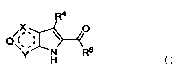Note: Descriptions are shown in the official language in which they were submitted.
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
FUSED HETEROCYCLIC INHIBITORS OF D-AMINO ACID OXIDASE
CROSS-REFERENCES TO RELATED APPLICATIONS
[0001] This application claims priority under 35 U.S.C. 119(e) to U.S.
Provisional
Patent Application No. 60/806,391 filed on June 30, 2006, U.S. Provisional
Patent
Application No. 60/842,465 filed on September 5, 2006, and U.S. Provisional
Patent
Application No. 60/914,293 filed on Apri126, 2007 each of which is
incorporated
herein by reference in its entirety for all purposes.
FIELD OF THE INVENTION
[0002] This invention relates to enzyme inhibitors and methods of treating
diseases
and conditions, wherein modulation of D-amino acid oxidase activity, D-serine
levels,
D-serine oxidative products and NMDA receptor activity in the nervous system
of a
mammalian subject is effective, along with a reduction in undesirable side
effects.
BACKGROUND OF THE INVENTION
[0003] Tne enzyme D-amino acid oxidase (DAAO) metabolizes D-amino acids, and
in particular, metabolizes D-serine in vitro at physiological pH. DAAO is
expressed
in the mammalian brain and periphery. D-Serine's role as a neurotransmitter is
important in the activation of the N-methyl-D-aspartate (NIvIDA) selective
subtype of
the glutamate receptor, an ion channel expressed in neurons, here denoted as
NMDA
receptor.
[0004] NMDA receptors mediate many physiological functions. NMDA receptors
are complex ion channels containing multiple protein subunits that act either
as
binding sites for transmitter amino acids and/or as allosteric regulatory
binding sites
to regulate ion channel activity. D-serine, released by glial cells, has a
distribution
similar to NMDA receptors in the brain and acts as an endogenous ligand of the
allosteric "glycine" site of these receptors (Mothet et al., PNAS, 97:4926
(2000)), the
occupation of which is required for NMDA receptor operation. D-serine is
synthesized in brain through serine racemase and degraded by D-amino oxidase
(DAAO) after release.
[0005] Small organic molecules, which inhibit the enzymatic cycle of DAAO, may
control the levels of D-serine, and thus influence the activity of the NMDA
receptor
1
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
in the brain. NMDA receptor activity is important in a variety of disease
states, such
as schizophrenia, psychosis, ataxias, ischemia, several forms of pain
including
neuropathic pain, and deficits in memory and cognition.
[0006] DAAO inhibitors may also control production of toxic metabolites of D-
serine oxidation, such as hydrogen peroxide and ammonia. Thus, these molecules
may influence the progression of cell loss in neurodegenerative disorders.
Neurodegenerative diseases are diseases in which CNS neurons and/or peripheral
neurons undergo a progressive loss of function, usually accompanied by (and
perhaps
caused by) a physical deterioration of the structure of either the neuron
itself or its
interface with other neurons. Such conditions include Parkinson's disease,
Alzheimer's disease, Huntington's disease and neuropathic pain. N-methyl-D-
aspartate (NMDA)-glutamate receptors are expressed at excitatory synapses
throughout the central nervous system (CNS). These receptors mediate a wide
range
of brain processes, including synaptic plasticity, that are associated with
certain types
of memory formation and learning. NMDA-glutamate receptors require binding of
two agonists to induce neurotransmission. One of these agonists is the
excitatory
amino acid L-glutamate, while the second agonist, at the so-called "strychnine-
insensitive glycine site", is now thought to be D-serine. In animals, D-serine
is
synthesized from L-serine by serine racemase and degraded to its corresponding
ketoacid by DAAO. Together, serine racemase and DAAO are thought to play a
crucial role in modulating NMDA neurotransmission by regulating CNS
concentrations of D-serine.
[0007] Known inhibitors of DAAO include benzoic acid, pyrrole-2-carboxylic
acids, and indole-2-carboxylic acids, as described by Frisell, et al., J.
Biol. Chem.,
223:75-83 (1956) and Parikh et al., JACS, 80:953 (1958). Indole derivatives
and
particularly certain indole-2-carboxylates have been described in the
literature for
treatment of neurodegenerative disease and neurotoxic injury. EP 396124
discloses
indole-2-carboxylates and derivatives for treatment or management of
neurotoxic
injury resulting from a CNS disorder or traumatic event or in treatment or
management of a neurodegenerative disease. Several examples of traumatic
events
that may result in neurotoxic injury are given, including hypoxia, anoxia, and
ischemia, associated with perinatal asphyxia, cardiac arrest or stroke.
Neurodegeneration is associated with CNS disorders such as convulsions and
2
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
epilepsy. U.S. Pat. Nos. 5,373,018; 5,374,649; 5,686,461; 5,962,496 and
6,100,289, to
Cugola, disclose treatment of neurotoxic injury and neurodegenerative disease
using
indole derivatives. None of the above references mention improvement or
enhancement of learning, memory or cognition.
[0008] WO 03/039540 to Heefner et al. and U.S. Patent Application Nos.
2005/0143443 to Fang et al. and 2005/0143434 to Fang et al. disclose DAAO
inhibitors, including indole-2-carboxylic acids, and methods of enhancing
learning,
memory and cognition as well as methods for treating neurodegenerative
disorders.
Patent Application No. WO/2005/089753 discloses benzisoxazole analogs and
methods of treating mental disorders, such as Schizophrenia. However, a need
for
additional drug molecules that are effective in treating memory defects,
impaired
learning, loss of cognition, and other symptoms related to NMDA receptor
activity,
remains. The present invention addresses this and other needs.
SUMMARY OF THE INVENTION
[0009) The invention provides novel inhibitors of D-amino acid oxidase that
are
useful in the prevention and treatment of a variety of diseases and/or
conditions
including neurological disorders, pain, ataxia, and convulsion.
[0010] In a first aspect, the present invention provides a compound having a
structure according to Formula (II):
R4
Q~..=
O
s
Y H R (II)
wherein Q is a member selected from 0, S, N and CR'. X is a member selected
from
0, S, N, NR3 and CR2a and Y is a member selected from 0, S, N, NR3 and CR2b,
wherein R' is a member selected from H, F, substituted or unsubstituted
arylalkyl,
substituted or unsubstituted heteroarylalkyl, substituted or unsubstituted C4-
C10
cycloalkyl, and substituted or unsubstituted C4-Cla heterocycloalkyl.
[0011[ In Formula (II), RZe is a member selected from H, F, Cl, Br, CN,
substituted
or unsubstituted C3-C6 alkyl, substituted or unsubstituted arylalkyl,
substituted or
unsubstituted heteroarylalkyl, substituted or unsubstituted C4-Clo cycloalkyl,
substituted or unsubstituted C4-C 10 heterocycloalkyl and alkenyl. R2b is a
member
3
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
selected from H, F, substituted or unsubstituted C3-C6 alkyl, substituted or
unsubstituted arylalkyl, substituted or unsubstituted heteroarylalkyl,
substituted or
unsubstituted C4-Cio cycloalkyl, and substituted or unsubstituted C4-CIo
heterocycloalkyl and alkenyl. R3 is a member selected from H, substituted or
unsubstituted Ci-C6 alkyl, substituted or unsubstituted arylalkyl, substituted
or
unsubstituted heteroarylalkyl, substituted or unsubstituted C4-C1o cycloalkyl,
and
substituted or unsubstituted C4-Clo heterocycloalkyl. R4 is a member selected
from H,
F, Cl, Br, CN, unsubstituted C1-C6 alkyl, substituted or unsubstituted
arylalkyl,
substituted or unsubstituted heteroarylalkyl, substituted or unsubstituted C4-
CIo
cycloalkyl and alkenyl. R6 is a member selected from O"X+ and OH, wherein X+
is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. In one embodiment, in which Q is CF and one member selected
from X
or Y is S and the other is CH, R4 is preferably other than H. In another
embodiment,
in which Q is CH, and Y is S, 0 or CH, at least one of RZa and R4 is other
than H. In
another embodiment, in which Q is CH, at least one of R28, R2b and R4 is
preferably
other than H.
[0012] In a second aspect, the invention provides a compound having a
structure,
which is a member selected from Formula (III) and Formula (IV):
R4 R4
Rl ~ (
R~
~ ~ \ o
H ~ 4R6
Yx (III) X H R6 (IV)
wherein X is a member selected from 0, S and NR3 and Y is a member selected
from
CR2 and N. R' and R2 are members independently selected from H, F, substituted
or
unsubstituted C3-C6 alkyl, substituted or unsubstituted arylalkyl, substituted
or
unsubstituted heteroarylalkyl, substituted or unsubstituted C4-C la
cycloalkyl, and
substituted or unsubstituted C4-C1o heterocycloalkyl and alkenyl. R3 is a
member
selected from H, substituted or unsubstituted C1-C6 alkyl, substituted or
unsubstituted
arylalkyl, substituted or unsubstituted heteroarylalkyl, substituted or
unsubstituted C4-
C io cycloalkyl, and substituted or unsubstituted C4-Cjo heterocycloalkyl. R4
is a
member selected from H, F, Cl, Br, CN, unsubstituted C1-C6 alkyl, substituted
or
unsubstituted arylalkyl, substituted or unsubstituted heteroarylalkyl,
substituted or
unsubstituted C4-CIo cycloalkyl and alkenyl. R6 is a member selected from O7C+
and
OH, wherein X+ is a positive ion, which is a member selected from inorganic
positive
4
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
ions and organic positive ions. In one embodiment, in which X is S, Y is CH
and R'
is F, R4 is preferably other than H. In another embodiment, in which in
Formula (III),
R' is H and Y is CH, R is preferably other than H. In yet another embodiment,
wherein in Forrnula (IV), R' is H, at least one of R2 and R4 is other than H.
[0013] In a third aspect, the invention provides a a pharmaceutical
composition
comprising a compound according to Formula (I) or a pharmaceutically
acceptable
salt, hydrate or prodrug thereof, and a pharmaceutically acceptable carrier:
R4
Z
Y A Re
wherein Z is a member selected from 0 and S, A is a member selected from NW, S
and O. Q is a member selected from 0, S, N, NR3a and CR'. X and Y are members
independently selected from 0, S, N, NR3 and CR2, provided that when X and Y
are
both CRZ, each R2 is independently selected. R3, R3a and R7 are members
independently selected from H, OR12, acyl, SOZR13, SOR13, substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl, wherein R'Z and R13 are members independently
selected from substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R', each R2 and R4 are
members
independently selected from H, F, Cl, Br, CN, CF3, acyl, OR14, S(O)20R14,
S(O)PR14,
NR14R15, SO2NR14R'5, substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R' and R2, together with
the atoms
to which they are attached, are optionally joined to form a 5- to 7-membered
ring.
The integer p is selected from 0 to 2. R14 and R15 are members independently
selected
from H, substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl,
substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl and
substituted or unsubstituted heterocycloalkyl. R14 and R15, together with the
nitrogen
atoms to which they are attached, are optionally joined to form a 5- to 7-
membered
ring. R6 is a member selected from O-X+ and OH, wherein X+ is a positive ion,
which
is a member selected from inorganic positive ions and organic positive ions.
In one
5
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
embodiment, in which R4 is H and A is NR7, R7 is preferably not a member
selected
from:
0
~-S-Ar
I-CH2-Ar ; and O
wherein Ar is substituted or unsubstituted phenyl. In another embodiment,
wherein
X is S and Y is CH, R4 is not C(O)-2-thiophenyl.
[00141 In a fourth aspect, the invention provides a pharmaceutical composition
comprising a compound according to Formula (VI) or Formula (VII), or a
pharmaceutically acceptable salt, hydrate or prodrug thereof, and a
pharmaceutically
acceptable carrier:
R4 R4
RX X~ O RI~Y O
Y A R6 (VI) X A R6 (VII)
wherein A is a member selected from NH and S. X is a member selected from 0, S
and NR3. Y is a member selected from CR2 and N. R3 and R7 are members
independently selected from H, OR1Z, acyl, SOZR13, SOR13, substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl, wherein R12 and R13 are members independently
selected from substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R1, RZ and R4 are members
independently selected from H, F, Cl, Br, CN, CF3, acyl, OR14, S(O)ZOR`,
S(O)pR14,
NR14R15, SO2NR14R15, substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl; and R' and R2, together
with the
atoms to which they are attached, are optionally joined to form a 5- to 7-
membered
ring. The integer p is selected from 0 to 2. R14 and R15 are members
independently
selected from H, substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R14 and R15, together with
the
nitrogen atoms to which they are attached, are optionally joined to form a 5-
to 7-
membered ring. R6 is a member selected from O'X+ and OH, wherein X' is a
positive
6
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
ion, which is a member selected from inorganic positive ions and organic
positive
ions. In one embodiment, in which in Formula (VI), X is S and Y is CH, R is
preferably not C(O)-2-thiophenyl.
[0015) In another aspect, the invention provides a method for treating or
preventing
a condition which is a member selected from a neurological disorder, pain,
ataxia and
convulsion, said method comprising administering to a subject in need thereof
a
therapeutically effective amount of a compound of Formula (I) or a
pharmaceutically
acceptable salt, hydrate or prodrug thereof:
R4
Z
Q
y A Rs
m
wherein Z is a member selected from 0 and S. A is a member selected from NR7,
S
and O. Q is a member selected from 0, S, N, NR3a and CR1. X and Y are members
independently selected from 0, S, N, NR3 and CR2, provided that when X and Y
are
both CR2, each RZ is independently selected. R3, R3a and R7 are members
independently selected from H, OR12, acyl, SO2R13, SOR13, substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl, wherein R'Z and R13 are members independently
selected from substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R', R2 and R4 are members
independently selected from H, F, Cl, Br, CN, CF3, acyl, OR14, S(O)20R14,
S(O)RR14,
NR'4 R15, S02NR'4 R15, substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R' and R2, together with
the atoms
to which they are attached, are optionally joined to form a 5- to 7-membered
ring.
The integer p is selected from 0 to 2. R14 and R15 are members independently
selected
from H, substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl,
substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl and
substituted or unsubstituted heterocycloalkyl. R14 and R'5, together with the
nitrogen
atoms to which they are attached, are optionally joined to fonm a 5- to 7-
membered
7
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
ring. R6 is a member selected from O"X+ and OH, wherein X+ is a positive ion,
which
is a member selected from inorganic positive ions and organic positive ions.
DETAILED DESCRIPTION OF THE INVENTION
I. Definitions
[0016] Where substituent groups are specified by their conventional chemical
formulae, written from left to right, they equally encompass the chemically
identical
substituents, which would result from writing the structure from right to
left, e.g.,
-CH2O- is intended to also recite -OCH2-.
[0017] The term "alkyl," by itself or as part of another substituent, means,
unless
otherwise stated, a straight or branched chain, or cyclic hydrocarbon radical,
or
combination thereof, which may be fully saturated, mono- or polyunsaturated
and can
include di- and multivalent radicals, having the number of carbon atoms
designated
(i.e. Cl-Clo means one to ten carbons). Examples of saturated hydrocarbon
radicals
include, but are not limited to, groups such as methyl, ethyl, n-propyl,
isopropyl, n-
butyl, t-butyl, isobutyl, sec-butyl, cyclohexyl, (cyclohexyl)methyl,
cyclopropylmethyl, homologs and isomers of, for example, n-pentyl, n-hexyl, n-
heptyl, n-octyl, and the like. An unsaturated alkyl group is one having one or
more
double bonds or triple bonds. Examples of unsaturated alkyl groups include,
but are
not limited to, vinyl, 2-propenyl, crotyl, 2-isopentenyl, 2-(butadienyl), 2,4-
pentadienyl, 3-(1,4-pentadienyl), ethynyl, 1- and 3-propynyl, 3-butynyl, and
the
higher homologs and isomers. The term "alkyl," unless otherwise noted, is also
meant to include those derivatives of alkyl defined in more detail below, such
as
"heteroalkyl" with the difference that the heteroalkyl group, in order to
qualify as an
alkyl group, is linked to the remainder of the molecule through a carbon atom.
Alkyl
groups that are limited to hydrocarbon groups are termed "homoalkyl".
[0018] The tenn "alkenyl" by itself or as part of another substituent is used
in its
conventional sense, and refers to a radical derived from an alkene, as
exemplified, but
not limited, by substituted or unsubstituted vinyl and substituted or
unsubstituted
propenyl. Typically, an alkenyl group will have from I to 24 carbon atoms,
with
those groups having from 1 to 10 carbon atoms being preferred.
8
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0019] The term "alkylene" by itself or as part of another substituent means a
divalent radical derived from an alkane, as exemplified, but not limited, by -
CH2CH2CH2CH2-, and further includes those groups described below as
"heteroalkylene." Typically, an alkyl (or alkylene) group will have from 1 to
24
carbon atoms, with those groups having 10 or fewer carbon atoms being
preferred in
the present invention. A "lower alkyl" or "lower alkylene" is a shorter chain
alkyl or
alkylene group, generally having eight or fewer carbon atoms.
[0020] The terms "alkoxy," "alkylamino" and "alkylthio" (or thioalkoxy) are
used in
their conventional sense, and refer to those alkyl groups attached to the
remainder of
the molecule via an oxygen atom, an amino group, or a sulfur atom,
respectively.
[0021] The term "heteroalkyl," by itself or in combination with another term,
means, unless otherwise stated, a stable straight or branched chain, or cyclic
hydrocarbon radical, or combinations thereof, consisting of the stated number
of
carbon atoms and at least one heteroatom selected from the group consisting of
0, N,
Si, S, B and P and wherein the nitrogen and sulfur atoms may optionally be
oxidized
and the nitrogen heteroatom may optionally be quaternized. The heteroatom(s)
may
be placed at any interior position of the heteroalkyl group or at the position
at which
the alkyl group is attached to the remainder of the molecule. Examples
include, but
are not limited to, -CH2-CHZ-O-CH3, -CH2-CH2-NH-CH3, -CH2-CH2-N(CH3)-CH3, -
CH2-S-CH2-CH3, -CH2-CH2,-S(O)-CH3, -CH2-CH2-S(O)2-CH3, -CH=CH-O-CH3, -
Si(CH3)3, -CH2-CH=N-OCH3, and -CH=CH-N(CH3)-CH3. Up to two heteroatoms
may be consecutive, such as, for example, -CH2-NH-OCH3 and -CH2-O-Si(CH3)3.
Similarly, the term "heteroalkylene" by itself or as part of another
substituent means a
divalent radical derived from heteroalkyl, as exemplified, but not limited by,
-CH2-
CH2-S-CH2-CH2- and -CH2-S-CH2-CH2-NH-CH2-. For heteroalkylene groups,
heteroatoms can also occupy either or both of the chain termini (e.g.,
alkyleneoxy,
alkylenedioxy, alkyleneamino, alkylenediamino, and the like). Still further,
for
alkylene and heteroalkylene linking groups, no orientation of the linking
group is
implied by the direction in which the formula of the linking group is written.
For
example, the formula -CO2R'- represents both -C(O)OR' and
-OC(O)R'.
9
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
100221 The terms "cycloalkyl" and "heterocycloalkyl", by themselves or in
combination with other terms, represent, unless otherwise stated, cyclic
versions of
"alkyl" and "heteroalkyl", respectively. Additionally, for heterocycloalkyl, a
heteroatom can occupy the position at which the heterocycle is attached to the
remainder of the molecule. A "cycloalkyl" or "heterocycloalkyl" substituent
may be
attached to the remainder of the molecule directly or through a linker,
wherein the
linker is preferably alkylene. Examples of cycloalkyl include, but are not
limited to,
cyclopentyl, cyclohexyl, 1-cyclohexenyl, 3-cyclohexenyl, cycloheptyl, and the
like.
Examples of heterocycloalkyl include, but are not limited to, 1-(1,2,5,6-
tetrahydropyridyl), 1-piperidinyl, 2-piperidinyl, 3-piperidinyl, 4-
morpholinyl, 3-
morpholinyl, tetrahydrofuran-2-yl, tetrahydrofiuan-3-yl, tetrahydrothien-2-yl,
tetrahydrothien-3-yl, 1-piperazinyl, 2-piperazinyl, and the like.
[00231 The terms "halo" or "halogen," by themselves or as part of another
substituent, mean, unless otherwise stated, a fluorine, chlorine, bromine, or
iodine
atom. Additionally, terms such as "haloalkyl," are meant to include
monohaloalkyl
and polyhaloalkyl. For example, the term "halo(C1-C4)alkyl" is mean to
include, but
not be limited to, trifluoromethyl, 2,2,2-trifluoroethyl, 4-chlorobutyl, 3-
bromopropyl,
and the like.
(00241 The term "aryl" means, unless otherwise stated, a polyunsaturated,
aromatic,
substituent that can be a single ring or multiple rings (preferably from 1 to
3 rings),
which are fused together or linked covalently. The term "heteroaryl" refers to
aryl
groups (or rings) that contain from one to four heteroatoms selected from N,
0, S, Si
and B, wherein the nitrogen and sulfur atoms are optionally oxidized, and the
nitrogen
atom(s) are optionally quatemized. A heteroaryl group can be attached to the
remainder of the molecule through a heteroatom. Non-limiting examples of aryl
and
heteroaryl groups include phenyl, 1-naphthyl, 2-naphthyl, 4-biphenyl, 1-
pyrrolyl, 2-
pyrrolyl, 3-pyrrolyl, 3-pyrazolyl, 2-imidazolyl, 4-imidazolyl, pyrazinyl, 2-
oxazolyl, 4-
oxazolyl, 2-phenyl-4-oxazolyl, 5-oxazolyl, 3-isoxazolyl, 4-isoxazolyl, 5-
isoxazolyl, 2-
thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 2-
pyridyl, 3-
pyridyl, 4-pyridyl, 2-pyrimidyl, 4-pyrimidyl, 5-benzothiazolyl, purinyl, 2-
benzimidazolyl, 5-indolyl, 1-isoquinolyl, 5-isoquinolyl, 2-quinoxalinyl, 5-
quinoxalinyl, 3-quinolyl, and 6-quinolyl. Substituents for each of the above
noted
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
aryl and heteroaryl ring systems are selected from the group of acceptable
substituents
described below.
[0025] For brevity, the term "aryl" when used in combination with other terms
(e.g.,
aryloxy, arylthioxy, arylalkyl) includes both aryl and heteroaryl rings as
defined
above. Thus, the term "arylalkyl" is meant to include those radicals in which
an aryl
group is attached to an alkyl group (e.g., benzyl, phenethyl, pyridylmethyl
and the
like) including those alkyl groups in which a carbon atom (e.g., a methylene
group)
has been replaced by, for example, an oxygen atom (e.g., phenoxymethyl, 2-
pyridyloxymethyl, 3-(1-naphthyloxy)propyl, and the like).
[0026] Each of the above terms (e.g., "alkyl," "heteroalkyl," "aryl" and
"heteroaryl") are meant to include both substituted and unsubstituted forms of
the
indicated radical. Preferred substituents for each type of radical are
provided below.
[0027] Substituents for the alkyl and heteroalkyl radicals (including those
groups
often referred to as alkylene, alkenyl, heteroalkylene, heteroalkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, cycloalkenyl, and heterocycloalkenyl) are
generically
referred to as "alkyl group substituents," and they can be one or more of a
variety of
groups selected from, but not limited to: substituted or unsubstituted aryl,
substituted
or unsubstituted heteroaryl, substituted or unsubstituted heterocycloalkyl, -
OR', =0,
=NR', =N-OR', -NR'R", -SR', -halogen, -SiR'R 'R", -OC(O)R', -C(O)R', -CO2R', -
CONR'R", -OC(O)NR'R", -NR"C(O)R', -NR'-C(O)NR"R`, -NR"C(O)2R', -NR-
C(NR'R"R"')=NR", -NR-C(NR'R")=NR`, -S(O)R', -S(O)2R', -S(O)2NR'R",
-NRSO2R', -CN and NO2 in a number ranging from zero to (2m'+1), where m' is
the
total number of carbon atoms in such radical. R', R", R"' and R"" each
preferably
independently refer to hydrogen, substituted or unsubstituted heteroalkyl,
substituted
or unsubstituted aryl, e.g., aryl substituted with 1-3 halogens, substituted
or
unsubstituted alkyl, alkoxy or thioalkoxy groups, or arylalkyl groups. When a
compound of the invention includes more than one R group, for example, each of
the
R groups is independently selected as are each R', R", R"' and R"" groups when
more
than one of these groups is present. When R' and R" are attached to the same
nitrogen atom, they can be combined with the nitrogen atom to form a 5-, 6-,
or 7-
membered ring. For example, -NR'R" is meant to include, but not be limited to,
1-
pyrrolidinyl and 4-morpholinyl. From the above discussion of substituents, one
of
11
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
skill in the art will understand that the term "alkyl" is meant to include
groups
including carbon atoms bound to groups other than hydrogen groups, such as
haloalkyl (e.g., -CF3 and -CH2CF3) and acyl (e.g., -C(O)CH3, -C(O)CF3, -
C(O)CH2OCH3, and the like).
[0028] Similar to the substituents described for the alkyl radical,
substituents for the
aryl and heteroaryl groups are generically referred to as "aryl group
substituents."
The substituents are selected from, for example: substituted or unsubstituted
alkyl,
substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl,
substituted or
unsubstituted heterocycloalkyl, -OR', =O, =NR', N-OR', -NR'R", -SR', -halogen,
-
SiR'R"R"', -OC(O)R', -C(O)R', -CO2R', -CONR'R", -OC(O)NR'R", -NR"C(O)R',
-NR'-C(O)NR"R"', -NR"C(O)ZR', -NR-C(NR'R"R`)-NR"", -NR-C(NR'R")-NR",
-S(O)R', -S(O)2R', -S(O)ZNR'R", -NRSO2R', -CN and -NOZ, -R', -N3, -CH(Ph)2,
fluoro(Ci-C4)alkoxy, and fluoro(C1 -C4)alkyl, in a number ranging from zero to
the
total number of open valences on the aromatic ring system; and where R', R",
R"' and
R"" are preferably independently selected from hydrogen, substituted or
unsubstituted
alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted
aryl and
substituted or unsubstituted heteroaryl. When a compound of the invention
includes
more than one R group, for example, each of the R groups is independently
selected
as are each R', R", R"' and R"" groups when more than one of these groups is
present.
[0029] Two of the substituents on adjacent atoms of the aryl or heteroaryl
ring may
optionally be replaced with a substituent of the formula -T-C(O)-(CRR')y-U-,
wherein T and U are independently -NR-, -0-, -CRR'- or a single bond, and q is
an
integer of from 0 to 3. Alternatively, two of the substituents on adjacent
atoms of the
aryl or heteroaryl ring may optionally be replaced with a substituent of the
formula -
A-(CH2),-B-, wherein A and B are independently -CRR'-, -0-, -NR-, -S-, -S(O)-,
-S(O)2-, -S(O)2NR'- or a single bond, and r is an integer of from I to 4. One
of the
single bonds of the new ring so formed may optionally be replaced with a
double
bond. Alternatively, two of the substituents on adjacent atoms of the aryl or
heteroaryl ring may optionally be replaced with a substituent of the formula -
(CRR')S-X-(CR"R"')d-, where s and d are independently integers of from 0 to 3,
and
X is -0-, -NR'-, -S-, -S(O)-, -S(O)2-, or -S(O)2NR'-. The substituents R, R',
R" and
12
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
R"' are preferably independently selected from hydrogen or substituted or
unsubstituted (CI-C6)alkyl.
[0030] As used herein, the term "acyl" describes a substituent containing a
carbonyl
residue, C(O)R. Exemplary species for R include H, halogen, substituted or
unsubstituted alkyl, substituted or unsubstituted aryl, substituted or
unsubstituted
heteroaryl, and substituted or unsubstituted heterocycloalkyl.
[0031] As used herein, the term "fused ring system" means at least two rings,
wherein each ring has at least 2 atoms in common with another ring. "Fused
ring
systems may include aromatic as well as non aromatic rings. Examples of "fused
ring
systems" are naphthalenes, indoles, quinolines, chromenes and the like.
[0032] As used herein, the term "heteroatom" includes oxygen (0), nitrogen
(N),
sulfur (S), silicon (Si) and boron (B).
100331 The symbol "R" is a general abbreviation that represents a substituent
group.
Exemplary substituent groups include substituted or unsubstituted alkyl,
substituted or
unsubstituted heteroalkyl, substituted or unsubstituted aryl, substituted or
unsubstituted heteroaryl, and substituted or unsubstituted heterocycloalkyl
groups.
[0034] The phrase "therapeutically effective amount" as used herein means that
amount of a compound, material, or composition comprising a compound of the
present invention which is effective for producing a desired therapeutic
effect, at a
reasonable benefit/risk ratio applicable to any medical treatment.
[0035] The term "pharmaceutically acceptable salts" includes salts of the
active
compounds which are prepared with relatively nontoxic acids or bases,
depending on
the particular substituents found on the compounds described herein. When
compounds of the present invention contain relatively acidic functionalities,
base
addition salts can be obtained by contacting the neutral form of such
compounds with
a sufficient amount of the desired base, either neat or in a suitable inert
solvent.
Examples of pharmaceutically acceptable base addition salts include sodium,
potassium, calcium, ammonium, organic amino, or magnesium salt, or a similar
salt.
When compounds of the present invention contain relatively basic
functionalities,
acid addition salts can be obtained by contacting the neutral form of such
compounds
with a sufficient amount of the desired acid, either neat or in a suitable
inert solvent.
13
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Examples of pharmaceutically acceptable acid addition salts include those
derived
from inorganic acids like hydrochloric, hydrobromic, nitric, carbonic,
monohydrogencarbonic, phosphoric, monohydrogenphosphoric,
dihydrogenphosphoric, sulfuric, monohydrogensulfuric, hydriodic, or
phosphorous
acids and the like, as well as the salts derived from relatively nontoxic
organic acids
like acetic, propionic, isobutyric, maleic, malonic, benzoic, succinic,
suberic, fumaric,
lactic, mandelic, phthalic, benzenesulfonic, p-tolylsulfonic, citric,
tartaric,
methanesulfonic, and the like. Also included are salts of amino acids such as
arginate
and the like, and salts of organic acids like glucuronic or galactunoric acids
and the
like (see, for example, Berge e[ al., Journal of Pharmaceutical Science, 66: 1-
19
(1977)). Certain specific compounds of the present invention contain both
basic and
acidic functionalities that allow the compounds to be converted into either
base or
acid addition salts.
[0036] When a residue is defined as "O u, then the formula is meant to
optionally
include an organic or inorganic cationic counterion. Preferably, the resulting
salt
form of the compound is pharmaceutically acceptable.
[00371 The neutral forms of the compounds are preferably regenerated by
contacting the salt with a base or acid and isolating the parent compound in
the
conventional manner. The parent form of the compound differs from the various
salt
forms in certain physical properties, such as solubility in polar solvents,
but otherwise
the salts are equivalent to the parent form of the compound for the purposes
of the
present invention.
100381 In addition to salt forms, the present invention provides compounds,
which
are in a prodrug form. Prodrugs of the compounds described herein are those
compounds that readily undergo chemical changes under physiological conditions
to
provide the compounds of the present invention. For instance, prodrugs for
carboxylic acid analogs of the invention include a variety of esters. In an
exemplary
embodiment, the pharmaceutical compositions of the invention include a
carboxylic
acid ester. In another exemplary embodiment, the prodrug is suitable for
treatment
/prevention of those diseases and conditions that require the drug molecule to
cross
the blood brain barrier. In a preferred embodiment, the prodrug enters the
brain,
where it is converted into the active form of the drug molecule. In another
example, a
14
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
prodrug is used to enable an active drug molecule to reach the inside of the
eye after
topical application of the prodrug to the eye. Additionally, prodrugs can be
converted
to the compounds of the present invention by chemical or biochemical methods
in an
ex vivo environment. For example, prodrugs can be slowly converted to the
compounds of the present invention when placed in a transdermal patch
reservoir with
a suitable enzyme or chemical reagent.
[0039] Certain compounds of the present invention can exist in unsolvated
forms as
well as solvated forms, including hydrated forms. In general, the solvated
forms are
equivalent to unsolvated forms and are encompassed within the scope of the
present
invention. Certain compounds of the present invention may exist in multiple
crystalline or amorphous forms ("polymorphs"). In general, all physical forms
are of
use in the methods contemplated by the present invention and are intended to
be
within the scope of the present invention. "Compound or a pharmaceutically
acceptable salt, hydrate, polymorph or solvate of a compound" intends the
inclusive
meaning of "or", in that materials meeting more than one of the stated
criteria are
included, e.g., a material that is both a salt and a solvate is encompassed.
[0040] Certain compounds of the present invention possess asymmetric carbon
atoms (optical centers) or double bonds; the racemates, diastereomers,
geometric
isomers and individual isomers are encompassed within the scope of the present
invention. Optically active (R)- and (S)-isomers and d and I isomers may be
prepared
using chiral synthons or chirai reagents, or resolved using conventional
techniques.
When the compounds described herein contain olefinic double bonds or other
centers
of geometric asymmetry, and unless specified otherwise, it is intended that
the
compounds include both E and Z geometric isomers. Likewise, all tautomeric
forms
are included.
[0041] The compounds of the present invention may also contain unnatural
proportions of atomic isotopes at one or more of the atoms that constitute
such
compounds. For example, the compounds may be radiolabeled with radioactive
isotopes, such as for example tritium (3H), iodine-125 (125 1) or carbon-14
(14C). All
isotopic variations of the compounds of the present invention, whether
radioactive or
not, are intended to be encompassed within the scope of the present invention.
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
100421 In the context of the present invention, compounds that are considered
to
possess activity as DAAO inhibitors are those displaying 50% inhibition of the
enzymatic activity of DAAO (IC50) at a concentration of not higher than about
100
M, preferably, not higher than about 10 M, more preferably not higher than
about
1 M, even more preferably not higher than about 100 nM and most preferably
not
higher than about 25 nM.
[0043] The term "neurological disorder" refers to any condition of the central
or
peripheral nervous system of a mammal. The term "neurological disorder"
includes
neurodegenerative diseases (e.g., Alzheimer's disease, Parkinson's disease and
amyotrophic lateral sclerosis), neuropsychiatric diseases (e.g. schizophrenia
and
anxieties, such as general anxiety disorder). Exemplary neurological disorders
include MLS (cerebellar ataxia), Huntington's disease, Down syndrome, multi-
infarct
dementia, status epilecticus, contusive injuries (e.g. spinal cord injury and
head
injury), viral infection induced neurodegeneration, (e.g. AIDS,
encephalopathies),
epilepsy, benign forgetfulness, closed head injury, sleep disorders,
depression (e.g.,
bipolar disorder), dementias, movement disorders, psychoses, alcoholism, post-
traumatic stress disorder and the like. "Neurological disorder" also includes
any
condition associated with the disorder. For instance, a method of treating a
neurodegenerative disorder includes methods of treating loss of memory and/or
loss
of cognition associated with a neurodegenerative disorder. Such method would
also
include treating or preventing loss of neuronal function characteristic of
neurodegenerative disorder.
[0044] "Pain" is an unpleasant sensory and emotional experience. Pain
classifications have been based on duration, etiology or pathophysiology,
mechanism,
intensity, and symptoms. The term "pain" as used herein refers to all
categories of
pain, including pain that is described in terms of stimulus or nerve response,
e.g.,
somatic pain (normal nerve response to a noxious stimulus) and neuropathic
pain
(abnormal response of a injured or altered sensory pathway, often without
clear
noxious input); pain that is categorized temporally, e.g., chronic pain and
acute pain;
pain that is categorized in tenns of its severity, e.g., mild, moderate, or
severe; and
pain that is a symptom or a result of a disease state or syndrome, e.g.,
inflammatory
pain, cancer pain, AIDS pain, arthropathy, migraine, trigeminal neuralgia,
cardiac
ischaemia, and diabetic peripheral neuropathic pain (see, e.g., Harrison's
Principles of
16
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Intemal Medicine, pp. 93-98 (Wilson et al., eds., 12th ed. 1991); Williams et
al., J. of
Med. Chem. 42: 1481-1485 (1999), herein each incorporated by reference in
their
entirety). "Pain" is also meant to include mixed etiology pain, dual mechanism
pain,
allodynia, causalgia, central pain, hyperesthesia, hyperpathia, dysesthesia,
and
hyperalgesia.
[0045] "Somatic" pain, as described above, refers to a normal nerve response
to a
noxious stimulus such as injury or illness, e.g., trauma, burn, infection,
inflammation,
or disease process such as cancer, and includes both cutaneous pain (e.g.,
skin, muscle
or joint derived) and visceral pain (e.g., organ derived).
[0046] "Neuropathic pain" is a heterogeneous group of neurological conditions
that
result from damage to the nervous system. "Neuropathic" pain, as described
above,
refers to pain resulting from injury to or dysfunctions of peripheral and/or
central
sensory pathways, and from dysfunctions of the nervous system, where the pain
often
occurs or persists without an obvious noxious input. This includes pain
related to
peripheral neuropathies as well as central neuropathic pain. Common types of
peripheral neuropathic pain include diabetic neuropathy (also called diabetic
peripheral neuropathic pain, or DN, DPN, or DPNP), post-herpetic neuralgia
(PHN),
and trigeminal neuralgia (TGN). Central neuropathic pain, involving damage to
the
brain or spinal cord, can occur following stroke, spinal cord injury, and as a
result of
multiple sclerosis. Other types of pain that are meant to be included in the
definition
of neuropathic pain include pain from neuropathic cancer pain, HIV/AIDS
induced
pain, phantom limb pain, and complex regional pain syndrome. In a preferred
embodiment, the compounds of the invention are of use for treating neuropathic
pain.
[0047] Common clinical features of neuropathic pain include sensory loss,
allodynia (non-noxious stimuli produce pain), hyperalgesia and hyperpathia
(delayed
perception, summation, and painful aftersensation). Pain is often a
combination of
nociceptive and neuropathic types, for example, mechanical spinal pain and
radiculopathy or myelopathy.
[0048] "Acute pain", is the normal, predicted physiological response to a
noxious
chemical, thermal or mechanical stimulus typically associated with invasive
procedures, trauma and disease. It is generally time-limited, and may be
viewed as an
appropriate response to a stimulus that threatens and/or produces tissue
injury.
17
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
"Acute pain", as described above, refers to pain which is marked by short
duration or
sudden onset.
[00491 "Chronic pain" occurs in a wide range of disorders, for example,
trauma,
malignancies and chronic inflammatory diseases such as rheumatoid arthritis.
Chronic pain usually lasts more than about six months. In addition, the
intensity of
chronic pain may be disproportionate to the intensity of the noxious stimulus
or
underlying process. "Chronic pain", as described above, refers to pain
associated
with a chronic disorder, or pain that persists beyond resolution of an
underlying
disorder or healing of an injury, and that is often more intense than the
underlying
process would predict. It may be subject to frequent recurrence.
[0050] "Inflammatory pain" is pain in response to tissue injury and the
resulting
inflammatory process. Inflammatory pain is adaptive in that it elicits
physiologic
responses that promote healing. However, inflammation may also affect neuronal
function. Inflammatory mediators, including PGE2 induced by the COX2 enzyme,
bradykinins, and other substances, bind to receptors on pain-transmitting
neurons and
alter their function, increasing their excitability and thus increasing pain
sensation.
Much chronic pain has an inflammatory component. "Inflammatory pain", as
described above, refers to pain which is produced as a symptom or a result of
inflammation or an immune system disorder.
[0051] "Visceral pain", as described above, refers to pain which is located in
an
internal organ.
[00521 "Mixed etiology" pain, as described above, refers to pain that contains
both
inflammatory and neuropathic components.
[0053] "Dual mechanism" pain, as described above, refers to pain that is
amplified
and maintained by both peripheral and central sensitization.
[0054] "Causalgia", as described above, refers to a syndrome of sustained
burning,
allodynia, and hyperpathia after a traumatic nerve lesion, often combined with
vasomotor and sudomotor dysfunction and later trophic changes.
[0055] "Central" pain, as described above, refers to pain initiated by a
primary
lesion or dysfunction in the central nervous system.
18
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0056] "Hyperesthesia", as described above, refers to increased sensitivity to
stimulation, excluding the special senses.
[0057] "Hyperpathia", as described above, refers to a painful syndrome
characterized by an abnormally painful reaction to a stimulus, especially a
repetitive
stimulus, as well as an increased threshold. It may occur with allodynia,
hyperesthesia, hyperalgesia, or dysesthesia.
[0058] "Dysesthesia", as described above, refers to an unpleasant abnormal
sensation, whether spontaneous or evoked. Special cases of dysesthesia include
hyperalgesia and allodynia,
[0059] "Hyperalgesia", as described above, refers to an increased response to
a
stimulus that is normally painful. It reflects increased pain on
suprathreshold
stimulation.
100601 "Allodynia", as described above, refers to pain due to a stimulus that
does
not normally provoke pain.
[0061] The term "pain" includes pain resulting from dysfunction of the nervous
system: organic pain states that share clinical features of neuropathic pain
and
possible common pathophysiology mechanisms, but are not initiated by an
identifiable lesion in any part of the nervous system.
[0062] The term "Diabetic Peripheral Neuropathic Pain".(DPNP, also called
diabetic neuropathy, DN or diabetic peripheral neuropathy) refers to chronic
pain
caused by neuropathy associated with diabetes mellitus. The classic
presentation of
DPNP is pain or tingling in the feet that can be described not only as
"buming" or
"shooting" but also as severe aching pain. Less commonly, patients may
describe the
pain as itching, tearing, or like a toothache. The pain may be accompanied by
allodynia and hyperalgesia and an absence of symptoms, such as numbness.
[0063] The term "Post-Herpetic Neuralgia", also called "Postherpetic
Neuralgia"
(PHN), is a painful condition affecting nerve fibers and skin. It is a
complication of
shingles, a second outbreak of the varicella zoster virus (VZV), which
initially causes
chickenpox.
[0064] The term "neuropathic cancer pain" refers to peripheral neuropathic
pain as
a result of cancer, and can be caused directly by infiltration or compression
of a nerve
19
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
by a tumor, or indirectly by cancer treatments such as radiation therapy and
chemotherapy (chemotherapy-induced neuropathy).
[0065] The term "HIV/AIDS peripheral neuropathy" or "HIV/AIDS related
neuropathy" refers to peripheral neuropathy caused by HIV/AIDS, such as acute
or
chronic inflammatory demyelinating neuropathy (AIDP and CIDP, respectively),
as
well as peripheral neuropathy resulting as a side effect of drugs used to
treat
HIV/AIDS.
[0066] The term "Phantom Limb Pain" refers to pain appearing to come from
where
an amputated limb used to be. Phantom limb pain can also occur in limbs
following
paralysis (e.g., following spinal cord injury). "Phantom Limb Pain" is usually
chronic
in nature.
[0067] The term "Trigeminal Neuralgia" (TN) refers to a disorder of the fifth
cranial (trigeminal) nerve that causes episodes of intense, stabbing, electric-
shock-like
pain in the areas of the face where the branches of the nerve are distributed
(lips, eyes,
nose, scalp, forehead, upper jaw, and lower jaw). It is also known as the
"suicide
disease".
[0068] The term "Complex Regional Pain Syndrome (CRPS)," formerly known as
Reflex Sympathetic Dystrophy (RSD), is a chronic pain condition. The key
symptom
of CRPS is continuous, intense pain out of proportion to the severity of the
injury,
which gets worse rather than better over time. CRPS is divided into type 1,
which
includes conditions caused by tissue injury other than peripheral nerve, and
type 2, in
which the syndrome is provoked by major nerve injury, and is sometimes called
causalgia.
[0069] The term "Fibromyalgia" refers to a chronic condition characterized by
diffuse or specific muscle, joint, or bone pain, along with fatigue and a
range of other
symptoms. Previously, fibromyalgia was known by other names such as
fibrositis,
chronic muscle pain syndrome, psychogenic rheumatism and tension myalgias.
[0070] The term "convulsion" refers to a CNS disorder and is used
interchangeably
with "seizure," although there are many types of seizure, some of which have
subtle
or mild symptoms instead of convulsions. Seizures of all types may be caused
by
disorganized and sudden electrical activity in the brain. Convulsions are a
rapid and
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
uncontrollable shaking. During convulsions, the muscles contract and relax
repeatedly.
II. Introduction
[0071J The present invention relates to novel inhibitors of the enzyme D-amino
acid
oxidase. These compounds are useful for treating or preventing any disease
and/or
condition, wherein modulation of D-serine levels, and/or its oxidative
products, is
effective in ameliorating symptoms. Inhibition of the enzyme can lead to
increases in
D-serine levels and a reduction in the formation of toxic D-serine oxidation
products.
Thus, the invention provides methods for the treatment or prevention of
neurological
disorders. For example, the invention provides methods of enhancing leaming,
memory and/or cognition, for treating or preventing loss of memory and/or
cognition
associated with neurodegenerative diseases (e.g., Alzheimer's disease) and for
preventing loss of neuronal function characteristic of neurodegenerative
diseases.
Further, methods are provided for the treatment or prevention of pain, ataxia,
and
convulsion.
III. Compositions
A. Fused Heterocycles
[0072] The heterocyclic inhibitors of the invention are characterized by a
variety of
core-moieties. In an exemplary embodiment, the core-moiety includes a fused
heterocyclic ring system of two 5-membered rings. Exemplary 5-membered rings
include heteroaromatic rings, such as oxazoles, isoxazoles, thiazoles,
isothiazoles,
imidazoles and pyrazoles and preferably pyrroles, thiophenes and furans.
[0073] In a first aspect, the present invention provides compounds having a
structure according to Formula (I):
R
Q'~Z
Y A Rs
(I)
wherein Q is a member selected from 0, S, CR' and N, NR3a. X and Y are members
independently selected from 0, S, NR3, CR2 and N. When both X and Y are CR2,
then each R2 is independently selected. Z is a member selected from 0 and S. Z
is
preferably O. A is a member selected from NR7, S and O. In a preferred
embodiment, A is selected from NH and S.
21
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0074] In Formula (I), R3a, R3 and R7 are members independently selected from
H,
OR12, acyl, SOZRt3, SOR13, substituted or unsubstituted alkyl, substituted or
unsubstituted heteroalkyl, substituted or unsubstituted aryl, substituted or
unsubstituted heteroaryl and substituted or unsubstituted heterocycloalkyl.
[0075] R'Z and R13 are members independently selected from substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl.
[0076] R', R2 and R4 are members independently selected from H, halogen (e.g.,
F,
Cl, Br), CN, CF3, acyl, OR14, S(O)20R14, S(O)RR14, NR14R15, SO2NR14R15,
substituted
or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted
or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl, wherein p is an integer selected from 0 to 2.
R' and
R2, together with the atoms to which they are attached, are optionally joined
to form a
5- to 7-membered ring. R14 and R'S are members independently selected from H,
substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted
or unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted
or
unsubstituted heterocycloalkyl. R14 and R15, together with the nitrogen atoms
to
which they are attached, are optionally joined to form a 5- to 7-membered
ring.
[0077] In one embodiment, R4 in Formula (I) is selected from H, F, Cl, Br and
unsubstituted Ci-C6 (preferably unsubstituted CI-C4 alkyl, more preferably
unsubstituted Ci-C3 alkyl, and most preferably unsubstituted CI-C2 alkyl).
[0078] R6 is a member selected from O'X, ORB, NR9R10, NR8NR9R'0, NR8OR9,
NRgSO2R", substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl, wherein wherein X+ is a
positive
ion, which is a member selected from inorganic positive ions and organic
positive
ions. R6 and R4, together with the atoms to which they are attached, are
optionally
joined to form a 5- to 7-membered ring. In a preferred embodiment, R6 is
selected
from O-X+, ORg. R8, R9 and R10 are members independently selected from H,
substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted
or unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted
or
22
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
unsubstituted heterocycloalkyl. R8 is preferably H or CI-C4 unsubstituted
alkyl, such
as methyl, ethyl, propy). R" is a member selected from substituted or
unsubstituted
alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted
aryl,
substituted or unsubstituted heteroaryl and substituted or unsubstituted
heterocycloalkyl. At least two of R8, R9, R10 and R", together with the atoms
to
which they are attached, are optionally joined to form a 5- to 7-membered
ring.
[0079] In one embodiment, wherein Q is C-R', X is S, Y is CRZ, R4 is H, A is
NH
and Z is 0, (i) R' and R2 are preferably not both H; (ii) R' and R2 are
preferably not
both halogen, unless at least one member selected from R' and R2 is fluoro;
and (iii)
when one member selected from R' and R2 is halogen other than fluoro, then the
other
member is preferably not H or unsubstituted CI-CZ alkyl.
[0080] In a related embodiment, wherein Q is C-R', X is CR2, Y is S, R4 is H,
A is
NH and Z is 0, (i) R' and R2 are preferably not both H; (ii) R' and RZ are
preferably
not both halogen, unless at least one member selected from R' and R2 is
fluoro; and
(iii) when one member selected from R' and R2 is halogen other than fluoro,
then the
other member is preferably not H or unsubstituted C1-C2 alkyl.
[0081] In another embodiment, wherein Q is C-R', X is S, Y is CH, R4 is H, A
is
NH and Z is 0, R' is preferably not a member selected from CN and C=CH. In yet
another embodiment, wherein Q is C-R', X is CH, Y is S, R4 is H, A is NH and Z
is
0, R' is preferably not a member selected from CN and C CH.
[0082] Other preferred compounds include those in which Q is C-R', X is S, Y
is
CH, A is NH, R' is H, Z is 0 and R4 is not C1-C3 alkyl substituted with
halogen; those
in which Q isC-Rt,XisCH,YisS,AisNH,R' isH,ZisOandR4isnotQ-C3
alkyl substituted with halogen; as well as those in which Q is C-R', R4 is H,
Z is 0, A
is NR' and R7 is not a member selected from:
0
~-S-Ar
J-CHZ Ar ; and 0
wherein Ar is substituted or unsubstituted phenyl. Those compounds in which Q
is
C-Rl, X is S, Y is CH, A is S, R' is H, Z is 0, R6 is OH, and R4 is not a
member
selected from H and unsubstituted CJ-C2 alkyl, as well as those compounds in
which
23
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Q is C-Rl, X is CH, Y is S, A isS,R' isH,ZisO,R6isOH,andR4isnotamember
selected from H and unsubstituted CI-C2 alkyl, are also preferred.
[00831 In a further embodiment, wherein Q is C-Rl, X is S, Y is CH, A is NH,
R' is
H, Z is 0, and R6 is ORg, in which R8 is unsubstituted Ci-C6 alkyl, R4 is
preferably not
unsubstituted CI-C2 alkyl. In another embodiment, wherein Q is C-R1, X is S, Y
is
CH, R4 is H, A is NH, Z is 0, and R6 is ORa, in which R8 is unsubstituted CI-
C6 alkyl,
R' is preferably not carboxylic acid ester. In yet another embodiment, wherein
X is S,
Y is CH, R4 is H, R' is H, Z is 0, R6 is OH, and A is NR7, R7 is preferably
not
cyclohexylmethyl.
[00841 It is also generally prefered that when Q is C-Rl, X is S, Y is CR2, R4
is H or
acyl, A is NR7, in which R7 is a member selected from H and acyl, and Z is 0,
then (i)
R' and RZ are not both unsubstituted CI-C2 alkyl, and (ii) when one member
selected
from R' and R2 is unsubstituted CI-CZ alkyl, another member is not H; and
when, in
Formulal,QisC-Rl,XisO,YisCR2,R4isH,AisNH,andZisO,then(i)R' and
R 2 are not both H, (ii) R' and R2 are not both unsubstituted Ci-C2 alkyl, and
(iii) when
one member selected from R' and R2 is unsubstituted C1-CZ alkyl, then the
other
member is not H.
100851 In one exemplary embodiment, wherein Q is C-Rl, X is S, Y is CH, Z is
0,
and R6 is OR8, R4 is preferably not C(O)-2-thiophenyl. In another embodiment,
wherein Q is C-Rl, X is 0, Y is CH, R4 is H, A is NH and Z is 0, R' is
preferably not
a member selected from Cl, Br, I, CN and unsubstituted Ci-C2 alkyl. In yet
another
embodiment, wherein, in Formula I, Q is C-R', X is 0, Y is CRZ, R' is H, R 4
is H, A
is NH, and Z is 0, R2 is preferably not Cl, Br or I.
[00861 In a further embodiment, wherein Q is C-Rl, X is 0 or S, Y is CH, R, is
H,
R4 is H, A is NR7, Z is 0 and R6 is OH, R7is preferably not methyl.
[00871 It is also generally preferred that when Q is C-Rl, X is CH, Y is S, R4
is Cl,
Br or I, A is NH and Z is 0, then R' is not a member selected from Cl, Br and
I; and
when Q is C-Rl, X is CR2, Y is S, A is NR7, in which R7 is phenyl, Z is 0, and
R6 is
ORB, in which R8 is unsubstituted CI-C6 alkyl, then R4 is not a member
selected from
phenyl, unsubstituted CI-C2 alkyl, and OH.
24
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Pyrrole Analoes
[0088] In one embodiment, in Formula (I), A is NR7 and preferably NH.
[00891 In one example according to this embodiment, Q is selected from N and C-
R1, and each of X and Y is a member selected from CR2, NR3 and N. In this
example
at least one of X and Y is preferably NR3. Exemplary fused pyrroles have the
general
structure:
R3 R4 RZ R4 R3 R4 R4
~! "
R~ N '\ O R' O Rl~N C O 4! N O
N N R6 N N IR6
N R6 t N N R6 I
RZ R3 H Ra H
RZ 4 R3 4
N~ o ~N1N
f H R N Rs
R3 ; and R2 H
wherein R6 is preferably a member selected from O-X+and ORB, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. R8 is preferably H or CI-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, iso-Bu).
Each of the preferred embodiments set forth herein above for compounds
according to
Formula (I) are optionally, equally applicable to the compounds of this
paragraph.
[0090] In another exemplary embodiment, Q is C-R' and each of X and Y is a
member selected from S, CR2 and N, with the proviso that at least one of X and
Y is
S. Exemplary fused pyrroles have the structure:
R4 R2 R4 R4
R' \ I\ O Ri ~ I\ O R1~S I\ O
N R6 S N R6 N N R6
R2 H ; H and
R4
N I 4!0
S N R6
H
wherein R6 is preferably a member selected from O-X+ and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. R8 is preferably H or CI-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, iso-Bu).
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Each of the preferred embodiments set forth herein above for compounds
according to
Formula (I) are optionally, equally applicable to the compounds of this
paragraph.
