Note: Descriptions are shown in the official language in which they were submitted.
CA 02659938 2009-03-25
=
SILICATES ADDITION IN BITUMEN FROTH TREATMENT
FIELD OF THE INVENTION
The present invention relates generally to a bitumen froth treatment process
for
reducing the fine solids concentration in hydrocarbon diluent-diluted bitumen
("dilbit"). In particular, silicates, such as sodium silicates, are added
during bitumen
froth treatment stage(s) to aid in the removal of fine solids such as clays
with the
water phase.
BACKGROUND OF THE INVENTION
Oil sand, as known in the Athabasca region of Alberta, Canada, comprises water-
wet, coarse sand grains having flecks of a viscous hydrocarbon, known as
bitumen,
trapped between the sand grains. The water sheaths surrounding the sand grains
contain very fine clay particles. Thus, a sample of oil sand, for example,
might
comprise 70% by weight sand, 14% fines, 5% water and 11% bitumen (all % values
stated in this specification are to be understood to be % by weight except
where
otherwise provided).
For the past 25 years, the bitumen in Athabasca oil sand has been commercially
recovered using a water-based process. In the first step of this process, the
oil sand
is slurried with process water, naturally entrained air and, optionally,
caustic (NaOH).
The slurry is mixed, for example in a tumbler or pipeline, for a prescribed
retention
time, to initiate a preliminary separation or dispersal of the bitumen and
solids and to
induce air bubbles to contact and aerate the bitumen. This step is referred to
as
"conditioning".
The conditioned slurry is then further diluted with flood water and introduced
into a
large, open-topped, conical-bottomed, cylindrical vessel (termed a primary
separation vessel or "PSV"). The diluted slurry is retained in the PSV under
quiescent conditions for a prescribed retention period. During this period,
aerated
bitumen rises and forms a froth layer, which overflows the top lip of the
vessel and is
WSLega1\053707\00247 5206300v1 1
CA 02659938 2009-03-25
conveyed away in a launder. Sand grains sink and are concentrated in the
conical
bottom. They leave the bottom of the vessel as a wet tailings stream
containing a
small amount of bitumen. Middlings, a watery mixture containing solids and
bitumen, extend between the froth and sand layers.
The wet tailings and middlings are separately withdrawn, combined and sent to
a
secondary flotation process. This secondary flotation process is commonly
carried
out in a deep cone vessel wherein air is sparged into the vessel to assist
with
flotation. This vessel is referred to as the TOR vessel. The bitumen recovered
by
flotation in the TOR vessel is recycled to the PSV. The middlings from the
deep
cone vessel are further processed in induced air flotation cells to recover
contained
bitumen.
The bitumen froths produced by the PSV and flotation cells are combined and
subjected to cleaning, to reduce water and solids contents so that the bitumen
can
be further upgraded. More particularly, it has been conventional to dilute
this
bitumen froth with a light hydrocarbon diluent, for example, with naphtha, to
increase
the difference in specific gravity between the bitumen and water and to reduce
the
bitumen viscosity, to thereby aid in the separation of the water and solids
from the
bitumen. This diluent diluted bitumen froth is commonly referred to as
"dilfroth". It is
desirable to "clean" dilfroth, as both the water and solids pose fouling and
corrosion
problems in upgrading refineries. By way of example, the composition of
naphtha-
diluted bitumen froth typically might have a naphtha/bitumen ratio of 0.65 and
contain 20% water and 7% solids. It is desirable to reduce the water and
solids
content to below about 3% and about 1`)/0, respectively.
Separation of the bitumen from water and solids may be done by treating the
dilfroth
in a sequence of scroll and disc centrifuges. Alternatively, the dilfroth may
be
subjected to gravity separation in a series of inclined plate separators
("IPS") in
conjunction with countercurrent solvent extraction using added light
hydrocarbon
diluent. However, these treatment processes still result in bitumen often
containing
undesirable amounts of solids and water.
WSLega1\053707\00247\ 5206300v1 2
CA 02659938 2009-03-25
More recently, a staged settling process (often referred to as Stationary
Froth
Treatment or SFT) for cleaning dilfroth was developed as described in U.S.
