pressed by the user (step
S001), and a power ON state is achieved (step S002).
The turning-on of power supply causes the power
indicator LED 11 to be lit. The controller 8 executes
.25 initial setting of the internal functions of the device
(step S003) After that, it is detected whether or not
an internal wall face constituting member 5 is fitted
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
14
according to an electric signal (.step S004). If no
internal wall face constituting member 5 can be
detected, it is determined to mean NO, and the
processing shifts to step S007 to.automatically turn
off power supply'and ends at step 5008.
If any internal wall face constituting member 5
is detected, it is determined to mean YES, and the
processing shifts to step S005. At step S005, the
patient's inhaling action is detected with the pressure.
sensor 7. If no inhaling action by the patient can be
detected, it is determined to mean NO, and the
processing returns to step S005. If an inhaling action
by the patient can be detected, it is determined to
mean YES, and the processing shifts to step S006. At
step S006, "Ejecting" is indicated on the display unit
9, and control is so effected as to eject a prescribed
dose of the medicine accommodated in the reservoir 6b
from the ejection head 6a, followed by a shift to step
S007. At step S007 the controller 8 turns off power
supply to the device, and the processing ends at step
S008. As power supply to the device is turned off, the
power indicator LED goes off, and every indication on
the LCD display is also turned off. After the end of
inhalation, the user detaches the ejection head 6a and
the internal wall face constituting member 5 having the
reservoir 6b built into it from the mouthpiece 2 and
the device body 1. Also, after the three rounds of
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
inhalation a day for instance, the user removes the
mouthpiece 2 from the device body 1 to end the
operation.
(Embodiment 2)
5 A perspective view of an inhaler.in a second
embodiment of the present invention is shown in Fig. 4.
Fig. 5 shows a section of the inhaler shown in Fig. 4.
In this embodiment, the internal wall face
constituting member 5 referred to in Embodiment 1 is
10 replaced with a thin film 12. The fi.lm 12 serving as
the internal wall face constituting member is in tight
contact with the internal wall face of the air flow
path which constitutes part of the surface of the flow
path member 3, and therefore scarcely affects the shape
15 of the air flow path. Accordingly, no matter whether
the air.flow path is provided with the film 12 or not,
the air flow generated by inhalation.does not vary, but
a similar state of inhalation can be realized.. After
the inhalation ends; when the user,peels off the film
12 to which the medicine has stuck, the stuck medicine
can be removed from the air flow path, and a new
internal wall face of the air flow path is formed after
the film is peeled off.
It is more preferable here for a plurality of
layers of the film 12 to be stacked in advance. By
peeling off one layer of film after each occasion of
inhalation, the frequency of replacing the flow path
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
16
member 3 and the mouthpiece 2 can be reduced.
As shown in Fig. 4, the inhaler in this
embodiment has the inhaler body 1, the flow path member
3 supported by the device body 1 and the film 12 in
tight contact with the internal wall face of the flow
path member 3 to serve as the internal wall face
constituting member. The ejection head 6a and the
reservoir 6b (together constituting a medicine ejection
cartridge 6) is fitted to the device body 1, and the
ejection head 6a is so oriented as to be able to eject
the medicine into the air flow path.
An air.flow is generated within the flow.path
member 3 by the user's inhalation.
The film 12 is rolled as shown in Fig. 4, and is
in tight contact with the internal wall face of the air
flow path which constitutes part of the surface of the
flow path member 3 in a plurality of layers (two layers
are shown in Fig. 4). So that the user can peel off
one layer of the film at a time, each layer of the film
12 is provided with a film pinch 14. The user peels
off an equivalent length of the film to one round of
the internal wall face of=the air flow path by pulling
the film pinch 14 after the end of inhalation: In this
way, it is possible to eject the medicine to a clean
air flow path on the next occasion of inhalation. It
is advisable to make a cut 13 to facilitate peeling off
one layer at a time.
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
17
And when the final part of the film has been
smeared by inhalation, the flow path member 3 is
replaced. It is sufficient to replace the medicine
ejection cartridge 6 with the ejection head 6a and the
reservoir 6b built'into it once a week if a quantity of
the medicine for a week's consumption is stored in the
reservoir 6b. For instance, if inhalation is to be
done three times a day, stocking 21 layers of the film
would enable the flow path member 3 and the medicine
ejection cartridge 6 to be replaced at the same time.
Description of the electrical configuration of
the inhaler and the operating sequence of the device
will be dispensed with as they are the same as in
Embodiment 1.
There-is not particular limitation of the
threading of the film. It may be continuous to
constitute.a roll via the cut 13 as shown in Fig. 4, or
the layers of film may be separated from one another.
The position of the film pinch 14 also can be freely
selected. Although it is disposed on the -suction port
2 side in the arrangement illustrated in Fig. 4, it can
as well be provided on the air inlet 4 side. This is a
more preferable arrangement because the film pinch 14
would not obstruct inhalation.