100911 In yet another exemplary embodiment, Q is C-Rl and each of X and Y is a
member selected from 0, CR2 and N, with the proviso that at least one of X and
Y is
O. Exemplary fused pyrroles have the general structure:
R4 R2 R R
R' \ I~ O R' O RI~O I\ O
N Rs O N R6 N N R6
R2
H H = H
and
R 4
N I
O N R6
wherein R 6 is preferably a member selected from O-X+ and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. R8 is preferably H or CI-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, fso-Bu).
Each of the preferred embodiments set forth herein above for compounds
according to
Formula (I) are optionally, equally applicable to the compounds of this
paragraph.
[0092] In yet another exemplary embodiment Q in Formula (I) is 0 or S.
Exemplary fused pyrroles have the general structure:
R2 R 4 R 4 R2 R R4
O N O O N O
S. ~ S _ ~ O O ~
2 H R6 H Rs H R6 2 H N R6
R2 ~ R2 ~ R2 ~~d R
wherein each R2 is independently selected. R6 is preferably a member selected
from
O-X+ and OR8, wherein X+ is a positive ion, which is a member selected from
inorganic positive ions and organic positive ions anfd RS is preferably H or
CI -C4
alkyl (e.g., Me, Et, Pr, iso-Pr, n-Bu, iso-Bu). Each of the preferred
embodiments set
forth herein above for compounds according to Formula (I) are optionally,
equally
applicable to the compounds of this paragraph.
Thiophene Analogs
[00931 In another embodiment, in Formula (I), A is S.
26
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0094] In one example according to this embodiment, Q is selected from N and C-
R', and each of X and Y is a member selected from CR2, NR3 and N with the
proviso
that at least one of X and Y is NR3. Exemplary fused thiophenes have the
structure:
R3 R RZ R4 R3 R4
O ~ ~ \ O N O
R' \ ~\ R ~ 6 Rl~ I\
S Rs N S R N S R4
s
R2 R3
> > ;
R R2 R R3 Ra
R'~N I\ O N~ I\ O NN I\
N S R6 N S Re ~ S Rs
R3 R3 R
wherein R6 is preferably a member selected from O-X+ and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. Rg is preferably H or CI-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, iso-Bu).
Each of the preferred embodiments set forth herein above for compounds
according to
Formula (I) are optionally, equally applicable to the compounds of this
paragraph.
100951 In another example, Q is C-Rl and each of X and Y is a member selected
from S, CR2 and N, with the proviso that at least one of X and Y is S.
Exemplary
fused thiophenes have the general structure:
R R2 R4 R4
S RB R, / I\ O R'~S I\ O
R' \ Jf3_/<
S S Rs N S Rs
RZ and
R 4
_ R,~N I \ O
S S Rs
wherein R6 is preferably a member selected from O-X+and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. R8 is preferably H or Ci-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, iso-Bu).
Each of the preferred embodiments set forth herein above for compounds
according to
Formula (I) are optionally, equally applicable to the compounds of this
paragraph.
27
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[00961 In yet another example, Q is C-Rl and each of X and Y is a member
selected
from 0, CR2 and N, with the proviso that at least one of X and Y is O.
Exemplary
fused thiophenes have the general structure:
R4 R2 R R
O O O
Ri ~\ O R1 7 I \ R~ ~\
\ S Rs O S R6 N S R6
R2 and
R4
R~~N I \ O
0 S Rs
wherein R6 is preferably a member selected from O-X+ and ORg, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. R8 is preferably H or CI-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, iso-Bu).
Each of the preferred embodiments set forth herein above for compounds
according to
Fonnula (I) are optionally, equally applicable to the compounds of this
paragraph.
[00971 In yet another exemplary embodiment Q in Formula (I) is 0 or S.
Exemplary
fused thiophenes have the general structure:
R2 R4 R4 R2 R R4
O N~ O
O N~ O
S \ S \ O \ O \
~ S R6 - S Rs S R6 f S R6
R2 R2 R2 , and R
,
wherein each R2 is independently selected and wherein R6 is preferably a
member
selected from O-X+ and OR8, wherein X+ is a positive ion, which is a member
selected
from inorganic positive ions and organic positive ions. R8 is preferably H or
CI-C4
alkyl (e.g., Me, Et, Pr, iso-Pr, n-Bu, iso-Bu). Each of the preferred
embodiments set
forth herein above for compounds according to Formula (I) are optionally,
equally
applicable to the compounds of this paragraph.
[00981 In a preferred embodiment, in Formula (1), A is NH and Z is O. Hence,
in
one aspect, the present invention provides a compound having a structure
according to
Fonnula (II):
28
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
R4
Q tX,, ~ O
Y H Rs
an.
[0099] In another embodiment, Q is CR' and the compound of the invention has a
structure according to Formula (IIa):
R4
0
i~
:
Y N Rs
(IIa)
wherein R', X, Y, R4 and R6 are defined as above for Formula (I) or Formula
(II).
Each of the preferred embodiments set forth herein above for compounds
according to
Formula (I) are optionally, equally applicable to the compounds of Formula
(II) and
(IIa).
101001 In one embodiment, in Formula (II), Q is a member selected from 0, S, N
and
CR'. X is a member selected from 0, S, N, NR3 and CR2a and Y is a member
selected
from 0, S, N, NR3 and CR2b, wherein R' is a member selected from H, F,
substituted
or unsubstituted arylalkyl, substituted or unsubstituted heteroarylalkyl,
substituted or
unsubstituted Ca-CIo cycloalkyl, and substituted or unsubstituted C4-CIO
heterocycloalkyl. R2a is a member selected from H, F, Cl, Br, CN, substituted
or
unsubstituted C3-C6 alkyl, substituted or unsubstituted arylalkyl, substituted
or
unsubstituted heteroarylalkyl, substituted or unsubstituted C4-CIO cycloalkyl,
substituted or unsubstituted C4-Clo heterocycloalkyl and alkenyl. R2b is a
member
selected from H, F, substituted or unsubstituted C3-C6 alkyl, substituted or
unsubstituted arylalkyl, substituted or unsubstituted heteroarylalkyl,
substituted or
unsubstituted C4-CIO cycloalkyl, and substituted or unsubstituted C4-CIO
heterocycloalkyl and alkenyl. R3 is a member selected from H, substituted or
unsubstituted CI-C6 alkyl, substituted or unsubstituted arylalkyl, substituted
or
unsubstituted heteroarylalkyl, substituted or unsubstituted C4-CIO cycloalkyl,
and
substituted or unsubstituted C4-Cjo heterocycloalkyl. R4 is a member selected
from H,
F, Cl, Br, CN, unsubstituted CI -C6 alkyl, substituted or unsubstituted
arylalkyl,
substituted or unsubstituted heteroarylalkyl, substituted or unsubstituted C4-
Clo
cycloalkyl and alkenyl. In a preferred embodiment, R4 is a member selected
from H,
F, Cl, Br, CN and unsubstituted CI-C4 alkyl. R6 is a member selected from O-X+
and
29
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
OH, wherein X+ is a positive ion, which is a member selected from inorganic
positive
ions and organic positive ions. In one embodiment, in which Q is CF and one
member selected from X or Y is S and the other is CH, R4 is preferably other
than H.
In another embodiment, in which Q is CH, and Y is S, 0 or CH, at least one of
R28
and R4 is other than H. Each of the preferred embodiments set forth herein
above for
compounds according to Formula (I) are optionally, equally applicable to the
compounds of this paragraph.
[0101] In one example, R', R28, R2b and R4 are members independently selected
from H and F.
[0102] In another example, Q is CR' and one member selected from X and Y is S
and the other member is CR2a, CRZb or N. Exemplary compounds have the formula:
R
R' \ ( \
Rg
R2b H
wherein R 4 is preferably a member selected from H, F, Cl, Br, CN and
unsubstituted
C1-C4 alkyl.
[0103] In a further example, Q is CR' and one member selected from X and Y is
0
and the other member is CRZB, CR2b or N. Exemplary compounds have the formula:
Ra
RI \ ~ \
t~ R6
R2b H
wherein R4 is preferably a member selected from H, F, Cl, Br, CN and
unsubstituted
CI -C4 alkyl.
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0104] Hence, the invention provides compounds having a structure selected
from:
R4 R R4 R R R4
O S O
s
R' \ I N Rs Ri /(\ R' O R' \ I N R
0
R2b H 0 H R6 S H R6 Rib H
R3 R4 R2a R4
O Ri N ~\ O R' / (\
~ 4 O i N ~ o
S s
R~ J -~ R~,~C, N Rs N N R
N N H
Rs S N Rs R~, H
H H R3
R3 R4 a Rza R4 R3 R4 %
% ~\ R'~N O N/ I\ 4
Ri N
NN I\
~~ N N R N R6 N ,14
Rs
N N Rs . R3 H . Rs H . R2b H
> > > >
R4 R4
R'-~~O ' 4Rr- O
N H --~~0 N R6
;and H
[0105] In another embodiment, the invention provides a compound having a
structure, which is a member selected from Formula (III) and Formula (IV):
R4 R4
Rl--\X r O RY O
Y Rs (IIn X N R6
H H (IV)
[0106] In Formula (III) and Formula (IV), X is a member selected from 0, S and
NR3. Y is a member selected from CR2 and N. R3 is a member selected from H,
OR12, acyl, SO2R13, SOR13, substituted or unsubstituted alkyl, substituted or
unsubstituted heteroalkyl, substituted or unsubstituted aryl, substituted or
unsubstituted heteroaryl and substituted or unsubstituted heterocycloalkyl,
wherein
Rt2 and R13 are members independently selected from substituted or
unsubstituted
alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted
aryl,
substituted or unsubstituted heteroaryl and substituted or unsubstituted
heterocycloalkyl.
[0107] R% R2 and R4 in Formulae (III) and (IV) are members independently
selected from H, F, Cl, Br, CN, CF3, acyl, OR14, S(O)POR14, S(O)2R14, NR14R15,
SO2NR14R15, substituted or unsubstituted alkyl, substituted or unsubstituted
31
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl, wherein p is an integer
selected
from 0 to 2. R' and R2, together with the atoms to which they are attached,
are
optionally joined to form a 5- to 7-membered ring. R14 and R15 are members
independently selected from substituted or unsubstituted alkyl, substituted or
unsubstituted heteroalkyl, substituted or unsubstituted aryl, substituted or
unsubstituted heteroaryl and substituted or unsubstituted heterocycloalkyl.
R14 and
R15, together with the nitrogen atoms to which they are attached, are
optionally joined
to form a 5- to 7-membered ring.
[0108] In one embodiment, R4 in Formula (I) is selected from H, F, Cl, Br and
unsubstituted CI-C6 (preferably unsubstituted C i-C4 alkyl, more preferably
unsubstituted CI -C3 alkyl, and most preferably unsubstituted CI -CZ alkyl).
[0109] R6 is a member selected from O-X+, ORg, NR9R'o, NR8NR9Ri , NRsOR9,
NR8SOZR", substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl, wherein wherein X+ is a
positive
ion, which is a member selected from inorganic positive ions and organic
positive
ions. R6 and R4, together with the atoms to which they are attached, are
optionally
joined to form a 5- to 7-membered ring. R8, R9 and R10 are members
independently
selected from H, substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R" is a member selected
from
substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted
or unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted
or
unsubstituted heterocycloalkyl. At least two of R8, R9, R10 and R", together
with the
atoms to which they are attached, are optionally joined to form a 5- to 7-
membered
ring.
[0110] R8, R9 and R10 are members independently selected from H, substituted
or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl and R" is a member selected from substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
32
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl. At least two of Rg, R9, R10 and R' 1, together
with the
atoms to which they are attached, are optionally joined to form a 5- to 7-
membered
ring.
[0111] In an exemplary embodiment, wherein X is S, and Y is CR2, (i) R' and R2
are preferably not both H, (ii) R' and R2 are preferably not both halogen,
unless at
least one member selected from R' and R2 is fluoro, and (iii) when one member
selected from R' and R2 is halogen other than fluoro, then the other member is
preferably not H or unsubstituted CI-C2 alkyl.
[0112] In another exemplary embodiment, wherein X is S and Y is CH, R' is
preferably not a member selected from CN and C CH. In a further embodiment,
wherein X is S, Y is CH, R' is H and R6 is OH, R4 is preferably not a member
selected from H and unsubstituted Cl-C2 alkyl.
[0113] Generally preferred compounds include those, in which, in Formula
(III), X
is S, Y is CH, R6 is OR8, wherein R8 is unsubstituted C1-C6 alkyl, and R' is
not
carboxylic acid ester; and those, in which, in Formula (III), X is S, Y is CR2
and (i) R'
and R2 are not both unsubstituted C1-C2 alkyl, (ii) when one member selected
from R'
and R2 is CI-C2 unsubstituted alkyl, then the other member is not H; and
(iii') when R'
is unsubstituted CI-C2 alkyl, then R2 is not acyl.
101141 In a further embodiment, wherein, in Formula (III), X is 0 and Y is
CR2, (i)
R' and R2 are preferably not both H, (ii) R' and R2 are preferably not both
unsubstituted CI-CZ alkyl, and (iii) when one member selected from R' and R2
is
unsubstituted Ci-C2 alkyl, then the other member is preferably not H.
[0115] When in Formula (III), X is 0 and Y is CH, then those compounds, in
which
R' is not a member selected from Cl, Br, I, CN, and unsubstituted CI-C2 alkyl
are
generally preferred; and when, in Formula (III), X is 0, Y is CR2 and R' is H,
then,
preferred compounds are those in which R2 is not Cl, Br or I.
[0116] In an exemplary embodiment, in Formual (II) and (IV), X is a member
selected from 0, S and NR3 and Y is a member selected from CR2 and N. R' and
R2
are members independently selected from H, F, substituted or unsubstituted C3-
C6
alkyl, substituted or unsubstituted arylalkyl, substituted or unsubstituted
33
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
heteroarylalkyl, substituted or unsubstituted C4-CIo cycloalkyl, and
substituted or
unsubstituted C4-CIo heterocycloalkyl and alkenyl. R3 is a member selected
from H,
substituted or unsubstituted CI -C6 alkyl, substituted or unsubstituted
arylalkyl,
substituted or unsubstituted heteroarylalkyl, substituted or unsubstituted C4-
CIo
cycloalkyl, and substituted or unsubstituted C4-CI0 heterocycloalkyl. R4 is a
member
selected from H, F, Cl, Br, CN, unsubstituted CI-C6 alkyl, substituted or
unsubstituted
arylalkyl, substituted or unsubstituted heteroarylalkyl, substituted or
unsubstituted C4-
CIo cycloalkyl and alkenyl. R6 is a member selected from O-X+ and OH, wherein
X+
is a positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. In one embodiment, in which X is S, Y is CH and R' is F, R4 is
preferably other than H. In another embodiment, in which in Formula (III), R'
is H
and Y is CH, R4 is preferably other than H. In yet another embodiment, wherein
in
Formula (IV), R' is H, at least one of R2 and R4 is other than H.
[0117] Exemplary compounds according to Formulae (III) and (IV) include:
R2 R2
Rl O ~\ O Ri / ,\ O RI / ~\ RI S ~\ O
\ N R6 O N Rg S N R6 \ N Rs
R2 H H H R2
R3 R2
O
RIS I O R' N I\ O R~ \ I\ O R' (
N N Rg 8 N Rg N Rs N H Rs
H R2 H
R3 R F~
N N O N~ ~\ O Nf V I\ O
R ~\ N N R6 N N Rg X N Rs
N H Re. R3 H . Rs H R2 H
~ . > >
Rl-\O O N O
N H Re and O H Rs
wherein R6 is preferably a member selected from O'X+ and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions. R8 is preferably H or Ci-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-
Bu, iso-Bu).
Each of the preferred embodiments set forth herein above for compounds
according to
Formulae (III) and (IV) are optionally, equally applicable to the compounds of
this
paragraph.
34
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0118] Preferred compounds of the invention include those in which, in
Formulae
(I), (II), (IIa), (III) and (IV), at least one of R', R2 and R3 includes an
aromatic ring or
a fused ring system including an aromatic ring. In one embodiment, at least
one of
R', R2 and R3 has the formula:
I-L1-Ar
wherein Ar is a member selected from substituted or unsubstituted aryl,
substituted or
unsubstituted heteroaryl and a fused ring system. Ll is a linker moiety, which
is a
member selected from substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. Particularly preferred
compounds
are those, in which R' represents a small group, such as H and F, and a member
selected from R 2 and R3 includes the aromatic moiety.
[0119] Exemplary linker moieties include Ci to CS substituted or unsubstituted
alkyl
chains wherein one or more carbon atoms are optionally replaced with a group
including one or more heteroatoms, forming e.g., ether, thioether, amines,
amides,
sulfonamides or sulfones.
[0120] In an exemplary embodiment, at least one of R~, R2 and R3 has a
formula,
which is a member selected from:
16 17
-(CR R )ri Ar ; and J-(CR16R17)rj--Q1_Ar
wherein n is an integer from 0 to 5, and Ql is a member selected from 0 and S.
Rt6
and R17 are members independently selected from H, substituted or
unsubstituted
alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted
aryl,
substituted or unsubstituted heteroaryl and substituted or unsubstituted
heterocycloalkyl. R16 and R17, together with the carbon to which they are
attached,
are optionally joined to form a 3- to 7-membered ring, which is a member
selected
from substituted or unsubstituted cycloalkyl and substituted or unsubstituted
heterocycloalkyl, and which is optionally fused to Ar.
[0121] In an exemplary embodiment, Ar is a phenyl ring and has the formula:
__(R5)m
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
wherein m is an integer from 0 to 5. Each R5 can be selected from a variety of
substituents. In an exemplary embodiment, each R5 is a member independently
selected from H, halogen, CN, halogen substituted alkyl (e.g., CF3), hydroxy,
alkoxy
(e.g., methoxy and ethoxy), acyl (e.g., acetyl), CO2R18, OC(O)R18, NR'8R'9,
C(O)NR18R19, NR'gC(O)R20, NR18S02RZ0, S(O)2R20, S(O)R 20, substituted or
unsubstituted alkyl (e.g., methyl, ethyl, propyl and isopropyl), substituted
or
unsubstituted heteroalkyl, substituted or unsubstituted aryl, substituted or
unsubstituted heteroaryl and substituted or unsubstituted heterocycloalkyl,
wherein
adjacent R5 are optionally joined to form a ring, wherein the ring is a member
selected
from substituted or unsubstituted cycloalkyl, substituted or unsubstituted
heterocycloalkyl, substituted or unsubstituted aryl and substituted or
unsubstituted
heteroaryl.
[01221 R'g and R'9 are members independently selected from H, substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl. R20 is a member selected from substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl. R'g and a member selected from R19 and R20,
together
with the atoms to which they are attached, are optionally joined to form a 5-
to 7-
membered ring.
[01231 In one embodiment, at least one of R2 and R3 has the structure:
1-(CR'sR17),i-Ar
wherein n is an integer from 0 to 5; and R16 and R'7 are as defined above.
[0124] Exemplary structures according to this embodiment include:
(R5)m
~I\
R~ S R4 (CH~n R4 R' C R4
Z (R
S)'" ~ ~ Z
(RS)"' Z Z R, ~NR'
S / ( \
~(CHz)n 47 R7 (CHz)n R~
> > e
36
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
(R5)m (R5)m
R 4 R2 R/ ()-(CH2) \(C; 2)n R4
Rt
Rt / I\ Z (R ~
~m N~I Z Rt N I\ Z
.
C R7 Rs / I\ (CH2)n R7 Rs R2 R7 Rs
(R5)m
z I
R R4 \(C;2)n R4 Rt S R4
(R5)m N Z Z N N I \ Z (R5)m ) Z
N s N Rs ()-(cH2) s
~ CH2n 7 R R2 'R7 n R 7 R
f a a
(R5)m (R5)m
Rt N
(CH2)n R4 ID (CH2)n ~NR.<F 4 R3 R4
Rt Z
/ I\ Z Rt Z (R5)m S N N ~ R3 R~ (CH2), R7 R Rs
a a s
(R5)m
Rt N R4 I\ (CH2)n R4 Rt S R4
N
(i5)m N ~ ~ Z Rt~ I Z ( i ~m ~ ~ Z N /V(CH2)n R7 Rs N N Rs (CHA S Rs
R7
(R5)m (RS)m
F ~ (CH2n R4 R4 (CHA R4
Rt O
\ Z
Rt / I\ Z (R5)m ;~ ~ Z Rt
S S Rs / ~~ \~7__~s O S Rs
(CH2)n R
a a v
(RS)m (R5)m
R2 R4 1 V (CH2)n Ra I \ (CH2)n R4
t
(R5)m R N Rt N ~\ Z R, / I\ Z
S \ S Rs N S Rs
(CHA Rs R2 R3
f f s
(R5)m
R3 F I (CH2)n 4
Rt N R4 R N R 4 R
(R5)m P~s Z (R5)m N Y~ Z Rt~N \ Z
(CH2)n Rs I\ (CH2)n S Rs N S R.
and
wherein m, n, Z, R', R2, R3, R4 and R6 are defined as above.
37
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0125] In one example, R 2 has the structure:
J-(CR16R17)r-Ar
wherein n is an integer from 0 to 5; and R16 and RI7 are as defined above.
[0126] Preferred compounds according to this example include:
s
COOH
\ ~ \ COOH r COOH
H H
~
~
COOH ~y \ COOH ~ \ O
S H Ci S H S H OH
[0127] In another exemplary embodiment, R" has the structure:
1-(CR16R17 )d--Ar
wherein n is an integer from 0 to 5; and R16 and RI7 are as defined above.
[0128] Exemplary analogs include:
(R5)m (R5)m (Rb)m
\R2 R ~/ R Q R2 R
Z S Z Z
(CH2) \ I \ N Rg O N RB
R2 R7 R7
> > >
(R5)m (R5)m (R5)m
R R R4
O S 2 N
(CH2) \ (CH2)C (CHZ) ~
N Re N N RB S N RB
R2 R? R7 R7
~ e e
(R5)m (R5)m (R)m
R R R2 R
(CH2)--~~ Z (CH2)N ~ \ Z (CH2) / \ Z
N N RB O N Re S N RB
R7 R7
R7
(R5)m (R5)m (R5).
R4 R R2 R4
S Z ~ N Z Z
(CH2n \ ~ \ (CH2)~ ~ (CI"12)n
N Re O N RB S S Re
R2 R7 R7
> > >
38
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
(R5)m (R`')m (RS)m
Qx/I R R2 R R
Z
Z Z O
(CH2)n \ I \ (CH2)11 ~ I \ (CH2) \
RB O S RB S RB
R2 R2
e v e
(R5)m (RS)m (RS)m
~~
\ / R ~ / R4 R4
S Z N Z O Z
(CH2)rr-~ 1 \ (CH2)n--~ I \ (CH2)fi--~. I
N S Re S S RB N S R8
> > ;
(R5)m
R
NZ
(CHz)i
O S Re
and
wherein m,n, Z, R2, R4 and R6 are as defined above.
Substituted Analogs
[0129] Certain compounds of the invention include substituents R1, R2 and R4
that
are halogen (e.g., F, Cl, Br), CN, CF3 or lower alkyl groups, such as
substituted or
unsubstituted (preferably unsubstituted) Ci-C4 alkyl, such as methyl and
ethyl.
[0130] Accordingly, the invention provides compounds having a structure
according to Formula (X):
R4
O
Y H Rs
(X)
wherein Q is a member selected from CR1, N and NR3a One member selected from
X and Y is 0, S or N and the other member is CRZ. R1, R2 and R4 are members
independently selected from H, F, Cl, Br, CN and CF3, provided that at least
one
member selected from R1, R2 and R4 is other than H. R6 is a member selected
from O'
X+ and OR8, wherein X+ is a positive ion, which is a member selected from
inorganic
positive ions and organic positive ions, and wherein R8 is preferably H or CI-
C4 alkyl
(e.g., Me, Et, Pr, iso-Pr, n-Bu, iso-Bu). The compound is preferably not a
member
selected from: 2-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid; 2-chloro-4H-
thieno[3,2-b]pyrrole-5-carboxylic acid; 2-bromo-4H-thieno[3,2-b]pyrrole-5-
carboxylic acid; 2-cyano-4H-thieno[3,2-b]pyrrole-5-carboxylic acid; 2,3-
dichloro-4H-
39
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
thieno[3,2-b]pyrrole-5-carboxylic acid; 3-chloro-4H-thieno[3,2-bjpyrrole-5-
carboxylic acid; 3-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid; 3-bromo-4H-
furo[3,2-b]pyrrole-5-carboxylic acid; 2-chloro-4H-furo[3,2-b]pyrrole-5-
carboxylic
acid; 2-bromo-4H-furo[3,2-b]pynrole-5-carboxylic acid; 2-cyano-4H-furo[3,2-
b]pyrrole-5-carboxylic acid; 2-fluoro-6H-thieno[2,3-b]pyn:ole-5-carboxylic
acid; 2-
chloro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid; 2-bromo-6H-thieno[2,3-
b]pyrrole-
5-carboxylic acid; 2-cyano-6H-thieno[2,3-b]pyrrole-5-carboxylic acid; 2,3-
dichloro-
6H-thieno[2,3-b]pyrrole-5-carboxylic acid; 2,4-dichloro-6H-thieno[2,3-
b]pyrrole-5-
carboxylic acid and esters of these compounds.
101311 In one example, the compound of Formula (X) has a structure according
to
Formula (XI):
R4
O
Ri~;% `}--~
Y N Rs
H (XI)
wherein one member selected from X and Y is 0 or S and the other member is
CR2.
[01321 In another example, in Formula (XI), R' and R2 are H and R4 is a member
selected from F, Cl, Br, CN, CH3 and CF3. Exemplary compounds include:
H3 CF 3 CI Br
O O
(1N C~OIRs \ ~ \ CIOIRs \ [ \ CIOlR6 \ [ \ ClOIR6
N N N
H H H H
CH3 F3 r
U ~\ C(O)RB \ ~\ C(O)Rs \ ~\ C(O)R6 \ ~\ C(O)R6
N N N N
H H H H
3 C F3 Br
ci5coR6 C(p)Rs C(p)Rs S N S N S N
H H H and H
[0133] In another example, in Formula (XI), R' and R4 are H and X is CR2,
wherein
R2 is a member selected from F, Cl, Br and CN. Exemplary compounds include:
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
\ , \ C(O)RB \ ~C(O)Re \ ~ C(O)R6
HsC H ; FsC H ; CI H
P H3C
C(O)R6 C(O)Rs
C(p)R6
N HH F; S H ;
FgC CI Br
/ I\ C(p)Rs / I\ C(O)Rs / I\ C(O)Re
S H = S H and S H
~
[0134] In yet another example, in Formula (XI), R2 and R4 are H and R' is CF3.
Fluoro-Substituted Analogs
[01351 In another embodiment, the invention provides fluoro-substituted
analogs. In
one embodiment, the invention provides fluoro-substituted compounds having a
structure according to Formula (XII):
R4
, \ O
QY A R6 WI)
wherein A, Q, X, Y, R4 and R6 are defined as in Formula (I), provided that at
least one
member selected from R', R2 and R4 is F.
[0136] In one embodiment, in which Q is CF, and one member selected from X and
Y is S and the other member is CH, R4 is preferably other than H. In another
embodiment, in which A is NH, Q is CF, X is S and Y is CH, R4 is preferably
other
than H. In another embodiment, in which A is NH, Q is CF, X is CH and Y is S,
R4 is
preferably other than H. In yet another embodiment, in which A is S, Q is CF,
Y is S
and X is CH, R4 is preferably other than H. In a further embodiment, in which
A is S,
Q is CF, X is S and Y is CH, R4 is preferably other than H.
[0137] In another embodiment, the fluoro-substituted compound of the invention
has a structure according to Formula (XIII):
R4
O
N Y H R6
(XIII)
wherein one member selected from X and Y is 0 or S and the other member is
CR2.
41
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[01381 In Formula (XIII), R', R 2 and R4 are members independently selected
from
H and F, provided that at least one member selected from R', R2 and R is F.
R6 is a
member selected from O-X+ and OR8, wherein X+ is a positive ion, which is a
member
selected from inorganic positive ions and organic positive ions, and wherein
R8 is
preferably H or CI-C4 alkyl (e.g., Me, Et, Pr, iso-Pr, n-Bu, iso-Bu). In one
embodiment, in which R' is F, X is S and Y is CH, R4 is preferably other than
H. In
another embodiment, in which R' is F, Y is S and X is CH, R4 is preferably
other than
H.
[01391 In yet another embodiment, the fluoro-substituted compound of the
invention has a structure according to Formula (XIV):
R
X O
~
R
N Re
R2 H (XIV)
wherein X is a member selected from 0 and S. R', R2 and R4 are members
independently selected from H and F, provided that at least one member
selected from
R', R2 and R4 is F. R6 is a member selected from O"X+ and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions, and wherein R8 is preferably H or Ci-C4 alkyl (e.g., Me, Et,
Pr, iso-Pr,
n-Bu, iso-Bu). In one example according to this embodiment, in which R' is F,
X is S
and R2 is H, R is preferably other than H.
[01401 Exemplary compounds according to this embodiment include:
4 R4 Ra Ra
N Rs \ JtI>-/6 RI \ I\ O RI S
6
RZ H RZ H Fi R H R
R~ ~ R' ~ ~
N Rs N Rs
RZ H R2 H
wherein R', R2 and R4 are selected from H and F.
42
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0141] In a further embodiment, the compound of the invention has a structure
according to Formula (XV):
RZ R4
R O
Y N Re
H (XV)
wherein Y is a member selected from 0 and S. R', R2 and R4 are members
independently selected from H and F, provided that at least one member
selected from
R1, RZ and R4 is F. R6 is a member selected from O'X+ and OR8, wherein X+ is a
positive ion, which is a member selected from inorganic positive ions and
organic
positive ions, and wherein R8 is preferably H or CI-C4 alkyl (e.g., Me, Et,
Pr, iso-Pr,
n-Bu, iso-Bu). In one example according to this embodiment, in which the
moiety -
C(O)R6 is an ester group, A is S, Q is CF, Y is S and X is CH, R4 is other
than H.
[01421 Exemplary compounds according to this embodiment include:
R4 R2 R4 R2 R4
R~ / 1\ O O R' O R' / I \ O
S N Rs S N Re S N Rs O N Rs
H H H H
2 R4 2
Ri
/ JtI>-~= \ O O
O N 6
H
wherein R', R2 and R4 are selected from H and F.
[0143] In an exemplary embodiment, in Formulae (X) to (XV), R' is F. Compounds
according to this embodiment include, for example:
I\ F~S O F / (\ O
F u I\ F \
S: 'p
N Re N Re N N RB O N Re
H H H ; H
F N I\ O F/ O S O
~ s s F F ~\
S H R S H R S Re = \ S Re .
. ~
F/ ~\ O F/ ~\ O
O S Re and S S Rs .
[0144] In another exemplary embodiment, in Formulae (X) to (XV), RZ is F.
Exemplary compounds according to this embodiment include:
43
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
p ~\ \
~>-~6~r'>-6 ~c>-R6 Sco H
H O I N Rs
F ; F ; F ; H ;
F F F
S I N RB
H ~SR6 / ; O and S ~S Rs.
101451 In yet another embodiment, in Formulae Formulae (X) to (XV), R4 is F.
Exemplary compounds according to this embodiment include:
F
F
0 ~ ~ 4R c<6;~L3
s s
H RH ; S Rs; S Rs;
F F
p/ ~\ O~
0 H N R6 S H N R6 S H R6
O S Rs and
> > .
' O
S S R6.
[0146] In a further embodiment, in Formulae Formulae (X) to (XV), at least two
of
R1, R 2 and R4 are F. Exemplary compounds according to this embodiment
include:
S I N C(p)Rs N C(O)Rs F \ \ C(O)Rs
F H : F H H
O
F s I\ C(O)Rs F/S I C(O)Rs F ~ N C(O)Rs
N \N N H
H H
S F ~\ C(O)Rs F C(O)Rs F~ ~ C(p)Rs
H S N S N
F H H
F F F F
F / I\ C(O)Rs ~J[._C(0)R6 / I\ C(O)Rs
O N S N 0 N
H ; H H
44
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
F
F F
F / I\ C(O)Re F / I\ C(O)RB S N 0 N S
~JIC(O)R6
H H F
F
~-(o)R6 0--
\,__:
S S F I\ C(0)Rs F I \ C(0)Rs
F
S S
F (C(0)R6 F S ~\ C(O)Rs
F~\ C(O)Rs S S
N S ; F ; F
F 41 C(O)Rs F~ l\ C(O)Rs F / ~\ C(O)Rs
S \ J$_Co)R6 \ C(p)R6 F / ' \ C(p)Rs
S S O S S S ; and
F / ~ \ C(O)Rg
O S
[0147] In another embodiment, in Formulae Formulae (X) to (XV), each of R', R2
and R4 is F. Exemplary compounds according to this embodiment include:
F F
F
F \ I C(O)R6 F I \ C(O)R6 F I C(p)Rs
H H S N
F ; F H
F F F
F / I C(O)RB F \`\ ~O)R6 F \ I\ C(O)R6
S S
0 H ; F ; F
F F F
F C(O)Re F 1\ C(O)RB
S S and 0 S
[0148] The inventors have discovered that certain fluoro-substituted (F-
substituted)
compounds of the invention are associated with unexpectedly high in vitro and
in vivo
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
activities. Some compounds of the invention, are significantly more active
than their
respective Cl- or Br-substituted counterparts. Compounds of the invention are
evaluated in Examples 8 and 9. Supporting data is summarized in Table 9.
[0149] In one embodiment, the F-substituted analog has an IC50 (DAAO
inhibition)
below about 1 M, preferably below about 100 nM and more preferably below
about
50 nM. In a particularly preferred embodiment, the F-substituted analog has an
IC50
below about 25 nM. In another example, the F-substituted analog has an IC50
that is
at least about one order of magnitude lower than the IC50 measured for at
least one of
the respective Br- or Cl-substituted analogs. In one example, the IC50 is
measured
using an in vitro DAAO enzyme inhibition assay described herein (Example 8).
[0150] In another example, the F-substituted compound of the invention
increases
D-serine levels in the cerebellum of a test animal. D-Serine levels may be
determined
following the experimental procedures described herein (e.g., Example 9). In
an
exemplary embodiment, the F-substituted analog (at 50 mg/kg) increases D-
serine
levels in the cerebellum of mice (measured 2 hours after i.p. dosing) between
about
1.5 fold and 2 fold and preferably more than 2 fold when compared to vehicle.
Several of the analyzed fluoro-substituted analogs of the invention (at 50
mg/kg)
increased D-serine levels by at least 2 fold, while none of the respective Cl-
or Br-
substituted analogs that were analyzed had this activity.
[0151] Particularly preferred are those F-substituted compounds of the
invention
that are capable of maintaining an elevated D-serine level for at least 6
hours. For
example, those F-substituted compounds that (at 50 mg/kg) increase D-serine
levels
between about 1.5 fold and 2 fold and preferably more than 2 fold even when
measured 6 hours after dosing, are generally preferred.
[0152] Even more preferred are those F-substituted compounds that increase D-
serine levels at a lower dose of 10 mg/kg between about 1.5 fold and 2 fold
and
preferably more than 2 fold when measured 2 hours after dosing. Most preferred
are
F-substituted compounds that increase D-serine levels (at a lower dose of 10
mg/kg)
between about 1.5 fold and about 2 fold and preferably more than 2 fold even
when
measured 6 hours after dosing.
[0153] When the increases in D-serine levels are significantly (e.g., at least
about
20%, preferably at least about 40%, more preferably about 60% and most
preferably
46
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
at least about 80% or at least about 100%) higher for the F-substituted
analogs when
compared to the increases measured for at least one of the respective Br- or
Cl-
substituted analogs, then those F-substituted analogs are generally preferred.
For
example, when under the same test conditions, the F-substituted analog causes
an
increase in the D-serine level of 2.7 fold, and the respective Cl-substituted
analog
causes an increase of 1.5 fold, then the F-substituted analog has an activity
that is
80% higher than the activity measured for the Cl-substituted analog.
101541 Also generally preferred are those compounds of the invention that show
activity in a pain model, such as those described herein (e.g., Chung model)
as well as
a model of cognition, such as those described herein (e.g., a contextual fear
conditioning model. Such experiments are described herein for compounds 1 and
11
(e.g., Examples 10 and 18) but are equally useful for the analysis of other
compounds
of the invention.
101551 For a fluoro-substituted compound of the invention to be useful as a
DAAO
inhibitor, which is suitable for pharmaceutical product development, candidate
compounds must demonstrate acceptable activity against the enzyme D-amino acid
oxidase (DAAO).
(0156] In one example, the compounds activity is measured using an in vitro
DAAO enzyme inhibition assay. Such assays are known in the art. An exemplary
assay format is described herein (e.g., Example 8). The fluoro-substituted
compounds
of the invention are judged to be sufficiently potent if they have an IC50
below about
nM. This level of activity is particularly important for the treatment of
pain, such
as neuropathic pain and other types of pain described herein.
(0157] In another example, the compounds activity is determined by measuring D-
25 serine levels in vivo. Elevation of the D-serine level in a certain brain
area (e.g., the
cerebellum) of a test animal (e.g., mouse, rat, pig and the like) is
indicative of DAAO
inhibition in vivo. An exemplary assay format, which measures D-serine levels
(LC/MS/MS) in the cerebellum of mice two hours and six hours after
intraperitoneal
(i.p.) dosing, is described herein (e.g., Example 9). Increases in D-serine
levels were
determined through comparison with vehicle. Useful variations of this assay
will be
apparent to those of skill in the art. Compounds of the invention are judged
to be
47
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
sufficiently active in this assay-when at least one, preferably at least two,
more
preferably at least three and most preferably all four of the following
criteria are met:
1) At a dose of 50 mg/kg, compounds must cause an elevation of D-serine level
(measured about 2 hours after dosing) of greater than about 2 fold when
compared to
vehicle.
2) At a dose of 50 mg/kg, compounds must cause an elevated D-serine level
(measured about 6 hours after dosing) of greater than about 2 fold when
compared to
vehicle.
3) At a dose of 10 mg/kg, compounds must cause an elevation of D-serine level
(measured 2 hours after dosing) of greater than about 2 fold when compared to
vehicle.
4) At a dose of 10 mg/kg, compounds must cause an elevation of D-serine level
(measured 6 hours after dosing) of greater than about 2 fold when compared to
vehicle.
[0158] Activity of the test compounds in this in vivo asay is particularly
important
for the treatment of pain, such as neuropathic pain and other types of pain
described
herein.
[0159] Particularly preferred for pharmaceutical development are those fluoro-
substituted compounds of the invention, which demonstrate sufficient activity
against
the enzyme DAAO both in vitro (e.g., DAAO enzyme inhibition assay) and in vivo
(e.g., elevation of D-serine levels in the cerebellum of mice).
Deuterated Analogs
[0160] In another exemplary embodiment, at least one of R', R2 and R4 in any
of
Formulae (I) to (VII) and (X) to (XV) is deuterium. Exemplary compounds
according
to this embodiment include:
0
H D N D w
N RB
~ Ra
/ D D
D 0 O D O H
and mixtures thereof, wherein R6 can include deuterium. In a preferred
embodiment,
R6 is a member selected from OH, and OD. The compounds can optionally be
labeled
48
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
with another isotope, such as C13. For example, the carbon atom of the
carboxylic
acid group is C13.
B. Synthesis
[0161] The compounds of the present invention, including compounds of Formula
(I) to Formula (VII), may be prepared by methods known in the art. One of
ordinary
skill in the art will know how to modify procedures to obtain the analogs of
the
present invention. Suitable procedures are described e.g., in W02004/031194 to
Murray, P. et al.; Yarovenko, V.N., Russian Chemical Bulletin, International
Edition
(2003), 52(2): 451-456; Krayushkin M.M et al., Organic Letters (2002), 4(22):
3879-
3881; Eras J. et al., Heterocyclic Chem. (1984), 21: 215-217, each of which is
incorporated herein by reference in its entirety. In addition, compounds may
be
prepared using the methods described below and in Examples 1 through 7 or
modified
versions thereof.
[0162] In an exemplary embodiment, the fused pyrrole analogs of the present
invention may be prepared according to Scheme 1 or Scheme 2, by condensation
of
an appropriate five-membered heteroaromatic aldehyde and 2-azidoacetate,
followed
by cyclization and saponification of the resulting ester to afford the
carboxylic acid
analog.
Scheme 1
o X rmxylene OX NaOH X
X CHO Na . ::oEt Q/ Q Q~ OH N, C02Et H COzEt H COzH
Scheme 2
y
HO O ~ m-xylene NaOH '
Q\\ fVa , N~~OEt X/N3~ COzEt reflux X /N\COZEt EtOH X N COzH
X EtOH H H
[0163] In Scheme 1 and Scheme 2, X, Y and Q are defined as above for Formula
(I). Exemplary compounds that may be prepared by the method of Scheme 12
include
the following:
O S R+~ R+ N
R N / N\ COOH R N\ l N COOH / N COOH N COOH
H H H H
R2 = H, Me, CF3, iPr, nBu, CI R2 = H, Me R+ = H. Me R = H, Me, CI, Br
49
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0164) Exemplary compounds that may be prepared by the method of Scheme 13
include the following:
R2
i ~ R N R~N
O A
~ S ' O ~ \ \ N N COOH N COOH N COOH S H COOH
H H H
RI = H. Me R, = H. Me Ri = H, Me, Br
[01651 In another exemplary embodiment, the fused thiophene analogs of the
invention can be prepared by condensation of the appropriate aldehyde and
rhodanine,
followed by hydrolysis of the rhodanine ring and cyclization. Substituted
aldehydes
may be prepared from a halogenated (e.g., Br, I) precursor through Suzuki
coupling
with an appropriate boronic acid.
Scheme 3
Rhodanine S
Y AcOH/NaOAc
refax H Hydrolysis \ H H Cydization y k OH
O.X / -i Q.X
o O o
Rhodanine S
AcOH / NaQAC S
~ reflux 6Nii Hydrolyeis ~; H H Cyelizetion ~ OH
Q.Y H Y / Y / -~ Y
0 0 0
B.1. Synthesis of Fused Pyrazole Pyrrole Analogs
[0166J In an exemplary embodiment, fused pyrrole-pyrazole analogs of the
invention are prepared following a procedure outlined in Scheme 4 or Scheme 5
below.
Scheme 4
~~ r O Na , N+~pg N COyEt rrrxylene N~ ~ COZEt NOC N~~ OyH
N FaOH. -S C to RT. R N N3 reflux N N' EtOH. 94 "C
R R H
Scheme 5
0 N N
~ Ne oEi ~ ~ N ~ CO2Et NaOHy N I / OZH
NN/ H EtOH. -5 qC to RT, reflux R EtOH, 94 C R
R 0 R COZET
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[01671 Generally, these compounds can be prepared by condensation of the
appropriate pyrazole aldehyde and 2-azidoacetate, followed by cyclization. The
resulting ester is then saponified to afford the carboxylic acid analog.
B.2. Synthesis of Fused Thiophene Pyrrole Analogs
[0168] Fused pyrrole-thiophene analogs of the present invention may be
prepared
using a procedure such as those outlined in Schemes 6 to 9 below.
Scheme 6
1. NalEtOH H
/\ OH Pd(OAch, PPFr3 ~\ N3CO,Et N CO2H
ICHO + RB, OH K3PO RCHO 2. Xylene s
ACN
3. KOH/MeOH
Scheme 7
I R 1. Na/EIOH
\ pH Pd(OAC)2, PPh3 /\ W~/COtEt R/ / COiH
S CHO + R1BT_ OH K3~4 S CHO 2. Xylene S
ACN
3 KOHlMeOH
Scheme 8
1 CHO R CHO ~= Na/EtOH R
~ OH Pd(OAc)2, PPh3 N,COZE- ~ COZH
~`- NH
+ RB, OH S K3P04 S 2. Xylene `.S
ACN 3 KOH/MeOH
Scheme 9
CHO 1' Na/EtOH
CHO OH Pd(OAc)Z. PPh3 N,,,
_/CO2EI COZFI
NH
+ R'B, OH I S K3PO R kS R 2. Xylene S
ACN 3KOH/MeOH
.
[0169] In an exemplary embodiment, the thiophene derivative, carrying a
desired R-
group, is prepared by Suzuki coupling of a halogenated thiophene aldehyde and
the
appropriate boronic acid analog. Condensation of the resulting thiophene
intermediate and 2-azidoacetate, followed by cyclization and saponification of
the
ester group affords the final carboxylic acid analog.
51
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
B.3. Synthesis of Fused Furan Pyrrole Analogs
[0170] In another exemplary embodiment, fused furan pyrrole analogs of the
present invention are prepared using a procedure such as those outlined in
Schemes
and 11 below.
5 Scheme 10
R 1- Na/EtOH R H
/\ OH Pd(OAc)Z. PPh3 N,CO,Et N/ COzH
O CHO + RB`OH K3Poa CHO O
ACN 2- Xylene
3_ KOHIMeOH
Scheme 11
1 = Na/EtOH
oH Pd(OAc)2, PPh3 n~~ N3~COZEt CO=H
I~ ~O~ 'CHO + R,TB' OH K3POq R^o~CHO R O"
ACN 2. Xylene
3= KOH/MeOH
[0171] In analogy to the corresponding thiophene analogs, the fused furan
pyrrole
10 derivatives of the invention may be prepared by Suzuki coupling of a
halogenated
furan aldehyde and an appropriate boronic acid. Condensation of the resulting
furan
intermediate and 2-azidoacetate, followed by cyclization and saponification of
the
ester group affords the desired carboxylic acid analog.
B.4. Synthesis of Fused Pyrrole Pyrrole Analogs
[0172] In another exemplary embodiment, fused pyrrole-pyrrole analogs of the
current invention are prepared using the synthetic approach outlined in Scheme
12
below. Similarly to the above described compounds, fused pyrrole-pyrrole
analogs
can be prepared by condensation of the appropriate pyrrole aldehyde and 2-
azidoacetate, followed by cyclization and saponification of the ester group.
Substituted pyrrole aldehydes may be prepared by Suzuki coupling of a
halogenated
pyrrole aldehyde and the appropriate boronic acid analog.
Scheme 12
H H
~ Ng1. N3 OEt m N N
xylene COZEt ~OH / / CO2H
R H EtOH, R retlux EtOH,
0 -5 C tD RT COZEt R 94 C tD RT R
52
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
B.5. Synthesis of Fused Thiazole Pyrrole Analogs
[01731 In another exemplary embodiment, fused thiazole-pyrrole analogs of the
current invention are prepared using the synthetic approach outlined in Scheme
13
below. Similarly to the above described compounds, fused thiazole-pyrrole
analogs
can be prepared by condensation of the appropriate thiazole aldehyde and 2-
azidoacetate, followed by cyclization and saponification of the ester group.
Substituted thiazole aldehydes may be prepared by Suzuki coupling of a
halogenated
thiazole aldehyde and the appropriate boronic acid analog.
Scheme 13
NaOH S H
~S ~ Nel, N3`'OEt 'S ~ m" S H
NH '~ xyien~ ~ ~ i-COZEt ~ COpH
EtOH, N 'r ~ reilux Nf~! EtOH, N
0 -5 C to RT CO2Et 94 C to RT
N Nal, N3` ~OEt N H N H
S~ S~ ~Ms xYlenQ II ,}-COzEt NaOH ~i 1:/ COzli
H EtOH. ~ ~1 EtOH, S
0 COZEt reftux C to
RT
-5 CtoRT 94
B.6. Synthesis of Fused Thiophene Thiophene Analogs
[01741 In a further embodiment, the fused thiophene-thiophene analogs of the
invention are synthesized using a procedure such as those outlined in Schemes
14 and
15.
Scheme 14
Rhodanine S
Br R-B(OH)Z R rAcOH eflux ~ NaOAc R S
NH
H Pd(OAc)2, TPP / S\ H
O
S K3P04.ACN 0
0 94 deg. C
36-48 hr
Hydrolysis using o
2M aq. NaOH R HS Cyclization R S
followed by 3N aq. HCI \ i OH Chlorine gas in ~ ~ OH
S 1,1,2-Vichloroethane s
0 reflux x 1 hr.
53
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Schemel5
OH ~s
S4
0 HO'B H Rhodanine
G-P~O~\ O AcOH / NaOAc ~^\~~NH
~ R~O_A- J ~ R H reflux q
R OH TEA, cat. DMAP 0 O Pd(OAc)2, TPP ~~ t\ O
1 K3P04,ACN S S
THF 0 dep. C to r.t. 90 deg. C
18 hr
Chlorine gas in
Hydrolysis using SH 1,1,2-trichloroethane O
2M eq. NeOH OH reflux x 1 hr. R
R / \ O ~ g OH
followed by 3N aq. HCI S
B.8. Synthesis of 1,5-dihydropyrrolo[2,3-clpyrrole Analogs
[01751 1,5-dihydropyrrolo[2,3-c]pyrrole-2-carboxylic acid analogs of the
invention
can be prepared following a procedure outlined in Scheme 17.
Scheme 17
(MeO)3CH HO ~~(C~th O)YCOOEt 1) KH. THF Et ~ ~ COOEt
TA
Et ~ I Et I piparldine. benzene~ COOEt 2 T 0 N 1 COOE1
O AH 0 B 0 H C Ts D
NBS. CC14 ECOOEt N~R NeOH
dlbenzoyi ~ EtOH EtO-'/ N-R EtOH HO,~N-R
COOEt
pemxlde O R= H, Me, /' N
N Bn, Ph. etc. O Ts O H
Ts E Br F ti
[0176] Generally, these compounds can be prepared from commercially available
compounds such as A and B. For example, formylation of A, such as with
trimethyl
orthoformate and trifluroactetic acid provides aldehyde B. Knoevenagel
condensation
of B provides C, which is protected by standard tosylation conditions to
provide
compounds such as D. Bromination of D, such as with N-bromosuccinimide and
dibenzoyl peroxide, provides E, which is then reacted with ammonia or with
amines
such as methyl amine or benzyl amine to form cyclized products such as F.
Standard
deprotection of the N-tosyl group and saponification affords the desired
carboxylic
acid analog. Relevant references, which are incorporated by reference, include
Sha,
Chin-Kang, et al. Heterocycles 1990, 31, 603-609.
B.9. Synthesis of 1H-thieno[3,4-b]pyrrole 1H-furo[3,4-binyrrole Analogs
[0177) In an exemplary embodiment, 1H-thieno[3,4-b]pyrrole-2-carboxylic acid
and 1H-furo[3,4-b]pyrrole-2-carboxylic acid analogs of the invention are
prepared
following a procedure outlined in Scheme 18.
54
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Scheme 18
RBr
1) Cs1C03, DMF
X" I EtOH X~ PhZP X! 2) [Pdl, K,CO,
NH I CH ~/ OOMe NaOH 1 NHBOC
X~ ~ isoamyl rutrite tBuOH
~ ~_~~l'C ~
OOMe OOH R = H. Me, Et,
A B C D Ph, COOEt, eta
X=0,S
CICOOMe i R OEt sflip f OEt N~H R
X OH
X~ \ (iPr~N)NU, THF X \ 1 mmH9 _ \ EtOH y X~
B~ B~ O H O H O
E F G H
[0178] Generally, these compounds can be prepared from appropriately
substituted
furans and thiophenes such as A, B, or C,which are easily synthesized using
standard
literature procedures such as those listed below. Curtius rearrangement of C
provides
D, which can be allylated and subjected to Heck conditions to afford bicyclic
compound E. Standard functional group manipulation, such as acylation, BOC
deprotection, and saponification affords the desired carboxylic acid analogs.
Relevant
references, which are incorporated by reference, include Yu, Shuyuan et al J.
Chem.
Soc., Perkin Transactions 1 1991, 10, 2600-2601. Wensbo, D.; et al Tetrahedron
1995, 51, 10323-10342; Wensbo, D.; Gronowitz, S. Tetrahedron 1996, 52, 14975-
14988, and references cited therein.
B.10. Synthesis of Fluoro-Substituted Analogs of the Invention
[0179] In an exemplary embodiment, fluoro-substituted analogs of the invention
may be prepared following procedures outlined in Schemes 19 to 24.
[0180] In an exemplary embodiment, fluoro-substituted analogs of the invention
may be prepared following procedures outlined in Schemes 19 to 24.
[01811 In an exemplary embodiment, fluoro-substituted fused pyrrole analogs of
the
invention may be prepared following adaptations to procedures outlined in
Schemes 1
to 18. Fluorine may be incorporated early, such as in the aldehyde starting
materials
of Scheme 1 and Scheme 2. Fluorinated five membered heteroaromatic aldehydes
may be prepared from the corresponding bromo, chloro- or iodo substituted
aldehydes, as shown in Schemes 19 and 20, by protecting the aldehyde as an
acetal,
then subjecting the bromo-, chloro-, or iodo-acetal to transmetalation
conditions (such
as, for example, with nBuLi or tBuLi) followed by fluorination (for example,
with N-
fluorobenzenesulfonimide (NFSI) or Selectfluor ). Deprotection of the acetal
under
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
standard conditions provides fluorinated aldehydes, which may be converted to
the
fused pyrrole analogs of the invention as outlined in Schemes 1 and 2.
Scheme 19
O X m-xylene Q'X NaOH Q'
X CHO
Na , ::oEt , Q~ OH Y N3 CO2Et Y H C02Et H
Scheme 20
OP OP
CHO 1. transmetalation CHO
/~ protection x \OP 2, fluorination IX~OP deprotection Q
n
Br. CI, or1 Y Br, CI, or~Y p /Y F~
P = protecting group
[0182] Fluorinated, five membered heteroaromatic aldehydes may also be
prepared
from the corresponding bromo- or iodo-substituted protected methyl alcohols
following the transmetalation, fluorination protocol used for acetals, as
shown in
Schemes 21 and 22. Standard deprotection of the alcohol, followed by oxidation
(such as, for example, with Mn02 or pyridinium chlorochromate) provides
fluorinated
five membered heteroaromatic aldehydes, which may be converted, as shown in
Schemes I and 2, to the fused pyrrole analogs of the invention.
Scheme 21
x
O,X CHO Na', ::0Et \ meEtOH
YCOzH
~ OH Y N3 COZEt H COZEt
H
Scheme 22
CHO 1= reductfon 1. transmetalation 1. deprotection CHO
x~ 2. protection XOP 2, fluorination x.- \~\OP 2. oxidation
CQ~
Br, CI, or IOxY Br, CI, or~Y FY F~Y
P = protecting group
[0183] Altematively, fluoro-substituted five membered heteroaromatic aldehydes
may be obtained by direct fluorination of a five-membered heteroaromatic
aldehyde,
protected five-membered heteroaromatic aldehyde, or protected five-membered
heteroaromatic methyl alcohol (such as, for example, with nBuLi or tBuLi, or
LDA),
followed by fluorination conditions (for example, with N-
fluorobenzenesulfonimide
(NFSI) or Selectfluor(&) and optional deprotection to provide fluorinated
aldehydes,
56
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
which may be taken on, as in Scheme I and Scheme 2, to the fused pyrrole
analogs of
the invention. Alternatively, fluorinated aldehydes may be obtained by
fluorodecarboxylation of a carboxylic acid containing five-membered
heteroaromatic
precursor.