Patent
No. 6,746,599, whereby dilfroth is first subjected to gravity settling in a
splitter vessel
to produce a splitter overflow (raw dilbit) and a splitter underflow (splitter
tails) and
then the raw dilbit is further cleaned by gravity settling in a polisher
vessel for
sufficient time to produce an overflow stream of polished dilbit and an
underflow
stream of polisher sludge. Residual bitumen present in the splitter tails can
be
removed by mixing the splitter tails with additional naphtha and subjecting
the
produced mixture to gravity settling in a scrubber vessel to produce an
overhead
stream of scrubber hydrocarbons, which stream is recycled back to the splitter
vessel.
The froth treatment product stream is naphtha-diluted bitumen (dilbit) that is
specified to contain less than 2 c/o water and less than 0.9 c/o. High solids
content in
the diluted bitumen is detrimental to upgrading process equipment. Fine solids
are
believed to be nucleation sites for coke formation that will foul equipment
such as
heat exchangers. Solids have been shown to plug the interstitial spaces
between
catalysts, accelerating their loss of activity. Some typical streams that are
fed to
process units that utilize a catalyst, and have been identified as being prone
to
carrying high solids content are the LGO drawn off the DRUs, the feed to the
LC-
Finer, and even the HGO coming off the Coker, which is due to fine clay solids
passing through the Coker. Solids in all of these streams can accelerate the
fouling
of the catalysts in their respective processing units.
The amount of solids present in dilbit can vary depending on the type of ore
used, as
some ores may have more solids and fines content than others. Without being
limited to theory, it is further believed that alterations in the wettability
of the fine clay
materials that are being processed in the froth treatment plant may also
contribute to
an increase in fines. Thus, in some instances, it appears that more fines may
reporting to the froth treatment product stream relative to the water content
¨ a
deviation from the 'rule of thumb' that states the water-to-solids ratio in
diluted-
bitumen rich streams is about four.
WSLega1\053707\00247\ 5206300v1 3
CA 02659938 2009-03-25
For a naphtha-based gravity settling froth treatment process, such as SFT,
fine clays
have been hypothesized to cause process instabilities, particularly the
formation of
emulsions stabilized by fine clays. In addition, these fine clay particles may
contribute to the rag layer formation, a persistent multiple emulsion that
forms as a
low quality neutral-density phase in the splitter. Further, fines in the
polisher vessel
underflow may assist in the development of complex rheology that encumbers
recovery of bitumen and the efficient transport of tailings. The adsorption of
surface-
active solids at the water-diluted bitumen interface is postulated as one of
the main
contributors to rag formation and stable water-in-oil emulsions in froth
treatment.
SUMMARY OF THE INVENTION
Oil sand clays (interburden clays) are found to consist mainly of the
kaolinite and
illite clays by X-ray diffraction analysis. It was originally believed that
oil sand clays
were water wet solids and thus would partition in water phase. Surprisingly,
however, it was discovered that a portion of the interburden clay material
also
appeared to preferentially partition into the oil. Without being bound to
theory, it is
believed that the reason for the interburden clays having a hydrophobic
behavior
might be due to the presence of naphthenic acid in oil sand. In general,
process
water contains 100 ppm or 0.01% naphthenic acid and oil sand contains up to
500
ppm. Thus, the presence of naphthenic acid may change the wettability of water
wet
clays such as kaolinite into oil wet clay.
The concept of using silicates such as sodium silicates as a process aid in
the oil
sand industry is not new. However, the prior patents and publications on
sodium
silicates addition for oil sand processing have only focused their use in the
primary
extraction process and not the upgrading of bitumen froth.
Thus, in one aspect, the present application is directed to the use of
silicates, such
as sodium silicates, in a continuous process as an additive in a diluted
bitumen froth
feed to promote the association of fine solids with the water phase.
WSLega1\053707\00247\ 5206300v1 4
CA 02659938 2009-03-25
A method for processing a bitumen froth comprising bitumen, water and solids
including fine solids for reducing the solids concentration is provided,
comprising:
= diluting the bitumen froth with a hydrocarbon diluent to form a dilfroth;
= adding a sufficient amount of a silicate to the dilfroth to cause a
substantial
amount of fine solids to associate with the water instead of the diluted
bitumen; and
= allowing the diluted bitumen to separate from the water containing the
substantial amount of fine solids to produce a dilbit having less than about
3%
by weight solids.