On the other hand, it is also possible to extend
the film 12 in tight contact with the internal wall
face of the air flow path to the area of the suction
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
18
port 2. A section of the inhaler in such an embodiment
is shown in Fig_. 6. As the film 12 is present even in
the part where the user's mouth comes into touch and
the film is peeled off after use, the suction port can
be kept clean. In this case, with a view to greater
ease of peeling off the film, a plurality of film
pinches may be provided for each layer (film pinches,.14
and 14').
(Embodiment 3)
A perspective view of an inhaler in a third
embodiment of the present invention is shown in Fig. 7.
Fig. 8 shows a section of the inhaler shown in Fig. 7.
The configuration in this embodiment does not
involve peeling-off of the film 12 as in Embodiment 2,
but is one folding back a sheet 15 serving as the
internal wall face constituting member within the air
flow path after inhalation. A plastic sheet having a
degree of softness permitting easy manual folding can
be used as the sheet 15. The sheet 15 is in tight
contact with the internal wall face of the air flow
path and hardly affects the shape of the air-flow path.
Therefore, no matter whether the air flow pathis
provided with the sheet 15 or not, the air flow
generated by inhalation does not vary, but a similar
state of inhalation can be realized. After the
.inhalation ends, when the userlifts a sheet pinch 16,
the sheet 15 is folded along a cut 17, and the faces
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
19
which constituted the internal wall face of the air
flow path during inflation come into contact. As a
result, the stuck medicine is removed from the internal
wall surface of the air flow path, and a new internal
wall face of the air flow path is formed. The air flow
which carries the medicine on the next occasion of
inhalation will arise on the clean internal wall face
free from the sticking of the medicine..
Fig. 9 illustrates the inhaler as viewed from the
air inlet.4 in a state in which the sheet 15 is folded
back. Cuts are made in two opposite positions within
the air flow path to enable the sheet 15 to be folded.
in the reverse direction in a stroke if lifted with a
force not weaker than a prescribed level.
Though not shown in the figure,.if not only the
sheet pinch 16 on the air inlet 4 side but also another
is disposed on the suction port 2 side and both pinches
are lifted, the sheet 15 can be folded more easily.
An adhesive 18 may be applied in advance onto the
upper half of the air flow path internal wall above the
cut 17. In this case, sheets 15 are adhered to one
another to prevent the trouble of the sheet 15 being
unfolded by a wrong action after inhalation without
being noticed. The material of the adhesive 18, though
not particularly required to be so, may preferably be
not so viscous as to let it stick to a finger which
might come into contact. Something like the glue on
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
the b,ack side of a postage stamp, made of polyvinyl
alcohol, could advisably be applied in advance. This
material, which is made adhesive by contact with liquid,
is not adhesive before inhalation, and is convenient
5 because the sticking of the ejected liquid medicine to
-the sheet 15 gives it an adhesive force.
A plurality of layers of the sheets 15 may be
provided and folded bac=k repeatedly. In that case,
each sheet needs to be provided with a pinch.
10 (Embodiment 4)
Embodiment 2 and Embodiment 3 use a method by
which a pinch i.s utilized for peeling off,or folding
back the film or the like, but a configuration which
allows the user to automat'ically peel off the film by a
15 simple action can be adopted as well.
A method of winding up the film in a roll shape
will be described as an example of such inhaler.
Fig. 10 shows a part of the section of the
inhaler as viewed from the air inlet 4 with the device.
20 body 1 being excluded. A take-up roll 19 and a roll
chamber 20 are arranged outside and above the flow path
member 3 and connected to the flow path member 3. The
used film is wound up into the roll 19 from inside the
air flow path. Thetransfer of the film from inside
the air flow path into the roll chamber 20 is carried
out in the following manner. Within the air flow path,
a support plate 22 turns to urge the film to transfer.
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
21
The roll 19 has a leader film 21, and the leader film
21 is kept in contact with the support plate 22 in
advance; the rotation of the support plate 22 moves the
used film to be spliced with the leader film. By
pressing a stud 23, a claw provided on the stud 23 is
caused to release the used film from its stacked state,
and the used film is spliced by the stud with the
leader film on the support plate. Advisably, a
splicing agent may be provided near an end of the film
to facilitate splicing of the used film then with the
leader film. The splicing agent may be any adhesive,
or splicing may as well be accomplished by an
electrostatic action. The passage for the stud 23 is
provided in advance in the external circumferential
face of the flow path member 3. This enables rotation
of the roll 19 to rotate the interlocked support plate
22 and the further interlocked used film to transfer to
be wound up bythe roll 19. The arrangement is such
that the stud 23 is then moved away from the film by a
block 24 and returns to its initial,position as it
rotates. When a series of repeated actions take place,
the previous used film serves as the leader film for
the next round.
The user himself or herself may control the roll
19, or a rotary mechanism such as a motor may be
provided to automatically control it via a controller
in accordance with a program. The roll 19 is provided
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514 - '
22
with a lever for controlling the rotation when the user
is to operate it, and the lever is turned to rotate the
roll 19, resulting in the execution of the operation
described above.