(0184] Fluoro-substituted five membered heteroaromatic aldehydes may also be
obtained by synthesis of the heteroaromatic ring following incorporation of
fluorine.
One example is described, in Example 2, for the synthesis of 4-fluorofuran-2-
carbaldehyde starting from (4-bromo-4,4-difluoro-but-2-ynyloxy)-tert-butyl-
dimethyl-silane.
[0185] Fluorine may also be incorporated into the azide intermediates of
Schemes 1
and 2, from the corresponding bromo-, chloro-, or iodo-compound, as described
above, or from the corresponding carboxylic acid, by fluorodecarboxylation
(such as
in the synthesis of ethyl 2-azido-3-(5-fluorofuran-2-yl)prop-2-enoate from 5-
(2-azido-
3-ethoxy-3-oxoprop-l-enyl)furan-2-carboxylic acid in Example 2.
101861 In addition, fluorine may be incorporated later in the synthesis, into
the
fused pyrrole esters or acids. As shown in Schemes 23 and 24, fused pyrrole
esters or
acids of Schemes 1 and 2 may be subjected to standard bromination,
chlorination or
iodination conditions (for example, Br2, KOH, IZ, KOH, NBS, NCS), followed by
transmetalation conditions (for example, nBuLi or tBuLi), then fluorination
conditions (e.g., N-fluorobenzenesulfonimide (NFSI) or Selectfluor ), to
provide
fluorinated fused pyrrole esters or acids. Alternatively, the fused pyrrole
esters or
acids of Schemes I and 2 may be subjected to direct deprotonation conditions
(e.g.,
nBuLi or tBuLi, or LDA), then fluorination conditions (e.g., N-
fluorobenzenesulfonimide (NFSI) or Selectfluor ), to provide fluorinated fused
pyrrole esters or acids.
57
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Scheme 23
0 X rrl-oX NaOH ,X
~/ 1
X CHO Na", N3`AOEt Q, e~l,xe 4 / \ EtOH
Q~ / Y/ 1 COZEt - Y CO2Et N C02H
y EtOH
~ N3 H H
Scheme 24
bromination, X IX
.X iodinaNon, or C" 1. transmetalation 3-
7 Y~ /\ chlorination Y/ \ C02R 2, ffuorination F/COzR
NCO2R Br, CI, or I H H
H
R = H, methyl, ethyl
[0187] In Schemes 1-24, X, Y and Q are defined as above for Formula (I). The
reagents and reaction conditions, such as those given in Schemes 1 to 24 are
exemplary and can be replaced with other suitable reagents and conditions,
known to
those of skill in the art. Representative examples for synthetic routes
incorporating
fluorine into fused pyrrole analogs may be found in Examples 1 and 2.
C. Pharmaceutical Compositions
[0188] While it may be possible for compounds of the present invention to be
administered as the raw chemical, it is preferable to present them as a
pharmaceutical
composition. According to a further aspect, the present invention provides a
pharmaceutical composition comprising a compound of Formula (I) to Formula
(VII)
or (X) to (XV) or a pharmaceutically acceptable salt, solvate, hydrate or
prodrug
thereof, together with one or more pharmaceutical carrier and optionally one
or more
other therapeutic ingredient. The carrier(s) must be "acceptable" in the sense
of being
compatible with the other ingredients of the formulation and not deleterious
to the
recipient thereof. The term "pharmaceutically acceptable cancier" includes
vehicles
and diluents.
[01891 The formulations include those suitable for oral, parenteral (including
subcutaneous, intradermal, intramuscular, intravenous and intraarticular),
rectal and
topical (including dermal, buccal, sublingual and intraocular) administration,
as well
as those for administration by inhalation. The most suitable route may depend
upon
the condition and disorder of the recipient. The formulations may conveniently
be
presented in unit dosage form and may be prepared by any of the methods well
known
in the art of pharmacy. All methods include the step of bringing into
association a
compound or a pharmaceutically acceptable salt or solvate thereof ("active
58
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
ingredient") with the carrier which constitutes one or more accessory
ingredients. In
general, the formulations are prepared by uniformly and intimately bringing
into
association the active ingredient with liquid carriers or finely divided solid
carriers or
both and then, if necessary, shaping the product into the desired formulation.
Oral
formulations are well known to those skilled in the art, and general methods
for
preparing them are found in any standard pharmacy school textbook, for
example,
Remington: The Science and Practice of Pharmacy., A.R. Gennaro, ed. (1995),
the
entire disclosure of which is incorporated herein by reference.
[0190] Pharmaceutical compositions containing compounds of Formula (I) to
Formula (VII) and (X) to (XV) may be conveniently presented in unit dosage
form
and prepared by any of the methods well known in the art of pharmacy.
Preferred
unit dosage formulations are those containing an effective dose, or an
appropriate
fraction thereof, of the active ingredient, or a pharmaceutically acceptable
salt thereof.
The magnitude of a prophylactic or therapeutic dose typically varies with the
nature
and severity of the condition to be treated and the route of administration.
The dose,
and perhaps the dose frequency, will also vary according to the age, body
weight and
response of the individual patient. In general, the total daily dose (in
single or divided
doses) ranges from about I mg per day to about 7000 mg per day, preferably
about I
mg per day to about 100 mg per day, and more preferably, from about 10 mg per
day
to about 100 mg per day, and even more preferably from about 20 mg to about
100
mg, to about 80 mg or to about 60 mg. In some embodiments, the total daily
dose
may range from about 50 mg to about 500 mg per day, and preferably, about 100
mg
to about 500 mg per day. It is further recommended that children, patients
over 65
years old, and those with impaired renal or hepatic function, initially
receive low
doses and that the dosage be titrated based on individual responses and/or
blood
levels. It may be necessary to use dosages outside these ranges in some cases,
as will
be apparent to those in the art. Further, it is noted that the clinician or
treating
physician knows how and when to interrupt, adjust or terminate therapy in
conjunction with individual patient's response.
[0191] It should be understood that in addition to the ingredients
particularly
mentioned above, the formulations of this invention may include other agents
conventional in the art having regard to the type of formulation in question,
for
example those suitable for oral administration may include flavoring agents.
59
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
101921 Formulations of the present invention suitable for oral administration
may be
presented as discrete units such as capsules, cachets or tablets each
containing a
predetermined amount of the active ingredient; as a powder or granules; as a
solution
or a suspension in an aqueous liquid or a non-aqueous liquid; or as an oil-in-
water
liquid emulsion or a water-in-oil liquid emulsion. The active ingredient may
also be
presented as a bolus, electuary or paste.
[0193] A tablet may be made by compression or molding, optionally using one or
more accessory ingredients. Compressed tablets may be prepared by compressing
in
a suitable machine the active ingredient in a free-flowing form such as a
powder or
granules, optionally mixed with a binder, lubricant, inert diluent,
lubricating, surface
active or dispersing agent. Molded tablets may be made by molding in a
suitable
machine a mixture of the powdered compound moistened with an inert liquid
diluent.
The tablets may optionally be coated or scored and may be formulated so as to
provide sustained, delayed or controlled release of the active ingredient
therein. Oral
and parenteral sustained release drug delivery systems are well known to those
skilled
in the art, and general methods of achieving sustained release of orally or
parenterally
administered drugs are found, for example, in Remington: The Science and
Practice
ofPharmacy, pages 1660-1675 (1995).
[0194] Formulations for parenteral administration include aqueous and non-
aqueous
sterile injection solutions which may contain anti-oxidants, buffers,
bacteriostats and
solutes which render the formulation isotonic with the blood of the intended
recipient.
Formulations for parenteral administration also include aqueous and non-
aqueous
sterile suspensions, which may include suspending agents and thickening
agents. The
formulations may be presented in unit-dose of multi-dose containers, for
example
sealed ampoules and vials, and may be stored in a freeze-dried (lyophilized)
condition
requiring only the addition of a sterile liquid carrier, for example saline,
phosphate-
buffered saline (PBS) or the like, immediately prior to use. Extemporaneous
injection
solutions and suspensions may be prepared from sterile powders, granules and
tablets
of the kind previously described. Formulations for rectal administration may
be
presented as a suppository with the usual carriers such as cocoa butter or
polyethylene
glycol. Formulations for topical administration in the mouth, for example,
buccally or
sublingually, include lozenges comprising the active ingredient in a flavored
basis
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
such as sucrose and acacia or tragacanth, and pastilles comprising the active
ingredient in a basis such as gelatin and glycerin or sucrose and acacia. .
[0195] The pharmaceutically acceptable carrier may take a wide variety of
forms,
depending on the route desired for administration, for example, oral or
parenteral
(including intravenous). In preparing the composition for oral dosage form,
any of
the usual pharmaceutical media may be employed, such as, water, glycols, oils,
alcohols, flavoring agents, preservatives, and coloring agents in the case of
oral liquid
preparation, including suspension, elixirs and solutions. Carriers such as
starches,
sugars, microcrystalline cellulose, diluents, granulating agents, lubricants,
binders and
disintegrating agents may be used in the case of oral solid preparations such
as
powders, capsules and caplets, with the solid oral preparation being preferred
over the
liquid preparations. Preferred solid oral preparations are tablets or
capsules, because
of their ease of administration. If desired, tablets may be coated by standard
aqueous
or nonaqueous techniques. Oral and parenteral sustained release dosage forms
may
also be used.
[0196] Exemplary formulations, are well known to those skilled in the art, and
general methods for preparing them are found in any standard pharmacy school
textbook, for example, Remington, THE SCIENCE AND PRACTICE OF PHARMACY, 21 st
Ed., Lippincott.
[0197] In an exemplary embodiment, the invention provides a pharmaceutical
composition including a pharmaceutically acceptable carrier and a compound
having
the formula:
R3
O
R2 N COR4
H
in which R' is a member selected from the group consisting of H, substituted
or
unsubstituted arylalkyl and substituted or unsubstituted heteroarylalkyl. R2
is a
member selected from the group consisting of H, substituted or unsubstituted
alkenyl,
substituted or unsubstituted arylalkyl and substituted or unsubstituted
heteroarylalkyl.
R3 is a member selected from the group consisting of H, CI-C6 substituted or
61
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
unsubstituted alkyl, substituted or unsubstituted arylalkyl and substituted or
unsubstituted heteroarylalkyl. R4 is a member selected from OH and O'X+
wherein X+ is a positive ion which is a member selected from organic positive
ions
and inorganic positive ions, wherein substituted or unsubstituted arylalkyl
and
substituted or unsubstituted heteroarylalkyl have the formula:
HCH2)n-Ar
in which Ar is a member selected from the group consisting of substituted or
unsubstituted aryl and substituted or unsubstituted heteroaryl, and
n is an integer from I to 4.
IV. Methods
A. Methods for Treatment or Prevention
[0198] In a further aspect the invention provides a method for treating or
preventing
a disease or condition which is a member selected from a neurological
disorder, pain,
ataxia and convulsion. The method includes administering to a subject in need
thereof a therapeutically effective amount of a compound of the invention
(e.g., those
of Formula (I) to (VIII) or Formula (X) to (XV)) or a pharmaceutically
acceptable
salt, solvate, hydrate or prodrug thereof.
[0199] In an exemplary embodiment, the method of the invention includes
administering to a subject in need thereof a therapeutically effective amount
of a
compound of Formula (I) or a pharmaceutically acceptable salt, solvate,
hydrate or
prodrug thereof:
R
Q {x,, ~ z
Y A Rs
m
wherein Q, X, Y, Z, R4 and R6 are defined as above for Formula (I). In an
exemplary
embodiment, Z is a member selected from 0 and S. A is a member selected from
NR7, S and O. Q is a member selected from 0, S, N, NR3a and CR'. X and Y are
members independently selected from 0, S, N, NR; and CR2; with the proviso
that -
when X and Y are both CRZ, each R2 is independently selected. R3, R 3a and R7
are
members independently selected from H, OR'Z, acyl, SOZR13, SOR13, substituted
or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
62
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
unsubstituted heterocycloalkyl, wherein R12 and R13 are members independently
selected from substituted or unsubstituted alkyl, substituted or unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl. R', R2 and R4 are members
independently selected from H, F, Cl, Br, CN, CF3, acyl, OR14, S(O)20R'4,
S(O)PR'4,
NR14R'5, SO2NR'4R'5, substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
and substituted or unsubstituted heterocycloalkyl, wherein R' and RZ, together
with
the atoms to which they are attached, are optionally joined to form a 5- to 7-
membered ring, wherein p is an integer selected from 0 to 2. In a preferred
embodiment, R', R2 and R4 are members independently selected from H, F, Cl, Br
and
unubstituted Ci-C4 alkyl. R14 and R15 are members independently selected from
H,
substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl,
substituted
or unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted
or
unsubstituted heterocycloalkyl. R14 and R15, together with the nitrogen atoms
to
which they are attached, are optionally joined to form a 5- to 7-membered
ring. R6 is
a member selected from O"X+ and OH, wherein X+ is a positive ion, which is a
member selected from inorganic positive ions and organic positive ions.
[02001 In another exemplary embodiment, the subject is preferably not in need
of
treatment for a condition, which is a member selected from a H4-receptor
mediated
disease, a monocyte chemoattractant protein-1 (MCP-1) receptor mediated
disease,
type-2 diabetes, insulin resistance, syndrome X, hyperinsulinaemia,
hyperglucagonaemia, cardiac ischemia, obesity, artherosclerosis, diabetic
neuropathy,
diabetic nephropathy, diabetic retinopathy, cataracts, hypercholesterolemia,
hypertriglyceridemia, hyperlipidemia, hyperglycemia, hypertension, tissue
ischemia
and myocardial ischemia.
[0201] In another embodiment, the subject is preferably not in need of
inhibiting
glycogen phospliorylase.
[0202] All compounds exemplified herein are useful in the methods of the
inventions. Preferred compounds of Formula (I) include those in which Z is 0
and R6
is a member selected from O-X+ and OR8, wherein R8 is preferably H or CI -C4
unsubstituted alkyl.
63
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0203] In an exemplary embodiment, the compound of Formula (I) has the
formula:
R4
X 0
Ri~ ~ ~
Y A Rs
wherein A is a member selected from NH, X is a member selected from 0, S and
NR3. Y is a member selected from CR2 and N. R6 is preferably a member selected
from O-X+ and OR8, wherein R8 is preferably H or Ci-C4 unsubstituted alkyl.
[0204] In another exemplary embodiment, the compound of Formula (I) has the
formula:
R4
R'~X I ~ O
Y A R6
wherein A is a member selected from NH and S. Y is a member selected from 0, S
and NR3 and X is a member selected from CR2 and N. R6 is preferably a member
selected from O"X+ and OR8, wherein R8 is preferably H or CI -C4 unsubstituted
alkyl.
[0205] In one exemplary embodiment, R6 is a member selected from O' and OH, A
is a member selected from S and NH and R' is a member selected from H, CN and
halogen (e.g., F, Cl or Br).
[02061 Preferred compounds of the invention include those in which the
substituents R', R2 and R4 are each independently selected from H and F.
Particularly preferred compounds include those in which, in Formula (I), R6 is
a
member selected from O-X+ and OH, A is NH, and wherein one or more of the
following selections are made:
a) Q is C-Rl, wherein R' is a member selected from H and F.
b) Y is C-R2, wherein R2 is a member selected from H and F.
c) R4 is a member selected from H and F.
[0207] Other preferred compounds of Formula (I) include those, in which X is a
member selected from S and 0 and Y is selected from N and CR2. In one
exemplary
embodiment, R2 is a member selected from H and methyl.
[0208] Furthermore, preferred compounds of Formual (I) include those, in which
Y
is a member selected from S and 0 and X is CR2. In one exemplary embodiment,
R2
is a member selected from H and methyl.
64
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
102091 Accordingly, preferred compounds useful in the methods of the invention
include:
\ I\ COOH \ I\ COOH F Z I\ COOH Br \ COOH
H H3C H H H
Br
S N COOH d/) COOH \ COOH \ ~\ COOH
H s N s s H
O
\ I\ COOH \ I\ COOH N COOH \ ~\ COOH
H N
- H % Br H H3C H
H3C
<\S I\ COOH I\ COOH ~/I\ COOH e I\ COOH
N H N H S H O H
F
~JCo0H <J_COOH tJ$'-COoH
H ; F H ; H ;
F
COOH \
COOH \ COOH COOH
N I N
JX'H S
Br H H ~ H H ~
O F F COOH \COOH COOH \ COOH
; H ; S H and S H
[0210] Other preferred compounds useful in the methods of the invention are
those in
which at least one of R~, R2 and R3 includes an aromatic ring or a fused ring
system
with at least one aromatic ring. In an exemplary embodiment, at least one of
Rl, R2
and R3 has the formula:
I-LI-Ar
wherein Ar is a member selected from substituted or unsubstituted aryl,
substituted or
unsubstituted heteroaryl and a fused ring system. Ll is a linker moiety, which
is a
member selected from substituted or unsubstituted alkyl, substituted or
unsubstituted
heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted
heteroaryl
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
and substituted or unsubstituted heterocycloalkyl. Particularly preferred
compounds
are those, in which R' represents a small group, such as H and F, and a member
selected from R2 and R3 includes the aromatic moiety.
[0211] Exemplary linker moieties include Ci to C5 substituted or unsubstituted
alkyl
chains wherein one or more carbon atoms are optionally replaced with a moiety
including one or more heteroatoms, forming e.g., ether, thioether, amines,
amides,
sulfonamides or sulfones.
[0212] In an exemplary embodiment, in Formula (I), at least one of R', RZ and
R3
has a formula, which is a member selected from:
1-(CRIsR17) r~-Ar. and 1 1CR1sR17 hp'-Ar
~
wherein n is an integer from 0 to 5, and Ql is a member selected from 0 and S.
R16
and R17 are members independently selected from H, substituted or
unsubstituted
alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted
aryl,
substituted or unsubstituted heteroaryl and substituted or unsubstituted
heterocycloalkyl. R16 and R17, together with the carbon to which they are
attached,
are optionally joined to form a 3- to 7-membered ring, which is a member
selected
from substituted or unsubstituted cycloalkyl and substituted or unsubstituted
heterocycloalkyl, and which is optionally fused to Ar.
[0213] In an exemplary embodiment, Ar is a phenyl ring and has the formula:
, \ ~ (R5)m
wherein m is an integer from 0 to S. Each R5 can be selected from a variety of
substituents. In an exemplary embodiment, each R5 is a member independently
selected from H, halogen, CN, halogen substituted alkyl (e.g., CF3), hydroxy,
alkoxy
,
(e.g., methoxy and ethoxy), acyl (e.g., acetyl), carbamate, sulfonamide, urea,
CO2R18
OC(O)RiB, NRisRi9, C(O)NR18R19, 11M18C(O)R20, NRi8S02R20, S(O)2R20' S(O)R 20,
substituted or unsubstituted alkyl (e.g., methyl, ethyl, propyl and
isopropyl),
substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl,
substituted
or unsubstituted heteroaryl and substituted or unsubstituted heterocycloalkyl,
wherein
adjacent R5 are optionally joined to form a ring, wherein the ring is a member
selected
from substituted or unsubstituted cycloalkyl, substituted or unsubstituted
66
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
heterocycloalkyl, substituted or unsubstituted aryl and substituted or
unsubstituted
heteroaryl.
[0214] R18 and R'9 are members independently selected from H, substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl. R20 is a member selected from substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted aryl, substituted or unsubstituted heteroaryl and substituted or
unsubstituted heterocycloalkyl. R'g and a member selected from R19 and R20,
together
with the atoms to which they are attached, are optionally joined to form a 5-
to 7-
membered ring.
[0215] Subjects for treatment according to the present invention include
humans
(patients) and other mammals in need of therapy for the stated condition.
[0216] Compounds of the invention possess unique pharmacological
characteristics
with respect to inhibition of DAAO and influence the activity of the NMDA
receptor
in the brain, particularly by controlling the levels of D-serine. Therefore,
these
compounds are effective in treating conditions and disorders (especially CNS-
related
disorders), which are modulated by DAAO, D-serine and/or NMDA receptor
activity.
In one embodiment, compounds of the invention are associated with diminished
side
effects compared to administration of the current standards of treatment.
[0217] Accordingly, the present invention relates to methods for increasing
the
concentration of D-serine and/or decreasing the concentration of toxic
products of D-
serine oxidation by DAAO in a mammal. Each of the methods comprises
administering to a subject in need thereof a therapeutically effective amount
of a
compound of the invention, for example those of Formula (I), Formula (II),
Formula
(III), Formula (IV), Formula (V), Formula (VI), or Formula (VII), or a
phannaceutically acceptable salt or solvate thereof.
[0218] Compounds of the invention are typically more selective than known DAAO
inhibitors, including indole-2-carboxylates, and demonstrate higher
selectivity for
DAAO inhibition relative to binding at the NMDA receptor's D-serine binding
site.
The compounds also exhibit an advantageous profile of activity including good
bioavailability. Accordingly, they offer advantages over many art-known
methods for
67
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
treating disorders modulated by DAAO, D-serine or NMDA receptor activity. For
example, unlike many conventional antipsychotic therapeutics, DAAO inhibitors
can
produce a desirable reduction in the cognitive symptoms of schizophrenia.
Conventional antipsychotics often produce undesirable side effects, including
tardive
dyskinesia (irreversible involuntary movement disorder), extra pyramidal
symptoms,
and akathesia, and these may be reduced or eliminated by administering
compounds
of the invention.
[0219] Compounds of the present invention may also be used in conjunction with
therapy involving administration of D-serine or an analog thereof, such as a
salt of D-
serine, an ester of D-serine, alkylated D-serine, D-cycloserine or a precursor
of D-
serine, or can be used in conjunction with therapy involving administration of
antipsychotics, antidepressants, psychostimulants, and/or Alzheimer's disease
therapeutics.
[0220] The compounds of the invention may also be used in conjunction with
therapy involving administration of antipsychotics (for treating schizophrenia
and
other psychotic conditions), psychostimulants (for treating attention deficit
disorder,
depression, or learning disorders), antidepressants, nootropics (for example,
piracetam, oxiracetam or aniracetam), acetylcholinesterase inhibitors (for
example,
the physostigmine related compounds, tacrine or donepezil), GABA analogs
(e.g.,
gabapentin) or GABA receptor modulators, Alzheimer's disease therapeutics
(e.g.,
nemantine hydrochloride) and/or analgesics (for treating of persistant or
chronic pain,
e.g. neuropathic pain). Such methods for conjoint therapies are included
within the
invention.
Conditions and Disorders
[02211 In one embodiment, the compounds of the present invention are useful
for
the treatment of neurological disorders, pain (e.g., neuropathic pain), ataxia
and
convulsion. Neurological disorders include neurodegenerative diseases (e.g.,
Alzheimers disease) and neuropsychiatric disorders (e.g., schizophrenia).
Neuropsychiatric Disorders
[0222] Neuropsychiatric disorders include schizophrenia, autism, and attention
deficit disorder. Clinicians recognize a distinction among such disorders, and
there
are many schemes for categorizing them. The Diagnostic and Statistical Manual
of
68
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Mental Disorders, Revised, Fourth Ed., (DSM-IV-R), published by the American
Psychiatric Association, provides a standard diagnostic system upon which
persons of
skill rely, and is incorporated herein by reference. According to the
framework of the
DSM-IV, the mental disorders of Axis I include: disorders diagnosed in
childhood
(such as Attention Deficit Disorder (ADD) and Attention Deficit-Hyperactivity
Disorder (ADHD)) and disorders diagnosed in adulthood. The disorders diagnosed
in
adulthood include (1) schizophrenia and psychotic disorders; (2) cognitive
disorders;
(3) mood disorders; (4) anxiety related disorders; (5) eating disorders; (6)
substance
related disorders; (7) personality disorders; and (8) "disorders not yet
included" in the
scheme.
[0223] ADD and ADHD are disorders that are most prevalent in children and are
associated with increased motor activity and a decreased attention span. These
disorders are commonly treated by administration of psychostimulants such as
methylphenidate and dextroamphetamine sulfate.
[0224] The compounds (and their mixtures) of the present invention are also
effective for treating disruptive behavior disorders, such as attention
deficit disorder
(ADD) and attention deficit disorder/hyperactivity (ADHD), which is in
accordance
with its accepted meaning in the art, as provided in the DSM-IV-TRTM. These
disorders are defined as affecting one's behavior resulting in inappropriate
actions in
leaming and social situations. Although most commonly occurring during
childhood,
disruptive behavior disorders may also occur in adulthood.
[0225] Schizophrenia represents a group of neuropsychiatric disorders
characterized
by dysfunctions of the thinking process, such as delusions, hallucinations,
and
extensive withdrawal of the patient's interests from other people.
Approximately one
percent of the worldwide population is afflicted with schizophrenia, and this
disorder
is accompanied by high morbidity and mortality rates. So-called negative
symptoms
of schizophrenia include affect blunting, anergia, alogia and social
withdrawal, which
can be measured using SANS (Andreasen, 1983, Scales for the Assessment of
Negative Symptoms (SANS), Iowa City, Iowa). Positive symptoms of schizophrenia
include delusion and hallucination, which can be measured using PANSS
(Positive
and Negative Syndrome Scale) (Kay et al., 1987, Schizophrenia Bulletin 13:261-
276).
Cognitive symptoms of schizophrenia include impainnent in obtaining,
organizing,
69
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
and using intellectual knowledge which can be measured by the Positive and
Negative
Syndrome Scale-cognitive subscale (PANSS-cognitive subscale) (Lindenmayer et
al.,
1994, J. Nerv. Ment. Dis. 182:631-638) or with cognitive tasks such as the
Wisconsin
Card Sorting Test. Conventional antipsychotic drugs, which act on the dopamine
D2
receptor, can be used to treat the positive symptoms of schizophrenia, such as
delusion and hallucination. In general, conventional antipsychotic drugs and
atypical
antipsychotic drugs, which act on the dopamine D2 and 5HT2 serotonin receptor,
are
limited in their ability to treat cognitive deficits and negative symptoms
such as affect
blunting (i.e., lack of facial expressions), anergia, and social withdrawal.
[0226] Disorders treatable with the compounds of the present invention
include, but
are not limited to, depression, bipolar disorder, chronic fatigue disorder,
seasonal
affective disorder, agoraphobia, generalized anxiety disorder, phobic anxiety,
obsessive compulsive disorder (OCD), panic disorder, acute stress disorder,
social
phobia, posttraumatic stress disorder, premenstrual syndrome, menopause,
perimenopause and male menopause.
[0227] Compounds and compositions of the present invention are also effective
for
treating eating disorders. Eating disorders are defined as a disorder of one's
appetite
or eating habits or of inappropriate somatotype visualization. Eating
disorders
include, but are not limited to, anorexia nervosa; bulimia nervosa, obesity
and
cachexia.
[0228J In addition to their beneficial therapeutic effects, compounds of the
present
invention provide the additional benefit of avoiding one or more of the
adverse effects
associated with conventional mood disorder treatments. Such side effects
include, for
example, insomnia, breast pain, weight gain, extrapyramidal symptoms, elevated
serum prolactin levels and sexual dysfunction (including decreased libido,
ejaculatory
dysfunction and anorgasmia).
Leaming, Memory and Cognition
[0229] Generally, compounds of the invention can be used for improving or
enhancing learning and memory in subjects without cognitive deficits or
patients
suffering from cognitive deficits. Patients, who may benefit from such
treatment,
include those exhibiting symptoms of dementia or learning and memory loss.
Individuals with an amnesic disorder are impaired in their ability to learn
new
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
information or are unable to recall previously learned information or past
events. The
memory deficit is most apparent on tasks to require spontaneous recall and may
also
be evident when the examiner provides stimuli for the person to recall at a
later time.
The memory disturbance must be sufficiently severe to cause marked impairment
in
social or occupational functioning and must represent a significant decline
from a
previous level of functioning. The memory deficit may be age-related or the
result of
disease or other cause. Dementia is characterized by multiple clinically
significant
deficits in cognition that represent a significant change from a previous
level of
functioning, including memory impairment involving inability to leam new
material
or forgetting of previously learned material. Memory can be formally tested by
measuring the ability to register, retain, recall and recognize information. A
diagnosis
of dementia also requires at least one of the following cognitive
disturbances: aphasia,
apraxia, agnosia or a disturbance in executive functioning. These deficits in
language,
motor performance, object recognition and abstract thinking, respectively,
must be
sufficiently severe in conjunction with the memory deficit to cause impairment
in
occupational or social functioning and must represent a decline from a
previously
higher level of functioning.
[0230] Compounds of the invention are useful for preventing loss of neuronal
function, which is characteristic of neurodegenerative diseases. Therapeutic
treatment
with a compound of the invention improves and/or enhances memory, learning and
cognition. In one embodiment, the compounds of the invention can be used to
treat a
neurodegenerative disease such as Alzheimer's, Huntington's disease,
Parkinson's
disease and amyotrophic lateral sclerosis, as well as MLS (cerebellar ataxia),
Down
syndrome, multi-infarct dementia, status epilecticus, contusive injuries (e.g.
spinal
cord injury and head injury), viral infection induced neurodegeneration, (e.g.
AIDS,
encephalopathies), epilepsy, benign forgetfulness, and closed head injury.
[02311 Compounds of the invention are useful for treating or preventing loss
of
memory and/or cognition associated with a neurodegenerative disease. The
compounds also ameliorate cognitive dysfunctions associated with aging and
improve
catatonic schizophrenia
[0232] Alzheimer's disease is manifested as a form of dementia that typically
involves mental deterioration, reflected in memory loss, confusion, and
disorientation.
71
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
In the context of the present invention, dementia is defined as a syndrome of
progressive decline in multiple domains of cognitive function, eventually
leading to
an inability to maintain normal social and/or occupational performance. Early
symptoms include memory lapses and mild but progressive deterioration of
specific
cognitive functions, such as language (aphasia), motor skills (apraxia) and
perception
(agnosia). The earliest manifestation of Alzheimer's disease is often memory
impairment, which is required for a diagnosis of dementia in both the National
Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's
Disease-and the Alzheimer's Disease and Related Disorders Association (NINCDS-
ADRDA) criteria (McKhann et al., 1984, Neurology 34:939-944), which are
specific
for Alzheimer's disease, and the American Psychiatric Association's Diagnostic
and
Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria,
which are
applicable for all forms of dementia. The cognitive function of a patient may
also be
assessed by the Alzheimer's disease Assessment Scale-cognitive subscale (ADAS-
cog; Rosen et al., 1984,.4m. J. Psychiatry 141:1356-1364). Alzheimer's disease
is
typically treated by acetylcholine esterase inhibitors such as tacrine
hydrochloride or
donepezil. Unfortunately, the few forms of treatment for memory loss and
impaired
learning available at present are not considered effective enough to make any
significant difference to a patient, and there is currently a lack of a
standard nootropic
drug for use in such treatment.
[0233] Other conditions that are manifested as deficits in memory and learning
include benign forgetfulness and closed head injury. Benign forgetfulness
refers to a
mild tendency to be unable to retrieve or recall information that was once
registered,
learned, and stored in memory (e.g., an inability to remember where one placed
one's
keys or parked one's car). Benign forgetfulness typically affects individuals
after 40
years of age and can be recognized by standard assessment instruments such as
the
Wechsler Memory Scale. Closed head injury refers to a clinical condition after
head
injury or trauma. Such a condition, which is characterized by cognitive and
memory
impairment, can be diagnosed as "amnestic disorder due to a general medical
condition" according to DSM-IV.
[0234] Compounds and compositions of the invention are also effective for
treating
cerebral function disorders. The term cerebral function disorder, as used
herein,
includes cerebral function disorders involving intellectual deficits, and may
be
72
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
exemplified by senile dementia, Alzheimer's type dementia, memory loss,
amnesia/amnestic syndrome, epilepsy, disturbances of consciousness, coma,
lowering
of attention, speech disorders, Parkinson's disease and autism.
Pain
[0235] The compounds of the invention are useful to treat any kind of acute or
chronic pain. In a preferred embodiment, the compounds of the invention are
useful
to treat chronic pain. In a particularly preferred embodiment, the compounds
of the
invention are useful to treat neuropathic pain. The term "pain" includes
central
neuropathic pain, involving damage to the brain or spinal cord, such as may
occur
following stroke, spinal cord injury, and as a result of multiple sclerosis.
It also
includes peripheral neuropathic pain, which includes diabetic neuropathy (DN
or
DPN), post-herpetic neuralgia (PHN), and trigeminal neuralgia (TGN). It also
includes dysfunctions of the nervous system such as Complex Regional Pain
Syndrome (CRPS), formerly known as Reflex Sympathetic Dystrophy (RSD), and
causalgia, and neuropathic pain symptoms such as sensory loss, allodynia,
hyperalgesia and hyperpathia. It further includes mixed nociceptive and
neuropathic
pain types, for example, mechanical spinal pain and radiculopathy or
myelopathy, and
the treatment of chronic pain conditions such as fibromyalgia, low back pain
and neck
pain due to spinal nerve root compression, and reflex sympathetic dystrophy.
102361 Other conditions and disorders include, but are not limited to, autism,
childhood learning disorders, depressions, anxieties and sleep disorders.
Compounds
of the invention may also be useful for the treatment of neurotoxic injury
that follows
cerebral stroke, thromboembolic stroke, hemorrhagic stroke, cerebral ischemia,
cerebral vasospasm, hypoglycemia, amnesia, hypoxia, anoxia, perinatal asphyxia
and
cardiac arrest.
[0237] The term "treating" when used in connection with the foregoing
disorders
means amelioration, prevention or relief from the symptoms and/or effects
associated
with these disorders and includes the prophylactic administration of a
compound of
the invention, a mixture thereof, a solvate (e.g., hydrate), prodrug (e.g.,
ethyl or
methyl esters of the current carboxylic acid inhibitors) or a pharmaceutically
acceptable salt of either, to substantially diminish the likelihood or
seriousness of the
condition.
73
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
B. Models of Disease
102381 In animals, several established models of learning and memory are
available
to examine the beneficial cognitive enhancing effects and potential related
side effects
of treatment. Descriptions of tests that may be employed to assess changes in
cognition in non-human species are given in the following references and
references
cited therein. Each of the following references is incorporated by reference
into this
application in their entirety: Sarter, M., Intern. J. Neuroscience, 1987,
32:765-774;
Methods and Findings in Experimental and Clinical Pharmacology 1998, 20(3),
249-277; Indian Journal of Pharmacology 1997, 29(4), 208-221. The tests
include
the Morris water maze (Stewart and Morris, In "Behavioral Neuroscience. A
Practical
Approach. Volume I", 1993, R. Saghal, Ed., 107-122; Morris, R. Journal of
neuroscience methods 1984, 11(1), 47-60), delayed non-match to sample
(Bontempi, B, et al, Journal of Pharmacology and Experimental Therapeutics
2001,
299(1), 297-306.; Alvarez, P; Zola-Morgan, S; Squire, L.R. Proc Natl Acad Sci
US
A. 1994 7;91(12), 5637-41.),delayed Alternation (also called delayed non-
matching to
position; Roux, S; Hubert, I; Lenegre, A; Milinkevitch, D; Porsolt, RD.
Pharmacol
Biochem Behav. 1994 49(3), 83-8; Ohta, H; Ni, X.H.; Matsumoto, K; Watanabe, H,
Jpn J Pharmacol. 1991, 56(3), 303-9), social discrimination models (Engelmann,
M;
Wotjak, C..T; Landgraf R. Physiol Behav. 1995, 58(2), 315-21), social
recognition
test (also called delay-induced forgetting; Lemaire, M; Bohme, G.A.; Piot. 0;
Roques,
B.P.; Blanchard, J.C. Psychopharmacology (Berl). 1994,115(4):435-40),
contextual
fear conditioning (Barad, M; Bourtchouladze, R; Winder, DG; Golan, H; Kandel,
E.
Proc Natl Acad Sci USA. 1998, 95(25), 15020-5; Bourtchouladze, R.; Frenguelli,
B.; Blendy, J.; Cioffi, D.; Schutz, G.; Silva, A.J. Cell, 1994, 79, 59-68),
and
conditioned fear extinction (Walker, DL; Ressler, KJ; Lu, K.T., Davis, M., J
Neurosci. 2002, 22(6), 2343-51; Davis, M.; Ressler, K.; Rothbaum, B.O.;
Richardson,
R. Biol. Psychiatry, 2006, 60, 369-375).
[0239] The Morris water maze is one of the best-validated models of learning
and
memory, and it is sensitive to the cognitive enhancing effects of a variety of
pharmacological agents. The task performed in the maze is particularly
sensitive to
manipulations of the hippocampus in the brain, an area of the brain important
for
spatial learning in animals and memory consolidation in humans. Moreover,
improvement in Morris water maze performance is predictive of clinical
efficacy of a
74
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
compound as a cognitive enhancer. For example, treatment with cholinesterase
inhibitors or selective muscarinic cholinergic agonists reverse learning
deficits in the
Morris maze animal model of learning and memory, as well as in clinical
populations
with dementia. In addition, this animal paradigm accurately models the
increasing
degree of impairment with advancing age and the increased vulnerability of the
memory trace to pre-test delay or interference which is characteristic of
amnesiac
patients.
[0240] Contextual fear conditioning is a form of associative learning in which
animals learn to fear a new environment (or an emotionally neutral conditioned
stimulus) because of its temporal association with an aversive unconditioned
stimulus
(US), such as a foot shock. When exposed to the same context or conditioned
stimulus at a later time, conditioned animals show a variety of conditioned
fear
responses, including freezing behavior. Because robust learning can be
triggered with
a single training trial, contextual fear conditioning has been used to study
temporally
distinct processes of short-term and long-term memory. Contextual fear
conditioning
is believed to be dependent on both the hippocampus and amygdale function.
[0241] Another example of learning is called fear extinction, a process
exhibited in
both human and animals, including rodents. Extinction of fear refers to the
reduction
in the measured level of fear to a cue previously paired with an aversive
event when
that cue is presented repeatedly in the absence of the aversive event.
Extinction of
fear is not the erasure of the original fear memory, but instead results from
a new
form of learning that acts to inhibit or suppress the original fear memory
(Bouton,
M.D.; Bolles, R.C. J. Exp. Psychol. Anim. Behav. Process. 1979, 5, 368-378;
Konorski, J. Inegrative Activity of the Brain: An Interdiscipinary Approach,
1967,
Chicago: The University of Chicago Press; Pavlov, I.P. Conditioned Reflexes.
1927,
Oxford, United Kingdom: Oxford University Press.). The literature also
suggests that
glutamate acting at the N-methyl D-aspartate (NMDA) receptor is critically
involved
in learning and memory (Bear, M.F. Proc. Nat. Acad. Sci. 1996, 93, 13453-
13459;
Castellano, C.; Cestari, V.; Ciamei, A. Curr. Drug Targets, 2001, 2, 273-283;
Morris,
R.G.; Davis, S.; Butcher, S.P. Philos. Trans. R Soc. Lond. B Biol. Sci. 1990.
329, 187-
204; Newcomer, J.W.; Krystal, J.H. Hippocampus, 2001, 11, 529-542.). There is
also
evidence that the NMDA receptor is involved with extinction of fear. For
example,
NMDA antagonists such as 2-amino-5-phosphopentanoic acid (APV) are known to
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
block fear extinction (Davis, M.; Ressler, K.; Rothbaum, B.O.; Richardson, R.
Biol.
Psychiatry, 2006, 60, 369-375; Kehoe, E.J.; Macrae, M.; Hutchinson, C.L.
Psychobiol. 1996, 24, 127-135; Lee, H.; Kim, J.J. J. Neurosci. 1998, 18, 8444-
8454;
Szapiro, G.; Vianna, M.R.; McGaugh, J.L.; Medina, J.H.; Izquierdo, I.
Hippocampus,
2003, 13, 53-58.), and NMDA agonists (such as the partial agonsist D-
cycloserine),
are known to facilitate fear extinction (Davis, M.; Ressler, K.; Rothbaum,
B.O.;
Richardson, R. Biol. Psychiatry, 2006, 60, 369-375; Ledgerwood, L.;
Richardson, R.;
Cranney, J. Behav. Neurosci. 2003,117 341-349; Walker, D.L.; Ressler, K.J.; Lu
K.-
T.; Davis, M. J. Neurosci. 2002, 22, 2343-2351). Additional experimental
conditions
for fear extinction tests may be found in the references cited in this
paragraph, and are
incorporated by reference.
[0242] In human exposure therapy, a patient is repeatedly exposed for
prolonged
periods to a feared object or situation in the absence of aversive
consequences. As a
result, the patient is often able to face their feared cues or situations with
less fear and
avoidance (extinction retention) due to the learning that took place during
exposure
therapy (extinction training). It has been shown that agents, such as D-
cycloserine,
that improve extinction in animals also improve the effectiveness of exposure-
based
psychotherapy. Examples of exposure based cognitive-behavioral therapy (CBT)
improved by agents that improve extinction include exposure to phobic objects
as
therapy for phobia disorders (For acrophobia, see Davis, M.; Ressler, K.;
Rothbaum,
B.O.; Richardson, R. Biol. Psychiatry, 2006, 60, 369-375; Ressler, K.J.;
Rothbaum,
B.O.; Tannenbaum, L.; Anderson, P.; Graap, K.; Zimand, E.; Hodges, L.; Davis,
M.
Archives Gen. Psychiatry 2004, 61, 1136-1144.), exposure to phobic situations
as
therapy for panic disorders (For social anxiety disorder, see Hoffmann, S.G.;
Meuret,
A.E.; Smits, J.A.; Simon, N.M.; Pollack, M.H.; Eisenmenger, K.; Shiekh, M.;
Otto,
M.W. Arch. Gen. Psychiatry 2006, 63, 298-304; Hofinann, S.G.; Pollack, M.H.;
Otto,
M.W. CNS Drug Reviews 2006, 12, 208-217), recollection of traumatic memories
as
therapy for Post-Traumatic Stress Disorder, exposure to cues associated with
drug
cravings as therapy for drug addiction, and exposure to cues associated with
smoking
as therapy for smoking cessation. Because of the cognitive, learning aspects
associated with psychotherapy based treatment for disorders such as phobias,
anxiety,
Post-Traumatic Stress Disorder, and Addiction, compounds of the invention are
useful
as an adjunct with psychotherapy for the treatment of these conditions.
Clinically,
76
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
compounds of the invention are useful as an adjunct to shorten the number of
therapy
sessions required or improve the therapeutic outcome of therapy.
[0243] In humans, methods for improving learning and memory may be measured
by such tests as the Wechsler Memory Scale and the Minimental test. A standard
clinical test for determining if a patient has impaired learning and memory is
the
Minimental Test for Learning and Memory (Folstein et al., J. Psychiatric Res.
12:185, 1975), especially for those suffering from head trauma, Korsakoffs
disease or
stroke. The test result serves as an index of short-term, working memory of
the kind
that deteriorates rapidly in the early stages of dementing or amnesiac
disorders. Ten
pairs of unrelated words (e.g., army-table) are read to the subject. Subjects
are then
asked to recall the second word when given the first word of each pair. The
measure
of memory impairment is a reduced number of paired-associate words recalled
relative to a matched control group. Improvement in learning and memory
constitutes
either (a) a statistically significant difference between the performance of
treated
patients as compared to members of a placebo group; or (b) a statistically
significant
change in performance in the direction of normality on measures pertinent to
the
disease model. Animal models or clinical instances of disease exhibit symptoms
which are by definition distinguishable from normal controls. Thus, the
measure of
effective pharmacotherapy will be a significant, but not necessarily complete,
reversal
of symptoms. Improvement can be facilitated in both animal and human models of
memory pathology by clinically effective "cognitive enhancing" drugs which
serve to
improve performance of a memory task. For example, cognitive enhancers which
function as cholinomimetic replacement therapies in patients suffering from
dementia
and memory loss of the Alzheimer's type significantly improve short-term
working
memory in such paradigms as the paired-associate task. Another potential
application
for therapeutic interventions against memory impairment is suggested by age-
related
deficits in performance which are effectively modeled by the longitudinal
study of
recent memory in aging mice.
[0244] The Wechsler Memory Scale is a widely used pencil-and-paper test of
cognitive function and memory capacity. In the normal population, the
standardized
test yields a mean of 100 and a standard deviation of 15, so that a mild
amnesia can be
detected with a 10-15 point reduction in the score, a more severe amnesia with
a 20-
30 point reduction, and so forth. During the clinical interview, a battery of
tests,
77
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
including, but not limited to, the Minimental test, the Wechsler memory scale,
or
paired-associate learning are applied to diagnose symptomatic memory loss.
These
tests provide general sensitivity to both general cognitive impairment and
specific loss
of learning/memory capacity (Squire, 1987). Apart from the specific diagnosis
of
dementia or amnestic disorders, these clinical instruments also identify age-
related
cognitive decline which reflects an objective diminution in mental function
consequent to the aging process that is within normal limits given the
person's age
(DSM IV, 1994). As noted above, "improvement" in learning and memory within
the
context of the present invention occurs when there is a statistically
significant
difference in the direction of normality in the paired-associate test, for
example,
between the performance of therapeutic agent treated patients as compared to
members of the placebo group or between subsequent tests given to the same
patient.
[0245] In animals, many established models of schizophrenia are available to
examine the beneficial effects of treatment; many of which are described in
the
following references, as well as references cited within, and are incorporated
by
reference: Saibo Kogaku 2007, 26(1), 22-27; Cartmell,J.; Monn, J.A.; Schoepp,
D.D. J. Pharm. Exp. Ther. 1999, 29](1), 161-170; Rowley, M; Bristow, L.J.;
Hutson, P.H. J. Med. Chem. 2001 15;44(4), 477-501; Geyer, M.A.; Ellenbroek, B;
Prog Neuropsychopharmacol Biol Psychiatry 2003, 27(7):1071-9; Geyer, M.A.;
Krebs-Thomson, K; Braff, D.L.; Swerdlow, N.R. Psychopharmacology (Berl). 2001
156(2-3):117-54; Jentsch, J.D.; Roth, R.H. Neuropsychopharmacology 1999,
20(3):201-25. The tests include Prepulse Inhibition (Dulawa, S.C.; Geyer, M.A.
Chin
JPhysiol. 1996, 39(3):139-46), PCP stereotypy test (Meltzer et al (In "PCP
(Phencyclidine): Historical and Current Perspectives", ed. E.F. Domino, NPP
Books,
Ann Arbor, 1981, 207-242), Amphetamine stereotypy test (Simon and Chermat, J.
Pharmacol. (Paris), 1972, 3, 235-238), PCP hyperactivity (Gleason, S.D.;
Shannon,
H.E. Psychopharmacology (Berl). 1997, 129(1):79-84) and MK-801 hyperactivity
(Corbett, R; Camacho, F; Woods, A.T.; Kerman, L.L.; Fishkin, R.J.; Brooks, K;
Dunn, R.W. Psychopharmacology (Berl). 1995, 120(1):67-74.
[0246] The prepulse inhibition test may be used to identify compounds that are
effective in treating schizophrenia. The test is based upon the observations
that
animals or humans that are exposed to a loud sound will display a startle
reflex and
that animals or humans exposed to a series of lower intensity sounds prior to
the
78
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
higher intensity test sound will no longer display as intense of a startle
reflex. This is
termed prepulse inhibition. Patients diagnosed with schizophrenia display
defects in
prepulse inhibition, that is, the lower intensity prepulses no longer inhibit
the startle
reflex to the intense test sound. Similar defects in prepulse inhibition can
be induced
in animals via drug treatments (scopolamine, ketamine, PCP or MK-801) or by
rearing offspring in isolation. These defects in prepulse inhibition in
animals can be
partially reversed by drugs known to be efficacious in schizophrenia patients.
It is felt
that animal prepulse inhibition models have face value for predicting efficacy
of
compounds in treating schizophrenia patients.
[0247] In animals, many established models of pain are available to examine
the
beneficial effects of treatment; many of which are reviewed in Methods in Pain
Research, CRC Press, 2001, Kruger, L. (Editor). Tests of acute pain include
the tail
flick (d'Amour and Smith, J. Pharmacol. Exp. Ther. 1941, 72, 74-79), hot plate
(Eddy, N.B.; Leimbach, D. J Pharmacol Exp Ther. 1953, 107(3):385-93), and paw
withdrawal tests. The phenylbenzoquinone writhing assay is a measure of
peritoneovisceral or visceral pain. Persistent pain tests, which use an
irritant or
foreign chemical agent as the nociceptive stimulus, include the formalin test
(Wheeler-Aceto, H; Cowan, A Psychopharmacology (Berl). 1991, 104(1):35-44),
Freund's adjuvant (Basile, A.S. et al Journal ofPharmacology and Experimental
Therapeutics 2007, 321(3), 1208-1225; Ackerman, N. R. et al ; Arthritis &
Rheumatism 1979, 22(12), 1365-74), capsaicin (Barrett, A.C. et al Journal of
Pharmacology and Experimental Therapeutics 2003, 307(1), 237-245), and
carrageenin models. These models have an initial, acute phase, followed by a
second,
inflammatory phase.
[0248] Neuropathic pain models are reviewed in Wang and Wang, Advanced Drug
Delivery Reviews 2003, and include the Spinal Nerve Ligation (SNL) model (also
called the Chung Model; Kim, S.H.; Chung, J.M. Pain 1992 50(3):355-63; Chaplan
et
al., Journal ofNeuroscience Methods 1994, 53(1):55-63; Chaplan SR, Bach FW,
Pogrel JW,.), Chronic Constriction Injury (CCI) model (also called the Bennett
Model; Bennett, G.J; Xie, Y.K Pain 1988 33(l):87-107.), Progressive Tactile
Hypersensitivity (PTH) model (Decosterd, I. Pain, 2002, 100(1), 15 5-162;
Anesth.
Analg. 2004, 99, 457-463), Spared Nerve Injury (SNI) model (Decosterd, I.
Pain,
2002, 100(1), 155-162; Anesth. Analg. 2004, 99, 457-463), the lumbar nerve
ligation
79
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
model (Ringkamp, M; Eschenfelder, S; Grethel, E.J.; Habler, H.J., Meyer, R.A.,
Janig, W., Raja, S.N. Pain, 1999, 79(2-3), 143-153), and streptozocin-or
chemotherapy induced diabetic neuropathy (Courteix, C.; Eschalier, A.;
Lavarenne, J.
Pain, 1993, 53(1), 81-88; Aubel, B. et al Pain 2004, 110(1-2), 22-32.).
[0249] Opioids, such as morphine, display robust efficacy in models of acute
pain,
such as the tail flick and hot plate tests, as well as in both the initial,
acute phase and
the second, inflammatory phase of persistent pain tests, such as the formalin
test.
Opioids also display efficacy in neuropathic pain models, such as the Spinal
Nerve
Ligation (SNL) model. The general analgesic effects of opiate compounds such
as
morphine in neuropathic pain models, however, are suggested by the increase in
paw
withdrawal threshold (PWT) in both the injured and the contralateral
(uninjured) paw.
Compounds that are useful specifically for the treatment of persistent or
chronic pain
states (e.g., neuropathic pain), such as gabapentin, tend to display efficacy
in models
of persistent inflammatory and neuropathic pain, such as the fonmalin (second
phase)
and SNL models. Compounds of this type, however, tend to increase PWT in the
SNL model in only the injured paw. In addition, these compounds fail to
display
efficacy in acute tests such as the tail flick test and the hot plate test,
and also fail to
display efficacy in the initial, acute phase of the formalin test. The lack of
effect of
compounds in the acute pain tests supports the notion that the antinociceptive
action
of these compounds is related to specific mechanisms associated with a central
sensitized state following injury. As a result, compounds that are efficacious
in
neuropathic pain model(s), such as the SNL (Chung) model, and the second phase
of
the formalin test, but are not efficacious in acute pain models, such as hot
plate and
tail flick, or in the first phase of the formalin test suggest that these
compounds are
more likely to be effective in persistent and chronic, rather than acute, pain
states (see
Table 1). In addition, their ability to increase PWT in the SNL model should
be
specific for the ipsilateral (injured) paw. Relevant references follow, and
are included
by reference. Singh, L. et al, Psychopharmacology, 1996,127, 1- 9. Field, M.J.
et
al Br. J. Pharmacol. 1997, 121, 1513-1522. Iyengar, S. et al, J. Pharmacology
and
Experimental Therapeutics, 2004, 311, 576-584. Shimoyama, N. et al
Neuroscience
Letters, 1997, 222, 65-67. Laughlin, T.M. et al J. Pharmacology and
Experimental
therapeutics, 2002, 302, 1168-1175. Hunter, J.C. et al European J. Pharmacol.
1997,
324, 153-160. Jones, C.K. et al J. Pharmacology and Experimental therapeutics,
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2005, 312, 726-732. Malmberg, A.B.; Yaksh, T.L. Anesthesiology, 1993, 79, 270-
281. Bannon, AW et al Brain Res., 1998, 801, 158-63.
102501 In a preferred embodiment, the compounds of the invention are useful
for
the treatment of persistent or chronic pain states (e.g., neuropathic pain).
As
described above, such compounds may be profiled in vivo by evaluating their
efficacy
in models of both acute and neuropathic pain. Preferred compounds demonstrate
efficacy in neuropathic pain models, but not in acute pain models.
Table 1: Profile of morphine and gabapentin in a variety of animal models
Animal Model Morphine Gabapentin
Acute Pain
Hot plate + -
Tail flick + -
Formalin (early phase) + -
Tissue Iniury/lnflammatory Pain
Formalin (second phase) + +
Carrageenan + +
Nerve iniury/Neuropathic Pain
Spinal Nerve Ligation (SNL; Chung) + +
Chronic Constriction Injury (CCI; Bennet) + +
[0251) There are various animal models with chronic brain dysfunctions thought
to reflect the processes underlying human epilepsy and seizures/convulsions,
such as
those described in Epilepsy Res. 2002 Jun;50(1-2):105-23 . Such chronic models
include the kindling model of temporal lobe epilepsy (TLE), post-status models
of
TLE in which epilepsy develops after a sustained status epilepticus, and
genetic
models of different types of epilepsy. Currently, the kindling model and post-
status
models, such as the pilocarpine or kainate models, are the most widely used
models
for studies on epileptogenic processes and on drug targets by which epilepsy
can be
prevented or modified. Furthermore, the seizures in these models can be used
for
testing of antiepileptic drug effects. A comparison of the pharmacology of
chronic
models with models of acute (reactive or provoked) seizures in previously
healthy
(non-epileptic) animals, such as the maximal electroshock seizure test,
demonstrates
that drug testing in chronic models of epilepsy yields data which are more
predictive
of clinical efficacy and adverse effects.