In one embodiment, the dilbit produced has a solids concentration of less than
about
1% by weight solids.
In one embodiment, the bitumen froth is diluted with naphtha to give a naphtha
to
bitumen ratio of about 0.5:1 to about 1:1. It is understood that the diluted
bitumen in
the dilfroth can be separated from the water containing the fine solids by any
number
of processes, such as gravity settling in staged gravity settlers, a sequence
of scroll
and disc centrifuges, gravity separation in a series of inclined plate
separators
("IPS"), optionally, in conjunction with countercurrent solvent extraction
using added
light hydrocarbon diluent, etc.
By "silicate" is meant any of a wide variety of compounds containing silicon,
oxygen
and one or more metals with or without hydrogen, for example, a sodium
silicate
having the general formula xNa2OTS102.
Without being bound to theory, it is believed that during processing of
diluted
bitumen froth, stable water-in-oil emulsions may persist due to the presence
of fine
clay solids. Further, much of the fine clay solids, for example, kaolinite and
illite,
were found to be partially oil-wet, thereby tending to associate with the
bitumen
phase rather than the water phase. The addition of silicates, for example,
sodium
meta silicate Na2SiO3, is believed to change the wettability of these clay
solids from
WSLega1\053707\00247\ 5206300v1 5
= CA 02659938 2009-03-25
oil-wet to water-wet. This allows the clay solids to settle into the aqueous
phase
rather than the oil phase. Further, it is believed that the silicates also may
break up
the water-in-oil emulsions, which may be stabilized by the partially oil-wet
clay
solids, and thus release more clay solids to the aqueous phase.
In addition, for gravity settling froth treatment processes, for example, SFT,
a rag
layer tends to form between the bitumen phase and the tailings phase. It is
believed
that this rag layer is stabilized by the presence of fine clays. The addition
of silicates
can reduce the rag layer, which may also result in better recovery and quality
of
dilbit.
DESCRIPTION OF THE DRAWINGS
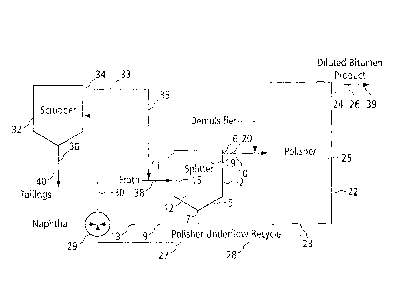
FIG. 1 is a schematic showing one embodiment of a bitumen froth treatment
process
useful in the present invention.
FIGS. 2(a), 2(b) and 2(c) are graphs showing the effect on the mass percent of
fine
solids in raw dilbit when dilfroth was treated with increasing amounts
(0.0001, 0.01
and 0.1%) of sodium silicate.
DESCRIPTION OF THE PREFERRED EMBODIMENT
In one aspect, the invention is concerned with a bitumen froth treatment
process for
reducing the fine solids content of hydrocarbon diluent-diluted bitumen. In
the
embodiment shown in FIG. 1, the hydrocarbon diluent is process naphtha. It is
understood, however, that other low molecular weight hydrocarbon diluents
could
also be used.
FIG. 1 shows a stationary bitumen froth treatment facility comprising gravity
settlers,
which can be used in one embodiment of the present invention. It is understood
that
other bitumen froth treatment facilities can also be used, for example,
facilities
comprising scroll centrifuges, disc centrifuges, inclined plate separators, or
various
combinations thereof. For example, a sequence of scroll and disc centrifuges
or a
series of inclined plate separators can be used.
WS Legal\ 053707\00247 5206300v1 6
CA 02659938 2009-03-25
Bitumen froth is initially received from an extraction facility (not shown),
which
extracts bitumen from oil sand using a water extraction process known in the
art.
The bitumen froth, as received, typically comprises about 60% bitumen, about
30%
water and about 10% solids.