A system of winding up.within the flow path
member 3 is shown in Fig. 11 as an example,of winding-
up system. As in the case of Fig. 10, the used film is
released by the-stud 23 from the stacked film, and the
used film is transferred by the stud 23 along the
external circumference of the support plate 22. The
passage of the stud 23 and the block 24 similarly
configured would enable the stud 23 to return to its
initial position.
This enables the used film to be transferred to
the external circumferential face of the support plate
22, and the series of repeated actions cause.the used
films to be stacked on the external circumferential
face of the support plate 22. This operation, too, may
be automatically accomplished by a rotary mechanism
such as a motor or a gear rail via a controller in
accordance with a program.
The system of winding up the film within the flow
path member 3 may as well have a double track structure
in which a rail or lead member for guiding the stud 23
is provided over one and half turns around the external
circumferential face of the flow path member 3 and a
half turn may be changed'over in a switchback way.
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
23
This enables the used film to transfer one full turn to
be replaced by a new face by only a half turn of the
stud 23 traveling along the external circUmferential
face of the flow path member 3.
(Embodiment 5)
A section of an inhaler in a fifth embodiment of
the present invention is shown in Fig. 12. The inhaler
in this embodiment has a wiper 25, in contact with the
internal wall face of the flow path, as a unit for
removing the medicine stuck to the internal wall face
of the air flow path formed by the flow path member 3.
The medicine stuck to the internal wall face of the air
flow path is wiped off with the wiper 25. There is no
particular limitation to the configuration of the wiper
25, which needs only the capability of wiping off the
medicine. The wiper 25 may have an absorber capable of
sucking the medicine. Or it may have a blade-shaped
structure provided with a separate medicine collecting
mechanism.
Incidentally, a suction pipe having a suction
pump may be used in place of the wiper 25.
The wiper 25 is supported by a wiper supporting
rod 26. The wiper supporting rod 26 has a spring
mechanism, which is the angled part in Fig. 12, and is
so urged that the wiper 25 comes in constant contact
with the internal wall face of the air flow path.
Incidentally, in Fig. 12, the state in which the wiper~
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
24
25 and the wiper supporting rod 26 are represented by
solid lines is a state of being in contact with the
internal wall face of the flow path, and the state in
which they are represented by broken lines is a state
of being accommodated in the save position to be
described afterwards with reference to Fig. 13. When
the user has finished inhalation and the ejection of
the medicine is stopped, the controller 8 transmits an
operation signal to a stepping motor 27 supported by
motor supporting pillars 28. The wiper 25 is caused by
the stepping motor 27 to rotate along the internal wall
face of the air flow path in a full turn to wipe the
stuck medicine. (Fig. 13 shows a block diagram of the
electrical configuration of the inhaler in this
embodiment, including the stepping motor 27.)
Fig. 13 illustrates the inhaler shown in Fig. 12
as viewed from the suction port 2 side. Part of the
flow path membe.r 3 and the suction port 2 is a
partially chipped structure to accommodate the wiper 25,
and a slide cover 29 is positioned there. While the
user is inhaling, the wiper 25 is accommodated in a
space near the slide cover 29 (Fig. 14)..
The controller 8 so controls the revolution of
the stepping motor 27 as to cause the wiper 25 to make
a full turn counterclockwise in Fig. 13,,while wiping
the internal wall face of the air flow path, from the
position of the wiper 25 shown in Fig. 14 (the save
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
position) This results in so controlling the wiper 25
as to wipe off the medicine stuck to the internal wall
face of the flow path and return to the save position.
The repetition of the rotating operation of the
5 wiper 25 every time the delivery ends enables the
interrial wall face of the air flow path to be kept in a
clean state.
And, after.three rounds of inhalation a day for
instance, the user holds a slide knob 30 and slides the
10 slide- cover 29 toward the suction port 2. The user
wipes the wiper 25 with a towel or the like through an
opening bored in the flow path member, and further
cleans it by washing with alcohol or the like. After
that, the user returns the slide cover 29 to its
15 original position. This enables the wiper 25 to be
returned to a clean state.
As the wiper 25 is intended for wiping off the
stuck medicine, it is adequate for the wiper to be so
arranged as to be able to wipe the area farther toward
20 the suction port 2 than the ejection head 6a. However,
if it is made long enough to be able to wipe the
ejection head 6a as well as shown in Fig. 12, it will
be more favorable because wiping of the ejection nozzle
face of the ejection head can be accomplished at the
25 same time as the wiping of the internal wall face of
the air flow path. Thus, it is preferable for the
wiper 25-to be as long as the distance from the
CA 02658094 2009-01-16
WO 2008/010425 PCT/JP2007/063514
26
position of the ejection head 6a to the suction port 2
within the flow path member 3.
While the present invention has been described
with reference to exemplary embodiments, it is to be
understood that the invention is not limited to the
disclosed exemplary embodiments. The scope of the
following claims is to be accorded the broadest
interpretation so as to encompass all such
modifications and equivalent structures and functions.
This application claims the benefit of Japanese
Patent Application No. 2006-196327, filed July 19, 2006
and No. 2007-150303, filed June 6, 2007, which are
hereby incorporated by reference herein in their
entirety.