81
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0252] The following examples are provided to illustrate selected embodiments
of
the invention and are not to be construed as limiting its scope.
82
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
EXAMPLES
General Procedures
General Procedure 1: Synthesis of Fused Pyrrole Analogs
N H
NaOEt, EtOH ~COzEt Xylene / reflux 02Et
+ N3',--ICO2Et --~ A q Ql
H H
[0253] In the above Scheme, ring A represents any substituted or unsubstituted
5-
membered, aromatic ring. Exemplary aromatic rings include thiophenes, furans,
thiazoles and pyrroles.
A) Condensation of an Aldehyde with Ethyl Azidoacetate
[0254] A solution of the aldehyde (e.g., 1.61 g, 8.41 mmol) and about 4 to
about 7
equivalents of ethyl azidoacetate (e.g., 4.34 g, 33.7 mmol) in anhydrous EtOH
(e.g.,
10.5 mL) was added dropwise to a solution of sodium (e.g., 0.8 g) in anhydrous
EtOH
(e.g., 50.0 mL) at a temperature between about 0 C and about -45 C (typically
between about -10 and about -5 C (e.g., NaCl/ice)). The reaction mixture was
stirred
for about 1 hour (h) while the temperature was maintained below 0 C and was
then
allowed to warm to ambient temperature (also called room temperature, rt)
(e.g.,
ovemight). The mixture was quenched with a cold solution of saturated aqueous
NH4CI or was diluted with water (e.g., 0.5 L). The product was extracted with
diethyl
ether or ethyl acetate (EtOAc) (e.g., 3 x 0.2 L) and the combined organic
phases were
washed with saturated aqueous NaCI solution (2 x 0.1 L), dried (e.g., over
Na2SO4)
and filtered. The solvent was removed in vacuo to give the ethyl
azidoacrylate.
Alternatively, the solvent was reduced in vacuo (e.g., to about 50 mL) and the
resulting solution was used in the next reaction step.
B) Cyclization of the Ethyl Azidoacrylate
[0255] A solution of the above ethyl azidoacrylate in o- or m-xylene (e.g.,
150 mL)
was heated to reflux for a time period between about 15 minutes (min) and 14 h
(typically about 1 h). The reaction mixture was then allowed to cool to
ambient
temperature. The solution was concentrated in vacuo and the crude product was
purified (e.g., silica gel column chromatography) to give the fused pyrrole
ethyl ester.
General Procedure 2: Saponification of Ethyl and Methyl-Esters
83
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
N CO2Et N COOH
CA CA'
[0256] To a solution or suspension of the ester (e.g., 0.33 g, 1.2 mmol) in
MeOH or
EtOH (e.g., 16.5 mL) was added an aqueous base, such as 10M NaOH (e.g., 0.6
mL, 6
mmol), 5M KOH (e.g., 1.2 mL, 6 mmol) or 1M LiOH (e.g., 6 mL). The solution was
heated to a temperature between about 80 C and refluxed for a time period
between
about 30 min and about 20 h (e.g., 5 h). The reaction mixture was cooled to rt
and
was then acidified. In one example, the mixture was poured into water (e.g.,
200 mL)
and the pH of the resulting mixture was adjusted to about pH 1-2 with HCI. In
another example, excess solvent was removed in vacuo and the residue was
dissolved
in 5% citric acid (e.g., 15 mL). In yet another example, the solvent was
removed in
vacuo and the residue was dissolved in a saturated solution of NH4C1(e.g., 15
mL).
The acidified solution was then extracted (e.g., 3 x 100 mL EtOAc) and the
combined
organic layers were washed (e.g., with brine), dried (e.g., over Na2SO4),
filtered and
concentrated in vacuo to give the carboxylic acid.
Example 1
Synthesis of Fused Thiophene Pyrrole Analogs
1.1. Synthesis o/'Intermediate Aldehydes
1.1.a) Synthesis of 4-(4-Chlorobenzyl)thiophene-2-carbaldehyde
O P(Et0)= pd(OA )2 TPP C
/
CHO
C~ K~Da CHgCN s
90 C. 16 h
[0257] A solution mixture of Pd(OAc)2 (144 mg, 0.64 mmol) and
triphenylphosphine (TPP) (136 mg, 0.52 mmol) were weighed into a vial,
dissolved in
acetonitrile and transferred into a 40 mL Wheaton vial containing diethyl 4-
chlorobenzyl phosphate (Org. Lett. 2005, 7, 4875-4878; 3.08 g, 11.6 mmol), 5-
formylthiophen-3-ylboronic acid (2.0 g, 12.8 mmol), K3PO4 (2.72 g, 12.8 mmol)
and
a stir-bar. Nitrogen gas was bubbled through the mixture. The vial was closed
tightly
and heated to 90 C and vigorously stirred for 16 h. The reaction was diluted
with
water and extracted with dichloromethane (DCM) (3 x 100 mL). The combined
84
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
extracts were washed with brine, dried over Na2SO4, filtered and concentrated.
Purification by flash chromatography (Isco CombiFlash) (0-20% heptane/EtOAc)
yielded 4-(4-chlorobenzyl)thiophene-2-carbaldehyde: 835 mg, 28% yield. 'H NMR
(400 MHz, CDC13) 8 ppm: 10.10 (d, 1H), 7.80'(d, 1H), 7.63 (m, 1H), 7.55 (m,
2H),
7.40 (m, 2H), 4.23 (s, 2H).
1.1.b) Synthesis of 4-Phenethylthiophene-2-carbaldehyde
C~%
Br ~pp~P~N}~Gz
/ \ PPIb CW CHO I~H
S 7o.C
%CHO
102581 Under a N2 atmosphere, 4-bromothiophene-2-carbaldehyde (1.0 g, 5.2
mmol) was taken up in diisopropylamine (20 mL). TPP (549 mg, 2.1 mmol),
bis(benzonitrile)palladium chloride ([Pd(PhCN)2)C12) (400 mg, 1.0 mmol), and
copper iodide (199 mg, 1.0 mmol) were added. The mixture was degassed with N2
before phenylacetylene (1.15 mL, 10.4 mmol) was added, and the reaction was
stirred
at 70 C for 16 h. The mixture was concentrated to a dark brown solid and
chromatographed in 0-15 % EtOAc in heptane to yield 4-(phenylethynyl)thiophene-
2-
carbaldehyde (981 mg, 88 %) .'H NMR (400 MHz, CDC13) S(ppm): 9.93 (d, 1H),
7.88 (t, 1 H), 7.85 (d, 1 H), 7.53 (m, 2H), 7.38 (m, 3H).
\\ -~
Ptl/C
EtOAc I \
g CHO S CHO
[02591 Under a N2 atmosphere, 4-(phenylethynyl)thiophene-2-carbaldehyde (386
mg, 1.8 mmol) was dissolved EtOAc (6 mL), and palladium on carbon (Pd/C) (44
mg) was added. The flask was evacuated and flushed with H2 (3x). The reaction
stirred at rt overnight with a balloon of H2. The mixture was filtered through
a plug of
Celite and the filtrate was concentrated to give 4-phenethylthiophene-2-
carbaldehyde (373 mg, 95 %). 'H NMR (400 MHz, CDC13) S(ppm): 9.87 (d, 1H),
7.56 (d, 1H), 7.33 (m, 1H), 7.29 (m, 2H), 7.23 (m, 1H), 7.16 (m, 2H), 2.97 (m,
4H).
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.1.c) Synthesis of 4-[2-(4-Chlorophenyl)-ethyl]-thiophene-3-carbaldehyde
H er - -O
~ c~OH
\ Pd(OAc)z I \
BT. /S -
~ TPP, K,PO,. CFi3Gd, ~ ~
CI 94 C, 24 h, 55% CI
[02601 To a 40-mL scintillation vial containing trans-2-(4-
chlorophenyl)vinylboronic
acid (0.42 g, 2.30 mmol), 3-bromo-4-formylthiophene (0.40 g, 2.09 mmol), K3PO4
(0.490 g, 2.30 mmol), TPP (22 mg, 0.08 mmol, 4 mol%), Pd(OAc)2 (4.7 mg, 0.02
mmol, 1 mol%) and a stir-bar, was added acetonitrile (2.5 mL). The vial was
purged
with N2i capped tightly and heated at 94 C (aluminum multi-reaction block)
while
vigorously stirred for 32 h. The reaction was diluted with water and extracted
with
EtOAc (3 x 50 mL). The combined extracts were washed with brine, dried over
Na2SO4i filtered and concentrated. Purification by flash chromatography (Isco
CombiFlash) 0-10% EtOAc in heptane afforded the desired 4-[2-(4-chlorophenyl)-
vinyl]-thiophene-3-carbaldehyde (285 mg, 54%, purity >85%). 'H NMR (400 MHz,
CDC13) S ppm 6.99 (d, J=16.38 Hz, 1 H), 7.31-7.36 (m, 2 H), 7.45-7.49 (m, 2
H), 7.50
(d,.T--3.20 Hz, 1 H), 7.76 (dd,.,F--16.34, 0.78 Hz, 1 H), 8.13 (d, J=3.20 Hz,
1 H), 10.07
(d, J=0.82 Hz, I H).
- -o o
/ \ liz. 10% Pd-C~ - / \
\/ S EtOAc, RT, 48 h \/ S
CI 72% CI
[0261] 4-(4-Chlorophenethyl)thiophene-3-carbaldehyde was synthesized from 4-
[2-(4-chlorophenyl)-vinyl)-thiophene-3-carbaldehyde (260 mg, 1.04 mmol)
following
the conditions used to hydrogenate 4-(phenylethynyl)thiophene-2-carbaldehyde
to 4-
phenethylthiophene-2-carbaldehyde (Example 1.1.b). Purification by flash
chromatography (0-10% EtOAc/heptane) yielded 4-(4-chlorophenethyl)thiophene-3-
carbaldehyde (188 mg, 72%). 'H NMR (400 MHz, CDC13) S ppm 2.86-2.92 (m, 2 H),
3.16-3.22 (m, 2 H), 6.91 (dd, J--3.20, 0.82 Hz, 1 H), 7.10-7.15 (m, 2 H), 7.22-
7.27 (m,
2 H), 8.11 (d, J=3.11 Hz, 2 H), 10.00 (d, J=0.82 Hz, I H).
86
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.1.d) Synthesis of 5-Phenethylthiophene-2-carbaldehyde
CHO PdiC /S` cHo
EtOAc
[0262] 5-Phenethylthiophene-2-carbaldehyde was synthesized from 5-
(phenylethynyl)thiophene-2-carbaldehyde (4.0 g, 18.8 mmol) following the
conditions
used to hydrogenate 4-(phenylethynyl)thiophene-2-carbaldehyde to 4-
phenethylthiophene-2-carbaldehyde (Example 1. l .b). 5-Phenethylthiophene-2-
carbaldehyde(3.8 g, 93 %) was used in the next step without further
purification 'H
NMR (400 MHz, CDC13) S(ppm): 9.83 (s, 1H), 7.60 (d, 1H), 7.30 (m, 2H), 7.23
(m,
1 H), 7.19 (m, 2H), 6.86 (dt, 111), 3.21 (t, 2H), 3.03 (t, 2H).
1.1.e) Synthesis of 5-(4-chlorobenzyl)thiophene-2-carbaldehyde
e /~
(a-+), g a,o ci
O-P(EtO)2 Pd(OAc)2, TPP
\
CI K3POa, CH3CN S CHO
90 C, 16 h
102631 The title compound was synthesized from 5-formylthiophen-2-ylboronic
acid and diethyl 4-chlorobenzyl phosphate using the conditions to synthesize 4-
(4-
chlorobenzyl)thiophene-2-carbaldehyde (Example 1.1.a). Purification by flash
chromatography (0-20% heptane/EtOAc) yielded 5-(4-chlorobenzyl)thiophene-2-
carbaldehyde (730 mg, 48%). 'H NMR (400 MHz, CDC13) S ppm 9.82 (s, 1H), 7.62
(d, 1 H), 7.31 (m, 2H), 7.18 (m, 2H), 6.90 (m, 1 H), 4.17 (s, 2H).
1.1.f) Synthesis of 4-Benzyl-thiophene-3-carbaldehyde
0
O o=s o
O,'P(EtO)2 S , Pd(OAc)z, TPP -O
/ \
K3PO4, CH3CN/IPA, s
80 C, 16 h, 46 %
102641 The title compound was synthesized from diethyl benzyl phosphate (Org.
Lett. 2005, 7, 4875-4878) and 4-formylthiophen-3-ylboronic acid using the
conditions
to synthesize 5-(4-chlorobenzyl)thiophene-2-carbaldehyde (Example l .l.a).
Purification by prep-TLC (10% heptane/DCM, eluting 3x) yielded 4-
benzylthiophene-3-carbaldehyde (204 mg, 46%). 'H NMR (400 MHz, CDC13) 8 ppm
87
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
4.29 (s, 2 H), 6.83-6.86 (m, 1 H), 7.20-7.26 (m, 3 H), 7.29-7.34 (m, 2 H),
8.12 (d,
J=3.22 Hz, 1 H), 9.98 (d, J=0.73 Hz, 1 H).
1.1.g) Synthesis of 4-phenylthiophene-3-carbaldehyde
OH , '-
HO-BO ~ , Pd(OA ~ ~ -O
/ \ K3P .. cH3CN/IFA. / \
s Bo'C, 18 h, 50 96
[0265] The title compound was synthesized from iodobenzene and 4-
formylthiophen-3-ylboronic acid using the conditions to synthesize 5-(4-
chlorobenzyl)thiophene-2-carbaldehyde. Double elution by prep-TLC (10%
heptane/DCM) allowed for the isolation of 4-phenylthiophene-3-carbaldehyde
(300
mg, 48% yield). 'H NMR (400 MHz, CDC13) S ppm 7.32 (d, ,I--3.29 Hz, 1 H), 7.39-
7.50 (m, 5 H), 8.27 (d, J=3.29 Hz, 1 H), 9.87 (s, 1 H); 13C NMR (100 MHz,
CDC13) S
185.80, 143.82, 138.91, 134.68, 134.28, 129.30, 128.58, 128.05, 124.76.
1.1.h) Synthesis of 4-(4-Chlorobenzyl)-thiophene-3-carbaldehyde
P
I CI [0266] The title compound was synthesized from 4-chlorobenzyl diethyl
phosphate
and 4-formylthiophen-3-ylboronic acid using the conditions to synthesize 5-(4-
chlorobenzyl)thiophene-2-carbaldehyde (Example 1.1.a). Purification by prep-
TLC
(50% heptane/DCM, double elution) yielded 266 mg of 4-(4-
chlorobenzyl)thiophene-
3-carbaldehyde (58%). 'H NMR (400 MHz, CDC13) S ppm 4.25 (s, 2 H), 6.84-6.88
(m, I H), 7.14-7.19 (m, 2 H), 7.25-7.30 (m, 2 H), 8.12 (d, .t=3.17 Hz, I H),
9.96 (s, 1
H); 13C NMR (100 MHz, CDC13) 5 185.52, 140.84, 140.28, 140.02, 138.06, 132.10,
130.31, 128.58, 124.75, 34.70; LCMS- MS (ESI+) 236.68 (M+H).
1.1.i) Synthesis of 4-fluoro-thiophene-2-carboxaldehyde and 5-fluoro-
thiophene-2-carboxaldehyde
Br TBDPS-Cl Br Ph
\ OH Imidazole / \ O-Si
CH2CI2 '
S 0 C to r.t. S Ph
[0267] To a 250-mL round bottom flask fitted with a magnetic stir bar under a
N2
atmosphere was added 4-bromo-thiophene-2-methanol (2.0 g, 10 mmol, 1 equiv)
and
88
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
30 mL of anhydrous DCM. The reaction flask was then cooled to 0 C and the tert-
butyl-diphenylsilyl chloride (3.4 g, 3.2 mL, 12.4 mmol, 1.2 equiv) was added
followed by imidazole (1.06 g, 15.5 mmol, 1.5 equiv). The reaction was stirred
for 16
h and was allowed to equilibrate to rt. The reaction mixture was subsequently
taken
up in 75 mL DCM and washed with water. The organic layer was then dried
(Na2SO4), filtered, and evaporated in vacuo. The resulting residue was
chromatographed over silica gel (0 - 10% EtOAc in heptane over 18 min.-
retention
time (tR) of product: 4- 12 min) to give the desired ((4-bromothiophen-2-
yl)methoxy)-tert-butyl diphenyl silane (4.3929 g, 98%). 'H-NMR (400 MHz,
CD3CN) S ppm 7.66 - 7.71 (m, 4 H), 7.39 - 7.51 (m, 6 H), 7.29 (d, J=1.46 Hz, 1
H),
6.77 - 6.81 (m, 1 H), 4.89 (d, J=0.93 Hz, 2 H), 1.06 (s, 9 H).
Br Ph NFSI F Ph Ph
b -ii~ HF + k 3 O 4i -~
S Ph -78 C to r.t S Ph F S Ph
[0268] To a 40-mL vial fitted with a magnetic stir bar under a N2 atmosphere
was
added ((4-bromothiophen-2-yl)methoxy)-tert-butyi diphenyl silane (2.9 g, 6.7
mmol,
1 equiv) and 15 mL of anhydrous tetrahydrofuran (THF). The reaction vial was
cooled to -78 C and n-BuLi (3.2 mL, 2.5 M, 8 mmol, 1.2 equiv) was added
slowly,
dropwise. Stirring was continued at -78 C for 1 h. N-fluorobenzenesulfonimide
(NFSI) (2.54 g, 8 mmol, 1.2 equiv) was dissolved in 7 mL of anhydrous 'I'HF
(0.9
mL/mmol reagent) in a separate vessel under inert atmosphere, and was then
added
dropwise over 10 to 15 min to the reaction vial. The reaction temperature was
maintained at -78 C for 4 h, and was subsequently allowed to equilibrate to
rt
overnight. The reaction was quenched by the addition of approx. 30 mL of
saturated
aqueous ammonium chloride solution. The resulting aqueous mixture was
extracted
with ether (4 x 20 mL). The combined organic layers were dried (Na2SO4),
filtered,
and evaporated. The resulting residue was chromatographed over silica gel (0 -
10%
EtOAc in heptane over 20 min; tR of product: 5- 15 min) to give a mixture
which was
qualitatively shown by 'H and ' 9F NMR to contain tert-butyl(((4-
fluorothiophen-2-
yl)methoxy)methyl)diphenylsilane and tert-butyl(((5-fluorothiophen-2-
yl)methoxy)methyl)diphenylsilane. 2.6 g isolated as a mixture. 'H NMR (400
MHz,
CD3CN) showed signature peaks at 7.68, 7.44, and 4.78 ppm that were indicative
of
the desired product. '9F NMR (376 MHz, CD3CN) showed a multiplet at approx. -
134 to 133 ppm. The material was carried on without further purification.
89
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
F.
Ph
O-Si + o-Si,~ TBAF OH + OH THF S Ph F S Ph 25 C S F S
[0269] To a 100 mL round bottom flask fitted with a magnetic stir bar under a
N2
atmosphere was added tert-butyl(((4-fluorothiophen-2-
yl)methoxy)methyl)diphenylsilane and tert-butyl(((5-fluorothiophen-2-
yl)methoxy)methyl)diphenylsilane mixture (2.6 g, 7 mmol, 1 equiv) and 20 mL of
anhydrous THF. A tetra n-butyl ammonium fluoride (TBAF) solution (14 mL, I M,
14 mmol, 2 equiv) in THF was then added in one portion and stirring continued
for 16
h at 25 C. The reaction mixture was taken up into an equal volume of ether
and
washed with water, brine, and dried over anhydrous Na2SO4. The mixture was
filtered
and evaporated. The resulting residue was chromatographed over silica gel
(gradient
of 0- 40% EtOAc in pentane over 20 min. (tR of product: 10 - 12 min.). The
isolated
fractions were consolidated and evaporated carefully to give a yellow oil
(0.791 g,
85%) as a mixture which was qualitatively shown by 'H and 19F NMR to contain
the
desired 4-fluorothiophene-2-methanol and 5-fluorothiophene-2-methanol. 'H NMR
(400 MHz, CD3CN) showed signature peaks at 6.97, 6.39, 4.71 and 3.37 ppm that
were indicative of the desired product. 19F NMR (376 MHz, CD3CN) showed a
strong signal at -130 ppm. The material was carried on without further
purification.
F / \ OH + oH M"~ = F / \ iC + 'O
F~ CH2CI2 ~ F~
S 25 0C S
[0270] To a 250-mL round bottom flask fitted with a magnetic stir bar under a
N2
atmosphere at 25 C was added the 4-fluoro-thiophene-2-methanol and 5-fluoro-
thiophene-2-methanol mixture (0.79 g, 6.05 mmol, 1 equiv) and 50 mL of
anhydrous
DCM. Manganese (IV) oxide (5.26 g, 60.5 mmol, 10 equiv) was added in one
portion, and stirring was continued ovemight at 25 C. The reaction material
was
subsequently filtered through a short pad of Celite , and the resulting plug
was
washed thoroughly with DCM. The organics were evaporated to give a light brown
oil (0.5998 g, 77%) as a mixture which was qualitatively shown by 'H and '9F
NMR
to contain 4-fluoro-thiophene-2-carboxaldehyde and 5-fluoro-thiophene-2-
carboxaldehyde. 'H NMR (400 MHz, CD3CN) showed a signature peak for the
aldehyde at 9.75 ppm and a similar aromatic pattern as well as disappearance
of the
hydroxy-methyl moiety of the starting material. '9F NMR (376 MHz, CD3CN)
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
showed a strong signal at -119.20 ppm. The material was carried on without
further
purification.
1.1.j) Synthesis of 5-phenethylthiophene-3-carbaldehyde
g Pd(OAc)2, PPh3,
+ B(OH)2 K3PO4, ACN
CHO
CHO
[0271] (E)-5-styrylthiophene-3-carbaldehyde was synthesized from 5-iodo-3-
thiophene carboxaldehyde and (E)-styrylboronic acid using the conditions to
synthesize 4-(4-chlorobenzyl)thiophene-2-carbaldehyde. The crude product was
chromatographed over silica gel (0 to 25% EtOAc in heptane over 30 min) to
give
(E)-5-styrylthiophene-3-carbaldehyde (0.115 g, 20% yield). 'H NMR (400 MHz,
CDC13) S ppm 9.86 (s, 1H), 7.97 (s, 1H), 7.48 (m, 31-1), 7.38 (m, 2H), 7.31
(m, 1H),
7.19 (d, J=16.2 Hz, 114), 6.99 (d,.,r----16.2 Hz, 1 H).
q\/ S1.) H2, Pd/C, EtOAc S
CHO 2.)PDC, DCM
CHO
[0272] Pd/C (25 % by weight) was added to a solution of (E)-5-styrylthiophene-
3-
carbaldehyde (0.300 g, 1.4 mmol) in EtOAc (5.0 mL). The reaction vessel was
evacuated and flushed (x 3) with H2. The reaction was stirred at rt ovennight
under a
balloon of H2. The mixture was filtered through a Celite plug, washed with
EtOAc
(0.2 L). The solution was concentrated in vacuo and chromatographed over
silica gel
(0 to 25% EtOAc in heptane over 30 min) to yield 0.245 g of 5-
phenethylthiophene-3-
methylalcohol.'H NMR (400 MHz, CDC13) S ppm 7.32 (m, 2H), 7.24 (m, 3H), 7.01
(m, l H), 6.80 (s, 1 H), 4.60 (d,.I--0.98 Hz, 211), 3.13 (m, 2H), 3.01 (m,
2H), 1.85 (s,
1H).
[0273] Pyridinium dichromate (PDC) (0.863 g, 2.30 mmol) was added to a
solution
of 5-phenethylthiophene-3-methylalcohol (0.200 g, 0.92 mmol) in DCM (5.0 mL).
The mixture stirred at rt for 5 h. The mixture was filtered through a Celite
plug and
washed with DCM (0.2 L). The solution was concentrated in vacuo and
chromatographed over silica gel (0 to 25% EtOAc in heptane over 30 min) to
give 5-
phenethylthiophene-3-carbaldehyde (0.045 g). 'H NMR (400 MHz, CDC13) S ppm
91
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
9.86 (s, 1 H), 7.97 (s, 1 H), 7.48 (m, 3H), 7.38 (m, 2H), 7.31 (m, 1 H), 7.19
(d,.T--16.2
Hz, 1 H), 6.99 (d,.T--16.2 Hz, 1 H).
1.1.k) Synthesis of 5-Fluorothiophene-3-carboxaldehyde
0 1) 1-NMP, nBuLi; 0
H TMEDA, sBuLi H
/ 1 THF, -78 C ~ `
S 2) NFSI, THF F S
-78aC-rt
[0274] To N-methyl piperazine (1-NMP) (0.54 g, 5.4 mmol) in anhydrous THF (15
mL) cooled to -78 C was added nBuLi (2.5 M in hexane, 2.0 mL, 4.9 mmol)
dropwise followed by 3-thiophenecarboxaldehyde (0.5 g, 4.5 mmol). The
resulting
mixture was stirred at -78 C for 15 min at which time
tetramethylethylenediamine
(TMEDA) (1.04 g, 8.9 mmol) and sec-butyllithium (sBuLi) (1.4 M cyclohexane,
3.8
ml, 5.4 mmol) were added in sequence, dropwise. After stirring 2 h at -78 C,
NFSI
(1.4 g, 4.5 mmol) was added dropwise as a solution in THF (5 mL). Upon
addition of
NFSI the dry ice bath was removed and the reaction was allowed to warm to 23
C
over I h. After 4 h, the reaction was quenched by the addition of H20 (20 mL)
and
extracted with Et20 (3 x 30 mL), and the combined organic extracts were washed
with brine, dried over Na2SO4, and filtered. The solvent was removed in vacuo.
Purification by flash column chromatography (20% EtOAc in hexanes) afforded
the
desired aldehyde 5-fluorothiophene-3-carboxaldehyde as a mixture with starting
material. The mixture was carried on to the next step without further
purification.
1.2. Synthesis of Intermediate Esters
[02751 The following ethyl esters were synthesized from the indicated aldehyde
according to General Procedure 1 A (to yield an intermediate acrylate)
followed by
General Procedure 1 B.
1.2.a) Synthesis of ethyl 2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
H
~ \ ~COzEt 1) NaOEt, EtOH / \N/ COZEt
Br +
g I N3 2) m-xylene reflux Br S
0
[0276] The title compound was synthesized from 5-bromothiophene-2-
carboxaldehyde (1.61 g, 8.41 mmol) in two steps. The crude product was
chromatographed over silica gel (gradient 0 to 25% EtOAc in heptane over 30
min) to
give ethyl 2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate as yellow needles
(0.330
92
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
g, 15%). Rf= 0.29 (25:75 heptane / EtOAc); 'H NMR (400 MHz, CDC13) S(ppm)
9.03 (s, 1 H) 7.05 (s, I H) 7.03 (s, 1 H) 4.37 (q,.T--7.1 Hz, 2 H) 1.39 (t, ,T-
-7.1 Hz, 3
H).
1.2.b) Synthesis of ethyl 2,3-dibromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
[02771 The title compound was synthesized from 4,5-dibromothiophene-2-
carboxaldehyde (2.0 g, 7.41 mmol) in two steps. The crude product was purified
by
silica gel colum chromatography (0-25% EtOAc/heptane over 30 min) to give
ethyl
2,3-dibromo-4H-thieno[3,2-b]pyrrole-5-carboxylate as a yellow solid (0.158 g,
6%).
Rf= 0.57 (50:50 heptane/EtOAc); 'H NMR (400 MHz, CDC13) S(ppm) 9.02 (s, 1 H)
7.09 (s, I H) 4.39 (q, J=7.1 Hz, 2 H) 1.41 (t,.T--7.1 Hz, 3 H).
1.2.c) Synthesis of ethyl 3-methyl-4H-thieno[3,2-b]pyrrole-5-carboxylate
Na EtOZC N
\ + N3 COZEt / \ ~ s
s CHO EtOH s
H
n2C xylene /N/
CO2Et
N3 -- S reflux S
[0278] A) Ethyl 2-azido-3-(4-methylthiophen-2-yl)acrylate (orange-red oil) was
synthesized from 4-methyl-2-thiophenecarbaldehyde (1.0 g, 7.9 mmol). 'H NMR
(400
MHz, CDC13) S(ppm): 7.15 (m, 1H), 7.10 (m, 1H), 7.09 (m, 1H), 4.35 (q, 2H),
2.26
(d, 3H), 1.39 (t, 3H).
[0279] B) The title compound was prepared from ethyl 2-azido-3-(4-
methylthiophen-2-yl)acrylate and was purified by flash column chromatography
(0-20
% EtOAc in heptane) and recrystallization from ether/heptane to give ethyl 3-
methyl-
4H-thieno[3,2-b]pyrrole-5-carboxylate as an orange solid (94 mg). LCMS m/e 210
(M+H). 'H NMR (400 MHz, CDC13) S(ppm): 9.04 (s, 1H), 7.08 (d, 1H), 6.94 (m,
1H), 4.38 (q, 2H), 2.35 (d, 3H), 1.40 (t, 3H).
93
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.2.d) Synthesis of ethyl2-methyl-4H-thieno[3,2-b]pyrrole-5-carboxylate
EtO~C ~ ~ CHO N ~COZEt E~ ~ p ~ ~ ~ N3
S S
Et02C N N C02Et
~ \ ~ a xylene ~ '
S S
[0280] A) Ethy12-azido-3-(5-methylthiophen-2-yl)acrylate (1.9 g) was
synthesized
from 5-methyl-2-thiophenecarbaldehyde (2.0 g, 15.9 mmol) and was isolated as
an
orange solid after purification by flash column chromatography (100 %
heptane). 'H
1VMR (400 MHz, CDC13) S(ppm): 7.14 (m, 1 H), 7.10 (s, 1 H), 6.74 (m, 1 H),
4.35 (q,
2H), 2.54 (d, 3H), 1.39 (t, 3H).
[0281] B) The title compound was prepared from ethyl 2-azido-3-(5-
methylthiophen-2-yl)acrylate and was isolated to give ethy12-methyl-4H-
thieno[3,2-
b]pyrrole-5-carboxylate as a pale yellow solid (965 mg). LCMS m/e 210 (M+H).
'H
NMR (400 MHz, CDC13) S(ppm): 8.95 (s, 1 H), 7.06 (dd, 1H), 6.65 (m, 1 H), 4.36
(q,
2H), 2.56 (d, 3H), 1.39 (t, 3H).
1.2.e) Synthesis of ethyl2-chloro-4H-thieno[3,2-b]pyrrole-5-carboxylate
Na Et02C
CI /S\ CHO + N^COZEt EtoH cl NA N3
S
H
EtOZC
N3 xylene N/ C02Et
/ ~ ~ ---
CI S CI /S
[0282] A) Ethy12-azido-3-(5-chlorothiophen-2-yl)acrylate (1.13 g) was
synthesized
from 5-chloro-2-thiophene-carboxaldehyde (2.0 g, 10.5 mmol) and was isolated
as an
orange solid after purification by flash column chromatography (100 %
heptane). 'H
NMR (400 MHz, CDC13) S(ppm): 7.06 (m, 1H), 7.02 (s, 1H), 6.89 (d, 1H), 4.36
(q,
2H), 1.39 (t, 3H).
[0283] B) The title compound was prepared from ethyl 2-azido-3-(5-
chlorothiophen-2-yl)acrylate and was purified by flash column chromatography
(0-20
% EtOAc in heptane) to give ethyl 2-chloro-4H-thieno[3,2-b]pyrrole-5-
carboxylate
(418 mg) as a yellow solid. 1 H NMR (400 MHz, CDC13) S(ppm): 9.10 (s, 1H),
7.05
(m, 1H), 6.90 (m, IH), 4.38 (q, 2H), 1.39 (t, 3H).
94
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.2.f) Synthesis of ethyl 3-bromo-6H-thieno[2,3-blpyrrole-5-carboxylate
COZEt
Br CHO Br
+ C s s
COZEt Br
Br ~ COzEt
~3 )"no NH
~ N3 ~e'"l^ux
S
[0284J A) Ethyl 2-azido-3-(4-bromothiophen-3-yl)acrylate was synthesized from
4-
bromo-3-thiophene-carbaldehyde (2.0 g, 10.5 mmol) and was isolated as an
orange oil
after purification by flash column chromatography (100 % heptane). 'H NMR (400
MHz, CDC13) S(ppm): 8.31 (m, 1H), 7.30 (m, 1H), 7.03 (m, IH), 4.40 (q, 2H),
1.42
(t, 3H).
[0285] B) The title compound was prepared from ethyl 2-azido-3-(4-
bromothiophen-3-yl)acrylate and was purified by flash column chromatography (0-
20
% EtOAc in heptane) to give ethyl 3-bromo-6H-thieno[2,3-b]pyrrole-5-
carboxylate
(971 mg) as a pale yellow solid. LCMS m/e 275 (M+H). 'H NMR (400 MHz,
CDC13) S(ppm): 9.38 (s, 1H), 7.07 (m, 1H), 6.85 (s, 1H), 4.39 (q, 2H), 1.41
(t, 3H).
1.2.g) Synthesis of ethyl 3-(4-chlorobenzyl)-4A-thieno[3,2-b]pyrrole-5-
carboxylate
CI N Na
~ ~ 3COz~t CI COZEt
S CHO EtOH S
N H
xylere
~ ~Et reflux / N/ COZEt
S
S
[0286] A) Ethyl 2-azido-3-(4-(4-chlorobenzyl)thiophen-2-yl)acrylate was
synthesized from 4-(4-chlorobenzyl)thiophene-2-carbaldehyde (835 mg, 3.5 mmol)
and was isolated as a yellow oil (657 mg, 54 %) after pu.rification by flash
column
chromatography (100 % heptane). 'H NMR (400 MHz, CDC13) S(ppm): 7.20 (m,
2H), 7.04 (m, 2H), 7.02 (s, 2H), 6.99 (s, 1H), 4.27 (q, 2H), 3.84 (s, 2H),
1.30 (t, 3H).
[02871 B) The title compound was synthesized from ethyl 2-azido-3-(4-(4-
chlorobenzyl)thiophen-2-yl)acrylate and was purified by flash column
chromatography (0-20 % EtOAc in heptane) to give ethy13-(4-chlorobenzyl)-4H-
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
thieno[3,2-blpyrrole-5-carboxylate (350 mg, 58 %). 'H NMR (400 Ivll-Iz, CDC13)
S
(ppm): 8.56 (s, 1 H), 7.31 (m, 2H), 7.19 (m, 2H), 7.10 (d, 1 H), 6.97 (m, 1
H), 4.34 (q,
2H), 4.04 (s, 2H), 1.37 (t, 3H).
1.2.h) Synthesis of ethyl 3-phenethyl-4H-thieno[3,2-b]pyrrole-5-carboxylate
Q
Na
N3 CO=Et N3
\ EtOH COiEt
S CHO
\ ~ L
"~e,e ~ Mrm` Ca,s
/S\ ~ O~t S [0288] A) Ethy12-azido-3-(4-phenethylthiophen-2-yl)acrylate (334
mg, 56 %) was
synthesized from 4-phenethyl-thiophene-2-carbaldehyde (373 mg, 1.7 mmol) and
was
isolated as a yellow solid after purification by flash column chromatography
(100 %
heptane). 'H NMR (400 MHz, CDC13) S(ppm): 7.29 (m, 2H), 7.22 (m, 1H), 7.17 (m,
3H), 7.10 (s, 1 H), 7.09 (s, 114), 4.36 (q, 21-1), 2.93 (s, 4H), 1.40 (t, 3H).
[0289] B) The title compound was prepared from ethy12-azido-3-(4-
phenethylthiophen-2-yl)acrylate and was purified by flash column
chromatography
(0-20 % EtOAc in heptane) to give ethyl 3-phenethyl-4H-thieno[3,2-b]pyrrole-5-
carboxylate (188 mg) as a yellow-orange solid. IH NMR (400 MHz, CDC13) S(ppm):
8.46 (s, IH), 7.31 (m, 2H), 7.25 (m, 1 H), 7.19 (m, 2H), 7.07 (d, 1 H), 6.95
(m, 1 H),
4.33 (q, 2H), 3.03 (m, 4H), 1.38 (t, 3H).
1.2.i) Synthesis of 3-[2-(4-chlorophenyl)-ethyl]-6H-thieno[2,3-b]pyrrole-5-
carboxylic acid ethyl ester
Ny
~'f
OEf'~;:rx
NaOEUEIOH, -5 rrin20 CI 58%
OEt m-xylene, reflux \
~ 8%
CI S N3 CI S H OEt
96
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
102901 A) Ethy12-azido-3-{4-[2-(4-chlorophenyl)-ethyl]-thiophen-3-yl}-acrylate
(142 mg, 58%) was synthesized from 4-[2-(4-chlorophenyl)-ethyl]-thiophene-3-
carbaldehyde (170 mg, 0.68 mmol) and isolated after purification by flash
chromatography (Isco CombiFlash, 0-5% EtOAc/heptane). 'H NMR (400 MHz,
CDC13) 8 ppm 1.41 (t, J--7.14 Hz, 3 H), 2.84-2.96 (m, 4 H), 4.38 (q, J=7.14
Hz, 2 H),
6.83 (d,.P--0.55 Hz, I H), 6.91 (d,.P--3.11 Hz, I H), 7.05-7.10 (m, 2 H), 7.23-
7.27 (m,
2 H), 8.26 (d, J=3.20 Hz, 1 H); LCMS- MS (ESI+) 333.71 (M-N2).
(0291J B) The title compound was prepared from ethy12-azido-3-{4-[2-(4-
chlorophenyl)-ethyl]-thiophen-3-yl}-acrylate and was purified by flash
chromatography (Isco CombiFlash, 0-5% EtOAc/heptane) to afford ethyl 3-[2-(4-
chlorophenyl)-ethyl]-6H-thieno[2,3-b]pyrrole-5-carboxylate(112 mg, 87%) as a
straw-colored solid. 'H NMR (400 MHz, CDC13) 8 ppm 1.41 (t,.P--7.14 Hz, 3 H),
2.97-3.01 (m, 4 H), 4.39 (q,.I--7.08 Hz, 2 H), 6.46 (s, 1 H), 7.05 (d,.T--1.92
Hz, 1 H),
7.08-7.12 (m, 2 H), 7.23-7.27 (m, 2 H), 9.37 (s, 1 H); LCMS- MS (ESI+) 333.71
(M+H).
1.2.j) Synthesis of ethyl2-phenethyl-4H-thieno[3,2-bJpyrrole-5-carboxylate
Na N3
cr CHO ~\/C02Et COZEt
s -r ~ S
EtOH
N3 xylene N
C02Et CO2Et
S reflux / S
[02921 A) Ethy12-azido-3-(5-phenethylthiophen-2-yl)acrylate was synthesized
from
5-phenethylthiophene-2-carbaldehyde (1.5 g, 6.9 mmol) and was isolated as an
orange
oil (832 mg, 37%) after purification by flash column chromatography (100 %
heptane). 'H NMR (400 MHz, CDC13) S(ppm): 7.30 (m, 2H), 7.22 (m, 3H), 7.14 (d,
1 H), 7.10 (s, 1 H), 6.73 (dt, 1 H), 4.36 (q, 2H), 3.16 (t, 2H), 3.02 (t,
2H),1.39 (t, 3H).
[0293] B) The title compound was prepared from ethy12-azido-3-(5-
phenethylthiophen-2-yl)acrylate and was purified by flash column
chromatography
(0-20 % EtOAc in heptane) to afford ethy12-phenethyl-4H-thieno[3,2-b]pyrrole-5-
carboxylate (502 mg, 66 %) as a pale yellow solid. 'H NMR (400 MHz, CDC13) 8
97
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
(ppm): 8.86 (s, 1 H), 7.30 (m, 2H), 7.22 (m, 3H), 7.07 (dd, 111), 6.62 (dd,
114), 4.36 (q,
2H), 3.17 (t, 2H), 3.03 (t, 2H), 1.38 (t, 3H).
1.2.k) Synthesis of ethyl 2-(4-chlorobenzyl)-4H-thieno[3,2-b]pyrrole-5-
carboxylate
CI
Na CI
^b--/C02Et C~t
/
CHO EtOH g
c:l
CO2Et xylene CI
// \ / reilux
COZEt
s
s
[0294] A) Ethy12-azido-3-(5-(4-chlorobenzyl)thiophen-2-yl)acrylate was
synthesized from 5-(4-chlorobenzyl)thiophene-2-carbaldehyde (730 mg, 3.1 mmol)
and was isolated as a yellow oil (84 mg, 8 %) after purification by flash
column
chromatography (100 % heptane). 'H NMR (400 MHz, CDC13) S(ppm): 7.30 (m,
2H), 7.19 (m, 2H), 7.15 (d, 1H), 7.08 (s, 1H), 6.76 (m, 1H), 4.35 (q, 2H),
4.14 (s, 2H),
1.39 (t, 3H).
[0295] B) The title compound was prepared from ethyl 2-azido-3-(5-(4-
chlorobenzyl)thiophen-2-yl)acrylate and was purified by flash column
chromatography (0-20 % EtOAc in heptane) to afford ethyl 2-(4-chlorobenzyl)-4H-
thieno[3,2-b]pyrrole-5-carboxylate (42 mg, 55 %). 'H NMR (400 MHz, CDC13) 8
(ppm): 8.86 (s, 1H), 7.30 (m, 2H), 7.21 (m, 2H), 7.06 (dd, 1H), 6.67 (d, 1H),
4.36 (q,
2H), 4.15 (s, 2H), 1.38 (t, 3H).
1.2.1) Synthesis of ethyl 3-benzyl-6H-thieno[2,3-b]pyrrole-5-carboxylate
~ ~
~ \ _p Na~ ~
OEt
--- I ~ OEt
/ \ C, ~ 45 ~ rtdnH, S N~o
O
ro-xytene, relhnc_ / \ O
oEt ~
- 4:
S N, s H OEt
[02961 A) Ethy12-azido-3-(4-benzylthiophen-3-yl)acrylate was synthesized from
4-
benzyl-thiophene-3-carbaldehyde (200 mg, 0.99 mmol) and was isolated after
purification by flash chromatography (Isco CombiFlash, 0-5% EtOAc/heptane)
(210
98
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
mg, 68%). 'H NMR (400 MHz, CDC13) 6 ppm 1.36 (t,.1=7.13 Hz, 3 H), 4.01 (s, 2
H),
4.32 (q,.T--7.16 Hz, 2 H), 6.86-6.91 (m, 2 H), 7.16-7.21 (m, 2 H), 7.21-7.25
(m, 1 H),
7.27-7.33 (m, 2 H), 8.28 (d, .F---3.17 Hz, 1 H).
[0297] B) The title compound was prepared from ethyl 2-azido-3-(4-
benzylthiophen-3-yl)acrylate and was purified by flash chromatography (Isco
CombiFlash, 0-5% EtOAc/heptane) to afford ethyl 3-benzyl-6H-thieno[2,3-
b]pyrrole-
5-carboxylate (169 mg, 88%) as an off-white solid. 'H NMR (400 MHz, CDC13) S
ppm 1.37 (t, J 7.14 Hz, 3 H), 4.04 (s, 2 H), 4.34 (q,.T--7.14 Hz, 2 H), 6.52
(t, .f=1.10
Hz, 1 H), 6.90 (d, J--1.92 Hz, I H), 7.20-7.26 (m, 1 H), 7.28-7.34 (m, 4 H),
9.11 (s, I
H); LCMS- MS (ESI+) 285.78 (M+H).
1.2.m) Synthesis of ethyl3-phenyl-6H-thieno[2,3-b]pyrrole-5-carboxylate
N'v 'OEt O
-O ---
Q NaOEVEtOH. / I ~ OEt
S C~45 min, S N3
& O
~e'reft!~. \ O
I OEt 71`~ S N OEt
S N3 H
[02981 A) Ethy12-azido-3-(4-phenylthiophen-3-yl)acrylate was synthesized from
4-
formylthiophen-3-ylboronic acid (300 mg, 1.59 mmol) and was isolated after
purification by flash chromatography (Isco CombiFlash, 0-5% EtOAc/heptane)
(270
mg, 60%). 'H NMR (400 MHz, CDC13) 8 1.30 (t,.f--7.13 Hz, 3 H), 4.29 (q, J=7.13
Hz, 2 H), 6.89 (s, 1 H), 7.25 (d, J=3.27 Hz, I H), 7.27 (s, 1 H), 7.34-7.37
(m, 2 H)
7.38-7.48 (m, 3 H), 8.38 (d, J=3.22 Hz, I H).
[02991 B) The title compound was prepared from ethyl 2-azido-3-(4-
phenylthiophen-3-yl)acrylate and was purified by flash chromatography (Isco
CombiFlash, 0-10% EtOAc/heptane) to afford ethyl 3-phenyl-6H-thieno[2,3-
b]pyrrole-5-carboxylate (170 mg, 71%) as an off-white solid. 'H NMR (400 MHz,
CD3OD) S ppm 1.40 (t, J=7.13 Hz, 3 H), 4.35 (q, J--7.13 Hz, 2 H), 7.19 (s, 1
H), 7.27
(s, 1 H), 7.28-7.34 (m, I H), 7.41-7.47 (m, 2 H), 7.73-7.78 (m, 2 H); LCMS- MS
(ESI+) 272.0 (M+H).
99
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.2.n) Synthesis of ethyl3-(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-
carboxylate
0 o cl / ~ 0
Na~ -
CI OEt
---~ / ~ OEt
S 5~ ~t~~ ~ N3
70%
CI O CI /
mxylene, reflux
OEt
S N3 7696 s H OEt
103001 A) Ethyl-2-azido-3-(4-(4-chlorobenzyl)thiophene-3-yl)acrylate (230 mg,
60%) was prepared from 4-(4-chlorobenzyl)-thiophene-3-carbaldehyde (260 mg,
1.1
mmol) and was isolated after purification by flash chromatography (Isco
CombiFlash,
0-5% EtOAc/heptane). 'H NMR (400 MHz, CDC13) & ppm 1.37 (t, J=7.15 Hz, 3 H),
3.98 (s, 2 H), 4.32 (q, .>=7.13 Hz, 2 H), 6.80 (s, I H), 6.89 (d, J=3.12 Hz, 1
H), 7.08-
7.13 (m, 2 H), 7.24-7.29 (m, 2 H), 8.29 (d, J-3.12 Hz, 1 H); ' 3C NMR (100
MHz,
CDC13) S 163.36, 140.46, 137.94, 132.58, 132.20, 130.01, 129.58, 128.69,
125.19,
122.45, 116.63, 62.10, 34.69, 14.16; LCMS- MS (ESI+) 319.75 (M-N2).
103011 B) The title compound was prepared from ethyl-2-azido-3-(4-(4-
chlorobenzyl)thiophene-3-yl)acrylate and was purified by flash chromatography
(Isco
CombiFlash, 0-5% EtOAc/heptane) to afford ethyl 3-(4-chlorobenzyl)-6H-
thieno[2,3-
b]pyrrole-5-carboxylate (158 mg, 76%) as a straw-colored solid. 'H NMR (400
MHz,
CDC13) 8 ppm 1.37 (t, J=7.14 Hz, 3 H), 4.00 (s, 2 H), 4.35 (q, J=7.14 Hz, 2
H), 6.53
(t, .F--1.10 Hz, 1 H), 6.87 (d, J=1.92 Hz, 1 H), 7.18-7.23 (m, 2 H), 7.25-7.30
(m, 2 H),
9.16 (s, 1 H);13C NMR (100 MHz, CDC13) 8 161.47, 137.90, 137.79, 132.08,
131.64,
131.08, 130.06, 128.56, 128.04, 116.59, 106.77, 60.71, 35.25, 14.43; LCMS- MS
(ESI+) 319.72 (M+H).
1.2.o) Synthesis of ethyl6H-thieno[2,3-b]pyrrole-5-carboxylate
~~::O
[' COzEt t N3 2) m xylene reflux S ~
[0302] A) Ethy12-azido-3-(thiophen-3-yl)acrylate was synthesized from
thiophene-
3-carboxaldehyde (4.50 g, 40.Ommol) and isolated after purification by silica
gel
100
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
column chromatography (0 to 25% EtOAc in heptane over 30 min.). 2.8 g of the
purified intermediate were used in the next step.
103031 B) The title compound was prepared from ethyl 2-azido-3-(thiophen-3-
yl)acrylate and was purified by recrystallization from DCM to give ethy16FI-
thieno[2,3-b]pyrrole-5-carboxylate (1.0 g, 13%) as a white solid. Rf= 0.51
(50:50
heptane / EtOAc); 1 H NMR (400 MHz, CDC13) S ppm 1.40 (t, J--7.15 Hz, 3 H)
4.39
(q, J--7.14 Hz, 2 H) 6.92 (d,.f---5.37 Hz, 1 H) 7.01 (d, J=5.37 Hz, I H) 7.11
(d, J=1.90
Hz, I H) 9.48 (s, 1 H).
1.2.p) Synthesis of ethyl 3-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate
Br 1) ethyl azidoacetate Br N CO Et
/ \ NaOMe. EtOH 7 ~ ~ z
S CHO 2) m-xylene s
[03041 A) Ethy12-azido-3-(4-bromothiophen-2-yl)acrylate was synthesized from 4-
bromothiophene-2-carboxaldehyde (2.0 g, 10.47 mmol) and was obtained as a dark
brown residue after purification by silica gel column chromatography (heptane
and
EtOAc) (1.8 g).
103051 B) The title compound was prepared from ethyl 2-azido-3-(4-
bromothiophen-2-yl)acrylate and was purified by silica gel column
chromatography
to give ethyl 3-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate (27.6 mg, 0.102
mmol,
1%). tH NMR (400 MHz, acetone) S ppm 1.34 (t,.I--7.13 Hz, 2 H) 3.88 (s, 2 H)
4.34
(q, J=7.13 Hz, I H) 7.70 (t, ,f--1.34 Hz, 1 H) 7.86 (dd,.f--3.90, 1.51 Hz, I
H).
1.2.q) Synthesis of 2-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylate ethyl ester
and 3-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylate ethyl ester
1. Ethy1 azidoaoetate/ H a
F. Na , EtOH F
\ AO + / ~ ~O 0 C to r.t. / ` / COOEt / COOEt
g F 2. m-X~tene S +
F S
145 C
[03061 A) The intermediate acrylates (ethyl 2-azido-3-(4-fluorothiophen-2-
yl)acrylate and ethyl 2-azido-3-(5-fluorothiophen-2-yl)acrylate) were obtained
from a
mixture of 4-fluoro-thiophene-2-carboxaldehyde and 5-fluoro-thiophene-2-
carboxaldehyde (1.4 g, 10.8 mmol, 1 equiv). The mixture was purified by silica
gel
column chromatography (0 - 15% EtOAc in heptane over 20 min, tR of product: 3-
5
min.) to give a pale oil (0.37 g, 14%). 'H NMR (400 MHz, CD3CN) showed
signature peaks in the aromatic region from 6.5 - 7.8 ppm and an ethyl ester
pattern at
101
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
4.3 ppm and 1.3 ppm. '9F NMR (376 MHz, CD3CN) showed a strong signal at -
127.60 ppm.
[0307J B) A mixture of ethy12-azido-3-(4-fluorothiophen-2-yl)acrylate and
ethy12-
azido-3-(5-fluorothiophen-2-yl)acrylate (0.37 g) was dissolved in m-xylene (--
10 mL)
and heated at 145 C for 20 min in a capped 40-mL vial. The m-xylene was
evaporated in vacuo and the resulting residue was chromatographed over silica
gel (0
to 40% EtOAc in heptane over 30 min) to give two products: (a) 0.15 g of an
impure
pale oil with an RJ= 0.25 (10:90 EtOAc/heptane), which stained a bright violet
color
when developed using anisaldehyde and heat, which was further purified via
preparative HPLC using a Chromeleon purification system (methanol/0.1% formic
acid-1% acetonitrile mixture in water, 50 mm Dynamax C~18, 28 mL/min (initial
gradient of 20% methanol and increasing to 100% over 7 min) to give ethyl 2-
fluoro-
4H-thieno[3,2-b]pyrrole-5-carboxylate (48.9 mg, 3%). tR of product: 4.2 - 4.4
min.
'H NMR (400 MHz, CD3CN) S ppm 10.10 (s, 1 H), 6.98 - 7.05 (m, 1 H), 6.69 (dd,
J=2.05, 0.49 Hz, I H), 4.29 (q, J=7.09 Hz, 2 H), 1.33 (t,.,,--7.13 Hz, 3 H).
'9F NMR
(376 MHz, CD3CN) 8 ppm -122.18 (d, J=2.29 Hz, 1 F). (b) 10.5 mg of an impure
pale
oil with an Rf= 0.30 (10:90 EtOAc/heptane), which stained a bright red color
when
developed using anisaldehyde and heat, was further purified via preparative
HPLC as
described above (40% - 100% methanol over 7 min) to give ethy13-fluoro-4H-
thieno[3,2-b]pyrrole-5-carboxylate (5.4 mg, 0.3%). tR of product: 3- 3.4 min.
'H
NMR (400 MHz, CD3CN) S ppm 10.30 (s, 1 H), 7.06 (t, ,JI-i-2.05 Hz, 1 H), 6.90
(d,
J=2.54 Hz, 1 H), 4.32 (q, J=7.09 Hz, 2 H), 1.34 (t, J--7.10 Hz, 3 H). 19F NMR
(376
IvIHz, CD3CN) S ppm -144.16 (t, ,t--2.29 Hz, 1 F).
1.2.r) Synthesis of ethyl2-pbenethyl-6H-thieno[2,3-b]pyrrole-5-carboxylate
H
S g N C02Et
+ N3---'C02Et 1.) NaOEt, EtOH \ \ ~
CHO 2=) nrxylene, reflux
[0308] A) Ethy12-azido-3-(5-phenethylthiophen-3-yl)acrylate was prepared from
5-
phenethylthiophene-3-carboxaldehyde (0.106 g, 0.49 mmol) in EtOH (2.0 mL) and
chromatographed over silica gel (0 to 10% EtOAc in heptane over 20 min).