With reference now to FIG. 1, a hydrocarbon diluent such as naphtha is mixed
with
bitumen froth, for example, in a mixer (not shown) to provide diluent-diluted
bitumen
froth (dilfroth). In one embodiment, the naphtha may at least partly be
supplied by
recycling scrubber naphtha, produced as described below. The naphtha is
supplied
in an amount such that the naphtha to bitumen ratio of the dilfroth is
preferably in the
range 0.5-1.0, most preferably about 0.65. A silicate, for example, sodium
silicate, is
also added to the dilfroth at a concentration ranging between about 0.0001 to
about
0.1% wt/wt or more.
The dilfroth 38 is then fed into the chamber of a gravity settler vessel,
referred to in
FIG. 1 as splitter 2, for example, through an inlet means (not shown). In this
embodiment, splitter 2 has a conical bottom 5. It has underflow and overflow
outlets
7, 6 at its bottom and top ends, respectively. The diluted bitumen froth is
temporarily
retained in the splitter 2 for a sufficient length of time to allow a
substantial potion of
the solids and water to separate from the diluted bitumen. The splitter
overflow is
referred to heretoforward as raw dilbit 20. Line 9 withdraws a stream of
splitter tails
13 through the underflow outlet 7. Splitter overflow line 10 collects an
overflow
stream of raw dilbit 20.
As previously mentioned, it is believed that silicates change the surface
properties of
the fine solids, causing them to associate with the water phase, rather than
the oil
phase. This will result in the fine solids leaving the primary settler with
the water in
the tailings streams, not the product, effectively reducing the solids content
in the
diluted bitumen (dilbit).
The rate at which dilfroth 38 is fed to the splitter 2 and the diameter of the
cylindrical
section 11 of the splitter 2 are selected to ensure a preferred flux of <10
m/h, for
example, in a range between about 3 to about 9 m/h. The bottom layer 12 of
splitter
V/SImgal\053707\00247\ 5206300v1 7
CA 02659938 2009-03-25
tails 13 comprises mainly sand and aqueous middlings, said tails containing
some
hydrocarbons, and the top layer 19 of raw dilbit 20 comprises mainly
hydrocarbons
containing some water and a reduced amount of fines (clay particles).
Preferably,
the incoming dilfroth 38 may be introduced into the middlings 15 across the
cross-
section of the splitter 2, at an elevation spaced below the top layer of raw
dilbit 20
and well above the underflow outlet 7.
Preferably, the rates of feeding dilfroth 38 and withdrawing splitter tails 13
are
controlled to maintain the elevation of the interface generally constant. It
is of
course desirable to keep the interface away from the bottom of the splitter 2,
to
minimize hydrocarbon losses with the splitter tails 13. For example, one may
monitor the composition of the splitter tails 13 and vary the rates with the
objective of
keeping the splitter tails hydrocarbon content below a predetermined value,
usually
less than 35% hydrocarbon (i.e., naphtha plus bitumen), or less than 20%
bitumen.
The raw dilbit 20 produced through the splitter overflow outlet 6 routinely
comprises
less that about 3% solids. However, it may be desirable to decrease the solids
concentration even more. Thus, in one embodiment, the raw dilbit 20 is pumped
through line 10 to a second gravity settler vessel, preferably a flat-
bottomed, vapor-
tight tank, referred to as the polisher 22, and subjected to further gravity
settling
therein. It is understood that a cone bottomed tank could also be used. A
demulsifier as known in the art may be added to the raw dilbit 20 as it moves
through the line 10. In this embodiment, the polisher 22 has a bottom
underflow
outlet 23 and a top overflow outlet 24.
The raw dilbit and optional demulsifier is temporarily retained for a
prolonged period
(for example, <24 hours) in the polisher chamber 25. Water droplets coalesce
and
settle, together with most of the remaining fine solids. Polisher dilbit 39 is
removed
as an overflow stream from the polisher 22 through line 26. The polisher
dilbit 39 is
found to comprise hydrocarbons, typically containing <3.0 wt. A) water and
<1.0 wt.
% solids. Polisher sludge 27, comprising water, solids and typically between
about
WSLega1\053707\00247\ 5206300v1 8
CA 02659938 2009-03-25
20-70% hydrocarbons, or 12-40% bitumen, is removed from the polisher 22 as an
underflow stream through line 28.