102
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0309] B) The title compound was synthesized from ethy12-azido-3-(5-
phenethylthiophen-3-yl)acrylate and purified by silica gel column
chromatography (0
to 25% EtOAc in heptane over 30 min) to give ethyl-2-phenethyl-6H-thieno[2,3-
b]pyrrole-5-carboxylate as yellow solid (0.013 g, 9%). tH 1VMR (400 MHz,
CDC13) S
(ppm) 9.09 (s, 1 H), 7.30 (m, 2 H), 7.22 (m, 3 H), 6.98 (d, J=1.95 Hz, 1 H),
6.66 (d,
J= 0.6 Hz, IH), 4.36 (q, .J=7.0 Hz, 2 H), 3.13 (m, 2H), 3.00 (m, 2H), 1.38
(t,.f--7. Hz,
3 H).
1.2.s) Synthesis of ethyl 2-fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylate
0
1) ethyl azidoacetate
H NaH, EtOH, -5 OC F /I O
F /S 2) mxylene, 1400C S H OEt
[0310] A) Ethyl 2-azido-3-(5-fluorothiophen-3-yl)acrylate was prepared from 5-
fluorothiophene-3-carbaldehyde (as a mixture with 3-thiophenecarboxaldehyde,
0.29
g, -2.2 mmol) in EtOH (8.5 mL) and used without purification in the next
reaction
step.
[0311] B) The title compound was synthesized from the above intermediate and
purified by preparative RP-HPLC (10-100% gradient 0.1% formic acid in H20 to
CH3CN over 10 min) to afford pure ethy12-fluoro-6H-thieno[2,3-b]pyrrole-5-
carboxylate as a white solid (0.030 g, 15%). 'H NMR (400 MHz, CD3OD) S(ppm)
6.99 (m, I H), 6.56 (m, 1 H), 4.31 (q,.,7---7.3 Hz, 2 H) 1.36 (t,.f--7.3 Hz, 3
H). '9F
NMR (282 MHz, CD3OD) S ppm -132.24 (1 F). LCMS m/e 214 (M+H).
1.2.t) Synthesis of methyl 3-fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylate
s s H
(/ N3~COzEt F 1500~ \ I N O~e
F~HO EtOHt Xylenes
OZNIe F
[03121 A) Methy12-azido-3-(4-fluorothiophen-3-yl)acrylate was prepared from 4-
fluorothiophene-3-carbaldehyde (Ozaki et al US 6,995,144 B2 (2006); 6.0 mmol
in
10 mL of DCM) and purified by chromatography (0.53g, 37%).
[0313] B) The title compound was synthesized from methyl 2-azido-3-(4-
fluorothiophen-3-yl)acrylate and purified by preparative RP-HPLC. The
acetonitrile
was removed under vacuum and the aqueous layer was extracted with methyl tert-
butyl ether (MTBE). The residue was then taken up in DCM and washed with
103
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
ammonium chloride solution, water, and brine. The organic layer was dried with
sodium sulfate, filtered, and the filtrate was evaporated to afford methyl 3-
fluoro-6H-
thieno[2,3-b]pyrrole-5-carboxylate (170 mg, 36%) as a pale-yellow solid. 'H
NMR
(400 MHz, CD3OD) S(ppm) 7.04 (d, J=5.5 Hz, 1 H), 6.90 (d, J=5.5 Hz, 1 H).
1.3. Synthesis of ethyl 2-(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-
carboxylate
Ci o
0 4Chlorobenmyl diloride
/ \ OEt im- / NH OEt
NH aCi, i oCE g
s 0
[0314] Under a N2 and at 0 C, to a 40-mL scintillation vial fitted with a
magnetic
stir bar was added aluminum chloride (0.7 g 5.28 mmol) and ethyl 6H-thieno[2,3-
b]pyrrole-5-carboxylate (0.61 g, 3.14 mmol, 0.9 equiv) in solution in 10 mL
dichloroethane (DCE). 4-Chlorobenzoyl chloride (0.92 g, 5.28 mmol) was then
added
at 0 C and stirring was continued for 2 h as the reaction was allowed to warm
to rt.
The reaction was cooled and was added to an ice-filled beaker. The aqueous
mixture
was extracted x 3 with EtOAc. The organic layers were combined, dried over
anhydrous sodium sulfate, filtered and evaporated in vacuo. The resulting
residue
was purified via ISCO Companion ( 0-30% gradient EtOAc / heptane over 30 min)
to
give ethyl 2-(4-chlorobenzoyl)-6H-thieno[2,3-b]pyrrole-5-carboxylate (0.34 g).
IH
NMR (400 MHz, CDC13) S ppm 1.42 (t, J=7.13 Hz, 3 H) 4.43 (q,.f--7.13 Hz, 2 H)
7.17 (d, J=1.81 Hz, 1 H) 7.50 (d, J--8.44 Hz, 2 H) 7.59 (s, 1 H) 7.77 - 7.86
(m, 2 H)
10.03 (s, 1 H).
NaBH4racb ~' / OEt
OEt
g NH THF/reflux \ NH
O S
[0315] Under a N2 and at rt, to a 40-mL scintillation vial fitted with a
magnetic stir
bar was added ethy12-(4-chlorobenzoyl)-6H-thieno[2,3-b]pyrrole-5-carboxylate
(0.203 g, 0.61 mmol) in solution in 5 mL in THF. A1C13 (0.22 g, 1.67 mmol,
2.75
equiv) and NaBH4 (0.116 g, 3.0 mmol, 5 eq.) are added in the same time. The
mixture was heated to reflux for 2 h. The reaction was cooled to rt and
solvent was
evaporated. The crude product was purified via ISCO Companion (0-30% EtOAc /
heptane over 30 min) to give ethy12-(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-
carboxylate (0.050 g). 'H NMR (400 MHz, CDC13) S ppm 1.39 (t, J=7.13 Hz, 3 H)
104
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
4.11 (s, 2 H) 4.37 (q, J=7.13 Hz, 2 H) 6.71 (s, 1 H) 7.00 (d, .P--1.76 Hz, I
H) 7.18 -
7.23 (m, 2 H) 7.27 - 7.32 (m, 2 H) 9.41 (s, 1 H).
1.4. Synthesis of methyl 6-methyl -4N-thieno[3,2-b]pyrrole-5-carboxylate
/
S S N
\ / \ (Me)2NH, CH2O \
N COOMe AcOH ~ COOMe
H
[0316] Under N2, to 9 mL of glacial acetic acid were added N,N-dimethylamine
(40%
aqueous solution) (437 mg, 9.94 mmol), formaldehyde (37% aqueous solution)
(283
mg, 9.90 mmol), and methyl4H-thieno[3,2-b]pyrrole-5-carboxylate (1.8 g, 9.94
mmol). The temperature was kept between 0- 5 C while the components were
added. The reaction mixture was heated at reflux for 1 h, and then allowed to
stand at
rt for 12 h. The mixture was poured onto 30 g of ice, and was brought to pH 10
by
careful addition of 10% sodium hydroxide. The temperature was not allowed to
exceed 10 C while the base was added. The gummy substance that precipitated
solidified when stored in the refrigerator overnight. The solid was collected
and dried
in a vacuum. It was recrystallized from petroleum ether (30 - 60 C) to yield
methyl
6-[(dimethylamino)methyl]-4H-thieno[3,2-b]pyrrole-5-carboxylate (1.65 g, 70%).
IH
NMR (400 NIHz, CDCl3) S ppm 2.36 (s, 6 H) 3.86 (s, 3 H) 3.89 (s, 2 H) 6.85 (d,
J=5.32 Hz, I H) 7.28 (d, J=5.32 Hz, 1 H) 9.84 (s, 1 H).
/ S
S N Me l, rt \ /\
\ ~ ~ NaBH4, MeOH N COOMe
N COOMe H
[0317] Under NZ,Hto methyl 6-[(dimethylamino)methyl]-4H-thieno[3,2-b]pyrrole-5-
carboxylate (0.34 g, 1.45 mmol) was added methyl iodide (1.48 mL, 2.37 mmol).
The
mixture was allowed to stand at rt for I h, and then the methyl iodide was
removed.
The resulting salt was dissolved in absolute methanol (5 mL). To this solution
was
carefully added sodium borohydride (1.23 g, 3.25 mmol) in small portions.
After the
addition was complete, the reaction mixture was diluted to a volume of 25 mL
by the
addition of 3N hydrochloric acid. The mixture was stored in the refrigerator
overnight,
and then the blue precipitate was dissolved in boiling methylcyclohexane, and
the
solution was treated with Darco (activated carbon) and filtered. The filtrate
was
evaporated and purified by chromatography over silica gel (0 to 40% EtOAc in
105
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
heptane over 30 min) to give methyl6-methyl-4H-thieno[3,2-b]pyrrole-5-
carboxylate
(0.12 g, 43%). 'H NMR (400 MHz, CDC13) S ppm 2.53 (s, 3 H) 3.91 (s, 3 H) 6.92
(d,
J=5.27 Hz, I H) 7.32 (d, J=5.32 Hz, 1 H) 8.81 (s, 1 H).
1.5. Synthesis of methyl 6-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylate
H TMSCHZN2 N
N CO2H MeOH , COZMe
g s
[0318] 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (3.0 g, 17.9 mmol) was
dissolved in
anhydrous MeOH (50.0 mL) and cooled to 0 C. A solution (2 M in hexanes,
Aldrich) of trimethylsilyldiazomethane (45 mL) was added in portions and the
yellow
color of the TMSCH2N2 remained. Stirring was continued for 10 min and then the
solvent was removed with N2 stream. The residue was chromatographed over
silica
gel (5%-40%, 30 min, EtOAc in heptane) to give methyl 4H-thieno[3,2-b]pyrrole-
5-
carboxylate(2.8 g, 86% yield). 'H N1VIR (400 MHz, CD3C1) S ppm 3.90 (s, 3 1-1)
6.95
(dd,.P--5.32, 0.78 Hz, I H) 7.13 (dd, J=1.88, 0.76 Hz, 1 H) 7.33 (d, .T--5.37
Hz, 1 H)
9.02 (br. s, 1 H).
;Sl~
o
I~ N CZMe SNaH ~. /\N/ Co2Me
s S
[0319] Methyl4H-thieno[3,2-b]pynole-5-carboxylate (2.8g, 15.45 mmol) was
dissolved in 150 mL anhydrous THF. NaH (3.0 g, 60% oil dispersion, 75 mmol)
was
added and the reaction stirred for 15 min. SEMCI [(2-trimethylsilyl)-
ethoxymethyl
chloride] (0.7 mL, 3.95 mmol) was added dropwise over 5 min. The reaction was
stirred 1 h at rt and then CAUTIOUSLY poured onto 25 g crushed ice with
stirring.
The aqueous was extracted with EtOAc, dried ( Na2SO4), filtered and evaporated
in
vacuo to give a green residue. The residue was chromatographed over silica gel
(EtOAc in heptane, 3%-10%, 3 h, TLC visualized with KMnO4 with heat) to give
methyl 4-(2-trimethylsilanyl-ethoxymethyl)-4H-thieno[3,2-b]pyrrole-5-
carboxylate
(3.85 g, 80% yield). 'H NMR (400 MHz, (CD3)2C0) S ppm -0.08 (s, 9 H) 0.84 (t,
J=7.83Hz,2H)3.54(t,J=7.88Hz,2H)3.83(s,3H)5.94(s,2H)7.21 - 7.25 (m, 1
H) 7.26 (s, 1 H) 7.55 (d, J=5.37 Hz, I H).
106
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
UOH
S S
N/ CO2Me EtOH,80OC N/ COzH
[0320] Methy14-(2-trimethylsilanyl-ethoxymethyl)-4H-thieno[3,2-b]pyrrole-5-
carboxylate (2.89 g, 9.27 mmol) was dissolved in 60 mL EtOH. A 2 M solution of
LiOH (46 mL) was added and the reaction was heated to 75 C for 30 min. EtOH
was
removed with a N2 stream. The residue was taken up in 300 mL water and
acidified
to pH 2 with conc. HC1 which gave a white precipitate. The precipitate was
extracted
into EtOAc. The solution was dried (Na2SO4), filtered and evaporated in vacuo
to
give 4-(2-trimethylsilanyl-ethoxymethyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic
acid
(2.57 g, 93% yield). 'H NMR (400 MHz, (CD3)2C0) S ppm -0.08 (s, 9 H) 0.77 -
0.91
(m, 2 H) 3.55 (t, 2 H) 5.96 (s, 2 H) 7.23 (d, J=5.37 Hz, 1 H) 7.31 (s, 1 H)
7.55 (d,
J=5.37 Hz, I H).
O 1. n-BuLi, -78 C 0
1 (C6H5S02)2NF
/ \
N/ COZH 2. TMSCH2N2 N N~ Co2Me
~ MeOH
F
103211 4-(2-Trimethylsilanyl-ethoxymethyl)-4H-thieno[3,2-b]pyrrole-5-
carboxylic
acid (1.9 g, 6.4 mmol) was dissolved in anhydrous THF (250 mL) and cooled to -
78
C. n-BuLi (1.6 M in hexanes, 12 mL, 19.2, 3 equiv) was added over 5 min and
stirred at -78 C for 60 min. A solution of NFSI (3.1 g, 9.6 minol, 1.5 equiv)
in 15
mL anhydrous THF was added over 15 min and the reaction was stirred at -78 C
for
5 h and then allowed to warm to rt ovemight. The reaction was cooled in an ice
bath,
quenched with 6N HCI, and then extracted with EtOAc and evaporated in vacuo to
give 5.5 g of dark residue. The residue was chromatographed over silica gel
(DCM in
EtOAc) to give a more pure residue. This residue was chromatographed via prep
reverse phase HPLC (RP-HPLC) to give 360 mg of the 2-fluoro isomer (2-fluoro-4-
((2-(trimethylsilyl)ethoxy)methyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic acid)
and a
separate mixture of starting material and 6-fluoro isomer (6-fluoro-4-((2-
(trimethylsilyl)ethoxy)methyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic acid).
This
latter mixture was converted to the corresponding methyl ester via TMSCH2N2.
The
107
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
mixture of esters was chromatographed over silica gel (EtOAc in heptane, 5%-
20%)
to give methyl 6-fluoro-4-((2-(trimethylsilyl)ethoxy)methyl)-4H-thieno[3,2-
b]pyrrole-
5-carboxylate (16 mg, 0.0485 mmol, 0.8% yield). 'H 1VMR (400 MHz, (CD3)2CO) 6
ppm -0.08 (s, 9 H) 0.80 - 0.87 (m, 2 H) 3.49 - 3.57 (m, 2 H) 3.87 (s, 3 H)
5.88 (s, 2 H)
7.29 (dd, J---5.32, 2.20 Hz, 1 H) 7.66 (d,.P--5.32 Hz, I H).
~
OI H
N/ CO2Me nBu4NF \ N~ CO2Me
`. Ethytenediamine ~\
S F DMF, 80 C S F
[0322] Methyl 6-fluoro-4-((2-(trimethylsilyl)ethoxy)methyl)-4H-thieno[3,2-
b]pyrrole-5-carboxylate (16 mg, 0.0485 mmol) was dissolved in 3 mL anhydrous
DMF. TBAF (1M THF, 0.485 mL, 10 equiv) and ethylenediamine (0.10 mL, 87.45
mg, 1.455 mmol, 30 equiv) were added, and the reaction was heated to 80 C for
1 h
and then allowed to cool to rt overnight. TLC (1/1 EtOAc in heptane,
visualized with
anisaldehyde and heat) indicated complete reaction. The product was
partitioned with
LiCI solution and EtOAc, dried (Na2SO4), filtered, evaporated in vacuo the
organic
layer to give a residue. The residue was passed through a 5 g silica gel
cartridge (1/1
EtOAc in heptane) to give methyl 6-fluoro-4H-thieno[3,2-b]pyrrole-5-
carboxylate (9
mg, 94% yield) as a white solid. The regiochemistry of fluorine was determined
via
NMR-NOE experiments. 'H NMR (400 MHz, (CD3)2C0) S ppm 3.86 (s, 3 H) 7.03
(dd, J=5.27, 2.29 Hz, 1 H) 7.55 (d, J=5.27 Hz, 1 H) 10.81 (br. s., 1 H). 19F
NMR (376
MHz, (CD3)2C0) S ppm -155.88 (dd,.P--27.47, 2.29 Hz, I F).
1.6. Synthesis of ethyl 4-bromo-6H-thieno[2,3-b)pyrrole-5-carboxylate
Br
S O TBAF crOEOEt N t
[0323] To a solution of ethy16H-thieno[2,3-b]pyrrole-5-carboxylate (0.06 g,
0.31
mmol) in dichloromethane (1 mL) was added TBAF (1M THF, 0.46 mL) followed by
NBS (0.07 g, 0.4 mmol). The resulting mixture was allowed to stir at 23 C for
16 h at
which time the entire reaction mixture was placed on a silica gel column.
Flash
column chromatography (20% EtOAc in hexanes) affords one major peak containing
108
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
a mixture of 4-bromo and 2,4-dibromo products. Separation of the desired
product
from the byproduct by RP-HPLC (10-100% gradient 0.1% formic acid in H20 to
CH3CN over 10 min) afforded ethy14-bromo-6H-thieno[2,3-b]pyrrole-5-carboxylate
(0.03 g, 35% yield).
1.7. Synthesis of ethyl4-bromo-6H-thieno[2,3-b]pyrrole-5-carboxylate
BOC O DIEA, O
/ ~ \ O DMAP O TBAF, NBS Br ~~ \
S N Et S N OEt S N OEt
H
tBuO O
tBuO O
103241 To ethyl 6H-thieno[2,3-b]pyrrole-5-carboxylate (0.12 g, 0.62 mmol)
dissolved in dichloromethane (4 mL) was added N,N-diisopropylethylamine
(DIPEA)
(0.32 mL, 1.85 mmol) followed by t-butyl dicarbonate (BOC2O) (0.20 g, 0.92
mmol)
and 4-(N,N-dimethylamino)pyridine (DMAP) (0.015 g, 0.12 mmol). The combined
reaction mixture was allowed to stir at 23 C for 3 h at which time the
reaction
mixture was transferred directly to a silica gel column. Flash column
chromatography
(20% ethyl acetate in hexanes) afforded the carbamate-protected intermediate 6-
tert-
butoxycarbonyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid ethyl ester in
quantitative
yield.
[0325] To 6-tert-butoxycarbonyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid
ethyl
ester (0.09 g, 0.31 mmol) as a solution in dichloromethane (1 mL) was added
TBAF
(1 M THF, 0.46 mL) followed by N-bromosuccinimide (NBS) (0.07 g, 0.4 mmol).
The
resulting mixture was allowed to stir at 23 C for 16 h, after which time the
entire
reaction mixture was placed directly on a silica gel column. Flash column
chromatography (20% ethyl acetate in hexanes) afforded 6-tert-butoxycarbonyl-2-
bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid ethyl ester (0.04 g, 36%
yield).
1.8. Synthesis of ethyl 3-chloro-4H-thieno[3,2-b]pyrrole-5-carboarylate
H H
Br N/ CO2Et CuCI, DMF C~ N/ CO2Et
S S
[0326] Ethyl 3-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylate (200 mg, 0.730
mmol)
was dissolved in 20 ml anhydrous DMF. Copper chloride (150 mg, 1.52 mmol, 2
equiv) was added, and the reaction was heated to 140 C for 16 h. The reaction
was
cooled, partitioned between water and EtOAc, and the organic layer was dried
(MgSO4), filtered, and evaporated in vacuo. Chromatography (silica gel,
109
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
heptane/ethyl acetate) yielded ethyl 3-chloro-4H-thieno[3,2-b]pyrrole-5-
carboxylate
(112 mg, 74% yield). 'H NMR (400 MHz, (CD3)2C(O)) S ppm 1.33 (t, J=7.13 Hz, 3
H) 4.31 (q, J=7.11 Hz, 2 H) 7.17 (s, I H) 7.39 (s, 1 H) 11.45 (br. s., 1 H).
1.9 Synthesis of ethyl 4-chloro-6H-thieno[2,3-b]pyrrole-5-carboxylate
CI
S O TBAF S~ O
OEt NCS OEt
The title compound was synthesized from ethyl 6hl-thieno[2,3-b]pyrrole-5-
carboxylate (0.20 g, 1.02 mmol) and NCS (0.17 g, 1.2 mmol) using the
halogenation
conditions to synthesize ethyl 4-bromo-6H-thieno[2,3-b]pyrrole-5-carboxylate.
Separation of the desired product by RP-HPLC (10-100% gradient 0.1% formic
acid
in H20 to CH3CN over 10 min) afforded ethy14-chloro-6H-thieno[2,3-b]pyrrole-5-
carboxylate (0.044 g, 19% yield). 'H NMR (400 MHz, CD3OD) S(ppm): 9.96 (br s,
1 H), 6.98 (d, J = 5.4Hz, 1 H), 6.92 (d, J = 5.4Hz, 1 H), 4.43 (q, J = 7.1 Hz,
2H), 1.43 (t,
J= 7.1Hz, 3H). 13C NMR (101 MHz, CD3OD) S(ppm): 161.1, 136.8, 131.3, 124.4,
123.5, 121.5, 116.5, 61.3, 14.6. LCMS m/e 230 (M+H).
1.10. Synthesis of Carboxylic Acids from Esters
[0327] The following compounds were synthesized via saponification of their
corresponding esters, for example according to General Procedure 2.
1.10.a) Synthesis of 3-methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (2)
H H
~~NC02Et NaOH CO2H
S MeOH S /
[0328] The title compound was synthesized from ethy13-methyl-4H-thieno[3,2-
b]pyrrole-5-carboxylate (94 mg, 1.1 mmol) according to General Procedure 2.
The
crude product was purified by silica gel chromatography to give 3-methyl-41Y-
thieno[3,2-b]pyrrole-5-carboxylic acid 2 (57 mg) in 100% purity (HPLC). LCMS
m/e
182 (M+H). 'H NMR (400 MHz, CD3OD) S(ppm): 7.04 (s, 1H), 6.94, (m, 1H), 2.32
(d, 3H).
110
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.10.b) Synthesis of 2-methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (3)
H H
N C02Et NaOH N C02Fi
/ / MeOH /
s s
[03291 The title compound was prepared from ethyl 2-methyl-4H-thieno[3,2-
b]pyrrole-5-carboxylate (250 mg, 1.2 mmol) according to General Procedure 2
and
was purified by silica gel chromatography to give 2-methyl-4H-thieno[3,2-
b]pyrrole-
5-carboxylic acid 3 (117 mg) in 100% purity (HPLC). LCMS m/e 182 (M+H). 'H
NMR (400 MHz, CD3OD) S(ppm): 6.98 (m, 1H), 6.68 (m, 111), 2.52 (d, 3H).
1.10.c) Synthesis of 2-chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (4)
H H
N C02Et NaOH N OCI / / MeOH /S
/
S CI
103301 The title compound was synthesized from ethyl 2-chloro-4H-thieno[3,2-
b]pyrrole-5-carboxylate (250 mg, 1.1 mmol) according to General Procedure 2
and
was purified by silica gel chromatography to give 2-chloro-4H-thieno[3,2-
b]pyrrole-
5-carboxylic acid 4(164 mg) in 100 % purity (HPLC). 'H NMR (400 MHz, CD3OD)
8 (ppm): 7.01 (m, 1 H), 6.97 (m, 1 H).
1.10.d) Synthesis of 2-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (5)
H H
N COZEt NaOH / \N/ COZH
gr S MeOH, reflux gr S
[03311 The title compound was prepared from ethyl 2-bromo-4H-thieno[3,2-
b]pyrrole-5-carboxylate (General Procedure 2) and was purified by silica gel
column
chromatography (25 to 100% EtOAc in heptane over 30 min) to give 2-bromo-4H-
thieno[3,2-b]pyrrole-5-carboxylic acid 5 as a light green solid in 97% purity
(HPLC)
(0.09 g, 30%). Rf= 0.06 (50:50 heptane / EtOAc); 'H NMR (400 MHz, (CD3)2S0) S
(ppm) 12.65 (s, 1 H) 12.04 (s, I H) 7.16 (s, 1 H) 6.99 (s, I H). LCMS m/e 246
(M+H).
111
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.10.e) Synthesis of 2,3-dibromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid
(6)
Br N / C02H
Br/S
[0332] The title compound was synthesized from ethyl 2,3-dibromo-4H-thieno[3,2-
b]pyrrole-5-carboxylate (0.158 g, 0.45 mmol) (General Procedure 2) and was
purified
by silica gel column chromatography (0-100% EtOAc /heptane) to give 2,3-
dibromo-
4H-thieno[3,2-b]pyrrole-5-carboxylic acid 6 as a light brown solid in 97%
purity by
HPLC (0.054 g, 38%). Rf= 0.07 (1:1 heptane/EtOAc); 'H NMR (400 MHz,
(CD3)2S0) S(ppm) 12.80 (s, I H) 12.55 (s, 1 H) 7.08 (s, I H). LCMS m/e 324
(M+H). Note:-
1.10.f) Synthesis of 6H-thieno[2,3-b]pyrrole-5-carboxylic acid (7)
H
KOH ; ~
\ MeOH, reflux N
S H S H
103331 The title compound was synthesized from ethyl 6H-thieno[2,3-b]pyrrole-5-
carboxylate (0.140 g, 0.72 mmol) according to General Procedure 2 and purified
by
silica gel column chromatography (0 to 100% EtOAc in heptane over 30 min) to
give
6H-thieno[2,3-b]pyrrole-5-carboxylic acid 7 as a white solid (9 mg, 7.5%). Rf=
0.15
(50:50 heptane / EtOAc) in 99% purity (HPLC). 'H NMR (400 MHz, CD3OD) S ppm
6.95 (dd,.P--5.42 Hz and.,'--8.0 Hz, 2 H) 7.01 (s, 1 H). LCMS m/e 168 (M+H).
1.10.g) Synthesis of 3-bromo-6H-thieno[2,3-bjpyrrote-5-carboxylic acid (8)
Br / S C0 NaO Br / CO2H
NH M8H S NH
[03341 The title compound was synthesized from ethyl 3-bromo-6H-thieno[2,3-
b]pyrrole-5-carboxylate (300 mg, 1.1 mmol) according to General Procedure 2.
The
crude product was purified by silica gel column chromatography to give 3-bromo-
6H-
thieno[2,3-b]pyrrole-5-carboxylic acid 8 (164 mg) in 100% purity (HPLC). 'H
NMR
(400 MHz, CD3OD) S(ppm): 6.96 (s, 1 H), 6.92 (s, 1 H).
112
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.10.h) Synthesis of 3-benzyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (9)
~\
NaOH, 96% EtOM O
\ O 7p C, 81% / \
S H OEt OH
[0335] The title compound was prepared from ethyl3-benzyl-6H-thieno[2,3-
b]pyrrole-5-carboxylate (167 mg, 0.585 mmol) according to General Procedure 2
and
was purified by flash chromatography (Isco CombiFlash, 0-100% EtOAc/heptane)
to
give 3-benzyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 9 (122 mg, 81%) as a
pale
yellow solid. 'H NMR (400 MHz, CD3OD) 8 ppm 4.00 (s, 2 H), 6.58 (t, ,T--1.00
Hz, 1
H), 6.79 (s, 1 H), 7.14-7.31 (m, 5 H); LCMS- MS (ESI+) 257.9 (M+H); HPLC (UV =
100%), (ELSD = 100%).
1.10.i) Synthesis of 3-phenyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (10)
O NaOH. 8596 EtOH 0
S H OEt 70"C. tH% S OH
(0336] The title compound was prepared from ethyl 3-phenyl-6lY-thieno[2,3-
b]pyrrole-5-carboxylate (165 mg, 0.61 mmol) according to General Procedure 2
and
was purified by flash chromatography (Isco CombiFlash, 0-100% EtOAc/heptane)
to
afford 3-phenyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 10 (120 mg, 81%) as
a
pale yellow solid. 'H NMR (400 MHz, CD3OD) 8 ppm 7.18 (s, 1 H), 7.27 (s, 1 H),
7.28-7.34 (m, I H), 7.44 (t, J=7.66 Hz, 2 H), 7.74-7.78 (m, 2 H); LCMS- MS
(ESI+)
244.0 (M+H); HPLC (UV = 100%), (ELSD = 100%).
1.10.j) Synthesis of 3-(4-chlorobenzyl)-4A-thieno[3,2-b]pyrrole-5-carboxylic
acid
(14)
H
G / N KOH CI ~ N C~
/ C~~ MeOH ~
S S
[0337] The title compound was synthesized from ethyl3-(4-chlorobenzyl)-4H-
thieno[3,2-b]pyrrole-5-carboxylate (170 mg, 0.53 mmol) according to General
Procedure 2. The crude product was purified by silica gel chromatography to
give 3-
(4-chlorobenzyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 14 in 100% purity
113
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
(HPLC). LC/MS: m/e 292 (M+II). 'H NMR (400 MHz, CD3OD) S(ppm): 7.25 (m,
4H), 7.06 (s, 1 H), 6.87 (m, IH), 4.04 (s, 2H).
1.10.k)Synthesis of 3-phenethyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (15)
H XOH H
N ~H N
C
/ Co,~ / COZH
s s
[03381 The title compound was synthesized from ethyl 3-phenethyl-4H-thieno[3,2-
b]pyrrole-5-carboxylate (188 mg, 0.63 mmol) according to General Procedure 2
and
was purified by silica gel column chromatography to give 3-phenethyl-4H-
thieno[3,2-
b]pyrrole-5-carboxylic acid 15 (118 mg, 69 %) in 95.5 % purity (HPLC). LCMS
m/e
272 (M+H). 'H NMR (400 MHz, CD3OD) S(ppm): 7.22 (m, 4H), 7.15 (M, 1H), 7.05
(s, I H), 6.92 (s, 1 H), 3.02 (m, 4H).
1.10.1) Synthesis of 3-(4-chlorophenethyl)-6H-thieno[2,3-b]pyrrole-5-
carboxylic
acid (16)
NaOH, 95 h EtOH 0
S N OEt 94 OC, 65% S
CI H CI OH
[03391 The title compound was synthesized from ethyl 3-(4-chlorophenethyl)-6H-
thieno[2,3-b]pyrrole-5-carboxylate (110 mg, 0.33 mmol) according to General
Procedure 2 and was purified by flash chromatography (Isco CombiFlash, 0-100%
EtOAc/heptane) to afford 3-(4-chlorophenethyl)-6H-thieno[2,3-b]pyrrole-5-
carboxylic acid 16 (66 mg, 65%) as an off-white solid. 'H NMR (400 MHz, CD3OD)
S ppm 2.93-3.03 (m, 4 H), 6.50 (s, 1 H), 7.01 (s, 1H), 7.12-7.17 (m, 2 H),
7.20-7.24
(m, 2 H); LCMS-MS (ESI+) 305.72 (M+H); HPLC (UV = 98%), (ELSD = 100%).
1.10.m) Synthesis of 2-phenethyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid
(18)
N~ CO2Et KOH N
~ C02H
MeOH 0--~ I
S
[0340] The title compound was synthesized from ethyl 2-phenethyl-4H-thieno[3,2-
b]pyrrole-5-carboxylate (290 mg, 0.97 mmol) according to General Procedure 2.
The
114
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
crude product was purified by silica gel chromatography and recrystallization
(EtOAc) to give 2-phenethyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 18 (70
mg) in
100% purity (HPLC). LC/MS: m/e 272 (M+H). 'H NMR (400 MHz, CD3OD) S
(ppm): 7.21 (m, 5H), 6.99 (d, 1H), 6.65 (dd, 1H), 3.14 (m, 2H), 2.99 (m, 2H).
1.10.n) Synthesis of 2-(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-carboxylic
acid (19)
ct
KOH / MeOH
/ OEt / OH
S H
S
NH
[0341] The title compound was prepared from ethy12-(4-chlorobenzyl)-6H-
thieno[2,3-b]pyrrole-5-carboxylate (50 mg, 0.15 mmol) according to General
Procedure 2. The crude product was purified by silica gel chromatography to
give 2-
(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 19 (9 mg) in 95 %
purity.
LC/MS: m/e 290 (M-H). 'H NMR (400 MHz, CD3OD) S ppm 4.13 (s, 2 H), 6.75 (s,
1 H), 6.94 (s, 1 H), 7.23 - 7.35 (m, 4 H).
1.10.o) Synthesis of 2-(4-chlorobenzyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic
acid
(20)
CI Ci
~ a NaOH
\ ~ COzEt MeOH COZH
S S
[0342] The title compound was prepared from ethyl 2-(4-chlorobenzyl)-4H-
thieno[3,2-b]pyrrole-5-carboxylate (42 mg, 0.13 mmol) according to General
Procedure 2. The crude product was purified by silica gel column
chromatography
and HPLC to afford 2-(4-chlorobenzyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic
acid 20
(12 mg) in 100% purity (HPLC). LC/MS: m/e 290 (M-H). 'H NMR (400 MHz,
CD3OD) S(ppm): 7.28 (m, 4H), 6.96 (d, 1 H), 6.73 (d, 1 H), 4.15 (s, 2H).
1.10.p) Synthesis of 3-(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-carboxylic
acid
(29)
ci / ci
aOH, 95% EIOH O
td
70 C, 73% /
H
S H OEt S H
[0343] The title compound was prepared from ethyl 3-(4-chlorobenzyl)-6H-
thieno[2,3-b]pyrrole-5-carboxylate (152 mg, 0.475 mmol) according to General
115
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Procedure 2 and was purified by flash chromatography (Isco CombiFlash, 0-100%
EtOAc/heptane) to give 3-(4-chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-carboxylic
acid 29 (102 mg, 73%) as a pale yellow solid. 'H NMR (400 MHz, CD3OD) 8 ppm
4.00 (s, 2 H), 6.62 (t, J--0.96 Hz, I H), 6.79 (s, I H), 7.23-7.30 (m, 4 H);
13C NMR
(100 MHz, CD30D) S 164.59, 140.11, 139.87, 133.12, 132.61, 132.37, 131.53,
129.55, 129.47, 117.51, 108.00, 36.19; LCMS- MS (ESI+) 291.72 (M+H); HPLC
(UV = 99.2%), (ELSD = 100%).
1.10.q) Synthesis of 3-bromo-4H-tbienot3,2-b]pyrrole-5-carboxylic acid (49)
Br N H
COzEt LjOH (2M) ~ N CO2H
~~s/ EtOH (80 C) ts\ /
[0344] The title compound was synthesized from ethyl 3-bromo-4hl-thieno[3,2-
b]pyrrole-5-carboxylate (27.6 mg, 0.102 mmol) according to General Procedure 2
to
give 3-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (15.6 mg, 62%) in 99%
purity (HPLC). 'H NMR (400 MHz, (CD3)ZCO) S ppm 7.22 (s, I H) 7.49 (s, I H)
11.33 (br. s., 0.05 H). LCMS m/e 246 (M+H).
1.10.r) Synthesis of 6-methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (52)
S
NaOH, MeOH N COOMe N H H
Q\7(COOH
[0345] The title compound was synthesized from methyl6-methyl-4H-thieno[3,2-
b]pyrrole-5-carboxylate (0.10 g, 0.5 mmol) according to General Procedure lA
and
was purified by silica gel column chromatography (0 to 100% EtOAc in heptane
over
30 min) to give 6-methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 52 as a
solid (19
mg, 20%) in 94.7% purity (HPLC). 'H NMR (400 MHz, CD3OD) S ppm 2.48 (s, 3
H) 6.93 (d, J=5.27 Hz, 1 H) 7.34 (d, J=5.27 Hz, 1 H). LCMS m/e 180 (M-H).
1.10.s) Synthesis of 6-tluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (54)
H H
N COZMe LOH N / C02H
g F EtOH. 80 OC S F
[03461 The title compound was synthesized from methyl 6-fluoro-4H-thieno[3,2-
b]pyrrole-5-carboxylate (9 mg, 0.0451 mmol) according to General Procedure 2
and
was purified using a 5 g silica gel cartridge (DCM/ EtOAc) to give 6-fluoro-4H-
thieno[3,2-b]pyrrole-5-carboxylic acid 54 (3.3 mg, 41%) in 100% purity (HPLC).
'H
116
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
NMR (400 MHz, CD3OD) S ppm 6.92 (dd, J=5.22, 2.25 Hz, 1 H) 7.35 (d,.F--5.27
Hz,
I H). '9F NMR (376 MHz, CD3OD) S ppm -158.76 (br. s., 1 F).
1.10.t) Synthesis of 2-fiuoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (55)
1. NaOH, MeOH
H 70 - 80 C H
IV COOH
COOEt 2. 10% v/v aq. HCI
F g F g
[0347] The title compound was synthesized from ethyl2-fluoro-4H-thieno[3,2-
b]pynrole-5-carboxylate (0.0489 g, 0.23 mmol) according to General Procedure 2
to
give 2-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 55 (38.6 mg, 91%) as a
cream-colored solid in 97.3% purity. LC/MS m/e 183.7 (M-H). 'H NMR (400 MHz,
CD3OD) S ppm 7.03 (s, I H), 6.64 (d, J=1.66 Hz, 1 H). '9F NMR (376 MHz,
CD3OD) S ppm -123.29 (d,.I--1.91 Hz, 1 F).
1.10.u) Synthesis of3-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (56)
1. NaOH, MeOH
H 70 - 80 C
F N / COOEt 2. 10% v/v aq. HCI F COOH
S S
[0348] The title compound was synthesized from ethyl3-fluoro-4H-thieno[3,2-
b]pyrrole-5-carboxylate (0.0054 g, 0.023 mmol) according to General Procedure
2
and was purified by preparative HPLC using a Chromeleon purification system
(30%
to 100% over 7 min methanol/0.1% formic acid-1% acetonitrile in water, 50 mm
Dynamax C-18, 28 mL/min) to give 3-fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic
acid 56 (0.8 mg, 17%) in 97% purity (HPLC, UV) and 100% (ELSD). LC/MS m/e
184 (M-H). Retention time of product: 2.5 - 2.8 min. 'H NMR (400 MHz, CD3OD)
5 ppm 7.01 (d, J--2.25 Hz, 1 H), 6.84 (d, J=2.49 Hz, 1 H). '9F NMR (376 MHz,
CD3OD) S ppm -145.73 (t,.P--2.29 Hz, 1 F).
117
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
1.10.v) Synthesis of 2-phenethyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid
(59)
H
N N CO2H
g C02Et
KOH ~ \
MeOH, reflux
[03491 The title compound was synthesized from ethyl 2-phenethyl-6H-thieno[2,3-
b]pyrrole-5-carboxylate (0.33 g, 1.2 mmol) according to General Procedure 2
and was
purified by silica gel column chromatography (25 to 100% EtOAc in heptane over
30
min) to give 2-phenethyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 59 (13 mg,
3 %)
as an off-white solid in 94 % purity (HPLC). 'H NMR (400 MHz, CD3OD) S(ppm)
7.21 (s, 1 H), 6.88 (s, 1 H), 6.61 (s, 1 H), 3.09 (m, 1 H), 2.97 (m, 1 H).
LCMS m/e
270 (M-H).
1.10.w) Synthesis of 4-bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (64)
Br Br
~ f \ O NaOH O
S N OEt MeOH S N OH
[0350] The title compound was synthesized from ethy14-bromo-6H-thieno[2,3-
b]pyrrole-5-carboxylate (0.03 g, 0.11 mmol) according to General Procedure 2,
and
was purified by RP-HPLC (10-100% gradient 0.1% formic acid in H20 to CH3CN
over 10 min) to give 4-bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 64 as
an
off-white solid (0.022 g, 78% yield). 'H NMR (400 MHz, CD3OD) S(ppm) 7.04 (d,
J-5.5 Hz, 1 H), 6.90 (d, ./=5.5 Hz, 1 H).
1.10.x) Synthesis of 2-bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (65)
Br / O NaOH Br / O
1\
S N OEt MeOH S H OH
tBuO
[0351] The title compound was synthesized from 6-tert-butoxycarbonyl-2-bromo-
6H-thieno[2,3-b]pyrrole-5-carboxylic acid ethyl ester (0.04 g, 0.11 mmol)
according
to General Procedure 2 and was purified by RP-HPLC (10-100% gradient 0.1%
formic acid in H20 to CH3CN over 10 min) afforded 2-bromo-6H-thieno[2,3-
b]pyrrole-5-carboxylic acid 65 as an off-white solid (0.020 g, 70% yield).
Note that
118
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
the tert-butyloxycarbonyl group was removed under the reaction conditions. 'H
NMR
(400 MHz, CD3OD) S(ppm) 7.07 (s, I H), 6.96 (s, 1 H).
1.10.y) Synthesis of 2-fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (66)
H H
S N COOEt Na0 - S N COOH
\ \ / MeOH F~
F
[0352] The title compound was synthesized from ethyl 2-fluoro-6H-thieno[2,3-
b]pyrrole-5-carboxylate (0.03 g, 0.14 mmol) according to General Procedure 2
and
was purified by RP-HPLC (10-100% gradient 0.1 % formic acid in H20 to CH3CN
over 10 min) to afford 2-fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 66
as a
light pink solid (0.019 g, 73%). 'H NMR (400 MHz, CD3OD) S(ppm) 6.98 (s, 1 H),
6.56 (d, J=2.6 Hz, 1 H). '9F NMR (282 MHz, CD3OD) S ppm -132.58 (1 F). LCMS
m/e 186 (M+H).
1.10.z) Synthesis of 3-chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (67)
CI N/ C02Et LiOH, EtOH CI N COZH
S S
[0353] The title compound was synthesized from ethy13-chloro-4H-thieno[3,2-
b]pyrrole-5-carboxylate (100 mg, 0.4353 mmol) according to General Procedure
2.
3-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 67 was isolated pure
without
purification (35.3 mg, 40% yield). 'H NMR (400 MHz, CD3OD) S ppm 7.08 (s, 1 H)
7.22 (s, I H).
1.10.aa) Synthesis of 3-fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (68)
NaOH
~1r1-co2Me 9:?--COH
F F
[0354] The title compound was synthesized from methyl 3-fluoro-6H-thieno[2,3-
b]pyrrole-5-carboxylate (53.7 mg, 0.2518 mmol) according to General Procedure
2
and was purified by RP-HPLC to afford 3-fluoro-6H-thieno[2,3-b]pyrrole-5-
carboxylic acid 68 (30 mg, 65%) as a slight pink solid. 'H NMR (300 MHz,
CD3OD)
S(ppm): 6.97 (d, J=0.48Hz, 1H), 6.43 (d, J=2.93, 1H), 4.9 (br s, 2H). '9F NMR
(282
119
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
MHz, CD3OD) S ppm: -134.56 (s, 1F). 13C NMR (75.4 MHz, CD3OD) S(ppm):
164.1, 152.5, 149.0, 105.9, 105.8, 98.9, 98.5. LCMS rn/e = 186 (M+H).
1.10.bb) Synthesis of 4-chloro-6H-thieno[2,3-b]pyrrole-5-carboxylate (69)
<K/ Et NaOH \ I ~ COZH
CI cl
The title compound was synthesized from ethyl 4-chloro-6H-thieno[2,3-b]pyrrole-
5-
carboxylate (0.04 g, 0.11 mmol) according to General Procedure 2 and was
purified
by RP-HPLC (10-100% gradient 0.1 % formic acid in H20 to CH3CN over 10 min).
The desired fraction was treated under vacuum to remove the acetonitrile, and
the
remainder was extracted with MTBE. The organic layer was washed with saturated
ammonium chloride, water, and brine; dried over sodium sulfate; filtered and
evaporated to give 4-chloro-6H-thieno[2,3-b]pyrrole-5-carboxylate 69 as a
white solid
(0.013 g, 37% yield). 'H NMR (400 MHz, CD3OD) S(ppm): 7.04 (d, J=5.5Hz, 1H),
6.94 (d, J=5.5Hz, 1 H). 13C NMR (101 MHz, CD3OD) S(ppm): 163.2, 138.1, 131.8,
124.6, 122.7, 116.8, 111.5. LCMS m/e = 202 (M+H).
1.11. Synthesis of 6-chloro-4H-thieno[3,2-blpyrrole-5-carboxylic acid (70)
N
COOH Ncs DMF / ` / COOH
S S CI
103551 To a 20 mL vial fitted with a magnetic stir bar at 25 C was added 4H-
thieno[3,2-b]pyrrole-5-carboxylic acid (0.1 g, 0.599 mmol, 1 equiv) and 2 mL
of
anhydrous DMF. N-chlorosuccinimide (NCS) (0.08 g, 0.599 mmol, 1 equiv) was
subsequently added and the reaction vessel contents stirred for 1 h at 25 C
before
heating the reaction vial to 55 C for 12 h. The reaction was then allowed to
cool to
C and was diluted with EtOAc (10 mL). The resulting mixture was then washed
with water (5 mL) x 3. The organic phase was dried over anhydrous MgSO4,
filtered,
and evaporated in vacuo. The resulting residue was dissolved in a small volume
of
25 methanol, filtered through a 0.45 micron syringe filter, and further
purified via
preparative HPLC using the Chromeleon purification system. A 0.1 % formic acid
/
1% acetonitrile mixture in water (aqueous phase) and methanol (no modifier
added -
organic phase) using a 50mm Dynamax HPLC C-18 column at 28 mL/min (initial
120
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
gradient of 40% methanol and increasing to 100% over 7 min) afforded the
desired 6-
chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 70 (5.5 mg, 5%) with purity
by
HPLC of 100% (UV) and 100% (ELSD). LC/MS m/e 199.9 (M-H). tR of product:
2.3 - 2.7 min. 'H NMR (400 MHz, CD3OD) S ppm 7.41 (d,./---5.32 Hz, 1 H), 6.97
(d,
J=5.27 Hz, 1 H).
1.12. Synthesis of 6-bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (71)
H
\ N/ COOH NBS. DMF_ COOH
S S Br
[0356] The title compound was synthesized from 4H-thieno[3,2-b]pyrrole-5-
carboxylic acid (0.1 g, 0.599 mmol, 1 equiv) and N-bromosuccinimide (NBS)
(0.107
g, 0.599 mmol, I equiv) according to the halogenation method reported in
Example
1.10 for the chlorination of 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (with
NCS) to
6-chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid, providing the desired 6-
bromo-
4H-thieno[3,2-b]pyrrole-5-carboxylic acid 71 (15.5 mg, 10.5%) with purity by
HPLC
of 100% (UV) and 100% (ELSD). LC/MS m/e 243.9 (M-H). tR of product: 2.5 - 2.8
min. 'H NMR (400 MHz, CD3OD) 8 ppm 7.42 (d,.T--5.32 Hz, 1 H), 7.01 (d,.T--5.32
Hz, 1 H).
Example 2
Synthesis of Fused Furan Pyrrole Analogs
2.1. Synthesis oflntermediate Aldehydes
2.1.a) Synthesis of 4-phenethyl-furan-2-carbaldehyde
0
O Cul, Pd(PhCN)2CI2 o
O + HP(t-butyl)~BF4, HN(!-Pr)z
Br dioxane. rt
/
[0357] A solid mixture of 4-bromo-2-furaldehyde (1.50 g, 8.57 mmol),
PdC12(PhCN)2 (197 mg, 0.514 mmol) and CuI (65.0 mg, 0.343 mmol) was flushed
under an argon stream for 1 min. A solution of HP(t-butyl)3BF4 (298 mg, 1.03
mmol)
and diisopropylamine (1.80 mL, 12.9 mmol) in dioxane (9 mL) was added to the
solid
mixture followed by phenylacetylene (1.13 mL, 10.3 mmol). The reaction was
allowed to stir at rt under an atmosphere of argon for 15 h before being
filtered
121
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
through a plug of silica gel with EtOAc. The solution was then concentrated in
vacuo
and chromatographed over silica gel to give 4-phenylethynyl-furan-2-
carbaldehyde as
a colorless oil (1.54 g, 92%). Rj= 0.35 (1:9 heptane / EtOAc); 'H NMR (400
MHz,
CDC13) S ppm 9.68 (d, J=0.5 Hz, 1 H) 7.90 (s, 1 H) 7.48 - 7.55 (m, 2 H) 7.35 -
7.40
(m, 3 H) 7.33 (d, J=0.7 Hz, 1 H).
0
\ / ~o o/ O
H2, Pd/Carbon
MeOH
[0358] To a solution of 4-phenylethynyl-furan-2-carbaldehyde (1.54 g, 7.84
mmol)
in MeOH was added Pd/C (154 mg, 10% Pd by weight). A vacuum was applied to
the reaction mixture and back filled (x4) with H2. The reaction was then
allowed to
stir at rt for 14 h under an atmosphere of H2 before being filtered through a
plug of
Celite with EtOAc. The reaction was then concentrated in vacuo to give 4-
phenethyl-furan-2-carbaldehyde as a colorless oil (1.53 g, 97%). 'H NMR (400
MHz, CDC13) S ppm 9.59 (d,.I--0.6 Hz, 1 H) 7.40 (d, J=0.8 Hz, 1 H) 7.28 - 7.34
(m, 2
H) 7.20 - 7.26 (m, I H) 7.14 - 7.20 (m, 2 H) 7.05 (d, J'--0.6 Hz, 1 H) 2.87 -
2.94 (m, 2
H) 2.78 - 2.85 (m, 2 H).
2.1.b) Synthesis of 5-benzyl-furan-2-carbaldehyde
H B O O,P Pd(OAc)2 , PPh3, K3PO4 O/ O
HO MeCN, f-PrOH, 80 C
[0359] The title compound was synthesized from 5-formylfuran-2-ylboronic acid
(0.80 g, 5.7 mmol) and benzyl diethyl phosphate (1.5 g, 6.3 mmol) using the
same
conditions used to synthesize 4-(4-chlorobenzyl)thiophene-2-carbaldehyde.
Purification by flash chromatography yielded 5-benzyl-furan-2-carbaldehyde as
a
brown solid (0.37 g, 65%). 'H NMR (400 MHz, CDC13) 8 ppm 9.56 (s, 1 H) 7.29 -
7.38 (m, 3 H) 7.24 - 7.28 (m, 2 H) 7.17 (d, J=3.5 Hz, 1 H) 6.19 (d,.P--3.6 Hz,
1 H)
4.07 (s, 2 H).
122
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.1.c) Synthesis of 4-benzyl-furan-2-carbaldehyde
\ / ~ O 0 PPh,, Pd(OAc)2 O
O-B + O P-0KP0' / \ O
~O 2:1 MeCN / IPA -
[0360] The title compound was synthesized from 5-formylfuran-3-boronic acid
pinacol ester (878 mg, 3.95 mmol), and benzyl diethyl phosphate (1.25 g, 5.14
mmol)
using the same conditions used to synthesize 4-(4-chlorobenzyl)thiophene-2-
carbaldehyde, with the exception that triphenylphosphine and Pd(OAc)2 were
dissolved in 2:1 CH3CN/isopropyl alcohol. Purification by flash chromatography
yielded 4-benzyl-furan-2-carbaldehyde as a white solid (300 mg, 41%). 1H NMR
(400 MHz, CDCI3) S ppm 9.56 (s, 1 H) 7.29 - 7.38 (m, 3 H) 7.24 - 7.28 (m, 2 H)
7.17
(d,.T--3.5 Hz, 1 H) 6.19 (d, J=3.6 Hz, 1 H) 4.07 (s, 2 H).
2.1.d) Synthesis of 4-vinylfuran-2-carbaldehyde
,OBu 3._CHO
~BOB- K3PO4, TPP
Br Pd(OAc)2, DMF,
100 C, (37%)
[0361] The title compound was synthesized from 4-bromo-furan-2-carbaldehyde
(1.1 g, 6.29 mmol) and vinylboronic acid dibutyl ester (1.67 mL, 7.54 mmol)
using
the same conditions used to synthesize 4-(4-chlorobenzyl)thiophene-2-
carbaldehyde,
with the exception that the reaction was run in DMF (20 mL). Purification by
flash
chromatography (0-30% EtOAc in heptane) provided 4-vinylfuran-2-carbaldehyde
as
an orange oil; Yield 282 mg (37%). 'H NMR (400 MHz, CDC13) S ppm 5.31 (dd,
J=10.88, 0.93 Hz, 1 H), 5.61 (dd,J=17.57, 0.54 Hz, 1 H), 6.56 (dd,.P--17.55,
10.91
Hz, 1 H), 7.37 (s, I H), 7.67 (s, I H), 9.66 (d, J=0.59 Hz, I H).
2.1.e) Synthesis of 4-cyclopropylfuran-2-carbaldehyde
OH Q
O CHO ~~OHK3~a. POY3 SYCHO
~ / -
Br Pd(OAc)2, totuene/HzO,
100 C, (31%)
[0362] The title compound was synthesized from 4-bromo-furan-2-carbaldehyde
(300 mg, 1.71 mmol) and cyclopropylboronic acid (171 mg, 1.99 mmol), using the
123
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
conditions to synthesize 4-(4-chlorobenzyl)thiophene-2-carbaldehyde, with the
exception that the reaction was run in toluene (7.5 mL) and water (0.5 mL),
and
triphenylphosphine was replaced with tricyclohexylphosphine (48 mg, 0.17
mmol).
Purification by flash chromatography (0-60% EtOAc in heptane) provided 4-
cyclopropylfuran-2-carbaldehyde as an orange oil 72 mg (31%). 'H NMR (400 MHz,
CDC13) S ppm 0.55-0.61 (m, 2 H), 0.90-0.97 (m, 2 H), 1.69-1.77 (m, 1 H), 7.00
(d,
./'--0.78 Hz, 1 H), 7.49 (d, J=0.59 Hz, 1 H), 9.58 (d, .t=0.49 Hz, I H).
2.1.f) Synthesis of 4-isopropylfuran-2-carbaldehyde
0 \ / CHO AICI_ CI 0 CHO
CSz,1696
[0363] To a suspension containing aluminium chloride (24 g, 180 mmol) in 100
mL
of CS2 was added 2-furaldehyde (9.8 mL, 156 mmol). To this mixture was added
dropwise isopropyl chloride (14.3 mL, 156 mmol), and the resulting mixture
stirred at
rt for 24 h. The dark mixture was carefully poured into a vigorously stirred
250 g of
ice, and then extracted with ether (5 x 100 mL). The combined organic layers
were
washed with water, brine, dried (NazSO4), filtered through a pad of silica
gel, and
concentrated. The residue was purified by flash chromatography (0-5% EtOAc in
heptane) to give 4-isopropylfuran-2-carbaldehyde as an orange oil (NMR purity -
85%): Yield 3.5 g (16%). 'H NMR (400 MHz, CDC13) S ppm 1.25 (d, J=6.88 Hz, 6
H), 2.80-2.91 (m, I H), 7.16-7.18 (m, I H), 7.47 (q, .t--0.91 Hz, I H), 9.61
(d,.P--0.59
Hz, I H).
2.1.g) Synthesis of (Z)-4-(prop-l-enyl)furan-2-carbaldehyde
Br
/ ~ /B(OH)' / ~
O HO Pd(OAc)2, Ph3P O CHO
K3P04, DMF
100 C
16 hr
[0364] The title compound was synthesized from 4-bromo-furan-2-carboxaldehyde
(1.1 g, 6.3 mmol, 1 equiv) and cis-propene boronic acid (0.65 g, 7.5 mmol, 1.2
equiv)
using the conditions to synthesize 4-(4-chlorobenzyl)thiophene-2-carbaldehyde,
with
the exception that the reaction was run in DMF (20 mL). The resulting residue
was
purified via ISCO Companion (0-25% EtOAc / heptane over 30 min, retention time
of
product: 23 - 26 min) to give (Z)-4-(prop-l-enyl)furan-2-carbaldehyde (0.4130
g,
124
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
48% yield). LC/MS m/e 136.8 (M+H). 'H NMR (400 MHz, CD3CN) S(ppm): 9.59
(d,.I--0.63 Hz, I H), 7.83 (s, 1 H), 7.42 (s, I H), 6.23 (dd,,I--11.40, 1.68
Hz, 1 H),
5.79 - 5.89 (m, I H), 1.87 (dd, J=7.10, 1.78 Hz, 3 H).
2.1.h) Synthesis of 4-(trifluoromethyl)furan-2-carbaldehyde
Br
O NBSIAIBN. CCI4 O
\ / reflUx \ /
F3 F3C
[03651 A solution of 2-methyl-4-trifluoromethyl-furan (J. Heterocyclic
Chemistry
1970, 7, 269-272) (340 mg, 2.26 mmol), N-bromosuccinimide (423 mg, 2.38 mmol)
and azobisisobutyronitrile (19 mg, 0.11 mmol) in carbon tetrachloride (10 mL)
was
refluxed for 1.5 h, then allowed to cool to rt and filtered through a cotton
plug. The
solvent was evaporated to give 2-(bromomethyl)-4-(trifluoromethyl)furan as an
orange oil (508 mg, 98%). The product was pure enough by proton NMR that no
further purification was necessary. 'H NMR (400 MHz, CDC13) S ppm 4.46 (d,
J=0.44 Hz, 2 H), 6.56 (d, .V0.49 Hz, I H), 7.77 (m, I H).
Br
/oJ 1.) HMTA, H20, reflux, 1h 0 CHO
2.) conc. HCI, reflux, lh
i i
F3C F3C
[0366] A mixture of 2-bromomethyl-4-trifluoromethyl-furan (500 mg, 3.57 mmol),
hexamethylenetetramine (HMTA) (637 mg, 4.54 mmol) and water (2.6 mL) were
placed in a 50 mL pear-shaped flask equipped with a vigreaux column atop of
which
is attached to dry-ice condenser chilled at -78 C. The mixture was heated at
reflux for
1 h, and then treated with concentrated HCl (1.7 mL). Reflux was maintained
for an
additional 1 h before the reaction was cooled to rt, diluted with water and
extracted
with DCM (4 x 50 mL). The combined organic extracts were washed with water,
brine, dried (Na2SO4) and carefully concentrated to give 4-
(trifluoromethyl)furan-2-
carbaldehyde. 'H NMR (400 MHz, CDC13) S ppm 7.37 (m, 1 H), 8.01 (m, I H), 9.74
(d, J=0.54 Hz, I H).
125
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.1.i) Synthesis of (E)-4-styrylfuran-2-carbaldehyde
R /
Br
\ B(OH)2
/O CHO O Pd(ORc)2, TPP 0 CHO
K3PO,~ . DMF
100 C
18 hr
[0367] The title compound was synthesized from 4-bromo-furan-2-carboxaldehyde
(1.1 g, 6.3 mmol, I equiv) and trans-phenylvinyl-boronic acid (1.4 g, 9.4
mmol, 1.5
equiv) using the conditions used to synthesize 4-(4-chlorobenzyl)thiophene-2-
carbaldehyde, with the exception that the reaction was run in DMF (25 mL). The
resulting residue was purified via ISCO Companion ( 0-30% EtOAc / heptane) and
preparative HPLC using the Chromeleon purification system (0.1% formic acid /
1%
acetonitrile mixture in water (aqueous phase) and methanol (no modifier added -
organic phase) using a 50 mm Dynamax HPLC C-18 column at 28mL/min (initial
gradient of 40% methanol and increasing to 100% over 7 min)) afforded a clean
product, retention time of product: 3.4 - 3.6 min. Amount of (E)-4-styrylfluan-
2-
carbaldehyde isolated: 89.1 mg (7% yield). 'H NMR (400 MHz, CD3CN) S(ppm):
9.62 (d, J=0.59 Hz, I H), 7.91 (s, 1 H), 7.63 (d, J=0.63 Hz, 1 H), 7.50 - 7.55
(m, 2 H),
7.35 - 7.42 (m, 2 H), 7.26 - 7.32 (m, 1 H), 7.08 (s, 2 H).
2.1.j) Synthesis of 4-methyl-2-furaldehyde
/ ~ n-BuLi (1.6 M), -78 C_
/ \
O CO2H DMF, THF OHC O CO2H
[0368) Under N2, a solution of 3-methyl-2-furoic acid (2.0 g, 15.9 mmol) in
THF
(80 mL) was cooled to -78 C and n-BuLi (1.6 M in hexane) (20.8 mL, 33.3 mmol,
2.1 equiv) was added dropwise. The mixture was kept for 30 min at -78 C, then
a
solution of DMF (6.11 mL, 79.4 mmol, 5 equiv) in THF (20 mL) was added. After
being stirred for 3 h at -78 C, the reaction mixture was allowed to warm to
rt. The
reaction was quenched with saturated aqueous ammonium chloride then the
reaction
mixture was partitioned between water and ether. The ether layer was washed
with
water, and then dried over sodium sulfate, filtered, and the solvent was
evaporated.
The residue was purified by chromatography over silica gel (0 to 30% EtOAc in
heptane over 30 min) to give 5-formyl-3-methyl-2-furoic acid (0.9 g, 37%). 'H
NMR
(400 MHz, CD3OD) 5 ppm 2.39 (s, 3 H) 7.29 (s, 1 H) 9.67 (s, I H).
126
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
~ ~ Cu / Quinoline ~ ~
OHC O CO2H Distilation at 260 C OHC O
[0369] Under N2, 5-formyl-3-methyl-2-furoic acid (0.83 g, 0.54 mmol) was
heated
in distillation apparatus at 250-260 C in presence of copper (0.17 g, 0.27
mmot, 0.5
equiv) and quinoline (1.5 mL). After 45 min, the system was cooled down and
the
distillate gave 4-methyl-2-furaldehyde (0.32 g, 54%). 'H NMR (400 MHz, CDC13)
S
ppm 2.04 - 2.18 (m, 3 H) 7.09 (s, 1 H) 7.46 (d, J=0.78 Hz, 1 H) 9.45 - 9.71
(m, 1 H).
2.1.k) Synthesis of 4-fluorofuran-2-carbaldehyde
~ 3( rrBuLi, CBr2F2 J( F F
Si' \ Si/ \
Br
THF, -78 C 6
[03701 To a solution of tert-butyl-dimethyl-prop-2-ynyloxy-silane (11.6 g,
6.78
mmol) in dry THF (190 mL) was added nBuLi (46.6 mL, 1.6 M solution in hexane)
dropwise (via an addition funnel) over 30 min at 0 C under N2. The reaction
mixture
was stirred at rt for 1.5 h before being cooled to -78 C. Then, CF2Br2 (18.8
mL, 20.3
mmol) was added dropwise over 30 min. After stirring for 2.5 h at -78 C, the
reaction
mixture was quenched with a saturated solution of NH4C1 and was extracted with
ether. The combined organic extracts were washed with brine, dried over
anhydrous
Na2SO4, filtered, and concentrated. Vacuum distillation (0.35 - 0.7 Torr)
provided (4-
bromo-4,4-difluoro-but-2-ynyloxy)-tert-butyl-dimethyl-silane (15.4 g, 76%
yield) as a
yellow liquid (55-70 C): 'H NMR (400 MHz, CDC13) 50.15 (s, 6 H), 0.93 (s, 9
H),
4.46 (t, J= 4.08 Hz, 2 H); 19F NMR (376.19 MHz, CDC13) 5-33.01 (t, J = 4.1 Hz,
2F).
F F F F
F F 37% CHyOaq, In \
-Si -Si +
Br HO ~
i / THFMZO O ~ OH OH
[0371] To a stirred solution of (4-bromo-4,4-difluoro-but-2-ynyloxy)-tert-
butyl-
dimethyl-silane (9.0 g, 30.1 mmol) and HCHO (37 wt % solution in water, 3.36
mL,
45.1 mmol) in THF/H20 (38.6 mL, 4/1, v/v) was added indium power (4.14 g, 36.1
mmol) at rt. After stirring vigorously for 22 h, the reaction mixture was
filtered
through Celite , and the filter cake was washed sequencially with NH4C1
solution
and EtOAc. After separation of the layers, the aqueous layer was extracted
with
127
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
EtOAc, and the combined organic extracts were washed with brine, dried over
anhydrous Na2SO4, filtered, and concentrated. The residue was purified by
flash
chromatography on silica gel eluting with 0-100% EtOAc in heptane to afford 5-
(tert-
butyldimethylsilyloxy)-2,2-difluoropent-3-yn-l-ol (3.3 g, 44%, light pale oil)
and
free propargyl alcohol 4,4-difluoropent-2-yne-1,5-diol (0.85 g 21 %, clear
pale oil).
Silylated alcohol 5-(tert-butyldimethylsilyloxy)-2,2-difluoropent-3-yn-l-ol:
'H NMR
(400 MHz, CDC13) 8 0.14 (s, 6 H), 0.92 (s. 9 H), 3.88 (t, J = 12.23, Hz, 2 H)
4.41 (t, J
= 4.47, 2 H); 19F NMR (376.19 MHz, CDC13) 5 -96.15 (tt, J = 12.21, 4.29, IF).
~F F AgN03 --Y O
Si~\
p ,
OH THF
F F
103721 AgNO3 (31 mg, 0.184 mmol) was added to a solution of 5-(tert-
butyldimethylsilyloxy)-2,2-difluoropent-3-yn-l-ol (0.46 g, 1.84 mmol) in THF
(18
mL) under N2. The resulting mixture was then refluxed for 2.5 h, cooled to rt
and
diluted with NH4C1 solution. The layers were separated and the aqueous phase
extracted with ethyl acetate (3 x 30 mL). The combined organic extracts were
washed
with water, brine, dried (Na2SO4), filtered and concentrated to a light oil
tert-
butyl((4,4-difluoro-4,5-dihydrofuran-2-yl)methoxy)dimethylsilane that was used
as is
without further purification. 'H NMR (400 MHz, CDC13) S 0.11 (s, 6 H), 0.93
(s, 9
H), 4.24 (tt, J = 3.69, 0.63 Hz, 2 H), 4.44 (td, J = 17.29, 0.46, Hz, 2 H),
5.29 (t, J
1.32, 1 H); 19F NMR (376.19 MHz, CDCl3) 5 -83.15 (tt, J = 17.28, 3.67, IF).
O
1 O
sio, 'Si OZ
F F
0i ~ ~
F
[0373] tert-Butyl((4,4-difluoro-4,5-dihydrofuran-2-yl)methoxy)dimethylsilane
was
diluted with DCM and treated with silica gel (5 g Si02/lg of compound). The
flask
was swirled around to ensure an even mix, DCM was allowed to air dry and the
flask
left at rt overnight. The silica gel was transferred to a fritted funnel and
eluted with
DCM until no more product could be detected by TLC. The filtrate was
concentrated
to provide an orange oil tert-butyl((4-fluorofuran-2-
yl)methoxy)dimethylsilane. 'H
NMR (400 MHz, CDC13) S 0.09 (s, 6 H), 0.91 (s, 9 H), 4.55 (br s, 2 H), 6.20
(m, 1
128
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
H), 7.31 (dd, J= 5.03, 0.63 Hz, I H); 19F NMR (376.19 MHz, CDC13) 8 -170.53
(dd,
J= 4.95, 1.32, IF).
O TBAF HO
~Si-O \ /
F
F
[0374] A solution of TBAF in THF (1 M, 2.5 mL, 2.54 mmol) was added to a
solution of tert-butyl-(4-fluoro-furan-2-ylmethoxy)-dimethyl-silane (0.39 g,
1.69
mmol) in THF (10 mL). After stirring for 4 h, the reaction was diluted with
1VH4C1
solution and extracted with EtOAc (3 x 50 mL). The combined extracts were
washed
with brine, dried (Na2SO4) filtered, and concentrated. Purification by flash
chromatography on silica gel 0-50% EtOAc/heptane afforded (4-fluorofuran-2-
yl)methanol (190 mg, 97%) as an orange oil: 'H NMR (400 MHz, CDC13) S 4.54 (s,
2
H), 6.27 (m, I H), 7.34 (dd, J= 5.08, 0.83 Hz, I H); '3C NMR (100 MHz, CDC13)
S
57.36 (d, J=1.3 Hz), 100.39 (d, J-- 19.8 Hz), 125.69 (d, ,F--29.4 Hz), 152.8
(d, J=7.5
Hz), 153.26 (d, J=249.6 Hz); '9F NMR (376.19 MHz, CDC13) 8 -170.17 (ddd, J
5.11, 1.49, 1.32 Hz, 1 F).
O O
HO MnOi O~ q\/
F F
103751 Activated Mn02 (1.68 g, 16.4 mmol, 85% pure) was added to a solution of
(4-fluorofuran-2-yl)methanol (0.19 g, 1.64 mmol) in DCM (15 mL). After
stirring the
heterogeneous mixture at rt overnight, an additional 500 mg of Mn02 was added.
The
reaction was continued for an additional h, then the oxidant was filtered off
over
Celite and the cake washed with DCM. The solvent was carefully stripped off
at 5
C to a residual volume of about 5 mL. This orange solution of 4-fluorofuran-2-
carbaldehyde in DCM was used without further purification: 'H 1VMR (400 MHz,
CDC13) S 7.10 (dd, J= 1.46, 0.98, 1 H); 7.63 (dd, J = 5.27, 0.49, 1 H), 9.59
(m, 1 H);
19F NMR (376.19 MHz, CDC13) 5 -166.04 (d, J = 5.28 Hz, 1 F).
129
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.2. Synthesis otEsters -
[0376] The following ethyl esters were synthesized from the indicated aldehyde
according to General Procedure 1 A (to yield an intermediate acrylate)
followed by
General Procedure 1B.
2.2.a) Synthesis of ethyl 4H-furo[3,2-b]pyrrole-5-carboxylate
[0377] The title compound was synthesized from 2-furaldehyde (1.44 g, 15.0
mmol)
and was purified by silica gel column chromatography (0 to 25% EtOAc in
heptane
over 25 min) to give ethy14H-furo[3,2-b]pyrrole-5-carboxylate as a pink solid
(0.330
g, 12%). Rf = 0.42 (50:50 heptane / EtOAc); 'H NMR (400 MHz, CDC13) S(ppm)
8.63 (s, I H) 7.53 (s, I H) 6.81 (s, I H) 6.47 (s, 1 H) 4.36 (q, J=7.1 Hz, 2
H) 1.38 (t,
J=7.1 Hz, 3 H).
2.2.b) Synthesis of ethyl3-phenethyl-4H-furo[3,2-b]pyrrole-5-carboxytate
0 0
\ / \ O /COzEt NaOEt i /
+ N3
N, EtOH d
O O
\ / N m-xylene /N~
Y
~ reflux H O
c d
[0378] A) Ethyl 2-azido-3-(4-phenethyl-furan-2-yl)-acrylate was synthesized
from
4-phenethyl-furan-2-carbaldehyde (1.53 g, 7.64 mmol) to give a colorless oil
(0.718 g,
30%) after purification by silica gel column chromatography. 'H NMR (400 MHz,
CDC13) S ppm 7.28 - 7.34 (m, 2 H) 7.17 - 7.25 (m, 4 H) 6.99 (s, 1 H) 6.81 (s,
I H)
4.35 (q, J=7.1 Hz, 2 H) 2.86 - 2.94 (m, 2 H) 2.73 - 2.80 (m, 2 H) 1.38 (t,.P--
7.1 Hz, 3
H).
[0379] B) The title compound was prepared from ethyl 2-azido-3-(4-phenethyl-
furan-2-yl)-acrylate and was purified by silica gel column chromatography to
give
ethyl 3-phenethyl-4H-furo[3,2-b]pyrrole-5-carboxylate as a white solid (613
mg, 94
%). 'H NMR (400 MHz, CDC13) S ppm 7.48 (br s., 1 H) 7.28 - 7.39 (m, 4 H) 7.23 -
7.26 (m, 2 H) 6.67 (d, J=1.8 Hz, 1 H) 4.30 (q, J=7.1 Hz, 2 H) 2.90 - 2.99 (m,
4 H)
1.36 (t, J=7.2 Hz, 3 H).
130
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.2.c) Synthesis of ethyl 2-benzyl-4H-furo[3,2-b]pyrrole-5-carboxylate
O 1 O Et02C) NaOEt a 1 \O/ \
Nl EtOH, -10 C ~~ N3 p
m-xylene c:rO-lJ
NS p reflux N
H 0
[03801 A) Ethy12-azido-3-(5-benzyl-furan-2-yl)-acrylate was prepared from 5-
benzyl-furan-2-carbaldehyde (295 mg, 1.58 mmol) and was purified by silica gel
column chromatography to give a brown oil (35.0 mg, 7 %). 'H NMR (400 MHz,
CDC13) S ppm 7.30 - 7.36 (m, 3 H) 7.24 (d,.P--0.6 Hz, 2 H) 7.09 (dd, J=3.4,
0.4 Hz, 1
H) 6.21 - 6.24 (m, I H) 6.05 - 6.08 (m, 1 H) 4.35 (q,.P--7.1 Hz, 2 H) 4.05 (s,
2 H) 1.35
- 1.39 (m, 3 H).
[03811 B) The title compound was prepared from ethy12-azido-3-(5-benzyl-furan-
2-yl)-acrylate and was purified by silica gel column chromatography to afford
ethyl 2-
benzyl-4H-furo[3,2-b]pyrrole-5-carboxylate as a tan solid (17 mg, 53 %). 'H
NMR
(400 MHz, CDC13) S ppm 8.61 (br. s., I H) 7.31 - 7.37 (m, 2 H) 7.23 - 7.31 (m,
3 H)
6.74 (dd, J=1.6, 0.9 Hz, 1 H) 6.10 (d, J=0.9 Hz, 1 H) 4.34 (q, J=7.1 Hz, 2 H)
4.07 (s,
2 H) 1.37 (t, j---7.1 Hz, 3 H).
2.2.d) Synthesis of ethyl 3-benzyl-4H-furo[3,2-b]pyrrole-5-carboxylate
0 0
Y / ` 0 + p NaOEt P
/\ N l EtOH /\ N.
~ \ ~
0 O O
\ / \ m-xylene Ol
NS reflux /\ N 1
H O
[03821 A) Ethy12-azido-3-(4-benzyl-furan-2-yl)-acrylate was synthesized from 4-
benzyl-furan-2-carbaldehyde (0.300 g, 1.61 mmol) and purified to give a pale
yellow
oil (135 mg, 28%). 'H NMR (400 MHz, CD3CN) S ppm 7.42 (d, J'--0.9 Hz, 1 H)
7.30
(d, J=7.1 Hz, 2 H) 7.19 - 7.28 (m, 3 H) 7.00 (s, 1 H) 6.75 (s, I H) 4.29 (q,.I-
'-7.1 Hz, 2
H) 3.79 (s, 2 H) 1.32 (t, J-7.1 Hz, 3 H).
[03831 B) The title compound was prepared from ethy12-azido-3-(4-benzyl-furan-
2-yl)-acrylate and was purified by silica gel column chromatography to afford
ethyl 3-
131
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
benzyl-4H-furo[3,2-b]pyrrole-5-carboxylate as a brown solid (52 mg, 43%). 'H
NMR
(400 MHz, CD3CN) 6 ppm 9.57 (br. s., 1 H) 7.40 (s, 1 H) 7.28 - 7.35 (m, 4 H)
7.19 -
7.27 (m, I H) 6.68 (d, .I--1.8 Hz, 1 H) 4.26 (q,.T--7.1 Hz, 2 H) 3.92 (s, 2 H)
1.27-1.34
(m, 3 H).
2.2.e) Synthesis of ethyl3-vinyl-4H-furo[3,2-b]pyrrole-5-carboxylate
0 0
j3_CHO NaOEUEtOH 3
52 / o
N O
/
O O
[0384] A) Ethyl 2-azido-3-(4-vinylfuran-2-yl)acrylate (398 mg, 52%) was
synthesized from 4-vinylfuran-2-carbaldehyde (0.4 g, 3.28 mmol) and was
purified by
flash chromatography (Isco CombiFlash, 0-5% EtOAc/heptane). 'H NMR (400 MHz,
CDC13) 8 ppm 1.39 (t, J--7.13 Hz, 3 H), 4.36 (q, J-7.13 Hz, 2 H), 5.23 (dd, .T-
-10.88,
1.22 Hz, I H), 5.58 (dd, J=17.52, 1.17 Hz, I H), 6.55 (dd, .T--17.57, 10.88
Hz, I H),
6.81 (s, 1 H), 7.25 (s, 1 H), 7.46 (s, I H); LCMS- MS (ESI+) 205.86 (M-N2).
[0385] B) The title compound was synthesized from ethyl 2-azido-3-(4-
vinylfuran-
2-yl)acrylate and was purified by flash column chromatography (Isco
CombiFlash, 0-
30% EtOAc/heptane) to afford ethyl 3-vinyl-4H-furo[3,2-b]pyrrole-5-carboxylate
as a
white solid (215 mg, 62 %). 'H NMR (400 MHz, CDC13) S ppm 1.40 (t, J=7.13 Hz,
3
H), 4.38 (q, J=7.13 Hz, 2 H), 5.35 (d, J=10.93, Hz, 1 H), 5.52 (d, J=17.57 Hz,
1 H),
6.63 (dd, J=17.57, 10.88 Hz, 1 H), 6.80 (d,.t=1.66 Hz, 1 H), 7.53 (s, 1 H);
LCMS-
MS (ESI+) 205.85 (M+H).
2.2.f) Synthesis of ethyl3-cyclopropyl-4H-furo[3,2-b]pyrrole-5-carboxylate
Q 0
CHO N3 ..J~ag 51oEt
NeOB/EtOH
58%
O O ~ OEt
O ~'ene - ~ N
OEt reflux, 30 min H O
88%
132
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[03861 A) Ethy12-azido-3-(4-cyclopropylfuran-2-yl)acrylate (148 mg, 56%) was
synthesized from 4-cyclopropylfuran-2-carbaldehyde (145 mg, 1.06 mmol) and was
purified by flash chromatography (Isco CombiFlash, 0-20% EtOAc/heptane). 'H
NMR (400 MHz, CDC13) S ppm 0.56-0.61 (m, 2 H), 0.85-0.91 (m, 2 H), 1.38 (t,
J--7.15 Hz, 3 H), 1.66-1.75 (m, 1 H), 4.34 (q, J=7.16 Hz, 2 H), 6.79 (s, 1 H),
6.87 (s, 1
H), 7.30 (s, 1 H); LCMS- MS (ESI+) 219.84 (M-NZ).
[03871 B) The title compound was synthesized from ethyl 2-azido-3-(4-
cyclopropylfuran-2-yl)acrylate and was purified by flash chromatography (Isco
CombiFlash) eluting with 0-15% EtOAc/heptane to afford ethyl 3-cyclopropyl-4H-
furo[3,2-b]pyrrole-5-carboxylate as a white solid (114 mg, 88%). 'H NMR (400
MHz,
CDC13) S ppm 0.66-0.71 (m, 2 H), 0.88-0.94 (m, 2 H), 1.38 (t, .>=7.13 Hz, 3
H), 1.72-
1.80 (m, I H), 4.36 (q, J=7.13 Hz, 2 H), 6.75 (d, .,F--1.66 Hz, 1 H), 7.31
(d,.T--0.88 Hz,
1 H); LCMS- MS (ESI+) 219.82 (M+H).
2.2.g) Synthesis of ethyl3-bromo-4H-furo[3,2-blpyrrole-5-carboxylate
Br Na/EtOH Br EtOZC
t/o\-CHO + N3 COEt / \ / ~
o
Br Eb0~,, O2C Br N
/ Ns xylena /CO~t
O
~
[03881 A) Ethyl 2-azido-3-(4-bromofuran-2-yl)acrylate was synthesized from 4-
bromo-2-furaldehyde (2.0 g, 11.4 mmol) and was purified by flash column
chromatography (100 % heptane) to give an orange oil. tH NMR (400 MHz, CDC13)
S(ppm): 7.47 (d, 1H), 7.17 (s, 1H), 6.77 (s, 1H), 4.36 (q, 2H), 1.39 (t, 3H).
[03891 B) The title compound was synthesized from ethy12-azido-3-(4-
bromofuran-2-yl)acrylate and was purified by flash column chromatography (0-20
%
EtOAc in heptane) to give ethy13-bromo-4H-furo[3,2-b]pyrrole-5-carboxylate
(400
mg).as a light brown solid. LCMS m/e 259 (M+H). 'H NMR (400 MHz, CDC13) S
(ppm): 8.71 (s, IH), 7.51 (s, 1H), 6.82 (d, 1H), 4.37 (q, 2H), 1.39 (t, 3H).
133
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.2.h) Synthesis of ethyl 3-isopropyl-4H-furo[3,2-b]pyrrole-5-carboxylate
0 0
CHO N3 OEt
' ~OEt
NaOEUEtOH N3
63%
O O OEt
\ rrrxytene
N OEt -~ N O
3 reflux. 30 rrdn
44 %
[03901 A) Ethy12-azido-3-(4-isopropylfuran-2-yl)-acrylate (1.36 g, 63%) was
synthesized from 4-isopropylfuran-2-carbaldehyde (1.2 g, 8.69 mmol) and was
purified by flash chromatography (Isco CombiFlash, 0-1% EtOAc/heptane) (NMR
purity: -80%). 'H NMR (400 MHz, CDC13) S ppm 1.22-1.25 (m, 6 H), 1.35-1.41 (m,
3 H), 2.82 (m, I H), 4.30-4.38 (m, 2 H), 6.82 (d, .F=0.44 Hz, 1 H), 7.04
(d,./'--0.34 Hz,
I H), 7.26 (t, J=0.90 Hz, 1 H); LCMS- MS (ESI+) 221.83 (M-N2).
[0391] B) The title compound was synthesized from ethyl 2-azido-3-(4-
isopropylfuran-2-yl)-acrylate (1.3 g, 5.22 mmol) and was purified by flash
chromatography (Isco CombiFlash, 0-5% EtOAc/heptane) and reverse phase semi-
preparative HPLC (MeOH:H20) to give a pure fraction of ethyl 3-isopropyl-4H-
furo[3,2-b]pyrrole-5-carboxylate(436 mg, 47% based on the purity of the
starting
material). 'H NMR (400 MHz, CDC13) 6 ppm 1.32 (d, J--6.88 Hz, 6 H), 1.39 (t,
J=7.15 Hz, 3 H), 2.92-3.01 (m, 1 H), 4.36 (q, J=7.09 Hz, 2 H), 6.76 (d, .F--
1.66 Hz, 1
H), 7.28 (d, J=1.12 Hz, I H), 8.79 (s, 1 H); LCMS- MS (ESI+) 221.83 (M+H).
2.2.i) Synthesis of ethyl 3-(tert-butyl-dimethyl-silanyloxymethyl)-4H-furo[3,2-
b] pyrrole-5-carboxylate
1) Na / EtOH HO-'
a
\--J b
COZEt
~ ~ N3~C02Et 2) m Xyl /flux ~ ~
O
0
[03921 A) Ethyl 2-azido-3-(4-hydroxylmethyl-furan-2-yl)-acrylate was
synthesized
from 4-benzoyloxymethyl-2-furaldehyde (J. Am. Chem. Soc. 2003, 125, 9740-9749)
(10.0 g, 43.4 mmol) and was purified by silica gel column chromatography (0 to
30%
EtOAc in heptane over 30 min) to give 5.0 g of a reddish solid.
[0393] B) Ethyl 2-azido-3-(4-hydroxylmethyl-furan-2-yl)-acrylate was converted
to
ethyl 3-hydroxymethyl-4H-furo[3,2-b]pyrrole-5-carboxylate according to General
134
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Procedure I B and was purified by silica gel column chromatography (0 to 40%
EtOAc in heptane over 30 min) to give a light reddish solid (0.50 g, 30 % in 2
steps).
'H NMR (400 MHz, CDC13) S ppm 1.38 (t, J---7.13 Hz, 3 H) 2.11 (t,.f--6.15 Hz,
1 H)
4.35 (q,.P--7.22 Hz, 2 H) 4.69 (d, J-5.86 Hz, 2 H) 6.38 (s, I H) 6.77 (dd, J--
1.66, 0.88
Hz, 1 H) 8.80 (br. s., I H).
I
HO N COZEt TBDMSCI / Et3N ~gi H
N CO Et
O Imidazote / CH2Ch / /
O
[0394] To a solution of ethyl 3-hydroxymethyl-4H-furo[3,2-b]pyrrole-5-
carboxylate
(1.75g, 8.37 mmol) in CH2C12 (50 mL) was added imidazole (0.85g, 12.55 mmol)
and
Et3N (1.16mL, 8.37 mmol) and then cooled to 0 C. t-butyldimethylsilyl chloride
(1.64g, 10.88 mmol) was added slowly and the mixture was stirred at rt for 3 h
and
then poured into 50 mL H20. The product was extracted with CH2C12 (3 x 50 mL)
and the combined organic layers were washed with saturated aq NaC1, dried over
NaZSO4, filtered and concentrated in vacuo to give ethyl3-(tert-butyl-dimethyl-
silanyloxymethyl)-4H-furo[3,2-b]pyrrole-5-carboxylate as a solid. The solid
was
clean enough to be used in next step. tH NMR (400 MHz, CDC13) S ppm 0.12 (s, 6
H)
0.93 (s, 9 H) 1.38 (t, J=7.13 Hz, 3 H) 4.35 (q,.f---7.13 Hz, 2 H) 4.72 (d,
J=0.59 Hz, 2
H) 6.33 (d, J=0.49 Hz, I H) 6.77 (dd, J=1.59, 0.85 Hz, 1 H) 8.63 (br. s., I
H).
2.2j) Synthesis of (Z)-ethyl 3-(prop-l-enyl)-4H-furo[3,2-b]pyrrole-5-
carboxylate
Ethyl azidoacetate/
Na / EtOH
/ \ 0 -c t r=t.
O CHO O COOEt
\ _ H
3 m-Xylene / \ N COOEt
0 COOEt 130 C
3 hours O
[0395] A) Ethy12-azido-3-(4-((Z)-prop-l-enyl)furan-2-yl)acrylate (663 mg, 87%)
was synthesized from (Z)-4-(prop-l-enyl)furan-2-carbaldehyde (0.4130 g, 3.7
mmol,
I eq.) and was purified via ISCO Companion (0-20% EtOAc / heptane over 19 min,
tR: 3-6 min). tH NMR (400 MHz, CD3CN) S(ppm): 7.63 (s, I H), 7.21 (s, 1 H),
6.78
(s, 1 H), 6.20 (dd, J=11.37, 1.61 Hz, I H), 5.71 - 5.82 (m, 1 H), 4.31 (q,
J=7.13 Hz, 2
H), 1.86 (dd, J=7.13, 1.76 Hz, 3 H), 1.33 (t, J=7.13 Hz, 3 H).
135
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0396] B) The title compound was synthesized from ethyl 2-azido-3-(4-((Z)-prop-
l-
enyl)furan-2-yl)acrylate (0.6633 g) and purified via ISCO Companion (0-30%
EtOAc/
heptane over 30 min, retention time: 26-29 min) to give (Z)-ethy13-(prop-l-
enyl)-
4H-furo[3,2-b]pyrrole-5-carboxylate (145 mg, 25%). LC/MS m/e 219.8 (M+H). 'H
NMR (400 MHz, CD3CN) S(ppm): 9.70 (s, 1 H), 7.65 (s, 1 H), 6.72 (d, J=1.71 Hz,
I
H), 6.30 - 6.37 (m, 1 H), 5.82 - 5.94 (m, 1 H), 4.24 - 4.34 (m, 2 H), 1.88
(dd, ,T--7.05,
1.78 Hz, 3 H), 1.30 - 1.36 (m, 3 H).
2.2.k) Synthesis of ethyl 3-(trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-
carboxylate
0
\O/ CHO NaOEt, N3--'-OEt O ~ ~OEt
F3C 0 C - RT ~ ( N3
F3
COOEt
\ I N xylene, reflux ~I\COOEt
3
CF3 H
CF3
[03971 A) Ethy12-azido-3-(4-(trifluoromethyl)fiuran-2-yl)acrylate (43 mg, 10%)
was synthesized from 4-trifluoromethyl-furan-2-carbaldehyde (373 mg, 2.27
mmol)
and was purified by flash chromatography (Isco CombiFlash, 0-40%
EtOAc/heptane).
'H NMR (400 MHz, CDCl3) S ppm 1.40 (t, J=7.15 Hz, 3 H), 4.38 (q, .t=7.13 Hz, 2
H), 6.80 (d, J=0.34 Hz, 1 H), 7.25 (s, I H), 7.78 (dd,.,--1.44, 0.85 Hz, 1 H);
LCMS-
MS (ESI+) 247.82 (M-N2).
[0398) B) The title compound was prepared from ethyl 2-azido-3-(4-
(trifluoromethyl)furan-2-yl)acrylate (45 mg, 0.16 mmol) and was purified by
flash
chromatography (Isco CombiFlash, 0-30% EtOAc/heptane) to afford ethyl 3-
(trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-carboxylate as a white solid (30 mg,
76%).
'H NMR (400 MHz, CDC13) S ppm 1.40 (t, J=7.13 Hz, 3 H), 4.39 (q,.F--7.13 Hz, 2
H), 6.85 (d, ,t--1.71 Hz, 1 H), 7.84 (q, J=1.56, 1 H), 9.08 (s, 1 H); LCMS- MS
(ESI+)
247.8 (M+H).
136
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.2.1) Synthesis of (E)-ethyl3-styryl-4H-furo[3,2-b]pyrrole-5-carboxylate
Ethyl azidoaEtcetate/
Na/ OH
- 0 'C to r.t. -
N,
O CHO COOEt
C_cOOEt
~ 130 'C
O COOEt 3 hours O
[0399] A) (E)-Ethyl 2-azido-3-(4-styrylfuran-2-yl)acrylate (36.1 mg, 26%) was
synthesized from (E)-4-styrylfuran-2-carbaldehyde (0.0891 g, 0.5 mmot) and was
purified via ISCO Companion (0-50%, EtOAc / heptane, over 35 min, retention
time:
3-8 min). 'H NMR (400 MHz, CD3CN) S(ppm): 7.71 (s, 1 H), 7.47 - 7.54 (m, 3 H),
7.34 - 7.40 (m, 2 H), 7.24 - 7.30 (m, 1 H), 6.99 - 7.10 (m, 2 H), 6.79 (s, 1
H), 4.32 (q,
J=7.13 Hz, 2 H), 1.34 (t, .t=7.10 Hz, 3 H).
[0400] B) The title compound was prepared from (E)-ethyl 2-azido-3-(4-
styrylfuran-2-yl)acrylate (36.1 mg) and was purified via preparative HPLC
using the
Chromeleon purification system (60-100% methanol/0.1 % formic acid-1%
acetonitrile in water, 50 mm Dynamax C-18 column at 28 mL/min over 7 min, tR _
3.5-
3.8 min) to give (E)-ethyl 3-styryl-4H-furo[3,2-b]pyrrole-5-carboxylate (18.1
mg,
55% yield). 'H NMR (400 MHz, CD3CN) S(ppm): 10.07 (s, I H), 7.75 (s, I H),
7.57
- 7.62 (m, 2 H), 7.40 (t, J=7.61 Hz, 2 H), 7.26 - 7.32 (m, 1 H), 7.09 - 7.22
(m, 2 H),
6.78 (d, J-1.71 Hz, 1 H), 4.33 (q, J--7.13 Hz, 2 H), 1.36 (t, J=7.13 Hz, 3 H).
2.2.m) Synthesis of ethyl3-methyl-4H-furo[3,2-b]pyrrole-5-carboxylate
H
1) Na / EtOH \\
+ N CO2Et
0 CHO N3 C02Et 2) rrrxylene, /`~ ~
reflux 0
[0401] A) Ethyl 2-azido-3-(4-methyl-2-faryl)acrylate (0.25 g, 42%) was
synthesized from 4-methyl-2-furaldehyde (0.3 g, 2.7mmol) and was purified by
silica
gel column chromatography (0 to 30% EtOAc/heptane over 30 min). 'H NMR (400
MHz, CD3OD) S ppm 1.33 (t, -7---7.13 Hz, 3 H) 2.02 (d, J=0.78 Hz, 3 H) 4.28
(q,
.f--7.13 Hz, 2 H) 6.69 (s, I H) 6.93 (s, 1 H) 7.31 (s, I H).
[04021 B) The title compound was synthesized from ethyl 2-azido-3-(4-methyl-2-
furyl)acrylate and was purified by silica gel column chromatography (0 to 40%
137
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
EtOAc in heptane over 30 min) to give ethyl 3-methyl-4H-ftuo[3,2-b]pyrrole-5-
carboxylate (0.17 g, 78%). 'H NMR (400 MHz, CD3OD) d ppm 1.36 (t, J=7.13 Hz, 3
H) 2.15 (d, .,,'--1.32 Hz, 3 H) 4.31 (q, J=7.13 Hz, 2 H) 6.65 (s, 1 H) 7.24 -
7.44 (m, 1
H). LCMS m/e 194 (M+H).
2.2.n) Synthesis of ethyl 2-(trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-
carboxylate
1) NaOEt, EtOH
FsC /O\ CHO + N3,,~CO2Et 2) rrrxylene reflux k\' / COOEt
F3C 0
[0403] A) Ethyl 2-azido-3-(5-(trifluoromethyl)furan-2-yl)acrylate was
synthesized
from 5-(trifluoromethyl)furan-2-carbaldehyde (1.00 g, 6.09 mmol) and was
purified
by silica gel column chromatography (0 to 25% EtOAc in heptane over 20 min) to
give a yellow oil (0.512 g, 30%). Rf= 0.63 (50:50 heptane / EtOAc); '9F NMR
(376
MHz, CDC13) fi(ppm) -64.63 (s, 3 F); 'H NMR (400 MHz, CDC13) S(ppm) 7.14 (m,
I H) 6.88 (m, 1 H) 4.37 (q,..."---7.1 Hz, 2 H) 1.40 (t,./-'-7.1 Hz, 3 H).
[04041 B) The title compound was synthesized from ethy12-azido-3-(5-
(trifluoromethyl)furan-2-yl)acrylate (0.512 g) and was purified by silica gel
column
chromatography (0 to 30% EtOAc in heptane over 20 min) to give ethyl 2-
(trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-carboxylate as yellow solid (0.250
g, 55).
Rf= 0.50 (50:50 heptane / EtOAc); 19F NMR (376 MHz, CDC13) S(ppm) -64.68 (s, 3
F); 'H NMR (400 MHz, CDC13) 6(ppm) 6.88 (m, 1 H) 6.84 (m, 1 H) 4.38 (q, .t=7.1
Hz, 2 H) 1.40 (t, J=7.1 Hz, 3 H).
2.2.o) Synthesis of ethyl 3-fluoro-4H-[3,2-b]pyrrole-5-carboxylate
F 1) NaOEt, EtOH H
/\ + N3',.IC02Et 2) m-xylene reflux F N COOEt
~~
O CHO 1 0
[0405] A) Ethy12-azido-3-(4-fluoro-furan-2-yl)-acrylate was synthesized from 4-
fluorofuran-2-carbaldehyde (-160 mg, 1.4 mmol) and was purified by silica gel
coiumn chromatography (0 to 30% EtOAc in heptane) to give 180 mg (91%). 'H
NMR (400 MHz, CDC13) S ppm 1.39 (t,.f--7.13 Hz, 3 H), 4.36 (q,.,'----7.13 Hz,
2 H),
6.72 (d, J=1.46 Hz, 1 H) 7.03 (s, I H), 7.41 (dd, J=5.08, 0.78 Hz, 1 H); '9F
NMR
(376.19 MHz, CDC13) 5 -167.30 (dt, J = 5.03, 1.61 Hz, I F). LCMS- MS (ESI+)
198.1 (M-N2).
138
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[0406] B) The title compound was synthesized from ethyl 2-azido-3-(4-fluoro-
furan-2-yl)-acrylate (190 mg, 0.84 mmol), and was purified by silica gel
column
chromatography (0 to 30% EtOAc in heptane) to give ethyl 3-fluoro-4H-[3,2-
b]pyrrole-5-carboxylate as white solid, 108 mg (65%). 'H NMR (400 MHz, CDC13)
S
ppm 1.40 (t, J=7.15 Hz, 3 H), 4.39 (q, J=7.13 Hz, 2 H), 6.74 (t, J=1.95, 1 H),
7.52 (d,
J=4.44 Hz, I H), 9.30 (s, I H); '9F NMR (376.19 MHz, CDC13) S-179.37-179.42
(m,
I F); LCMS- MS (ESI+) 198.0 (M+H).
2.2.p) Synthesis of ethyl2-fluoro-4H-furo[3,2-b]pyrrole-5-carboxylate
1)Na/EtOH N3
HO O ~ O + N~~COZEt HO /\ CO2Et
O 2) m-xylene / reflux O
N3 NaHCO3/ HZO N3
HO ~ ~ COZEt COZEt
O O Setectfluor / Hexane / EtOAc F O
O
N3 O-Xylene / 1300C / 5min N
F COZEt ~ ~ OEt
O F O
(0407] A) 5-(2-azido-3-ethoxy-3-oxoprop-l-enyl)furan-2-carboxylic acid was
prepared from 5-formyl-2-furancarboxylic acid (2.0 g, 14.28 mmol) and was
purified
by silica gel column chromatography (0 to 30% EtOAc in heptane over 20 min) to
give a yellow solid (2.40 g, 67%). 'H NMR (400 MHz, CD3OD) S ppm 1.38 (t,
J=7.13 Hz, 3 H) 4.36 (q,.P--7.11 Hz, 2 H) 6.82 (s, 1 H) 7.22 (d, J=3.71 Hz, 1
H) 7.27
(d,.P--3.71 Hz, I H).
[0408] To 5-(2-azido-3-ethoxy-3-oxoprop-l-enyl)furan-2-carboxylic acid (0.50
g,
2.03 mmol) was added a mixture of NaHC03 (0.34 g, 4.06 mmol) and Selectfluor
(1.08 g, 3.05 mmol), followed by water (4.0 mL), hexane (5.0 mL) and EtOAc
(2.0
mL). The mixture was stirred at rt for 5 min. The organic layer was separated,
dried
(Na2SO4), filtered, and concentrated in vacuo. Purification by silica gel
chromatography (0 to 30% EtOAc in heptane over 20 min) yielded pure ethyl 2-
azido-3-(5-fluorofuran-2-yl)prop-2-enoate as a reddish oil (0.20 g, 45%). 'H
NMR
(400 MHz, CD3OD) 8 ppm 1.35 (t, J=7.15 Hz, 3 H) 4.32 (q, J=7.16 Hz, 2 H) 5.74
139
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
(dd, J=6.83, 3.66 Hz, I H) 6.63 (s, I H) 7.05 (t,.)--3.59 Hz, 1 H). '9F NMR
(376 MHz,
CD3OD) 8 ppm -115.12 (dd, J=6.60, 3.30 Hz).
[04091 B) The title compound was prepared from ethyl 2-azido-3-(5-fluorofuran-
2-
yl)prop-2-enoate (0.20 g, 0.88 mmol) and was purified by silica gel column
chromatography (0 to 40% EtOAc in heptane over 20 min) to give pure ethyl 2-
fluoro-4H-furo[3,2-b]pyrrole-5-carboxylate as white solid (0.13 g, 74%). 'H
NMR
(400 MHz, CD3OD) 8 ppm 1.35 (t,.f--7.13 Hz, 3 H) 4.30 (q, .t=7.11 Hz, 2 H)
5.86 (d,
J-6.30 Hz, I H) 6.72 (s, 1 H). '9F NMR (376 MHz, CD3OD) 5 ppm -108.54 (d,
.,'--6.60 Hz). LCMS m/e 198 (M+H).
2.3. Synthesis of ethyl2-chloro-4H-furo[3,2-b]pyrrole-5-carboxylate
H H
N/J COZEt S0202 N C02Et
E120
O a O
[04101 Under a N2 atmosphere, sulfuryl chloride (0.15 mL, 1.85 mmol ) was
added
dropwise over 10 min to a stirring solution of ethyl 4H-furo[3,2-b]pyrrole-5-
carboxylate (300 mg, 1.67 mmol) in ether (7.5 mL). The reaction was stirred at
rt for
4 h. The solvent was removed in vacuo. The residue was taken up in DCM and
washed with H20 (1 x) and brine (1 x), then dried with NaZSOa, filtered and
concentrated. Purification by HPLC gave 160 mg of ethyl 2-chloro-4H-furo[3,2-
b]pyrrole-5-carboxylate. 'H NMR (400 MHz, CDC13) S(ppm): 8.98 (s, 1H), 6.76
(s,
1H), 6.34 (s, 1H), 4.35 (q, 2H), 1.38 (t, 3H).
2.4. Synthesis of ethyl3-formyl-4H-furo[3,2-b)pyrrole-5-carboxylate
HO i N i / CO2Et MnO2 / CH2CI2 O- N i
COZEt
O O
104111 To a solution of ethy13-hydroxymethyl-4H-fiuo[3,2-b]pyrrole-5-
carboxylate
(l.lg, 5.26 mmol) in CHZCIZ (100 mL) was added Mn02 (4.6g, 52.6 mmol). The
reaction mixture was stirred at rt overnight and was then filtered through
Celite and
washed with CH2C12 (3 x 5OmL). The organic solution was concentrated in vacuo
and chromatographed over silica gel (0 to 40% EtOAc in heptane over 30 min) to
give
ethyl 3-formyl-4H-furo[3,2-b]pyrrole-5-carboxylate (1.0 g, 92%) as light
yellow
140
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
solid. 'H NMR (400 MHz, CDCl3) S ppm 1.41 (t,.T--7.13 Hz, 3 H) 4.40 (q, J=7.13
Hz, 2 H) 6.83 (dd, J--1.54, 1.00 Hz, I H) 7.23 (d, J=0.88 Hz, 1 H) 8.98 (br.
s., 1 H)
9.67 (s, 1 H).
2.5. Synthesis of methyl 2-methyl-4H-furo[3,2-b]pyrrole-5-carboxylate
--N
~ ~ ~ (Me)ZNH / CH2O ~ ~ ~
H COOMe AcOH N COOMe
Fi
[0412] Under N2, to 9 mL of glacial acetic acid were added N,N-dimethylamine
(40% aqueous solution) (437 mg, 9.94 mmol), formaldehyde (37% aqueous
solution)
(283 mg, 9.90 mmol), and methyl 4H-thieno[3,2-b]pyrrole-5-carboxylate (1.64 g,
9.94
mmol). The temperature was kept between 0 and 5 C while the components were
added. The reaction mixture was heated at reflux for 1 h, and was then allowed
to
stand at rt for 12 h. The mixture was poured onto 30 g of ice, and it was
brought to pH
10 by careful addition of 10% sodium hydroxide. The temperature was not
allowed to
exceed 10 C while the base was added. The gummy substance that precipitated
solidified when stored in the refrigerator overnight. The solid was collected
and dried
in vacuo. It was recrystallized from petroleum ether (30 - 60 C) to yield
methyl2-
[(dimethylamino)methyl]-4H-fiuo[3,2-b]pyrrole-5-carboxylate (0.80 g, 36%). IH
NMR (400 MHz, CDC13) S ppm 2.36 (s, 6 H) 3.71 (s, 2 H) 3.81 (s, 3 H) 6.33 (s,
1 H)
6.69 (s, I H).
NI/
O O
Mel, rt
N rLCOOMe NaBH4, MeOH N \ COOMe
H
[0413] Under N2, tFoi methyl 2-[(dimethylamino)methyl]-4H-furo[3,2-b]pyrrole-5-
carboxylate (0.58 g, 2.61 mmol) was added methyl iodide (3 mL, 4.82 mmol). The
mixture was allowed to stand at rt for 1 h, and then the methyl iodide was
removed.
The resulting salt was dissolved in absolute methanol (5 mL). To this solution
was
carefully added sodium borohydride (2.21 g, 5.84 mmol) in small portions.
After the
addition was complete, the reaction mixture was dilute to a volume of 25 mL by
the
addition of 3N hydrochloric acid. The mixture was stored in the refrigerator
overnight,
and then the blue precipitate was dissolved in boiling methylcyclohexane, and
the
solution was treated with Darco and filtered. The filtrate was evaporated and
purified
141
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
by chromatography over silica gel (0 to 40% EtOAc/heptane over 30 min) to give
methyl 2-methyl-4H-furo[3,2-b]pyrrole-5-carboxylate (0.25g, 53%). 'H NMR (400
MHz, CDC13) d ppm 2.42 (s, 3 H) 3.87 (s, 3 H) 6.09 (d, J=0.49 Hz, 1 H) 6.74
(s, 1 H)
8.56 (s, I H).
2.6. Synthesis of ethyl 3-ethyl-4H-furo[3,2-b]pyrrole-5-carboxylate
~
N 10% Pd/C, H N
COOEt 2 - / ~ / COOEt
O 1 atm, EtOAc, RT, 6 h, 0
[04141 A solution of ethy13-vinyl-4H-furo[3,2-b]pyrrole-5-carboxylate (105 mg,
0.51 mmol) in EtOAc (8 mL) in a 40-mL scintillation vial was treated with 10%
Pd/C
(-15 mg) and a balloon of H2. The system was evacuated and refilled three
times with
H2 before hydrogenating at rt for 6 h. The catalyst was removed by filtration
over
Celite and the filtrate was concentrated. The crude product was purified by
flash
chromatography (0-10% EtOAc/heptane) to give ethyl 3-ethyl-4H-furo[3,2-
b]pyrrole-
5-carboxylate (96 mg, 91 %). 'H NMR (400 MHz, CDC13) S ppm 1.30 (t, J=7.54 Hz,
3
H), 1.36-1.42 (m, 3 H), 2.57-2.64 (m, 2 H), 4.33-4.40 (m, 2 H), 6.76 (d,
J=1.66 Hz, 1
H), 7.31 (t, J=1.12 Hz, 1 H); LCMS- MS (ESI+) 207.83 (M+H).
2.7. Synthesis of inethyl6-bromo-4H-furo[3,2-b]pyrrole-5-carboxylate
H NBS, TBAF H
/ N / Co2Me CH,CHõ r.t cBrC0e
\ N/ zBflO [0415] To a cold solution (ice-water bath) of inethyl4H-furo[3,2-
b]pyrrole-5-
carboxylate (1.0 g, 6.05 mmol) in DCM (10 mL) was added TBAF (1.0 M in THF,
9.0 mL, 9.0 mmol) and NBS (1.5 g, 7.9 mmol). The resulting dark colored
solution
was stirred from 0*C to rt overnight. The reaction mixture was diluted with 50
mL of
CH2C12 and washed with water (100 mL) and brine (100 mL) and dried (Na2SO4).
After filtration, the filtrate was concentrated by evaporation and the crude
product
was purified by silica gel chromatography (0-5% EtOAc/hexane) to afford a
white
solid. 'H NMR (400 MHz, CDC13) S(ppm) 8.84 (broad, 1 H, NH), 7.54 (d, J = 2.2
Hz, 1 H), 6.48 (d, J = 1.83 Hz, 1 H), 3.92 (s, 3H) ppm; m+/z 244 (100%), 246
(100%).
142
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.8. Synthesis of 4-tert-butoxycarbonyl-2-bromo-4H-furo[3,2-b]pyrrole-5-
carboxylic acid methyl ester
H B c
N 02Me BoczO, Et3N, CH2CI N COzMe
~
O O
[0416] To a solution of methyl 4H-furo[3,2-b]pyrrole-5-carboxylate (1.0 g,
6.06
mmol) in CH2C12 (10 ml) was added triethyl amine (1.85 g, 18.2 mmol) and DMAP
(148 mg1.22 mol). Then BOC2O (2.0 g, 9.1 mmol) was added. The resulting
mixture
was stirred overnight. After the reaction was complete as judged TLC analysis
(10%
EtOAc /hexane), the reaction mixture was washed with water and brine and dried
over
Na2SO4. Affter filtration, the filtrate was concentrated and the crude product
was
purified by silica gel chromatography (20% EtOAc in hexane) to give 4-tert-
butoxycarbonyl-4H-fiuo[3,2-b]pyrrole-5-carboxylic acid methyl ester as a white
solid (987 mg). 'H NMR (400 MHz, CDC13) S(ppm) 7.45 (d, J = 1.47 Hz, 1H), 6.82
(s, 1H), 6.59 (s, IH), 3.80 (s, 3H), 1.55 (s, 9H).
Boc
N NBS, TBAF, Boc
COZMe CH2CI2, r.t------ N CO zMe
O / O \ / Br
To a solution of 4-tert-butoxycarbonyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid
methyl ester (100 mg, 0.38 mmol) in DCM (1 mL) was added a solution of TBAF in
THF (1.0 M, 0.57 ml, 0.57 mmol) followed by the addition of NBS (87 mg, 0.49
mmol). The resulting mixture was stirred at rt overnight. The reaction mixture
was
diluted with DCM (10 ml), washed with 10 mL of water and then with 10 mL of
brine
and dried with Na2SO4= The solid was removed by filtration. The filtrate was
concentrated by evaporation. The crude product was purified by chromatography
(0-
20% EtOAc in hexane) to give 85 mg of 4-tert-butoxycarbonyl-2-bromo-4H-
furo[3,2-
b]pyrrole-5-carboxylic acid methyl ester (65%). 'H NMR (400 MHz, CDC13) S(ppm)
6.81 (s, 1 H), 6.61 (s, 1 H), 3.83 (s, 3H), 1.59 (s, 9H).
143
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.9. Synthesis of inethyl6-iodo-4H-furo(3,2-b]pyrrole-5-carboxylate
H H
NA COzMe IZ,KOH,DMF- C \NZ COZMe
O O 1
104171 A mixture of methyl 4H-fiuo[3,2-b]pyrrole-5-carboxylate (5.00 g, 30.3
mmol) and KOH (3.40 g, 60.6 mmol) in DMF (100 mL) was cooled to -10 C. Iodine
(7.31 g, 28.8 mmol) in DMF (40 mL) was charged via an addition funnel over 30
min.