The splitter tails 13 produced through the splitter underflow outlet 7 are
pumped
through line 9, optionally first to a mixer/vessel 29, where it is mixed with
polisher
sludge 27 and naphtha to produce a gravity settler feed 30 preferably having a
naphtha:bitumen ratio in the range 4:1 to 10:1, more preferably about 6:1 to
about
8:1 or greater. Additional sodium silicate may be added to the gravity settler
feed 30
prior to introducing the feed to a third gravity settler vessel, scrubber 32.
In one
embodiment, less than 0.1% wt/wt sodium silicate is added. The gravity settler
feed
30 (with or without additional sodium silicate) is then temporarily retained
in the
scrubber 32 (for example for 20 to 30 minutes) and subjected to gravity
settling
therein.
The scrubber overflow stream 33 of hydrocarbons, mainly comprising naphtha and
lighter bitumen, is removed through an overflow outlet 34 and in one
embodiment
may be recycled through line 35 to splitter 2. Scrubber underflow stream of
scrubber
tails 36, comprising water and solids containing some hydrocarbons, is removed
via
line 40 and forwarded to a naphtha recovery unit (not shown).
Example 1
A bench-scale pilot plant of a continuous naphtha-based froth treatment
gravity
setting operation with a configuration similar to that shown in FIG. 1 was
used in the
following example. Varying amounts of sodium silicate (0.0001, 0.01 and 0.1%
sodium silicate, wt/wt based on bitumen froth) were added to bitumen froth
diluted
with naphtha. In this example, bitumen froth contained an average of about
60.1
wt% bitumen, about 28.4 wt% water and about 11.5 wt% solids. The naphtha to
bitumen ratio was 0.6.
The treated dilfroth was then fed to a splitter vessel and the splitter
overflow (raw
dilbit) was analyzed as to the mass percent of fine solids that are present in
the
splitter overflow versus the diameter (pm) of the fines. FIGS. 2(a) to 2(c)
show that
WSLega1\053707\00247\ 5206300v1 9
CA 02659938 2009-03-25
the addition of sodium silicate resulted in less fine solids (e.g., solids
having a
diameter less than 10 pm) present in the raw dilbit when compared to no
addition of
sodium silicate. Further, the reduction in fine solids was shown to be dose
dependent. Thus, a total reduction in solids was shown to be ¨0.1% absolute or
¨10% solids reduction in splitter overflow product (raw dilbit). The amount of
solids
in the raw dilbit is further reduced in the polisher, resulting in a polisher
overflow
product (polished dilbit) having less than 3%, and more likely, less than 1 A)
solids.
Example 2
Bench-scale batch tests were also performed to see what effect the addition of
sodium silicates would have on the stable rag layer that forms between the
diluted
bitumen layer and the water layer in the splitter vessel during gravity
settling of the
diluted bitumen froth. It is believed that the rag layer may be a result of
stable water-
in-oil emulsions persisting, primarily due to the clay solids present in the
diluted
bitumen froth. The rag layer is a mixture of partially oil-wet solids, oil and
water-in-oil
emulsions. Much of the clays solids are kaolinite and illite. The formation of
such a
rag layer prevents complete separation of the diluted bitumen from the water
and
solids.
In this example, bitumen froth containing water and fine solids including
clays
(approximately 60% bitumen, 30% water and 10% fine solids) was first diluted
with
naphtha to give a dilfroth having a naphtha to bitumen ratio of about 0.7:1.
Sodium
meta silicate (Na2SiO3) from a 10-4M solution was then added to the dilfroth
to
increase the pH from about 8.2 to about 8.5 and the dilfroth was allowed to
stand for
several minutes to allow the diluted bitumen to collect at the top and the
water and
fine solids collect at the bottom.
When compared to untreated dilfroth, Na2S103-treated dilfroth had a much less
pronounced rag layer. Without being bound to theory, it is believed that the
addition
of Na2SiO3 makes the clay solids more water-wet, which can enhance the
coalescence of emulsions. Further, if the clay solids become more water-wet,
in the
rag layer, some of the adsorbed oil on the solid surface can be replaced by
water,
WSLega1\053707\00247\ 5206300v1 10
CA 02659938 2015-09-29
and the density of the rag material will be greater than that of water,
causing it to
settle to the bottom.
Waega1\053707100247\ 5206300v1 11