The resulting mixture was warmed to rt and stirred for additional 12 h. The
reaction
mixture was poured into water, adjusted with HCI (2 N) to pH 6-7, and
extracted with
EtOAc. The crude product was purified by flash chromatography (silica gel, 0
to 30 %
ethyl acetate in hexanes) to give a light tan solid methyl 6-iodo-4H-furo[3,2-
b]pyrrole-5-carboxylate (3.85 g, 44 % yield). 'H NMR (400 MHz, CDC13) S(ppm)
8.98 (br, s, 1 H); 7.55 (d, J = 2 Hz, 1 H); 6.52 (d, J = 2 Hz, 1 H); 3.91 (s,
3H). MS (m/z
291).
2.10. Synthesis of methyl 6-fluoro-4H-furo[3,2-b]pyrrole-5-carboxylate
H 1. NaN, TMSCI
/ \N/ CO2Me 2. tguLi. NFSI C02Me
O 1 O F
104181 To a suspension of sodium hydride (95 %, 0.130 g, 5.16 mmol) in THF (15
mL) cooled to -20 C was added a solution methyl 6-iodo-4lY-furo[3,2-b]pyrrole-
5-
carboxylate (1.00 g, 3.44 mmol) in THF (15 mL). Chlorotrimethylsilane (0.46
mL,
3.61 mmol) was added after 20 min. The resulting mixture was slowly warmed up
to
0 C over I h, and then recooled to -78 C. t-Butyllithium (1.7 M in pentane,
4.45
mL, 7.57 mmol) was added. After 40 minutes, a solution of NFSI (1.09 g, 3.44
mmol)
in THF (5 mL) was added. The resulting mixture was stirred at -78 C for 1 h,
then
quenched with methanol/water, and warmed to rt. The mixture was diluted with
brine
and extracted with EtOAc. GCMS of the crude showed 50:50 of inethyl6-fluoro-4H-
furo[3,2-b]pyrrole-5-carboxylate: methyl 6-iodo-4H-furo[3,2-b]pyrrole-5-
carboxylate,
which were separated by column chromatography. 'H NMR (400 MHz, (CD3)2C(O))
S ppm 3.83 (s, 3 H) 6.60 (s,.P--2.17, l H) 7.75 (d,.1--2.20 Hz, 1 H) 10.32
(br. s., 1 H).
144
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.11. Synthesis of ethyl3-chloro-4H-furo[3,2-b]pyrrole-5-carboxylate
Br N CO2Et CuCI, DMF CI N COZEt
O O /
[0419] The title compound was synthesized from ethyl3-bromo-4H-furo[3,2-
b]pyrrole-5-carboxylate (200 mg, 0.774 mmol) using the conditions to
synthesize
ethyl 3-chloro-4H-thieno[3,2-b]pyrrole-5-carboxylate. Chromatography (silica
gel,
heptane/EtOAc) yielded ethyl3-chloro-4H-furo[3,2-b]pyrrole-5-carboxylate (70
mg,
42% yield).
2.12. Syntfiesis of Carboxylic Acids from Esters
2.12.a) Synthesis of 4H-furo[3,2-b]pyrrole-5-carboxylic acid (11)
H
N C02H
~ ~
O
[0420] The title compound was synthesized from ethy141Y-furo[3,2-b]pyrrole-5-
carboxylate (0.33 g, 1.84 mmol) according to General Procedure 2 and was
purified
by silica gel column chromatography (0 to 100% EtOAc in heptane over 30 min)
to
give 4H-furo[3,2-b]pyrrole-5-carboxylic acid 11 as a light pink solid (0.200
g, 72%).
Rf= 0.07 (1:1 heptane / EtOAc); 'H NMR (4001v1I4z, (CD3)2S0) S(ppm) 12.34 (s,
1
H) 11.48 (s, I H) 7.75 (s, 1 H) 6.68 (s, 1 H) 6.57 (s, 1 H).
2.12.b) Synthesis of 3-phenethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid
(17)
0 0
Y O-/ NaOH j O-H
N N
H O
- H O MeOH, reflux o
~ ~ 20 [0421] The title compound was prepared from ethyl 3-phenethyl-4H-
furo[3,2-
b]pyrrole-5-carboxylate (265 mg, 0.935 mmol) according to General Procedure 2
to
give 3-phenethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 17 as a tan solid
(117 mg,
49%). 'H NMR (400 MHz, (CD3)2S0) S ppm 12.34 (br s., 1 H) 11.68 (s, 1 H) 7.51
(s,
1 H) 7.25 - 7.32 (m, 4 H) 7.15 - 7.22 (m, I H)6.63(d,.t=1.7Hz, 1 H) 2.91 -
2.99(m,
2 H) 2.73 - 2.81 (m, 2 H).
145
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.12.c) Synthesis of 2-chloro-4H-furo[3,2-b]pyrrole-5-carboxylic acid (23)
H H
N N
~ CO2Et KOH COZH
C MeOH CI
[04221 The title compound was prepared from ethyl 2-chloro-4H-furo[3,2-
b]pyrrole-5-carboxylate (186 mg, 0.87mmol) according to General Procedure 2.
The
crude product was purified by silica gel chromatography to afford 2-chloro-4H-
furo[3,2-b]pyrrole-5-carboxylic acid 23 (50 mg, 31%). LCMS m/e 184 (M-H).
Purity by HPLC: 97.5%. 'H NMR (400 MHz, CD3OD) S(ppm): 6.70 (d, 1 H), 6.45
(d, 1 H).
2.12.d) Synthesis of 2-benzyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (24)
\ MeOH O \ O_H NaOH H O~ reflux H O
c:r-co;r--L(
[0423] The title compound was prepared from ethyl 2-benzyl-4H-fiuo[3,2-
b]pyrrole-5-carboxylate (17 mg, 63 mol) according to General Procedure 2 to
give 2-
benzyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 24 (13 mg, 87 %) as a tan
solid. IH
NMR (400 MHz, (CD3)ZSO) S ppm 12.17 (br. s., I H) 11.36 (s, I H) 7.19 - 7.36
(m, 5
H) 6.59 (dd, J=1.7, 0.9 Hz, 1 H) 6.29 (d,.T--0.8 Hz, I H) 4.04 (s, 2 H).
2.12.e) Syntheis of 3-benzyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (26)
O
\ O NaOH O-H
H O~ MeOH ~ H O
J:t[04241 The title compound was prepared from ethyl 3-benzyl-4H-furo[3,2-
b]pyrrole-5-carboxylate (52 mg, 0.19 mmol) according to General Procedure 2 to
give
3-benzyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 26 as a tan solid (41 mg,
87%). 'H
NMR (400 MHz, (CD3)2S0) S ppm 12.32 (br. s., 1 H) 11.60 (s, 1 H) 7.57 (s, I H)
7.33-7.38(m,2H)7.25-7.31(m,2H)7.15-7.21(m,1H)6.63(d,J=1.5Hz,1H)
3.84 (s, 2 H). HPLC 99%. LCMS 242 (M+H).
146
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.12.f) Synthesis of 3-bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid (30)
Br N/ CO2Et ~~ r)N/ COZH
MeOH
[0425] The title compound was synthesized from ethyl 3-bromo-4H-furo[3,2-
b]pyrrole-5-carboxylate (100 mg, 0.39 mmol) according to General Procedure 2
and
was purified by silica gel column chromatography to give 3-bromo-4lY-furo[3,2-
b]pyrrole-5-carboxylic acid 30 (46 mg) in 99.6 % purity (HPLC). LCMS m/e 229
(M-
H). 'H NMR (400 MHz, CD3OD) S(ppm): 7.65 (s, 1H), 6.74 (s, 1H).
2.12.g) Synthesis of 3-cyclopropyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid
(31)
O OEt O
N O LiOF~,/EtOH
\ N O H
H 95 C, 39%
[0426] The title compound was synthesized from ethyl 3-cyclopropyl-4H-furo[3,2-
b]pyrrole-5-carboxylate (110 mg, 0.50 mmol) according to General Procedure 2
and
was purified by flash chromatography (Isco CombiFlash, 0-60% EtOAc/heptane) to
afford 3-cyclopropyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 31 (34 mg, 35%).
IH
NMR (400 MHz, CD3OD) S ppm 0.67-0.72 (m, 2 H), 0.86-0.92 (m, 2 H), 1.75-1.84
(m, 1 H), 6.64 (s, 1 H), 7.34 (d, J=0.83 Hz, I H); LCMS- MS (ESI-) 189.8 (M-
H);
HPLC (UV = 95.9%), (ELSD =100%).
2.12.h) Synthesis of 3-vinyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (32)
0 OEt 0 0
\ \ LiOHeylEtOH \ 3C N 0 N OH
H 95 oC, 42% ~ H
[0427] The title compound was synthesized from ethyl 3-vinyl-4H-furo[3,2-
b]pyrrole-5-carboxylate (100 mg, 0.49 mmol) according to General Procedure 2
and
was purified by flash chromatography (Isco CombiFlash, 0-40% EtOAc/heptane) to
give 3-vinyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 32 (36 mg, 42%). 'H NMR
(400
MHz, CD3OD) S ppm 5.29 (dd, J--11.03, 0.73 Hz, 1 H), 5.81-5.88 (m, I H), 6.59-
6.68
(m, 1 H), 6.72 (s, 1 H), 7.63 (s, 1 H); LCMS- MS (ESI-) 175.8 (M-H); HPLC (UV
=
99.2%), (ELSD = 100%).
147
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.12.i) Synthesis of 3-isopropyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (40)
\ \ OEt uOH.H20
p O EtOH, 72% gq , C N OH
[0428] The title compound was synthesized from ethyl 3-isopropyl-4H-furo[3,2-
b]pyrrole-5-carboxylate (120 mg, 0.54 mmol) according to General Procedure 2
and
was purified through a plug of silica to give 3-vinyl-4hl-furo[3,2-b]pyrrole-5-
carboxylic acid 40 (76 mg, 72 %). 'H NMR (400 MHz, CD3OD) S ppm 1.31 (d,
J=6.88 Hz, 6 H), 2.91-3.00 (m, 1 H), 6.66 (s, I H), 7.33 (d,.f--0.98 Hz, 1 H);
LCMS-
MS (ESI-) 191.8 (M-H); HPLC (UV = 100%), (ELSD = 100%).
2.12.j) Synthesis of 3-hydroxymethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid
(42)
H
Si H NaOH / MeOH HO N
CO2H
N COZEt / /
/ / O
O
[0429] The title compound was synthesized from ethy13-(tert-butyl-dimethyl-
silanyloxymethyl)-4H-furo[3,2-b]pyrrole-5-carboxylate (0.30 g, 0.93 mmol)
according to General Procedure 2 and was purified by silica gel column
chromatography (25 to 100% MeOH in CH2CI2 over 30 min) to give 3-
hydroxymethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 42 as a white solid (20
mg,
12%) in 99 % purity (HPLC). 'H NMR (400 MHz, (CD3)2S0) S ppm 4.41 (s, 2 H)
6.33 (d, J=0.49 Hz, 1 H) 6.43 (s, I H) 8.46 (s, I H) 10.95 (br. s., I H). LCMS
m/e
180 (M-H).
2.12.k) Synthesis of 3-formyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (43)
O- / N/CO NaOH / MeOH O- N/ CO~
~~ ZEt _
[04301 The title compound was synthesized from ethyl3-formyl-4H-furo[3,2-
b]pyrrole-5-carboxylate (0.14 g, 0.67 mmol) according to General Procedure 2
and
was purified by silica gel column chromatography (10 to 100% MeOH in CH2C12
over 30 min) to give 3-formyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 43 (30
mg,
148
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
25%) as a light green solid in 99 % purity (HPLC). 'H NMR (400 MHz, CD3OD) 8
ppm 6.62 (s, I H) 7.42 (s, 1 H) 9.45 (s, 1 H). LCMS m/e 178 (M-H).
2.12.1) Synthesis of (Z)-3-(Prop-l-enyl)-4H-furo[3,2-b]pyrrole-5-carboxylic
acid
(46)
1. NaOH/ MeOH
70 'C
- N ~Et 2. 1036 v/v aq. HCI / COOH
/ ~ /
O O
[04311 The title compound was synthesized from (Z)-ethyl 3-(prop-l-enyl)-4H-
furo[3,2-b]pyrrole-5-carboxylate (0.1445 g, 68 mmol) according to General
Procedure
2 and was purified by preparative HPLC using a Chromeleon purification system
(50-
100% over 7 min methanol/0.1 % formic acid-1 % acetonitrile in water, 50 mm
Dynamax C-18, 28 mL/min) to give (Z)-3-(prop-l-enyl)-4H-furo[3,2-b]pyrrole-5-
carboxylic acid 46 (40.4 mg, 32% yield). LC/MS m/e 189.8 (M-H). Purity by
HPLC:
99.1% (UV); 100% (ELSD). 'H NMR (400 MHz, CD3OD) S(ppm): 7.64 (s, 1 H),
6.72 (s, 1 H), 6.32 - 6.38 (m, 1 H), 5.81 - 5.91 (m, I H), 1.91 (dd, J=7.03,
1.76 Hz, 3
H).
2.12.m) Synthesis of 3-(trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-
carboxylic acid (47)
COOEt VOH=H20 0 ( ~ COOH
H Et~OH. 94 C H
F3 F3
[04321 The title compound was synthesized from ethyl3-(trifluoromethyl)-4H-
furo[3,2-b]pyrrole-5-carboxylate (108 mg, 0.44 mmol) according to General
Procedure 2 and was purified through a plug of silica to remove baseline
impurities to
give 3-(trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-carboxylic acid 47 (89 mg, 93
%).
'H NMR (400 MHz, CD3OD) 8 ppm 6.80 (s, 1 H), 8.08 (q, .f--1.58 Hz, I H); 13C
NMR (100 MHz, CDC13) 8 97.53 (dd, J=180.7, 1.3 Hz), 108.78 (qd, .t=39.2, 11.7
Hz),
123.79 (q, J=265.4 Hz), 124.73 (m), 127.92 (d, J=5.8 Hz), 148.96 (dq,.P--
208.7, 5.8
Hz), 150.32 (d, ,P--8.0 Hz), 164.57 (s); LCMS- MS (ESI-) 217.8 (M-H); HPLC (UV
=
99.3%), (ELSD = 100%).
149
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.12.n) Synthesis of (E)-3-styryl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (48)
/ 1. NaOH/ MeOH
H 70 C
N C~t 2. 1096 v/v aq. HCI
t ~ ~ COOH
O O
[04331 The title compound was synthesized from (E)-ethyl 3-styryl-4H-furo[3,2-
b]pyrrole-5-carboxylate (0.0181 g, 0.071 mmol) according to General Procedure
2A
and was purified via preparative HPLC (Chromeleon purification system, 40-100%
over 7 min, methanol/0.1 % formic acid-1 % acetonitrile in water, 50 mm
Dynamax C-
18, 28 mL/min, retention time of product: 3.9-4.0 min) to give (E)-3-styryl-
41Y-
furo[3,2-b]pyrrole-5-carboxylic acid 48 (4.9 mg, 30%). LC/MS m/e 251.9 (M-H).
Purity by HPLC: 97.9% (UV); 100% (ELSD). 'H NMR (400 MHz, CD3OD) S
(ppm): 8.40 (s, 1 H), 7.76 (s, 1 H), 7.58 - 7.62 (m, 2 H), 7.34 - 7.39 (m, 2
H), 7.31 (d,
.F=16.40 Hz, 1 H), 7.22 - 7.27 (m, 1 H), 7.12 (d,.P-- 16.40 Hz, I H), 6.76 (s,
1 H).
2.12.o) Synthesis of 3-met6yl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (50)
H H
/\ N/ C02Et NaOH / MeOH /\ N/ CO2H
O O
104341 The title compound was synthesized from ethyl 3-methyl-4H-furo[3,2-
b]pyrrole-5-carboxylate (0.17 g, 0.88 mmol) according to General Procedure 2
and
was purified by silica gel column chromatography (0 to 100% EtOAc in heptane
over
30 min) to give 3-methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 as a solid
(90
mg, 62%). 'H NMR (400 Ml-Iz, CD3OD) d ppm 2.15 (d, .F--1.27 Hz, 3 H) 6.65 (s,
1 H)
7.34 (d, J=1.27 Hz, 1 H). LCMS m/e 164 (M-H). 99.5% pure by HPLC.
2.12.p) Synthesis of 2-met6y1-4H-furo[3,2-b]pyrrole-5-carboxylic acid (57)
0 NaOH/MeOH 0
2cooH
N COOMe H H
[04351 The title compound was synthesized from methyl 2-methyl-4H-furo[3,2-
b]pyrrole-5 -carboxylate (0.15 g, 0.84 mmol) according to General Procedure 2
and
was purified by silica gel column chromatography (0 to 100% EtOAc in heptane
over
30 min) to give 2-methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 57 as a solid
(35
150
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
mg, 25%). 'H NMR (400 MHz, CD3OD) d ppm 2.37 (d,.I--0.83 Hz, 3 H) 6.12 (s, 1
H)
6.61 (d, ,I'--0.59 Hz, 1 H). LCMS m/e 164 (M-H). 99% pure by HPLC.
2.12.q) Synthesis of 3-ethyl-4H-furo[3,2-bJpyrrole-5-carboxylic acid (58)
H H
0
N LiOHadEtOH N
/ COOEt -~ ~ ( / COOH
95 C, 90% O
[0436] The title compound was synthesized from ethyl 3-ethyl-4H-furo[3,2-
b]pyrrole-5-carboxyl ate (95 mg, 0.46 mmol) according to General Procedure 2
and
was purified through a plug of silica to give 3-ethyl-4H-furo[3,2-b]pyrrole-5-
carboxylic acid 58 (74 mg, 90 %). 'H 1VMR (400 MHz, CD3OD) S ppm 1.28 (t,
J=7.52 Hz, 3 H), 2.55-2.63 (m, 2 H), 6.66 (s, 1 H), 7.35 (t, .t=1.15 Hz, 1 H);
LCMS-
MS (ESI-) 177.8 (M-H); HPLC (UV = 100%), (ELSD = 100%).
2.12.r) Synthesis of 6-bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid (72)
Me LiOH, THF-H20 / N/ COZH
N/ CO2 ~
O Br
104371 The title compound was synthesized from methyl6-bromo-4H-fiuo[3,2-
b]pyrrole-5-carboxylate (40 mg, 0.16 mmol) according toGeneral Procedure 2 and
was purified by reverse phase HPLC to give 15 mg of 6-bromo-4lY-furo[3,2-
b]pyrrole-5-carboxylic acid 72. 'H NMR (400 MHz, CD3OD) 5(ppm) 7.64 (d, J =
2.2
Hz, IH), 6.5 5(d, J = 2.2 Hz, 1 H).
2.12.s) Synthesis of 2-bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid (73)
qoc
N C02Me 1= VOH, THF-H2O CO H
2.2 N HCI tS 2
Br O Br O
104381 The title compound was synthesized from 4-tert-butoxycarbonyl-2-bromo-
4H-furo[3,2-b]pyrrole-5-carboxylic acid methyl ester (78 mg, 0.226 mmol)
according
to General Procedure 2 and was purified by reverse phase HPLC to give 2-bromo-
4H-
furo[3,2-b]pyrrole-5-carboxylic acid 73 (14 mg). 'H NMR (400 MHz, CD3OD) S
(ppm) 6.69 (s, IH), 6.55 (s, 1H).
151
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.12.t) Synthesis of 3-fluoro-4H-furo[3,2-bJpyrrole-5-carboxylic acid (74)
LiOH-H20 (10 equiv)
H THF-EtOH-H20 (1:1:1) H
F/ \ N/ COOEt 50 C. 5 hrs F `N / COOH
O O
[0439] The title compound was synthesized from ethyl 3-fluoro-4H-furo[3,2-
b]pynole-5-carboxylate (40 mg, 0.203 mmol) according to General Procedure 2
and
was purified by silica gel chromatography (0-50% EtOAc in hexane) to yield 3-
fluoro-4H-furo[3,2-b]pyrroie-5-carboxylic acid 74 (23 mg, 68%) as a white
solid. 'H
NMR (400 MHz, CD3OD) 8 ppm 6.68 (t, J=2.25, I H), 7.67 (d, J=4.30 Hz, 1 H);19F
NMR (376.19 MHz, CD3OD) 8 -182.87 (dd,.P=4.29, 2.30 Hz, I F); LCMS- MS
(ESI+) 170.1 (M+H); HPLC (UV = 100%).
2.12.u) Synthesis of 6-fluoro-4H-furo[3,2-b]pyrrole-5-carboxylic acid (75)
H H
N COZMe NaOH, EtOH N
croH
ao\ O F
F
[0440] The title compound was synthesized from methyl 6-fluoro-4H-furo[3,2-
b]pyrrole-5-carboxylate (5 mg, 0.0295 mmol) according to General Procedure 2.
Purification was not required, and 4.2 mg (84% yield) of 6-fluoro-4Fl-furo[3,2-
b]pyrrole-5-carboxylic acid 75 was obtained. 19F NMR (376 MHz, CD3OD) S ppm -
168.28 (d, J=1.53 Hz, I F). 'H NMR (400 MHz, CD3OD) S ppm 6.50 (t, J=2.16 Hz,
I H) 7.62 (d, J=2.20 Hz, 1 H).
2.12.v) Synthesis of 3-chloro-4H-furo[3,2-b]pyrrole-5-carboxylic acid (76)
N COZH
Ci N CO2Et LiOH, EtOH Ci
/O ~ / ~ / O
[04411 The title compound was synthesized from ethyl 3-chloro-4H-furo[3,2-
b]pyrrole-5-carboxylate (30 mg, 0.1404 mmol) according to General Procedure 2.
Purification was not required, and 13 mg (50% yield) of 3-chloro-4H-furo[3,2-
b]pyrrole-5-carboxylic acid 76 was obtained. 'H NN1R (400 MHz, CD3OD) S ppm
6.72 (s, I H) 7.66 (s, 1 H).
152
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
2.12.w) Synthesis of 2-trifluoromethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid
(77)
N C02Et KOH, MeOH_ COZH
/ / /
F3C O F3C O
[04421 The title compound was synthesized from ethyl 2-trifluoromethyl-4H-
furo[3,2-b]pyrrole-5-carboxylate (0.05 g, 0.20 mmol) according to General
Procedure
2, and was purified by chromatography over silica gel (reverse phase gradient
20 to
100% MeOH in H20 w/ 0.1 % formic acid over 7 min) to give 2-trifluoromethyl-
4FI-
furo[3,2-b]pyrrole-5-carboxylic acid 77 as an off-white solid in >99% purity
(HPLC)
(0.07 g, 16%). Rf= 0.08 (50:50 heptane / EtOAc); 19F NMR (376 MHz, CDC13) S
(ppm) -66.13 (s, 3 F) 'H NMR (400 MF-lz, CD3OD) S(ppm) 7.05 (m,.I---0.8 Hz, 1
H)
6.75 (s, I H). LCMS m/e 218 (M-H).
2.12.x) Synthesis of 2-fluoro-4H-furo[3,2-b]pyrrole-5-carboxylate acid (78)
H O H
LiOH/EtOH/THF N
OEt OH
F / O 750C / 2hrs F / O
[0443] The title compound was synthesized from ethyl 2-fluoro-4H-furo[3,2-
b]pyrrole-5-carboxylic acid ethyl ester (0.040 g, 0.203 mmol) according to
General
Procedure 2, and was purified by chromatography over silica gel (0 to 100%
EtOAc
in heptane over 20 min) to give a pure 2-fluoro-4H-fiuo[3,2-b]pyrrole-5-
carboxylic
acid 78 as an off white solid (0.020g, 59%). 'H NMR (400 MHz, CD3OD) S ppm
5.85
(dd, J--6.30, 0.63 Hz, 1 H) 6.71 (s, 1 H), '9F NMR (376 MHz, CD3OD) S ppm -
108.82
(d, J=5.94 Hz). LCMS m/e 168 (M-H). 100.0% pure by HPLC.
2.13. Synthesis of 3-cyano-4H-furo[3,2-b]pyrrole-5-carboxylic acid (51)
O- N COOH H2NOH=HCI NC N COOH
DMF, 1250C
O O
[0444] To a solution of 3-formyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (0.20
g,
0.2 M, 1.12 mmol) in DMF (6.0 mL) was added hydroxylamine hydrochloride (0.16
g, 2.24 mmol). The reaction mixture was heated at 125 C overnight, then
cooled to rt.
The mixture was partitioned between EtOAc (20 mL) and H20 (20 mL). The aqueous
phase was extracted with EtOAc (3 x 20 mL). The combined organic phases were
153
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
washed with H20 and saturated aq NaCI, filtered and concentrated in vacuo. The
crude product was chromatographed over silica gel (0 to 40% MeOH in CH2CI2
over
30 min) to give 3-cyano-4H-furo[3,2-b]pyrrole-5-carboxylic acid 51 (4 mg,
2.1%) as
a brown solid in 92.1% purity (HPLC). 'H NMR (400 MHz, CD3OD) S ppm 6.65 (d,
J-0.68 Hz, I H) 7.33 (d,.P--0.68 Hz, I H). LCMS m/e 175 (M-H).
2.14. Synthesis of 6-chloro-4H-furo[3,2-b]pyrrole-5-carboxylic acid (79)
p
N CI
O O
I a
H\ OH DMF, 55 C H OH
N2, 4h
[04451 A stirred solution of 4H-furo[3,2-b]pyrrole-5-carboxylic acid (5.00 g,
33.09
mmol) in anhydrous DMF (40.0 mL) was cooled to 0 C under nitrogen. Solid N-
chlorosuccinimide (4.86 g, 36.39 mmol, 1.10 equiv) was added in several
portions
over 10 min while monitoring the internal reaction temperature. The reaction
was
stirred at 0 C for 30 min, then allowed to warm to rt, followed by heating at
55 C for
a period of 4 h. The progress of the reaction was followed by TLC (8:2
heptane/EtOAc, R f= 0.6) and LCMS m/e 184 (M-1). After 4 h, the reaction would
progress no further and the black reaction mixture was poured into water (600
mL)
and extracted with EtOAc (4 x 500 mL). The combined organic extracts were
passed
through a large Celite /Silica-gel pad to remove the solid material, flushing
with
more EtOAc to afford a dark brown, clear solution which was a very complex
mixture
by TLC. Celite 521 (50 g) was added to the solution and the solvent was
removed in
vacuo. The dried material was loaded into a cartridge and flushed onto a
silica-gel
column (120 g, ISCO preloaded flash SG) with 5% EtOAc/heptane, then
chromatographed using a 5% - 20% EtOAc/heptane gradient to obtain 5.20 g of a
three-component co-eluting mixture, consisting solely of the 4H-furo[3,2-
b]pyrrole-5-
carboxylic acid starting material, the desired 6-chloro-4H-furo[3,2-b]pyrrole-
5-
carboxylic acid in approximately 8-10% of the total material isolated, and a
considerable quantity of the 2-chloro-4H-furo[3,2-b]pyrrole-5-carboxylic acid
as the
major component. The material was reverse-phase purified, using a 95/5%
MeCN/H20 0.05% TFA : 5/95% MeCN/HZO 0.05% TFA elution system, to isolate
154
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
49.7 mg of the desired 6-chloro derivative with an 88% purity after extraction
with
EtOAc (2 L total volurne) and washing with a copious amount of water (3 L
total
volume) to facilitate the removal of any trace amount of TFA. After drying in
vacuo
at rt, the reddish-brown material obtained was further purified by normal
phase, silica-
gel chromatography using 10% MeOH/DCM to achieve 92% purity by HPLC. This
material was dissolved in 0.5 mL MeOH, 1.0 mL of EtOAc was added, then the
solution triturated with heptane to precipitate a brown, clumpy impurity that
was
filtered away to yield a clear, light yellow filtrate. The solvent was again
removed in
vacuo at rt to afford 10.3 mg (0.056 mmol, 1.68% yield) of a pale reddish-
orange
solid which was of 98.3% purity by HPLC. 'H NMR (400 MHz, CD3OD) 8 6.53 (d,
J=2.15 Hz, 1 H) 7.64 (d,.P--2.34 Hz, 1 H). LCMS m/e 184 (M-1).
Example 3:
Synthesis of Fused Pyrrole Pyrrole Analogs
3.1. Syntkesis olIntermediate Atdeh ydes
3.1.a) Synthesis of 1-benzyl-lH-pyrrole-2-carbaldehyde
/N\ COZMe
/N\ CO,Me NaH.BnBr
H DMF
[0446] To a cooled (0 C) solution of methyl-2-pyrrole carboxylate (8.00 g,
63.9
mmol) in DMF (320 mL) was added NaH (60 % by weight 5.10 g, 128 mmol). After
min, benzylbromide (11.4 mL, 95.9 mmol) was added and the reaction was
20 warmed to rt. Stirring was continued for 2 h before quenching with
saturated aq
NH4CI (0.5 L). The mixture was extracted 3x with EtOAc and the combined
organic
layers were washed with H20 (3x) and brine, dried over MgSO4i filtered and
concentrated in vacuo to give a yellow oil. The crude product was
chromatographed
over silica gel (0 to 10% EtOAc in heptane over 25 min) to give methyl 1-
benzyl-llY-
pyrrole-2-carboxylate as a colorless oil (7.75 g, 56%). Rf= 0.48 (25:75
heptane /
EtOAc); 'H NMR (400 MHz, CDC13) S(ppm) 7.28 - 7.34 (m, 2 H) 7.23 - 7.27 (m, 1
H) 7.09 - 7.13 (m, 2 H) 7.01 (dd, J=4.0, 1.8 Hz, 1 H) 6.88 - 6.91 (m, 1 H)
6.19 (dd,
J=4.0, 2.6 Hz, 1 H) 5.57 (s, 2 H).
155
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
/ \ OH
N CO=Me DIBALH N
. I ~ CHzCIz I /
[0447] To a solution of methyl l-benzyl-lH-pyrrole-2-carboxylate (3.00 g, 13.9
mmol) in DCM (70 mL) at -78 C was added a 1M solution of diisobutylaluminum
hydride (DIBAL-H) in heptane (35.0 mL, 34.8 mmol). After 45 min the reaction
was
quenched with saturated aq NH4Cl (20 mL) and Rochell's salt (100 g). The
mixture
was allowed to warm to rt and was stirred for 2.5 h. The reaction mixture was
extracted 3x with EtOAc. The combined organic layers were washed with H20,
saturated aq NaCI, dried over MgSO4, filtered and concentrated in vacuo to
give a
pale yello-w oil. The crude product was chromatographed over silica gel (0 to
20%
EtOAc in heptane over 20 min) to give (1-benzyl-lH-pyrrol-2-yl)-methanol as a
colorless oil (2.30 g, 88%). Rf= 0.47 (1:1 heptane / EtOAc); 'H NMR (400 MHz,
CDC13) S(ppm) 7.27 - 7.35 (m, 4 H) 7.08 - 7.10 (m, I H) 7.06 - 7.08 (m, 1 H)
6.73
(dd, J=2.7, 1.8 Hz, 1 H) 6.19 (dd, J=3.5, 1.8 Hz, 1 H) 6.12 - 6.16 (m, 1 H)
5.21 - 5.23
(s, 2 H) 4.53 (d, J=5.1 Hz, 2 H).
OH O
N TPAP. NMO N
I j CHCIZ
104481 To a mixture of (1-benzyl-lH-pyrrol-2-yl)-methanol (3.08 g, 16.4 mmol)
and powdered 4 A molecular sieves (3.0 g) in DCM (33 mL) was added NMO (2.89
g, 24.7 mmol) along with tetrapropylammonium perruthenate (TPAP) (289 mg,
0.822
mmol). The mixture turned black and exothermed. After 20 min, the crude
mixture
was filtered through a plug of silica gel (EtOAc) to give a red solution. The
solution
was concentrated in vacuo and the resulting oil was chromatographed over
silica gel
(0 to 35% EtOAc in heptane over 35 min) to give 1-benzyl-lH-pyrrole-2-
carboxaldehyde as a colorless oil (2.09 g, 69%). 'H NMR (400 MHz, CDCl3) 8
(ppm) 9.58 (s, 1 H) 7.24 - 7.35 (m, 3 H) 7.16 (dd,.,'--7.7, 1.1 Hz, 2 H) 6.98
(d, J=3.5
Hz, 2 H) 6.26 - 6.31 (m, I H) 5.58 (s, 2 H).
156
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
3.2. Syntbesis of Esters
104491 Unless otherwise indicated, the following ethyl esters were synthesized
from
the indicated aldehyde according to General Procedure lA (to yield an
intermediate
acrylate) followed by General Procedure I B.
3.2.a) Synthesis of ethyl 4-methyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-
carboxyiate
O H
/COzEt 1) NaOEt, EtOH / \N / CO2Et
/f + I
N N, 2) o-xylene reflux N
I
[0450] The title compound was synthesized from N-methyl-2-
pyrrolecarboxaldehyde (3.00 g, 27.4 mmol). The crude product was
chromatographed
over silica gel (0 to 20% EtOAc in heptane over 45 min) to give ethy14-methyl-
1,4-
dihydropyiiolo[3,2-b]pyrrole-2-carboxylate as a white solid (0.870 g, 16%). Rf
0.34 (25:75 heptane / EtOAc); 'H NMR (400 MHz, CDC13) S(ppm) 8.46 (s, 1 H)
6.80
(d, J=2.9 Hz, 1 H) 6.75 (s, 1 H) 5.94 (dd, J=2.9, 0.8 Hz, 1 H) 4.35 (q,.I---
7.1 Hz, 2 H)
3.69 (s, 3 H) 1.3 8(t, J=7. l Hz, 3 H).
3.2.b) Synthesis of ethyl 4-benzyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-
carboxylate
O H
N COZEt
N ~CO=Et 1) NaOEt. EtOH N
+
N3 2) o-xylene reflux
I I \
[0451] The title compound was synthesized from 1-benzyl-lH-pyrrole-2-
carbaldehyde (2.09 g, 11.2 mmol). The crude product was purified by silica gel
column chromatography (0 to 20% EtOAc in heptane over 55 min) to give a brown
solid (0.393 g, 13%). 'H NMR (400 MHz, CDC13) S(ppm) 8.46 (s, 1 H) 7.28 - 7.36
(m, 3 H) 7.16 - 7.21 (m, 2 H) 6.91 (d, J=3.0 Hz, 1 H) 6.58 (dd, J=1.5, 0.7 Hz,
1 H)
6.00 (dd, J=3.0, 0.7 Hz, 1 H) 5.13 (s, 2 H) 4.31 (q, J--7.1 Hz, 2 H) 1.35
(t,.,F--7.1 Hz, 3
H).
157
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
3.3. Syntftesis olCarboxylic Acids from Esters
3.3.a) Synthesis of 4-methyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-carboxylic
acid
(12)
H
/ \/ CO2Et KOH N/ CO=
N MeOH, reflux N
1 1
[0452] The title compound was synthesized from ethyl4-methyl-l,4-dihydro-
pyrrolo[3,2-b]pyrrole-2-carboxylate (0.35 g, 1.8 mmol) according to General
Procedure 2 and was purified by silica gel column chromatography (0 to 50%
EtOAc
in heptane over 11 min) to give 4-methyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-
carboxyiic acid 12 (0.26 g, 88%) as an off white solid in 95% purity by HPLC.
Rf
0.08 (50:50 heptane / EtOAc); 'H NMR (400 MHz, (CD3)2S0) S(ppm) 11.92 (s, 1 H)
10.82 (s, 1 H) 6.91 (d, ,t=2.9 Hz, l H) 6.59 (dd, ./=1.7, 0.8 Hz, 1 H) 5.78
(dd,.T--2.9,
0.8 Hz, I H) 3.62 (s, 3 H). LCMS m/e 165 (M+H).
3.3.b) Synthesis of 4-methyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-carboxylate
potassium salt (12a)
H H
\N/ CO2H K2C03 N COZK
N H2OMeOH N
I I
[0453] To a suspension of K2C03 (0.110 g, 0.798 mmol) in H20 (0.4 mL) and
MeOH (2 mL) was added a solution of 4-methyl-1,4-dihydropyrrolo[3,2-b]pyrrole-
2-
carboxylic acid 12 (262 mg, 1.60 mmol) in MeOH (2 mL). The solution was
stirred
for 20 min and was then concentrated in vacuo to give potassium-4-methyl-1,4-
dihydro-pyrrolo[3,2-b]pyrrole-2-carboxylate 12a as a grey solid in 95 % purity
by
HPLC (294 mg, 91%). 'H NMR (400 MHz, (CD3)2S0) S(ppm) 9.80 (s, 1 H) 6.58 (d,
.1--2.8 Hz, 1 H) 6.10 (s, I H) 5.70 (dd, J=2.8, 0.8 Hz, I H) 3.55 - 3.57 (m, 3
H).
158
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
3.3.c) Synthesis of 4-benzyl-1,4-dihydro-pyrrolo[3,2-b]pyrrole-2-carboxylic
acid
(13)
H H
\ N/ COzEt / \N/ COZH
/
~ KOH
MeOH, reflux
[0454J The title compound was synthesized from ethyl 4-benzyl-1,4-
dihydropyrrolo[3,2-b]pyrrole-2-carboxylate (158 mg, 0.589 mmol) according to
General Procedure 2 and was purified by silica gel column chromatography (0 to
50%
EtOAc in heptane over 12 min) to give 4-benzyl-1,4-dihydro-pyrrolo[3,2-
b]pyrrole-2-
carboxylic acid 13 as an off white solid (82 mg, 58%) in 97% purity by HPLC.
RJ=
0.06 (1:1 heptane / EtOAc); 1 H NMR (400 MHz, (CD3)ZSO) S(ppm) 11.91 (s, 1 H)
10.86 (s, 114) 7.29 - 7.36 (m, 2 H) 7.22 - 7.28 (m, 3 H) 7.11 (d, J=2.9 Hz, 1
H) 6.44
(dd, J--1.7, 0.8 Hz, 1 H) 5.84 (dd, .t=3.0, 0.7 Hz, 1 H) 5.13 (s, 2 H).
3.3.d) Synthesis of 4-benzyl-1,4-dihydro-pyrrolo[3,2-b]pyrrole-2-carboxylate
potassium salt (13a)
H H
N CO=H KCO, \N/ CO2K
N -- N
I / H20 MeOH
104551 To a suspension of K2CO3 (24 mg, 0.17 mmol) in H20 (0.2 mL) and MeOH
(1 mL) was added a solution of 4-benzyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-
carboxylic acid 13 (82 mg, 0.34 mmol) in MeOH (2 mL). The solution was stirred
for
35 min and then concentrated in vacuo to give potassium 4-benzyl-1,4-dihydro-
pyrrolo[3,2-b]pyrrole-2-carboxylate 13a as a grey solid (93 mg, 98%) in 95%
purity
by HPLC. 'H NMR (400 MHz, (CD3)2S0) S(ppm) 9.61 (s, I H) 7.27 - 7.33 (m, 2 H)
7.19 - 7.26 (m, 3 H) 6.74 (d, J--2.9 Hz, 1 H) 5.90 (s, I H) 5.73 (dd,.,[----
2.9, 0.8 Hz, 1
H) 5.04 (s, 2 H).
159
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Example 4
Synthesis of Fused Pyrazole Pyrrole Analogs
4.1. Synthesis oflntermediate Aldehydes
4.1.a) Synthesis of 1-Benzyl-lH-pyrazole-4-carbaldehyde
NaH, BiBr N' OEt
NN ~ oet TM
[0456] To a stirred suspension of NaH (53 mg, 1.33 mmol, 60% dispersion in
mineral oil) in THF (5 mL), was added dropwise over 3 min a solution of ethyl
11Y-
pyrazole-4-carboxylate (155 mg, 1.11 mmol). The mixture was stirred at rt for
45
min and then treated with benzyl bromide (neat). After 2 h, the reaction was
quenched with saturated solution of NH4C1 and extracted with EtOAc (3 x 50
mL).
The combined organic layers were washed with water, brine, dried (Na2SO4),
filtered
and concentrated. Purification by flash chromatography (Isco CombiFlash) 0-60%
EtOAc/heptane provided ethyl 1-benzyl-lH-pyrazole-4-carboxylate (256 mg, 98%).
'H NMR (400 MHz, CDC13) 8 ppm 1.33 (t, J=7.09 Hz, 3 H), 4.28 (q, J=7.08 Hz, 2
H), 5.31 (s, 2 H), 7.24-7.28 (m, 2 H), 7.31-7.42 (m, 3 H), 7.86 (s, 1 H), 7.95
(s, 1 H);
LCMS- MS (ESI+) 230.80 (M+H).
oEt Lawr-+F Nf/ O
/ \ s~a%T
(04571 To a stirred suspension of lithium aluminum hydride (LAH) (68 mg, 1.79
mmol) in THF (8 mL) at 0 C was added dropwise over 5 min a solution of ethyl 1-
benzyl-lH-pyrazole-4-carboxylate (250 mg, 1.1 mmol). After stirring for I h at
0 C,
it was warmed to rt for 30 min and then quenched with 1N HCI until a clear
solution
was obtained. Extraction with EtOAc (3 x 50 mL) and washings of the combined
organic layers with water, and then brine, provided the crude ( l-benzyl-1 H-
pyrazol-4-
yl)methanol after drying and evaporation of the solvent. Crude 'H NMR was
clean
enough to be used as is without further purification: crude yield 192 mg
(94%). 1H
NMR (400 MHz, CDC13) S ppm 4.58 (s, 2 H), 5.29 (s, 2 H), 7.21-7.26 (m, 2 H),
7.29-
7.38 (m, 3 H), 7.39 (s, 1 H), 7.55 (s, I H); LCMS- MS (ESI+) 188.90 (M+H).
160
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Dess-lulartin
oxidation
Dcnn.
/ \
[0458] (1-Benzyl-lH-pyrazol-4-yl)methanol (190 mg, 1.0 mmol) in DCM (8 mL)
at rt was treated with Dess-Martin periodinane (670 mg, 1.58 mmol). After 1.5
h, the
reaction was quenched with a mixture of saturated solution of sodium
thiosulfate and
10% NaHCO3 (1:1) at rt, stirred for 30 min before extraction with DCM (3 x 30
mL).
The combined extracts were washed with.NaHCO3i brine, dried (Na2SO4), filtered
and concentrated. Purification by flash chromatography (Isco CombiFlash) 0-40%
EtOAc/heptane provided 1-benzyl-IH-pyrazole-4-carbaldehyde (86 mg, 46%). IH
NMR (400 MHz, CDC13) S ppm 5.35 (s, 2 H), 7.27-7.30 (m, 2 H), 7.36-7.43 (m, 3
H),
7.88 (s, 1 H), 8.01 (s, 1 H), 9.85 (s, 1 H); LCMS- MS (ESI+) 186.90 (M+H).
4.1.b) Synthesis of 1-phenethyl-lH-pyrazole-4-carbaldehyde
NeH, caL Nal
Pne-,etr,yl-ar
N / I THF. RT-80 . C
N 79'i6
[0459] To a stirred suspension of NaH (125 mg, 3.12 mmol, 60% dispersion in
mineral oil) in THF (10 mL), was added dropwise over 5 min, a solution of 1H-
pyrazole-4-carbaldehyde (250 mg, 2.60 mmol). The mixture was stirred at rt for
45
min; sodium iodide (10 mg) was added before the addition of phenethyl bromide
(0.42 mL, 3.12 mmol). After 15 min, the reaction was heated at 80 C for 4 h,
then
cooled to rt, quenched with saturated solution of NH4C1 and extracted with
EtOAc (3
x 50 mL). The combined organic layers were washed with water, brine, dried
(NaZSO4), filtered and concentrated. Purification by flash chromatography
(Isco
CombiFlash) 0-40% EtOAc/heptane provided 1-phenethyl-llY-pyrazole-4-
carbaldehyde: Yield 410 mg (79%). 'H NMR (400 MHz, CDC13) S ppm 3.20 (t,
.P--7.03 Hz, 2 H), 4.39 (t,.f---7.05 Hz, 2 H), 7.06 (dd,.F--7.91, 1.46 Hz, 2
H), 7.22-7.32
(m, 3 H), 7.63 (s, I H), 8.00 (s, 1 H), 9.79 (s, 1 H); LCMS- MS (ESI+) 200.87
(M+H).
Cottineau, B. et al., J. Bioorg. Med. Lett. 2002, 12, 2105.
161
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
4.1.c) Synthesis of 2-phenethyl-2H-pyrazole-3-carbaldehyde
I NaOEtr N,
N phenehyl Br
RT,25".6
[0460] To a solution prepared by dissolving sodium (1.01 g, 44.07 mmol) in
absolute EtOH (25 mL), was added IH-pyrazole (2.5 g, 36.72 mmol). The solution
was heated to gentle reflux, then allowed to cool to about 50 C and treated
with a
catalytic amount of NaI (25 mg) followed by a slow addition of phenethyl
bromide
(6.0 mL, 44.07 mmol). The reaction was returned to reflux and after a few min,
a
white solid precipitated out of solution. After refluxing for 16 h, the
solvent was
removed by evaporation and the residue dissolved in water (30 mL) and
extracted
with EtOAc (4 x 50 mL). The combined organic extracts were washed with water
and brine, dried (Na2SO4), filtered and concentrated. The crude product was
purified
by flash chromatography (Isco CombiFlash) 0-20% EtOAc/heptane to afford 1-
phenethyl-IH-pyrazole (1.56 g, 25%). 'H NMR (400 MHz, CDC13) S ppm 3.18 (t,
J=7.28 Hz, 2 H), 4.34-4.39 (m, 2 H), 6.18 (t, J-2.06 Hz, I H), 7.07-7.11 (m, 2
H),
7.17 (d, ./=2.20 Hz, I H), 7.20-7.31 (m, 3 H), 7.55 (d, J--1.74 Hz, 1 H); LCMS-
MS
(ESI+) 172.86 (M+H).
nBui- N
cfb ~ ,p + 78 C, 43% ~ N ~
[04611 To a stirred, pre-cooled solution of 1-phenethyl-1 H-pyrazole (1.10 g,
6.39
mmol) in THF (30 mL) at -78 C, was added dropwise n-BuLi (4.8 mL, 7.66 mmol;
1.6 M in hexane) at such a rate that the internal temperature stayed below -70
C.
Following the addition, the mixture was stirred at -78 C for 1.5 h, during
which time
the anion precipitated out as a yellow solid. Then DMF (1.25 mL, 15.97 mmol)
was
added neat and dropwise, and the reaction stirred at -78 C for 90 min when
TLC
indicated the reaction was not progressing any further. It was quenched with
NH4C1
solution (10 mL), allowed to warm to rt and extracted with EtOAc (4 x 50 mL).
The
combined organic extracts were washed with water, brine, dried (Na2SO4),
filtered
and concentrated. Purification by flash chromatography (Isco CombiFlash) 0-10%
EtOAc/heptane provided 2-phenethyl-2H-pyrazole-3-carbaldehyde (540 mg, 43%).
'H NMR (400 MHz, CDC13) S ppm 3.09-3.15 (m, 2 H), 4.74-4.80 (m, 2 H), 6.88 (d,
J=2.10 Hz, I H), 7.16-7.20 (m, 2 H), 7.20-7.32 (m, 3 H), 7.58 (d, J=2.01 Hz, 1
H),
9.77 (s, I H); LCMS- MS (ESI+) 200.88 (M+H).
162
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
4.2. Svnthesis of Esters
[04621 Unless otherwise indicated, the following ethyl esters were synthesized
from
the indicated aldehyde according to General Procedure IA (to yield an
intermediate
acrylate) followed by General Procedure 1B.
4.2.a) Synthesis of ethyl 1-benzyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-
carboxylate
0
N. ~ ~ ~t
EtOH. -5 C RT,~ Na
7796
m-xylene ~
~ COyEt
~J ~2Et reB N N N
N, 0 H
[04631 A) Ethyl 2-azido-3-(1-benzyl-IH-pyrazol-4-yl)acrylate (248 mg, 78%) was
synthesized from 1-benzyl-IH-pyrazole-4-carbaldehyde (200 mg, 1.07 mmol) and
was purified by flash chromatography (Isco CombiFlash, 0-40% EtOAc/heptane);
'H
NMR (400 MHz, CDC13) S ppm 1.37 (t, J=7.14 Hz, 3 H), 4.33 (q,.P--7.14 Hz, 2
H),
5.33 (s, 2 H), 6.83 (s, 1 H), 7.25 (dd, J=7.87, 1.65 Hz, 2 H), 7.31-7.40 (m, 3
H), 7.82
(s, 1 H), 7.94 (s, I H); LCMS- MS (ESI+) 269.86 (M-NZ).
[04641 B) The title compound was prepared from ethyl2-azido-3-(1-benzyl-IH-
pyrazol-4-yl)acrylate and was purified by flash chromatography (Isco
CombiFlash, 0-
30% EtOAc/heptane) to afford ethyl 1-benzyl-1,6-dihydropyrrolo[2,3-c]pyrazole-
5-
carboxylate (137 mg, 62%) as a straw-colored solid. 1H NMR (400 MHz, CDC13) S
ppm 1.34 (t, ./'--7.14 Hz, 3 H), 4.29 (q, J=7.14 Hz, 2 H), 5.40 (s, 2 H), 6.85
(d, J=1.65
Hz, I H), 7.31-7.35 (m, 2 H), 7.39-7.44 (m, 3 H), 7.60 (d, .--0.64 Hz, 1 H),
7.72 (s, I
H); LCMS- MS (ESI+) 269.84 (M+H).
4.2.b) Ethyl 1-phenethyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-carboxylate
- N] /~ . OEt
N~' N~ . o~ C
~~ EIOH, 0 C- RT, IV N3
74 h
\ COZEt ~~ggg~, Q~N ~ COZEt
N H
163
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
[04651 A) Ethy12-azido-3-(I-phenethyl-lH-pyrazol-4-yl)acrylate (462 mg, 74%)
was prepared from 1-phenethyl-lH-pyrazole-4-carbaldehyde (400 mg, 2.0 mmol)
and
was purified by flash chromatography (Isco CombiFlash 0-40% EtOAc/heptane). 'H
NMR (400 MHz, CDC13) S ppm 1.38 (t, .F=7.15 Hz, 3 H), 3.19 (t,.,F--7.27 Hz, 2
H),
4.30-4.39 (m, 4 H), 6.80 (s, 1 H), 7.09-7.12 (m, 2 H), 7.22-7.32 (m, 3 H),
7.72 (s, 1
H), 7.80 (s, 1 H); LCMS- MS (ESI+) 283.88 (M-N2).
[0466] B) The title compound was prepared from ethyl2-azido-3-(1-phenethyl-lhl-
pyrazol-4-yl)acrylate and was purified by flash chromatography (Isco
CombiFlash 0-
30% EtOAc/heptane) to afford ethyl 1-phenethyl-1,6-dihydropyrrolo[2,3-
c]pyrazole-
5-carboxylate (198 mg, 48%) as a white solid. 'H NMR (400 MHz, CDC13) S ppm
1.35 (t, J=7.13 Hz, 3 H), 3.17 (t, J--6.78 Hz, 2 H), 4.28 (q, ,>=7.13 Hz, 2
H), 4.45 (t,
J=6.78 Hz, 2 H), 6.80 (d, ./-'-1.56 Hz, 1 H), 7.08-7.13 (m, 2 H), 7.22-7.31
(m, 3 H),
7.53 (s, 1 H), 7.71 (s, I H); LCMS- MS (ESI+) 283.84 (M+H).
4.2.c) Synthesis of ethyl 1-phenethyl-1,4-dihydropyrrolo[3,2-c]pyrazole-5-
carboxylate
NeOOH
N ~0 "'`~oEI 389~ COZEt
N=~ _ N=1 N3
COZEt m'xyllene N ~
'N ,~-~
reflux 30 min N OEt
=JJ N3 20% H
[0467] A) Ethy12-azido-3-(I-phenethyl-lH-pyrazol-5-yl)acrylate (306 mg, 38%)
was prepared from 2-phenethyl-2H-pyrazole-3-carbaldehyde (530 mg, 2.65 mmol)
and was purified by flash chromatography (Isco CombiFlash 0-20%
EtOAc/heptane).
'H NMR (400 MHz, CDC13) 8 ppm 1.41 (t, J=7.15 Hz, 2 H), 3.11 (t,.T--7.17 Hz, 2
H),
4.35 (q, J=7.13 Hz, 2 H), 4.41 (t, .F--7.15 Hz, 2 H), 6.46 (s, 1 H), 6.93 (d,
J=2.05 Hz, 1
H), 7.01-7.06 (m, 2 H), 7.18-7.29 (m, 3 H), 7.58 (dd, J--2.07, 0.71 Hz, 1 H);
LCMS-
MS (ESI+) 283.86 (M-N2).
[0468] B) The title compound was synthesized from ethyl 2-azido-3-(1-phenethyl-
1H-pyrazol-5-yl)acrylate and was purified by flash chromatography (Isco
CombiFlash 0-30% EtOAc/heptane) to afford ethyl 1-phenethyl-1,4-
dihydropyrrolo[3,2-c]pyrazole-5-carboxylate (50.6 mg, 19 %) as a white solid.
'H
164
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
NMR (400 MHz, CDC13) S ppm 1.40 (t, .1--7.13 Hz, 3 H), 3.18-3.25 (m, 2 H),
4.37 (q,
J=7.13 Hz, 2 H), 4.45 (dd,.f---8.15, 7.03 Hz, 2 H), 6.53-6.57 (m, 1 H), 7.13-
7.18 (m, 2
H), 7.19-7.31 (m, 3 H), 7.39 (s, I H), 8.49 (s, I H); LCMS- MS (ESI+) 283.86
(M+H).
4.3. Synthesis of Carboxylic Acids from Esters
4.3.a) Synthesis of 1-benzyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-carboxylic
acid
(21)
N ' COZH
N." :) COZEt ~~H+ N H N
N N EtO 8% 4C,
()_J
[0469] The title compound was prepared from ethyl 1-benzyl-1,6-dihydro-
pyrrolo[2,3-c]pyrazole-5-carboxylate (118 mg, 0.44 mmol) according to General
Procedure 2. The crude product was purified by flash chromatography (Isco
CombiFlash, 0-60% MeOH/DCM) and preparative TLC on silica with 10%
MeOH/DCM to afford 1-benzyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-carboxylic
acid
21 (40 mg, 38%) as an off-white solid. 'H NMR (400 MHz, CD3OD) 8 ppm 5.40 (s,
2 H), 6.78 (s, 1 H), 7.18-7.22 (m, 2 H), 7.22-7.33 (m, 3 H), 7.49 (s, 1 H);
LCMS- MS
(ESI+) 241.79 (M+H); HPLC (UV = 97%), (ELSD = 100%).
4.3.b) Synthesis of 1-phenethyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-carboxylic
acid (22)
QTO-co2Et QI)-co2H
N H EtOH 94 C, N H
55.296
[0470] The title compound was synthesized from ethyl 1-phenethyl-1,6-
dihydropyrrolo[2,3-c]pyrazole-5-carboxylate (190 mg, 0.67 mmol) according to
General Procedure 2. The crude product was purified through a silica plug (10%
MeOH/EtOAc) to give 1-phenethyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-carboxylic
acid 22 (94.4 mg, 55.2%). 'H 1VMR (400 MHz, CD3OD) S ppm 3.12 (t, J--7.27 Hz,
2
H), 4.41 (t, ,.----7.27 Hz, 2 H), 6.79 (s, 1 H), 7.09-7.12 (m, 2 H), 7.13-7.22
(m, 3 H),
7.47 (s, 1 H); LCMS- MS (ESI+) 255.82 (M+H); HPLC (UV = 97.8%), (ELSD =
100%).
165
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
4.3.c) Synthesis of 1-Phenethyl-1,4-dihydro-pyrrolo[3,2-c]pyrazole-5-
carboxylic acid (28)
NN C UGH=Hz i~~ NfJ \ O
\ H Et eo ~ ~~'H
[0471] The title compound was prepared from ethyl 1-phenethyl-1,4-
dihydropyrrolo[3,2-c]pyrazole-5-carboxylate (50 mg, 0.18 mmol) according to
General Procedure 2. The crude product was purified through a plug of silica
(EtOAc) to give 1-phenethyl-l,4-dihydropyrrolo[3,2-c]pyrazole-5-carboxylic 28
(40.6
mg, 90 %). 'H NMR (400 MHz, CD3OD) S ppm 3.15 (t,.T--7.05 Hz, 2 H), 4.43 (t,
J=7.08 Hz, 2 H), 6.48 (d, J=0.54 Hz, 1 H), 7.07 - 7.11 (m, 2 H), 7.12 - 7.23
(m, 3 H),
7.34 (s, 1 H); LCMS- MS (ESI+) 255.82 (M+H); HPLC (UV = 100%), (ELSD =
100%).
Example 5
Synthesis of Fused Thiazole Pyrrole Analogs
5.1. Synthesis of Esters
5.1.a) Synthesis of ethyl4H-Pyrrolo[3,2-d]thiazole-5-carboxylate
O N rCOZEt NaOEt, EtOH Et02C N
s + N3 0 C to RT Na I s~
EtOZC N~ I N m xylene Et0 C- ~
/ ~
3 reflux Z N s
S H
[04721 A) Ethyl 2-Azido-3-thiazol-4-yl-acrylate (400 mg, 67%) was synthesized
from thiazole-4-carbaldehyde (300 mg, 2.6 mmol) according to General Procedure
1A
and was purified by flash chromatography (Isco CombiFlash 0-40%
EtOAc/heptane).
'H NMR (400 MHz, CDC13) 8 ppm 1.40 (t,.T--7.13 Hz, 3 H), 4.38 (q, J=7.14 Hz, 2
H), 7.27 (s, 1 H), 8.23 (d, J=1.95 Hz, I H), 8.81 (d, J--2.00 Hz, 1 H); LCMS-
MS
(ESI+) 196.84 (M-N2).
[0473] B) The title compound was prepared from ethy12-azido-3-thiazol-4-yl-
acrylate (400 mg, 1.78 mmol) according to General Procedure I B and was
purified by
flash chromatography (Isco CombiFlash 0-30% EtOAc/heptane) to afford ethyl4hf-
pyrrolo[3,2-d]thiazole-5-carboxylate as a white solid (350 mg, 53%). 'H NMR
(400
166
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
MHz, CDC13) S ppm 1.41 (t,.P--7.13 Hz, 3 H), 4.40 (q,.P--7.13 Hz, 2 H), 7.33
(d,
J=1.95, I H), 8.56 (s, 1 H), 9.39 (s, 1 H); LCMS- MS (ESI+) 196.85 (M+H).
5.1.b) Synthesis of ethyl 4H-pyrrolo[2,3-d]thiazole-5-carboxylate
O-; S rCOZEt NaOEt, EtOH Et02C S~
\ -~
I N// + N3 0 C to RT N3 N
EtO2C ~ S ,R.Xylene S
( N~ EtOZC e X i reflux ` N N
~
FI
[0474] A) Ethyl 2-azido-3-thiazol-5-yl-acrylate (246 mg, 41%) was synthesized
from thiazole-5-carbaldehyde (300 mg, 2.6 mmol) according to General Procedure
IA
and was purified by flash chromatography (Isco CombiFlash 0-40%
EtOAc/heptane).
'H NMR (400 MHz, CDC13) S ppm 1.41 (t, J=7.13 Hz, 3 H), 4.39 (q, .F--7.13 Hz,
2
H), 7.19 (s, 1 H), 8.08 (s, 1 H), 8.88 (s, 1 H); LCMS- MS (ESI+) 196.81 (M-
N2).
[0475] B) The title compound was prepared from ethy12-azido-3-thiazol-5-yl-
acrylate (240 mg, 1.1 mmol) according to General Procedure 1 B and was
purified by
flash chromatography (Isco CombiFlash 0-30% EtOAc/heptane) to afford ethyl 4H-
pyrrolo[2,3-d]thiazole-5-carboxylate as a white solid (191 mg, 91%). 'H NMR
(400
MHz, CDC13) 8 ppm 1.42 (t,.t--7.15 Hz, 3 H), 4.41 (q,.I--7.14 Hz, 2 H), 7.16
(d,
J=1.95, 1 H), 8.76 (s, 1 H), 9.86 (s, 1 H); LCMS- MS (ESI+) 196.82 (M+H).
5.2. Synthesis olCarboxylic Acids from Esters
5.2.a) Synthesis of 4H-pyrrolo[3,2-d]thiazole-5-carboxylic acid (41)
N LiOH-H2O N
EtOZC~S EtOH, 94 oC HOOC~N CS~
H H
[0476] The title compound was synthesized from ethyl 4H-pyrrolo[3,2-dJthiazole-
5-
carboxylate (180 mg, 0.95 mmol) according to General Procedure 2 to give 41Y-
pyrrolo[3,2-d]thiazole-5-carboxylic acid 41 (83 mg, 54%) as a beige solid. 'H
NMR
(400 MHz, CD3OD) S ppm 7.14 (s, 1 H), 8.68 (s, 1 H); LCMS- MS (ESI-) 166.7 (M-
H); HPLC (UV = 99.5%), (ELSD = 100%).
167
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
5.2.b) Synthesis of 4H-pyrrolo[2,3-d]thiazole-5-carboxylic acid (44)
/S~ LiOH HZO HOOC~S\
Et02C-,H~N EtOH, 94 OC H N N
[0477] The title compound was synthesized from ethy14H-pyrrolo[2,3-d]thiazole-
5-
carboxylate (190 mg, 0.97 mmol) according to General Procedure 2 to give 4H-
pyrrolo[2,3-d]thiazole-5-carboxylic acid 44 (170 mg, 86%) (HCl salt) as a
beige solid.
'H NMR (400 MHz, CD3OD) S ppm 7.14 (s, 1 H), 8.87 (s, 1 H); LCMS- MS (ESI-)
166.8 (M-H); HPLC (UV = 100%), (ELSD = 100%).
Example 6:
Synthesis of Fused Thiophene Thiophene Analogs
6.1. Synzhesis of Carboxylic Acids
6.1.a) Synthesis of 6-(4-chlorobenzyl)-thieno[3,2-b]thiophene-2-carboxylic
acid
(25)
Rhodamne ~
AcOH / NaOAc
~ reflux ~
NH
ci I~ / s\ H-y s
o
[0478] A) To a 20-mL scintillation vial fitted with a magnetic stir bar was
added 3
mL of glacial acetic acid (AcOH). The vial was capped tightly and heated to 80
C.
To the hot AcOH was added 4-(4-chlorobenzyl)thiophene-2-carbaldehyde (example
1.1.a); 0.37 g, 1.56 mmol, 1 equiv) and rhodanine (0.23 g, 1.7 mmol, 1.1
equiv) with
stirring until a solution was formed. To the mixture was then added anhydrous
sodium acetate (0.45 g, 5.5 mmol, 3.5 equiv), and the vial was capped tightly
and
heated to 110 C for approx. 1 h. The reaction vial was cooled to rt and the
contents
were poured into water. The resulting precipitate was filtered, washed with
water and
a cold mixture of 1:1 water/ethanol. The solid was dried thoroughly in vacuo
at 40 C
to give 5-((4-(4-chlorobenzyl)thiophen-2-yl)methylene)-2-thioxothiazolidin-4-
one
(451 mg, 81%). 'H NMR (400 MHz, (CD3)2S0) S(ppm): 7.70 (s, 1 H), 7.67 (s, 1
H),
7.46 (s, 1 H), 7.34 - 7.38 (m, 2 H), 7.25 - 7.29 (m, 2 H), 3.96 (s, 2 H).
168
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
~ Hydrolysis H
~ \NH H
CI ~ 1. 2M aq. NaoH a p
0 60 deg. C
2. 1096 v/v aq. HCI
[0479] B) To a 20-mL scintillation vial fitted with a magnetic stir bar under
a N2
atmosphere was added 3.5 mL of a 2 M aq. NaOH solution heated to 45 C. 5-((4-
(4-
chlorobenzyl)thiophen-2-yl)methylene)-2-thioxothiazolidin-4-one was added to
the 2
M NaOH solution. After complete dissolution, the temperature of the reaction
vial
was increased to 60 C over a 30 min period. The vial was subsequently cooled
to 5
C and cold 10% (v/v) aq. HCl solution was added until a precipitate formed
(approx.
pH 2-3). The resulting precipitate was collected by filtration, washed several
times
with water, and dried thoroughly under vacuum at 40 C to give 3-(4-(4-
chlorobenzyl)thiophen-2-yl)-2-mercaptoacrylic acid (379 mg, 95% yield). Note:
'H
NMR showed a number of peaks in the aromatic region. The presence of the
signal
for the vinyl proton and the loss of the rhodanine moiety (i.e.; absence of
the proton
attached to the nitrogen in the rhodanine moiety) was used as an indicator of
the
desired compound. The material was used in the next step without further
purification.
Gyclization
H ' ~ ~ OH
p cl ~ S
0 Chlorine gas in
1,1,2-trichloroethane
reflux x 1 hr.
[04801 C) To a 100-mL three-necked round bottom flask fitted with a reflux
condenser, an addition funnel, and a magnetic stir bar was added 3-(4-(4-
chlorobenzyl)thiophen-2-yl)-2-mercaptoacrylic acid (0.38 g, 1.3 mmol, I equiv)
and 8
mL of 1,1,2-trichloroethane. In a separate vessel, a solution of chlorine
(using
approx. 0.1 g of C12 gas) was formed using 20 mL of 1,1,2-trichloroethane in a
40 mL
scintillation vial. The C12 solution was added to the main reaction vessel
dropwise
over 45 min at 25 C. Stirring was continued for I h at 25 C before heating
the
reaction vessel to reflux (approx. 110-115 C) for 1 h. The reaction was
cooled to rt,
the contents filtered, and the collected solid was washed with a small volume
of 1,1,2-
trichloroethane. The crude product was purified by preparative HPLC using a
Chromeleon purification system (60% to 100% over 7 min methanol/0.1% formic
acid-1% acetonitrile in water, 50 mm Dynamax C-18, 28 mL/min) to give 6-(4-
169
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
chlorobenzyl)-thieno[3,2-b]thiophene-2-carboxylic acid 25 (16 mg, 5%). LC/MS
m/e
341 (M+Na). Purity: 95.8% (HPLC, UV); 100% (ELSD). 'H NMR (400 MHz,
CD3OD) S(ppm): 7.96 (s, I H), 7.48 (s, 1 H), 7.27 - 7.36 (m, 4 H), 4.10 (s, 2
H).
6.1.b) Synthesis of 5-chloro-4-(4-chlorobenzyl)-thieno[2,3-b]thiophene-2-
carboxylic acid (27)
I ~ OH
s
C~ ~ CI S
104811 The title compound was prepared from 4-(4-chlorobenzyl)thiophene-3-
carbaldehyde according to procedures A-C outlined above in Example 6.2.a) to
afford
5-chloro-4-(4-chlorobenzyl)-thieno[2,3-b]thiophene-2-carboxylic acid 27 (12
mg,
10% for the final step). Under these conditions, a chlorine substituent was
added to
the 5-position. LC/MS m/e 343 (M+H). Purity: 100% (HPLC, UV); 100% (ELSD).
'H NMR (400 MHz, CD3OD) S(ppm): 7.65 (s, 1 H), 7.29 - 7.33 (m, 2 H), 7.23 -
7.28
(m, 2 H), 4.17 (s, 2 H). J. Med. Chem. 1985, 28(12): 1896-1903.
6.2.c) Synthesis of 6-phenethylthieno[3,2-blthiophene-2-carboxylic acid (60)
[04821 The title compound was synthsized from 4-phenethylthiophene-2-
carbaldehyde (Example 1.1.b)) in three steps according to the procedures
outlined
above in Example 6.l .a).
Rhodanine KII_ cCHO / AcOH / NaOAc ~ NH
reflux, j
~ g S\
O
[04831 A) (Z)-5-((4-phenethylthiophen-2-yl)methylene)-2-thioxothiazolidin-4-
one
(343 mg, 82%). 'H NMR (400 MHz, (CD3)ZSO) S(ppm): 7.80 (s, I H), 7.70 (s, 1
H),
7.56 (s, I H), 7.15 - 7.31 (m, 5 H), 2.91 (s, 4 H).
1. 2M aq. NaOH
60 C
~ 2. 10% v/v aq. HCI
NH j H OH
S O g O
104841 B) (Z)-2-mercapto-3-(4-phenethylthiophen-2-yl)acrylic acid (0.2675 g
(89%
yield). The 'H NMR showed a number of peaks in the aromatic region, presence
of
170
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
the vinyl proton and loss of the rhodanine moiety. The material was used in
the next
step without further purification.
%IS C12
1,1,2-trichloroethane
SH OH reflux, 1 hr. S COOH
~ 0 s
[04851 C) The title compound was synthesized from (Z)-2-mercapto-3-(4-
phenethylthiophen-2-yl)acrylic acid (0.2675 g, 0.93 mmol) and was purified by
preparative HPLC as described above to give 6-phenethylthieno[3,2-b]thiophene-
2-
carboxylic acid 60 (52 mg, 20%). LC/MS m/e 289 (M+H). Purity: 93.4% (BPLC,
UV); 100% (ELSD). 'H NMR (400 MHz, CD3OD) S(ppm): 8.09 (s, 1 H), 7.30 (d,
J=6.39 Hz, 2 H), 7.25 (d,.P--7.17 Hz, 2 H), 7.16 - 7.20 (m, 3 H), 2.91 (s, 4
H).
Example 7
Synthesis of Fused Pyrrole Thiophene Analogs
7.1. Synthesis of 4H-thieno[3,2-blpyrrole-2-carboxylic acid (53)
02N
c-COOH \
q~0 S COOH
[0486] Under N2, fuming nitric acid (4.7 mL, 112.0 mmol) was added slowly over
10
min to acetic anhydride (16.6 mL, 175.6 mmol) cooled in a dry ice/acetone bath
to -78
C. 5-methyl-2-thiophene carboxylic acid (5.0 g, 35.2 mmol) was added in 1 g
portions over 10 min to the solution. The reaction was kept at -20 C for 1 h
before
quenching over ice. The yellow solid was filtered off and washed with water
(200
mL). The crude product was recrystallized from 95 % EtOH to give 5-methyl-4-
nitro-
2-thiophene carboxylic acid as a pale yellow solid (4.6 g, 70 %). 'H NMR (400
MHz,
CD3OD) S(ppm) 8.13 (s, 1H) 2.82 (s, 3H).
OMe N 02N
02N MeO~N' N
\ U N ~ / S COOMe
g COOH DMF
[04871 To a solution of 5-methyl-4-nitro-2-thiophene carboxylic acid (4.6 g,
24.6
mmol) in DMF (14.5 mmol) was added N, N-dimethylformamide dimethyl acetal (3.8
mL, 28.5 mmol) and pyrrolidine (2 drops). The mixture was refluxed for 3 h,
171
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
concentrated in vaczro and the residue taken up in EtOAc (0.2 L). The organic
phase
was washed with water, saturated aq NaCI, dried over Na2SO4, filtered and
concentrated in vacuo. The crude product was chromatographed over silica gel
(0 to
40% EtOAc/heptane over 60 min) to give methyl 5-(2-dimethylaminovinyl)-4-
nitrothiophene-2-carboxylate as a dark red solid (1.0 g, 16 %). 'H NMR (400
MHz,
CDC13) S(ppm) 8.10 (s, IH) 7.31 (d, J=13.1 Hz, IH) 6.56 (d, J-13.1 Hz, IH)
3.87 (s,
3H) 3.07 (s, 6H). LCMS m/e 279 (M+Na).
0)8CO2Me N S C02Me
HCO2NH4, Pd/C MeOH H
[04881 To a solution of methyl 5-(2-dimethylaminovinyl)-4-nitro-thiophene-2-
carboxylate (0.693 g, 2.73 mmol) in MeOH (15.0 mL) were added ammonium
formate (0.332 g, 5.26 mmol) and Pd/C (10 % by weight). The mixture was
refluxed
for 6 h. Additional ammonium formate (0.664 g, 10.53 mmol) was added to the
reaction'and the mixture was refluxed for 20 h. Additional ammonium formate
(0.664
g, 10.53 mmol) and Pd/C (30 % by weight) were added and the reaction mixture
was
refluxed for another 8 h. Additional Pd/C was added and the mixture was
refluxed for
another 16 h. The reaction was cooled and filtered through a Celite plug. The
filtrate was concentrated in vacuo, taken up in EtOAc (0.2 L) and washed with
water,
saturated aq NaCI, dried over Na2SO4, filtered and concentrated in vacuo. The
crude
product was purified by HPLC to obtain methyl 4H-thieno[3,2-b]pyrrole-2-
carboxylate as a yellow solid (0.078 g, 16 %). 'H NMR (400 MHz, CDC13) S(ppm)
8.40 (s, 1H) 7.71 (s, IH) 7.20 (t,J--2.7 Hz, 1H) 6.50 (m, 1H), 3.90 (s, 3H).
S CO2Me KOH S CO2H
C5 / MeOH, reflux / ~ ~/
H H
[04891 The title compound was synthesized from methyl 4H-thieno[3,2-b]pyrrole-
2-carboxylate (0.078 g, 0.43 mmol) according to General Procedure 2 and was
purified by silica gel column chromatography (gradient 25 to 100%
EtOAc/heptane
over 30 min) to give 4H-thieno[3,2-b]pyrrole-2-carboxylic acid 53 as an off-
white
solid in 100 % purity (HPLC) (0.030 g, 42 %). 'H NMR (400 MHz, CD3OD) S(ppm)
7.66 (d, J=0.6 Hz, 1 H) 7.22 (d, J=2.9 Hz, I H) 6.39 (dd, J=2.9, 0.6 Hz, 1 H).
LCMS
m/e 166 (M-H).
172
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Example 8
D-Amino Acid Oxidase Inhibition
8.1. D-Amino Acid Oxidase Enzyme Assay
[0490] DAAO enzyme activity was measured using the substrate D-serine at its
Michaelis-Menton K,,, of 5mM. The rate of oxidation is measured as a rate of
production of hydrogen peroxide, which was detected using the enzyme
horseradish
peroxidase (Sigma cat. No. P-8375). This coupled reaction uses the enzyme
substrate
Amplex Red.(Molecular Probes), which is converted to the fluorescent reaction
product, resorufin (excitation 530-560nm; emission -590nm). Although DAAO has
a
higher pH optimum, all reagents were prepared in 50mM sodium phosphate buffer
at
pH 7.4 and inhibition curves were generated at this pH.
[0491] The final concentrations of components in 200 l total volume per well
(black clear-bottom 96-well plate, Costar) were:
(a) Horseradish peroxidase: 4 Units per mL
(b) D-serine: 5 mM
(c) Test Compound: 100 - 0.0064 uM for IC50
(d) Amplex Red reagent: 50 uM
(e) DMSO: 1.6%
[0492] The reactions were initiated by addition of DAAO enzyme while the
fluorescence was monitored. H202 was added at 16uM final concentration to a
control well on each plate to test for compound interference with a coupled
enzyme.
Inhibition curves were generated in the presence of varying concentrations of
the
inhibitor and IC50 values were calculated for each inhibitor.
8.Z. Results oJDAAO Inhibition Assay
104931 ICSO values were determined for compounds 78, 23, 73, 55, 4, 5, 66, 80,
65,
74, 76, 30, 56; 67, 49, 68, 81, 8, 75, 79, 72, 54, 70, 71, 82, 69, 64, 84, and
6 and are
summarized in Table 2 below.
173
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 2: Human and Porcine DAAO Inhibition [IC5o]
Compound Human
No. Compound Name DAAO
( M)
1 4H-Thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
2 3-Methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
3 2-Methyl-4h/-thieno[3,2-b]pyrrole-5-carboxylic acid (+)
4 2-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+)
2-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (++)
6 2,3-Dibromo-4H-thieno[3,2-b]pynrole-5-carboxylic acid (+)
7 6H-Thieno[2,3-b]pyrrole-5-carboxylic acid (+++)
8 3-Bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (++)
9 3-Benzyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (++)
3-Phenyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+)
11 4H-Furo[3,2-b]pyrrole-5-carboxylic acid (+++)
( )
12a 4-Methyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-carboxylate +
potassium salt
13a 4-Benzyl-1,4-dihydropyrrolo[3,2-b]pyrrole-2-carboxylate (++)
potassium salt
14 3-(4-Chlorobenzyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (++)
3-Phenethyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (++)
16 3-(4-Chlorophenethyl)-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (++)
17 3-Phenethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (++)
18 2-Phenethyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+)
19 2-(4-Chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+)
2-(4-Chlorobenzyl)-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+)
21 1-Benzyl-1,6-dihydropynrolo[2,3-c]pyrazole-5-carboxylic acid (+)
22 1-Phenethyl-1,6-dihydropyrrolo[2,3-c]pyrazole-5-carboxylic acid (+)
23 2-Chloro-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
24 2-Benzyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
6-(4-Chlorobenzyl)-thieno[3,2-b]thiophene-2-carboxylic acid (+)
26 3-Benzyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
174
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 2. continued: Human and Porcine DAAO Inhibition [ICsol
27 5-Chloro-4-(4-chlorobenzyl)-thieno[2,3-b]thiophene-2-carboxylic (+)
acid
28 1-Phenethyl-1,4-dihydro-pyn=olo[3,2-c]pyrazole-5-carboxylic (+)
acid
29 3-(4-Chlorobenzyl)-6h/-thieno[2,3-b]pyrrole-5-carboxylic acid (++)
30 3-Bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+++)
31 3-Cyclopropyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
32 3-Vinyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+++)
33 3-Methylthieno[3,2-b]thiophene-2-carboxylic acid (+)
34 2,3-Dimethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid porcine: (+)
35 Thieno[3,2-b]thiophene-2-carboxylic acid (+)
36 Thieno[2,3-b]thiophene-2-carboxylic acid (++)
37 3-Methylthieno[2,3-b]thiophene-2-carboxylic acid (+)
38 4-Methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
40 3-Isopropyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
41 4H-Pyrrolo[3,2-d]thiazole-5-carboxylic acid (++)
42 3-Hydroxymethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
43 3-Formyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
44 4H-Pyrrolo[2,3-dJthiazole-5-carboxylic acid (+++)
46 (Z)-3-(Prop-l-enyl)-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
47 3-(Trifluoromethyl)-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
48 3-styryl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
49 3-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
50 3-Methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+++)
51 3-Cyano-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
52 6-Methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (++)
53 4H-Thieno[3,2-b]pyrrole-2-carboxylic acid (+)
175
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 2, continued: Human and Porcine DAAO Inhibition [IC50]
54 6-Fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
55 2-Fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
56 3-Fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
57 2-Methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
58 3-Ethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (++)
59 2-Phenethyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+)
60 6-Phenethylthieno[3,2-b]thiophene-2-carboxylic acid (+)
63 1,3-Dimethyl-lH-thieno[2,3-c]pyrazole-5-carboxylic acid (-)
64 4-Bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (++)
65 2-Bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+)
66 2-Fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+++)
67 3-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
68 3-Fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+++)
69 4-Chloro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+++)
70 6-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (+++)
71 6-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid (++)
72 6-Bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid (++)
73 2-Bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid (+)
74 3-Fluoro-4H-furo[3,2-b]pynrole-5-carboxylic acid (+++)
75 6-Fluoro-4H-furo[3,2-b]pynrole-5-carboxylic acid (+++)
76 3-Chloro-4H-furo[3,2-b]pyrrote-5-carboxylic acid (+++)
77 2-Trifluoromethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid (-)
78 2-Fluoro-4H-furo[3,2-b]pyrrole-5-carboxylate acid (+++)
79 6-Chloro-0H-furo[3,2-b]pynrole-5-carboxylic acid (+++)
80 2-Chloro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+)
81 3-Chloro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+++)
82 4-Fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (+++)
84 2,4-dibromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid (-)
IC5o <_ 100 nM =(+++); ICso 51 M =(++); IC50< 100 M =(+); IC50 > 100 M =(-)
Example 9
Measurements of NMDA Receptor Affinity and Other Activities
[0494J Compound 1 was tested for in vitro activities in a panel screen of
receptors
and enzyme targets. Of particular interest is the activity versus the NMDA
receptor.
To measure the affinity of the compound for D-Serine's binding site on the
NMDA
176
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
receptor (also known as the "Glycine site" or the "strychnine-insensitive
glycine
site"), a radioligand-binding assay was performed with membranes prepared from
rat
cerebral cortex. The radioactive ligand was [3H]MDL-105519. The amount of
radioactivity displaced by the compounds was assessed by scintillation
counting.
Non-specific binding is accounted for in the presence of 1mM Glycine.
Affinities are
calculated from the values of % inhibition of specific [3H]MDL-105519 binding
by
the test compounds. Compound 1 (10 M) inhibited 23% of specific binding of
the
radiolabeled compound.
Example 10
Chung Model Data for Compounds 1 and 11
10.1. Met/lods
[0495] Adult male Sprague-Dawley rats, weighing 200-230 g at the time of
surgery,
were used. They were housed in groups of 4 in an air-conditioned room on a 12
h
light/dark cycle. Food and water were available ad libitum. The animals were
allowed
to acclimatize to the experimental environment for three days by leaving them
on a
lifted metal mesh for at least 40 min. The baseline paw withdrawal threshold
(PWT)
was examined using a series of graduated von Frey hairs for 3 consecutive days
before surgery and re-assessed on the 7th day after surgery and on the 11th to
14th day
before drug dosing. The rat Chung model was prepared as described by Kim and
Chung (1992). The-rat was anaesthetized with 5% isoflurane mixed with oxygen
(2 L
per min) followed by an i.p. injection of sodium pentobarbitone at 50 mg/kg.
The
back was shaved and sterilized with 75 % ethanol. The animal was placed in a
prone
position and a para-medial incision was made on the skin covering L4-6 level.
The
L5 spinal nerve was carefully isolated and tightly ligated with 6/0 silk
suture. The
wound was then closed in layers after a complete hemostasis. A single dose of
antibiotics (Amoxipen 15 mg/rat, ip) was routinely given for prevention of
infection
after surgery. The animals were placed in a temperature controlled recovery
chamber
until fully awake before being returned to the home cage. The animals were
placed in
individual Perspex boxes on a raised metal mesh for at least 40 min before the
test.
Starting with the filament of lowest force, each filament was applied
perpendicularly
to the centre of the ventral surface of the paw until slightly bent for 6
seconds. If the
animal withdrew or lifted the paw upon stimulation, then a hair with force
177
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
immediately lower than that tested was used. If no response was observed, then
a hair
with force immediately higher was tested. The lowest amount of force required-
to
induce reliable responses (positive in 3 out of 5 trials) was recorded as the
value of
PWT. Only those animals with significant allodynia (PWTS 3.5 g) were selected
for
drug dosing experiments. The rats in a neuropathic pain state were randomly
divided
into experimental groups: Vehicle group and 1 group had 8 rats and the
gabapentin
group had 9 rats. The drug test was carried out 12 to 14 days after surgery.
Isotonic 50 mM phosphate buffer (PB), dosed orally at 3 mL/kg, served as the
vehicle control.
Gabapentin was dissolved in normal saline and given orally at 100 mg/kg. 1 was
dissolved in PB to 10mg/mL and given orally at 30 mg/kg. The PWT was assessed
at
1, 3, 6 and 24 h following drug or vehicle administration. The animals were
returned
to their come cage for a break (about 30 min) between two neighboring testing
time
points. One-way analysis of variance (ANOVA) (SPSS software) was used for
statistical analysis to compare different groups on the same time points.
Paired
Student -t test was used to compare different time points in the same group.
The
significance level was set at P<0.05.
10.2. Results for 4H-thienoJ3,2-bJpyrrole-5-carboxylic acid (1)
[0496] In naive rats before surgery, the PWT ranged from 8.6 to 20 g, with an
average value around 10-13 g (12.53 1.53 g and 12.63 1.49 for the left and
right
limbs, respectively, in the vehicle group on the day before surgery, 11.71
1.05 g and
11.62 f 1.07 g for both the left aiid right sides, respectively in the
gabapentin group
and 11.4 t 1.06 g and 11.30 f 1.09 g for both the left and right sides,
respectively, in
the 1 group). There was no statistical difference between the groups (one-way
ANOVA). On day 7 after surgery, the PWT on the side ipsilateral to the ligated
nerve
was significantly lower that of pre-surgical levels (2.26 f 0.64 g for the
vehicle
control group, 1.62 f 0.23 g for the gabapentin group and 1.76 t 0.21 g for
the 1
group, P<0.001 for all groups compared to pre-surgery values, paired Student-t
test).
On day 12 to 14, before dosing, the PWT on the ipsilateral side were further
decreased. The animals also showed some degree of disuse of the affected limb
or
limping. However, the general behavior of animals was not remarkably different
from their naive counterparts. After surgery, the PWT on the operated side was
significantly lower compared to the contralateral side. Prior to vehicle
administration
on the day of experiment, the PWT was 1.34 t 0.30 g on the ipsilateral side
versus
178
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
8.15 t 0.19 g on the contralateral side (n=8). After vehicle treatment, the
PWT were
not significantly changed in either hind limb over a period of 24 hours
(P>0.05,
compared to the pre-dosing level). On the ipsilateral side, the PWT was 1.09 +
0.10 g,
1.18 0.27 g, 1.30 f 0.34 g and 1.19 f 0.20 g at the 1, 3, 6 and 24 hour time
points,
respectively. On the contralateral side, the PWT was 8.95 f 0.97 g, 9.05
0.97 g, 9.15
f 0.97 g and 8.86 f 1.09 g at the 1, 3, 6 and 24 hour time points,
respectively.
Gabapentin, after oral dosing, significantly increased the PWT on the
ipsilateral side.
The effect became significant 1 hour after dosing (from 1.48 f 0.22 g before
dosing to
3.77 + 0.42 g 1 hour after dosing, P<0.001, n = 9). Three hours after dosing,
the effect
reached a peak (6.27 t 0.76 g, P<0.001 compared to pre-dosing level). At 6 and
24
hours after gabapentin, the PWT was 2.38 f 0.29 g and 2.69 t 0.60 g,
respectively
(P<0.01 a:rid P>0.05, respectively, paired Student's t-test, compared to the
pre-drug
level). The PWT at 1, 3 and 24 hour time points were significantly higher than
those
observed in the vehicle group at the same time points (P<0.001 in general and
from
P<0.05 to P<0.001 at different time points, one way ANOVA). In contrast, the
PWT
on the side contralateral to the nerve ligation were not significantly changed
over the
whole observation period in general. The PWTs were 9.67 f 0.68 g before drug
dosing and 10.11 t 0.93 g, 10.11 0.93 g, 8.29 t 0.42 g and 9.40 0.71 g at
1, 3, 6
and 24 hours after drug dosing, respectively (P>0.05 for all time points,
compared to
the pre-dosing level, paired Student's t-test). Compound 1, at 30 mg/kg,
induced a
significant increase in PWT in the ipsilateral side of Chung model rats. The
effect
was observed 1 hour after dosing and reached a peak 6 hours after dosing. The
PWT
were 1.25 f 0.18 g before drug dosing and 2.50 f 0.33 g an hour after dosing
(P<0.01,
compared to pre-dosing control level, paired Student's t-test). From 3 hours
onward,
the PWT gradually increased to reach a maximum level at 6 hours after drug
administration (4.44 0.27 g and 5.71 0.66 g at 3 and 6 hours,
respectively,
P<0.001 for both time points, compared to the pre-dosing level, paired
Student's t-
test). At 24 hours after dosing, the PWT declined to near the pre-dosing
control level
(1.90 f 0.38 g, P>0.05). At all of the time points observed from 1 to 24
hours, the
PWT were significantly (P<0.001 and 0.01) higher than those recorded at the
same
time points in the vehicle control group. The PWT on the contralateral side
were not
significantly changed over the whole observation period. The PWT observed at
1, 3, 6
and 24 hours after dosing were 8.15 t 0.45 g, 8.90 f 0.15 g, 9.70 0.77 g and
8.35 f
0.50 g, respectively (P>0.05, compared to pre-drug level of 8.80 f 0.13 g).
179
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
10.3. Results for 4H furo[3,2-bJpyrrole-5-carbozylic acid (11)
[0497] In rats that were dosed orally with vehicle, there were no significant
changes
in PWT from the baseline value over the 24-hour observation period.
Gabapentin, as a
positive control, orally dosed at 100 mg/kg, significantly increased the PWT,
with
effects commencing the first hour after oral dosing and reaching a peak 3
hours after
dosing. The effect of gabapentin gradually declined from 6 hours onwards. 11,
at an
oral dose of 10 mg/kg, also significantly elevated the PWT. Similar to
gabapentin, the
increase in PWT was first observed 1 hour after dosing. The effect reached a
peak at 6
hours after dosing.
Example 11
Hot-Plate Data for 4H-Thieno[3,2-b]pyrrole-5-carboxylic acid (1)
11.1. Methods
[04981 The method, which detects analgesic activity, followed that described
by
Eddy and Leimbach (J. Pharmacol. Exp. Ther., 107, 385-393, 1953).
Rats were placed onto a hot metal plate maintained at 52 C surrounded by a
Plexiglas
cylinder (Height: 26 cm; Diameter: 19 cm) (Apelex : Model DS37). The latency
to
the first foot-lick was measured (maximum : 30 seconds). 10 rats were studied
per
group. The test was performed blind. 1 was evaluated at 2 doses (10 and 30
mg/kg),
administered i.p. 30 minutes before the test, and compared with a vehicle
control
group. Morphine (8 mg/kg i.p.), administered under the same experimental
conditions, was used as the positive control. The experiment therefore
included 4
groups. Data were analyzed by comparing treated groups with vehicle control
using
unpaired Student's t tests.
11.2. Results
[0499] The results for 4H-thieno[3,2-b]pyrrole-5-carboxylic acid 1 are
summarized
in Table 3. In summary, unlike morphine at 8 mg/kg i.p., 4H-thieno[3,2-
b]pyrrole-5-
carboxylic acid did not increase foot-licking latency at either 10 or 30 mg/kg
i.p.
180
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 3: Effects of 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) and morphine
in the hot plate test in the rat (10 rats per group)
Compound I FOOT-LICKING LATENCY (#)
(mg/kg) (s)
i.p. -2 h
mean t s.e.m. p % change
value from control
Vehicle 18.5 t 2.5 - -
13.2 t 2.2 NS 0.1352 -29%
30 16.0 * 2.3 NS 0.4846 -14%
MORPHINE
8 mg/kg 25.4 t 2.1 0.0455 +37%
l.p. -30 min
Student's t test: NS = Not Significant; p < 0.05; (#): cut-off = 30 seconds.
Example 12
5 Tail Flick Data for 4H-Thieno[3,2-b]pyrrole-5-carboxylic acid (1)
12.1 Metliods
[0500] The method, which detects analgesic activity, followed that described
by
d'Amour and Smith (J. Pharmacol. Exp. Ther. 72, 74-79, 1941). The rat's tail
was
heated by means of an infrared radiant energy source (Ugo Basile: type 7360)
(setting
10 20 IR). The latency before the animal withdraws its tail was measured
(maximum: 30
seconds). 10 rats were studied per group. The test was performed blind. 1 was
evaluated at 2 doses (10 and 30 mg/kg), administered i.p. 30 minutes before
the test,
and compared with a vehicle control group. Morphine (8 mg/kg i.p.),
administered
under the saine experimental conditions, was used as the positive control. The
experiment therefore included 4 groups. Data were analyzed by comparing
treated
groups with vehicle control using unpaired Student's t tests.
12.2 Results
105011 The results for 4H-thieno[3,2-b]pyrrole-5-carboxylic acid 1 are
summarized
in Table 4. In summary, unlike morphine at 8 mg/kg i.p., 4H-thieno[3,2-
b]pyrrole-5-
carboxylic acid did not increase tail-flick latency at either 10 or 30 mg/kg
i.p.
181
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 4: Effects of 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) and morphine
in the tail-flick test in the rat (10 rats per group)
Compound I TAIL-FLICK LATENCY (#)
(mg/kg) (s)
i.p. -2 h
mean t s.e.m. p % change
value from control
Vehicle 4.2 t 0.9 - -
3.5 t 0.3 NS 0.4486 -17%
30 5.5 3 0.9 NS 0.3380 +31%
MORPHINE
8 mg/kg 24.7 t 2.8 <0.0001 +488%
i.p_ -30 min
StudenCs t test NS = Not Significant; p < 0.001; (#): cut-off = 30 seconds.
5 Example 13
Formalin Paw Test (early phase) Data for 41Y-Thieno[3,2-b]pyrrole-
5-carboxylic acid (1)
13.1 Methods
[0502] The method, which detects analgesic/anti-inflammatory activity,
followed
10 that described by Wheeler-Aceto et al (Psychopharmacology, 104, 35-44,
1991). Rats
were given an intraplantar injection of 5% formalin (50 l) into the posterior
left paw.
This treatment induces a recognizable flinching response in control animals.
The
number of flinches was counted for 10 minutes, beginning immediately after
injection
of formalin. 10 rats were studied per group. The test was performed blind. 1
was
evaluated at 2 doses (10 and 30 mg/kg), administered i.p. 30 minutes before
formalin,
and compared with a vehicle control group. Morphine (8 mg/kg i.p.),
administered
under the same experimental conditions, was used as the positive control. The
experiment therefore included 4 groups. Data were analyzed by comparing
treated
groups with vehicle control using unpaired Mann-Whitney U tests.
13.2 Results
[0503] The results for 4H-thieno[3,2-b]pyrrole-5-carboxylic acid 1 are
summarized
in Table 5. In summary, unlike morphine at 8 mg/kg i.p., 4H-thieno[3,2-
b]pyrrole-5-
carboxylic acid at either 10 or 30 mg/kg i.p.did not significantly reduce the
number of
flinches observed during the first 10 minutes after administration of
formalin.
182
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 5: Effects of 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) and morphine
in the formalin paw test (early phase) in the rat (10 rats per group)
Compound I NUMBER OF FLINCHES
(mg/kg) (0 to 10 min after formalin)
i.p. 2 h before formafin
mean t s.e.m. p % change
value from control
Vehicle 25.8 3.1 - -
31.1 3.0 NS 0.2263 +21%
30 25.6 3.5 NS 0.9096 -1%
MORPHINE
8 mg/kg 4.4 0.9 0.0002 -83%
i.p. 30 min before formalin
Mann-Whitney U test NS = Not Significant; p< 0.001
5
Example 14
Formalin Paw Test (late phase) Data for 4H-Thieno[3,2-b]pyrrole-5-carboxylic
acid (1)
14.1 Methods
10 105041 The method, which detects analgesic/anti-inflammatory activity,
followed
that described by Wheeler-Aceto et al (Psychopharmacology, 104, 35-44, 1991).
Rats
were given an intraplantar injection of 5% formalin (50 l) into the posterior
left paw.
This treatment induced a recognizable flinching response in control animals.
The
number of flinches was counted for 15 minutes, beginning 20 minutes after
injection
of formalin. 8 rats were studied per group. The test was performed blind. 1
was
evaluated at 2 doses (10 and 30 mg/kg), administered i.p. 30 minutes before
the test
(i.e. 10 minutes before formalin), and compared with a vehicle control group.
Morphine (8 mg/kg i.p.), administered under the same experimental conditions,
was
used as reference substance. The experiment therefore included 4 groups. Data
were
analyzed by comparing treated groups with vehicle control using unpaired Mann-
Whitney U tests.
14.2 Results
[0505] The results for 4H-thieno[3,2-b]pyrrole-5-carboxylic acid 1 are
summarized
in Table 6. In summary, 4H-thieno[3,2-b]pyrrole-5-carboxylic acid dose
dependently
reduced the number of flinches observed during the late phase (20 - 25 minutes
after
formalin) of the formalin test.
183
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 6.: Effects of 4H-thieno[3,2-bJpy.rrole-5-carboxylic acid (1) and
morphine
in the formalin paw test (late phase) in the rat (10 rats per group)
Compound I NUMBER OF FLINCHES
(mg/kg) (20 to 35 rnin after formalin)
i.p. 2 h before fonnaiin
mean t s.e.m. p % change
value from control
Vehicle 119.1 t 14.2 - -
83.6 t 11.8 NS 0.0657 -30%
30 48.9 3 12.2 0.0063 -59%
MORPHINE
8 mg/kg 6.3 t 2.5 0.0008 -95%
l.p. 30 min before formalin
Mann-Whitney U test: NS = Not Significant; "' = p < 0.01; '"' = p < 0.001
5 Example 15
Rat Forced Swim Test data for 4H-thieno[3,2-bJpyrrole-5-carboxylic acid (1)
15.1 Methods
105061 The method, which detects antidepressant activity, followed that
described
by Porsolt et al (Eur. J. Pharmacol., 47, 379-391, 1978). Rats forced to swim
in a
10 situation from which they cannot escape rapidly become immobile.
Antidepressants
decrease the duration of immobility. Rats were individually placed in a
cylinder
(Height = 40 cm; Diameter = 20 cm) containing 13 cm water (25 C) for 15
minutes
on the first day of the experiment (Session 1) and were then put back in the
water 24
hours later for a 5 minute test (Session 2). The duration of immobility during
the 5
minute test was measured. 6 rats were studied per group. The test was
performed
blind. Compound 1 was evaluated at 2 doses (10 and 30 mg/kg), administered
i.p. 3
times : 24 hours, 4 hours and 30 minutes before the test (Session 2), and
compared
with a vehicle control group. Imipramine (32 mg/kg i.p.), administered under
the
same experimental conditions, was used as reference substance. The experiment
therefore included 4 groups. Data were analyzed by comparing treated groups
with
vehicle control using unpaired Student's t tests.
15.2 Results
[0507] The results for 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) are
summarized in Table 7. In summary, 4H-thieno[3,2-b]pyrrole-5-carboxylic acid
at
184
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
30 mg/kg reduced the duration of immobility by 35% (p = 0.0059) compared to
vehicle.
Table 7: Effects of 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) and
imipramine in the behavioral despair test in the rat (6 rats per group)
1 DURATION OF IMMOBILITY
(mgfkg) (s)
i.p.-24h,-4 hand-2h
mean t s.e.m. p % change
value from control
Vehicle 187.2 t 7.5 - -
178.5 13.0 NS 0.5755 -5%
30 122.5 t 17.0 0.0059 -35%
IMIPRAMINE
32 mg/kg 79.0 19.5 0.0004 -58%
i.p. -24 h, -4 h and -30 min
5 Student's t test: NS = Not Significant; "= p < 0.01; p < 0.001
Example 16
Amphetapine Stereotypy Test Data for 4H-thieno[3,2-b]pyrrole-5-carboxylic
acid (1)
16.1 Methods
10 [0508] The method, which detects antipsychotic activity, followed that
described by
Simon and Chermat (J. Pharmacol. (Paris), 3, 235-238, 1972). Amphetamine
induces stereotyped behavior characterized by sniffmg and head movements.
Stereotypies are antagonized by established antipsychotics, acting mainly on
dopaminergic systems at the striatal level. Rats were placed in individual
Plexiglas
enclosures (20 x 10 x 10 cm). They were injected with d-amphetamine (3 mg/kg
i.p.)
and scored for the intensity of stereotypies on a 4 point scale (0 - 3).
Observations
were performed at 10 minute intervals for 3 hours. A total stereotypy score
per animal
was obtained by cumulating the stereotypy scores obtained at each interval. 6
rats
were studied per group. The test was performed blind. 1 was evaluated at 2
doses (10
and 30 mg/kg), administered i.p. 30 minutes before amphetamine, and compared
with
a vehicle control group. Haloperidol (1 mg/kg i.p.), administered under the
same
experimental conditions, was used as reference substance. The experiment
therefore
included 4 groups. Data were analyzed by comparing treated groups with vehicle
control using unpaired Student's t tests.
185
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
16.2 Results
[0509] The results for 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) are
summarized in Table 8. In summary, 4H-thieno[3,2-b]pyrrole-5-carboxylic acid
at
30 mg/kg significantly reduced the stereotypy intensity, compared to vehicle,
at the
70-120 minute interval, 130 to 180 minute interval, and over 180 minute
interval, with
the greatest reduction (69%, p < 0.0001) occurring during the 130 to 180
minute
interval.
Table 8: Effects of 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) and
haloperidol
in the amphetamine stereotypy test in the rat (6 rats per group)
STEREOTYPY tNTENSfTY
1~01 Taal ecare Teq1 eeas 7ob1 eeaa Cu'nJaroO seae
L0.-2n 10-90min 70-120min 170.180min o=arlBOnin
mean s e.e.e+. P %a n0O nxan : e.e.m. p x~0O meen t we.m p %~ meen a e.e.m. p
%"~e
vew ~min wwa vuus tran t ~ma van. nan Ca,Ca =aN. hmm mMd
VOMpO 13.520.5 - - 112 : 0.e - - 72 t 0.3 - - S].a ! 1.7 - -
/0 13.3 e 0.0 NS 0.9727 -1% 13.2 0 1.3 NS 1.0000 0% 0.2 s 1.0 NS 0.6058 -14%
22.7 a 2-7 NS 0.7190 -7%
30 11.7 t 0.8 NS 0.1646 -13% 10.3 ! 0.3 ^ 0.0081 .22% 2.2 ! 0.5 '^ <0.0001
=80% 2e.2 t 1.2 0.0010 -28%
)/AL pCRlDOL
1 mOhO 1.8 ! 0.5 A.0001 .87% 0.2 ! 0.2 Ø0001 -08% 0.2 s 0.2 ^- -0001 -07% 22
= 0.7 M.0001 -03%
7.p. -70 min
lo Haeeneele...eNS=NO151pN0ant--=0- 0.01;^=0<0.001.
Example 17
In vivo Elevation of D-Serine Levels in the Cerebellum
17.1 Methods
[0510] Mice (C57BL/6, 8-9 weeks of age) are dosed intraperitoneally at 10
mL/kg
with 50 mg/kg of compound suspended in 45% (w/v) hydroxy-(3-cyclodextrin
vehicle.
Animals are sacrificed at either 2 or 6 hours post compound administration
with an
N=3 per time point. At sacrifice, trunk blood is collected into tubes
containing
potassium EDTA, which are then centrifuged to permit isolation of plasma. The
cerebellum is dissected from each animal. Plasma and cerebellum samples are
stored
at -80 C until samples are analyzed (LC/MS/MS).
17.2 Results
[0511] The results for compounds 1, 2, 3, 4,5, 7, 8, 9,11, 12a, 13a, 16,17,29,
30,
32, 35, 36, 41, 44, 49, 50, 52, 54, 55, 56, 64, 66, 67, 68, 70, 71, 74, 75, 76
and 77 are
summarized in Table 9. In summary, a number of compounds, dosed either 10 or
50
mg/kg i.p., are effective at increasing cerebellar D-serine levels at the two-
hour time
186
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
point, as compared to vehicle. A smaller subset of compounds is also effective
at
maintaining elevated D-serine levels through the 6-hour time point.
187
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 9: In vivo Elevation of D-Serine Levels in the Cerebellum
Dose Avg D-serine
Cmpd Compound Name (mgtkg) Time l.evel in
No. (h) Cerebellum
(nmol/g)
Vehicle 2 2.3 (-)
I 4H-Thieno[3,2-b]pyrrole-5-carboxylic acid 50 ++
6 ++
2
2 3-Methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 +
6
3 2-Methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 2
6
4 2-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 2
6
2 +
2-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50
6 +
7 6H-Thieno[2,3-b]pyrrole-5-carboxylic acid 50 2 ++
6 +
8 3-Bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 50 2 +
6 +
9 3-Benzyl-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 50 2
6 _
11 4H-Furo[3,2-b]pyrrole-5-carboxylic acid 50 2 ++
6 Hi
12a Potassium- 4-methyl-1,4-dihydro-pyrrolo[3,2- 50 2 +
b]pyrrole-2-carboxylate 6 -
13a Potassium 4-benzyl-1,4-dihydro-pyrrolo[3,2- 50 2 +
b]pyrrole-2-carboxylate 6 -
16 3-[2-(4-Chlorophenyl)-ethyl]-6H-thieno[2,3- 50 2
b]pyrrole-5-carboxylic acid 6 -
17 3-Phenethyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 2
6 -
29 3-(4-Chlorobenzyl)-6H-thieno[2,3-b]pyrrole-5- 50 2
carboxylic acid 6 -
30 3-Bromo-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 2 +
6 +
32 3-Vinyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 2 +
6
35 Thieno[3,2-b]thiophene-2-carboxylic acid 50 2 +
6
188
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Table 9: Continued, In vivo elevation of D-serine levels in the cerebellum
36 Thieno[2,3-b]thiophene-2-carboxylic acid 50 2 ++
6 +
41 4H-Pyrrolo[3,2-d]thiazole-5-carboxylic acid 50 2 +
6 +
2
44 4H-Pyrrolo[2,3-d]thiazole-5-carboxylic acid 50 ++
6 +
2 -
49 3-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50
6 -
50 3-Methyl-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 2 +
6
+
52 6-Methyl-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 2
6 +
2 ++
54 6-Fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 10
6 +
55 2-Fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 2 ++
6 +
56 3 Fluoro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 10 2 +
6 +
2 +
64 4-Bromo-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 50 6
2 ++
66 2-Fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 50 6 +
+
67 3-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 2
6 +
+
68 3-Fluoro-6H-thieno[2,3-b]pyrrole-5-carboxylic acid 50 2
6 +
70 6-Chloro-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 10 2
6 -
71 6-Bromo-4H-thieno[3,2-b]pyrrole-5-carboxylic acid 50 2 +
6 +
++
74 3-Fluoro-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 2
6 ++
75 6-Fluoro-4H-furo[3,2-b]pyrrole-5-carboxylic acid 50 2
6 +
-
76 3-Chloro-4H-furo[3,2-b]pyrrole-5-carboxylic acid 10 2
6 -
> 10=(+++); 5-9.9=(++);2.5-4.9=(+);<2.5=(-)
189
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
Example 18
Contextual Fear Conditioning Data for 4H-furo[3,2-b]pyrrole-5-carboxylic acid
(11) and 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1)
18.1. Methods
[0512] Young-adult C57BL/6 male mice were used. Mice were received at 6-7
weeks of age. Upon arrival, mice were assigned unique identification numbers
(tail
marked) and were group housed in polycarbonate cages with filter tops. All
mice
were acclimated to the colony room for at least four weeks prior to testing
and were
subsequently tested at an average age of 10-12 weeks of age. During the period
of
acclimation, mice were examined on a regular basis, handled, and weighed to
assure
adequate health and suitability. Mice were maintained on a 12 /12 light/dark
cycle
with the light on at 6:00 a.m. The experiments were always conducted during
the light
phase of the cycle. The day before the initiation of the experiment, mice were
housed
single in individual cages and maintained so till the end of the experiment.
Animals
were randomly assigned across treatment groups. With the exception of testing
times,
the mice had ad lib access to food and water. Rolipram (0.1 mg/kg) was
dissolved in
1% DMSO i.p. 20 min prior to training at a dose volume of 8 ml/kg. To assess
contextual conditioning, we use a standardized contextual fear conditioning
task
originally developed for evaluation of memory in CREB mutant mice
(Bourtchouladze, R. et al.; Cell 1994, 79, 59-68). Specifically, on the
training day,
the mouse is placed into the conditioning chamber for 2 minutes before the
onset of
the unconditioned stimulus (US), a 0.75 mA foot shock of 2 seconds duration.
The
US is repeated two times with a I min inter-trial interval between shocks.
Training is
performed using an automated software package. After the last training trial,
a mouse
is left in the conditioning chamber for another 30 sec and then placed back in
its
home cage. Contextual memory is tested 24 hours after training. The mouse is
placed into the same training chamber and conditioning is assessed by scoring
freezing behavior. Freezing is defined as the complete lack of movement in
intervals
of 5 seconds (Kim et al., 1993; Phillips & LeDoux, 1992; Bourtchouladze et
al.,
1994; 1998; Abel et al., 1997; Kogan et al., 1997). Total testing time lasted
3
minutes. After each experimental subject, the experimental apparatus is
thoroughly
cleaned with 75% ethanol, water, dried, and ventilated for a few minutes. To
evaluate the effects of compounds on contextual memory, we injected mice with
a
compound or vehicle 2 hours before training and trained them with 2 training
trials.
190
CA 02659060 2008-12-10
WO 2008/005456 PCT/US2007/015396
In parallel, a separate group of mice was injected with a reference compound,
Rolipram or vehicle alone, 20 minutes before training. Mice were tested in the
same
context 24 hours after training.
18.2. Results
[0513] Compound 11 was dissolved in vehicle A and administered p.o. 2 hrs
prior
to training at a dose volume of 10 ml/kg. 10 mg/kg of 11-injected mice froze
significantly more than vehicle injected mice (69.7% +/- 3.0% and 33.3% +/-
5.1%
for a compound- and vehicle-injected, respectively; p<0.001; n=10 per dose).
Similarly, Rolipram injected mice froze significantly more than their
corresponding
vehicle-injected mice (44.4% +/-4.4% vs. 27.2% +/- 3.6% for Rolipram and
vehicle,
respectively; p<0.05). Importantly, there was no effect of drug-compound
injections
on immediate freezing responses measured 30 sec after training.
[0514] 4H-thieno[3,2-b]pyrrole-5-carboxylic acid (1) was active at 10 mg/kg
P.O.
[0515] 'A11 publications and patent documents cited in this application are
incorporated by reference in their entirety for all purposes to the same
extent as if
each individual publication or patent document were so individually denoted.
By
their citation of various references in this document, Applicants do not admit
any
particular reference is "prior art" to their invention.
191